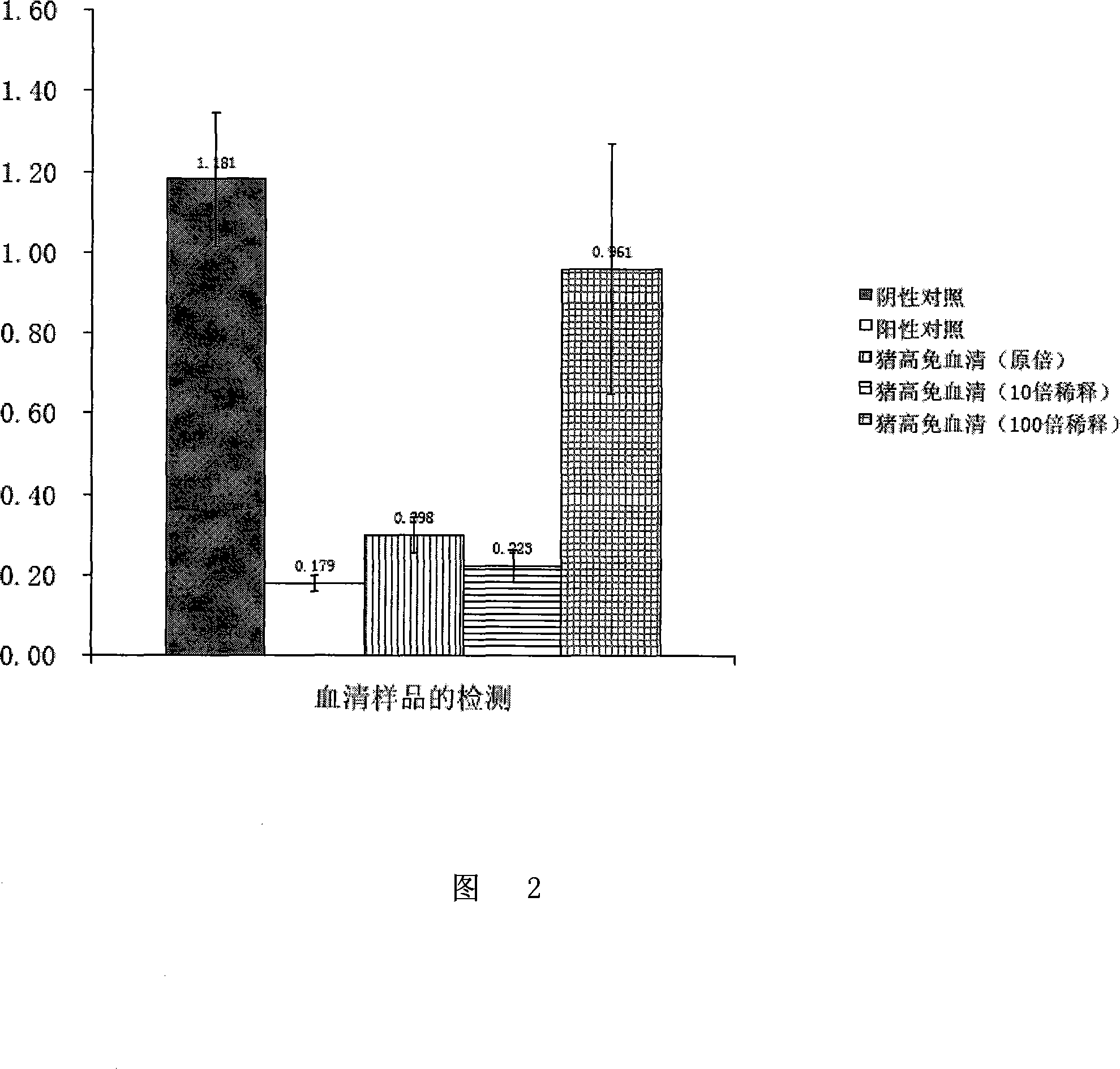
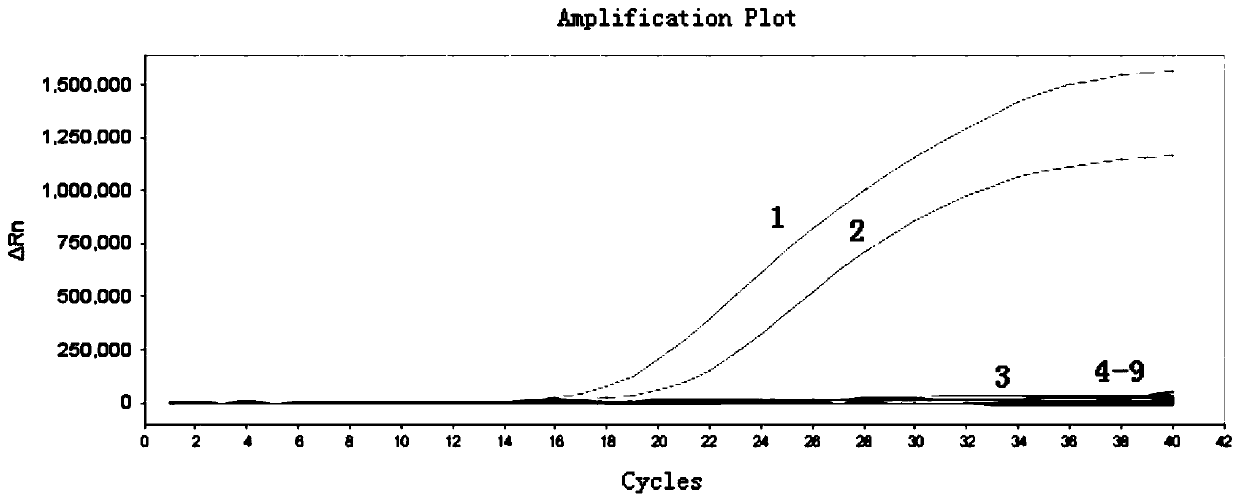
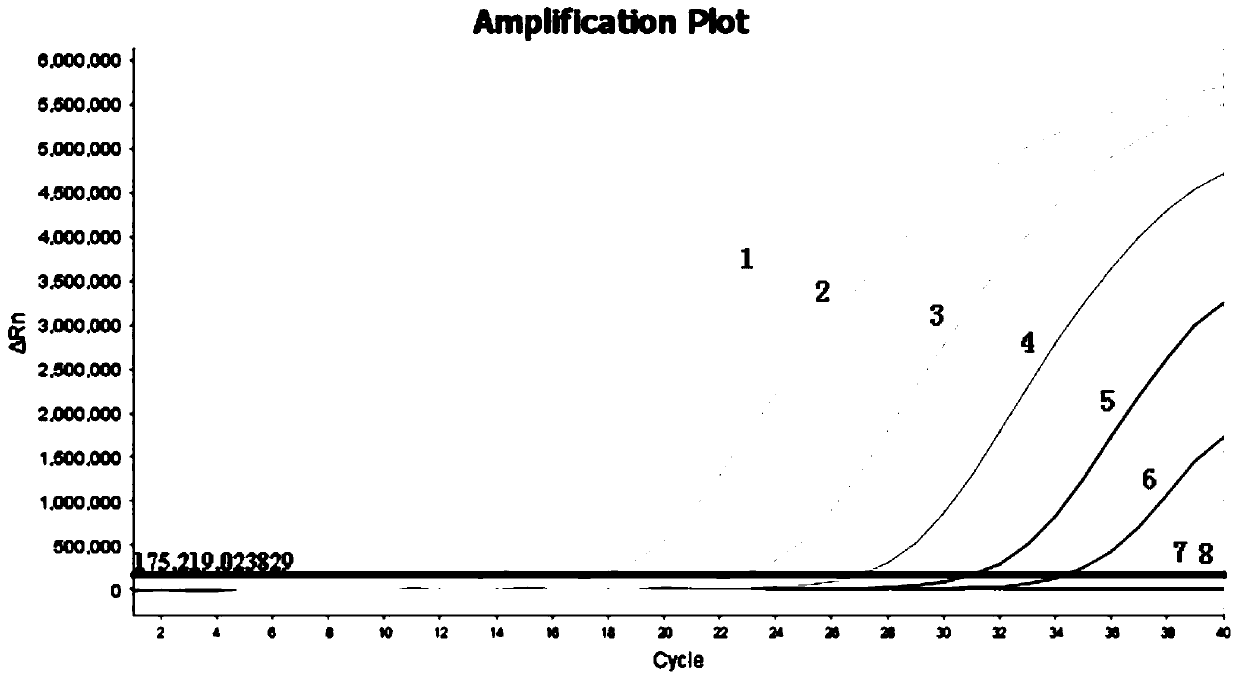
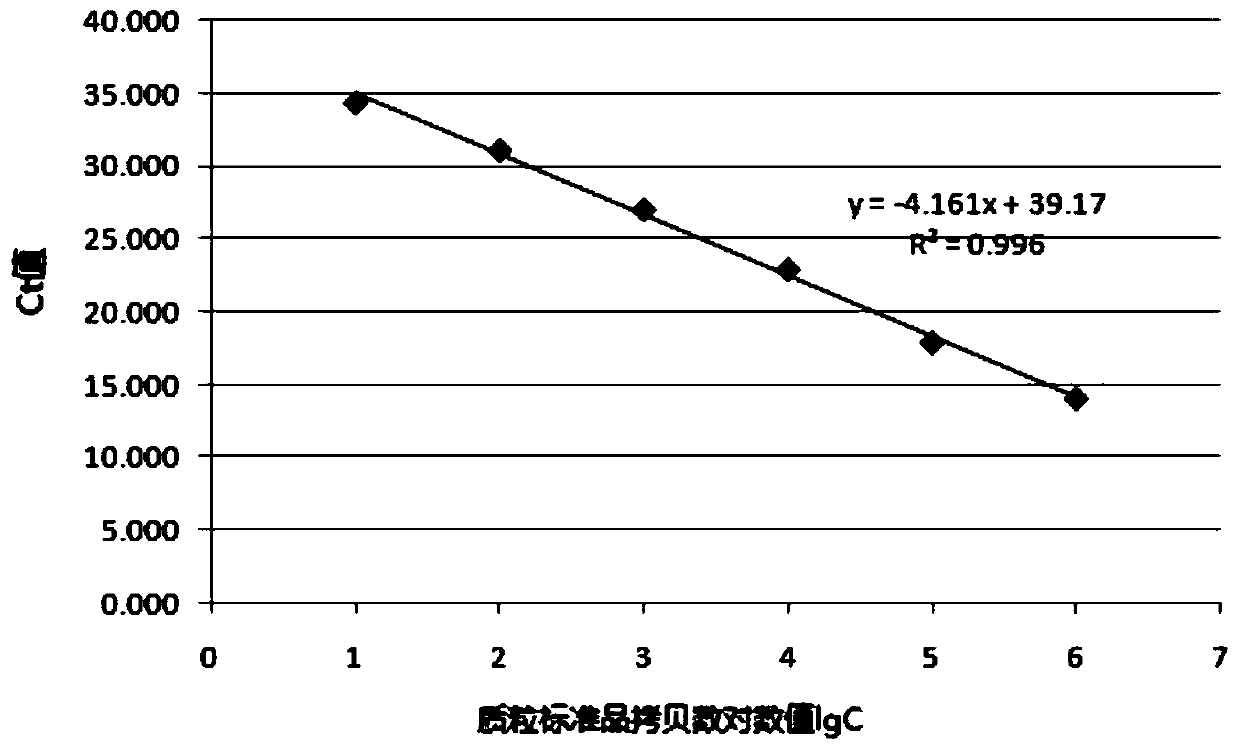
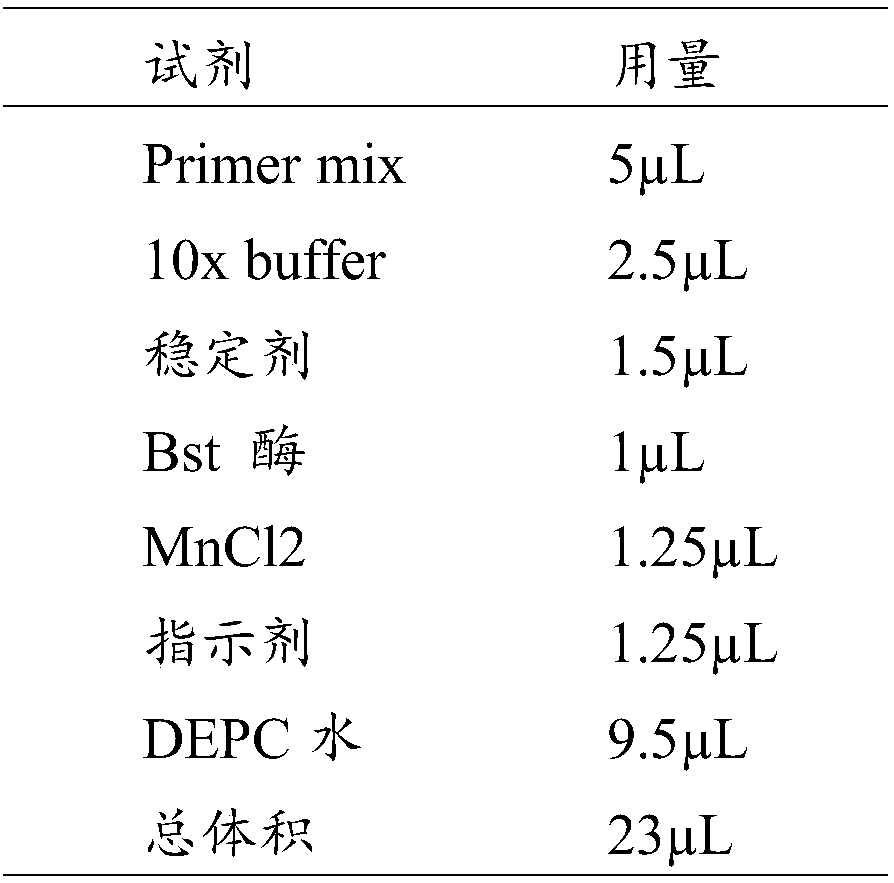
Patents
Literature
243 results about "Swine Fever Virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rapid fluorescence PCR detection kit for ASFV (African swine fever virus)
PendingCN109593893ADetection fitReduce manual operationsMicrobiological testing/measurementDNA/RNA fragmentationSerum igeFluorescence
The invention discloses a rapid fluorescence PCR detection kit for ASFV (African swine fever virus). The kit comprises specific primers ASFV-F and ASFV-R as well as a TaqMan probe ASFV-P, the 5' end of the probe labels fluorescence dye as FAM and the 3' end labels a fluorescence quenching group as BHQ-1. The kit can be used for detecting the ASFV in nasal swabs, blood, serum, plasma and tissue ofswine to realize rapid detection of the ASFV. In the whole ASFV detection process, only 40 min is taken from DNA extraction to obtaining of detection results, so that the detection time is greatly shortened, and the detection efficiency is improved.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD +2
Cpf1 reagent kit and detection method for quickly detecting nucleic acid of African swine fever virus
ActiveCN110551846AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverFluorescence
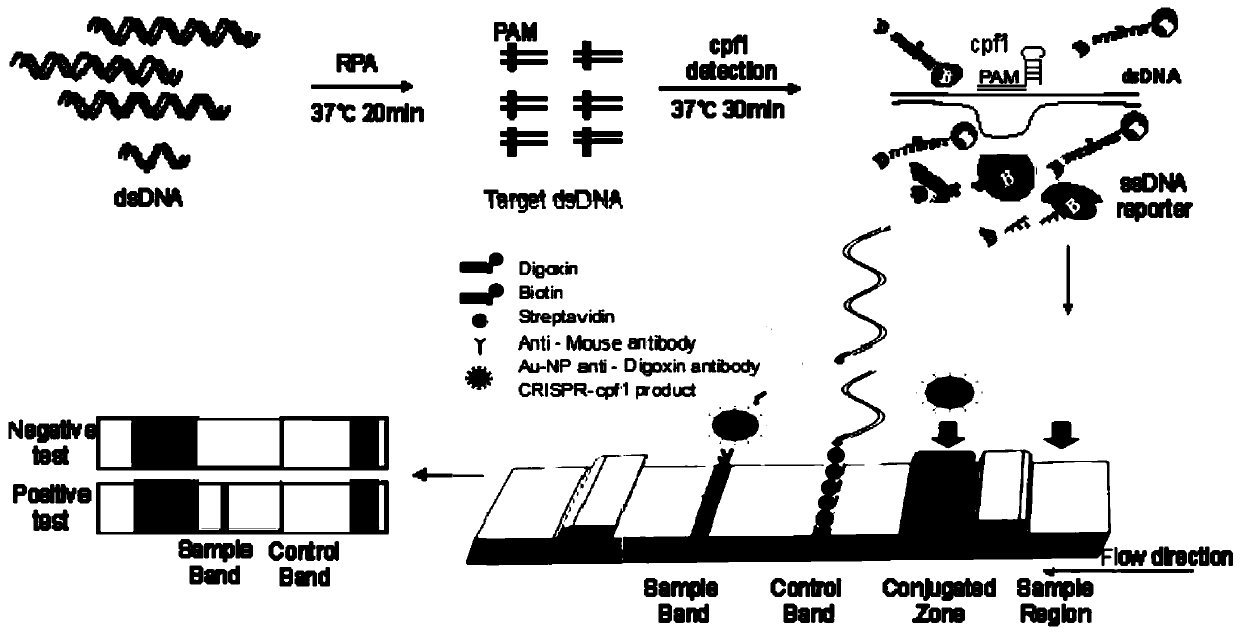
The invention discloses a Cpf1 reagent kit for quickly detecting nucleic acid of an African swine fever virus. The Cpf1 reagent kit comprises a Cpf1 detection system suitable for quickly detecting theAfrican swine fever virus, and an immune colloidal gold test strip, wherein the Cpf1 detection system comprises specific crRNA protein, specific Cpf1 protein and a single-chain DNA(ssDNA) reporting system in accordance with a p72 gene of the African swine fever virus, the specific crRNA is one or more of crRNAs from ASFV P72 crRNA1 to ASFV P72 crRNA10, and the sequence of the specific crRNA is SEQ NO.4 to SEQ NO.13; and the single-chain DNA(ssDNA) reporting system comprises ssDNA FQreporter for fluorescence detection of a microplate reader and / or ssDNA DB reporter for detecting the immune