Patents
Literature
109 results about "Muscular spasticity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Spasticity (from Greek spasmos-, meaning 'drawing, pulling') is a feature of altered skeletal muscle performance with a combination of paralysis, increased tendon reflex activity and hypertonia. It is also colloquially referred to as an unusual "tightness", stiffness, or "pull" of muscles.
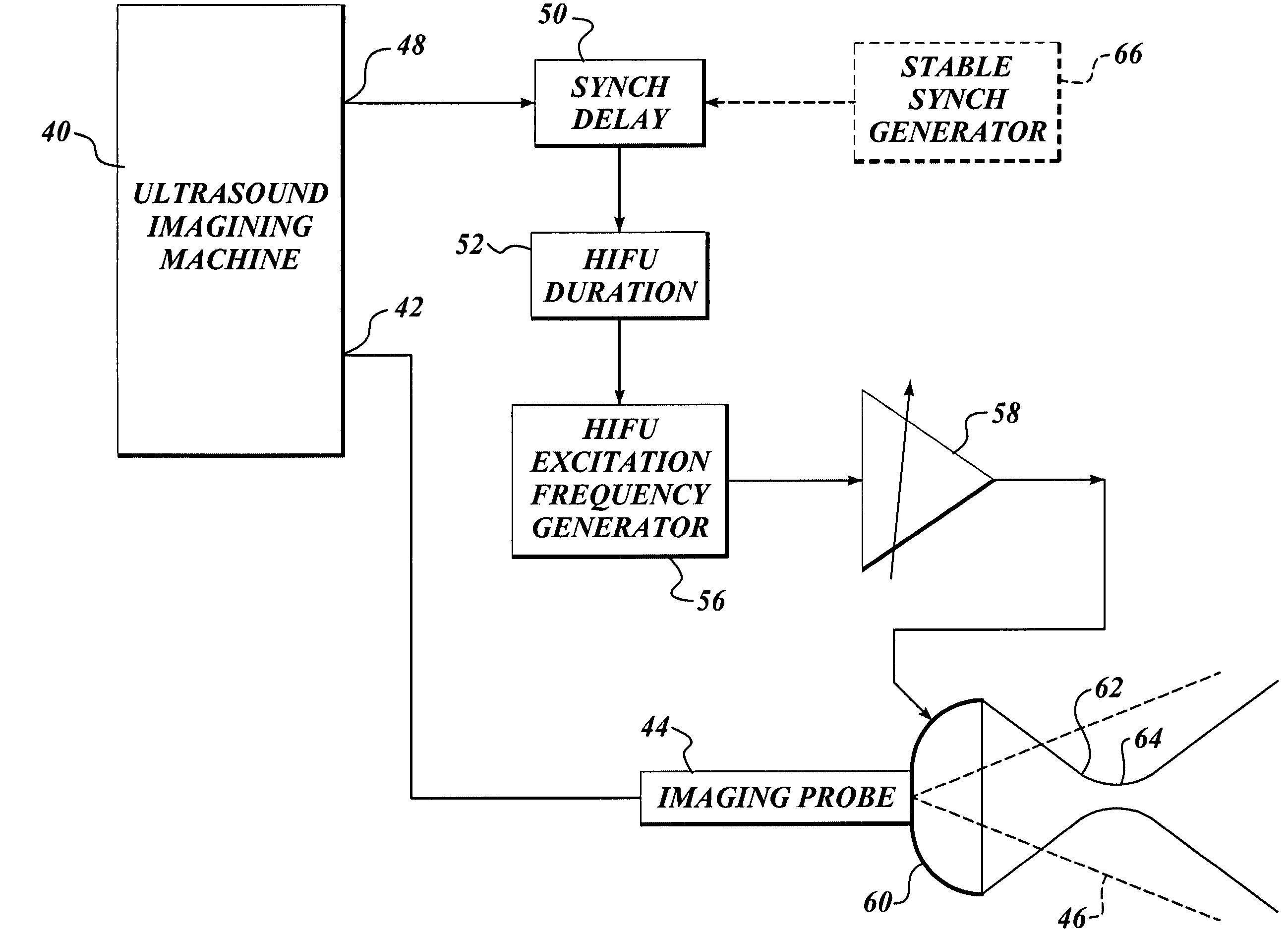
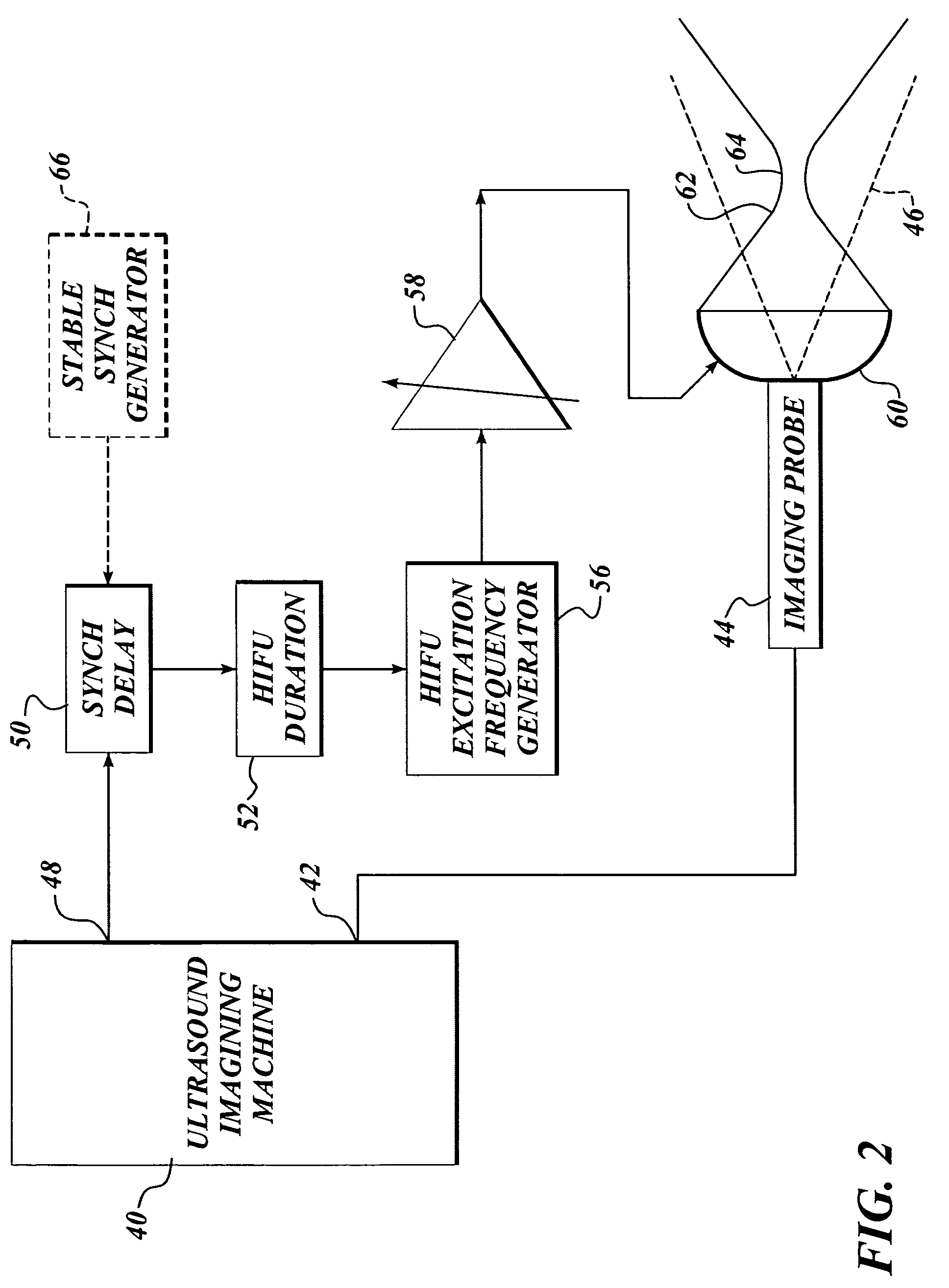
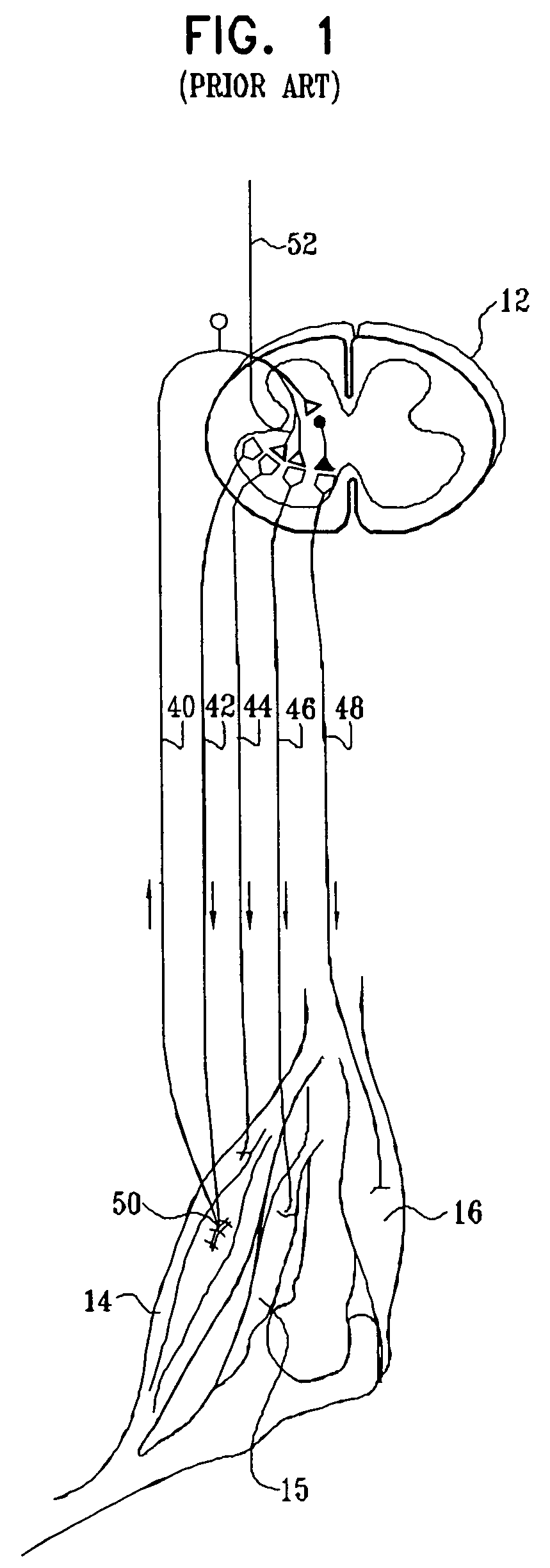
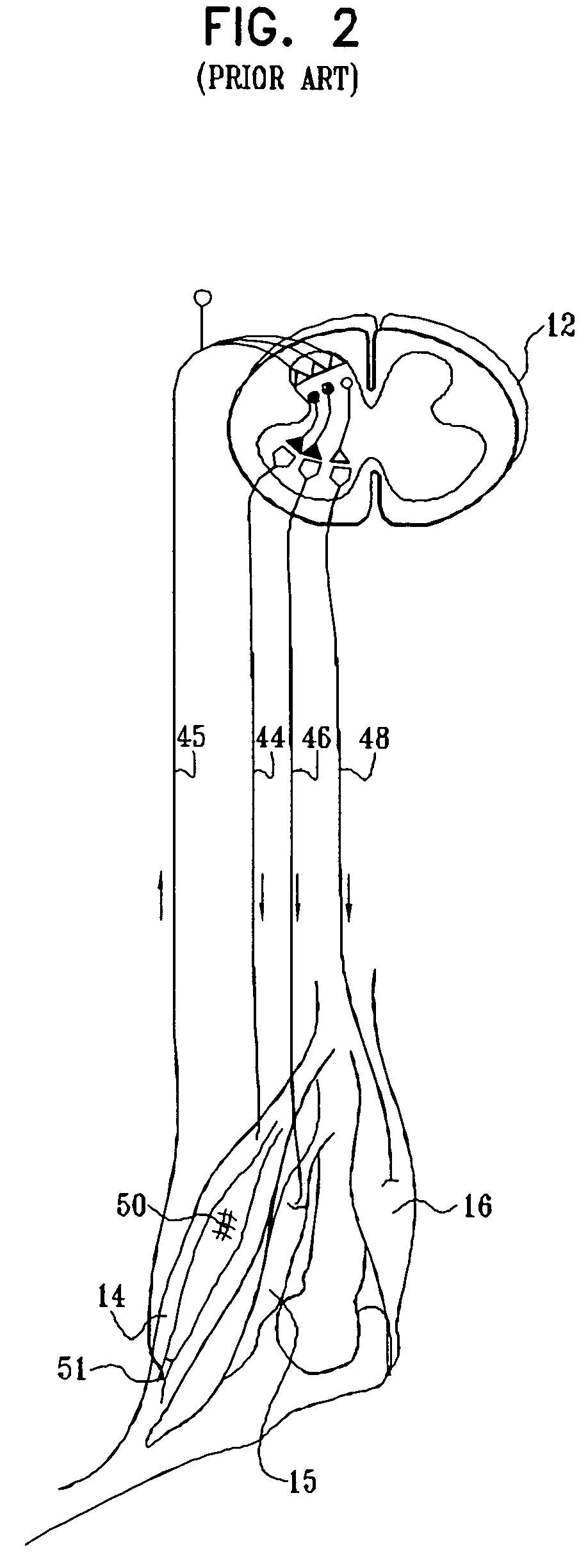
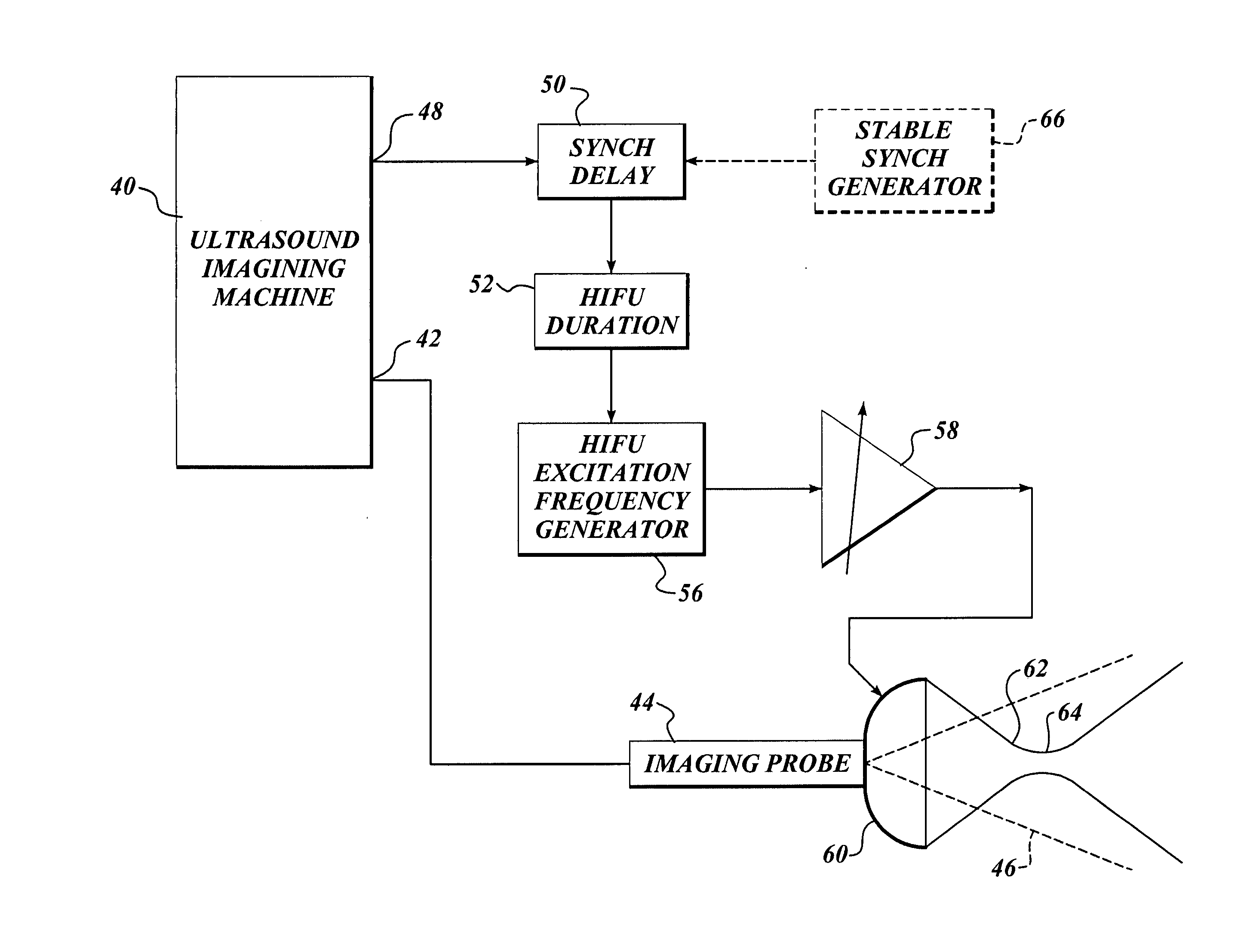
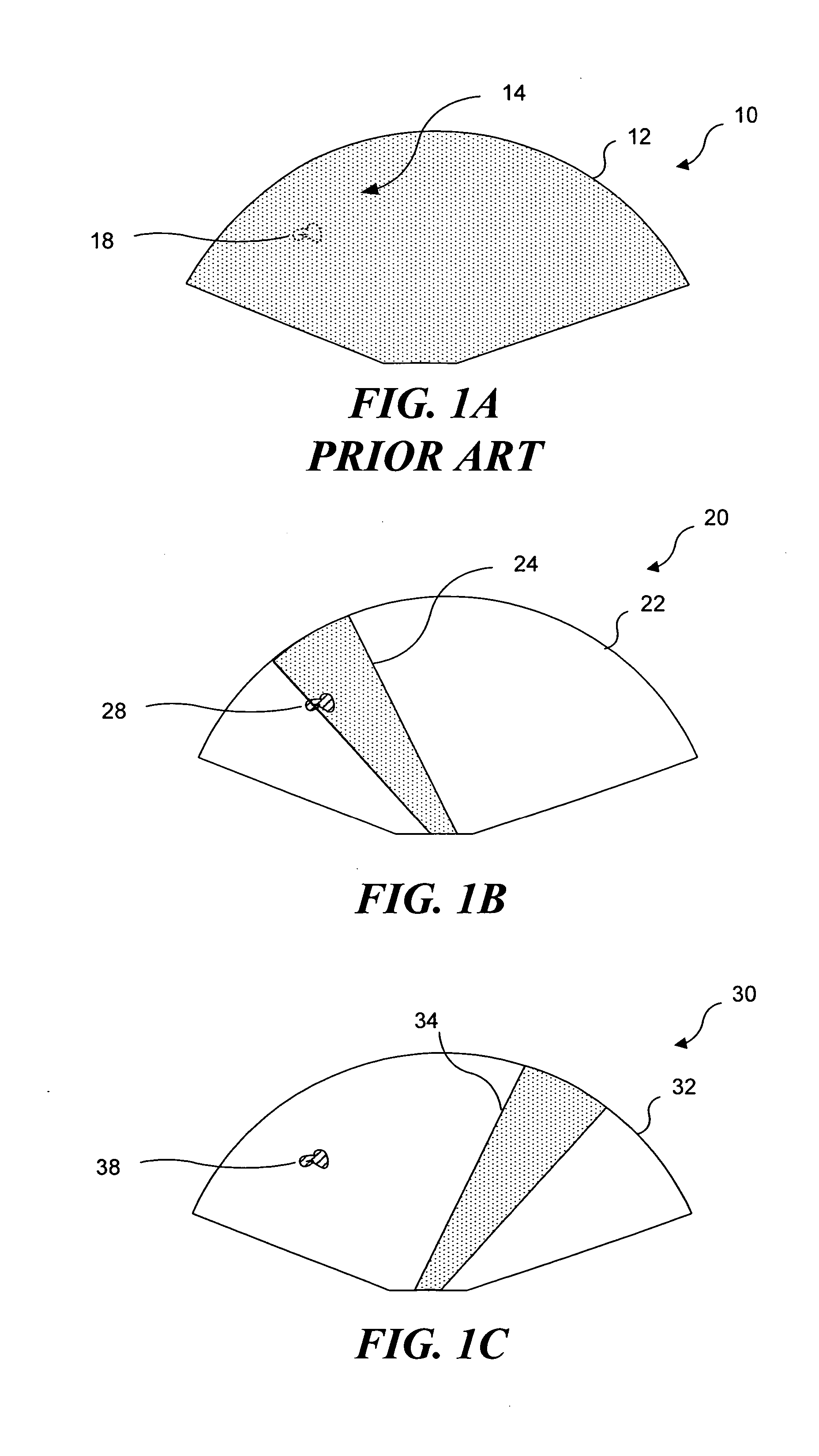
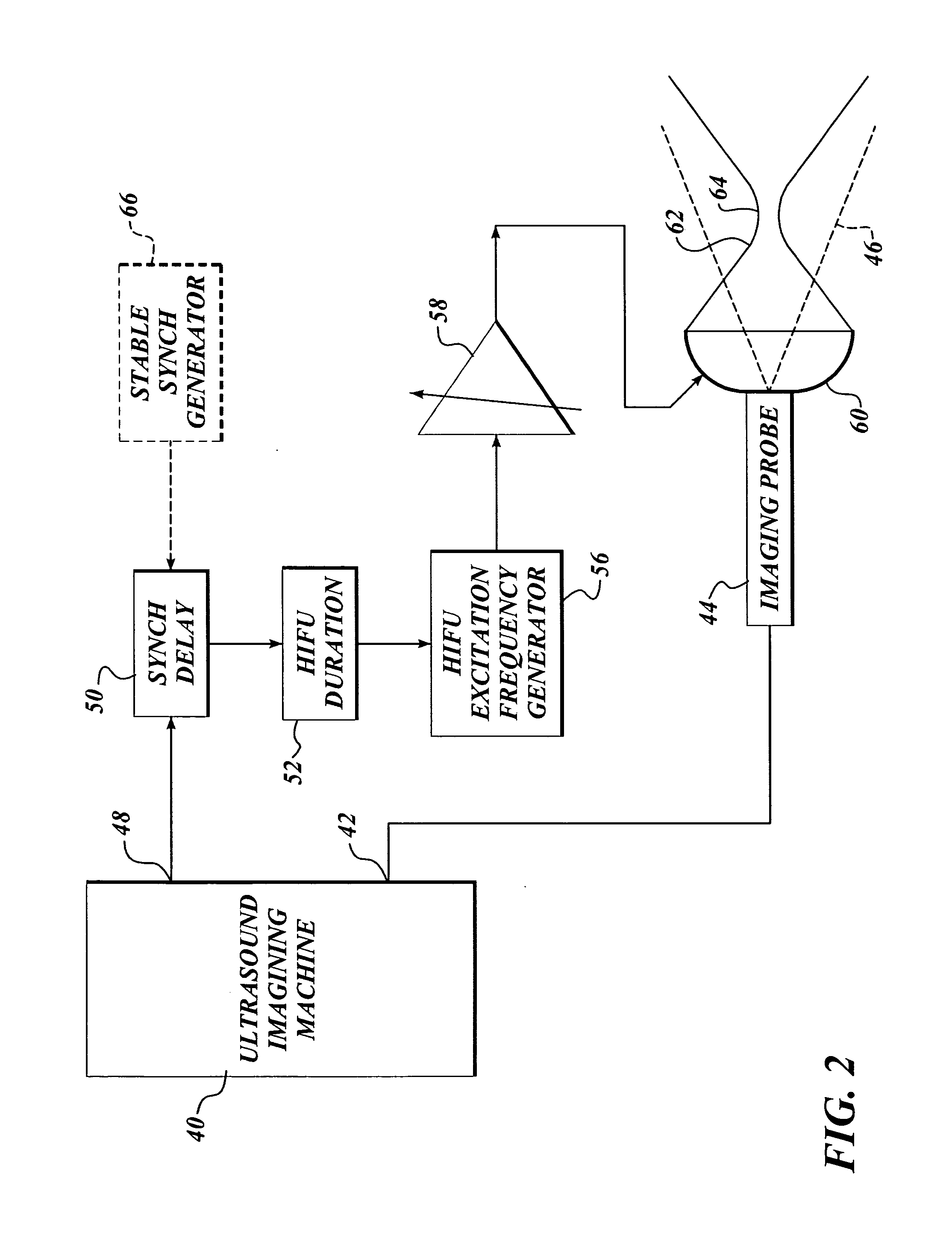
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS7510536B2Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesSonificationHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
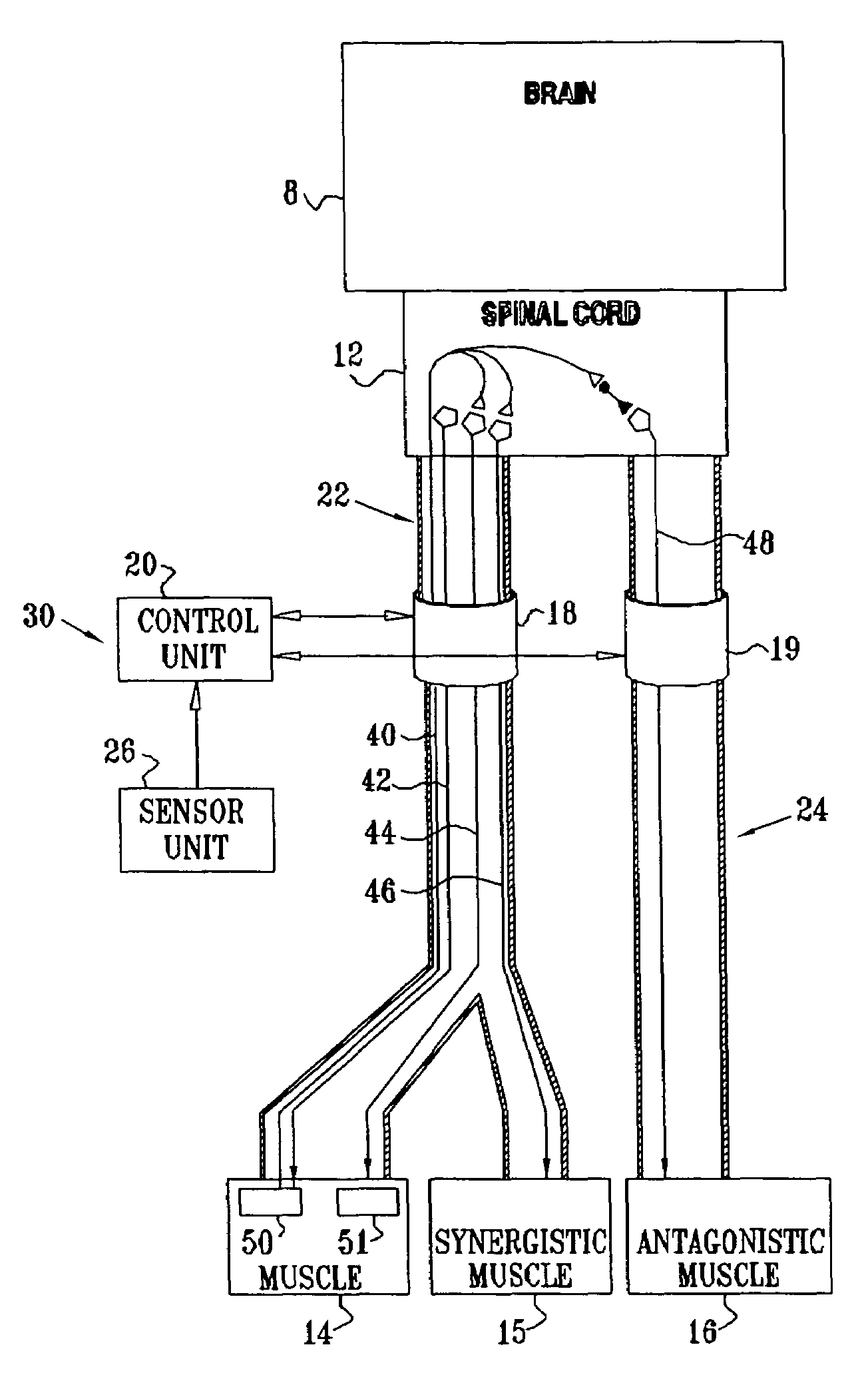
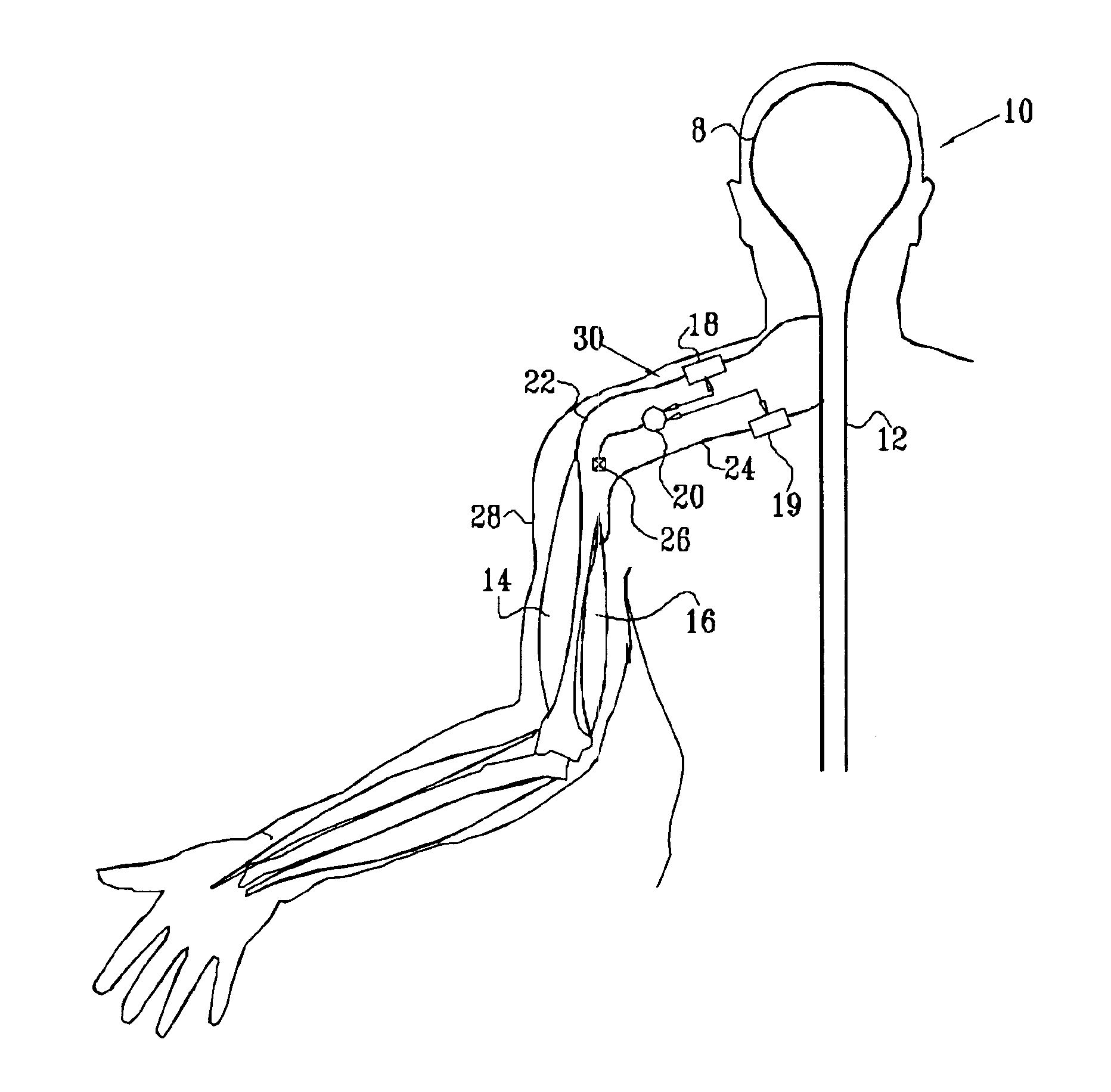
