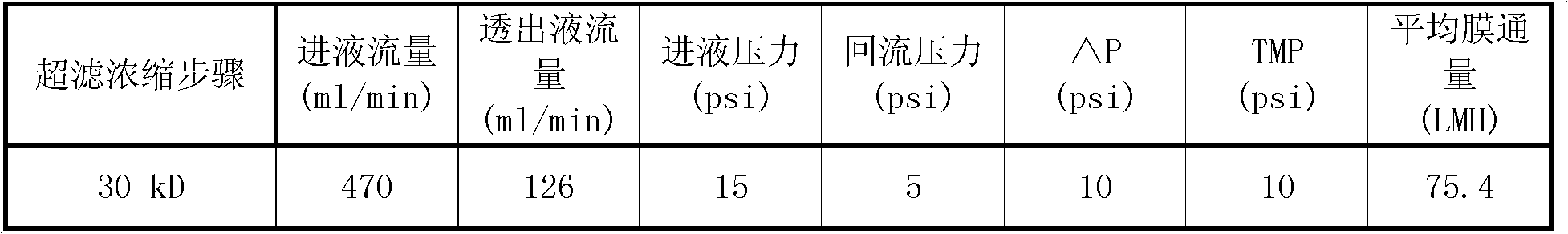
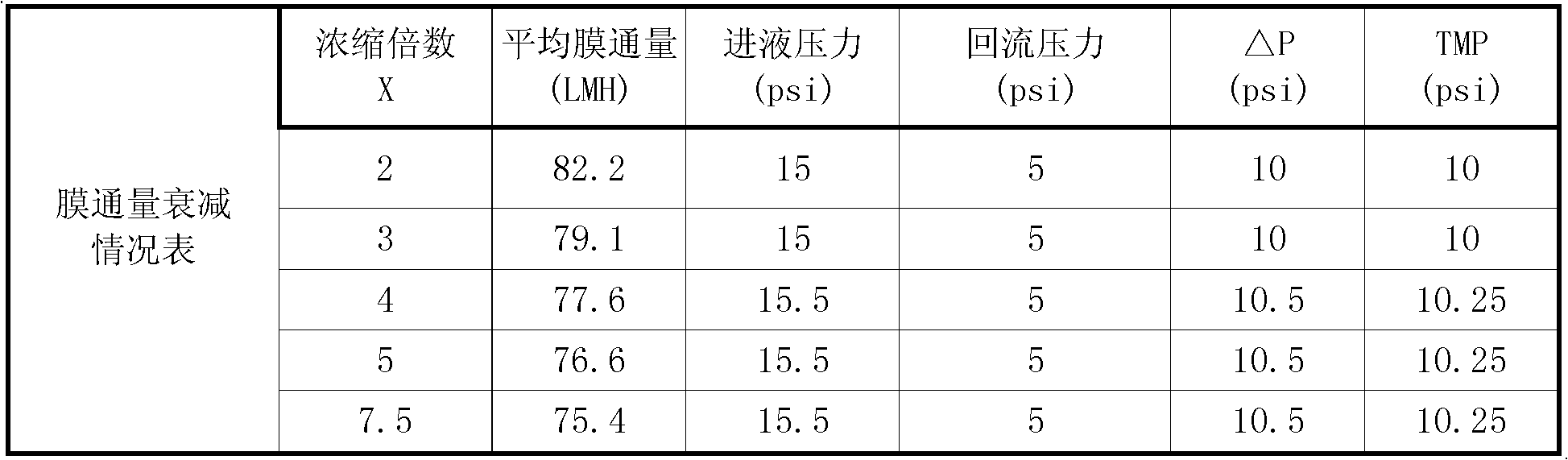
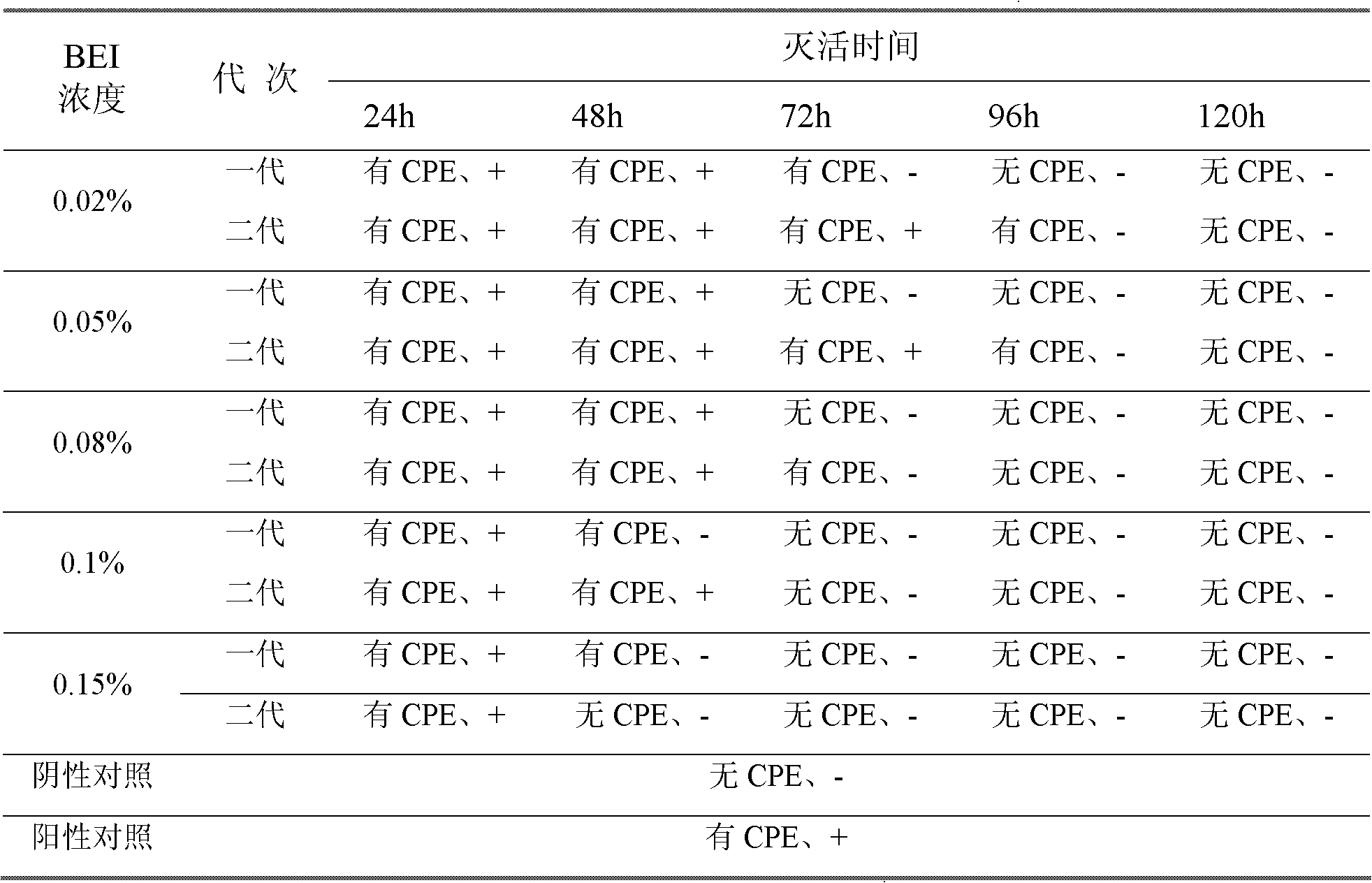
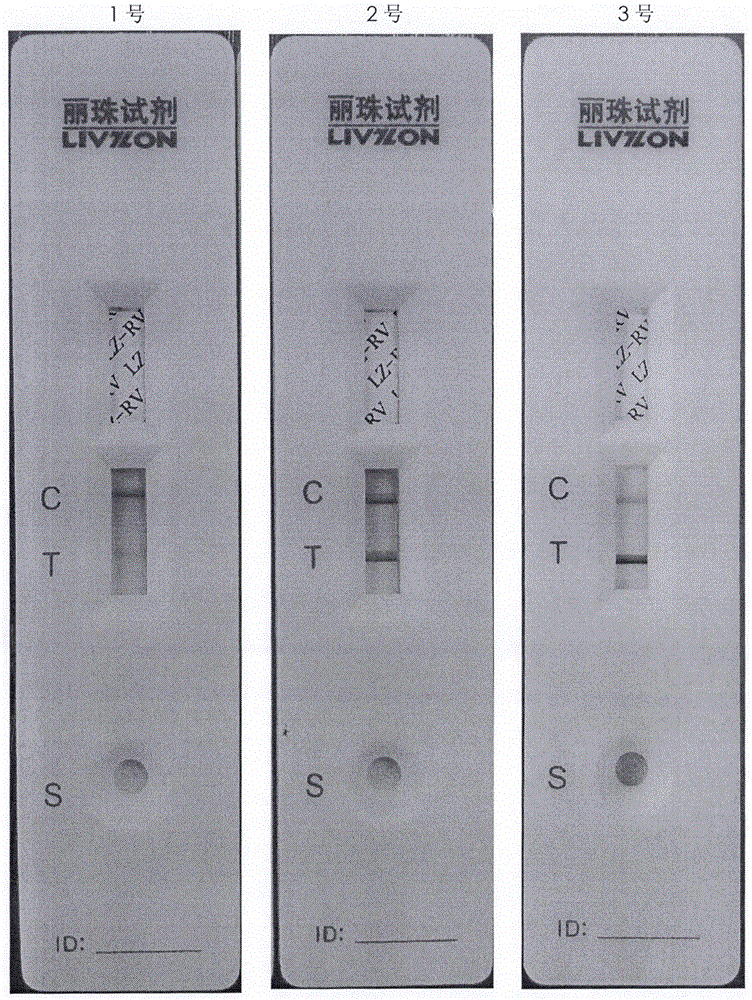
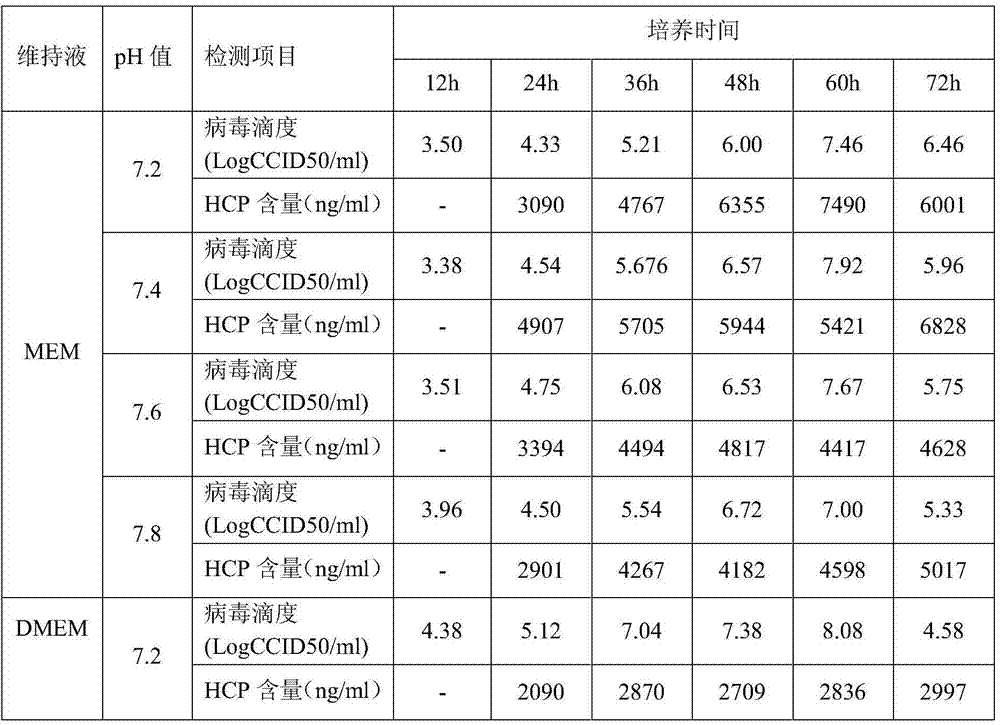
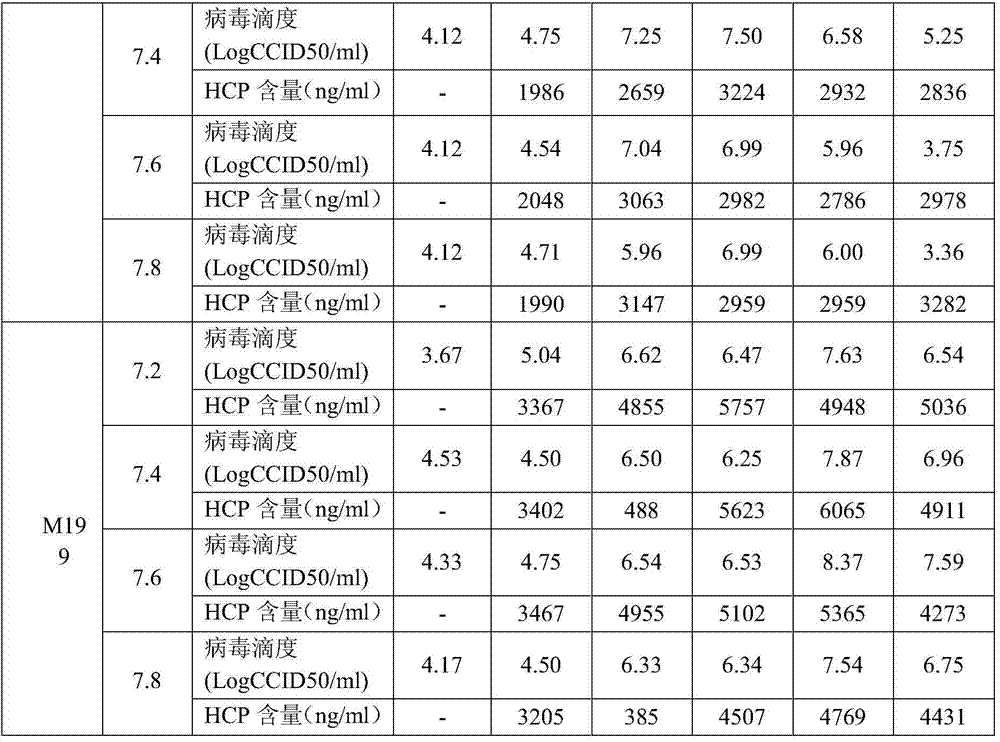
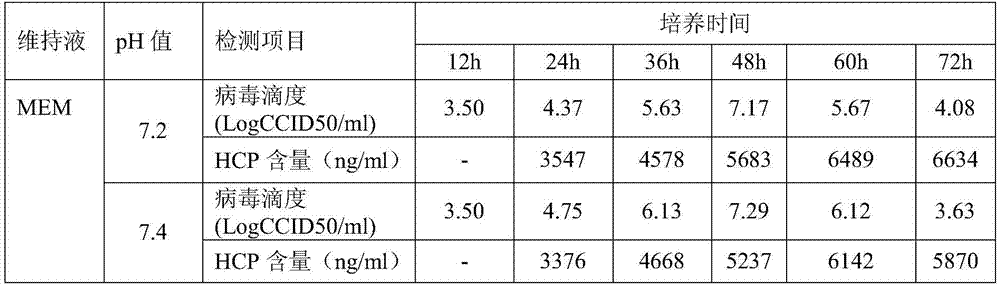
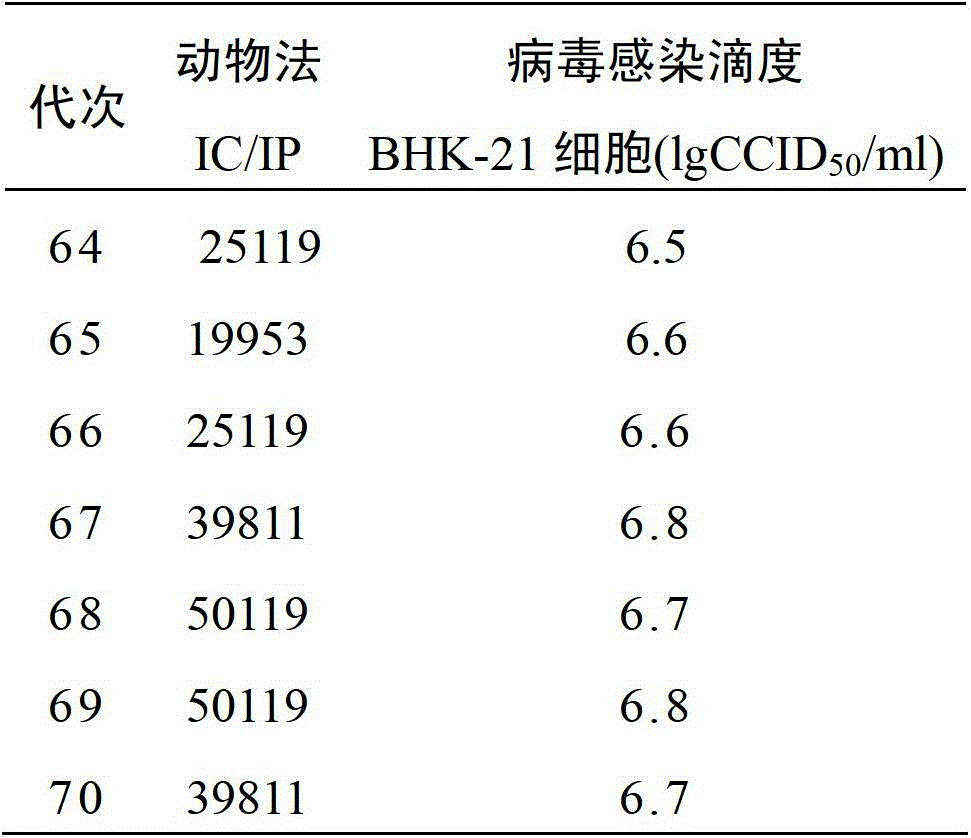
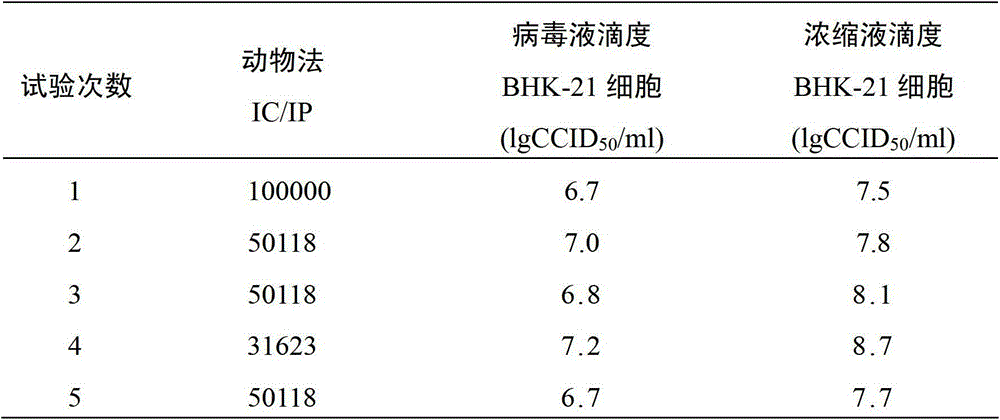
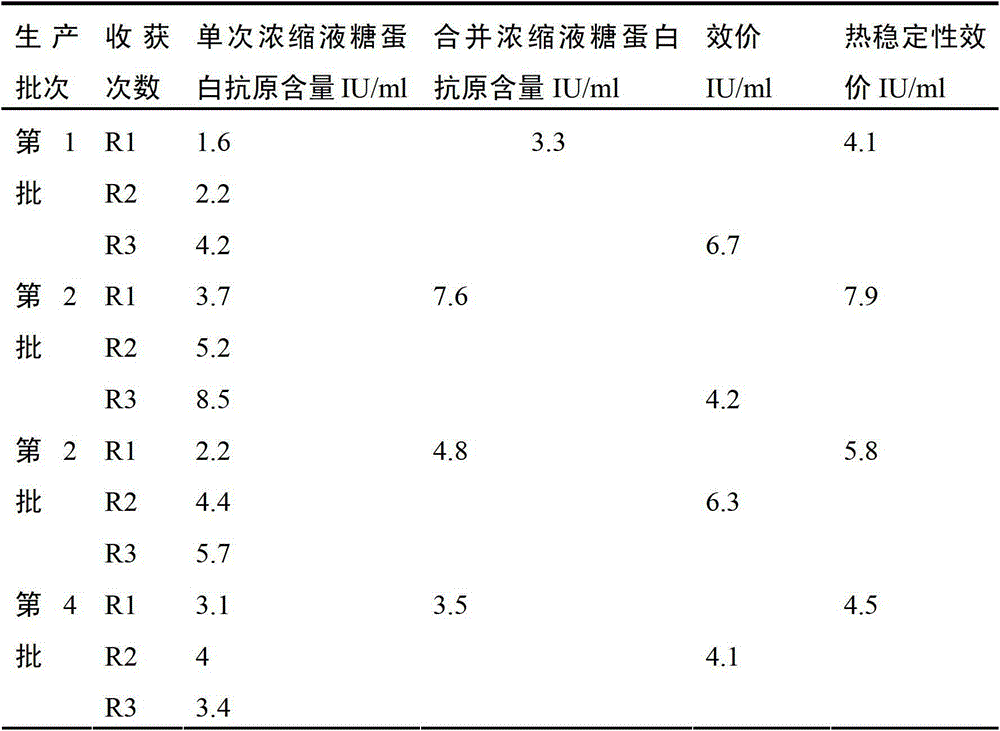
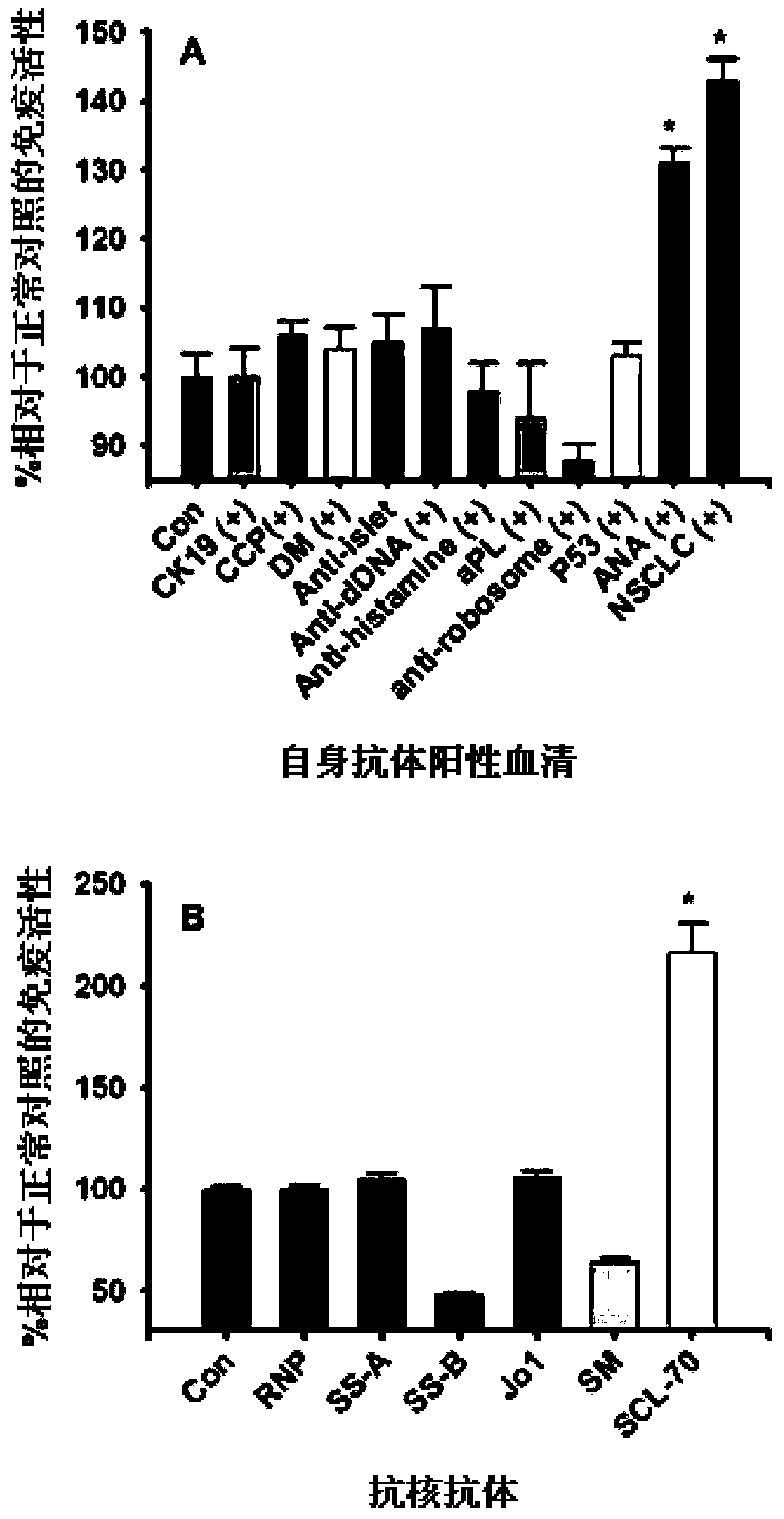
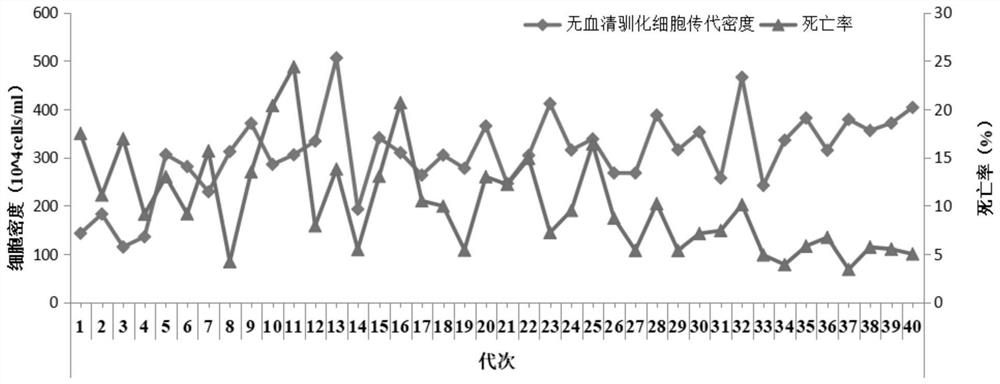
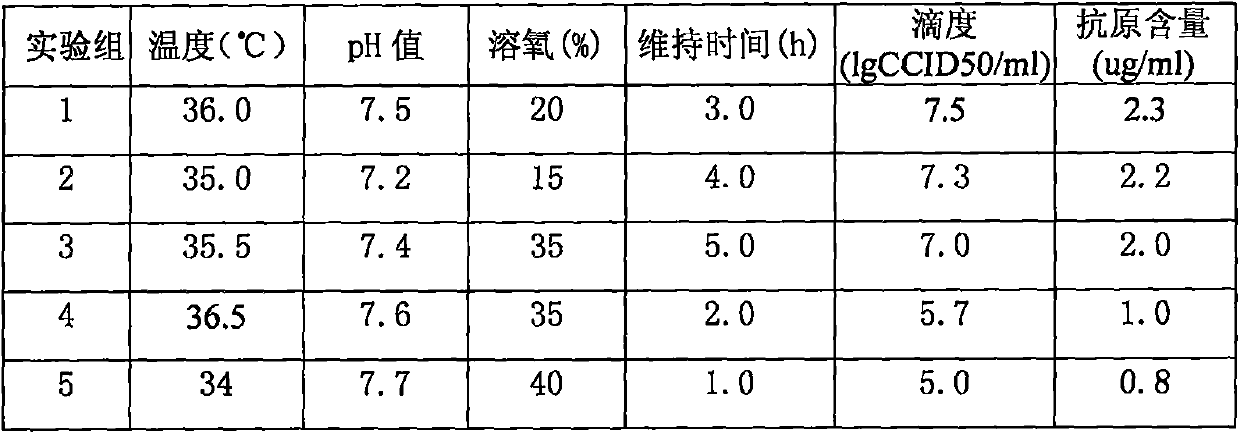
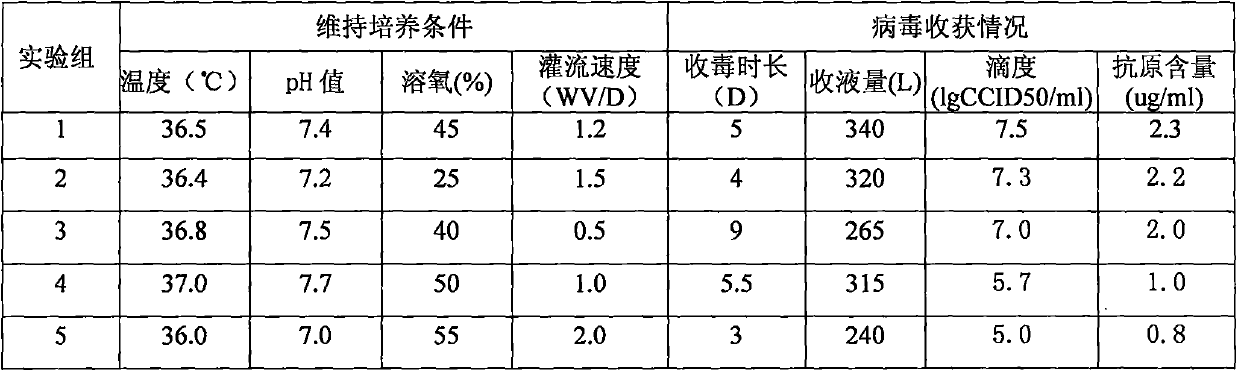
Patents
Literature
42results about How to "High antigen content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof
ActiveCN106946995AHigh antigen contentImprove securitySsRNA viruses negative-senseViral antigen ingredientsDiseaseInclusion bodies
