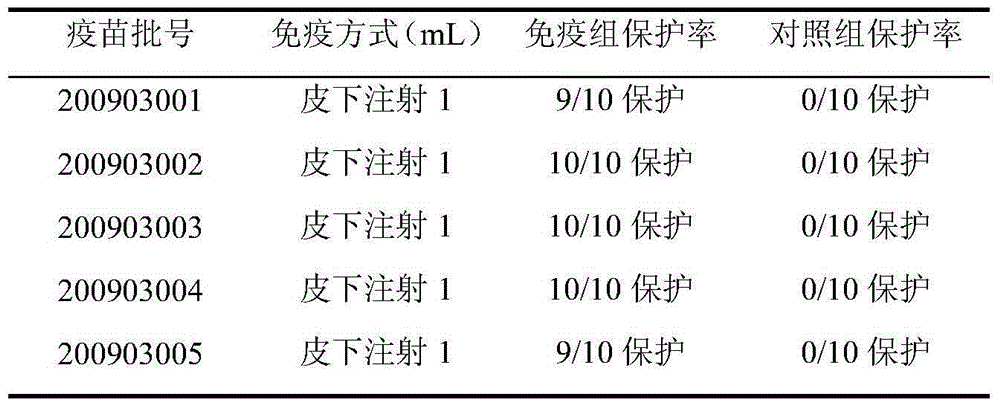
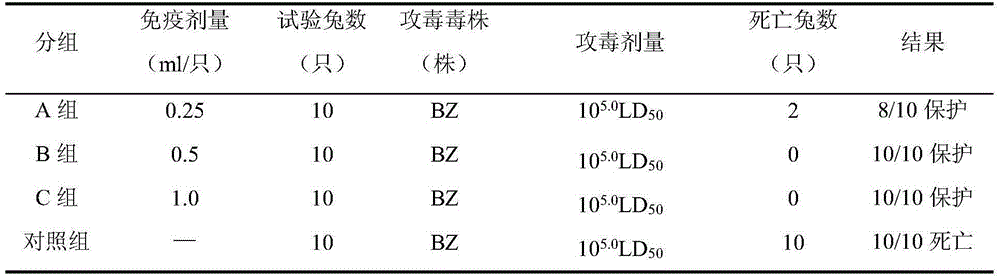
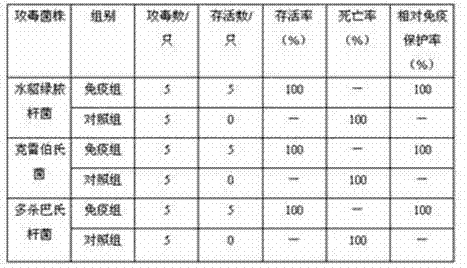
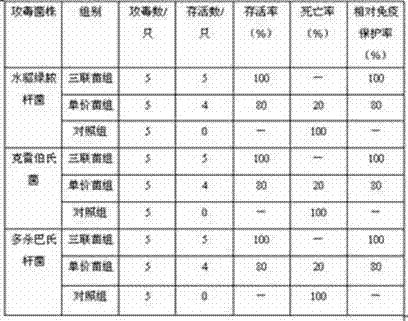
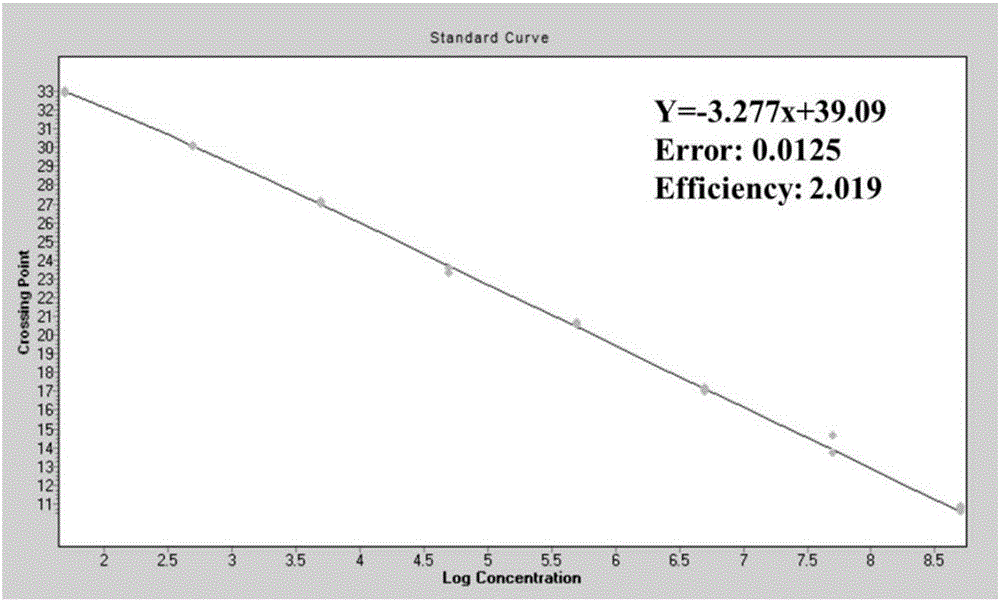
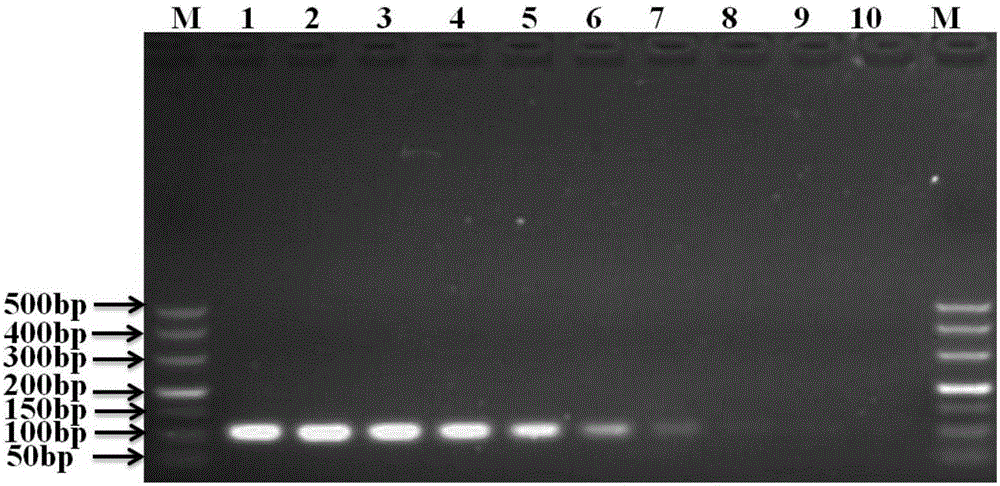
Patents
Literature
96 results about "Pasteurellosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pasteurellosis is an infection with a species of the bacterial genus Pasteurella, which is found in humans and other animals. Pasteurella multocida (subspecies P. m. septica and P. m. multocida) is carried in the mouth and respiratory tract of various animals, including pigs. It is a small, Gram-negative bacillus with bipolar staining by Wayson stain. In animals, it can originate in fulminant septicaemia (chicken cholera), but is also a common commensal.
