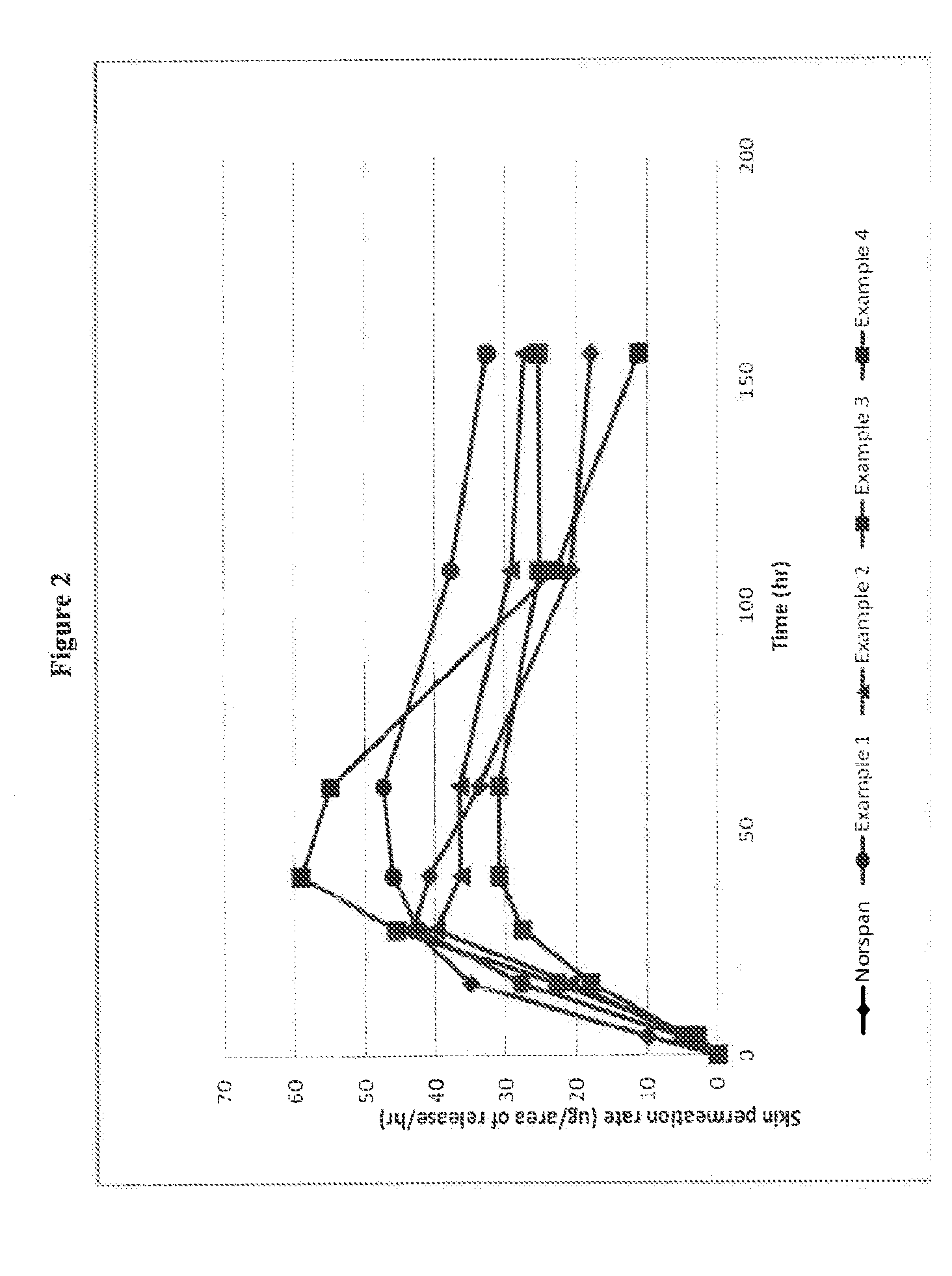
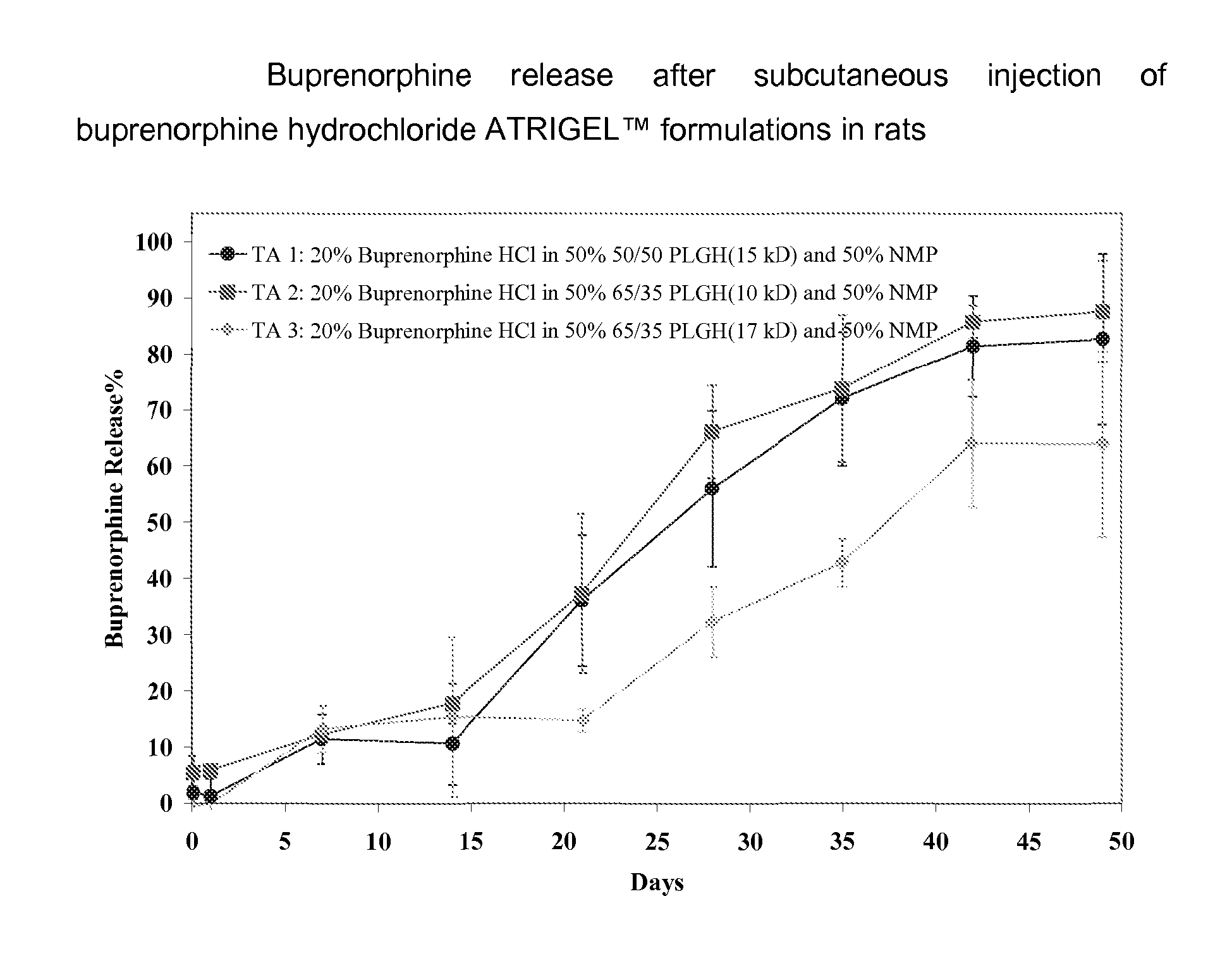
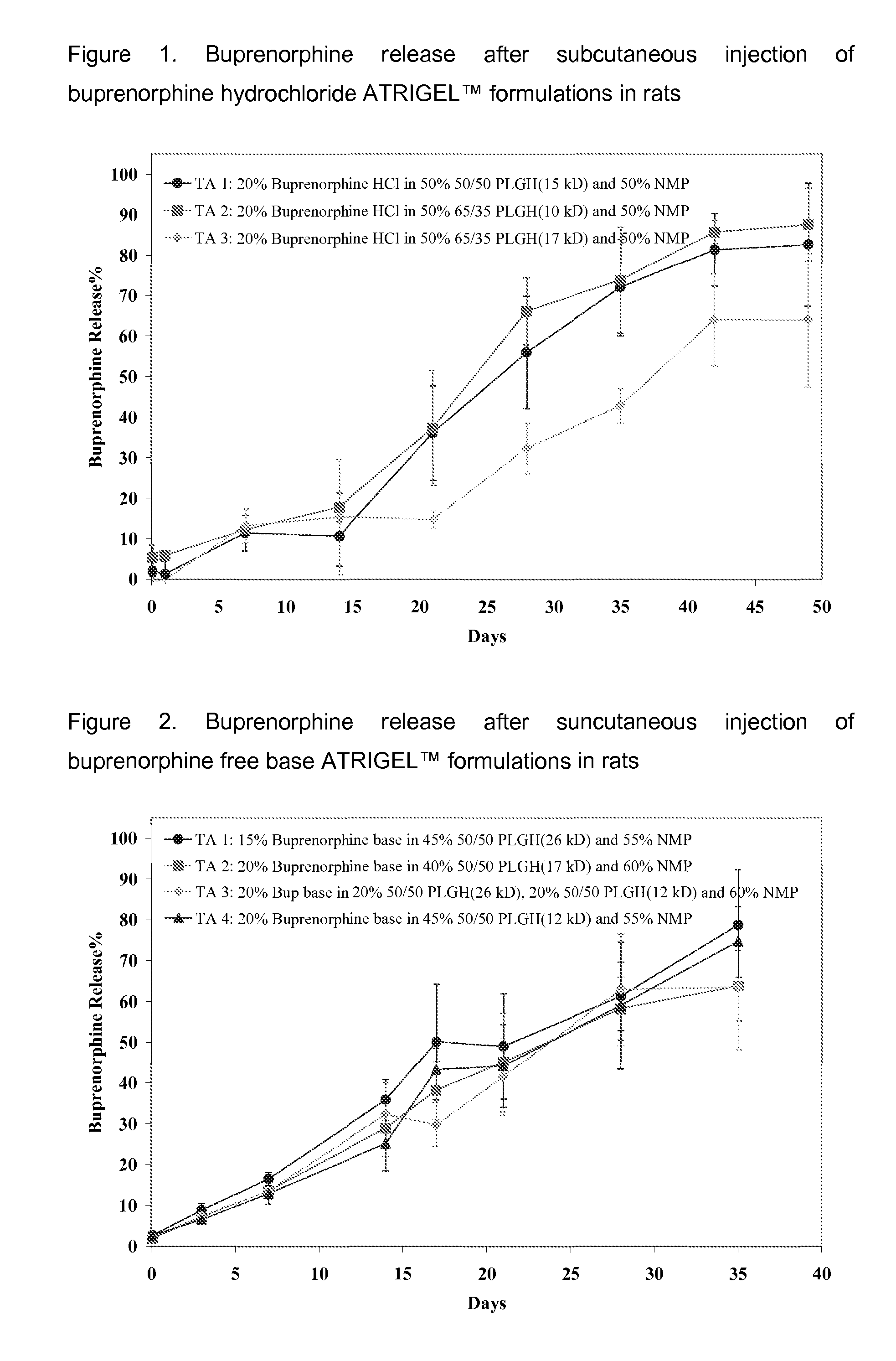
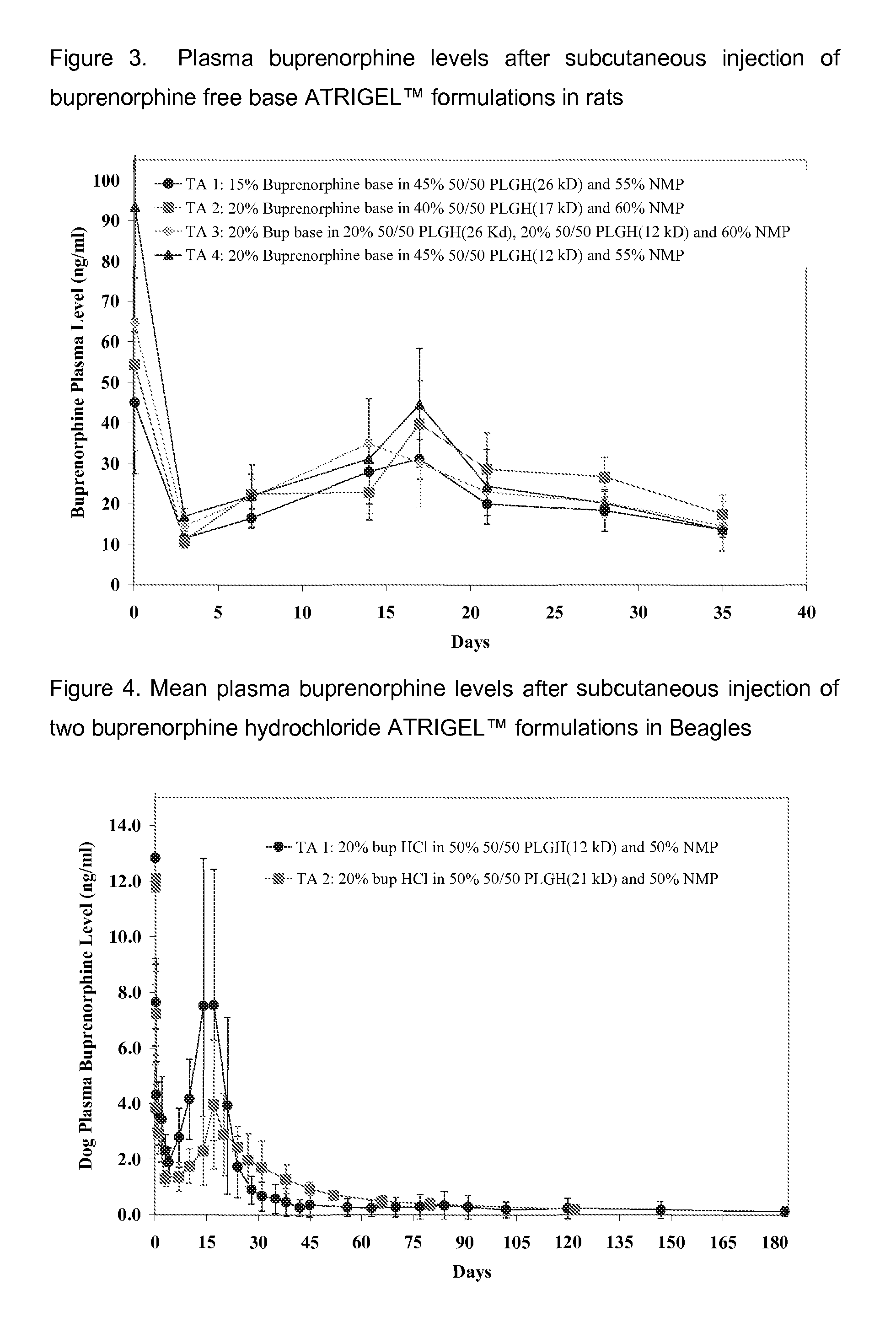
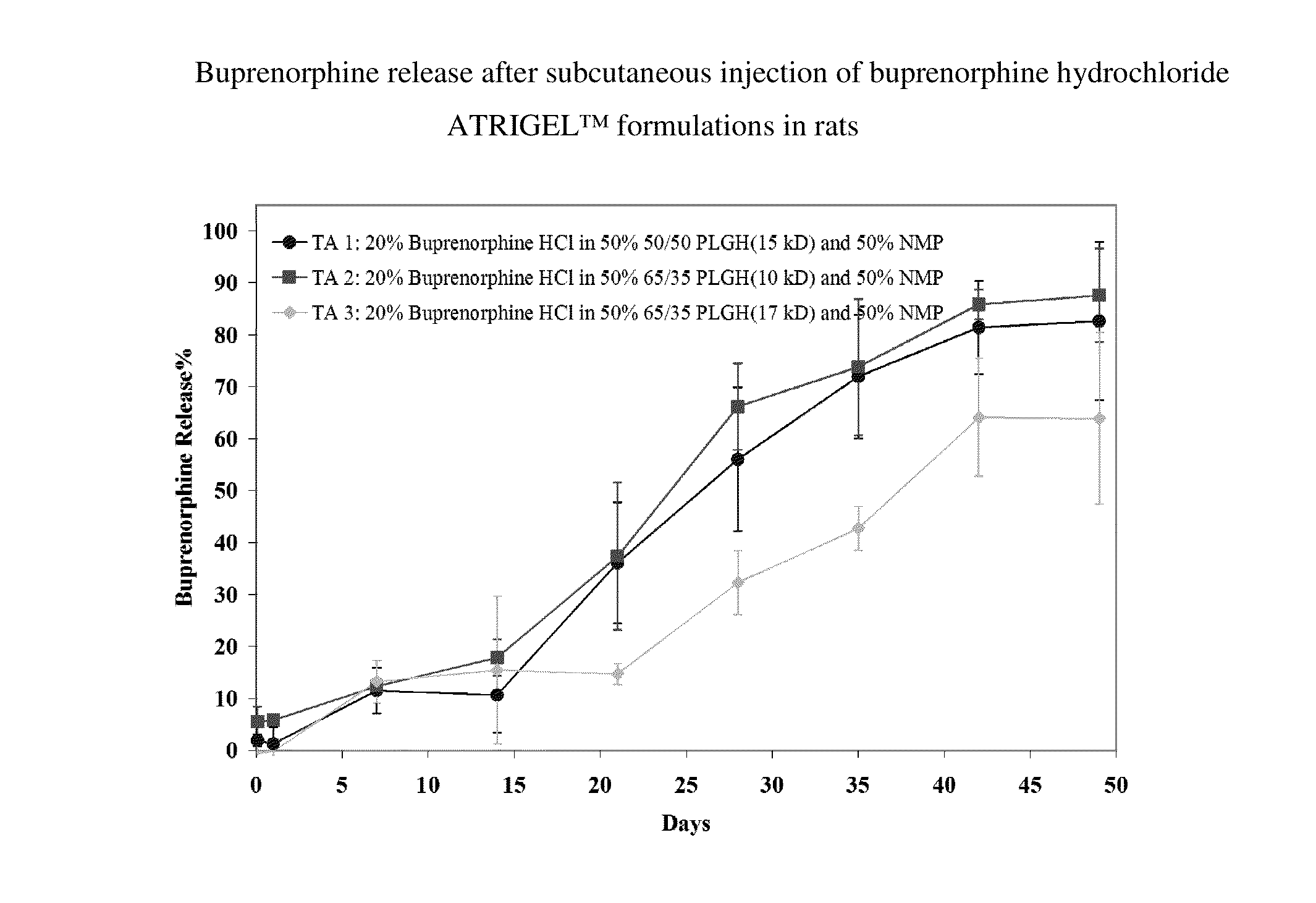
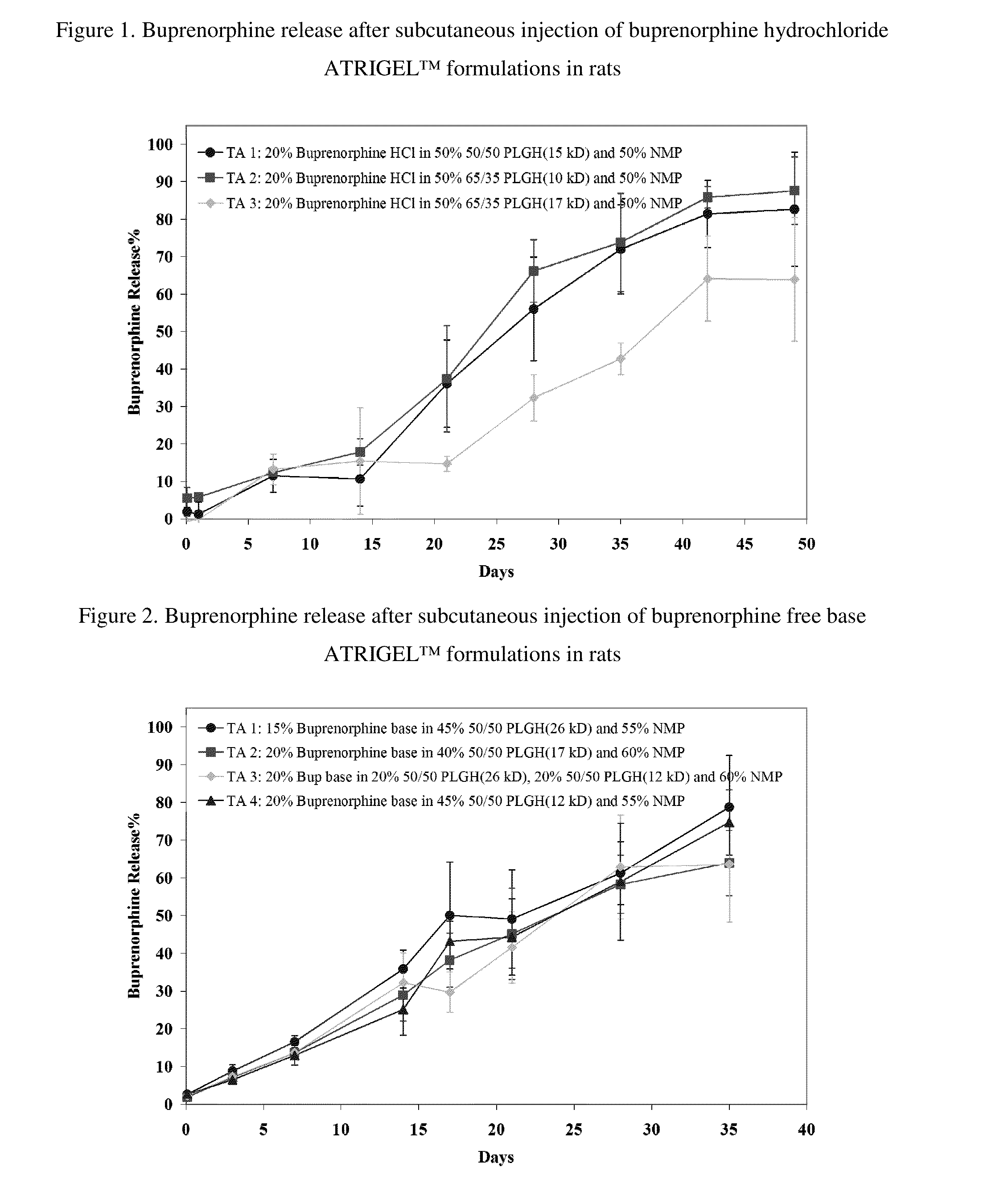
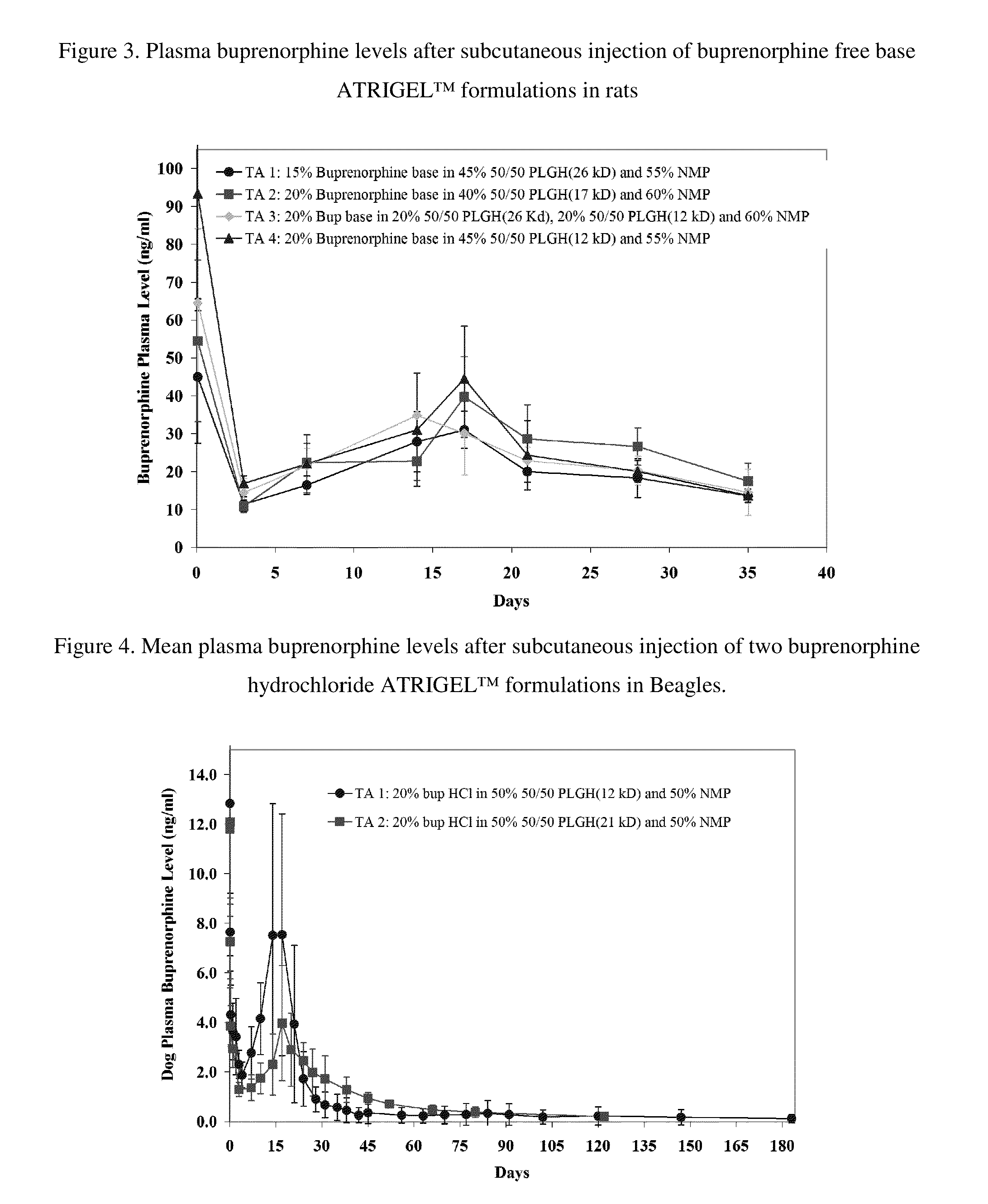
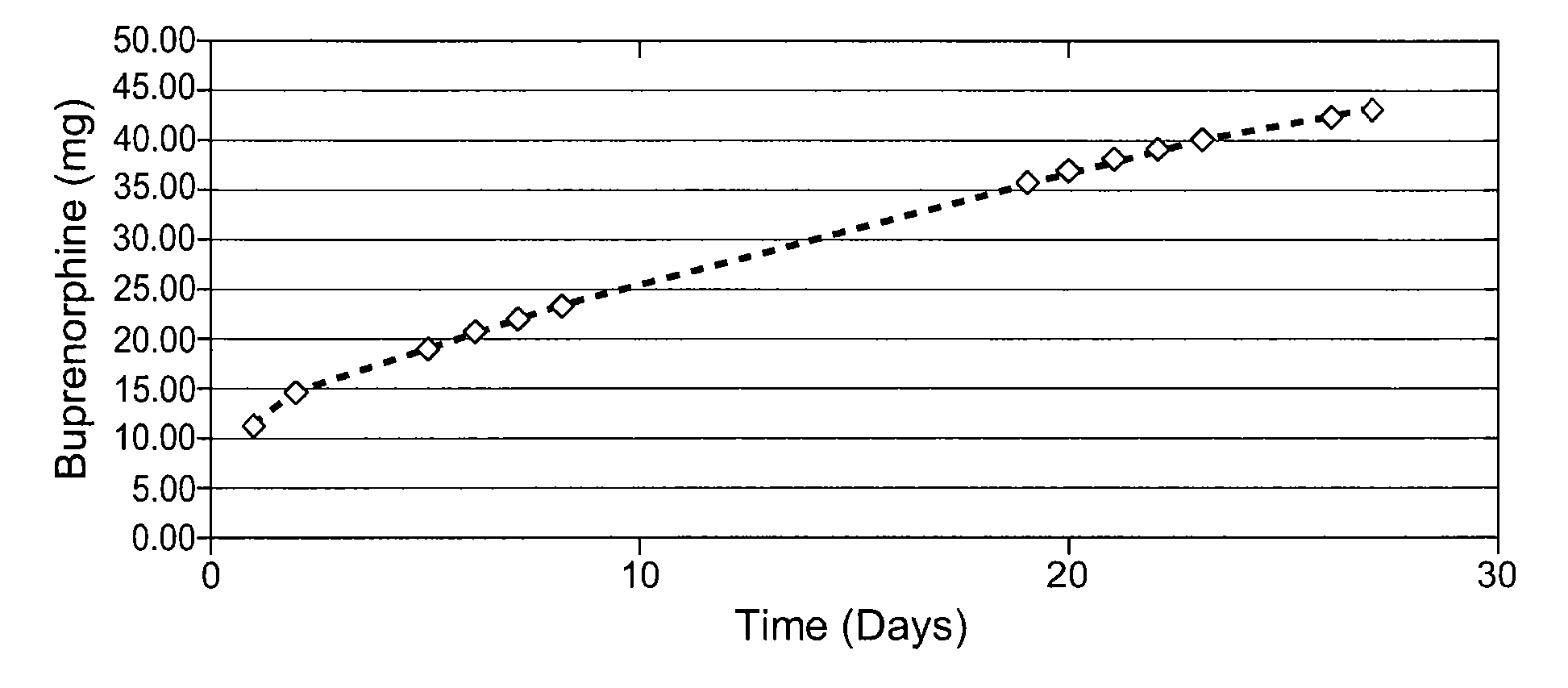
Patents
Literature
123 results about "Buprenorphine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to help relieve severe ongoing pain (such as due to arthritis, chronic back pain).
Analgesic compositions containing buprenorphine
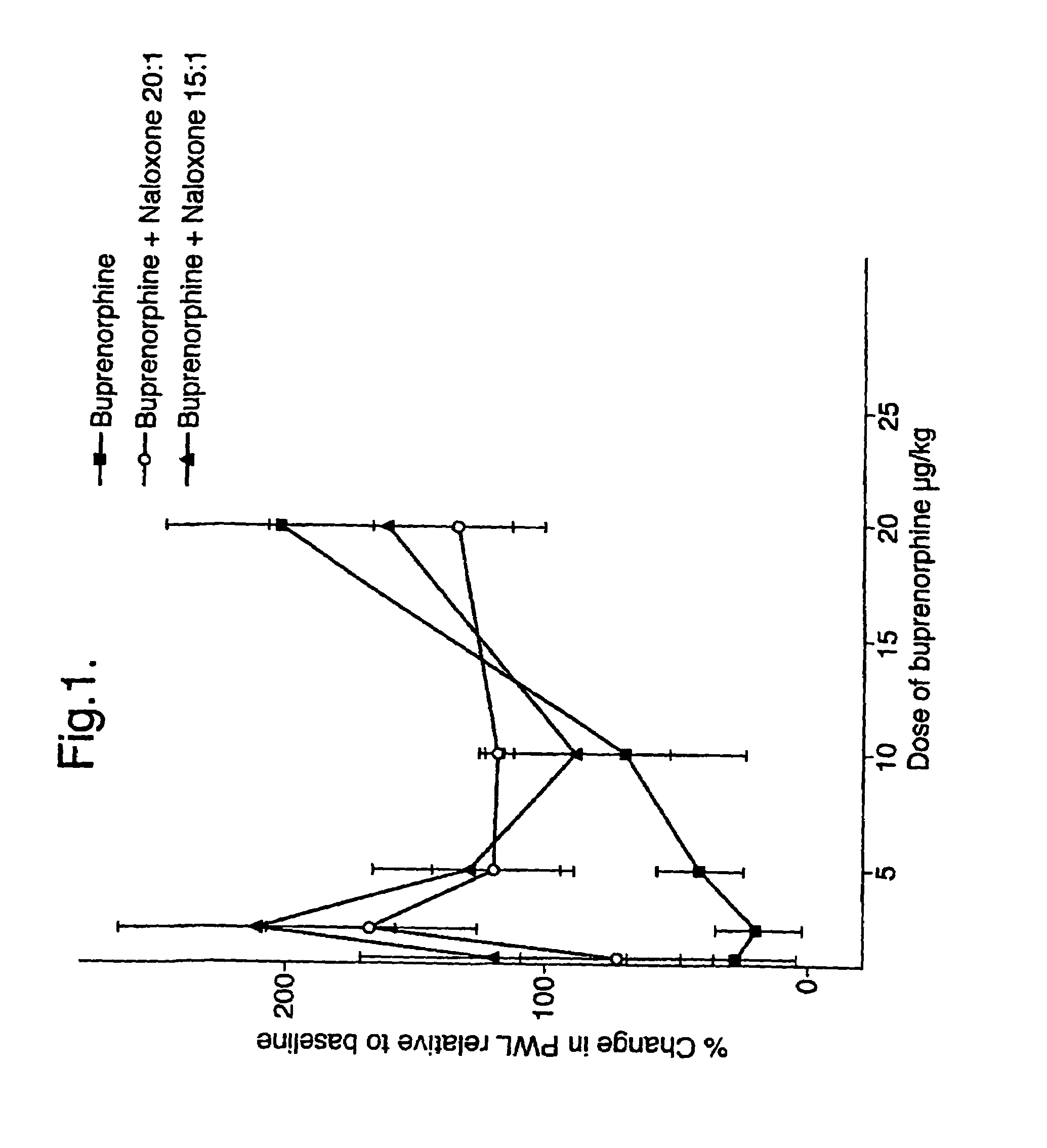
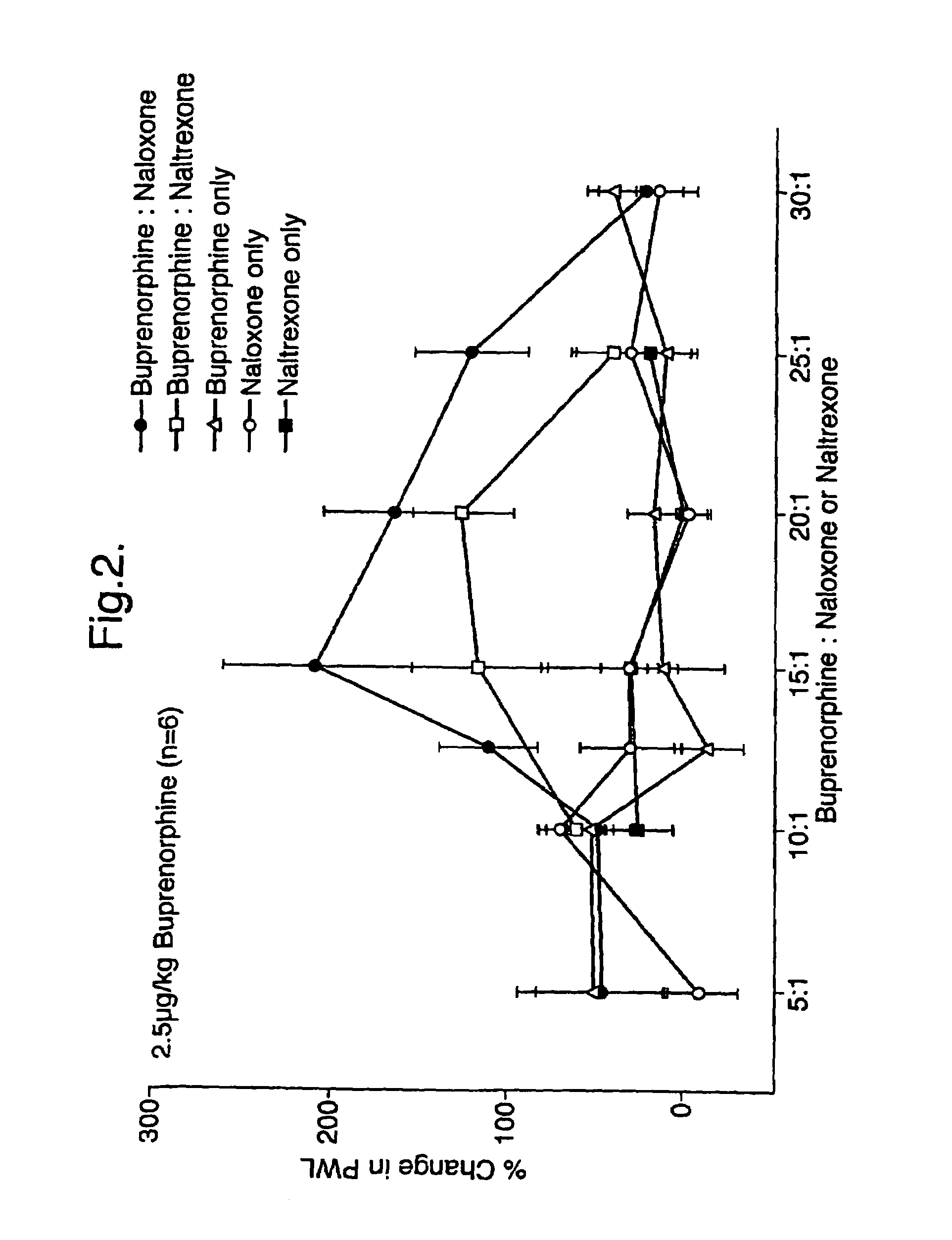
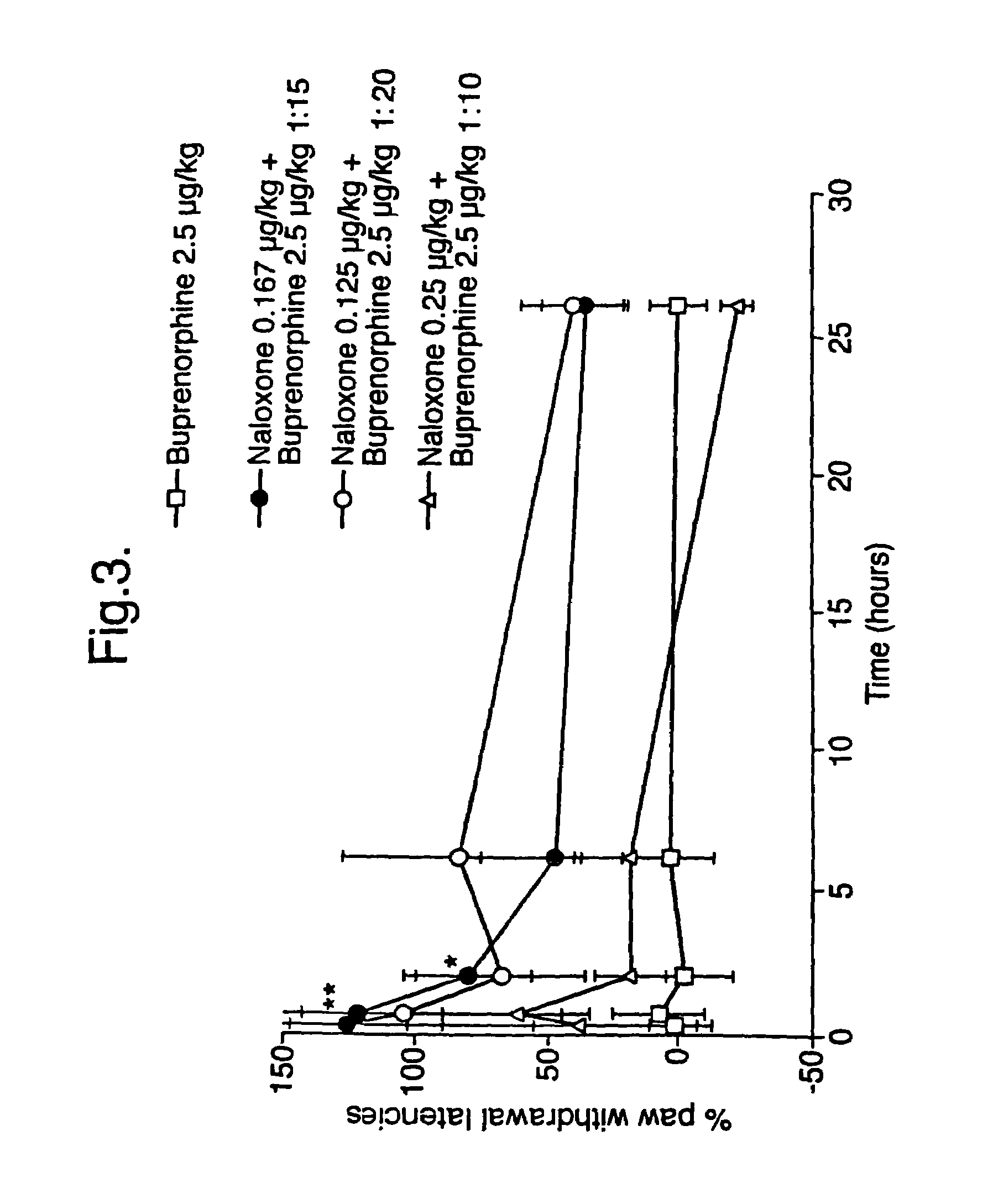
