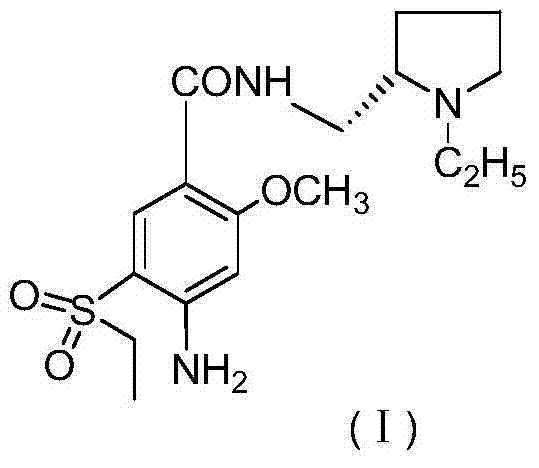
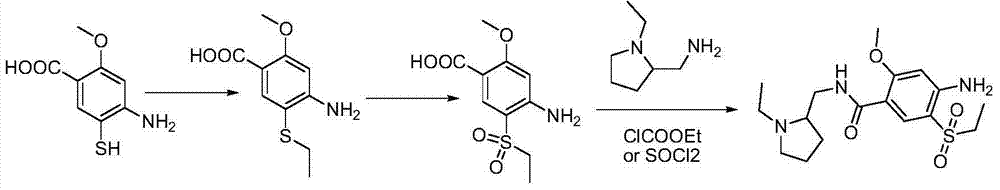
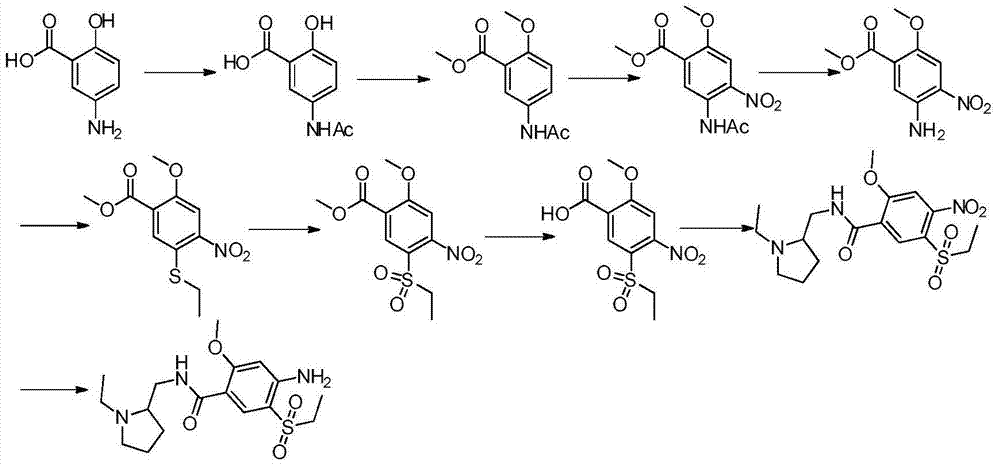
Preparation method of (S)(-)-amisulpride
The technology of amisulpride and compound is applied in the field of preparation of antipsychotic drug-amisulpride, can solve the problems of slow reaction speed, difficult to industrialized production, and high reaction temperature, and achieves mild reaction conditions, short reaction time, raw materials, etc. low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of (S)-1-ethylpyrrolidine-2-carboxylic acid
[0040] Put 75g of L-proline, 131g of potassium hydroxide and 500ml of absolute ethanol into a 1000ml four-neck flask, stir at 25°C until completely dissolved, then slowly add dropwise a solution of 85g of bromoethane and 100ml of absolute ethanol. After the dropwise addition was completed, the stirring reaction was continued for 4 h, and then the temperature was raised to 35° C. for 1.5 h. After the reaction was completed, adjust the pH to 4-5 with concentrated hydrochloric acid, filter with suction, recover ethanol under reduced pressure, and precipitate a white solid, which was dried to obtain 88.1 g of the target product, with a yield of 94.7%.
Embodiment 2
[0041] Example 2: Preparation of (S)-N-(2,4-dimethoxybenzyl)-1-ethylpyrrolidine-2-carboxamide
[0042] Add 112g of N,N-carbonyldiimidazole (CDI) and 400ml of dichloromethane into a 1000ml four-neck flask, stir to dissolve, and add (S)-1-ethylpyrrolidine-2-carboxylic acid obtained in the previous step in batches 86g, stirred at room temperature for 3h, then added dropwise to a solution of 110g of 2,4-dimethoxybenzylamine and 100ml of dichloromethane at a controlled temperature of 0-10°C. Add 300ml of water, stir, stand still, and separate layers. The organic layer is washed with 300ml of 5% sodium bicarbonate in water, dried over anhydrous sodium sulfate, filtered, and the organic solvent is evaporated under reduced pressure to obtain 151.2g of a light yellow solid. The rate is 86.3%.
Embodiment 3
[0043] Example 3: Preparation of (S)-(2,4-dimethoxyphenyl)-N-[(1-ethylpyrrolidin-2-yl)methyl]methanamine
[0044] Under nitrogen protection, 146 g of (S)-N-(2,4-dimethoxybenzyl)-1-ethylpyrrolidine-2-carboxamide and 500 ml of anhydrous tetrahydrofuran (THF) obtained in the previous step were added to In a 2000ml four-neck flask, cool down to -15~-10°C, add dropwise 750ml of 1mol / L diborane tetrahydrofuran solution, after the drop is complete, heat up to 35°C and stir to react overnight, then cool down to -15°C, add dropwise 200ml Ice water, release a lot of gas. The mixed solution was adjusted to pH 10-11 with 10% potassium hydroxide, extracted three times with 500ml ether, combined the organic layers, washed twice with 50ml of saturated aqueous sodium chloride solution, washed once with 500ml of water, dried over anhydrous sodium sulfate, After filtration, the solvent was distilled off under reduced pressure at 25°C to obtain 118.3 g of light yellow oil. Yield 85.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com