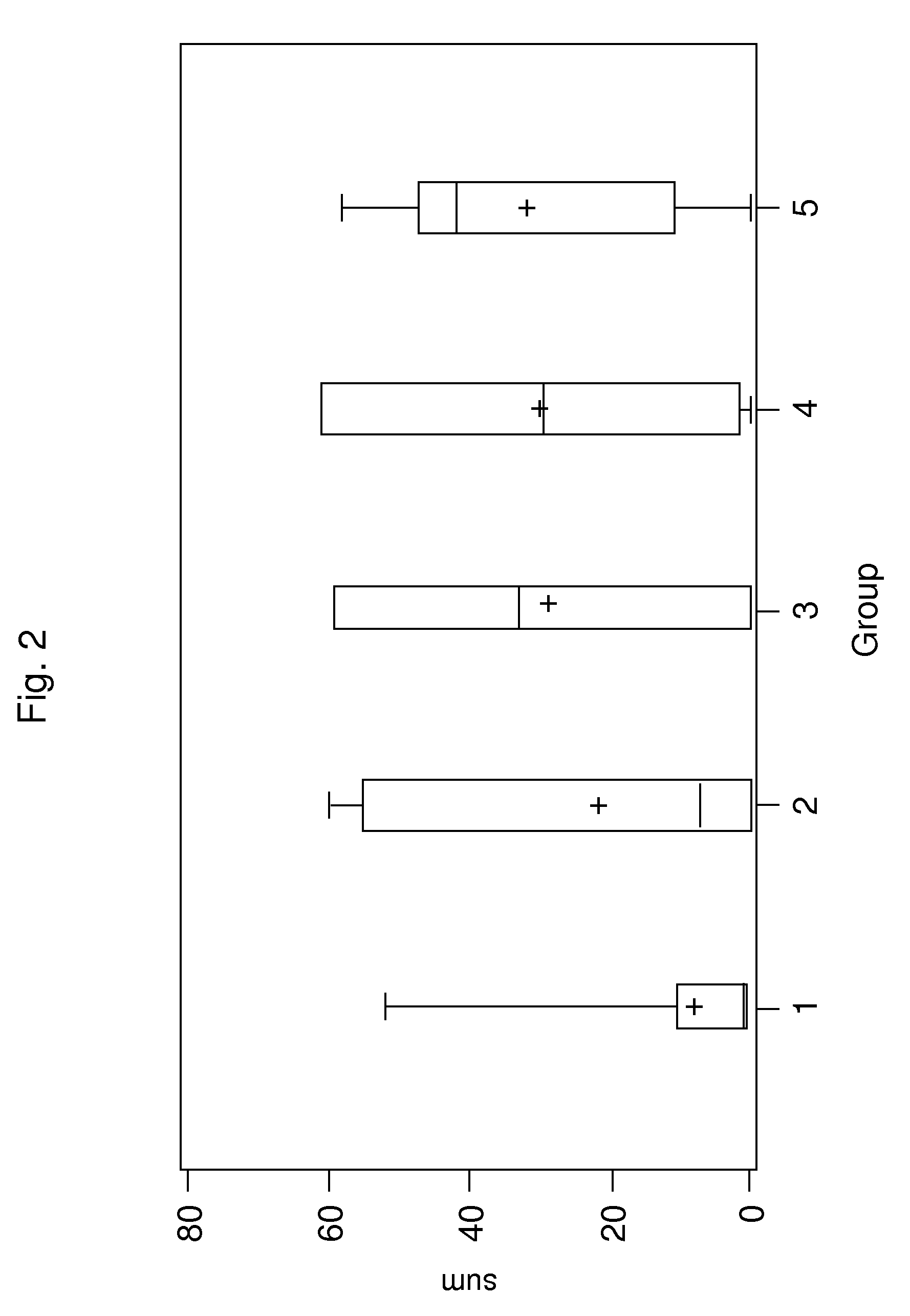
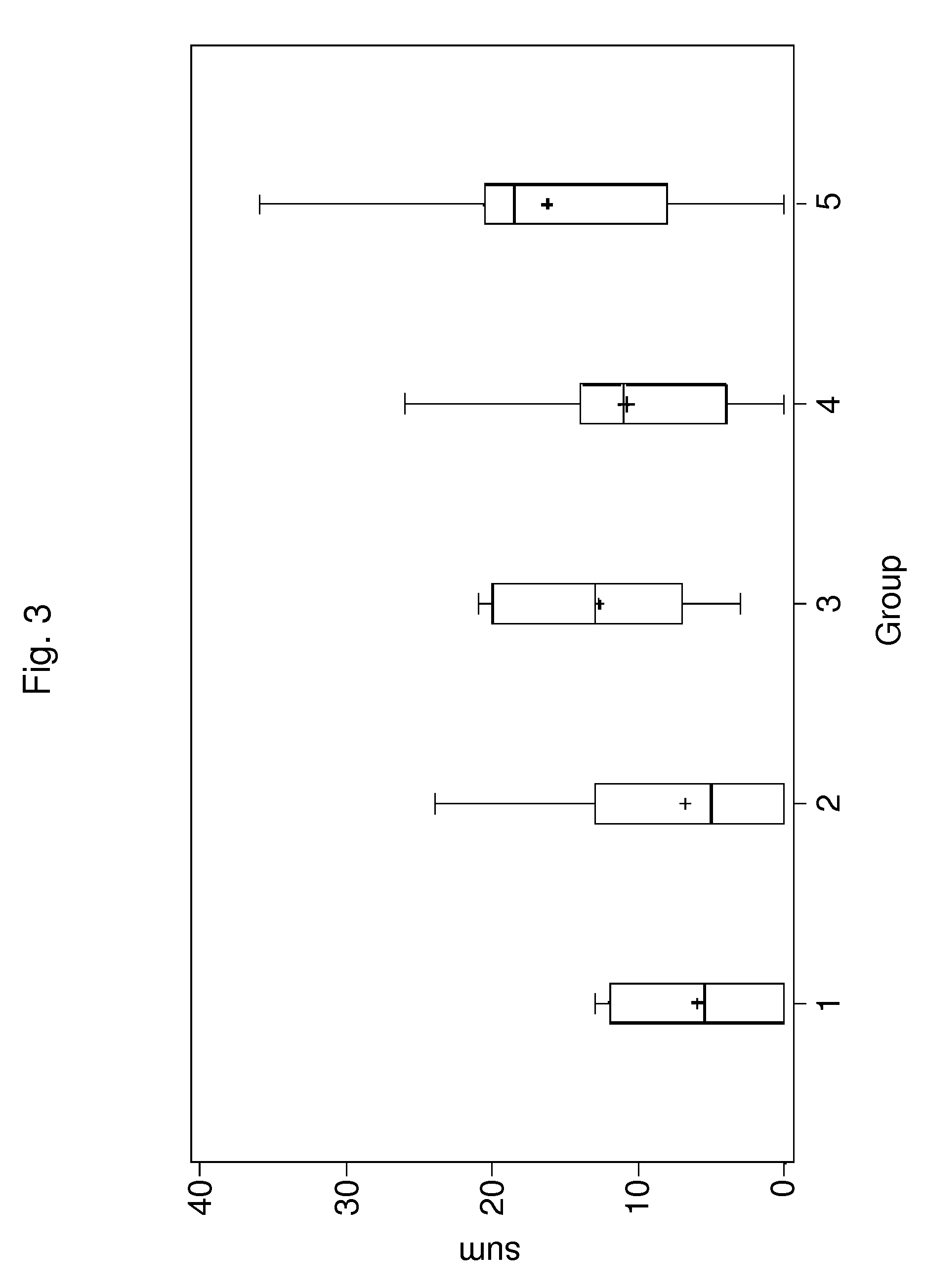
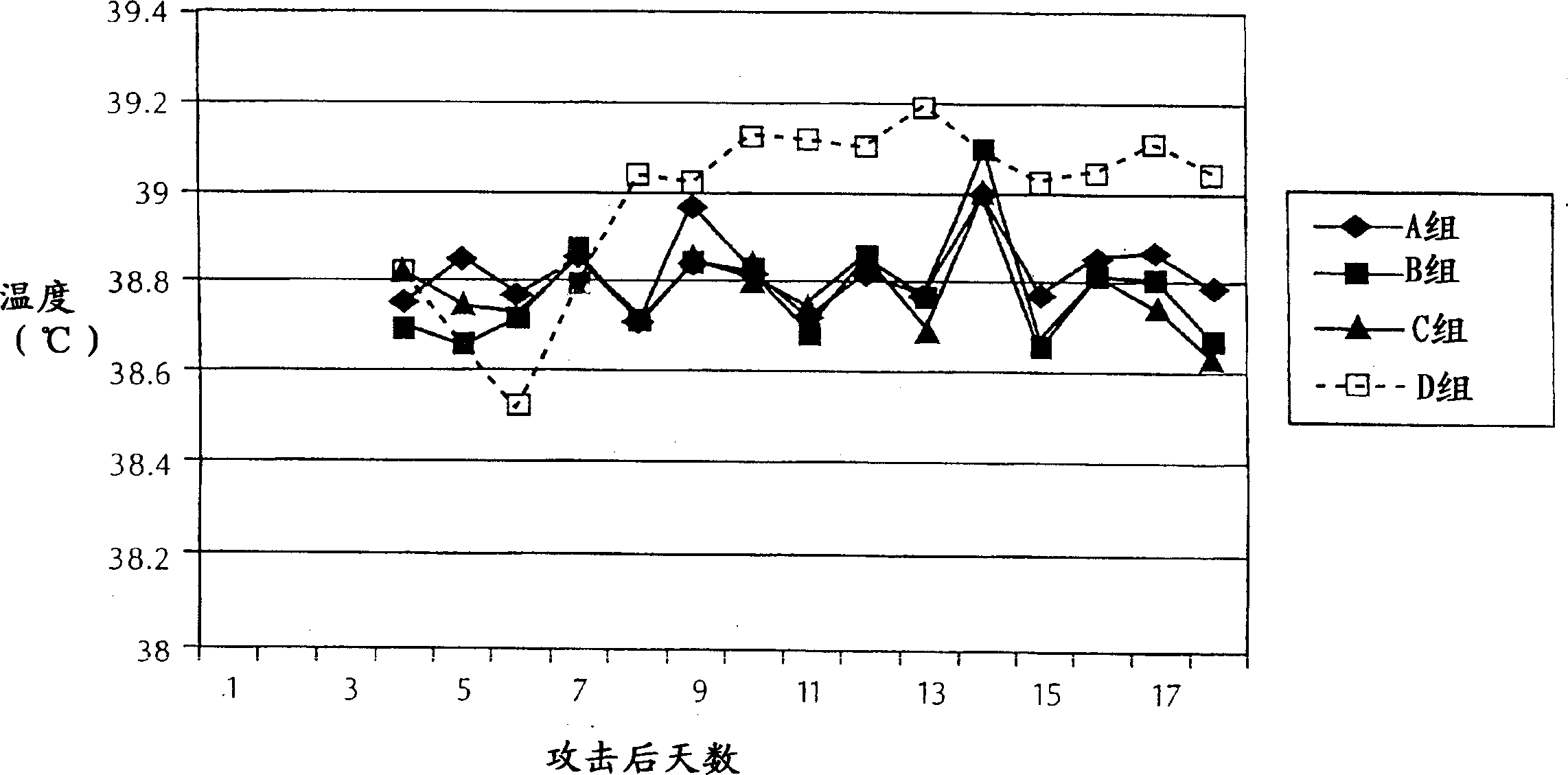
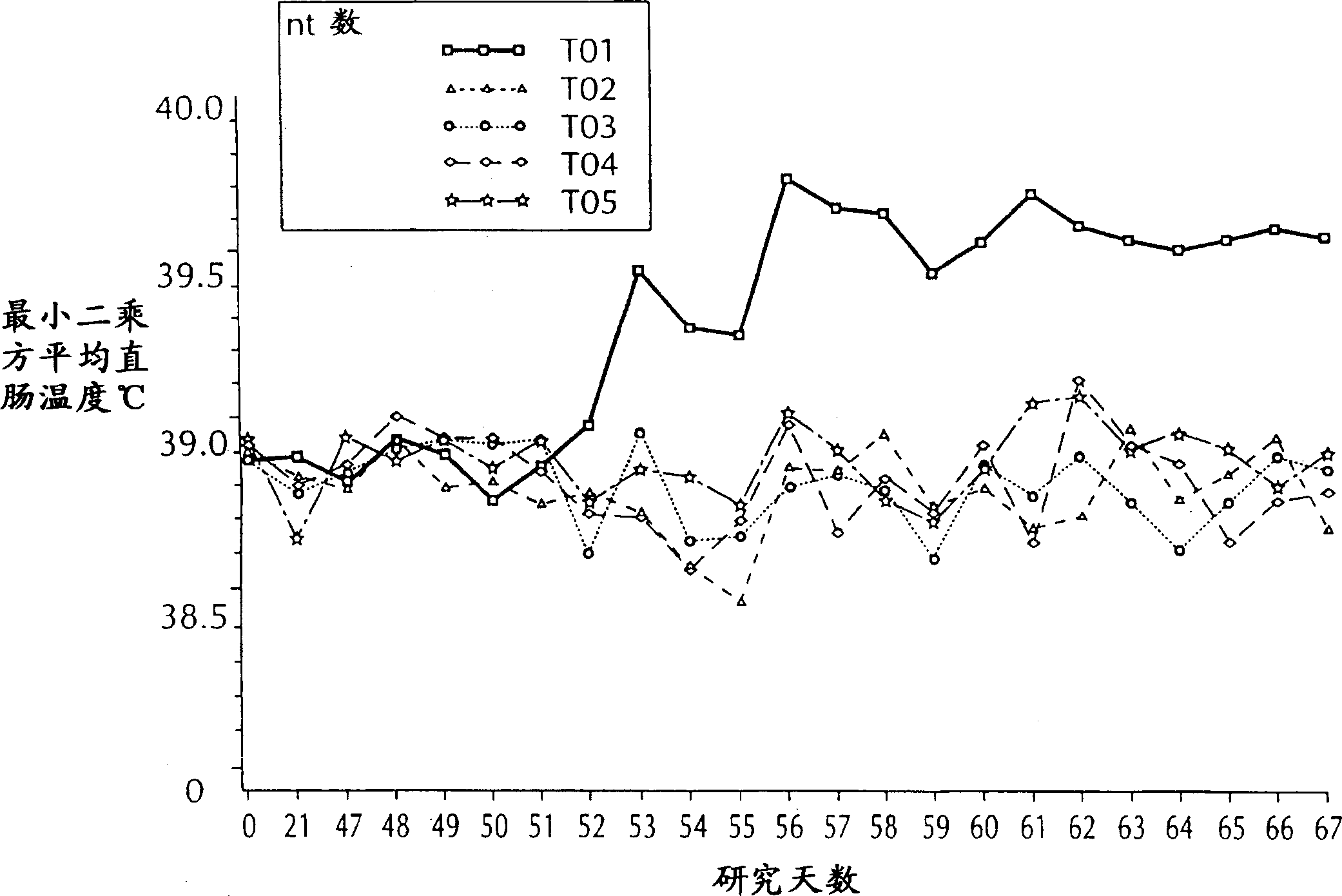
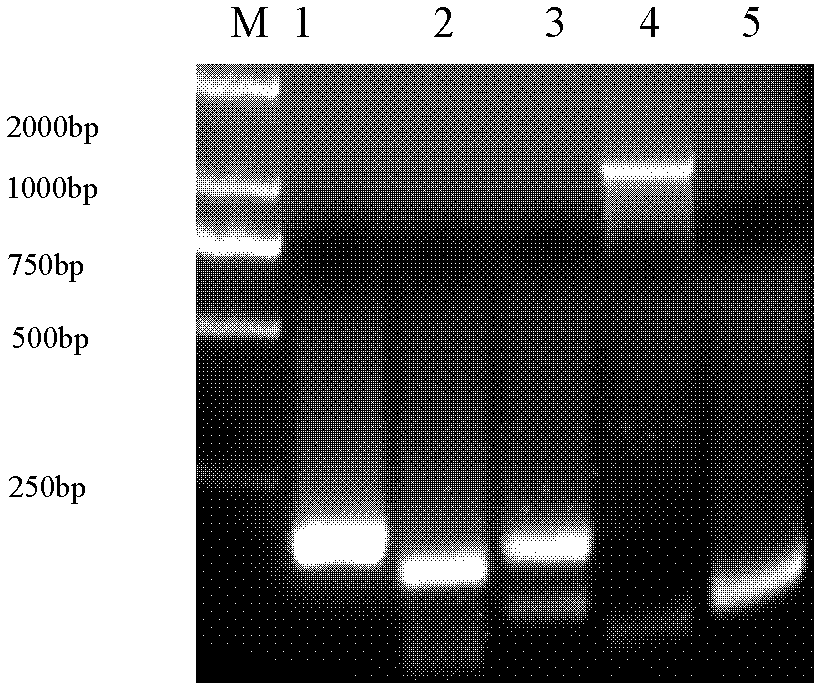
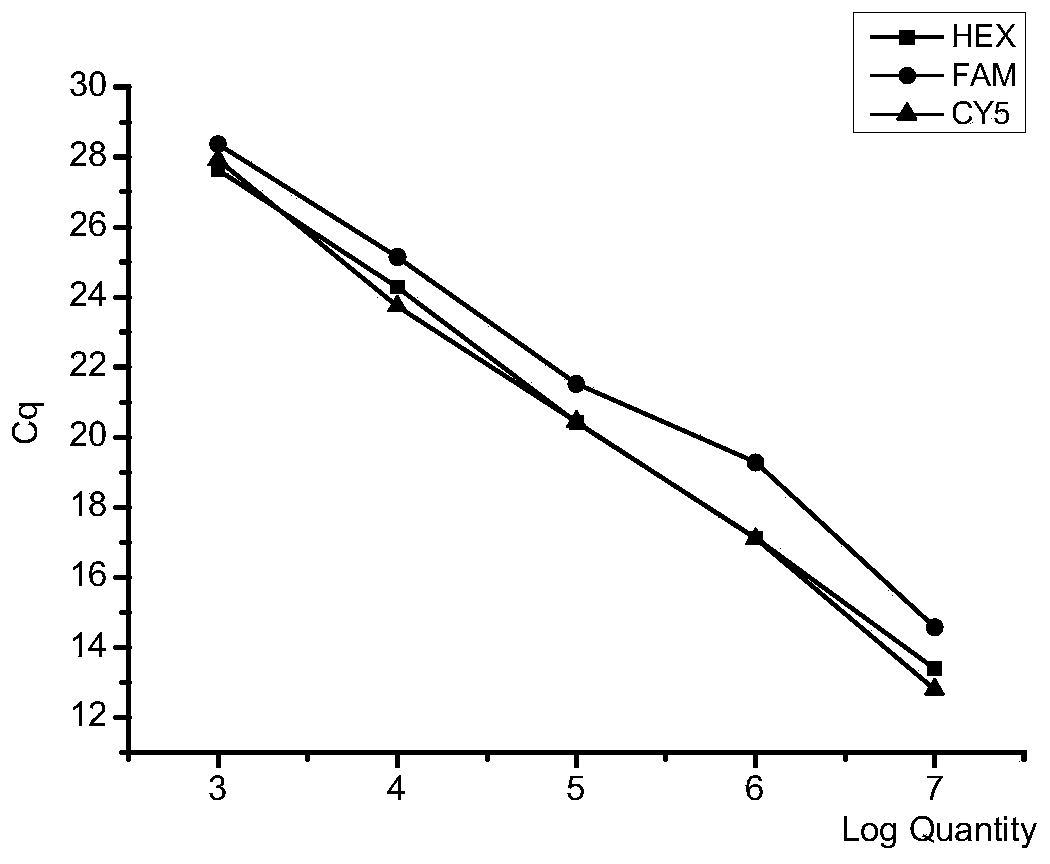
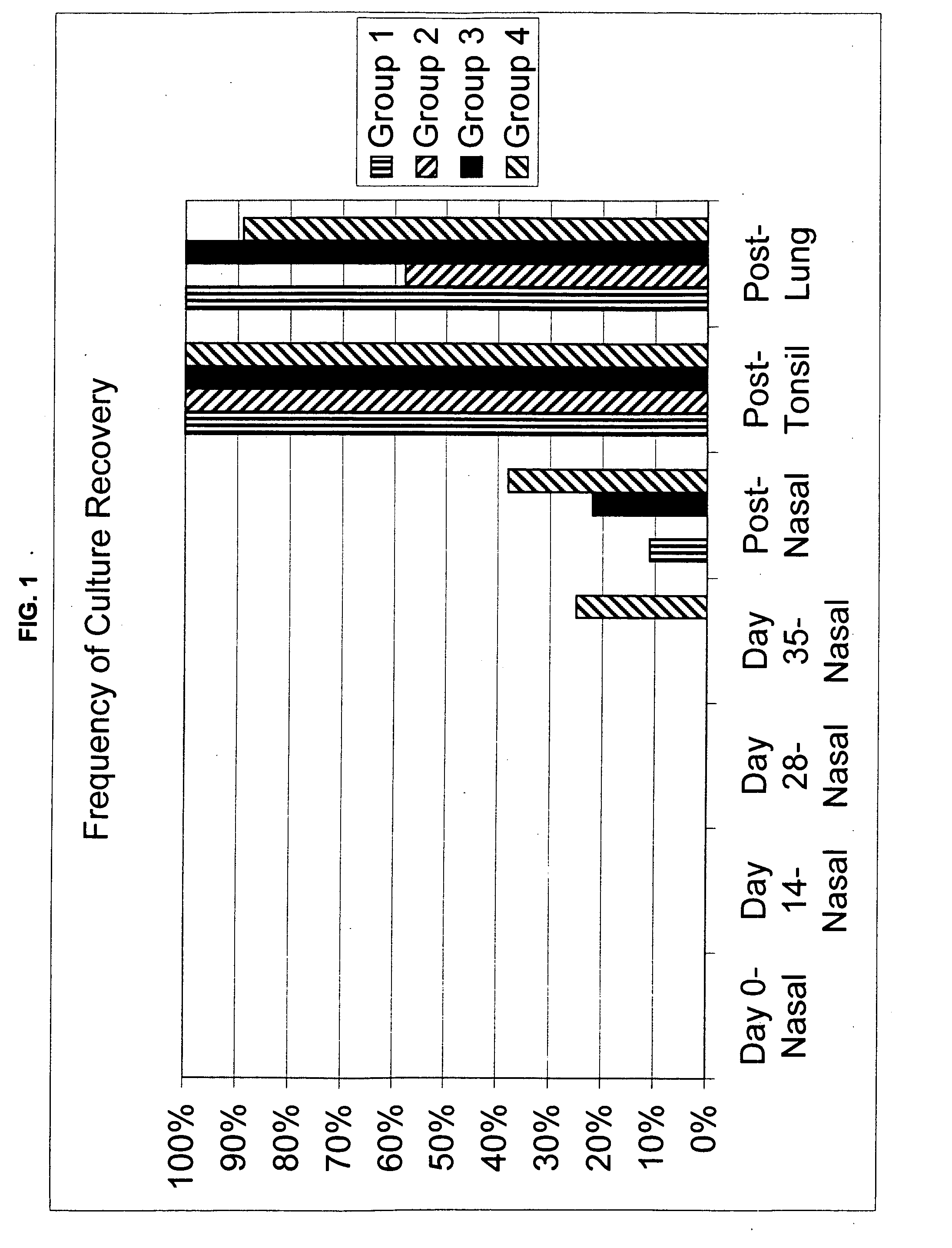
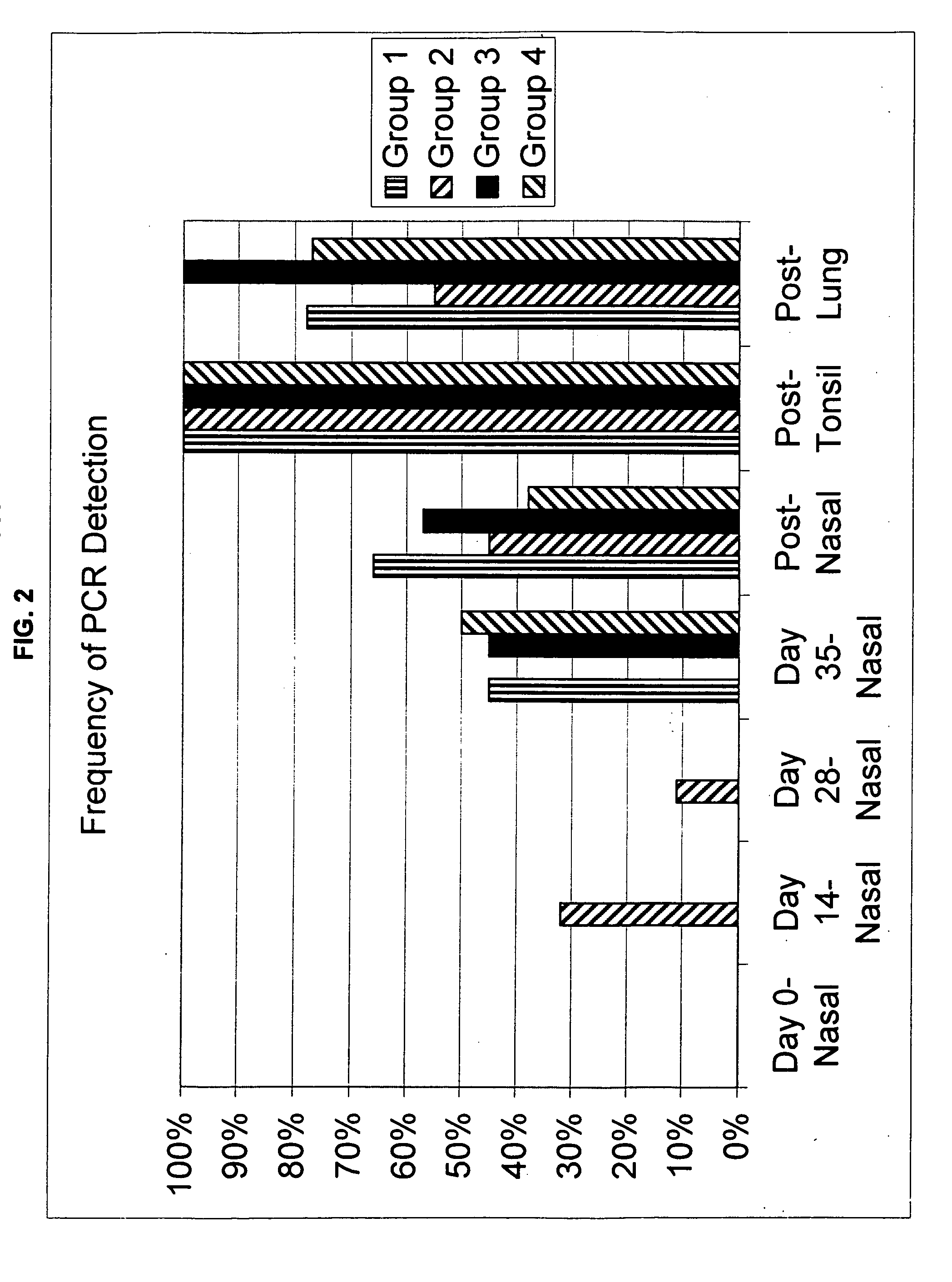
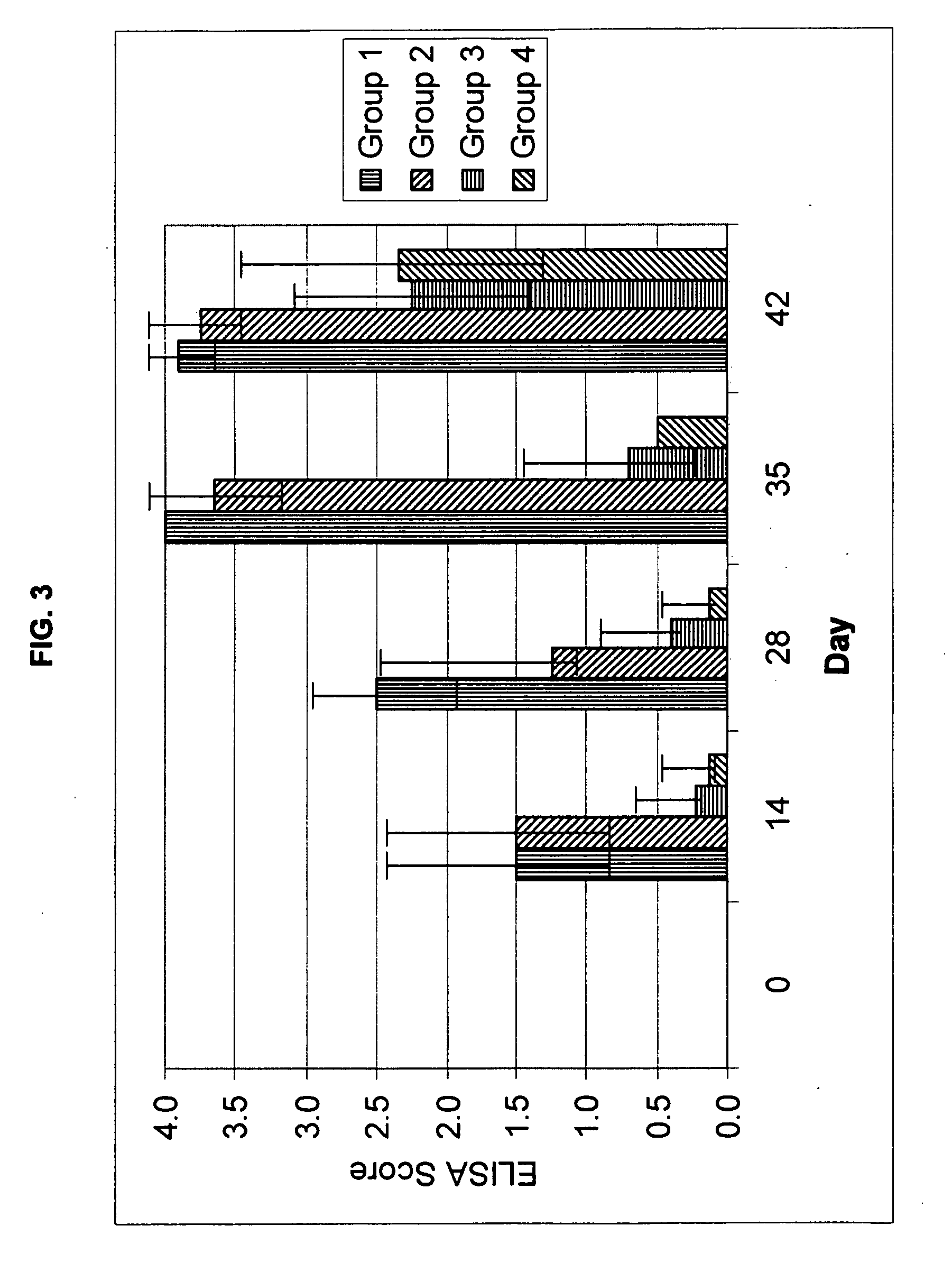
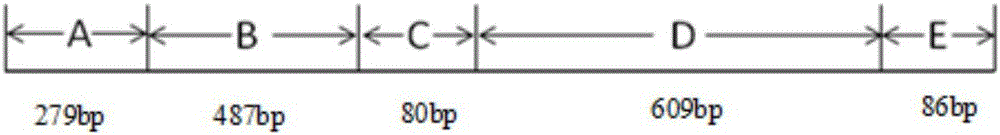
Patents
Literature
137 results about "Mycoplasma bovis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma bovis is one of 126 species of genus Mycoplasma. It is the smallest living cell and anaerobic in nature. It does not contain any cell wall, and is therefore resistant to penicillin and other beta lactam antibiotics.
