Primers, probes and kit for rapidly detecting mycoplasma bovis on site
A technology of Mycoplasma bovis and a kit is applied in the field of microbial detection to achieve the effects of high sensitivity, strong specificity and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
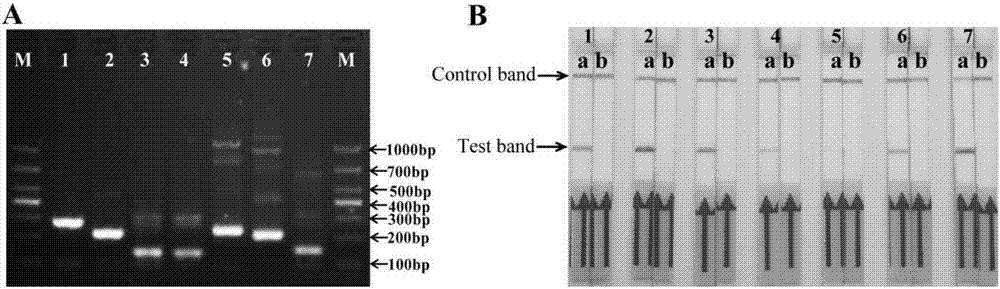
[0075] Design and screening of embodiment 1 primers and probes
[0076] At present, there are no specific rules for the design of RPA-nfo primers and probes, and the specificity and amplification efficiency must be tested after the RPA reaction, in order to screen and obtain primers and probes that can be used in clinical testing. In the experiment, it is necessary to design multiple pairs of primers and probes from both ends of the target sequence for optimization and screening, and the substitution or increase or decrease of individual bases will have an important impact on the test results.
[0077] The present invention designs forward primers, reverse primers and probes respectively according to the uvrC gene (GengBank No.AF003959) and oppD-oppF gene (GengBank No.AF130119) specific to Mycoplasma bovis, see Table 1 respectively. When designing primers, the conservation of uvrC gene and oppD-oppF gene in the primer design region was firstly BLASTed on the Genebank data, bot...
Embodiment 2
[0084] The establishment of embodiment 2 Mycoplasma bovis RPA-LFD detection method
[0085] 1. Experimental steps
[0086] (1) Preparation of positive quality control standard:
[0087] ①Extraction of template DNA: Genomic DNA was extracted from the lung tissue of dairy cows positive for Mycoplasma bovis detected by PCR in our laboratory.