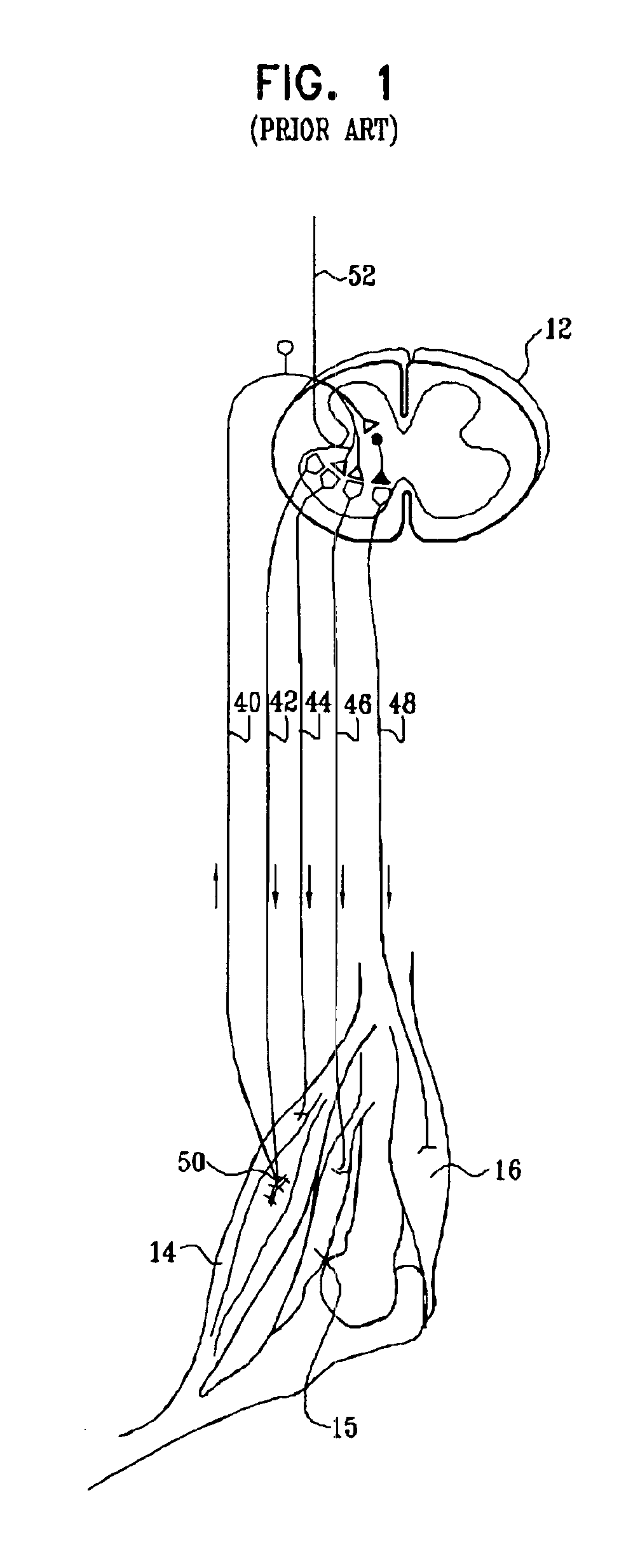
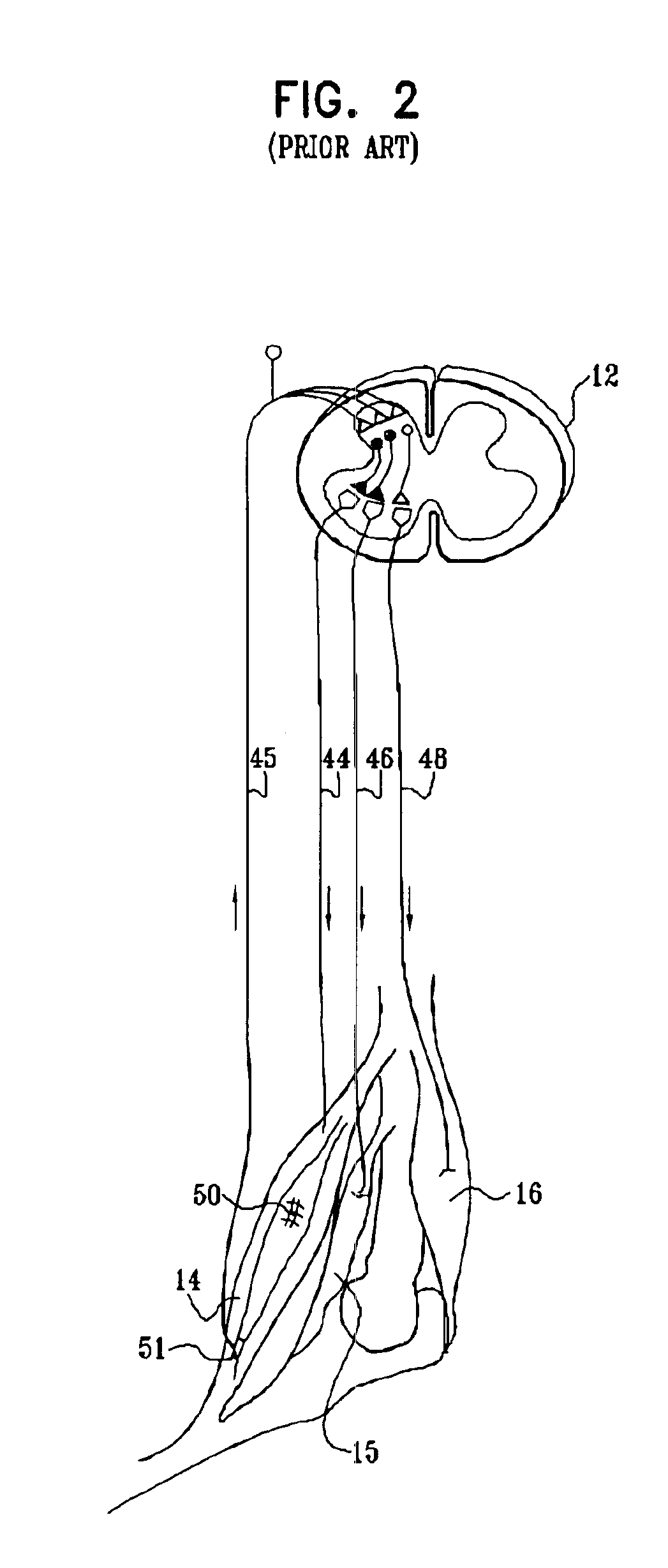
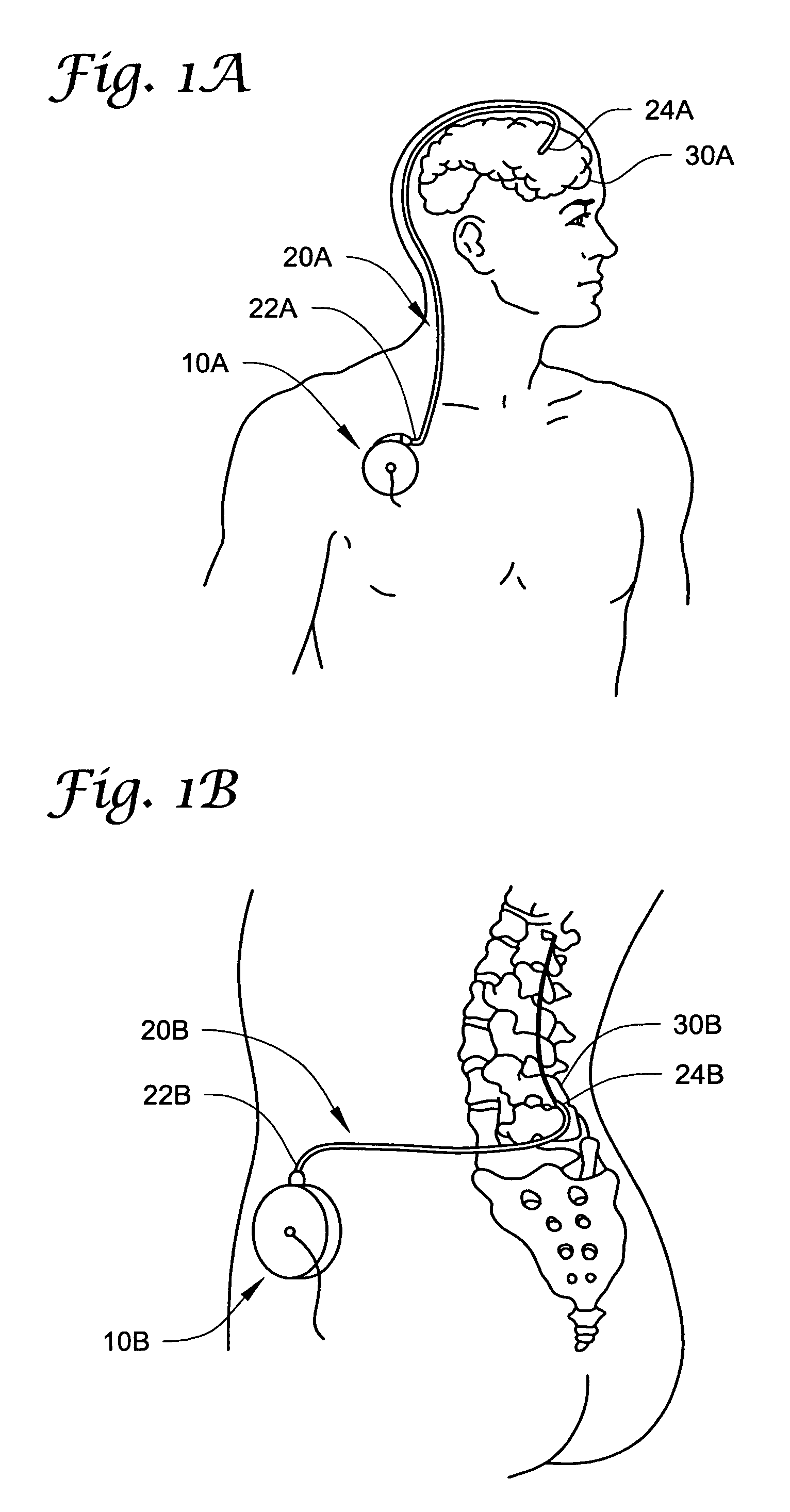
Nerve stimulation for treating spasticity, tremor, muscle weakness, and other motor disorders
InactiveUS7324853B2Induce antagonistic musclesReduce spasmsElectrotherapyArtificial respirationFiberTruncal muscle weakness
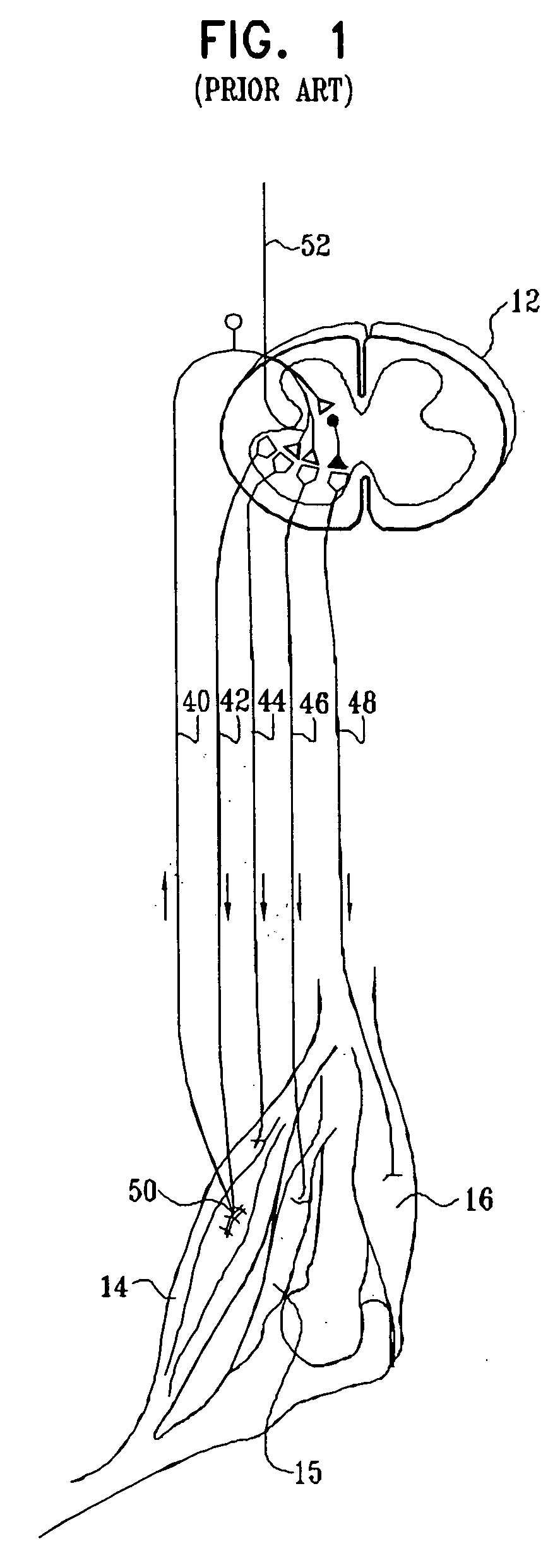
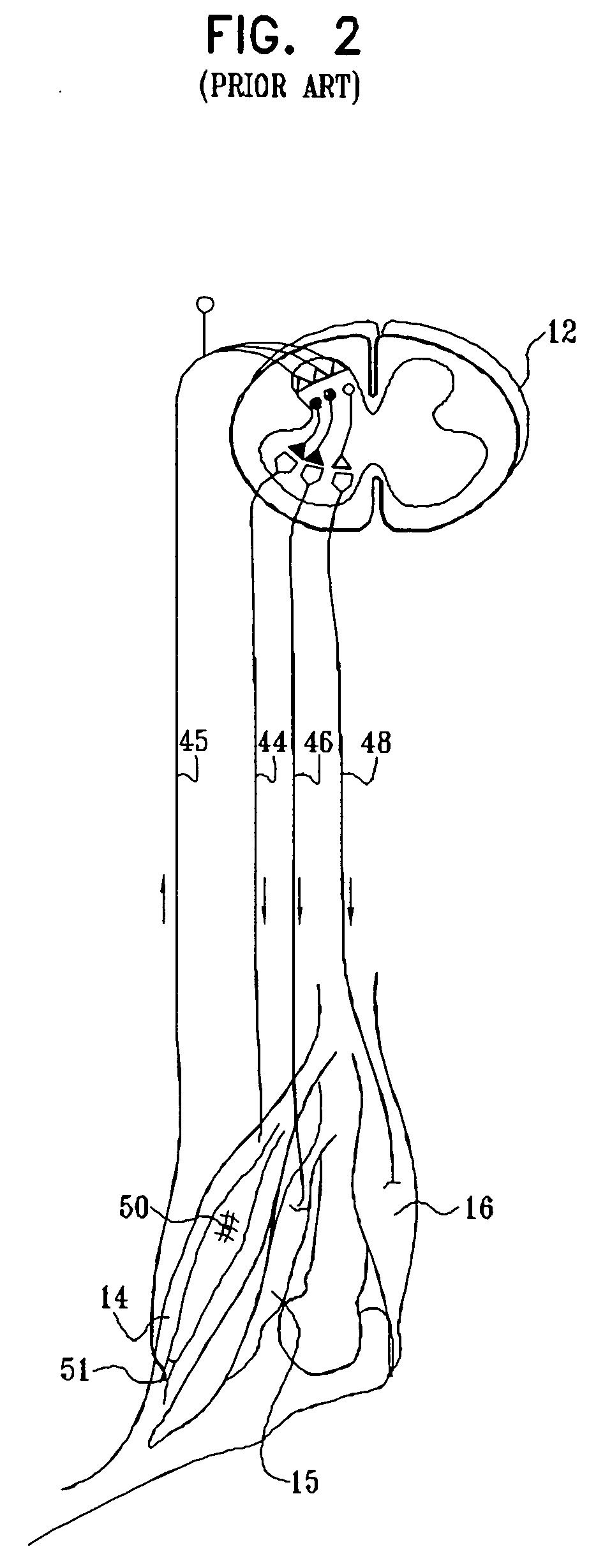
A method for treating spasticity of a subject is provided, including driving a current into a nerve of the subject that includes one or more sensory fibers, and configuring the current so as to inhibit propagation of action potentials in one or more of the sensory fibers, so as to treat the spasticity. In a preferred embodiment, the sensory fibers include one or more Ia sensory fibers, and configuring the current includes configuring the current so as to inhibit propagation of the action potentials in at least one of the Ia sensory fibers.
