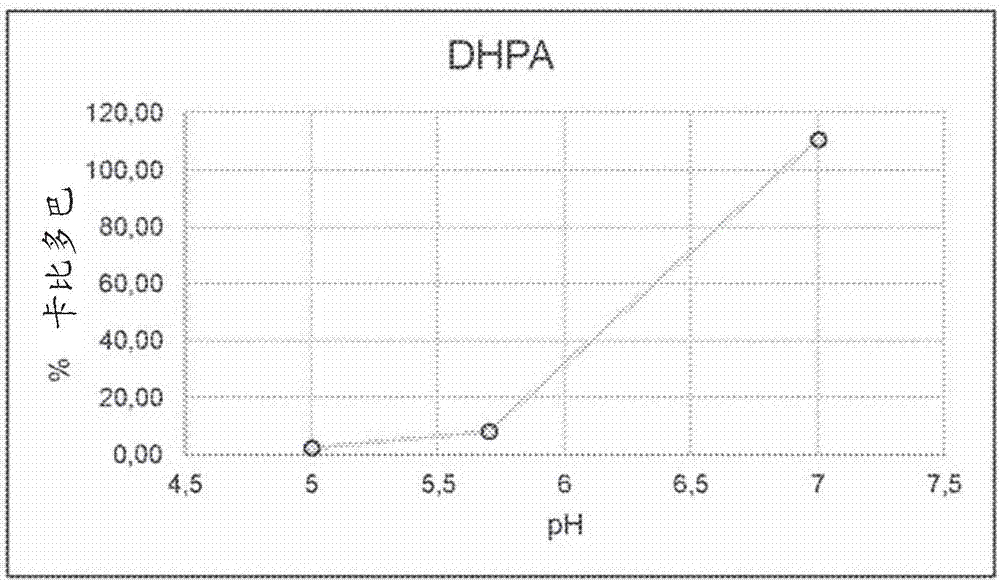
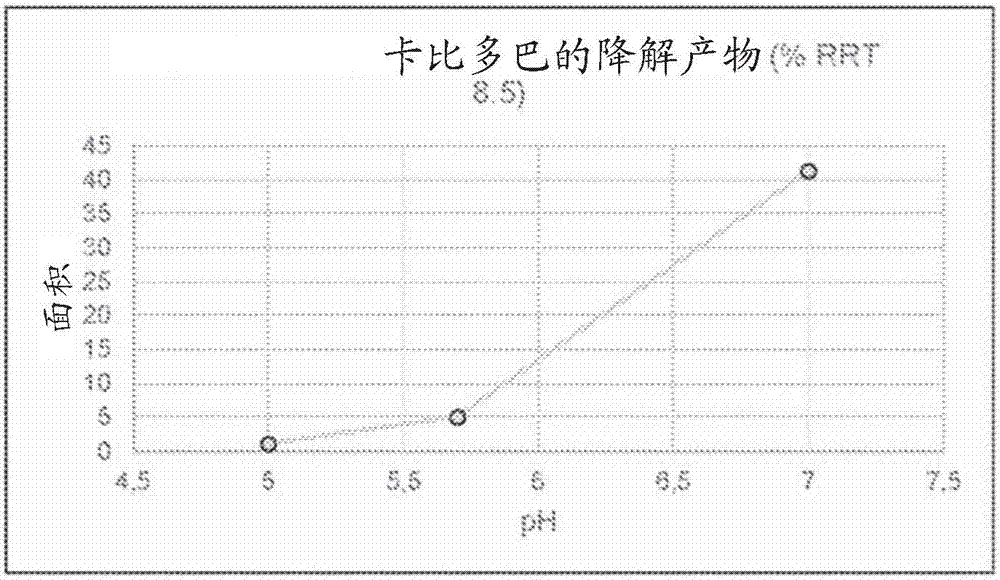
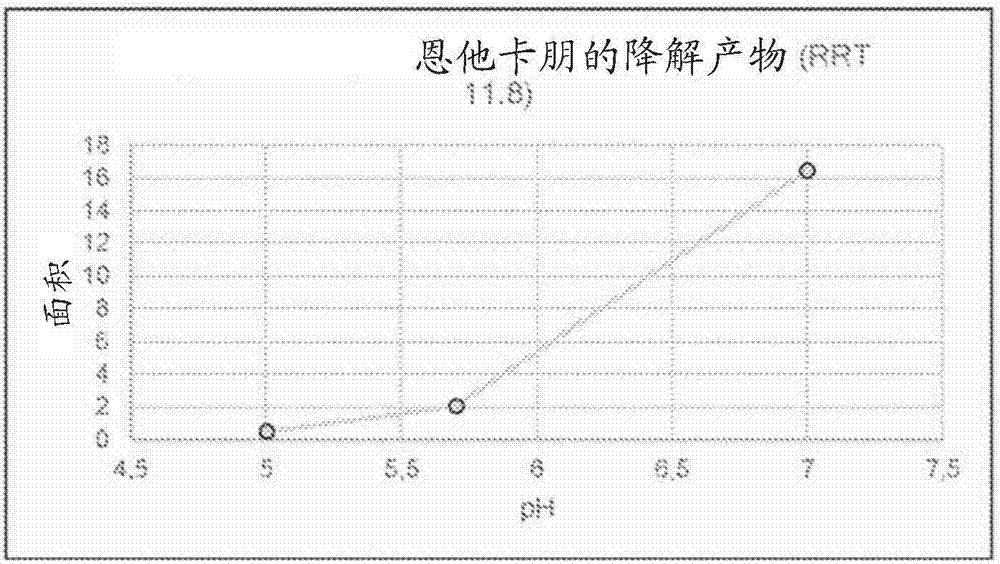
Patents
Literature
37 results about "Decarboxylase inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any substance or agent which suppresses, prevents or opposes the activity of decarboxylase.
Drug treatment for restless leg syndrome
A method for the treatment of Restless Leg Syndrome (RLS), which comprises administering an alpha2-agonist and a second agent selected from the group consisting of the dopamine agonists, opioids, benzodiazepines and the combination of L-DOPA plus a decarboxylase inhibitor.
Owner:BRECHT HANS MICHAEL
Piperidine compounds useful as malonyl-CoA decarboxylase inhibitors
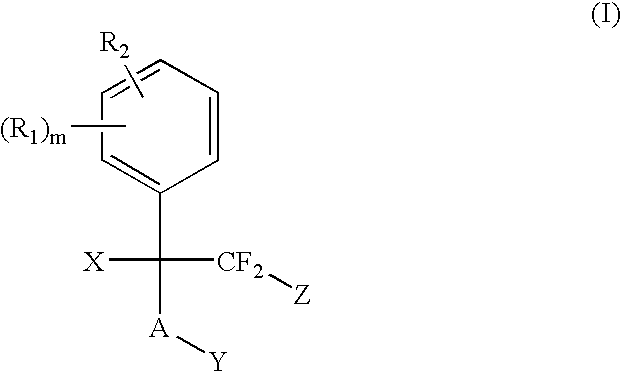
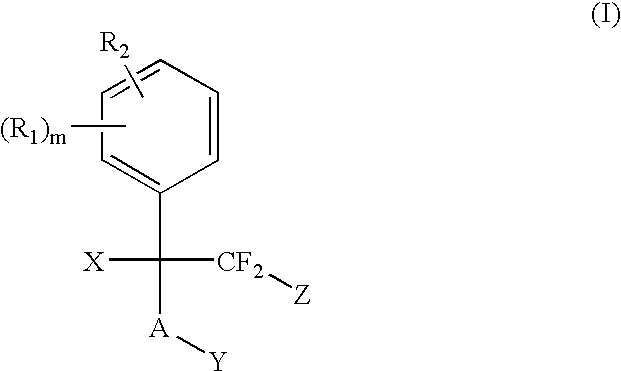
The present invention provides methods for the use of compounds as depicted by structure I, pharmaceutical compositions containing the same, and methods for the prophylaxis, management and treatment of metabolic diseases and diseases modulated by MCD inhibition. The compounds disclosed in this invention are useful for the prophylaxis, management and treatment of diseases involving in malonyl-CoA regulated glucose / fatty acid metabolism pathway. In particular, these compounds and pharmaceutical composition containing the same are indicated in the prophylaxis, management and treatment of cardiovascular diseases, diabetes, cancer and obesity.
Owner:CHUGAI PHARMA CO LTD
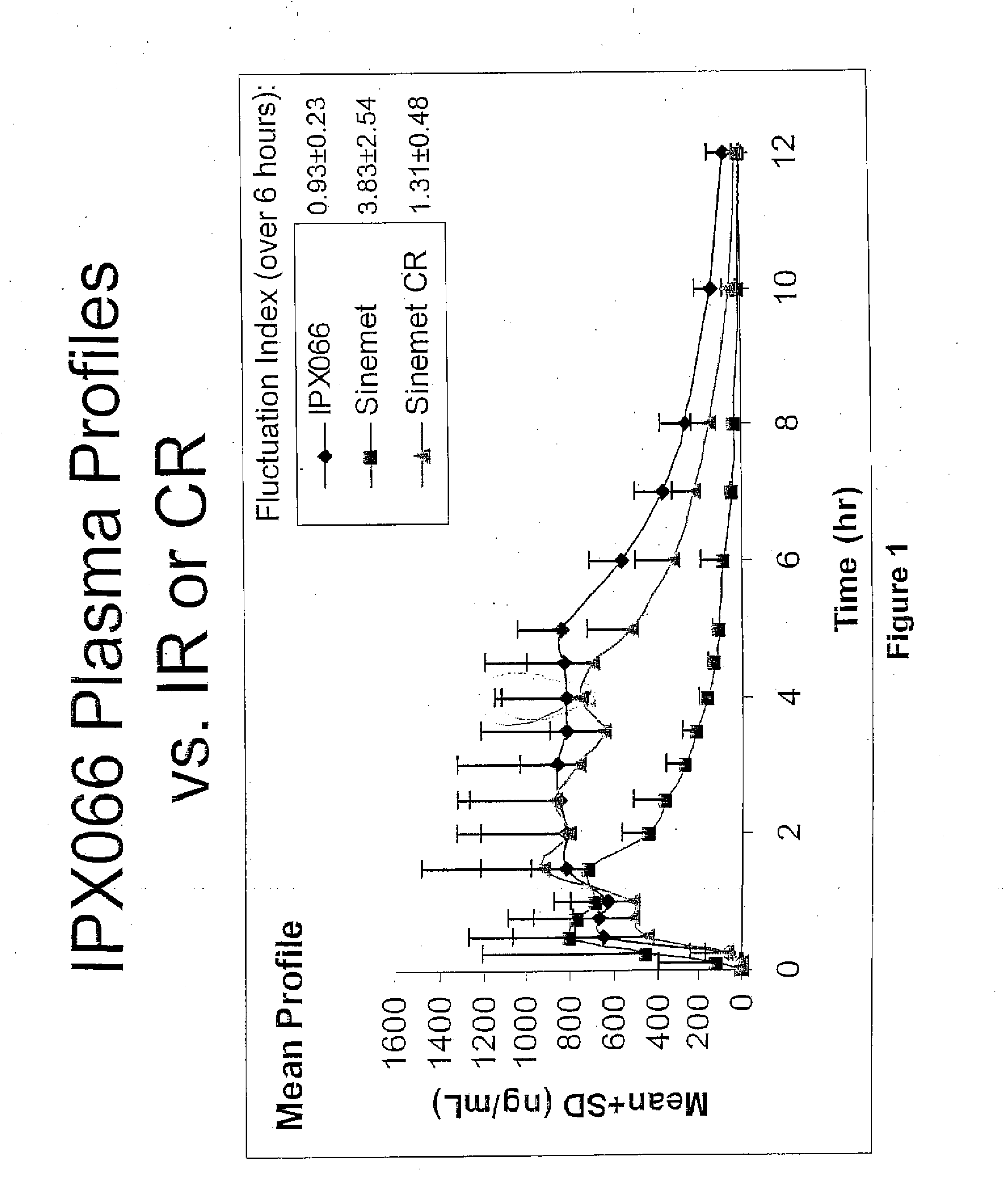
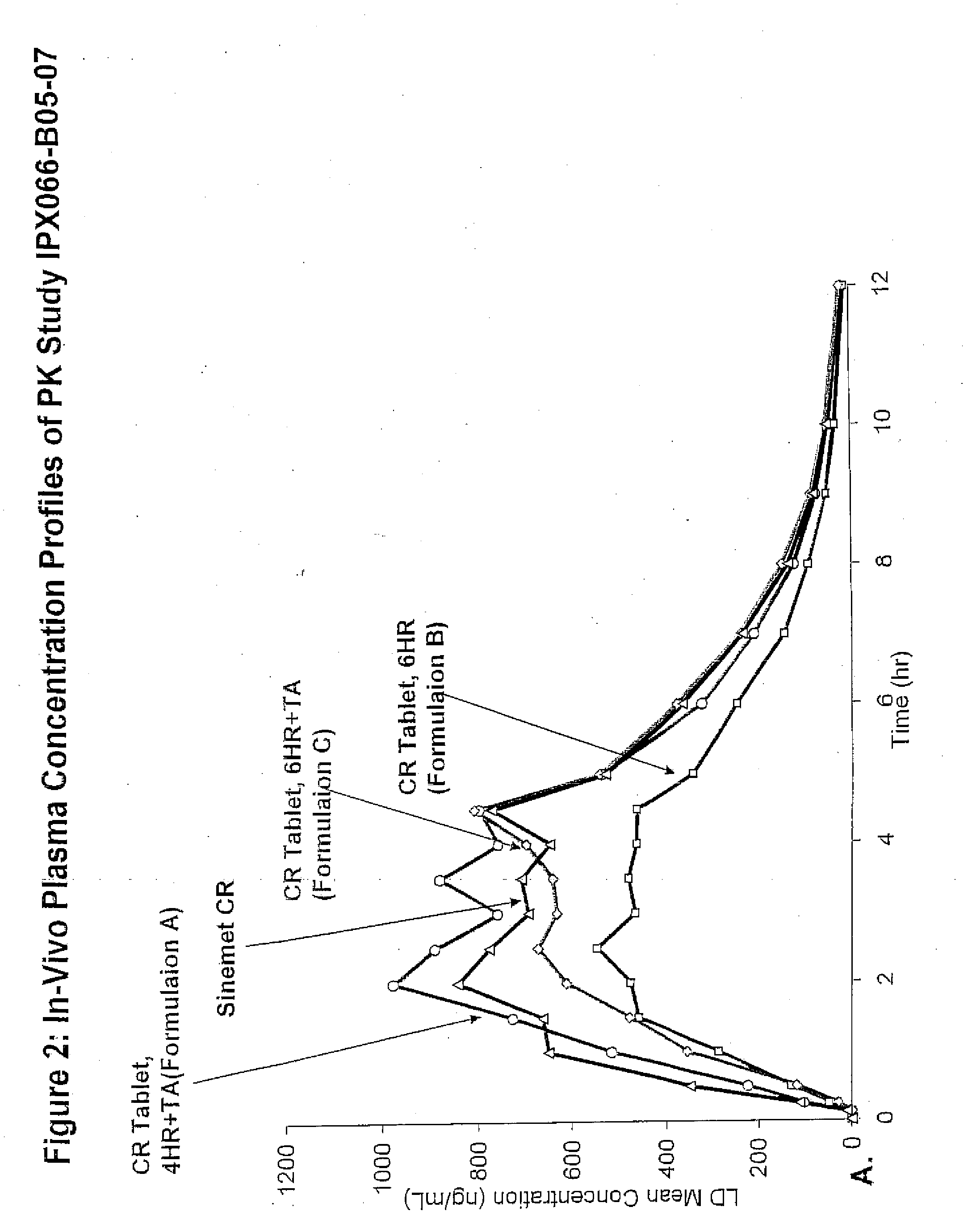
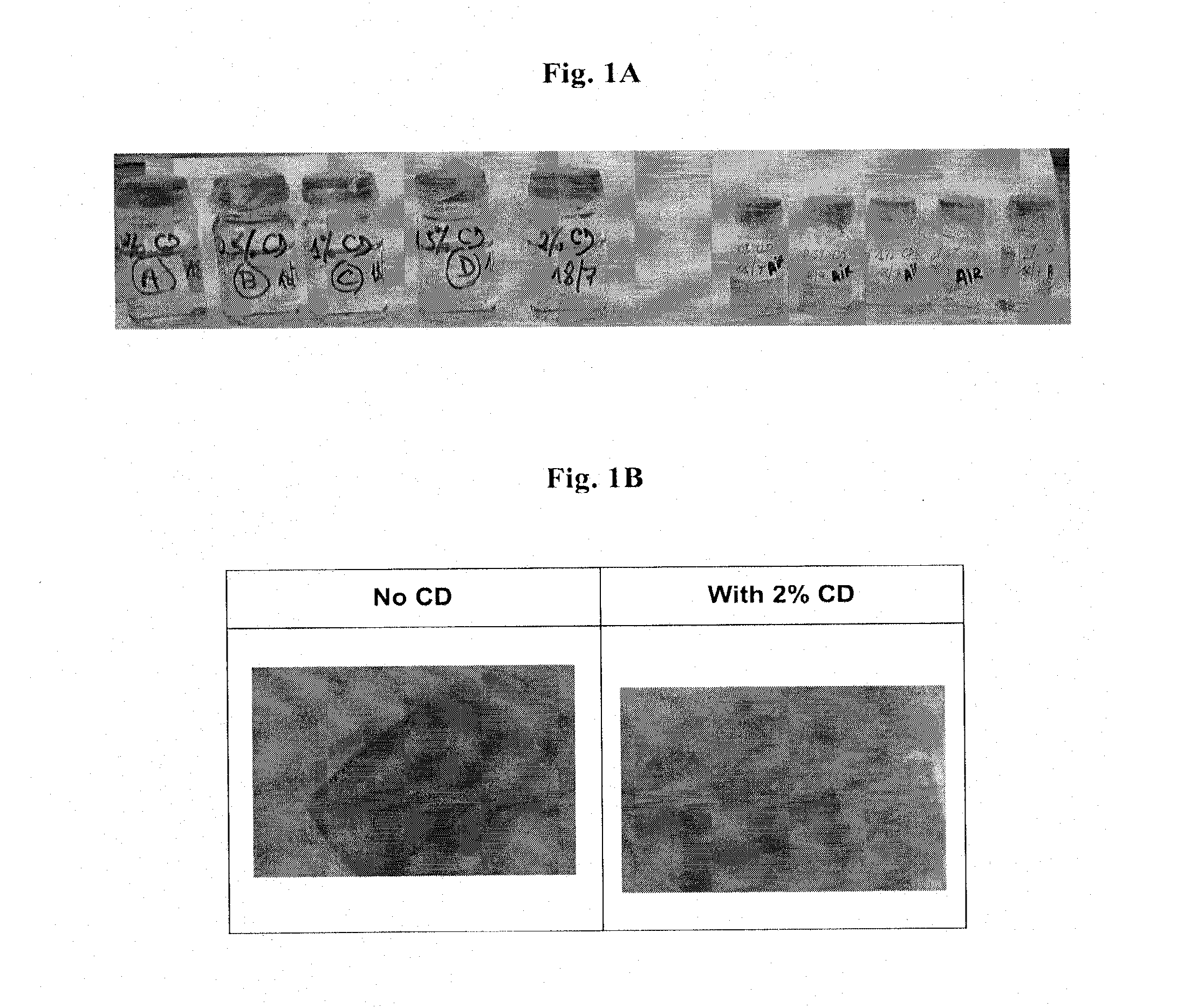
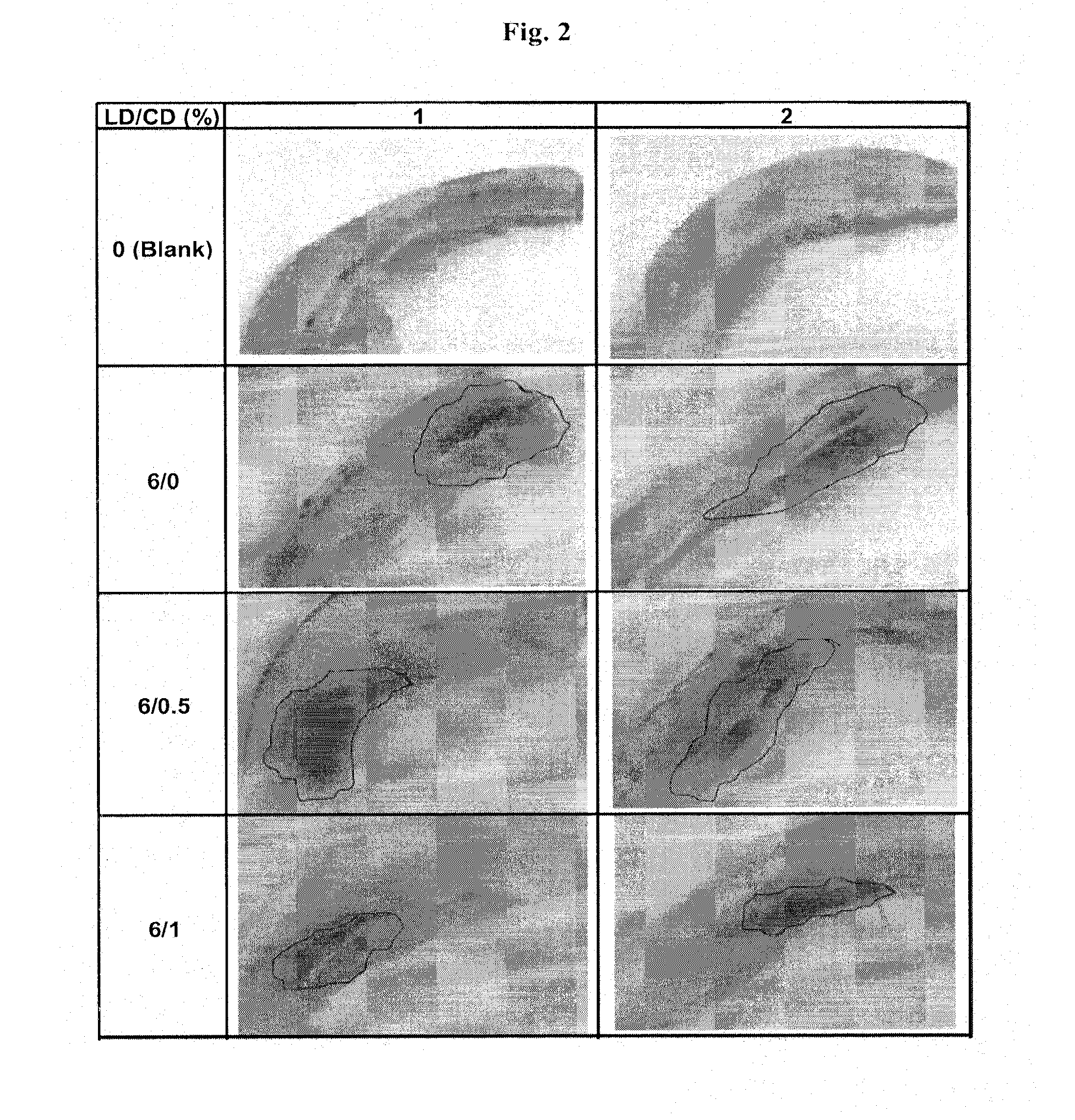
