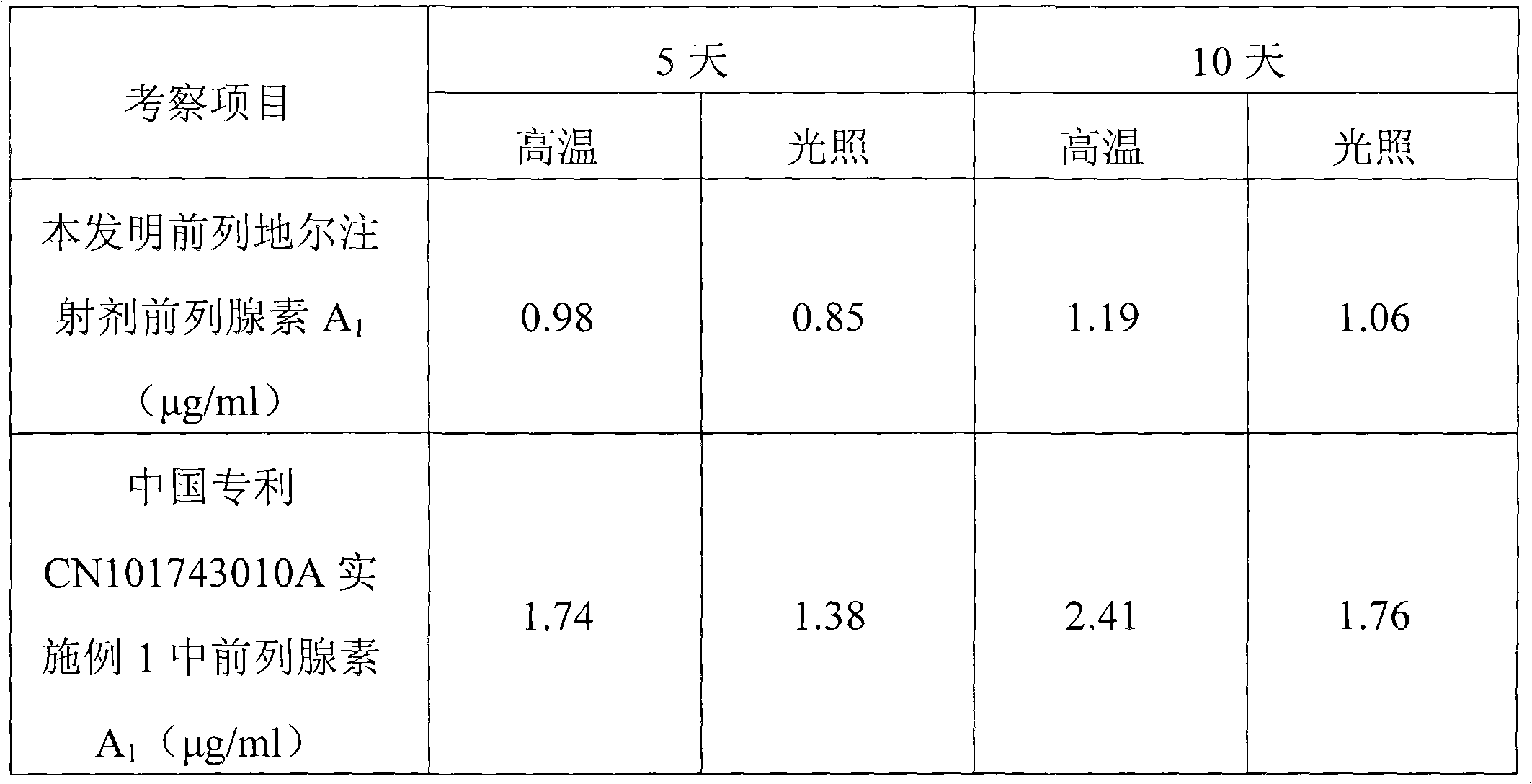
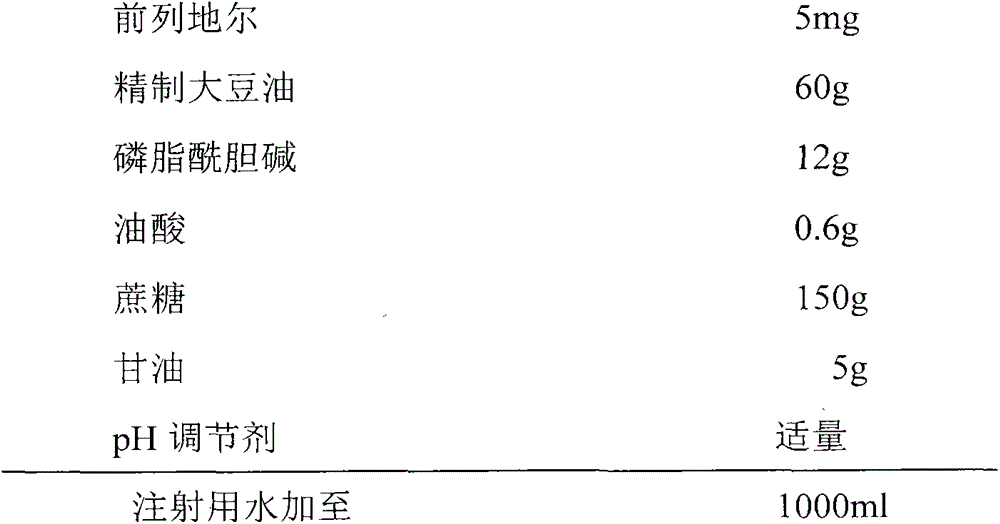
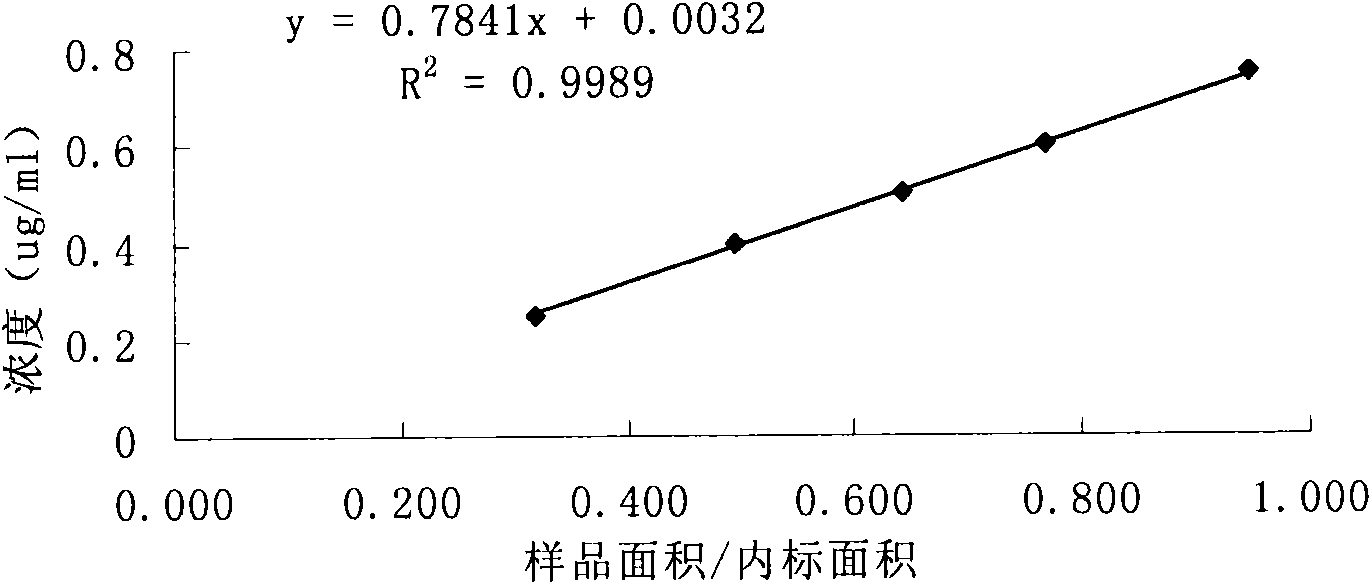
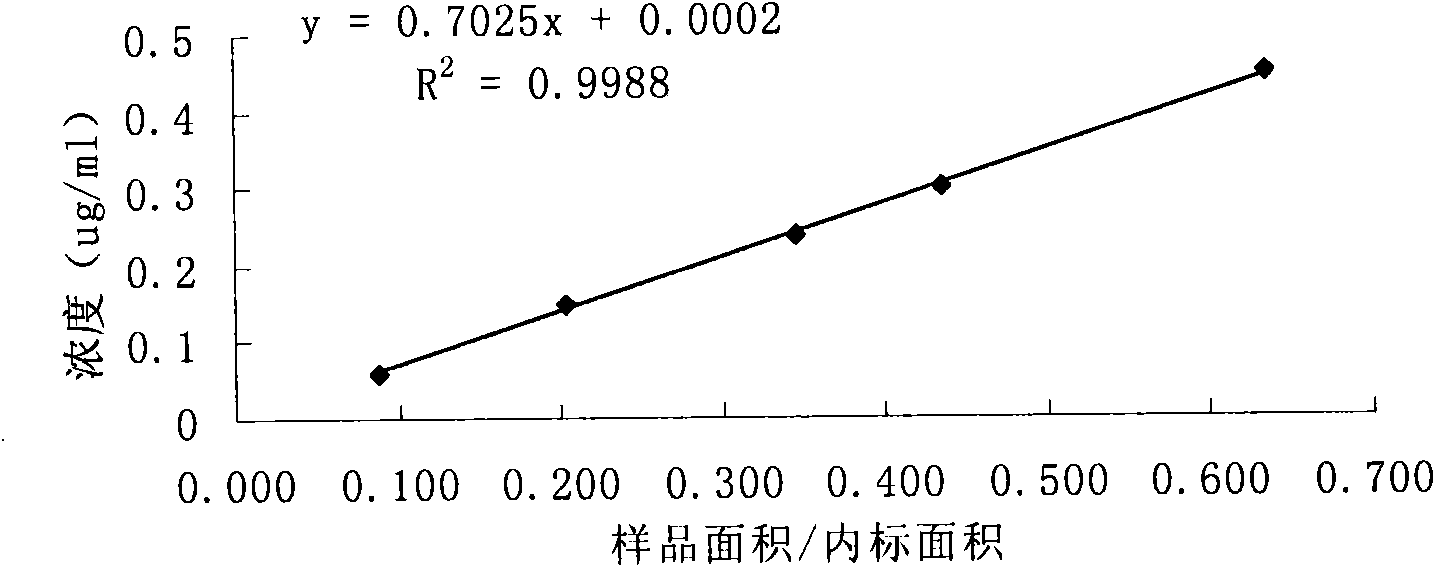
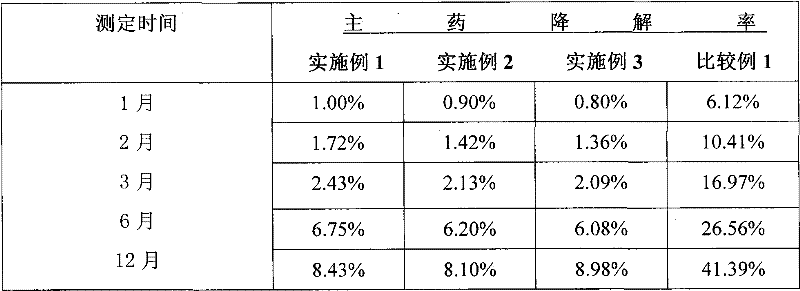
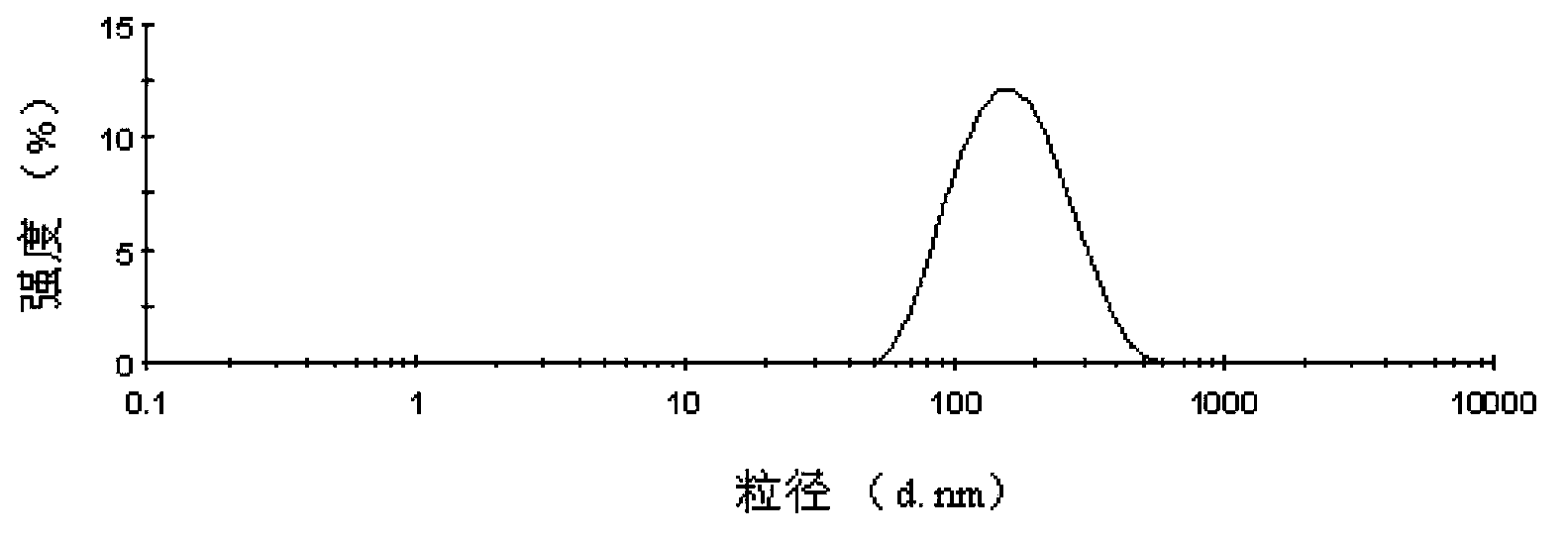
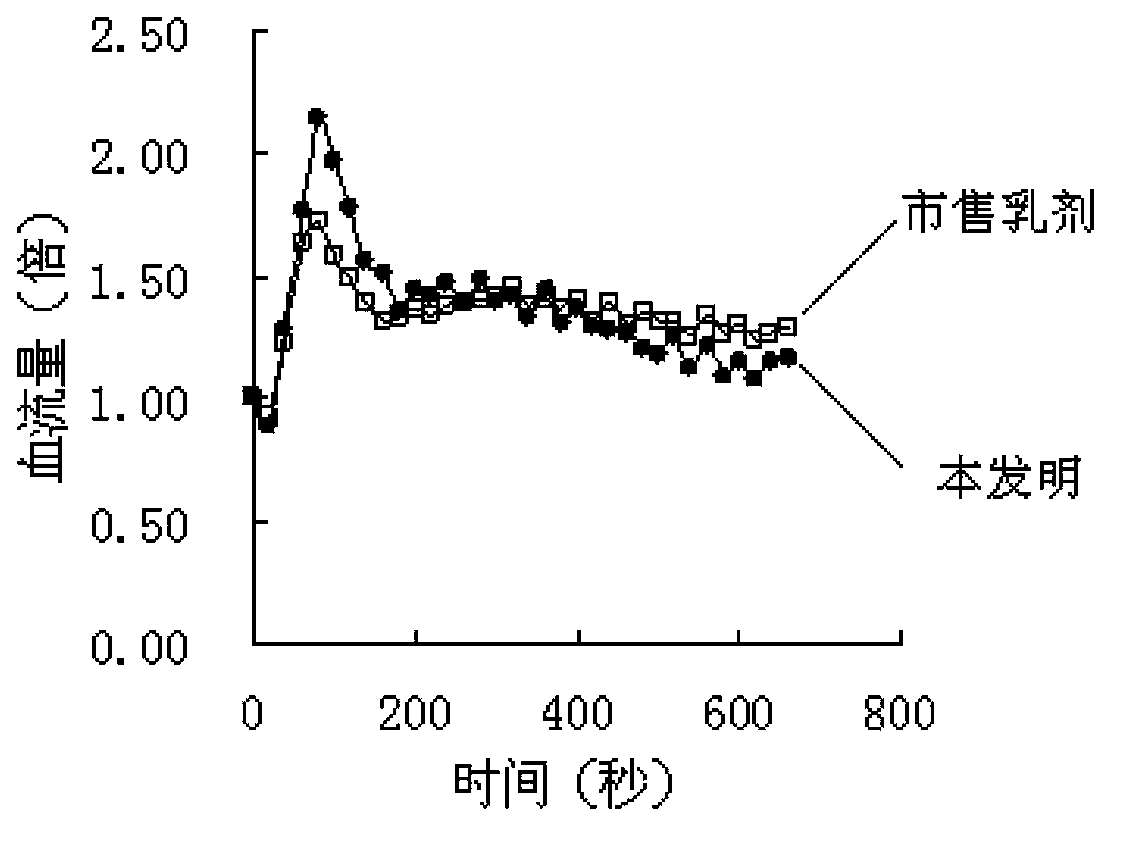
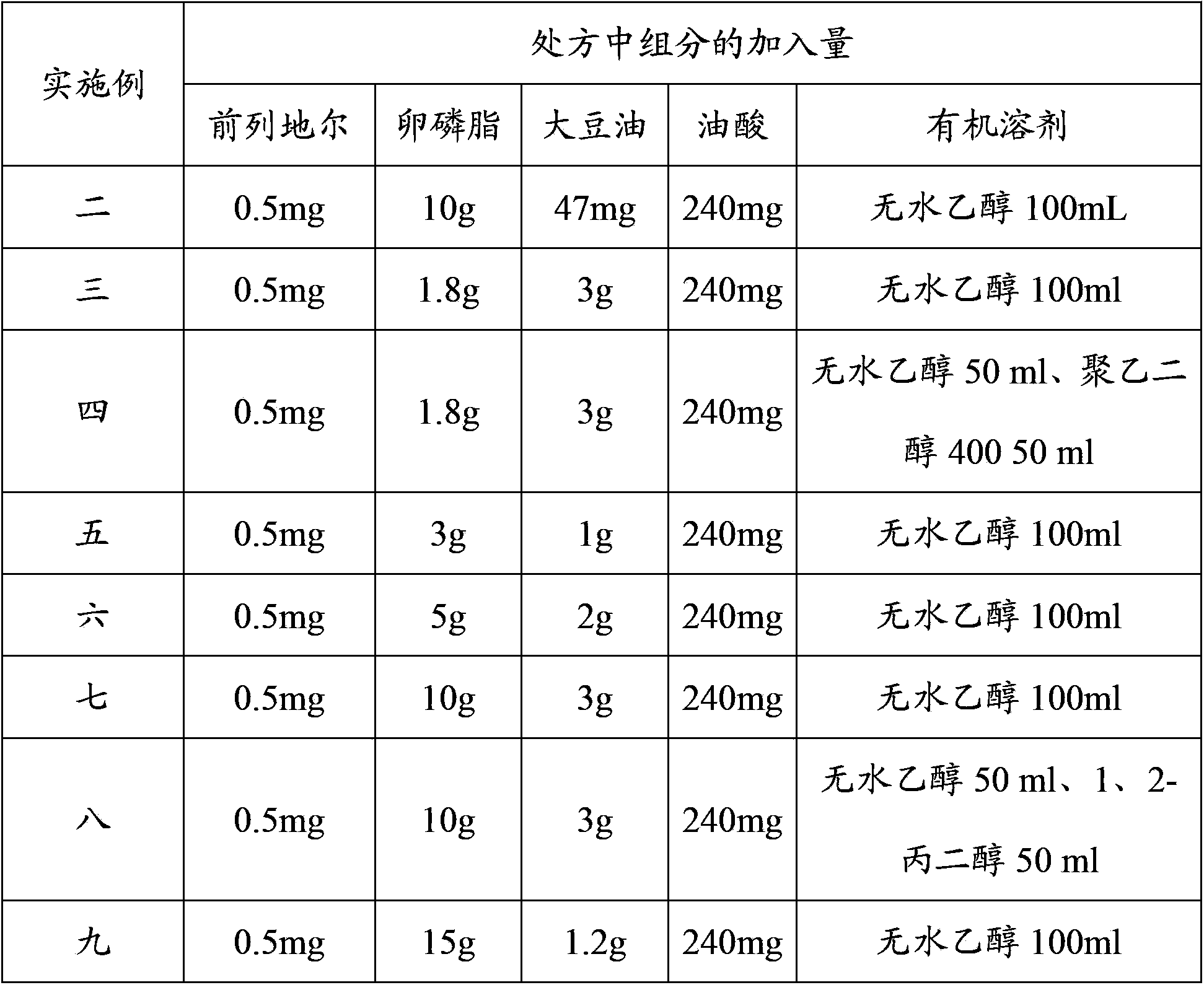
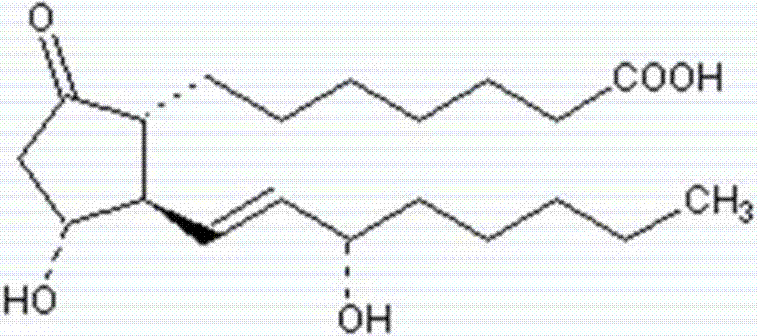