colloidal gold test strip. According to the Cpf1 reagent kit disclosed by the invention, for the first time, the Cpf1 is used for detecting the African swine fever virus, and has the advantages of beinghigh in sensitivity, high in specificity, short in time consumption, high in flux, independent of large-scale experiment equipment and the like. The advantages enable a detection method based on the immune colloidal gold test strip developed by the invention to be conveniently used in basic laboratories and breeding enterprises to be used for performing detection, identification and diagnosis on basic quick detection of the African swine fever.
Owner:SHANGHAI TECH UNIV
Method for detecting pig plague virus specific antibody and its ELISA reagent kit
InactiveCN101144818AGuaranteed specificityGuaranteed Differential DiagnosisMaterial analysis by observing effect on chemical indicatorElisa kitSwine Fever Virus
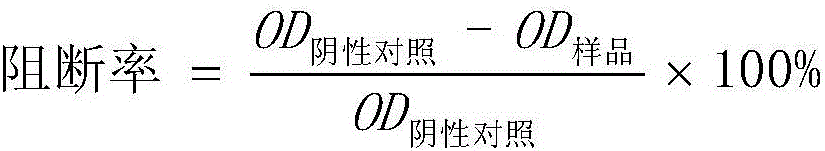
The invention discloses a method for detecting the specific antibody of classical swine fever virus and a special ELISA kit thereof. The kit includes a classical swine fever virus antigen and an enzyme-labeled classical swine fever virus single-epitope specific antibody; the said swine fever virus antigen is a polypeptide containing one or more than one amino acid residue sequence described in sequence 1. The detection reagent of the classical swine fever virus specific antibody of the present invention can carry out effective detection to the classical swine fever virus specific antibody by solid-phase antigen competition ELISA (blocking method); The B cell epitope ensures the differential diagnosis, and the high degree of conservation of the epitope among various strains ensures the specificity of detection.
Owner:TSINGHUA UNIV +2
Amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains
PendingCN110184390ARapid identificationImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationTonsilAfrican swine fever
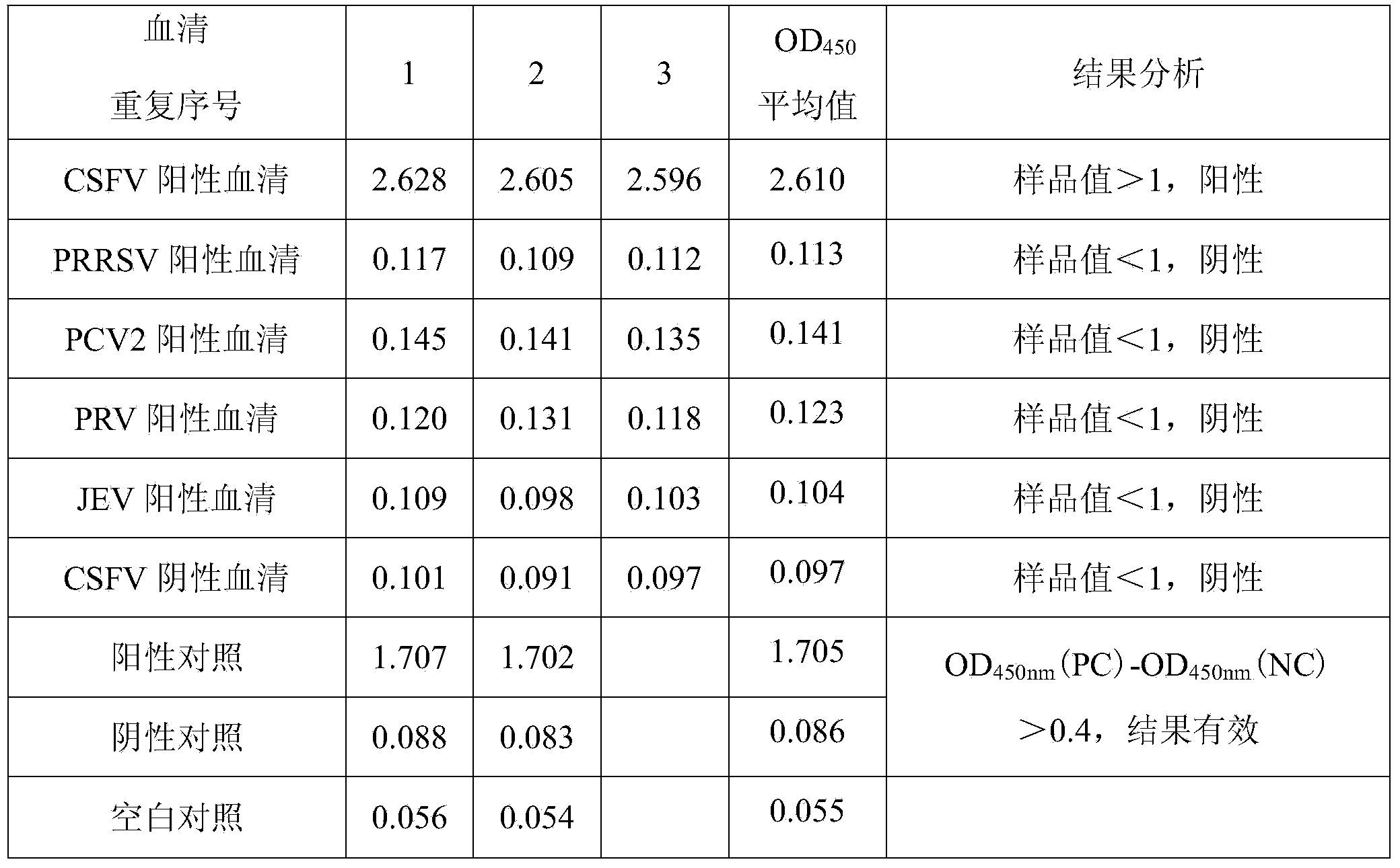
