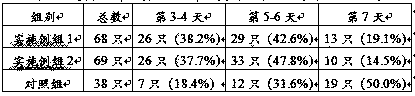
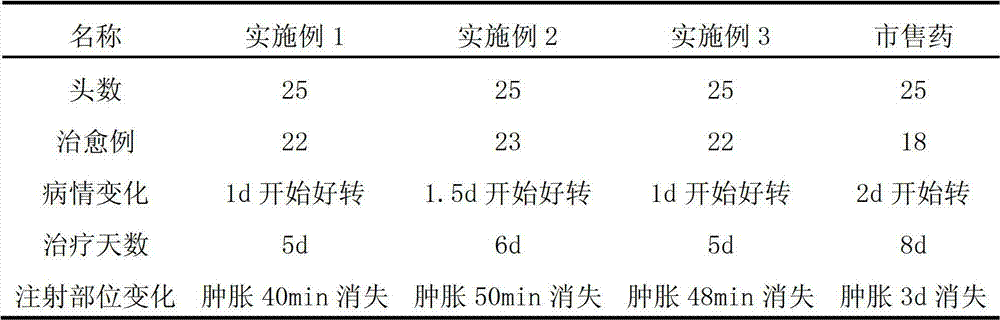
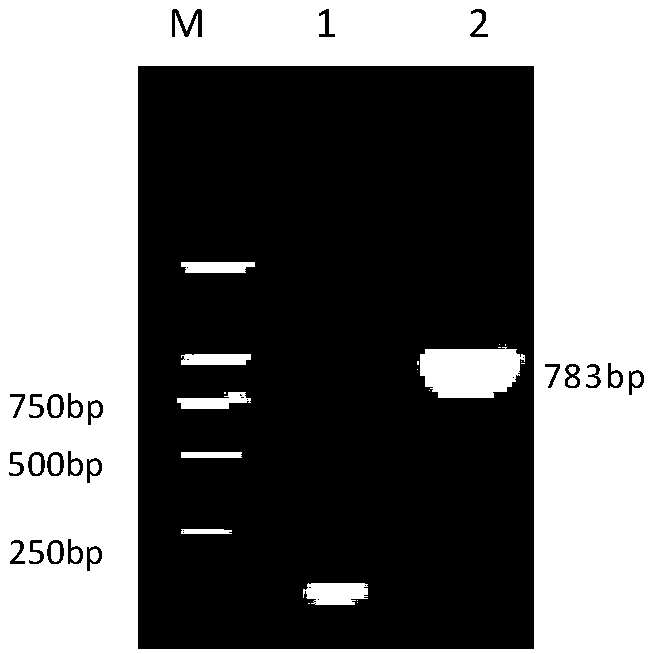
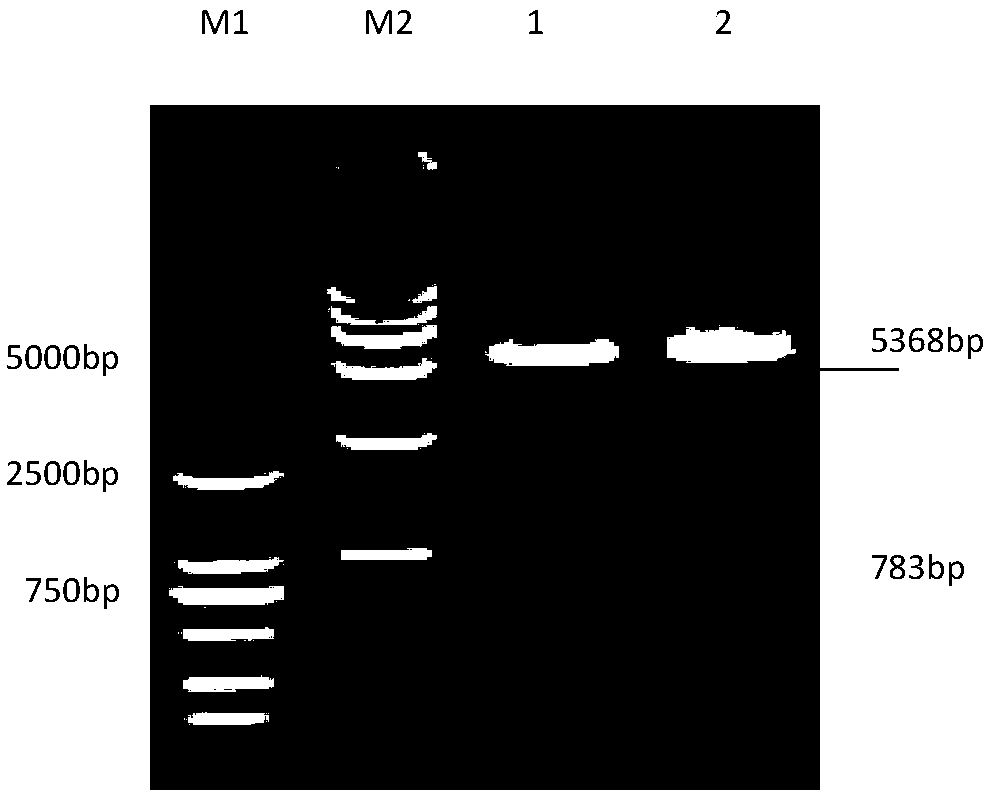
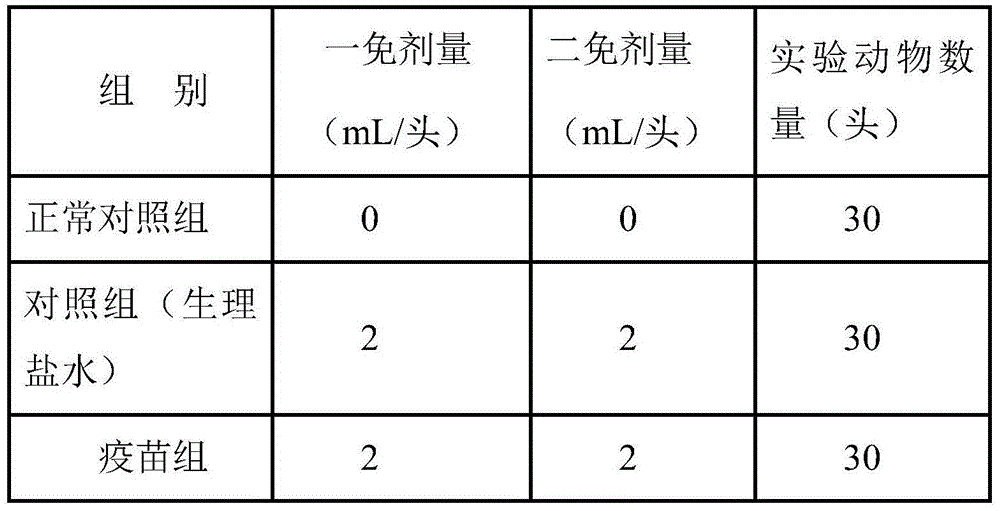
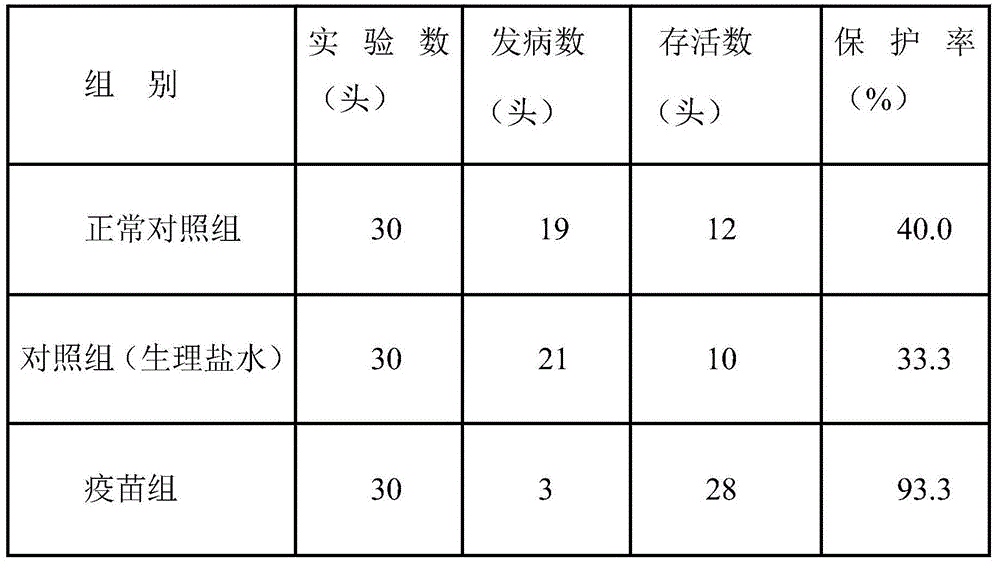
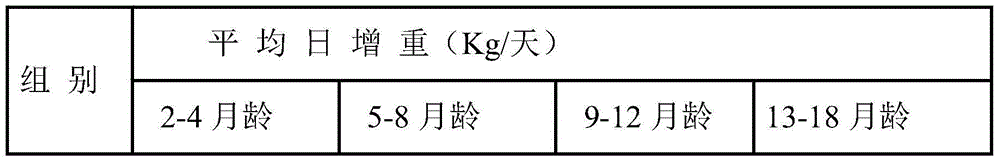
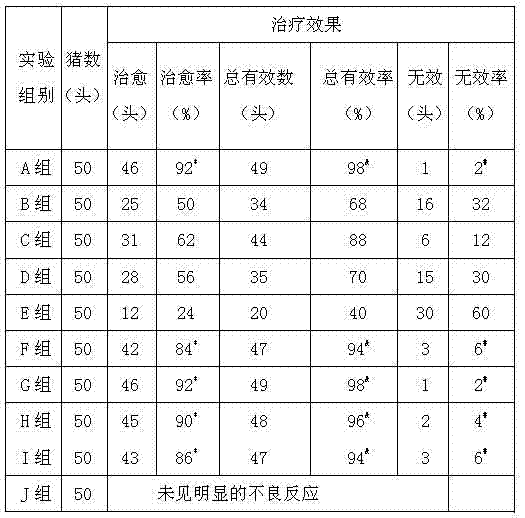
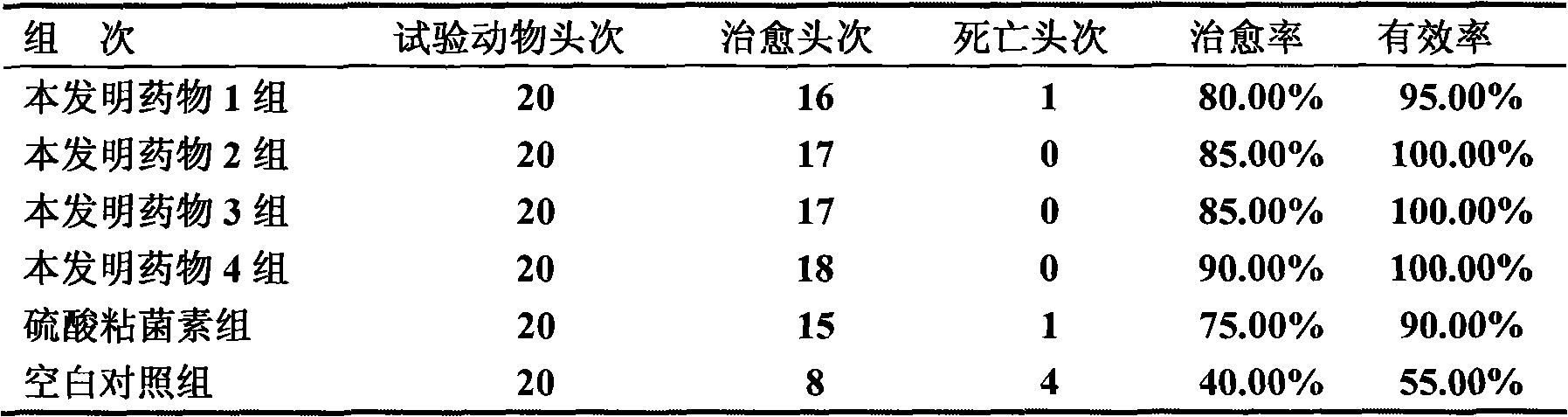
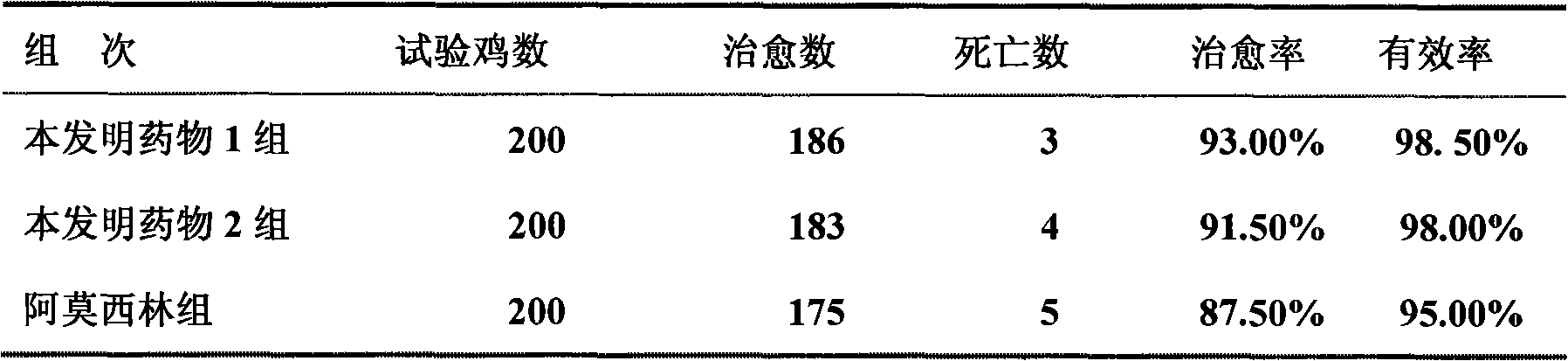
Patents
Literature
140 results about "Pleuropneumonia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pleuropneumonia is inflammation of the lungs and pleura, pleurisy being the inflammation of the pleura alone.
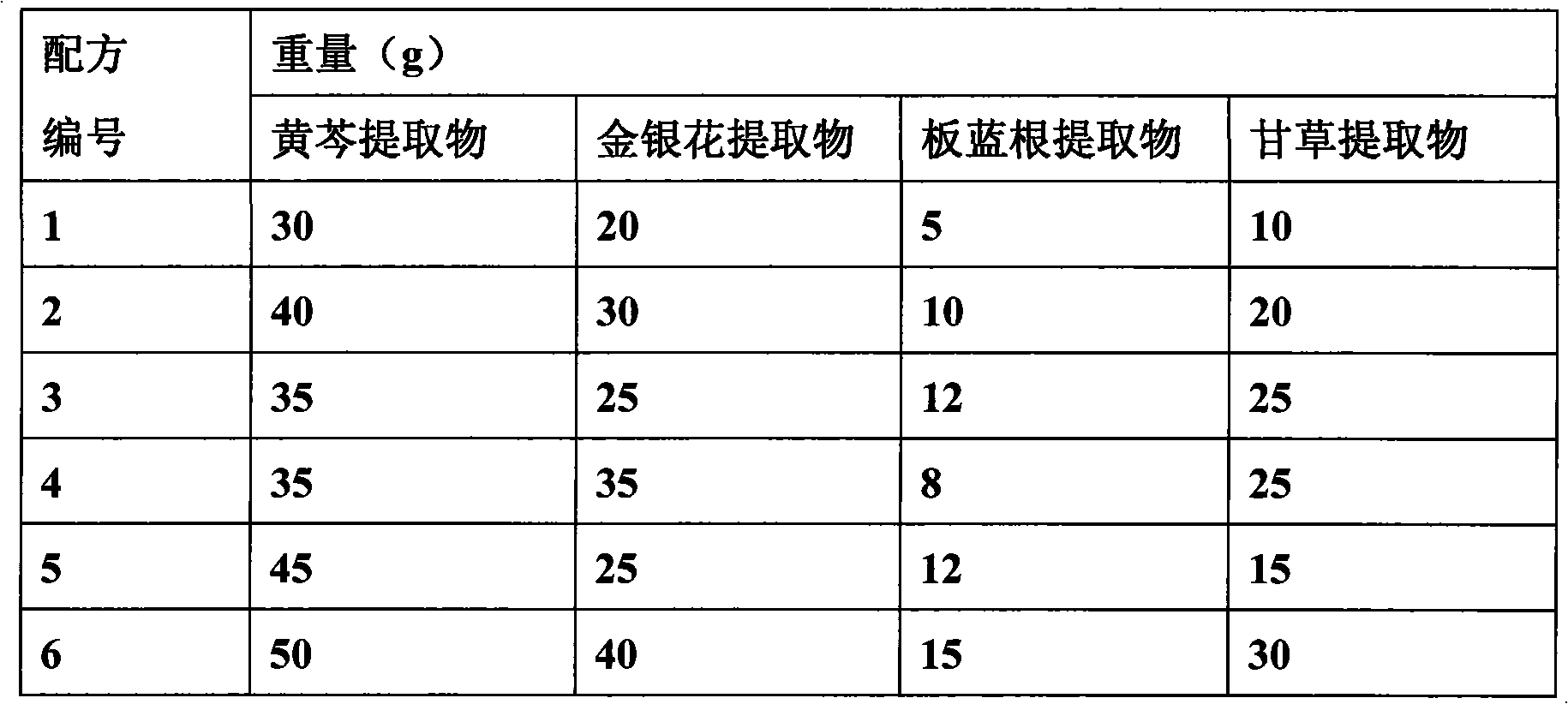
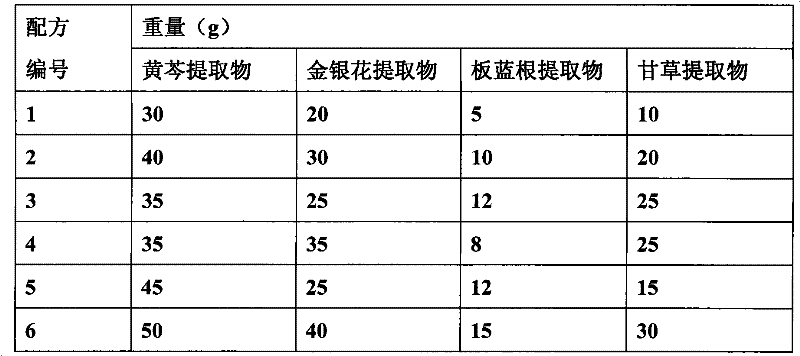
Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease
InactiveCN101695342APrevention and treatment of asthmaPrevention and treatment of contagious pleuropneumoniaFood processingAnimal feeding stuffMedicinal herbsBaical Skullcap Root
The invention provides a Chinese herbal medicinal feed additive for preventing and controlling swine infectious disease. The swine feed additive consists of 37 Chinese medicinal herbs, namely, gypsum, rehmannia root, rhinoceros horn, golden thread, cape jasmine fruit, tree peony bark, baical skullcap root, red paeony root, figwort root, common anemarrhena rhizome, forsythia suspensa , platycodon root, liquorice root, common lophatherum herb, amur corktree bark, honeysuckle flower, Chinese pulsatilla root, indigowoad root, heartleaf houttuynia herb, astragalus, szechwon tangshan root, hawkthorn fruit, medicated leaven, barley sprout, radish seed, chicken's gizzard -membrane, Chinese thorowax root, common andrographis herb, philippine violet herb, tuber fleeceflower root, massa medicata fermentata fujianensis, cyrtomium rhizome, tung leaf, tangerine peel, white paeony root, pine needle and indigowoad leaf through scientific compatibility. The feed additive is added into swine feed in the proportion; under the condition of not using any vaccine, the feed additive can effectively prevent and cure severe mixed flu symptoms, infection and other syndromes caused by swine respiratory disease, asthma, contagious pleuropneumonia, swine virus mixed flu, high swine fever, porcine circovirus, swine fever, flu, pseudorabies, salmonellosis, bacillosis, streptococcus, erysipelas, paratyphoid, eperythrozoon, toxoplasm and other multi-pathogeny and provides genuine green food for the market.
