Polypeptide sequence combined with bovine viral diarrhea E2 protein and application of polypeptide sequence
A bovine viral diarrhea, polypeptide sequence technology, applied in the fields of peptides, material testing products, instruments, etc., can solve the problems of cattle industry loss, persistent infection, etc., and achieve the effect of low detection cost and good affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1 Screening of phage random peptide library
[0021] Coat the polyvinyl chloride plate with the purified expressed BVD E2 protein, 100 μl per well (100 μl / ml), place it at 4°C overnight, wash with PBST and then block with 5% BSA solution.
[0022] Add 100 μl of the diluted phage peptide library to the corresponding wells, incubate at room temperature for 2 hours, wash with TBST solution 8-10 times, add 200 μl of eluent to each well, shake for 5-8 minutes at room temperature, aspirate and elute liquid and neutralized to neutral.
[0023] The eluate after neutralization can be used for titer determination and the next round of culture; the phage after LB culture are collected by centrifugation for the next round of screening.
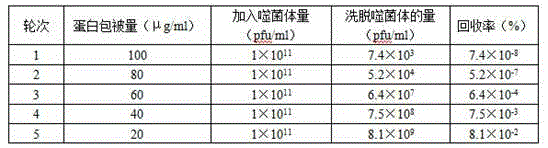
[0024] The enrichment and identification of phages in different screening rounds are shown in Table 1, and the titer determination results of the eluate are shown in Table 2.
example 2
[0025] Example 2 Identification of Screening Peptides
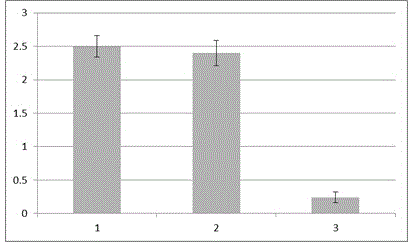
[0026] (1) Sonicate the inoculated MDBK cell culture solution of bovine viral diarrhea virus, and then coat the ELISA plate at 2 μg / ml (protein amount); in the same way, artificially express the bovine viral diarrhea virus E2 protein Coated with MDBK not inoculated with virus as a control. When performing specificity tests, different viral proteins were coated.
[0027] (2) Add the artificially synthesized polypeptide KRLREL coupled with horseradish peroxidase at an amount of 100 ng / ml to 50 μl per well of the above ELISA plate, mix well on a shaker, and incubate at 37°C in the dark for 30 minutes;
[0028] (3) Wash 6 times with a microplate washer, or manually shake off the liquid in the wells of the microplate, fill each well with diluted buffer solution, and then spin dry, repeat the washing process 6 times;
[0029] (4) Add TMB solution to the corresponding microwell according to the required amount, add 100 μl to...
example 3
[0036] Example 3 Affinity Identification of Screening Peptides
[0037] (1) Sonicate the inoculated MDBK cell culture solution of bovine viral diarrhea virus, and then coat the ELISA plate at 2 μg / ml (protein amount); in the same way, artificially express the bovine viral diarrhea virus E2 protein Coated with MDBK not inoculated with virus as a control. When performing specificity tests, different viral proteins were coated.
[0038] (2) Add the artificially synthesized polypeptide KRLREL coupled with horseradish peroxidase, and add 50 μl of the enzyme-labeled polypeptide at different concentrations to the above ELISA plate, mix well on a shaker, and incubate at 37°C in the dark for 30 minutes;
[0039] (3) Wash 6 times with a microplate washer, or manually shake off the liquid in the wells of the microplate, fill each well with diluted buffer solution, and then spin dry, repeat the washing process 6 times;
[0040] (4) Add TMB solution to the corresponding microwell accor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com