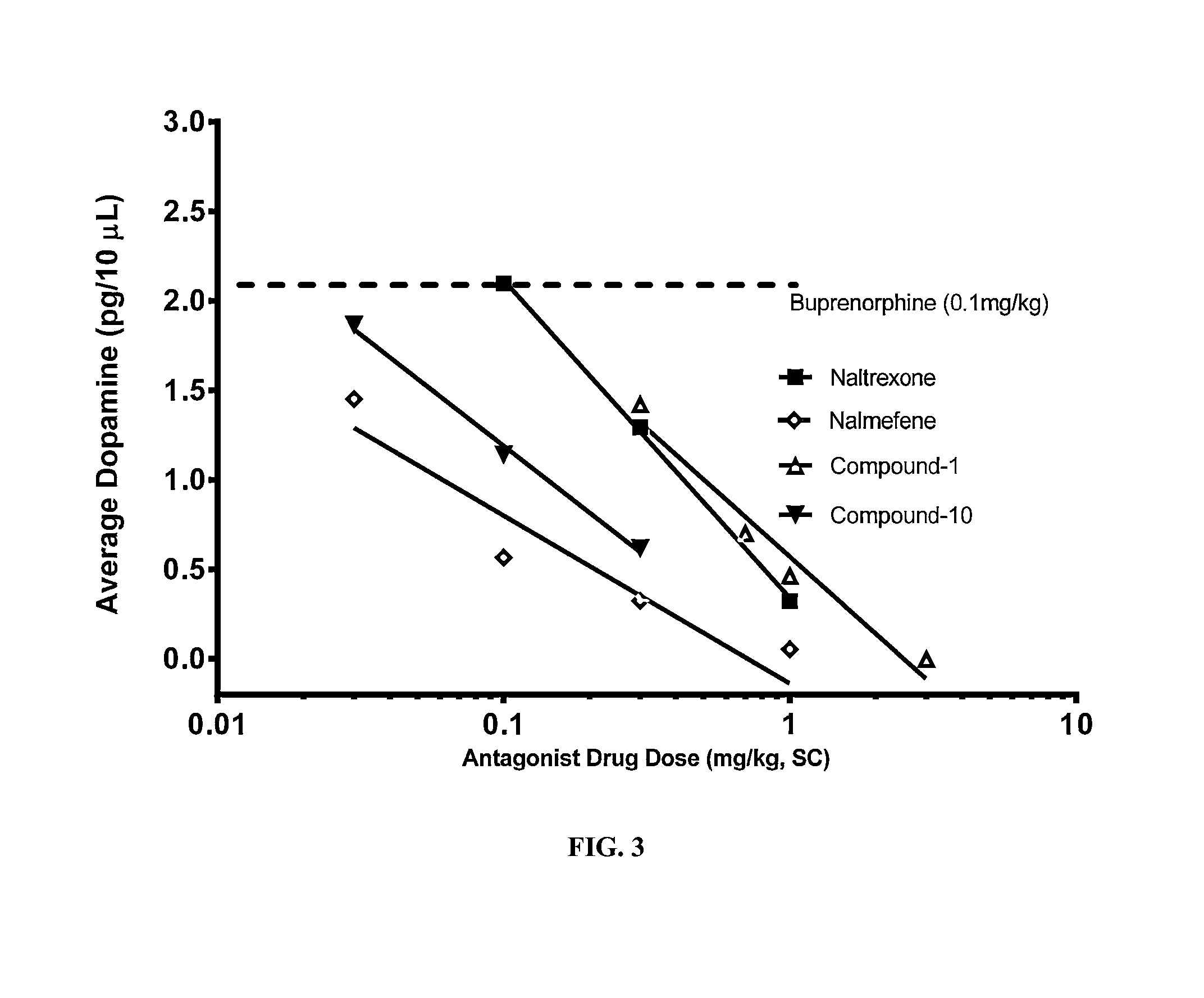
An analgesic composition in parenteral unit dosage form or in a unit dosage form suitable for delivery via the mucosa comprising an amount of buprenorphine which is less than the clinical dose required to achieve pain relief and an amount of naloxone such that the ratio by weight of buprenorphine to naloxone is in the range of from 12.5:1 to 27.5:1, or an amount of naltrexone or nalmefene such that the ratio by weight of buprenorphine to naltrexone or nalmefene is in the range of from 12.5:1 to 22.5:1. The analgesic action of the buprenorphine is potentiated by the low dose of naloxone, naltrexone or nalmefene.
