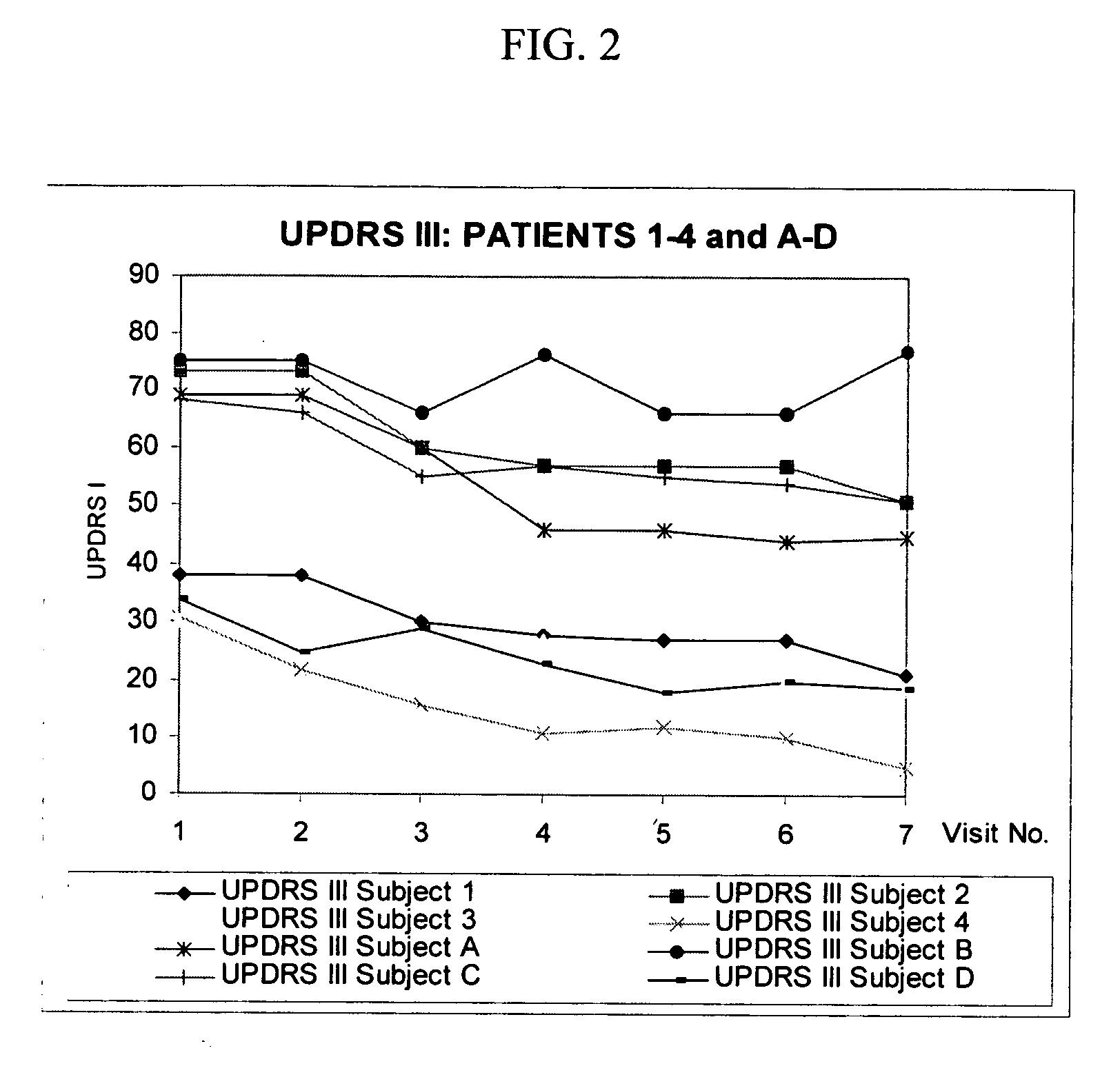
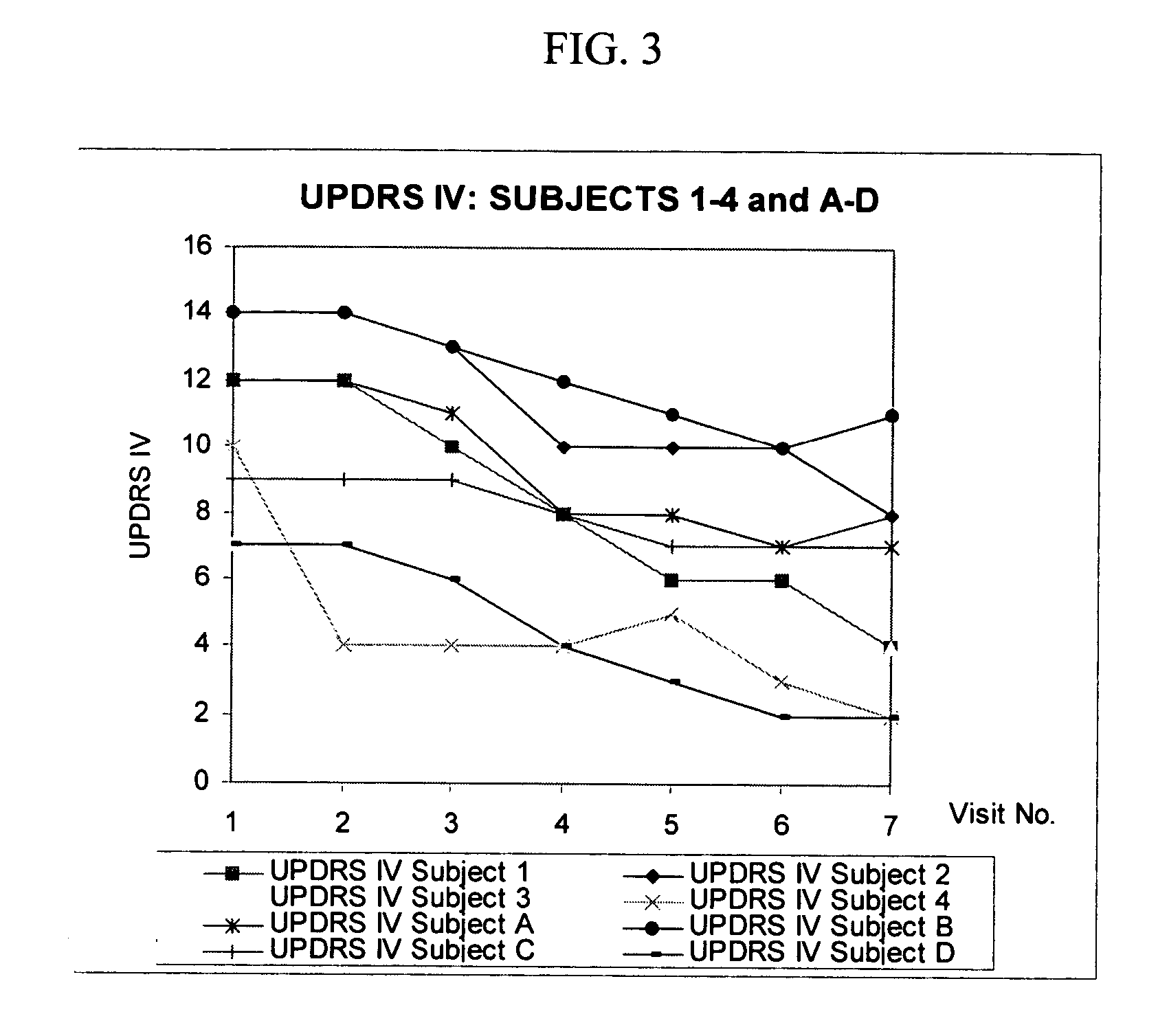
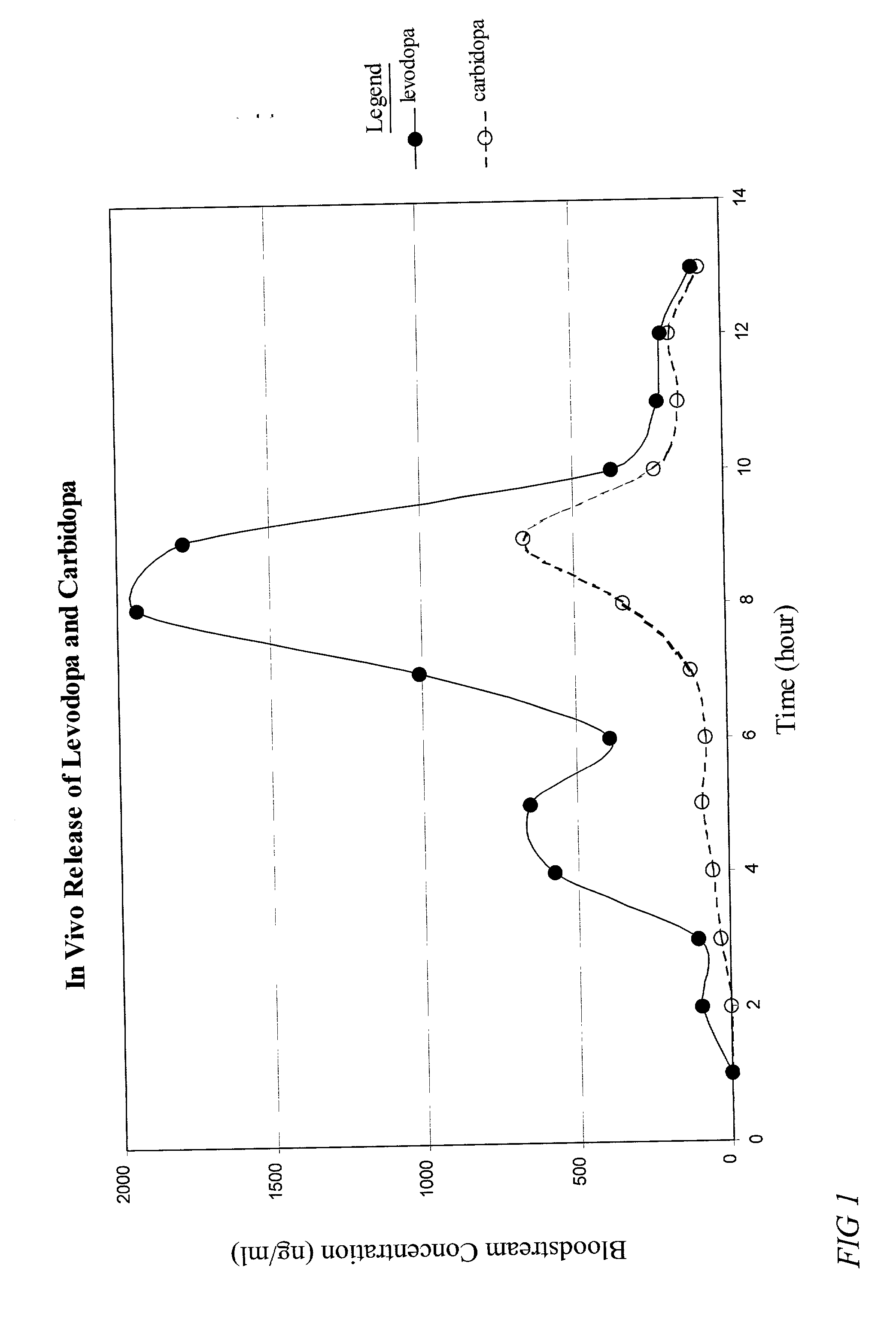
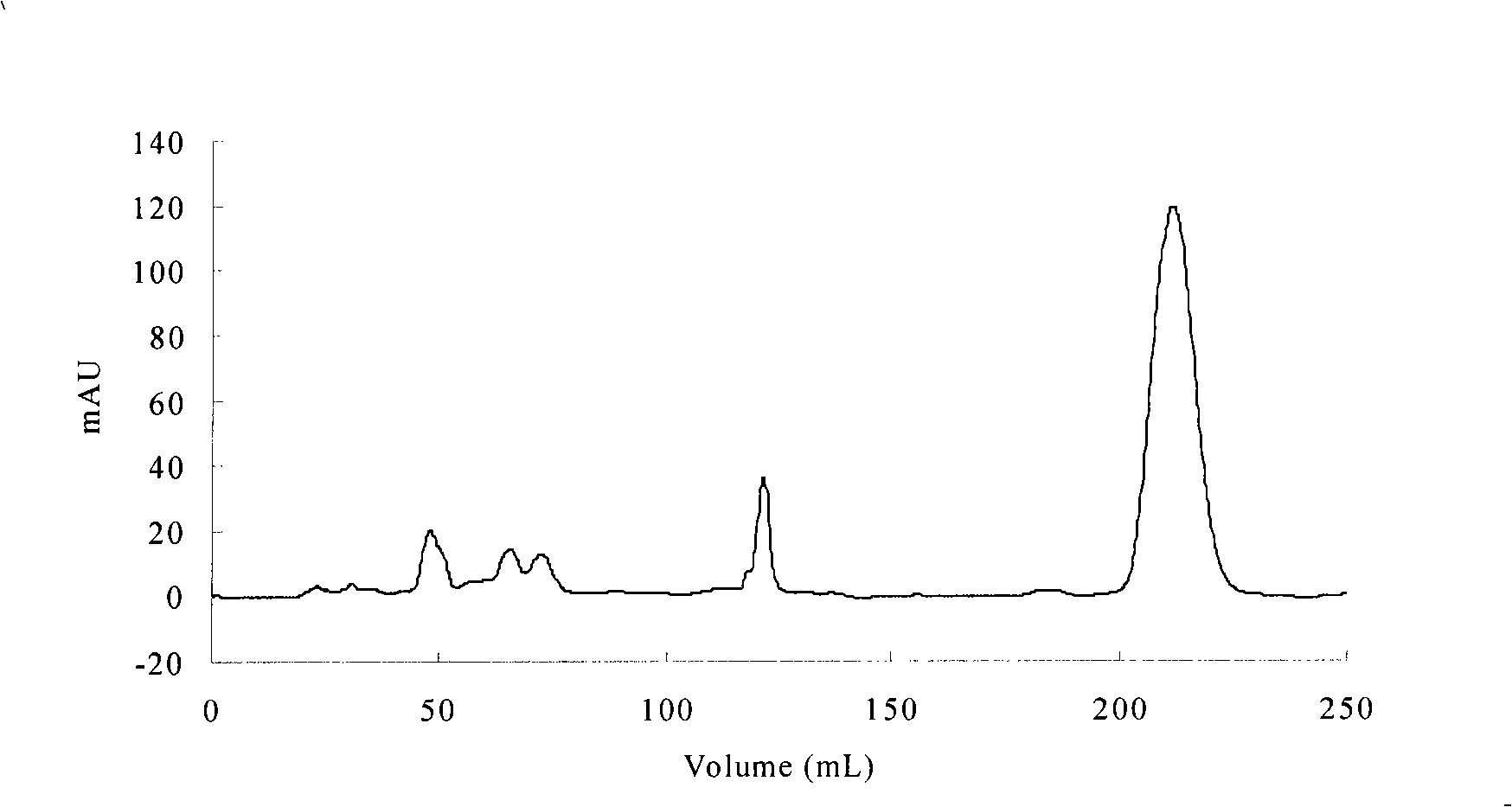
Patents
Literature
328 results about "Levodopa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
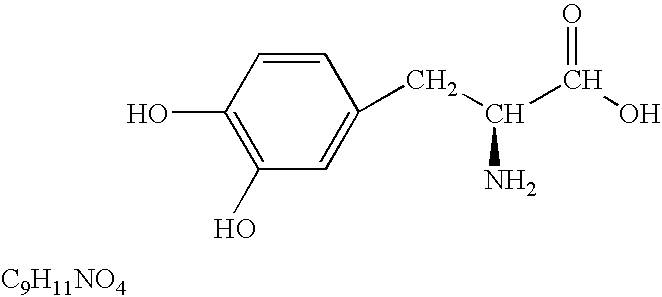
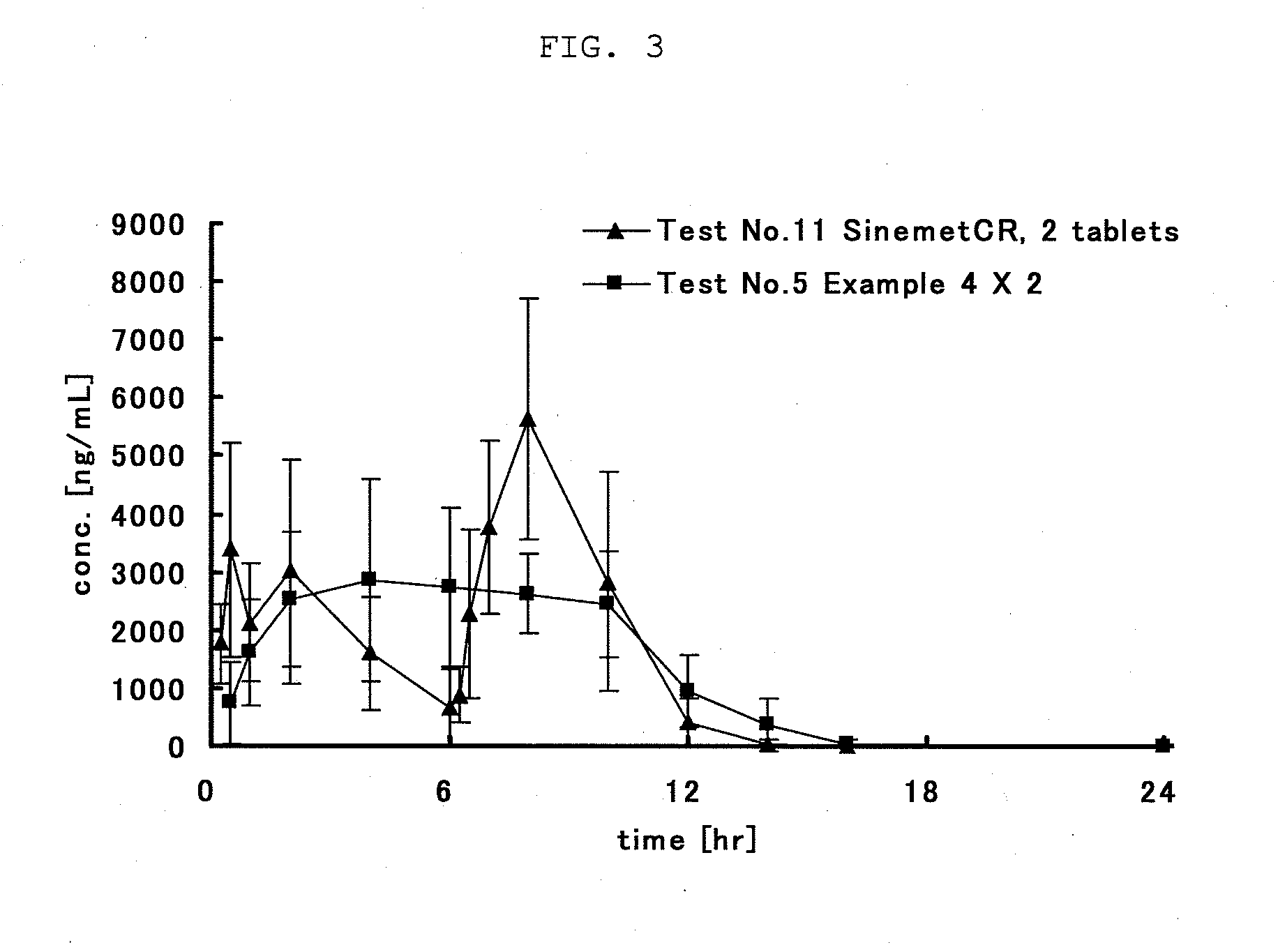
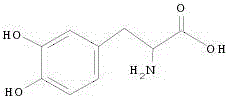
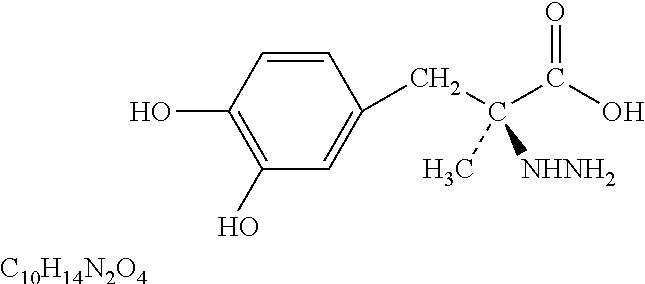
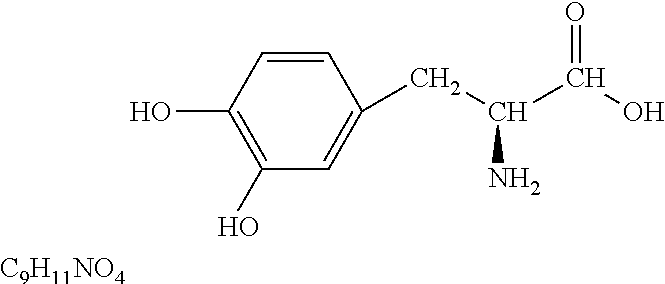
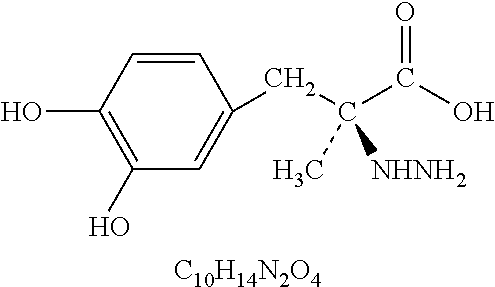
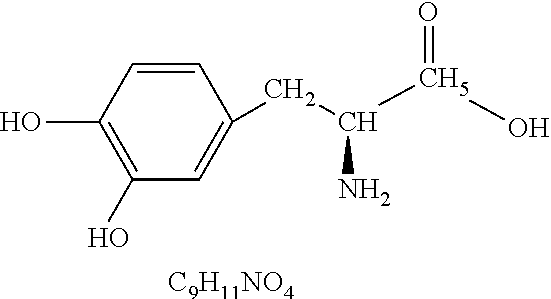
L-DOPA, also known as levodopa and l-3,4-dihydroxyphenylalanine, is an amino acid that is made and used as part of the normal biology of humans, as well as some animals and plants. Humans, as well as a portion of the other animals that utilize l-DOPA in their biology, make it via biosynthesis from the amino acid l-tyrosine. l-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Furthermore, l-DOPA itself mediates neurotrophic factor release by the brain and CNS. l-DOPA can be manufactured and in its pure form is sold as a psychoactive drug with the INN levodopa; trade names include Sinemet, Pharmacopa, Atamet, Stalevo, Madopar, and Prolopa. As a drug, it is used in the clinical treatment of Parkinson's disease and dopamine-responsive dystonia.
Treatment regimen for parkinson's disease
InactiveUS20120295960A1Reduce potential side effectsReduce maintenanceNervous disorderNucleic acid vectorSide effectCombination therapy
Provided is an improved treatment for Parkinson's Disease where the efficacy of L-Dopa treatment is increased by including gene therapy in the treatment regimen. The combination therapy results in long-term improvements in response to L-Dopa and diminished side effects caused by L-Dopa.
Owner:OXFORD BIOMEDICA (UK) LTD
Combination treatment for impaired motor function in parkinson's disease
