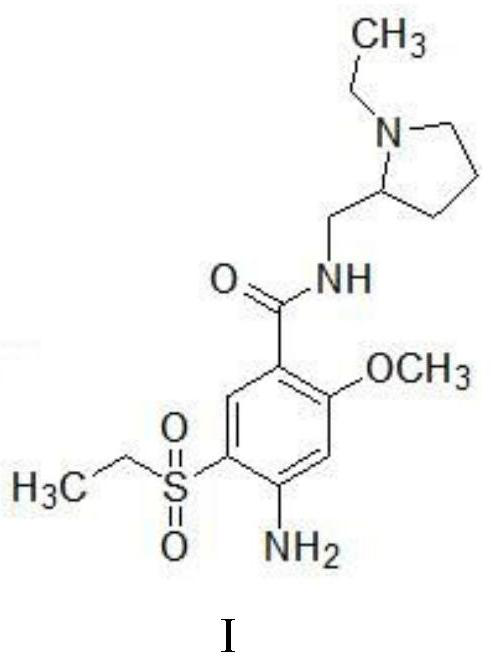
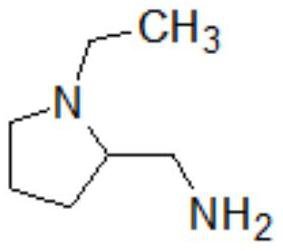
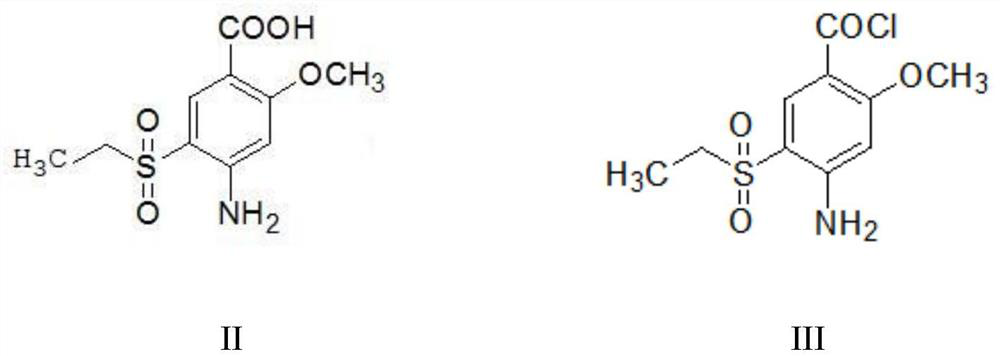A kind of preparation method of amisulpride
A technology of amisulpride and compounds, applied in the field of pharmaceutical chemical synthesis, can solve problems such as unfavorable industrial production, long reaction time, and tediousness, and achieve the effects of high yield of target products, short preparation cycle, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A preparation method of amisulpride, comprising the steps of:
[0041] (1) Add 300g of dichloromethane and 50g of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid (II) into the reaction flask, stir evenly, and add 2.5g of N,N-dimethylformamide , add 24.1g of thionyl chloride dropwise at a temperature of 20-25°C, raise the temperature to 35-40°C after the dropwise addition, and reflux for 4 hours to obtain 2-methoxy-4-amino-5-ethanesulfonylbenzyl The reaction liquid of acyl chloride (III); The reaction liquid directly carries out the next step reaction without processing;
[0042] (2) The temperature of the reaction solution obtained in step (1) is lowered to 20-25°C, and the temperature is controlled at 20-25°C, and a mixed solution of 49.5g of N-ethyl-2-aminomethylpyrrolidine (IV) and 100g of dichloromethane is added dropwise After completion of the dropwise addition, the temperature was raised to 35-40°C for 4 hours, and the TLC test was qualified; the obtained reactio...
Embodiment 2
[0046] A preparation method of amisulpride, comprising the steps of:
[0047](1) Add 300g of dichloromethane and 50g of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid (II) into the reaction flask, stir well, and add 2.5g of N,N-dimethylformamide , add 24.1g of thionyl chloride dropwise at a temperature of 20-25°C, raise the temperature to 35-40°C after the dropwise addition, and reflux for 6 hours to obtain 2-methoxy-4-amino-5-ethanesulfonylbenzyl The reaction liquid of acyl chloride (III); The reaction liquid directly carries out the next step reaction without processing;
[0048] (2) The temperature of the reaction solution obtained in step (1) was lowered to 20-25°C, and the temperature was controlled at 20-25°C, and a mixed solution of 61.9g N-ethyl-2-aminomethylpyrrolidine (IV) and 100g dichloromethane was added dropwise , the dropwise addition was completed, and the heat preservation was raised to 35-40° C. for 5 hours, and the TLC test was qualified; the obtained react...
Embodiment 3
[0050] A preparation method of amisulpride, comprising the steps of:
[0051] (1) Add 300g of dichloromethane and 50g of 2-methoxy-4-amino-5-ethanesulfonylbenzoic acid (II) into the reaction flask, stir well, and add 2.5g of N,N-dimethylformamide , add 24.1g of thionyl chloride dropwise at a temperature of 20-25°C, raise the temperature to 35-40°C after the dropwise addition, and reflux for 8 hours to obtain 2-methoxy-4-amino-5-ethanesulfonylbenzyl The reaction liquid of acyl chloride (III); The reaction liquid directly carries out the next step reaction without processing;
[0052] (2) The temperature of the reaction solution obtained in step (1) was lowered to 20-25°C, and a mixed solution of 74.3g of N-ethyl-2-aminomethylpyrrolidine (IV) and 100g of dichloromethane was added dropwise at a temperature of 20-25°C , the dropwise addition was completed, and the temperature was raised to 35-40°C for 6 hours, and the TLC test was qualified; the obtained reaction solution was was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com