Multi-fluorescent quantitative PCR reagent kit capable of synchronously detecting three bovine respiratory pathogens, and multi-fluorescent quantitative PCR detection method capable of synchronously detecting three bovine respiratory pathogens
A multiplex fluorescence quantitative detection kit technology, applied in the field of livestock disease detection, can solve the problems of the multiplex fluorescence quantitative PCR method that has not been reported yet, time-consuming, low sensitivity and accuracy, etc., and achieves high sensitivity, strong specificity, good repetitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 Primer and probe design
[0073] According to the specific sequence 229bp sequence of Mycobacterium bovis registered on GenBank (accession number: AJ003103), the uvrC gene of Mycoplasma bovis (accession number: AF003959) and the khe gene of Klebsiella bovis (accession number: AF293352) using Oligo7 .0 software designed specific primers and TaqMan probes, and selected the following specific primers and TaqMan probes.
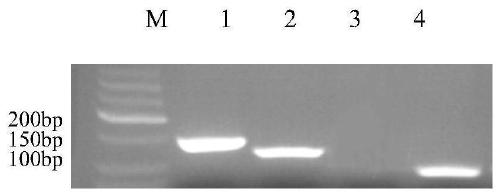
[0074] Using the genomic DNA of Mycobacterium bovis, Mycoplasma bovis and Klebsiella bovis as templates and the corresponding specific primers to amplify, the corresponding specific product size fragments were displayed after electrophoresis, such as figure 1 .
[0075] The amplified products were purified, cloned and then sequenced. The sequencing results were blasted on NCBI. As a result, each pair of primers was consistent with the theoretical sequence in the database, and there was only a single base difference. This difference may be due...
Embodiment 2
[0079] Example 2 Simultaneous detection of multiple fluorescent quantitative PCR detection kits for three bovine respiratory pathogens
[0080] In addition to the primers and TaqMan probes described in Example 1, the above kit also includes the following reagents: PremixEx Taq TM (2×), template DNA and with sterile ddH2O.
[0081] Further, the amplification reaction system is 20 μL, including the following: Premix Ex TaqTM (2×) 10 μL, upstream and downstream primers (10 μM / L) each 0.5 μL, probe (10 μM / L) each 0.5 μL, template DNA 2 μL, Make up to 20 µL with sterile ddHO water.
[0082] Amplification was carried out according to the following reaction parameters: 95°C for 600s, 95°C for 10s, 60°C for 10s, 72°C for 20s, 45 cycles, 95°C for 10s, 65°C for 60s, 97°C for 1s, 37°C for 30s.
Embodiment 3
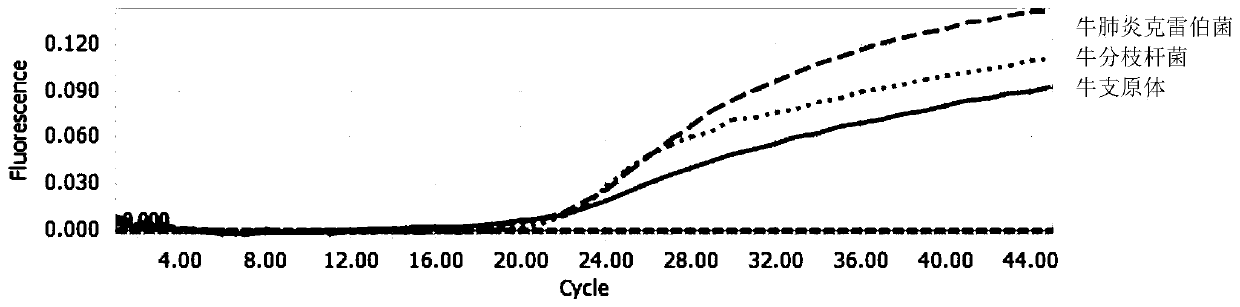
[0083] Example 3 Simultaneous detection of multiple fluorescent quantitative PCR detection methods for three bovine respiratory pathogens
[0084] Using the primers and TaqMan probes in Example 1, and the kit in Example 2, multiplex fluorescent quantitative PCR detection was performed.