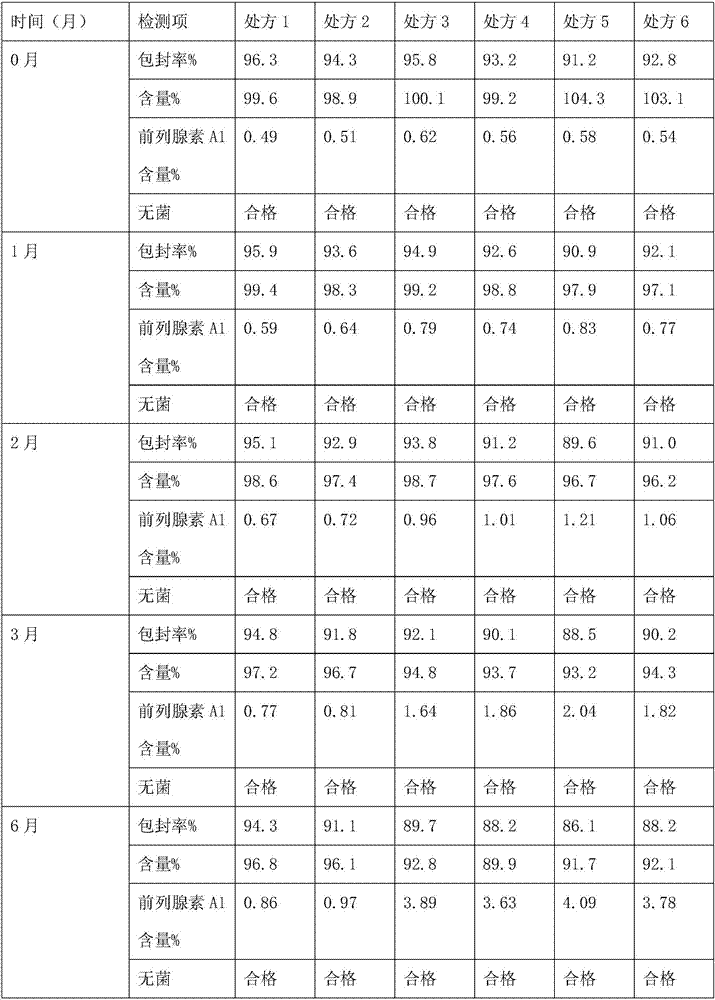
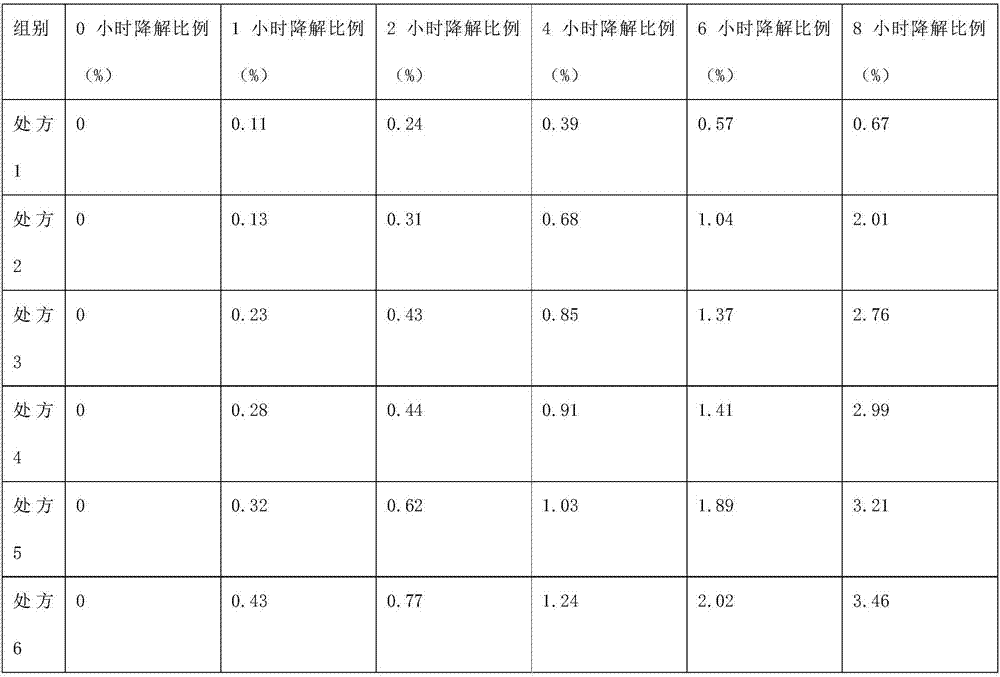
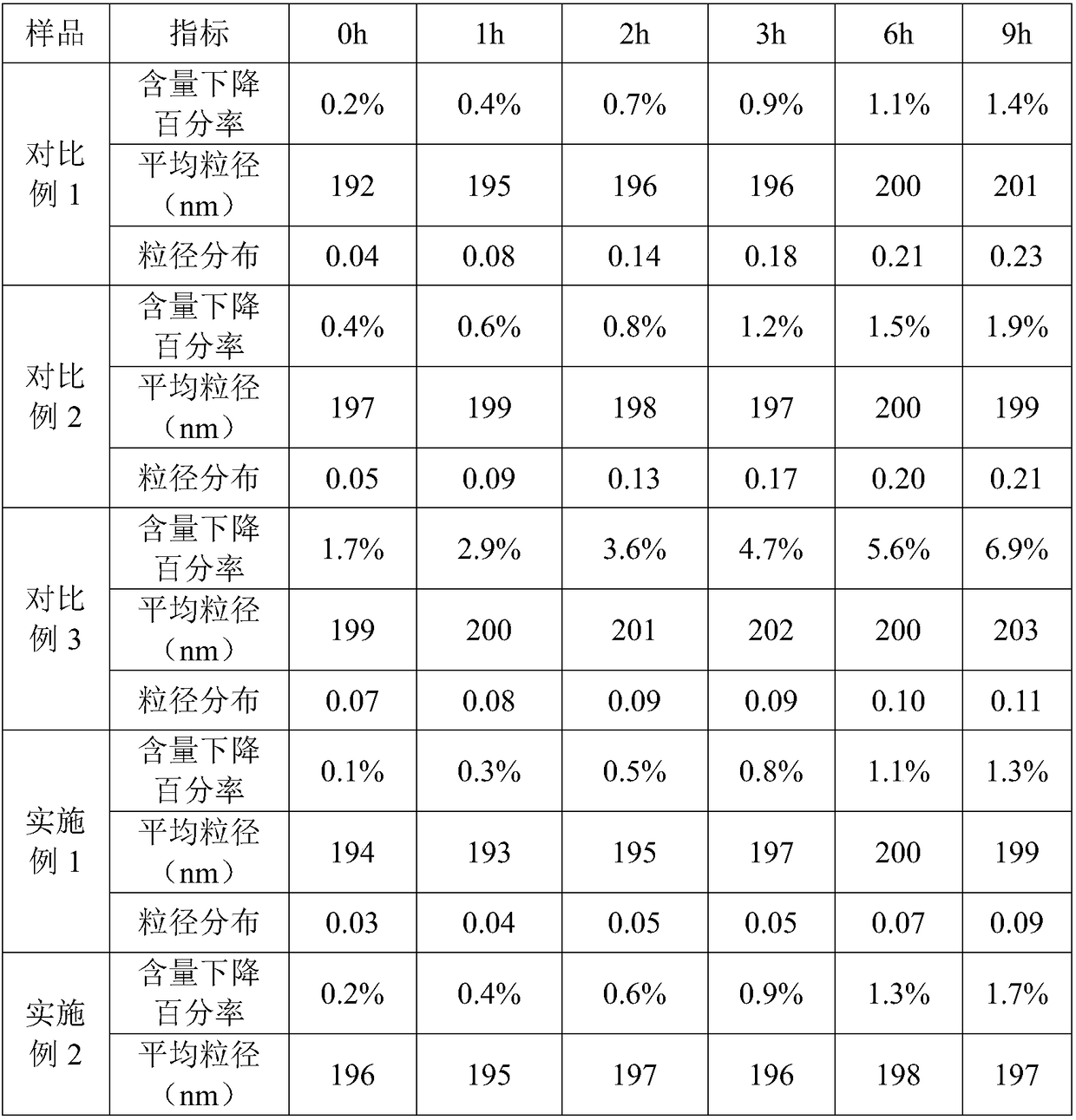
Patents
Literature
30 results about "Alprostadil Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This is not a list of all drugs or health problems that interact with alprostadil injection. Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take alprostadil ...
