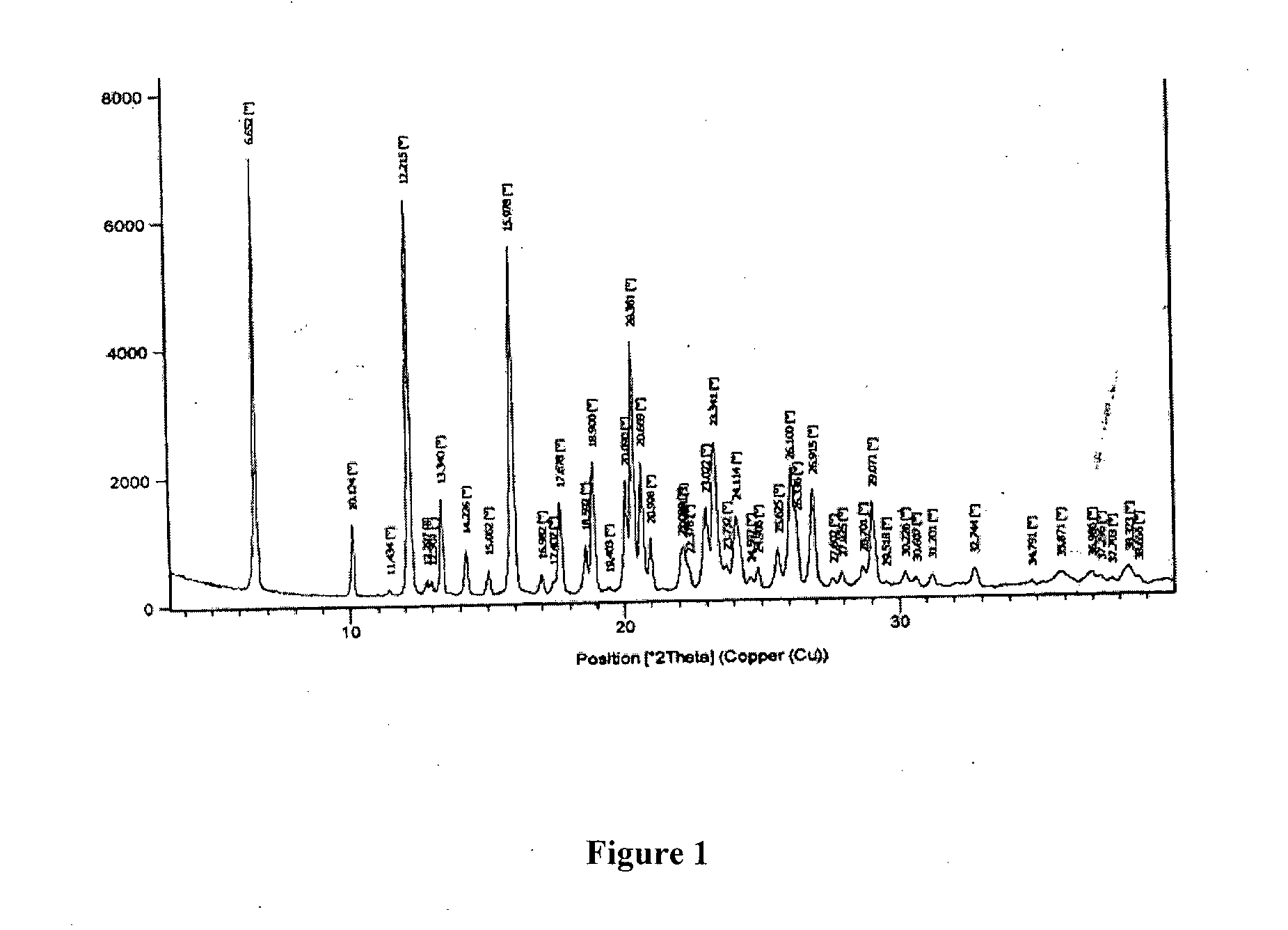
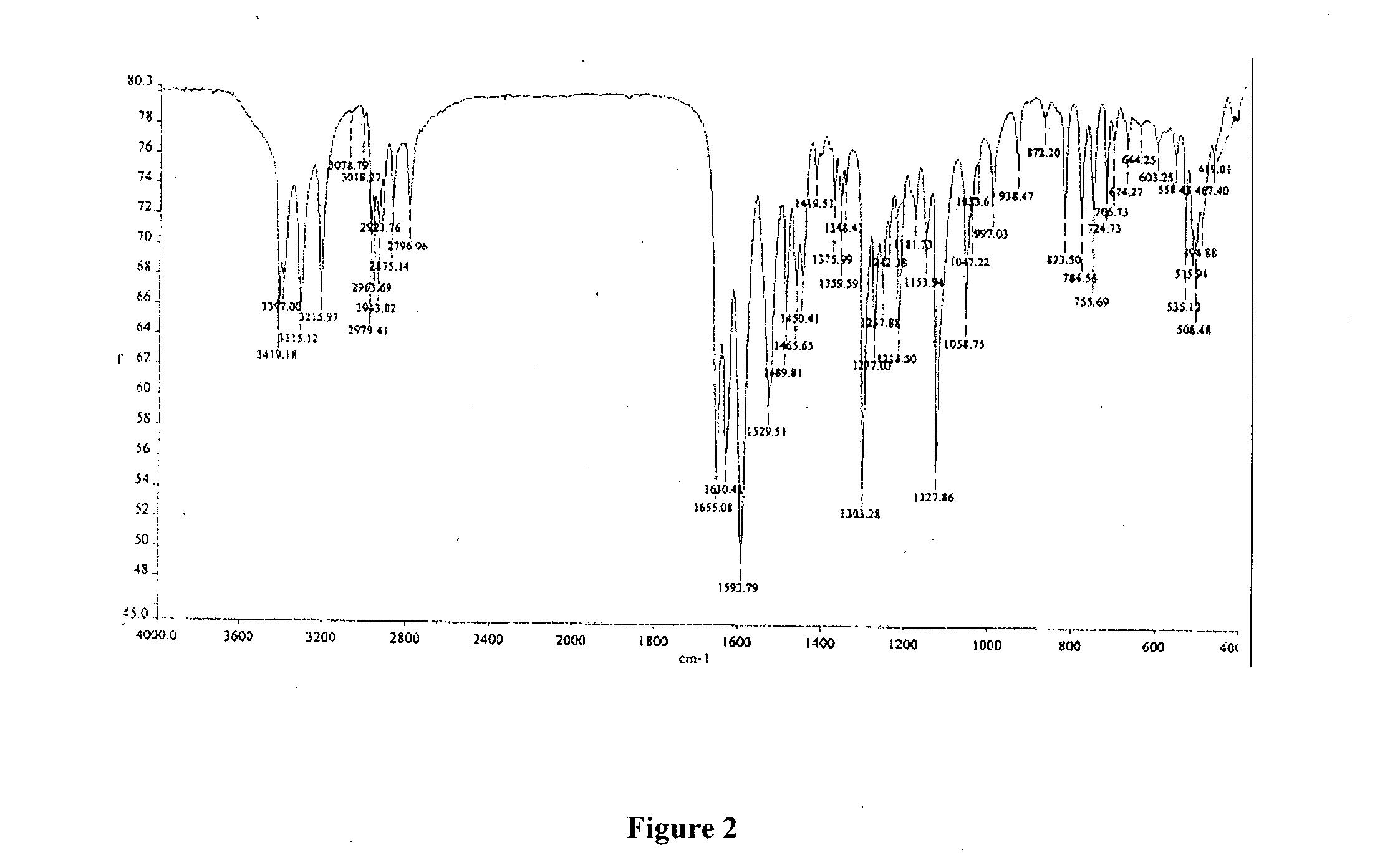
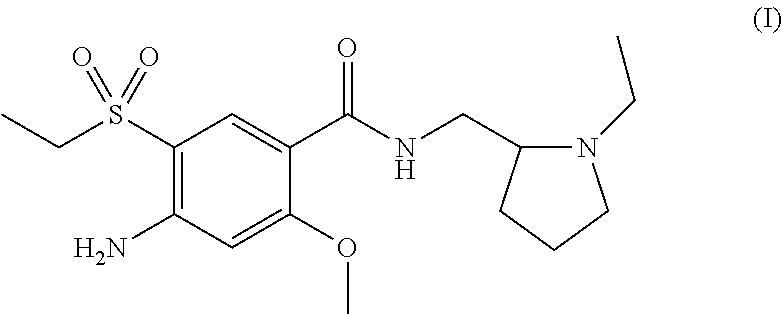
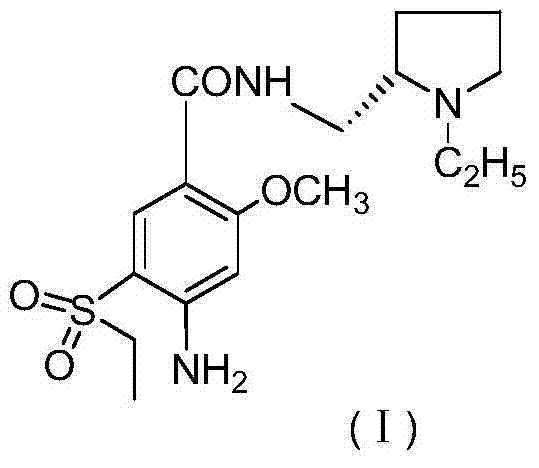
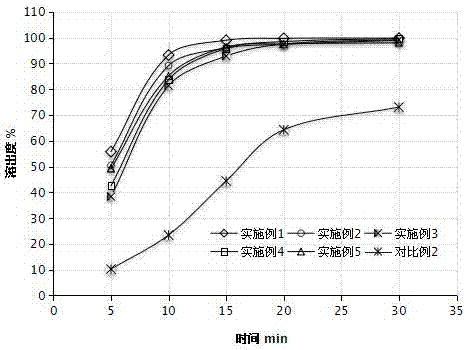
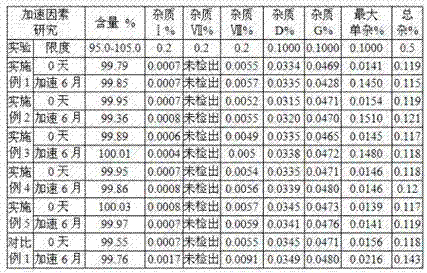
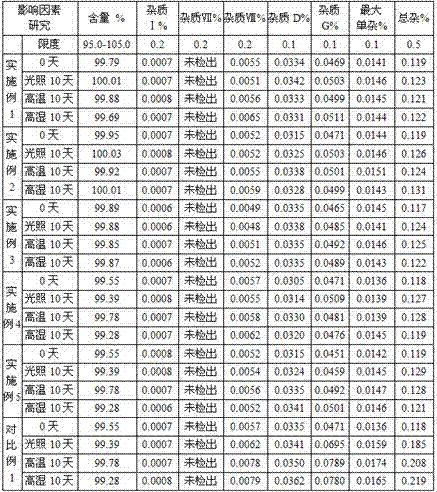
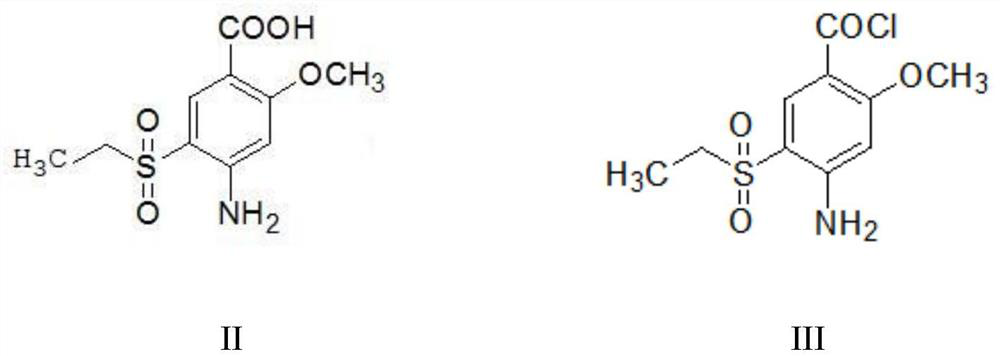Patents
Literature
42 results about "Amisulpride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
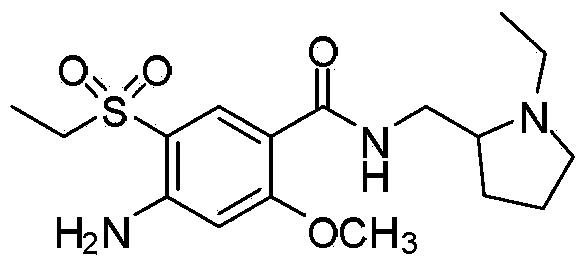
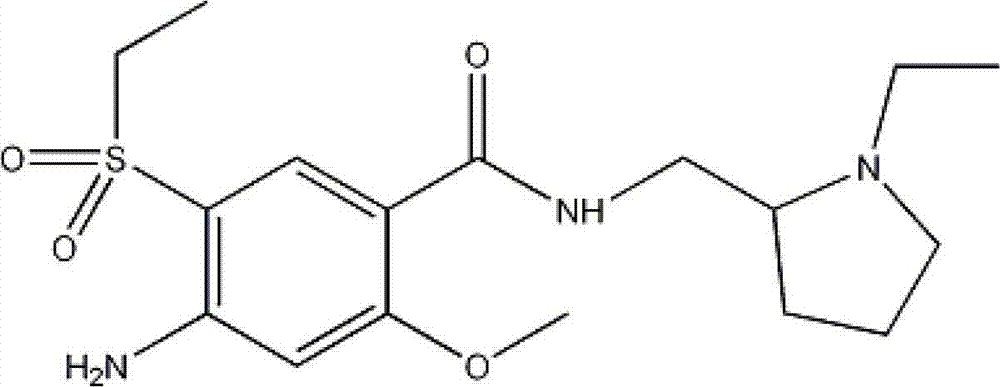
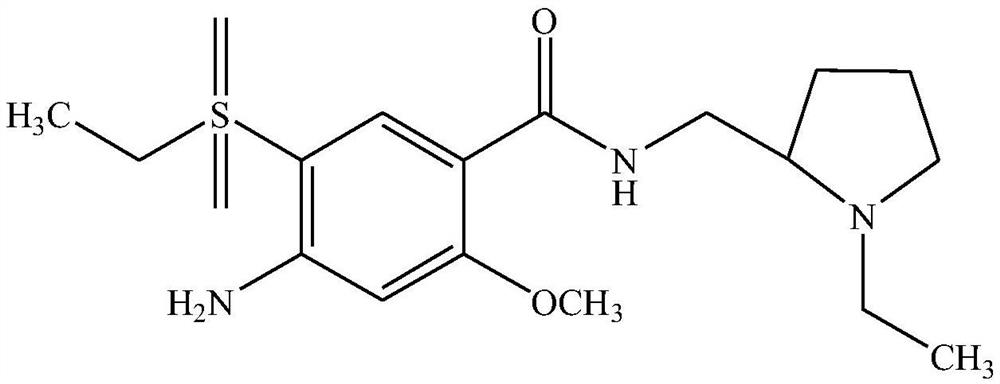
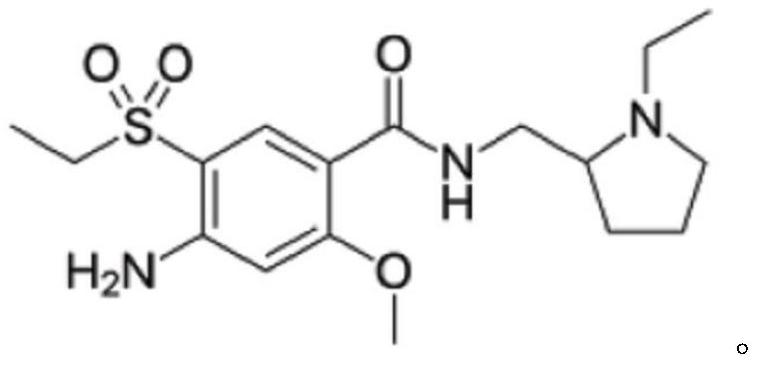
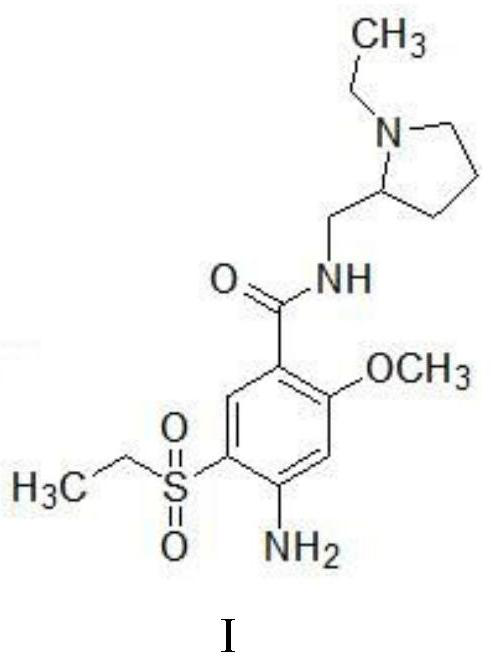
Amisulpride, sold under the brand name Solian among others, is an antipsychotic medication used to treat schizophrenia. It is also used to treat dysthymia. It is usually classed with the atypical antipsychotics. Chemically it is a benzamide and like other benzamide antipsychotics, such as sulpiride, it is associated with a high risk of elevating blood levels of the lactation hormone, prolactin (thereby potentially causing the absence of the menstrual cycle, breast enlargement, even in males, breast milk secretion not related to breastfeeding, impaired fertility, impotence, breast pain, etc.), and a low risk, relative to the typical antipsychotics, of causing movement disorders. It has also been found to be modestly more effective in treating schizophrenia than the typical antipsychotics.
