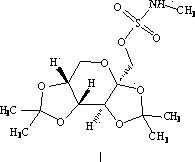
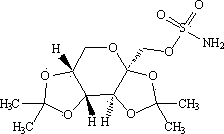
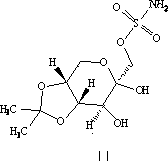
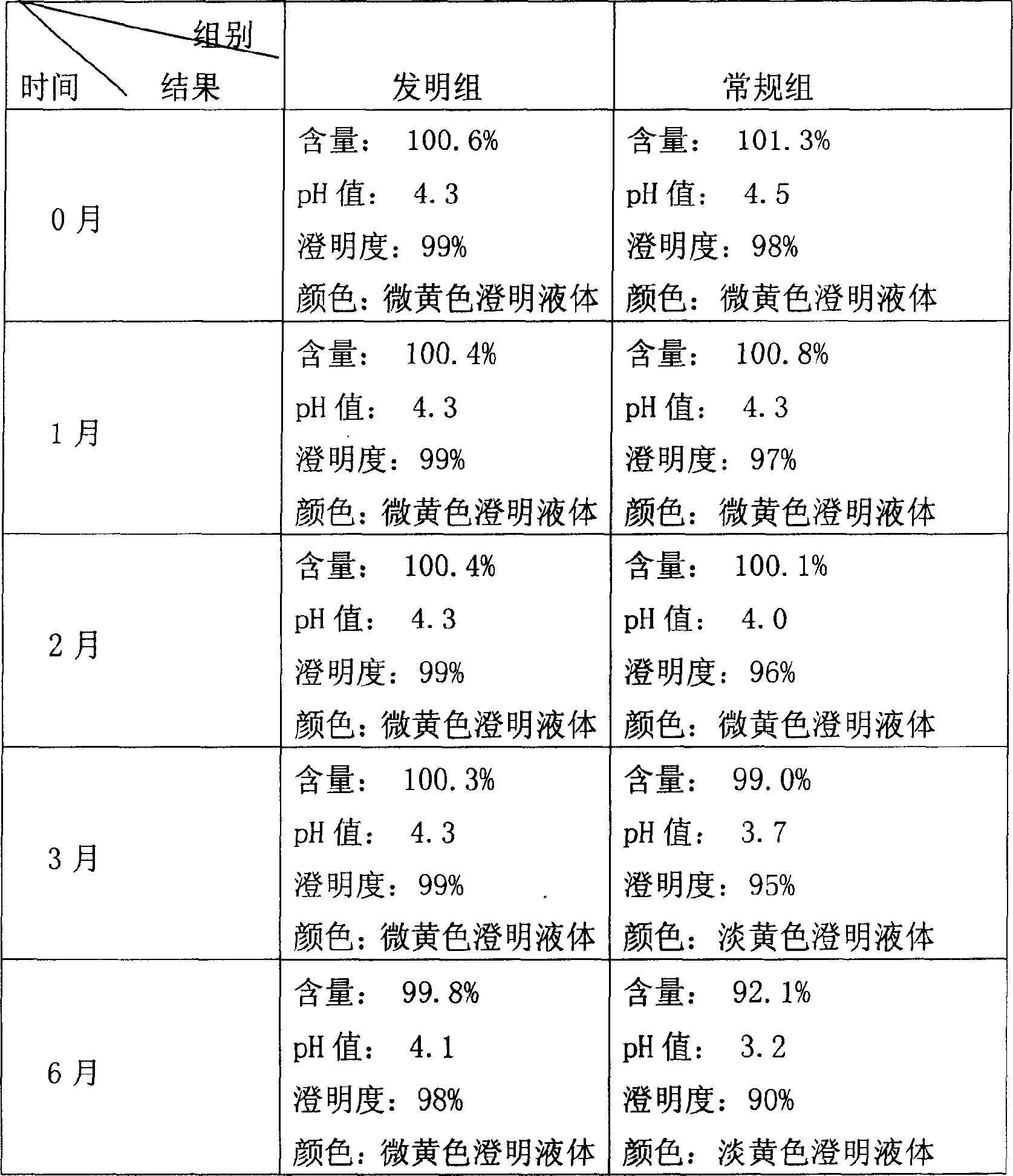
Patents
Literature
46results about How to "Low related substances" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
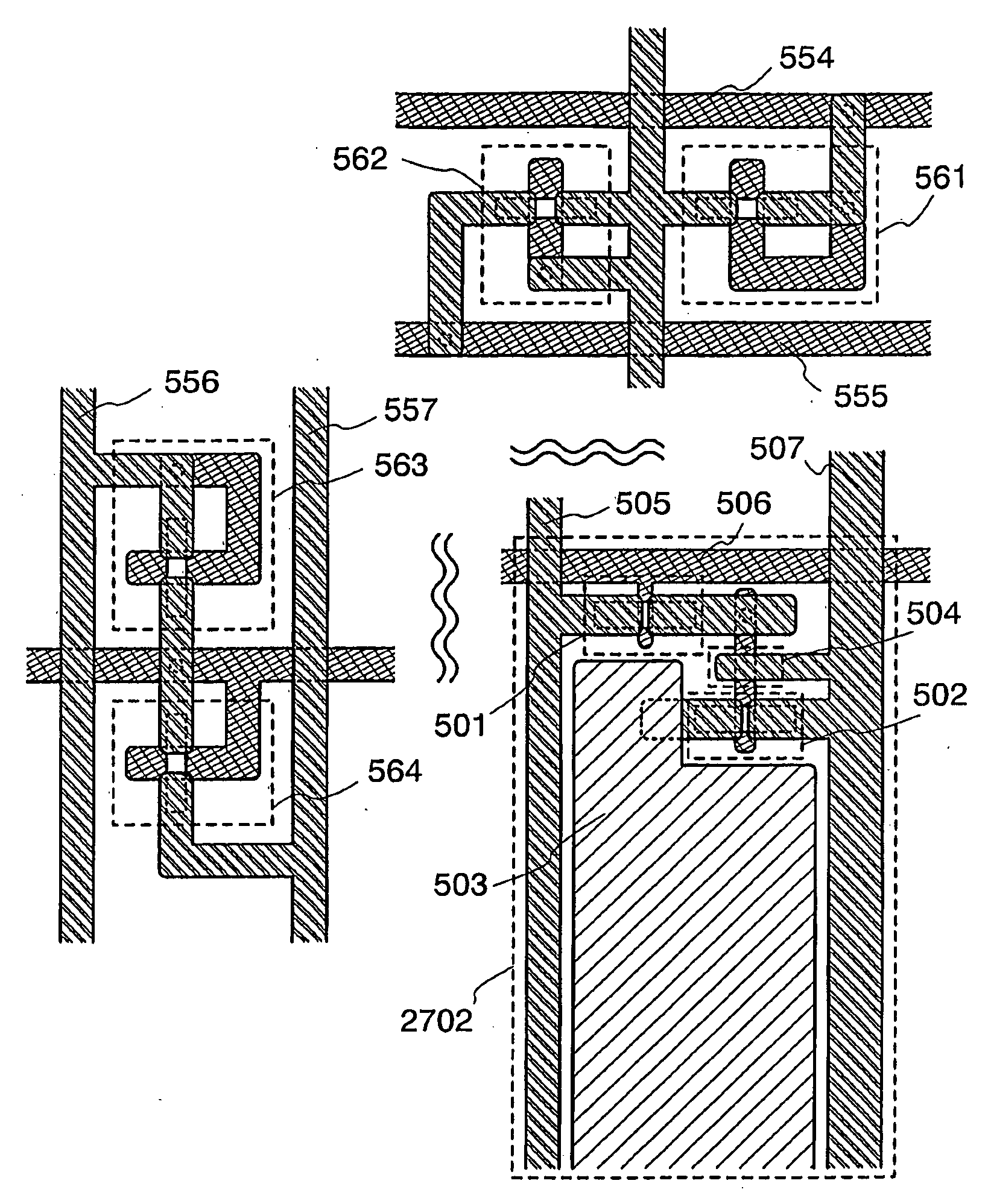
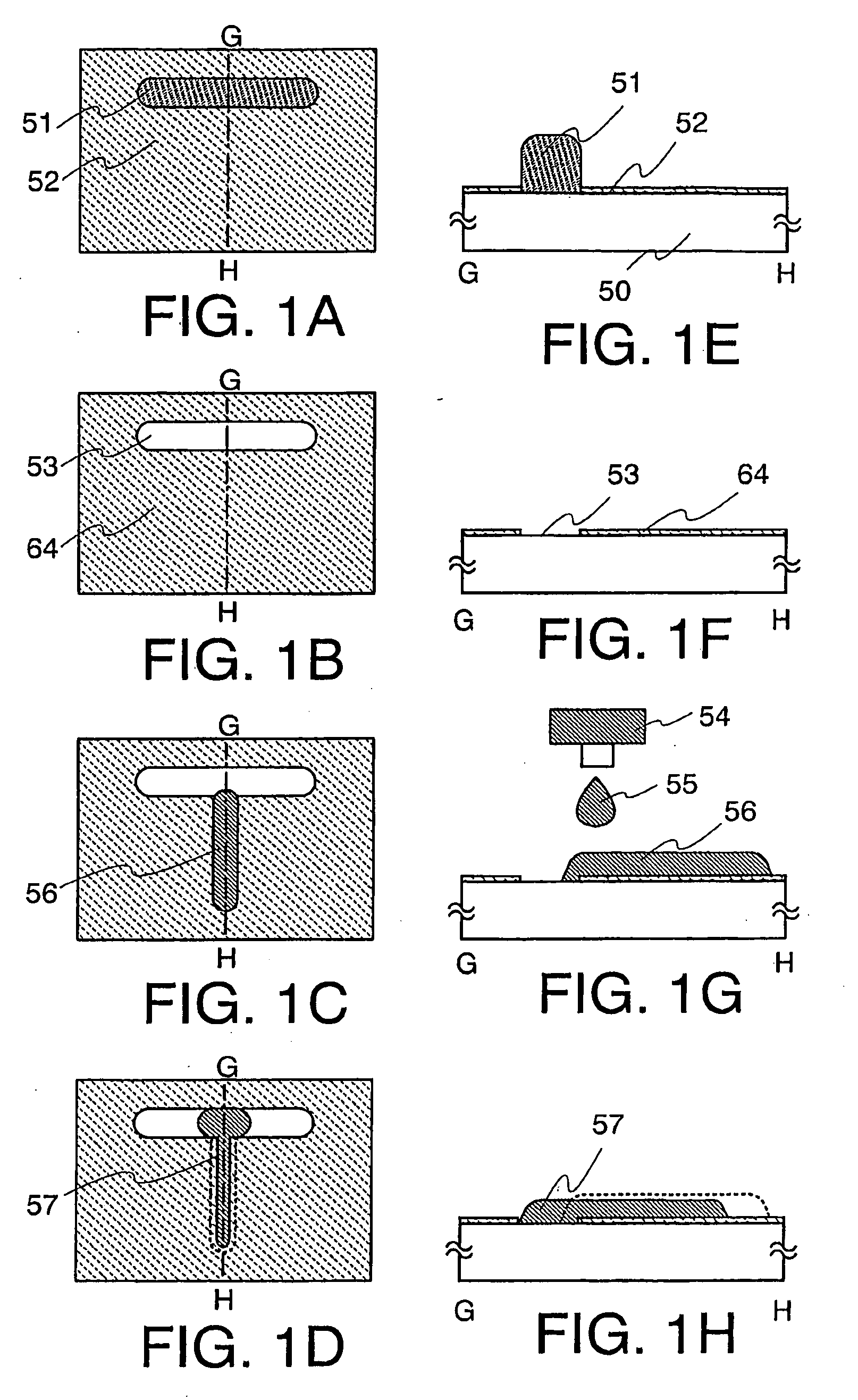
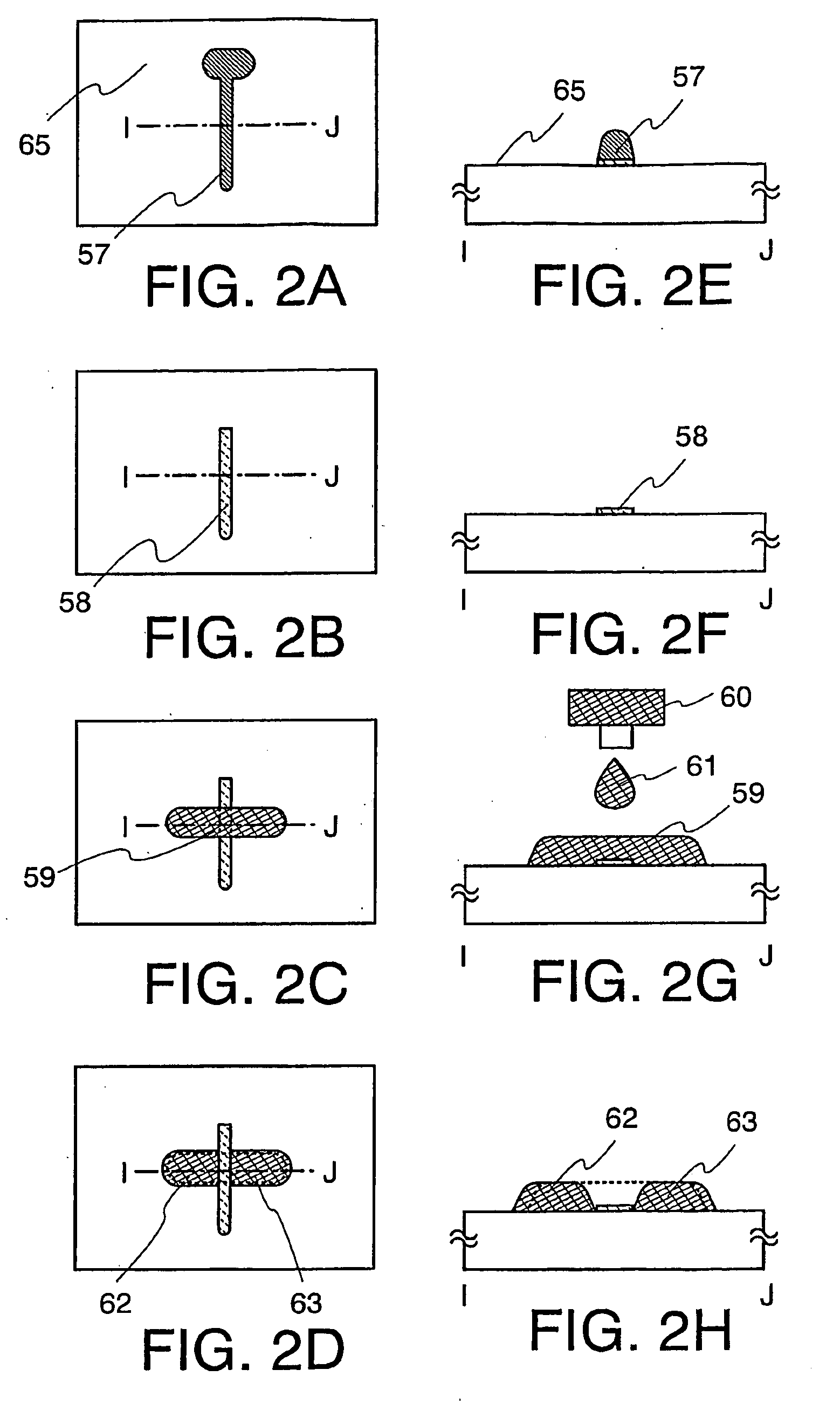
Thin Film Transistor and Display Device, and Method for Manufacturing Thereof
InactiveUS20080315428A1Low wettabilityLow related substancesTransistorLiquid surface applicatorsDisplay deviceEngineering
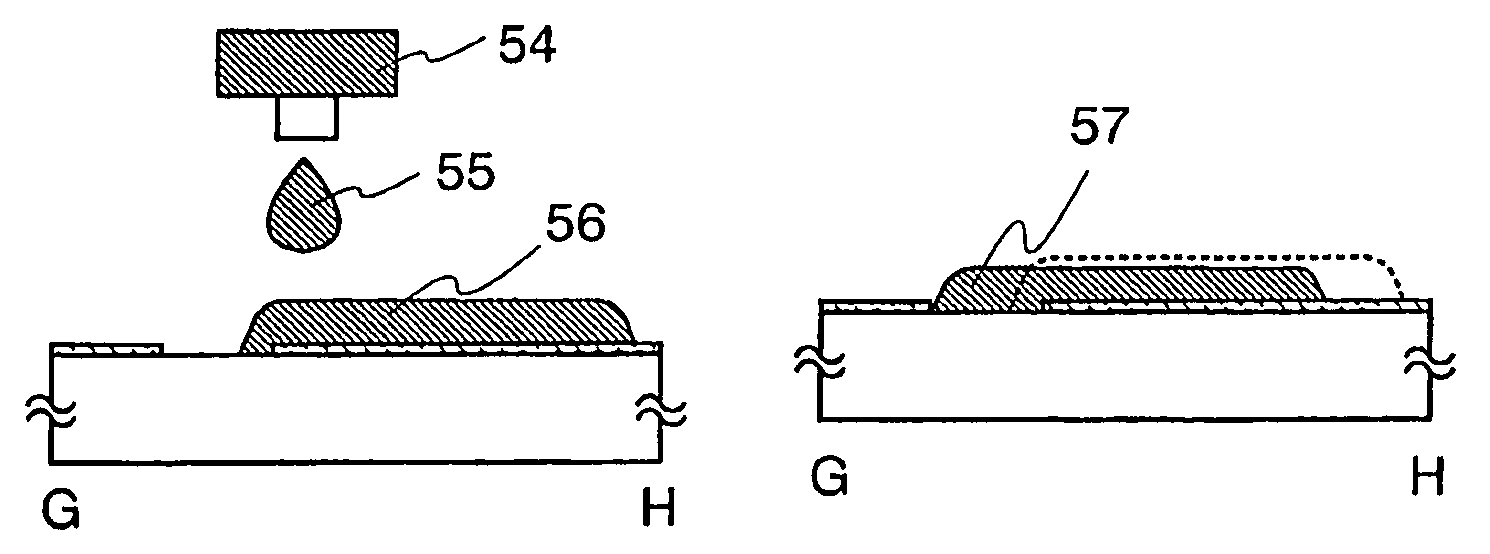
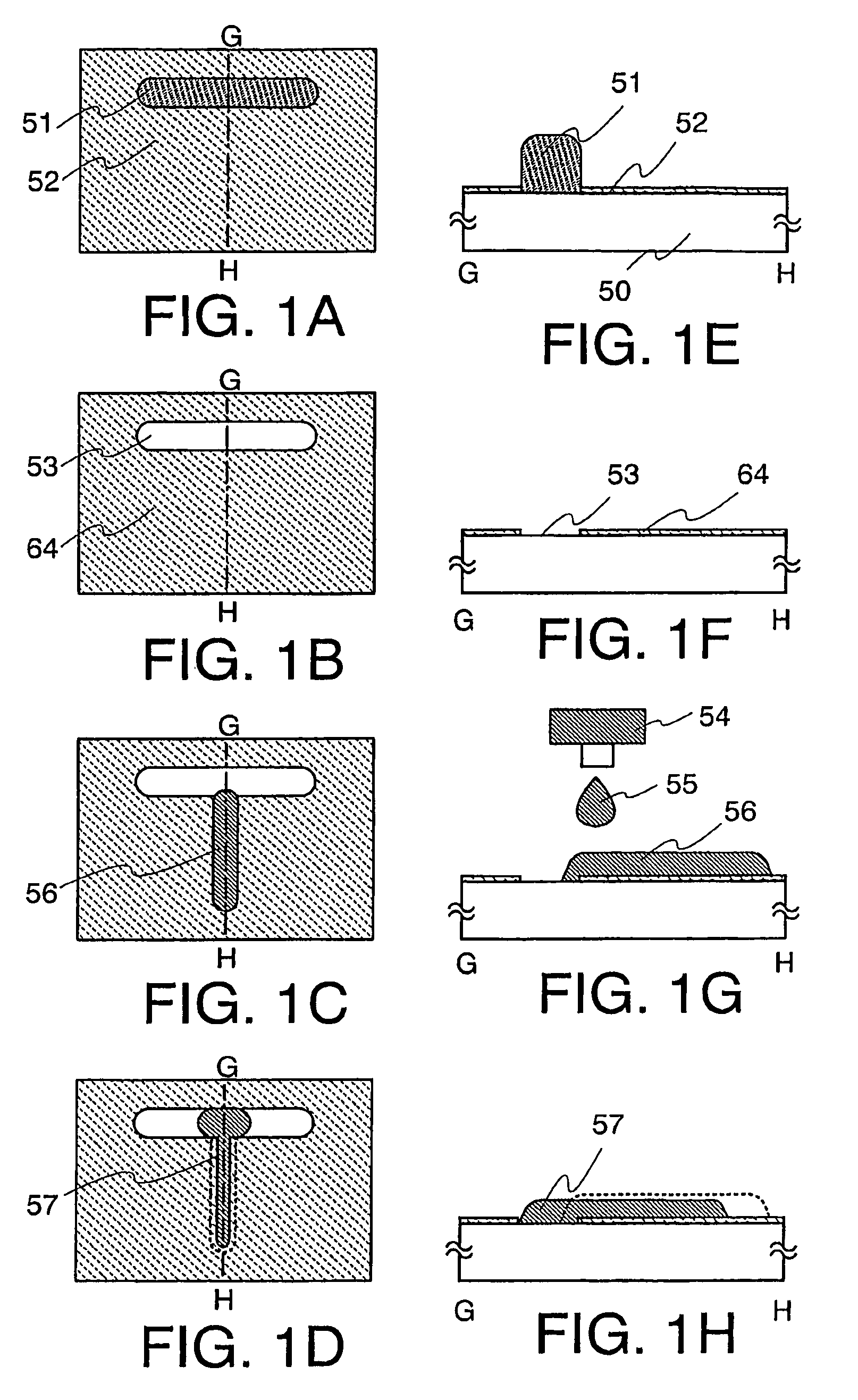
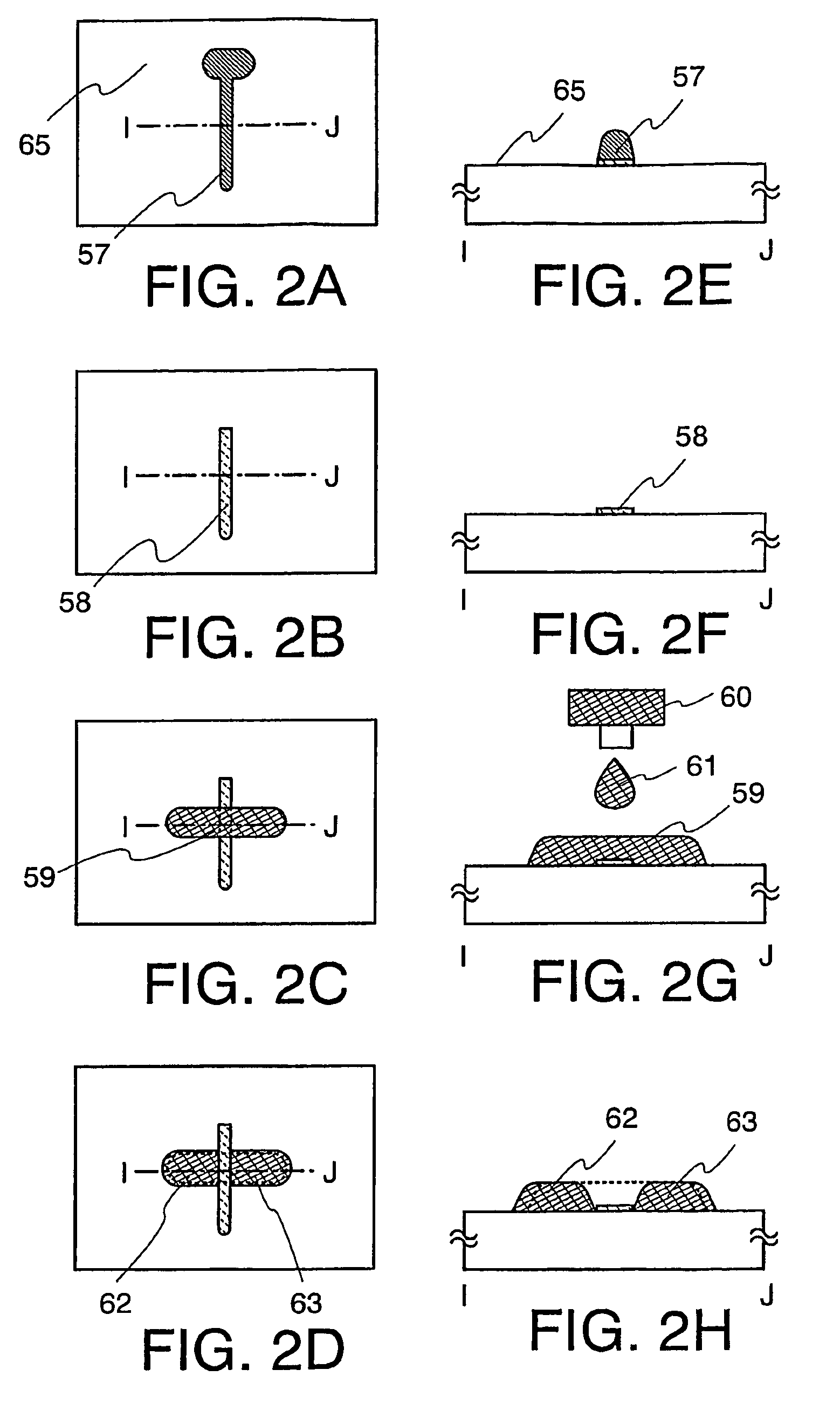
The present invention discloses a display device and a manufacturing method thereof by which a manufacturing process can be simplified. Further, the present invention discloses technique for manufacturing a pattern such as a wiring into a desired shape with good controllability. A method for forming a pattern for constituting the display device according to the present invention