Stable alprostadil injection emulsion and preparation method thereof
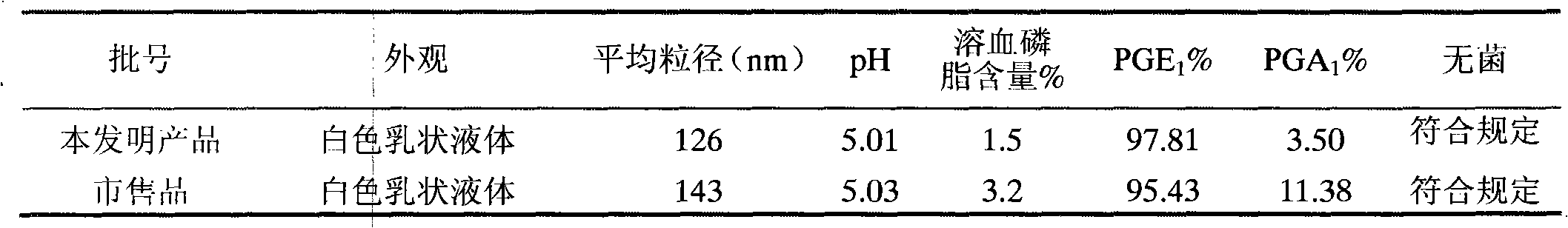
InactiveCN101474150AGood chemical stabilityReduce metabolic inactivationOrganic active ingredientsRespiratory disorderEmulsionBioavailability
The invention discloses a stable alprostadil injection latex which takes alprostadil as active ingredient, contains oil for injection, emulsifier, glycerin for injection and water for injection which are accepted by pharmacy, and is prepared by emulsification technology. Compared with the existing alprostadil injection latex on the market, the stable alprostadil injection latex prepared by the invention can remarkably enhance the thermal stability of the alprostadil, prolong the period of validity of the alprostadil injection latex while reducing the degradation of the alprostadil at the lung and being beneficial to improving the bioavailability, thus further improving the drug effect.
Owner:四川思达康药业有限公司 +1
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787AClinical application safetyEnsure safetyOrganic active ingredientsDigestive systemChemical structureLipid formation
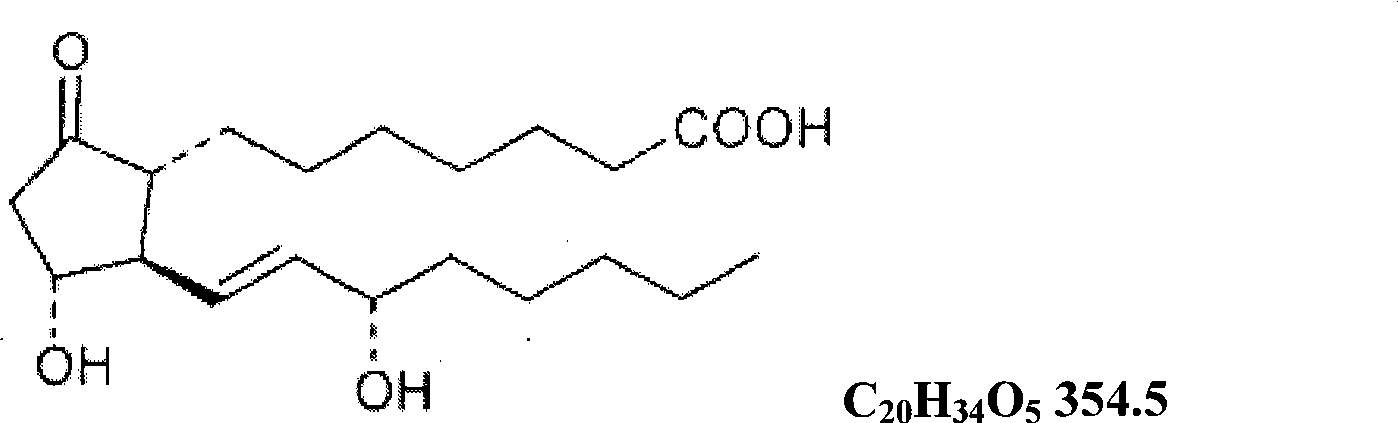
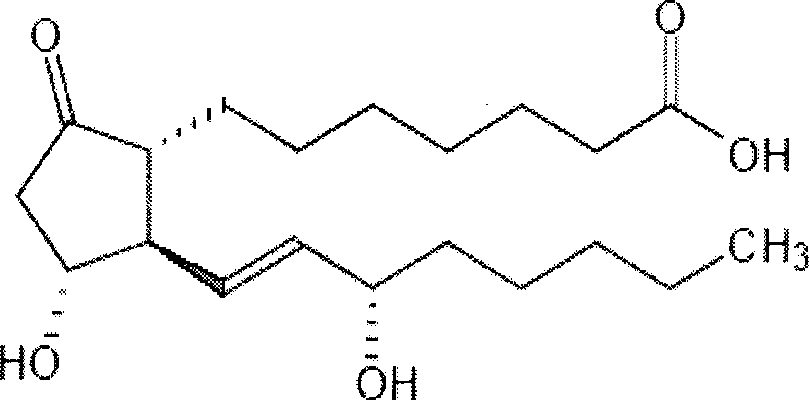
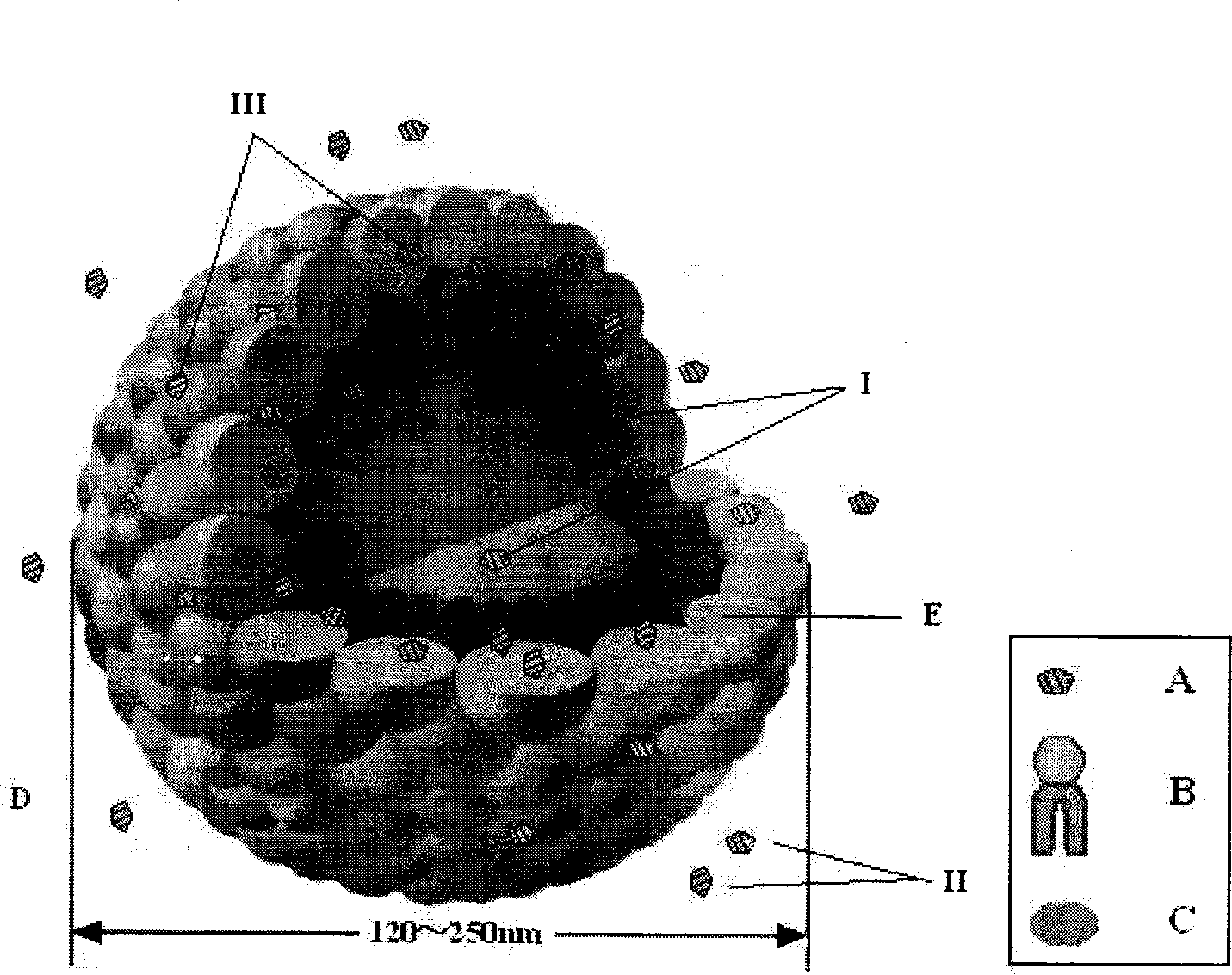
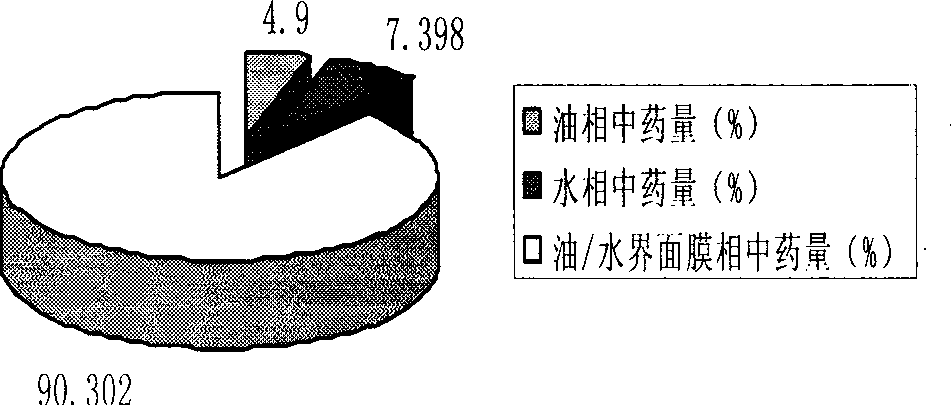
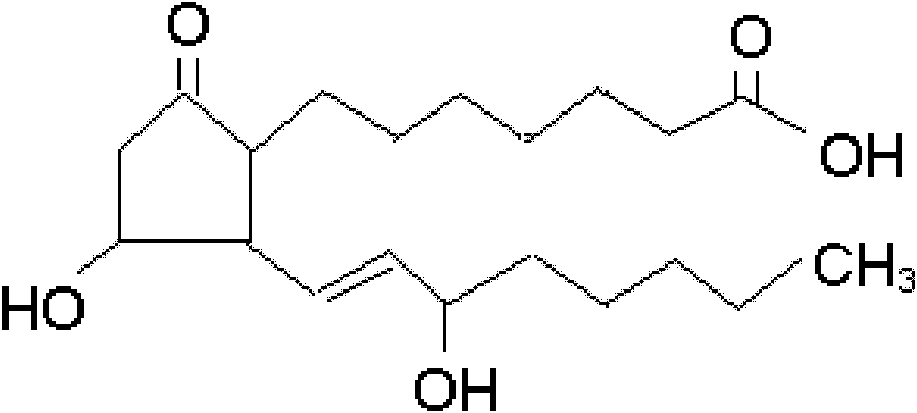
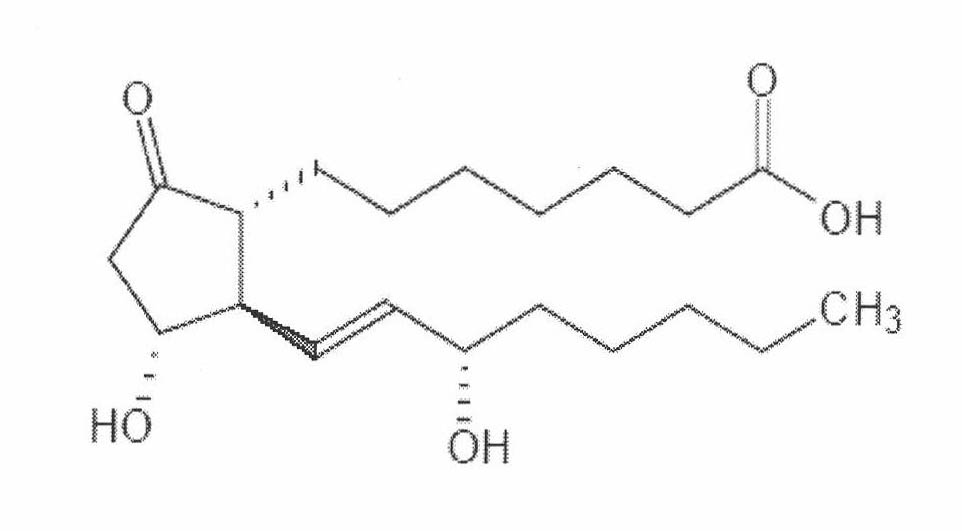
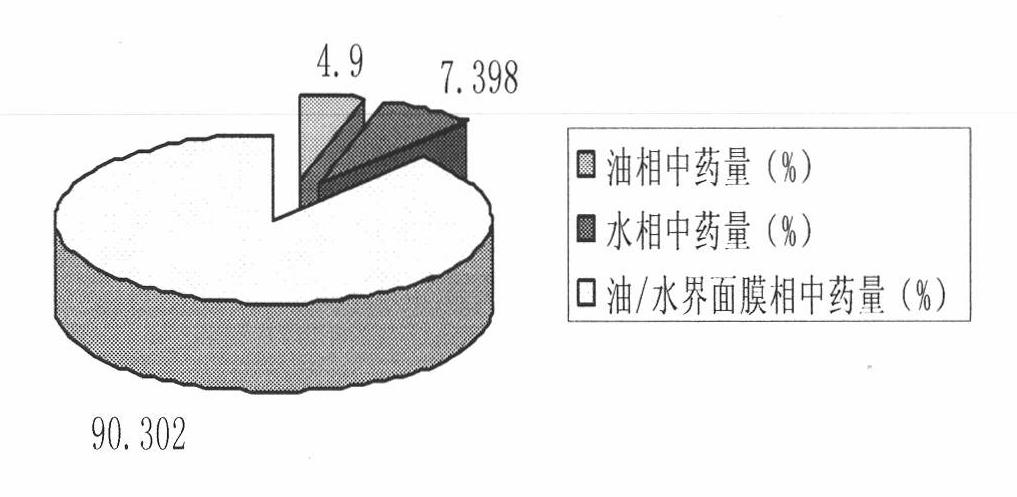
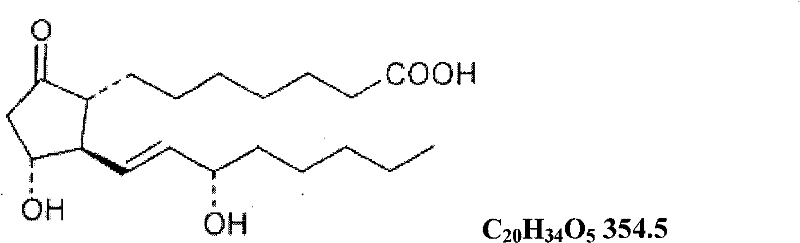
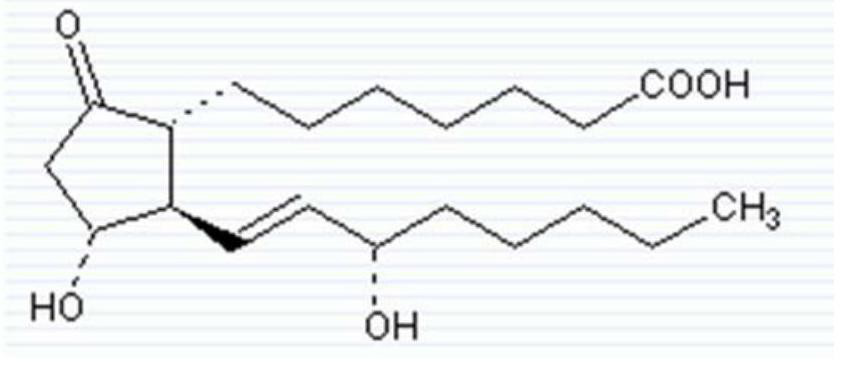
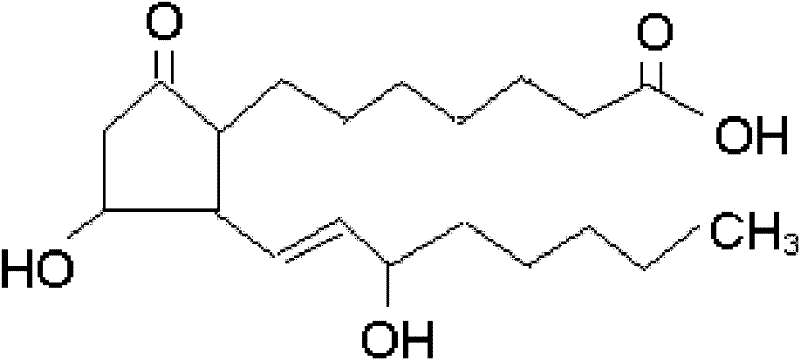
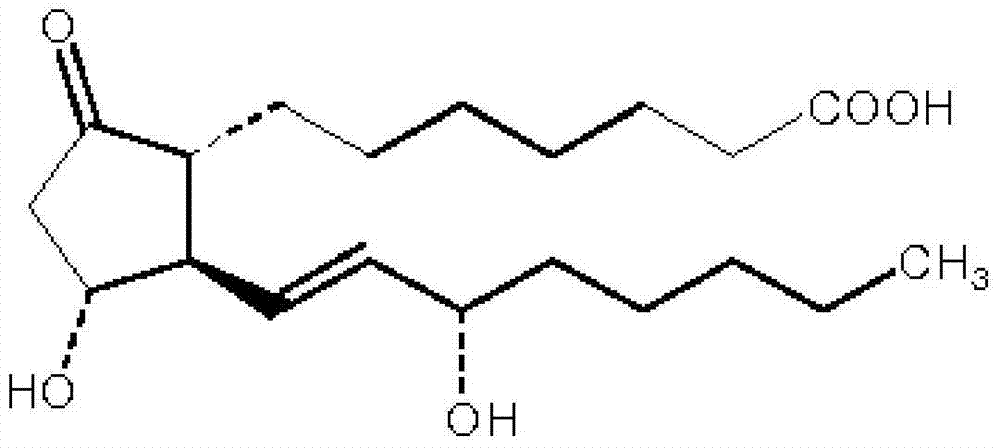
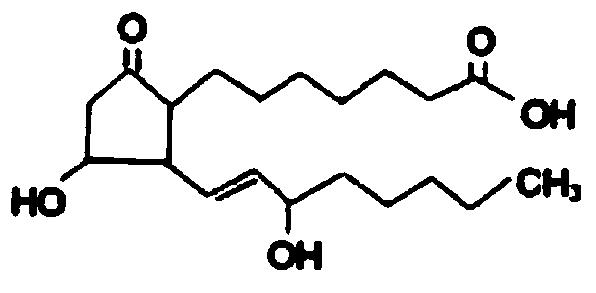
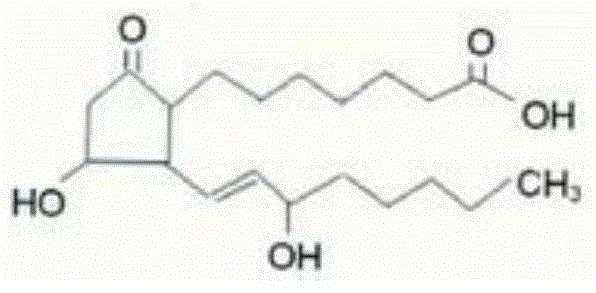
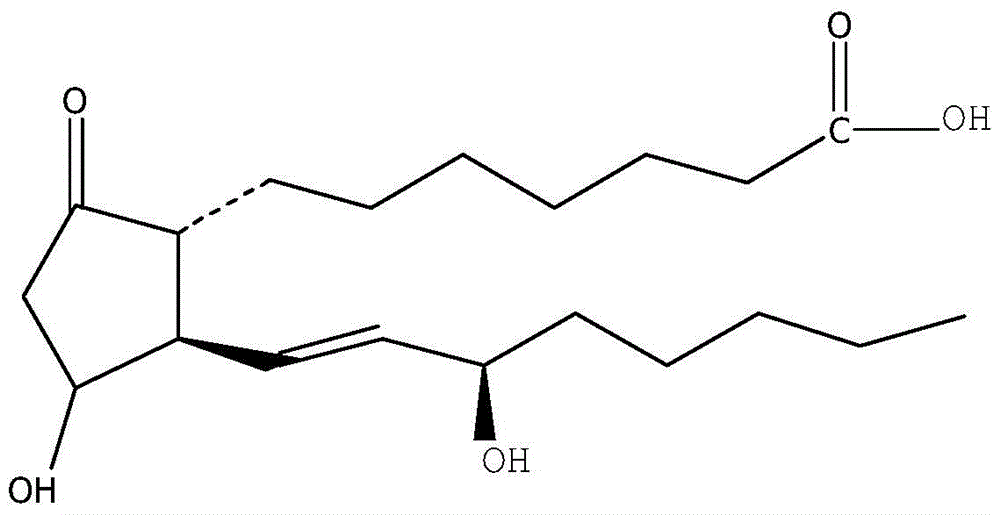
The invention relates to a method for preparing a prostaglandin E1 lipid microsphere injection of a charging non-homogeneous phase (comprising a water phase, an oil / water interfacial film phase and an oil phase) dispersion system, of which the surface of the lipid microsphere can be charged with positive electricity or negative electricity. The prostaglandin E1 is alprostadil, of which the chemical structure comprises a basic skeleton of 20-carbon fatty acid with a 5-carbon ring and two side chains, wherein one side chain is provided with a hydrophilic carboxylic acid group, so that the prostaglandin E1 has the characteristic of light surface activity action. By utilizing the characteristic, and according to the formula and