InactiveUS20060063810A1Shorten the timeIncreased “ on ” timeBiocideNervous disorderNR1 NMDA receptorNMDA receptor
The invention provides a method, and dosage form therefor, of treating impaired motor function associated with Parkinson's disease, anti-Parkinson's drug treatment, e.g. L-Dopa therapy, and / or dementia associated with Parkinson's disease. The invention includes the combined administration of an NMDA receptor antagonist and an antidepressant, e.g., the combination of amantadine and citalopram or venlafaxine, or an NMDA receptor antagonist and an anxiolytic agent, e.g., amantadine and buspirone or trazodone, for the amelioration of undesired tremors, akinesia, dyskinesia, or bradykinesia associated with one or more different disorders or diseases. The drugs can be included in a single dosage form. One embodiment includes a combination dosage form containing each drug in controlled release forms. Another embodiment includes a combination dosage form providing a controlled release of an NMDA receptor antagonist and a rapid release of a neuroactive agent after administration to a subject.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Rapidly expanding composition for gastric retention and controlled release of therapeutic agents, and dosage forms including the composition
InactiveUS20040234608A1Improved gastric retentionHigh retention ratePowder deliveryOrganic active ingredientsGastric fluidAttention deficits
The present invention provides a pharmaceutical composition for use in a dosage form for oral administration to a patient. The composition expands upon contact with gastric fluid and promotes retention of the dosage form in the patient's stomach for a prolonged period of time. The present invention further provides pharmaceutical dosage forms containing an active ingredient, and the pharmaceutical composition. The forms are adapted for immediate or controlled release of the active ingredient. The dosage forms may be used advantageously in the treatment of Parkinson's disease with levodopa and hyperactivity and attention deficit disorder with methylphenidate.