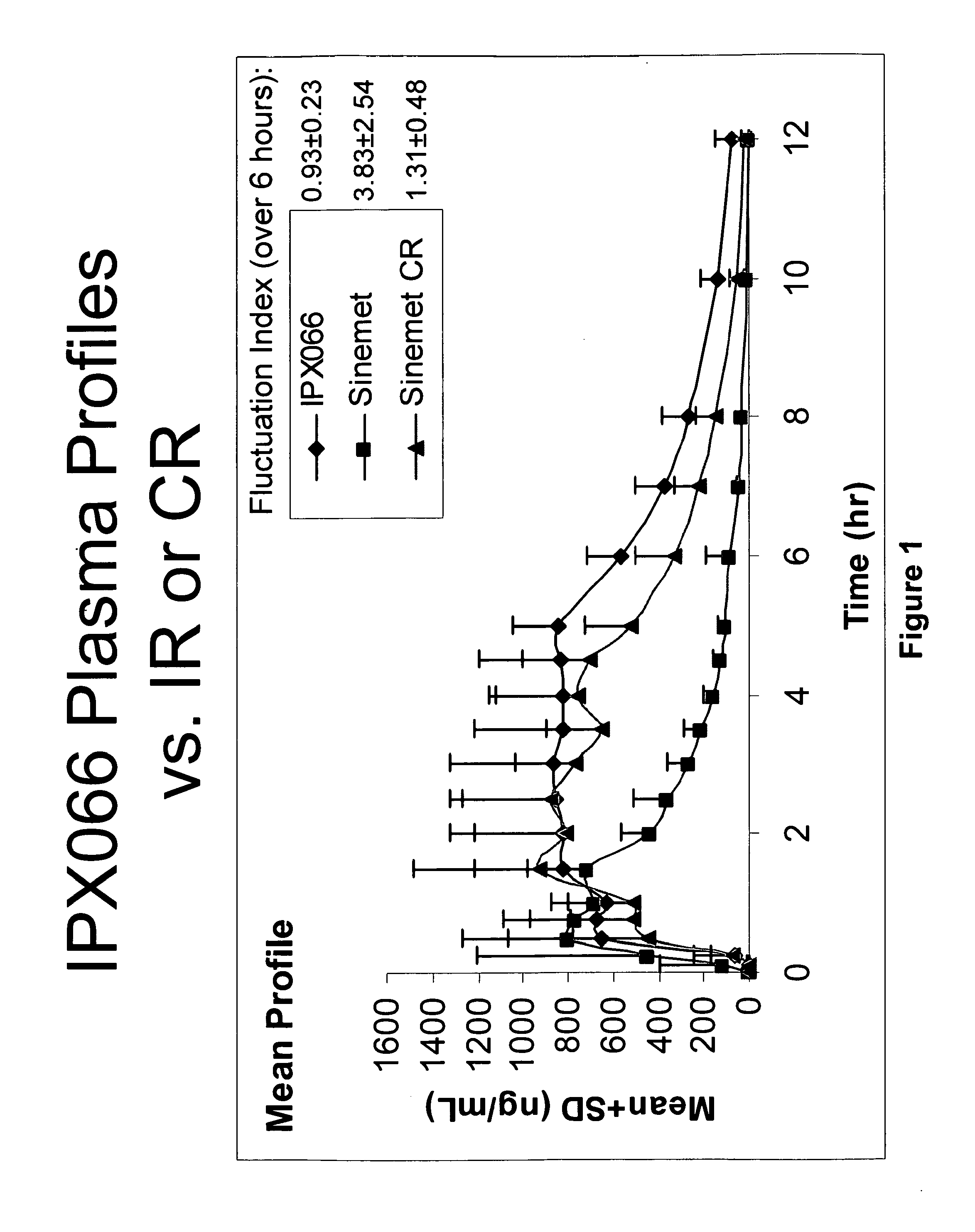
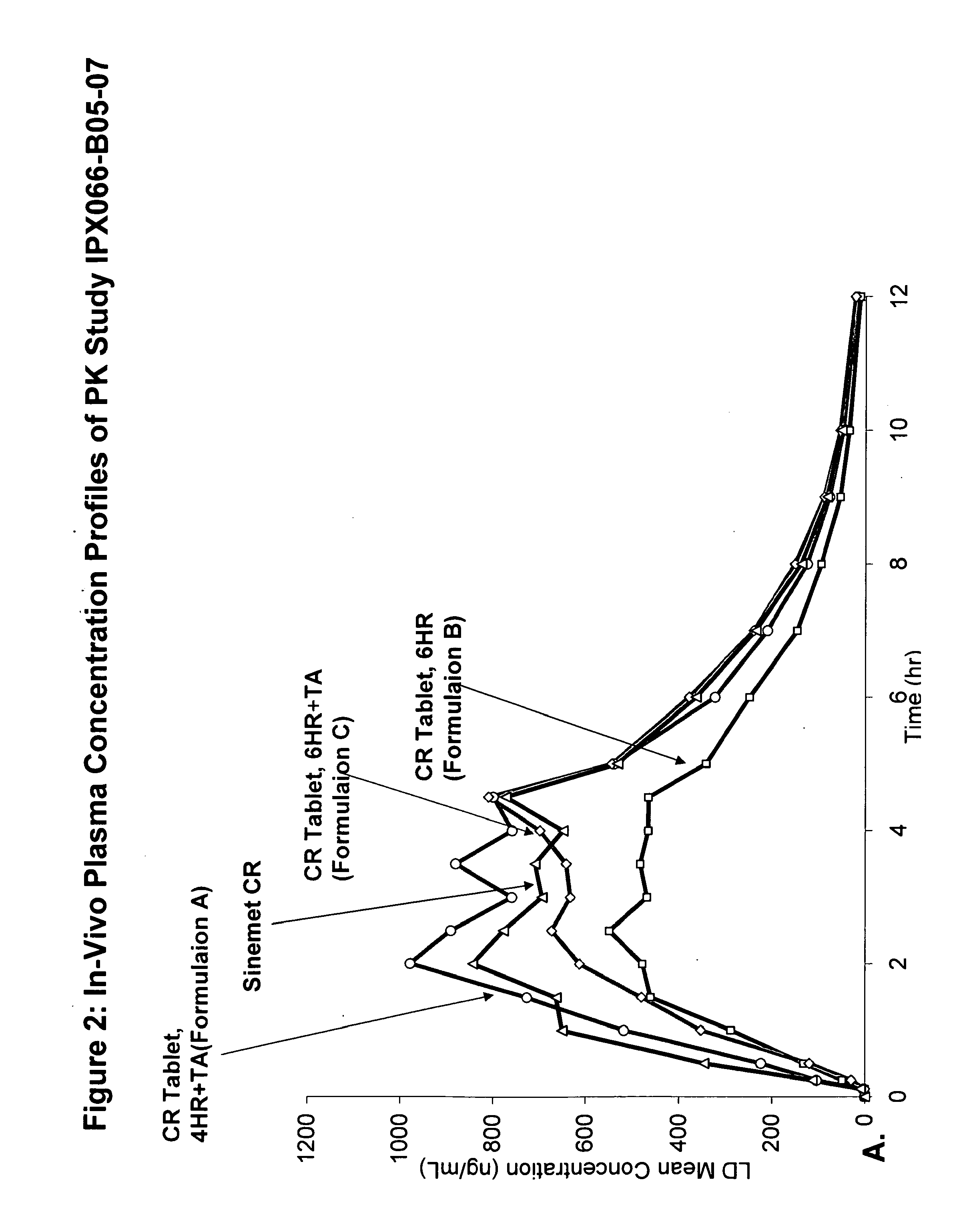
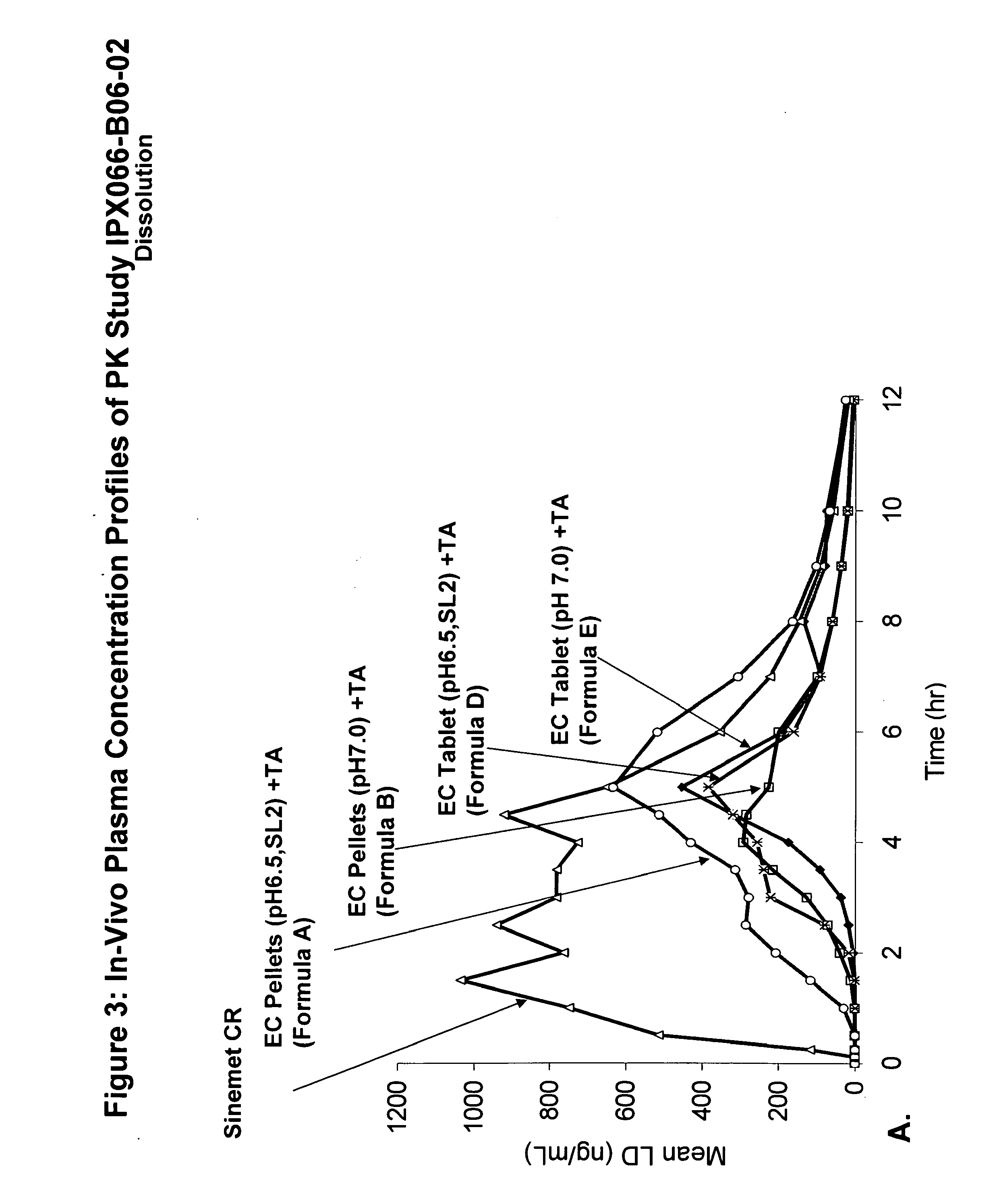
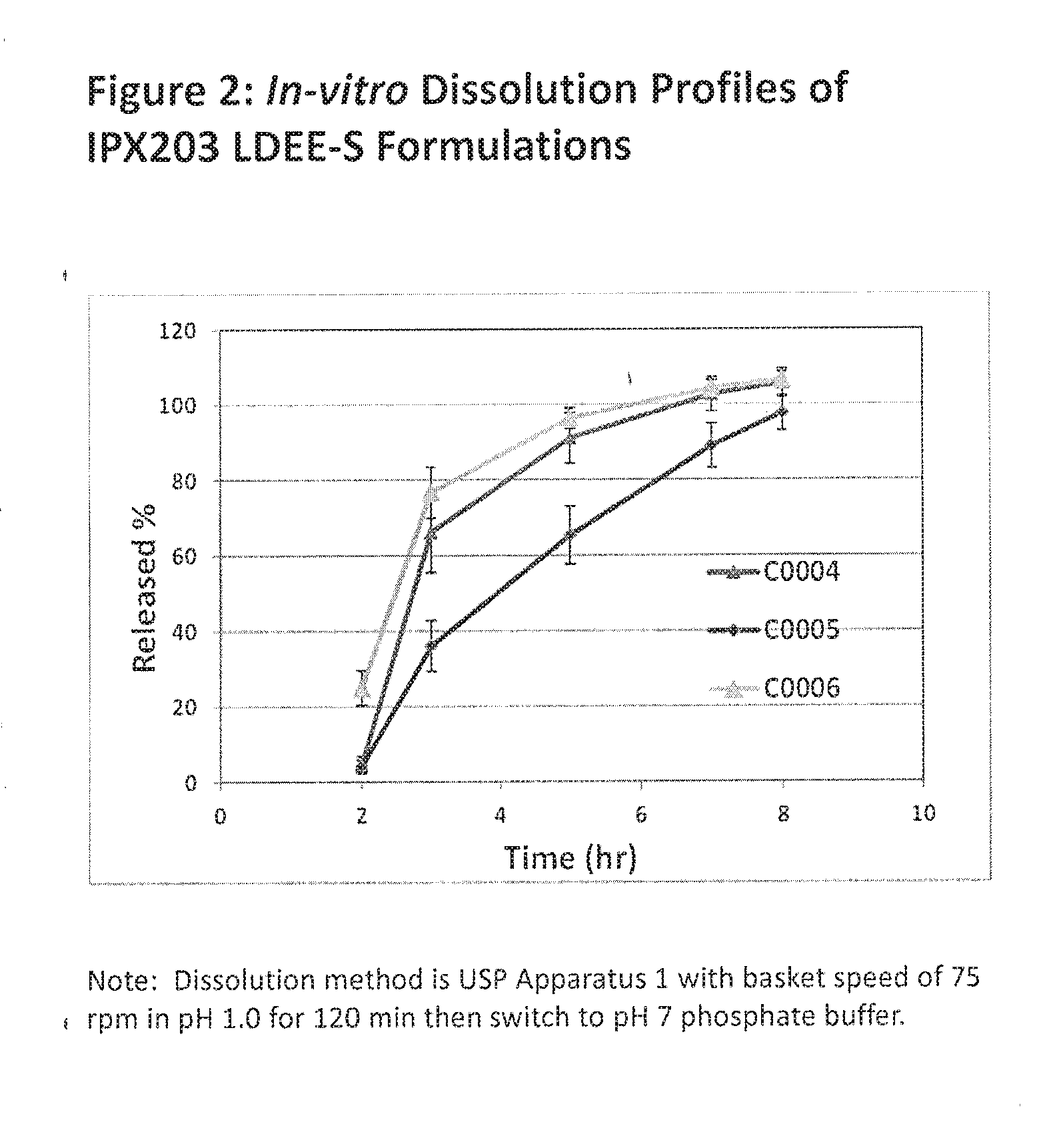
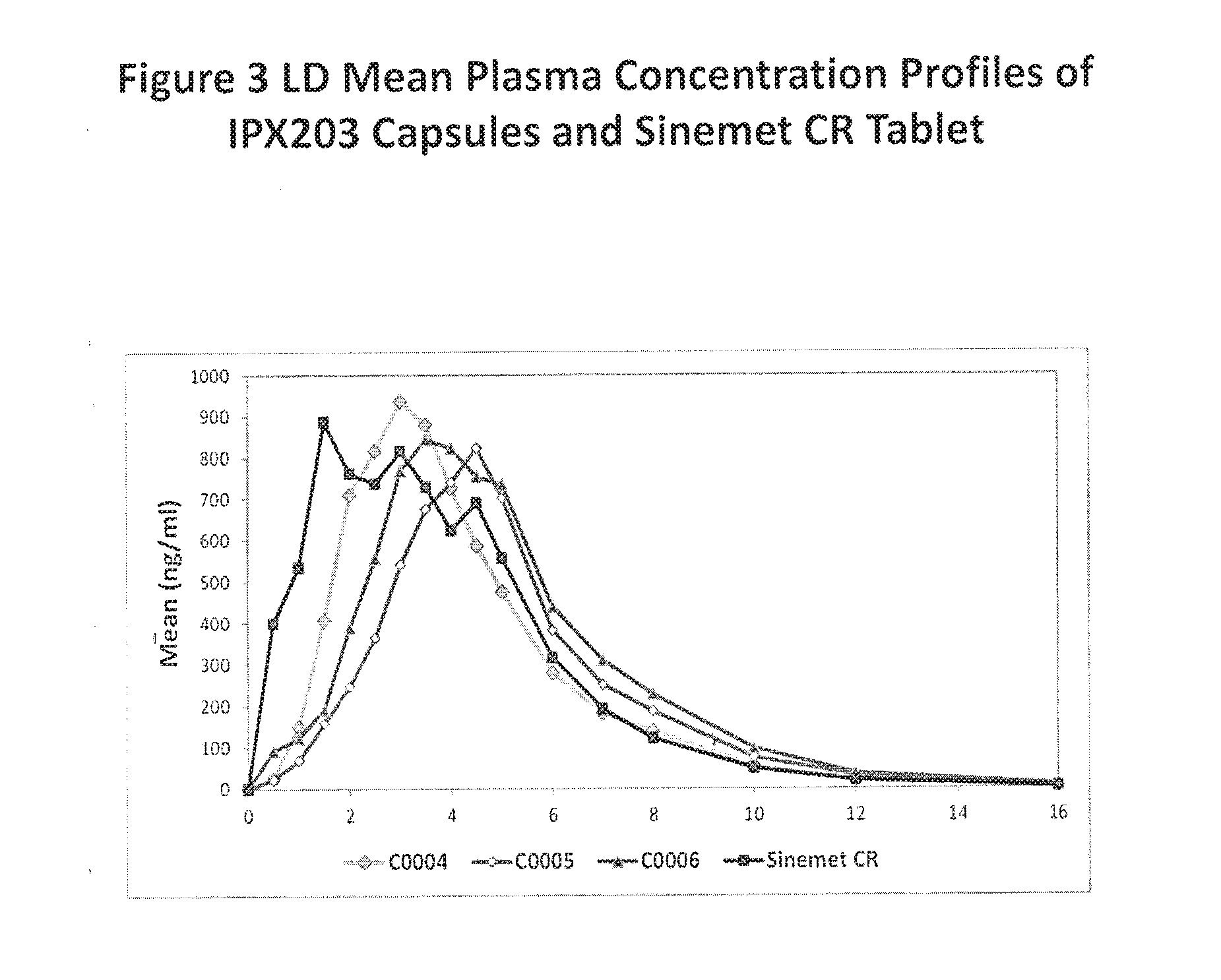
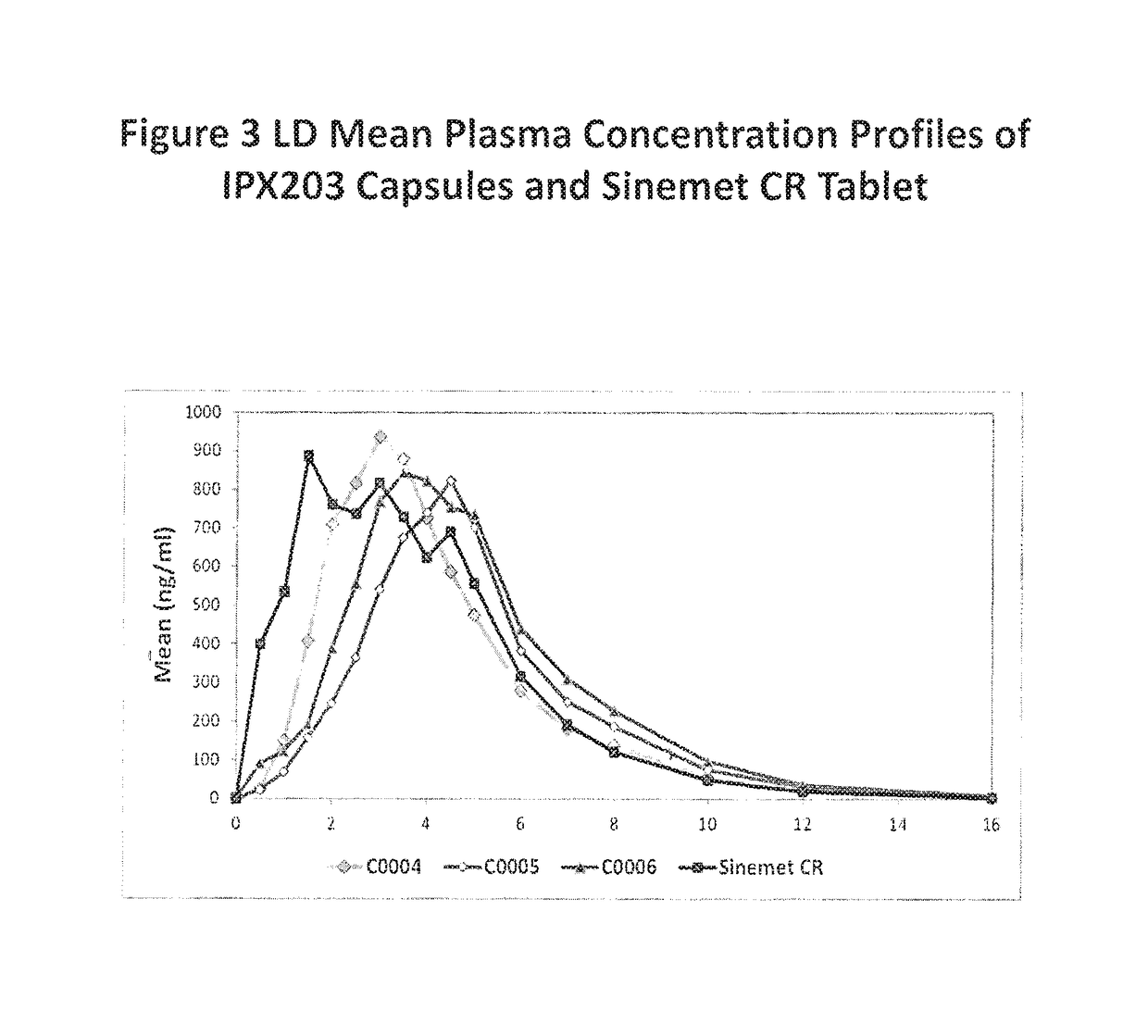
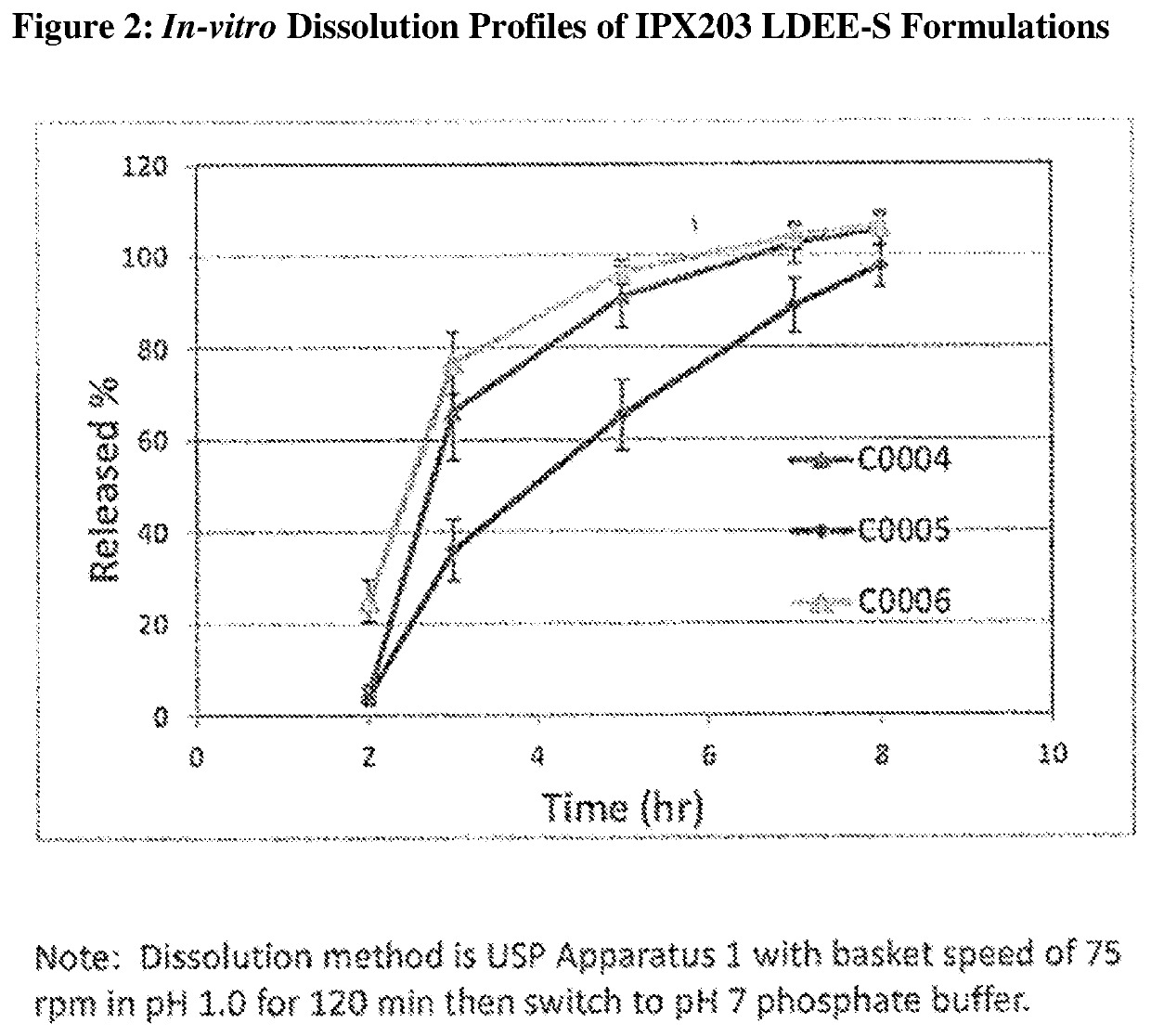
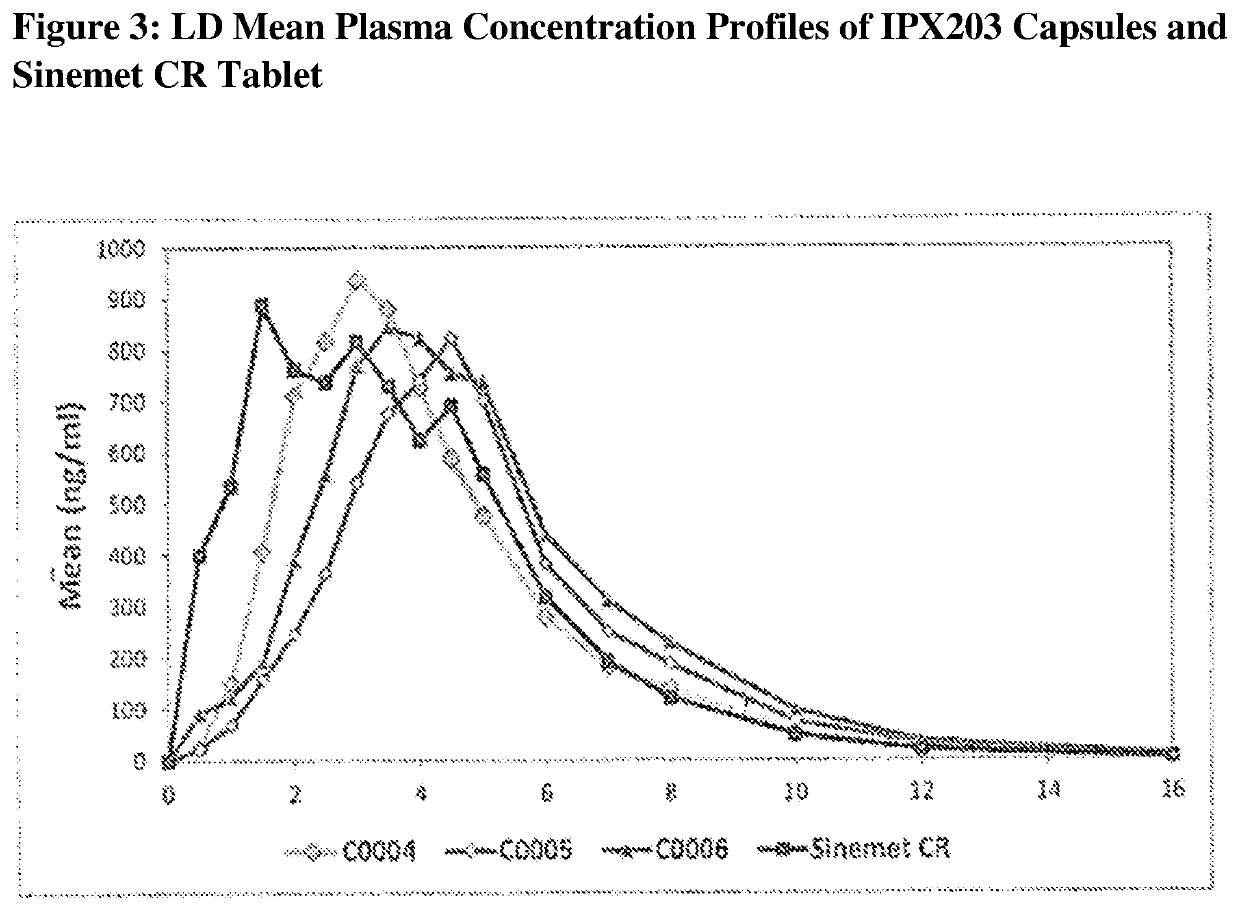
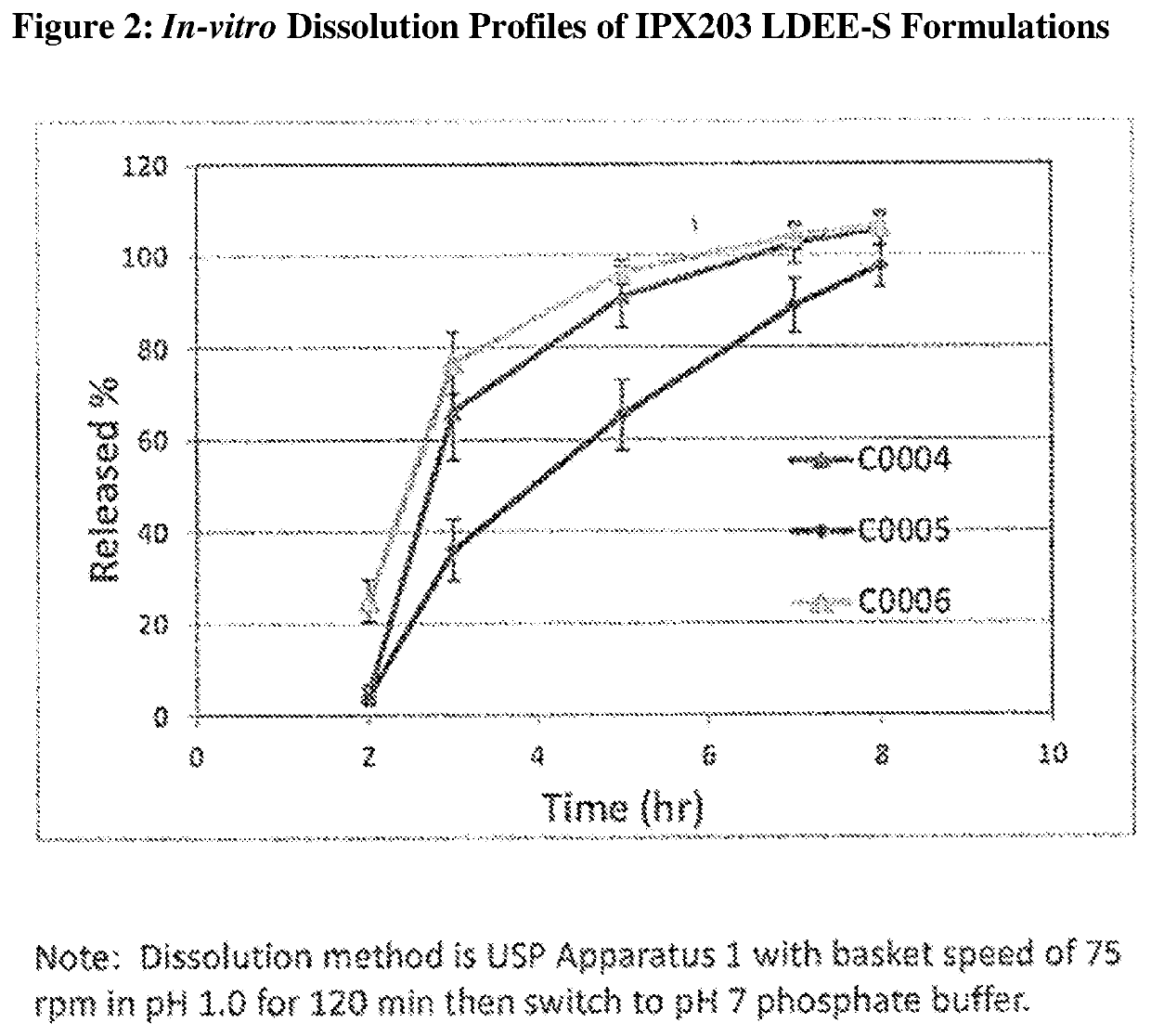
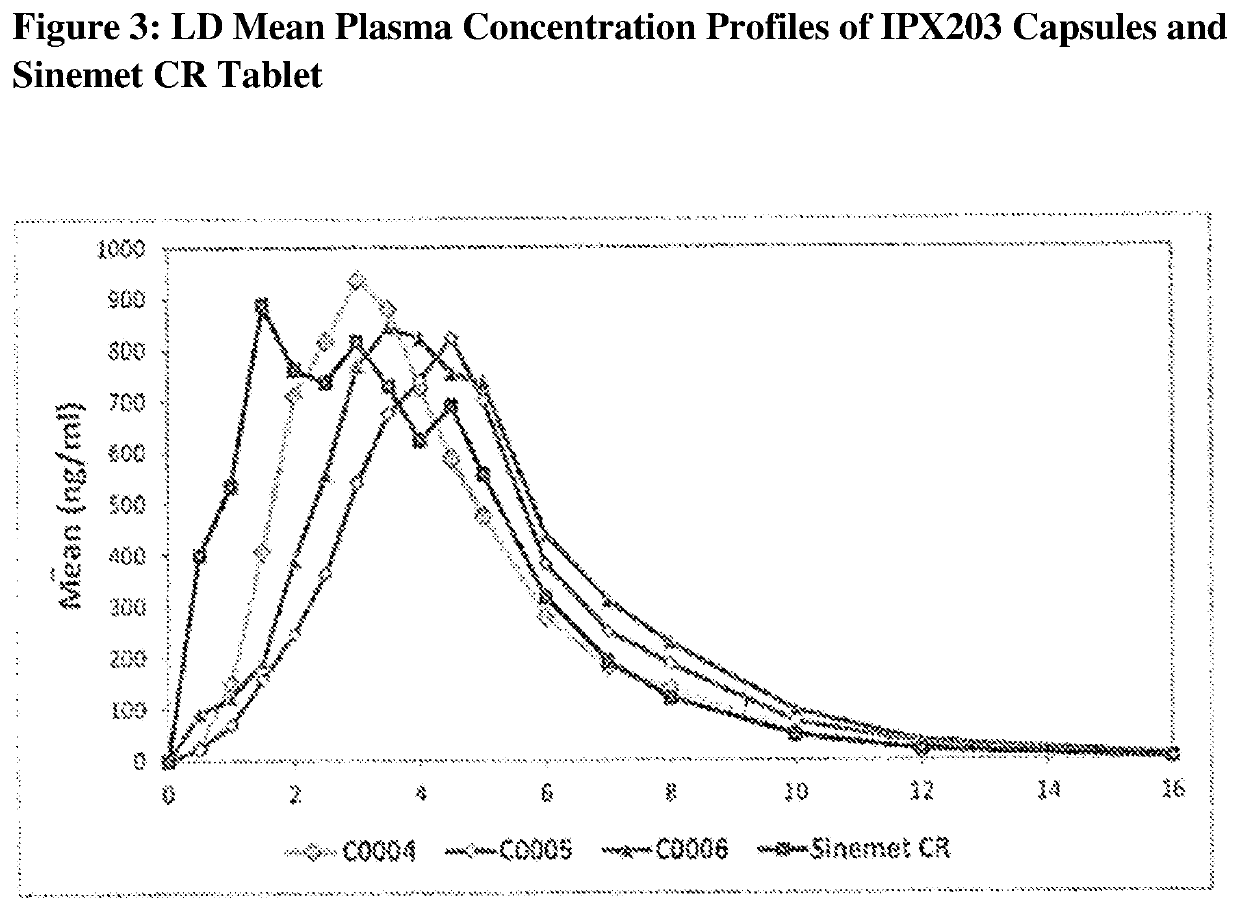
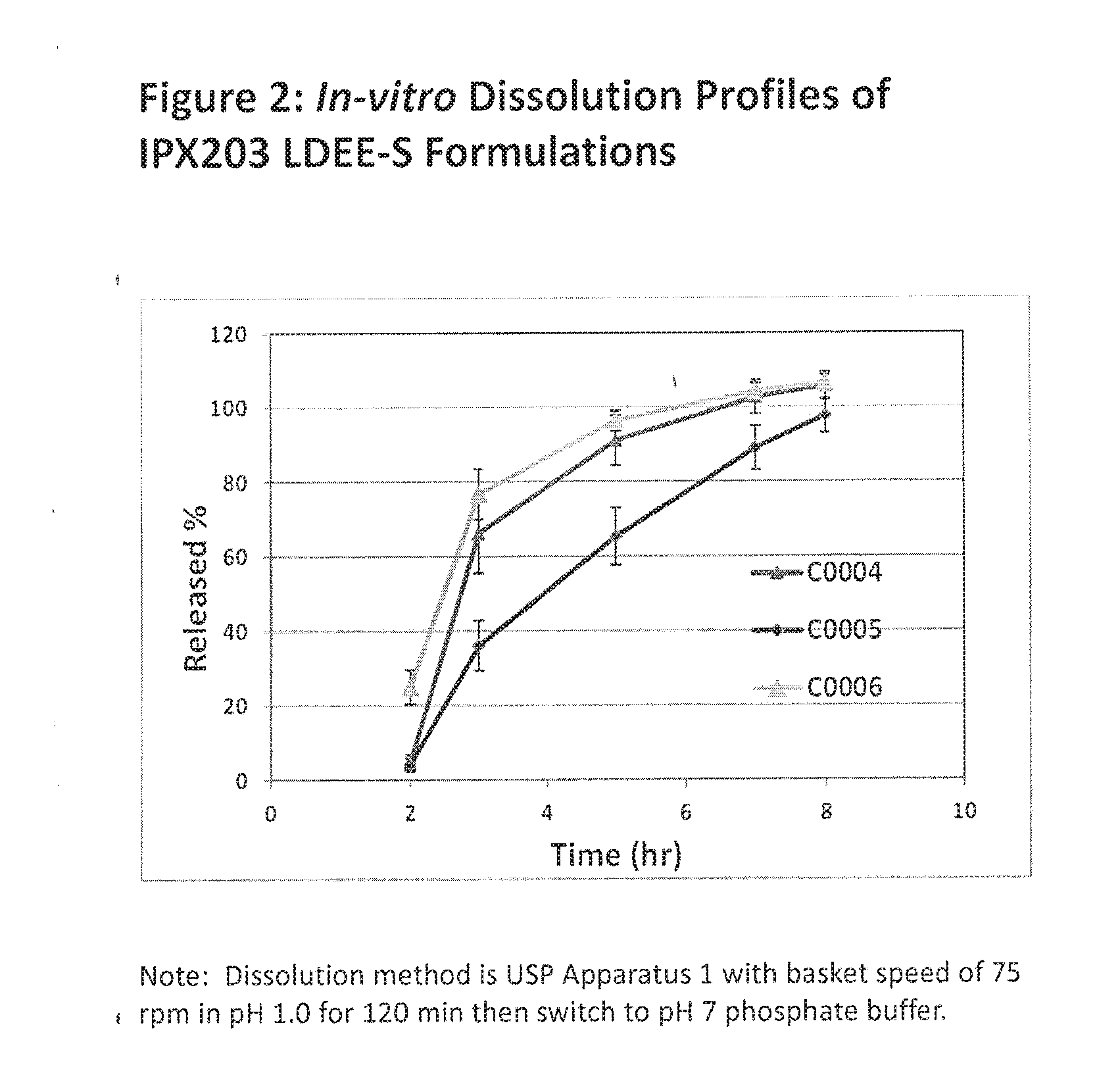
Controlled release formulations of levodopa and uses thereof
ActiveUS20120177731A1Reduce volatilityExtension of timeBiocideNervous disorderControl releaseImmediate release
The current invention provides a controlled release oral solid formulation of levodopa comprising levodopa, a decarboxylase inhibitor, and a carboxylic acid. Also provided by this invention is multiparticulate, controlled release oral solid formulations of levodopa comprising: i) a controlled release component comprising a mixture of levodopa, a decarboxylase inhibitor and a rate controlling excipient; ii) a carboxylic acid component; and iii) an immediate release component comprising a mixture of levodopa and a decarboxylase inhibitor.
Owner:IMPAX LAB LLC
Cyanoamide compounds useful as malonyl-CoA decarboxylase inhibitors
The present invention provides methods for the use of compounds as depicted by structure I, pharmaceutical compositions containing the same, and methods for the prophylaxis, management and treatment of metabolic diseases and diseases modulated by MCD inhibition. The compounds disclosed in this invention are useful for the prophylaxis, management and treatment of diseases