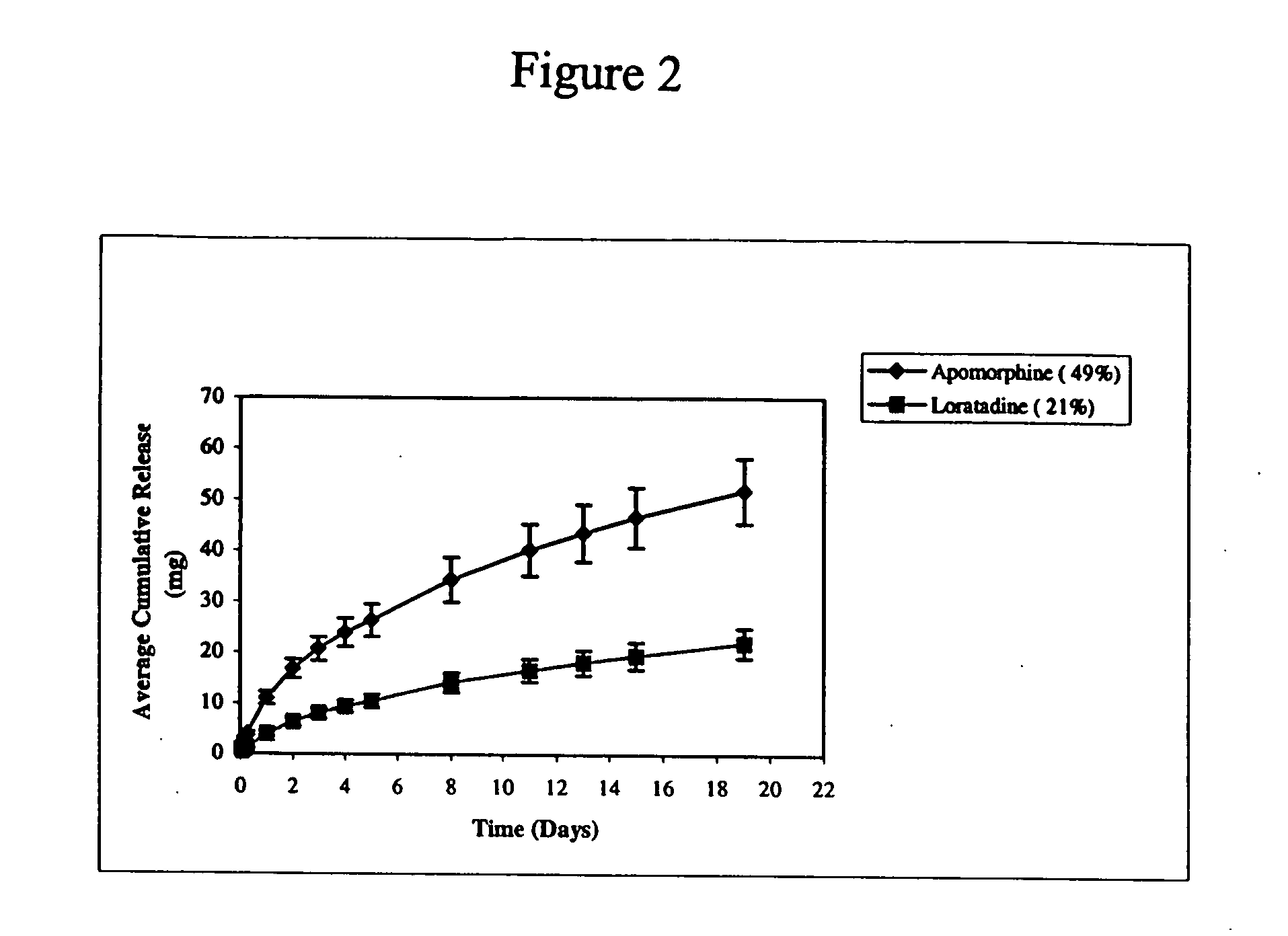
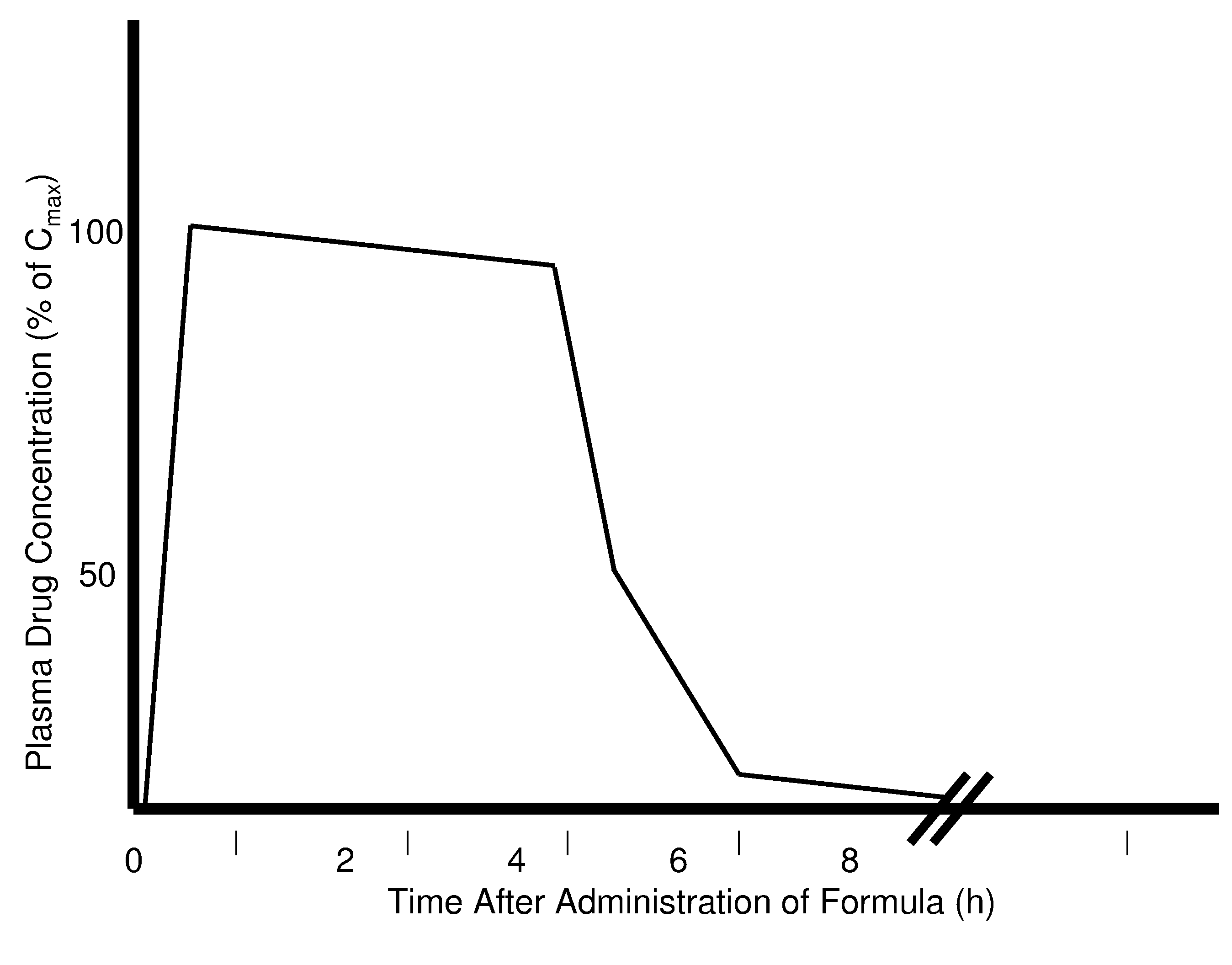
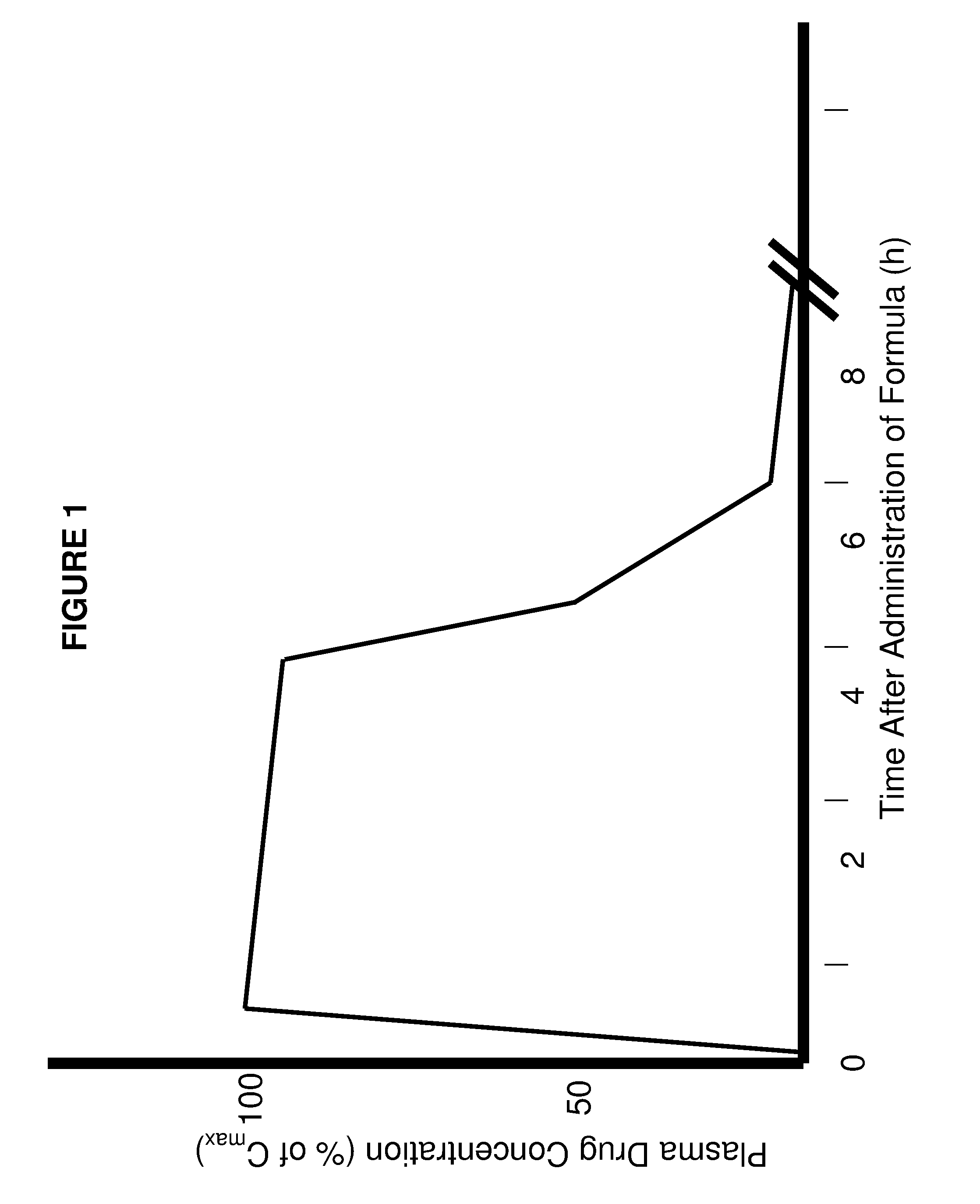
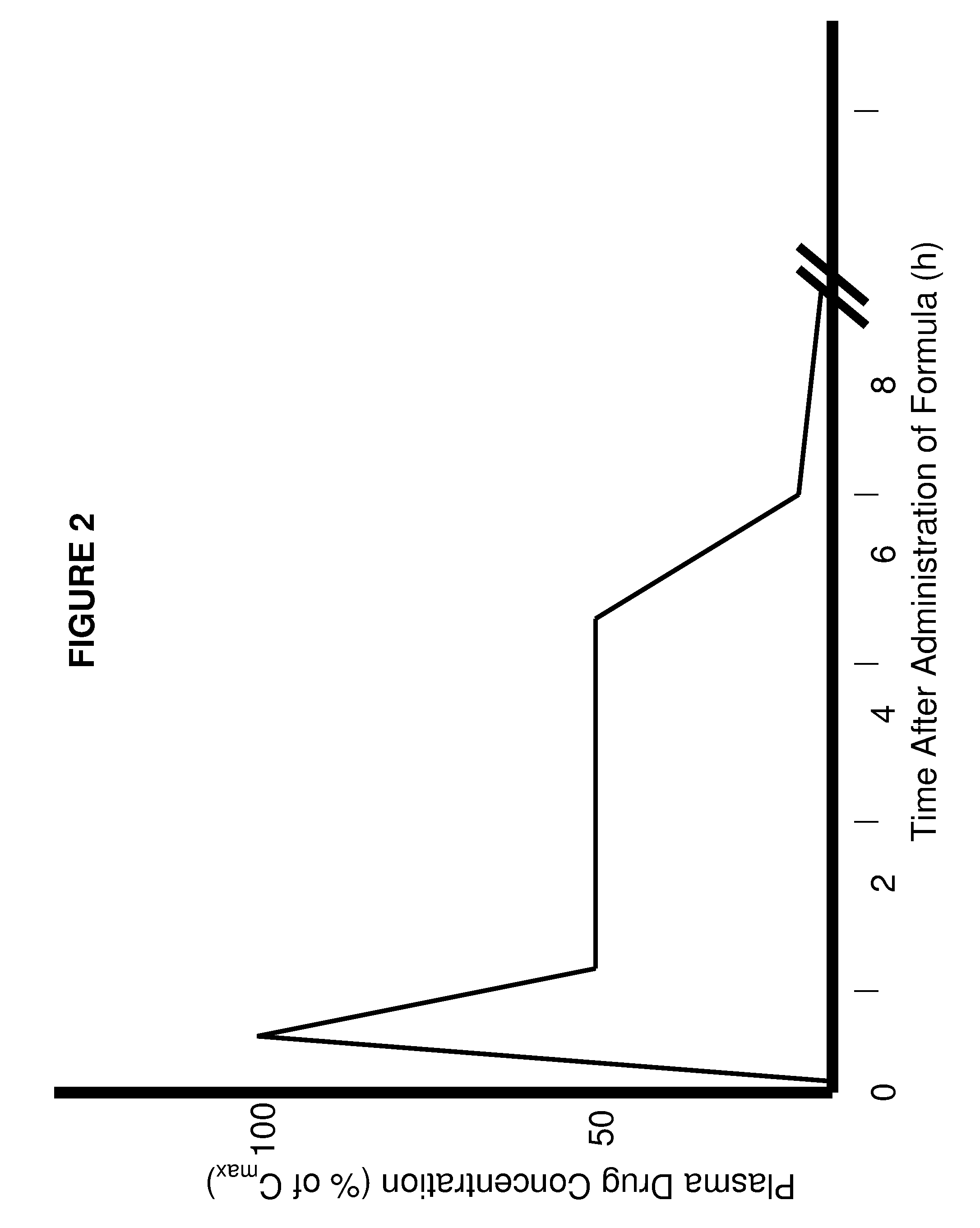
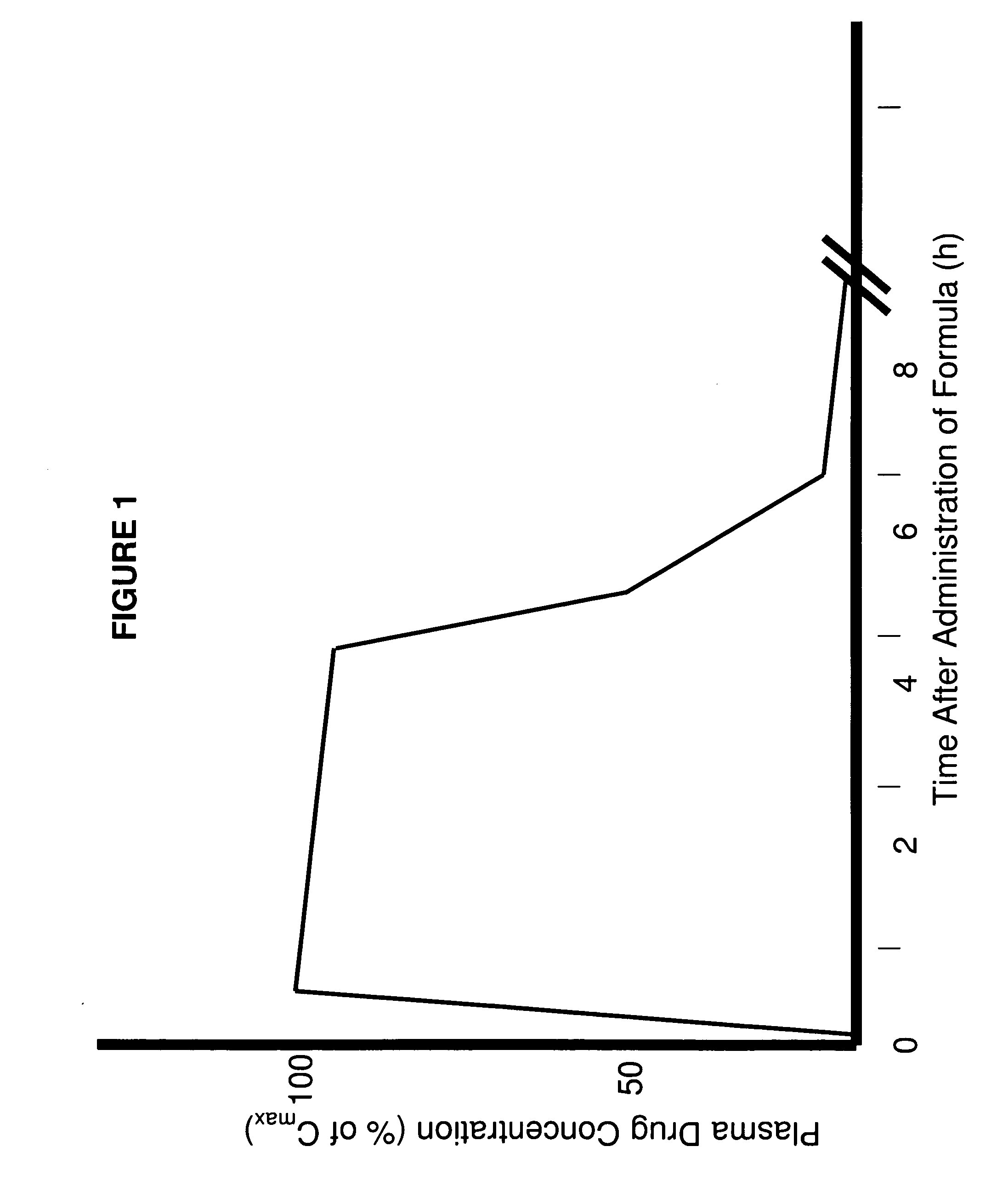
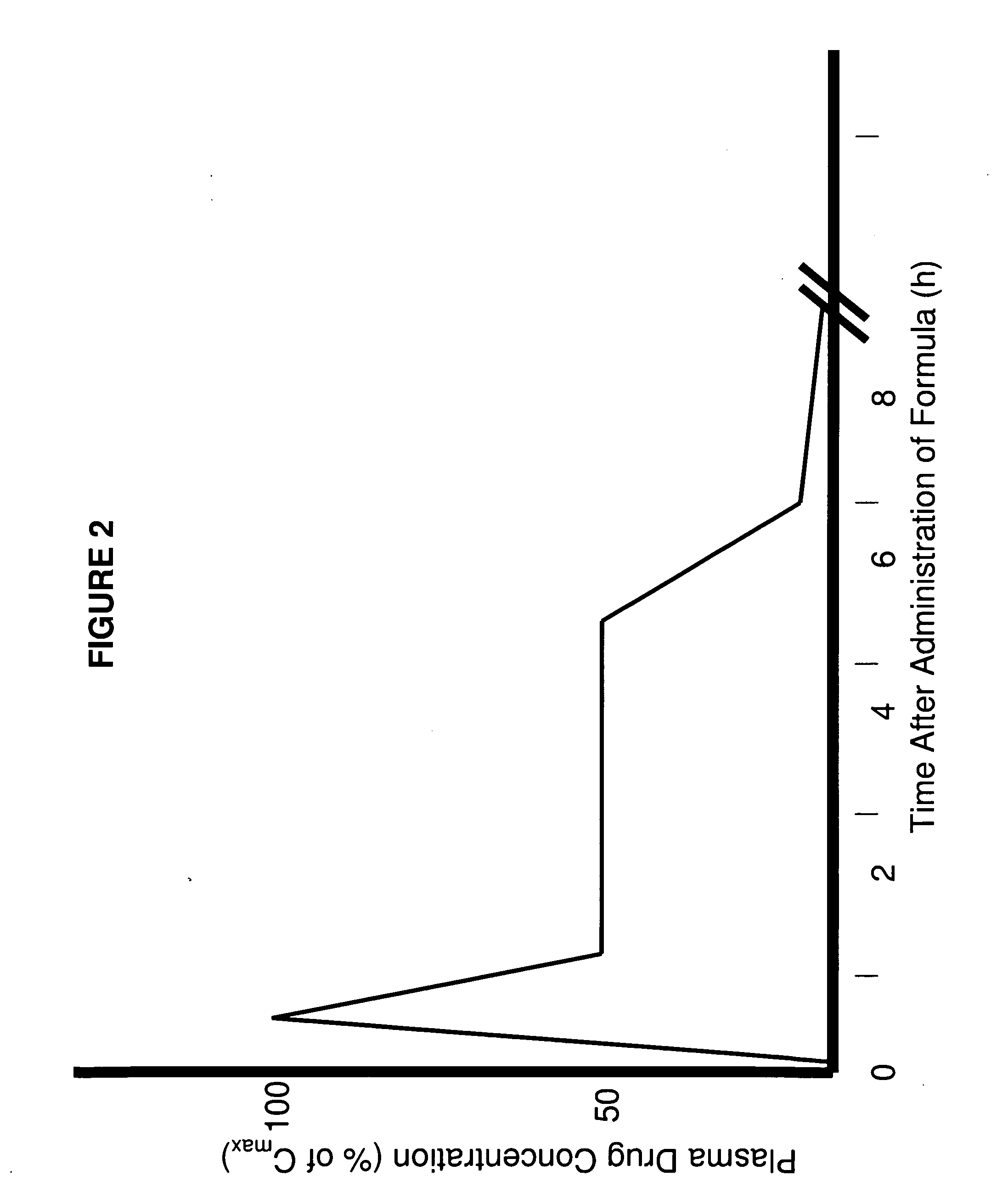
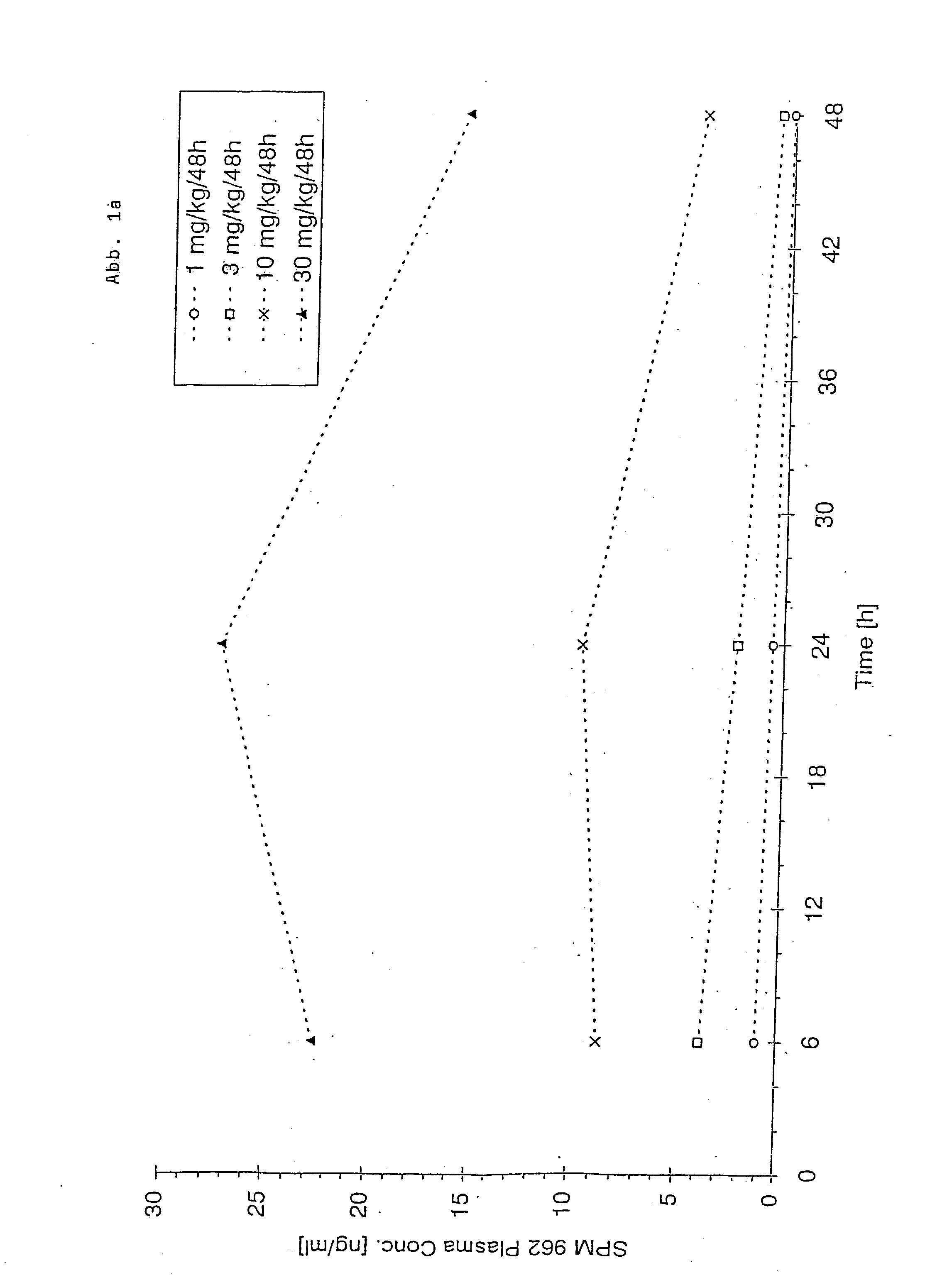
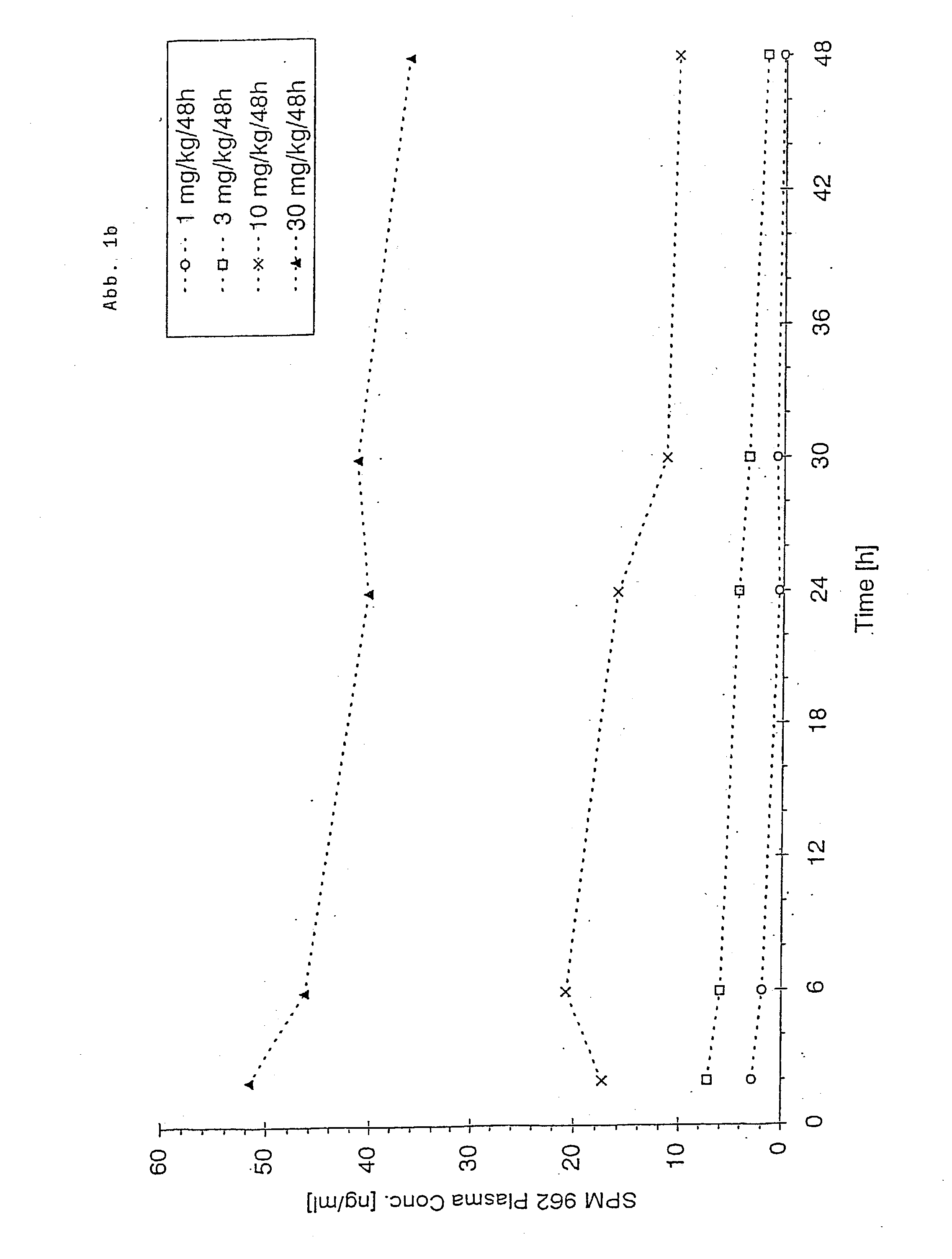