Owner:INDIVIOR UK
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
Implantable polymeric device for sustained release of buprenorphine
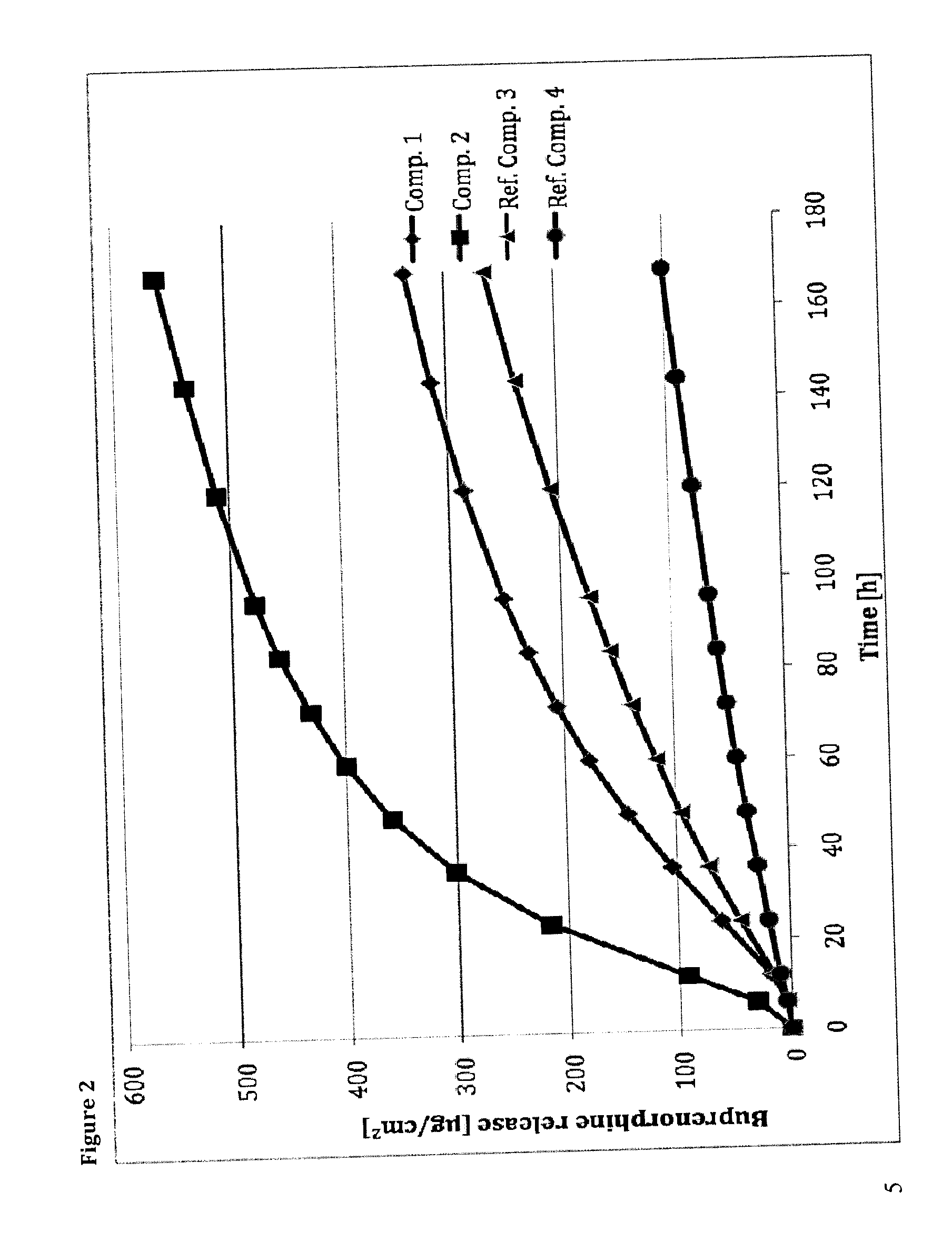
The present invention provides compositions, methods, and kits for treatment of opiate addiction and pain. The invention provides a biocompatible nonerodible polymeric device which releases buprenorphine continuously with generally linear release kinetics for extended periods of time. Buprenorphine is released through pores that open to the surface of the polymeric matrix in which it is encapsulated. The device may be administered subcutaneously to an individual in need of continuous treatment with buprenorphine.
Owner:FEDSON INC
Compositions of buprenorphine and μ antagonists
Owner:ALKERMES PHARMA IRELAND LTD
Buprenorphine-Wafer for Drug Substitution Therapy
The present invention relates to oral pharmaceutical dosage forms comprising buprenorphine with the dosage form releasing buprenorphine instantly upon oral, preferably sublingual, application of the dosage form. The present invention also relates to the use of such dosage forms for treating pain in a human or animal or for drug substitution therapy in drug-dependent human subjects.
Owner:RHODES PHARMA LP
Opioid-receptor antagonists in transdermal systems having buprenorphine
Transdermal systems with an active agent such as buprenorphine and an opioid receptor antagonist are provided. The opioid receptor antagonist may include a μ, κ or δ opioid receptor antagonist. Methods of treatment using such a system are also provided.
Owner:GRUNENTHAL GMBH
Methods of providing sustained treatment with opioids
InactiveUS6231886B1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationPlasma glucose
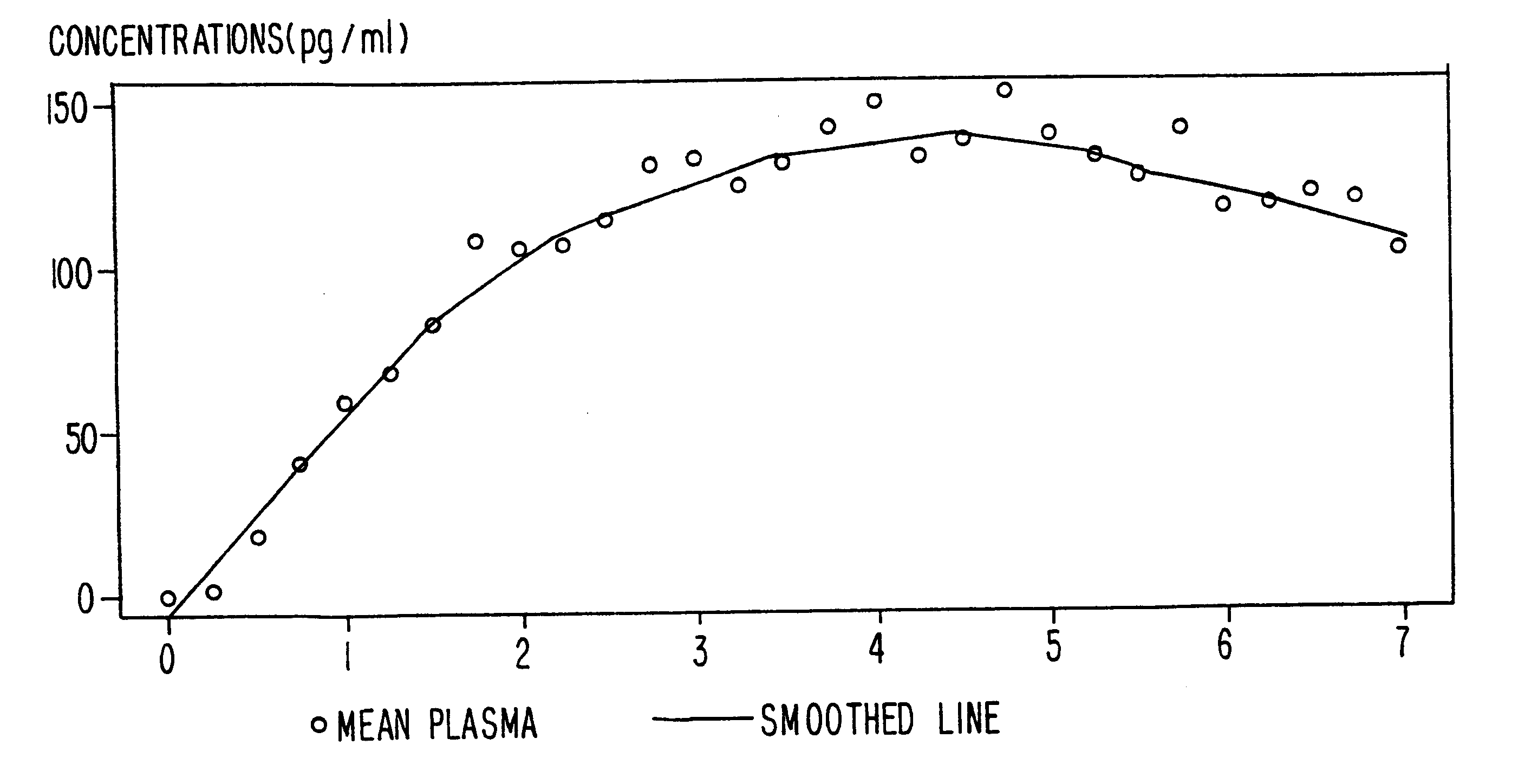
