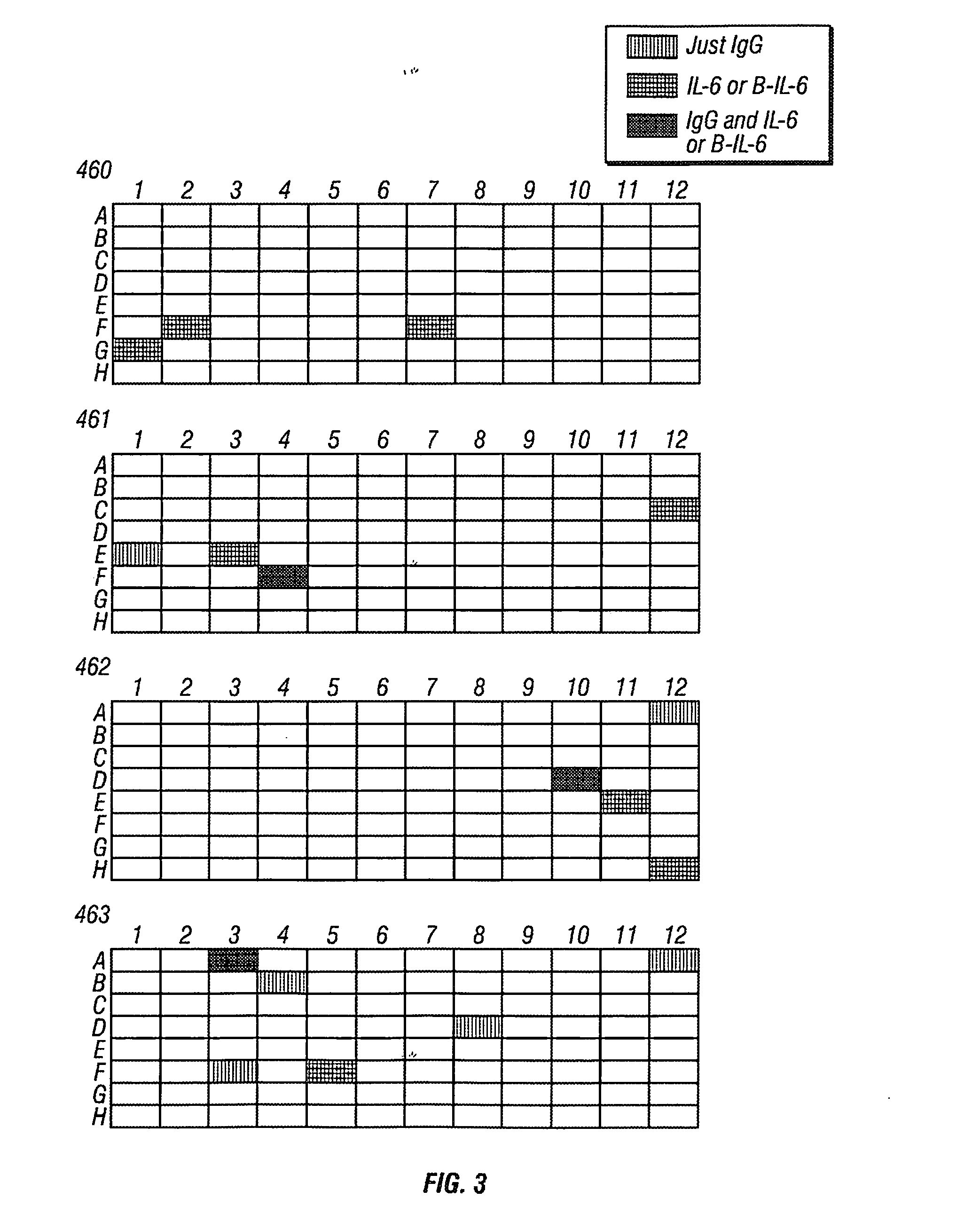
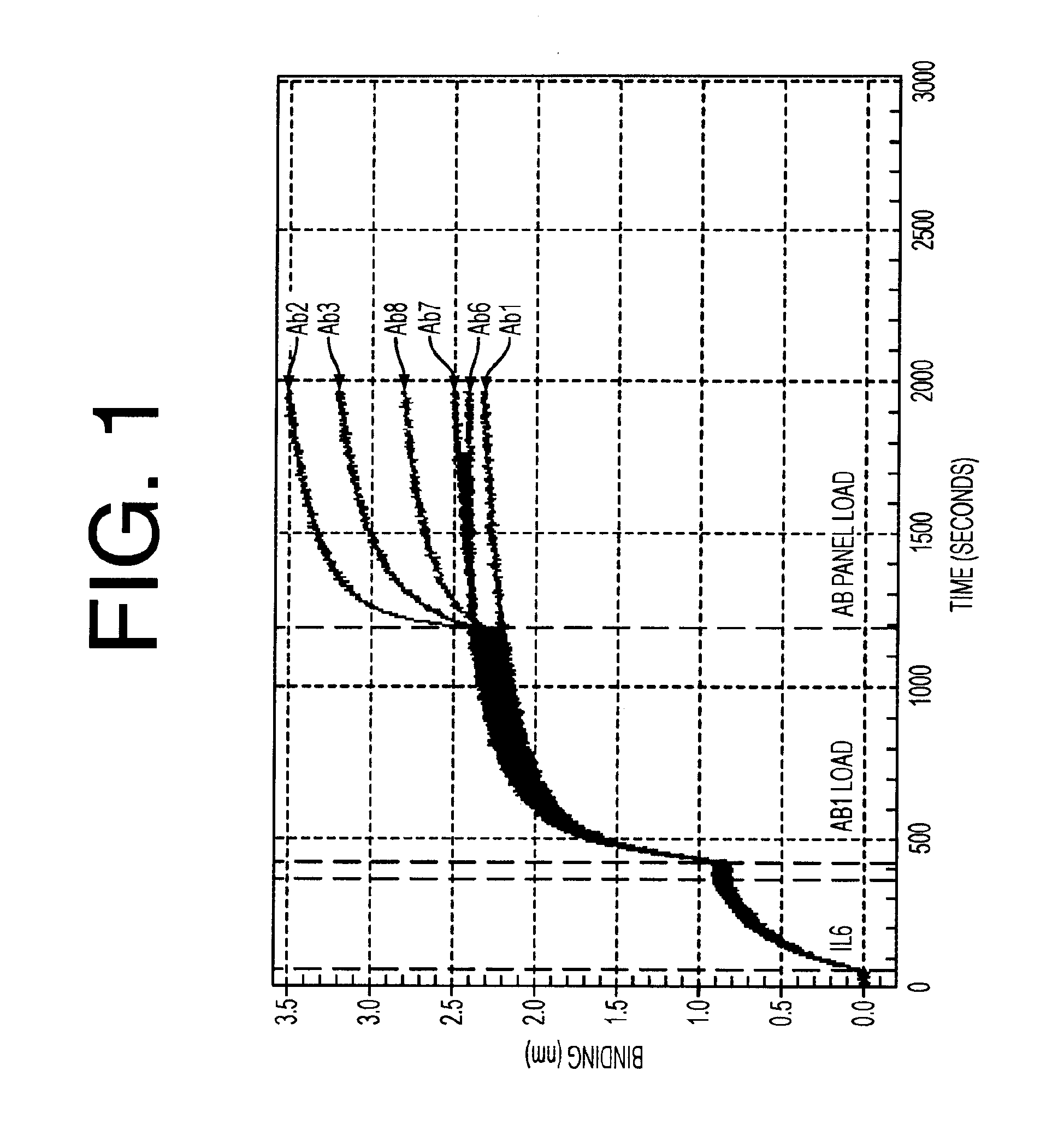
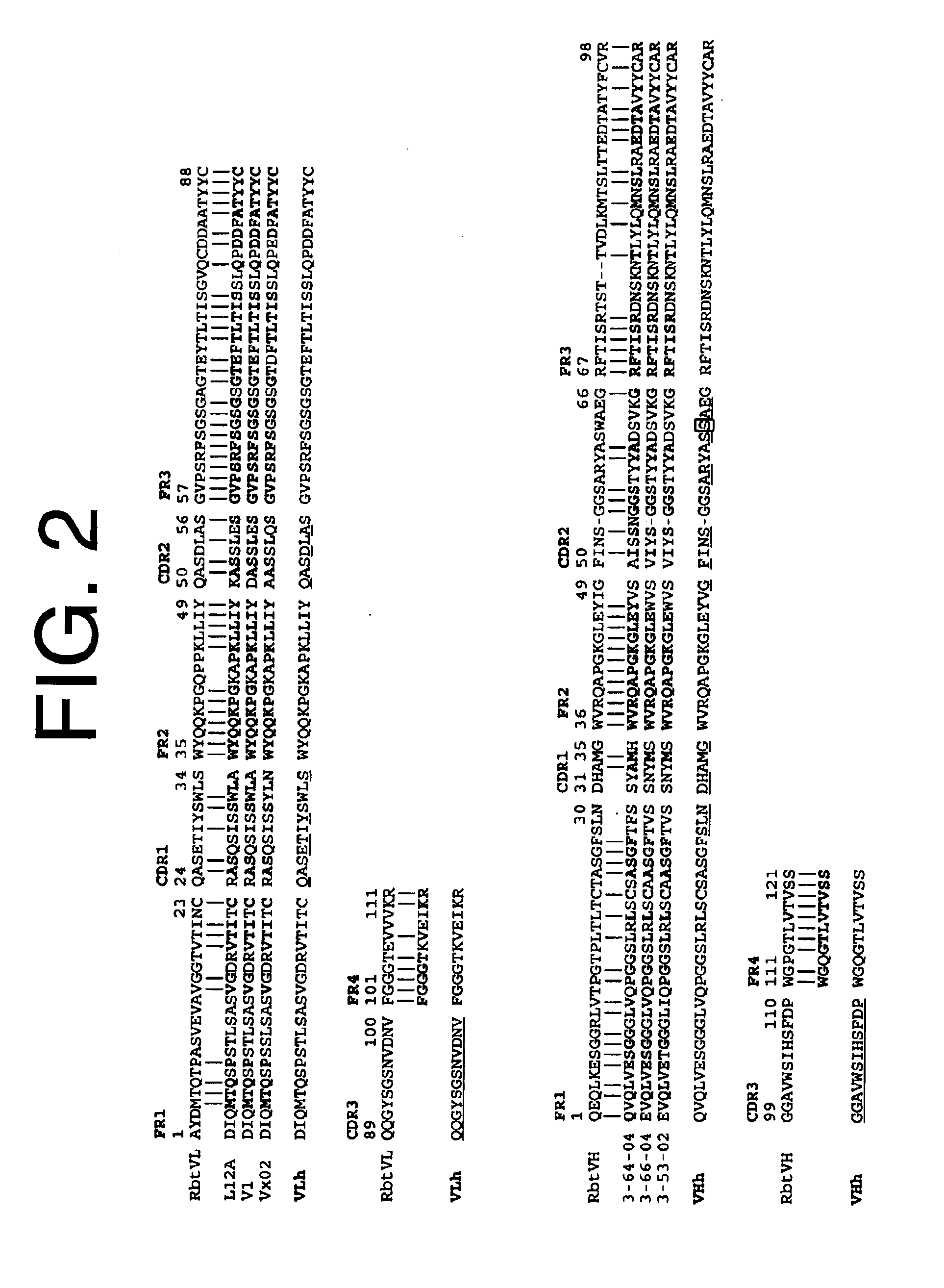
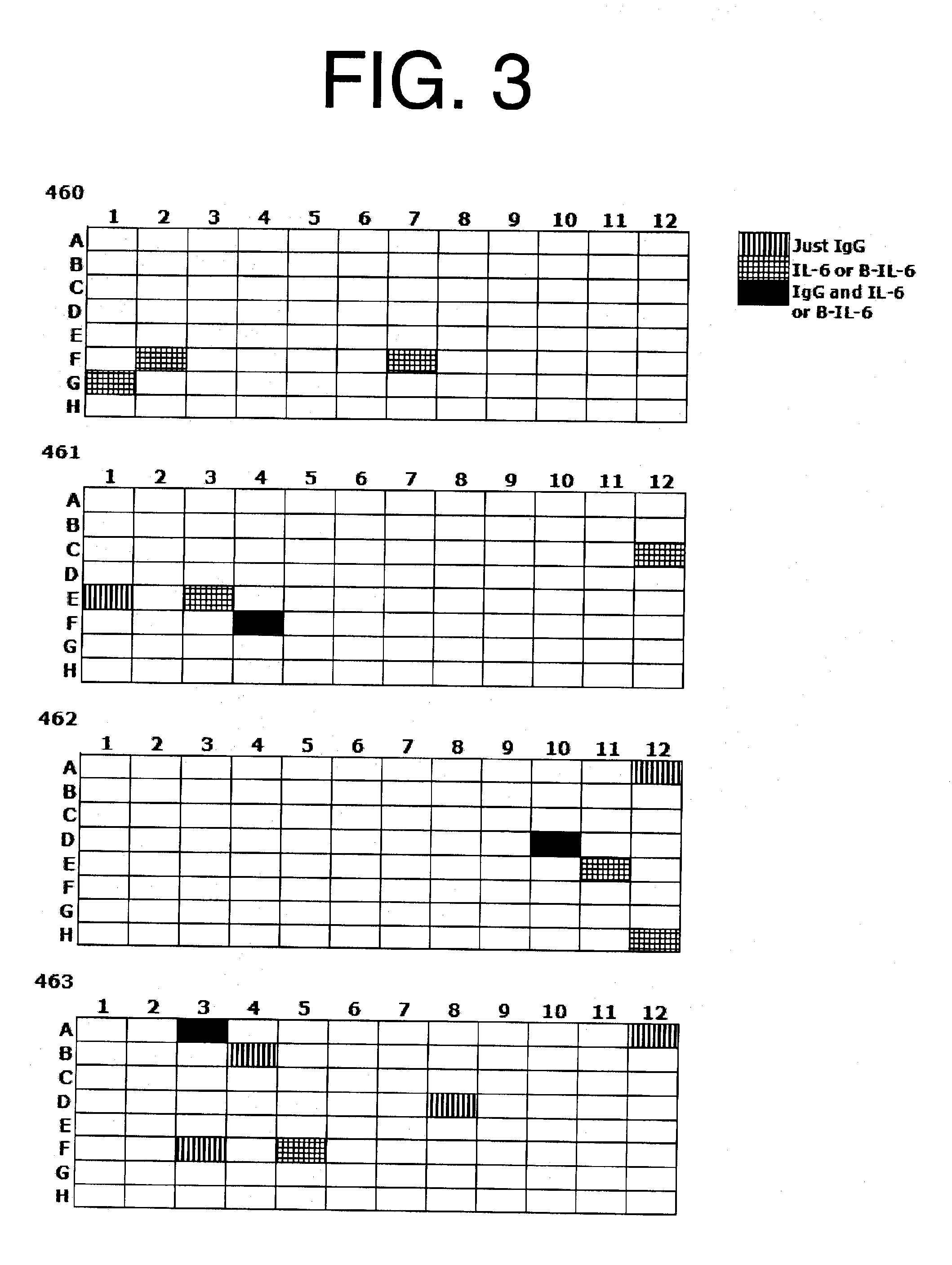
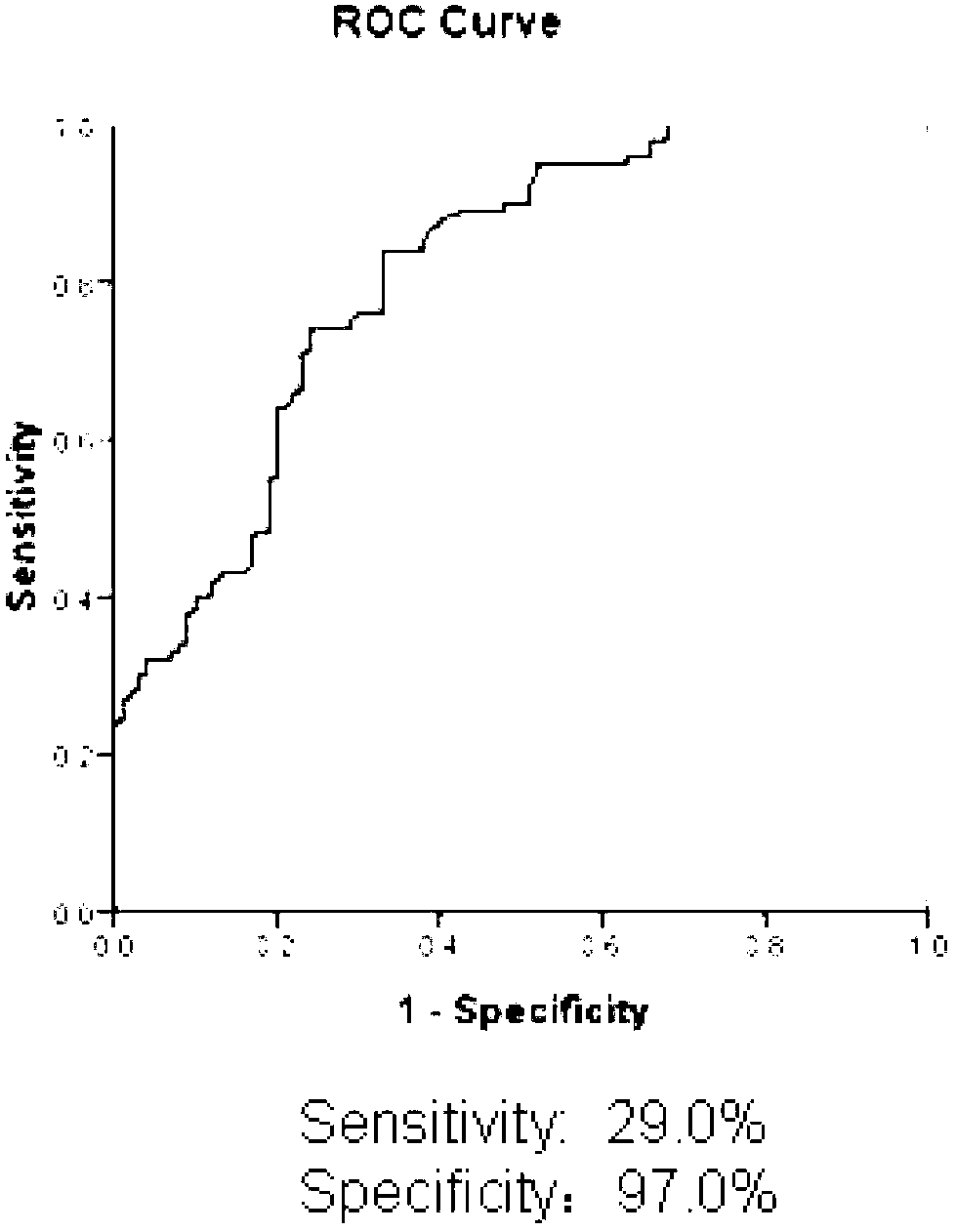
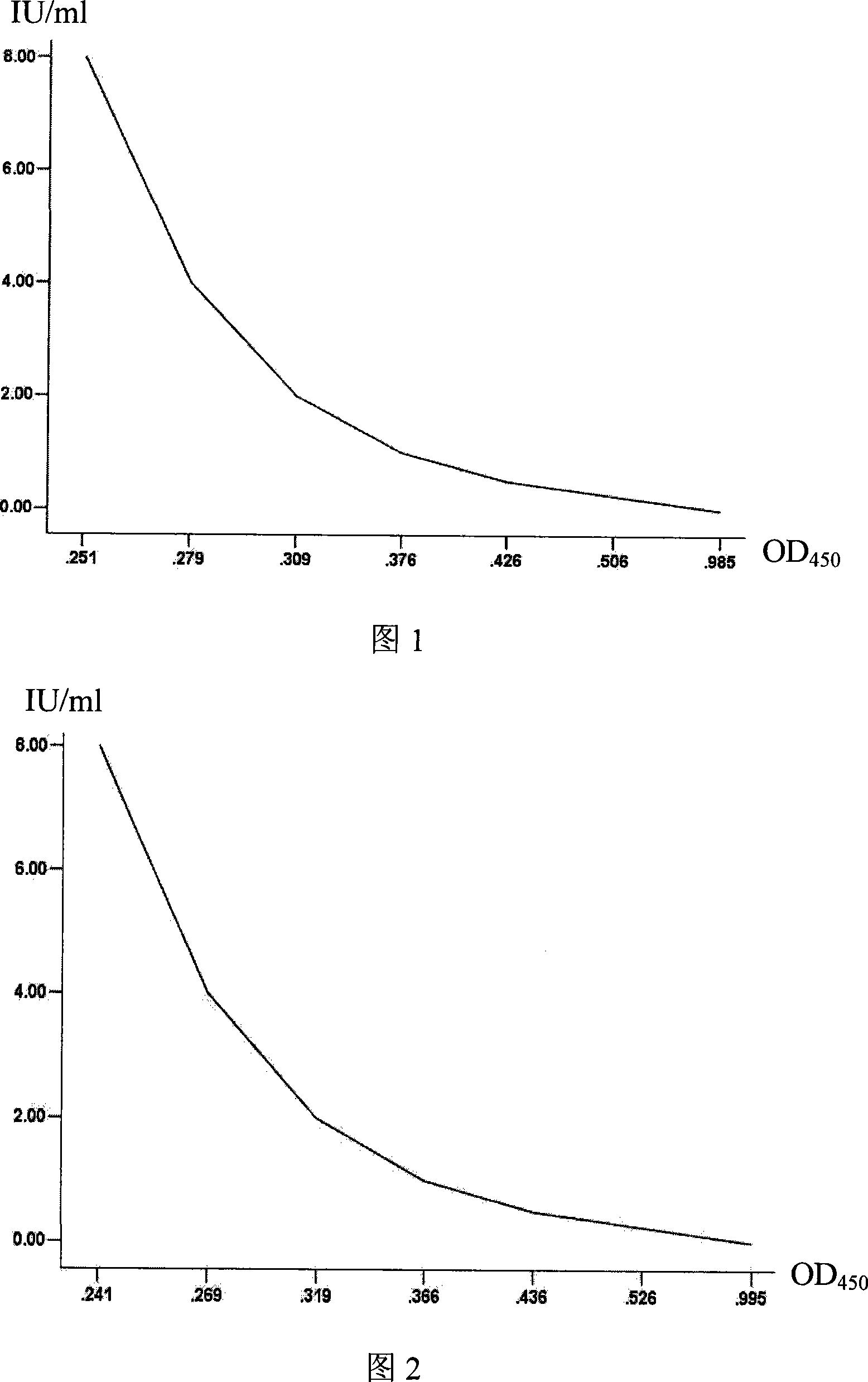
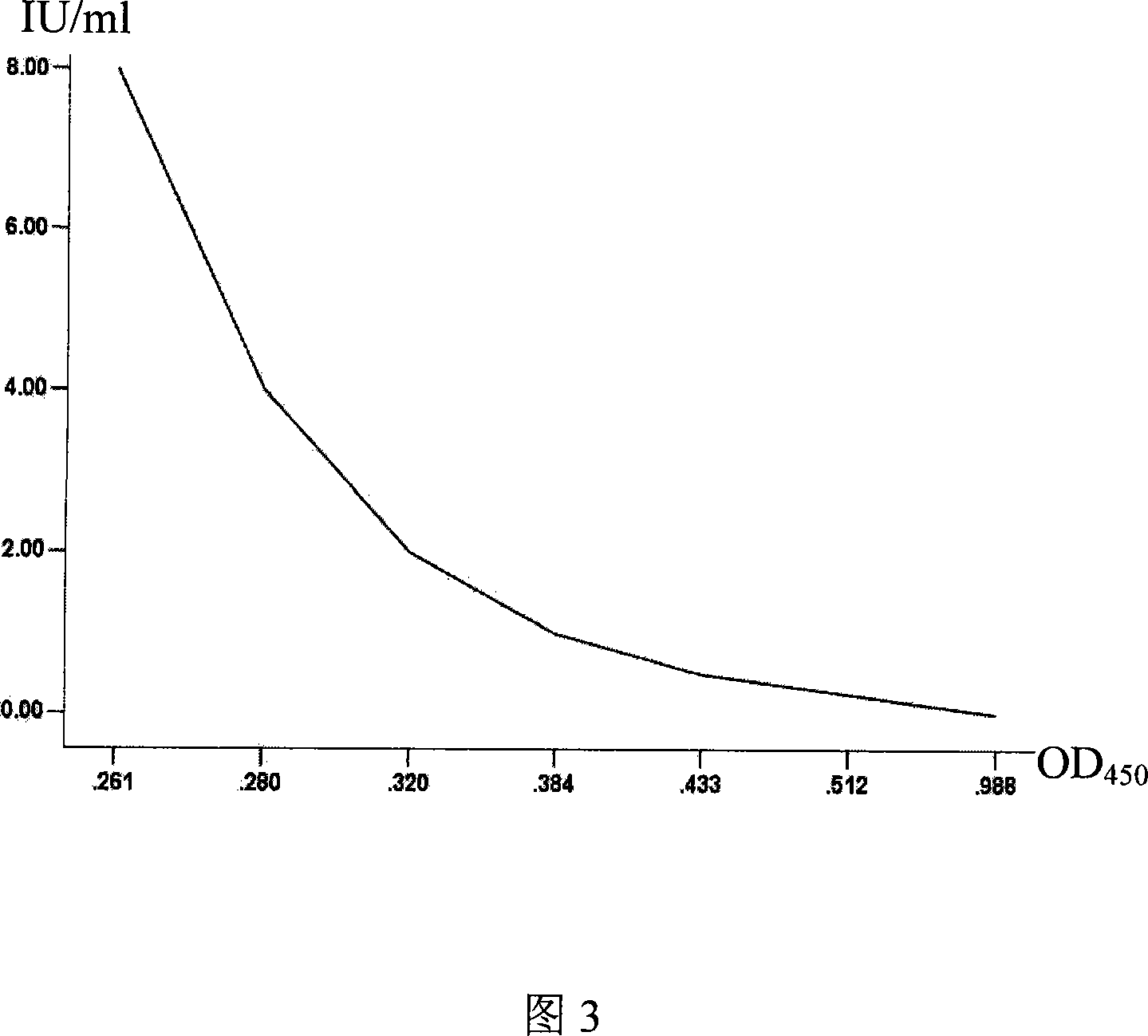
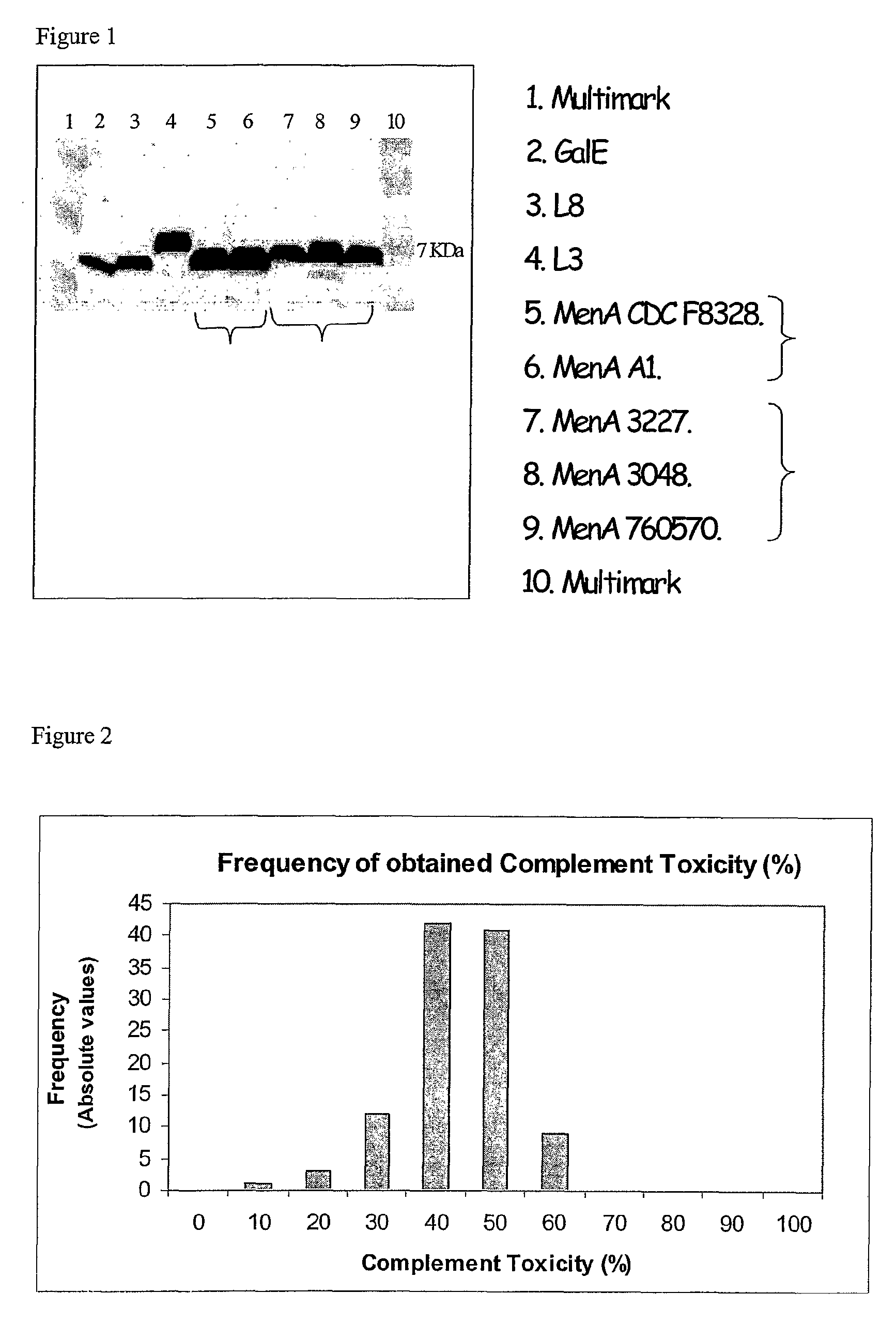
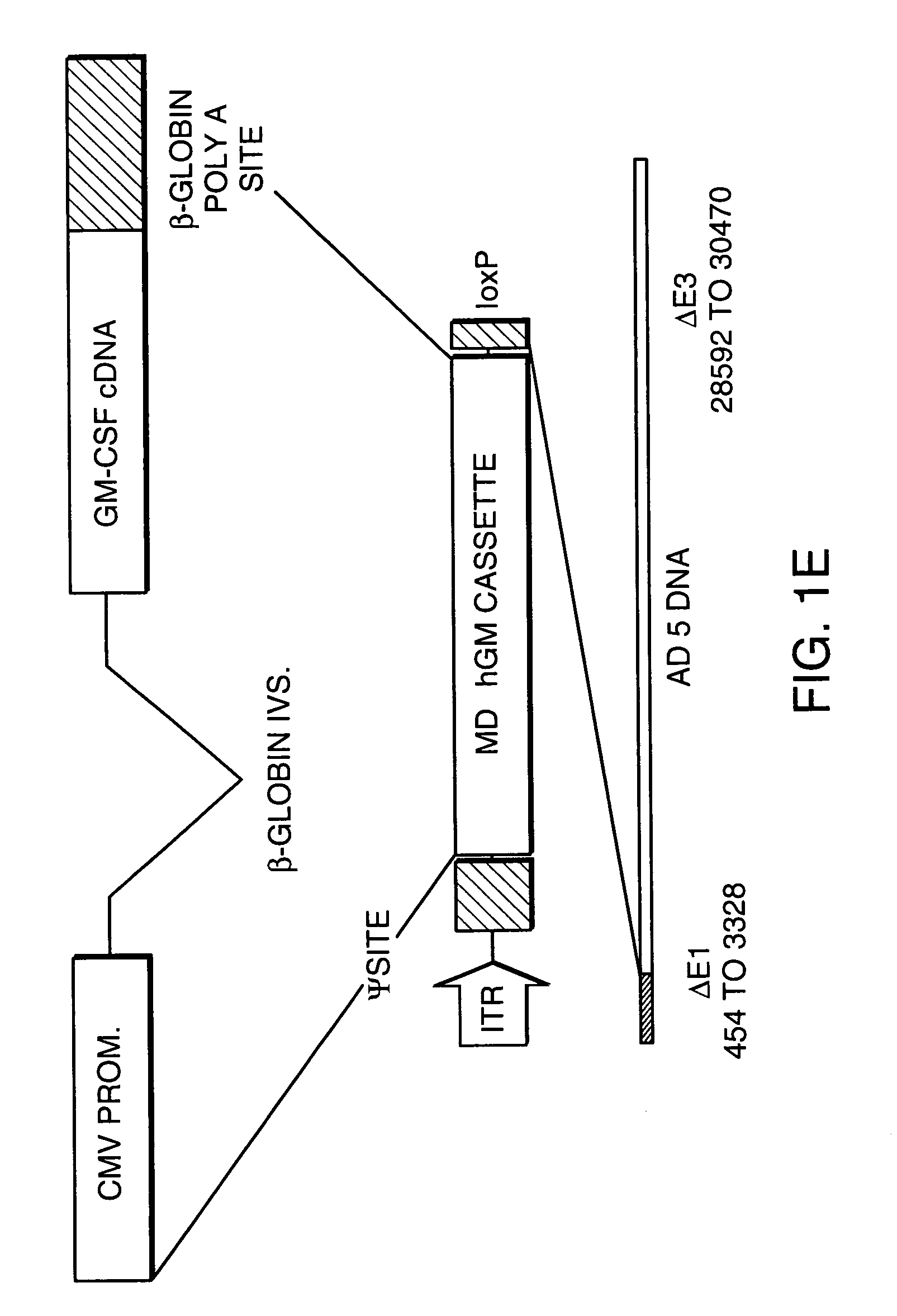