Owner:TEVA PHARM USA INC
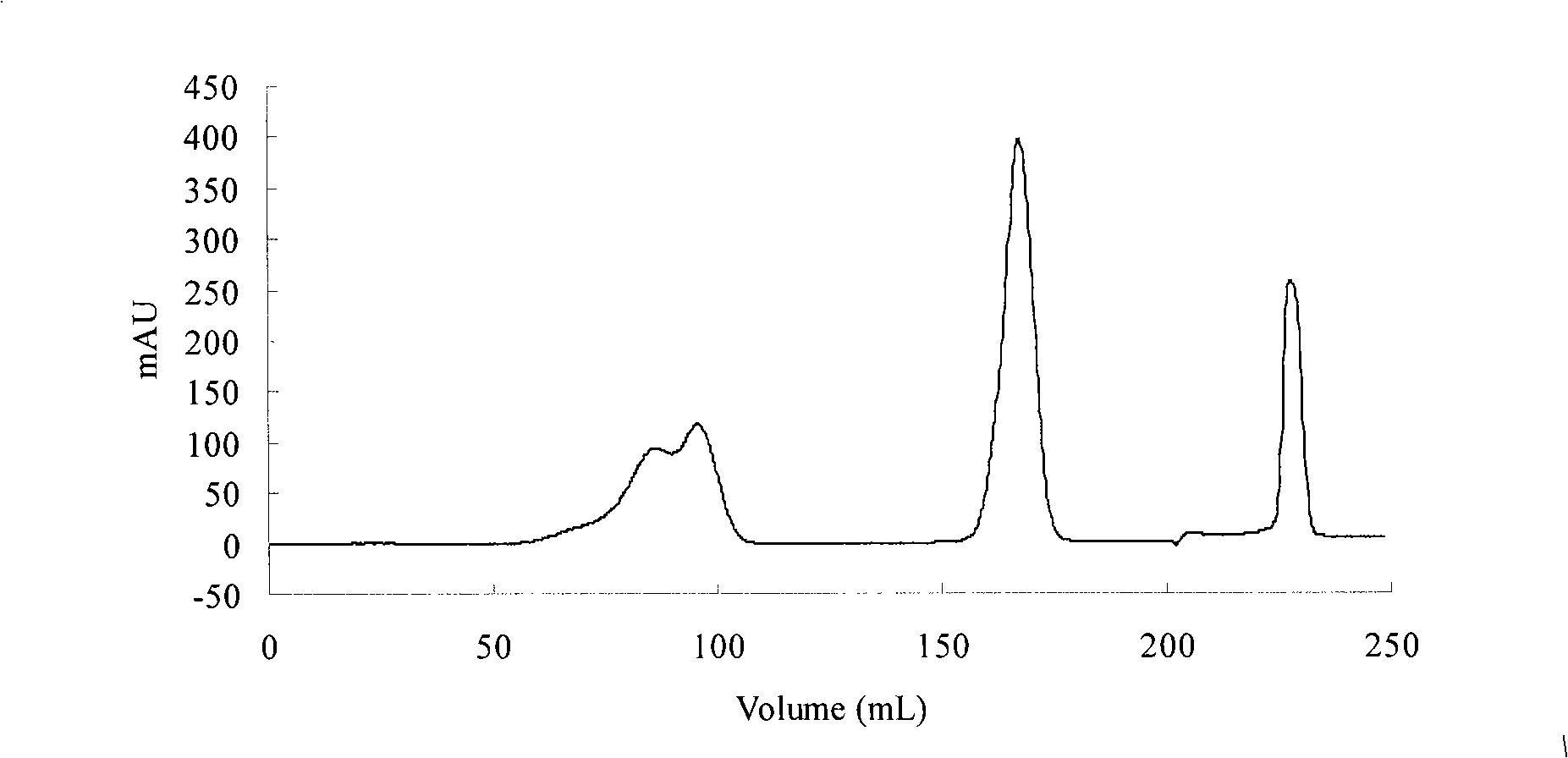
Method for separating and purifying sea-mussel mucin by using mixing adsorption chromatography
ActiveCN101348520AOvercoming the problem of low yieldHigh yieldPeptide preparation methodsAnimals/human peptidesHigh concentrationChromatographic separation
The invention relates to a method for separating and purifying mussel mucin by using a mixed adsorption chromatography. Mussel mucin contains a group of L-3,4- -Dihydroxyphenylalanine (L-DOPA), a phenohydroxyl group thereof can act as the supplier for hydrogen bond, the benzene ring thereof can generate a hydrophobic effect, and the lysine thereof with strong positive charges is capable of forming a static bond. On the basis of the properties of mussel mucin, a mixed adsorption chromatography (i.e. the adsorption chromatography based on three principles of adsorption with hydrogen bond, adsorption with hydrophobic effect, and static adsorption) is adopted to overcome the problem of low yielding rate of mussel mucin in the prior art for separating and purifying mussel mucin. An strong acid extraction is adopted to eliminate small-molecular compounds from a desalting column, an argar medium with high concentration and high cross-linking degree to separate and purify mussel mucin, and an acetic acid-urea- polyacrylamide gel electrophoresis is used to differentiate mussel mucin through specific chromogenesis with nitro blue tetrazolium. Three principles adopted with one separation medium to separate mussel mucin achieve high selectivity, simplify the purification technology, and decrease production cost.
