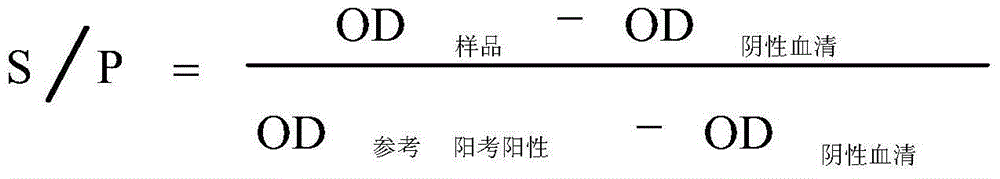
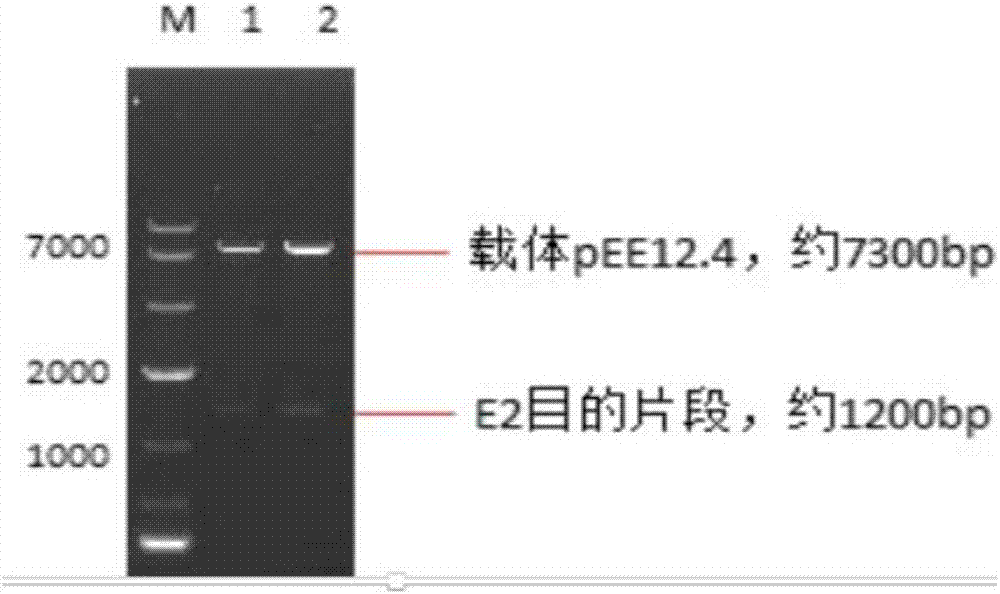
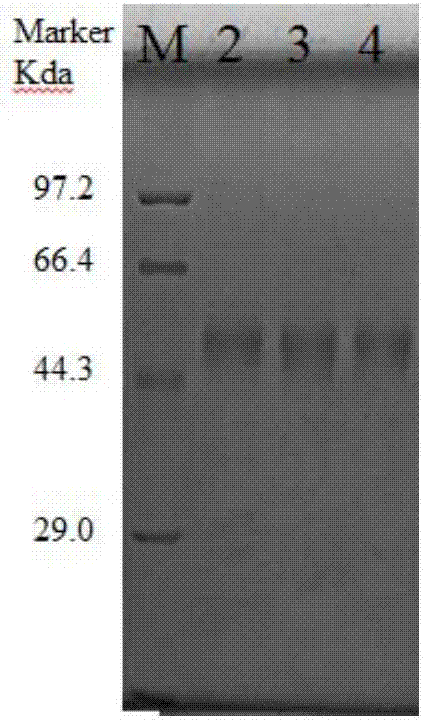
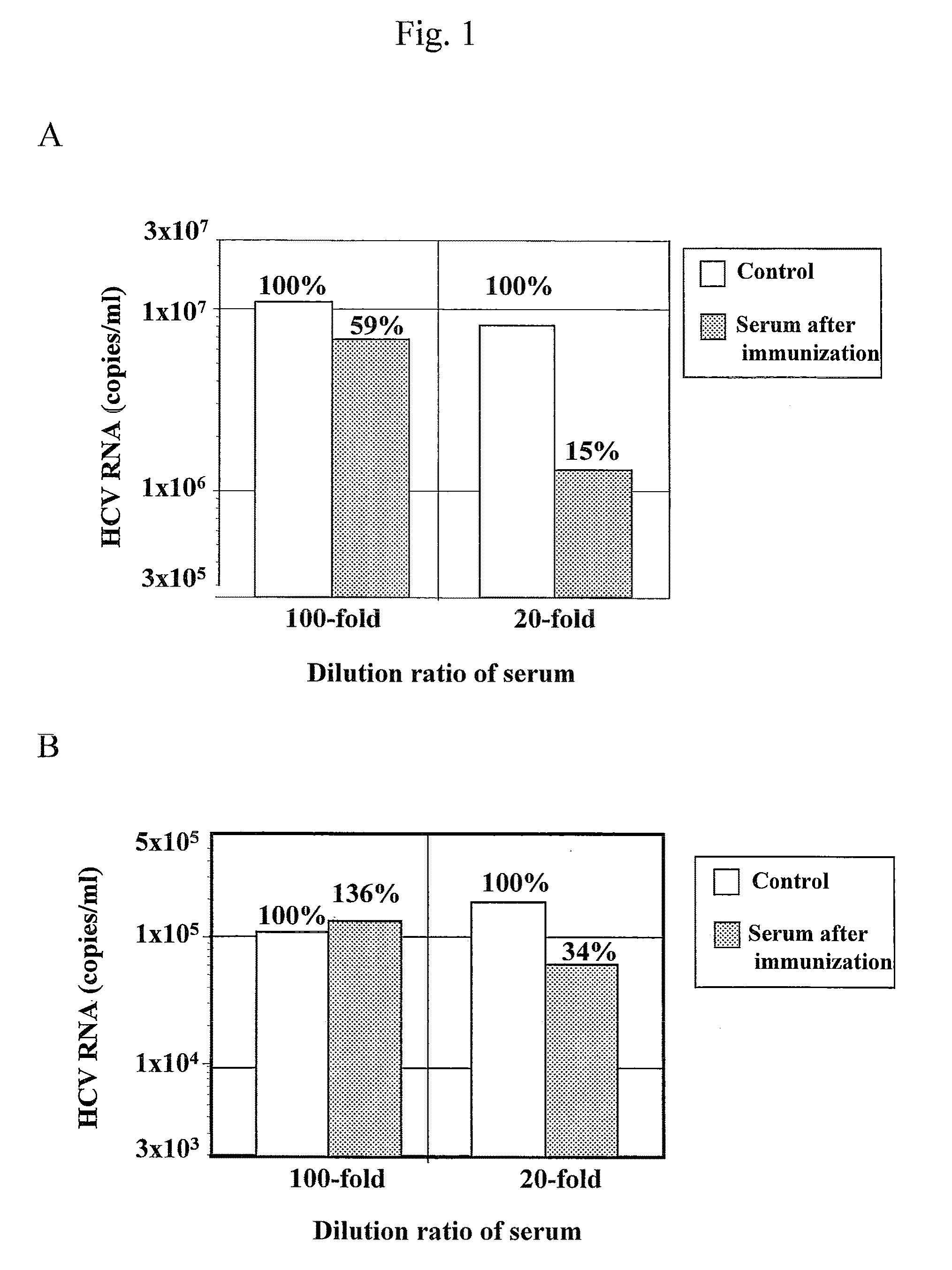
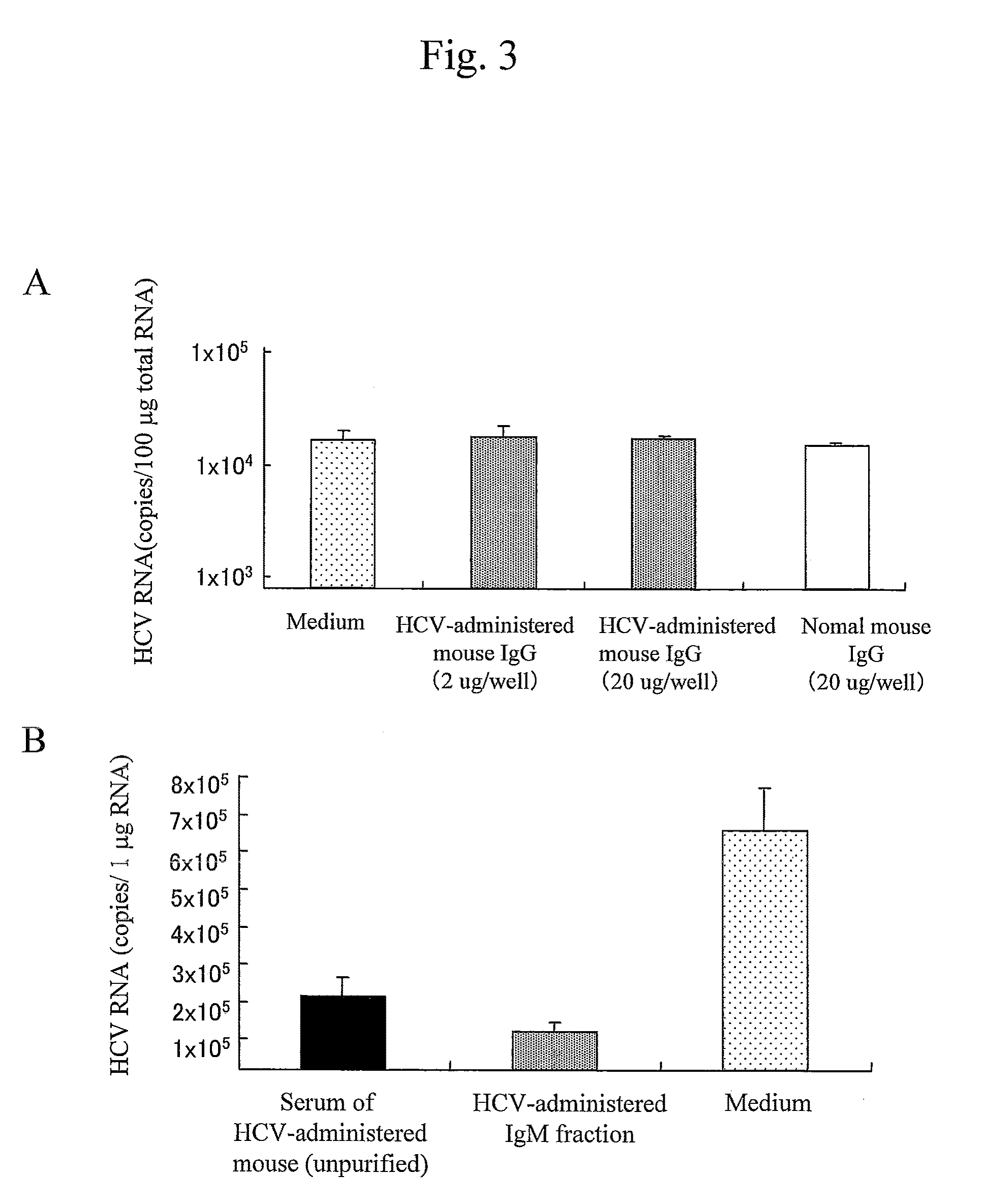
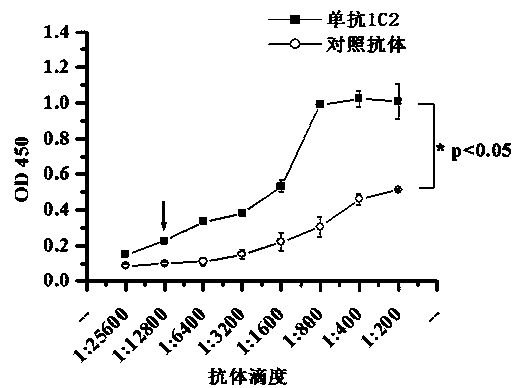
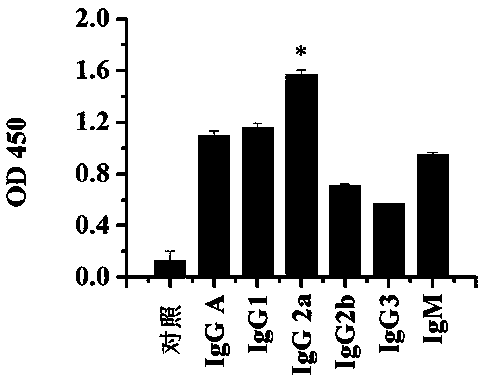
Patents
Literature
181 results about "E2 protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
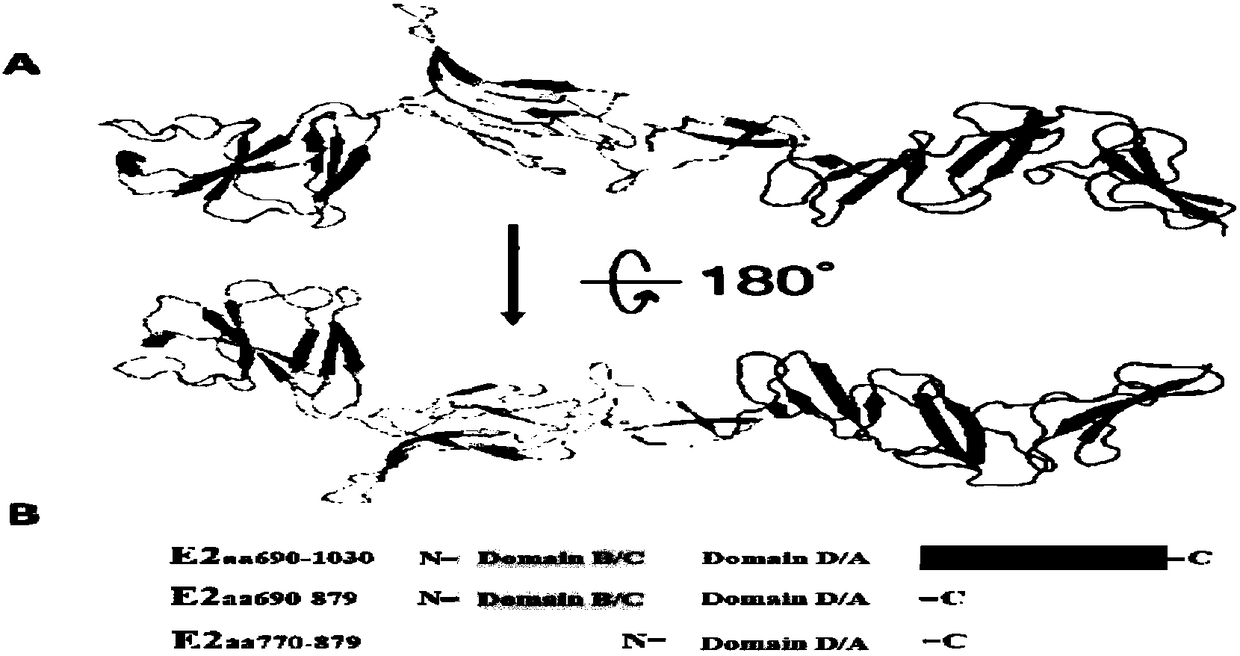
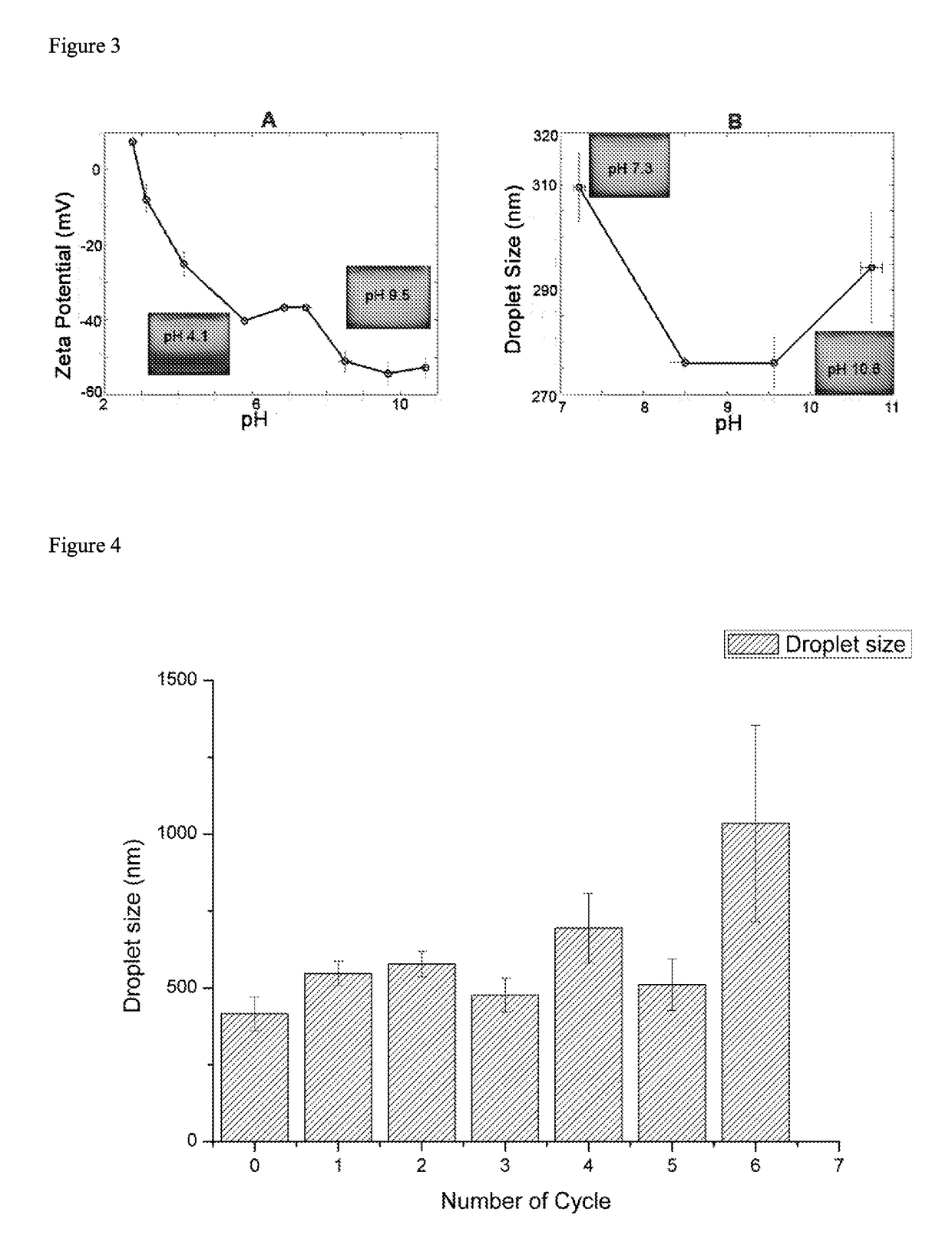
E2, the receptor binding protein, contains residues critical for immunogenicity, host range, and tissue/cell tropism ( Voss et al, 2010 ). The E2 protein consists of three domains (A, B, and C), of which A and B have been found to contain the majority of residues that affect cell attachment and or tissue/cell tropism.
Prevention and treatment of HCV infection employing antibodies that inhibit the interaction of HCV virions with their receptor
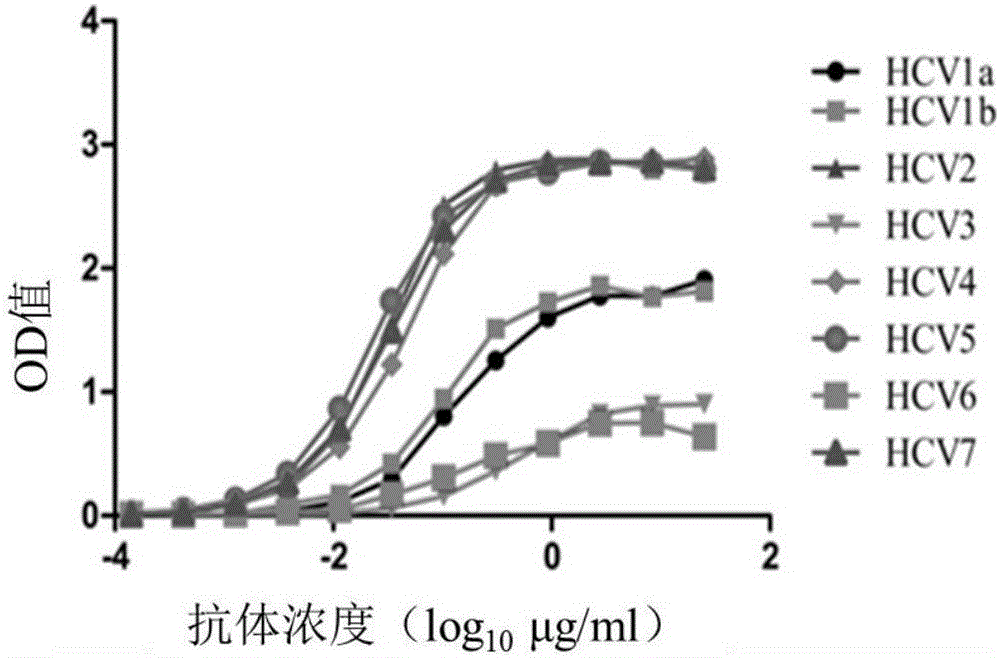
Human monoclonal antibodies binding to epitopes common to type 1 and 2 HCV are provided, as well as conformationally conserved HCV E2 2a and 2b proteins. Compositions comprising the antibodies find use in diagnosis and therapy. The antibodies recognize conformational epitopes that are conserved across multiple genotypes of HCV. Thus the antibodies have the potential to be useful in the prevention and treatment of the majority of HCV infections. A subset of the antibodies (CBH-2, CBH-5, CBH-7, CBH-8C, CBH-8E, and CBH-11) have the ability to prevent the binding of HCV E2 proteins of multiple genotypes to human CD81, a possible co-receptor for HCV infection. A subset of the antibodies (CBH-2 and CBH-5) have been shown to inhibit the binding of HCV virions (as opposed to purified E2 protein) to human CD81. A further subset of the antibodies (CBH-4D, CBH4B, CBH-8C, and CBH-9) have been shown to prevent HCV envelope mediated fusion using an HCV psuedotype system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Hepatitis C virus asialoglycoproteins
InactiveUS6074852AFacilitate secretionFacilitated releaseNanotechFungiSialic acid aldolaseVirus-like particle
Two Hepatitis C Virus envelope proteins (E1 and E2) are expressed without sialylation. Recombinant expression of these proteins in lower eukaryotes, or in mammalian cells in which terminal glycosylation is blocked, results in recombinant proteins which are more similar to native HCV glycoproteins. When isolated by GNA lectin affinity, the E1 and E2 proteins aggregate into virus-like particles.
