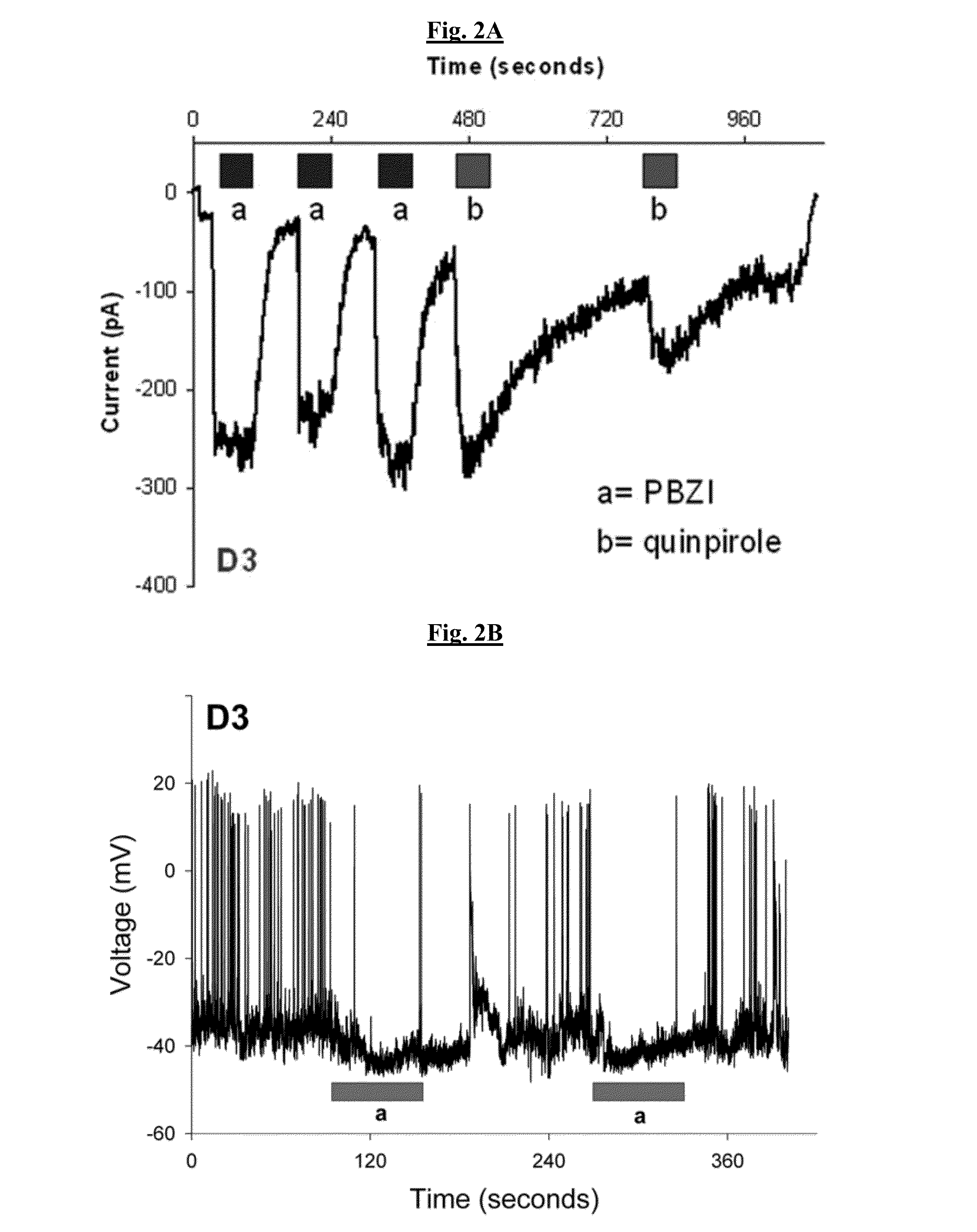
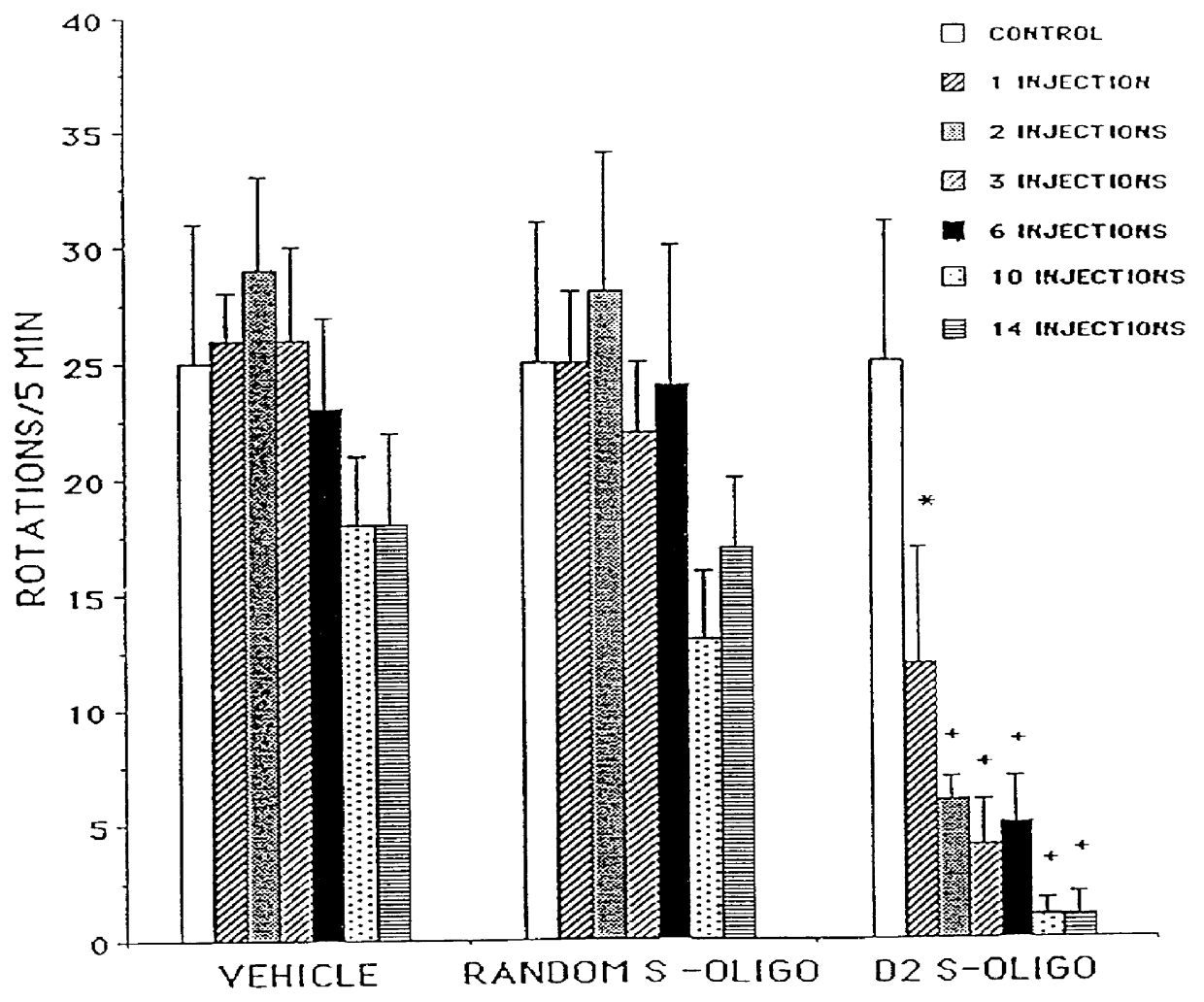
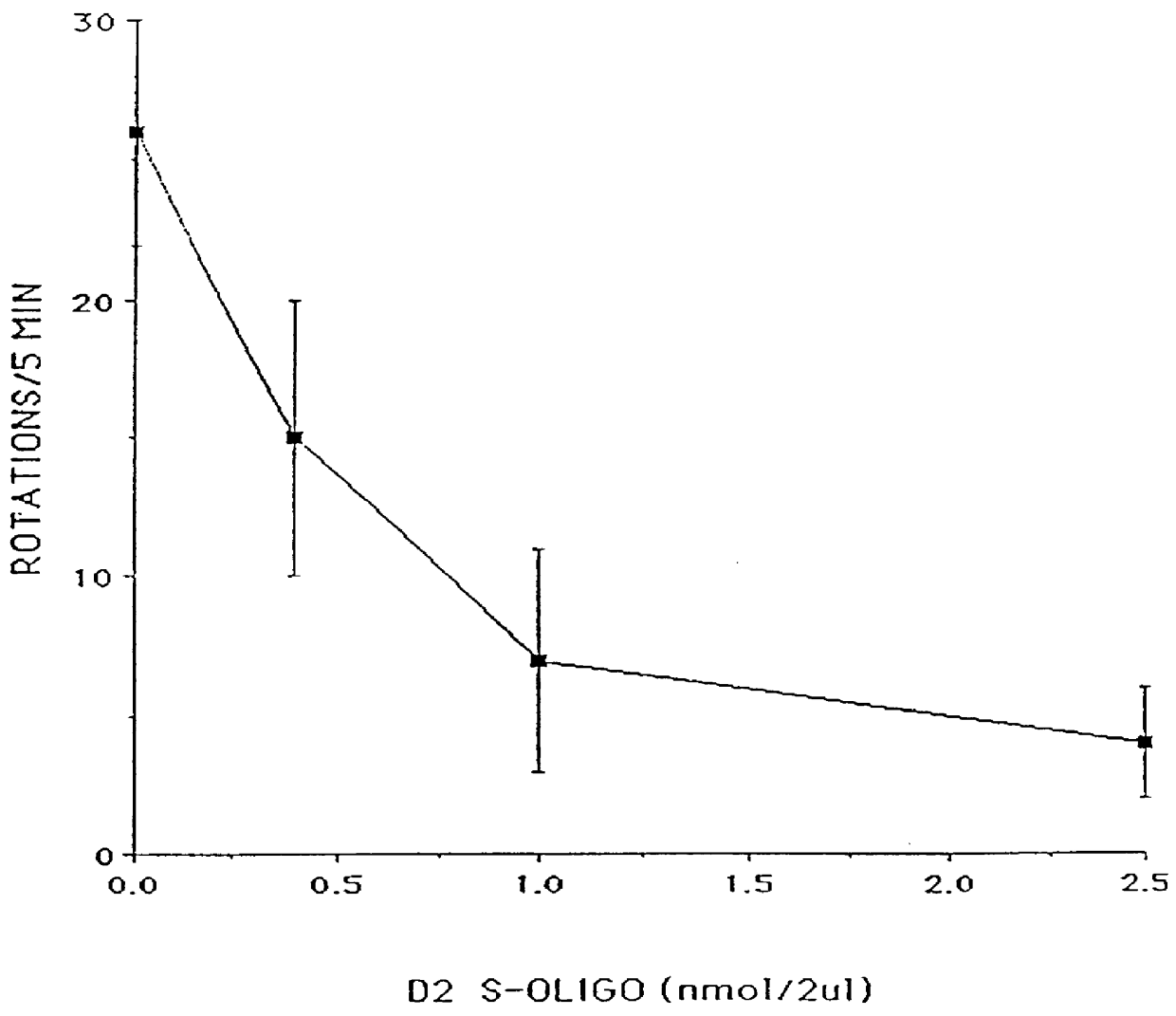
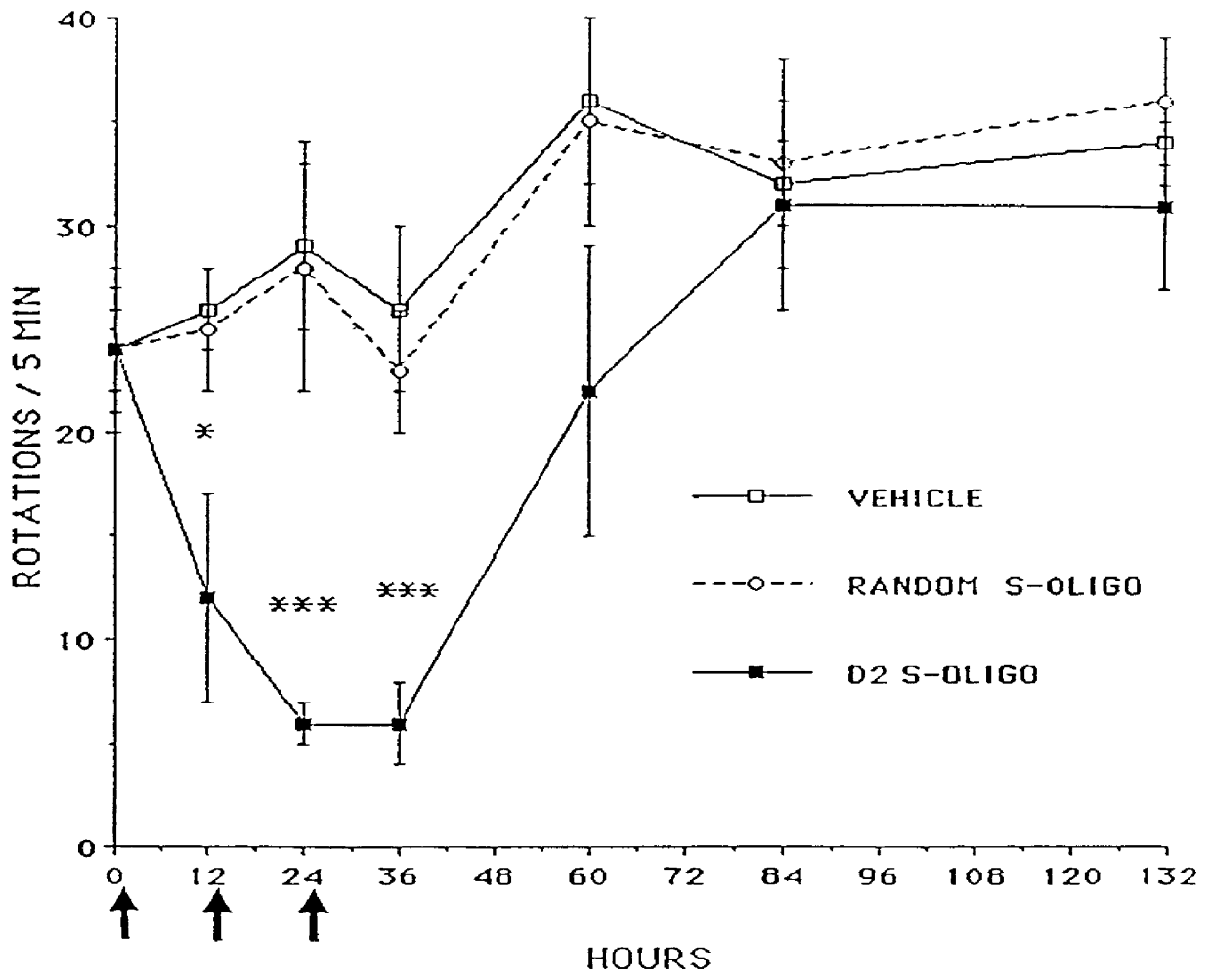
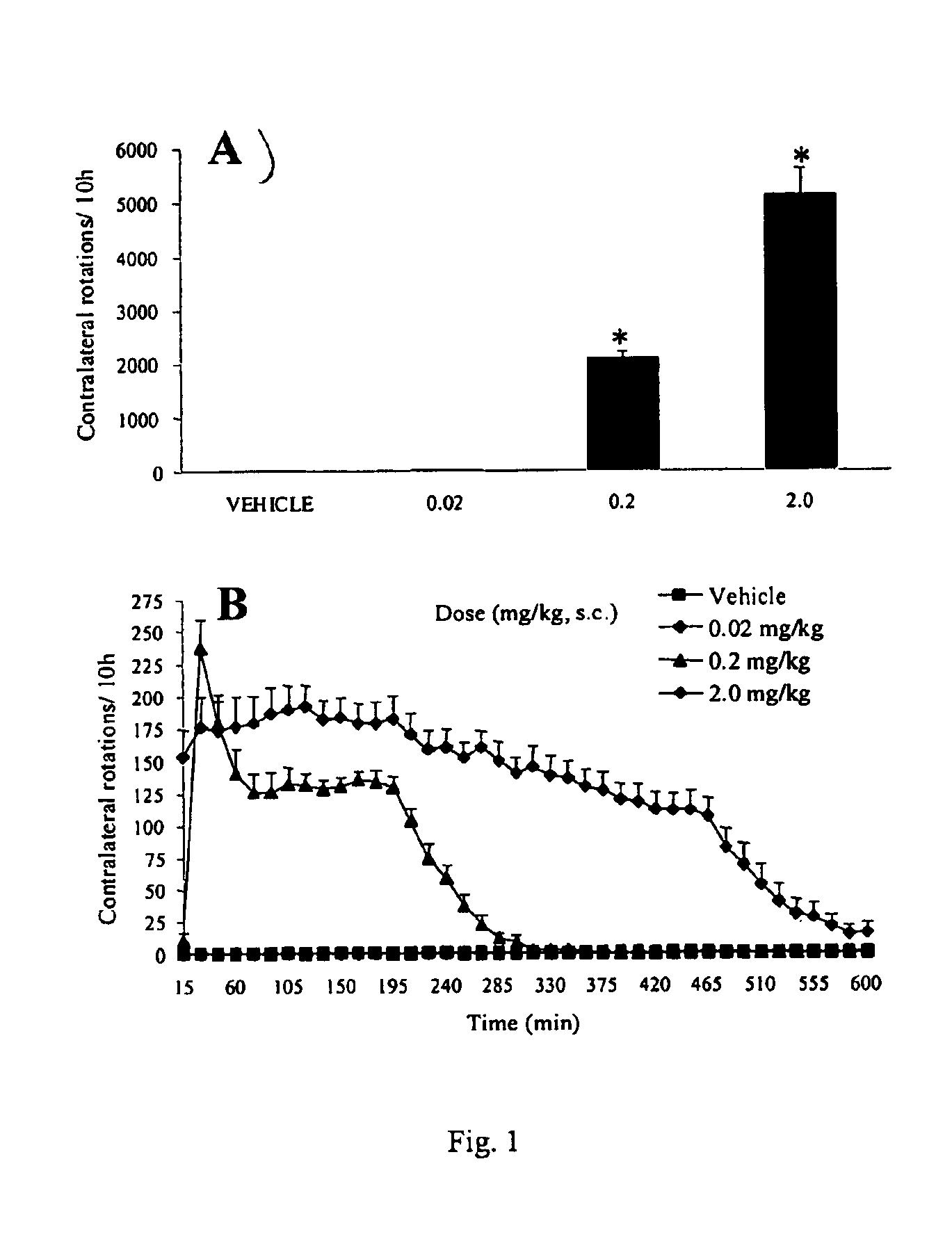
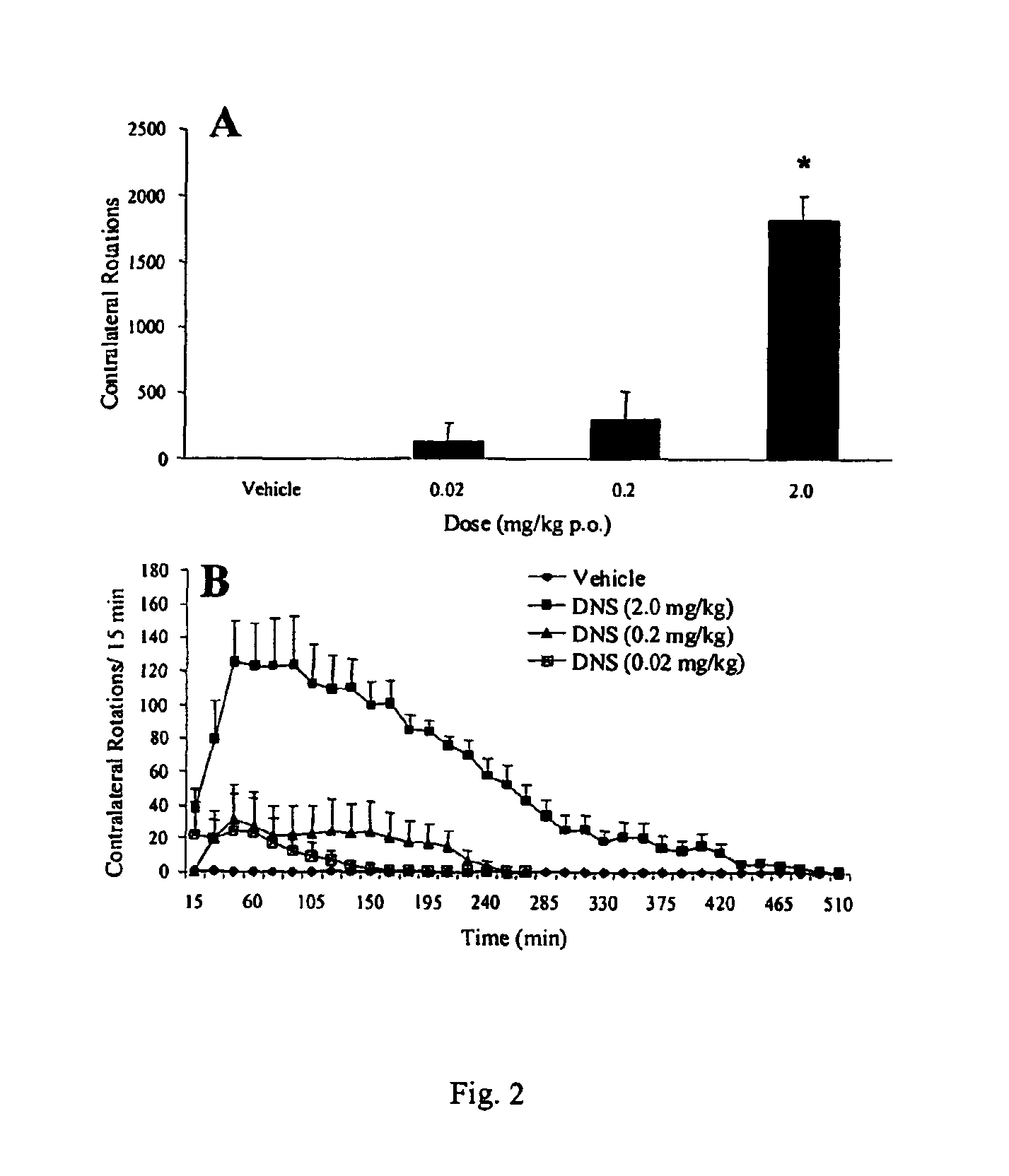
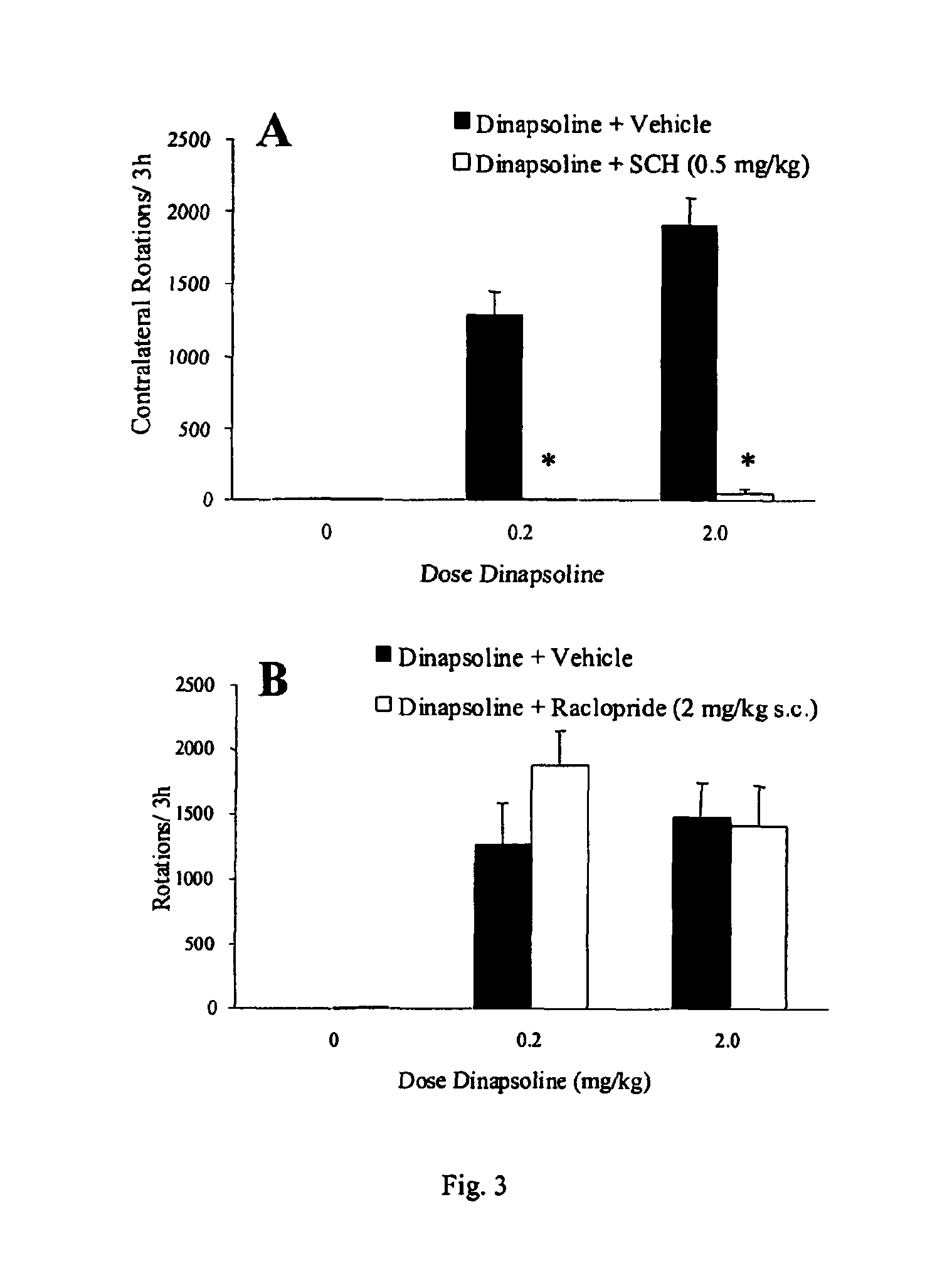
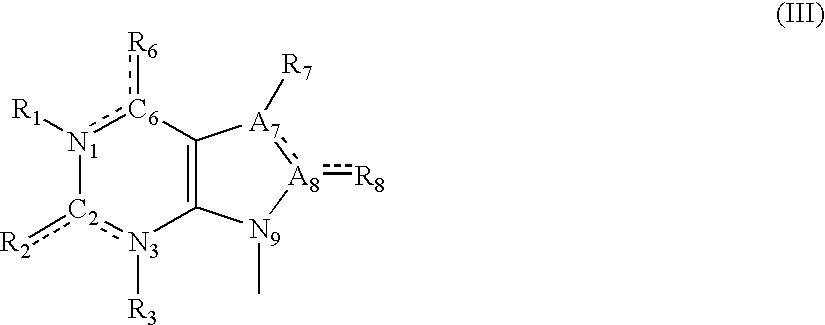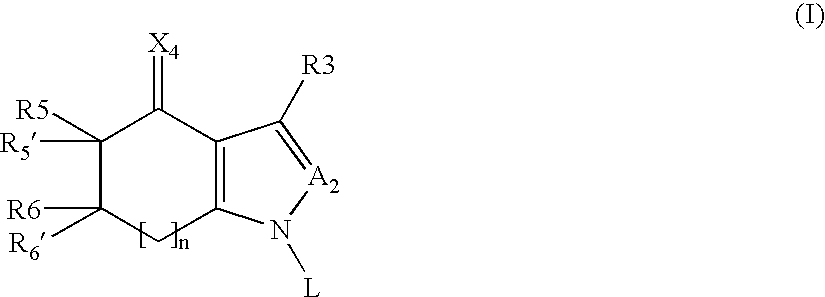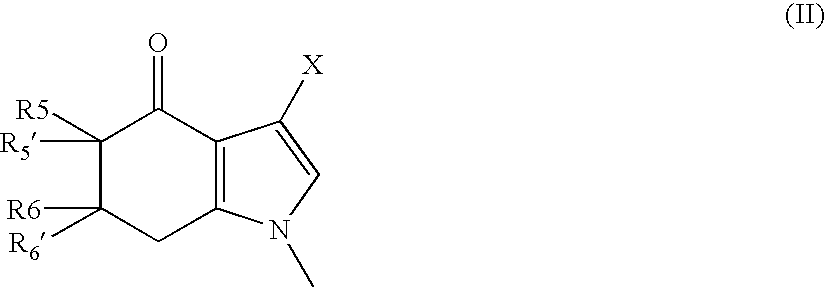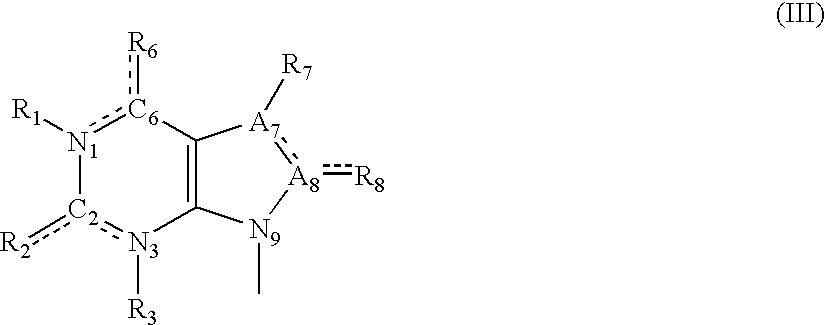Patents
Literature
181 results about "D2 dopamine receptors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