Owner:JIANGYIN USUN BIOCHEMICAL TECH CO LTD
Pulmonary delivery for levodopa
In one aspect, the invention is related to a method of treating a patient with Parkinson's disease, the method including administering to the respiratory tract of the patient particles that include more than about 90 weight percent (wt %) of levodopa. The particles are delivered to the patient's pulmonary system, preferably to the alveoli or the deep lung.
Owner:CIVITAS THERAPEUTICS
Subcutaneously infusible levodopa prodrug compositions and methods of infusion
InactiveUS20140088192A1Reduce morbidityAdequate operational stabilityBiocideNervous disorderInfusion pumpLevodopa
Owner:SYNAGILE CORP
Treatment of pain
A method of treating pain by the co-administration of an antidepressant together with one or more precursors or inducers of neurotransmitters, particularly amino acids selected from L-phenylalanine, L-tyrosine, L-tryptophan and L-DOPA.
Owner:AMARIN NEUROSCI
Compositions and uses
According to the invention there is provided a method of treating and / or preventing the symptoms of Parkinson's disease comprising delivering apomorphine, optionally in combination with levodopa and / or a dopamine agonist that is not apomorphine, wherein apomorphine is administered by inhalation.
Owner:VECTURA LTD
Matrices for drug delivery and methods for making and using the same
In one aspect, biocompatible matrices such as sol-gels encapsulating a reaction center may be administered to a subject for conversion of prodrugs into biologically active agents. In certain embodiments, the biocompatible matrices of the present invention are sol-gels. In one embodiment, the enzyme L-amino acid decarboxylase is encapsulated and implanted in the brain to convert L-dopa to dopamine for treatment of Parkinson's disease.
Owner:BABICH JOHN W +2
Mucoadhesive Oral Formulations of High Permeability, High Solubility Drugs
Solid oral dosage formulations, such as tablet, mini-tab, multiparticulates or osmotic delivery systems, are coated with a mucoadhesive polymeric coating or formed of a mucoadhesive polymer to increase oral bioavailability of Biopharmaceutical Classification System (BCS) Class I drugs. Representative BCS I drugs include valacyclovir, gabapentin, furosemide, levodopa, metformin, and ranitidine HCl. The inclusion of mucoadhesives in the solid oral dosage form brings the dosage form into close proximity with the target epithelium and facilitates diffusion of drug into intestinal tissue. The mucoadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. Preferred mucoadhesive polymers include poly(adipic)anhydride “P(AA)” and poly(fumaric-co-sebacic)anhydride “P(FA:SA)”. Other preferred mucoadhesive polymers include non-erodable polymers such as DOPA-maleic anhydride co polymer; isopthalic anhydride polymer; DOPA-methacrylate polymers; and DOPA-cellulosic based polymers.
