Lipid-emulsion eye drops with dexamethasone and preparation method thereof
A technology of dexamethasone lipid and dexamethasone, applied in the field of medicine, can solve the problems of subconjunctival scarring, loss, adhesion and the like, and achieve the effects of increasing drug concentration, increasing drug concentration and prolonging residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
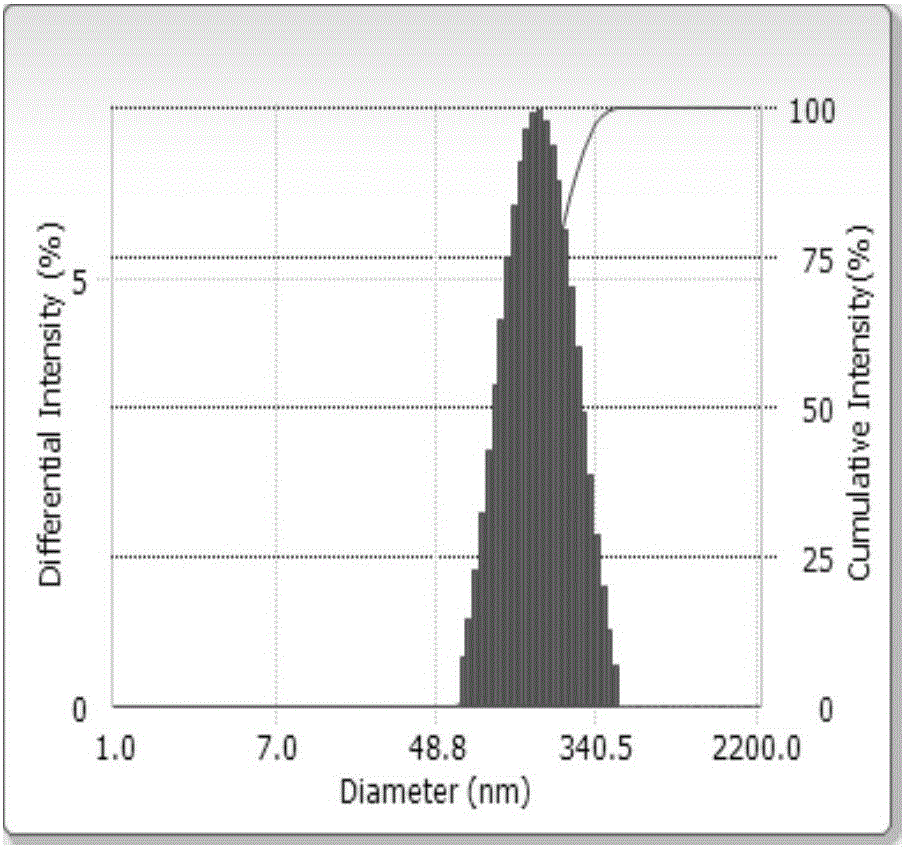
Embodiment 1
[0051]
[0052]
[0053]Preparation method: Dissolve the prescription amount of egg yolk lecithin PL-100M and dexamethasone in a mixed solvent of ethanol and acetone (1:1, v / v), the mixed solvent is 200g; evaporate under reduced pressure at 40°C to remove the solvent to form the drug and A mixture of phospholipids, the mixture is dispersed into the prescription amount of medicinal soybean oil to form an oil phase, another prescription amount of Poloxamer 188 emulsifier is dissolved in an appropriate amount of water, and then the aqueous solution containing glycerin is mixed with the oil phase , 800rpm shear emulsification for 10min, vested colostrum. Adjust the pressure of the secondary valve of the high-pressure homogenizer to 200-300 bar, homogenize for 2 minutes to make the distribution of the refined particles more uniform, then adjust the primary valve between 500-600 bar, and cycle 10 times of homogenization treatment to make the material fully In the narrow area c...