comprises the steps of forming a first region and a second region; discharging a composition containing a pattern formation material to a region across the second region and the first region; and flowing a part of the composition discharged to the first region into the second region; wherein wettability with respect to the composition of the first region is lower than that of the second composition.
Owner:SEMICON ENERGY LAB CO LTD
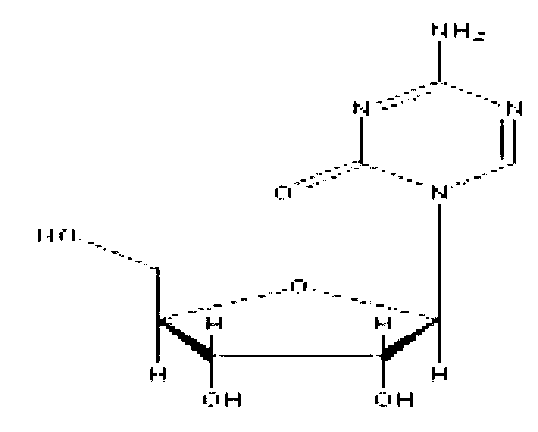
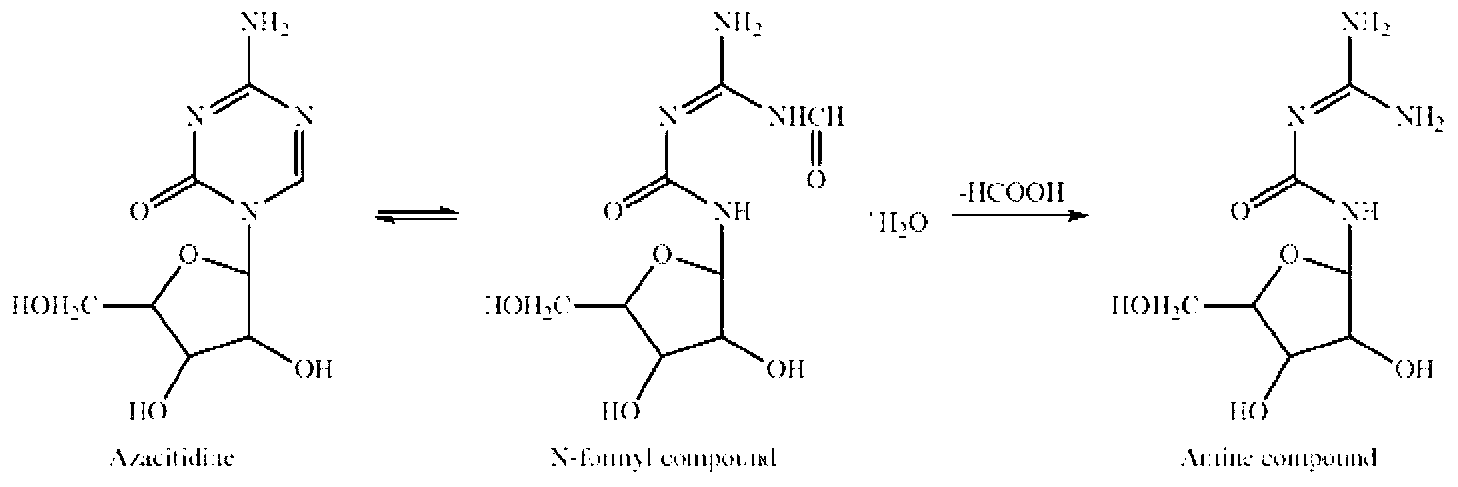
Azacitidine for injection and preparation method thereof
InactiveCN103251564AInhibit rapid hydrolysisAvoid hydrolysisPowder deliveryOrganic active ingredientsFreeze-dryingMannitol
Azacitidine for injection and a preparation method thereof are disclosed. Main components of the azacitidine for injection contain main drugs of azacitidine and mannitol. Mainly by controlling pH value of an azacitidine aqueous solution, hydrolysis of azacitidine is effectively inhibited, related substances of freeze-dried product are minimized, and product quality of the azacitidine freeze-drying preparation is raised.