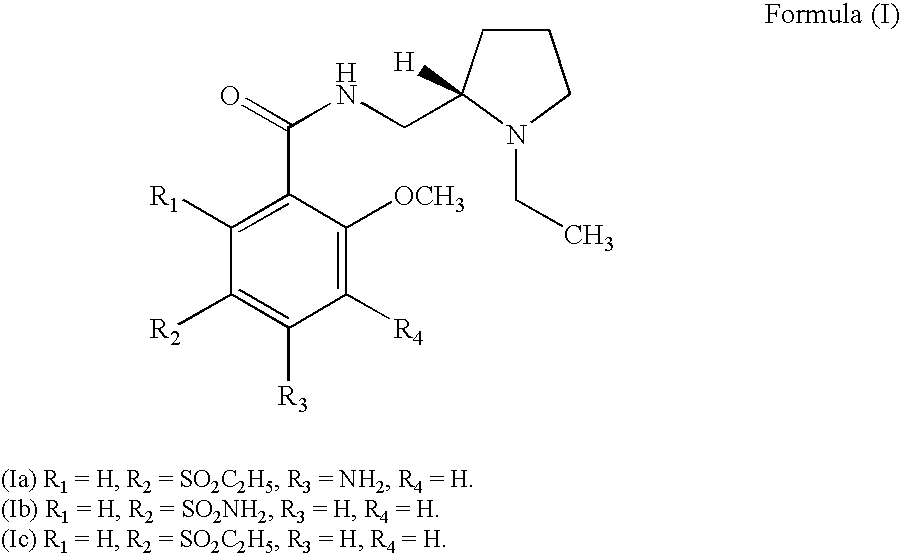
Method of treating bipolar depression with a benzamide derivative
InactiveUS20080188537A1Effective treatmentBiocideNervous disorderBipolar mood disorderBipolar I disorder
A benzamide derivative, especially amisulpride, is used to prevent or treat bipolar depression of a patient suffering from bipolar disorder I or bipolar disorder II.
Owner:COPHARM
R-amisulpride medicine salt, preparation method, crystal form and application thereof
ActiveCN106995397AGood curative effectLower blood sugarMetabolism disorderOrganic chemistry methodsPyrrolidineTriethylamine
The invention concretely relates to R-amisulpride medicine salt, a preparation method, a crystal form and application thereof in preparing a medicine for treating diabetes. The preparation method comprises the following steps: by adopting 2-aminomethyl-N-ethyl pyrrolidine as a raw material, and adopting L-tartaric acid, for splitting to obtain (R)-2-aminomethyl-N-ethyl pyrrolidine; directly condensing amisulpride acid and the (R)-2-aminomethyl-N-ethyl pyrrolidine under the catalysis of isopropyl chlorocarbonate and triethylamine, thus obtaining R-amisulpride; reacting the R-amisulpride obtained in the step (2) and acid to obtain the R-amisulpride medicine salt. The preparation method has the advantages of simplicity in operation, high safety, good product quality, low cost and the like, and is convenient for scale production.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
Compositions and methods for treating metabolic disorders
InactiveUS20150018360A1Preventing and treating and ameliorating effectModulating blood glucose levelBiocideMetabolism disorderMedicineBlood sugar
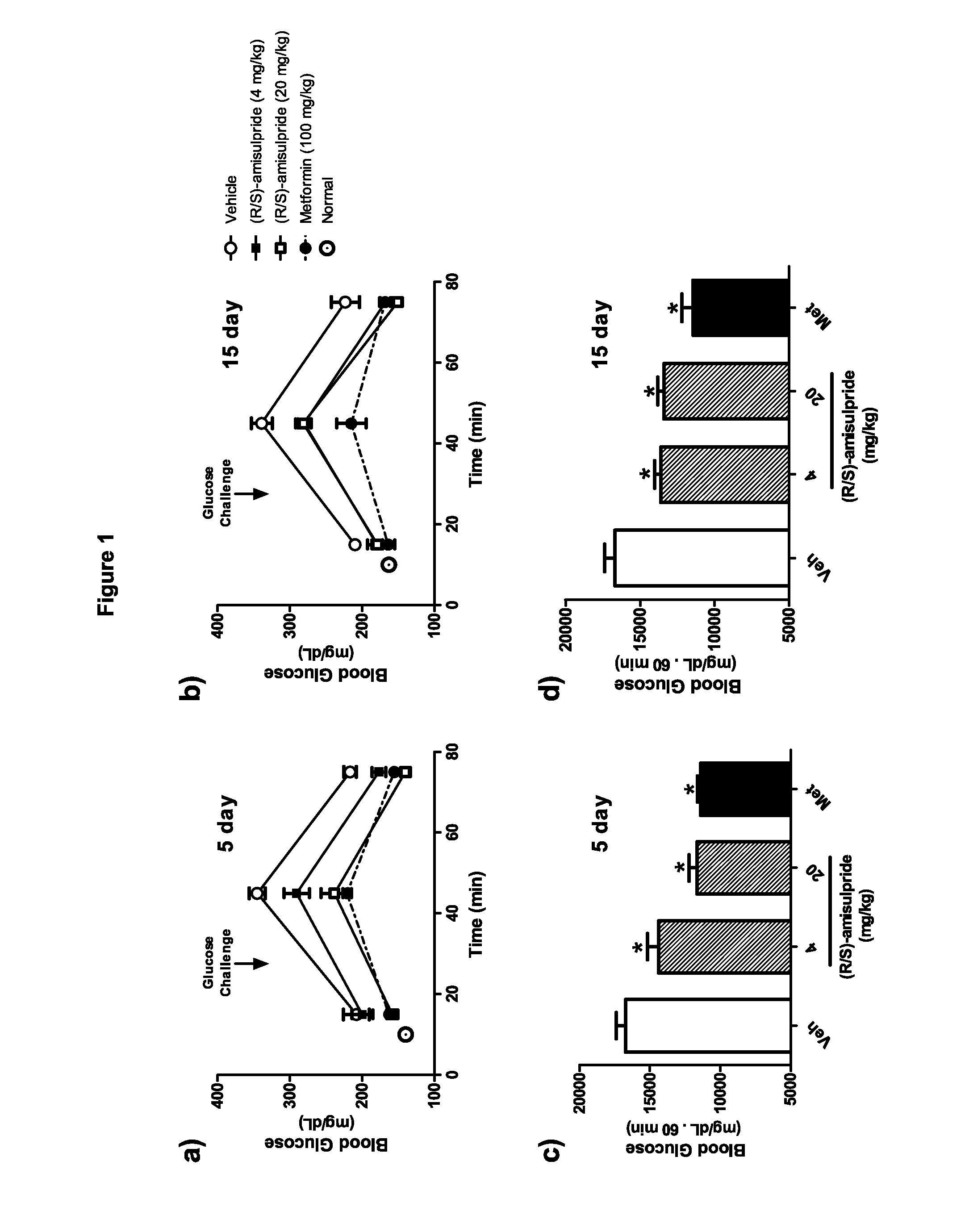
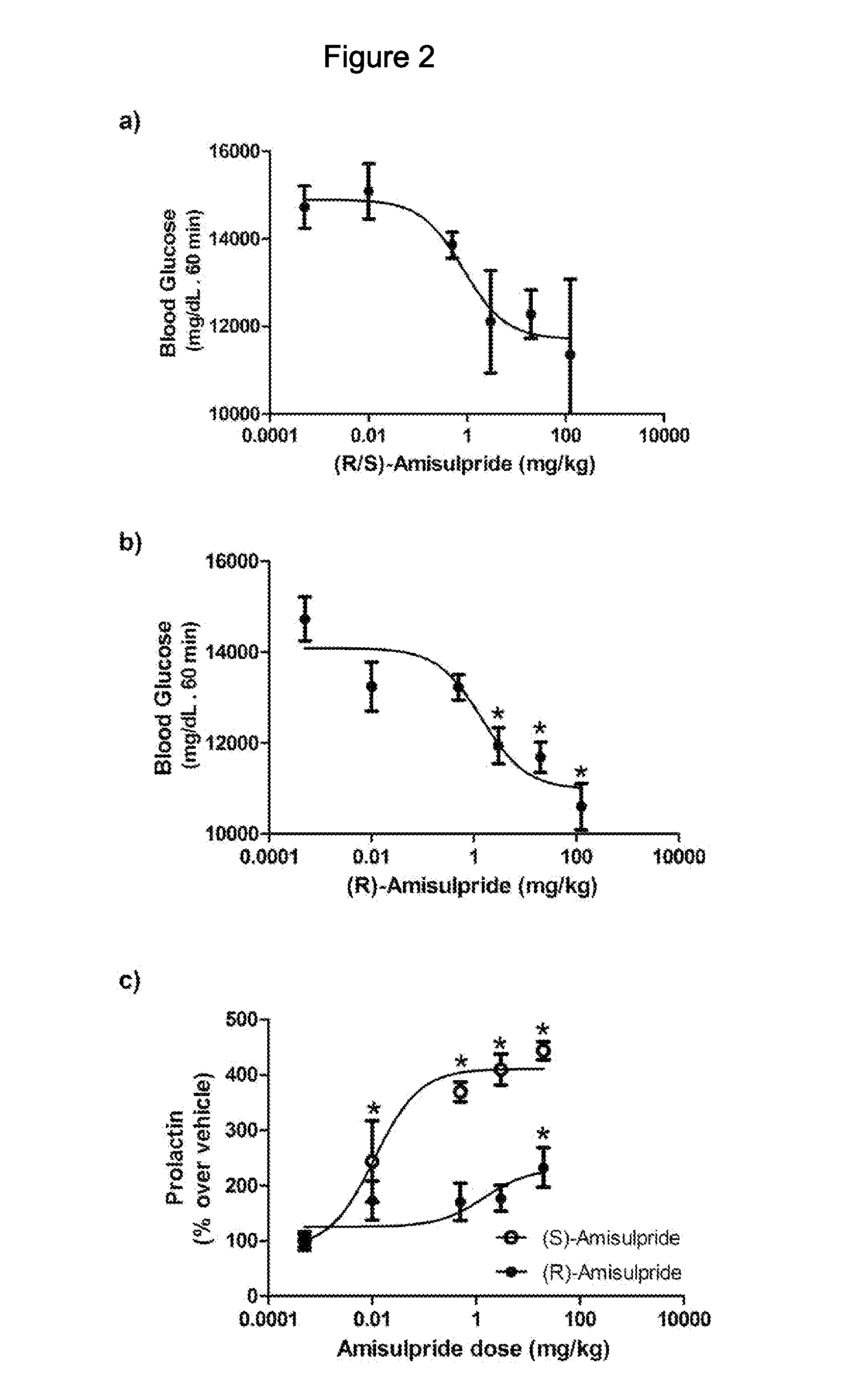
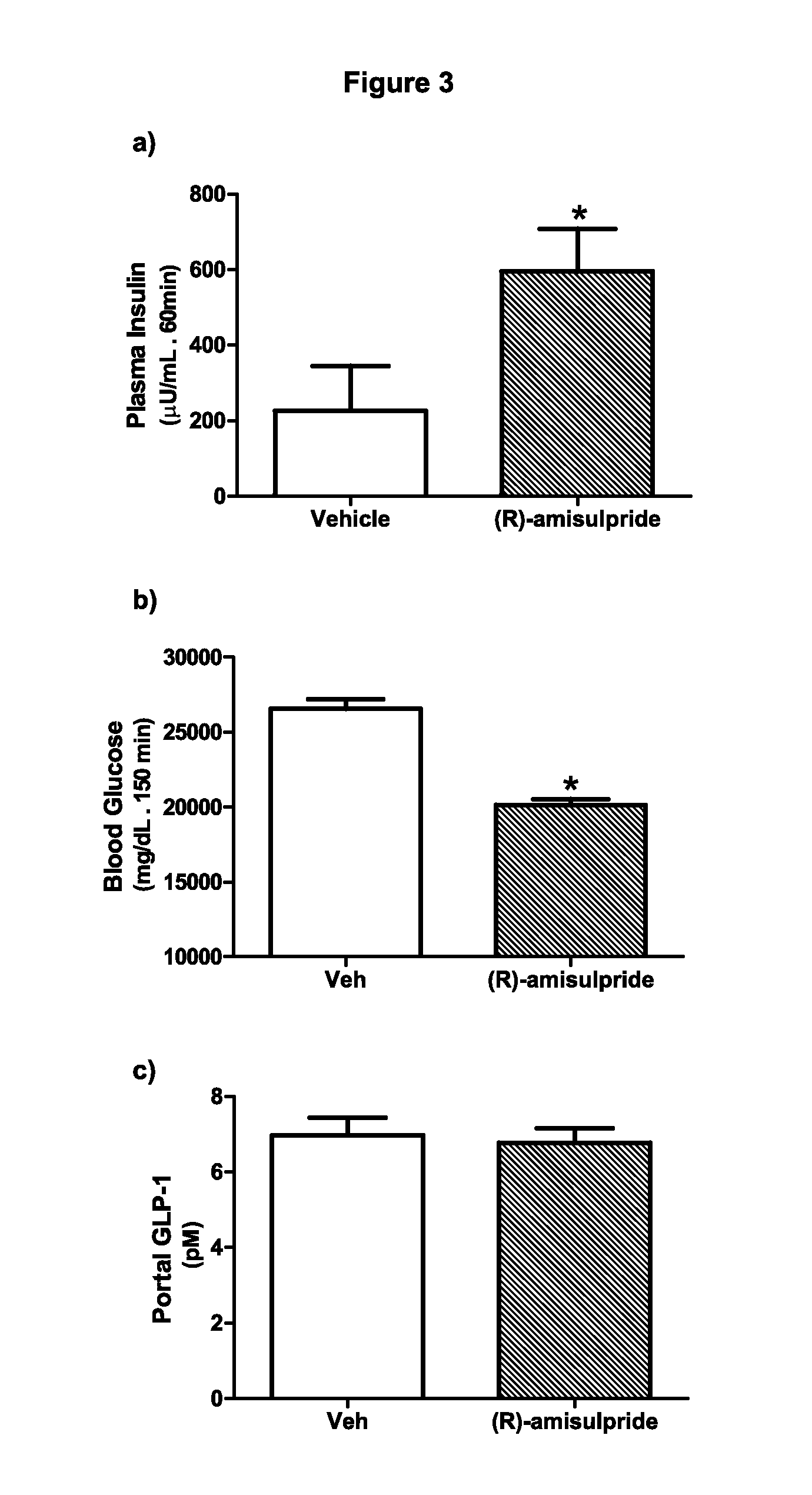
The present invention provides, inter alia, compositions containing enantiomerically pure (R)(+)-amisulpride or enantiomerically pure (R)(+)-sulpiride, optionally with dopamine receptor modulators. The present invention also provides compositions containing racemic (RS)-amisulpride or (RS)-sulpiride in combination with dopamine receptor modulators. Methods for preventing, treating, or ameliorating the effects of a metabolic disorder or key element thereof, for modulating blood glucose levels, and for preventing, treating, or ameliorating the effects of diabetes in a subject are also provided. Additionally, the present invention provides methods for counter-acting the dopamine antagonist activity of (S)-amisulpride in racemic (RS)-amisulpride, or the dopamine antagonist activity of (S)-sulpiride in racemic (RS)-sulpiride, administered to a subject to prevent, treat, or ameliorate the effects of a metabolic disorder.
Owner:BIOMED VALLEY DISCOVERIES INC
Novel solid pharmaceutical composition comprising amisulpride
The invention relates to a solid pharmaceutical composition for oral administration of amisulpride, which comprises at least one coated amisulpride particle and at least one pharmaceutically acceptable excipient suitable for an orodispersible administration of the composition.
Owner:SANOFI AVENTIS SA
Process for preparation of amisulpride
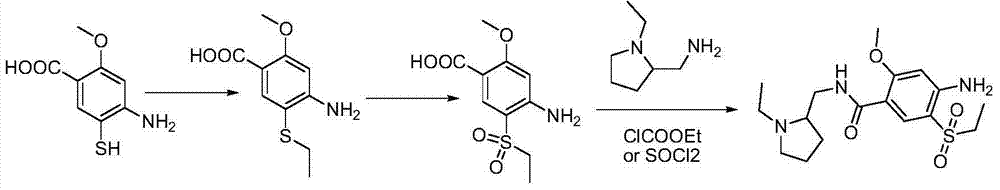
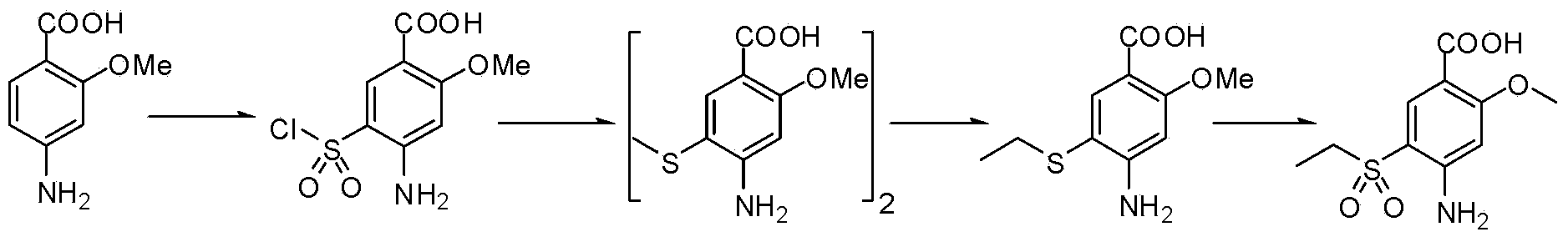