The invention provides an amphimorphic FQ-PCR detection reagent kit for identifying African swine fever and swine fever virus wild strains. A P72 gene of ASFV and a 5'UTR noncoding region of CSFV arerespectively used as an amplification target area, a pair of specific primers and a TaqMan MGB probe (SEQ ID NO:1-6) are designed, a real-time fluorescent quantitation PCR(FQ-PCR) technique is used, and identification and detection of ASFV and CSFV are realized. The detection reagent kit provided by the invention is suitable for detecting viral nucleic acid in samples of serum, spleen, lymph nodes, tonsil, kidney and the like of suspected ASFV or CSFV infected pigs, the sensitivity can reach 1.0*10<1>copy / [mu]L, and the detection reagent kit does not have any cross reactions with other pathogens which are liable to be in mixed infection with the ASFV and the CSFV or of which the infection symptoms are similar such as PRRSV, PRV, PCV2, PPV, JEV and HPS.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Swine fever vaccine heat-resistant freeze-drying protective agent, and preparation method and application thereof
The invention discloses a swine fever vaccine heat-resistant freeze-drying protective agent, and a preparation method and an application thereof. The heat-resistant freeze-drying protective agent comprises the following components: trehalose, polyvinylpyrrolidone (PVP), gelatin, sodium glutamate, D-sorbic alcohol, polypeptone, L-arginine, vitamin C and water for injection and is prepared by dissolving and degerming. The heat-resistant freeze-drying protective agent is mixed with swine fever virus antigen liquid according to the volume ratio of 1:(08-1.2) and is frozen and dried after being packaged to obtain swine fever vaccine. The prepared heat-resistant freeze-drying protective agent is simple in formula, is easy to prepare, is suitable for large-scale production and has good protection efficacy on the vaccine. The prepared vaccine has the characteristics of safety and sterility, stable quality, heat resistance and long saving time. The swine fever vaccine heat-resistant freeze-drying protective agent solves the problems that the vaccine is required to be frozen at a low temperature, is not convenient to store and the like in the transportation process. Through the swine fever vaccine heat-resistant freeze-drying protective agent, the swine fever vaccine can be saved for above 30 months at a temperature of 2-8 DEG C.