Owner:FUKANGREN BIO PHARMA
Edaravone crystal form and preparation method thereof
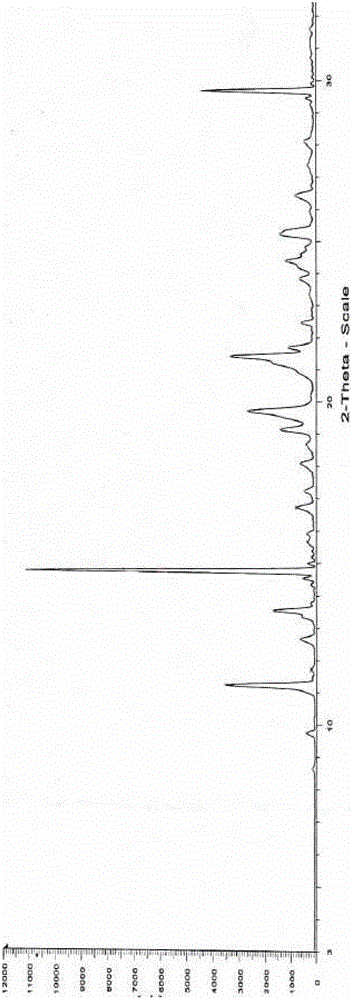
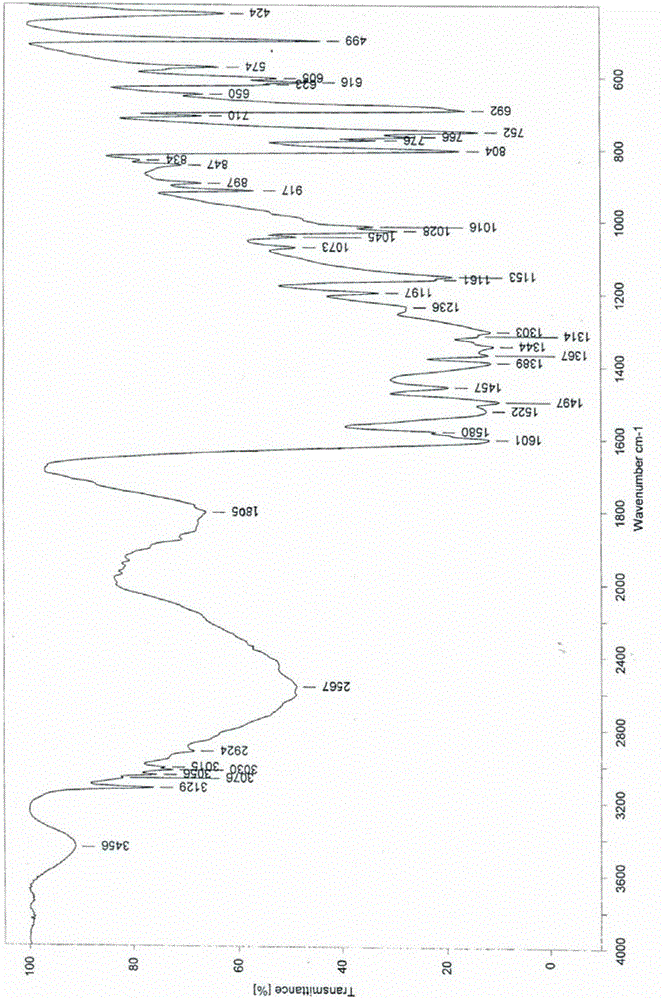
InactiveCN102060771AGood water solubilityLow related substancesOrganic chemistrySolubilityWater soluble
The invention relates to an Edaravone crystal form and a preparation method thereof. The Edaravone crystal form is characterized by X-ray diffractogram of powder. The crystal form can obviously improve the water solubility of Edaravone, thereby being more beneficial to preparation of injections. Besides, related substances are further decreased, and the safety of the preparation is improved.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD +1
Microemulsions and use thereof as a fuel
InactiveUS20070028507A1Effectively solubilizedEfficient combustionOther chemical processesMixing methodsPollutantCombustion
The invention relates to bicontinuous microemulsions and to the use thereof as a fuel, combustion or heating fluid. Said fuels permit an increased efficiency of internal combustion systems and heating installations of any type and, simultaneously, a minimized emission of pollutants, associated with combustion, to be obtained.
Owner:UNIV OF COLOGNE
Method for absorbing acrylic acid and method for purifying acrylic acid
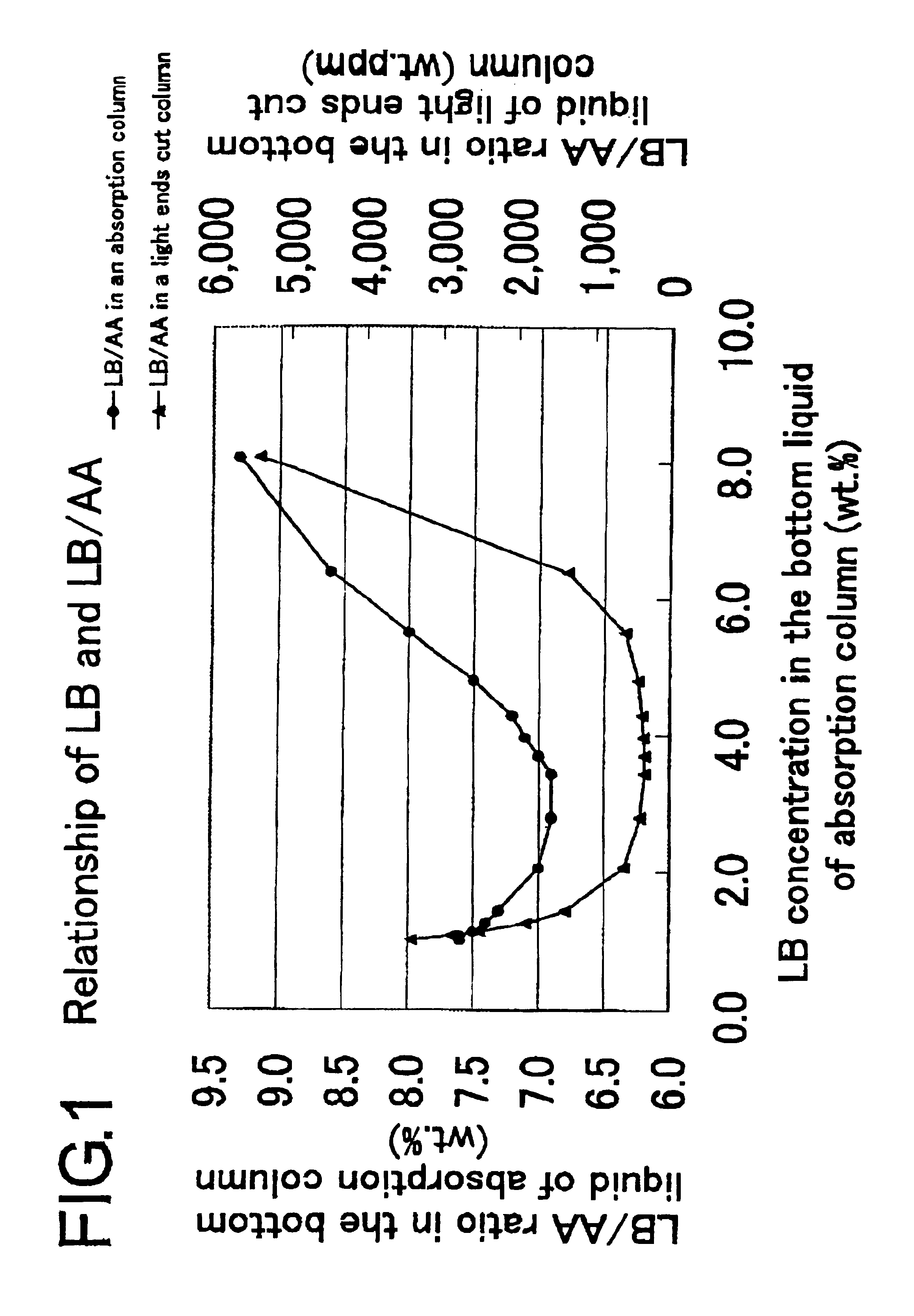
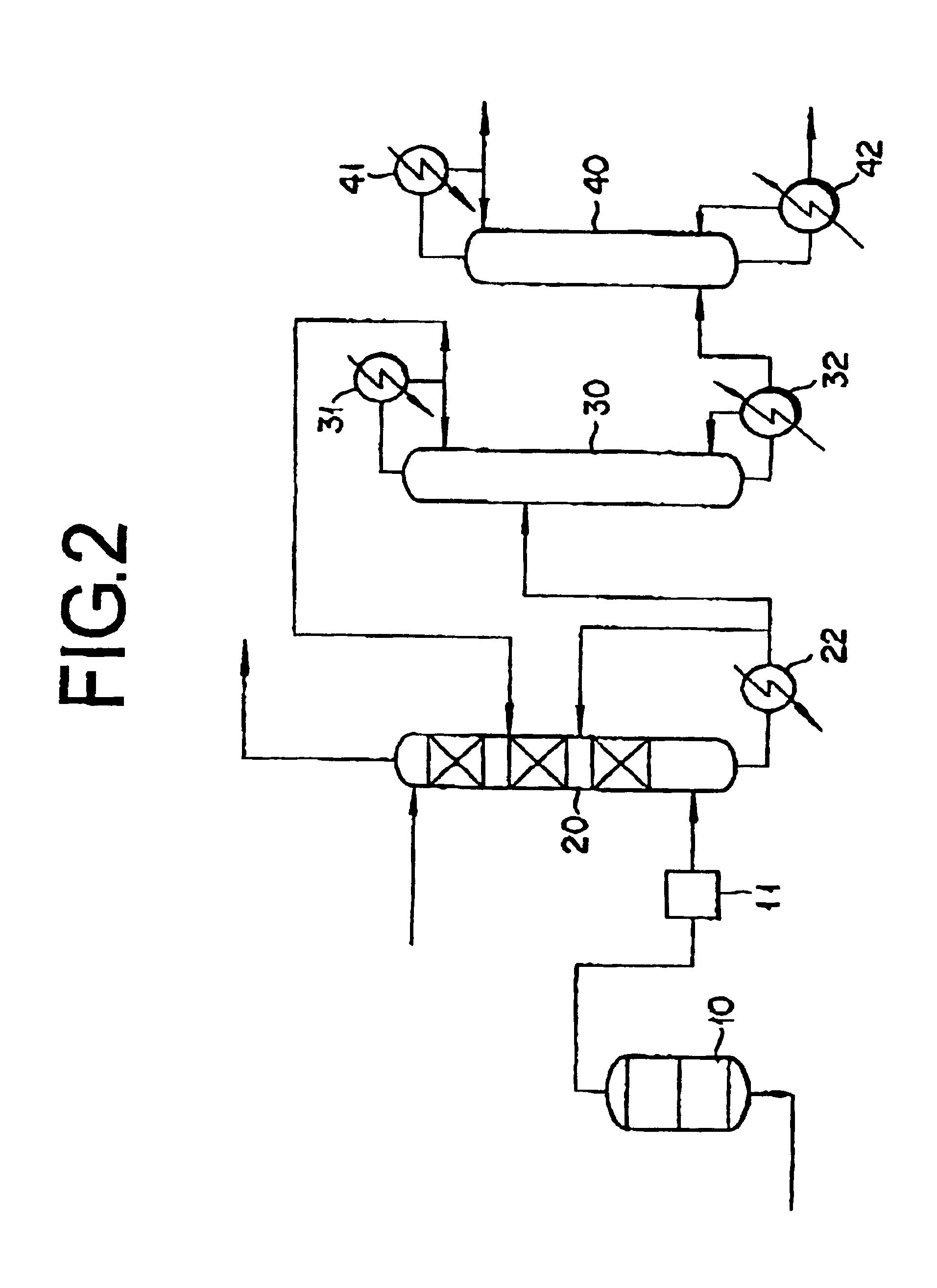
InactiveUS6888025B2Absorption coefficientLow related substancesOrganic compound preparationDistillation separationCounter flowAbsorption column
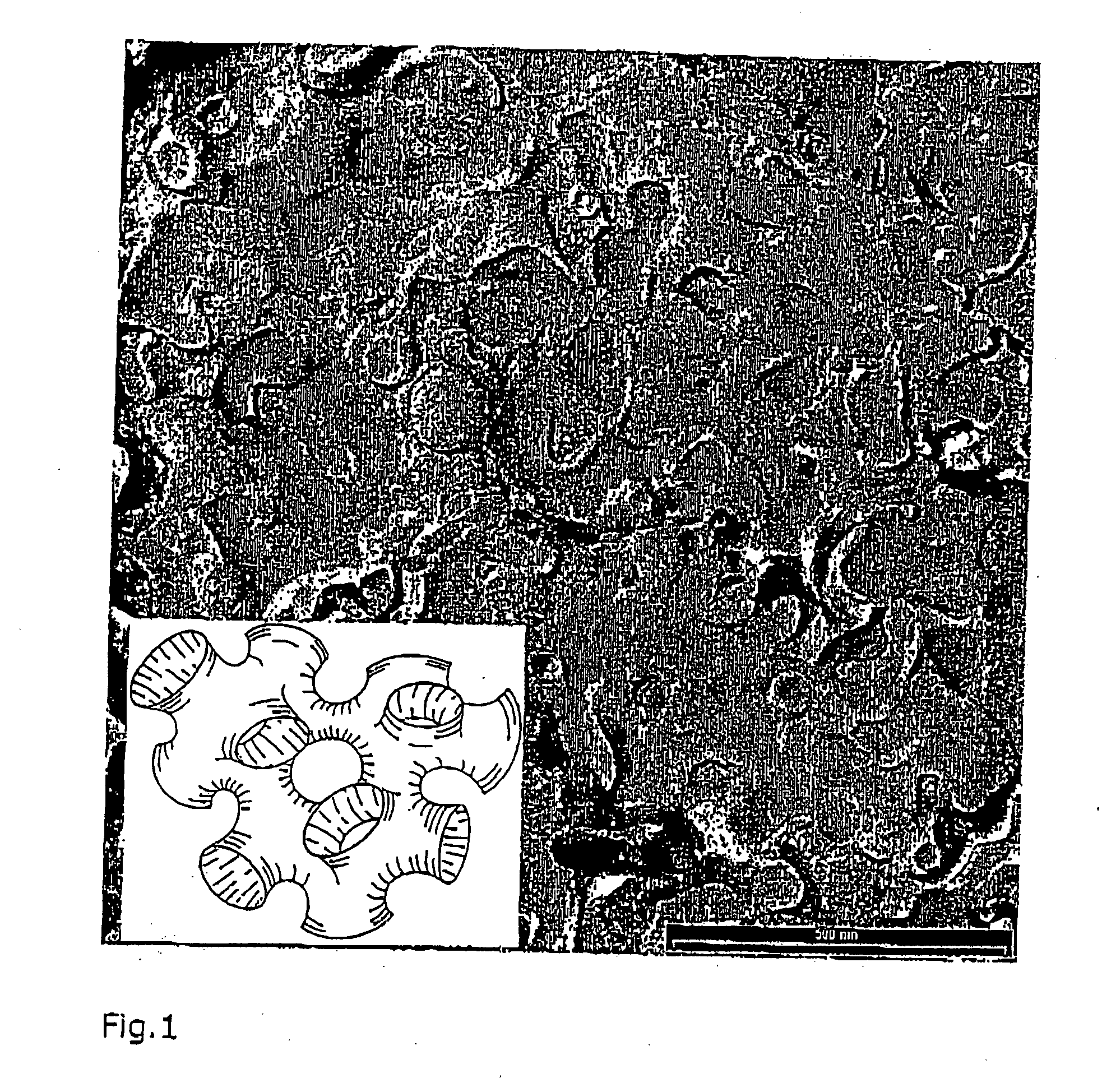
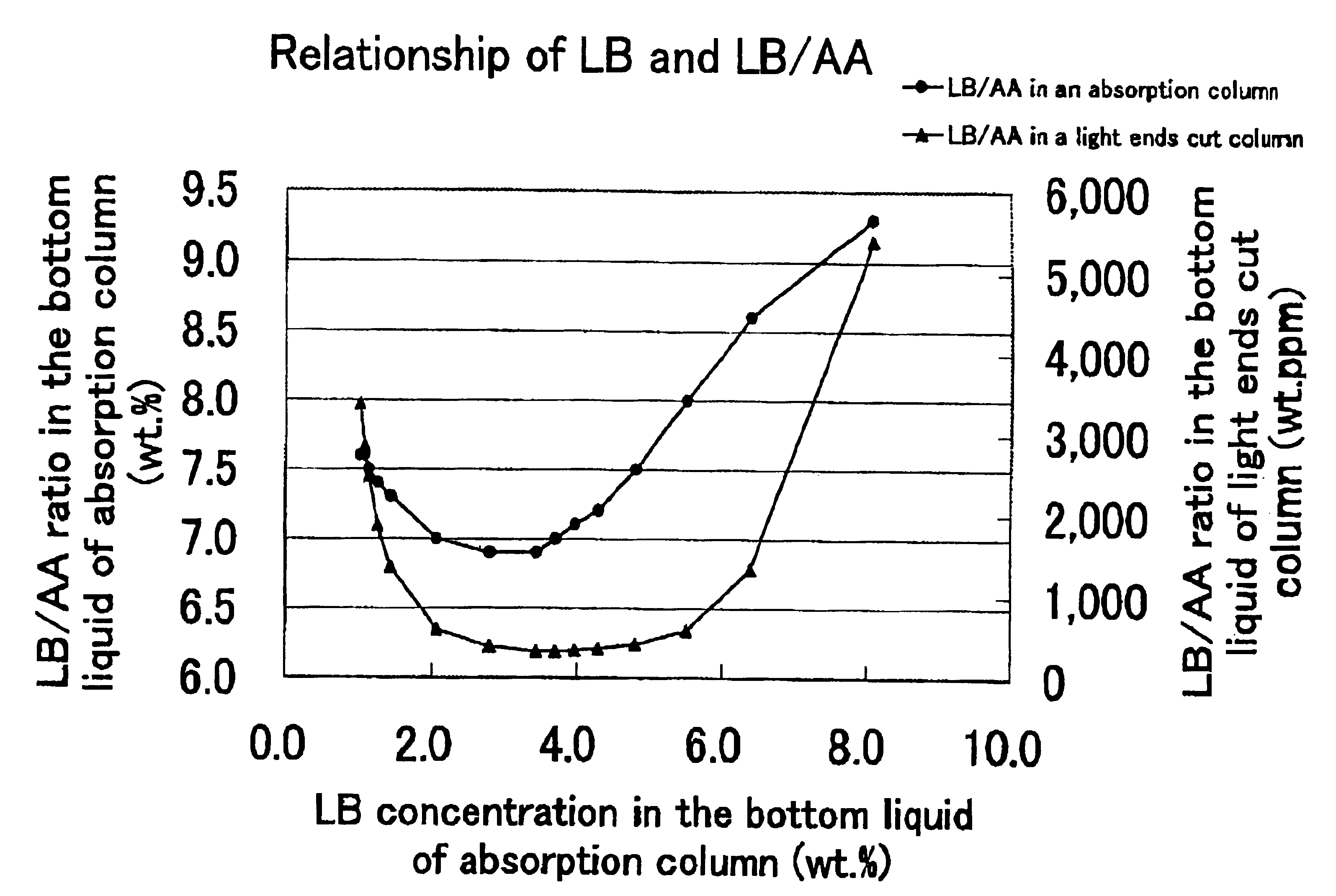
This invention concerns a method for absorbing acrylic acid, characterized by supplying an acrylic acid-containing reaction gas component obtained by the reaction of catalytic gas phase oxidation to an acrylic acid absorption column and advancing a high boiling inert hydrophobic organic liquid into counter-flow contact with said reaction gas in said acrylic acid absorption column with the mass flow rate of the organic liquid fixed in the range of 0.2-7.0 times the mass flow rate of the acrylic acid in the reaction gas thereby absorbing acrylic acid in said organic liquid and obtaining an acrylic acid-containing solution including a low boiling substance in the range of 0.7-7.5 wt % based on the weight of bottom liquid of absorption column.