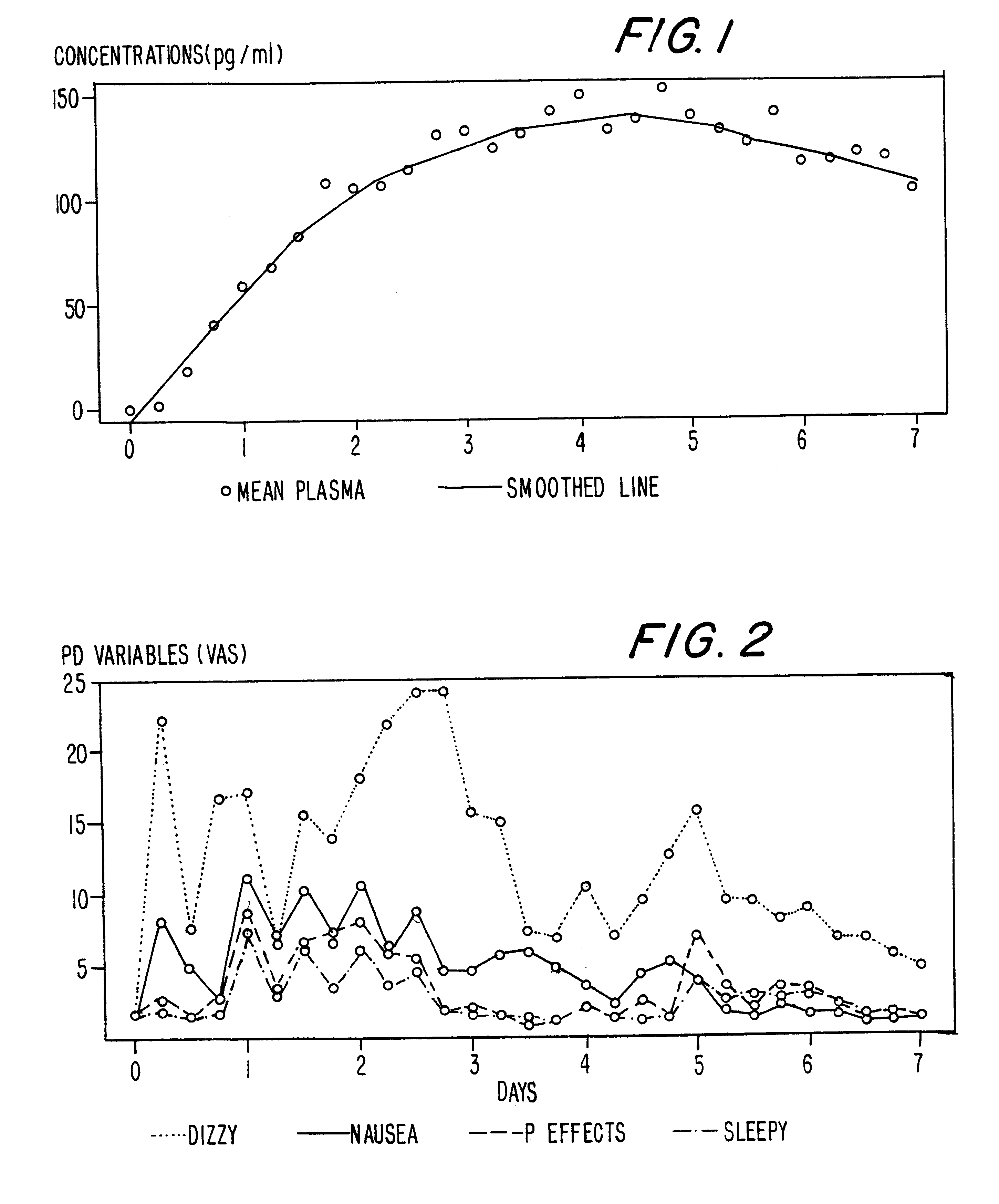
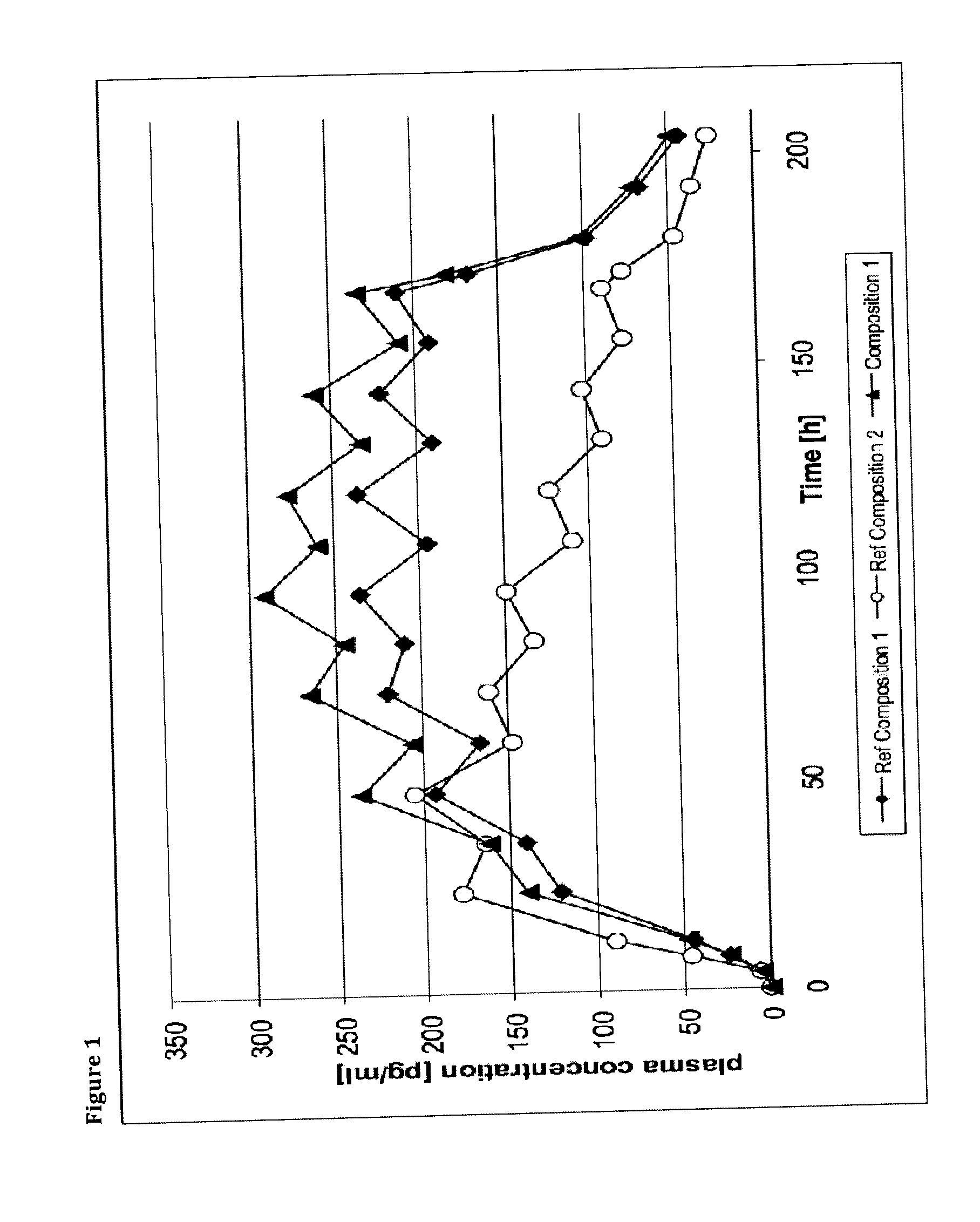
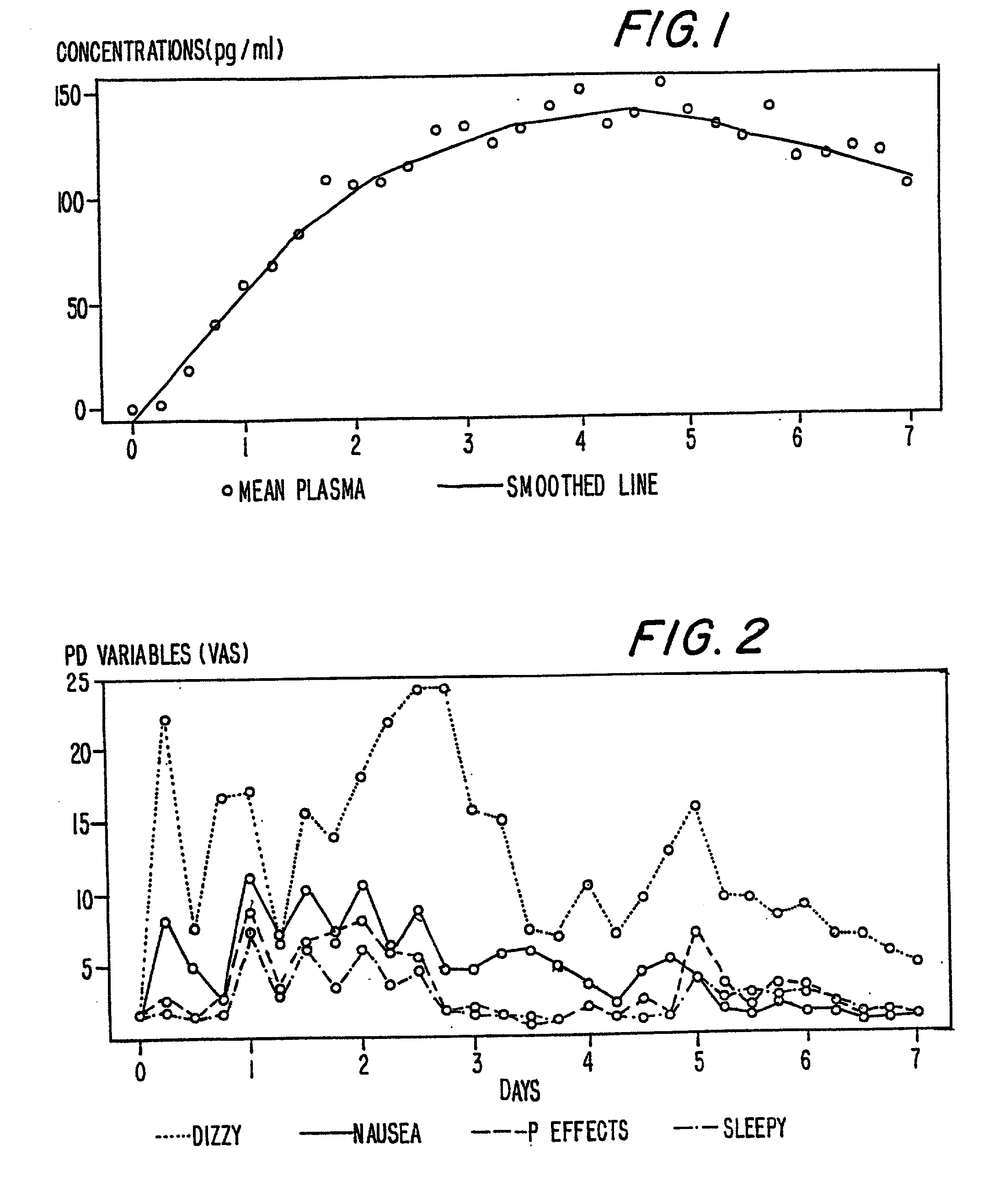
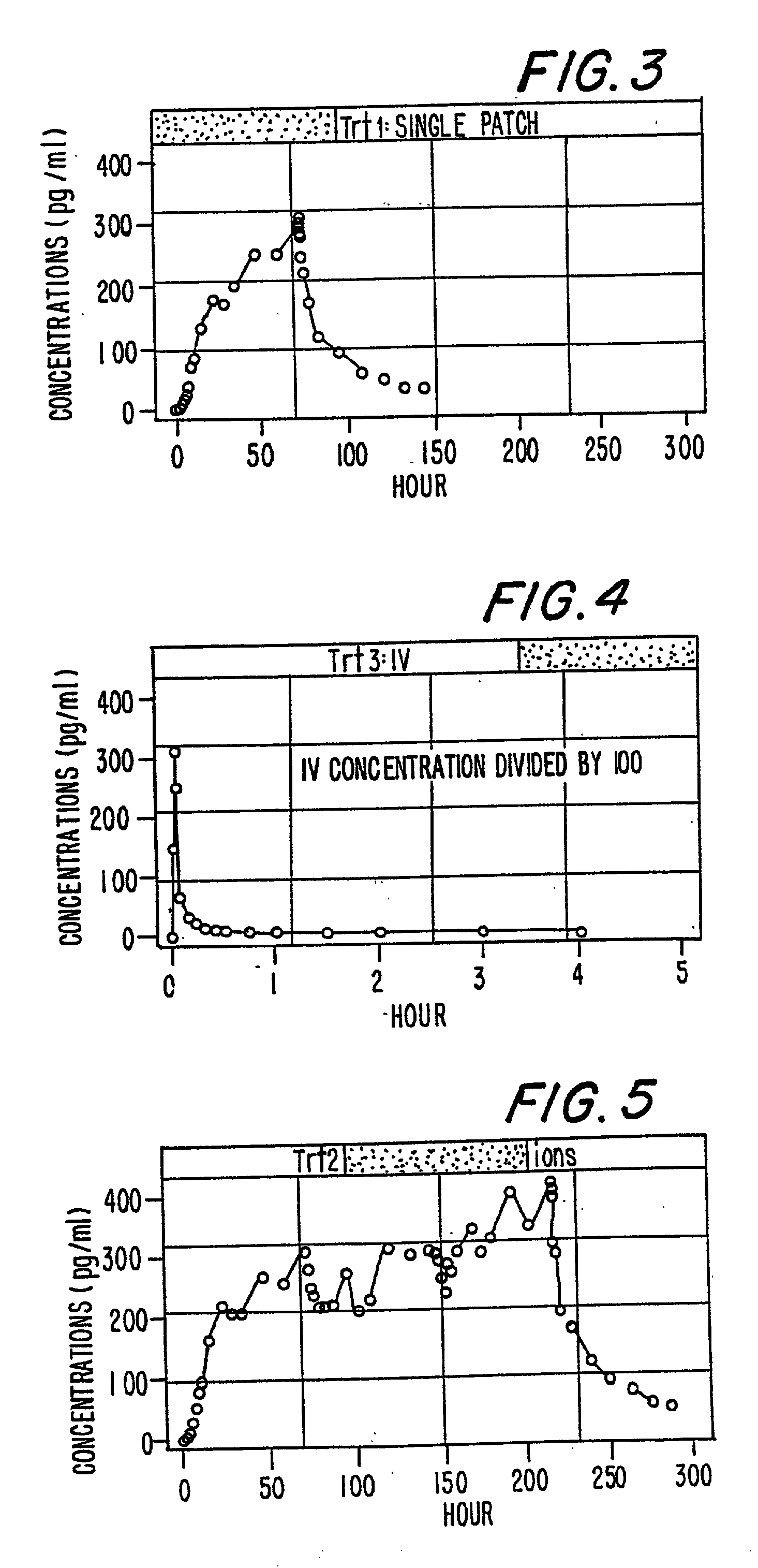
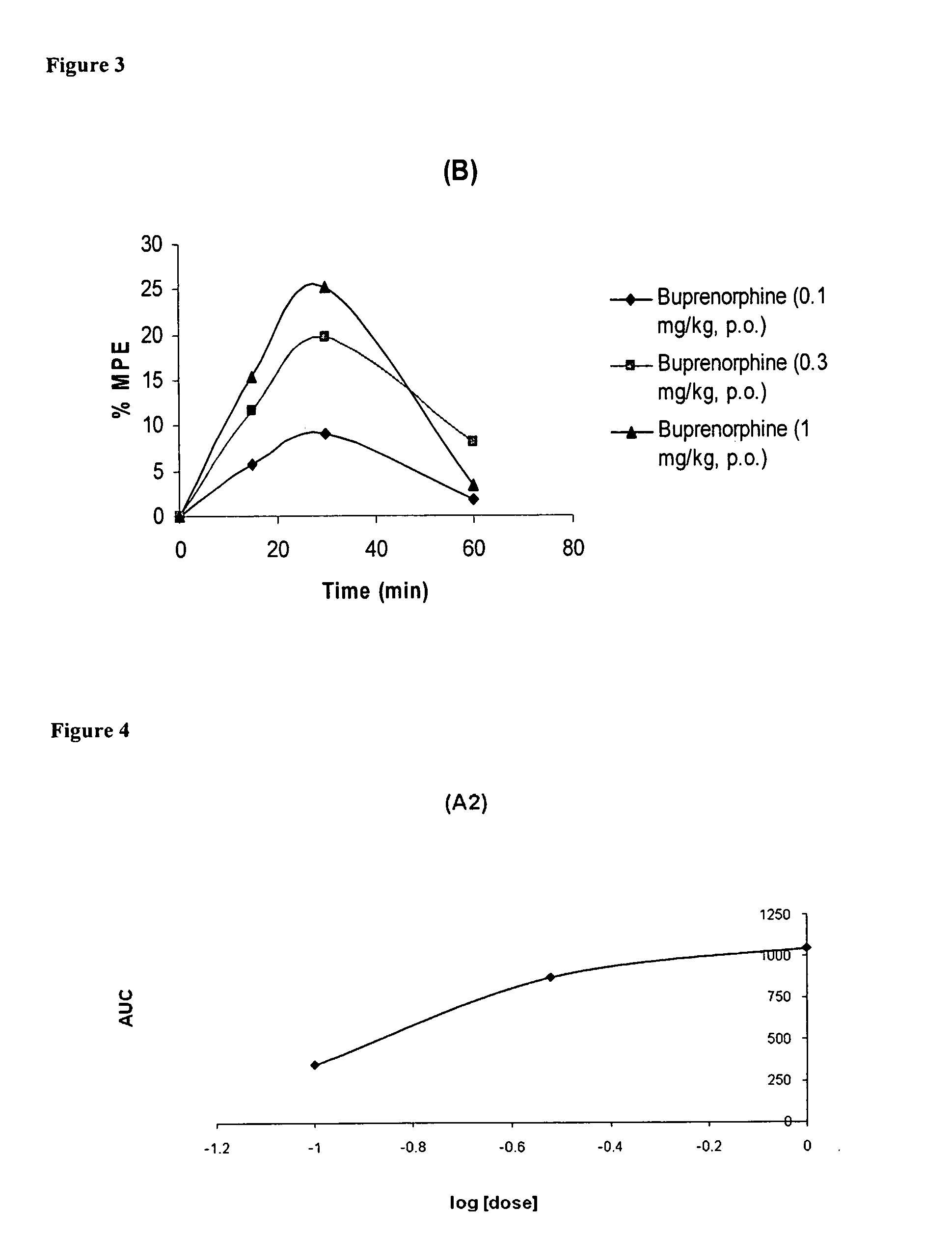
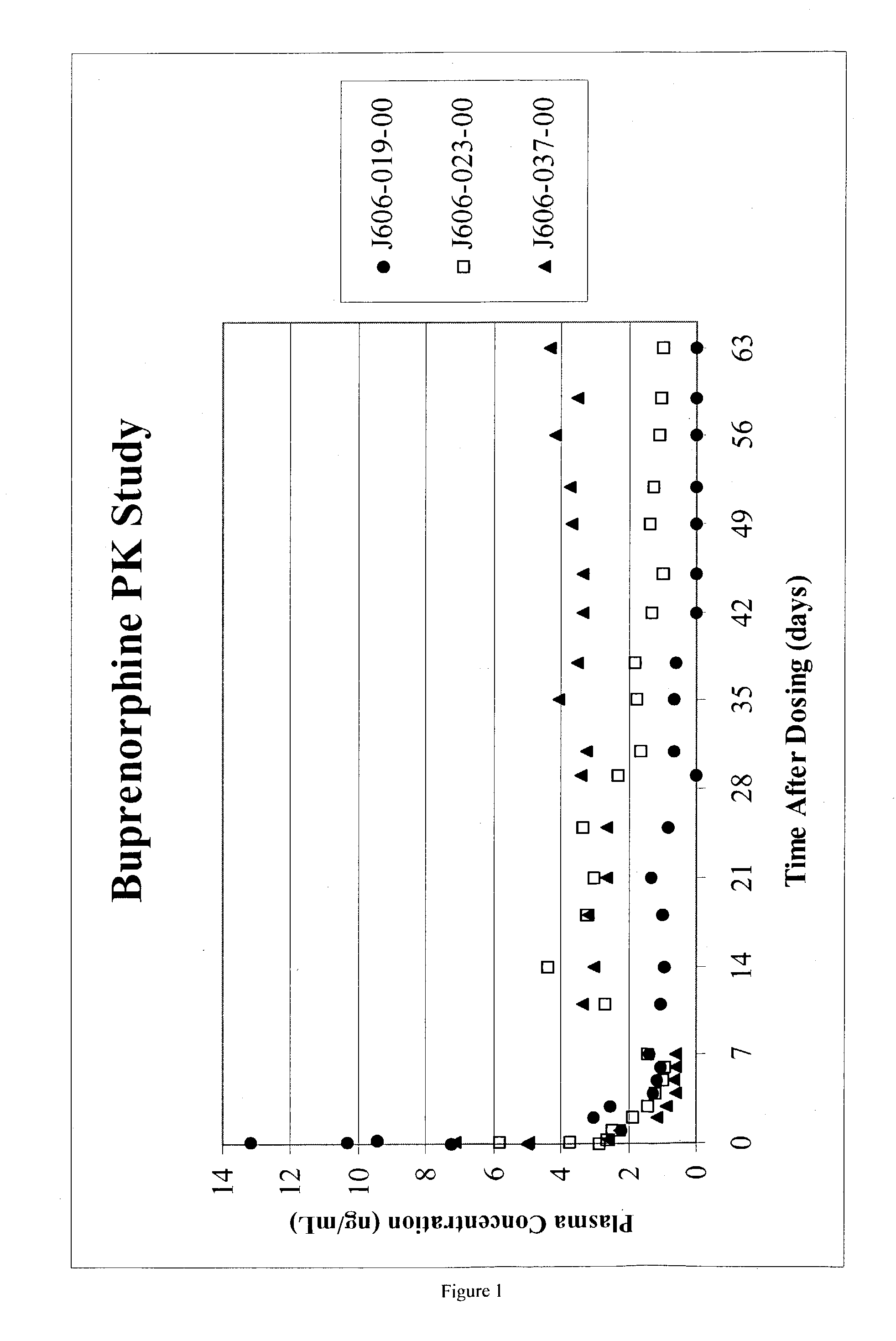
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an addition two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Analogs and prodrugs of buprenorphine
Owner:PURDUE PHARMA LP
Method for reducing pain
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
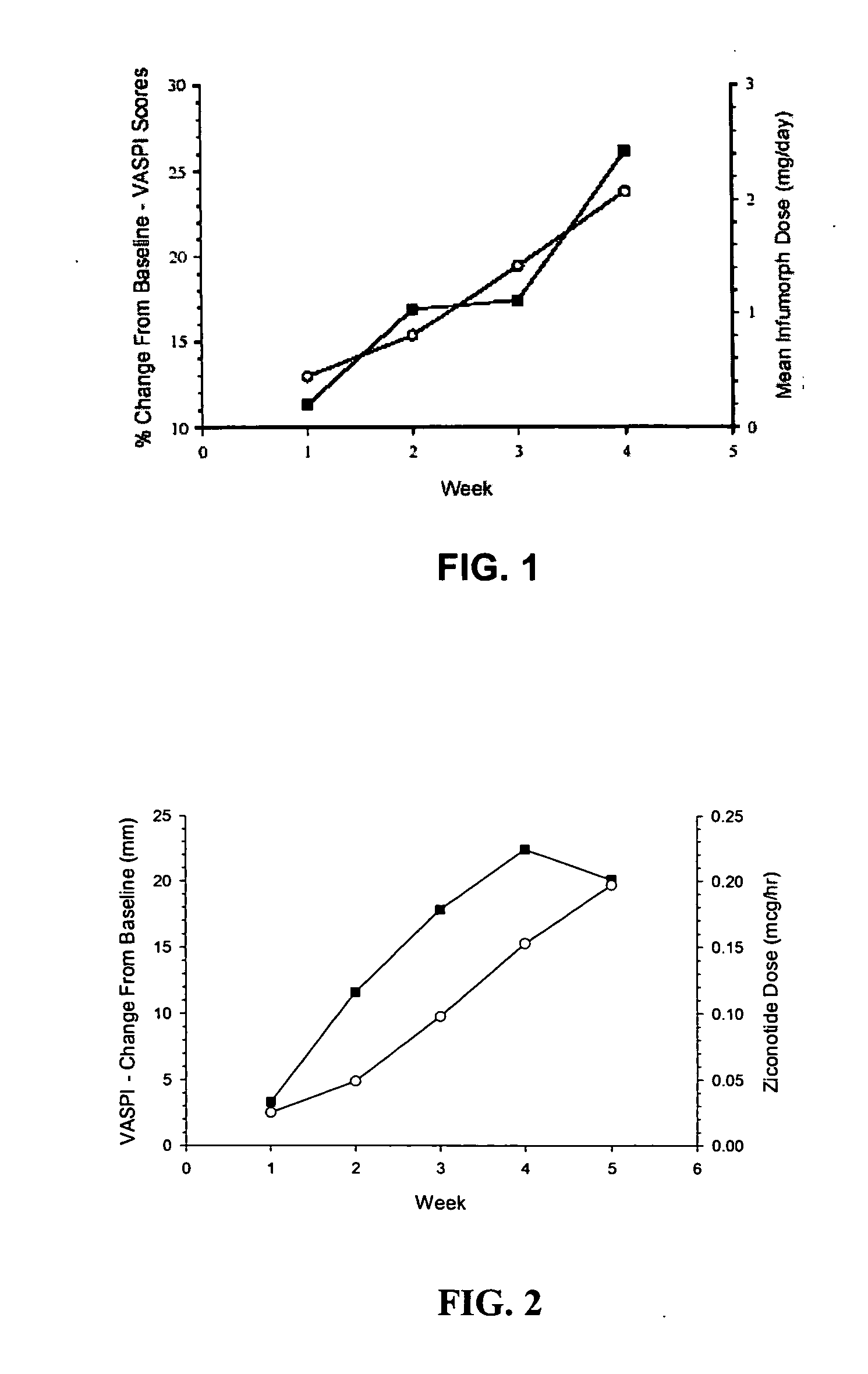
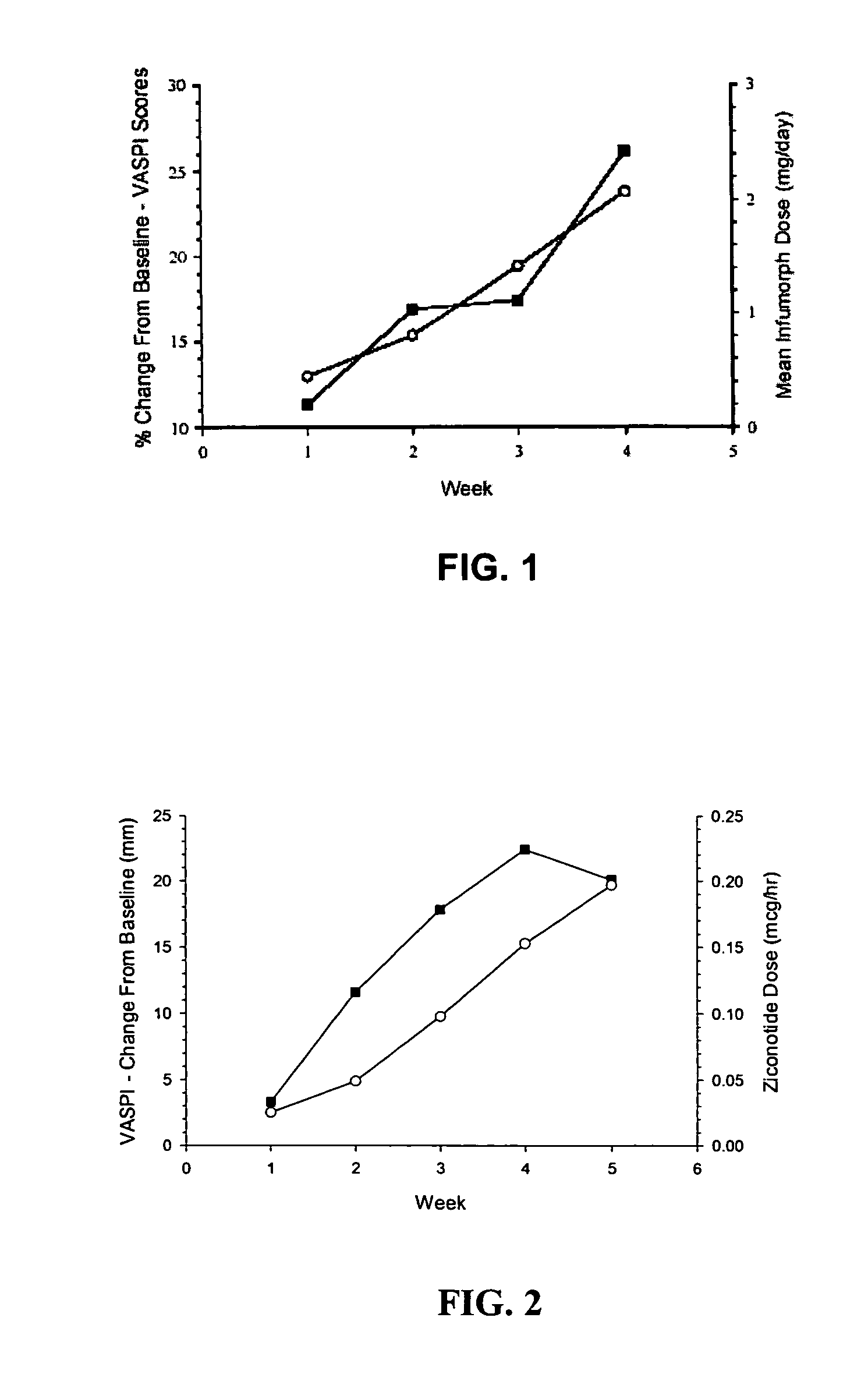
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Transdermal therapeutic system comprising buprenorphine
ActiveUS20130331803A1Improve comfortBiocideAdhesive dressingsTransdermal medicationPenetration enhancer
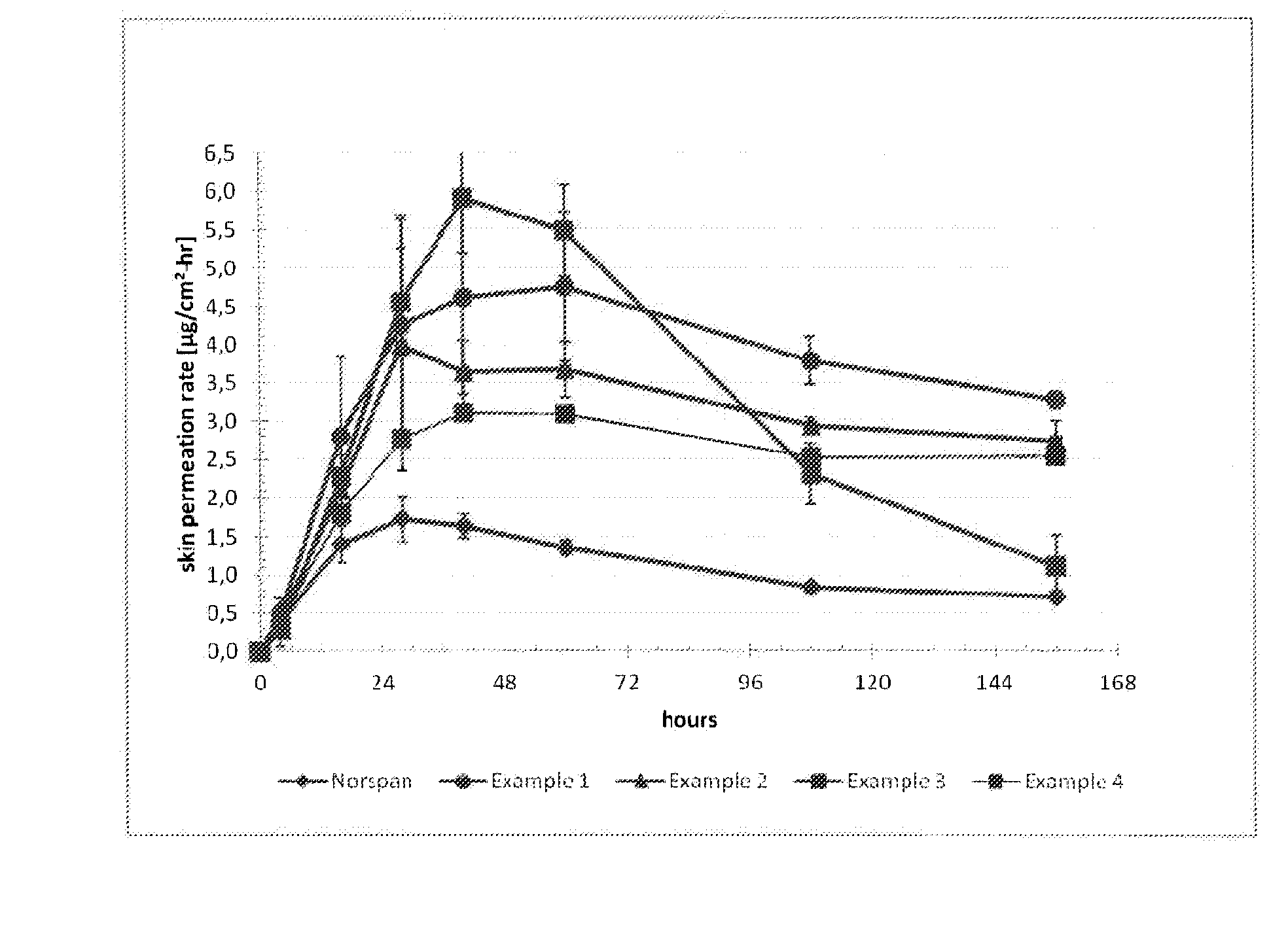
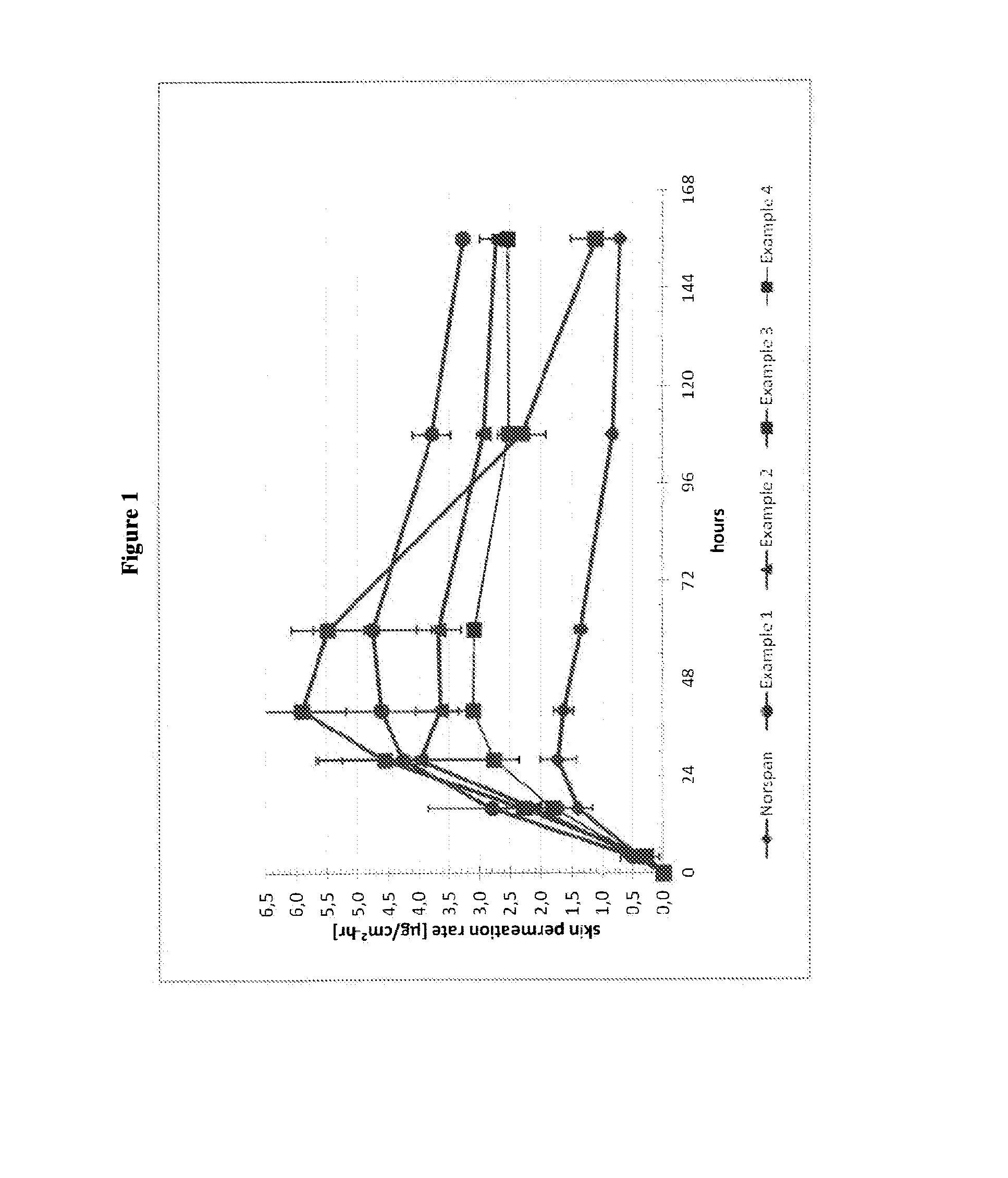
The invention is concerned with a transdermal therapeutic system (TTS) comprising buprenorphine and a method of manufacturing such a TTS. The transdermal therapeutic system is used for the transdermal administration of buprenorphine and analogues thereof. In particular, the invention relates to the use of a transdermal therapeutic system (TTS) for analgesic purposes. The TTS according to the invention comprises a transdermal drug delivery composition comprising buprenorphine and an adhesive component, which is a mixture of a crosslinked and a non-crosslinked acrylic polymer and a penetration enhancer comprising a keto acid.
Owner:HEXAL AG
Method of providing sustained analgesia with buprenorphine
InactiveUS20010002259A1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationZero order kinetics
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an additional two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Transdermal delivery system comprising buprenorphine