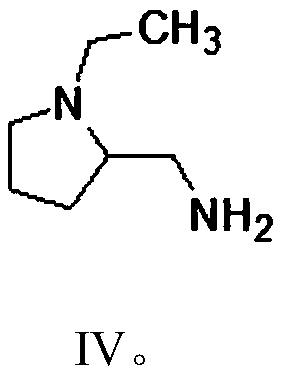
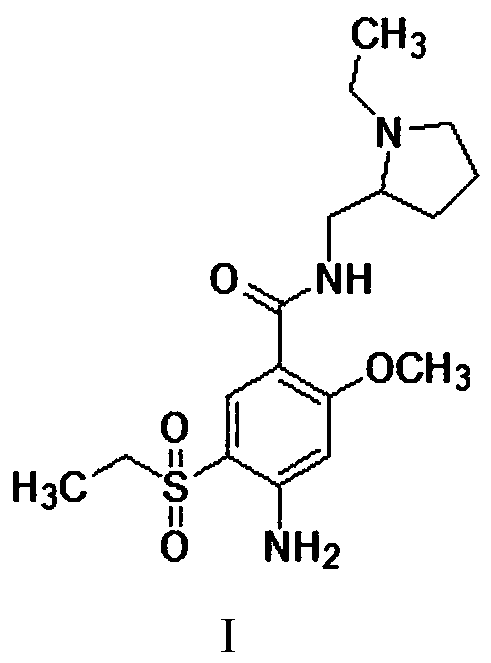
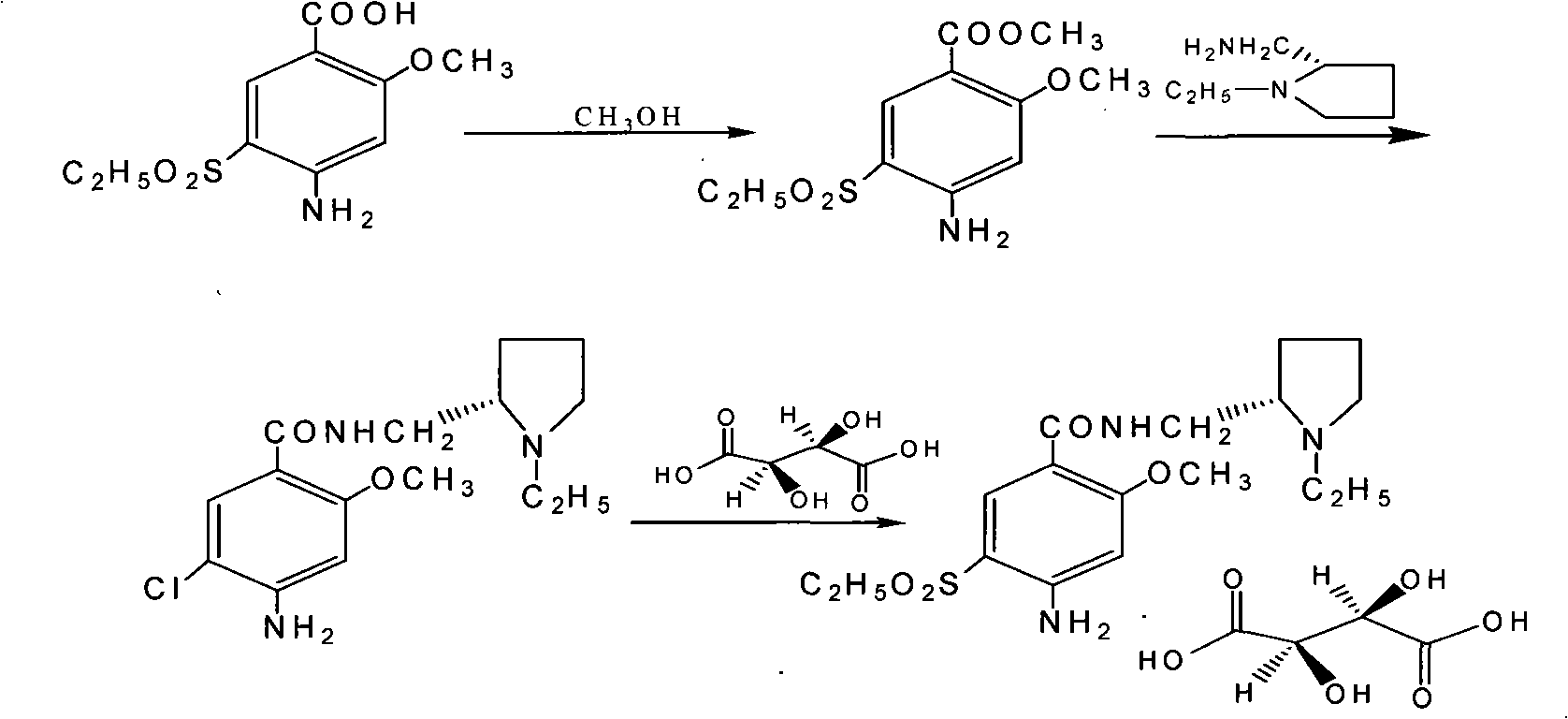
The present invention is related to a novel process for the preparation of amisulpride (I) which involves: methylation of 4-amino-salicylic-acid (VI) with dimethyl sulphate and base, optionally in presence of TBAB to obtain 4-amino-2-methoxy methyl benzoate (VII) and (ii) oxidation of 4-amino-2-methoxy-5-ethyl thio benzoic acid (IX) or 4-amino-2-methoxy-5-ethyl thio methyl benzoate (X) with oxidizing agent in the presence of sodium tungstate or ammonium molybdate to give 2-methoxy-4-amino-5-ethyl-sulfonyl benzoic acid (IV) or 2-methoxy-4-amino-5-ethyl-sulfonyl methyl benzoate (XI) respectively.
Owner:LUPIN LTD
Preparation method of (S)(-)-amisulpride
InactiveCN104725292AShort reaction timeRaw materials are low-toxic and easy to obtainOrganic chemistryCombinatorial chemistrySide reaction
The invention relates to a preparation method of (S)(-)-amisulpride. According to the preparation method, by introducing an R2 group which is selected from benzyl, p-methoxybenzyl, 2,4-dimethoxybenzyl, p-nitrobenzyl and p-methylbenzyl, on one hand, the steric hindrance is increased, the side reaction is avoided, on the other hand, amino is activated, the nucleophilicity of amino is increased, and the condensation reaction can be easily carried out at mild conditions. The preparation method has the characteristics that the raw materials are low in toxicity and easily available, and the reaction conditions are mild; the generation of chiral isomer dextroisomers is avoided; the preparation method is applicable to the industrial production.
Owner:HUBEI JINGJIANGYUAN PHARMA
Amisulpride tablet and method for preparing same
ActiveCN107126422AImprove stabilityEasy to take medicineNervous disorderInorganic non-active ingredientsDissolutionCherry flavor
The invention discloses an amisulpride tablet and a method for preparing the same. The amisulpride tablet comprises raw materials and excipients, and is characterized in that film coating is carried out on the surfaces of the amisulpride tablet by the aid of film coating premixes; the film coating premixes comprise opadry gastric-soluble coating powder and flavoring agents; the flavoring agents comprise sweeteners, taste masking agents and aromatizers; the sweeteners are selected from a type of aspartame and acesulfame potassium; each taste masking agent is sodium chloride; the aromatizers are selected from a type of cherry flavor essence, apple flavor essence, honey peach flavor essence, sweet orange flavor essence, strawberry flavor essence and mint flavor essence. The amisulpride tablet and the method have the advantages that film coating is carried out on the amisulpride tablet, accordingly, bitter taste of amisulpride can be masked to a certain extent while the stability of the amisulpride tablet which is a medicine is improved, the medication compliance of patients can be improved, and medicine taking pretending possibility of the patients can be reduced to a certain extent; usage of lubricants is controlled, accordingly, the dissolution of the medicine can be greatly improved, and the medicine can be stably and quickly released in bodies.
