Patents
Literature
92 results about "Mucosal disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mucosal disease. mucosal disease --> bovine virus diarrhoea. A specific infectious disease of cattle, caused by a togavirus; characterised by ulceration of the mouth, pharynx, oesophagus, and sometimes the stomachs and intestines; may or may not be accompanied by severe diarrhoea. Synonym: mucosal disease.
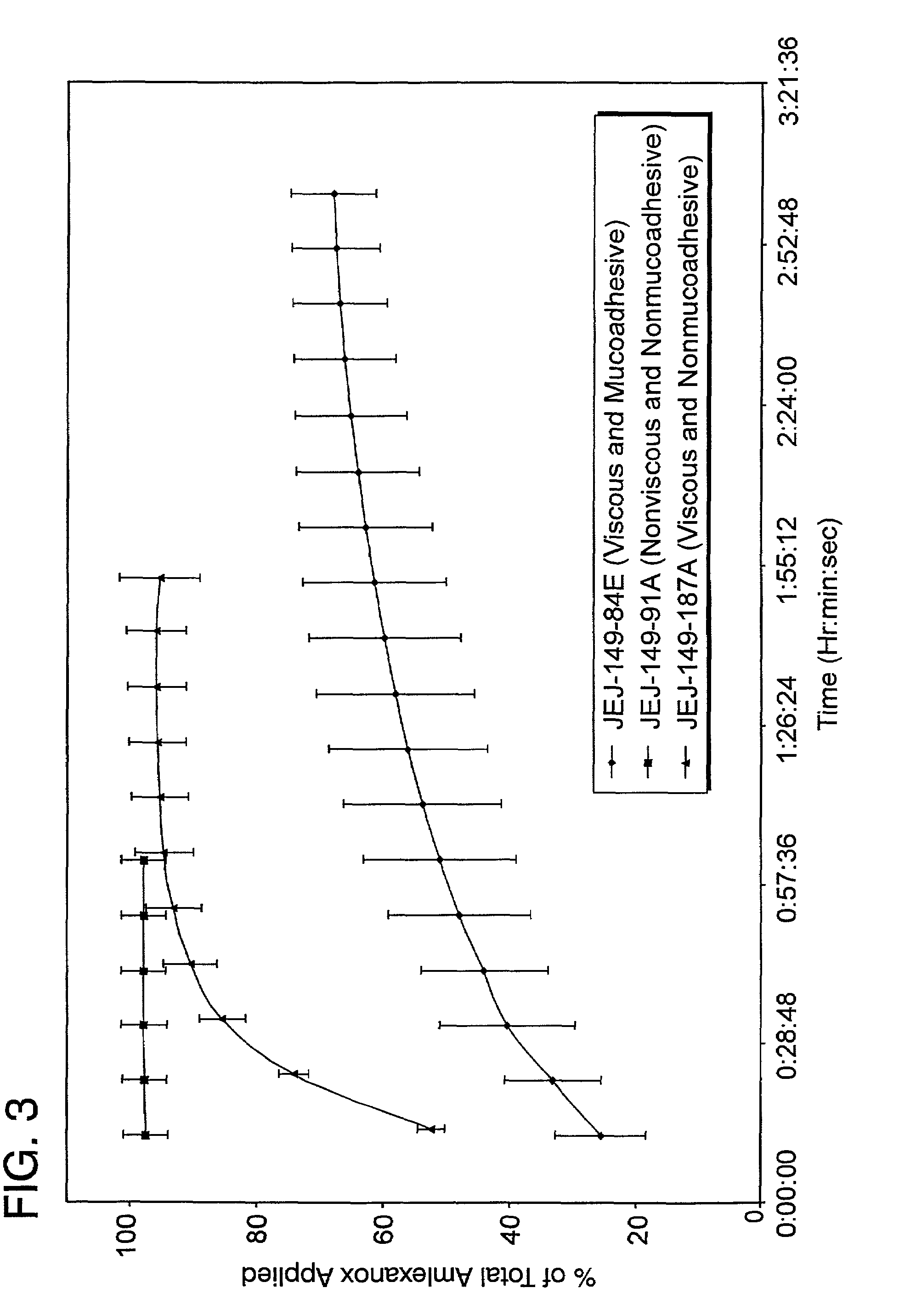
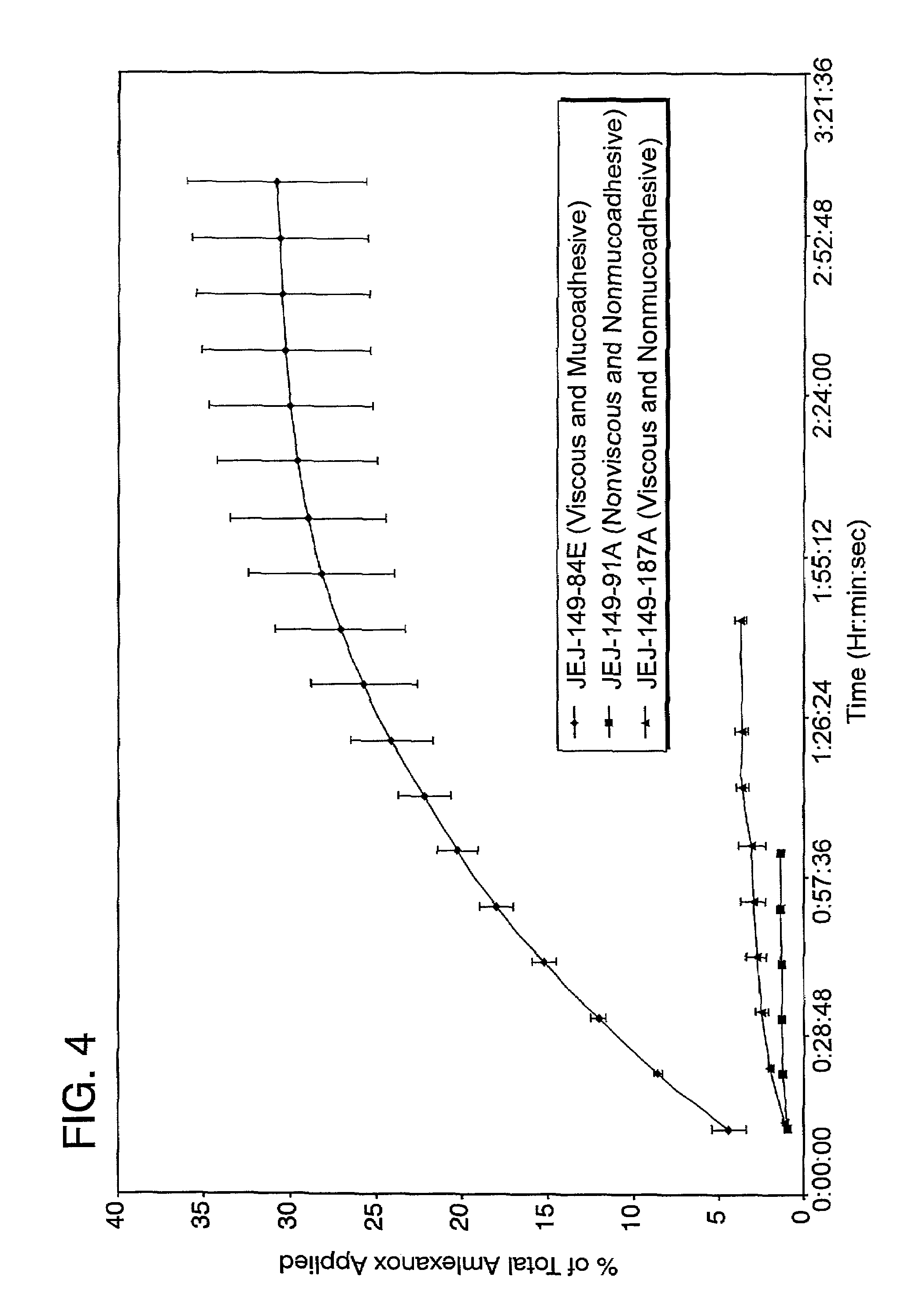
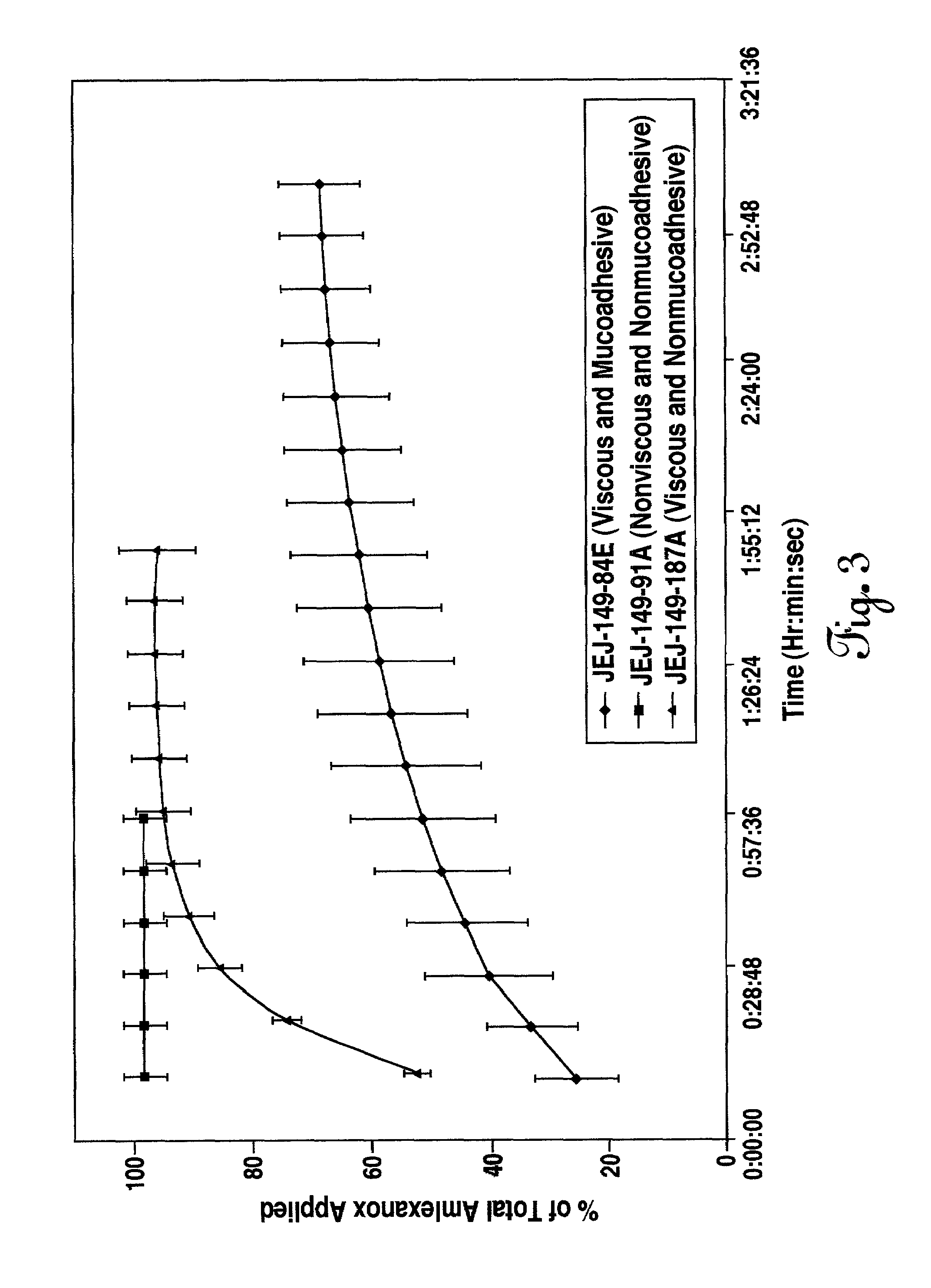
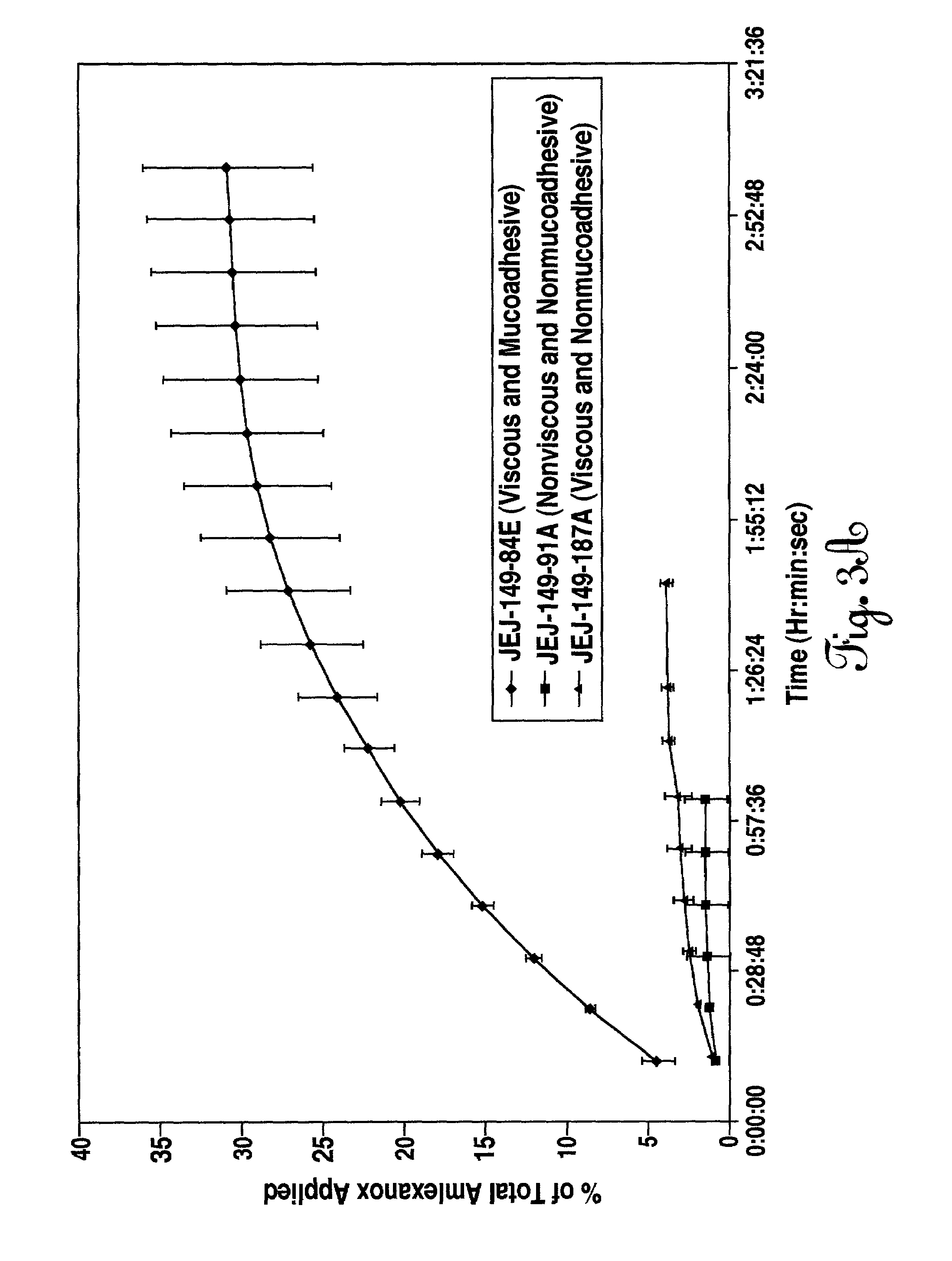
Liquid formulations for the prevention and treatment of mucosal diseases and disorders
Stable, viscous, mucoadhesive aqueous compositions which are useful for the prevention and treatment of ulcerative, inflammatory, and / or erosive disorders of mucous membranes and / or the delivery of pharmaceutically active compounds to mucosal surfaces for topical treatment or transfer to the systemic circulation.