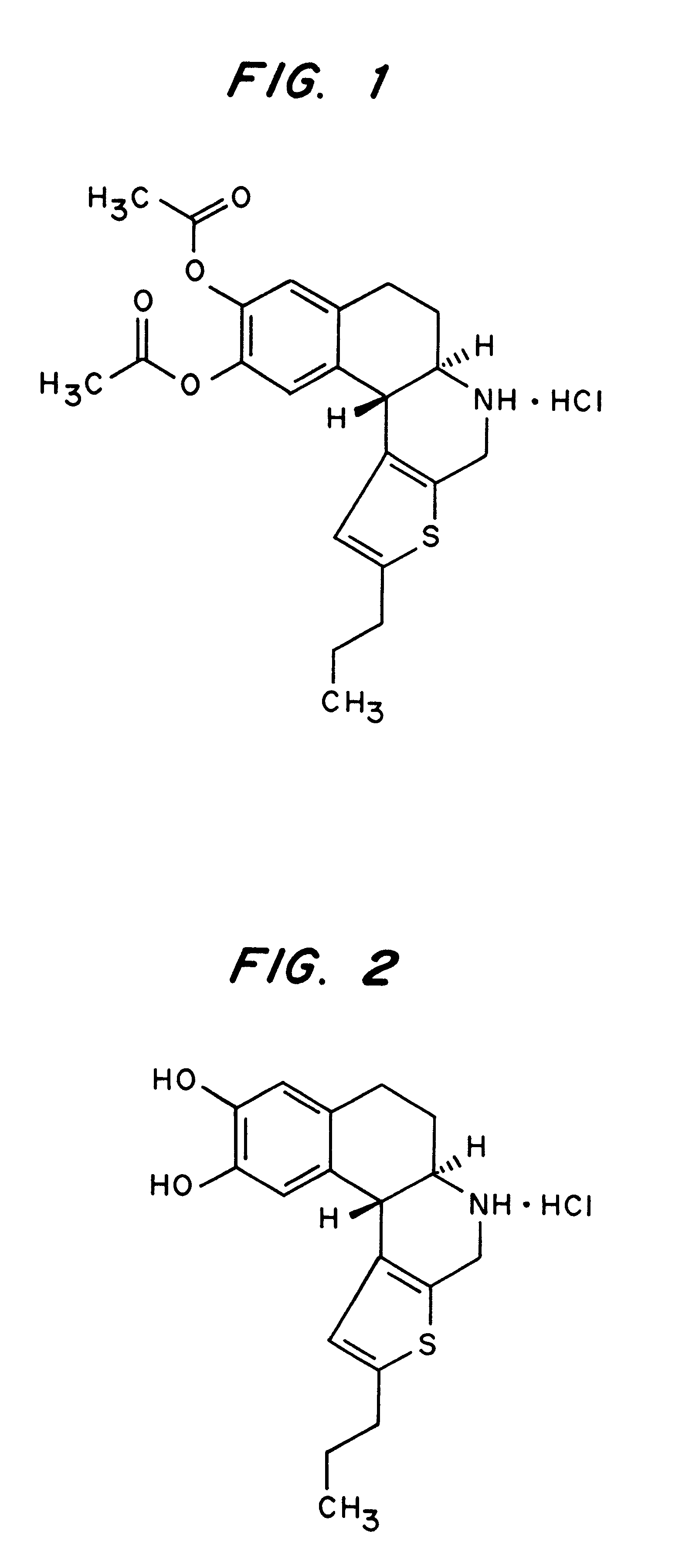
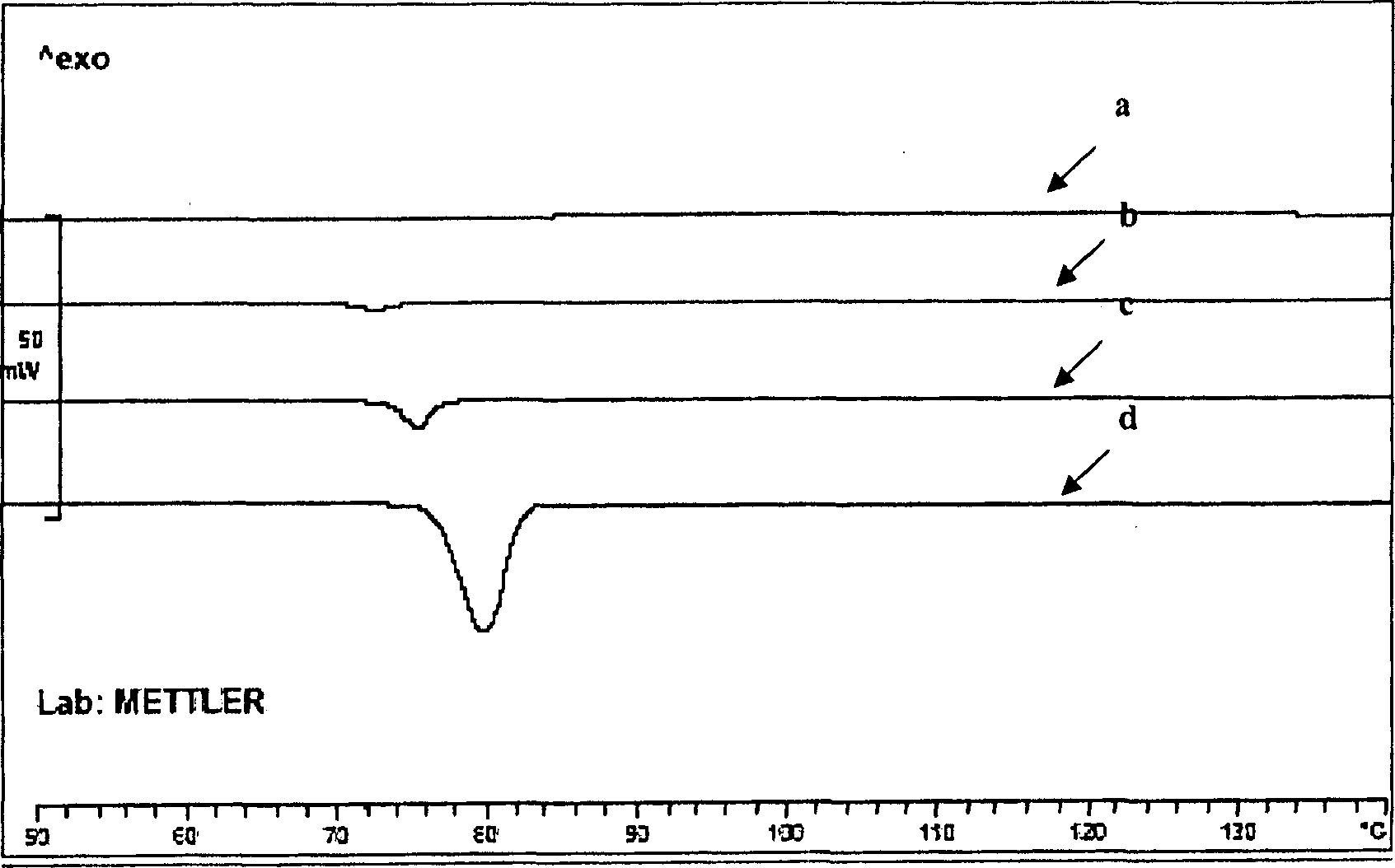
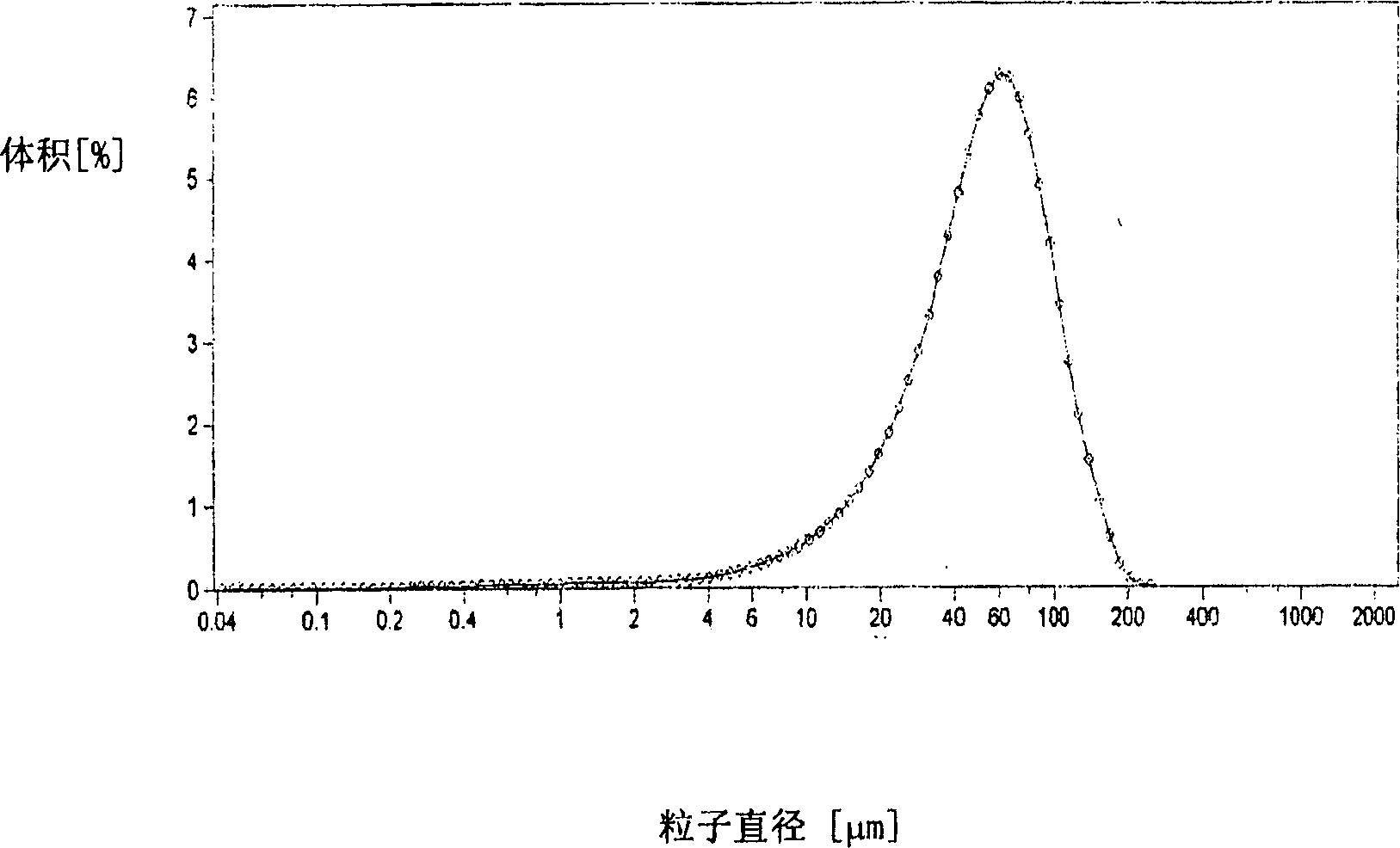
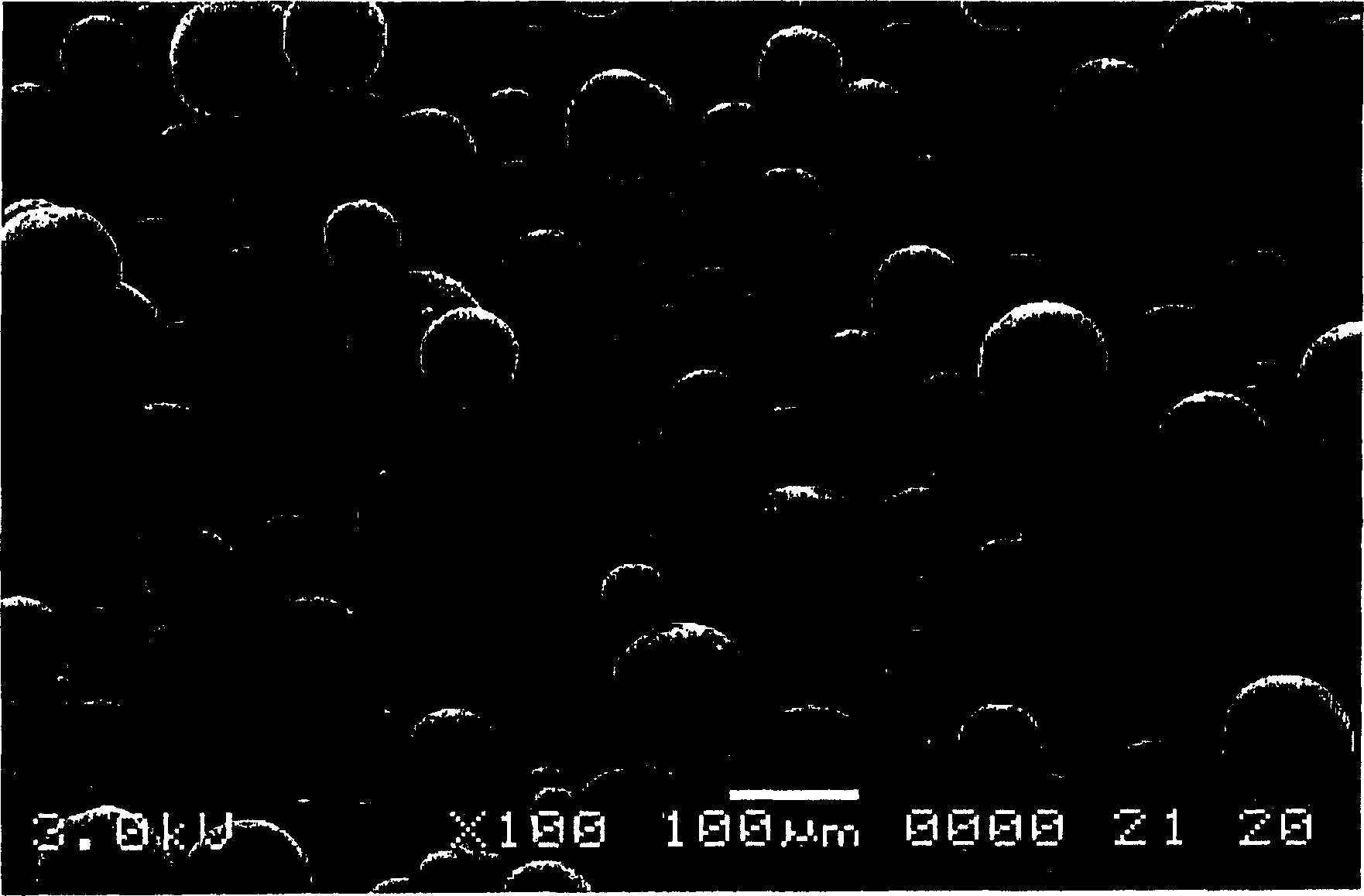
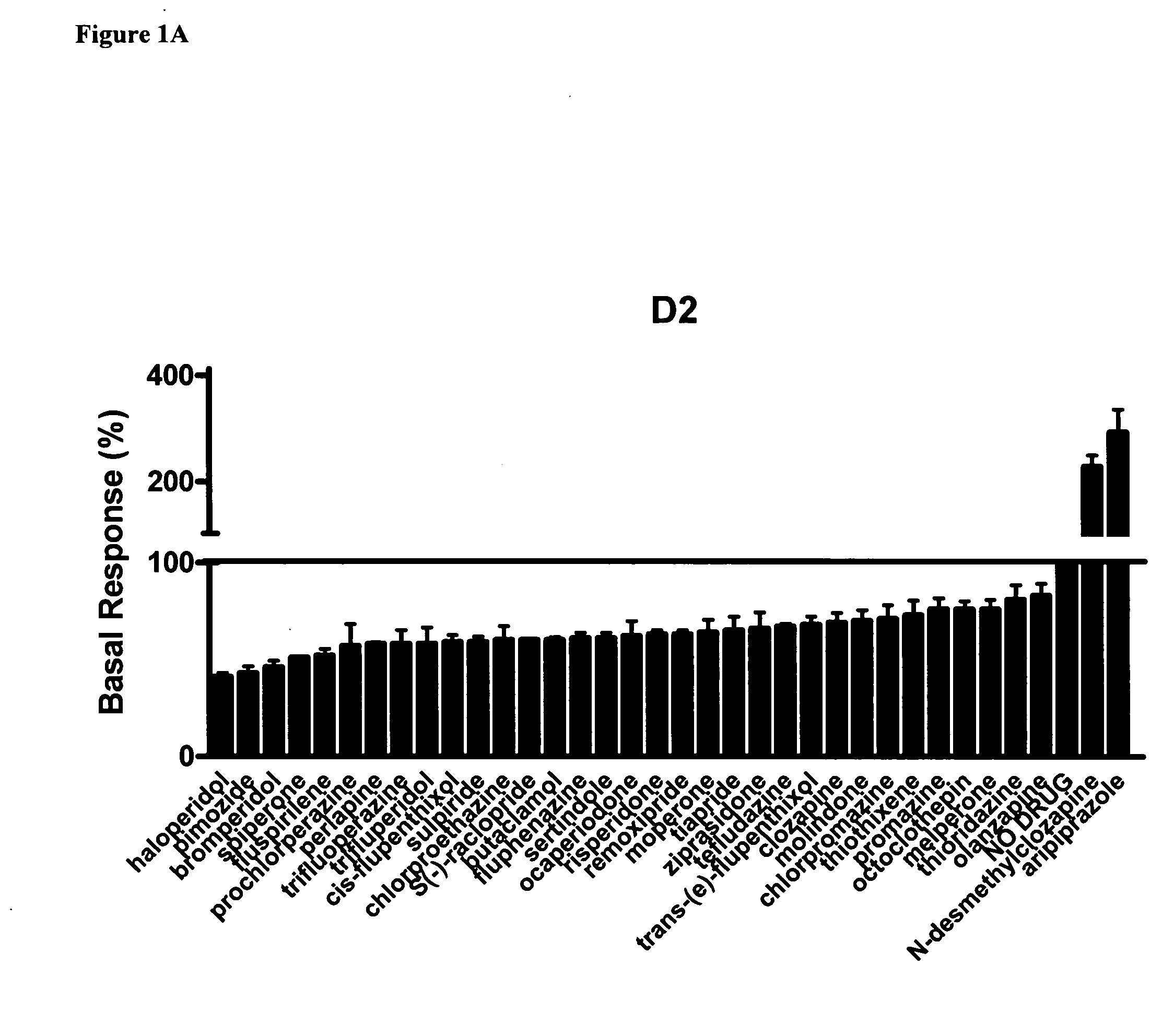
The existence of multiple types of receptors for dopamine was first proposed in 1976. There are at least five subtypes of dopamine receptors, D1, D2, D3, D4, and D5. The D1 and D5 receptors are members of the D1-like family of dopamine receptors, whereas the D2, D3 and D4 receptors are members of the D2-like family.