the preparation process provided in the invention, the prostaglandin E1 has an unique drug-carrying mode in a solution of lipid microsphere with the non-homogeneous phase dispersion system, and the prepared lipid microsphere injection is fundamentally different from an alprostadil injection(Kaishi, and is prepared by adopting the technology of the Japanese business corporation LTT Bio-Pharma Co., Ltd. already sold in markets, and the difference lies in that the drug-carrying mode is completely different, the content of degradation products in the preparation such as impurities is more than 50 percent lower than that of in the Kaishi, so that the prostaglandin E1 lipid microsphere injection and the alprostadil injection are fundamentally different. The invention relates to a method for preparing the prostaglandin E1 lipid microsphere injection and the drug-carrying characteristics thereof in a three-phase system; in the formula, 0.0001 to 0.1 weight portion of prostaglandin E1 is used as a drug, the prostaglandin E1 is added with auxiliary materials for medical purpose to prepare the prostaglandin E1 lipid microsphere injection, and the auxiliary materials for medical purpose comprises the following materials in portion by weight: 5 to 20.
Owner:李淑斌
Preparation method of alprostadil injection
ActiveCN101627968AQuality assuranceReduce incidenceOrganic active ingredientsDigestive systemMedicineAlprostadil Injection
The invention relates to a preparation method of an alprostadil injection. The alprostadil injection consists of alprostadil, oil for injection, emulsifier, stabilizer and other agents, pH regulator and water for injection. Compared with the commercially available products, the quality standard of the prepared product is significantly improved, and the safety of clinical medication is increased.
Owner:辽宁中海康生物制药股份有限公司
Alprostadil composition as well as preparation method thereof
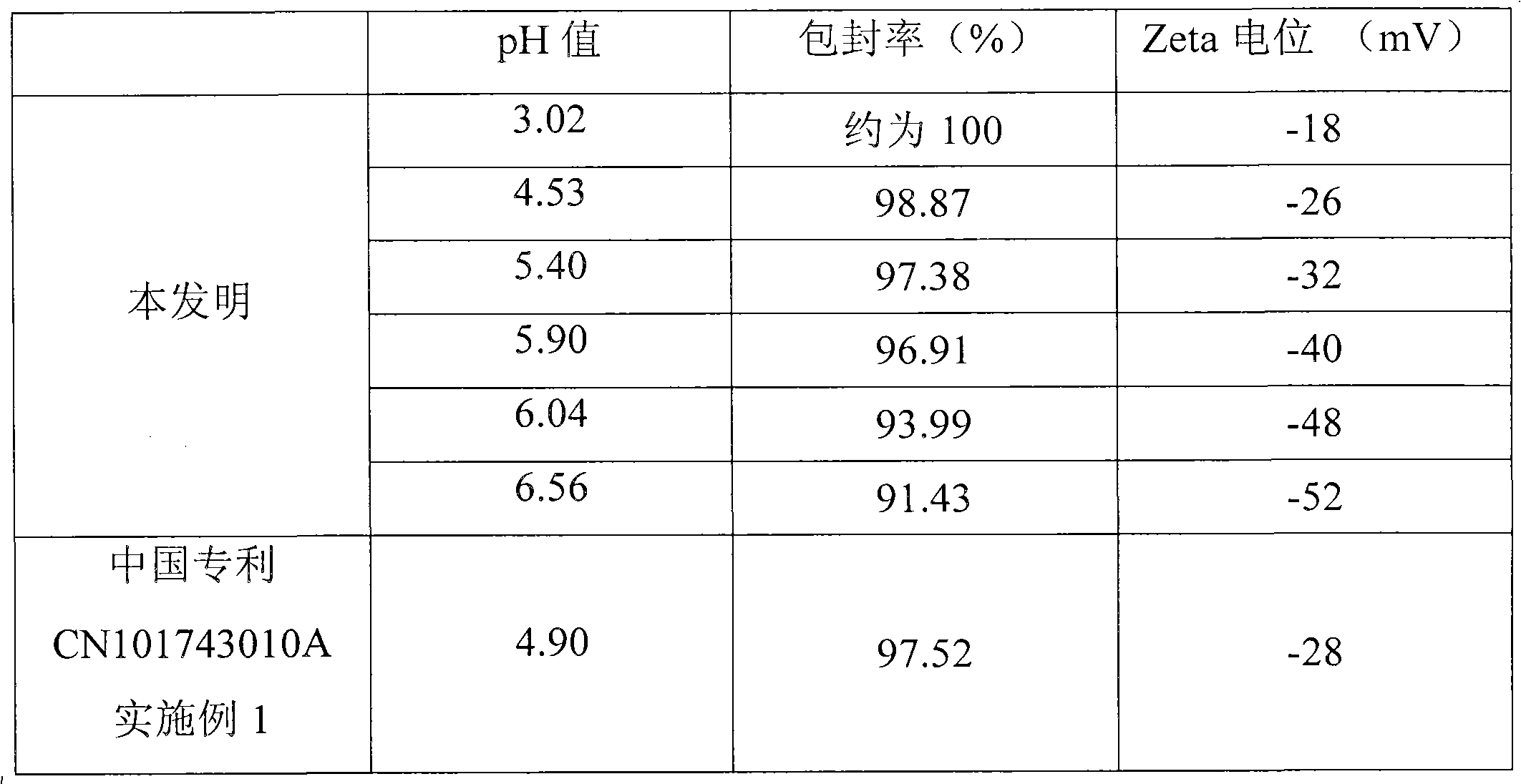
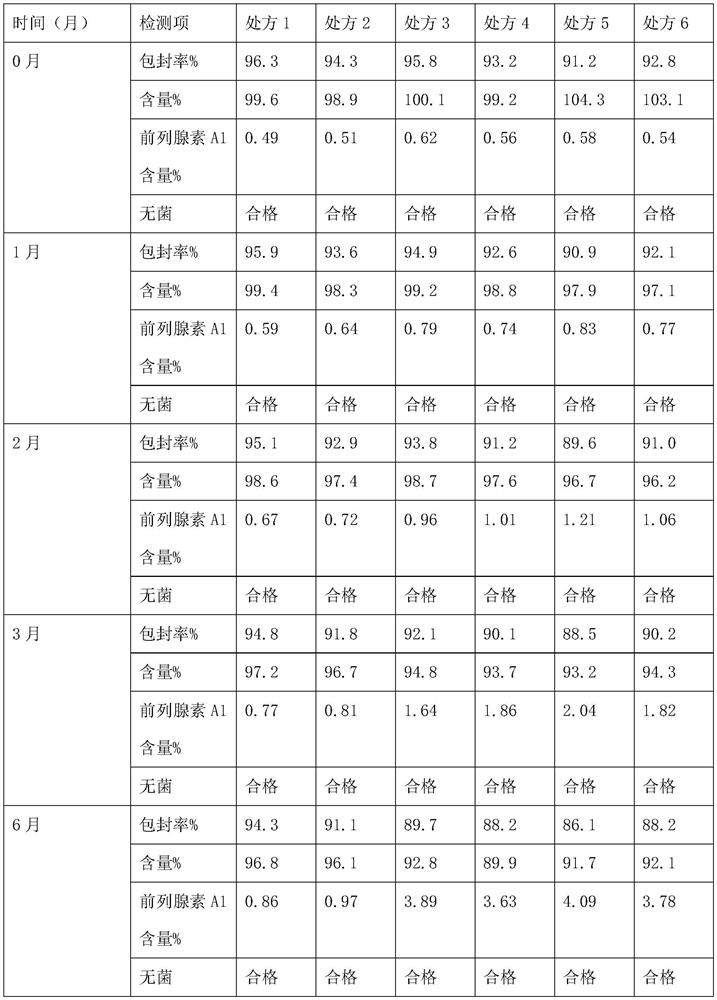
The invention relates to an alprostadil composition which comprises alprostadil, oil for injection, phospholipid and water for injection, wherein the concentration of alprostadil in the formula is 6-11 micrograms / ml, preferably 7-9 micrograms / ml, the concentration of oil for injection is 100mg / ml and the concentration of phospholipid is 18mg / ml. The encapsulation efficiency of the alprostadil injection prepared by the invention is over 95%.
