Preparation method of porcine parvovirus inactivated vaccine
A parvovirus and inactivated vaccine technology, applied in the field of bioengineering, can solve the problem of low antibody titer, achieve the effect of increasing antigen content, extensive social and economic benefits, and improving immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] This implementation case illustrates the method for porcine parvovirus venom preparation:
[0027] 1. Cell preparation Take the cells out of the liquid nitrogen tank and place them in a 37°C water bath to melt them quickly. Transfer the cells into a cell bottle containing 10% fetal bovine serum cell growth medium and culture them at 37°C. When they grow into a good monolayer, use pancreatic Digest the cells with enzymatic digestion solution, and expand step by step according to 1:2 passaging. Inoculate the expanded seed cells into 15000ml spinner bottles, add 1000ml of cell growth solution containing 10% newborn bovine serum to each bottle, culture at 37°C, spin the bottle at a speed of 6-12 rpm, and grow into a good monolayer Afterwards, the cells were digested with trypsin solution, and divided into bottles at a ratio of 1:3 for passage.
[0028] 2. Virus propagation Take F13 generation BJ-2 strain PPV to produce seed virus, inoculate well-grown ST cells at 2% inocul...
Embodiment 2
[0031] This implementation case illustrates the method of purifying and concentrating porcine parvovirus:
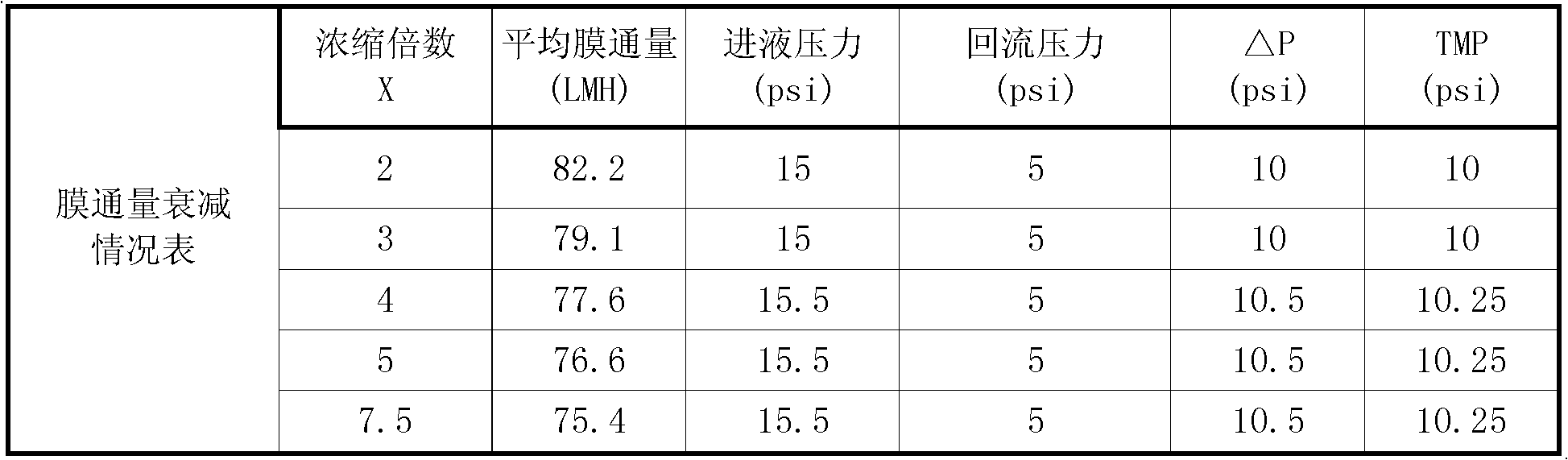
[0032] Take 20,000 milliliters of porcine parvovirus liquid with a hemagglutination value of 28, purify, concentrate, measure the hemagglutination value, and compare the difference before and after concentration. Specific steps are as follows:
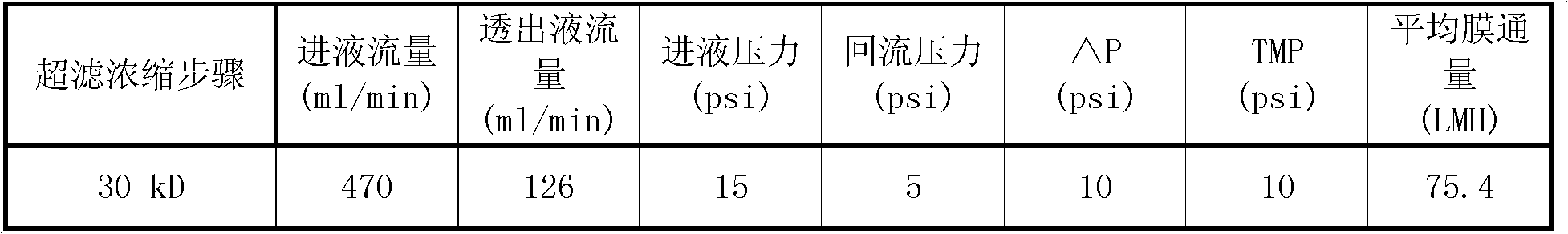
[0033] (1) Add the raw material solution to a microfiltration system with a pore size of 0.22 microns for filtration, return the concentrated solution to the raw material tank, and discharge the raw material solution after about 10 minutes; collect the osmotic pressure, and observe the permeate at this time, it is clear and bright, and it has been removed Impurities such as cell debris enter the next step of ultrafiltration concentration.