Owner:孟祥合
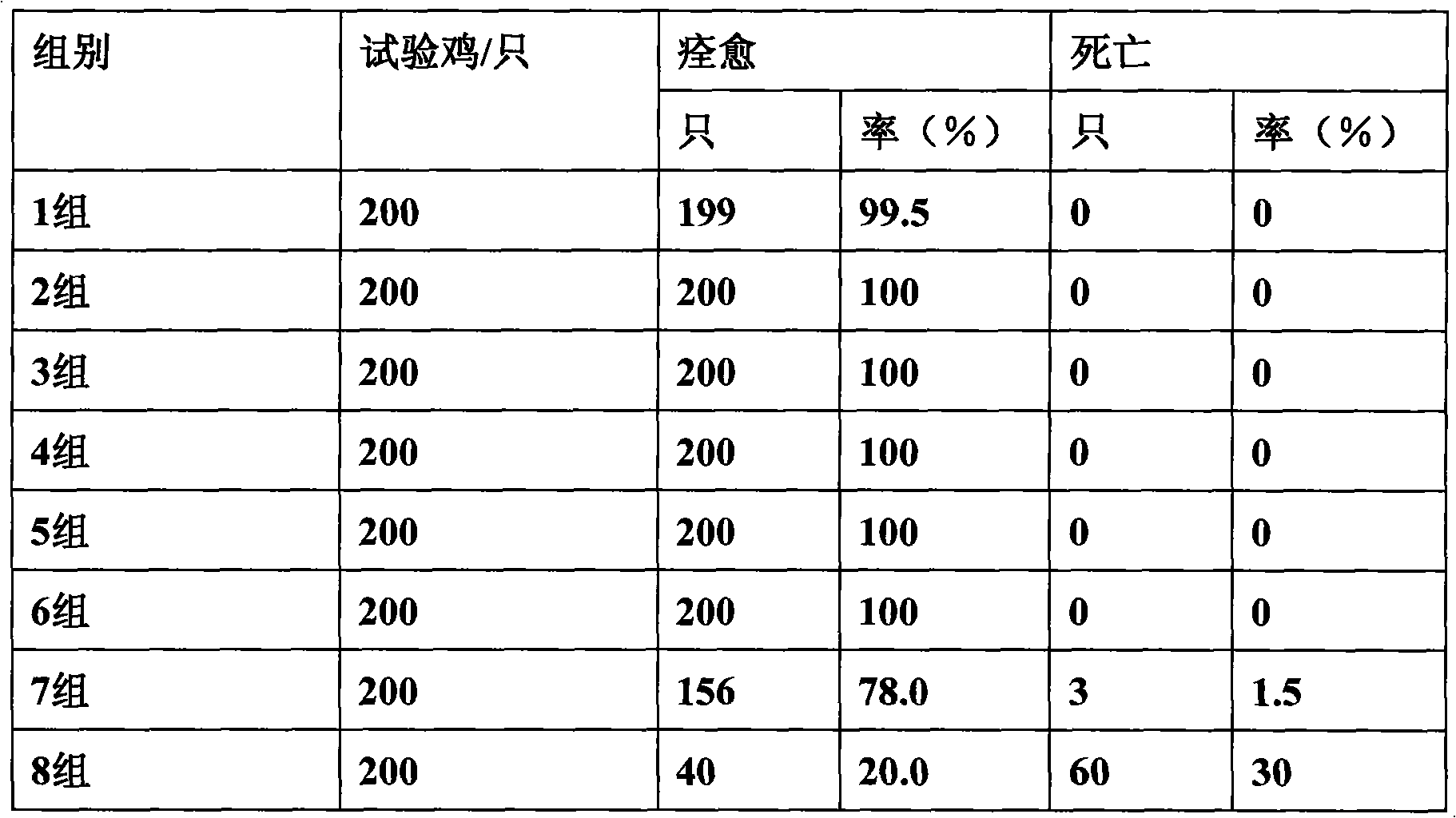
Antibacterial antivirus Chinese medicinal composition for animal
ActiveCN101584747AReasonable compositionStrong broad-spectrum antibacterialAntibacterial agentsFood processingPoultry diseaseBacteroides
The invention discloses an antibacterial antivirus Chinese medicinal composition for the animal, comprising the following main medicament ingredients: scutellaria extract, honeysuckle extract, isatis root extract and glycyrrhiza extract. the pharmaceutical composition of the invention can be made into premix, injection, oral liquid or granula, also can be made into feedstuff premix adding into the feedstuff. the chinese medicinal composition of the invention is a pure Chinese medicinal compound preparation which has the antibacterial, anti-inflammation, antivirus and immunity-reinforced functionsand wide application range, can effectively prevent and treat various livestock and poultry diseases caused by the virus and the bacteria, such as piggyyellow-white dysentery, swine plague, infectious pleuropneumonia, asthma, canker, circovirus, swine influenza, white diarrhea, newcastle disease, bursa of Fabricius, egg drop syndrome, duck serositis and the like, and has fast effect and remarkable curative effect.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Traditional Chinese medicine for treating respiratory diseases and promoting growth for swine and preparation method thereof
ActiveCN101698024AGood curative effectQuick resultsAnimal feeding stuffRespiratory disorderDiseaseTrichosanthes kirilowii
The invention discloses a traditional Chinese medicine for treating respiratory diseases and promoting growth for swine and a preparation method thereof. The traditional Chinese medicine is composed of plantain herb, herba patriniae, hindu datura flower, perilla leaf, trichosanthes kirilowii maxim and gelsmium elegans. The traditional Chinese medicine of the invention can effectively prevent and treat livestock respiratory diseases, such as swine enzootic pneumonia, infectious pleuropneumonia of swine, porcine reproductive and respiratory syndrome (PRRS), swine plague, swine influenza and thelike; meanwhile, the traditional Chinese medicine of the invention can improve animal productivity and promote animal growth. The traditional Chinese medicine of the invention has wide raw material resources, is safe and environmentally-friendly and has the advantages of simple preparation technology, low production cost and the like.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
Primers for triple PCR of three types of sheep pathogenic mycoplasmas and detection method
InactiveCN106434919AOvercome limitationsQuick checkMicrobiological testing/measurementDNA/RNA fragmentationEpidemiologic surveyMycoplasma pneumonia
The invention provides primers for triple PCR of three types of sheep pathogenic mycoplasmas and a detection method. The three pairs of special primers MO, MCC and MCCP are synthesized respectively according to the designs of sheep mycoplasma pneumoniae, a goat mycoplasma mycoide subspecies and a goat mycoplasma pneumonia subspecies, and a triple PCR optimum reaction system and reaction conditions for the three types of sheep pathogenic mycoplasmas are provided. The simultaneous pathogenic detection of the sheep mycoplasma pneumoniae, the goat mycoplasma mycoide subspecies and the goat mycoplasma pneumonia subspecies can be rapidly carried out without cloning, sequencing and sequence comparison, and the detection method has the advantages of being rapid, accurate, strong in specificity, good in repeatability and the like, suitable for rapid detection of the sheep mycoplasma pneumoniae, the goat mycoplasma mycoide subspecies and the goat mycoplasma pneumonia subspecies and large-scale epidemiological investigation and has great economic and social benefits.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
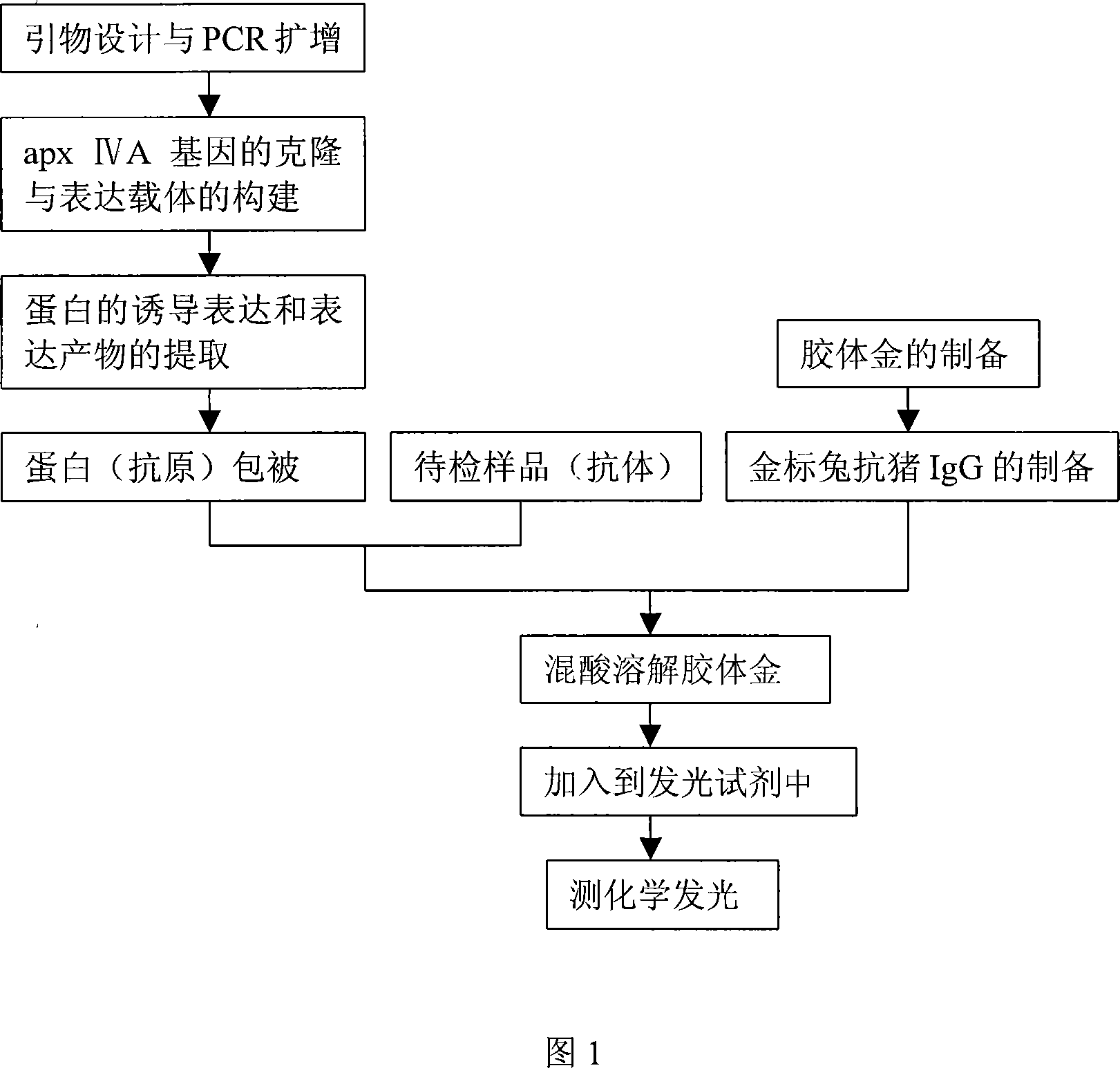
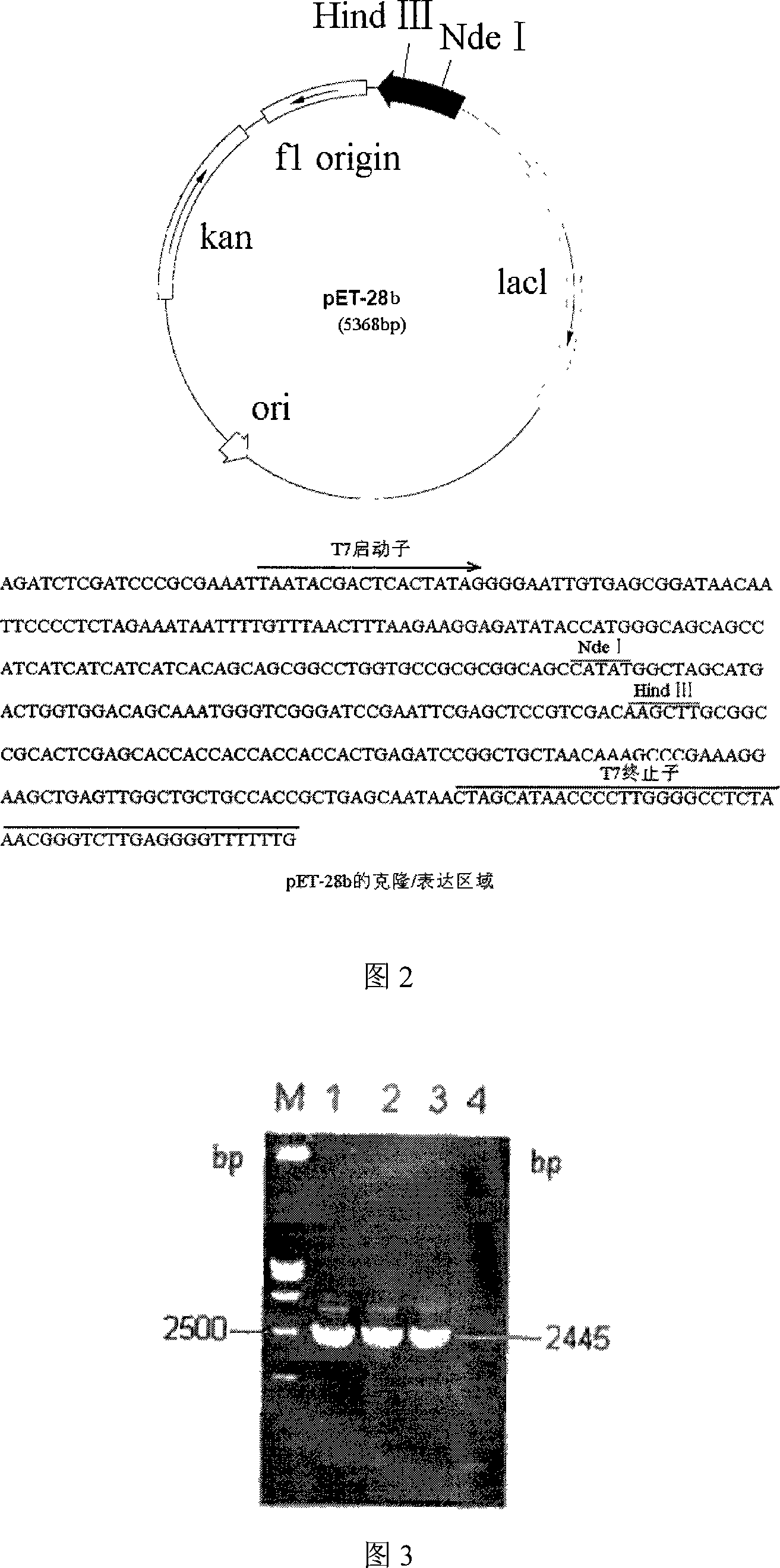
Colloidal gold chemiluminescence immune analysis method for detecting pig pleuropneumonia antibody
InactiveCN101113980ASuitable for testingHigh sensitivityChemiluminescene/bioluminescenceAnimal virusAntigen
The invention discloses a colloidal gold chemiluminescence immune assay method of detecting pig peripneumonia antibody. The method relates to animal virus immunodetection and chemiluminescence immune assay field. The main points of the invention are hereinafter: fixing the pig peripneumonia Apx IVA antigen in 96-microtitor plate, blocking the spare active site in the plate, add sample product to be examined, thus the pig peripneumonia Apx IVA antigen is binded. The gold-labeled rabbit anti pig IgG is added and the specific reaction with the sample can take place. The chemiluminescence reagent is added with the dissolved solution after the colloidal gold labeled on rabbit anti pig IgG is dissolved by violet acid and then detect chemiluminescence intensity. The chemiluminescence intensity is in direct ratiot to antibody concentration to be detected, thus linear correlation is available between chemiluminescence intensity and pig peripneumonia Apx IVA antibody. Compared with the prior art, the method has more accuracy and high specificity to better meet the need of clinical detection of peripneumonia.
Owner:HUAZHONG AGRI UNIV
Medication for preventing and treating diseases of pigs
InactiveCN1626106AEasy to prepareMedication convenienceAntibacterial agentsOrganic active ingredientsDiseaseMetritis
A veterinary medicine for preventing and treating the frequently encountered diseases of pig, such as metritis, mammitis, acyesis, sterility, dysentery, epidemic enteritis, paratyphoid, asthma, etc is prepared from 15 raw materials including florfenicol, amoxicillin, lucomycin, doxycycline, etc.
Owner:杨联华
Pig functional feed and preparation method thereof
ActiveCN104256168AQuick effectDefinite curative effectAnimal feeding stuffVegetable oilAnimal science