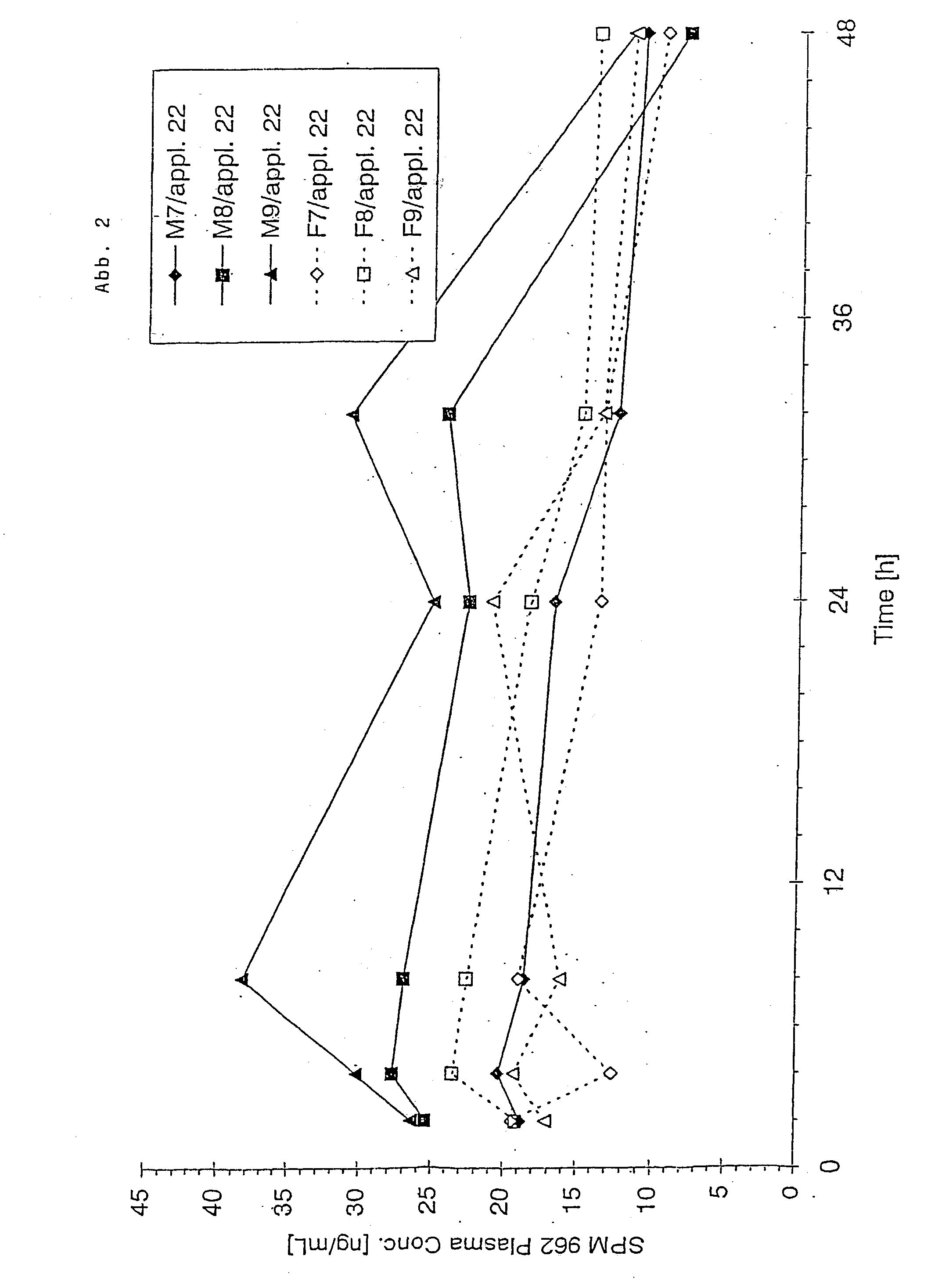
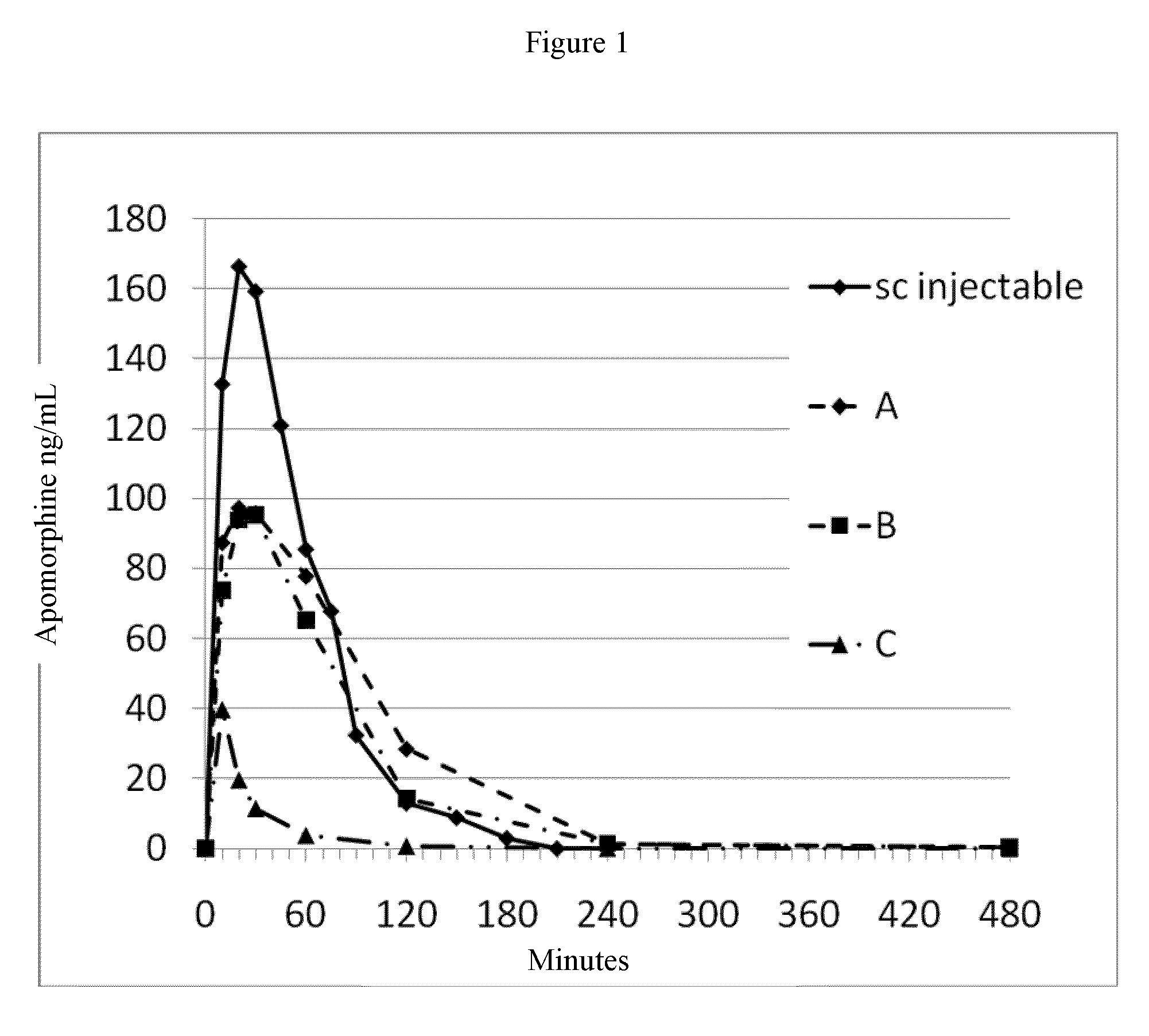
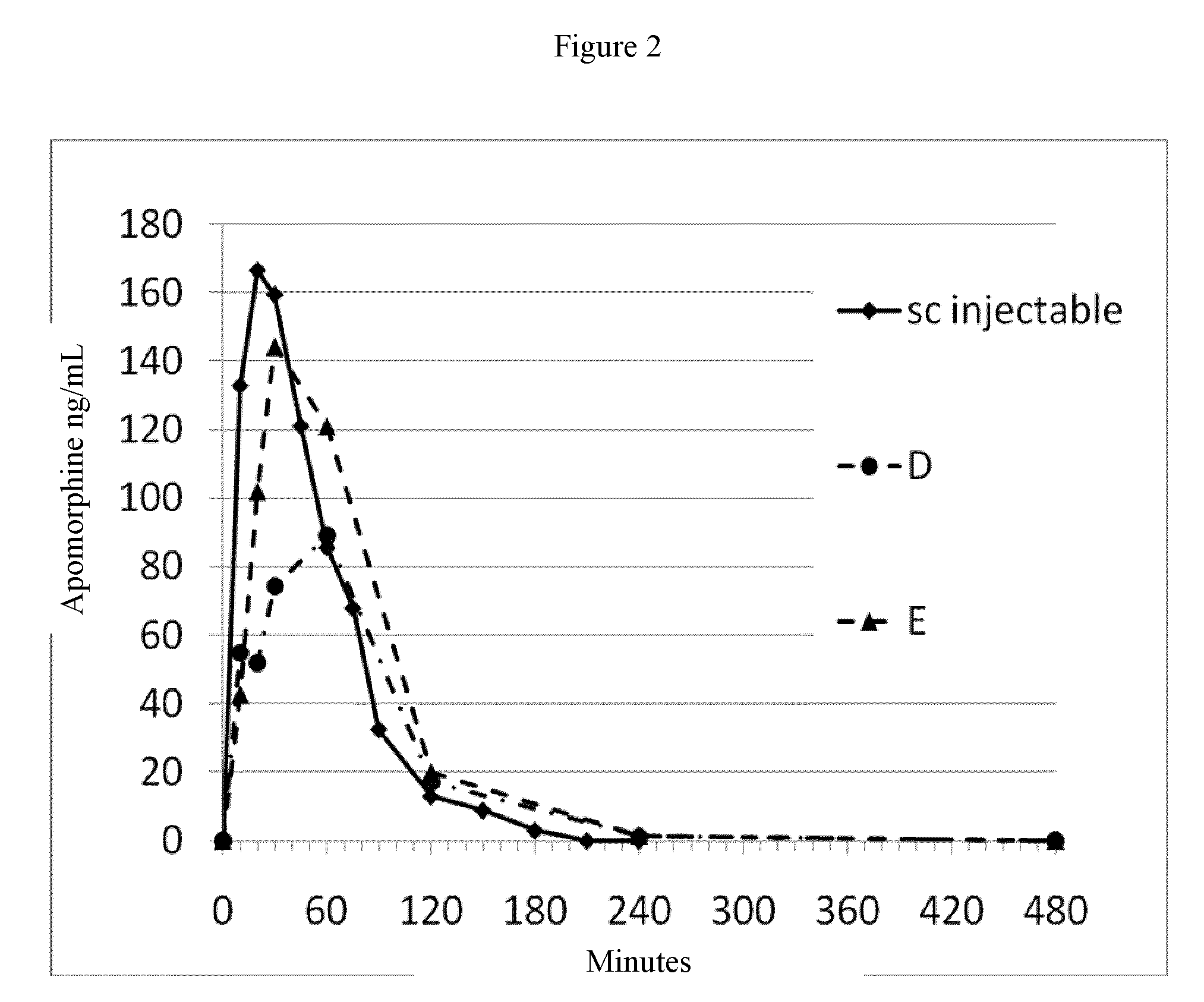
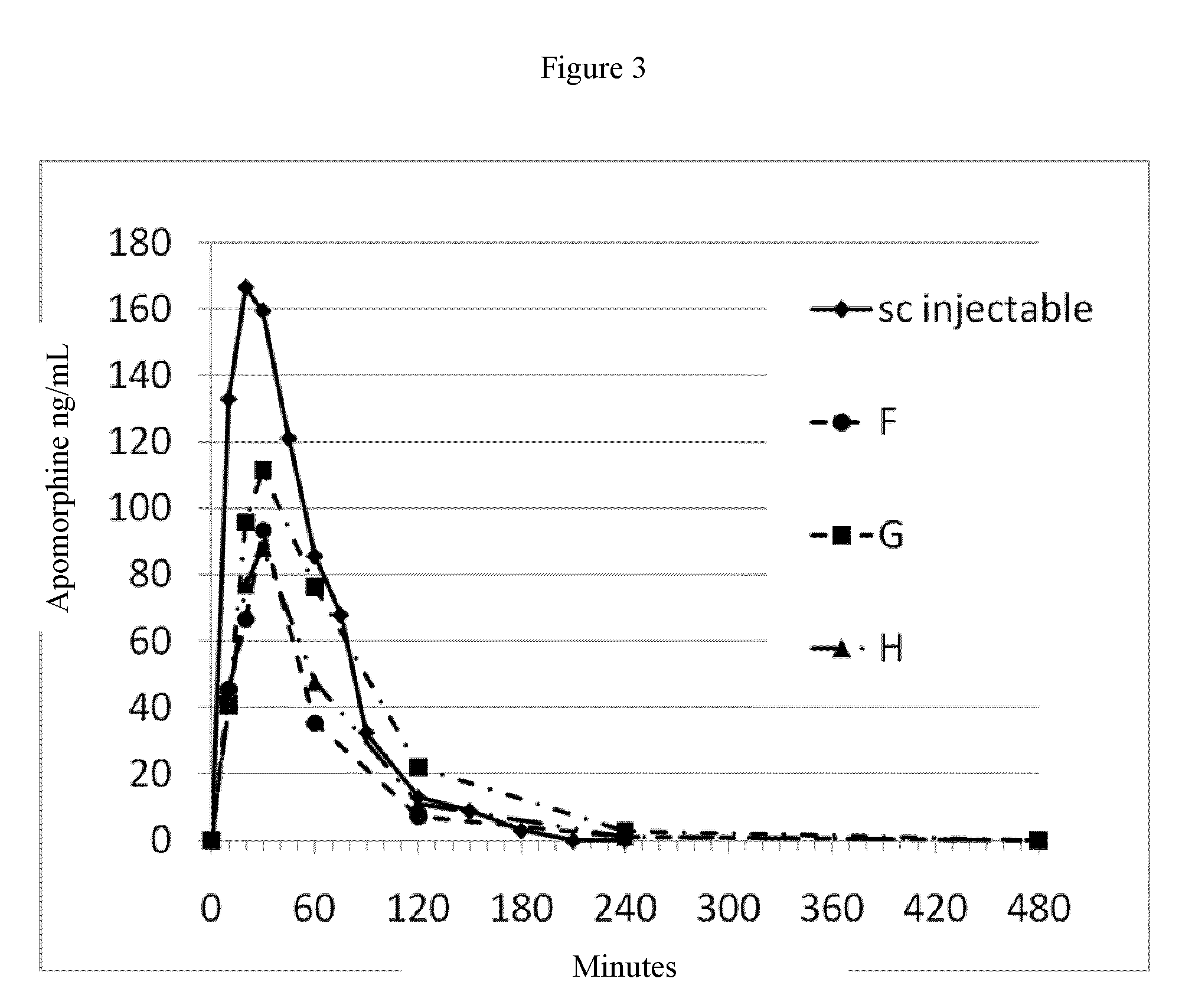
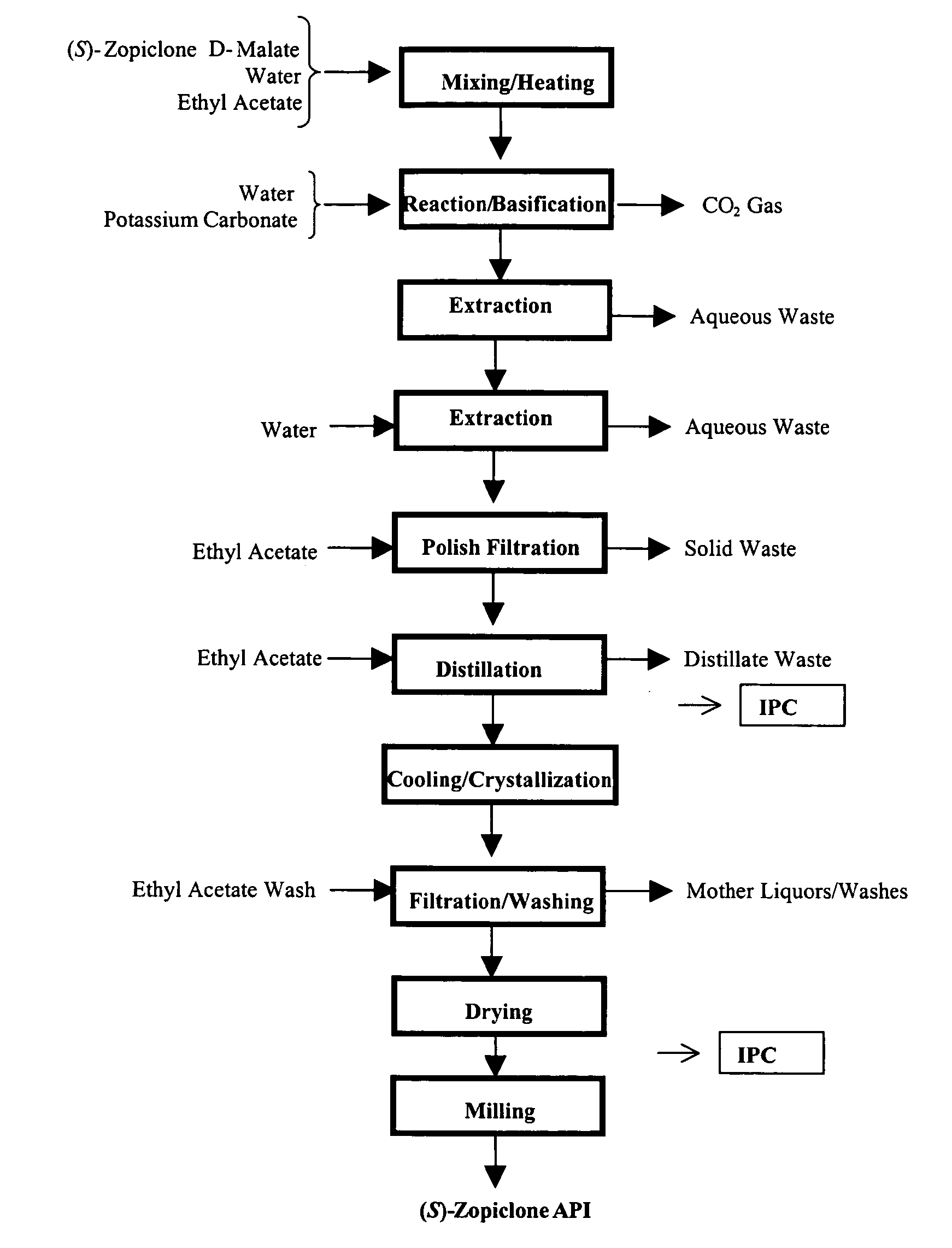
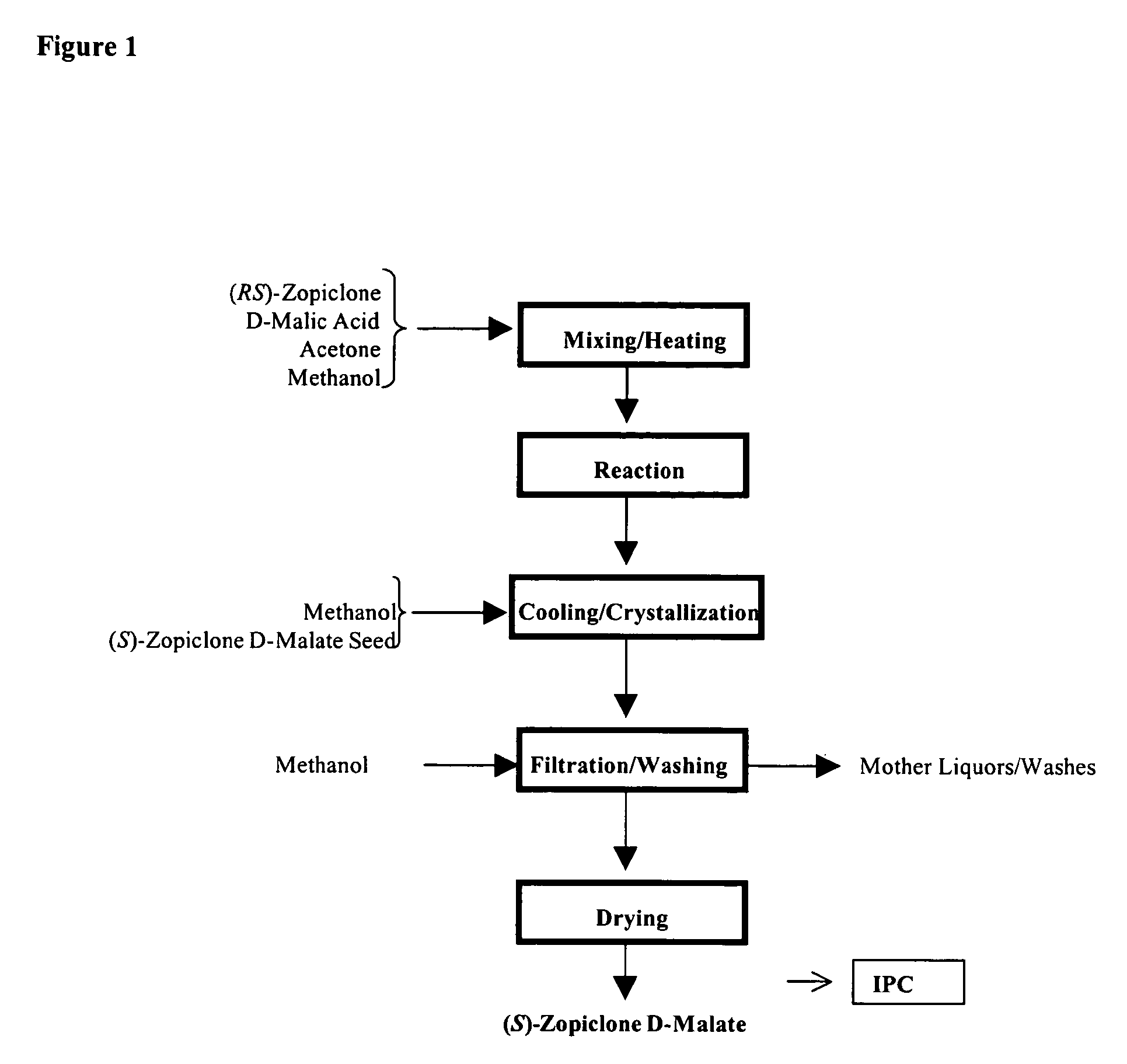
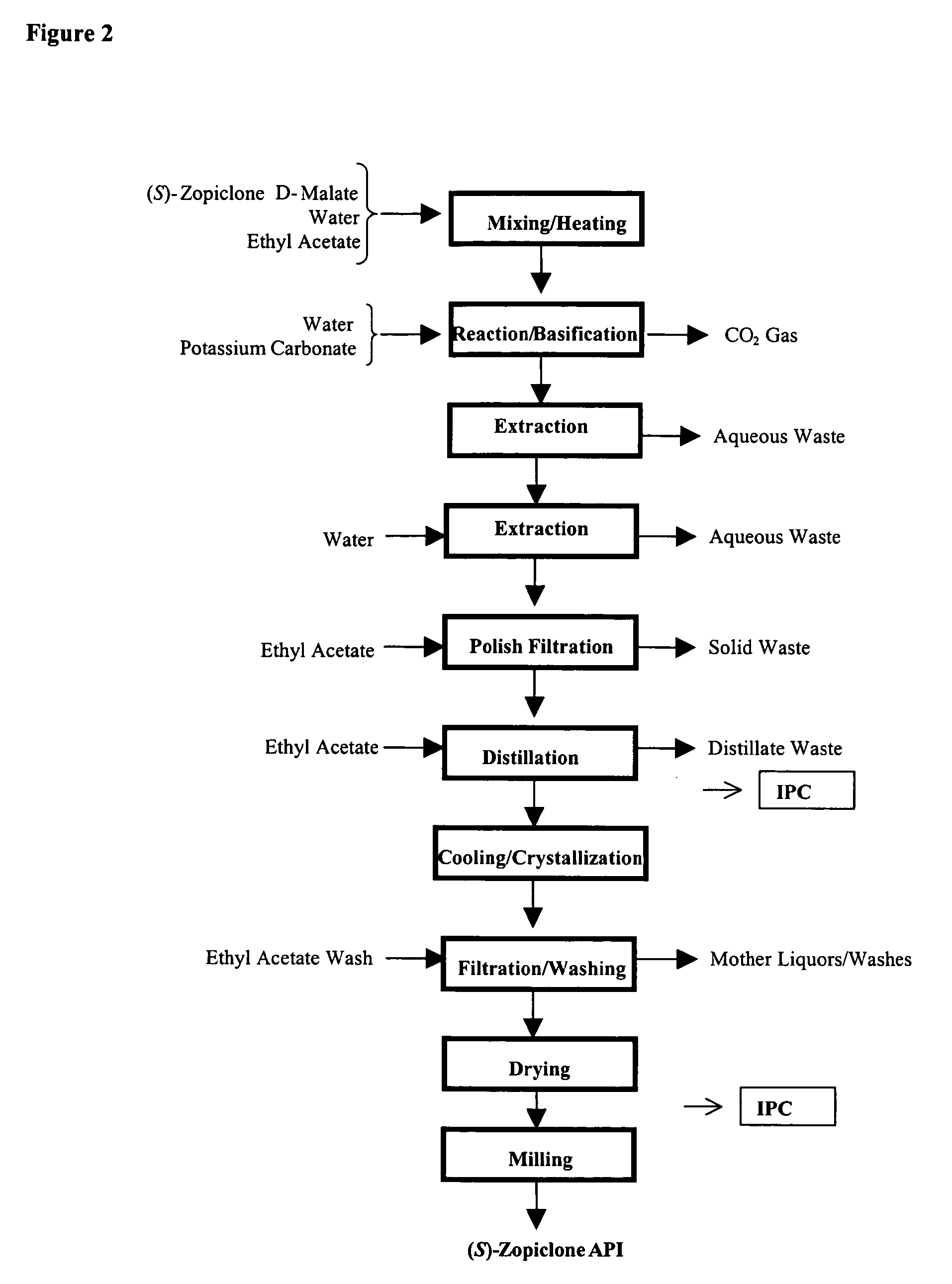