Owner:河南后羿生物工程股份有限公司
Indirect ELISA kit for detecting African swine fever virus antibody and application thereof
InactiveCN102236017AImmunoglobulinsMaterial analysisAfrican swine fever virus AntibodyPositive control
The invention discloses an indirect ELISA kit for detecting an African swine fever virus antibody and an application thereof, and belongs to the technical field of biology. The kit adopts prokaryotic expression recombinant P30 protein as a coating antigen, and detects the antibody of African swine fever virus in porcine serum based on the indirect ELISA principle. The coating antigen in a 96-well plate of the kit is prokaryotic expression recombinant P30 protein which has good antigenicity. The enzyme-linked immunoassay kit provided by the invention comprises a 96-well plate coated with P30 protein, a positive control, a negative control, a horseradish peroxidase-labeled rabbit anti-porcine IgG polyclonal antibody, a concentrated washing liquid, a serum diluent, a TMB substrate, and a terminating liquid. The kit of the invention is applicable to the screening of large quantities of samples, and main reagents in the kit are provided in a form of operating fluid which is convenient for use.
Owner:陈文刚
Recombinant virus for expressing swine fever virus E2 gene, and preparation method and application thereof
ActiveCN104178505AImprove expression levelHigh expressionViral antigen ingredientsAntiviralsPig farmsSwine Fever Virus
Owner:HUAZHONG AGRI UNIV
Multiplex real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2
InactiveCN102071259AEasy to identifyEasy diagnosisMicrobiological testing/measurementMicroorganism based processesAgricultural scienceFluorescence
The invention discloses a multiplex SYBR Green I real-time fluorescence PCR (polymerase chain reaction) detection primer and method for porcine rabies virus, porcine parvovirus and porcine circovirus type 2. The primer is obtained through synthesis according to design. The multiplex SYBR Green I real-time fluorescence PCR detection method for detecting porcine rabies virus, porcine parvovirus and porcine circovirus type 2 by utilizing the primer comprises the following steps: extracting the DNA of a sample, and then, detecting the sample by utilizing a SYBR Green I real-time fluorescence PCR reaction system and a SYBR Green I real-time fluorescence PCR amplification program. The invention has the beneficial effects that three types of viruses, namely the porcine rabies virus, the porcine parvovirus and the porcine circovirus type 2, can be simultaneously and effectively diagnosed and detected; non-specific swine fever virus, porcine reproductive and respiratory syndrome virus and swine influenza virus can not be detected; and the invention is beneficial to identification and diagnosis of the breeding disorder virus of a pregnant swine, and has better sensitivity, repeatability and stability.
Owner:HENAN AGRICULTURAL UNIVERSITY
Triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and preparation method and application thereof
InactiveCN104745731AMicrobiological testing/measurementMicroorganism based processesPositive controlAfrican swine fever
The invention discloses a triple fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection reagent for African swine fever viruses, swine fever viruses and respiratory syndrome viruses and a preparation method and application thereof. Three sets of specific primers and Taqman probes as well as positive controls specific to African swine fever virus CP530R genes, swine fever virus 5 minute-UTR genes and swine respiratory syndrome virus NSP2 genes respectively are designed and synthesized, and a rapid, easy and convenient triple fluorescent RT-PCR detection system with high specificity and high sensitivity is established by using the three sets of primers and probes, so that nucleic acids of the African swine fever viruses, the swine fever viruses and the respiratory syndrome viruses can be detected synchronously from a detected sample within 3-4 hours in a rapid, accurate, specific, safe, easy and convenient way. The detection reagent can be applied to synchronous detection of the nucleic acids of trace African swine fever viruses, swine fever viruses and respiratory syndrome viruses in hogs and relevant samples thereof.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Dual-fluorescence PCR detection reagent, kit and detection method for classical swine fever virus and African swine fever virus
PendingCN110760620AReduce workloadLow costMicrobiological testing/measurementDNA/RNA fragmentationClassical swine fever virus CSFVSwine Fever Virus
The invention discloses a dual fluorescence PCR detection reagent, a kit and a detection method for swine fever virus and African swine fever virus, and belongs to the field of animal pathogen detection. Aiming at a 5'-UTR gene of the swine fever virus and a P72 gene of the African swine fever virus, primers and probes capable of covering all strains and suitable for double detection are separately designed and screened, including two pairs of specific primers and two specific probes; the invention also describes a kit containing the primers and the probes and a PCR detection method using theprimers and the probes; the invention, through the design of the primers and the adjustment of each component of PCR, realizes purposes of one-time analysis and simultaneous detection and differentiation of the swine fever virus and the African swine fever virus on the premise of no reduction in sensitivity and specificity, which not only reduces the workload and cost of detection, but also greatly saves the detection time, thus gaining valuable time for epidemic disease prevention and treatment.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY +1
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Classic swine fever virus (CSFV) C strain E2 truncated protein and its preparation method and use
ActiveCN103588864ASimplifyEasy to purifySsRNA viruses positive-senseVirus peptidesInclusion bodiesElisa kit
The invention discloses a classic swine fever virus (CSFV) C strain E2 truncated protein and its preparation method and use. The main antigen region of the E2 gene is expressed and three pairs of disulfide bonds for maintaining the antigen space structure are expressed simultaneously so that the expressed protein has a certain space structure and satisfies natural protein characteristics and the inclusion body is conducive to protein purification. The E2 truncated protein obtained by the invention is used for building a CSFV IgG antibody ELISA detection method. A result shows that the recombinant protein has good antigenicity. The built ELISA kit adopting the CSFV C strain E2 truncated protein has good specificity, accuracy, sensitivity and repeatability after use. A substrate coloration solution used in the invention has an indication effect, and after several hours to 4 nights after the reaction, the detection result still has credibility. Therefore, the coloration system can reduced human factor influences, avoids false positive appearance, makes a negative result stable and guarantees good detection on CSFV antibodies.
Owner:SOUTH CHINA AGRI UNIV
Detection reagent and kit for African swine fever virus and application of detection reagent
PendingCN109136408AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesSwine Fever VirusAfrican swine fever virus
The invention discloses a detection reagent and kit for an African swine fever virus and application of the detection reagent. The detection reagent for the African swine fever virus comprises a primer pair, comprising upstream primer and a downstream primer which have sequences as shown by SEQ ID NO: 1 and SEQ ID NO: 2; the end 5' of the upstream primer is labeled with a first nucleic acid marker, and the end 5' of the downstream primer is labeled with a second nucleic acid marker. The method for detecting the African swine fever virus, provided by the invention, is to amplify a gene segmentin a proper length by a labeled specific primer to guarantee high specificity of detection, and the accuracy of a detection result is improved; furthermore, the detection method provided by the invention avoids processes of diluting an amplification product, hybridizing probes and the like and maintains the advantages of intuitiveness, quickness, convenience, maturation, low cost and the like of an immune colloidal gold test strip.
