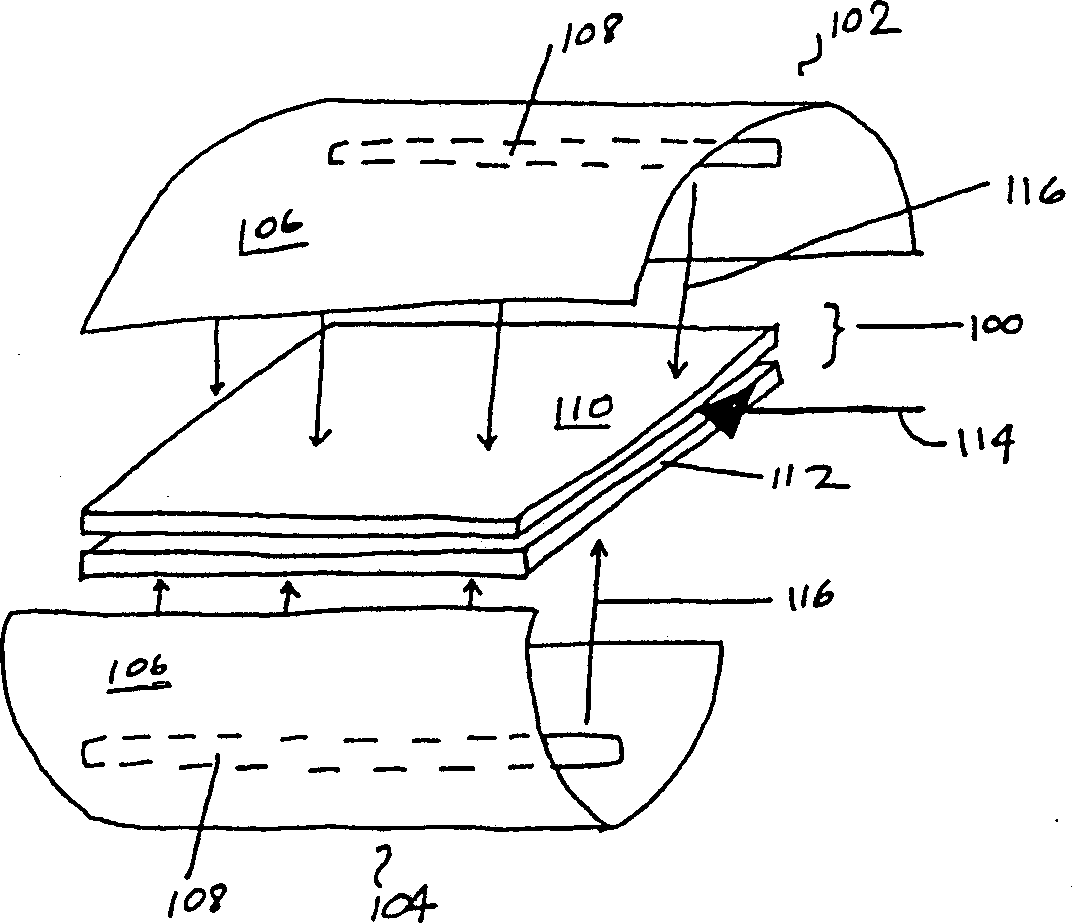
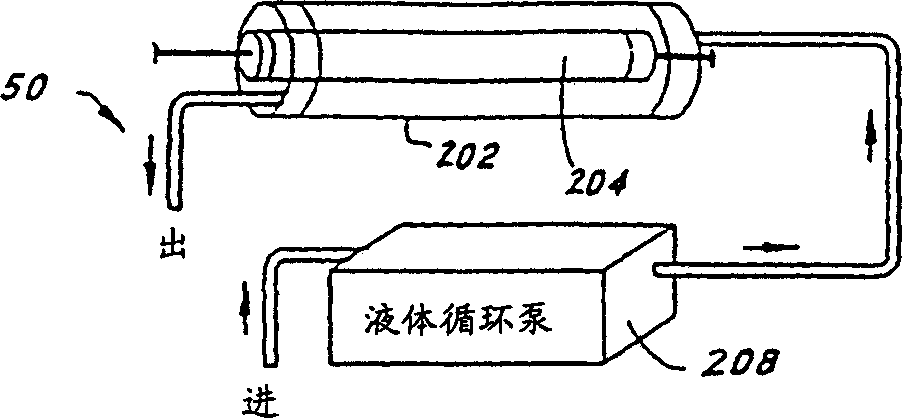
Patents
Literature
96 results about "Bovine viral diarrhea virus BVDV" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
BVD virus or BVDV is the causative organism of the disease. During the 1970s, it was learned that bovine viral diarrhea virus (BVDV) was closely related to the hog cholera virus. Today the BVDV has been successful in infecting cattle of all ages.
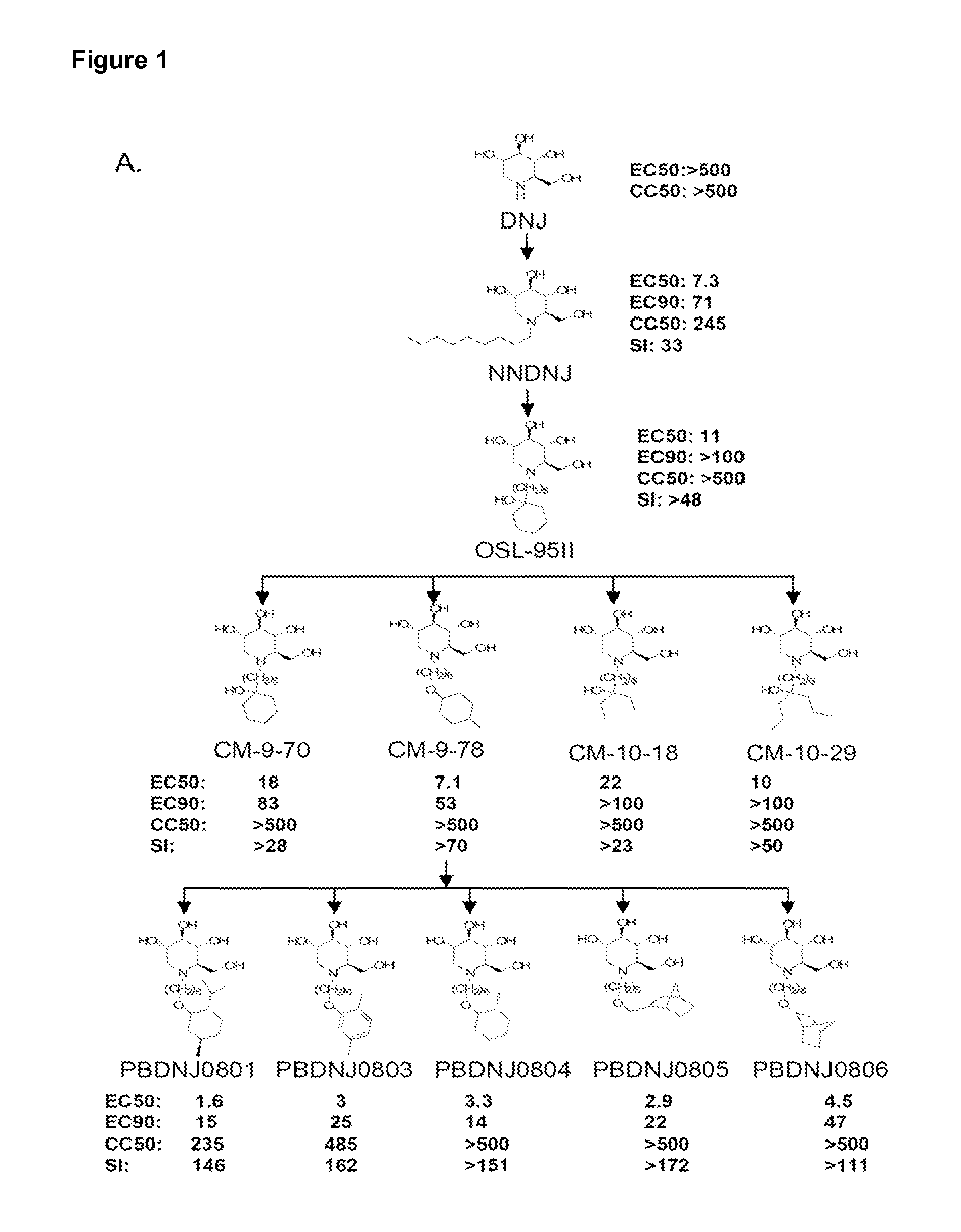
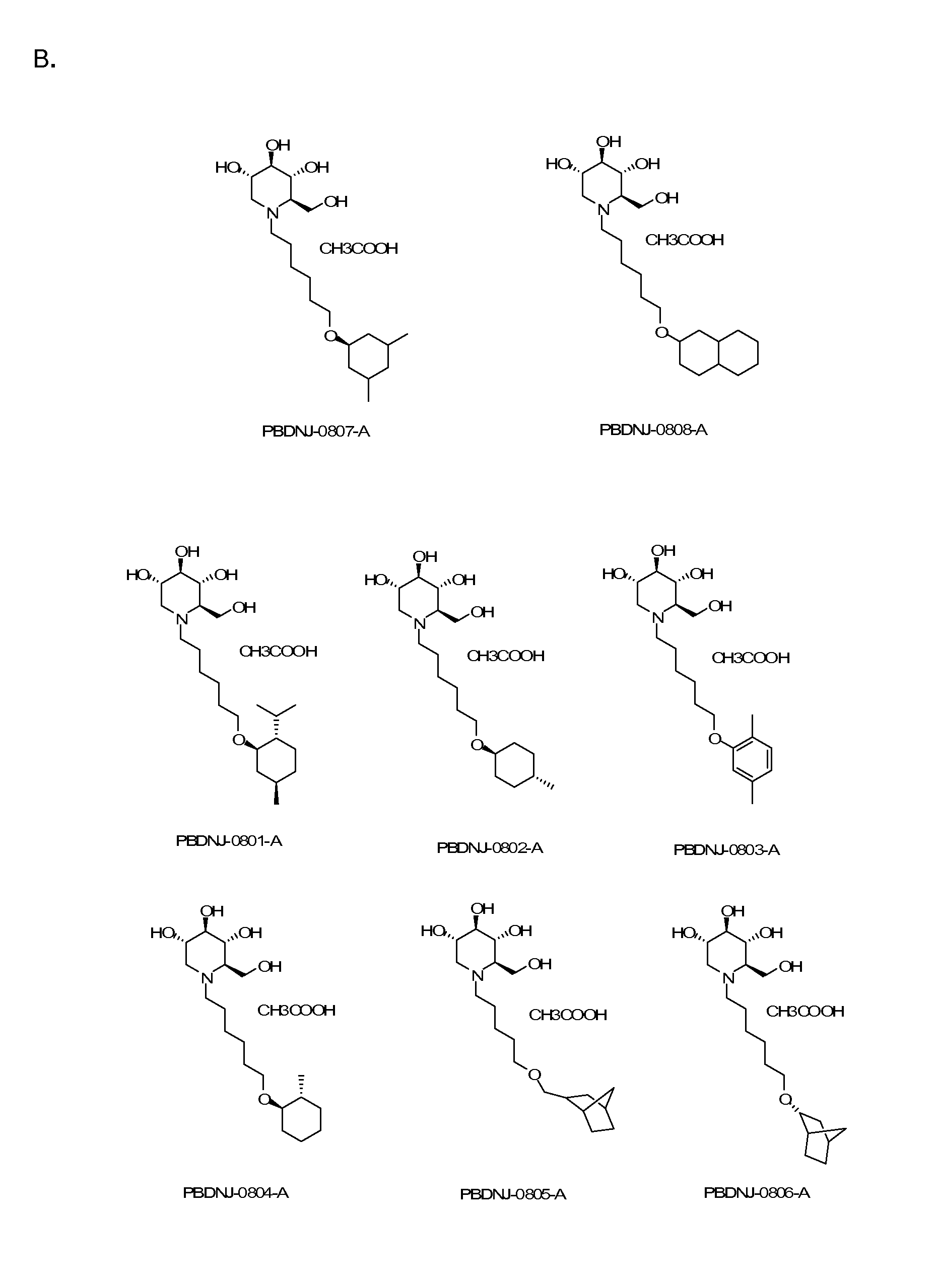
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
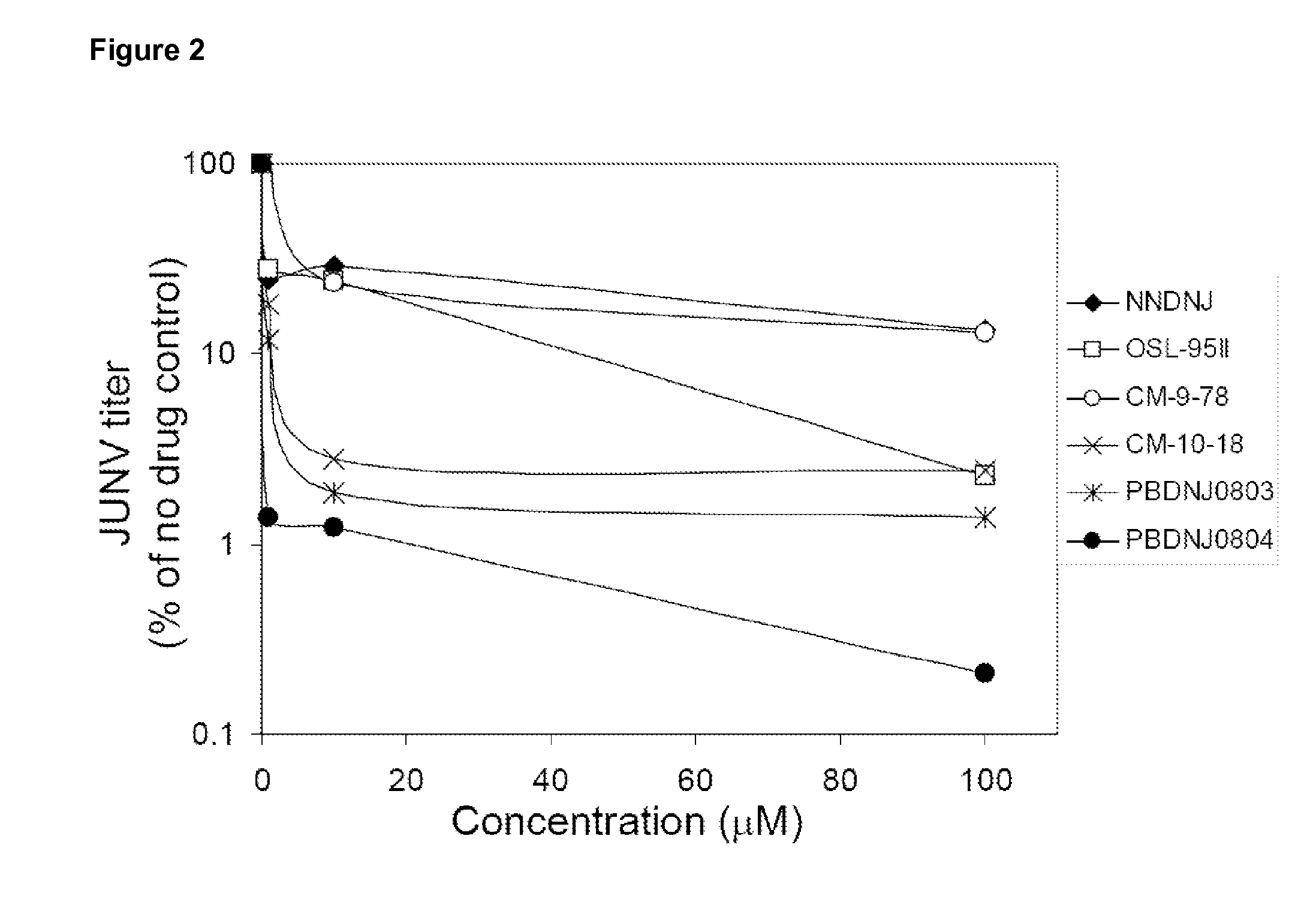
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
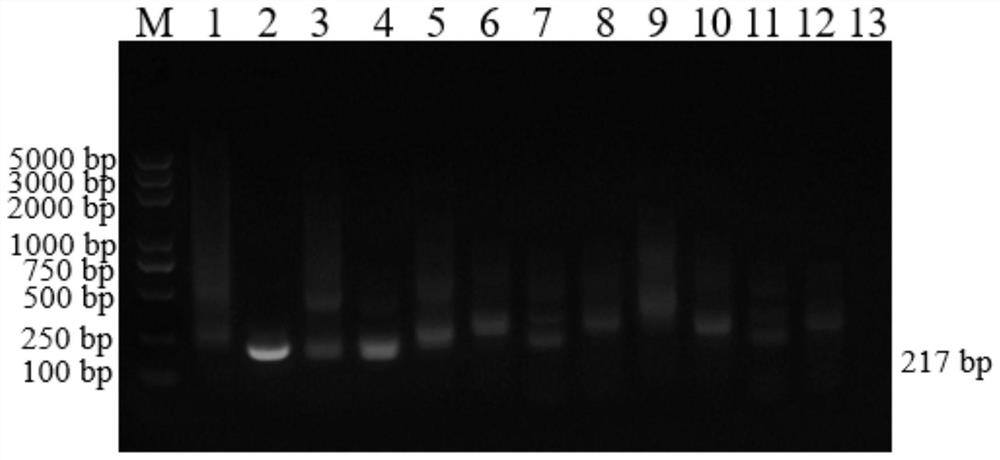
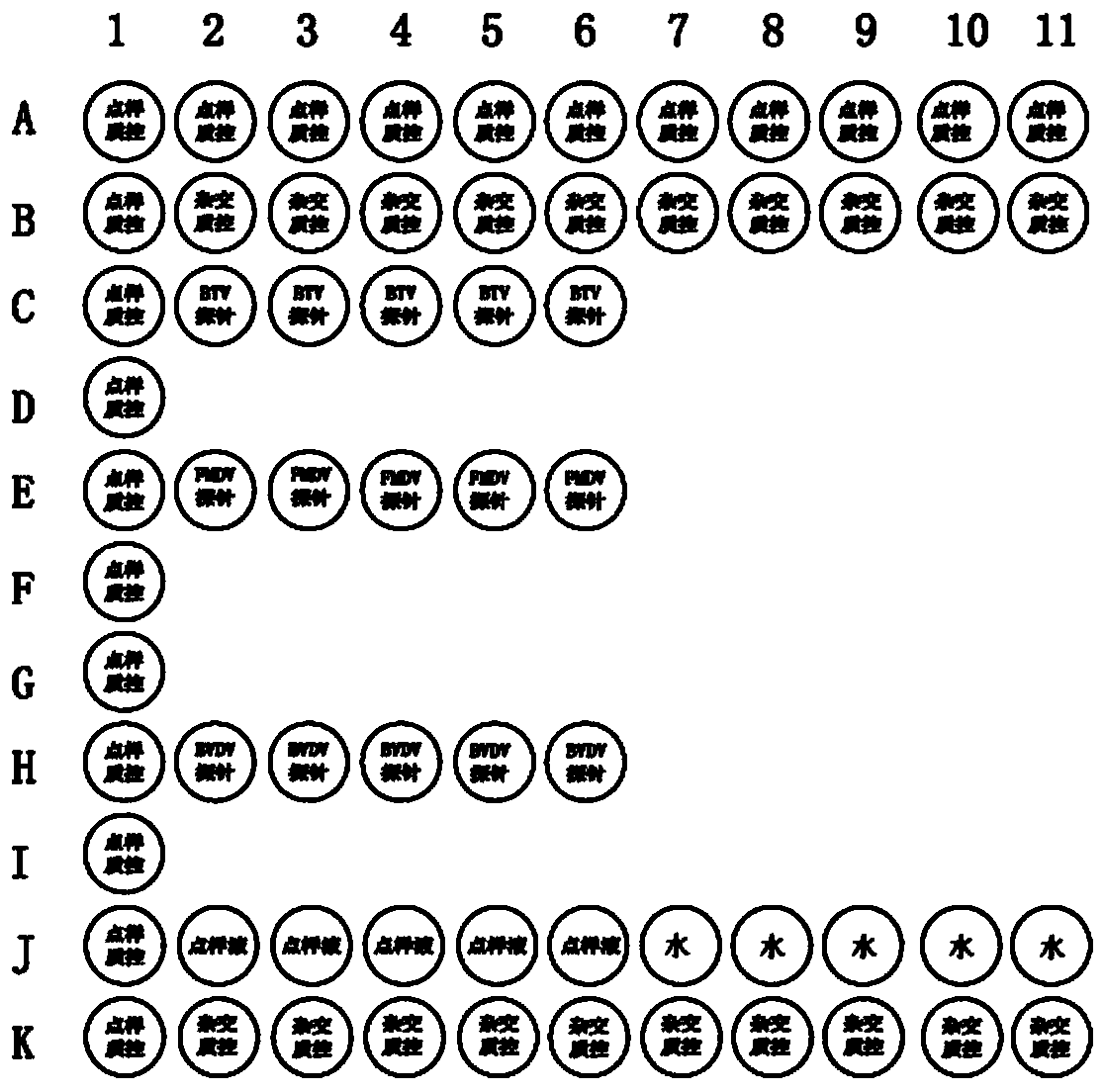
Multiplex PCR (polymerase chain reaction) primer, probe and gene chip for detecting bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus
InactiveCN103695566AImprove throughputShorten diagnostic timeMicrobiological testing/measurementDNA/RNA fragmentationForward primerMultiplex
The invention relates to a multiplex PCR (polymerase chain reaction) primer, a probe and a gene chip for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus. The multiplex PCR primer and probe have the nucleotide sequences shown by SEQ ID No.1 to SEQ ID and No.9. The gene chip comprises a solid-phase carrier, a sample application quality control probe, a positive hybrid quality control probe and a multiplex PCR primer for detecting the bluetongue virus, foot and mouth disease virus and bovine viral diarrhea virus and the corresponding probe. In the invention, the forward primers of three viruses are marked with fluorescence, a gene chip detection technology carrying three viruses in animal fur is established based on multiplex RT-PCR (reverse transcription-polymerase chain reaction), and the RNA virus in the fur can be sensitively and specifically detected with high flux; the three viruses are screened at the same time in detection once, and the situation that a specific method is required for each virus before is changed, thereby saving the diagnosis time, meeting the needs for quick detection of mass imported / exported fur samples of the exit-entry inspection and quarantine departments and the fur import and export enterprises, and realizing relatively high application values.
Owner:徐超
Double-antibody biotin-Avidin ELISA (enzyme-linked immuno sorbent assay) detection kit for cattle viral diarrhea virus and application method thereof
InactiveCN102023217AAvoid missing detectionStrong specificityMaterial analysisBovine virus diarrhea virus AntigenAssay
