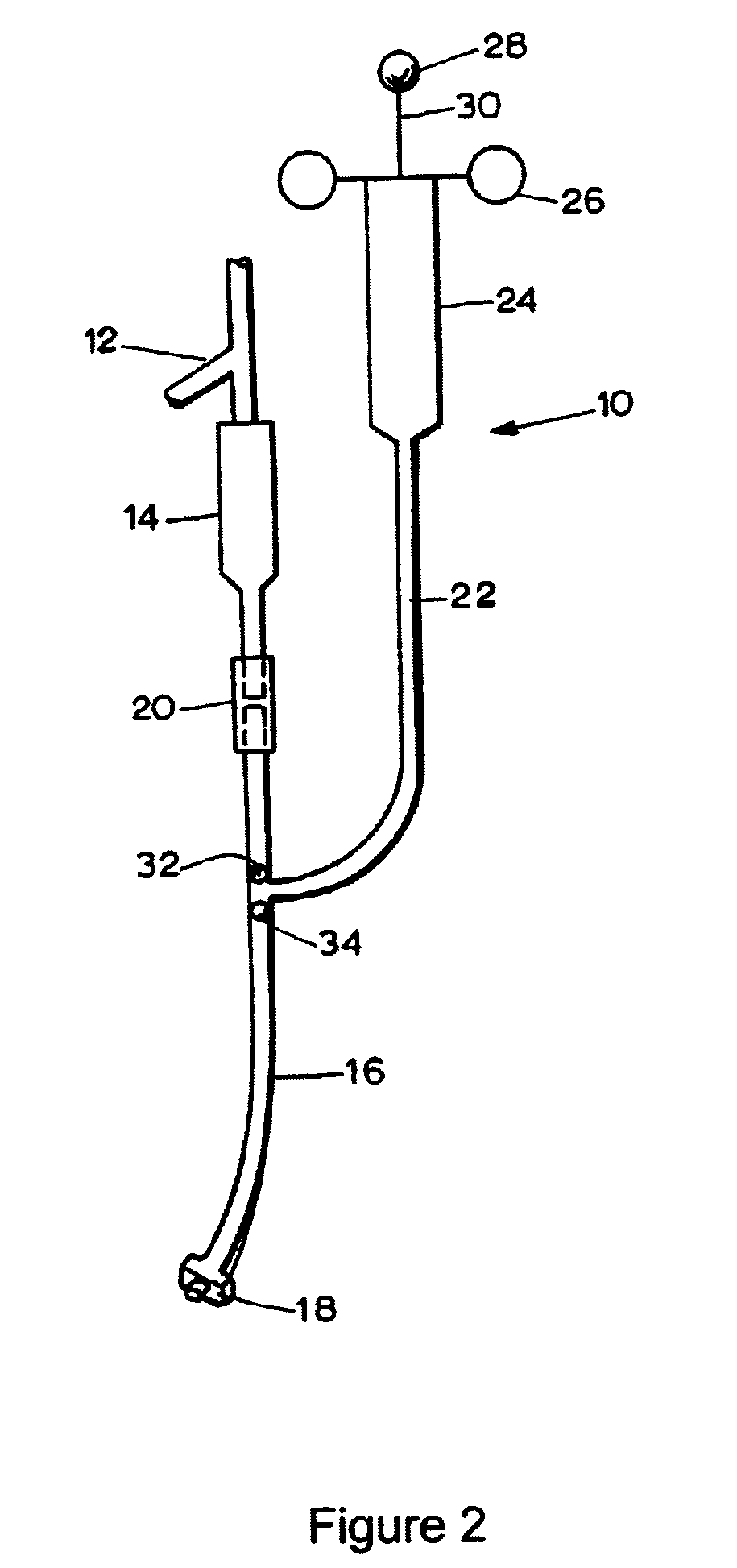
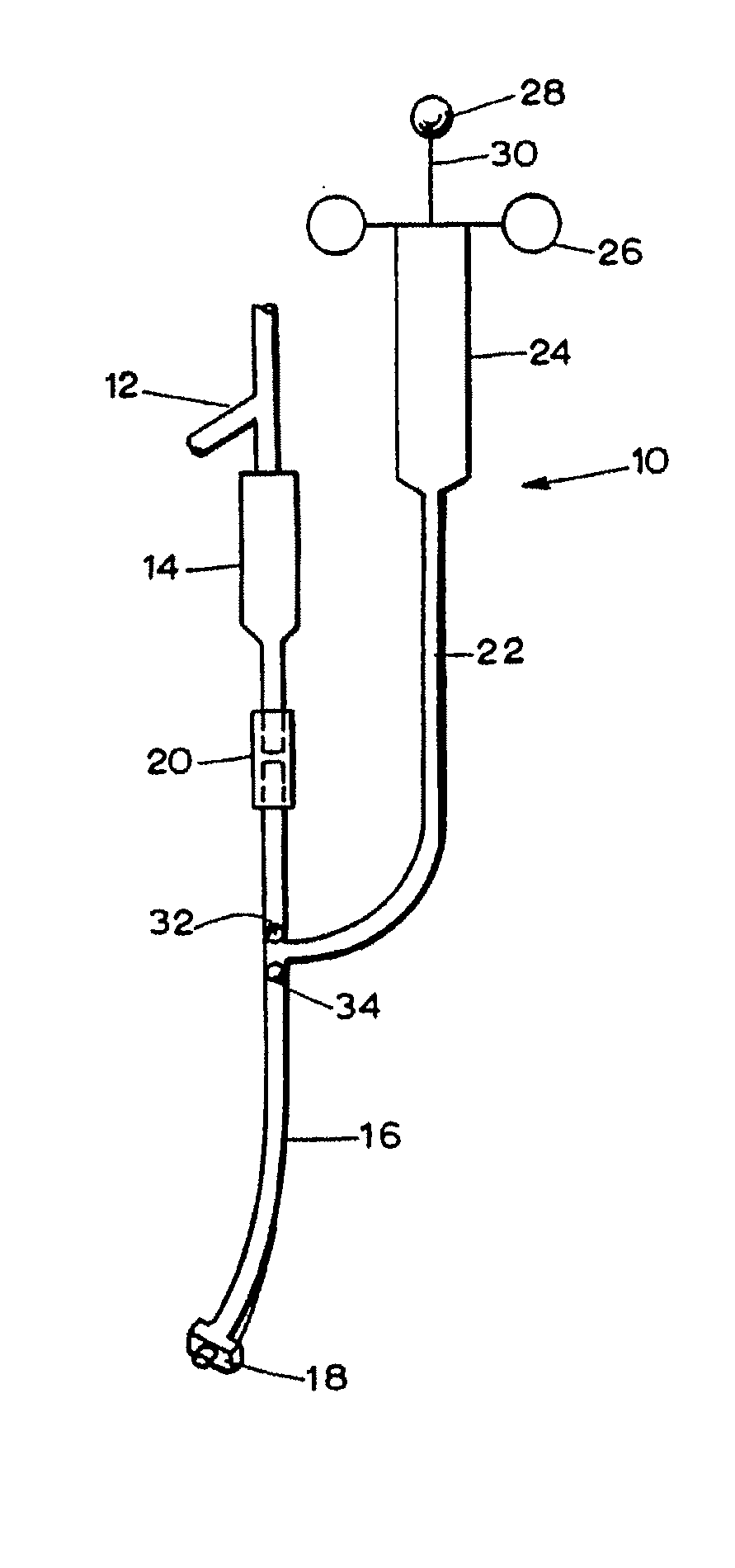
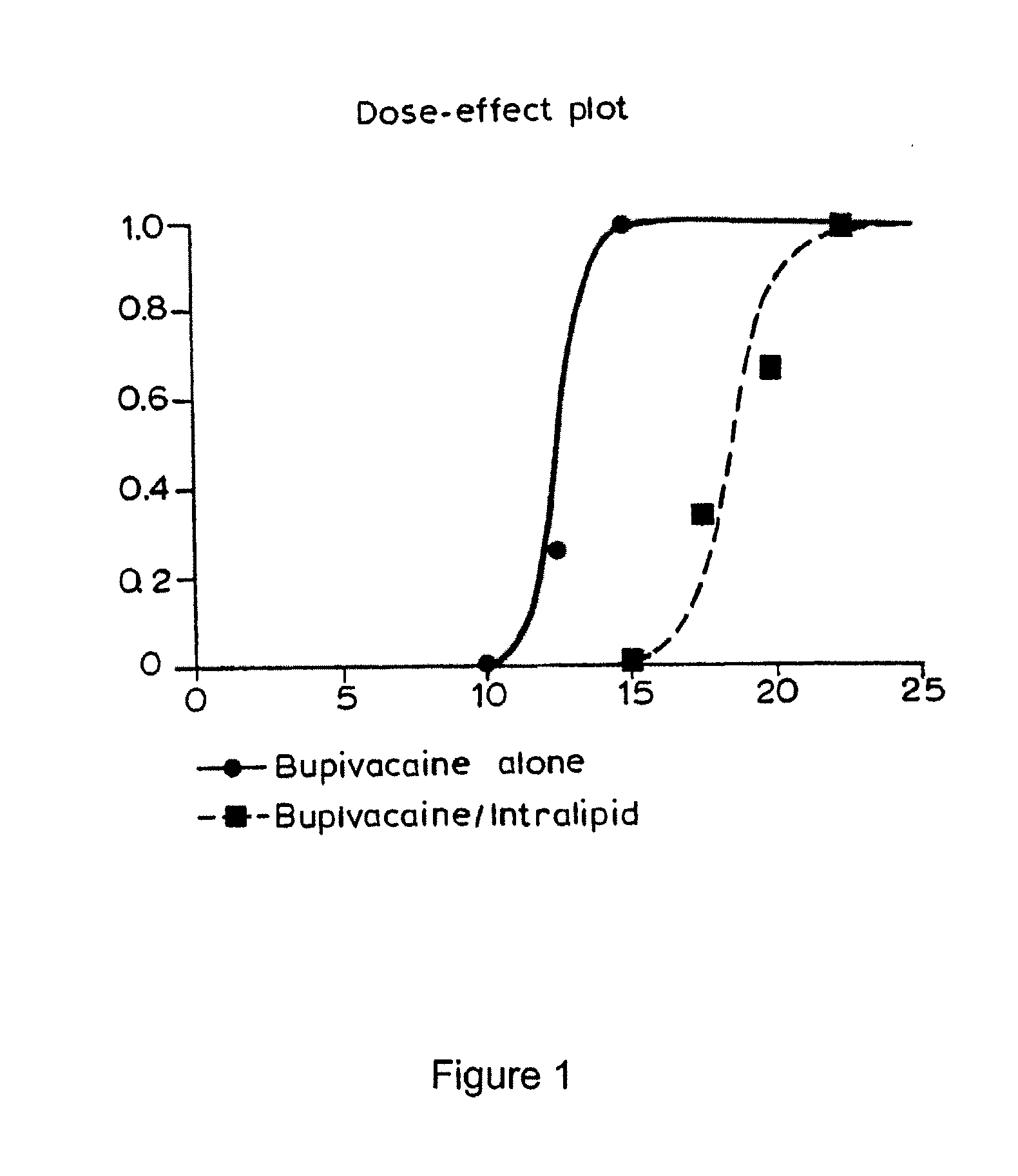
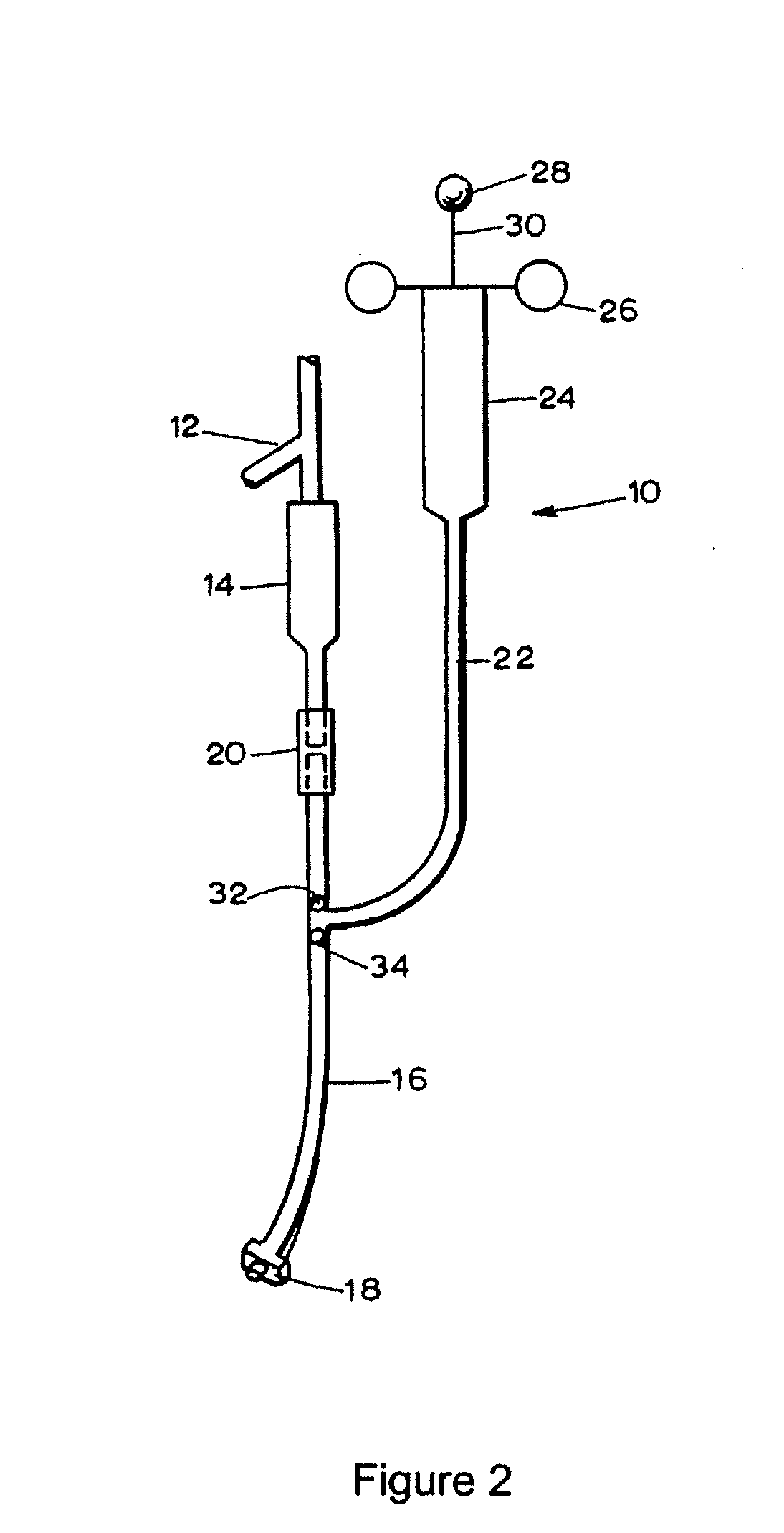
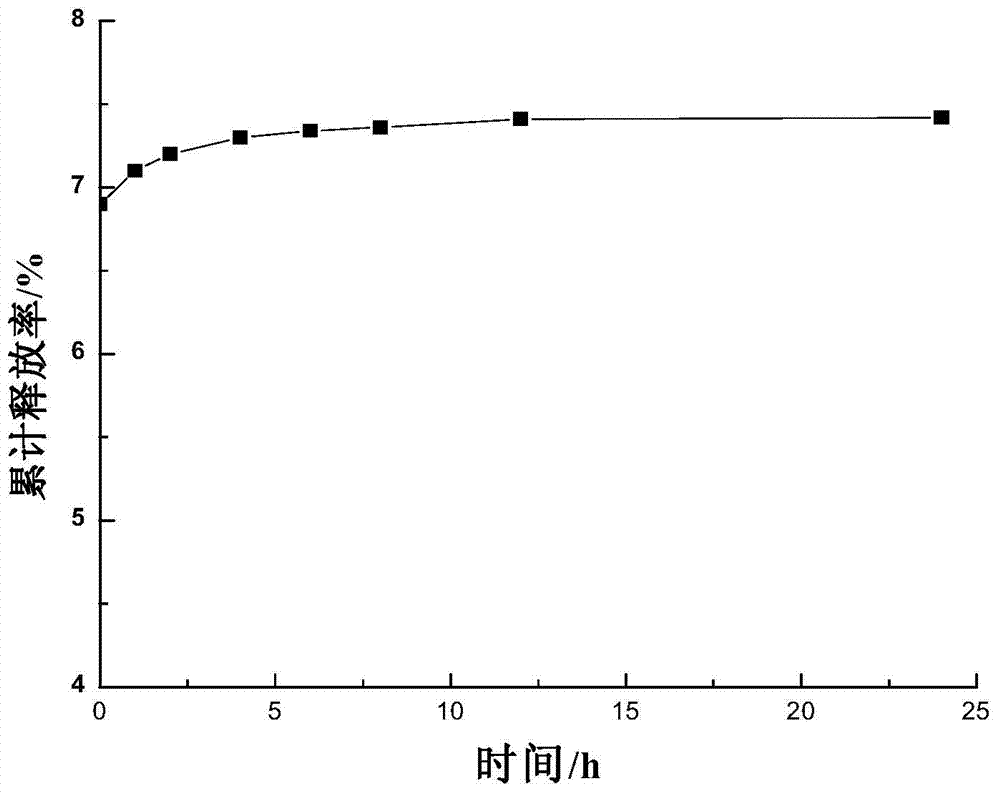
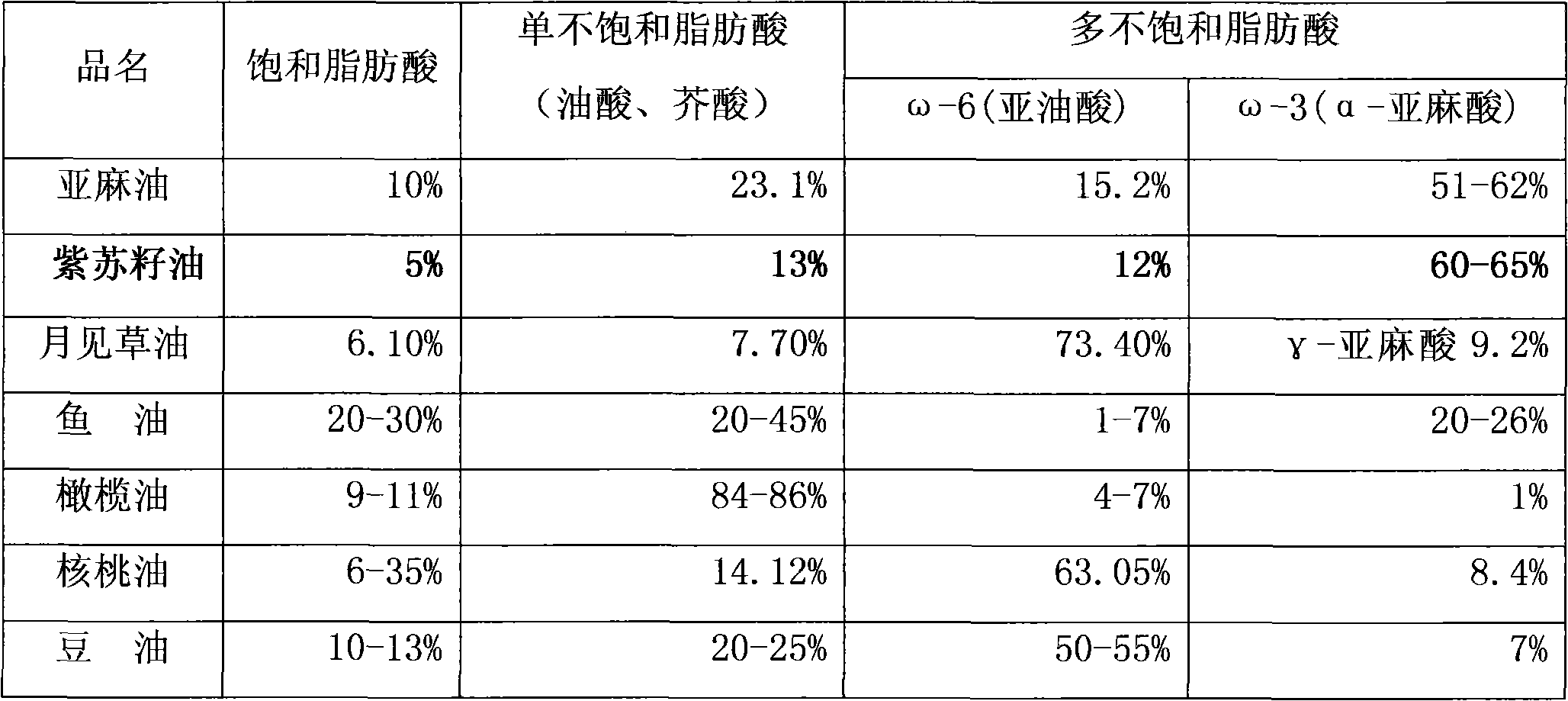
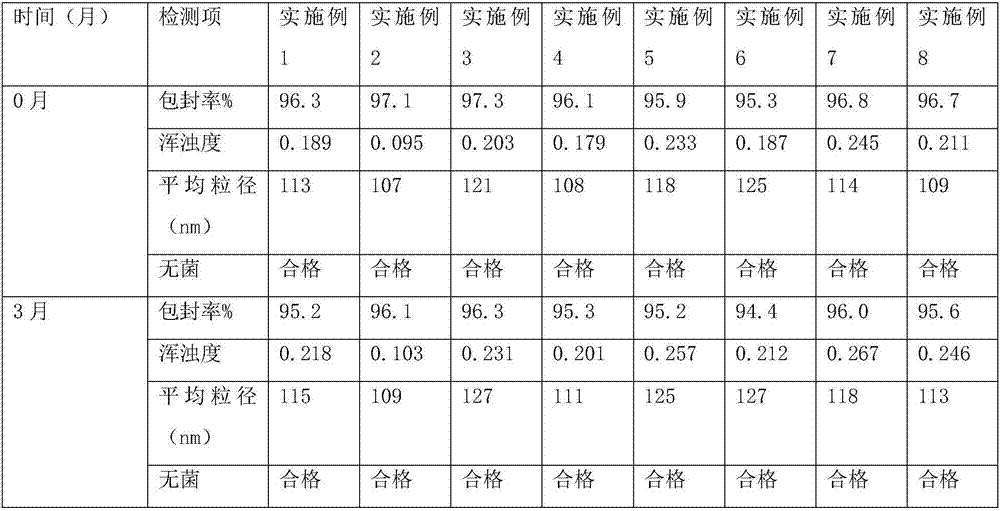
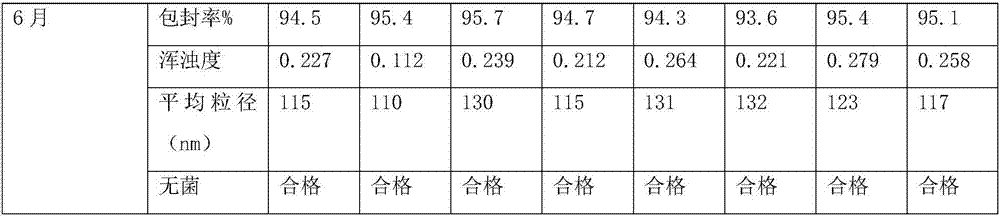
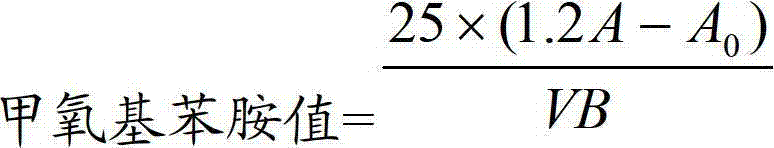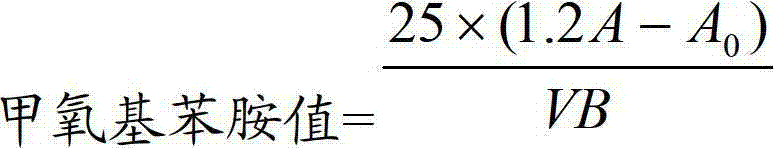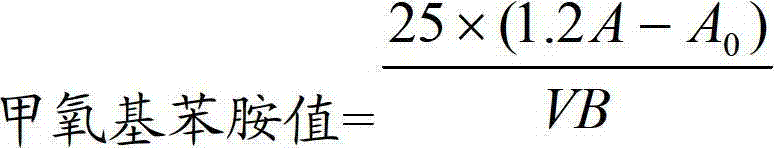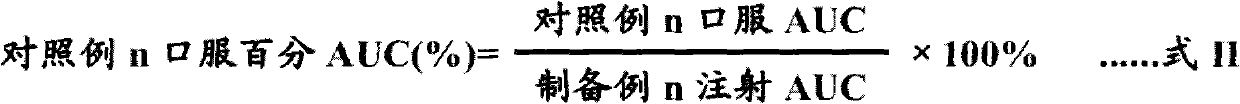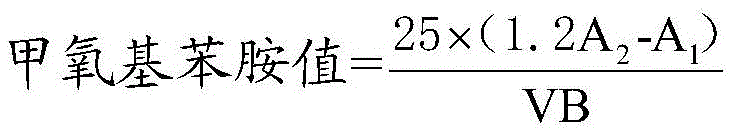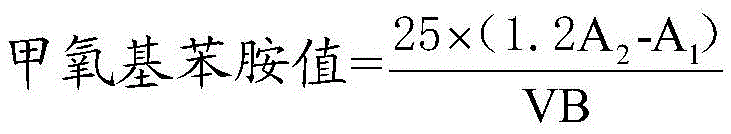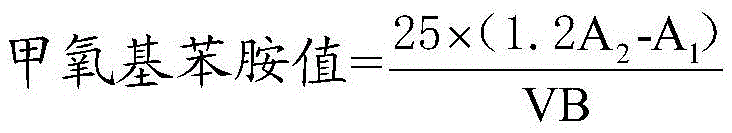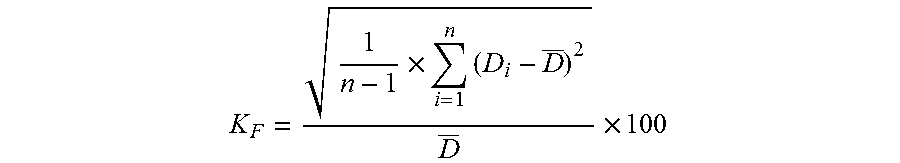Patents
Literature