Owner:JINAN LANDAN MEDICINE SCI & TECH CO LTD
Alprostadil injection and preparation method thereof
ActiveCN102178650AImprove securityImprove stabilityOrganic active ingredientsEmulsion deliveryGlycerolOil phase
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Alprostadil injection preparation
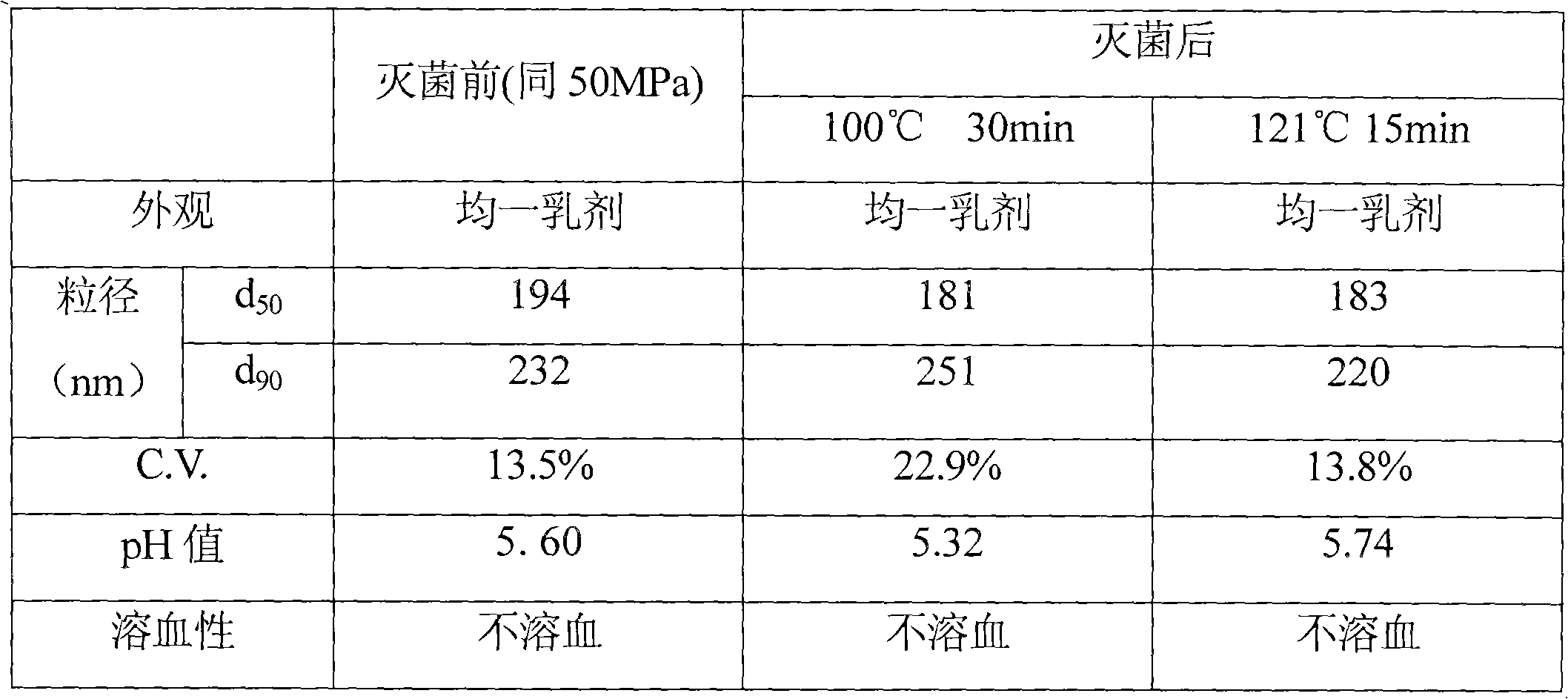
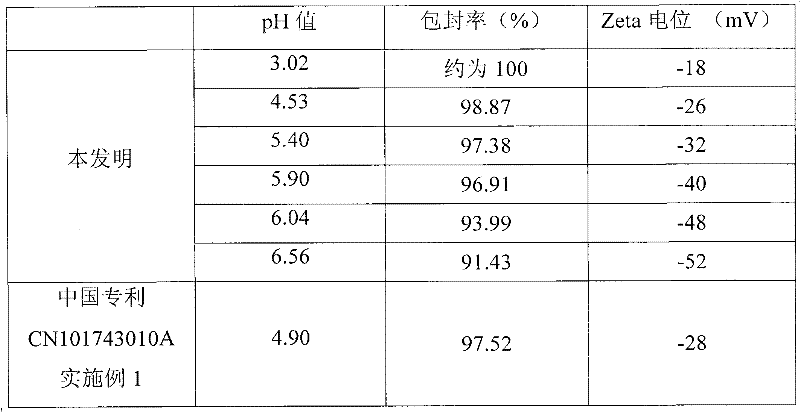
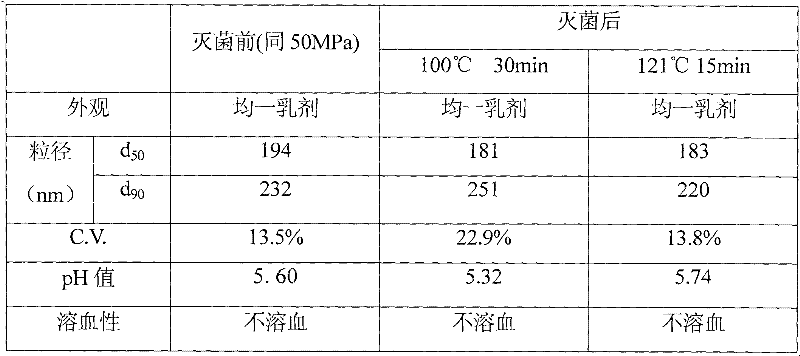
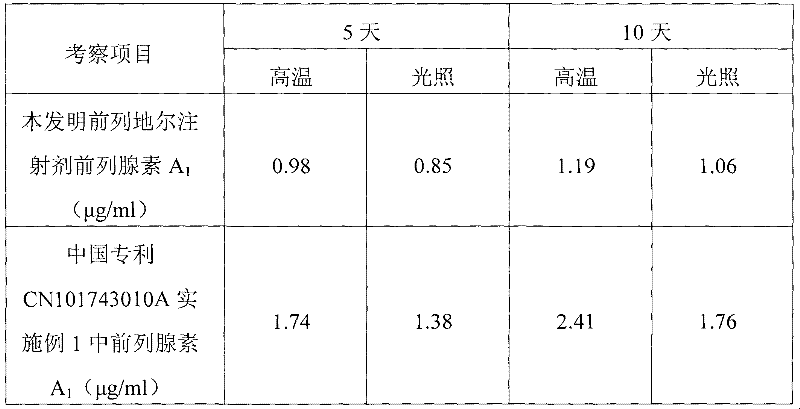
The invention belongs to the technical field of medicines and discloses an injection preparation prepared from alprostadil serving as a raw material. The injection preparation is characterized by comprising the following components in part by weight: 0.01 part of alprostadil, 200 parts of soybean oil, 36 parts of phospholipid, 50 parts of glycerin and 4.8 parts of oleic acid. Through a preferable test of a preparation process, the preparation process of adjusting the pH value of foremilk to be between 5.4 and 6.0 and treating the foremilk by using a nanomachine for 5 times under the pressure of 50MPa so as to prepare lipid microspheres with the average uniform particle size of between 100 and 280nm and performing flowing steam sterilization at the temperature of 121 DEG C for 15 minutes is determined. The preparation process has the advantages of high stability, small impurity quantity and the like.
Owner:HAINAN BIKAI PHARM CO LTD
Injection alprostadil fat emulsion and preparing method thereof
ActiveCN105012248AReduce security risksEasy to operateOrganic active ingredientsPowder deliveryFat emulsionsAlprostadil Injection
An alprostadil lipid emulsion for injection and a preparation method therefor. The lipid emulsion contains alprostadil, phosphatidylcholine, phosphatidylglycerol, oil for injection and a lyophilized protective agent. Based on 1 part by weight of the alprostadil, the content of the phosphatidylcholine is 1200 to 4000 parts by weight, the content of the phosphatidylglycerol is 12 to 120 parts by weight, the content of the oil for injection is 2000 to 20000 parts by weight, and the content of the lyophilized protective agent is 16000 to 60000 parts by weight.