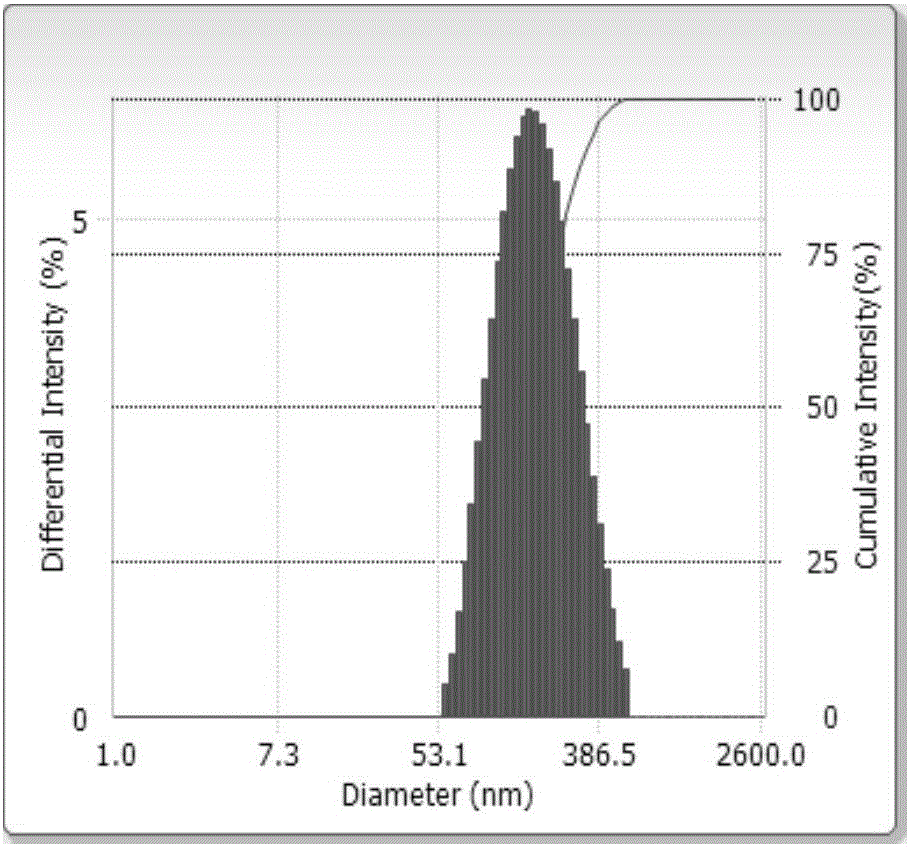
Embodiment 2
[0055]
[0056]
[0057] Preparation method: Dissolve the prescribed amount of LIPOID E 80 and dexamethasone in a mixed solvent of ethanol and acetone (1:3, v / v), the mixed solvent is 100 g; evaporate the solvent under reduced pressure at 40°C to form a mixture of drug and phospholipid , disperse the mixture into the prescribed amount of medicinal soybean oil to form an oil phase, and dissolve another prescribed amount of Solutol HS15 emulsifier in an appropriate amount of water, then mix the aqueous solution containing the emulsifier with the oil phase, and emulsify at 800rpm for 10min. Vested colostrum. Adjust the pressure of the secondary valve of the high-pressure homogenizer to 200-300 bar, homogenize for 2 minutes to make the distribution of the refined particles more uniform, then adjust the primary valve between 500-600 bar, and cycle 10 times of homogenization treatment to make the material fully In the narrow area composed of the valve core, valve seat and impa...
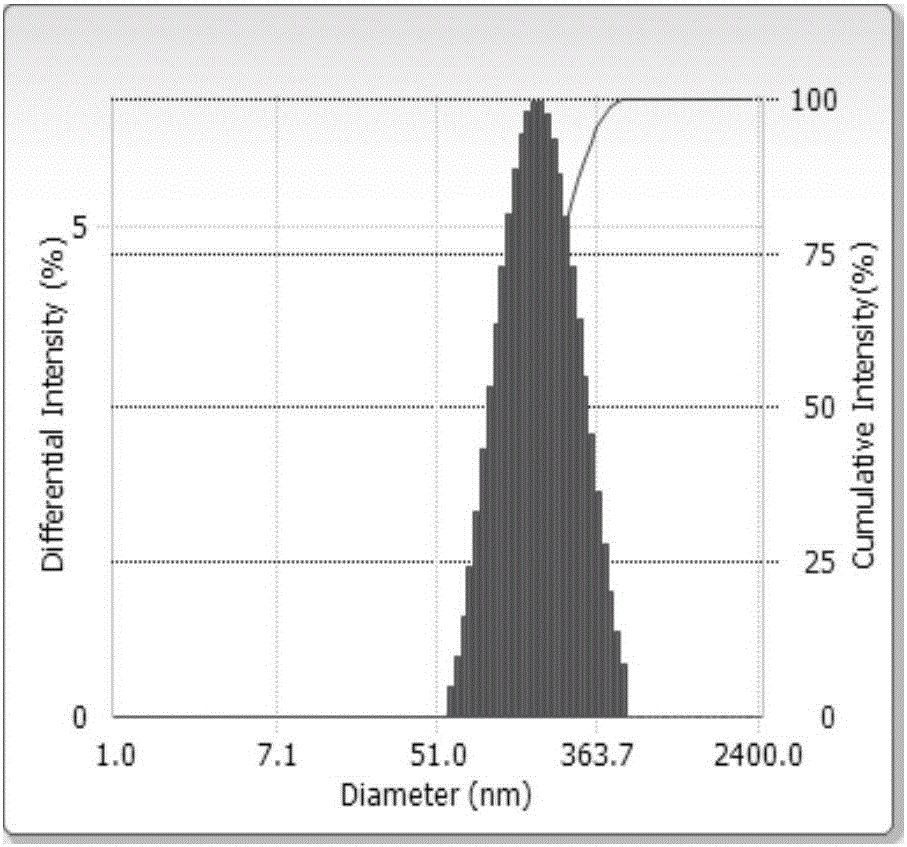
Embodiment 3
[0059]
[0060] Preparation method: Dissolve the prescribed amount of LIPOID S 75 and dexamethasone in a mixed solvent of ethanol and acetone (1:2, v / v), the mixed solvent is 140 g; evaporate the solvent under reduced pressure at 40°C to form a mixture of drug and phospholipid , disperse the mixture into the medicinal soybean oil of the prescribed amount to form an oil phase, and dissolve the Cremophor EL emulsifier of the prescribed amount in an appropriate amount of water, then mix the aqueous solution containing the emulsifier with the oil phase, and emulsify at 800rpm for 10min. Vested colostrum. Adjust the pressure of the secondary valve of the high-pressure homogenizer to 200-300 bar, homogenize for 2 minutes to make the distribution of the refined particles more uniform, then adjust the primary valve between 500-600 bar, and cycle 10 times of homogenization treatment to make the material fully In the narrow area composed of the valve core, valve seat and impact ring,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com