The invention discloses a pig functional feed, which is characterized by comprising the following raw materials: corn, sticky rice, bean pulp, peanut meal, lotus root starch, fish meal, seaweed meal, honey, boiled egg white, vegetable oil, salt and vitamin premix. The pig functional feed disclosed by the invention is applied to pigs suffering from contagious pleuropneumonia, and is capable of treating contagious pleuropneumonia and also capable of satisfying nutritional requirements of pigs and keeping normal production performance.
Owner:江门市六和饲料有限公司
Florfenicol compound composition and preparation method thereof
ActiveCN102846650AImprove stabilityAchieve long-term goalsAntibacterial agentsOrganic active ingredientsTreatment effectSwine plague
The invention discloses a florfenicol compound composition and a preparation method thereof. The composition is composed of florfenicol, azithromycin, tylosin, propylene glycol, dimethyl formamide, alpha-pyrrolidone, anhydrous ethanol and polyvinylpyrrolidone (PVP). The preparation method of the composition comprises steps of: 1) adding dimethyl formamide to florfenicol, stirring and dissolving; and sequentially adding propylene glycol, alpha-pyrrolidone and anhydrous ethanol, and stirring to mix evenly; 2) adding azithromycin and tylosin, and stirring to mix evenly; 3) heating with stirring at 40 DEG C, adding polyethylene pyrrole pyrrolidone, dissolving and continuing to stir for 15min; and 4) filtering the obtained solution, inflating nitrogen and encapsulating to obtain the florfenicol compound composition. The compound composition provided by the invention is mainly used in the treatment of swine respiratory diseases, and especially has specific long-term treatment effect and no recurrence on respiratory diseases, such as infectious pleuropneumonia, Haemophilus parasuis, swine plague and asthma.
Owner:河南后羿制药有限公司
Method for preparing actinobacillus pleuropneumoniae (App) bacterial ghost and method for preparing subunit vaccine by loading pasteurella antigen with App bacterial ghost
InactiveCN101934072APrevention of swine pleuropneumoniaPrevention of PasteurellosisAntibacterial agentsBacterial antigen ingredientsAntigenPleuronectes pinnifasciatus
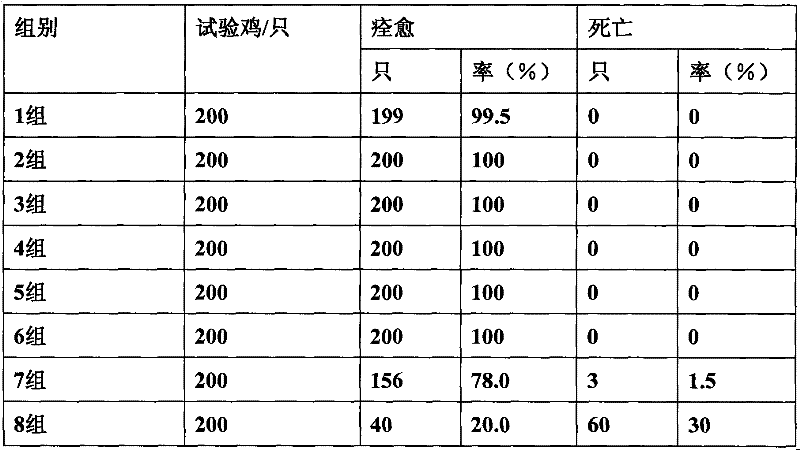
The invention discloses a method for preparing an actinobacillus pleuropneumoniae (App) bacterial ghost and a method for preparing a subunit vaccine by loading a pasteurella antigen with the App bacterial ghost. A recombinant swine App bacterial ghost is prepared by controllable double-cracking technology and a pasteurella protection gene is introduced into an App bacterial ghost carrier, so that swine pleuropneumonia and a pasteurella bigeminal gene vaccine for preventing and treating swine pasteurellosis and swine pleuropneumonia are obtained. The preparation of the bacterial ghost carrier and the application of the bacterial ghost carrier to the prevention and treatment of important animal epidemic diseases are realized and a method is provided for the research of a multi-geminal gene vaccine at the same time. An animal experiment indicates that the protection rates of the bigeminal vaccine on infectious swine pleuropneumonia and pasteurellosis are up to 99 percent and 99.2 percent respectively.
Owner:TIANJIN AGRICULTURE COLLEGE
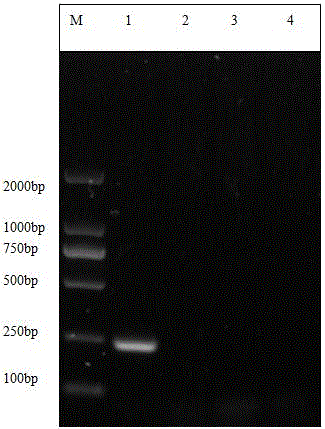
PCR diagnostic kit for porcine infectious pleuropneumonia
InactiveCN101724709AThe test result is accurateThe detection method is simpleMicrobiological testing/measurementMicroorganism based processesPositive controlTE buffer
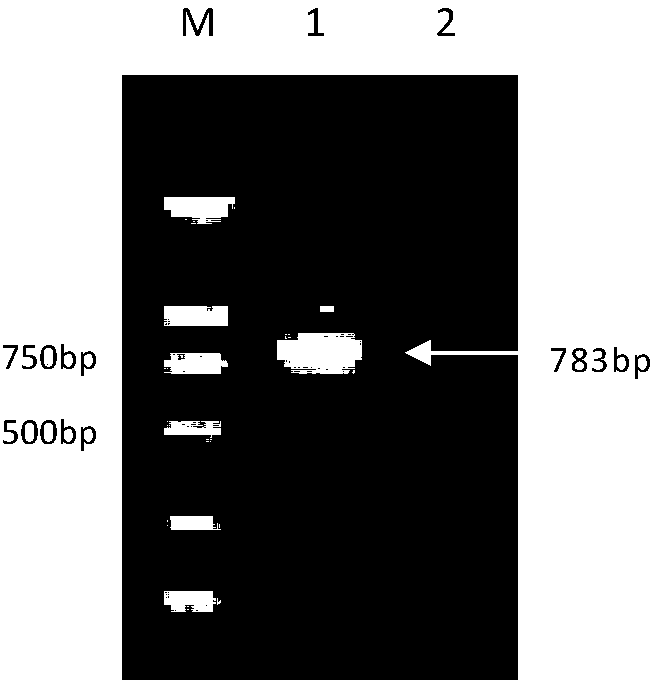
The invention discloses a PCR diagnostic kit for porcine infectious pleuropneumonia, which comprises 400mu L of proteinase K with the concentration of 20mg / mL, 1000mu L of cracking solution, 1500mu L of TE buffer solution, 250mu L of PCR enzyme, 170mu L of ultra-pure water, 50mu L of MarkerDL2000, 40mu L of primer P1 and primer P2 which are mixed in the same volume and have the same concentration of 20mu M, 20mu L of negative control and 20mu L of positive control. By optimizing the PCR reaction conditions, the invention develops a PCR kit for detecting the actinobacillus of the porcine infectious pleuropneumonia; and by comparing the PCR detection result with the negative control and the positive control, detection conclusions can be obtained, thereby achieving the aim of quickly detecting whether a sample has porcine infectious pleuropneumonia or not. The invention has accurate detection result, quick and sensitive detection process, simple detection mode and good using effect.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
Preparation method of triple inactivated vaccine for pigs
InactiveCN104998256AImprove immunityHighlight immune functionAntibacterial agentsBacterial antigen ingredientsAntigenProtective antigen
The invention provides a preparation method of a triple inactivated vaccine for pigs. The method determines an antigen composition with excellent immunization effects by selection of the antigen. The prepared polyvalent vaccine has outstanding immunization effects. The prepared vaccine contains a PCP immunization protective antigen exotoxin (Aps), has cross immunization protection effects better than those of a whole cell inactivated vaccine, greatly reduces side reaction, and can simultaneously prevent haemophilus parasuis, swine streptococcosis and actinobacillus pleuropneumonia by combined immunization with inactivated haemophilus parasuis and streptococcus suis. Compared with haemophilus parasuis and streptococcus suis inactivated vaccines sold on the market, the triple inactivated vaccine has the same corresponding pathogen immune protection force. Compared with the actinobacillus pleuropneumonia inactivated vaccine sold on the market, the triple inactivated vaccine has a cross immunization protecting force on diseased pigs with different serotypes and realizes multiple protection purposes.