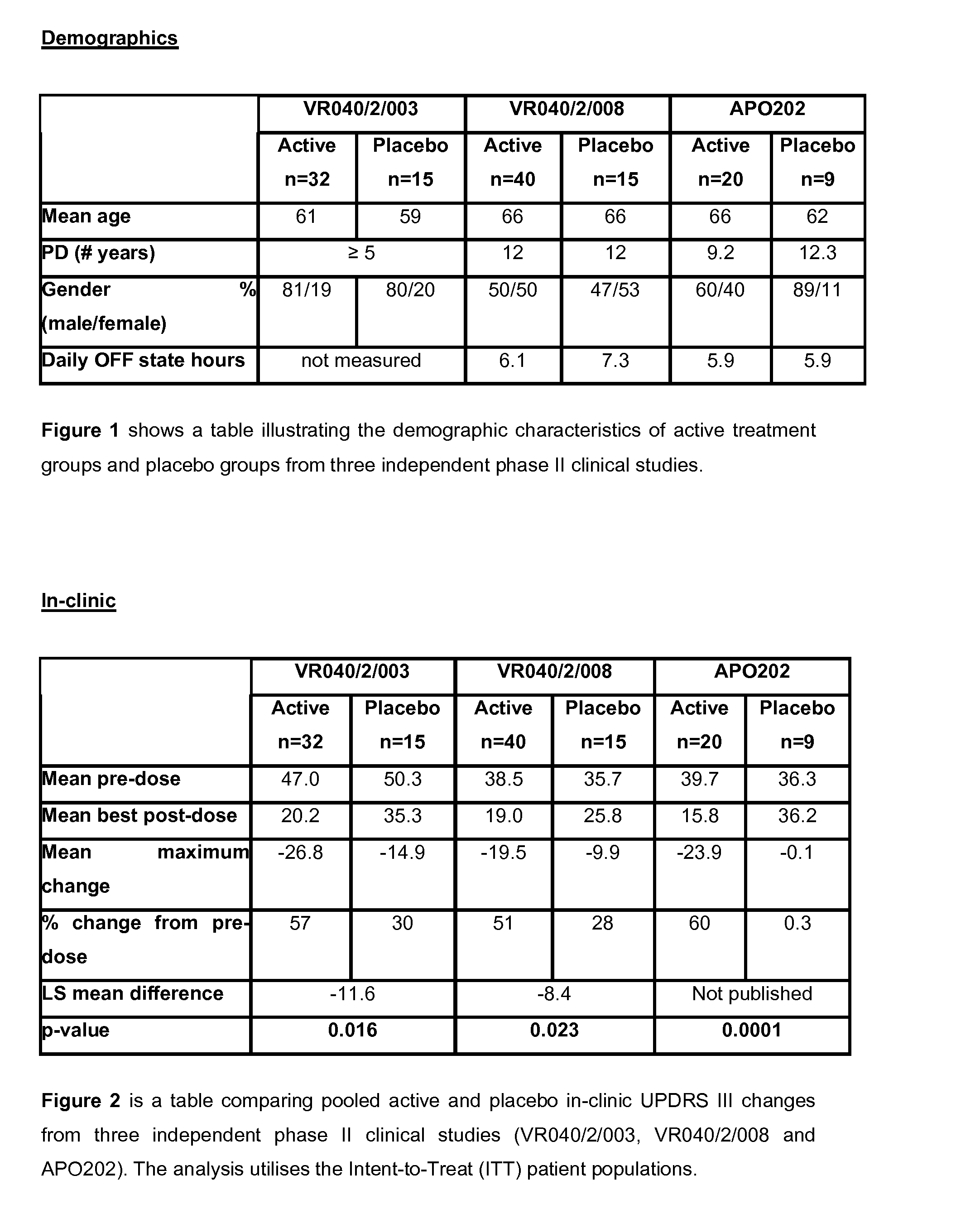
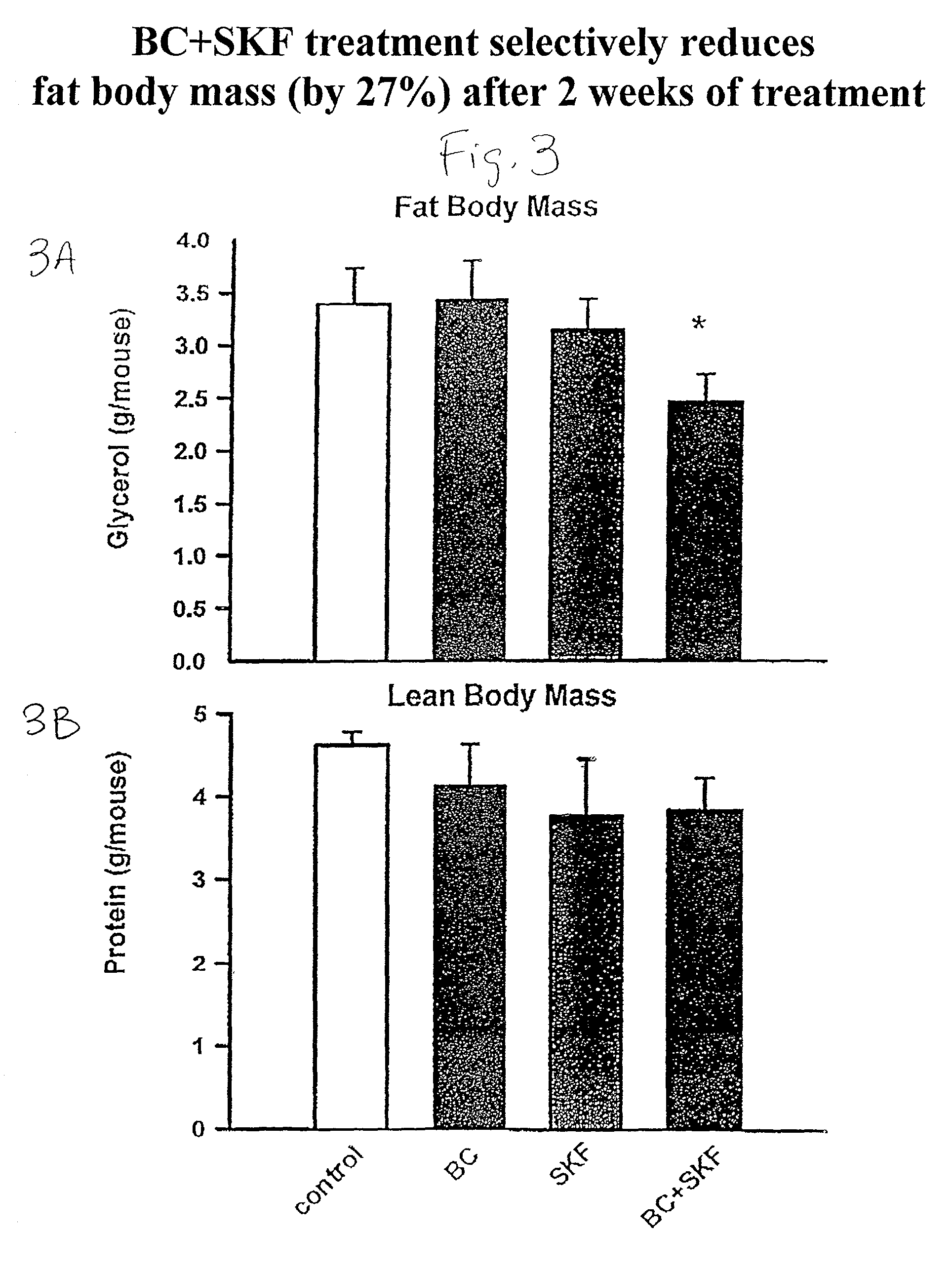
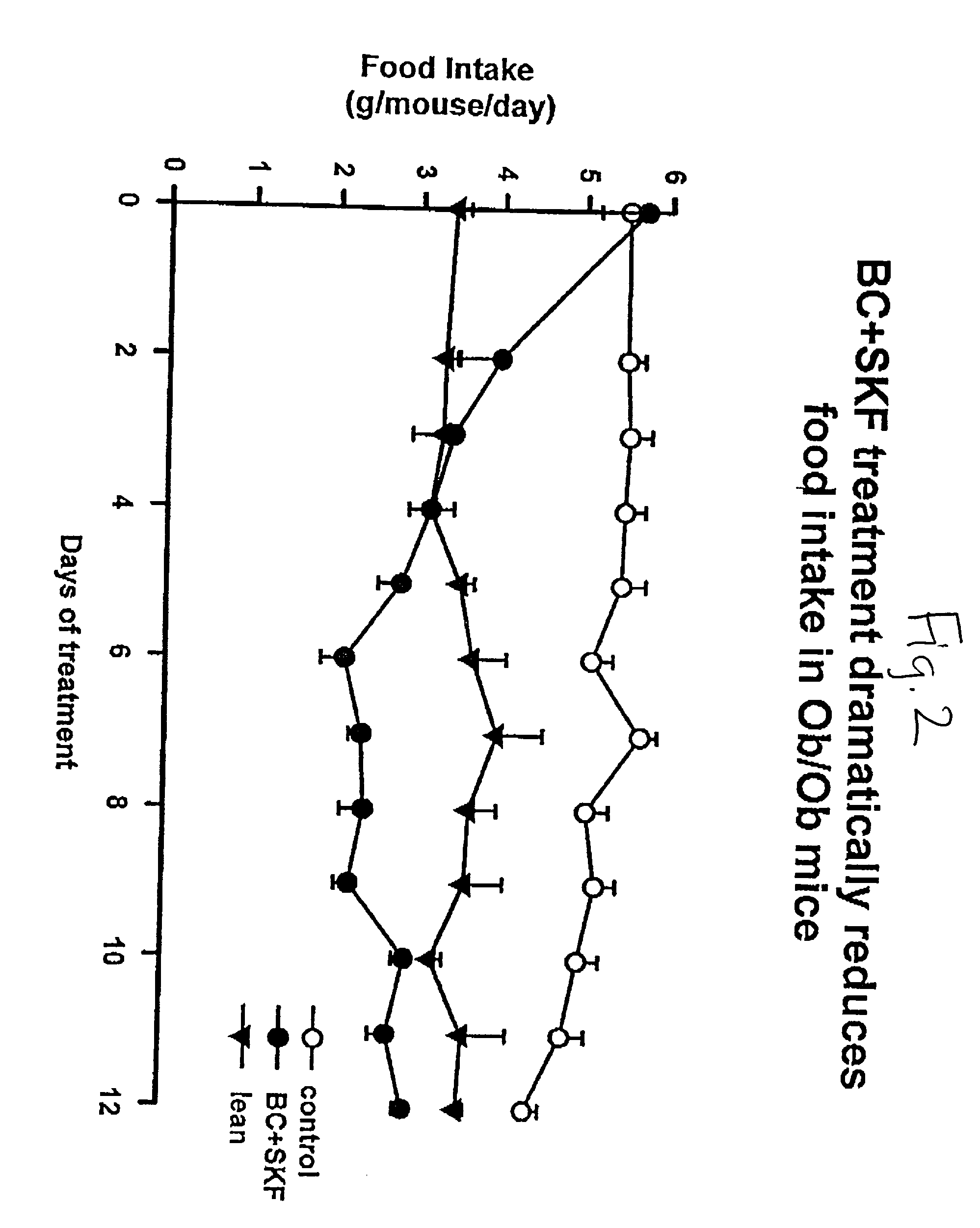
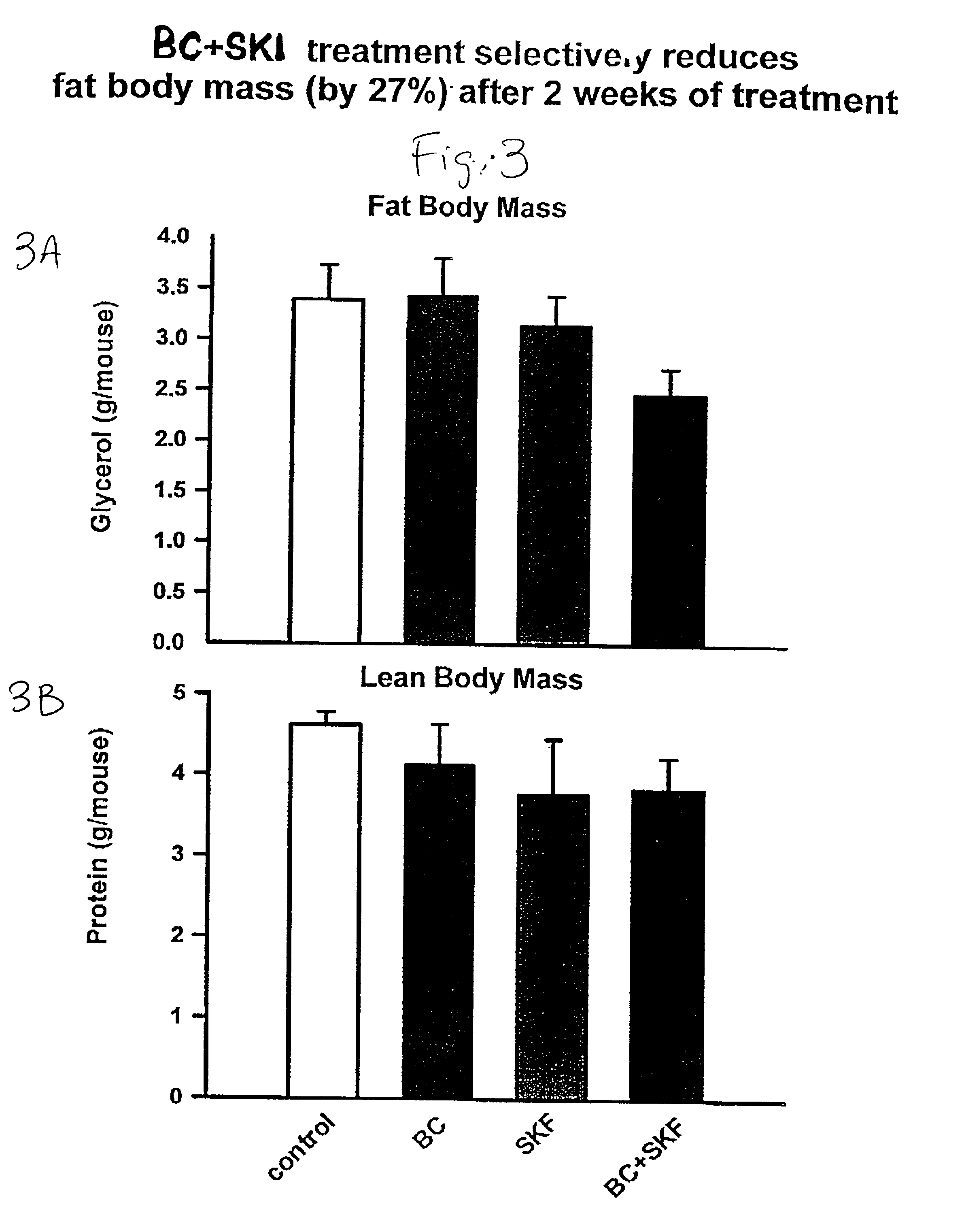
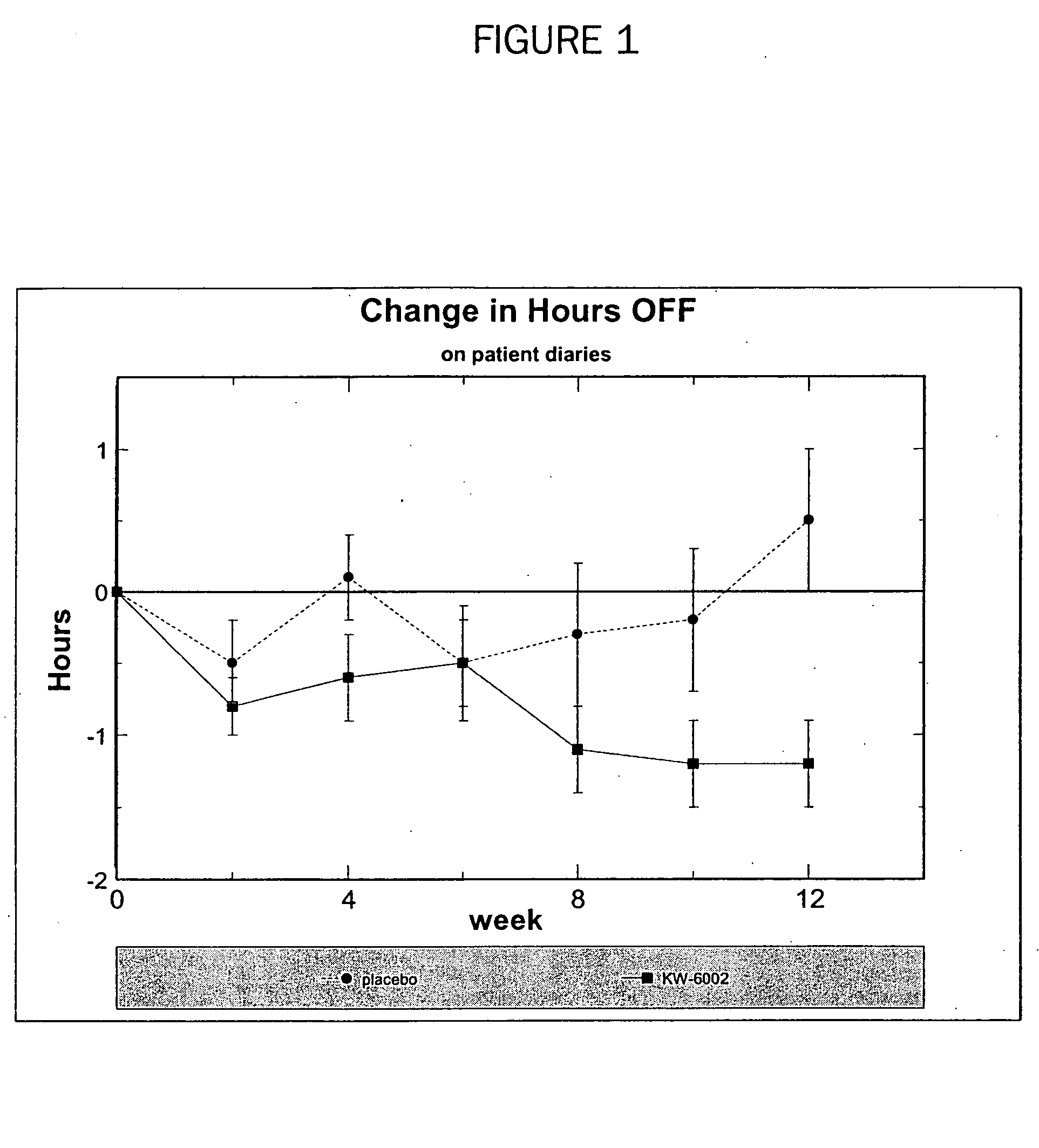
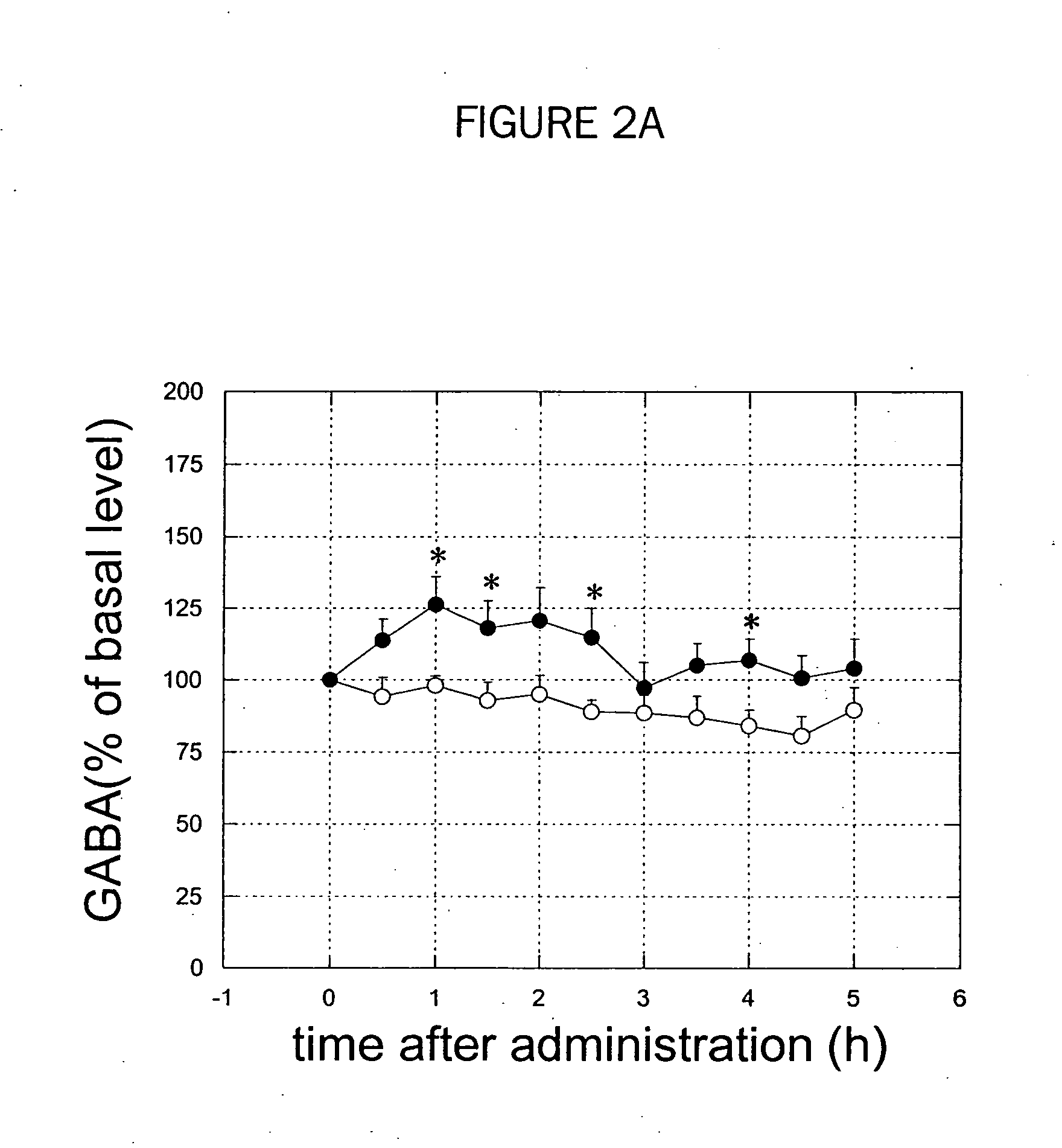
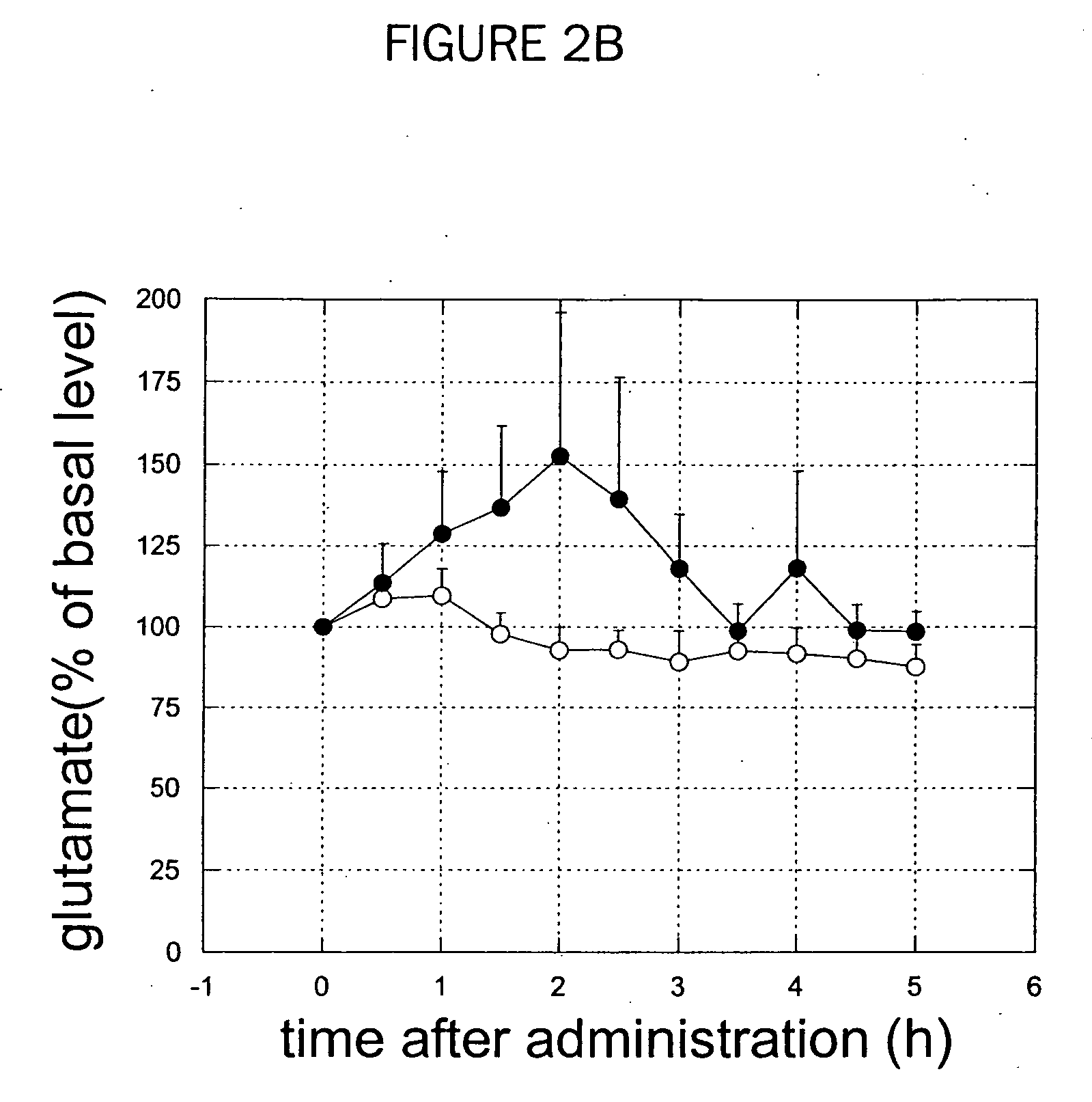
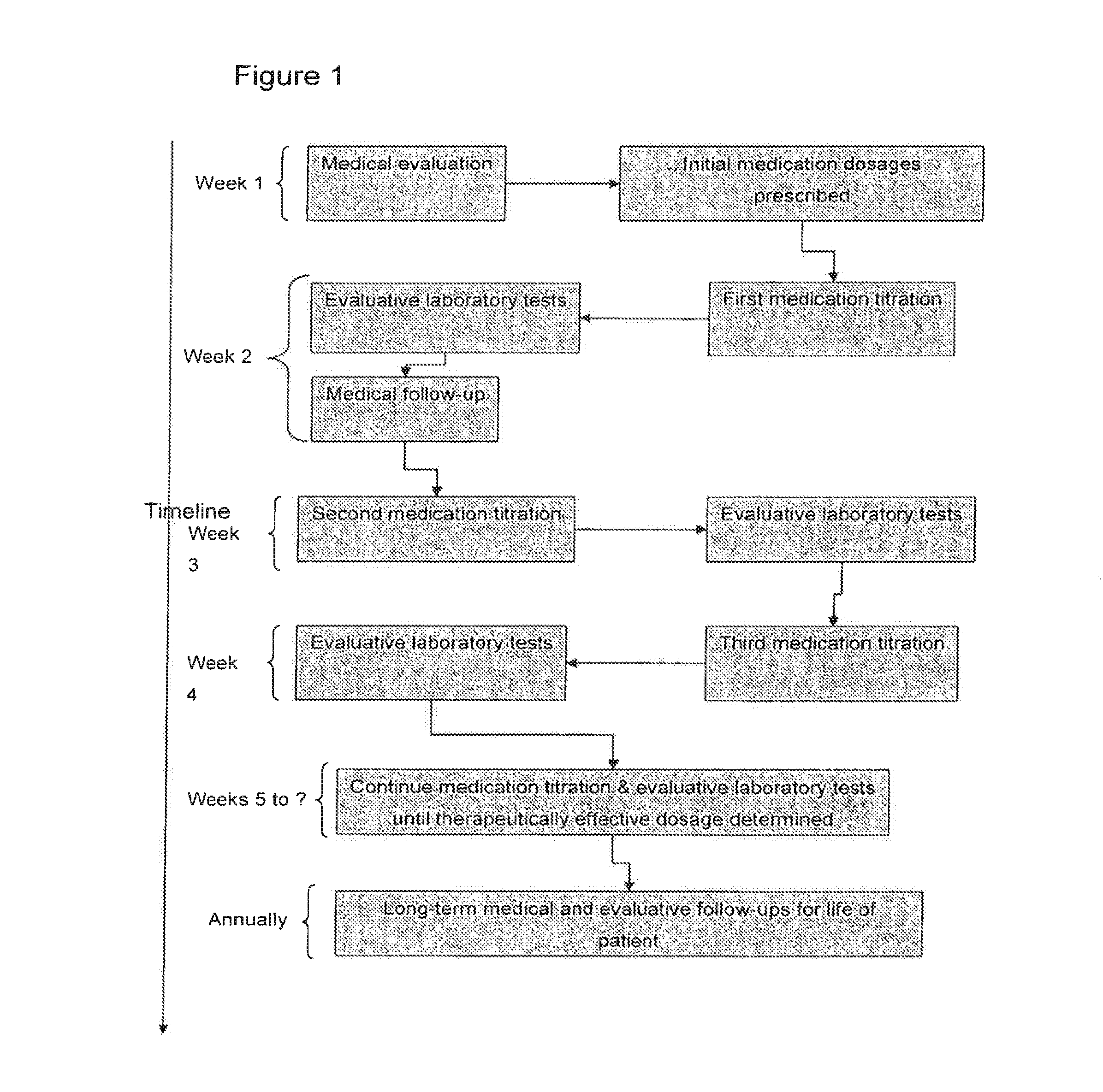