Owner:HEBEI LONGHAI PHARMA
Use of amisulpride as an anti-emetic
ActiveUS8686019B2Strong and difficult to alleviateEffect can be minimised and avoidedHeavy metal active ingredientsBiocideAnti-emeticAmisulpride
Amisulpride is used in the therapy of nausea, vomiting or retches. The therapy may utilize a novel injectable formulation, in unit dosage form, comprising less than 50 mg amisulpride.
Owner:ACACIA PHARMA
Use of Amisulpride as an Anti-Emetic
ActiveUS20130022688A1Effect can be minimised and avoidedStrong and difficult to alleviateBiocideHeavy metal active ingredientsAnti-emeticAmisulpride
Amisulpride is used in the therapy of nausea, vomiting or retches. The therapy may utilize a novel injectable formulation, in unit dosage form, comprising less than 50 mg amisulpride.
Owner:ACACIA PHARMA
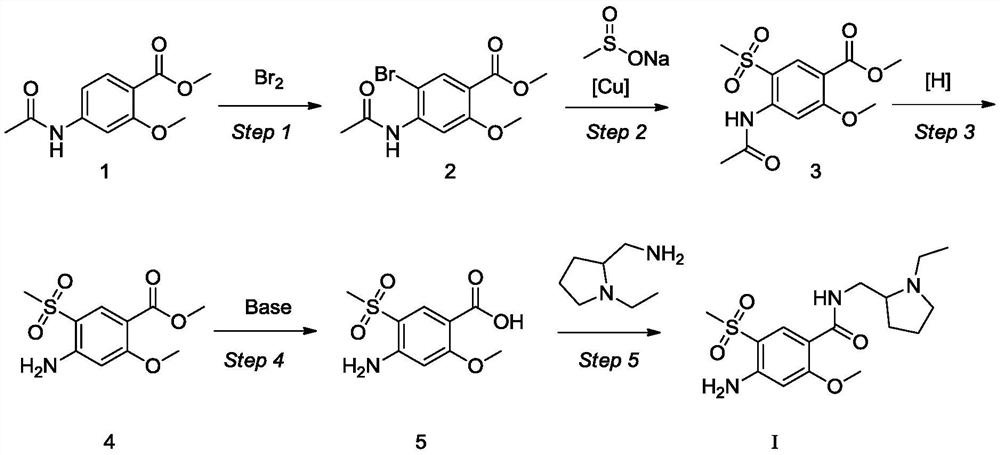
Synthesis method for amisulpride
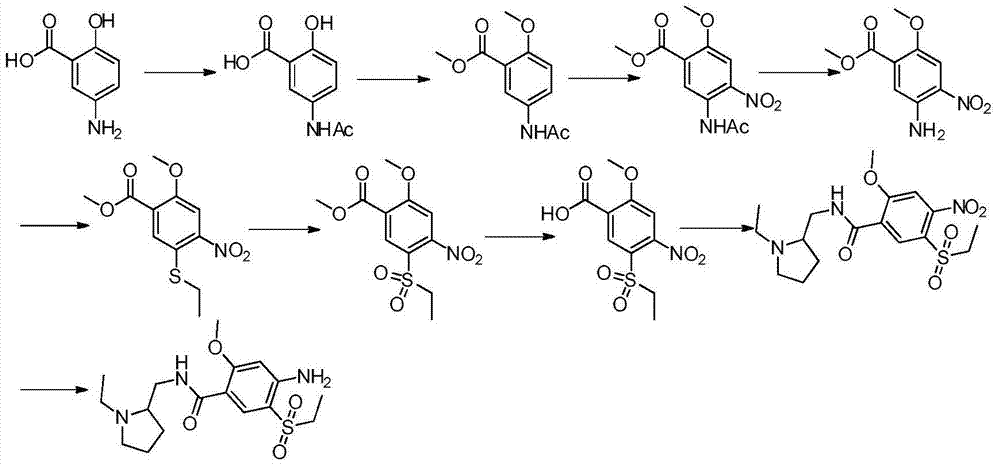
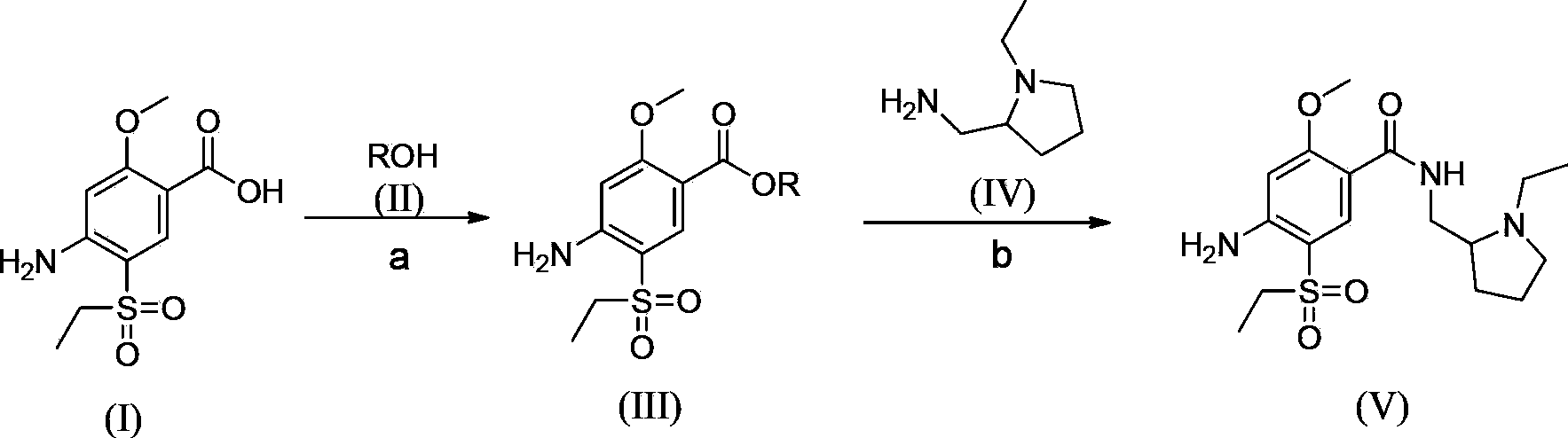
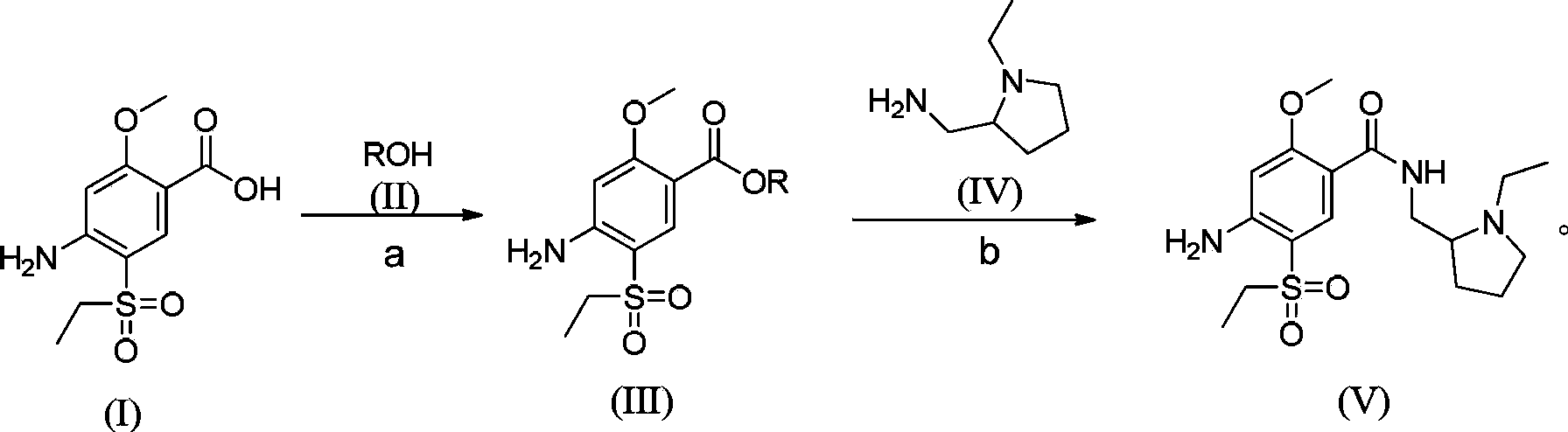
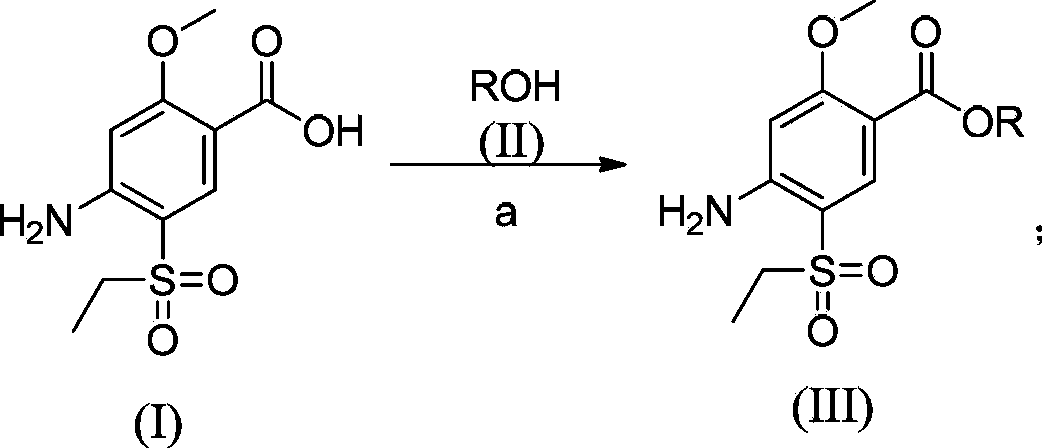
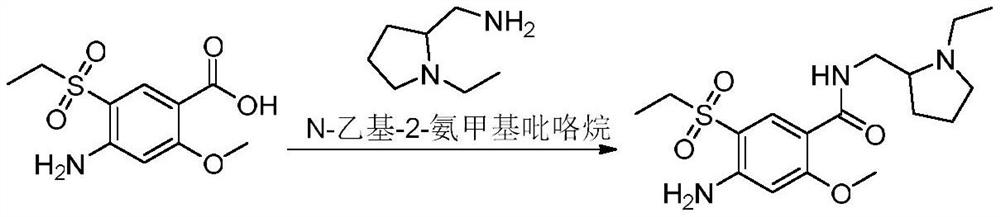
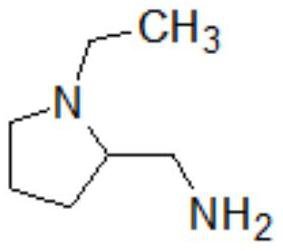
The invention relates to a synthesis method for amisulpride. The method comprises the following steps: a, esterifying 2-methoxy-4-amino-5-ethylsulfonyl benzoic acid (I) and low alcohol (II) under the catalysis of thionyl chloride to obtain low alcohol ester (III), b, performing condensation on the low alcohol ester (III) obtained in the step a and N-ethyl-2-aminomethylpyrrolidine (IV) to obtain the amisulpride (V). The synthesis method is short in synthesis route (a target compound can be obtained by two steps), high in yield and atom utilization rate and easy to operate, reaction conditions are mild, a product purification post-treatment process is simple, the use of a catalyst harmful to the environment during the reaction is avoided, and a common recyclable solvent can be used as a reaction solvent.
Owner:SHANGHAI MEDICILON INC
Method or preparing amisulpride acid
ActiveCN103450058ASimple and fast operationHigh yieldOrganic chemistryOrganic compound preparationDiethyl sulfideBenzoic acid
The invention discloses a method for preparing a key intermediate of amisulpride. 2- methoxyl-4-amino-5-diethyl sulfide benzoic acid is oxidized in an alkaline condition so as to obtain the amisulpride acid (key intermediate of amisulpride). Compared with the existing methods, the method can produce products with higher quality, the yield is improved remarkably, the operation is simpler, safer, and has environment-friendly effect, and the method is especially suitable for industrial production.
Owner:广安凯特制药有限公司
Psychotropic agents and uses thereof
The R enantiomer of amisulpride and amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for antagonizing serotonin (e.g., 5-HT2a, 5-HT7) receptor in a subject, either individually or in combination with one or more other CNS active agents. The R enantiomer of amisulpride and amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more conditions responsive to modulation of serotonin (e.g., 5-HT2a, 5-HT7) receptor in a subject, either individually or in combination with one or more other CNS active agents. The R enantiomer of amisulpride and amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more disorders associated with an abnormality in levels of serotonin in the brain, either individually or in combination with one or more other CNS active agents.