Owner:TIANJIN RINGPU BIO TECH
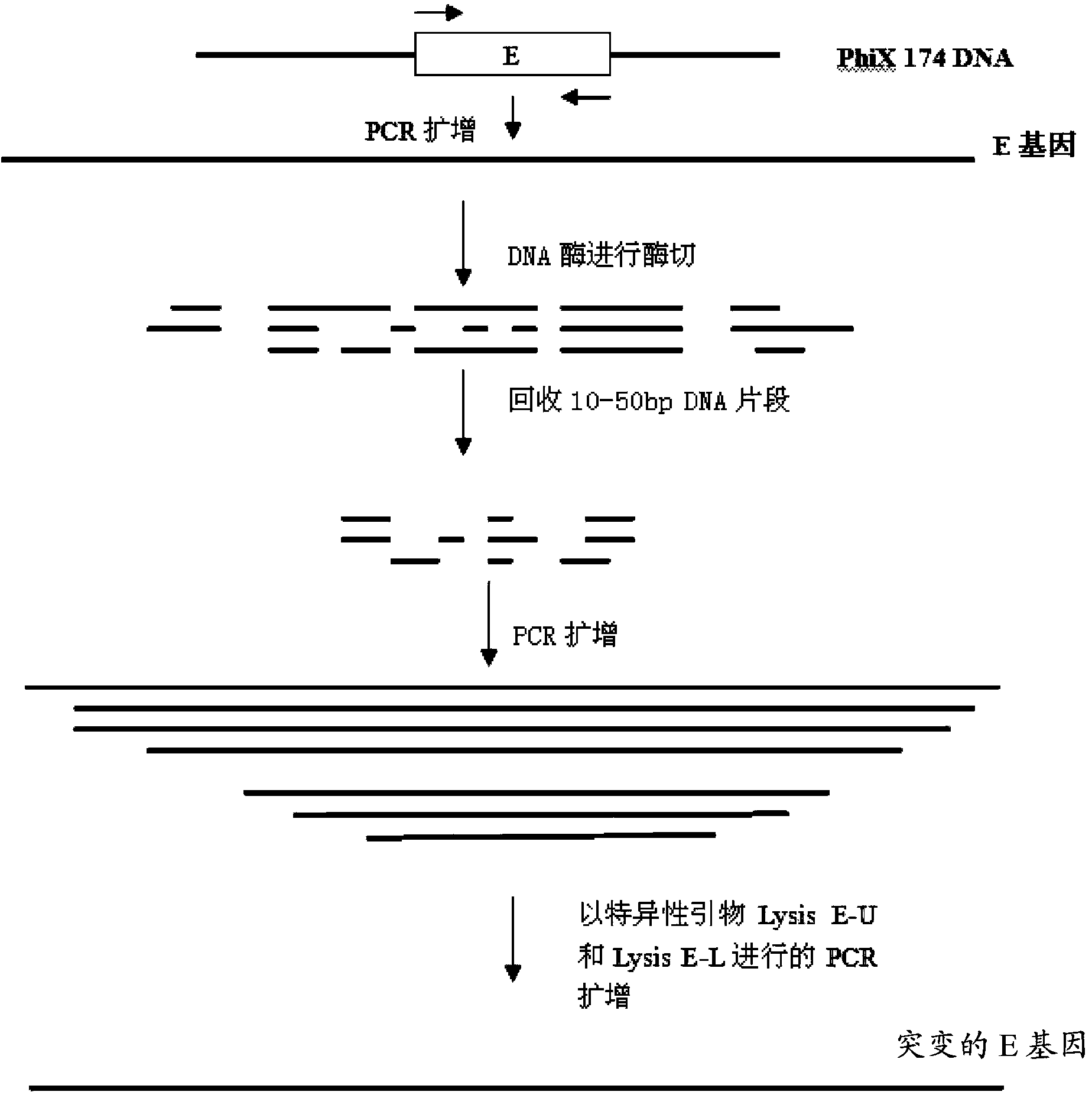
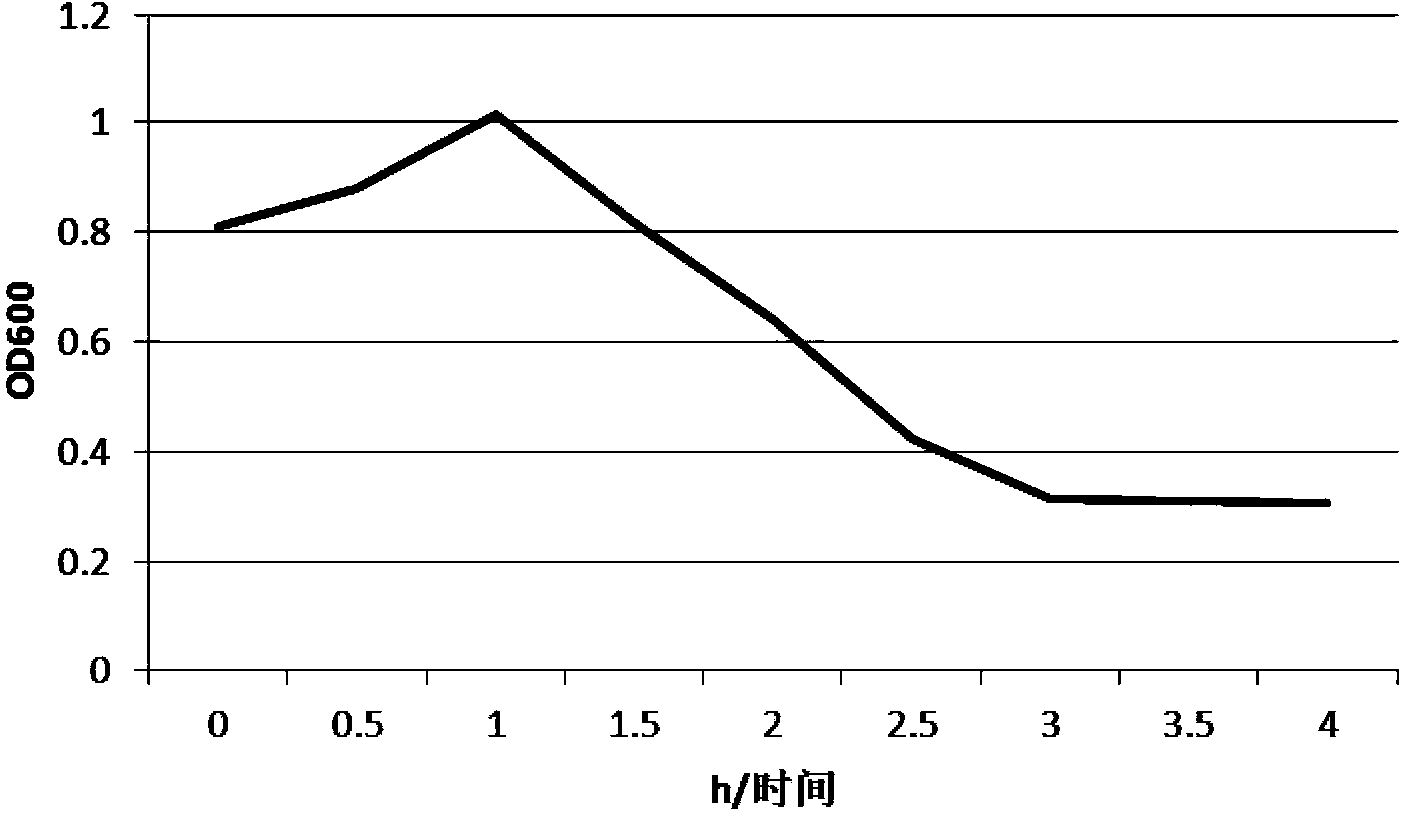
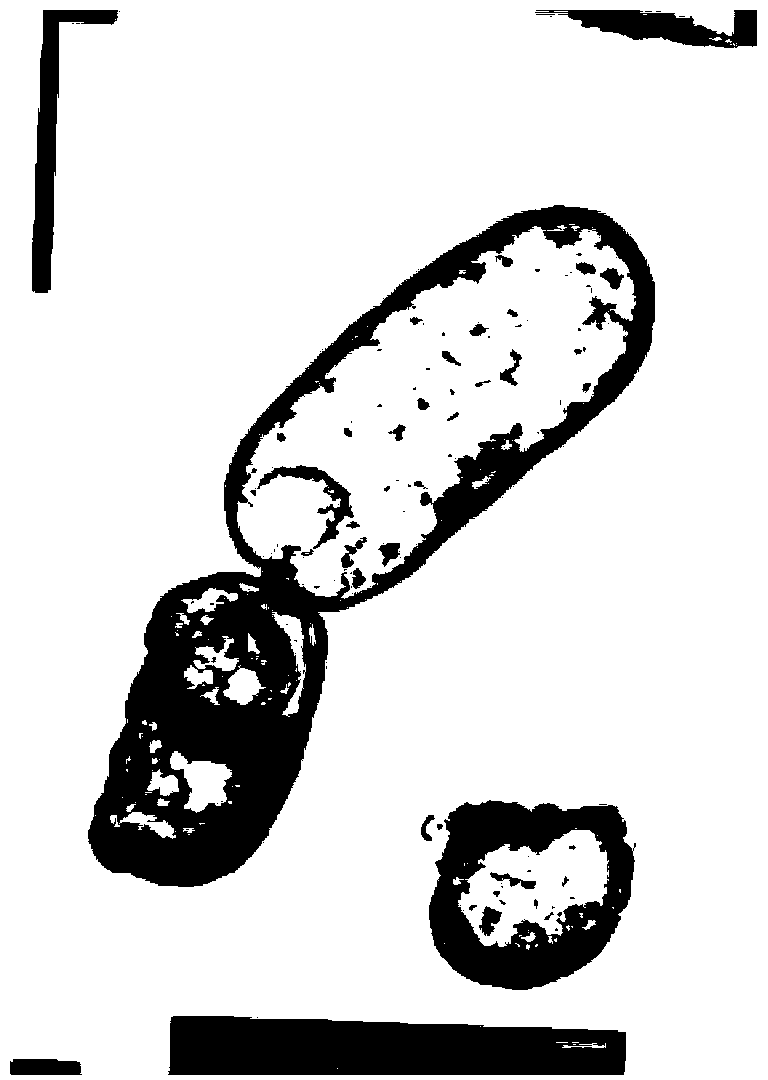
Mutant phage lysis gene E, lysis plasmid vector containing lysis gene and application in preparation of bacterial ghost vaccines
InactiveCN103451195ARepress transcriptionImprove cracking efficiencyAntibacterial agentsBacterial antigen ingredientsCulture bacteriaOrganism
The invention discloses a mutant phage lysis gene E, a lysis plasmid vector containing the lysis gene and an application in preparation of bacterial ghost vaccines. The mutant phage lysis gene E (Eprom) disclosed by the invention is obtained after mutation of a promoter region of the lysis gene E of a phage phiX174, the E gene after mutation can turn the temperature of culture bacteria from the existing 28 DEG C to 37 DEG C, the mutant phage lysis gene E further has higher lysis efficiency, higher starting induction concentration and large-scale production capacity, and the culture lysis efficiency of a fermentation tank is as high as 99.99997%. After the Eprom is connected with pBV220, the high-efficient lysis plasmid vector pBV-Eprom can be obtained. The pBV-Eprom is transformed into actinobacillus pleuropneumoniae to induce the gene expression of the Eprom so as to get a bacterial ghost of the actinobacillus pleuropneumoniae. Porcine transmissible pleuropneumonia bacterial ghost vaccines disclosed by the invention has good safety and immune protection efficacy and can stimulate organisms to produce high-titer antibodies and further provide good cross immunoprotection against attacks of different serotypes of actinobacillus pleuropneumoniae virulent strains.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Compound preparation for treating diseases caused by poultry sensitive bacteria and preparation method thereof
InactiveCN101987105ALess irritatingProlong the action timeAntibacterial agentsTetracycline active ingredientsEscherichia coliAntioxidant
The invention relates to a compound preparation for treating diseases caused by poultry sensitive bacteria and a preparation method thereof. The compound preparation is a long-acting injectable suspension prepared from florfenicol and occrycetin as main medicines and comprises 2.0-20.0% (W / V) of florfenicol, 5.0-30.0% (W / V) of occrycetin, 0.05-0.40% (W / V) of antioxidant, 1-10% (W / V) of antiallergic factor synergist, 0.01-0.04% (W / V) of complexing agent, 0.01-15% (W / V) of suspending agent, 1.0-10.0% (W / V) of flocculating agent, 0.01-0.5% (W / V) of preservative and 60-100% (W / V) of water for injection. The liquid medicine of the invention has stable performance and can effectively overcome the defects of instable performance, easy color change, precipitation and failure and the like of injections. The compound preparation has small irritativeness, low cost and simple process and can prolong the residence time of medicine in body and increase the bioavailability of the medicine. The compound preparation can be used for preventing and treating infectious diseases caused by poultry sensitive bacteria, such as pasteurellosis, colibacillosis, salmonellosis, contagious pleuropneumonia, mycoplasmlpneumonia of swine, acute respiratory infection and the like.
Owner:TIANJIN RINGPU BIO TECH
Method for preparing tiamulin fumarate effervescent granules for livestock and poultry
InactiveCN101897679AImprove bioavailabilityPreparation Technology ScienceAntibacterial agentsPharmaceutical delivery mechanismBiotechnologySodium bicarbonate
