Patents
Literature
1214 results about "Pain relief" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
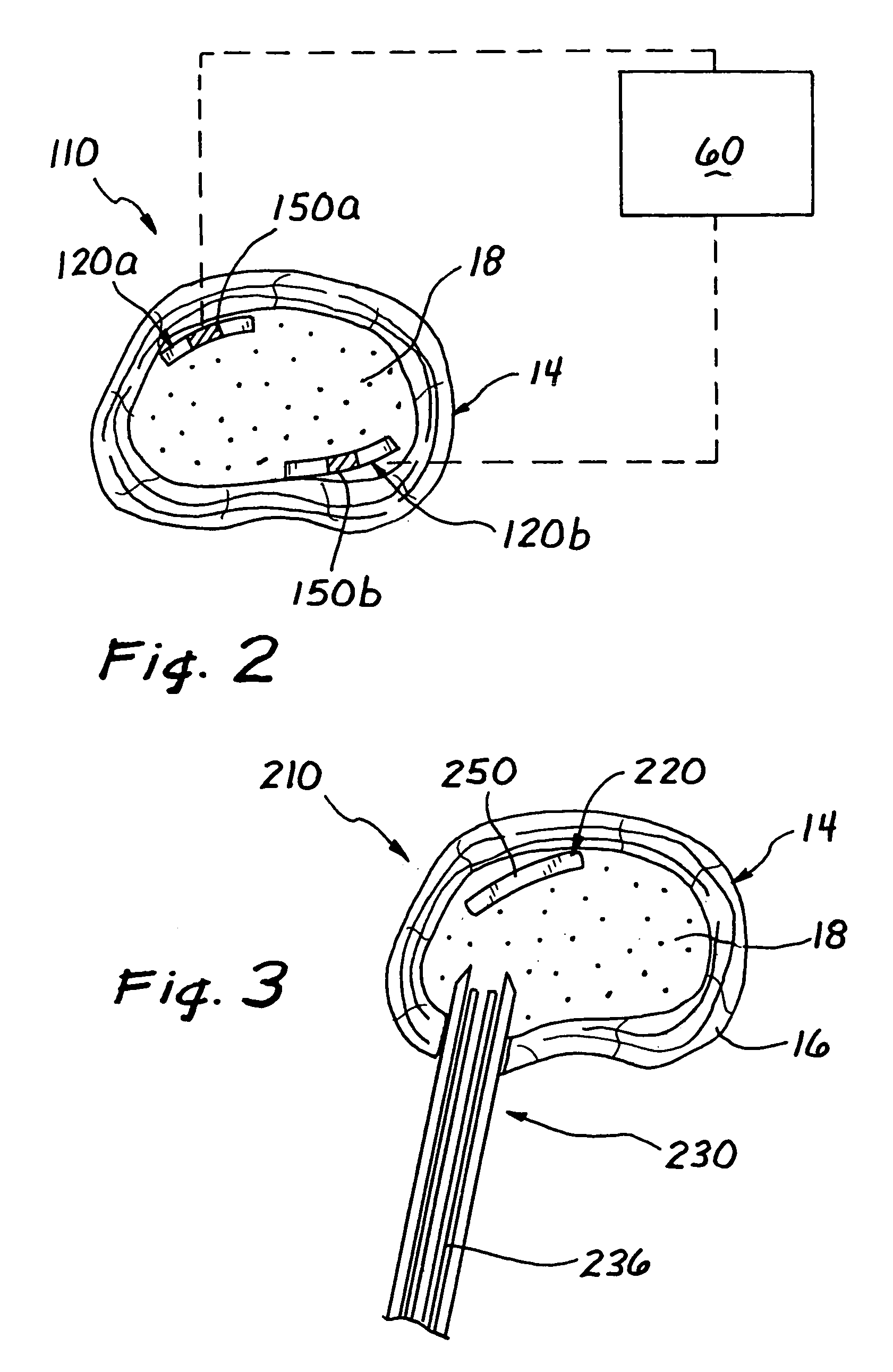
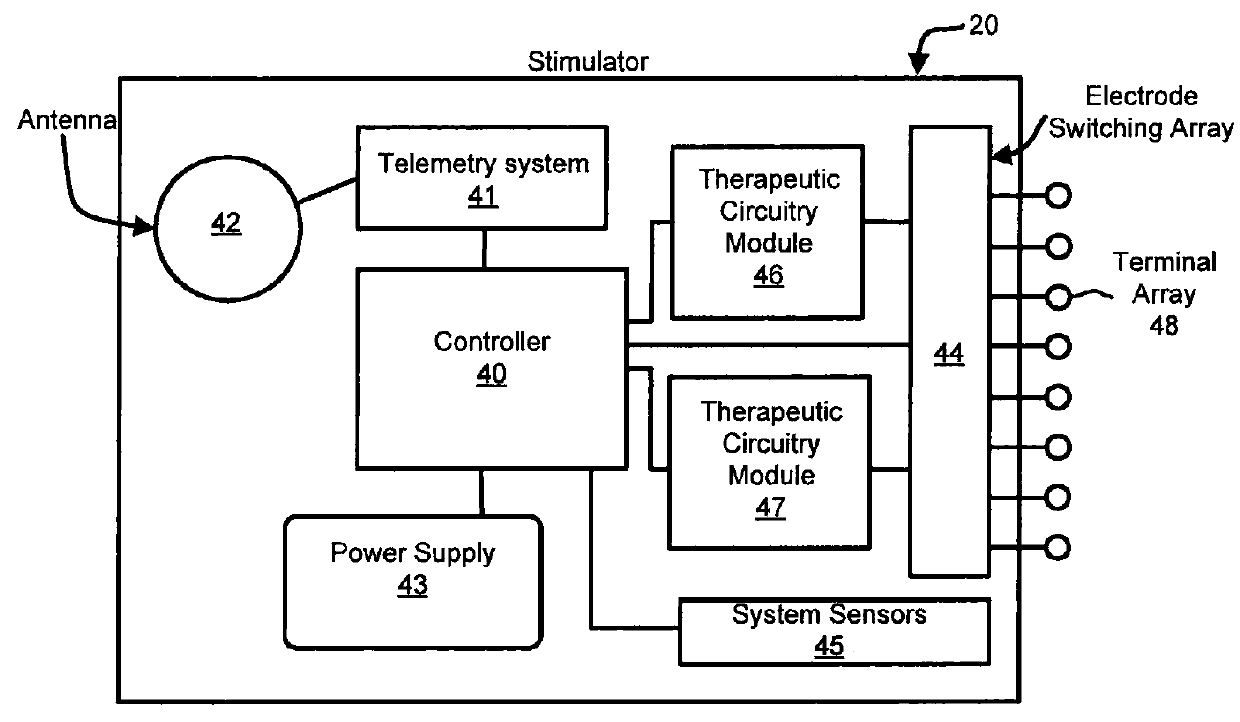
Apparatus and method for bioelectric stimulation, healing acceleration, pain relief, or pathogen devitalization
ActiveUS7117034B2Minimal stressPromote healingElectrotherapyArtificial respirationEngineeringAnimal body
An method and method for generating an electrical signal for use in biomedical applications, including two timing-interval generators, each optionally driving a multistep sequencer; analog, digital or hybrid means for combining the resulting timed signals into a complex electrical signal; optional filtering means for blocking direct current, removing selected frequency components from the resulting signal, and / or providing voltage step-up if needed; and conductive means for coupling the resulting signal to a human or animal body, food, beverage or other liquid, cell or tissue culture, or pharmaceutical material, in order to relieve pain, stimulate healing or growth, enhance the production of specific biochemicals, or devitalize selected types of organisms.
Owner:HEALTHONICS INC
Portable ultrasound device for the treatment of wounds
InactiveUS7878991B2Ultrasonic/sonic/infrasonic diagnosticsUltrasound therapyFocus ultrasoundMedicine
Device and methods for the treatment of wounds using ultrasound energy are disclosed. The portable wound treatment device may deliver ultrasound energy to a wound through direct contact with the ultrasound tip and / or through a liquid coupling medium. Several ultrasound tips specially designed to concentrate and focus ultrasound energy onto a wound are also disclosed. The ultrasound tip may also possess an abrasive peripheral boundary to aid in debriding the wound and / or removing necrotic tissue. The disclosed invention may have multiple beneficial effects in treating a wound such as sterilizing a wound, reducing external bleeding, and / or providing pain relief.
Owner:BACOUSTICS LLC
Modular stimulator for treatment of back pain, implantable RF ablation system and methods of use