Owner:内蒙古多肽科技有限公司
Sterilization technology of prostadil fatty emulsion and method of determination
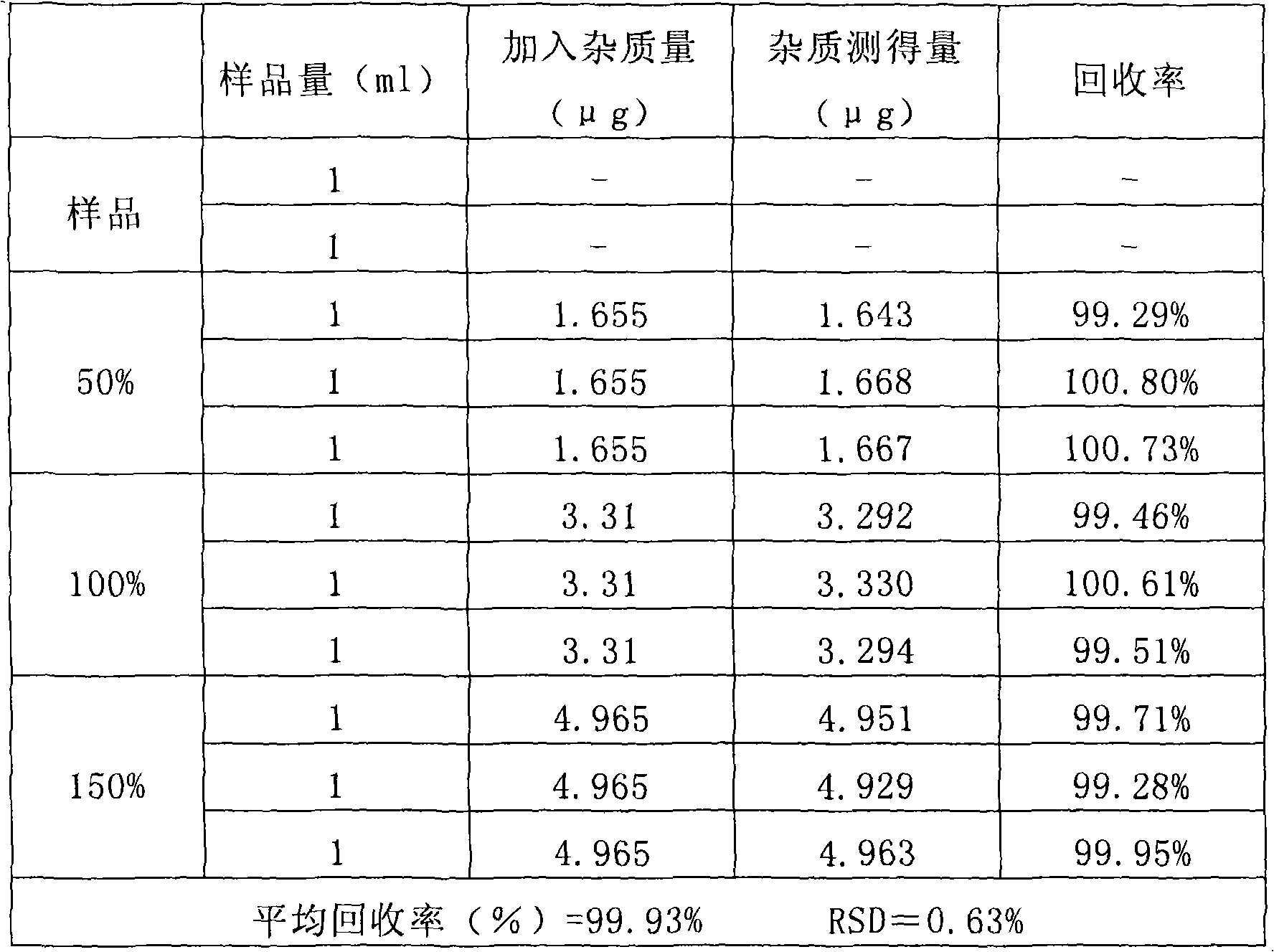
InactiveCN1823786AHigh recovery rateMeet sterility requirementsOrganic active ingredientsMetabolism disorderEmulsionMicrowave
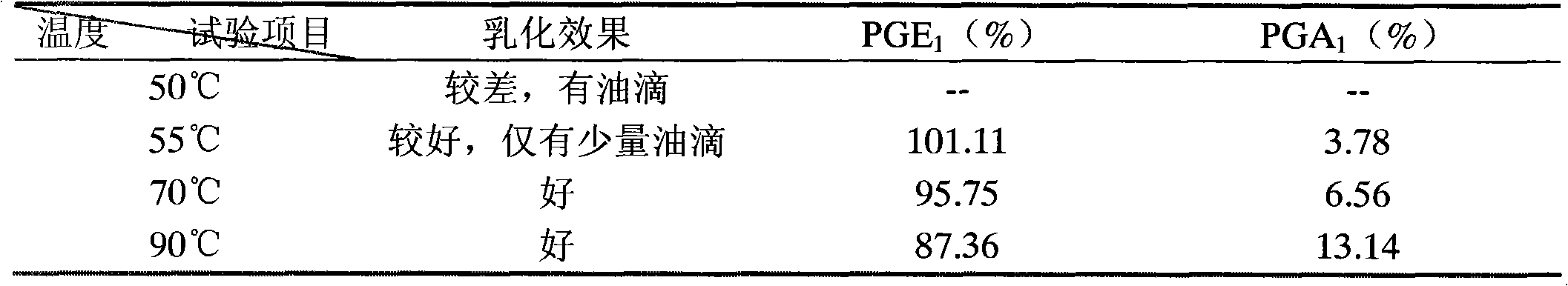
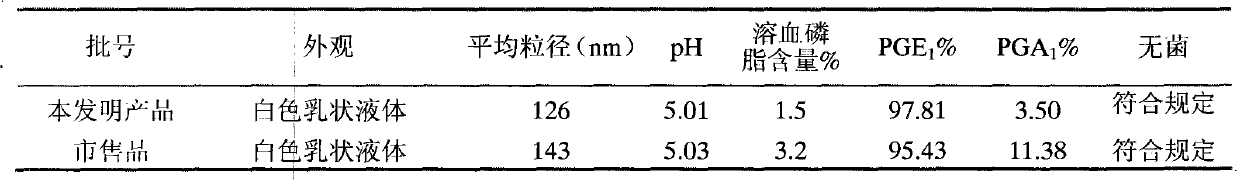
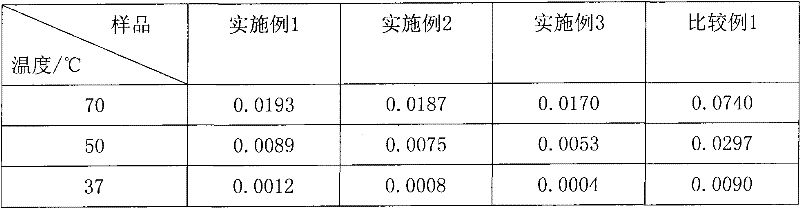
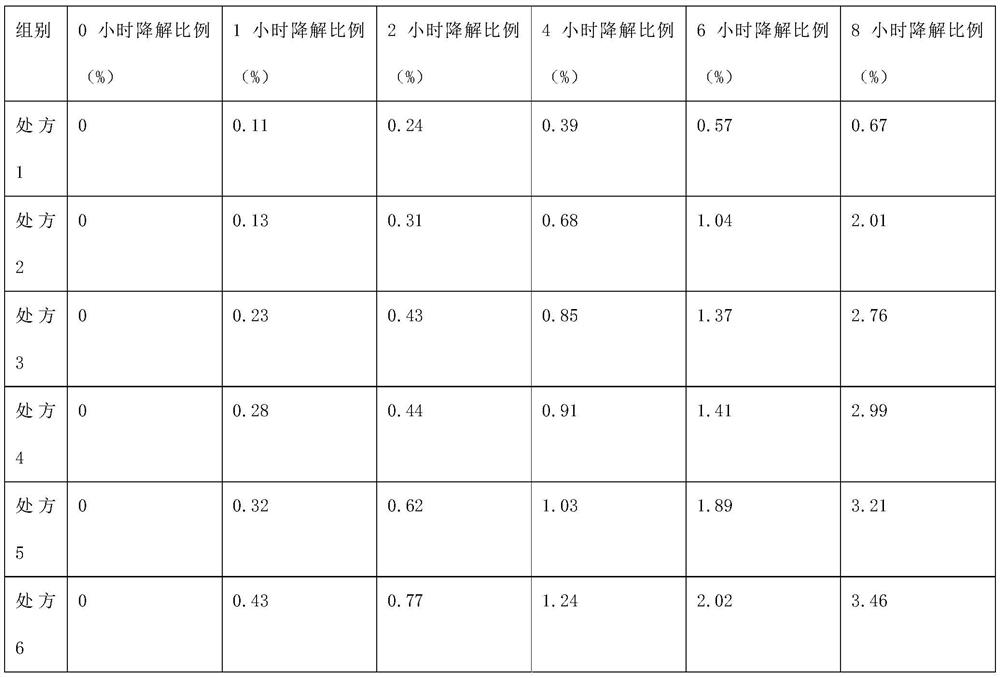
A bactericiding process for the fatty emulsion of prostaglandin E1 features that said fatty emulsion in the ampules is heated to 65-140 deg.C for 10s-5 min by microwaves at 915 or 2450 MH2. A method for measuring its main component PGE1 and impurity PGA1 includes such steps as freeze drying for demulsifying to obtain oily sample, extracting in organic solvent, and efficient liquid-phase chromatography at 204-214 nm in wavelength to measure PGE1 and PGA1.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Method for controlling quality of alprostadil injection
InactiveCN101581702AComponent separationPreparing sample for investigationIsoprostaglandin E1Silica gel
The invention discloses a method for measuring prostaglandin E1 and / or prostaglandin A1 in alprostadil fat emulsion injection, which comprises the following steps: (1) ultrasonically processing the alprostadil fat emulsion injection, obtaining an alprostadil solution of which the fat phase is removed; and (2) performing high performance liquid chromatography measurement to the alprostadil solution of which the fat phase is removed, obtaining the content of the prostaglandin E1 and / or prostaglandin A1, wherein the conditions of the high performance liquid chromatography measurement are as follows: stationary phase: octadecyl ether-bonded monolithic silica is filling agent, mobile phase: the ratio of phosphate buffer to acetonitrile is equal to 1-6:1, and the detection wavelength is 278 nm. The method can accurately measure the alprostadil and the degradation products thereof in the alprostadil fat emulsion injection.
Owner:上海万特医药科技(集团)有限公司
Prostaglandin E1 lipid microsphere injection with charge effect and preparation method thereof
InactiveCN101496787BGood physiological compatibilitySolve the problem of high doseOrganic active ingredientsDigestive systemLipid formationChemical structure
Owner:李淑斌
A kind of alprostadil injection preparation and preparation method thereof
The invention provides an alprostadil injection preparation and a preparation method thereof. The alprostadil injection preparation is prepared from alprostadil, dioleoyl phosphatidylglycerole, cholesterol, polyethylene glycol 2000 and trehalose. Compared with the prior art, the preparation greatly improves the stability and bioavailability of the preparation, drug release is stable, the quality of the preparation product is improved, and the curative effect is more significant.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Content detection method of alprostadil injection
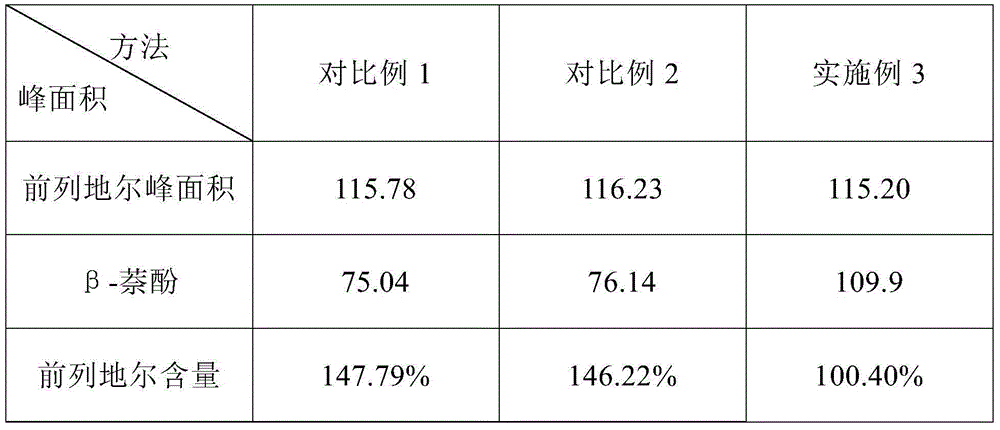
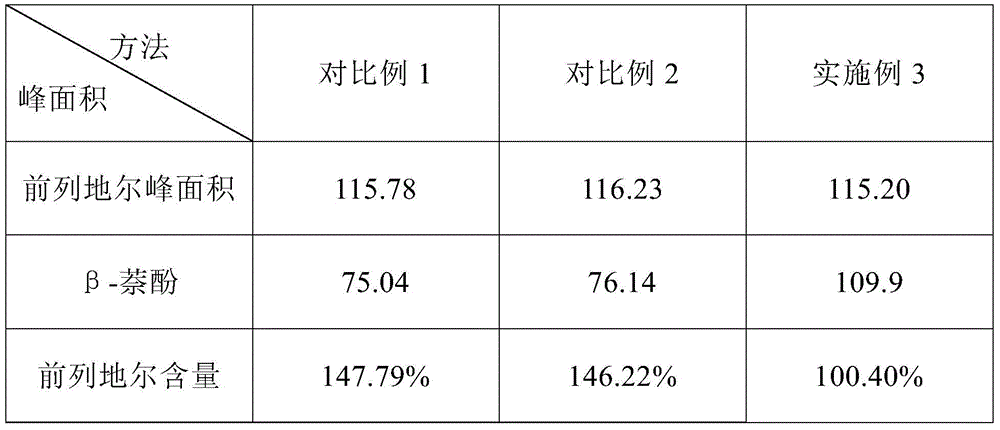
ActiveCN104614455ASolve the problem of β-naphthol peak area reductionHigh precisionComponent separationPost column derivatizationInternal standard
The invention relates to a content detection method of an alprostadil injection. The content detection method of the alprostadil injection comprises the following steps: preparing a beta-naphthol internal standard solution; preparing beta-naphthol internal standard solutions with the same concentration from a test solution and a reference solution, then carrying out column separation on alprostadil by using a high performance liquid chromatography, taking 1 mol / L of a KOH solution as a reaction solution by using a post-column derivatization method, controlling the temperature of a reaction tank to be 60 DEG C, finally detecting alprostadil and beta-naphthol peak area on a wavelength of 278 nm, and calculating the content of alprostadil by using an internal standard method. By adopting the method provided by the invention to detect the content of the alprostadil injection, the problem of small beta-naphthol peak area in the existing method for detecting the content of the alprostadil injection is effectively solved, the detection accuracy of the content of the alprostadil injection is improved, and the content detection method of the alprostadil injection is applicable to detection of the content of the alprostadil injection in different prescriptions.