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof are disclosed. The sequence of an antigen protein in the vaccine is shown as SEQ ID NO:1. The antigen protein has advantages of high safety, high immunity, no pathogenicity for chickens or other animals, and the like. The subunit vaccine can prevent chicken hydropericardium syndrome, inclusion body hepatitis and other diseases which are caused by infection of fowl adenovirus group I serum type 4.
Owner:苏州沃美生物有限公司
PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as preparation method and applications thereof
ActiveCN103033622AImprove expression efficiencyImproving immunogenicityMicroorganism based processesGenetic engineeringSorbentMonoclonal antibody
The invention relates to a PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as a preparation method and applications thereof, wherein the detection kit comprises an elisa plate of a polyclonal antibody of peridium anti-PCV2-Cap (nucleocapsid) protein, seal liquids, sample diluent, an antigen standard product, a second antibody of a monoclonal antibody of HRP marked anti-PCV2-Cap protein, a concentrated washing liquid, an enzyme substrate solution A, an enzyme substrate solution B and a stop solution, wherein the antigen standard product is purified reconstructed PCV2-Cap protein. The specificity of the kit provided by the invention achieves 100%, and the sensitivity is as high as 4ng / ml, and the kit can be used for swinery PCV2 antigen detection and PCV2 vaccine product quantitative detection.