Owner:NANJING AGRICULTURAL UNIVERSITY
Monoclone antibody and application thereof
ActiveCN105837686AImprove accuracyMake up for single preventionImmunoglobulins against virusesAntiviralsHeavy chainSwine Fever Virus
The invention provides a monoclone antibody and application thereof. The monoclone antibody comprises a heavy chain as shown in SEQ ID No. 2 and a light chain as shown in SEQ ID No. 4. An enzyme-linked immunosorbent assay (ELISA) antibody detection kit (a blocking method) prepared with the monoclone antibody is good in sensitivity and high in specificity, and pigs infected with hog cholera virus can be detected more accurately. An ELISA antibody detection kit (a competition method) prepared with the monoclone antibody is short in detection time and can be used for quickly preliminarily screening pigs infected with the hog cholera virus and support further purification against the hog cholera virus. The monoclone antibody further has neutralizing activity and can further be used for preparing medicines for preventing and / or treating hog cholera relevant diseases.
Owner:LUOYANG PULIKE WANTAI BIOTECH
BAS-ELISA kit for detecting hog cholera virus Erns and E2 antigen
ActiveCN103760365AHigh sensitivityImprove stabilityBiological material analysisBiological testingBiotin-streptavidin complexElisa kit
The invention discloses a BAS-ELISA kit for detecting hog cholera virus Erns and E2 antigen, and belongs to the field of biological technologies and diagnosis and research of animal-borne diseases. The kit comprises an ELISA plate coated with a CSFV-Erns monoclonal antibody and a resistant-CSFV-E2 monoclonal antibody, a sample diluent, CSFV positive control and CSFV negative control, a scrubbing solution, a biotinylation rabbit-resistant CSFV-Erns polyclonal antibody and a biotinylation rabbit-resistant CSFV-E2 polyclonal antibody mixed solution, ELISA streptavidin, an enzyme developing substrate and a stop solution, wherein the two monoclonal antibodies and corresponding polyclonal antibodies have different epitopes. The kit adopts a biotin-avidin detecting system, and implements the combined detection for the hog cholera virus Erns and E2 protein, so that the sensitivity, specificity and repeatability in the detection of the hog cholera virus are greatly improved; the kit can be applied to the diagnosis and research of the hog cholera.
Owner:WUHAN CHOPPER BIOLOGY
Fluorescent quantitative PCR kit for rapid and quantitative detecting hog choleravirus and hog cholera lapinized vaccine and its use
InactiveCN1405328AQuantitatively accurateThe detection process is fastMicrobiological testing/measurementFluorescenceSwine Fever Virus
Owner:WUHAN UNIV
African swine fever virus fluorescent type RAA detecting kit
PendingCN110358867ARealize detectionEfficient amplificationMicrobiological testing/measurementDecompositionSwine Fever Virus
The invention discloses an African swine fever virus fluorescent type RAA detecting kit which comprises specific primers ASFV-F and ASFV-R and an RAA-exo probe ASFV-P. The basic groups in a target amplification subsequence are substituted by tetrahydrofuran (THF residues), dT-fluorophore and dT-quenching groups, a marked reported fluorescent dye is FAM, a fluorescent quenching group is BHQ-1, splitting decomposition can be conducted on the probe at the THF site by exonuclease in an agent, the fluorescent group can be separated from the quenching groups, and fluorescent signals are generated. By means of the kit, at the constant temperature of 37-42 DEG C, the African swine fever virus is detected within 20 minutes, the specificity is 100%, and the detection sensitivity is 10 copies per microliter. Thus, the kit has the advantages of being simple, rapid and sensitive in operation and provides effective technological means for on-site rapid detecting and screening of the African swine fever virus.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD +2
Swine fever negative and positive serum reference materials and preparation method thereof
InactiveCN101915850APreparation Program ScienceRigorous preparation proceduresBiological testingFreeze-dryingSwine Fever Virus
The invention relates to swine fever negative and positive serum reference materials and a preparation method thereof. The swine fever negative and positive serum reference materials are prepared by using China-domesticated, separated and identified swine fever viruses to immunize negative swine and adopting processes of sampling blood, separating serum, irradiating, filtering, split charging, freezing-drying, melting sealing, inspecting and the like, and by final cooperative calibration and constant value preparation. The swine fever negative and positive serum reference materials prepared by the method has the advantages that: the preparation process is scientific, strict and complete; the specificity is high; the application range is wide; and the accuracy of the constant value is high.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Prokaryotic expression protein of VP73 gene from African swine fever virus and preparation method thereof