Owner:SHANGHAI JINGFENG PHARMA
Alprostadil injection and preparation method thereof
InactiveCN105287376AOvercome the defects of traditional emulsification processUniform particle size distributionOrganic active ingredientsEmulsion deliverySize changeAlprostadil Injection
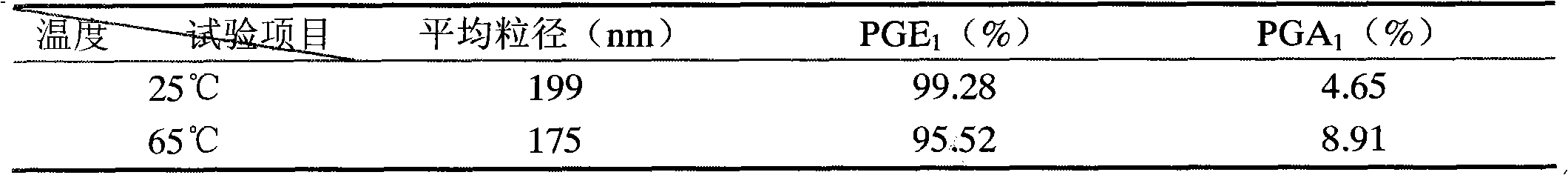
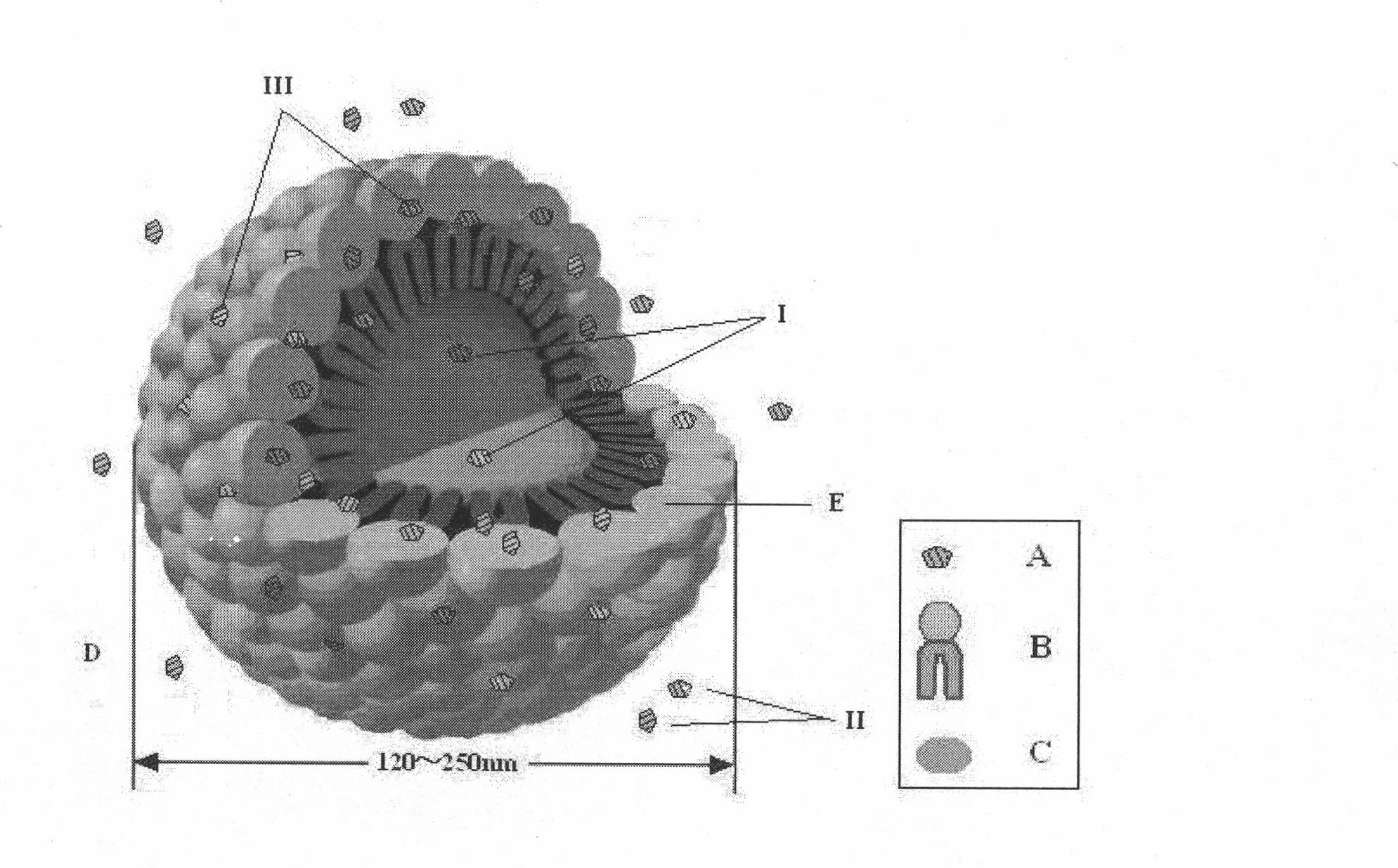
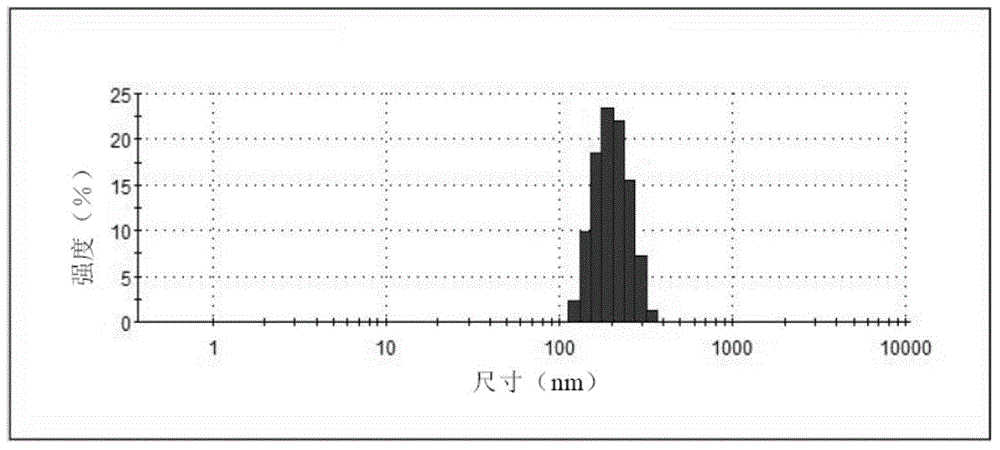
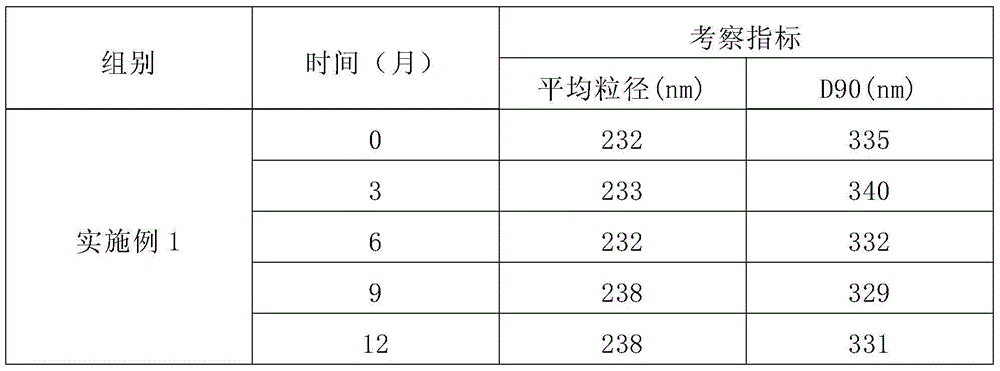
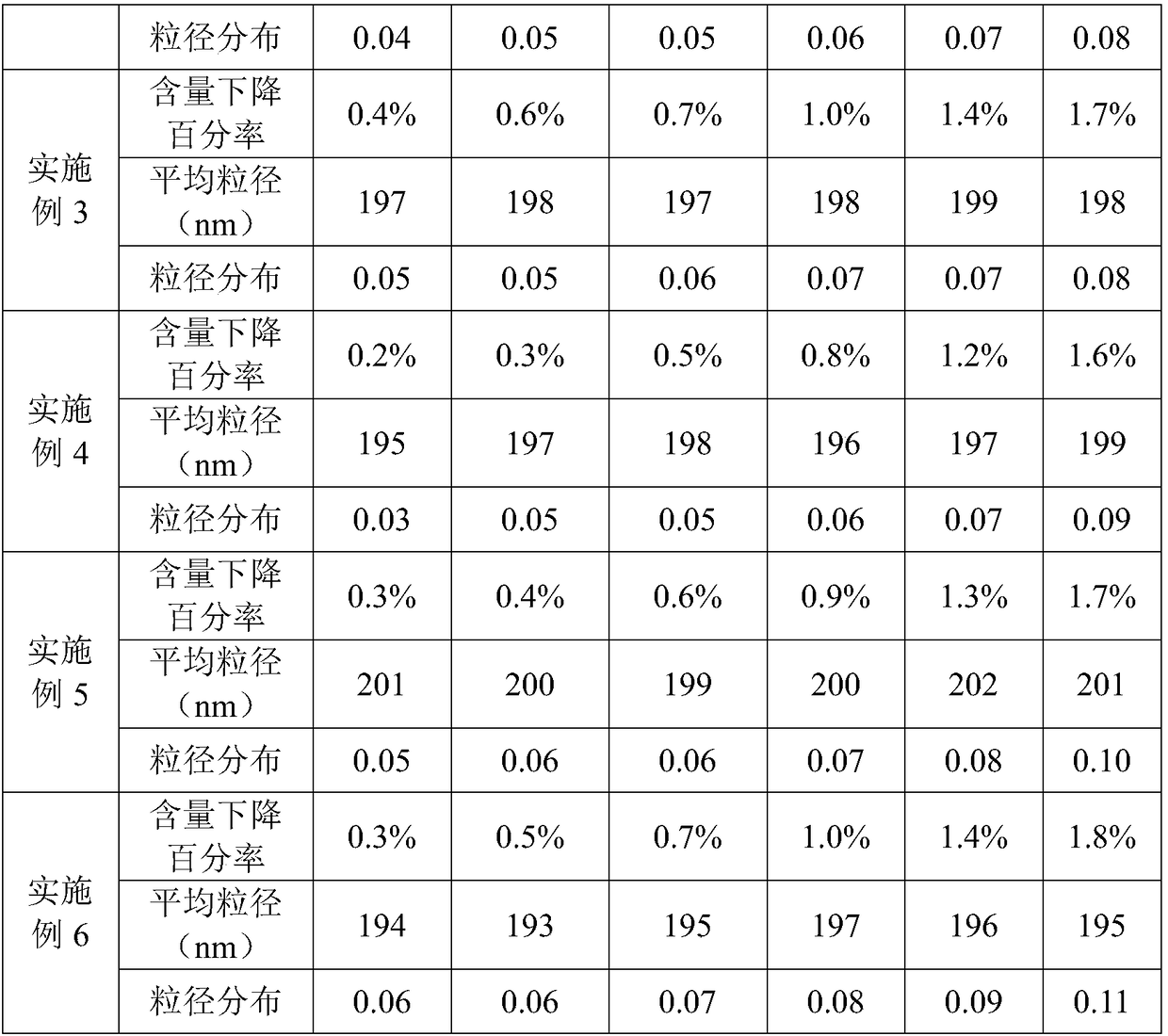
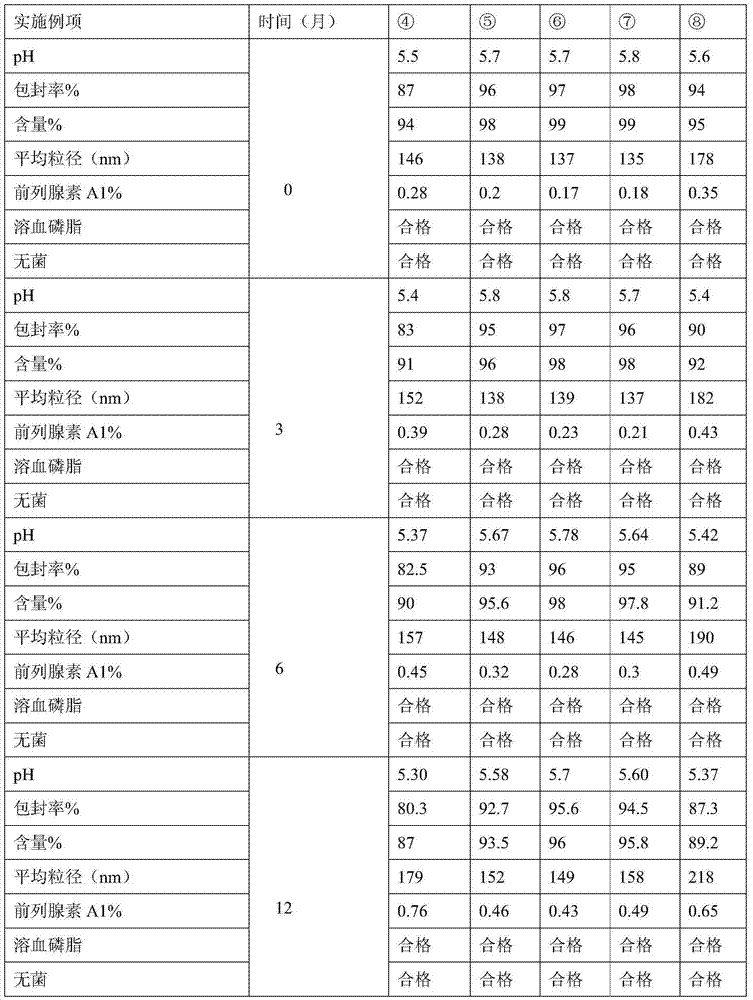
The invention provides an alprostadil injection and a preparation method thereof. The alprostadil injection is composed of alprostadil, oil for injection, an oleophylic emulsifying agent, an isotonic agent for injection and a stabilizing agent. The alprostadil injection contains alprostadil particles with an average particle size being 100-300nm and D90 being 150-350nm, and particle size change of the alprostadil injection is smaller than or equal to 10% after the alprostadil injection is placed for 12 months at the temperature of 2-8 DEG C.
Owner:SHANGHAI SINE PHARMA LAB
Preparation method of alprostadil injection
ActiveCN101627968BGood lookingReduce contentOrganic active ingredientsDigestive systemMedicineAlprostadil Injection
The invention relates to a preparation method of an alprostadil injection. The alprostadil injection consists of alprostadil, oil for injection, emulsifier, stabilizer and other agents, pH regulator and water for injection. Compared with the commercially available products, the quality standard of the prepared product is significantly improved, and the safety of clinical medication is increased.
Owner:辽宁中海康生物制药股份有限公司
Stable alprostadil injection emulsion and preparation method thereof
InactiveCN101474150BGood chemical stabilityReduce metabolic inactivationOrganic active ingredientsRespiratory disorderEmulsionBioavailability
The invention discloses a stable alprostadil injection latex which takes alprostadil as active ingredient, contains oil for injection, emulsifier, glycerin for injection and water for injection which are accepted by pharmacy, and is prepared by emulsification technology. Compared with the existing alprostadil injection latex on the market, the stable alprostadil injection latex prepared by the invention can remarkably enhance the thermal stability of the alprostadil, prolong the period of validity of the alprostadil injection latex while reducing the degradation of the alprostadil at the lungand being beneficial to improving the bioavailability, thus further improving the drug effect.
Owner:四川思达康药业有限公司 +1
Alprostadil injection
ActiveCN103110579BImprove stabilitySimple preparation processOrganic active ingredientsEmulsion deliveryOrganic solventPhospholipid
The invention belongs to the field of a pharmaceutical preparation, and in particular relates to an alprostadil injection. The injection consists of 0.001-0.05mg / ml of alprostadil, 0.01-100mg / ml of an oil phase, 10-500mg / ml of phospholipid and the balance of an organic solvent. The injection disclosed by the invention is constantly an anhydrous system in storage process, and is used right as long as being ready, so that the injection can prevent oxidization reaction of the phospholipid and degradation reaction of the medicine, and can improve the stability of the alprostadil injection. An alprostadil composition provided by the invention can prepare the alprostadil injection which is excellent in stability, and the preparation process is quite simple.
Owner:北京德立英捷医药科技有限公司
Preparation method of alprostadil injection
ActiveCN107184550ALarge particle sizeHigh affinityOrganic active ingredientsMetabolism disorderYolkSide effect
The invention relates to a preparation method of an alprostadil injection. The preparation method comprises the following steps: adding egg yolk lecithin and polyethylene glycol 2000 into an organic solvent to dissolve, adding alprostadil to stir and dissolve, decompressing and drying to obtain alprostadil compound powder, and further preparing into an emulsion injection. Active ingredients have high affinity with an oil phase, the alprostadil is enabled to be wrapped in emulsion stably, at the same time the metabolic inactivation of the alprostadil injection emulsion in alkaline environment is reduced, so that the stability and safety of drugs are improved, and the toxic and side effects of the drugs are reduced.