Owner:LB PHARMA INC
Intermediate in amisulpride and method for preparing amisulpride by using intermediate
InactiveCN102807516AQuality improvementAvoid it happening againOrganic chemistryOrganic compound preparationBenzoic acidPyrrolidine
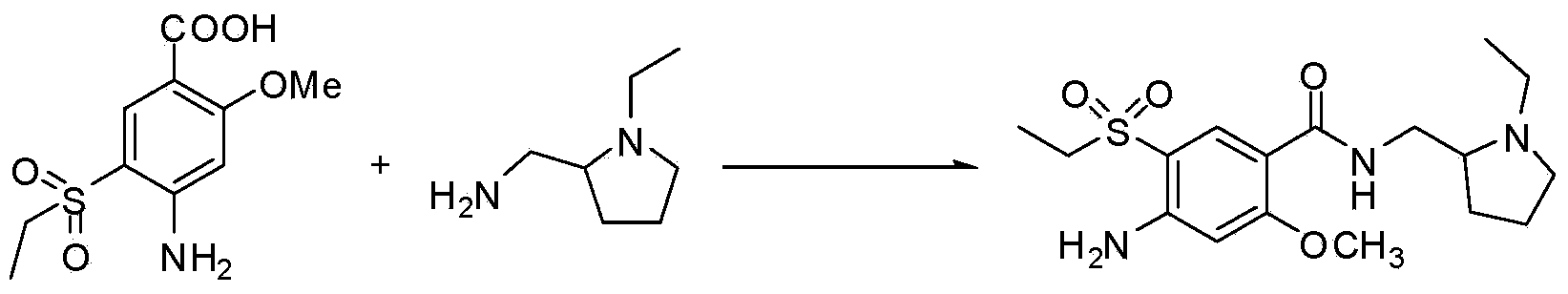
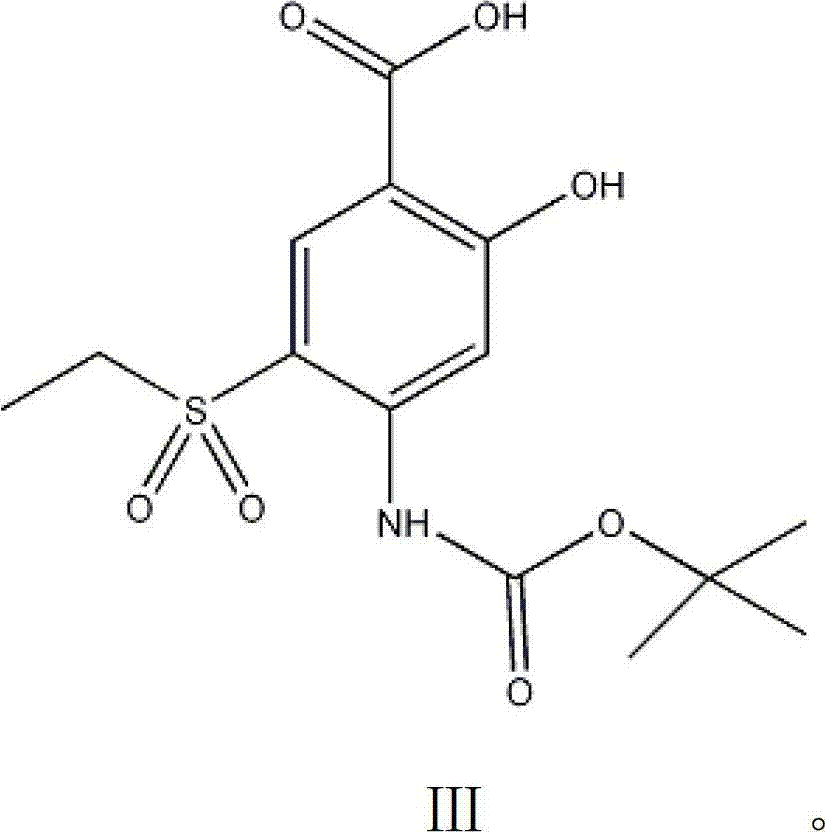
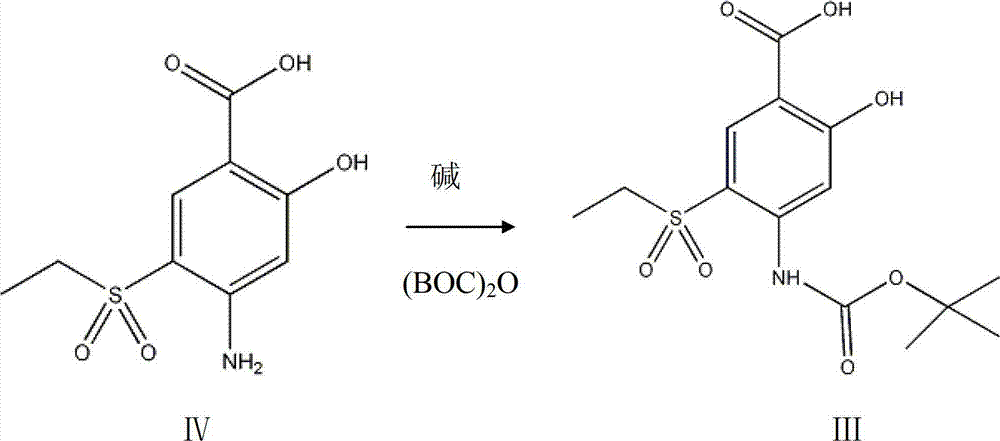
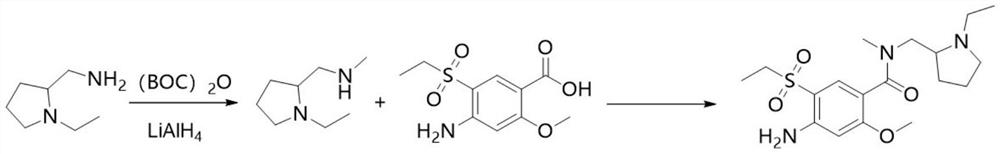
The invention discloses an intermediate in amisulpride and a method for preparing amisulpride by using an intermediate. The method comprises the following steps: reacting 4-amino-2-methoxy group-5-ethylsulfonyl benzoic acid and di-tert-butyl dicarbonate ester so as to obtain the intermediate (4-(N-tert-tert-butoxycarbonyl)amino-2-methoxy group-5-ethylsulfonyl benzoic acid) of the amisulpride; and carrying out condensation reaction on the intermediate (4-(N-tert-butoxycarbonyl)amino-2-methoxy group-5-ethylsulfonyl benzoic acid) of the amisulpride and N-ethyl-2-aminomethyl pyrrolidine so as to obtain 4-(N-tert-tert-butoxycarbonyl)amino-N-((1-ethyl-2-pyrrolidyl)methyl)-5-ethylsulfonyl-2-methoxy benzamide, and carrying out deprotection of tert-butoxycarbonyl group (BOC) so as to obtain the amisulpride. According to the method, the process processing is simple, impurities which are hard to remove are avoided, the reaction yield is high and can be up to 76%, the purity of the obtained amisulpride is high, and the medical quality of the amisulpride is greatly improved.
Owner:四川省百草生物药业有限公司
Oral preparation containing amisulpride
ActiveCN102600132BMask bitternessImprove medication complianceNervous disorderMacromolecular non-active ingredientsSolubilityWater soluble
The invention relates to an oral preparation containing amisulpride. The amisulpride which is active medicine is prepared into cyclodextrin inclusion compound, is sieved, is uniformly mixed with pharmaceutically acceptable auxiliary materials, and is granulated by a wet method or a dry method, obtained granules are squashed to form tablets, filled into capsules to form a capsule preparation or is directly separately packaged to obtain a granule preparation. The water solubility and the stability of the oral preparation containing the amisulpride can be strengthened, bitter taste of the amisulpride is covered, medication compliances of patients are improved, and bioavailability of the oral preparation is improved to a great extent.
Owner:QILU PHARMA CO LTD
Amisulpride oral-administration solution
The invention provides a drug solution applicable to oral dosing. The drug solution comprises Amisulpride, cyclodextrin or derivatives thereof, an appropriate medicinal solvent system and other adjuvants. The cyclodextrin or derivatives thereof and a drug cosolvent system can be used for remarkably improving the solubility of the Amisulpride in the solvent system, promoting drug absorption and enhancing a treatment effect. The Amisulpride oral liquid preparation provided by the invention is high in absorption rate, convenient to take and easy in divided dose, can rapidly exert a drug effect and can simultaneously meet various requirements such as clinical medication, sufferer compliance and industrialized mass production.
Owner:BEIJING VENTUREPHARM BIOTECH
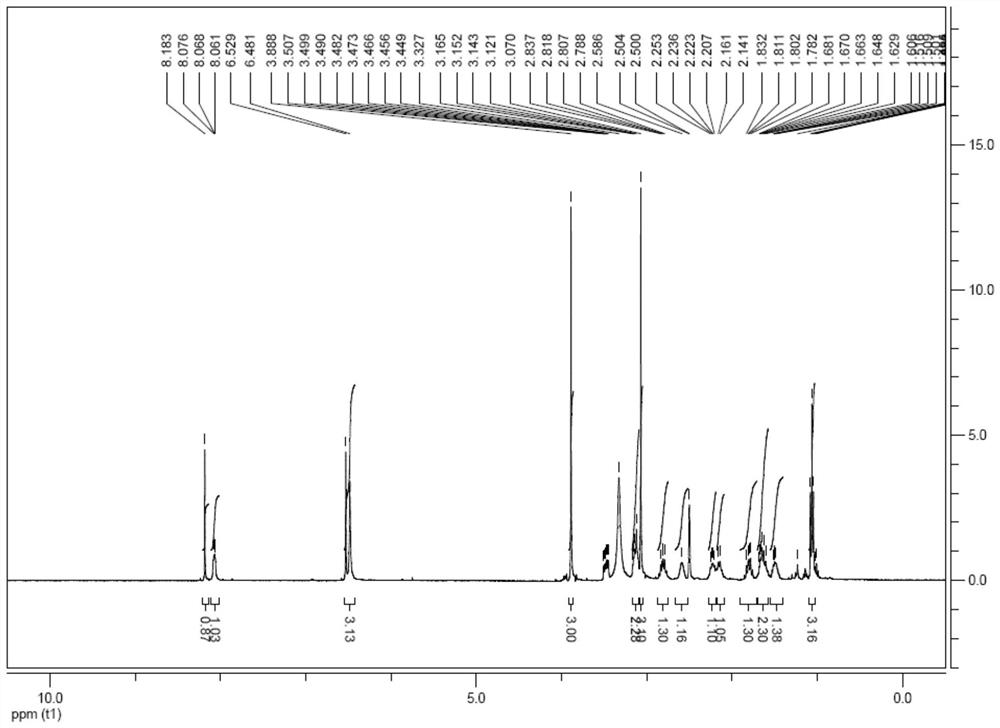
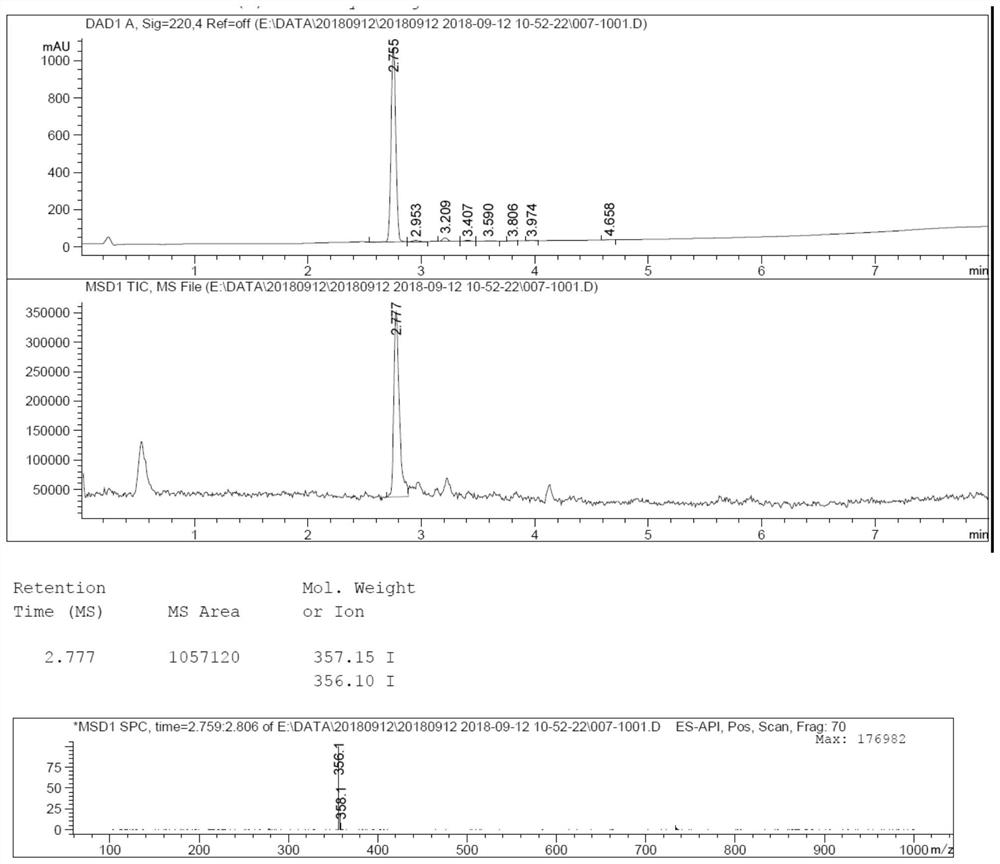
Preparation method of amisulpride
ActiveCN112624951ASimple post-processingSuitable for industrial productionOrganic chemistryChemical recyclingBenzoic acidPtru catalyst
The invention relates to a preparation method of amisulpride, which comprises the steps of carrying out condensation reaction on 4-amino-2-methoxy-5-ethyl sulfonyl methyl benzoate, N-ethyl-2-aminomethylpyrrolidine and a solvent at the temperature of 50-100 DEG C under the condition of taking organic alkali as a catalyst, and after the reaction is finished, concentrating the reaction liquid to remove the solvent, and filtering and drying to obtain the amisulpride. The organic alkali is sodium methoxide, potassium methoxide, sodium ethoxide, potassium ethoxide, sodium isopropoxide, potassium isopropoxide, potassium tert-butoxide or sodium tert-butoxide, and the specific synthetic route is shown in the description. By adopting the preparation method disclosed by the invention, a catalyst harmful to the environment is not used in the reaction process, the post-treatment is simple, the solvent is a common recyclable solvent, the yield reaches 90% or above, the purity can reach 99.7%, the single impurity content is less than 0.1%, the requirements of medicinal preparations are met, and the preparation method is suitable for industrial production.