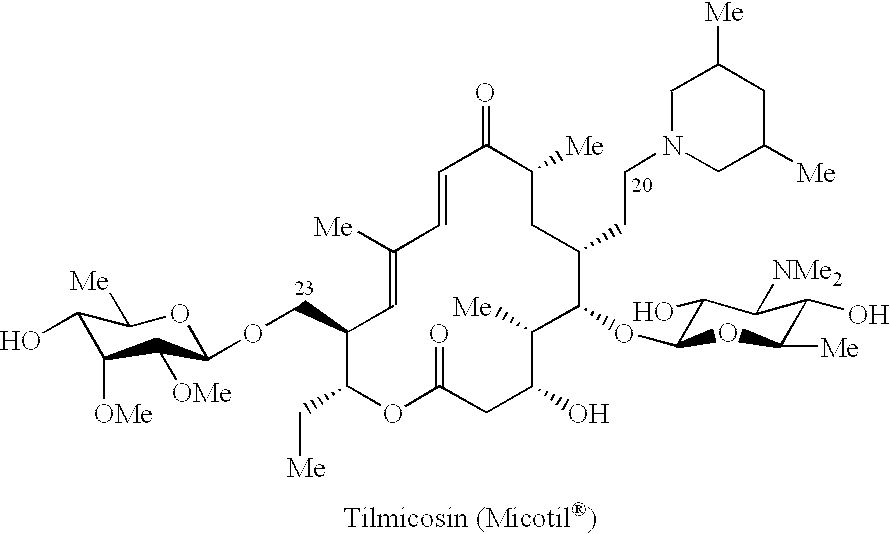
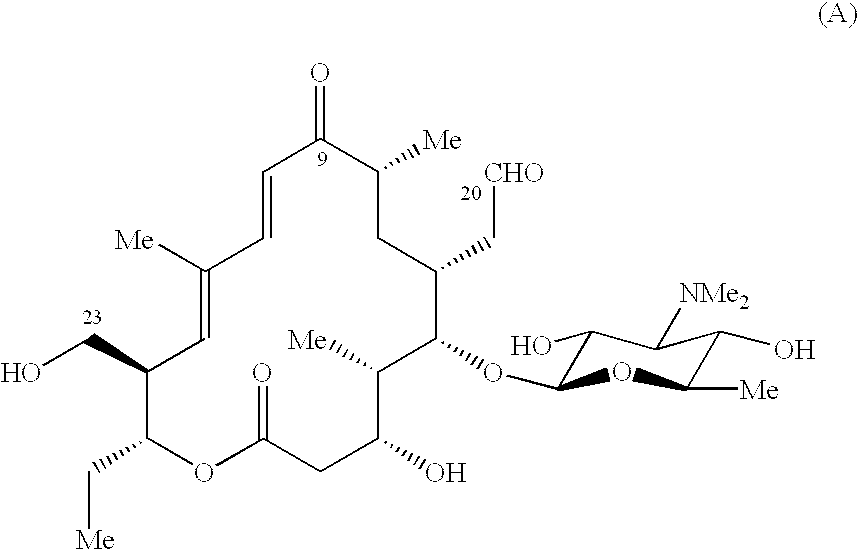
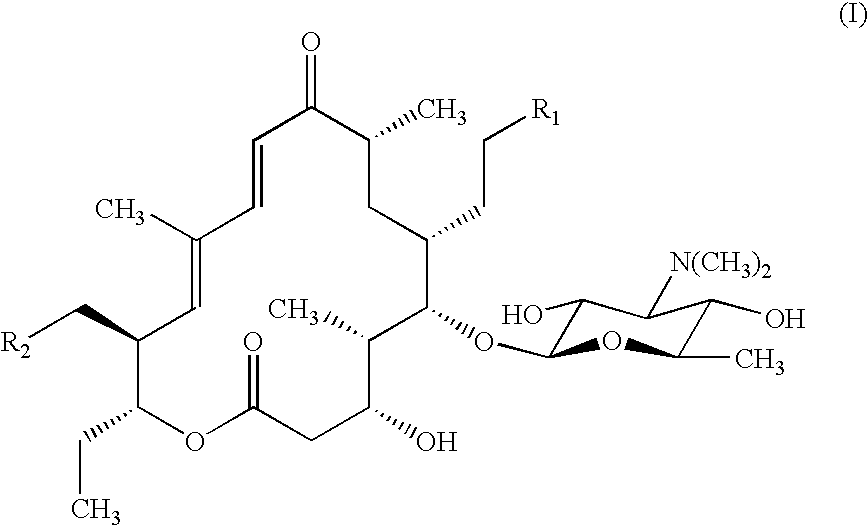
Macrolide antibiotics and treatment and prophylaxis of pasteurellosis using the same
ActiveUS6514946B1High antibacterial activityReduced activityAntibacterial agentsBiocideTreatment fieldAntibacterial activity
20,23-disubstituted mycaminosyltylonolide derivatives and use of the same in the field of the prophylaxis and treatment of pasteurellosis are disclosed. The di-substituents are peperidino optionally substituted with one or two methyl groups. The derivatives have selective antibacterial activity against Pasteurella.
Owner:ZH BISEIBUTSU KAGAKU KENYKU KAI
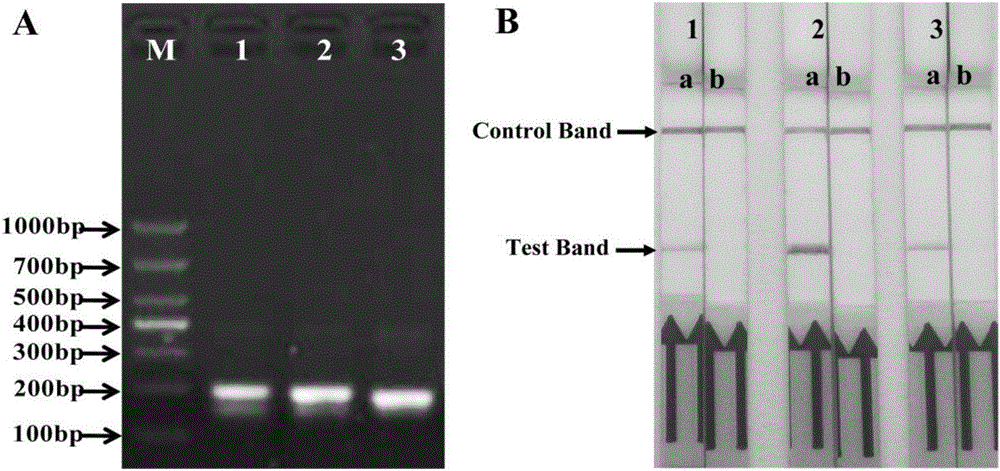
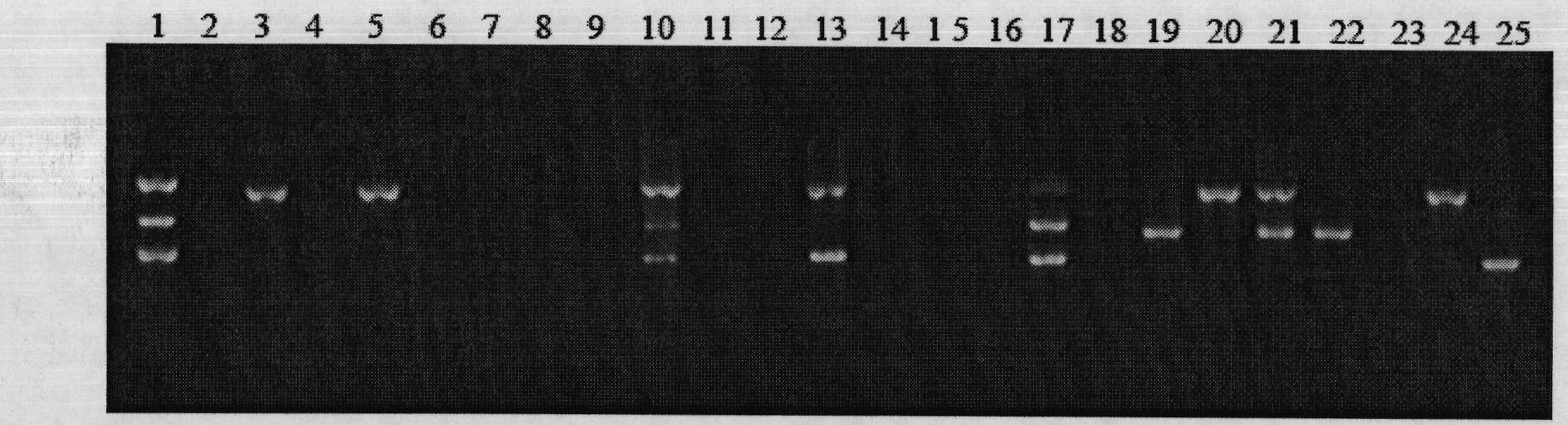
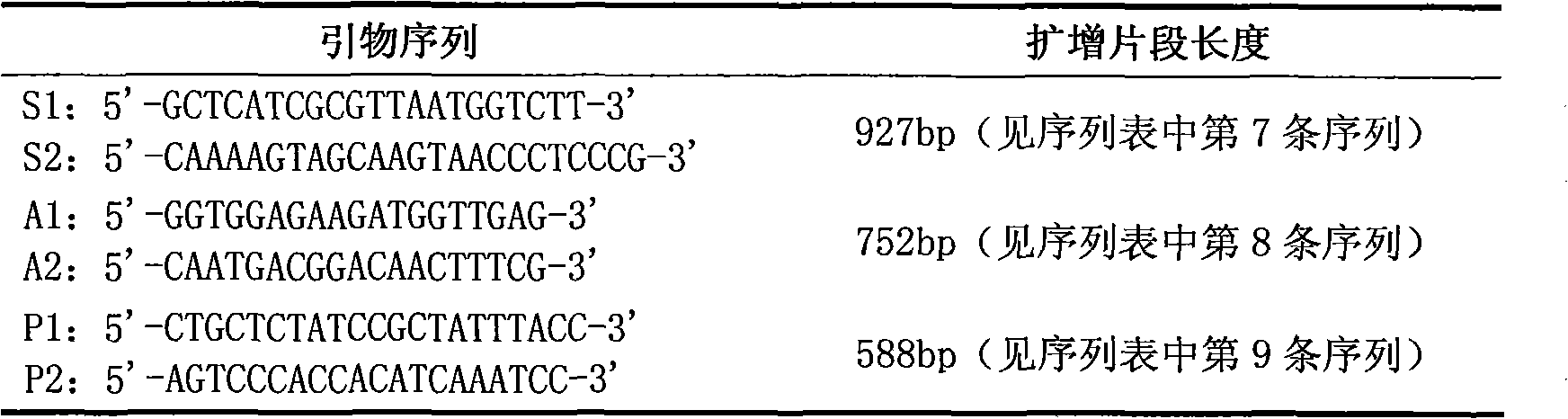
Primer, probe and kit for rapidly detecting pasteurella mutocida on site
InactiveCN106811541AExcellent detection timeNo cross reactionMicrobiological testing/measurementMicroorganism based processesForward primerCrude lysate
The invention discloses a combination of primer and probe for rapidly detecting pasteurella multocida on site by RPA-LFD. A forward primer sequence is shown in SEQ ID No. 1; a reverse primer sequence is shown in SEQ ID No. 2; a probe sequence is shown in SEQ ID No. 3. The invention also discloses a kit for detecting pasteurella multocida. The pasteurella multocida RPA-nfo detection combination of primer and probe and kit have high sensitivity and strong specificity, at least can detect six copied / reacted Pasteurella multocida DNAs, and can perform sensitive, specific and rapid detection of Pasteurella multocida DNA on crude lysate of a sample to be detected within 25 min by means of a constant-temperature water bath kettle or human armpit temperature without special instrument and equipment, so as to be suitable for the diagnosis of the pasteurella mutocida disease on site or base.
