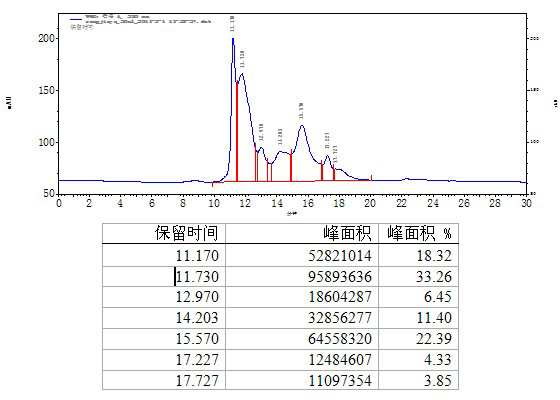
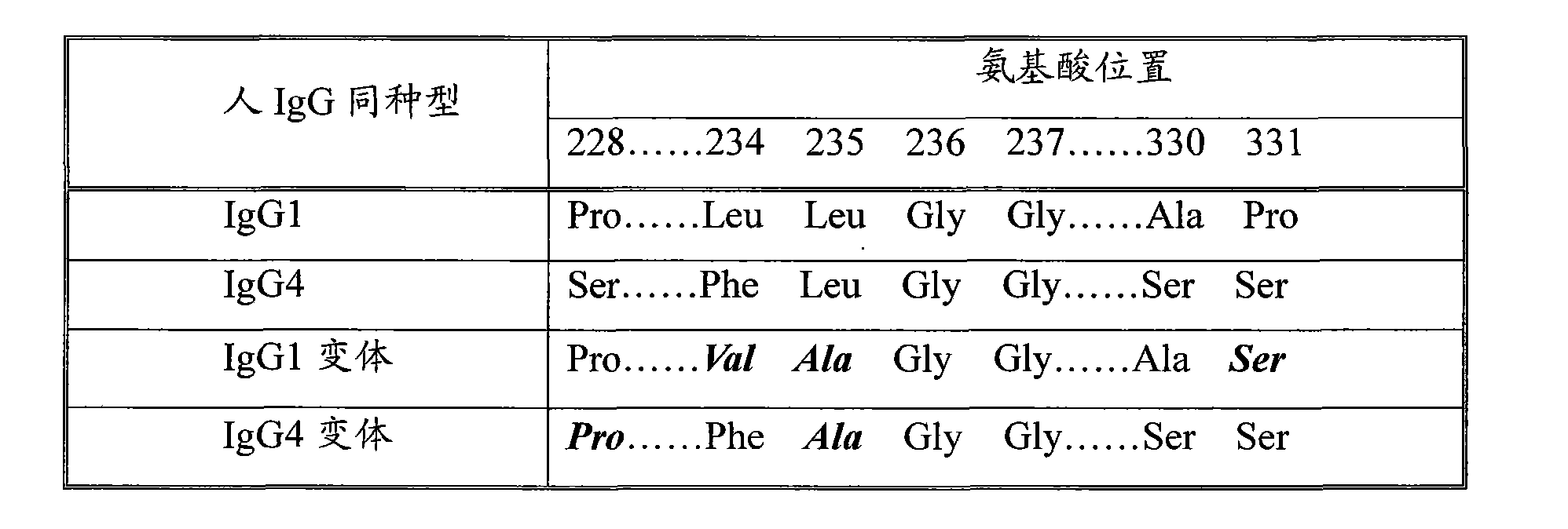
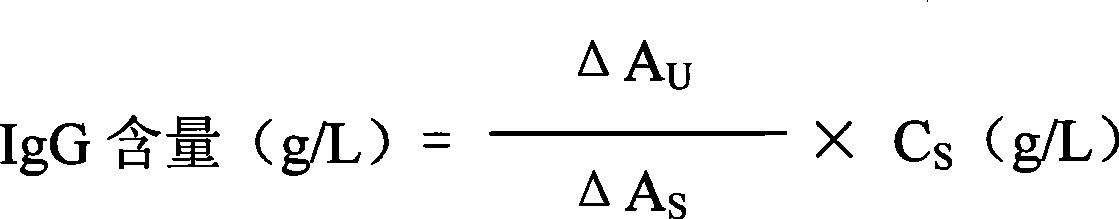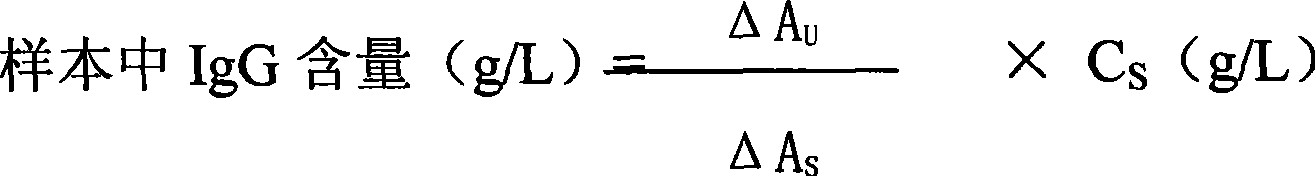Patents
Literature
366 results about "Immunoglobulin G" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
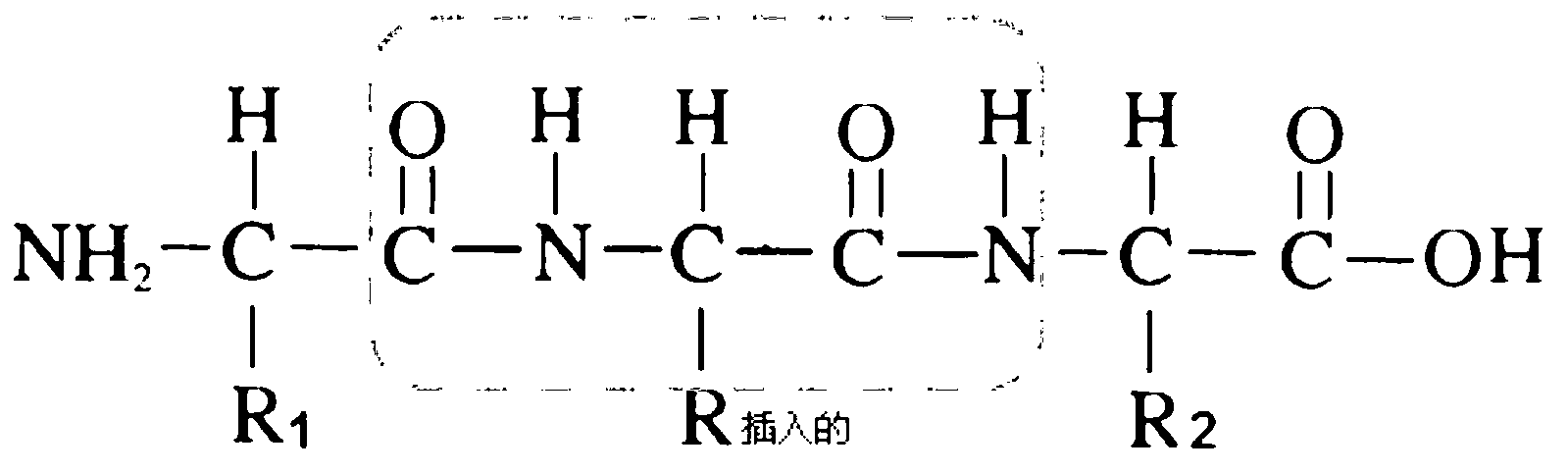
Immunoglobulin G (IgG) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG has two antigen binding sites.
A kind of infant formula milk powder that does not get angry and its preparation process
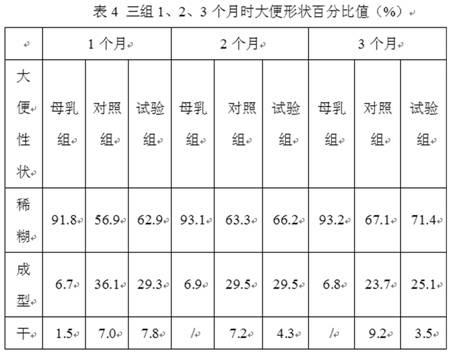
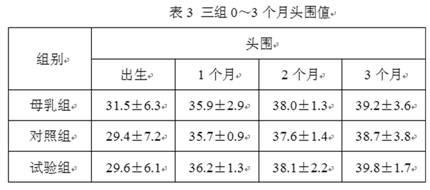
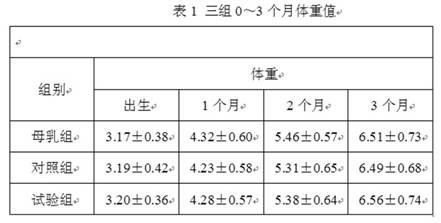
ActiveCN102283289ANot ediblePromote digestion and absorptionMilk preparationVegetable oilFructooligosaccharide
The invention relates to an anti-inflaming infant formula milk powder and a preparation process thereof. The anti-inflaming infant formula milk powder comprises the following components in percentage by weight: 22%-50% of lactose, 10%-20% of goat milk whey protein concentrate, 9.8%-20% of structural grease 1,3-dioleoyl 2-palmitoyl triglyceride, 10%-13.5% of non-fat goat milk powder, 8%-10% of vegetable oil, 2%-8% of beta-casein, 0.8%-1.6% of fructooligosaccharides, 0.5%-1.5% of mineral premix, 0.3%-1% of galactooligosaccharide, 0.2%-1% of immunoglobulin G, 0.2%-0.5% of lactulose, 0.10%-0.45% of arachidonic acid, 0.1%-0.6% of docosahexaenoic acid, 0.06%-0.12% of vitamin premix, 0.04%-0.06% of lactoferrin, and 0.01%-0.06% of nucleotide. The invention also includes the preparation process ofthe infant formula milk powder. The nutrition constituents and the functions of the infant formula milk powder are close to those of breast milk, the infant formula milk powder is easy to assimilate,and infants do not get inflamed after eating the milk powder.
Owner:AUSNUTRIA DAIRY CHINA
Purified antigen for Alzheimer's disease and methods of obtaining and using same
