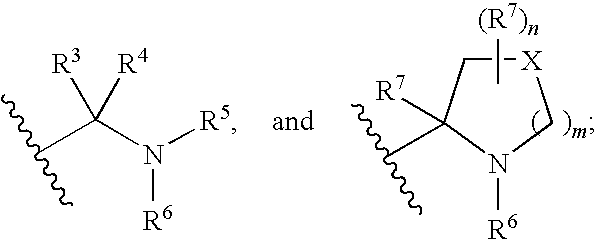
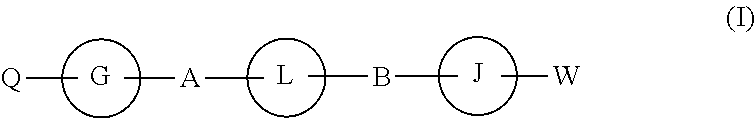Patents
Literature
2107results about "Inactivation/attenuation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
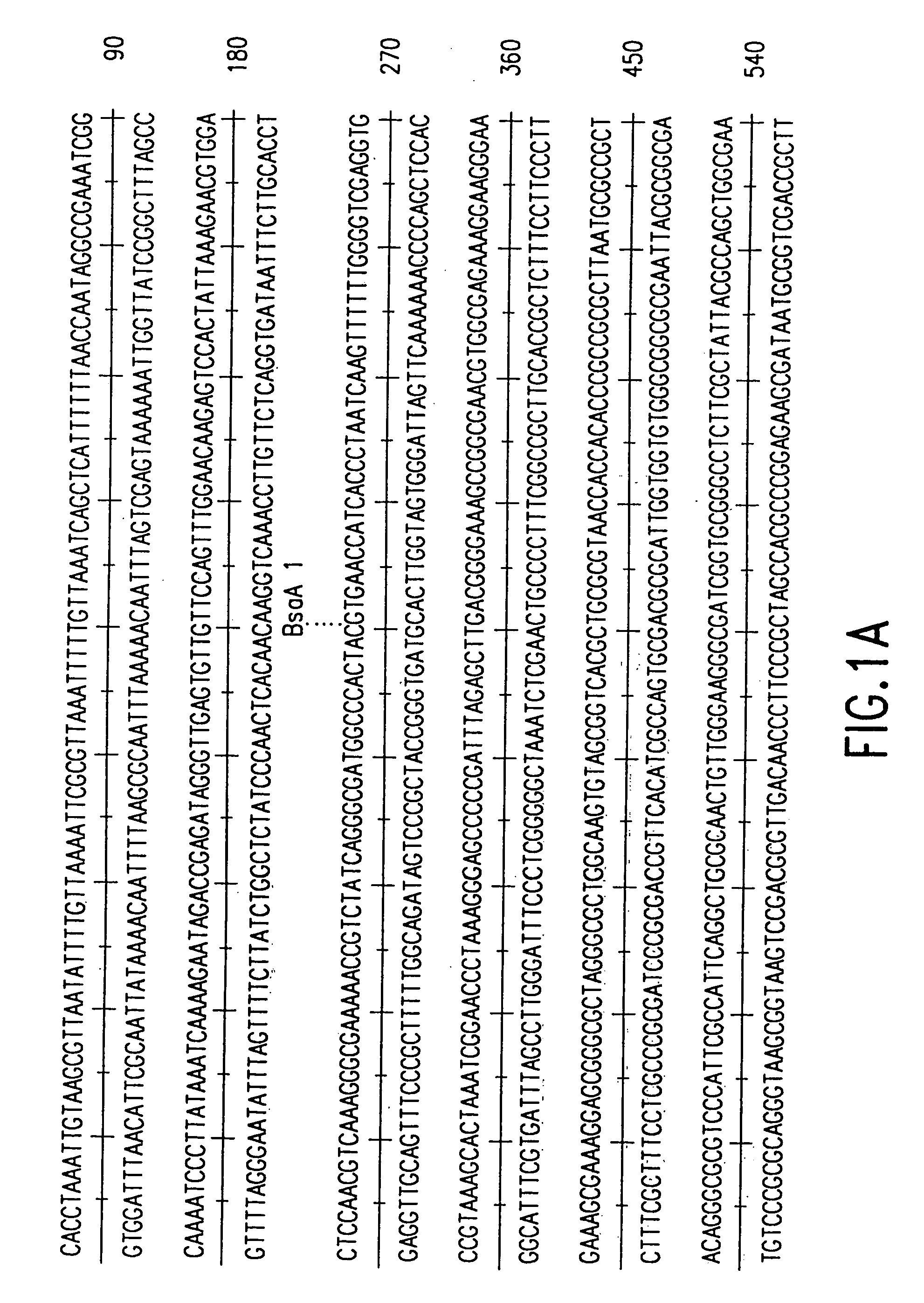
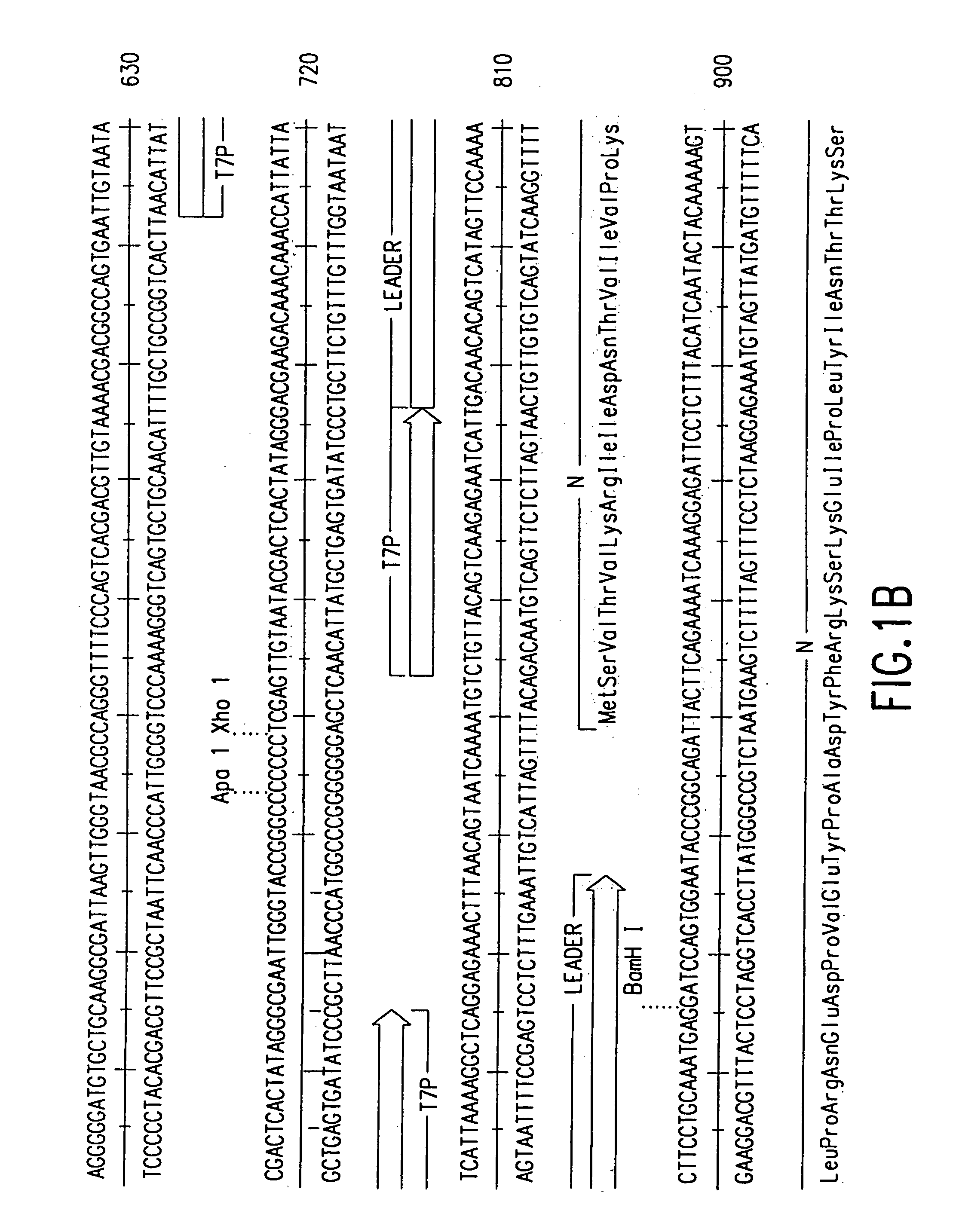
DNA transfection system for the generation of infectious influenza virus
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Reduction of porcine circovirus-2 viral load with inactivated PCV-2
InactiveUS6517843B1Improving immunogenicityImprove performanceBiocideGenetic material ingredientsDiseaseStaining
Porcine circovirus-2 (PCV-2) is a recently identified agent wherein the potential spectrum of PCV-2-associated disease has been expanded by evidence of vertical and sexual transmission and associated reproductive failure in swine populations. PCV-2 was isolated from a litter of aborted piglets from a farm experiencing late term abortions and stillbirths. Severe, diffuse myocarditis was present in one piglet associated with extensive immunohistochemical staining for PCV-2 antigen. Variable amounts of PCV-2 antigen were also present in liver, lung and kidney of multiple fetuses. Inoculation of female pigs with a composition including an immunogen from PCV-2 or an epitope of interest from such an immunogen or with a vector expressing such an immunogen or epitope of interest prior to breeding, such as within the first five weeks of life, or prior to the perinatal period, or repeatedly over a lifetime, or during pregnancy, such as between the 6th and 8th and / or the 10th and 13th weeks of gestation, can prevent myocarditis, abortion and intrauterine infection associated with porcine circovirus-2. In addition, innoculation of male and / or female pigs with the aforementioned compositions can be carried out to prevent transmission of PCV-2 from male to female (or vice versa) during mating. Thus, the invention involves methods and compositions for preventing myocarditis, abortion and intrauterine infection associated with porcine circovirus-2.