Owner:SHANDONG NORMAL UNIV
Bivalent inactivated vaccine for bovine multocida pasteurellosis and preparation method of bivalent inactivated vaccine
ActiveCN107569681AImprove securityImprove packaging utilizationAntibacterial agentsBacterial antigen ingredientsImmune effectsCapsular type
The invention relates to a bivalent inactivated vaccine for bovine multocida pasteurellosis, belonging to the technical field of preparation of veterinary biological products. The bivalent inactivatedvaccine provided by the invention is composed of antigens and a vaccine adjuvant, wherein the antigens are a bovine multocida pasteurellosis capsular type-A Pm-TJ strain and a bovine multocida pasteurellosis capsular type-B C45-2 strain. For the bivalent inactivated vaccine prepared by adopting the method provided by the invention, the concentration purifying process is adopted, the fermentationculture process of the bovine multocida pasteurellosis capsular type-B C45-2 strain is optimized, so that the fermentation time is shortened by one half, and the production efficiency is improved. Forthe bivalent inactivated vaccine provided by the invention, the bovine fibrinous and suppurative pneumonia and bovine hemorrhagic septicemia caused by bovine pasteurella multocida infection can be prevented through one-time injection immunization, and the bivalent inactivated vaccine has the features of safety, reliability and good immune effect.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Method for preparing actinobacillus pleuropneumoniae (App) bacterial ghost and method for preparing subunit vaccine by loading pasteurella antigen with App bacterial ghost
InactiveCN101934072APrevention of swine pleuropneumoniaPrevention of PasteurellosisAntibacterial agentsBacterial antigen ingredientsAntigenPleuronectes pinnifasciatus
The invention discloses a method for preparing an actinobacillus pleuropneumoniae (App) bacterial ghost and a method for preparing a subunit vaccine by loading a pasteurella antigen with the App bacterial ghost. A recombinant swine App bacterial ghost is prepared by controllable double-cracking technology and a pasteurella protection gene is introduced into an App bacterial ghost carrier, so that swine pleuropneumonia and a pasteurella bigeminal gene vaccine for preventing and treating swine pasteurellosis and swine pleuropneumonia are obtained. The preparation of the bacterial ghost carrier and the application of the bacterial ghost carrier to the prevention and treatment of important animal epidemic diseases are realized and a method is provided for the research of a multi-geminal gene vaccine at the same time. An animal experiment indicates that the protection rates of the bigeminal vaccine on infectious swine pleuropneumonia and pasteurellosis are up to 99 percent and 99.2 percent respectively.
Owner:TIANJIN AGRICULTURE COLLEGE
Novel antibacterial antiviral feed additive
InactiveCN102845607AImprove antibacterial propertiesLow inhibitory concentrationAntibacterial agentsAnthropod material medical ingredientsEscherichia coliMycotoxin
The invention discloses a novel antibacterial antiviral feed additive for a livestock and poultry breeding industry. The novel antibacterial antiviral feed additive is composed of plume poppy, lightyellow sophora root, macleaya microcarpa, yucconin, tea saponin, Origanum vulgare L., narcissus seed and pagodatree flower bud powder and also comprises a hydrophilic aluminosilicate as a carrier. The novel antibacterial antiviral feed additive has the advantages that specific active components of natural plants can directly or indirectly kill viruses, have strong antibacterial effects and a wide antibacterial spectrum and compared with the common drugs such as berberine hydrochloride, penicillin and aureomycin, the specific active components have stronger activities of resisting some bacteria; in feeding, antibiotic drugs are avoided and mycotoxins are reduced and good effects of treating and preventing avian pasteurellosis, escherichia coli, bacillus rhusiopathiae suis, cholera fowl, white scour of piglets, and pullorum disease are obtained; and the novel antibacterial antiviral feed additive can resist stress and coccidium and remove heavy metals and drug residues.
Owner:王茜 +1
Swine pasteurellosis bivalent inactivated vaccine and preparation method thereof
InactiveCN101766814AAvoid infectionPreventive Immunization SafetyAntibacterial agentsBacterial antigen ingredientsSerotypeP. multocida
The invention provides a swine pasteurellosis bivalent inactivated vaccine, which can prevent infection caused by two different capsule serotypes of pasteurella multocida, reaches the effect that one stitch of vaccine can prevent multiple infections, reduces the cost, does not have the hidden trouble of dispersing toxin and is safe and reliable. The invention also provides a preparation method for the swine pasteurellosis bivalent inactivated vaccine.
Owner:广东永顺生物制药股份有限公司
Compound preparation for treating diseases caused by poultry sensitive bacteria and preparation method thereof
InactiveCN101987105ALess irritatingProlong the action timeAntibacterial agentsTetracycline active ingredientsEscherichia coliAntioxidant
The invention relates to a compound preparation for treating diseases caused by poultry sensitive bacteria and a preparation method thereof. The compound preparation is a long-acting injectable suspension prepared from florfenicol and occrycetin as main medicines and comprises 2.0-20.0% (W / V) of florfenicol, 5.0-30.0% (W / V) of occrycetin, 0.05-0.40% (W / V) of antioxidant, 1-10% (W / V) of antiallergic factor synergist, 0.01-0.04% (W / V) of complexing agent, 0.01-15% (W / V) of suspending agent, 1.0-10.0% (W / V) of flocculating agent, 0.01-0.5% (W / V) of preservative and 60-100% (W / V) of water for injection. The liquid medicine of the invention has stable performance and can effectively overcome the defects of instable performance, easy color change, precipitation and failure and the like of injections. The compound preparation has small irritativeness, low cost and simple process and can prolong the residence time of medicine in body and increase the bioavailability of the medicine. The compound preparation can be used for preventing and treating infectious diseases caused by poultry sensitive bacteria, such as pasteurellosis, colibacillosis, salmonellosis, contagious pleuropneumonia, mycoplasmlpneumonia of swine, acute respiratory infection and the like.
Owner:TIANJIN RINGPU BIO TECH
Rumen-protected coated enrofloxacin mini-pill and preparation method thereof
ActiveCN105106175ADoes not affect ruminationEasy to useAntibacterial agentsOrganic active ingredientsDiseaseWhole body
The invention discloses a rumen-protected coated enrofloxacin mini-pill and a preparation method thereof, and solves the problems of conventional medicines that animals are easily caused to not ruminate and have digestive disorders. The rumen-protected coated enrofloxacin mini-pill is prepared from the following raw materials by weight: 5-15% of enrofloxacin, 60-80% of an auxiliary material and 10-25% of coating layer. The invention further provides the preparation method for the rumen-protected coated enrofloxacin mini-pill. Through oral medication, a medicinal component is not released in the rumen, and the medicinal component, namely enrofloxacin, is released when being dissolved after reaching the true stomach, so that an antibacterial action is directly played in intestinal tracts; enrofloxacin can also be used for treatment of general infection diseases of mycoplasma, pasteurellosis and the like after being absorbed into blood through intestinal tracts; animal rumination is completely unaffected, and the use is very convenient.