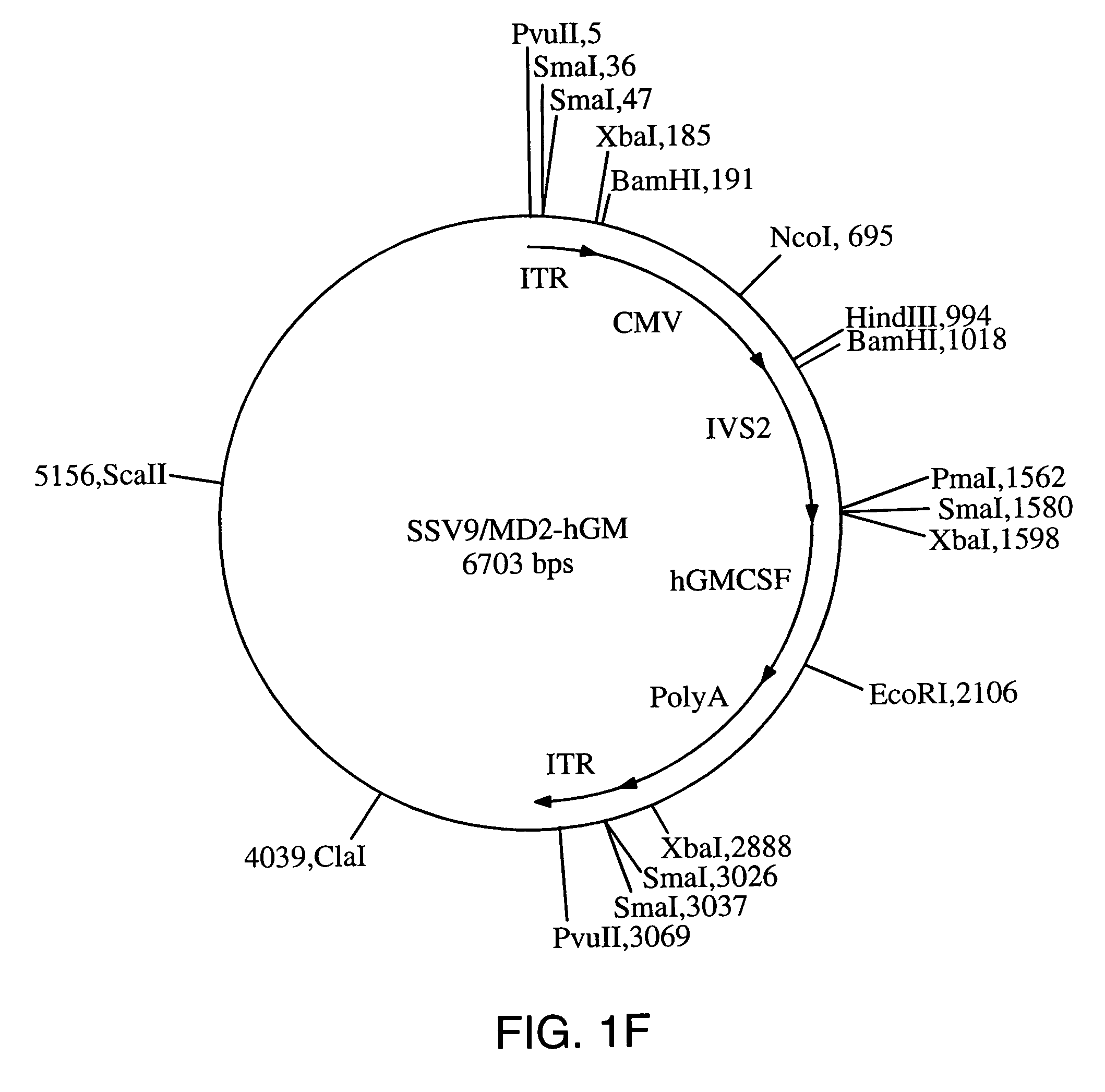
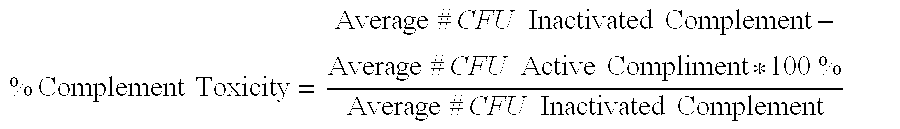Patents
Literature
65 results about "Serum reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serum sickness is a reaction that is similar to an allergy. The immune system reacts to medicines that contain proteins used to treat immune conditions.
