Mineral salt-sulfonic acid compositions and methods of use
a technology of mineral salt and composition, applied in the field of mineral salt-sulfonic acid compositions and methods of use, can solve the problems of severe malabsorption of nutrients, pain, infection, and ulceration, and achieve the effects of reducing the number of syringes, and improving the permeability of the syringes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Bactericidal Activity of Compositions Comprising Zinc Gluconate and Taurine
[0179]This example illustrates the microbicidal activity of a composition comprising zinc gluconate and taurine.
[0180]GelX™ ORAL GEL was formulated as a viscous gel comprised of purified water, polyvinyl pyrrolidone (PVP), taurine, zinc gluconate, PEG-40 hydrogenated castor oil, sodium saccharin, sodium hydroxide, and flavoring. GelX™ ORAL GEL has a mechanical action which provides pain relief by adhering to the mucosal surface of the mouth and throat soothing lesions.
[0181]Antimicrobial activity of a composition comprising zinc gluconate and taurine was determined by a method typically used to demonstrate whether one or more agents may be included as a preservative in a composition formulated under conditions such that the composition will be considered a non-sterile composition according to regulatory authorities.
[0182]The minimum inhibitory concentration of solutions containing differing amounts of zinc gl...
example 2
Treatment of Oral Aphthous Stomatitis
[0193]This example describes the effectiveness of using a composition comprising zinc gluconate and taurine and polyvinyl pyrrolidone (PVP) for treatment of oral aphthous stomatitis.
[0194]A painful ulcer inside the oral cavity caused by a rupture of the membrane is defined by the term aphthous. Anaphthous ulcer is also called a canker sore. When multiple aphthous ulcers occur and / or when the condition is chronic (or recurrent), the condition is called aphthous stomatitis (see, e.g., Natah et al., Int. J. Oral. Maxillofac. Surg. 33:221-34 (2004)).
[0195]Over a period of eleven months, in a dentist's surgery in Italy, 150 patients who were affected by minor aphthous were recruited to participate in a clinical study. The patients (87 female and 63 male) were between the ages of 18 and 71 and provided informed consent to participate. The patients were randomly divided into five groups, thirty people each.[0196]Group A: Patients were treated with 100% ...