Owner:JACOB JULES S +4
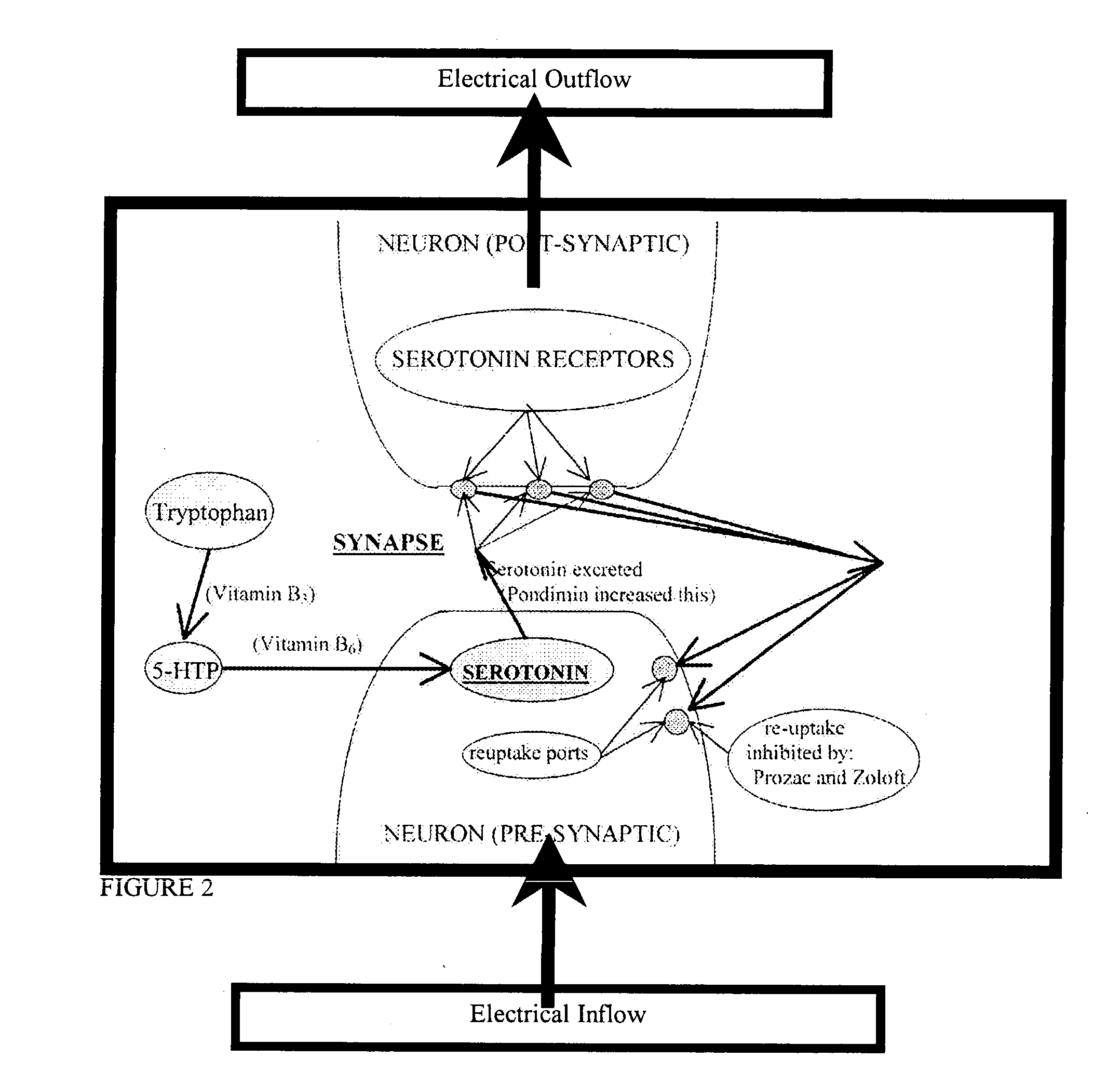
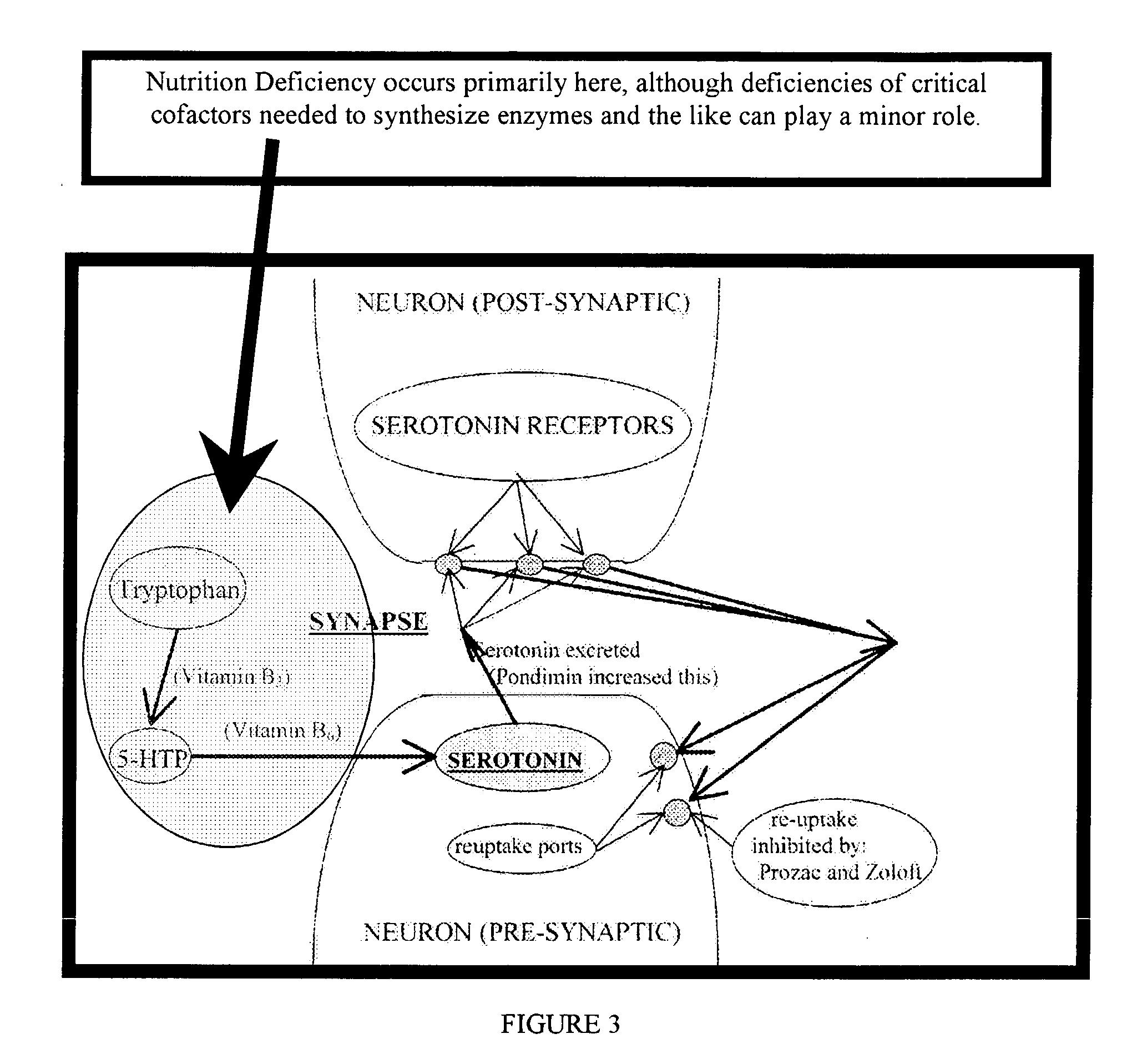
Serotonin and catecholamine system segment optimization technology
InactiveUS20030181509A1Practical and reliable and accurate and efficientBiocideNervous disorderSerotoninCatecholamine
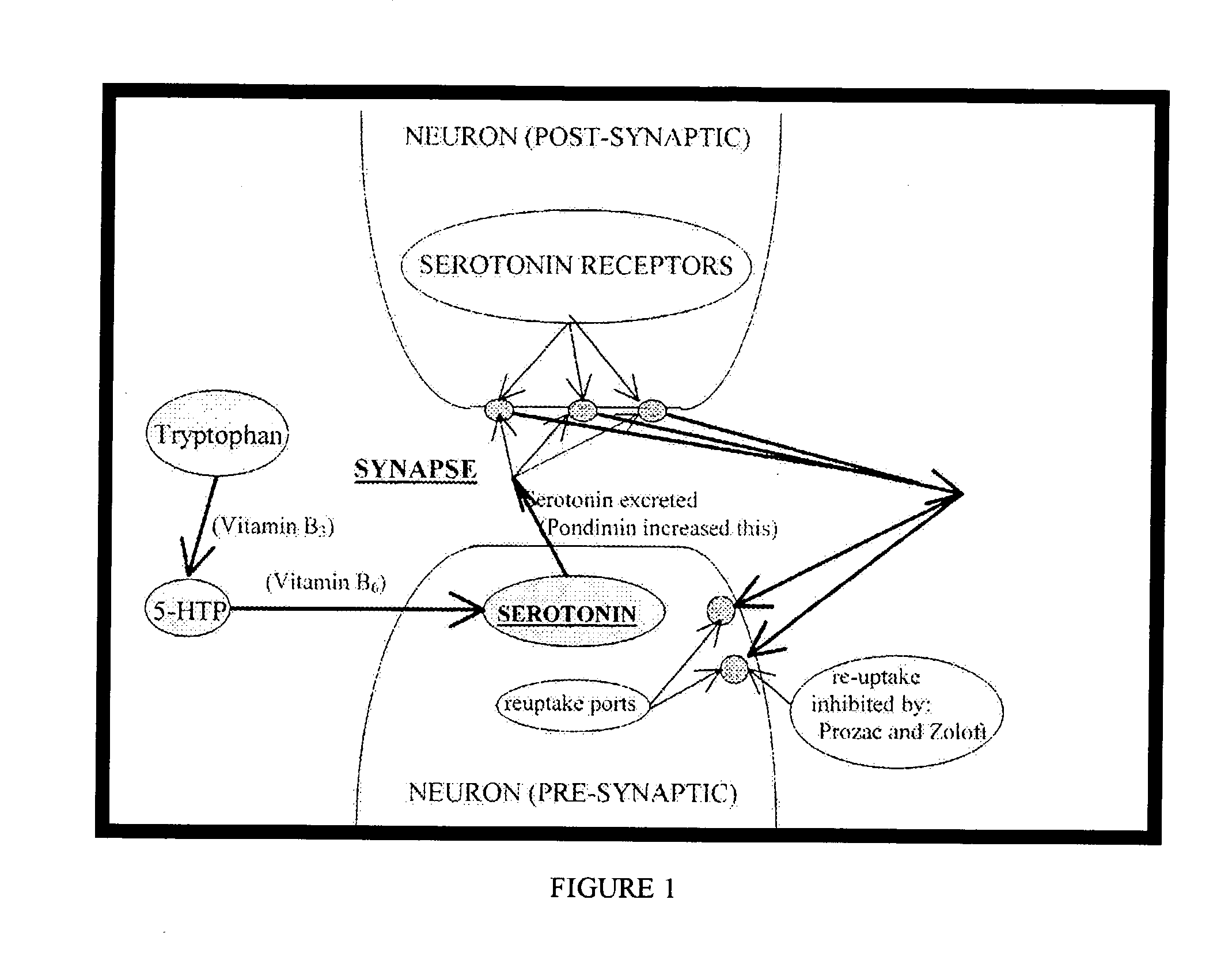
A method of treating neurotransmitter dysfunction in a patient. The method includes the step of administering an amino acid precursor of a catecholamine in a balanced and effective therapeutic range. The catecholamine precursor is preferably L-dopa, but may alternatively be tyrosine, D,L-Phenylalanine or an active isomer thereof, and N-acetyl-L-tyrosine or other amino acid precursor of L-dopa. An amino acid precursor of serotonin in an effective therapeutic range, is also administered. The serotonin precursor is preferably 5-HTP, but may alternatively be tryptophan. At least one cofactor is also preferably administered. Cofactor options include Vitamin B6, Vitamin C, Calcium, Folate, and Cysteine. A method of periodic administration and patient checking is also disclosed.
Owner:HINZ MARTIN C
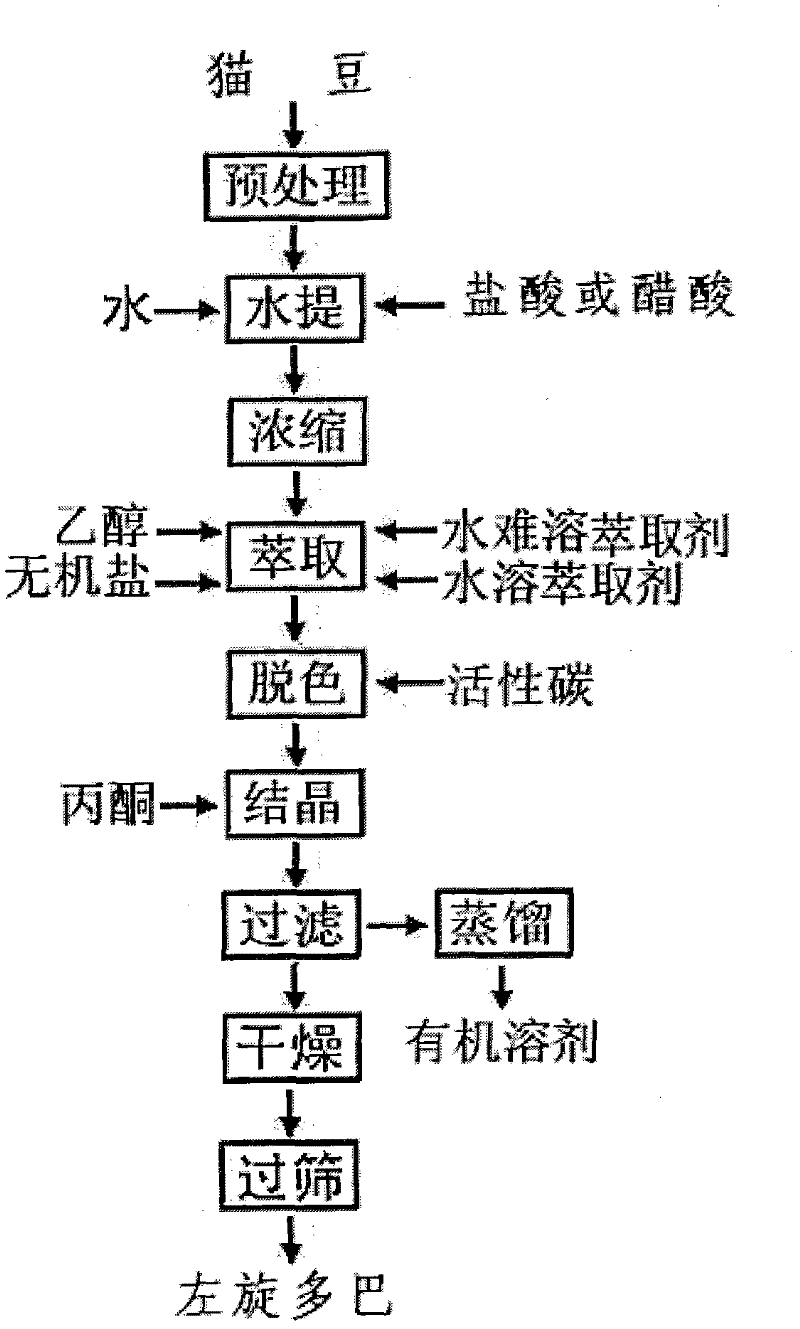
A kind of preparation method of cat bean levodopa
InactiveCN102267919AImprove separation efficiencyLarge amount of processingOrganic compound preparationAmino-carboxyl compound preparationSolventImpurity
The invention discloses a preparation method of cat bean levodopa. In the method, cat bean is used as a raw material, and an acid aqueous solution is used as an extraction solvent. In the water phase system, the average concentration in the solution reaches the distribution equilibrium of 20-40%, and then extracts with an extractant that is incompatible with the two water phases to remove impurities respectively, and the remaining part is decolorized, crystallized and dried to obtain a mass fraction of 99.9% Levodopa products. The invention has the advantages of reduced process, no pollutant discharge, high levodopa product yield and purity, less solvent consumption, low cost and easy industrial production, and the obtained product can be used as a raw material medicine.