Owner:CHIRON CORP
Modified Human Hepatitis C Virus Genomic RNA That can be Autonomously Replicated
ActiveUS20090176200A1Easy to useSsRNA viruses positive-senseGenetic material ingredientsNucleotideGenetics
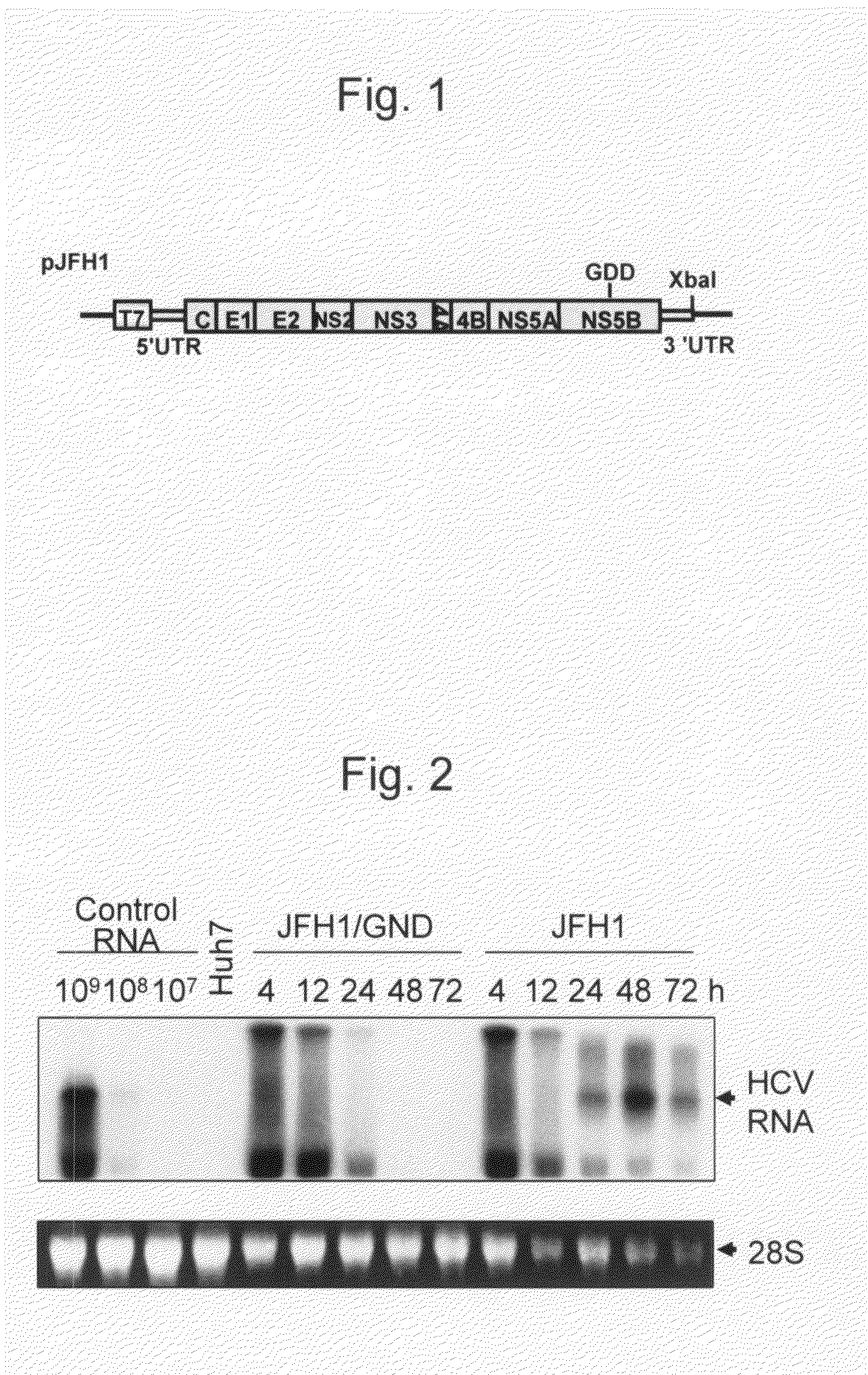
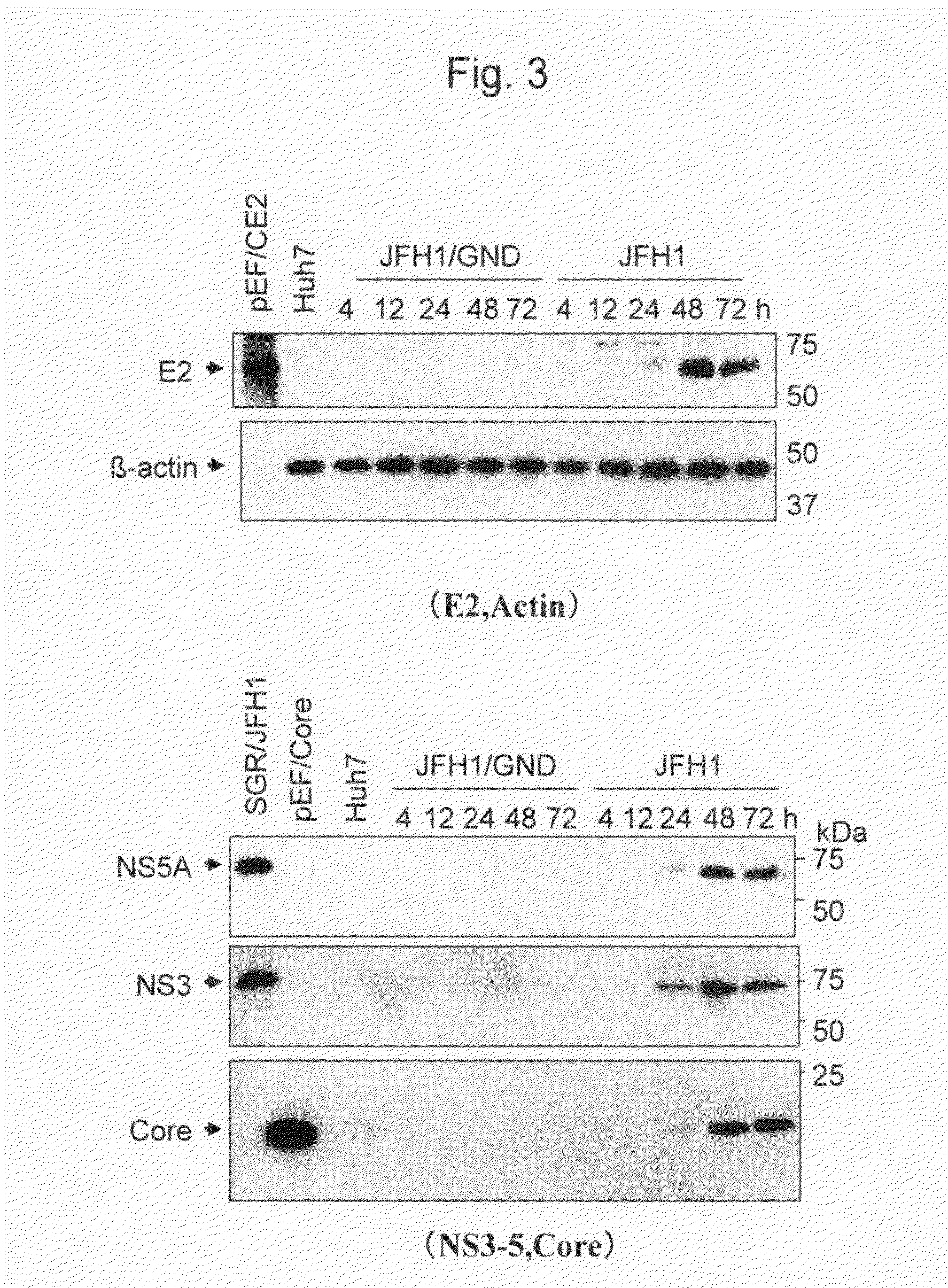
The present invention provides modified hepatitis C virus genomic RNA, comprising nucleotide sequences of genomic RNA portions of two or more types of hepatitis C viruses, which comprises a 5′ untranslated region, a core protein coding sequence, an E1 protein coding sequence, a p7 protein coding sequence, an E2 protein coding sequence, an NS2 protein coding sequence, an NS3 protein coding sequence, an NS4A protein coding sequence, an NS4B protein coding sequence, an NS5A protein coding sequence, an NS5B protein coding sequence, and a 3′ untranslated region, and which can be autonomously replicated. In particular, the present invention relates to modified hepatitis C virus genomic RNA, which can be autonomously replicated by substitution of the RNA sequence portion encoding NS3, NS4, NS5A, and NS5B proteins of hepatitis C virus genomic RNA with a partial RNA sequence encoding NS3, NS4, NS5A, and NS5B proteins of a JFH1 strain shown in SEQ ID NO: 1.
Owner:TORAY IND INC +1
Human papillomavirus inhibitors
The present invention provides systems for identifying anti-viral agents. In particular, the invention encompasses reagents and strategies for identifying agents that inhibit or disrupt key protein-protein interactions that are important in the life cycle of papillomaviruses. The invention allows identification, production, and / or use of agents that reduce or inhibit the replication of HPV by inhibiting (e.g., precluding, reversing, or disrupting) the formation of the E1-E2 protein-protein complex. The invention also provides specific inhibitory agents, pharmaceutical compositions, and methods of using these inhibitors and pharmaceutical compositions for inhibiting viral replication in vitro. Methods are also described for the treatment and prevention of HPV infections and HPV-related diseases in patients.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
CSFV antibody detection system and preparation method thereof
ActiveCN105527442AHigh detection rate sensitivityImprove capture efficiencyBiological testingSerum igeE2 protein
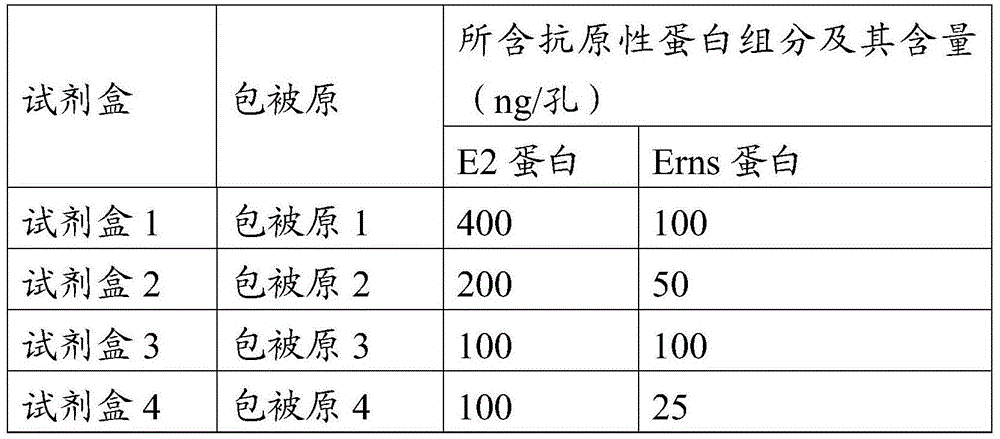
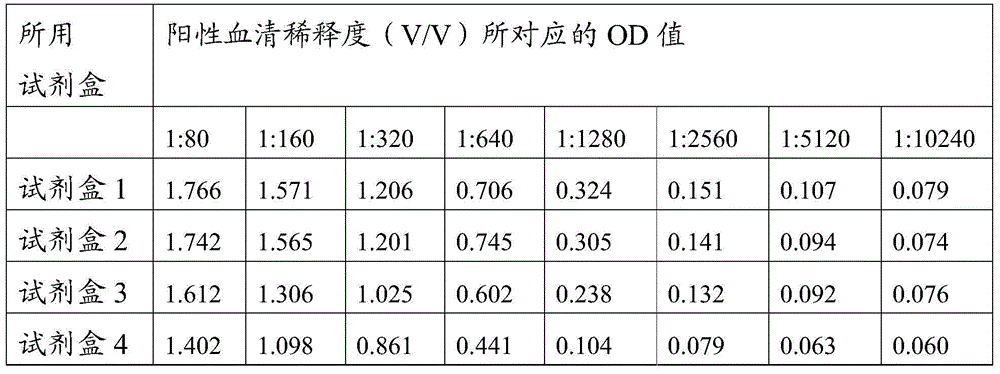
The invention provides a CSFV antibody detection system and a preparation method thereof. A coating antigen of the detection system contains CSFV E2 protein and Erns protein. The CSFV E2 protein and Erns protein are recombinant proteins expressed by eucaryon, correct spatial conformation and posttranslational modification process can be guaranteed, antigen is capable of effectively combining with the antibody in serum, and specificity, sensitivity and repeatability of detection can be increased. The system and the method can be sued for diagnosis of CSFV antibody in prevention and control of CSFV as well as immunization evaluation of a CSFV vaccine.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Recombinant virus for expressing swine fever virus E2 gene, and preparation method and application thereof
ActiveCN104178505AImprove expression levelHigh expressionViral antigen ingredientsAntiviralsPig farmsSwine Fever Virus
Owner:HUAZHONG AGRI UNIV
Test paper card for detecting swine transmissible gastroenteritis virus antibody and preparation and detection method of test paper card
InactiveCN107942061AImprove stabilityHigh sensitivityMaterial analysisCelluloseTransmissible gastroenteritis virus Antibody
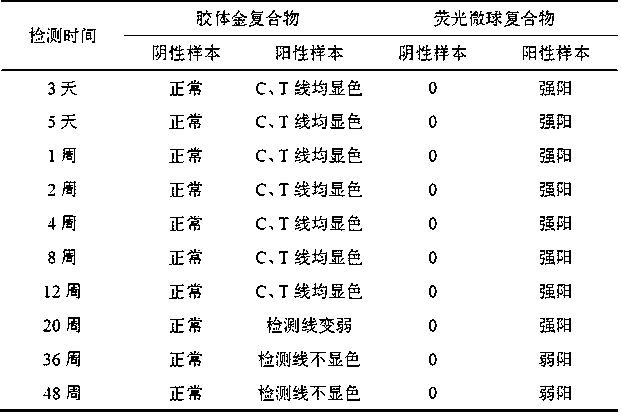
The invention relates to a test paper card for detecting porcine transmissible gastroenteritis virus antibody, preparation and detection method thereof, and belongs to the field of immunological detection. The test paper card includes a jammed case and a test strip, and the test strip includes a bottom plate and sequentially lapped and pasted on the Absorbent pad, detection pad, binding pad and sample pad on the base plate; the detection pad is a nitrocellulose membrane with a quality control line C and a detection line T, the quality control line C is coated with goat anti-mouse monoclonal antibody, and the detection line T is Coated with inactivated porcine transmissible gastroenteritis virus; the binding pad is a glass cellulose membrane embedded with time-resolved fluorescent microsphere-labeled anti-E2 protein monoclonal antibody; the sample pad is dried glass after soaking in the sample treatment solution Cellulose film. The test paper card prepared by the invention has better stability and higher sensitivity, and can achieve the purpose of semi-quantitative detection through fluorescence signal analysis.