The invention relates to a double-antibody biotin-Avidin ELISA (enzyme-linked immuno sorbent assay) detection kit for cattle viral diarrhea virus antigen and an application method thereof. The kit comprises washing liquid for ELISA detection, developing liquid, stopping liquid, Streptavidin-HRP, positive control and negative control and is characterized by further comprising an ELISA plate coating cattle viral diarrhea virus single antibody 3D8 and a cattle viral diarrhea virus single antibody 3F9 marked by biotin. The detection without cross reaction is performed by using the kit, thereby being capable of preventing leak detection caused by low titer in the antibody and having high specificity; a biotin-affine sensitizer is used for amplification, thereby increasing the flexibility, improving the detection rate, and simultaneously reducing the amount of second antibody; and the kit is suitable for being used widely.
Owner:YANGZHOU UNIV
Primer combination for simultaneously identifying 8 kinds of cattle pathogens and GeXP detection method
InactiveCN106191309APromote healthy developmentImprove throughputMicrobiological testing/measurementMicroorganism based processesVesicular StomatitisPathogen
The invention discloses a primer combination for simultaneously identifying 8 kinds of cattle pathogens and a GeXP detection method. The primer combination provided by the invention consists of a primer pair I, a primer pair II, a primer pair III, a primer pair IV, a primer pair V, a primer pair VI, a primer pair VII and a primer pair VIII. The invention also provides the GeXP detection method for simultaneously identifying foot and mouth disease viruses, blue tongue viruses, vesicular stomatitis viruses, bovine viral diarrhoea viruses, bovine rotaviruses, enterotoxigenic escherichia coli, infectious bovine rhinotracheitis viruses and peste des petits ruminants viruses. The GeXP detection method built by the invention can be used for simultaneously identifying 8 kinds of cattle infectious disease pathogens. The method has the characteristics of high flux, specificity and high sensitivity, and can be used for the cattle disease epidemiology monitoring and the emergent epidemic situation identification and diagnosis, and guarantees the healthy development of cattle rearing industry.
Owner:GUANGXI VETERINARY RES INST
Polypeptide sequence combined with bovine viral diarrhea E2 protein and application of polypeptide sequence
ActiveCN104597256AHigh affinityImprove bindingBiological material analysisPeptidesBiotinBacteriophage
