Patents
Literature
669 results about "Pain duration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Others apply acute to pain that lasts less than 30 days, chronic to pain of more than six months' duration, and subacute to pain that lasts from one to six months. A popular alternative definition of chronic pain, involving no arbitrarily fixed durations, is "pain that extends beyond the expected period of healing".
Methods and compositions for the treatment of pain and other alpha 2 adrenergic-mediated conditions
InactiveUS20050059744A1Improve effectivenessHigh activityBiocideOrganic active ingredientsSedative EffectsSide effect
Owner:ALLERGAN INC
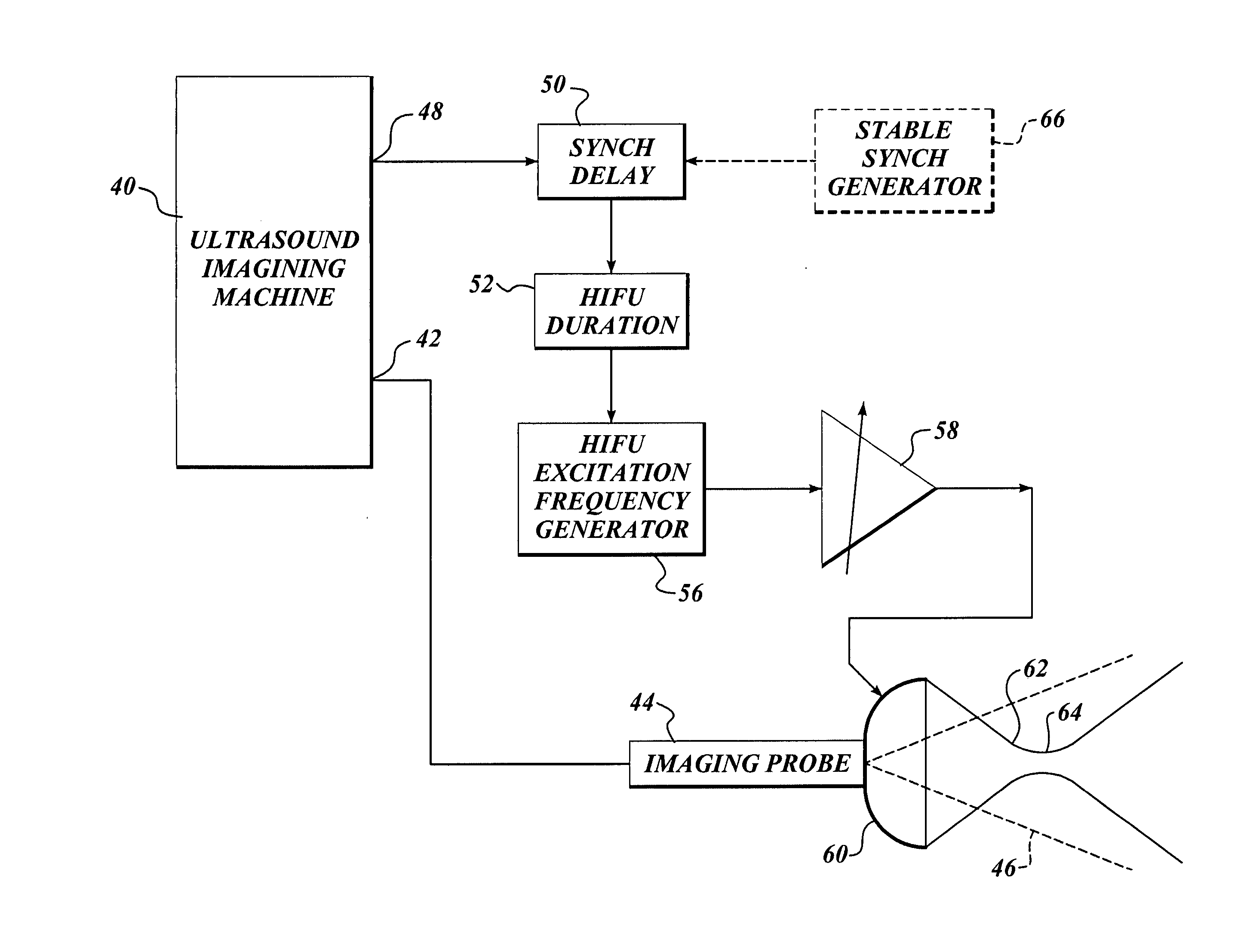
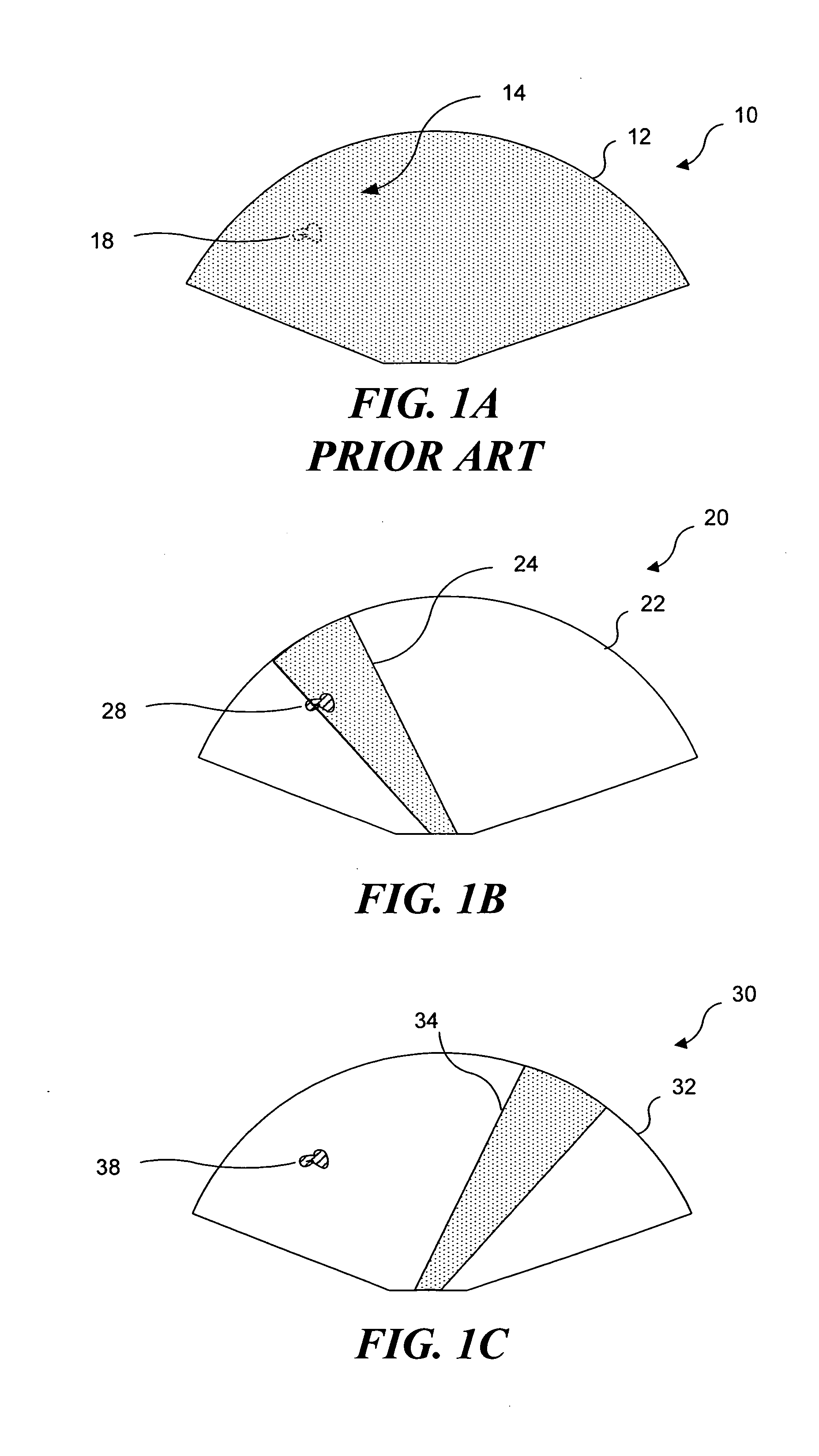
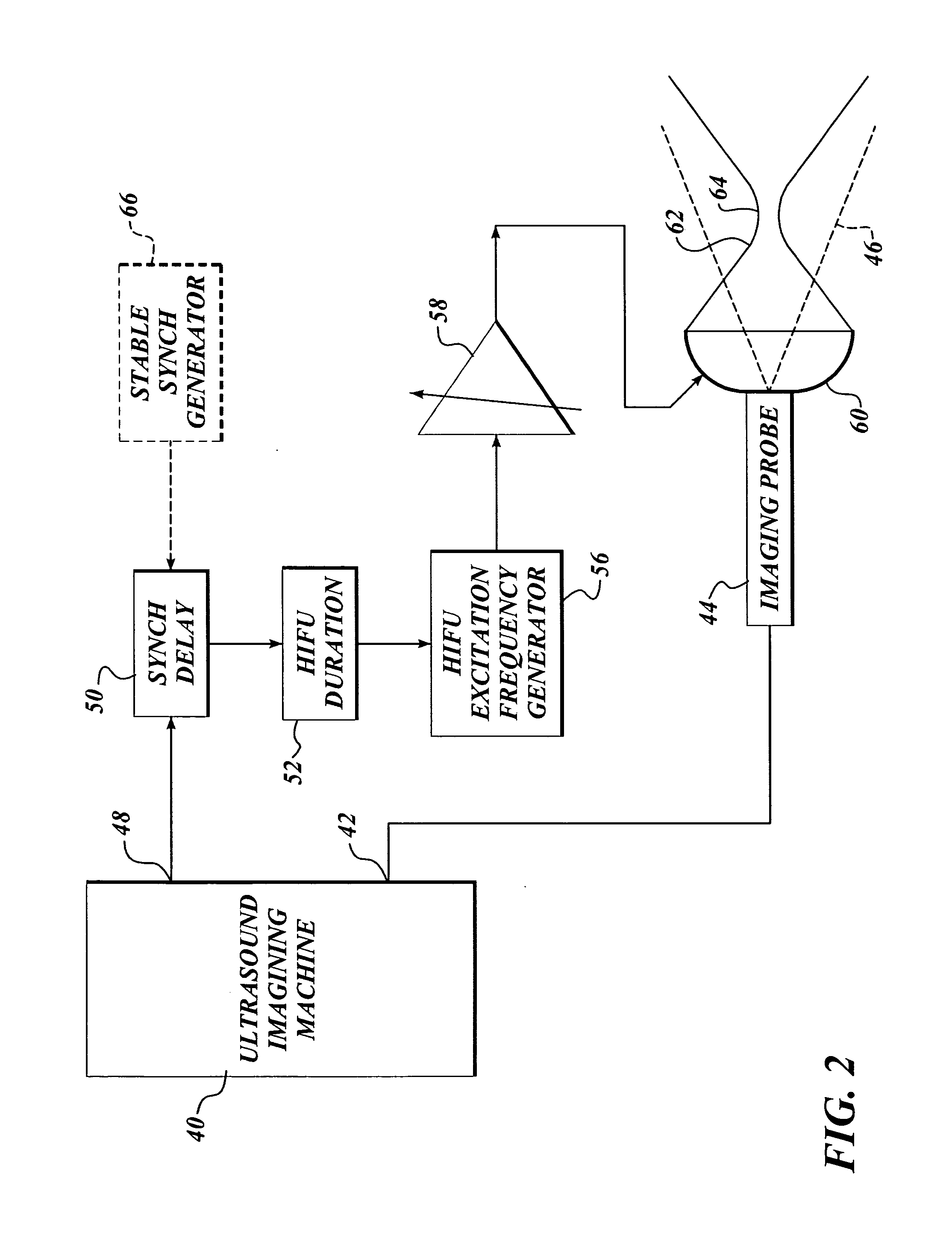
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS20050240126A1Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesAbnormal tissue growthHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
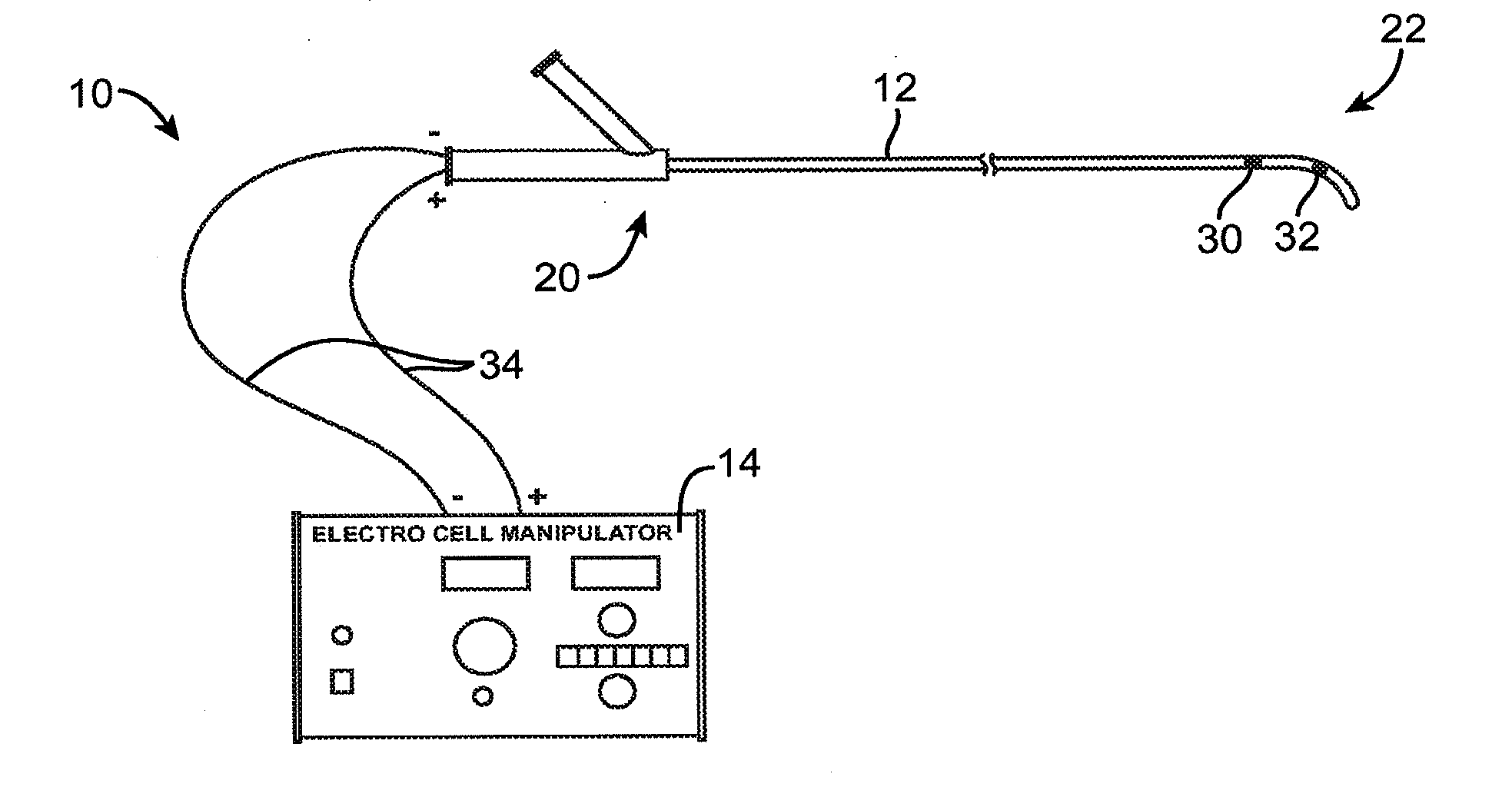
Systems and methods for neuromodulation for treatment of pain and other disorders associated with nerve conduction
ActiveUS20100010567A1Good effectSusceptible to injurySpinal electrodesSurgical needlesDiseaseConduction pathway
Methods and apparatus are provided for selective destruction or temporary disruption of nerves and / or conduction pathways in a mammalian body for the treatment of pain and other disorders. Apparatus comprises catheters having electrodes for targeting and affecting nerve tissue at a cellular level to reversible and irreversible nerve poration and incapacitation.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Microneedle devices and microneedle delivery apparatus
InactiveUS20050261631A1Reduces tip fractureEffective perforationSurgical needlesMicroneedlesStratum corneumPain experience
Microneedle devices with microneedles having a truncated tapered shape are disclosed. The microneedles of microneedle devices may also have a controlled aspect ratio. Microneedle delivery apparatus are disclosed that include drivers designed to deliver microneedles at velocities that may enhance perforation of the stratum corneum while limiting the sensation of pain experienced at the delivery site.
