Oral preparation containing amisulpride
A technology for oral preparations of amisulpride, applied in the field of pharmaceutical preparations, can solve the problems of large specifications, difficulty in orally dispersible tablets, difficulty in achieving rapid dissolution and rapid onset of effect, etc., to improve water solubility, facilitate rapid dissolution, improve The effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
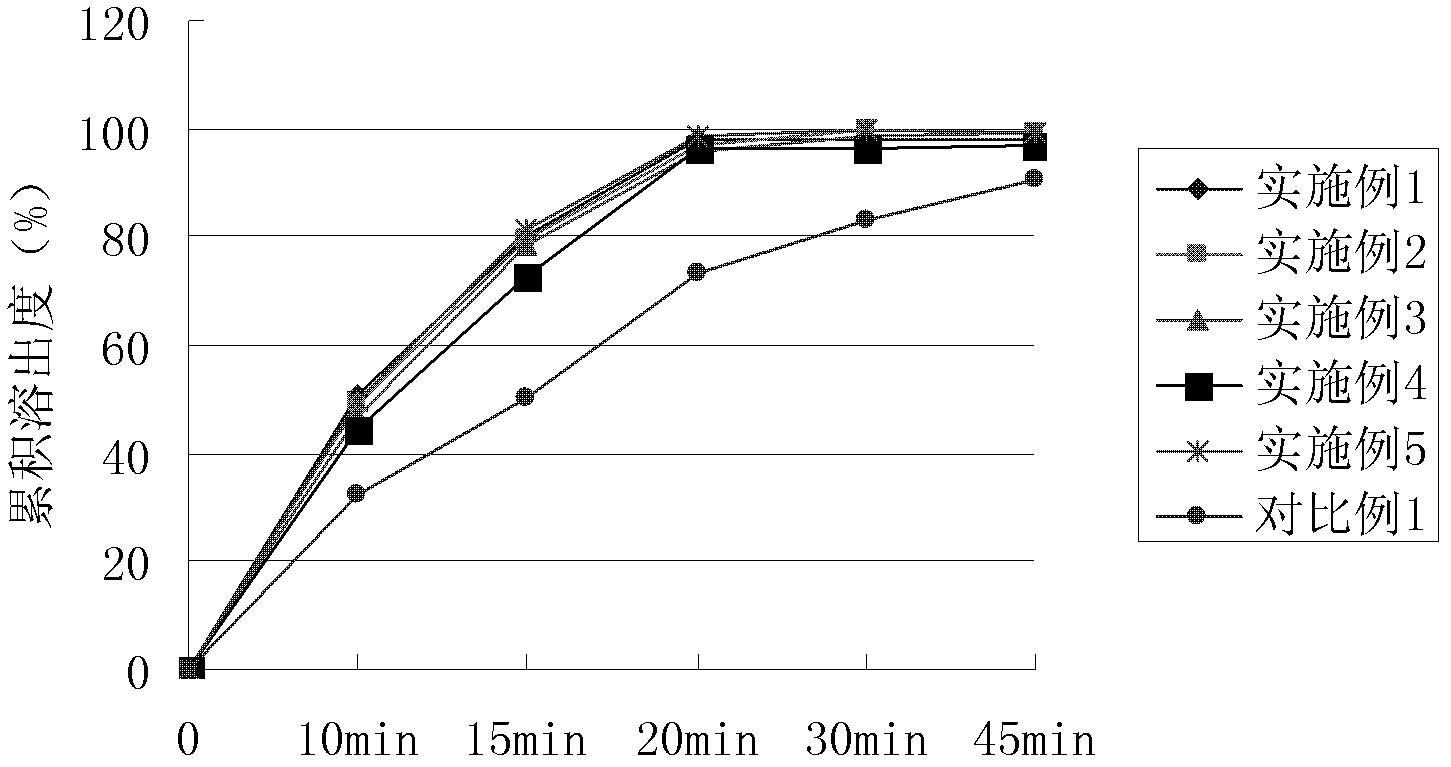
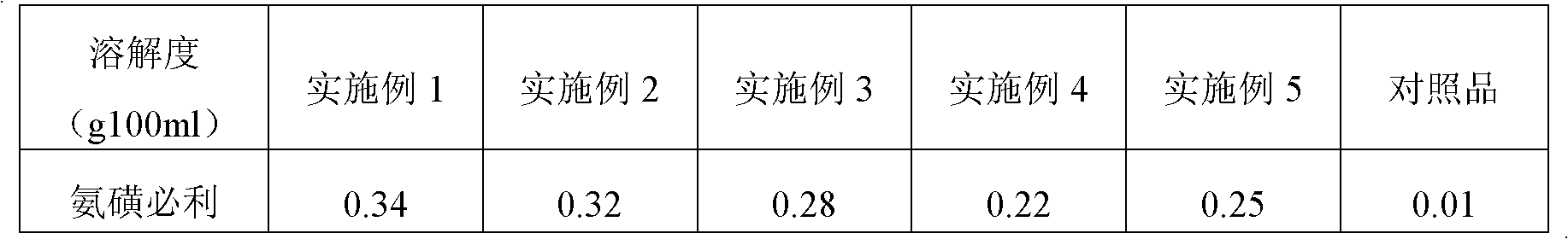
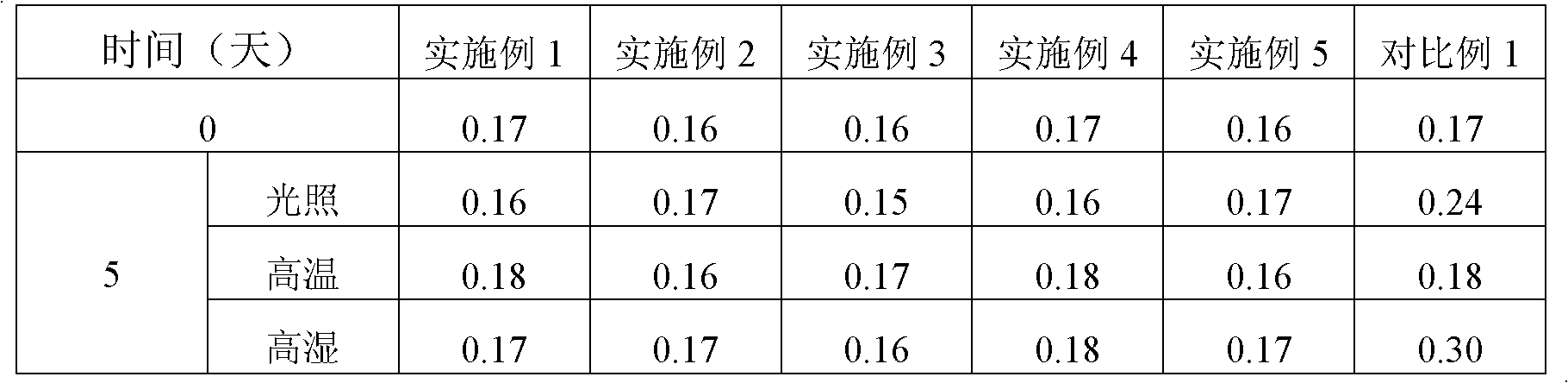
Image
Examples
Embodiment 1
[0063] Embodiment 1: amisulpride oral preparation prescription:
[0064] Raw material name dosage
[0065] Amisulpride 100g,
[0066] β-cyclodextrin 350g,
[0067] Microcrystalline Cellulose 63g,
[0068] Crospovidone 18g,
[0069] Polyvinylpyrrolidone 16g is made into 10wt% polyvinylpyrrolidone aqueous solution and added,
[0071] Silica 3g,
[0072] A total of 1000 units were made.
[0073] Preparation Process:
[0074] 1) Prepare 50°C saturated aqueous solution by weighing 350g of β-cyclodextrin, then place it in a high-speed mixer, start stirring at a speed of 1000 rpm; dissolve 100g of amisulpride in 3000ml of ethanol, and then add it dropwise to In the stirred saturated aqueous solution of β-cyclodextrin, the dropping rate is 2ml / min, and stirred at constant temperature for 1 hour; stop heating and continue stirring to room temperature, stop stirring after 3 hours, and spray dry to obtain β-cyclodextrin inclusion compound ;
[00...
Embodiment 2
[0078] Embodiment 2: amisulpride oral preparation prescription:
[0079] Raw material name dosage
[0080] Amisulpride 100g,
[0081] β-cyclodextrin 250g,
[0082] Lactose 199g,
[0083] Croscarmellose Sodium 21g,