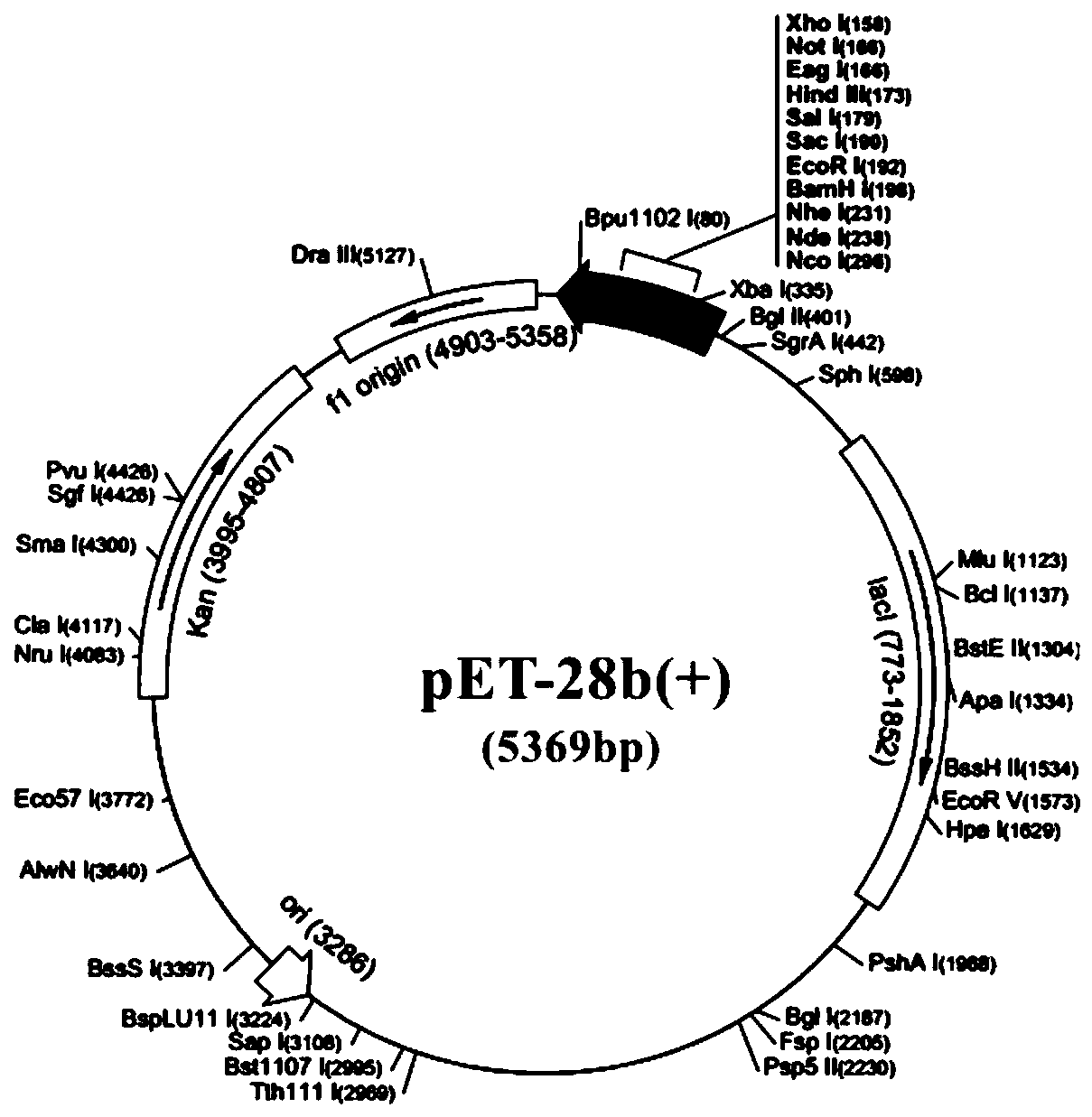
Mycoplasma bovis monoclonal antibody, and preparation method and application thereof
ActiveCN103509756AStrong variabilityEasy to operateImmunoglobulins against bacteriaMicroorganism based processesMycoplasma bovis antigenMycoplasma antigen
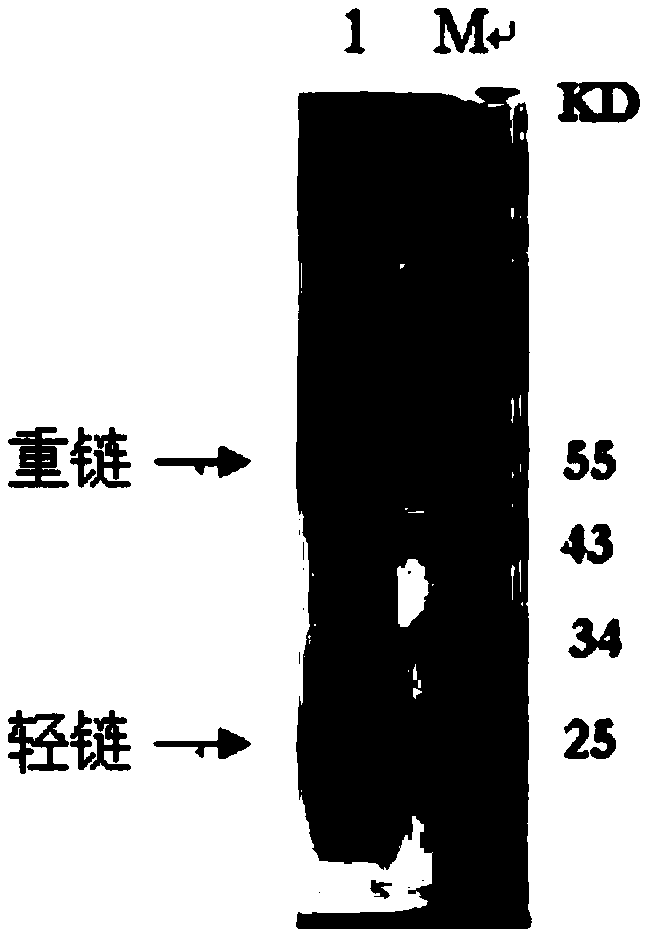
The invention discloses a mycoplasma bovis monoclonal antibody, and a preparation method and an application thereof. The preparation method of the mycoplasma bovis monoclonal antibody comprises the following steps: 1, preparing a mycoplasma bovis antigen; 2, immunizing mice by the antigen; 3, preparing a hybridomas cell secreting the mycoplasma bovis monoclonal antibody and monoclonal antibody ascites; and 4, purifying the above obtained monoclonal antibody. In the invention, mice are immunized by a mycoplasma bovis geographical strain HB0801 antigen, a hybridomas cell strain 1C11, CCTCC NO:C201218, which can efficiently secrete the mycoplasma bovis monoclonal antibody, is obtained through a hybridomas cell technology, the generated monoclonal antibody can be specifically combined with mycoplasma bovis and mycoplasma agalactiae, and the monoclonal antibody is utilized to establish sandwich ELISA for detecting the mycoplasma bovis antigen. The method has the advantages of simple operation, short required time and high sensitivity.
Owner:HUAZHONG AGRI UNIV
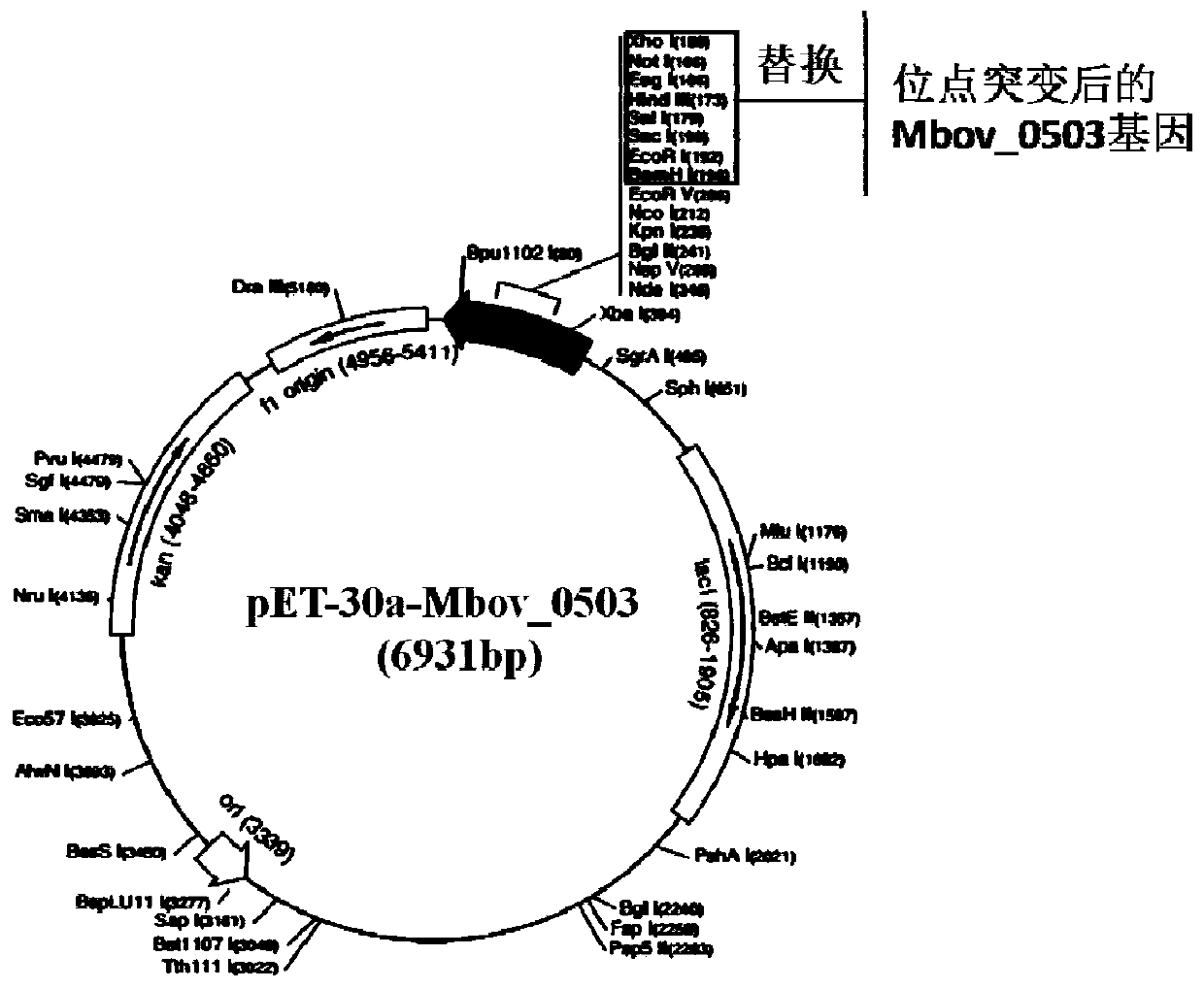
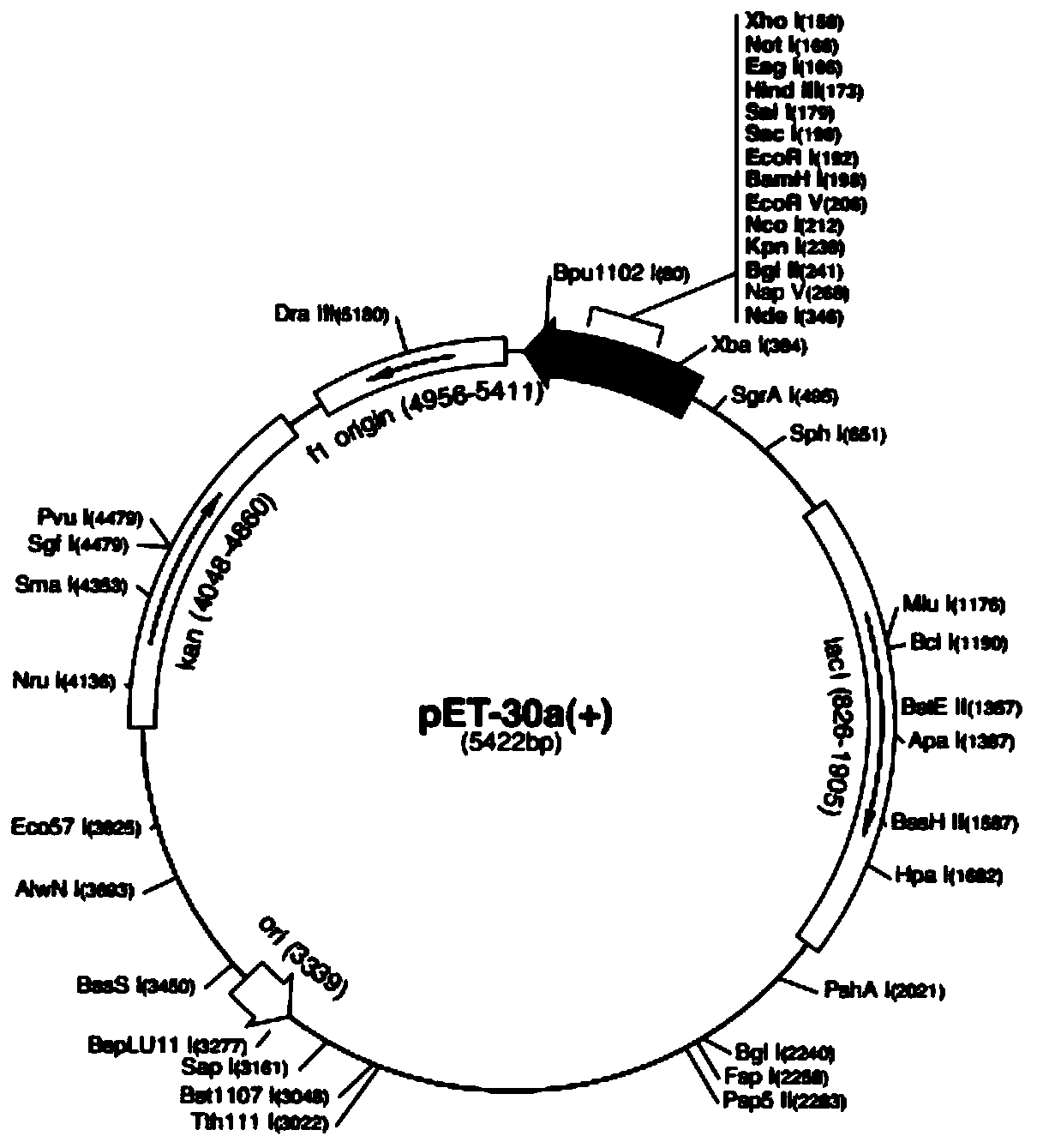
Mycoplasma bovis gene mutation strain having reduced adhesion capacity and adhesion protein
ActiveCN109837226AReduce adhesionDemonstrated adhesionBacteriaMicrobiological testing/measurementDiseaseEscherichia coli
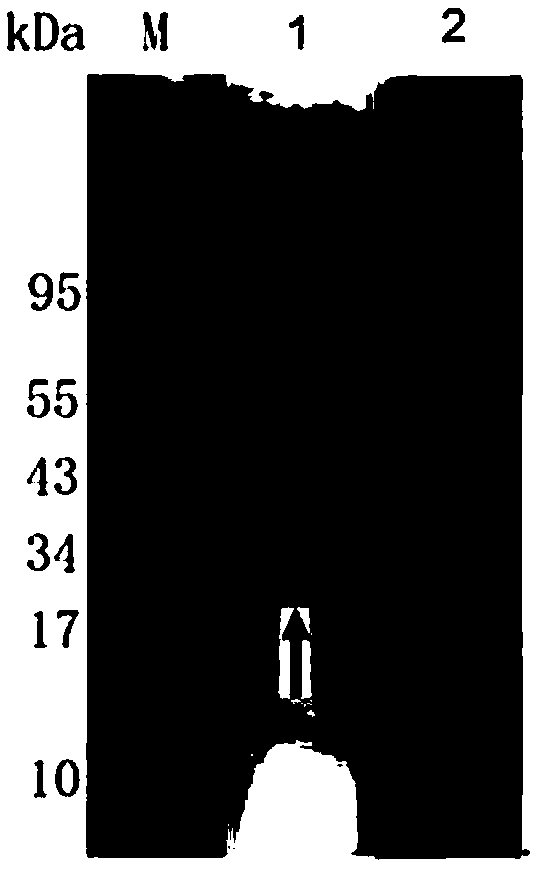
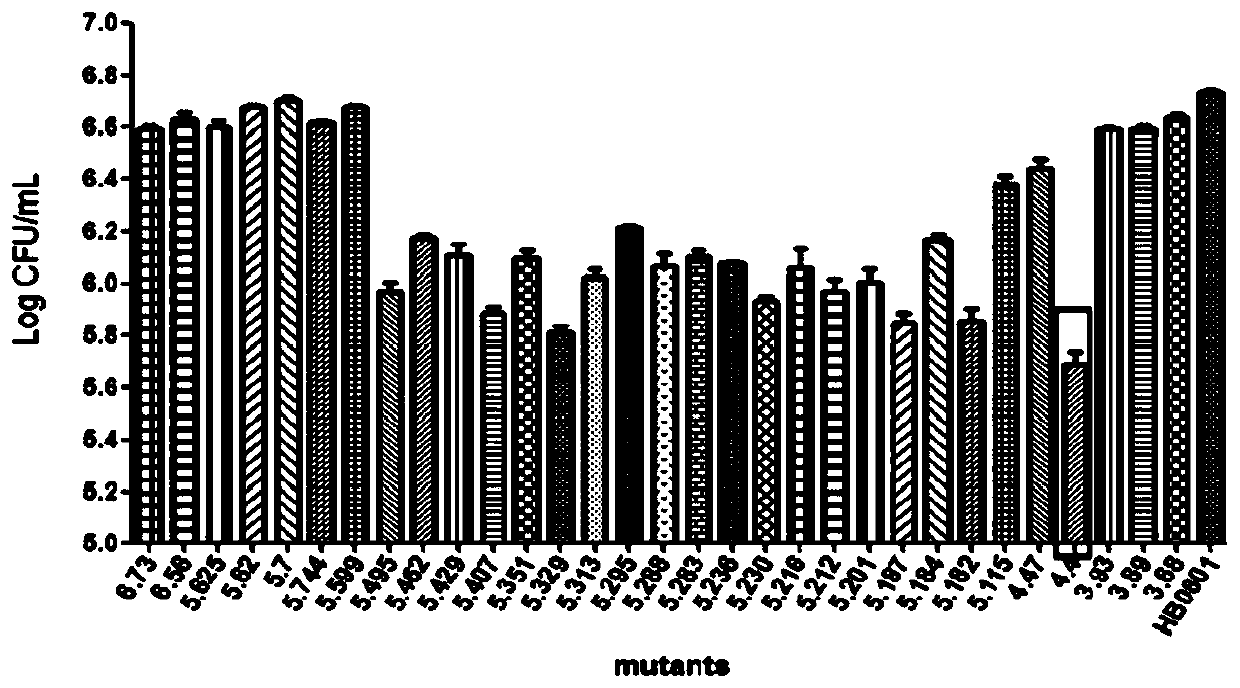
The invention belongs to the technical field of prevention and treatment of animal borne diseases, and relates to a mycoplasma bovis gene mutation strain having reduced adhesion capacity and adhesionprotein. The protein gene Mbov_0503 is cloned from a mycoplasma bovis HB0801 genome. According to the partiality properties of escherichia coli to codons, the Mbov_0503 gene is modified, and mycoplasma bovis tryptophan codons UGA are mutated into codons UGG for coding tryptophan in the escherichia coli, so that recombination protein Mbov0503 is obtained. The nucleotide sequence of the protein geneis shown as SEQID NO:13, and the coded protein sequence is shown as SEQID NO:14. The mutation strain is the adhesion defect strain screened from a mutant library. Compared with wild strains, the mutation stain has the advantages that the adhesion capacity to host EBL cells, the cross-membrane transmission capacity to MDBK cells and destructivity to tight connection between cells of the mutation strain are notably reduced. The mycoplasma bovis gene mutation strain can be used for prevention and treatment of mycoplasma bovis diseases.