Owner:NIPPON SHOKUBAI CO LTD
Process for preparing naloxone hydrochloride injection
InactiveCN101590014APrevent oxidative deteriorationReduce oxygen contentOrganic active ingredientsNervous disorderMedicineOxygen content
The invention provides a process for preparing naloxone hydrochloride injection. The pH of the naloxone hydrochloride injection can be unchangeable during placing by strictly controlling the pH range in production; and simultaneously, a method for inert gas protection adopted in the production reduces the oxygen content in liquid medicine and an ampoule to the minimum so as to prevent oxidative deterioration of the naloxone hydrochloride injection to reduce related substances. The technology greatly ensures the product quality.
Owner:SHANDONG XINHUA PHARMA CO LTD
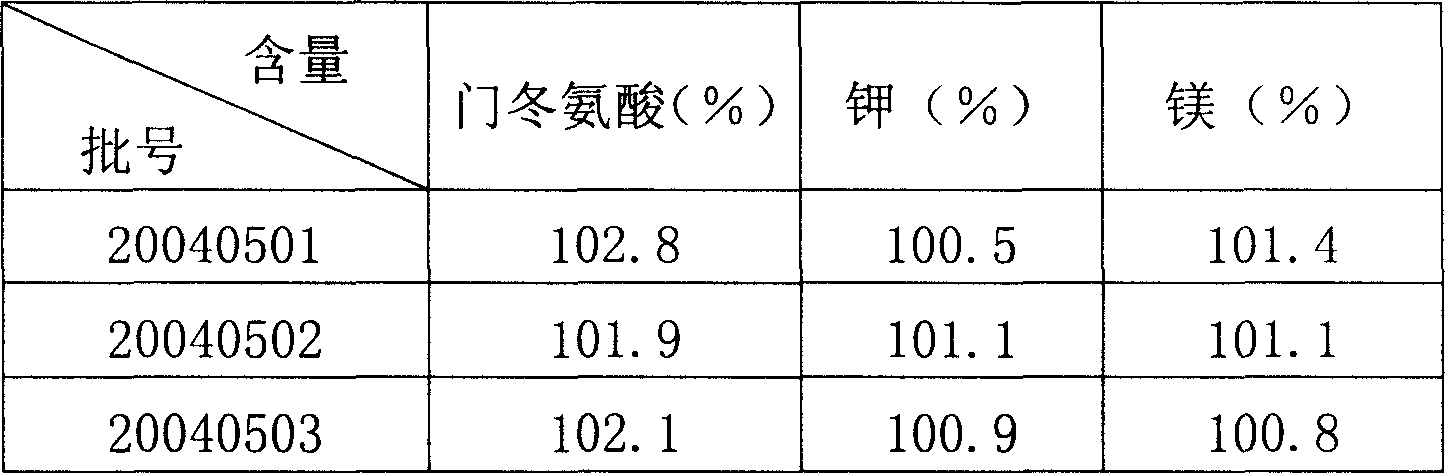
Preparation method of potassium magnesium aspartate freeze dried powder injection
InactiveCN1830431ALow related substancesLoose texturePowder deliveryOrganic active ingredientsCongestive heart failure chfUltrafiltration
A freeze-dried powder injection of spartase for supplementing electrolyte and treating arrhythmia, myocarditis sequelae, congestive heart failure, myocardial infarction, etc is prepared through reaction between L-aminobutanedioic acid and magnesium oxide, ultrasonic treating, cold storage, ultrafiltration, adding the filtered liquid prepared from potassium hydroxide and mannitol, filling in containers in dark condition, freeze drying and sealing.
Owner:巴里莫尔制药(通化)有限公司
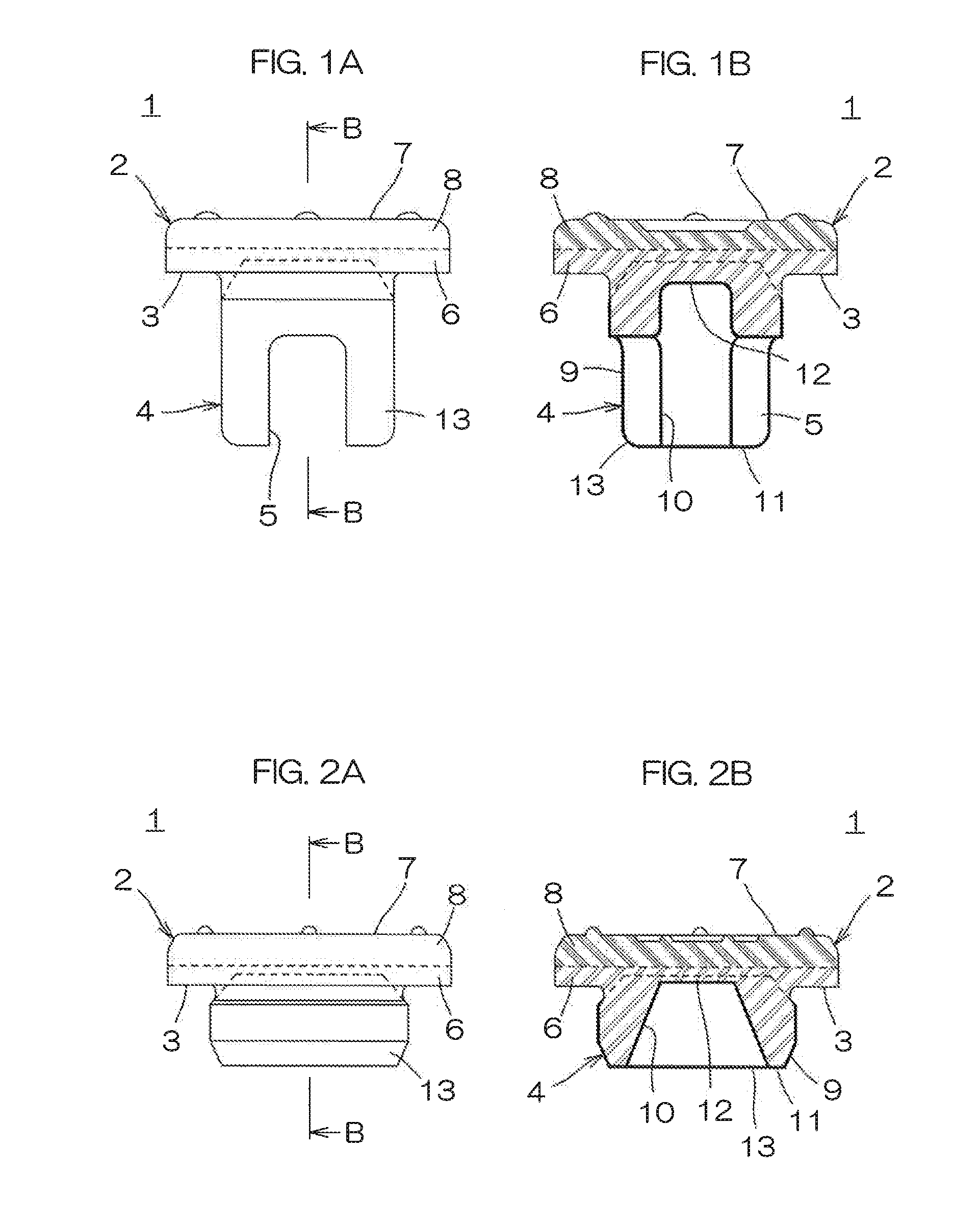
Medical rubber closure and method for manufacturing the same
ActiveUS20160075485A1Good oil resistanceSatisfactory moisture permeation resistanceClosuresMouldsEngineeringMechanical engineering
A medical rubber closure according to the present invention includes a disc-shaped flange portion and a leg portion formed to be continuous to a lower surface side of the flange portion, and a region at the lower surface side of the flange portion and the leg portion are made of a nitrile based rubber, a region at a top surface side of the flange portion is made of a butyl based rubber, and the leg portion is laminated with a coating layer made of a fluorine based resin film.