Owner:WUHAN CHOPPER BIOLOGY
2 type subunit vaccine for porcine circovirus as well as preparation method and application thereof
InactiveCN102517331AQuick responseHigh activityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
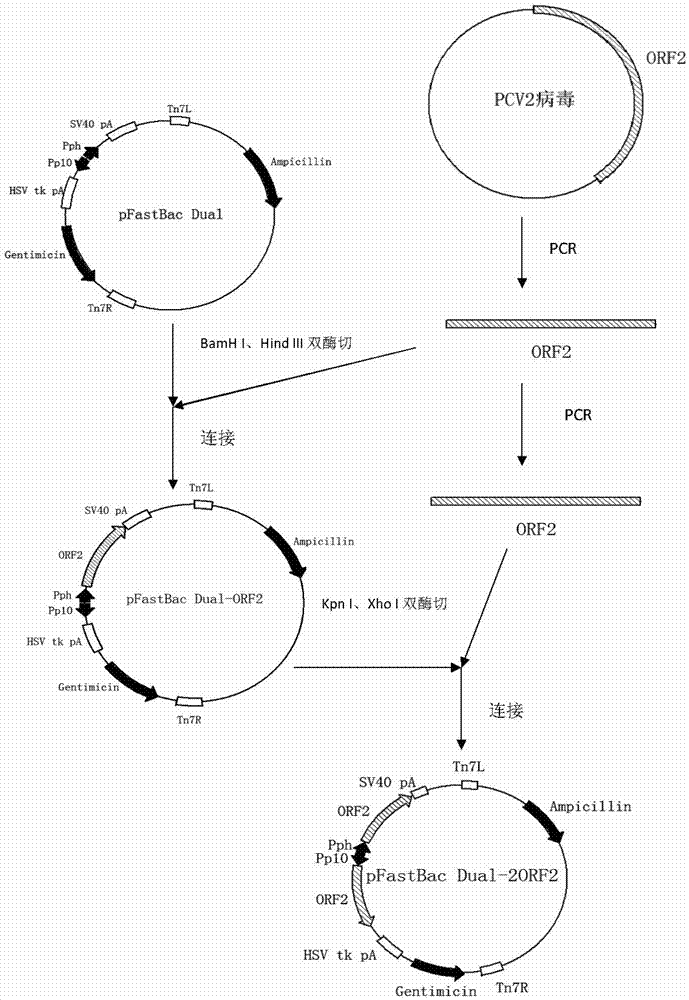
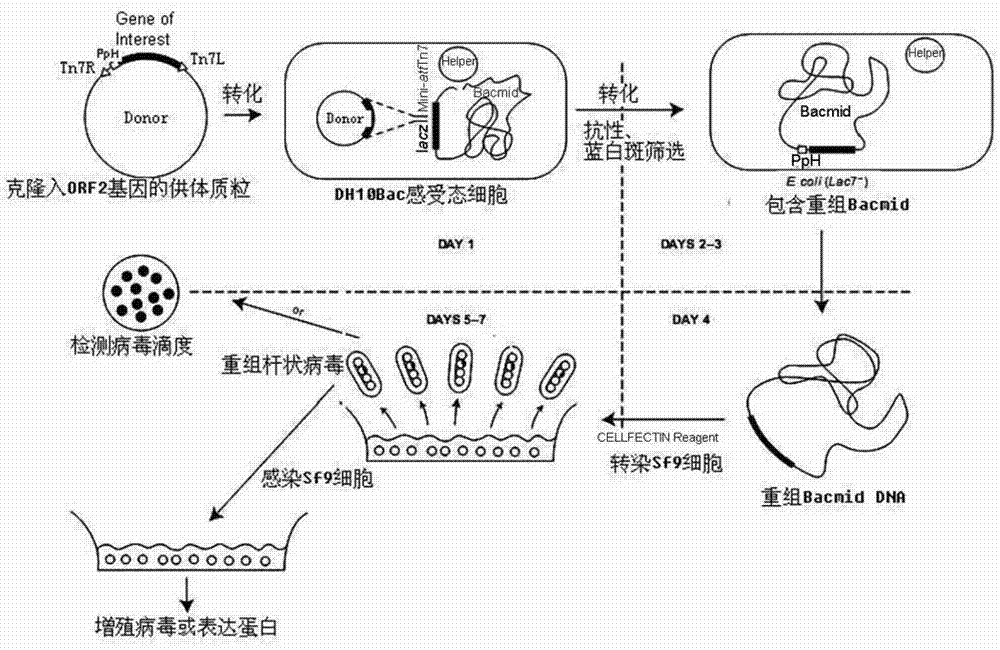
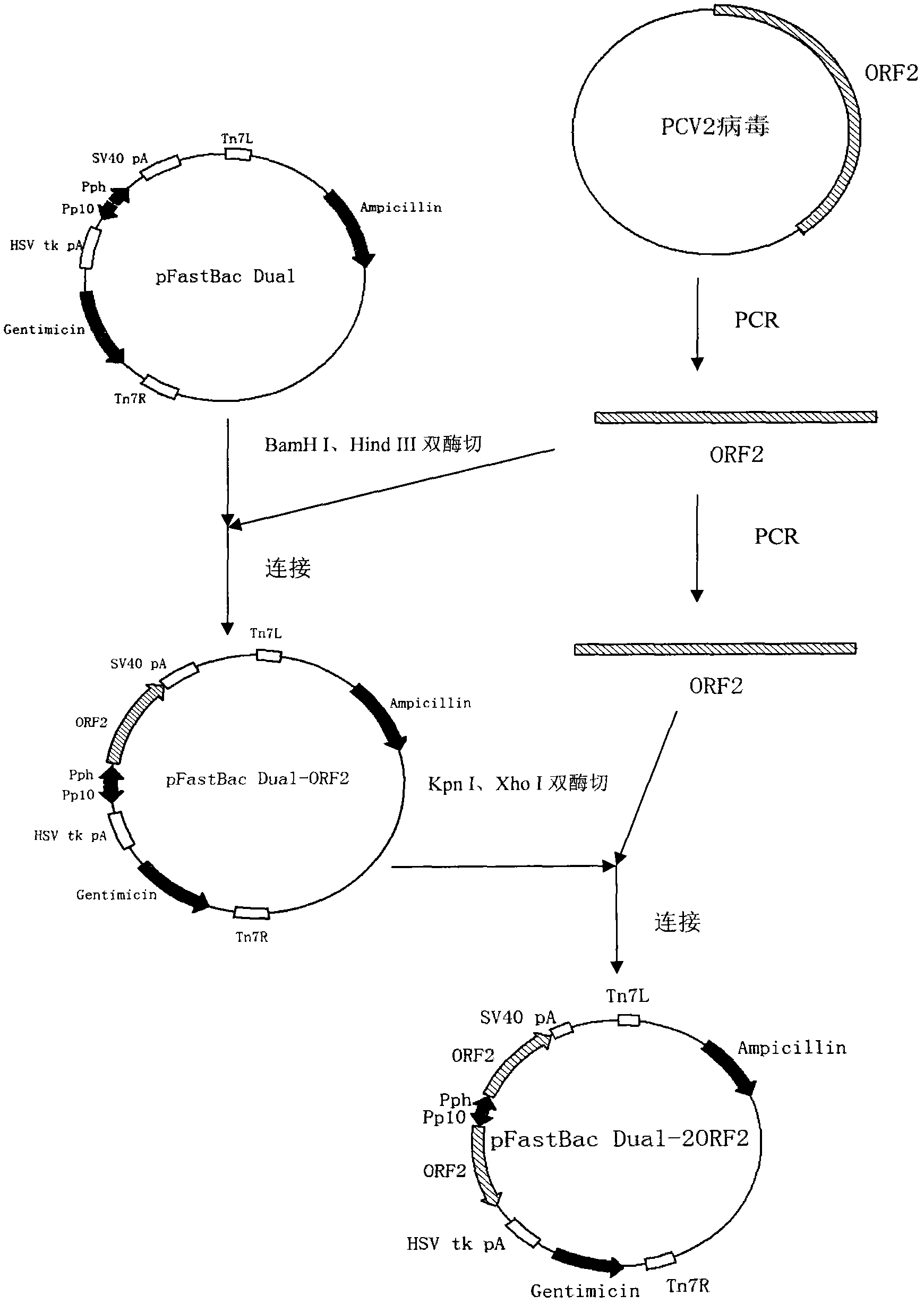
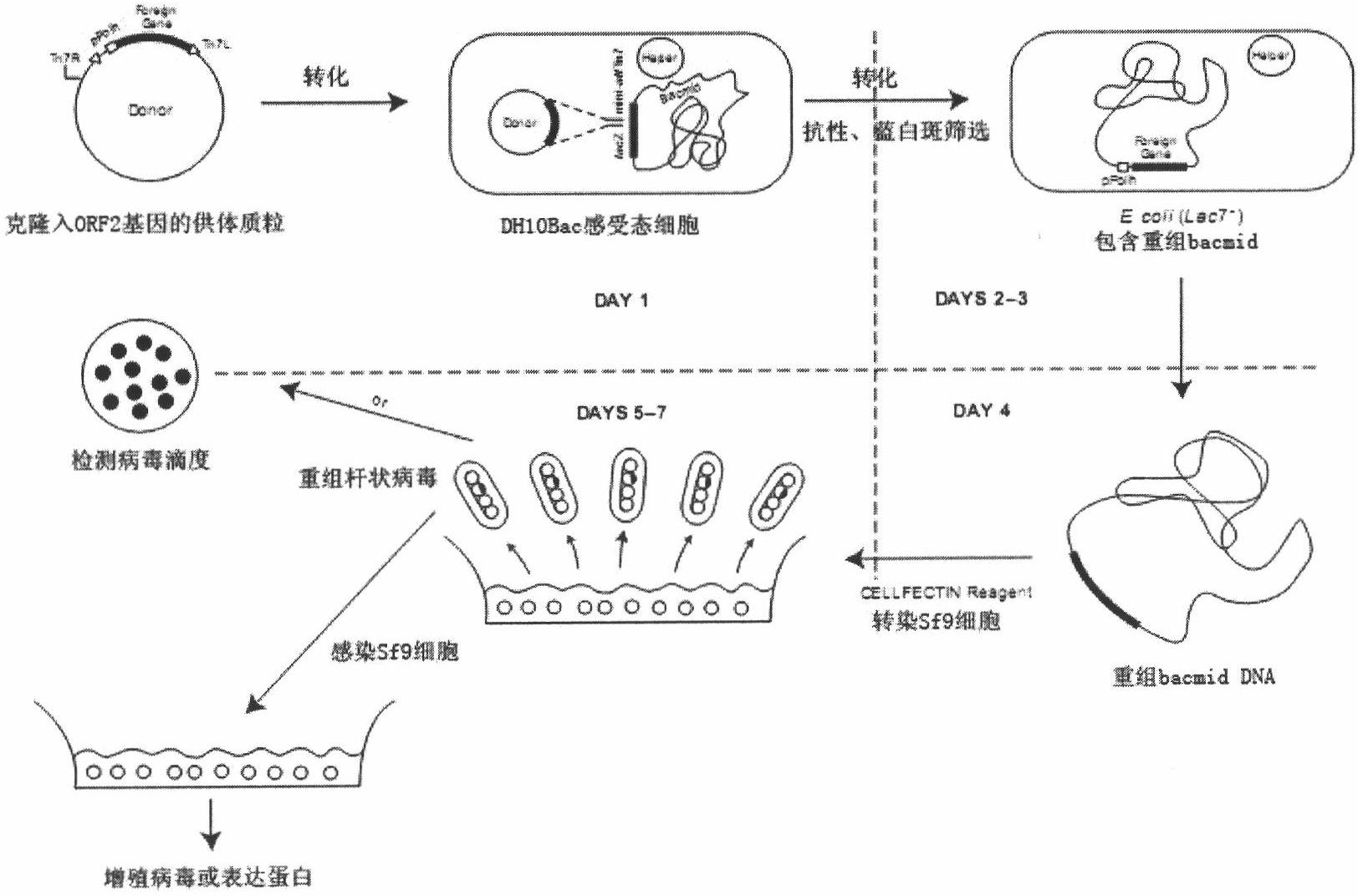
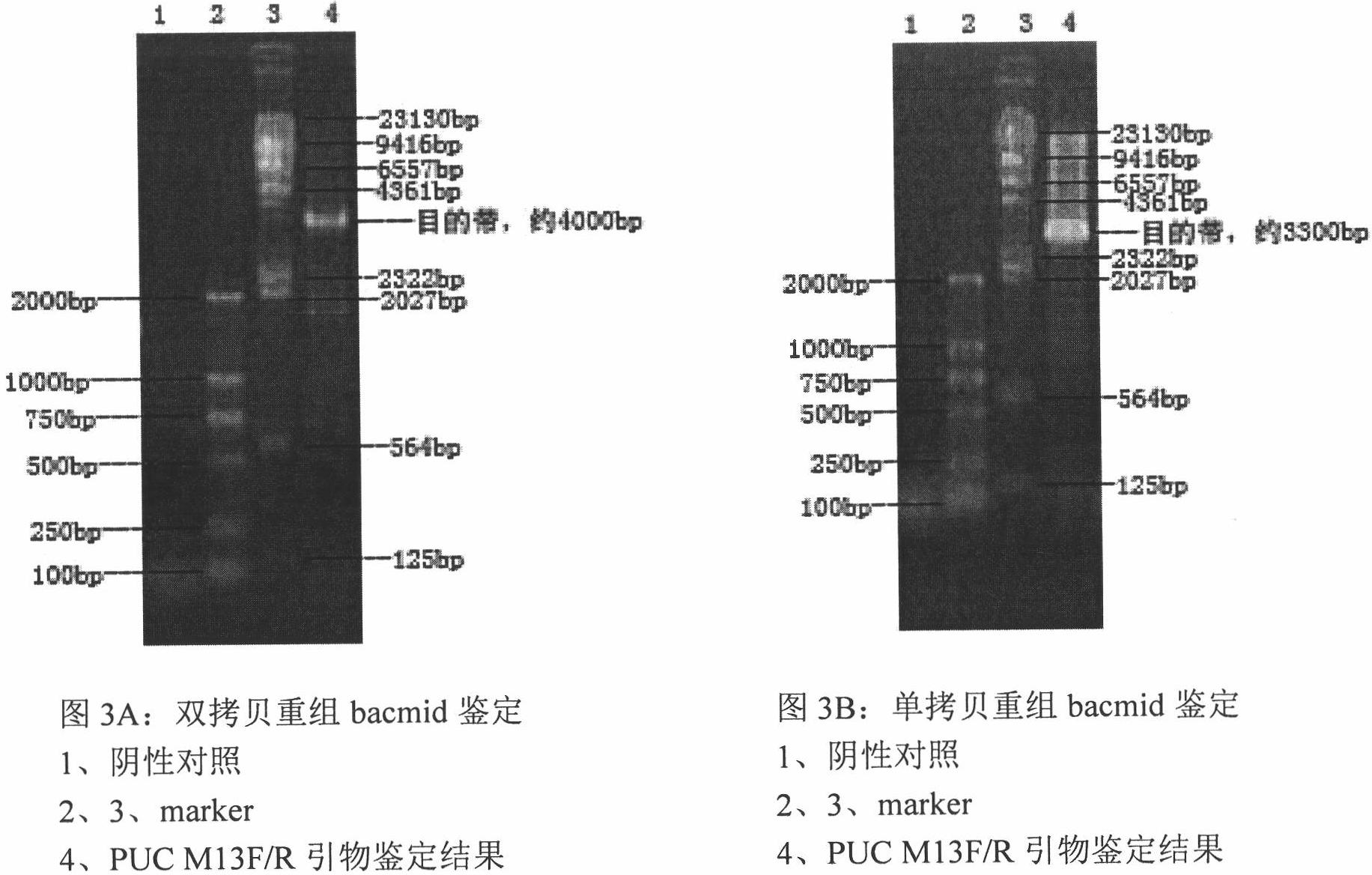
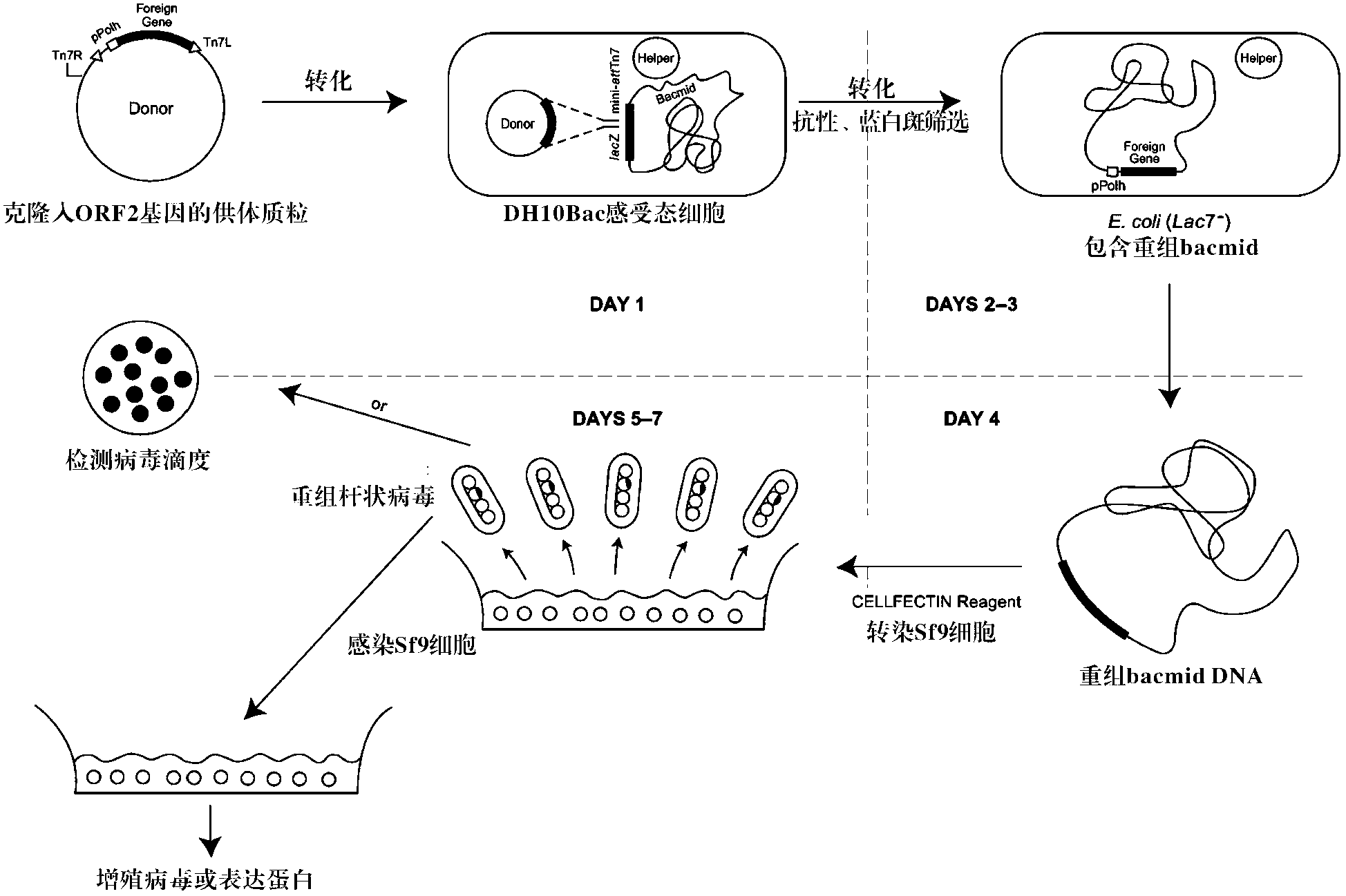
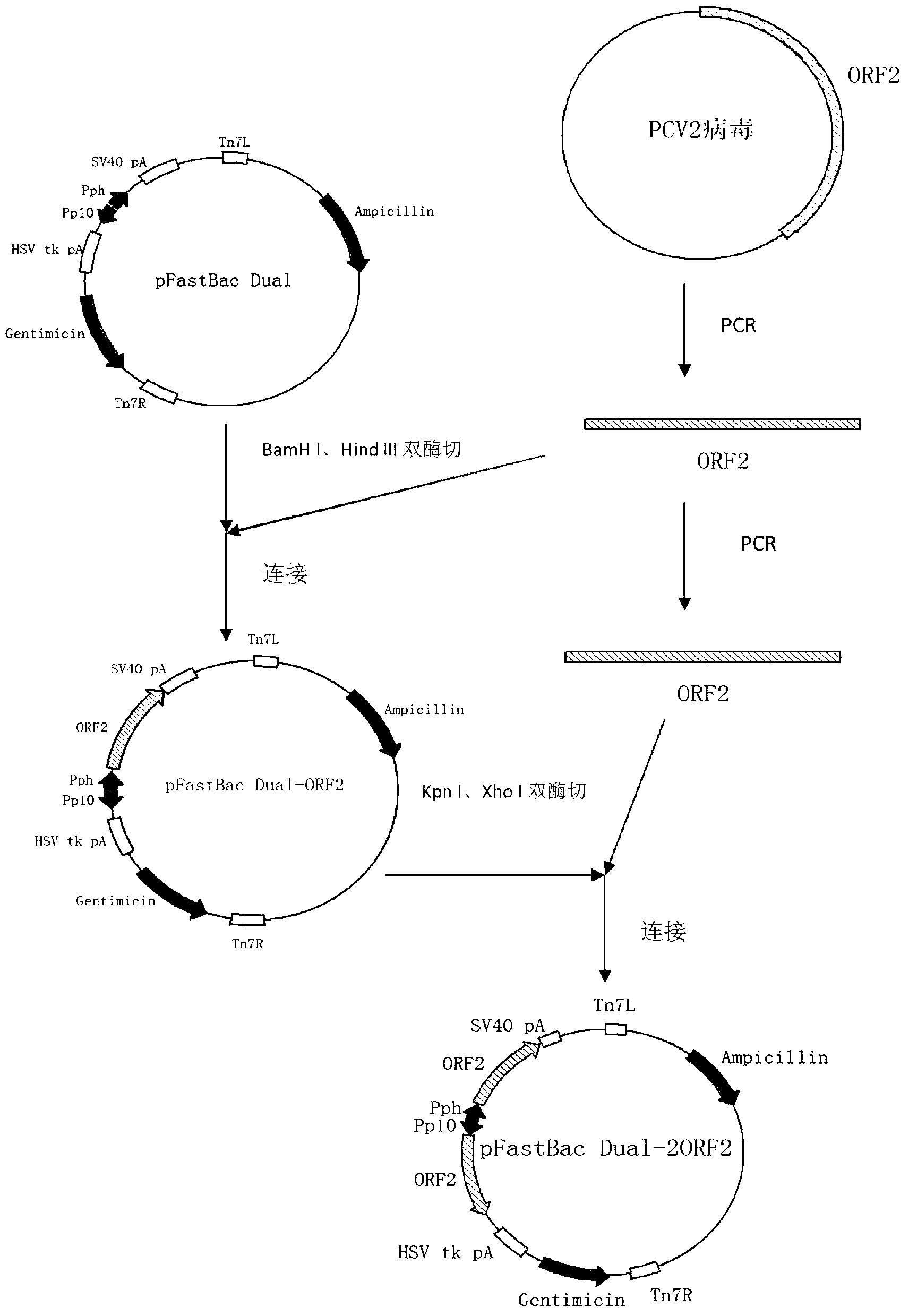
The invention relates to a 2 type subunit vaccine for a porcine circovirus as well as a preparation method and application thereof. A recombinant bacilliform virus contains double promoters (a polyhedrin promoter and a P10 promoter), a coding gene of a Cap protein with double copying can be expressed, and the expression efficiency of the protein is obviously enhanced; moreover, the Cap protein expressed by an inserted foreign gene does not contain an excess sequence, virus-like particles (VLPs) can be effectively formed, and the immunogenicity of an expressed protein is enhanced; furthermore, a produced antigen has high content; and according to the 2 type subunit vaccine for the porcine circovirus, which is disclosed by the invention, the productivity ratio and the quality of a viral protein of the 2 type subunit vaccine for the porcine circovirus are obviously enhanced, and a prepared vaccine composition has the advantages of stable and persistent immune effect, high safety and the like.