involving in malonyl-CoA regulated glucose / fatty acid metabolism pathway. In particular, these compounds and pharmaceutical composition containing the same are indicated in the prophylaxis, management and treatment of cardiovascular diseases, diabetes, cancer and obesity
Owner:CHUGAI PHARMA CO LTD
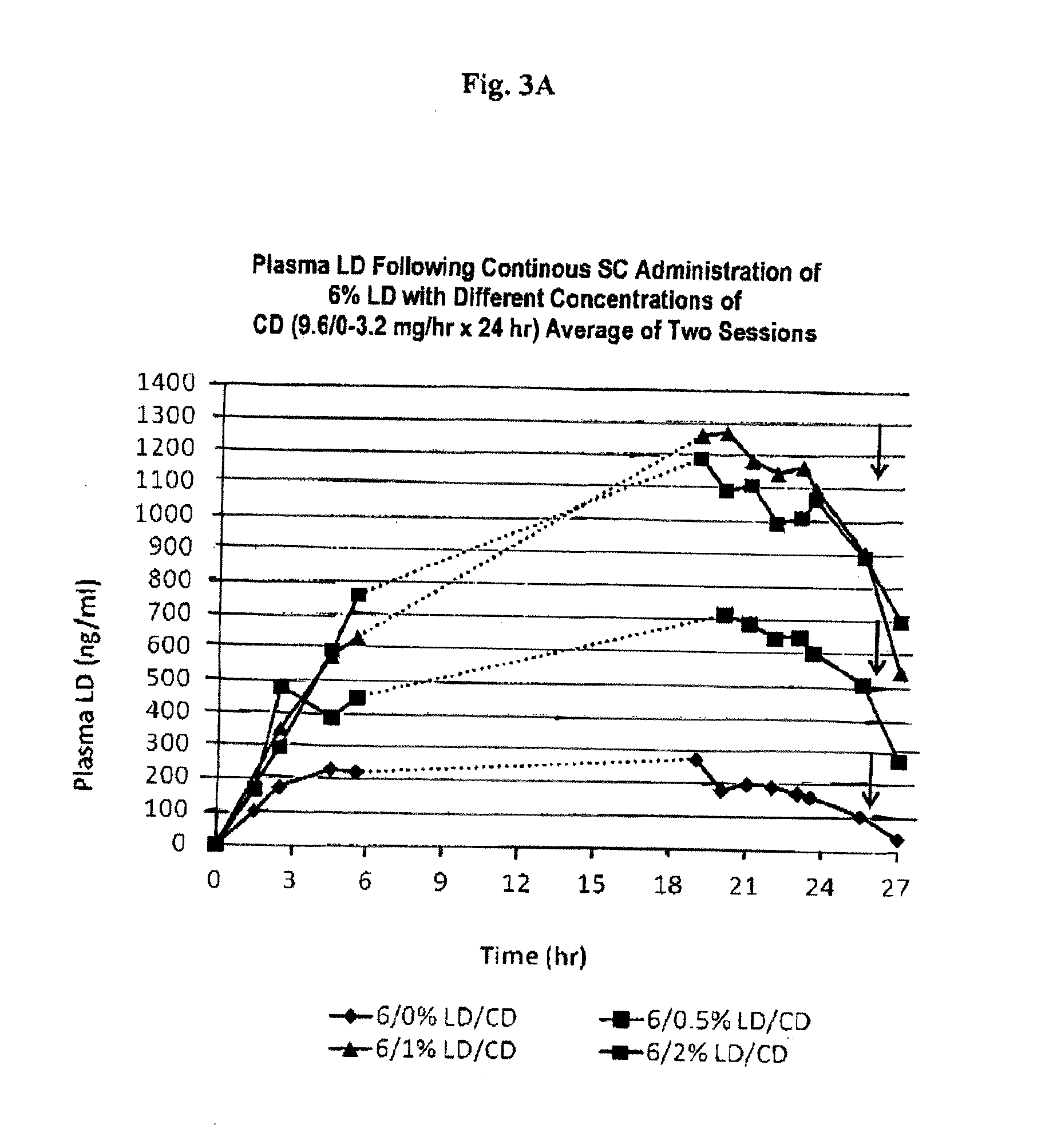
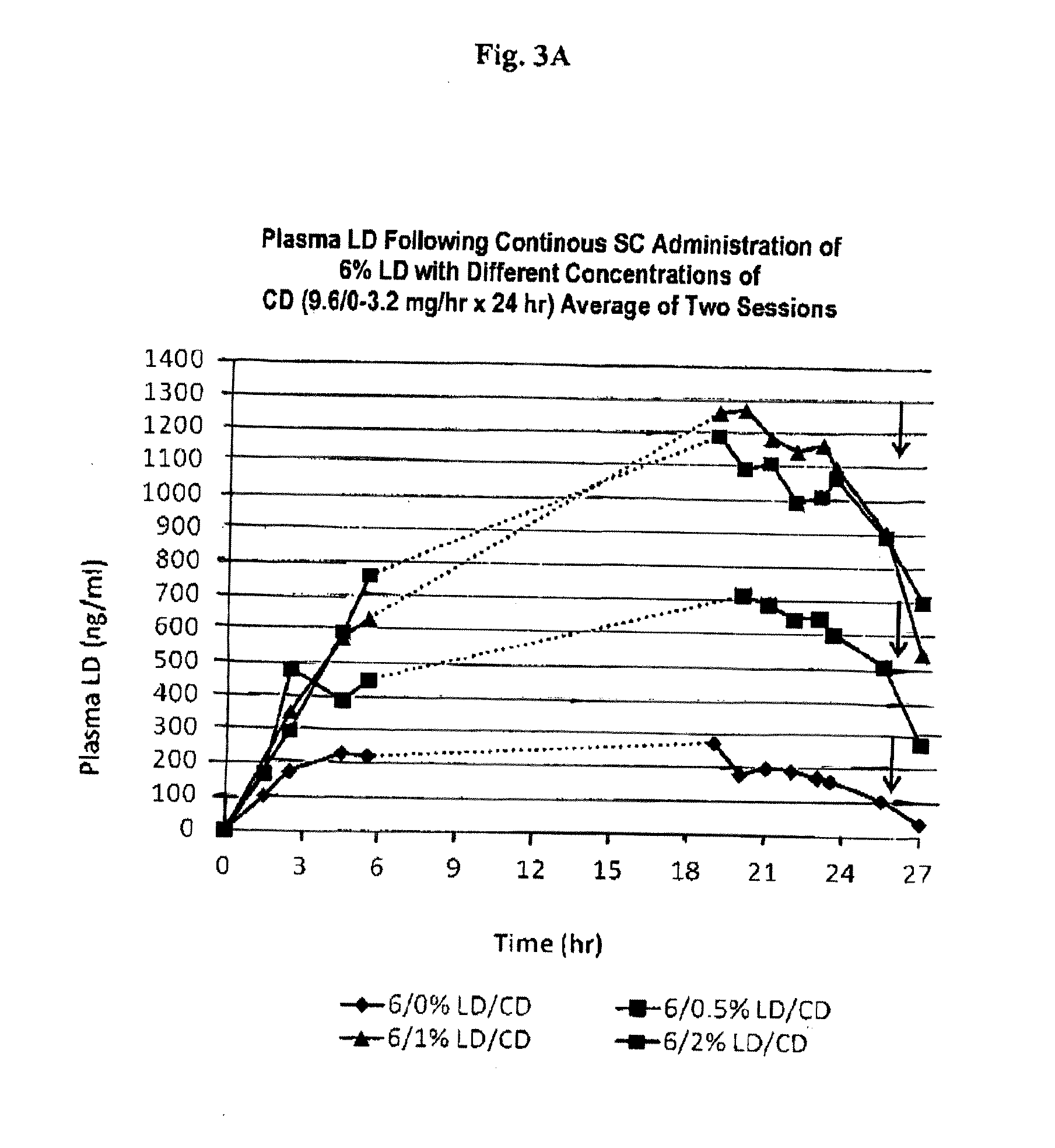
Continuous administration of l-dopa, dopa decarboxylase inhibitors, catechol-o-methyl transferase inhibitors and compositions for same
ActiveUS20140249228A1Improve efficiencyReduce the daily dosage of levodopaBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
Continuous administration of l-dopa, dopa decarboxylase inhibitors, catechol-o-methyl transferase inhibitors and compositions for same
ActiveUS20140249229A1Improve efficiencyReduce the daily dosage of levodopaBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
Controlled release formulations of levodopa and uses thereof
ActiveUS20100298268A1Reducing motor fluctuationReducing “ on-off ” effectBiocideNervous disorderImmediate releaseCarboxylic acid
The current invention provides a controlled release oral solid formulation of levodopa comprising levodopa, a decarboxylase inhibitor, and a carboxylic acid. Also provided by this invention is multiparticulate, controlled release oral solid formulations of levodopa comprising: i) a controlled release component comprising a mixture of levodopa, a decarboxylase inhibitor and a rate controlling excipient; ii) a carboxylic acid component; and iii) an immediate release component comprising a mixture of levodopa and a decarboxylase inhibitor.