Owner:SUMITOMO RUBBER IND LTD
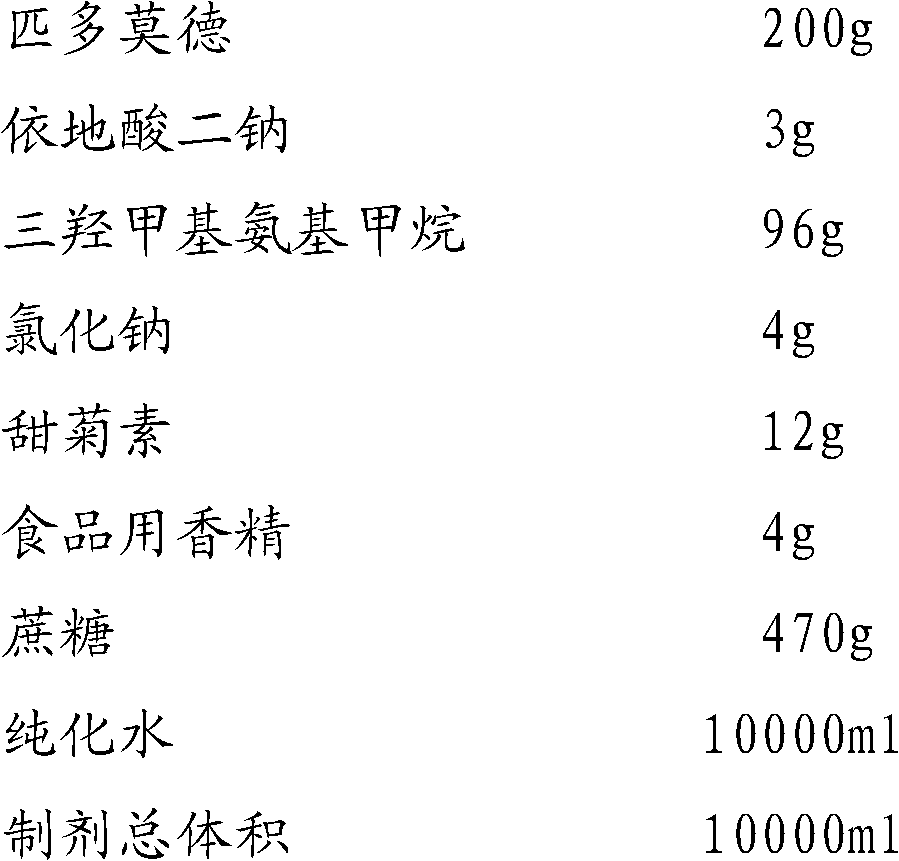
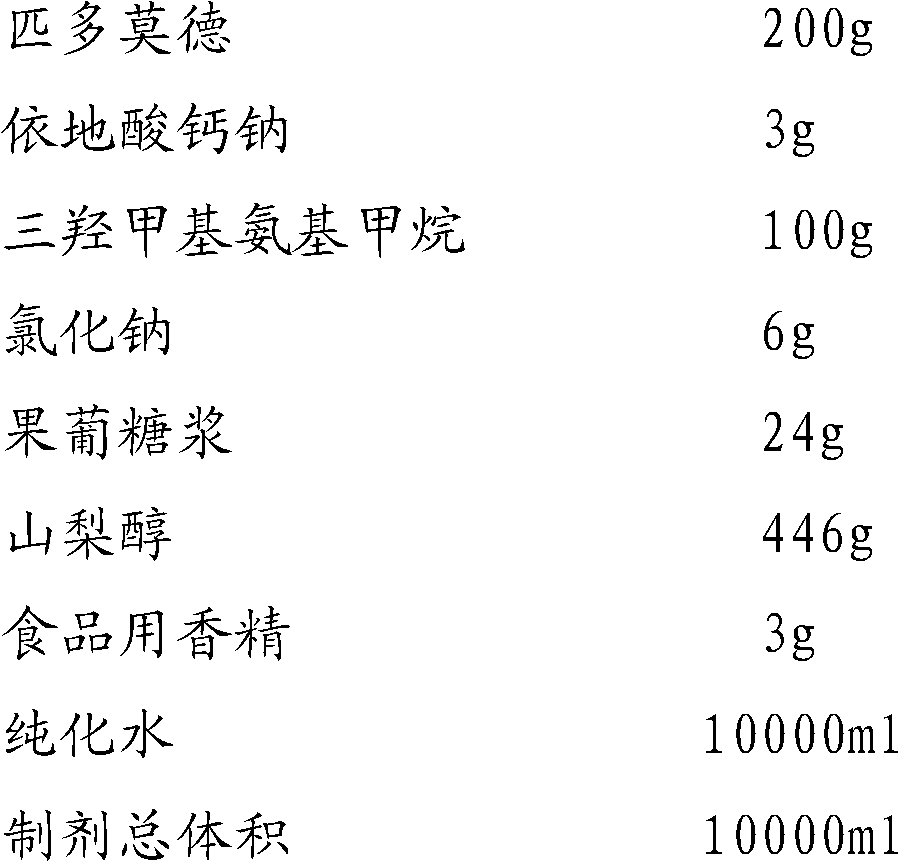
Oral liquid preparation of pidotimod
ActiveCN102525903AStable dissolutionStable storageDipeptide ingredientsPharmaceutical delivery mechanismDiseaseInduced infections
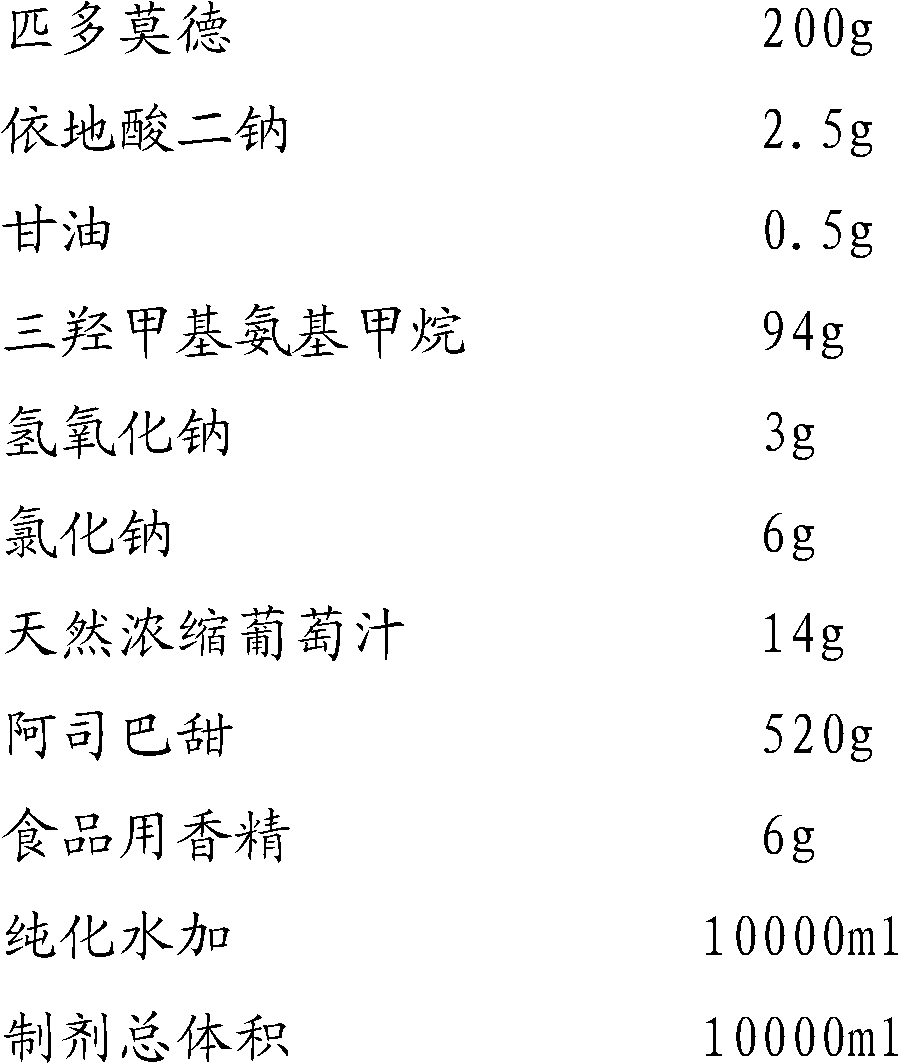
The invention provides an oral liquid preparation of pidotimod, which can promote immunoreaction of a human body, effectively reduces times and degree of treating acute attack of bacteria or virus induced infection, shortens the course of the disease, can serve as an auxiliary medicine of antibiotic during acute infection and belongs to the field of medicines. The oral liquid preparation comprises the pidotimod, a chemical stabilizer, a pH regulator, a flavoring agent and the like, wherein trihytdroxy methyl-aminomethane serves as the pH regulator; and stability of the solution can be effectively improved through quantitative proportion and synergistic effect of each ingredient. The oral liquid preparation has stable quality, is convenient to store for a long time and is particularly suitable for old people or children to take.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Method and device for controlling an internal combustion engine