Owner:3M INNOVATIVE PROPERTIES CO
Drug depot implant designs and methods of implantation
ActiveUS20070243225A1Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
Implantable system enabling responsive therapy for pain
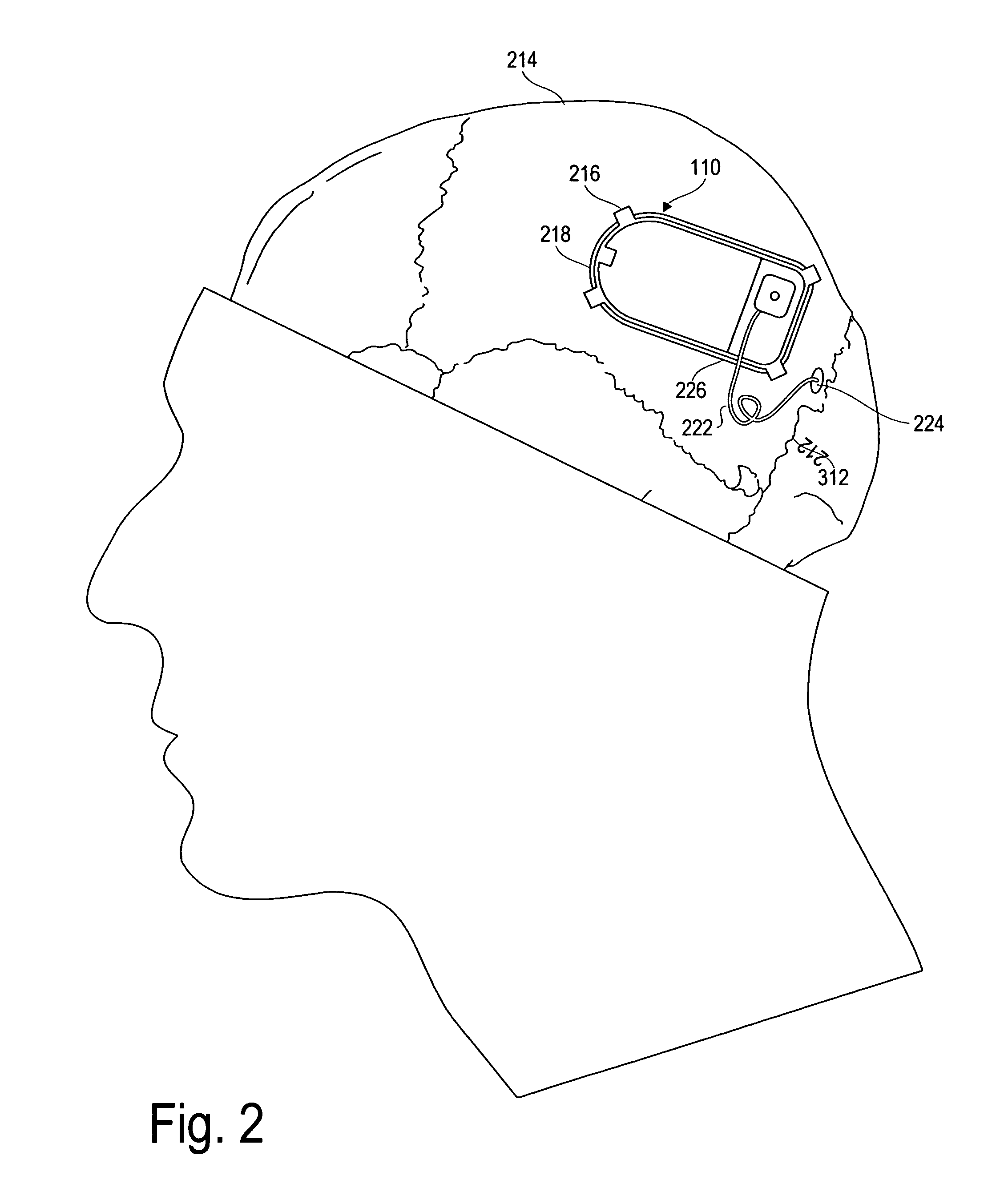
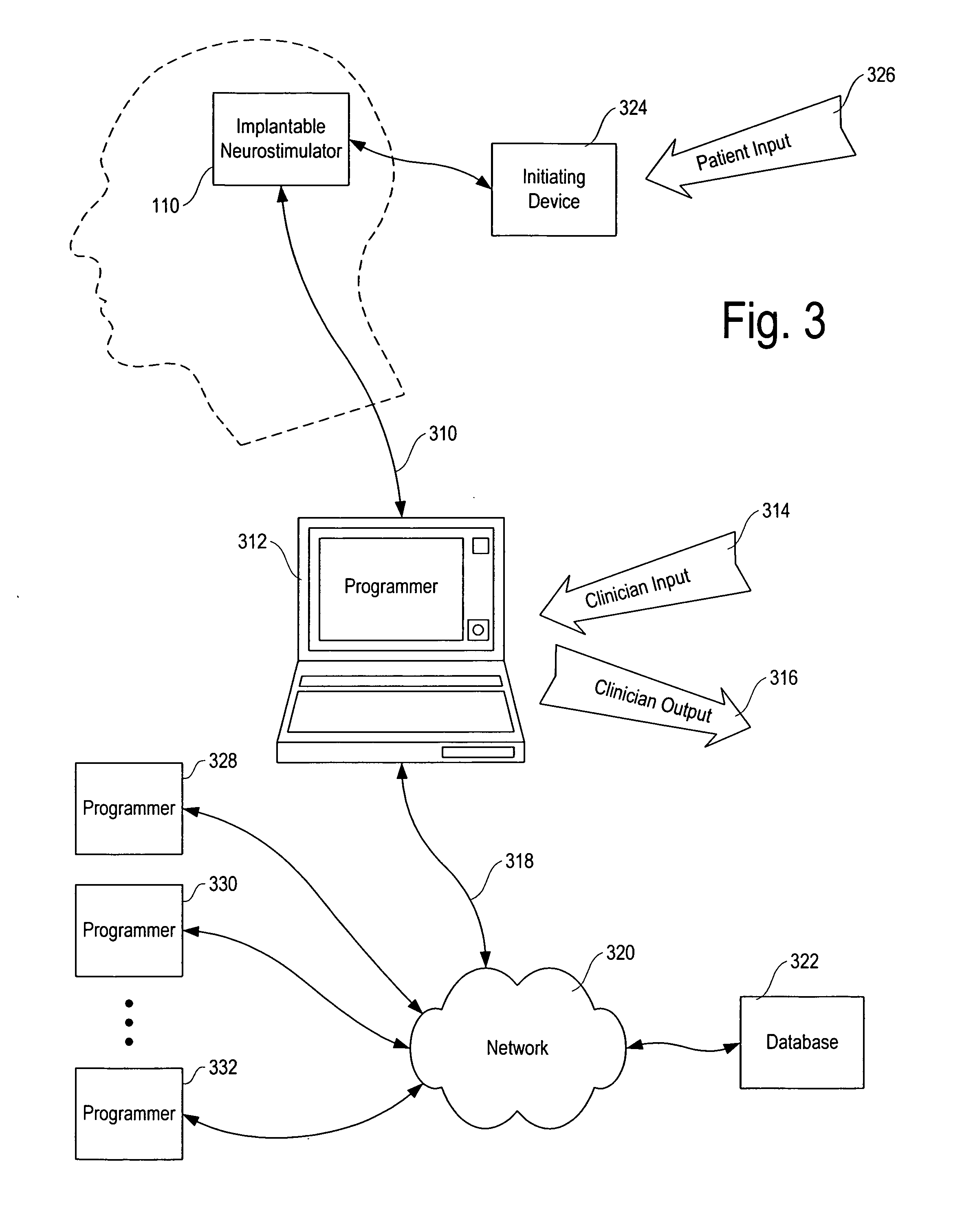
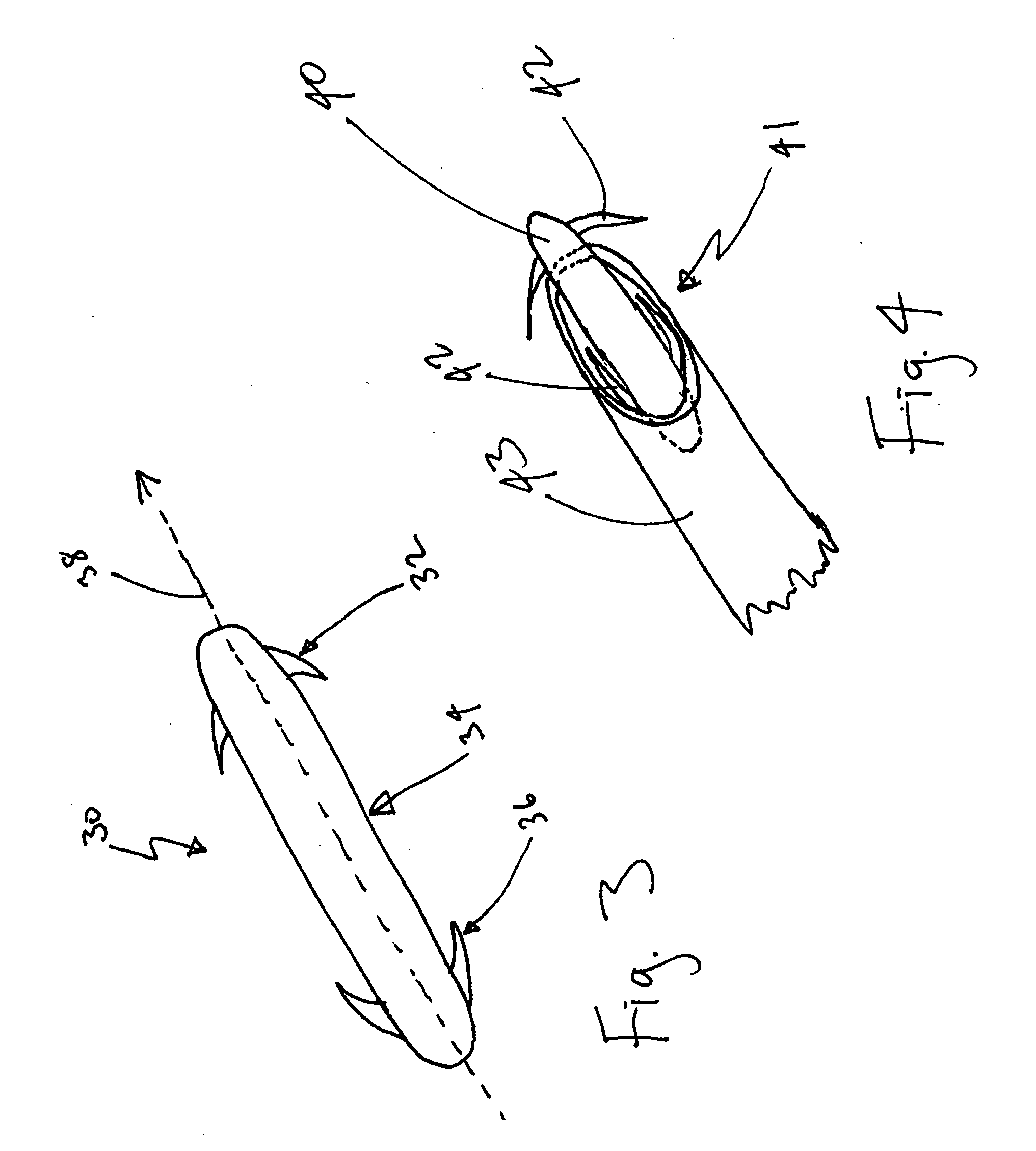
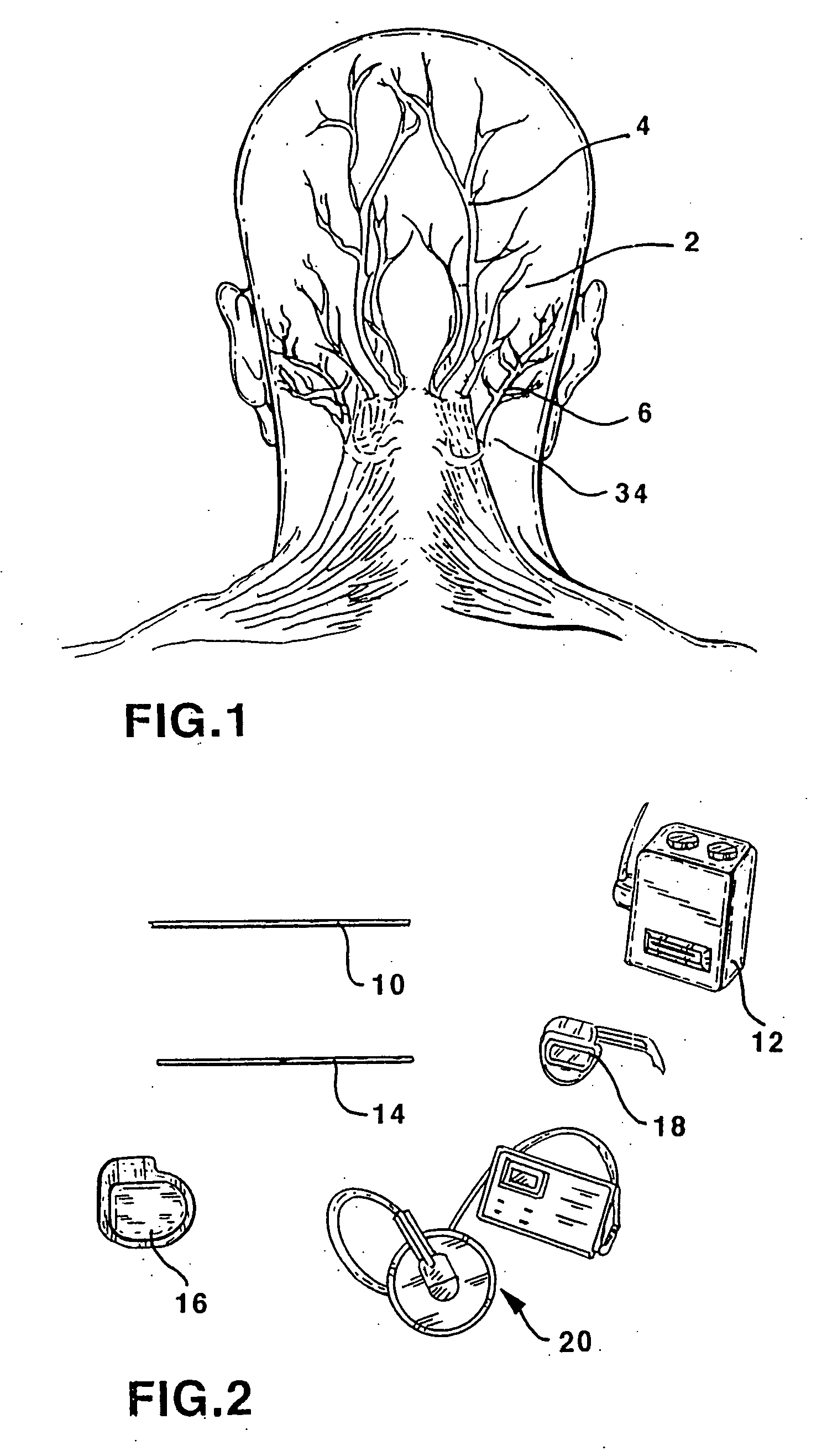
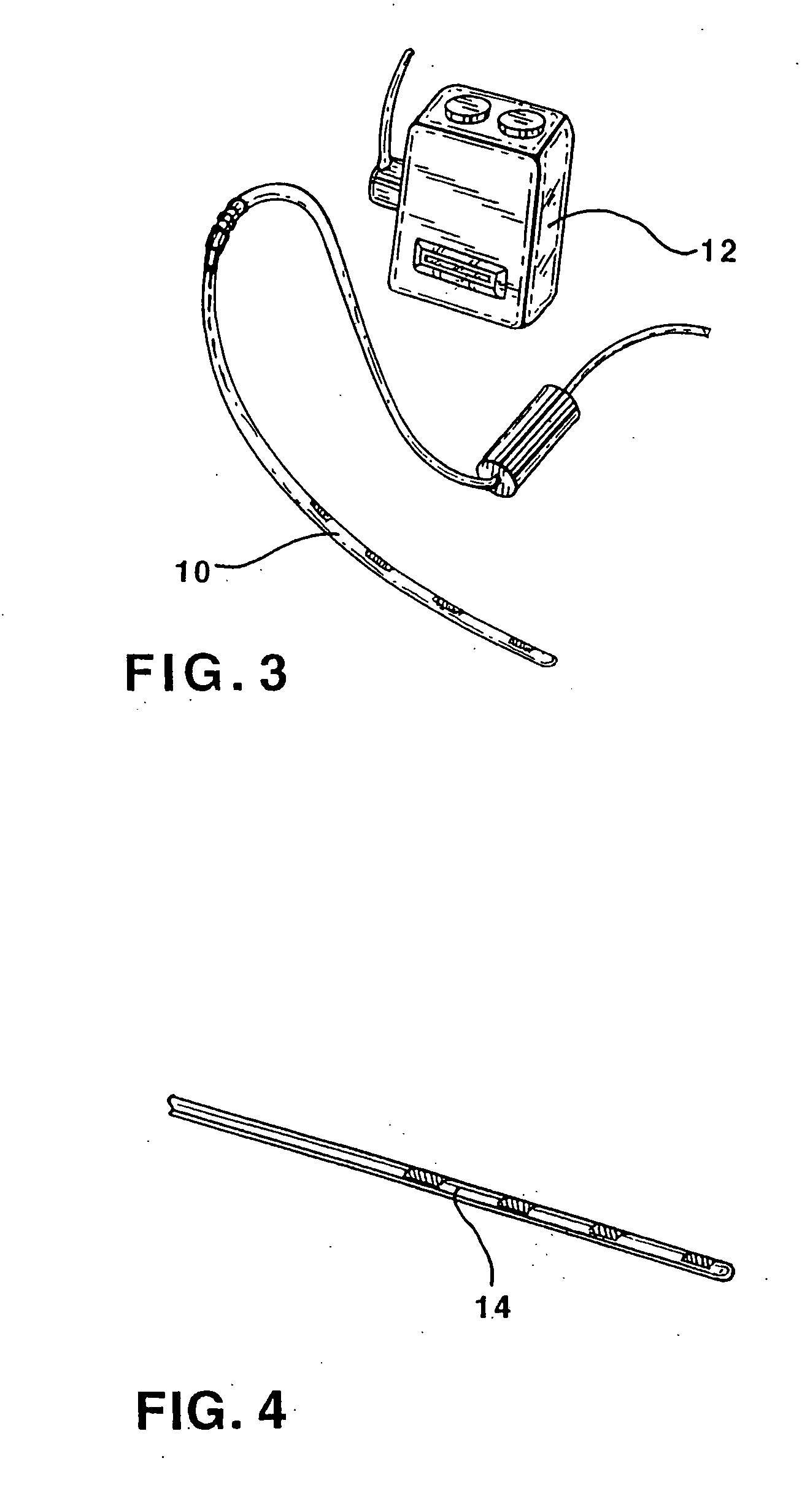
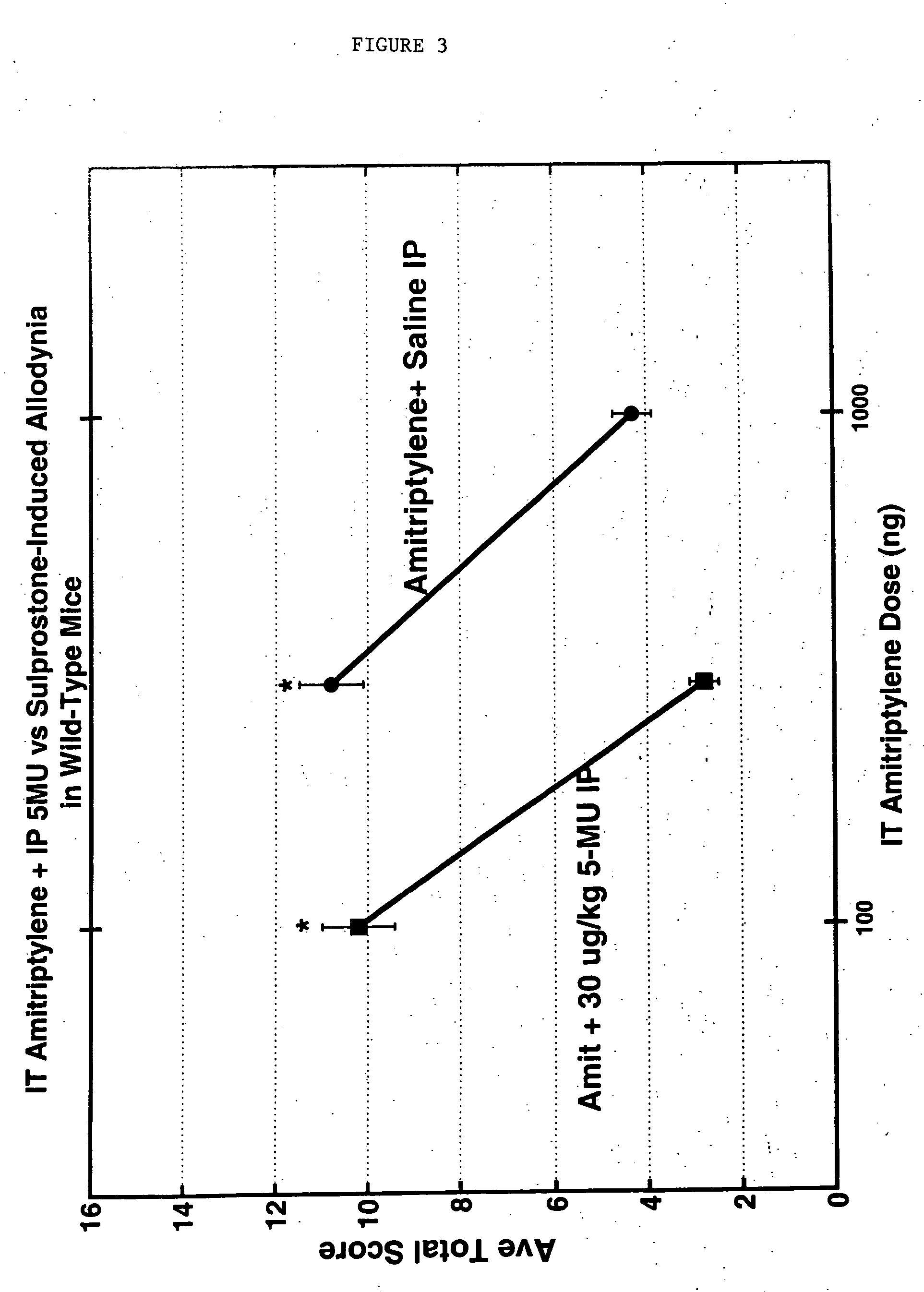
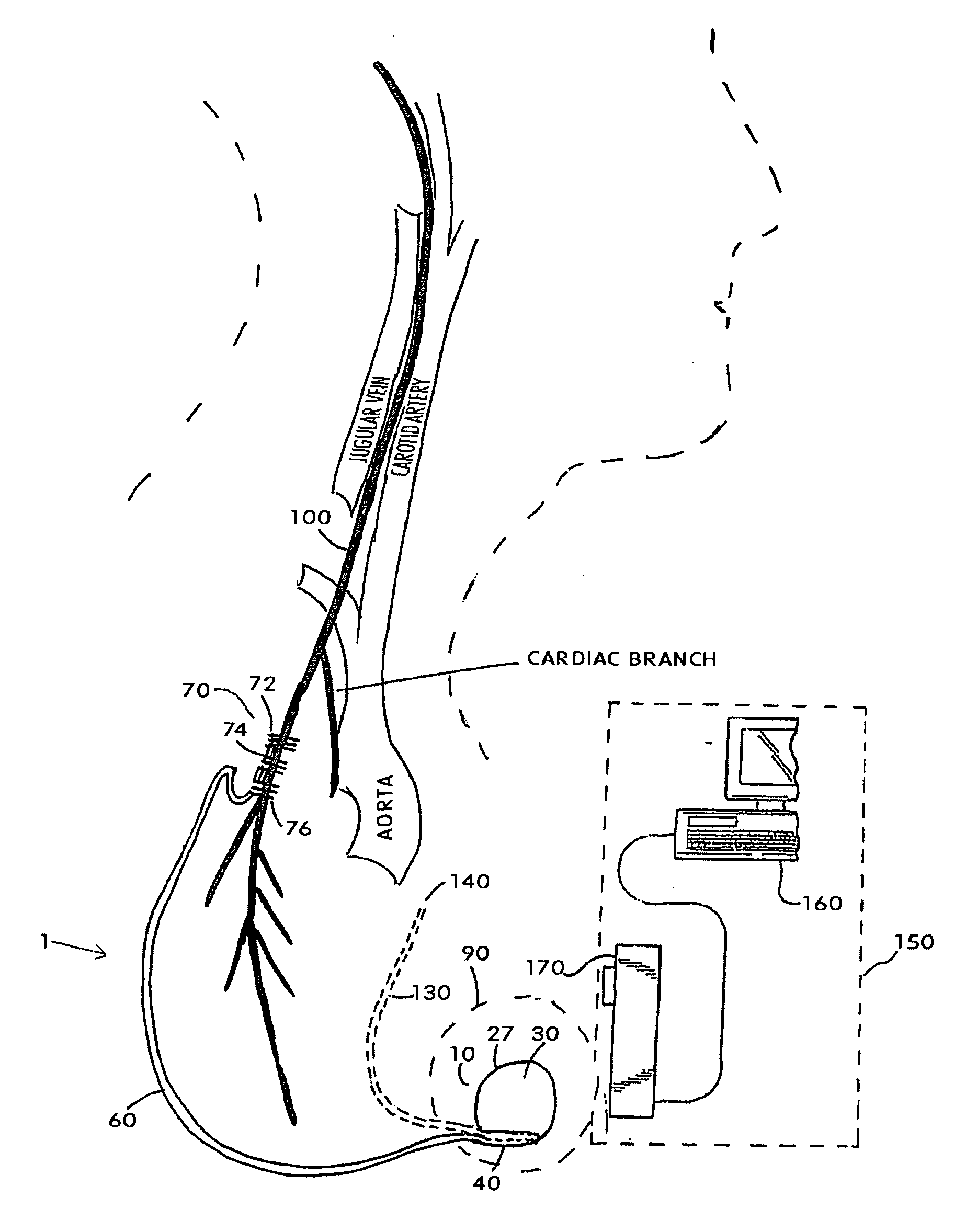
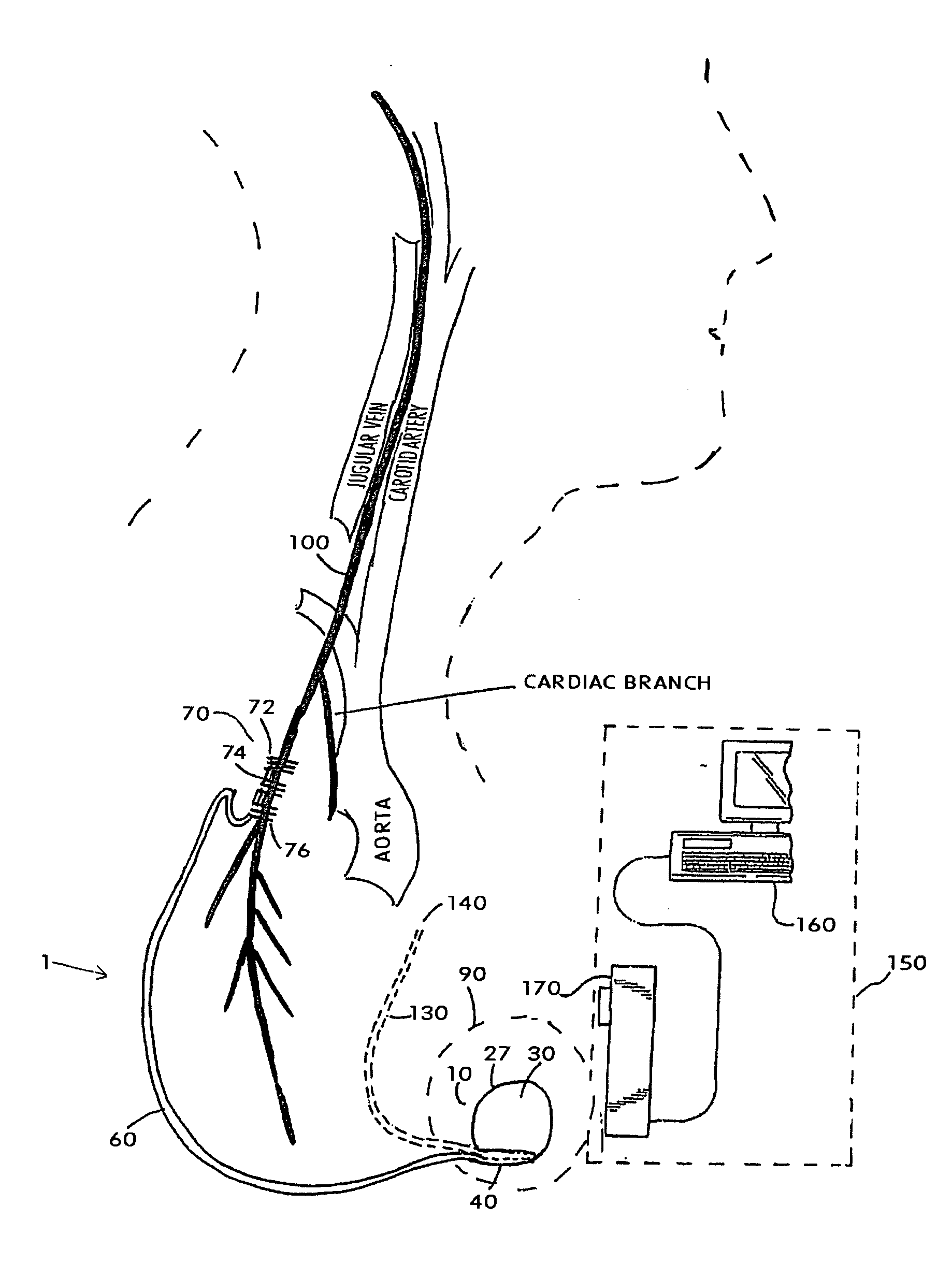
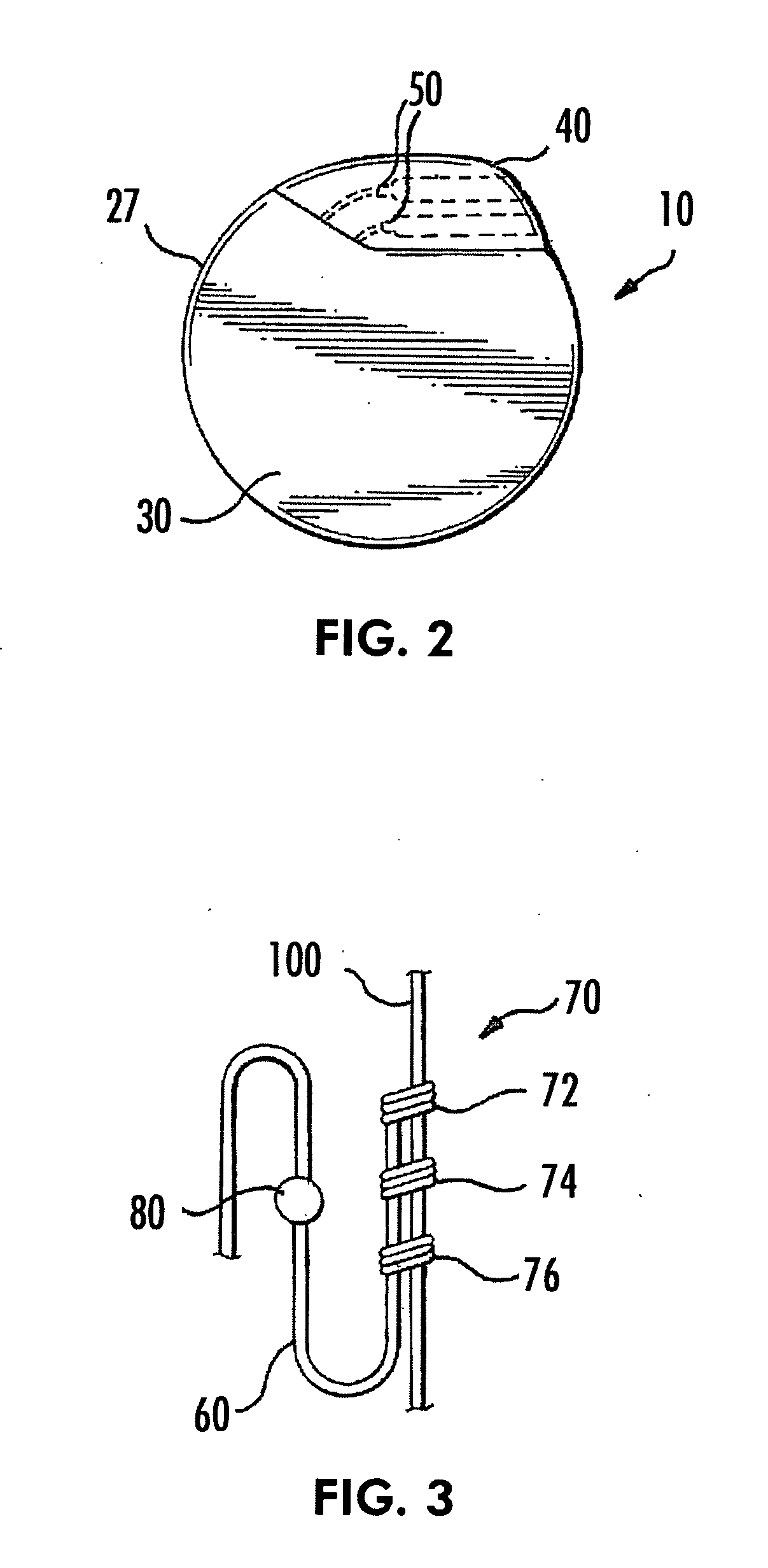
ActiveUS20070213783A1Relieve symptomsQuality improvementElectrotherapyFlow monitorsNervous systemPain duration
An implantable neurostimulator system for treating pain includes scheduled and responsive therapy capabilities including responsive stimulation applied to the brain and peripheral sections of the nervous system. Methods for treating chronic nociceptive, neuropathic, and psychogenic pain employ an inventive system to advantageously reduce multiple symptoms and components of pain and to address underlying causes of pain.