Owner:IMPAX LAB LLC
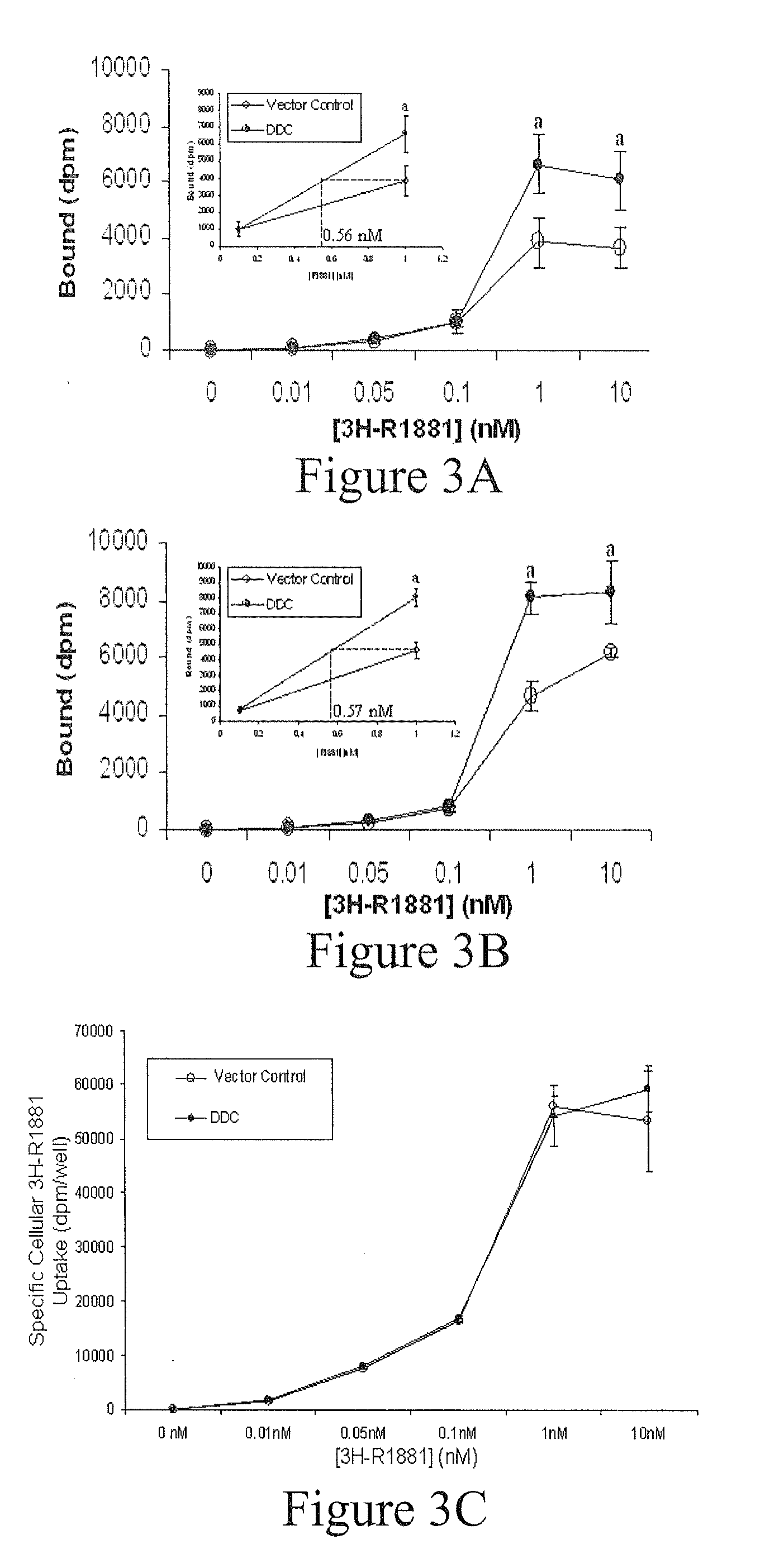
Treatment of Prostate Cancer with DDC Inhibitor
ActiveUS20100048709A1Low affinityBiocidePeptide/protein ingredientsMultiple formsDihydroxyphenylalanine
Owner:THE UNIV OF BRITISH COLUMBIA
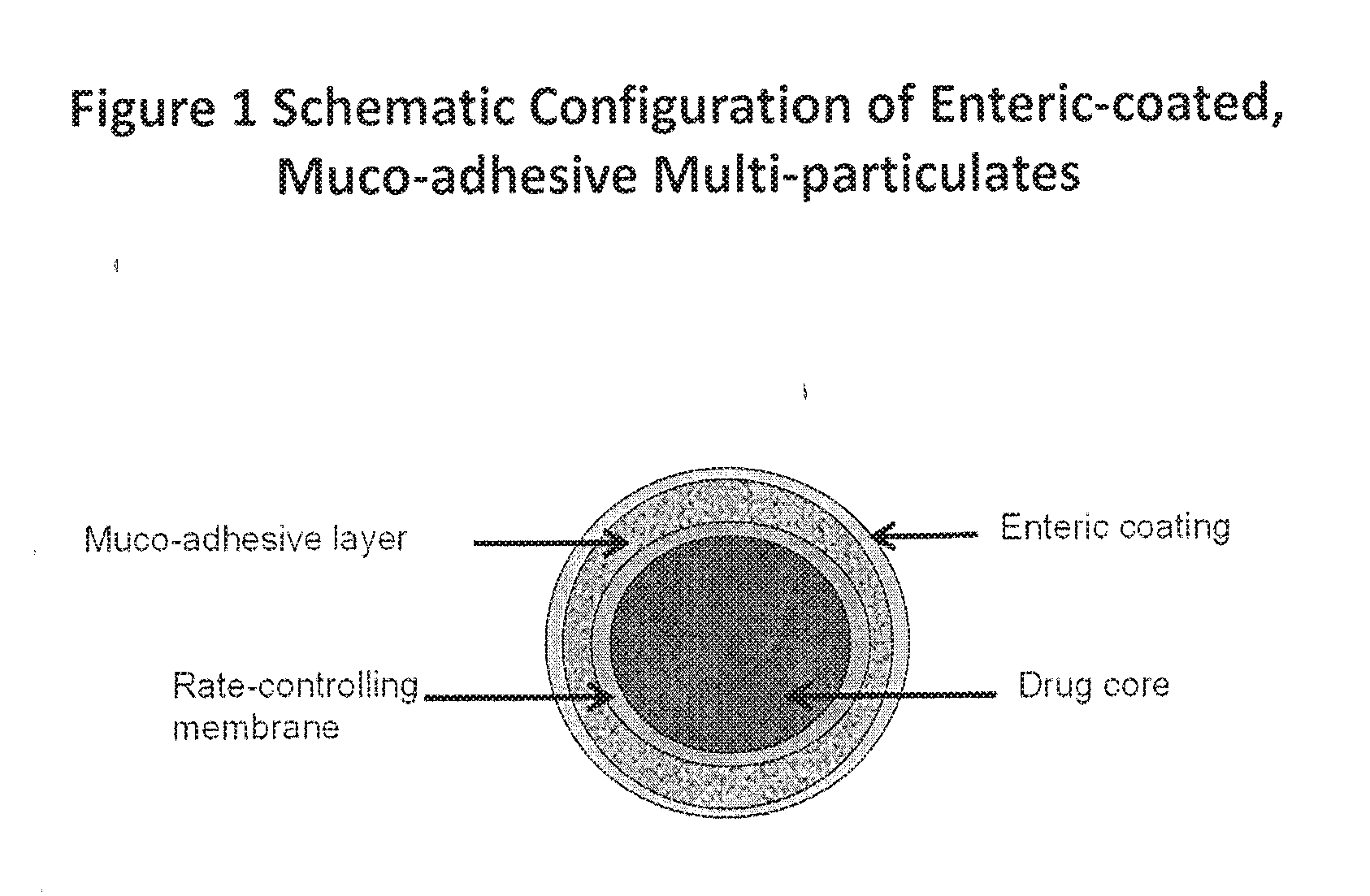
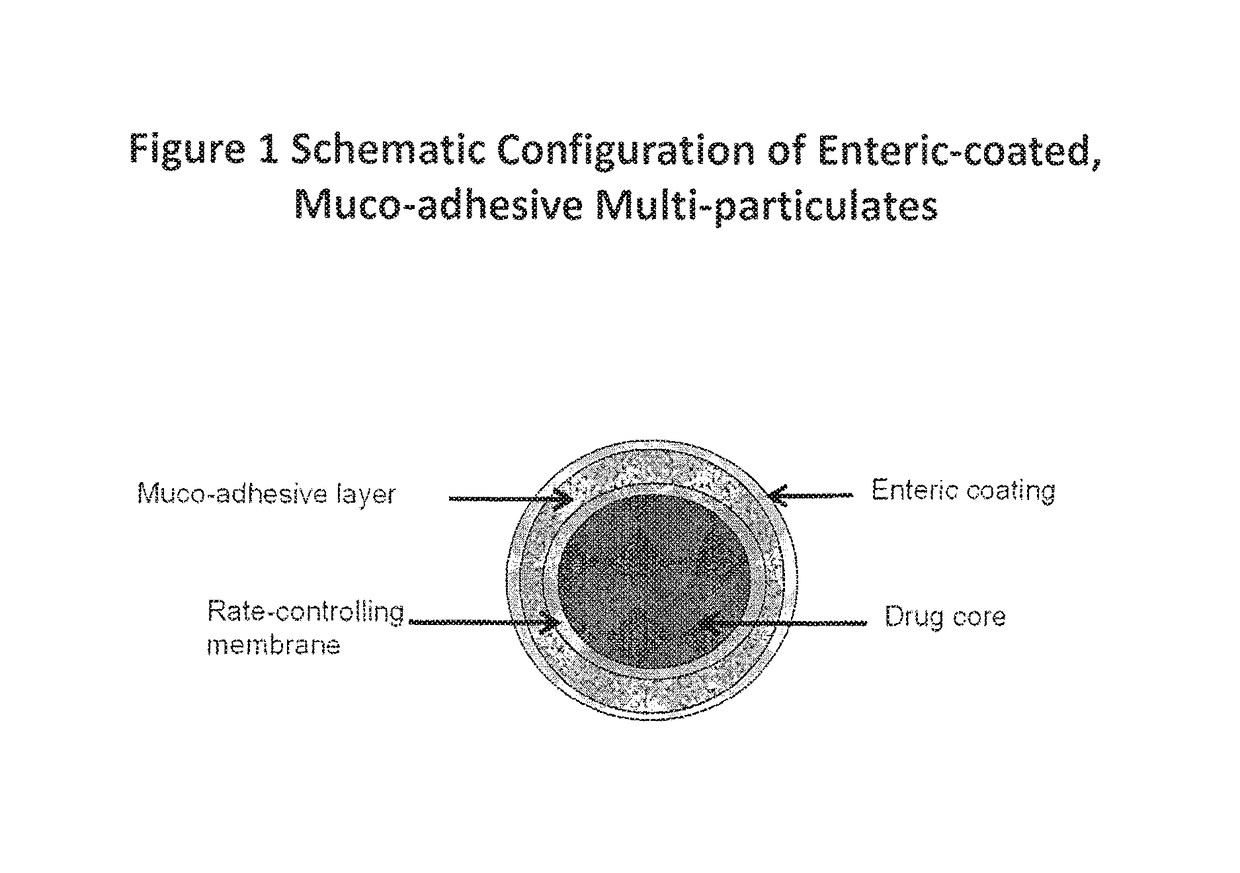
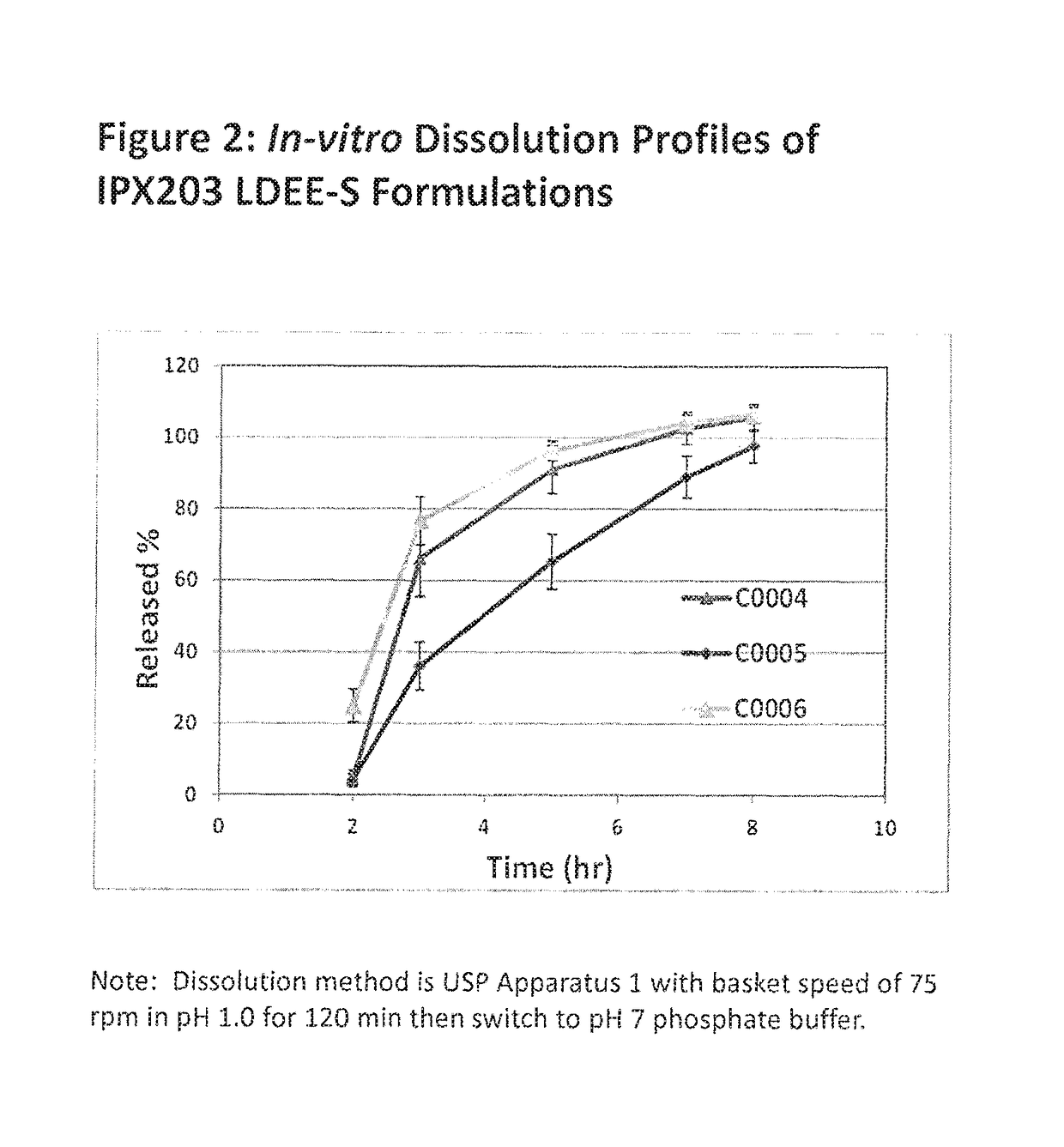
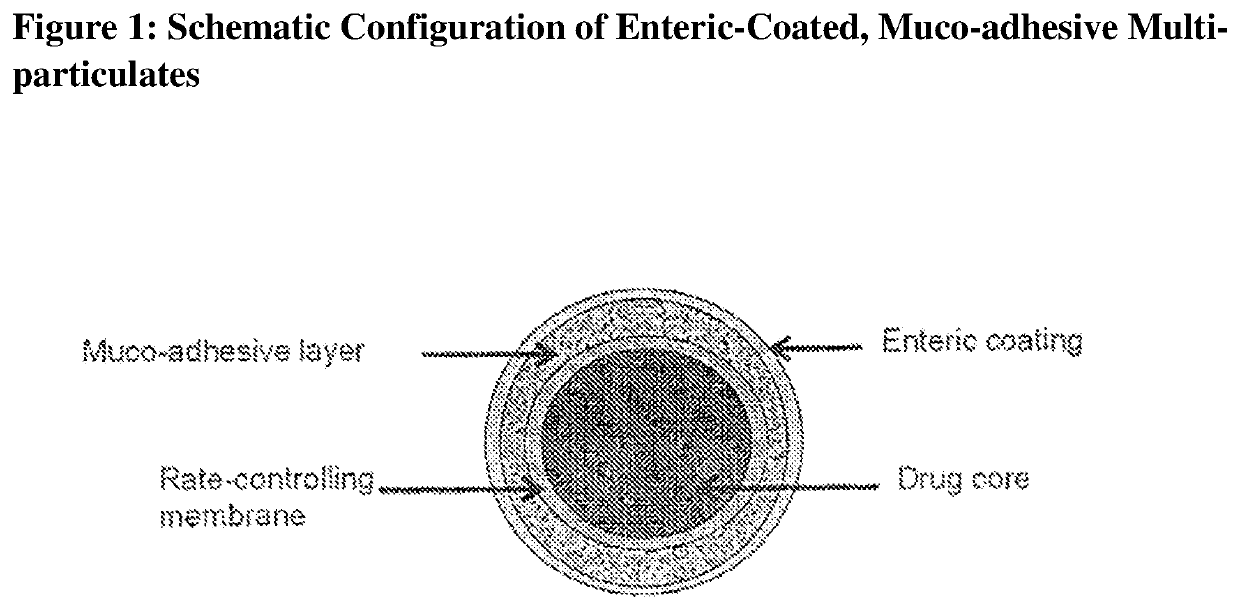
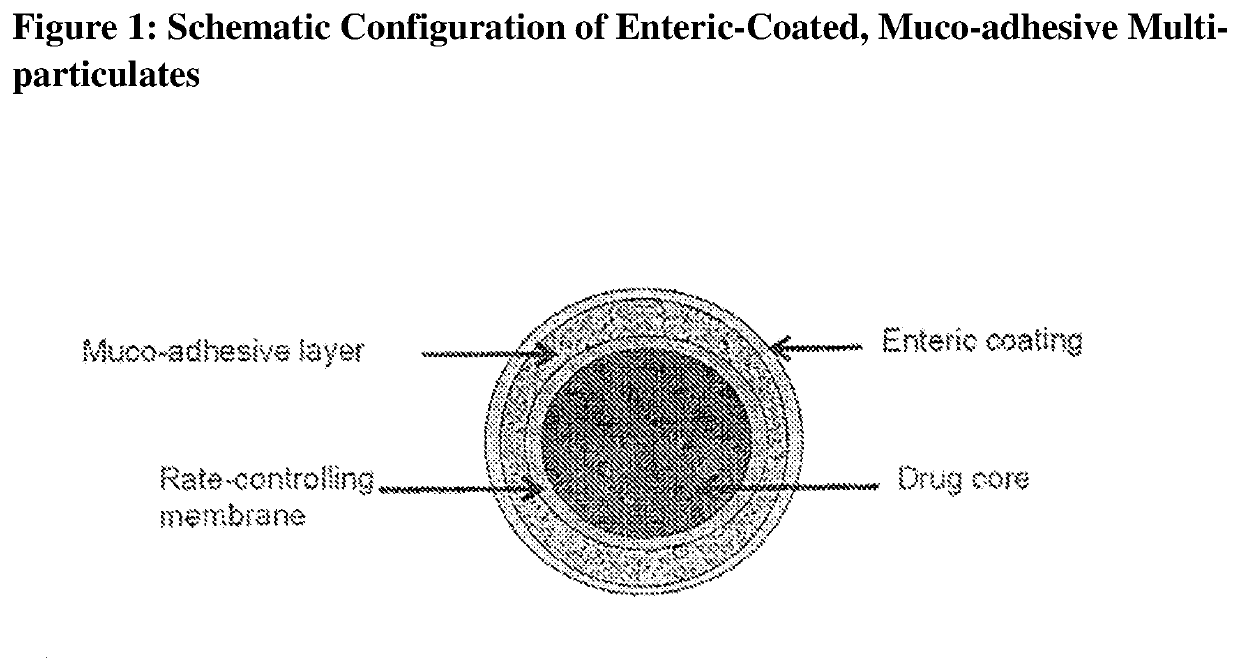
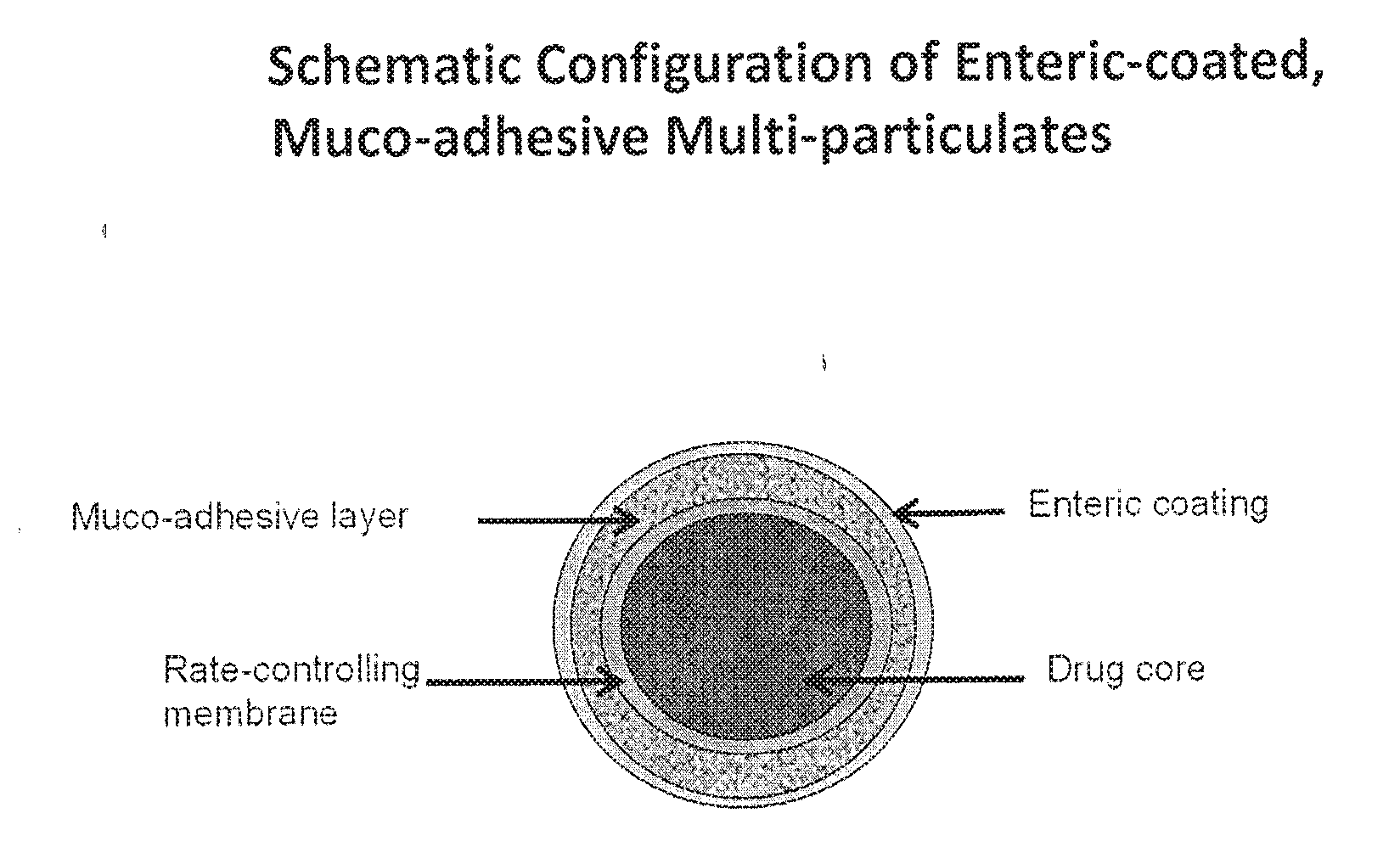
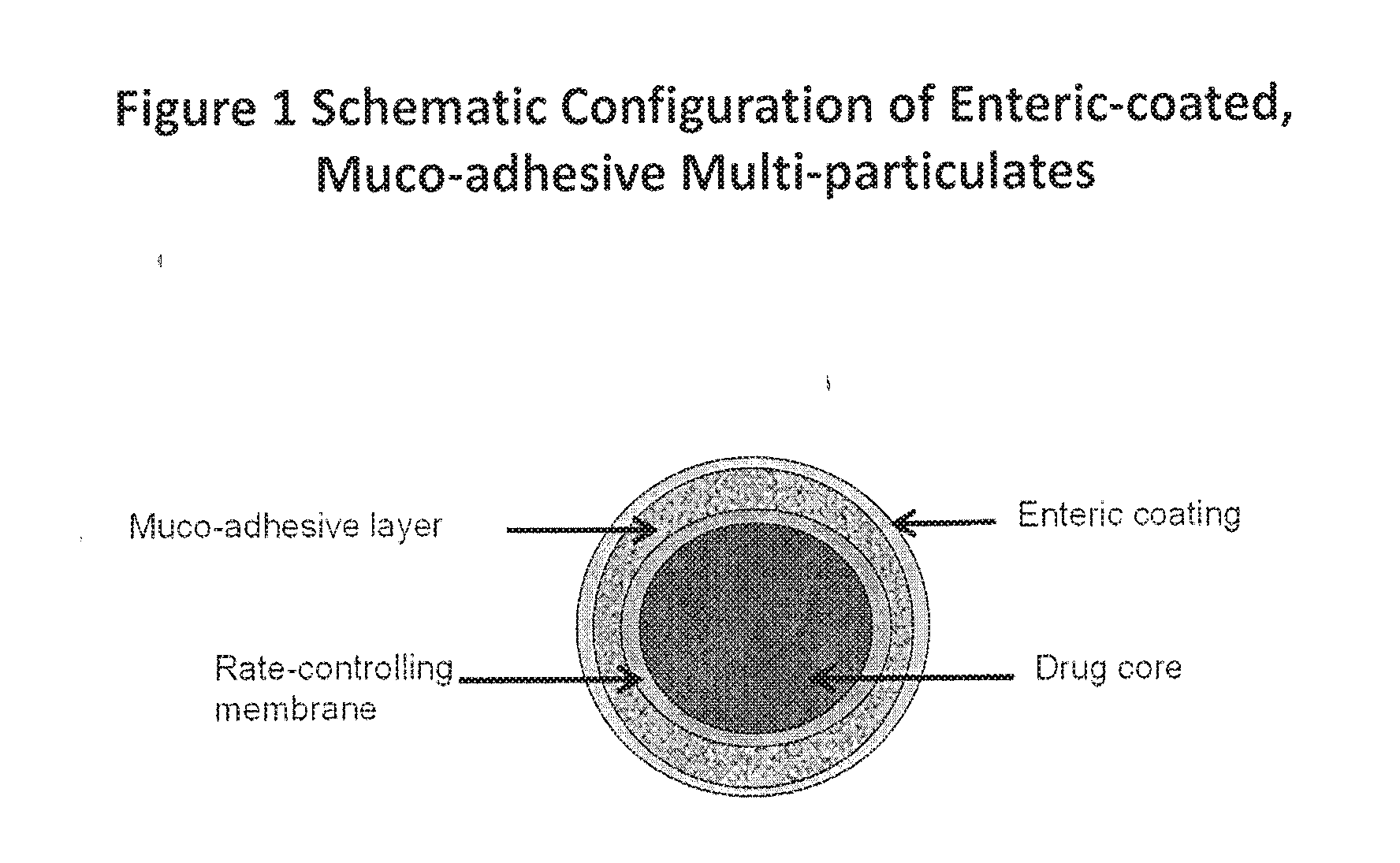
Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof
ActiveUS20160287523A1Effective amountOrganic active ingredientsNervous disorderControl releaseAdhesive
The invention provides a controlled release oral solid formulation comprising (a) a controlled release component comprising core comprising levodopa and / or an ester of levodopa or salts thereof, wherein the core is coated with a layer of a muco-adhesive polymer and externally coated with a layer of an enteric coated polymer; and (b) a decarboxylase inhibitor component.
Owner:IMPAX LAB LLC
Continuous administration of dopa decarboxylase inhibitors and compositions for same
ActiveUS9040589B2Improve efficiencyReduce daily dosageBiocideNervous disorderDiseaseHuntingtons chorea
Disclosed herein are compositions that include for example the arginine salt of carbidopa, and methods for treating neurological or movement diseases or disorders such as restless leg syndrome, Parkinson's disease, secondary parkinsonism, Huntington's disease, Parkinson's like syndrome, PSP, MSA, ALS, Shy-Drager syndrome and conditions resulting from brain injury including carbon monoxide or manganese intoxication, using substantially continuous administration of carbidopa or salt thereof together with administration of levodopa.