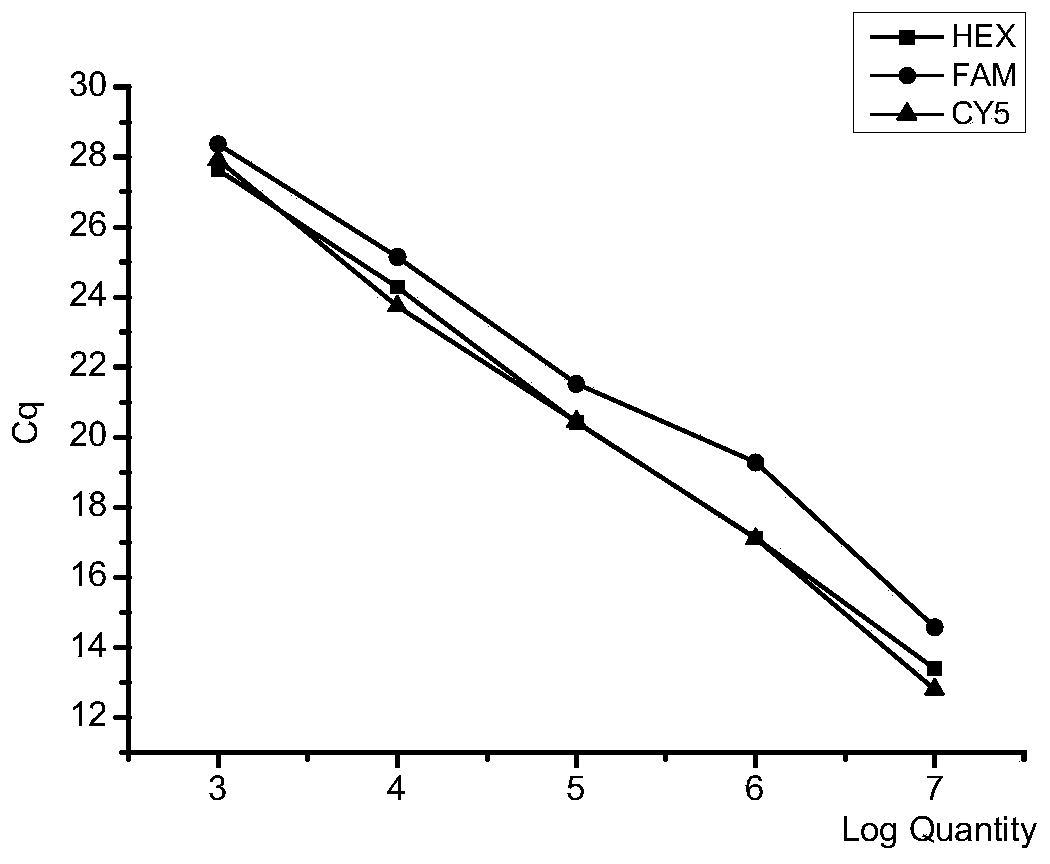
[0085](1) Establish a standard curve: respectively construct recombinant plasmids carrying Mycobacterium bovis, Mycoplasma bovis and Klebsiella bovis specific sequences (specific sequences are as shown in Example 1), prepare DNA standard items, and multiply by 10 times respectively Serially diluted to 1×10 3 , 1×10 4 , 1×10 5 , 1×10 6 , 1×10 7 Copy / μL, with different concentrations of standard products as templates, fluorescent quantitative PCR was carried out under the guidance of the primers in Example 1 and TaqMan probes. The amplification reaction system of fluorescent quantitative PCR is 20 μL, including the following: Premix Ex TaqTM (2×) 10 μL, upstream and downstream primers (10 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com