Owner:NEUROPACE
Drug depot implant designs and methods of implantation
ActiveUS20070243228A1Uniform drug distributionMinimal disruptionBiocidePeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
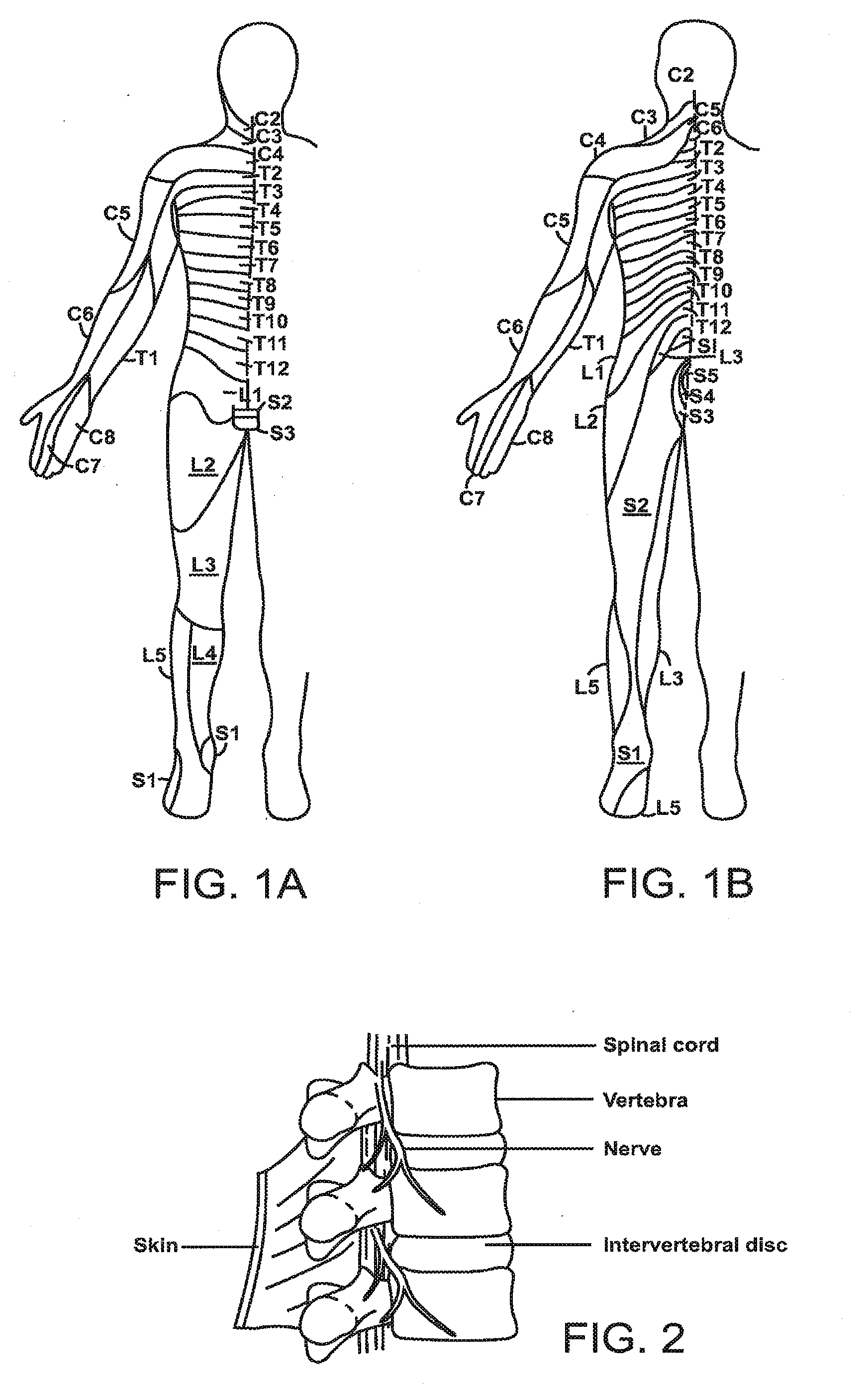
Peripheral nerve stimulation
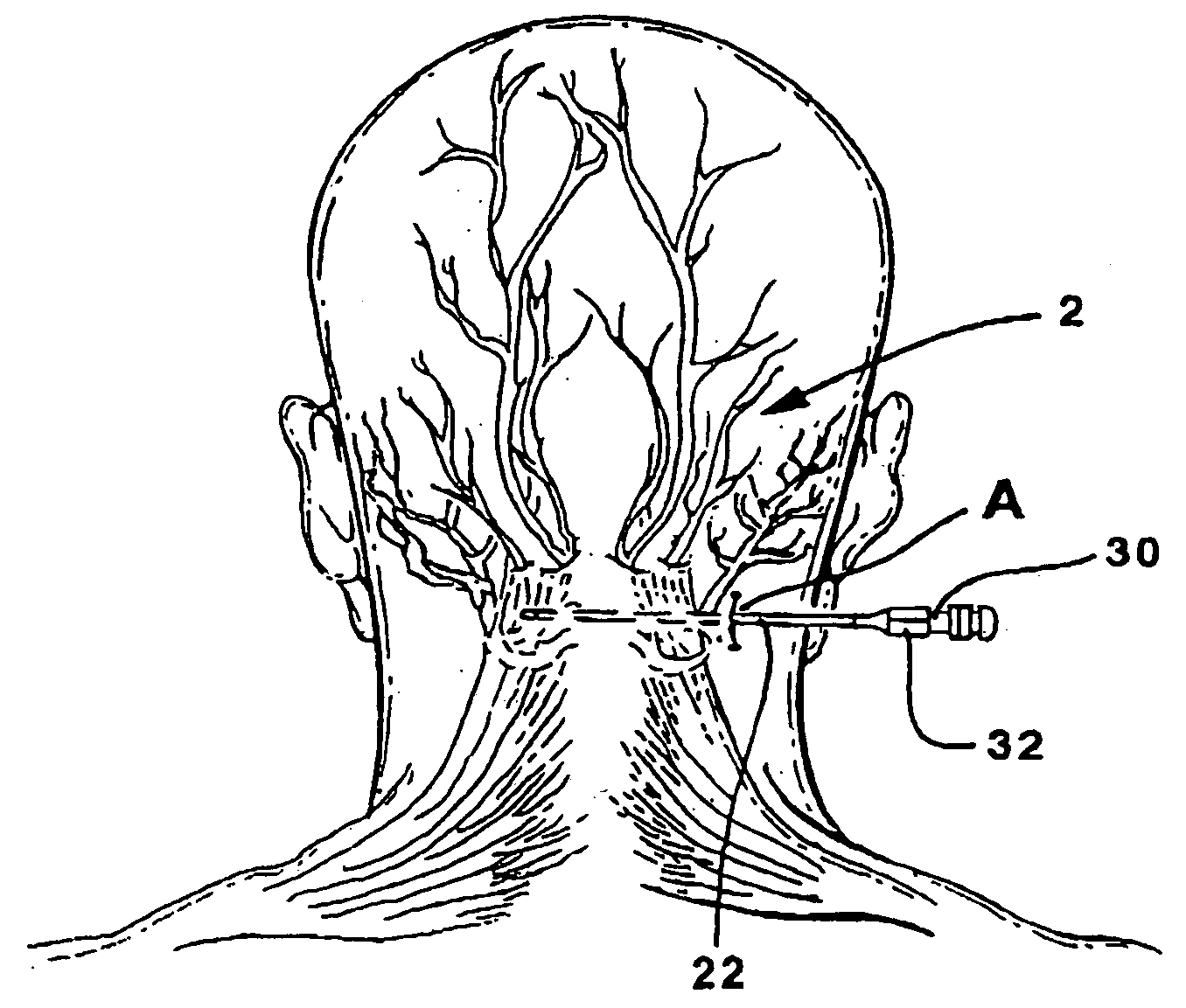
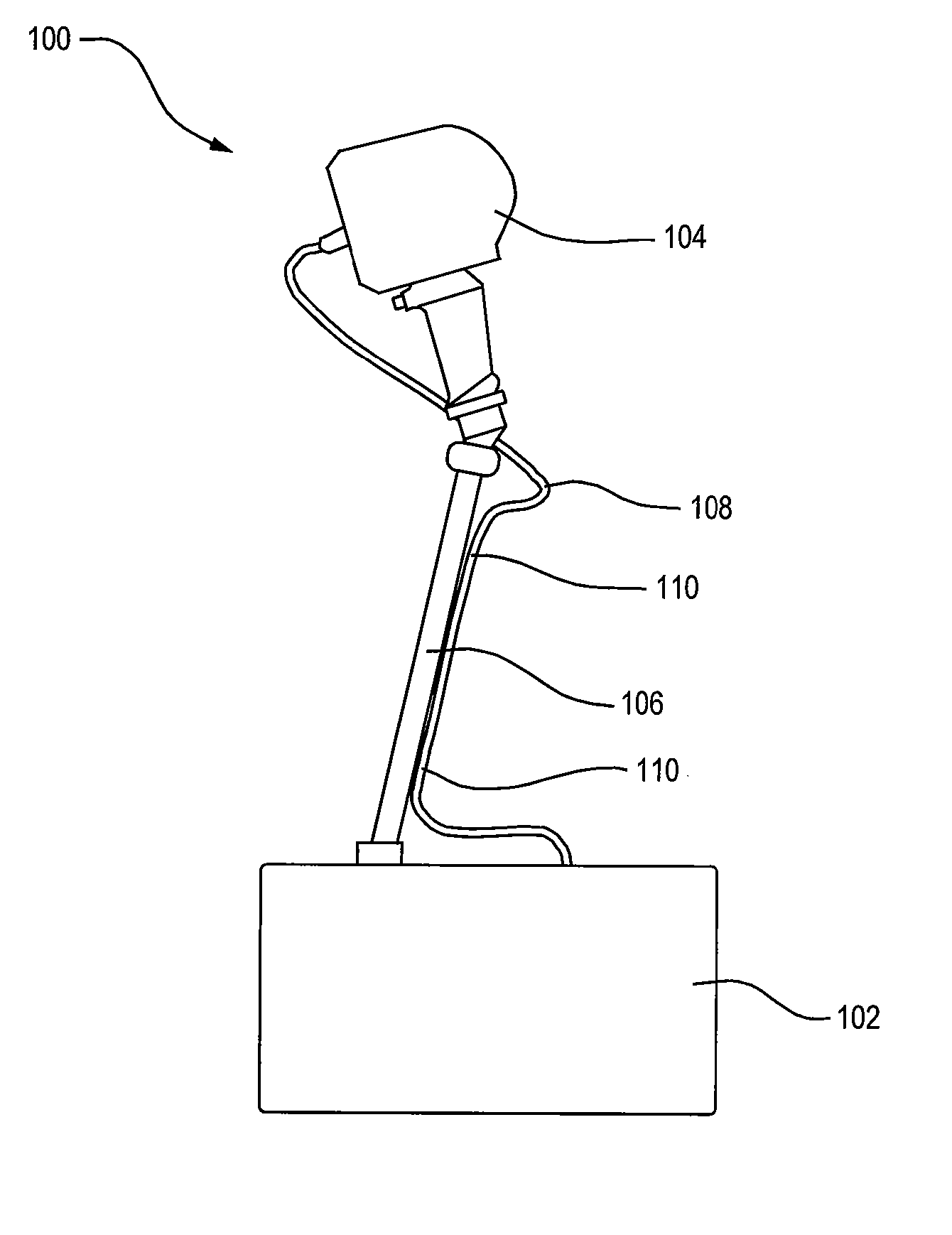
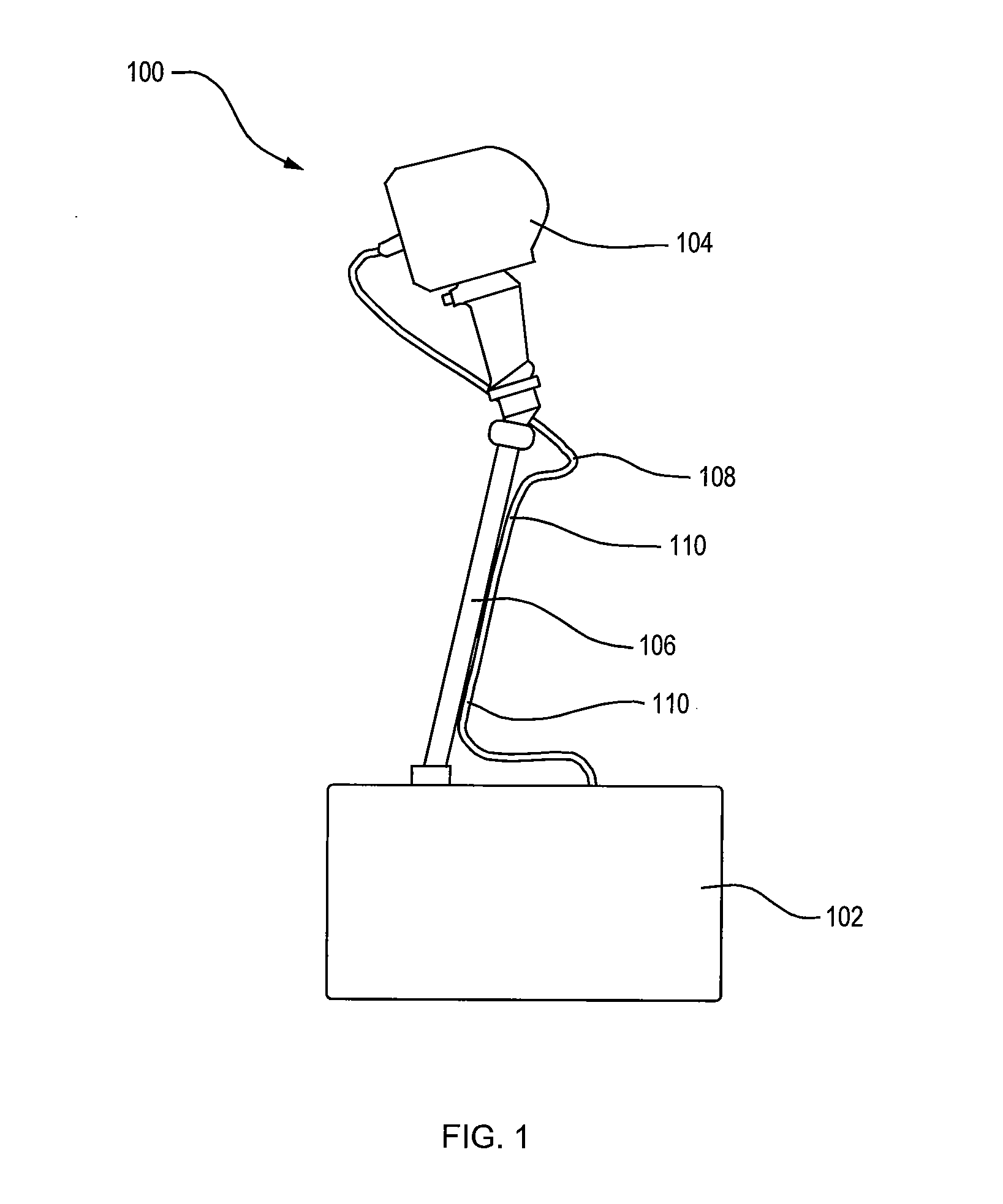
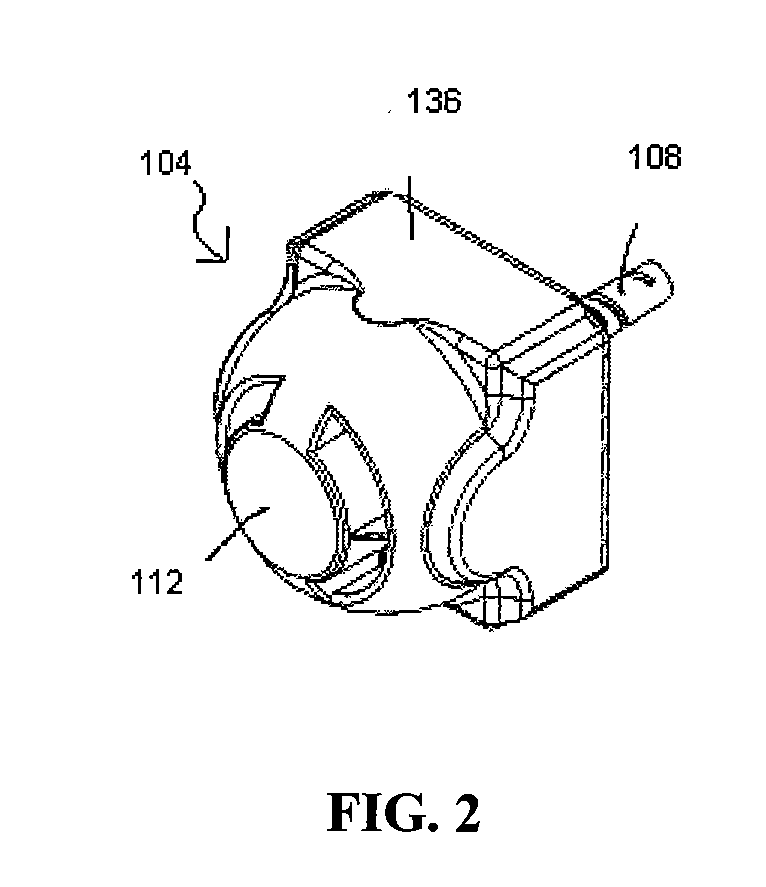
An apparatus for treating pain by electrical stimulation is disclosed. A lead is placed subcutaneously in the region of pain. The subcutaneous tissue is electrically stimulated to cause paresthesia. The method encompasses subcutaneous placement of an electrical lead near the region of pain and subsequent electrical stimulation of the tissue to cause paresthesia. In particular, an apparatus for treating intractable occipital neuralgia using percutaneous electrostimulation techniques is disclosed.