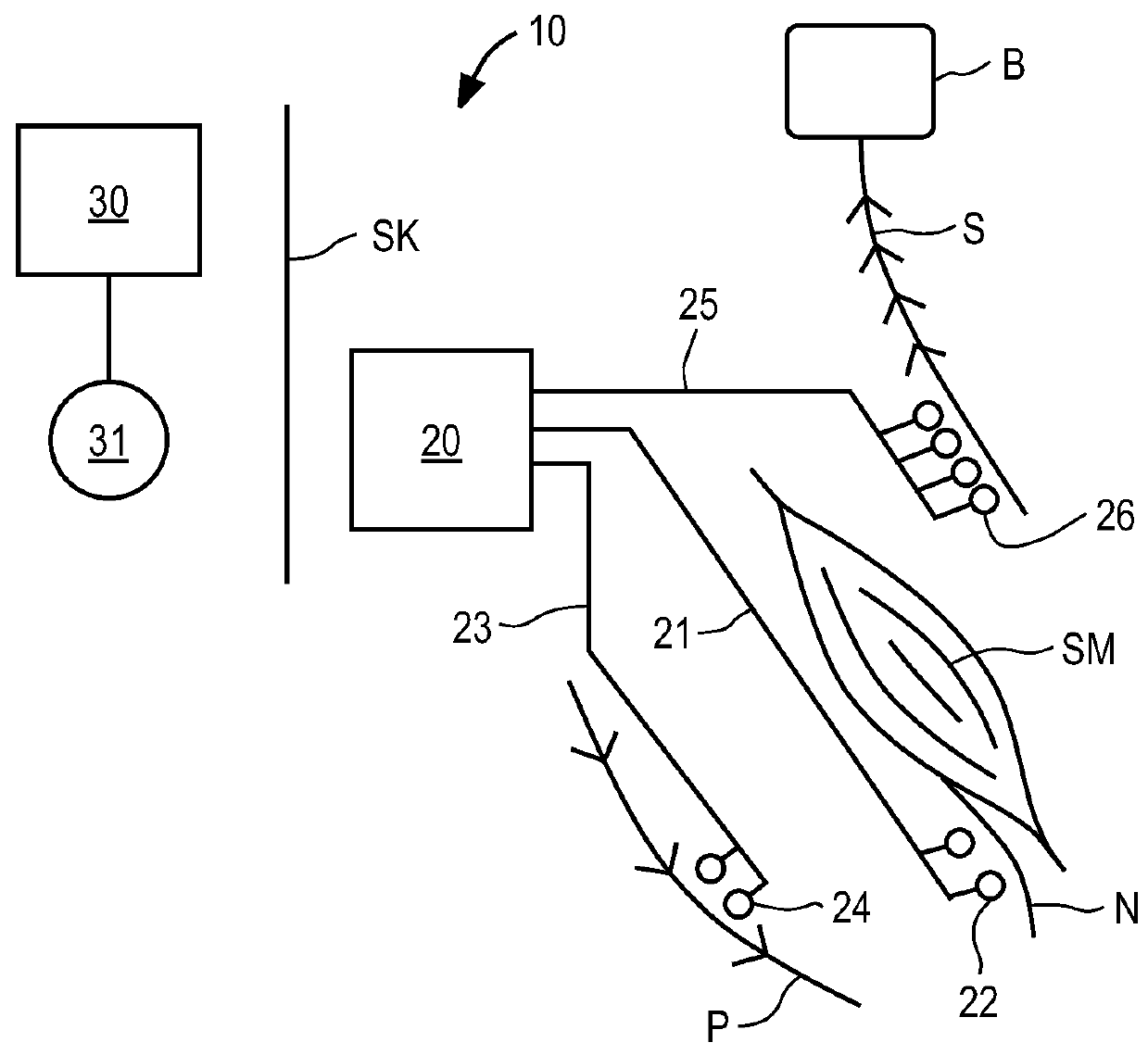
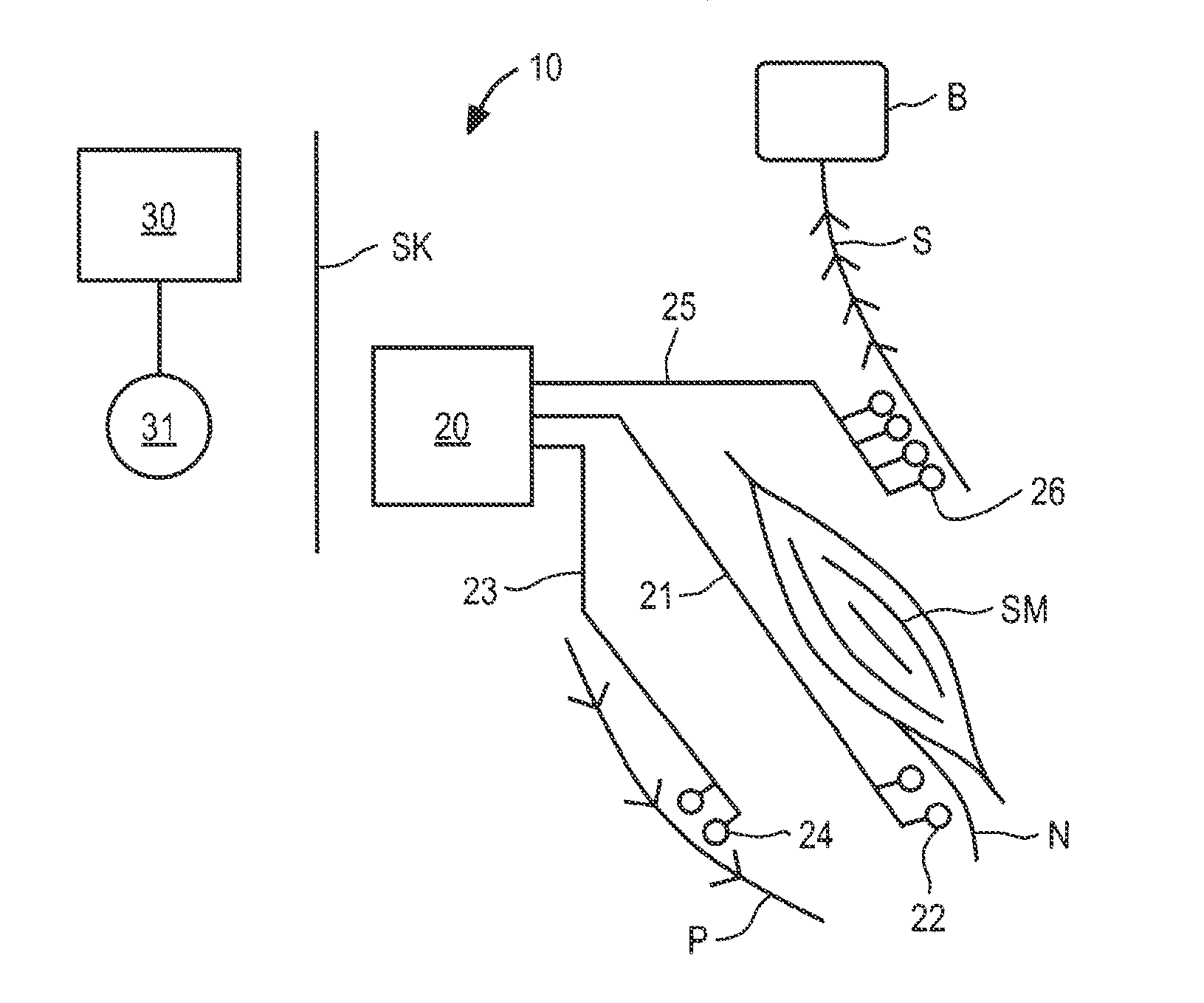
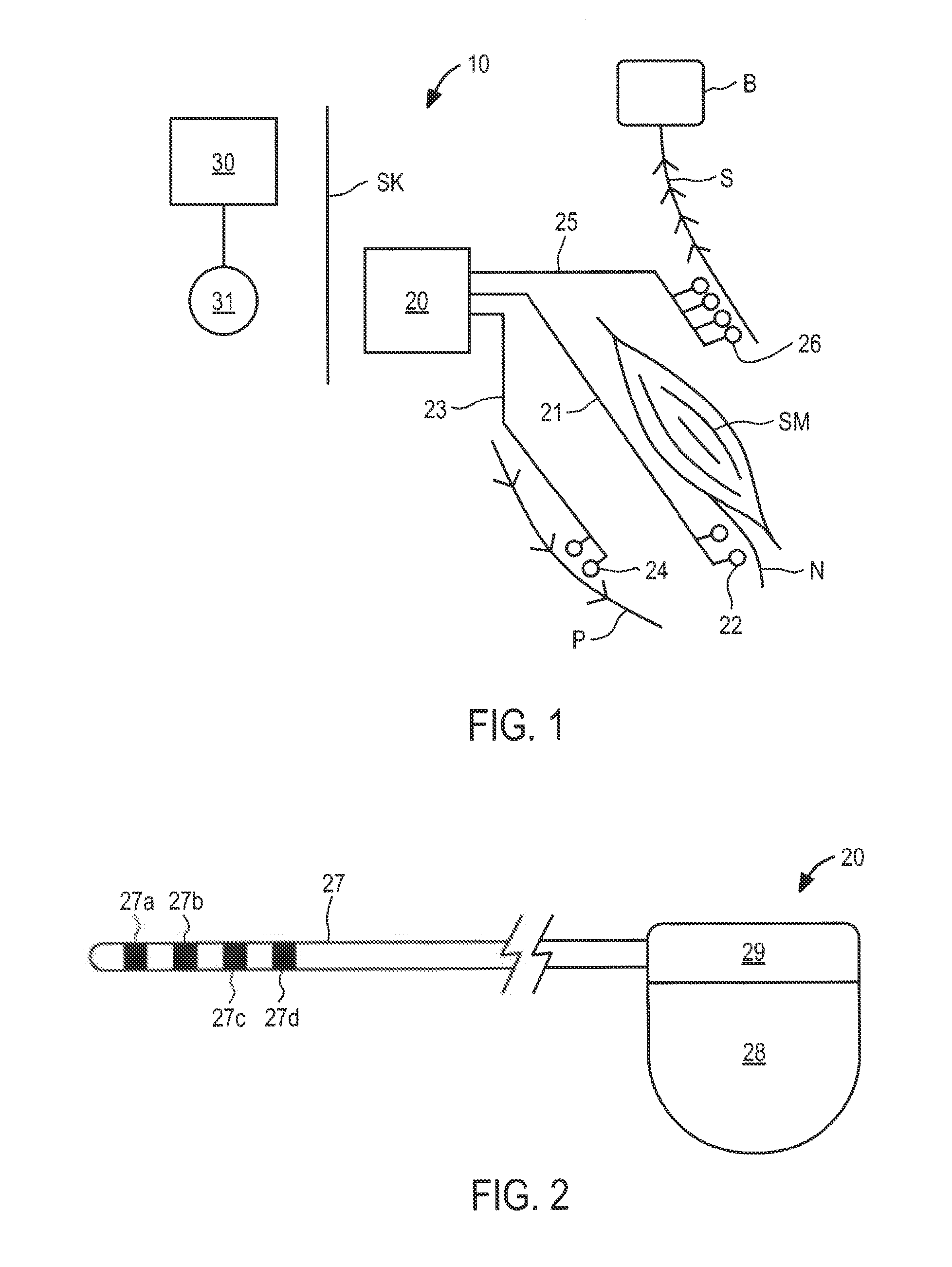
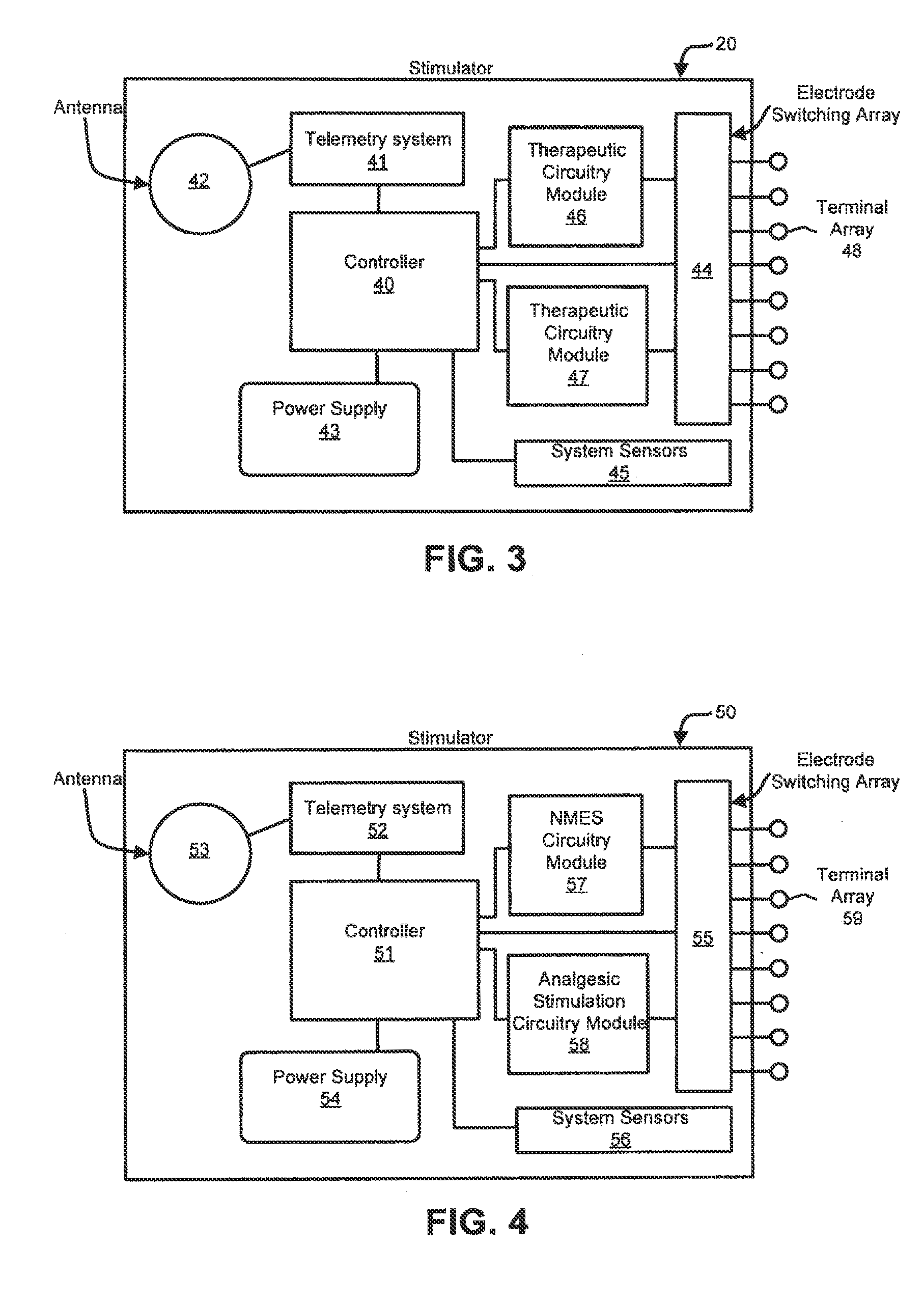
ActiveUS20110224665A1Rehabilitate spinal stabilityRestore neural driveSpinal electrodesDiagnosticsRf ablationMuscle contraction
Apparatus and methods for treating back pain are provided, in which an implantable stimulator is configured to communicate with an external control system, the implantable stimulator providing a neuromuscular electrical stimulation therapy designed to cause muscle contraction to rehabilitate the muscle, restore neural drive and restore spinal stability; the implantable stimulator further including one or more of a number of additional therapeutic modalities, including a module that provides analgesic stimulation; a module that monitors muscle performance and adjusts the muscle stimulation regime; and / or a module that provides longer term pain relief by selectively and repeatedly ablating nerve fibers. In an alternative embodiment, a standalone implantable RF ablation system is described.
Owner:MAINSTAY MEDICAL
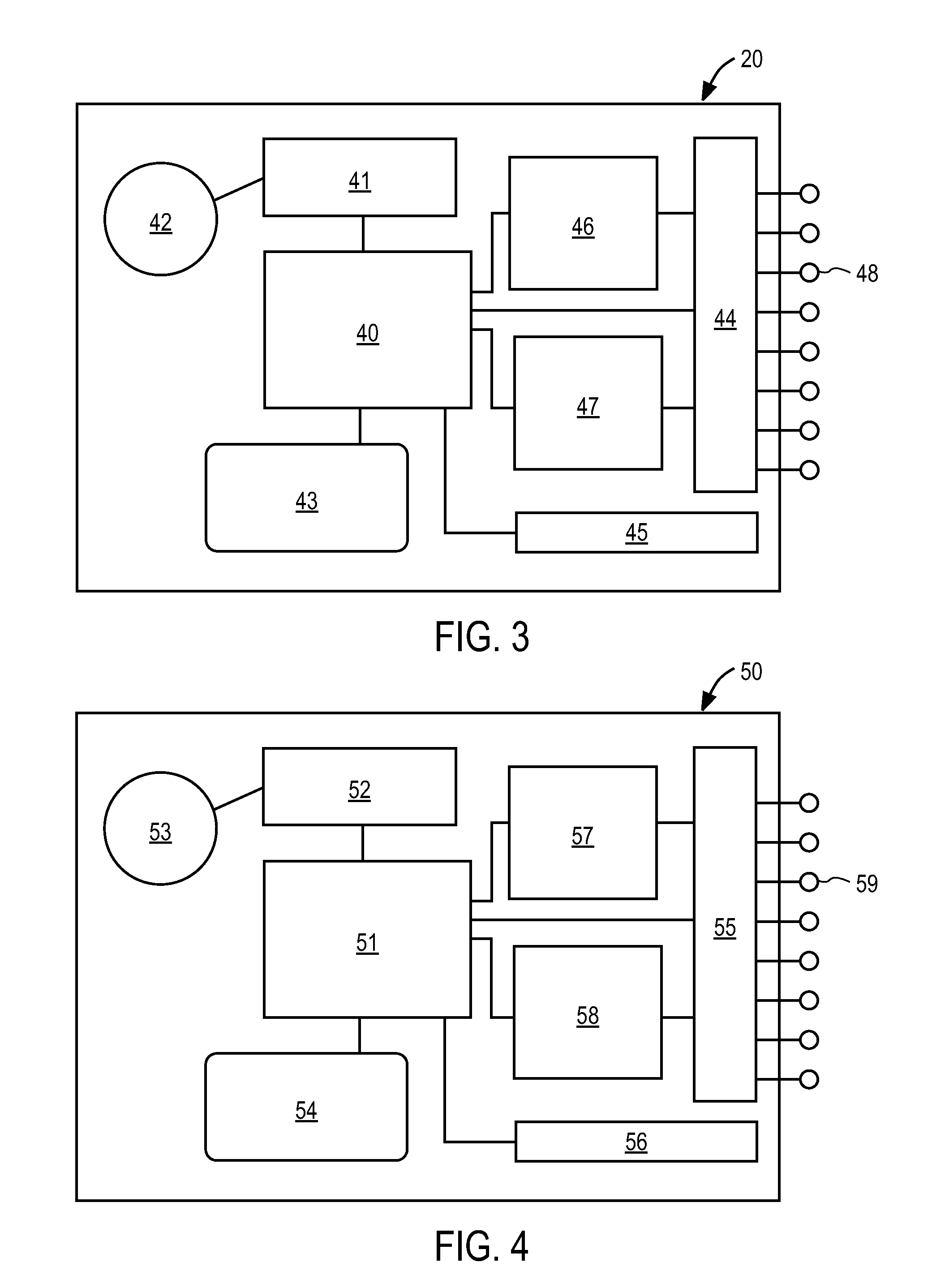
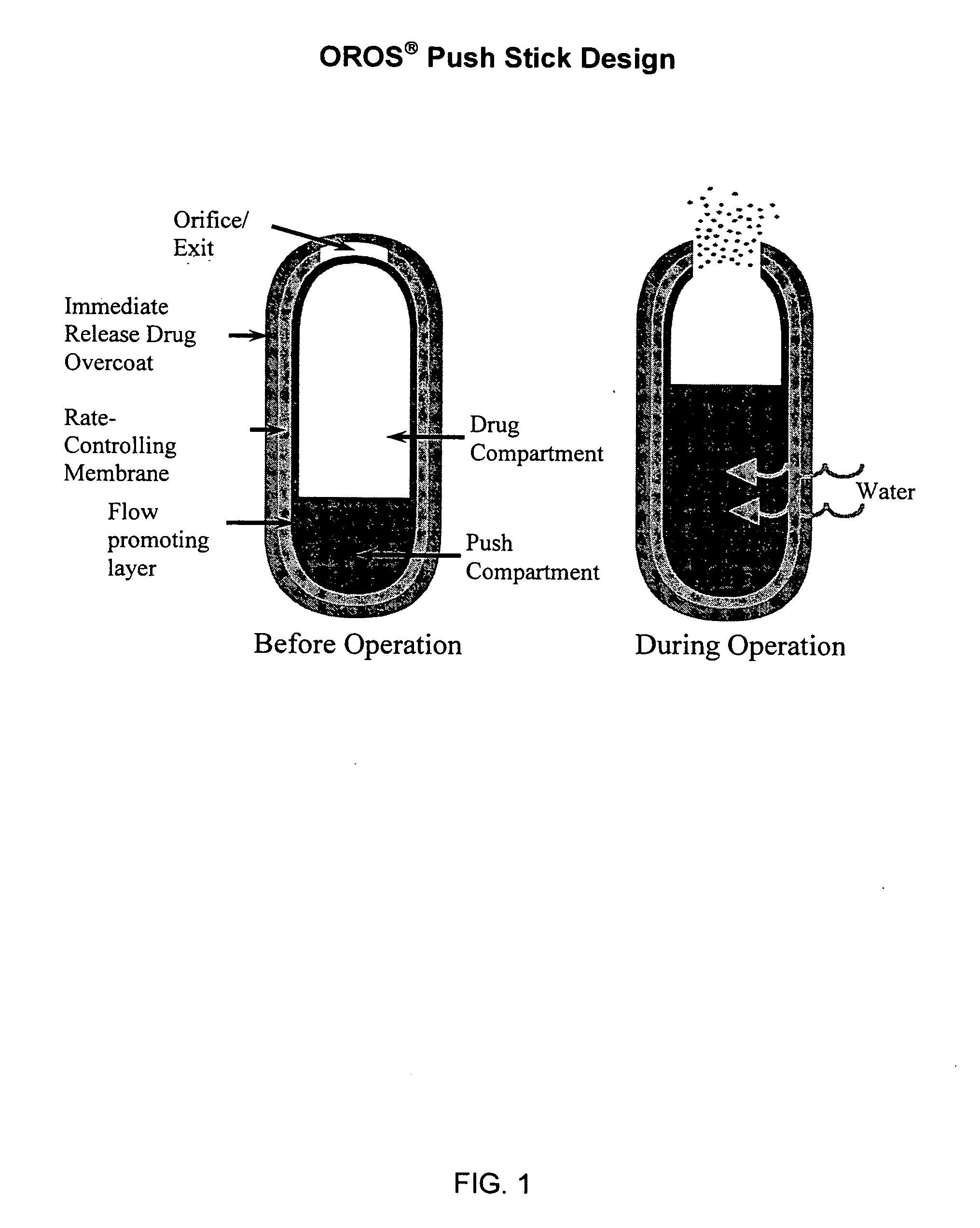
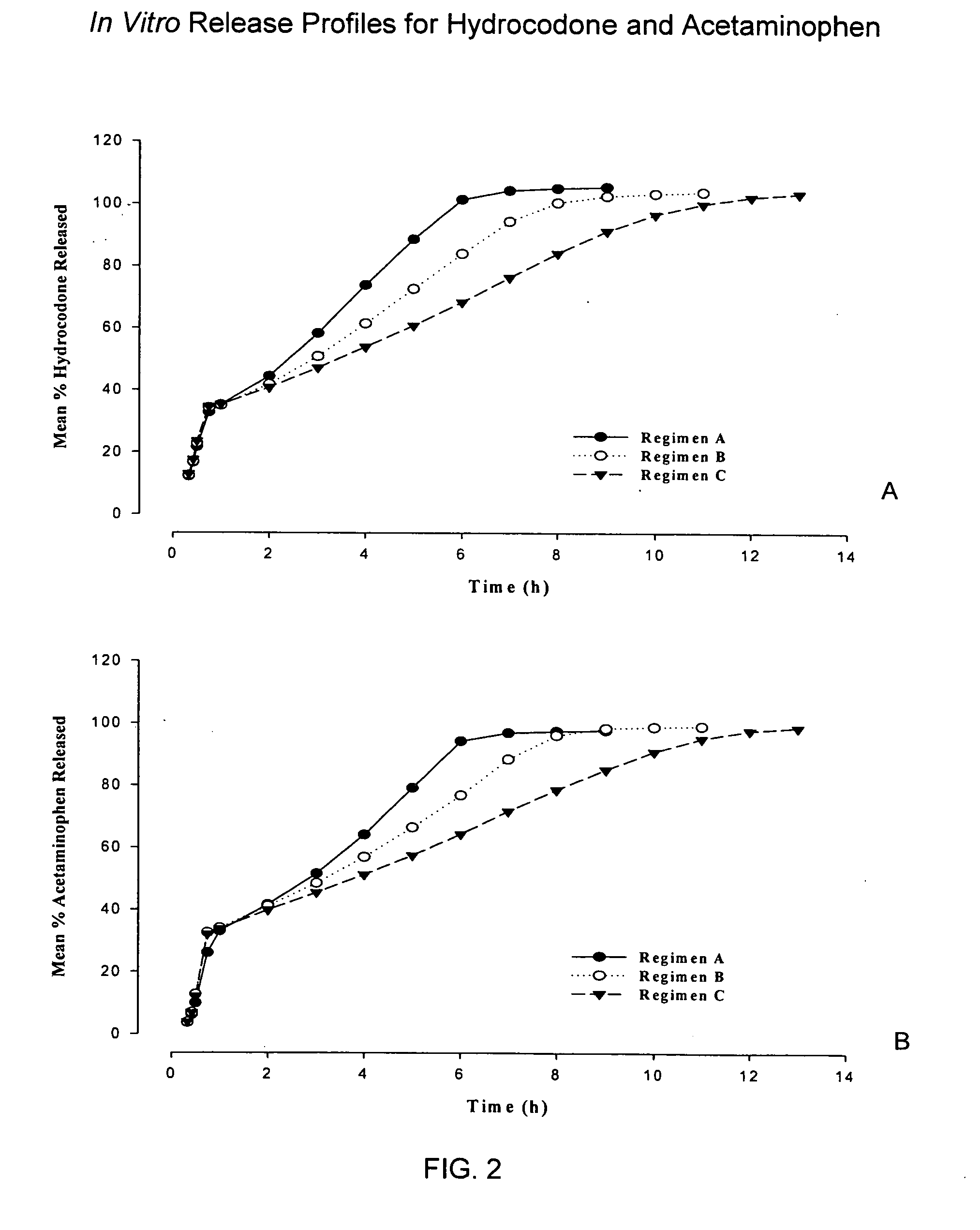
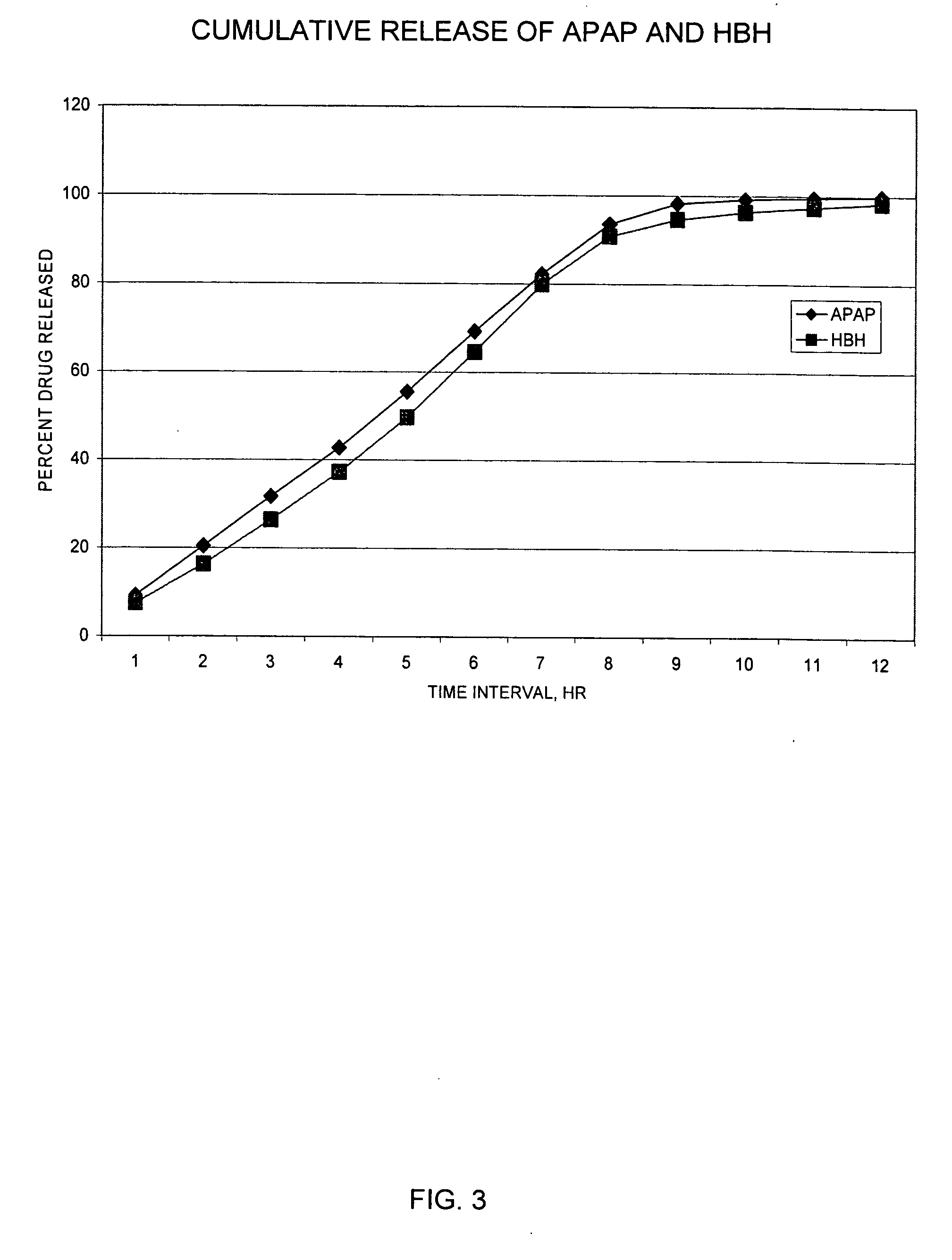
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
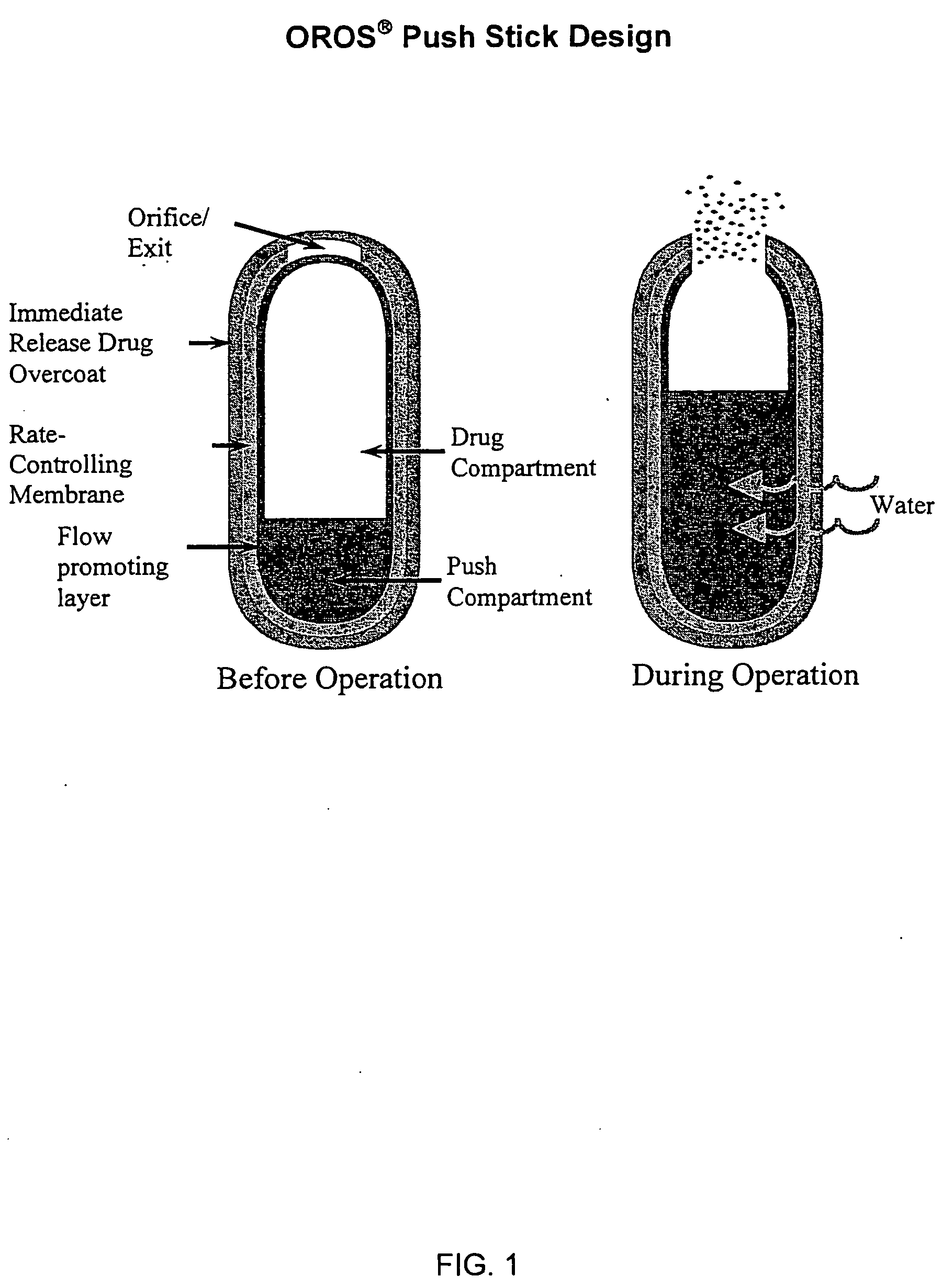
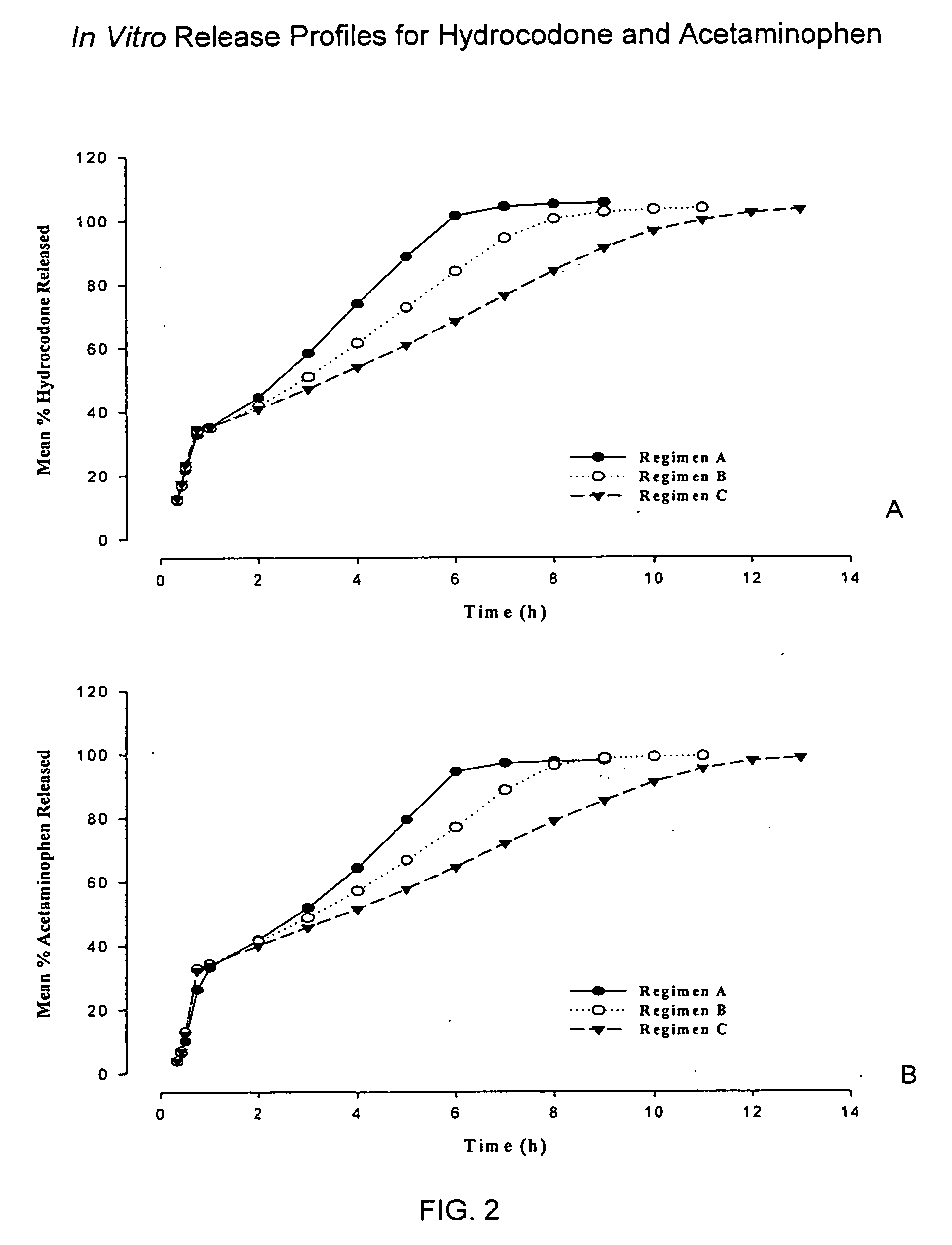
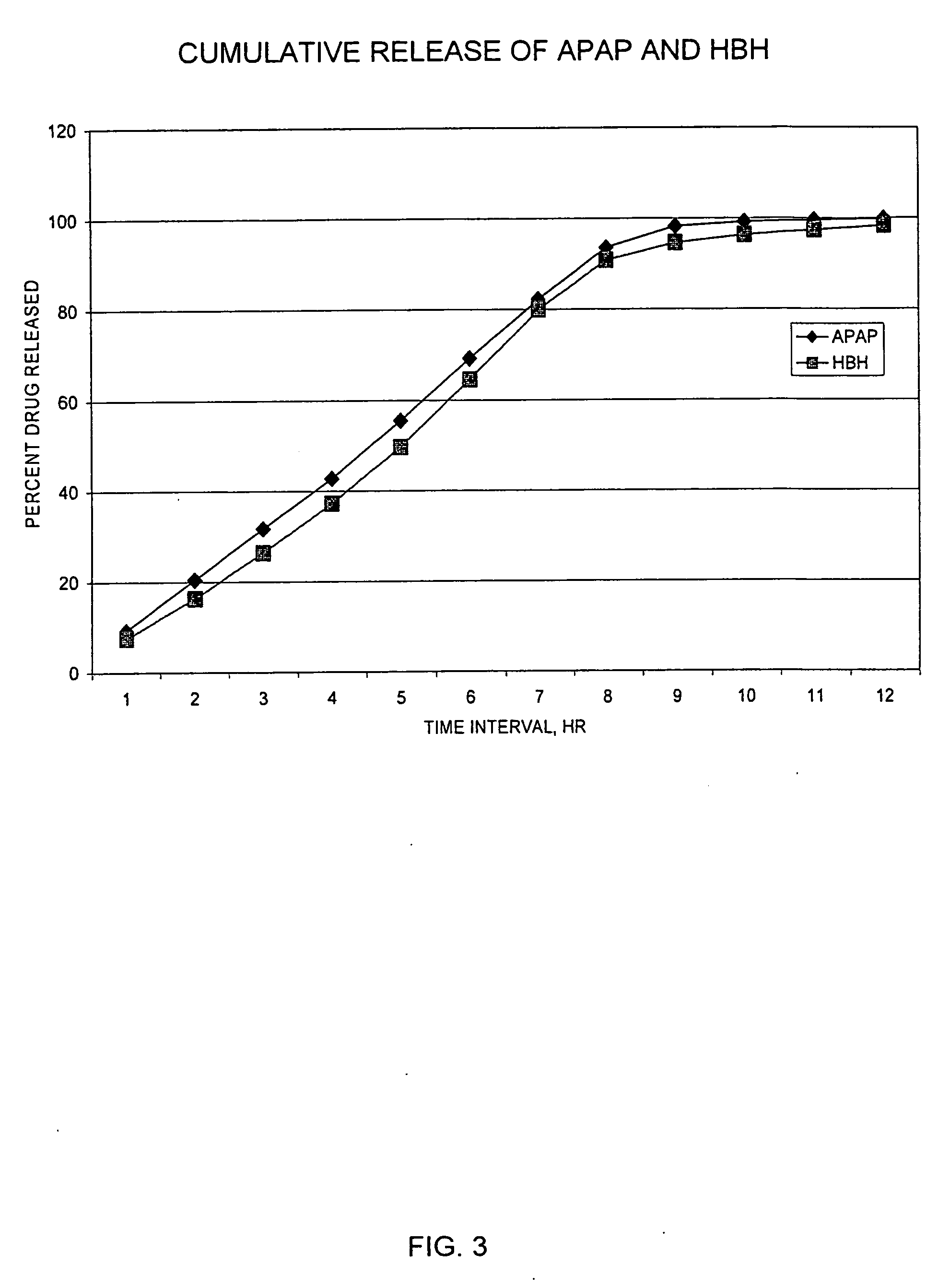
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Vibrating therapy device
InactiveUS20070255187A1Easy to disassembleChiropractic devicesVibration massageHands freePhysical therapy
A device for providing therapy to a user including vibration and temperature modulation. The device provides mobile temperature modulation capabilities, mobile vibration capabilities, or a combination of the two. The device provides advantages to a person or animal including the ability to utilize the device in a hands-free manner. The device may also provide, under certain circumstances, pain relief or healing therapy to a person or animal.
Owner:BRANCH ALAN P
Spinal disc therapy system