InactiveUS20140363487A1Small area of releaseImprove adhesionBiocideNervous disorderLevulinic acidSkin contact
The invention relates to transdermal therapeutic system for the transdermal administration of buprenorphine, comprising a buprenorphine-containing self-adhesive layer structure comprising A) a buprenoφhine-impermeable backing layer, and B) a buprenorphine-containing pressure-sensitive adhesive layer on said buprenorphine-impermeable backing layer, the adhesive layer comprising a) at least one polymer-based pressure-sensitive adhesive, b) an analgesically effective amount of buprenorphine base or a pharmaceutically acceptable salt thereof, and c) a carboxylic acid selected from the group consisting of oleic acid, linoleic acid and linolenic acid, levulinic acid and mixtures thereof, in an amount sufficient so that said analgesically effective amount of buprenorphine is solubilized therein to form a mixture, and the carboxylic acid buprenorphine mixture forms dispersed deposits in the said pressure-sensitive adhesive, wherein said buprenorphine-containing pressure-sensitive adhesive layer is the skin contact layer.
Owner:LTS LOHMANN THERAPIE-SYST AG
Injectable flowable composition comprising buprenorphine
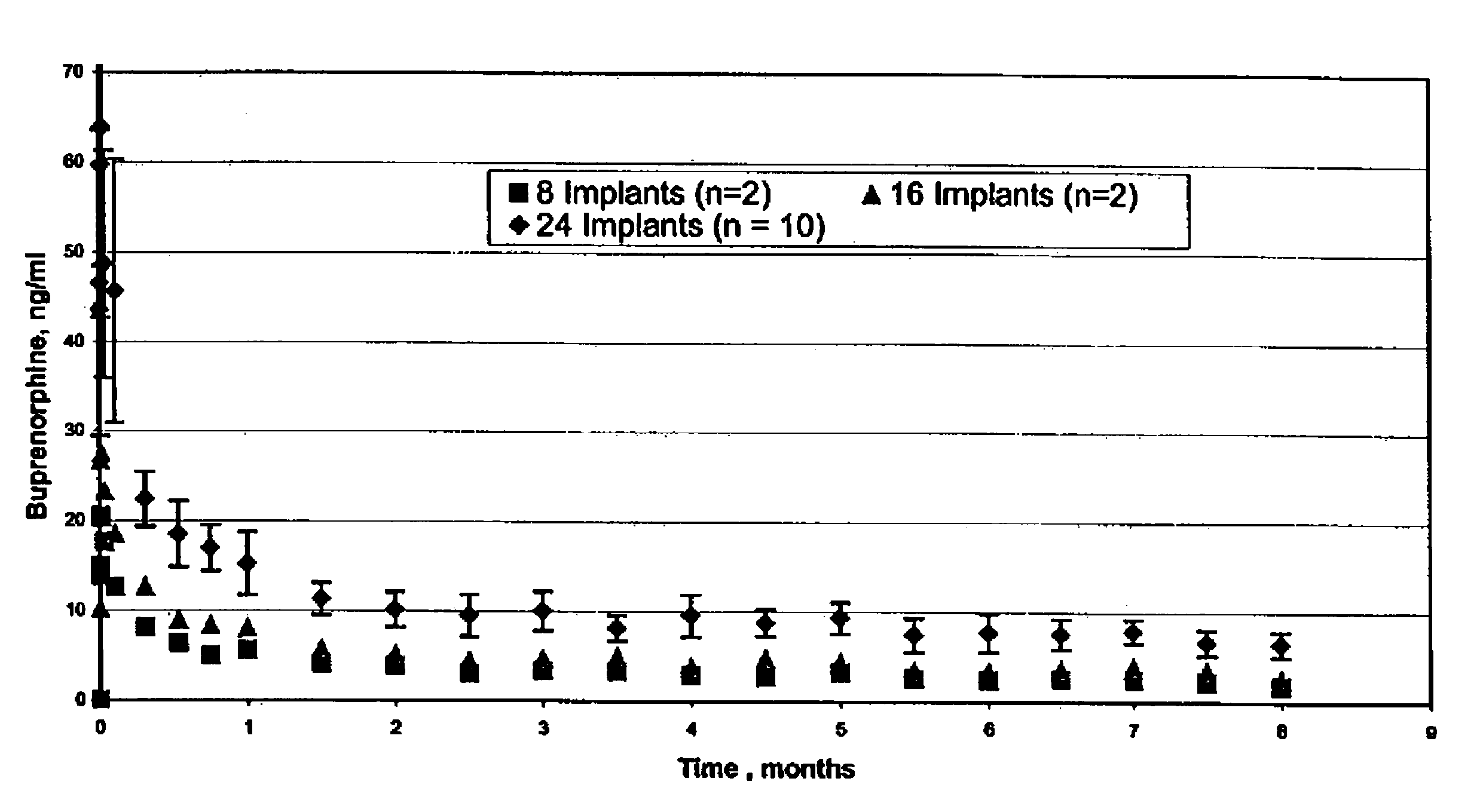
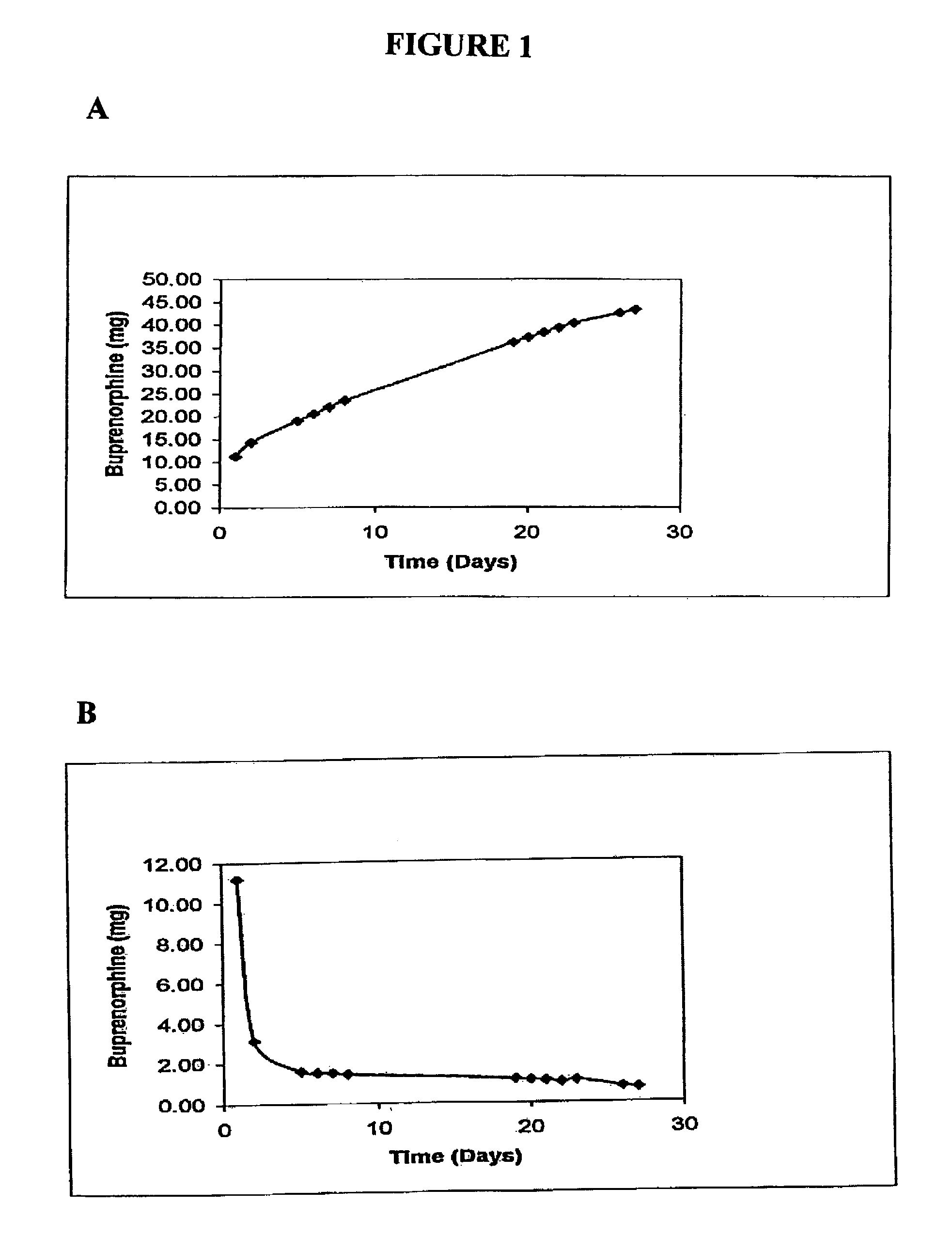
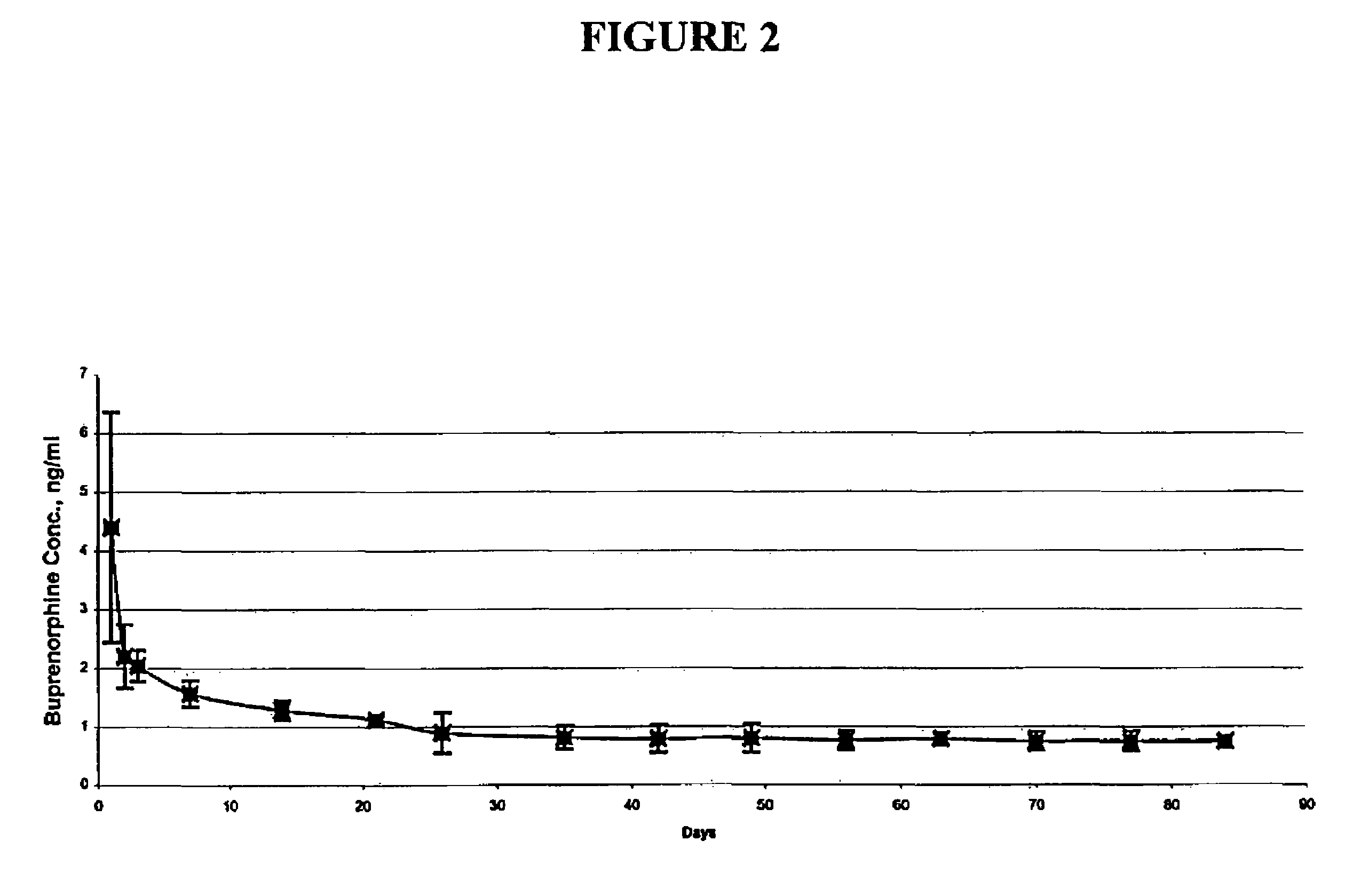
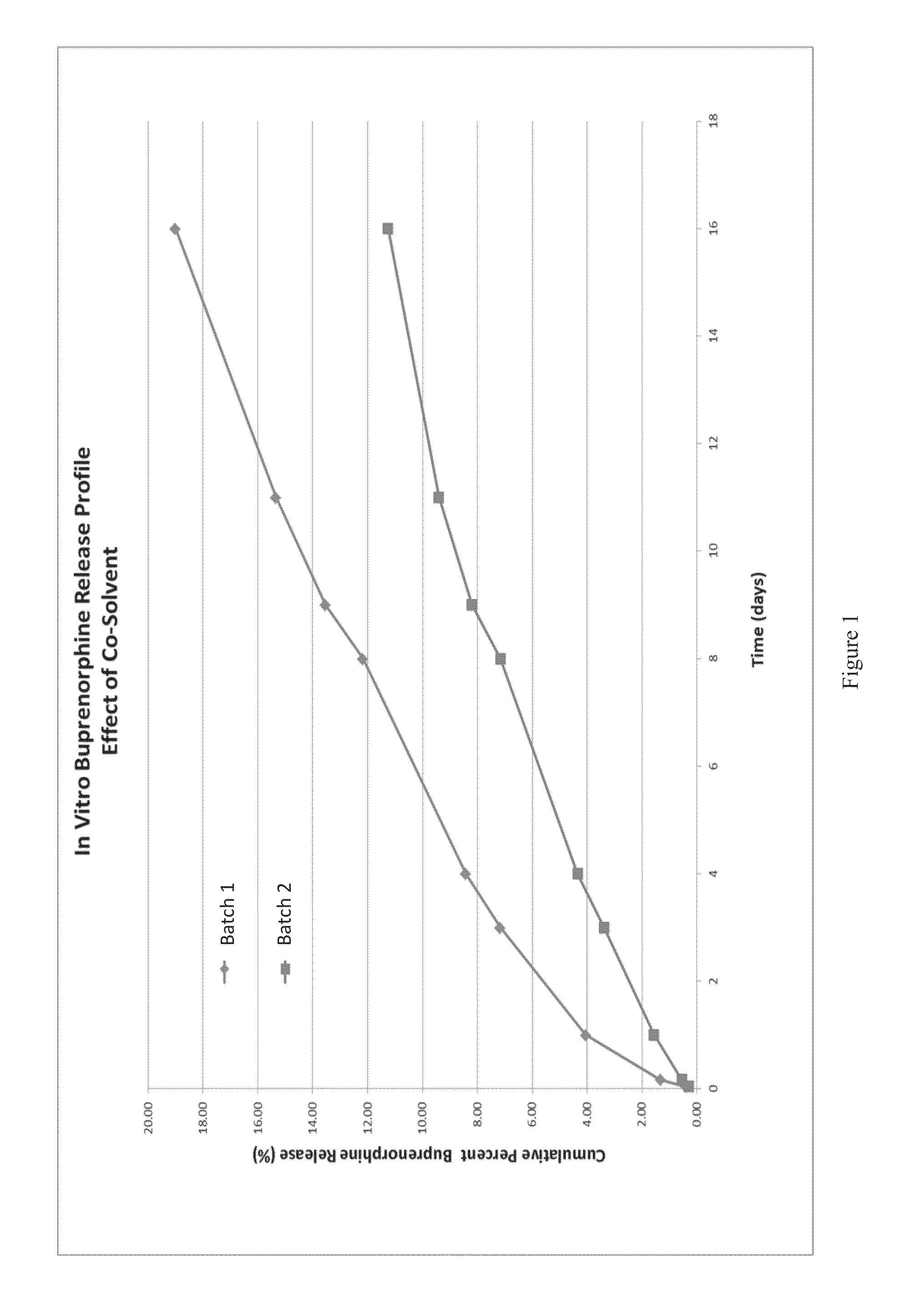
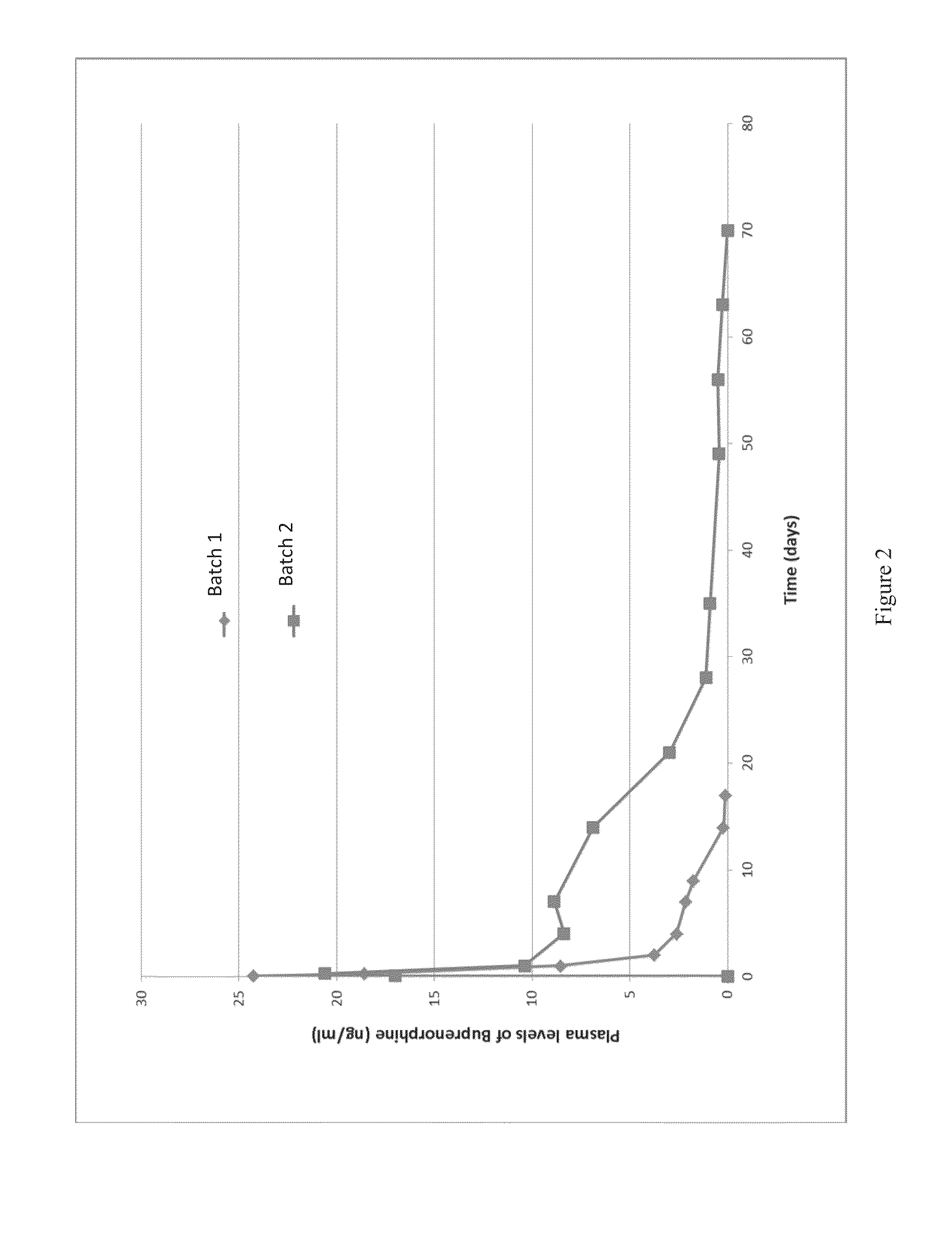
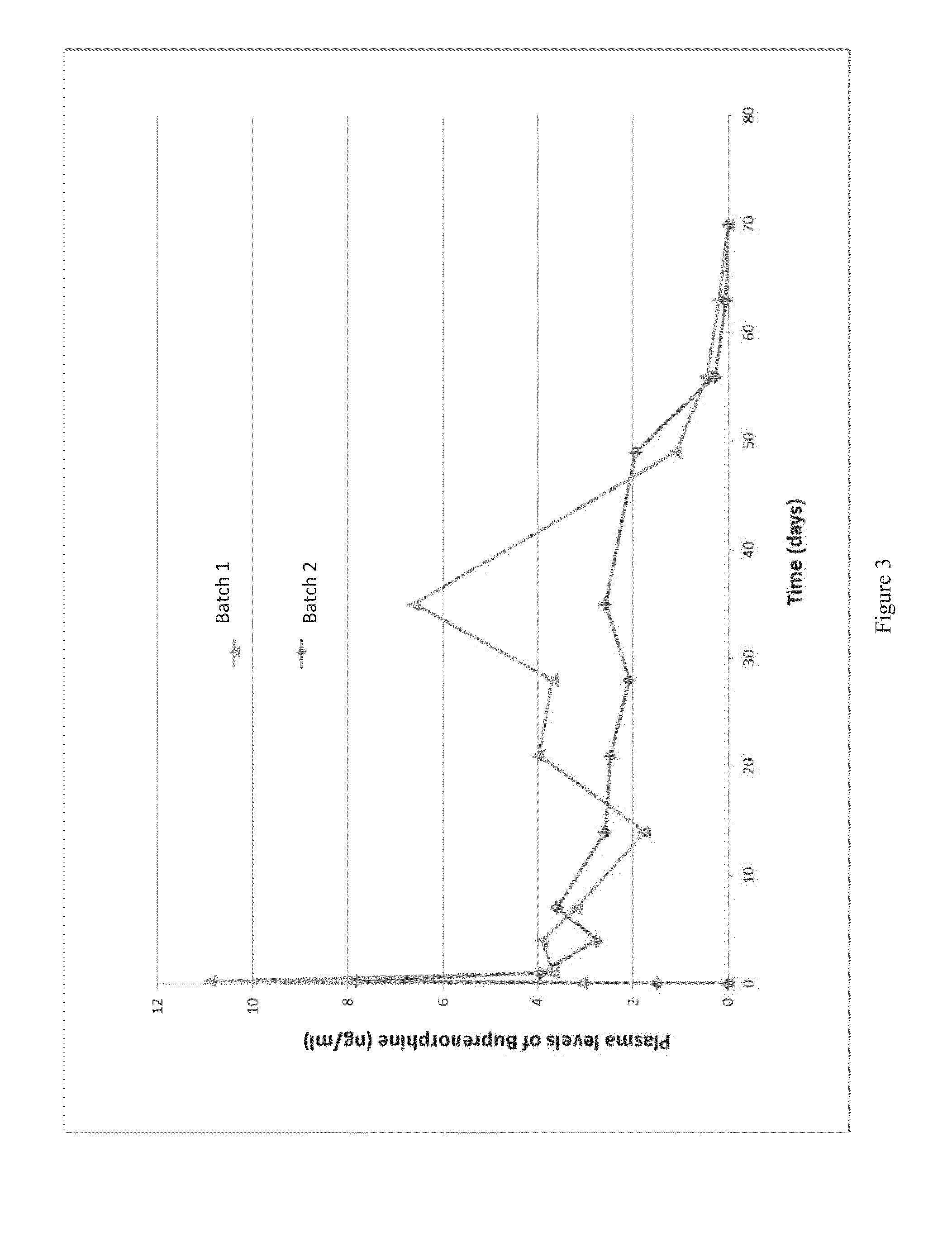
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
High drug load buprenorphine microspheres and method of producing same
ActiveUS20140271869A1Reduce the amount requiredLarge batchBiocidePowder deliverySolubilityMicrosphere