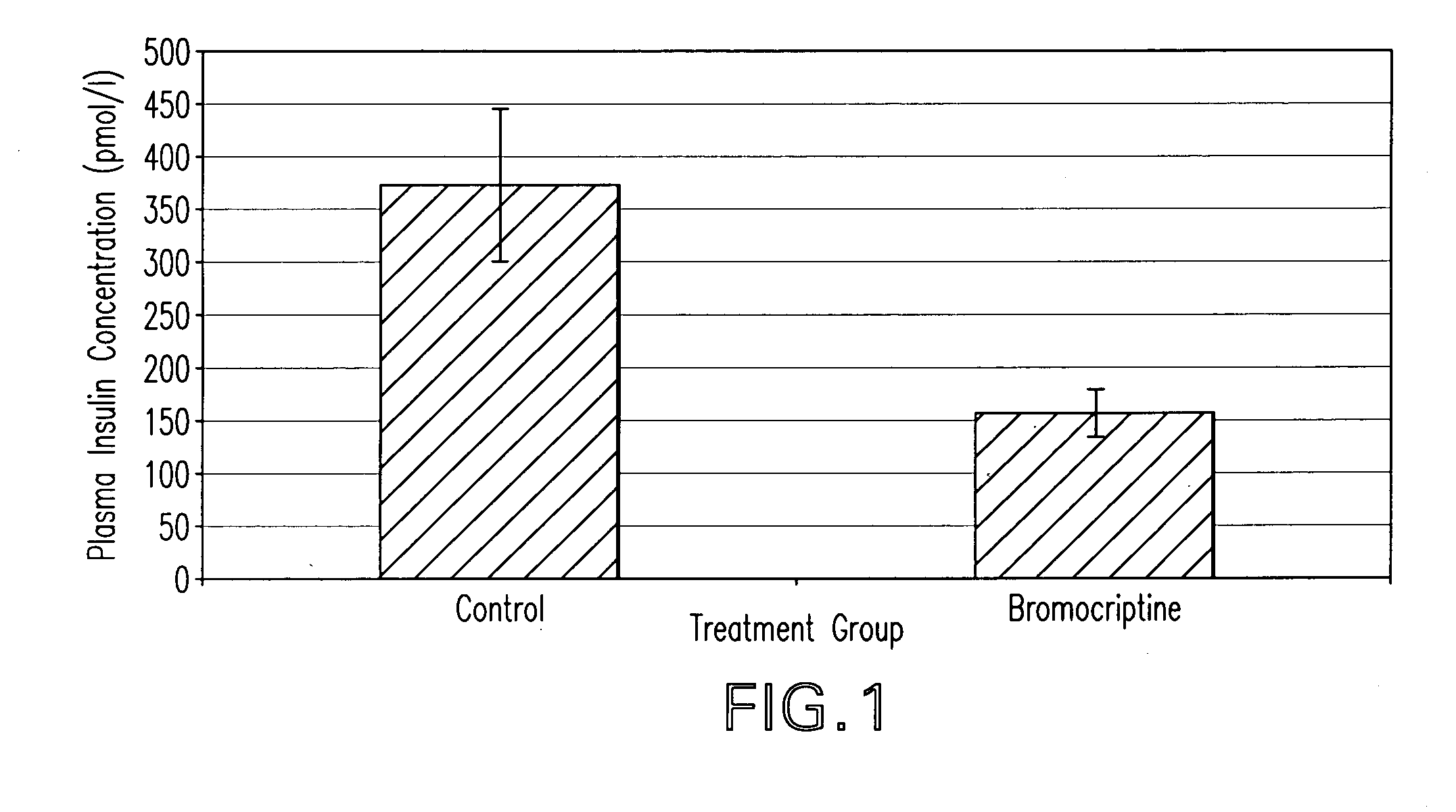
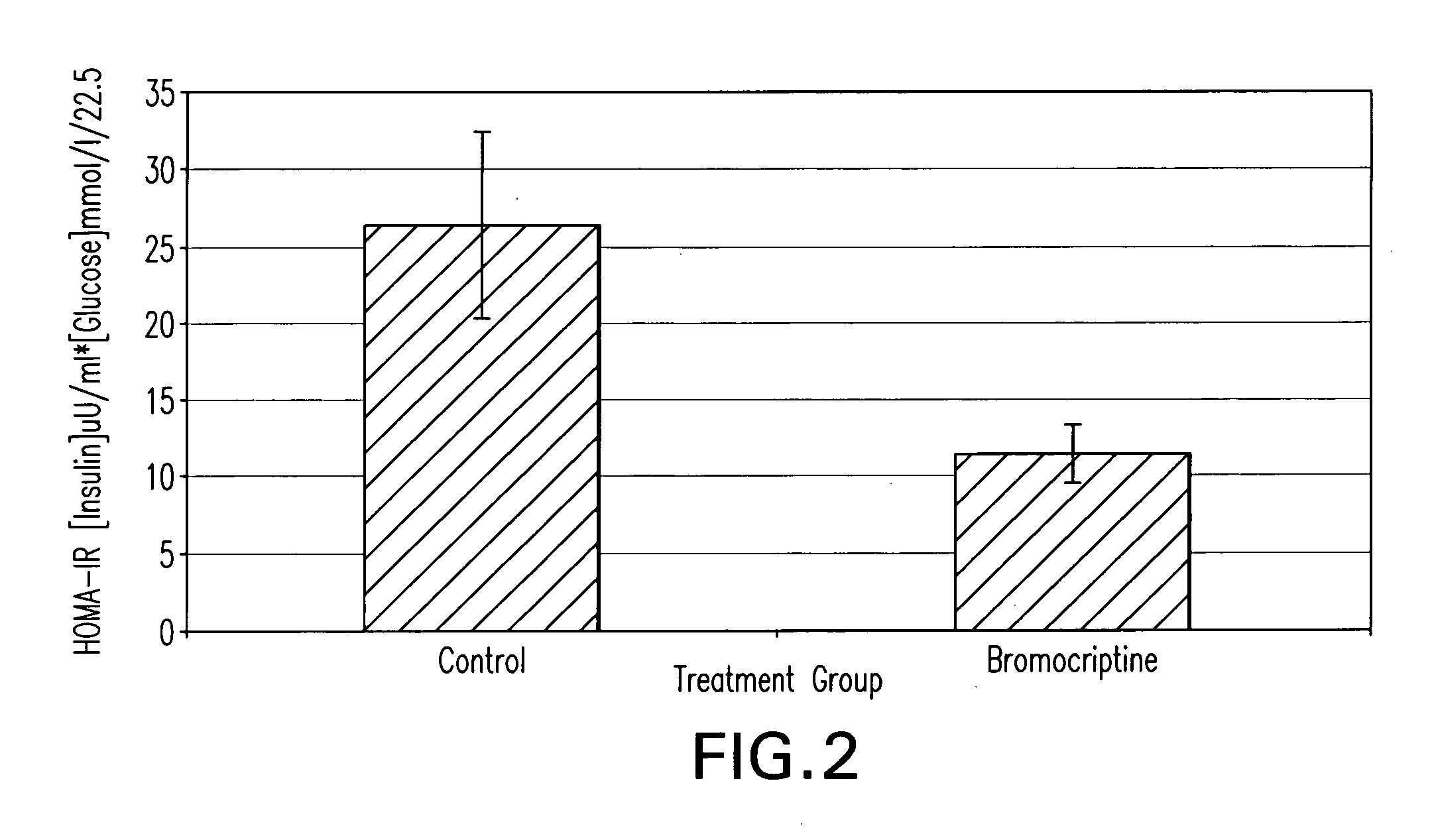
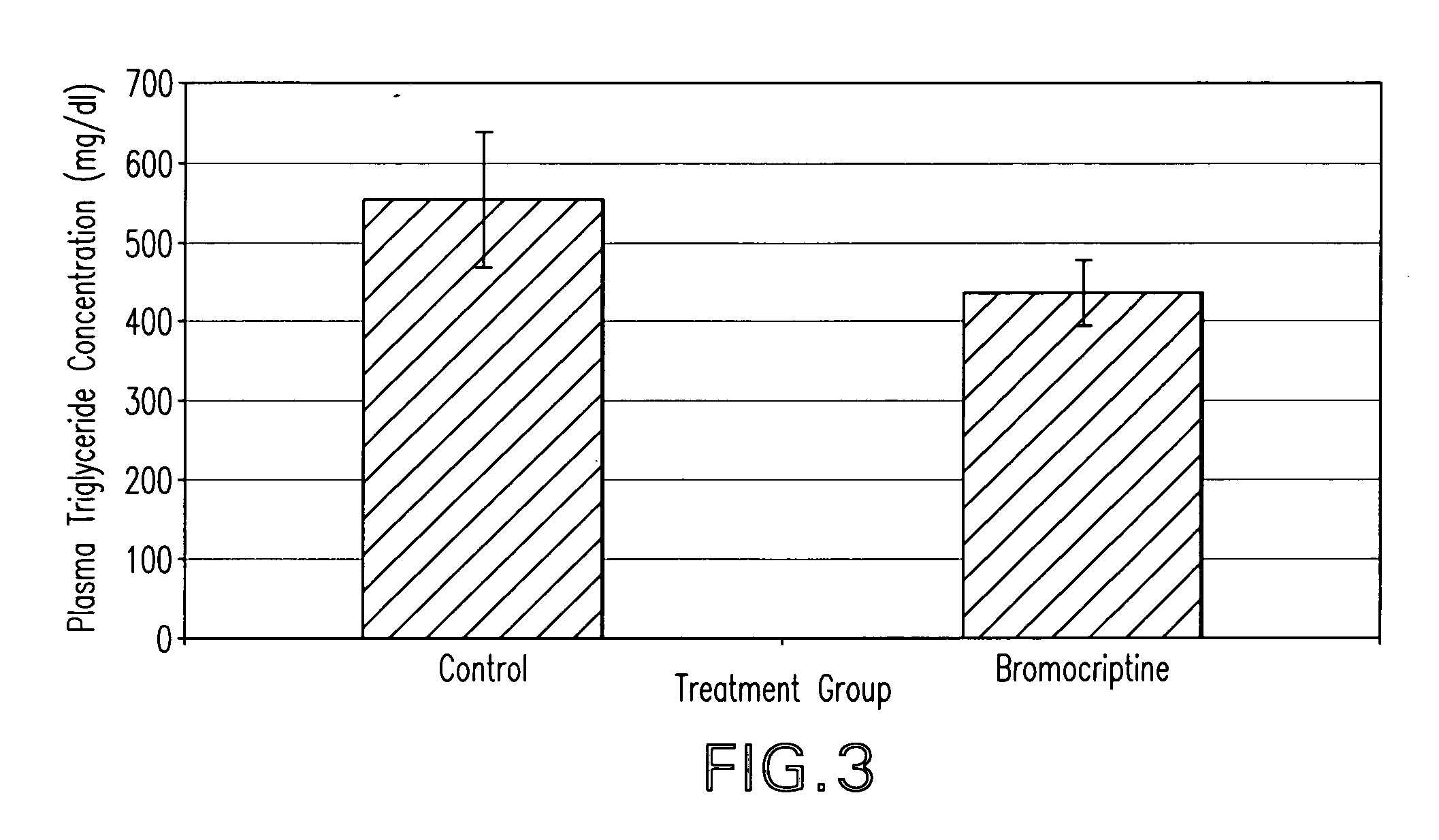
Methods of treating metabolic syndrome using dopamine receptor agonists
InactiveUS20080200453A1Relieve symptomsEffective treatmentBiocideMetabolism disorderDiseaseVascular disease
The present invention is directed to a method of simultaneously treating hypertension, hypertriglyceridemia, a pro-inflammatory state, a pro-coagulative state, and insulin resistance (with or without treating obesity or endothelial dysfunction), associated with or independent from Metabolic Syndrome, as well as vascular disease such as cardiovascular, cerebrovascular, or peripheral vascular disease comprising the step of administering to a patient suffering from such disorders a therapeutically effective amount of a central acting dopamine agonist. In one embodiment, the central acting dopamine agonist is bromocriptine, optionally combined with a pharmaceutically acceptable carrier.
Owner:VEROSCI
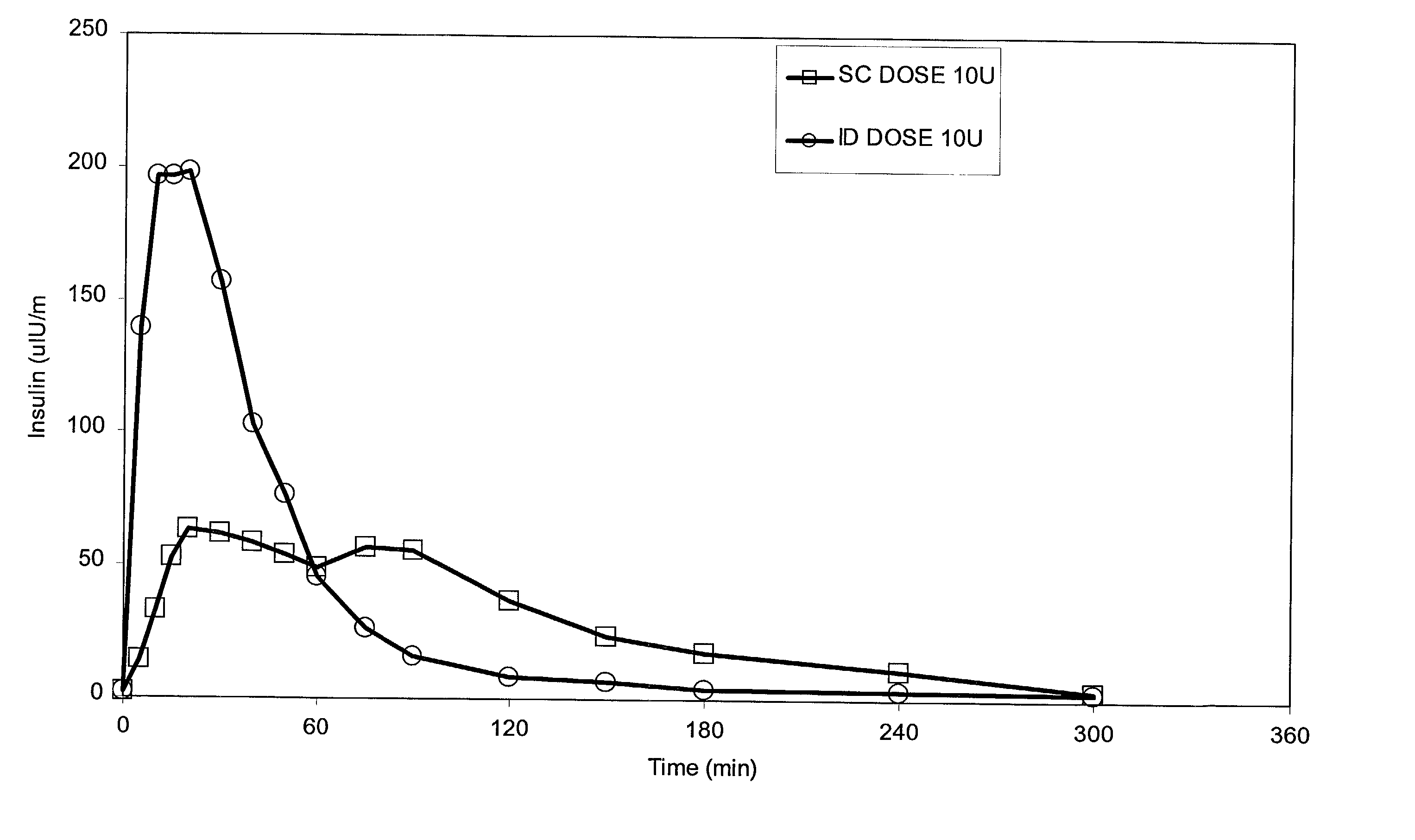
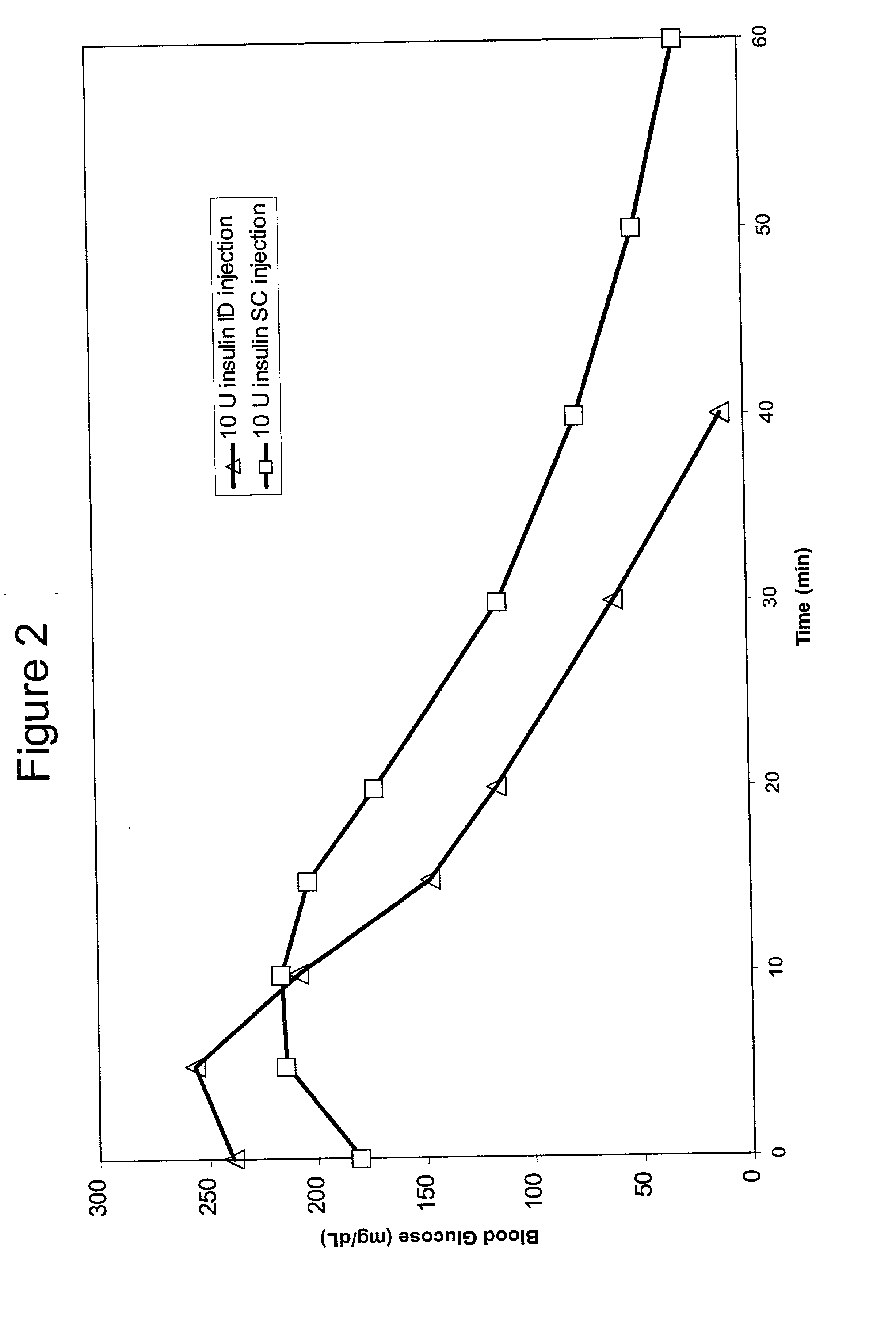
Intradermal delivery of substances
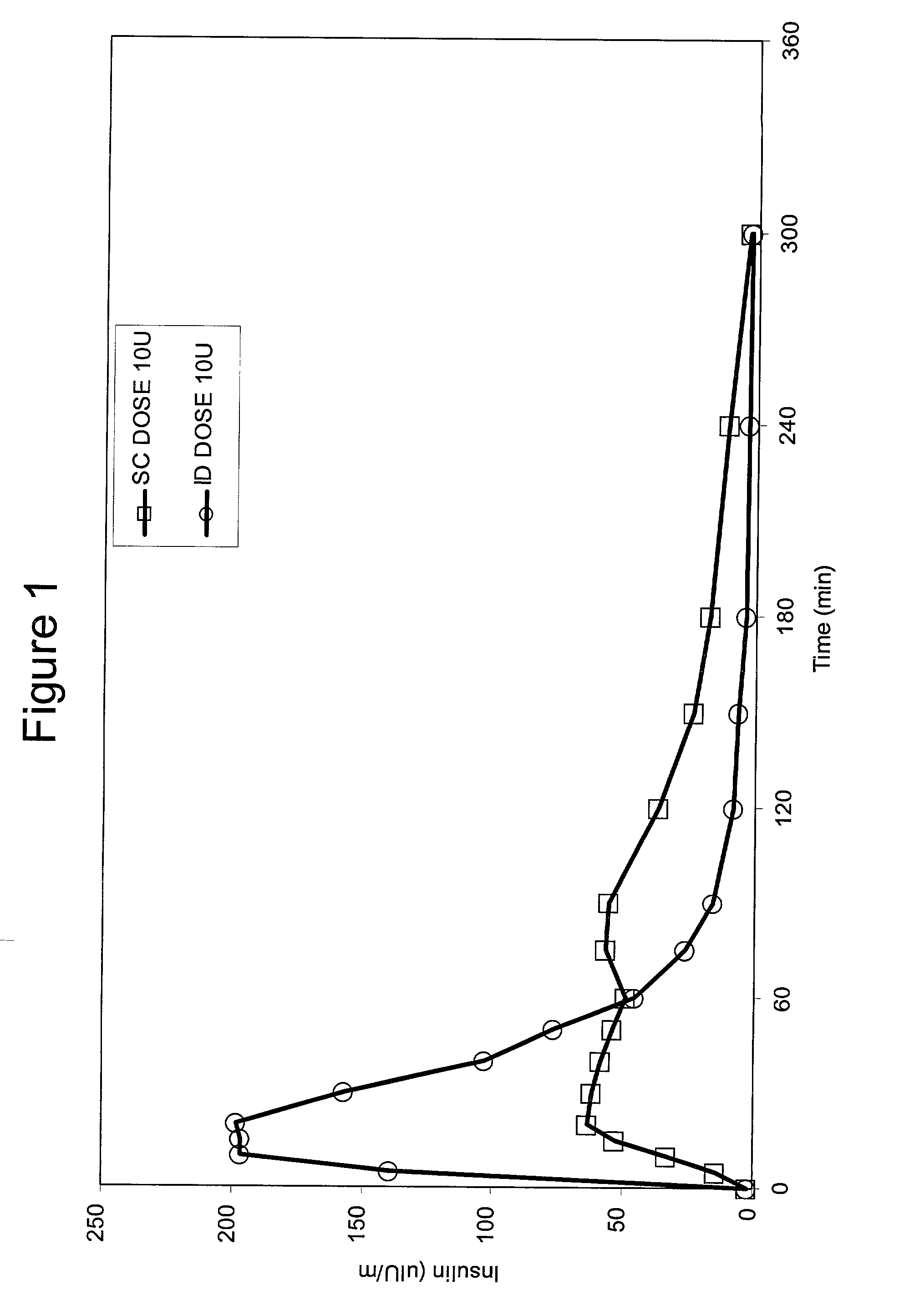
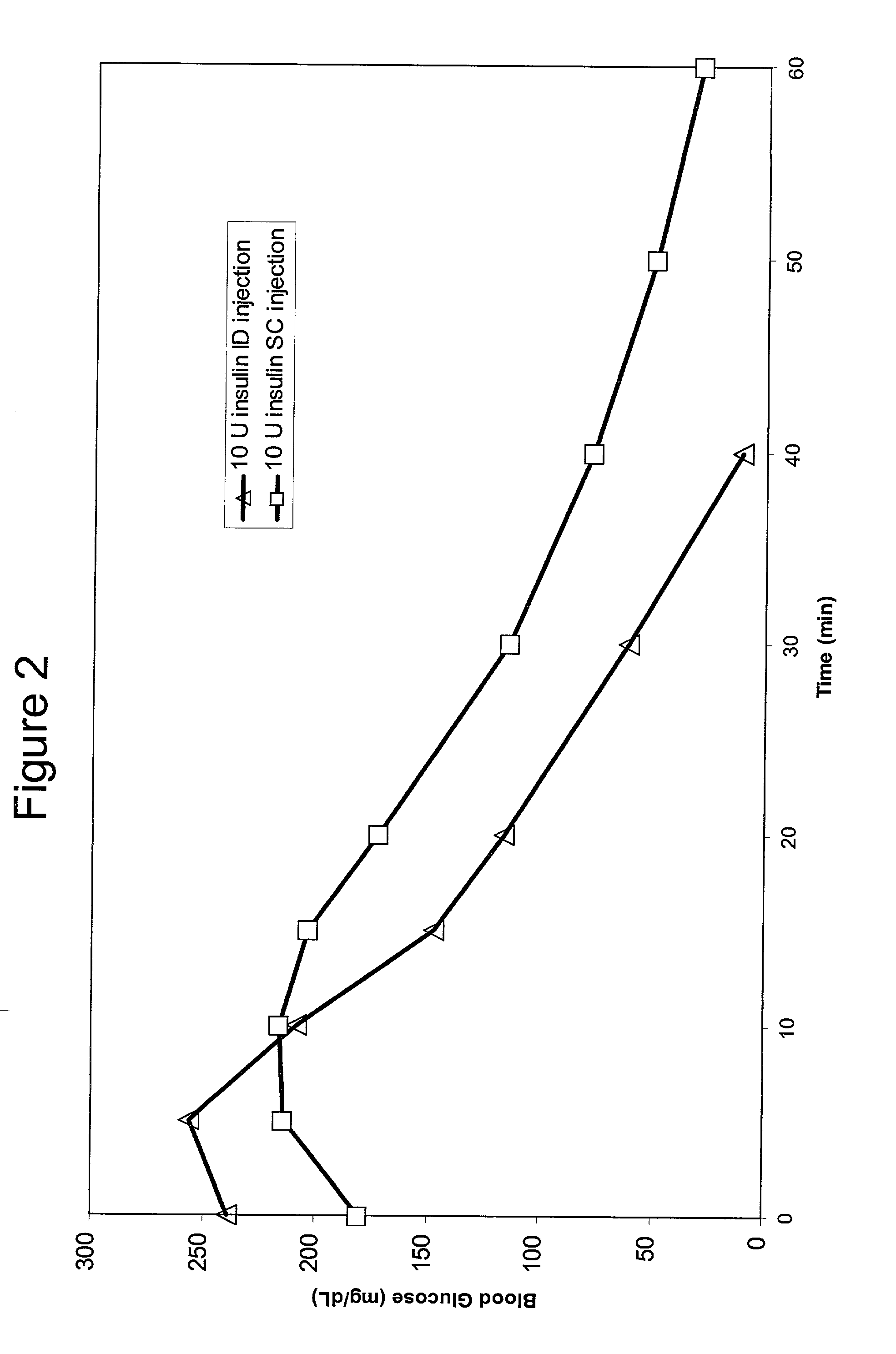
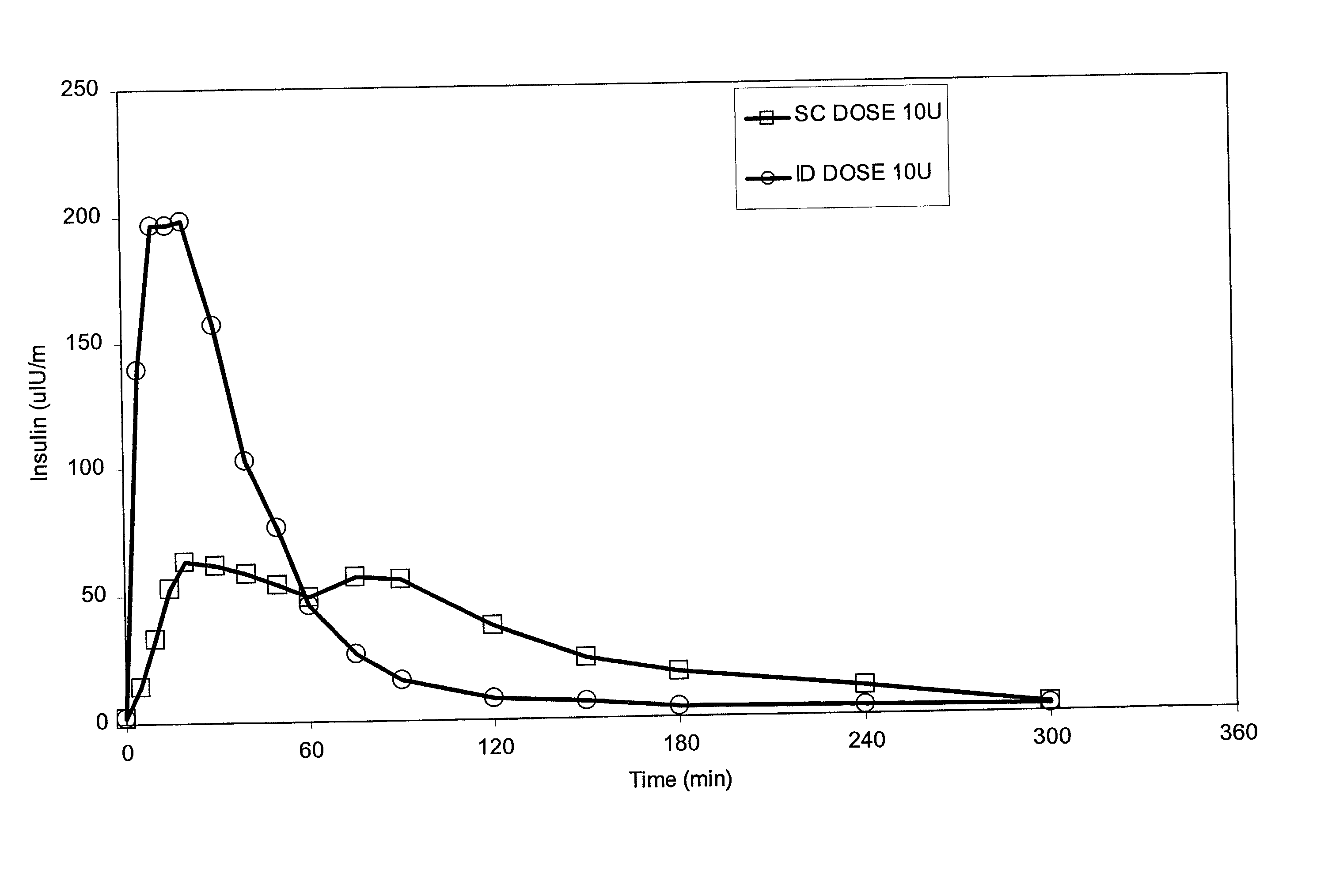
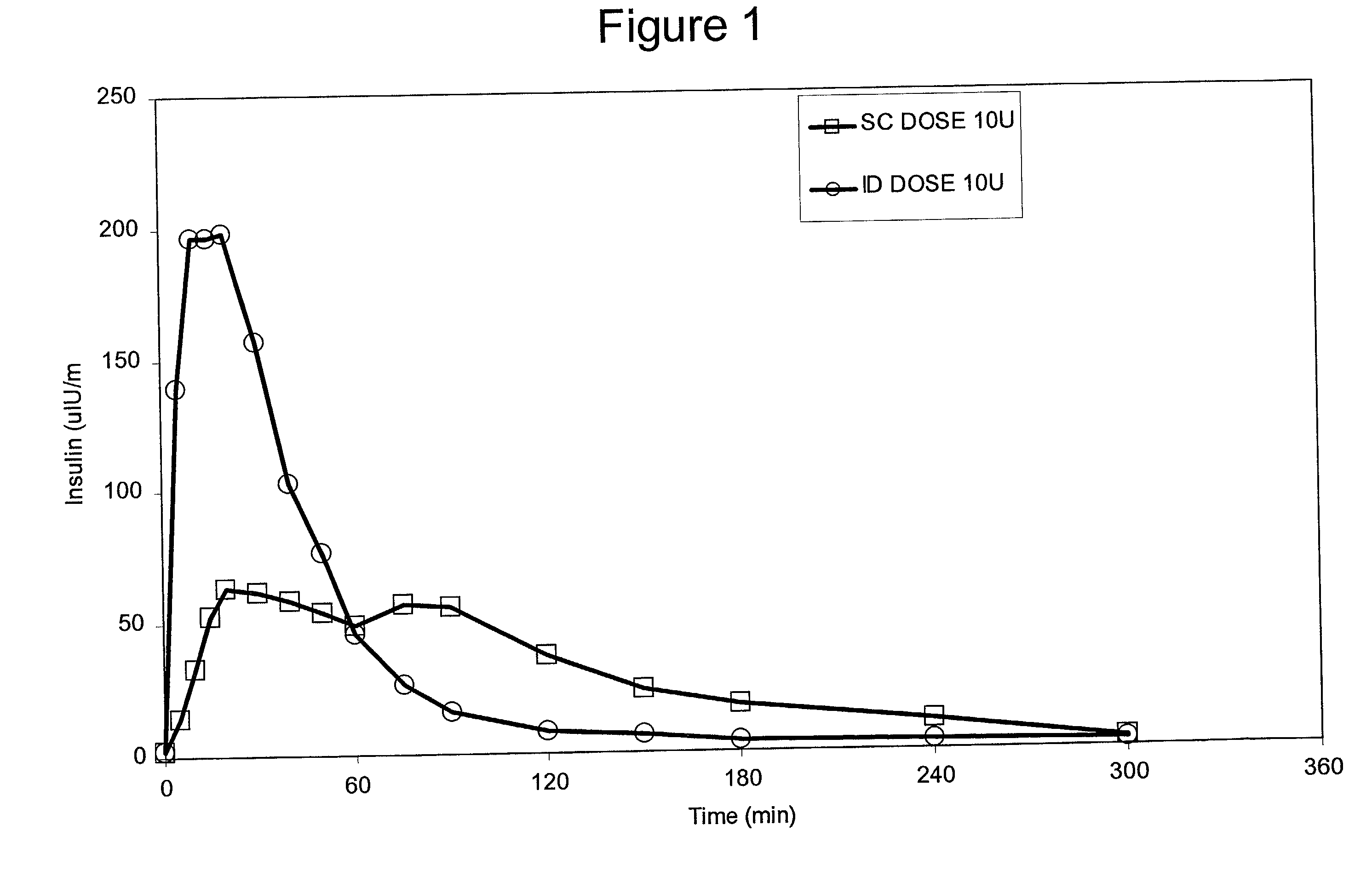
InactiveUS20040073160A1Increase uptakeRapid uptake rateJet injection syringesPeptide/protein ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
Enhanced pharmacokinetic profile of intradermally delivered substances
InactiveUS20030073609A1Increase uptakeEnhanced iontophoresisPowder deliveryOrganic active ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
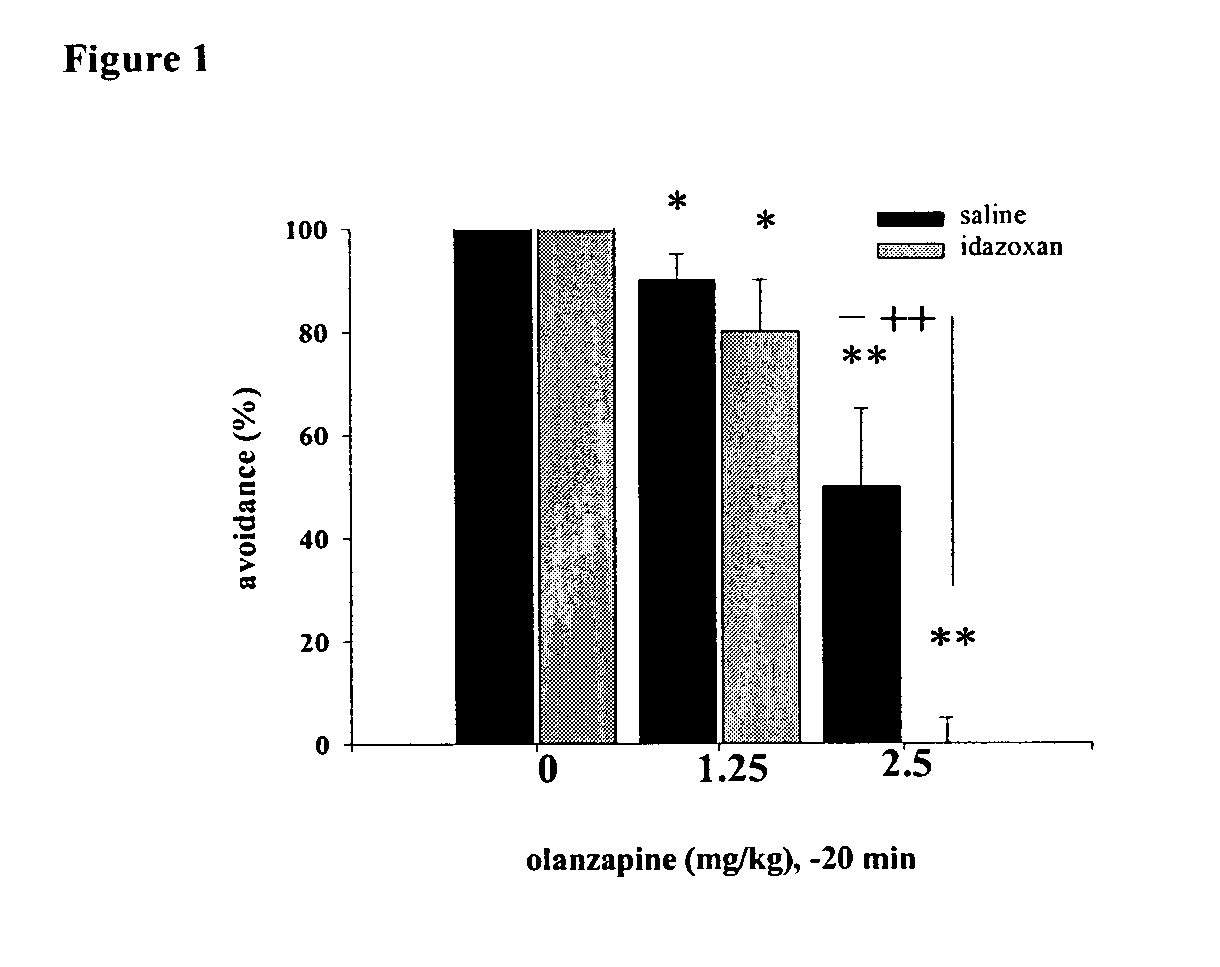
Novel antipsychotic combination therapies and compositions useful therein
InactiveUS20040127489A1Minimize adverse eventReduce doseBiocideNervous disorderAdrenergicCombination therapy
The present invention provides novel antipsychotic therapies and compositions useful therein and provides methods for identifying new candidate molecules for the treatment of psychosis based on the proportional binding affinities for alpha2 adrenergic and D2 dopamine receptors.
Owner:THE STANLEY MEDICAL RES INST
Novel D3 Dopamine Receptor Agonists to Treat Dyskinesia in Parkinson's Disease
The present invention provides a method of inhibiting, suppressing or preventing levodopa-induced dyskinesia in a patient suffering from Parkinson's Disease, comprising the step of administering to the patient a pharmaceutical composition comprising at least one compound of the invention. The present invention further provides a method of inhibiting, suppressing or preventing Parkinson's Disease in a patient, comprising the step of administering to the patient a pharmaceutical composition comprising at least one compound of the invention.
Owner:RUTGERS THE STATE UNIV +1
Methods of treating metabolic syndrome using dopamine receptor agonists
InactiveUS20050054652A1Prevent desensitizationMinimize desensitizationBiocideAnimal repellantsDiseaseHypertriglyceridemia