Owner:AMAG PHARMA
Liquid formulations for the prevention and treatment of mucosal diseases and disorders
Stable, viscous, mucoadhesive aqueous compositions which are useful for the prevention and treatment of ulcerative, inflammatory, and / or erosive disorders of mucous membranes and / or the delivery of pharmaceutically active compounds to mucosal surfaces for topical treatment or transfer to the systemic circulation.
Owner:AMAG PHARMA
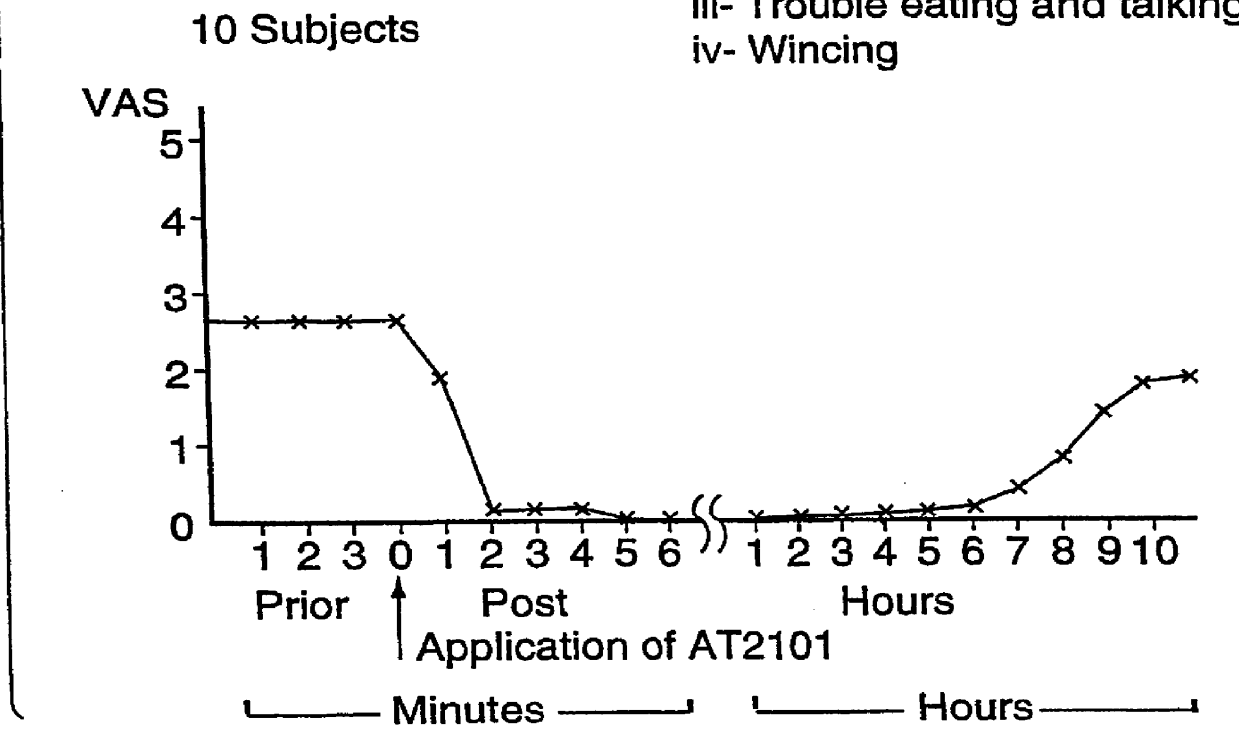
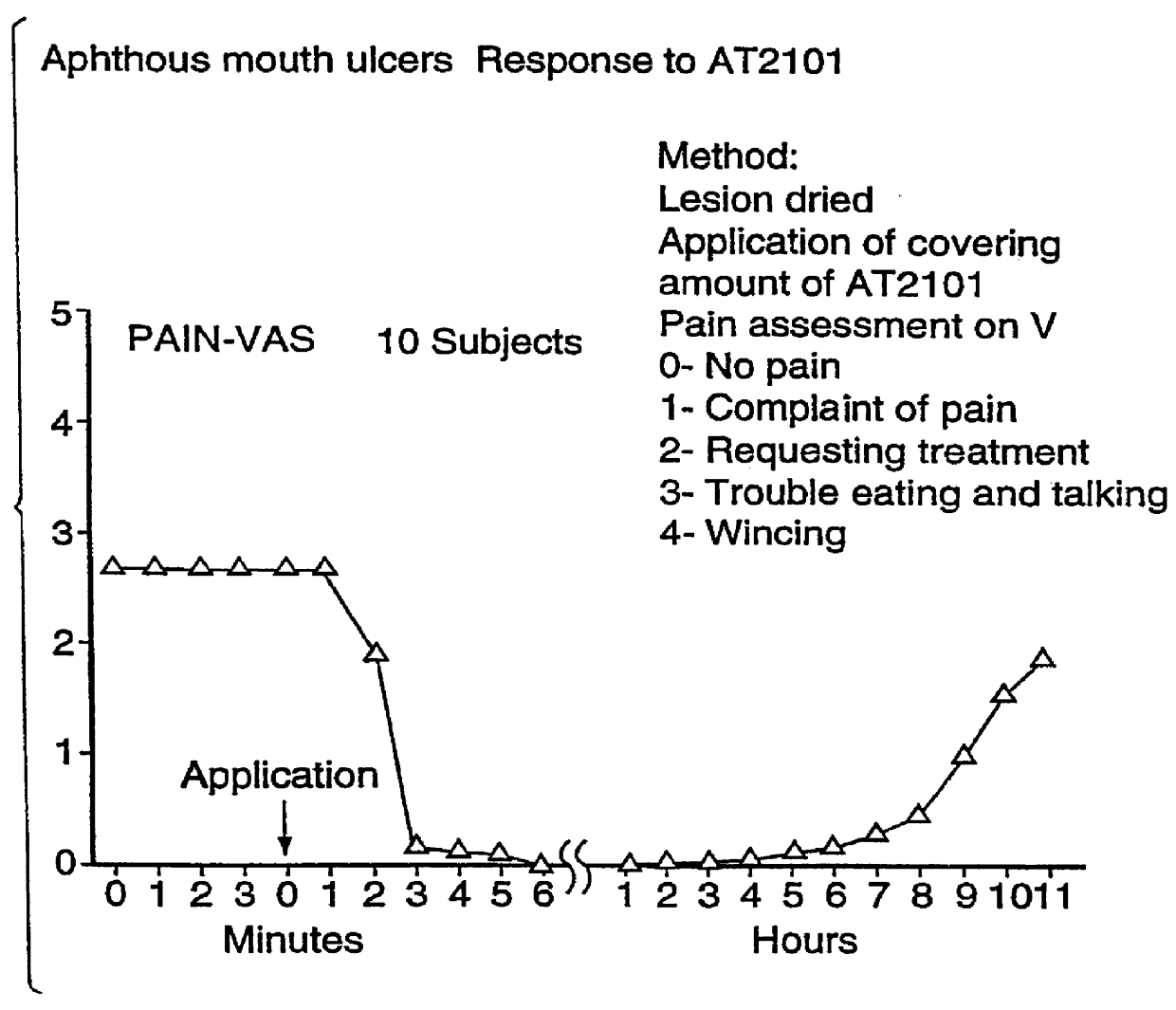
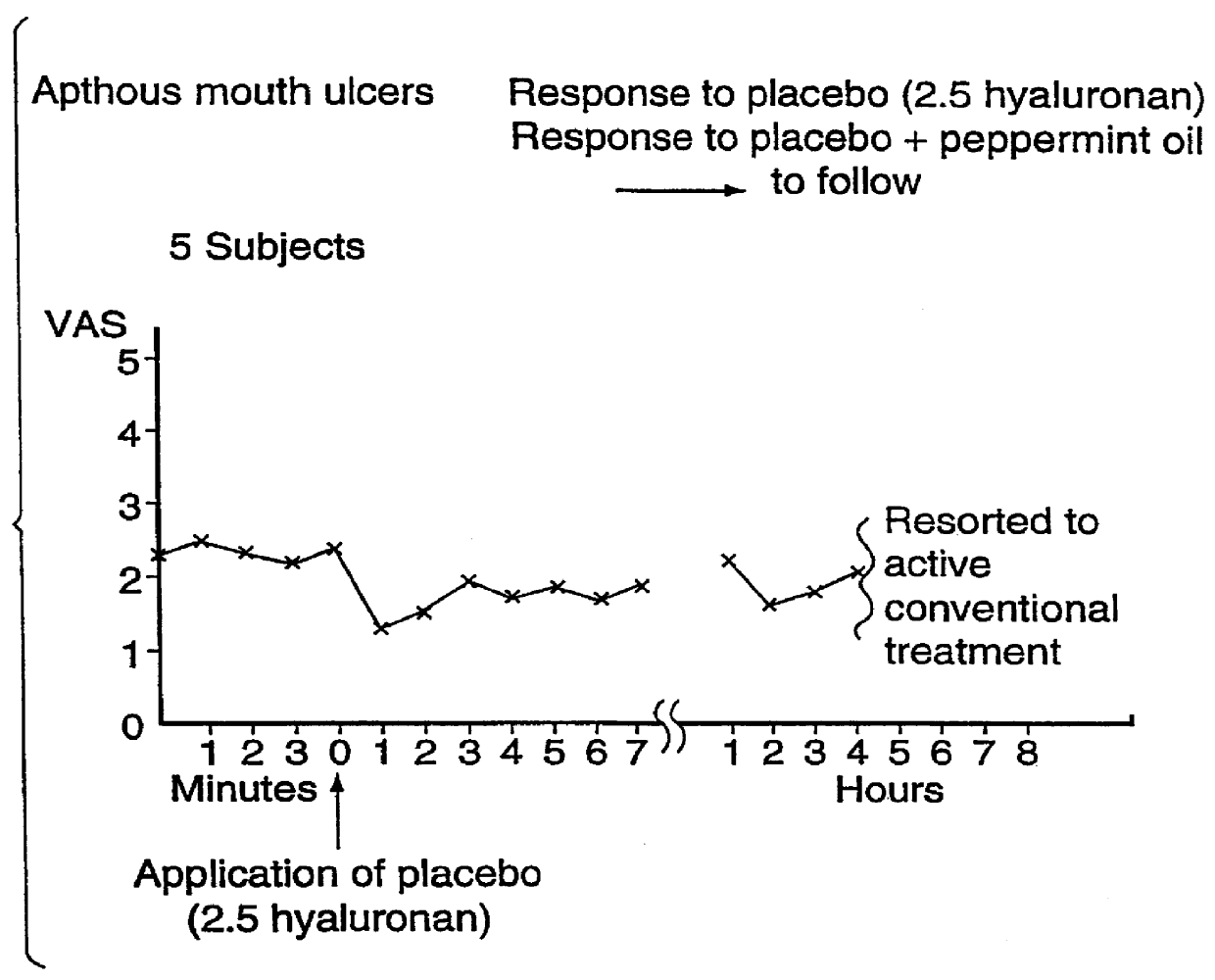
Use of hyaluronic acid and a NSAID for the manufacture of a medicament for the treatment of mucosal diseases
