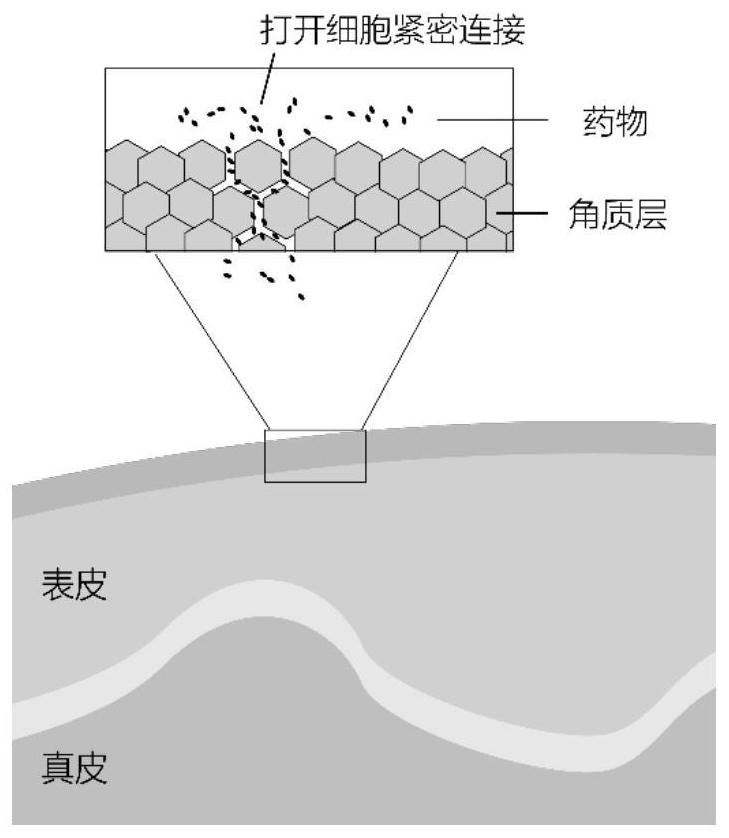
Application of fluorine-containing compound modified chitosan in preparation of transdermal delivery preparation
A technology for transdermal drug delivery and chitosanization, which is applied in the field of cationic polymers as drug transdermal carriers, can solve the problems of high toxicity, limited transdermal effect, low drug bioavailability and generalizability, etc. The effect of long, reduced administration area, and fast action speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Preparation of perfluoroheptanoic acid-modified chitosan as a carrier for transdermal application, transdermal delivery of insulin, and treatment of diabetes. The specific fluorinated chitosan preparation process of this embodiment is referred to embodiment 5.
[0071] specific method:
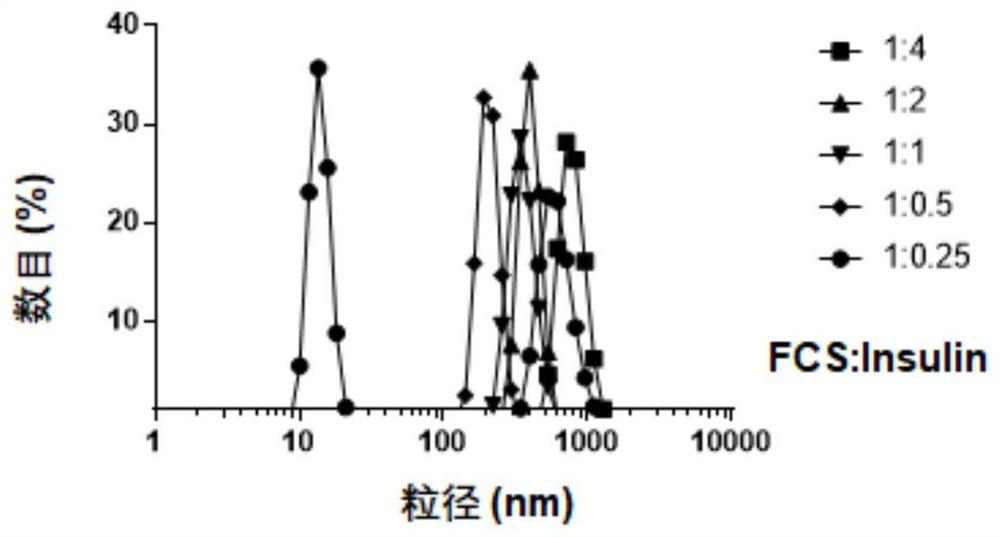
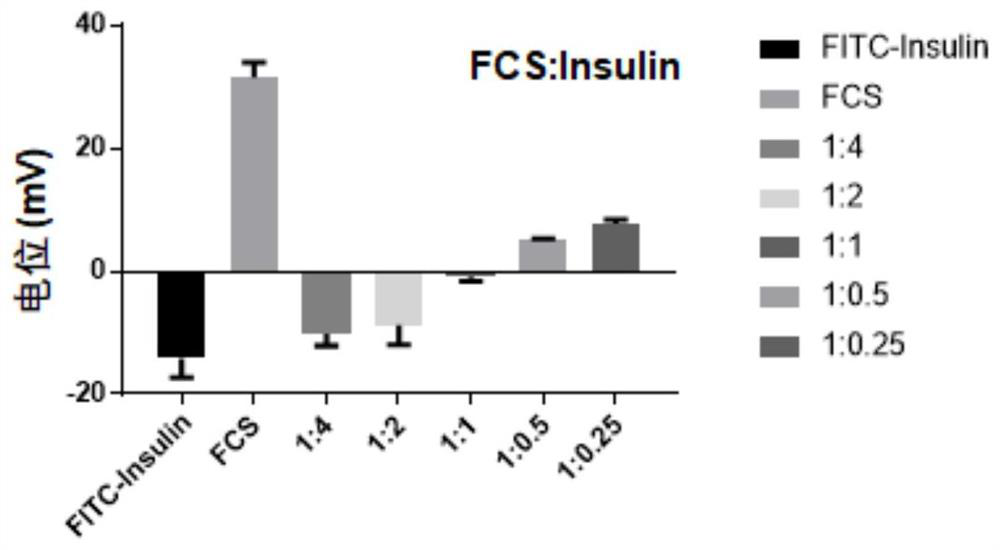
[0072] 1. Preparation of perfluoroheptanoic acid-modified chitosan-insulin complex: respectively dissolve perfluoroheptanoic acid-modified chitosan and insulin in a weak acid solution environment to dissolve evenly, mix well and add dropwise while stirring Weak base solution, adjust the pH to 6-7. Under neutral conditions, perfluoroheptanoic acid-modified chitosan and insulin combine together due to electrostatic adsorption to form a stable complex, in which perfluoroheptanoic acid-modified chitosan The preferred reaction mass ratio of sugar to insulin is 1:0.25-4, more preferably 1:0.5-2. After the reaction is complete, take it out, pre-add a freeze-drying protective agent...
Embodiment 2
[0080] Example 2: Preparation of perfluoroheptanoic acid-modified chitosan as a carrier transdermal ointment for transdermal delivery of apoptosis-ligand 1 antibody for treatment of melanoma on the body surface.
[0081] specific method:
[0082] 1. Prepare the chitosan-immunoglobulin G complex (hereinafter referred to as FCS-IgG) modified by perfluoroheptanoic acid: since immunoglobulin G has the same structure as the apoptosis-ligand 1 antibody, at the material level Immunoglobulin G was used as a template to study the preferred ratio of perfluoroheptanoic acid-modified chitosan binding to apoptosis-ligand 1 antibody. Add different amounts of immunoglobulin G into the aqueous solution of perfluoroheptanoic acid-modified chitosan, and stir at room temperature for 1 hour to form a stable complex. Wherein, the reaction mass ratio of perfluoroheptanoic acid-modified chitosan to immunoglobulin G is 1:0.25-4, and is further preferably 1:1 through dynamic light scattering particle...
Embodiment 3
[0099] Embodiment 3: prepare the chitosan (deacetylation degree ≥ 95%, viscosity 100-200mpa.s) of the different modification degree of 3-fluorobenzoic acid, wherein the feeding molar ratio of 3-fluorobenzoic acid and N-glucosamine unit is respectively 1:2.1, 1:4.2, 1:8.4, 1:16.8.
[0100] Synthetic method: (1) preparation of chitosan acetic acid aqueous solution: take by weighing 200mg fully dried chitosan and add in 10ml 1% acetic acid aqueous solution, also can adopt hydrochloric acid aqueous solution certainly, stir 30min to fully dissolve, then slowly add dropwise 1.6ml0. 5M sodium hydroxide, stirred until the solution was clear and the pH was around 6.5. From the point of view of alkalization solution, sodium hydroxide can be replaced by alkalis such as ammonia water and triethylamine, but the by-product of using sodium hydroxide is sodium chloride from the perspective of product technology, which is more suitable for industrialization. Prepare 4 parts of chitosan acetic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| degree of deacetylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com