Owner:NEURODERM
Methods for treating GI tract disorders
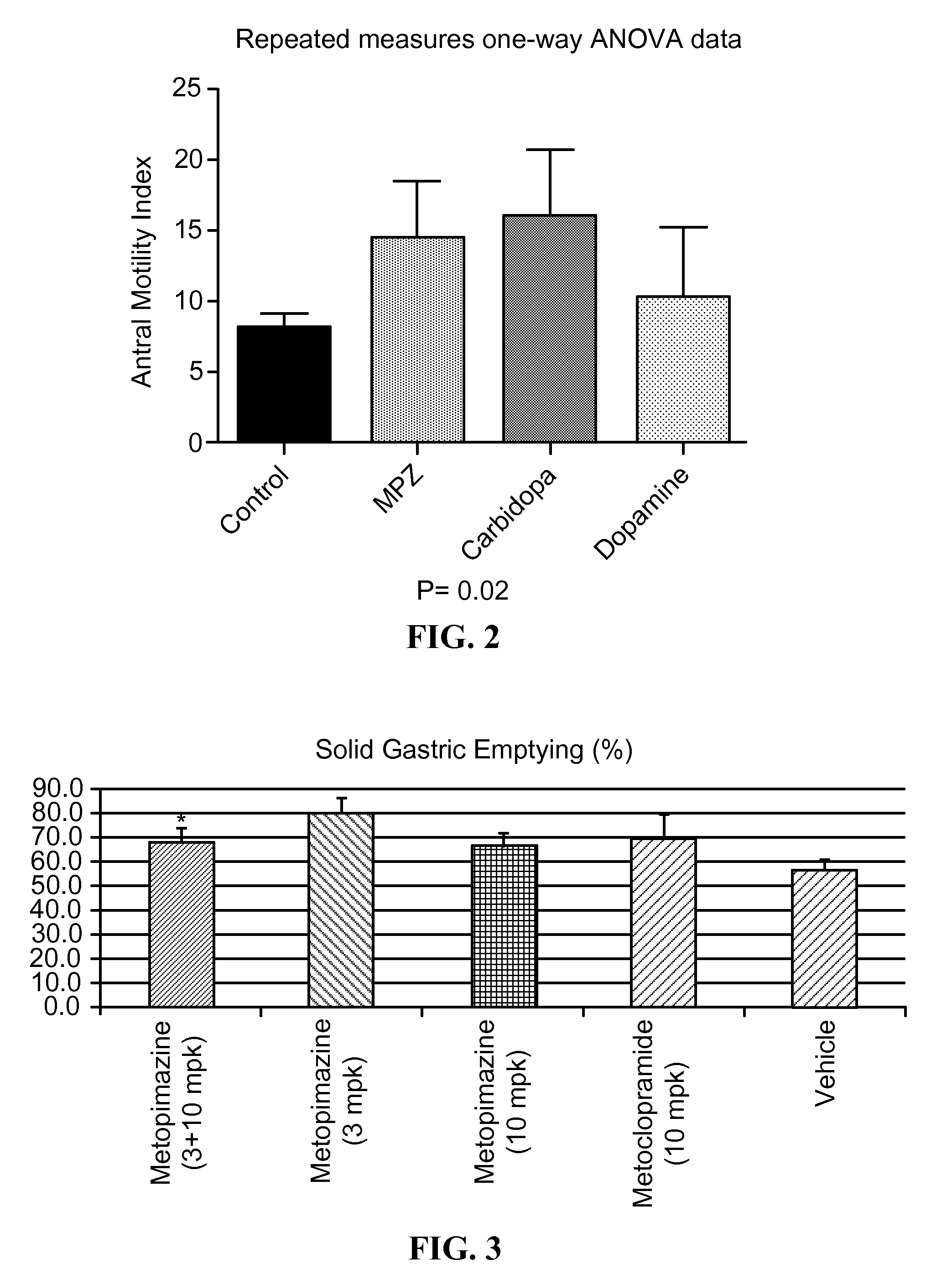
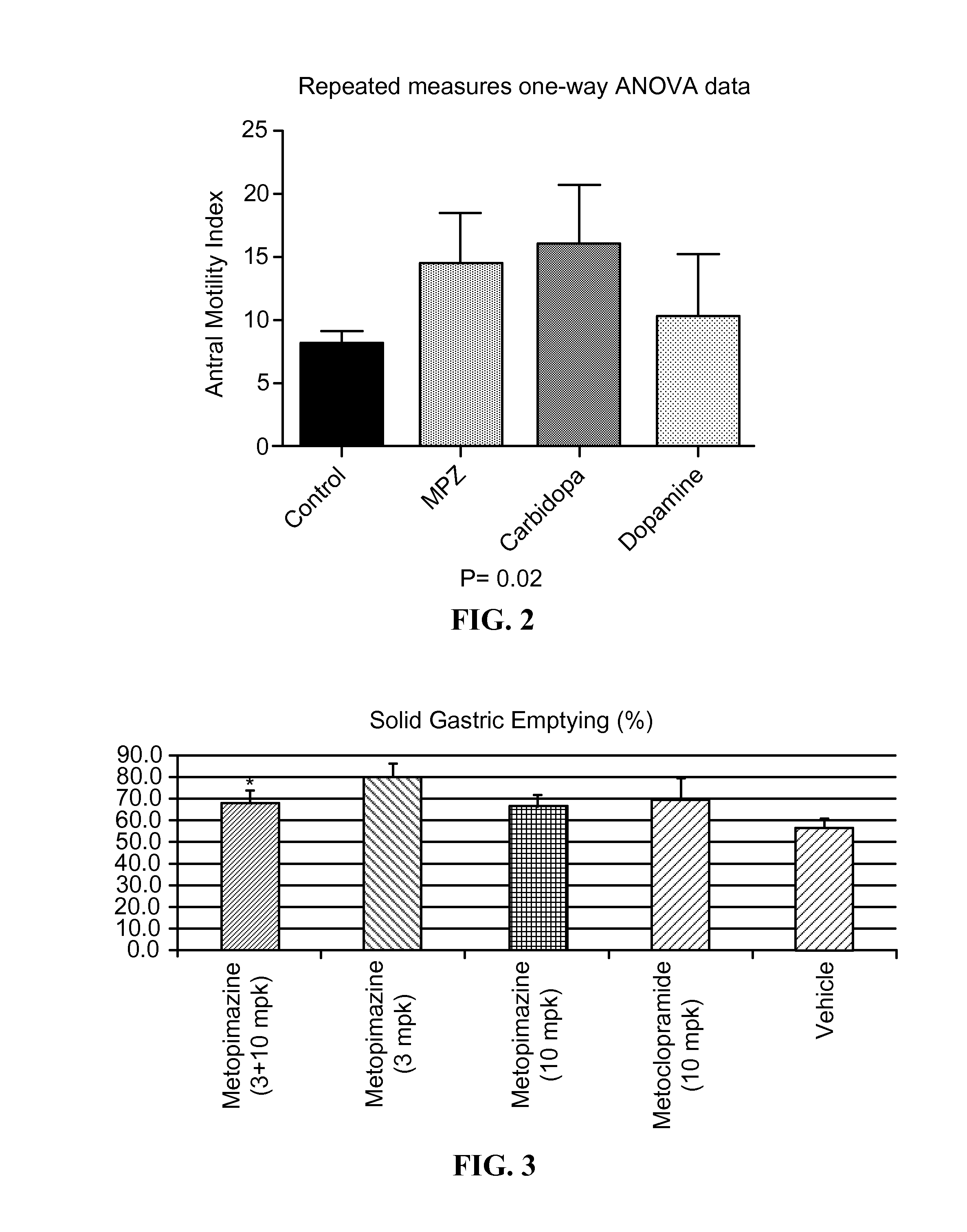
ActiveUS9132134B2Improving gastric emptyingAntibacterial agentsNervous disorderDiseaseDisease irritable bowel
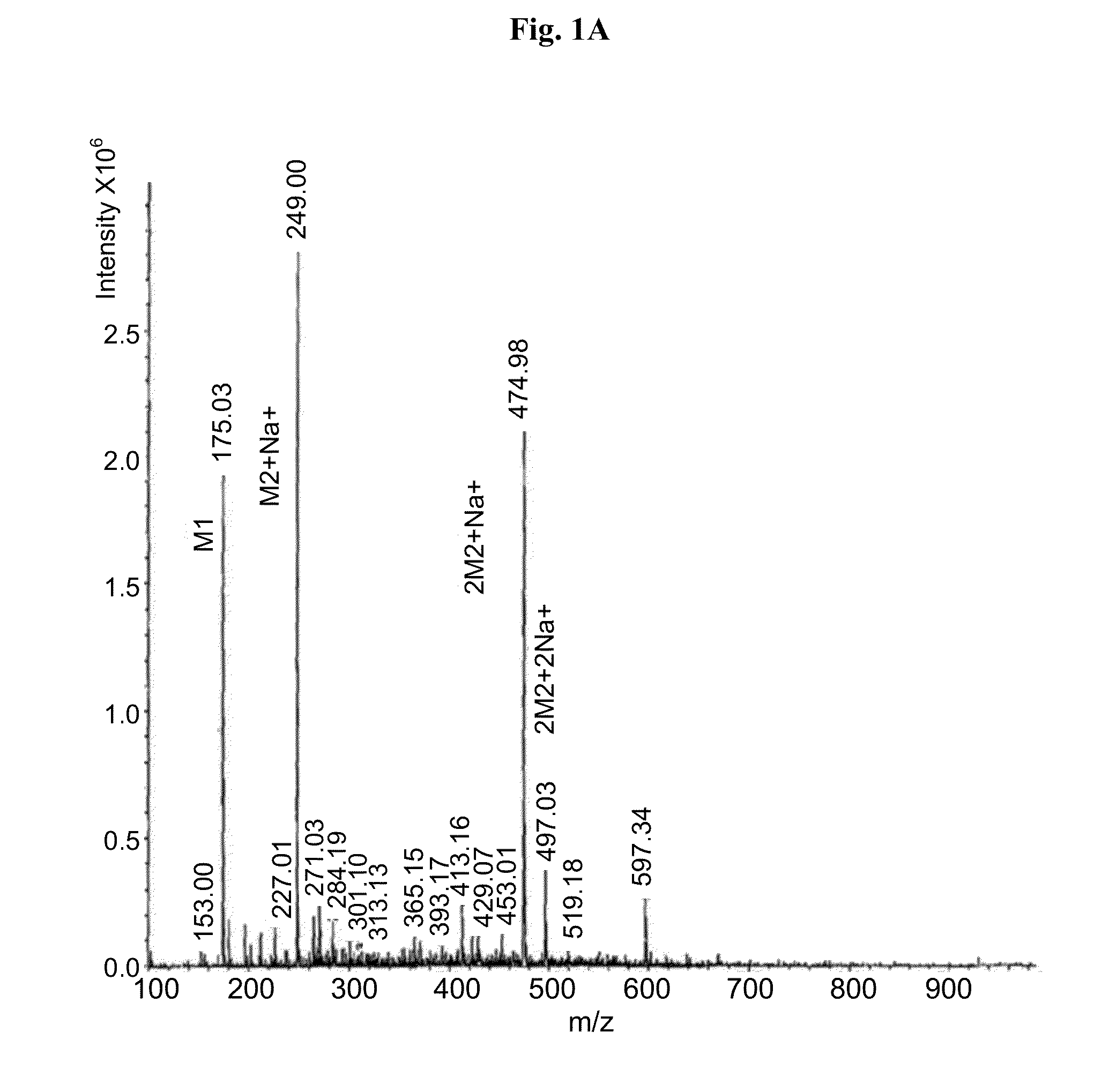
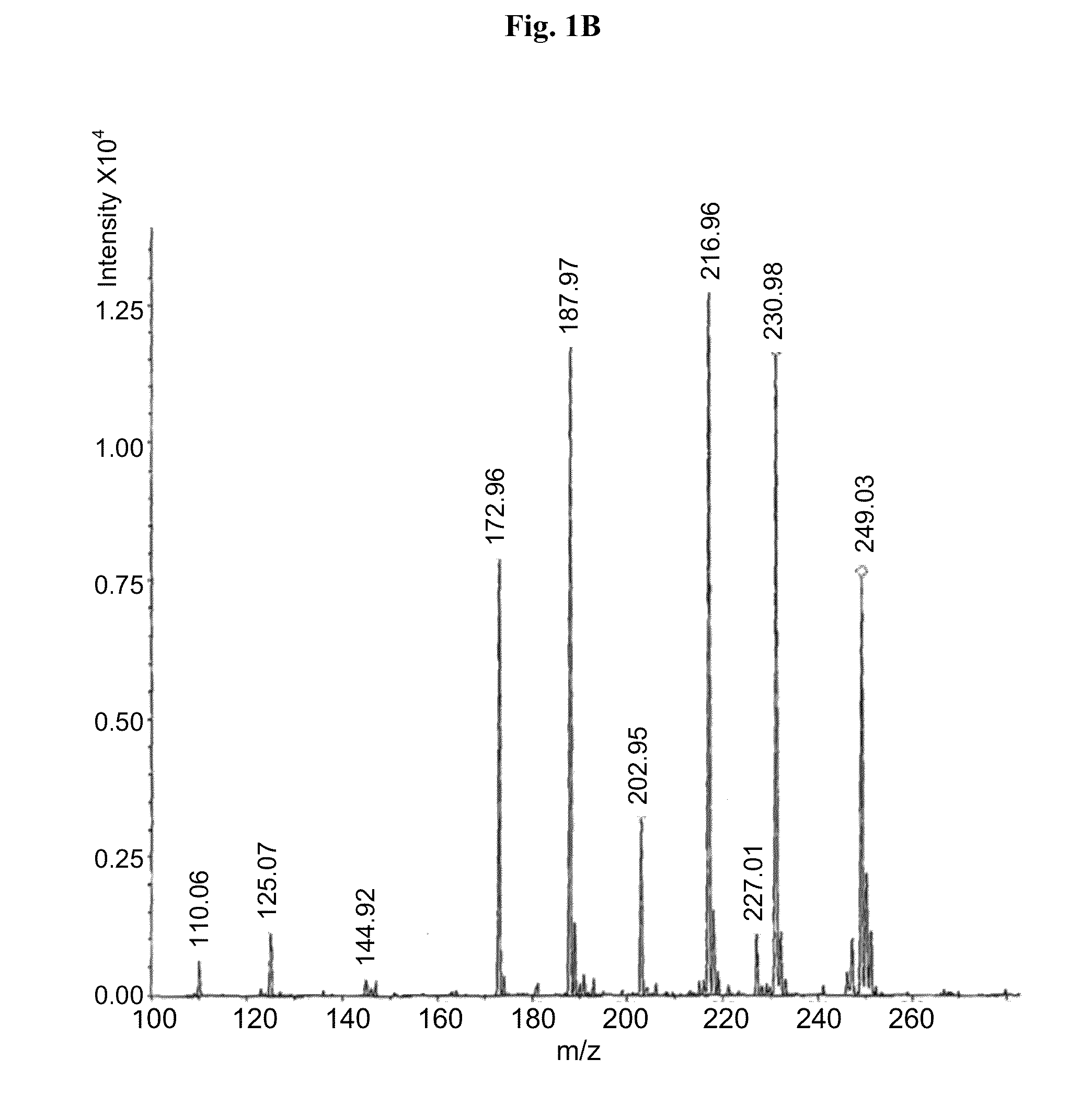
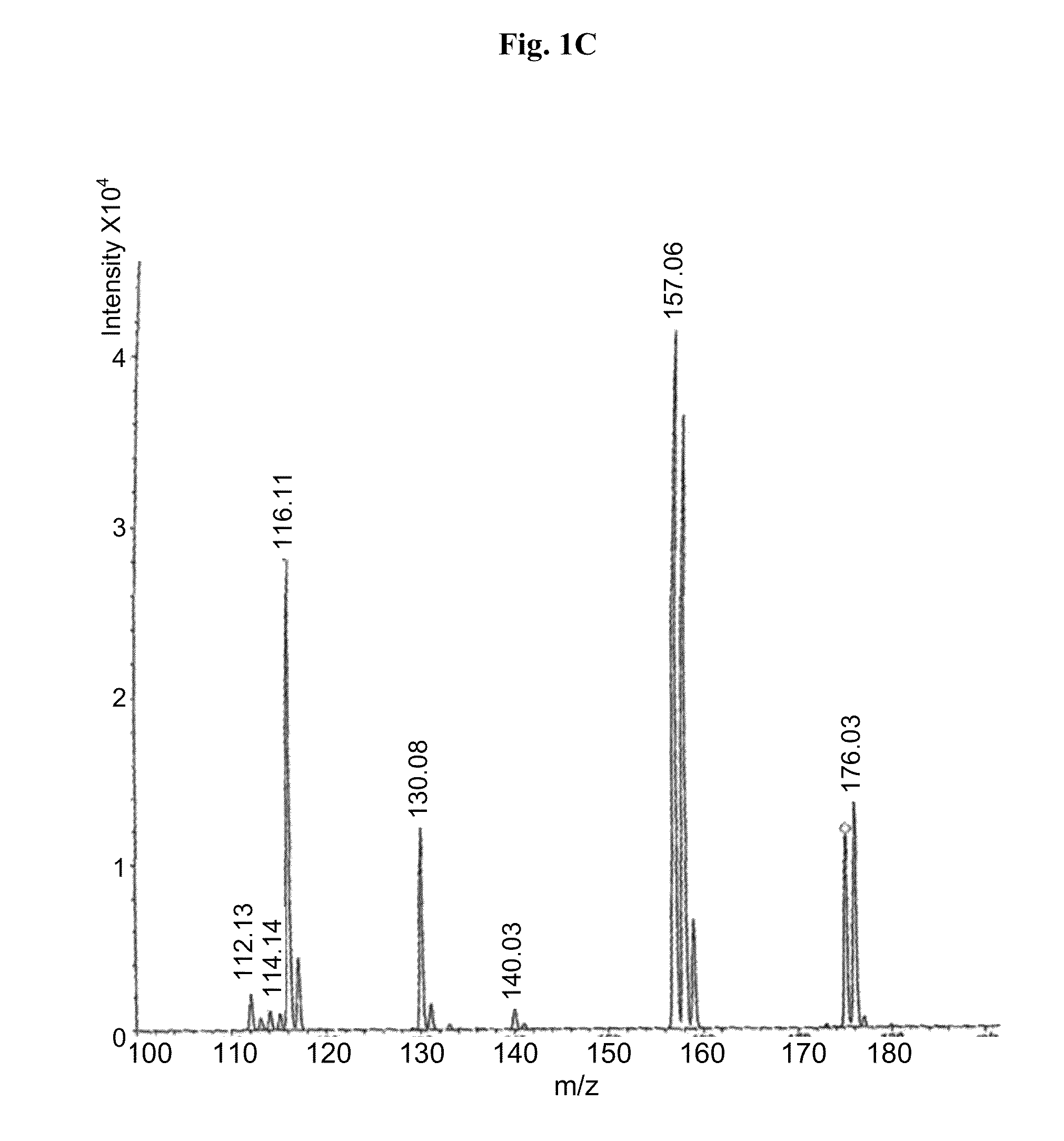
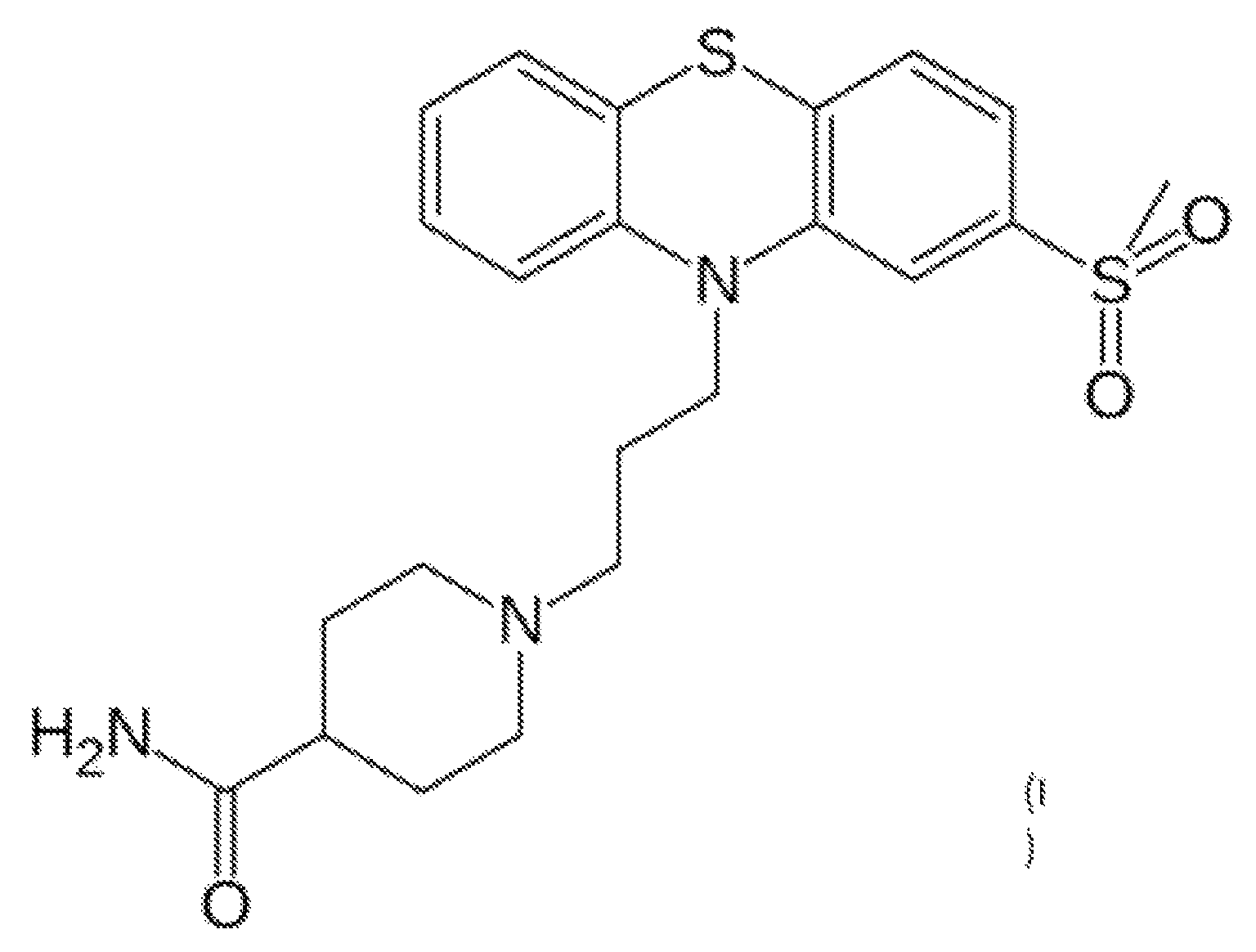
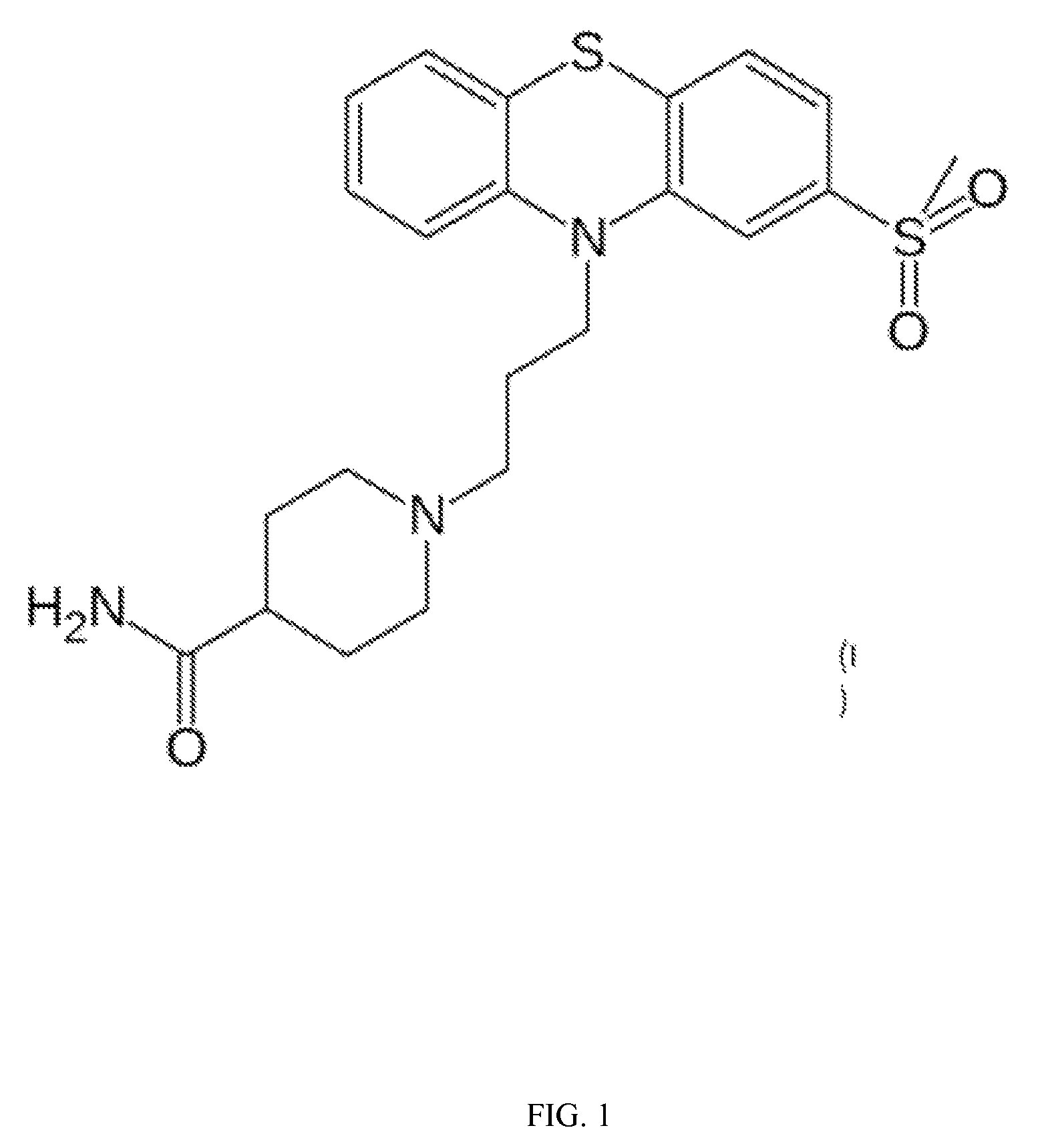
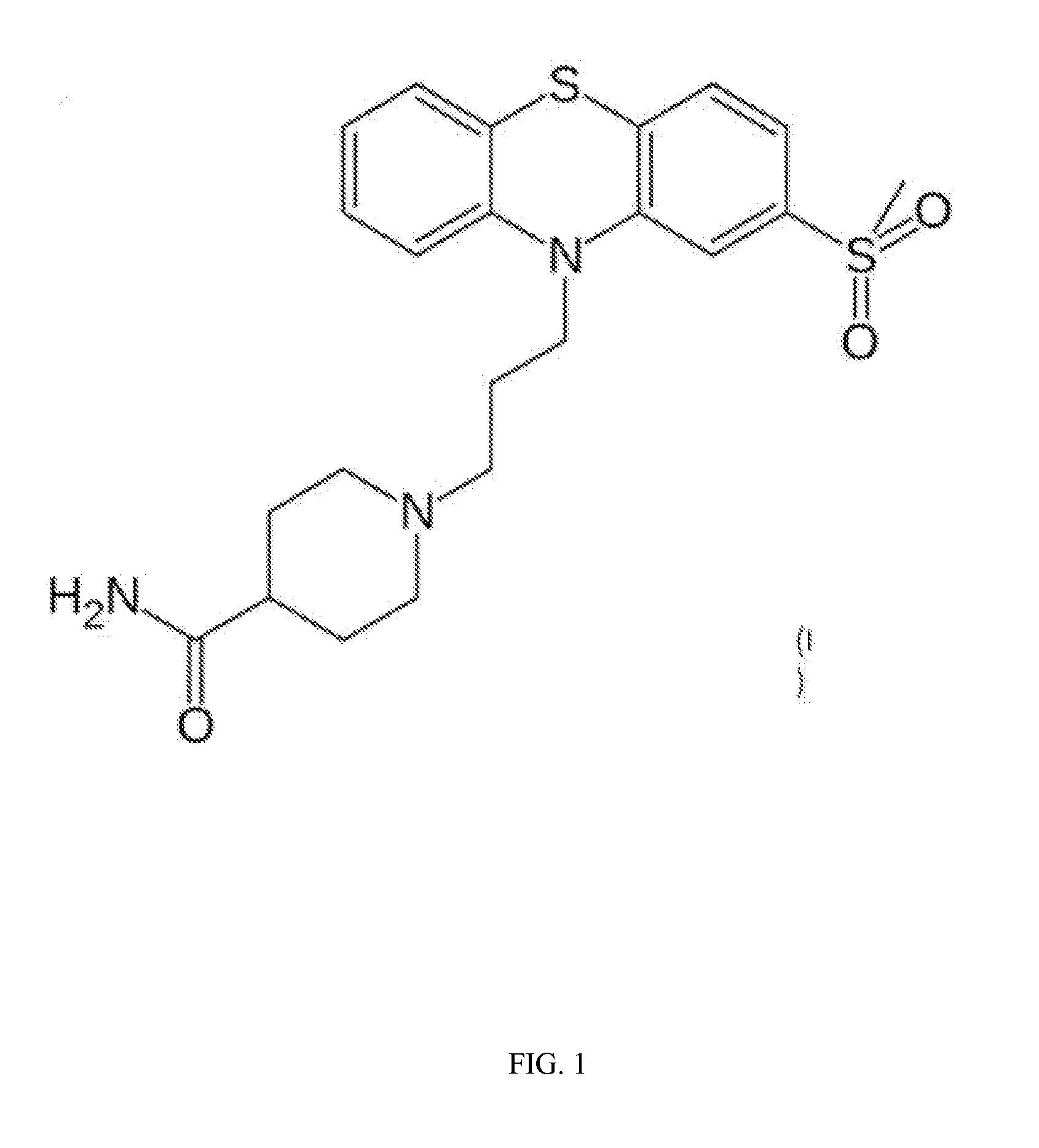
Provided herein are methods, compositions, and kits for the treatment of an enteric nervous system disorder. Such methods may comprise administering to a subject an effective amount of a phenothiazine compound, a peripherally restricted dopamine decarboxylase inhibitor, and / or a peripherally restricted dopamine D2 receptor antagonist that does not substantially inhibit hERG channels.