example 3
Inhibition of IL-6 and IL-8 Production in Cells by Zinc and Taurine
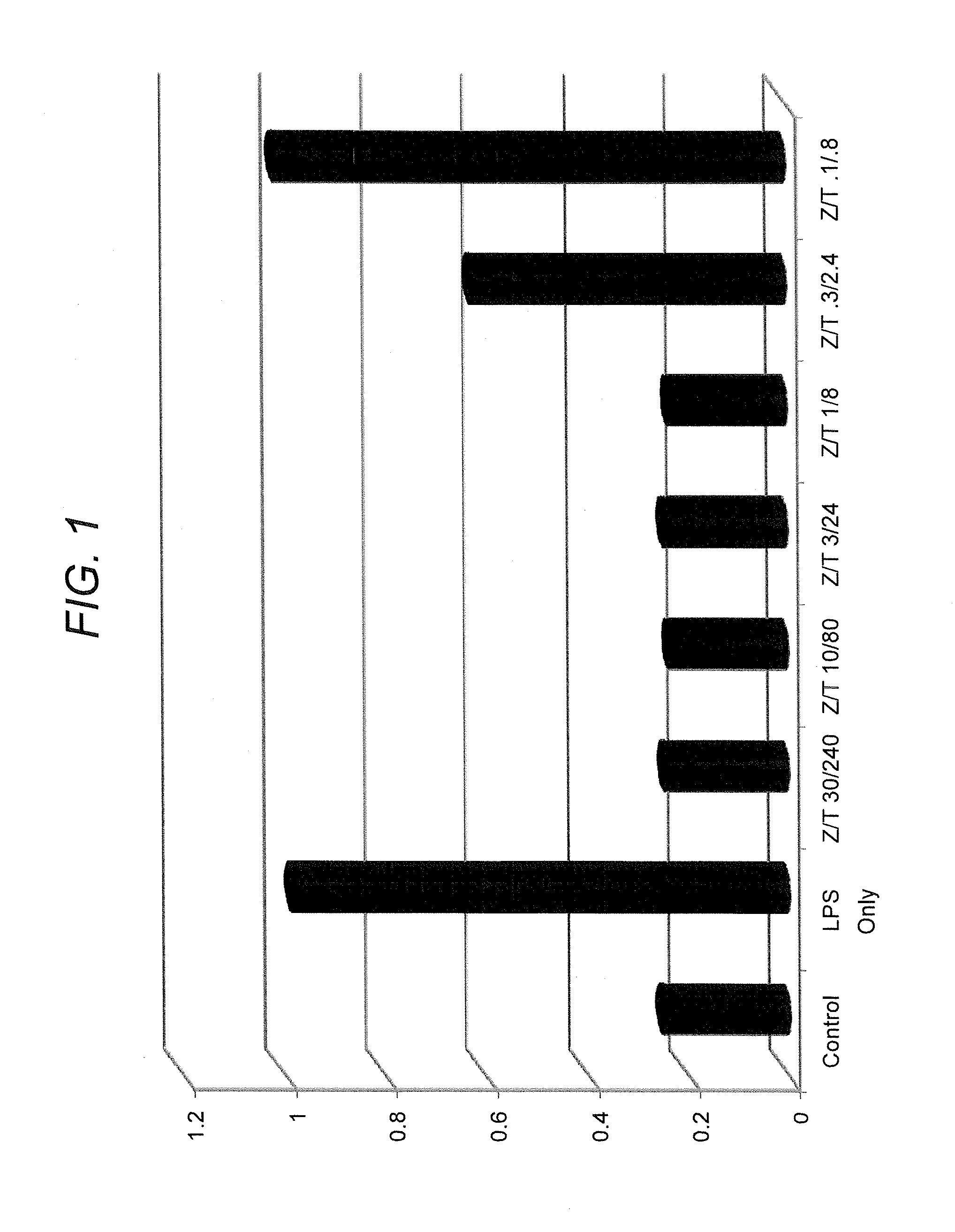
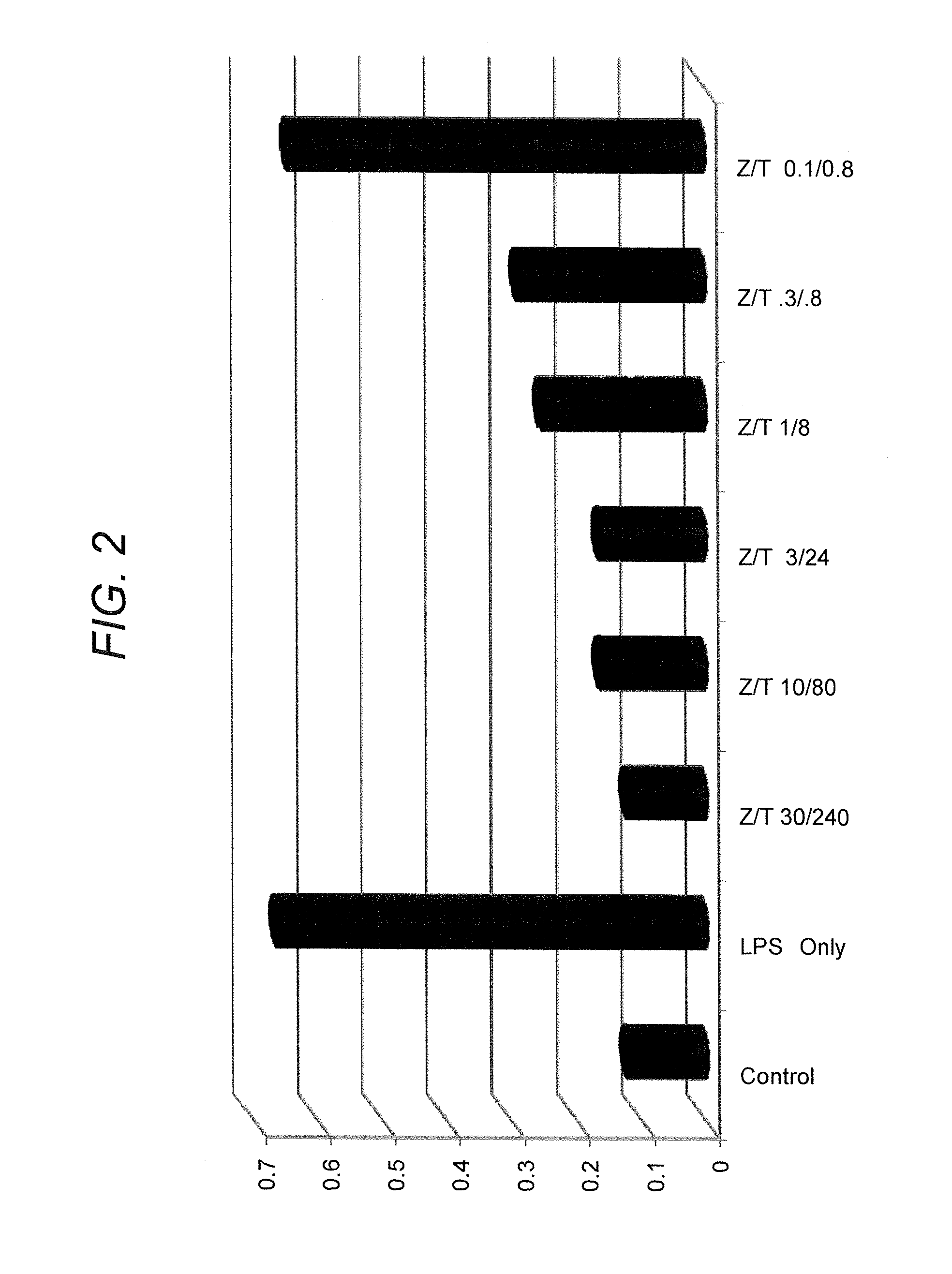
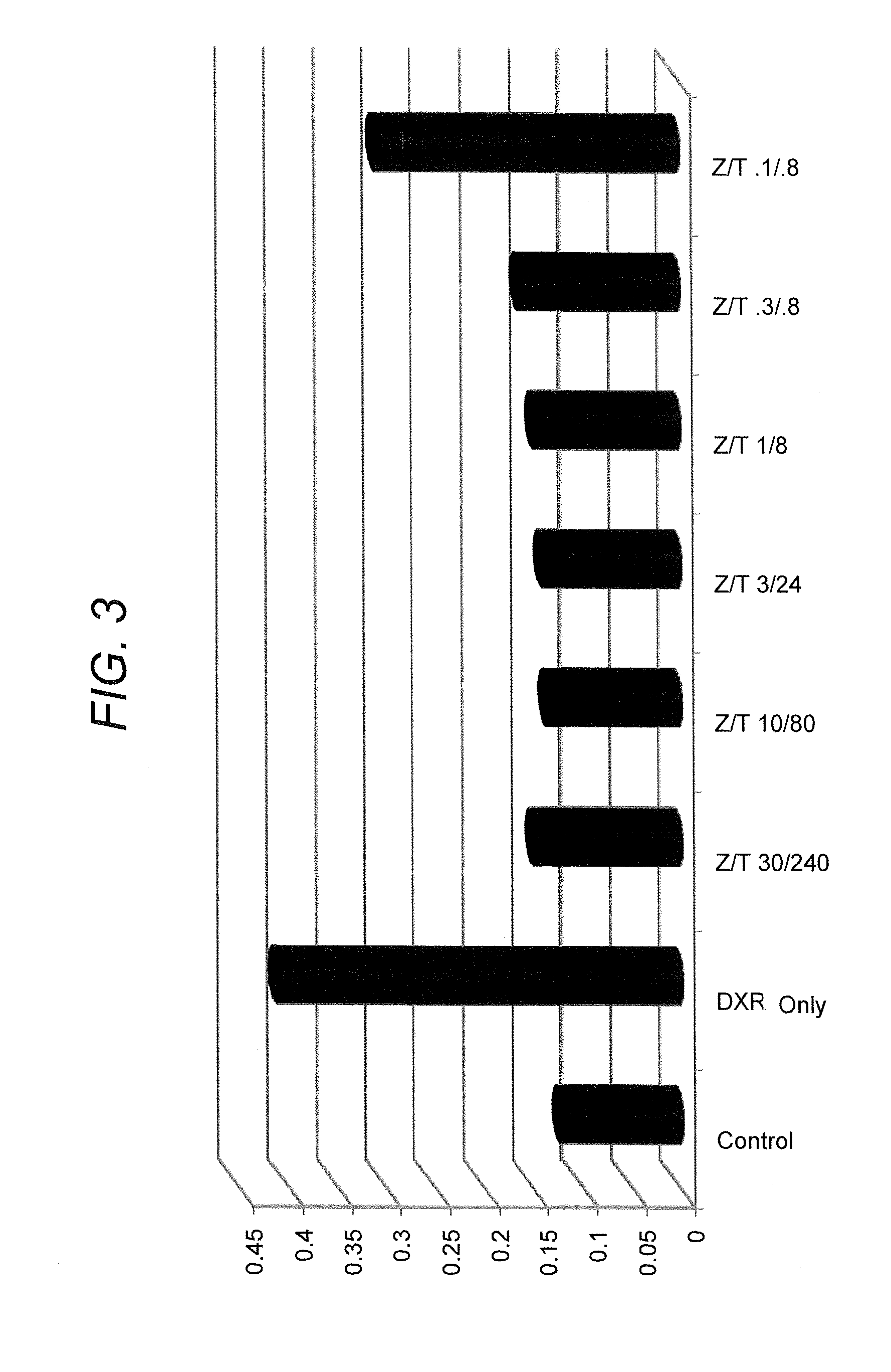
[0203]This example describes the capability of zinc (e.g., zinc gluconate) in combination with taurine to inhibit production of cytokines, IL-6 and IL-8, in cells stimulated by either lipopolysaccharide (LPS) or doxorubicin, a chemotherapeutic agent.
[0204]CaCo2 cells (human intestinal (colonic) epithelial cells) were grown in culture in 24-well tissue culture dishes according to methods routinely practiced in the cell culture art. Zinc gluconate and taurine were combined at varying concentrations and added to the cells in the absence and presence of proinflammatory agents (lipopolysaccharide, doxorubicin, dextran-sulfate sodium (DSS)). In one series, zinc gluconate and taurine were combined with lipopolysaccharide (LPS) (1 μg / ml) for six hours. In a second series, zinc gluconate and taurine were combined with doxorubicin (3 μM) for 24 hours. In a third series, zinc gluconate and taurine were combined with DSS (5% w / w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com