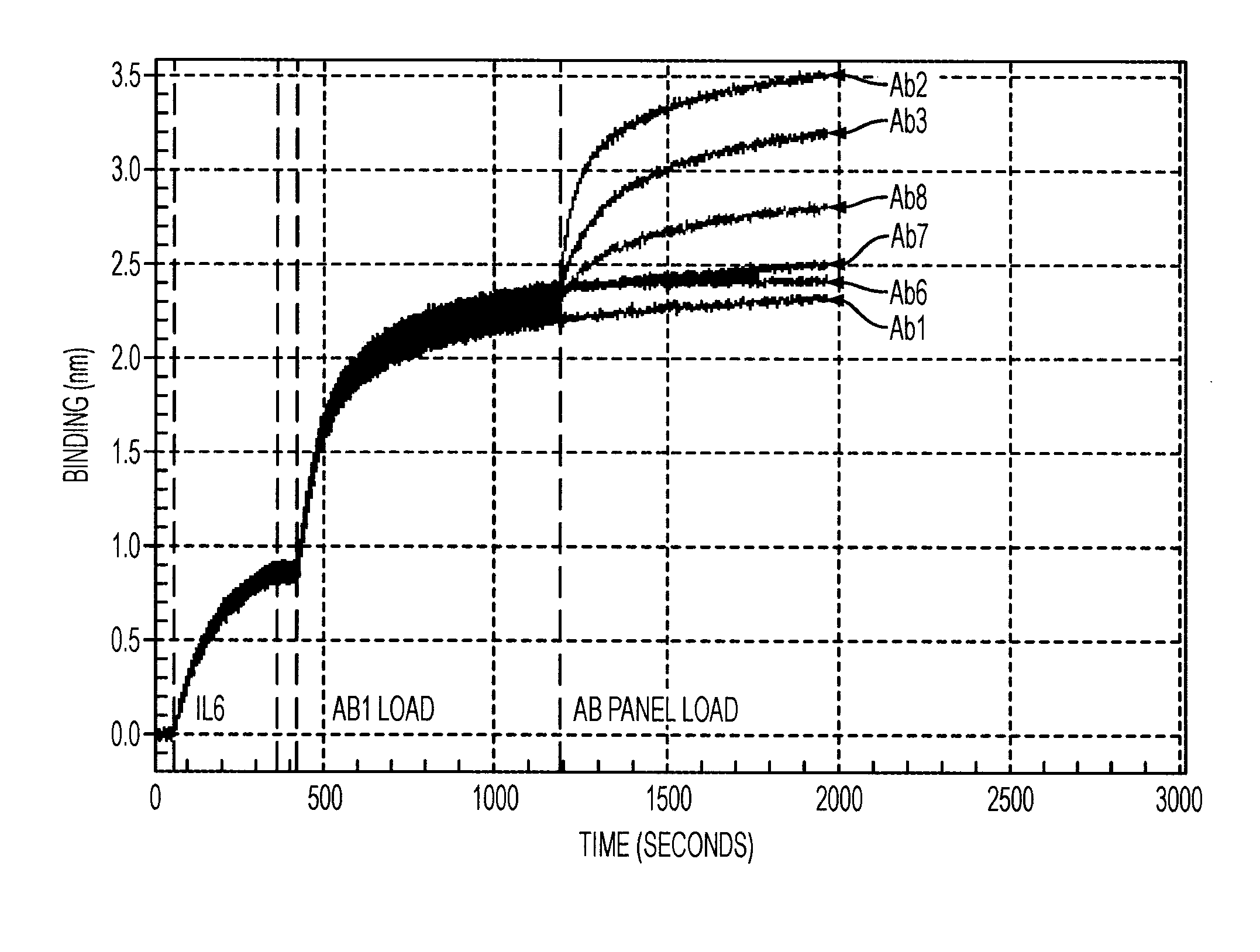
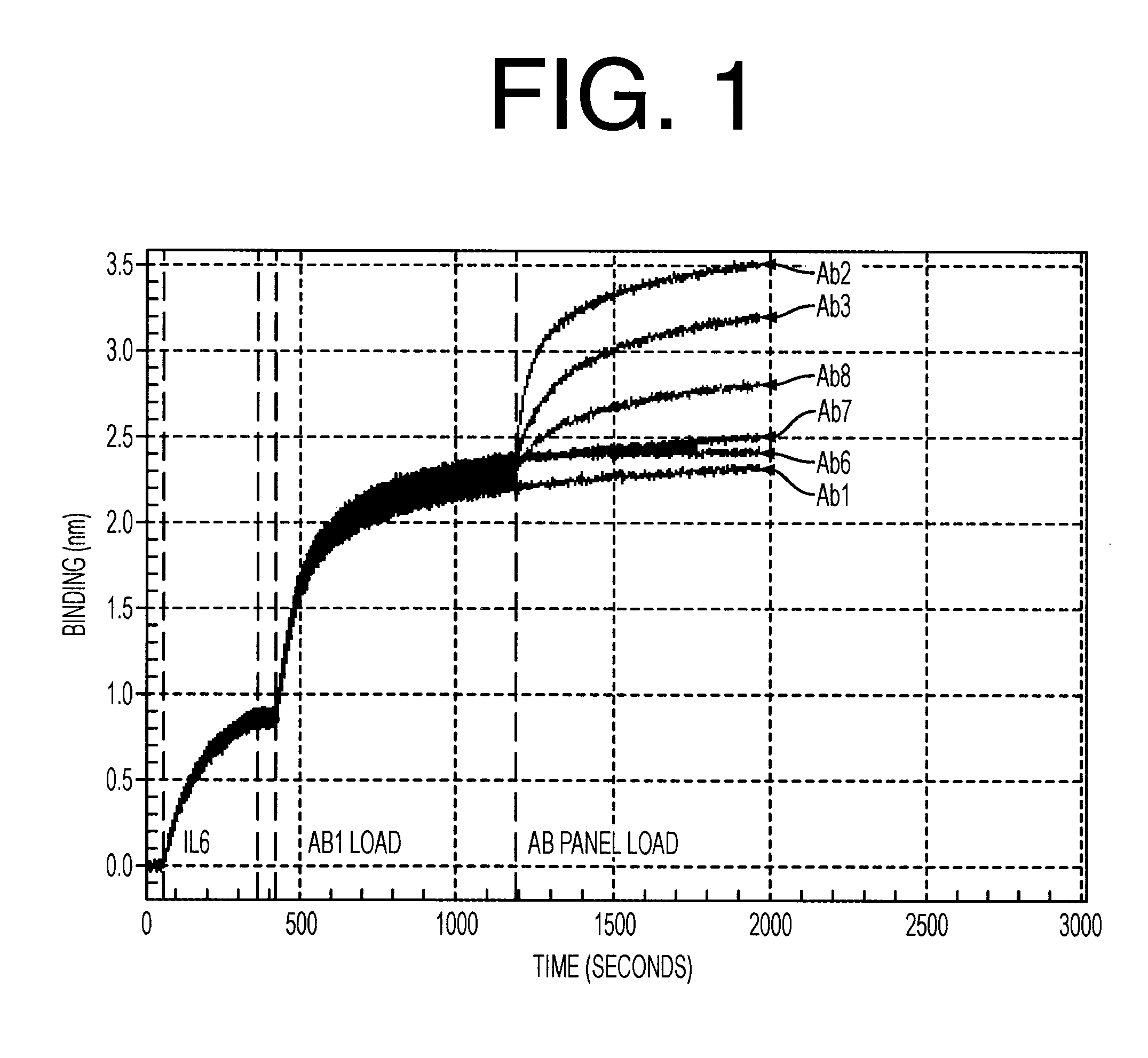
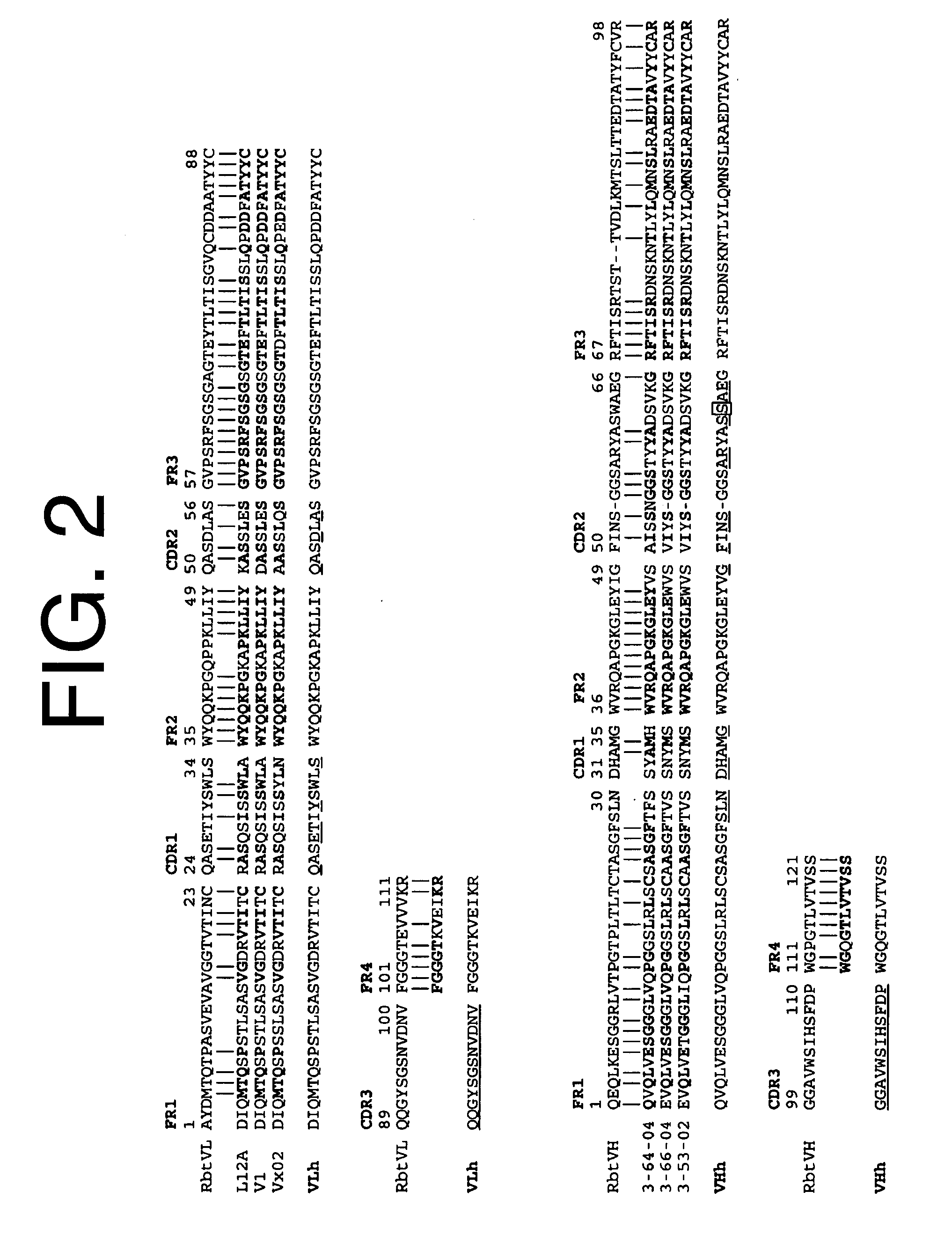
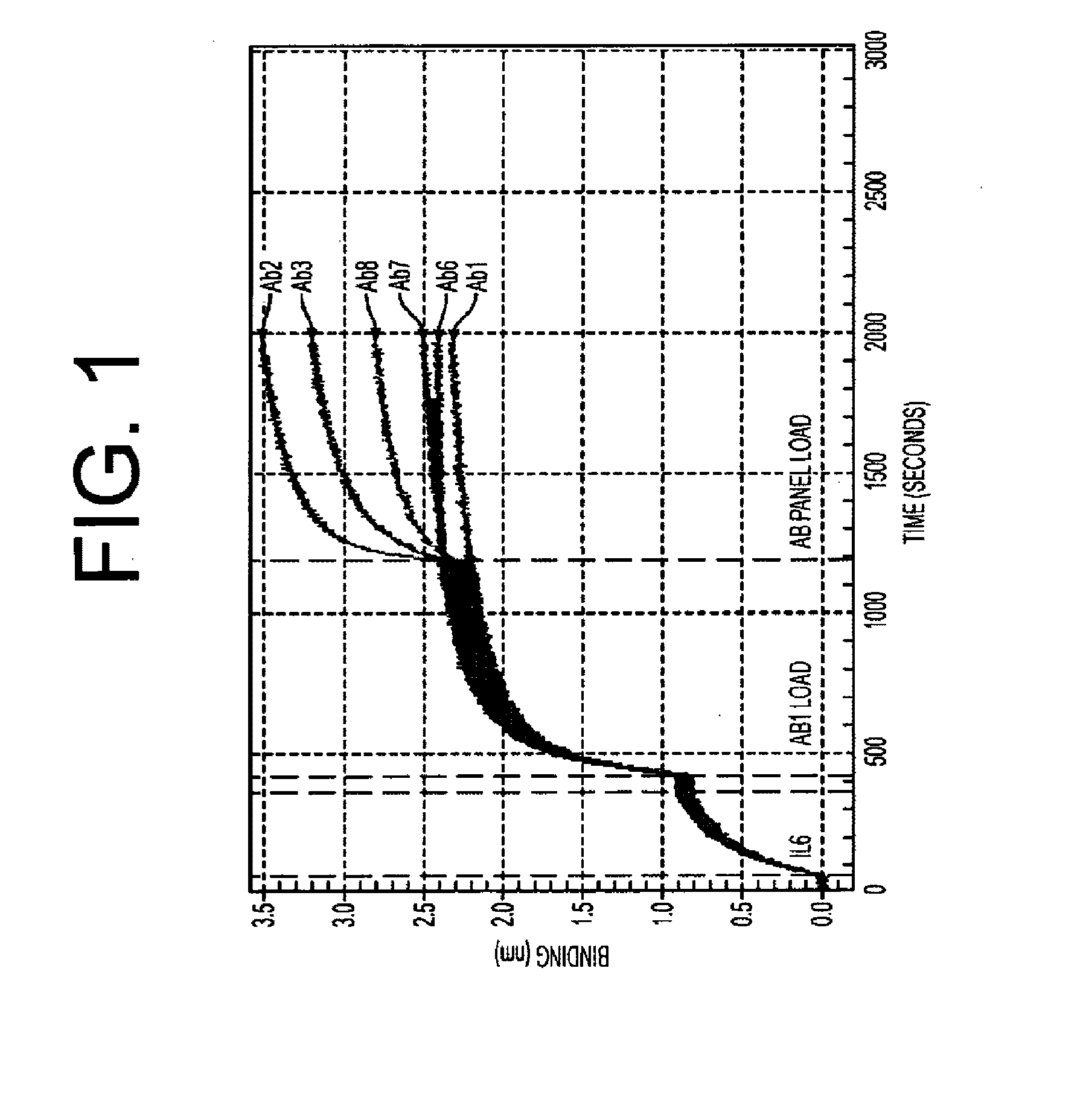
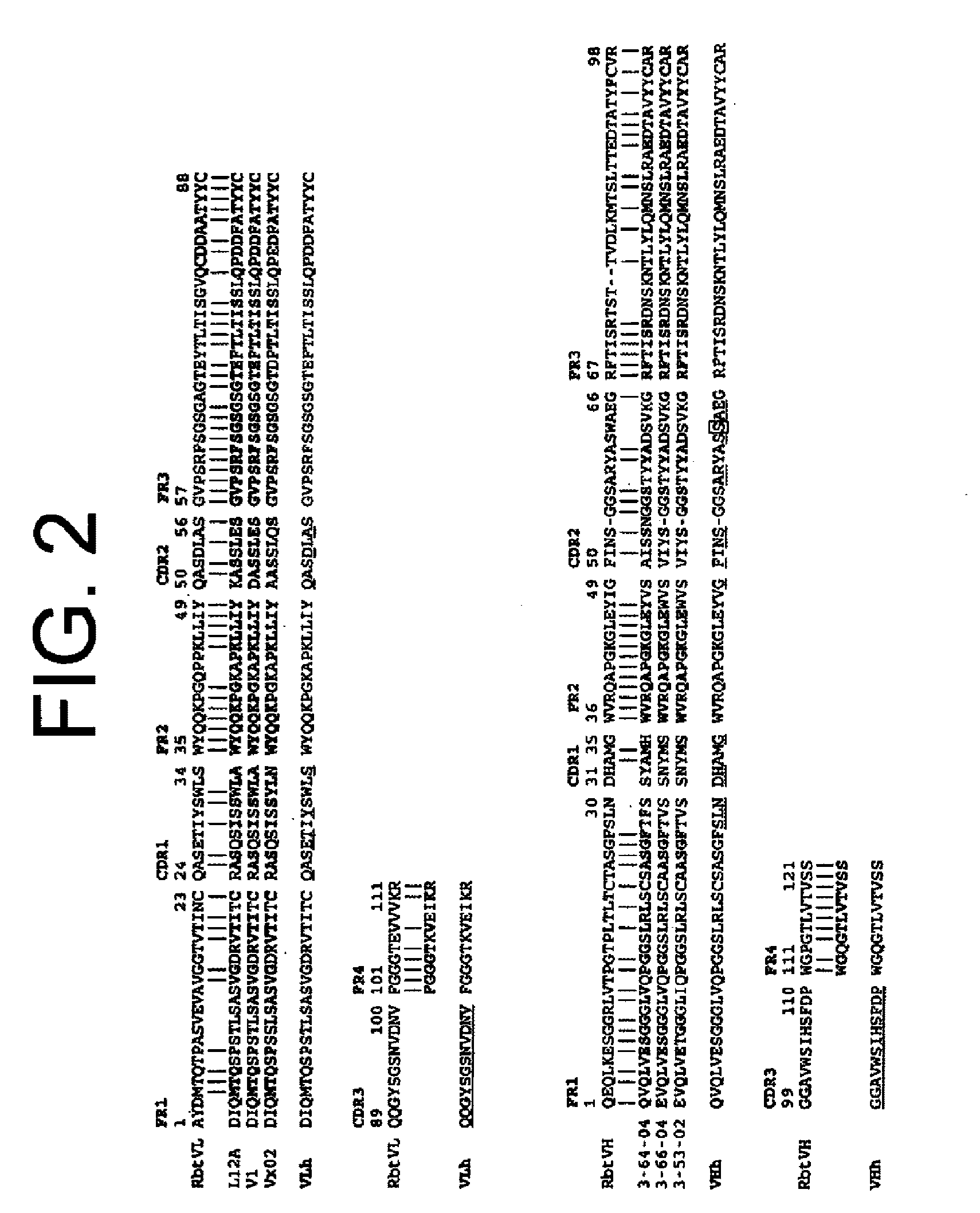
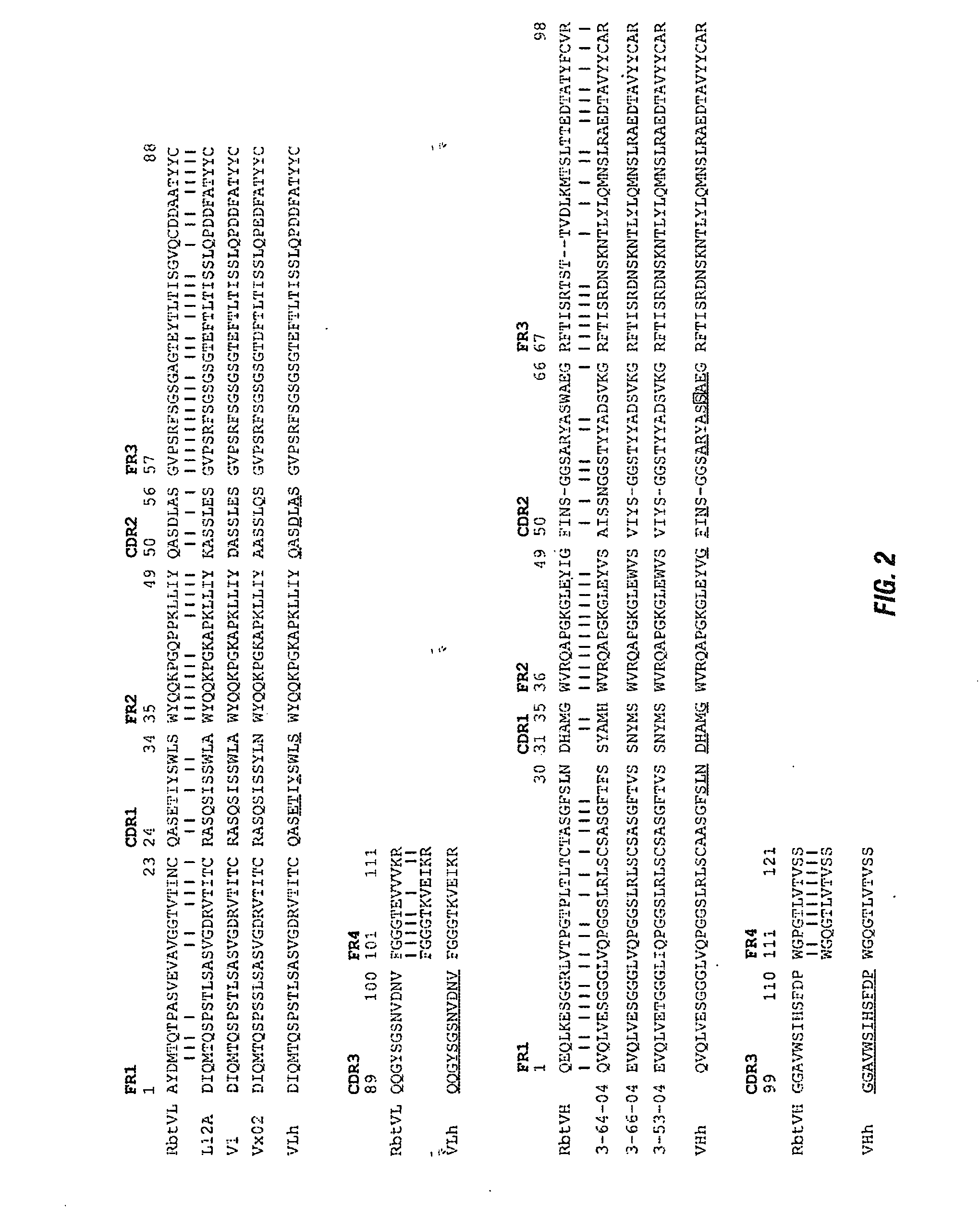
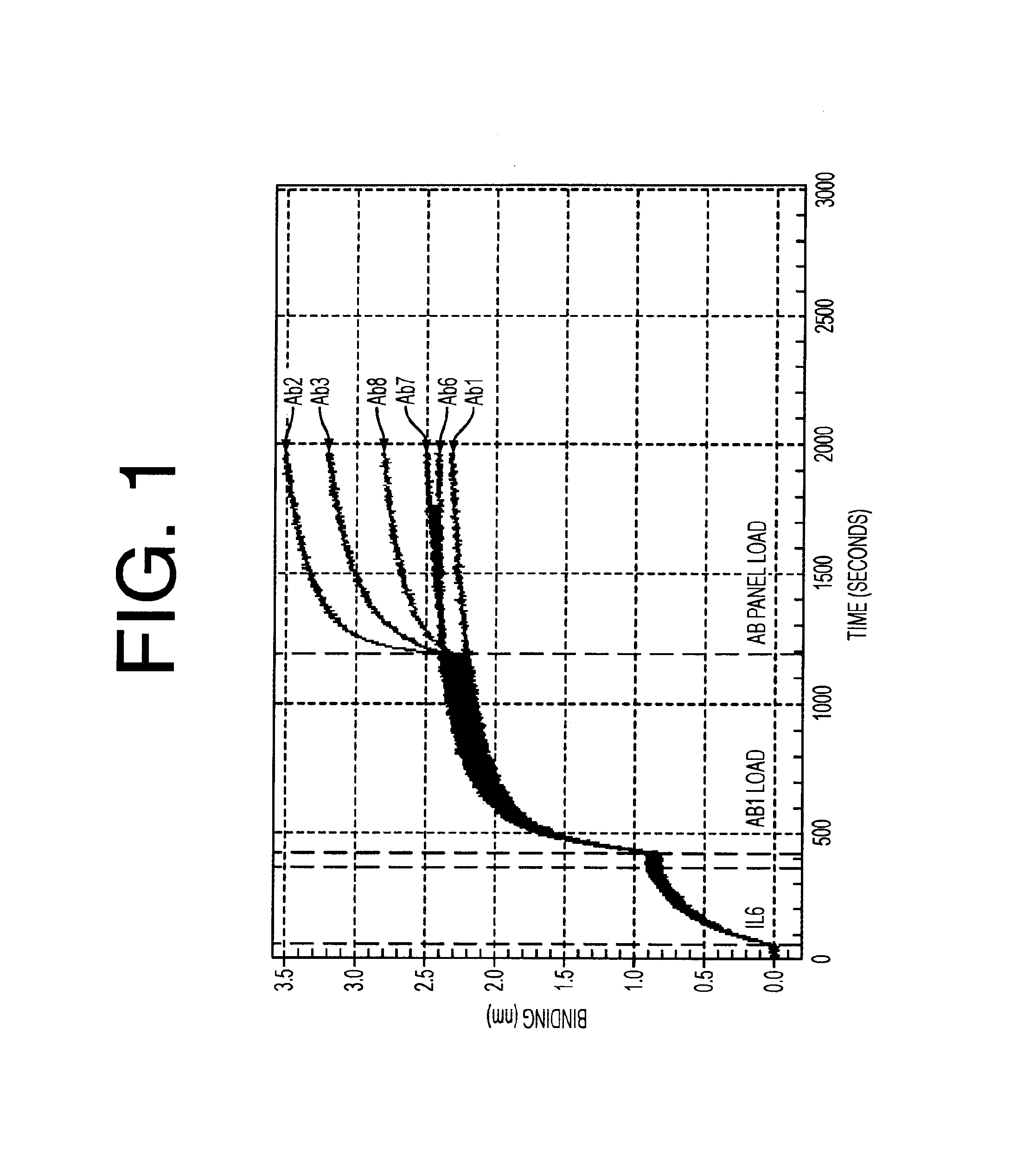
Antibodies to il-6 and use thereof
ActiveUS20100129357A1Eliminate—the risk of thrombosisPrevent thrombosisPeptide/protein ingredientsImmunoglobulins against animals/humansDiseaseAntibody fragments
The present invention is directed to therapeutic methods using IL-6 antagonists such as an Ab1 antibody or antibody fragment having binding specificity for IL-6 to prevent or treat disease or to improve survivability or quality of life of a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level, reduced serum albumin level, elevated D-dimer or other cogulation cascade related protein(s), cachexia, fever, weakness and / or fatigue prior to treatment. The subject therapies also may include the administration of other actives such as chemotherapeutics, anti-coagulants, statins, and others.
Owner:VITAERIS INC +1
Antagonists of il-6 to prevent or treat cachexia, weakness, fatigue, and/or fever
ActiveUS20110217303A1Easy to keepImprove the quality of lifeMetabolism disorderMuscular disorderSerum reactionProtein level
The present invention is directed to therapeutic methods using antibodies and fragments thereof having binding specificity for IL-6 to prevent or treat cachexia, fever, weakness and / or fatigue in a patient in need thereof. In preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level. In another preferred embodiment, the patient's survivability or quality of life will preferably be improved.
Owner:VITAERIS INC +1
Antibodies to il-6 and use thereof
