Group I serotype 4 avian adenovirus genetically engineered subunit vaccine, its preparation method and application
A gene and vaccine technology, applied in the field of genetic engineering subunit vaccines of group I serotype 4 avian adenovirus, can solve the problems of group I avian adenovirus prevention and control loopholes, difficulty in culturing chicken embryo liver cells, and difficulty in popularization and application, and achieves Low cost, easy to scale up, strong immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The construction of embodiment 1 recombinant baculovirus:
[0065] Using the Bacto Bac system to construct recombinant baculovirus,
[0066] According to the hexon gene sequence published by GeneBank, the following primers were designed to amplify the gene of the hexon antigen region
[0067] HX-P1: GGATCCATGGGAAGCTACTTTGACTTGAAAAAC
[0068] HX-P2: GAATTCGTATTCGGTGCCCGCGTTATTC
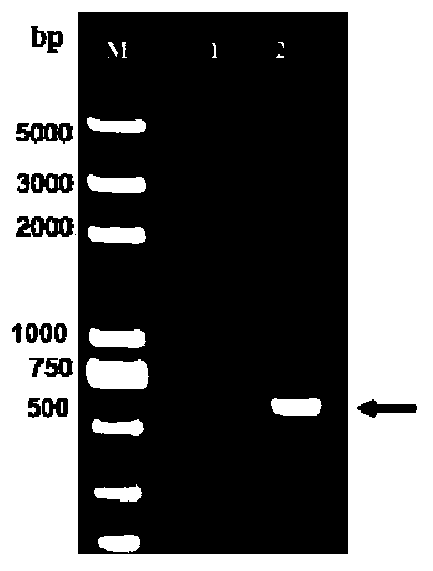
[0069] The genome of the isolated group I serotype 4 avian adenovirus MD-4 strain was extracted, and its genome was used as a template, and HX-P1 and HX-P2 were used as primers to amplify the gene fragment Hx of the main antigen region of the hexon, and the The gene was cloned into the pMD-19T vector to obtain the recombinant vector pMD-Hx. Then pMD-Hx was digested by BamHI and EcoRI, and the Hx gene fragment was cloned into the transfer vector pFastBac 1 to construct the recombinant vector pF-Hx.
[0070] According to the E. coli heat-sensitive toxin B subunit gene sequence and the E. coli ...
Embodiment 2
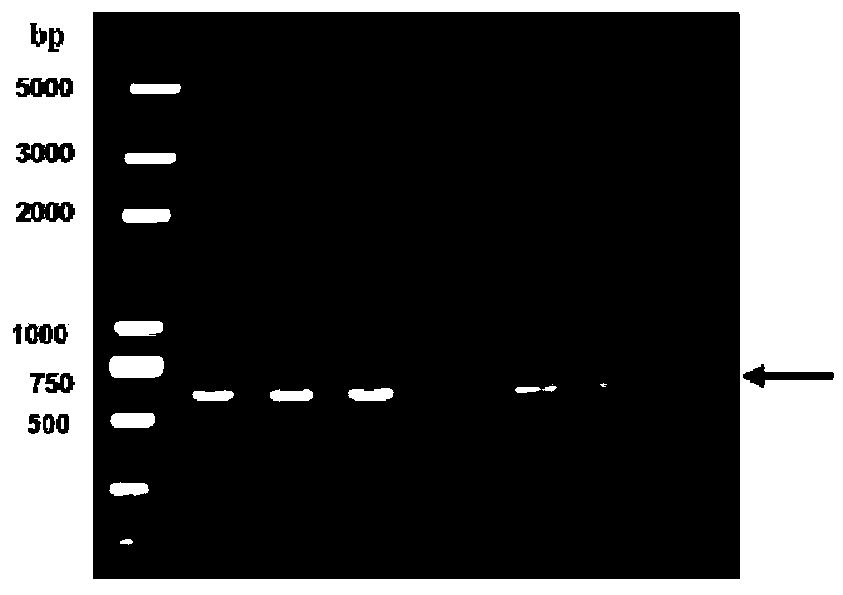
[0082] Transform the recombinant transfer vector pMD-Hx-STLT-Pt into Escherichia coli DH10Bac to obtain the recombinant plasmid Bacmid-Hx-STLT-Pt inserted into the fusion antigen protein gene of group I serotype 4 avian adenovirus, and transfect the recombinant plasmid into insects In Sf9 cells, the recombinant baculovirus rBac-Hx-STLT-Pt was obtained, and the recombinant baculovirus rBac-Hx-STLT-Pt was amplified as a seed virus for future use.
[0083] SDS-PAGE detection: The harvested cell cultures were subjected to SDS-PAGE detection, and Sf9 cells infected with empty baculovirus were used as a negative control. The specific operation is as follows: take 40 μl of harvested cell culture, add 10 μl of 5×loadingbuffer, bathe in boiling water for 5 minutes, centrifuge at 12000 r / min for 1 minute, take the supernatant and carry out SDS-PAGE gel (12% concentration gel) electrophoresis, electrophoresis Afterwards, the gel was stained and decolorized to observe the target band. Su...
Embodiment 3
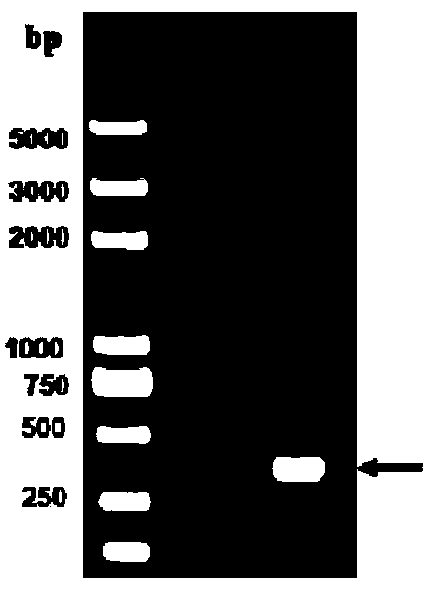
[0088] Example 3 Bioreactor Serum-Free Suspension Culture of Insect Cells and Quantitative Expression of Hx-STLT-Pt Protein and Determination of Agar Expansion Titer
[0089] Aseptically culture sf9 insect cells in a 1000ml shake flask for 3-4 days until the concentration reaches 3-5×10 6 cell / mL, when the viability is greater than 95%, inoculate the cells into a 5L bioreactor with an inoculation concentration of 3-8×10 5 cell / mL. When the cell concentration reaches 3-55×10 6 When cell / mL, inoculate the cells into a 50L bioreactor, and wait for the cells to grow to a concentration of 3-55×10 6 cell / mL, inoculate into a 500L bioreactor until the cell concentration reaches 2-85×10 6 When cell / mL, the recombinant baculovirus rBac-Hx-STLT-PT was inoculated, and the reactor culture conditions were pH 6.0-6.5, temperature 25-27°C, dissolved oxygen 30-80%, and stirring speed 100-180rpm. Considering the optimal conditions for cell culture, the preferred pH is 6.2, the temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com