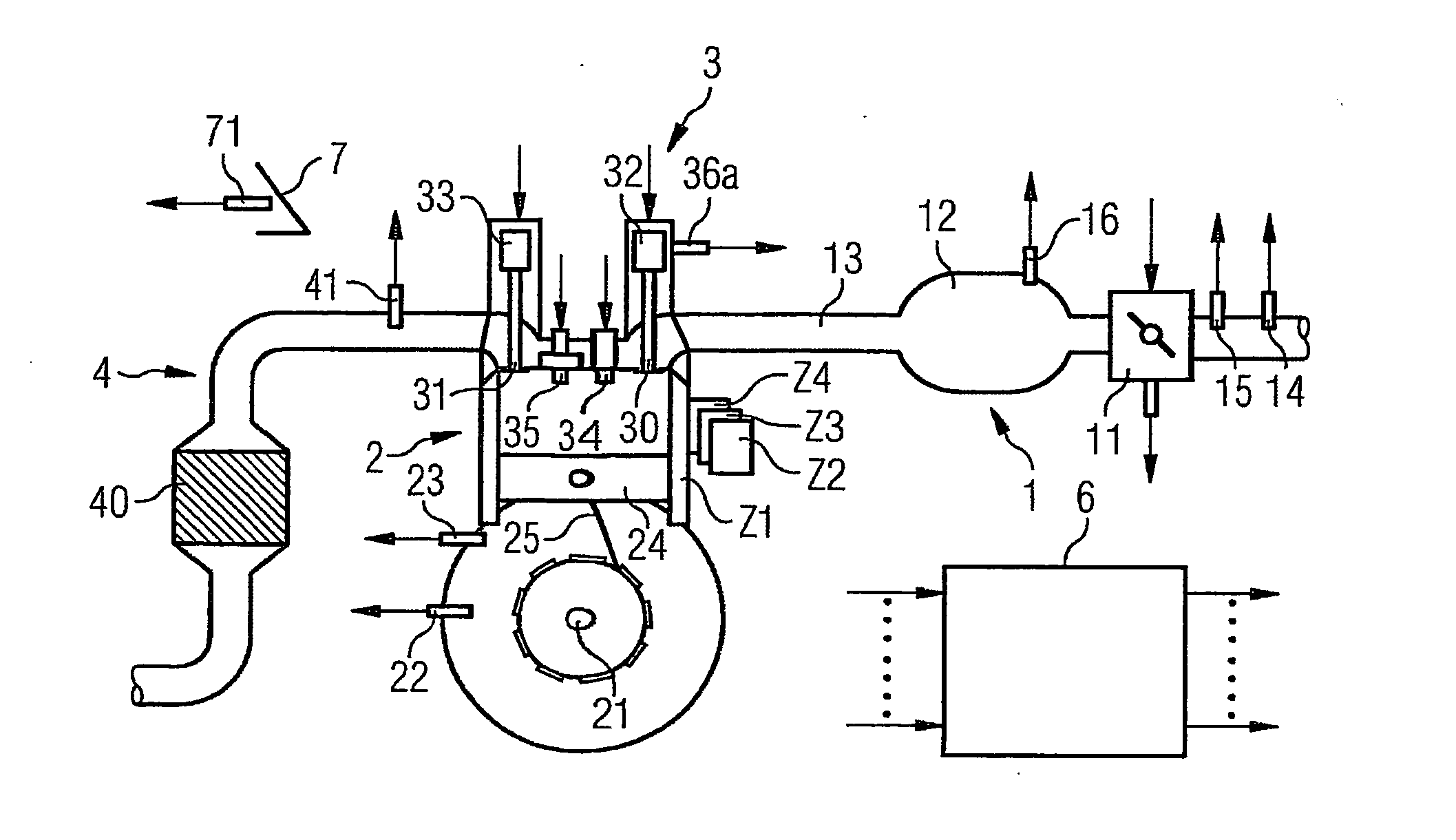
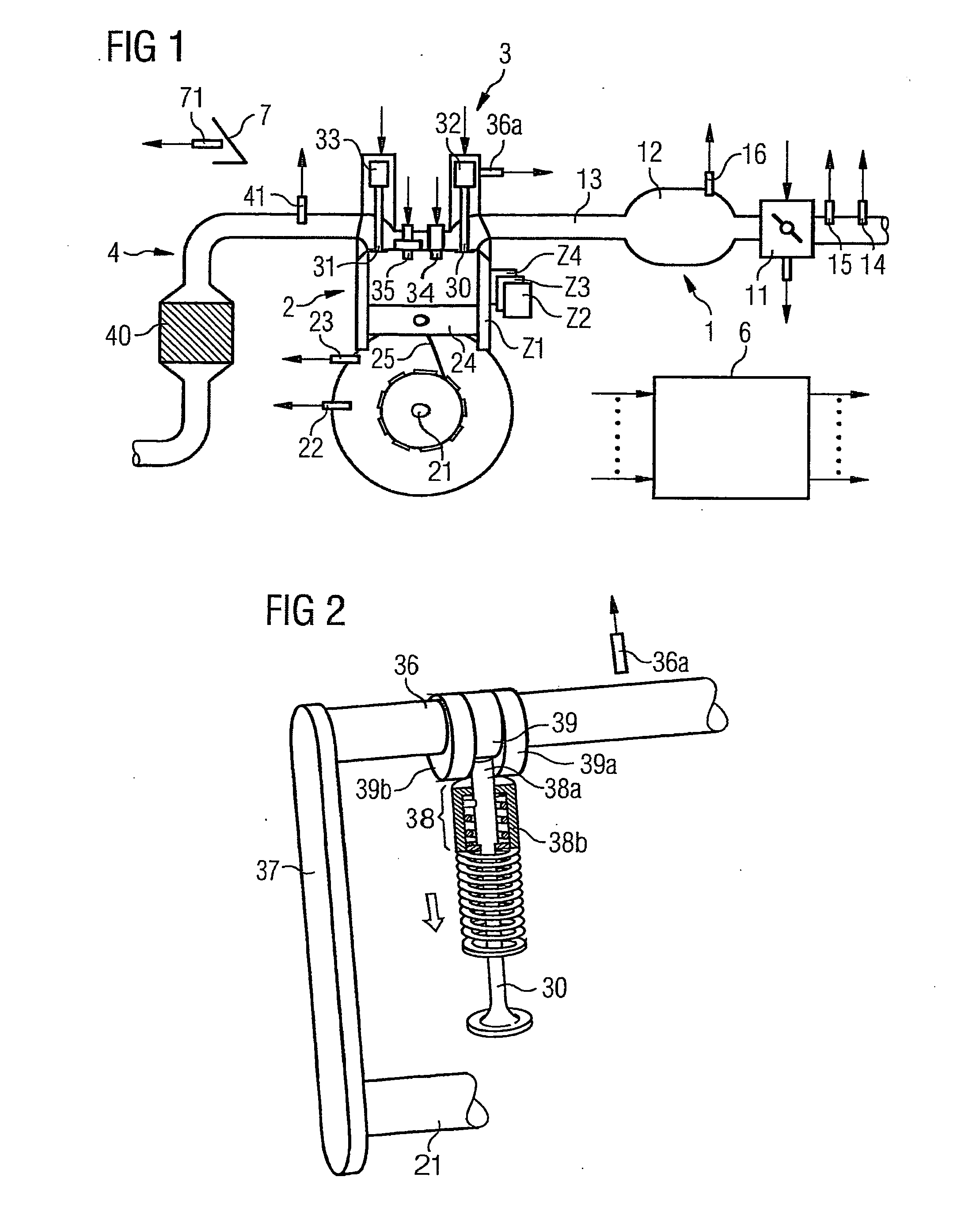
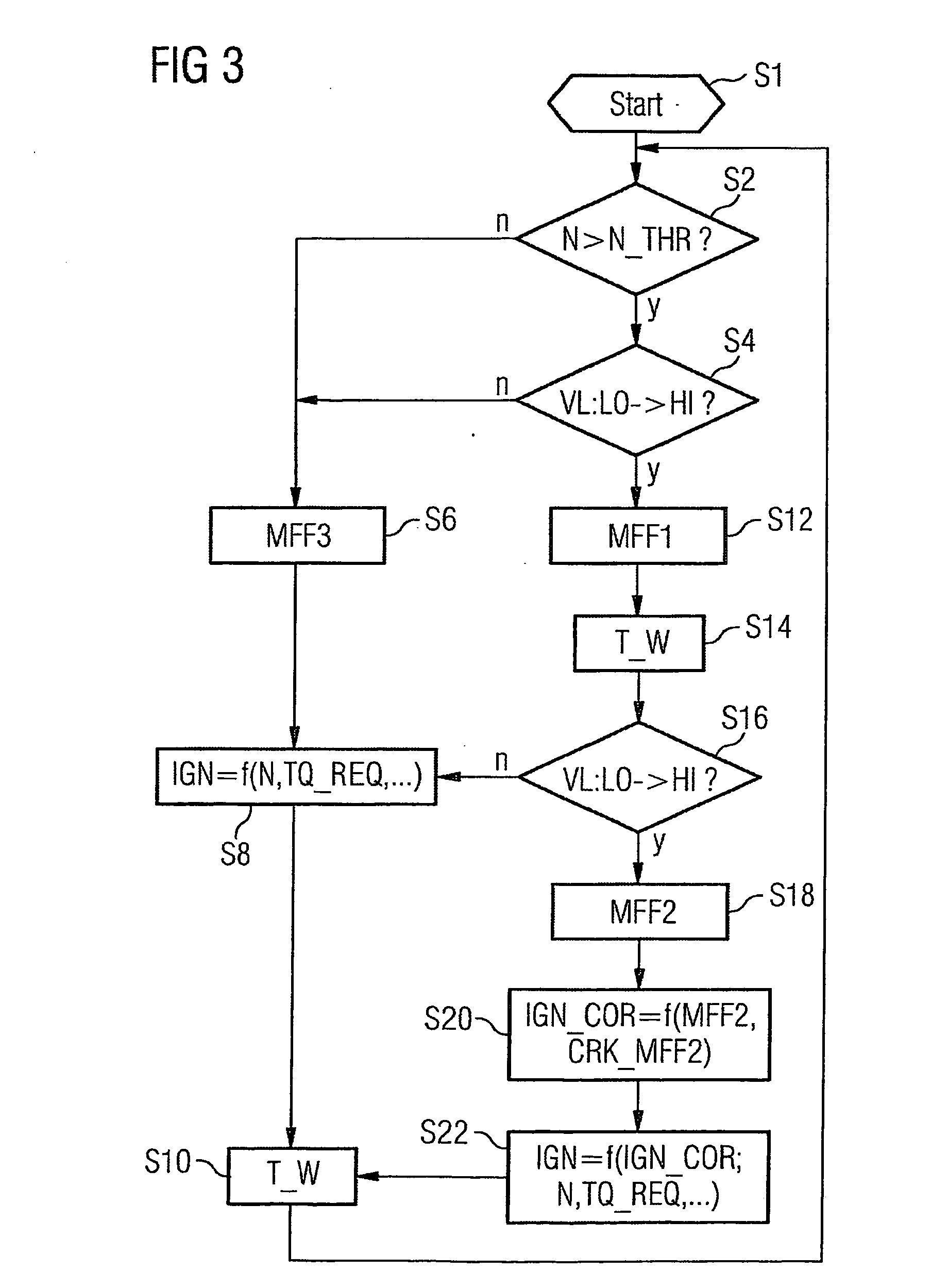
ActiveUS7398749B2Low related substancesEmission reductionValve arrangementsElectrical controlExternal combustion engineEngineering
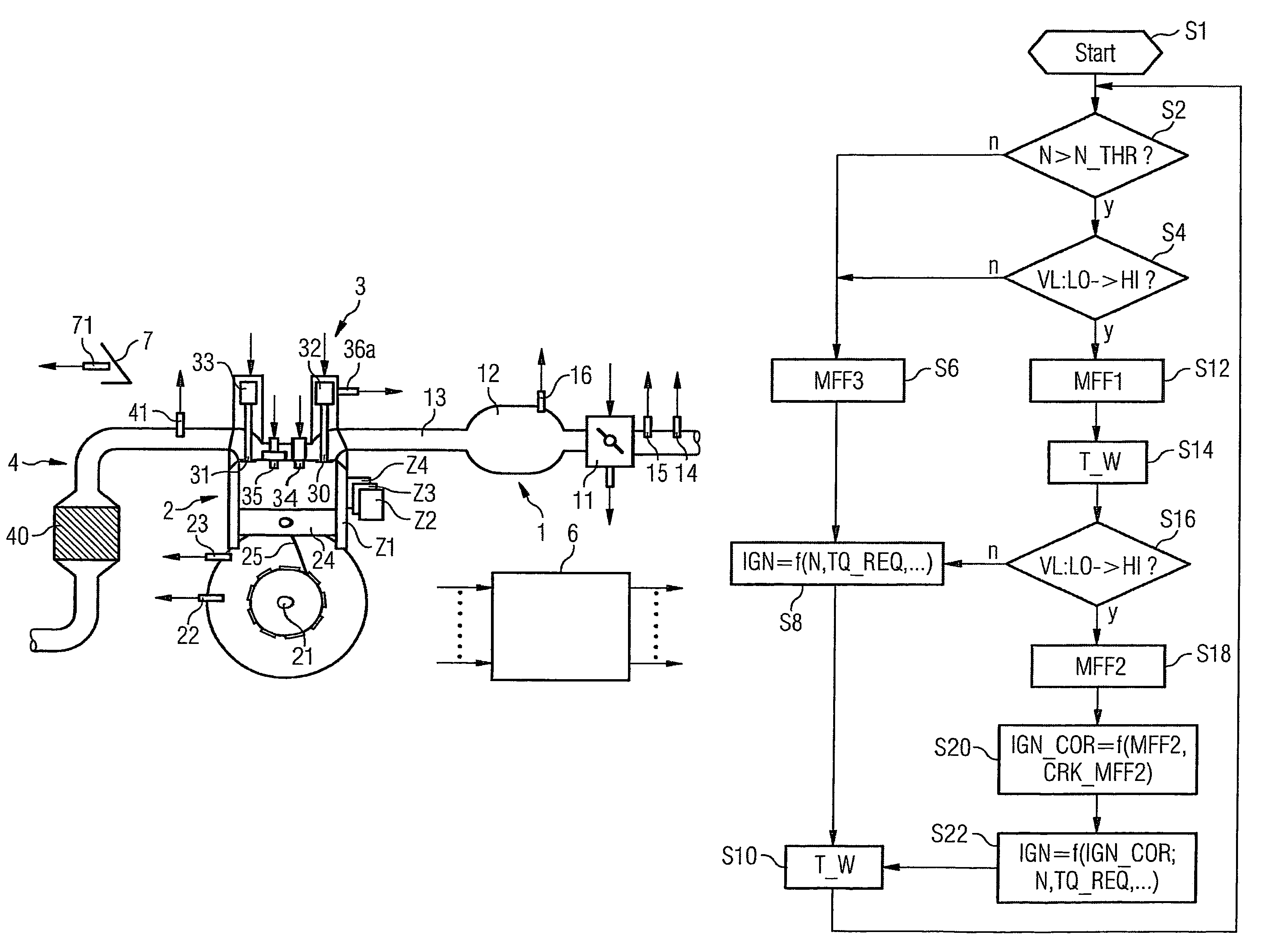
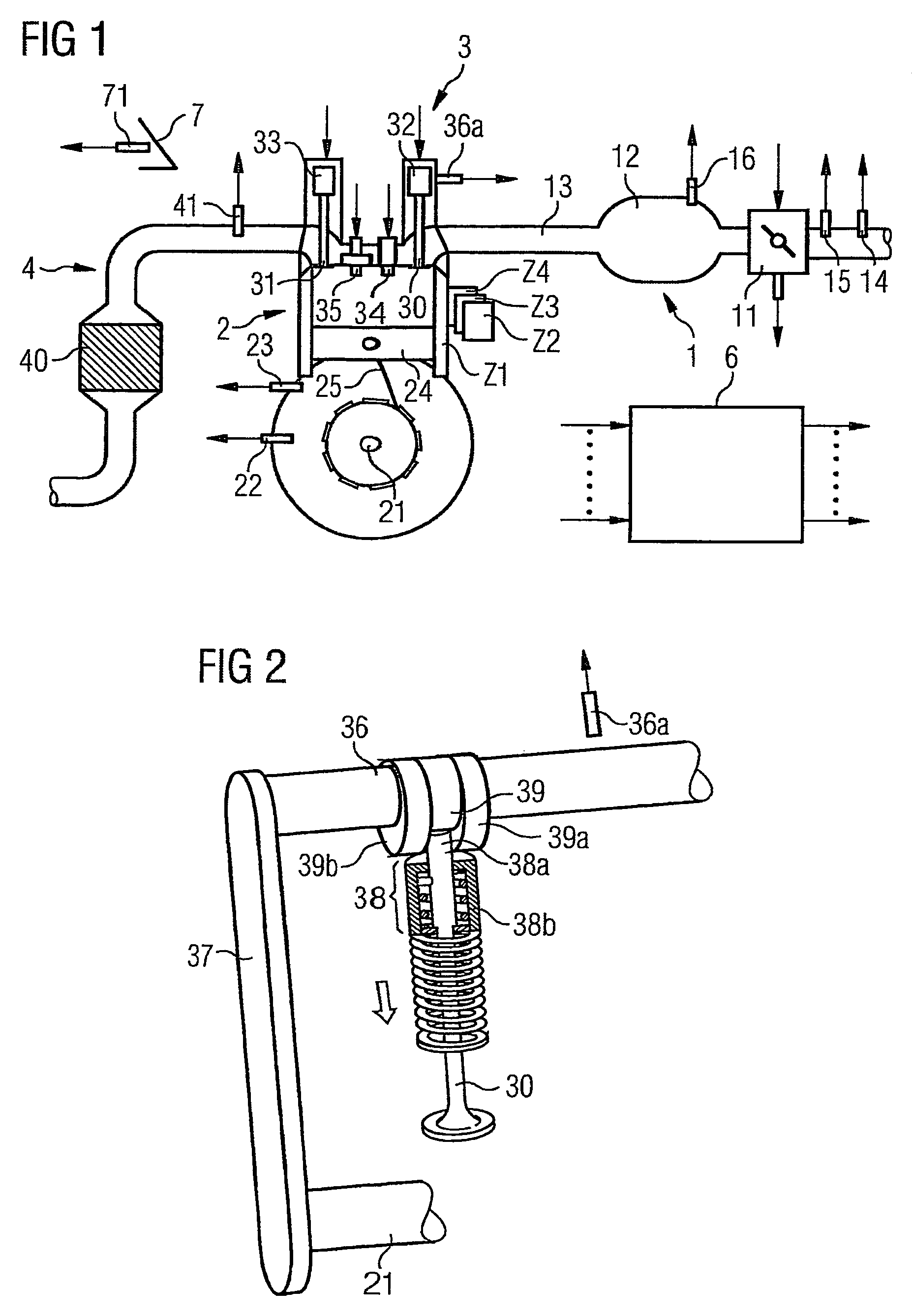
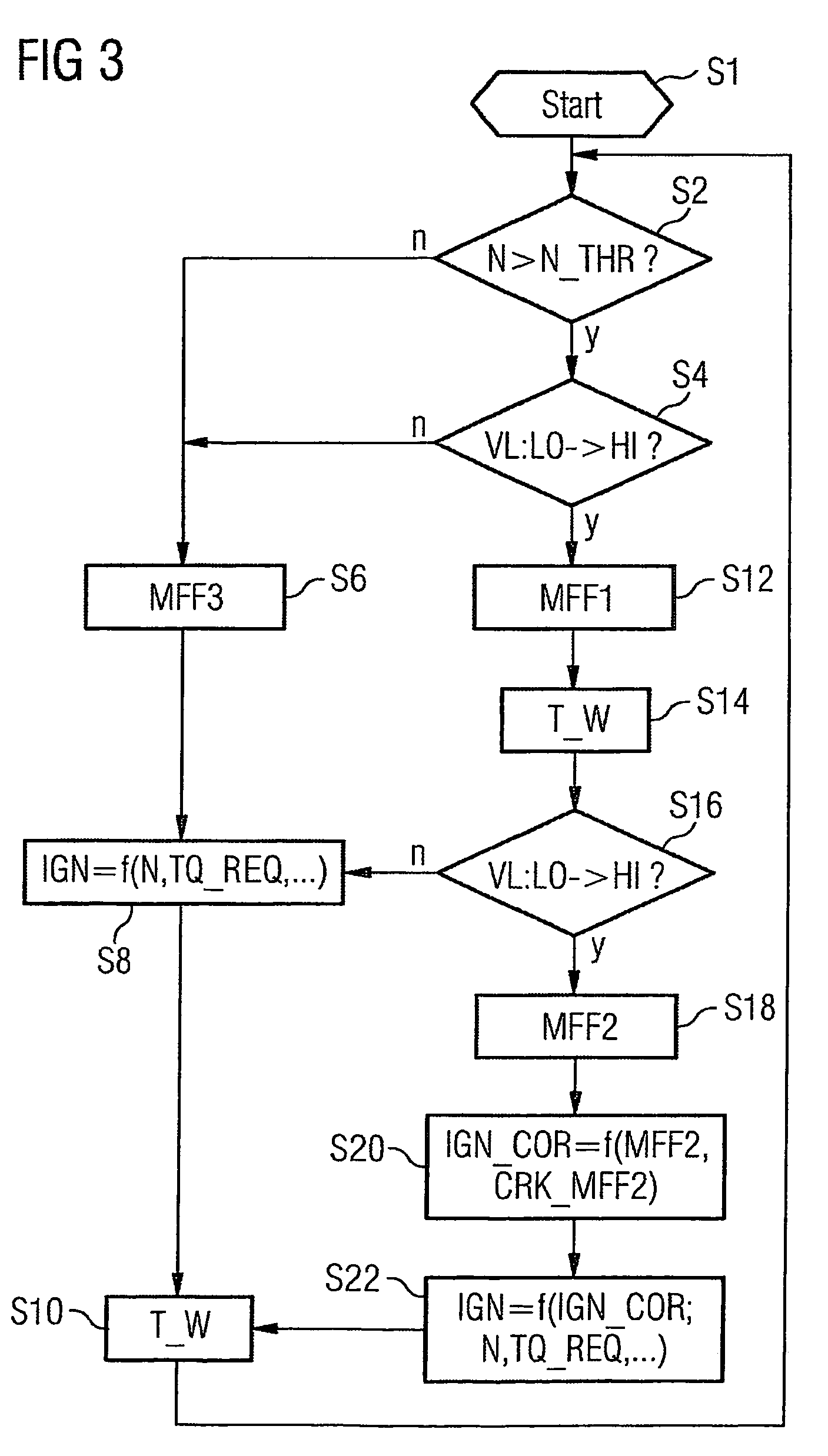
The invention relates to a method and device for controlling an internal combustion engine comprising an inlet pipe leading to a cylinder input where a gas input valve is placed. Said engine also comprises a drive for the gas input valve which makes it possible to adjust a gas input valve lift for at least two values. The engine also comprises an injection valve for metering fuel and a spark plug which controls the crankshaft angle of air-fuel mixture ignition. Said internal combustion engine is controlled in a following manner: a fuel is metered at least once during the intake stroke of a cylinder when the valve lift (VL) passes from one value to the other and at least one final injection is carried out in a dosing manner only when the valve lift (VL) is really carried out.