Owner:南宁市一锋生物科技有限公司
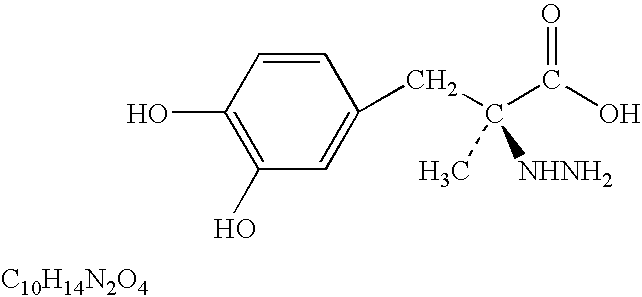
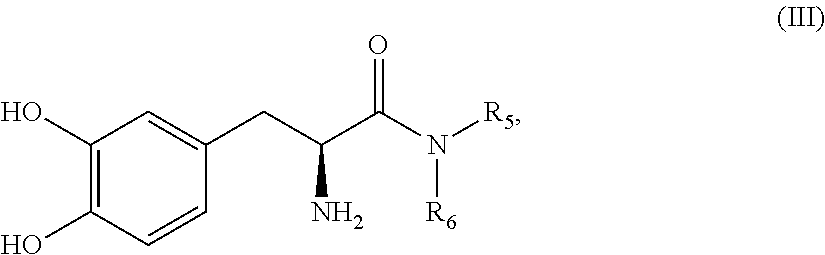
L-DOPA amide derivatives and uses thereof
L-DOPA amide derivatives, pharmaceutical compositions containing same and their use in the treatment of conditions associated with impaired dopaminergic activity / signaling (e.g., Parkinson disease) are disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Gastric retention-type sustained-release levodopa preparation
The present invention is to provide a levodopa preparation which is able to persistently release levodopa in the stomach, has a sufficient gastric residence time, is in an easily ingestible size and is able to be easily manufactured industrially with an object of maintaining a sustained concentration of levodopa in blood. It is a gastric retentive preparation for levodopa, characterized in that, the preparation contains a gastric resident layer showing a sufficient retentive property due to a high mechanical strength in the stomach and showing a good disintegrating property in an intestinal tract in addition to a drug releasing layer containing levodopa.
Owner:KISSEI PHARMA
Trosine phenol lyase engineering bacteria as well as construction method thereof and application thereof
The invention relates to trosine phenol lyase engineering bacteria as well as a construction method thereof and application thereof. A tyrosine phenol lyase gene is constructed to express plasmids; chaperonin is introduced to express the plasmids to obtain the trosine phenol lyase engineering bacteria which are identified as escherichia coli FPLF8 (Escherichia coli HPLF8) preserved in China Center for Type Culture Collection on February 19, 2016, with a preservation number being CCTCCNO:M 2016065. The engineering bacteria can be synthesized into levodopa by fermentation and conversion, and are efficiently expressed; the expressed product is stable and high in activity, and can be synthesized into levodopa by conversion in the presence of related substrates; and the process is simple, the cost is low, the yield is high, emission of three wastes is a little, and the application value for industrial production is achieved.
Owner:ZHEJIANG UNIV OF TECH +2
Pulmonary delivery for levodopa
ActiveUSRE43711E1Reduce in quantityIncrease contentBiocidePowder deliveryIntensive care medicineLevodopa
In one aspect, the invention is related to a method of treating a patient with Parkinson's disease, the method including administering to the respiratory tract of the patient particles that include more than about 90 weight percent (wt %) of levodopa. The particles are delivered to the patient's pulmonary system, preferably to the alveoli or the deep lung.
Owner:CIVITAS THERAPEUTICS
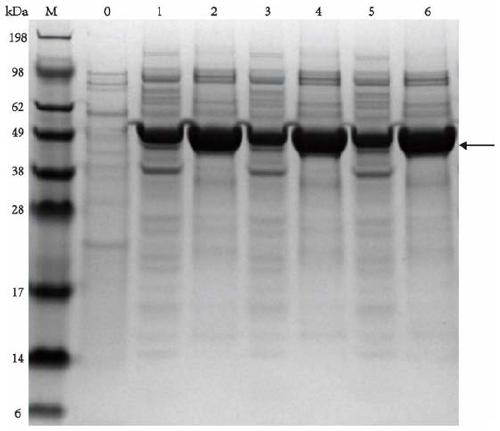
Tyrosine phenol-lyase (TPL) mutant of fusobacterium nucleatum, gene, carrier, engineered bacteria strain, and applications of TPL mutant of fusobacterium nucleatum
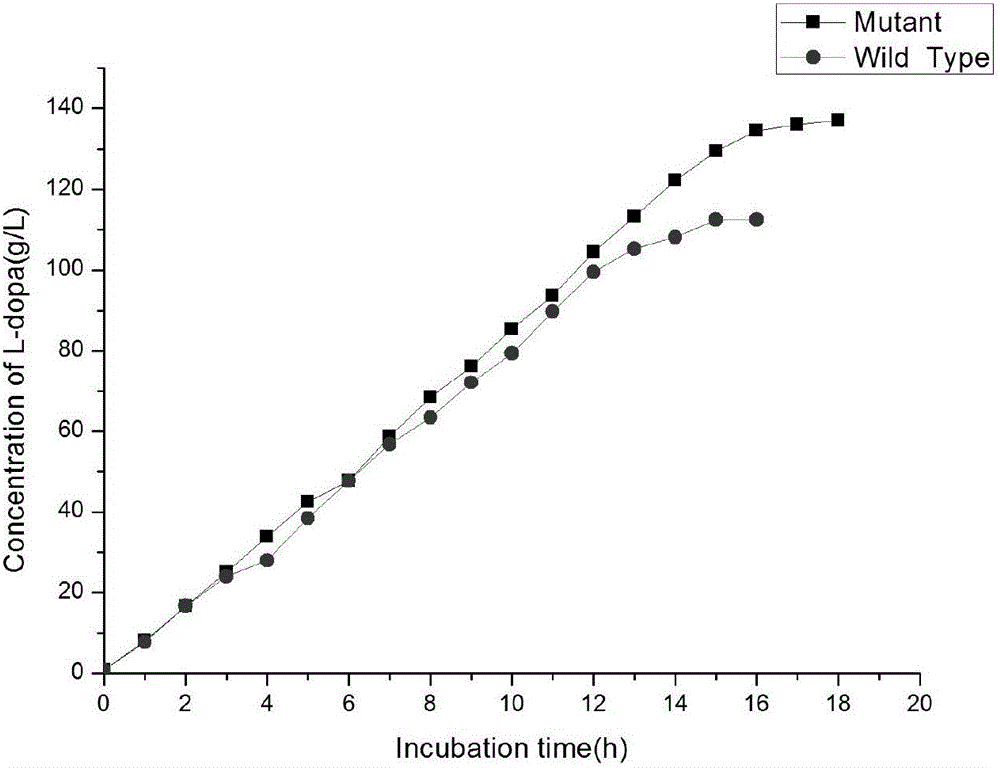
ActiveCN106754846AHigh cumulative concentration of synthetic levodopaHigh cumulative concentrationBacteriaMicroorganism based processesWild typeFusobacterium nucleatum
The invention discloses tyrosine phenol-lyase (TPL) mutant of fusobacterium nucleatum, a gene, a carrier, an engineered bacteria strain, and applications of the TPL mutant of fusobacterium nucleatum in synthetizing levodopa. Compared with the wild type, the TPL mutant of fusobacterium nucleatum provided by the invention has the more excellent catalytic performance. The accumulated concentration in synthesizing levodopa of the TPL mutant of fusobacterium nucleatum achieves 140g / L or above, compared with that of the wild type, the accumulated concentration is improved by 17-25 %, and the optical purity is greater than 99.5%; the conversion rate of the substrate, namely, catechol achieves 99.8% or above, and compared with that of the wild type, the conversion rate is improved by 15-20 %.
Owner:ZHEJIANG UNIV OF TECH
Production of tyrosine hydroxylase positive neurons
The present invention relates to a method of producing neurons that express the enzyme tyrosine hydroxylase (TH) by subjecting neural stem cells to FGF-1, a protein kinase A activator, a protein kinase C activator, and dopamine / L-DOPA. Surprisingly, when forskolin is used as a protein kinase A activator, it requires only low levels of FGF-1 and forskolin to efficiently produce TH positive neurons from fetal or adult neural stem cells. Also provided are compositions used to produce TH positive neurons and the resulting neural cell culture, as well as a method of treating disease or conditions which are associated with dopamine neuron loss or dysfunction.
Owner:STEM CELL THERAPEUTICS
Composition and dosage form for sustained effect of levodopa
InactiveUS20050113452A1Decrease dopamine eliminationAvoid movement disordersBiocideNervous disorderDopamine transportDopamine
The present invention encompasses compositions for the treatment of Parkinson's disease comprising a therapeutically effective amount of levodopa or a metabolic precursor thereof and at least one dopamine transport inhibitor in sufficient amount to decrease dopamine degradation, wherein the dopamine transport inhibitor is administered to avoid dyskinesia.