InactiveUS7223227B2Simple structureEasy to placeElectrotherapyPharmaceutical delivery mechanismSpinal columnTreatment effect
Spinal disc therapy systems in accordance with the present invention generally include an implant element structured to have a therapeutic effect on a human or animal body when implanted into an intervertebral disc annulus or intervertebral disc nucleus. The implant element may include a biochemically active agent that provides pain relief, inflammation relief or other benefit to the human or animal body. The implant element may be mechanically active or mechanically activatable in being effective in providing a therapeutic effect to the human or animal body when implanted in the intervertebral disc. For example, the implant element may include a mechanically active or mechanically activatable component that radiates wave energy, for example, in the form of electrical or magnetic energy, into the body.
Owner:PFLUEGER D RUSSELL
Topical dermal anaesthetic
A liquid composition applied transdermally for relief of pain comprising alcohol in an amount by weight of about 57 to about 91 percent; glycerin in an amount by weight of about 1 to about 12 percent; an analgesic agent in an amount by weight of about 2 to about 28 percent, the analgesic agent comprising a derivative of salicylic acid; methylsulfonylmethane in an amount by weight of about 0.02 to 5 percent; and emu oil in an amount by weight of about 0.01 to 3 percent, the liquid composition permeating skin to relieve pain. The composition further comprising, as an additional feature, aloe vera in an amount by weight of at least about 0.05 percent and having an amount by weight of about 0.05 to 4 percent. The composition features transdermal pain relief such that a patient can apply the analgesic agent directly to an area of pain without such side effects as stomach irritation which is normally associated with aspirin. The composition may be sprayed or rolled directly onto the painful area. Because of the unique formula, the composition is safe to vital internal organs, requires no mixing before use, and is shelf stable for marketing purposes.