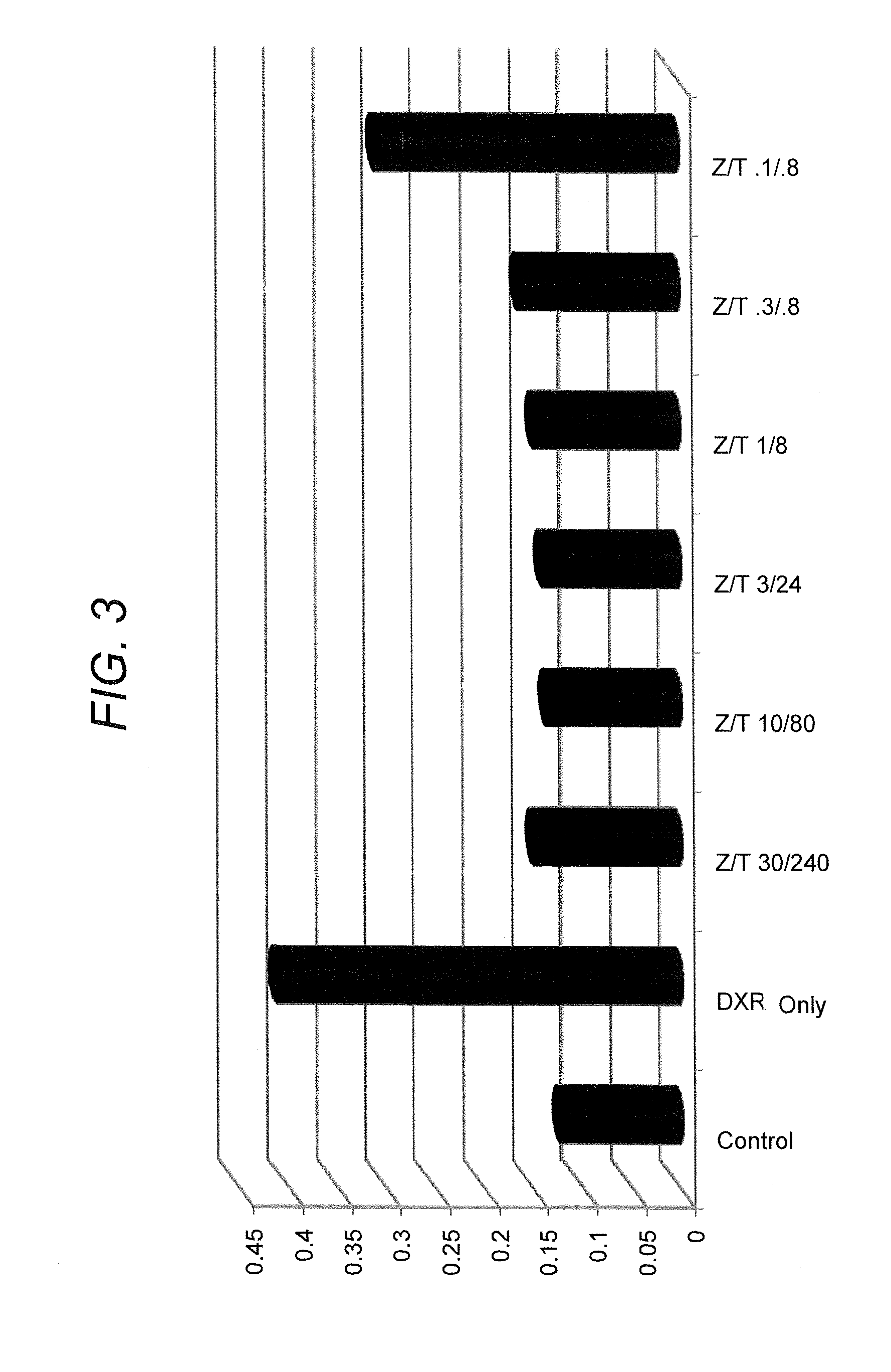
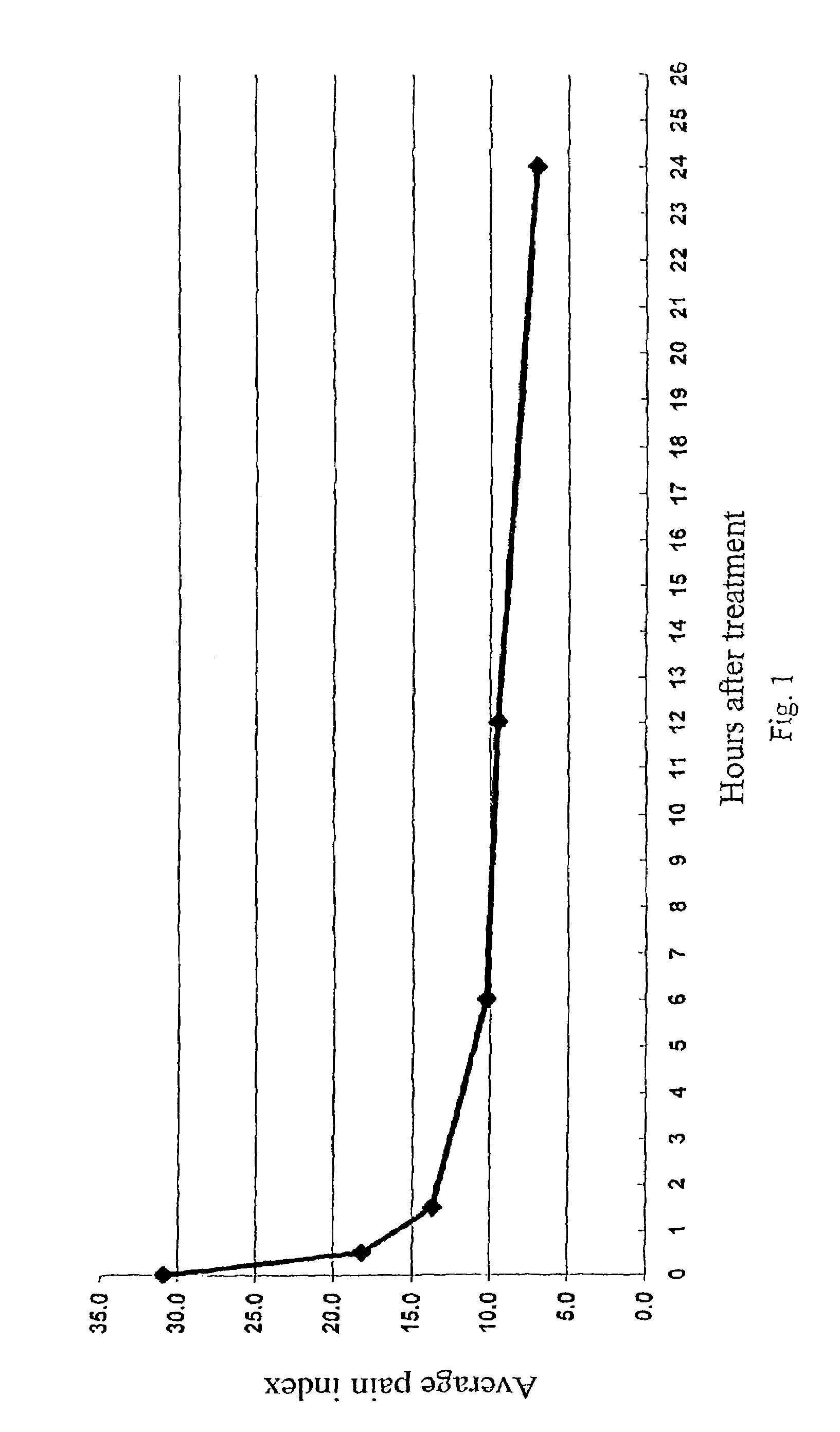
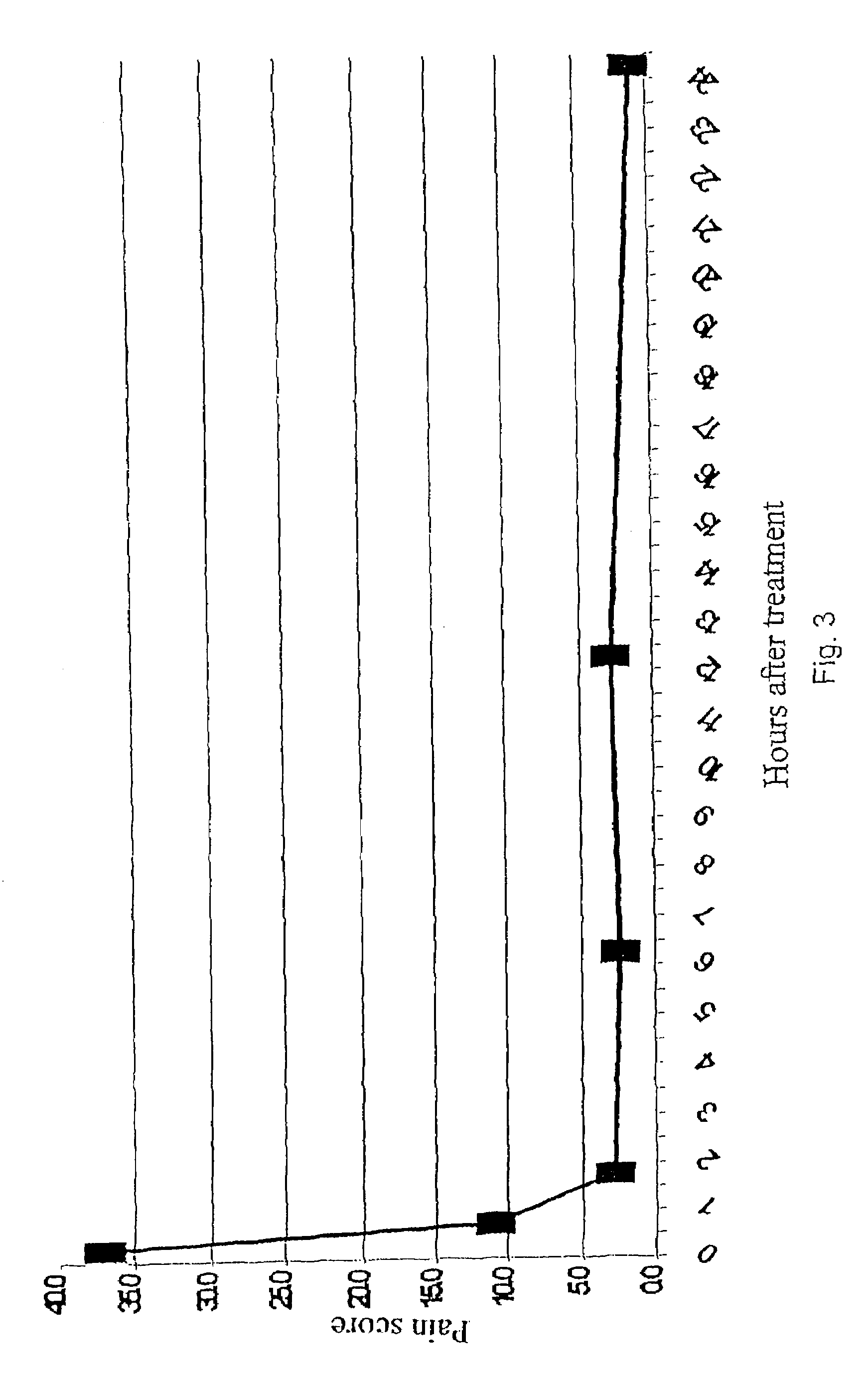
InactiveUS6159955AConvenient treatmentRelieve painBiocidePeptide/protein ingredientsDiseaseMucosal disease
PCT No. PCT / CA96 / 00488 Sec. 371 Date Dec. 24, 1997 Sec. 102(e) Date Dec. 24, 1997 PCT Filed Jul. 18, 1996 PCT Pub. No. WO97 / 03699 PCT Pub. Date Feb. 6, 1997The use of an effective amount of a composition comprising an N.S.A.I.D. and a form of hyaluronic acid selected from hyaluronic acid, pharmaceutically acceptable salts thereof, fragments thereof and / or subunits thereof for mucous membrane trauma, disease, and / or pain relief.