Owner:洛阳现代生物技术研究院有限公司
Vectors, cell lines and their use in obtaining extended episomal maintenance replication of hybrid plasmids and expression of gene products
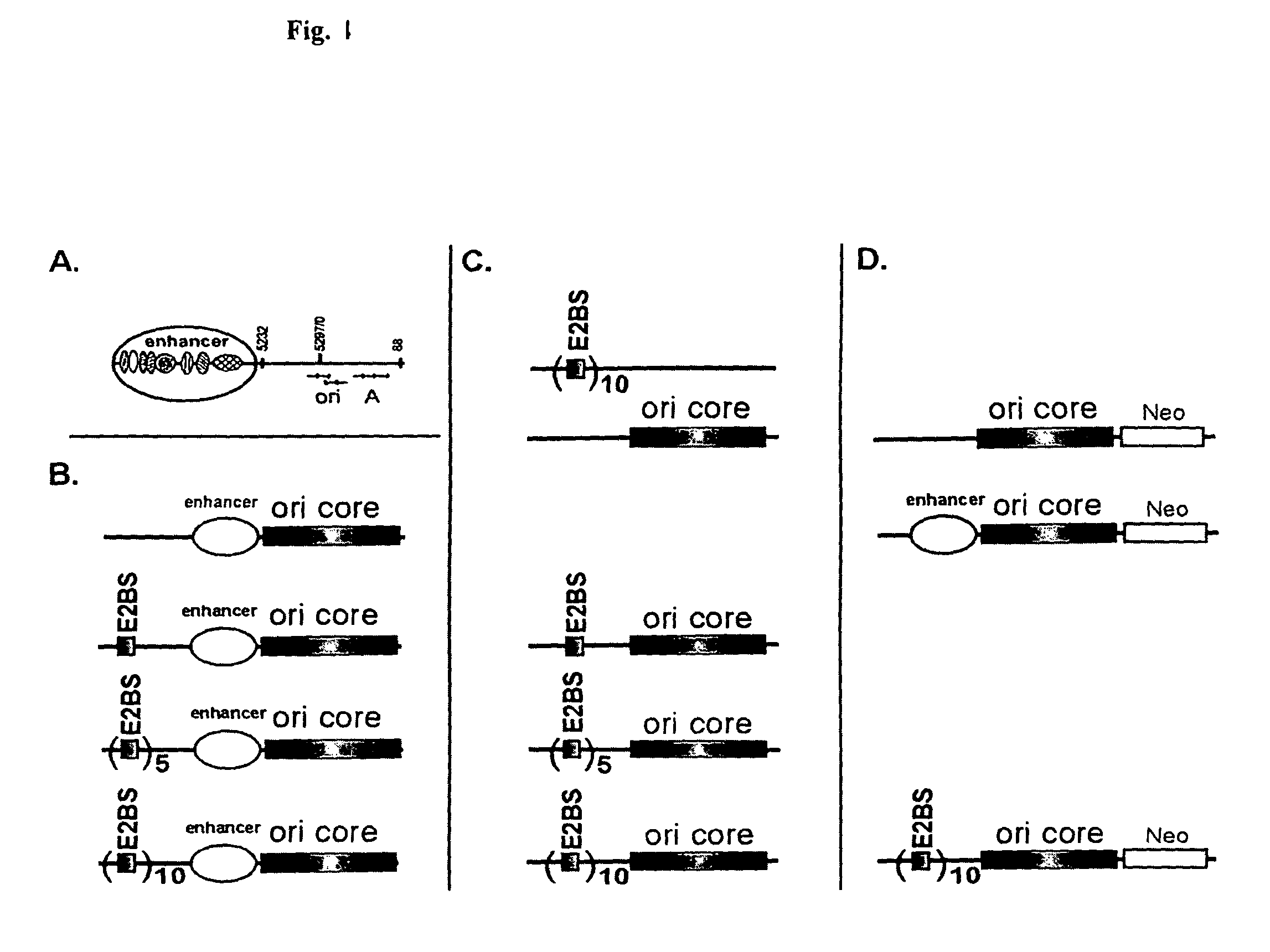
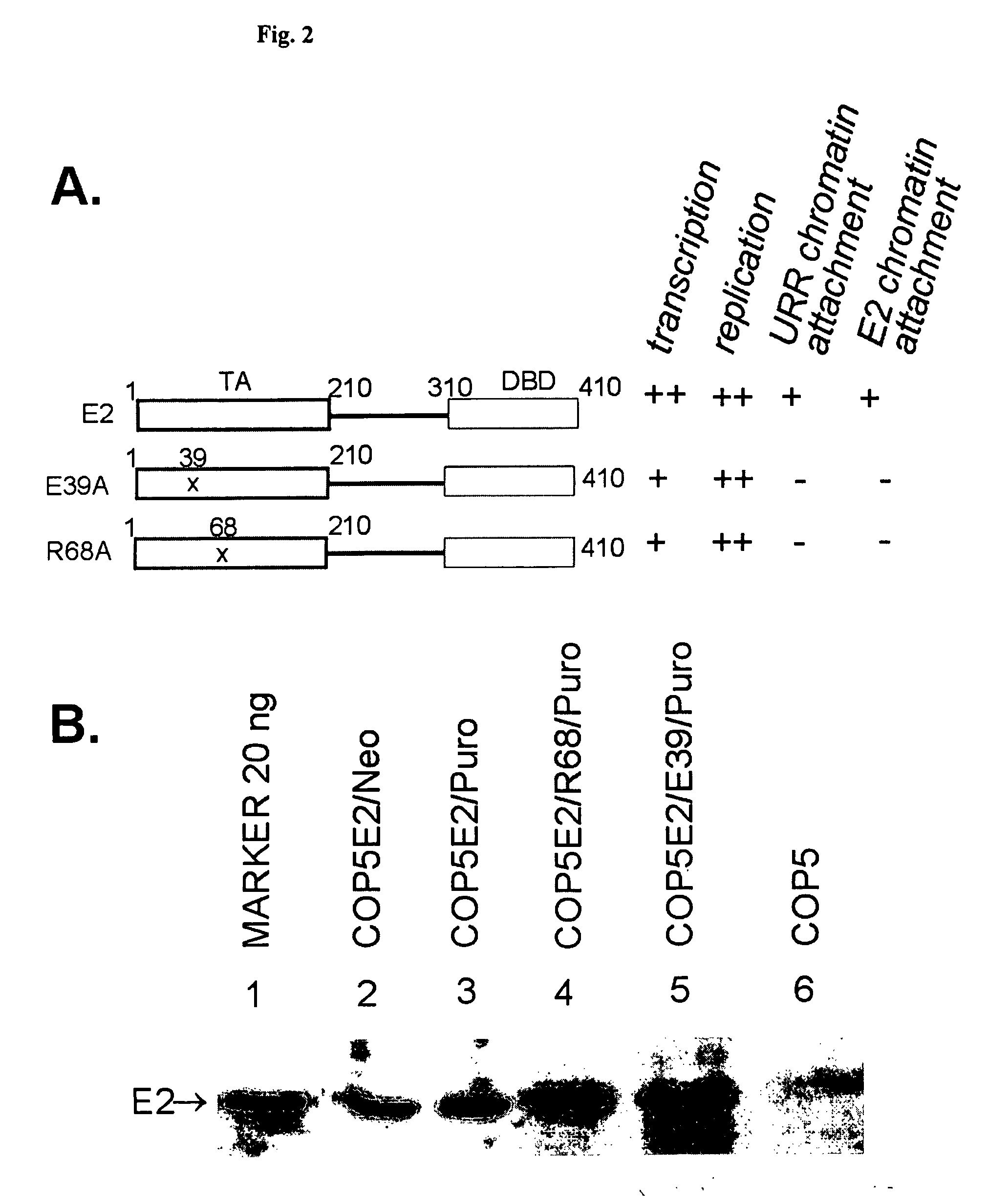
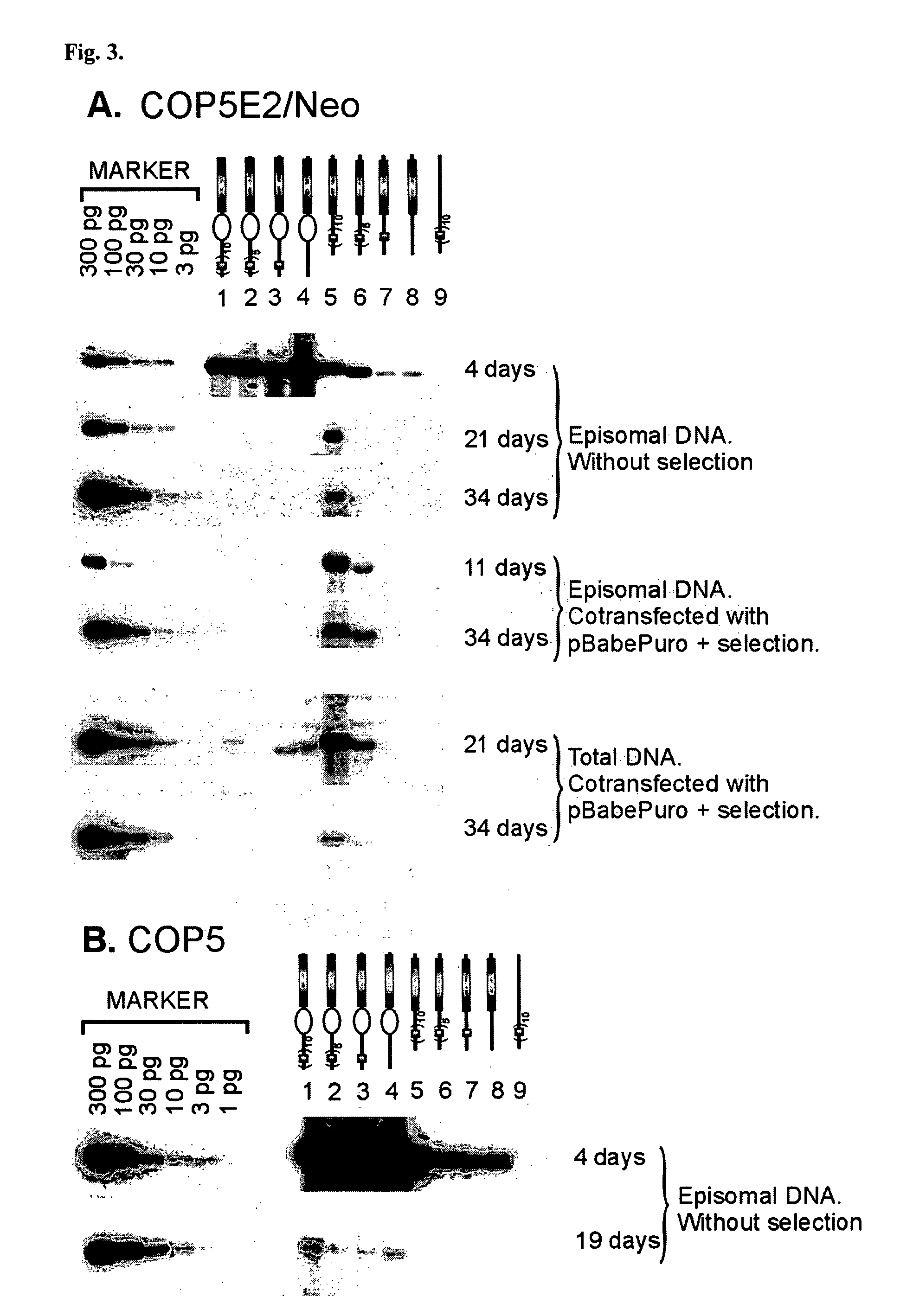
This disclosure shows that the BPV-1 E2 protein-dependent minichromosome maintenance element (MME) comprised of E2 multimeric binding sites can provide the stable maintenance replication function to the mouse polyomavirus (PyV) core origin plasmids in the presence of BPV-1 E2 protein and PyV large T-antigen (LT). MME dependent plasmids are lost with the frequency of 6% per generation. Significantly long stable maintenance replication can also be provided without selection pressure. We also demonstrate that PyV core origin maintenance function / replication activation could be provided by Epstein-Barr virus Family of repeats and EBNA1 protein. The maintenance of the Polyomavirus core origin plasmid was characterized by 13% loss of the plasmid during one cell generation in the case of EBV FR harboring plasmids. Our data clearly indicate that maintenance functions from different viruses can provide segregation / partitioning function to different heterologous origins in variety of cells and can be used in expression of gene products.
