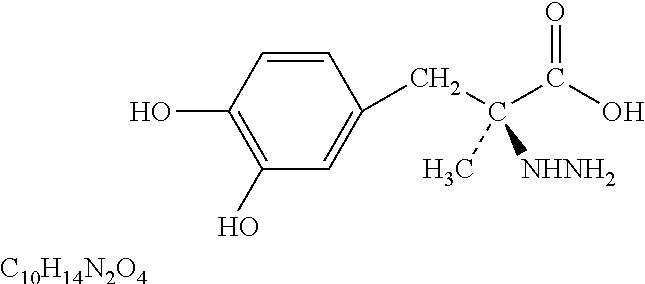
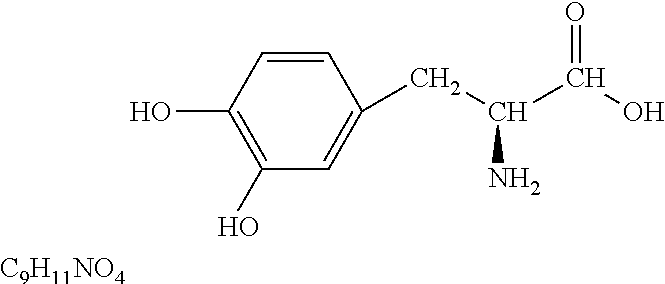
Pulmonary delivery for levodopa
a technology of levodopa and pulmonary veins, which is applied in the direction of spray delivery, peptide/protein ingredients, aerosol delivery, etc., can solve the problems of decreased dopamine storage capacity, patients tend to develop problems involving gastric emptying, and tend to become less effective, so as to reduce the number of puffs required, increase the amount of drug that can be contained and administered from a given inhaler capsule, and reduce the effect of puffs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Particles Comprising L-Dopa and Trehalose
[0096]Particles with a formulation containing L-Dopa and trehalose were prepared as follows: The aqueous solution was formed by adding 2.375 g L-Dopa and 125 mg trehalose to 700 ml of USP water. The organic solution comprised 300 ml of ethanol. The aqueous solution and the organic solution were combined in a static mixer. A 1 L total combination volume was used, with a total solute concentration of 2.5 g / L in 30 / 70 ethanol / water. The combined solution flowed from the static mixer into a 2 fluid atomizer and the resulting atomized droplets were spray dried under the following process conditions:
[0097]
Inlet temperature~135° C.Outlet temperature from the drying drum~49 to 53° C.Nitrogen drying gas =95 kg / hrAtomization rate =14 g / min2 Fluid internal mixing nozzle atomizerLiquid feed rate =70 ml / minPressure in drying chamber =−2.0 in water
[0098]The resulting particles had a FPF(5.6) of 33%, and a FPF(3.4) of 12%, both measured using a 2-stage ACI....
example 2
Particles Comprising L-Dopa, Trehalose and Sodium Chloride
[0101]Particles with a formulation containing L-Dopa, trehalose and sodium chloride were prepared as follows: The aqueous solution was formed by adding 2.325 g L-Dopa, 125 mg trehalose and 50 mg sodium chloride to 700 ml of USP water. The organic solution comprised 300 ml of ethanol. The aqueous solution and the organic solution were combined in a static mixer. A 1 L total combination volume was used, with a total solute concentration of 2.5 g / L in 30 / 70 ethanol / water. The combined solution flowed from the static mixer into a 2 fluid atomizer and the resulting atomized droplets were spray dried under the following process conditions:
[0102]
Inlet temperature~135° C.Outlet temperature from the drying drum~49 to 53°C.Nitrogen drying gas =95 kg / hrAtomization rate =14 g / min2 Fluid internal mixing nozzle atomizerLiquid feed rate =70 ml / minLiquid feed temperature~50° C.Pressure in drying chamber =−2.0 in water
[0103]The resulting part...
example 3
Particles Comprising L-Dopa and DPPC
[0106]Particles with a formulation containing L-Dopa and DPPC were prepared as follows: The aqueous solution was formed by adding 1.1875 g L-Dopa to 300 ml of USP water. The organic solution comprised 62.5 mg DPPC in 700 ml of ethanol. The aqueous solution and the organic solution were combined in a static mixer. A 1 L total combination volume was used, with a total solute concentration of 1.25 g / L in 70 / 30 ethanol / water. The combined solution flowed from the static mixer into a 2 fluid atomizer and the resulting atomized droplets were spray dried under the following process conditions:
[0107]
Inlet temperature~108° C.Outlet temperature from the drying drum~49 to 53° C.Nitrogen drying gas =95 kg / hrAtomization rate =18 g / min2 Fluid internal mixing nozzle atomizerLiquid feed rate =70 ml / minLiquid feed temperature~50° C.Pressure in drying chamber =−2.0 in water
[0108]The resulting particles had a FPF(5.6) of 29%, and a FPF(3.4) of 10%, both measured usi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com