100 results about "Lipid emulsion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lipid emulsion or fat emulsion refers to an emulsion of lipid for human intravenous use. It is often referred to by the brand name of the most commonly used version, Intralipid, which is an emulsion of soy bean oil, egg phospholipids and glycerin, and is available in 10%, 20% and 30% concentrations.
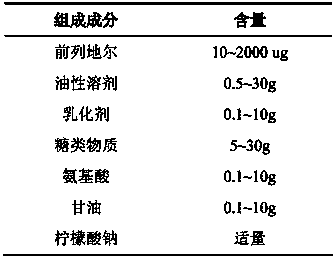
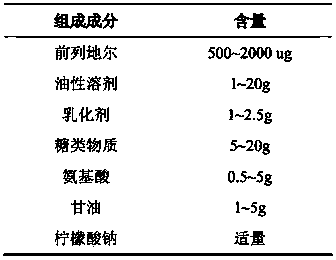
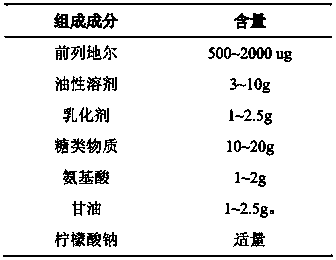
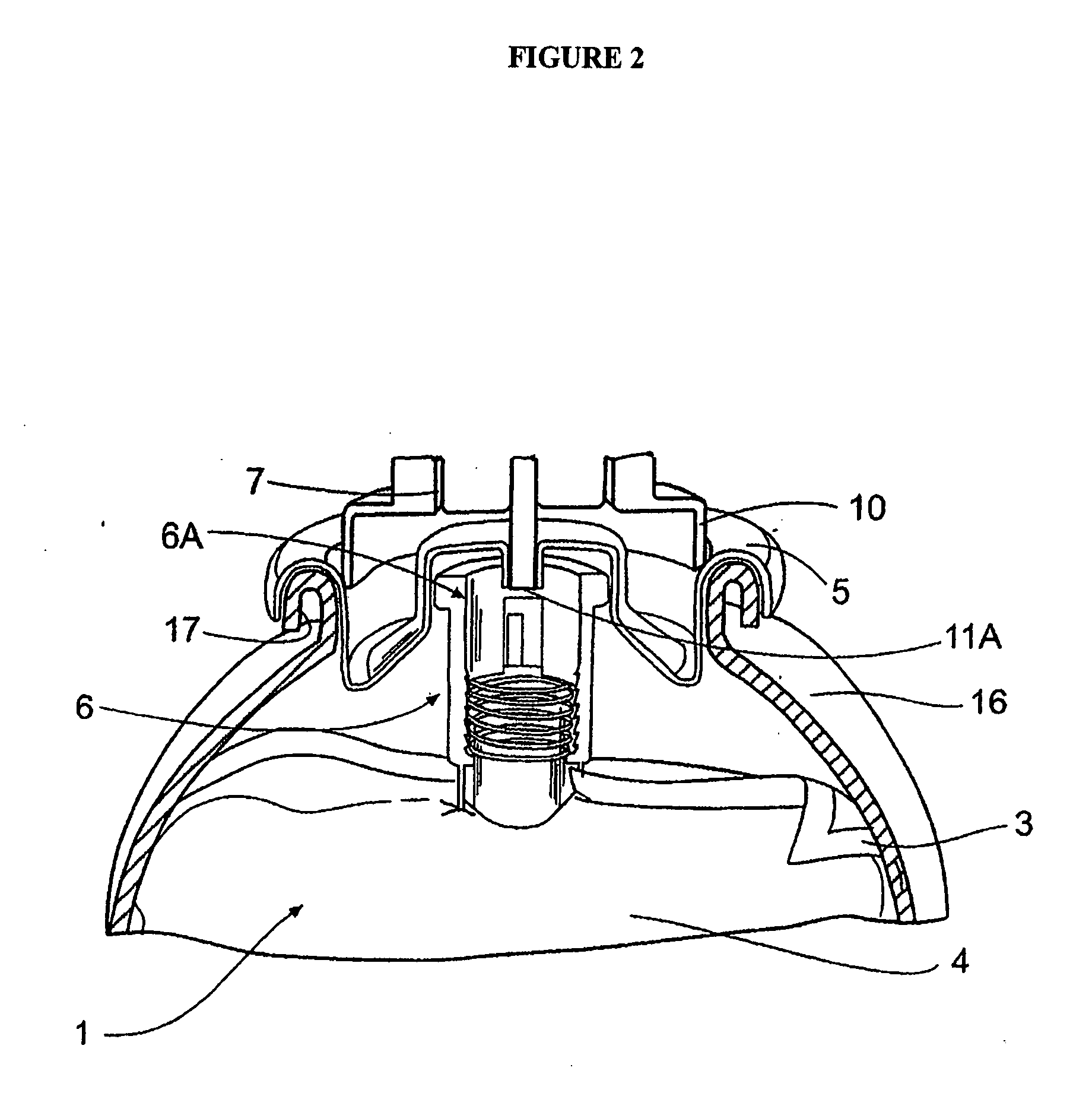
Superregulated long-cycled lipid emulsion carrying medicine reagent for mainline
ActiveCN101032467AImprove stabilityImprove bioavailabilityEmulsion deliveryOil/fats/waxes non-active ingredientsRetention periodAntioxidant
The present invention relates to high stability medicine carrying fat emulsion preparation for intravenous injection and its preparation process. The high stability medicine carrying fat emulsion preparation consists of: clinically effective liposoluble medicine, oil with medicinal effect or as medicine solvent, lecithin as the surfactant of the emulsion, polyethylene glycol phospholipid derivative as the emulsion stabilizer and emulsifier to increase the half life of the fat emulsion in blood, oleic acid or oleate as the emulsion stabilizer and emulsifier, vitamin E as the antioxidant, and complexing agent for controlling metal ion. The fat emulsion preparation of the present invention has long retention period in blood, increased passive medicine targeting function, high stability, raised bioavailability of the liposoluble medicine and raised clinical therapeutic effect.