The invention mainly relates to a polypeptide sequence combined with bovine viral diarrhea E2 protein and application of the polypeptide sequence. The polypeptide sequence is KRLREL and is a linear combined polypeptide, the polypeptide sequence can be prolonged and modified by taking the polypeptide sequence as a core, and modification materials can be but not limited to nano materials, fluorescent materials, enzymes, biotin and specific protein. By adopting a phage peptide library screening technique, the expressed and purified bovine viral diarrhea E2 protein can be screened for multiple rounds, and polypeptide clone strains which can be specifically combined with the bovine viral diarrhea E2 protein can be ultimately obtained; the polypeptide clone strains can be selected for sequencing, the core sequence of the polypeptide is KRLREL, the manually synthesized polypeptide used for ELISA combination experiment shows that the synthesized polypeptide can be well combined with the bovine viral diarrhea E2 protein; the process is simple, and the operation is relatively convenient compared with the operation that expression protein is manually expressed and is further immunized to obtain a protein antibody; by marking the polypeptide, a kit or a test paper bar (card) for quantitative and qualitative detection on the bovine viral diarrhea E2 protein can be rapidly produced.
Owner:HENAN ACAD OF AGRI SCI
Application of andrographolide C15 substitution derivative in manufacturing anti-hepatitis drug
ActiveCN102600129ATo clarify the anti-BVDV activity in vitroOrganic active ingredientsDigestive systemBovine Viral Diarrhea VirusesCytopathic effect
The invention discloses the application of an andrographolide C15 substitution derivative in manufacturing an anti-hepatitis drug, which belongs to the technical field of pharmaceutical chemistry. According to the invention, MDBK (mardin-darby bovine kidney cells) and BVDV (bovine viral diarrhea virus) are utilized to study the inhibitory action of the compound on cytopathic effect caused by the virus through an external screening model. Through the screening of a large number of andrographolide derivatives, the compound with a structure of general formula 1 is found to significantly inhibit MDBK cytopathic effect caused by the BVDV; the andrographolide C15 substitution derivative has high efficiency and low toxicity, thereby being used for manufacturing medicaments for treating and preventing hepatitis and having good prospect in development and application. The general formula 1 is shown in the description.