Owner:MEDTRONIC INC
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS20050240126A1Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesAbnormal tissue growthHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
Nerve stimulation for treating spasticity, tremor, muscle weakness, and other motor disorders
InactiveUS6892098B2Induce antagonistic musclesReduce spasmsElectrotherapyArtificial respirationFiberTruncal muscle weakness
A method for treating spasticity of a subject is provided, including driving a current into a nerve of the subject that includes one or more sensory fibers, and configuring the current so as to inhibit propagation of action potentials in one or more of the sensory fibers, so as to treat the spasticity. In a preferred embodiment, the sensory fibers include one or more Ia sensory fibers, and configuring the current includes configuring the current so as to inhibit propagation of the action potentials in at least one of the Ia sensory fibers.
Owner:BIO CONTROL MEDICAL B C M +1
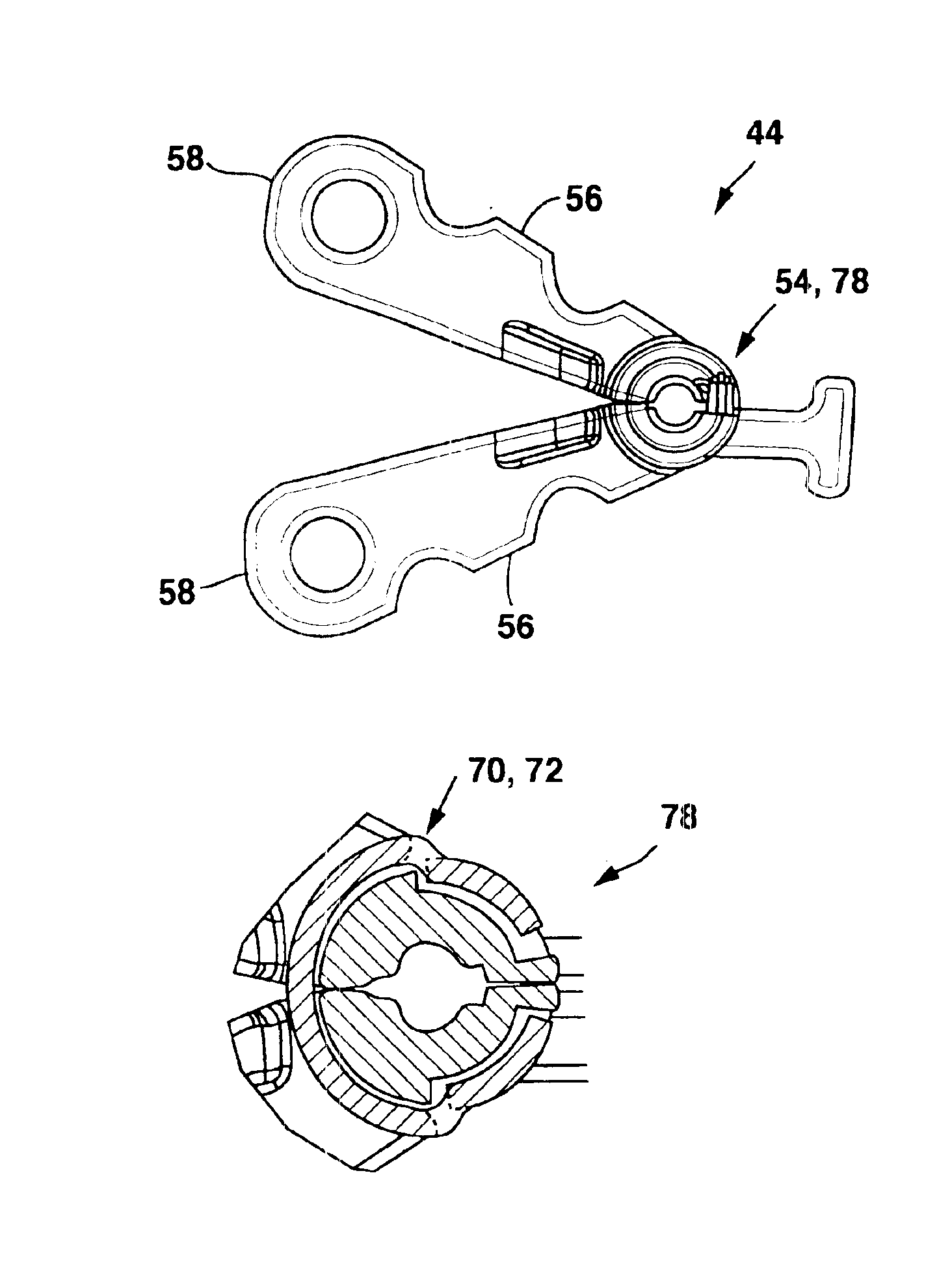
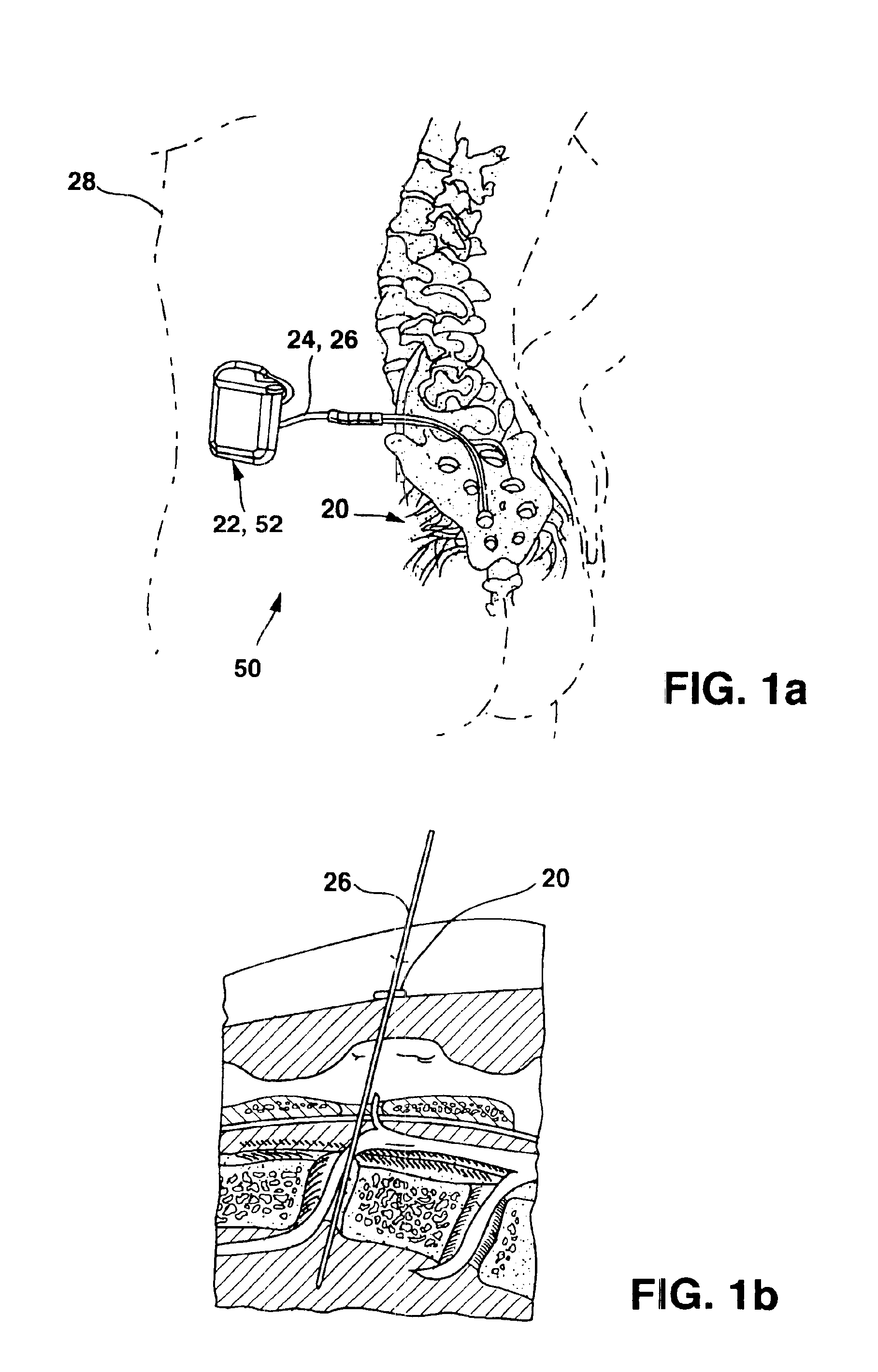
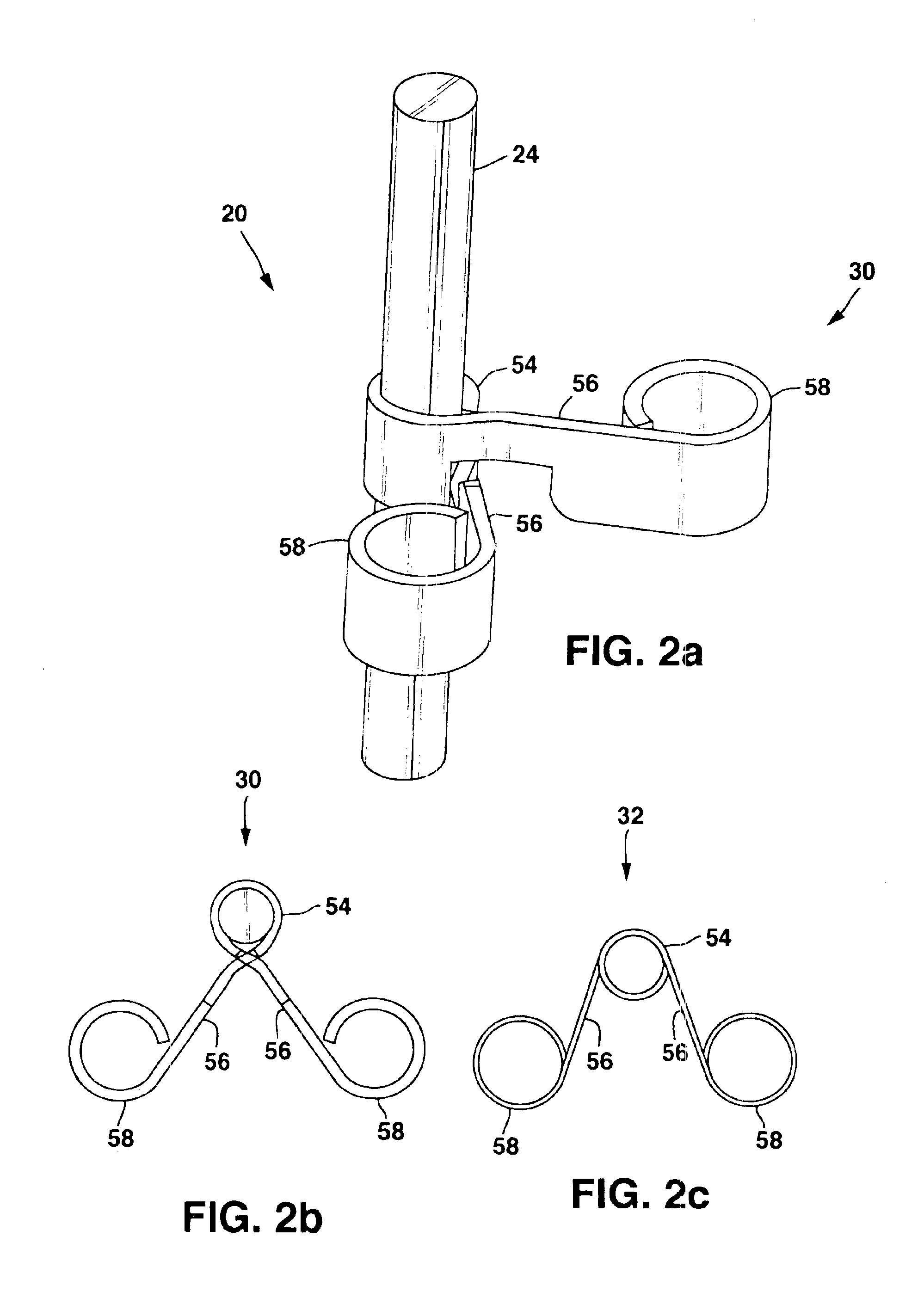
Implantable therapy delivery element adjustable anchor
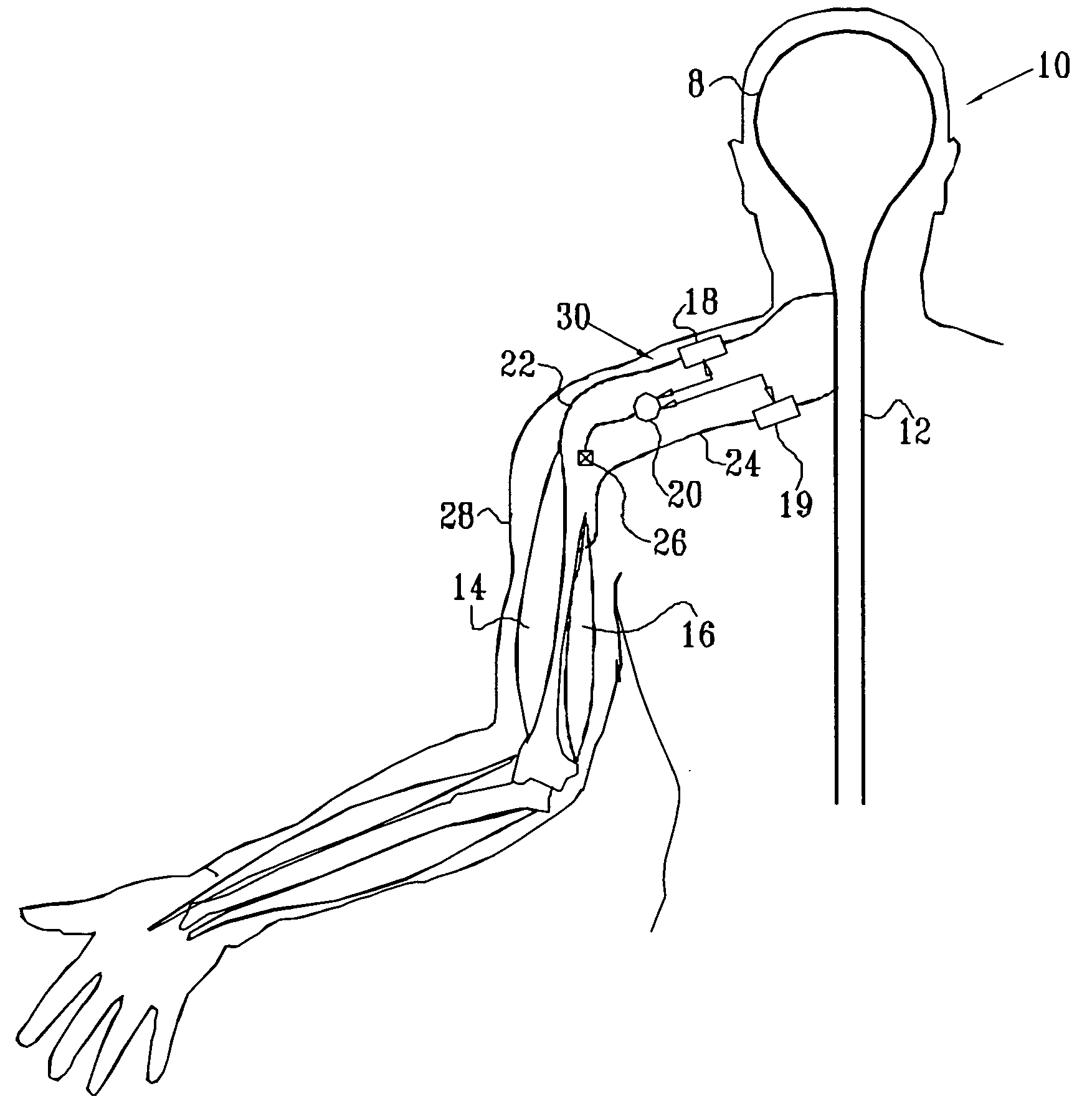
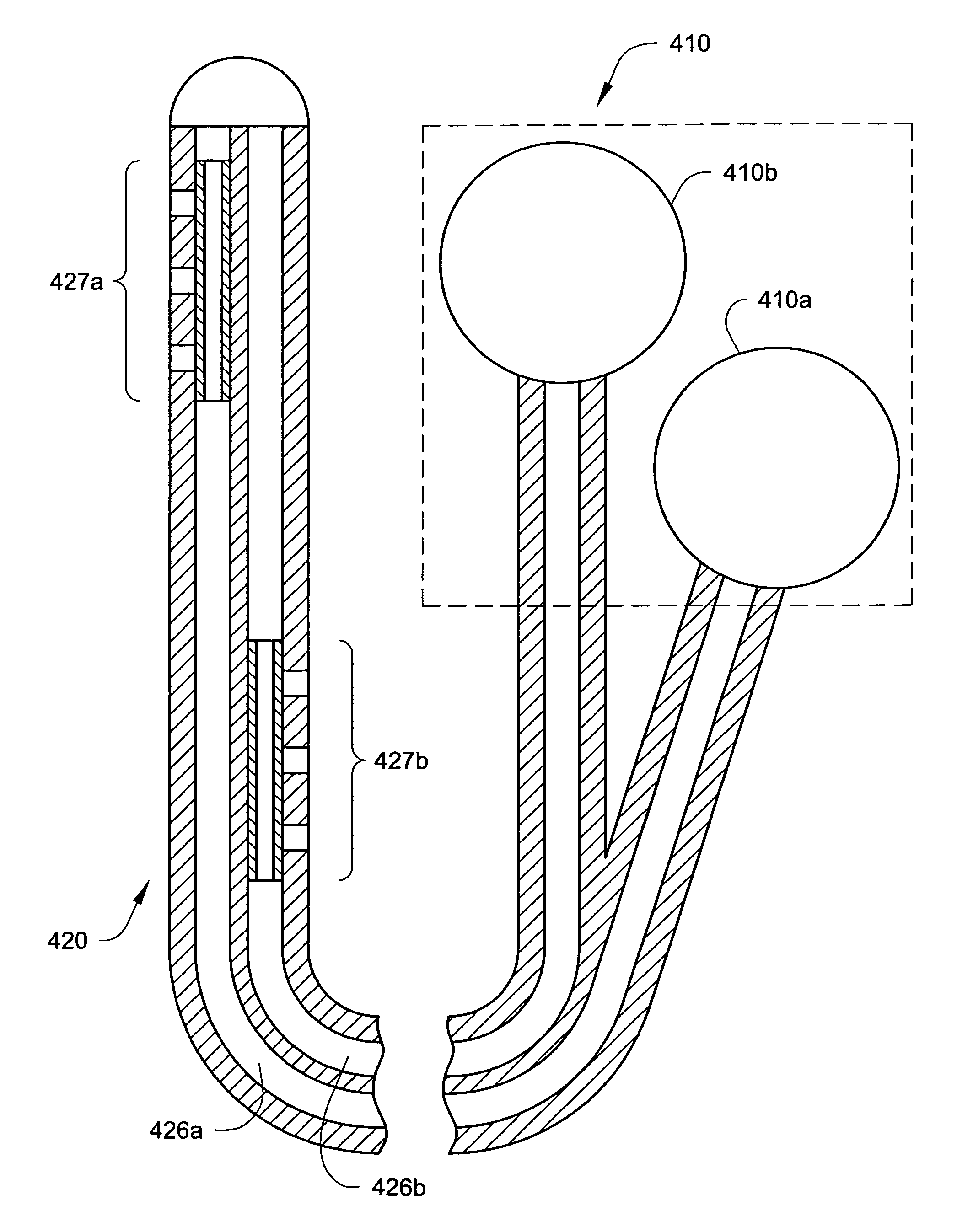
InactiveUS6901287B2Facilitates invasive procedureQuick placementSpinal electrodesDiagnostic recording/measuringHuman bodySacral nerve stimulation
An implantable therapy delivery system has a therapy delivery element that is inserted or implanted into a human body and anchored or fixed to tissue to delivery a therapy to a patient. In one embodiment an implantable neurostimulator uses an electrical stimulation lead to delivery a therapy such as sacral nerve stimulation, peripheral nerve stimulation, and the like. In another embodiment the implantable therapeutic substance delivery device, also known as a drug pump, is connected to a catheter to deliver a therapy to treat conditions such as spasticity, cancer, pain, and the like. The therapy delivery element is anchored to tissue using an adjustable anchor having a therapy grip element, at least two extension elements connected to the therapy grip element, and a tissue fixation element connected to the extension elements. The extensions project substantially perpendicular in relation to the therapy delivery element and are configured to actuate the therapy grip element to an opened position and a closed position. A tissue fixation element is connected to the extensions and configured for fixation to a tissue location from an axial direction to the therapy delivery element. The adjustable anchor facilitates minimally invasive procedures, facilitates securing the therapy delivery element in the same plane as the therapy delivery element was inserted, facilitates rapid placement to reduce procedure time, and provides a wide range of other benefits. The adjustable anchor and its methods of operation have many embodiments.
Owner:MEDTRONIC INC
Topical therapy for the treatment of migranes, muscle sprains, muscle spasms, spasticity and related conditions
InactiveUS20070065463A1Improve efficiencyGood patient acceptanceBiocideBacterial antigen ingredientsMuscular spasticityTopical treatment
The invention is directed to topical formulations and methods of treating a migraines and / or cluster headaches, muscle sprains, muscle spasms, spasticity, tension headaches, tension related migraines and related conditions associated with muscle tension and pain with a therapeutically effective amount of an ergot alkaloid, skeletal muscle relaxant, serotonin agonist, combinations thereof, pharmaceutically acceptable salt thereof, prodrugs thereof or derivative thereof.
Owner:AFGIN PHARMA
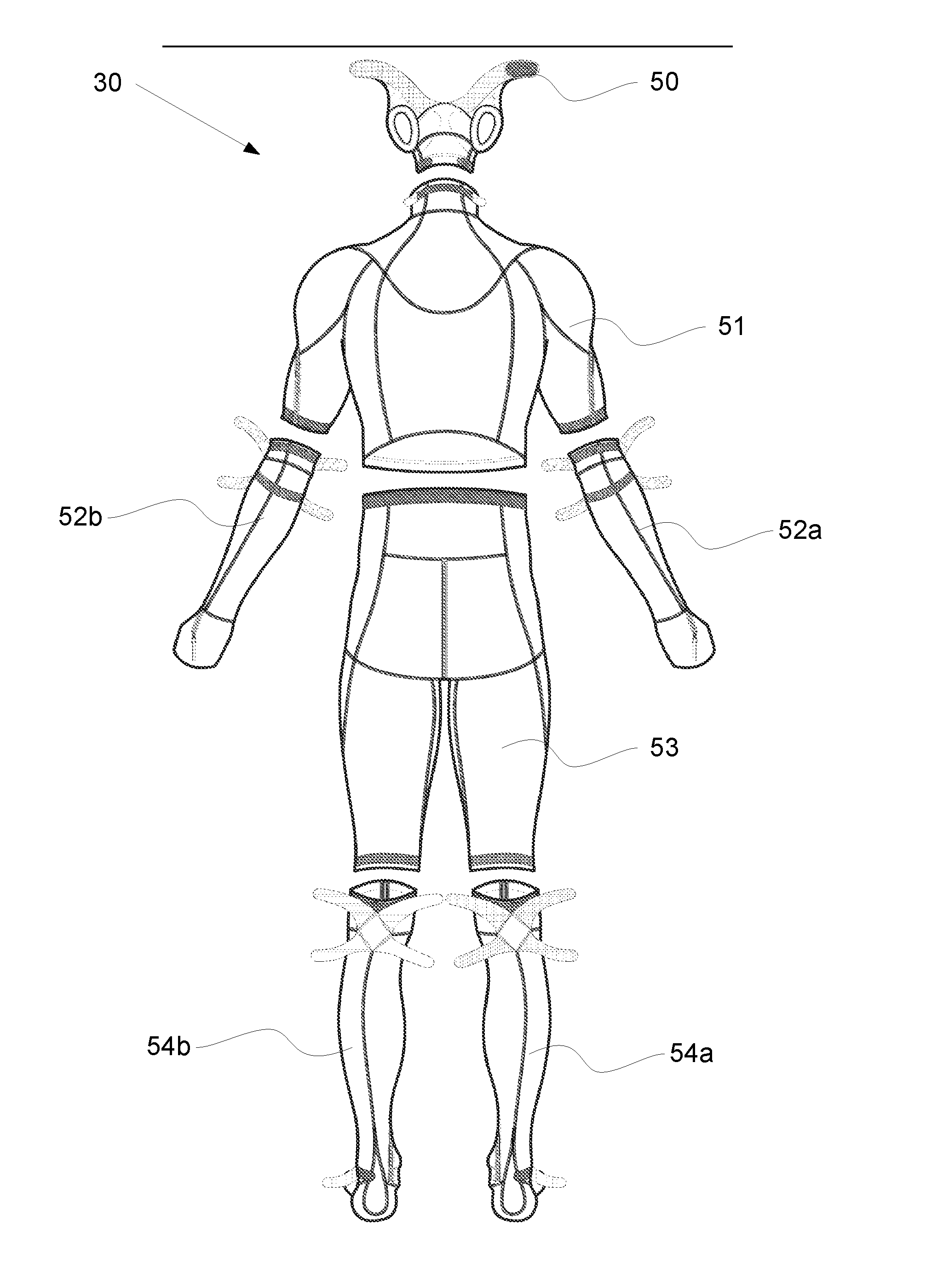
System And Garment For Muscle Relaxation Of A Spastic Muscle
InactiveUS20120245483A1Improve muscle relaxationMitigate, alleviate or eliminate one or moreElectrotherapyElectromyographyDiseaseMuscle relaxation
The present invention relates generally to muscle relaxation. Muscle relaxation is desired in many disease states, including spastic paresis and biomechanical and neuromuscular dysfunction. More specifically, the invention relates to a system that causes muscle relaxation by reducing muscular spasticity through the stimulation of joints and muscles. The system consists of a garment with electrodes, a hardware unit and software controlling the stimulation.
