Swine fever-porcine circovirus combined subunit vaccine, as well as preparation method and application thereof
A pig ring and dual subunit technology, which is applied in the field of swine fever-porcine ring dual subunit vaccine and its preparation, can solve the problems of difficulty in rational arrangement of immunization procedures for various diseases, hidden dangers of biological safety, immune interference, etc. Prevention and protection of infection, strong immunogenicity, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of classical swine fever virus E2 protein and porcine circovirus type 2 Cap protein
[0030] 1.1 Preparation of swine fever virus E2 protein: refer to the preparation of swine fever protein in the invention patent method or other preparation method of swine fever virus E2 in the application number 200810178235.4 or 200310103408.3 or 200810150304.0 or 201310300549.8 or 200810178235.4 or 201610625392.X or 201510187995.1 For E2 protein, full-length CSF E2 protein or truncated CSF E2 protein can be prepared according to actual needs.
[0031] 1.2 Preparation of porcine circovirus type 2 Cap protein: refer to the preparation method of porcine circovirus type 2 Cap protein in the invention patent with application number 201310050003.1 or 201210270504.6 or 201110100331.9 or 201110053536.6 or 201010618223.6 or the preparation method in other patents or literatures to prepare pigs The circovirus type 2 Cap protein can prepare full-length porcine circovi...
Embodiment 2
[0032] Embodiment 2: the preparation of swine fever-porcine circular double subunit vaccine (illustrate with preparation 1ml / head part, totally 220g as example)
[0033] The consumables and materials used for the preparation of vaccines need to undergo aseptic treatment in advance, and the preparation process is completed in a biological safety cabinet or other instruments or environments that can ensure that the entire preparation process is sterile.
[0034] 1. Preparation of Montanide GEL 01PR (adjuvant): According to the mass ratio of antigen solution and adjuvant of 10:1, weigh 20g (about 20ml) of adjuvant and place it in a pre-prepared reagent bottle, seal it, and wait for use.
[0035] 2. According to the mass ratio of antigen solution and adjuvant of 10:1, the total mass of antigen solution is 200g (about 200ml). According to the concentration of classical swine fever virus E2 protein and porcine circovirus type 2 Cap protein concentration and the content of each prot...
Embodiment 3
[0040] Embodiment 3: Immunization experiment of swine fever-porcine circular double subunit vaccine
[0041] 3.1 Vaccine preparation: Proteins and vaccines were prepared according to the methods of Examples 1 and 2, and the specific vaccine information is shown in Table 2 below:
[0042] Table 2
[0043]
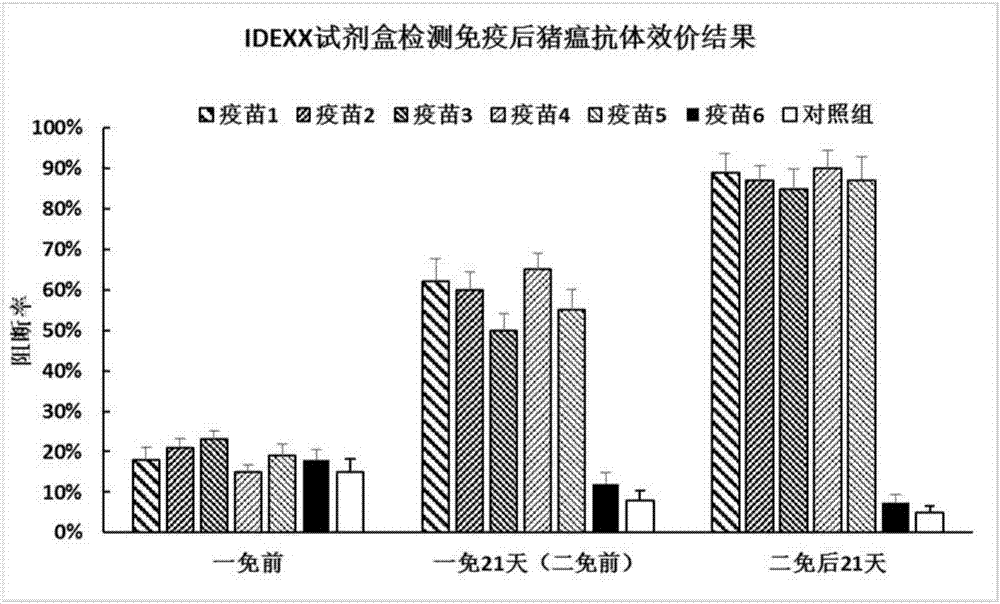
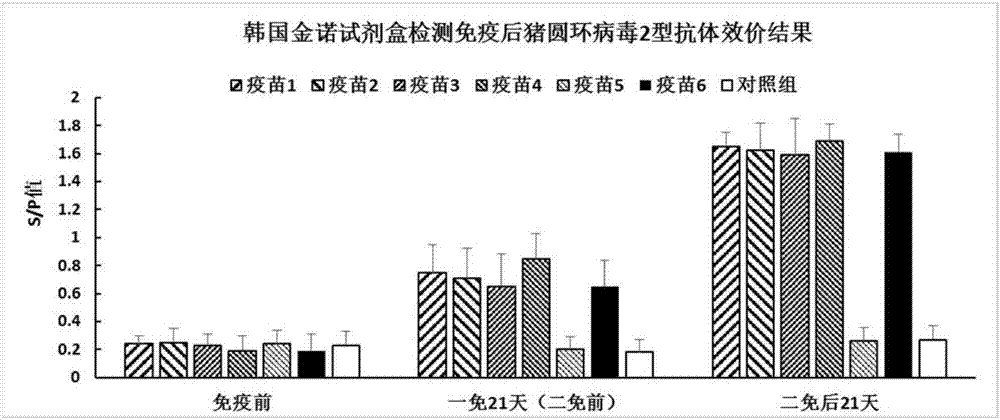
[0044] 3.2 Immunization experiment: 35 piglets aged 28-35 days (negative for CSFV and PCV2 antigen antibodies) were randomly divided into 7 groups with 5 pigs in each group. The blank control group was injected intramuscularly with 1ml of normal saline each time, and the other 6 groups of immunized groups were injected intramuscularly with 1ml of the corresponding vaccine each time. A booster immunization was given three weeks after the initial immunization. Serum was collected before immunization, before the second immunization and 21 days after the second immunization, and tested. Antibody titer.
[0045] Experimental results such as Figure 1a As shown, the test resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com