Owner:NEUROGASTRX
Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof
The invention provides a controlled release oral solid formulation comprising (a) a controlled release component comprising core comprising levodopa and / or an ester of levodopa or salts thereof, wherein the core is coated with a layer of a muco-adhesive polymer and externally coated with a layer of an enteric coated polymer; and (b) a decarboxylase inhibitor component.
Owner:IMPAX LAB LLC
Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof
ActiveUS10987313B2Effective amountOrganic active ingredientsGranular deliveryMedicinal chemistryEnzyme inhibitor
The invention provides a controlled release oral solid formulation comprising (a) a controlled release component comprising core comprising levodopa and / or an ester of levodopa or salts thereof, wherein the core is coated with a layer of a muco-adhesive polymer and externally coated with a layer of an enteric coated polymer; and (b) a decarboxylase inhibitor component.
Owner:IMPAX LAB LLC
Methods for Treating GI Tract Disorders
ActiveUS20150087638A1Improving gastric emptyingAntibacterial agentsNervous disorderDiseaseNervous system
Provided herein are methods, compositions, and kits for the treatment of an enteric nervous system disorder. Such methods may comprise administering to a subject an effective amount of a phenothiazine compound, a peripherally restricted dopamine decarboxylase inhibitor, and / or a peripherally restricted dopamine D2 receptor antagonist that does not substantially inhibit hERG channels.
Owner:NEUROGASTRX
Method for improving stability of benserazide hydrochloride oral administration solid composition
PendingCN112741808AImprove liquidityReduce collisionOrganic active ingredientsNervous disorderBenserazideBENSERAZIDE HYDROCHLORIDE
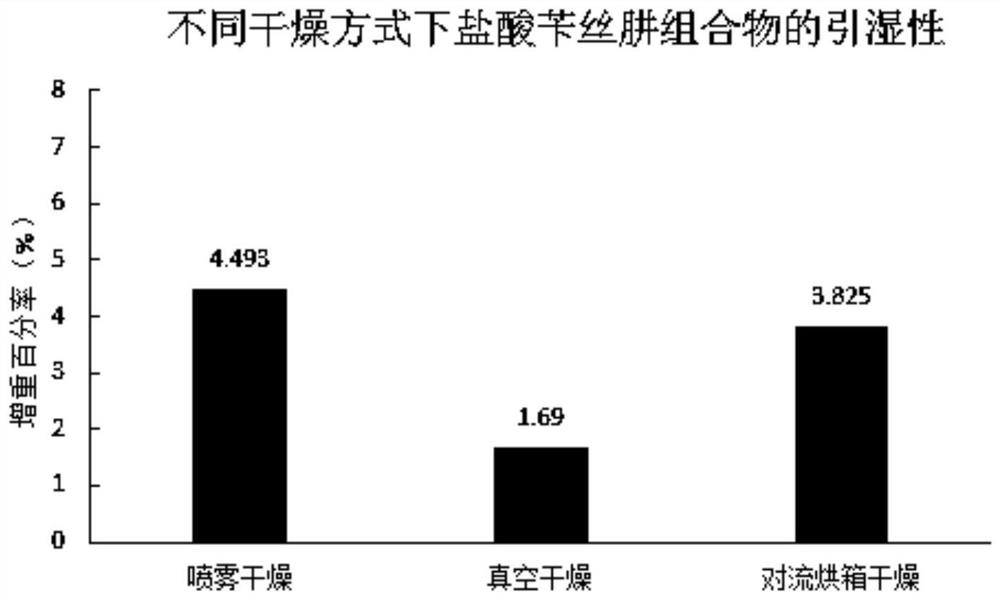
The invention belongs to the field of drug preparations, and particularly relates to a method for improving the stability of an oral administration solid composition containing benserazide hydrochloride. The benserazide hydrochloride is a peripheral decarboxylase inhibitor, and the benserazide hydrochloride and levodopa are always prepared into a compound preparation and are combined to be mainly used for treating the Parkinson' s disease (essential paralysis agitans), and postencephalitic or the symptomatic Parkinson' s syndrome (non-drug-induced paralysis agitans syndrome) combined with cerebral arteriosclerosis. The property of the benserazide hydrochloride is extremely unstable, and the benserazide hydrochloride is extremely sensitive to change of PH, ray, temperature and humidity. Since the ways of wet granulation and vacuum drying are adopted, the stability of the benserazide hydrochloride in an oral administration solid preparation containing the benserazide hydrochloride can be improved.
Owner:NANJING GRITPHARMA CO LTD
Pharmaceutical compositions comprising levodopa, a dopamine decarboxylase inhibitor and a COMT inhibitor and a method of administration thereof
A pharmaceutical gel composition for intra-intestinal administration is provided and comprises (i) a dopamine replacement agents, (ii) a dopamine decarboxylase inhibitor (DDI), and (iii) a COMT inhibitor.
Owner:LOBSOR PHARM AB
Dopa decarboxylase inhibitor compositions
The present invention provides highly stable carbidopa-based pharmaceutical compositions comprising an antioxidant combination consisting of ascorbic acid and at least one additional antioxidant, wherein said combination strongly inhibits carbidopa degradation. These compositions may further comprise levodopa and / or one or both of arginine and meglumine, and are beneficial in treatment of a disease, disorder or condition associated with loss of dopamine or dopaminergic neurons, e.g., Parkinson's disease.
Owner:NEURODERM
Method and Composition for the Treatment of Parkinson's Disease
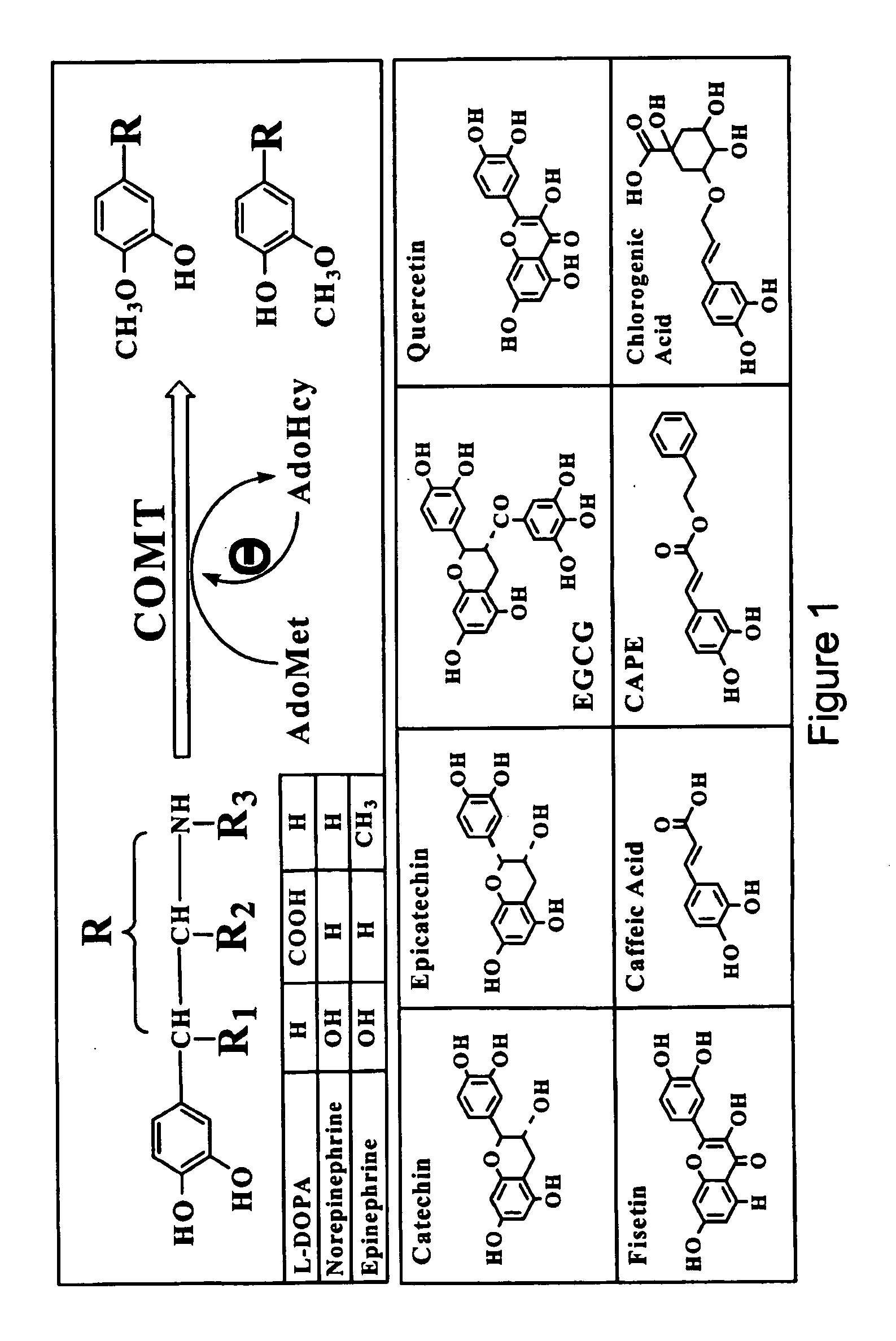
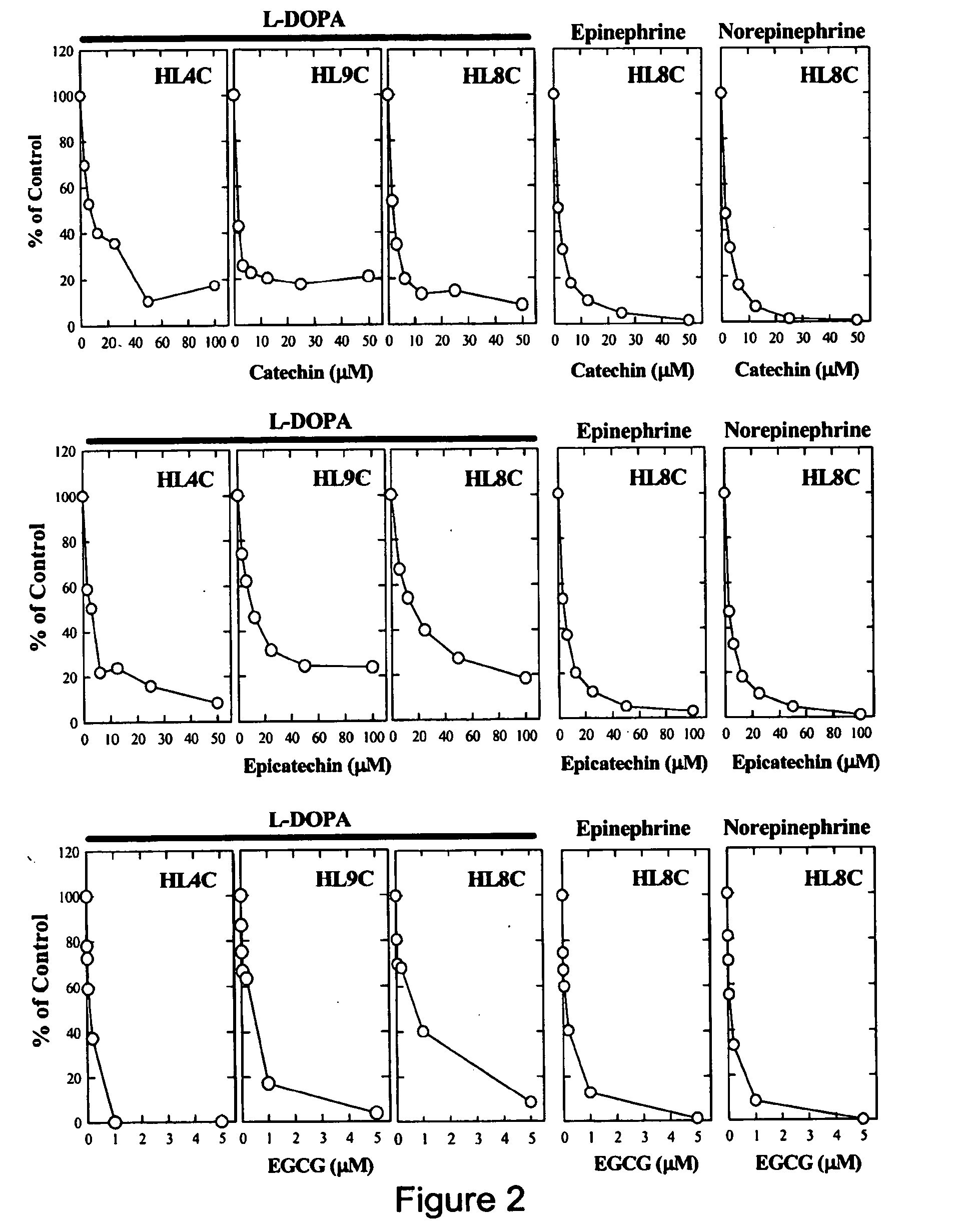
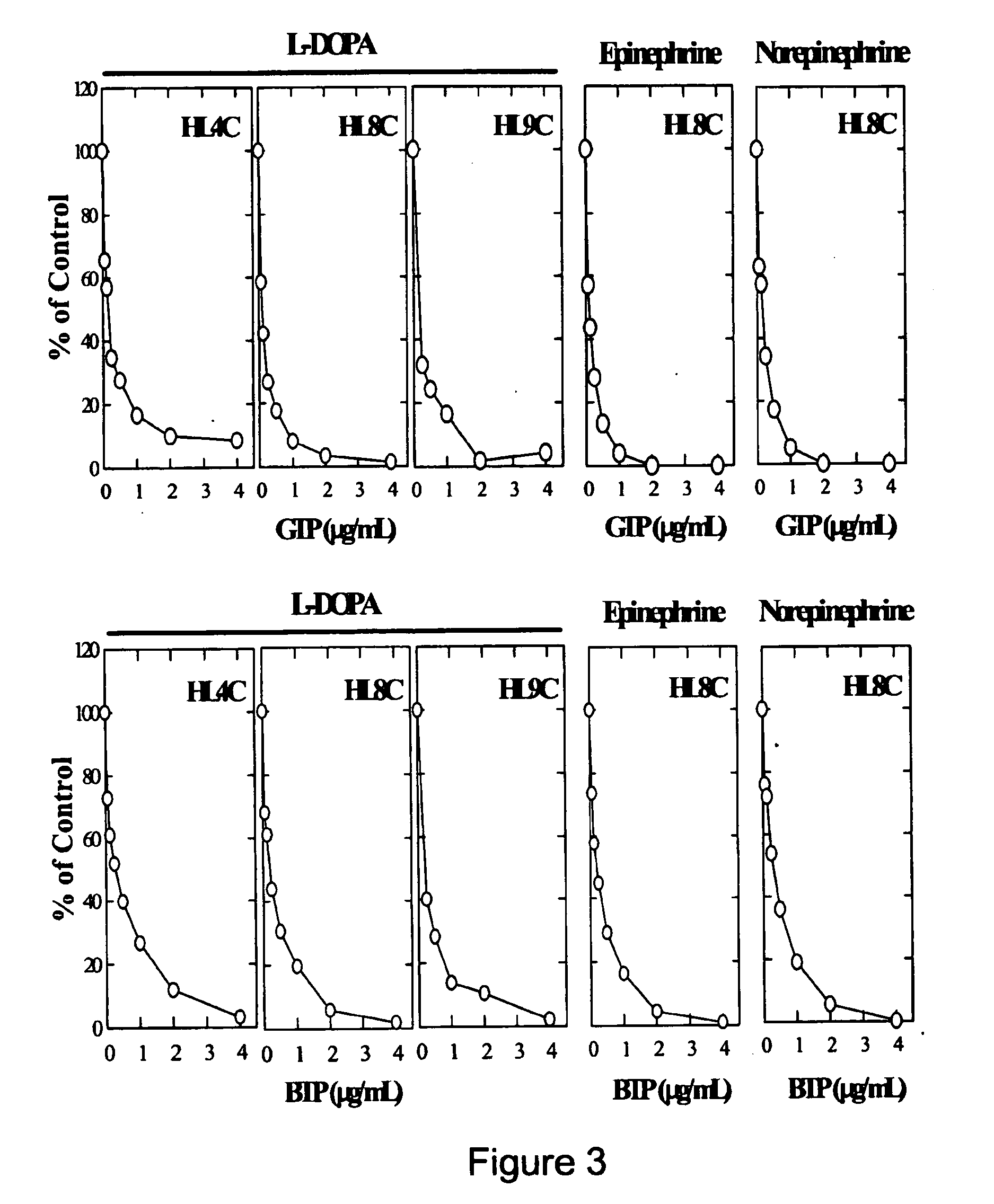
It has been discovered that polyphenols are effective as a co-pharmaceutical in combination with traditional dual drug therapies of catecholamines and decarboxylase inhibitors for the treatment of Parkinson's disease. Accordingly, a method of treating Parkinson's disease comprising administering to a subject suffering from Parkinson's disease a pharmaceutical composition comprising at least one catecholamine, at least one decarboxylase inhibitor, and at least one polyphenol is provided.
Owner:ZHU BAO TING
Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof
ActiveUS20200009062A1Limit practiceLimit scopeOrganic active ingredientsGranular deliveryMedicinal chemistryEnzyme inhibitor
The invention provides a controlled release oral solid formulation comprising (a) a controlled release component comprising core comprising levodopa and / or an ester of levodopa or salts thereof, wherein the core is coated with a layer of a muco-adhesive polymer and externally coated with a layer of an enteric coated polymer; and (b) a decarboxylase inhibitor component.
Owner:IMPAX LAB LLC
Muco-adhesive, controlled release formulations of levodopa and/or esters of levodopa and uses thereof
ActiveUS20160250170A1Limit practiceLimit scopeOrganic active ingredientsNervous disorderControl releaseAdhesive
The invention provides a controlled release oral solid formulation comprising (a) a controlled release component comprising core comprising levodopa and / or an ester of levodopa or salts thereof, wherein the core is coated with a layer of a muco-adhesive polymer and externally coated with a layer of an enteric coated polymer, and (b) a decarboxylase inhibitor component.