Owner:XIAN LIBANG PHARMA
Glycopyrrolate in cosmetic preparations
Glycopyrronium bromide, derivatives and / or isomers thereof in combination with one or more active substances selected from a list of substances as recited in the claims and / or in the form of a W / Si emulsion, an O / W gel, a soap gel stick, and / or a surfactant-containing cleansing formula, and corresponding cosmetic preparations, in particular deodorant / antiperspirant preparations.
Owner:BEIERSDORF AG
Composition and method for modifying the fatty acid composition of cell membranes of organs and tissues
InactiveUS7560486B2Rapid and efficient uptake/enrichmentRestore balanceBiocideMetabolism disorderTG - TriglycerideCell membrane
The present invention relates to a composition and method for rapidly modifying the fatty acid composition of cell membranes of organs and tissues, in particular to increase the amount of omega-3 fatty acids of cell membranes of organs and tissues by parenterally administering to the human or animal body a supply of fatty acids in the form of an isotonic lipid emulsion comprising fatty acid triglycerides.
Owner:CARPENTIER YVON +1
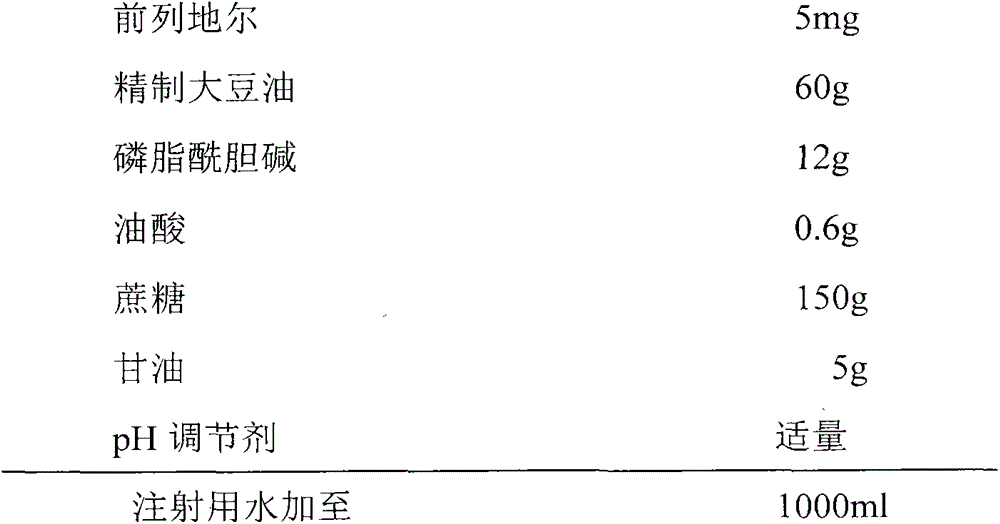
Alprostadil frozen-drying lipid emulsion and preparation method thereof
ActiveCN103301076AGood effectAvoid negative effectsPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention provides an alprostadil frozen-drying lipid emulsion and a preparation method thereof. The emulsion comprises the following compositions in percentage by mass: 0.0005-0.01% of alprostadil, 1-10% of oil for injection, 0.01-5% of an emulsifier, 0.02-2% of a stabilizer, 0-0.5% of an antioxidant, 1-20% of a freeze-drying protecting agent, and proper pH regulator and water for injection; the preparation method comprises the steps of: stirring alprostadil and oil for injection, and evenly mixing to obtain an oil phase; stirring freeze-drying protecting agent and water for injection to obtain a water phase; respectively the emulsifier, antioxidant and stabilizer into the water phase or the oil phase according to the solubility; mixing the water phase and the oil phase, carrying out high-speed stirring and dispersion, carrying out constant volume on the water for injection, regulating the pH value to obtain initial emulsion; transferring the initial emulsion into a high-pressure homogenizer for homogenizing for many times, then filtering, subpackaging, freeze-drying to obtain the alprostadil frozen-drying lipid emulsion. The alprostadil frozen-drying lipid emulsion is more stable and more uniform, the preparation technology and method can be simplified, and the economic efficiency can be improved.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
Lipid emulsions in the treatment of systemic poisoning
InactiveUS7261903B1Reduced bioavailabilityLow toxicityDrug compositionsEmulsion deliveryLipid formationWhole body
Lipid emulsion compositions and methods of using such composition via intravenous infusion to reduce the bioavailability and toxicity of poisonous agents in the bloodstream.
Owner:WEINBERG GUY +1
Lipid emulsion with low anisidine value and preparation method thereof
InactiveCN102805727AAchieve antioxidantReduce the value of methoxyanilineEmulsion deliveryOil/fats/waxes non-active ingredientsEdetic AcidDisodium Edetate
The invention relates to a lipid emulsion with a low anisidine value, which contains oily constituents, water, an emulsifying agent and an osmotic pressure regulator, wherein the lipid emulsion contains a metal ion chelant but not an antioxidant. The content of the metal ion chelant is below 100mg / ml. The metal ion chelant is one or more of sodium calcium edentate, edetate disodium, edetate sodium and edetic acid. The invention relates to a method for preparing the lipid emulsion with the low anisidine value as well. According to the invention, in the process of preparing the lipid emulsion, other antioxidants are not needed to add, such as Vitamin E (VE), the antioxidation can be implemented only by using the metal ion chelant, and the low anisidine value can be greatly reduced. The low anisidine value of the lipid emulsion cannot be reduced by adding other antioxidants.
Owner:JIANGSU JIUXU PHARMA +1
Lipid Emulsions in the Treatment of Systemic Poisoning
InactiveUS20080021411A1Reduced bioavailabilitySevere systemic toxicityBiocideInfusion syringesLipid formationTricyclic antidepressant
The present invention provides lipid emulsions and methods of intravenously administering lipid emulsions to treat systemic toxicity caused by foreign lipophilic and amphiphilic substances. In particular, methods are provided to treat cardiovascular impairment, such as cardiotoxicity, asystole and ischemia of the brain and heart, and neurological impairments, such as seizures and comas, caused by foreign lipophilic and amphiphilic substances, including cardiovascular impairment caused by local anesthetics, tricyclic antidepressants, sodium channel blockers, and calcium channel blockers.
Owner:RESQ PHARMA INC
Injection alprostadil freeze-dried emulsion
The invention relates to injection alprostadil freeze-dried emulsion. A freeze-drying protective additive of the emulsion consists of special amino acid and arbohydrate, wherein the amino acid and the arbohydrate are added to alprostadil lipid emulsion, so as to prepare the injection alprostadil freeze-dried emulsion which is quite high in stability through a certain freeze-drying process. The freeze-dried emulsion not only meets requirements of storing a preparation at normal temperature, but also effectively improves safety of the preparation and reduces adverse reaction incidence through replacing other freeze-drying protective additives by the amino acid.
Owner:XIAN LIBANG PHARMA
Long-cycled lipid emulsion profofol preparation
ActiveCN101032468AImprove stabilitySmall toxicityHydroxy compound active ingredientsAnaestheticsVegetable oilHalf-life
The present invention relates to propofol carrying fat emulsion preparation with long circulating time and its preparation process. The propofol carrying fat emulsion preparation consists of: propofol or liposoluble propofol derivative, vegetable oil as medicine solvent, lecithin as the surfactant of the emulsion, polyethylene glycol phospholipid derivative as the emulsion stabilizer and emulsifier to increase the half life of the fat emulsion in blood, oleic acid or oleate as the emulsion stabilizer and emulsifier, vitamin E as the antioxidant, and complexing agent for controlling metal ion. The propofol carrying fat emulsion preparation of the present invention has long retention period in blood, increased passive medicine targeting function, high stability, raised anesthetic action of propofol.