The present invention is directed to a method of simultaneously treating hypertension, hypertriglyceridemia, a pro-inflammatory state, a pro-coagulative state, and insulin resistance (with or without treating obesity or endothelial dysfunction), associated with or independent from Metabolic Syndrome, comprising the step of administering to a patient suffering from such disorders a therapeutically effective amount of a central acting dopamine agonist. In one embodiment, the central acting dopamine agonist is bromocriptine, optionally combined with a pharmaceutically acceptable carrier.
Owner:CINCOTTA ANTHONY H
Methods and compositions for diagnosis and treatment of pathological conditions related to abnormal dopamine receptor expression
InactiveUS6025193ANot limitedProducing selective changeNervous disorderPeptide/protein ingredientsEukaryotic plasmidsLiposome
Methods and compositions are provided for diagnosing and treating pathological conditions related to a dopamine receptor abnormality. The methods comprise administering to a patient having such a pathological condition a plasmid encoding an oligonucleotide, antisense to one or more RNA molecules encoding one of the several dopamine receptors, thereby selectively controlling expression of one or more dopamine receptor subtypes, and alleviating the pathological conditions related to their expression. The vectors are targeted to specific regions of the brain via complexation with antibody studded liposomes.
Owner:ALLEGHENY UNIV OF THE HEALTH SCI
Composition for the administration of a D1-agonists
Owner:WEST PHARMA SERVICES DRUG DELIVERY & CLINICAL RES CENT
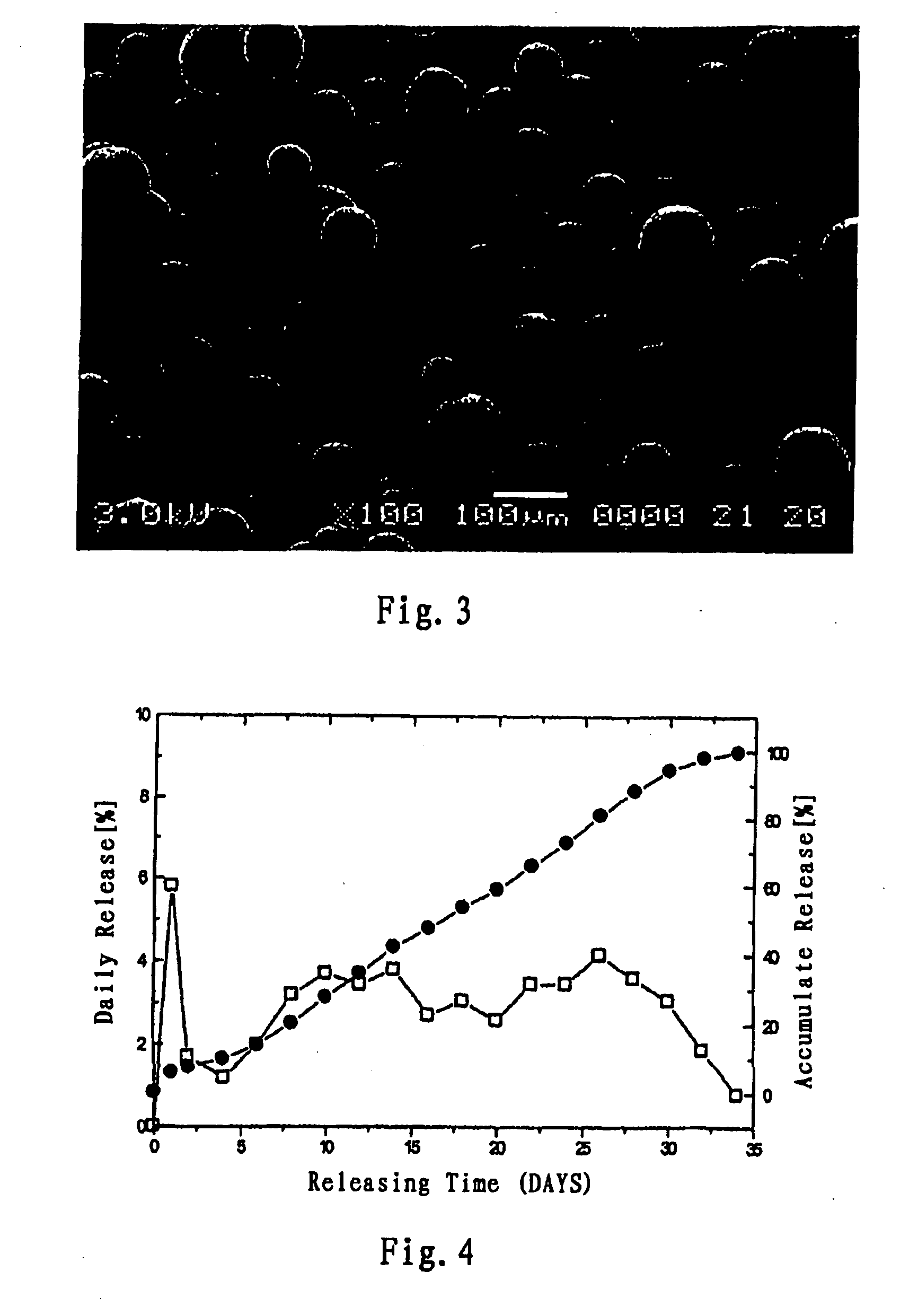
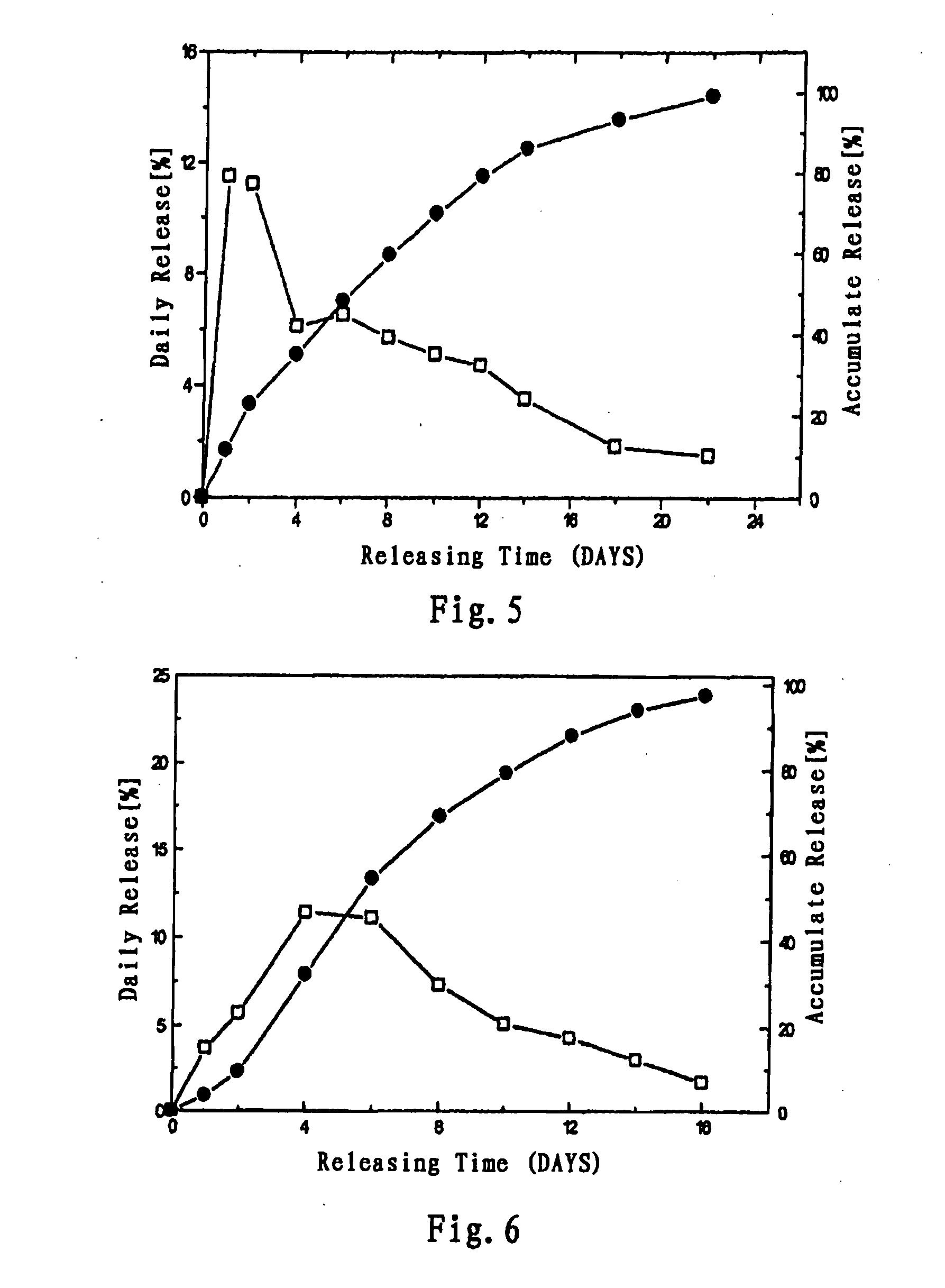
Long acting sustained-release formulation containing dopamine-receptor stimulant medicine and its preparation process
ActiveCN1762495AReduce frequency of useImprove bioavailabilityNervous disorderPharmaceutical non-active ingredientsStimulantSustained Release Formulations
The invention relates to a long acting sustained-release formulation containing dopamine-receptor stimulant medicine, which comprises 5-5- wt% of effective dose of dopaminergic acceptor medicaments, and 50-95 wt% of medicinal macromolecular auxiliary materials.
Owner:SHANDONG LUYE PHARMA CO LTD
Pramipexole once-daily dosage form
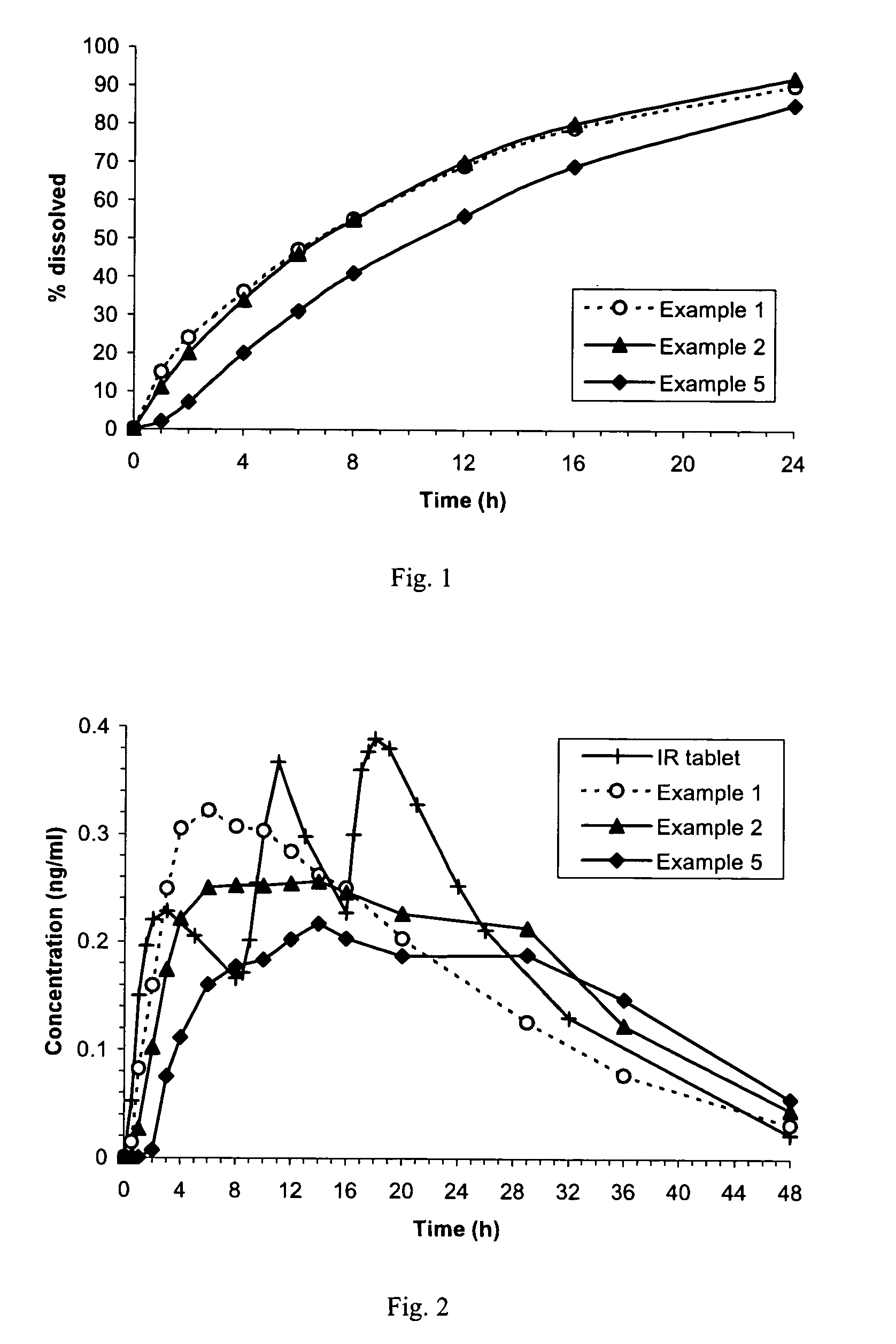
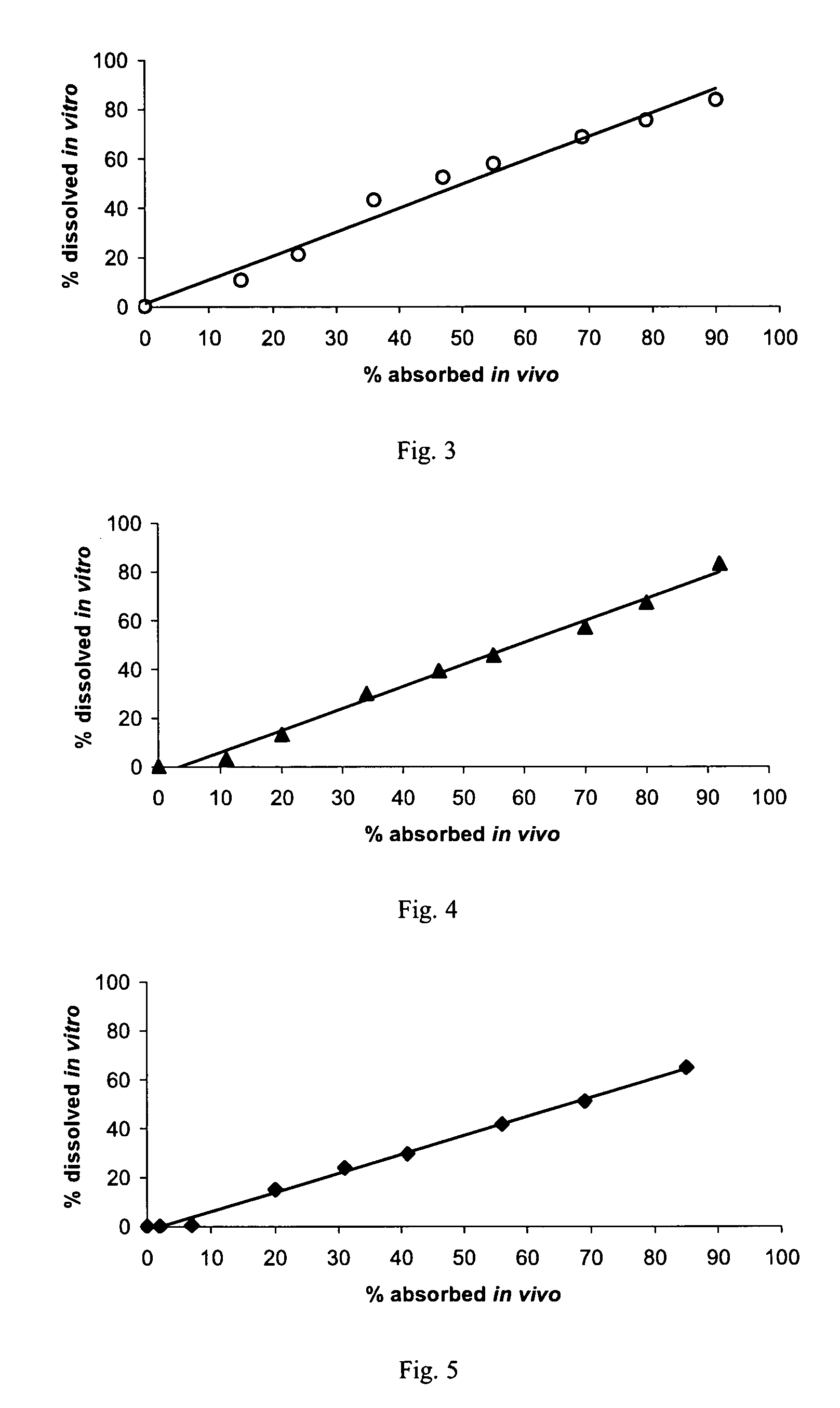
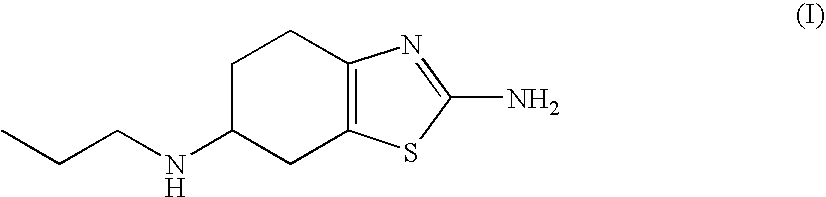
An orally deliverable pharmaceutical composition comprises a therapeutically effective amount of pramipexole or a pharmaceutically acceptable salt thereof and at least one pharmaceutically acceptable excipient, said composition exhibiting at least one of (a) an in vitro release profile wherein on average no more than about 20% of the pramipexole is dissolved within 2 hours after placement of the composition in a standard dissolution test; and (b) an in vivo pramipexole absorption profile following single dose administration to healthy adult humans wherein the time to reach a mean of 20% absorption is greater than about 2 hours and / or the time to reach a mean of 40% absorption is greater than about 4 hours. The composition is useful for oral administration, not more than once daily, to a subject having a condition or disorder for which a dopamine receptor agonist is indicated.