InactiveCN102101889ANo risk of poisoningHigh detection sensitivityMicroorganism based processesPeptide preparation methodsAfrican swine fever virus AntibodySwine Fever Virus
The invention relates to a method for preparing a genetic engineering product, in particular to a prokaryotic expression protein of VP73 gene from African swine fever virus (ASFV) and a preparation method thereof. The preparation method comprises: artificially synthesizing the whole-length sequence of VP73 gene according to the sequence of the VP73 gene from ASFV in GenBank, constructing a recombinant expression vector pET32a-VP73, sequencing, verifying, transforming prokaryotic expression recipient bacteria E.coli BL21(DE3), and inducing expression by isopropyl-1-thio-beta-d-galactopyranoside (IPTG), wherein the molecular weight of the recombinant fusion protein is about 65KD. Protein purified by nickel column affinity chromatography can undergo a specific immune imprinting reaction with ASFV positive serum and avoid cross reaction with viruses such as swine fever virus, hog cholera virus, porcine circovirus, porcine reproductive and respiratory syndrome virus, swine influenza virus and pseudorabiesvirus. Experiments show the expressed protein has high detection sensitivity, and high specificity. When the antigen is used for detection, risk of spreading poison is avoided. And the antigen can be used as a detection antigen for use in an enzyme-linked immuno sorbent assay (ELISA) method for identifying an ASFV antibody.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Primer group and kit for African swine fever virus LAMP detection and application
InactiveCN109628644AEasy to operateHigh sensitivityMicrobiological testing/measurementMicroorganism based processesSwine Fever VirusColor changes
Owner:许昌佰柯蛋白与基因工程研究院有限公司
Recombinant swine fever virus E2 protein swine source monoclonal antibody and preparation method and application thereof
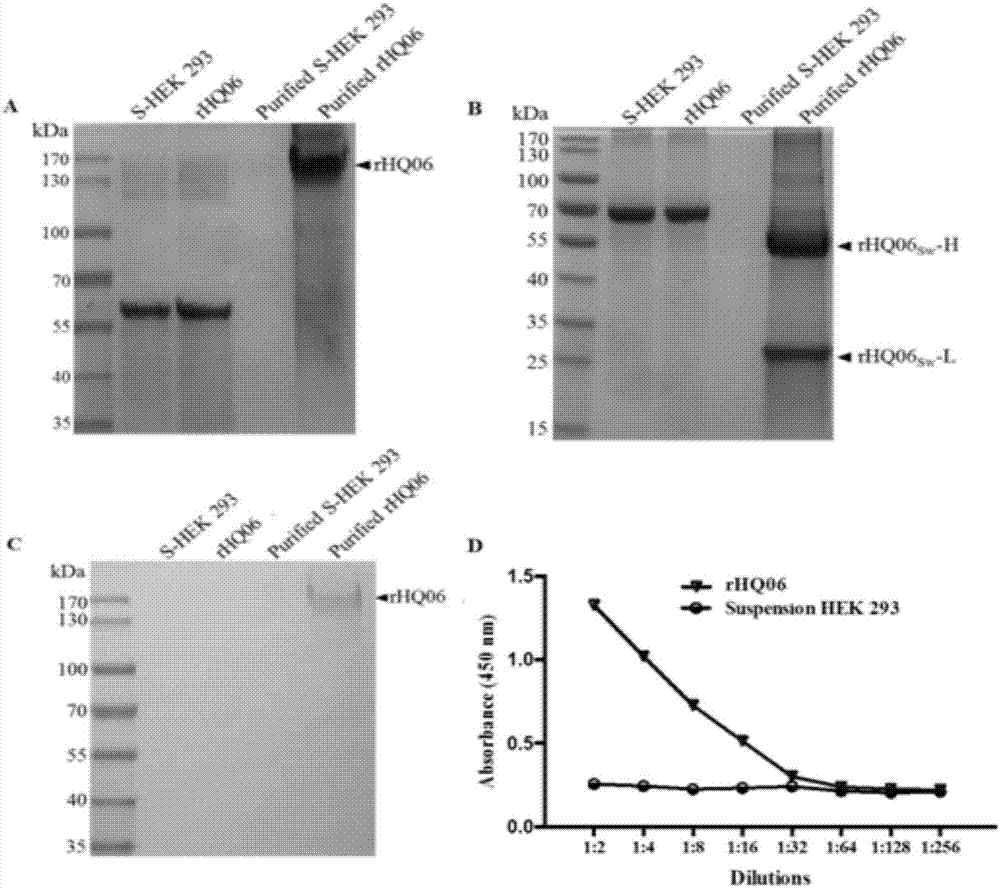
ActiveCN107973850AEasy to prepareImprove responseImmunoglobulins against virusesAntiviralsHeavy chainSwine Fever Virus
The invention discloses a recombinant swine fever virus E2 protein swine source monoclonal antibody and a preparation method and application thereof. Firstly, the recombinant swine fever virus E2 protein swine source monoclonal antibody is disclosed, an amino acid sequence of a heavy chain is shown in SEQ ID NO.1, and an amino acid sequence of a light chain is shown in SEQ ID NO.2. The invention also discloses a suspension HEK293 cell line which can stably express the recombinant swine fever virus E2 protein swine source monoclonal antibody. The recombinant swine fever virus E2 protein swine source monoclonal antibody has the advantages that the coding genes of the heavy chain and light chain are cloned into an eukaryotic expression vector, and the stable and high-efficiency expression ofthe recombinant swine fever virus E2 protein swine source monoclonal antibody is realized by the suspension HEK293 cell line; the reactogenicity and neutralizing activity are good, and the important development value is realized in development of novel swine fever virus diagnosing and treating preparations.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
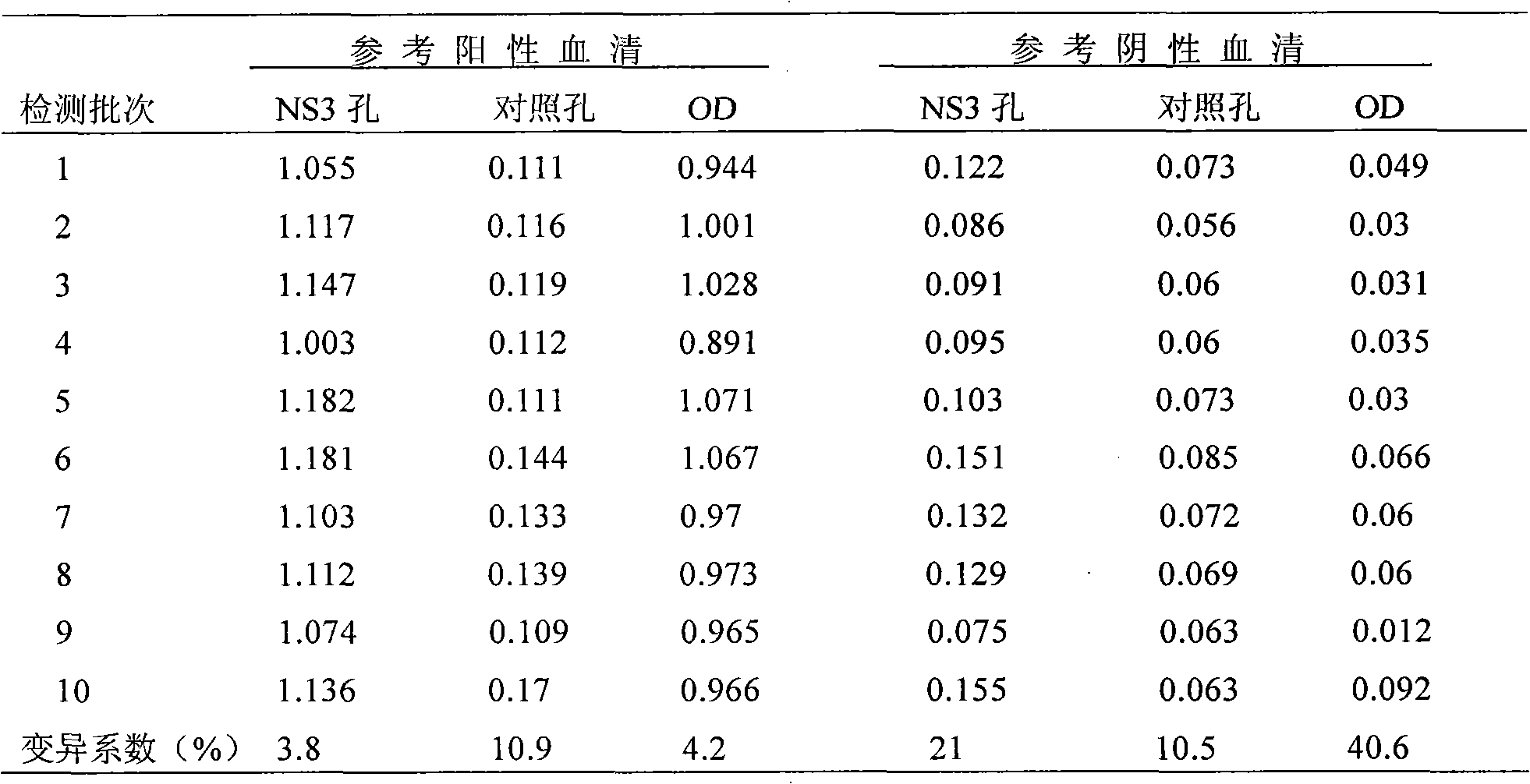
Indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) method established based on classical swine fever virus NS3
The invention relates to an indirect ELISA (Enzyme-Linked Immuno Sorbent Assay) method established based on classical swine fever virus (CSFV). The swine fever virus recombination protein NS3 is obtained by a gene clone expression technique and the indirect ELISA method is established by using the recombination protein NS3 as the envelope antigen. The method comprises the steps of preparing the antigen recombination protein NS3, establishing indirect ELIS, establishing a judging standard and carrying out clinical serology detection. The invention can be used for detecting specific antibody of CSFV NS3 in the swinery so as to judge the antibody level of CSFV NS3 in the swinery and the condition of natural infection of CSFV in the swinery, and provides a detection method for identifying ELISA in the marker vaccine in the CSFV E2 gene engineering. At present, the method firstly adopts CSFV NS3 to establish the ELISA for detecting the CSFV antibody in the swinery worldwide. The sensitivity and the specificity are obviously higher than the ELISA established based on Ems.