Owner:JAGOTEC AG +1
Liquid formulations for the prevention and treatment of mucosal diseases and disorders
InactiveUS7544348B2Low viscosityIncrease liquid viscosityBiocideCosmetic preparationsDiseaseMucosal disease
Stable, viscous, mucoadhesive aqueous compositions which are useful for the prevention and treatment of ulcerative, inflammatory, and / or erosive disorders of mucous membranes, especially mucositis.
Owner:AMAG PHARMA
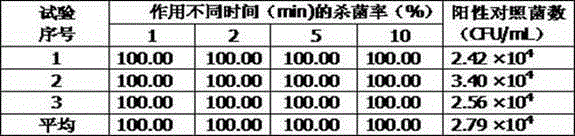
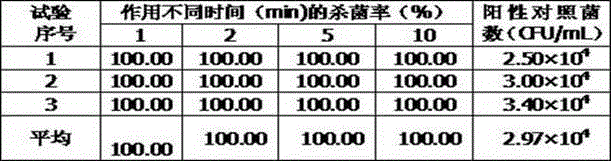
Antibacterial composition based on natural plant raw material and application of antibacterial composition
InactiveCN104688810AImprove securityEfficient bactericidal effectAntibacterial agentsCosmetic preparationsBiotechnologyDisease
The invention relates to an antibacterial composition based on a natural plant raw material and an application of the antibacterial composition, especially prevention and treatment of skin and mucosal diseases and infection of people and animals. The medicine tolerance problem of an antibiotic is an increasingly serious problem in global attention. The invention aims at providing a safe and effective natural antibacterial composition to avoid the problem of the medicine tolerance of the antibiotic instead of the antibiotic. The natural antibacterial composition provided by the invention has a broad-spectrum efficient sterilization effect, and is effective for gram-positive bacterium, gram-negative bacterium and fungus; candida albicans, staphylococcus aureus and escherichia coli can be completely killed within one minute; and meanwhile, the antibacterial composition has good safety. In addition, the composition also has the efficacy of resisting viruses, inflammation and allergy. The composition can be used for preventing and treating skin and mucosal diseases and infection of people and animals. The problem of the medicine tolerance generated by functional antibiotics is avoided.
Owner:KUNMING BIKAI TECH
Anti-microbial dental formulations for the prevention and treatment of oral mucosal disease
InactiveUS7972137B2Preventing and/or treating gingival diseaseBiocideCosmetic preparationsDiseaseMucosal disease
Compositions and methods are directed to antimicrobial compositions and articles that release ●NO from the composition and / or article in an amount effective to prevent gingival and other oral mucosal diseases.
Owner:ROSEN GERALD M
Medicine for curing oral mucosal disease
The invention provides a medicine for curing an oral mucosal disease. The medicine is prepared by the following raw traditional Chinese medical materials: mint, mulberry leaves, chrysanthemums, roots of straight ladybell, radix scrophulariae, ophiopogon roots, radix rehmanniae recen, angelica, rhizoma anemarrhenae, fiveleaf akebia, lophatherum gracile, atractylodes, Tuckahoe, honeysuckle flowers, scutellaria, trichosanthes roots, gardenia, balloon flowers and liquorice. All components in the invention are used to jointly take the efficacy of nourishing Yin, reducing fever, clearing lung and stomach, dispelling wind, reducing pathogenic fire and enriching the blood to restore normal menstruation. The invention differentially cures the vulvar leukoplakia by traditional Chinese medical science, has ideal curing effect and achieves a curative ratio up to 85 percent so that patients can be cured for 6-10 days.
Owner:辛保山
Mineral salt-sulfonic acid compositions and methods of use
The present disclosure generally relates to the medical use of compositions comprising a mineral salt and a sulfonic acid for prevention and / or treatment of one or more mucosal diseases, disorders, or conditions or one or more dermal diseases, disorders, or conditions.
Owner:BMG PHARMA
Dendrimer like amino amides possessing sodium channel blocker activity for the treatment of dry eye and other mucosal diseases
InactiveUS8980898B2More potent and/or absorbed less rapidlyLess reversibleOrganic active ingredientsSenses disorderDendrimerDisease
Owner:PARION SCI DURHAM NC
Topical formulation(s) for the treatment of inflammation, skin and mucosal disorders and other diseases thereof
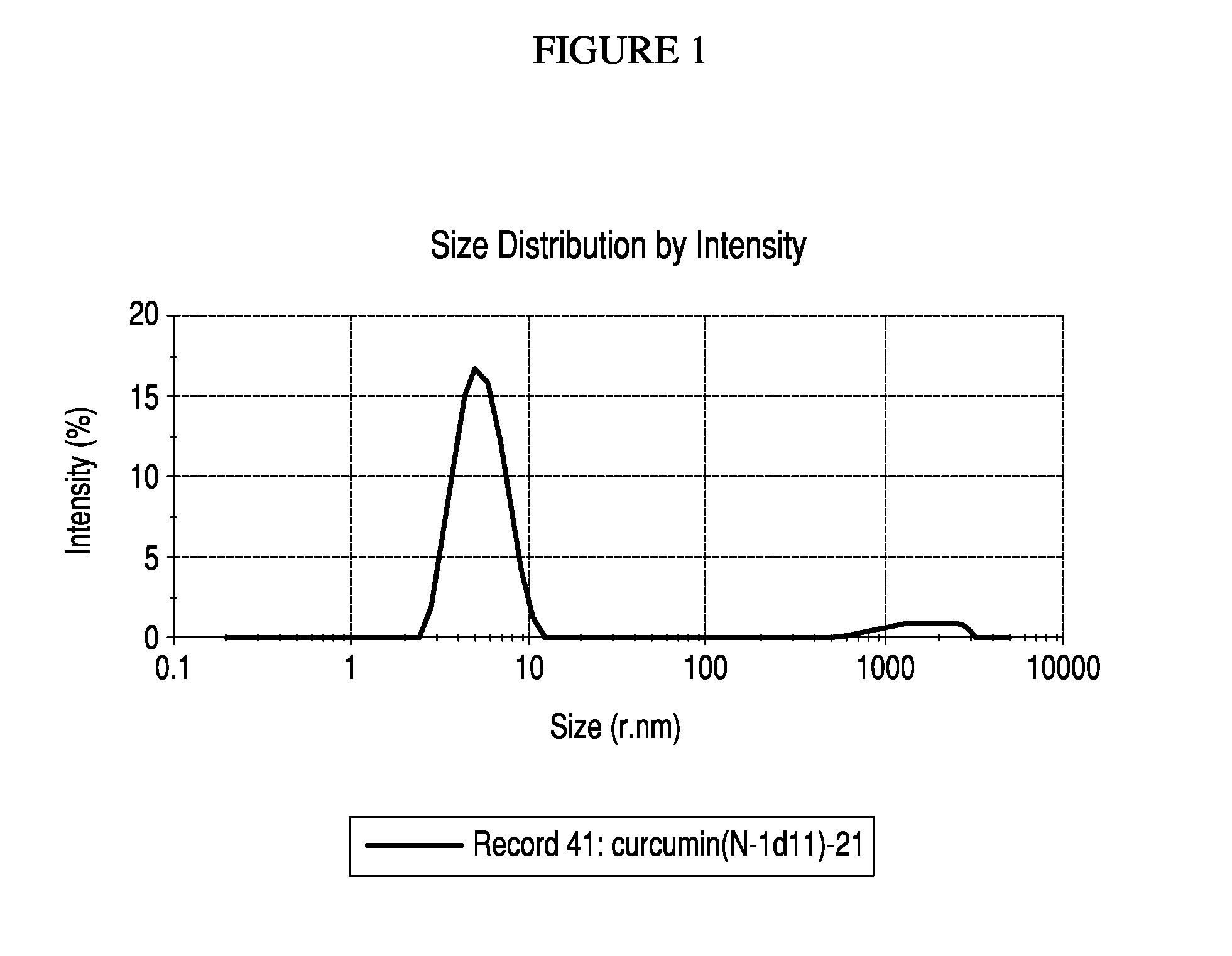
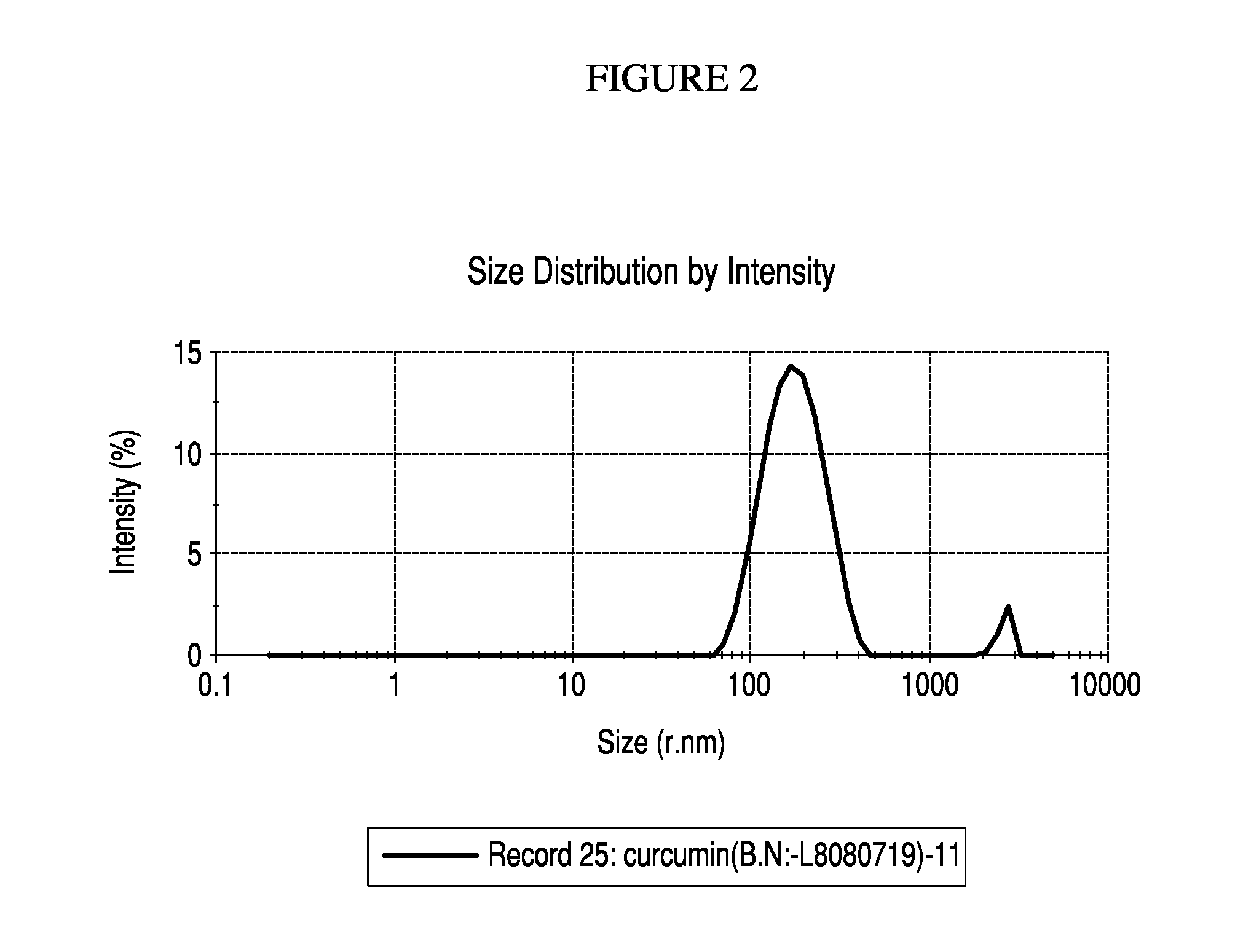
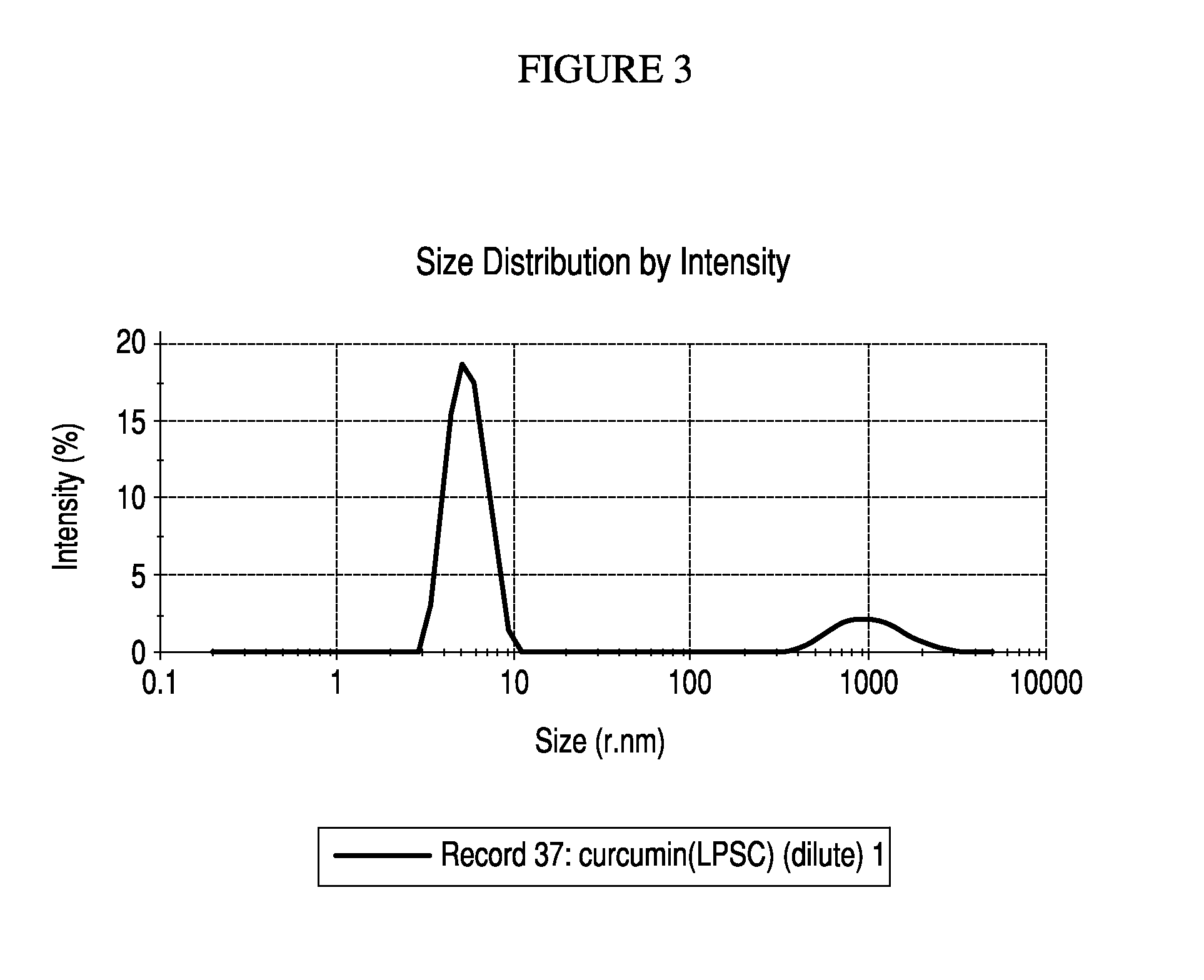
Nanoemulsified topical formulation comprising curcumin, tetrahydroxycurcumin (BDMC) and curcuminoid either alone or in combinations in an amount of 0.001% to 50% together with one or more pharmaceutically, nutraceutically or dietically acceptable excipient(s) or inactive ingredients, useful for the treatment of inflammation, various skin and oral disorders, mucosal disorders and other diseases associated or related thereof.