Owner:WUHAN CHOPPER BIOLOGY
Porcine circovirus type 2 subunit vaccine, and preparation method and application thereof
ActiveCN102925486AImprove expression efficiencyImproving immunogenicityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a porcine circovirus type 2 subunit vaccine, and a preparation method and an application thereof. The recombinant baculovirus contains double promoters (a polyhedrin protein promoter and a P10 promoter), and can express double copies of Cap protein coding genes, such that protein expression efficiency is substantially improved. Also, Cap protein expressed by an inserted exogenous gene does not contain excess sequences, such that virus-like particles (VLPs) can be effectively formed, expressed protein immunogenicity is improved, and the content of produced antigen is high. According to the porcine circovirus type 2 subunit vaccine provided by the invention, protein yield and quality of porcine circovirus type 2 subunit vaccine are substantially improved, and prepared vaccine compositions have the advantages of stable and long-lasting immune effect, high safety, and the like.
Owner:WUHAN CHOPPER BIOLOGY
Vibrio harveyi recombined outer-membrane protein Ompk microspheres vaccine and preparation method thereof
InactiveCN101507815AHigh antigen contentLong release timeAntibacterial agentsPharmaceutical non-active ingredientsAntigenSide effect
The invention discloses a microsphere vaccine and a preparation method thereof. The vaccine is the microsphere vaccine which is prepared from recombinant outer-membrane protein Ompk of vibrio harveyi as a common mariculture fish pathogen, as well as biodegradable polymer materials through emulsification and drying. The microsphere vaccine is simple in preparation process, convenient for factory production, stable and reliable in production, safe and convenient to use, and the product yield reaches more than 80 percent. The microsphere vaccine has the advantages of high antigen content, long sustained release time, few toxic-side effects, convenience for large-scale application and the like. The prepared microsphere vaccine which is orally taken for immunization can stimulate an immune system to produce immune response in a long period of time, has high relative protection ratio to tested fishes, and can effectively prevent mariculture fish vibriosis.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Method for producing rotavirus vaccines in large scale by utilizing bioreactor
ActiveCN106047821AIncrease productionQuality improvementViral antigen ingredientsAntiviralsMicrobiologyVero cell
The invention relates to a method for producing rotavirus vaccines in large scale by utilizing a bioreactor, and in particular relates to a method for culturing rotavirus inactivated vaccines in large scale by utilizing a 75L bioreactor. The method comprises the following steps: (1) carrying out reviving and passage on Vero cells; (2) collecting the cells and inoculating the cells into the bioreactor; (3) culturing the cells in the bioreactor; (4) changing the liquid and washing the cells; (5) activating and infecting rotaviruses; and (6) carrying out maintenance culture and harvesting on the rotaviruses. The method aims at overcoming the defect that in the large-scale production, the rotaviruses can not release cells easily, so that the obtained viruses are low in toxicity titer, and the inactivated rotavirus vaccines with high titer and high yield are obtained; the method also aims at overcoming the defect that in the large-scale production, the adsorption force and infection force of the rotaviruses are poor, and thus the yield and quality of the rotaviruses are greatly improved.
Owner:LIVZON GROUP VACCINE ENG
Preparation method of porcine parvovirus inactivated vaccine
InactiveCN102688485AHigh antigen contentRemove impuritiesAntiviralsAntibody medical ingredientsAntigenChemistry
The invention relates to a preparation method of a porcine parvovirus inactivated vaccine. The preparation method comprises the following preparation steps of: (1) taking a porcine parvovirus fluid out of a raw material tank, placing the porcine parvovirus fluid into a microfiltration system, filtering to obtain a penetrating fluid and a concentrated solution, returning the concentrated solution to the raw material tank, and discharging the porcine parvovirus fluid after the solid content of the porcine parvovirus fluid is larger than or equal to 1g / L; (2) carrying out ultrafiltration treatment on the penetrating fluid, and further concentrating the porcine parvovirus fluid until the virus hemocoagulate value is up to 28-213 to obtain a concentrated solution; (3) filtering the concentrated solution again by using the microfiltration system to obtain a parvovirus fluid with the hemocoagulate value of 28-213 after the treatment; and (4) inactivating the parvovirus fluid and preparing the concentrated and inactivated parvovirus fluid into a vaccine product. The invention provides a safe and feasible vaccine production method so that vaccine production is not dependent on the traditional process again, high-titer parvovirus fluid is produced, the antigen content is increased, and the requirement for immune production is met.
Owner:扬州优邦生物药品有限公司
Large-scale production method of rotavirus vaccine
ActiveCN105969737AIncrease productionQuality improvementViral antigen ingredientsAntiviralsRotavirus RNABiological activation
The invention relates to a large-scale production method of a rotavirus vaccine, particularly a large-scale culture method of a rotavirus inactivated vaccine by using a 14L bioreactor. The method comprises the following steps: 1) revival and subculture of Vero cells; 2) cell collection and bioreactor inoculation; 3) bioreactor culture of cells; 4) solution exchange and cell washing; 5) rotavirus activation and infection; and 6) sustaining culture and collection of rotavirus. The invention aims to overcome the defect of low infectivity titer in the obtained virus since the rotavirus can not easily release cells in rotavirus vaccine large-scale production, thereby obtaining the high-titer rotavirus, and greatly enhancing the rotavirus yield. The invention also aims to overcome the defect of low adsorption infectivity of rotavirus in the mass production process, thereby greatly enhancing the rotavirus quality and further enhancing the antigenicity and immunogenicity of the rotavirus inactivated vaccine.
Owner:LIVZON GROUP VACCINE ENG
Preparation method for microencapsulated oral live vaccine of gosling plague
The present invention discloses a preparation method for a microencapsulated oral live vaccine of gosling plague. The preparation method comprises: preparing a gosling plague SYG strain into a vaccine half finished product by goose embryo proliferation culture; adding a 5% sucrose and skimmed milk solution after a sterility test is qualified, and uniformly stirring; adding 20-40% porous starch tothe half finished product solution, stirring for 20-40 minutes at a certain temperature, wherein the temperature is controlled to 37 DEG C; mixing the resulting liquid and a 1-2.5% sodium alginate solution, completely stirring, and then carrying out dehydration and drying by a fluidized bed to prepare the microencapsulated vaccine dry powder of the gosling plague, wherein the volume of the resulting liquid is the same as the volume of the sodium alginate solution. The method of the present invention ha characteristics of science, simpleness, stable and reliable production, less loss of vaccine titer. With adopting the vaccine microencapsulation technology, the live vaccine of the gosling plague can be adopted for immunization by the oral route, the stress reaction is reduced, the sustained release function is provided for the live vaccine of the present invention, and the breeder goose in the laying period can use the live vaccine as usual.
Owner:HANGZHOU JIANLIANG VETERINARY BIOLOGICAL PREPARATIONS CO LTD
Preparation method for human diploid cell rabies vaccine virus solution
InactiveCN103060276AStable titerReduce dosageMicroorganism based processesViruses/bacteriophagesAntigenDiploid cells
The invention provides a preparation method for human diploid cell rabies vaccine virus solution, and relates to the field of biotechnology. The preparation method comprises the step of inoculating rabies vaccine fixed virus PM-1503-3M strain in MRC-5 cell to generate the human diploid cell rabies vaccine virus solution. The method disclosed by the invention is simple in process and cost-saving; and the prepared virus solution is high in antigen content, high in virus valence and titer, suitable for large-scale industrialized production, and capable of meeting the needs of domestic and foreign markets on human diploid cell rabies vaccine.
Owner:BEIJING MINHAI BIOTECH
Swine mycoplasma hyopneumoniae culture medium and preparation method and application thereof
InactiveCN106434502ALow serum levelsLow side reaction rateBacteriaMicroorganism based processesMycoplasma cultureArginine
The invention provides a swine mycoplasma hyopneumoniae culture medium and a preparation method and application thereof and belongs to the technical field of bioengineering. The swine mycoplasma hyopneumoniae culture medium is prepared from a basal culture medium and an auxiliary culture medium, which are mainly prepared from ingredients such as MEM, beef extract powder, yeast leachate powder, lactoalbumin hydrolysate, gastric mucin, an arginine solution, pig blood serum and chicken blood serum. The swine mycoplasma hyopneumoniae culture medium is prepared through subjecting the basal culture medium and the auxiliary culture medium to sterile treatment, then, carrying out volume determination by using injection water, and adjusting the pH value of the solution. The culture medium provided by the invention is low in blood serum content and is applied to the preparation of vaccine antigens, the growth speed of swine mycoplasma hyopneumoniae is high, the culture cycle is short, the fungus content of a semi-finished product fungus solution is high, the production cost is low, and the prepared vaccines are good in immunization effect and low in side reaction occurrence probability, so that the culture medium is suitable for being industrially produced on a large scale.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Bivalent vaccine for pig and preparation method thereof
InactiveCN101332298ASolve the problem of poor immunogenicityHigh antigen contentAntiviralsAntibody medical ingredientsAntigenAdjuvant
The invention provides a divalent vaccine for pigs, wherein, the active ingredients are porcine aftosa inactivation antigen and porcine reproductive and respiratory obstrucion syndrome inactivation antigen with the proportion of 1 to 0.6 - 1.5. The invention also provides a preparation method of the divalent vaccine for pigs, which comprises the processes that monolayer cell is cultured by a rolling bottle, the basic virus and the virus culture fluid are accessed, the Ph value of the culture fluid is regulated to be 7.4 plus or minus 0.2 by adopting an HEPES or NaHCO3 buffer system and the accession amount of the virus and the culture fluid are 300 to 800 ml / 15L per bottle; the virus fluid is collected after the cytopathic effect appears and the virus is inactivated after the potency of the virus is measured; 50V of adjuvant and the mixed inactivation antigen are emulsified according to the volume ratio of 1 to 1, and an 11 model white oil or Marcol 52 model oil and the mixed inactivation antigen are emulsified according to the volume ratio of 1 - 2.0 to 1 for the purpose of confecting the divalent vaccine for pigs. The divalent vaccine of the invention can be put into super-large scale production without restricting the use of virus strain, and can ensure the immune efficacy while the immune dosage is reduced.
Owner:薛景山
Method for preparing pig porcine reproductive and respiratory syndrome inactivated vaccine
InactiveCN106729687AHigh antigen contentImproving immunogenicityViral antigen ingredientsAntiviralsMolecular sieveAdjuvant
The invention provides a method for preparing a pig porcine reproductive and respiratory syndrome inactivated vaccine. Through steps of virus clarification, concentration, molecular sieve chromatography, inactivation, freeze-drying and the like, the method is capable of effectively ensuring that the content of impurities in antigen for preparing the vaccine is reduced to the minimum extent, so that the phenomenon that side effects are caused after the product is used can be avoided, and meanwhile the inactivation effect of the vaccine can be ensured. The PRRSV inactivated vaccine prepared by using the method is high in antigen content, high in immunogenicity, high in antigen purity, good in security, small in side effect, free of adjuvant, rapid in antibody protection generation, easy to inject and easy to absorb.
