Implantable polymeric device for sustained release of buprenorphine
a polymer device and buprenorphine technology, applied in the direction of biocide, prosthesis, drug composition, etc., can solve the problems of unsuitable use as a take-home medication, poor compliance with the dosing scheme, and inability to tolerate enteral drug delivery in patients with particular indications,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0040] Materials
[0041] The following materials were used: [0042] Buprenorphine HCl, 99.3%, supplied by Macfarlan Smith Limited, Wheatfield Road, Edinburgh, EH 11 2QA [0043] Buprenorphine base, supplied by Diosynth B.V. Vlijtseweg 130, 7317 AK Apeldoorn, The Netherlands [0044] Buprenorphine HCl, supplied by Diosynth B.V. Vlijtseweg 130, 7317 AK Apeldoorn, The Netherlands [0045] Methanol-ChromAR, HPLC grade, supplied by Mallinckrodt, St. Louis, Mo. [0046] Acetonitrile, HPLC grade, supplied by Mallinckrodt, St. Louis, Mo. [0047] Sodium lauryl sulfate, 99%, supplied by Sigma Chemicals, St. Louis, Mo. [0048] EVA, 33% vinyl acetate, supplied by Equistar Chemicals, LP, Houston, Texas [0049] Tower Plasti-Peel pouches, Catalog #30307PN, supplied by DRG Medical Packaging, Mundelein, Ill.