Owner:PHARMACIA CORP
Heteroaryl Amide Analogues
Owner:H LUNDBECK AS
Method of treating metabolic disorders and depression with dopamine receptor agonists
This invention relates to methods and formulations for treating metabolic disorders and depression. In some embodiments, the methods comprise administering a dopamine receptor agonist and an anti-depressant.
Owner:VEROSCI
Tetrahydroindolone and purine derivatives linked to arylpiperazines
InactiveUS6770638B2Effective treatmentImprove propertiesBiocideOrganic chemistryPurinePurine derivative
Pharmaceutical composite compositions comprising tetrahydroindolones linked to arylpiperazines and derivatives thereof are disclosed. Specifically, composite compositions useful in treating anti-psychotic disorders are disclosed. The composite compositions disclosed herein can effectively ameliorate symptoms and treat psychotic disorders without causing a decrease in cognitive function. Generally, the composite compounds consist of two moieties, moiety A and B in which a tetrahydroindolone comprises a moiety A linked through a linker L to a moiety B, where B is an arylpiperazinyl moiety. The composite compound provides anti-psychotic actively by interaction with GABA, seratoninne and dopamine receptors. The composite molecules with the combined activities will provide treat psychiatric and neurological diseases without cognitive impairment.
Owner:SPECTRUM PHARMA INC
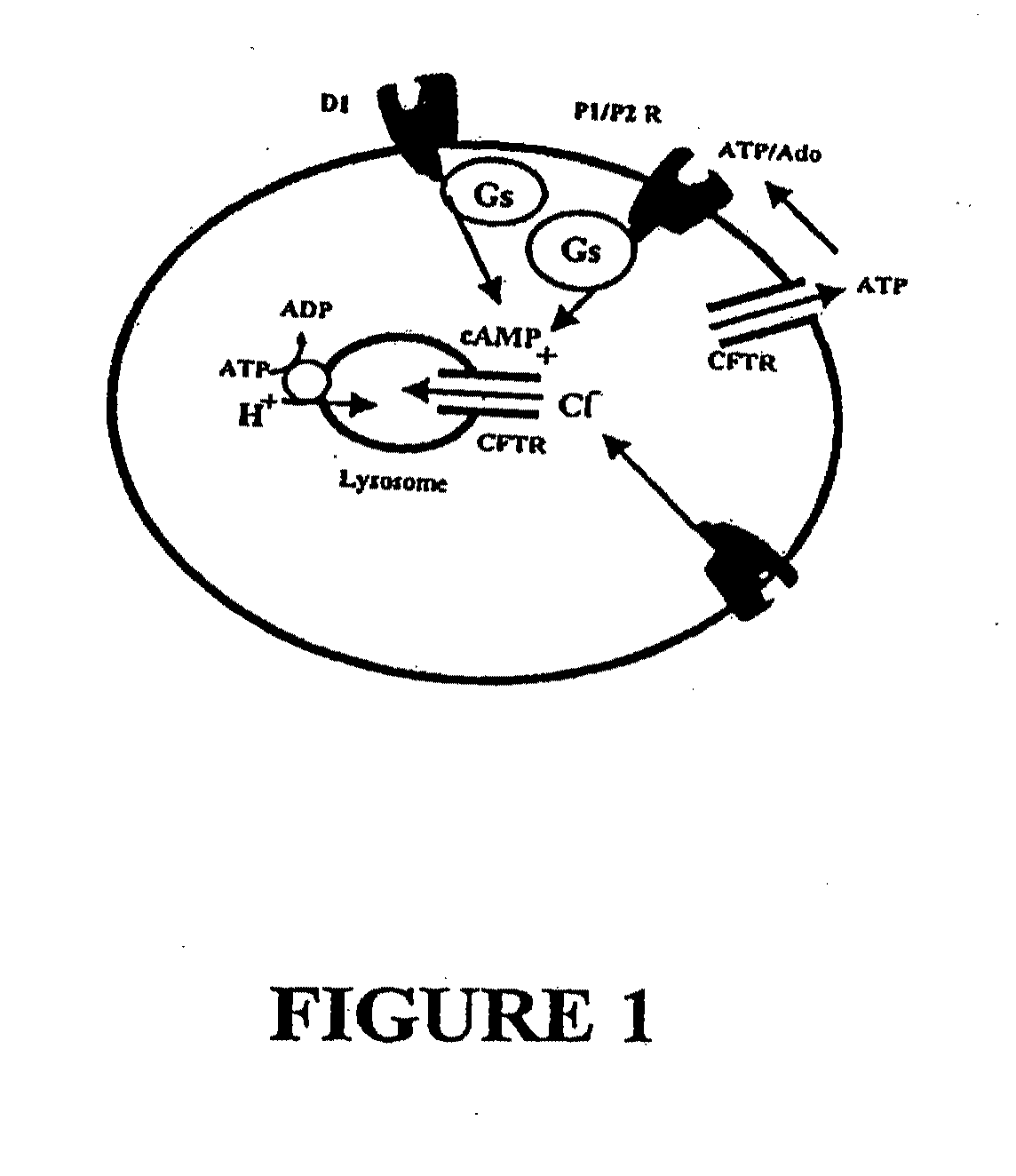
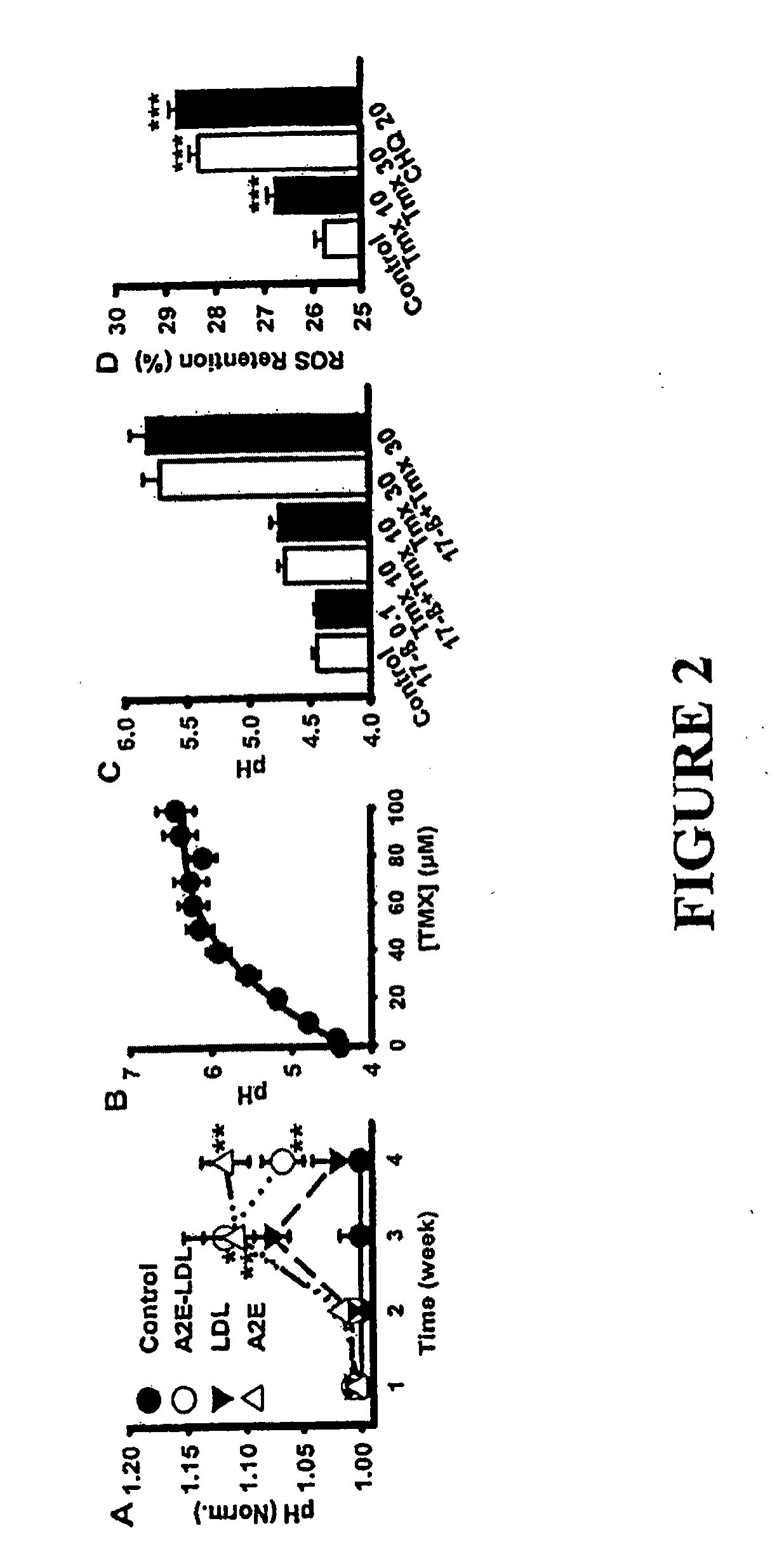
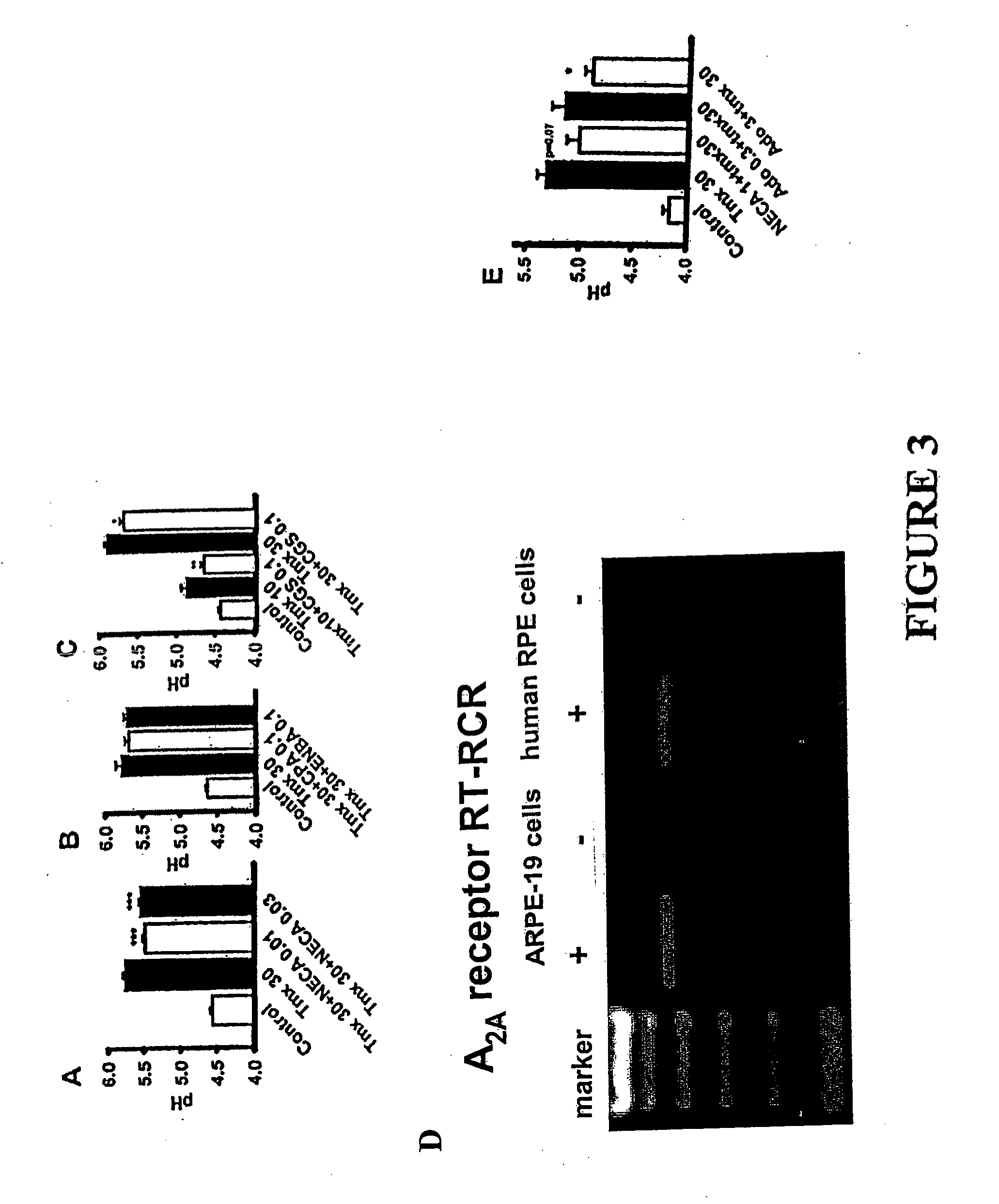
Method for Treatment of Macular Degeneration
Provided is a method of treating or preventing age-related macular degeneration (AMD) in a patient subject to, or symptomatic of the disease, wherein the method comprises restoring normal lysosomal pH (pHL), or acidifying an abnormally elevated pHL, thus decreasing or preventing a damaging accumulation of lipofuscin or waste products in the retinal pigment epithelium (RPE) cells of the eye of the patient. Further, this method is achieved by elevating cAMP by administering or stimulating receptors coupled to a Gs protein in an amount sufficient to decrease the elevated pHL or restore acidity of said lysosomes, specifically by administering or stimulating receptors comprising D1-like dopamine receptors by the use of D1-like dopamine receptor agonists. Methods for selecting and quantifying the effectiveness of drugs to restore pHL and determine outer segment clearance rates is also provided using a high through-put screening protocol.
Owner:MITCHELL CLAIRE +1
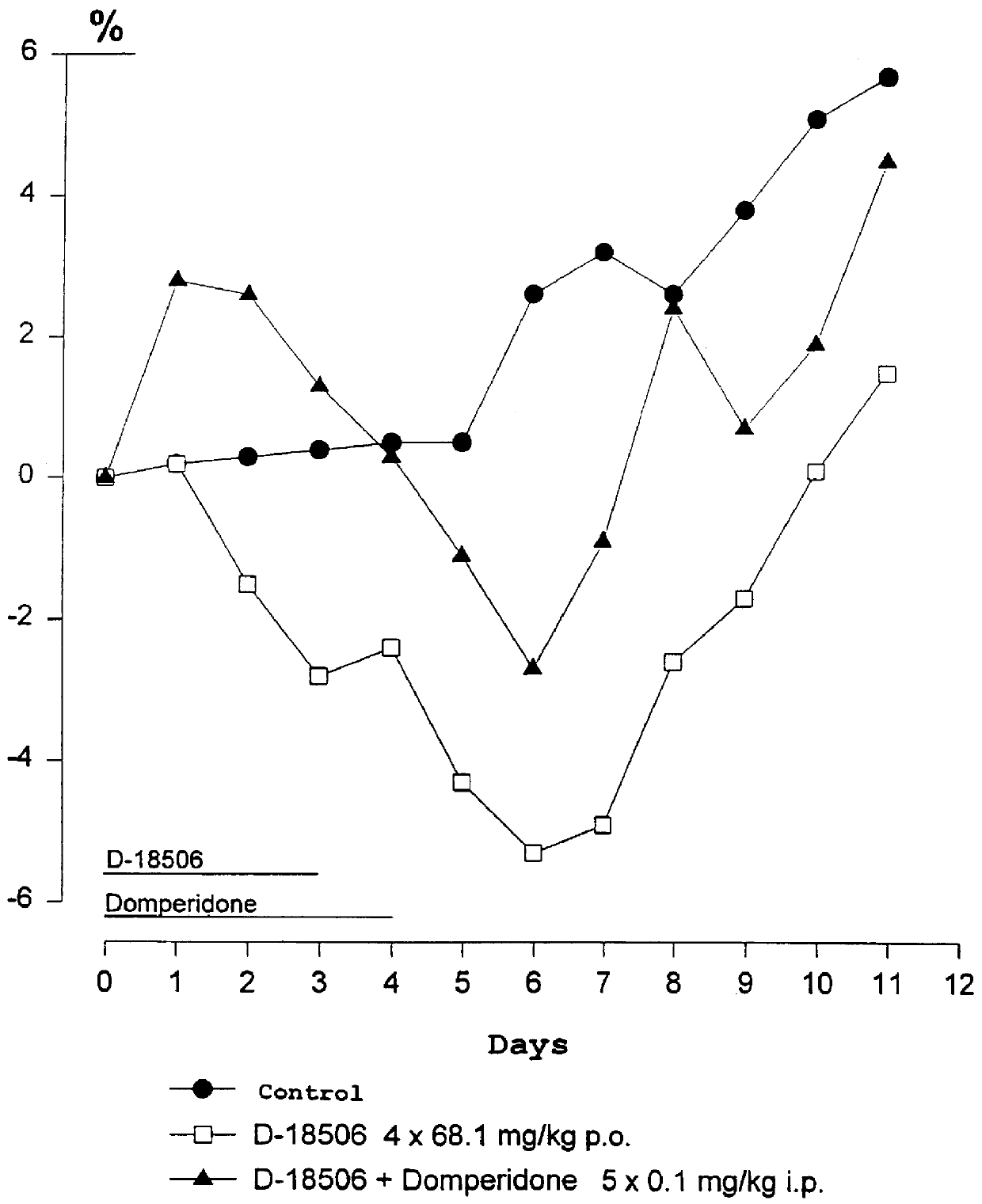
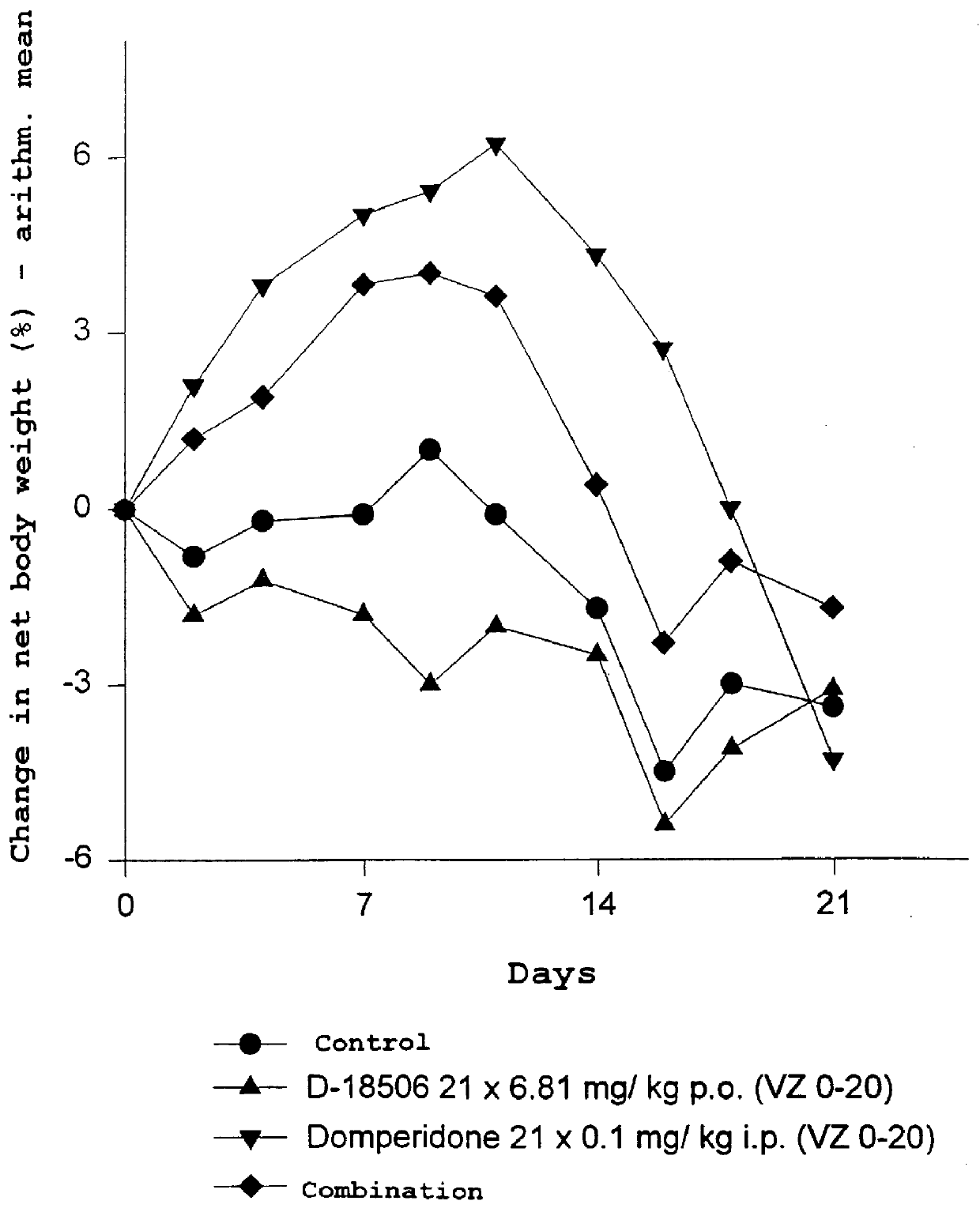
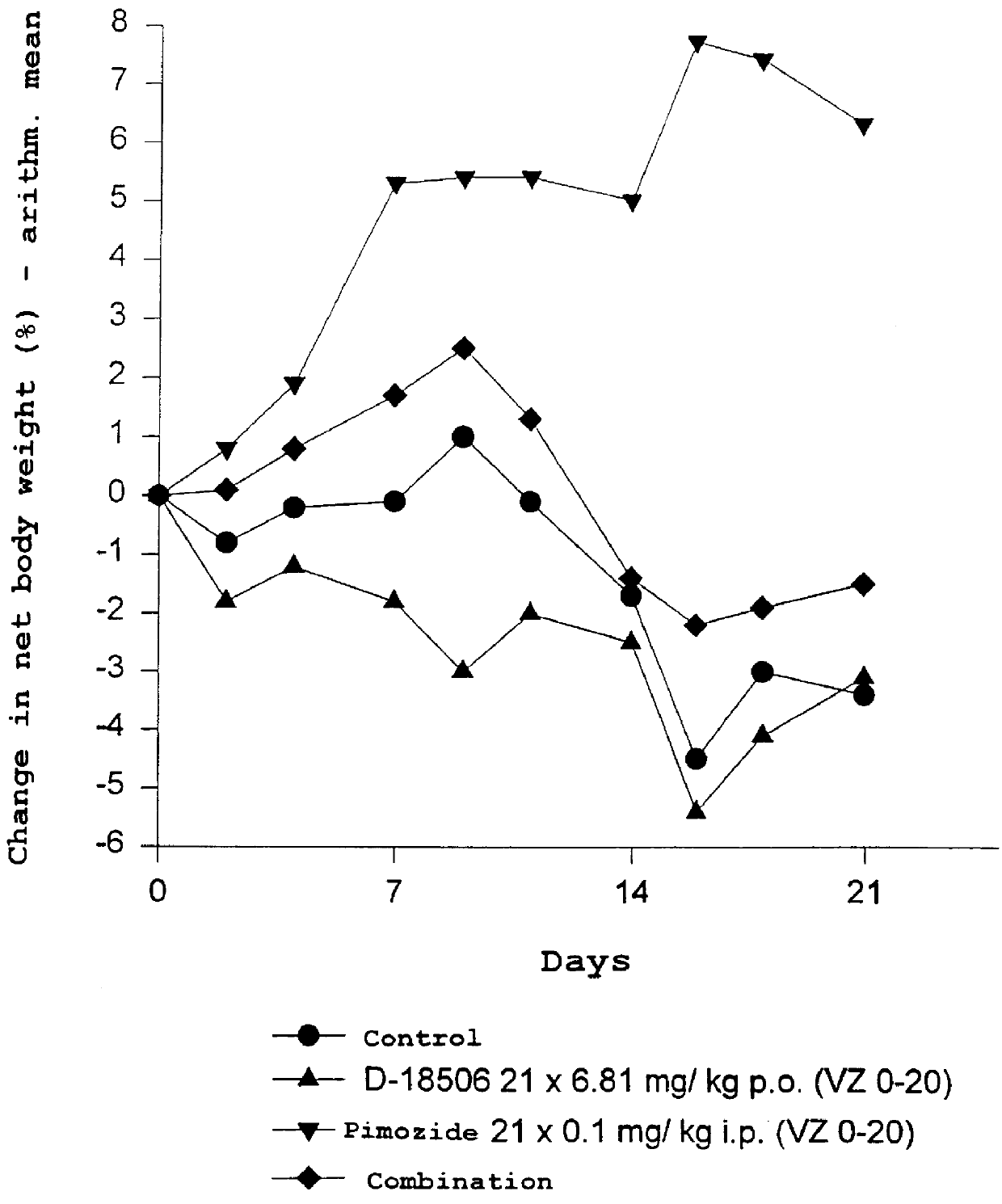
Use of dopamine receptor antagonists in palliative tumor therapy
The side effect of decrease in body weight caused by the alkylphosphocholines such as miltefosine can be antagonized by certain acetylcholine receptor antagonists such as domperidone and pimozide. The combination of alkylphosphocholine plus the antagonist does not have any effect on the anti-tumor action of the alkylphosphocholine. The combination also caused no new side effects in the animals.