The invention relates, among other things, a preparation comprising Alzheimer's disease antigen (A68), as well as methods of obtaining this purified antigen, and methods of using this purified antigen, for instance, for diagnosing Alzheimer's disease and for detecting human autoantibodies to the Alzheimer disease antigen. The antigen preparation according to the invention is purified in that it is substantially free of immunoglobulin G. The invention further relates to methods of making Alzheimer disease antigens that can be used instead of or along with the A68 antigen preparation (e.g., for diagnosing AD), such as recombinant human tau, tau isolated from various species including human, and phosphorylated recombinant human tau or isolated tau, as well as A68 anti-idiotypic antibodies.
Owner:MOLECULAR GERIATRICS
Stabilising formulation for immunoglobulin g compositions in liquid form and in lyophilised form
This invention is related to a stabilising formulation for immunoglobulins G compositions comprising a sugar alcohol, glycine and a non-ionic detergent, which is suitable for the stabilization of immunoglobulins G compositions in liquid form and in lyophilised form. The invention also relates to an immunoglobulins G composition in liquid form or in lyophilised form comprising said stabilising formulation.
Owner:YEDA RES & DEV CO LTD
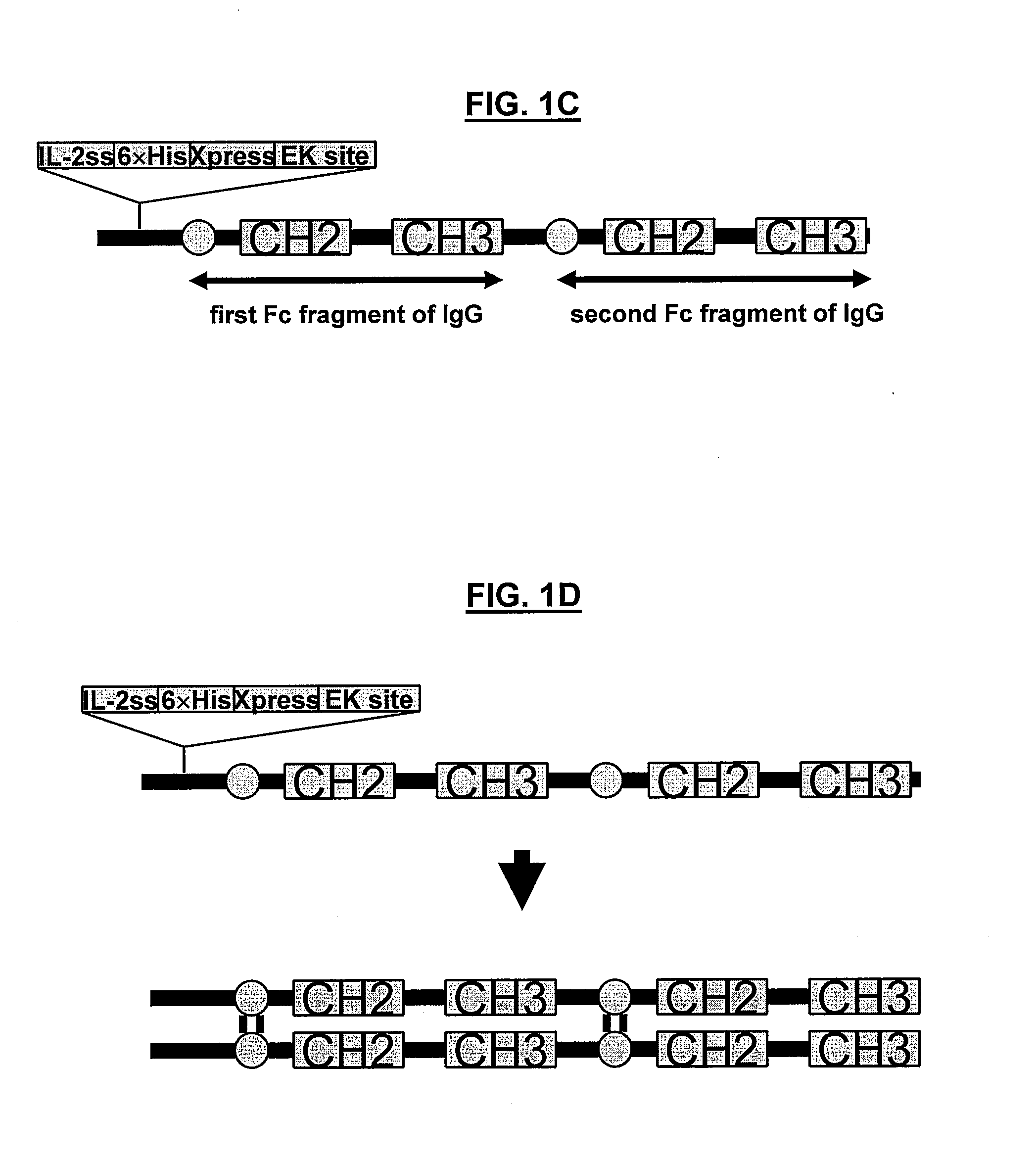
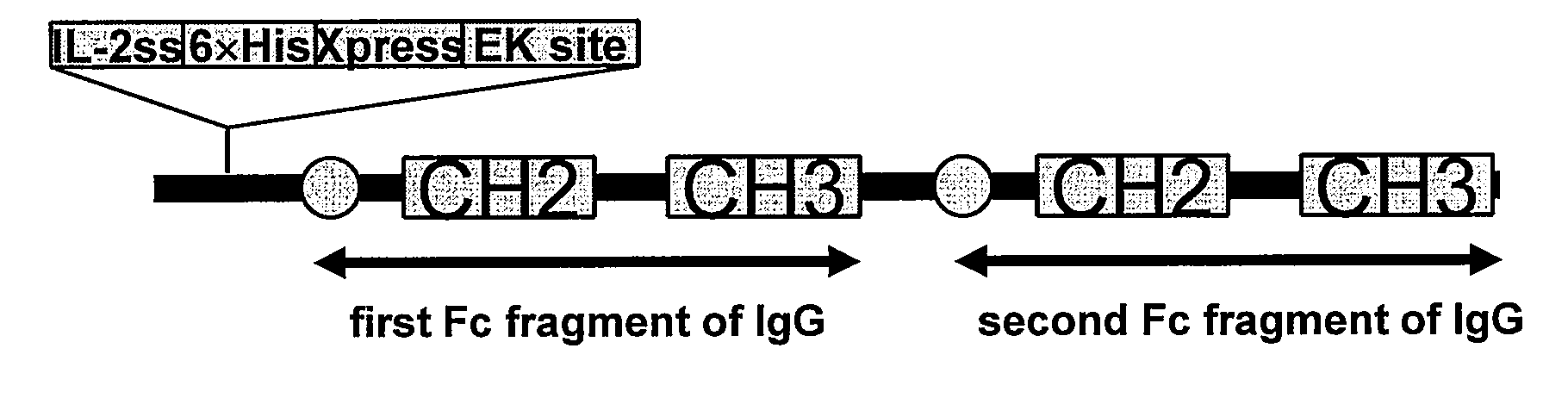
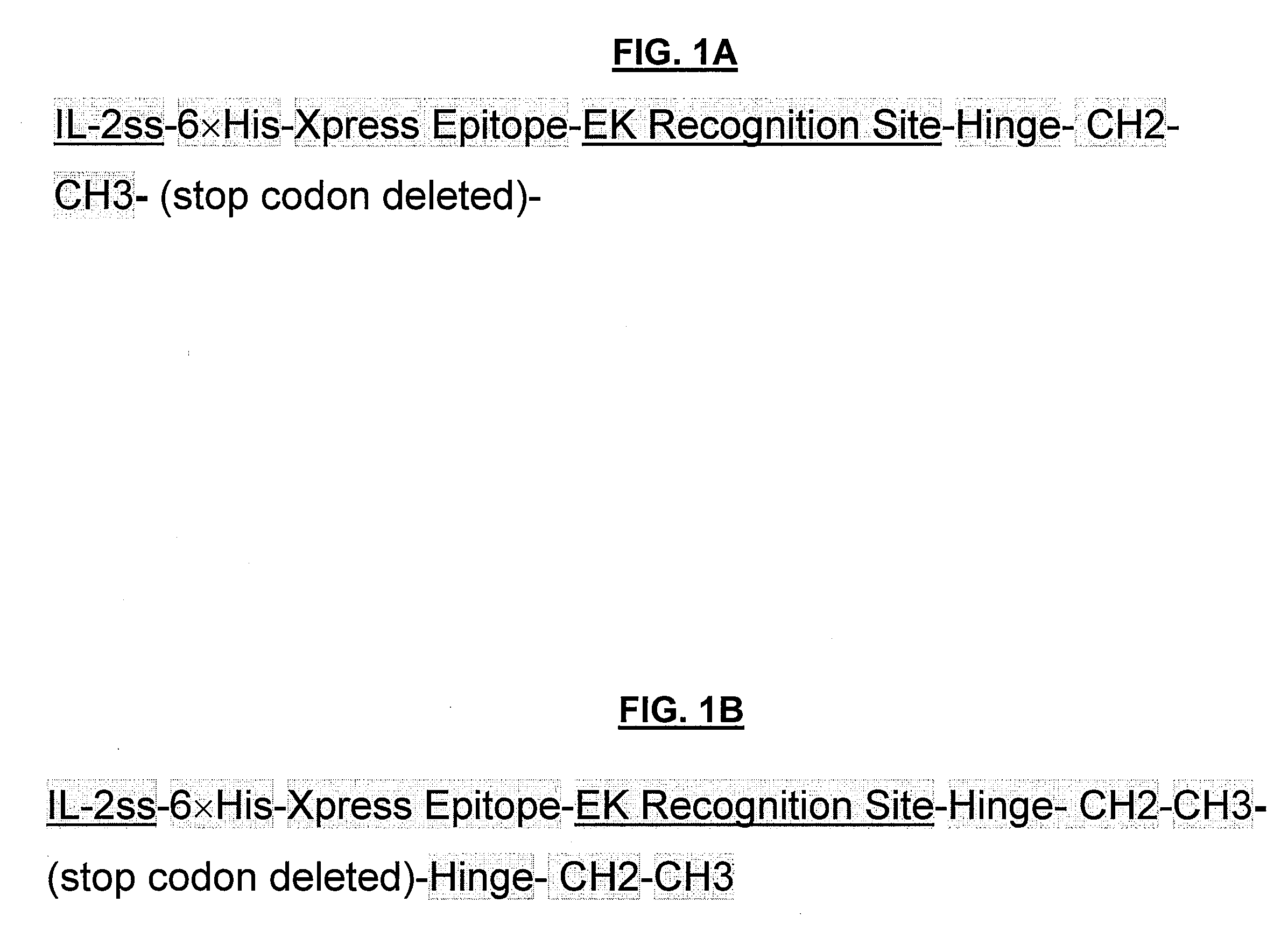
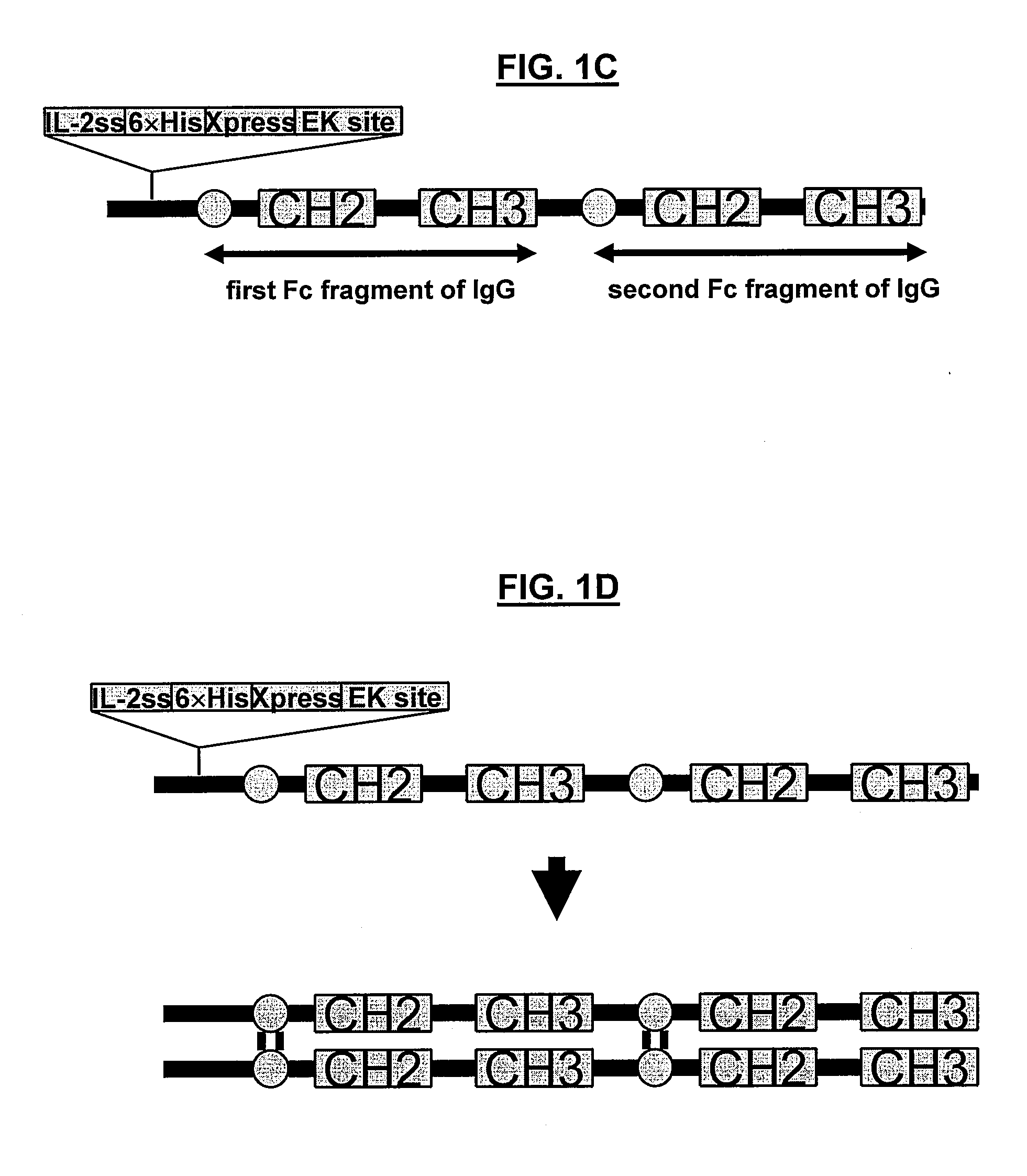
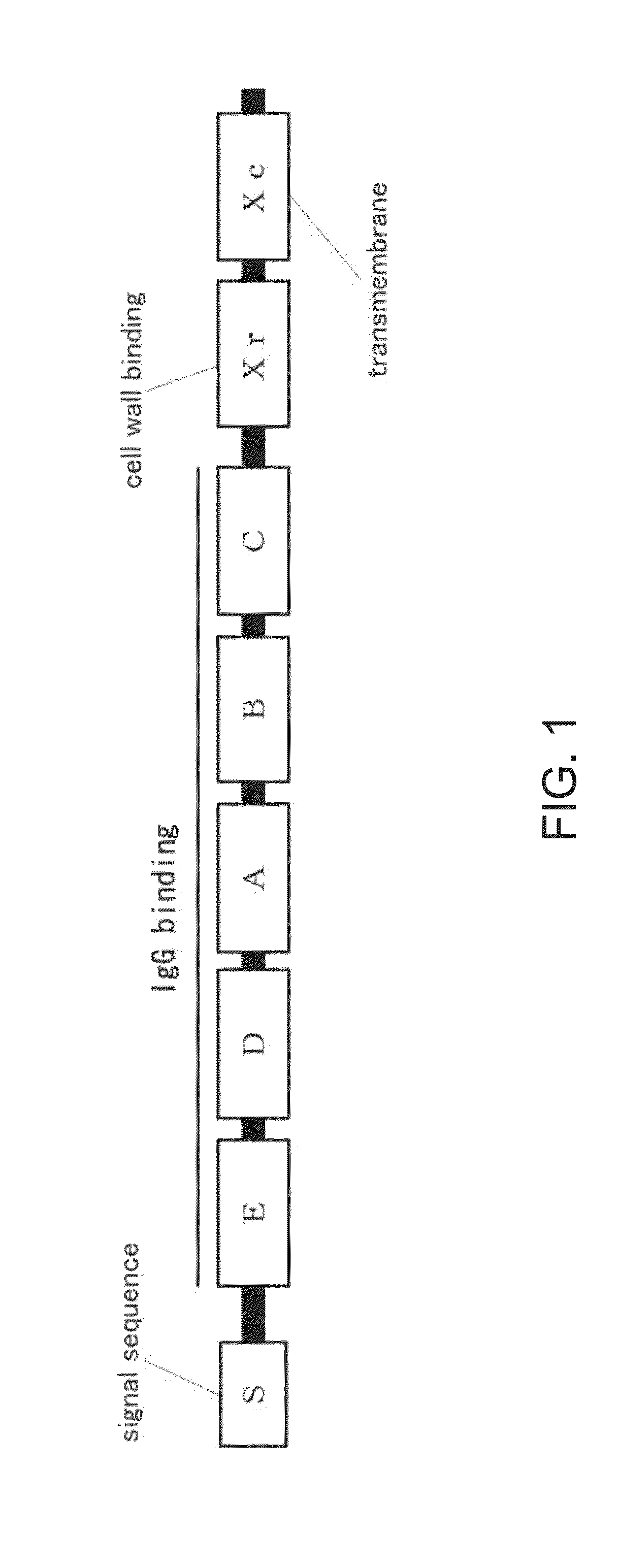
POLYPEPTIDES COMPRISING Fc FRAGMENTS OF IMMUNOGLOBULIN G (lgG) AND METHODS OF USING THE SAME
Polypeptides comprising at least a first and second Fc fragment of IgG that can be used to induce a stimulated cell to produce the anti-inflammatory cytokine Interleukin-10 and methods of using the same are disclosed herein.