Owner:QUEENS UNIV OF BELFAST +4
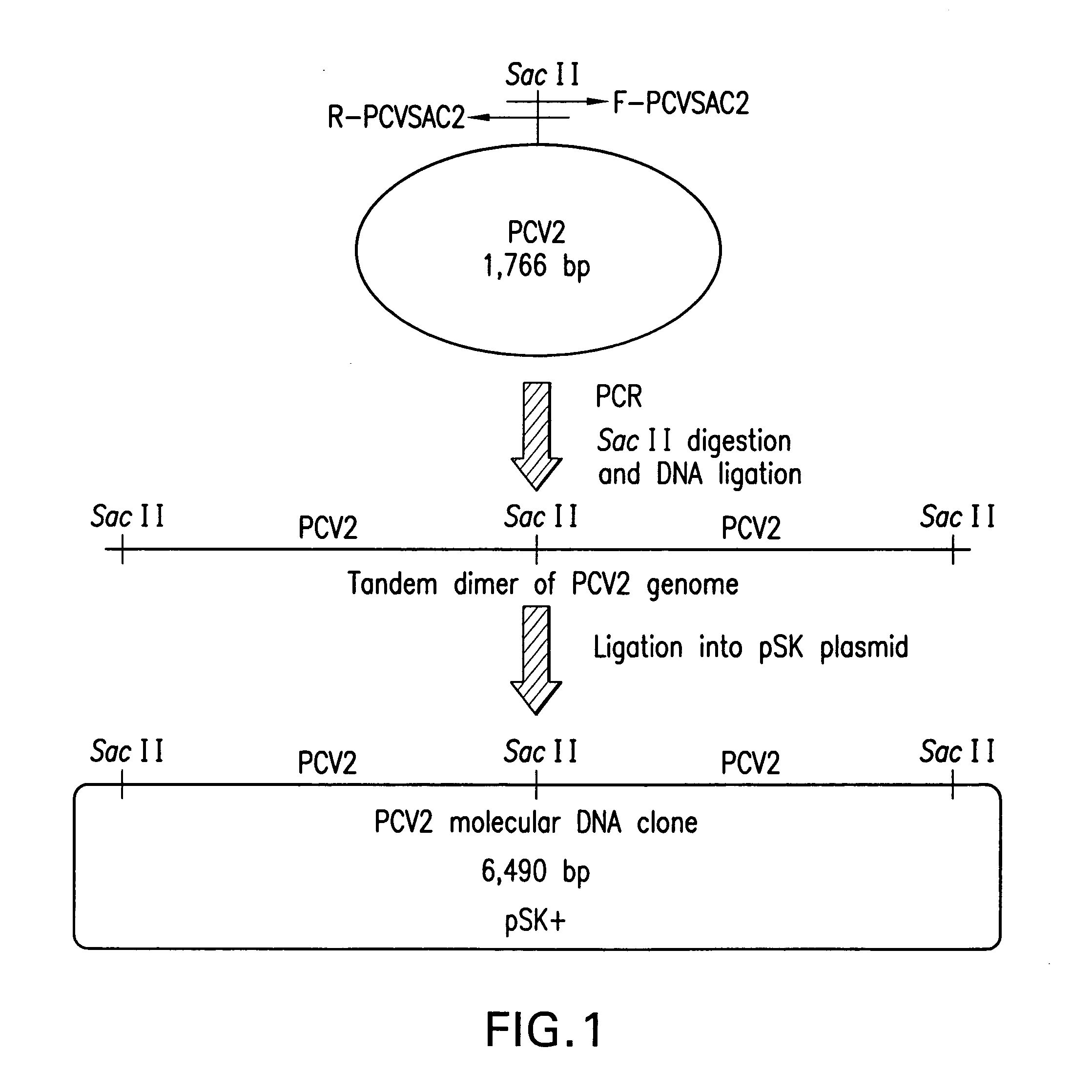
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
Multi plasmid system for the production of influenza virus
InactiveUS20050266026A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggCold adapted
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. A method of producing a cold adapted (ca) influenza virus that replicates efficiently at, e.g., 25° C. (and immunogenic compositions comprising the same) is also provided.
Owner:MEDIMMUNE LLC
Screening for west nile virus antiviral therapy
InactiveUS20050058987A1Improve efficiencySsRNA viruses positive-senseVectorsHigh-Throughput Screening MethodsImmunogenicity
The instant invention provides stable and novel lineage I WNV reverse genetics systems, and methods for making the reverse genetics systems, specifically, a fully-infectious lineage I WNV cDNA or replicon system engineered with one or more nucleotide sequences each encoding a reporter gene to be used in high throughput cell-based screening assays for the identification of novel antiflaviviral chemotherapeutics and / or vaccines effective to treat and / or immunize against infections by WNV and other emerging flaviviruses, such as, for example, JEV, SLEV, AV, KV, JV, CV, YV, TBEV, DENV-1, DENV-2, DENV-3, DENV-4, YFV and MVEV. The present invention further provides methods of high throughput screening of antiflaviviral compounds or improved derivatives thereof using novel lineage I WNV reverse genetics systems and / or cell lines stably containing the reverse genetics systems. Also, the invention provides novel pharmaceutical compositions comprising an attenuated lineage I WNV that is less virulent but similarly immunogenic as the parent WNV and is capable of providing a protective immune response in a host.
Owner:HEALTH RES INC
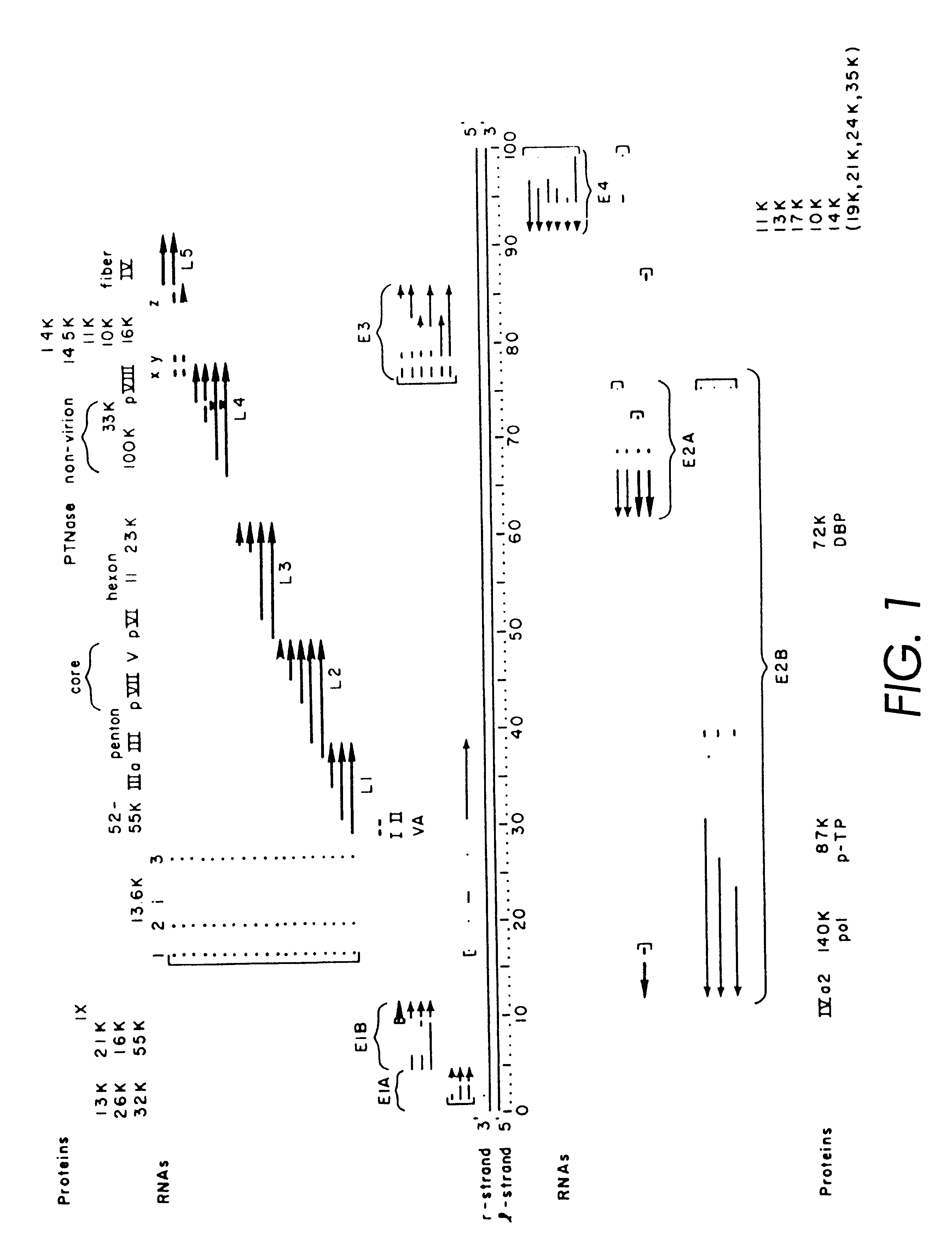
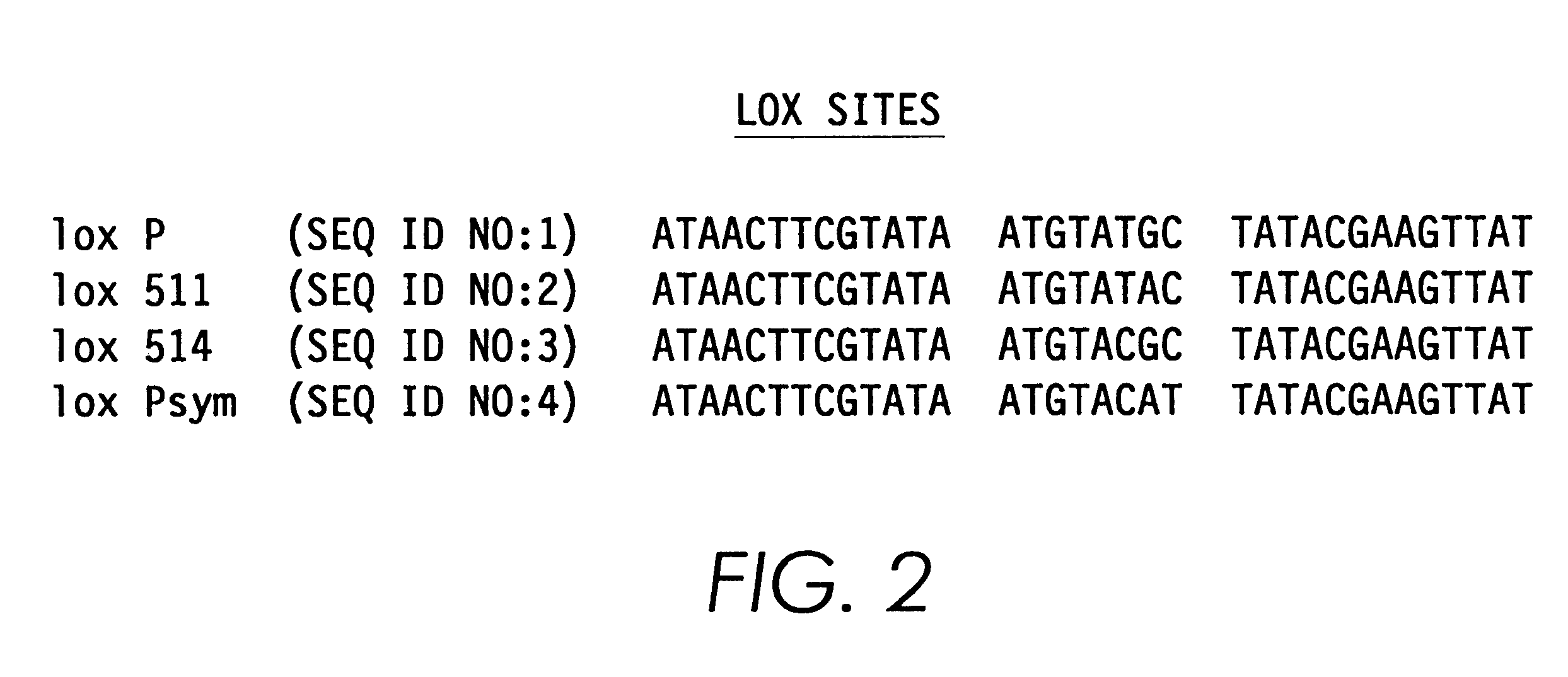
Helper-free, totally defective adenovirus for gene therapy
InactiveUS6228646B1Encapsidation efficiencyMaximal recombinationInactivation/attenuationNucleic acid vectorIn vivoHelper virus
A method for producing in vivo packaged recombinant adenovirus vectors is provided. The recombinant Ad vectors do not contain any Adenovirus genes and are therefore useful for gene therapy. The recombinant Adenovirus vectors are packaged in vivo using a helper virus which is itself very inefficiently packaged, providing a recombinant viral preparation with very little or no contamination with helper virus. In particular, the method makes use of a helper virus in which the packaging site can be easily excised in vivo by recombination mediated by a recombinase. The helper virus is also useful for the in vivo construction of new recombinant adenovirus vectors containing substitutions in the E1 or other adenoviral region.