Owner:AETERNA ZENTARIS GMBH
Use of N-desmethylclozapine and related compounds as dopamine stabilizing agents
InactiveUS20060252744A1Reduce exposureMany symptomBiocideNervous disorderPsychosis drugNeuropsychiatric disease
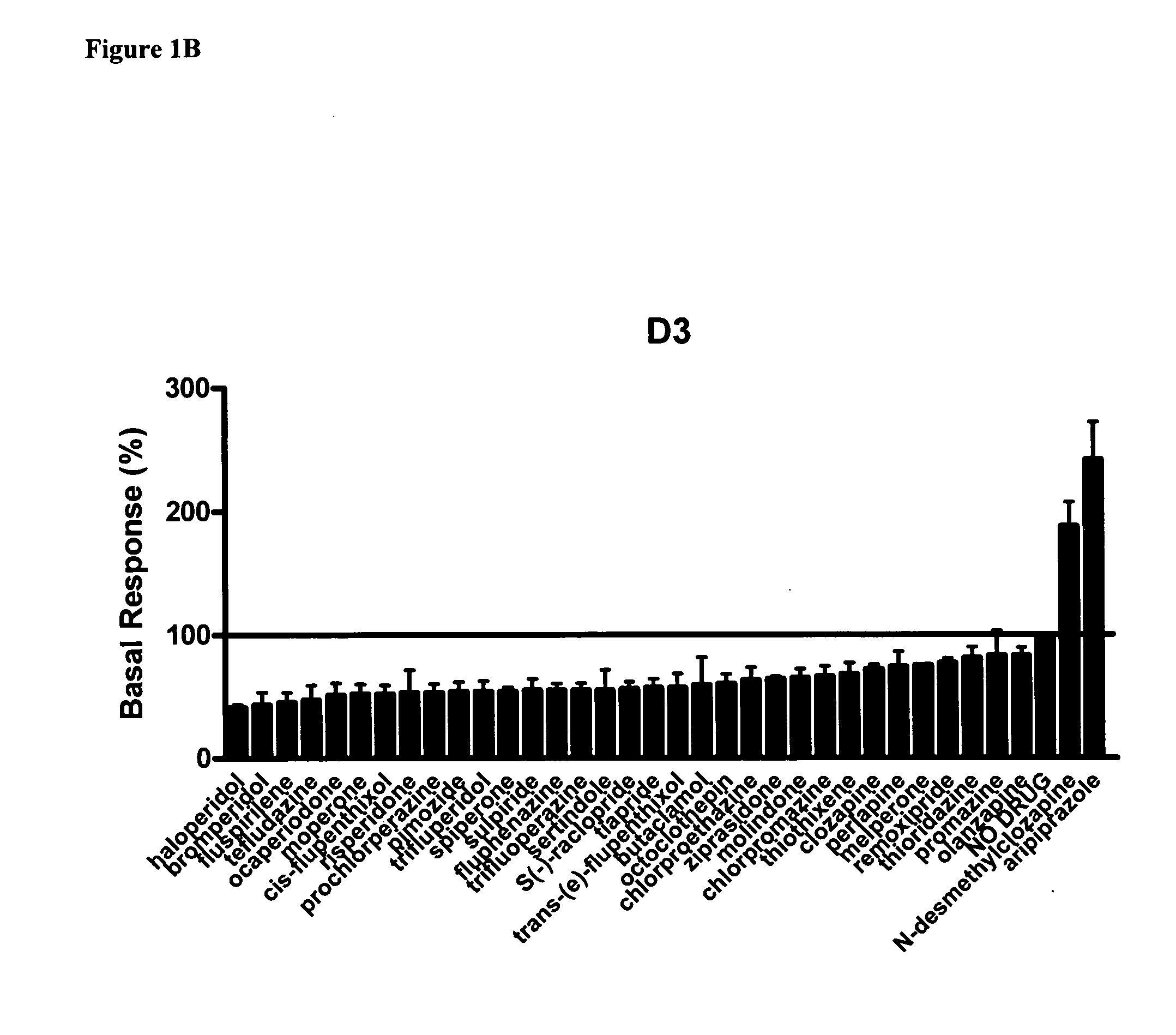
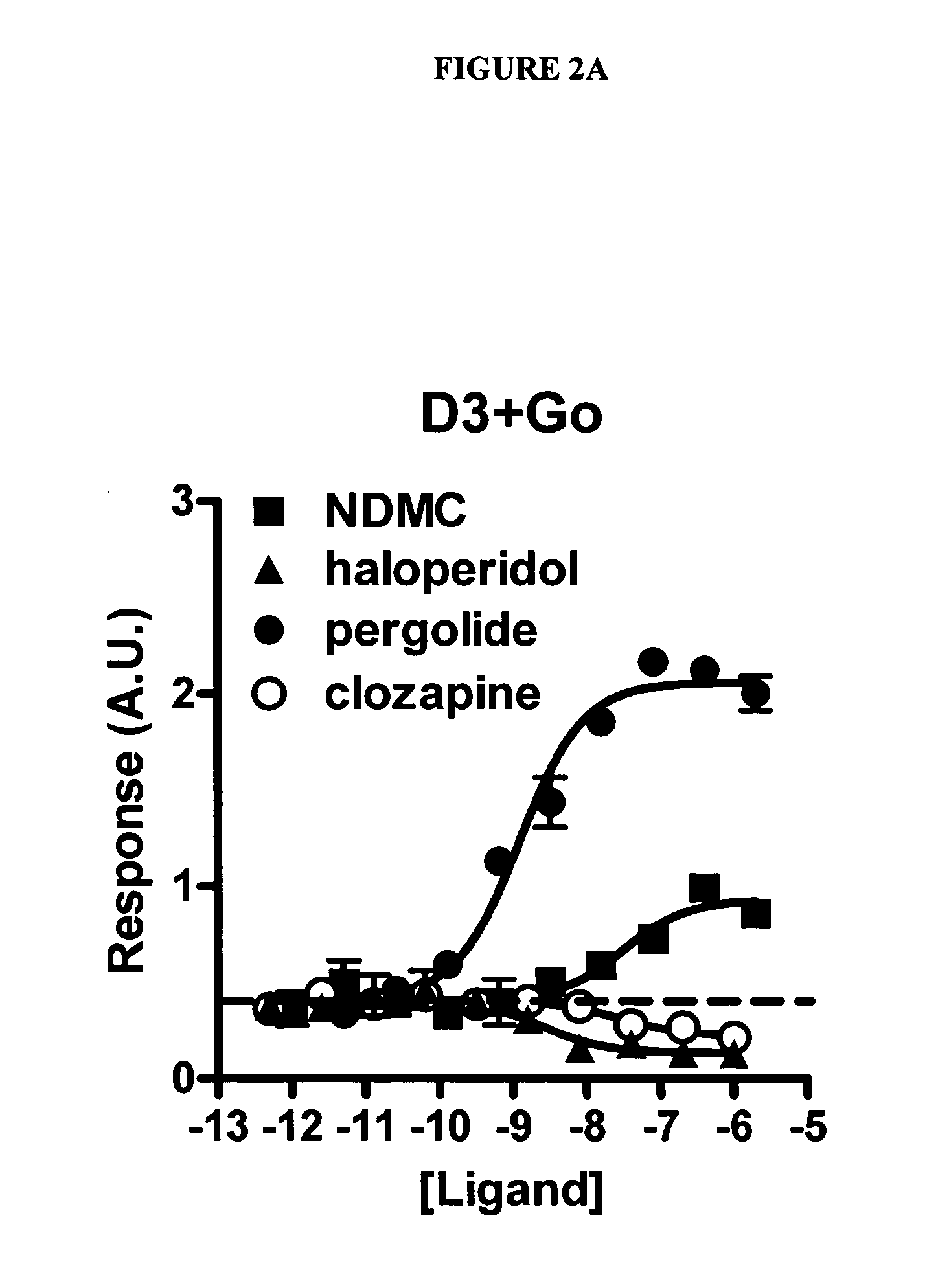
Disclosed herein is the use of N-desmethylclozapine (NDMC) and related compounds to treat a variety of neuropsychiatric diseases including psychosis. It is shown that NDMC and related compounds are agonists or partial agonists at D2 and D3 dopamine receptors and thus may be effective as a dopamine stabilizing agent, allowing it to be used to treat or provide reduced incidence of Extrapyramidal symptoms (EPS) and / or tardive dyskinesias (TD). Also disclosed is administering NDMC and related compounds in combination with other anti-psychotic agents.
Owner:ACADIA PHARMA INC
Methods and compositions for the induction of hypothermia
InactiveUS20120282227A1Short amount of timeReduce rateBiocideNervous disorderAntioxidantBlood vessel
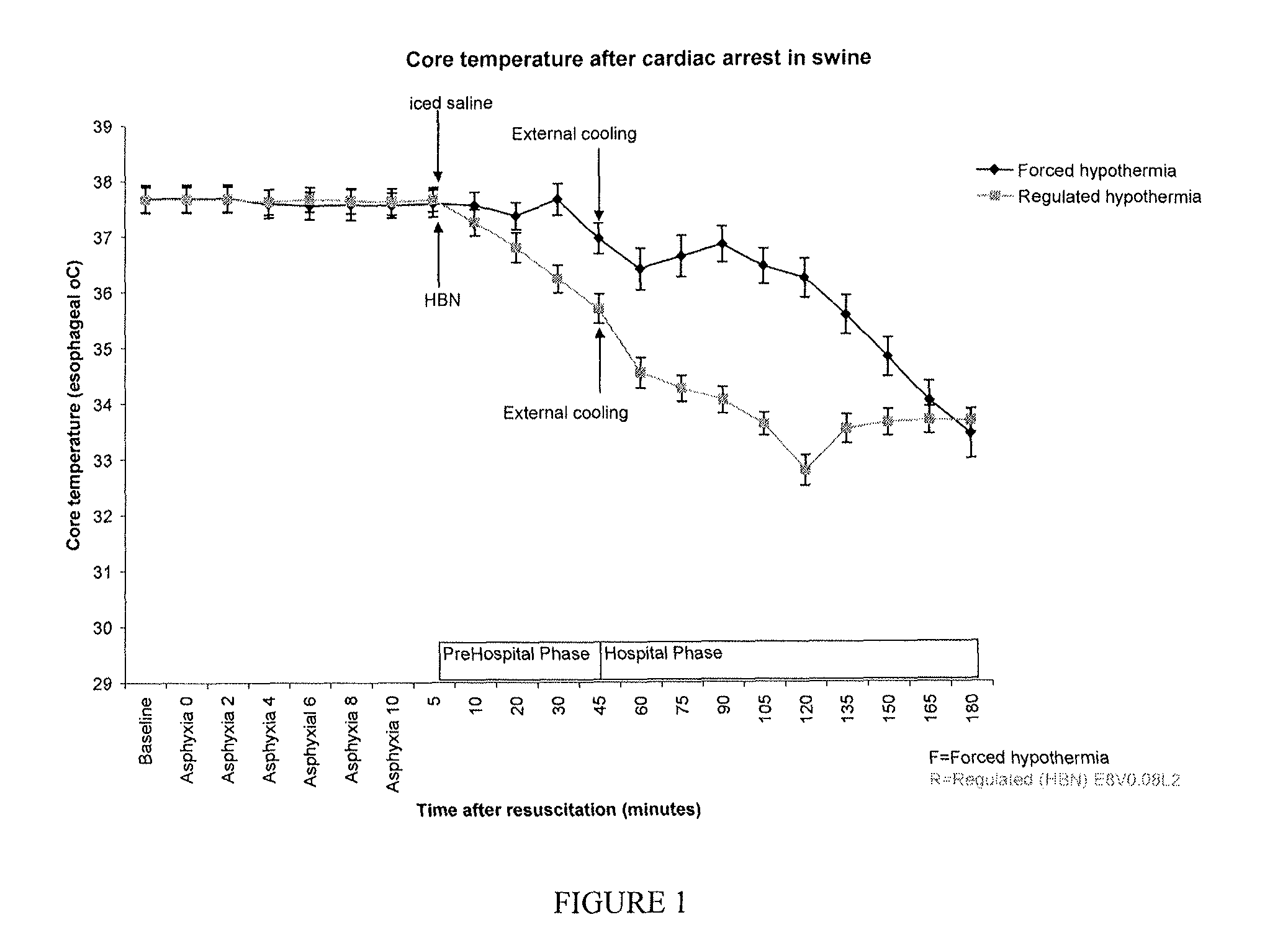
Provided herein are methods for the induction of hypothermia and the treatment of clinical insults in a subject through the administration of a regulated hypothermic multidrug combination. The compositions or multidrug combinations of the invention comprise a regulated hypothermic compound or a dopamine receptor agonist; a vasoactive compound; and an antiarrhythmic compound or a serotonin 5-HT3 receptor antagonist. Additional agents can be included in the composition including at least one of an antioxidant, a vitamin, N-acetylcysteine, and an antihyperglycemic compound. The invention further recognizes that a two phase delivery of multidrug combinations, a rapid infusion of the composition to induce hypothermia followed by a period of slow infusion to maintain the hypothermic state for a sustained period of time. Using the two phase delivery method of the invention, the composition may comprise ethanol and, optionally, at least one of a vasoactive compound, an antiarrhythmic compound, a serotonin 5-HT3 receptor antagonist, an antioxidant, a vitamin, N-acetylcysteine, and an antihyperglycemic compound. This two phase delivery method can be used to deliver any of the compositions of the invention and provides significant benefits to a patient.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Compositions and methods for treating metabolic disorders
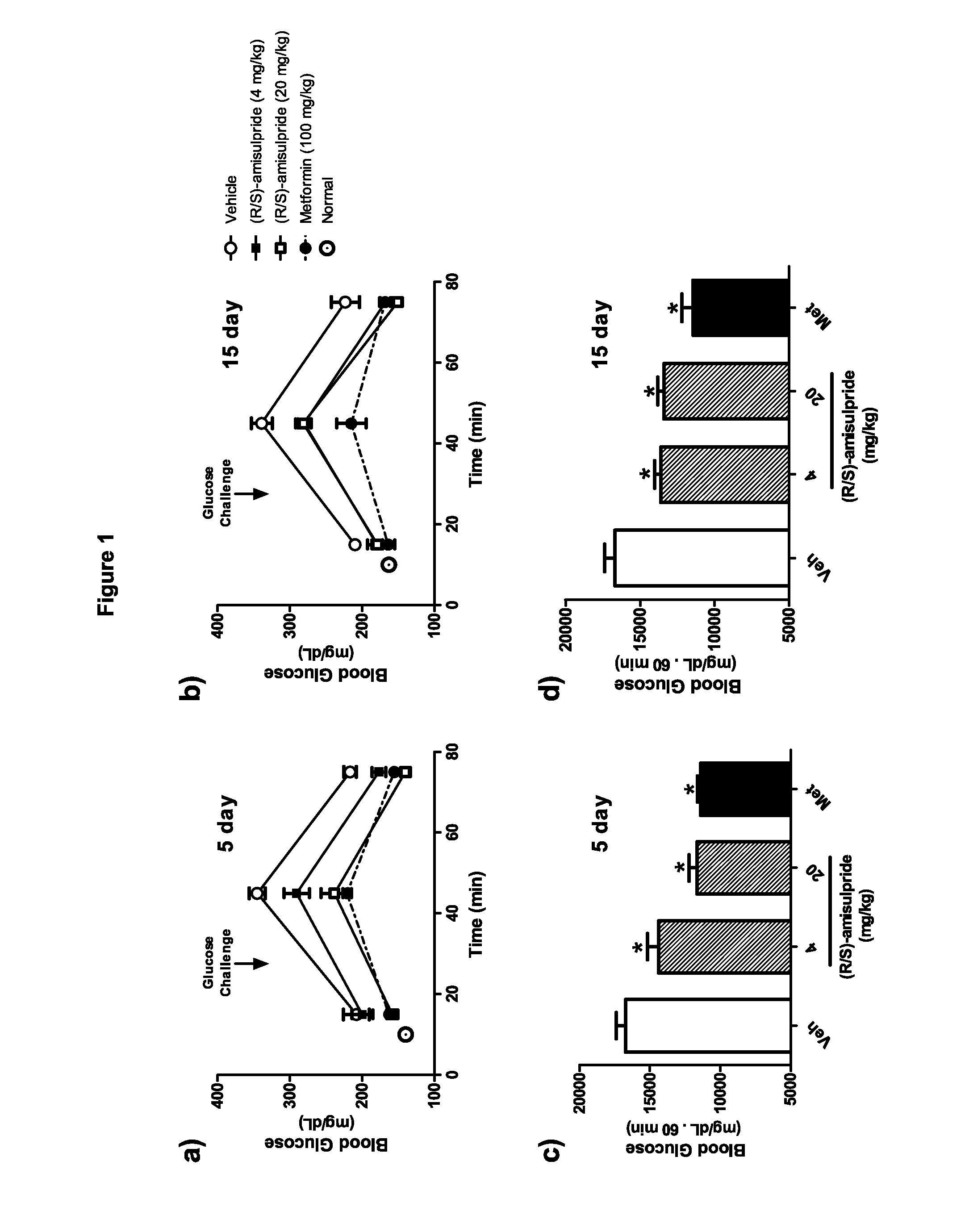
InactiveUS20150018360A1Preventing and treating and ameliorating effectModulating blood glucose levelBiocideMetabolism disorderMedicineBlood sugar
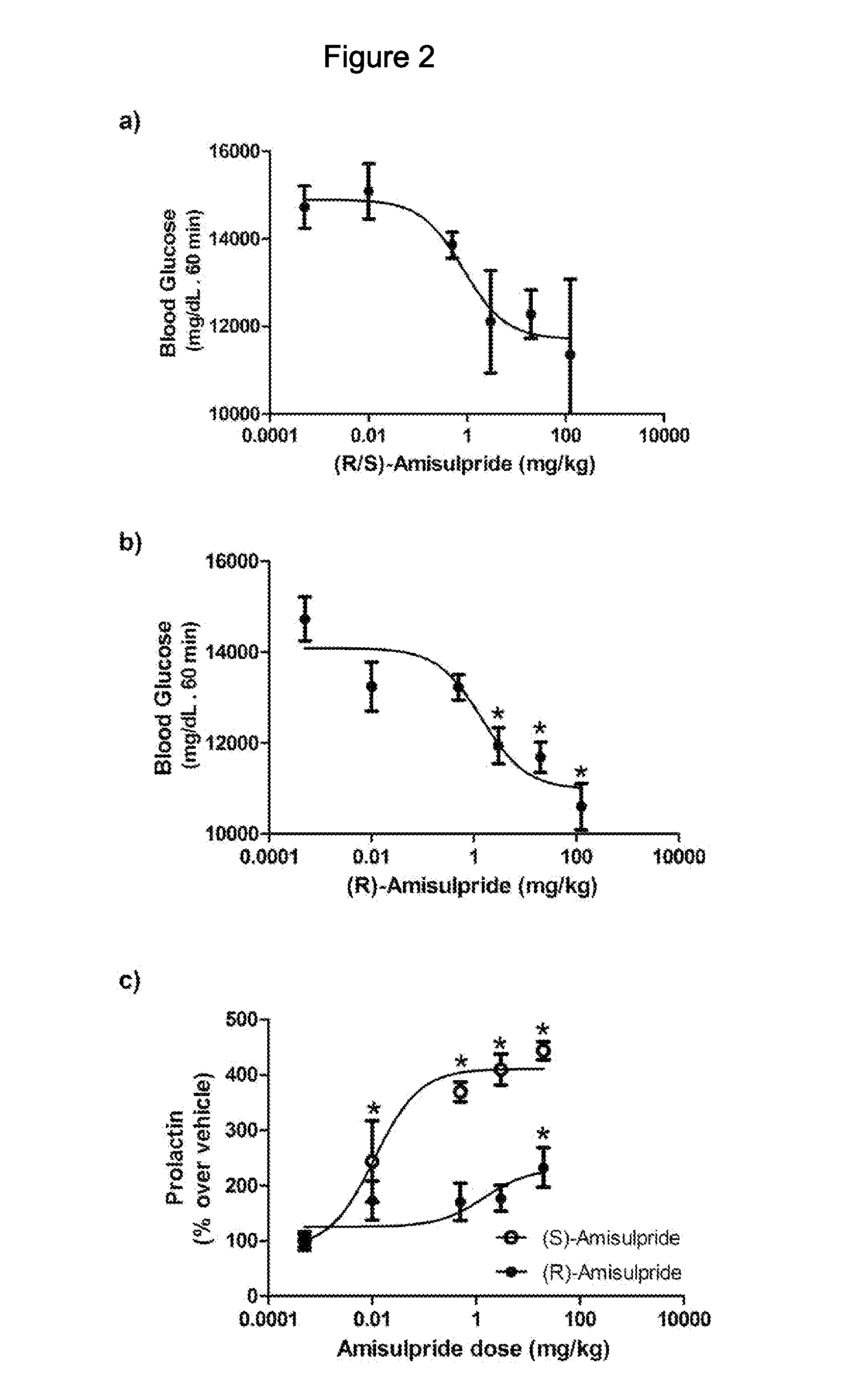
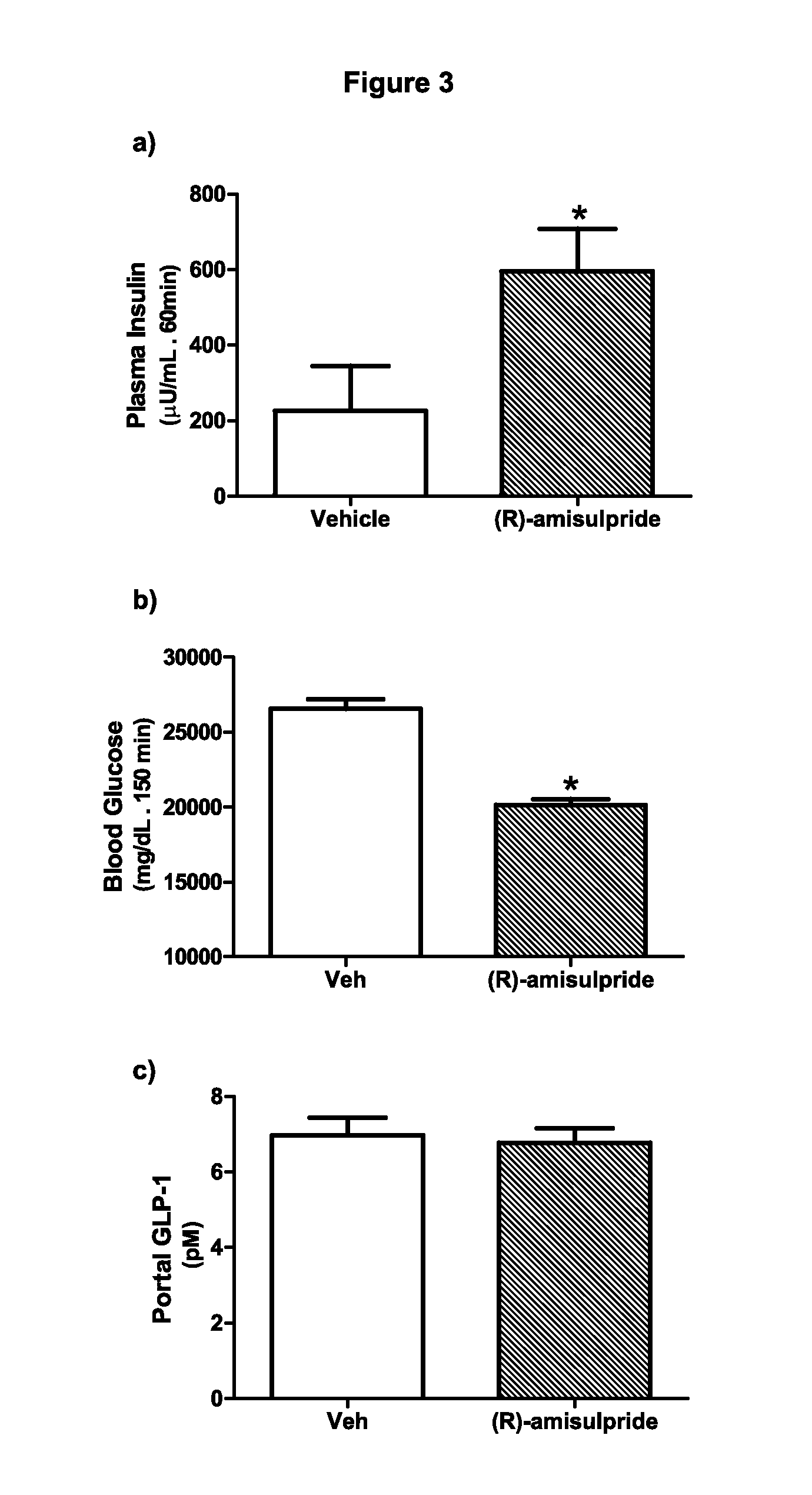
The present invention provides, inter alia, compositions containing enantiomerically pure (R)(+)-amisulpride or enantiomerically pure (R)(+)-sulpiride, optionally with dopamine receptor modulators. The present invention also provides compositions containing racemic (RS)-amisulpride or (RS)-sulpiride in combination with dopamine receptor modulators. Methods for preventing, treating, or ameliorating the effects of a metabolic disorder or key element thereof, for modulating blood glucose levels, and for preventing, treating, or ameliorating the effects of diabetes in a subject are also provided. Additionally, the present invention provides methods for counter-acting the dopamine antagonist activity of (S)-amisulpride in racemic (RS)-amisulpride, or the dopamine antagonist activity of (S)-sulpiride in racemic (RS)-sulpiride, administered to a subject to prevent, treat, or ameliorate the effects of a metabolic disorder.