Owner:JIANGSU ALPHA PHARM CO LTD
Preparation method of amisulpride
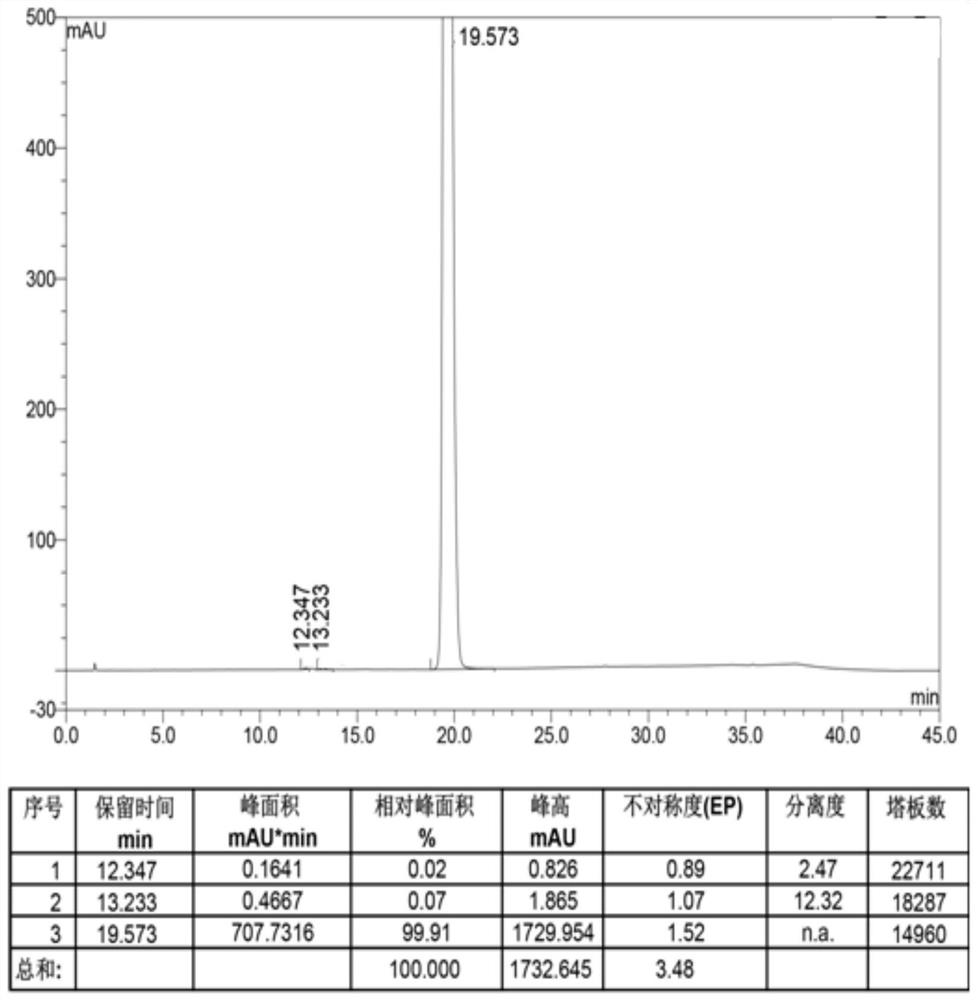
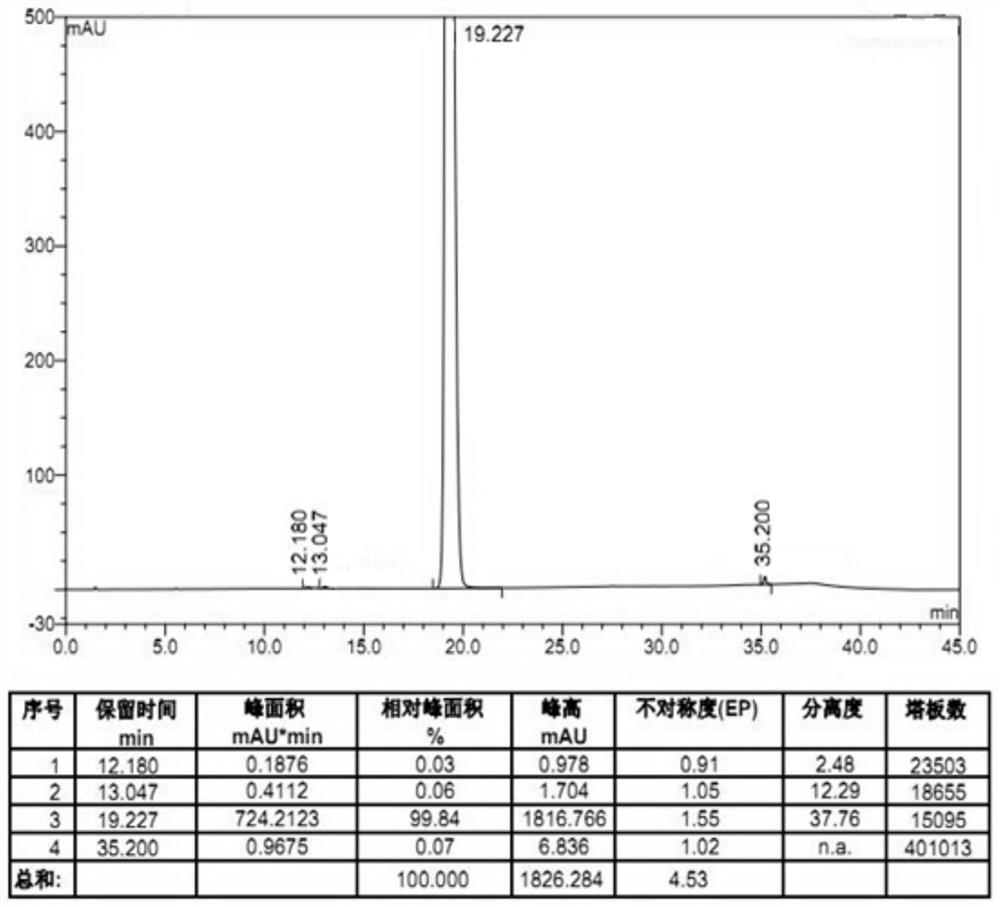
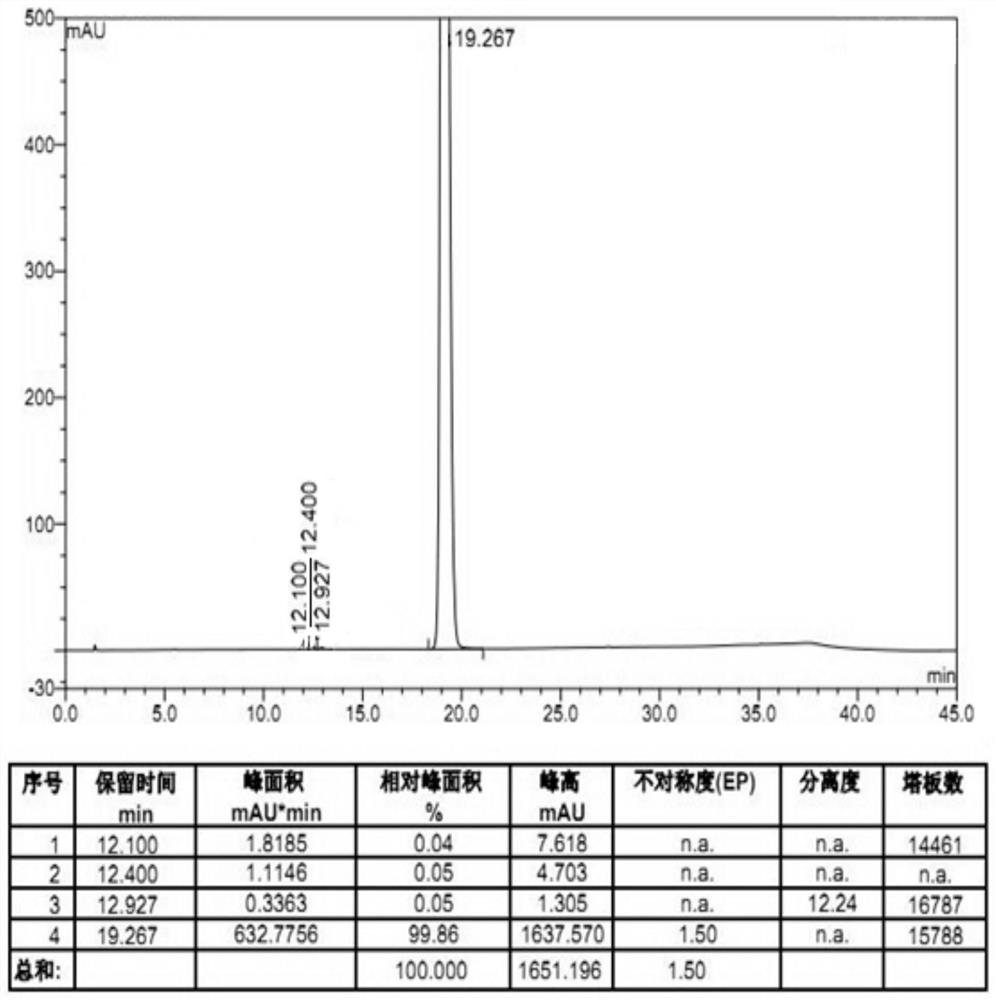
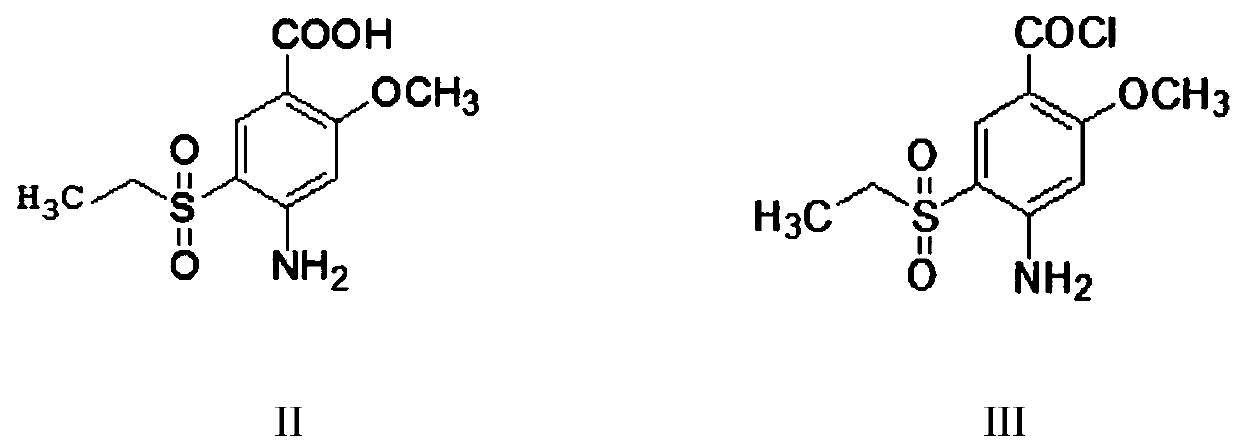
The invention provides a preparation method of amisulpride. The preparation method comprises the following steps: with 2-methoxy-4-amino-5-ethylsulfonyl benzoic acid and thionyl chloride as raw materials, carrying out an acylation reaction to obtain 2-methoxy-4-amino-5-ethylsulfonyl benzoyl chloride; and then, subjecting 2-methoxy-4-amino-5-ethylsulfonyl benzoyl chloride and N-ethyl-2-aminomethylpyrrolidine to an amidation reaction on to obtain amisulpride. The preparation method has the following advantages: raw materials are easy to obtain; highly toxic products are not used; the preparation method is simple; preparation period is short; side reactions are few; the yield and purity of the target product are high; and the target product has market competitiveness.
Owner:山东安弘制药有限公司
Rescue treatment of post operative nausea and vomiting
Amisulpride is useful in the treatment of postoperative nausea and / or vomiting in a patient, wherein the patient has already been administered a prophylaxis drug for postoperative nausea and / or vomiting, and wherein the dose of amisulpride is 7.5 to 15 mg.
Owner:ACACIA PHARMA
Psychotropic agents and uses thereof
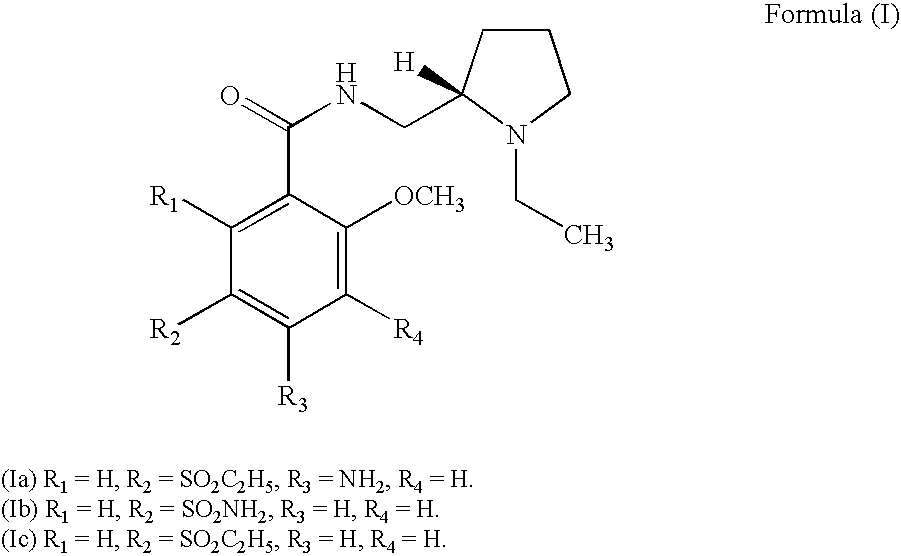
Novel amisulpride derivatives and pharmaceutical compositions thereof are disclosed. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may have better membrane permeability compared to amisulpride. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for antagonizing dopamine and / or serotonin (e.g., 5-HT2a) and / or α2 receptor in a subject, either individually or in combination with other CNS active agents. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more conditions responsive to modulation of dopamine and / or serotonin (e.g., 5-HT2a) and / or α2 receptor in a subject, either individually or in combination with other CNS active agents. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more disorders associated with an abnormality in levels of dopamine and / or serotonin in the brain, either individually or in combination with other CNS active agents.