ActiveUS20130034554A1Reduce riskIncrease apoptosisCompounds screening/testingAntipyreticEGFR inhibitorsAnti-coagulants
Owner:VITAERIS INC +1
Antagonists of IL-6 to raise albumin and/or lower crp
ActiveUS20130183264A1Organic active ingredientsNervous disorderSerum reactionAntiendomysial antibodies
The present invention is directed to therapeutic methods using IL-6 antagonists such as antibodies and fragments thereof having binding specificity for IL-6 to improve survivability or quality of life of a patient in need thereof. In preferred embodiments, the anti-IL-6 antibodies will be humanized and / or will be aglycosylated. Also, in preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level or a reduced serum albumin level prior to treatment. In another preferred embodiment, the patient's Glasgow Prognostic Score will be increased and survivability will preferably be improved.
Owner:VITAERIS INC +1
Antagonists of IL-6 to raise albumin and/or lower CRP
ActiveUS8420089B2Improve survivabilityQuality improvementOrganic active ingredientsNervous disorderElevated serumAnti-IL-6
The present invention is directed to therapeutic methods using IL-6 antagonists such as antibodies and fragments thereof having binding specificity for IL-6 to improve survivability or quality of life of a patient in need thereof. In preferred embodiments, the anti-IL-6 antibodies will be humanized and / or will be aglycosylated. Also, in preferred embodiments these patients will comprise those exhibiting (or at risk of developing) an elevated serum C-reactive protein level or a reduced serum albumin level prior to treatment. In another preferred embodiment, the patient's Glasgow Prognostic Score will be increased and survivability will preferably be improved.