Owner:BIOMED VALLEY DISCOVERIES INC
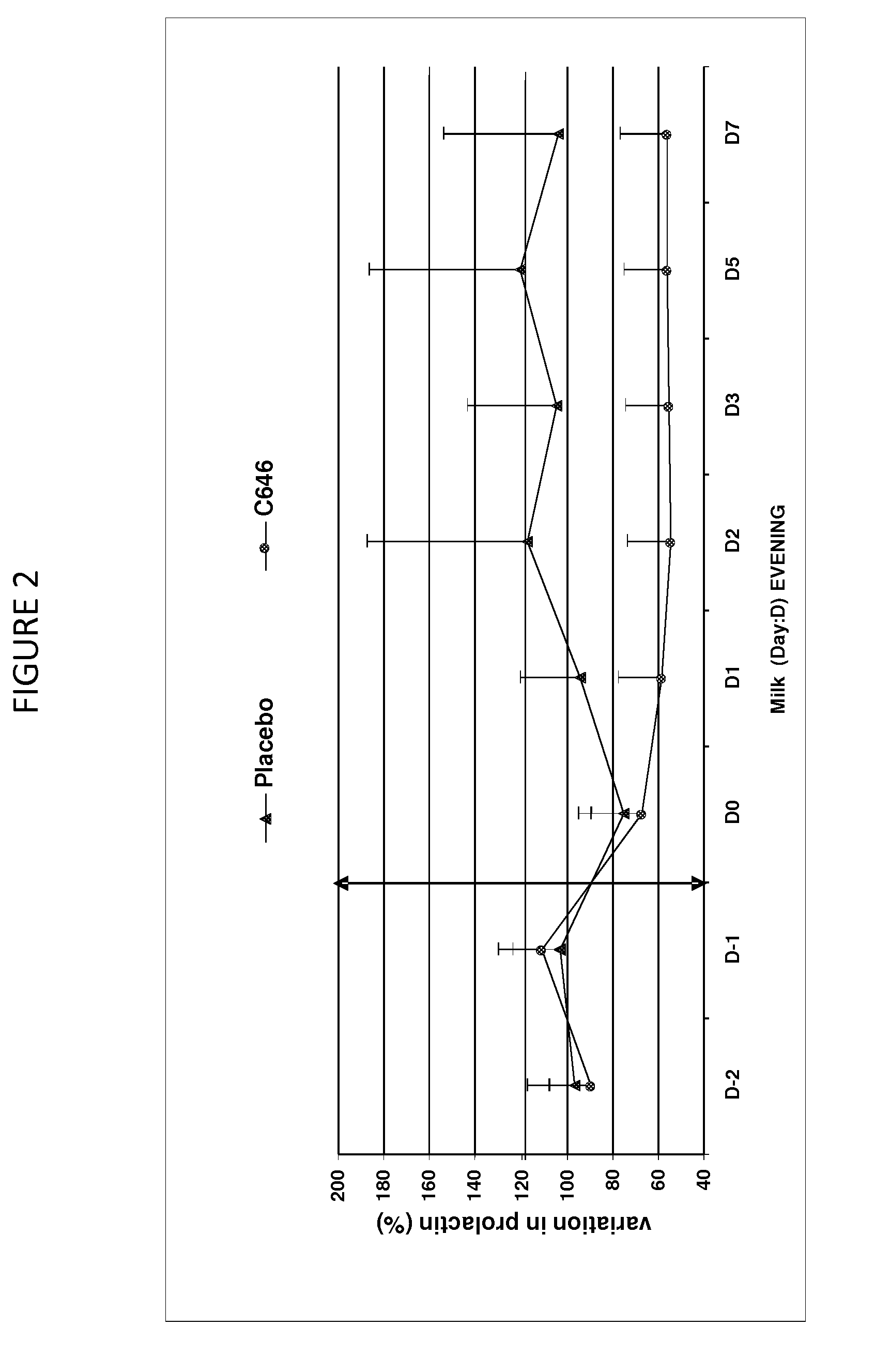
Antiprolactinic veterinary composition for ruminants
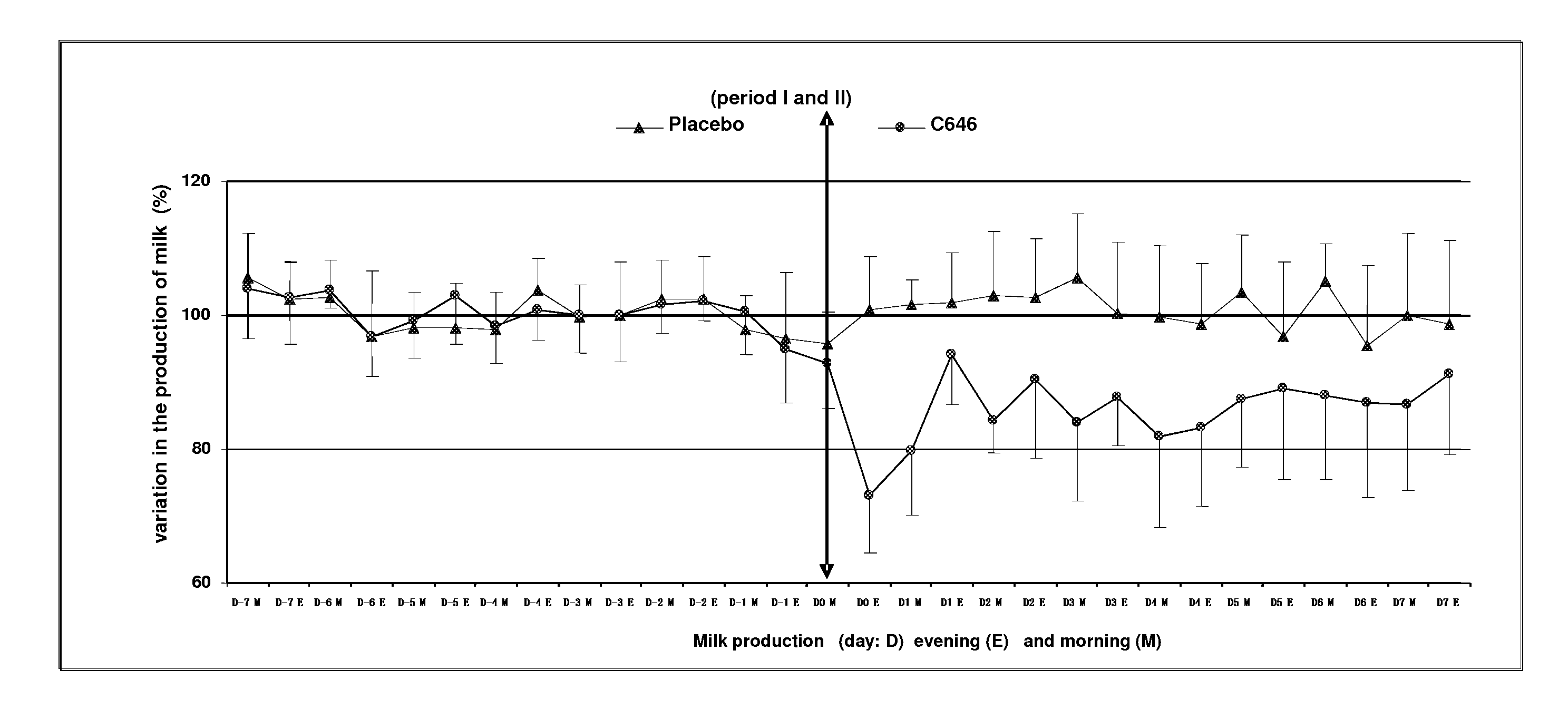
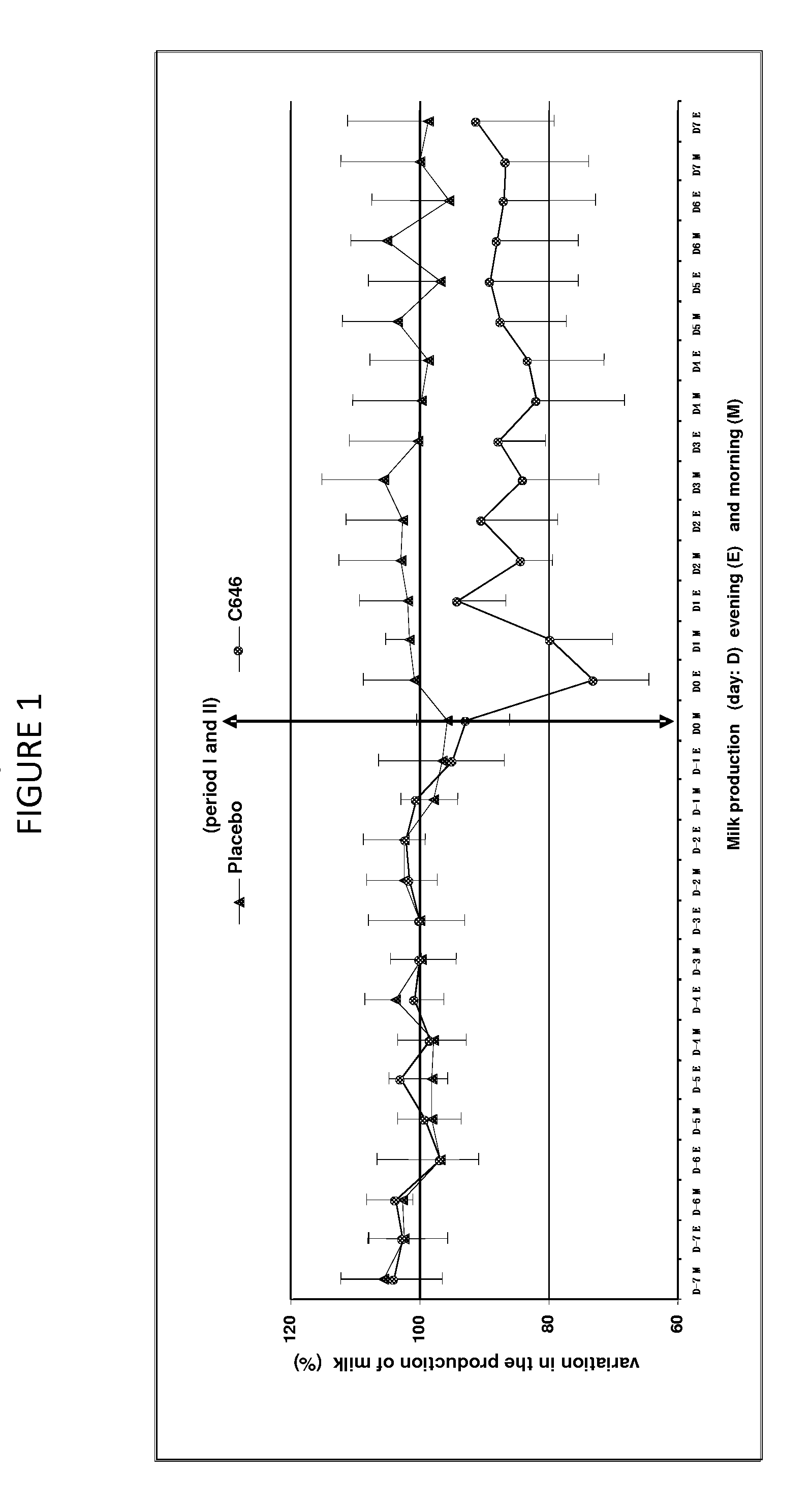
This present invention relates to an antiprolactinic veterinary composition to be administered to ruminants. Said composition comprises at least one antiprolactinic compound which is an agonist of dopamine receptors, and is particularly useful for promoting a substantial reduction of lactation, mammary involution, and for treating and / or intra-mammary diseases or infections of ruminants.
Owner:CEVA SANTE ANIMALE
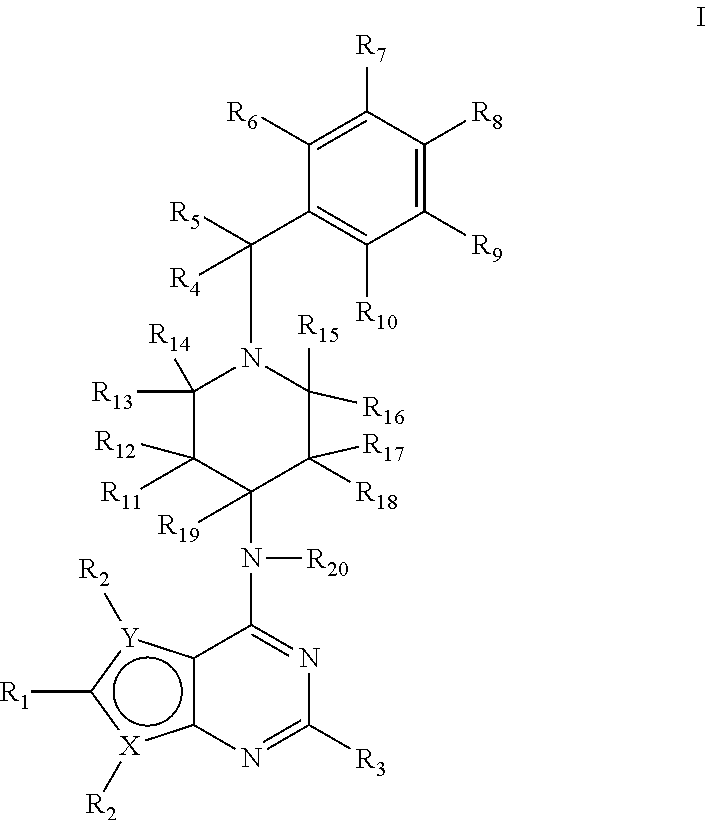
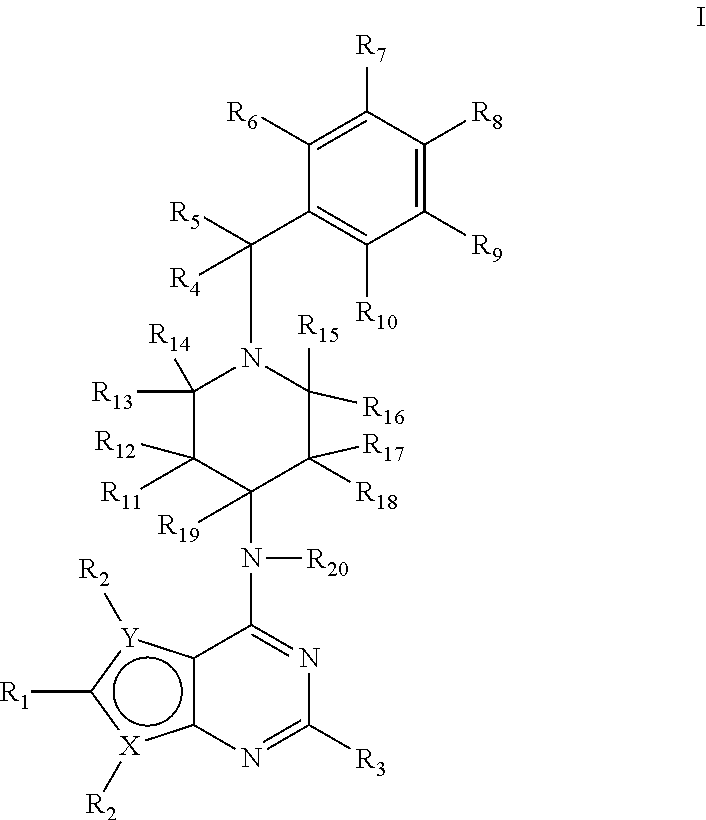
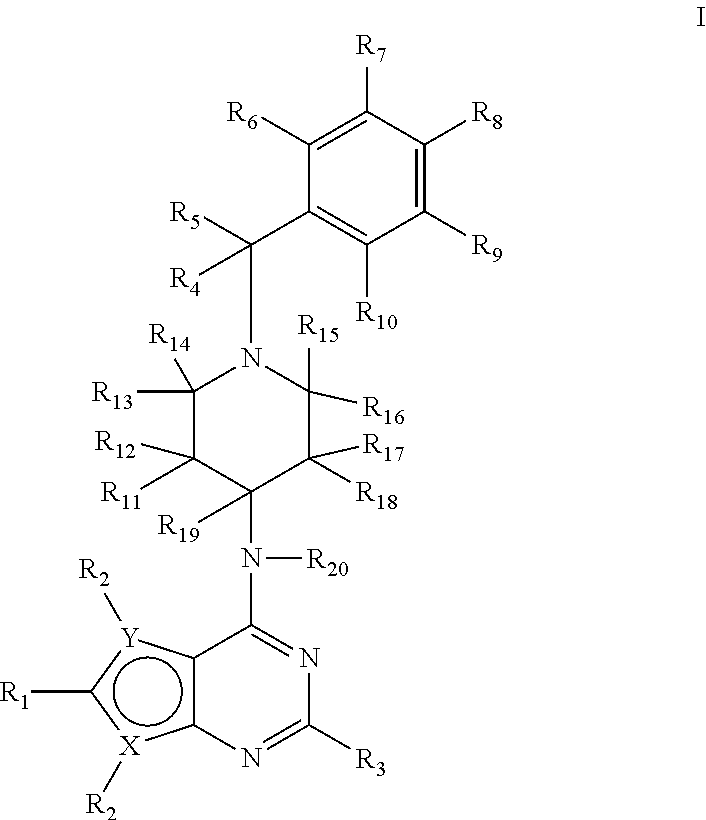
Deuterium-Enriched Pyrimidine Compounds and Derivatives
The present invention is concerned with deuterium-enriched pyrimidine compounds of formula I, their derivatives, enantiomers, diastereomers, solvates and pharmaceutical salts thereof,and their uses in the treatment, prevention and modulation of various diseases including chronic liver diseases, liver cirrhosis, liver fibrosis, hepatocellular carcinoma, liver cancer, renal cell carcinoma, kidney cancer, colorectal cancer, brain cancer, breast cancer, blood cancer, lung cancer, thyroid cancer, ovarian cancer, pancreas cancer, prostate cancer, stomach cancer, testicular cancer, uterus cancer, intestinal cancer, skin cancer, and other forms of cancer, carcinoid tumors, teratocarcinoma, tumor progression, metastasis and fibrosis in the neuroendocrine neoplasia, fibrotic processes as well as a disease state modulated directly or indirectly with 5-HT receptors, 5-HT1, 5-HT1A, 5-HT2 receptors, 5-HT2A and 5-HT2B receptors, dopamine receptors and multiple kinase pathways.
Owner:DHANOA DALJIT SINGH
Method of treatment of dopamine-related dysfunction
The present invention relates to the treatment of dopamine-related dysfunction using full D1 dopamine receptor agonists in an intermittent dosing protocol with a short, but essential, “off-period.” The D1 agonist concentration is reduced during the “off-period” to obtain a plasma concentration of agonist that suboptimally activates D1 dopamine receptors for a period of time to prevent induction of tolerance. Specifically, the method comprises the steps of periodically administering to a patient a full D1 agonist with a half-life of up to about 6 hours at a dose resulting in a first plasma concentration of agonist capable of activating D1 dopamine receptors to produce a therapeutic effect. The dose is reduced at least once every 24 hours to obtain a second lower plasma concentration of agonist that results in suboptimal activation of D1 dopamine receptors for a period of time sufficient to prevent induction of tolerance.