The invention relates to a method for preparing tiamulin fumarate effervescent granules for livestock and poultry, which comprises the following steps of: taking raw and auxiliary materials in part by weight for later use; mixing the weighed and crushed tiamulin fumarate, citric acid, polyethylene glycol 6000 in an amount which is one second of the total amount, dextrin and sucrose uniformly in a groove-shaped mixer, adding purified water into the mixture to prepare a soft material, granulating and drying the soft material, and settling the granules; weighing crushed sodium bicarbonate, mixing the reset auxiliary materials in the groove-shaped mixer uniformly, adding purified water into the mixture to prepare a soft material, granulating and drying the soft material, and settling the granules; and mixing the two kinds of dried granules uniformly in a three-dimensional mixer, and packing the mixed granules. The prepared tiamulin fumarate effervescent granules have the advantages of good mouthfeel and stable quality after oral administration of sick livestock and poultry, and are an ideal preparation for treating mycoplasma pneumonia, actinomycetic pleuropneumonia and treponema dysentery at present.
Owner:SRICK TIANJIN BIO TECH
A rapid detection kit for goat infectious pleuropneumonia and its preparation method
ActiveCN102277439AResolution timeSolve the workloadMicrobiological testing/measurementMicroorganism based processesTrue positive rateLoop-mediated isothermal amplification
The invention discloses a rapid detection kit for goat contagious pleuropneumonia and a preparation method thereof. The kit is established on the basis of molecular biology. The kit comprises 2*loop-mediated isothermal amplification (LAMP) reaction liquid, a primer mixer, BstDNA polymerase, a color-developing agent, a standard positive template and ddH2O, wherein the primer mixture comprises a forward inner primer FIP, a downward inner primer BIP, a forward outer primer F3 and a downward outer primer B3; and the ratio of the FIP to the BIP to the F3 to the B3 is 8:8:1:1. The kit has the characteristics of high sensitivity and specificity and the like, can react quickly, is easy to operate, does not need special instrument equipment and overcomes the defects of long pathogeny separation time, high workload, fussy detection method, complicated operation and the like of goat contagious pleuropneumonia, and the detection method is complicated, is difficult to operate and the like, and can be used for detecting whether clinical samples are infected with Mycoplasma capricolum subsp. Capripneumonia as well as performing early diagnosis and molecular epidemiological survey of goat contagious pleuropneumonia.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, hybridoma cell strain and application
InactiveCN102876635ANo cross reactionStrong specificityImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliBordetella
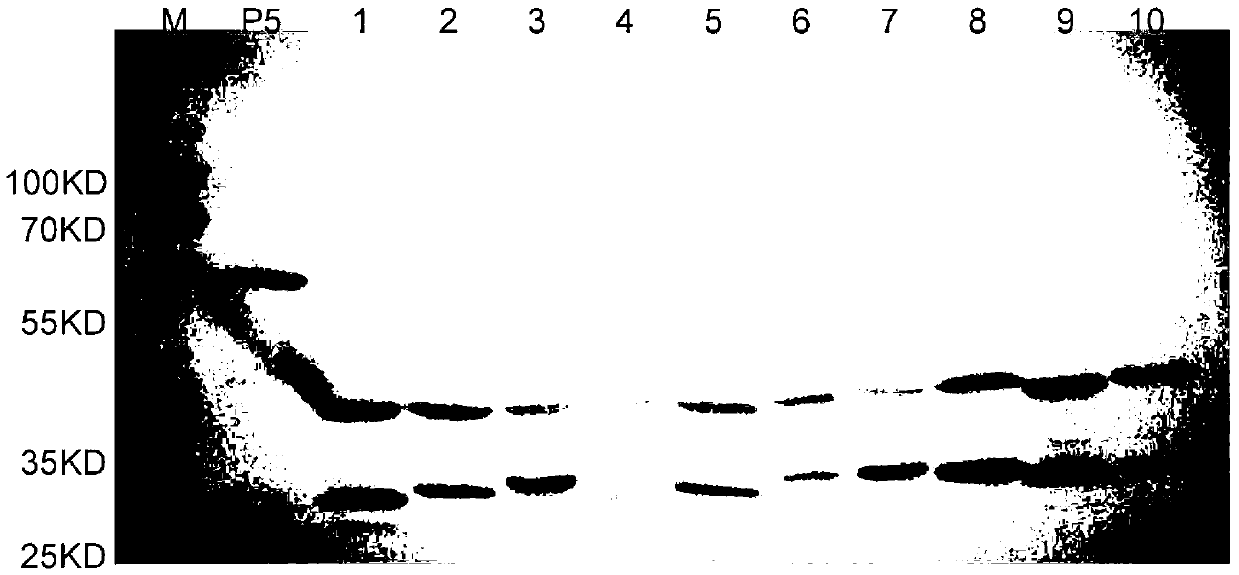
The invention discloses a haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, a hybridoma cell strain and an application. The hybridoma cell strain is preserved in the China center for type culture collection (CCTCC), and the preservation serial number is CCTCCC2012135. The monoclonal antibody prepared by the hybridoma cell strain is good in specificity, high in valence, high in generality, free from cross reaction with swine Escherichia coli, swine pasteurella, swine pleuropneumonia actinobacillus, streptococcus suis and swine bordetella bacilli, capable of detecting haemophilus parasuis with different serotypes and widely applicable to etiology diagnosis, serology detection and immunology detection and prevention of haemophilus parasuis diseases, and the enzyme-linked immuno sorbent assay (ELISA) antibody valence can reach 1:204800 after purification.