Owner:INERVENTIONS
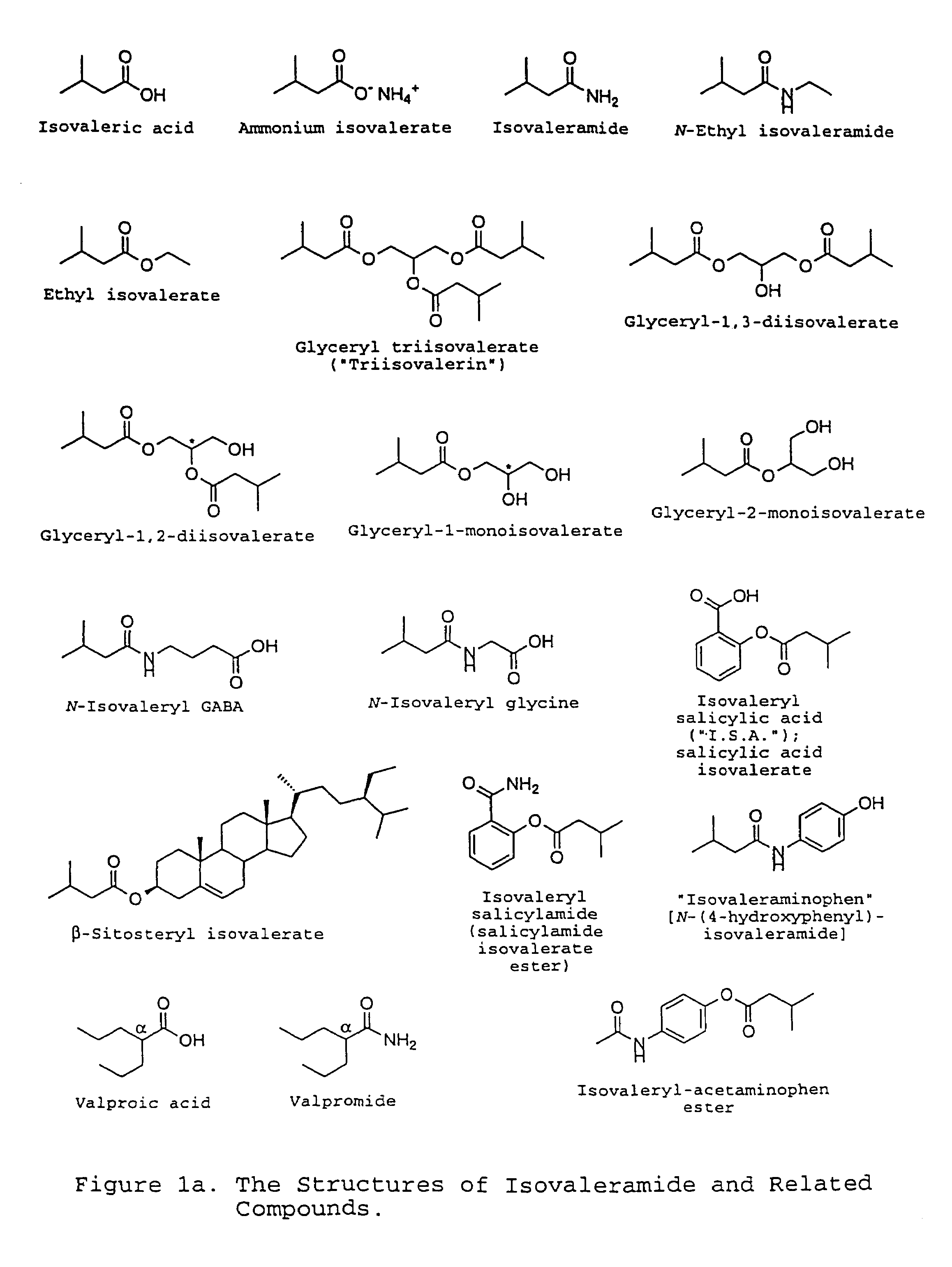
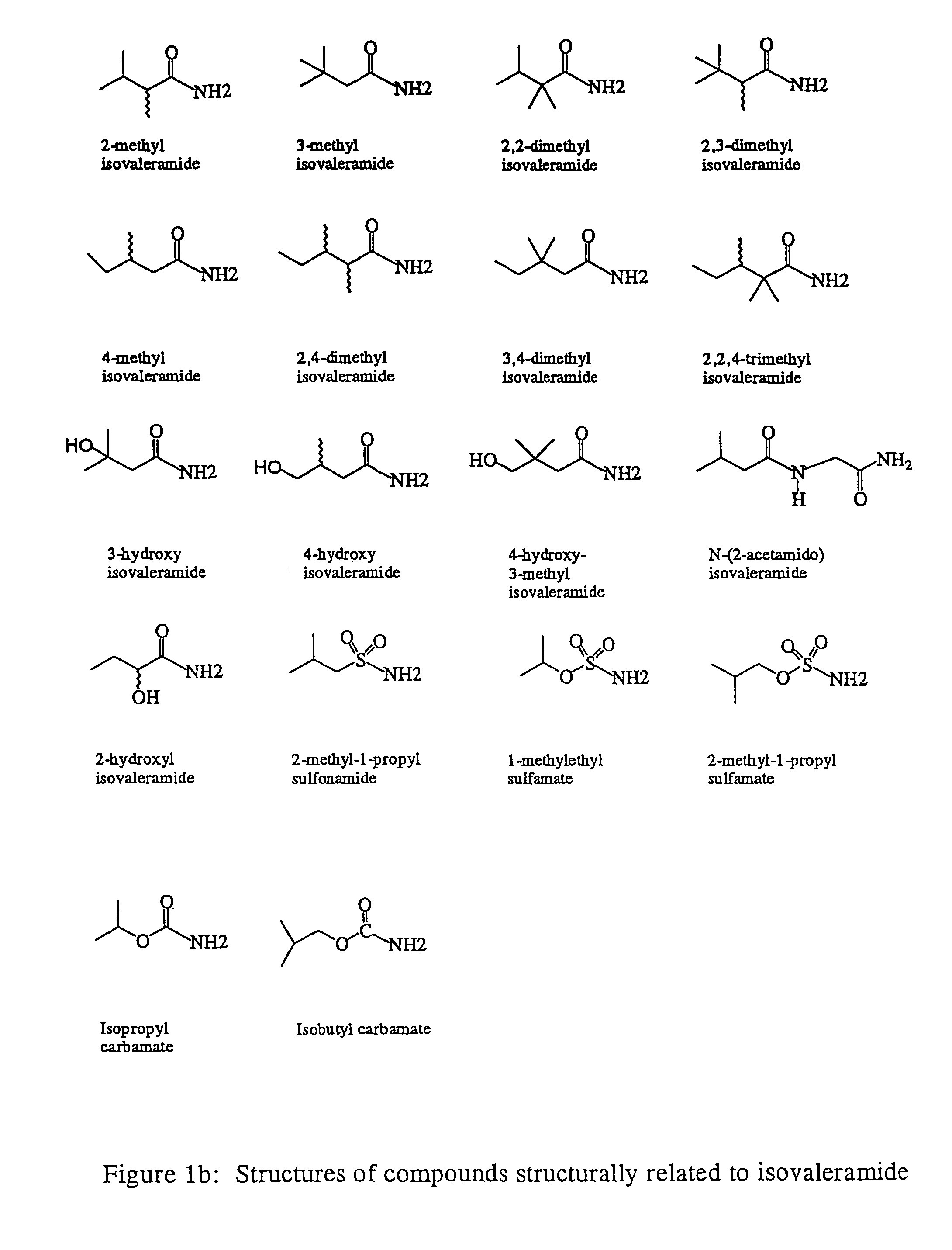
Treating a variety of pathological conditions, including spasticity and convulsions, by effecting a modulation of CNS activity with isovaleramide, isovaleric acid, or a related compound
InactiveUS20040072900A1Many symptomDecrease in muscle toneBiocideSalicyclic acid active ingredientsConvulsionCytotoxicity
Preparations and extracts of valerian, as well as isovaleramide, isovaleric acid, and certain structurally related compounds exhibit clinically significant pharmacological properties which implicate a treatment for a variety of pathological conditions, including spasticity and convulsions, which are ameliorated by effecting a modulation of CNS activity. The compositions in question generally are non-cytotoxic and do not elicit weakness or sedative activity at doses that are effective for the symptomatic treatment of such pathological conditions.
Owner:NPS PHARM INC
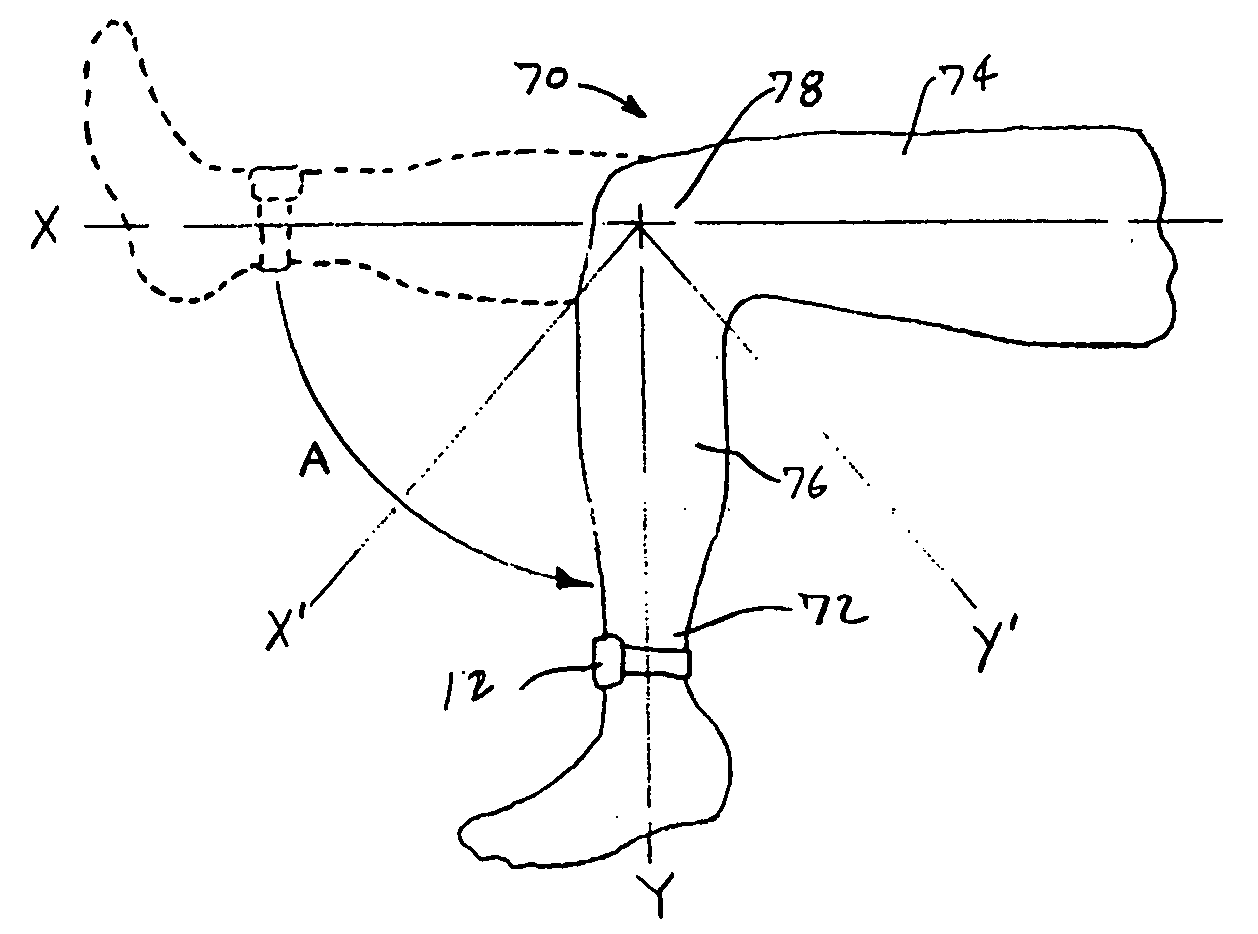
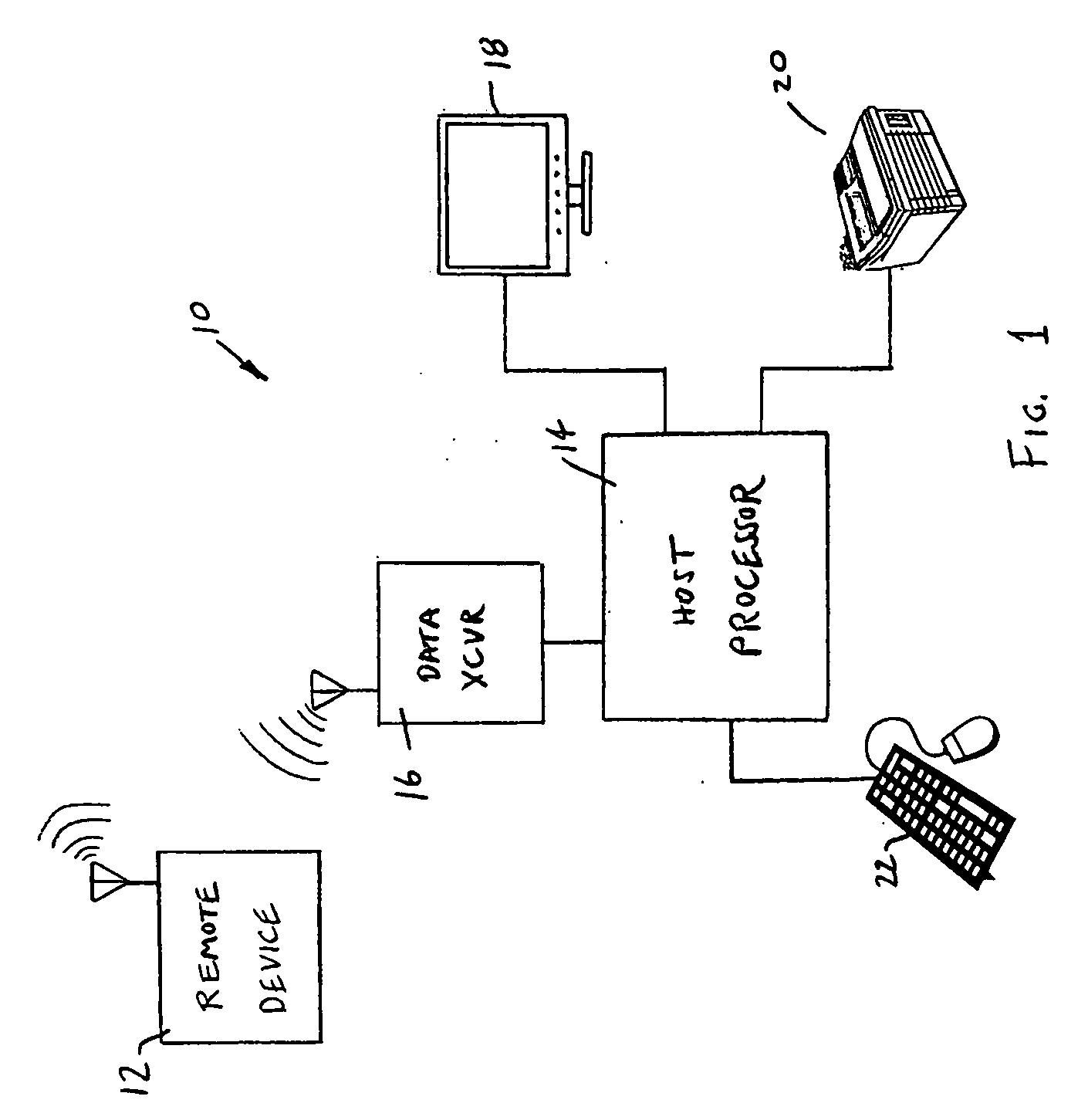
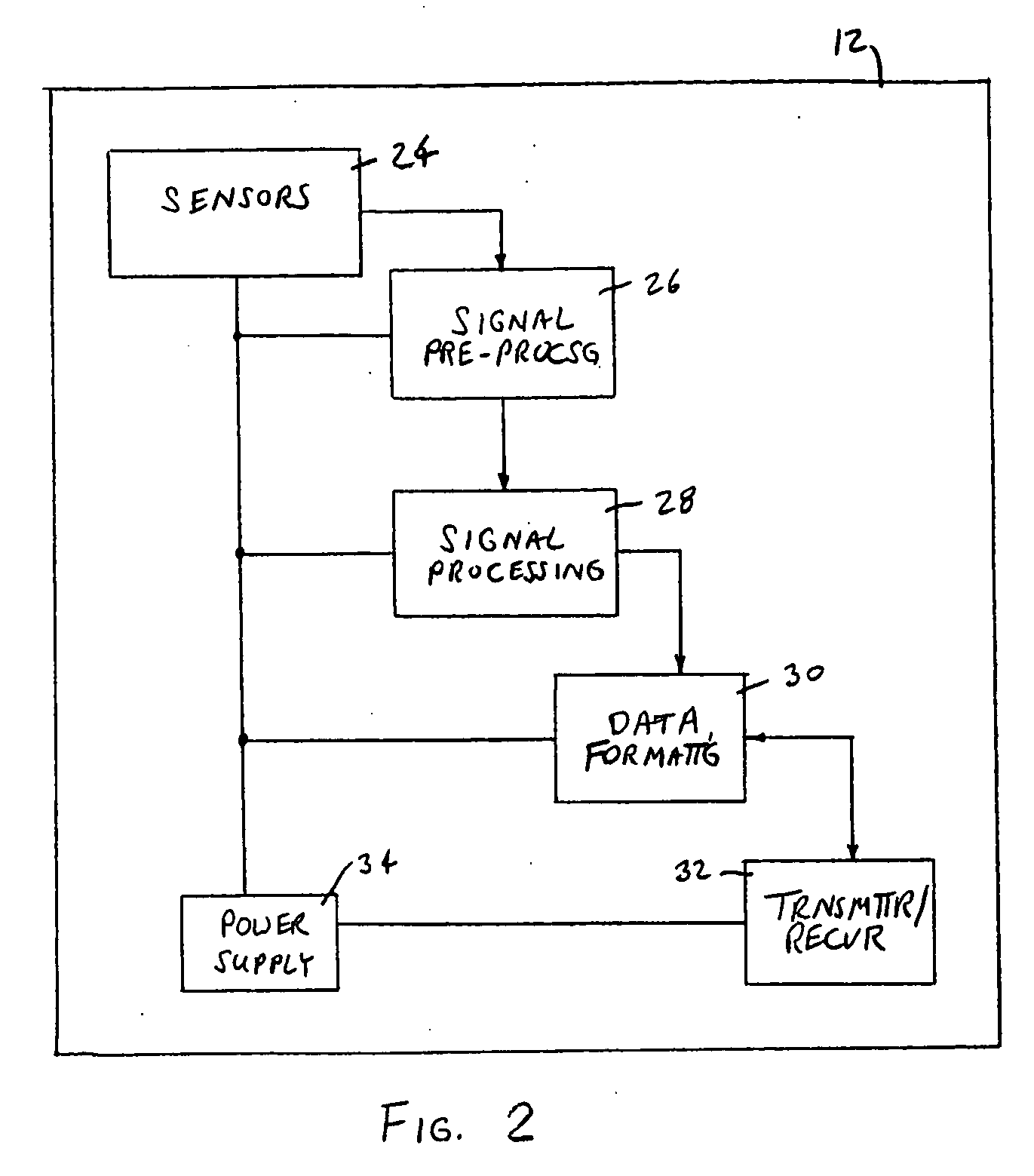
Apparatus and method for evaluating a hypertonic condition
InactiveUS20070027631A1Provide real-time feedbackVolume/mass flow measurementPerson identificationGyroscopeAccelerometer
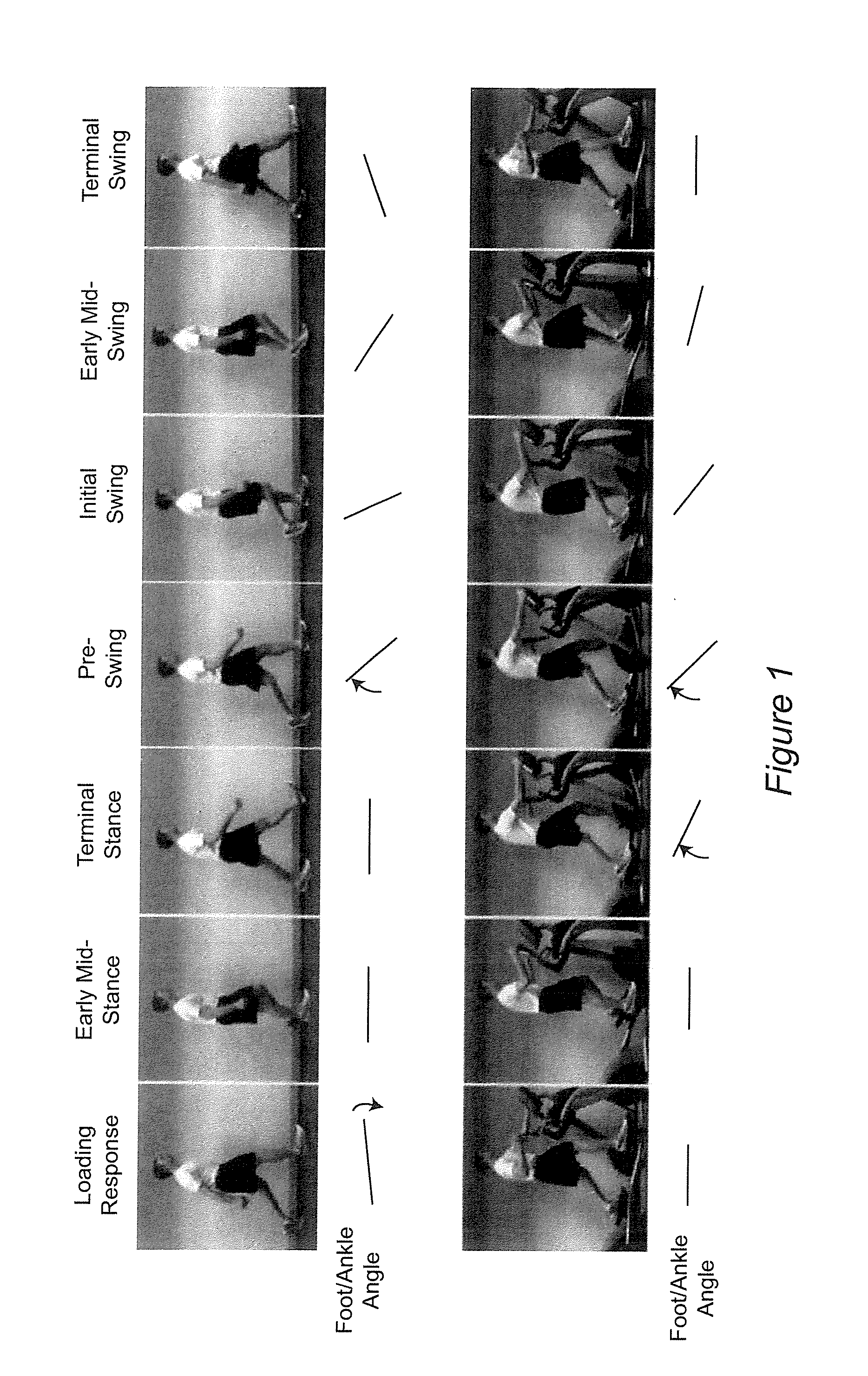
An apparatus and method for evaluating a hypertonic condition such as spasticity in a movable extremity are described. The apparatus includes an accelerometer, a gyroscope, and a sensor adapted for quantifying force or pressure. The apparatus further includes a data communication device adapted for transmitting data signals obtained from the accelerometer, said gyroscope, and said sensor for processing and real-time display. The method includes the steps of moving the extremity through a range of motion about an axis of rotation. During the movement of the limb parameters such as acceleration, angular velocity, the force applied to the extremity, and the time duration to move the extremity through the range of motion are all measured. The measured parameters are then transmitted to a data processor which processes the data to generate information that characterizes the hypertonic condition of the extremity for immediate feedback to the examiner or storage in a database for monitoring of the patient's condition. In accordance with a further aspect of the present invention there is described an apparatus and method for simulating movement of a limb that has a hypertonic condition based on characterizing information from a real limb.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Nerve stimulation for treating spasticity, tremor, muscle weakness, and other motor disorders
InactiveUS20050102007A1Induce antagonistic musclesReduce spasmsElectrotherapyArtificial respirationFiberTruncal muscle weakness
A method for treating spasticity of a subject is provided, including driving a current into a nerve of the subject that includes one or more sensory fibers, and configuring the current so as to inhibit propagation of action potentials in one or more of the sensory fibers, so as to treat the spasticity. In a preferred embodiment, the sensory fibers include one or more Ia sensory fibers, and configuring the current includes configuring the current so as to inhibit propagation of the action potentials in at least one of the Ia sensory fibers.