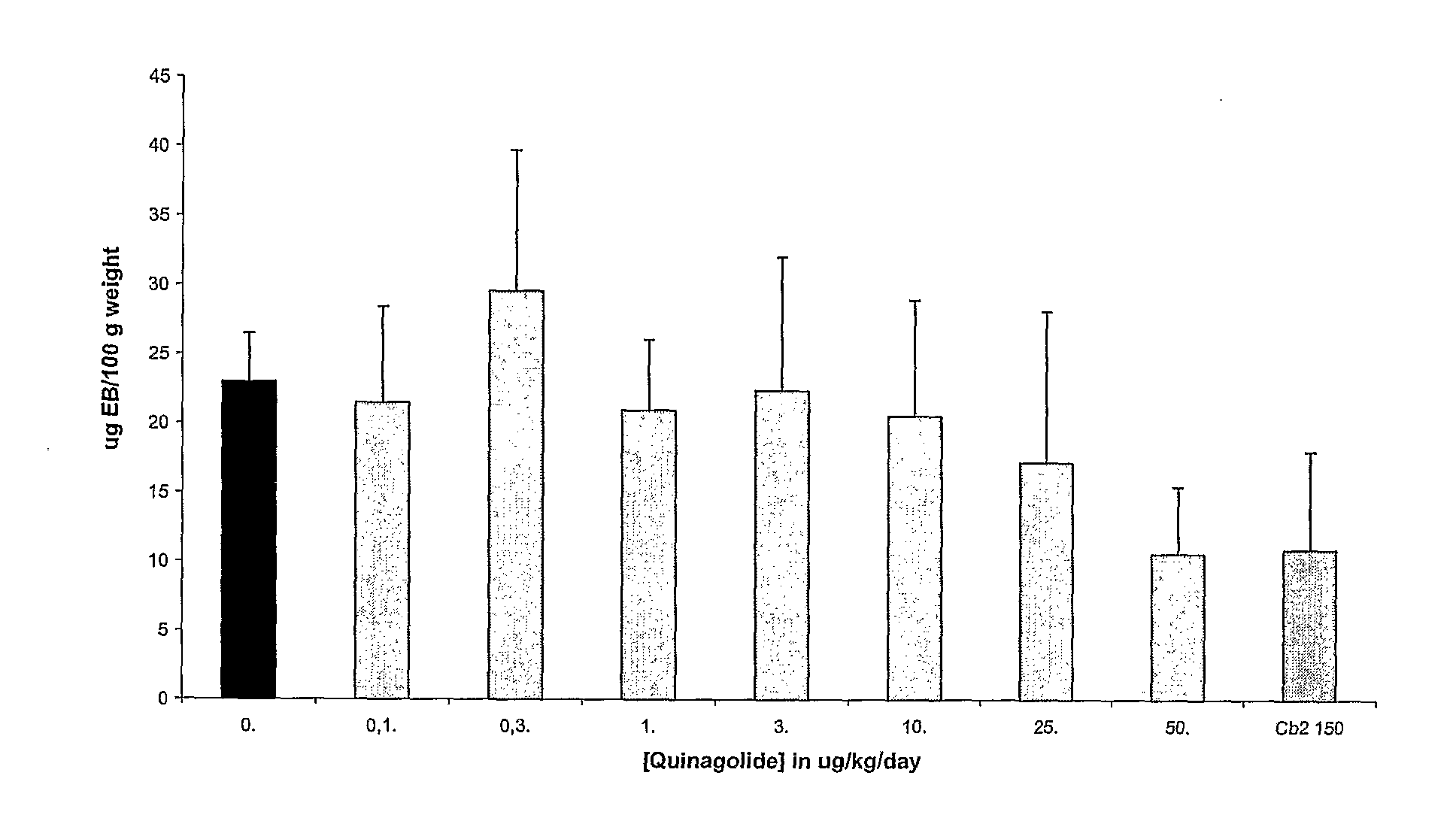
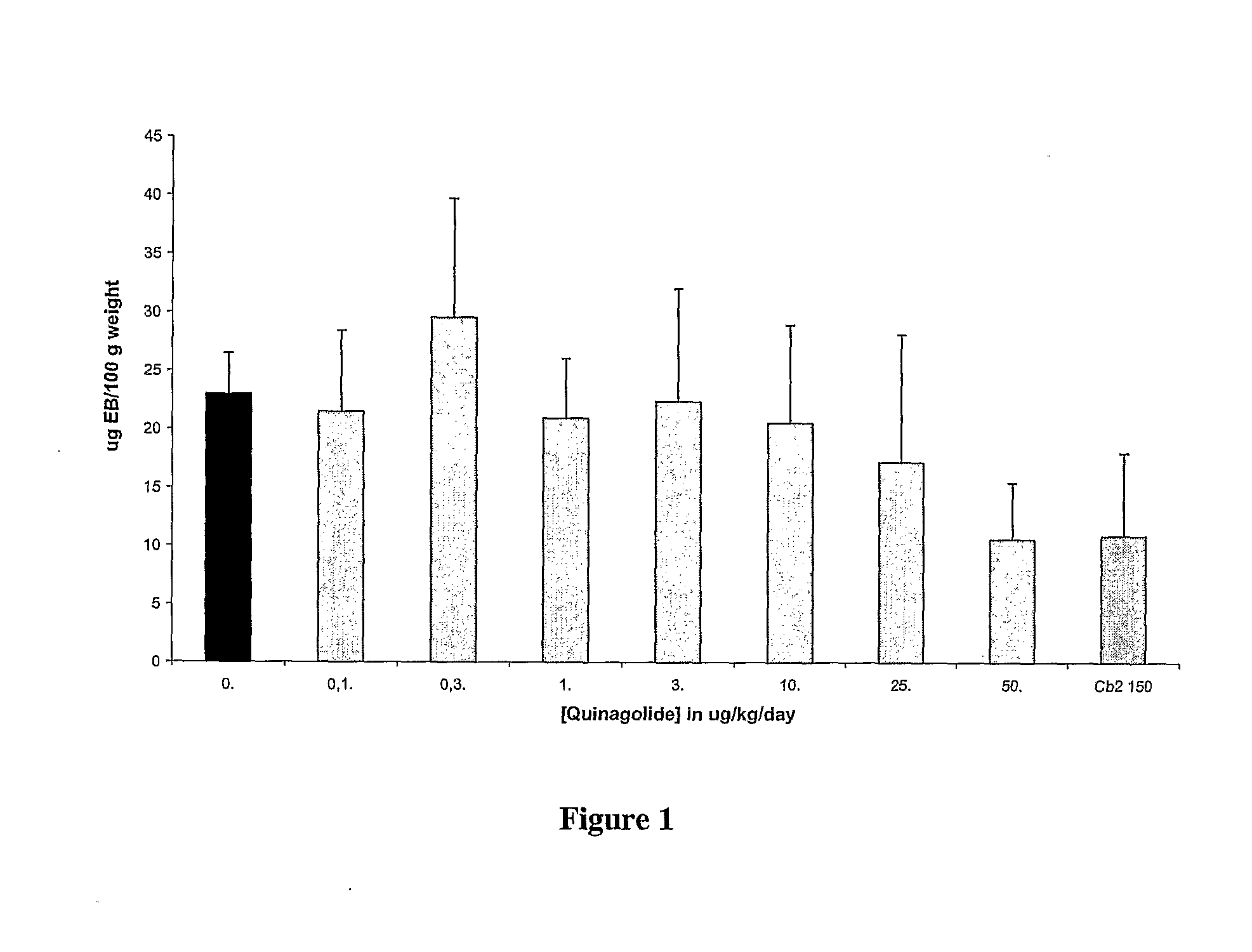
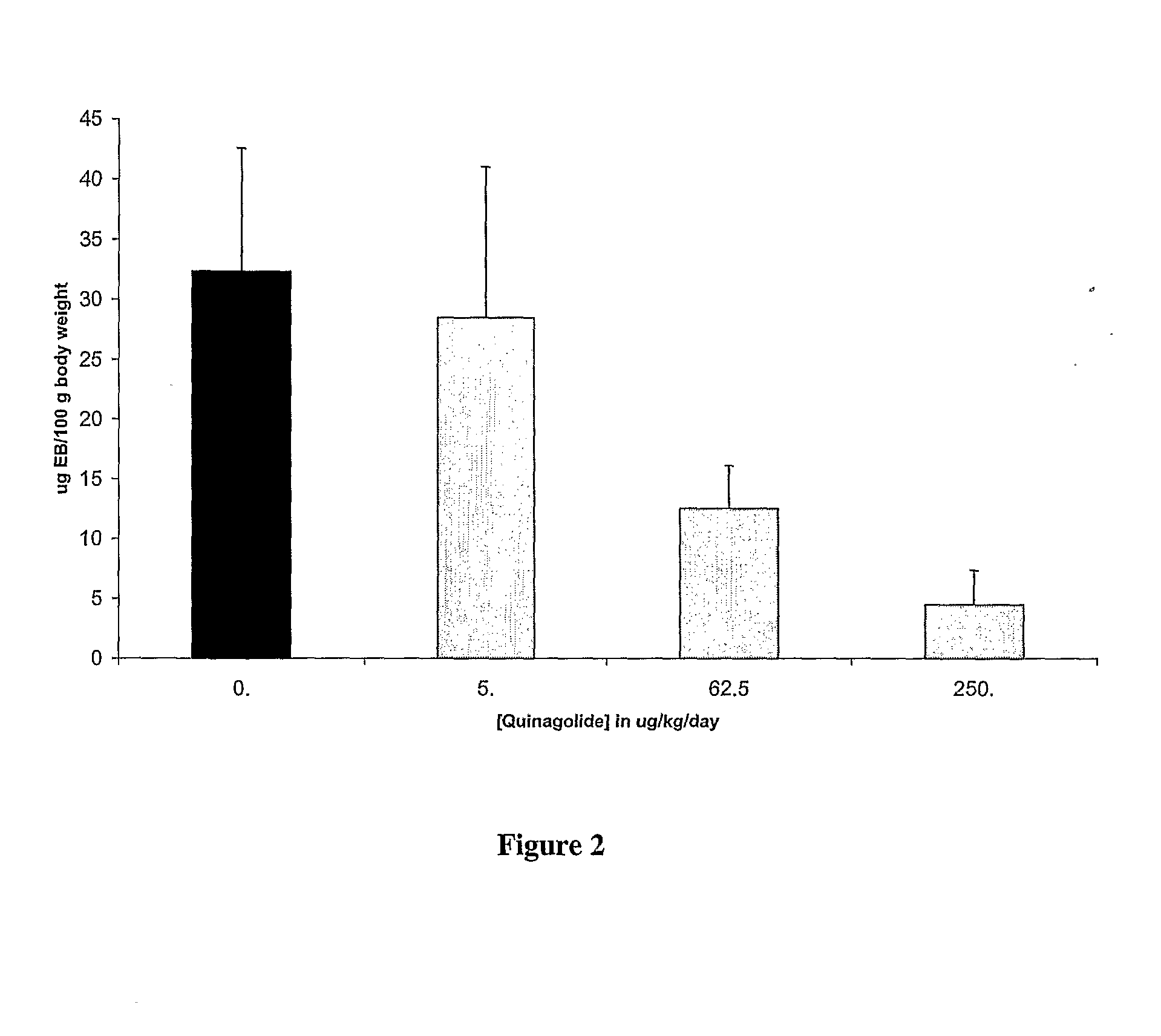
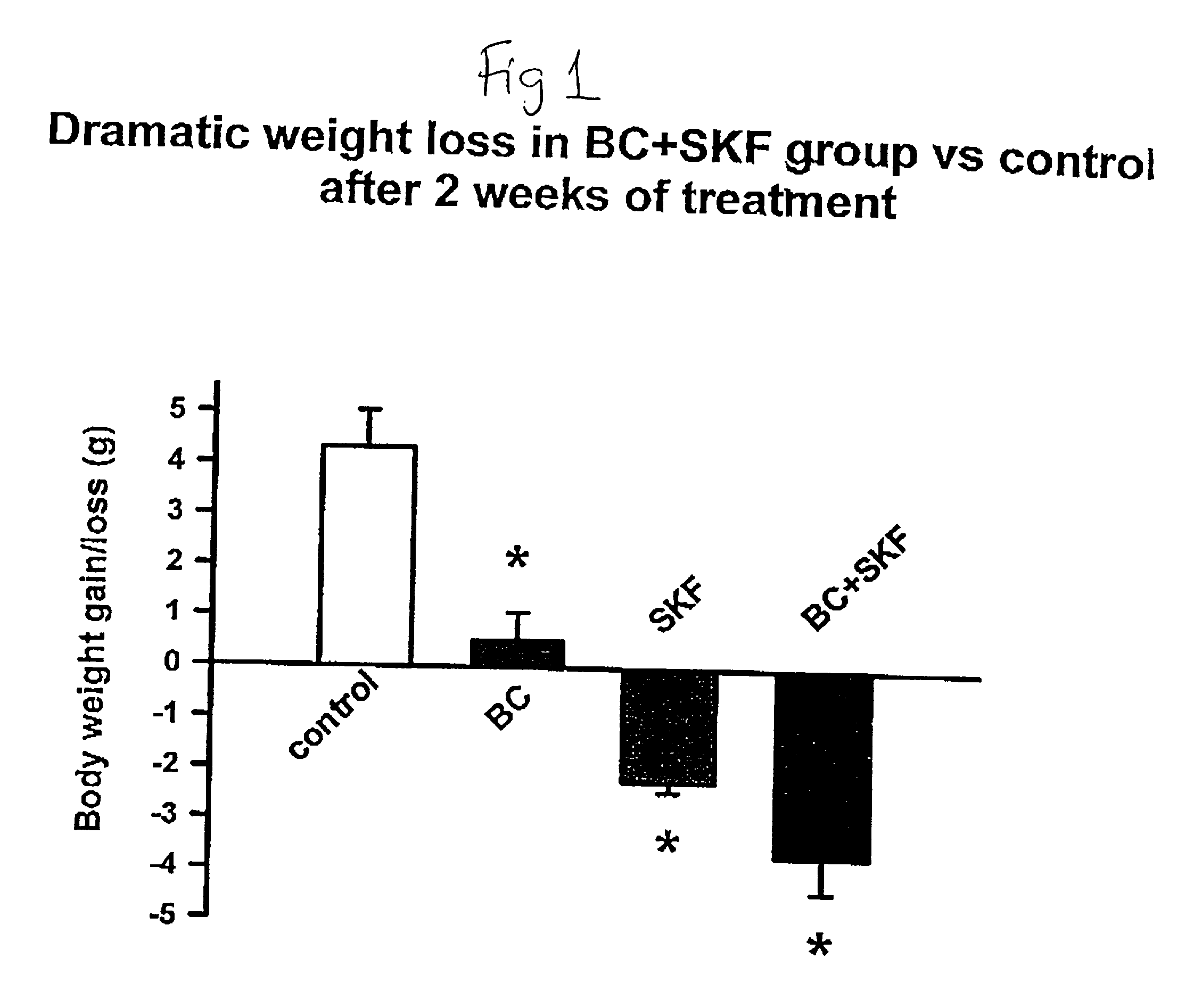Patents
Literature
94 results about "Dopaminergic Agonists" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