Owner:WEINER RICHARD L
Bipolar tissue treatment system
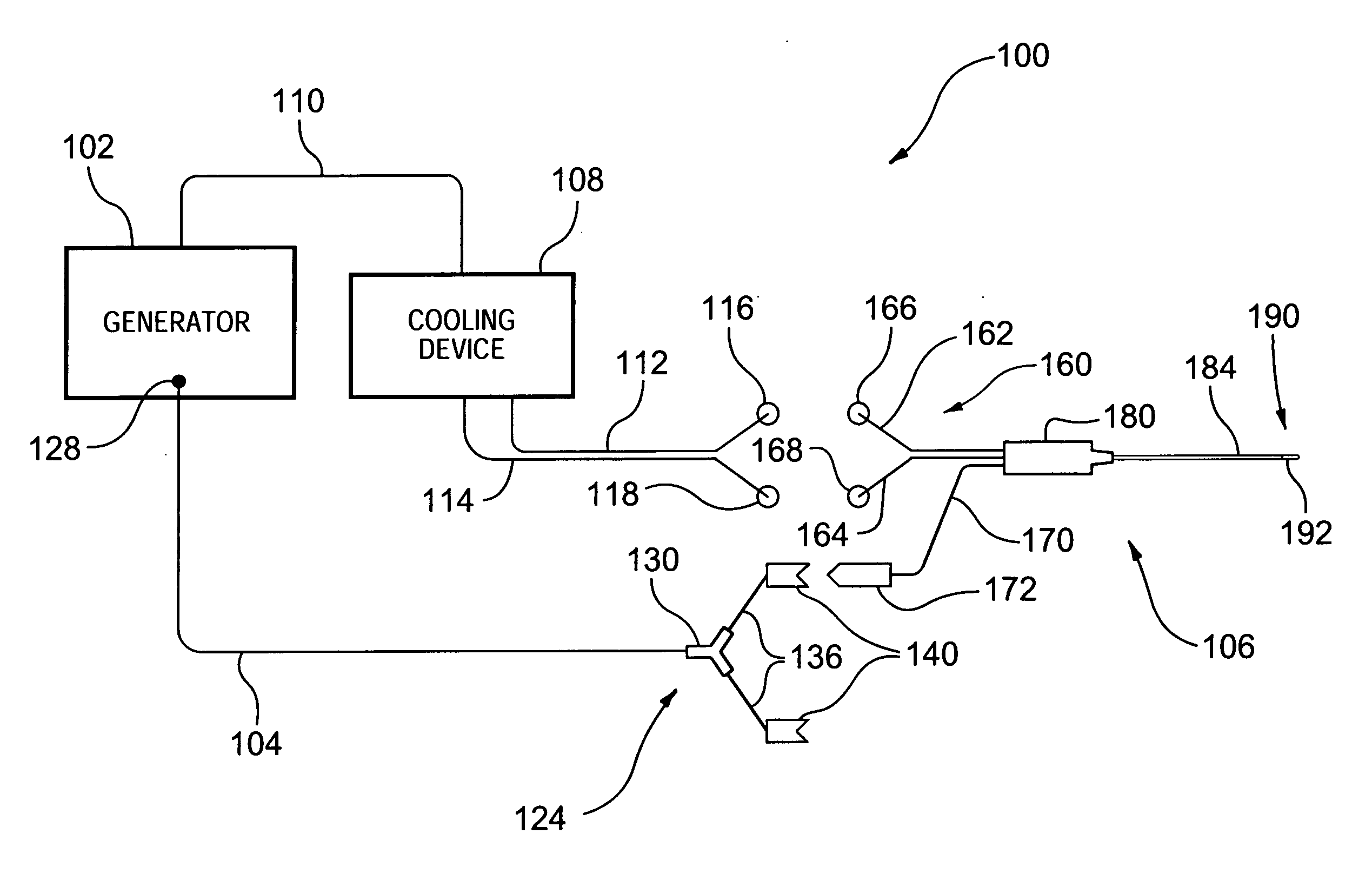
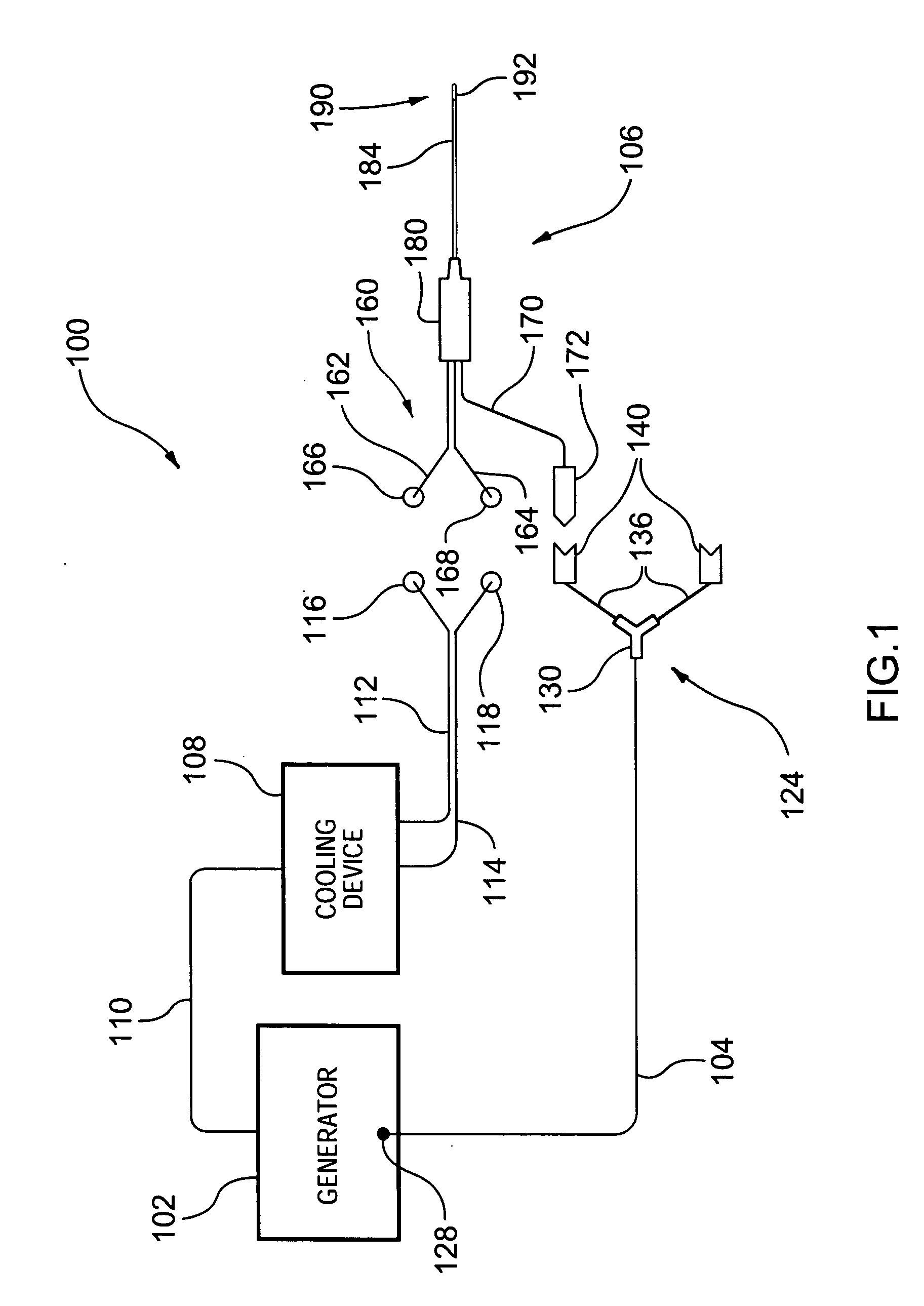
InactiveUS20050177209A1Reduce the temperatureSurgical instruments for heatingTherapeutic coolingRadio frequency energyCardiac Ablation
A novel medical probe assembly, system, and methods for the use thereof to treat tissue are described. The system optionally comprises an energy source, two probe assemblies, and one or more cooling devices to provide cooling to at least one of the probe assemblies. The probe assemblies may be configured in a bipolar mode, whereby current flows preferentially between the probe assemblies. The probe assemblies and system described herein are particularly useful to deliver radio frequency energy to a patient's body. RF energy delivery may be used for various applications, including the treatment of pain, tumor ablation and cardiac ablation.
Owner:BAYLIS MEDICAL
Devices and Methods for Beating Heart Cardiac Surgeries
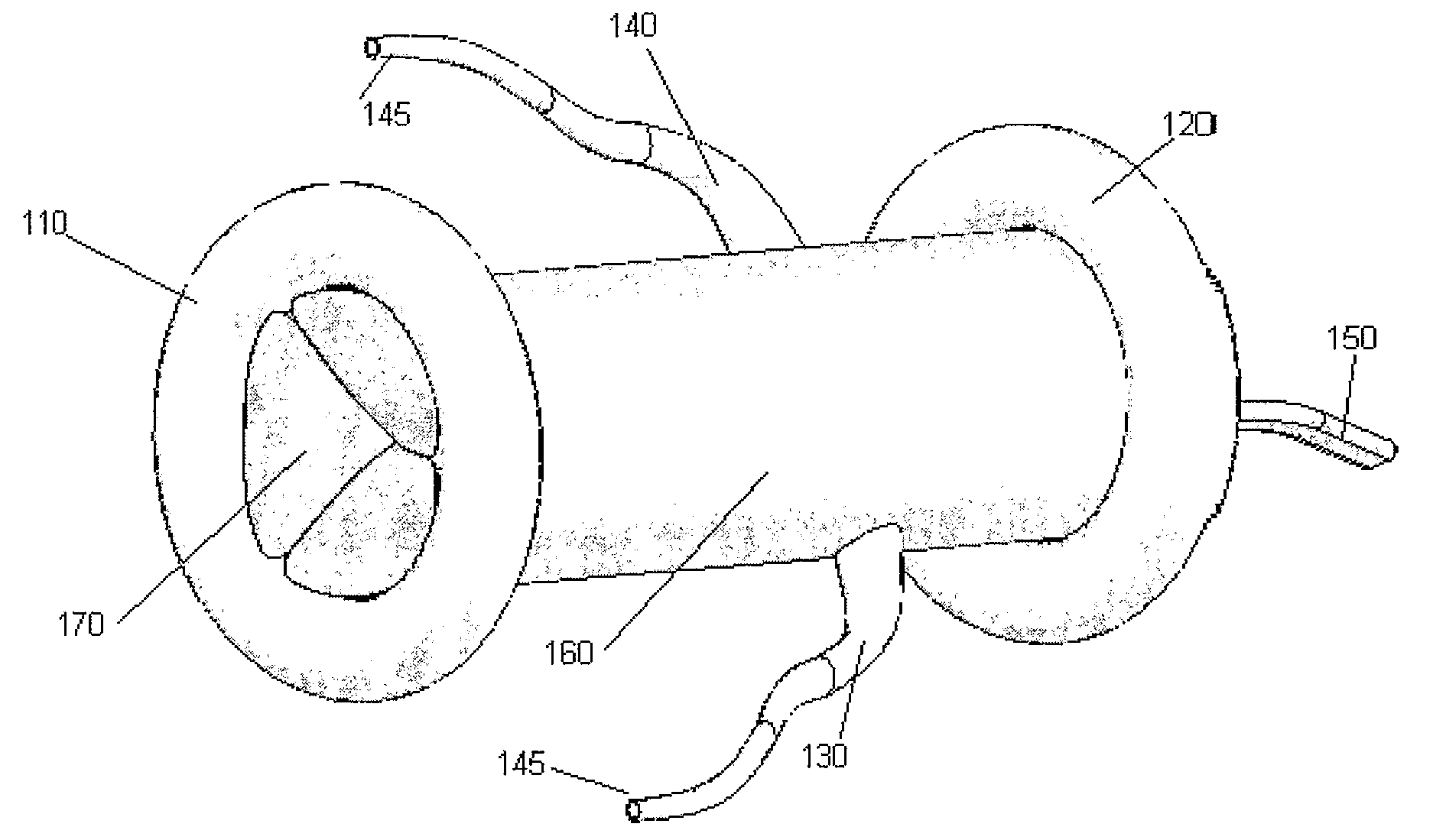
InactiveUS20070219630A1Reduce shakingImprove efficiencyVenous valvesCoronary arteriesCoronary artery guide catheter
The present invention provides devices for beating heart surgery. The device separates the valve and the surrounding area from the rest of the vascular system so the operation procedure can be carried out while the heart is beating during the entire course of the procedure. This is made possible through a temporary valve (170) and two coronary artery conducts (130, 140) incorporated in the balloon-catheter system. The system provides better view and ease of operation and thus, reduces surgery related complication and pains.
Owner:CHU XI
Flexible visually directed medical intubation instrument and method
Owner:PERCUVISION
Capsaicinoid gel formulation and uses thereof
InactiveUS20060148903A1Relieve painReduce the amount requiredBiocideNervous disorderSurgical sitePost-Procedural Pain
The present invention provides capsaicinoid gel formulations and methods for relieving pre- and post-surgical pain at a site in a human or animal by administering at a surgical site in a human or animal in need thereof a dose of capsaicinoid gel in an amount effective to attenuate post-surgical pain at the surgical site, the dose of capsaicin ranging from 100 μg to 10,000 μg.
Owner:ALGORX PHARMA INC
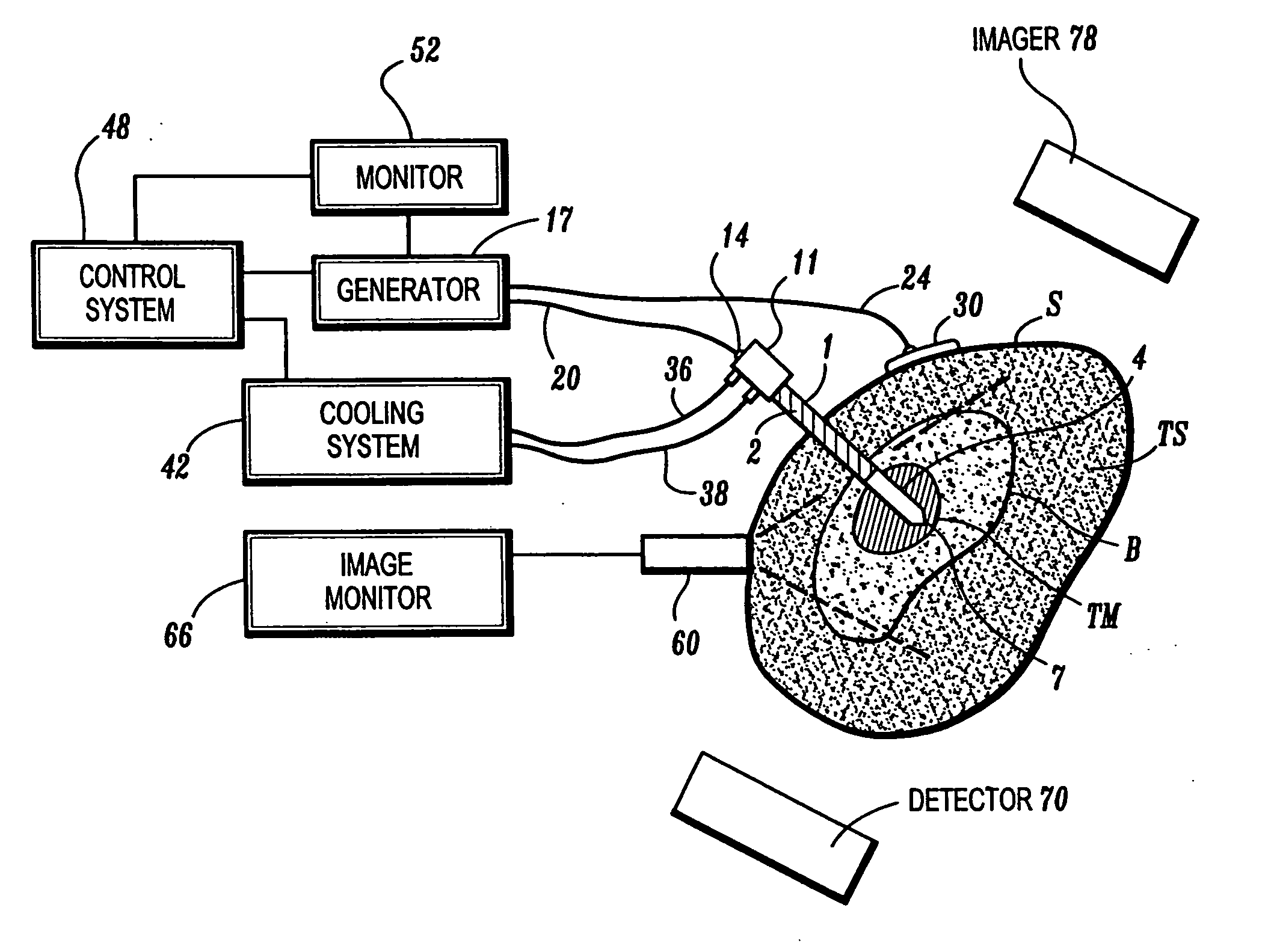
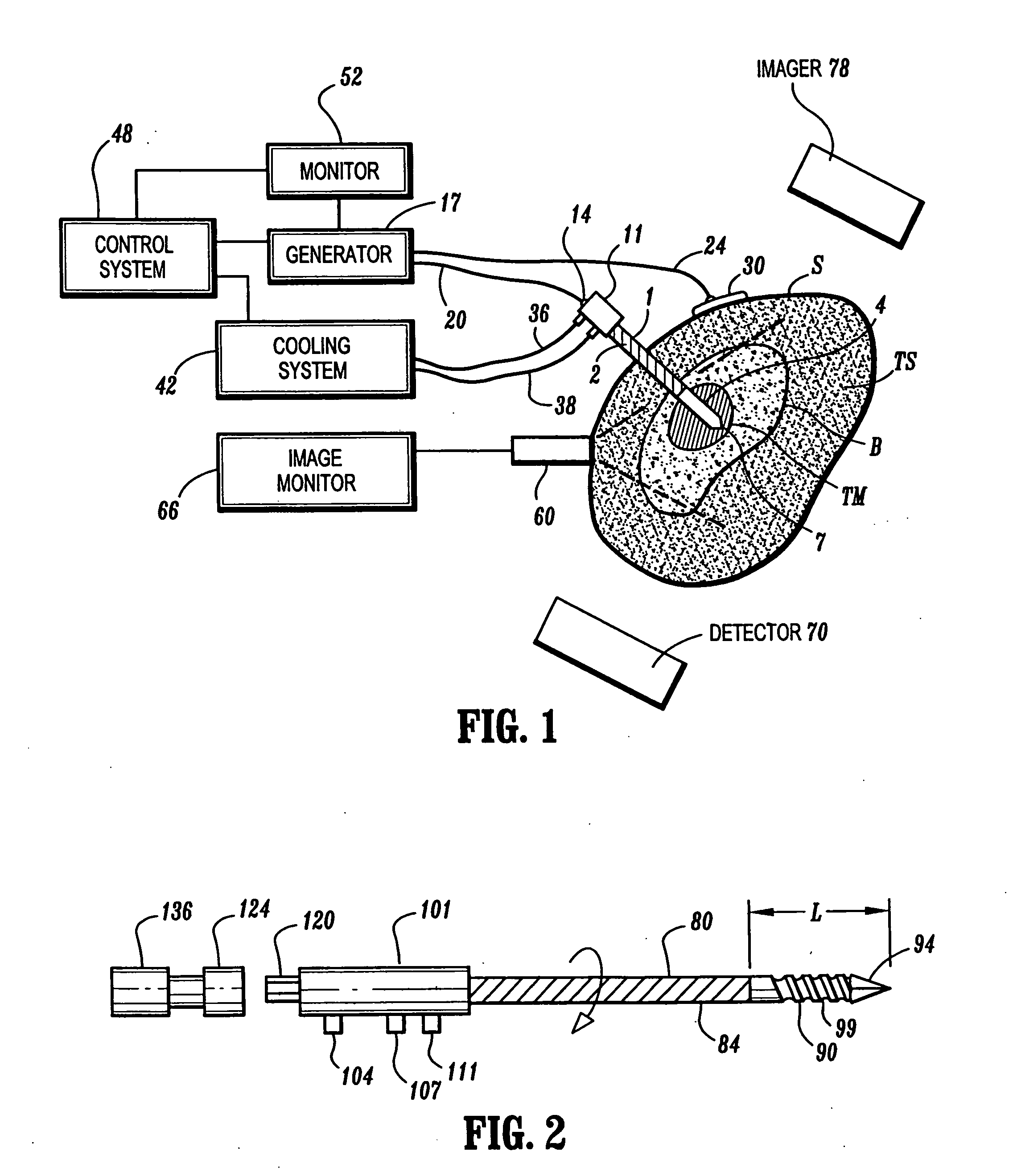
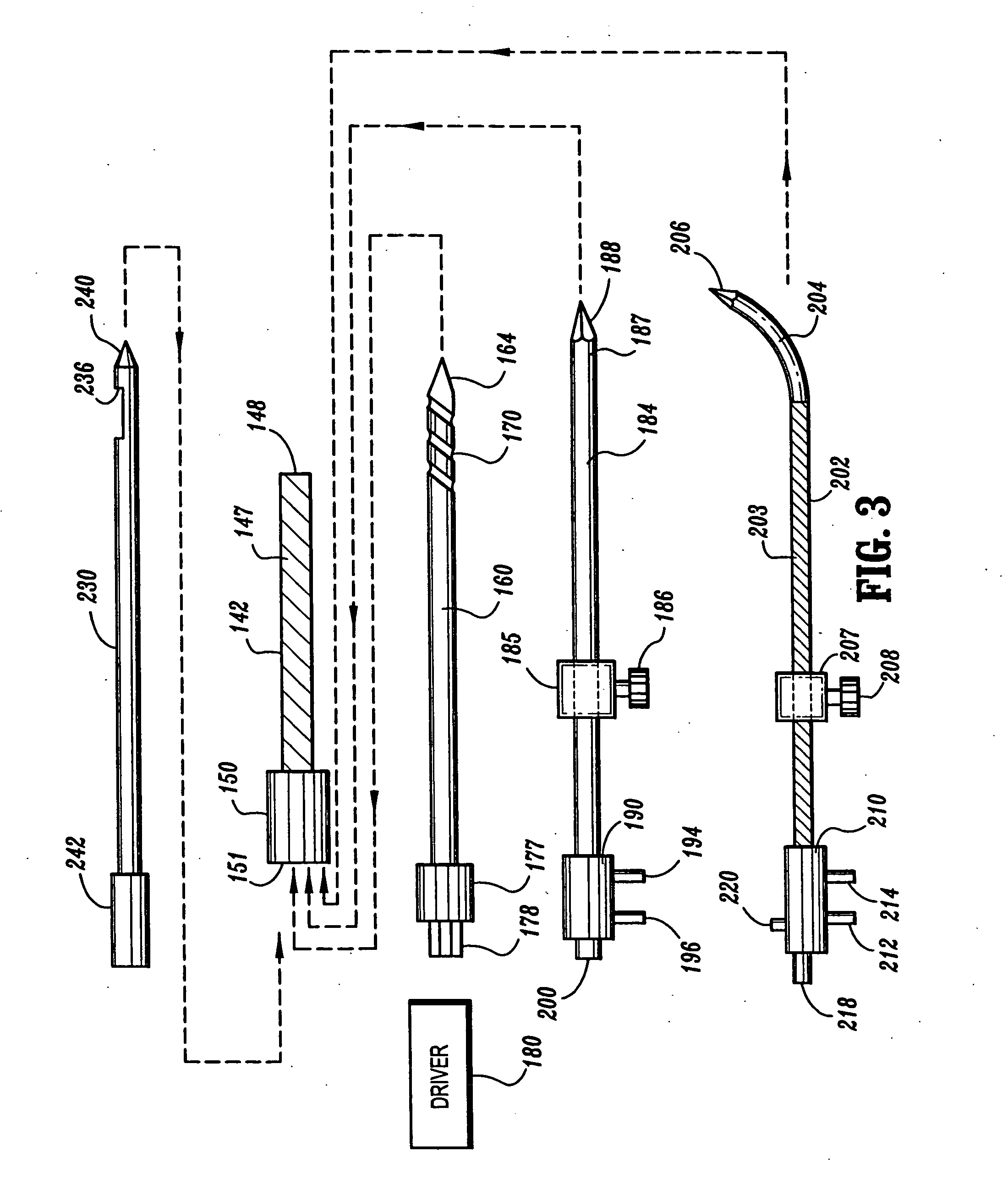
Ablation treatment of bone metastases
InactiveUS20050192564A1Easily toleratedReduce the dependency of the patientDiagnosticsSurgical needlesElectrode placementAbnormal tissue growth
Ablative treatment of metastatic bone tumors and relief of pain associated with metastatic bone tumors is achieved by heat ablation of the bone tumor or tissue near the bone tumor by an ablation probe. In one form the probe is an electrode coupled to a high frequency power supply to provide ablative heating of tissue proximate to an electrode that is placed in or near the bone tumor. Cooling of the electrode by fluid circulation from a cooling apparatus outside the patient's body may be used to enlarge the region of high frequency heating around the electrode. Image guidance of the electrode placement may be monitored by an imaging device. Tracking of the electrode by an image-guided navigator helps in placement of the electrode with respect to the configuration of the bone and bone metastasis. A set of tools accommodates biopsy and various shapes of electrodes according to clinical requirements. Several forms of electrodes, energy delivery and cooling apparatus and methods accommodate the specific objectives.