Owner:VITAERIS INC +1
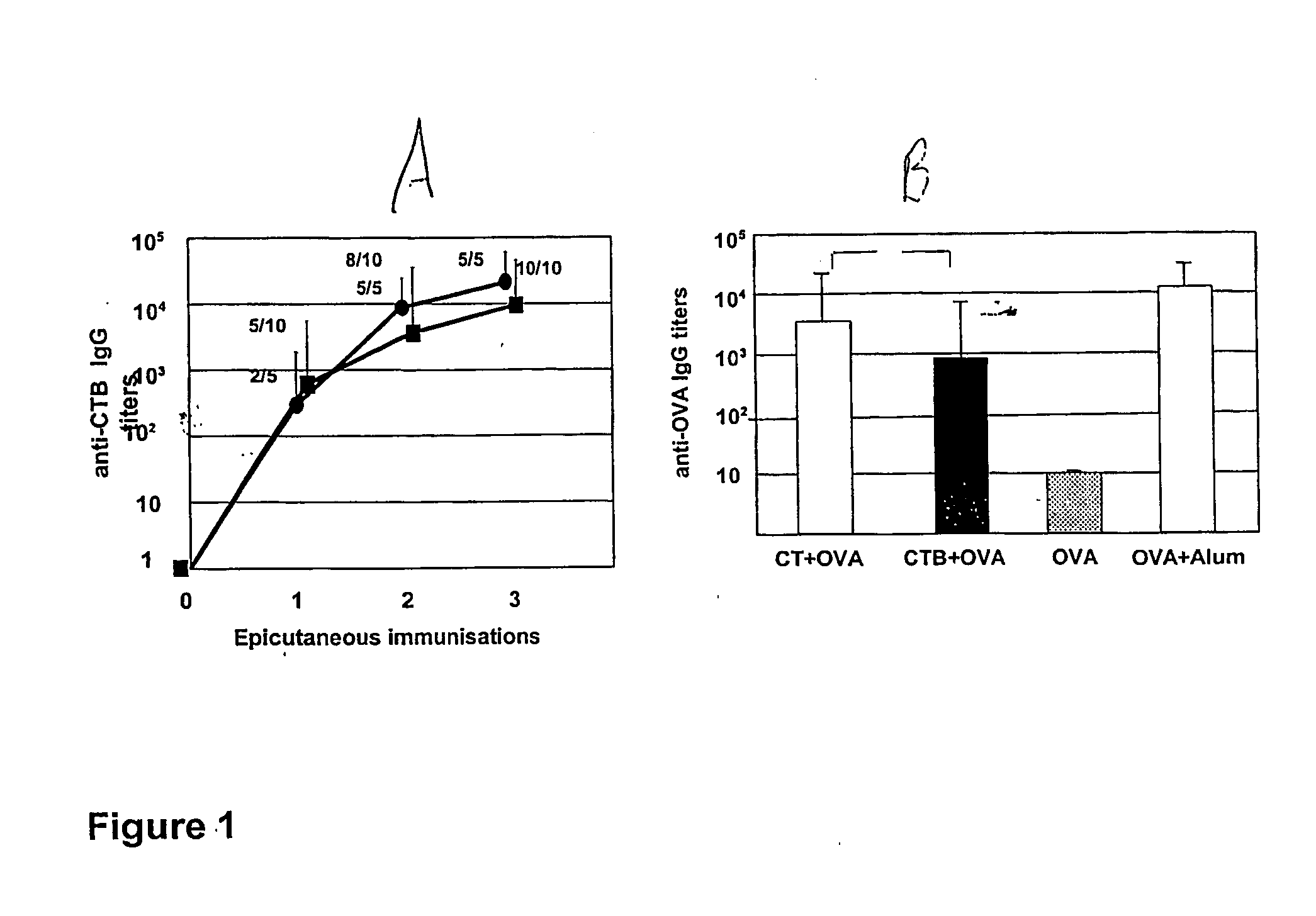
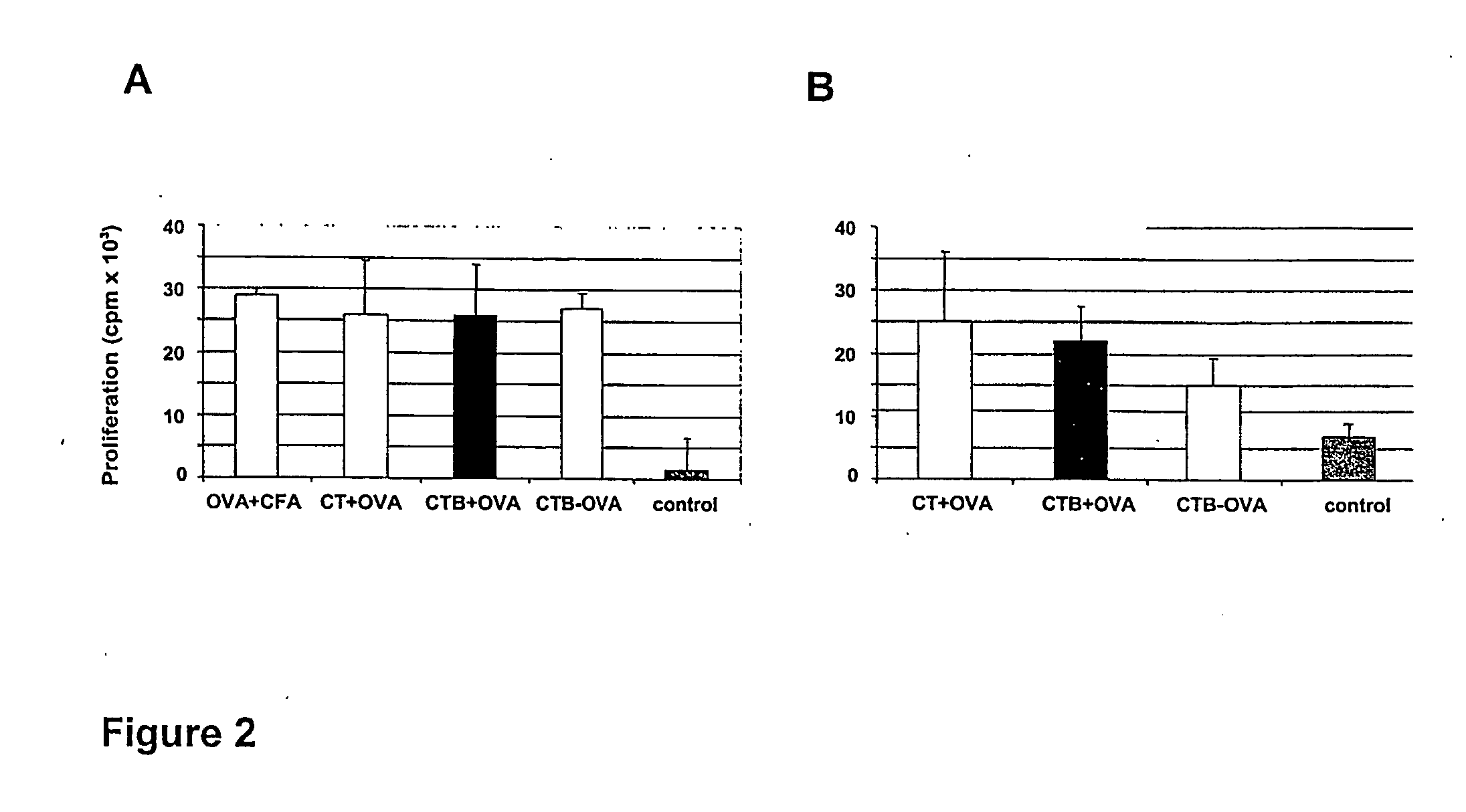
Supression of allergic reactions by transdermal administration of allergens conjugated to cholera toxin or fragments thereof
InactiveUS20050074462A1Suppress allergic reactionsBacterial antigen ingredientsPeptide/protein ingredientsAdjuvantCo administration
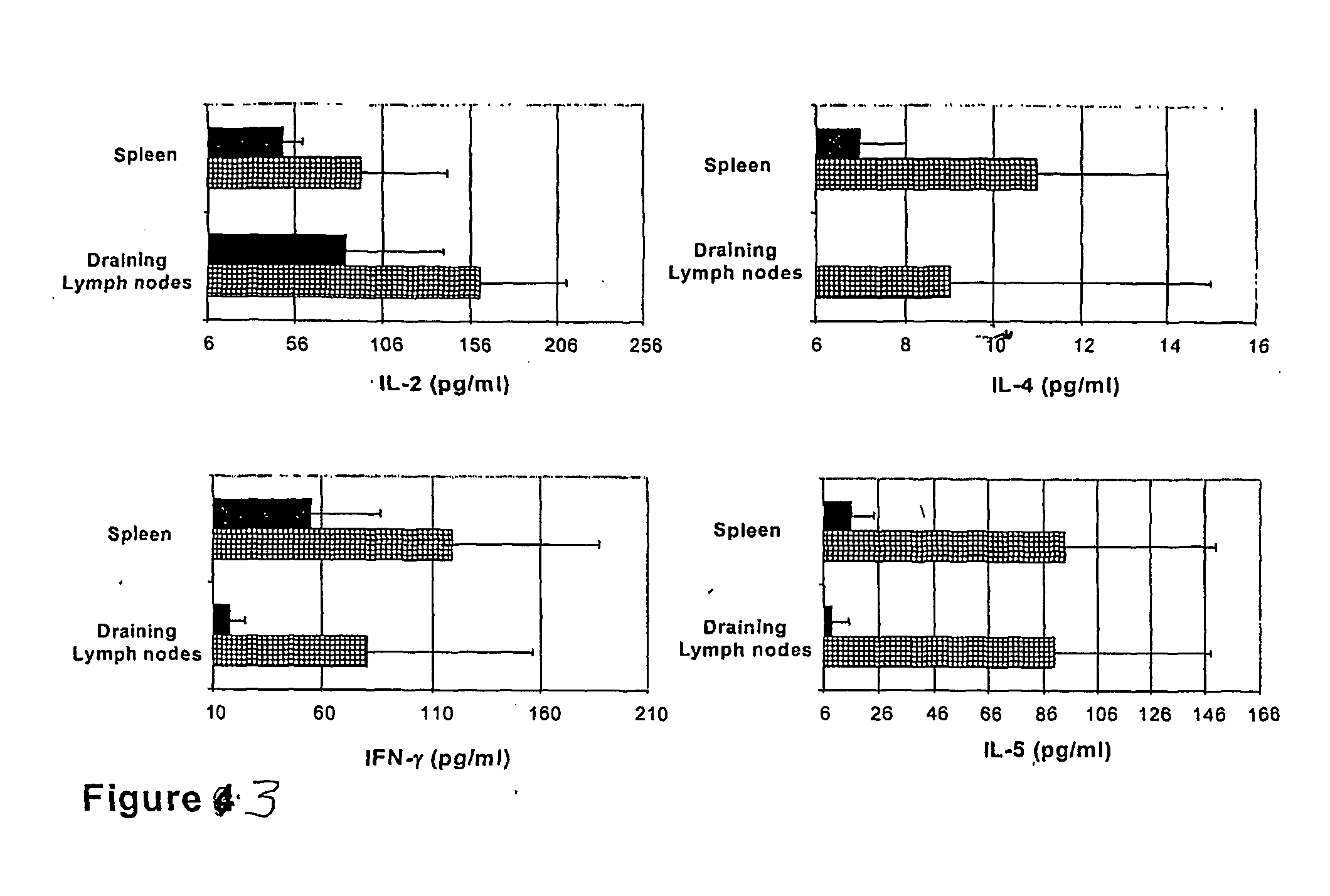
The present invention discloses the use of the non-toxic cell-binding B subunit of CT (CTB), and holotoxin CT that is devoid of ADP-ribosylating activity, as adjuvants for enhancing transcutaneous immune response to a co-administered protein allergen. It was found that topical administration of CTB to mice induced serum antibody response against itself comparable to those evoked by CT, but was inefficient at promoting systemic antibody responses against an admixed prototype protein allergen. To the contrary co-administration of either CT or CTB with allergen led to vigorous antigen-specific T cell proliferative responses in lymph nodes draining the cutaneous site of administration and at distant systemic sites. Consistent with these observations, it was found that CTB selectively potentiated Th1-driven responses without affecting Th2-dependent responses. Cutaneously applied CT enhanced serum IgE responses to a co-administered allergen, while CTB partially suppressed epicutaneously induced IgE responses to the same allergen.
Owner:DUOTOL
Epitope of rheumatoid arthritis and application thereof
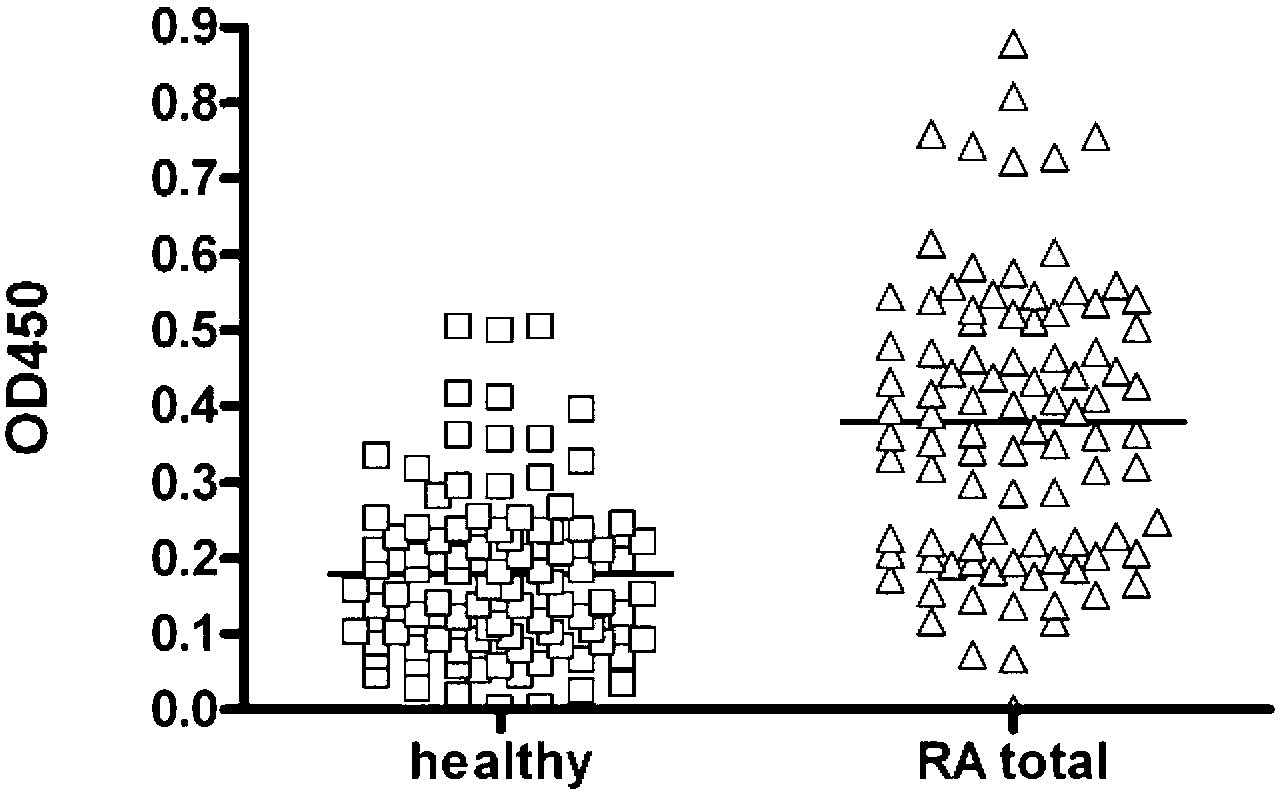
The invention discloses an epitope of rheumatoid arthritis and application thereof, belonging to the technical field of immunologic diagnosis. The epitope is a polypeptide, and the amino acid sequence is disclosed as SEQ ID NO:1 in the sequence table. ELISA detection and patient serum reaction condition indicate that the epitope polypeptide can be specifically combined with IgG in the patient serum, and does not react with the serum of a health person. The epitope polypeptide can be used for preparing medicines for diagnosing rheumatoid arthritis.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Methods for diagnosis and treatment of crohn's disease
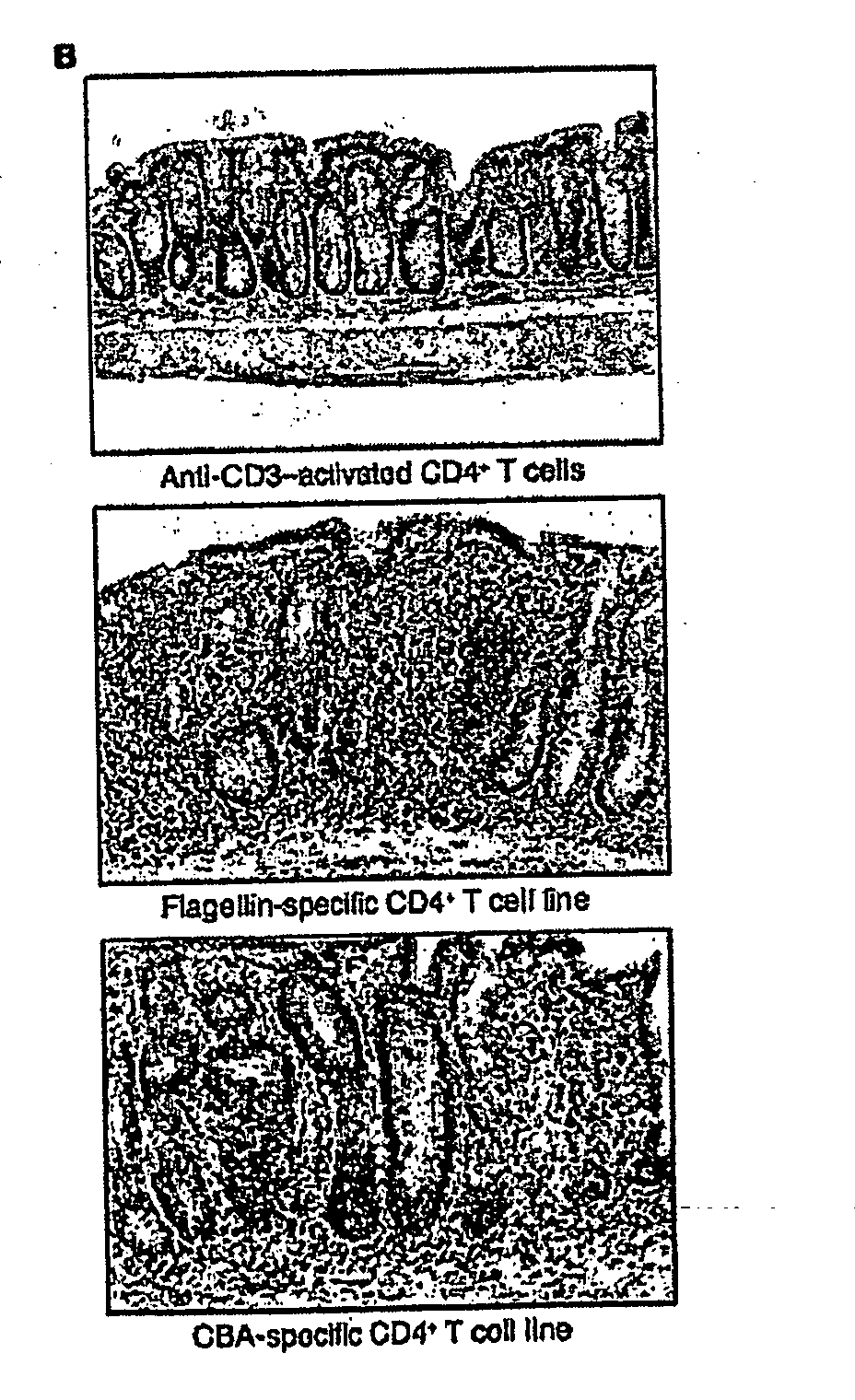
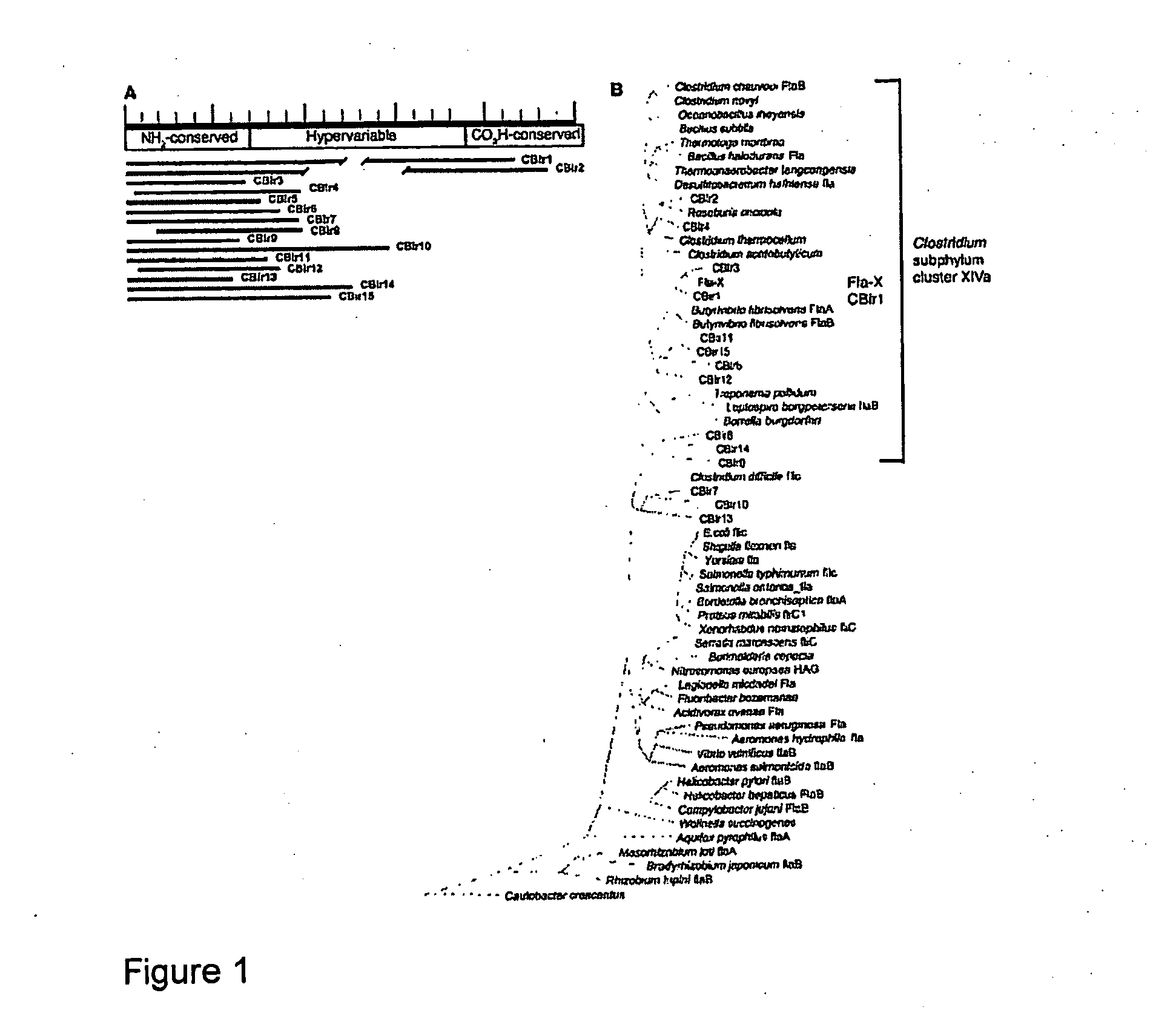
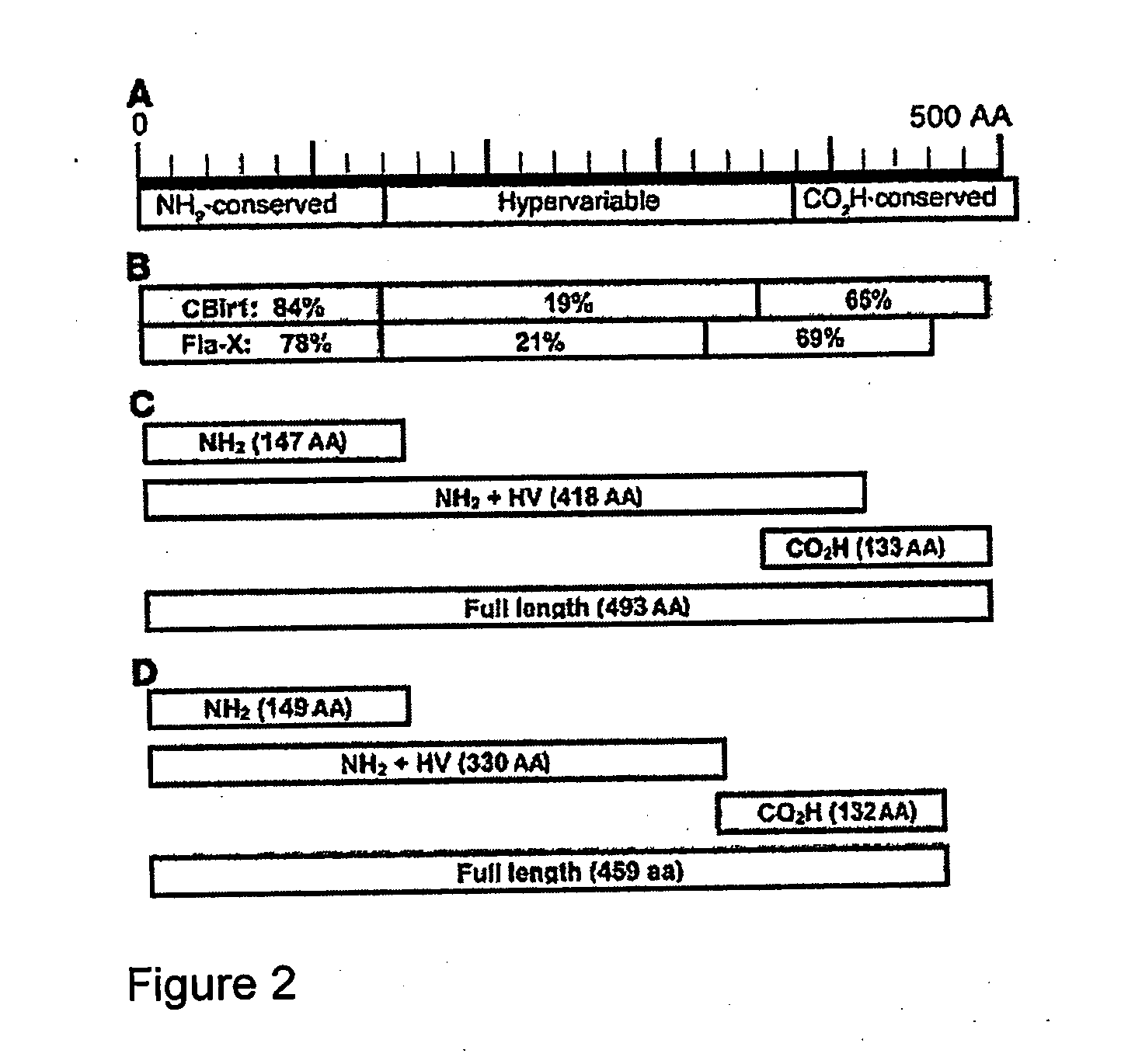
The inventors have discovered an elevated serum response to CBir1 flagellin in Crohn's disease patients. The present invention relates to methods for diagnosis and treatment of Crohn's disease and / or subtypes of Crohn's disease. Diagnosis is accomplished by determining the presence of the anti-CBir1 expression or determining the presence of anti-CBir1 expression and detection of the presence of pANCA. Treatment methods include antigen-directed therapy targeting CBir1 flagellin and manipulating the bacteria in the colon and / or small intestine.
Owner:CEDARS SINAI MEDICAL CENT
Cancer-associated antigens and methods of their identification and use
The present invention provides novel, isolated, tumor-associated antigens, and methods for identifying such antigens in a biological sample, and of screening for the presence of such an antigen in a biological specimen, wherein the tumor antigen identified reacts with serum from a subject treated with a vaccine comprising a cytokine and proliferation-incompetent tumor cells which express the tumor-associated antigen. Also provided are kits for carrying out the methods of the invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Preparation method and application of a high-throughput fully human antibody
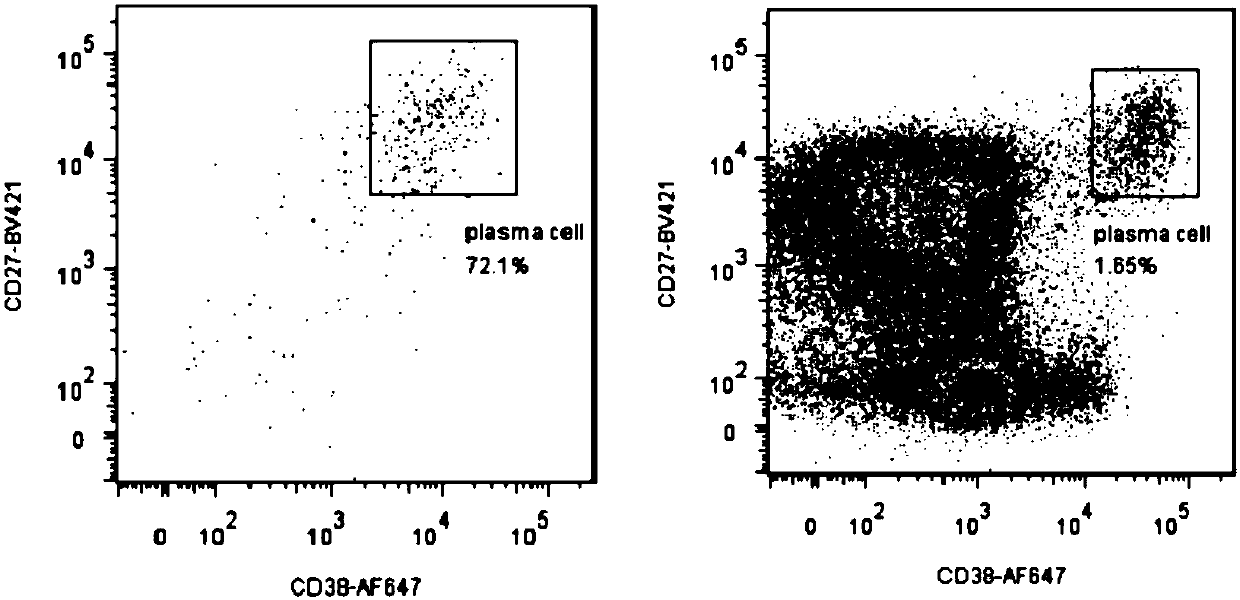
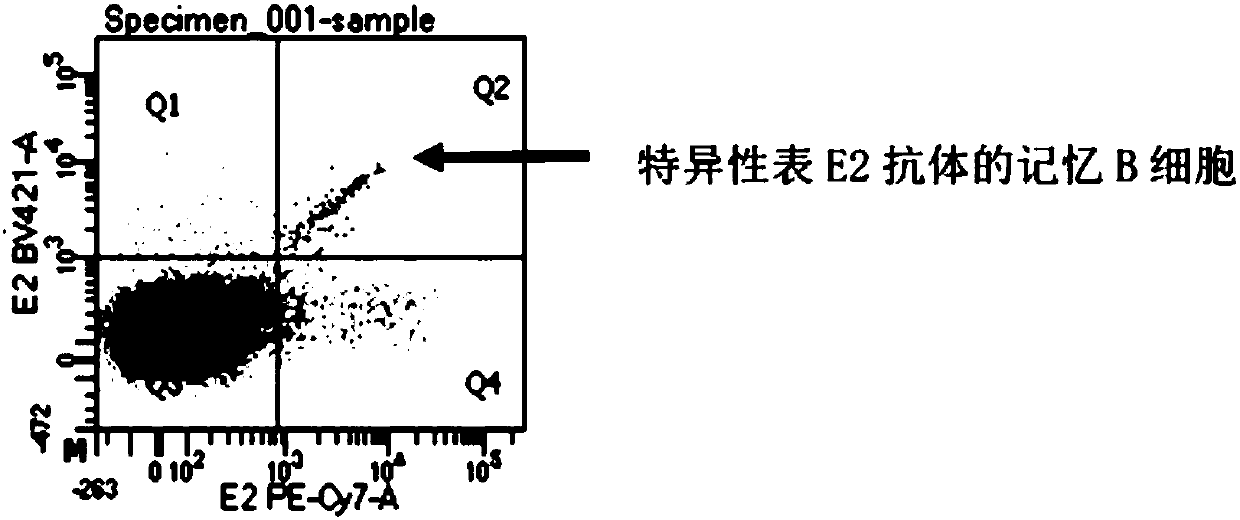
ActiveCN107760690BHigh affinityHigh potencyMicrobiological testing/measurementImmunoglobulinsPlasma cellHumanized antibody
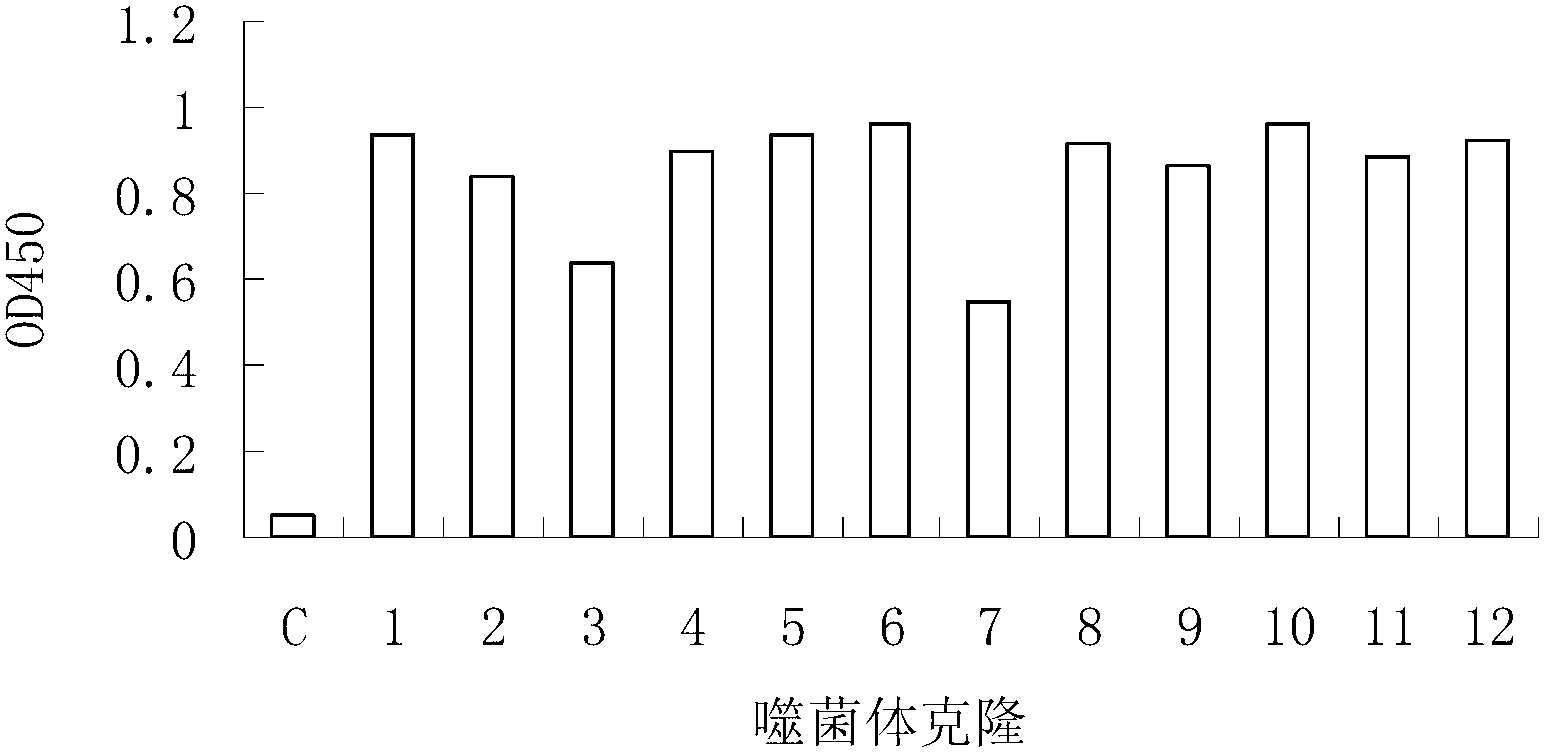
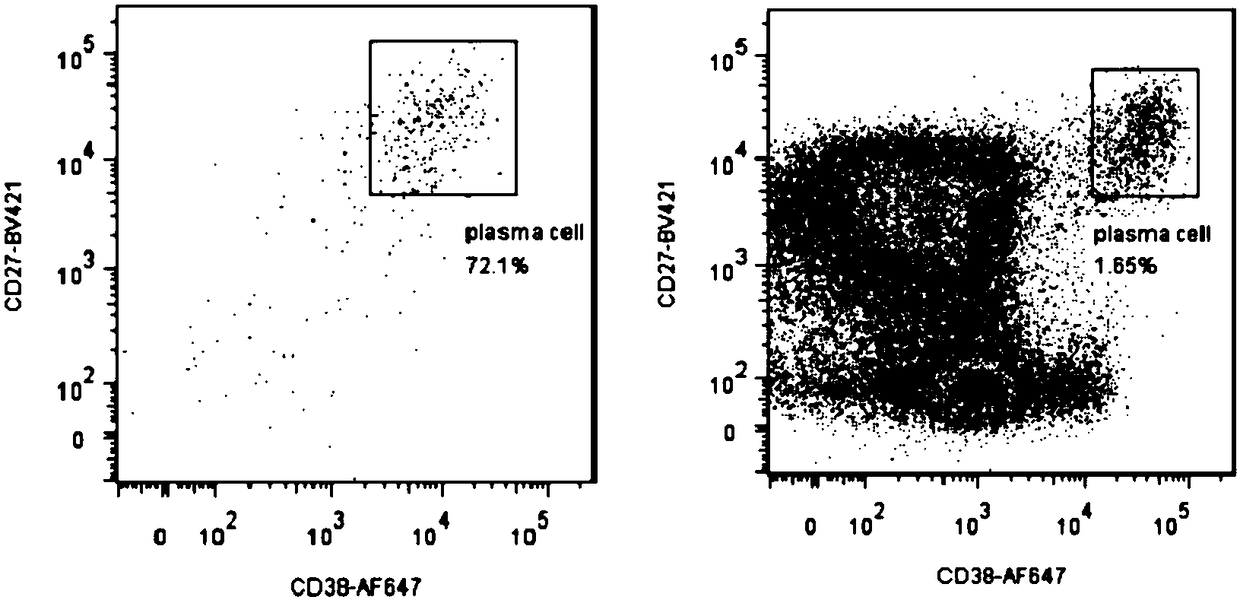
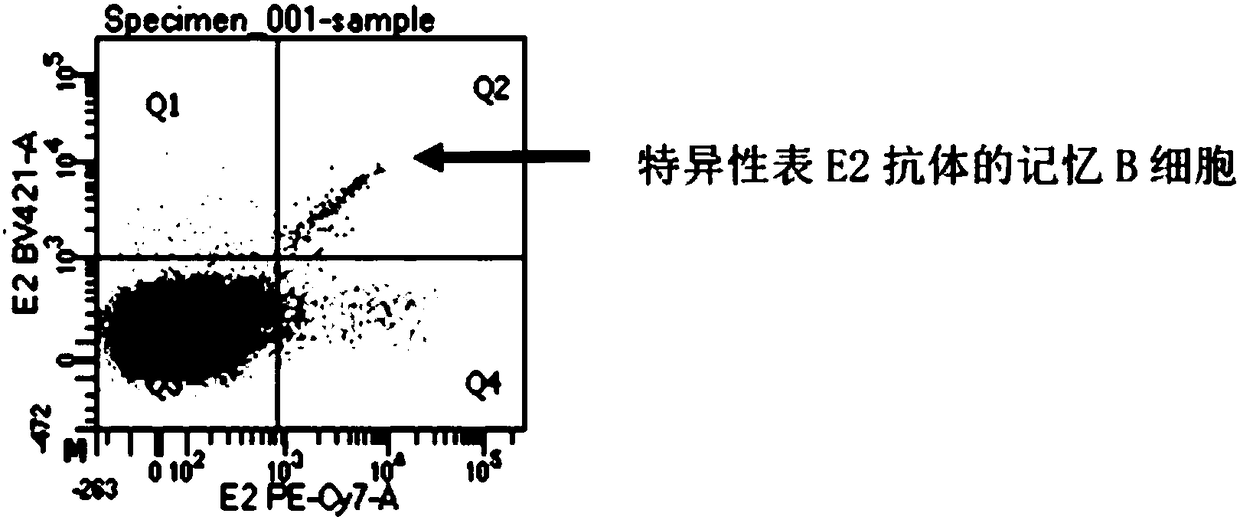
The invention relates to gene engineering and antibody preparation and specifically discloses a preparation method and application of a high-throughput fully-humanized antibody. The preparation methodcomprises the following steps: separating peripheral blood monouclear cells; separating single cells of plasma cells or antigen-specificity memory B blymphocytes; amplifying the heavy-chain and light-chain gene variable regions of single B cell antibody by use of a primer provided by the inventor; establishing an expression system containing antibody heavy-chain and light-chain genes by use of alinear expression system containing a heavy-chain fragment or light-chain fragment constant area; finally, separating and purifying a fully humanized monoclonal antibody. By adopting the primer combination provided by the invention to amplify the genes in the antibody heavy-chain and light-chain variable areas, natural pairing of the light-chain and heavy-chain variable areas can be maintained, and the preparation method has the advantages of high gene diversity, high titer, full humanization, high antibody affinity, strong specificity, no heterologous serum reaction, no risk of propagating other infectious diseases, etc.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