Owner:PURDUE RES FOUND INC +1
Sustained-release tablet composition comprising a dopamine receptor agonist
InactiveUS20050020589A1Simple methodBiocideAnimal repellantsSustained Release TabletCompound (substance)
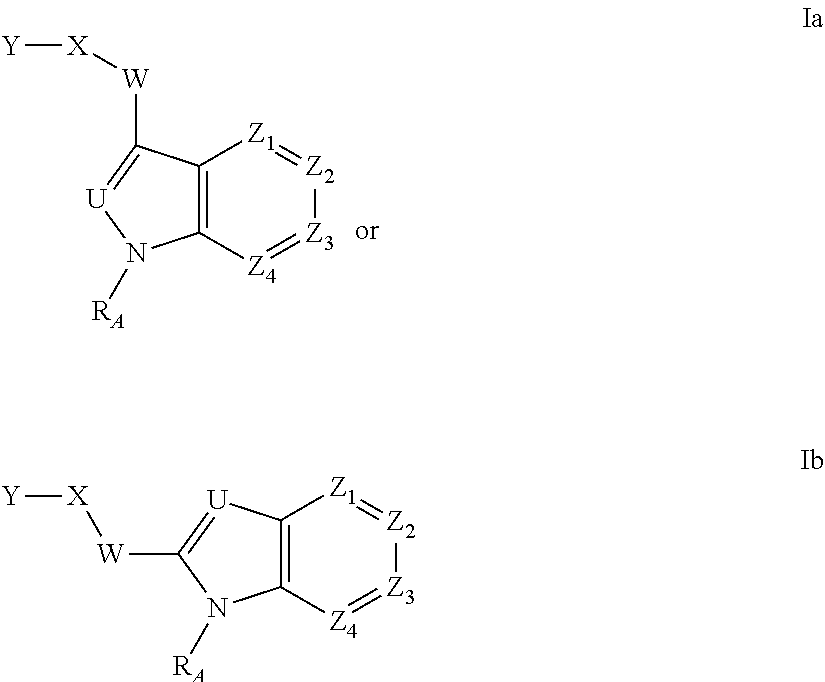
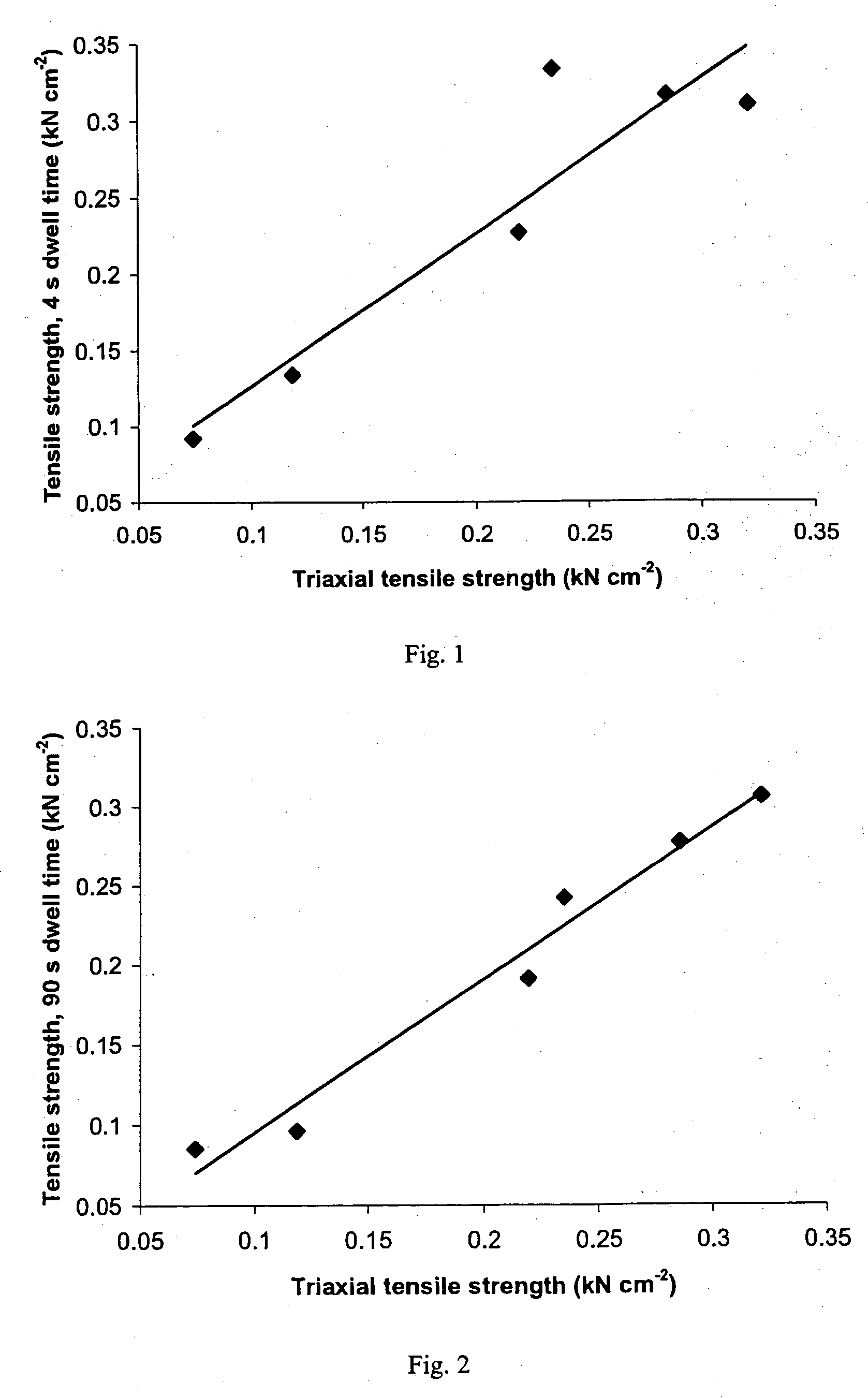
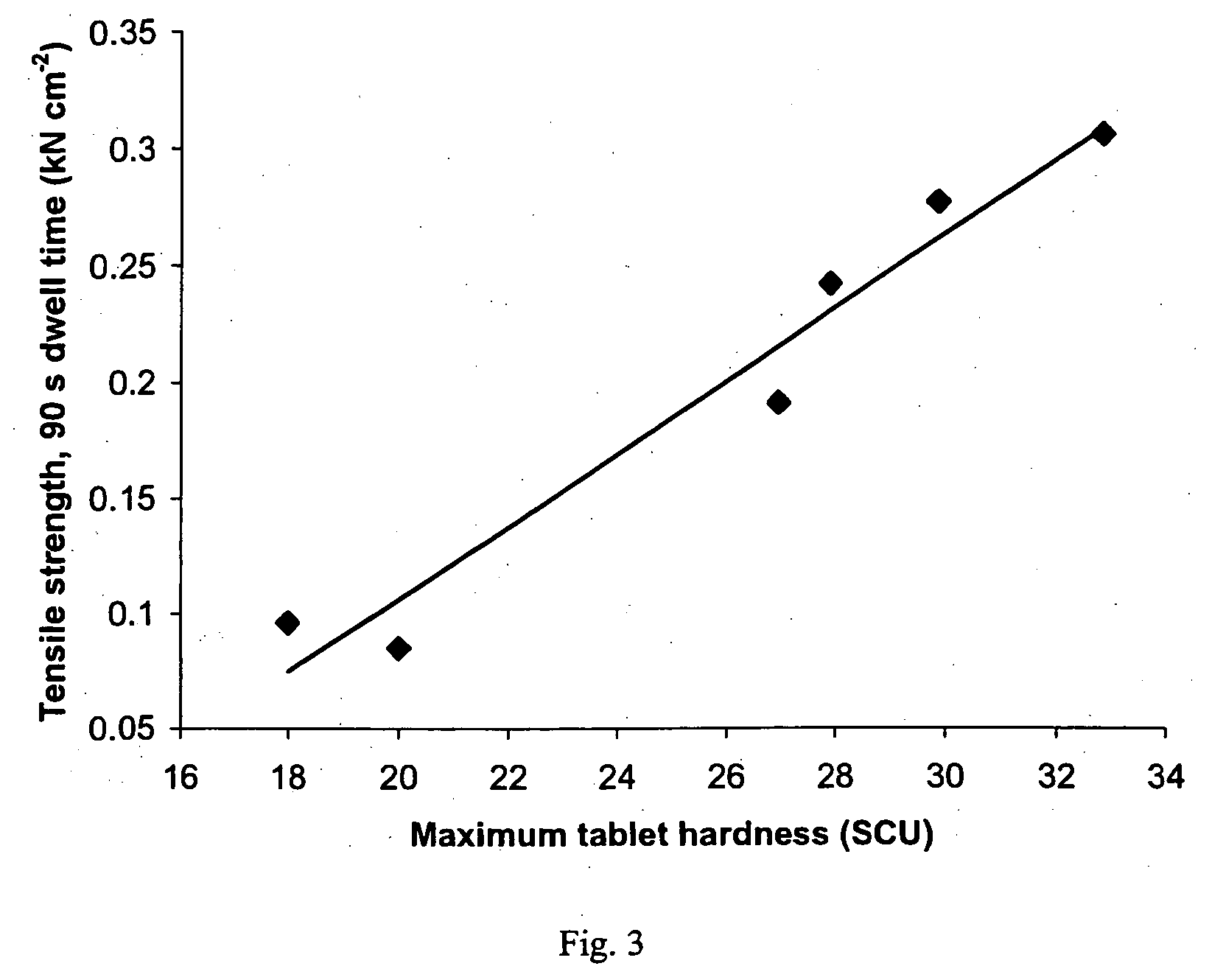
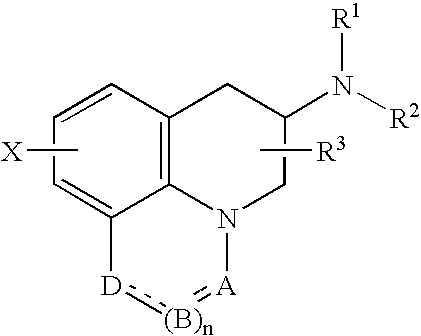
A sustained-release pharmaceutical composition in a form of an orally deliverable tablet comprises as active pharmaceutical agent a compound of formula or a pharmaceutically acceptable salt thereof, wherein R1, R2 and R3 are the same or different and are H, C1-6 alkyl (optionally phenyl substituted), C3-5 alkenyl or alkynyl or C3-10 cycloalkyl, or where R3 is as above and R1 and R2 are cyclized with the attached N atom to form pyrrolidinyl, piperidinyl, morpholinyl, 4-methylpiperazinyl or imidazolyl groups; X is H, F, Cl, Br, I, OH, C1-6 alkyl or alkoxy, CN, carboxamide, carboxyl or (C1-6 alkyl)carbonyl; A is CH, CH2, CHF, CHCl, CHBr, CHI, CHCH3, C═O, C═S, CSCH3, C═NH, CNH2, CNHCH3, CNHCOOCH3, CNHCN, SO2 or N; B is CH, CH2, CHF, CHCl, CHBr, CHI, C═O, N, NH or NCH3, and n is 0 or 1; and D is CH, CH2, CHF, CHCl, CHBr, CHI, C═O, O, N, NH or NCH3. The agent is. dispersed in a matrix comprising a hydrophilic polymer and a starch having a tensile strength of at least about 0.15 kN cm−2 at a solid fraction representative of the tablet. The composition exhibits sustained-release properties effective for treatment of Parkinson's disease. The tablet is optionally coated. Tablets of the invention have improved resistance to attrition or erosion during manufacture, packaging and handling.
Owner:PFIZER INC
Multi-purpose targeting molecule and applications thereof
ActiveCN107029239AEfficient targetingAchieve targeted deliveryMacromolecular non-active ingredientsIn-vivo testing preparationsVascular endotheliumBiology
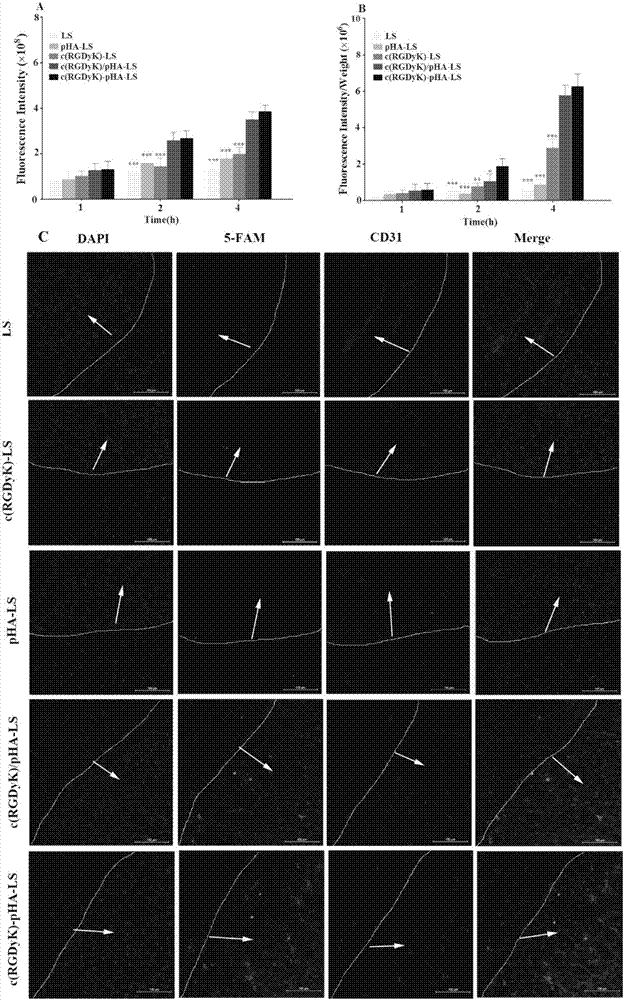
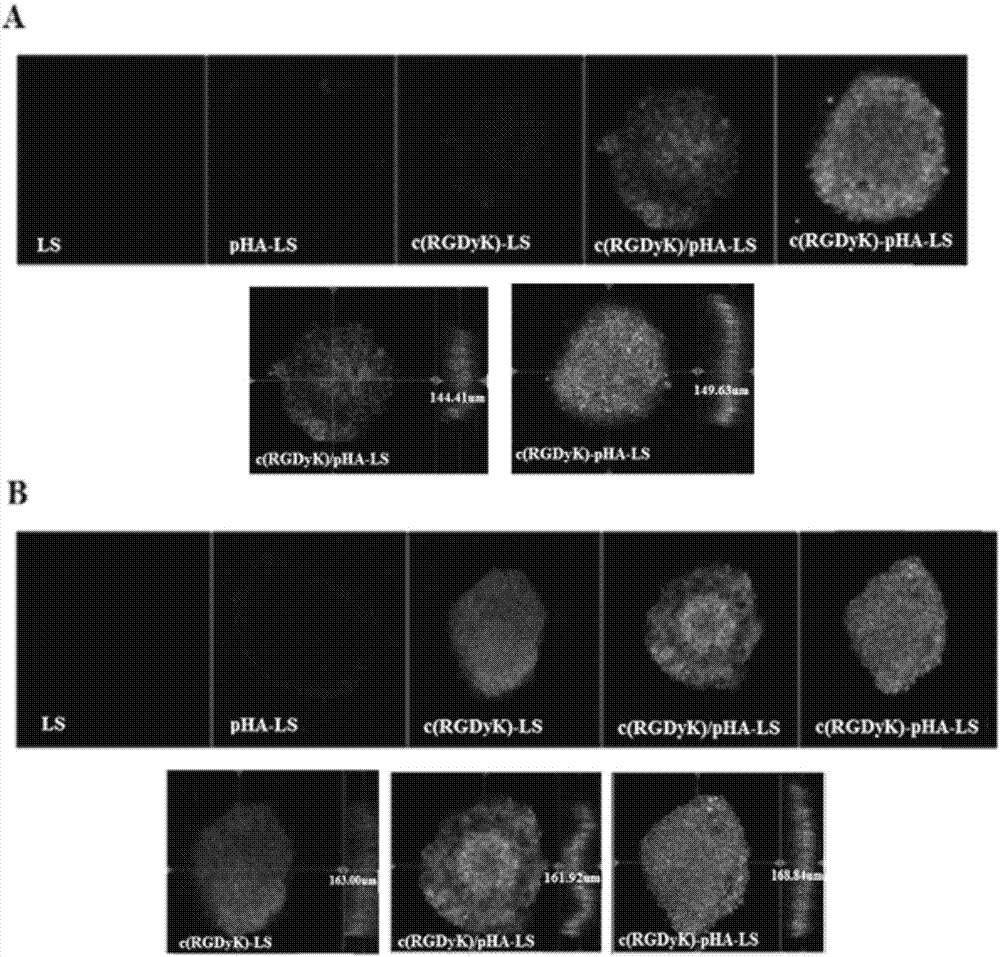
The invention belongs to the field of pharmacy, and relates to a multi-purpose targeting molecule, and applications of a compound and a drug delivery system modified by the multi-purpose targeting molecule in brain tumor diagnosis and treatment. According to the invention, the multi-purpose targeting molecule RGD-pHA being compatible to brain capillary endothelial cells and crossing the blood-brain barrier, being highly compatible to integrin and crossing the blood-brain tumor barrier, and targeting to the brain tumor cells is prepared by adopting a molecule splicing method; the invention further relates to preparation of fluorescein and a medicine modified by RGD-pHA, and a macromolecular carrier material, and applications of RGD-pHA in construction of the drug delivery system. The experimental result shows that the RGD-pHA can be specifically taken by brain capillary endothelial cells expressing the dopamine receptor for mediating the model drug or the nanometer drug delivery system to enter the brain, so that the model drug or the nanometer drug delivery system is specifically taken by the positive cells and the tumor sphere tissue expressing the integrin, the good brain tumor targeting effect is shown in vitro and vivo, and the efficiency in resisting brain tumor is obviously improved.
Owner:FUDAN UNIV
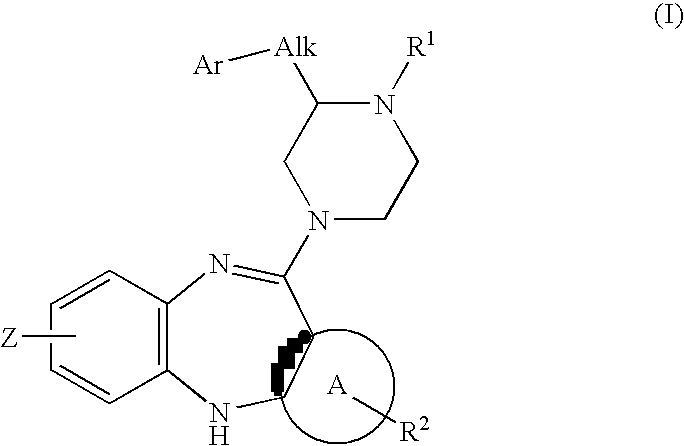
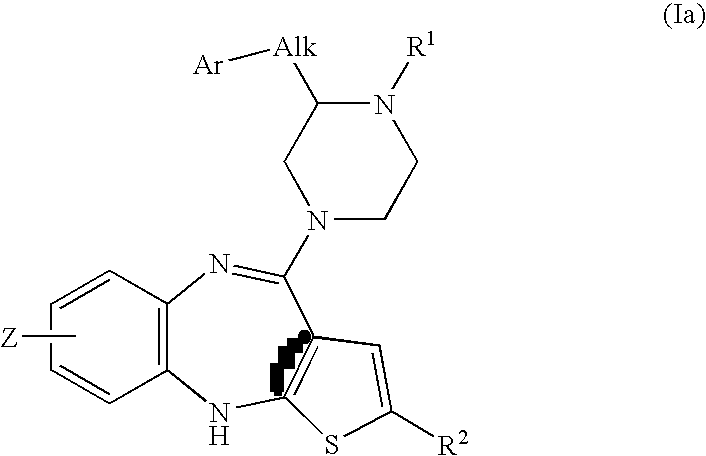
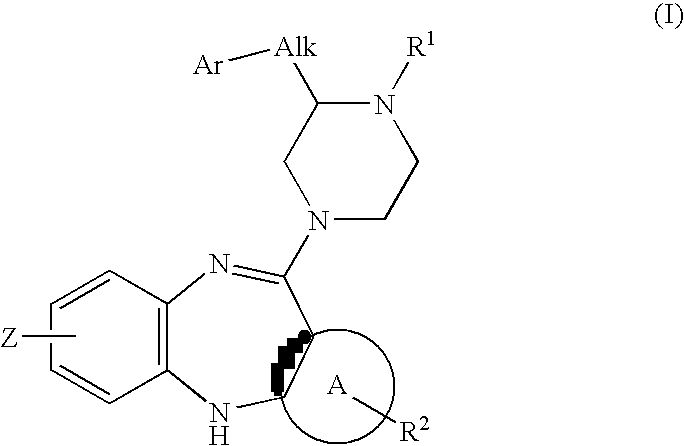
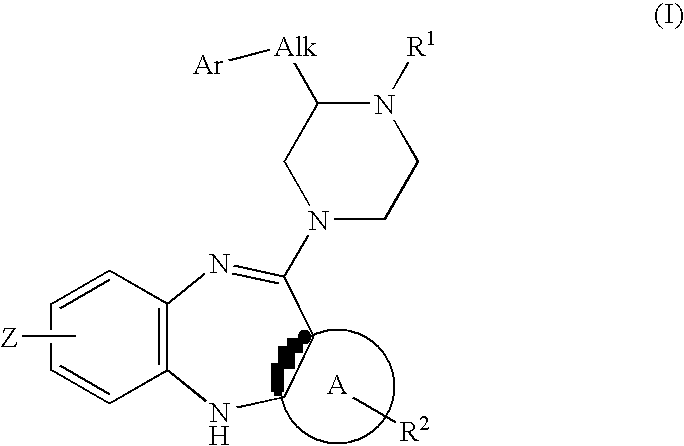
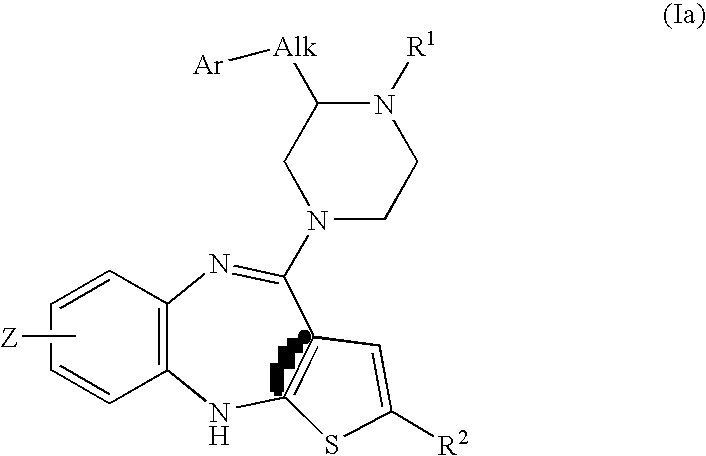
Piperazine substituted aryl benzodiazepines and their use as dopamine receptor antagonists for the treatment of psychotic disorders
Owner:ELI LILLY & CO
Methods of treating CNS disorders
The present invention relates to methods of treating various CNS disorders, e.g., mania, bipolar disorder and schizophrenia, by administering NMDA receptor antagonists, alone or in combination with dopamine receptor antagonists.
Owner:FOREST LAB HLDG LTD
Muscarinic receptor antagonists
The present invention relates generally to muscarinic receptor antagonist, which are useful, among other uses, for the treatment of various diseases of the respiratory, urinary and gastrointestinal systems mediated through muscarinic receptors. The invention also relates to the process for the preparation of disclosed compounds, pharmaceutical compositions containing the disclosed compounds and the method for treating diseases mediated through muscarinic receptors. Also provided herein are pharmaceutical composition comprising one or more muscarinic receptor antagonists and at least one other active ingredients include, but are not limited to, corticosteroids, beta agonists, leukotriene antagonists, 5-lipoxygenase inhibitors, anti-histamines, antitussives, dopamine receptor antagonists, chemokine inhibitors, p38 MAP Kinase inhibitors, and PDE-IV inhibitors.
Owner:RANBAXY LAB LTD
Piperazine substituted aryl benzodiazepines and their use as dopamine receptor antagonists for the treatment of psychotic disorders
Described herein are antipyschotic compounds of formula (I) wherein, A is an optionally benzo-fused five or six member aromatic ring having zero to three hetero atoms independently selected from N, O, and S; Alk is (C1-4) alkylene optionally substituted with OH, methoxy, ethoxy, or F; Ar is optionally substituted phenyl, naphthyl, monocyclic heteroaromatic, or bicyclic heteroaromatic; R1 is hydrogen or (C1-4) alkyl optionally substituted with OH, OR3, or OCH2CH2OH, wherein R3 is (C1-2) alkyl; R2 is H, (C1-6) alkyl, halogen, fluorinated (C1-6) alkyl, OR4, SR4, NO2, CN, COR4, CONR5R6, SO2NR5R6, NR5R6, NR5COR4, NR5SO2R4, or optionally substituted phenyl, wherein R4 is hydrogen, (C1-6) alkyl, fluorinated (C1-6) alkyl, benzyl, or optionally substituted phenyl, R5 and R6 are independently hydrogen, (C1-6) alkyl, or optionally substituted phenyl; Z is one or two substituents independently selected from hydrogen, halogen, (C1-6) alkyl, fluorinated (C1-6) alkyl, OR7, SR7, NO2, CN, COR7, CONR8R9, SO2NR8R9, NR8SO2R7, NR8R9, or optionally substituted phenyl, wherein R7 is hydrogen, (C1-6) alkyl, fluorinated alkyl, benzyl, or optionally substituted phenyl, R8 and R9 are independently hydrogen, (C1-6) alkyl, or optionally substituted phenyl; and salts, solvates, and crystal forms thereof. Also described are the use of the compounds of formula (a) as antagonists of the dopamine D2 receptor and as agents for the treatment of psychosis and bipolar disorders, and pharmaceutical formulations of the compounds of formula (I). Also described are compounds useful as intermediates for the synthesis of the compounds of formula (I).
Owner:ELI LILLY & CO
Combination treatment of sglt2 inhibitors and dopamine agonists for preventing metabolic disorders in equine animals
ActiveUS20170239281A1Increase insulin sensitivityReduce weightMetabolism disorderDigestive systemPhysiologyEquine metabolic syndrome
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Tetrahydroindolone and purine derivatives linked to arylpiperazines
InactiveUS20030114463A1Improve cognitive functionImproved therapeutic propertyBiocideOrganic chemistryPurinePurine derivative
Pharmaceutical composite compositions comprising tetrahydroindolones linked to arylpiperazines and derivatives thereof are disclosed. Specifically, composite compositions useful in treating anti-psychotic disorders are disclosed. The composite compositions disclosed herein can effectively ameliorate symptoms and treat psychotic disorders without causing a decrease in cognitive function. Generally, the composite compounds consist of two moieties, moiety A and B in which a tetrahydroindolone comprises a moiety A linked through a linker L to a moiety B, where B is an arylpiperazinyl moiety. The composite compound provides anti-psychotic actively by interaction with GABA, seratoninne and dopamine receptors. The composite molecules with the combined activities will provide treat psychiatric and neurological diseases without cognitive impairment.
Owner:SPECTRUM PHARMA INC
Long Acting Sustained-Release Formulation Containing Dopamine Receptor Agonist and the Preparation Method Thereof
ActiveUS20080260846A1Improve bioavailabilityGood effectBiocideNervous disorderDiseaseDopamine receptor D1
The present invention relates to a long-acting sustained-release dosage form for treatment of Parkinson Disease, comprising a dopamine receptor agonist and a pharmaceutically acceptable biodegradable polymer accessories, wherein the content of the dopamine receptor agonist in the sustained-release dosage form is 5-50% by weight, and the content of the pharmaceutically acceptable polymer accessories is 50-95% by weight.
Owner:GENEORA PHARMA (SHIJIAZHUANG) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com