Owner:LB PHARMA INC
Method for predicting the response of antipsychotic drugs
PendingUS20220290237A1Promote resultsIncrease powerOrganic active ingredientsMicrobiological testing/measurementVitamin B6 synthesisAmisulpride
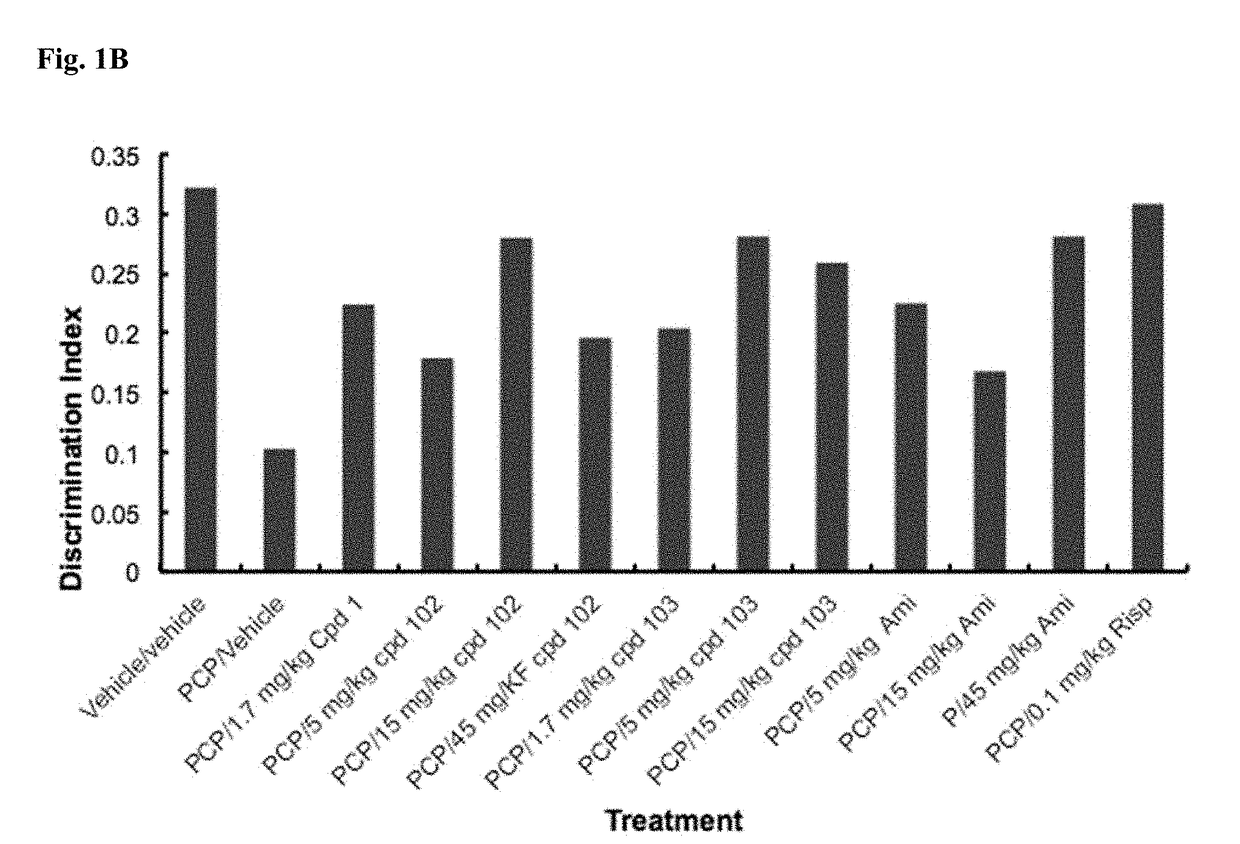
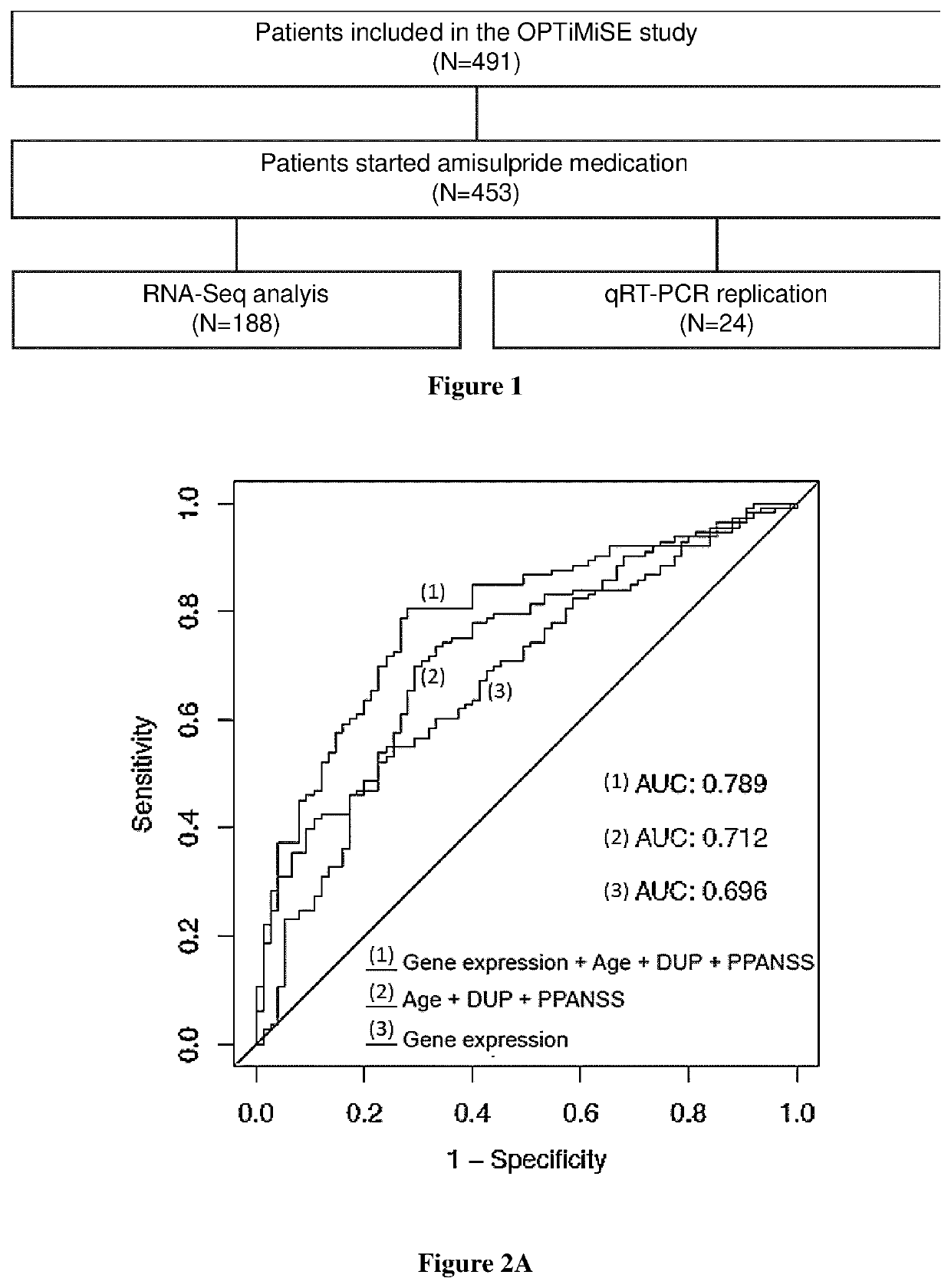
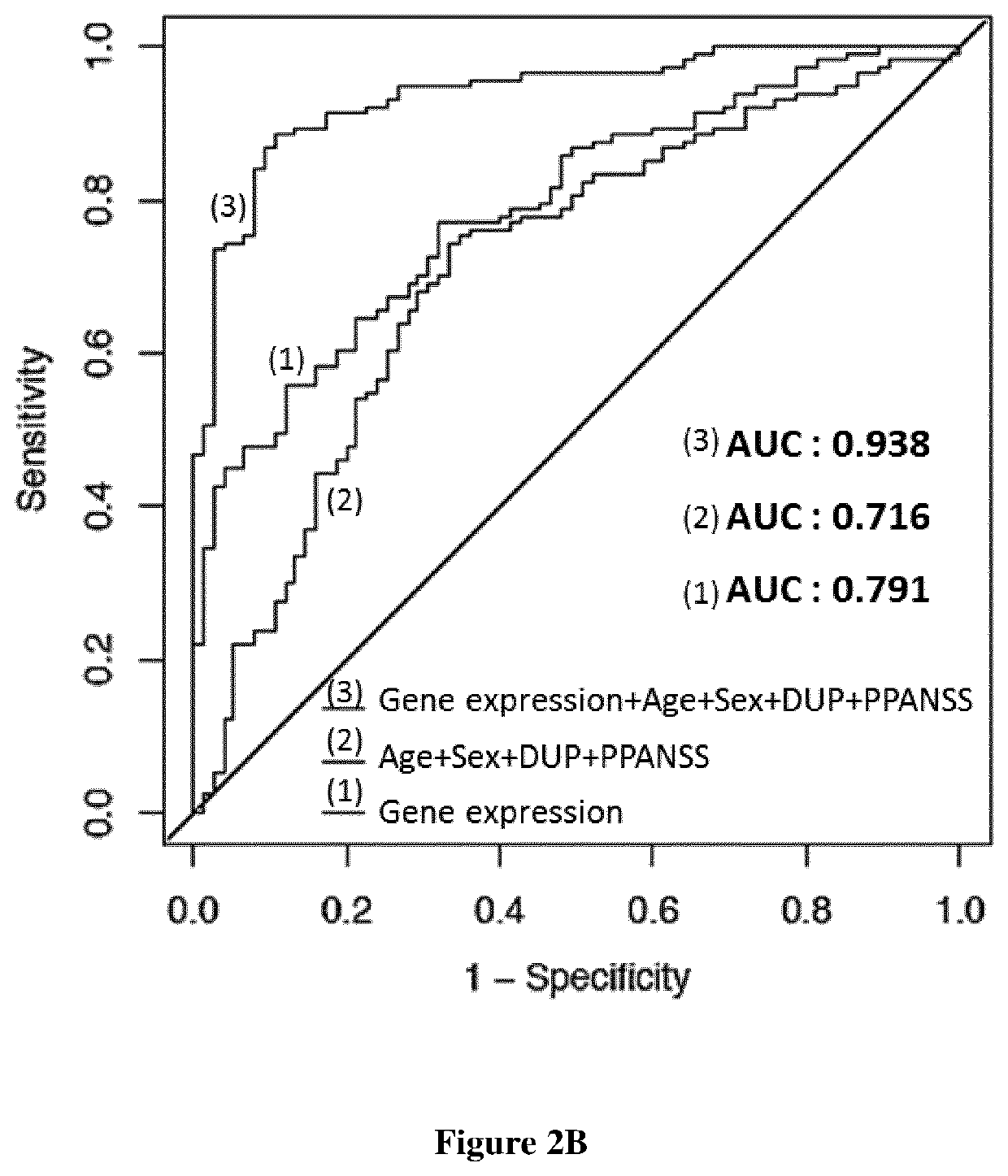
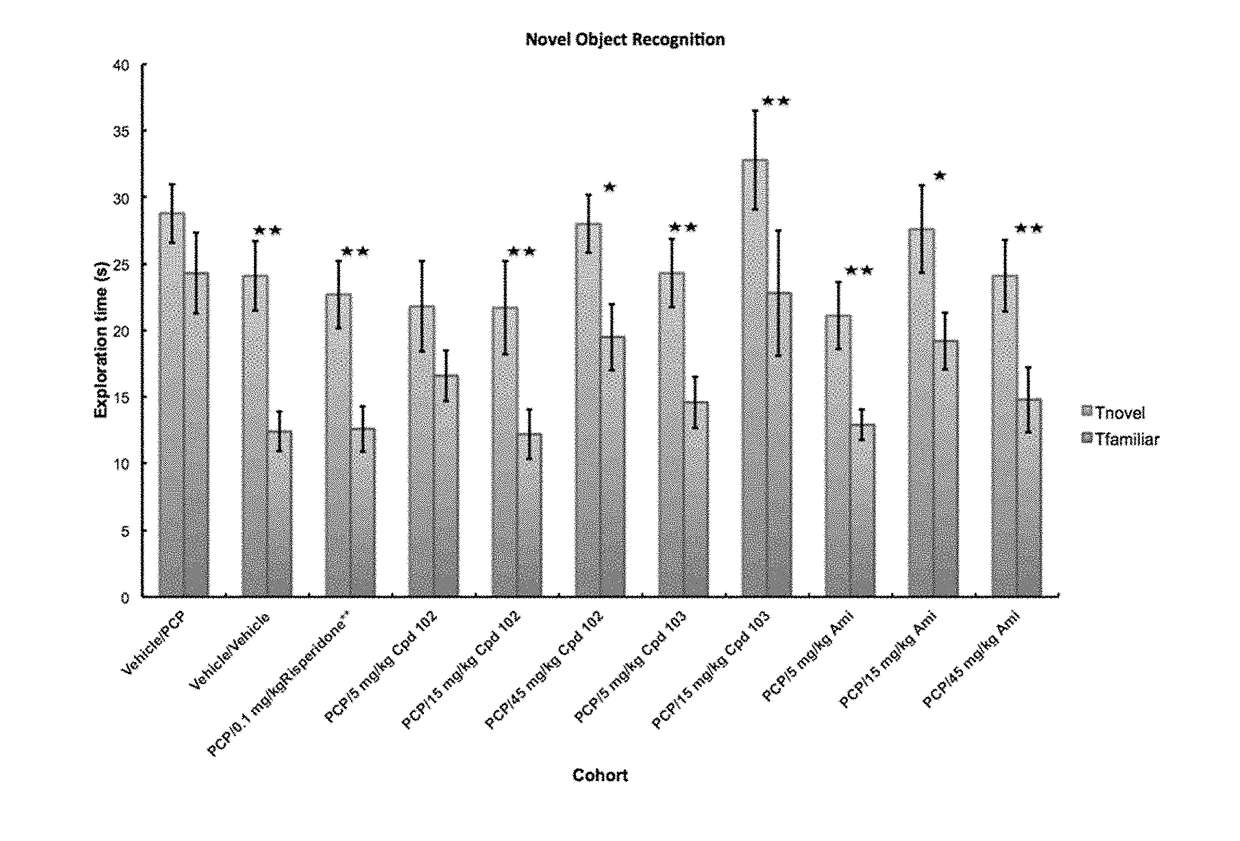
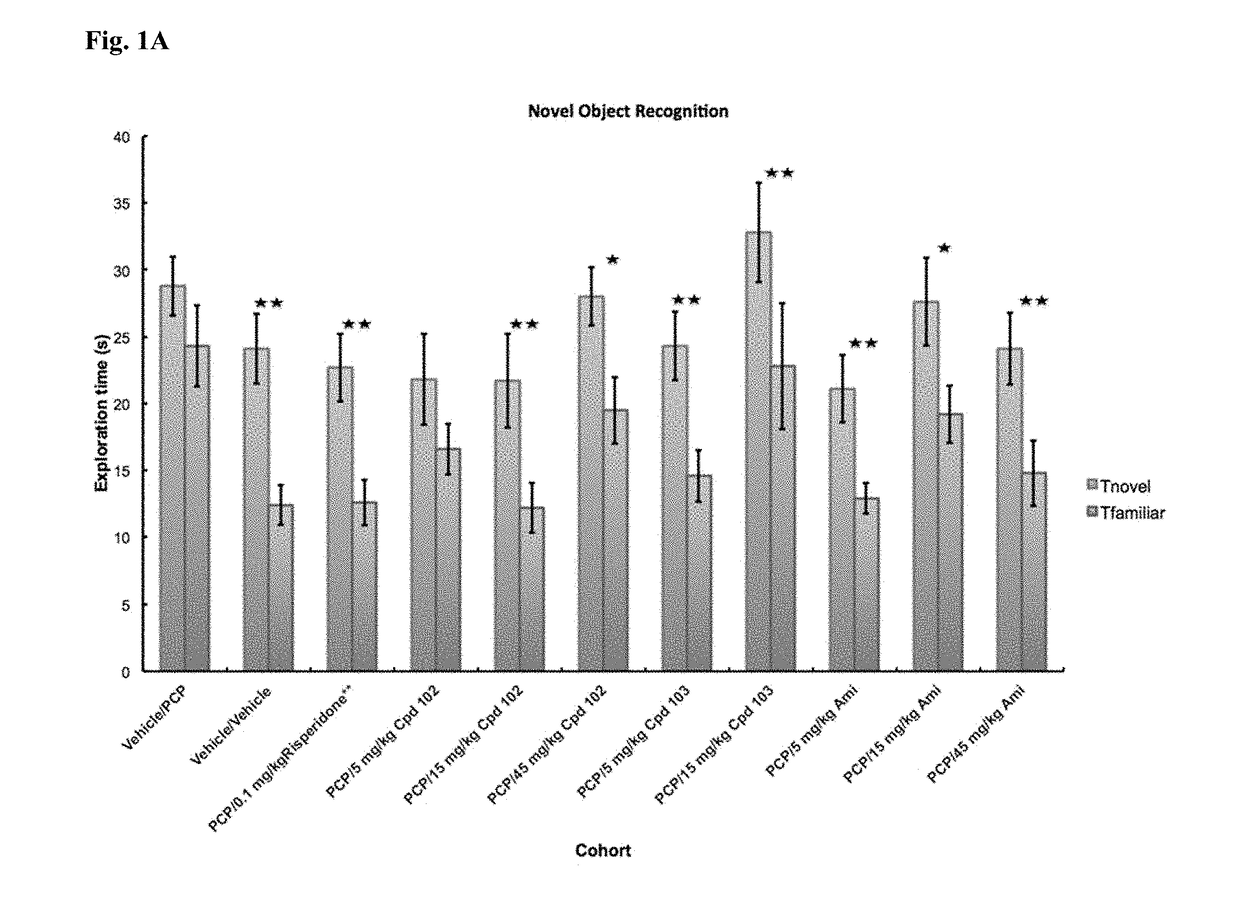
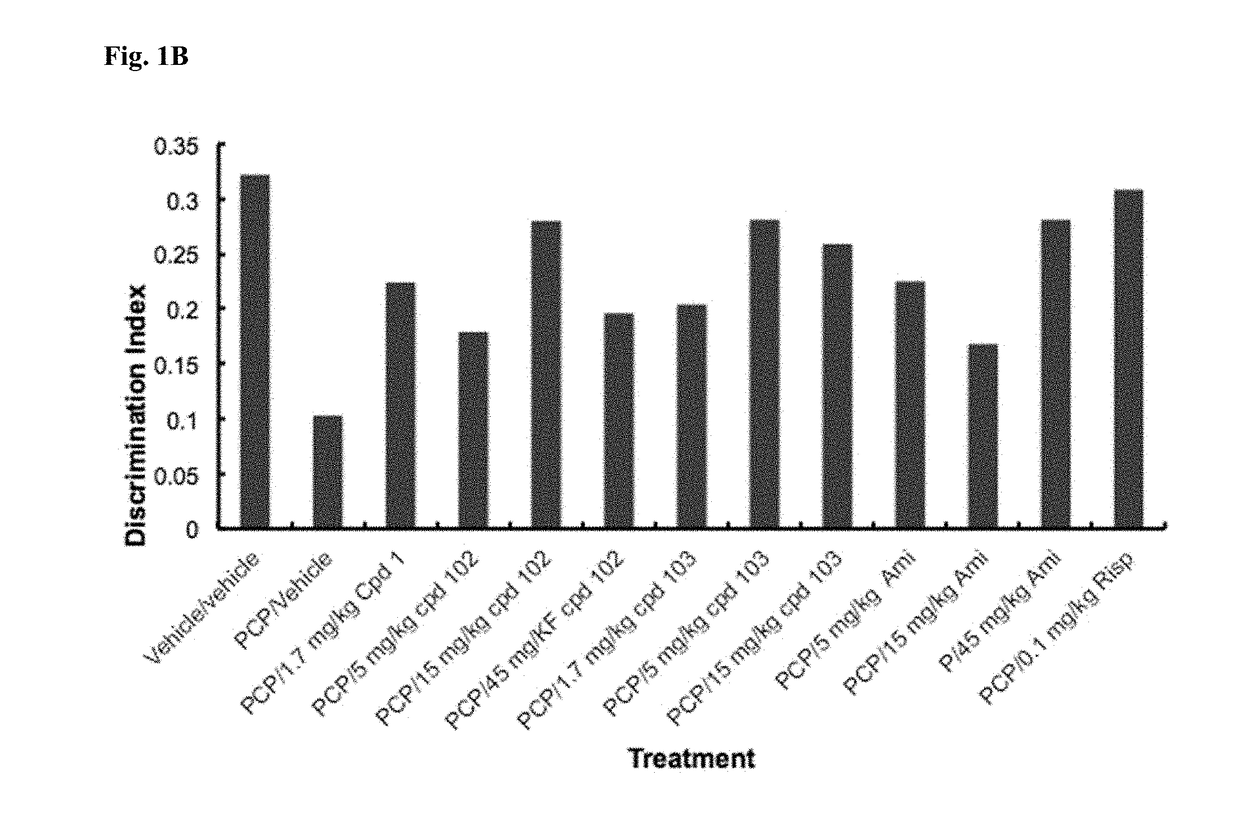
A fundamental shortcoming in the current treatment of schizophrenia is the lack of valid criteria to predict who will respond to antipsychotic treatment. The identification of blood-based biological markers of the therapeutic response would enable clinicians to identify the subgroup of patients in whom conventional antipsychotic treatment is ineffective and offer alternative treatments. As part of the Optimization of Treatment and Management of Schizophrenia in Europe (OPTiMiSE) programme, the inventors conducted a transcriptome analysis on 188 subjects with first episode psychosis, all of whom were subsequently treated with amisulpride for 4 weeks. They identify 32 genes for which the expression changed after treatment in good responders only. Among these genes, the expression of ALPL, a gene involved in vitamin B6 metabolism, as well as CA4, DGTA2, DHRS13, HOMER3 and WLS showed a significant difference in expression level between good and poor responders before starting treatment, allowing to predict treatment outcome with a predictive value of 93.8% when combined with clinical features Collectively, these findings identified new mechanisms to explain symptom improvement after amisulpride medication and highlight the potential of combining gene-expression profiling with clinical data to predict treatment response in first episode psychoses.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Synthesis method of (S) (-)-amisulprideD-(-)-tartrate
ActiveCN101898991BThe process operation is simple and feasibleMild conditionsOrganic chemistryAlcoholSynthesis methods
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Psychotropic agents and uses thereof
The R enantiomer of amisulpride and amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for antagonizing serotonin (e.g., 5-HT2a, 5-HT7) receptor in a subject, either individually or in combination with one or more other CNS active agents. The R enantiomer of amisulpride and amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more conditions responsive to modulation of serotonin (e.g., 5-HT2a, 5-HT7) receptor in a subject, either individually or in combination with one or more other CNS active agents. The R enantiomer of amisulpride and amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more disorders associated with an abnormality in levels of serotonin in the brain, either individually or in combination with one or more other CNS active agents.
Owner:LB PHARMA INC
Amisulpride injection
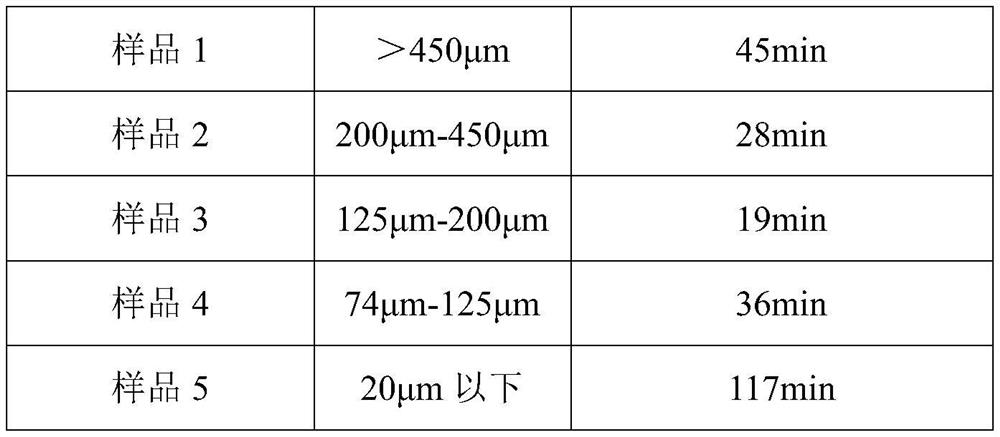
PendingCN114732781AShorten the dissolution timeImprove solubilityDigestive systemPharmaceutical delivery mechanismActive ingredientMedicinal chemistry
The invention provides an amisulpride injection and a preparation method thereof, the particle size of the used bulk drug amisulpride is not less than 20 [mu] m; preferably not less than 74 [mu] m; further, preferably, the particle size is 74-450 [mu] m; furthermore, the particle size of the particles is preferably 125-450 [mu] m. According to the amisulpride injection and the preparation method thereof provided by the invention, the preparation efficiency can be improved, and the content of a finished product is more stable.
Owner:BEIJING PUDEKANGLI PHARMA TECH DEV CO LTD
Amisulpride transdermal patch
PendingCN112716921ASustained and stable drug releaseMedication convenienceNervous disorderPharmaceutical non-active ingredientsTransdermal patchMenthol
The amisulpride transdermal patch comprises a medicine carrying layer, a backing layer and an anti-sticking layer, and the medicine carrying layer is composed of amisulpride, a penetration enhancer and a pressure-sensitive adhesive matrix. The weight percentage content of amisulpride in the medicine carrying layer is 3%-5%. The weight percentage content of the penetration enhancer in the medicine carrying layer is 4-6%, and the penetration enhancer is propylene glycol, oleic acid and menthol in a weight ratio of 10: (1-3): (0.1-0.2). The pressure-sensitive adhesive matrix is a hot-melt pressure-sensitive adhesive matrix.