Owner:VELTRAN LP
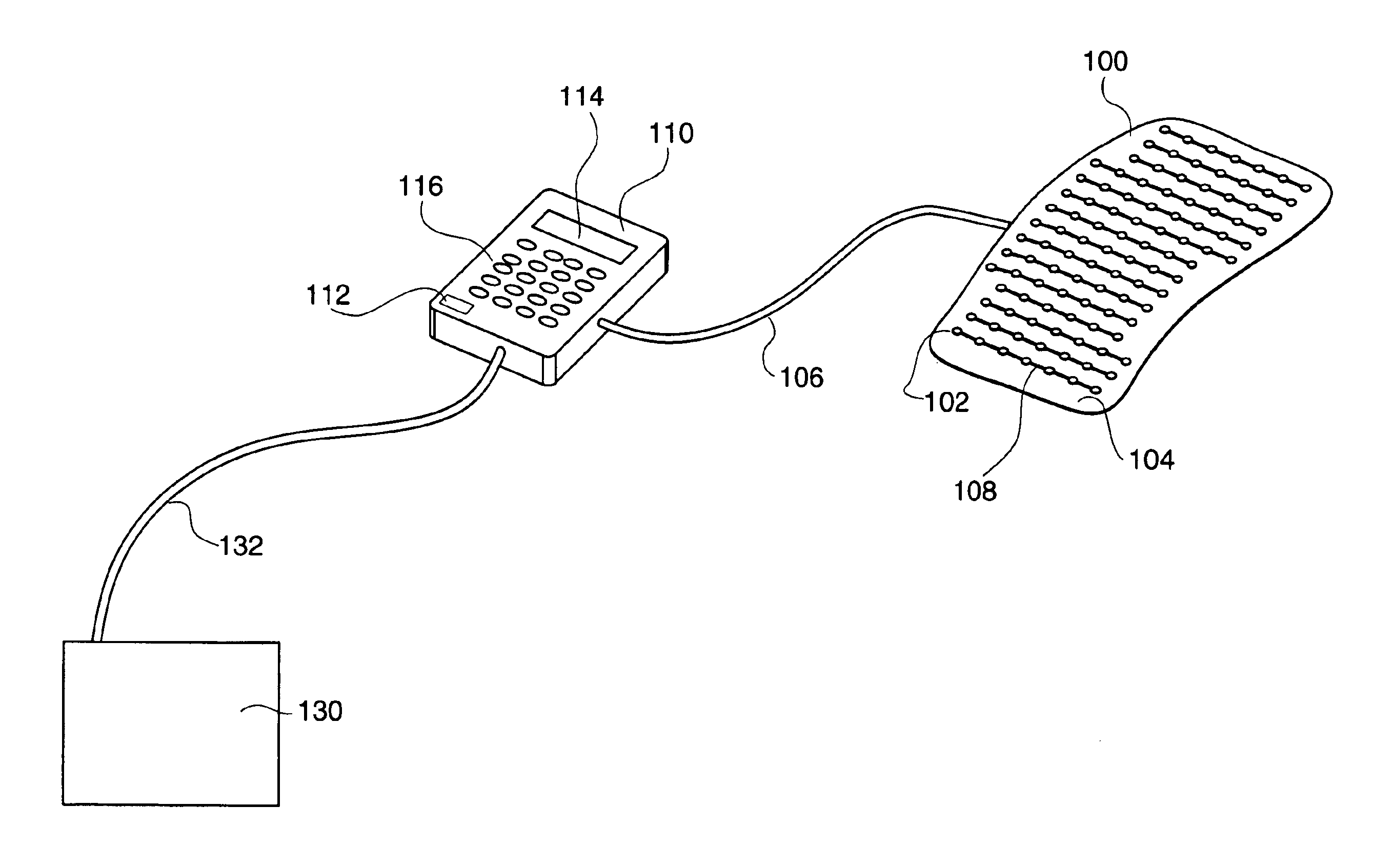
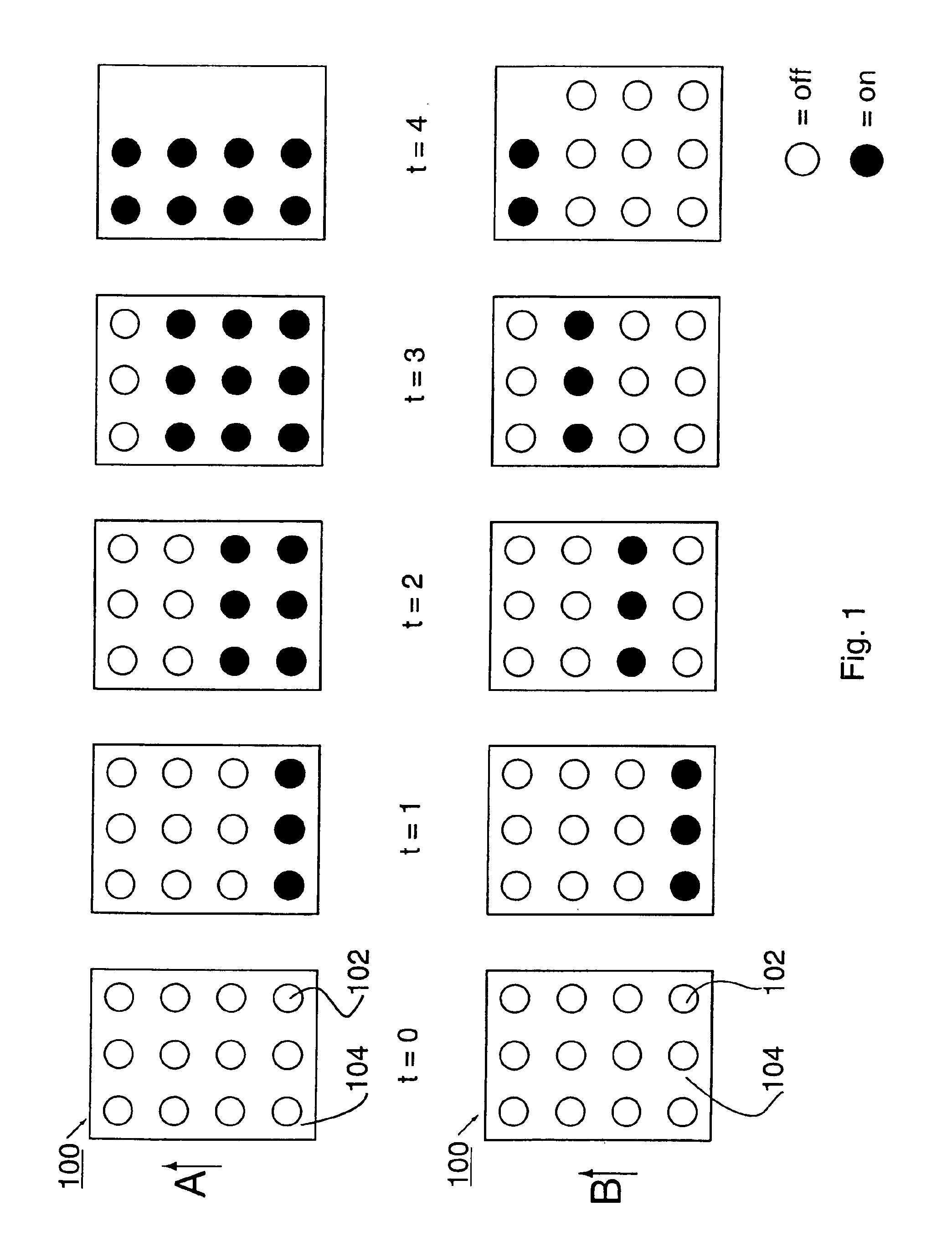
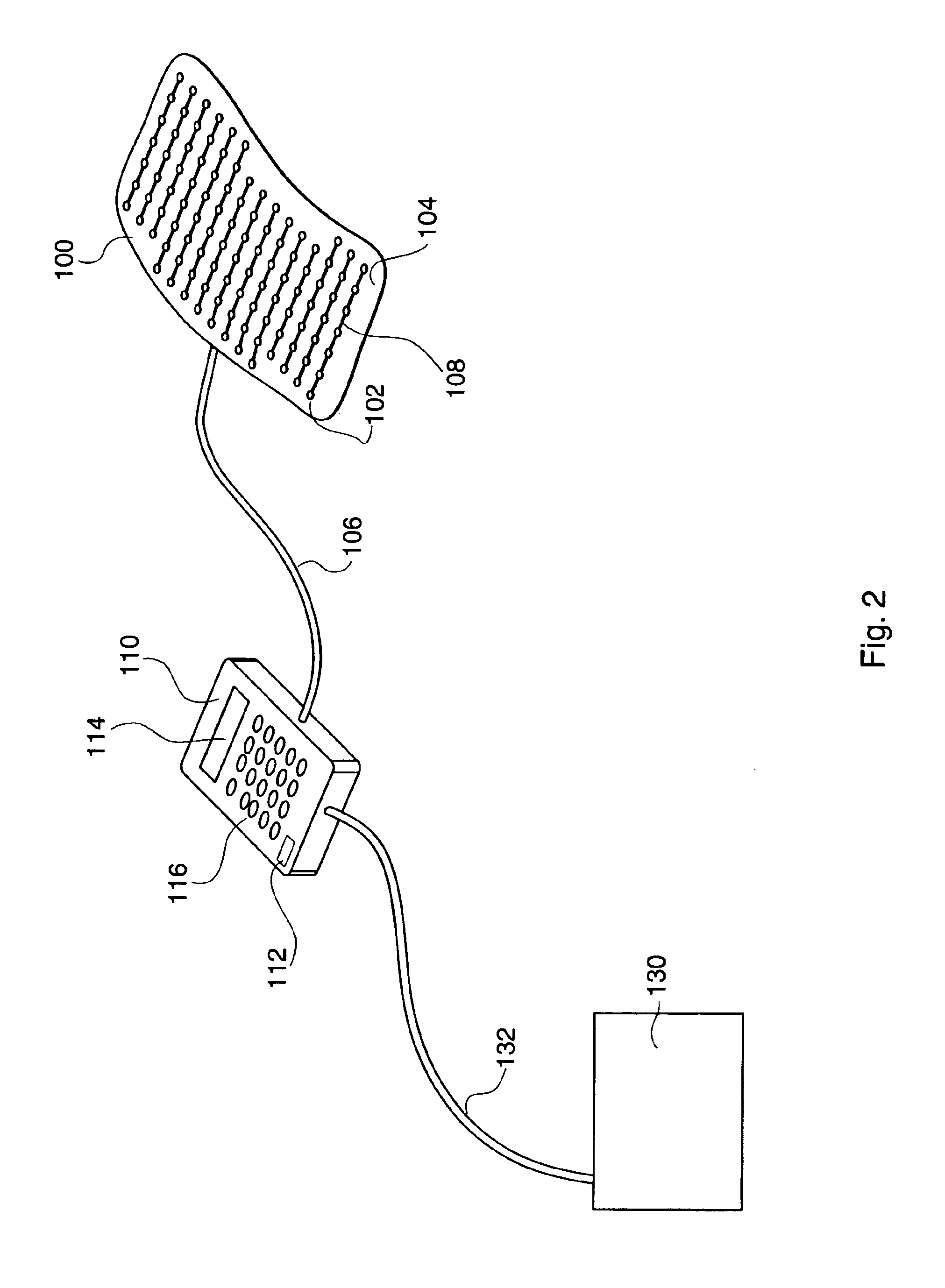
Therapeutic method and apparatus
InactiveUS6860896B2Increase blood flowRelieve painDiagnosticsHeart defibrillatorsMedicineAcupuncture
An energy therapy device is provided that utilizes an array of energy-emitting elements to stimulate Qi energy flow along acupuncture meridians. Energy-emitting elements are activated and deactivated sequentially to produce an energy wave. The energy wave is brought into contact with, or in close proximity to, anatomical sites on a patient's body that have underlying acupuncture meridians. The energy wave produced by the energy therapy device stimulates the flow of Qi energy resulting in a number of therapeutic benefits including pain relief, and reduction of inflammation.
Owner:SAMSON JEFFREY T
Topical Composition for Treating Pain
ActiveUS20080311167A1Ameliorate and eliminate painFree from painBiocideHydrocarbon active ingredientsSequelaPreventing pain
Topical compositions having as the active ingredient a lipid, fatty acid ester, natural wax, sterol, or combinations thereof referred to herein as “lipophilic vehicle” or “LV” and methods of use, have been developed for the amelioration or prevention of pain or the sequelae of pain. The composition may be in the form of an ointment, cream, gel, lotion, spray, foam, paste, patch, suspension or dispersion. In the preferred embodiment, the formulation is a gel. The LV may contain a penetration enhancer, most preferably one with membrane disruptive properties. The formulation may be applied to or impregnated into a gauze, wrap, bandage, cotton-tipped stick, adhesive bandage strip, or other support wrap or medical bandage or wound cover. For example, the compositions may be are incorporated onto or into disposables such as hemorrhoid wipes, sponge, mouth guards, dental trays; needles or catheters; adult diapers; gloves, socks or wrist bands, for ease of application. The composition is applied topically to a site at or adjacent to a painful region. The composition is reapplied as necessary. Pain relief is typically obtained within minutes and lasts for periods of variable duration ranging from minutes to several hours and even, in some cases, days. The composition is variably effective to treat visceral, somatic and neuropathic pain both acute and chronic as well as muscle pain and stiffness and joint pain and stiffness.
Owner:EPICENTRX
Preparation of topical regional compositions for the relief of pain
Owner:FROME BRUCE M
Pain relief lollipop compositions and methods
InactiveUS20090191257A1Without pain and invasivenessFast pain reliefOrganic active ingredientsNervous disorderGabapentinOpioid Agonist
A pain relief lollipop comprises a candy matrix comprising (a) an opioid agonist, (b) an N-methyl-D-aspartate receptor antagonist different from the opioid agonist, (c) gabapentin, or a pharmaceutically acceptable salt thereof, and, optionally, a muscle relaxant, sedative, anxiolytic, and / or antidepressant. A patient can self-administer small amounts of the pain relief drug as needed by simply licking or sucking on the lollipop in response to his subjective experience of pain.
Owner:INNOVATIVE PHARMA
Methods and devices for inflammation treatment
InactiveUS20130066237A1Improve the level ofImprove abilitiesUltrasound therapyHeart defibrillatorsNon ablativePain relief
Methods and devices are disclosed for controlled mediation and / or improvement of inflammation, inflammation associated with pain, and pain by delivering non-ablative thermal tissue damage to portions of a region of tissue including a volume of inflamed tissue, thereby activating the immune systems pain relief response to the tissue damage.
Owner:PALOMAR MEDICAL TECH
Myoblast transfer therapy for relieving pain and for treating behavioral and perceptive abnormalities
An analgesic benefit is realized by continuously supplying a peptide in vivo that activates an opioid receptor or that interferes with the binding of substance P to its receptors. The long-term, continuous provision of such a peptide can be accomplished by (a) transducing myogenic cells with DNA expressing the peptide and (b) administering the transduced myogenic cells to a patient, such that the cells continuously produce the peptide.
Owner:LAW PETER K
Homeopathic formulations useful for treating pain and/or inflammation
ActiveUS7229648B2Lose their effectivenessLow efficiencyBiocideNervous disorderHypericum perforatumRuta plant
Homeopathic formulations (a) comprising tinctures and / or diluted extracts preferably subjected to potentization of at least 8 or 9 herbs selected from Bellis Perennis, Calendula Officinalis, Hamamelis Virginiana, Arnica Montana, Hypericum Perforatum, Aconitum Napellus, Ledum Palustre, Bryonia Alba and Ruta Graveolens; or (b) consisting of, as active ingredients, tinctures and / or diluted extracts subjected to potentization of 5, 6 or 7 herbs selected from Bellis Perennis, Calendula Officinalis, Hamamelis Virginiana, Arnica Montana, Hypericum Perforatum, Aconitum Napellus, Ledum Palustre, Bryonia Alba and Ruta Graveolens. The potentized homeopathics are in a penetrating base, preferably clear gel base. The homeopathic formulations are highly effective in treating or relieving pain and inflammation. Also, a method of treating or relieving pain and inflammation by administering any of the homeopathic formulations of the invention to a subject, preferably a subject in need of such treatment or relief. Further, a method of making the homeopathic formulation by mixing the homeopathically prepared herbal active ingredients with a base, preferably a clear gel base.
Owner:NUTRITION RES
Multilayer conductive appliance having wound healing and analgesic properties
InactiveUS6861570B1Increase ion migrationImprove concentrationFinger bandagesNon-adhesive dressingsElectroless platingPain relief
A dressing for promoting healing and pain relief of the body of a living organism having a pathologic condition has at least one layer of conductive material having a resistance no greater than 1000 Ω / cm2. When placed proximate a portion of the body of the living organism suffering from the pathologic condition, the dressing alters the electrodynamic processes occurring in conjunction with said pathologic condition to promote healing and pain relief in the living organism. When used as a wound dressing, the conductive material is placed in contact with tissue around the periphery of the wound and with the wound, lowering the electrical potential and resistance of the wound and increasing the wound current. In an exemplary embodiment, the conductive material is a multi-ply nylon fabric plated with silver by an autocatalytic electroless plating process and with the plies in electrical continuity. The dressing provides an antimicrobial and analgesic effect. The dressing may be provided for numerous applications and may include other layers such as an absorbent layer, a semi-permeable layer and additional layer of conductor material. Multilaminate embodiments of the present invention exhibit conductive material concentration gradients and, potentially, a capacitive effect when sequential conductor layers are insulated by intervening layers.