Owner:FUBICHENG SHANGHAI PHARMA TECH CO LTD
A kind of alprostadil fat emulsion for injection and preparation method thereof
ActiveCN105012248BReduce security risksEasy to operatePowder deliveryOrganic active ingredientsFat emulsionAlprostadil Injection
An alprostadil lipid emulsion for injection and a preparation method therefor. The lipid emulsion contains alprostadil, phosphatidylcholine, phosphatidylglycerol, oil for injection and a lyophilized protective agent. Based on 1 part by weight of the alprostadil, the content of the phosphatidylcholine is 1200 to 4000 parts by weight, the content of the phosphatidylglycerol is 12 to 120 parts by weight, the content of the oil for injection is 2000 to 20000 parts by weight, and the content of the lyophilized protective agent is 16000 to 60000 parts by weight.
Owner:内蒙古多肽科技有限公司
Sterilization technology of prostadil fatty emulsion and method of determination
InactiveCN100551374CMeet sterility requirementsHigh recovery rateOrganic active ingredientsMetabolism disorderFreeze-dryingMicrowave irradiation
The invention relates to a sterilization process of alprostadil fat emulsion. Use 915MHz or 2450MHz frequency microwave equipment to irradiate the 1ml and 2ml ampoule alprostadil injection placed in the microwave equipment to raise the temperature of the ampoule to 65-140°C and maintain it for 10 seconds to 5 minutes for sterilization , can completely kill the microorganisms in the preparation. The determination of the main drug PGE1 and impurity PGA1 in the preparation adopts the method of freeze-drying to de-emulsify, so that the sample becomes an oily substance, and then extracts the oily substance with an organic solvent to obtain a test solution. At the place, PGE1 and PGA1 were determined according to the external standard method or internal standard method. The invention effectively reduces the main drug degradation of the alprostadil fat emulsion caused by the traditional sterilization process.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Alprostadil injection solution
InactiveCN108653208AImprove stabilityLess irritatingOrganic active ingredientsPeptide/protein ingredientsOctreotide acetateMedicine
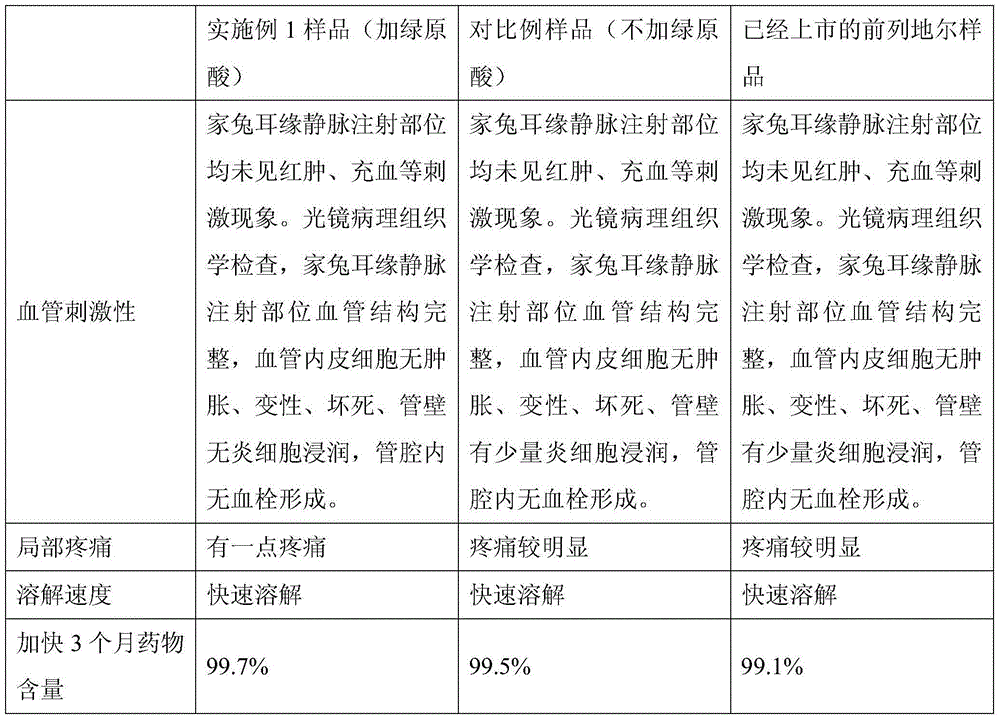
The invention provides an alprostadil injection solution, which consists of alprostadil, phospholipid, injection oil and injection water, wherein the contents of the various components are as follows:4-8[mu]g / ml of the alprostadil, 4-20mg / ml of the phospholipid and 100-300mg / ml of the injection oil, wherein the phospholipid consts of phosphatidylcholine PC and phosphatidyl glycerol PG at the ratio of (95.5-99.7) to 0.3, and the phospholipid also consists of tocopherol in terms of the total amount of the phospholipid. The alprostadil injection solution provided by the invention can achieve good compatibility stability with an octreotide acetate injection solution; and it is accidentally discovered that the alprostadil injection solution prepared by the invention can obviously reduce vascular stimulation and injection pain.
Owner:BEIJING LANDAN PHARMA TECH
A kind of preparation method of alprostadil injection
ActiveCN107184550BLarge particle sizeHigh affinityOrganic active ingredientsMetabolism disorderBiotechnologyPolyethylene glycol
The invention relates to a preparation method of an alprostadil injection. The preparation method comprises the following steps: adding egg yolk lecithin and polyethylene glycol 2000 into an organic solvent to dissolve, adding alprostadil to stir and dissolve, decompressing and drying to obtain alprostadil compound powder, and further preparing into an emulsion injection. Active ingredients have high affinity with an oil phase, the alprostadil is enabled to be wrapped in emulsion stably, at the same time the metabolic inactivation of the alprostadil injection emulsion in alkaline environment is reduced, so that the stability and safety of drugs are improved, and the toxic and side effects of the drugs are reduced.
Owner:FUBICHENG SHANGHAI PHARMA TECH CO LTD
Alprostadil injection and preparation method thereof
ActiveCN102178650BImprove qualitySimple production processOrganic active ingredientsEmulsion deliveryGlycerolOil phase
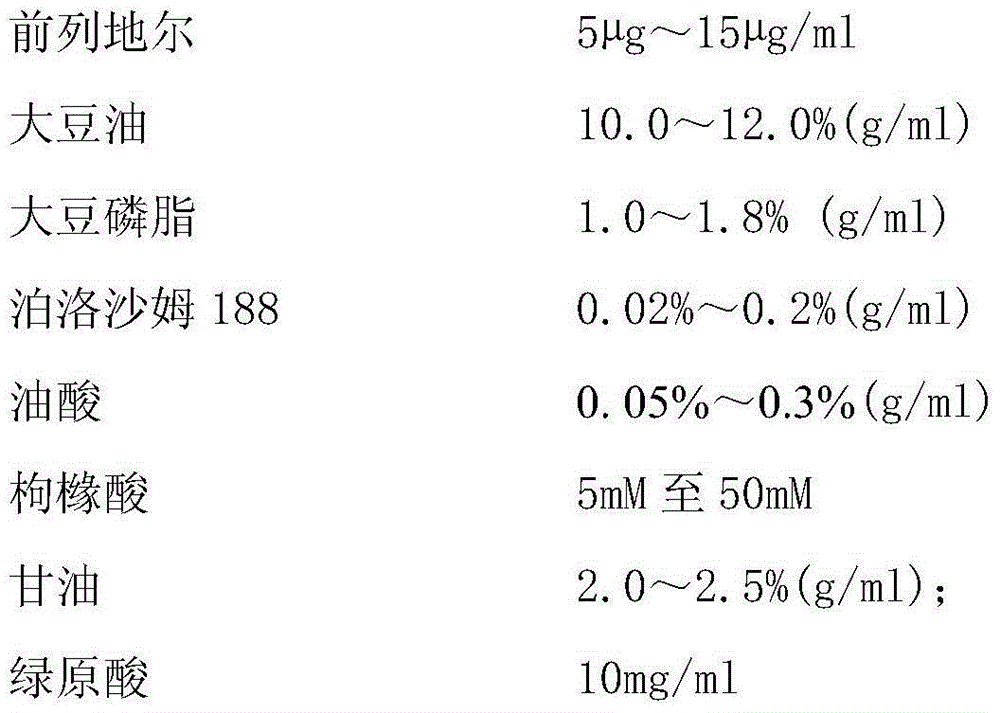
The invention relates to an alprostadil injection and a preparation method thereof. The alprostadil injection has a prescription as follows: a, a main drug is alprostadil with concentration of 5mu g-15mu g / ml; b, an oil phase is soybean oil with concentration of respectively 10.0-12.0 percent; c, soyabean lecithin and poloxamer 188 are selected as emulsifying agents and have concentrations of respectively 1.0-1.8 percent and 0.02-0.2 percent; d, citric acid is adopted as a pH regulating agent with concentration of 5nM to 50Mm and pH of 5.0-6.0; e, glycerol is adopted as an osmotic pressure regulating agent with concentration of respectively 2.0-2.5 percent; and f, the balance is injection water.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Alprostadil injection and preparation method thereof
ActiveCN109820820AIncreased release rateImprove stabilityOrganic active ingredientsMetabolism disorderBioavailabilityAlprostadil Injection
The invention discloses a preparation method of an alprostadil injection. The method comprises the following steps: preparing an alprostadil inclusion compound; preparing alprostadil inclusion compound liposome suspension liquid; preparing an alprostadil inclusion compound liposome; preparing an alprostadil injection. In addition, the invention also discloses the alprostadil injection obtained bythe method. The alprostadil injection is small in average particle size and narrow in particle size distribution; meanwhile, high encapsulation efficiency can be realized; moreover, the alprostadil injection has a slow release effect and can obviously improve the bioavailability.
Owner:浙江长典药物技术开发有限公司
Alprostadil injection and preparation method thereof
ActiveCN103599066BHigh encapsulation efficiencyHigh content of the main drugOrganic active ingredientsNervous disorderMedicineOleic Acid Triglyceride
The invention provides an alprostadil injection. Every 1000ml of alprostadil injection comprises the following raw materials: 5mg of alprostadil, 90-110g of soybean oil, 15-20g of phosphatide, 2-3g of oleic acid, 22.1-25g of isoosmotic agent, and appropriate pH modifier, wherein the content of the pH modifier is enough to regulate the pH of the injection to be 5.0-6.0. The invention also provides a preparation method for the alprostadil injection. Compared with the prior art, the alprostadil injection prepared by the method has higher encapsulation efficiency and content of main drug, contains fewer related substances, has good stability, and provides safety guarantee for clinical medication.
Owner:SICHUAN KELUN PHARMA CO LTD
Alprostadil injection preparation
The invention belongs to the technical field of medicines and discloses an injection preparation prepared from alprostadil serving as a raw material. The injection preparation is characterized by comprising the following components in part by weight: 0.01 part of alprostadil, 200 parts of soybean oil, 36 parts of phospholipid, 50 parts of glycerin and 4.8 parts of oleic acid. Through a preferabletest of a preparation process, the preparation process of adjusting the pH value of foremilk to be between 5.4 and 6.0 and treating the foremilk by using a nanomachine for 5 times under the pressure of 50MPa so as to prepare lipid microspheres with the average uniform particle size of between 100 and 280nm and performing flowing steam sterilization at the temperature of 121 DEG C for 15 minutes is determined. The preparation process has the advantages of high stability, small impurity quantity and the like.
Owner:HAINAN BIKAI PHARM CO LTD
Alprostadil injection preparation and preparation method thereof
The invention provides an alprostadil injection preparation and a preparation method thereof. The alprostadil injection preparation is prepared from alprostadil, dioleoyl phosphatidylglycerole, cholesterol, polyethylene glycol 2000 and trehalose. Compared with the prior art, the preparation greatly improves the stability and bioavailability of the preparation, drug release is stable, the quality of the preparation product is improved, and the curative effect is more significant.
Owner:GUANGZHOU YIPINHONG PHARMA +4
Medicine composition containing alprostadil and preparation method of medicine composition
The invention relates to a medicine composition containing alprostadil and a preparation method of the medicine composition. The prescription of the composition containing alprostadil injection comprises the following components: balance of water for injection.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Medicine composition containing alprostadil and preparation method of medicine composition
ActiveCN103690484BOrganic active ingredientsDigestive systemMedical prescriptionAlprostadil Injection
The invention relates to a medicine composition containing alprostadil and a preparation method of the medicine composition. The prescription of the composition containing alprostadil injection comprises the following components: balance of water for injection.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
A kind of detection method of alprostadil injection
The invention discloses a detection method of alprostadil injection. The method comprises: taking 20 μL of alprostadil reference substance solution for HPLC sample analysis; Used for HPLC injection analysis; calculate the encapsulation efficiency of alprostadil injection according to the external standard method. The detection method of the invention has high precision, good reproducibility and good stability, and can accurately measure the encapsulation efficiency of the alprostadil injection.
Owner:浙江长典药物技术开发有限公司
A kind of content detection method of alprostadil injection
ActiveCN104614455BSolve the problem of β-naphthol peak area reductionHigh precisionComponent separationPost column derivatizationInternal standard
The invention relates to a content detection method of an alprostadil injection. The content detection method of the alprostadil injection comprises the following steps: preparing a beta-naphthol internal standard solution; preparing beta-naphthol internal standard solutions with the same concentration from a test solution and a reference solution, then carrying out column separation on alprostadil by using a high performance liquid chromatography, taking 1 mol / L of a KOH solution as a reaction solution by using a post-column derivatization method, controlling the temperature of a reaction tank to be 60 DEG C, finally detecting alprostadil and beta-naphthol peak area on a wavelength of 278 nm, and calculating the content of alprostadil by using an internal standard method. By adopting the method provided by the invention to detect the content of the alprostadil injection, the problem of small beta-naphthol peak area in the existing method for detecting the content of the alprostadil injection is effectively solved, the detection accuracy of the content of the alprostadil injection is improved, and the content detection method of the alprostadil injection is applicable to detection of the content of the alprostadil injection in different prescriptions.
Owner:SHANGHAI JINGFENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com