Owner:XIAN LIBANG PHARMA
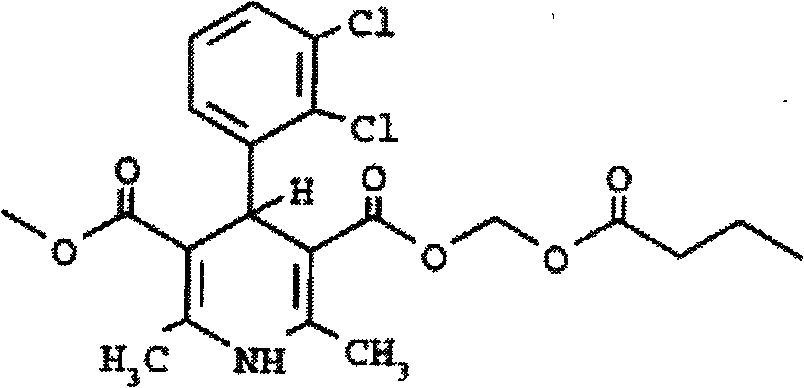
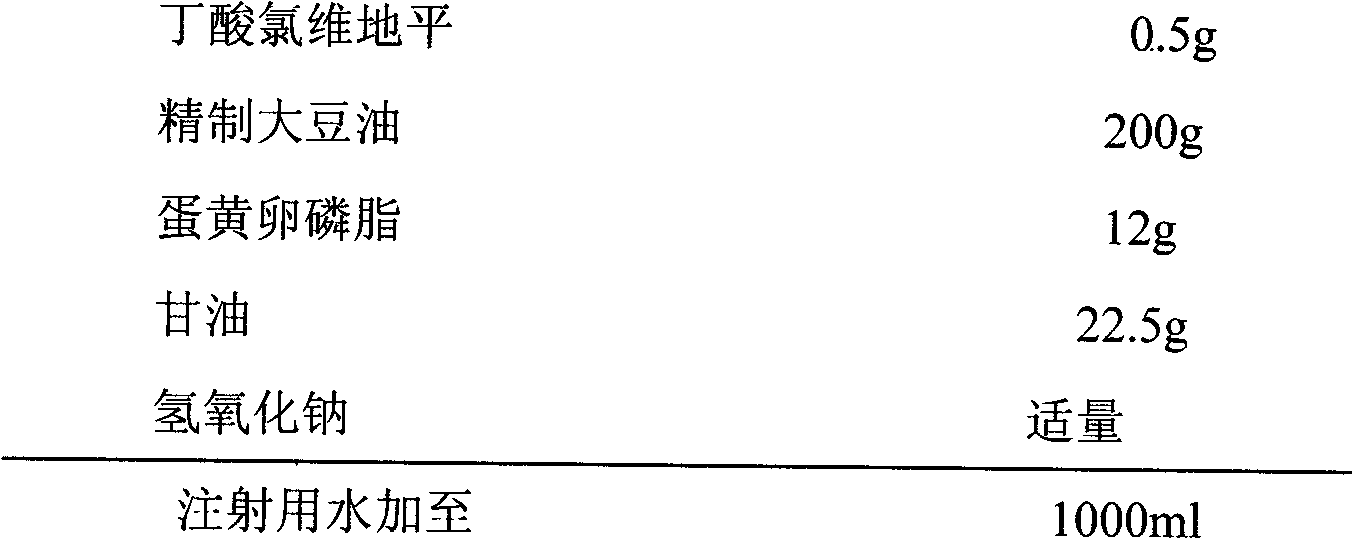
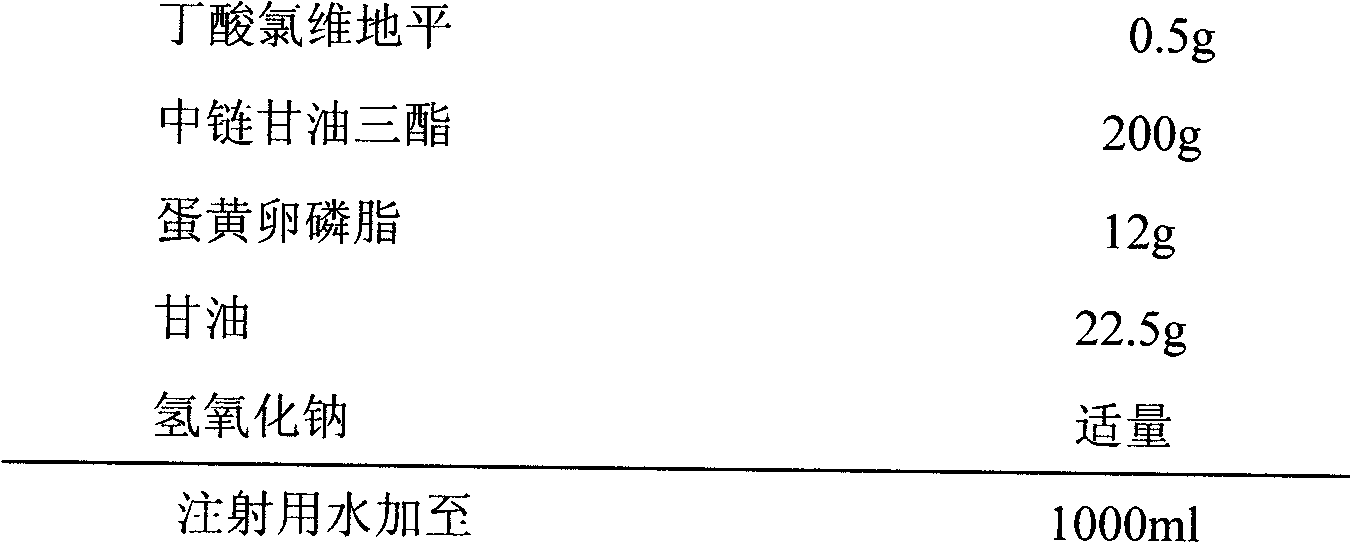
Clevidipine butyrate structured lipid emulsion and preparation method thereof
InactiveCN102319212ATo promote metabolismImprove technical effectOrganic active ingredientsEmulsion deliveryEmulsionMedicine
The invention relates to a clevidipine butyrate structured lipid emulsion which comprises clevidipine butyrate, structured triglyceride, an emulsifier, an isotonic agent, a pH regulator, and injection water, wherein the weight volume percentage of clevidipine butyrate is 0.05-0.1% (w / v); the weight volume percentage of structured triglyceride is 5-30% (w / v); the weight volume percentage of the emulsifier is 1.0-2.0% (w / v); and the weight volume percentage of the isotonic agent is 1.0-5.0% (w / v).
Owner:辽宁中海康生物制药股份有限公司
Devices containing lipid emulsions
InactiveUS20080031826A1Easily oxidizablePrevent oxidationBiocideSugar food ingredientsLipid formationChemistry
The present invention relates to compositions and methods for the storage and consumption of a lipid emulsion. In particular, the present invention relates to the storage of an omega-3 lipid emulsion for consumption in a container under non-oxidizing conditions.
Owner:AKER BIOMARINE ASA
Injection alprostadil fat emulsion and preparing method thereof
ActiveCN105012248AReduce security risksEasy to operateOrganic active ingredientsPowder deliveryFat emulsionsAlprostadil Injection
An alprostadil lipid emulsion for injection and a preparation method therefor. The lipid emulsion contains alprostadil, phosphatidylcholine, phosphatidylglycerol, oil for injection and a lyophilized protective agent. Based on 1 part by weight of the alprostadil, the content of the phosphatidylcholine is 1200 to 4000 parts by weight, the content of the phosphatidylglycerol is 12 to 120 parts by weight, the content of the oil for injection is 2000 to 20000 parts by weight, and the content of the lyophilized protective agent is 16000 to 60000 parts by weight.
Owner:内蒙古多肽科技有限公司
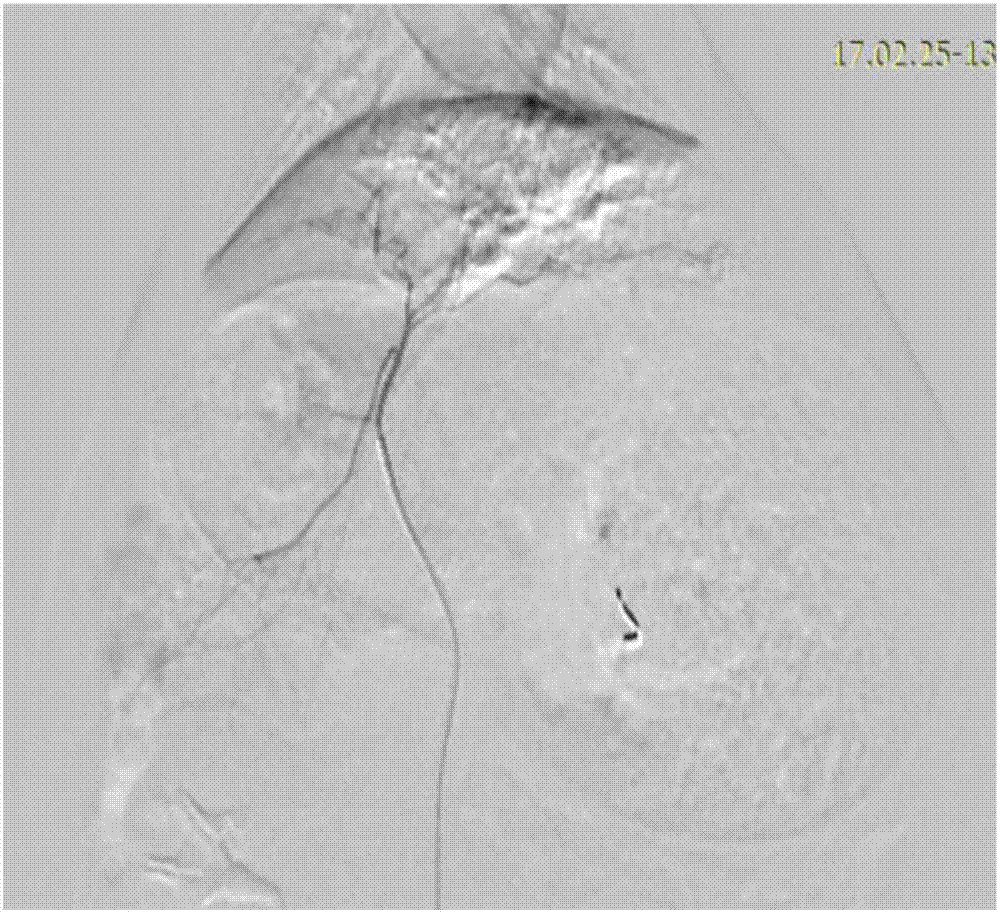
Iodinated lipid emulsion vascular thrombosis material, preparation method and application thereof
ActiveCN107261197ALow viscositySmall doseSurgical adhesivesPharmaceutical delivery mechanismLipiodolLipid emulsion
The invention relates to an iodinated lipid emulsion vascular thrombosis material, a preparation method and an application thereof, and belongs to the technical field of vascular thrombosis materials used in medical appliance intervention. The iodinated lipid emulsion vascular thrombosis material includes, by mass, 20-80% of iodinated lipid emulsion, 15-75% of temperature sensitive nano-hydrogel and 0.04-3.0% of a gelator; on the basis of the mass of the iodinated lipid emulsion, the iodinated lipid emulsion includes, by mass, 50-90% of lipiodol, 0.1-0.6% of a co-surfactant and the balanced water. The vascular thrombosis material is low in viscosity and is excellent in capability of resisting blood scouring, can form firm and durable thrombosis, can be image-developed for long period, brings convenience to reexamination and diagnosis of patients at any time, has better curative effects and is safe and controllable.