Owner:RAKUVABRIK LTD
Preparation methods and application of recombinant swine fever E2 protein and subunit vaccine of recombinant swine fever E2 protein
PendingCN107674883AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetVaccine Production
The invention discloses preparation methods and application of recombinant swine fever E2 protein and a subunit vaccine of the recombinant swine fever E2 protein. The preparation method of the recombinant swine fever E2 protein comprises the following steps that (1) a swine fever E2 protein coding gene is cloned into an eukaryotic expression vector to obtain recombinant plasmid containing the swine fever E2 protein coding gene; (2) then, the recombinant plasmid containing the swine fever E2 protein coding gene is transfected into a CHO cell strain; (3) the CHO cell strain obtained in the step(2) is cultured, screened and domesticated; and (4) the cell strain in the step (3) is fermented and cultured; and the recombinant swine fever E2 protein is obtained after purification. The methods provided by the invention have the advantages that the target protein can be obtained from cell culture supernatant; the yield reaches up to 1g / L; the protein purification time is shortened; the vaccineproduction steps are simplified; and the vaccine production cost is also greatly reduced.
Owner:NOVO BIOTECH CORP
E2 subunit vaccine comprising recombinant pestivirus E2 protein
InactiveUS6919085B2Increased and improved yieldProduce improveSsRNA viruses positive-senseViral antigen ingredientsCell culture mediaProtein C
The invention relates to a method of increasing protein expression in baculo vector virus expression systems. The invention provides a method to produce a recombinant protein in insect cell culture which comprises selecting a recombinant baculovirus expressing said protein, growing insect cells in growth medium in a culture vessel and infecting the cells with an inoculum of at least one baculovirus at a cell density of 1×105 to 5×106 cells / ml with an m.o.i of <0.01. The invention also provides a method to produce recombinant pestivirus E2 or Em9 protein or fragments thereof in insect cell culture characterized by a final concentration of the protein fragments in the growth medium at harvest of at least 100 μg / ml. The invention also provides a method of producing recombinant FSH, α-units and / or β-units, and complexes and fragments thereof, at a concentration in the growth medium at harvest of at least 15 μ / ml.
Owner:STICHTING INST VOOR DIERHOUDERIJ & DIERGEZONDHEID +1
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and subunit vaccine and application
ActiveCN107973841AIncrease productionImprove securitySsRNA viruses positive-senseViral antigen ingredientsProtein targetBovine Viral Diarrhea Viruses
The invention discloses preparation methods for CHO cell expressed recombinant bovine viral diarrhea virus protein E2 and a subunit vaccine and applications and belongs to the technical fields of animal vaccines and veterinary biologicals. The object of the invention is to provide a preparation method capable of industrially producing the bovine viral diarrhea virus recombinant subunit vaccine ona large scale. The preparation method for the recombinant subunit vaccine, provided by the invention, comprises the following steps: 1) cloning an eukaryotic expression vector containing a protein E2coding gene; 2) transfecting CHO cells, and performing selection, screening and acclimatizing to obtain suspending CHO cell strains, which stably and efficiently express the protein E2; 3) subjectingthe cell strains obtained in the step 2) to fermentation culture, and carrying out purification, so as to obtain recombinant protein E2; and 4) uniformly mixing the recombinant protein E2 and ISA 201VG thoroughly, thereby obtaining the recombinant subunit vaccine. According to the method provided by the invention, target protein can be obtained from cell culture supernatant, the yield reaches upto 500mg / L, the protein purification time is shortened, the vaccine production steps are simplified, and the vaccine production cost is greatly reduced.
Owner:NOVO BIOTECH CORP
Antibody having inhibitory activity on infection with hepatitis c virus (HCV) and use thereof
The objection of the invention is to provide an antibody that inhibits infection with hepatitis C virus (HCV). To this end, this invention provides an antibody that recognizes the hepatitis C virus (HCV) particle obtained from the hepatitis C virus (HCV) genome comprising the following (i) and (ii) ligated to each other as an antigen and has an inhibitory activity on infection with hepatitis C virus (HCV): (i) (a) the 5′-untranslated region, the core protein-encoding sequence, the E1 protein-encoding sequence, the E2 protein-encoding sequence, and the p7 protein-encoding sequence of the JFH-1 strain of the hepatitis C virus (HCV) or (b) the 5′-untranslated region, the core protein-encoding sequence, the E1 protein-encoding sequence, the E2 protein-encoding sequence, and the p7 protein-encoding sequence of the J6CF strain the hepatitis C virus (HCV); and (ii) the NS2 protein-encoding sequence, the NS3 protein-encoding sequence, the NS4A protein-encoding sequence, the NS4B protein-encoding sequence, the NS5A protein-encoding sequence, the NS5B protein-encoding sequence, and the 3′-untranslated region of the JFH-1 strain.
Owner:TORAY IND INC +1
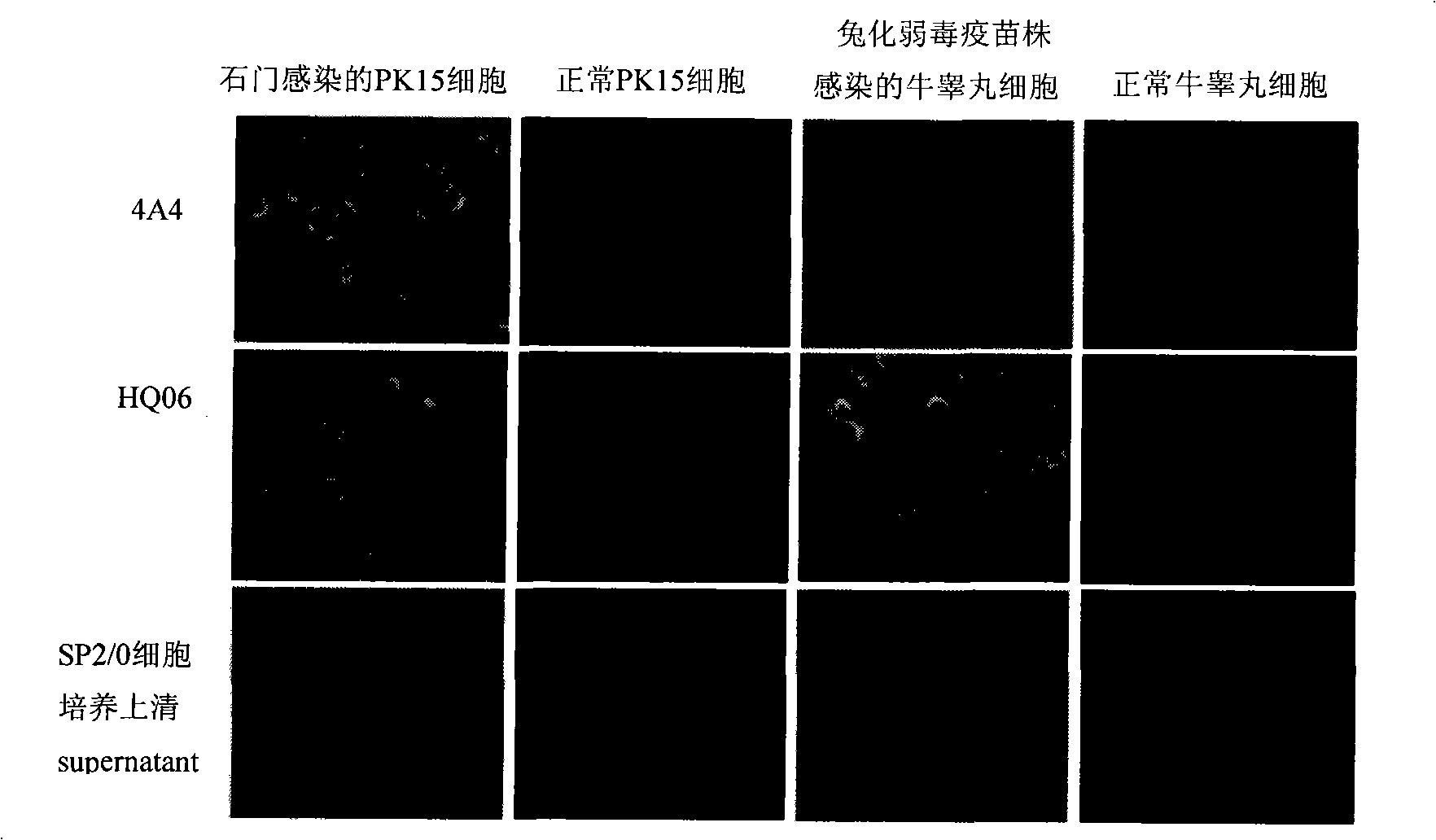
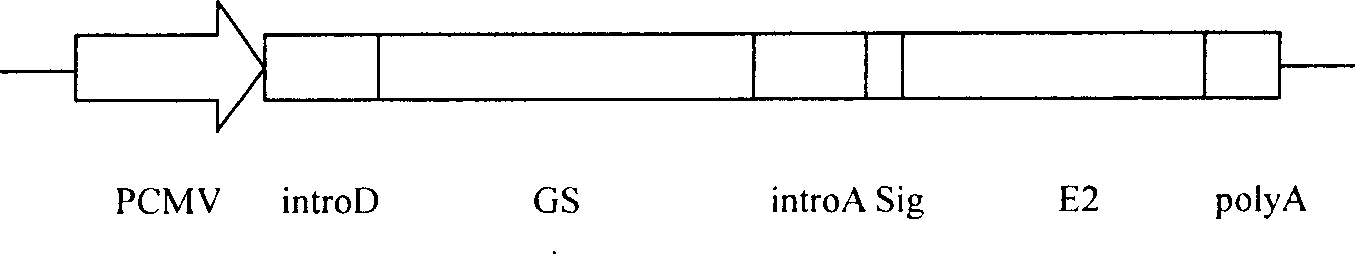
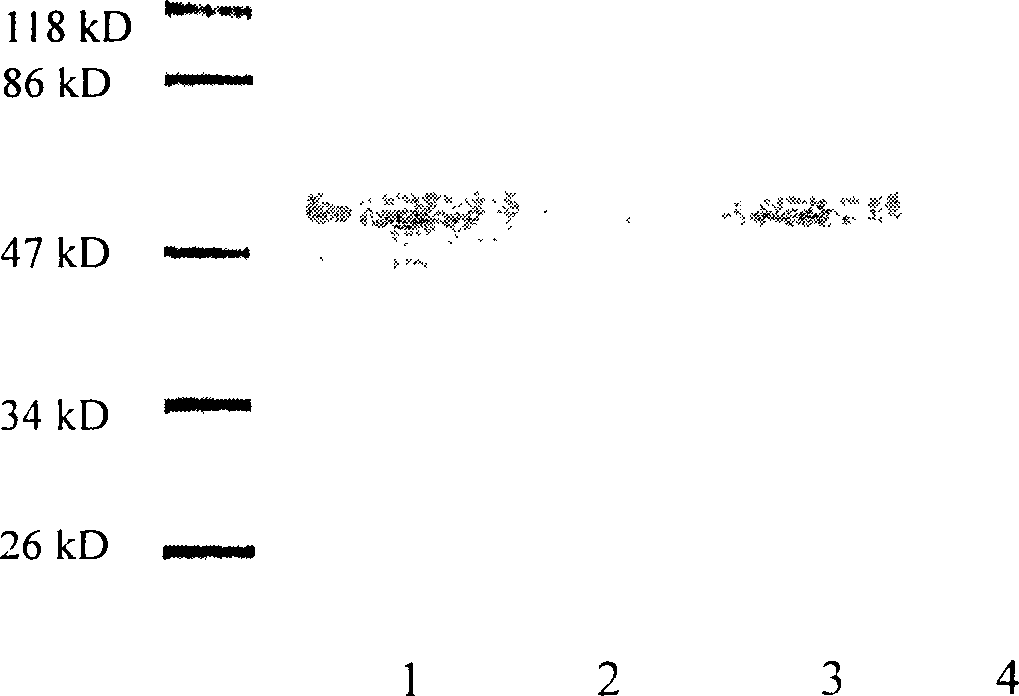
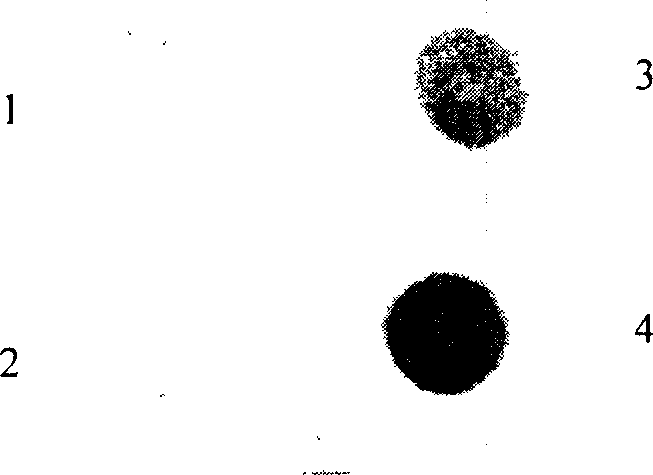
Method for expressing hepatitis C virus envelope protein E2 by mammal cell with high efficient secretion
The present invention relates to biomedicine technology. HCV envelope protein E2 mediates the combination between HCV and target cell and is key protein relates to HCV infection and one kind of low expression protein hard to obtain in gene recombination process. The present invention aims at provides high efficiency secretion method for mammal to express HCV envelope protein E2. The method constitutes one new type of mammal cell expressing plasmid, which expresses target gene E2 protein in high level while expressing glutamine synthetase as the screening marker in low level. The present invention makes it possible to batch prepare recombinant HCV envelope protein E2 with the natural biological function and antigenicity of HCV envelope protein, lays the foundation for development of serological HCV infection detecting reagent and HCV vaccine, and provides HCV molecular virological research with important material.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hog cholera virus truncated E2 protein and application of same
ActiveCN108107217APreserve antigenicityReduce manufacturing costBiological testingBovine Viral Diarrhea VirusesBiology
The invention discloses a hog cholera virus truncated E2 protein which is designed on the basis of protein spatial structure, and an application of the same. In the invention, according to the crystalstructure of bovine viral diarrhea virus E2 protein, the spatial structure of the hog cholera virus E2 protein is simulated, and then the hog cholera virus E2 protein is subjected to truncated expression, wherein the amino acid sequence of the truncated protein E2B / C / D / A is represented as the SEQ ID No.1. The truncated protein can maintain the complete antigenicity of the E2 protein, and has no cross reaction with a bovine viral diarrhea virus antibody. The invention further constructs a CHO cell line which stably expresses the truncated protein E2B / C / D / A and is assigned the accession numberof CGMCC No.14722. The invention also discloses an indirect ELISA kit which is used for detection of a hog cholera virus antibody, wherein the enveloped antigen is the hog cholera virus truncated protein E2B / C / D / A. The kit is used for specifically detecting the hog cholera virus antibody with high specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Classical swine fever E2 subunit vaccine and application thereof
InactiveCN106139139AThe effective amount of antigen is stableLess side effectsViral antigen ingredientsVirus peptidesImmune effectsAdjuvant
The invention provides a classical swine fever E2 subunit vaccine and a preparation method thereof. The preparation method specifically comprises the steps that a Baculovirus Carrier Expression System is used for expressing a large amount of recombination classical swing fever E2 protein in insect cells, and the classical swine fever E2 subunit vaccine with a good immune effect is developed. The Bac-to-Bac Baculovirus Carrier Expression System is used for expressing the classical swine fever E2 protein. The classical swine fever E2 protein and an adjuvant 201R are emulsified into the novel subunit vaccine, and by means of piglet immune challenge tests, it is verified that the subunit vaccine has a good immune protection effect.