Owner:鼎正生物药业(天津)有限公司
Duck hepatitis bivalent live vaccines and preparation method thereof
ActiveCN105727275AHigh antigen contentImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsYolkMicroorganism
The invention relates to duck hepatitis bivalent live vaccines and a preparation method thereof. According to the invention, 1) SPF chicken embryos are inoculated with vaccine strains of the duck hepatitis bivalent live vaccines, namely DHV-1 QL1 strains, through allantoic cavities for culture, and are inoculated with DHV-3 QL3 strains through chorioallantoic membranes or yolk sacs for culture, the antigen contents of the prepared vaccines are high, and all not less than 10<8.5>ELD50 / ml, so that the duck hepatitis bivalent live vaccines have the characteristics of good safety and excellent immunogenicity, and break through limitations of DHV-3 type virus strains which only can be proliferated in duck embryos; 2) virus solutions cultured by the SPF chicken embryos lower risks of exogenous pathogenic microbial contamination, improve labor efficiency and reduce production costs, thereby being suitable for mass production; 3) the bivalent live vaccines disclosed by the invention can prevent DHV-1 type and DHV-3 type virulent viruses attacking on ducklings; 4) the duck hepatitis bivalent live vaccines are simple in production process, easy in quality control and small in batch difference, thereby providing a guarantee for producing safe and qualified vaccines.
Owner:QILU ANIMAL HEALTH PROD
Poliomyelitis inactivated vaccine and its production method
ActiveCN104312981BHigh titerHigh antigen contentViral antigen ingredientsMicroorganism based processesAntigenBalance salt
Owner:BEIJING BIOLOGICAL PROD INST CO LTD
Method for preparing poultry cholera microcapsule vaccine
InactiveCN100566751CHigh antigen contentLong release timeAntibacterial agentsAntibody medical ingredientsAntigenFreeze-drying
The invention discloses a preparation method of fowl cholera microcapsule vaccine. The preparation method of the vaccine is to prepare the seed bacterial liquid after the freeze-dried strain of Pasteurella multocida type A is resuscitated and subcultured, and obtain a semi-finished product containing more than 9 billion bacteria / ml through culture and proliferation; the semi-finished bacterial liquid is sterilized After passing the biopsy test, it is homogenized with an equal volume of 2% sodium alginate solution through a disperser for primary emulsification; the above emulsion and an equal volume of 5% chitosan solution are subjected to secondary emulsification through a high-pressure emulsifier; the final product The emulsion is dehydrated by a spray dryer to make a microcapsule vaccine dry powder. The preparation method is scientific, simple and reasonable, the production is stable and reliable, and the product yield reaches more than 85%. Using microencapsulation technology, the finished product has the advantages of high antigen content, long sustained release time, low toxic and side effects, low cost, convenient use, and long shelf life.
Owner:JIANGSU INST OF POULTRY SCI
Fluorescence detection kit for simultaneous detection of three kinds of breast cancer tumor markers and detection method thereof
InactiveCN107831152AReduce the binding forceHigh antigen contentFluorescence/phosphorescenceFluorescenceWavelength
The invention relates to a testing or inspection method containing enzymes or microbes, in particular to a fluorescence detection kit for simultaneous detection of three kinds of breast cancer tumor markers and a detection method thereof. The kit for the simultaneous detection of three kinds of breast cancer tumor markers is characterized in that the three kinds of breast cancer tumor markers arerespectively CEA, CA153 and CA125; the kit comprises ten kinds of solutions and respectively G1 solution, G2 solution, G3 solution, Q1 solution, Q2 solution and Q3 solution. Compared with the prior art, the method provided by the invention has the advantages that the simultaneous detection on three kinds of breast cancer markers can be realized through switching different maximum emitting wave lengths.
Owner:TIANJIN UNIV
Preparation method for human diploid cell rabies vaccine virus solution
InactiveCN103060276BStable titerReduce dosageMicroorganism based processesViruses/bacteriophagesAntigenDiploid cells
The invention provides a preparation method for human diploid cell rabies vaccine virus solution, and relates to the field of biotechnology. The preparation method comprises the step of inoculating rabies vaccine fixed virus PM-1503-3M strain in MRC-5 cell to generate the human diploid cell rabies vaccine virus solution. The method disclosed by the invention is simple in process and cost-saving; and the prepared virus solution is high in antigen content, high in virus valence and titer, suitable for large-scale industrialized production, and capable of meeting the needs of domestic and foreign markets on human diploid cell rabies vaccine.
Owner:BEIJING MINHAI BIOTECH
Porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine
PendingCN112957460AReduce the chance of side effectsHigh antigen contentAntibacterial agentsBacterial antigen ingredientsUltrafiltrationVirus Protein
The invention discloses a porcine circovirus, porcine pseudorabies virus and mycoplasma triple inactivated vaccine which comprises an antigen and a vaccine adjuvant, the antigen is composed of a porcine circovirus type 2 antigen, a porcine pseudorabies virus antigen and a mycoplasma antigen, the porcine circovirus type 2 antigen is a purified, concentrated and inactivated porcine circovirus type 2 protein antigen solution, and the content of Cap protein is more than or equal to 160 [mu]g / ml; the porcine pseudorabies virus antigen is a purified, concentrated and inactivated porcine pseudorabies virus protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; the mycoplasma antigen is an inactivated mycoplasma protein antigen solution, and the content of the Cap protein is more than or equal to 160 [mu]g / ml; and the vaccine adjuvant is composed of a water-based high-molecular polymer adjuvant and a composite polysaccharide immunopotentiator. Foreign protein is removed through clarification filtration and ultrafiltration concentration, and the side reaction probability of the vaccine is greatly reduced; and three-proofing can be achieved through one needle, so that the number of immunization times and stress are reduced. The method is economical and practical, the immunization procedure is simplified, and the epidemic prevention cost is reduced.
Owner:JIANGXI ZHENGBANG TECHNOLOGY CO LTD +1
A kind of tumor-specific antigen and its application
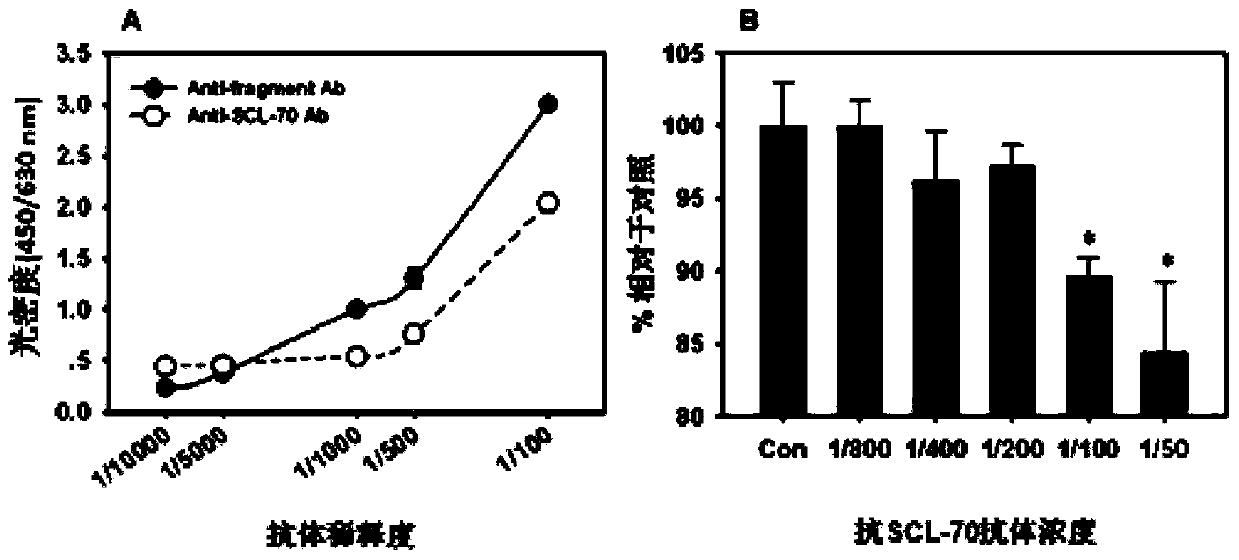
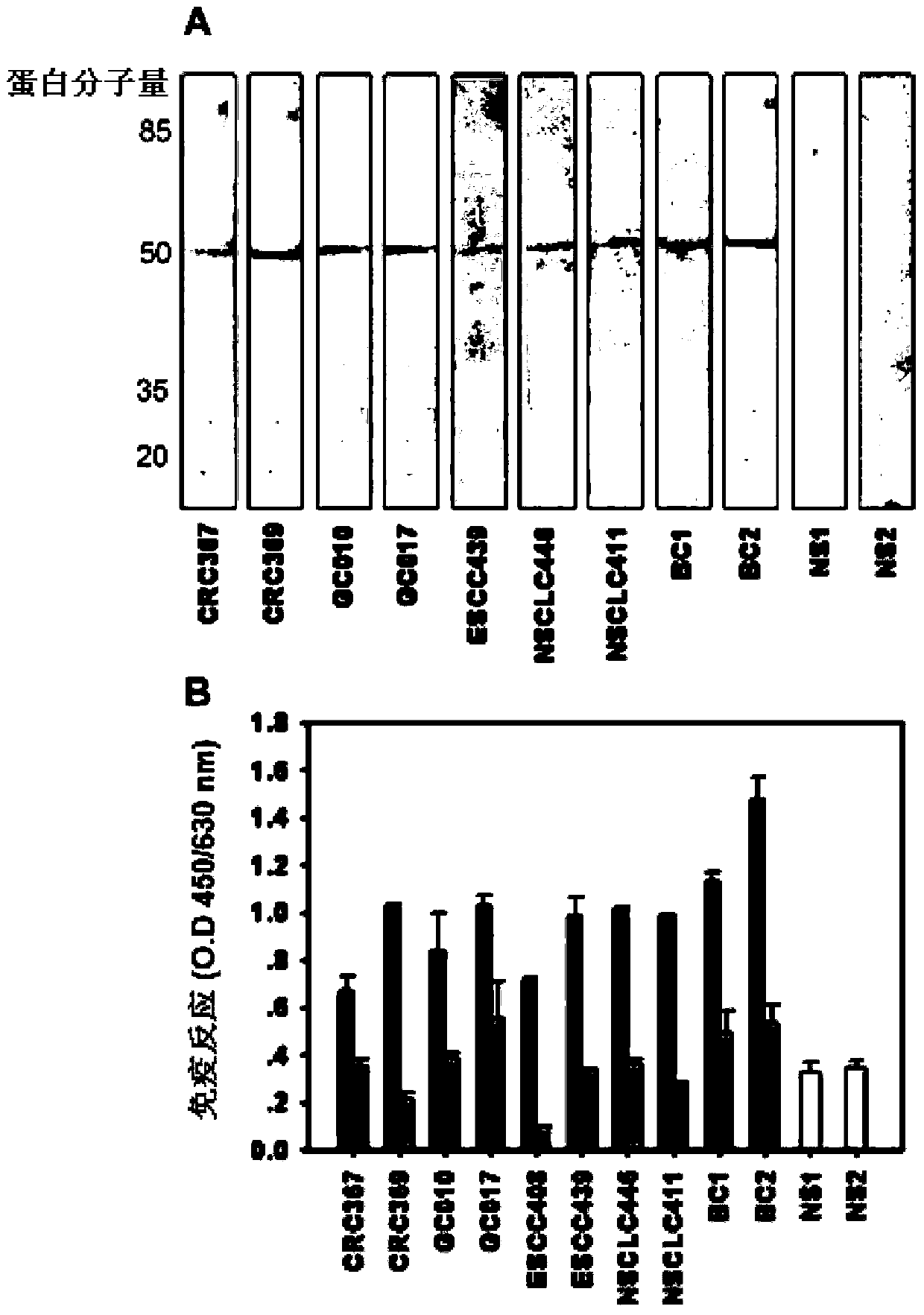
ActiveCN106191022BHigh antigen contentHas clinical applicationBiological testingIsomerasesCancer cellAutoantibody
The invention discloses a tumor specific antigen and application thereof. The amino acid sequence of the tumor specific antigen TOP-1-40 is shown as SEQ ID NO:1. A protein dimensional electrophoresis method is used for being combined with a cancer serological immunoblotting and antibody capture enzyme-linked immunoassay method for separating and obtaining the tumor specific antigen from cancer cell strain cells and cancer tissues. Through the specific polyclonal antibody for resisting the antigen, the antigen is determined to be a protein fragment, with the molecular weight being about 40kd, of DNA topoisomerase I by using the methods of a recombinant protein method, an immunoblotting method, a fluorescent-immunohistochemistry method, an antibody capture enzyme-linked immunoassay method and the like; the antigen is named as TOP-1-40. The antigen content is generally increased in common cancer tissues; the antigen cannot be detected in corresponding normal tissues, or the content is very low. The self antibody concentration of the antigen in the serum is detected by the antibody capture enzyme-linked immunoassay method; the antigen can be used for the early stage screening of common cancer. The detection method has 95 to 100 percent of specificity and 61 to 66 percent of sensibility. The tumor specific antigen has good prospects in clinic application.