Owner:ANEW MED LIFE SCI WUHAN CO LTD
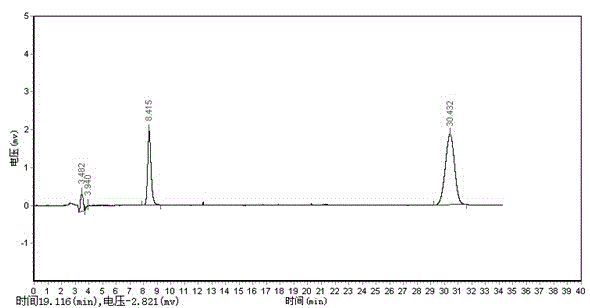
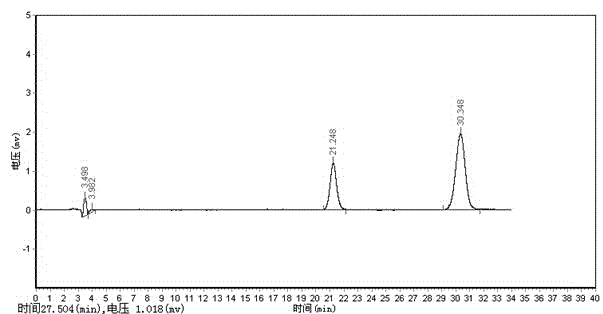
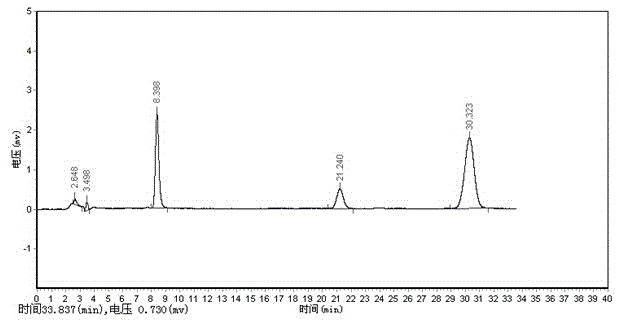
Method for determining anisidine value of lipid emulsion
ActiveCN102809503ATo achieve the separation effectAvoid disadvantagesPreparing sample for investigationColor/spectral properties measurementsDistillationUltraviolet absorption
The invention relates to a method for determining an anisidine value of lipid emulsion. The method comprises the following steps: extracting the lipid emulsion with ethyl acetate and drying the ethyl acetate phase by low-temperature vacuum rotation distillation to obtain a residue 1 as a sample; or adding hexyl hydride to dissolve the residue 1, carrying out extraction with acetonitrile, enabling the hexyl hydride phase for standby use, and drying the acetonitrile phase by low-temperature vacuum rotation distillation to obtain a residue 2; dissolving the residue 2 with alcohol, sequentially adding a sodium hydrogen sulfite solution and ethyl acetate, carrying out extraction with water, carrying out extraction with ethyl acetate after adding hydrochloric acid in the water phase, combining the ethyl acetate phase with the hexyl hydride phase, and carrying out low-temperature vacuum rotation distillation for drying to obtain a residue 3 as a sample. According to the method for determining the anisidine value of the lipid emulsion, the interference to the detection result resulting from high-temperature operation and ultraviolet absorption of a main drug is effectively avoided; and the method is especially applicable to determination of the anisidine value of the lipid emulsion with the main drug having ultraviolet absorption at the wavelength of 350nm.
Owner:JIANGSU JIUXU PHARMA
Lipid emulsion of magnolol and preparation method thereof
InactiveCN103083237AGood effectFormulation ScienceNervous disorderHydroxy compound active ingredientsMagnololDrug loading dose
The invention relates to a lipid emulsion of magnolol and a preparation method thereof. According to the lipid emulsion of the magnolol and the preparation method thereof, the problem of poor water-solubility of the magnolol can be effectively solved. The following technical proposal is that the lipid emulsion of the magnolol is prepared from the following raw materials by weight percentage: 0.01%-5% of magnolol, 2-30% of oil for injection, 0.1-20% of an emulsifier, 1-10% of an isoosmotic adjusting agent, 0.001-1% of a stabilizer, 0.001-3% of an antioxidant, 0.00001-0.5% of a pH regulator and the balance of water for injection. The lipid emulsion of the magnolol, provided by the invention, is scientific in formulation, simple in formula, reliable in quality, good in stability, high in drug loading capacity, stable in long-term storage and good in safety, thus being a great innovation on the pharmaceutic preparation.
Owner:ZHENGZHOU UNIV
Composition and method for early bloom thinning of fruit trees and controlling cracking of fruits
InactiveUS20010039246A1Effective thinningSatisfactory applicationCosmetic preparationsBiocideFruit treeEmulsion
A composition and method for early bloom thinning of fruit trees and controlling cracking, wherein the composition provides that glyceride type of lipids are effective compounds for bloom thinning of fruit trees and controlling cracking of fruits and the method comprises making an aqueous emulsion with these lipids as active ingredients and spraying the emulsion on fruit trees at appropriate phenophases for blossom thinning or fruit cracking control. The composition further provides that copper compounds act as blossom thinning agents but cause phytotoxicity to trees and a mixture of the said lipid emulsion and copper compounds displays higher thinning effect than each applied alone and does not cause phytotoxicity to trees.
Owner:DUAN YOUSHENG +3
Alprostadil medium-and-long-chain lipid emulsion for injection and preparation method thereof
ActiveCN103610640AExtensive developmentWide range of applicationsOrganic active ingredientsPharmaceutical non-active ingredientsTG - TriglycerideGlycerol
The invention discloses an alprostadil medium-and-long-chain lipid emulsion for injection and a preparation method thereof, belonging to the technical field of biological medicines. The lipid emulsion is composed of the following components in parts by weight: 0.01-0.1 part of alprostadil, 10-100 parts of long-chain triglycerides, 10-100 parts of medium-chain triglycerides, 1-20 parts of phospholipids, 0.01-1 part of oleic acid, 5-20 parts of glycerol and 300-2500 parts of water for injection. The particle size of the lipid emulsion is 50-200 nm, the lipid emulsion has good stability while the pH value is 5.0-8.0, and the contents of degradation products including prostaglandin A1 and prostaglandin B1 of alprostadil measured by using an HPLC (High Performance Liquid Chromatography) method are respectively below 1%. Compared with traditional alprostadil long-chain lipid emulsions, aqueous-phase alprostadil is significantly decreased, so that the vascular stimulation is reduced, and the liver load is reduced, therefore, the lipid metabolism can be accelerated, and the triglyceride level of plasma can be reduced.
Owner:JILIN UNIV
Astragalus glycoside fatty emulsion and its preparation technology
A lipid emulsion of astragaloside A is proportional prepared from astragaloside A, oil, surfactant, isotonic regulator, cosolvent and water for injection. Its preparing process is also disclosed.
Owner:JIANGSU QINGJIANG PHARMA
Purple perilla seed oil lipid emulsion oral solution, beverage and manufacturing method thereof
The invention provides purple perilla seed oil lipid emulsion oral solution, a beverage and a manufacturing method thereof. The purple perilla seed oil lipid emulsion oral solution is prepared from refined purple perilla seed oil, refined lecithin, glycerin, sterile pure water, an antioxidant, a stabilizer, a pH regulator, flavorings, species and the like serving as raw materials. A manufacturing process comprises the following steps of: under the protection of nitrogen, heating the refined purple perilla seed oil and the refined lecithin to dissolve the mixture into oil phase liquid; mixing the glycerin, the antioxidant, the stabilizer, the flavorings, the spices and the sterile pure water and dissolving the solution into water phase liquid; adding the oil phase liquid into the strongly stirred water phase liquid slowly; shearing the solution to form primary emulsion at a high speed; regulating the pH value of the primary emulsion to be between 6.5 and 8.5 with a little pH regulator; emulsifying the primary emulsion with a high pressure homogenizer; filtering the solution with a microporous membrane filter; bottling and sealing the obtained solution; sterilizing the solution by microwave; and heating the solution for sterilization to obtain the purple perilla seed oil lipid emulsion oral solution which has 1 to 50 percent of the purple perilla seed oil. The purple perilla seed oil lipid emulsion oral solution is applicable to the field of healthy food; the purple perilla seed oil lipid emulsion oral solution having a high concentration of 5 to 50 percent can serve as a functionally nutritious health beverage; and the purple perilla seed oil lipid emulsion oral solution having a low concentration of 1 to 20 percent can serve as drinks for dinner and popular drinks.