Owner:MEDTRONIC INC
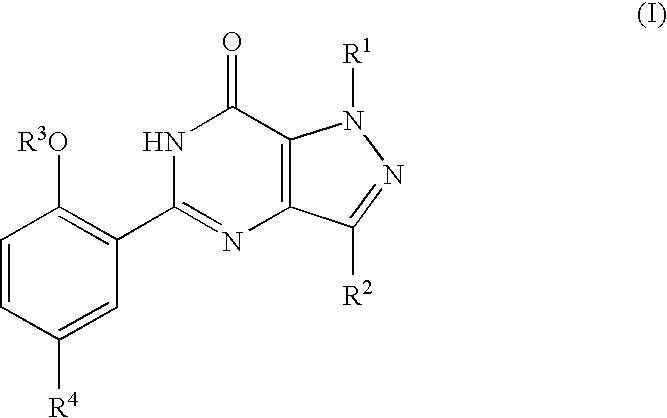
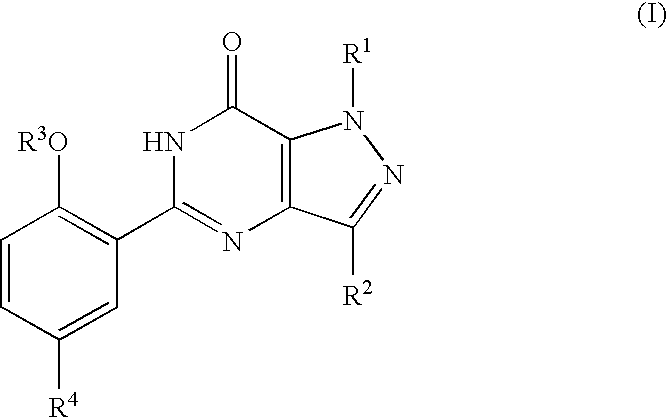
Thieno-pyridine derivatives as allosteric enhancers of the GABAB receptors
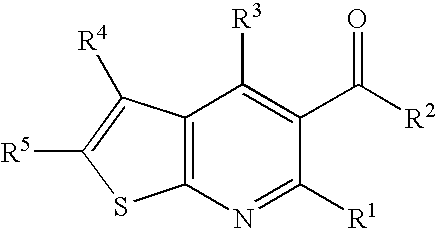
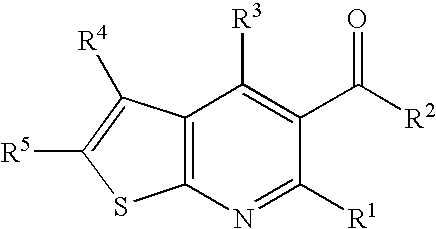
InactiveUS20060135552A1Valuable therapeutic propertyBiocideNervous disorderThienopyridine DerivativesMuscular spasticity
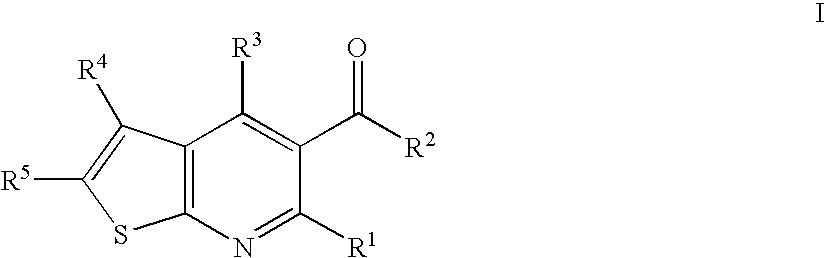
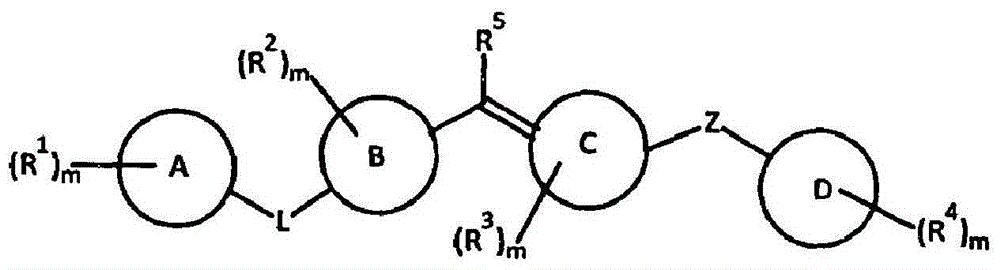
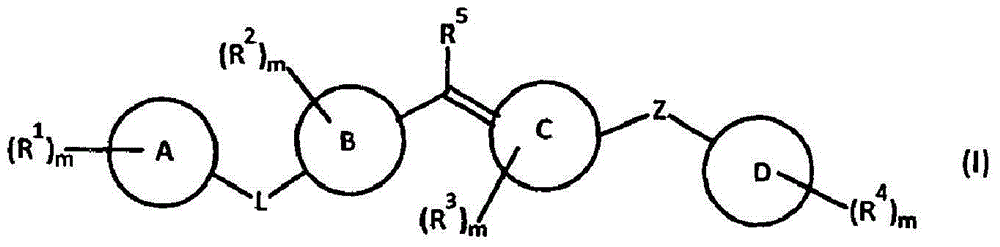
The present invention relates to compounds of formula I wherein R1, R2, R3, R4, and R5 are as defined in the specification. Compounds of the invention are active on the GABAB receptor and are useful for treating a variety of CNS disorders, including anxiety, depression, epilepsy, schizophrenia, cognitive disorders, spasticity-and skeletal muscle rigidity, spinal cord injury, multiple sclerosis, amyotrophic lateral sclerosis, cerebral palsy, neuropathic pain and craving associated with cocaine and nicotine, psychosis, panic disorder, posttraumatic stress disorders and gastrointestinal disorders.
Owner:F HOFFMANN LA ROCHE & CO AG
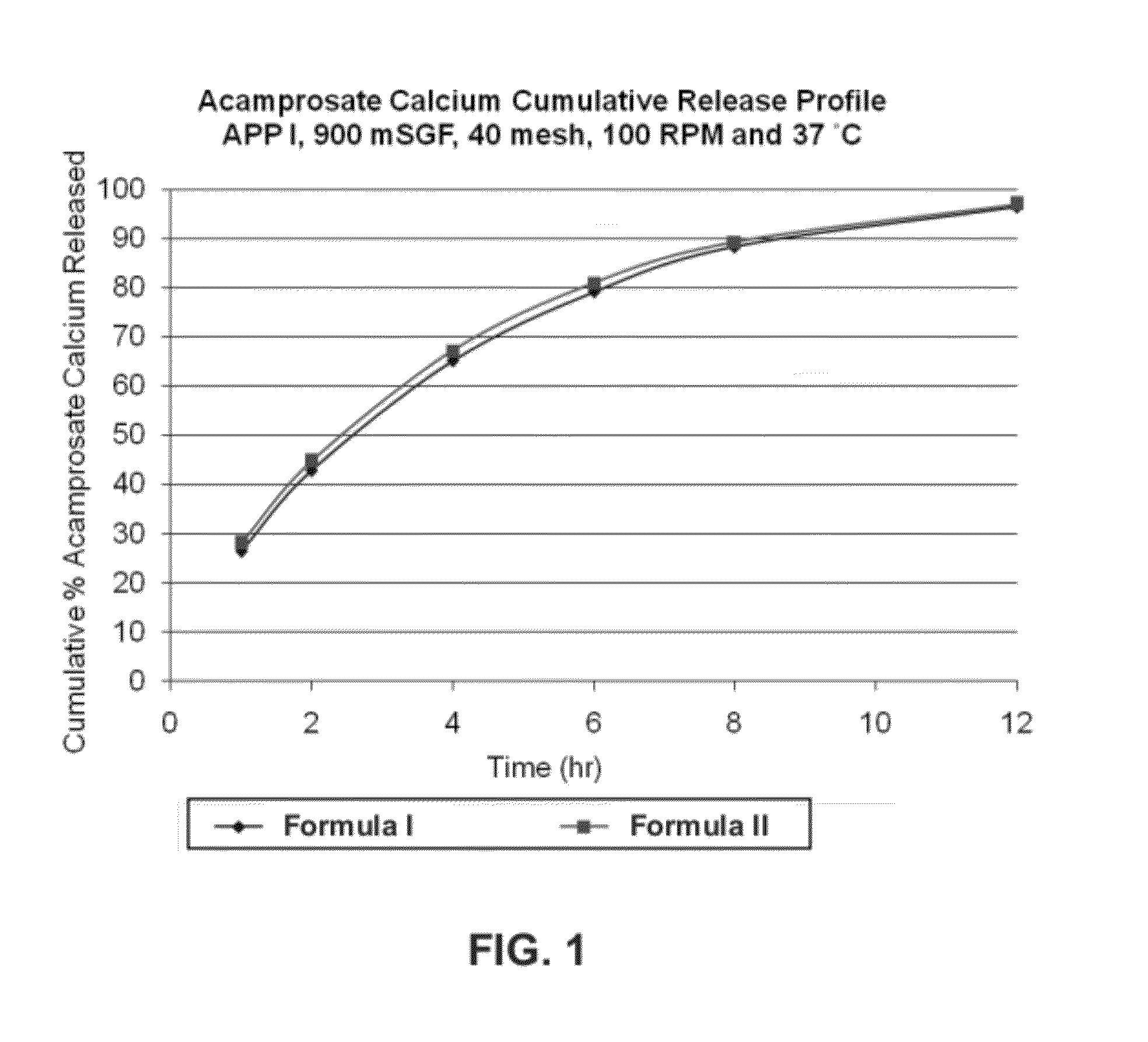
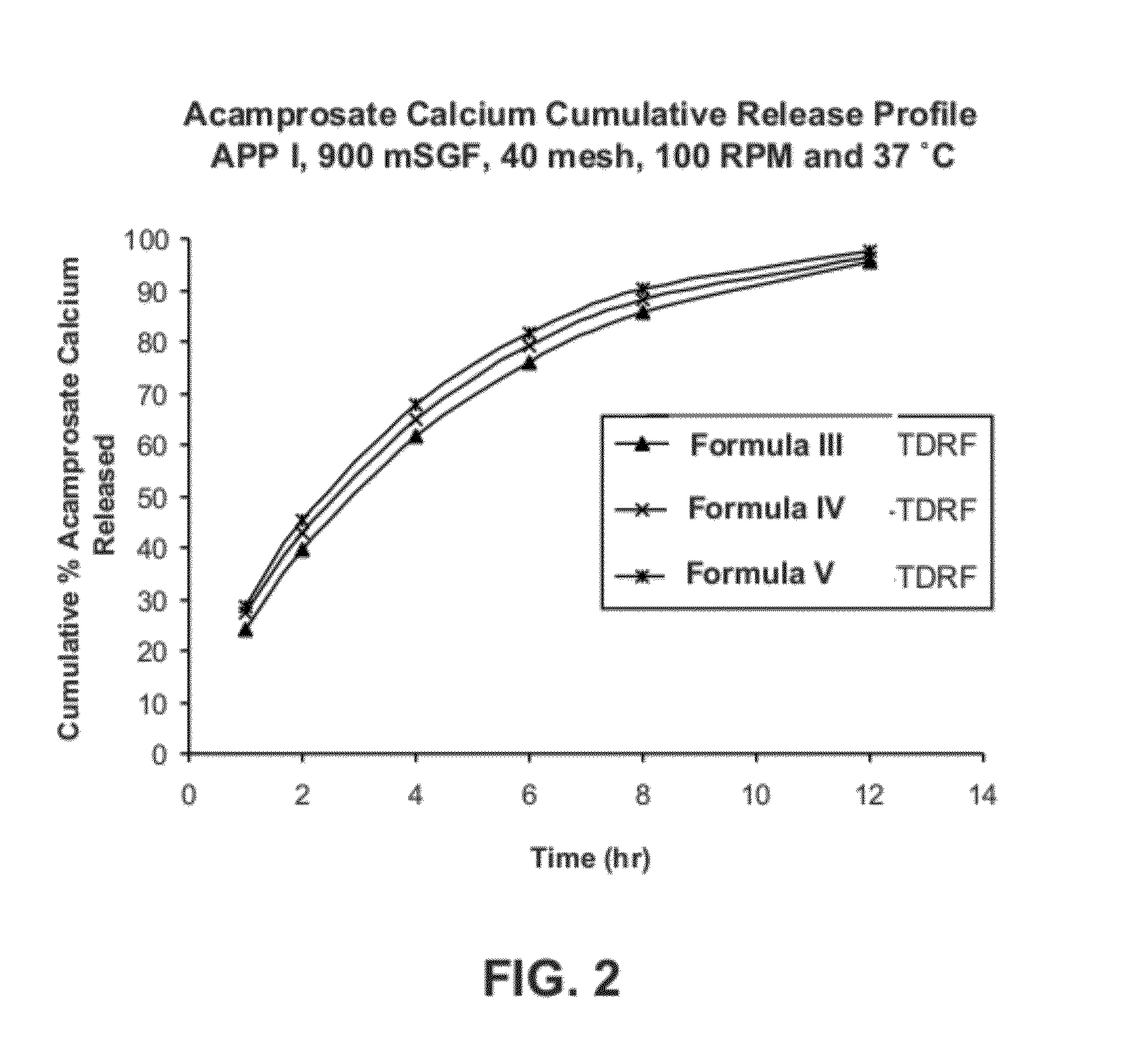
Gastric retentive dosage forms for extended release of acamprosate into the upper gastrointestinal tract
Gastric retentive dosage forms for sustained release of acamprosate are described which may allow once- or twice-daily dosing for both acute and long-term treatment of a disorder including alcohol dependence, tinnitus, sleep apnea, Parkinson's disease, levodopa-induced dyskinesias in Parkinson's disease, Alzheimer's disease, Huntington's disease, Amyotrophic lateral sclerosis, Cortical spreading depression, migraine, schizophrenia, anxiety, tardive dyskinesia, spasticity, multiple sclerosis, various types pain, or binge eating. Methods of treatment using the dosage forms and methods of making the dosage forms are also described.
Owner:DEPOMED SYST INC
Method and device for reducing symptomatic relapse of spasticity
InactiveUS20130116606A1Degree of reductionMitigate any symptomatic relapseChiropractic devicesVibration massageMaintenance therapyElectrical control
A wearable maintenance therapy device (10, 12, 14) imparts vibratory stimuli to appendicular muscles of a patient's limb during daily life activity of the patient to reduce symptomatic relapse of spasticity. A vibrator actuator (20) contained in a sup port housing (24) and configured for placement in operational contact with appendicular muscles of a limb (28) of a patient imparts localized vibration to one or more of the patient's appendicular muscles. The localized vibration produces proprioceptive input from the vibrated muscle or muscles (90t) to activate sensory areas of the patient's central nervous system. Programmable electrical control circuitry (50) controls vibration characteristics of the localized vibration and includes memory sites for storing operating values of the vibration characteristics. The vibration characteristics include pattern and timing of vibration specified to produce the proprioceptive input during the patient's daily life activity to mitigate any symptomatic relapse of spasticity.
Owner:OREGON HEALTH & SCI UNIV
Systemically and locally administered cells for neuropathic pain
InactiveUS20100159025A1Relieve painBlock ectopic neuronal firingNervous disorderAntipyreticWhole bodyUmbilical cord tissue
Methods for treating chronic pain, neuropathic pain or spasticity are provided. Some embodiments are to methods for treatment comprising administering cells obtained from human umbilical cord tissue, or administering pharmaceutical compositions comprising such cells or prepared from such cells. In some embodiments, administering the cells promotes repair and regeneration of nerves in the patient to decrease chronic pain, neuropathic pain or spasticity. Pharmaceutical compositions for use in the inventive methods, as well as kits for practicing the methods are also provided.
Owner:DEPUY SYNTHES PROD INC
Compositions and methods for the prevention or treatment of pain and other nervous system disorders
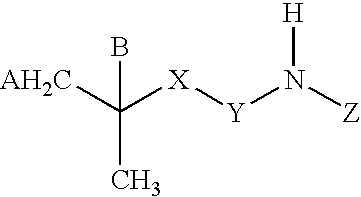
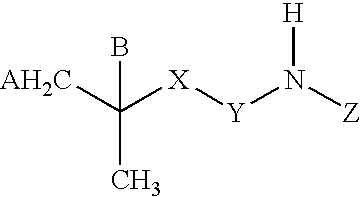
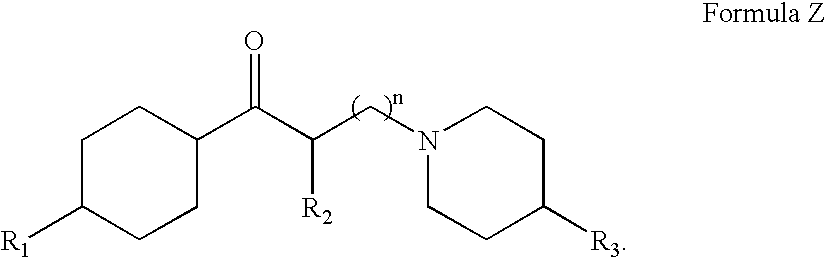
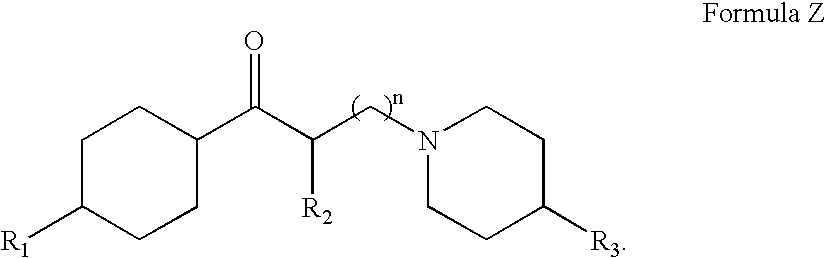
A tolperisone-related compound or a compound of Formula Z is administered for the prevention and treatment of periodic paralyses and myotonias of several types, long QT syndrome, Brugada syndrome, malignant hyperthermia, myasthenia, epilepsy, ataxia, migraine, Alzheimer's Disease, Parkinson's Disease, schizophrenia, and hyperekplexia, neuropathic pain, and pain associated with nervous system disorders including, but not limited to, painful diabetic neuropathy, postherpetic neuralgia, trigeminal neuralgia, complex regional pain syndrome, Guillain-Barre syndrome (GBS), Charcot-Marie-Tooth (CMT) disease, complex regional pain syndrome, type 1 (CRPS-1), ischemic neuropathy, fibromyalgia, chronic fatigue syndrome, painful spasticities, and other nervous system disorders that have pain as an attendant sign and / or symptom.