Owner:广东省农业科学院兽医研究所
Antibacterial antivirus Chinese medicinal composition for animal
ActiveCN101584747BAvoid defects with large fluctuations in qualityQuality assuranceAntibacterial agentsFood processingBiotechnologyPoultry disease
The invention discloses an antibacterial antivirus Chinese medicinal composition for the animal, comprising the following main medicament ingredients: scutellaria extract, honeysuckle extract, isatis root extract and glycyrrhiza extract. the pharmaceutical composition of the invention can be made into premix, injection, oral liquid or granula, also can be made into feedstuff premix adding into thefeedstuff. the chinese medicinal composition of the invention is a pure Chinese medicinal compound preparation which has the antibacterial, anti-inflammation, antivirus and immunity-reinforced functionsand wide application range, can effectively prevent and treat various livestock and poultry diseases caused by the virus and the bacteria, such as piggyyellow-white dysentery, swine plague, infectious pleuropneumonia, asthma, canker, circovirus, swine influenza, white diarrhea, newcastle disease, bursa of Fabricius, egg drop syndrome, duck serositis and the like, and has fast effect and remarkable curative effect.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Mycoplasma bovis immune related protein, detection kit containing same and application thereof to detection of mycoplasma bovis antibody
The invention discloses a mycoplasma bovis immune related protein, a detection kit containing the same and application thereof to detection of a mycoplasma bovis antibody. The mycoplasma bovis immunerelated protein is named p28 protein, and the amino acid sequence of the protein is shown in SEQ ID NO.2. A sensitivity test proves that the mycoplasma bovis serum antibody ELISA detection kit (MbH kit) provided by the invention can detect positive serum with the dilution multiple of 1:2560 at the minimum; and a specificity test proves that the kit has the specificity of 97.8% and has no specificreaction with the positive serums of contagious bovine pleuropneumonia (CBPP), foot-and-mouth disease (FMD), bovine tuberculosis (MB), bovine viral diarrhea (BVDV) and infectious bovine rhinotracheitis (IBRV), and has good stability and high accuracy. The invention provides a new technical means for detecting a mycoplasma bovis antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method, formula and use method of bovine mycoplasma pneumonia inactivated vaccine
InactiveCN104857509AStrong targetingReduce Lesion IndexAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention provides a preparation method, a formula and a use method of a bovine mycoplasma pneumonia inactivated vaccine. The preparation method of the bovine mycoplasma pneumonia inactivated vaccine comprises the following steps: 1, selecting diseased cattle which naturally infect mycoplasma pneumonia and is dying, taking diseased lungs, lymph glands and spleens to homogenize and removing precipitates and grease through filtering and centrifugation to obtain an antigen solution; 2, adding formaldehyde into the antigen solution to inactivate for 72 hours, adding 1,000 units of penicillin and 1,000 units of streptomycin into the antigen solution per millimeter and uniformly mixing to obtain an inactivated antigen solution; 3, mixing the inactivated antigen solution with an aluminum hydroxide adjuvant according to the proportion of 4:1 to obtain the inactivated vaccine. The vaccine prepared by the preparation method is safe in application, strong in pertinence and indeed in immune protection effect on mycoplasma causing bovine pleuropneumonia by infection, the lung lesion score of the infected cattle can be obviously reduced, the material weight ratio is improved, and the good effects of prevention and disease control can be achieved.
Owner:福清市默克兽医院
Veterinary compound hydrochloric acid injection and preparation method thereof
InactiveCN102151263AGood effectEasy to prepareAntibacterial agentsOrganic active ingredientsProtozoaDisease
The invention relates to veterinary compound hydrochloric acid injection and a preparation method thereof. The veterinary compound hydrochloric acid injection consists of oxytetracycline dihydrate injection, imidocarb and diclofenac sodium; the veterinary compound hydrochloric acid injection per 100 ml comprises the required raw materials of: 10-25g of oxytetracycline dihydrate injection, 1.5-5g of diclofenac sodium, 0.1-0.2g of imidocarb, 0.2-0.6g of sodium formaldehyde sulphoxylate, 5-15g of magnesium chloride, 5-13 ml of ethanolamine and 60-81 ml of organic solvents, wherein the allowance is water for injection. The veterinary compound hydrochloric acid injection has remarkable effect when being used for treating acute respiratory infection caused by eperythrozoon suis, babesiosis and other blood protozoa diseases, porcine respiratory disease complex (PRDC) and swine influenza virus (SIV), airway inflammation induced by porcine reproductive and respiratory syndrome (PRRS), haemophilus parasuis, pasteurella, pleuropneumonia and mycoplasma diseases; and the preparation method is simple and easy to operate, and is suitable for batch production.
Owner:XUCHANG TIANYUAN BIOLOGICAL TECH CO LTD
Preparation method for compound tilmicosin enteric-coated granules
ActiveCN104586875AIncrease payEffective infectionAntibacterial agentsOrganic active ingredientsOral glucoseDisease
The invention belongs to the technical field of veterinary medicines, and specifically relates to a preparation method for compound tilmicosin enteric-coated granules. The preparation method sequentially comprises the steps of: preparing materials, mixing the materials, granulating, preparing a coating solution, coating and the like, wherein the raw materials of the granules are tilmicosin, colistin, gatifloxacin, a sweetening agent, a flavouring agent, bromhexine hydrochloride, oral glucose, corn starch, saccharose powder, dextrin, talcum powder and starch slurry; the raw materials of the coating solution are HPMC, Tween-80, polyethylene glycol 6000, talcum powder and ethanol. The method disclosed by the invention enables the tilmicosin effective ingredient not to be broken in gastric acid, and to safely arrive at intestinal tracts to be absorbed; the obtained compound tilmicosin enteric-coated granules are capable of preventing and treating respiratory diseases such as mycoplasma diseases, pleuropneumonia and swine plague, capable of reducing the occurrences of bacterial diseases such as diarrhoea and salmonellosis, and capable of increasing the feed conversion.
Owner:ZHENGZHOU DOURIN VETERINARY TECH
Compound florfenicol injection and preparation method thereof
ActiveCN102755337AToxicShould not be used in combinationAntibacterial agentsTetracycline active ingredientsDoxycycline HyclateOfloxacinum
The invention discloses compound florfenicol injection and relates to the field of veterinary medicine preparation. The compound florfenicol injection is prepared from florfenicol, doxycycline hyclate, ofloxacin, dimethylacetamide (DMAC), propylene glycol and absolute ethyl alcohol. The invention also provides a preparation method of the compound florfenicol injection. As a novel veterinary compound preparation, the compound florfenicol injection has the characteristics of strong synergistic effect and low dosage, can effectively treat porcine infectious pleuropneumonia and has the important significance for sustainable development of the animal husbandry.
Owner:山东中牧兽药有限公司
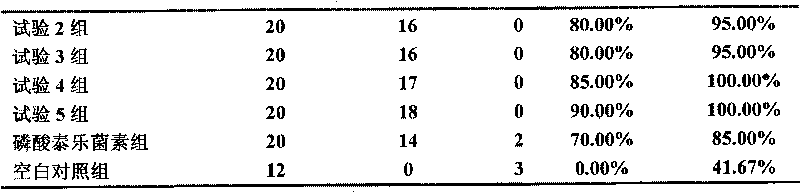
Traditional Chinese medicine for treating respiratory disease of livestock and preparation method thereof
ActiveCN101664485AGood curative effectQuick resultsClimate change adaptationAnimal feeding stuffDiseaseInfectious laryngotracheitis
The invention discloses a traditional Chinese medicine for treating respiratory disease of livestock and a preparation method thereof. The traditional Chinese medicine comprises five traditional Chinese medicinal materials of herba violae, turkey corn, aster, herba ephedra and radix scutellariae. The traditional Chinese medicine can be effectively used for treating diseases of swine enzootic pneumonia, swine contagious pleuropneumonia, porcine reproductive and respiratory syndrome, swine plague, swine influenza, infectious bronchitis of chicken, infectious laryngotracheitis of chicken and thelike, and has the advantages of environment protection without toxic and side effects, simple preparation process, low production cost and the like.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Drug for curing porcine contagious pleuropneumonia and preparation method thereof
ActiveCN103656315AGood curative effectQuick resultsAntibacterial agentsAmphibian material medical ingredientsSide effectStephania