Owner:北京大北农科技集团股份有限公司动物医学研究中心 +3
Preparation and application of neutralizing monoclonal antibody of anti-hepatitis C virus
ActiveCN103642792ATrigger immune responseAvoid difficultiesImmunoglobulins against virusesAntiviralsSpleenHepacivirus
The invention discloses preparation of a neutralizing monoclonal antibody of an anti-hepatitis C virus and application thereof. E2 protein is utilized to strengthen immunity after mice are immunized by pVAX-CpG-N2N8; spleen of the mice with the highest valence aiming at the E2 antibody is taken as an antigen-sensitized B cell and fused with a myeloma cell SP2 / 0 strain, and the fused cell is screened in HAT culture medium, so as to obtain the fused cell, and the fused grows and is cloned to obtain the hybridoma cell strain generating the monoclonal antibody disclosed by the invention. The neutralizing monoclonal antibody of the anti-hepatitis C virus E2 disclosed by the invention is generated by the hybridoma cell strain; epitope combined with the monoclonal antibody is at F550GCTWMNSTGFTKVCGAPPCVIG572 part of E2 glycoprotein. The neutralizing monoclonal antibody of the anti-hepatitis C virus disclosed by the invention has good capability of neutralizing HCV infection, can be used as a hepatitis C virus therapeutic antibody, and has a great application prospect in hepatitis C diagnosis and treatment.
Owner:WUHAN UNIV
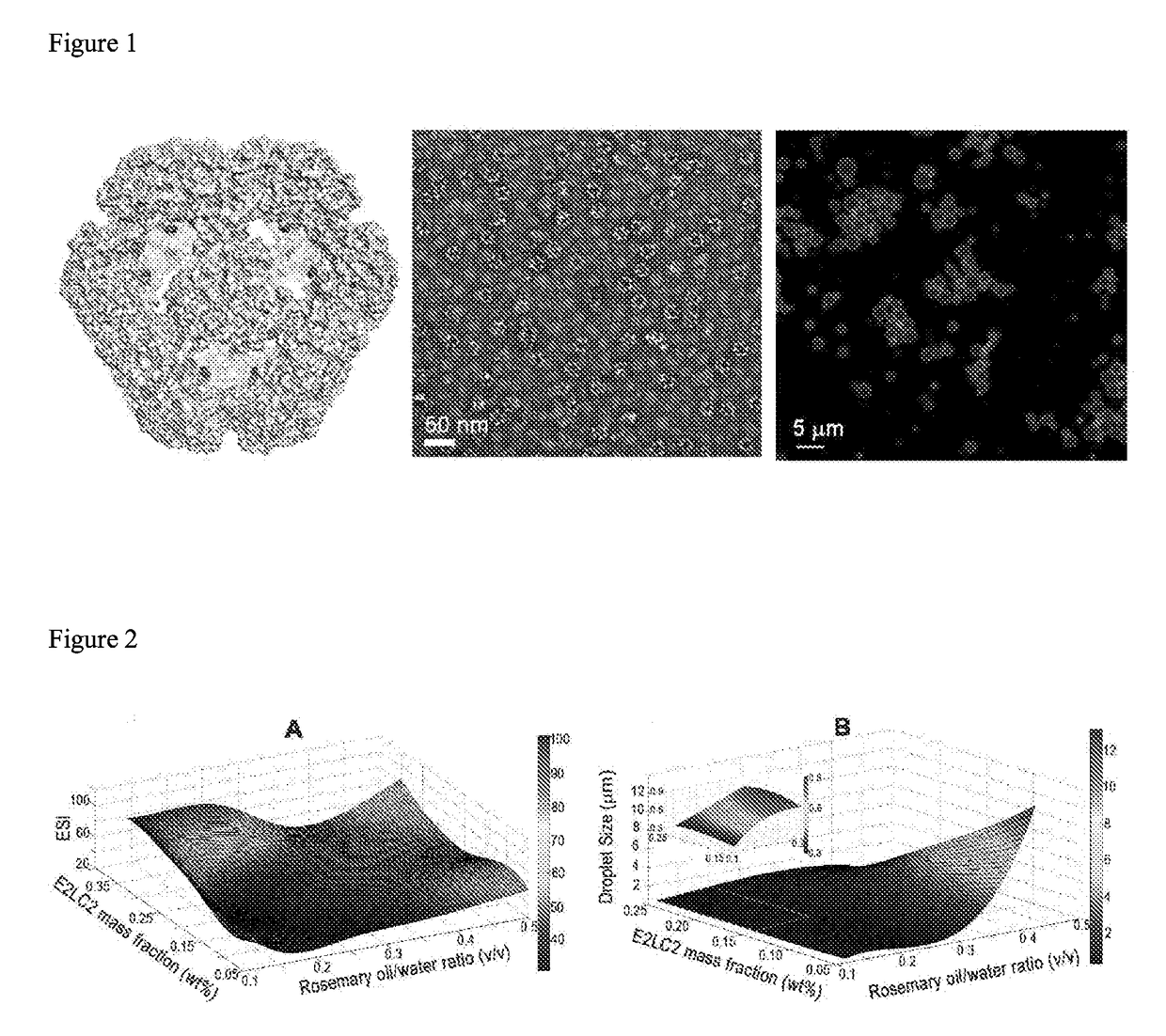
Protein cage-stabilized pickering emulsions and the use thereof
The present invention relates to a Pickering emulsion comprising an aqueous phase, an oil phase and a nanoparticle, wherein the nanoparticle is a protein cage. The protein cage is preferably a Bacillus stearothermophilus E2 protein of pyruvate hydrogenase multi-enzyme complex or an E2LC2 protein. Preferably the aqueous or oil phase of the Pickering emulsion comprises an agent, or the protein cage is coupled to or loaded with an agent, wherein the agent is a therapeutic, nutritional, nutraceutical or cosmetic agent. The invention further includes use of the Pickering emulsions disclosed herein in pharmaceutical, cosmetic, or food applications, or as a controlled release delivery system, and use of protein cages as emulsifiers in Pickering emulsions.
Owner:NANYANG TECH UNIV +1
Recombinant swine fever virus E2 protein swine source monoclonal antibody and preparation method and application thereof
ActiveCN107973850AEasy to prepareImprove responseImmunoglobulins against virusesAntiviralsHeavy chainSwine Fever Virus
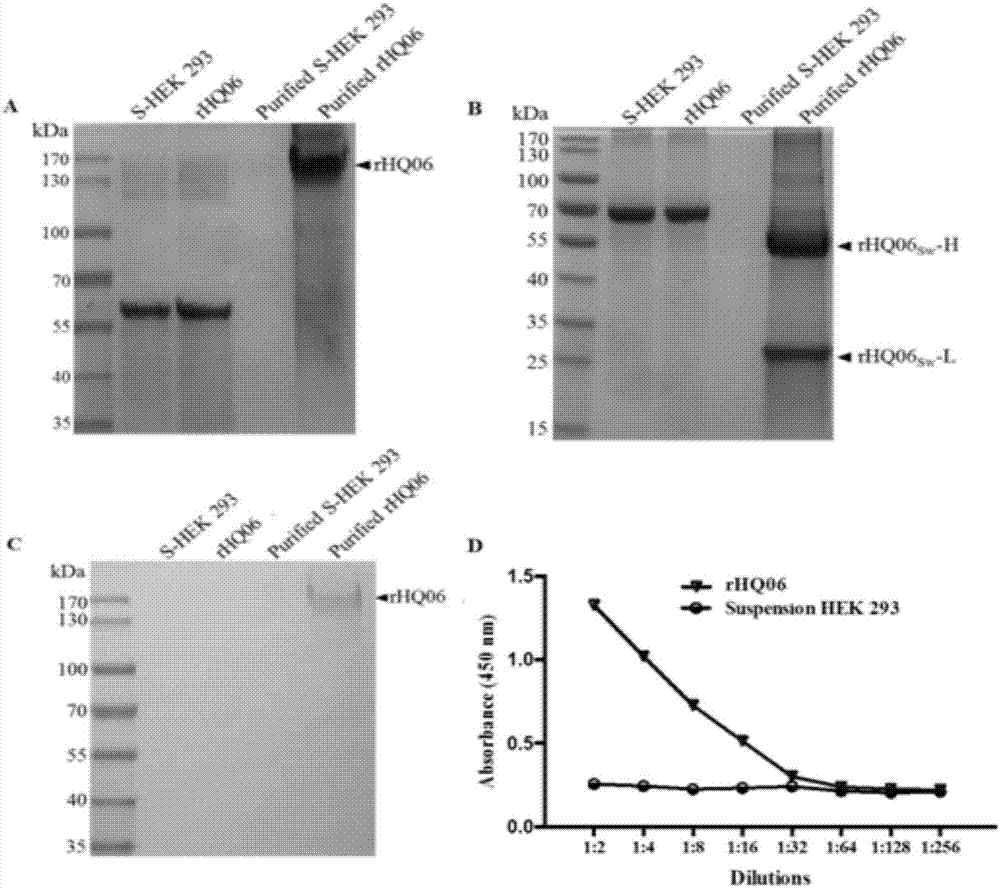
The invention discloses a recombinant swine fever virus E2 protein swine source monoclonal antibody and a preparation method and application thereof. Firstly, the recombinant swine fever virus E2 protein swine source monoclonal antibody is disclosed, an amino acid sequence of a heavy chain is shown in SEQ ID NO.1, and an amino acid sequence of a light chain is shown in SEQ ID NO.2. The invention also discloses a suspension HEK293 cell line which can stably express the recombinant swine fever virus E2 protein swine source monoclonal antibody. The recombinant swine fever virus E2 protein swine source monoclonal antibody has the advantages that the coding genes of the heavy chain and light chain are cloned into an eukaryotic expression vector, and the stable and high-efficiency expression ofthe recombinant swine fever virus E2 protein swine source monoclonal antibody is realized by the suspension HEK293 cell line; the reactogenicity and neutralizing activity are good, and the important development value is realized in development of novel swine fever virus diagnosing and treating preparations.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Novel expression vectors and uses thereof
InactiveUS20050026137A1Increase the number ofAvoiding a severe drawbackGenetic material ingredientsVirus peptidesEpstein-Barr Virus Nuclear AntigensOrigin of replication
The present invention relates to novel vectors, to DNA vaccines and gene therapeutics containing said vectors, to methods for the preparation of the vectors and DNA vaccines and gene therapeutics containing the vectors, and to therapeutic uses of said vectors. More specifically, the present invention relates to novel vectors comprising (a) an expression cassette of a gene of a nuclear-anchoring protein, which contains (i) a DNA binding domain capable of binding to a specific DNA sequence and (ii) a functional domain capable of binding to a nuclear component and (b) a multimerized DNA sequence forming a binding site for the anchoring protein, and optionally (c) one or more expression cassettes of a DNA sequence of interest. In particular the invention relates to vectors that lack a papilloma virus origin of replication. The nuclear-anchoring protein might be the E2 protein of Bovine Papilloma Virus type 1 or Epstein-Barr Virus Nuclear Antigen 1. The invention also relates to vectors that lack an origin of replication functional in a mammalian cell. The invention further relates to methods for expressing a DNA sequence of interest in a subject.