Owner:ZHENGZHOU UNIV
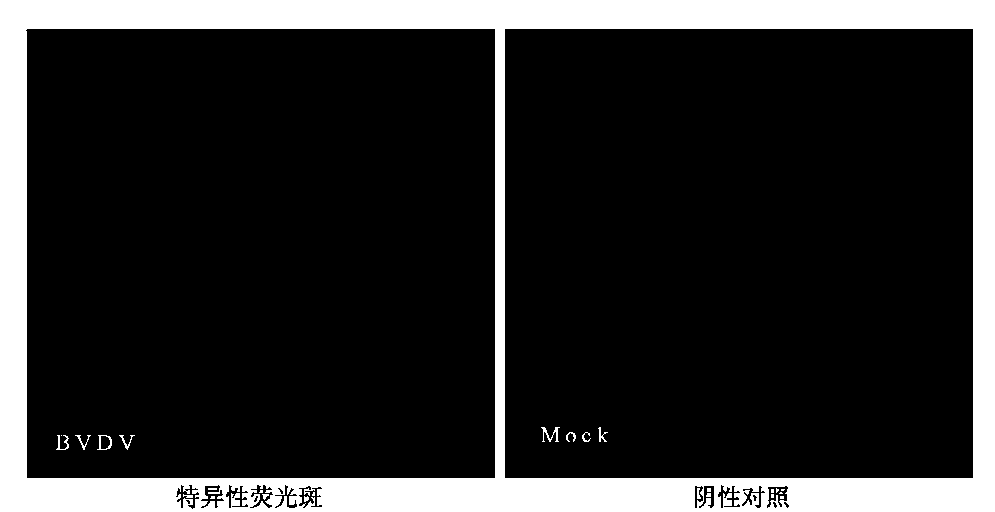
Method for detecting bovine viral diarrhea virus by virtue of indirect immunofluorescence
Owner:成都史纪生物制药有限公司
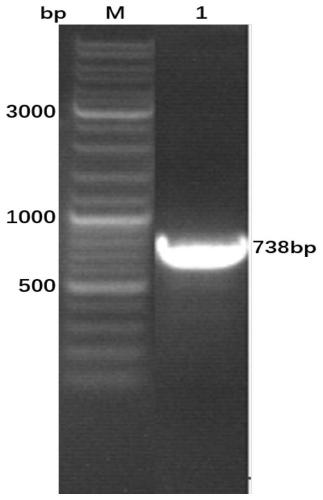
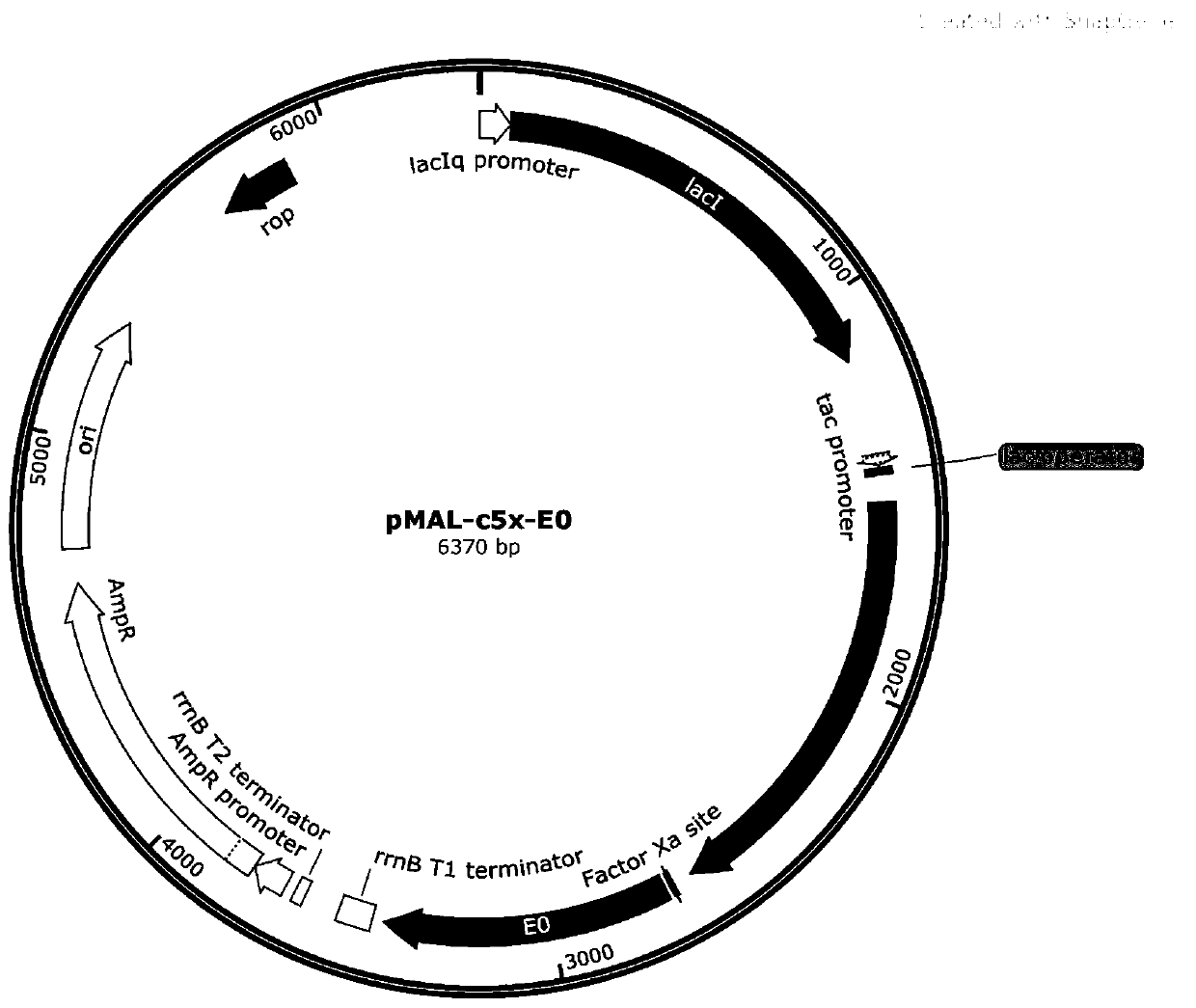
Bovine viral diarrhea virus E0 protein amino acid and preparation method thereof
ActiveCN111057132AEasy to purifyImprove solubilitySsRNA viruses positive-senseVirus peptidesBovine Viral Diarrhea VirusesTarget gene
The invention provides a bovine viral diarrhea virus E0 protein amino acid and a preparation method thereof. The amino acid sequence of the bovine viral diarrhea virus E0 protein amino acid is represented by SEQ ID NO:3. The preparation method comprises the following steps:S1, obtaining a BVDV NADL whole genome sequence from NCBI GenBank, and finding out a BVDV E0 gene sequence from the BVDV NADLwhole genome sequence; S2, optimizing and synthesizing the E0 gene sequence obtained in step S1; and S3, selecting a prokaryotic expression vector pMal-c5X with an MBP label, connecting the E0 gene with the pMal-c5X, inserting an E0 fragment into the pMal-c5X, converting the connected expression vector pMAL-c5x-E0 into an expression host bacterium BL21 (DE3), inducing the expression of the obtained recombinant protein, and separating and purifying the target protein. The target gene E0 is optimized, and is subjected to fusion expression with the MBP tag protein to finally achieve the efficientsoluble expression of the E0 protein, so the bovine viral diarrhea virus E0 protein amino acid has the advantages of high expression yield, good solubility, and convenience in expression protein purification; and the solubility of the Escherichia coli expression protein can be improved during the fusion expression of the foreign protein and the MBP so as to provide convenience for the purification of the expression protein.
Owner:SICHUAN AGRI UNIV
Kit, primer and probe for simultaneously detecting bovine viral diarrhea virus, bovine rotavirus and bovine coronavirus
ActiveCN112094953ASimple and fast operationStrong specificityMicrobiological testing/measurementMicroorganism based processesBovine rotavirusBovine Viral Diarrhea Viruses
The invention relates to the field of virus detection, and particularly relates to a kit, a primer and a probe for simultaneously detecting bovine rotavirus, bovine rotavirus and bovine coronavirus. The nucleotide sequences of the primer and the probe in the kit are shown as SED ID NO: 1-SEQ ID NO: 9. The kit has the technical advantages of being easy and convenient to operate, high in specificity, high in sensitivity, good in repeatability and capable of simultaneously achieving qualitative detection and accurate quantification of BVDV, BRV and BCoV.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Infectious bovine viral diarrhea virus clone
The invention belongs to the field of animal health and in particular Bovine Viral Diarrhea Virus (BVDV). The invention provides infectious BVDV clones and methods to produce said BVDV clones. The invention further relates to methods of attenuating said clones, attenuated BVDV clones and vaccines comprising said attenuated clones.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Cattle reproductive disease vaccines
InactiveUS20070298053A1Avoid infectionAntibacterial agentsSsRNA viruses negative-senseBovine Viral Diarrhea VirusesVirus type
The present invention relates to combination vaccines and methods for treating or preventing diseases or disorders in an animal caused by infection by Bovine Viral Diarrhea Virus (BVDV) Types 1 and 2, Bovine Herpes Virus Type-1 (BHV-1), Bovine Respiratory Syncytial Virus (BRSV), Parainfluenza Virus (PI3), Campylobacter fetus, Leptospira canicola, Leptospira grippotyphosa, Leptospira hardj-prajitno, Leptospira icterohaemmorrhagiae, Leptospira hardjo-bovis and Leptospira pomona by administering to the animal an effective amount of a combination vaccine. The combination vaccine can be a whole or partial cell inactivated or modified live preparation.