Owner:ARGENTUM INT
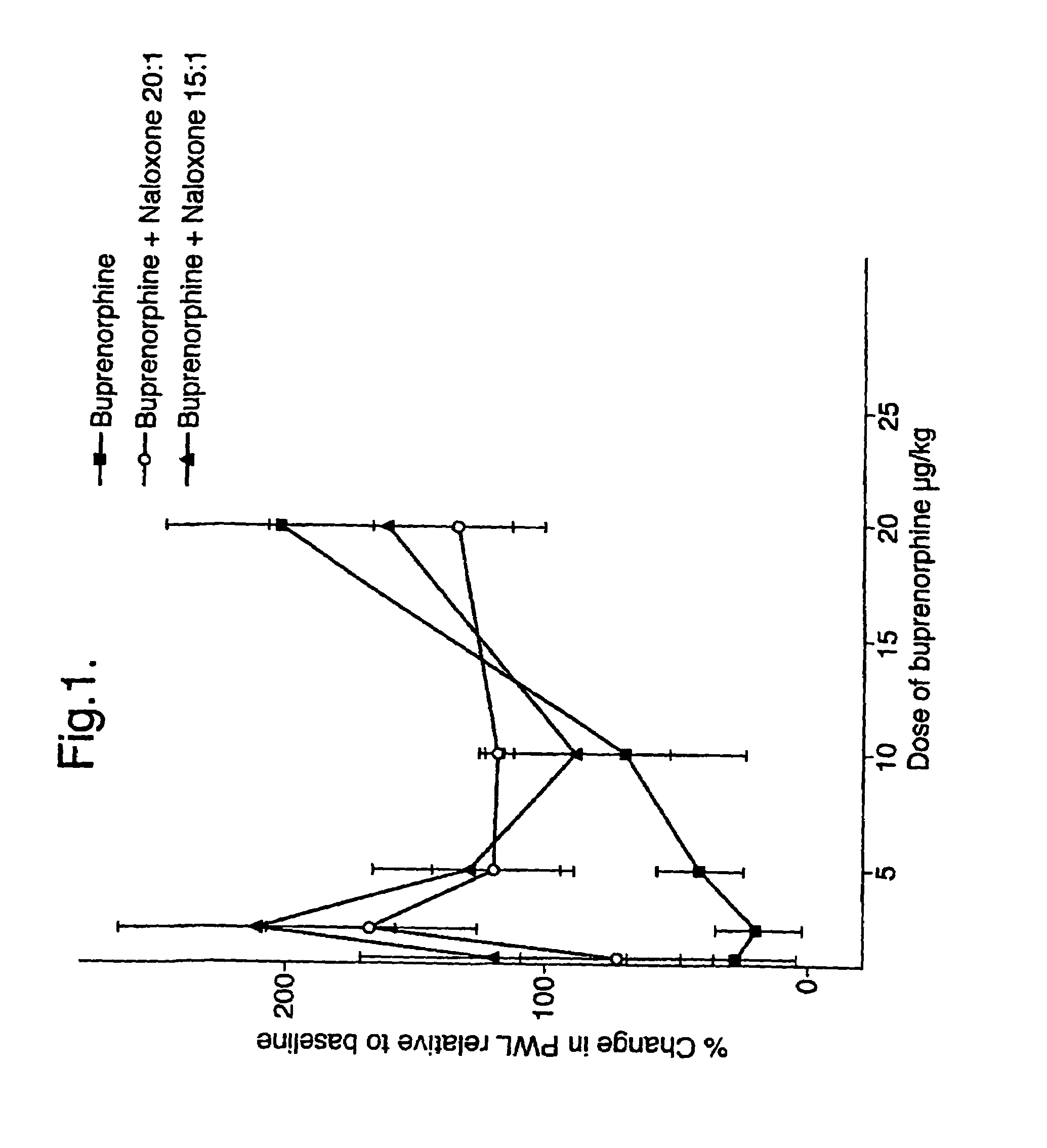
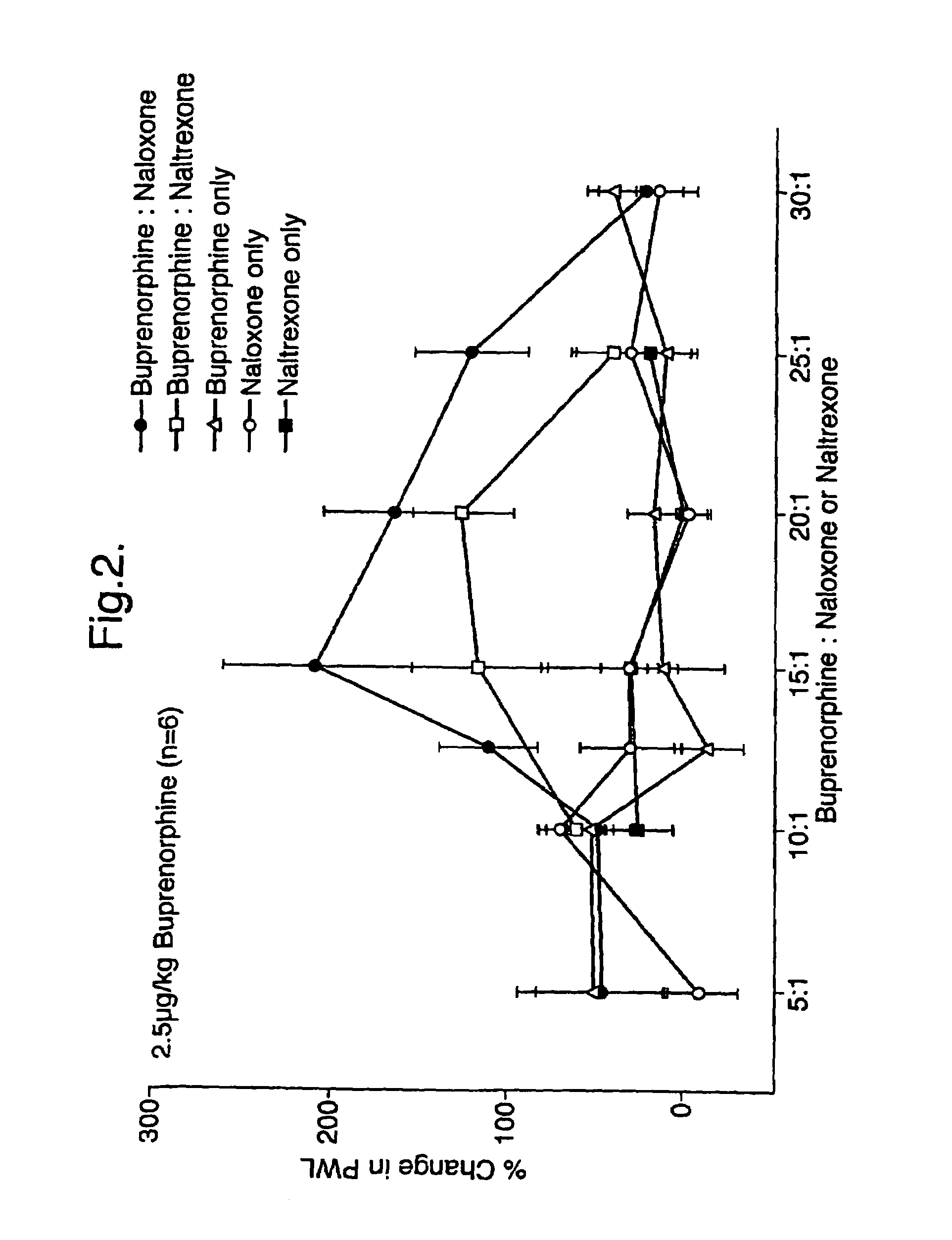
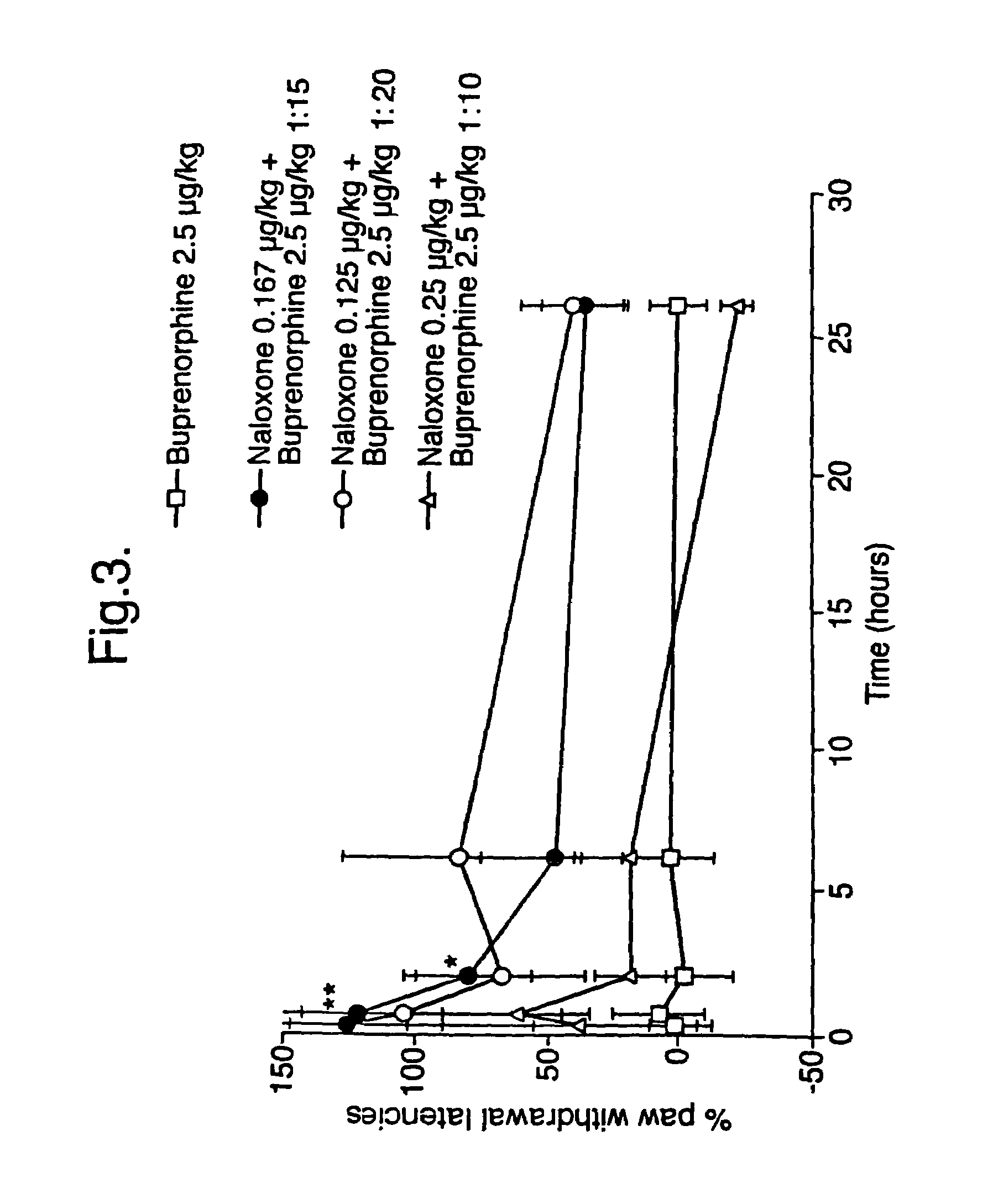
Analgesic compositions containing buprenorphine
An analgesic composition in parenteral unit dosage form or in a unit dosage form suitable for delivery via the mucosa comprising an amount of buprenorphine which is less than the clinical dose required to achieve pain relief and an amount of naloxone such that the ratio by weight of buprenorphine to naloxone is in the range of from 12.5:1 to 27.5:1, or an amount of naltrexone or nalmefene such that the ratio by weight of buprenorphine to naltrexone or nalmefene is in the range of from 12.5:1 to 22.5:1. The analgesic action of the buprenorphine is potentiated by the low dose of naloxone, naltrexone or nalmefene.
Owner:INDIVIOR UK
Internal and external disc shunts alleviate back pain
InactiveUS20110098628A1Relieve back painReduce loadSurgical needlesIntravenous devicesInstabilityBlood plasma
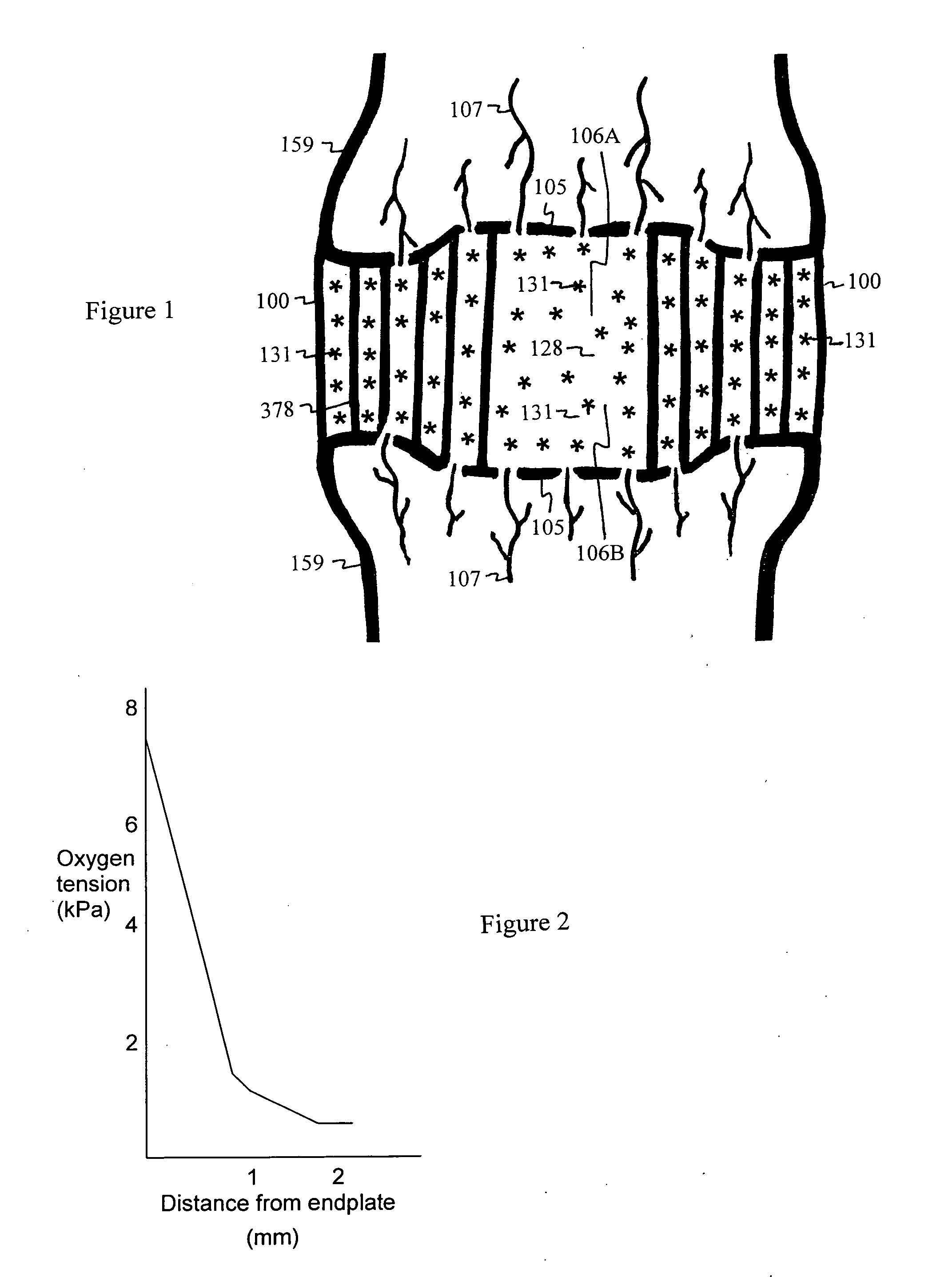
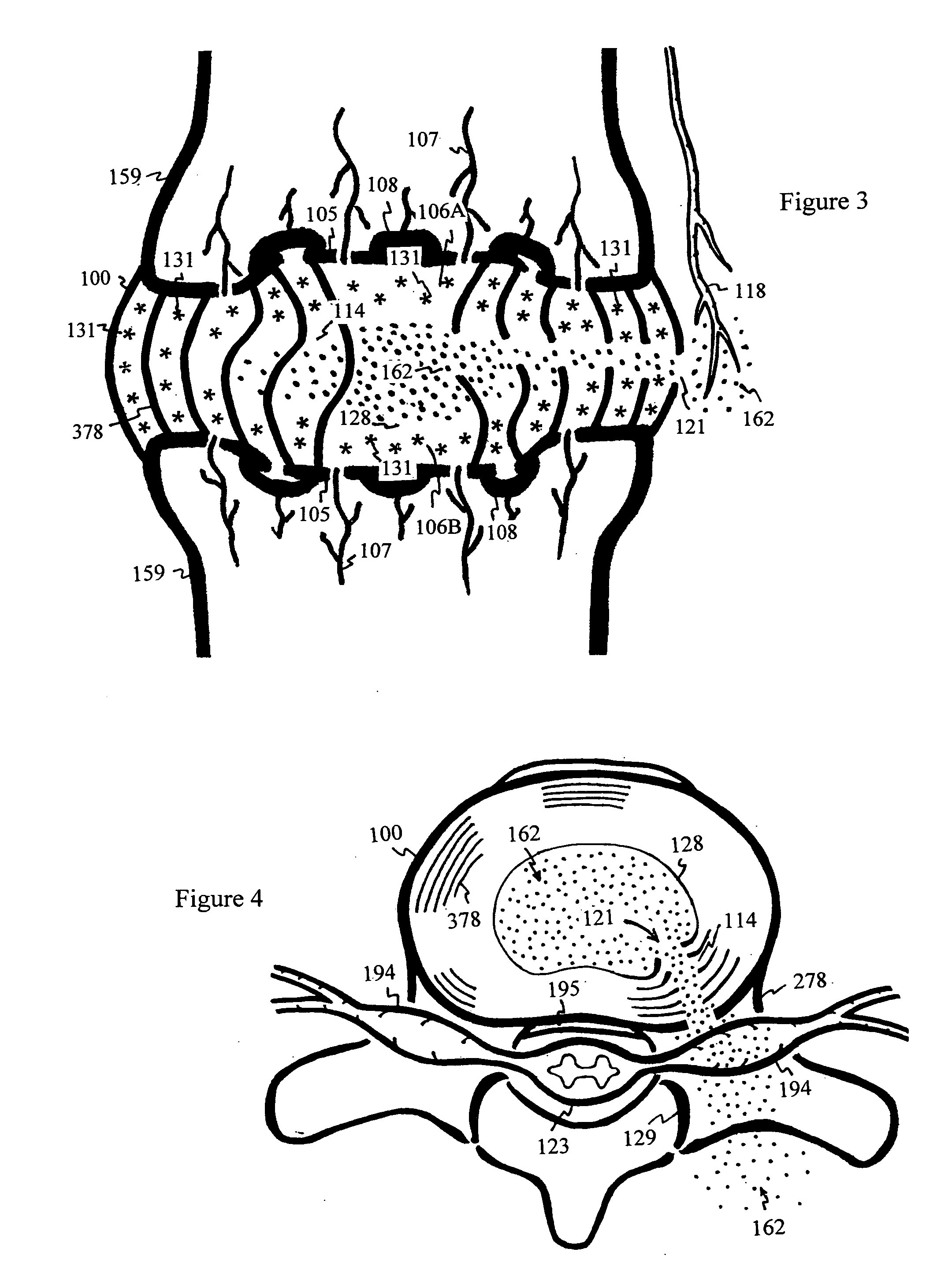
The intervertebral disc is avascular. Nutrients and waste are diffused through adjacent vertebral bodies into the disc. As we age, calcified layers form between the disc and vertebral bodies, blocking diffusion of nutrients, oxygen and pH buffer in blood. Under anaerobic conditions, lactic acid is produced, irritating nerve endings and causing nonspecific pain. In addition, the disc begins to starve and flatten. The weight shifts abnormally from disc to the facet joints causing strain and back pain.Shunt coils are formed and spiraled over the distal shaft of a twistable needle, then deployed into the nucleus of a degenerated disc by a sliding sleeve. The coils serve as an internal shunt, drawing nutrients, oxygen and buffering solute from the superior and inferior diffusion zones to neutralize lactic acid in the mid layer of the degenerated disc. The coils also serve as a bulking agent within the repaired disc to sustain compression and reduce facet loading and segmental instability. The end strands of the shunt coils can also extend from the disc to draw blood plasma from muscle or bodily circulation to expedite neutralization of lactic acid and rebuild disc matrix for pain relief and disc regeneration.
Owner:YEUNG JEFFREY E +1
Portable Ultrasound Device for the Treatment of Wounds
InactiveUS20080051693A1Less painful treatmentUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyFocus ultrasoundCoupling
Device and methods for the treatment of wounds using ultrasound energy are disclosed. The portable wound treatment device may deliver ultrasound energy to a wound through direct contact with the ultrasound tip and / or through a liquid coupling medium. Several ultrasound tips specially designed to concentrate and focus ultrasound energy onto a wound are also disclosed. The ultrasound tip may also possess an abrasive peripheral boundary to aid in debriding the wound and / or removing necrotic tissue. The disclosed invention may have multiple beneficial effects in treating a wound such as sterilizing a wound, reducing external bleeding, and / or providing pain relief.