A sustained release microsphere formulation with a high drug load may be formed by a continuous oil-in-water emulsion process by combining an organic dispersed phase with an aqueous continuous phase. The dispersed phase may include an encapsulating polymer, a primary solvent, such as dichloromethane, a pharmaceutically effective amount of an active agent having a solubility relative to the dispersed phase, and a co-solvent, such as benzyl alcohol, which is capable of increasing the solubility of the active agent relative to the dispersed phase. The continuous phase may include an aqueous solution of polyvinyl alcohol and water.
Owner:OAKWOOD LAB LLC
Implantable polymeric device for sustained release of buprenorphine with minimal initial burst
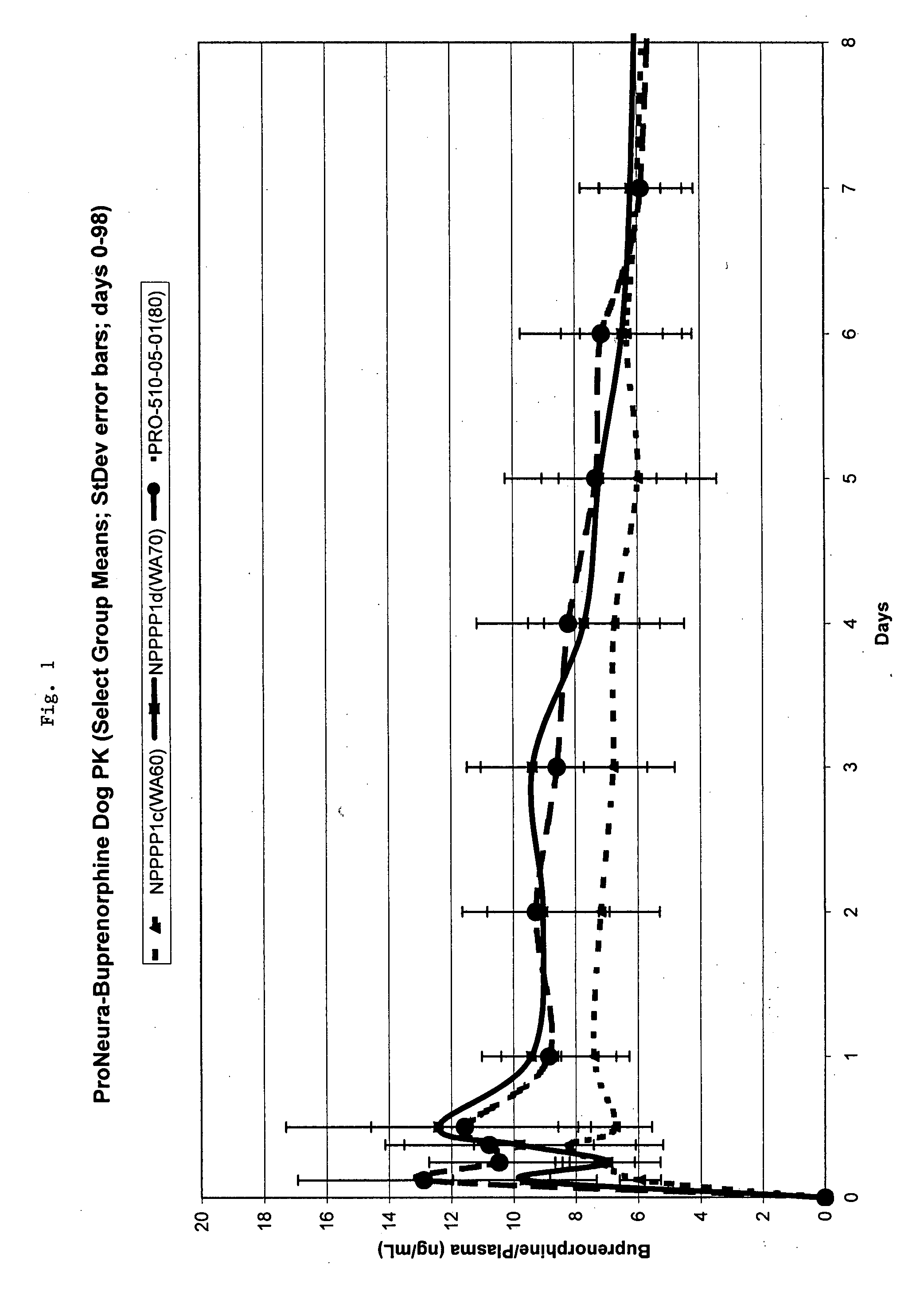
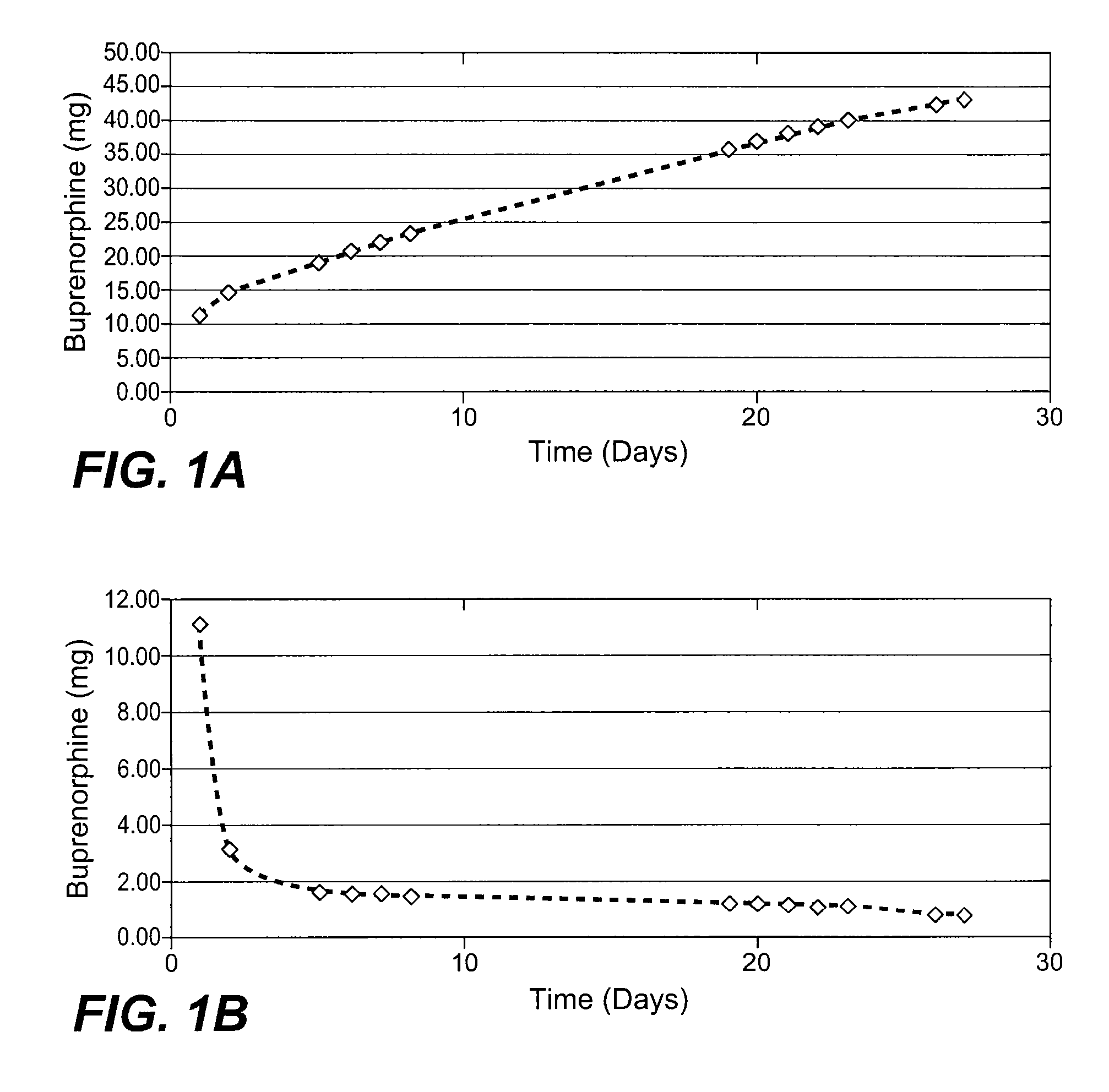
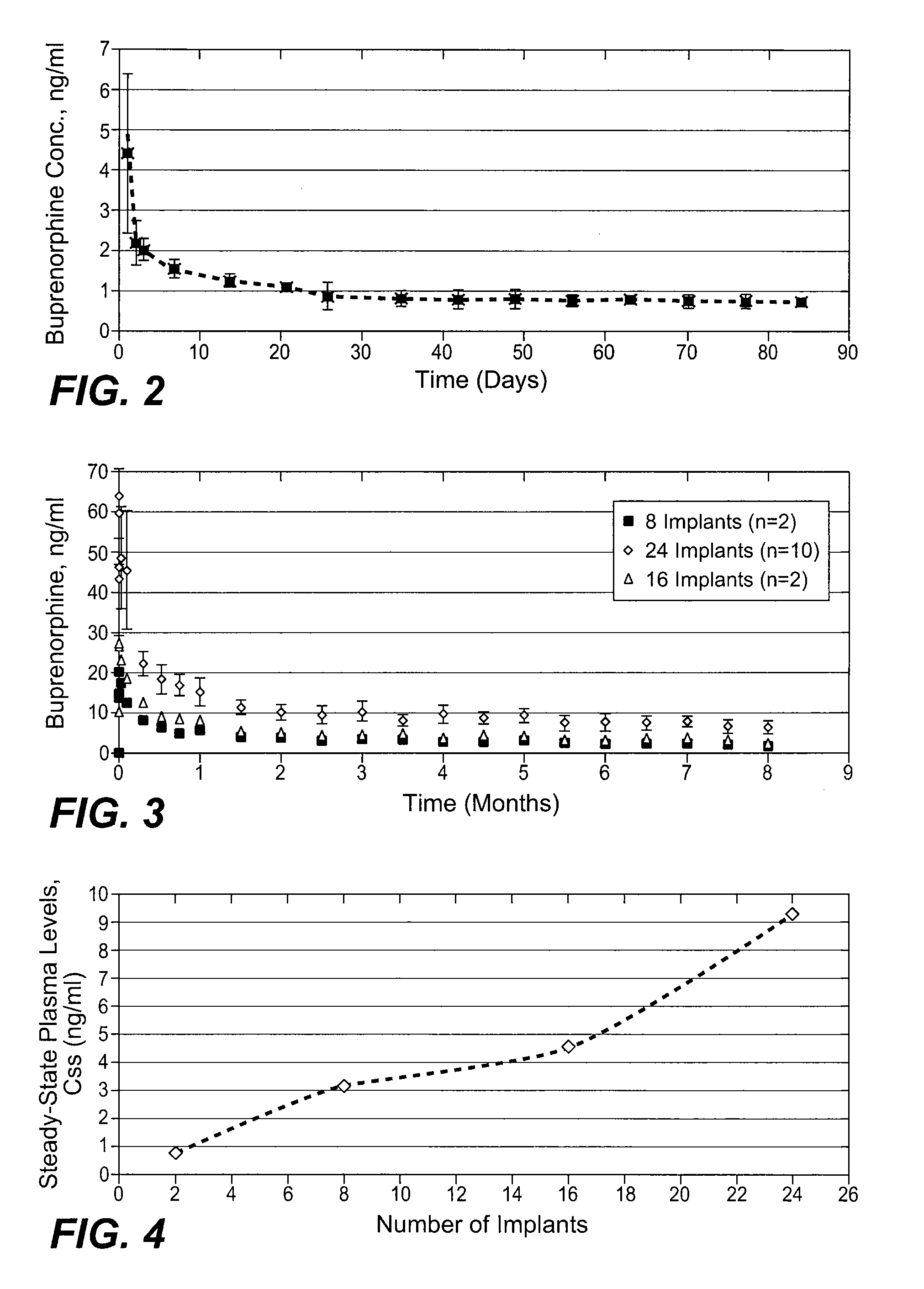
InactiveUS20070275031A1Low in steady stateLow initial burstBiocideGranular deliverySubcutaneous implantationBlood plasma
The present invention provides compositions, methods, and kits for treatment of pain. The invention provides a biocompatible nonerodible polymeric device which releases buprenorphine continuously with generally linear release kinetics for extended periods of time, and exhibits minimal initial burst upon subcutaneous implantation. Buprenorphine is released through pores that open to the surface of the polymeric matrix in which it is encapsulated. The device may be administered subcutaneously to an individual in need of continuous treatment with buprenorphine. Devices of the invention are washed with ethanol for greater than 30 minutes prior to subcutaneous implantation or have release characteristics of a device that has been washed with ethanol for greater than 30 minutes, such as a low peak to steady state plasma level ratio.