Owner:PFIZER INC
CRISPR-Cas13 a-based bovine viral diarrhea virus detection method
ActiveCN111979357AQuick checkStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBovine Viral Diarrhea VirusesNucleic acid detection
The invention discloses a primer for detecting bovine viral diarrhea virus, a kit prepared by using the primer and used for detecting BVDV, and a CRISPR-Cas13 a-based bovine viral diarrhea virus detection method. The primer is 5'-GGGGAUUUAGACUACCCCAAAAACGAAGGGGACUAAAACGCCAUCCAACGAACUCACCACUGUUGCU-3'; the kit at least comprises the primer; a sample to be detected is added into the kit for detection. According to the CRISPR-Cas13 a-based bovine viral diarrhea virus detection method, a CRISPR-Cas13a system is successfully applied to nucleic acid detection of the BVDV for the first time, and whether the BVDV is contained or not can be identified only by adding a micro-scale sample. The CRISPR-Cas13 a-based bovine viral diarrhea virus detection method has high specificity and sensitivity. The method provides a wide prospect for early diagnosis of the BVDV.
Owner:SHIHEZI UNIVERSITY
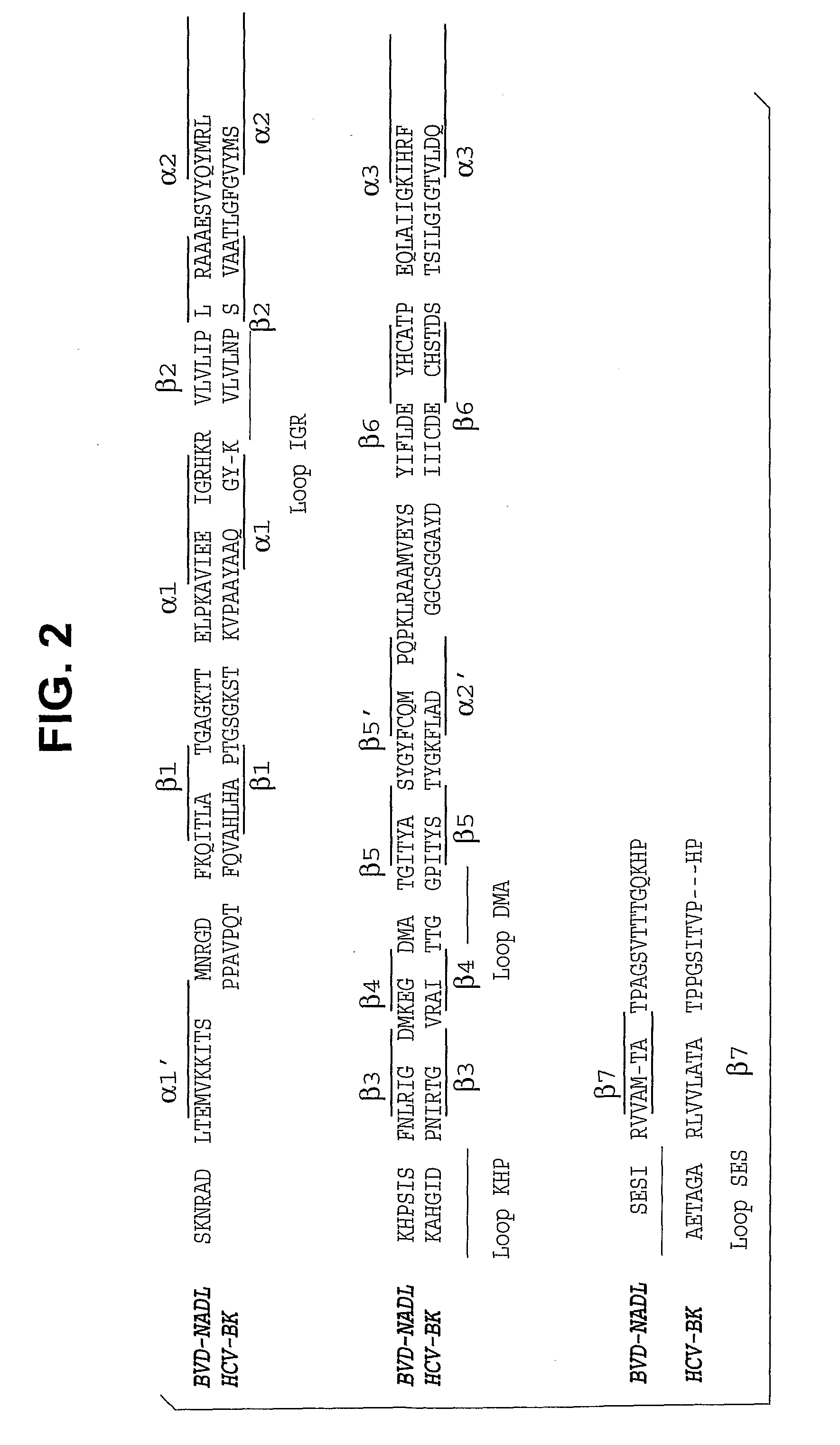
Imidazo [1, 2-a] pyrrolo [3, 2-c] pyridine compounds useful as pestivirus inhibitors
Owner:UNIV CLERMONT AUVERGNE +2
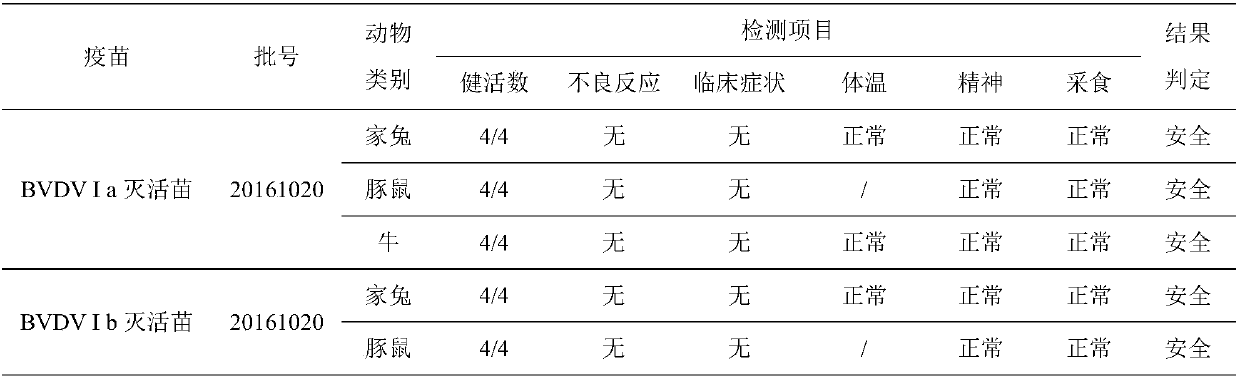
Bovine viral diarrhoea virus inactivated vaccine and preparation method thereof
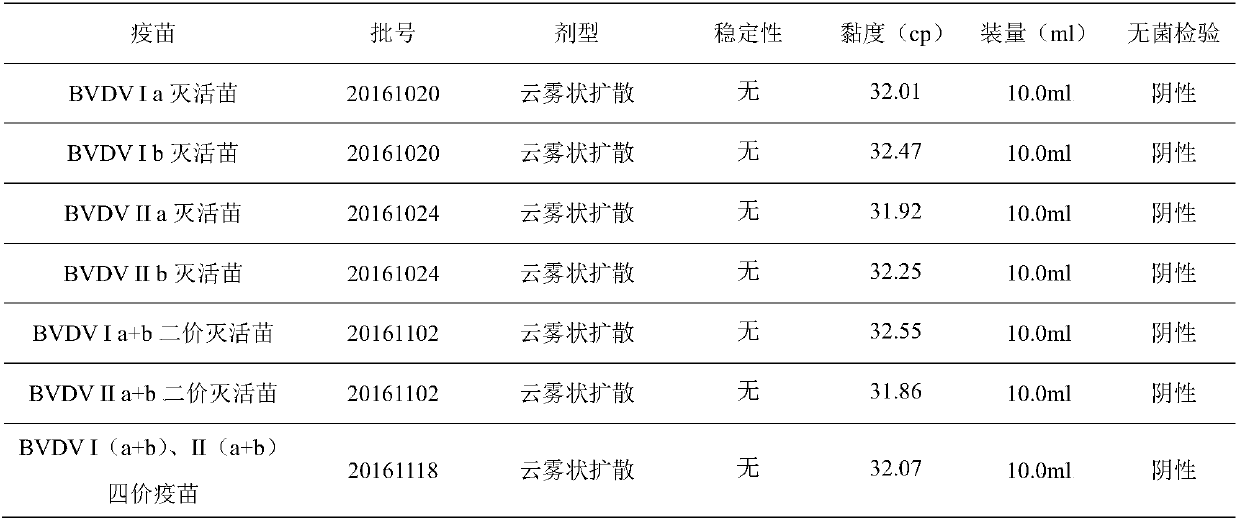
The invention relates to an inactivated vaccine of a bovine viral diarrhoea virus (BVDV) and a preparation method of the inactivated vaccine, and belongs to the field of veterinary biological products. The vaccine is composed of four gene subtypes including BVDV I a, BVDV I b, BVDV II a and BVDV II b. The bovine viral diarrhoea inactivated vaccine is prepared by carrying out virus proliferation byusing a continuous cell line cultured by cell spinner bottles, full suspension and micro carriers, and optimizing a vaccine preparation process and the like. Safety and effectiveness testing resultsof the vaccine show that bovines are free of local and systemic adverse reactions after the bovine is immunized by the bovine viral diarrhoea virus inactivated vaccine; all the bovines receive immuneprotection; the vaccine is safe and reliable, and is capable of preventing diseases caused by different BVDV gene subtypes. The vaccine also comprises various types of monovalent inactivated vaccines,bivalent inactivated vaccines and tetravalent inactivated vaccines prepared from the four gene subtypes above.