Owner:LAILA PHARMA PVT LTD
Method for preparing compound double-layer sustained-release medicinal membrane for treating oral mucosal disease
InactiveCN101468039AAvoid side effectsAvoid gastrointestinal reactionsHydroxy compound active ingredientsDigestive systemDiseaseMucosal disease
The invention provides a preparation method of a compound double-layer sustained release emulsion for treating diseases of oral mucosa made from the following raw materials: borneol, calculus bovis artifactus, tindazole tinidazole, dyclonine hydrochloride, Tween-80, glycerol, saccharin sodium, sodium carboxymethyl cellulose (CMC-Na), polyvinyl alcohol PVA17-18 etc. The compound double-layer sustained release emulsion has heat-cleaning and detoxifying, swelling-dispersing and ache-relieving, bacteria-resisting and inflammation-diminishing, putridity-impelling and tissue regeneration-promoting, ulcer face and lesion place-effectively protecting and curing functions, shortens course of the disease, overcomes deficiencies of single-layer emulsion of Chinese medicine or western medicine, is durable with obvious curative effect. When a double-layer emulsion is compared with a single-layer emulsion, medicine is released from a single layer in the single-layer emulsion and from double layers in the double-layer emulsion, thus medicine locally contacts the lesion places, the medicine flowing outside is decreased and foreign odour of medicine in oral cavity is decreased. The compound double-layer sustained release emulsion is made by combining advantages of Chinese medicine and western medicine which are rationally matched, has low dosage, direct effect, convenient use, quick effect, low side effect, determined and good curative effect.
Owner:赵呈利
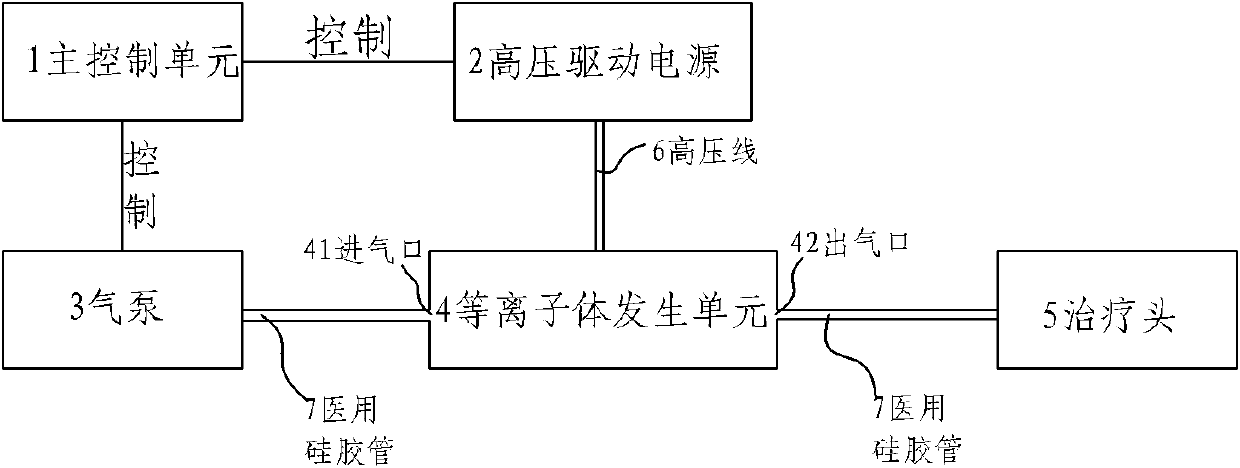
Plasma mucocutaneous disease therapeutic apparatus
InactiveCN102553075ASuitable for self-treatment at homeQuick cureElectrotherapyDiseaseMucosal disease
The invention relates to a plasma mucocutaneous disease therapeutic apparatus. The apparatus comprises a master control unit, a drive power supply, a miniature air pump, a plasma generation unit and a therapeutic head, wherein the plasma generation unit is provided with an air inlet and an air outlet; the master control unit is respectively connected with the drive power supply and the miniature air pump; the drive power supply is connected with the plasma generation unit; the air pump is connected with the air inlet of the plasma generation unit; and the therapeutic head is connected with the air outlet of the plasma generation unit. The plasma mucocutaneous disease therapeutic apparatus solves the technical problems of high cost, large size and complex operation of a conventional plasma mucocutaneous disease therapeutic apparatus, has the advantages of smart size, simpleness in operation and convenience for carrying, and is suitable for home self-treatment of patients.
Owner:西安航科等离子体科技有限公司
Mineral salt-sulfonic acid compositions and methods of use
The present disclosure generally relates to the medical use of compositions comprising a mineral salt and a sulfonic acid for prevention and / or treatment of one or more mucosal diseases, disorders, or conditions or one or more dermal diseases, disorders, or conditions.
Owner:BMG PHARMA
Therapeutic agent for infectious skin or mucosal disease
Owner:TAIKO PHARMA
Herbal compositions for the treatment of mucosal lesions
The present invention provides therapeutic compositions comprising extracts of the plant species Echinacea purpurea and Sambucus nigra and the extract(s) of at least one further plant selected from the group consisting of Hypericum perforatum, Commiphora molmol and Centella asiatica. The compositions of the invention are of particular utility in the management of inflammatory mucosal diseases of both viral and non-viral origin.
Owner:IZUN PHARMA
Compositions for the prophylaxis and treatment of dermatological/mucosal diseases, and uses thereof
InactiveUS20110217358A1Enhanced barrier functionStable chargingPowder deliveryCosmetic preparationsDiseaseMucosal disease
The present invention provides a composition containing a) clay material with a negative surface charge; b) magnesium ions and calcium ions both either incorporated within the clay material and / or added separately; c) water and / or non-aqueous solvent; d) substantially non-cationic carrier; where i) the mean particle size of the clay material is at least 5 nm; and ii) the total molar amount of magnesium ions is higher than the total molar amount of calcium ions. The present invention further relates to the use of said composition in the manufacture of a medicament for the prophylaxis and treatment of skin diseases, skin conditions, or mucosal diseases where the formation and / or recovery of epidermal / mucosal barrier function has beneficial effects.
Owner:DESPHARMA
Absorbable solid compositions for topical treatment of oral mucosal disorders
InactiveUS20110244039A1Stop progressEffective treatmentAntibacterial agentsBiocideDiseaseMucosal disease
The invention provides a solid, self-bioadhesive composition for topical application that adheres to the oral mucosal tissue comprising a therapeutically effective amount of at least one herbal or homeopathic active agent; and a pharmaceutically acceptable solid bioadhesive carrier in an amount from about 40 to 99 percent based on the weight of the whole composition.
Owner:AXIOMEDIC
Mucosal disease treatment system and application thereof
InactiveCN108478544ASolve the incurableResolve strong recurrencePeptide/protein ingredientsDigestive systemDiseaseMucosal disease
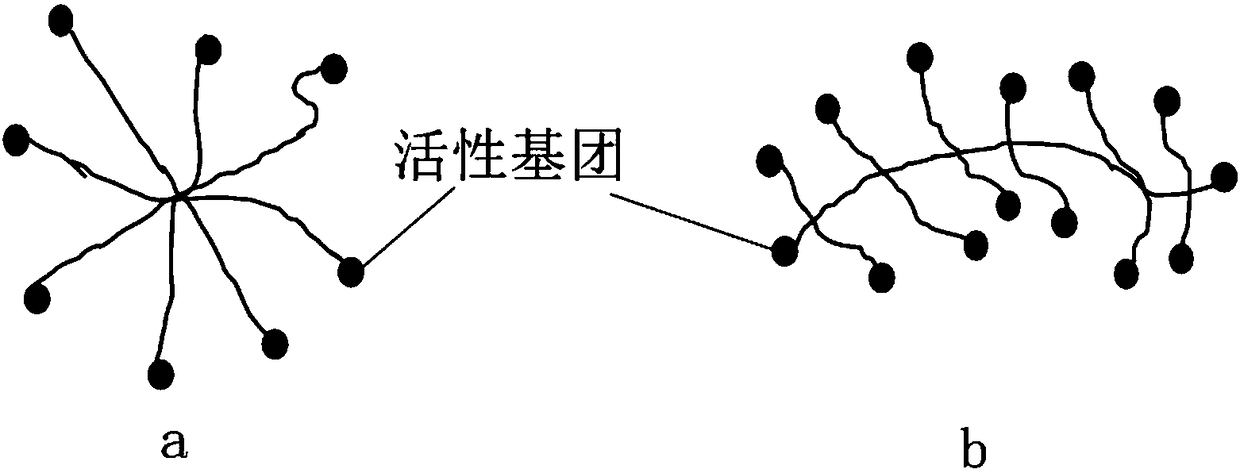
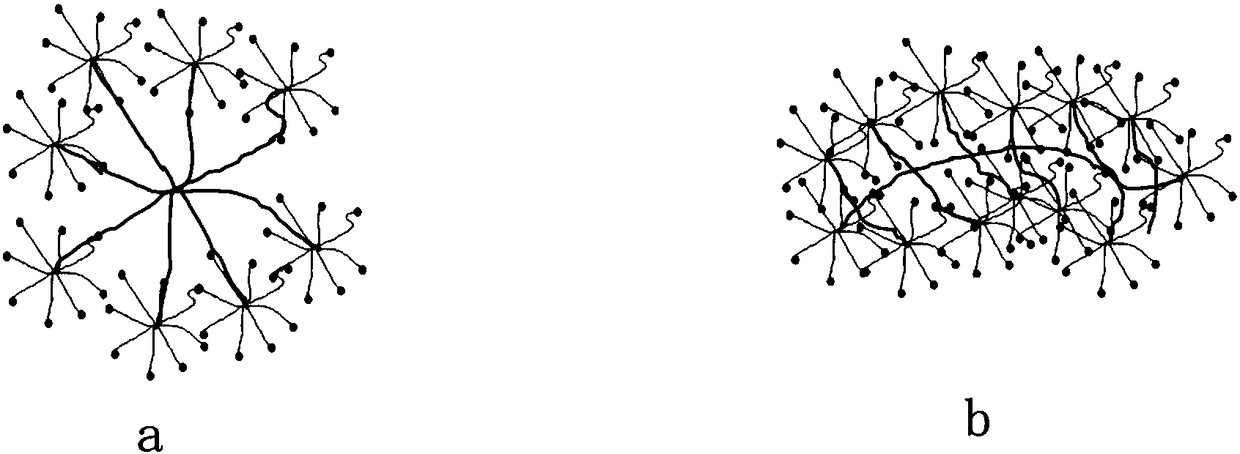
The invention discloses a mucosal disease treatment system. The mucosal disease treatment system comprises a biological adhesive, a biological medical material, and medical instruments, wherein the biological adhesive comprises the following components: a component a, namely a functionalized star polymer or / and a functionalized comb polymer, and a component b, namely functional polysaccharide; anactive group of the component a and an active group of the component b can chemically react to form a new chemical bond; the biomedical materials, which are selected according to different mucosal diseases, are mixed in the component a of the biological adhesive, or in the component b of the biological adhesive, or partially mixed in the component a of the biological adhesive and partially mixed in the component b of the biological adhesive. When the system is in use, one of the components a and b is covered on a mucous membrane to be treated with the aid of a corresponding medical instrument,and the other component is covered on the previous component; the biological adhesive is firmly adhered to the mucous membrane and seals and repairs the mucous membrane; and the biological medical material is continuously released into adhesive tissues, thereby treating mucosal diseases.