[0034] (2) Ultrafiltration concentration of parvovirus venom
[0035] Concentrate the pretreated permeate with a 1m2 polyethersulfone flat membrane bag with a molecular weight cut-off of 30K...
Embodiment 3
[0050] This implementation case illustrates the method of porcine parvovirus inactivation:
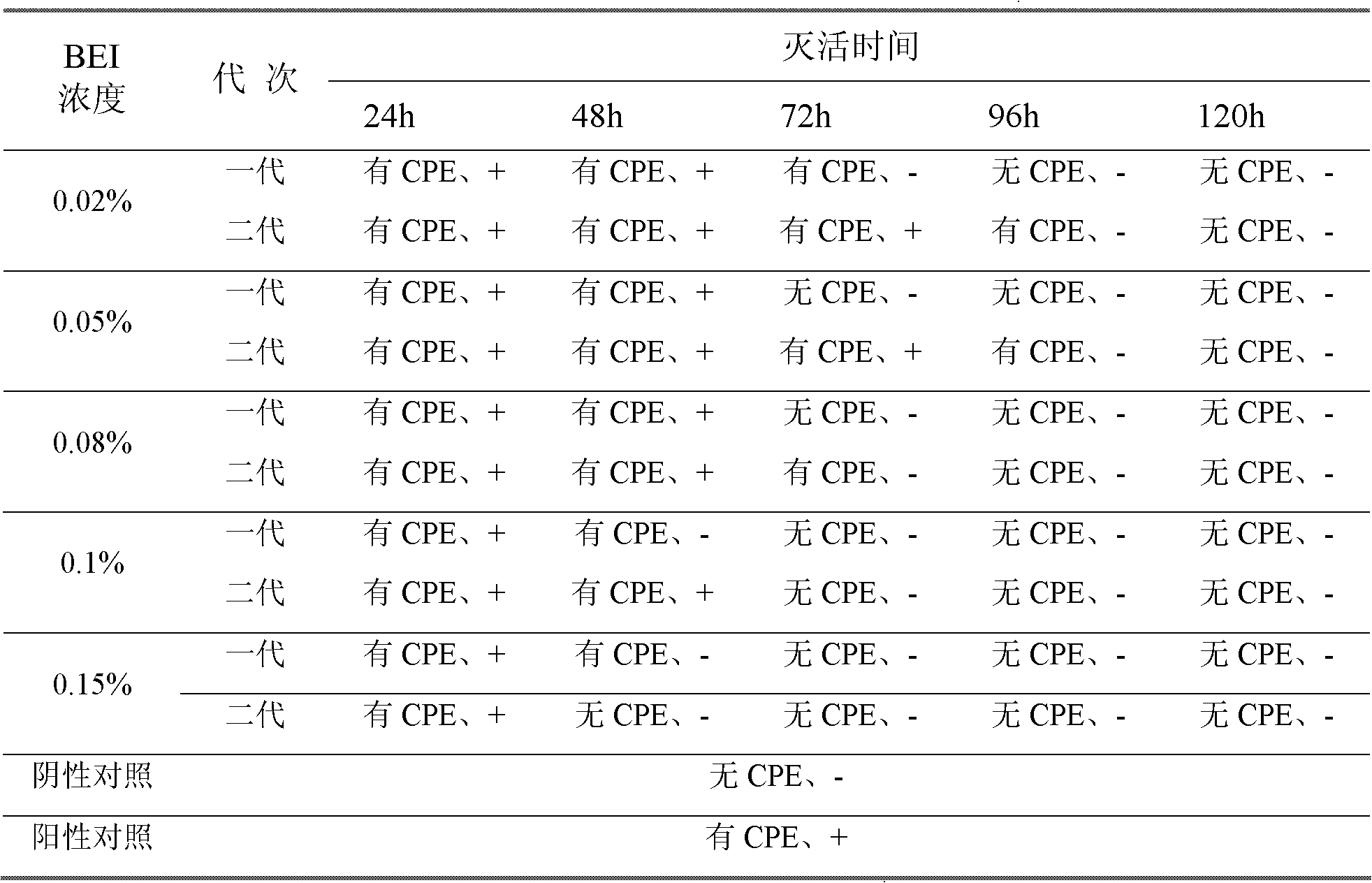
[0051] Diethyleneimine (BEI) inactivation test Take 5 bottles of porcine parvovirus antigen solution, add 0.02%, 0.05%, 0.08%, 0.1%, 0.15% final concentration of BEI (made by BEA cyclization), 32 ℃ Inactivation, samples were taken at 24, 48, 72, 96, and 120 hours of inactivation for inactivation testing, and the inactivated venom samples were diluted 10 times with serum-free cell nutrient solution, and then inoculated with cells that had grown into a single layer. Porcine testicular cells (ST cells), 1ml / bottle, after 1 hour of adsorption, replace with cell growth medium containing 2% fetal bovine serum and culture for 5 days, observe cell morphology and growth status, and determine whether there is cytopathic disease (CPE), The culture was harvested, frozen and thawed 3 times, and continuously passed on ST cells for 2 generations. At the same time, HA was detected to observe whether t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com