Preparation method and application of high-throughput fully-humanized antibody
ActiveCN107760690AHigh affinityHigh potencyMicrobiological testing/measurementImmunoglobulinsDiseaseAntibody Affinities
The invention relates to gene engineering and antibody preparation and specifically discloses a preparation method and application of a high-throughput fully-humanized antibody. The preparation methodcomprises the following steps: separating peripheral blood monouclear cells; separating single cells of plasma cells or antigen-specificity memory B blymphocytes; amplifying the heavy-chain and light-chain gene variable regions of single B cell antibody by use of a primer provided by the inventor; establishing an expression system containing antibody heavy-chain and light-chain genes by use of alinear expression system containing a heavy-chain fragment or light-chain fragment constant area; finally, separating and purifying a fully humanized monoclonal antibody. By adopting the primer combination provided by the invention to amplify the genes in the antibody heavy-chain and light-chain variable areas, natural pairing of the light-chain and heavy-chain variable areas can be maintained, and the preparation method has the advantages of high gene diversity, high titer, full humanization, high antibody affinity, strong specificity, no heterologous serum reaction, no risk of propagating other infectious diseases, etc.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
Epitope of systemic lupus erythematosus and application thereof
The invention discloses an epitope of systemic lupus erythematosus and an application thereof, belonging to the technical field of immunology diagnosis. The epitope is a polypeptide of which the amino acid sequence is shown as a sequence table SEQ ID NO:1. As proved by ELISA (Enzyme-Linked Immunosorbent Assay) detection and the serum reaction condition of a patient, the epitope polypeptide can be specifically combined with IgG (Immunoglobulin G) in the serum of the patient without reacting with the serum of a healthy person. The epitope polypeptide can be used for preparing a medicament for diagnosing systemic lupus erythematosus.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Kit for testing neutralizing antibody racing ELISA in human and animal rabies
InactiveCN101251537AAccurate quantitative determinationEasy to operateBiological testingAntigenRabies
The invention discloses a reagent box for detecting hydrophobia neutralizing antibody competition ELISA of human beings and animals, wherein the reagent box can easily, quickly, accurately and quantitatively detect the hydrophobia neutralizing antibody in blood serums of human beings and animals by marking the hydrophobia neutralizing antibody, the standard serum and the envelope antigen. By using hydrophobia virosome or virus glycoprotein to coat enzyme synapticulae, the enzyme labeling hydrophobia neutralizing antibody is mixed with the blood serum to be tested and the standard serum respectively according to a certain ratio and reacts with the hydrophobia virus glycoprotein antigen coated on the enzyme synapticulae, a standard curve is drawn according to the OD value of the standard blood serum reaction and the known neutralizing titer after the color development, and the titer of the corresponding neutralizing antibody is obtained from the standard curve according to the OD value of the reaction of the blood serum to be tested. The reagent box has the advantages of accurately and quantitatively detecting the neutralizing antibody of the hydrophobia virus, along with simple operation and short time; moreover, the test result of the invention keeps a good consistence with test results of neutralizing test methods recommended by WHO and OIE.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Rheumatosis autoantibody immunoblotting reagent kit
InactiveCN101021533AMaterial analysis by observing effect on chemical indicatorAntigenSerum reaction