Owner:叶尚勉
Method for culturing avian reovirus
InactiveCN104388395AProliferation is suitable forExcellent cultivation processMicroorganism based processesViruses/bacteriophagesAntigenViral replication
The invention provides a method for culturing an avian reovirus. According to the method, because a vero cell which has excellent performance and is conducive to the proliferation of the avian reovirus is used as a host cell to be used for proliferating the avian reovirus, an optimized process for culturing the avian reovirus can be determined. A bioreactor is adopted to culture and replicate the avian reovirus, and due to the adhesion of the cell to a carrier, the suspension culture of the cell can be realized, the number of vero cells in unit volume can be increased, and further, the yield of the avian reovirus can be improved. Meanwhile, because the avian reovirus culturing process can be monitored timely and controlled quantitatively, the stability of the production process can be guaranteed. Moreover, because the content of avian viral arthritis viruses in an avian viral arthritis virus liquor cultured by adopting the method is high, the content of antigens in the prepared inactivated vaccine is high, and the effect of the inactivated vaccine is good. Furthermore, due to the way of proliferating the avian reovirus by using the bioreactor through the microcarrier, the cost can be lowered, the labor can be saved, and the stability of the product can be improved.
Owner:TIANJIN RINGPU BIO TECH
Newcastle disease virus chimeric virus-like particle, vaccine and preparation method
ActiveCN106867975BResolve mismatchQuality improvementSsRNA viruses negative-senseViral antigen ingredientsPost translationalStructural protein
The invention discloses virus like particles of newcastle disease virus, a vaccine and a preparing method. The vaccine is a virus like particle vaccine. The virus like particles are chimeric newcastle disease virus like particles. The vaccine is a novel vaccine prepared by the adoption of modern biology principles and methods. The vaccine adopts currently popular virulent newcastle disease virus strain gene type VII as a vaccine strain, and solves the problem that existing vaccine strains are not matched with epidemic strains; besides, the vaccine is high in antigen content and easy to produce; post-translational processing of an expression product is similar to that of structural protein of newcastle disease virus, and newcastle disease virus membrane protein on the surfaces of the particles maintains a natural structure, biological activity and immunogenicity.
Owner:NOVARTIS BIOTECH WUHAN +1
Method for preparing porcine circovirus 2-type inactivated vaccine
ActiveCN103285385BIncreased sensitivityUniform stateViral antigen ingredientsAntiviralsImmune effectsPublic health
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Newcastle disease and H9N2 subtype avian influenza bivalent inactivated vaccine and preparation method thereof
ActiveCN102805864BImproving immunogenicityImprove the level ofViral antigen ingredientsAntiviralsNewcastle disease virus NDVImmunogenicity
The invention provides a newcastle disease and H9N2 subtype avian influenza bivalent inactivated vaccine and a preparation method of the bivalent inactivated vaccine, which relate to the field of biological engineering. The bivalent inactivated vaccine comprises a newcastle disease virus LaSota strain and an avian influenza virus A / chicken / HeNan / 03 / 2009 / (H9N2) strain. The preparation method of the vaccine comprises the steps of preparing, concentrating and deactivating virus liquid, performing water phase preparation and oil phase preparation, and emulsify, thereby obtaining the bivalent inactivated vaccine. The newcastle disease and H9N2 subtype avian influenza bivalent inactivated vaccine has good immunogenicity, creates a high antibody level, and can withstand not only the attack of homologous viruses, but also the attack of JX02 and SH01 of H9N2 subtype avian influenza viruses, which are prevalent since 2011; the bivalent inactivated vaccine can create high level antibodies and has a long persistent period; and the preparation method of the bivalent inactivated vaccine is simple and safe.
Owner:JIANGSU ACAD OF AGRI SCI
Bovine infectious rhinotracheitis virus and mycoplasma bovis dual inactivated vaccine and its preparation method and suspension mdbk cells used
ActiveCN111671893BGuaranteed stabilityQuality improvementAntibacterial agentsBacterial antigen ingredientsAntigenDisease
Owner:CHINA AGRI UNIV
A method for large-scale production of rotavirus vaccine using bioreactor
ActiveCN106047821BIncrease productionQuality improvementViral antigen ingredientsAntiviralsBiotechnologyMicrobiology
The invention relates to a method for producing rotavirus vaccines in large scale by utilizing a bioreactor, and in particular relates to a method for culturing rotavirus inactivated vaccines in large scale by utilizing a 75L bioreactor. The method comprises the following steps: (1) carrying out reviving and passage on Vero cells; (2) collecting the cells and inoculating the cells into the bioreactor; (3) culturing the cells in the bioreactor; (4) changing the liquid and washing the cells; (5) activating and infecting rotaviruses; and (6) carrying out maintenance culture and harvesting on the rotaviruses. The method aims at overcoming the defect that in the large-scale production, the rotaviruses can not release cells easily, so that the obtained viruses are low in toxicity titer, and the inactivated rotavirus vaccines with high titer and high yield are obtained; the method also aims at overcoming the defect that in the large-scale production, the adsorption force and infection force of the rotaviruses are poor, and thus the yield and quality of the rotaviruses are greatly improved.
Owner:LIVZON GROUP VACCINE ENG
Porcine epidemic diarrhea virus variant strain 2a, 2b bivalent inactivated vaccine and preparation method thereof
ActiveCN111041002BSmall batchSmall batch-to-batch varianceSsRNA viruses positive-senseViral antigen ingredientsAntigenEpidemic diarrhea
The invention provides a porcine epidemic diarrhea virus variant strain 2a, 2b bivalent inactivated vaccine and a preparation method thereof. The bivalent inactivated vaccine includes two currently popular porcine epidemic diarrhea variant strains 2a and 2b subtypes, which can prevent diarrhea caused by these two different variant strains. One injection is dual-purpose, safe and effective, and reduces animal immunity. And stress times, piglets can be protected by immunizing sows. The preparation method comprises: fully suspending and culturing porcine epidemic diarrhea virus variant strains 2a and 2b without serum, mixing the inactivated porcine epidemic diarrhea virus variant strain 2a and 2b virus liquids in proportion, adding adjuvant and fully emulsifying to obtain the product. The invention adopts the full-suspension serum-free ST cell culture process to prepare the virus liquid with high antigen content, easy scale-up culture, large batch size and small batch-to-batch difference. The process reduces the risk of pollution and low cost, and provides an effective means for the prevention and control of PEDV.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Group I serotype 4 avian adenovirus genetically engineered subunit vaccine, its preparation method and application
ActiveCN106946995BHigh antigen contentImprove securitySsRNA viruses negative-senseViral antigen ingredientsDiseaseInclusion bodies
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof are disclosed. The sequence of an antigen protein in the vaccine is shown as SEQ ID NO:1. The antigen protein has advantages of high safety, high immunity, no pathogenicity for chickens or other animals, and the like. The subunit vaccine can prevent chicken hydropericardium syndrome, inclusion body hepatitis and other diseases which are caused by infection of fowl adenovirus group I serum type 4.
Owner:苏州沃美生物有限公司
After treatment method for attenuated strain polio inactivated vaccine
ActiveCN1297313CMeet production needsStable production processViral antigen ingredientsInactivation/attenuationAntigenVaccine Immunogenicity
The present invention is after-treatment process of deactivated vaccine of attenuated polio strain. The large scale cultured virus liquid is three stage filtered in filtering columns of 06-1.0 micron, 0.45-0.6 micron and 0.22-0.45 micron pore size separately, clarified, ultrafiltered and concentrated, chromatographic column purified and deactivated to obtain semi-finished vaccine product with high purity, good immunogenicity, high safety and stable quality for the requirement of producing the attenuated polio strain. The semi-finished vaccine product can meet the requirement of product and may be used to produce Aabin IPV vaccine with stable quality, high safety and excellent immunogenicity.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
A kind of duck hepatitis bivalent live vaccine and preparation method thereof
ActiveCN105727275BHigh antigen contentImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsYolkAntigen
The invention relates to duck hepatitis bivalent live vaccines and a preparation method thereof. According to the invention, 1) SPF chicken embryos are inoculated with vaccine strains of the duck hepatitis bivalent live vaccines, namely DHV-1 QL1 strains, through allantoic cavities for culture, and are inoculated with DHV-3 QL3 strains through chorioallantoic membranes or yolk sacs for culture, the antigen contents of the prepared vaccines are high, and all not less than 10<8.5>ELD50 / ml, so that the duck hepatitis bivalent live vaccines have the characteristics of good safety and excellent immunogenicity, and break through limitations of DHV-3 type virus strains which only can be proliferated in duck embryos; 2) virus solutions cultured by the SPF chicken embryos lower risks of exogenous pathogenic microbial contamination, improve labor efficiency and reduce production costs, thereby being suitable for mass production; 3) the bivalent live vaccines disclosed by the invention can prevent DHV-1 type and DHV-3 type virulent viruses attacking on ducklings; 4) the duck hepatitis bivalent live vaccines are simple in production process, easy in quality control and small in batch difference, thereby providing a guarantee for producing safe and qualified vaccines.
Owner:QILU ANIMAL HEALTH PROD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com