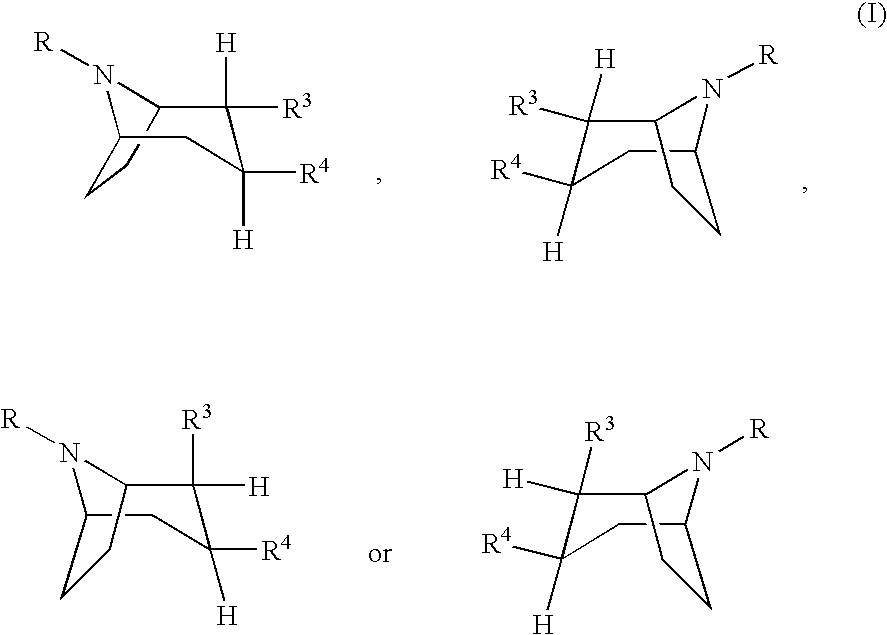
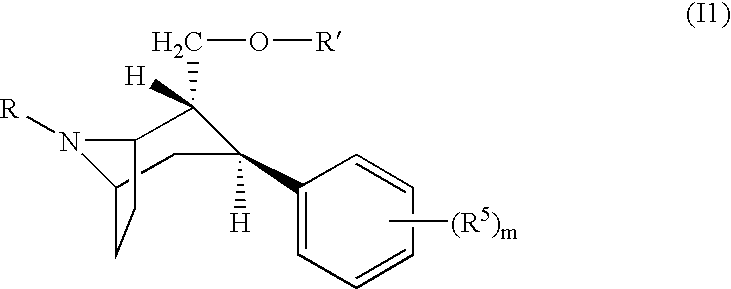
Dopamine agonist. A dopamine receptor agonist is a compound that activates dopamine receptors. Dopamine receptor agonists activate signaling pathways through trimeric G-proteins and β-arrestins, ultimately leading to changes in gene transcription.