Owner:HUAZHONG AGRI UNIV
Vaccines for Mycoplasma bovis and methods of use
The invention of novel, effective vaccines against Mycoplasma bovis for use in cattle is described. These vaccines demonstrate no undesirable side effects and protect against M. bovis related disease, such as contagious mastitis, respiratory pneumonia, joint infections, keratoconjunctivitis and middle ear infections. The novel vaccines also lessen the effect M. bovis infections on milk production, weight gain and animal health. Methods of diagnosing characterizing and treating M. bovis infections as specific biotypes are also disclosed. Vaccine compositions made in accordance with the invention may be either of the attenuated or inactivated variety. Vaccines may also include antigens from other pathogens so as to provide a protective immunogenic response to diseases other than those caused by M. bovis.
Owner:BIOMUNE
Mycoplasma bovis attenuated strain and application thereof
ActiveCN102220263AGreat clinical valueEasy to makeAntibacterial agentsBacterial antigen ingredientsSocial benefitsUltrasound attenuation
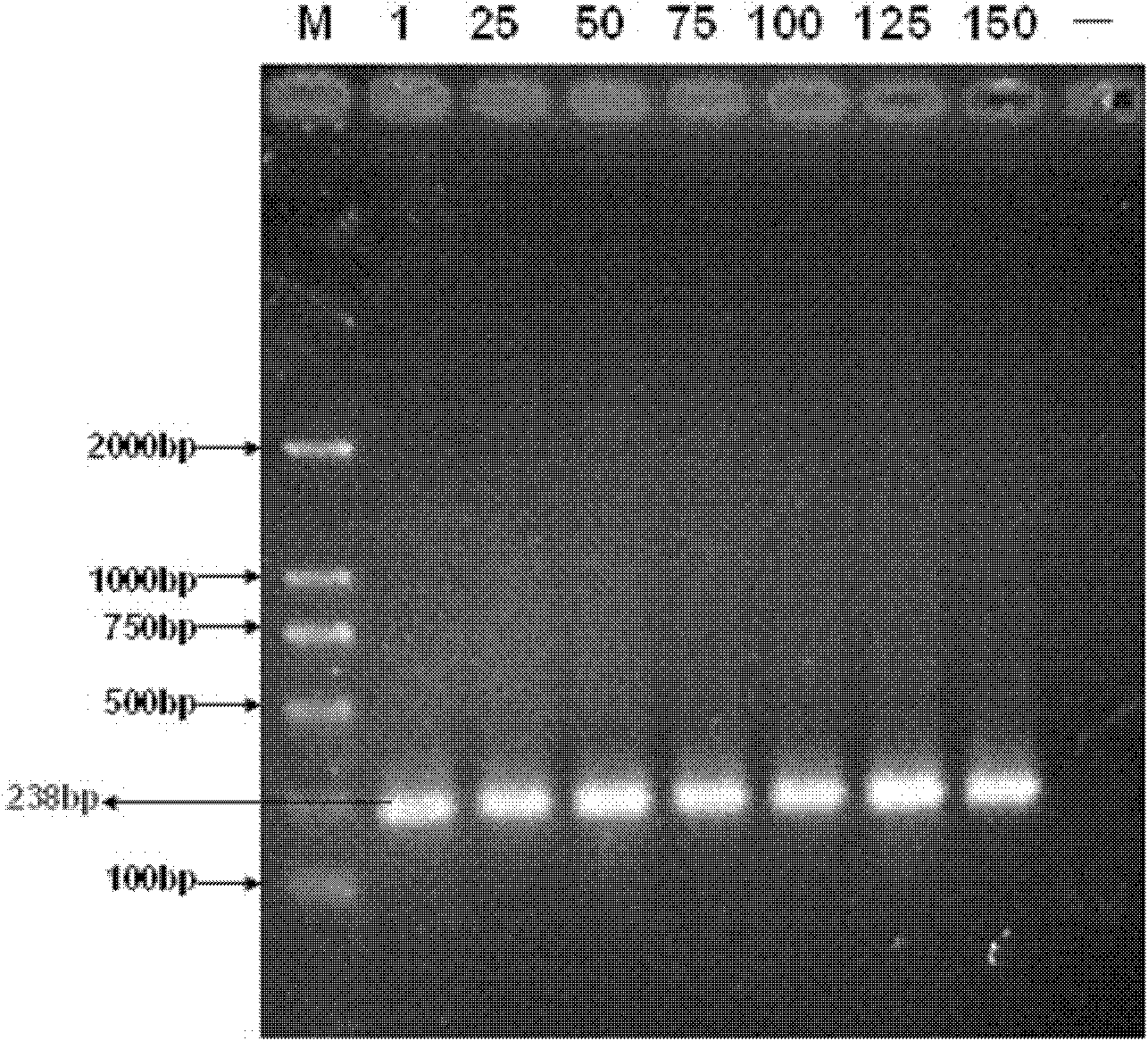
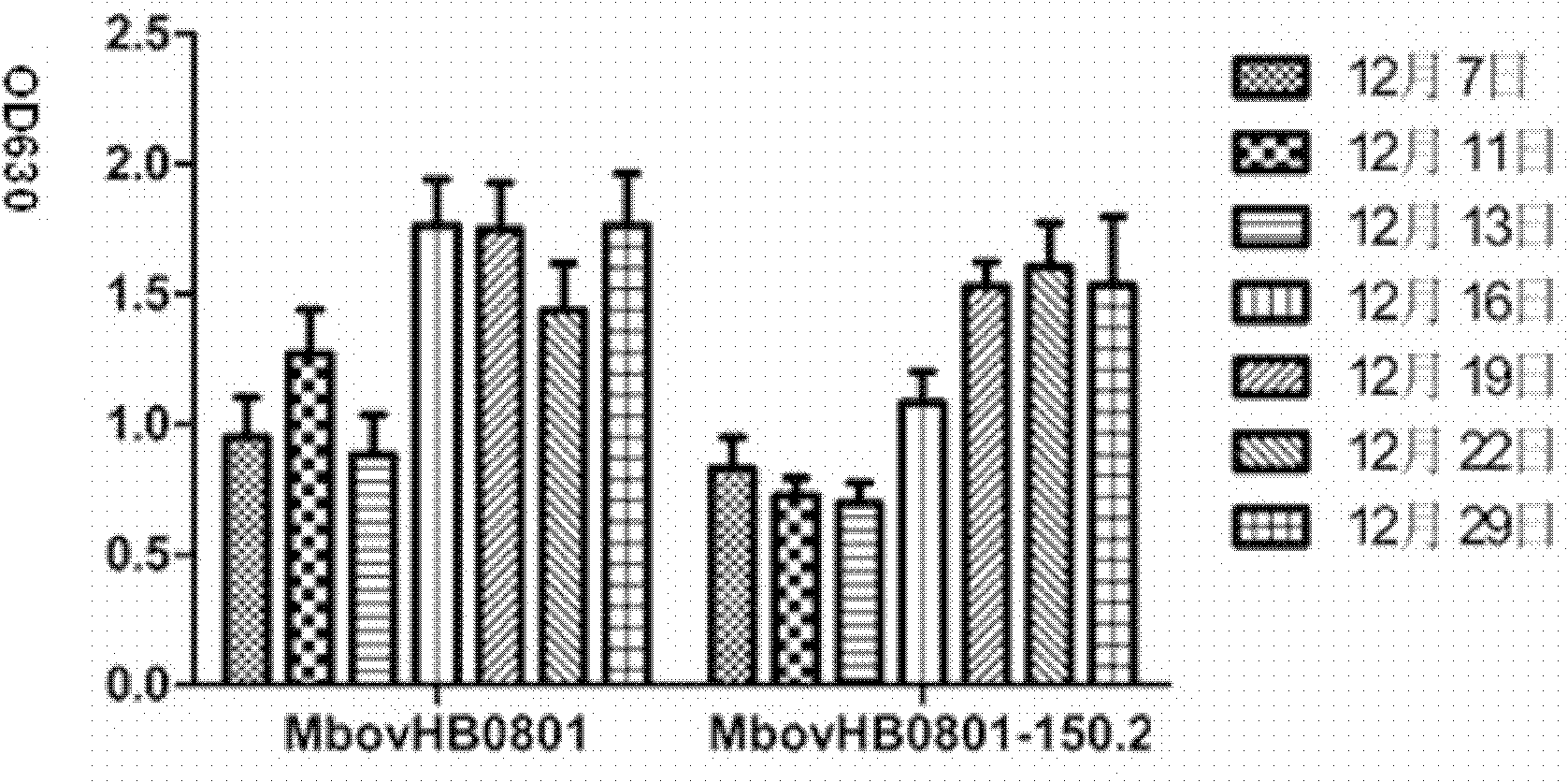
The invention discloses a mycoplasma bovis attenuated strain and application thereof. The mycoplasma bovis attenuated strain is prepared by the following steps: A. a mycoplasma bovis virulent strain is separated and determined: by pathogeny separation culture and PCR (Polymerase Chain Reaction) detection, the pathogeny is determined to be mycoplasma bovis; B. the mycoplasma bovis virulent strain is cultured: the mycoplasma bovis which is obtained by separation is inoculated with a liquid drug culture medium, and is then cultured in an incubator, wherein the culture medium becomes bright yellow from red, and the previous generation of bacterium liquid of 1mul is taken for inoculating PPLO (pleuropneumonia-like organism) culture medium and culturing; C. the full attenuation of mycoplasma bovis can be shown by the experiments, and by the detection of morphology, and the generation 150 of strain Mbov HB0801-150.2 cultured by mycoplasma bovis Mbov HB0801 can be verified by the experiment result; and D. the preservation number of the mycoplasma bovis Mbov HB0801-150.2 is CCTCC M20111102. The invention has good vaccine development prospective, wide clinic application value, low cost and small irritant to animals, and can produce huge economic benefits and social benefits.