Owner:COVIDIEN AG
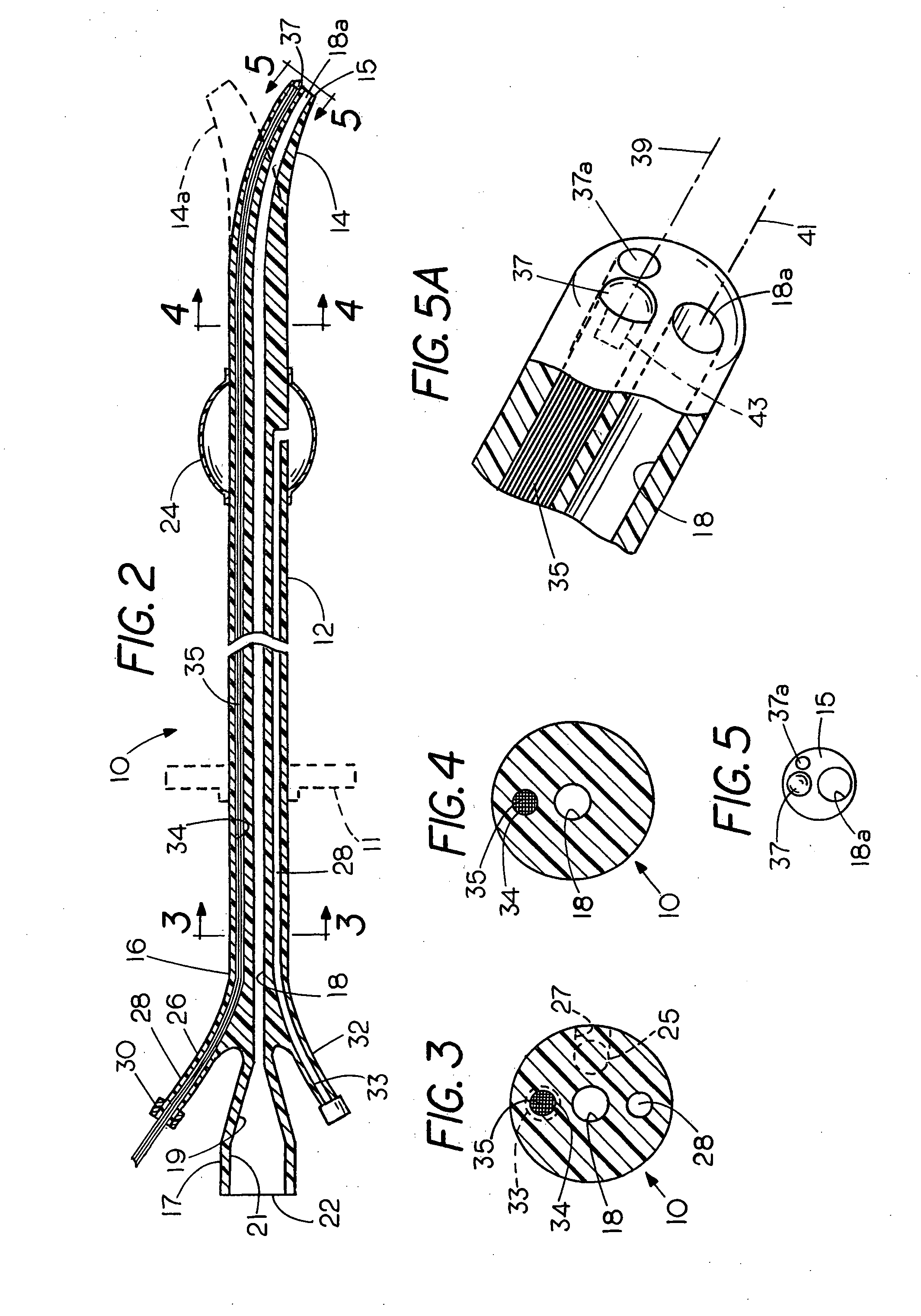
Administration instrument for medical use
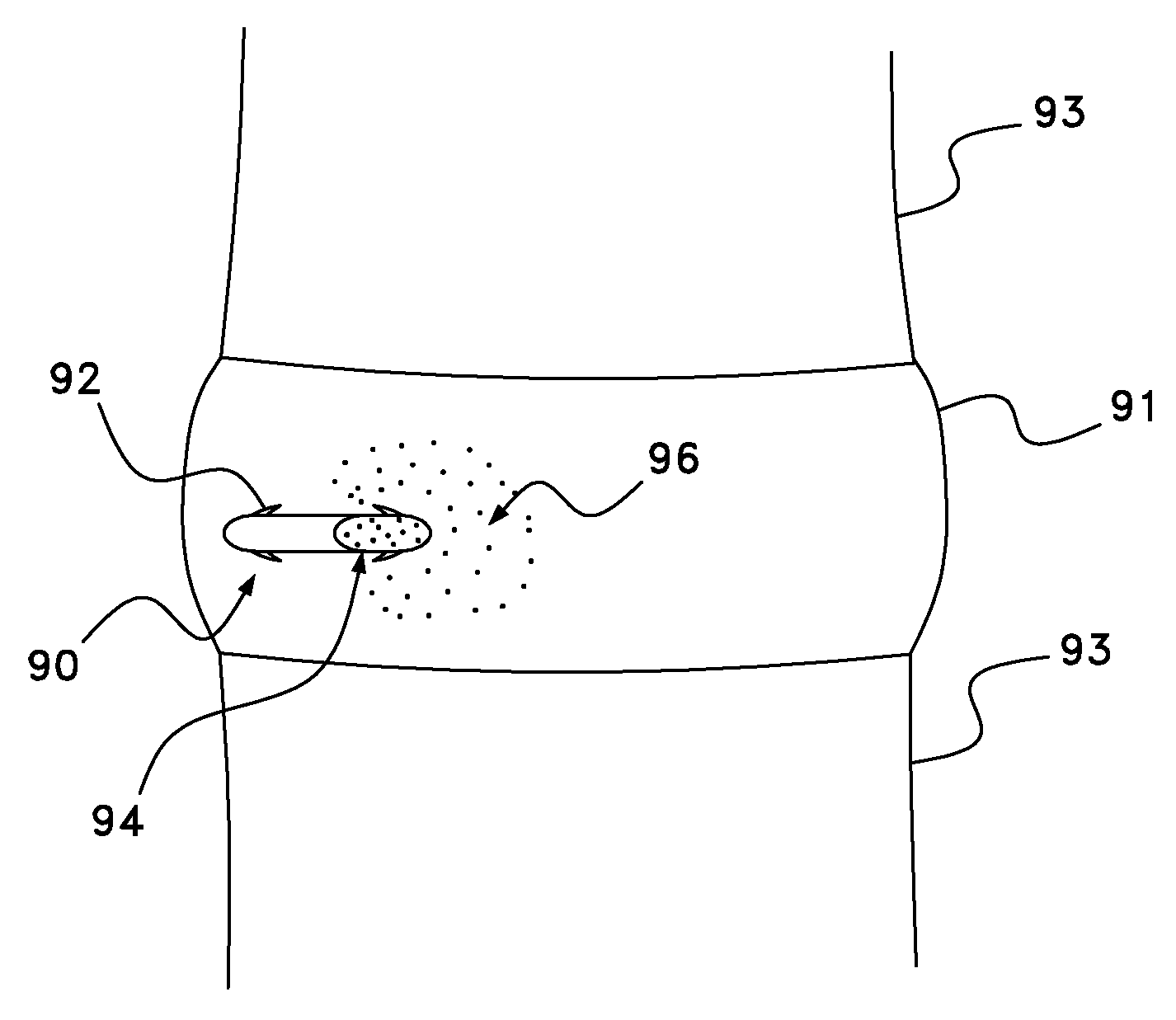
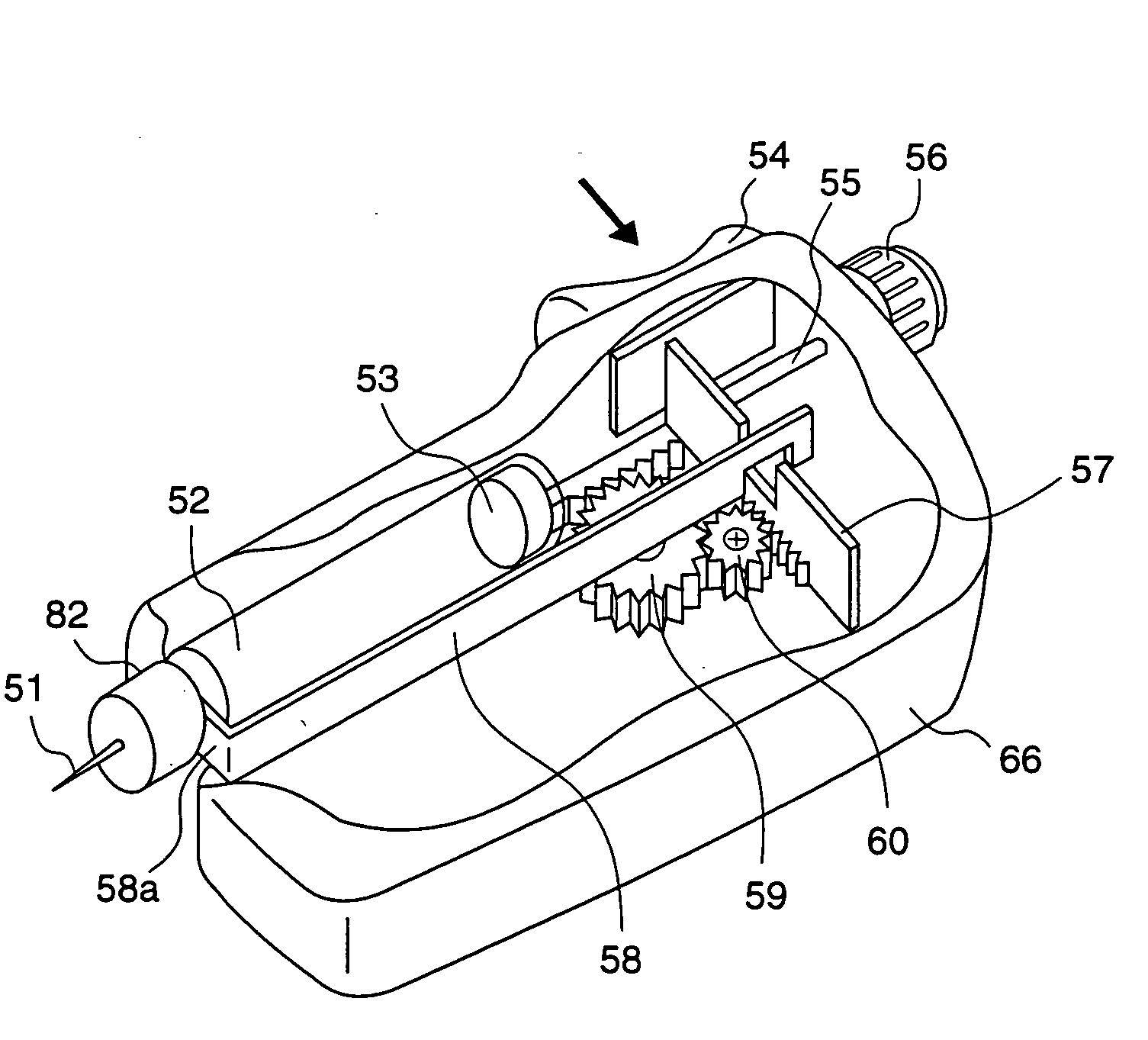
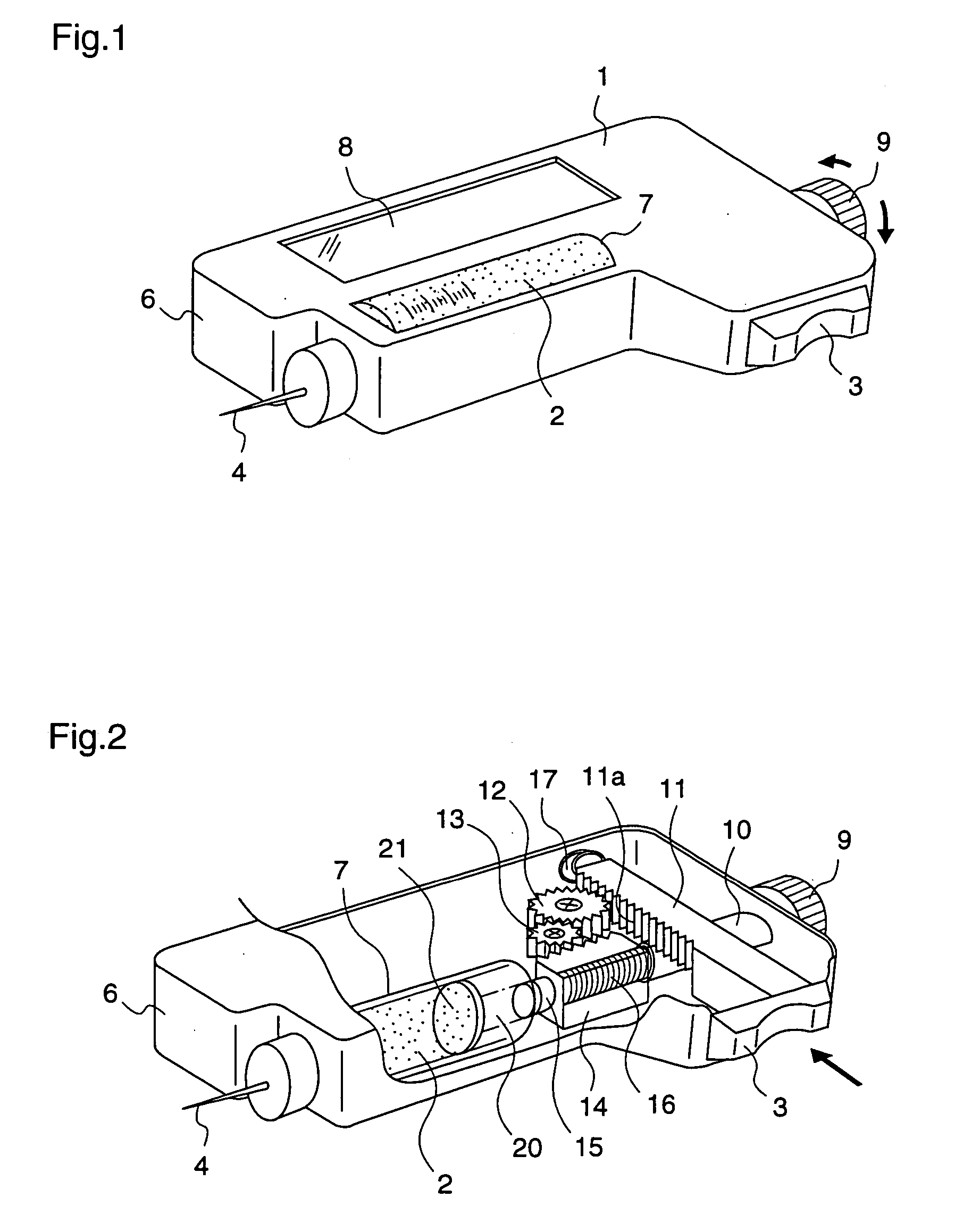
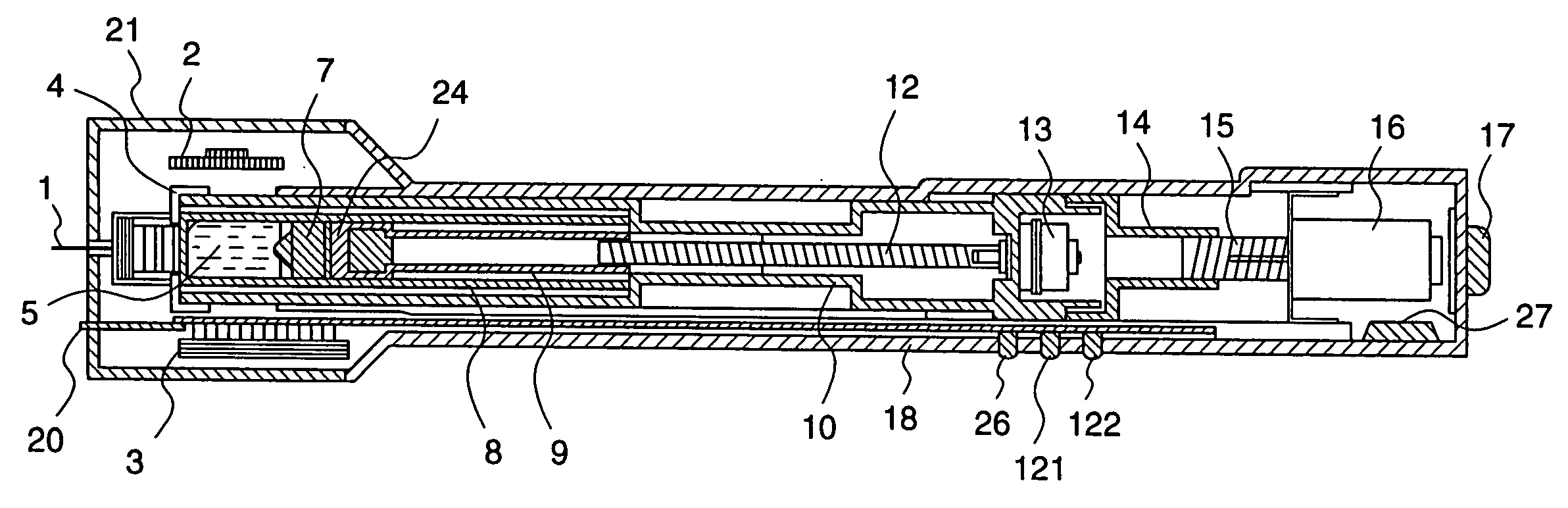
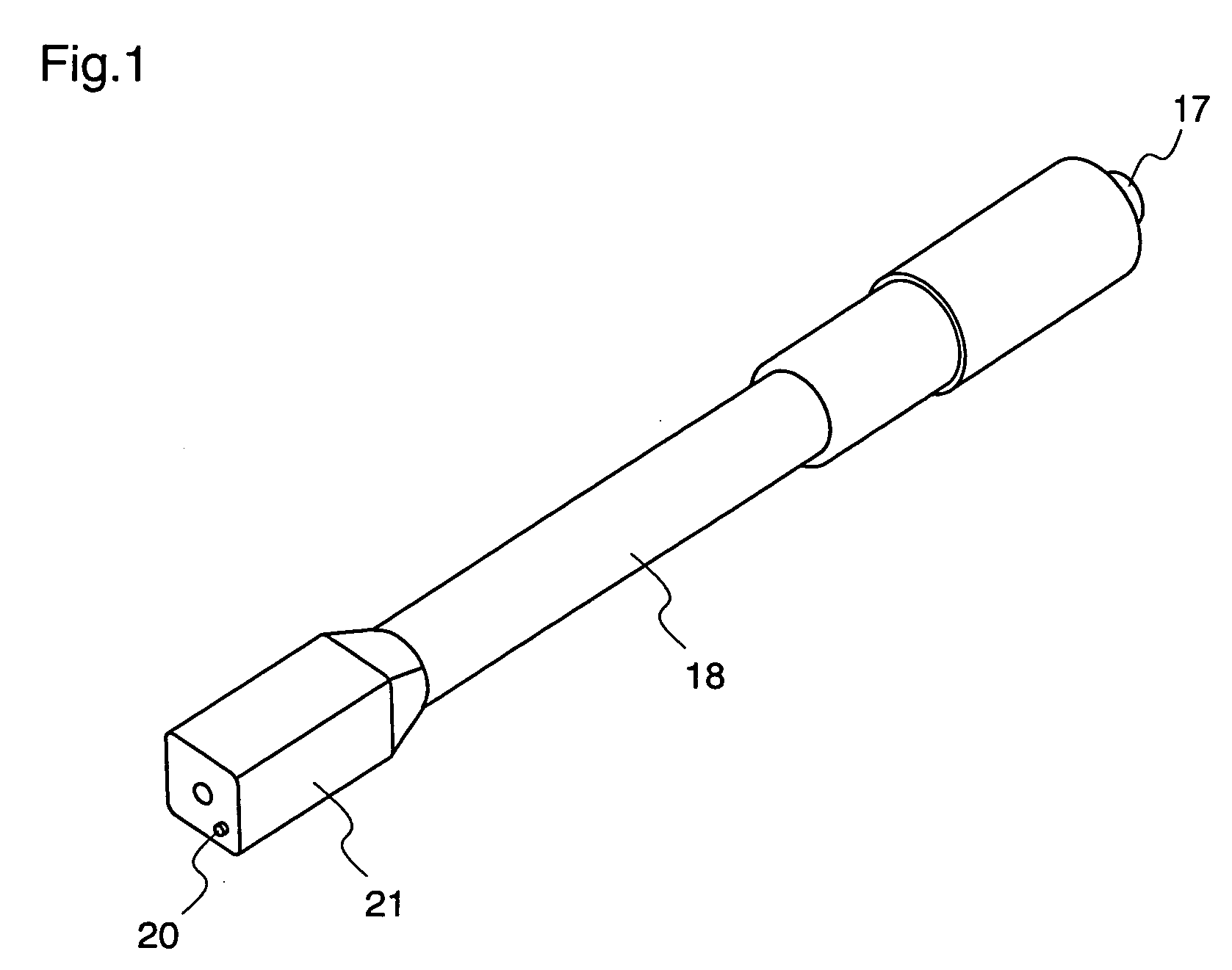
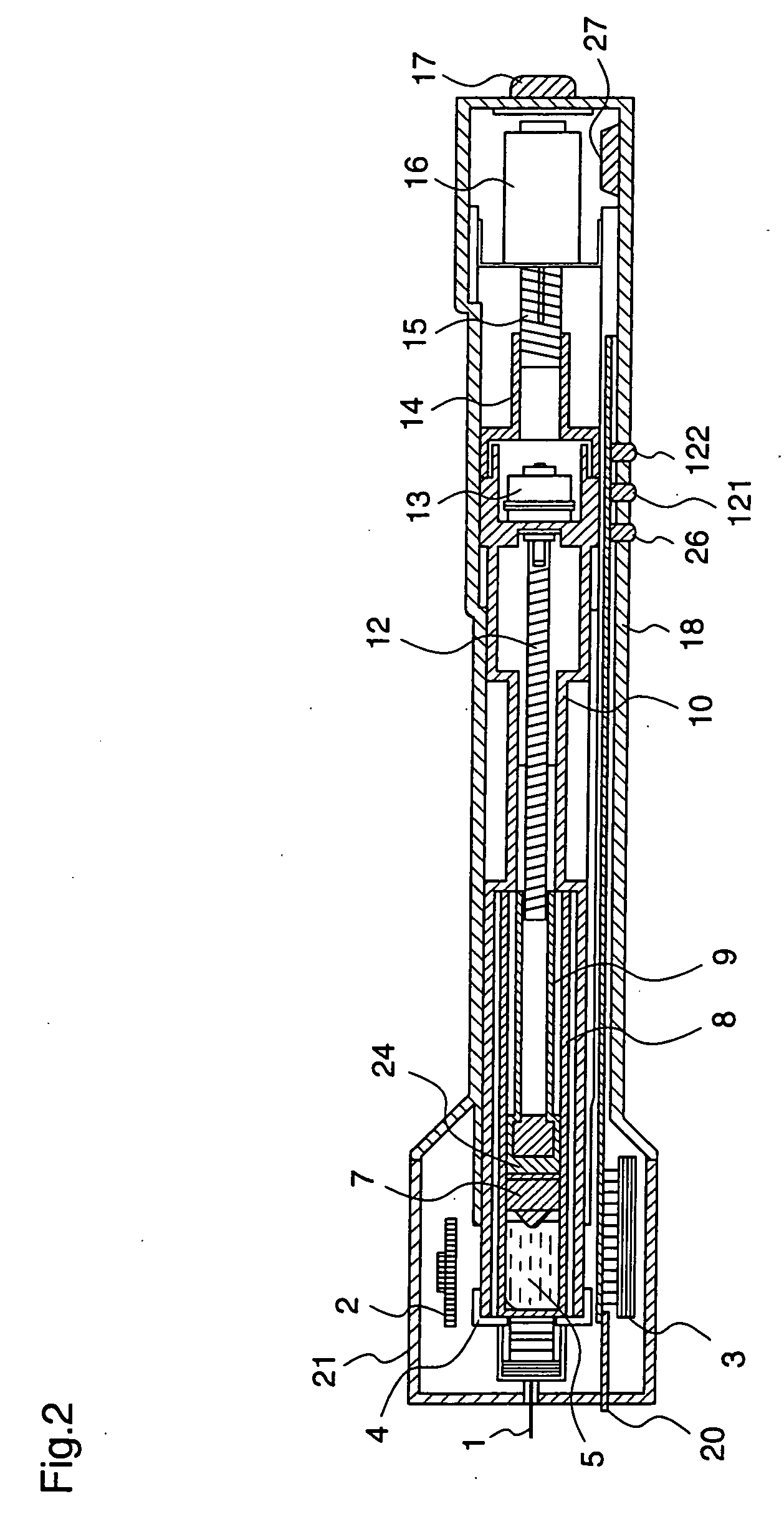
ActiveUS20050090781A1Not increasing physical and mental painAmpoule syringesMedical devicesPain durationDrugs solution
The present invention relates to an administration instrument for medical use that can perform injection of a drug solution with stability and with great reliability. For example, at the administration, it is possible to prevent a force that presses the injection button from acting in a direction of inserting the needle that is inserted into the skin deeper, and enables the administration under a stable state where the needle does not wobble, thereby alleviating physical and mental pain of the administration patient. With a structure in which an injection button (3) is pressed at an angle that is not parallel to the needle with respect to a direction in which the needle (4) is inserted into the skin, it is possible to prevent the force of pressing the injection button from being transmitted in a direction of inserting the needle into the skin deeper than the initial insertion of the needle, thereby achieving an administration under a stable state.
Owner:PHC HLDG CORP
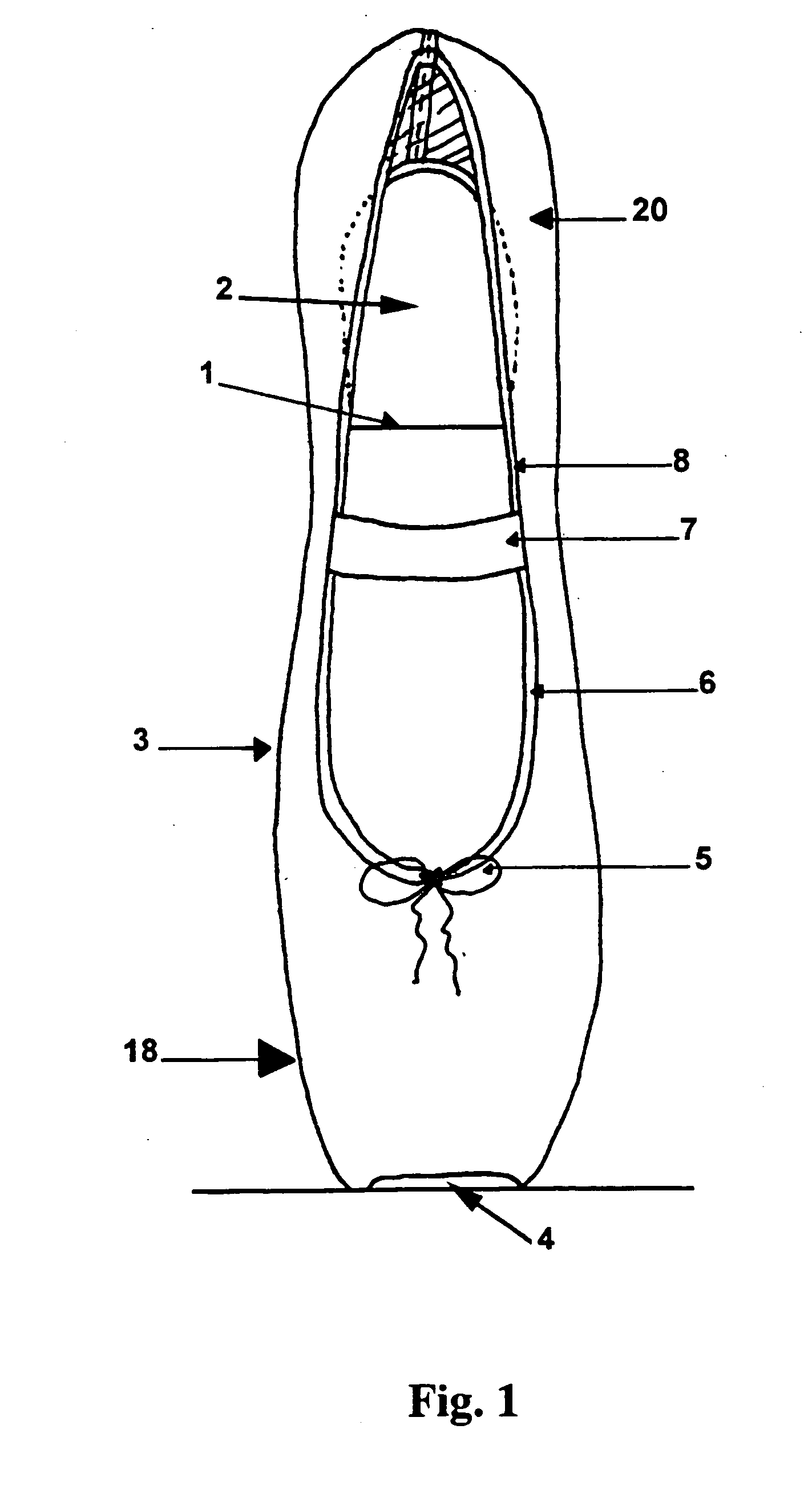
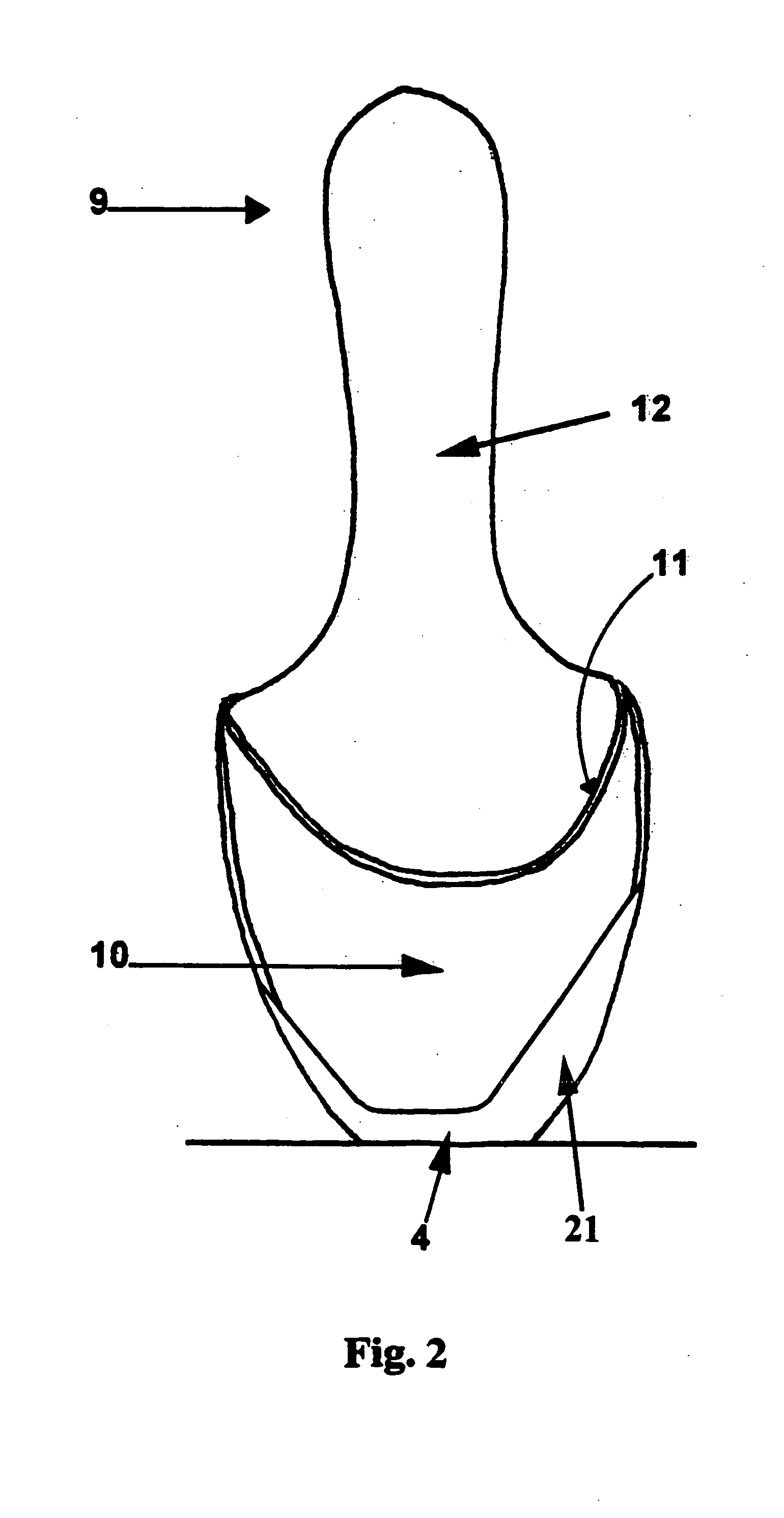
Ballet pointe shoe
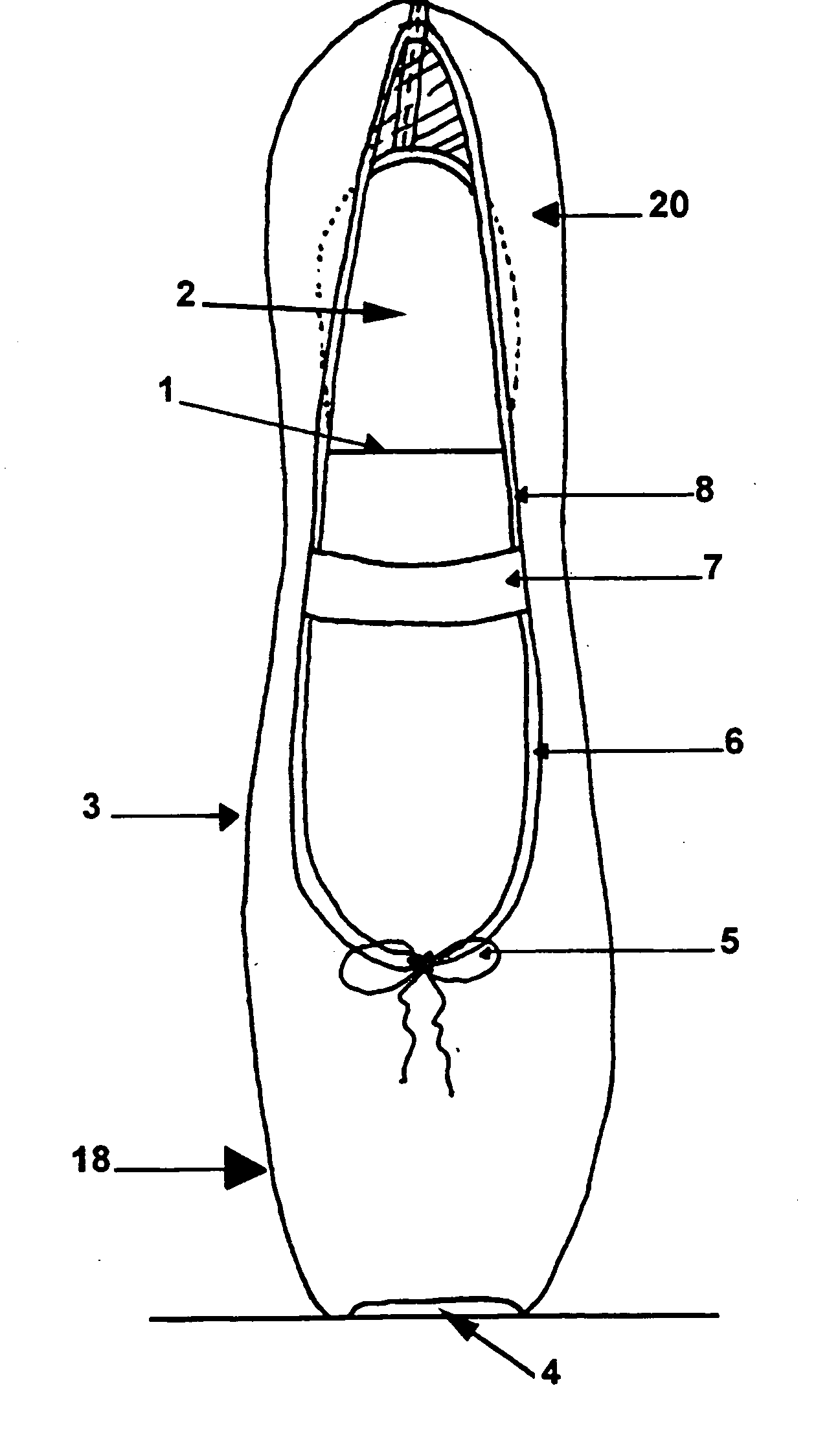
InactiveUS20050022421A1Extended service lifeRedistribute evenlyWear-resisting attachmentsUpperCustom fittingEngineering