Owner:杭州易敏生物医药科技有限公司
Externally applied medicine for treating skin and mucosa disease caused by human papilloma virus
ActiveCN1840116AGood priceGood curative effectAnthropod material medical ingredientsAntiviralsMedicinal herbsDisease
The invention relates to an externally-applied medicine for treating skin and mucosa disease caused by human papilloma virus, which is prepared from Chinese medicinal herbs including cnidium fruit, dysosmapleiantha woods, brucea fruit, poria cocos, cordate houttuynia, flavescent sophora root and wasp's nest. The preferred dosage form of the preparation is soft electuary.
Owner:BEIJING ZHONGKE PAITE BIOTECH +1
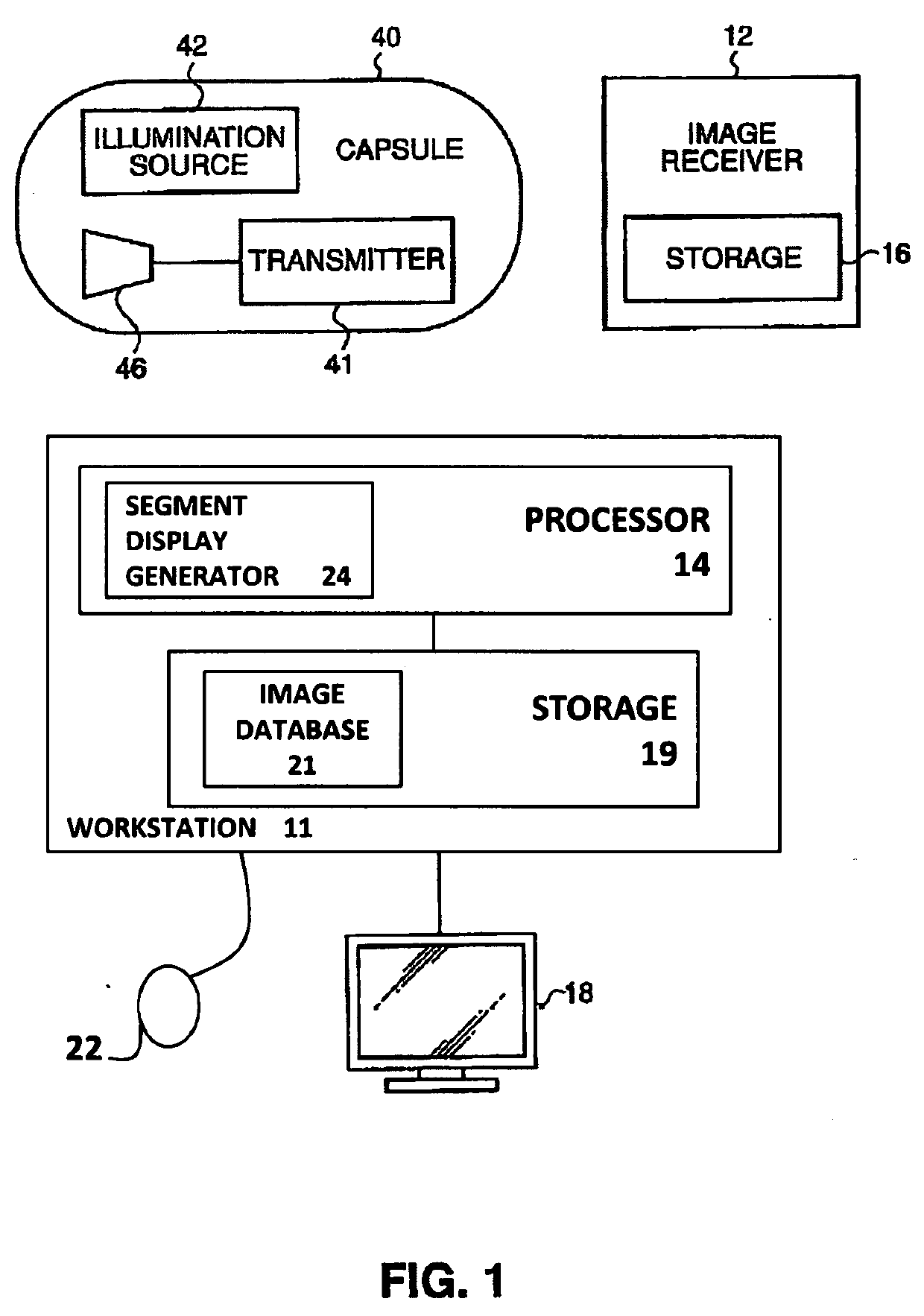
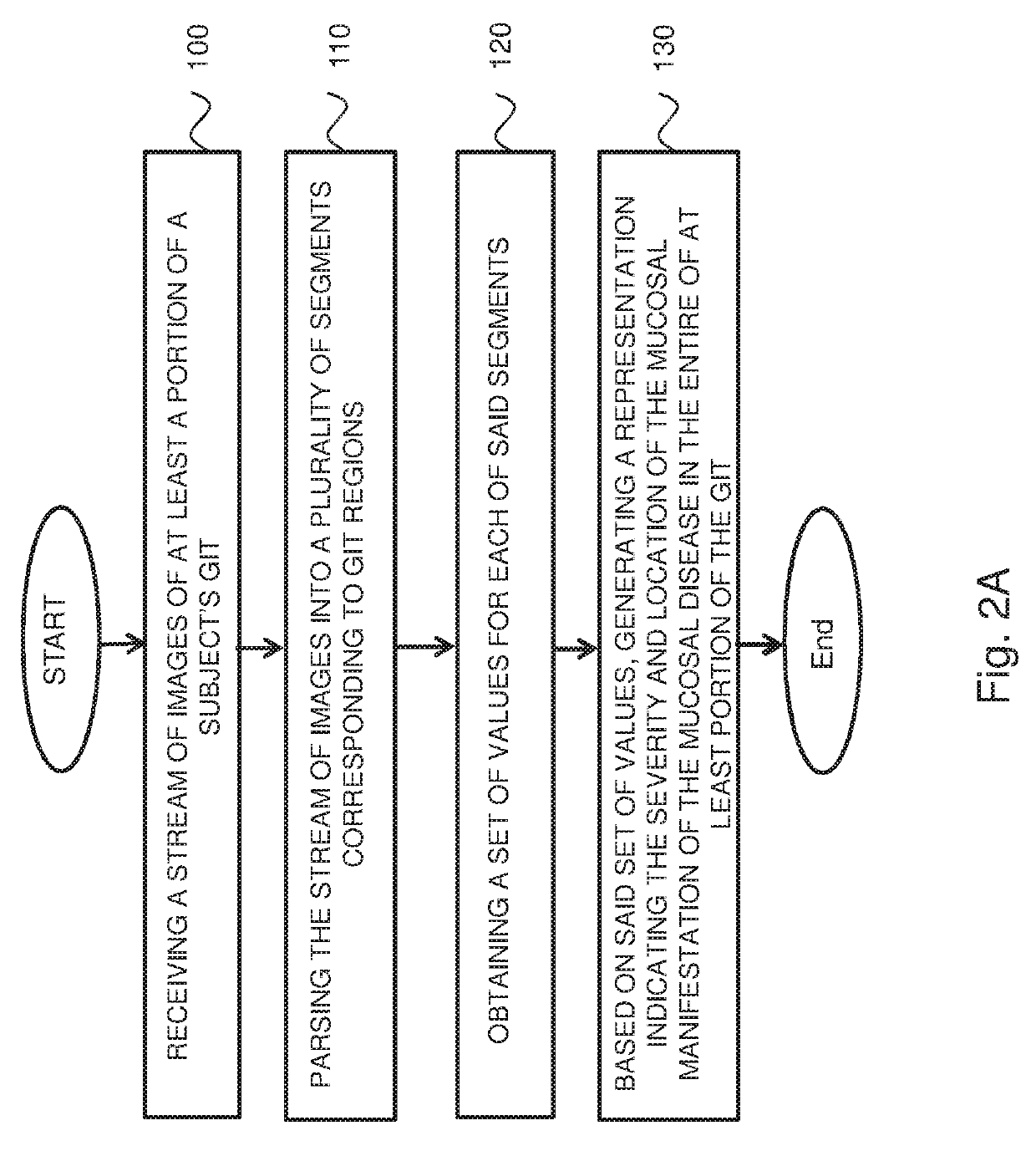
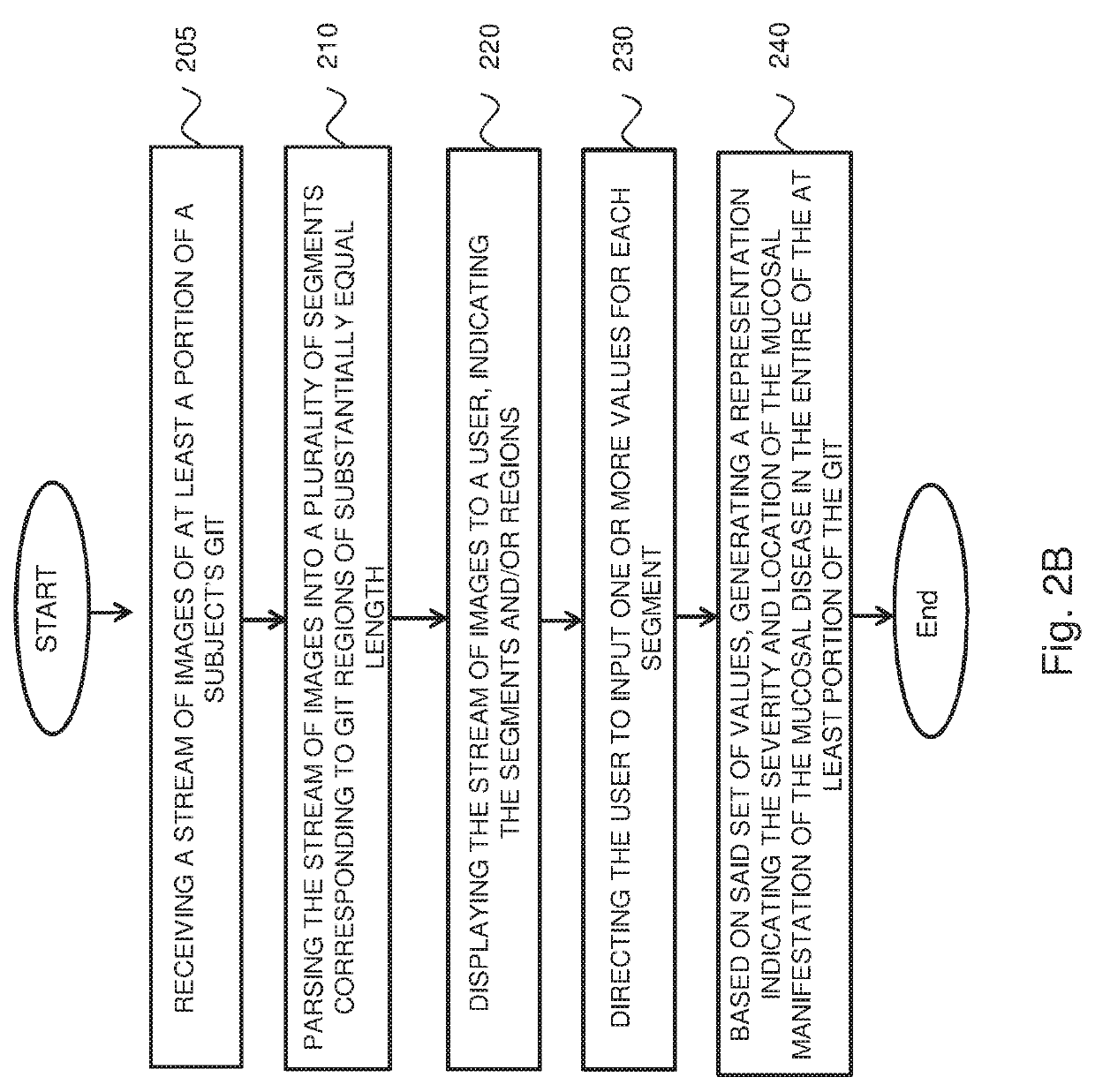
Systems and methods for assessment and monitoring of a mucosal disease in a subject's gastrointestinal tract
A computerized-method for a mucosal assessment of a mucosal disease in a Gastrointestinal Tract (GIT) of a subject, including receiving a stream of images of at least a portion of the GIT, parsing the stream into a plurality of segments, wherein each segment corresponds to a region of the at least portion of the GIT, obtaining a set of values for each segment, wherein the set of values refers to the pathological involvement of the segment in the mucosal disease and to severity of mucosal manifestation of the mucosal disease in the segment, and based on said set of values for each segment, generating a representation indicating the location and severity of the mucosal manifestation of the mucosal disease in the entirety of the at least portion of the subject's GIT, thereby allowing to assess the condition of the mucosal disease in the at least portion of the GIT.