Methods of treating metabolic syndrome using dopamine receptor agonists
InactiveUS20080200453A1Relieve symptomsEffective treatmentBiocideMetabolism disorderDiseaseVascular disease
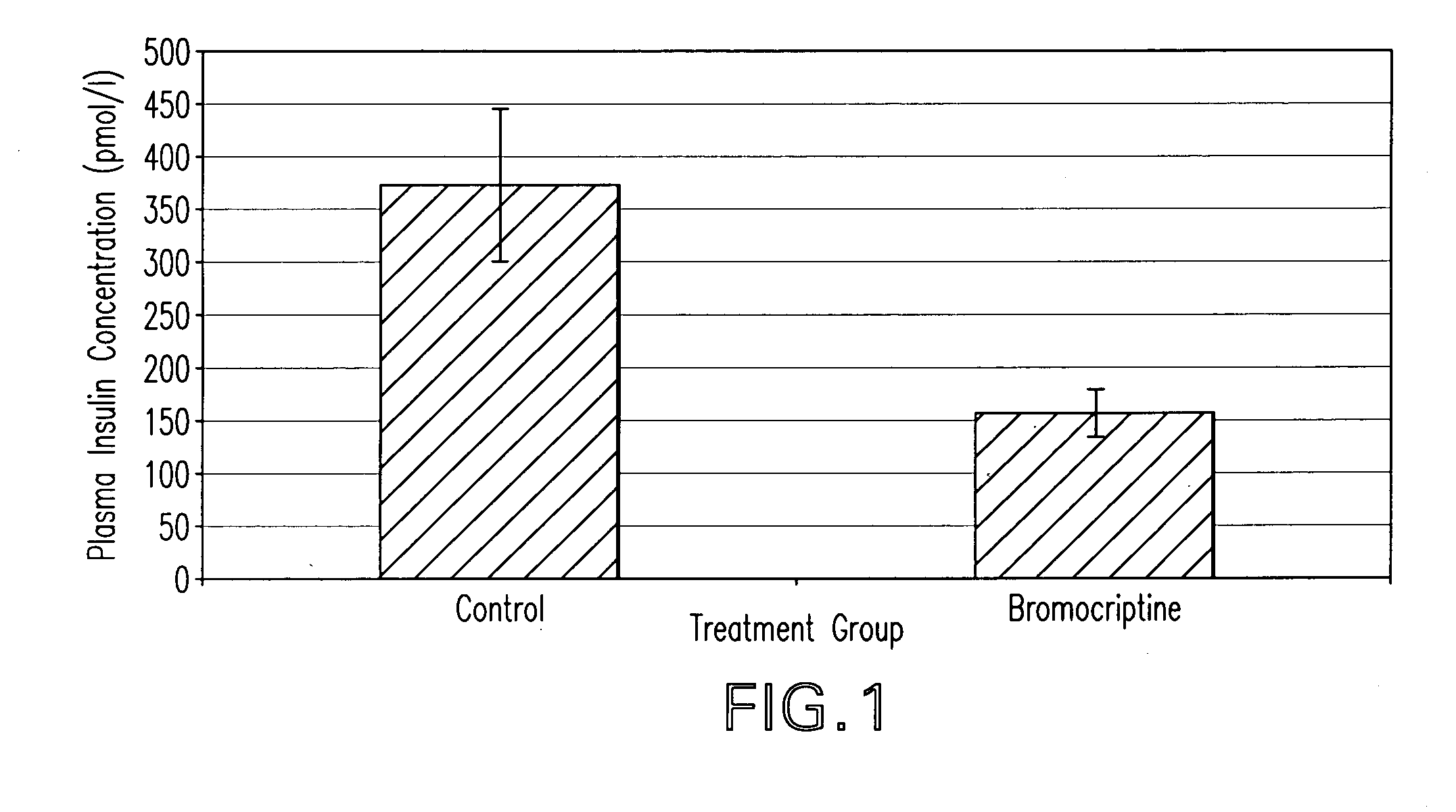
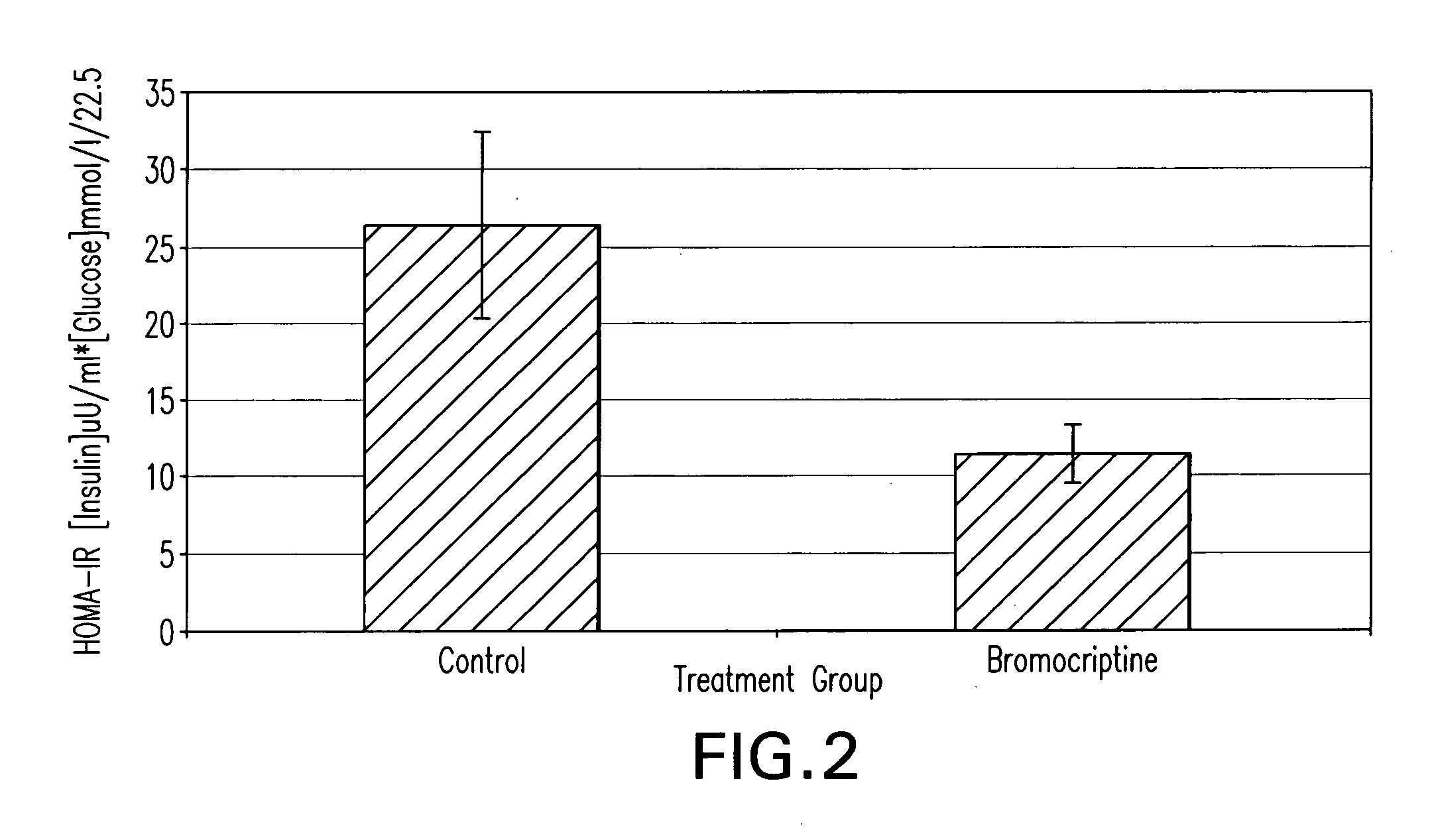
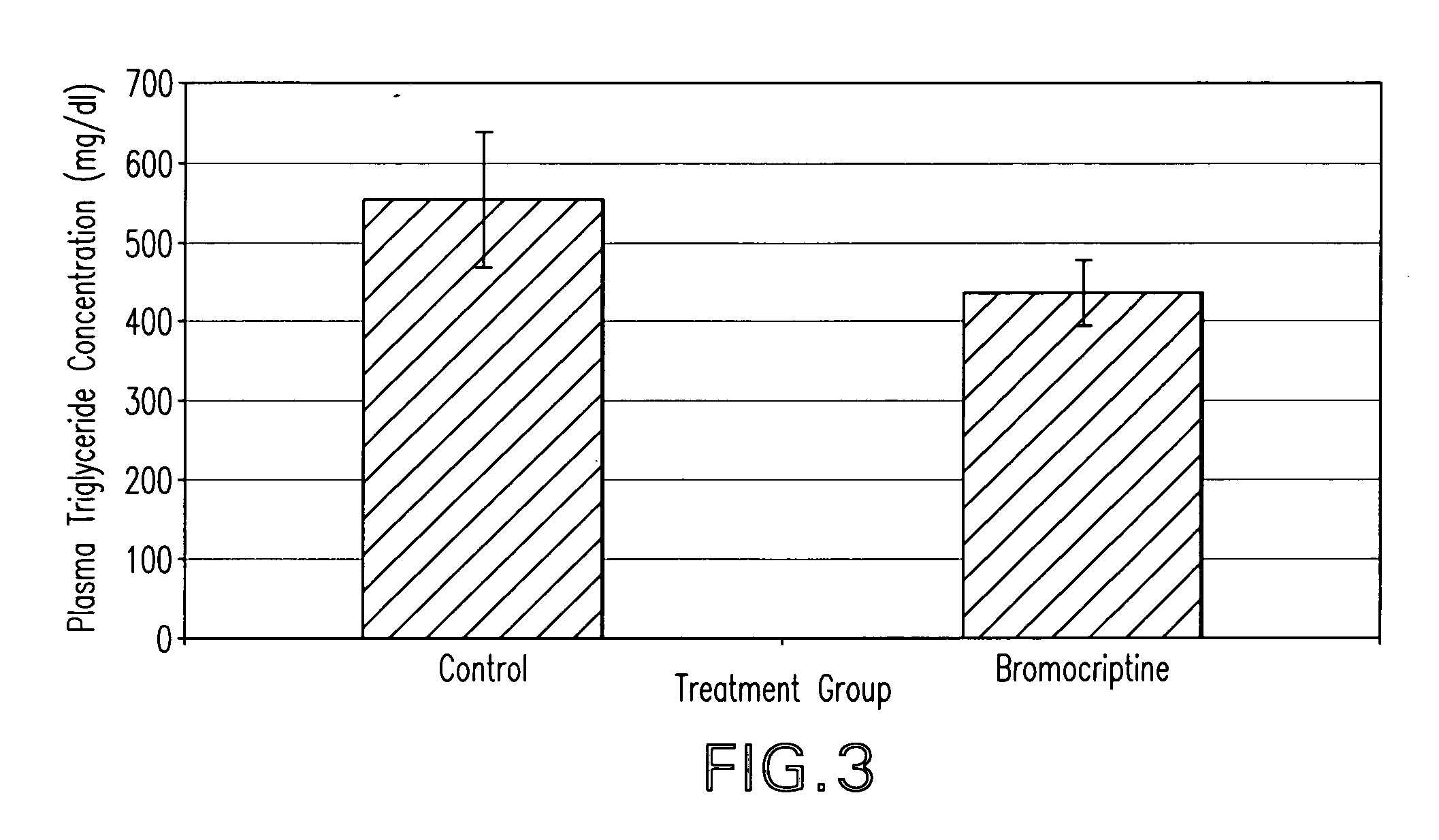
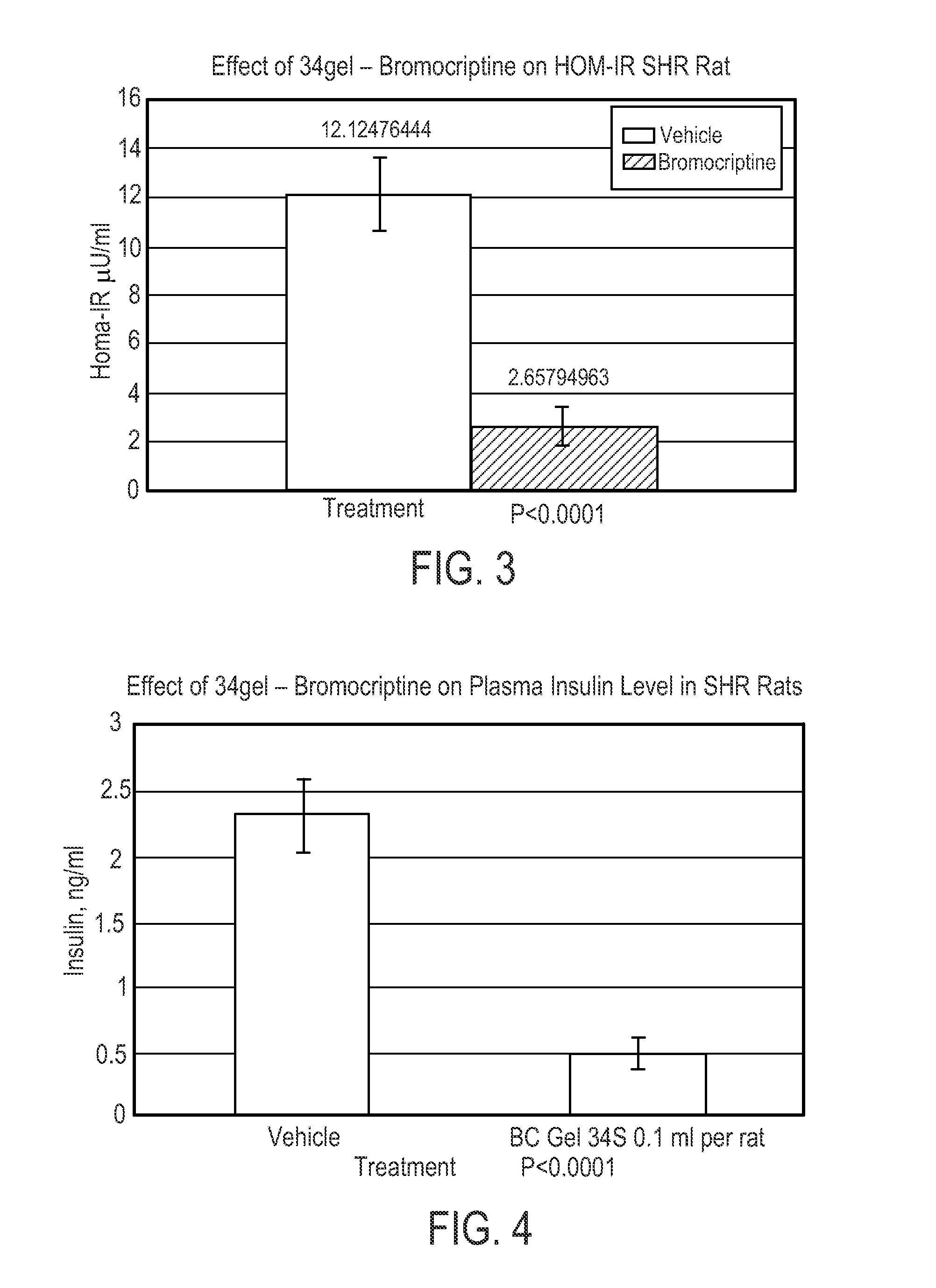
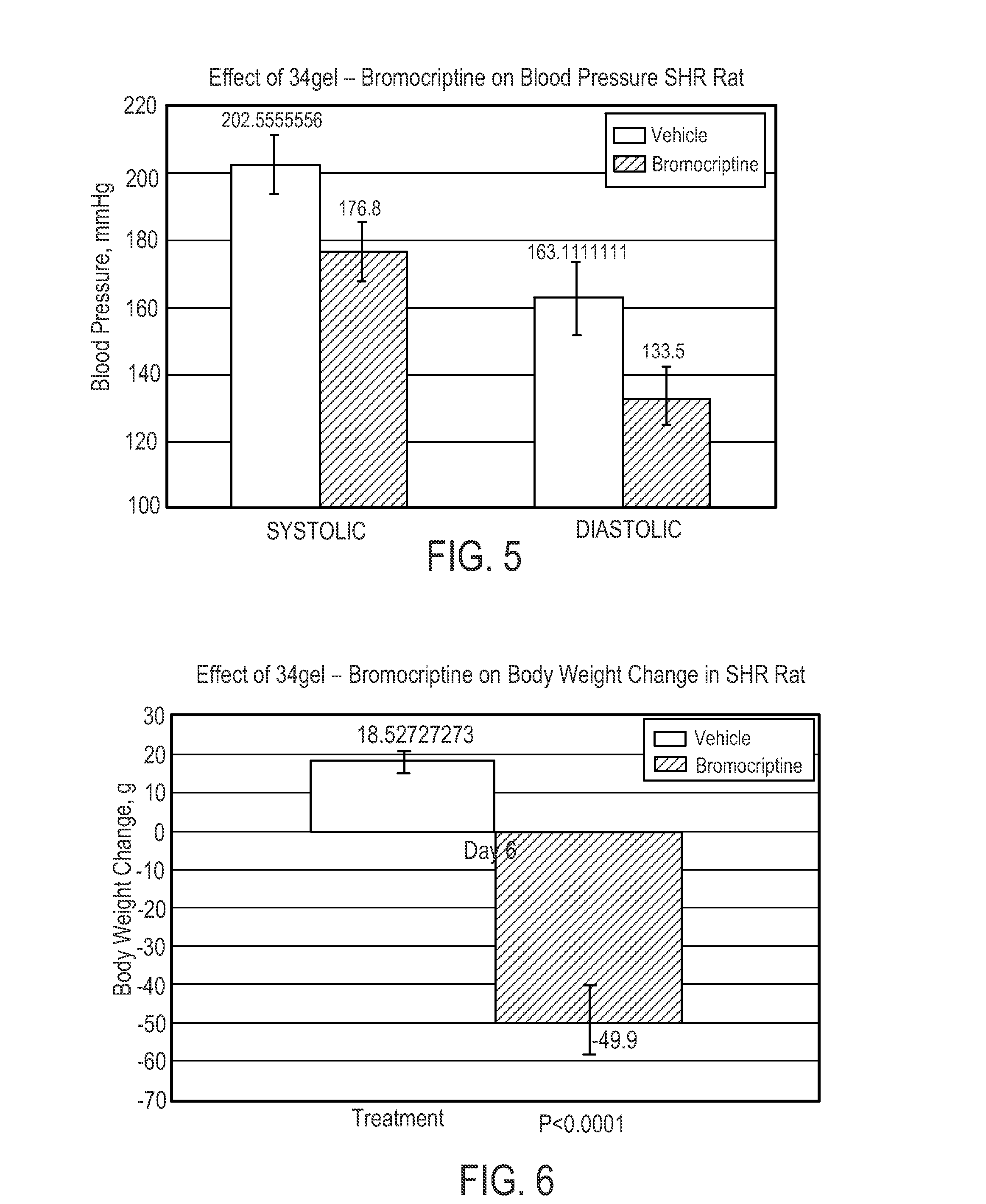
The present invention is directed to a method of simultaneously treating hypertension, hypertriglyceridemia, a pro-inflammatory state, a pro-coagulative state, and insulin resistance (with or without treating obesity or endothelial dysfunction), associated with or independent from Metabolic Syndrome, as well as vascular disease such as cardiovascular, cerebrovascular, or peripheral vascular disease comprising the step of administering to a patient suffering from such disorders a therapeutically effective amount of a central acting dopamine agonist. In one embodiment, the central acting dopamine agonist is bromocriptine, optionally combined with a pharmaceutically acceptable carrier.
Owner:VEROSCI
Implantable polymeric device for sustained release of dopamine agonist
The present invention provides compositions, methods, and kits for treatment of Parkinson's disease and other conditions for which treatment with a dopamine agonist is therapeutically beneficial. The invention provides a biocompatible nonerodible polymeric device which releases dopamine agonist continuously with generally linear release kinetics for extended periods of time. Dopamine agonist is released through pores that open to the surface of the polymeric matrix in which it is encapsulated. The device may be administered subcutaneously to an individual in need of continuous treatment with dopamine agonist.
Owner:TITAN PHARMA
Parenteral formulations of dopamine agonists
InactiveUS20100035886A1Reducing elevated cardiovascular-related inflammatory factorReducing elevated cardiovascular-related inflammatory factorsBiocideMetabolism disorderParenteral nutritionParenteral Dosage Form
This invention relates to stable pharmaceutical compositions for parenteral administration comprising dopamine agonists and peripheral acting agents useful for treatment of metabolic disorders or key elements thereof. The parenteral dosage forms exhibit long stable shelf life and distinct pharmacokinetics.