The invention discloses a drug for curing contagious pleuropneumonia and a preparation method thereof. The drug comprises the following raw crude drugs: magnolia bark, vernonia cinerea, oviductus ranae, peppergrass, scutellaria root, goldthread root, humble plant, conyzae herba, rhodiola root, bamboo shavings, rice distillate, common reed rhizome, semen raphani, Japanese raspberry root, mezoneuron, small fruit fig aerial root, head stephania root, lycoperdales, castanea seguinii and thermopsis lanceolata. The drug has the benefits that the drug takes heat clearing, fire purging, antisepsis, anti-inflammation, lung nourishing, yin nourishing, and cough and asthma relieving as therapeutic principles, has a good curative effect on curing contagious pleuropneumonia, is quick to become effective, has small toxic and side effects, and is low in cost.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Traditional Chinese medicinal composition for treating infectious pleuropneumonia of goats and preparation method of traditional Chinese medicinal composition
InactiveCN107349402AReduce appetiteClear boundariesAntibacterial agentsRespiratory disorderNitraria sibiricaSide effect
The invention discloses a traditional Chinese medicine composition for treating goat infectious pleuropneumonia and a preparation method thereof. 6-18 parts by weight of saxifrage, 9-27 parts by weight of Gingeria chinensis, and 10-30 parts by weight of Nitraria small fruit. The traditional Chinese medicine composition of the invention has the advantages of treating both symptoms and root causes, high overall curative effect, short course of treatment, not easy to produce drug resistance, low residue, small toxic and side effects, etc., and has very important economic and social benefits for promoting the healthy development of sheep raising industry.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Porcine actinobacillus pleuropneumoniae attenuated strain and porcine pleuropneumonia-preventing product prepared from same
InactiveCN104726387AReduced toxicityLow toxicityAntibacterial agentsBacterial antigen ingredientsBiotechnologyImmunogenicity
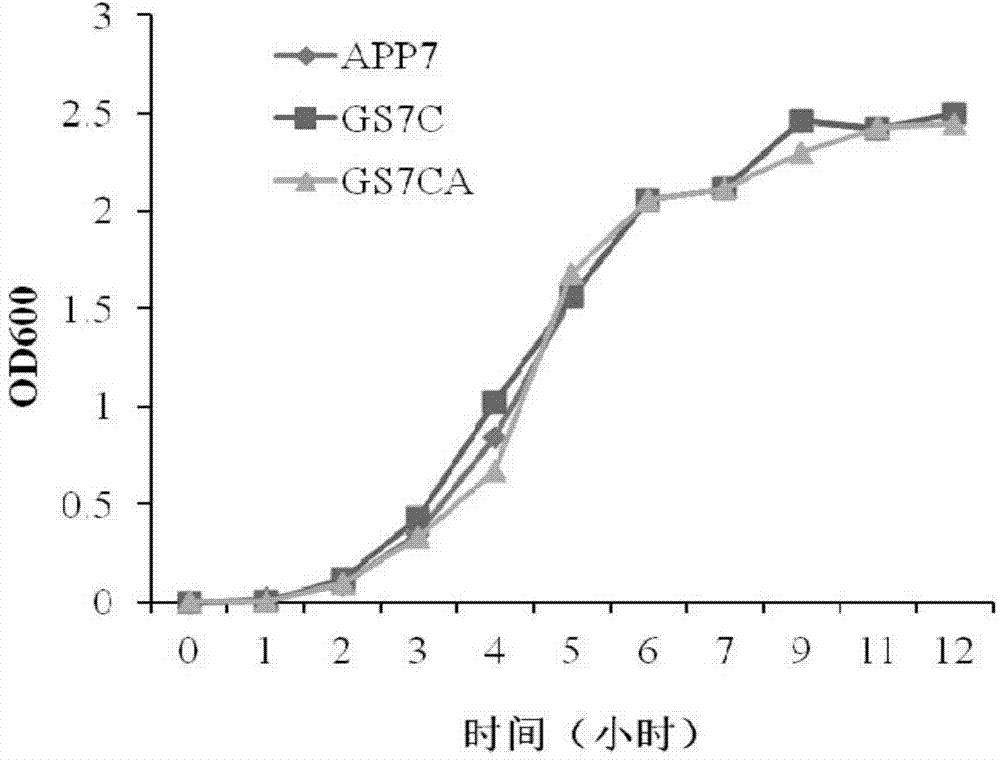
The invention discloses a porcine actinobacillus pleuropneumoniae attenuated strain and a porcine pleuropneumonia-preventing product prepared from the same, and relates to the fields of microorganisms and immunology. According to the porcine actinobacillus pleuropneumoniae attenuated strain and the porcine pleuropneumonia-preventing product prepared from the same disclosed by the invention, on the basis of a single mutant strain GS7C, a ureC gene segment in a genome is further deleted, and an apxIII-N gene segment with immunogenicity is inserted, and then the double mutant strains (GS7CA) of apxIIC- / apxIA+ and ureC- / apxIII+ which successfully express ApxIII-N proteins are screened by virtue of a sacB negative gene screening system, and the collection number is CGMCC NO. 10016. Experimental data indicates that, the hemolytic activity and urease activity of the double mutant strains are completely lost, the toxicity is greatly reduced, and stable inheritance can be realized; the attenuated live vaccine prepared by the porcine actinobacillus pleuropneumoniae attenuated strain disclosed by the invention has low toxicity, cross protection activity, high bio-safety and stable quality.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Triple fluorescent quantitative PCR primer and probe for detecting three sheep pathogenic mycoplasmas
InactiveCN109666753AEasy to operateStrong specificityMicrobiological testing/measurementMicroorganism based processesFluorescenceMycoplasma ovis
The invention provides a triple fluorescent quantitative PCR primer and a probe for detecting three sheep pathogenic mycoplasmas, wherein specific detection primers Mo-F, Mo-R and a fluorescent probeMo-P aiming at mycoplasma ovis are respectively designed, specific detection primers Mmc-F, mmc-R and probe Mmc-P aiming at mycoplasma filamentous subspecies of goat are designed, specific detection primers Mccp-F, Mccp-R and fluorescent probe Mccp-P for Mycoplasma goat subspecies of pneumonia. The triple fluorescent quantitative PCR method can simultaneously detect and identify three mycoplasmas,namely mycoplasma sheep pneumonia, mycoplasma goat pneumonia subspecies and mycoplasma filamentous goat subspecies, has the advantages of rapidness, accuracy, strong specificity, good repeatability and the like, can also save cost. The method is suitable for rapid detection of the three mycoplasmas and epidemiological investigation of mycoplasma ovis pneumonia and goat infectious pleuropneumoniacaused by the three mycoplasmas.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
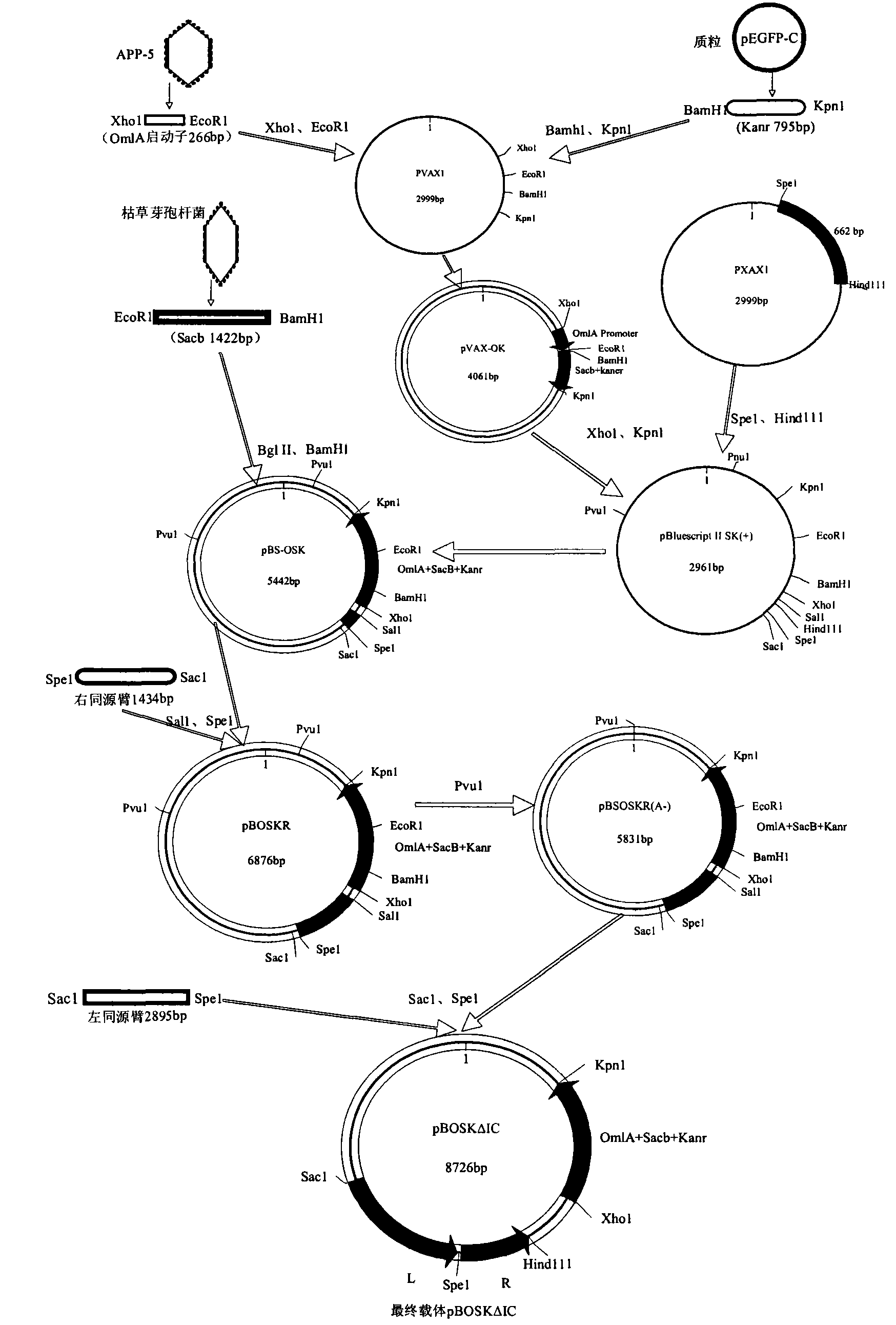
Apx I C gene deleted mutant strain of porcine infectious actinobacillus pleuropneumonia, construction method, vaccine and application
InactiveCN102618453ALow toxicityGood immune protectionAntibacterial agentsBacterial antigen ingredientsGenetic engineeringLong evans rats
The invention discloses a resistance marker-free Apx I C gene deleted mutant strain of porcine infectious actinobacillus pleuropneumonia. The number of the vaccine candidate strain is No. SW1delta I C. The strain is preserved in China Center for Type Culture Collection (CCTCC) on August 31, 2011, with a preservation number of CCTCC M 201130. The activating gene C of the toxin Apx I is deleted by means of genetic engineering, and the size of the deleted fragment is 475bp. The gene deleted strain constructed in the invention has genetic stability, and cannot reverse to virulence. In the invention, the gene deleted strain is also utilized to prepare a gene deleted attenuated vaccine. Meanwhile, immune efficacy tests of laboratory rats and piglets prove that the vaccine can induce good immune protection.
Owner:SICHUAN AGRI UNIV
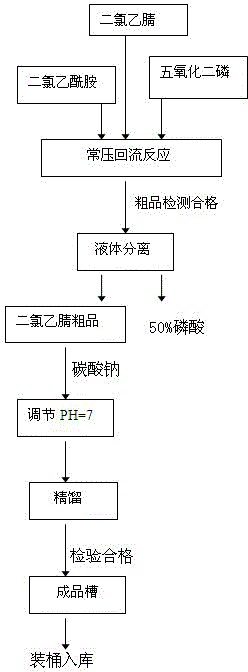
Novel production process for dichloroacetonitrile
InactiveCN106278945AReduce the temperatureLower reaction conditionsCarboxylic acid nitrile purification/separationReaction temperatureBacterial disease
The invention relates to a novel production process for dichloroacetonitrile, specifically to a novel synthetic process for a key intermediate dichloroacetonitrile of a fine chemical engineering product, i.e., florfenicol. Dichloroacetonitrile is a key intermediate of florfenicol, and florfenicol is a novel special wide-spectrum veterinary chloramphenicol antibiotic successively developed in later 80s and is used as a feed additive for a pig for prevention and treatment of swine bacterial diseases. When same dosages of florfenicol and spiramycin are used for treatment of respiratory diseases, the cure rate (91%) of florfenicol is substantially higher than the cure rate (41%) of spiramycin; and when 50 ppm of florfenicol is mixed with a feed for treatment of man-induced swine actinobacillus pleuropneumonia, a cure rate reaches 100%. The process provided by the invention mainly overcomes the problems of severe reaction conditions, high danger, high reaction temperature, slow reaction rate, low yield, poor controllability and the like of conventional processes. According to the process, dichloroacetamide and dichlorine pentoxide are subjected to a reflux reaction in a liquid environment with dichloroacetonitrile so as to obtain a crude product, and the crude product undergoes rectification so as to obtain a qualified product. The process has the advantages of mild reaction conditions, no pollution, high yield, high content, high added value of a byproduct and high economic benefits and environmental protection benefits.
Owner:HUBEI UNIV OF ARTS & SCI
Medicinal composition, as well as preparation process and application thereof
ActiveCN102846605AReduce decompositionRestore sensitivityAntibacterial agentsRespiratory disorderDiseasePharmaceutical Substances
The invention discloses a medicinal composition, which comprises the following components in parts by weight: 5 to 15 parts of amoxicillin, 1 to 5 parts of clavulanate potassium, and 1 to 5 parts of ambroxol hydrochloride. The invention discloses an application of the medicinal composition in preparing medicaments for treating animal respiratory infectious diseases, in particular for preparing porcine infectious pleuropneumonia and haemophilusparasuis. The invention also discloses a preparation method of compound amoxicillin soluble powder. The compound amoxicillin soluble powder comprises the following components in parts by weight: 5 to 15 parts of amoxicillin, 1 to 5 parts of clavulanate potassium, 1 to 5 parts of ambroxol hydrochloride, and 75 to 93 parts of glucosum anhydricum. The raw materials are uniformly mixed according to an equivalent incremental principle to obtain the compound amoxicillin soluble powder. The medicinal composition disclosed by the invention is reasonable and scientific in prescription, the bioavailability of amoxicillin can be increased compared with an amoxicillin preparation, the drug resistance is reduced, and the curative effect is improved.
Owner:JIUJIANG DACHENG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com