Owner:GIVEN IMAGING LTD
Slowly-released traditional Chinese medicine adhering tablet for treating mouth mucous diseases and its prepn. method
A Chinese medicine in the form of slow-releasing oral adhesive tablet for treating mouth mucosa disease is prepared from 7 Chinese-medicinal materials including natural indigo, catechu, borneol, liquorice root, etc. Its advantages are high curative effect and low toxic by-effect.
Owner:CHINA PHARM UNIV +1
Special fungicide for livestock and poultry, its production method and application
InactiveCN102266314AAnti-inflammatoryWith convergenceAntibacterial agentsBiocideDiseaseSodium bicarbonate
The invention relates to a fungicide specially used for livestock and poultry, its production method and application. Take 0.01-5 parts of chlorhexidine acetate, 0.002-10 parts of polyethylene glycol octylphenyl ether, and 63-99.958 parts of tap water, put them into a container, mix and stir evenly, heat to 65-70°C, and then add benzene 0.02-12 parts of ammonium chloride, stir and heat to 92-94°C, then cool to 18-26°C, add 0.01-10 parts of glutaraldehyde and stir evenly, then adjust the pH value with sodium hydroxide or sodium bicarbonate , so that the pH value is 6-10, which is obtained. The invention is specially used for the sterilization and disinfection of livestock and poultry fur, oral cavity, endometrium, inner and outer vulva, breast, barn, air and object surface, as well as the treatment of fur and mucous membrane diseases. Compared with the existing sterilizing and disinfecting liquid, the present invention has the characteristics of many types of sterilizing, high sterilizing rate, simple manufacturing process, low cost, clear curative effect when applied to livestock and poultry, and safe and reliable use.
Owner:崔秀梅
Hydrogel for treating female cervical erosion and preparation method thereof
InactiveCN106692307AFacilitate fixes and updatesImprove disease resistanceOrganic active ingredientsAerosol deliveryMucosal diseaseForsythia
The invention discloses hydrogel for treating female cervical erosion. The hydrogel uses natural medicines as main components, wherein the natural medicines include the following components in parts by weight: 1.5-7.0 parts of bletilla polysaccharide, 5.0-10.0 parts of sea buckthorn oil, 0.5-2.0 parts of forsythia oil, 0.5-2.0 parts of angelica oil and 1.0-5.0 parts of centella asiatica total glycoside. The hydrogel provided by the invention is prepared according to the steps of mixing the materials, dissolving, stirring / emulsifying, filling and the like, has the efficacies of removing slough, promoting tissue regeneration, detoxifying, dispelling dampness, relieving swelling and pains, dispersing blood stasis and expelling pus, can promote mucosal repair and renewal and improve mucosal disease resistance through antibacterial, antiviral, anti-inflammatory and analgesic effects, and has an exact effect of treating both symptoms and root causes of the female cervical erosion in ways of reducing cervical secretions, improving vaginal cleanliness and recovering the balance of vaginal endogenous beneficial bacterial florae and the like.
Owner:陈宇津
Composition for preventing or treating mucous membrane diseases and preparation method thereof
InactiveCN106310266ABioadhesiveEfficient killingAntibacterial agentsAntimycoticsEscherichia coliNasal cavity
The invention discloses a composition for preventing and treating mucous membrane diseases with staphylococcus aureus, escherichia coli and candida albicans as pathogenic bacteria and a preparation method thereof. The composition is characterized by being prepared from the following components in percentage by mass: 0.001-35 percent of an antimicrobial agent, 0-10.0 percent of an analgesic, 0.001-20.0 percent of a bio-adhesive, 0-30.0 percent of a humectant, 0-5.0 percent of a pH regulator, 0-8.0 percent of a flavoring agent, 0-5.0 percent of an emulsifier, 0-8.0 percent of an auxiliary antimicrobial agent and the balance of pure water, totally 100 percent. The components in the composition can be well combined to achieve functions of inhibiting bacteria, isolating, relieving pain and moistening, and the effects of isolating microbial infection, preventing secondary infection and accelerating wound healing can be achieved. The composition is especially suitable for preventing and treating mucous membrane diseases in the oral cavity, nasal cavity, gastrointestinal tracts, respiratory tracts, vagina, urinary bladder, genitals, epidermis and other positions.
Owner:王强 +1
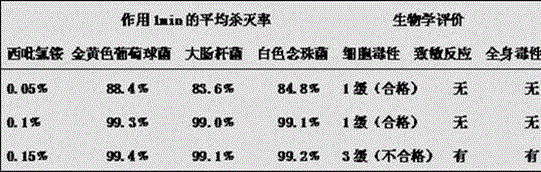
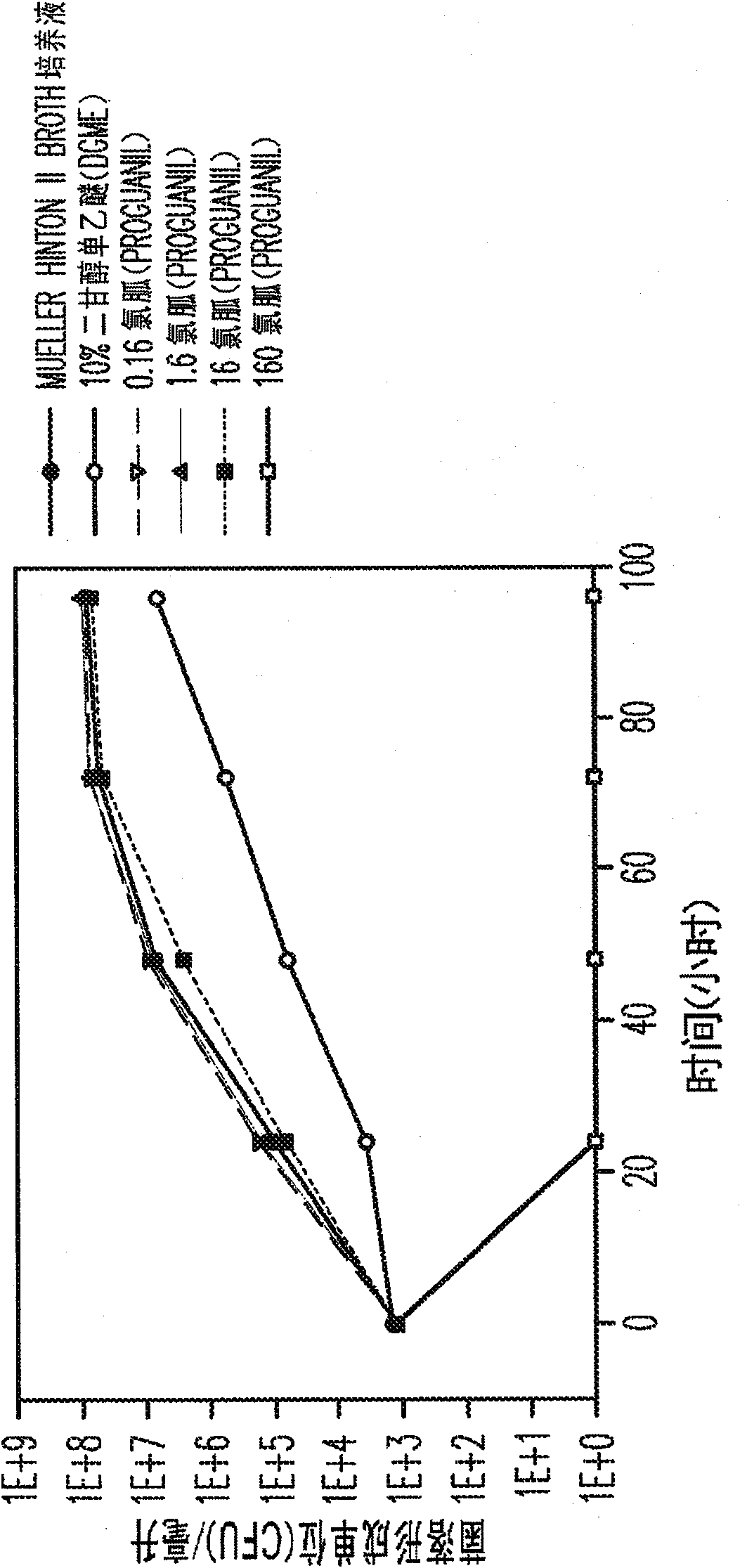
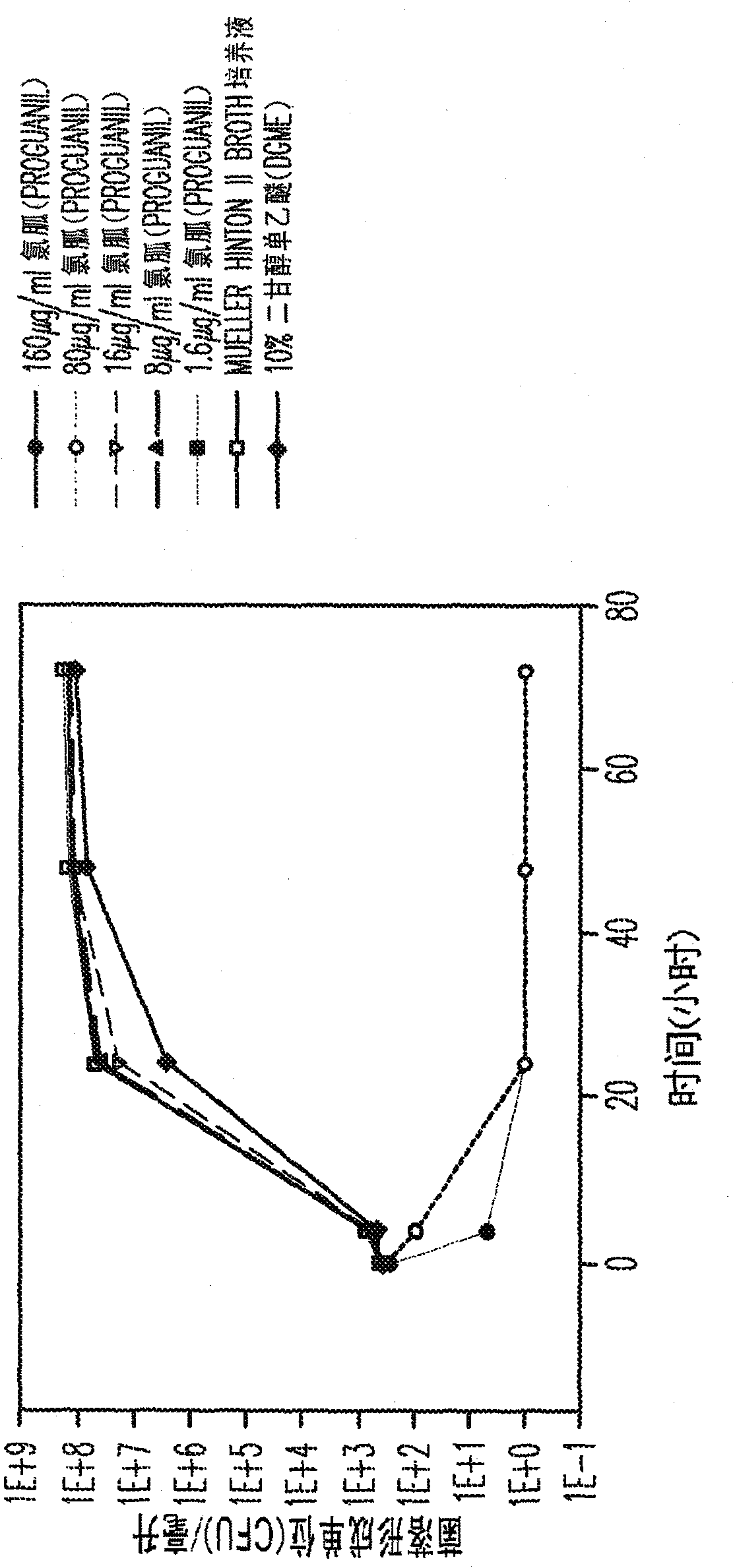
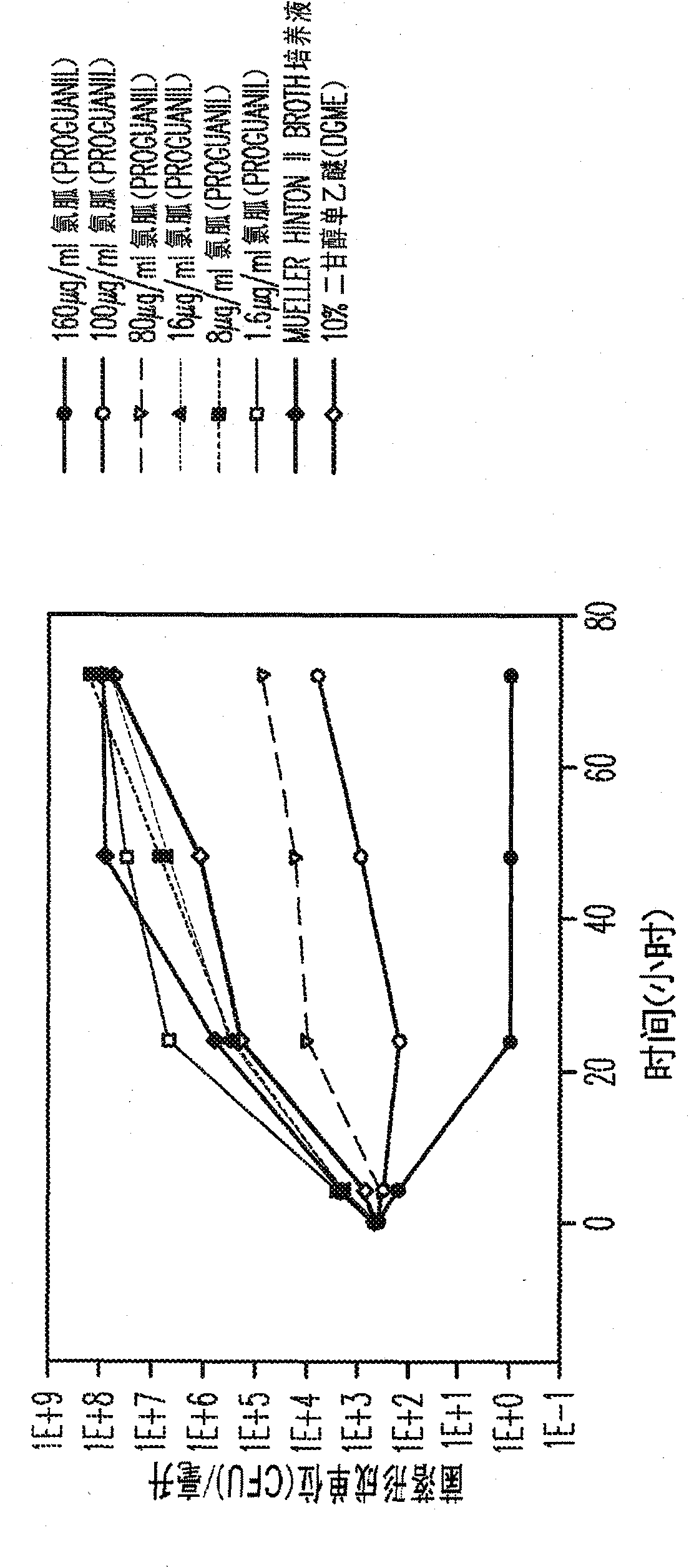
Proguanil to treat skin/mucosal diseases
Proguanil has been found to have rapid and effective killing activity against a variety of disease-causing micro organisms. For example, when applied topically, proguanil is particularly effective against Propionibacterium acnes, a bacteria that causes acne; Corynebacterium minutissimum, a bacteria that causes erythrasma, Gardnerella vaginalis, a bacteria that causes vaginosis; Trichomonas vaginalis, a protozoan that causes trichomoniasis and C. albicans, a fungus (a form of yeast).