Owner:SPEICHER BRIAN T +1
Method and Use of Peripheral Theta-Burst Stimulation (PTBS) for Improving Motor Impairment
InactiveUS20140031605A1Improve motor controlElectrotherapyMagnetotherapy using coils/electromagnetsMuscular spasticityBiological activation
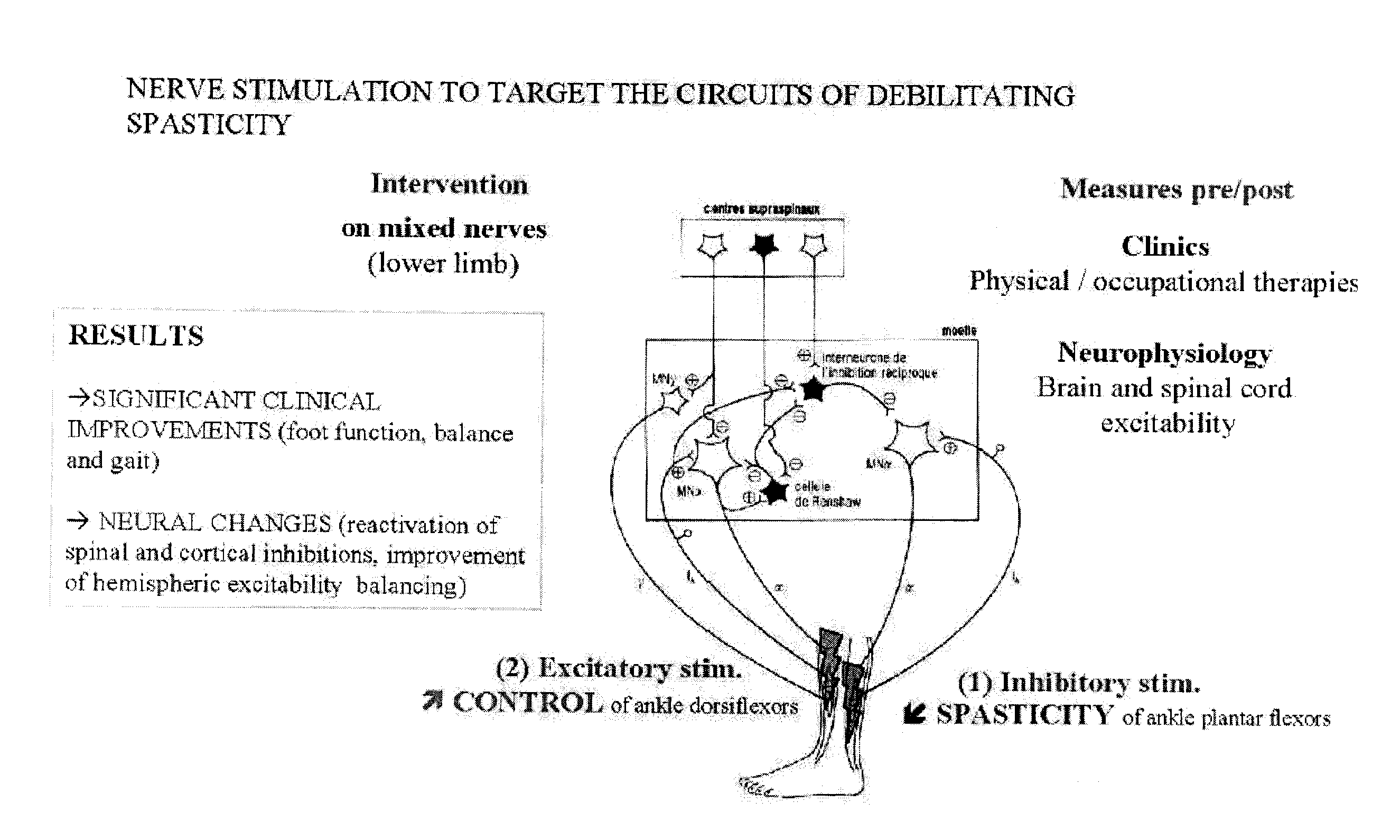
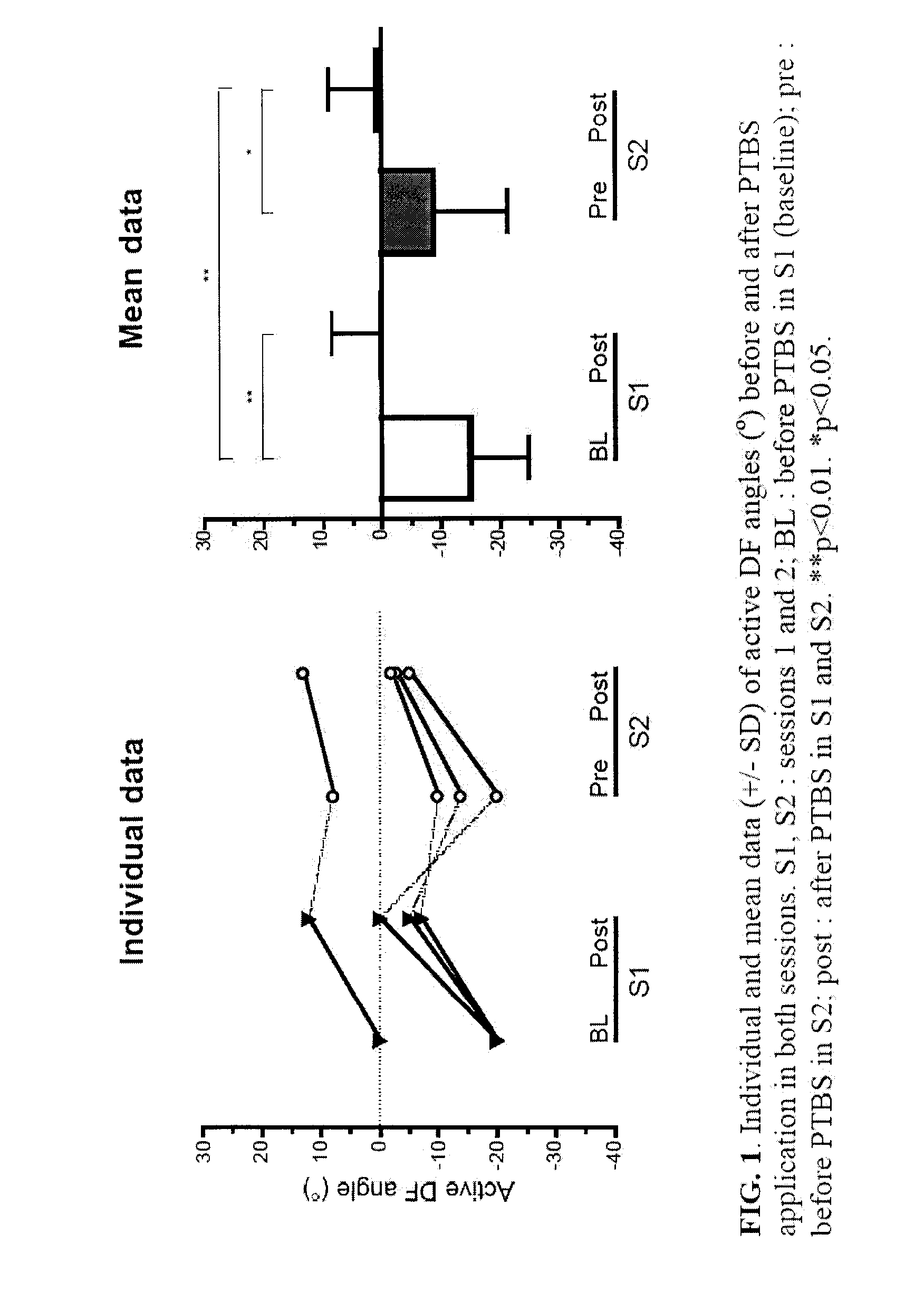
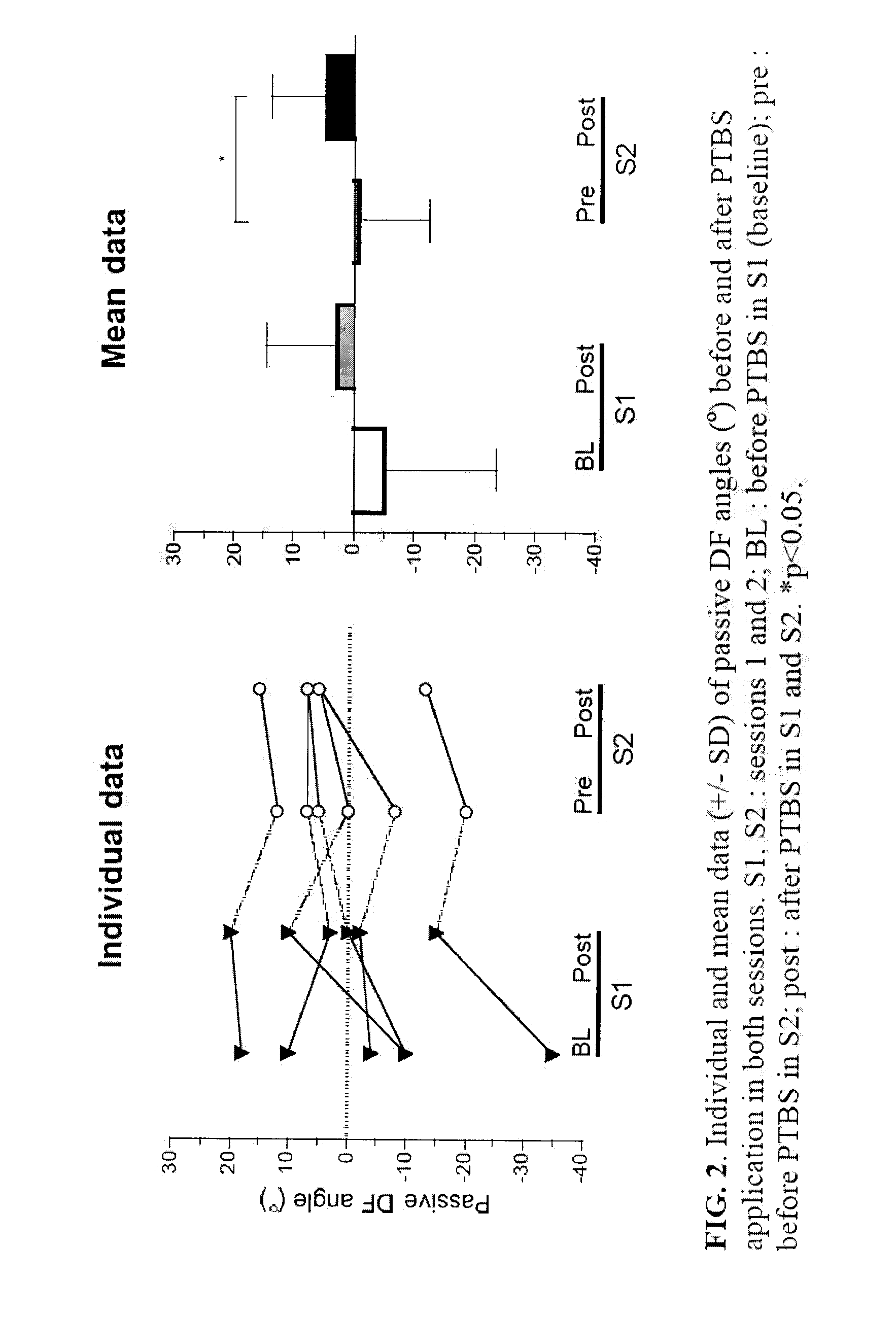
The present invention provides new evidences to support that repetitive peripheral magnetic stimulation (theta-burst en stimulation, TBS) over nerve / muscle improves sensorimotor control. Chronic low back pain (CLBP) is associated to a faulty volitional activation of transversus abdominis muscle (TrA) and its delayed contraction during anticipatory postural adjustment (APA), in correlation with maladaptive reorganization of primary motor cortex (M1). Repetitive magnetic stimulation of nerves can influence brain excitability and even reduce rigidity (Parkinson's disease), spasticity (stroke, ABI, cerebral palsy), and contribute to improvement of motor-control and function in stroke, chronic low back pain, ABI, cerebral palsy, prematurity and Parkinson's disease. We hereby test—for the first time—the after-effects of TBS applied over nerves or muscles (peripheral TBS, PTBS) on the motor abdominal-function of chronic low back pain sufferers and on the foot function of brain-injured subjects and to adjust TBS protocol per subject relative to the clinical profile. These pilot studies demonstrate the long-term influence of peripheral neurostimulation in chronic pain, rigidity and spasticity associated to motor impairment.
Owner:UNIV LAVAL
Method for alleviating signs and symptoms of spasticity
ActiveUS8426470B2Lower Level RequirementsAlleviation of signBiocideOrganic active ingredientsSedative EffectsImmediate release
A method of alleviating signs and symptoms of spasticity in human patient comprising orally administering to said human patients once in a day a controlled drug delivery system comprising an effective daily dose of baclofen or its pharmaceutically acceptable salt. The controlled drug delivery system is operable to produce a level of sedation lower than a sedation produced by three times a day immediate release tablets. A total daily dosage of the controlled release tablets and a total daily dosage of the three times a day immediate release tablets remain same.
Owner:SUN PHARMA INDS
Topical medicament
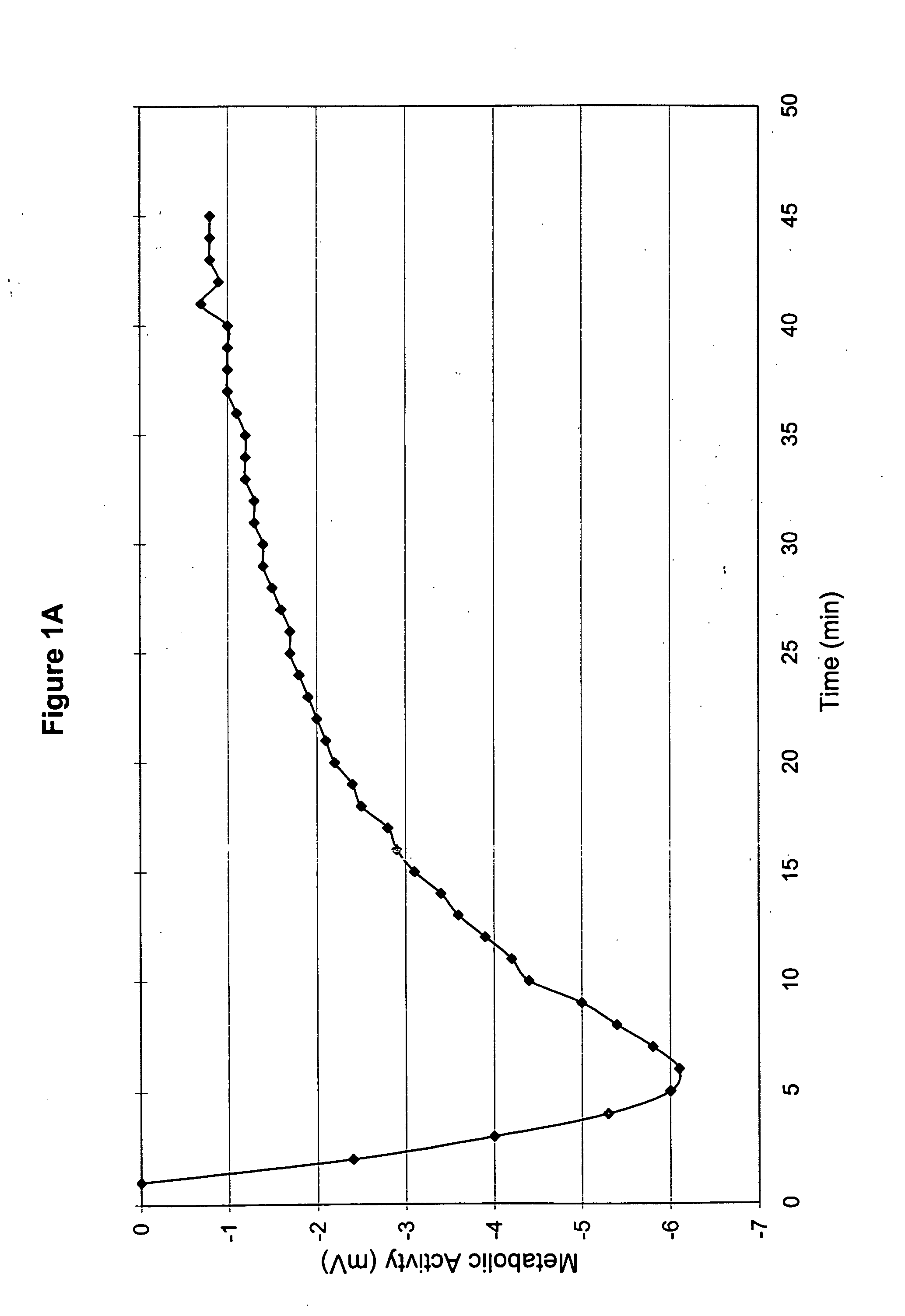
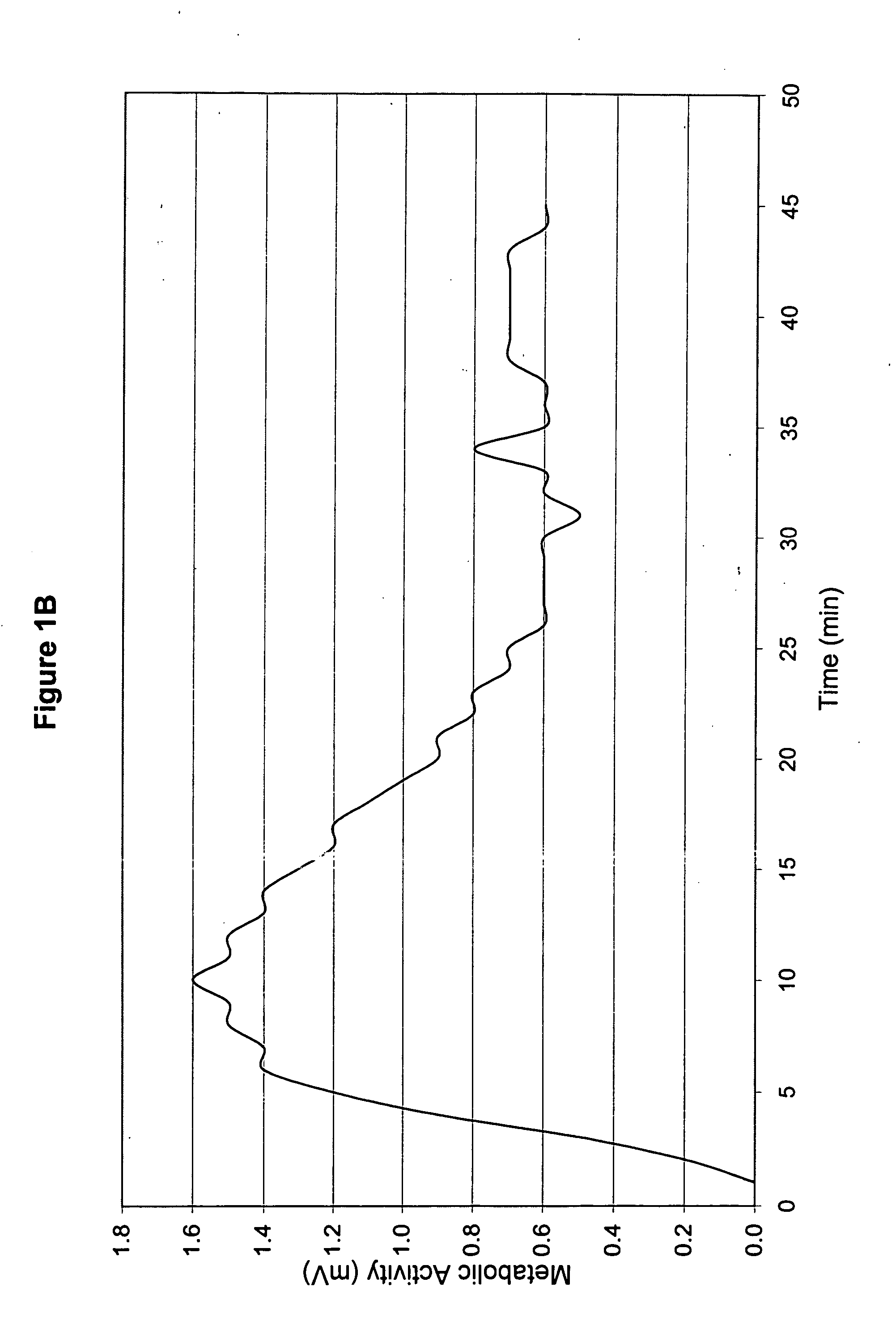
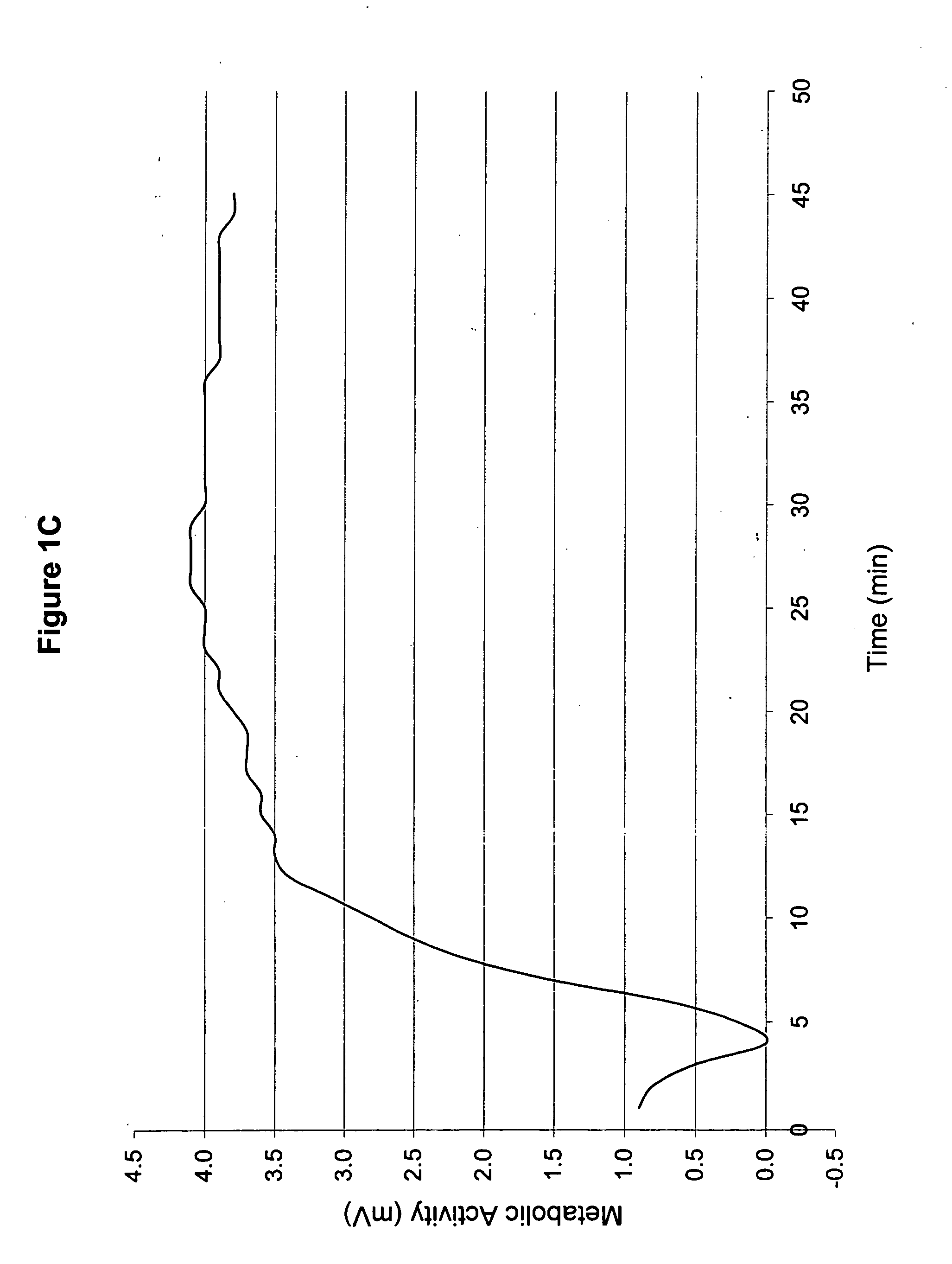
InactiveUS20060051432A1Improve metabolic activityStimulates the nervous systemBiocideNervous disorderDiseaseSinusitis
New topical medicaments are provided. The medicaments comprise menthol and camphor, preferably provided as part of a base gel, supplemented with potassium and a source of oxygen. The most preferred base gel is sold under the name SOMBRA, while the most preferred source of oxygen is a chlorite (e.g., sodium chlorite) and / or spirulina. The medicaments provide high metabolic activities and sustain those activities over prolonged periods of time, thus being useful for treating a large variety of ailments, including diabetic neuropathy, post hepatic neuralgia, scleroderma, psoriasis, strain, spasticity, headaches, neuropathy secondary to drugs, peripheral neuropathy, leg pain, muscle cramps, muscle aches and pains, bruise, sinusitis, sprain, arthritis, joint pain (arthralgia), and edema.
Owner:MORGAN CLYDE
Spasticity reducing closed-loop force-feedback control for post-stroke gait training
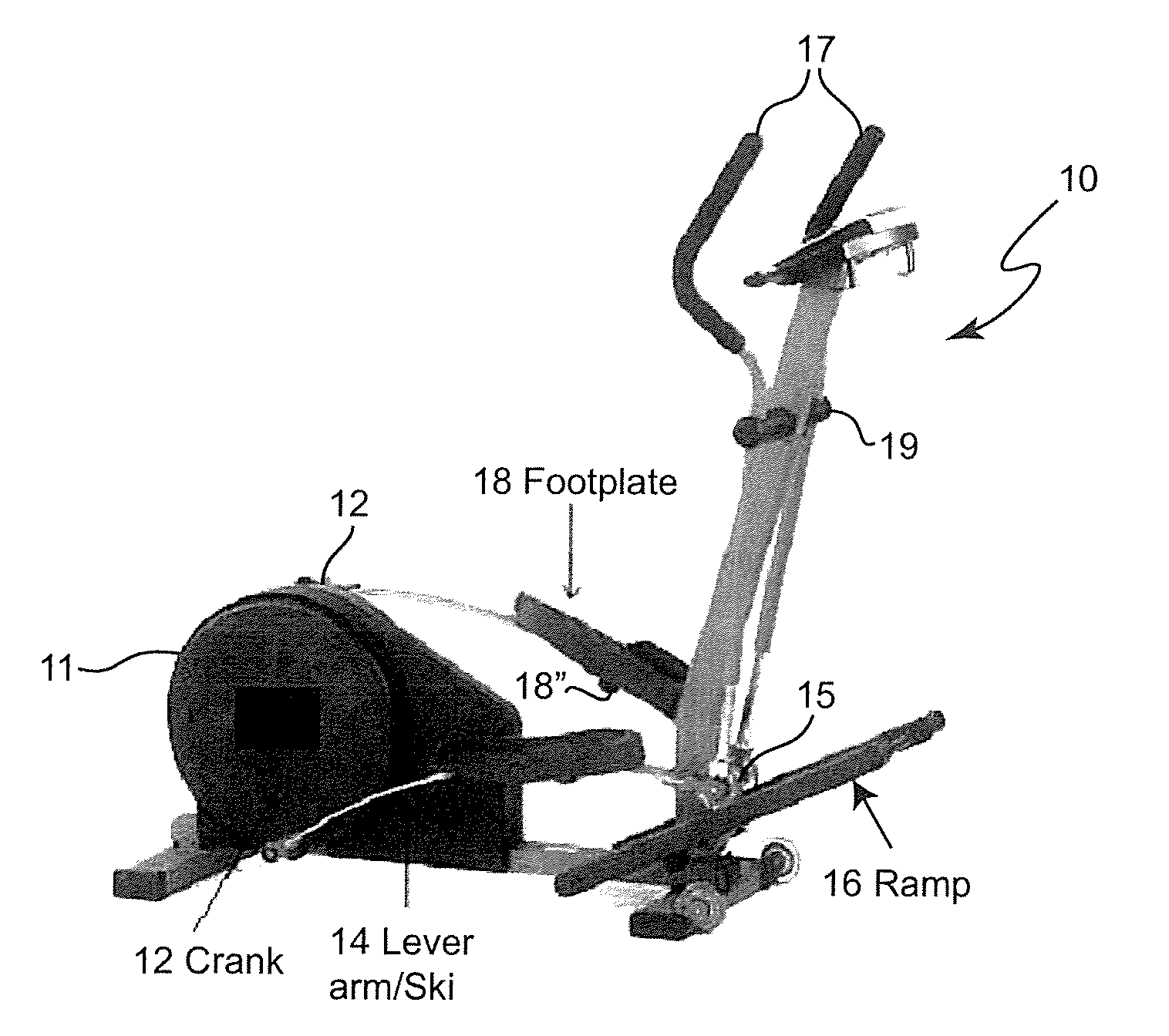
InactiveUS20130245511A1Regain muscle tone and coordinationReadily and easily alteredChiropractic devicesEye exercisersDiseaseControl system
A robotic module which can be attached to an exercise apparatus provides enhanced physical therapy for victims of stroke or other maladies or accidents by simulating normal or arbitrarily modified profiles of angular position of an extremity such as a foot in accordance with a position of that extremity along a locus of repetitive motion. A closed-loop control system which is included in the module provides regulation which reduces or avoids spastic responses often found in poststroke patients during gait rehabilitation.