Owner:VEROSCI
Dopamine-agonist combination therapy for improving sleep quality
InactiveUS20050267176A1Useful in treatmentOrganic active ingredientsBiocideLimb dyskinesiaSedative agent
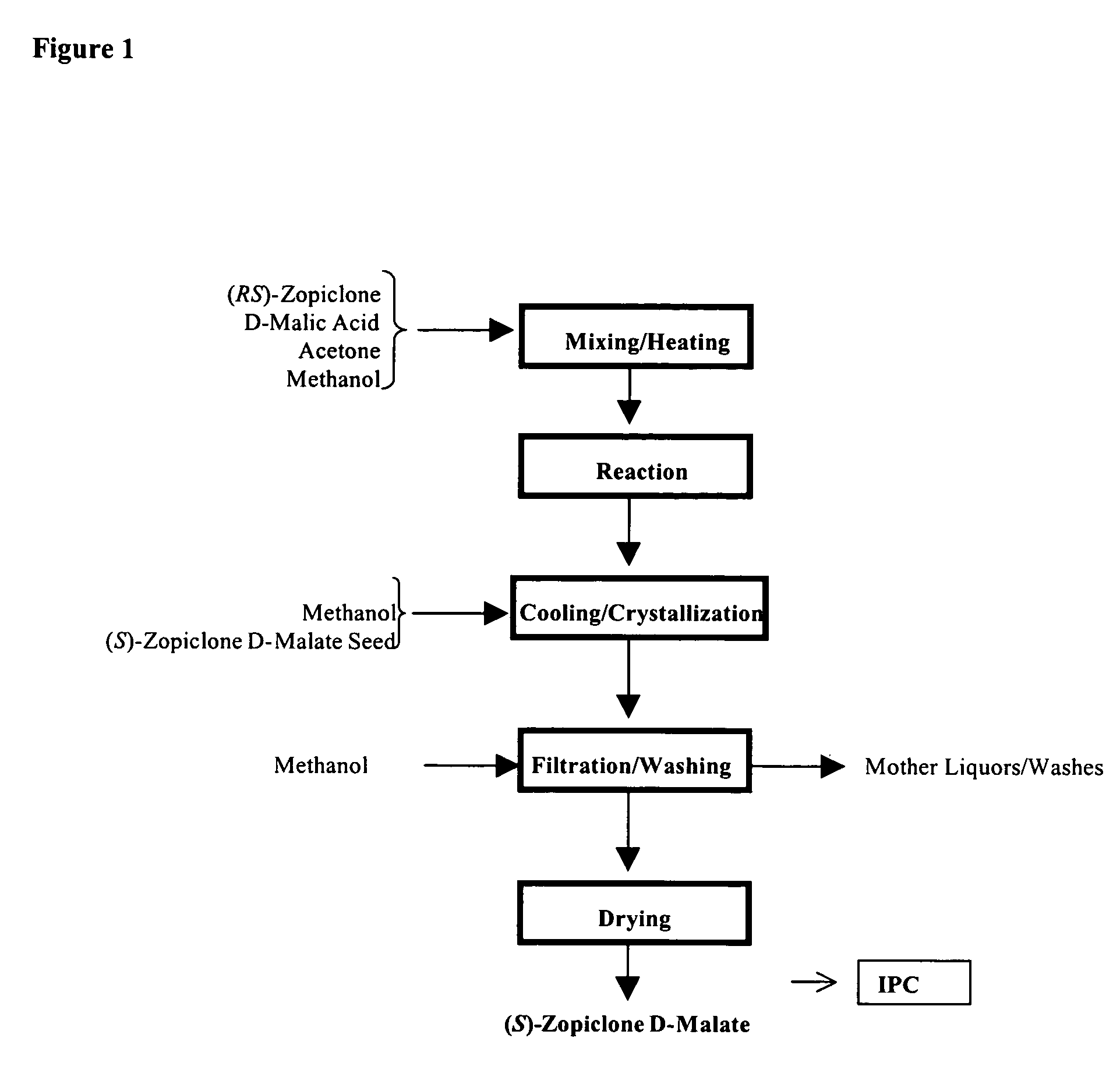
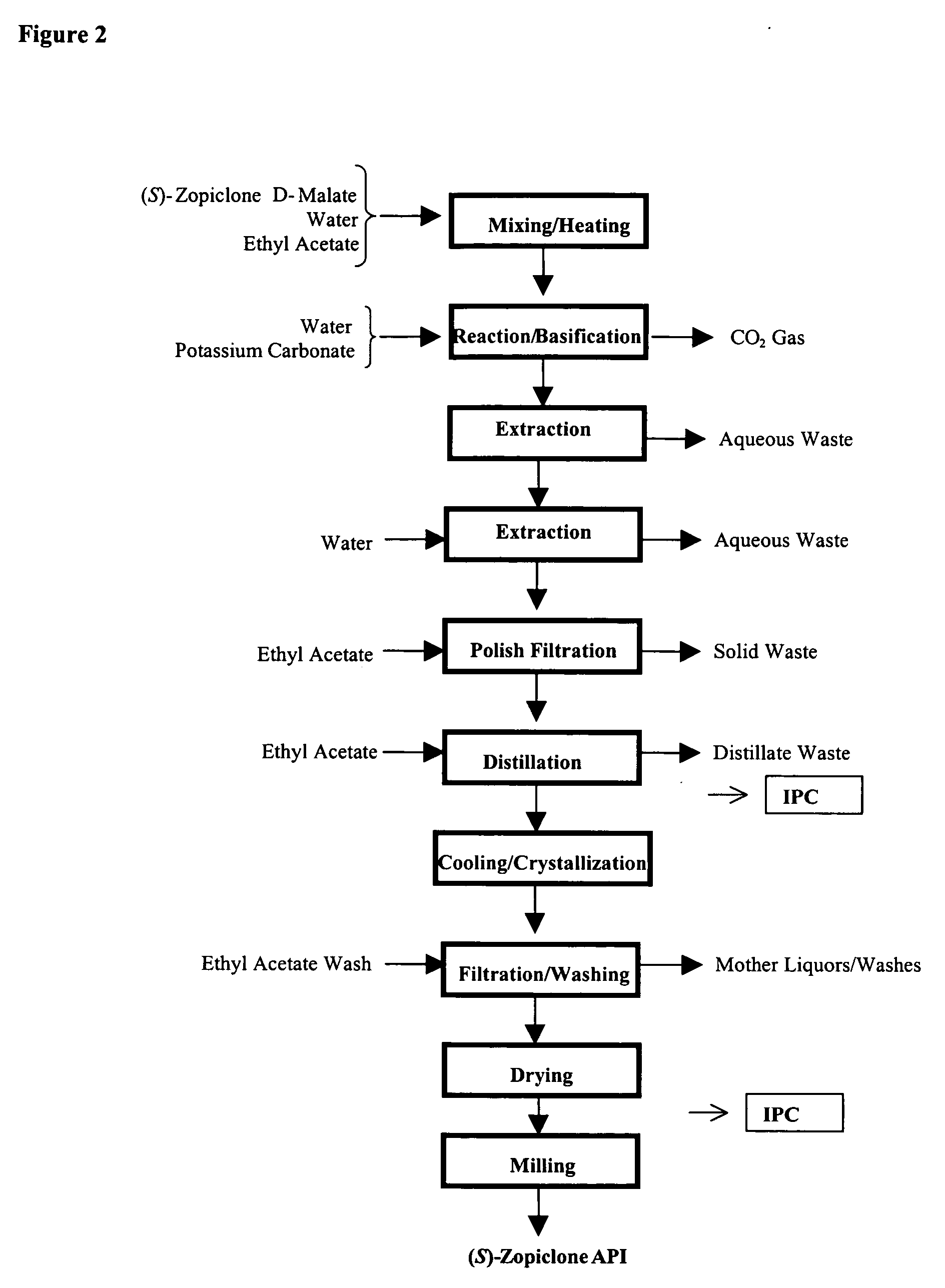
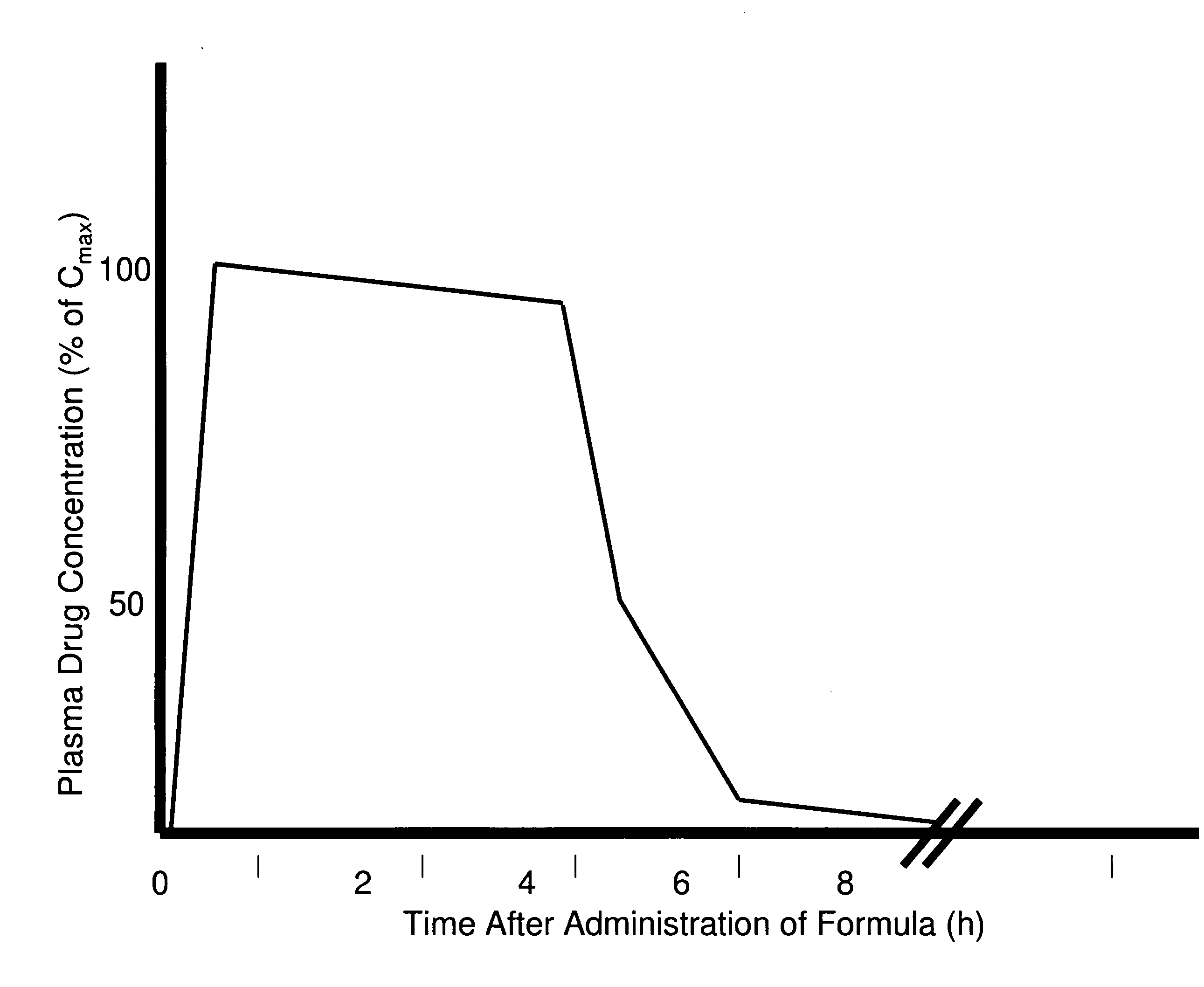
The present invention generally relates to pharmaceutical compositions comprising a dopamine agonist and sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone. The pharmaceutical compositions of the invention are useful in the treatment of restless-leg syndrome and periodic-limb-movement disorder, as well as various sleep disorders. In addition, the present invention relates to a method of treating a patient suffering from restless-leg syndrome, periodic-limb-movement disorder, a sleep abnormality, or insomnia, comprising coadministering a therapeutically effective amount of a dopamine agonist and a therapeutically effective amount of a sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone.
Owner:SEPACOR INC
Parenteral Formulations of Dopamine Agonists
ActiveUS20090143390A1Reducing elevated cardiovascular-related inflammatory factorReducing elevated cardiovascular-related inflammatory factorsBiocideAntipyreticEnteral administrationParenteral Dosage Form
This invention relates to stable pharmaceutical compositions for parenteral administration comprising dopamine agonists and peripheral acting agents_useful for treatment of metabolic disorders or key elements thereof. The parenteral dosage forms exhibit long stable shelf life and distinct pharmacokinetics.
Owner:VEROSCI
Novel pharmaceutical compositions administering n-0923
The invention relates to a pharmaceutical composition for administering the dopamine agonist N-0923 in depot form. The invention makes available for the first time a depot form of N-0923, which achieves a therapeutically significant plasma level over a period of at least 24 hours after administration to a patient. As a result of poor oral bio-availability and the short plasma half-life, N-0923 was previously administered either by an intravenous drip or by transdermal systems. Preferred embodiments of said invention are oily suspensions, containing the active ingredient N-0923 in a solid phase, in addition to anhydrous pharmaceutical preparations of N-0923.
Owner:UCB SA
Drug treatment for restless leg syndrome
A method for the treatment of Restless Leg Syndrome (RLS), which comprises administering an alpha2-agonist and a second agent selected from the group consisting of the dopamine agonists, opioids, benzodiazepines and the combination of L-DOPA plus a decarboxylase inhibitor.
Owner:BRECHT HANS MICHAEL
Methods of treating metabolic syndrome using dopamine receptor agonists
InactiveUS20050054652A1Prevent desensitizationMinimize desensitizationBiocideAnimal repellantsDiseaseHypertriglyceridemia
The present invention is directed to a method of simultaneously treating hypertension, hypertriglyceridemia, a pro-inflammatory state, a pro-coagulative state, and insulin resistance (with or without treating obesity or endothelial dysfunction), associated with or independent from Metabolic Syndrome, comprising the step of administering to a patient suffering from such disorders a therapeutically effective amount of a central acting dopamine agonist. In one embodiment, the central acting dopamine agonist is bromocriptine, optionally combined with a pharmaceutically acceptable carrier.
Owner:CINCOTTA ANTHONY H
Sublingual films
ActiveUS8414922B2Alleviating dyskinesiaEffectively alleviatedBiocideNervous disorderSexual functioningDopamine
The invention features sublingual film formulations of dopamine agonists and methods of treating Parkinson's disease, tremors, restless leg syndrome, sexual dysfunction, and depressive disorders therewith.
Owner:SUNOVION PHARMA INC
Treatment of psychosis associated with parkinson's disease and subcortical dementias using a combination of an atypical antipsychotic with a dopamine agonist
InactiveUS20070015763A1Opportunities decreaseSimple processBiocidePeptide/protein ingredientsAtypical antipsychoticZiprasidone
This invention relates to combinations of an atypical antipsychotic, for example ziprasidone, and a dopamine agonist, kits containing such combinations, pharmaceutical compositions comprising such combinations, and methods of using such combinations to treat patients suffering from psychosis and movement disorders associated with Parkinson's disease and subcortical dementias.
Owner:PFIZER INC +1
Methods of identifying responders to dopamine agonist therapy
The present invention is directed to a method of identifying patients to be treated by dopamine agonist therapy comprising the step of analyzing a plasma or urine sample from said patient for concentrations of norepinephrine (NE), norepinephrine metabolites (NE metabolites), dopamine, dopamine metabolites, serotonin, serotonin metabolites, or fasting triglycerides, wherein one or more of: (a) NE metabolites, (b) NE / NE metabolites: dopamine / dopamine metabolites, (c) NE and serotonin, (d) NE / NE metabolites and serotonin, (e) NE and serotonin metabolites, (f) NE / NE metabolites and serotonin metabolites, or (g) NE is / are greater than about 30% over normal level; or dopamine / dopamine metabolites are less than about 30% below normal; or said patient has hypertriglyceridemai and / or hypertension . The present invention is also directed to treating identified patients with dopamine agonist therapy.
Owner:VEROSCI
Dopamine-Agonist Combination Therapy For Improving Sleep Quality
The present invention generally relates to pharmaceutical compositions comprising a dopamine agonist and sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. The pharmaceutical compositions of the invention are useful in the treatment of restless-leg syndrome and periodic-limb-movement disorder, as well as various sleep disorders. In addition, the present invention relates to a method of treating a patient suffering from restless-leg syndrome, periodic-limb-movement disorder, a sleep abnormality, or insomnia, comprising coadministering a therapeutically effective amount of a dopamine agonist and a therapeutically effective amount of a sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone.
Owner:WOODWARD SPECIALTY LLC
Compositions and uses
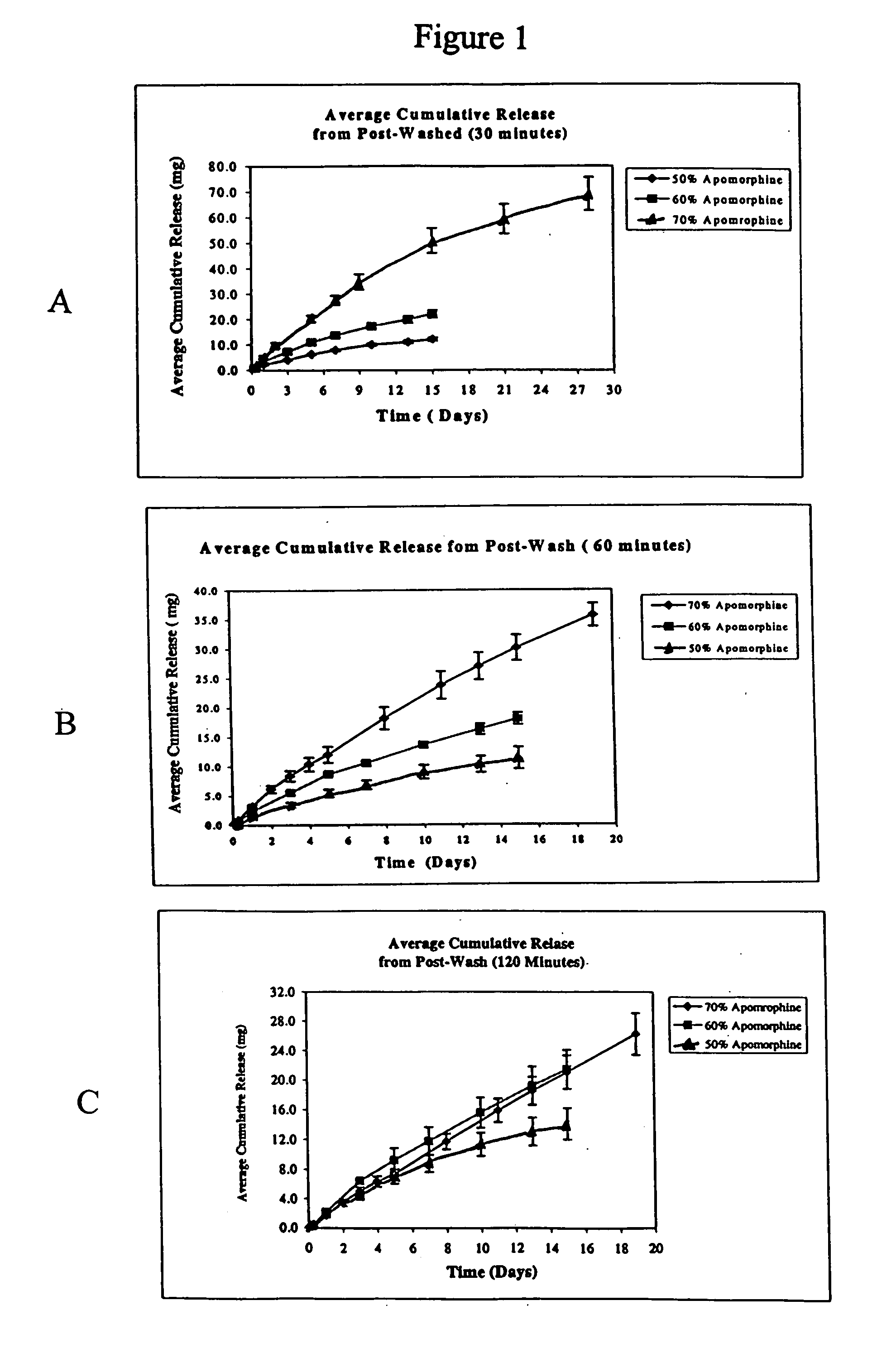
According to the invention there is provided a method of treating and / or preventing the symptoms of Parkinson's disease comprising delivering apomorphine, optionally in combination with levodopa and / or a dopamine agonist that is not apomorphine, wherein apomorphine is administered by inhalation.
Owner:VECTURA LTD
Method and composition for the treatment of lipid and glucose metabolism disorders
InactiveUS20020187985A1Improved modification and regulationImprovement of one or more metabolic indicesBiocideAnimal repellantsBlood plasmaPlasma insulin
Disclosed are methods for modifying or regulating at least one of glucose or lipid metabolism disorders which comprises administering to a human or vertebrate subject a D1 dopamine agonist in conjunction with a dopamine D2 agonist where the conjoined administration is effective to improve at least one of the following lipid and glucose metabolic indices: body weight, body fat, plasma insulin, plasma glucose and plasma lipid, and plasma lipoprotein. In preferred embodiments, the administration of the D1 dopamine agonist and the D2 dopamine agonist is conducted at a predetermined time.