Owner:BACOUSTICS LLC
Mesh Pouches for Implantable Medical Devices
ActiveUS20080132922A1Reduces and prevents implantReduces and prevents and surgery-related complicationElectrotherapyDiagnosticsFiberSide effect
Biodegradable polymer-coated surgical meshes formed into pouches are described for use with cardiac rhythm management devices (CRMs) and other implantable medical devices. Such meshes are formed into a receptacle, e.g., a pouch or other covering, capable of encasing, surrounding and / or holding the cardiac rhythm management device or other implantable medical device for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other implantable medical device. Preferred embodiments include surgical mesh pouches coated with one or more biodegradable polymers that can act as a stiffening agent by coating the filaments or fibers of the mesh to temporarily immobilize the contact points of those filaments or fibers and / or by increasing the stiffness of the mesh by at least 1.1 times its original stiffness. The pouches of the invention can also provide relief from various post-operative complications associated with their implantation, insertion or surgical use, and, optionally, include one or more drugs in the polymer matrix of the coating to provide prophylactic effects and / or alleviate side effects or complications associated with the surgery or implantation of the CRM or other implantable medical device.
Owner:MEDTRONIC INC
Chinese medicine label for eliminating ache and preparation thereof
InactiveCN101244252AGood curative effectLittle side effectsHeavy metal active ingredientsAnthropod material medical ingredientsClematisMyrrh
The invention relates to a Chinese herbal medicine paste for relieving pain, comprising a plurality of medicine materials of angelica, cassia twig, rhizoma chuanxiong, olibanum, myrrh, sylvestris, pubescent angelica, milettia reticulate, pheretima, siegesbeckiae, balsamine, japan yam rhizome, futokadsura stem, orientavine, a millet liquor flavored with the root bark, nuxvomica, Chinese polyphaga, homalomena rhizome, Chinese clematis radix clematidis with fixed weight proportions. The preparation method comprises the following steps: immerse the Chinese herbal medicine materials with fixed weight proportions into sesame oil; 1 kilogram medicine consumes 0.5kg sesame oil; after sever days of immersion, heat mixture for boiling and deslag the mixture; place the residual material on cloth to form a plaster. When in use, slightly heat the plaster and adhere the plaster to the affected part; and each plaster can be used for four days. The Chinese herbal medicine paste has the advantages of effective pain relief and elimination, simple application, rapid and persistent therapeutic effect and low side effect.
Owner:张金星
Resorbable Pouches for Implantable Medical Devices
ActiveUS20080128315A1Reduces and prevents implantReduces and prevents and surgery-related complicationBiocideElectrotherapyResorbable polymersSide effect
Biodegradable and resorbable polymer pouches are described for use with cardiac rhythm mamagent devices (CRMs) and other implantable medical devices (IMDs), i.e., a pouch, covering, or other receptacle capable of encasing, surrounding and / or holding the CRM or other IMD for the purpose of securing it in position, inhibiting or reducing bacterial growth, providing pain relief and / or inhibiting scarring or fibrosis on or around the CRM or other IMD. Optionally, the biodegradable and resorbable pouches of the invention include one or more drugs in the polymer matrix to provide prophylactic effects and alleviate side effects or complications associated with the surgery or implantation of the CRM or other IMD.
Owner:MEDTRONIC INC
Chemical ablation and method of treatment for various diseases
ActiveUS20160310200A1Improve treatment safetyImprove efficacyUltrasonic/sonic/infrasonic diagnosticsBalloon catheterAbnormal tissue growthDamages tissue
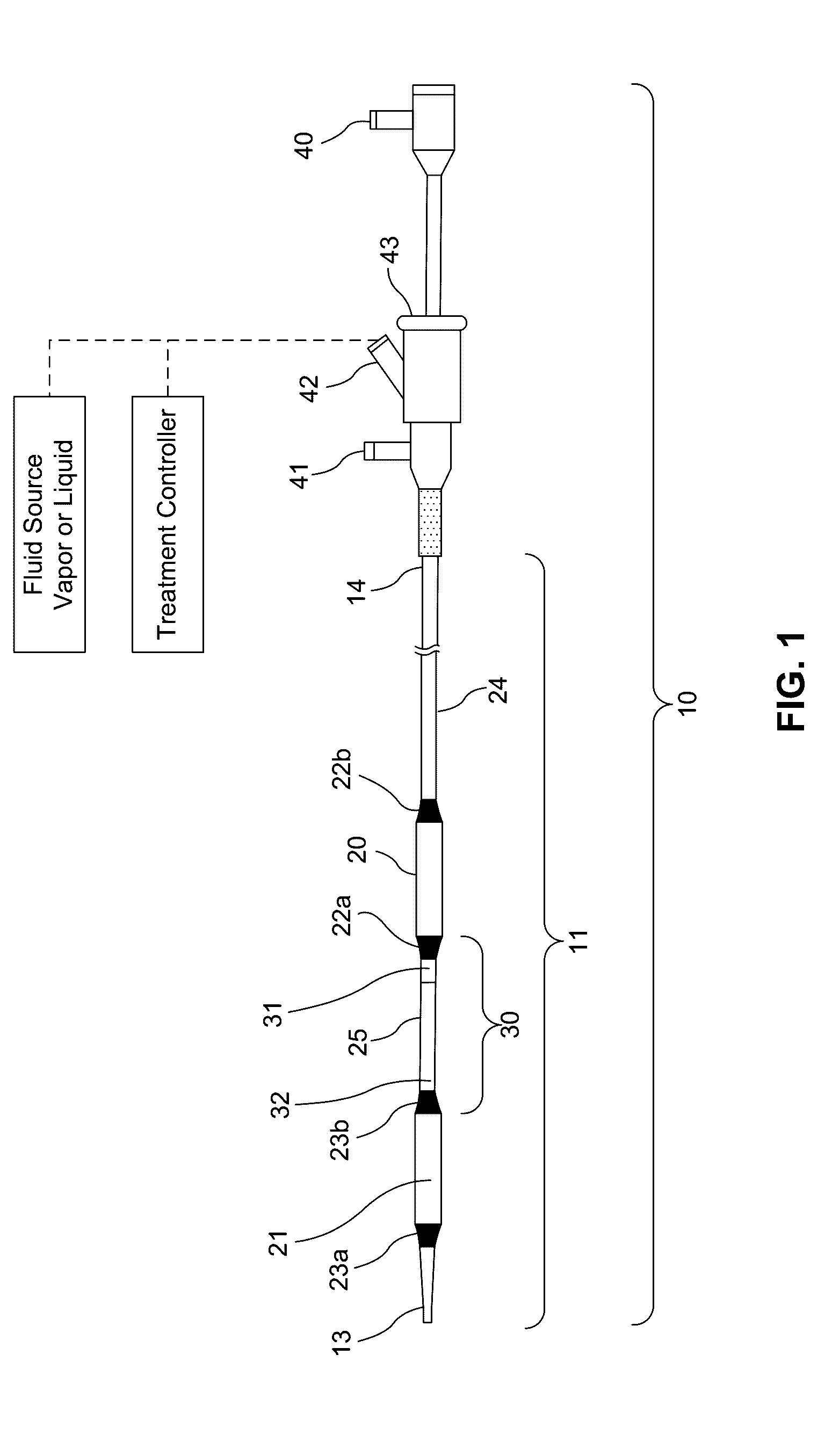
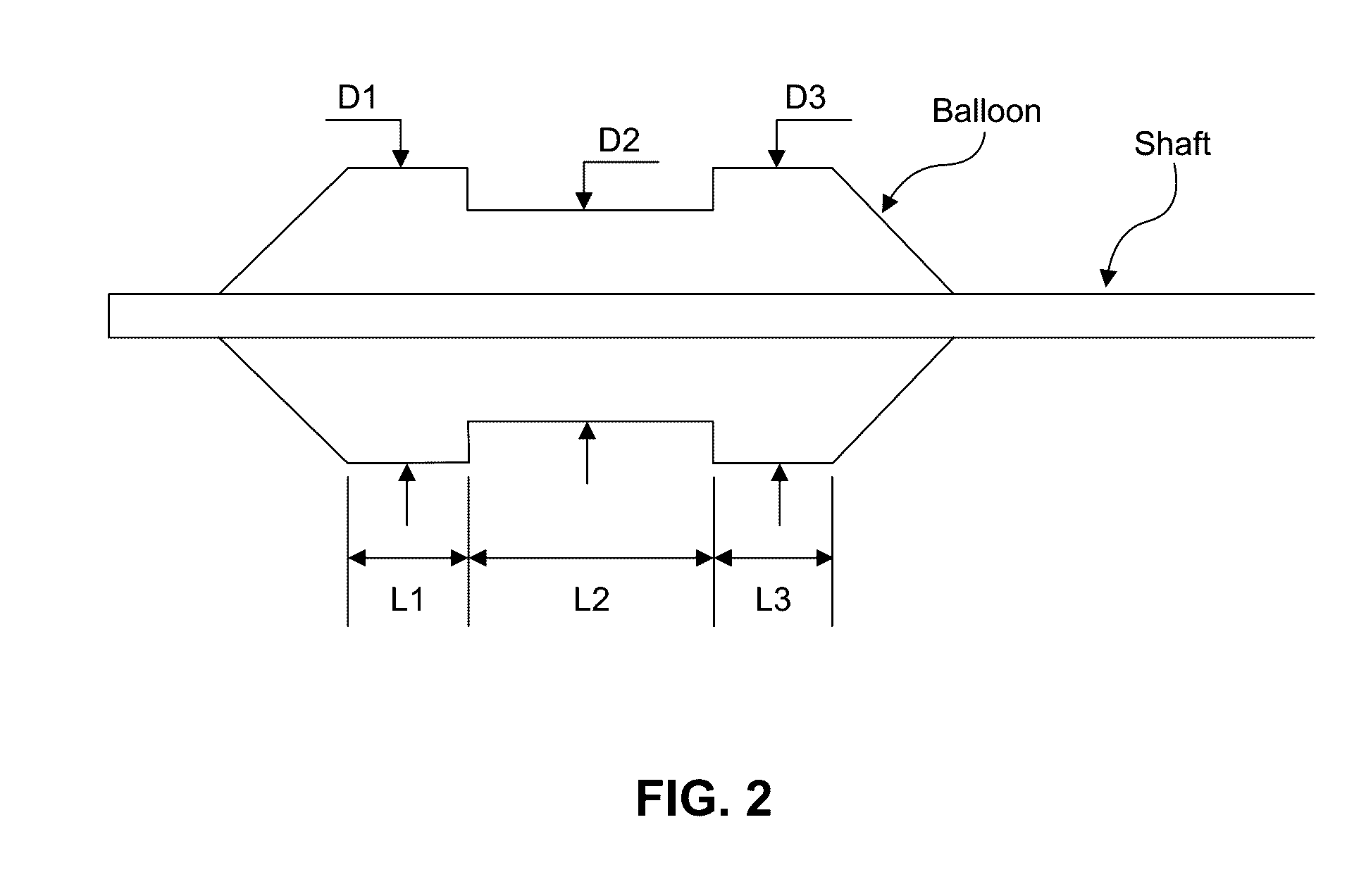
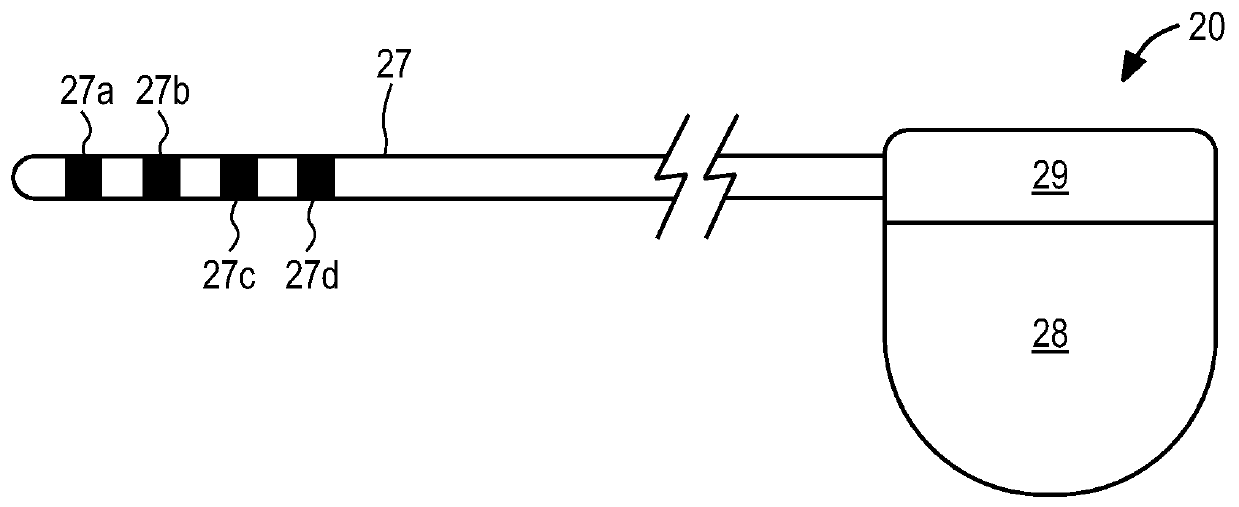
Embodiments of the present invention provide a device and a method for treating at least one of hypertension, pulmonary arteries, diabetes, obesity, heart failure, end-stage renal disease, digestive disease, nonalcoholic fatty liver disease, urological disease, cancers, tumors, pain, asthma or chronic obstructive pulmonary disease by delivering an effective amount of a formulation to a tissue. In embodiments of the present invention, the formulation may include at least one of a gas, a vapor, a liquid, a solution, an emulsion, or a suspensions of one or more ingredients. In embodiments of the present invention, amounts of the formulation and or energy are effective to injure or damage tissue, nerves, and nerve endings in order to relieve disease symptoms.
Owner:NEUROTRONIC
Modular stimulator for treatment of back pain, implantable RF ablation system and methods of use
ActiveUS9248278B2Rehabilitate spinal stabilityRestore neural driveSpinal electrodesDiagnosticsNerve fiber bundleElectricity
Apparatus and methods for treating back pain are provided, in which an implantable stimulator is configured to communicate with an external control system, the implantable stimulator providing a neuromuscular electrical stimulation therapy designed to cause muscle contraction to rehabilitate the muscle, restore neural drive and restore spinal stability; the implantable stimulator further including one or more of a number of additional therapeutic modalities, including a module that provides analgesic stimulation; a module that monitors muscle performance and adjusts the muscle stimulation regime; and / or a module that provides longer term pain relief by selectively and repeatedly ablating nerve fibers. In an alternative embodiment, a standalone implantable RF ablation system is described.