Owner:QILU ANIMAL HEALTH PROD
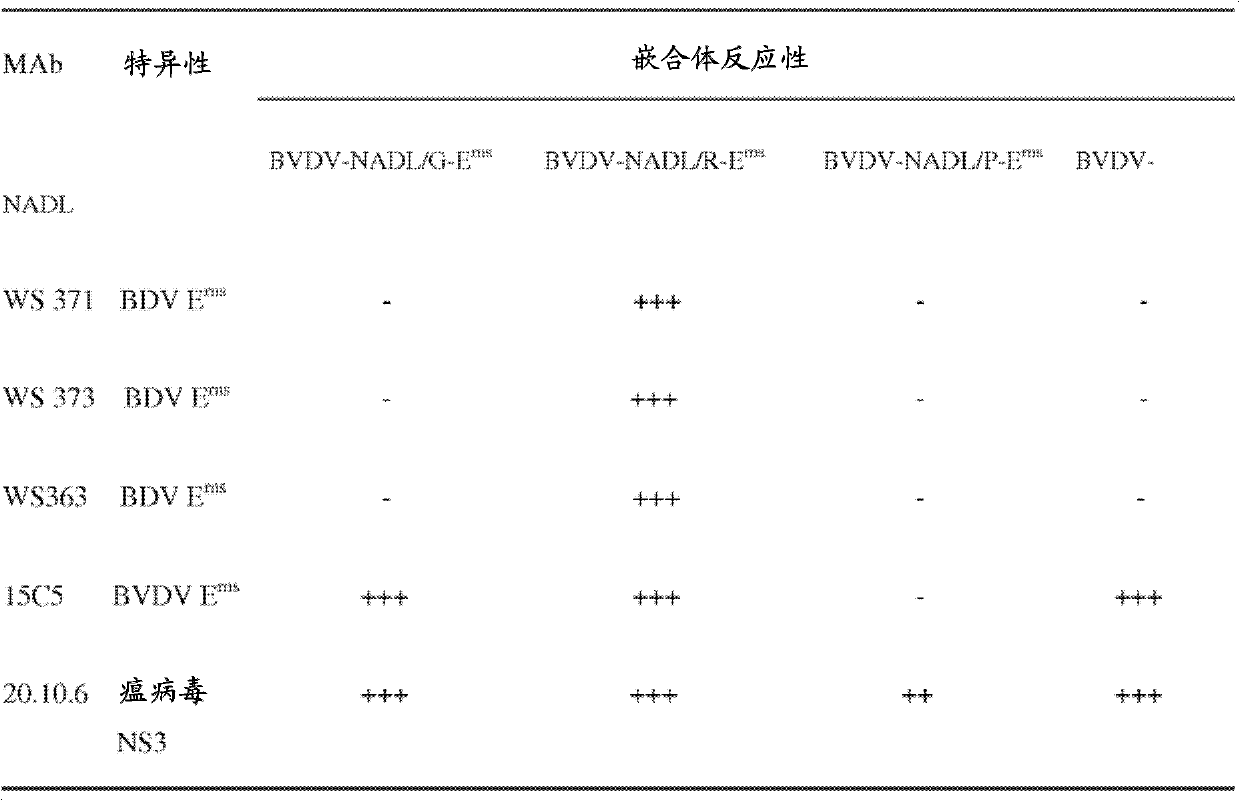
Bovine viral diarrhea virus with a modified erns protein
The present invention relates to chimeric pestiviruses having utility as immunogenic compositions and vaccines wherein said chimeric pestivirus comprises a bovine viral diarrhea virus which does not express its homologous Ems protein, further wherein said chimeric pestivirus expresses a heterologous Ems protein derived from another pestivirus, or a natural, synthetic or genetic variant of said heterologous Erns protein. Also described herein are methods and kits for treating or preventing the spread of bovine viral diarrhea virus infection, as well as methods and kits for differentiating between vaccinated and wild-type infected animals.
Owner:ZOETIS SERVICE LLC
RAA primer, probe and method for detecting knopvelsiekte virus
ActiveCN110592286AAccurate detectionNo cross reactionMicrobiological testing/measurementDNA/RNA fragmentationBovine parainfluenza virusBovine Viral Diarrhea Viruses
The invention discloses a primer, a probe and a method for detecting knopvelsiekte virus by a RAA fluorescence method. The primer and the probe are suitable for the detection by the RAA fluorescence method, can accurately detect knopvelsiekte virus plasmids without cross reaction with mycoplasma, bovine infectious rhinotracheitis virus, bovine viral diarrhea virus, bovine parainfluenza virus, bovine respiratory syncytial virus, goat pox virus and sheep pox virus, and have a specificity of 100%. The method is fast and easy to achieve high throughput, and can reduce time and cost for detection.The method for rapid detection of DNA of the knopvelsiekte virus based on the RAA fluorescence method has high sensitivity reaching 10 copies / reaction.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT +1
Bovine viral diarrhea-mucosal virus with modified erns protein
The invention relates to a chimeric plague virus that can be used for an immunogenicity composition and a vaccine. The chimeric plague virus contains a bovine viral diarrhea-mucosal virus which is not expressing Erns protein with the same source, and expression of the chimeric plague virus is from allos Erns protein of another plague virus or a natrual, synthetic or genetic variant of the allos Erns protein. The invention also describes a method and a kit for treating or preventing transmission of infection of the bovine viral diarrhea-mucosal virus, and a method and a kit for differentiating vaccinal animals and wild infected animals.
Owner:PFIZER INC
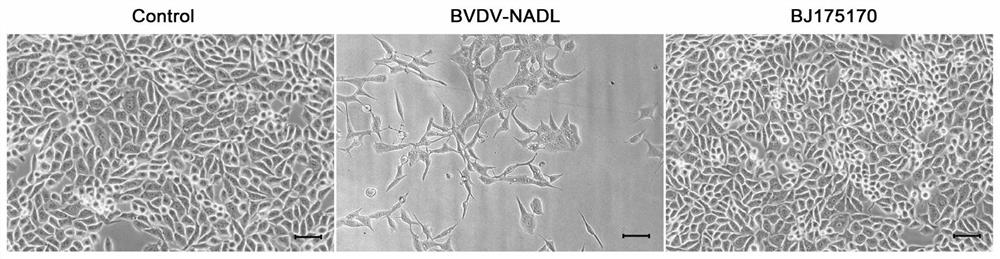
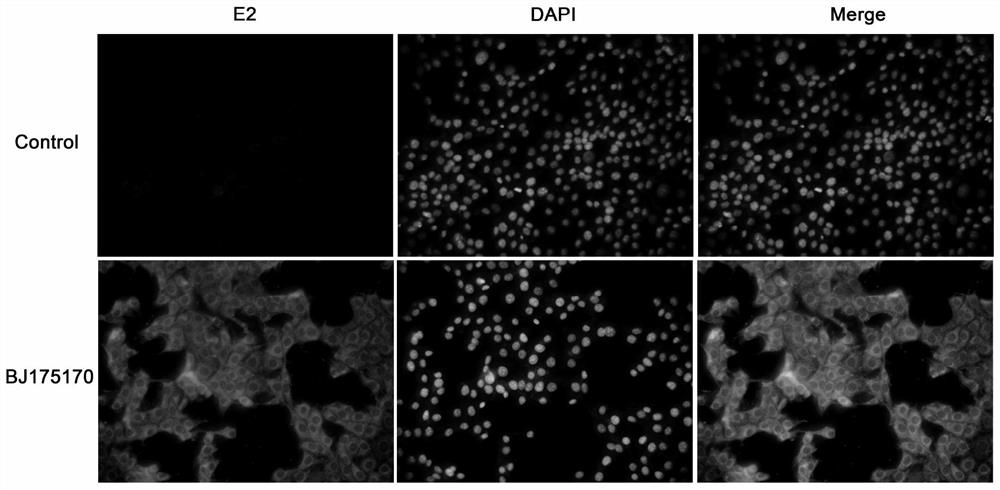
Bovine viral diarrhea virus BVDV-BJ175170 and application thereof
InactiveCN112359023AEasy to operateLow costSsRNA viruses positive-senseViral antigen ingredientsBovine Viral Diarrhea VirusesSerum dilution
The invention provides a bovine viral diarrhea virus BVDV-BJ175170 and application of the bovine viral diarrhea virus BVDV-BJ175170, wherein the preservation number of the BVDV-BJ175170 is CGMCC NO. 18339. According to the invention, closing and expressing of the E<rns> protein of the bovine viral diarrhea virus BVDV-BJ175170 is used for ELISA detection of BVDV and preparation of a kit; the kit disclosed by the invention is convenient to operate, low in cost and good in repeatability, can be used for detecting the viral diarrhea virus antibody of which the serum dilution reaches 1:6400, overcomes the defect of lack of a single antigen for BVDV-PI cattle diagnosis at present, has relatively high sensitivity, reduces the omission ratio, is relatively high in precision, and can be used for antibody level monitoring; and according to the method, the high-specificity reaction of the antigen and the antibody is combined, the E<rns> protein antibody in serum can be detected more accurately, asensitive detection means is provided for BVDV epidemiological investigation in China, and the method can be used for early diagnosis of bovine viral diarrhea virus infection.