Owner:LEUKOSIGHT +1
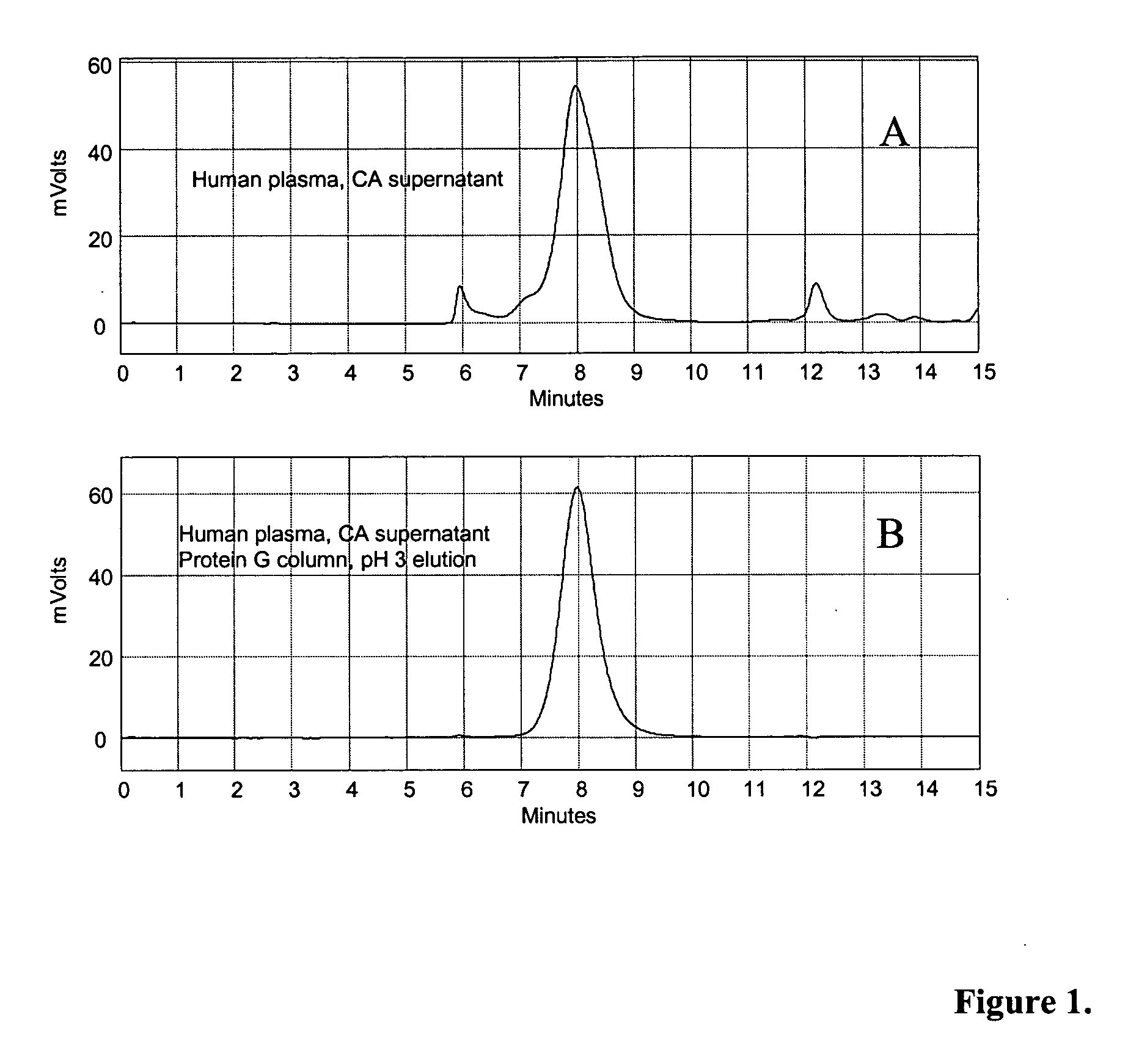
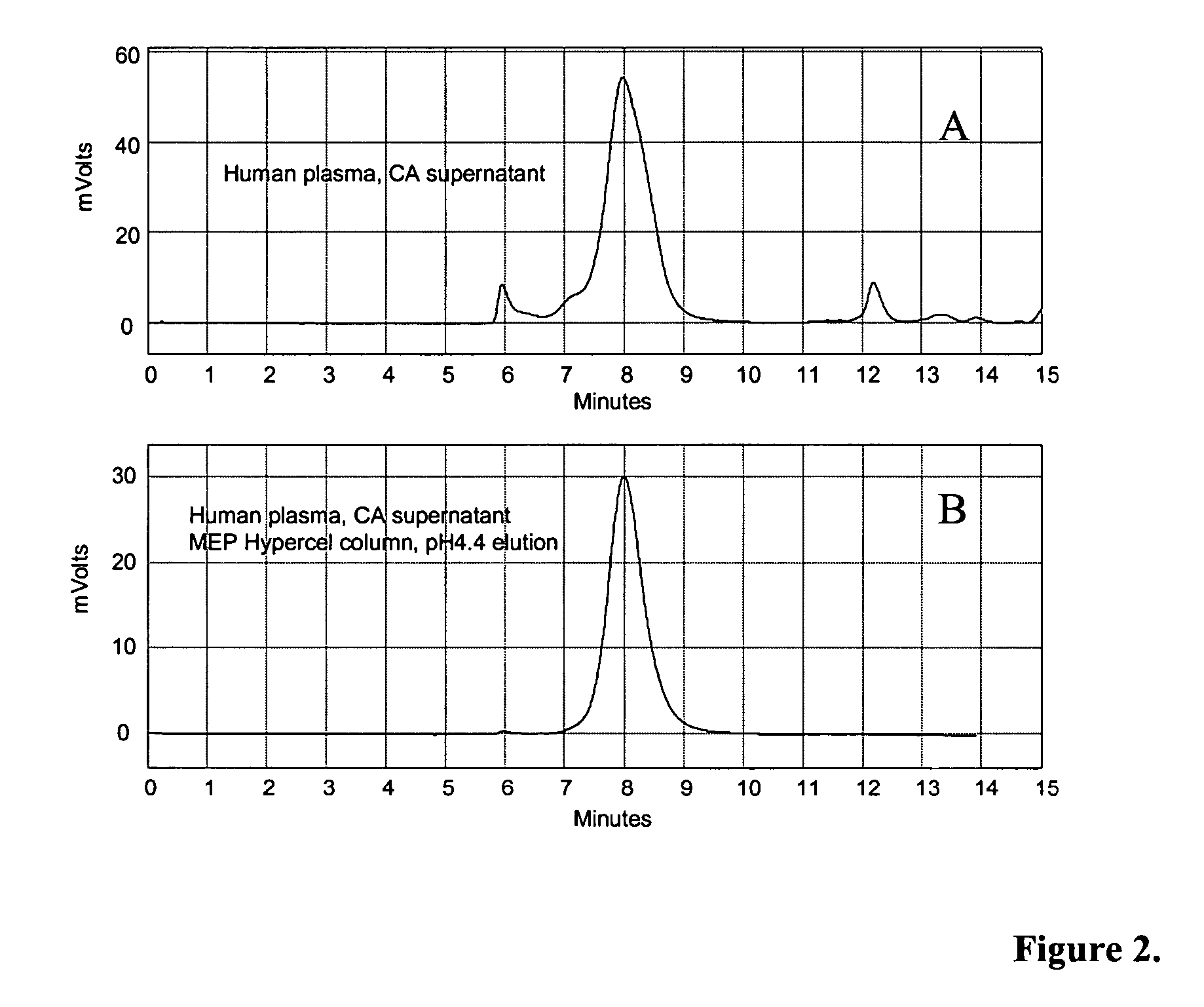
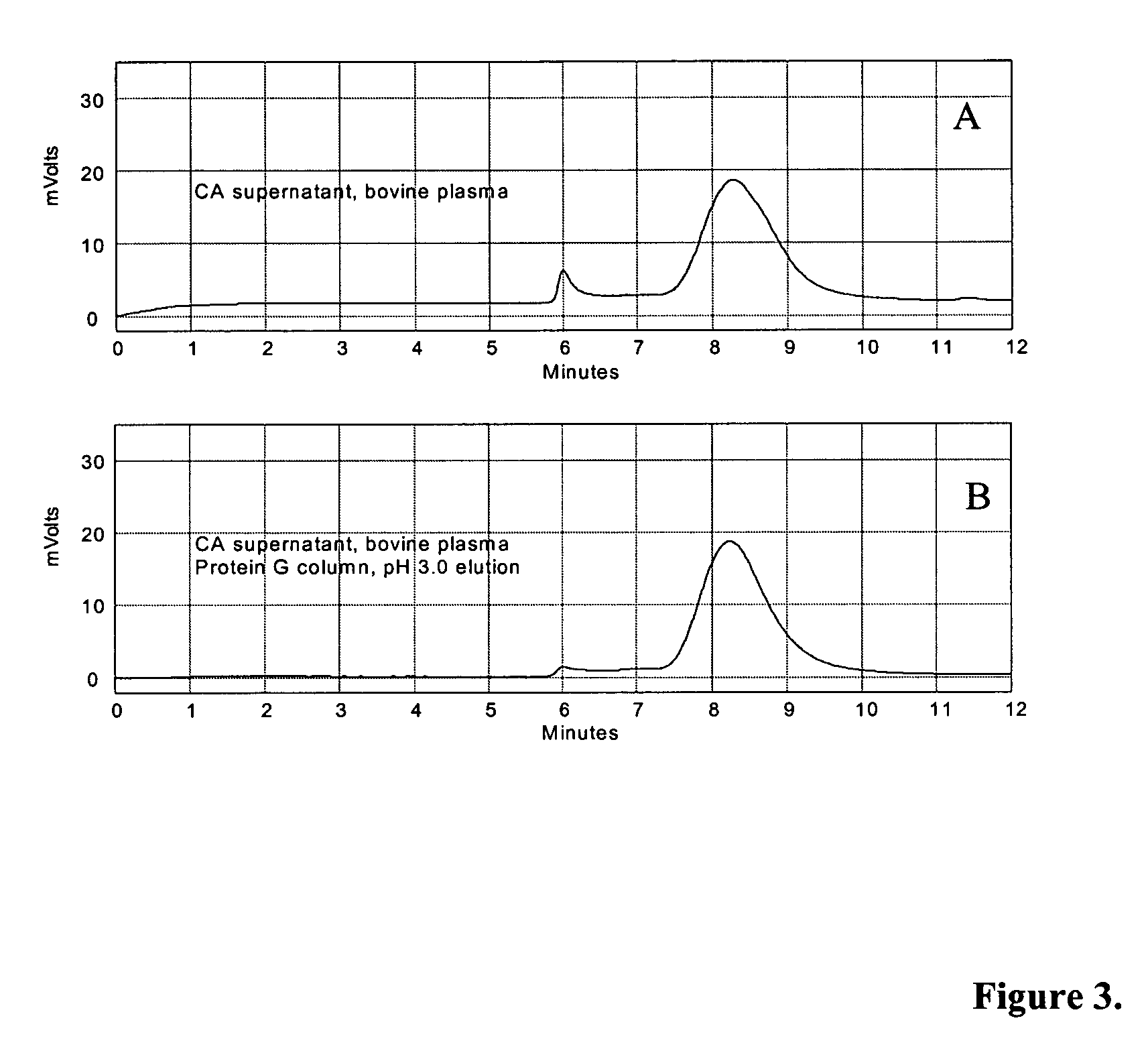
Methods for immunoglobulin purification
InactiveUS20050272917A1Serum immunoglobulinsImmunoglobulins against animals/humansImmunglobulin eBovine serum albumin
Disclosed herein are methods for purifying immunoglobulin G (IgG). The methods feature the use of particular buffers and reagents to isolate and purify human IgG or to remove host contaminating proteins, non-human or chimeric IgG, IgG dimers, IgG aggregates, bovine serum albumin, transmissible spongiform encephalopathy, DNA, viral DNA, or viral particles from a feedstock. IgG purified by the methods described herein can be used for research, diagnostic, or therapeutic purposes.
Owner:KYOWA HAKKO KIRIN CO LTD
Long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc)
ActiveCN103539860AImprove biological activitySmall toxicityPeptide/protein ingredientsAntibody mimetics/scaffoldsRecombinant human follicle stimulating hormoneSide effect
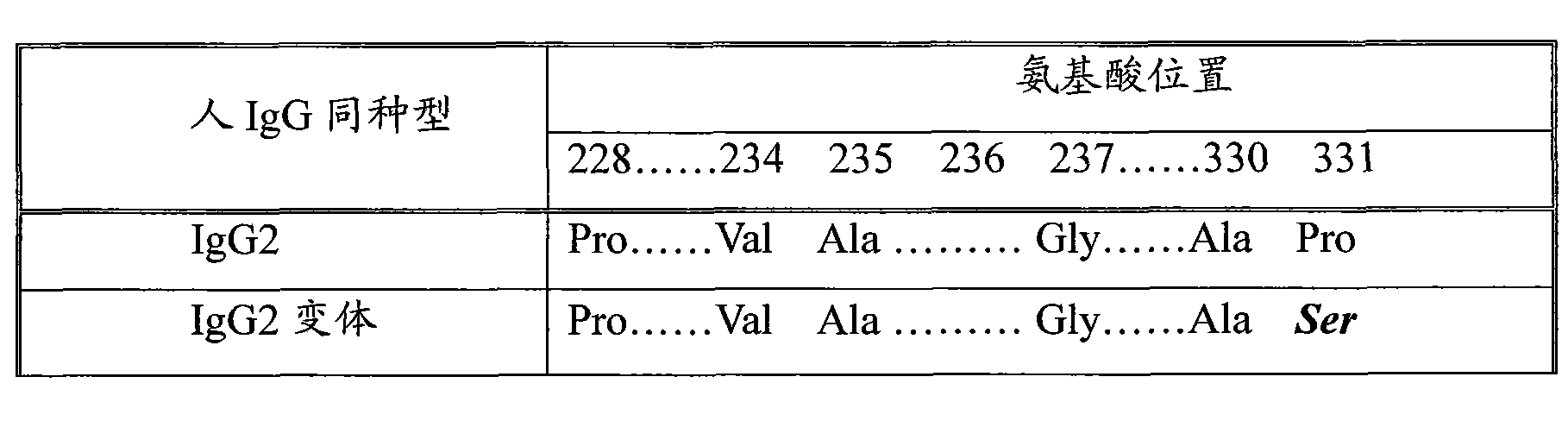
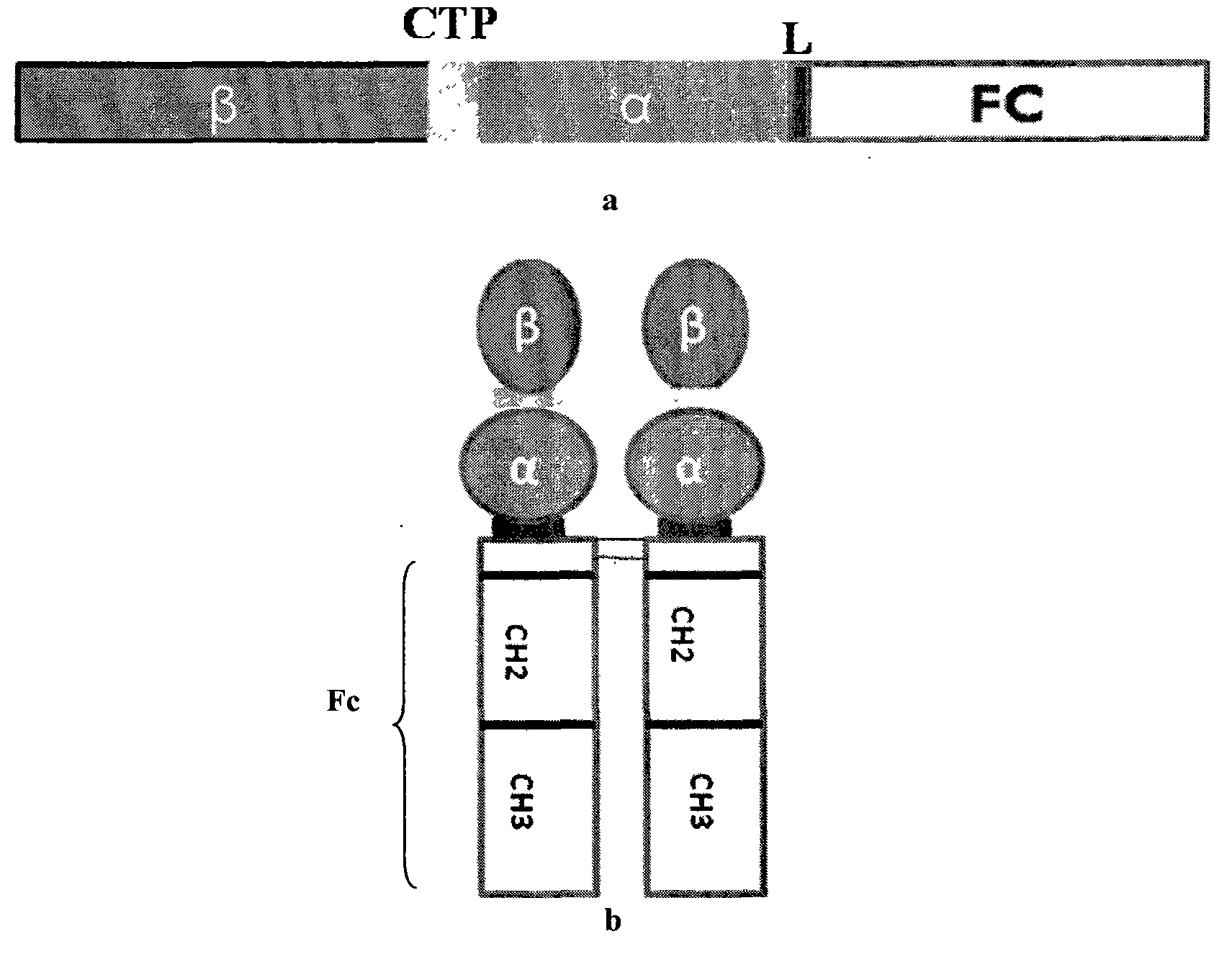
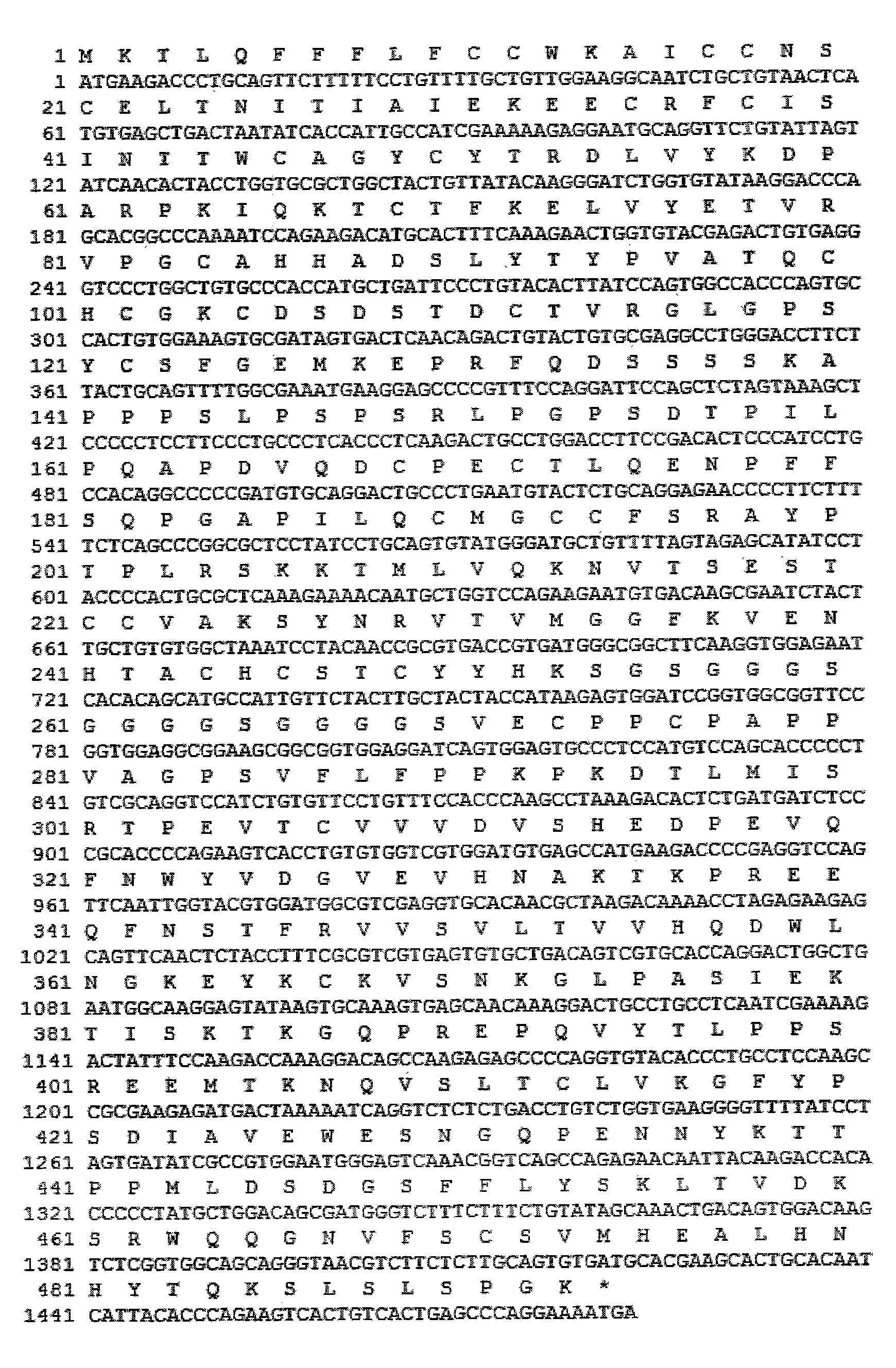
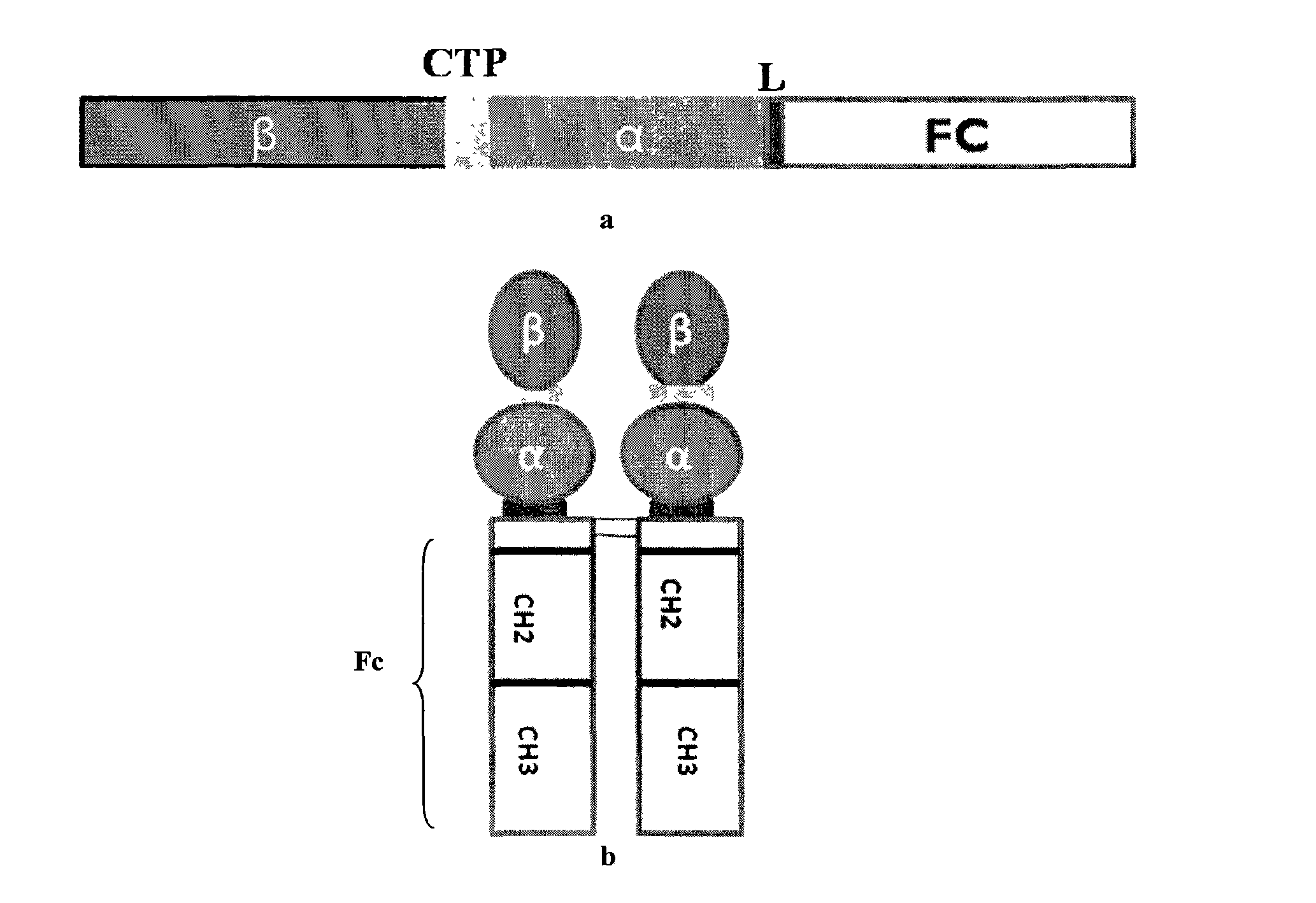
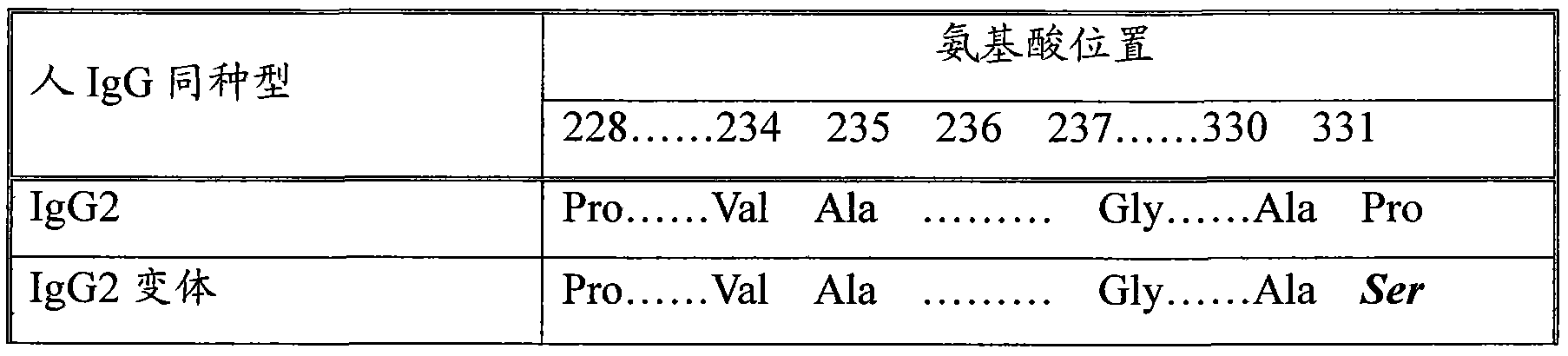
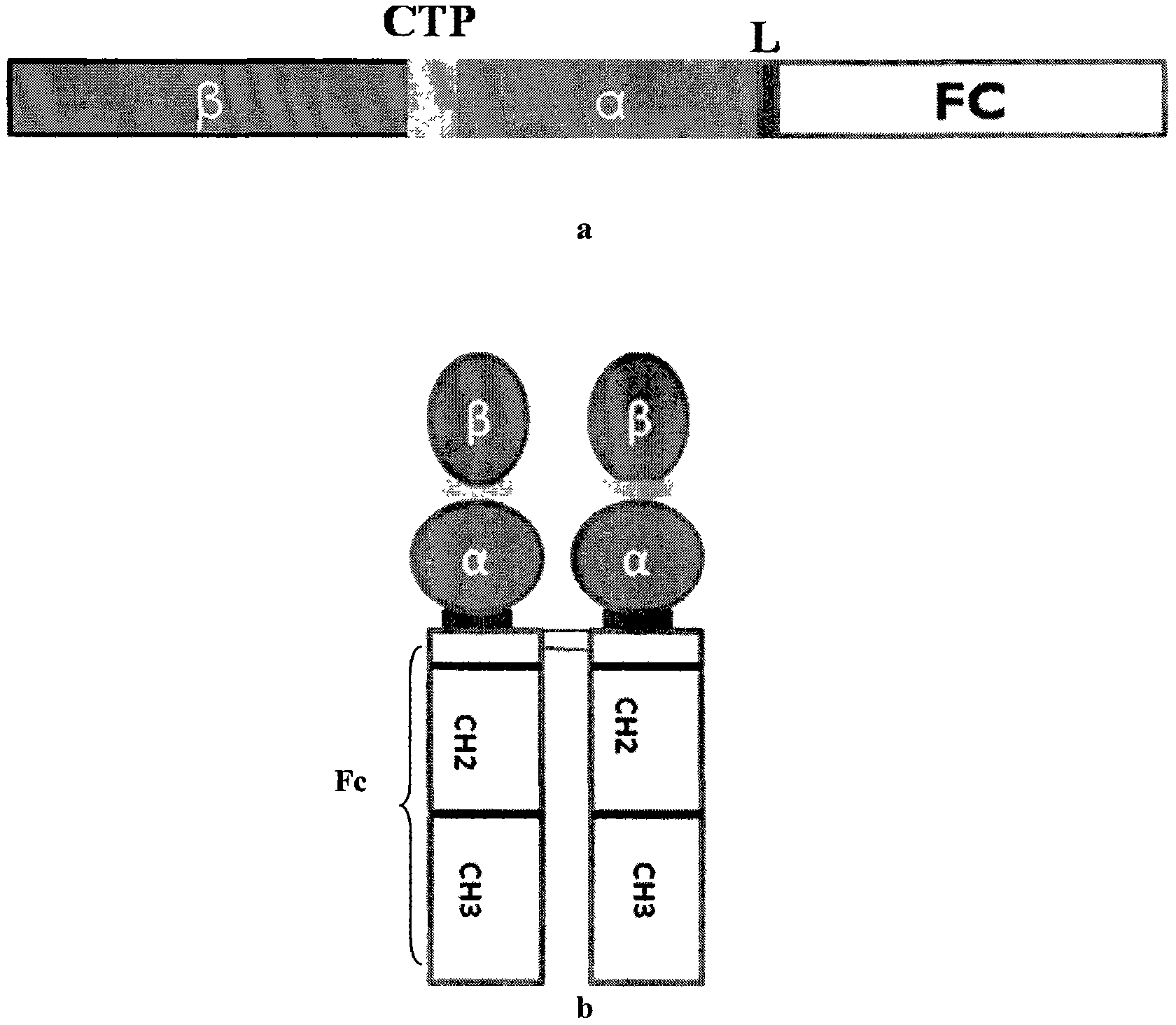
The invention discloses a long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc) and a preparation method thereof. The recombinant hFSH-Fc is a dimerization fusion protein. An amino acid sequence of the hFSH-Fc comprises an hFSHbeta subunit, CTP (carboxy-terminal peptide), an hFSHalpha subunit, a flexible peptide linker and a human IgG (immunoglobulin G)2 Fc variant from the N terminal to the C terminal in sequence. The hFSH-Fc has longer half-life in vivo and smaller side effects than existing hFSH. The invention also relates to an application of a recombinant hFSH-Fc composition to preparation of drugs for treating and / or preventing infertility.
Owner:UNICOHEALTH CO LTD
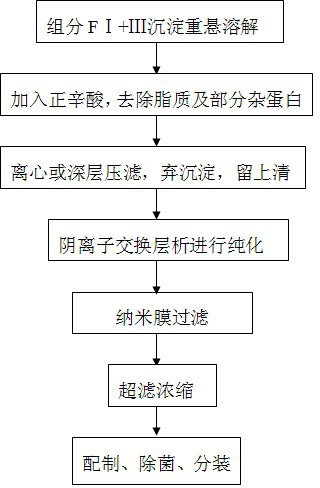
Method for purifying human immunoglobulin from separated component I+III of blood plasma
ActiveCN102250240ARealize comprehensive utilizationAvoid pollutionPeptide preparation methodsImmunoglobulinsBiotechnologyUltrafiltration