Owner:HUAZHONG AGRI UNIV
Real-time fluorescence quantification PCR detecting kit for cow mycoplasma and special primers and TaqMan probe thereof
InactiveCN105420379ANo follow-up work requiredReduce workloadMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMycoplasma bovis
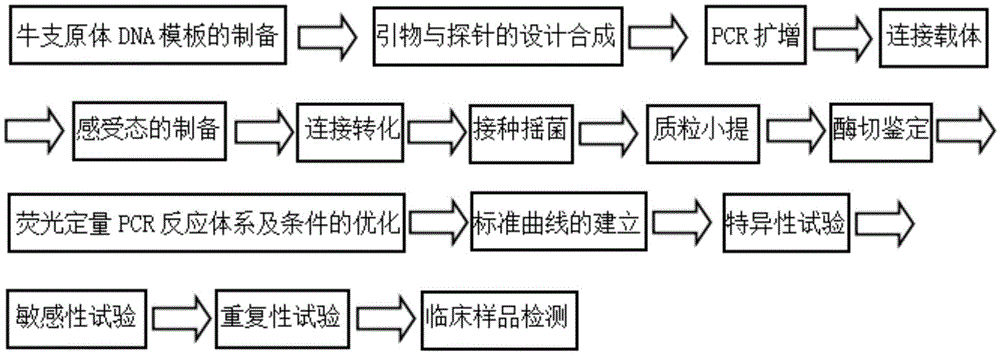
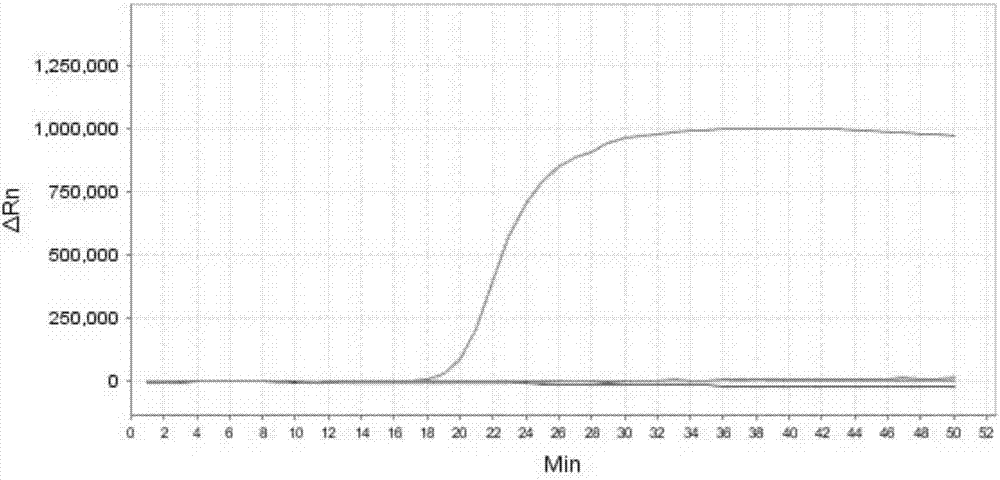
The invention discloses a real-time fluorescence quantification PCR detecting kit for cow mycoplasma, special primers and a TaqMan probe thereof and application of the detecting kit in detection of cow mycoplasma. The primers and the TaqMan probe for real-time fluorescence quantification PCR detecting of the cow mycoplasma are designed according to a specific conserved sequence of an OPPD / F gene of the cow mycoplasma and are used for detecting the cow mycoplasma of a sample to be detected qualitatively and quantitatively. The nucleotide sequence of the upstream primer (OF-A) is shown as SED ID NO:1 in a sequence table, the nucleotide sequence of the downstream primer (OF-B) is shown as SED ID NO:2 in the sequence table, and the nucleotide sequence of the TaqMan probe (OF-P) is shown as SEQ IDNO:3 in the sequence table. By means of the detecting kit, the special primers and the TaqMan probe thereof, the cow mycoplasma can be detected quickly, conveniently, efficiently, highly specifically and highly sensitively, and a novel technical platform is provided for detection of the cow mycoplasma.
Owner:JINYUBAOLING BIO PHARM CO LTD +1
LAMP primer combination for detecting 6 infectious pathogens of dairy cow mastitis and application thereof
ActiveCN107541509AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationStreptococcus pyogenesStaphylococcus cohnii
The invention discloses an LAMP primer combination for detecting 6 infectious pathogens of dairy cow mastitis and application thereof. The invention first provides a primer combination consisting of 36 DNA molecules shown in sequence 1 to sequence 36. The primer combination can be used for detecting whether a to-be-detected bacterium is Mycoplasma bovis, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pyogenes, Corynebacterium bovis or Staphylococcus epidermidis, and can be used for detecting whether a to-be-detected sample contains Mycoplasma bovis and / or Staphylococcus aureusand / or Streptococcus agalactiae and / or Streptococcus pyogenes and / or Corynebacterium bovis and / or Staphylococcus epidermidis. The primer combination provided by the invention is used for identifying and detecting the 6 infectious pathogens of dairy cow mastitis, has high specificity and high sensitivity, and can realize simple, rapid and accurate detection, thus having great popularization value.
Owner:CAPITALBIO CORP
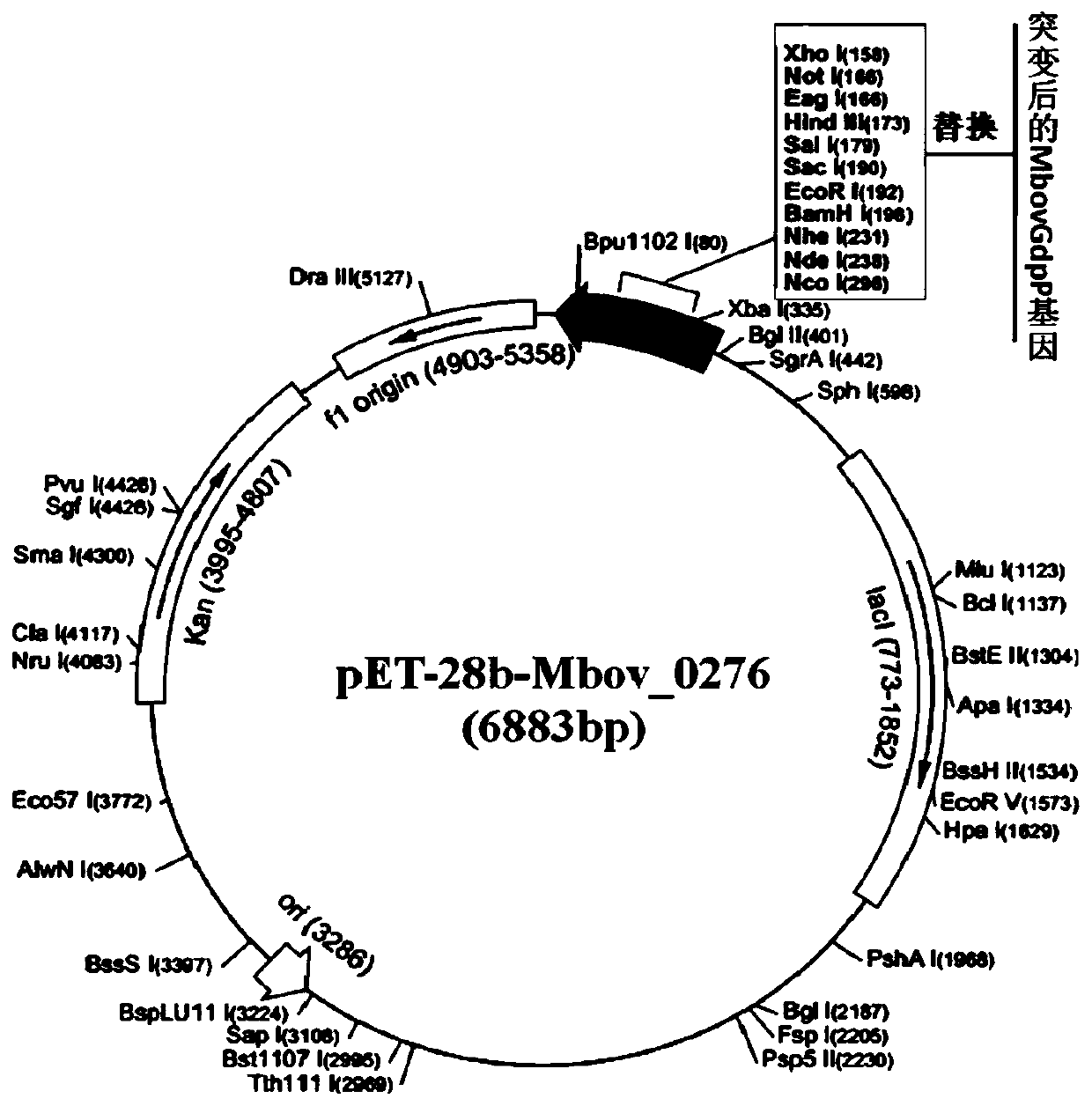
Mycoplasma bovis protein gene MbovGdpP and application thereof
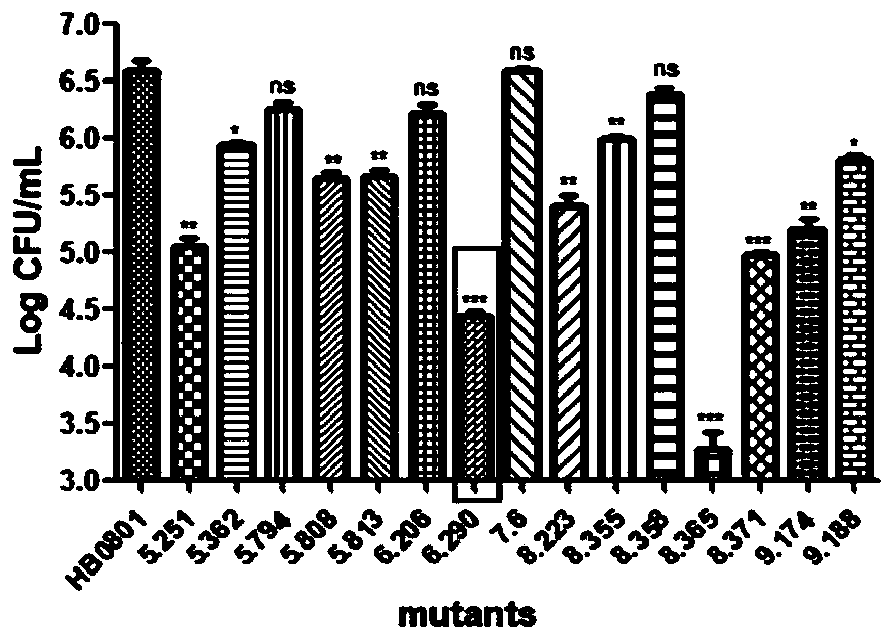
The invention belongs to the technical field of zoonosis prevention, and particularly relates to a mycoplasma bovis protein gene MbovGdpP and application thereof. Mbov_0276 is artificially synthesizedaccording to the mycoplasma bovis HB0801 genome sequence. An Mbov_0276 gene is modified for the preference of escherichia coli for codons; mycoplasma bovis tryptophan codons UGA are mutated into codons UGG for encoding tryptophan in escherichia coli to obtain escherichia coli recombinant proteins rMbovGdpP. The Mbov_0276 gene has the nucleotide sequence shown in SEQ ID NO:1, and the ended proteinhas the sequence shown in SEQ ID NO:2. A mutant strain T6.290 has the growth defect phenotype, has the small colony morphology on a PPLO culture medium, reduces the adhesion of EBL cells and improvesthe sensitivity to saline ions. The mutant strain can be expected to be applied in the mycoplasma bovis pathogenesis and the preparation of immunosuppressive medicines.
Owner:HUAZHONG AGRI UNIV
Mycoplasma hyopneumoniae vaccine and methods for reducing mycoplasma bovis pneumonia in cattle
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma bovis (M. bovis) by administering to the animal an effective amount of a Mycoplasma hyopneumoniae (M. hyo) vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Modified live vaccine of mycoplasma bovis, methods of producing modified live mycoplasma bovis vaccines, combination vaccines and methods of treatment
ActiveUS20100272759A1Reduce incidenceReduce severityAntibacterial agentsBacterial antigen ingredientsBacteroidesMycobacterium Infections
The present invention relates to new attenuated M. bovis bacteria strains passaged at least 110 times. Moreover, the present invention also provides immunogenic compositions comprising live bacteria of any of those attenuated M. bovis bacteria strain, their manufacture and use for the treatment and prophylaxis of M. bovis infections and combinations with other veterinary vaccines or medicaments.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Anti-mycoplasma bovis monoclonal antibody, hybridoma cell strain secreting monoclonal antibody and application
InactiveCN103436496AQuick checkRapid and Specific DetectionMicroorganism based processesTissue cultureMycoproteinSpecific immunity
The invention discloses an anti-mycoplasma bovis monoclonal antibody, a hybridoma cell strain secreting the monoclonal antibody and an application. When the monoclonal antibody reacts with different mycoplasma strain mycoproteins, Western Blotting results indicate that the monoclonal antibody 3G11 only performs specific immune reaction with a mycoplasma bovis isolate, and does not react with mycoplasma mycoides SC (MmmSC), mycoplasma mycoides LC (MmmLC), mycoplasma capricolum pneumonia (Mccp), M.agalactiae, and M.ovipneumoniase. Therefore, the anti-mycoplasma bovis monoclonal antibody has good specificity, can be used for diagnosing and testing mycoplasma bovis infection, and provides an effective approach to prevent and control the disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Application of mycoplasma bovis MbovP730 protein in natural infection and vaccine immunity identification
Owner:HUAZHONG AGRI UNIV
Mycoplasma bovis mutant strain with growth defect under cell coculture and application
ActiveCN109652357AMarked growth defectSignificantly small colony phenotypeBacteriaHydrolasesPhosphodiesteraseBovine embryo
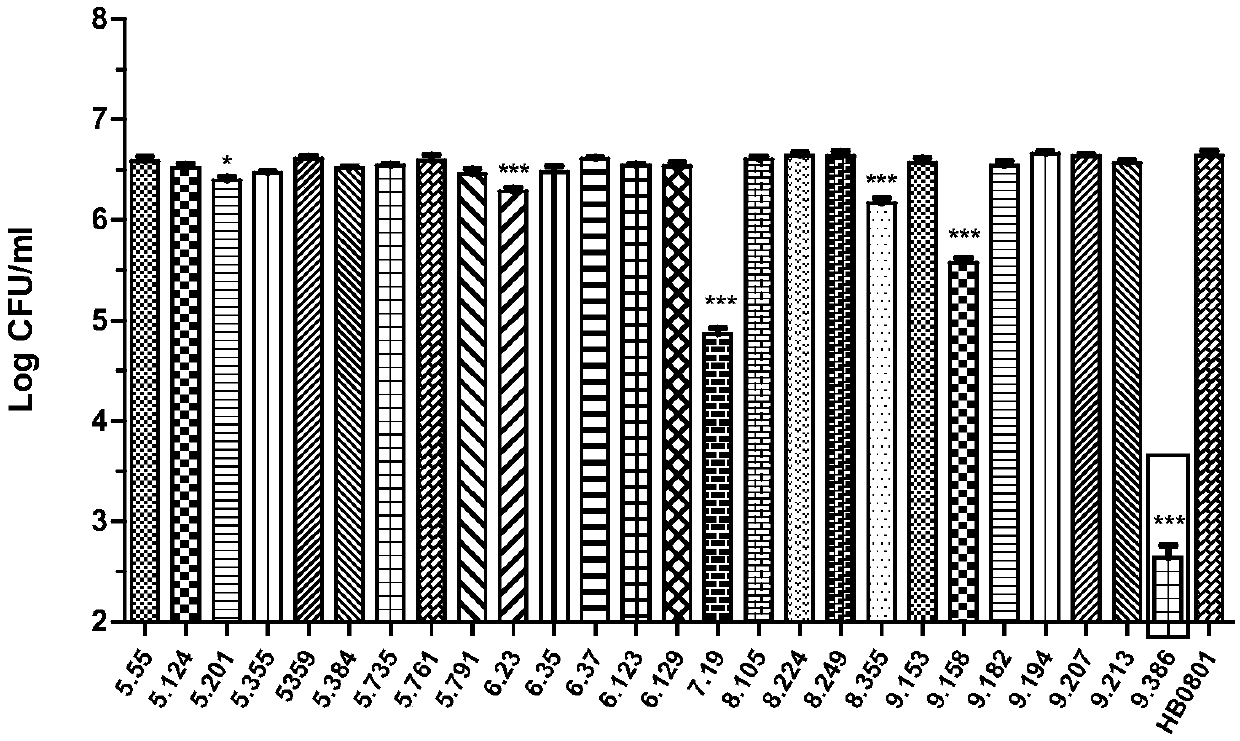
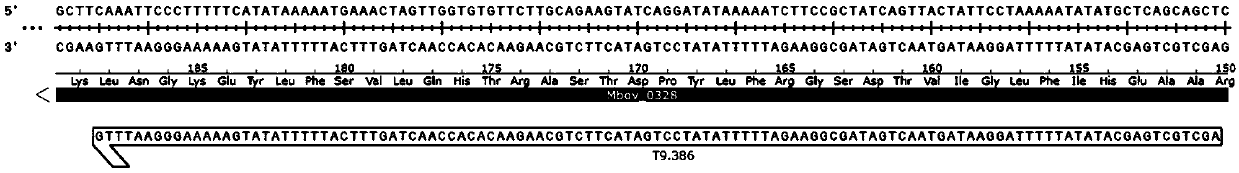
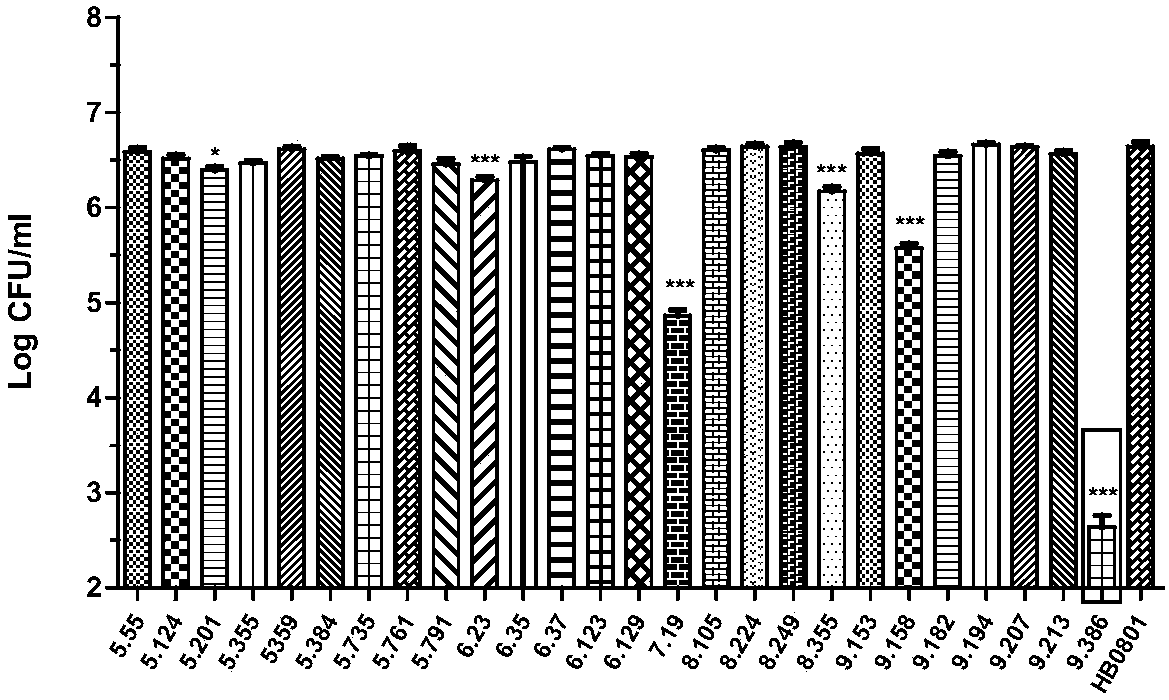
The invention belongs to the technical field of zoonosis prevention and treatment, and particularly relates to a mycoplasma bovis mutant strain with a growth defect under cell coculture and application. The mycoplasma bovis Mbov-0328 gene deletion mutant strain T9.386 is sifted out from a mycoplasma bovis gene mutant library, and the gene is coded to form cyclic dinucleotide phosphodiesterase. When the mutant strain and a bovine embryo pneumonocyte are co-cultured, the remarkable growth defect phenotype is shown; the mutant strain shows small colonial morphology on a PPLO solid culture medium.Proteomics between the mutant strain and a wild strain shows a remarkable difference expression spectrum. The mutant strain has 38 differential expression proteins, wherein 30 proteins are in up-regulated expression, and 8 proteins are in down-regulated expression. The mutant strain can be applied to the field of mycoplasma bovis metabolic physiology, pathopoiesia and immune prevention.