[0084] Hydroxypropyl methylcellulose 3g, made into 2wt% hydroxypropyl methylcellulose aqueous solution and added,
[0085] Talcum powder 30g,
[0086] A total of 1000 units were made.
[0087] Preparation Process
[0088] 1) Weigh β-cyclodextrin according to the prescription amount to make a 50°C saturated aqueous solution, then put it in a high-speed mixer, start stirring at a speed of 1500 rpm; dissolve the prescription amount of amisulpride in 3000ml of ethanol, and then Add dropwise into the stirred saturated aqueous solution of β-cyclodextrin at a speed of 2 ml / min, and stir at constant temperature for 30 minutes. Stop heating and continue stirring to room temperature, stop stirring after 2.5 hours, and spray dry to obtain β-cyclodextrin inclusion comp...
Embodiment 3
[0091] Embodiment 3: raw material name consumption
[0092] Amisulpride 100g,
[0093] α-Cyclodextrin 200g,
[0094] Pregelatinized starch 240g,
[0095] Sodium carboxymethyl starch 30g,
[0096] Hydroxypropyl cellulose 5g is made into the solution containing 5wt% hydroxypropyl cellulose with the ethanol of concentration 75wt% and adds,
[0097] Talcum powder 30g,
[0098] A total of 1000 units were made.
[0099] Preparation Process
[0100] 1) Weigh α-cyclodextrin according to the prescription amount, add appropriate amount of water and grind it into a paste, pour it into the colloid mill, and turn on the machine; dissolve the prescription amount of amisulpride in 3000ml of ethanol, and then add it dropwise to the ground In the pasty α-cyclodextrin, the speed is 3ml / min, continue to grind for 30 minutes, and spray dry to obtain the α-cyclodextrin inclusion compound;
[0101] 2) Pass the prepared α-cyclodextrin inclusion compound through a 100-mesh sieve, then mix it e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com