The invention relates to a method for separating and purifying human immunoglobulin from a component I+III of blood plasma, and aims to provide a high-efficiency method for recovering high-purity human immunoglobulin. According to the technical scheme provided by the invention, the method comprises the following steps of: a, fully dissolving component I+III precipitate; b, precipitating with octylic acid and removing lipid and a part of impurity protein to prepare IgG (Immunoglobulin G); c, purifying through anion exchange column chromatography; and d, collecting flow-through liquid, performing membrane nanofiltration, ultrafiltration and concentration, preparing the human immunoglobulin, sterilizing and packaging. The method has the beneficial effects of capability of being operated at the room temperature, simple and short steps, high yield, low energy consumption and high output and is suitable for mass production; comprehensive utilization of the blood plasma is fully realized; the time of the entire production process is shortened; the cost is reduced; extremely considerable economic benefit can be produced; the safety of a product is guaranteed by using two virus inactivation / elimination methods of different mechanisms; the environmental pollution is avoided; and the method has high economic and social values.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc)
ActiveCN103539861AImprove biological activityHigh activityPeptide/protein ingredientsPeptide preparation methodsSide effectRecombinant human follicle stimulating hormone
The invention discloses a long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc) and a preparation method thereof. The hFSH-Fc is a dimerization fusion protein. An amino acid sequence of the hFSH-Fc comprises an hFSHbeta subunit, CTP (carboxy-terminal peptide), an hFSHalpha subunit, a flexible peptide linker and a human IgG (immunoglobulin G) Fc variant from the N terminal to the C terminal in sequence. The hFSH-Fc has longer half-life in vivo and smaller side effects than existing hFSH. The invention also relates to an application of a recombinant hFSH-Fc composition to preparation of drugs for treating and / or preventing infertility.
Owner:UNICOHEALTH CO LTD
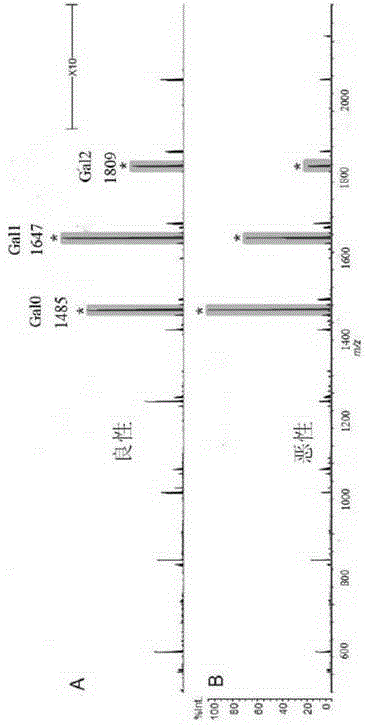
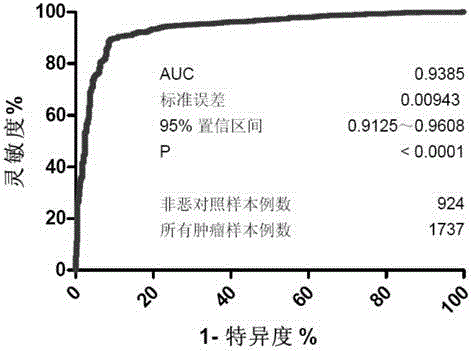
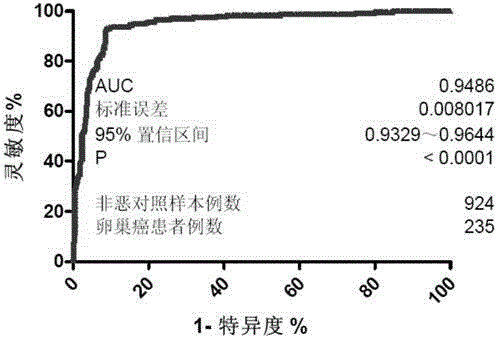
Product for malignant tumor related screening and assessing, and application and method thereof
The invention provides a product for malignant tumor related screening and assessing, and an application and a method thereof, and concretely relates to a product for carrying out screening, early stage diagnosis, prognosis assessment, risk assessment, condition monitoring and / or curative effect assessment on malignant tumors through detecting the change of double antenna complex N-linked sugar chain end galactose on the surface of immunoglobulin G in blood, and an application and a method thereof.