Owner:四川省川龙动科药业有限公司
Preparation method of spleen byproducts for producing homology anti-serum blood and transfer factor from fox, raccoon dog, mink
InactiveCN101422485AEasy to manufactureControl spreadAntibacterial agentsPeptide/protein ingredientsDiseaseBlood collection
The invention relates to a preparation method for obtaining blood used for preparing homologous antiserums and a spleen byproduct used for preparing transfer factors from the bodies of fur bearing animals such as foxes, raccoon dogs and minks; the furs of the foxes, the raccoon dogs and the minks are taken concentratedly in December and confirmed according to the fur-taking dates of different areas; healthy individuals are picked up 45 days before a predicated fur-taking date; the booster immunization of univalent vaccines or polyvalent vaccines of canine distemper, Canine parvovirus enteritis, adenovirus disease, corona virus laxness, parainfluenza, and pasteurellosis is carried out for 3 times by purified and condensed antigens on the foundation of carrying out immunization for once in summer; when the furs are taken, aseptic anticoagulation blood collection is carried out to a heart to improve the blood serum yield; and the spleens of the animals are collected for extracting specific or common transfer factors simultaneously. The blood can be conveniently prepared into the blood serums which resist communicable diseases, and the transfer factors can be extracted the spleen, thus providing guarantee for the healthy development of the breeding in the next year; the invention is used for curing the corresponding communicable diseases of the wild animals of the same species; and when a small amount of communicable disease cases appear in a breeding crowd, the corresponding pathogeny hyper-immune serums and transfer factors are mainly injected to a supposed healthy crowd after the pathogeny is confirmed so as to cure the affected animals in the early period of disease and control the spreading of the epidemic situations.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
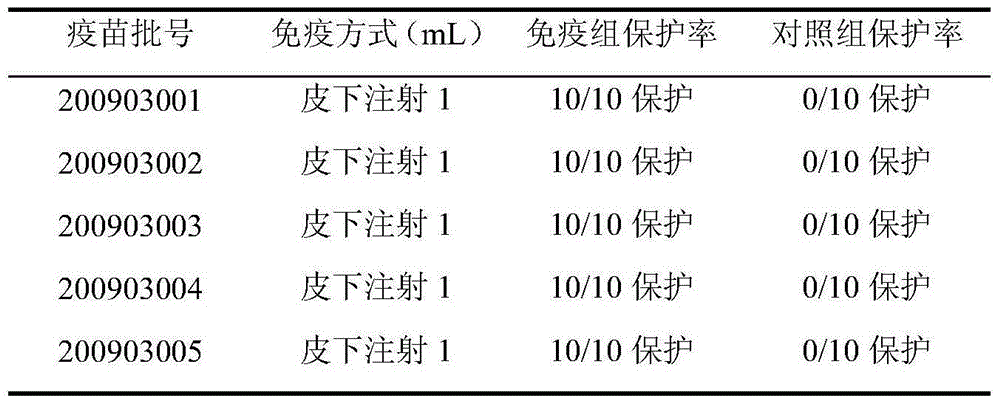
Fowl multi-killed pasteurellosis bacillus live vaccine
InactiveCN101081297ASimple processing methodProcess method scienceAntisepticsAntibody medical ingredientsSaccharumFreeze-drying
The present invention discloses one kind of live vaccine of fowl Pasteur's multocidum bacterium and its preparation process, and belongs to the field of biological product technology. The live vaccine is prepared with natural low virulent strain P7810 of fowl Pasteur's multocidum bacterium, and through steps of anabiosis and passage of the bacterium seed to form seed bacterium liquid, culturing to proliferate, adding gelatin in 1.5 % and cane sugar in 5 %, packing and freeze drying to obtain live vaccine of fowl Pasteur's multocidum bacterium P7810. The live vaccine is fed to fowl for immunizing to prevent fowl cholera, and has immunizing period as long as 6 months, high protecting rate, low cost and other advantages.
Owner:JIANGSU INST OF POULTRY SCI
Bivalent gene engineering vaccine of fowl infectious bursal disease and pasteurellosis
InactiveCN1522760AGood preventive immunityImprove securityAntibacterial agentsViral antigen ingredientsDiseaseP. multocida
The present invention discloses an avian infectious cloaced bursa disease and pasteurellosis bivalent gene engineering vaccine, relating to avian gene engineering vaccine. Said new vaccine is a pasteurella multocida C47-16 / p GEX-KG-VP2, CCTCC No:M203056, on pGEX-KG plasmid is connected an infectious cloacal bursa VP2 protein gene, after induced expression said vaccine can be used for immuniza chicken by means of oral administration or injection mode to make chicken body interior produce antibody for resisting infectious cloacal bursa virus, and when a standard strong strain is used to challenge virus, it can provide protection action for chicken. At the same time said carrier bacterium self-body is a safe effective low viulent vaccine strain for preventing pasteurellosis, so that said vaccine strain also can provide protection for resisting avian pasteurellosis.
Owner:YANGTZE UNIVERSITY
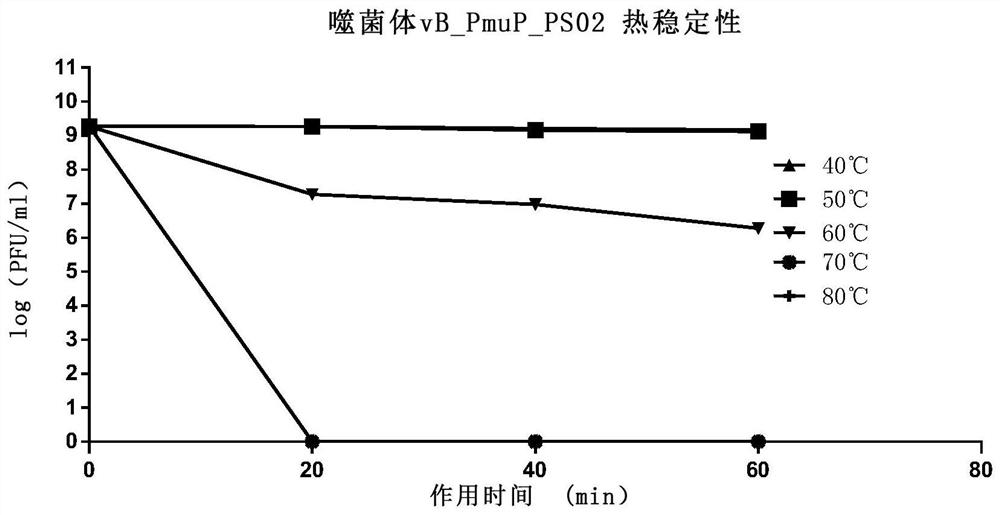
Pasteurella bacteriophage vB_PmuP_PS02 and bacteriophage composition and application thereof
ActiveCN111705042AStrong cracking abilityPrevention and Control of PasteurellosisAntibacterial agentsBiocideBiotechnologyDisease
The invention discloses a pasteurella bacteriophage vB_PmuP_PS02 and a bacteriophage composition and application thereof. The pasteurella bacteriophage vB_PmuP_PS02 is collected in the China General Microbiological Culture Collection Center on May 15, 2020, and has a collection number of CGMCC No.19972. The bacteriophage and the bacteriophage composition obtained by compounding the bacteriophage not only can be used for preparing medicines for preventing and treating various livestock diseases caused by pasteurella infection, especially have remarkable effects on swine plague and swine progressive atrophic rhinitis caused by swine pasteurella diseases, but also can be used for preparing swine feed additives, environment and feed disinfectants and the like. The bacteriophage and the bacteriophage composition thereof are safe to use and free of side effects, and the problems of antibiotic residues caused by traditional use of antibiotics and induction of drug-resistant pasteurella are effectively avoided.