Owner:RGT UNIV OF CALIFORNIA
Method of inducing a CTL response
Disclosed herein are methods for inducing an immunological CTL response to an antigen by sustained, regular delivery of the antigen to a mammal so that the antigen reaches the lymphatic system. Antigen is delivered at a level sufficient to induce an immunologic CTL response in a mammal and the level of the antigen in the mammal's lymphatic system is maintained over time sufficient to maintain the immunologic CTL response. Also disclosed is an article of manufacture for delivering an antigen that induces a CTL response in an animal.
Owner:MANNKIND CORP
Method of producing a vaccine which raises an immunological response against a virus causing a porcine respiratory and reproductive disease
InactiveUS6251404B1Efficient responseAntibacterial agentsSsRNA viruses positive-senseInfectious agentRespiratory disease
The present invention provides a vaccine which protects pigs from a virus and / or an infectious agent causing a porcine respiratory and reproductive disease, a method of protecting a pig from a disease caused by a virus and / or an infectious agent which causes a respiratory and reproductive disease, a method of producing a vaccine against a virus and / or an infectious agent causing a porcine reproductive and respiratory disease, and a biologically pure sample of a virus and / or infectious agent associated with a porcine respiratory and reproductive disease, particularly the Iowa strain of porcine reproductive and respiratory syndrome virus (PRRSV), and an isolated polynucleotide which is at least 90% homologous with a polynucleotide obtained from the genome of a virus and / or infectious agent which causes a porcine respiratory and reproductive disease.
Owner:ZOETIS WHC 2
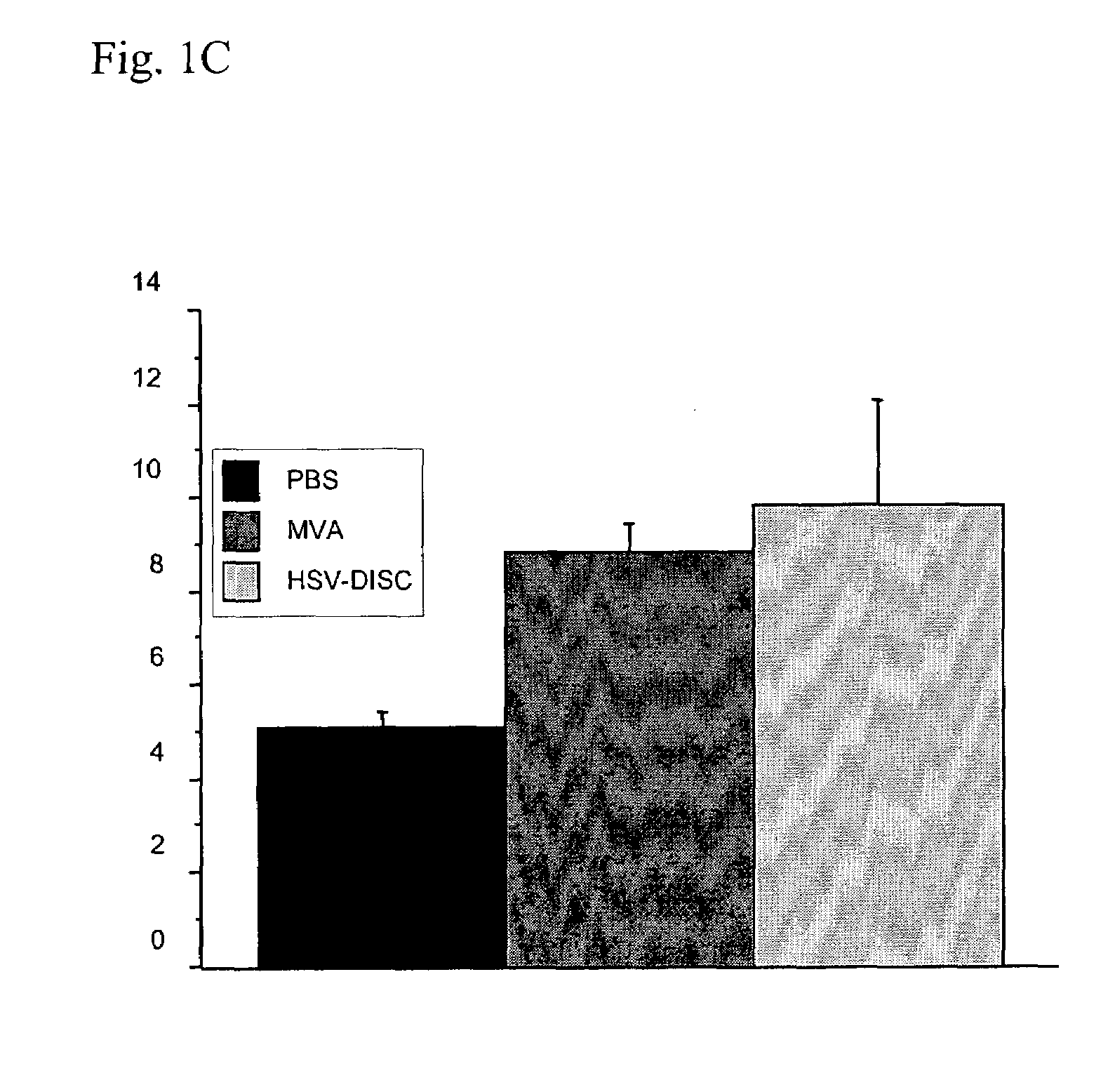
Modified vaccinia virus ankara for the vaccination of neonates
Owner:BAVARIAN NORDIC AS
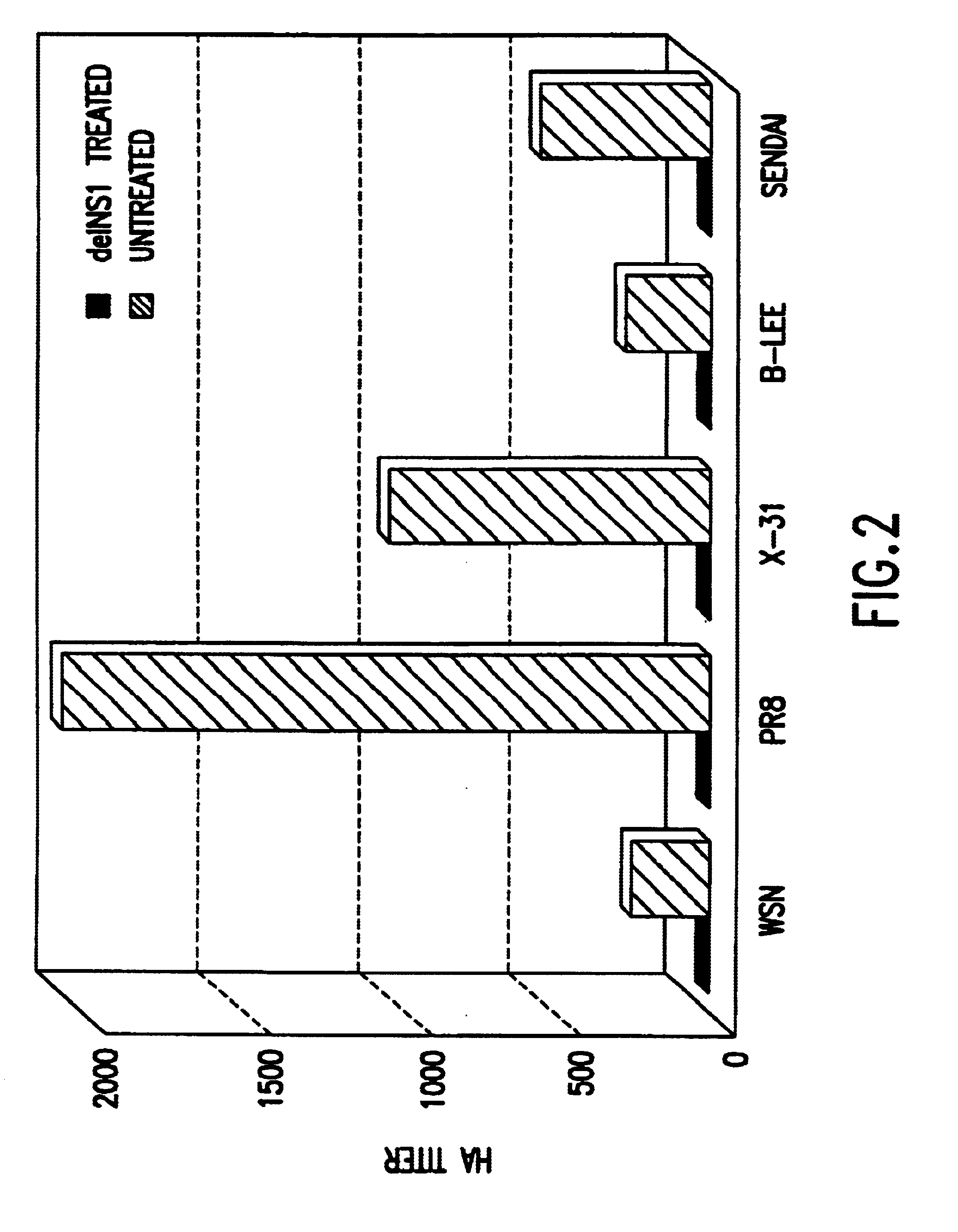
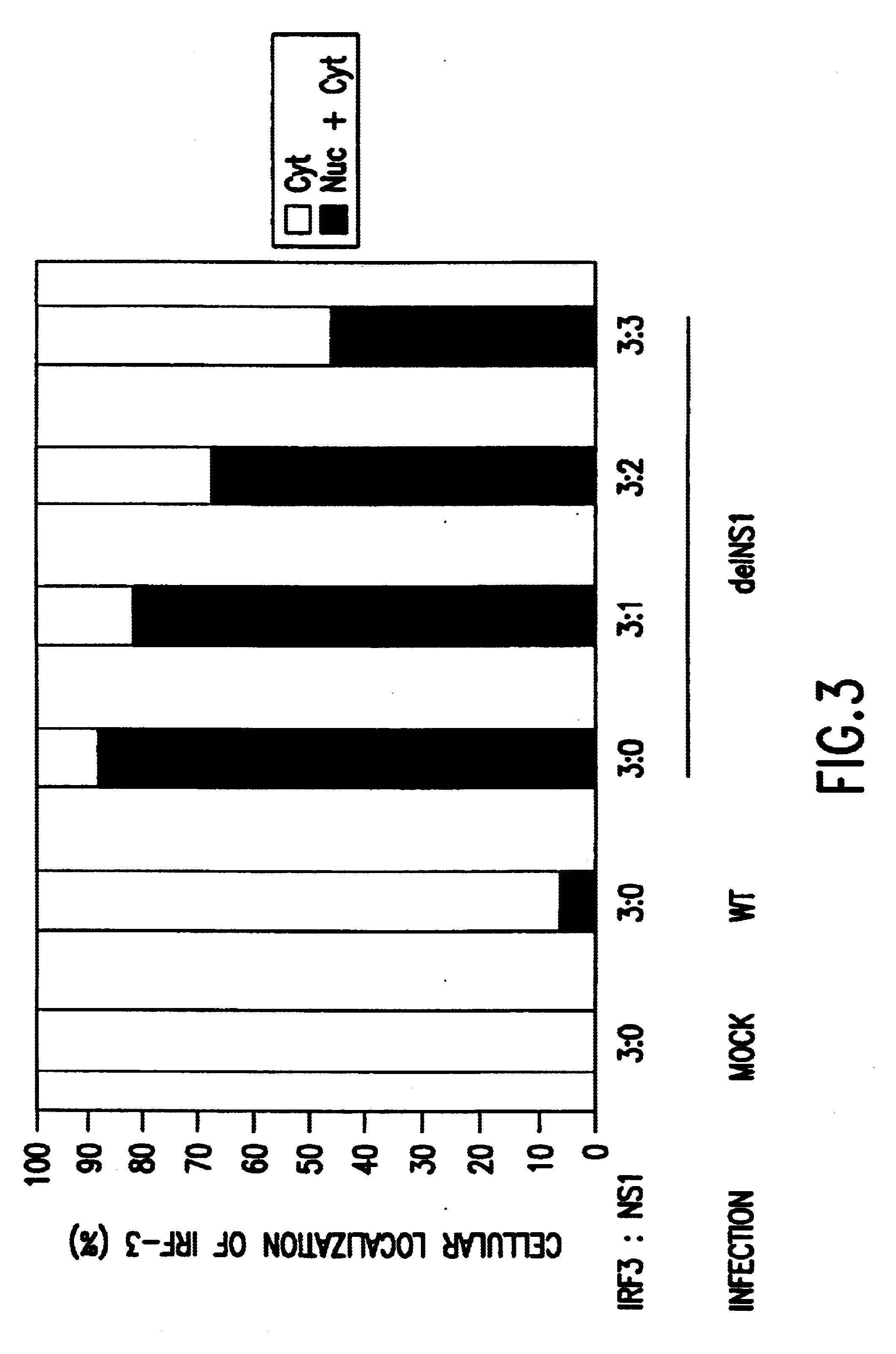
Interferon inducing genetically engineered attenuated viruses
InactiveUS6468544B1Reduce in quantityReduced characteristicsSsRNA viruses negative-senseVectorsGenetic engineeringRecombinant DNA
The present invention relates to genetically engineered attenuated viruses and methods for their production. In particular, the present invention relates to engineering live attenuated viruses which contain a modified NS gene segment. Recombinant DNA techniques can be utilized to engineer site specific mutations into one or more noncoding regions of the viral genome which result in the down-regulation of one or more viral genes. Alternatively, recombinant DNA techniques can be used to engineer a mutation, including but not limited to an insertion, deletion, or substitution of an amino acid residue(s) or an epitope(s) into a coding region of the viral genome so that altered or chimeric viral proteins are expressed by the engineered virus.