Owner:MAINSTAY MEDICAL
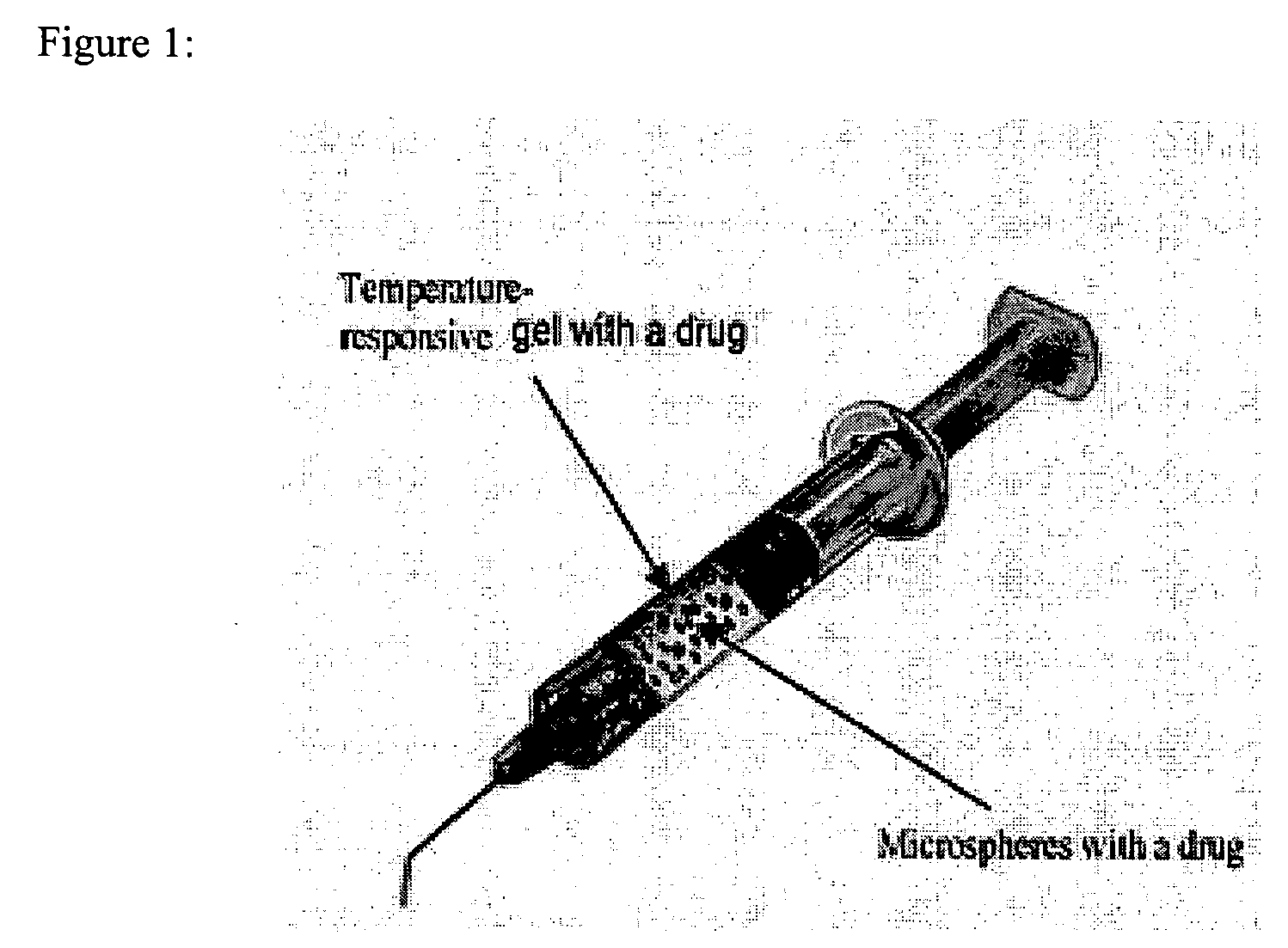
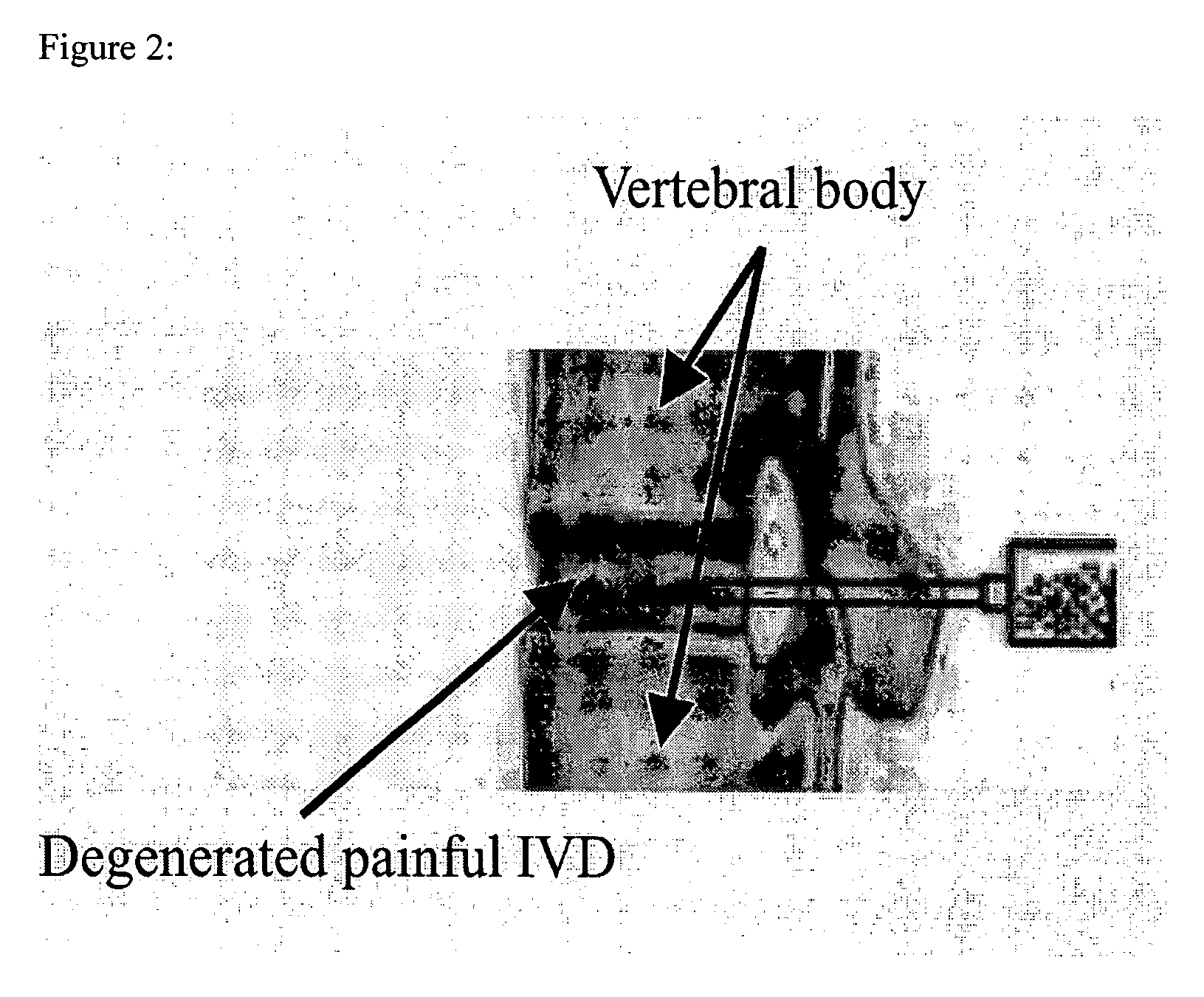
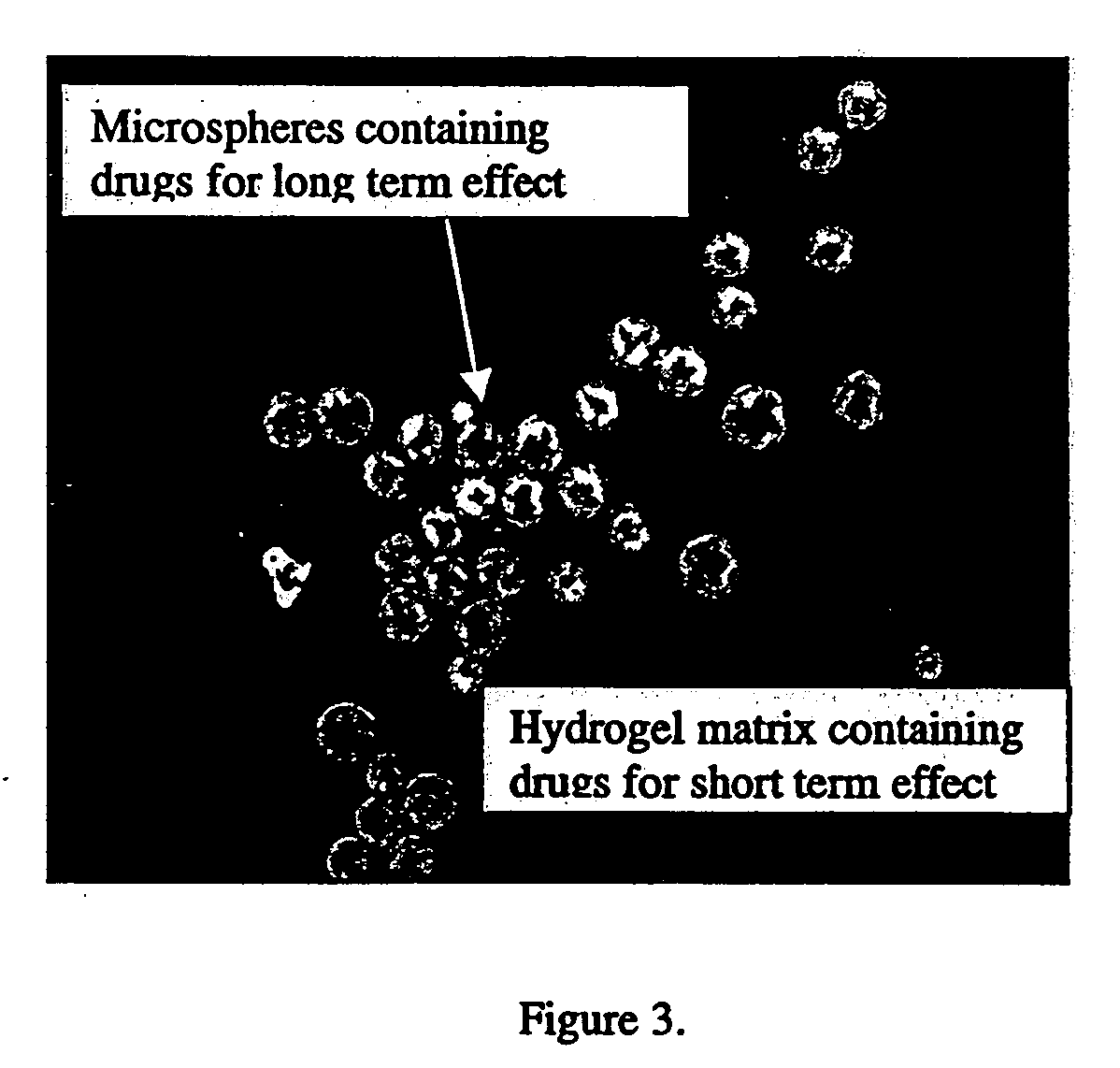
In situ controlled release drug delivery system
ActiveUS20060188583A1Less frequent administrationAvoidance of surgical interventionNervous disorderAntipyreticAbnormal tissue growthMicrosphere
A system is described for long-term controlled release delivery of a drug or a therapeutic agent. According to the invention, one or more drugs or therapeutic agents contained in microspheres are mixed with a temperature sensitive hydrogel which is then introduced directly to the desired situs of the drug or therapeutic agent. The temperature sensitive hydrogel may also contain a drug or a therapeutic agent, for example, a pain relieving drug, for a short-term controlled release. The temperature sensitive hydrogel is in liquid state at room temperature, but upon injection, shortly becomes gelatinous. This system is particularly suitable for treatment of diseases, disorders, or conditions, for example, tumors, discogenic back pain, or arthritis, warranting localized administration of a drug or a therapeutic agent. In addition, the specification provides a method for production of a drug—or therapeutic agent-containing microspheres.
Owner:UNIV OF IOWA RES FOUND
Neuropathy cream
Owner:OZTURK BINNUR +1
Modular stimulator for treatment of back pain, implantable RF ablation system and methods of use
ActiveUS20150374992A1Rehabilitate spinal stabilityRestore neural driveSpinal electrodesSurgical instrument detailsSpinal columnMuscle contraction
Apparatus and methods for treating back pain are provided, in which an implantable stimulator is configured to communicate with an external control system, the implantable stimulator providing a neuromuscular electrical stimulation therapy designed to cause muscle contraction to rehabilitate the muscle, restore neural drive and restore spinal stability; the implantable stimulator further including one or more of a number of additional therapeutic modalities, including a module that provides analgesic stimulation; a module that monitors muscle performance and adjusts the muscle stimulation regime; and / or a module that provides longer term pain relief by selectively and repeatedly ablating nerve fibers. In an alternative embodiment, a standalone implantable RF ablation system is described.
Owner:MAINSTAY MEDICAL
Multilayer conductive appliance having wound healing and analgesic properties
InactiveUS7291762B2Promote migrationImprove concentrationFinger bandagesNon-adhesive dressingsCapacitive effectWound dressing