Owner:VIRGINIA COMMONWEALTH UNIV
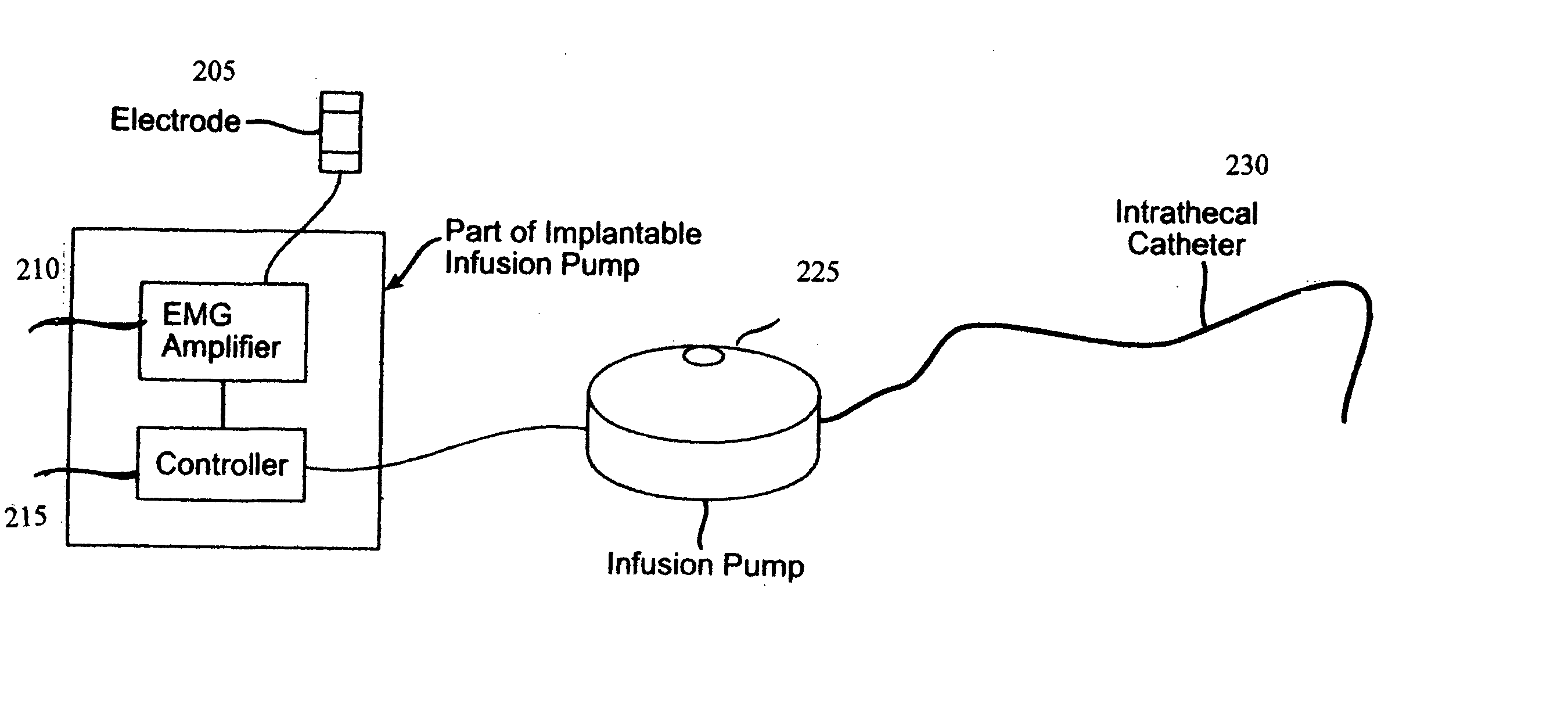
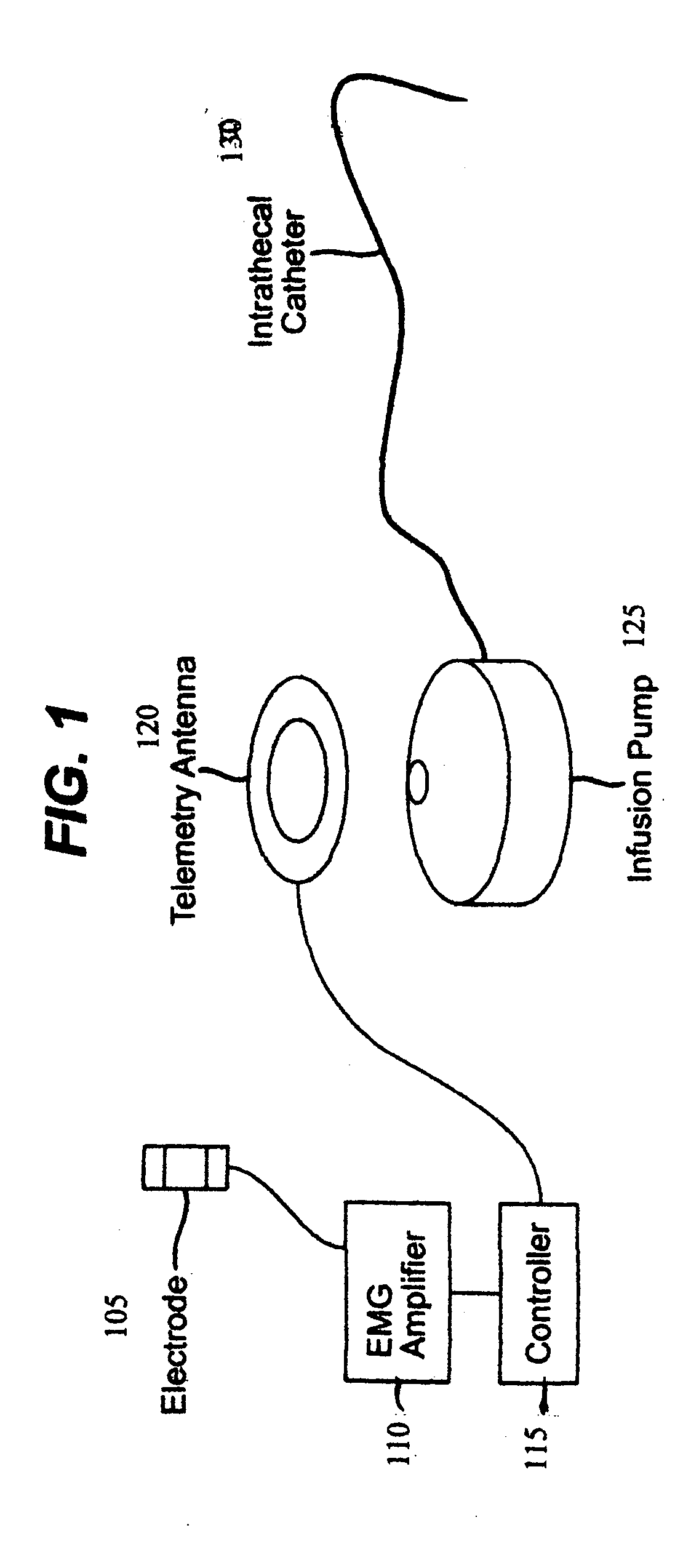
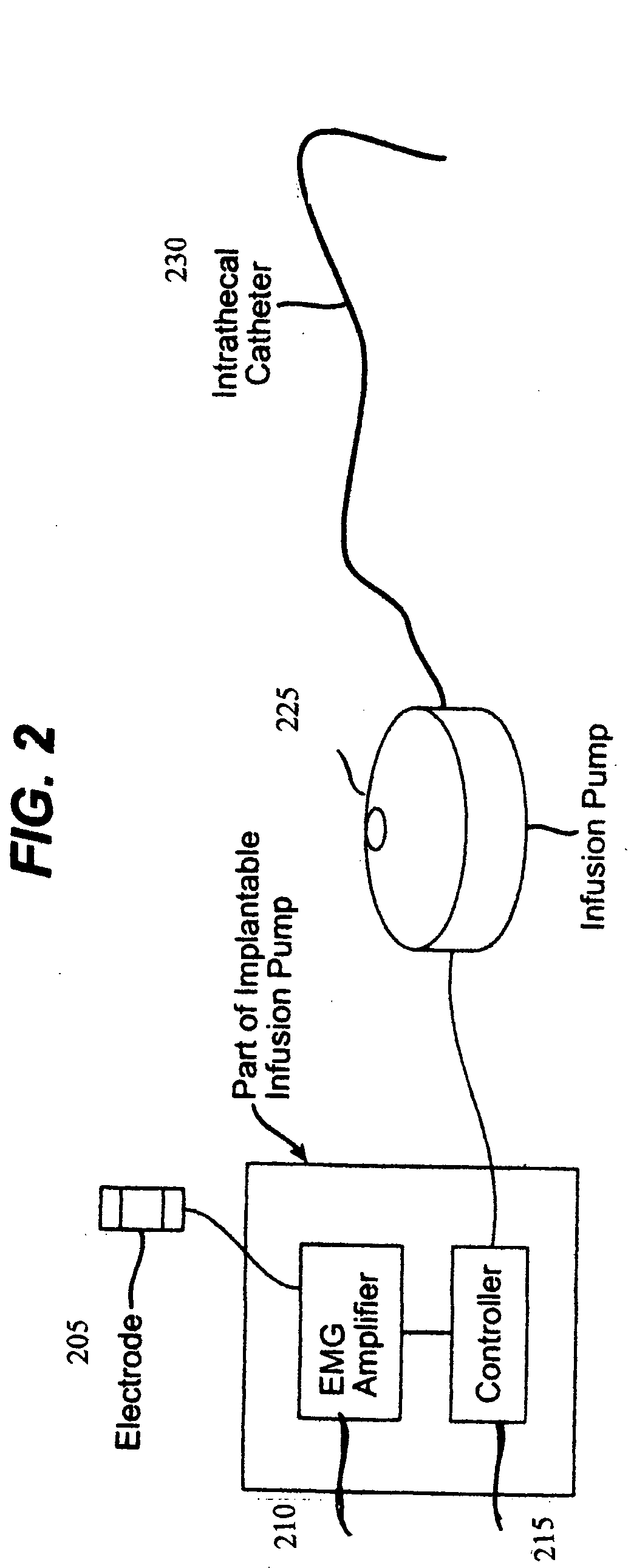
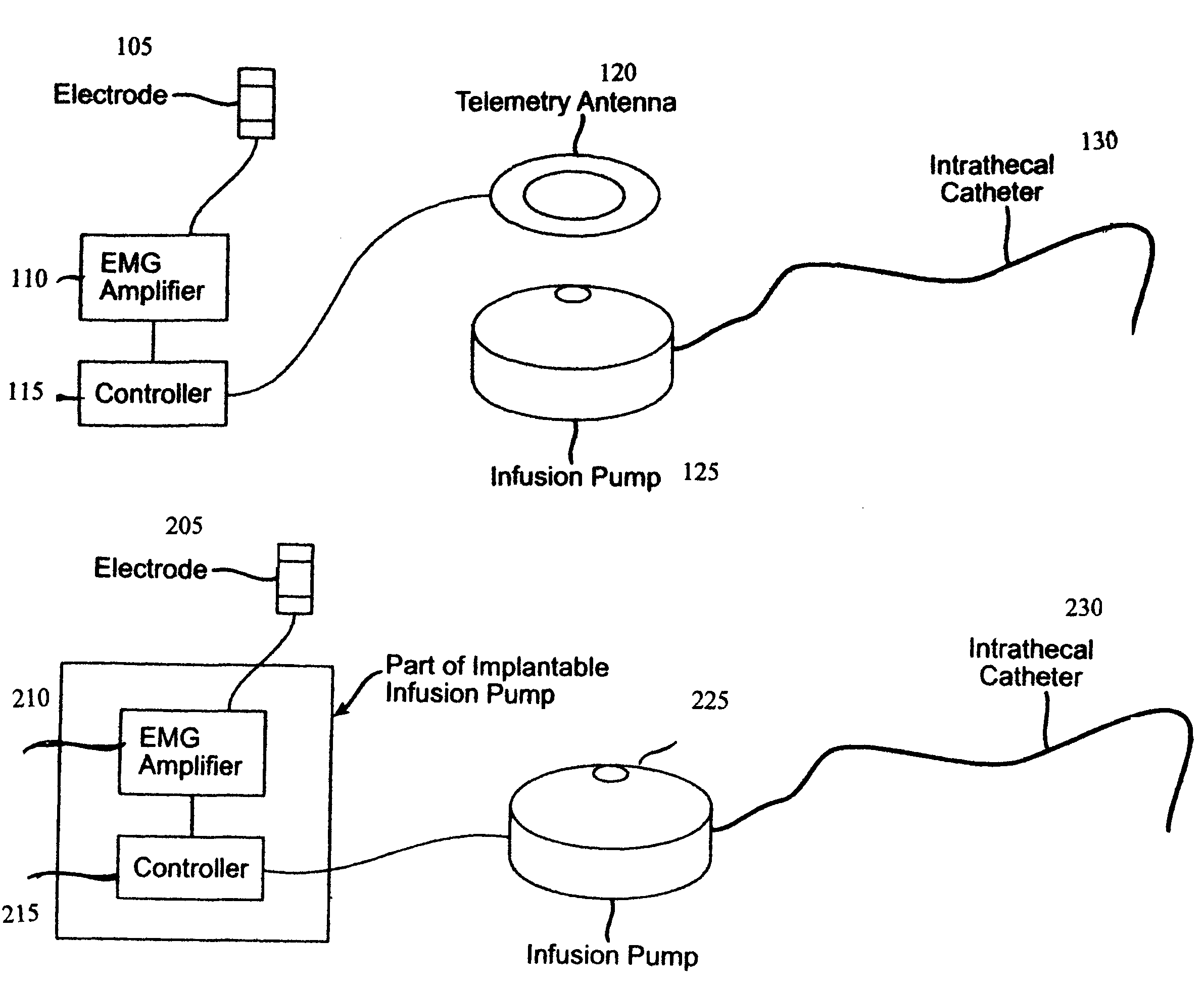
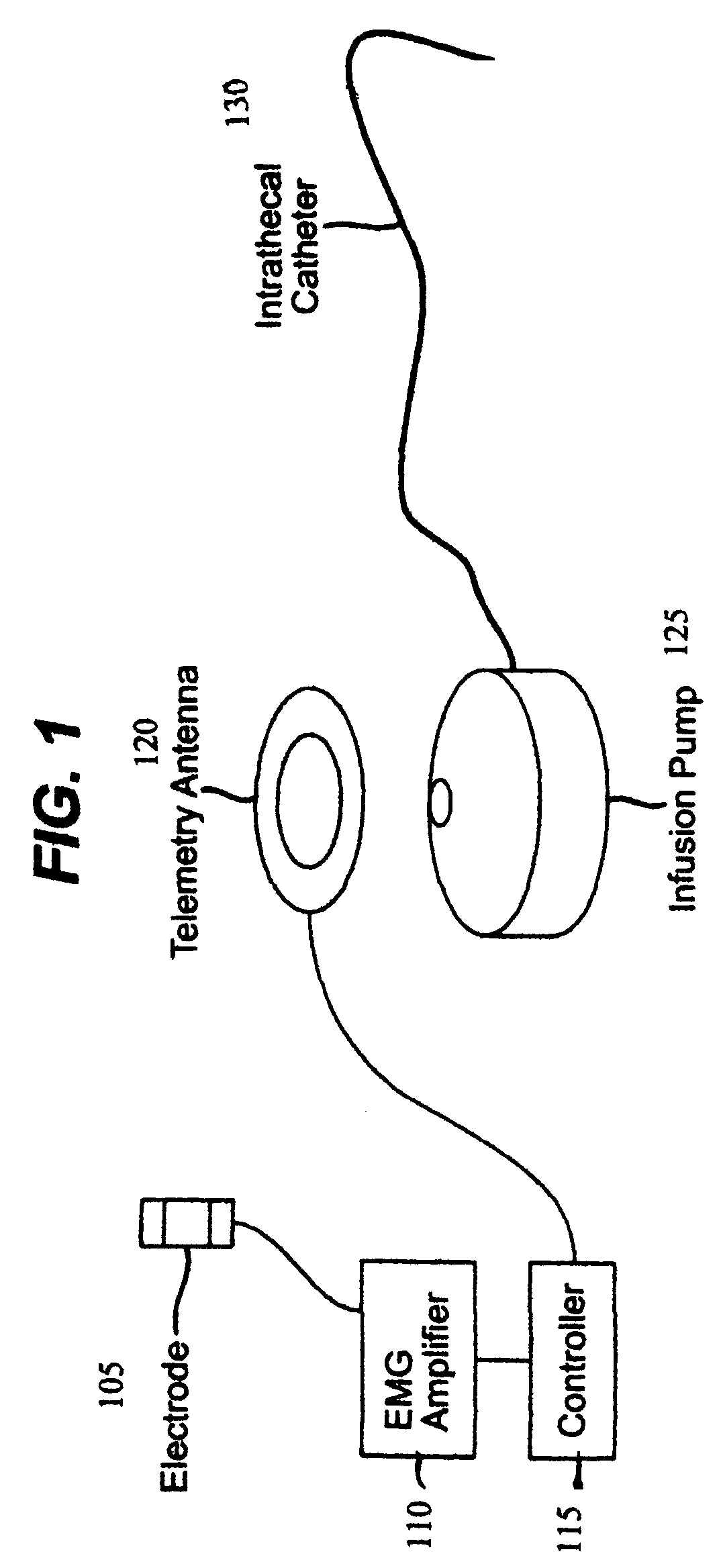
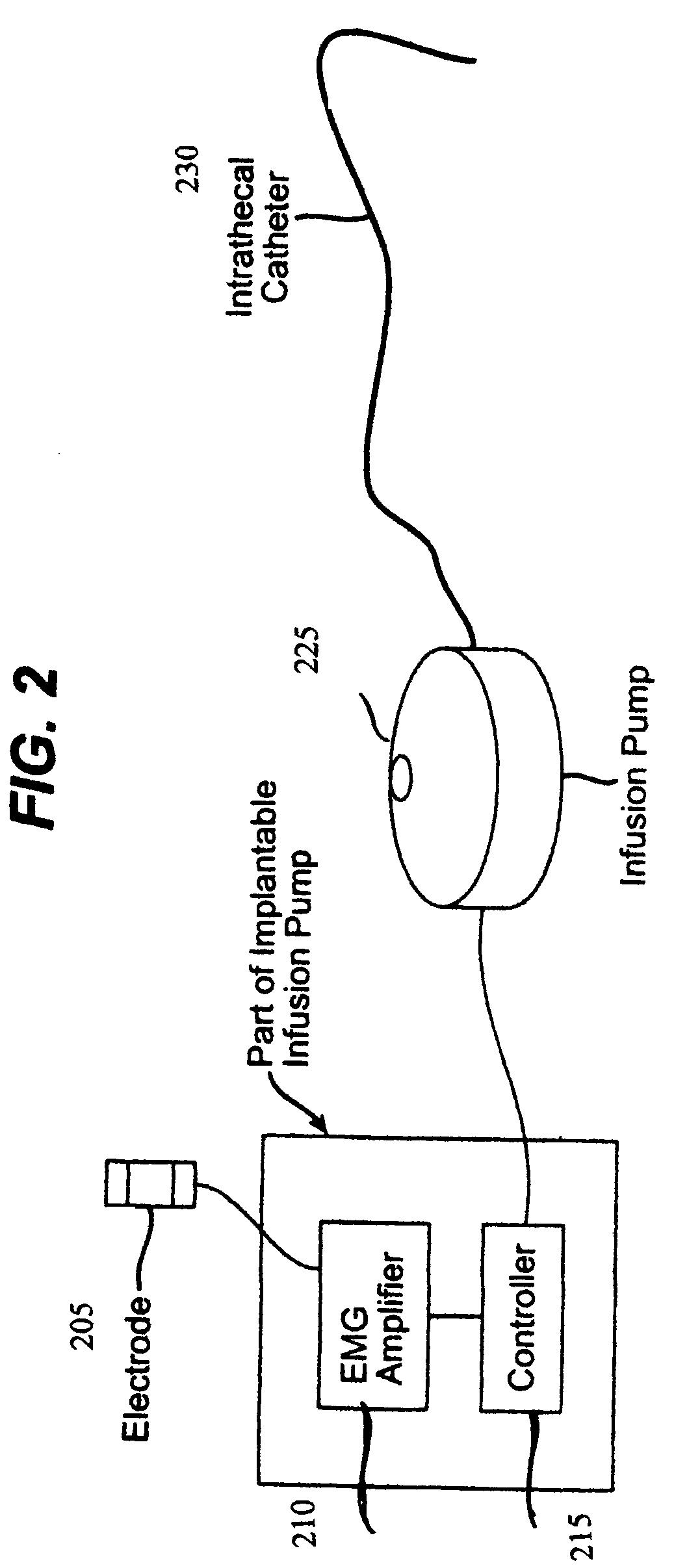
Closed loop system and method for controlling muscle activity via an intrathecal catheter
A closed loop feedback drug delivery system for controlling muscle activity, e.g., spasticity. The system includes a sensor, e.g., an EMG sensor, for monitoring muscle activity and generating a detected muscle activity signal. A controller automatically adjusts at least one of timing and dosage of a drug administered to control muscle activity based on the detected muscle activity signal and produces a control signal. Administering of the drug, e.g., an antispasmodic drug, for controlling muscle activity based on the control signal is performed by an infusion pump. The drug after being emitted from the pump is then delivered to the spinal cord of the patient using an intrathecal catheter.
Owner:CODMAN & SHURTLEFF INC
Sustained-release formulations for treating CNS-mediated disorders
Sustained-release compositions for delivering therapeutic concentrations of isovaleramide, isovaleric acid, and certain structurally related compounds are provided for the treatment for a variety of pathological conditions, including epilepsy and spasticity, which are ameliorated by effecting a modulation of CNS activity. The ability of the compositions to sustain relatively constant levels of the drug at a therapeutic dose in the serum for extended periods of time enables a once or twice daily administration schedule.