Owner:FIT BIOTECH OY PLC
Recombinant BHK cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the same in preparation of vaccines and diagnosis reagents of classical swine fever
ActiveCN103751773AFully emulsifiedAvoid infectionAntiviralsBiological testingStructural proteinMicrobiological culture
The present invention discloses a recombinant cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and applications of the recombinant cell line in preparation of vaccines and diagnosis reagents of classical swine fever, wherein the recombinant cell line is BCSFV-E012, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7720. In addition, the present invention further discloses an establishment method for the cell line for stably expressing classical swine fever virus E0-E1-E2 protein, and a method for preparing a classical swine fever prevention vaccine composition by using the cell line. The present invention further discloses applications of the E0-E1-E2 protein stably expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents. The classical swine fever vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no classical swine fever virus non-structural protein antibody production so as to identify the vaccinated animal and the virus infected animal.
Owner:HARBIN WEIKE BIOTECH DEV +1
Polypeptide sequence combined with bovine viral diarrhea E2 protein and application of polypeptide sequence
ActiveCN104597256AHigh affinityImprove bindingBiological material analysisPeptidesBiotinBacteriophage
The invention mainly relates to a polypeptide sequence combined with bovine viral diarrhea E2 protein and application of the polypeptide sequence. The polypeptide sequence is KRLREL and is a linear combined polypeptide, the polypeptide sequence can be prolonged and modified by taking the polypeptide sequence as a core, and modification materials can be but not limited to nano materials, fluorescent materials, enzymes, biotin and specific protein. By adopting a phage peptide library screening technique, the expressed and purified bovine viral diarrhea E2 protein can be screened for multiple rounds, and polypeptide clone strains which can be specifically combined with the bovine viral diarrhea E2 protein can be ultimately obtained; the polypeptide clone strains can be selected for sequencing, the core sequence of the polypeptide is KRLREL, the manually synthesized polypeptide used for ELISA combination experiment shows that the synthesized polypeptide can be well combined with the bovine viral diarrhea E2 protein; the process is simple, and the operation is relatively convenient compared with the operation that expression protein is manually expressed and is further immunized to obtain a protein antibody; by marking the polypeptide, a kit or a test paper bar (card) for quantitative and qualitative detection on the bovine viral diarrhea E2 protein can be rapidly produced.
Owner:HENAN ACAD OF AGRI SCI
Test paper for rapidly detecting hog cholera antibody and method for making same
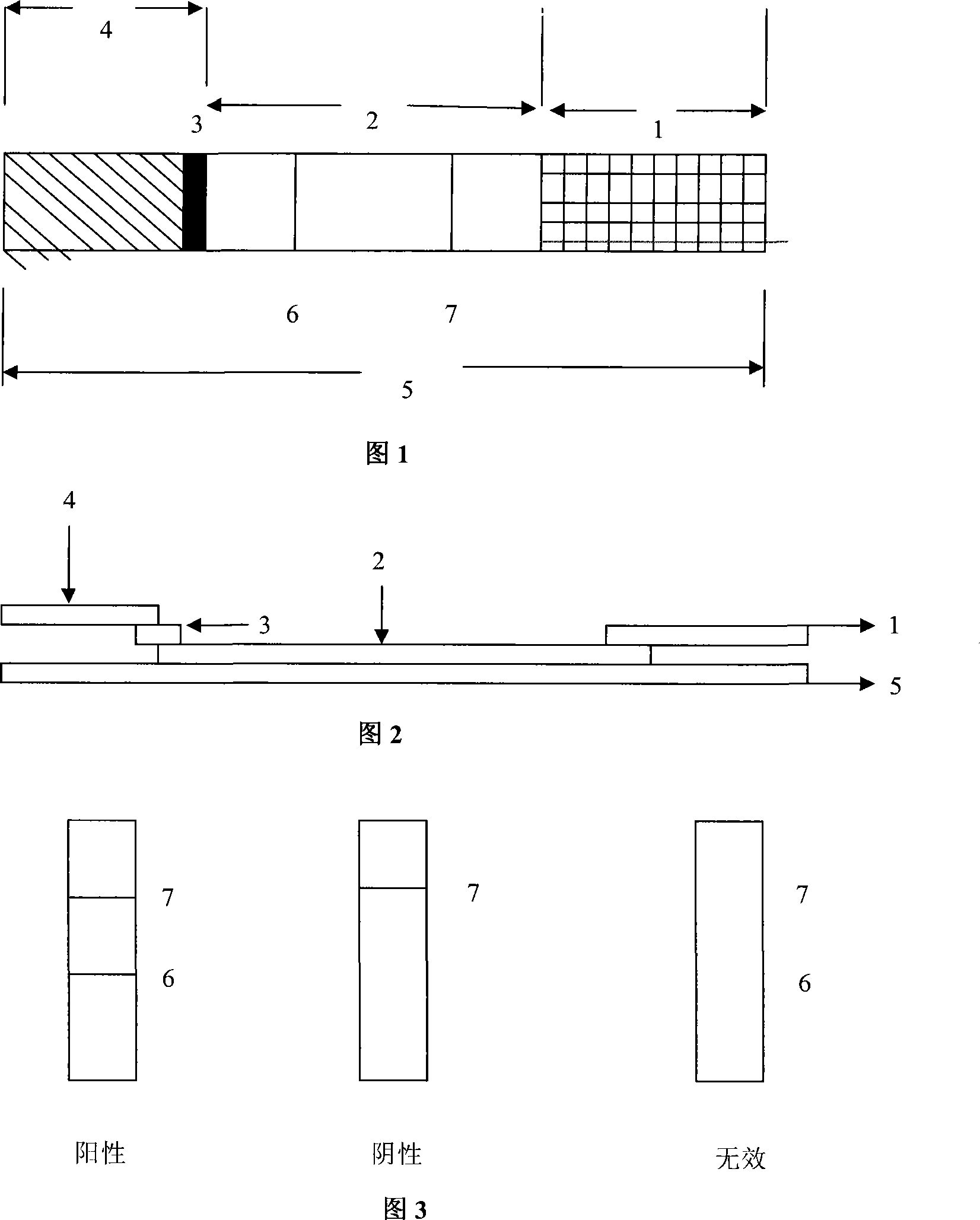
The invention discloses a test paper for rapidly detecting swine fever antibody and a preparation method thereof. The inventive test paper consists of a sample pad (4), a glass fiber member (3), a cellulose nitrate membrane (2), a water absorbent pad (1) and a support (5); wherein the cellulose nitrate membrane contains a detection line (6) coated by swine fever virus E2 protein and a reference line (7) coated with rabbit anti swine fever antibody; and the glass fiber member is combined with swine fever virus E2 protein marked by colloidal gold. The test paper can rapidly detect possibly present swine fever antibody in a sample to achieve rapid detection and timely epidemic control, thus creating favorable conditions for further separation and identification. The test paper has the advantages of convenient, rapid and easy usage, clear result, easy generalization, and no need for special instrument and equipment as well as professionals, and is suitable for large-batch onsite detection for base layer and for sudden events. The test paper is suitable for epidemic inquisition and performs assistant effect on swine fever virus infection diagnosis.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Kit for quickly detecting swine fever antibody and preparation method thereof
The invention relates to a kit for quickly detecting a swine fever antibody. In the method, a pair of specific primers is designed, a relatively conservative gene sequence is cloned, an antigen aiming at a swine fever E2 protein is expressed through a pronucleus expression technology, and, on the basis, the kit containing an enzyme linked plate coated with a high-purity and high activity swine fever virus specificity antigen, an enzyme conjugate of rabbit-anti-swine monoclonal antibody containing an HRP (Horseradish Peroxidase) marker, a TMB (Tetramethylbenzidine) color developing liquid and the like is prepared. The kit can quickly detect the swine fever antibody in blood serum or blood plasma and has strong specificity and high sensitivity.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU +2
Swine fever-porcine circovirus combined subunit vaccine, as well as preparation method and application thereof
ActiveCN107233566AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantAnimals vaccines
The invention discloses a swine fever-porcine circovirus combined subunit vaccine, as well as a preparation method and an application thereof, and belongs to the technical fields of animal vaccines and veterinary biological products. The vaccine is prepared from swine fever virus E2 protein, porcine circovirus type 2 cap protein and a pharmaceutically acceptable adjuvant. The preparation method comprises the following steps: (1) preparing swine fever virus E2 protein and porcine circovirus type 2 cap protein; (2) preparing an antigen solution from the swine fever virus E2 protein and porcine circovirus type 2 cap protein prepared in the step (1); and (3) mixing and stirring the antigen solution and Montanide GEL 01PR adjuvant in a mass ratio of 10:1. The vaccine has the advantages of strong immunogenicity, good safety, no immune interference and the like, can be used for preventing potential biological safety hazard for virus variation and fundamentally purifying swine fever virus and porcine circovirus type 2, and can achieve the effect of dual prevention with one injection by immunizing the vaccine to achieve the aims of saving time, labor and cost.