Owner:HUAZHONG AGRI UNIV
Mycoplasma bovis vaccine and methods of reducing pneumonia in animals
The present invention relates to Mycoplasma bovis vaccines and methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma bovis by administering to the animal an effective amount of a Mycoplasma bovis vaccine. The Mycoplasma bovis vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The Mycoplasma bovis vaccine administered in accordance with the present can be synthesized or recombinantly produced. The invention also relates to combination vaccines, methods of preparing Mycoplasma bovis vaccines and kits.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Mycoplasma bovis immunity-related protein P22, nucleotide sequence for coding same and application thereof
The invention discloses a mycoplasma bovis immunity-related protein P22, a nucleotide sequence for coding the same and an application thereof. A mycoplasma bovis Hubei strain is taken as a sample, and an efficient two-dimensional electrophoresis system of a mycoplasma bovis whole protein is established, so that a two-dimensional electrophoretogram with high resolution and high repeatability is obtained; an immunity-related protein is screened in combination with Western blot, and is named P22 protein; and as proved by a prokaryotic expression result of the protein, a Western blot rest result of a recombinant protein and a mycoplasma bovis positive serum is negative, and a Dot blot test result is positive. The protein is proved to be possibly a conformation-dependent immunity-related protein of mycoplasma bovis, and the mycoplasma bovis immunity-related protein P22 plays a guidance role in diagnosing, preventing and treating a mycoplasma bovis disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
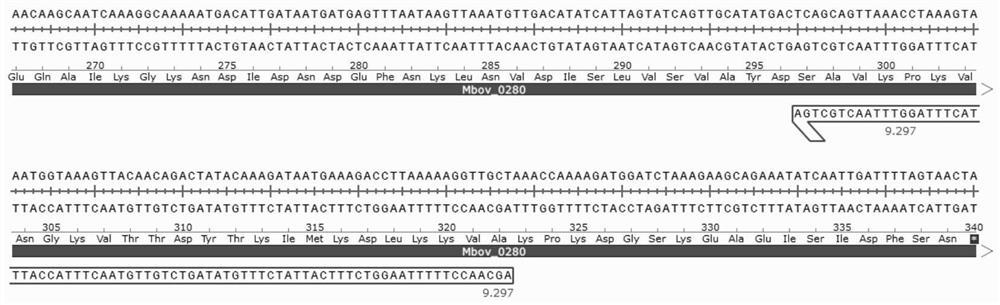
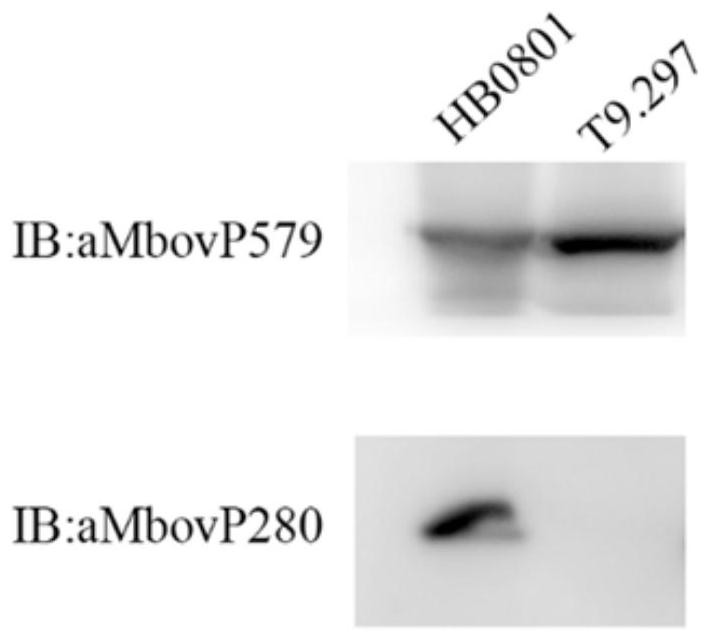
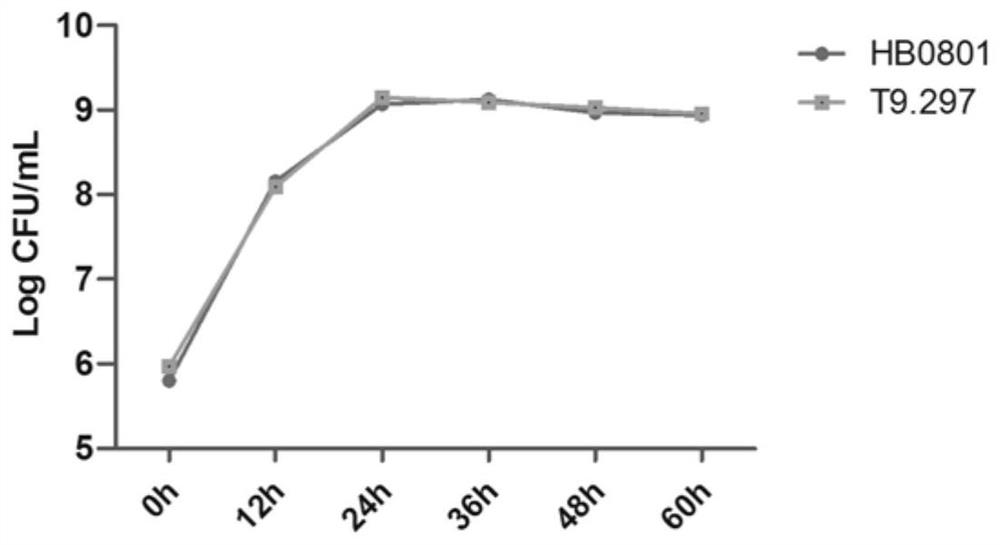
Mycoplasma bovis Mbov_0280 gene mutant and application thereof
ActiveCN111705026AReduce apoptosisAntibacterial agentsBacterial antigen ingredientsInfectious DisorderNucleotide
The invention discloses a mycoplasma bovis Mbov_0280 gene mutant and belongs to the technical field of animal infectious disease prevention and treatment. The mutant is named Mycoplasma bovis T9.297 is preserved in the China Center for Type Culture Collection and has a preservation number of CCTCC NO:M 2020085. The Mbov_0280 gene has a nucleotide sequence as shown in SEQ ID NO:1. The strain has the performance for inducing host macrophages apoptosis is weakened in comparison with a wild strain HB0801; the mutant has low toxicity since the bovine macrophages serve as a critical immune cell forresisting adventitious infection of a host; and the mutant favors a body to resisting mycoplasma bovis infection, and is expected to achieve a vital function in the field of mycoplasma bovis immunological control.
Owner:HUAZHONG AGRI UNIV
Mycoplasma bovis and application thereof
ActiveCN106929452AHigh proliferative titerStrong pathogenicityAntibacterial agentsBacterial antigen ingredientsBacteroidesRespiratory symptom
The invention discloses a Mycoplasma bovis and application thereof. The Mycoplasma bovis is Mycoplasma bovis HNMB1 and is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC NO:13295, the preservation data of November 28th, 2016 and the preservation address of No.3, Courtyard 1, Beichen West Road, Chaoyang District of Beijing. The strain HNMB1 is separated from a diary calf dead due to occurrence of typical respiratory symptoms, no other bacteria grow during separation on a PPLO solid culture medium, only mycoplasmas grow, and proliferation titer in a PPLO liquid culture medium is high and reaches up to more than 10<9>CFU / mL. The strain HNMB1 disclosed by the invention has stronger pathogenicity on the calf, results in pathogenesis and death of the calf and has good immunogenicity.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Use of mycoplasma bovis antigen
ActiveUS20120093854A1Antibacterial agentsBacterial antigen ingredientsMycoplasma bovis antigenImmunogenicity
The present invention relates to combination vaccines and / or the combined use of immunogenic compositions for the treatment and / or prophylaxis of cattle against microbiological infections, wherein the infections are caused by M. bovis and at least one further cattle relevant pathogen. The combination vaccine as described herein comprises at least one M. bovis antigen, preferably the attenuated, avirulent M. bovis as provided herewith and one or more further immunologically active components effective for the treatment and / or prophylaxis of infections caused by a further pathogen of cattle.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Primers, probes and kit for rapidly detecting mycoplasma bovis on site
InactiveCN106868167AExcellent detection timeStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationForward primerBacteroides
The invention discloses primers, probes and a kit for rapidly detecting mycoplasma bovis on site. The kit comprises a forward primer sequence shown as SEQ No.1, a reverse primer sequence shown as SEQ No.2, and a probe sequence shown as SEQ No.3, wherein the 5'-terminal of the reverse primer sequence is labeled by biotin; the 5'-terminal of the probe sequence is labeled by FAM, dSpacer is arranged at the position away from the 5'-terminal by 30 basic groups, and the 3'-terminal is blocked by C3-spacer. The primers, the probe assembly and the kit for detecting the mycoplasma bovis RPA-nfo are high in sensitivity and strong in specificity, the mycoplasma bovis DNA of 10 copies / reaction can be detected at the minimum, and the primers and the probes for detecting RPA-nfo respectively have no cross reaction with other mycoplasmas, as well as the bacteria including pasteurella multocida, mannheimia haemolytica, arcanobacterium pyogenes, histophilus somni, and streptococcus pneumoniae.
Owner:SHANDONG NORMAL UNIV
Mycoplasma bovis and application thereof
ActiveCN105441368AThe immune protection effect is indeedSimple preparation processAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
The invention relates to a mycoplasma bovis and application thereof. A pathogen is determined to be the mycoplasma bovis through the steps of isolated culture of the pathogen, animal regression testing and 16S rRNA gene sequence determination and is named as a mycoplasma bovis MbovFJ1201 strain, and the preservation number is CCTCC NO: M2015772. The strain is inoculated to a culture medium for expanding cultivation, a culture is obtained and is inactivated, an ISA-206 adjuvant is added in proportion of 1:1 after inactivation, and mixing is performed to obtain an inactivated vaccine. The vaccine is good in targeting property and good in immune protection effect, can remarkably reduce lung lesions caused by mycoplasma bovis infection and improve the average daily gain and can achieve the good effect of preventing and controlling related diseases of the mycoplasma bovis.
Owner:福清市默克兽医院
Composition, reagent kit and method for detecting mycoplasma bovis
ActiveCN110229919AGood technical effectStrong specificityMicrobiological testing/measurementMicroorganism based processesReaction temperaturePathogenic bacteria
The invention discloses a composition, reagent kit and method for detecting mycoplasma bovis. Through designing a specific primer and probe of an LppA gene of mycoplasma bovis, precise detection of the mycoplasma bovis is completed. The primer and probe combination can achieve intraspecific conservative of a mycoplasma bovis standard strain and a wild strain and interspecific specificity of the mycoplasma bovis and other pathogenic bacteria. The optimal reaction time, the optimal reaction temperature, the detection specificity, the detection sensibility, the detection repeatability and the detection stability in the detection process are explored, so that the optimal reaction condition is found out, a mycoplasma bovis fast detection method good in specificity, high in sensibility and capable of being stably repeated is established, and is simple to operate, convenient and time-saving, large experimental instrument equipment is not needed, and the fast detection method is especially suitable for staff without experiment foundation to operate.