Owner:VITESCO TECH GERMANY GMBH
Method for manufacturing thin film transistor and display device
InactiveUS7951710B2Low wettabilityLow related substancesTransistorLiquid surface applicatorsDisplay deviceControllability
The present invention discloses a display device and a manufacturing method thereof by which a manufacturing process can be simplified. Further, the present invention discloses technique for manufacturing a pattern such as a wiring into a desired shape with good controllability. A method for forming a pattern for constituting the display device according to the present invention comprises the steps of forming a first region and a second region; discharging a composition containing a pattern formation material to a region across the second region and the first region; and flowing a part of the composition discharged to the first region into the second region; wherein wettability with respect to the composition of the first region is lower than that of the second composition.
Owner:SEMICON ENERGY LAB CO LTD
Preparation method of acetyl glutamine injection
InactiveCN1830425AFunction increaseImprove neurological functionNervous disorderAmide active ingredientsClinical efficacyPhosphate
An acetylglutamide injection for improving brain function and treating coma, palsy, hypointelligence, dysmnesia, etc is prepared through mixing acetylglutamide and phosphate buffering liquid, ultrasonic treating, cold storage, ultrafiltration, mixing with Na2EDTA solution in dark condition, pouring in containers, sterilizing and lamp examining.
Owner:巴里莫尔制药(通化)有限公司
Preparation method of multi-kind micro-element injection
InactiveCN1830455AEnsure complete removalGood curative effectHeavy metal active ingredientsMetabolism disorderUltrafiltrationTrace element
An injection of trace elements for providing external nutrients is prepared through depositing in water, cold storage, ultrasonic treating, ultrafiltration, adding filtered sorbitol in dark condition and N2 atmosphere, pouring in containers, sterilizing and lamp examining.
Owner:巴里莫尔制药(通化)有限公司
Preparation method of clindamycin hydrochloride freeze dried powder injection
InactiveCN1830452AAvoid degradationEnsure complete removalAntibacterial agentsOrganic active ingredientsAcute bronchitisUltrafiltration
A freeze-dried powder injection of clindamycin hydrochloride for treating various infectious diseases, tonsillitis, tympanitis, acute bronchitis, pneumonia, etc is prepared from clindamycin hydrochloride through ultrafiltration, mixing with filtered mannitol in dark condition and N2 atmosphere, pouring in containers, freeze drying, and sealing.
Owner:巴里莫尔制药(通化)有限公司
Method and device for controlling an internal combustion engine
ActiveUS20060196479A1Low related substancesEmission reductionValve arrangementsElectrical controlGas cylinderCrankshaft
The invention relates to a method and device for controlling an internal combustion engine comprising an inlet pipe leading to a cylinder input where a gas input valve is placed. Said engine also comprises a drive for the gas input valve which makes it possible to adjust a gas input valve lift for at least two values. The engine also comprises an injection valve for metering fuel and a spark plug which controls the crankshaft angle of air-fuel mixture ignition. Said internal combustion engine is controlled in a following manner: a fuel is metered at least once during the intake stroke of a cylinder when the valve lift (VL) passes from one value to the other and at least one final injection is carried out in a dosing manner only when the valve lift (VL) is really carried out.
Owner:VITESCO TECH GERMANY GMBH
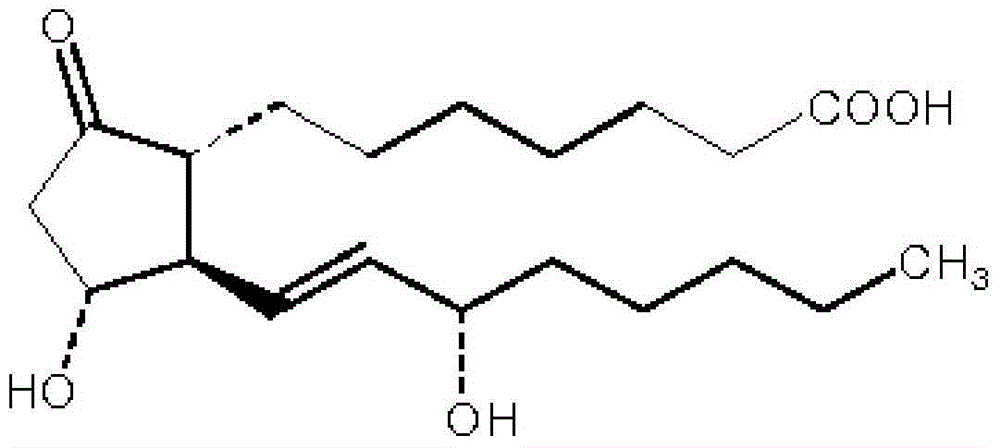
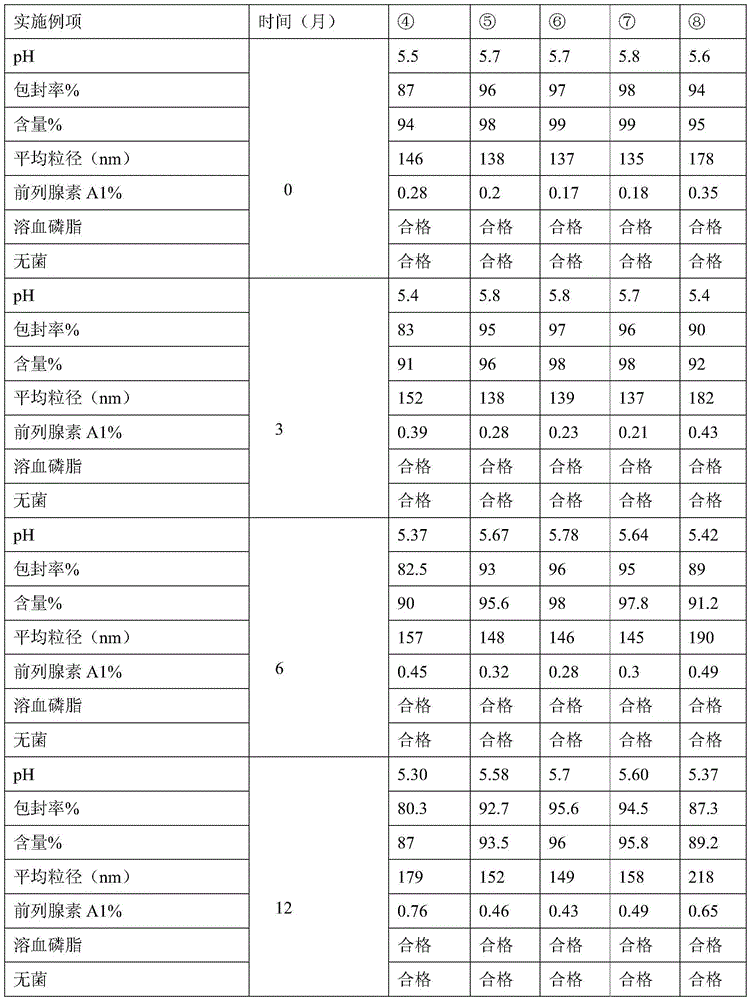
Alprostadil injection and preparation method thereof
ActiveCN103599066AHigh content of the main drugLow related substancesOrganic active ingredientsNervous disorderDrugSOYBEAN SEED OIL
The invention provides an alprostadil injection. Every 1000ml of alprostadil injection comprises the following raw materials: 5mg of alprostadil, 90-110g of soybean oil, 15-20g of phosphatide, 2-3g of oleic acid, 22.1-25g of isoosmotic agent, and appropriate pH modifier, wherein the content of the pH modifier is enough to regulate the pH of the injection to be 5.0-6.0. The invention also provides a preparation method for the alprostadil injection. Compared with the prior art, the alprostadil injection prepared by the method has higher encapsulation efficiency and content of main drug, contains fewer related substances, has good stability, and provides safety guarantee for clinical medication.
Owner:SICHUAN KELUN PHARMA CO LTD
Preparation method of vitamin C freeze dried powder injection
InactiveCN1830438AGuaranteed not to be oxidizedLow related substancesOrganic active ingredientsPowder deliveryVitamin CHydrogen Sulfate
A freeze-dried powder injection of VC for preventing and treating scurvy, acute and chronic infectious diseases, purpura, Keshan disease, etc is prepared from VC, sodium hydrogen sulfate, and disodium versenate through ultrasonic treating, adding CO2 saturated water for injection in dark condition and N2 atmosphere, regulating pH value and temp, etc.
Owner:阿尔贝拉医药控股(通化)有限公司
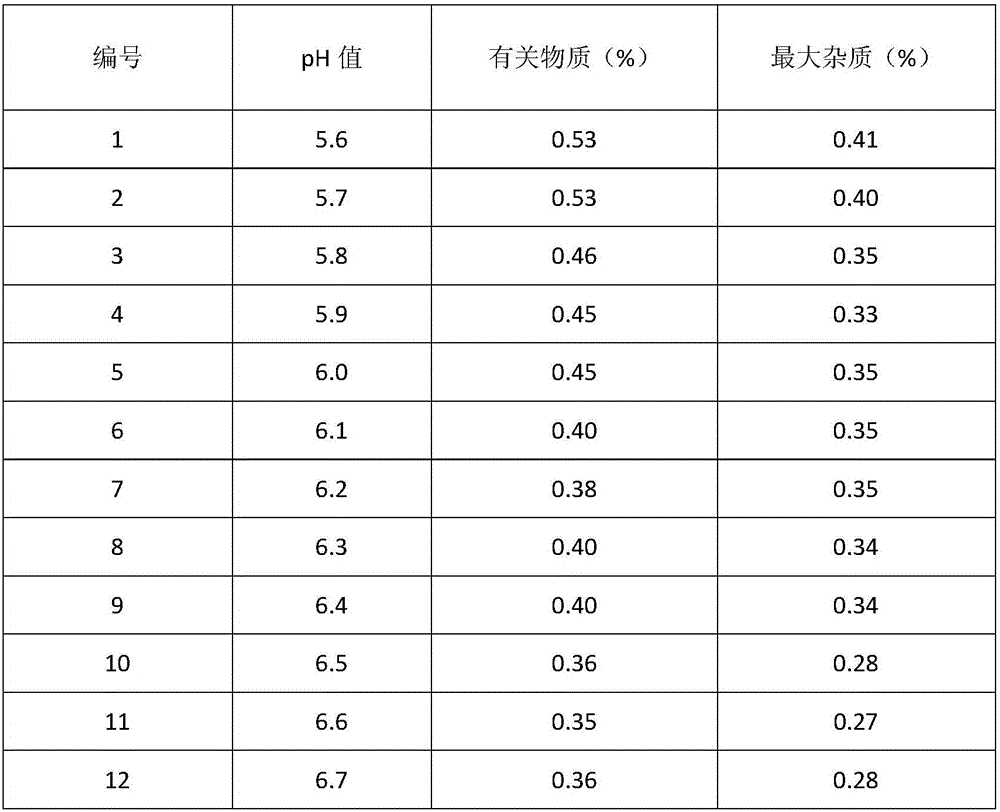
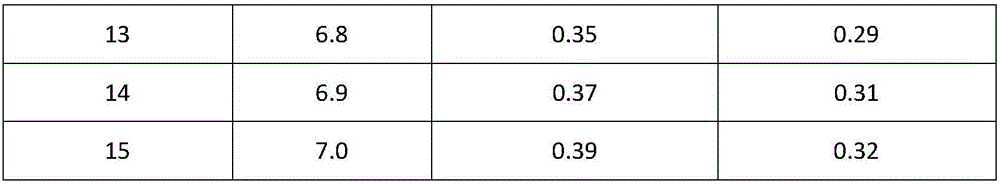
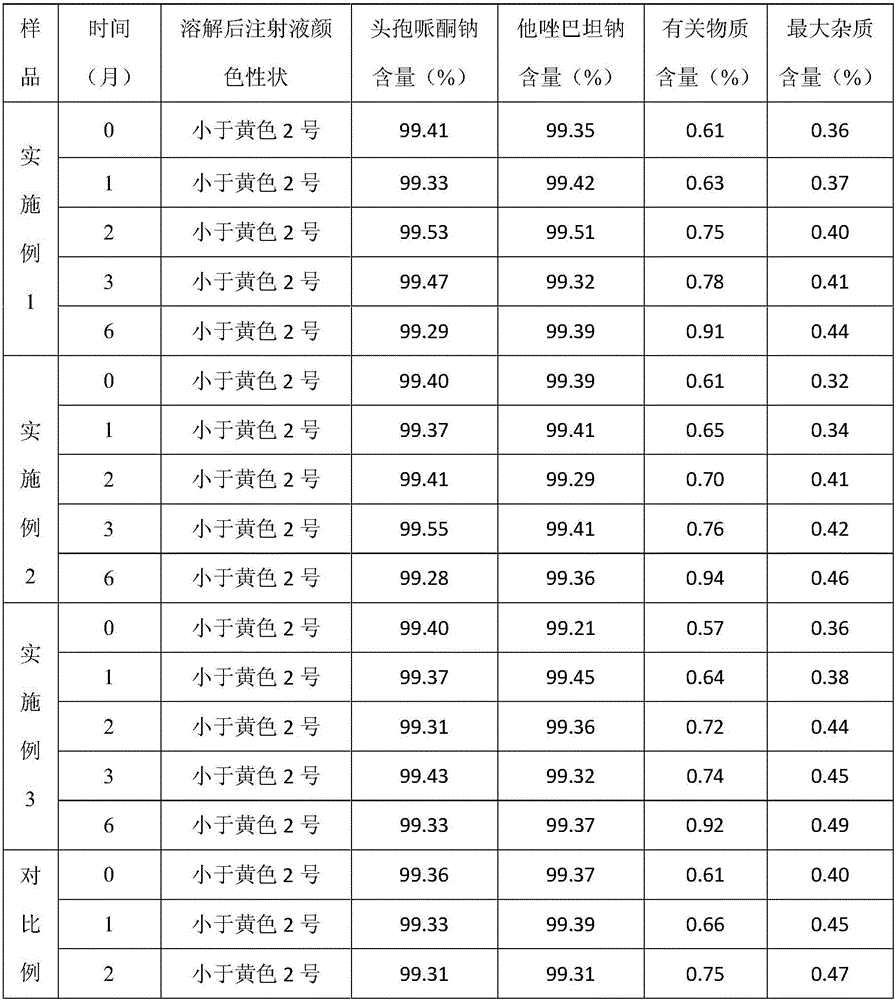
Cefoperazone sodium and tazobactam sodium pharmaceutical composition for injection
ActiveCN105748482AImprove solubilityLight colorAntibacterial agentsPowder deliverySodium bicarbonateSolubility
The invention belongs to the technical field of medicines, and particularly relates to cefoperazone sodium and tazobactam sodium pharmaceutical composition for injection and a preparation method of the cefoperazone sodium and tazobactam sodium pharmaceutical composition. The pharmaceutical composition is a freeze-dried powder preparation. The mass ratio of cefoperazone sodium to tazobactam sodium in the pharmaceutical composition is 4:1. The preparation method comprises the steps as follows: firstly, tazobactam is refined; then, tazobactam sodium is prepared from refined tazobactam and sodium bicarbonate; finally, cefoperazone sodium and prepared tazobactam sodium are uniformly mixed for preparation of freeze-dried powder. Compared with like products sold in the market, the pharmaceutical composition has the advantages of good solubility, light color, few related substances, small polymer content and low adverse reaction rate; besides, effective constituents in the pharmaceutical composition are stable, and when the pharmaceutical composition is preserved for a long term, few effective constituents are degraded, the content of impurities is low, the quality performance of products is relatively good, and the medication safety of patients is guaranteed accordingly.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Topiramate granule and preparation method thereof
InactiveCN104188920ALow related substancesImprove stabilityOrganic active ingredientsNervous disorderPharmaceutical drugPharmaceutical medicine
The invention provides a topiramate granule and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The preparation method comprises the steps of firstly, preparing a cyclodextrin inclusion compound from topiramate serving as an active drug; then, screening the inclusion compound, and next, uniformly mixing the screened inclusion compound and pharmaceutically-acceptable auxiliary materials; and granulating by using a wet process or dry process to obtain the granule. The granule is good in uniformity and stability and is easily dissolved into water; the bitter taste of topiramate is completely covered; the taste of the topiramate granule is remarkably improved; the dosage of the topiramate granule can be conveniently selected according to the age and bodyweight of a patient; and the compliance of the old, children and patients having difficulty in swallowing during drug use is improved.