Owner:QINGDAO PHAGEPHARM BIO TECH CO LTD
Compound florfenicol injection as well as preparation method and application thereof
ActiveCN103520211AFormulation ScienceStable in natureAmphibian material medical ingredientsAntibacterial agentsDiseaseSide effect
The invention provides compound florfenicol injection as well as a preparation method and application thereof. Each 1000ml of injection comprises 50-300g of florfenicol, 2.5-5g of venenum bufonis, 5-15g of oxymatrine, 5-15g of 654-2, 5-10g of vitamin B1, 0.1-1g of antioxidant, 100ml-800ml of first organic solvent, 100ml-800ml of second organic solvent and 0.1-1g of antioxidant. The compound florfenicol injection is prepared in a step-by-step dissolution mixing manner. The compound florfenicol injection is scientific in formula and has stable property; the components of the compound florfenicol injection have mutual enhancing effects so as to make up for the deficiency. The compound florfenicol injection has a relatively good antibacterial effect, relatively low toxic and side effects and a relatively short treatment course and can be used for preventing and treating diseases with respiratory symptoms, digestive tract symptoms and / or hyperpyrexia symptoms, which are caused by colibacillosis, salmonellosis, pasteurellosis and the like of beast and birds.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Bivalent inactivated vaccine for rabbit pasteurellosis and preparation method thereof
The invention relates to a bivalent inactivated vaccine for rabbit pasteurellosis and a preparation method thereof. The inactivated vaccine contains a rabbit type A pasteurella multocida PmA04 (CCTCC NO: M 2021202), and a rabbit type D pasteurella multocida PmD01 (CCTCC NO: M 2021201). The bivalent inactivated vaccine is prepared by using pasteurella multocida PmA04 and pasteurella multocida PmD01 as antigens for inactivation, and then adding a commercial MONTANIDETM GEL 02 PR adjuvant with the volume final concentration of 10% from the French Seppic Company. The bivalent inactivated vaccine can prevent infection of type A pasteurella multocida and typeD pasteurella multocida on rabbits at the same time, has the effect of preventing two diseases by one injection, is safe and reliable, and does not have the hidden danger of toxin dispersion.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Preparation method of egg yolk antibody for treating multocida pasteurellosis
InactiveCN102875673ANo pollution in the processHigh homologyEgg immunoglobulinsImmunoglobulins against bacteriaPasteurella multocida vaccineAntibiotic drug
The invention discloses a preparation method of an egg yolk antibody for treating multocida pasteurellosis. The preparation method is characterized by including the steps: (1) preparing a pasteurella multocida antigen; (2) immunizing an egg laying hen; and (3) extracting the egg yolk antibody. The preparation method has the advantages that combination of an acidified water extraction method with an octylic acid extraction method is preferable for preparing a pasteurella multocida vaccine, the preparation method is free from environmental pollution, and the extracted product is safe and effective to animals; the egg yolk antibody vaccine enables the egg yolk antibody to be directly acted on a hen body, and homology and effectiveness of the egg yolk antibody are higher than those of other biological agents; and the problem of residues of antibiotic drugs on meat, poultry and egg products is solved, production cost is low, and the quality of the egg yolk antibody is effectively improved.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
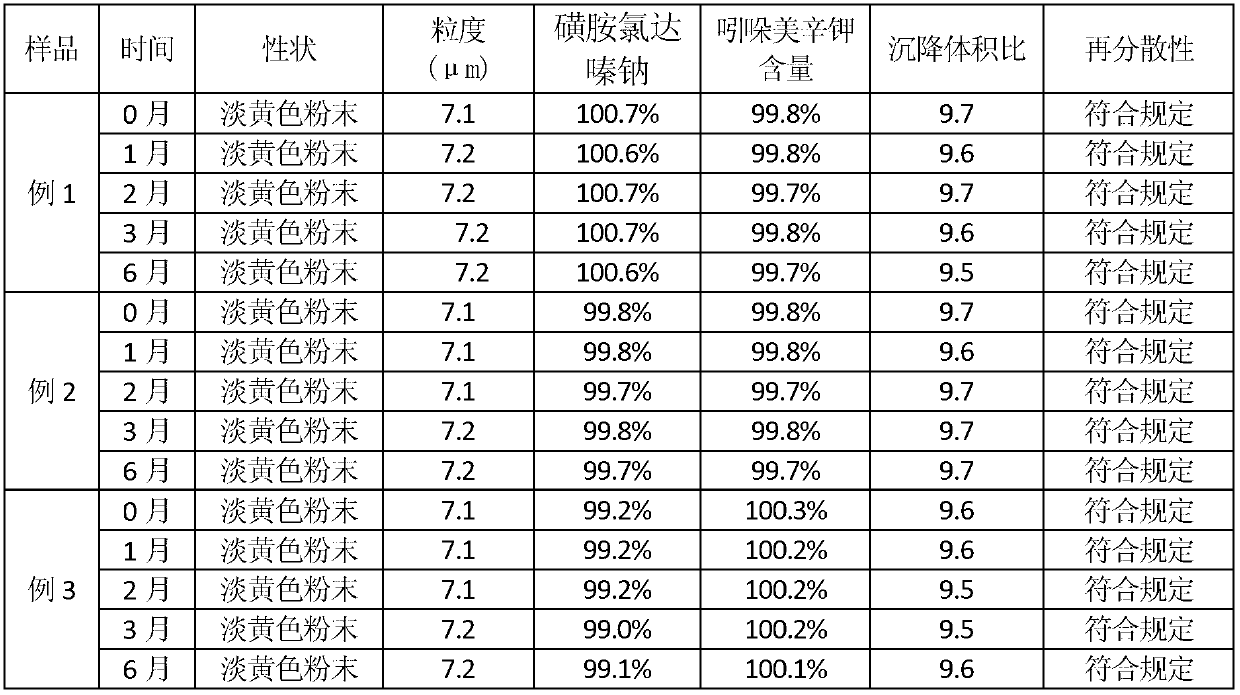
Compound sulfachlorpyridazine sodium powder for preventing and treating pasteurella infection of livestock and poultry
InactiveCN102846641ARapid drug actionPromote absorptionAntibacterial agentsOrganic active ingredientsPotassiumSuspending Agents
The invention relates to compound sulfachlorpyridazine sodium powder for preventing and treating pasteurella infection of livestock and poultry. The compound sulfachlorpyridazine sodium powder comprises the following components by weight: 60-65 parts of sulfachloropyridazine sodium, 2-4 parts of indometacin potassium, 10-25 parts of suspending agents, 2-4 parts of flavoring agents and acceptable carriers accounting for parts added to be 100 parts. The compound sulfachlorpyridazine sodium powder has definite effect on pasteurellosis of various animals and is suitable for being popularized and applied to breeding of livestock and poultry. According to the compound sulfachlorpyridazine sodium powder, the sulfachloropyridazine sodium and the indometacin potassium are compatible for use, so that when antibacterial and anti-inflammatory effects are achieved, the indometacin potassium serving as the antibacterial synergist of the sulfachloropyridazine sodium can have an synergistic effect to strengthen the drug effect of the main drug. The compound sulfachlorpyridazine sodium powder can be taken orally or mixed in feeds or drinking water, is good in absorption and safe in usage, does not produce drug resistance easily, can be used for health protection, prevention and treatment for a long time, has good palatability, flowability and suspension water-soluble performance, and is various in clinical dosing mode and convenient to dose.
Owner:金河牧星(重庆)生物科技有限公司
Triple inactivated vaccine for rabbit viral hemorrhagic disease, pasteurellosis and bordetella disease and preparation method of vaccine
ActiveCN110201153AProduced fastHigh immune attack protection rateAntibacterial agentsBacterial antigen ingredientsDiseaseBordetella
The invention relates to a triple inactivated vaccine for rabbit viral hemorrhagic disease, pasteurellosis and bordetella disease and a preparation method of the vaccine. The effective components of the vaccine comprise VP60 protein antigen of rabbit viral hemorrhagic disease virus, inactivated pasteurella multocida QLT-1 strain and bordetella bronchiseptica JN01 strain antigen. The triple inactivated vaccine has fast generation of protective antibody after immunization and high immune attack protection rate, with the attack protection rate for rabbit viral hemorrhagic disease virus AV-34 strain, pasteurella multocida QLT-1 strain and bordetella bronchiseptica JN01 strain all reaching above 80%. The results show that the vaccine is safe and reliable, and can be used for preventing the occurrence of rabbit viral hemorrhagic disease (rabbit plague), rabbit pasteurella multocida disease (type A) and rabbit bronchisepticaemia Bordetella disease.