Owner:MT SINAI SCHOOL OF MEDICINE +1
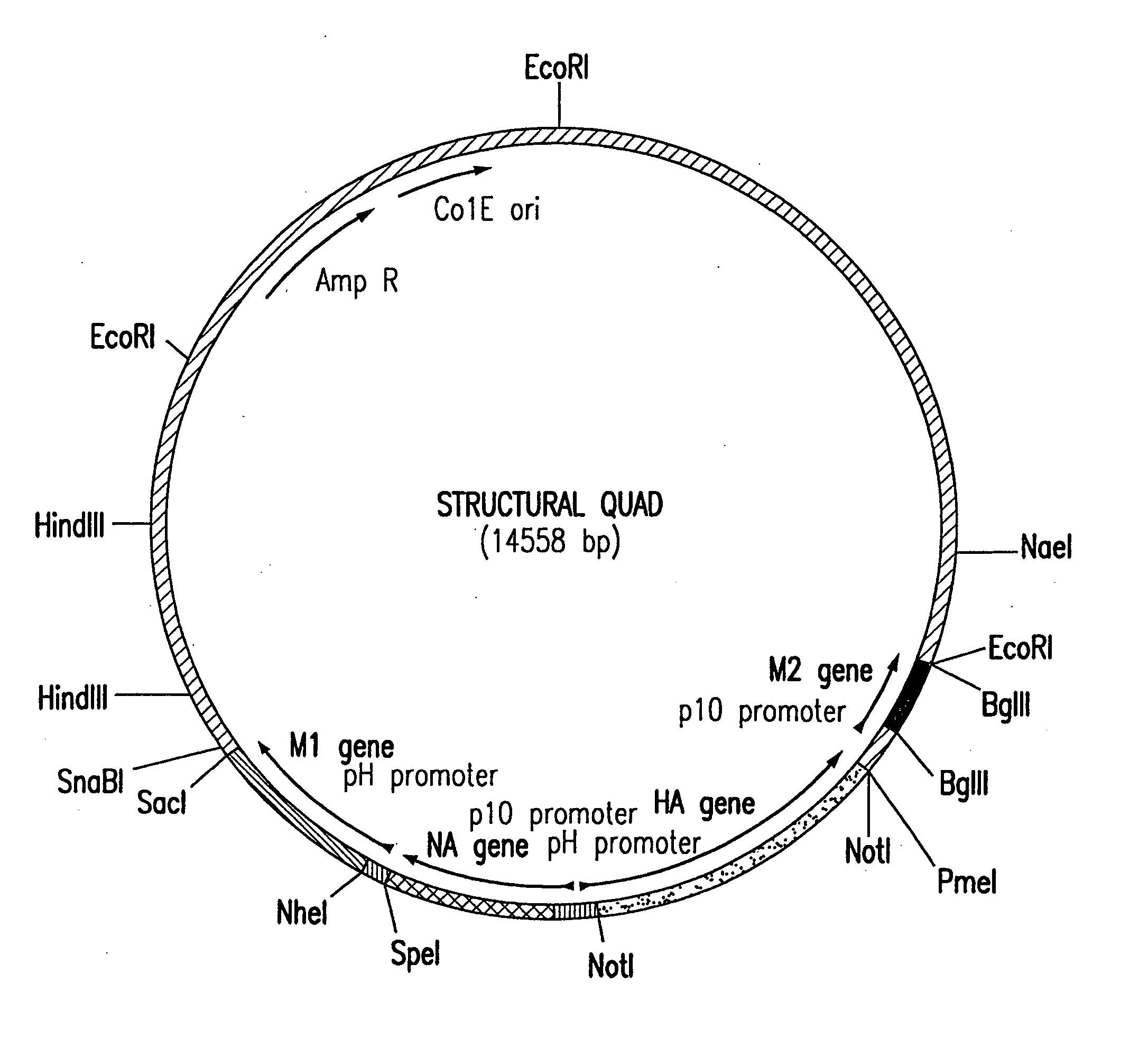
Assembly of wild-type and chimeric influenza virus-like particles (VLPs)
InactiveUS20050186621A1Minimal numberSsRNA viruses negative-senseFungiHeterologousVirus-like particle
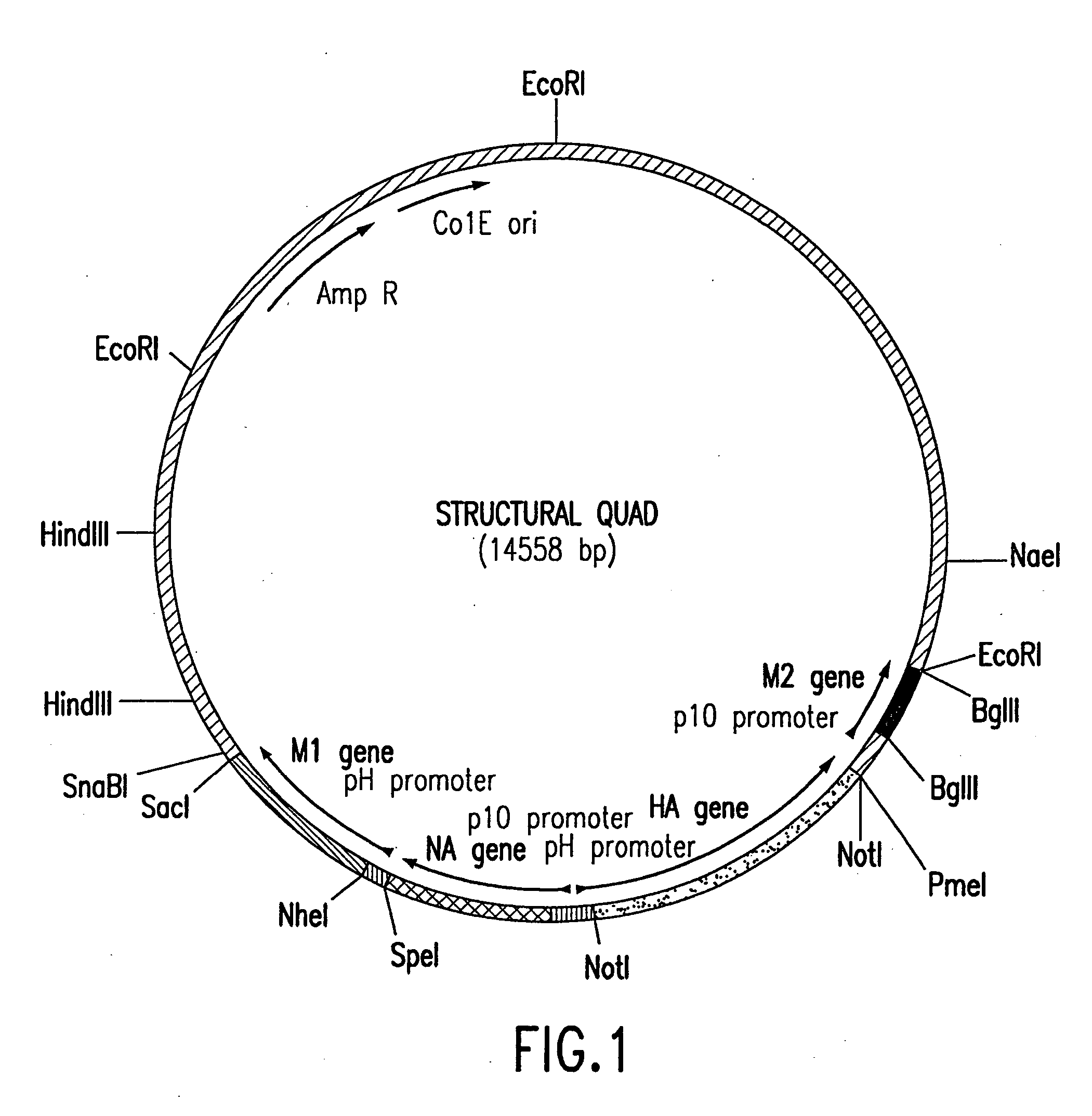
Influenza virus-like particles (VLPs) comprising the structural proteins HA, NA, M1 and M2 are described. VLPs are also generated containing M1 alone, as are VLPs with M1 and any one or two of HA, NA and M2. VLPs with HA from one influenza subtype and NA from a different influenza subtype are also described, as are VLPs in which a portion or all of HA or NA is replaced by a heterologous moiety not produced by influenza virus, so as to comprise chimeric VLPs.
Owner:WYETH HOLDINGS LLC
Method of inducing a CTL response
InactiveUS20020007173A1Thorough eradicationEasy to useVirusesPeptide/protein ingredientsAntigenMammal
Disclosed herein are methods for inducing an immunological CTL response to an antigen by sustained, regular delivery of the antigen to a mammal so that the antigen reaches the lymphatic system. Antigen is delivered at a level sufficient to induce an immunologic CTL response in a mammal and the level of the antigen in the mammal's lymphatic system is maintained over time sufficient to maintain the immunologic CTL response. Also disclosed is an article of manufacture for delivering an antigen that induces a CTL response in an animal.