Owner:TOLMAR INC
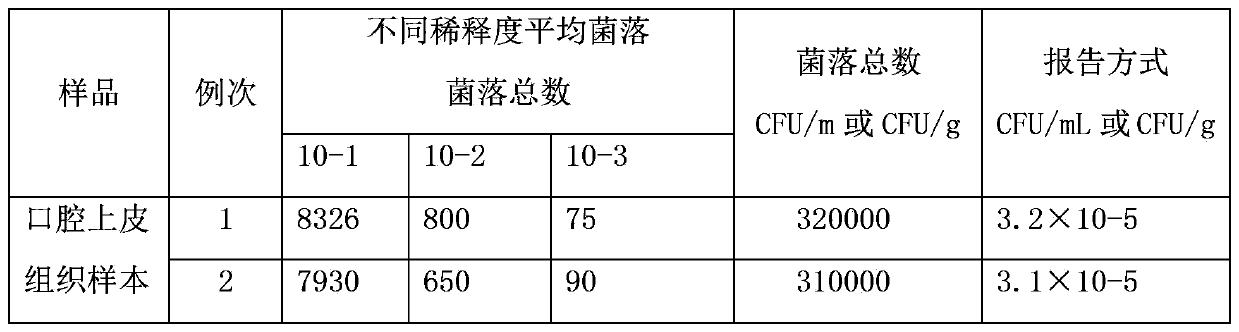
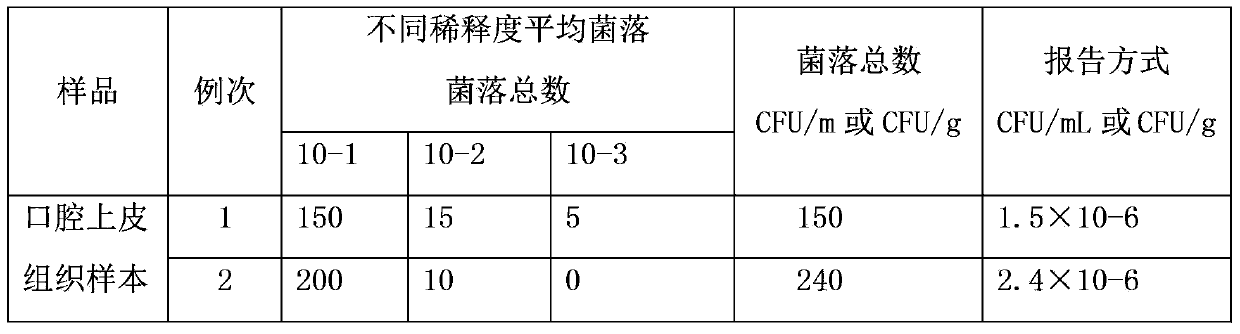
Nano-silver mouth wash for preventing and treating oral diseases and preparation method thereof
InactiveCN103385804AGood control effectLong-lasting and effectiveAntibacterial agentsCosmetic preparationsOral diseaseMucosal disease
The invention relates to the technical field of daily consumer goods for killing malignant bacteria, viruses and funguses in oral cavity and throat, cleaning the oral cavity and preventing and treating oral diseases, and in particular relates to nano-silver mouth wash for preventing and treating oral diseases and a preparation method thereof. The mouth wash is characterized by comprising 0.1ppm-1000ppm nano silver and the following components in percentage by weight: 0.01-5% of a hemostatic, 0.01-5% of a topical anaesthetic, 0.01-10% of a bacteriostatic agent, 0.1-25% of a dispersant, 0.1-25% of a thickener, 0.1-15% of a wetting agent, 0.1-10% of an algefacient, 0.1-35% of an adhesive agent, 0.01-5% of a corrigent and 0.01-5% of a colorant. The principle is as follows: silver particles with diameter of 10-50 nanometers can be absorbed to cytomembranes of harmful microorganisms to damage protein capsids and DNA (Deoxyribonucleic Acid) molecules of the harmful microorganisms to lead to death of the harmful microorganisms by interfering metabolism of the harmful microorganisms, so that the nano-silver mouth wash has broad spectrum sterilizing and bacteriostatic effect, is durable and effective in effect, free from toxic and side effects as well as drug resistance, safe and reliable, convenient to use, simple in production and preparation, and has very good preventing and treating effect to periodontal disease, acute and chronic aryngitis and oral mucosal diseases.
Owner:阎昭良
Treatment of skin or mucosal pathology
InactiveUS20150290173A1Increase in mucoadhesivityInhibit progressBiocideCosmetic preparationsDiseaseMucosal disease
The invention generally relates to compositions comprising a soluble chitosan derivative and an H2 receptor antagonist, and the use of the compositions for treating skin or mucosal diseases, such as inflammatory diseases or infectious disease.
Owner:ZARZATECH
Therapeutic agent for infectious skin and mucosal disease
InactiveUS20110052725A1Ameliorate a skin diseaseAntibacterial agentsBiocideDiseasePathogenic microorganism
A therapeutic agent for infectious skin and mucosal diseases to be applied to an infected area for ameliorating a symptom of the infected area caused by infection with a pathogenic microorganism includes: a chlorine dioxide solution including a dissolved chlorine dioxide gas.
Owner:TAIKO PHARMA
Herbal-based nasal solution and method of use thereof
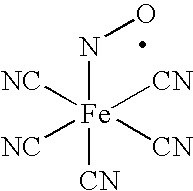
A nasal solution and method for treating nasal and sinus inflammation. The nasal solution includes vitamin C, an extract of scutellaria radix, an extract of eleutherococcus radix, an extract of chamomile, and a pharmaceutically acceptable carrier which is suitable for administering the vitamin C, the scutellaria radix extract; the eleutherococcus radix extract; and the chamomile to the nose of a patient. The method includes administering the nasal solution which suppresses early phase and late phase mediators of allergic mucosal disease in order to maintain long-term inhibition of nasal and sinus inflammation.
Owner:ZNOVA
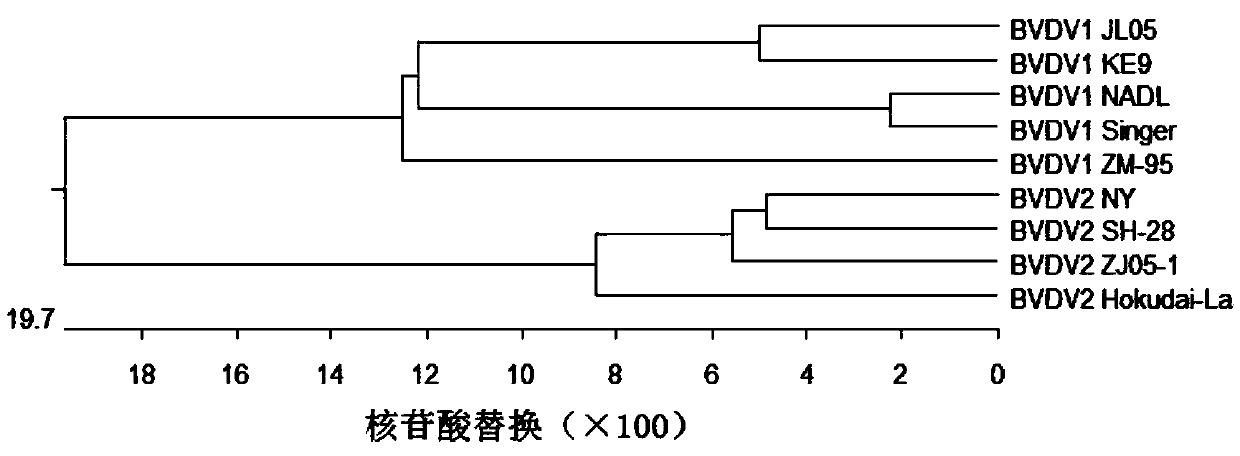
Deer-derived bovine viral diarrhea inactivated vaccine and preparation method thereof
InactiveCN108300703AMeet the virus requirements for seedling productionSsRNA viruses positive-senseViral antigen ingredientsBacteroidesMicroorganism
The invention discloses a deer-derived bovine viral diarrhea inactivated vaccine and a preparation method thereof, relates to the technical field of vaccines, and fills the blank of a vaccine specialfor viral diarrhea / mucosal diseases of sika deer. The deer-derived bovine viral diarrhea inactivated vaccine JL05 is preserved in the CGMCC on January 11, 2018, with a collection number of CGMCC No.15283. The deer-derived BVDVJL05 virus is subjected to passage by using MDBK cells and then is subjected to plaque cloning to purify the virus; detection shows that the content of the deer-derived BVDVJL05 virus is 107.2 TCID50 / ml or more, and no germ, mold, mycoplasma or exogenous virus pollution is caused; the deer-derived BVDV JL05 virus meets a virus requirement for vaccine preparation. The virus is inactivated by BEI and then is mixed and emulsified with a 206 adjuvant to prepare the vaccine; safety and potency inspection shows that the vaccine is safe to the sika deer, and the challenge protection rate can be up to 100 percent.
Owner:JILIN AGRICULTURAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com