Owner:TITAN PHARMA
Transdermal Therapeutic System For Administering Analgesics
InactiveUS20070298091A1Reduce usageImprove wearing comfortOrganic active ingredientsNervous disorderAnalgesic agentsBuprenorphine
Transdermal therapeutic systems for administering analgesics, preferably buprenorphine or one of its pharmaceutically acceptable salts or pro-drugs, and processes for the production of such systems.
Owner:GRUNENTHAL GMBH
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Systems and methods for treating an opioid-induced adverse pharmacodynamic response
Owner:PURDUE PHARMA LP
Buprenophine analogs
ActiveUS8530494B2Effective analgesiaEffective anti-hyperalgesiaBiocideNervous disorderMedicinal chemistryBuprenorphine
Owner:PURDUE PHARMA LP
Injectable flowable composition comprising buprenorphine
ActiveUS8975270B2Least riskImprove bioavailabilityBiocidePharmaceutical delivery mechanismMetaboliteMUSCLE NECROSIS
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Implantable polymeric device for sustained release of buprenorphine
The present invention provides compositions, methods, and kits for treatment of opiate addiction and pain. The invention provides a biocompatible nonerodible polymeric device which releases buprenorphine continuously with generally linear release kinetics for extended periods of time. Buprenorphine is released through pores that open to the surface of the polymeric matrix in which it is encapsulated. The device may be administered subcutaneously to an individual in need of continuous treatment with buprenorphine.
Owner:TITAN PHARMA
Oral Pharmaceutical Compositions of Buprenorphine and Method of Use
InactiveUS20110097395A1Improve efficiency and qualityDiscourages improper usagePowder deliveryBiocideBuprenorphineDrug
The present invention is directed to oral pharmaceutical compositions of buprenorphine and it pharmaceutically acceptable salts and the use thereof.
Owner:RELMADA THERAPEUTICS
Injectable opioid partial agonist or opioid antagonist microparticle compositions and their use in reducing consumption of abused substances
InactiveUS20030152638A1Avoid prolonged useReadily injected intramuscularlyBiocidePowder deliveryOpioid antagonistControl manner
An injectable slow-release partial opioid agonist or opioid antagonist formulation is provided comprising a partial opioid agonist or opioid antagonist in a poly(D,L-lactide) excipient with a small amount of residual ethyl acetate. Upon intramuscular injection of the composition, a partial opioid agonist or opioid antagonist is released in a controlled manner over an extended period of time. The composition finds use in the treatment of heroin addicts and alcoholics to reduce consumption of the abused substances. Of particular interest are the drugs buprenorphine, methadone and naltrexone.
Owner:EVONIK CORP
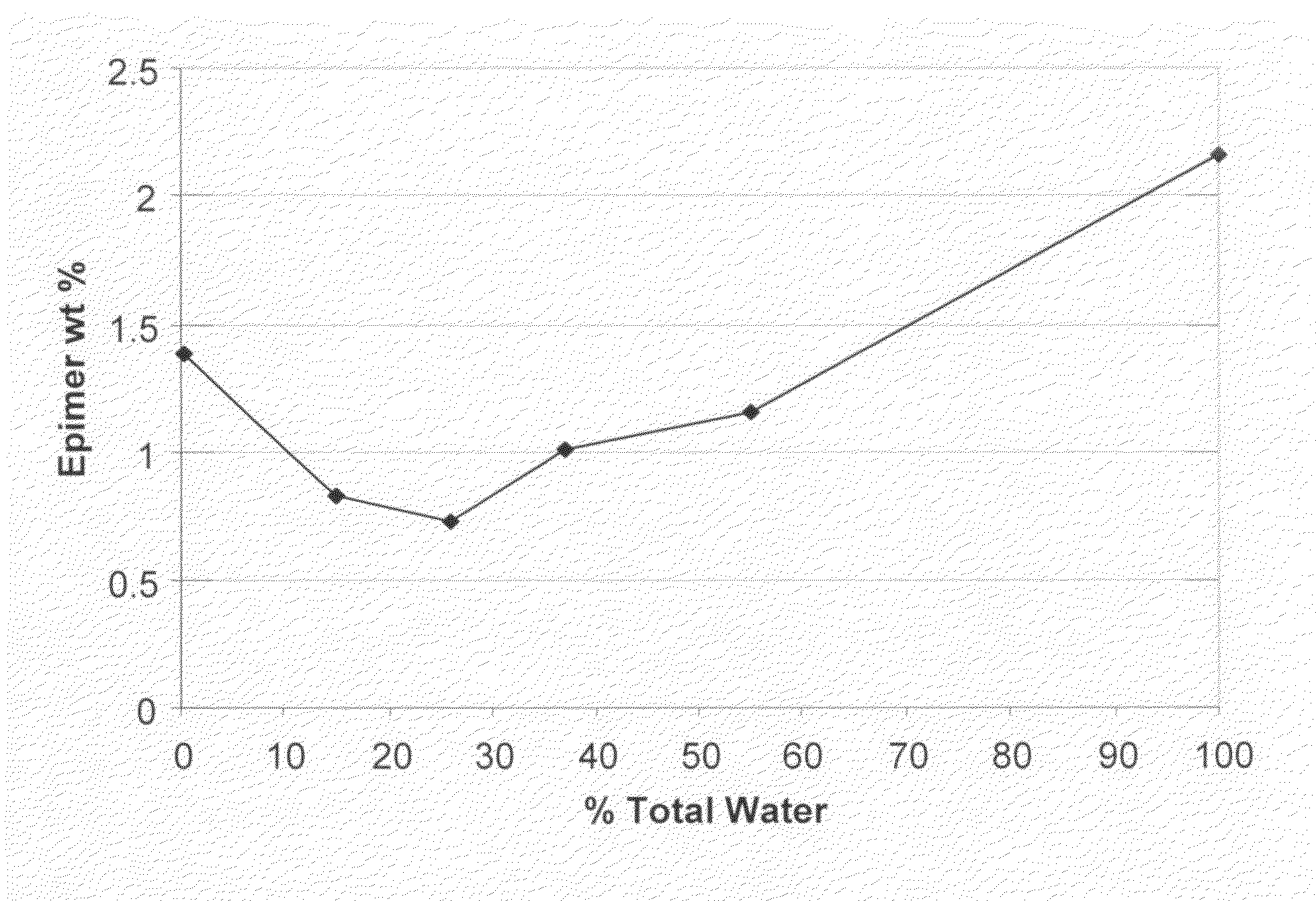
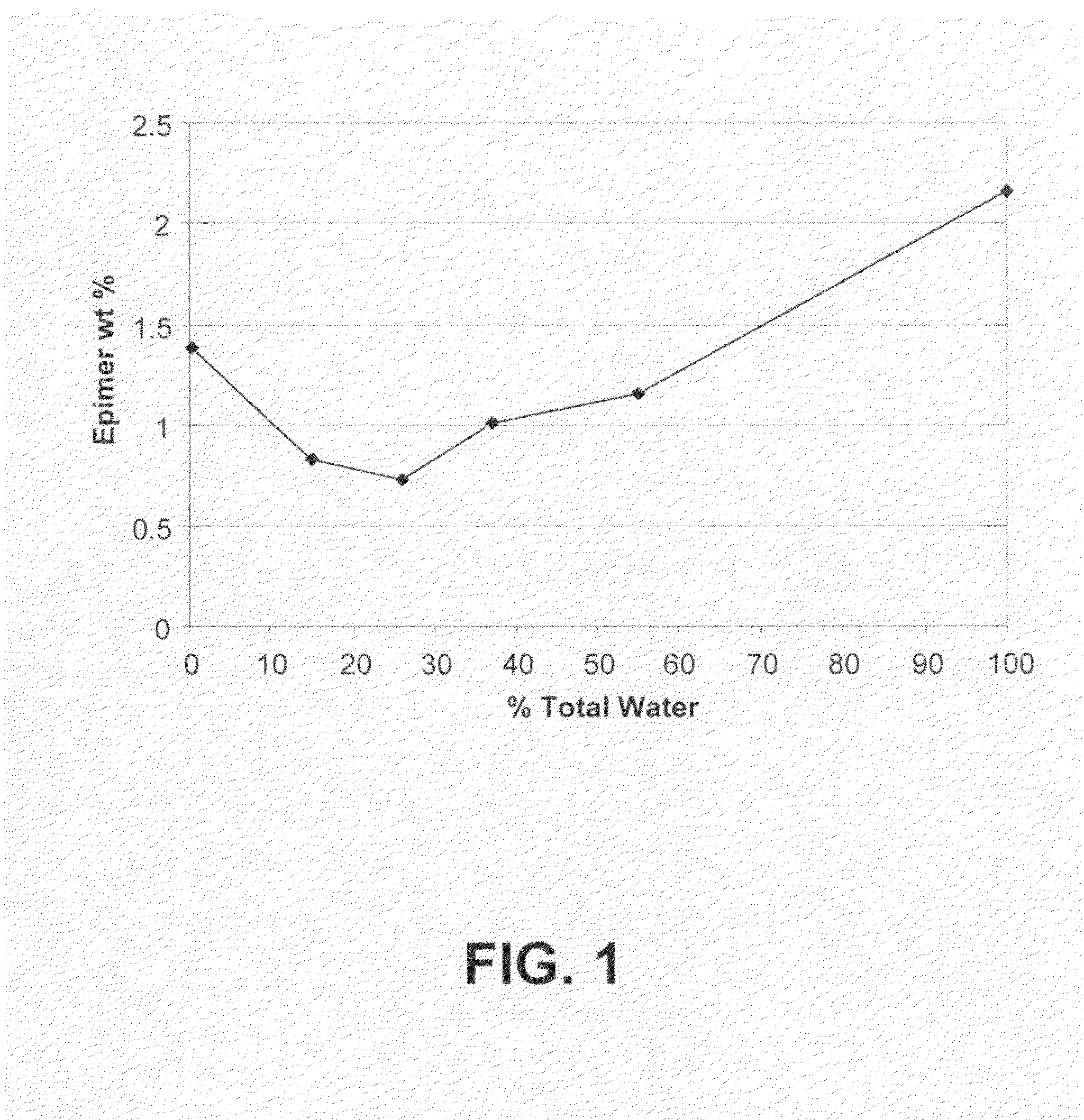
Processes for the production of buprenorphine with reduced impurity formation
The present invention provides process for the production of opiate alkaloids. In particular, the present invention provides processes for the production of buprenorphine or a derivative of buprenorphine that minimizes the formation of impurities.