Owner:MANNKIND CORP
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
Attenuated negative strand viruses with altered interferon antagonist activity for use as vaccines and pharmaceuticals
InactiveUS6669943B1Impaired ability to antagonizeAvoiding and minimizing side effectSsRNA viruses negative-senseVirus peptidesNegative strandPharmaceutical drug
Owner:AVIR GREEN HILLS BIOTECH RES DEVMENT TRADE +1
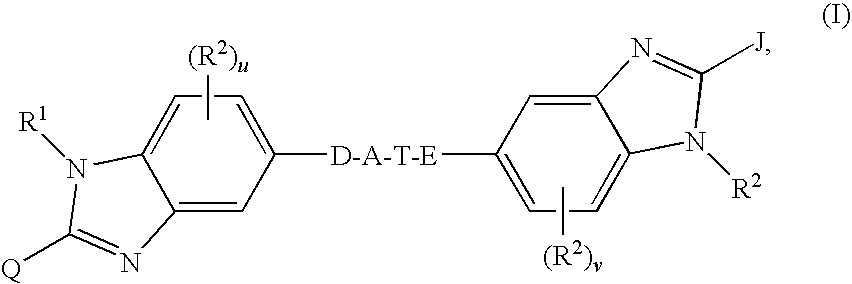
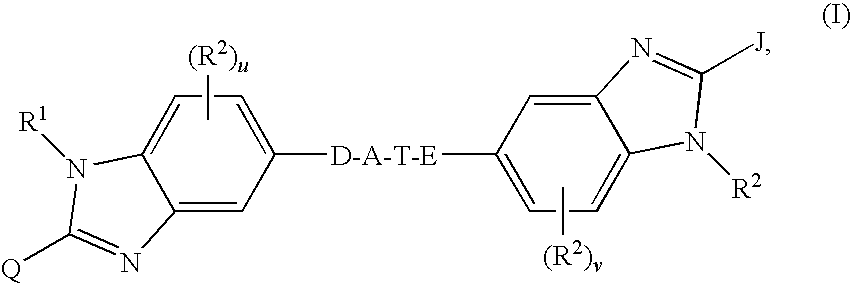
Linked dibenzimidazole derivatives
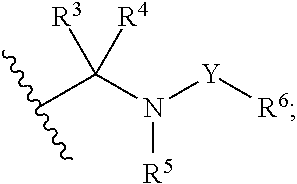
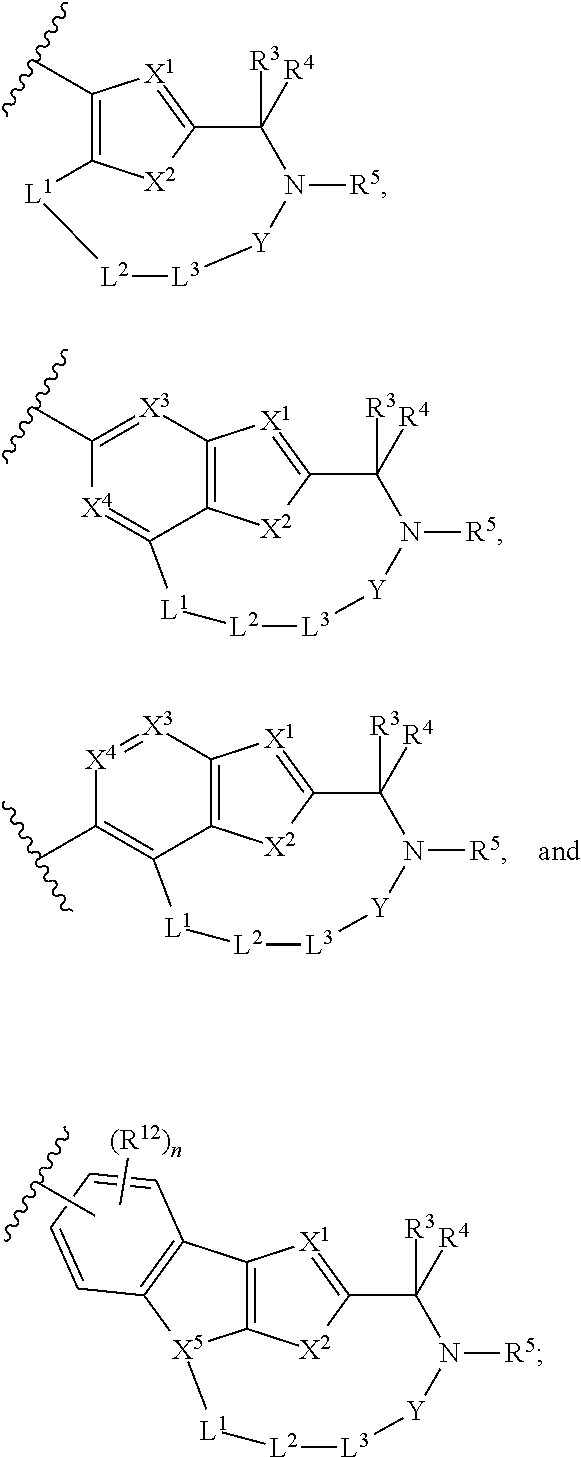
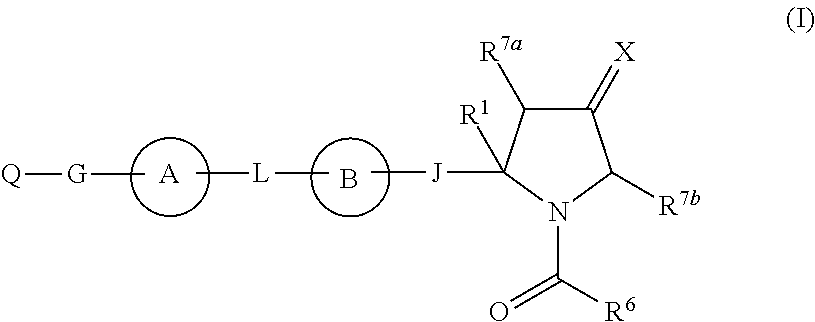
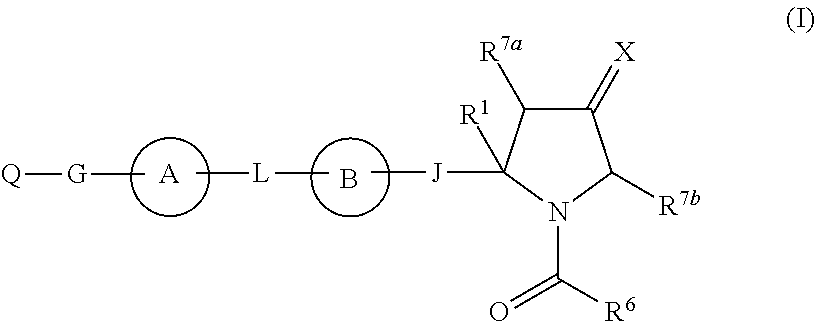
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit RNA-containing virus, particularly the hepatitis C virus (HCV). Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Isolated porcine respiratory and reproductive virus, vaccines and methods of protecting a pig against a disease caused by a porcine respiratory and reproductive virus
InactiveUS6110467AEfficient responseAntibacterial agentsSsRNA viruses positive-senseInfectious agentRespiratory disease
The present invention provides a vaccine which protects pigs from a virus and / or an infectious agent causing a porcine respiratory and reproductive disease, a method of protecting a pig from a disease caused by a virus and / or an infectious agent which causes a respiratory and reproductive disease, a method of producing a vaccine against a virus and / or an infectious agent causing a porcine reproductive and respiratory disease, and a biologically pure sample of a virus and / or infectious agent associated with a porcine respiratory and reproductive disease, particularly the Iowa strain of porcine reproductive and respiratory syndrome virus (PRRSV), and an isolated polynucleotide which is at least 90% homologous with a polynucleotide obtained from the genome of a virus and / or infectious agent which causes a porcine respiratory and reproductive disease.
Owner:ZOETIS WHC 2
Recombinant vesiculoviruses and their uses
The invention provides recombinant replicable vesiculoviruses. The invention provides a method which, for the first time, successfully allows the production and recovery of replicable vesiculoviruses, as well as recombinant replicable vesiculoviruses, from cloned DNA, by a method comprising expression of the full-length positive-strand vesiculovirus antigenomic RNA in host cells. The recombinant vesiculoviruses do not cause serious pathology in humans, can be obtained in high titers, and have use as vaccines. The recombinant vesiculoviruses can also be inactivated for use as killed vaccines.
Owner:YALE UNIV
Influenza virus vaccines and uses thereof
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
Hepatitis c virus inhibitors
ActiveUS20110189129A1Improve pharmacokineticsImproving the pharmacokinetics of drugsBiocidePeptide/protein ingredientsHepatitis c viralPharmaceutical drug
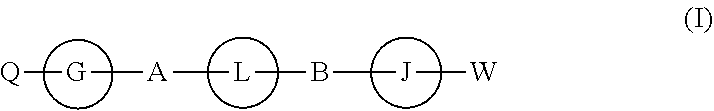
The present invention discloses compounds of Formula (I), and pharmaceutically acceptable salts, esters, or prodrugs thereof:Q-G-A-L-B—W (I),which inhibit RNA-containing virus, particularly the hepatitis C virus (HCV). Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Human immunodeficiency virus type 1 nucleic acids devoid of long terminal repeats capable of encoding for non-infectious, immunogenic, retrovirus-like particles
InactiveUS6080408AImprove efficiencyLow backgroundSugar derivativesViral antigen ingredientsPol genesReverse transcriptase activity
Non-infectious, retrovirus-like particles contain mutations to reduce gag-dependent RNA-packaging of the gag gene product, eliminate reverse transcriptase activity of the pol gene product, eliminate integrase activity of the pol gene product and eliminate RNase H activity of the pol gene product through genetic manipulation of the gag and pol genes. The corresponding nucleic acid molecules are described. The non-infectious, retrovirus-like particles have utility in diagnosis.