The invention discloses a immune imprinting regent box of the rheumatism self antibody which includes the print film in the reacting slot, the enzyme linked regent, the color regent A liquid, the color regent B liquid, the stop liquid, the condense washing solution and the standard band. Firstly, the ENA is divided by the SDS-polyacrylamide gel electrophoresis according to the molecular weight, then transfers it into the print membrane by the print technology which includes the ENA arrayed by the molecular weight; if the detected blood serum has the self antibody, it will disappear the color band by adding the enzyme linked and the color regent after combining with the according antigen. So it can detect that if the serum has the antibody of Sm, U1RNP,ssA,ssB,Scl-70,Jo-1,Ro60,Rib to diagnose and identify the rheumatism.
Owner:SHENZHEN BLOT BIOTECH
Serum bactericidal assay for N. meningitidis specific antisera
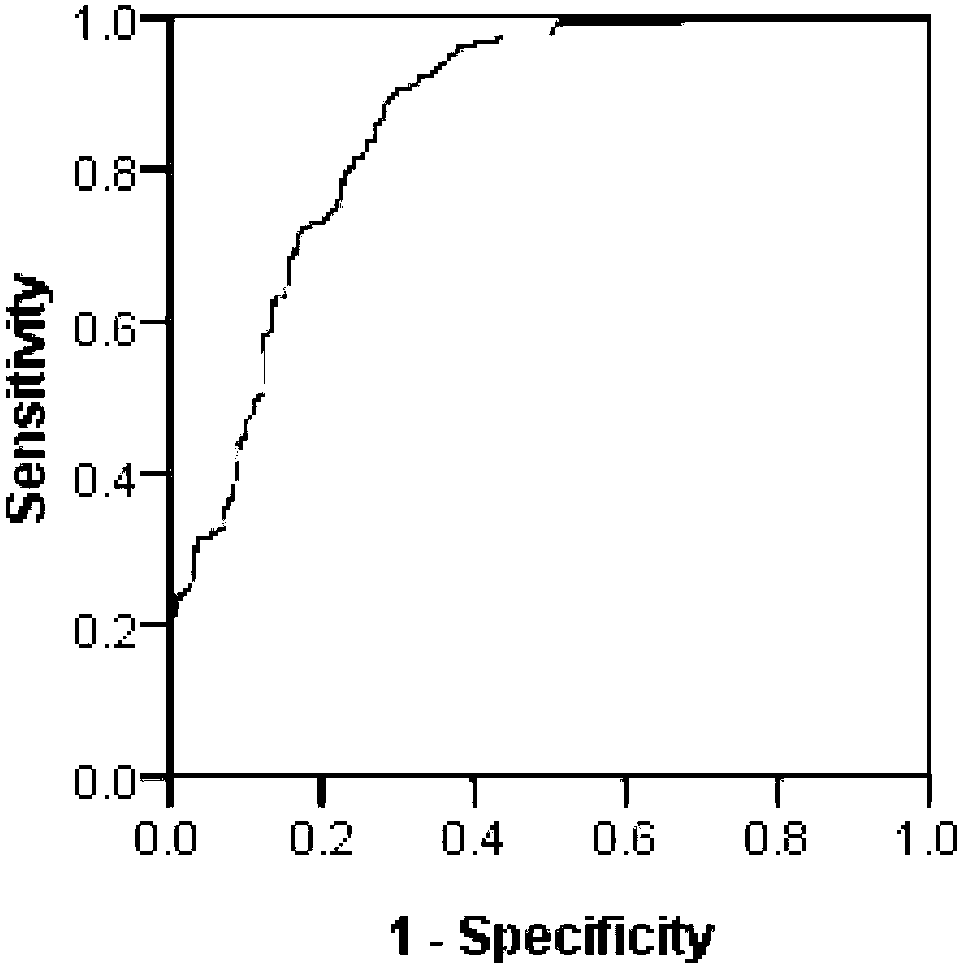
The present invention relates to the field of Serum Bactericidal Activity (SBA) assays for Gram negative bacteria, in particular N. meningitidis. The SBA assay is the most important method for measuring functional activity of serum antibodies against meningococcus. In order to determine whether a subject or a population is seropositive against invasive meningococcus the SBA test should ideally be both sensitive and specific. The inventors have found the standard N. meningitidis serotype A and W SBAs can be significantly improved in this regard.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
i(Ehrlichia canis) 120-KDa immunodominant antigenic protein and gene
The present invention provides a 120-kDa protein gene of Ehrlichia canis, amplified by PCR using primers derived from the DNA sequences flanking the Ehrlichia chaffeensis 120-kDa protein gene. The recombinant E. canis 120-kDa protein contains 14 tandem repeat units with 36 amino acids each. The repeat units are hydrophilic and predicted to be surface-exposed. Also disclosed is that the recombinant E. canis 120-kDa protein is antigenic and reacts with sera from dogs convalescent from canine ehrlichiosis.
Owner:RES DEVMENT FOUND
Methods and compositions for the treatment of diseases or conditions associated with increased C-reactive protein, interleukin-6, or interferon-gamma levels
InactiveUS20080003213A1Reduce IL- levelLower Level RequirementsBiocidePeptide/protein ingredientsInterleukin 6Adenosine
The invention features methods and compositions for reducing the serum C-reactive protein (CRP), IL-6, and / or IFN-γ levels in a patient in need thereof, and for treating diseases and conditions associated with an increased serum CRP, IL-6, and / or IFN-γ levels. The invention also features methods and compositions for treating a patient diagnosed with, or at risk of developing, periodontal disease by administering a corticosteroid or an analog thereof and / or a tetra-substituted pyrimidopyrimidine or an adenosine analog upregulator.
Owner:COMBINATORX
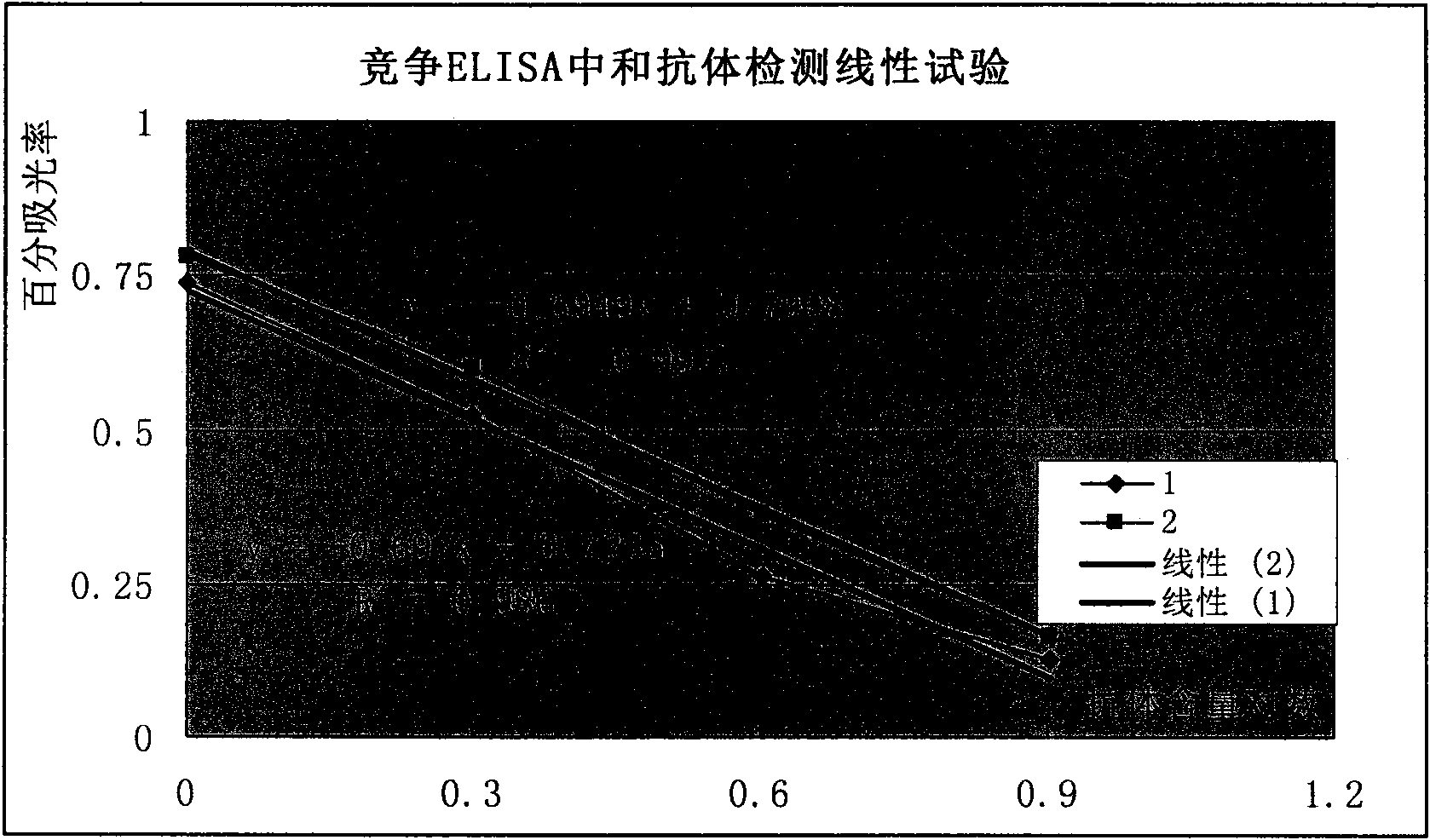
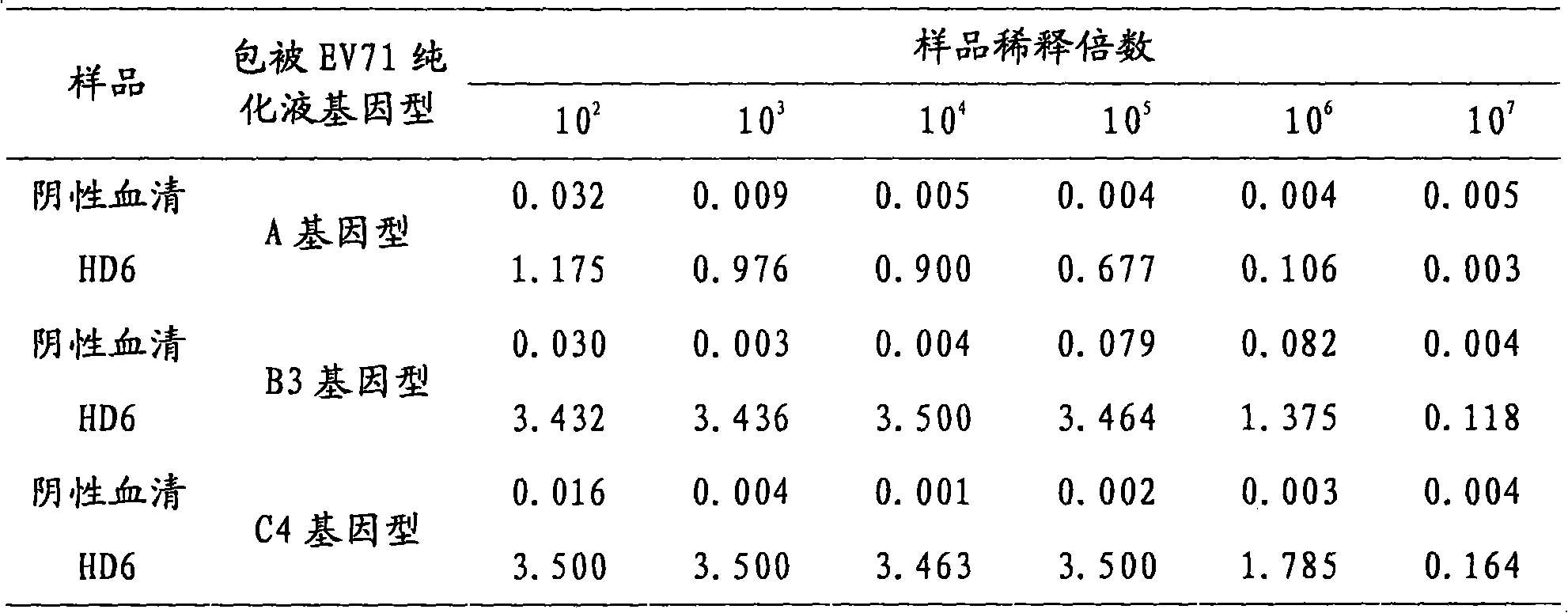
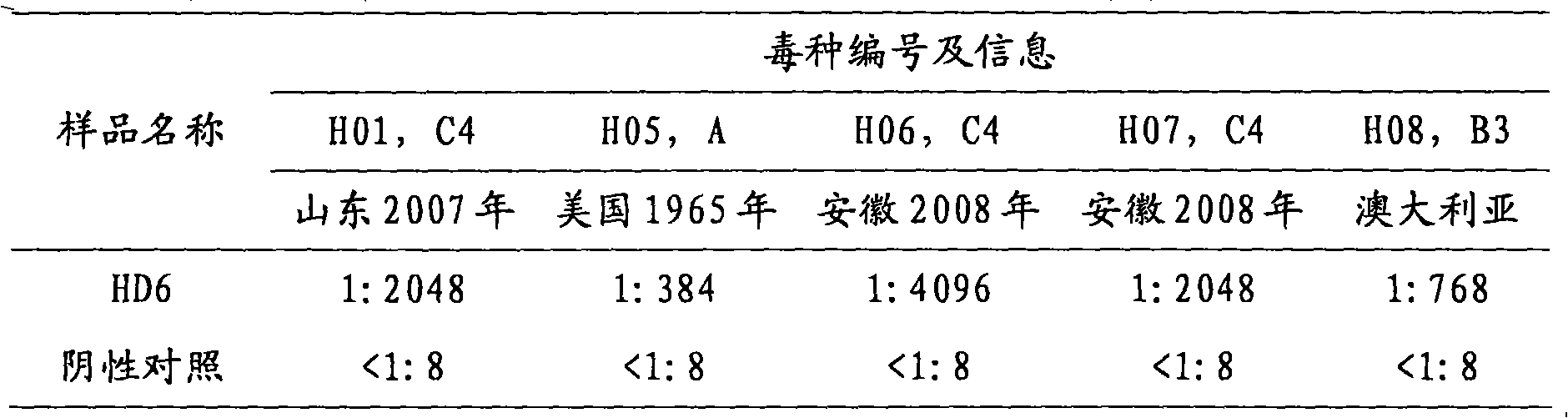
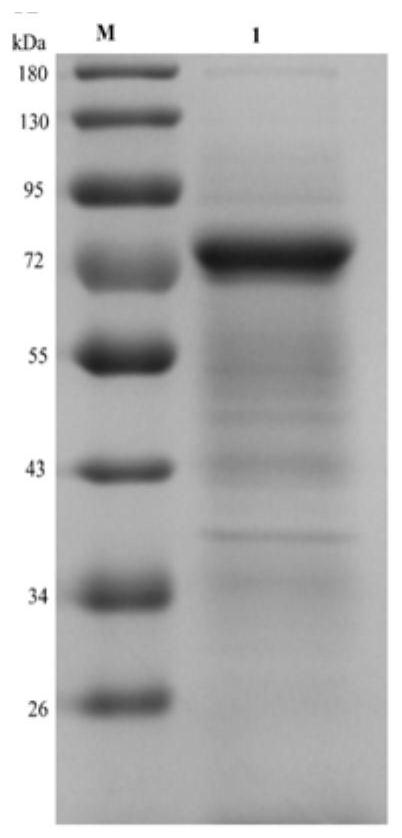
Competitive enzyme-linked immunosorbent assay method of EV71 neutralizing antibody, kit or reagent and prepration method thereof
ActiveCN101609097AGuaranteed specificityOvercome the inability to carry out the shortcomingsFermentationMaterial analysisAntigenSerum reaction
The invention discloses a competitive enzyme-linked immunosorbent assay method of an EV71 neutralizing antibody, a kit or a reagent and a preparation method thereof. The kit utilizes the labeled monoclonal antibody which has higher neutralizing potency on three genes of A, B and C of EV71 virus, a reference material of the EV71 neutralizing antibody and a coated plate and can simply, rapidly and accurately carry out qualitative and quantitative assay on the content of the EV71 neutralizing antibody in a human sample or an animal sample. EV71 virus particles are coated on the ELISA plate, the enzyme-labeled EV71 neutralizing antibody is diluted according to a certain proportion, then respectively mixed with the sample to be assayed and the reference material of the EV71 neutralizing antibody and reacted with an EV71 antigen coated on the ELISA plate, a semi-logarithmic standard curve is drawn according to the absorptance percentage of OD value of the reference serum reaction and the logarithm of the potency of the reference material of the EV71 neutralizing antibody after color development, and the absorptance percentage of the OD value of the sample to be assayed is substituted ina standard curve equation to calculate the corresponding neutralizing potency.