The invention relates to a custom-fitting, asymmetric ballet shoe with a shell (9) and liner (13) that enables the dancer to stand en pointe and perform the extreme movements required by ballet choreography with minimal discomfort, pain, and injury to the foot caused by the various aerial ballet maneuvers called for by both traditional and modern choreography. The shoe of the present invention is designed to redistribute the dancer's weight and the ground force reactions associated with dancing en pointe evenly across the toes while translating some of the force away from the distal aspect of the dancer's toes to the rest of the foot. The shoe has a removable cosmetic cover (18) to enable, inter alia, the color and design to be varied according to costume design and the cover's replacement when the fabric is worn, thereby extending the useful life of the remainder of the shoe.
Owner:BRUCKNER JANICE S
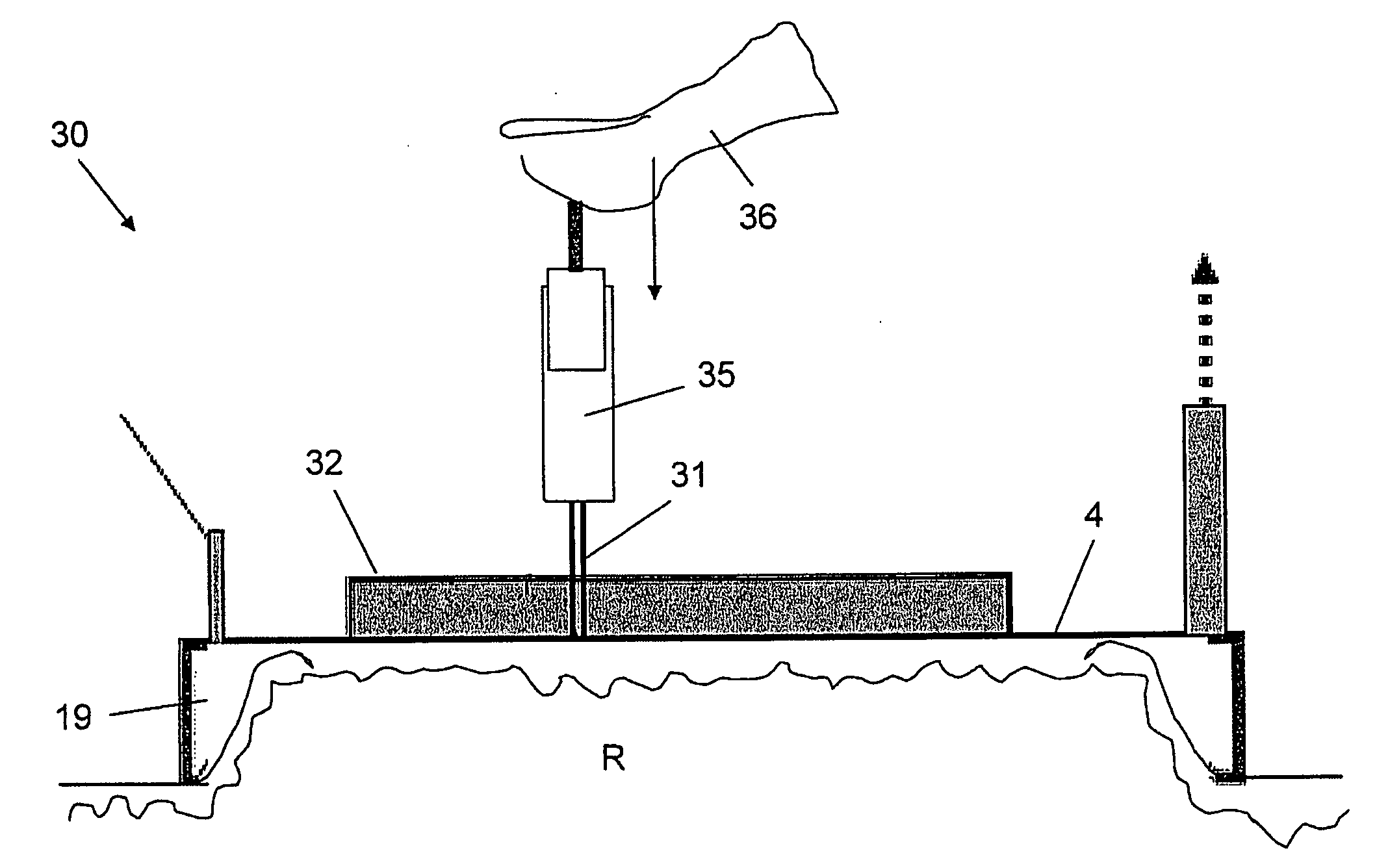
Apparatus and method for inhibiting pain signals transmitted during a skin related medical treatment
InactiveUS20060293722A1Improve skin appearanceChange effectUltrasound therapyElectrotherapyVacuum levelSKIN REGIONS
An apparatus adapted to inhibit pain signals generated by pain receptors in the skin during a skin related medical treatment such as an injection. An evacuation chamber is provided with an essentially rigid interface element larger than a threshold surface area through which a medical treatment can be administered to a selected skin region, one or more walls which are placeable in the vicinity of the skin region, an interior defined by the walls and by the interface element, and an opening at the bottom of the interior which is sealable by the skin region. A device generates a vacuum within the evacuation chamber interior to a level greater than the threshold vacuum level suitable for drawing the skin region through the opening towards, and in a compressing relation against, the interface element, to inhibit the transmission of a pain signal generated by pain receptors located within the skin region.