Owner:SHANGHAI ZHIXIAN BIOLOGICAL TECH CO LTD
Immune colloidal gold test strip for detecting porcine epidemic diarrhea virus as well as preparation method and application thereof
ActiveCN103454419AIncreased sensitivityStrong specificityMicroorganism based processesImmunoglobulins against virusesCelluloseNitrocellulose
The invention discloses an immune colloidal gold test strip for detecting porcine epidemic diarrhea virus as well as a preparation method and application thereof. The test strip sequentially comprises a sample pad, a colloidal gold pad, a nitrocellulose membrane, an absorbent paper and a PVC (poly vinyl chloride) base plate arranged below and used as an assembling platform according to a connection sequence, wherein the colloid gold pad comprises a glass cellulose membrane of a PEDV (porcine epidemic diarrhea virus) monoclonal antibody adsorbed with colloidal gold marks, the nitrocellulose membrane is provided with a goat rat resisting IgG (immunoglobulin G) polyclonal antibody coated quality control line and a rabbit resisting PEDV S protein polyclonal antibody coated detection line, and the PEDV monoclonal antibody is generated in secretion of hybridoma cells with a preservation number CCTCC C201392. Experiments prove that the test strip has the advantages of strong specificity and good stability; the operation is simple, technicists do not need special training, no special equipment is needed, the detection cost is low, the detection speed is fast and the results can be read in 5-10 minutes, and the test strip is applicable to field test.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Novel affinity ligand polypeptide library of immunoglobulin G constructed based on protein A affinity model and application of design method
The invention discloses a novel affinity ligand polypeptide library of immunoglobulin G (IgG) constructed based on a protein A affinity model and an application of a design method. According to a molecular mechanics / Poisson-Boltzmann solvent-accessible surface area method, a key residue of protein A with a higher affinity interaction with human IgG is analyzed and obtained based on the available human IgG-protein A compound structure; the simplified protein A affinity model is constructed; the affinity polypeptide molecule library of the IgG is constructed based on the simplified protein A affinity model. On the basis of the peptide library, an amino acid type represented by X is ascertained by further utilizing an amino acid location method. Then, candidate polypeptides are screened gradually by applying molecular docking and molecular dynamic simulation means. Finally, polypeptide affinity ligand capable of effectively separating and purifying the IgG is ascertained through an affinity chromatography experimental method.
Owner:TIANJIN UNIV
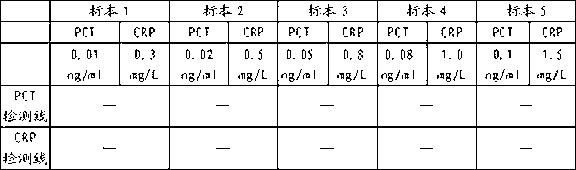
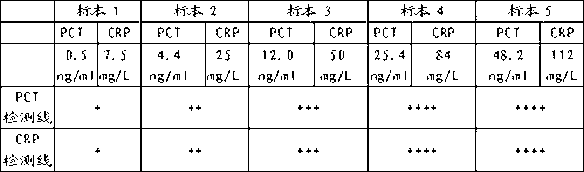
Colloidal gold test strip for combined detection of procalcitonin (PCT)/C-reactive protein (CRP) and preparation method thereof
The invention belongs to the field of clinical medical examination, particularly relates to a colloidal gold test strip for the combined detection of procalcitonin (PCT) / C-reactive protein (CRP). The colloidal gold test strip comprises a test strip bottom lining, and a sample pad, a polyester film on which gold-labeled antibodies are coated, a coating film and absorbent paper which are sequentially overlapped with and adhered to each other on the test strip bottom lining, wherein the coating film is provided with a control line which coats a rabbit antimouse immunoglobulin G (IgG) antibody, and two detection lines which are parallel to the control line and coat an antibody which can be in specific binding with a to-be-detected antigen PCT and an antibody which can be in the specific binding with a to-be-detected antigen CRP respectively; and two kinds of gold-labeled antibodies are provided, namely the antibody which is labeled by colloidal gold and can be in the specific binding with the to-be-detected antigen PCT and the antibody which is labeled by the colloidal gold and can be in the specific binding with the to-be-detected antigen CRP. By the colloidal gold test strip, the PCT / CRP can be detected simultaneously, the accuracy of diagnosing an inflammatory reaction is improved, and the gold test strip is easy to operate.
Owner:GETEIN BIOTECH
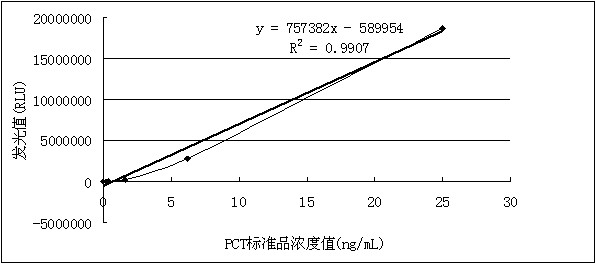
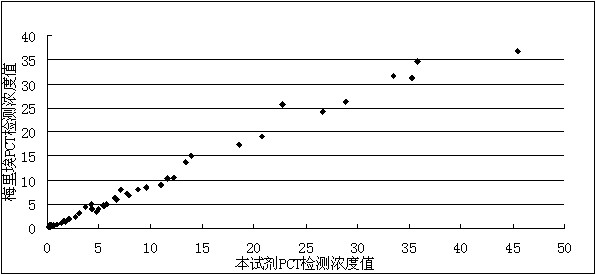
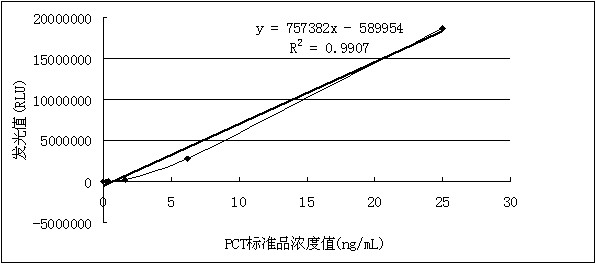
Kit for detecting procalcitonin
ActiveCN102305858AReduce dosageImprove responseChemiluminescene/bioluminescenceBiotin-streptavidin complexProtein molecules
The invention discloses a kit for detecting procalcitonin. The kit comprises a magnetic particle separation reagent, a PCT (procalcitonin) detection antibody, an enzyme conjugate and a luminescent substrate, wherein the protein molecule coupled with the magnetic particles in the magnetic particle separation reagent is goat anti-mouse immunoglobulin G or streptavidin; the PCT detection antibody isa mouse anti-PCT monoclonal antibody; and the enzyme conjugate is a horseradish peroxidase-labeled goat anti-PCT polyclonal antibody. The kit has the beneficial effects of high detection sensitivity and low cost.
Owner:SHENZHEN GOLDSITE DIAGNOSTICS
Immunomodulatory proteins
InactiveUS20150218236A1Easy to assembleSenses disorderNervous disorderFc(alpha) receptorCysteine thiolate
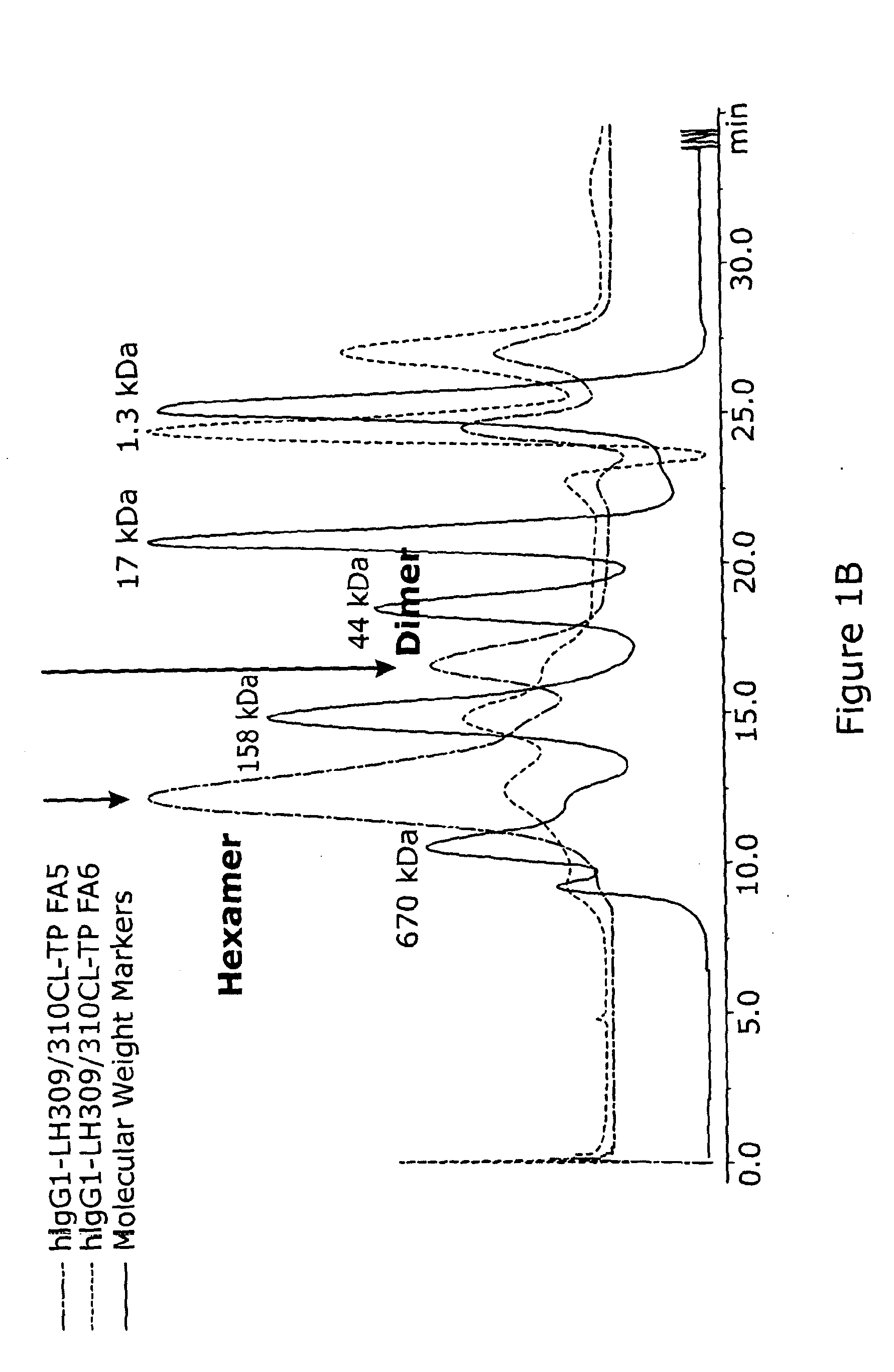
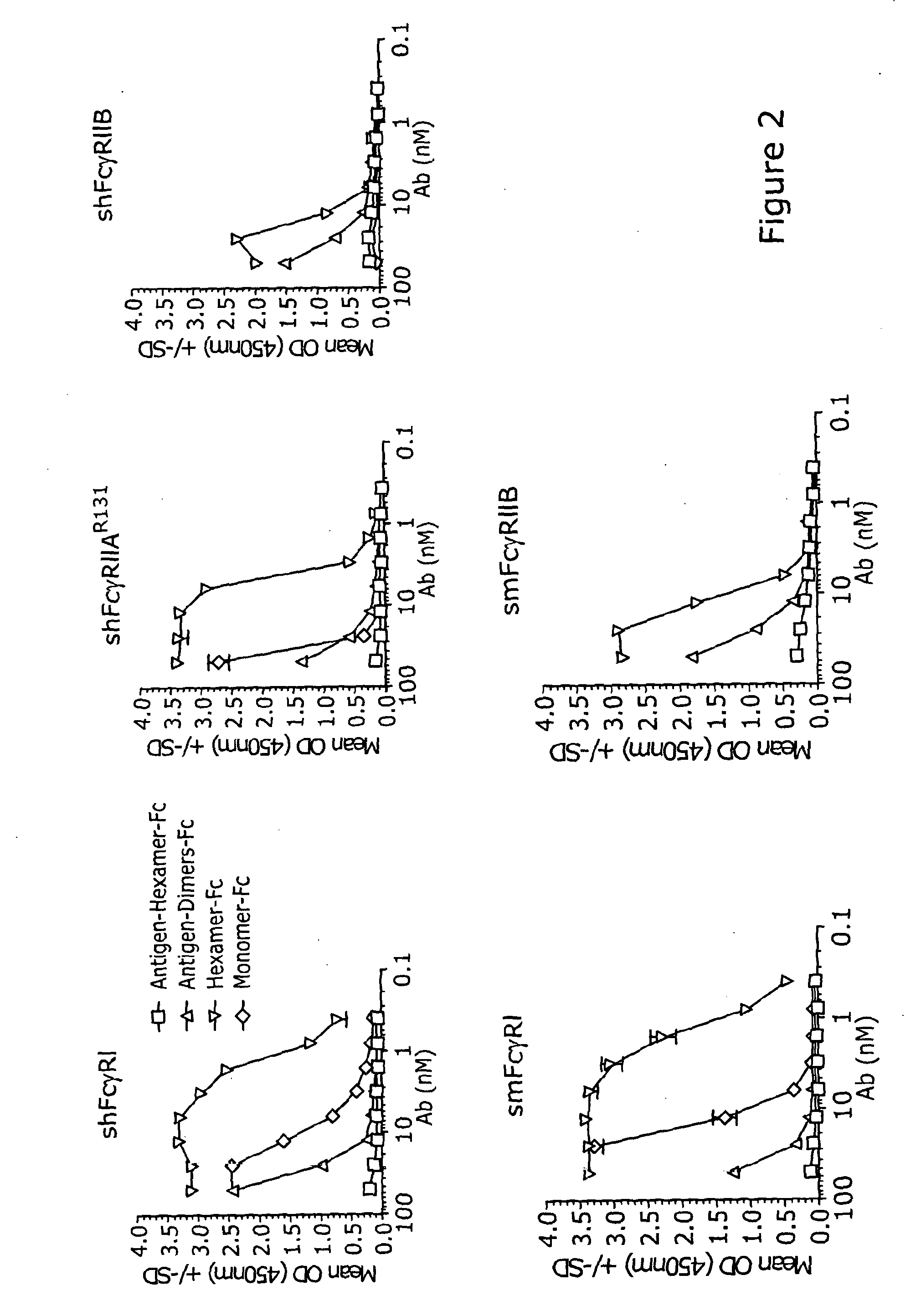
A method for treatment of a mammalian subject for an autoimmune or inflammatory disease, the method comprising: administering to the mammalian subject an effective amount of a polymeric protein comprising five, six or seven polypeptide monomer units; wherein each polypeptide monomer unit comprises an Fc receptor binding portion comprising two immunoglobulin G heavy chain constant regions; wherein each immunoglobulin G heavy chain constant region comprises a cysteine residue which is linked via a disulfide bond to a cysteine residue of an immunoglobulin G heavy chain constant region of an adjacent polypeptide monomer unit; wherein the polymeric protein does not comprise a further immunomodulatory portion; or an antigen portion that causes antigen-specific immunosuppression when administered to the mammalian subject.
Owner:LIVERPOOL SCHOOL OF TROPICAL MEDICINE
Kit for detecting cyclic citrullinated peptide (CCP) and immunoglobulin G (IgG) resistant bispecific antibody
InactiveCN101957365ANo pollution threatSimple and fast operationColor/spectral properties measurementsSerum igeBispecific antibody
The invention discloses a kit for detecting a cyclic citrullinated peptide (CCP) and immunoglobulin G (IgG) resistant bispecific antibody, which belongs to the field of clinical laboratory science. The kit of the invention is developed based on an enzyme-linked immunosorbent assay technology, utilizes a double-antigen sandwich method, comprises two antigens of CCP and IgG and can detect a naturalbispecific antibody existing in the serum of a rheumatoid arthritis patient, is convenient for use and can simply and easily detect the bispecific antibody.