Owner:QILU ANIMAL HEALTH PROD
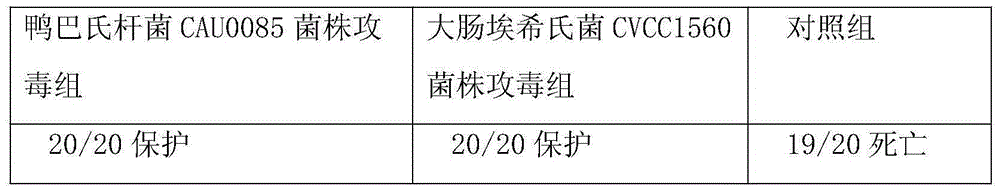
Pasteurellosis and duck colibacilosis combined propolis inactivated vaccine and preparation method thereof
InactiveCN105012946AImprove survival rateAntibacterial agentsAntibody medical ingredientsImmune effectsPropolis
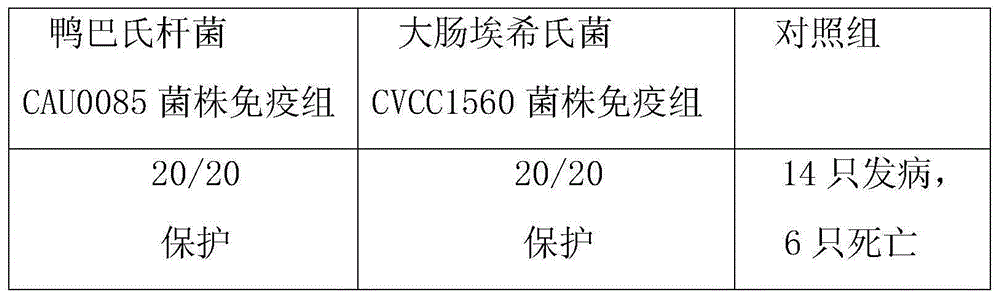
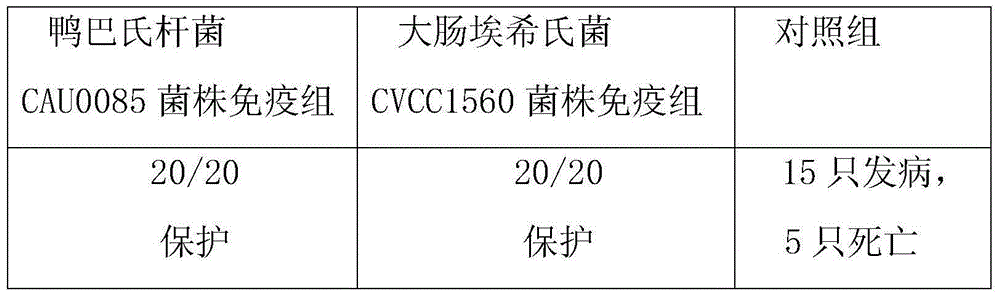
The invention belongs to the technical field of animal vaccine preparation, and particularly relates to a pasteurellosis and duck colibacilosis combined propolis inactivated vaccine and a preparation method thereof. Preparation of the combined propolis inactivated vaccine is completed by conducting propolis stock preparation, preparation of bacterial strains used for production, antigen liquid preparation, viable count, inactivation, ultrafiltration and concentration, vaccine security examination and protency examination on duck pasteurella CAU0085 bacterial strains and duck escherichia coli CVCC1560 bacterial strain antigens. The combined propolis inactivated vaccine is good in immune effect.
Owner:山东华宏生物工程有限公司
Rabbit complete formula granulated feed for preventing rabbit haemorrhagic disease
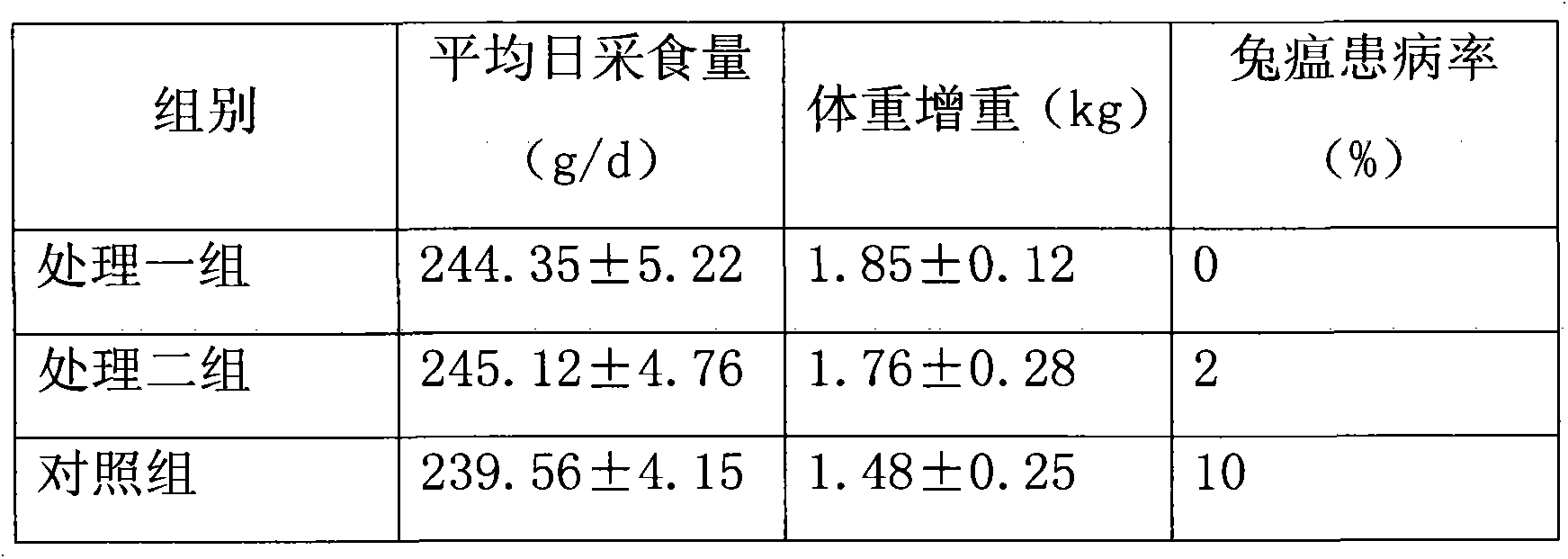
InactiveCN103349159AImprove immunityPromote healthy growthAnimal feeding stuffPasteurellosisSide effect
The invention discloses a rabbit complete formula granulated feed for preventing rabbit haemorrhagic disease. The feed comprises the following substances in percentage by weight: 26 percent of groundnut stem meal, 25 percent of bean cake, 22 percent of corn flour, 14 percent of wheat bran, 4 percent of sorghum, 2 percent of bone meal, 2 percent of yeast meal, 0.5 percent of edible salt, 0.3 percent of lysine, 0.2 percent of sodium sulfate, 2 percent of pine needle meal, 1.95 percent of traditional Chinese medicinal additive, and 0.05 percent of sulphaguanidine, wherein the traditional Chinese medicinal additive is isatis root or folium isatidis powder. The rabbit complete formula granulated feed for preventing rabbit haemorrhagic disease has the advantages of small toxic or side effect, no drug resistance, residue remaining difficulty and the like, the content of protein and vitamin in the feed is rich, the components are combined reasonably, and the isatis root or folium isatidis powder can be used for effectively preventing rabbit haemorrhagic disease, pasteurellosis and other fulminating infectious diseases; rabbits eating the feed are large in food intake, high in growing speed, and low in prevalence rate of rabbit haemorrhagic disease.
Owner:于琨
Bivalent propolis inactivated vaccine for rabbit hemorrhagic disease and multocida pasteurellosis and preparation method of bivalent propolis inactivated vaccine
InactiveCN105169380AReduce irritabilityExcellent immune protection rateAntibacterial agentsViral antigen ingredientsAntigenPropolis
The invention belongs to the technical field of animal vaccine preparation and particularly relates to a bivalent propolis inactivated vaccine for rabbit hemorrhagic disease and multocida pasteurellosis and a preparation method of the bivalent propolis inactivated vaccine. The preparation of the bivalent propolis inactivated vaccine is finished by using a rabbit hemorrhagic disease pathological tissue suspension and a multocida pasteurellosis liquid antigen through the steps of preparing a rabbit hemorrhagic disease production virus seed; preparing the rabbit hemorrhagic disease pathological tissue suspension; preparing a multocida pasteurellosis production seed; preparing a multocida pasteurellosis vaccine preparation liquid; preparing a propolis ethanol leachate; and preparing the bivalent propolis inactivated vaccine. The bivalent propolis inactivated vaccine disclosed by the invention has the immune protective rate of 100% for the rabbit hemorrhagic disease and the immune protective rate of 90% for the multocida pasteurellosis and is long in storage period; and meanwhile, due to the combination of the vaccine, the immune procedure is simplified, the labor is saved, and the immune cost is reduced.