A dressing for promoting healing and pain relief of the body of a living organism having a pathologic condition has at least one layer of conductive material having a resistance no greater than 1000 Ω / cm2. When placed proximate a portion of the body of the living organism suffering from the pathologic condition, the dressing alters the electrodynamic processes occurring in conjunction with said pathologic condition to promote healing and pain relief in the living organism. When used as a wound dressing, the conductive material is placed in contact with tissue around the periphery of the wound and with the wound, lowering the electrical potential and resistance of the wound and increasing the wound current. In an exemplary embodiment, the conductive material is a multi-ply nylon fabric plated with silver by an autocatalytic electroless plating process and with the plies in electrical continuity. The dressing provides an antimicrobial and analgesic effect. The dressing may be provided for numerous applications and may include other layers such as an absorbent layer, a semi-permeable layer and additional layer of conductor material. Multilaminate embodiments of the present invention exhibit conductive material concentration gradients and, potentially, a capacitive effect when sequential conductor layers are insulated by intervening layers.
Owner:ARGENTUM INT
Compositions and minimally invasive methods for treating incomplete tissue repair
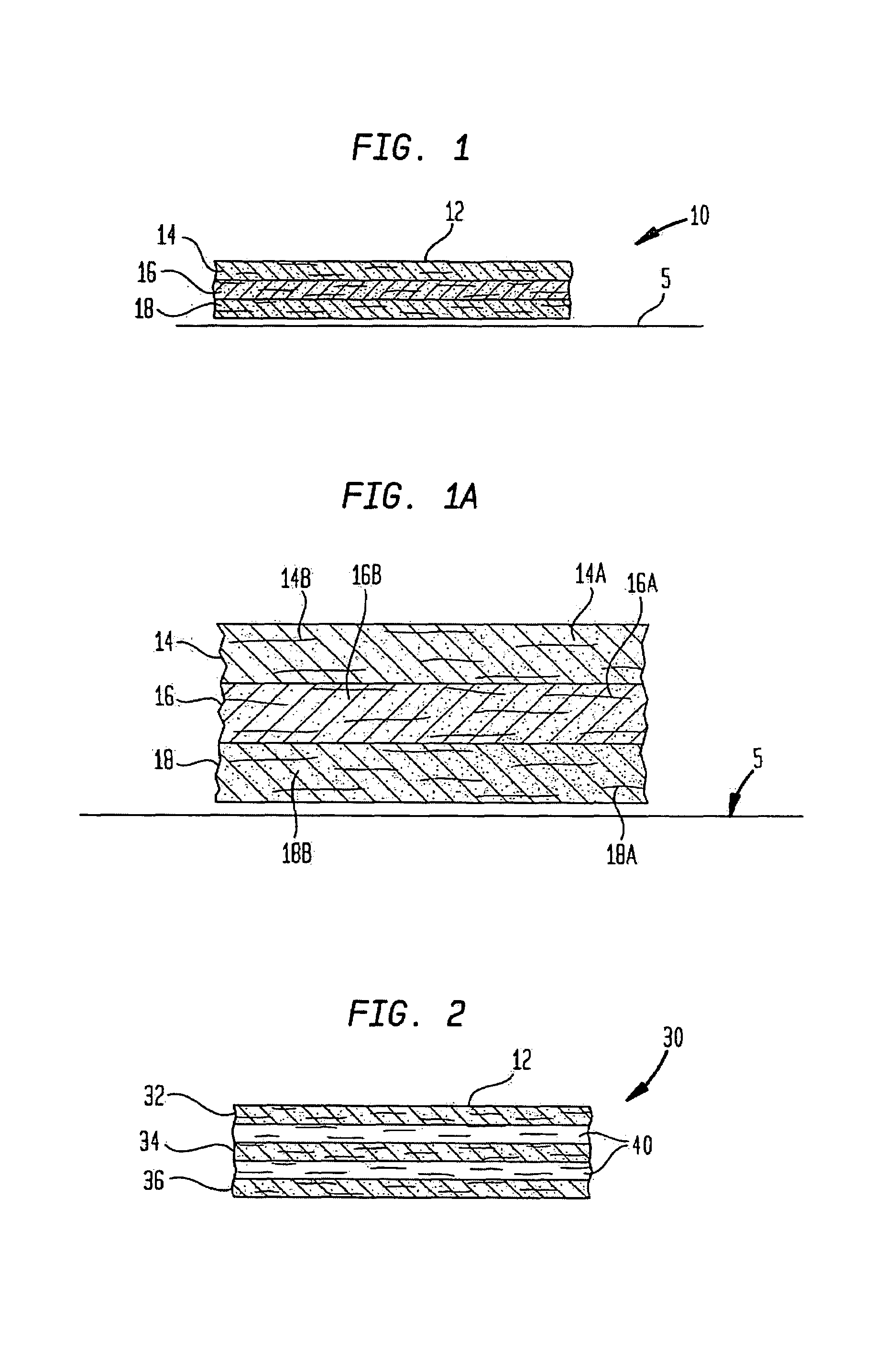
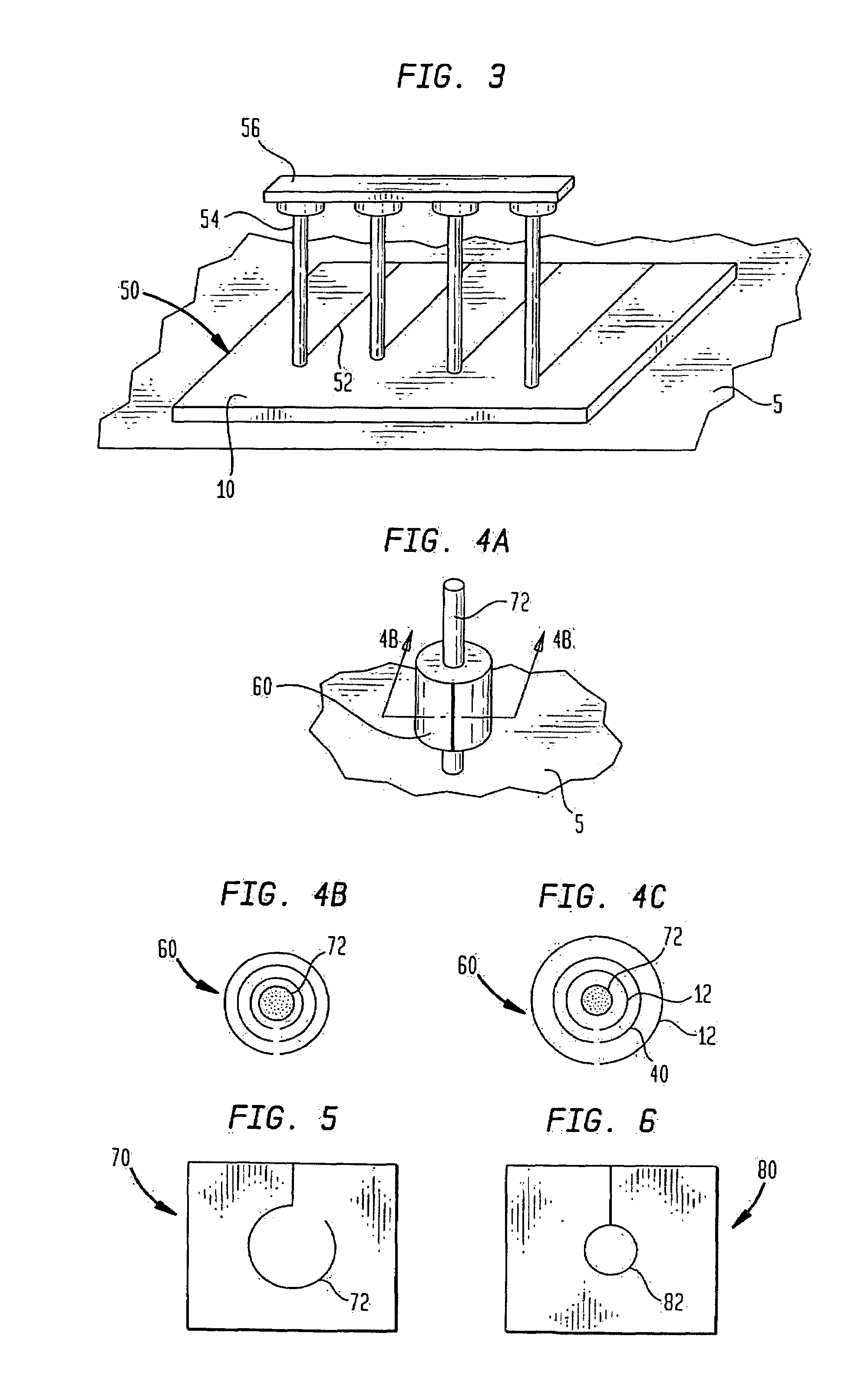
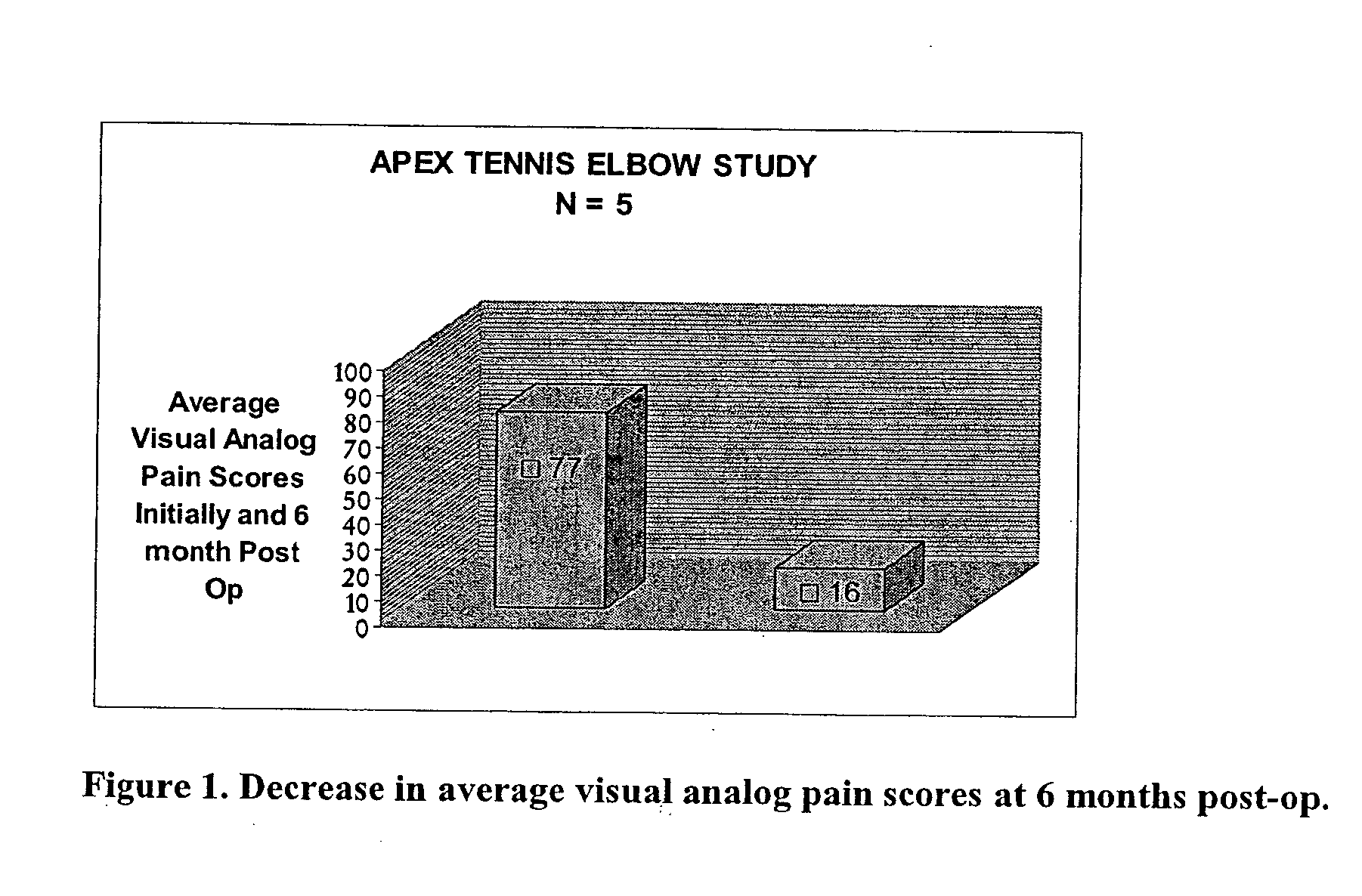
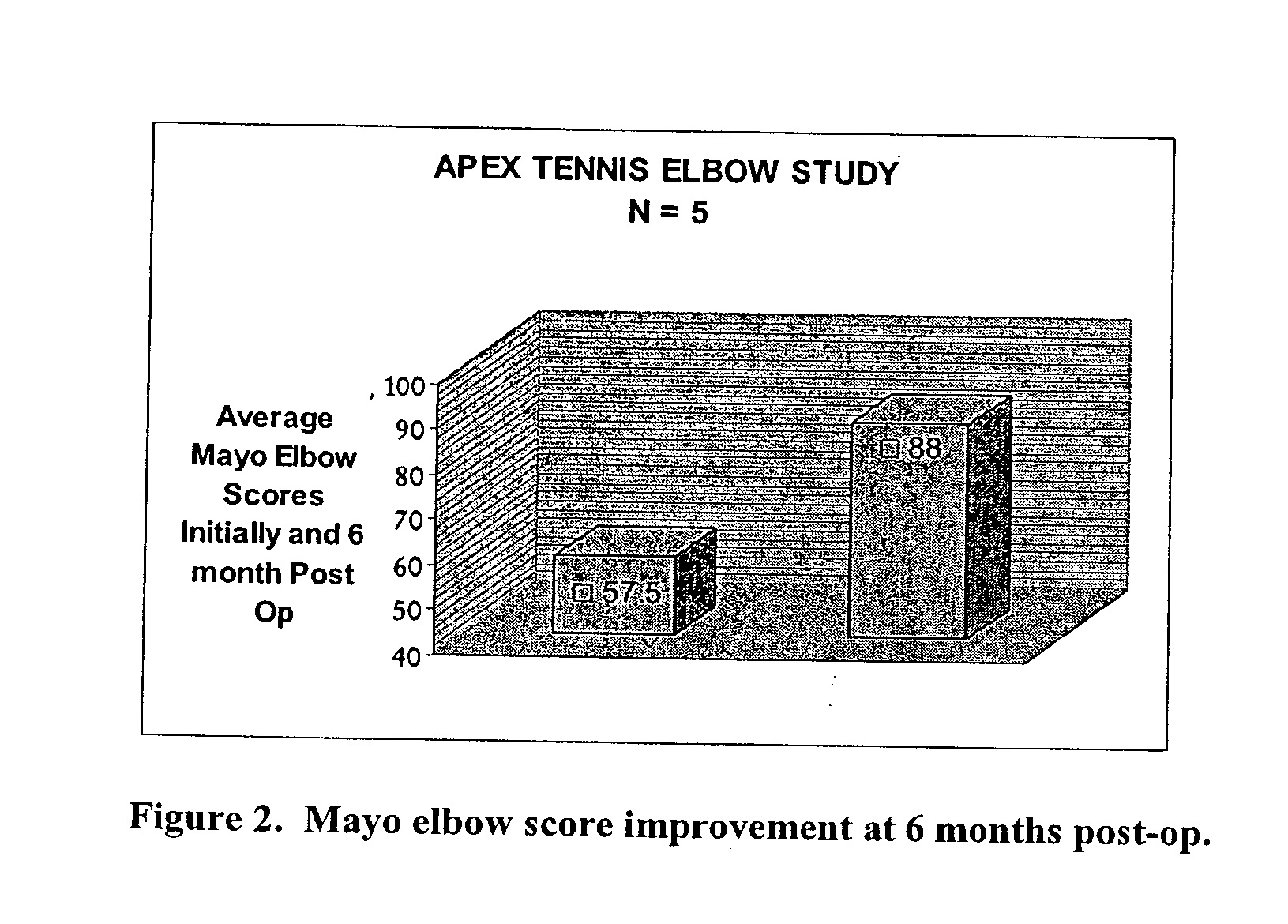
ActiveUS20050100536A1Extended storage timeCause effectsBiocideOrganic active ingredientsEpicondylitisTissue repair
Methods are described for using compositions containing platelet-rich plasma for the treatment of a variety of tissue lesions. Particularly, delivery of platelet-rich plasma to connective tissue is described. The described method and compositions have been shown to provide both pain relief and improved mobility in treatment of lateral epicondylitis.
Owner:BLUE ENGINE BIOLOGICS LLC
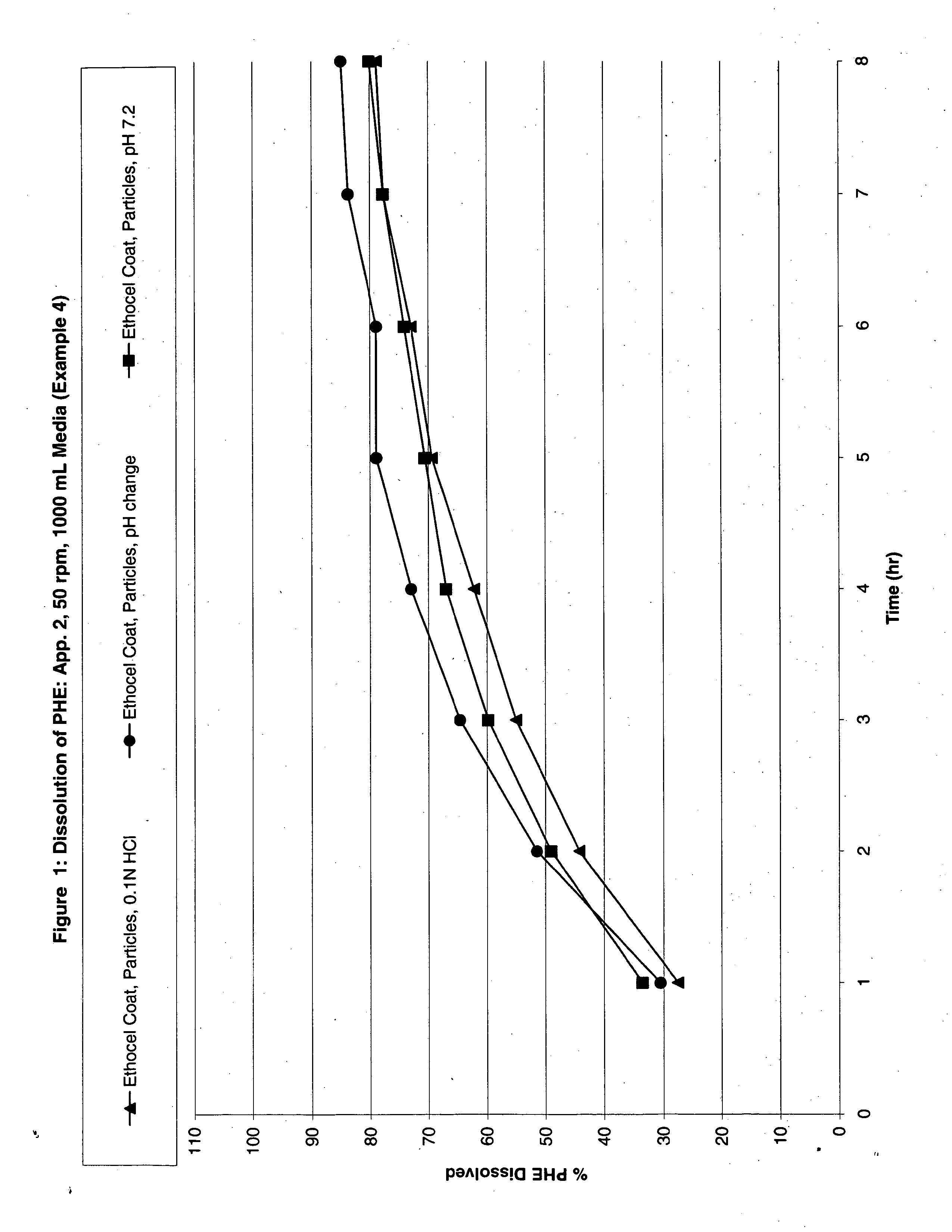
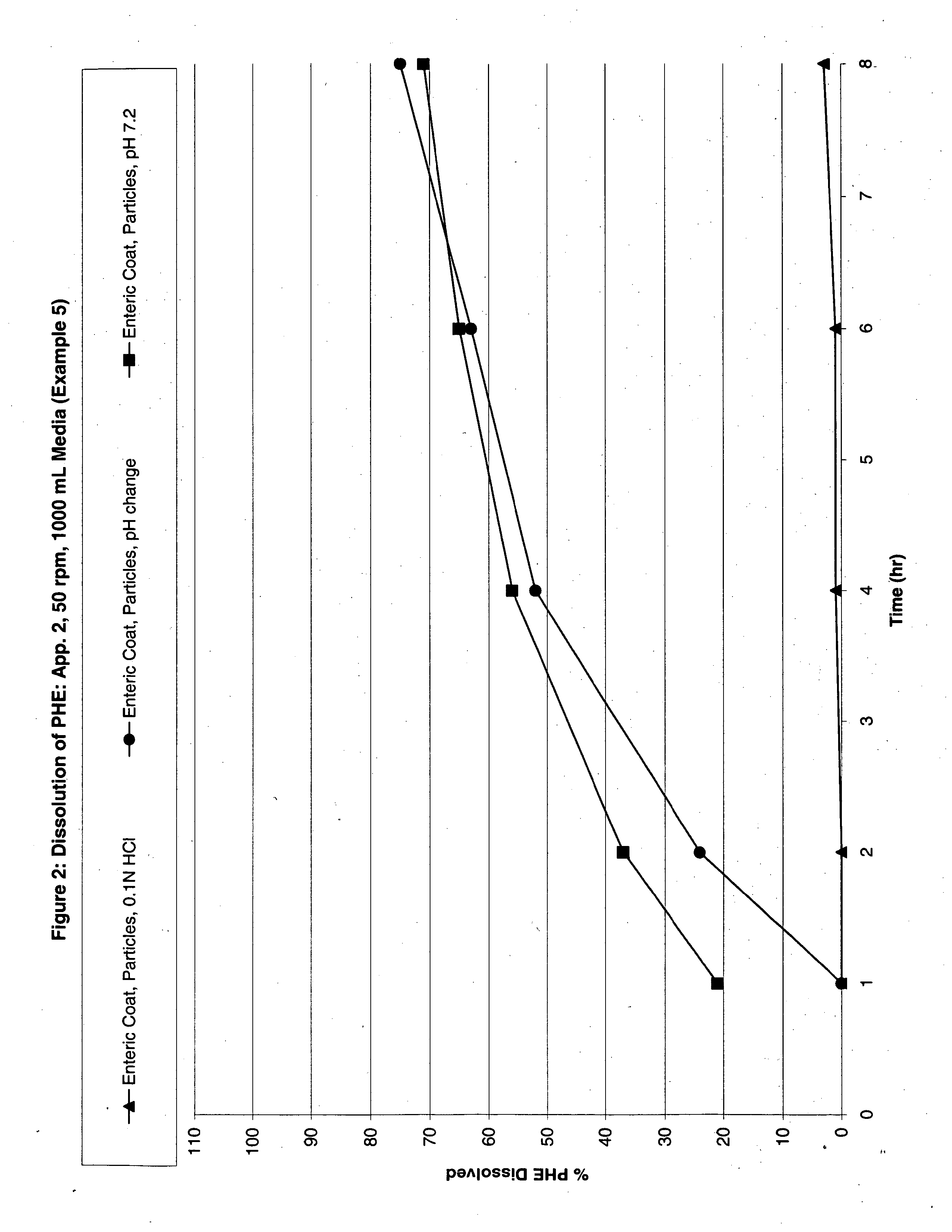
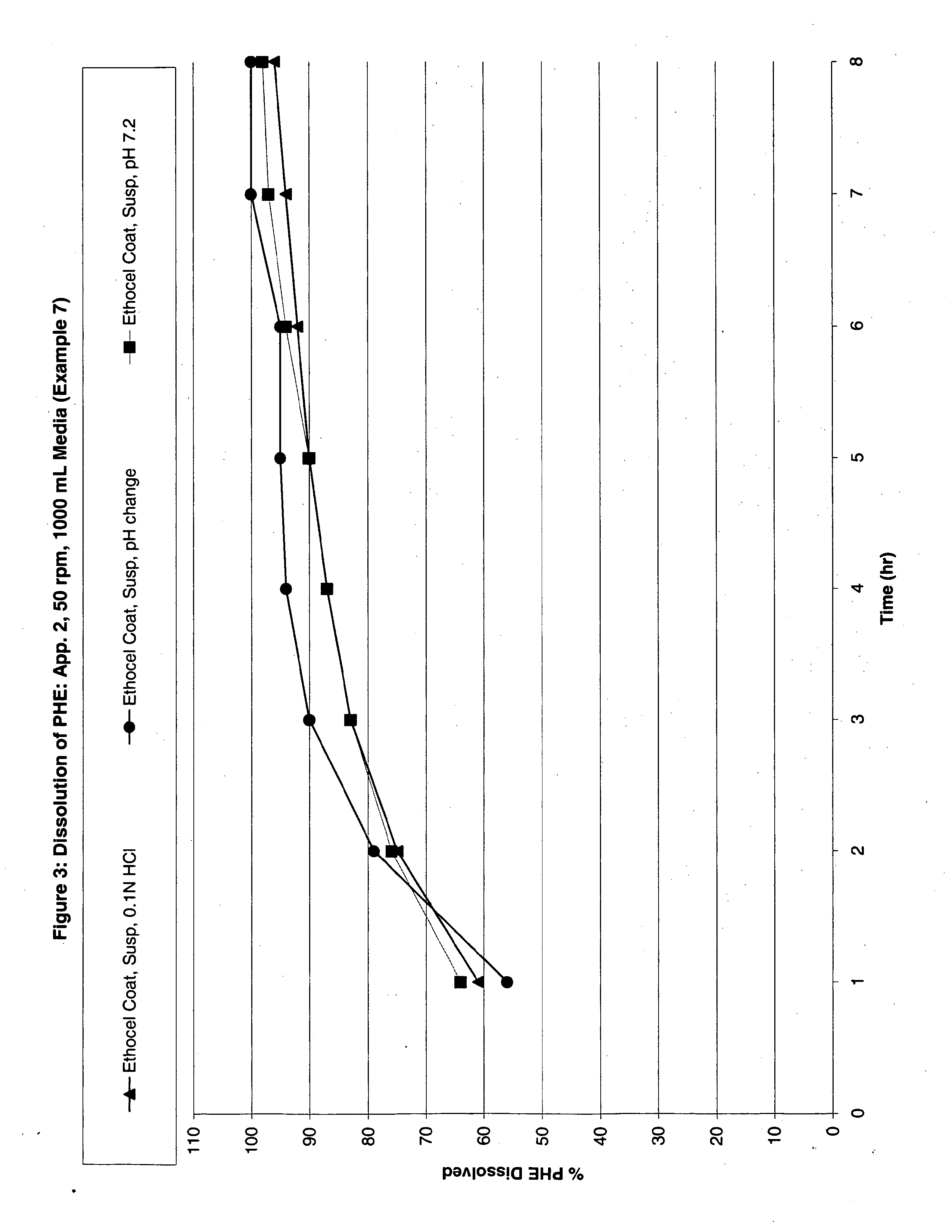
Modified release analgesic suspensions
A pharmaceutical dosage form comprising non-steroidal-anti-inflammatory drugs, in particular propionic acid derivatives such as ibuprofen, along with a second active ingredient having a shorter therapeutically effective plasma concentration duration, such as phenylephrine, and methods of administering the same are provided. This method provides improved therapeutic effect, in particular pain relief along with decongestant relief, over extended time periods.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com