Owner:BEIJING HOSPITAL
Kit for detecting creatine kinase isoenzyme and preparation and use methods thereof
The invention relates to a kit for detecting creatine kinase isoenzymes and preparation and use methods thereof. The kit for detecting creatine kinase isoenzymes comprises a substrate, as well as a sample pad, a conjugate pad, a coupling pad, a nitrocellulose membrane and water absorbent paper, which are arranged from one end to the other end on the substrate, wherein the conjugate pad is coated with a conjugate of fluorescence silicon dioxide nanoparticle-anti-creatine kinase isoenzyme MB monoclonal antibody, the nitrocellulose membrane is provided with a test line and a quality control line, the test line is arranged on the side close to the coupling pad and contains an anti-creatine kinase isoenzyme MB monoclonal antibody, and the quality control line is arranged on the side away from the coupling pad and contains goat anti-mouse IgG (Immunoglobulin G). The kit is low in price and has simple preparation process, and works with a high detection speed to provide the detection result within 15 minutes. The portable fluorescence detection device is adopted to achieve the purpose of accurate quantification of a target analyte. The kit is convenient to operate, can be applied to on-site detection, and is suitable for operators who do not need training. The kit has the advantages of high sensitivity, good specificity and accurate result, and is convenient for generalization and application.
Owner:王迎峰
Detection method and detection kit of antigen-specific IgG (immunoglobulin G) antibody of staphylococcus aureus SpA5 mutant
ActiveCN103645318AEliminate distractionsStrong specificityBiological material analysisSpecific iggStaphylococcus aureus
The invention belongs to the technical field of biology, and relates to a detection method and a detection kit of an antigen-specific IgG (immunoglobulin G) antibody of a staphylococcus aureus (SA) SpA5 mutant. By adopting the method disclosed by the invention, the specificity of the method is improved, and the method is simple and fast in operation, and good in repeatability.
Owner:CHENGDU OLYMVAX BIOPHARM +1
Semi-quantitative rapid detection test paper for immunoglobulin G in bovine colostrum and products thereof and preparation method thereof
InactiveCN101915833AAchieve semi-quantitative determinationExpand the detection rangeMaterial analysisGlass fiberCellulose
The invention discloses semi-quantitative rapid detection test paper for immunoglobulin G (IgG) in bovine colostrum and products thereof and a preparation method thereof, which relate to the semi-quantitative detection test paper for the IgG and the preparation method thereof and solve the problems of the complex detection operating procedure, long detection time and unsuitability for field operation of IgG in the conventional bovine colostrum and the products thereof. The test paper consists of a back lining, a sample absorbing pad, glass fiber, a cellulose nitrate membrane and a water absorbing pad, wherein the cellulose nitrate membrane is provided with 1 to 4 detection lines and 1 quality control line. The method comprises the following steps of: 1, preparing a rabbit anti-bovine IgG-colloidal gold marker and enveloping the marker on the glass fiber; 2, enveloping the detection lines and the quality control line on the cellulose nitrate membrane; and 3, assembling the test paper. The test paper has the advantages of high detection flexibility, minimum detectable amount of 10mu g / mL, high specificity, no cross reaction with IgG in ovine colostrum and products thereof, simple procedure, high detection speed, detection time of generally 15 to 20 minutes for one sample and suitability for field detection.
Owner:NORTHEAST INST OF GEOGRAPHY & AGRIECOLOGY C A S
Premature rupture of membrane fast examination tool using ICAM-1 as examination index and examination kit
The invention discloses a premature rupture of membrane (PROM) fast examination tool using an intercellular adhesion molecular (ICAM)-1 as an examination index. The tool comprises a gasket, an absorbent pad, a nitrocellulose membrane, a conjugate pad and a sample pad, wherein the absorbent pad, the nitrocellulose membrane, the conjugate pad and the sample pad are connected from top to bottom and adhered to the gasket; the conjugate pad is covered by the sample pad part; the nitrocellulose membrane is provided with a goat-anti-human ICAM-1 polyclonal antibody coated examination line and a goat-anti-mouse immunoglobulin G polyclonal antibody coated control line; the examination line is below and away from the control line at intervals; and the conjugate pad is a water absorbing fibber coated with conjugated mouse-anti-human ICAM-1 monoclonal antibody conjugate. A PROM fast examination kit using the ICAM-1 as the examination index consists of a kit body and the examination tool arranged in the kit body.
Owner:ORIGISSAY BIOLOGICS TECH
Fluorescent probe and method for rapidly detecting staphylococcus aureus by using same
InactiveCN102053151ASimple processStable and controllable fluorescence characteristicsFluorescence/phosphorescenceLuminescent compositionsUv vis absorbanceStaphylococcus aureus
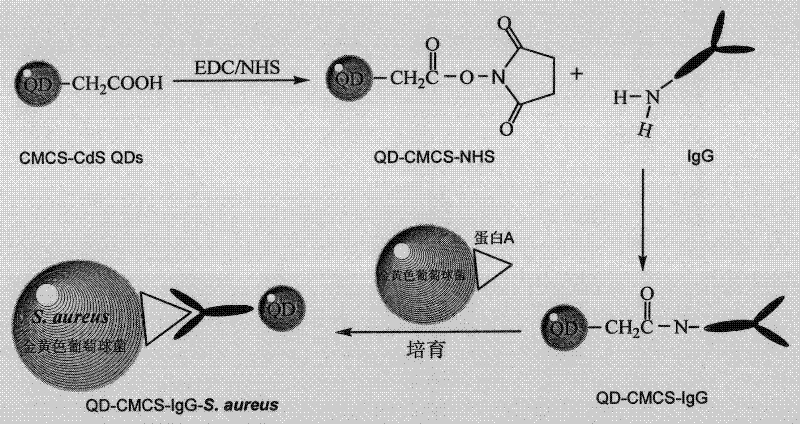
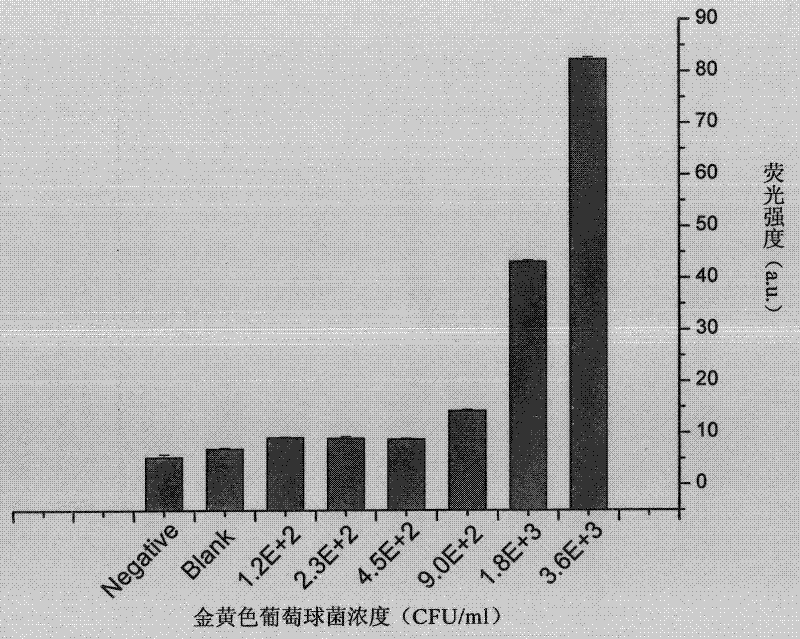
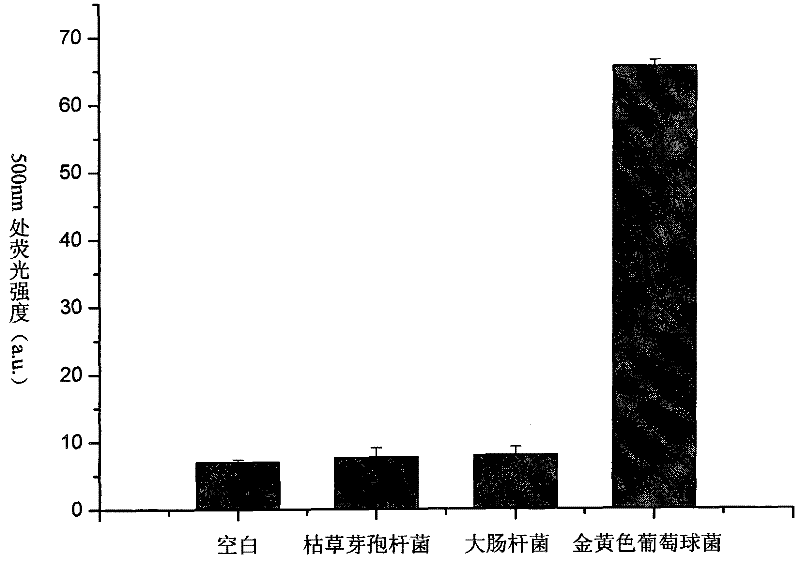