Owner:NINGXIA UNIVERSITY
Mycoplasma bovis multifunctional protein CDNPase
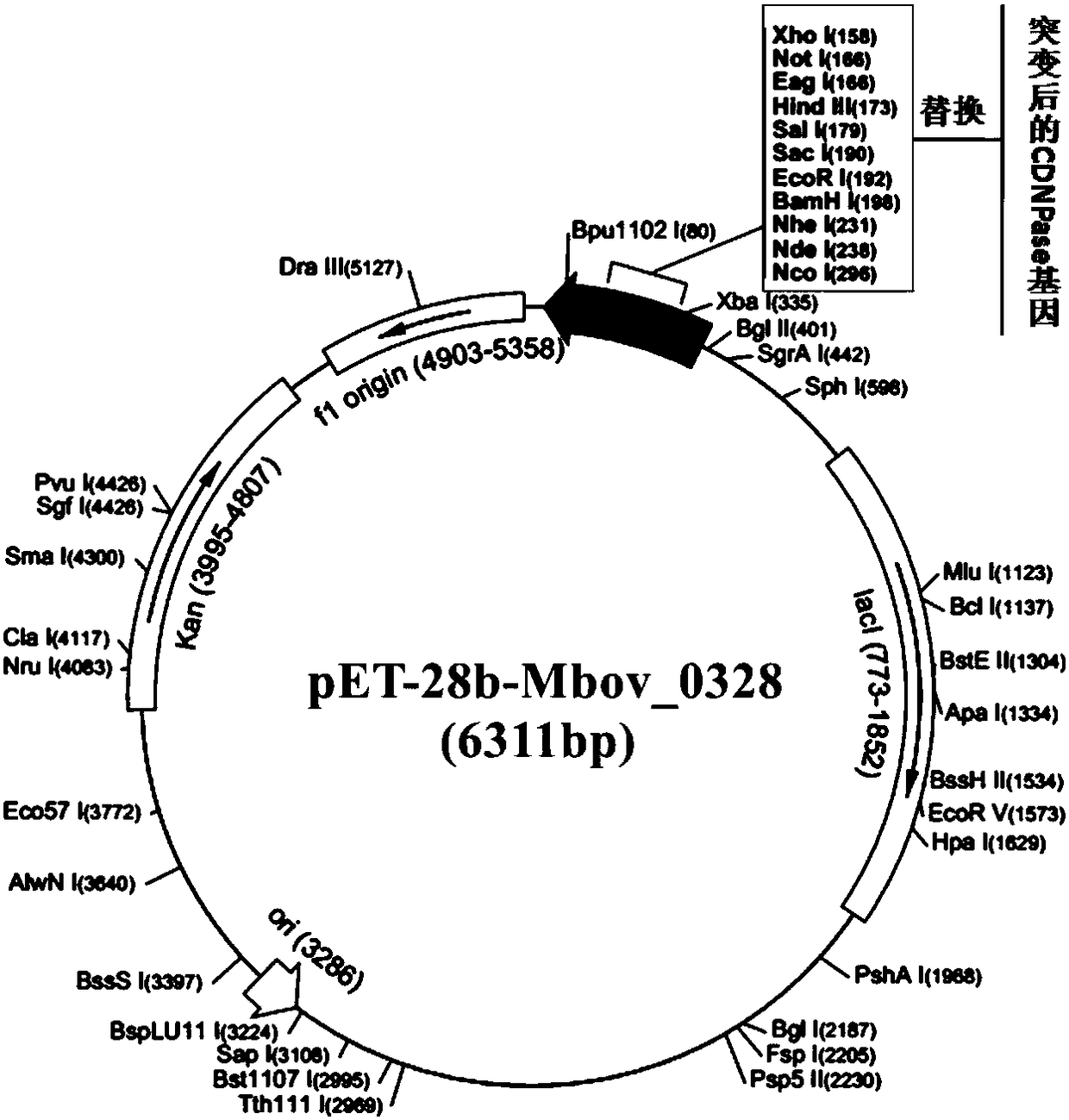
InactiveCN108660144ACyclic dinucleotide phosphodiesteraseCtiveBacteriaHydrolasesPhosphodiesteraseEscherichia coli
The invention belongs to the technical field of prevention and control of animal infectious diseases, and in particular relates to a mycoplasma bovis multifunctional protein gene CDNPase. The proteingene Mbov-0328 is obtained by conducting cloning from mycoplasma bovis HB0801 genome. In accordance with preference of escherichia coli to codon, the Mbov-0328 gene is modified by the inventor, and mycoplasma bovis tryptophan codon UGA is mutated into codon UGG for coding tryptophan in escherichia coli, so that recombinant protein rCDNPase, which is expressed by the escherichia coli, is finally obtained. A nucleotide sequence of the protein coding gene cloned by the invention sis shown as the 1-969th base groups in SEQ ID NO:11, and a sequence of the protein is shown as SEQ ID NO:12. The recombinant protein has activities of phosphodiesterase and nuclease of an ultra-small fragment.
Owner:HUAZHONG AGRI UNIV
Mutant having reduced adhesive ability and with deleted mycoplasma bovis gene
ActiveCN111235082AReduce adhesionDemonstrated adhesionBacteriaMicrobiological testing/measurementEscherichia coliTransmissible disease
The invention belongs to the field of control of animal borne diseases, and relates to a mutant having reduced adhesive ability and with a deleted mycoplasma bovis gene. The protein gene Mbov_0503 isobtained by cloning from a mycoplasma bovis HB0801 genome. According to the partiality of escherichia coli for codons, the Mbov_0503 gene is modified, and mycoplasma bovis tryptophan codon UGA is mutated into a codon UGG for coding tryptophan in the escherichia coli to obtain a recombinant protein Mbov0503. The sequence of the protein gene which is cloned is shown as SEQID NO:13, and the sequenceof the coded protein is shown as SEQID NO:14. The mutant provided by the invention is an adhesion deficient strain screened from a mutant bank. The mutant has adhesive ability for host EBL cells, andcompared with a wild strain, for the mutant disclosed by the invention, the transmembrane transmission capacity for MDBK cells and the destructivity for tight connection between cells are notably reduced. The mutant can be applied to pathopoiesis and control of the mycoplasma bovis.
Owner:HUAZHONG AGRI UNIV
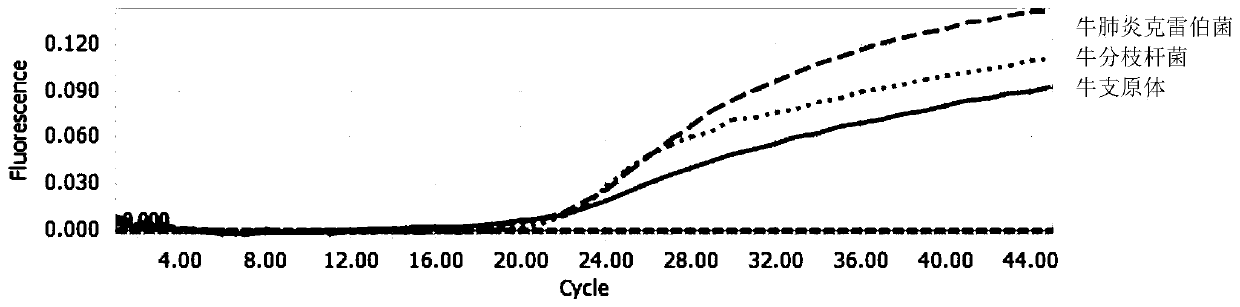
Multi-fluorescent quantitative PCR reagent kit capable of synchronously detecting three bovine respiratory pathogens, and multi-fluorescent quantitative PCR detection method capable of synchronously detecting three bovine respiratory pathogens
InactiveCN110016512AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesDiseaseBovine respiratory disease
The invention belongs to the technical field of livestock disease detection, and discloses a multi-fluorescent quantitative PCR reagent kit capable of synchronously detecting three bovine respiratorypathogens as well as a multi-fluorescent quantitative PCR detection method capable of synchronously detecting the three bovine respiratory pathogens, wherein the three bovine respiratory pathogens arerespectively mycobacterium bovis, mycoplasma bovis and Klebsiella pneumoniae. According to the multi-fluorescent quantitative PCR detection method capable of synchronously detecting the three bovinerespiratory pathogens, 3 groups of specific primers and probes are designed and synthesized on basis of a specific sequence 229bp of the mycobacterium bovis, a uvrC gene of the mycoplasma bovis and aKhe gene of the Klebsiella pneumoniae, thereby establishing the multi-fluorescent quantitative PCR detection method capable of synchronously detecting the mycobacterium bovis, the mycoplasma bovis andthe Klebsiella pneumoniae which are associated with respiratory diseases in cattle. The multi-fluorescent quantitative PCR detection method is capable of synchronously detecting the 3 bacteria; and sensitivity, repeatability and specificity tests have proven that the detection method is high in sensitivity, strong in specificity and relatively good in repeatability.
Owner:CHINA AGRI UNIV
Mycoplasma bovis vaccine and methods of use thereof
ActiveUS20090130148A1Reduce morbidityReduce severityAntibacterial agentsSenses disorderBacteroidesMycobacterium Infections
The present invention relates to new attenuated M. bovis bacteria strains. Moreover, the present invention also provides immunogenic compositions comprising live bacteria of an of those attenuated M. bovis bacteria strain, their manufacture and use for the treatment and prophylaxis of M. bovis infections.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
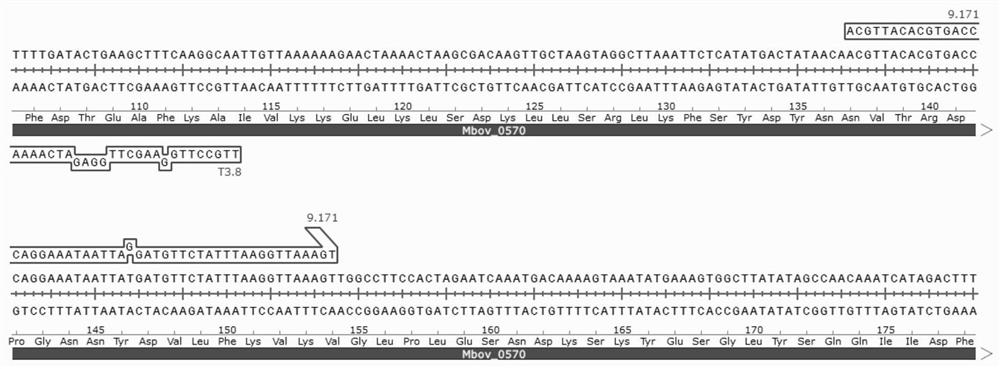
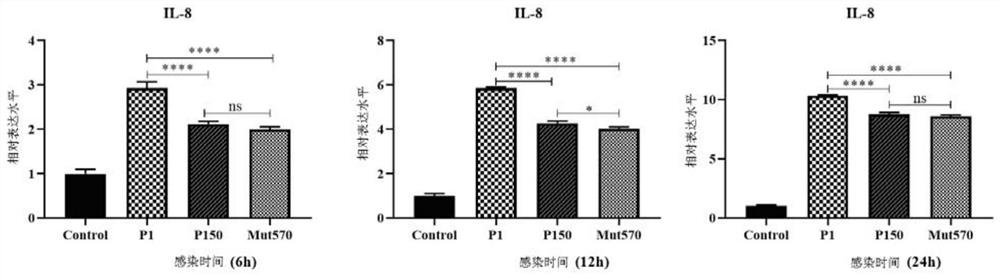
Mycoplasma bovis Mbov_0570 gene mutant strain and application thereof
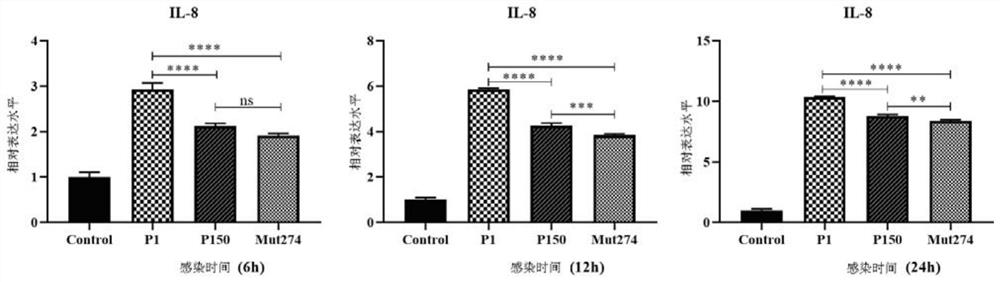
The invention discloses a Mycoplasma bovis Mbov_0570 gene mutant strain, which belongs to the technical fields of prevention and treatment of animal infectious diseases, is named as Mycoplasma bovis T9.171, and is preserved in China Center for Type Culture Collection (CCTCC). The preservation number of the Mbov_0570 gene is CCTCC NO: M 2020083, and the nucleotide sequence of the Mbov_0570 gene isshown as SEQ ID NO: 1. According to the invention, qRT-PCR is used for detecting the capacity of T9.171 strain for stimulating BoMac cells to express IL-8, which shows that the capacity of the mutantstrain for inducing the BoMac cells to express cytokines is reduced, so that the toxicity of the mutant strain is reduced, and the mutant strain is expected to play an important role in the fields ofmycoplasma bovis immune prevention and treatment as a vaccine strain.
Owner:HUAZHONG AGRI UNIV
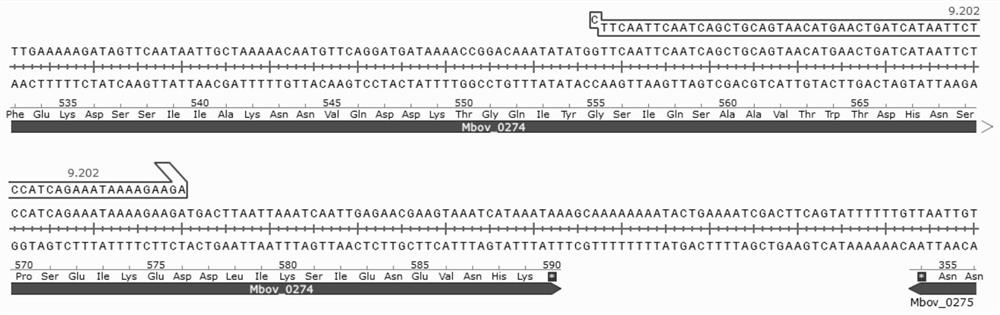
Mycoplasma bovis Mbov_0274 gene mutant and application thereof
ActiveCN111778177AAntibacterial agentsBacterial antigen ingredientsGenes mutationInfectious Disorder
The invention discloses a mycoplasma bovis Mbov_0274 gene mutant, and belongs to the technical field of animal borne disease prevention and treatment. The mutant is named as mycoplasma bovis T9.202, is preserved in China Center for Type Culture Collection with the preservation No. of CCTCC NO:M 2020084, and the nucleotide sequence of the Mbov_0274 gene is shown as SEQID NO:1. The capacity of a T9.202 strain for stimulating expression of IL-8 by BoMac cells is detected by qRT-PCR, results show that the capacity of the mycoplasma bovis bacterial strain T9.202 for inducing the expression of cytokine by the BoMac cells is reduced, and the mycoplasma bovis Mbov_0274 gene mutant is hopeful to development of important effects in the field of mycoplasma bovis immunization control.
Owner:HUAZHONG AGRI UNIV
Preparing method and application of mycoplasma bovis inactivated vaccine
The invention discloses a preparing method of a mycoplasma bovis inactivated vaccine. By studying the formaldehyde inactivation condition for preparing the mycoplasma bovis inactivated vaccine, the immunizing dose of the mycoplasma bovis inactivated vaccine, the immunization way of the mycoplasma bovis inactivated vaccine, the adjuvant adopted by the mycoplasma bovis inactivated vaccine, and clinical application of the mycoplasma bovis inactivated vaccine, the optimal mycoplasma bovis formaldehyde inactivation condition, the optimal immunizing dose of the mycoplasma bovis inactivated vaccine, the optimal immunization way of the mycoplasma bovis inactivated vaccine, and the optimal immunization adjuvant are found out, then clinical animal experiments are conducted, and the results show that the mycoplasma bovis inactivated vaccine has a good effect on prevention of mycoplasma bovis diseases in clinical application, can be used as a clinically popularized vaccine, and lays a scientific foundation for prevention of mycoplasma bovis diseases.