Owner:山东华宏生物工程有限公司
A kind of preparation method of egg yolk antibody with high titer
InactiveCN106810607AHigh purityHigh potencyEgg immunoglobulinsImmunoglobulins against bacteriaYolkTreatment effect
The invention provides a method for preparing a high-titer egg yolk antibody. The method of the invention is used to obtain a higher titer egg yolk antibody by adjusting the process characteristics, which has specificity, good therapeutic effect and rapid curative effect on piglet Pasteurella disease. The preparation can effectively prevent and treat pasteurellosis in piglets; its preparation method adopts octanoic acid precipitation method to extract refined egg yolk antibodies against Pasteurella piglets, which is safe, low in price, suitable for large-scale production, and the prepared egg yolk antibodies are pure High, high potency, stable properties, easy to store.
Owner:TIANJIN ZHENBANG AQUACULTURE
Pseudomonas aeruginosa, klebsiella and pasteurella triple-inactivated vaccine for mink
ActiveCN104740622APrevention of mixed infectionHigh immune protection rateAntibacterial agentsAntibody medical ingredientsDiseaseMicrobiology
The invention discloses a pseudomonas aeruginosa, klebsiella and pasteurella triple-inactivated vaccine for a mink. The vaccine is composed of inactivated pseudomonas aeruginosa, klebsiella and pasteurella, and is capable of simultaneously preventing a pseudomonas aeruginosa disease, a klebsiella disease and a pasteurella disease, and mixed infection with pseudomonas aeruginosa, klebsiella disease and pasteurella disease. The vaccine is small in side effect, has synergistic effect, has relatively good immunogenicity and safety, and can reach multiple prevention effects; the immune procedure for vaccination is reduced; and the protection efficiency is improved.
Owner:JILIN HEYUAN BIOENG LIMITED
Gene diagnosis kit and detection method for major cattle pathogenic bacteria-Pasteurella multocida
ActiveCN104073567ASpecific detectionGuaranteed accuracyMicrobiological testing/measurementMicroorganism based processesFarming environmentP. multocida
The invention provides a gene diagnosis kit and detection method for major cattle pathogenic bacteria-Pasteurella multocida and belongs to the technical field of bioscience. The kit and the detection method are designed by taking a pair of primers as the main body, and the primers are designed according to the Pasteurella multocida gene conservative area sequence. According to the invention, the polymerase chain reaction (PCR) technology is adopted for qualitatively detecting the specificity DNA fragments of cattle pathogenic bacteria-Pasteurella multocida simply, conveniently and quickly, the specificity is good and the sensitivity is high; the kit and the detection method can be used for clinical detection of cattle pasteurellosis, bacteria tracking and detecting in all cattle farming periods and monitoring of the cattle farming environment and avoid pathogen transmission and outbreak, thereby achieving a very high utility value.
Owner:KUNMING DIANLONG BIOLOGICAL MEDICINE SCI & TECH
Multiple PCR detection method for porcine bacteria
InactiveCN101984073AQuick checkAccurate detectionMicrobiological testing/measurementP. multocidaMultiplex pcrs
The invention provides a multiple PCR detection method for porcine bacteria. Streptococcus suis type 2, Actinobacillus pleuropneumoniae and pasteurella multocida positive samples are detected by extracting the DNA of test samples, carrying out PCR amplification with PCR testing kit and analyzing the amplified products.The method of the invention can rapidly and accurately detect the samples infected with one or more of the above three bacteria. The template DNA preparation step of the method is simple and the cost is low. The method can exclude the interferences of bacteria and impurity particles, thus greatly improving diagnostic accuracy and reducing false positive rate. The method can also be used for the molecular epidemiological investigation and the efficacy monitoring of Streptococcus suis type 2, Actinobacillus pleuropneumoniae and pasteurella multocida.
Owner:ZHEJIANG UNIV
Preparation method of envelope protein A gene vaccine for fowl cholera
InactiveCN102139116BEasy to makeEasy to prepareGenetic material ingredientsGene therapyEscherichia coliEnzyme digestion
The invention discloses a preparation method of a nucleic acid vaccine for fowl cholera, which comprises the following steps of: designing primers by a fowl cholera bacterium genome sequence; carrying out PCR (polymerase chain reaction) amplification by taking a fowl cholera bacterium CVCC474 strain genome as a template to obtain a target gene; carrying out enzyme digestion on the target gene and an eukaryotic expression vector pcDNA3.1(+) by utilizing a restriction enzyme KpnI and a restriction enzyme EcoRI; then connecting the target gene with the eukaryotic expression vector pcDNA3.1(+); converting into the Escherichia coli competence JM83; extracting plasmids; and carrying out enzyme digestion identification to obtain the nucleic acid vaccine for fowl cholera. The animal experiment detection shows that the nucleic acid vaccine for fowl cholera prepared by the method disclosed by the invention can reduce the occurrence of fowl cholera and can reduce the intrusion of fowl cholera bacteria on fowls; and the preparation method of the nucleic acid vaccine is simple and easy to operate.
Owner:HENAN UNIV OF SCI & TECH
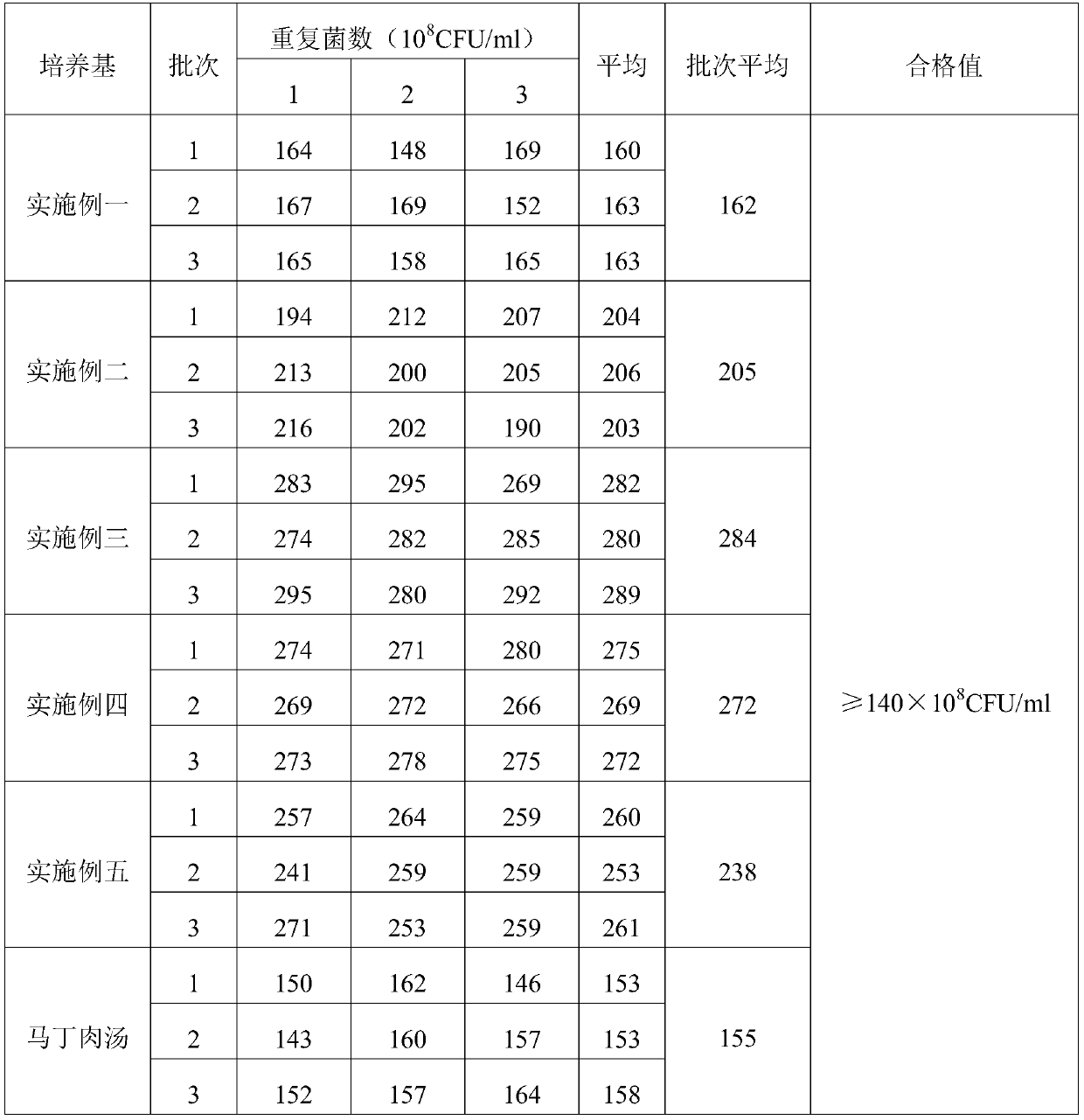
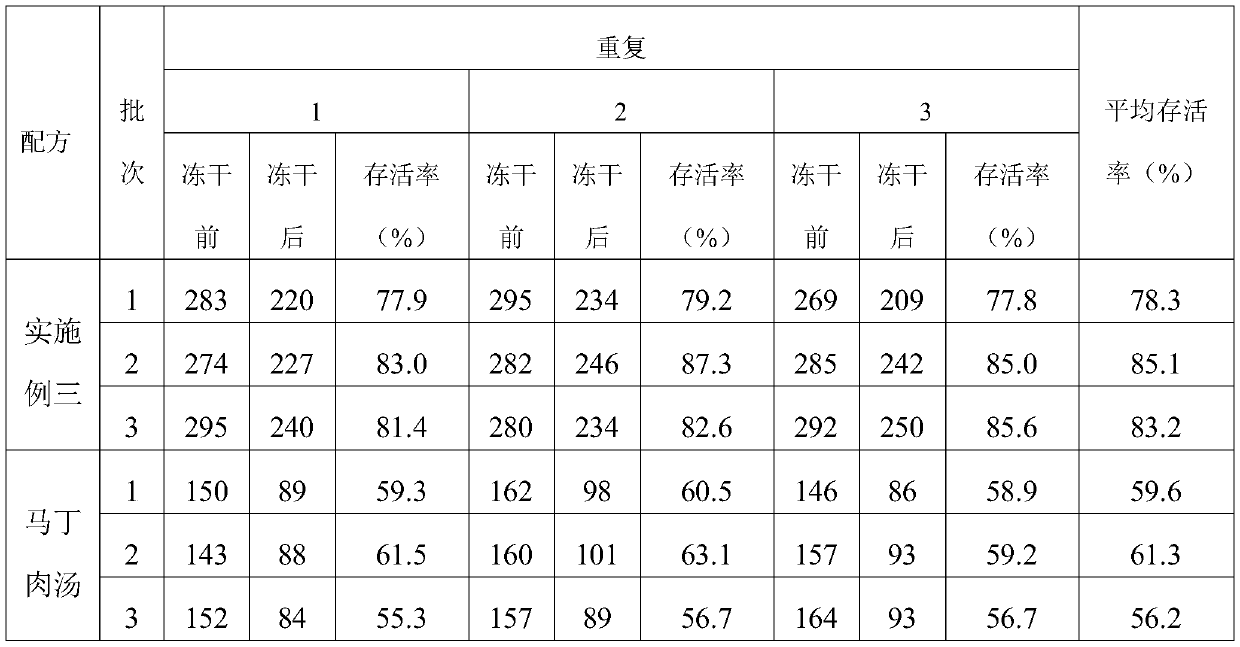
Swine pasteurellosis vaccine enrichment medium
PendingCN111040971APromote growth and reproductionRich in nutrientsBacteriaMicroorganism based processesBiotechnologyVaccine Production