The invention discloses a fluorescent probe, which is prepared by the following method. The method comprises the steps of: firstly, purifying fluorescence quantum dots synthesized in a carboxymethyl chitosan template, and connecting the fluorescence quantum dots with IgG (Immunoglobulin G) antibodies of a human by coupling effect of carbodiimide and N-hydroxysuccinimide eater; and then separating and purifying by using a sephadex column to obtain the fluorescent probe of the fluorescence quantum dots synthesized by immune-modified carboxymethyl chitosan. The invention further discloses a method for detecting staphylococcus aureus by using the fluorescent probe, comprising the detailed steps of: culturing the fluorescent probe and bacteria to be detected for 30 minutes, removing the fluorescent probe with nonspecific absorption by a plurality of times of centrifugal washing, finally, suspending bacteria marked by the fluorescent probe in a phosphate buffer solution, determining the fluorescence intensity and absorption intensity of bacterial suspension by adopting fluorescence spectrum and ultraviolet and visible absorption spectrum, and realizing quantitative determination of the staphylococcus aureus according to linear relation of the fluorescence intensity and the concentration of the bacterial suspension. The method disclosed by the invention is clean, simple and rapid and is suitable for being applied in rapid detection under emergency.
Owner:SOUTH CHINA UNIV OF TECH
Long-acting recombinant follicle-stimulating hormone and application thereof
ActiveCN103539862AImprove biological activityHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsRecombinant human follicle stimulating hormoneHalf-life
The invention discloses a long-acting recombinant human follicle-stimulating hormone-Fc fusion protein (hFSH-Fc) and a preparation method thereof. The hFSH-Fc is a dimerization fusion protein. An amino acid sequence of the hFSH-Fc comprises an hFSHbeta subunit, CTP (carboxy-terminal peptide), an hFSHalpha subunit, a flexible peptide linker and a human IgG (immunoglobulin G)2 Fc variant from the N terminal to the C terminal in sequence. The hFSH-Fc has longer half-life in vivo and better efficacy than existing FSH. The invention also relates to an application of a recombinant hFSH-Fc composition to preparation of drugs in the field of animal breeding.
Owner:GUANGZHOU VBIO PHARM CO LTD
Epitope of systemic lupus erythematosus and application thereof
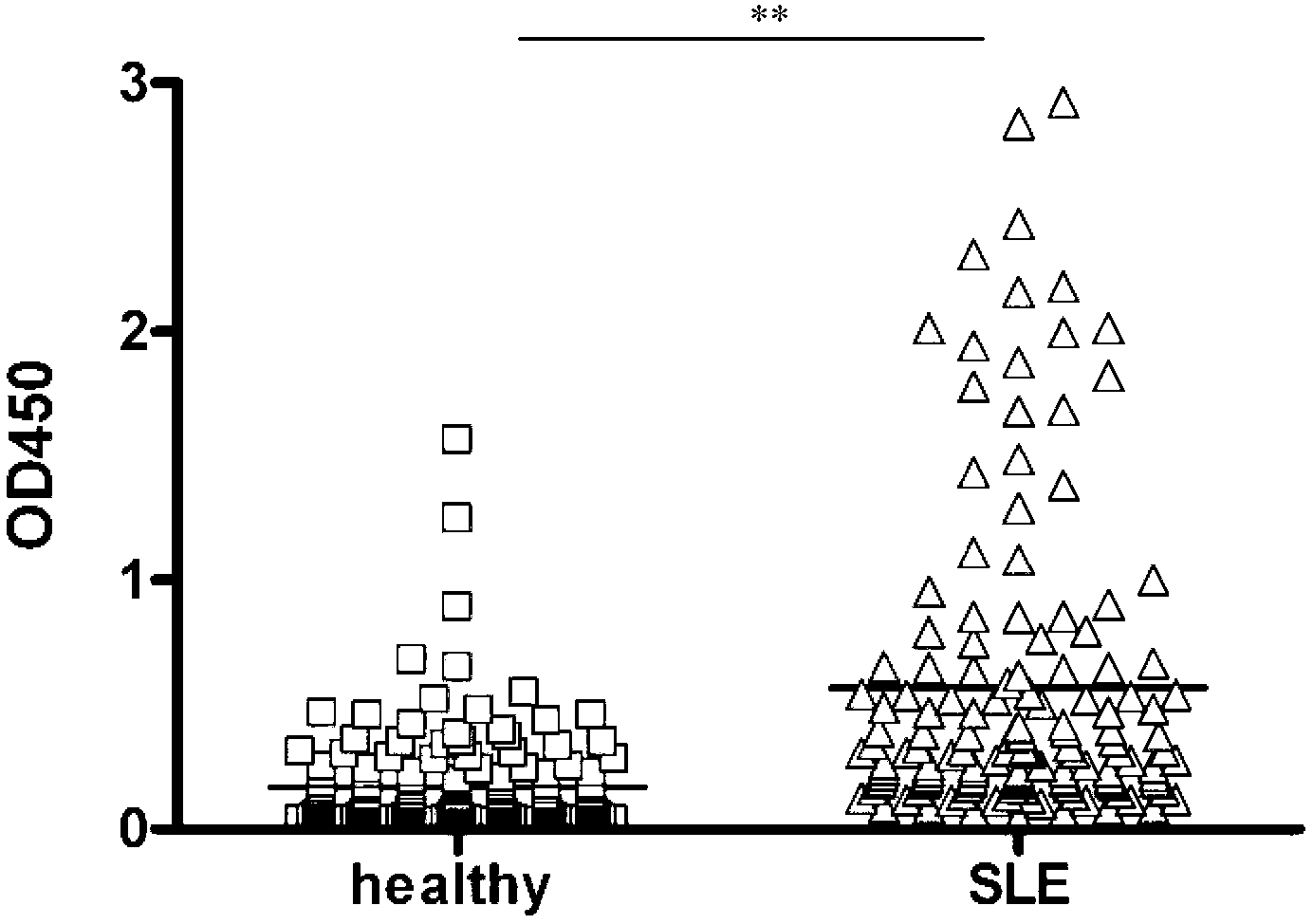
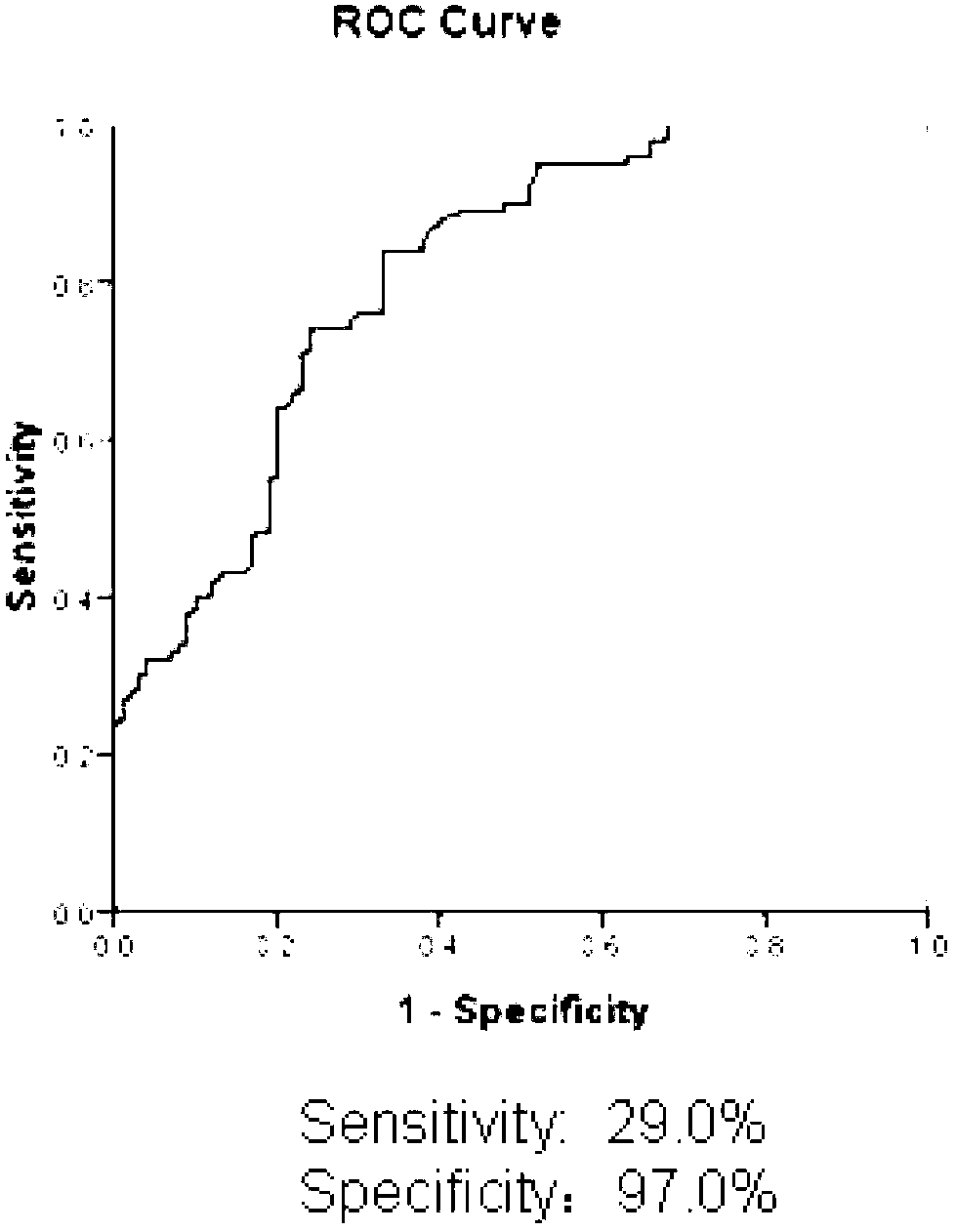
The invention discloses an epitope of systemic lupus erythematosus and an application thereof, belonging to the technical field of immunology diagnosis. The epitope is a polypeptide of which the amino acid sequence is shown as a sequence table SEQ ID NO:1. As proved by ELISA (Enzyme-Linked Immunosorbent Assay) detection and the serum reaction condition of a patient, the epitope polypeptide can be specifically combined with IgG (Immunoglobulin G) in the serum of the patient without reacting with the serum of a healthy person. The epitope polypeptide can be used for preparing a medicament for diagnosing systemic lupus erythematosus.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
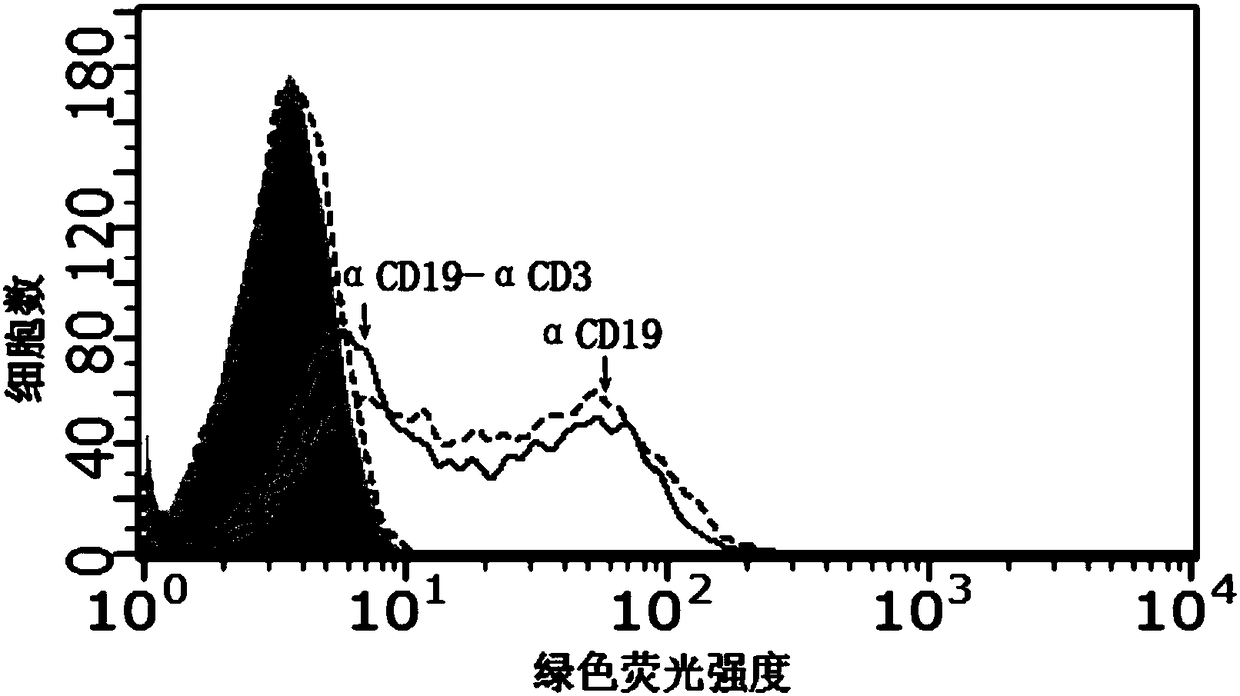
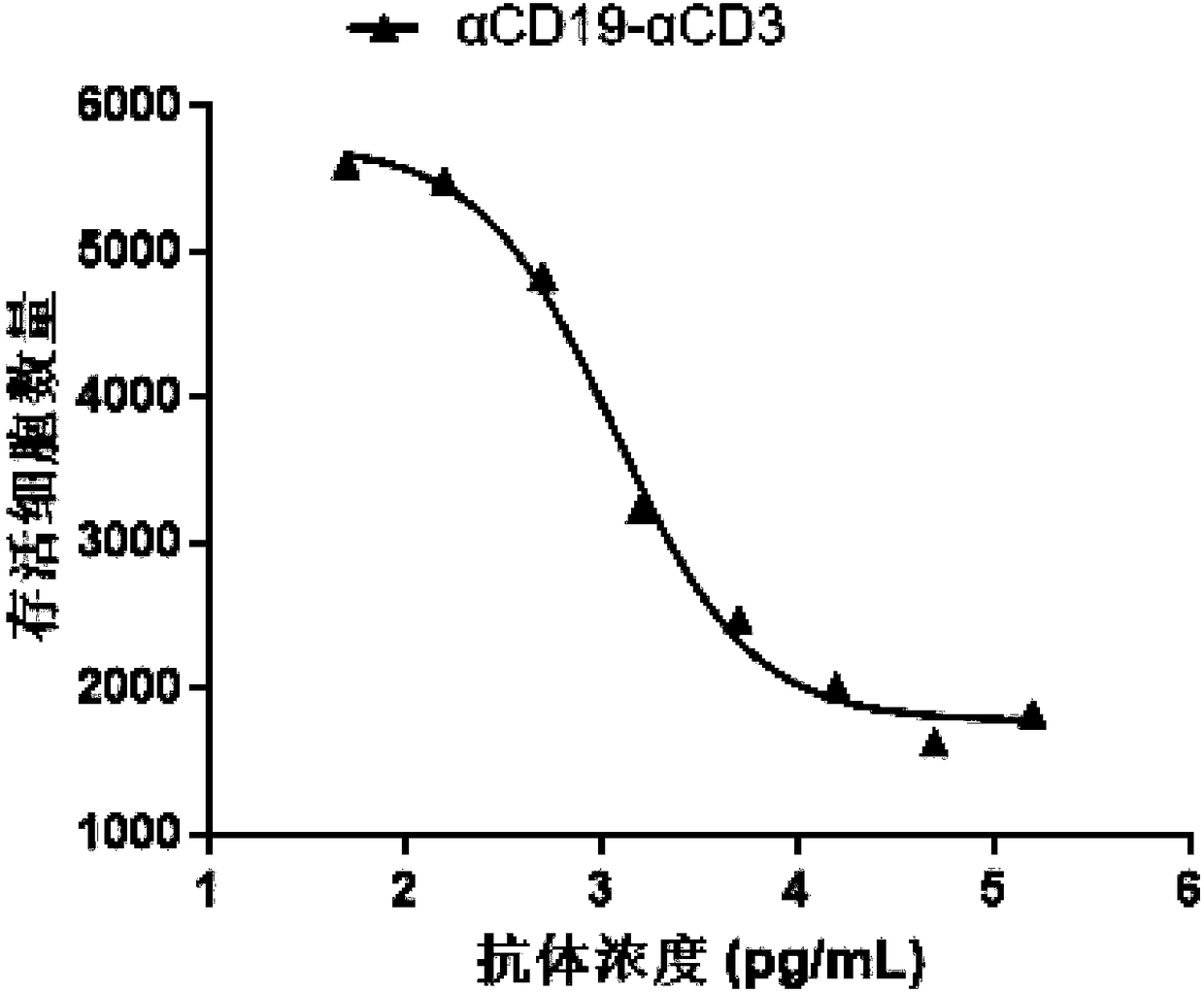
Bispecific antibody capable of binding to human CD19 or CD20 and human CD3, and application thereof
The invention discloses a bispecific antibody capable of binding to human CD19 or CD20 and human CD3. The bispecific antibody comprises a single-chain antibody domain (scFv), an immunoglobulin G domain (IgG) and an interdomain peptide linker sequence (Link1), and is formed by fusing above three components in one of the following ways: linkage of the carboxyl end of the scFv and the amino end of the light chain of the IgG by the Link1, linkage of the amino end of the scFv and the carboxyl end of the light chain of the IgG by the Link1; linkage of the carboxyl end of the scFv and the heavy chainof the IgG by the Link1, and linkage of the amino end of the scFv and the carboxyl end of the heavy chain of the IgG by the Link1. The invention also discloses a polynucleotide, a vector, a host cell, a medicinal composition related with the bispecific antibody, and a use of the bispecific antibody.
Owner:GMAX BIOPHARM
POLYPEPTIDES COMPRISING Fc FRAGMENTS OF IMMUNOGLOBULIN G (IgG) AND METHODS OF USING THE SAME
Polypeptides comprising at least a first and second Fc fragment of IgG that can be used to induce a stimulated cell to produce the anti-inflammatory cytokine Interleukin-10 and methods of using the same are disclosed herein.
Owner:UNIV OF MARYLAND +1
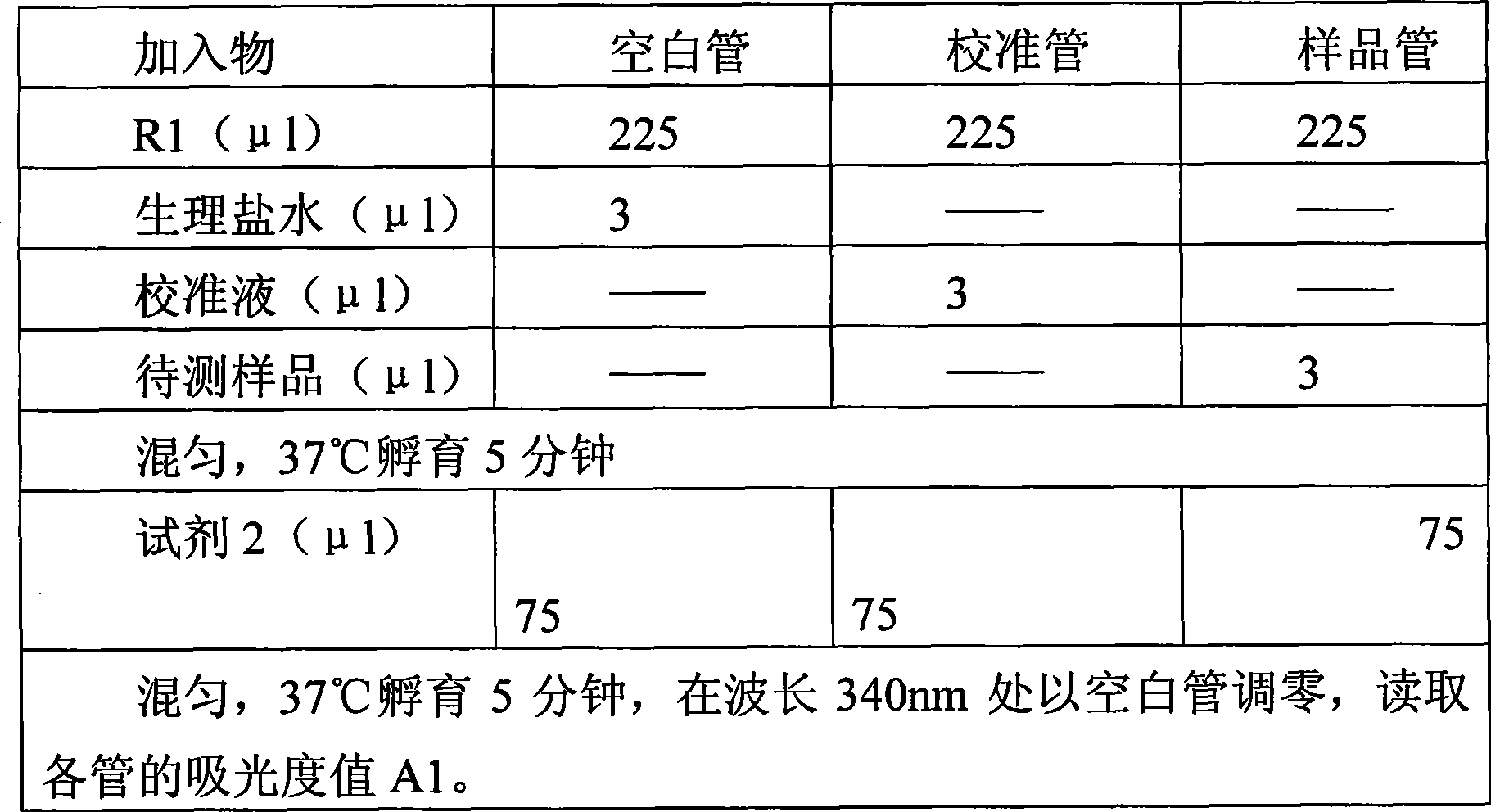
Immunoglobulin G detection reagent
The invention discloses a detecting reagent for immunoglobulin G, which comprises an IgG reactant, an anti-IgG antibody reagent and a liquid serological type constant-value calibrating agent, wherein, the IgG reactant can cause IgG antigen sites in a sample to be fully exposed so as to facilitate the full combination between the IgG antigens and the anti-IgG antibody reagent; the anti-IgG antibody reactant has high idiosyncrasy with IgG antigens in human serum; and the liquid serological type constant-value calibrating agent is used for sample comparison and result calculation. The anti-IgG antibody is from mammals of sheep, horses, mice, rabbits, or the like. The IgG reactant and the anti-IgG antibody reagent can be used as two independent reagents to form a double-reagent form of a product and also can be mixed according to certain proportions to form a single-reagent form of the product.
Owner:王贤理
Mutated protein of protein a having reduced affinity in acidic region and antibody-capturing agent
ActiveUS20140179898A1Reduced ability to bindEasy to eluteBacteriaPeptide/protein ingredientsMutated proteinProteome
A modified protein of an extracellular domain of protein A, which has the reduced ability to bind to immunoglobulin in an acidic region, compared with the wild-type extracellular domain of protein A, without impairing a selective antibody-binding activity in a neutral region. On the basis of three-dimensional structure coordinate data on a complex of the extracellular domain of protein A bound with the Fc region of immunoglobulin G, the modified protein is obtained by the substitution of amino acid residues that are located within the range of 10 angstroms from the Fc region and have a 20% or more ratio of exposed surface area, by histidine residues. Preferably, the modified protein is obtained by the substitution of amino acid residues at sites identified from the analysis of sequences selected from a library constituted by the protein group, by histidine residues. These substitutions may be combined.