Owner:SINOVAC BIOTECH
Diabetes autoantibody immunoblotting reagent kit
InactiveCN101042399AMaterial analysis by observing effect on chemical indicatorLangerhan cellDisease
This invention discloses one diabetes disease self antigen print agent case, whose islets of Langerhans cell extracts mixture protein composed of Langerhas element, glutamic acid decarboxylase and Langerhas cell; using protein print technique through SDS-polyacrylamide gel electrophoresis ranked by molecule size then into print film; its film bar comprises molecule of each Langerhans self antigen element to be put into reaction tank and serum; if it has antigen, then processing antigen combination and then adding enzyme cross and development agent in the zone; comparing with standard to judge whether serum has the antigen etc.
Owner:SHENZHEN BLOT BIOTECH
Application of mycoplasma bovis secretory protein MbovP274
The invention discloses application of mycoplasma bovis secretory protein MbovP274 in preparation of mycoplasma bovis diagnostic reagents, drugs or vaccines, and belongs to the technical field of animal infectious disease prevention and treatment. The mycoplasma bovis secretory protein MbovP274 has an amino acid sequence as shown in SEQ ID NO: 2; gene annotation information shows that the amino acid sequence is a functionally-unknown protein; and experiments show that the MbovP274 protein has antigenicity and can react with mycoplasma bovis positive serum instead of mycoplasma bovis negative serum; the MbovP274 protein is identified as a secretory protein through western blot; it is further verified that the protein can induce bovine macrophages to highly express IL-8 and IFN-gamma, the IL-8 is an important neutrophil activating factor, and the IFN-gamma is a wide immunomodulatory factor. According to the research, the MbovP274 is considered to be an important pathogenic factor of mycoplasma bovis and can activate host immunity, and has important potential in research and development of new drugs and vaccines.
Owner:HUAZHONG AGRI UNIV
Application of mycoplasma bovis secretory protein MbovP570
ActiveCN111850002AAntibacterial agentsBacterial antigen ingredientsInfectious DisorderNatural Killer Cell Inhibitory Receptors
The invention discloses application of mycoplasma bovis secretory protein MbovP570 in preparation of mycoplasma bovis diagnostic reagents, drugs or vaccines, and belongs to the technical field of animal infectious disease prevention and treatment. The mycoplasma bovis secretory protein MbovP570 has a sequence as shown in SEQ ID NO: 1. 2 is an amino acid sequence shown in the specification; test proves that the mycoplasma bovis secretory protein MbovP570 has antigenicity and can be used for detecting mycoplasma bovis. The protein can react with mycoplasma bovis positive serum instead of mycoplasma bovis negative serum, the protein can induce bovine macrophages to highly express IL-8 and IL-12, IL-8 can activate neutrophils, and IL-12 can activate NK cells and T cells. Therefore, it is believed that MbovP570 can activate a host immune system and has important potential in research and development of new drugs and vaccines.
Owner:HUAZHONG AGRI UNIV
Value setting method for C reactive proteins in serum
ActiveCN110850099ARegardless of the extraction rateReliable valueComponent separationMaterial analysis by electric/magnetic meansSerum reactionIsotopic labeling
The invention discloses a value setting method for C reactive proteins in serum. The method comprises the following steps of (1) purification and enrichment of C reactive proteins (CRP)in serum; (2) purity detection of the C reactive proteins; (3) digestion; and (4) quantitative analysis of the C reactive proteins. The method does not need to consider the factors such as protein extraction rate, digestion efficiency and instrument stability, and only needs to consider the correctness of pure CRP concentration in the whole experiment; the used pure CRP solution is a national primary standard substance (developed by the National Institute of Measurement) which is reliable in numerical value and can be traced to an SI unit; and the raw material is nature CRP which does not need to consider the differences of structures and is more excellent than the isotope labeled whole proteins.
Owner:NAT INST OF METROLOGY CHINA
Viral prophylaxis treatment methods and pre-exposure prophylaxis kits
ActiveUS20200397791A1Improve bioavailabilityMinimal protein bindingAntiviralsTamponsPre-exposure prophylaxisSerum reaction
The present disclosure provides compositions and methods for the prevention of HSV infection in an HSV seronegative individual and for the treatment and prevention of recurrence in an HSV seropositive individual.
Owner:ELIAN LLC
Method for detecting animal pathogenic microorganisms and special protein chip thereof
InactiveCN101629957ALarge capacityImprove detection efficiencyMaterial analysisAntigenPathogenic microorganism
The invention belongs to the field of biomedicine, providing a method for detecting animal pathogenic microorganisms and a special protein chip thereof. The detecting method essentially comprises the following steps of: a. preparing the protein chip, selecting a plurality of different groups of specific antigen of many kinds of pathogenic microorganisms by means of an experiment to be fixed on a properly modified carrier; and b. detecting: reacting blood serum of a animal to be detected with the protein chip, hybridizing with a marked secondary antibody, detecting a hybrid positive signal in a proper detection way, and obtaining a hybrid result of the blood serum and the chip by means of scanning and analyzing, wherein the blood serum infected with the different pathogenic microorganisms can show different positive signal combination modes after the reaction, so as to ensure whether the animal to be detected is infecting or is infected with the pathogenic microorganisms marked on the protein chip. The invention can simultaneously detect many kinds of the pathogenic microorganisms, improves detecting efficiency, selects many groups of different antigen with a certain specificity aiming at each pathogenic microorganism, leads the detection to have good specificity, and has good application prospect in detecting the animal pathogenic microorganisms.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Cancer-associated antigens and methods of their identification and use
The present invention provides novel, isolated, tumor-associated antigens, and methods for identifying such antigens in a biological sample, and of screening for the presence of such an antigen in a biological specimen, wherein the tumor antigen identified reacts with serum from a subject treated with a vaccine comprising a cytokine and proliferation-incompetent tumor cells which express the tumor-associated antigen. Also provided are kits for carrying out the methods of the invention.
Owner:ADURO GVAX
Use of protein TolC in preparing immunological formulation and vaccine
InactiveCN1876180AImproving immunogenicityAntibacterial agentsBiological testingProtective antigenSerum reaction
The invention discloses the usage of TolC protein. The TolC has strong serum reaction, is used to detect and diagnose dysentery, and is used as protective antigens to prepare vaccine.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
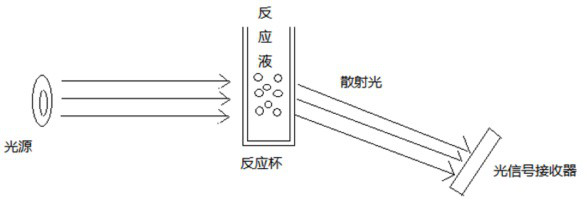
Specific protein reaction detection method and device
ActiveCN112485438AEnsure consistencyScattering properties measurementsBiological testingProtein detectionBlood plasma
A specific protein reaction detection method comprises the following steps: respectively carrying out C-reactive protein detection on a serum sample, a plasma sample and a blood sample of the same collection source to obtain specific protein reaction curves of the serum sample, the plasma sample and the blood sample, respectively acquiring curve characteristics of the serum sample, the plasma sample and the blood sample and inputting the curve characteristics into a serum reaction mathematical model, a plasma reaction mathematical model and a blood reaction mathematical model so as to acquireC-reactive protein concentration values cS and cP output of the serum sample and the plasma sample, and then acquiring an HCT detection value Hb of the blood sample; and performing HCT correction on the C-reactive protein concentration value cb' output by the blood reaction mathematical model, and taking the C-reactive protein concentration value cb' obtained after HCT correction as output. The C-reactive protein concentration values of different samples are obtained by obtaining the characteristics of the specific protein reaction curves of the different homologous samples, so that the different homologous samples keep consistent C-reactive protein detection results on the same detection equipment.
Owner:SHENZHEN COMEN MEDICAL INSTR
Serum Bactericidal Assay for N. Meningitidis Specific Antisera
The present invention relates to the field of Serum Bactericidal Activity (SBA) assays for Gram negative bacteria, in particular N. meningitidis. The SBA assay is the most important method for measuring functional activity of serum antibodies against meningococcus. In order to determine whether a subject or a population is seropositive against invasive meningococcus the SBA test should ideally be both sensitive and specific. The inventors have found the standard N. meningitidis serotype A and W SBAs can be significantly improved in this regard.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Application of protein to preparation of medicine for preventing trueperella pyogenes infection
ActiveCN110642927AFree from lethal infectionAntibacterial agentsBacterial antigen ingredientsTGE VACCINESpecific antibody
The invention belongs to the field of a veterinary biological product, and discloses an antigen protein of trueperella pyogenes, preparation of the antigen protein, application of the antigen proteinto a vaccine, and an antibody detection method. A gene of a highly conservative trueperella pyogenes Fe ABC (ATP Binding Cassette) transporter SBP (Substrate-Binding Protein) is cloned to a pGEX-4T-1plasmid; a BL21(DE3) recombinant strain is built; IPTG is used for induction expression of the recombinant protein SBP (GST-SBP); the soluble expression of the target protein is realized; and the recombinant protein is subjected to affinity purification by a Gluthathione-Sepharose 4B chromatography method. The recombinant SBP (GST-SBP) protein provided by the invention has high reactionogenicity and immunogenicity, and can react with the blood serum of trueperella pyogenes immunized or infected animals (mice and goats); the animals (mice and goats) can be stimulated to generate high-level specific antibodies; and the immune protective rate of the mice is 90 percent. The novel antigen protein is provided for the preparation of medicine for preventing the trueperella pyogenes infection.
Owner:CHONGQING ACAD OF ANIMAL SCI
Vaccination by circumventing preexistent immunity
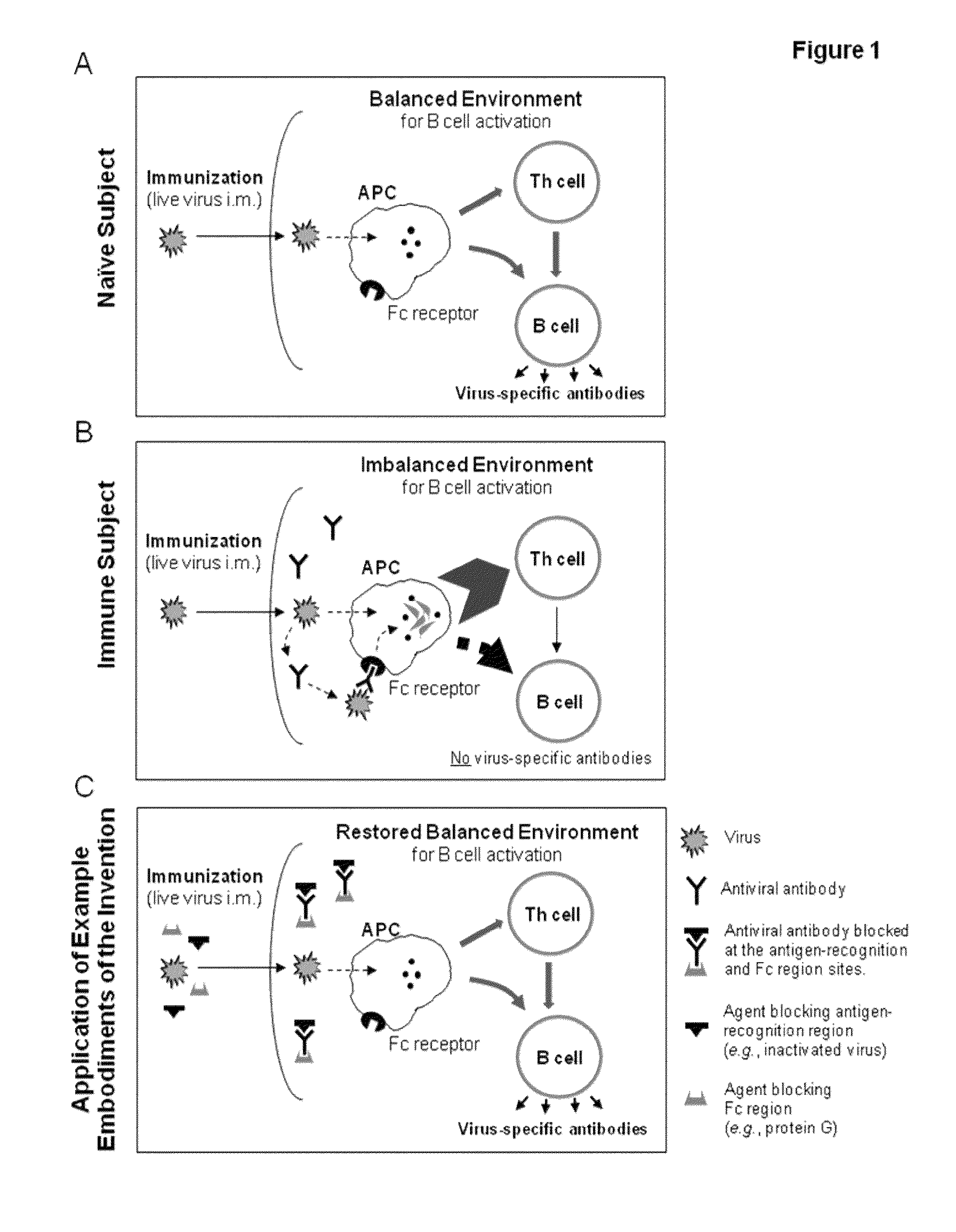
ActiveUS9149508B1Minimise imbalanceEnhance immune responseSsRNA viruses negative-sensePeptide/protein ingredientsSerum reactionVaccination
Methods and compositions are disclosed for improving vaccination in a subject with preexistent antibodies to the antigenic component of the immunizing vaccine. By temporarily sequestering, disabling, and / or suppressing preexistent antibodies and / or Fc-mediated mechanisms according to the various example embodiments, the target antigenic component of a vaccine has an increased opportunity to enter antigen-presenting cells through the same pathway that it would use in seronegative subjects. A balanced immune environment is thereby restored and B cells are properly activated to produce antigen-specific antibodies following vaccination.
Owner:SIGMOVIR BIOSYST
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com