LAMP primer combination for detecting 6 infectious pathogens of dairy cow mastitis and application thereof
A primer combination and primer set technology, applied in recombinant DNA technology, microbial measurement/testing, biochemical equipment and methods, etc., can solve problems such as time-consuming, poor specificity, and low throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] Embodiment 1, the preparation of kit
[0188] The kit consists of six LAMP primer sets, each for the detection of one infectious agent of cow mastitis.
[0189] The primer set for detecting Mycoplasma bovis is as follows (5'→3'):
[0190] Outer primer F3 (SEQ ID NO: 1): GGACGACGTCAAATCATCA;
[0191] Outer primer B3 (SEQ ID NO: 2): CCGTAGCGTAGCTGATCT;
[0192] Internal primer FIP (SEQ ID NO: 3): CCATGTCACCACTTCGCTTTCTCTTCCTCTTACGAGTGGGGCTA;
[0193] Internal primer BIP (SEQ ID NO: 4): CAAACCTCAAAAAACCGTTCTCAGACGATTACTAGCGATTCCGACT;
[0194] Loop primer LF (SEQ ID NO: 5): ACCGTCCATTGTAGCACGTGTG;
[0195] Loop primer LB (SEQ ID NO: 6): AAGTCTGCAACTCGACTTCATG.
[0196] The primer set used to detect Staphylococcus aureus is as follows (5'→3'):
[0197] Outer primer F3 (SEQ ID NO: 7): GCAACTGAAACAACAGAAGC;
[0198] Outer primer B3 (SEQ ID NO: 8): TTTTGTGTTGGGCGAGC;
[0199] Internal primer FIP (SEQ ID NO: 9): TCACGGATACCTGTACCAGCATCTCTATGGTCCGAGACCGCAATT;
[0200] In...
Embodiment 2
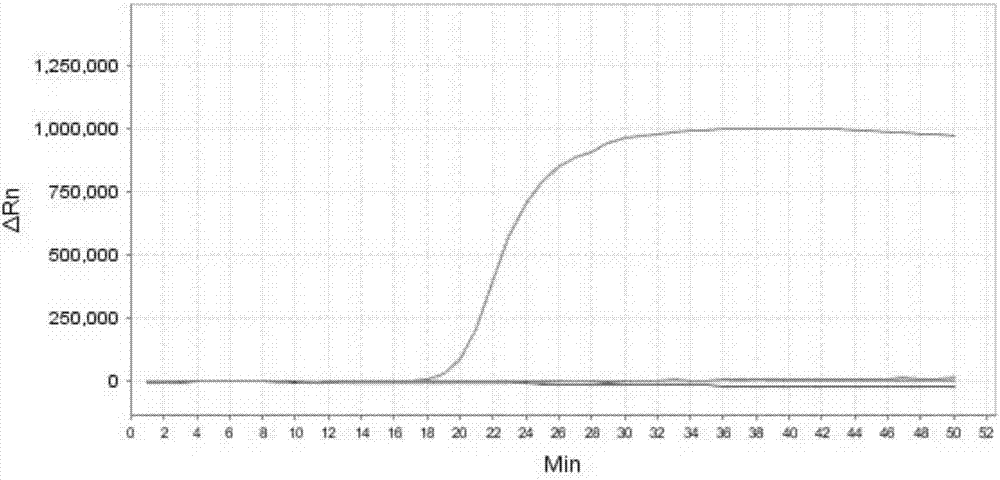
[0232] Embodiment 2, specificity
[0233] Test sample 1: Mycoplasma bovis ( 25523 TM ).
[0234] Test sample 2: Staphylococcus aureus (CVCC 545).
[0235] Test sample 3: Streptococcus agalactiae (CVCC 586).
[0236] Test sample 4: Streptococcus pyogenes (CGMCC1.8868).
[0237] Test sample 5: Corynebacterium bovis (CVCC CAU0107).
[0238] Test sample 6: Staphylococcus epidermidis ( 12228 TM ).
[0239] Each sample to be tested carries out the following steps respectively:
[0240] 1. Extract the genomic DNA of the sample to be tested.
[0241] 2. Using the genomic DNA extracted in step 1 as a template, each primer set prepared in Example 1 was used to perform loop-mediated isothermal amplification.
[0242] Reaction system (10 μL): 7.0 μL reaction solution (product of Boao Bio Group Co., Ltd., catalog number CP.440020), 1 μL primer mixture, 1 μL template DNA (5pg-50pg), make up to 10 μL with water. The primer mixture is the mixture of each primer in the primer set....
