Preparation method of acetyl glutamine injection
A technology of acetylglutamine and injection, applied in the direction of amide active ingredients, nervous system diseases, drug combination, etc., can solve the problems of poor product quality stability, clarity failure rate, strong irritation of liquid medicine, etc., and achieve clinical curative effect Effects of exacting, protecting and repairing nerve cells, improving clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
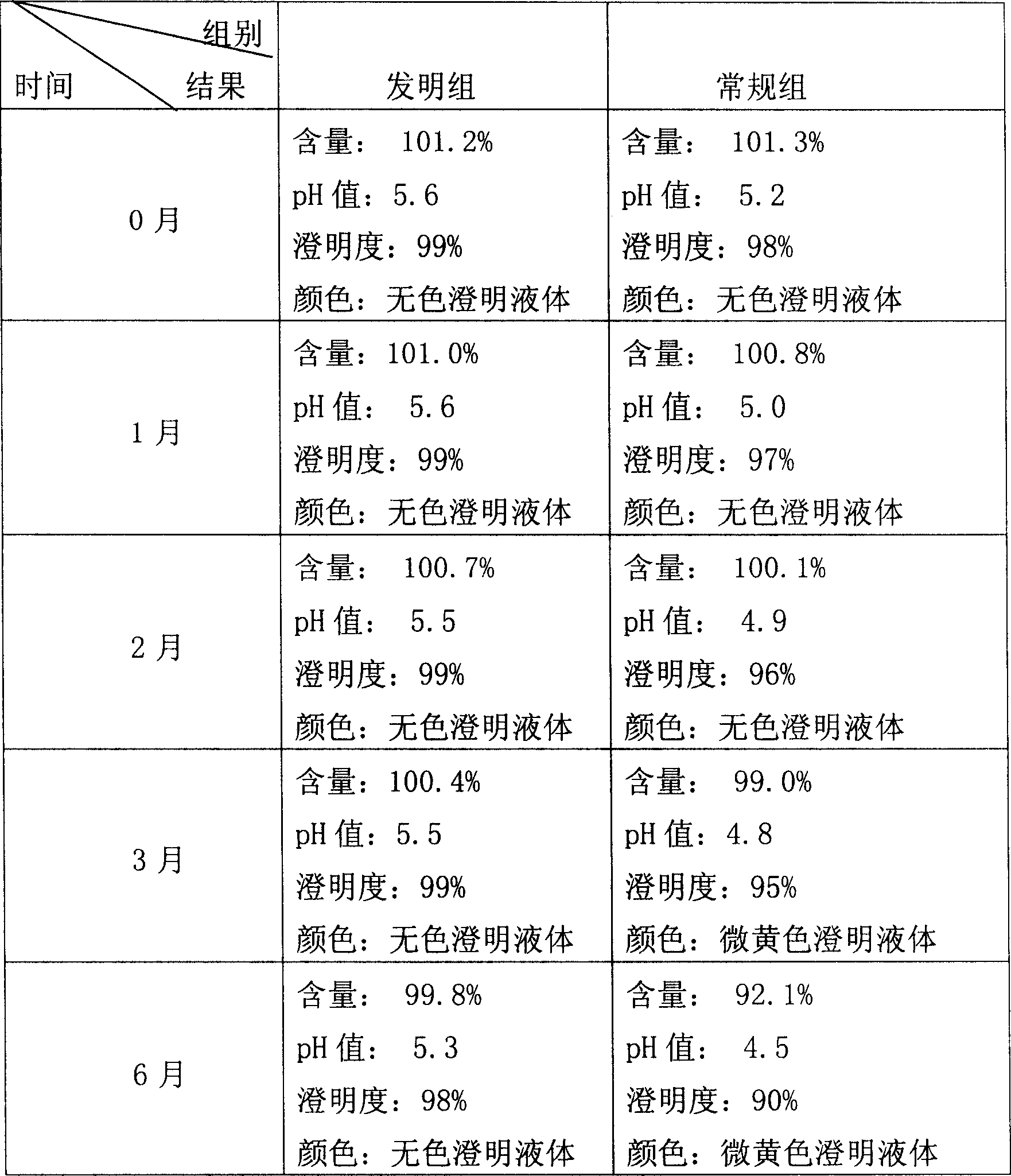
[0023] A) Take 70% fresh water for injection at 80°C, add disodium hydrogen phosphate and sodium dihydrogen phosphate in the prescription, stir to dissolve, cool down to 15°C, adjust pH to 6.0, avoid light, add acetylglutamine in the prescription , sonicated for 15 minutes, refrigerated at 4°C for 24 hours, and ultrafiltered with an ultrafiltration membrane with a molecular weight cut-off of 10,000 to obtain an ultrafiltrate.
[0024] B) Take fresh water for injection with a preparation volume of 15% at 80°C, add disodium ethylenediaminetetraacetate in the prescription, stir to dissolve, add 0.1% activated carbon, heat and boil for 25 minutes, cool to 40°C, filter with 0.45μm Membrane decarbonization and filtration to obtain ultrafiltrate.
[0025] C) Mix the above two medicinal liquids under the condition of avoiding light, sonicate for 15 minutes, adjust the volume to 5.8, adjust the pH value to 5.8, pass the inspection of the intermediate product, filter the medicinal liqui...
Embodiment 2
[0027] A) Take 80% fresh water for injection at 90°C, add disodium hydrogen phosphate and sodium dihydrogen phosphate in the prescription, stir to dissolve, cool down to 25°C, adjust pH to 5.7, avoid light, add acetylglutamine in the prescription , sonicated for 30 minutes, refrigerated at 6° C. for 12 hours, and ultrafiltered with an ultrafiltration membrane with a molecular weight cut-off of 10,000 to obtain an ultrafiltrate.
[0028] B) Take fresh water for injection with a preparation volume of 15% at 80°C, add disodium ethylenediaminetetraacetate in the prescription, stir to dissolve, add 0.2% activated carbon, heat and boil for 15 minutes, cool to 50°C, filter with 0.45μm Membrane decarbonization and filtration to obtain ultrafiltrate.
[0029] C) Mix the above two medicinal liquids under the condition of avoiding light, sonicate for 10 minutes, adjust the volume to 6.2, adjust the pH value to 6.2, pass the inspection of the intermediate product, filter the medicinal liq...
Embodiment 3
[0031] A) Take 75% fresh water for injection at 85°C, add disodium hydrogen phosphate and sodium dihydrogen phosphate in the prescription, stir to dissolve, cool down to 20°C, adjust pH to 5.0, avoid light, add acetylglutamine in the prescription , sonicated for 25 minutes, refrigerated at 6° C. for 12 hours, and ultrafiltered with an ultrafiltration membrane with a molecular weight cut-off of 10,000 to obtain an ultrafiltrate.
[0032] B) Take 5% fresh water for injection at 85°C, add disodium ethylenediaminetetraacetate in the prescription, stir to dissolve, add 0.4% activated carbon, heat and boil for 30 minutes, cool to 60°C, filter with 0.45μm Membrane decarbonization and filtration to obtain ultrafiltrate.
[0033] C) Mix the above two medicinal liquids under the condition of avoiding light, sonicate for 25 minutes, adjust the volume to 5.7, adjust the pH value to 5.7, pass the inspection of the intermediate product, filter the medicinal liquid through a 0.22 μm filter m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com