Owner:CHINA AGRI UNIV
Use of 17-ketosteroid compounds, as well as derivatives, metabolites and precursors for treatment of hapatitis C type virus and other togavirus infections
The present invention provides 17-keto steroid compounds and their derivatives, metabolites and precursors and pharmaceutically acceptable salts thereof for the treatment or prevention of hepatitis C virus and / or hepatitis G virus in patients requiring such treatment, these compounds Collectively referred to as "compounds of the present invention". In addition, the present invention provides methods of treating or preventing togavirus infections including one or more of alphaviruses, flaviviruses (such as yellow fever virus), hepatitis C virus, and hepatitis G virus, Infection with rubella virus or pestiviruses (such as bovine viral diarrhea virus). In addition, the present invention provides combination therapy comprising the administration of one or more compounds described herein and the administration of one or more compounds selected from the group consisting of plasma concentration-enhancing compounds, macrophage stimulating factors, oxidative agents, ribavirin, and alpha interferon compounds and / or oxygen supply. The compounds of the invention may also be used to alleviate or alleviate one or more symptoms associated with togavirus infection.
Owner:HOLLIS EDEN PHARMA
E2 recombinant protein and application thereof
PendingCN110746495AImprove purification effectStrong neutralizing activitySsRNA viruses positive-senseVirus peptidesBovine Viral Diarrhea VirusesBlot
The invention provides an E2 recombinant protein. The E2 recombinant protein has an amino acid sequence of a sequence table SEQ.ID.No.1, and the E2 recombinant protein has a base sequence of a sequence table SEQ.ID.No.2. The invention also provides application to an indirect ELISA kit for detecting bovine viral diarrhea virus antibodies. The E2 recombinant protein provided by the invention has high accuracy, not only is the neutrality of the protein ensured, but also the trouble of high mutation rate is avoided, and the recombinant protein is more beneficial to the development of a BVDV antibody detection technology. The E2 recombinant protein uses a traditional protein prokaryotic expression technology, the cost is low, the expression quantity is large, and after the successfully expressed E2 recombinant protein is subjected to urea gradient renaturation, the West blot detection reactionogenicity is strong. The E2 recombinant protein is applied to the indirect ELISA kit for detectingbovine viral diarrhea virus antibodies, and an established BVDV antibody ELISA detection method is high in sensitivity and good in specificity, is more beneficial to popularization and application ingrass-roots farmers, and is more beneficial to purification of BVDV in cattle herds in China.
Owner:SHIHEZI UNIVERSITY
Multi-nano-PCR primer group for detecting bovine rotavirus (BRV), bovine parvovirus (BPV) and bovine viral diarrhea virus (BVDV) and application of multi-nano-PCR primer group
ActiveCN110423845AShort stayAvoid crossbreedingMicrobiological testing/measurementAgainst vector-borne diseasesBovine rotavirusRotavirus RNA
The invention discloses a multi-nano-PCR primer group for detecting bovine rotavirus (BRV), bovine parvovirus (BPV) and a bovine viral diarrhea virus (BVDV) and application of the multi-nano-PCR primer group. The primer group contains dual-priming oligonucleotide (DPO) primer pairs used for detecting the BRV, the BPV and the BVDV correspondingly. A multi-DPO-nano-PCR detection method capable of simultaneously detecting the BRV, the BPV and the BVDV is established by combining DPO primers with a nano PCR technology. Compared with a conventional PCR method, the multi-DPO-nano-PCR detection method is time-saving and labor-saving, can quickly, accurately and specifically detect pathogens, and achieves high sensitivity on the basis of ensuring specificity. By providing the multi-nano PCR primergroup, the new method is provided for diagnosis of early infection and inapparent infection of the BRV, the BPV and the BVDV, a reliable technical means is provided for detection of clinical mixed infection of the BRV, the BPV and the BVD, and technical support is provided for epidemic disease testing, screening purification and comprehensive prevention and control.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Methods for inactivating pathogens using broad-spectrum pulsed light
InactiveCN1344170AImprove methodReliable methodPeptide/protein ingredientsInactivation/attenuationBovine Viral Diarrhea VirusesWhole blood product
A method of reducing pathogen content in a biologically derived composition by irradiating it with at least one high-intensity short-duration pulse of broad-spectrum incoherent polychromatic light. The biomolecule of interest retains biological activity in the resulting treated composition. Biologically derived compositions, such as serum, plasma or other blood products, including insulin, transferrin, heparin, collagen, coagulation factor VIII and / or coagulation factor IX, or containing monoclonal antibodies, or genetically engineered cell lines, etc. The content of pathogens, such as viruses, bacteria, pyrogens, fungi and / or prions present in the resulting protein composition is reduced by irradiation with broad spectrum pulsed light. A single pulse of broad spectrum light significantly reduces the amount of HIV-1, SV40, canine parvovirus or bovine viral diarrhea virus in a biologically derived composition.
Owner:PUREPULSE TECH
Primer combination and GeXP detection method for simultaneously identifying 5 bovine viral dermatitis viruses
InactiveCN105969913APromote healthy developmentImprove throughputMicrobiological testing/measurementMicroorganism based processesDisease epidemiologyInfectious laryngotracheitis virus
The invention discloses a primer combination and GeXP detection method for simultaneously identifying 5 bovine viral dermatitis viruses. The primer combination is composed of a primer pair I, a primer pair II, a primer pair III, a primer pair IV and a primer pair V. The invention also discloses a GeXP detection method for simultaneously identifying foot-and-mouth disease virus, bluetongue virus, vesicular stomatitis virus, bovine viral diarrhoea virus and infectious bovine rhinotracheitis virus. The GeXP detection method can simultaneously identify the 5 bovine viral dermatitis viruses. The method has the characteristics of higher flux, higher specificity and higher sensitivity, can be used for monitoring of bovine disease epidemiology and differential diagnosis of unexpected epidemic situations, and ensures the healthy development of cattle raising industry.
Owner:GUANGXI VETERINARY RES INST
Bovine viral diarrhea-bovine infectious rhinotracheitis bivalent subunit vaccine and preparation method and application thereof
PendingCN107174660AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsAntigenAdjuvant
The invention discloses a bovine viral diarrhea-bovine infectious rhinotracheitis bivalent subunit vaccine and a preparation method and application thereof, and belongs to the technical field of animal vaccines and animal biological products. The vaccine comprises bovine viral diarrhea virus E2 protein, bovine infectious rhinotracheitis virus gD protein and pharmaceutically acceptable adjuvant. The preparation method for the vaccine comprises the following steps that: 1) preparing the bovine viral diarrhea virus E2 protein and the bovine infectious rhinotracheitis virus gD protein; 2) mixing the bovine viral diarrhea virus E2 protein and the bovine infectious rhinotracheitis virus gD protein prepared in the 1) to prepare antigen liquid; 3) carrying out mixing emulsion on the anti-agent liquid with ISA 201VG at an volume ratio of 46:54. The vaccine has the advantages of high immunogenicity, high safety and no immunity interference; in addition, cows can be effectively prevented and protected from the infection of the bovine viral diarrhea virus and the bovine infectious rhinotracheitis virus, an effect of two prevention functions by one injection can be achieved, time and labor are saved, and cost is saved.
Owner:NOVO BIOTECH CORP
Vaccine diagnostics
ActiveUS9291624B2Microbiological testing/measurementImmunoassaysBovine Viral Diarrhea VirusesPestivirus
The present invention relates to improved diagnostic methods and kits for differentiating between (a) animals administered a chimeric pestivirus, and (b) animals infected with a wild-type bovine viral diarrhea virus (BVDV) or immunized with a conventional BVDV vaccine.
Owner:ZOETIS SERVICE LLC
Preparation and application of type 1 bovine viral diarrhea virus virus-like particles (BVDV-VLPs)
ActiveCN112961224AStrong humoral immune responseStrong immune responseSsRNA viruses positive-senseVirus peptidesBaculovirus expressionBovine Viral Diarrhea Viruses
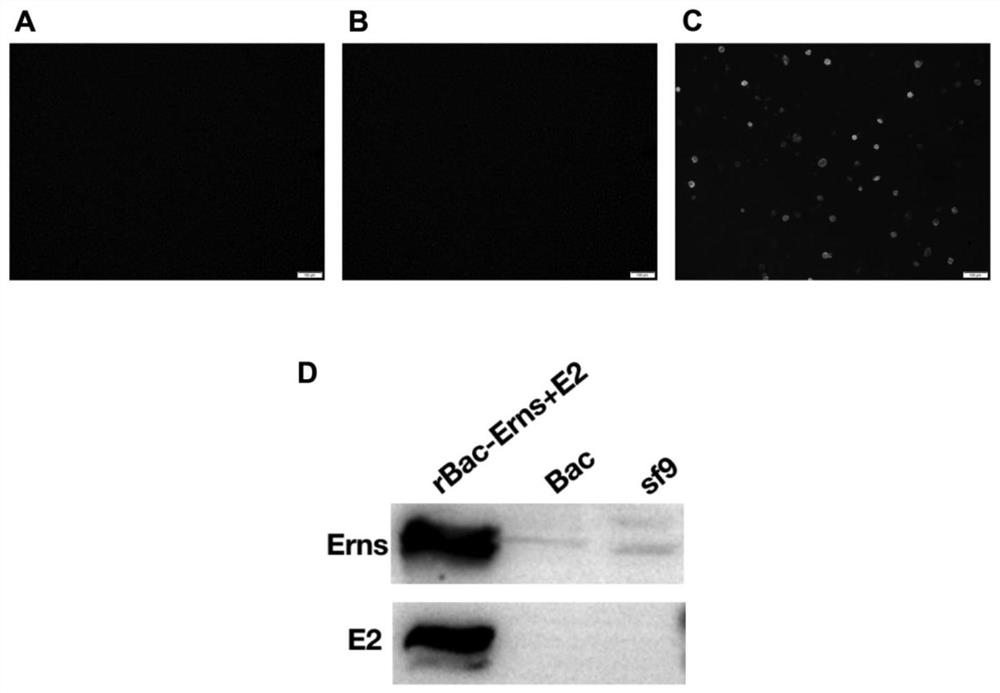
The invention discloses preparation and application of type 1 bovine viral diarrhea virus virus-like particles (BVDV-VLPs). The invention provides the type 1 BVDV-VLPs whcih are non-infectious virus particles composed of bovine viral diarrhea virus Erns protein and E2 protein. The BVDV-VLPs are prepared by using a baculovirus expression system (BEVS), the BVDV-VLPs are circular particles with the diameter of about 50 nm, and Erns and E2, which participate in assembly, exist in the form of homodimers. After a mouse is immunized by the BVDV-VLPs, the mouse can be induced to generate a humoral immune response level which is the same as that of an inactivated vaccine, and the humoral immune response level is higher than the cellular immune response level of the inactivated vaccine.