Owner:NINGXIA UNIVERSITY
Pcr-based genotyping
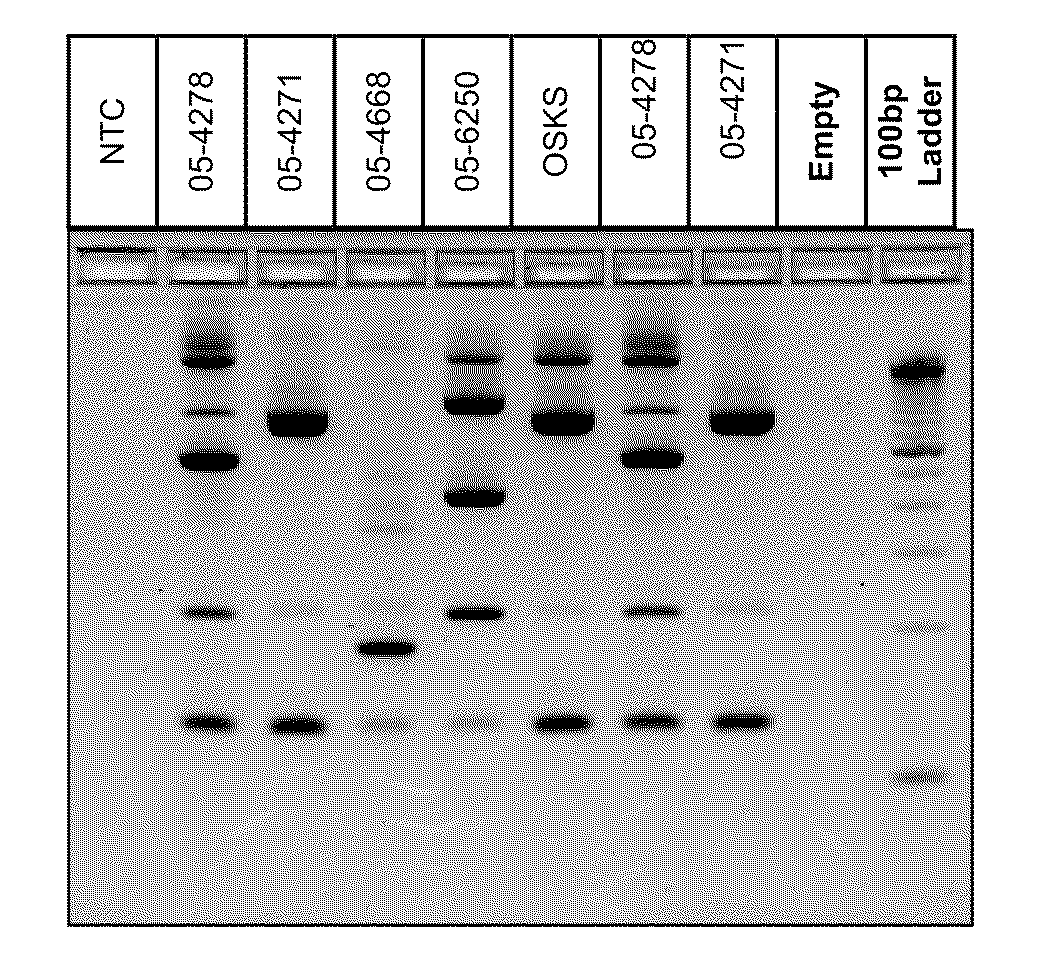
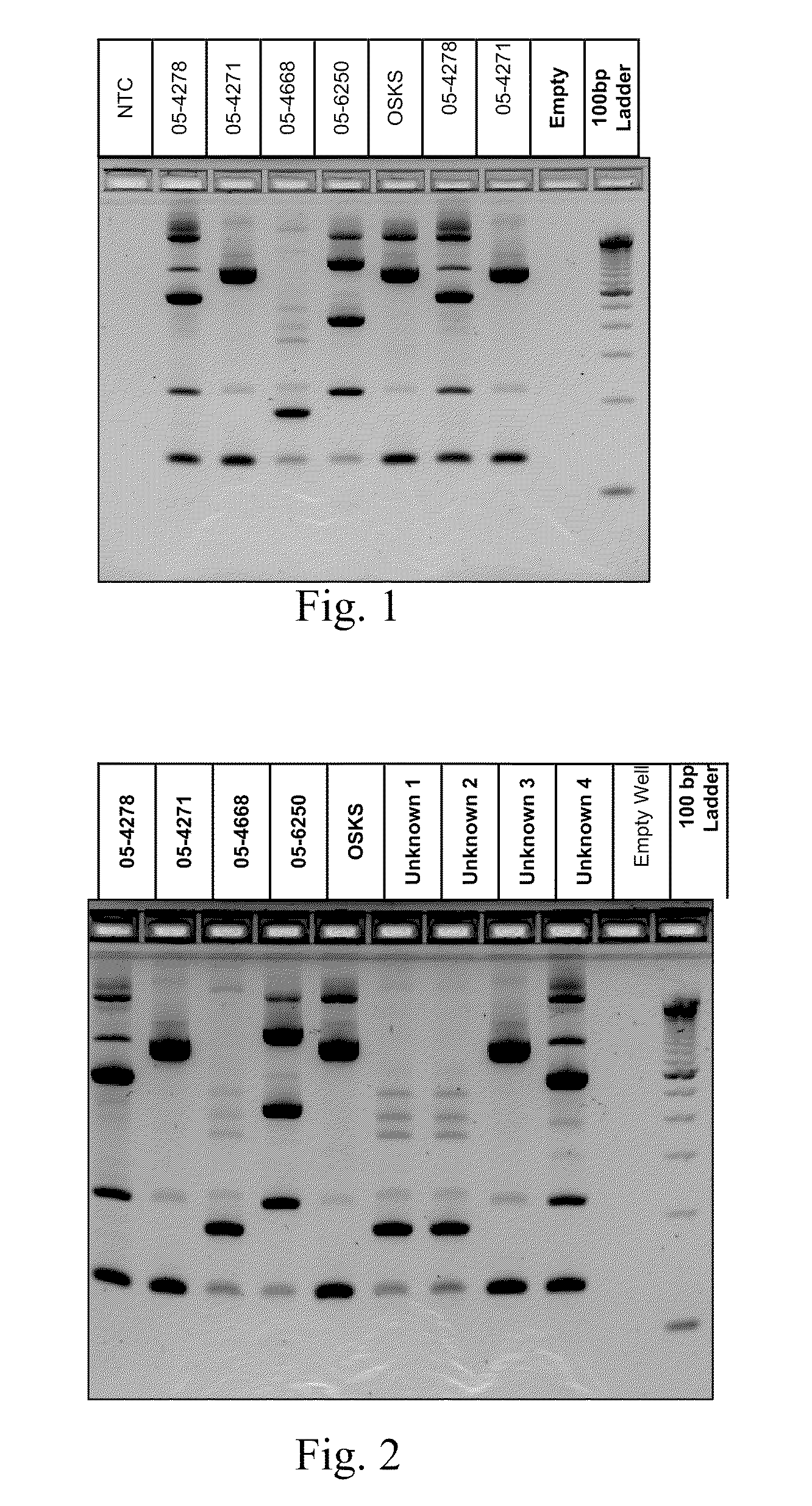
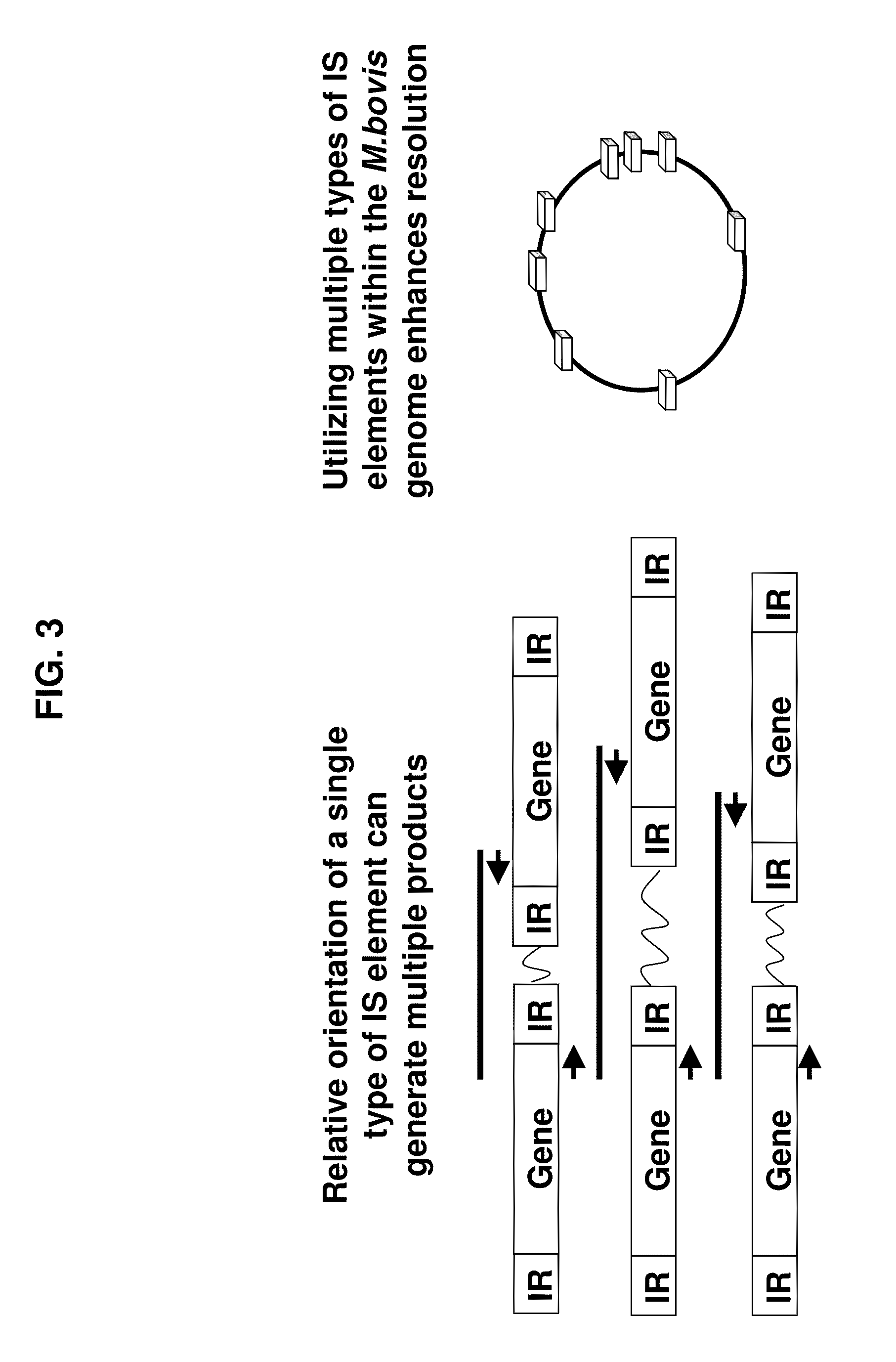
InactiveUS20110059437A1Improve analysisHigh sample throughputSugar derivativesMicrobiological testing/measurementInsertion sequenceBiology
A Mycoplasma bovis PCR-based genotyping method was developed that exploits the proximity of insertion sequences (IS) within the genome by using outward facing primers that selectively amplify sequences between IS elements. The method was applied to 16 field isolates of M. bovis, originating from pneumonic lung or arthritic joints, collected from the United States (Iowa or Kansas) between 2004 and 2005. The genomic fingerprints generated 14 distinct amplification profiles consisting of 4-8 fragments ranging in size from 200-3000 bp. Three isolates presented identical patterns and were isolated from two calves (one calf with pneumonic lung and the other with both pneumonic lung and arthritic joint) from a single farm during an outbreak and probably represent multiple infections with the same genotype. To demonstrate the stability of IS markers for molecular fingerprinting, 3 of the 16 field isolates were subjected to high number passage which resulted in patterns identical to the initial isolates. The results of these studies demonstrate the method can be used for simple and rapid molecular fingerprinting and differentiating M. bovis isolates with extension to epidemiology.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Mycoplasma bovis medium and preparation method
ActiveCN106399206AGrowth status can be judgedAvoid pollutionAntibacterial agentsBacterial antigen ingredientsPenicillinHydrolysate
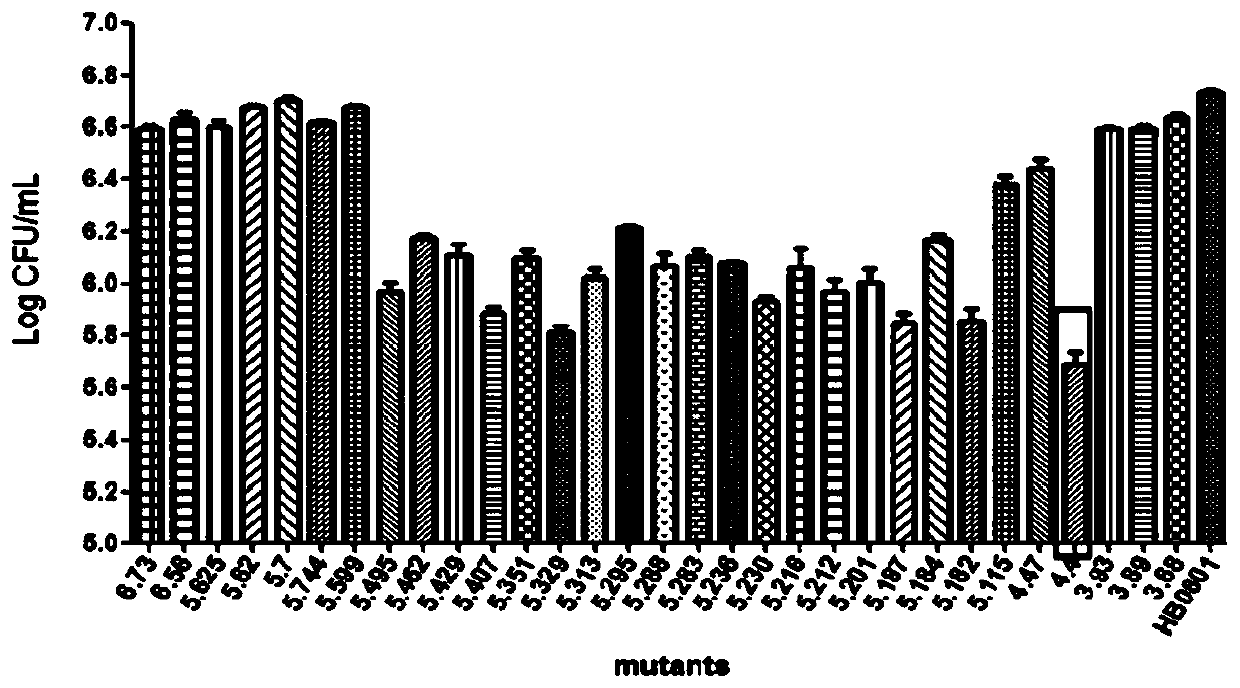
The invention discloses a mycoplasma bovis medium and a preparation method thereof. The mycoplasma bovis medium comprises a basic medium and an auxiliary medium through mixing and is prepared from Friis media premix, sodium pyruvate, a fresh yeast extract liquid, glucose, phenol red, deionized water, L-glutamine, L-cysteine, lactoalbumin hydrolysate, transferrin, insulin, penicillin and a small amount of horse serum. The medium not only can reduce the use amount of serum, but also remarkably increases the titer of living bacteria of mycoplasma bovis and lays a foundation for preparation of high quality vaccines of mycoplasma bovis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Hybridoma cell strain capable of secreting anti-mycoplasma bovis monoclonal antibody and application thereof
InactiveCN105695417AEfficient identificationStrong specificityImmunoglobulins against bacteriaTissue cultureAntiendomysial antibodiesMicrobiology
The invention provides a hybridoma cell strain capable of secreting an anti-mycoplasma bovis monoclonal antibody and application thereof.The hybridoma cell strain can produce the anti-mycoplasma bovis monoclonal antibody and is used for detecting mycoplasma bovis.The preservation number of the hybridoma cell strain is CCTCC C2015183, and the preservation date is November 8, 2015.The hybridoma cell strain has the advantages that the hybridoma cell strain can specifically secrete the anti-mycoplasma bovis monoclonal antibody and has better sensitivity and higher specificity.The hybridoma cell strain further has the advantages that the antibody produced by the cell strain can be applied in a variety of ways and has a good application value during actual production.
Owner:GANSU AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com