The invention provides a swine pasteurellosis vaccine enrichment medium, and relates to the field of biology. The swine pasteurellosis vaccine enrichment medium is prepared from the following raw materials by weight: 20.0g of meat liver and stomach membrane digestive juice powder, 8.0g of bovine pancreas digestive juice, 10.0g of peptone, 5.0g of NaCl, 1.0g of a growth promoting factor, and purified water added to 1000ml. A preparation method of the swine pasteurellosis vaccine enrichment medium comprises the following steps: adding all the raw materials for preparing the swine pasteurellosisvaccine enrichment medium into purified water, fully dissolving the raw materials, and carrying out sterilization at 121 DEG C for 15 minutes for later use. The invention relates to an application ofa synthetic dry powder culture medium in swine pasteurellosis vaccine production. The synthetic dry powder culture medium has the beneficial effects that commercial raw materials can be selected, theculture medium is clear, the number of cultured bacteria is high, and the freeze-drying survival rate of the bacteria is increased.
Owner:青岛高科技工业园海博生物技术有限公司
Method for preparing poultry cholera microcapsule vaccine
InactiveCN101062409AHigh antigen contentLong release timeAntibacterial agentsAntibody medical ingredientsAntigenFreeze-drying
The invention discloses a preparing method of cholera fowl microcapsule vaccine, which comprises the following steps: recovering and passing A type multiple killing property pasteurellosis freeze-drying bacterial; producing seed bacterial liquid; culturing; breeding; getting half-finished product with bacterial number above 9 billion / ml; deactivating the half-finished product bacteria liquid; testing; proceeding isotrope with equivoluminal 2% sodium alginate solution through dispersing device; proceeding first emulsify; proceeding twice emulsify with the emulsion and equivoluminal 5% chitose solution through high pressure emulsifying device; dewatering the final emulsion through spray dryer; producing microcapsule vaccine dry powder. This method possesses scientific method, high antigen content, long slow release time, low toxic side action and low cost.
Owner:JIANGSU INST OF POULTRY SCI
Doxycycline hydrochloride soluble powder and preparation method thereof
InactiveCN109700767APrevention and treatment of complicationsPrevention and treatment of secondary infectionsAntibacterial agentsPowder deliveryDiseaseEscherichia coli
The present invention provides doxycycline hydrochloride soluble powder. The doxycycline hydrochloride soluble powder is composed of the following raw materials in mass percentages: 45-55% of doxycycline hydrochloride and 45-55% of anhydrous glucose. The present invention also provides a preparation method of the doxycycline hydrochloride soluble powder. The doxycycline hydrochloride soluble powder can effectively treat respiratory diseases caused by treatment of pig and chicken gram-positive bacteria and negative bacteria of escherichia coli, salmonellosis, pasteurellosis and mycoplasma, hasa good therapeutic effect, is quick in effects, can address both symptoms and root causes, reduces stress response when diseases occur, prevents and treats concomitant and secondary infections of themycoplasma and chlamydia, uses conventional equipment during preparation, and is simple in production method, low in material cost, and suitable for industrialized mass production of antibiotic premixes of poultry and livestock.
Owner:江西九信药业有限公司
Kit for detecting pasteurella multocida in environmental aerosol sample, and application of kit
InactiveCN106834516APracticalStrong specificityMicrobiological testing/measurementMicroorganism based processesFluorescenceP. multocida
The invention discloses a primer and probe combination for detecting pasteurella multocida in an environmental aerosol sample. The primer and probe combination is characterized by comprising a positive primer, a negative primer and a probe, wherein the sequence of the positive primer is as shown in SEQ ID NO.1, the sequence of the negative primer is as shown in SEQ ID NO.2, and the sequence of the probe is as shown in SEQ ID NO.3. The invention also discloses a kit for detecting the pasteurella multocida in the environmental aerosol sample. The content and the distribution condition of the pasteurella multocida in the environmental aerosol sample are subjected to real-time fluorescence quantitative PCR detection by using TaqMan, the occurrence risk of pasteurellosis is predicted, and reference is provided for determination of selection, use times and key disinfection areas of sanitary disinfection reagents of a field area.
Owner:SHANDONG NORMAL UNIV
Method for producing combined inactivate vaccine of escherichia coli disease and pasteurellosis in yak
InactiveCN105833261ASimple production processEasy to useAntibacterial agentsBacteriaDiseaseEscherichia coli
The invention relates to a method for producing a combined inactivate vaccine of escherichia coli disease and pasteurellosis in yak. The method disclosed by the invention, which makes use of a self-prepared culture medium, is simple in production process and low in cost; safety tests and immunity tests on the yak prove that the combined inactivate vaccine disclosed by the invention has an immunizing effect on the escherichia coli disease and pasteurellosis; the combined inactivate vaccine is more convenient to use than a single vaccine, and the combined inactivate vaccine is capable of preventing the two diseases just by immunizing once, so that the workload of immunization is reduced, and immunity paralysis and immunity failure caused by frequent immunization are avoided; and the combined inactivate vaccine has the advantages of being good in safety, high in immunizing efficacy, long in shelf life and the like.
Owner:青海生物药品厂有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com