Embodiment 3
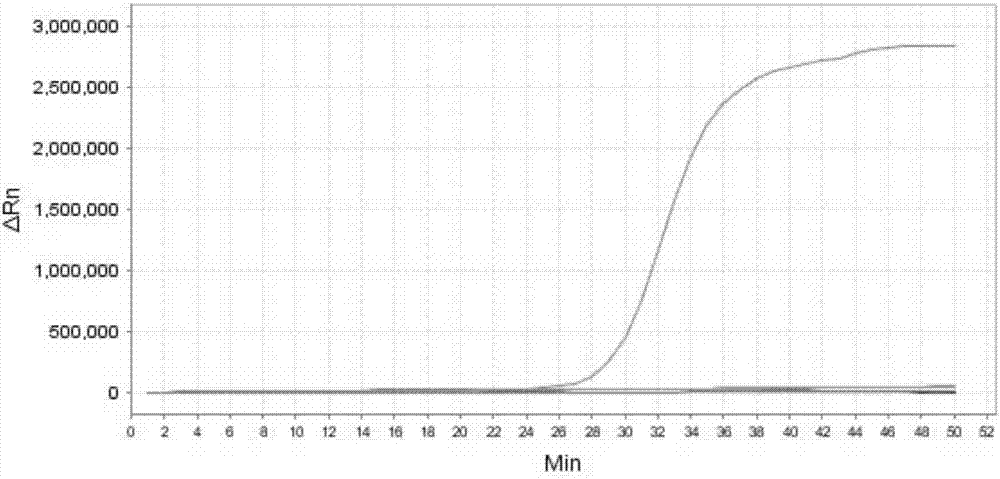
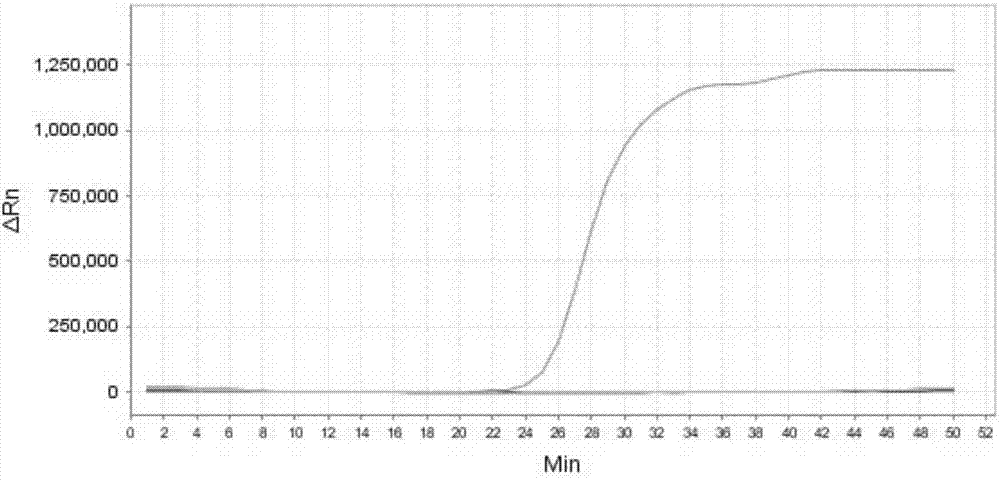
[0252] Embodiment 3, sensitivity
[0253] Test sample 1: Mycoplasma bovis ( 25523 TM ).
[0254] Test sample 2: Staphylococcus aureus (CVCC 545).
[0255] Test sample 3: Streptococcus agalactiae (CVCC 586).
[0256] Test sample 4: Streptococcus pyogenes (CGMCC1.8868).
[0257] Test sample 5: Corynebacterium bovis (CVCC CAU0107).
[0258] Test sample 6: Staphylococcus epidermidis ( 12228 TM ).
[0259] 1. Extract the genomic DNA of the sample to be tested, and perform gradient dilution with sterile water to obtain each dilution.
[0260] 2. Using the dilution obtained in step 1 as a template, the primer sets prepared in Example 1 were used to perform loop-mediated isothermal amplification.
[0261] When the sample to be tested is the sample to be tested 1, the loop-mediated isothermal amplification is performed using primer set I. When the sample to be tested is the sample to be tested 2, loop-mediated isothermal amplification is performed using primer set II. When...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com