Owner:NOVO BIOTECH CORP
Competitive Alpha LISA (linked immuno sorbent assay) detection kit for classical swine fever virus (CSFV) antibody and detection method thereof
InactiveCN103499693AStrong specificityHigh sensitivityBiological testingImmunoassaysAntigenSerum ige
The invention discloses a competitive Alpha LISA (linked immuno sorbent assay) detection kit for a classical swine fever virus (CSFV) antibody and a detection method thereof. The detection kit comprises donor microspheres, receptor microspheres, a swine fever virus E2 protein monoclonal antibody and an E2 protein antigen with a His label. A competitive Alpha LISA detection method for the CSFV antibody is created by optimizing test reaction conditions such as the donor microspheres, the receptor microspheres, the monoclonal antibody, the antigen and serum. The kit for detecting the CSFV antibody is good in specificity, high in sensitivity, low in usage amount of the serum, low in detection cost and short in detection time, does not need to be washed and can not be influenced by hemolysis.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Yeast expressed classical swine fever virus glycoprotein e2 and use thereof
InactiveUS20100028384A1Induce productionProtection against CSFV infectionSsRNA viruses positive-senseViral antigen ingredientsEnationBULK ACTIVE INGREDIENT
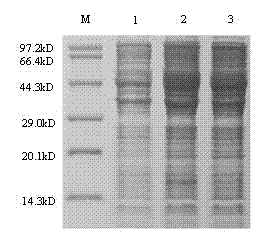
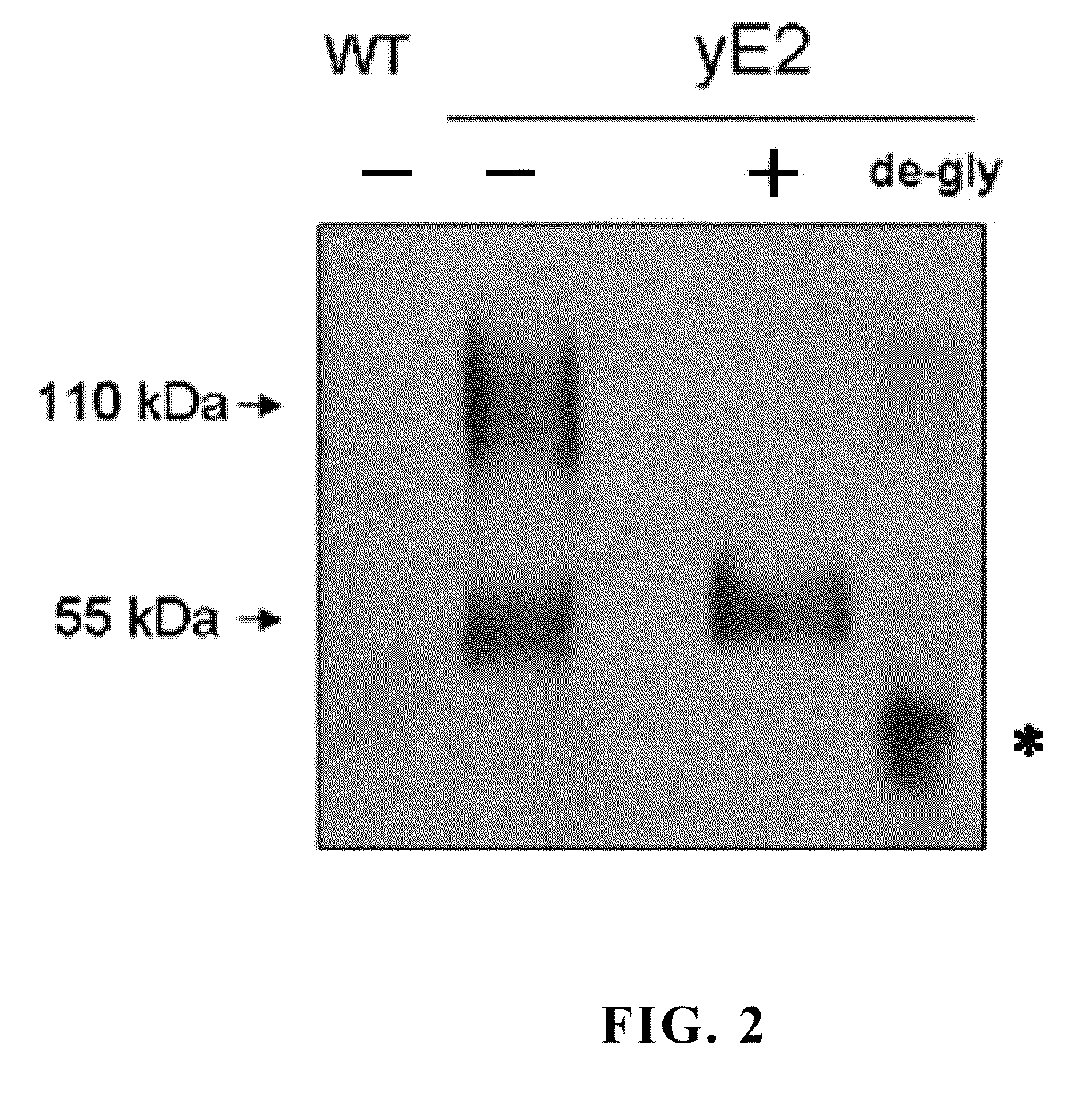
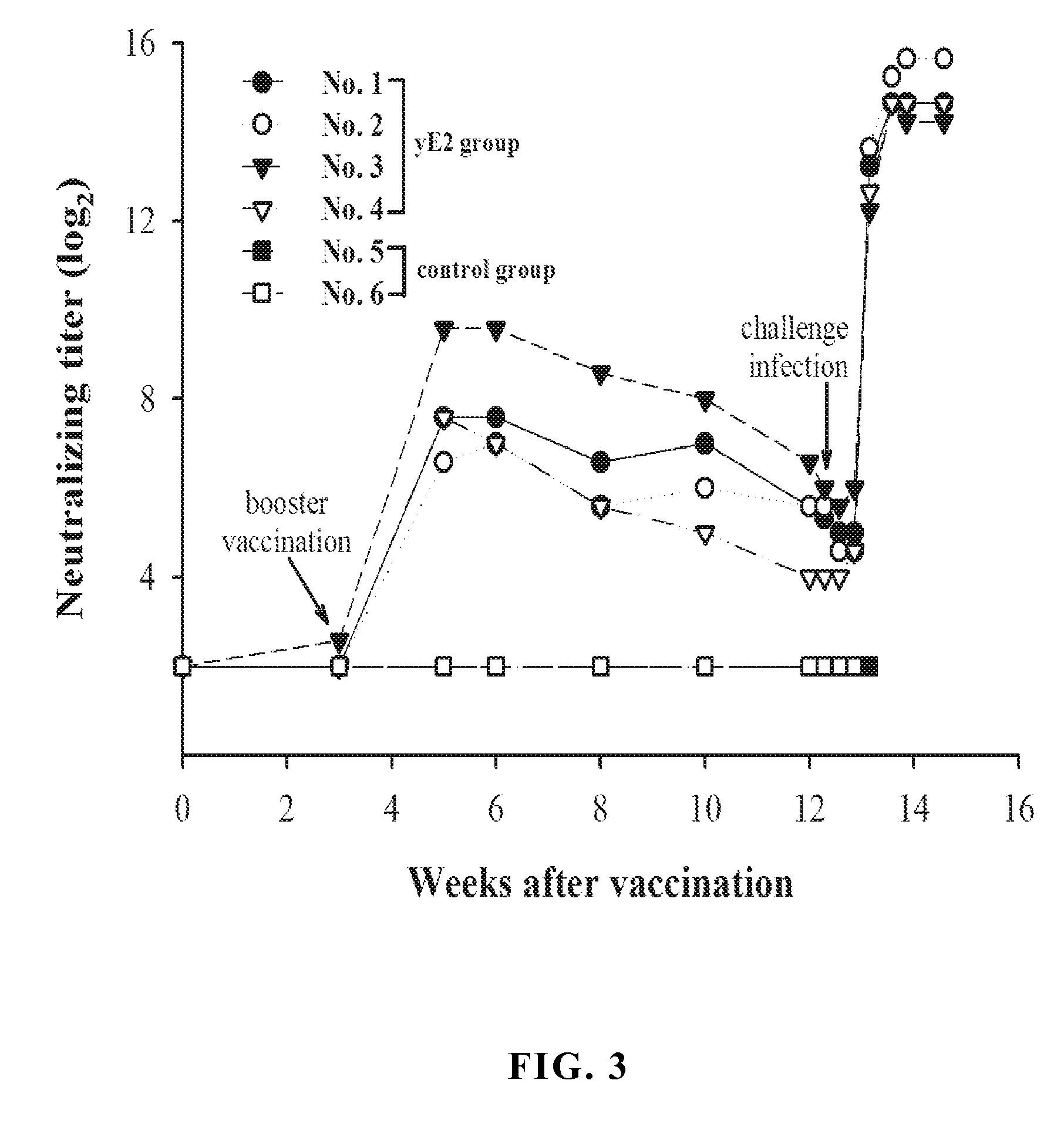
A glycoprotein E2 of classical swine fever virus (CSFV) expressed in a recombinant yeast system. The recombinant E2 protein (yE2) is able to form a homodimer, exhibits glycosylation conformation and possesses correct immunogenicity. An anti-CSFV vaccine can be provided with yE2 as a major active ingredient to induce high titers of neutralizing antibody in vaccinated pigs, and to induce a protection against CSFV infection.
Owner:MAO XING BIOLOGICAL TECH
Cd4+ human papillomavirus (hpv) epitopes
InactiveUS20070037151A1Improving immunogenicityViral antigen ingredientsMicrobiological testing/measurementAbnormal tissue growthHPV vaccines
The present invention provides CD4+ T-cell epitopes in E6, E7 and E2 proteins from various strains of human papillomavirus (HPV). In some preferred embodiments, the present invention provides means for the development of HPV vaccines, in particular multivalent vaccines for the prevention of infection with high-risk HPV strains. In additional embodiments, the present invention provides means for the development of therapeutic vaccines against high-risk HPV types that prevent the development of benign and / or malignant tumors in infected individuals. The present invention further provides epitopes suitable for use in prophylactic and therapeutic vaccines.
Owner:GENENCOR INT INC
Inhibitors of papilloma virus
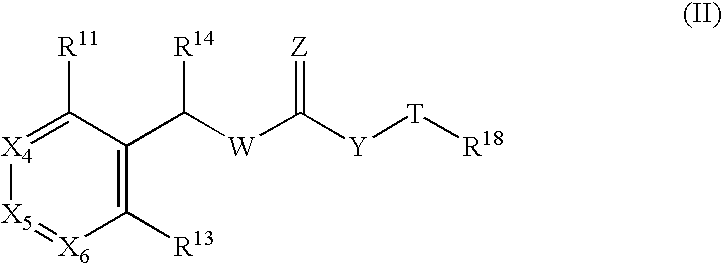
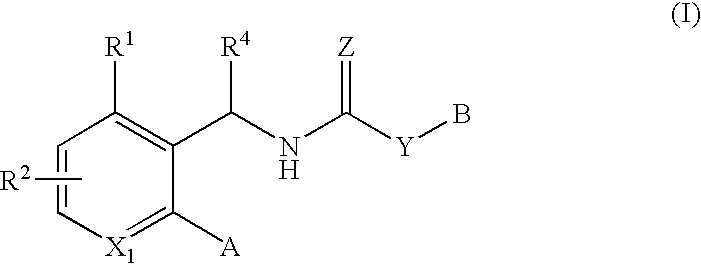
The use of a compound of formula (II): or its enantiomers or diastereoisomers thereof, or salts or pharmaceutically-acceptable esters thereof, in the treatment or prevention of a papilloma virus infection, particularly human papilloma virus in a mammal, wherein R11; X4; X5; X6; R13; R14; W; Z; Y; T; and R18 are defined herein. The present invention also provides novel compounds, pharmaceutical compositions and methods for using these compounds and compositions in the treatment or prevention of papilloma virus infection. More particularly, the present invention provides compounds, compositions and methods for inhibiting papilloma virus DNA replication by interfering with the E1-E2 protein-protein interaction essential for viral DNA replication.
Owner:BOEHRINGER INGELHEIM INT GMBH
Fully-human-derived anti-HCV (hepatitis C virus) neutralizing antibody-TRN1001
ActiveCN106749644AHigh potencyHigh cross activityImmunoglobulins against virusesAntiviralsSide effectMonoclonal antibody
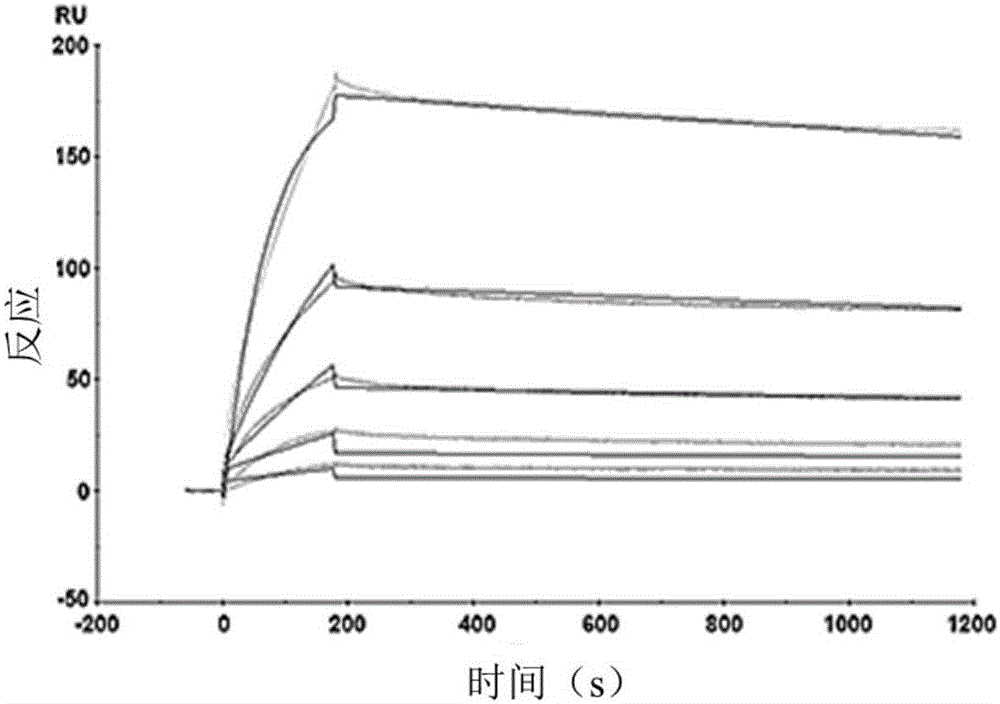
The invention discloses a fully-human-derived monoclonal antibody for neutralizing HCV (hepatitis C virus) and an application thereof and particularly discloses an antibody capable of recognizing and combining HCV envelope protein E2, a coding gene thereof, an expression vector and an application. The monoclonal antibody can stop the HCV from infecting susceptible cells and is fully-human-derived, and the antibody has greatly reduced immunogenicity, good affinity, good treatment effect and low side effects as compared with other animal-derived anti-HCV molecules.
Owner:JINAN UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com