Owner:HUNAN AGRICULTURAL UNIV
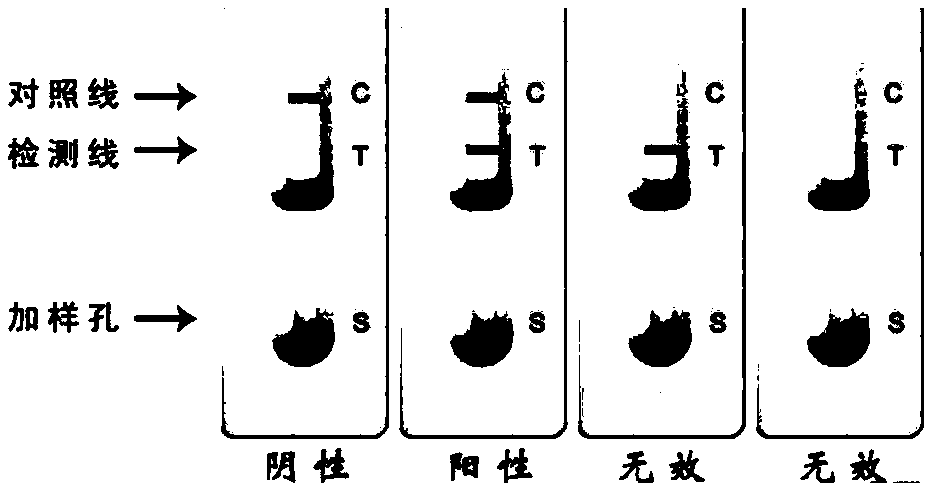
Test paper for rapidly detecting hog cholera antibody and method for making same
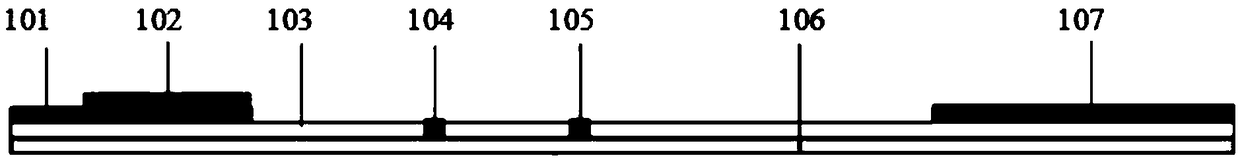
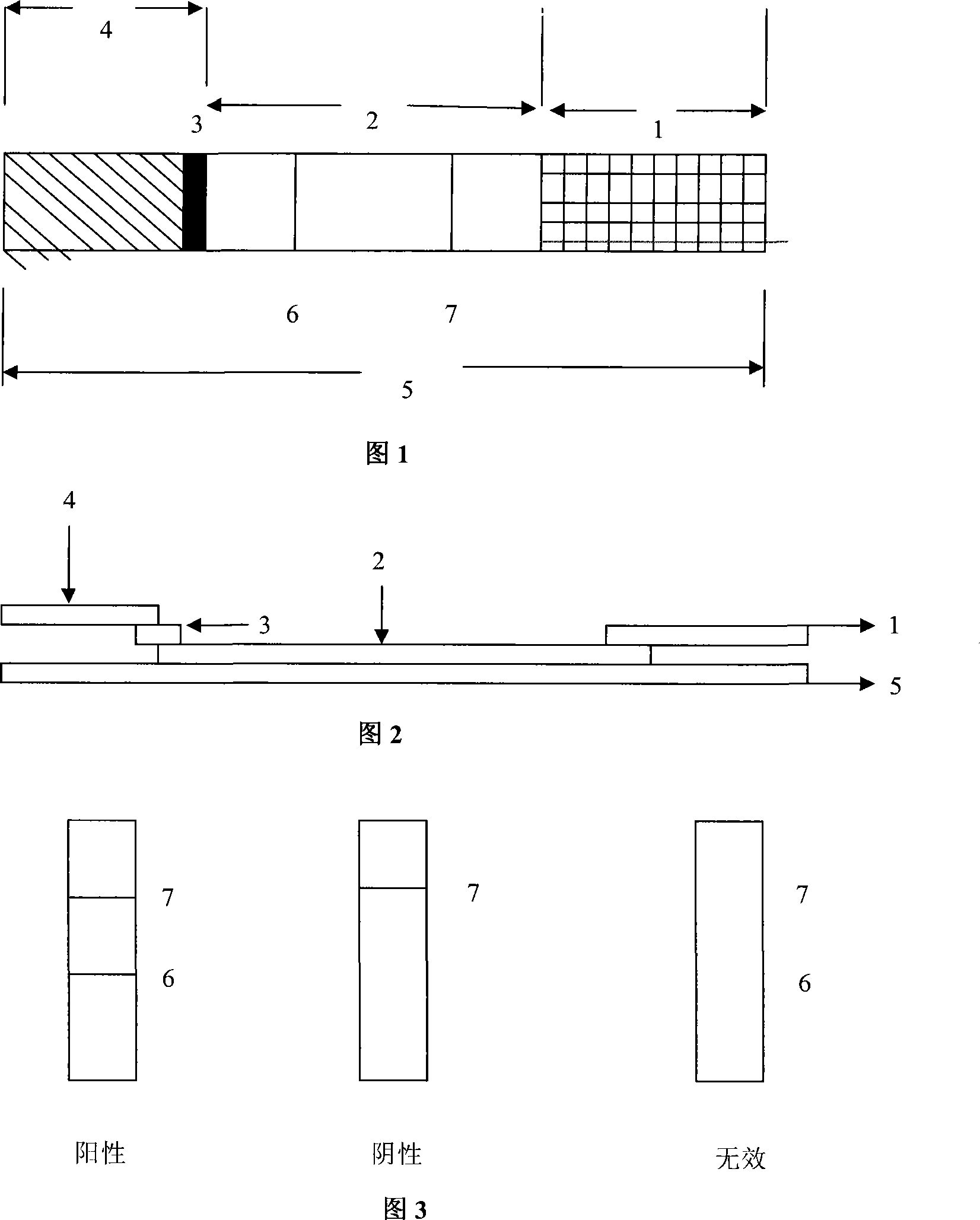
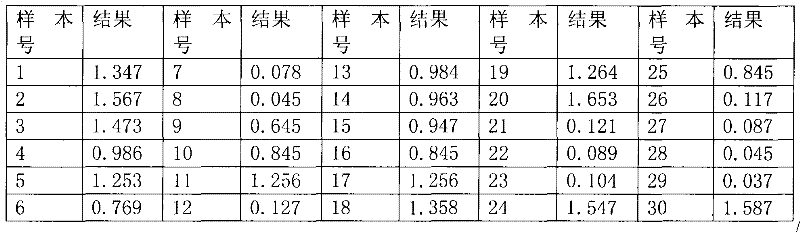
The invention discloses a test paper for rapidly detecting swine fever antibody and a preparation method thereof. The inventive test paper consists of a sample pad (4), a glass fiber member (3), a cellulose nitrate membrane (2), a water absorbent pad (1) and a support (5); wherein the cellulose nitrate membrane contains a detection line (6) coated by swine fever virus E2 protein and a reference line (7) coated with rabbit anti swine fever antibody; and the glass fiber member is combined with swine fever virus E2 protein marked by colloidal gold. The test paper can rapidly detect possibly present swine fever antibody in a sample to achieve rapid detection and timely epidemic control, thus creating favorable conditions for further separation and identification. The test paper has the advantages of convenient, rapid and easy usage, clear result, easy generalization, and no need for special instrument and equipment as well as professionals, and is suitable for large-batch onsite detection for base layer and for sudden events. The test paper is suitable for epidemic inquisition and performs assistant effect on swine fever virus infection diagnosis.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Kit for quickly detecting swine fever antibody and preparation method thereof
The invention relates to a kit for quickly detecting a swine fever antibody. In the method, a pair of specific primers is designed, a relatively conservative gene sequence is cloned, an antigen aiming at a swine fever E2 protein is expressed through a pronucleus expression technology, and, on the basis, the kit containing an enzyme linked plate coated with a high-purity and high activity swine fever virus specificity antigen, an enzyme conjugate of rabbit-anti-swine monoclonal antibody containing an HRP (Horseradish Peroxidase) marker, a TMB (Tetramethylbenzidine) color developing liquid and the like is prepared. The kit can quickly detect the swine fever antibody in blood serum or blood plasma and has strong specificity and high sensitivity.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU +2
Two-color fluorescence quantitative polymerase chain reaction (PCR) combined detection method of swine fever virus and blue ear disease virus and kit thereof
ActiveCN102212623AQuantitatively accurateAccurate detectionMicrobiological testing/measurementFluorescence/phosphorescenceDiseaseFluorescence
The invention discloses a two-color fluorescence quantitative polymerase chain reaction (PCR) combined detection method of swine fever virus and blue ear disease virus. By adopting the method, swine fever virus and blue ear disease virus in one sample to be detected can be detected and the loading level of the two viruses can be detected quantitatively. The invention also provides a kit used for the two-color fluorescence quantitative PCR combined detection of swine fever virus and blue ear disease virus. The detection method and kit are convenient and fast to operate and have high specificity and sensitive and reliable detection effect; and the minimum detection concentration is 1*10<2>copy / mu l.