Owner:VEROSCI
Method and composition for the treatment of lipid and glucose metabolism disorders
InactiveUS20010016582A1Improved modification and regulationImprovement of one or more metabolic indicesBiocideCarbohydrate active ingredientsBlood plasmaPlasma insulin
Disclosed are methods for modifying or regulating at least one of glucose or lipid metabolism disorders which comprises administering to a human or vertebrate subject a D1 dopamine agonist in conjunction with a dopamine D2 agonist where the conjoined administration is effective to improve at least one of the following lipid and glucose metabolic indices: body weight, body fat, plasma insulin, plasma glucose and plasma lipid, and plasma lipoprotein. In preferred embodiments, the administration of the D1 dopamine agonist and the D2 dopamine agonist is conducted at a predetermined time.
Owner:CINCOTTA ANTHONY H
Parenteral formulations of dopamine agonists
ActiveUS8741918B2Reducing elevated cardiovascular-related inflammatory factorsBiocideAntipyreticParenteral nutritionParenteral Dosage Form
This invention relates to stable pharmaceutical compositions for parenteral administration comprising dopamine agonists and peripheral acting agents useful for treatment of metabolic disorders or key elements thereof. The parenteral dosage forms exhibit long stable shelf life and distinct pharmacokinetics.
Owner:VEROSCI
Methods of identifying responders to dopamine agonist therapy
The present invention is directed to a method of identifying patients to be treated by dopamine agonist therapy comprising the step of analyzing a plasma or urine sample from said patient for concentrations of norepinephrine (NE), norepinephrine metabolites (NE metabolites), dopamine, dopamine metabolites, serotonin, serotonin metabolites, or fasting triglycerides, wherein one or more of: (a) NE metabolites, (b) NE / NE metabolites:dopamine / dopamine metabolites, (c) NE and serotonin, (d) NE / NE metabolites and serotonin, (e) NE and serotonin metabolites, (f) NE / NE metabolites and serotonin metabolites, or (g) NE is / are greater than about 30% over normal level; or dopamine / dopamine metabolites are less than about 30% below normal; or fasting triglycerides are greater than about 150 mg / dl and / or said patient has blood pressure of greater than about 135 / 85 mm Hg. The present invention is also directed to treating identified patients with dopamine agonist therapy.
Owner:CINCOTTA ANTHONY H
Methods of identifying responders to dopamine agonist therapy and treating metabolic conditions thereof
The present invention is directed to a method of identifying patients to be treated by dopamine agonist therapy comprising the step of analyzing a plasma or urine sample from said patient for concentrations of norepinephrine (NE), norepinephrine metabolites (NE metabolites), dopamine, dopamine metabolites, serotonin, serotonin metabolites, or fasting triglycerides, wherein one or more of: (a) NE metabolites, (b) NE / NE metabolites: dopamine / dopamine metabolites, (c) NE and serotonin, (d) NE / NE metabolites and serotonin, (e) NE and serotonin metabolites, (f) NE / NE metabolites and serotonin metabolites, or (g) NE is / are greater than about 30% over normal level; or dopamine / dopamine metabolites are less than about 30% below normal; or said patient has hypertriglyceridemai and / or hypertension . The present invention is also directed to treating identified patients with dopamine agonist therapy.
Owner:VEROSCI
Combination of dopamine agonists plus first phase secretagogues for the treatment of metabolic disorders
ActiveUS8877708B2Eliminate side effectsImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderPhases of clinical researchDopamine agonist
Owner:VEROSCI
Methods of identifying responders to dopamine agonist therapy and treating metabolic conditions thereof
The present invention is directed to a method of identifying patients to be treated by dopamine agonist therapy comprising the step of analyzing a plasma or urine sample from said patient for concentrations of norepinephrine (NE), norepinephrine metabolites (NE metabolites), dopamine, dopamine metabolites, serotonin, serotonin metabolites, or fasting triglycerides, wherein one or more of: (a) NE metabolites, (b) NE / NE metabolites: dopamine / dopamine metabolites, (c) NE and serotonin, (d) NE / NE metabolites and serotonin, (e) NE and serotonin metabolites, (f) NE / NE metabolites and serotonin metabolites, or (g) NE is / are greater than about 30% over normal level; or dopamine / dopamine metabolites are less than about 30% below normal; or fasting triglycerides are greater than about 150 mg / dl and / or said patient has hypertension. The present invention is also directed to treating identified patients with dopamine agonist therapy.
Owner:VEROSCI
Parenteral Formulations Of Dopamine Agonists
ActiveUS20130197005A1Reducing elevated cardiovascular-related inflammatory factorsBiocideMetabolism disorderParenteral Dosage FormMedicine
This invention relates to stable pharmaceutical compositions for parenteral administration comprising dopamine agonists and peripheral acting agents useful for treatment of metabolic disorders or key elements thereof. The parenteral dosage forms exhibit long stable shelf life and distinct pharmacokinetics.
Owner:VEROSCI
Pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor and a dopamine agonist
InactiveUS20050182090A1Reduce pruningEliminate side effectsBiocideNervous disorderTropaneDopamine agonist agent
The invention relates to a pharmaceutical composition comprising a monoamine neurotransmitter re-uptake inhibitor comprising a 2,3-disubstituted tropane moiety, or a tautomer, a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof, and at least one dopamine agonist or a pharmaceutically acceptable salt, solvate, or physiologically functional derivative thereof.
Owner:BOEHRINGER INGELHEIM PHARM KG
Delivery system and method for supporting and promoting healthy sexual function and prevention and treatment of sexual dysfunction
InactiveUS20060110478A1Increase in cGMPGood curative effectFood ingredient as antioxidantBiocideSexual functionVardenafil
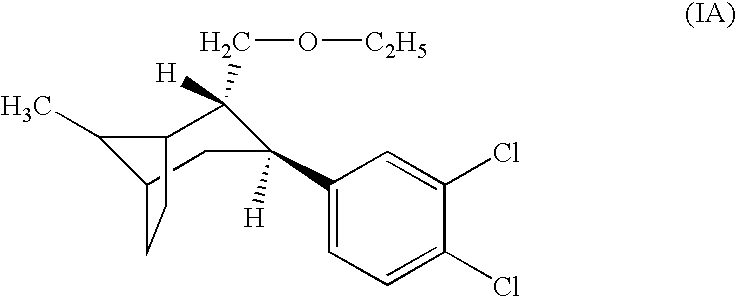
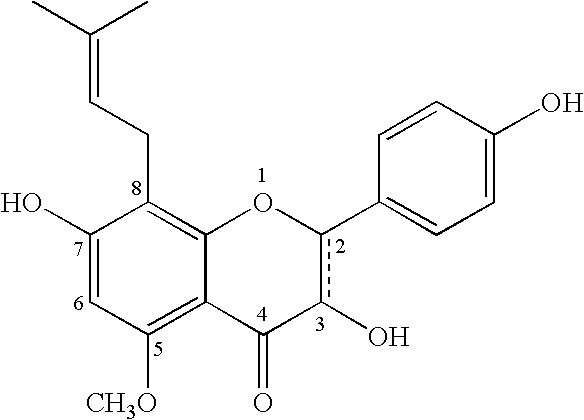
Improved delivery systems and delivery methods for supporting and promoting healthy sexual function, for preventing sexual dysfunction, or for treatment of sexual dysfunction. A compositions including one or more cGMP-specific PDE5 inhibitors and / or dopaminergic agonists is administered in the form of a breath-care strip, mint or lozenge, or a food or beverage product. The cGMP-specific PDE5 inhibitor comprises an ingredient selected from the group consisting of sophoflavescenol, vardenafil, tadalafil, and sildenafil. The dopaminergic agonist comprises apomorphine. Vitex agnus-castus extract, and one or more of lipoic acid, L-Arginine, folic acid, trimethylglycine, policosanol, carnitine, biotin, and acetyl L-Carnitine may also be included in the delivery vehicle.
Owner:MCCLEARY EDWARD LARRY +2
Methods of treating patients suffering from movement disorders
ActiveUS20060178379A1Reducing and suppressing adverse effectiveness of L-DOPAShorten closing timeBiocideNervous disorderAdenosineDisease patient
The present invention is directed to methods of treating movement disorders by administering an effective amount of one or more adenosine A2A receptor antagonists to a patient in need thereof. The present invention also provides methods of decreasing the adverse effects of L-DOPA in patients receiving L-DOPA therapy in the treatment of Parkinson's disease. The present invention further provides methods and compositions for treating Parkinson's disease patients with sub-clinically effective doses of L-DOPA by combining L-DOPA treatment with an effective amount of one or more adenosine A2A receptor antagonists (i.e., L-DOPA sparing effect). The present invention further provides methods of effective treatment of Parkinson's disease by co-administering at least one adenosine A2A receptor antagonist, L-DOPA and a dopamine agonist and / or a COMT inhibitor and / or a MAO inhibitor. The present invention further provides methods of prolonging effective treatment of Parkinson's disease by administering an adenosine A2A receptor antagonist singly or together with a dopamine agonist, and / or a COMT inhibitor, and / or a MAO inhibitor without prior or subsequent administration of L-DOPA, delaying or removing on-set of L-DOPA motor complication.
Owner:KYOWA HAKKO KIRIN CO LTD
Novel compositions comprising a phosphodiesterase-5 inhibitor and their use in methods of treatment
InactiveUS20130296324A1Enhance health and appearanceFacilitating accelerating healingBiocideNervous disorderDiseaseSynaptic cleft
The invention relates generally to novel pharmaceutical methods for the treatment of various conditions. Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; a serotonin-norepinephrine reuptake inhibitor; a cholinesterase inhibitor; a dopamine agonist; or a medication suitable to increase the chemical concentrations of the neurotransmitters, selected from amino acids, monoamines, neuropeptides and other agents capable of primary neurotransmission in the synaptic clefts, and their use for treating a neurodegenerative disease in a subject. The invention also relates to: Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; or a cholinesterase inhibitor, and their use for treating damaged skin in a subject.
Owner:HELD JERRY M
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
ActiveUS9352025B2Eliminate side effectsImprove blood sugar controlPeptide/protein ingredientsHeterocyclic compound active ingredientsPhases of clinical researchDopamine agonist
The present invention is directed to a method of treating a metabolic disorder or key elements of a metabolic disorder such method comprising the use of an agent(s) that increases central dopaminergic activity plus a first-phase insulin secretagouge.
Owner:VEROSCI
Combination Of Dopamine Agonists Plus First Phase Insulin Secretagogues For The Treatment Of Metabolic Disorders
ActiveUS20150024995A1Good blood pressureImpaired glucoseBiocidePeptide/protein ingredientsDopamine agonistMetabolic disorder
The present invention is directed to a method of treating a metabolic disorder or key elements of a metabolic disorder such method comprising the use of an agent(s) that increases central dopaminergic activity plus a first-phase insulin secretagouge.
Owner:VEROSCI
Treatment or Prevention of Ovarian Hyperstimulation Syndrome (Ohss) Using a Dopamine Agonist
InactiveUS20080293693A1Preventing ovarian hyperstimulation syndromeBiocideOrganic chemistryPhysiologyDopamine
Disclosed herein is a method for preventing ovarian hyperstimulation syndrome in a subject by administering an effective amount of a dopamine agonist in a pharmaceutically acceptable carrier. Also disclosed herein is a method for treating ovarian hyperstimulation syndrome by administering an effective amount of a dopamine agonist in a pharmaceutically acceptable carrier.
Owner:FERRING INT CENT SA
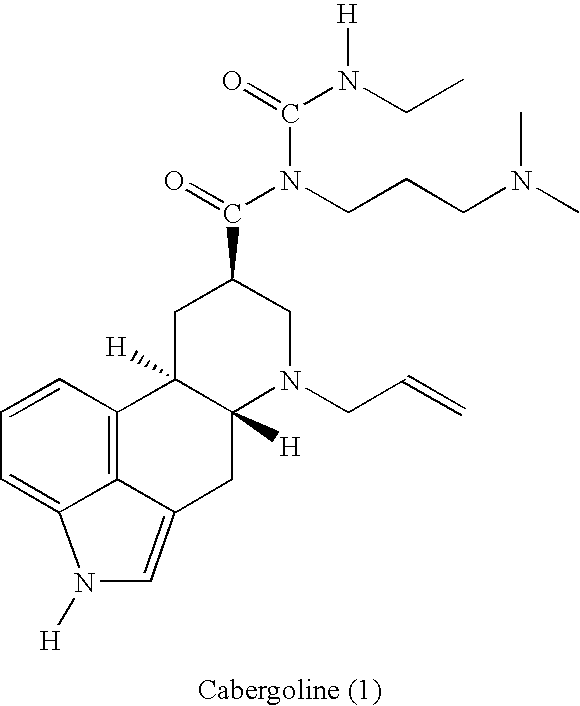
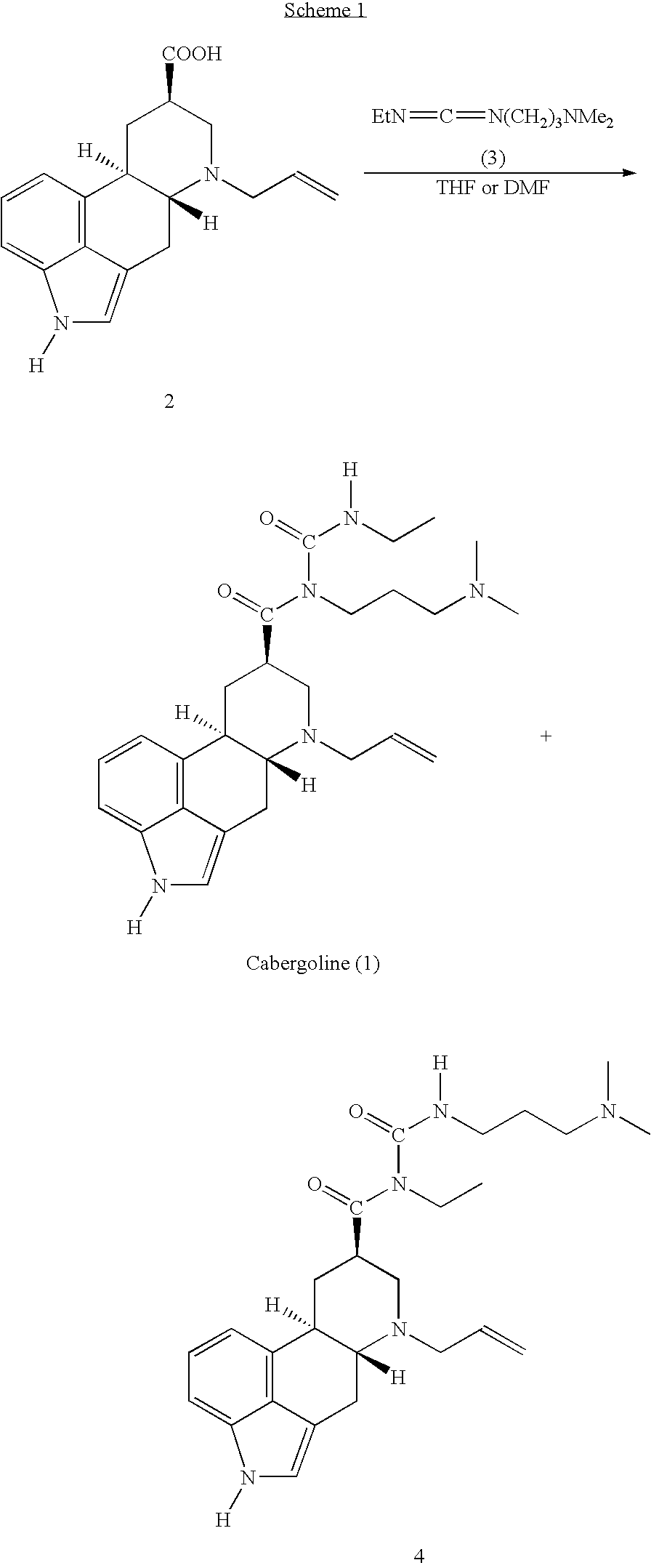
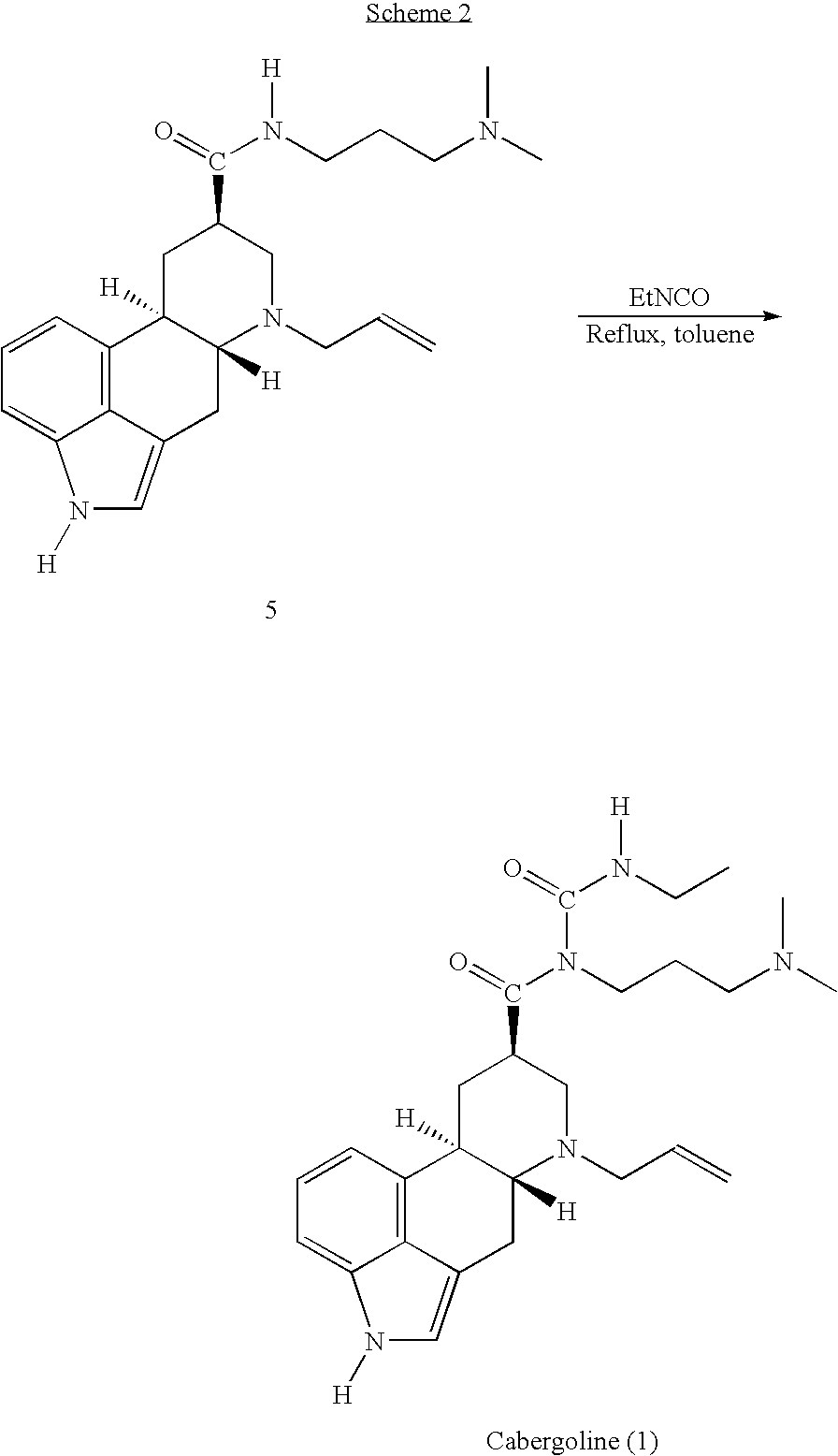
New and efficient process for the preperation of cabergoline and its intermediates
This invention relates to a new and efficient process for the production of dopamine agonists such as Cabergoline and the intermediates thereof.
Owner:APOTEX PHARMACHEN INC
Novel therapeutic method
InactiveUS20030060499A1Preserving dopaminergic functionReduce the populationBiocideAnimal repellantsMedicinePharmacology
A therapeutic method for preserving the dopaminergic function of patients suffering from Parkinson's disease, which method comprises administering an effective amount of ropinirole or a pharmaceutically acceptable salt or solvate thereof to a human or animal patient in need thereof. Typically, said patient has had Parkinson's disease for a period of less than three years since diagnosis. Preferably the invention comprises administering to said patient an effective amount of ropinirole or a pharmaceutically acceptable salt or solvate thereof, optionally in combination with one or more other dopamine agonists, in the absence of levodopa or any other dopamine precursor, and thereafter treating the patient with levodopa.
Owner:TULLOCH IAN FREDERIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com