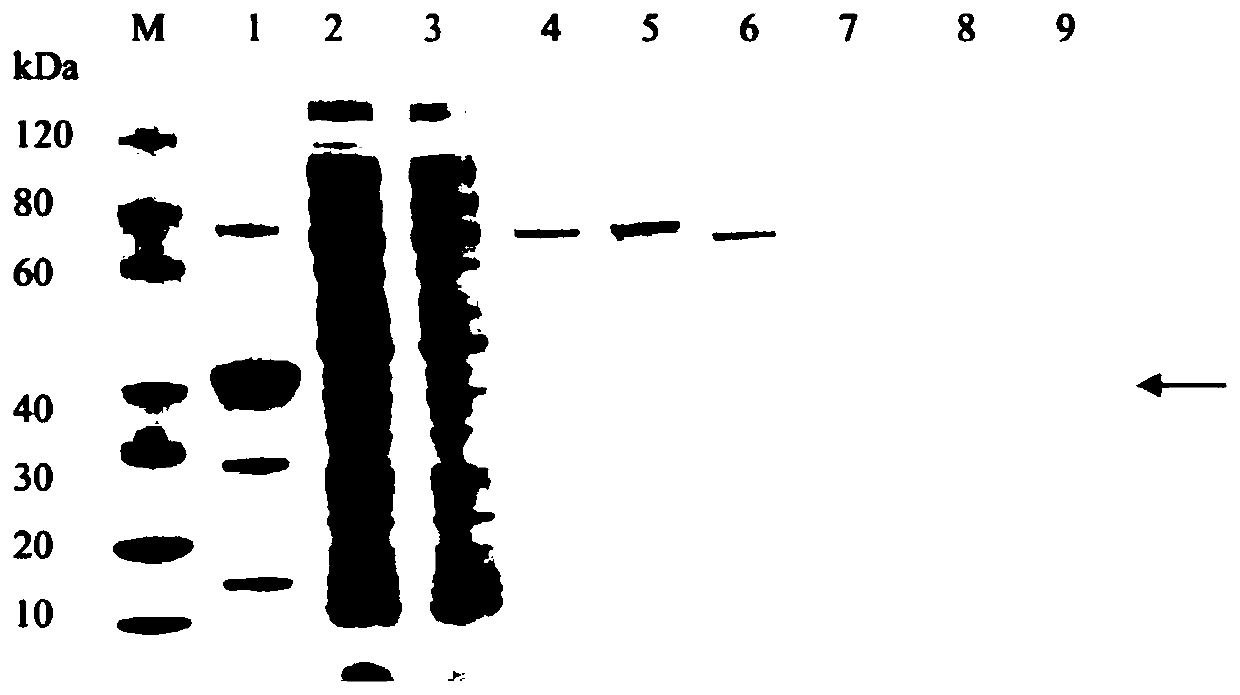
E2 recombinant protein and application thereof
A recombinant protein and sequence listing technology, applied to recombinant protein E2 and its application fields, can solve problems such as high mutation, achieve the effects of high accuracy, high sensitivity, and avoid high mutation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Amplification of E2 protein sequence and construction of prokaryotic expression plasmid pET30a-E2:
[0028] Use the codon optimization software MaxCodonTM Optimization Program (V13) to optimize the amino acid sequence of the E2 protein, and use the whole gene to synthesize the recombinant E2 protein gene. The amino acid sequence list of the E2 recombinant protein is SEQ.ID.No.1, and the recombinant The nucleotide sequence list of the E2 protein gene is SEQ.ID.No.2. Insert the recombinant E2 protein gene into the expression vector pET30a through the restriction sites Nde I and Hind III, and add 4.5 μL of the recombinant E2 protein gene fragment, 0.5 μL of the pET-30a expression vector, and 5 μL of the DNA ligation kit were mixed thoroughly, and placed in a low-temperature ligation instrument at a temperature of 16°C for the ligation reaction for 30 minutes to obtain the ligation product, which was passed through a restriction The accuracy of the final expression vecto...
Embodiment 2
[0052] This embodiment is the application of the recombinant protein E2 described in Example 1, the recombinant protein E2 is used to detect the indirect ELISA kit of bovine viral diarrhea virus antibody, and the indirect ELISA kit includes a coated microplate, negative Control serum, positive control serum, enzyme-labeled secondary antibody, enzyme-labeled secondary antibody diluent, concentrated washing solution, sample diluent, color development solution and stop solution, the coated microtiter plate uses recombinant protein E2 as the coating antigen.
[0053] 1. Preparation of enzyme-labeled secondary antibody: Rabbit anti-bovine IgG (produced by Jackson ImmunoResearch, USA) was diluted 100 times with HRP conjugate stabilizer / diluent I (lettuce from Huzhou Yingchuang Biotechnology Co., Ltd.).
[0054] 2. Preparation of coating buffer, blocking solution, sample diluent, enzyme-labeled secondary antibody diluent, and washing solution:
[0055] Coating buffer is the 0.05M car...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com