Owner:NPS PHARM INC
Closed loop system and method for controlling muscle activity via an intrathecal catheter
Owner:CODMAN & SHURTLEFF INC
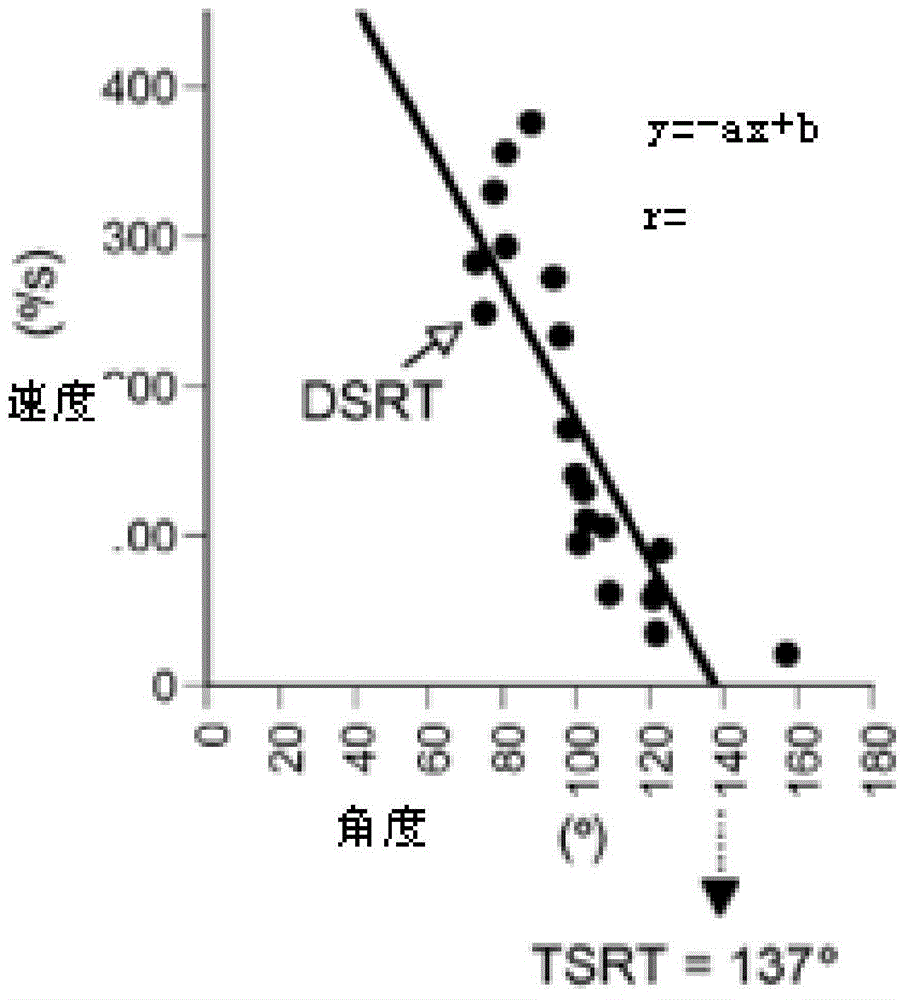
Spasticity evaluation system and device based on myotatic reflex threshold value and resistance variable
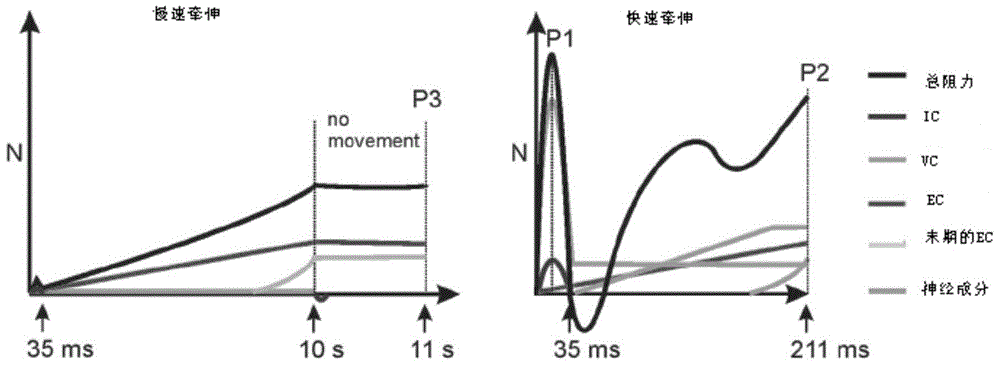
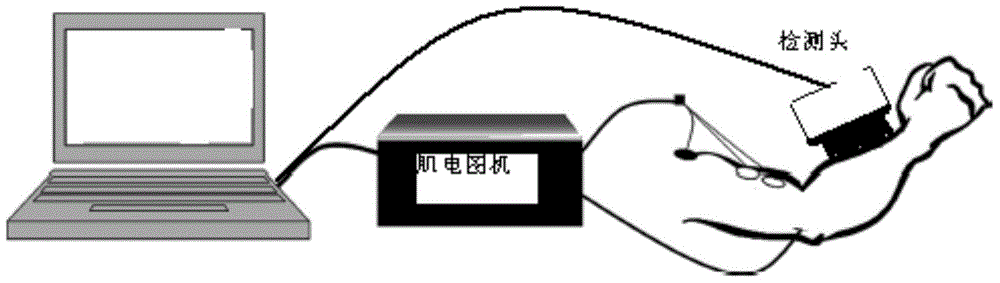
InactiveCN105266806ASolving the Difficulty of Accurately Evaluating Upper Motor Neuron Syndrome (UMNS)Helps to filterDiagnostic recording/measuringSensorsMyotatic reflexMuscular spasticity
The invention relates to the field of medical instruments and relates to a spasticity evaluation system and device based on the myotatic reflex threshold value and the resistance variable. The device comprises a detection head, a surface electromyogram, a computer and a display screen. The detection head integrates pressure, angle, speed and acceleration sensors and is connected with the computer through a data line or Bluetooth. The surface electromyogram records the electromyographic signals of measured muscles through surface electrodes. The computer analyzes the limb resistance variable, the stretching activity speed and the acceleration generated during a stretching activity and records the joint angle of triggering of myotatic reflex. A linear regression equation of the stretching speed-joint angle of triggering of myotatic reflex is established and the joint angle-myotatic reflex threshold value on the condition of the theoretical stretching speed 0 is obtained. The display screen can display the stretching speed animation preview, the myoelectric activity and the stretching resistance change. The evaluation system and device can solve the problem in accurate evaluation of the upper motor neuron syndrome (UMNS) in rehabilitation medicine, can better perform quantitative evaluation of spasticity therein, and facilitate further selection of reasonable intervention projects.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Systems and methods for spasticity treatment using spinal nerve magnetic stimulation
ActiveUS20170296837A1Improved spasticity reductionImprove muscle relaxationElectrotherapyMagnetotherapy using coils/electromagnetsMedicineSpinal nerve
Described are methods, devices, and systems for a novel, easy to use treatment for spasticity that does not involve medication. Methods and devices herein use low-frequency repetitive magnetic fields to enhance communication in the spinal nerve, thereby allowing improved relaxation, control, and coordination in a muscle.
Owner:WAVE NEUROSCI INC
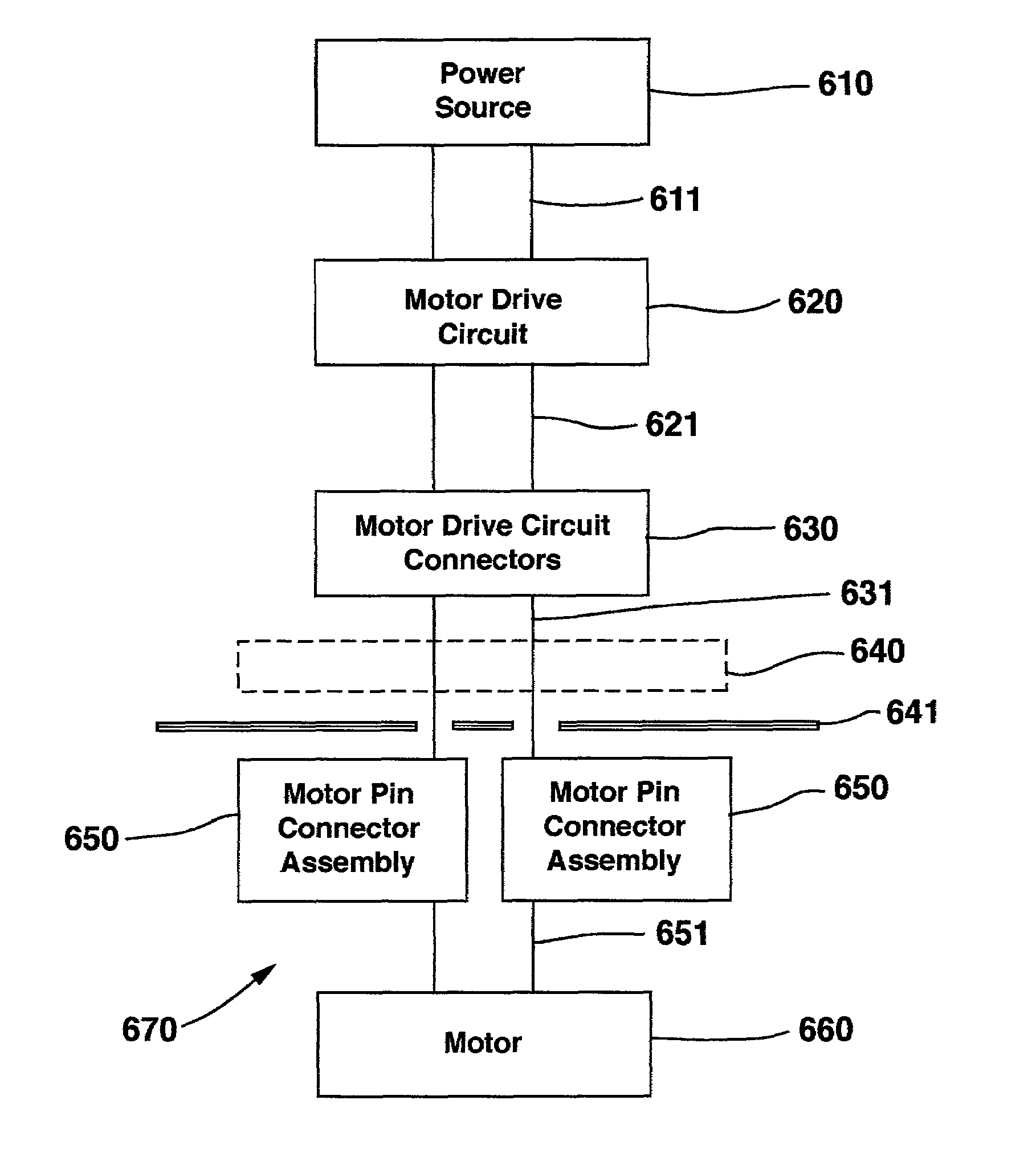
Implantable infusion device with motor connection and seal system
ActiveUS7264611B2High resistance connectionMedical devicesPharmaceutical delivery mechanismElectrical connectionMuscular spasticity
A medical device known as an implantable infusion device is configured for implanting in humans to deliver a therapeutic substance such as pharmaceutical compositions, genetic materials, and biologics to treat a variety of medical conditions such as pain, spasticity, cancer, and many other conditions. The infusion device incorporates a motor coil connector and mechanical sealing system between the clean motor compartment and the potentially corrosive pump compartment that provides a reliable electrical connection between the motor coil and the motor drive electronics. The motor coil connector provides for bonding of a very small diameter coil wire to one end of the connector and a highly corrosion resistant connection at the other end of the connector. Additionally, the motor coil connector and mechanical sealing system provides a seal against harmful corrosion materials that could emanate from the pump compartment and reach the motor compartment and cause malfunction of the motor. The infusion device has a housing, a power source, a therapeutic substance reservoir, a therapeutic substance pump, and electronics.
Owner:MEDTRONIC INC
Reduction of inflammatory mass with spinal catheters
ActiveUS8137334B2Improve efficacyReduce the amount requiredPharmaceutical delivery mechanismMedical devicesSpinal columnDisease
Devices, systems and methods for delivering one or more drugs to one or more internal body locations (such as the cerebrospinal fluid) are disclosed. In various aspects, the systems and methods may involve catheters having infusion sections with permeable membranes that develop significant back pressure to enhance uniform delivery of the drug over an infusion section; catheters that have two or more infusion sections spaced apart along the length of the same catheter, catheters that include two or more infusion sections serviced by independent lumens (such that, e.g., different drug solutions can be delivered to the different infusion sections); implantable drug delivery systems with pumps and multiple reservoirs from which drugs can be delivered; systems that are capable of delivering drug solutions with selected densities; etc. Methods for treating diseases, including pain and spasticity are also discussed, as well as methods for screening patients and optimizing therapies. In addition, methods for delivering a drug to a brain through a spinal canal are described.
Owner:MEDTRONIC INC
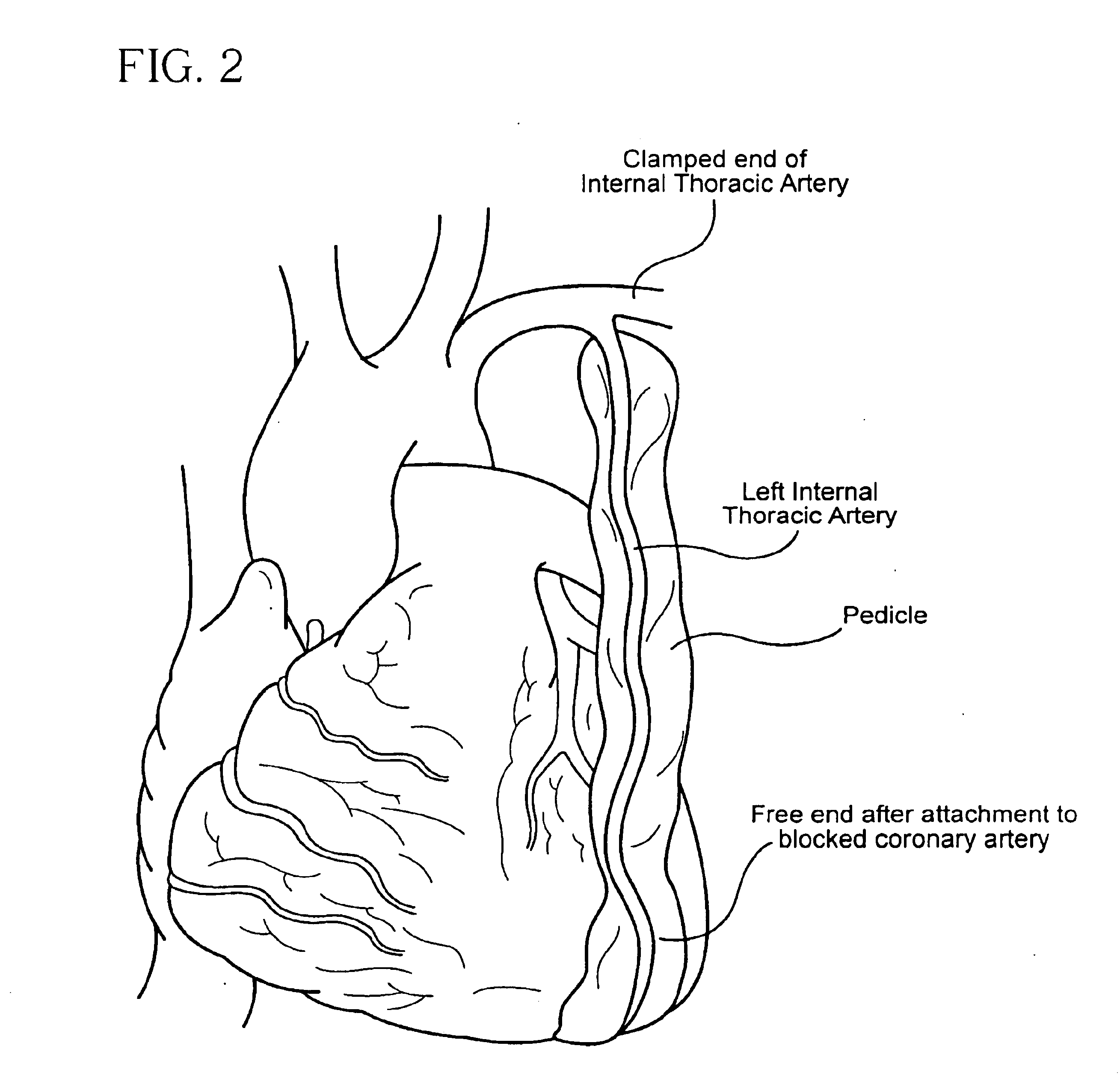
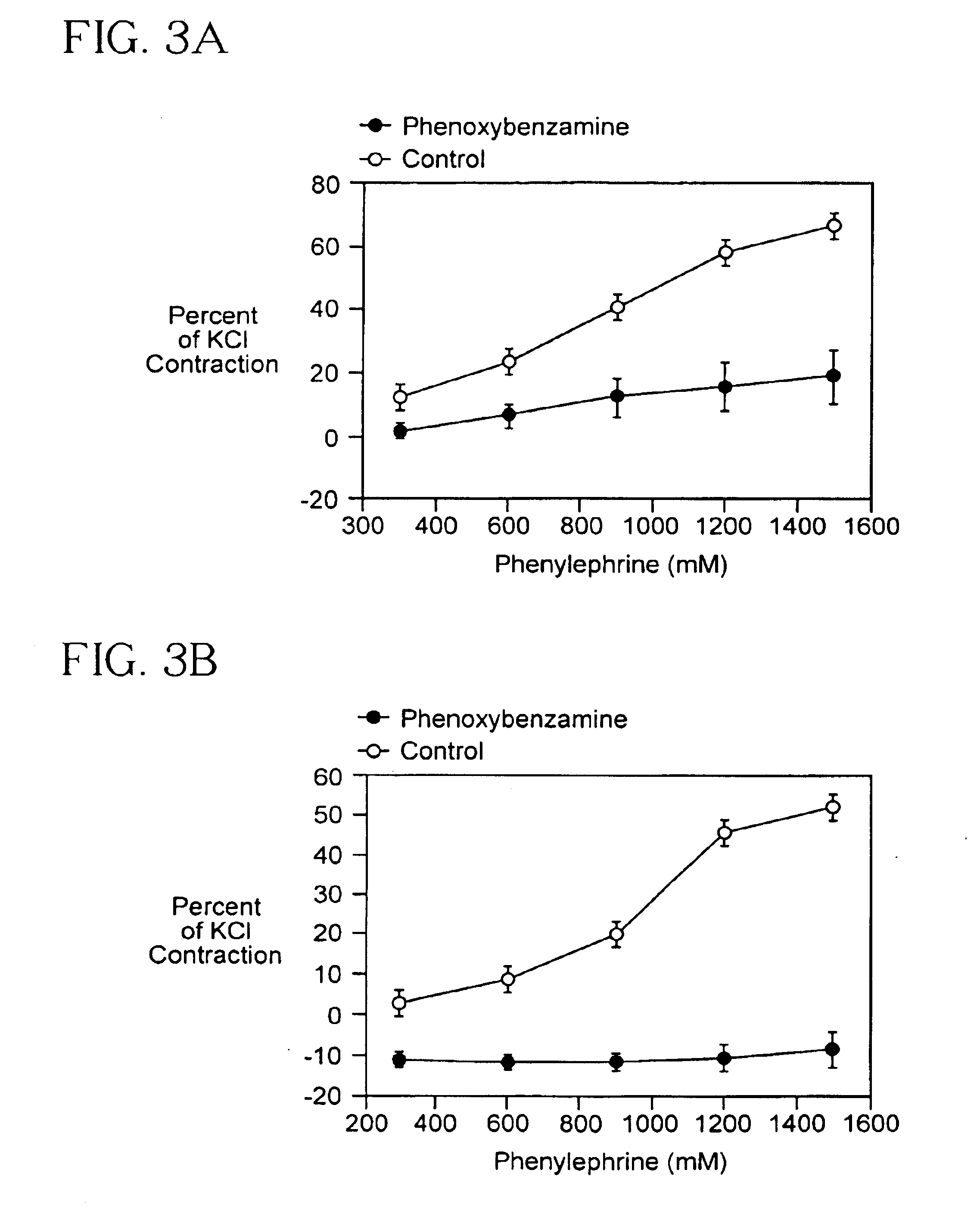
Methods and formulations for minimizing spasticity in blood vessel grafts
InactiveUS6900008B2Reduce usageReduces) spasticityBiocidePeptide/protein ingredientsBlood vessel spasmMuscular spasticity
The present invention relates to me methods for minimizing spasticity in blood vessels during transplantation and more particularly for minimizing spasticity in arterial transplants, for both ex-vivo and in-vivo procedures. The invention also relates to formulations, which can be used in these methods.
Owner:EMORY UNIVERSITY
Pharmaceutical composition for alleviating pain or spasticity in a patient suffering from spinal cord injury
To provide a novel pharmaceutical composition for alleviating pain or spasticity in a patient suffering from spinal cord injury. The present invention relates to a method for alleviating pain or spasticity in a patient suffering from spinal cord injury comprising administering to the patient an effective amount of cGMP PDE5 inhibitor, to a pharmaceutical composition comprising such an effective amount of the inhibitor, and to a use of the inhibitor in the manufacture of such a pharmaceutical composition.
Owner:PFIZER INC
Method and apparatus for resistive characteristic assessment
A simulated limb for training an evaluator to assess a resistive characteristic of an animal. The limb includes a first member simulating a first portion of the animal, and a second member simulating a second portion of the animal rotatably connected to the first member. The limb also includes a resistance element having a variable resistance that varies an amount of torque required to rotate the second member relative to the first member. A method for training an evaluator to assess a resistive characteristic using a simulated limb includes adjusting the resistance of the resistance element over a range of rotation thereby providing a resistance profile for the simulated limb mimicking the resistive characteristic of the animal. A method for simulating a resistive characteristic of an animal using a simulated limb includes applying a resistance profile to the simulated limb mimicking spasticity or muscle strength over a range of rotation.
Owner:BARNES JEWISH HOSPITAL
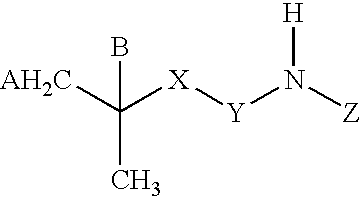
Amide compounds, compositions and applications thereof
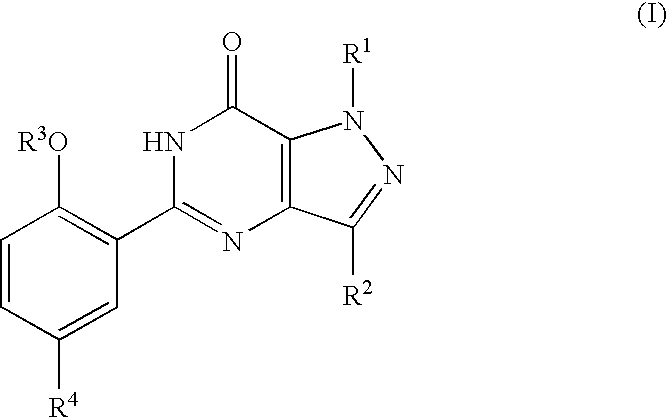
The present disclosure relates to substituted amide compounds that are inhibitors of Fatty Acid Amide Hydrolase (FAAH), their stereoisomers, tautomers, prodrugs, polymorphs, solvates, pharmaceutically acceptable salts, and pharmaceutical compositions containing them. These compounds are useful in the treatment, prevention, prophylaxis, management, or adjunct treatment of all medical conditions related to inhibition of Fatty Acid Amide Hydrolase (FAAH), such as pain including acute and post operative pain, chronic pain, cancer pain, cancer chemotherapy induced pain, neuropathic pain, nociceptive pain, inflammatory pain, back pain, pain due to disease of various origin such as: diabetic neuropathy, neurotropic viral disease including human immunodeficient virus (HIV), herpes zoster such as post herpetic neuralgia; polyneuropathy, neurotoxicity, mechanical nerve injury, carpal tunnel syndrome, immunologic mechanisms like multiple sclerosis; sleep disorders, anxiety and depression disorders, inflammatory disorders, weight and eating disorders, Parkinson's disease, addiction, spasticity, hypertension or other disorders. The disclosure also relates to the process of preparation of the amide compounds. Formula (1). The present disclosure also relates to methods for the preparation of such compounds, and to pharmaceutical compositions containing them.
Owner:ADVINUS THERAPEUTICS PVT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com