Preparation of Implantable Devices
[0050] Buprenorphine is available as a salt (HCl) and as a free base. The implantable device is intended to release drug over a 3-month period or lon...
example 2
In Vitro Characterization of Extruded Implantable Devices
[0061] Extruded rods prepared as described above were characterized for total drug load and for rate of drug release.
Assessment of Drug Loading
[0062] Extruded rods were accurately weighed, placed in tightly closed borosilicate glass jars containing 50 ml of methanol, and continuously stirred at room temperature. Sample aliquots were removed periodically and analyzed by HPLC. Initial experiments indicated that the incorporated compounds were completely extracted within 24 hr. Irradiated and nonirradiated rods were compared side by side.
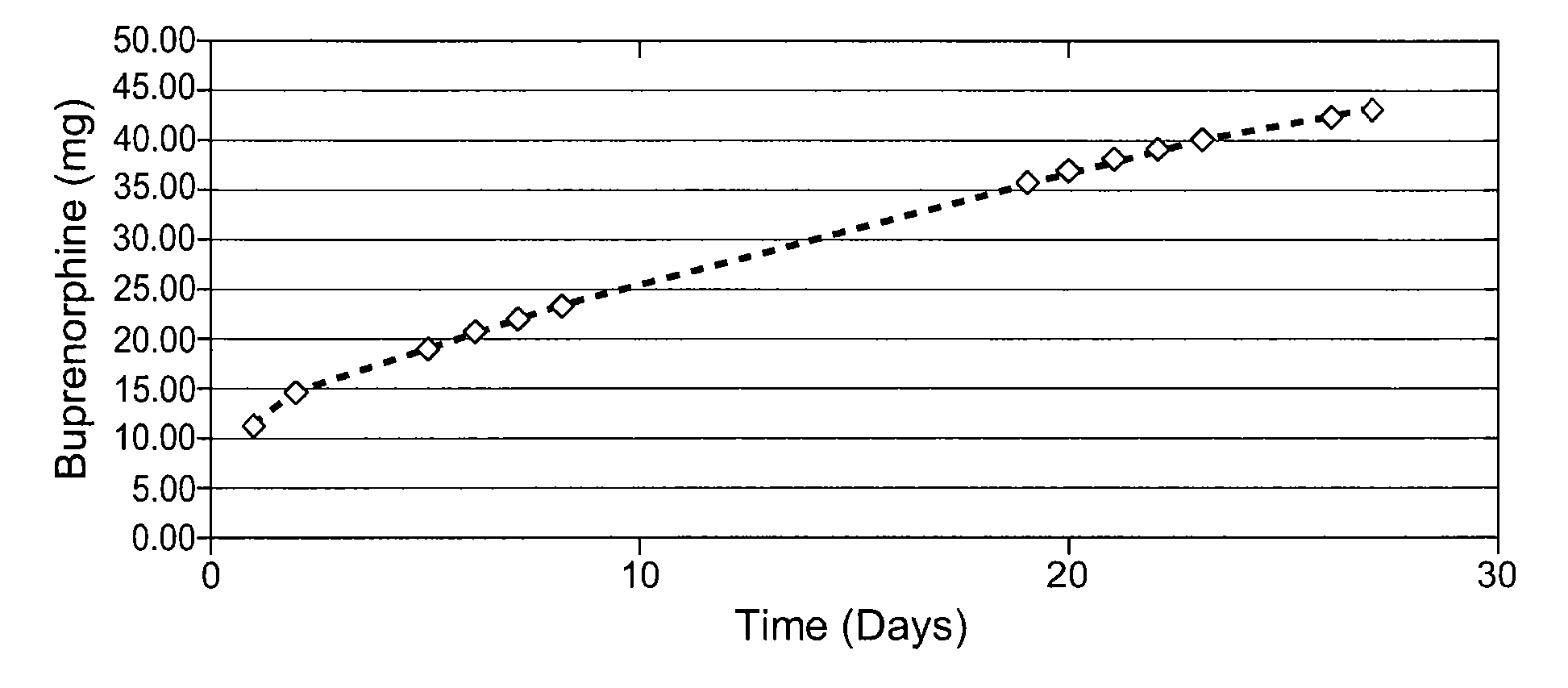
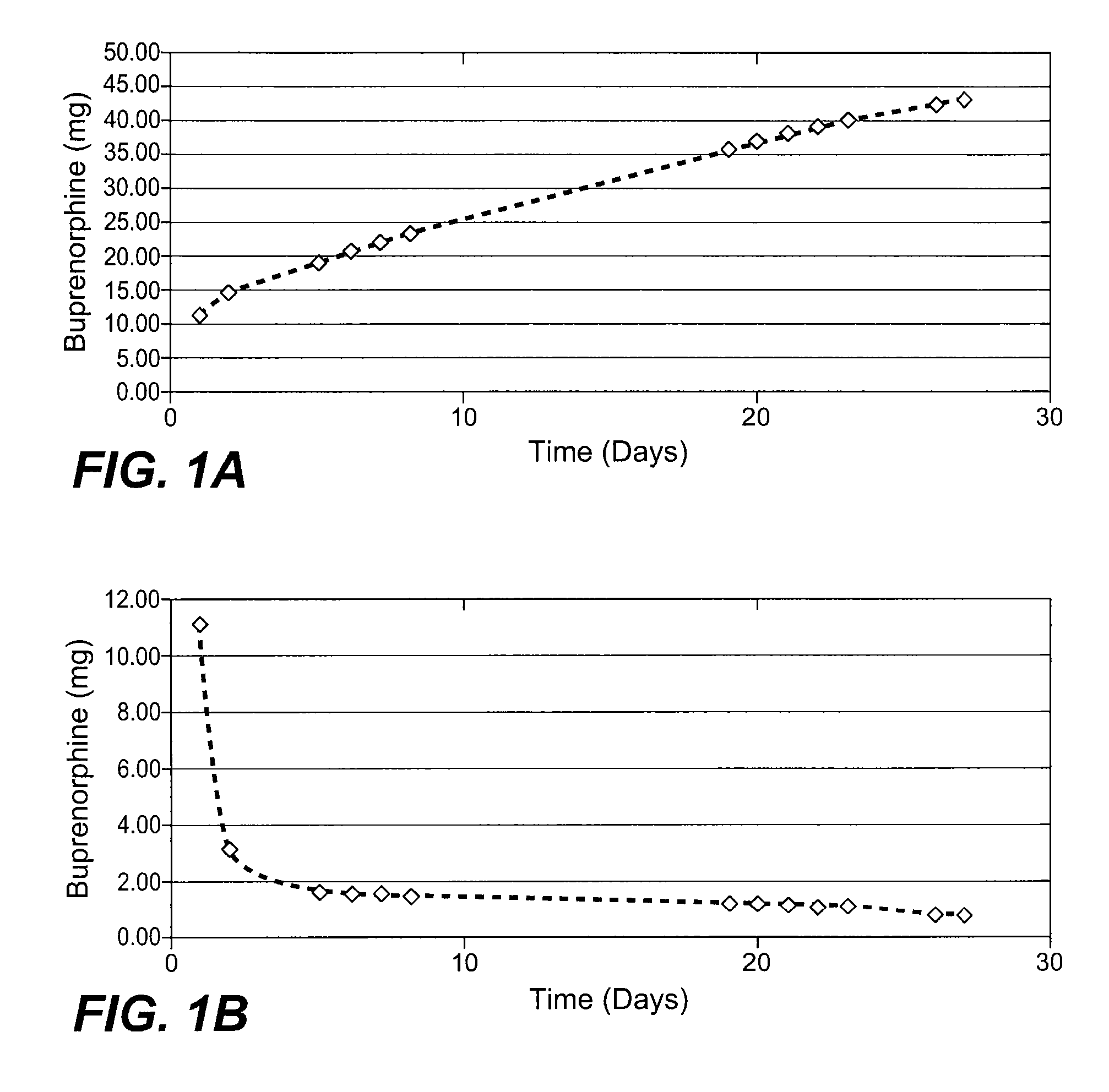
[0063] Drug loading studies indicated that the physical mixtures were homogeneous and contained the estimated quantities of buprenorphine. Table 1 shows the recovery for the free base (FB) and HCl salt at 50% loading. Table 2 shows the recovery of buprenorphine HCl at 75% loading, and indicates that the average drug load before and after gamma radiation was>75%.