Owner:ANHUI YIXINMING PHARMA TECH
Preparation method of garlicin injection
InactiveCN1830423AHas a broad-spectrum antibacterial effectInhibit aggregationAntibacterial agentsOrganic active ingredientsAlcoholIrritation
A garlicin injection for preventing and treating acute and chronic bacillary dysentery and enteritis, pertussis, fungus infection of lung and digestive tract, etc is prepared through ultrasonic treating to garlicin and tween-80, mixing with alcohol, ultrafiltration, pouring it in containers containing N2 in dark condition, sterilizing, and lamp examining.
Owner:巴里莫尔制药(通化)有限公司
Process for refining bendazac lysine and analogs thereof
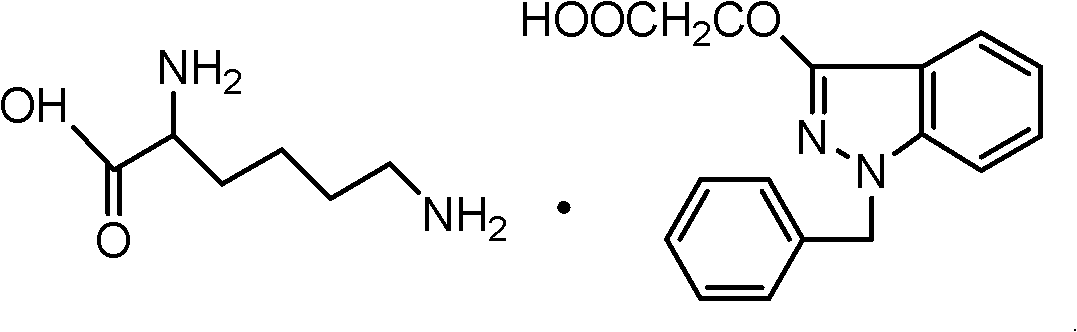
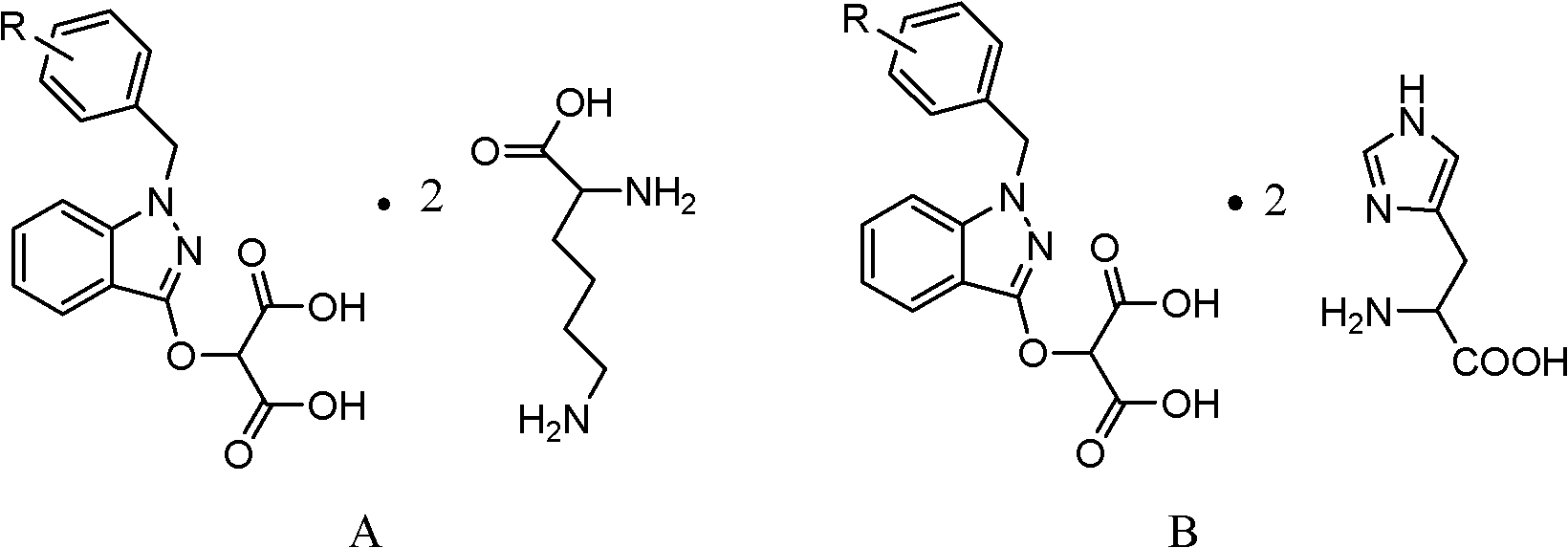
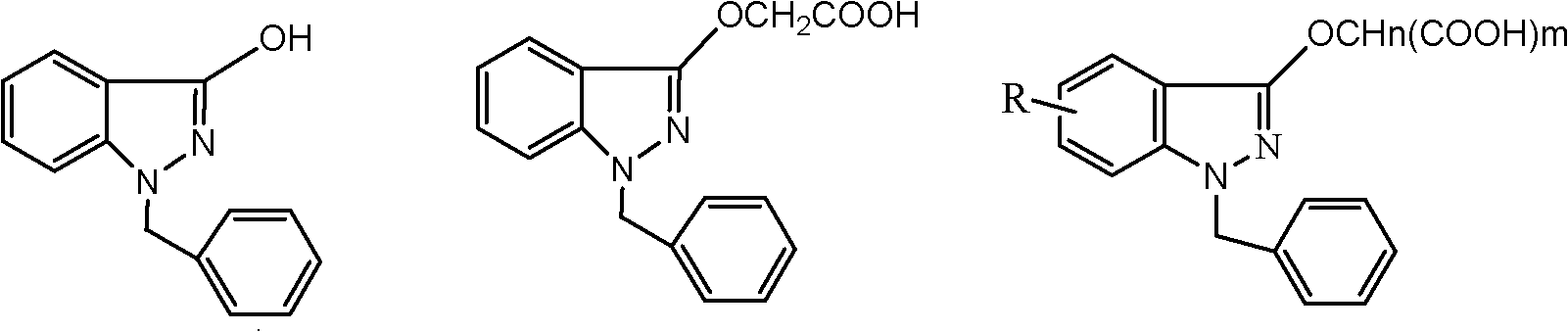
InactiveCN102206185AIncrease in sizeHigh yieldOrganic compound preparationAmino-carboxyl compound preparationHistidineImpurity
The invention provides a process for refining bendazac lysine and analogs thereof, belonging to the technical field of chemical drug preparation. The process comprises the steps of adopting bendazac or bendazac analogs and L-lysine or L-histidine as raw materials; reacting the raw and salifying the materials in an aqueous solution of a polar aprotic solvent so as to obtain a crude product; and then recrystallizing the crude product in an aqueous solution of a polar protic solvent, wherein the polar aprotic solvent preferably selected from acetonitrile while the polar protic solvent is preferably selected from ethanol. The bendazac lysine and the analogs thereof which are finally prepared by using the invention are both white crystalline powders with yield of more than 80% and content of 99.6-101%, wherein the content of 3-hydroxyl-1-benzyl indazole is lower than 0.1%; or even no 3-hydroxyl-1-benzyl indazole is detected; and the total content of impurities does not exceed 0.2%. The refining process provided by the invention has the advantages of simple method, mild condition, short reaction time, low product cost, high purity and high yield, is beneficial for large-scale production and can be also can be used for refining commercial bendazac lysine raw materials.
Owner:JILIN UNIV
Preparation of Ginkgo Damo injection
ActiveCN1939357BReduce permeabilityImprove deformation abilityOrganic active ingredientsSenses disorderIschemic retinopathyDipyridamole
Owner:通化谷红制药有限公司
Oral preparation containing amisulpride
ActiveCN102600132BMask bitternessImprove medication complianceNervous disorderMacromolecular non-active ingredientsSolubilityWater soluble
The invention relates to an oral preparation containing amisulpride. The amisulpride which is active medicine is prepared into cyclodextrin inclusion compound, is sieved, is uniformly mixed with pharmaceutically acceptable auxiliary materials, and is granulated by a wet method or a dry method, obtained granules are squashed to form tablets, filled into capsules to form a capsule preparation or is directly separately packaged to obtain a granule preparation. The water solubility and the stability of the oral preparation containing the amisulpride can be strengthened, bitter taste of the amisulpride is covered, medication compliances of patients are improved, and bioavailability of the oral preparation is improved to a great extent.
Owner:QILU PHARMA CO LTD
Microemulsions and use thereof as a fuel
InactiveUS7977389B2Low related substancesImprove efficiencyOther chemical processesMixing methodsCombustion systemThermodynamics
The invention relates to bicontinuous microemulsions and to the use thereof as a fuel, combustion or heating fluid. Said fuels permit an increased efficiency of internal combustion systems and heating installations of any type and, simultaneously, a minimized emission of pollutants, associated with combustion, to be obtained.
Owner:UNIV OF COLOGNE
Biological substance related article and method of manufacturing the same, and biological substance adsorption preventive coating composition and method of using the same
InactiveUS7569622B2Low protein adsorptionExcellent strength and water resistancePretreated surfacesAntithrombogenic treatmentSolventAmount of substance
Owner:JSR CORPORATIOON
Thiamphenicol glycinate hydrochloride freeze-dried powder injection and preparation method thereof
ActiveCN101904823ALow related substancesStable qualityAntibacterial agentsOrganic active ingredientsFreeze dryHydrochloride
The invention relates to a thiamphenicol glycinate hydrochloride freeze-dried powder injection and a preparation method thereof, belonging to the technical field of medicines. The proper pH value range and the proper freeze-drying conditions of an intermediate solution are screened out by researching the thiamphenicol glycinate formulation technology, therefore, the obtained freeze-dried powder injection has the advantages of low related substances, stable quality, easy transportation and storage and high safety.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Preparation method of tanshinone IIA sodium sulphonate small volume injection
InactiveCN1830433AImprove collateral circulationImprove local blood supplyOrganic active ingredientsPharmaceutical delivery mechanismVentricular premature contractionsCoronary artery disease
A low-dosage injection of tanshinone IIA-sodium sulfonate for treating coronary heart disease, angina pectoris, myocardial infarction and ventricular premature contraction is prepared from the tanshinone IIA-sodium sulfonate through depositing in water, cold storage, ultrafiltration, adding additives in dark condition, pouring in containers in Na atmosphere, sterilizing and lamp examining.
Owner:巴里莫尔制药(通化)有限公司
A kind of preparation method of compound paracetamol capsule
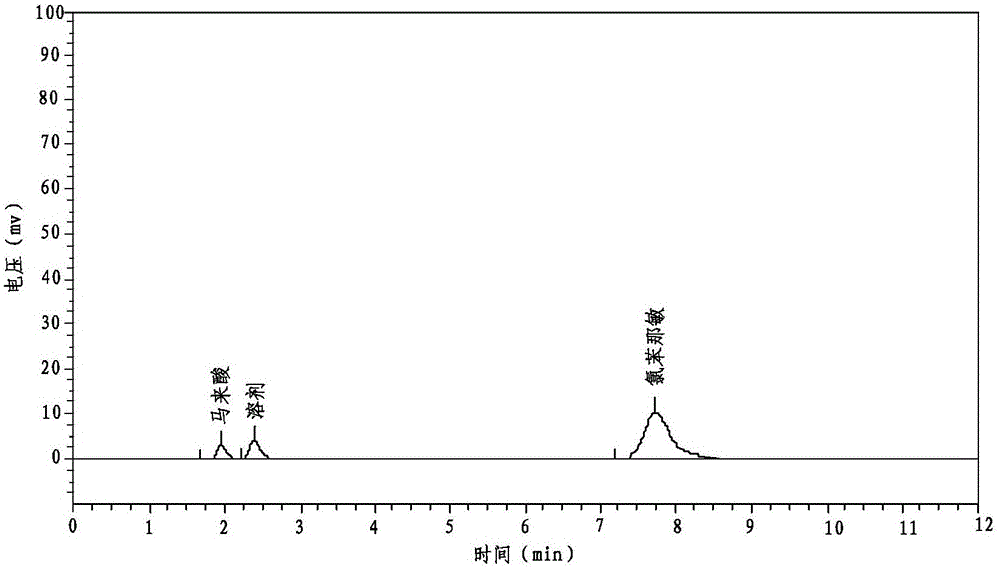
The invention relates to the technical field of the pharmacy, and especially relates to a preparation method of a compound paracetamol and amantadine hydrochloride capsule. The method is characterized in that smaller amounts of components comprising dextrin, calculus bovis factitious, chlorphenamine maleate and caffeine in a prescription are uniformly mixed to obtain mixed powder I, and larger amounts of compounds comprising viregyt hydrochloride and paracetamol in the prescription are uniformly mixed with the mixed powder I to realize uniform mixing and the uniformity of the content of the chlorphenamine maleate in the whole capsule. Double-tapered rotary vacuum drying is adopted, the physical and chemical properties of a material are not changed after the drying of the material, and the content of relevant substances in a finally finished product is low.
Owner:HAINAN ASIA PHARM CO LTD
A compound pharmaceutical composition of mezlocillin sodium and sulbactam sodium for injection
ActiveCN105963263BImprove solubilityWell mixedAntibacterial agentsPowder deliverySolubilityCompounding drugs
The invention relates to the technical field of medicines and discloses a mezlocillin sodium and sulbactam sodium compound drug composition for injection. The mezlocillin sodium and sulbactam sodium compound drug composition comprises mezlocillin sodium, sulbactam sodium and sodium benzoate. The mezlocillin sodium and sulbactam sodium compound drug composition realizes higher product performance by less auxiliary materials, has the advantages of being good in solubility, shallow in color, uniform in main drug mixing, low in related substance, low in polymer content, low in incidence rate of adverse reactions and the like and ensures that patients are safer in the clinical use, and the treatment effect is more reliable.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
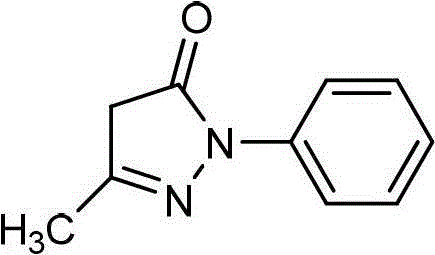
Edaravone A-type crystal and preparation method thereof
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com