Owner:TEVA PHARM USA INC
Method for extracting L-DOPA from velvet beans
ActiveCN103467328ASimple methodFew stepsOrganic compound preparationAmino-carboxyl compound preparationDevitrificationInorganic salts
The invention discloses a method for extracting L-DOPA from velvet beans. The method includes the steps that after the velvet beans are crushed, hydrochloric acid with the mass ratio being 1%.-3%. is used for conducting subcritical extraction, after an extracting solution is concentrated, standing, staying overnight and devitrification are carried out, and accordingly a crude extract is obtained; after the crude extract is dissolved by diluted hydrochloric acid, activated carbon is added for conducting reflux, then the activated carbon is filtered and removed, the pH of the filter liquor is adjusted to be 3.0-4.0 by using an alkaline agent, standing, staying overnight and devitrification are carried out, and after drying is completed, a finished product can be obtained. The method for extracting the L-DOPA from the velvet beans is simple in method, few in step, short in time, and a reagent is saved; the yield of the finished product is 2.8%-3.0%, the content can reach up to 98%, a great number of substances such as inorganic salt do not need to be added in the extracting process, exceeding of heavy metal and the like is avoided, and therefore product quality can not be affected.
Owner:三原金瑞生物工程有限公司
L-DOPA amide derivatives and uses thereof
L-DOPA amide derivatives, pharmaceutical compositions containing same and their use in the treatment of conditions associated with impaired dopaminergic activity / signaling (e.g., Parkinson disease) are disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Treatment of Motor and Movement Disorder Side Effects Associated with Parkinson's Disease Treatments
InactiveUS20130331399A1Not affect efficacyPreventing and attenuating and treating PDOrganic active ingredientsNervous disorderEltoprazineSide effect
This invention provides methods of treating motor disorder side effects associated with the administration of levodopa to a subject having Parkinson's disease, by administering a dose of eltoprazine or a pharmaceutically acceptable acid addition salt thereof. In particular, the invention provides methods for reducing dyskinesia associated with Parkinson's disease treatments, and effective doses of eltoprazine or a pharmaceutically acceptable acid addition salt thereof.
Owner:PSYCHOGENICS
Dosing regimens for subcutaneously infusible acidic compositions
The invention features methods, compositions, dosing regimens, and infusion pumps for subcutaneously infusing acidic solutions of L-DOPA prodrugs, such as esters and amides of L-DOPA, for the treatment of Parkinson's disease. The methods and acidic compositions of the invention can reduce the severity and rate of occurrence of transient local swelling, erythema, and persistent subcutaneous granulomas associated with subcutaneous delivery of certain agents used in the treatment of Parkinson's disease.
Owner:SYNAGILE CORP
Method for improving biosynthesis of levodopa
InactiveCN103122361AOptimizing BioprocessingIncrease productionBacteriaMicroorganism based processesEscherichia coliBiotechnology
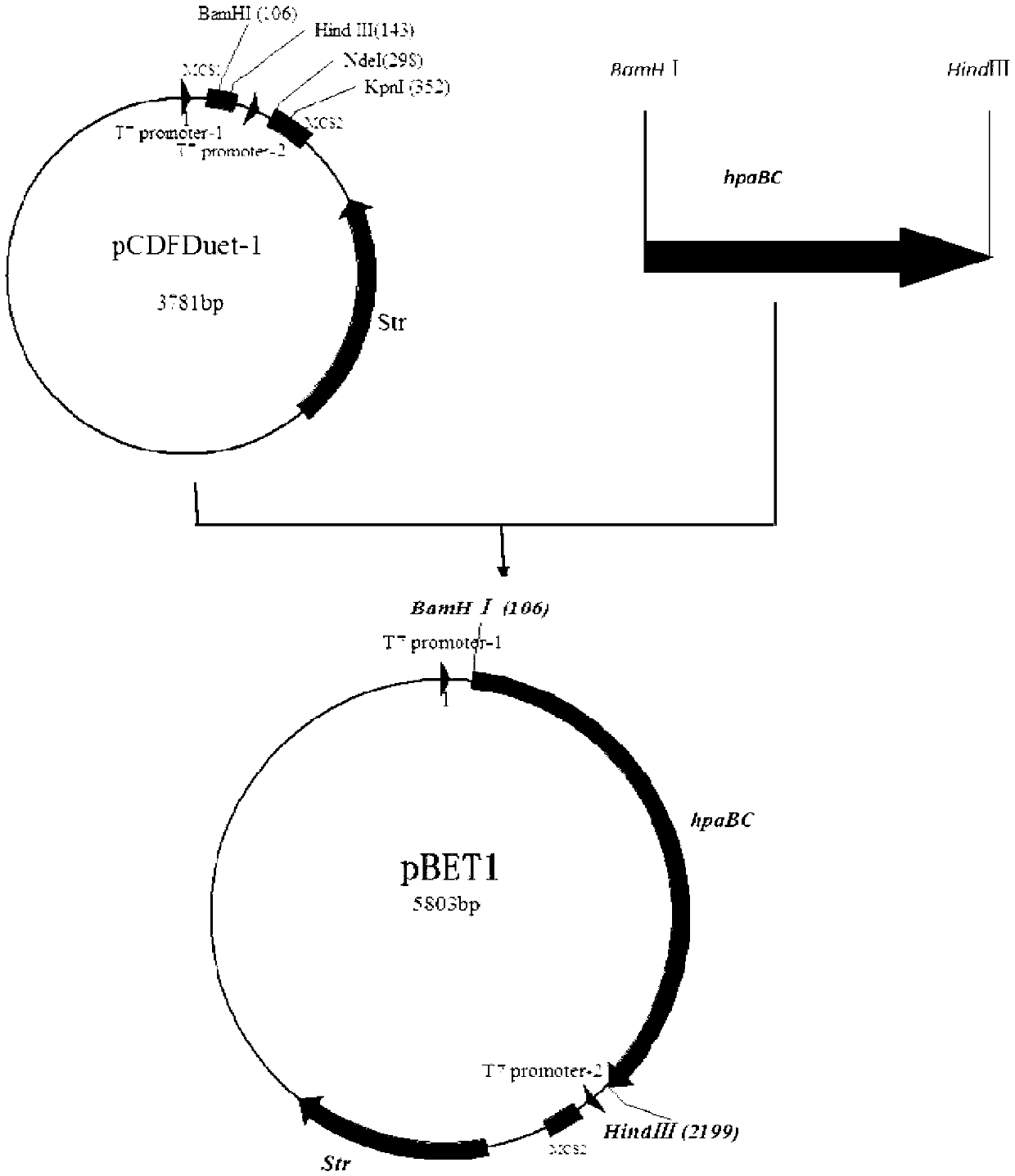
The invention discloses a method for improving biosynthesis of levodopa. The method comprises the following steps of: (1) using SEQ ID No.1 and SEQ ID No.2 as the primers, using an E.coli BL21 strain as the template, amplifying and recycling so as to obtain a hpaBC gene segment, conducting double digestion on a pCDFDuet-1 plasmid and the hpaBC gene segment, connecting and preparing a recombinant plasmid pBET1; (2) introducing the recombinant plasmid pBET1 into host escherichia coli so as to obtain transformed host cells; and (3) selecting the transformed host cells so as to obtain a levodopa production engineering bacteria single colony, taking 1ml of the levodopa production engineering bacteria single colony and putting into 100ml of a first culture medium, cultivating and measuring OD600, adding an isopropyl-beta-D-sulfo-pyran galactoside water solution and ascorbic acid, continuing cultivating and measuring the content of the levodopa. By utilizing the method disclosed by the invention, the output of the levodopa is increased, the production cost is lowered, and the operation process is simplified.
Owner:TIANJIN UNIV
Method for preparing levodopa by virtue of enzymic method
ActiveCN104726513ARich sourcesReduce manufacturing costMicroorganism based processesFermentationBiotechnologyStenotrophomonas maltophilia
The invention discloses a method for preparing levodopa by virtue of an enzymic method. The method comprises the following steps: picking up an annular stenotrophomonas maltophilia strain slant for inoculation into a seed culture medium for culturing, thereby obtaining a primary seed liquid; inoculating a fermentation culture medium with the primary seed liquid at the inoculum size of 3-10% for fermentation culturing, centrifuging after the fermentation is ended and collecting bacterial cells; and adding tyrosine and the bacterial cells to a buffer solution to have an enzymatic reaction under the conditions of 18-30 DEG C and the pH of 5.0-6.0, thereby converting the tyrosine into the levodopa. According to the method, due to the resting cell transformation technology, and the preferred intermittent weak ventilation technology, adopted in the transformation process, the catalytic efficiency of the enzyme is improved; the concentration of the levodopa is 27g / L and the molar transformation rate of the tyrosine is above 99%; and besides, the method is mild in conditions, high in enantio-selectivity and free from racemization effect, is more suitable for industrial production, and has remarkable economic benefit and industrial application value.
Owner:SHANDONG YANGCHENG BIOLOGY TECH CO LTD
Pulmonary Delivery for Levodopa
In one aspect, the invention is related to a method of treating a patient with Parkinson's disease, the method including administering to the respiratory tract of the patient particles that include more than about 90 weight percent (wt %) of levodopa. The particles are delivered to the patient's pulmonary system, preferably to the alveoli or the deep lung.
Owner:CIVITAS THERAPEUTICS
Capsules containing high doses of levodopa for pulmonary use
The present invention provides a capsule containing an inhalable powder composition wherein the composition comprises about 75% by weight or more levodopa, dipalmitoylphosphatidylcholine (DPPC) and a salt characterized by a working density of less than about 100 g / L. The invention further provides a capsule containing an inhalable powder composition wherein the composition comprises about 75% by weight or more levodopa, dipalmitoylphosphatidylcholine (DPPC) and a salt characterized by a working density of less than about 100 g / L wherein the capsule material comprises hydroxypropylmethylcellulose (HPMC) and titanium dioxide.