Owner:CANDELA CORP
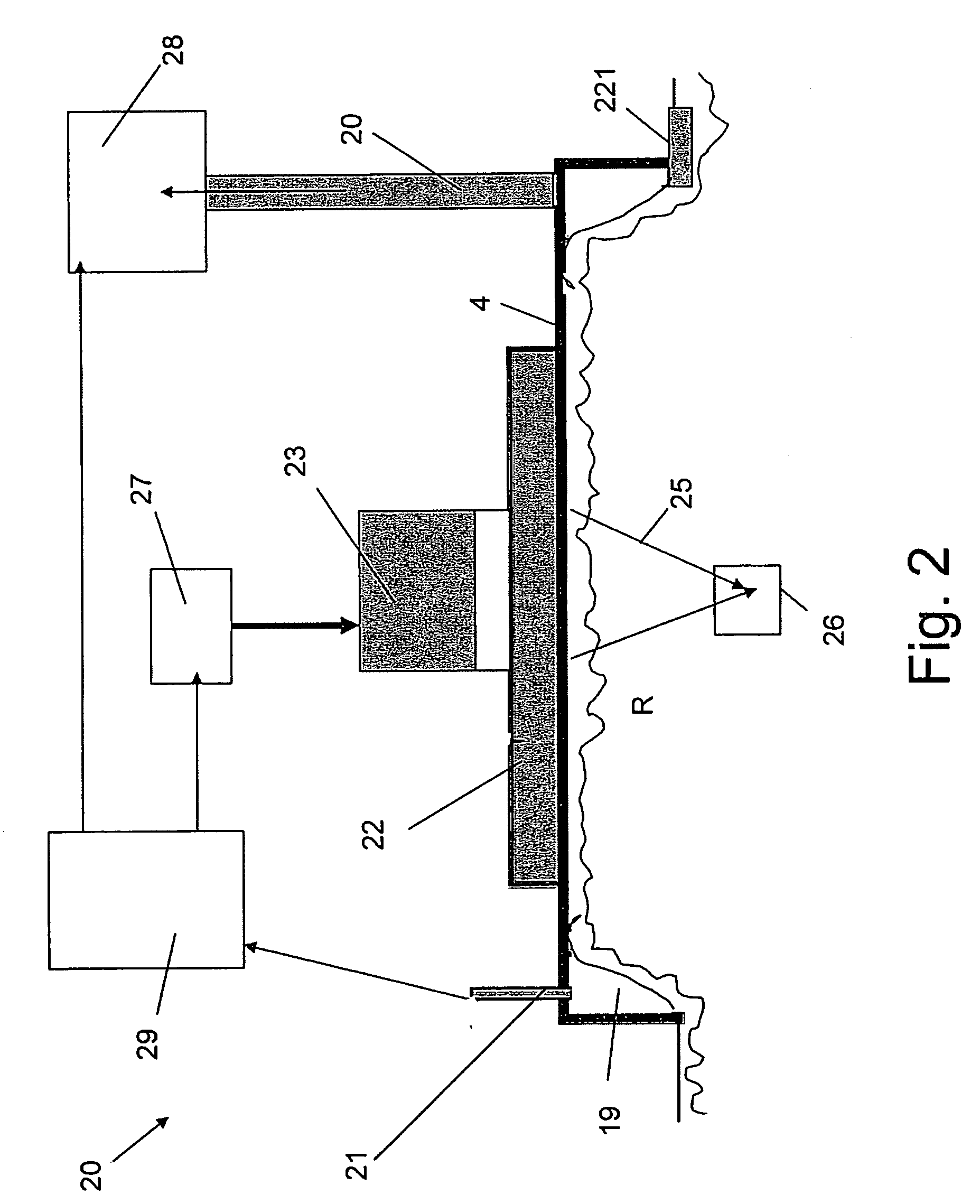
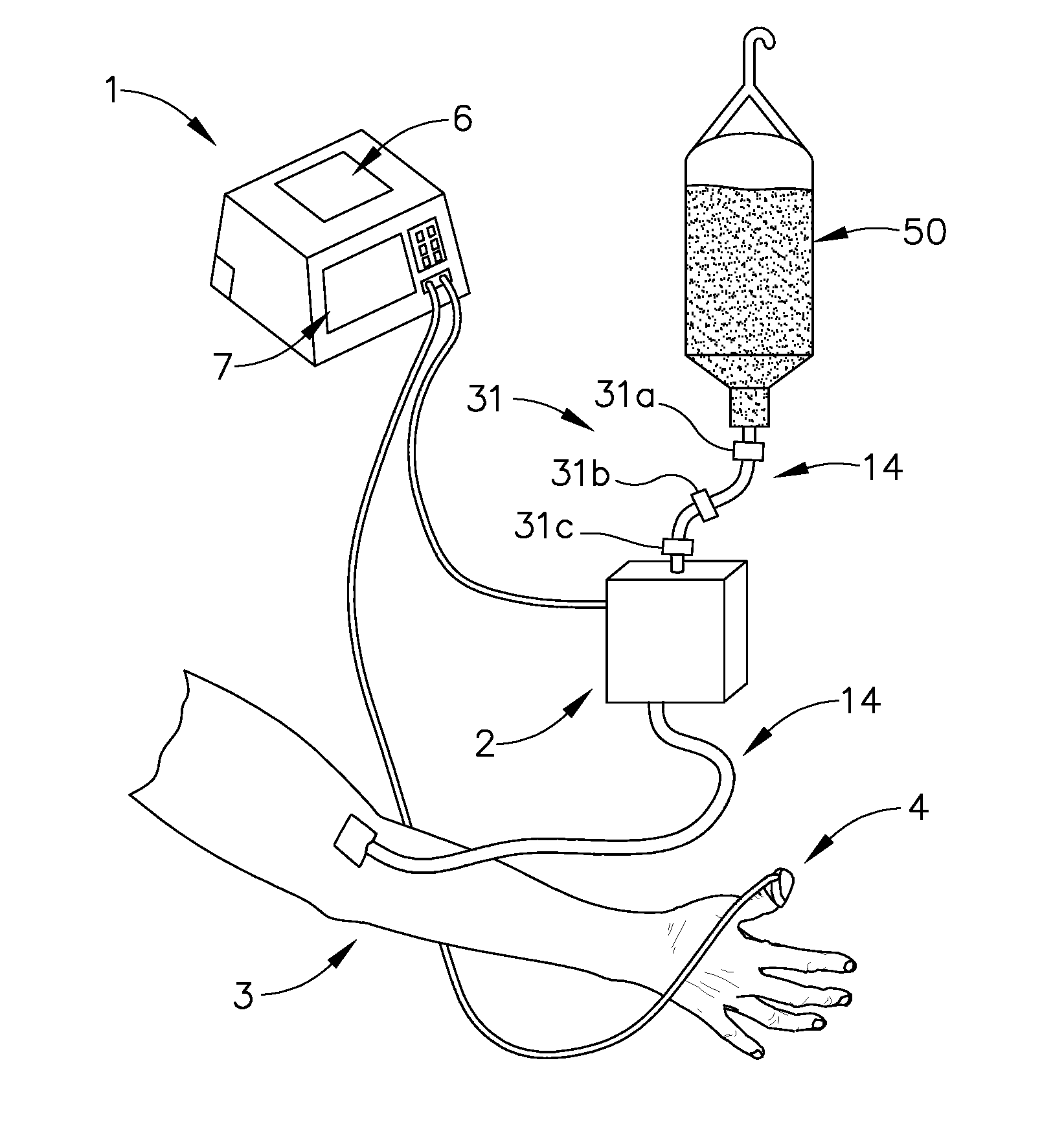
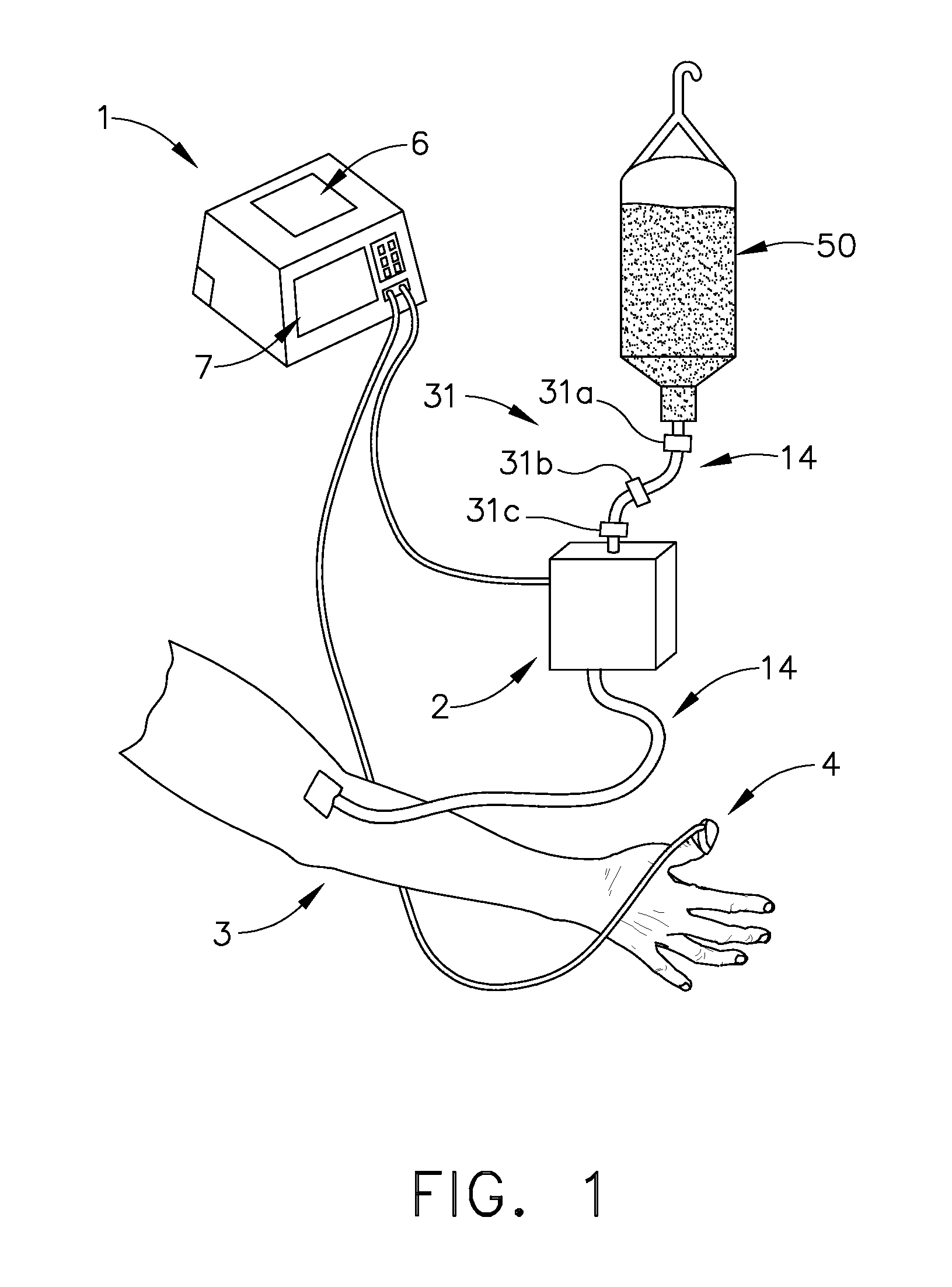
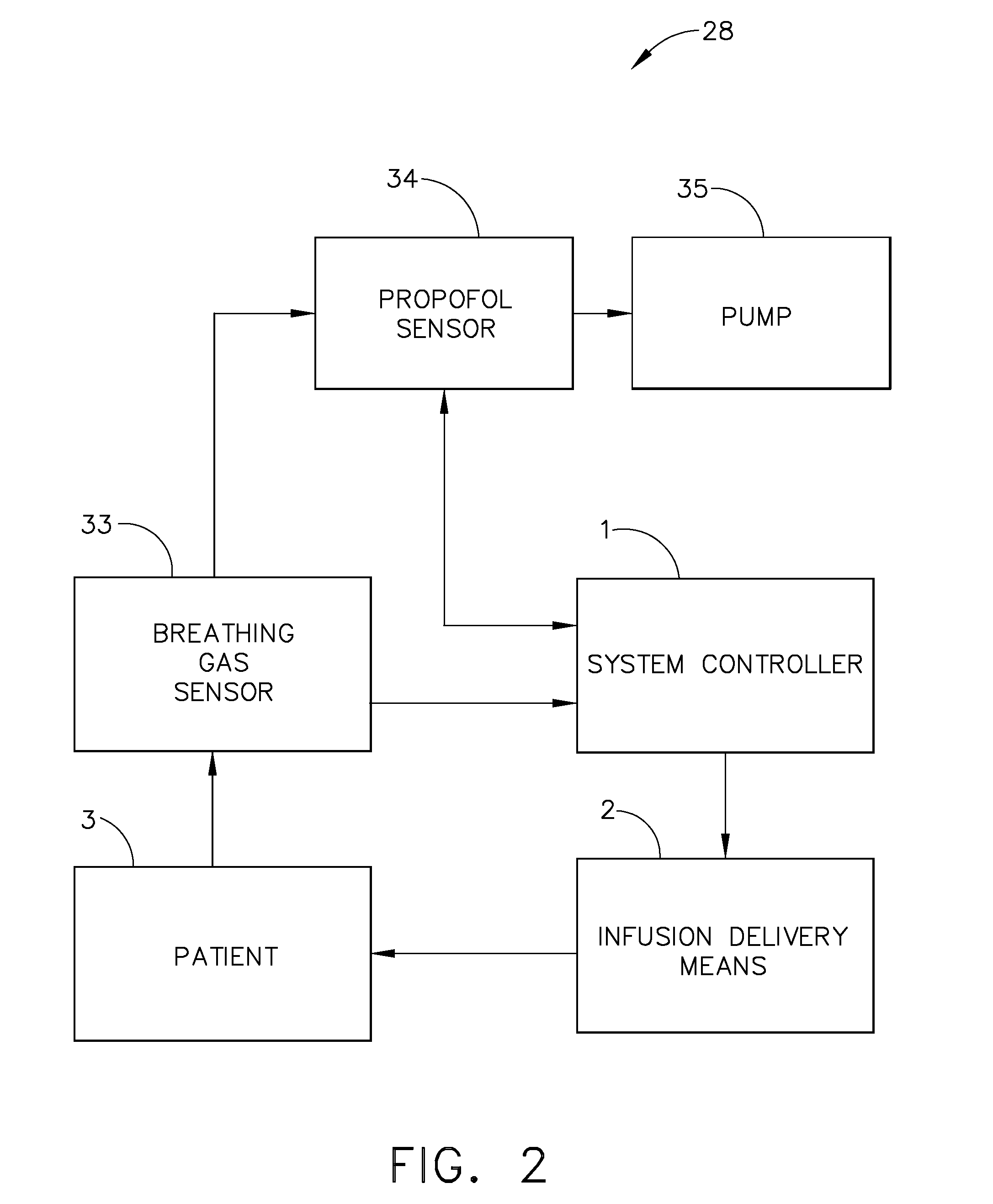
Apparatus and methods for controlling and automating fluid infusion activities
The present invention provides apparatuses and methods to safely and economically deliver infusion fluid to a patient during a medical procedure. The infusion fluid may be a sedative, analgesic, amnestic or other pharmaceutical agent (drug) for alleviating a patient's pain and anxiety before, during and / or after a medical or surgical procedure. In general the apparatus comprises a microprocessor-based controller that receives inputs from a plurality of physiological monitors attached to a patient. The system controller processes the data from the physiological monitors and based upon a fluid infusion algorithm delivers infusion fluid to a patient. The physiological monitors monitor the patient throughout the course of the procedure and depending upon the health of the patient, drug delivery may be adjusted to optimize the procedure while ensuring the patient's health is maintained. Functionality detectors such as an occlusion sensor, air-in-line sensor and a fluid detection sensor alert a clinician to such hazards as a pressure build up in the infusion line, air-bubbles in the infusion line, and the absence of fluid in the infusion line.
Owner:ETHICON ENDO SURGERY INC
Methods and compositions for the treatment, prevention or management of dysfunctional sleep and dysfunctional sleep associated with disease
Methods of treating, preventing and / or managing dysfunctional sleep, including but not limited to, dysfunctional sleep associated with chronic neurological or inflammatory condition such as pain and neurodegenerative disorders, which comprise the administration of one or more immunomodulatory compounds or a pharmaceutically acceptable salt, solvate, stereoisomer, clathrate or prodrug thereof, alone or in combination with known therapeutics are disclosed. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Medical automatic medicator
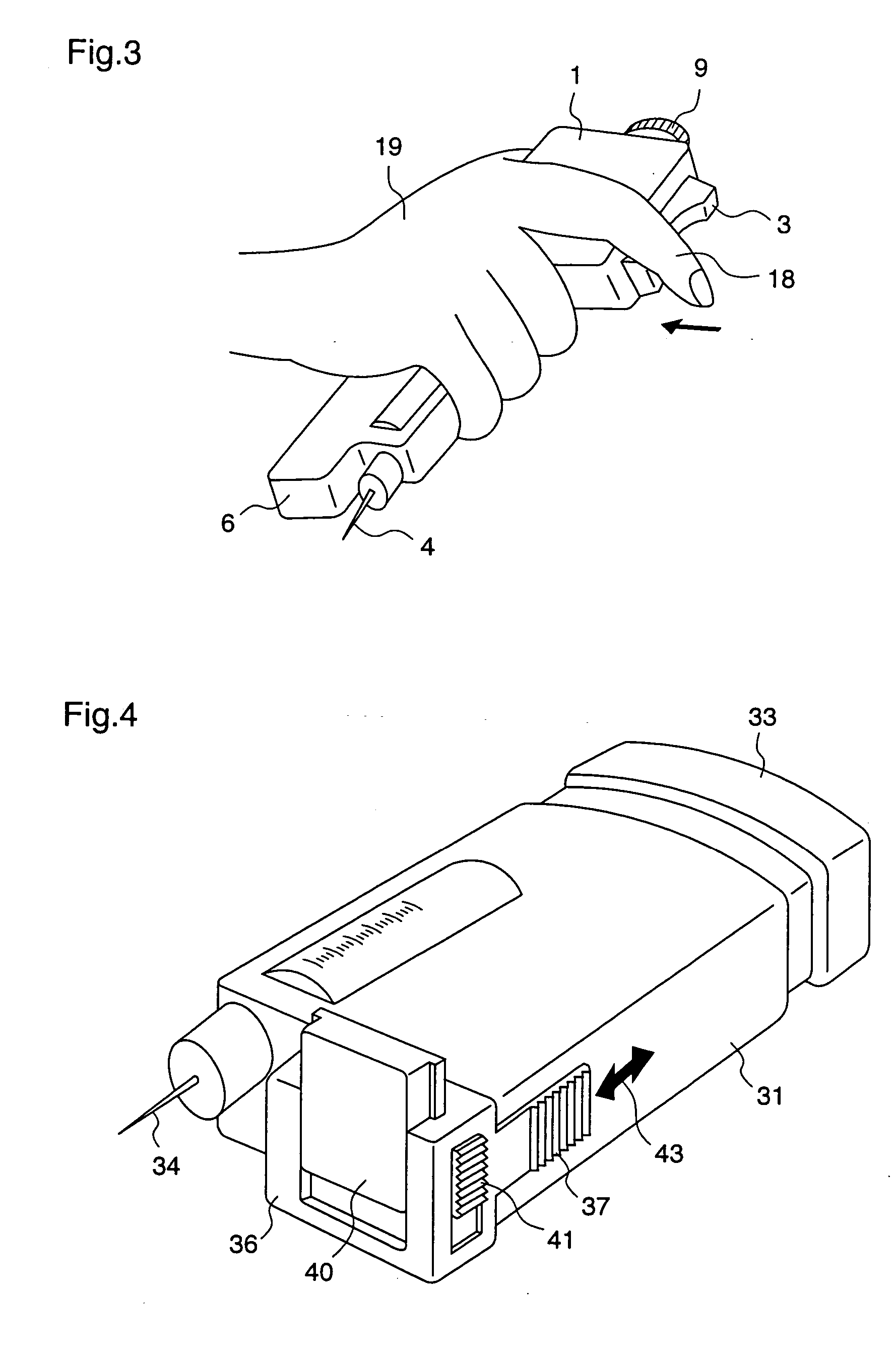
ActiveUS20050209569A1Control depthReduce painAmpoule syringesAutomatic syringesDrugs solutionNeedle insertion
After pressing a part of the exterior of a body of an administration instrument against a body region of a patient to which a drug solution is to be administered, an injection needle that is housed in the instrument body is automatically protruded from the body to insert the needle into the body region, and further, the injection needle being inserted into the body region is automatically housed in the instrument body to remove the needle from the body region. Thereby, the pain of the patient during needle insertion and needle removal is reduced, and administration at a constant speed is possible during injection of the drug solution. Furthermore, even when two kinds of drug solutions or a dissolving and mixing type drug solution are / is used, mixing can be easily and reliably carried out.