Owner:IMPAX LAB LLC
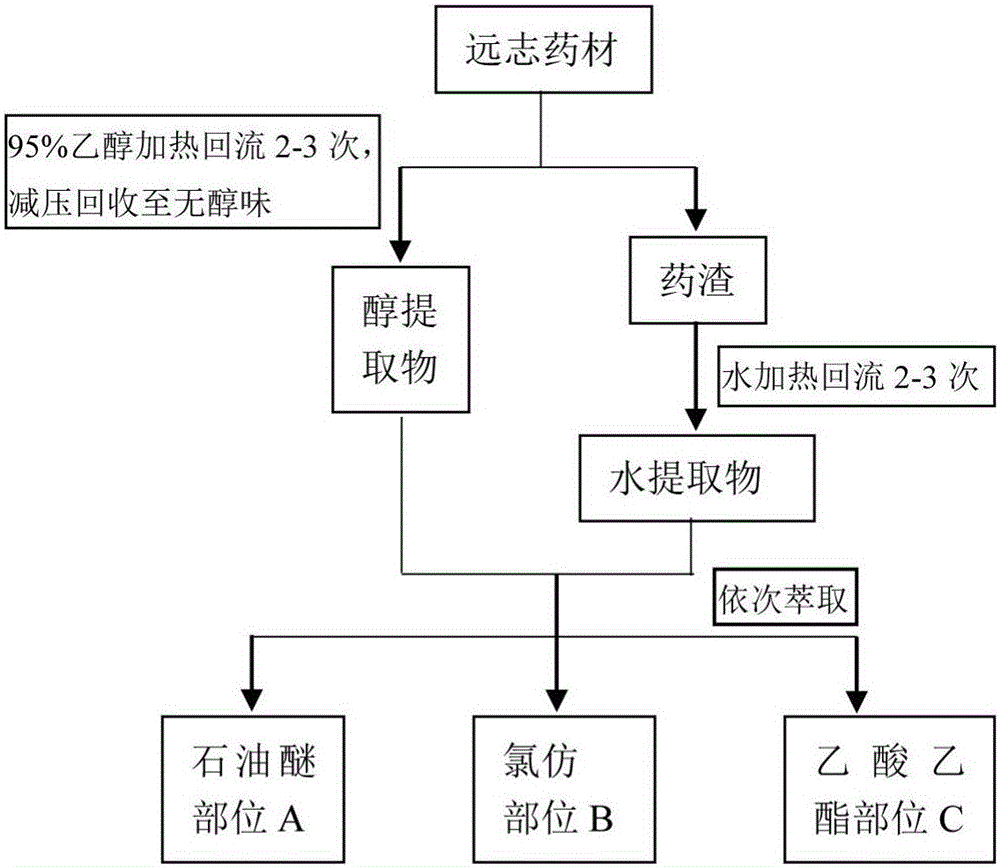
Polygala tenuifolia extract for parkinson's disease and preparation method and application of polygala tenuifolia extract
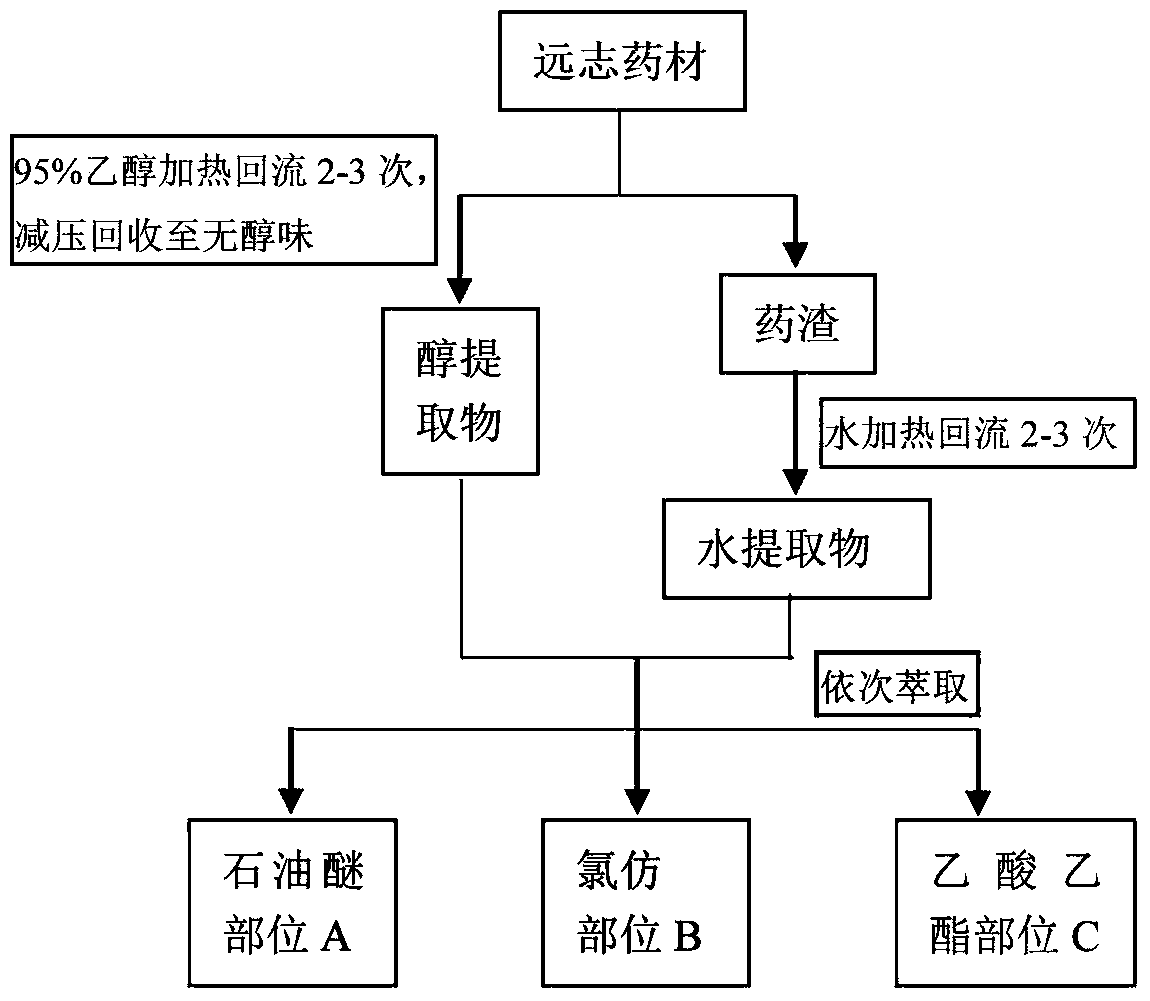
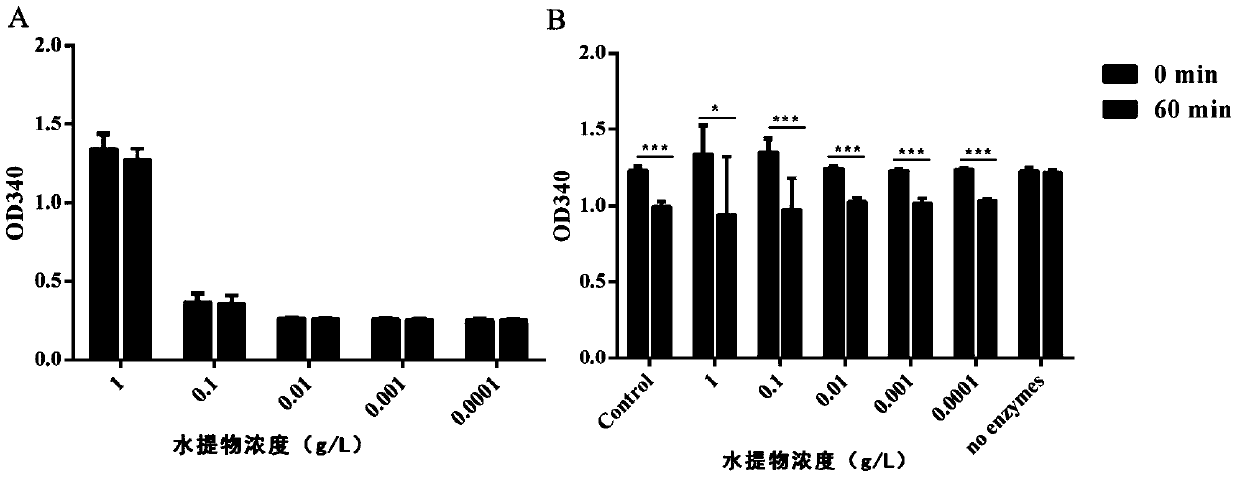
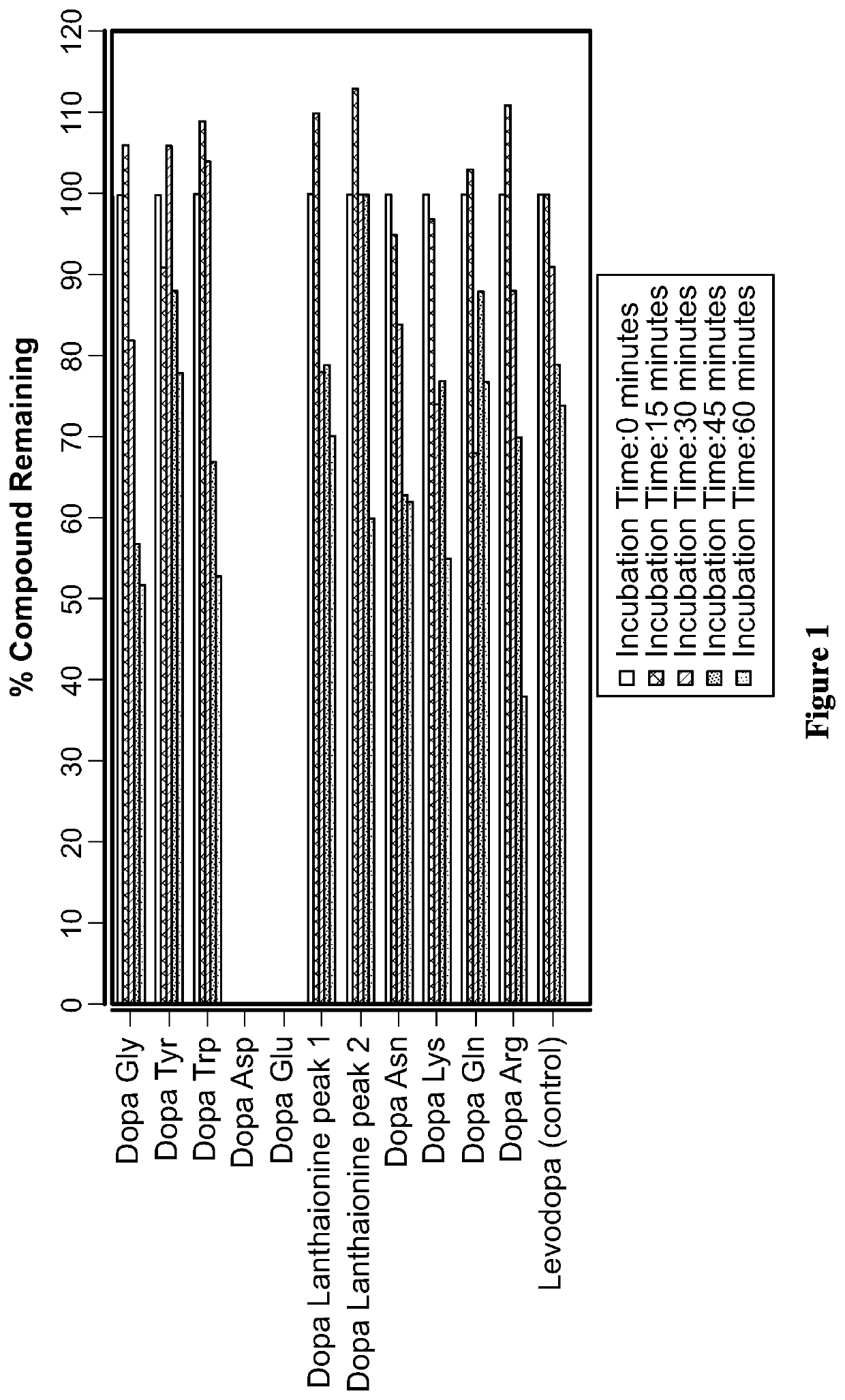
The invention provides a polygala tenuifolia extract for a parkinson's disease and a preparation method and an application of the polygala tenuifolia extract. The method comprises the following specific steps: taking a polygala tenuifolia material, adding 95% ethanol to soak over the night, carrying out heating reflux extraction and filtration and recovering ethanol until no alcohol taste in a vacuum condition to obtain an alcohol extract; adding water to soak the residual filter residues after filtration over the night, carrying out heating reflux extraction, filtration and vacuum concentration until a liquid extract to obtain a water extract; mixing the alcohol extract and the water extract at equal volume; adding petroleum ether, carrying out extracting and separating to remove the petroleum ether part; adding chloroform to the residual water solution, extracting and collecting a chloroform extracting solution; and concentrating the chloroform extracting solution until an extract, drying the extract in vacuum and crushing the product to obtain the effective part B. Through an in vitro anthropogenic dopa decarboxylase inhibitor screening model, the screened polygala tenuifolia effective part B has obvious anti-PD activity and can be applied to preparation of anti-PD medicines.
Owner:SHANXI UNIV
Method and composition to individualize Levodopa/Carbidopa therapy using a breath test
InactiveUS20070003481A1Maximize efficacyEasy to useOrganic active ingredientsIn-vivo radioactive preparationsMetaboliteDihydroxyphenylalanine
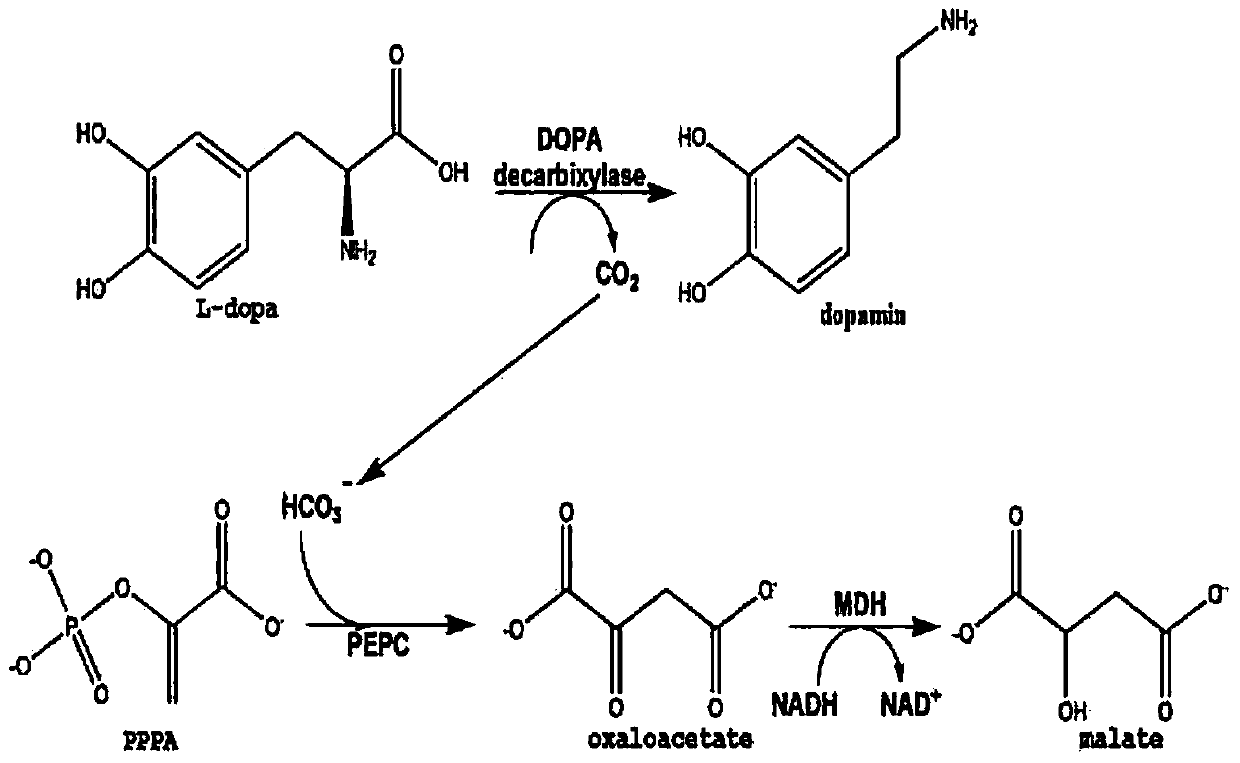
The present invention relates, generally to a method of determining and assessing L-3,4-dihydroxyphenylalanine (a.k.a., Levodopa; L-dopa; or LD) metabolic capacity in an individual mammalian subject via a breath assay, by determining the relative amount of 13CO2 exhaled by the subject upon intravenous or oral administration of a 13C-labeled substrate, such as levodopa. The present invention is useful as an in vivo phenotype assay for individualizing LD / Carbidopa(CD) therapy in Parkinsons disease patients by optimizing the dose and timing of the dose of dopamine decarboxylase (DDC) inhibitor like CD for systemic suppression of dopamine metabolism by evaluating DDC enzyme activity using the metabolite 13CO2 in expired breath.
Owner:OTSUKA AMERICA PHARMA INC
Pulsatile drug delivery system for treating morning akinesia
PendingCN109689036AImprove nighttime sleep patternsOrganic active ingredientsNervous disorderDepressantPharmaceutical Substances
Provided herewith is a pharmaceutical composition comprising, separately or together, a pulsatile release component comprising levodopa and a DOPA decarboxylase inhibitor for the management of OFF-time episodes in patients with Parkinson's disease.
Owner:康特拉医药股份有限公司
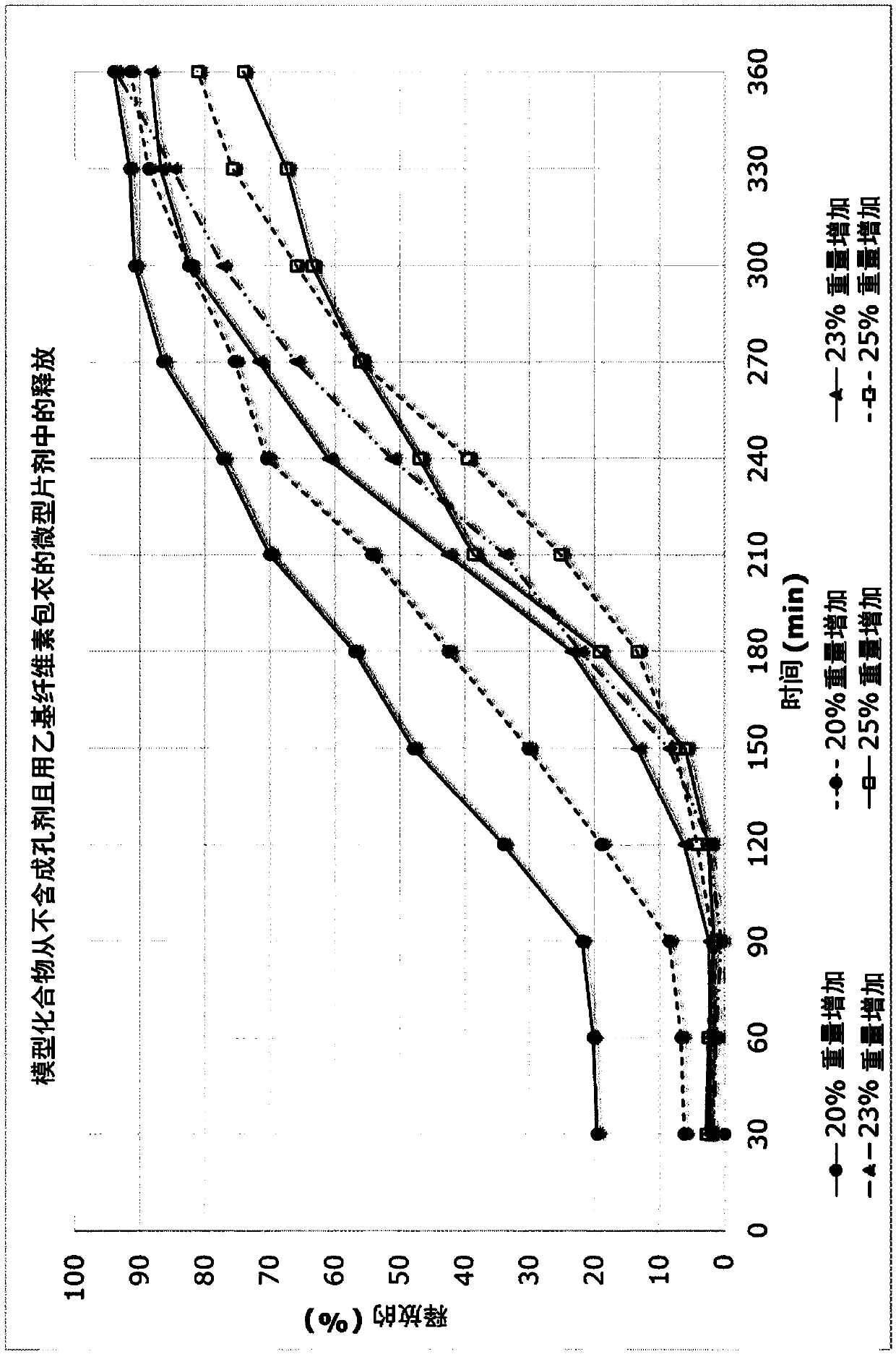
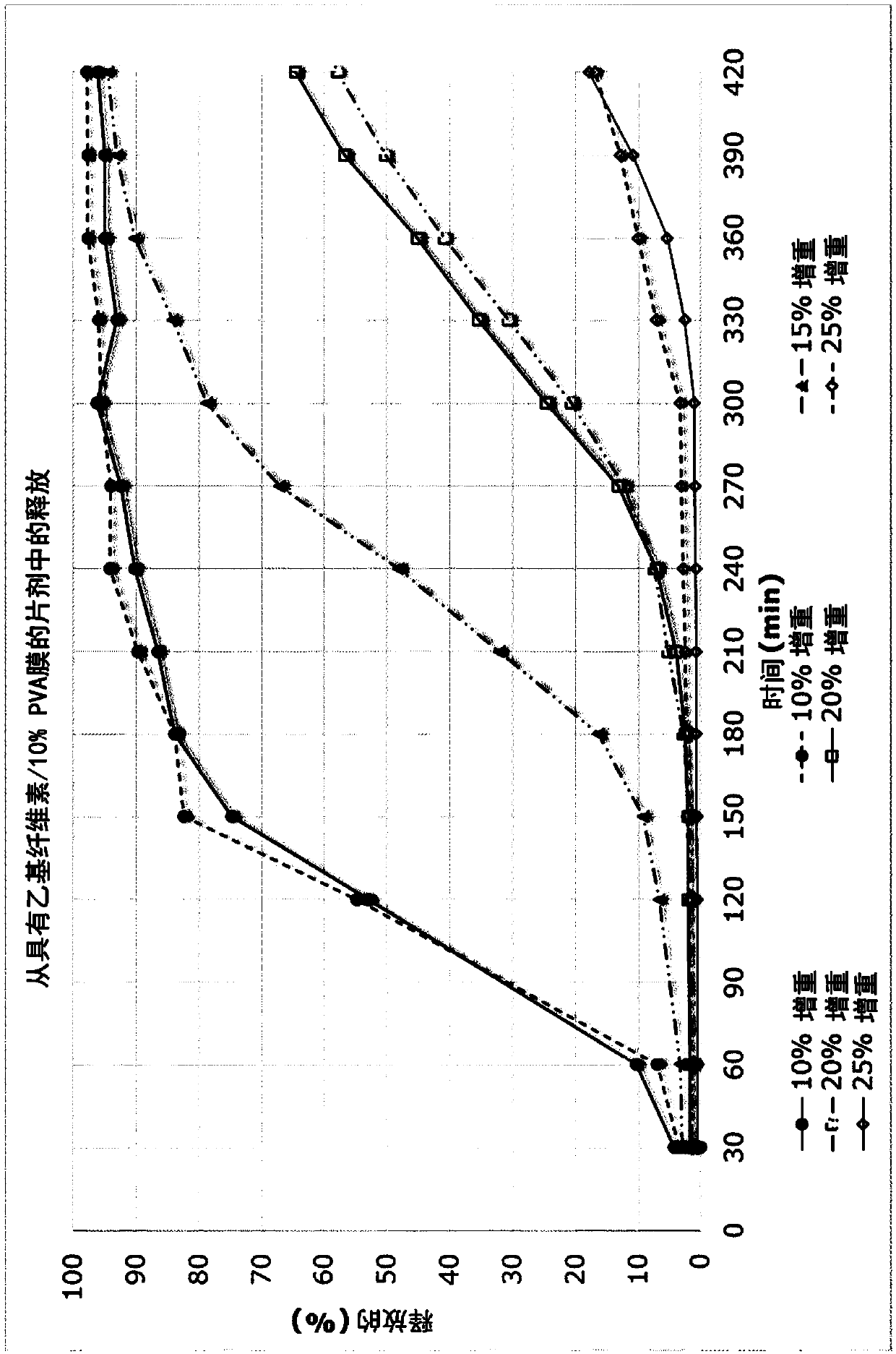
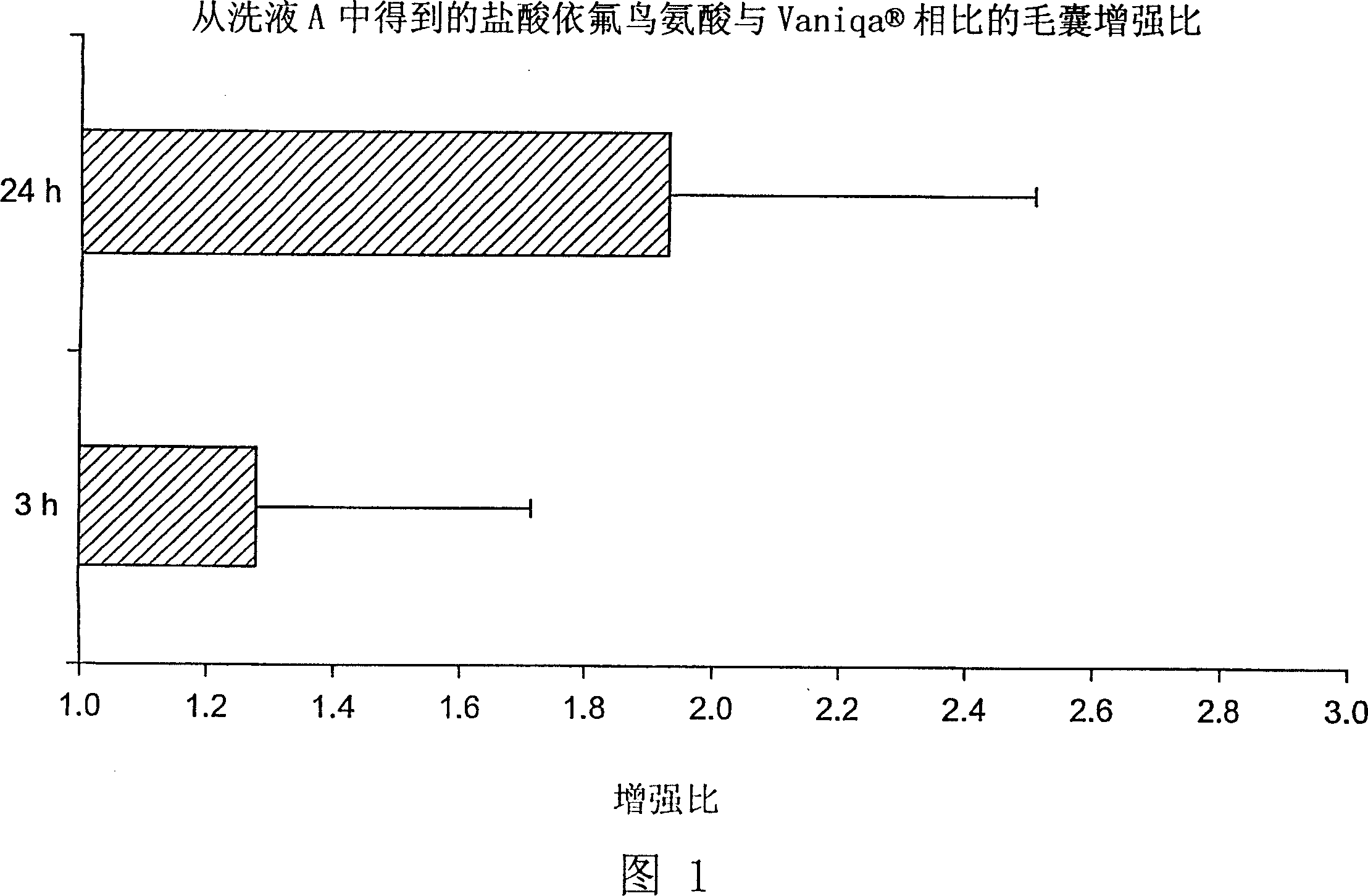
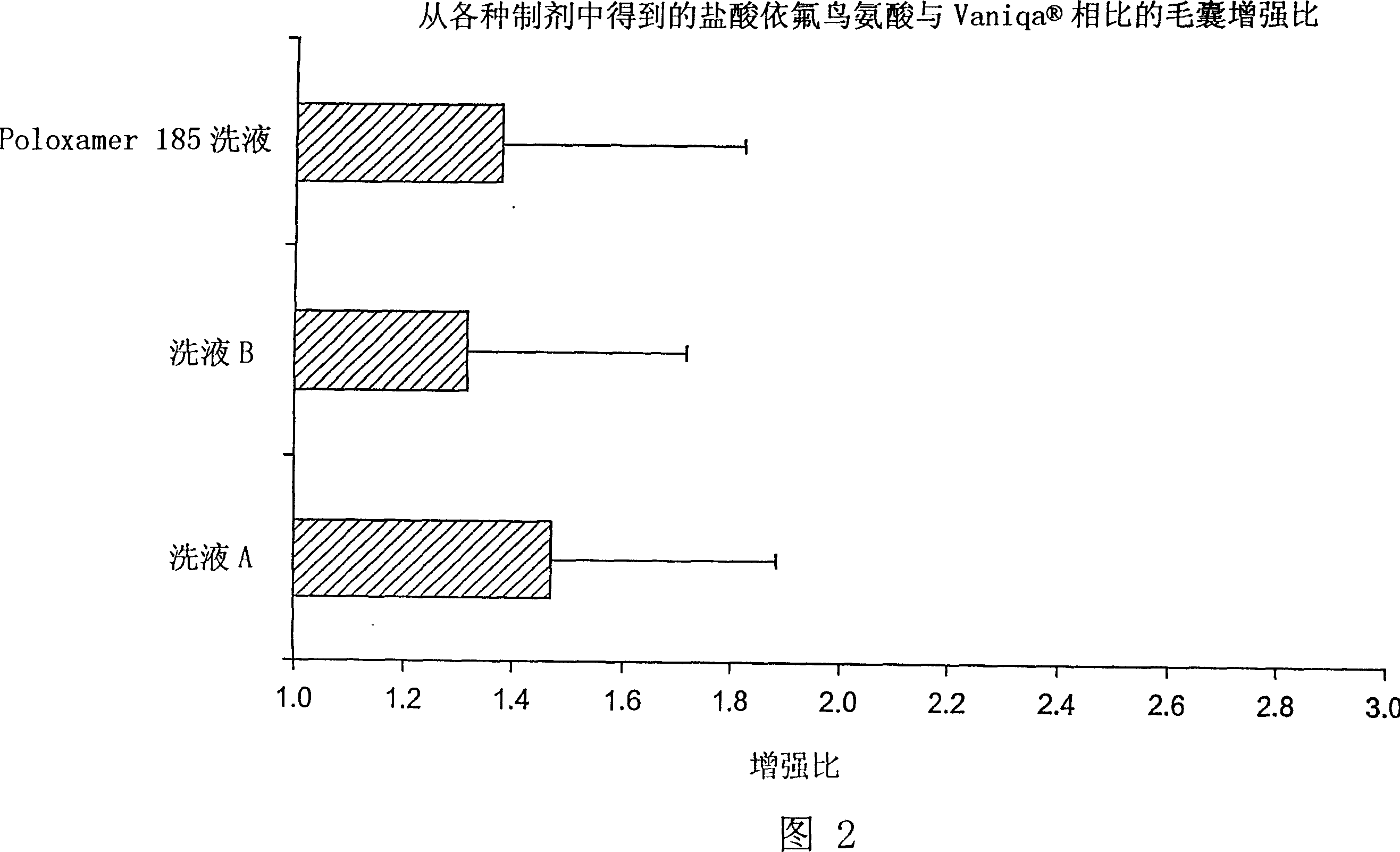
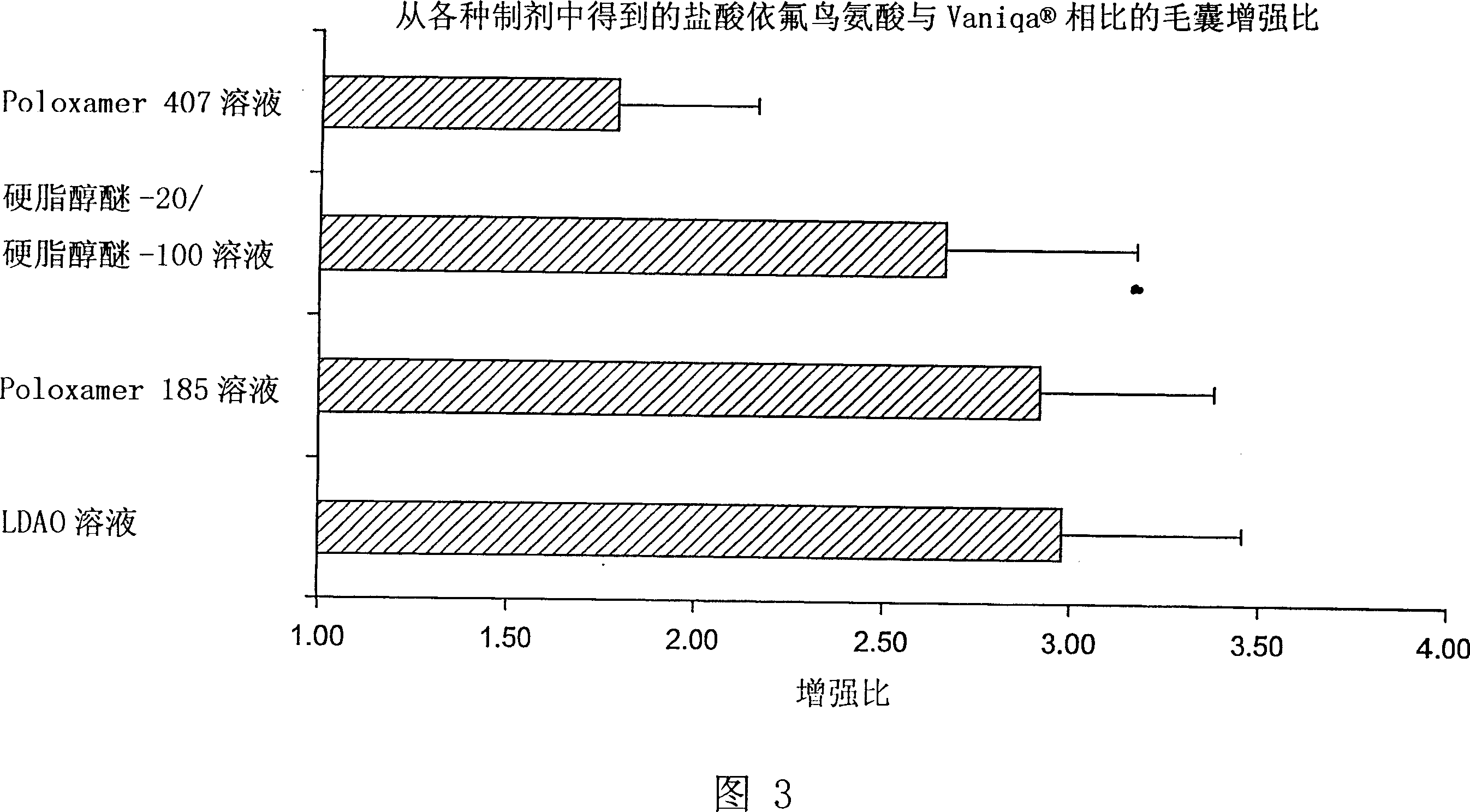
Topical composition for follicular delivery of an ornithine decarboxylase inhibitor
Owner:SKINMEDICA INC
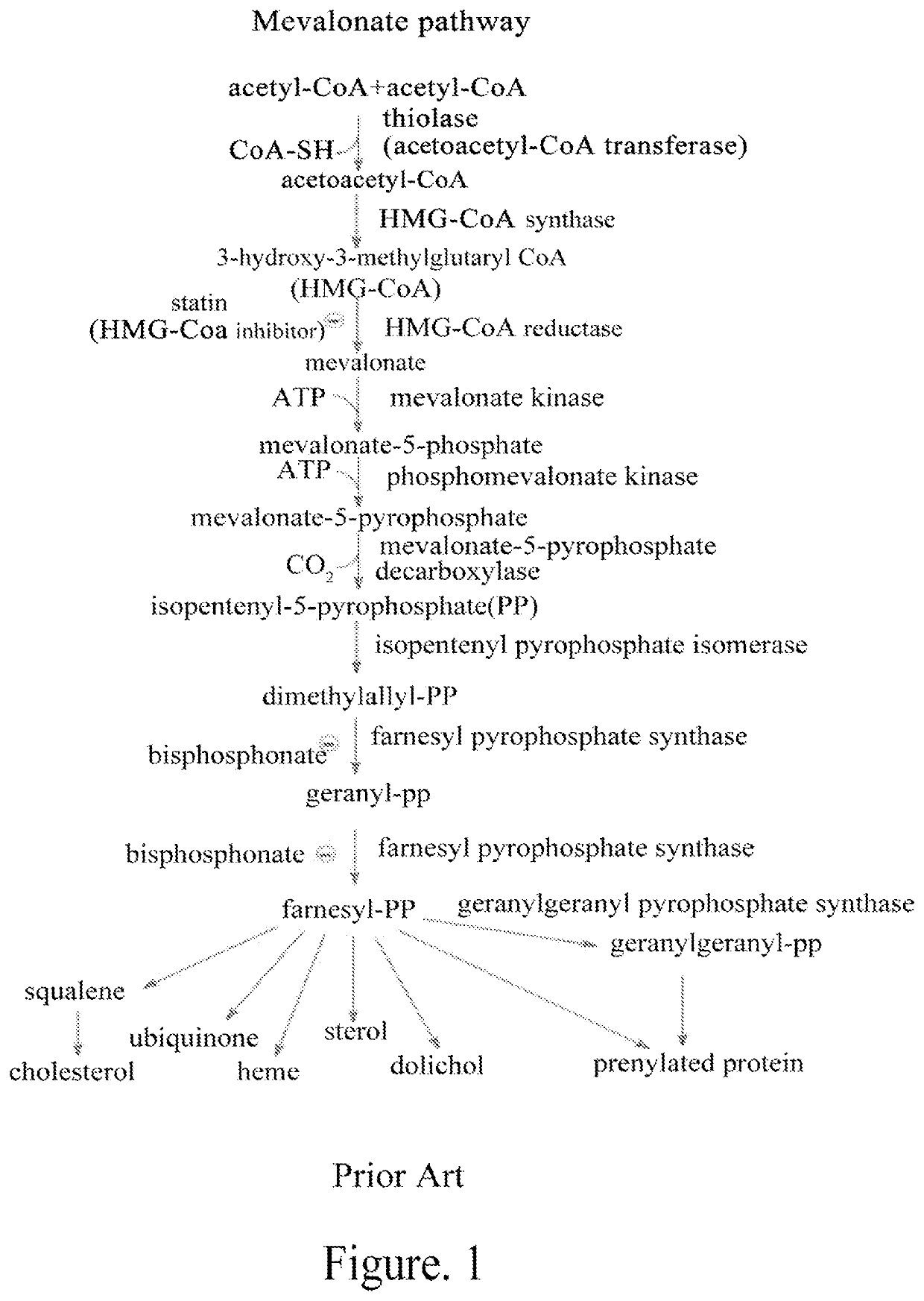
Mevalonate pathway inhibitor as highly-efficient vaccine adjuvant