Owner:广东省农业科学院兽医研究所
Swine fever-porcine circovirus combined subunit vaccine, as well as preparation method and application thereof
ActiveCN107233566AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantAnimals vaccines
The invention discloses a swine fever-porcine circovirus combined subunit vaccine, as well as a preparation method and an application thereof, and belongs to the technical fields of animal vaccines and veterinary biological products. The vaccine is prepared from swine fever virus E2 protein, porcine circovirus type 2 cap protein and a pharmaceutically acceptable adjuvant. The preparation method comprises the following steps: (1) preparing swine fever virus E2 protein and porcine circovirus type 2 cap protein; (2) preparing an antigen solution from the swine fever virus E2 protein and porcine circovirus type 2 cap protein prepared in the step (1); and (3) mixing and stirring the antigen solution and Montanide GEL 01PR adjuvant in a mass ratio of 10:1. The vaccine has the advantages of strong immunogenicity, good safety, no immune interference and the like, can be used for preventing potential biological safety hazard for virus variation and fundamentally purifying swine fever virus and porcine circovirus type 2, and can achieve the effect of dual prevention with one injection by immunizing the vaccine to achieve the aims of saving time, labor and cost.
Owner:NOVO BIOTECH CORP
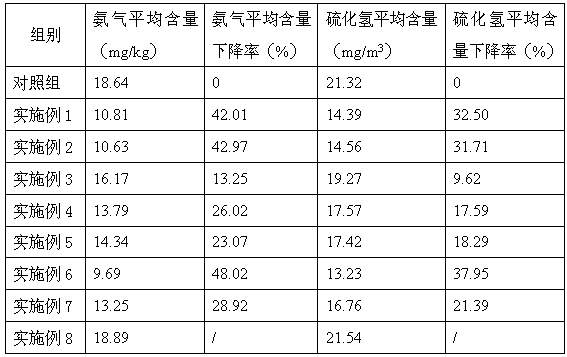
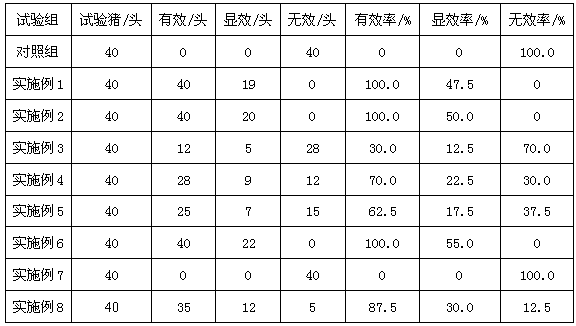
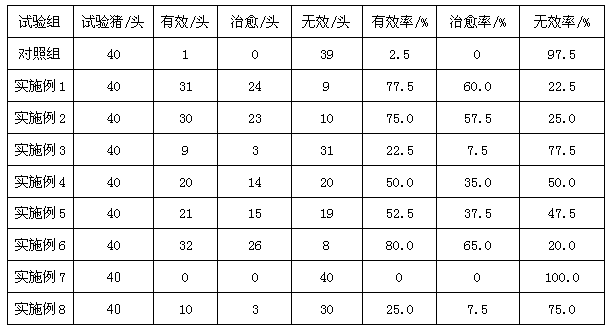
Swine feed additive with effects of enhancing immunity and deodorizing
The invention discloses a swine feed additive with effects of enhancing immunity and deodorizing. Through oligosaccharide in a formula, intestinal environment of the swine is regulated, and release of ammonia gas and hydrogen sulfide is reduced, so as to improve the environment of a pigsty; and an echinacea extract and other materials in the formula can combine to enhance the immunity of the swine and to take a quite strong effect on inhibiting swine fever viruses, so as to prevent and treat the swine fever. The swine feed additive disclosed by the invention can remarkably reduce contents of nitrogen, phosphor, hydrogen sulfide, organic amine and the like in the swine manure, and has a good deodorizing effect. The feed additive further can enhance the immunity of the swine, and take a quite strong effect on inhibiting swine fever viruses, so as to prevent and treat the swine fever, improve the health degree of the swine, reduce disease possibility of the healthy swine and reduce the death rate of the sick swine.
Owner:韶关金苹果饲料有限公司
Competitive Alpha LISA (linked immuno sorbent assay) detection kit for classical swine fever virus (CSFV) antibody and detection method thereof
InactiveCN103499693AStrong specificityHigh sensitivityBiological testingImmunoassaysAntigenSerum ige
The invention discloses a competitive Alpha LISA (linked immuno sorbent assay) detection kit for a classical swine fever virus (CSFV) antibody and a detection method thereof. The detection kit comprises donor microspheres, receptor microspheres, a swine fever virus E2 protein monoclonal antibody and an E2 protein antigen with a His label. A competitive Alpha LISA detection method for the CSFV antibody is created by optimizing test reaction conditions such as the donor microspheres, the receptor microspheres, the monoclonal antibody, the antigen and serum. The kit for detecting the CSFV antibody is good in specificity, high in sensitivity, low in usage amount of the serum, low in detection cost and short in detection time, does not need to be washed and can not be influenced by hemolysis.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
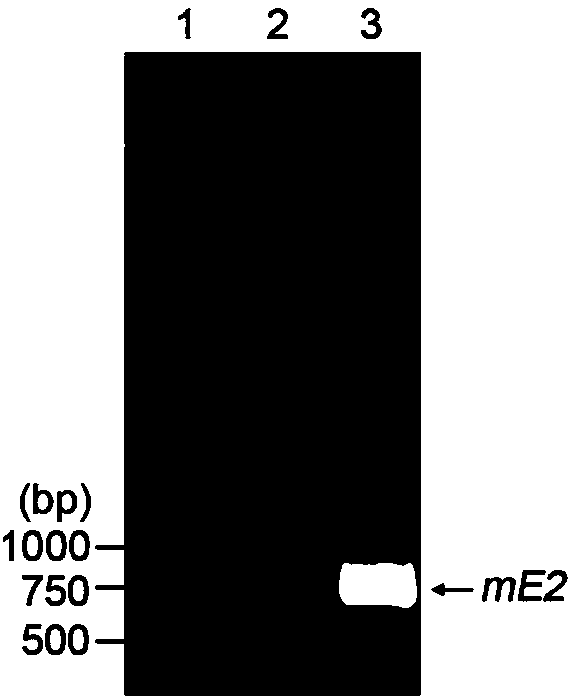
Swine fever virus subunit vaccine and preparation method and purpose thereof
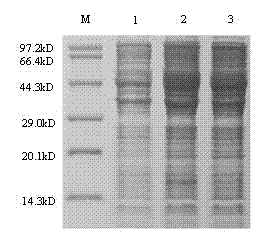
The invention belongs to the technical field of biological medicine, and concretely relates to a swine fever virus subunit vaccine and a preparation method and a purpose thereof. The invention provides a kluyveromyces marxianus yeast recombination strain used for preparing the swine fever virus subunit vaccine. The recombination strain is constructed by the following steps: swine fever virus envelope protein E2 is intercepted, through codon optimization, a coding sequence of the swine fever virus mE2 protein is obtained, and then is cloned to a kluyveromyces marxianus yeast expression vector,and the kluyveromyces marxianus yeast host strain is transformed. The invention also provides the method for preparing the swine fever virus subunit vaccine, which comprises the following steps: the kluyveromyces marxianus yeast host strain is subjected to recombination expression by using mE2, steps of culture, centrifugation, cell disruption, and separating purifying are carried out to obtain the swine fever virus mE2 protein antigen, and the purified antigen and an adjuvant are subjected to complex formulation to prepare the swine fever virus subunit vaccine. The injection immunotherapy ofswine fever virus mE2 protein recombination subunit vaccine can obtain a protective IgG antibody, and the subunit vaccine can reduce and prevent the swine fever virus infection-related disease.
Owner:FUDAN UNIV
Citric acid composite disinfectant
ActiveCN102228058AReduce pollutionImprove the bactericidal effectBiocideDisinfectantsEscherichia coliDisease
The invention relates to a citric acid composite disinfectant, which is composed of mixed components of, by weight: 12 to 32 parts of organic chlorine, 3 to 13 parts of citric acid, and 55 to 85 parts of sodium sulfate. When in use, according to different demands, the composite disinfectant can be dissolved with water, and can be diluted to a required concentration. After the composite disinfectant is dissolved, good sterilizing efficacy is maintained, disinfection effect is substantially improved, irritating odor is small, and stability of the product is enhanced. The product is safe and environment protecting, and has characteristics of broad spectrum and high efficiency. The product has substantial killing effects against pathogenic microorganisms such as foot-and-mouth disease virus, swine fever virus, swine vesicular disease virus, Newcastle disease virus, bursa disease virus, escherichia coli, and staphylococcus.
Owner:SHANXI XINYUAN HUAKANG CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com