Owner:PHC HLDG CORP
Electrosurgical tissue treatment method
InactiveUS20050177210A1Surgical instruments for heatingTherapeutic coolingCardiac AblationBiomedical engineering
A medical probe assembly, system, and methods for the use thereof to treat tissue are described. The system optionally comprises an energy source, two internally-cooled probe assemblies, and one or more cooling devices to provide cooling to at least one of the probe assemblies. The probe assemblies may be configured in a bipolar mode, whereby current flows preferentially between the probe assemblies. The probe assemblies and system described herein are particularly useful to deliver radio frequency energy to a patient's body. RF energy delivery may be used for various applications, including the treatment of pain, tumor ablation and cardiac ablation.
Owner:AVANOS MEDICAL SALES LLC
Intracutaneous injection
ActiveUS20060211982A1Minimize injectionMinimize medicationPeptide/protein ingredientsAutomatic syringesSlurryIntracutaneous injection
The delivery of biopharmaceutical and other therapeutic agents parenterally to an animal via a minimally invasive, low pain administration is provided. The agents are delivered to the patient via, e.g., the epidermal, dermal, or subcutaneous layer of the skin in a concentrated form of injectable paste of slurry.
Owner:XERIS PHARMA
Apparatus and method for a global model of hollow internal organs including the determination of cross-sectional areas and volume in internal hollow organs and wall properties
InactiveUS20090062684A1Ease of evaluationCorrection errorCatheterDiagnostic recording/measuringThree vesselsVisceral organ
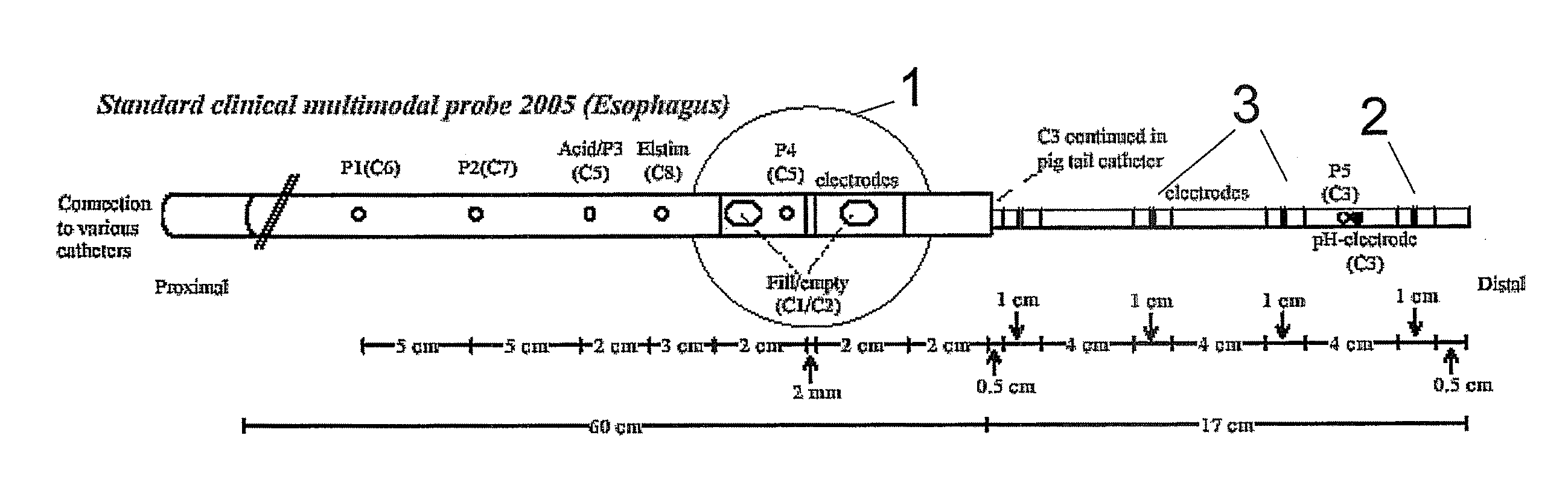
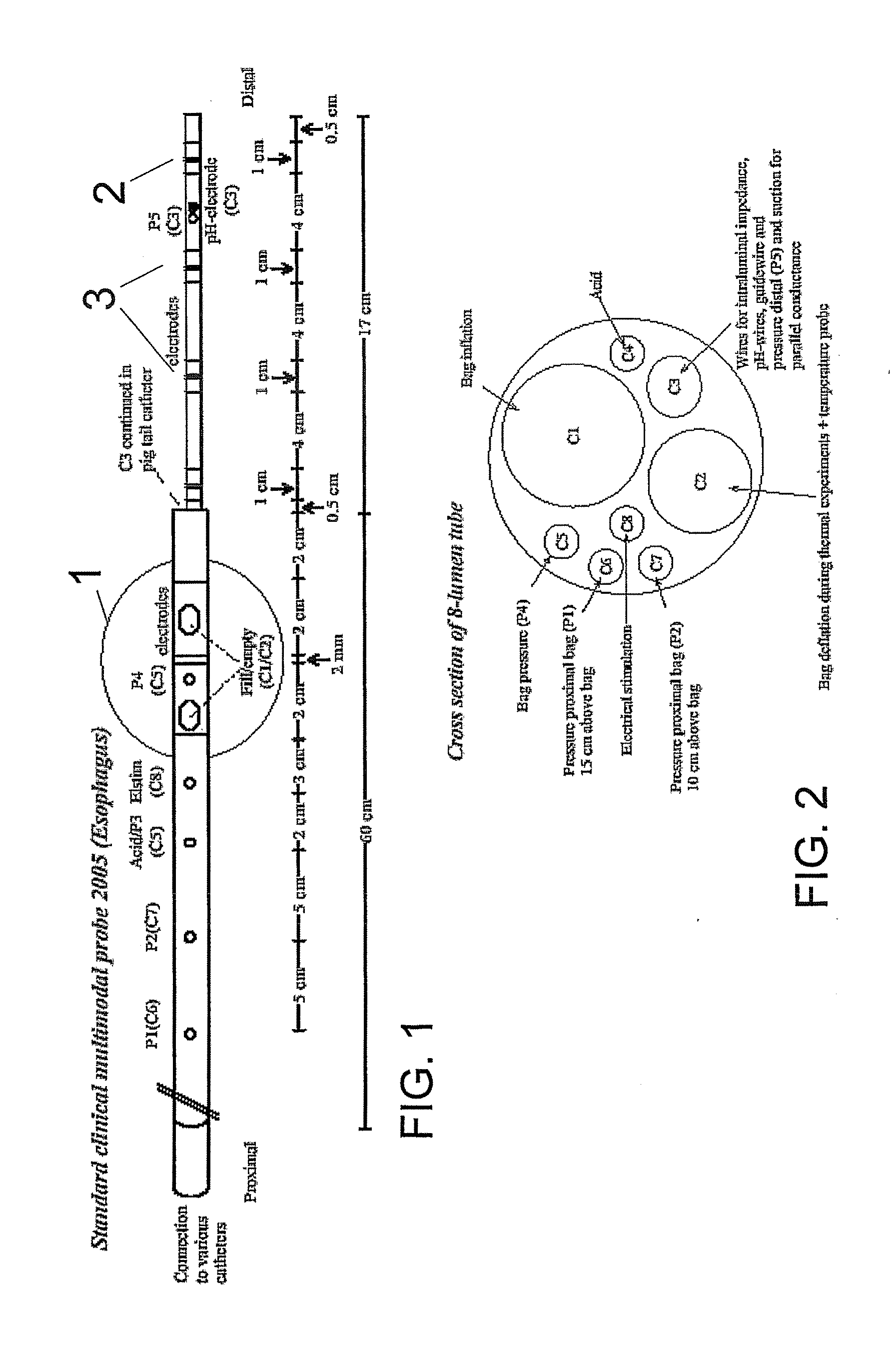
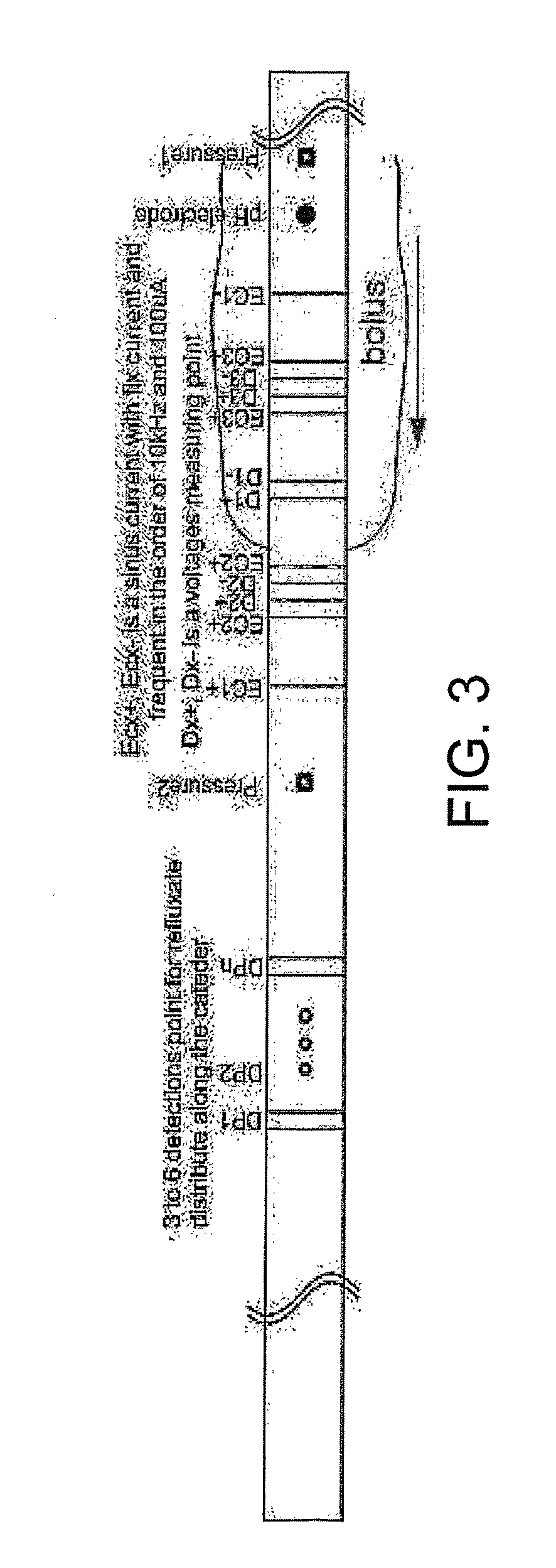
The present invention relates generally to medical measurement systems for evaluation of organ function and understanding symptom and pain mechanisms. This model takes into account a number of factors such as volume and properties of the fluid and the surrounding tissue. Particular emphasis is on a multifunctional probe that can provide a number of measurements including volume of refluxate in the esophagus and to what level it extents. The preferred embodiments of the invention relate to methods and apparatus for measuring luminal cross-sectional areas of internal organs such as blood vessels, the gastrointestinal tract, the urogenital tract and other hollow visceral organs and the volume of the flow through the organ. It can also be used to determine conductivity of the fluid in the lumen and thereby it can determine the parallel conductance of the wall and geometric and mechanical properties of the organ wall.
Owner:GREGERSEN ENTERPRISES 2005
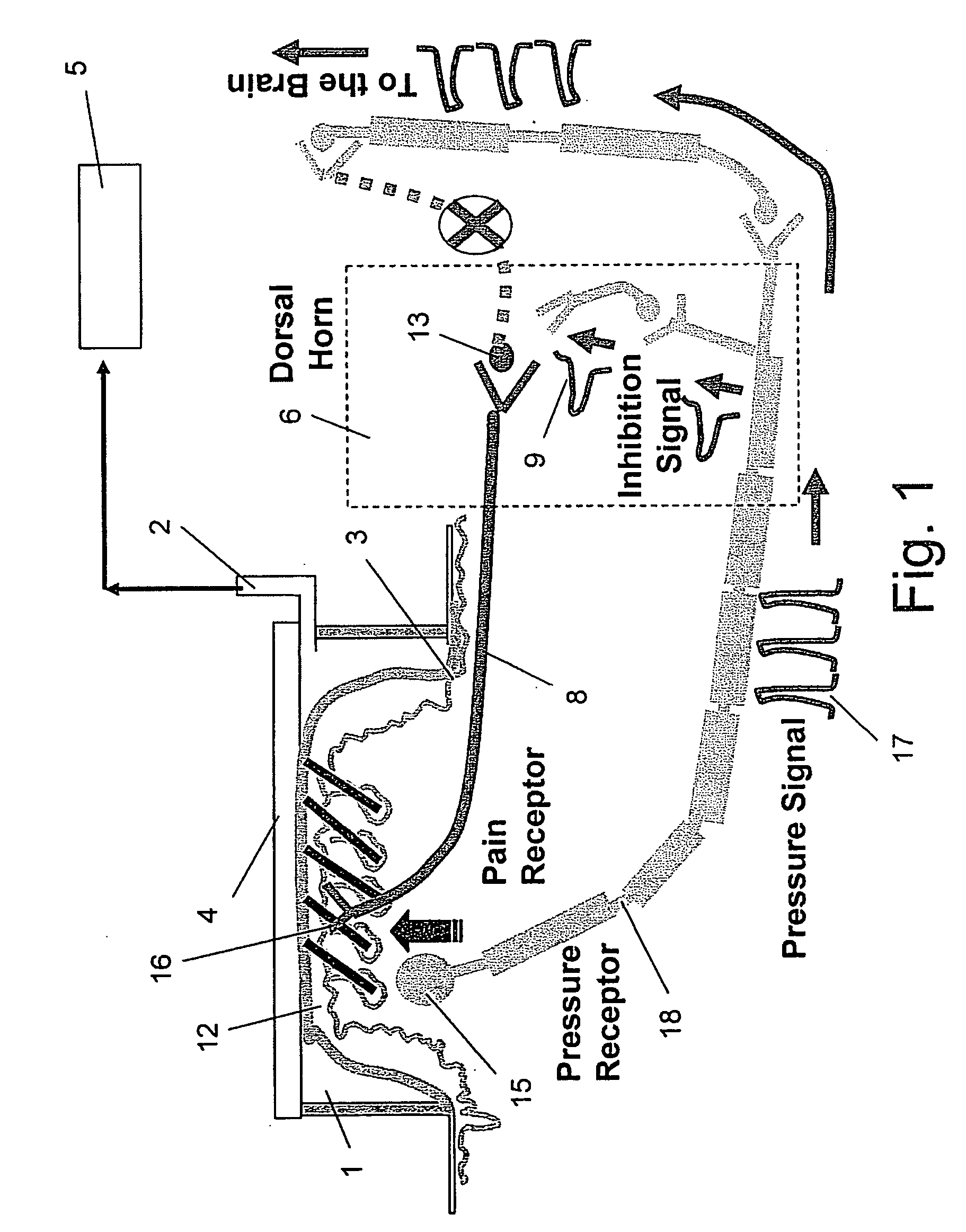
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
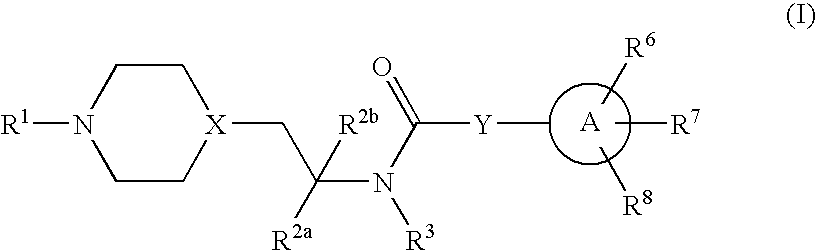
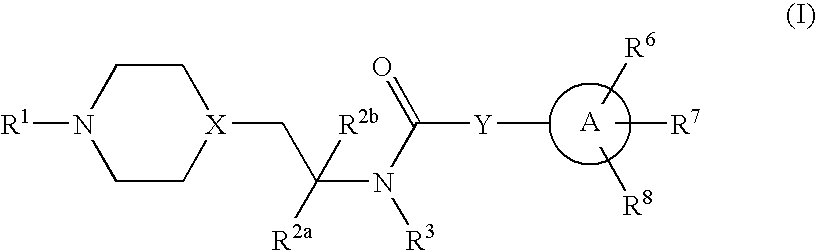
Inhibitors of P2X3
Compounds of formula 1 are modulators of P2X3 useful for the treatment of pain and genito-urinary, gastrointestinal, and respiratory disorders: wherein R1 is —C(═S)CH3, pyridyl, pyrimidinyl, pyrazinyl, thiazolyl, furyl, furylcarbonyl, acetyl, or carbamoyl; R2a and R2b are independently H, methyl, or ethyl; R3 is H or methyl; Y is a bond, —(CR4R5)n— or —CR4═CR5—; wherein R4 and R5 are each independently H or methyl and n is 1 or 2; X is N or CH; A is phenyl, 5-membered heterocyclyl, or 6-membered heterocyclyl; R6, R7 and R8 are each independently H, halo, lower alkyl, cycloalkyl, alkylthio, alkylthio-lower alkyl, alkylsulfonyl-lower alkyl, di(lower alkyl)amino-lower alkyl, morpholinyl-lower alkyl, 4-methyl-piperazinyl-methyl, trifluoromethyl, pyridyl, tetrazolyl, thiophenyl, phenyl, biphenyl, or benzyl (where thiophenyl, phenyl and benzyl are substituted with 0-3 lower alkyl, halo, sulfonamido, trifluoromethyl, lower alkoxy or lower alkylthio) or R6 and R7 together form a 5-membered or 6-membered carbocyclic or heterocyclic ring substituted with 0-3 substituents selected from the group consisting of lower alkyl, lower alkoxy, oxo, halo, thiophenyl-lower alkyl, phenyl, benzyl (where phenyl and benzyl are substituted with 0-3 lower alkyl, halo, sulfonamido, trifluoro-methyl, lower alkoxy, lower alkylthio, amino-lower alkyl, lower alkylamino-lower alkyl, or di(lower alkyl)amino-lower alkyl); and pharmaceutically acceptable salts thereof; wherein when R1 is pyrimidin-2-yl, X is N, Y is a bond and A is oxazol-5-yl the carbon atom at position 4 in said oxazol-5-yl is not substituted by propyl when the carbon atom at position 2 in said oxazol-5-yl is substituted by substituted phenyl and the carbon atom at position 4 in said oxazol-5-yl is not substituted by phenyl when the carbon atom at position 2 is substituted by unsubstituted or substituted phenyl.
Owner:ROCHE PALO ALTO LLC
Methods and compositions for the treatment of pain and other alpha 2 adrenergic-mediated conditions
InactiveUS20050058696A1Improve effectivenessHigh activityOrganic active ingredientsSenses disorderAdrenergicSide effect
Owner:ALLERGAN INC
Treatment Of Tissue Volume With Radiant Energy
InactiveUS20070213792A1Reduce sensitivityLower power settingsUltrasound therapyDiagnostics using lightForms of energyMedicine
Owner:PALOMAR MEDICAL TECH
Selective nerve stimulation for the treatment of angina pectoris
InactiveUS20070021786A1Alleviate and deter onsetof painModulate electrical activityElectrotherapyCoronary arteriesMedicine
A method is disclosed for electrically stimulating a cranial nerve, especially a vagus nerve, to treat or alleviate angina pectoris. Pain is lessened or prevented by application of predetermined therapeutic electrical signal to a selected location on the cranial nerve of a patient using an implanted neurostimulating device. Such method employs selective application of electrical signals to a predetermined location on the nerve to alter the activity of the nerve and cause dilation of a coronary artery in the patient, which in turn provides complete or partial relief of chest pain or deters the onset of such pain.
Owner:LIVANOVA USA INC
Controlled and directed local delivery of anti-inflammatory compositions
InactiveUS20060046961A1Relieve painLimiting bone lossOrganic active ingredientsPeptide/protein ingredientsSkeletal injuryControl release
The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by controlled and directed delivery of one or more biological response modifiers to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by implantable or infusion pumps, implantable controlled release devices, or by sustained release compositions comprising biological response modifiers.
Owner:SDGI HLDG
Spine distraction implant
InactiveUS20080086212A1Increase volumeReduce restrictionsInternal osteosythesisSpinal implantsDistractionDevice implant
A spine distraction implant alleviates pain associated with spinal stenosis and facet arthropathy by expanding the volume in the spine canal and / or neural foramen. The implant provides a spinal extension stop while allowing freedom of spinal flexion. An interspinous process implant with a selectably expandable spacer can be placed between adjacent spinous processes. a device implanted between the spinous processes of adjacent vertebrae of the spine can be used for relieving pain associated with the vertebrae and surrounding tissues and structures by maintaining and / or adding distraction between adjacent vertebrae. A tissue expander can be adapted to move from a first insertion position, for ease of implantation between spinous processes, to a second retention position that prevents displacement of the implant. An embodiment of a system can include an implant having a spacer with a thickness and a wing, wherein a first configuration of the wing has a first height substantially similar to the thickness and wherein the wing is adapted to be selectably arranged in a second configuration such that the wing has a second height greater than the first height. A periphery of the implant has a shape generally conformal with a shape of an inner surface of a cannula and a cross-sectional diameter smaller than an inner diameter of the cannula. The cannula is inserted such that a proximal end of the cannula is arranged between the adjacent spinous processes. The implant is then urged into position between the adjacent spinous processes by way of the cannula, and subsequently arranged in a second configuration to fix the implant in position.
Owner:KYPHON
Oral pharmaceutical formulations containing non-steroidal anti-inflammatory drugs and acid inhibitors
InactiveUS20070154542A1AntipyreticDigestive systemSide effectNonsteroidal Antiinflammatory Drugs/NSAIDs
The present disclosure provides enteric coated capsules and orally dissolving films comprising non-steroidal anti-inflammatory drugs and acid inhibitors, as well as methods of treating treatment humans for pain and / or inflammation while reducing gastrointestinal side effects.
Owner:HORIZON PHARMA USA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com