Owner:王京南
Nanometer lipid emulsion
InactiveCN107441044AImprove stabilitySafe useEmulsion deliveryOil/fats/waxes non-active ingredientsSolubilitySide effect
The invention relates to a nanometer lipid emulsion which comprises a drug, oil, an emulgator and water, wherein the solubility of the drug is 0-0.0005g / ml and the drug is difficult to dissolve in water. The emulsion is clear and is capable of reducing the drug degradation and the emulsion droplet agglutination caused by ultrahigh pressure and high temperature. The drug is capable of being stably packed in the emulsion droplet, so that the toxic or side effect of the drug is reduced.
Owner:FUBICHENG SHANGHAI PHARMA TECH CO LTD
Fatty emulsion injection of seal oil, method for preparation and the use in manufacturing intravenous injection
The present invention relates to a seal oil based lipid emulsion injection, and the main ingredients of which contains 190-210 g / l of refined OMEGA3 seal oil, 11-13 g / l of refined lecithin, 24-26 g / l of glycerol for injection, and balance amount of water. The preparation of the seal oil based lipid emulsion injection comprising the steps of stir and dispersion, high-pressure homogenization, vacuum filtration, antisepsis and encapsulation. The OMEGA3 seal oil lipid based emulsion injections have high content of energy, and thus are highly absorbable to human body, and can not only provide human body with caloricity, but also supply the fatty acids necessary for human body, and can enhance body's immunizing ability, reduce the content of cholesterol, adjust the blood concentration and then be used for anti-inflammation, in particular, it is highly useful for a postoperative patient to restore his strength.
Owner:LIU WEI +5
Phospholipid complex of natural Baikal skullcap root active ingredients as well as preparation method and preparation thereof
ActiveCN102988484AImprove hydrophilicityImprove lipophilicityAntibacterial agentsDigestive systemSolubilityWater dispersible
The invention discloses a phospholipid complex of natural Baikal skullcap root active ingredients and a preparation method thereof. The natural Baikal skullcap root active ingredients mean an active fraction (the baicalein content is more than 50 percent) or an active ingredient (the baicalein content is more than 90 percent) of baicalein extracted and separated from Baikal skullcap roots serving as a Chinese medicament and an active fraction (the wogomin content is more than 50 percent) or an active ingredient (the wogomin content is more than 90 percent) of wogomin. The water solubility and lipid solubility of the natural Baikal skullcap root active ingredients are poor, the dissolubility of the active ingredients is increased along with rise of the pH value of a solution, and the active ingredients are easily chemically degraded under an alkali condition. Due to the insufficiency of the physical and chemical properties of the natural Baikal skullcap root active ingredients, the active ingredients cannot be prepared into injection, and the bioavailability is low after the active ingredients are orally taken. Through a phospholipid complex technology, the water dispersibility and lipophilic property of the Baikal skullcap root active ingredients are obviously improved, and then the Baikal skullcap root active ingredients can be prepared into high-bioavailability oral preparation, freeze-dried injection or lipid emulsion to meet the requirement for injection or mucosa administration.
Owner:BEIJING WEHAND BIO PHARMA CO LTD
Lipid-emulsion eye drops with dexamethasone and preparation method thereof
InactiveCN106727308AThrough highImprove distributionOrganic active ingredientsSenses disorderDiseaseDexamethasone
The invention provides lipid-emulsion eye drops with dexamethasone. The lipid-emulsion eye drops are in an oil-in-water sub-micro emulsion system; grease in the emulsion drops is coated with lipid nano particles formed by lipid carriers and the dexamethasone. The invention aims at providing the lipid-emulsion eye drops with the dexamethasone and a preparation method thereof so as to increase tissue distribution in the eyes, promote permeation into the eyes and further achieve the purposes of safely and effectively treating intraocular diseases and fundus oculi diseases.
Owner:GUANGDONG PHARMA UNIV
Orally taken vitamin K1 lipid emulsion
The invention relates to an orally taken vitamin K1 lipid emulsion, in particular to an emulsion compound. The orally taken vitamin K1 lipid emulsion comprises 0.5-2 percent of vitamin K1, 5-15 percent of soybean oil, 0.5-2 percent of phospholipid, 1-5 percent of low alcohol, 1-5 percent of saccharides and water. The invention also relates to a method for preparing the emulsion compound. The vitamin K1 emulsion compound provided by the invention meets the properties of common pharmaceutics, can be orally administered, is enough in bioavailability and can be suitable for a wider range of people including infants.
Owner:广东安健医药有限公司
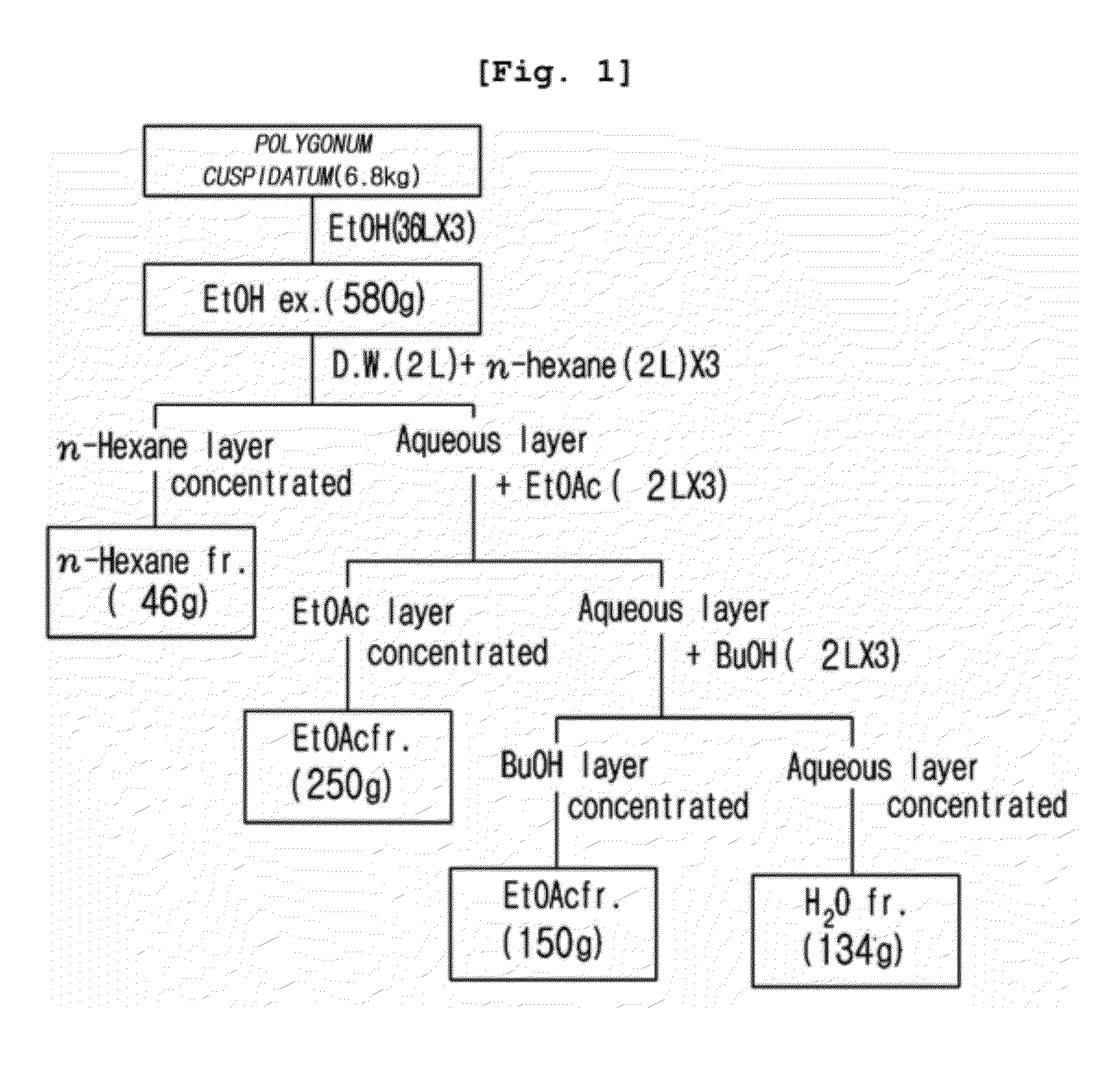
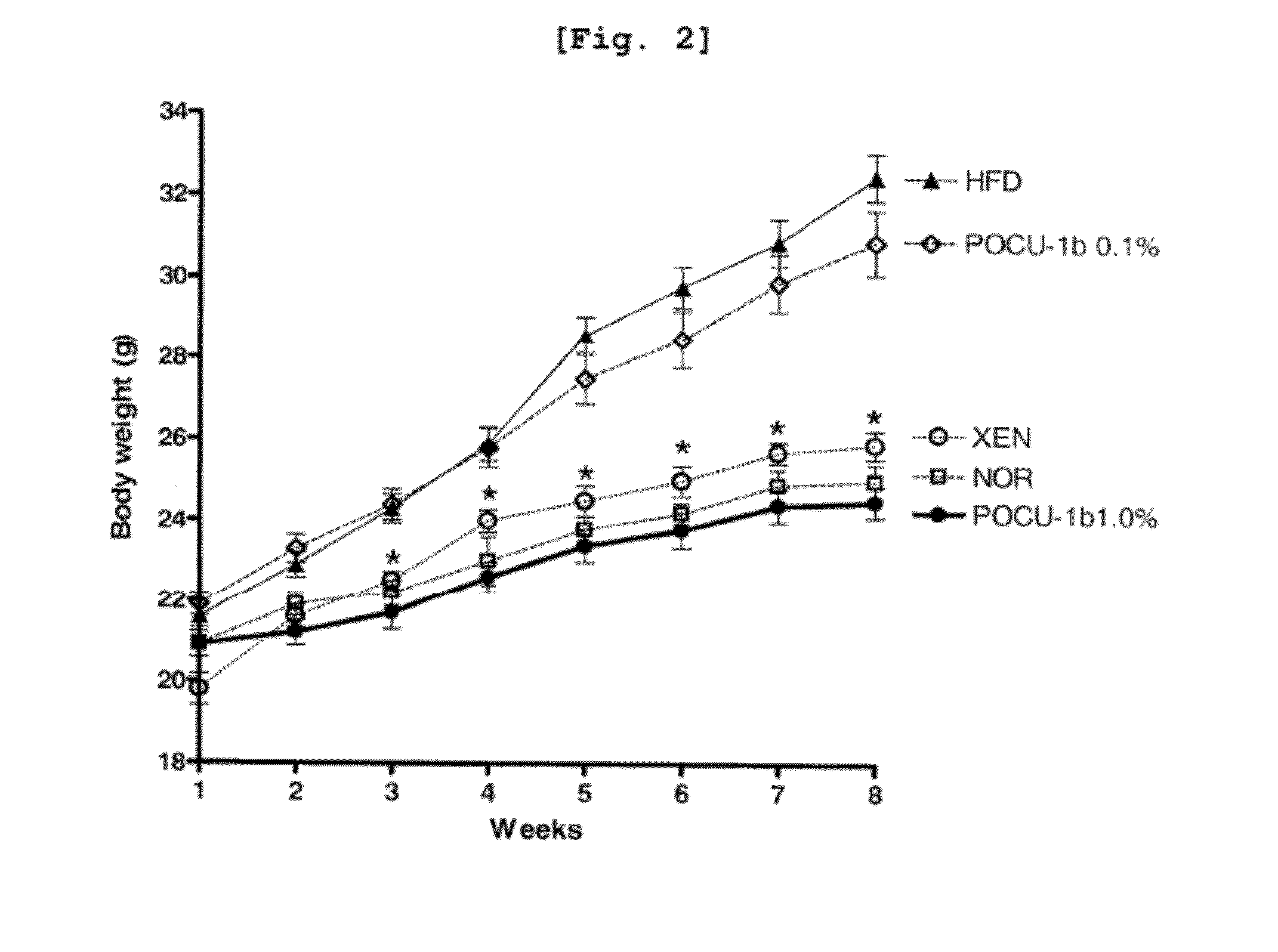
Compositions and functional foods for treating and preventing obesity using polygonum cuspidatum butanol fraction and ethyl acetate fraction
ActiveUS20120183633A1Reduce obesityToxic reductionBiocideMetabolism disorderEthyl acetateBULK ACTIVE INGREDIENT
The present invention relates to the obesity-curative or obesity-preventive effect of a Polygonum cuspidatum butanol fraction and a Polygonum cuspidatum ethylacetate fraction, and more particularly, to a pharmaceutical composition and a functional food for treating obesity, the pharmaceutical composition and the functional food comprising a Polygonum cuspidatum butanol fraction (POCU-1b) and an ethylacetate fraction as active ingredients, wherein the Polygonum cuspidatum butanol fraction and the ethylacetate fraction inhibit effectively the activity of pancreatic lipase, an important enzyme involving in fat absorption in a living body, and have excellent inhibitory effect on fat absorption in the short term fat absorption-inhibitory animal experiments using lipid emulsions.
Owner:KOREA INST OF ORIENTAL MEDICINE
Method for detecting alprostadil freeze-dried lipid emulsion