Owner:CHINA AGRI UNIV
Bovine viral diarrhea-infectious bovine rhinotracheitis bivalent subunit fusion vaccine and identification method thereof
ActiveCN112125961AImprove securityNo immune interferenceSsRNA viruses positive-senseViral antigen ingredientsAntigen epitopeAntigen
The invention relates to the technical field of biology, and particularly provides a bovine viral diarrhea-infectious bovine rhinotracheitis bivalent subunit fusion vaccine and an identification method thereof. The inventor optimizes genes of BVDV-NS3 and IBRV-VP8, and performs fusion expression on antigen epitope proteins of the BVDV-NS3 and the IBRV-VP8 to obtain polypeptide fragment compositions coded by SEQ ID NO.1 and SEQ ID NO.2. The polypeptide fragment compositions can be used for preparing bivalent subunit fusion vaccines, good protective efficacy can be generated after animals are immunized, the purpose of preventing bovine viral diarrhea and infectious bovine rhinotracheitis at the same time is achieved, and the effect of preventing two diseases by one injection is achieved. Meanwhile, a detection kit provided by the invention can be used for rapidly identifying and diagnosing whether cattle are immunized with vaccine strains or infected wild strains, so that the purification of bovine viral diarrhea virus and infectious bovine rhinotracheitis virus is realized.
Owner:天康制药股份有限公司
Triple RPA (recombinase polymerase amplification) detection kit for bovine viral diarrhea virus, bovine coronavirus and bovine rotavirus
ActiveCN112853000AConvenient and quick screeningLower requirementMicrobiological testing/measurementAgainst vector-borne diseasesBovine rotavirusBovine Viral Diarrhea Viruses
The invention discloses a triple RPA (recombinase polymerase amplification) detection kit for a bovine viral diarrhea virus, a bovine coronavirus and a bovine rotavirus. The triple RPA detection kit comprises RPA detection primer groups for the bovine viral diarrhea virus, the bovine coronavirus and the bovine rotavirus, wherein the RPA detection primer groups are respectively composed of nucleotide sequences shown as SEQ ID NO.1 and SEQ ID NO.5, SEQ ID NO.8 and SEQ ID NO.13, and SEQ ID NO.15 and SEQ ID NO.18. The kit is based on a recombinase polymerase amplification method, cDNA formed by RNA reverse transcription is used as a template for amplification reaction, and a detection result is obtained by nucleic acid gel electrophoresis. The kit can be used for simultaneously detecting the bovine viral diarrhea virus, the bovine coronavirus and the bovine rotavirus, is simple to operate, has relatively high sensitivity and specificity, and provides a rapid detection reagent for on-site screening of the bovine diarrhea virus.
Owner:NORTHWEST A & F UNIV
BVDV (Bovine Virus Diarrhea Virus) SMU-Z6/1a/SC/2016 isolate and application thereof
ActiveCN108707589AHigh titerImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantAttenuated vaccine
The invention discloses a BVDV (Bovine Virus Diarrhea Virus) SMU-Z6 / 1a / SC / 2016 isolate and application thereof. A preservation number of BVDV is CCTCC (China Center for Type Culture Collection) NO: V201813, and the BVDV SMU-Z6 / 1a / SC / 2016 isolate is preserved in CCTCC. The BVDV has higher virus titer, a BVDV strain has good immunogenicity, an inactivated vaccine can be prepared from inactivated BVDV and an 201 adjuvant and is capable of immunizing calf and is capable of generating a higher antibody, and the BVDV strain is a good BVDV vaccine candidate strain. A diagnostic reagent, the inactivated vaccine and an attenuated vaccine which are relevant to the BVDV can be prepared on the basis of the BVDV, and the BVDV SMU-Z6 / 1a / SC / 2016 isolate has a wide market application prospect in diagnosison BVDV regional epidemic diseases and vaccine prevention and control.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Marked Bovine Viral Diarrhea Virus Vaccines
InactiveUS20080305130A1SsRNA viruses positive-senseViral antigen ingredientsDNA unwinding enzymeWild type
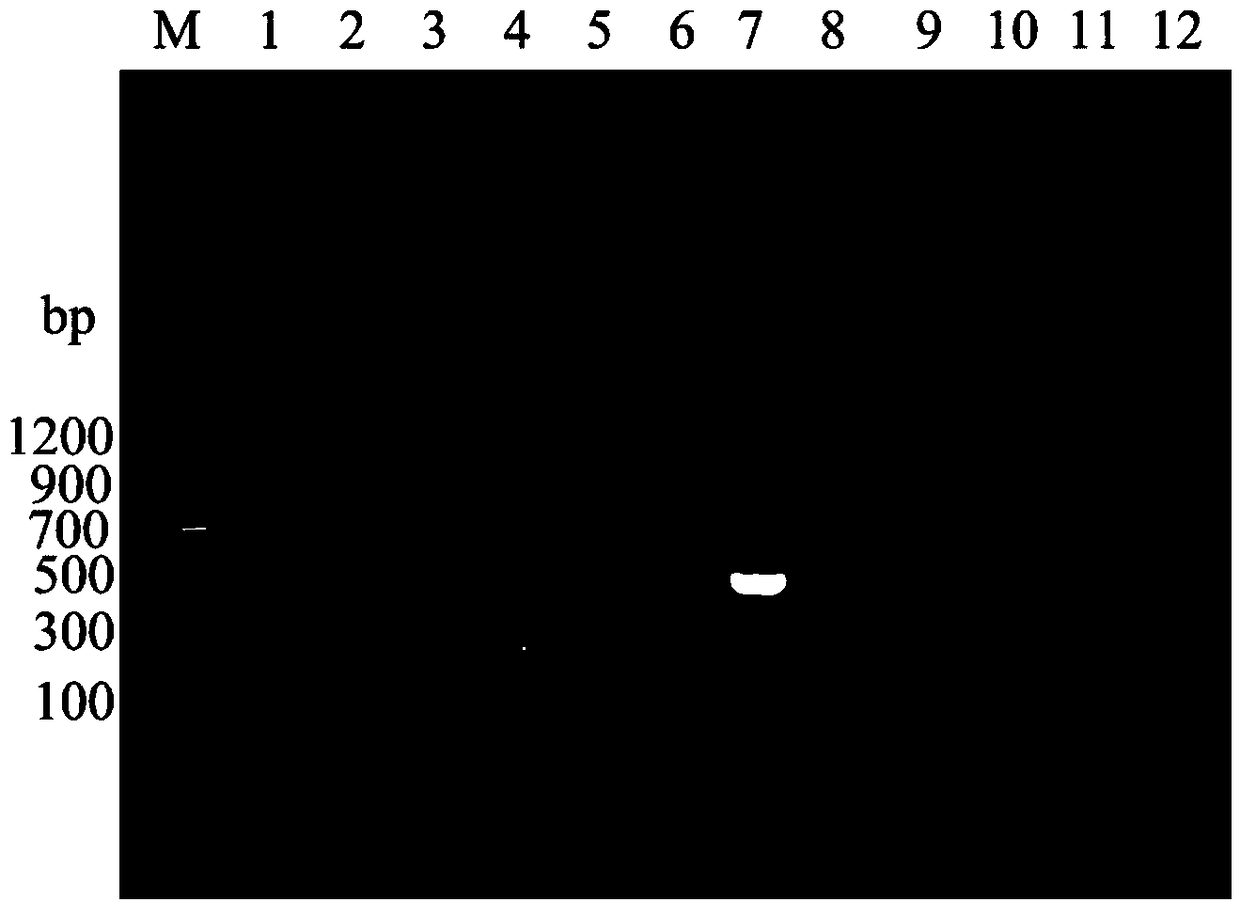
The present invention is directed to a bovine viral diarrhea virus comprising at least one helicase domain amino acid mutation wherein the mutation in the NS3 domain results in a loss of recognition by a monoclonal antibody raised against wild-type NS3 but wherein viral RNA replication and the generation of infectious virus is retained. The present invention is useful, for example, to produce a marked bovine viral diarrhea virus vaccine or to differentiate between vaccinated and infected or unvaccinated animals.
Owner:PFIZER INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00001.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00002.png)
![Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors Imidazo [1, 2-<i>a</i>] pyrrolo [3, 2-<i>c</i>] pyridine compounds useful as pestivirus inhibitors](https://images-eureka.patsnap.com/patent_img/cac4df94-e797-4eb0-8e30-473f04013ff1/US08404707-20130326-C00003.png)