Owner:NAT INST OF ADVANCED IND SCI & TECH
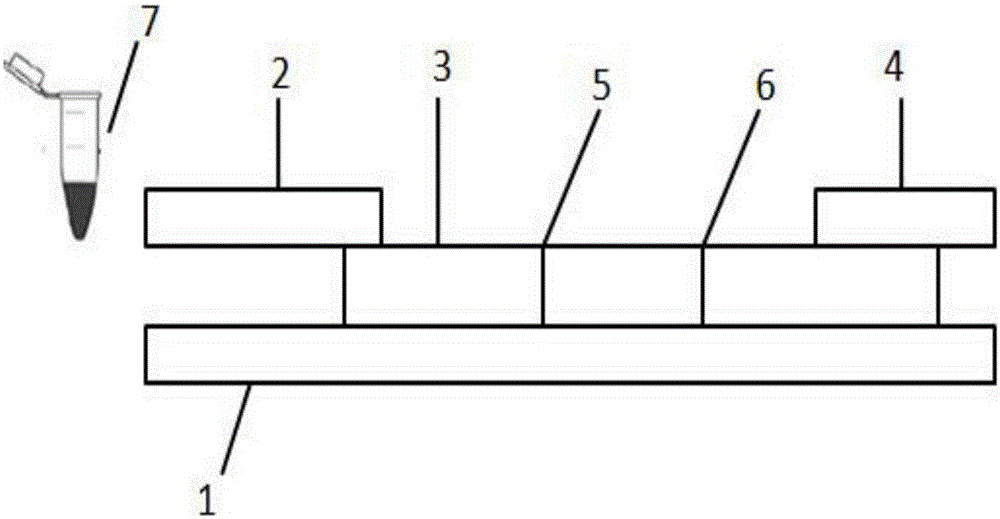
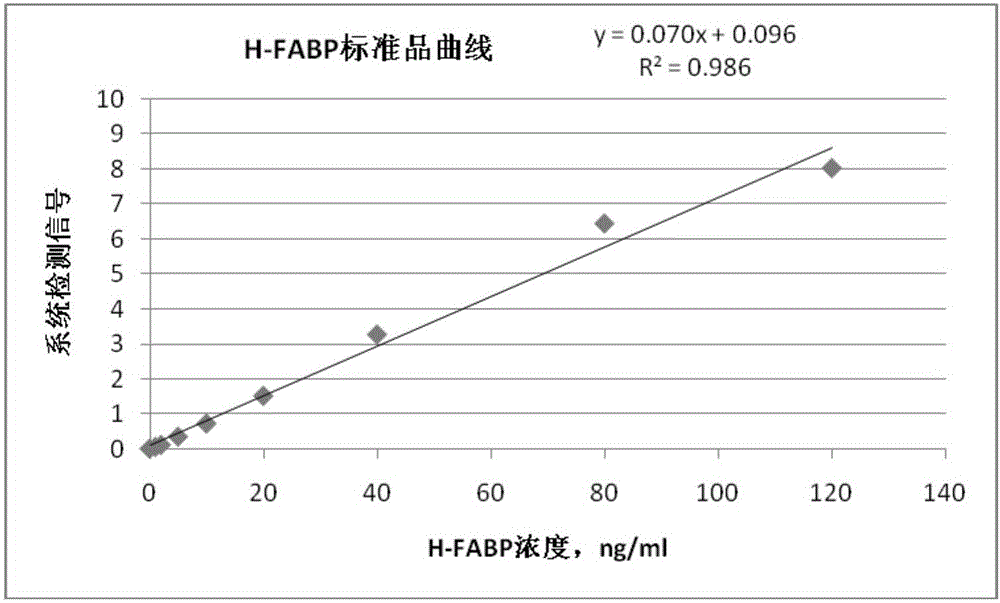
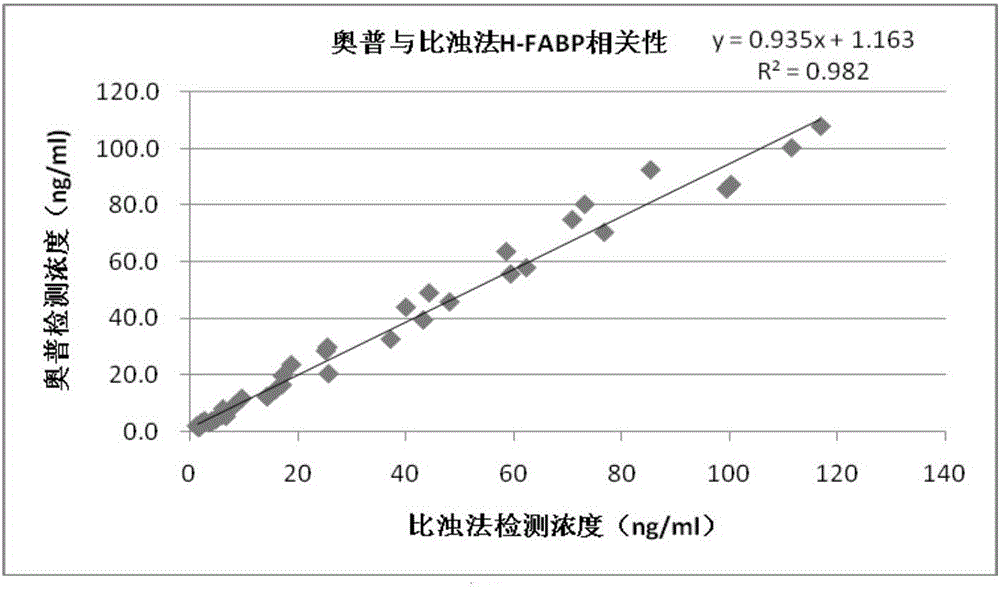
TRF (time-resolved fluorescence) immunochromatography reagent for rapidly and quantitatively detecting H-FABP (heart fatty acid-binding protein) and preparation method
InactiveCN105891508AHigh densityImprove accuracyDisease diagnosisBiological testingMicrospherePoint-of-care testing
The invention discloses a TRF (time-resolved fluorescence) immunochromatography reagent for rapidly and quantitatively detecting H-FABP (heart fatty acid-binding protein) and a preparation method and belongs to the field of clinical medical diagnosis. The reagent comprises two parts including a test strip and a fluorescent liquid, wherein the test strip comprises a bottom plate, Fusion5, a nitrocellulose membrane and a water absorbent pad; the Fusion5, the nitrocellulose membrane and the water absorbent pad are horizontally and sequentially connected and fixed onto the bottom plate; the nitrocellulose membrane is coated with a detection line for H-FABP monoclonal antibodies 1 and a quality control line comprising rabbit IgG (immunoglobulin G) antibodies; the fluorescent liquid contains TRF microspheres labeled by H-FABP monoclonal antibodies 2 and TRF microspheres labeled by goat anti-rabbit antibodies. According to the reagent, the fluorescence intensity is improved by the aid of the TRF microspheres, background signals are reduced, meanwhile, the content of the H-FABP in whole blood, serum or plasma is quantitatively detected, and only 10-20 microliters of samples are required. The test strip is convenient, rapid, simple to operate, short in detection time, high in specialty, high in sensitivity, more accurate in detection result and applicable to rapid diagnosis for clinical POCT (point-of-care testing).
Owner:SHANGHAI UPPER BIO TECH PHARMA
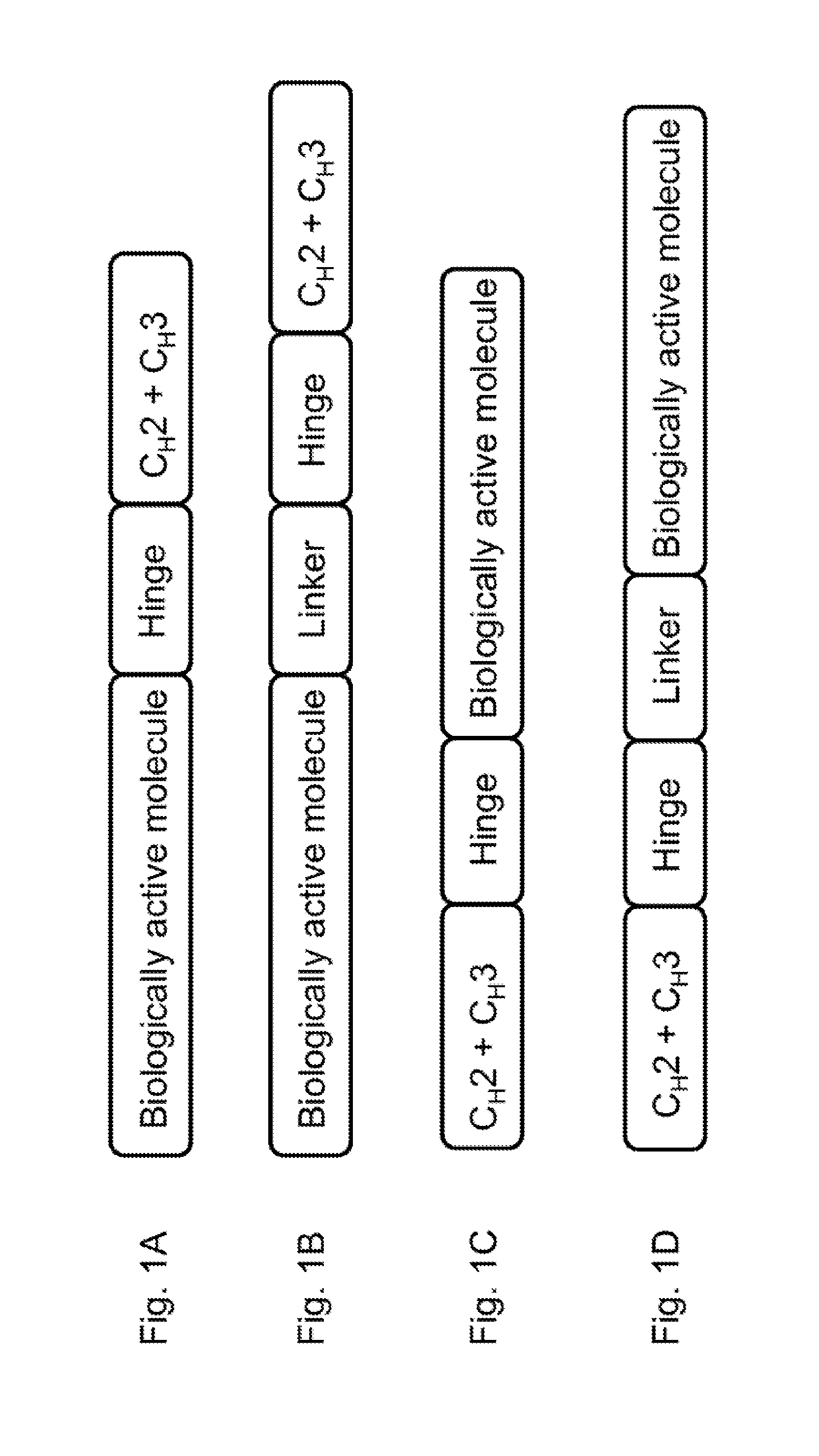
Immunoglobulin fusion proteins and uses thereof
PendingUS20160362474A1Increased serum half-lifeAntibody mimetics/scaffoldsDepsipeptidesDisulfide bondAmino acid
A fusion protein is disclosed. The fusion protein of the invention comprises an Fc fragment of an immunoglobulin G and a bioactive molecule, wherein the Fc is a single chain Fc. The amino acids in the hinge of the Fc is mutated, substituted, or deleted so that the hinge of Fc cannot form disulfide bonds. Methods for producing and using the fusion protein of the invention are also provided.
Owner:UBI PHARMA INC
Use of the endoglycosidase endos for treating immunoglobulin g mediated diseases
The invention provides use of an EndoS polypeptide, or a polynucleotide encoding an EndoS polypeptide, in the manufacture of a medicament for the treatment or prevention of a disease or condition mediated by IgG antibodies.
Owner:ハンサバイオファルマアクチボラゲット
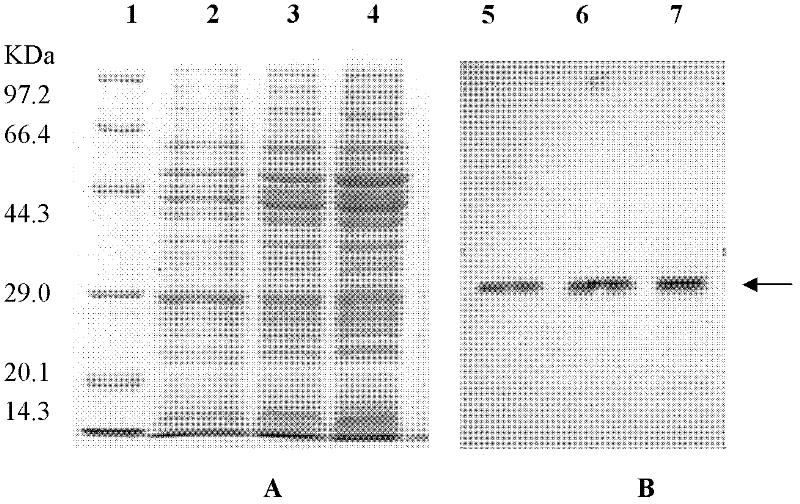
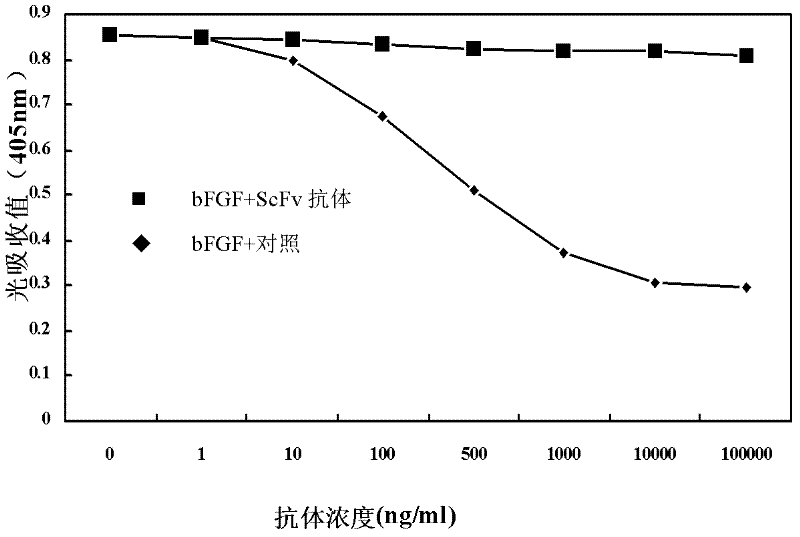
Anti to human alkaline fibroblast growth factor human s c F v antibody and application thereof
ActiveCN102558351AHigh affinityImprove featuresDigestive systemImmunoglobulins against growth factorsSingle-Chain AntibodiesFibrosis
The invention discloses a human single-chain antibody fragment (scFv) antibody of anti-recombination basic fibroblast growth factor and an application thereof. An amino acid sequence of heavy chain variable area of the human scFv antibody is shown in SEQ ID NO. 1, and an amino acid sequence of light chain variable area of the human scFv antibody is shown in SEQ ID NO. 2. A gene sequence encoding the heavy chain variable area is shown in SEQ ID NO. 3. A gene sequence encoding the light chain variable area is shown in SEQ ID NO. 4. The human scFv antibody has the characteristics of high affinity and specificity, and can be directly developed to be used as an antibody drug for human because the human scFv antibody is fully human antibody. The gene encoding the human scFv antibody can be constructed and expressed to obtain various forms of micromolecular genetic engineering antibodies, such as, fragment antigen-binding (Fab) antibody, F(ab)2, single chain antibody, Nanobody, antibody fusion protein, immunoglobulin G (IgG) complete antibody, etc., which can be used for preparing antibody drugs for diagnosing and treating tumor and / or inhibiting viscera fibrosis.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com