The invention provides a method for detecting an alprostadil freeze-dried lipid emulsion. The method comprises the steps: 1) adding water to the alprostadil freeze-dried lipid emulsion, and re-dissolving to obtain a sample aqueous solution; 2) demulsifying the sample aqueous solution obtained in the step 1) with a demulsifier, to obtain a demulsified sample solution, wherein the volume ratio (ml:ml) of the demulsifier to the sample aqueous solution is 5:1-5:6; and 3) determining the contents of alprostadil and prostaglandin A1 in the demulsified sample solution obtained in the step 2) by high performance liquid chromatography, wherein the demulsifier is composed of an organic solvent A and an organic solvent B, the organic solvent A is selected from the group consisting of alcohols, and the organic solvent B is selected from the group consisting of alkanes or tetrahydrofuran. The method for detecting the alprostadil freeze-dried lipid emulsion has the advantages of high recovery rate and accurate measurement results, enhances quality detection standards of the alprostadil freeze-dried lipid emulsion, reinforces the quality controllability of drugs, thereby ensuring the effectiveness and the safety of clinical medication.
Owner:PENGLAI NUOKANG PHARMA CO LTD
Determination method of methoxyaniline value of fat emulsion
ActiveCN103712936BPrevent oxidationAvoid degradationPreparing sample for investigationColor/spectral properties measurementsDemulsifierAcetic acid
The invention relates to the technical field of determination methods for anisidine value, and discloses a determination method for the anisidine value of lipid emulsion. The determination method comprises the steps of performing extraction treatment on the lipid emulsion by using an alkali metal salt as a demulsifier and diethyl ether as an extracting agent to obtain a diethyl ester phase and a water phase; carrying out vacuum rotary evaporation treatment on the diethyl ester phase at a temperature lower than 40 DEG C to obtain a residue I; dissolving the residue I with ethyl acetate to obtain an ethyl acetate phase; carrying out vacuum rotary evaporation treatment on the ethyl acetate phase at the temperature lower than 40 DEG C to obtain a residue II which is to be used as a sample. In a technical solution provided by the invention, vacuum rotary evaporation is carried out at the temperature lower than 40 DEG C, so that the problems of oxidation and degradation of unsaturated fatty acid esters caused by high temperature can be prevented; and accuracy for measurement of the anisidine value can be increased.
Owner:JIANGSU JIUXU PHARMA
Tacrolimus lipid emulsion for injection and preparation method thereof
ActiveCN104274406AGood chemical stabilityImprove stabilityOrganic active ingredientsAntimycoticsAntioxidantOil phase
The invention discloses a tacrolimus lipid emulsion for injection, and the tacrolimus lipid emulsion contains tacrolimus, an oily solvent for injection, a surfactant, amphiphilic block copolymers, an osmotic pressure regulator for injection, an antioxidant and a stabilizer. In addition, the invention also discloses a preparation method of the lipid emulsion. The tacrolimus lipid emulsion is capable of effectively increasing the chemical stability of the medicines; most of medicines in the tacrolimus lipid emulsion are distributed in an oil phase or an oil-water interface; and the medicines are prevented from directly contacting water, are solubilized into the oil phase, and are isolated, so that the medicine dosage of the water phase is reduced, the hydrolysis amount of the medicines is greatly reduced, and the stability on the medicines is greatly improved. The tacrolimus lipid emulsion for injection disclosed by the invention can be used for wrapping a part of medicines into the oil phase or an interfacial film, and preventing the medicines from directly contacting body fluid, so that the local and vascular stimulation which can be generated by the medicines is reduced.
Owner:西安远大德天药业股份有限公司
Application of large-dose glycerinum in freeze-thawing tolerable lipid emulsion
InactiveUS20170128362A1Lower requirementLower drug costsHydroxy compound active ingredientsMetabolism disorderHigh concentrationEmulsion
The invention relates to fat emulsions, particularly to a use of high-concentration glycerol in freeze-thaw resistant emulsions and a free-thaw resistant emulsion thereof. The said high-concentration glycerol is the glycerol that is greater than or equal to 3 w / v % in the emulsion composition. The maximum percentage of the glycerol in the emulsion is 50 w / v %. When the percentage of the oil in the emulsion is 2%-30 w / v %, the glycerol is more than or equal to ⅓ of the oil in the emulsion. The invention comprises a drug-contained emulsion through including drugs. Compared with prior arts, the invention provides a freeze-thaw resistant emulsion which tolerates the low-temperature freeze-thaw experiments, avoiding the pharmaceutical stability issues due to the temperature changes during the emulsion transport, storage and utilization, ensuring medicine quality, meanwhile it drastically reduces the requirements of the transport and storage conditions as well as the medicine costs.
Owner:DELI WEI BEIJING BIOLOGICAL TECH +1
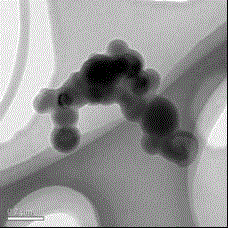
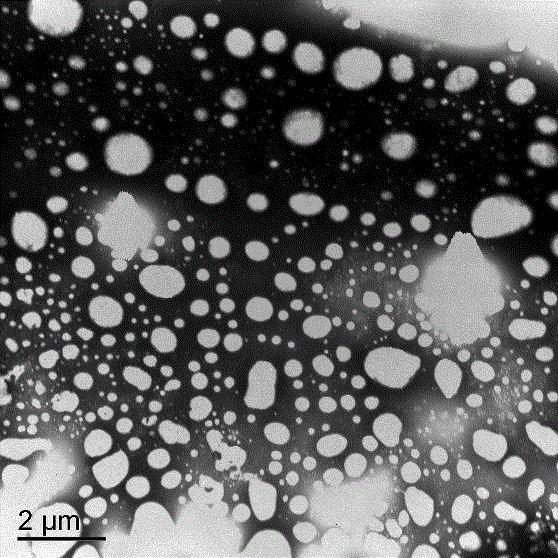
Method for determining microstructure of lipid microsphere/lipid emulsion
InactiveCN102944569APreparing sample for investigationMaterial analysis by measuring secondary emissionBeam energyMicrosphere
The invention discloses a method for determining the micromorphology of a lipid microsphere / lipid emulsion with a soft shell structure by use of a scanning electron microscope. The method comprises the following steps of: diluting and dispersing a to-be-tested sample, treating the sample by an anion device so as to positively charge the to-be-tested sample or a sample container carrying the to-be-tested sample, and scanning the microstructure of the surface of the sample by the change of electron beam energy, thus obtaining the good-reproducibility, stable and clear microstructure of the lipid microsphere / lipid emulsion, wherein the spectrogram of a scanning TEM (transmission electron microscope) is adopted in the determination method.
Owner:BEIJING TIDE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com