[0088] ②PCR amplification: using the extracted DNA as a template, use the upstream primer uvrC-S: 5'-TAAATGAGCGCAGTGCTGAT-3' and the downstream primer uvrC-A: 5'-AACTTGAATTTGAACTAAGT-3' to amplify. The reaction system is 50 μL, including 2 ×PCR mix: 25 μL, 2 μL of upstream and downstream primers, 2 μL of template, ddH 2 O 19 μL. The reaction conditions are: 94°C for 3 min, 94°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec, 35 cycles, 72°C for 10 min.
[0089] ③Construction of recombinant plasmids and establishment of positive quality control standards: PCR products were gel-purified and recovered, then connected to the pEASY-T3 vector (...
Embodiment 3
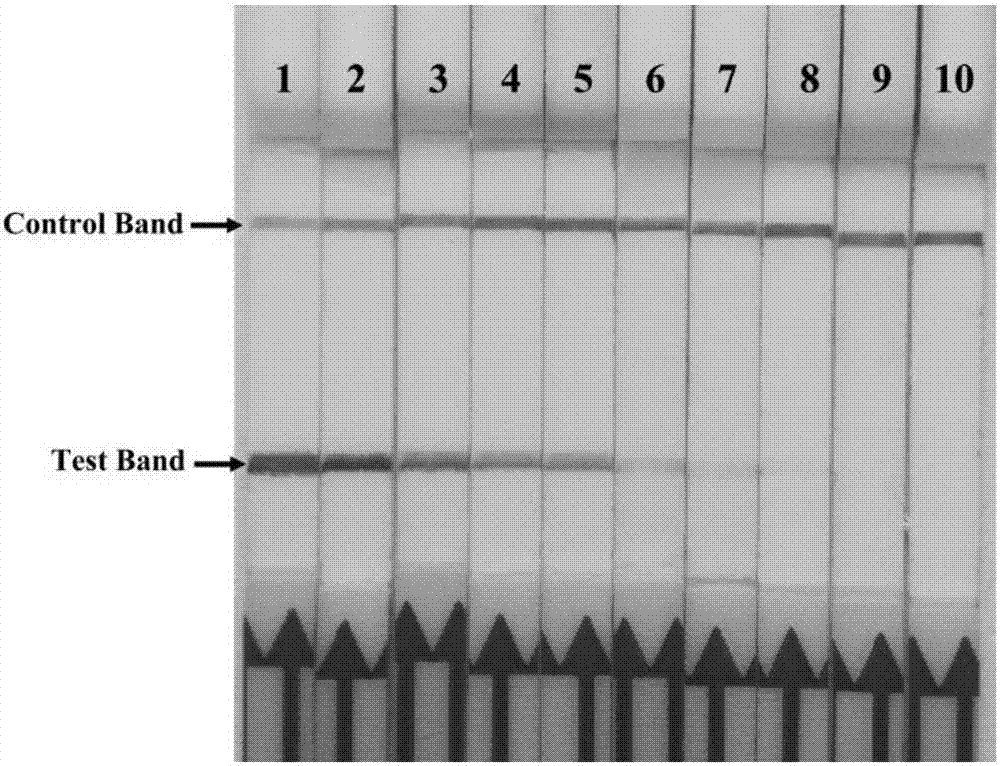
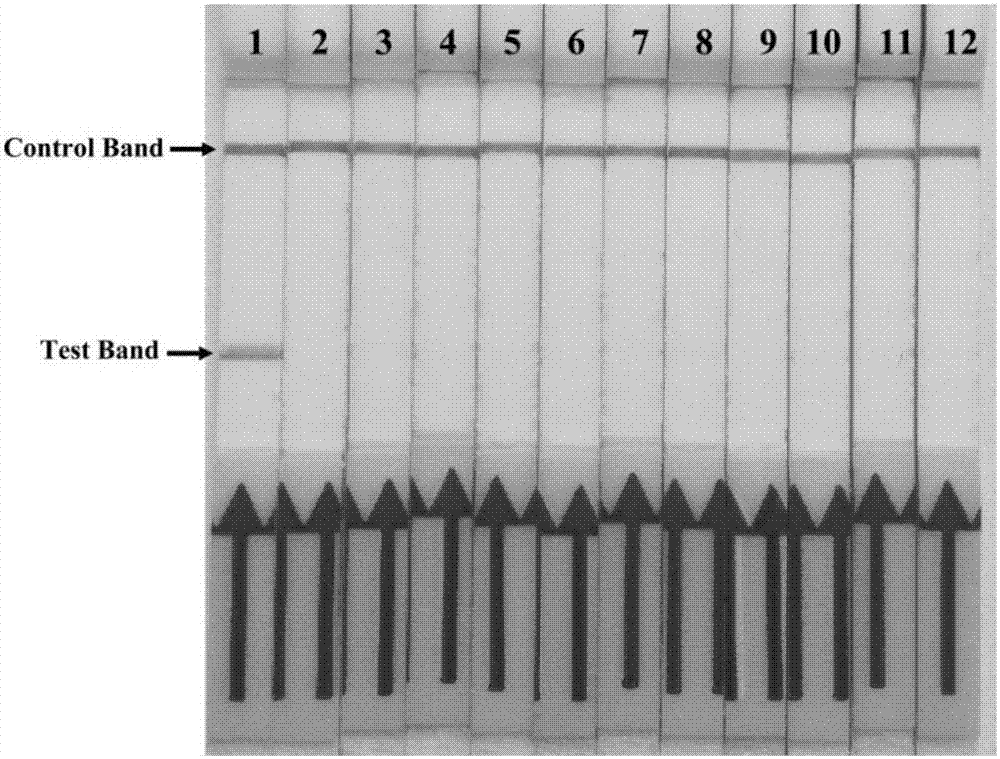
[0108] Application of embodiment 3 Mycoplasma bovis RPA-LFD detection method
[0109] 1. Experimental steps
[0110] (1) On-site preparation of clinical samples
[0111] Clinical samples such as lung tissue, aerosol centrifugation, and milk sample centrifugation were washed with 200 μL TE buffer [1.0MTris-HCl (pH8.0) 10mL, 0.5M Na 2 EDTA·2H 2 O (pH8.0) 2mL, add distilled water to 1000mL] to resuspend, take 200μL of nasal swab solution, blood and other liquid samples directly, then add 30μL 10% (w / v) SDS and 3μL 2% (w / v) For proteinase K, mix and incubate at 37°C for 1 hour, during which time it is inverted several times.
[0112] (2) Detection of clinical samples
[0113] According to the on-site lysing method of clinical samples in step (1), 9 nasal swab samples, 1 bovine lung tissue sample, 1 Aerosol samples, 1 blood sample and 1 milk sample were tested for Mycoplasma bovis RPA-LFD, and at the same time, 1 negative sample was taken from each of the above-mentioned diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com