Owner:CIVITAS THERAPEUTICS
Method for extracting levodopa from conversion solution
InactiveCN107141229ARelieve stressEasy to handleOrganic compound preparationAmino-carboxyl compound preparationSewage treatmentInorganic acids
The invention relates to a method for extracting levodopa from a conversion solution. The method comprises the steps of adding inorganic acid into the conversion solution in a flow manner, then, carrying out adsorption with absorbent resin, carrying out eluting, and the like. According to the method, the existing process of firstly carrying out levodopa extraction and then treating pyrocatechin-containing wastewater is abandoned, an extraction process for recovering pyrocatechin while carrying out levodopa extraction is invented, subsequent wastewater treating pressure is alleviated, and unexpended pyrocatechin is recovered while the levodopa is purified, so that subsequent wastewater treatment becomes simple, the extraction cost is greatly reduced, and the subsequent sewage treatment cost is reduced.
Owner:SHANDONG LUKANG PHARMA
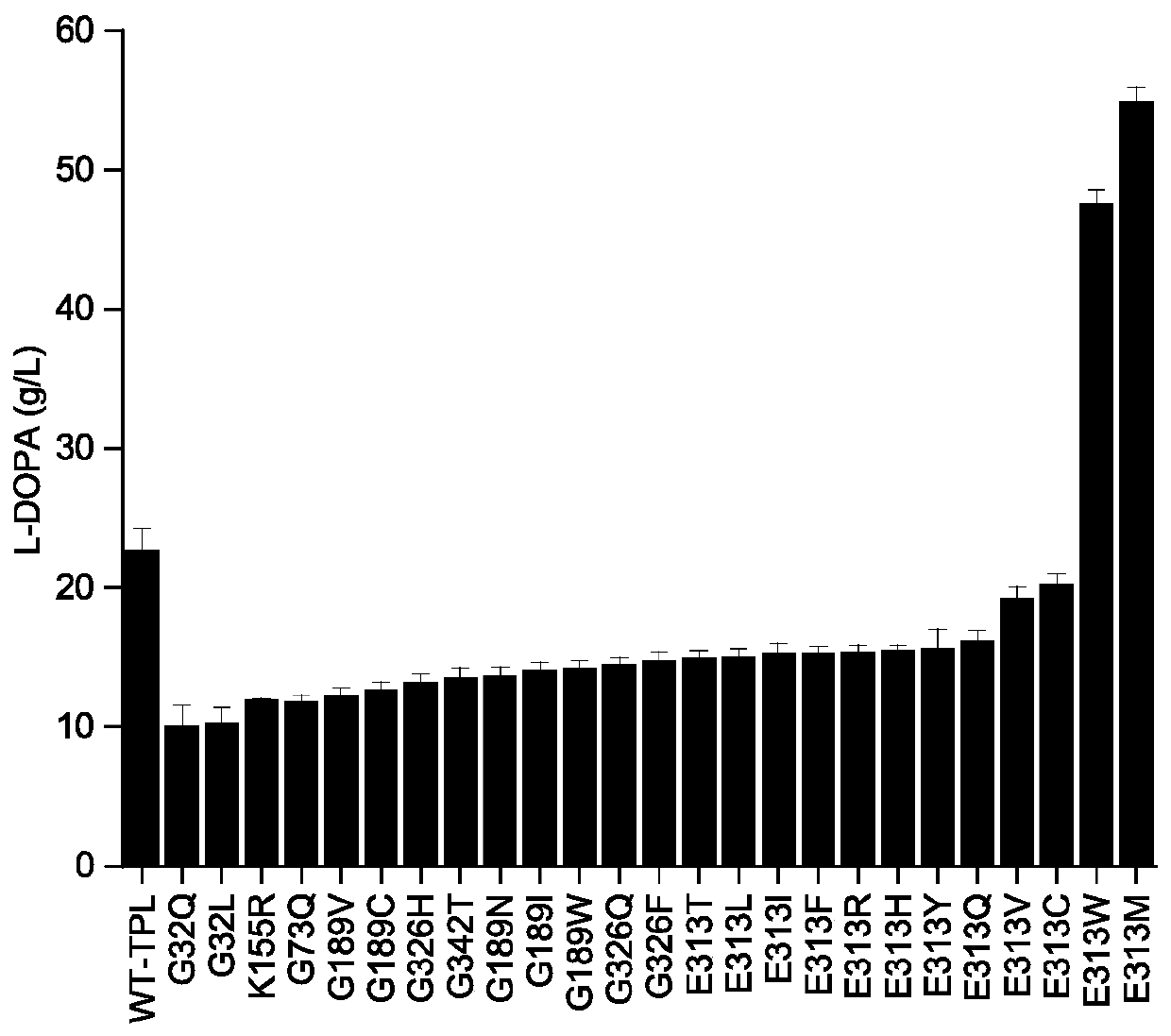
Escherichia coli expressing thermostable tyrosine phenolase and application thereof
The invention discloses escherichia coli expressing thermostable tyrosine phenolase and application thereof, and belongs to the technical field of bioengineering. According to the invention, escherichia coli BL21 is taken as a host, recombinant plasmids pET-28(a)-TPL and 25 tyrosine phenolase mutants are expressed, recombinant escherichia coli containing mutant plasmids for producing L-DOPA is obtained, whole-cell transformation is carried out on the obtained recombinant bacteria to produce the L-DOPA, the yield of the L-DOPA produced by the recombinant bacteria expressing the pET-28(a)-TPL (E313M) reaches 54.9 g / L, compared with a control strain, the yield increases by 142.9%, the half-life period of the expressed tyrosine phenolase is improved to 37.9 min, 17.1 min and 14.6 min respectively under the conditions of 20 DEG C, 40 DEG C and 60 DEG C. The escherichia coli lays the foundation for the L-DOPA production by the escherichia coli modified by metabolic engineering.
Owner:JIANGNAN UNIV
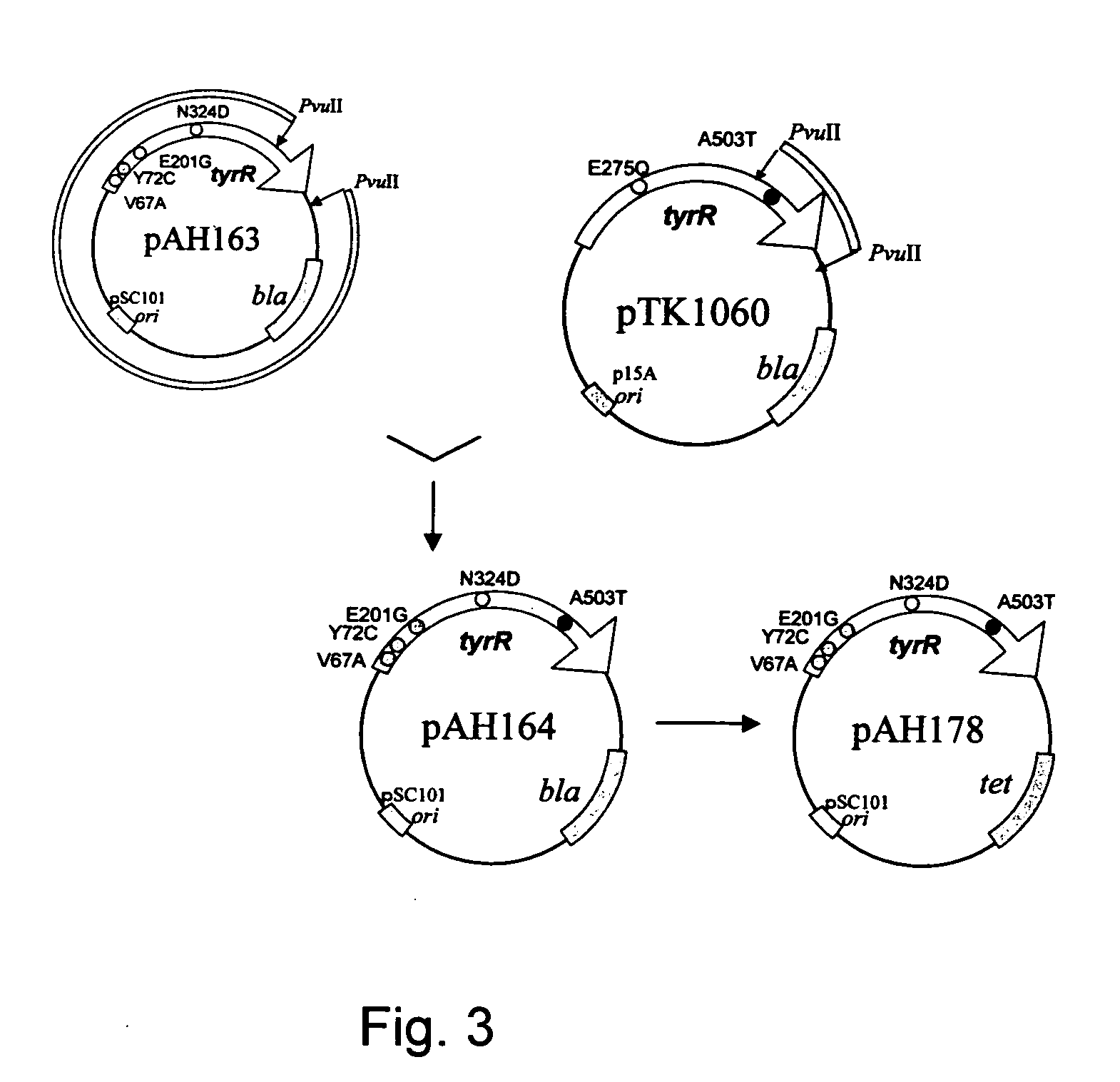
Mutant tyrosine repressor, a gene encoding the same, and a method for producing L-DOPA
A mutant tyrosine repressor that does not require tyrosine to induce expression of tyrosine phenol-lyase gene is obtained by introducing a mutation into a tyrosine repressor. A microorganism which is able to express large amounts of tyrosine phenol-lyase is obtained by introducing the mutant tyrosine repressor into the microorganism. The microorganism is useful for producing L-DOPA.
Owner:AJINOMOTO CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com