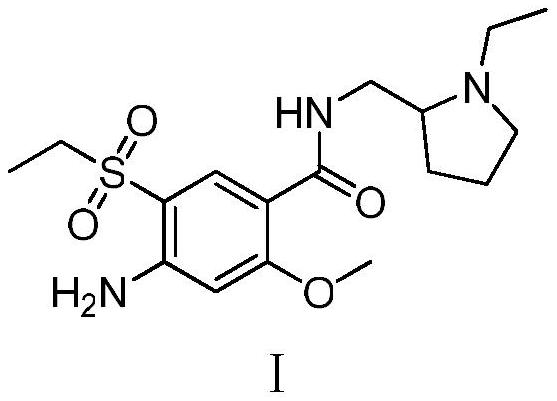
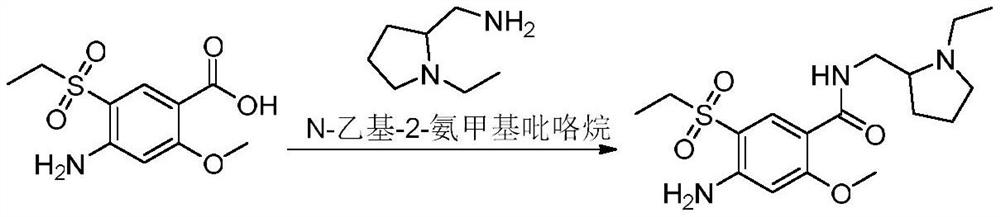
Synthesis method of amisulpride impurity H
A technology for amisulpride and impurities, which is applied in the field of synthesis of amisulpride impurity H, can solve the problems of unmentioned product purity, long steps, etc., and achieves strong practical application value, few by-products, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
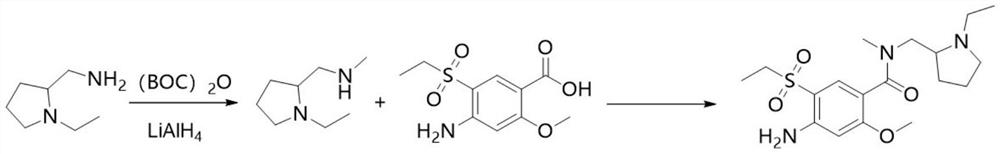
[0032] Synthesis of N-ethyl-2-methylaminomethylpyrrolidine
[0033] Control the temperature at 0°C and add 12.8g (0.10mol) N-ethyl-2-aminomethylpyrrolidine into 100ml of dichloromethane, then add 20.2g (0.20mol) triethylamine, add 28.3g ( 0.13mol) di-tert-butyl dicarbonate, keep 0 DEG C and react for 12 hours, wash with 100ml 10% aqueous citric acid solution, wash the organic layer with 100ml drinking water, dry the organic layer with 5g magnesium sulfate for 30 minutes, remove magnesium sulfate by filtration, The solution was cooled to 0-10°C, slowly added dropwise to the lithium aluminum hydride solution composed of 7.8g (0.2mol) lithium aluminum hydride and 100ml tetrahydrofuran, which had been cooled to 0°C in advance, heated and refluxed for 3 hours, and cooled to 0-10°C ℃, slowly added 15ml of 15% sodium hydroxide solution, stirred for 30 minutes, filtered to remove insoluble matter, and evaporated the solvent to obtain 11.4g of N-ethyl-2-methylaminomethylpyrrolidine, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com