Owner:CONNAUGHT LAB
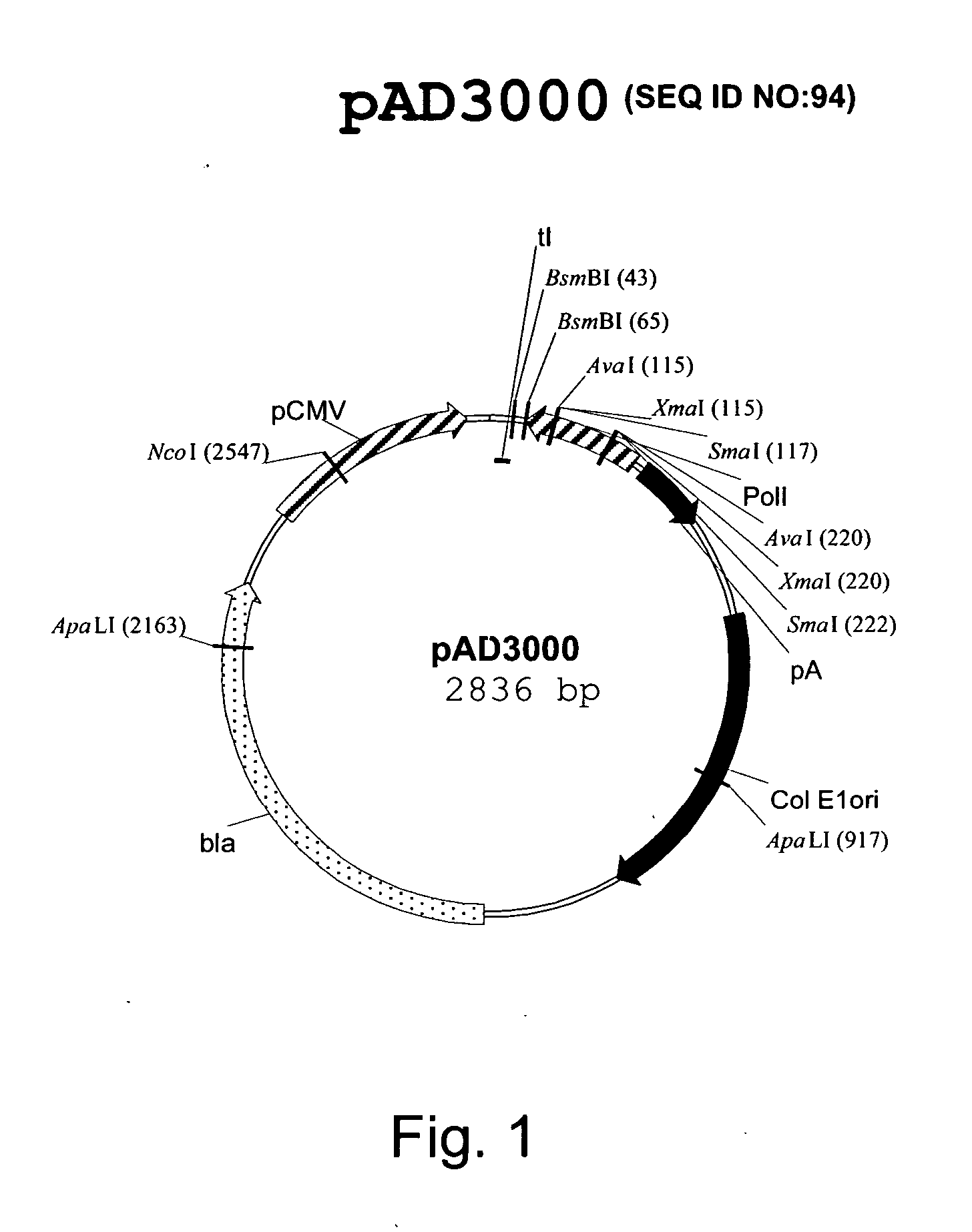
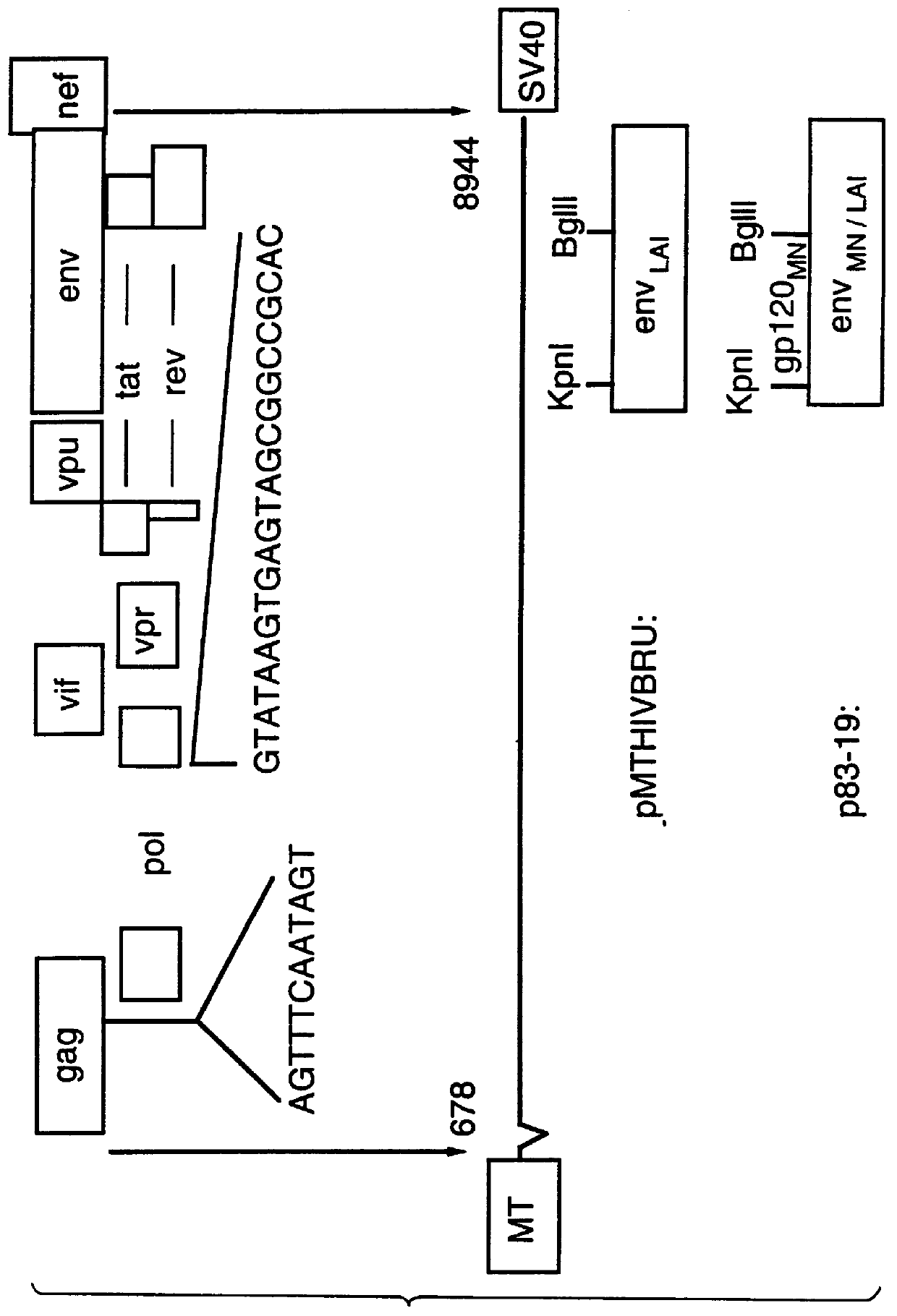
DNA transfection system for the generation of infectious influenza virus
InactiveUS20050186563A1Improve effectivenessElicit protective immunitySsRNA viruses negative-senseFungiDual promoterSingle-Stranded RNA
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuraminidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Alphavirus particles and methods for preparation
ActiveUS7078218B2Improve salt wash recoveryIncrease ionic strengthSsRNA viruses positive-senseGenetic material ingredientsHigh densityPotassium
Provided herein are methods for producing alphavirus replicon particles in high yield; replicon RNAs are electroporated into permissive cells, where the cells are at a relatively high density, together with at least one helper nucleic acid providing the necessary functions for packaging. After a growth period in appropriate medium, alphavirus replicon particles are harvested from the surfaces of the cells in which they were produced using a salt wash in which the salt concentration is from about 0.2 to about 5 M sodium chloride, calcium chloride, magnesium chloride, potassium chloride, ammonium acetate, ammonium bicarbonate, among others. After dilution, if necessary, the particles can be purified by a suitable chromatographic technique.
Owner:ALPHAVAX INC
Functional influenza virus-like particles (VLPs)
Recombinant influenza virus proteins, including influenza capsomers, subviral particles, virus-like particles (VLP), VLP complexes, and / or any portions of thereof, are provided as a vaccine for influenza viruses. The invention is based on the combination of two vaccine technologies: (1) intrinsically safe recombinant vaccine technology, and (2) highly immunogenic, self-assembled protein macromolecules embedded in plasma membranes and comprised of multiple copies of influenza virus structural proteins exhibiting neutralizing epitopes in native conformations. More specifically, this invention relates to the design and production of functional homotypic and heterotypic recombinant influenza virus-like particles (VLPs) comprised of recombinant structural proteins of human influenza virus type A / Sydney / 5 / 94 (H3N2) and / or avian influenza virus type A / Hong Kong / 1073 / 99 (H9N2) in baculovirus-infected insect cells and their application as a vaccine in the prevention of influenza infections and as a laboratory reagent for virus structural studies and clinical diagnostics.
Owner:NOVAVAX
Hepatitis c virus inhibitors
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit RNA-containing virus, particularly the hepatitis C virus (HCV). Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products.