ActiveUS11382971B2Organic active ingredientsGroup 5/15 element organic compoundsIsopentenyl pyrophosphateEnzyme Inhibitor Agent
Owner:TSINGHUA UNIV
Anti-Parkinson's disease polygala extract and its preparation method and application
Owner:SHANXI UNIV
Liquid compositions comprising a levodopa amino acid conjugate and uses thereof
Disclosed herein are liquid pharmaceutical formulations comprising levodopa amino acid conjugates that may further comprise a decarboxylase inhibitor, such as carbidopa, an antioxidant, a solvent, or any other pharmaceutically acceptable excipient. Further disclosed are methods of treating generative conditions and / or conditions characterized by reduced levels of dopamine in the brain, such as Parkinson's disease, comprising administering the disclosed liquid pharmaceutical formulations. Disclosed also are LDAA conjugate compounds.
Owner:NEURODERM
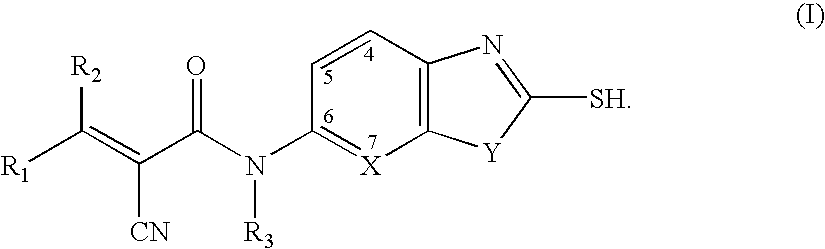
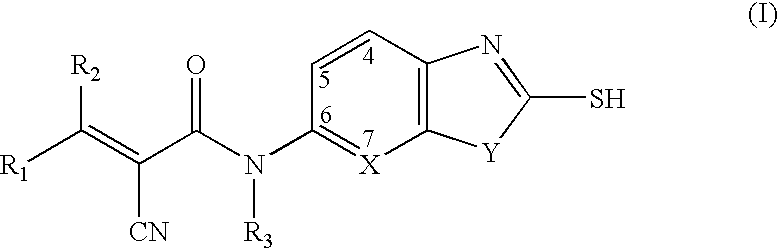
Malonyl-CoA decarboxylase inhibitors useful as metabolic modulators
The present invention relates to novel compounds (I), their prodrugs, and the pharmaceutically acceptable salts as well as pharmaceutical compositions containing such compounds useful in treating certain metabolic diseases and diseases modulated by the inhibition of the enzyme malonyl-coenzyme A decarboxylase (malonyl-CoA decarboxylase, MCD). In particular, the invention relates to compounds and compositions and the methods for the prophylaxis, management and treatment of cardiovascular diseases, diabetes, acidosis, cancers, and obesity through the inhibition of malonyl-coenzyme A decarboxylase.
Owner:CHUGAI PHARMA CO LTD
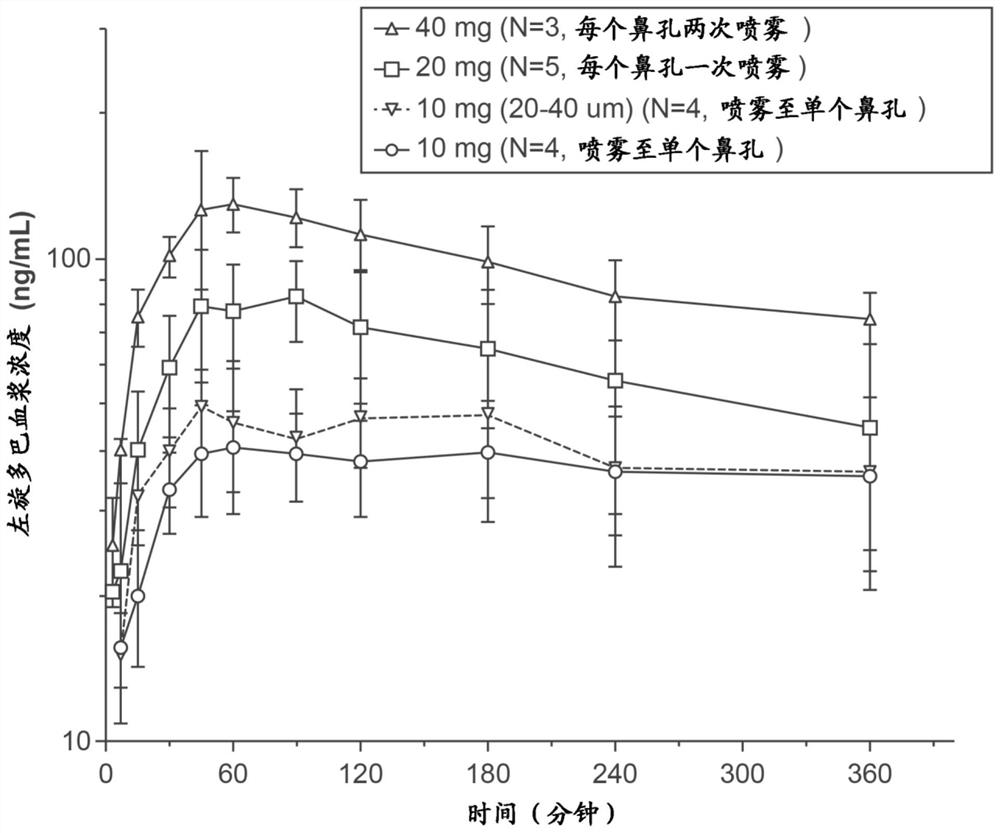
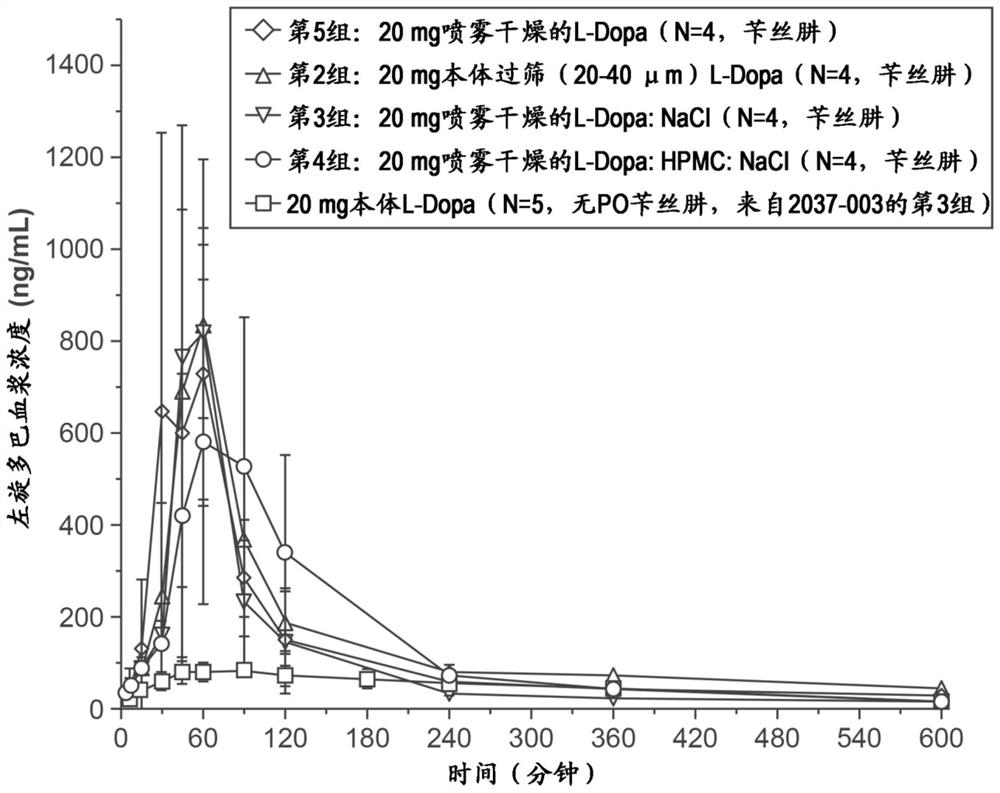
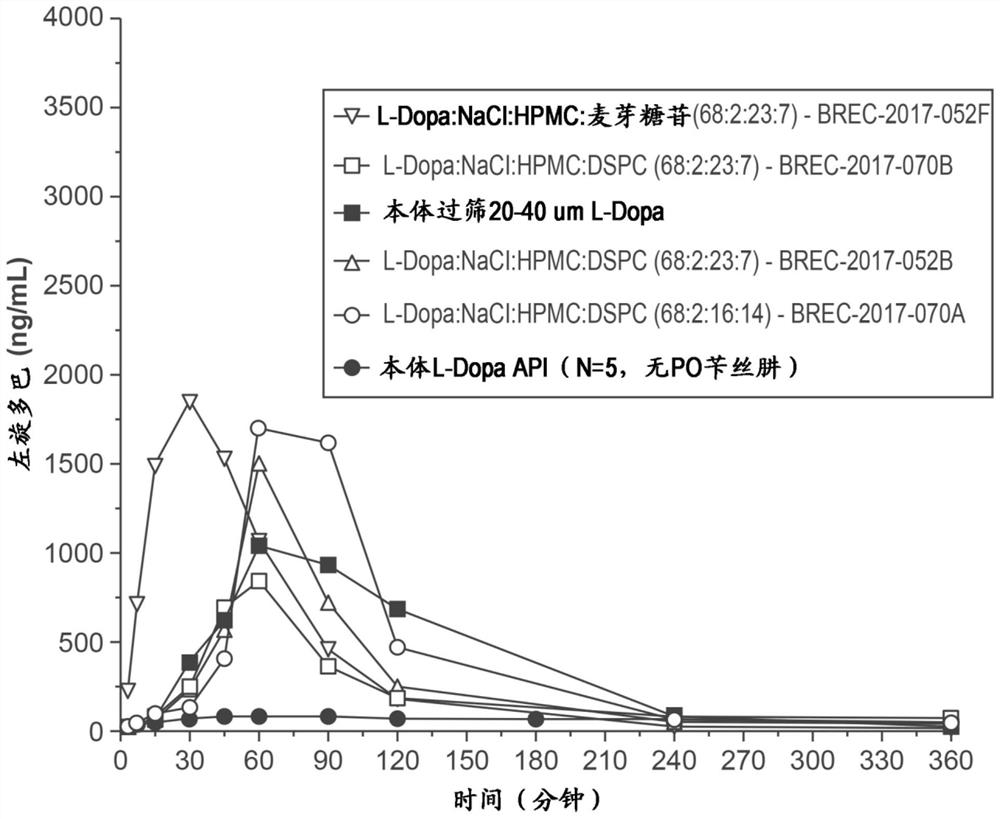
Respiratory tract delivery of levodopa and dopa decarboxylase inhibitor for treatment of parkinson's disease
A dry pharmaceutical composition is provided that is suitable for respiratory tract delivery of levodopa and DDI for treatment of Parkinson's disease or Parkinson syndrome. The dry pharmaceutical composition comprises levodopa, a dopa decarboxylase inhibitor (DDI) and at least one excipient. A unit dosage form of the dry pharmaceutical composition and a method of treating a patient with Parkinson's disease or Parkinson syndrome by administering the dry pharmaceutical composition are also provided.
Owner:IMPEL NEUROPHARMA INC
Azoles as malonyl-CoA decarboxylase inhibitors useful as metabolic modulators
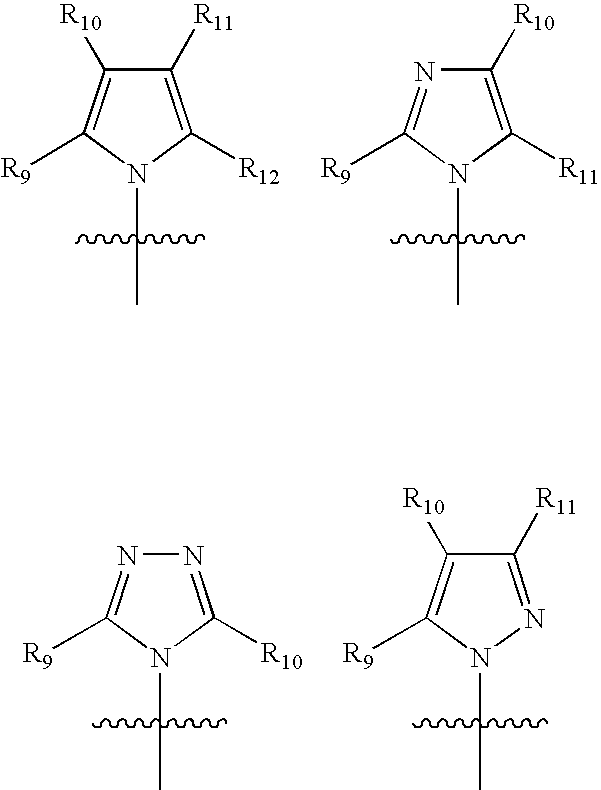
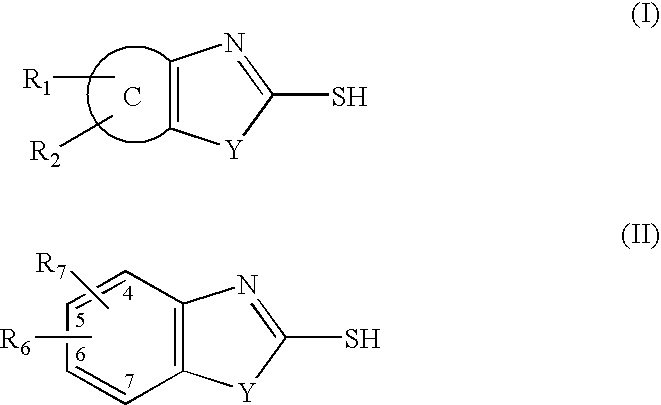
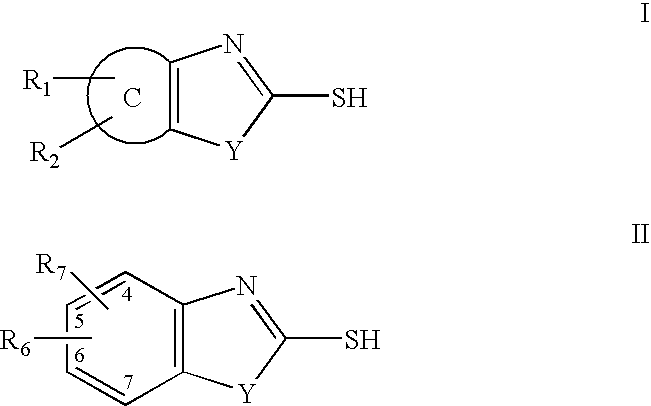
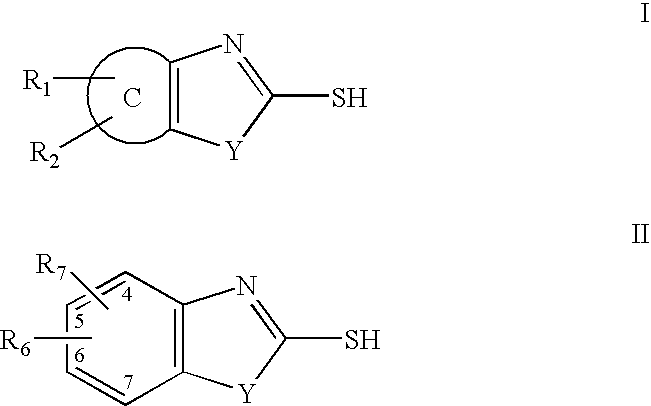
InactiveUS7709510B2Increase malonyl CoA concentrationProfound effectBiocideMetabolism disorderDiabetes mellitusObesity
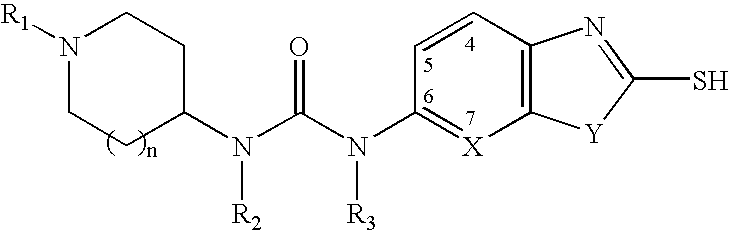
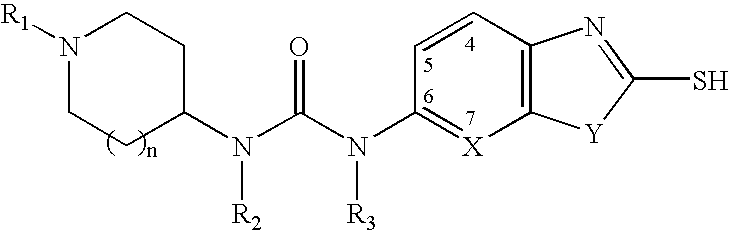
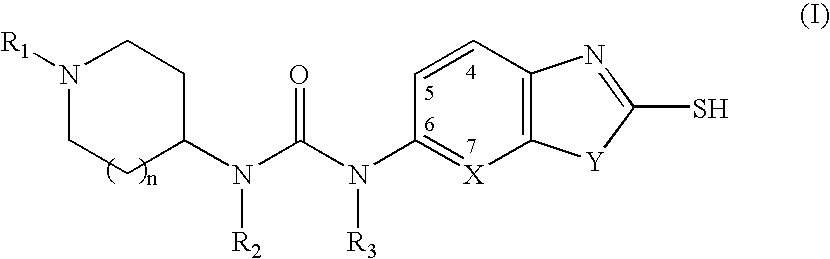
The present invention relates to methods of treatment of certain metabolic diseases, and to novel compounds and their prodrugs, and / or pharmaceutically acceptable salts, pharmaceutical compositions containing such compounds useful in treating such diseases. In particular, this invention relates to the use of novel compounds and compositions for treatment of cardiovascular diseases, diabetes, cancers, acidosis, and obesity through the inhibition of malonyl-CoA decarboxylase (MCD). These compounds have the formulae (I) and (II), wherein Y, C, R1, R2, R6, and R7 are defined herein.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com