Owner:SPECGX LLC
Buprenorphine analogs
ActiveUS8969358B2Eliminate the effects ofReduce and prevent constipationBiocideNervous disorderStereoisomerismConstipation
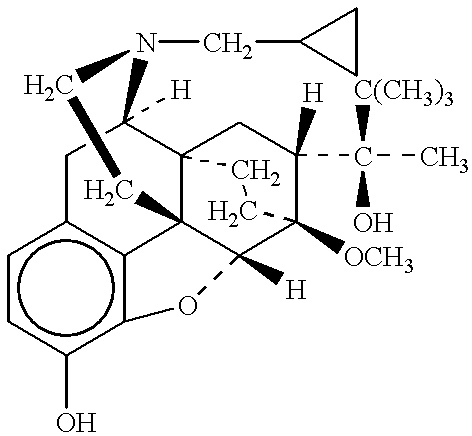
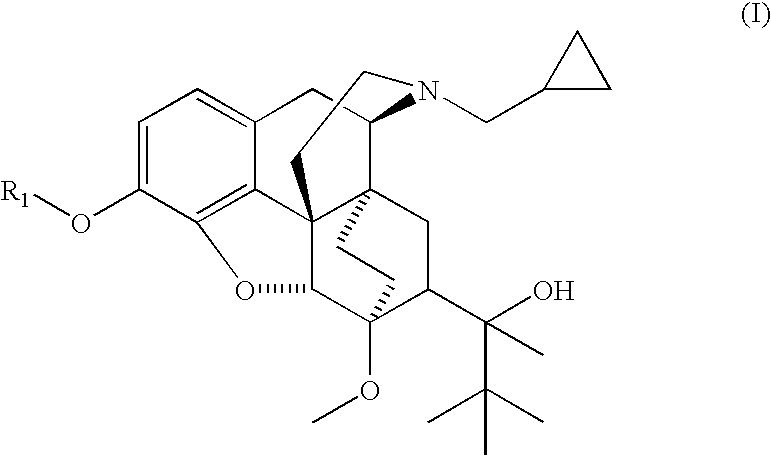
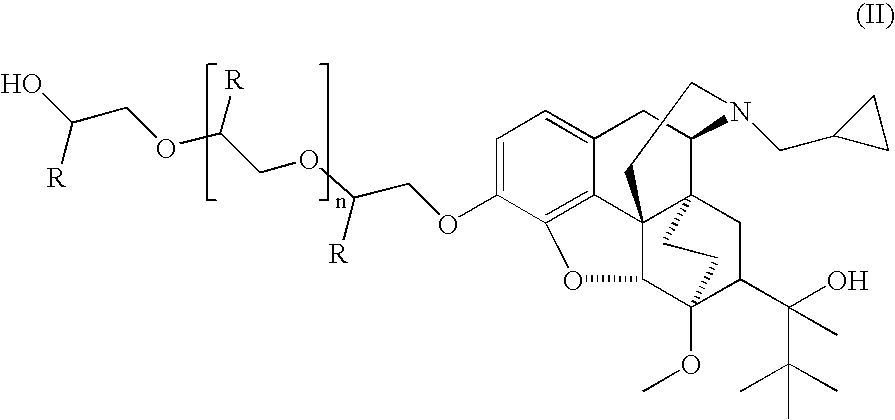
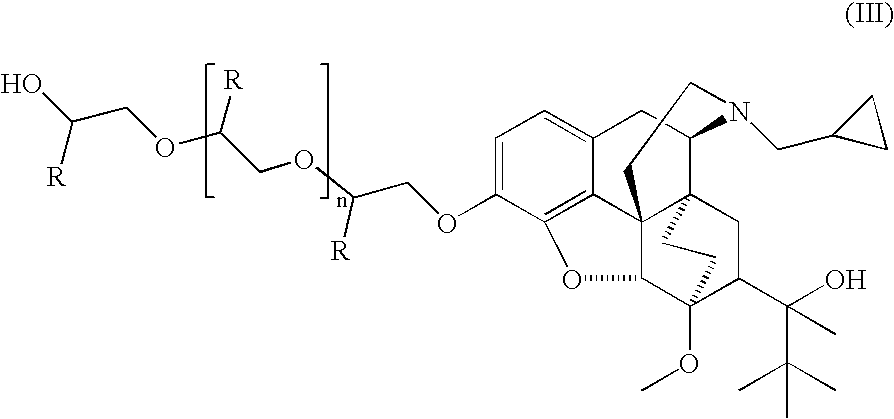
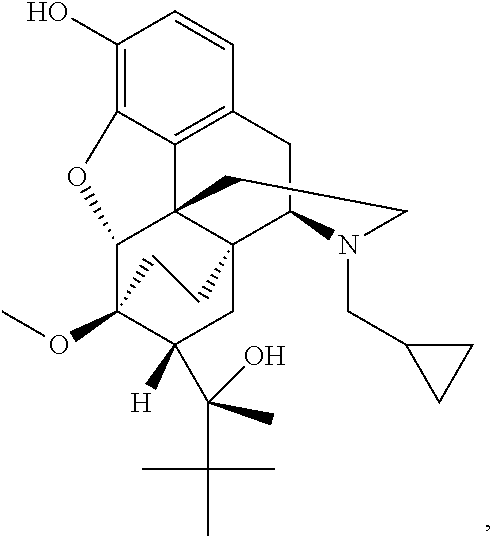
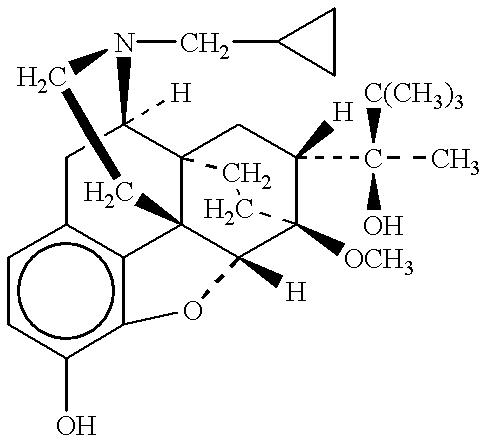
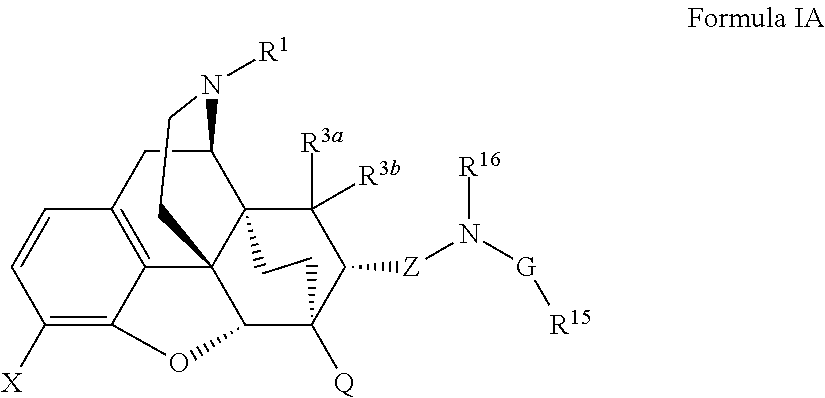
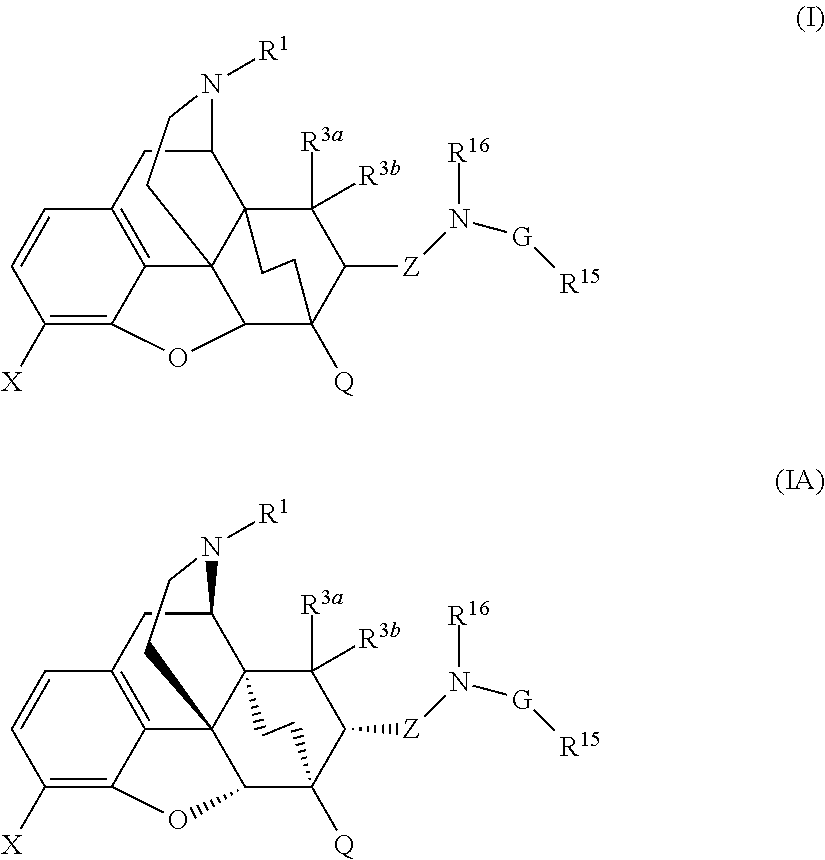
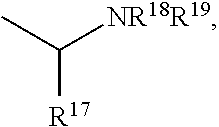
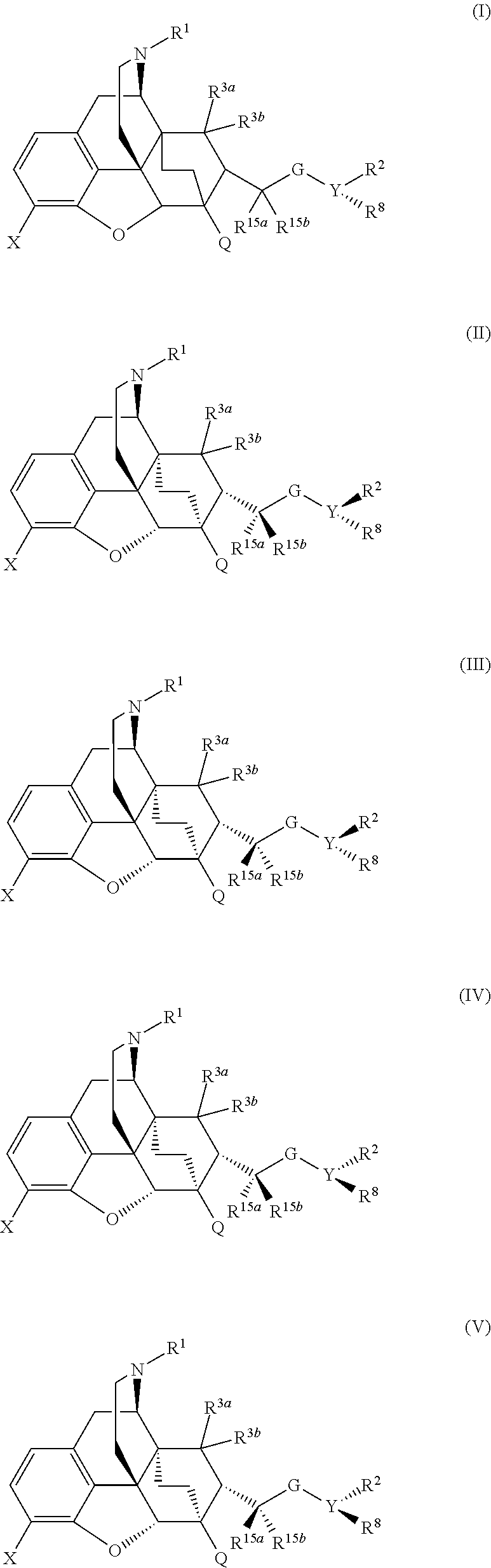
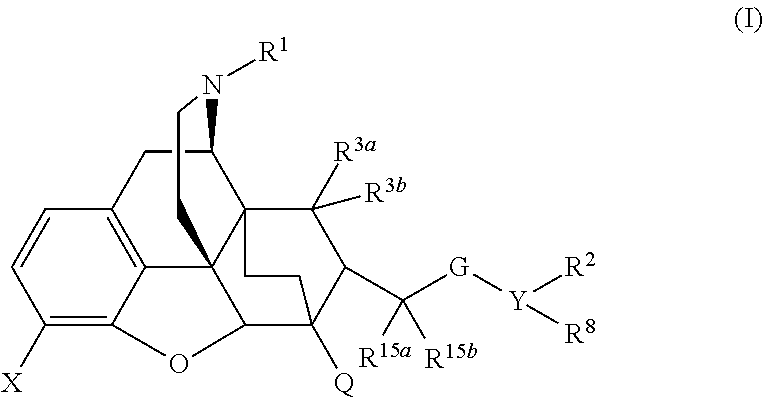
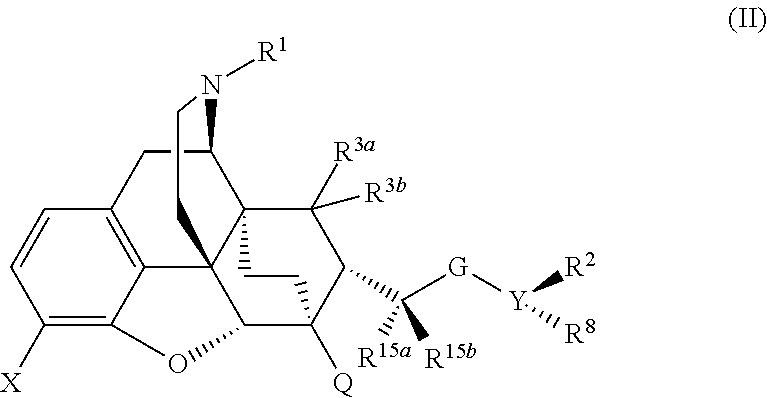
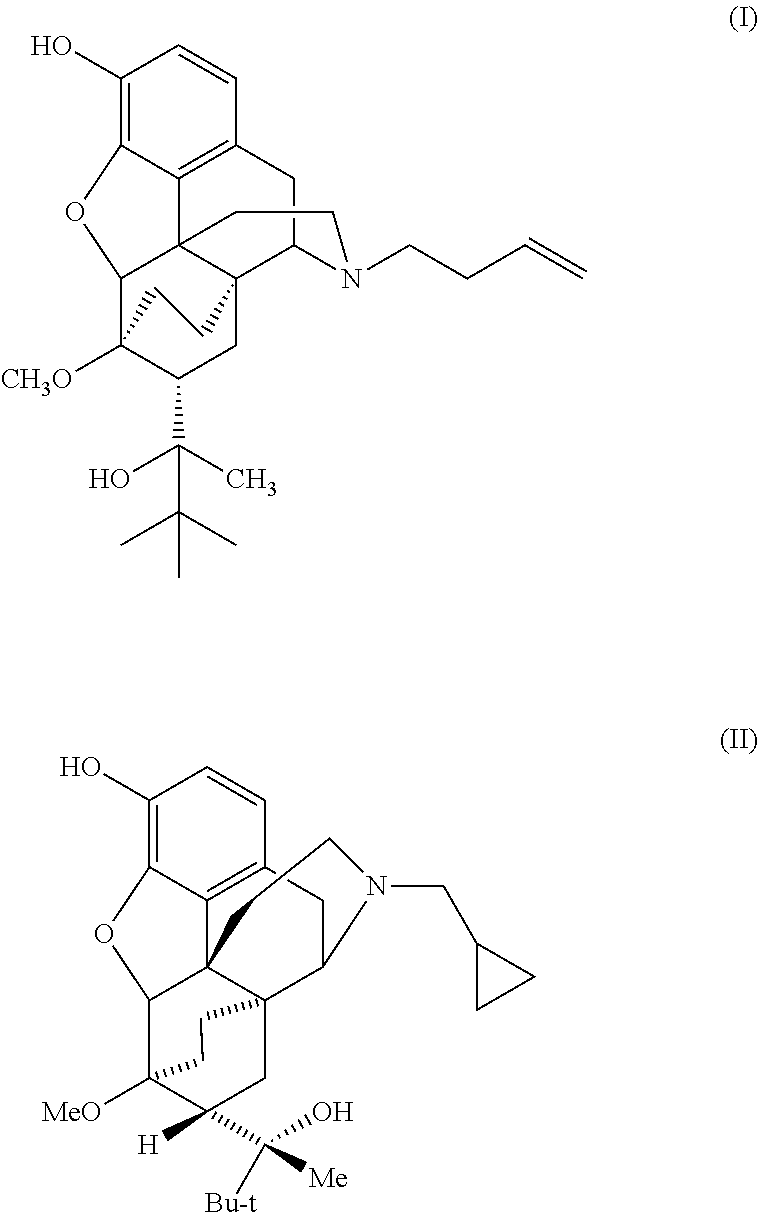
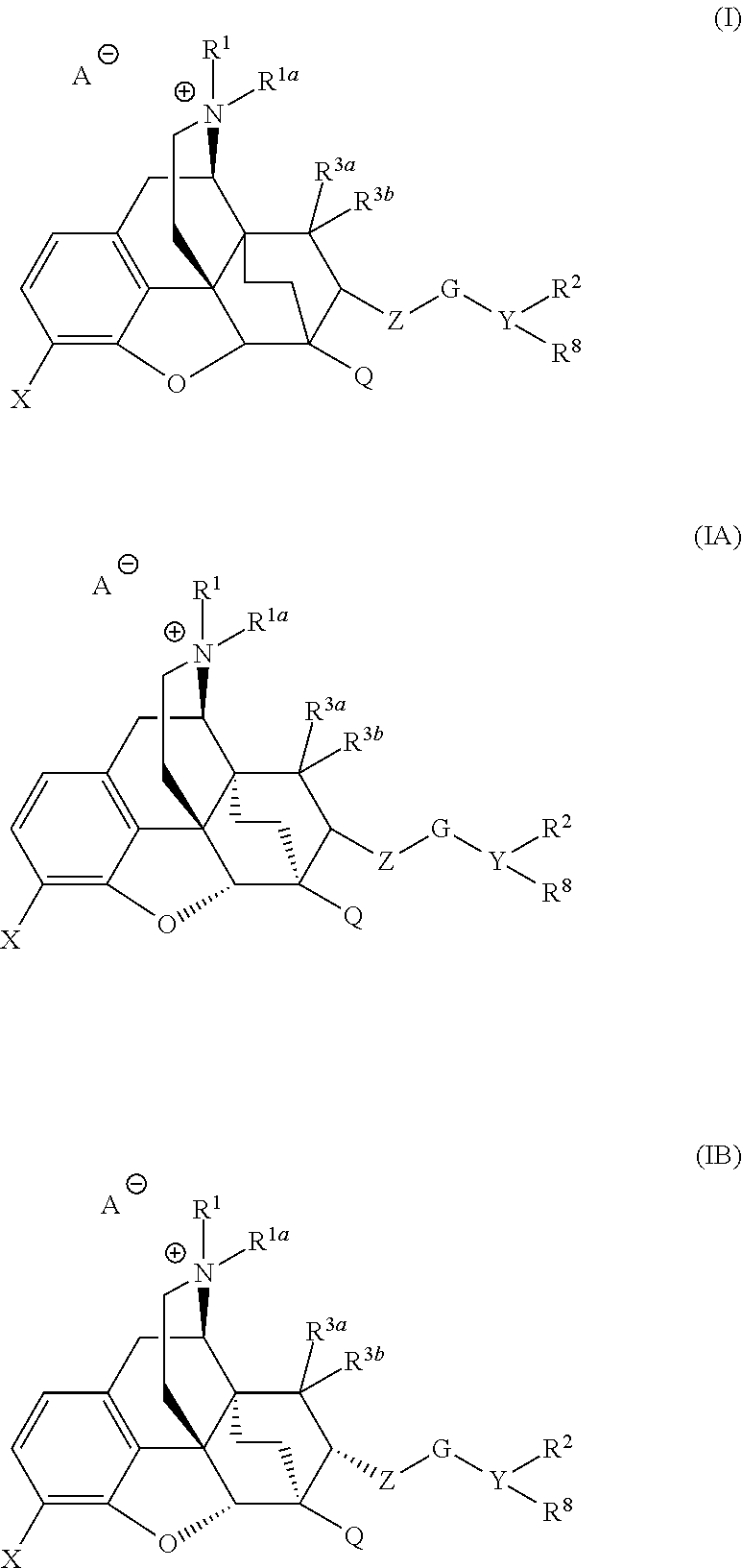
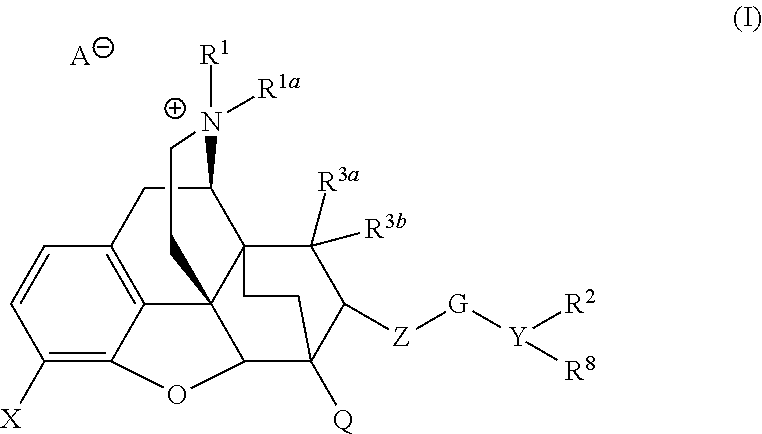
The present invention is directed to Buprenorphine Analog compounds of Formula I, II, III, IV or V and including various stereoisomers (such as Formula IA shown below), wherein R1, R3a, R3b, R16, R15, G, Q, X, A and Z are as defined herein.Compounds of the Invention may be useful for preparing medicaments useful for treating pain, constipation, and other conditions modulated by activity of opioid and ORL-1 receptors. Compounds of the Invention may be useful for treating Conditions such as pain, constipation, and others modulated by activity of opioid and ORL-1 receptors.
Owner:PURDUE PHARMA LP
Novel buprenorphine ester derivatives and process for preparing the same and medical compounds with long-acting analgesic effect
InactiveCN1500786AExtended duration of actionImprove securityOrganic active ingredientsNervous disorderMedicineAlkaloid
The present invention discloses novel monocarboxilic ester derivative of buprenorphine and dicarboxilic ester derivative of dibuprenorphine, which exhibit ever lasting pain relieving effect than the hydrochloride of buprenorphine. The present invention discloses also the synthesis process of these novel buprenorphine ester derivatives and long-acting pain relieving medicinal composition containing compound selected from buprenorphine alkaloid and these novel buprenorphine ester derivatives.
Owner:CHI MEI MEDICAL CENT
Treatment of dependence withdrawal
ActiveUS20060240085A1Alleviate withdrawal symptomsBiocideNervous disorderRegimenDrug withdrawal syndrome
Dosage regimens of buprenorphine to treat withdrawal or abstinence syndrome in a drug dependent or opioid tolerant patient who is pregnant are described. The method includes treating withdrawal or abstinence syndrome of the patient by transdermal administration of an amount of buprenorphine effective to reduce withdrawal symptoms. For example, a first buprenorphine-containing transdermal dosage form for a second dosing period that is no more than about 5 days, the second dosage form comprising the same or a greater dosage of buprenorphine than the first dosage form; and a third buprenorphine-containing transdermal dosage form for a third dosing period that is at least 2 days, the third dosage form comprising the same or a greater dosage of buprenorphine than the second dosage form.
Owner:PURDUE PHARMA LP
Buprenorphine analogs
ActiveUS20140163058A1Reduce and prevent constipationReduction and prevention of constipationBiocideNervous disorderOpioid receptorMedicinal chemistry
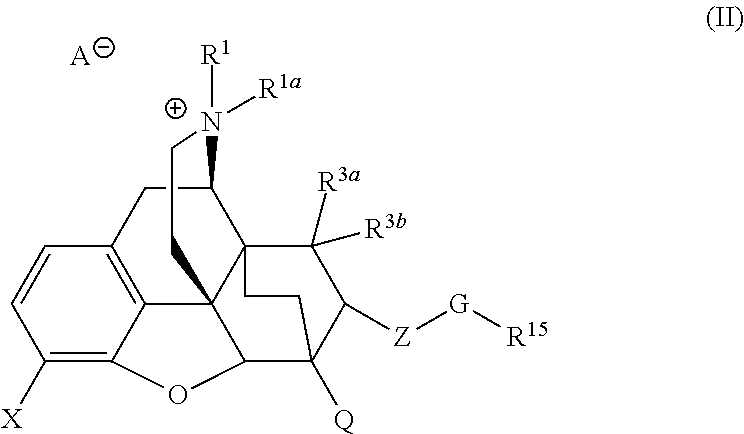
The present invention is directed to Buprenorphine Analog compounds of the Formula I, Formula II, Formula III, Formula IV, and Formula V, wherein R1, R2, R3a, R3b, R15a, R15b, X, Q, G, and Y are as defined herein.Compounds of the Invention are useful for treating pain and other conditions modulated by activity of opioid receptors.
Owner:PURDUE PHARMA LP
Method for the Enrichment of Buprenorphine using Chromatographic Techniques
The present invention provides processes for the enrichment of buprenorphine in a product. In particular, the present invention provides processes for the enrichment of buprenorphine in a product using chromatographic techniques.
Owner:SPECGX LLC
Quarternized buprenorphine analogs
ActiveUS9096606B2Eliminate the effects ofReduce and prevent constipationOrganic active ingredientsNervous disorderMedicinal chemistryBuprenorphine
Owner:PURDUE PHARMA LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com