Owner:IOWA STATE UNIV RES FOUND +1
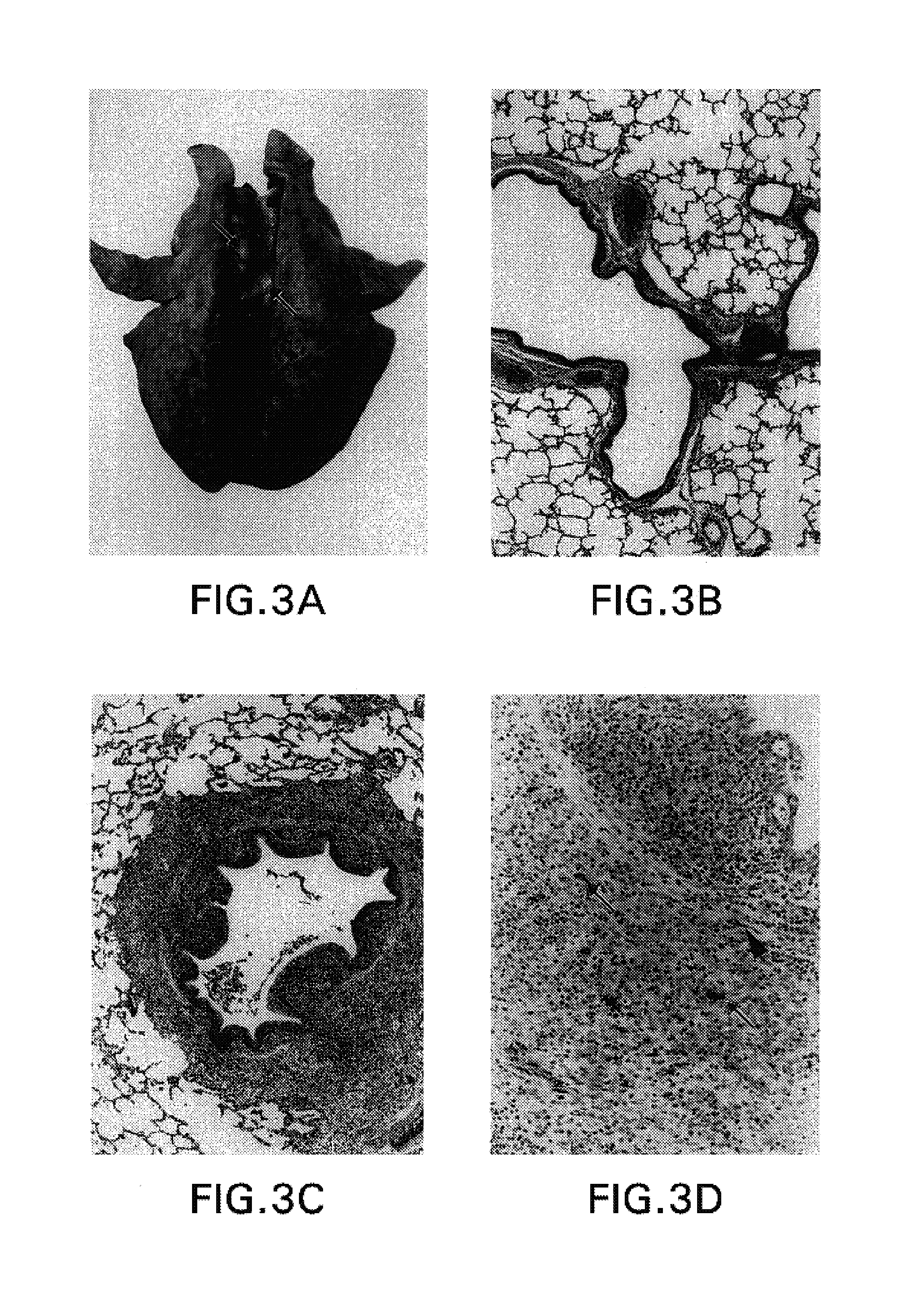
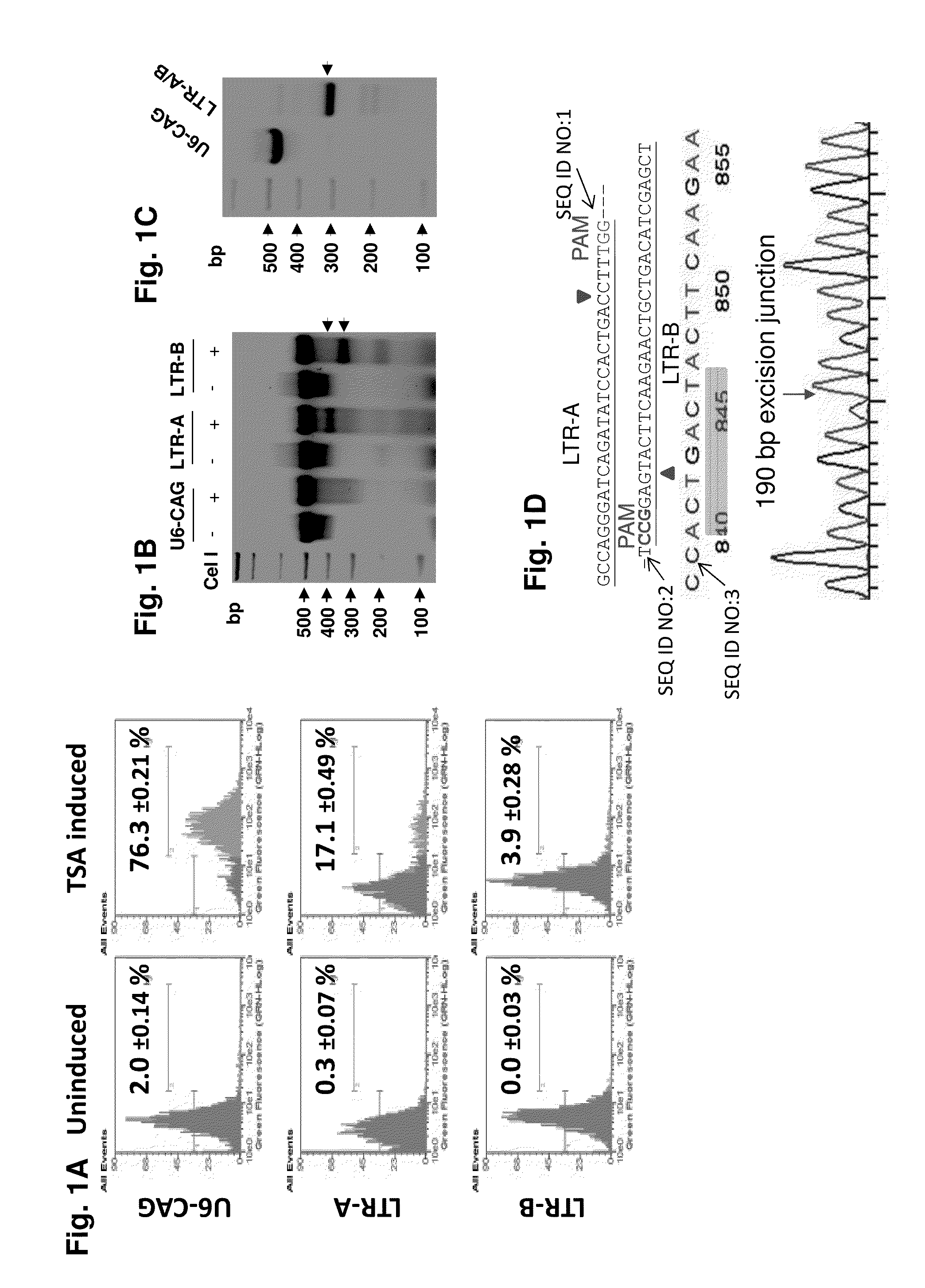
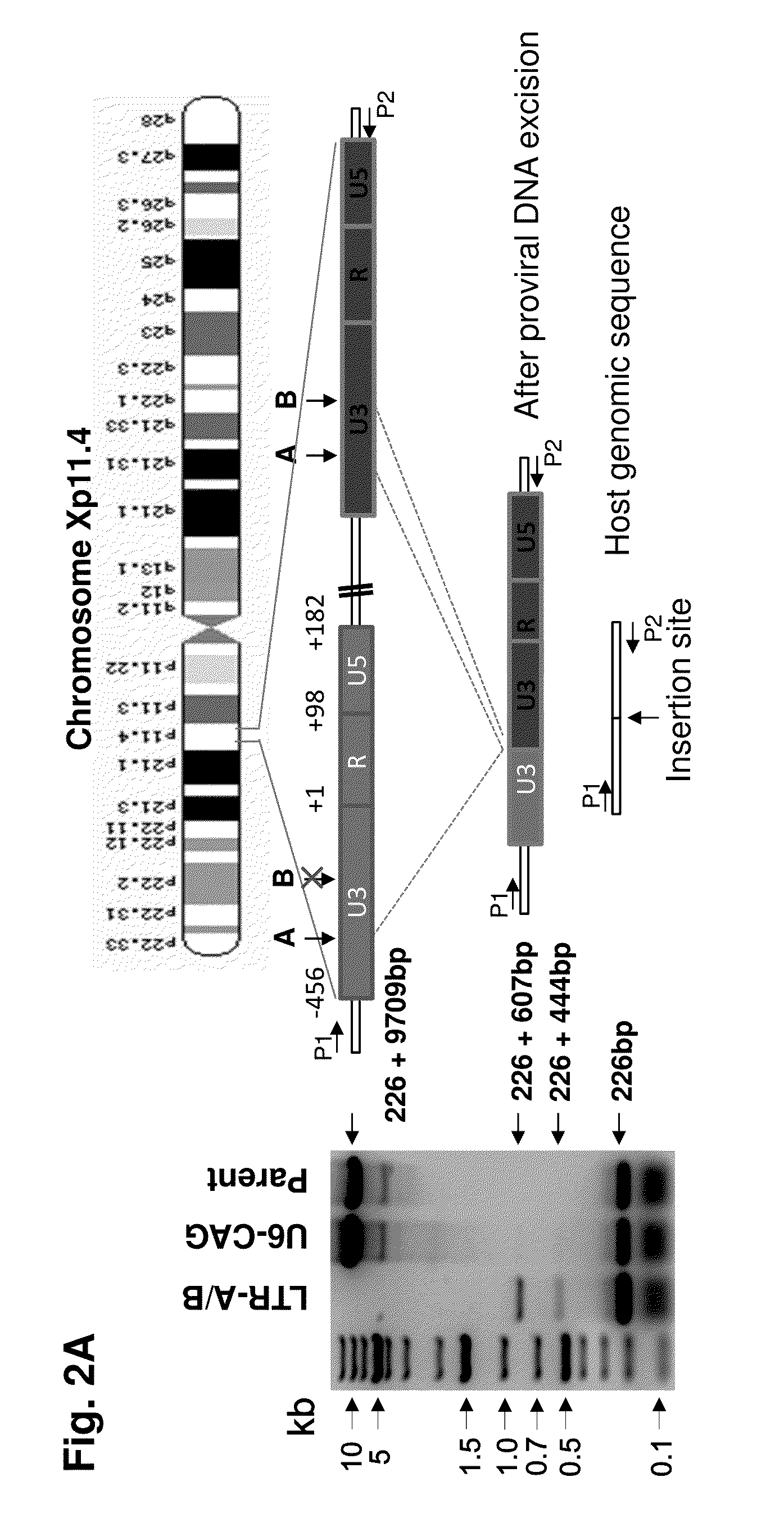
Methods and compositions for rna-guided treatment of HIV infection
A method of inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus by treating the host cell with a composition comprising a Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR)-associated endonuclease, and two or more different guide RNAs (gRNAs), wherein each of the at least two gRNAs is complementary to a different target nucleic acid sequence in a long terminal repeat (LTR) in the proviral DNA, and inactivating the proviral DNA. A composition for use in inactivating a proviral DNA integrated into the genome of a host cell latently infected with a retrovirus including isolated nucleic acid sequences comprising a CRISPR-associated endonuclease and a guide RNA, wherein the guide RNA is complementary to a target sequence in a human immunodeficiency virus.
Owner:TEMPLE UNIVERSITY
Compositions containing bacteriophages and methods of using bacteriophages to treat infections
InactiveUS6942858B1Efficient and economically feasibleHigh frequencyBiocideOrganic active ingredientsBacteroidesPhage therapy
Purified, host-specific, non-toxic, wide host range and virulent bacteriophage preparations that are effective in killing bacterial organisms in vivo are disclosed. Also disclosed are compositions containing these bacteriophages, methods of making the bacteriophage preparations and methods of treating bacterial infections using the compositions. Methods of treating bacterial infections using the compositions containing the bacteriophages in combination with conventional antibiotics also are disclosed.