TABLE 1Recovery of buprenorp...
example 3
In Vivo Evaluation of Drug Loaded Implantable Devices
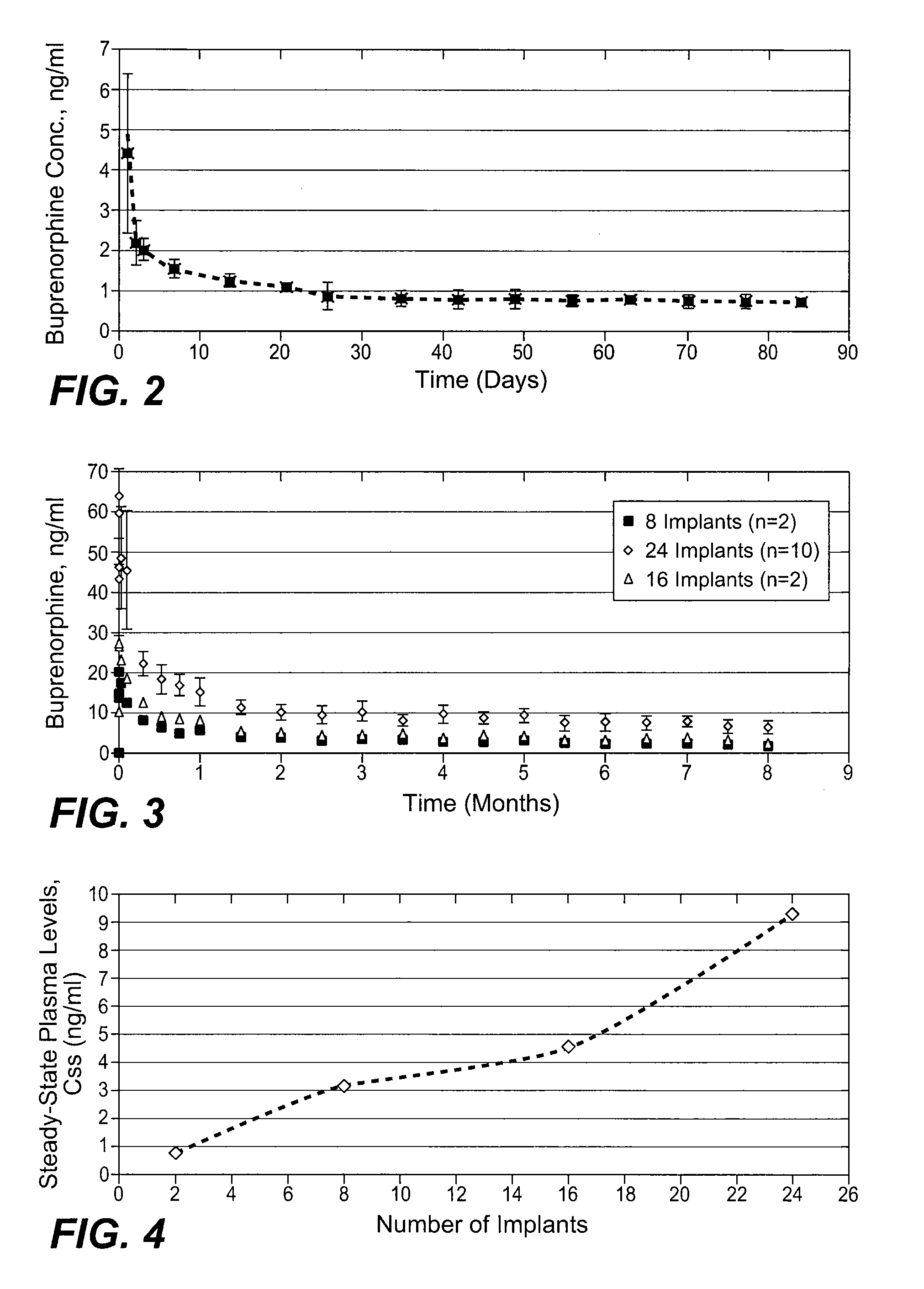
[0067] Three female beagle dogs were administered two sterilized implantable devices each containing 75% buprenorphine HCl in EVA containing 33% vinyl acetate, as described above (2 mm diameter×2.6 cm length rods). Implantable devices were administered by subcutaneous injection to the dorsal region of each dog using a trocar. Blood samples were withdrawn periodically over a period of 84 days and frozen. The frozen samples were analyzed by LC / MS / MS for content of buprenorphine and norbuprenorphine (the major metabolite).
[0068] After an initial burst, the mean buprenorphine plasma levels were maintained around 0.75-1.0 ng / ml through day 84. The mean buprenorphine and norbuprenorphine levels were similar in all three dogs. The peak concentration of buprenorphine in the plasma ranged from 2.41 to 6.32 ng / ml. After the initial burst effect, a slow decrease in plasma concentration of buprenorphine was observed for all the dogs. On day...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com