Owner:安徽京茗药业有限公司
Synthesis method of amisulpride impurity H
PendingCN114507174AMild reaction conditionsHigh purityOrganic chemistryPhysical chemistryEthyl group
The invention belongs to the field of pharmaceutical chemicals, and relates to a synthesis method of an amisulpride impurity H. According to the invention, N-ethyl-2-methylaminomethylpyrrolidine is prepared by using a one-step method, and an impurity H is prepared by constructing an amido bond by using chloroformate and an acid-binding agent system. The method is mild in reaction condition and high in product purity, and the prepared impurity H can be used for impurity research of amisulpride.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Amisulpride tablet and preparation method thereof
PendingCN112089698AIncreased release rate in vivoImprove complianceNervous disorderPill deliveryPharmacyActive agent
The invention belongs to the technical field of medicines, particularly relates to the field of pharmacy, and more particularly relates to an amisulpride tablet and a preparation method thereof. The amisulpride tablet is prepared from the following components in percentage by mass: 39.6 to 43.8 percent of amisulpride, 0.1 to 2.0 percent of a surfactant and the balance of pharmaceutically acceptable auxiliary materials. By adding the surfactant, the in-vivo release rate of the amisulpride during use can be remarkably increased, and drug absorption is guaranteed. Meanwhile, according to the preparation method disclosed by the invention, a dry granulation process is taken as a basis, and the problem of poor raw material fluidity of the amisulpride tablet is effectively solved through materialadding sequence design; and the problems of bitter taste and the like of the amisulpride are effectively solved in a preparation process, and the medication compliance of a patient is remarkably improved.
Owner:JIANGSU ALPHA PHARM CO LTD
Psychotropic agents and uses thereof
ActiveUS20180155282A1Strengthen membraneNervous disorderOrganic chemistry methodsMembrane permeabilitySerotonin
Novel amisulpride derivatives and pharmaceutical compositions thereof are disclosed. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may have better membrane permeability compared to amisulpride. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for antagonizing dopamine and / or serotonin (e.g., 5-HT2a) and / or α2 receptor in a subject, either individually or in combination with other CNS active agents. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more conditions responsive to modulation of dopamine and / or serotonin (e.g., 5-HT2a) and / or α2 receptor in a subject, either individually or in combination with other CNS active agents. The amisulpride derivative disclosed herein or a pharmaceutical composition thereof may be used for treating one or more disorders associated with an abnormality in levels of dopamine and / or serotonin in the brain, either individually or in combination with other CNS active agents.
Owner:LB PHARMA INC
Preparation method for pharmacopoeia impurity of amisulpride
The invention reports a preparation method for a pharmacopoeia impurity of amisulpride, and particularly relates to a preparation method for the pharmacopoeia impurity D of amisulpride. The method is simple and easy to operate, mild in condition, high in yield, low in energy consumption and pollution and suitable for laboratory-level standard substance preparation.
Owner:SHANGHAI SYNCORES TECH INC +1
A kind of preparation method of amisulpride
The invention provides a preparation method of amisulpride. The preparation method comprises the following steps: with 2-methoxy-4-amino-5-ethylsulfonyl benzoic acid and thionyl chloride as raw materials, carrying out an acylation reaction to obtain 2-methoxy-4-amino-5-ethylsulfonyl benzoyl chloride; and then, subjecting 2-methoxy-4-amino-5-ethylsulfonyl benzoyl chloride and N-ethyl-2-aminomethylpyrrolidine to an amidation reaction on to obtain amisulpride. The preparation method has the following advantages: raw materials are easy to obtain; highly toxic products are not used; the preparation method is simple; preparation period is short; side reactions are few; the yield and purity of the target product are high; and the target product has market competitiveness.
Owner:山东安弘制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com