Owner:NYMOX CORP
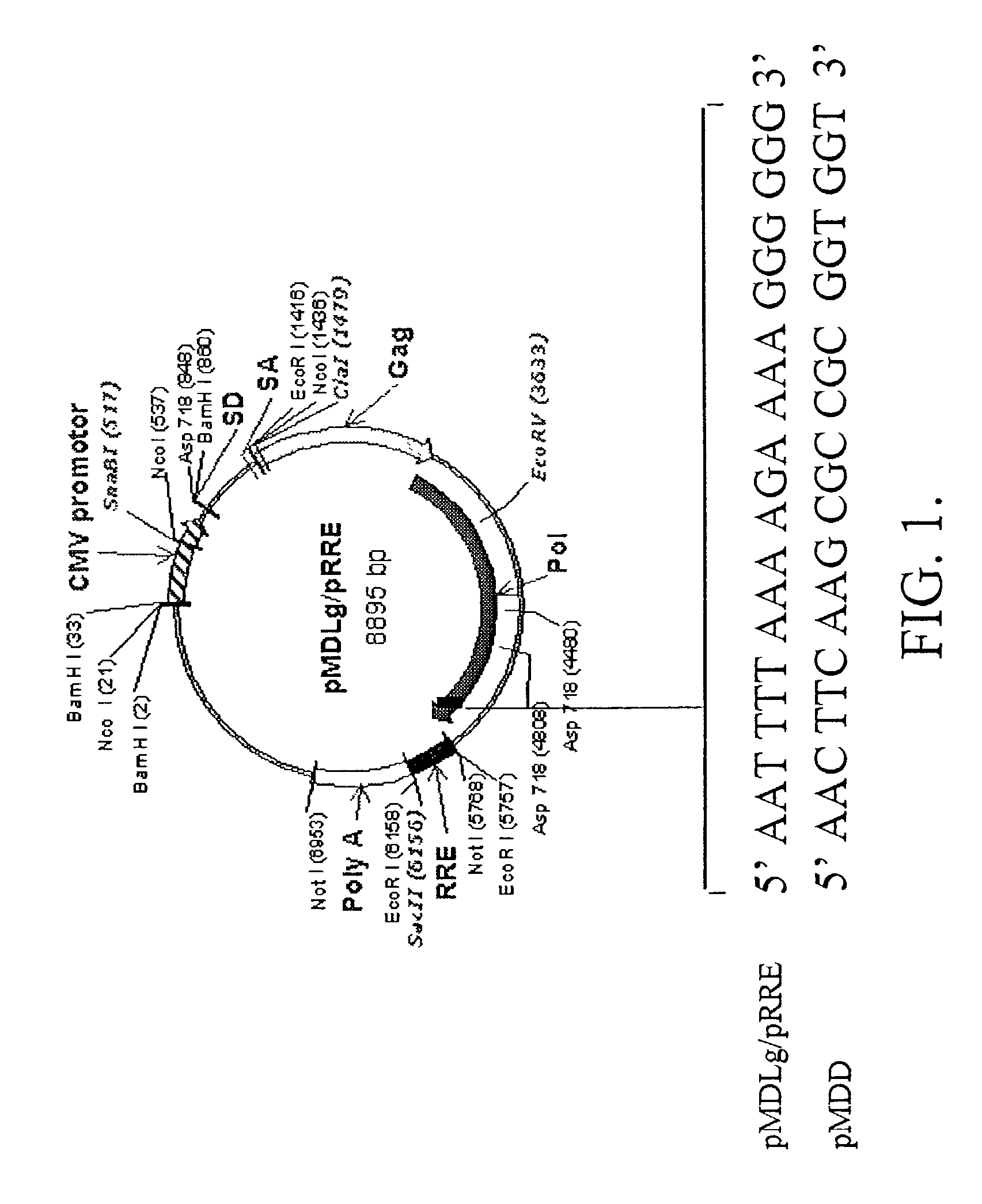
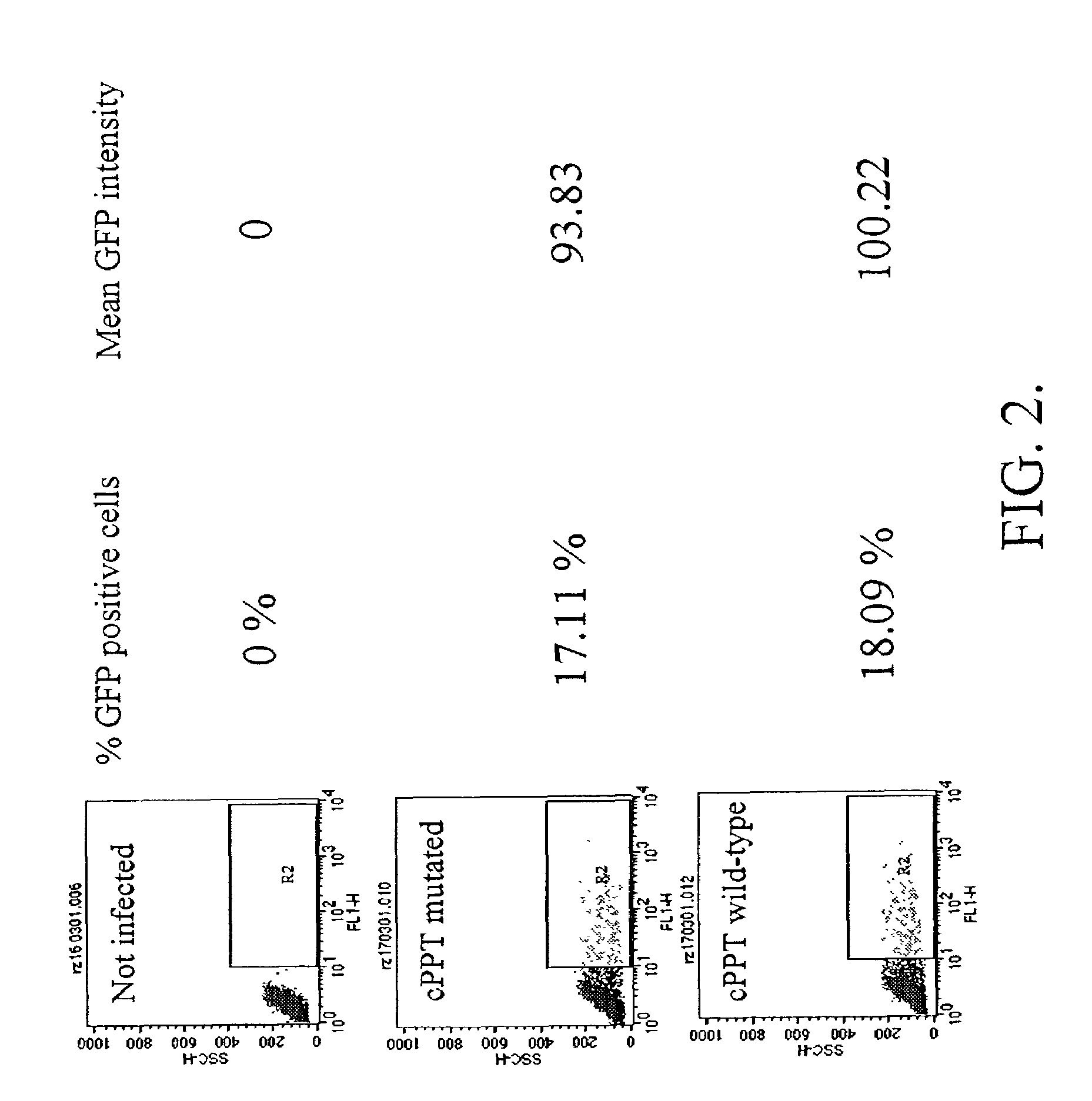
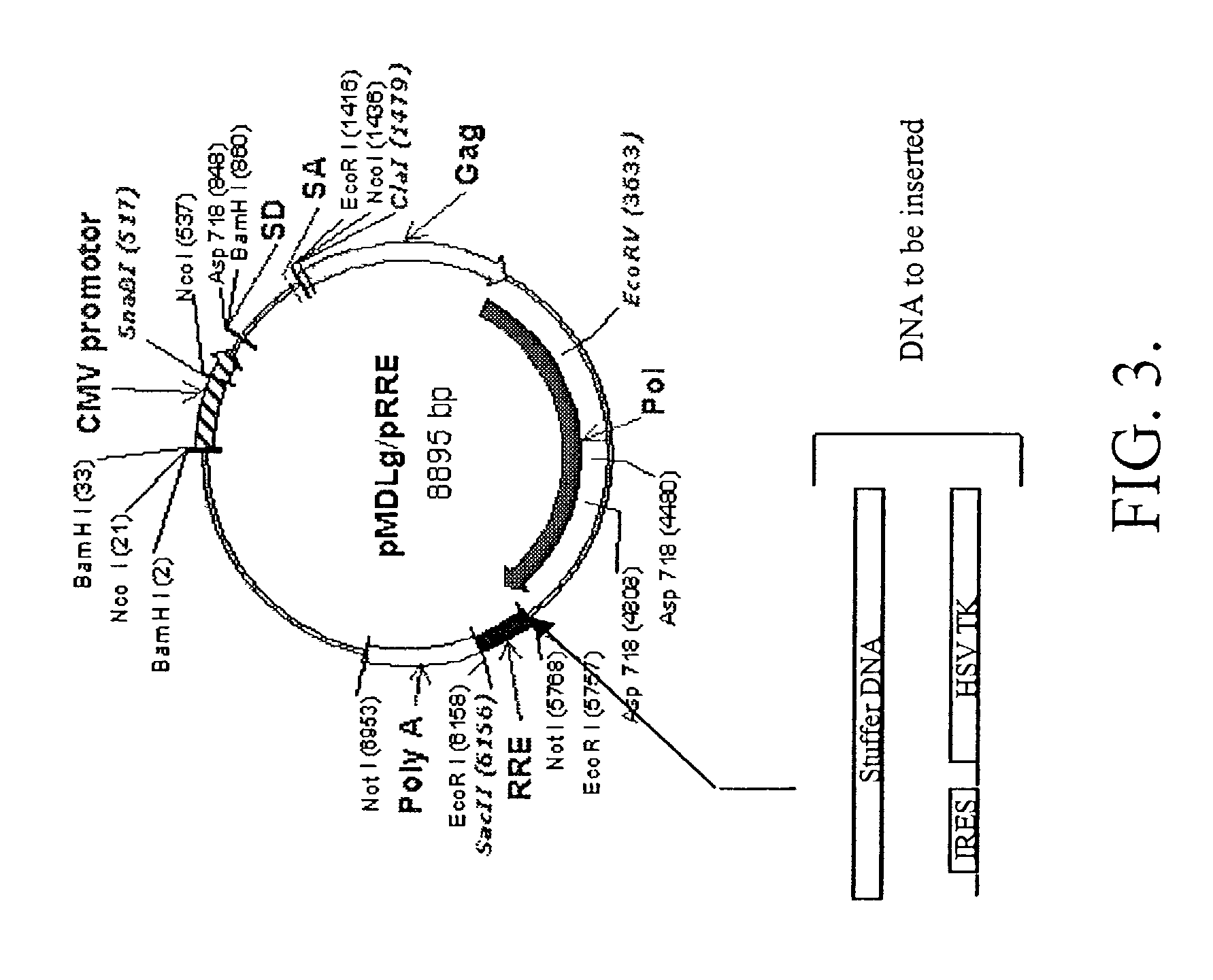
Methods and compositions relating to improved lentiviral vector production systems
ActiveUS7629153B2Increasing its biosafetySafe transfection and transductionVectorsGenetic material ingredientsTranscriptional Regulatory ElementsTransgene
The present invention provides HIV-derived lentivectors which are multiply modified to create highly safe, efficient, and potent vectors for expressing transgenes for gene therapy. The lentiviral vectors comprise various combinations of an inactive central polypurine tract, a stuffer sequence, which may encode drug susceptibility genes, and a mutated hairpin in the 5′ leader sequence that substantially abolishes replication. These elements are provided in conjunction with other features of lentiviral vectors, such as a self-inactivating configuration for biosafety and promoters such as the EF1α promoter as one example. Additional promoters are also described. The vectors can also comprise additional transcription enhancing elements such as the wood chuck hepatitis virus post-transcriptional regulatory element. These vectors therefore provide useful tools for genetic treatments for inherited and acquired disorders, gene-therapies for cancers and other disease, the creation of industrial and experimental production systems utilizing transformed cells, as well as for the study of basic cellular and genetic processes.
Owner:RES DEVMENT FOUND
Vitro method for disassembly/reassembly of papillomavirus virus-like particles (VLPS)
A method of disassembly / reassembly of papillomavirus VLPs is provided. The resultant VLPs have enhanced homogeneity, present conformational, neutralizing PV epitopes, and therefore are useful prophylactic and diagnostic agents. Further, these VLPs can be used to encapsulate desired moieties, e.g., therapeutic or diagnostic agents, or "marker" DNAs, and the resultant VLPs used as in vivo delivery vehicles or as pseudovirions for evaluating vaccine efficacy.
Owner:MEDIMMUNE LLC
Hepatitis c virus inhibitors
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit RNA-containing virus, particularly the hepatitis C virus (HCV). Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com