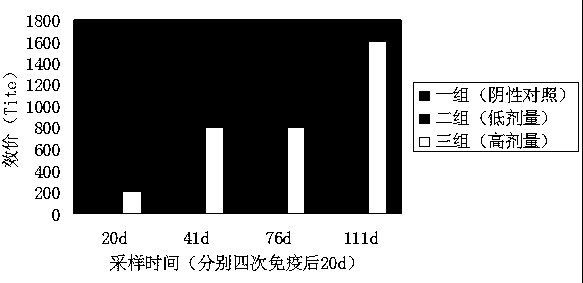
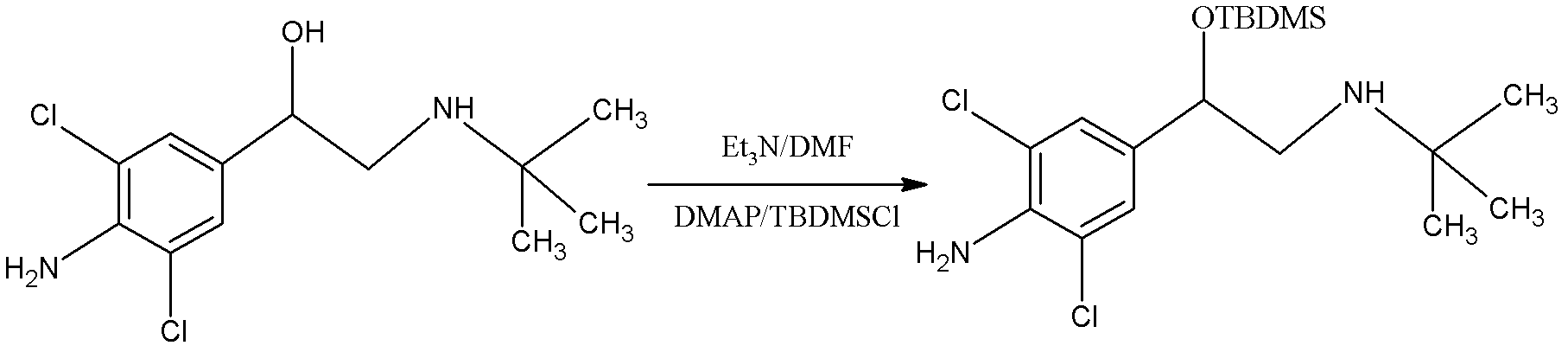
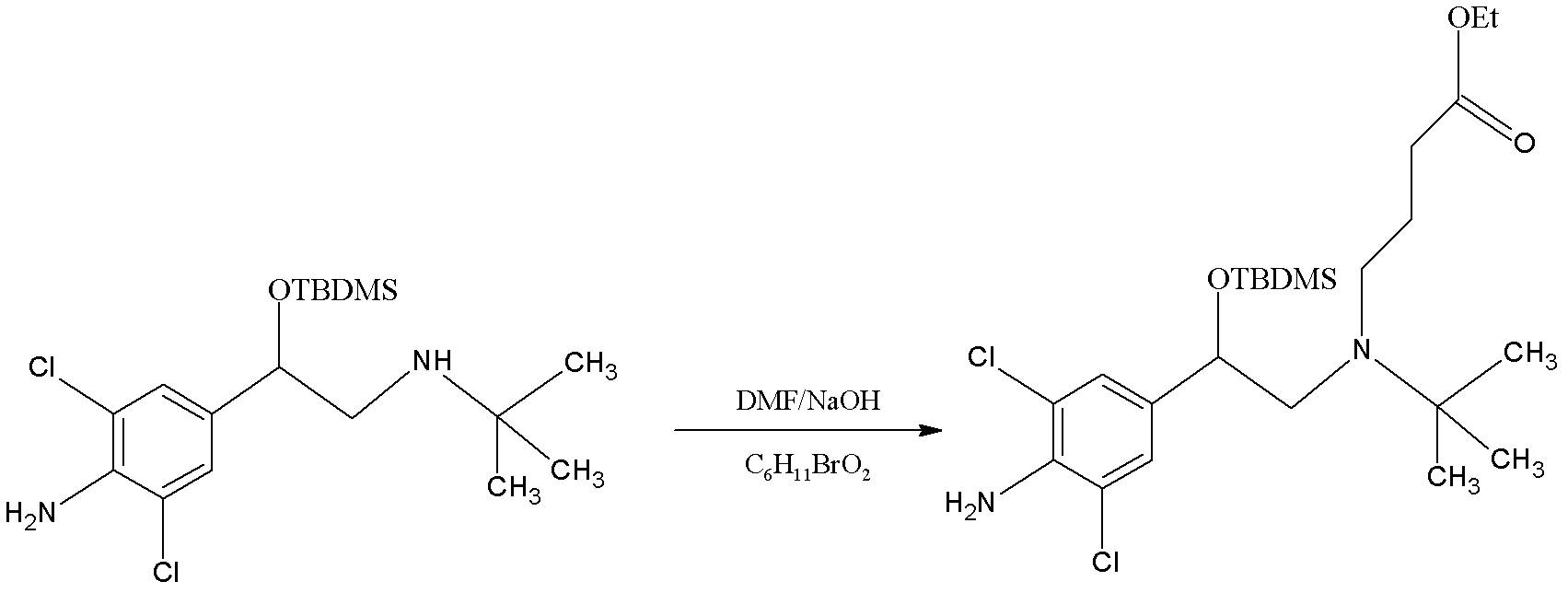
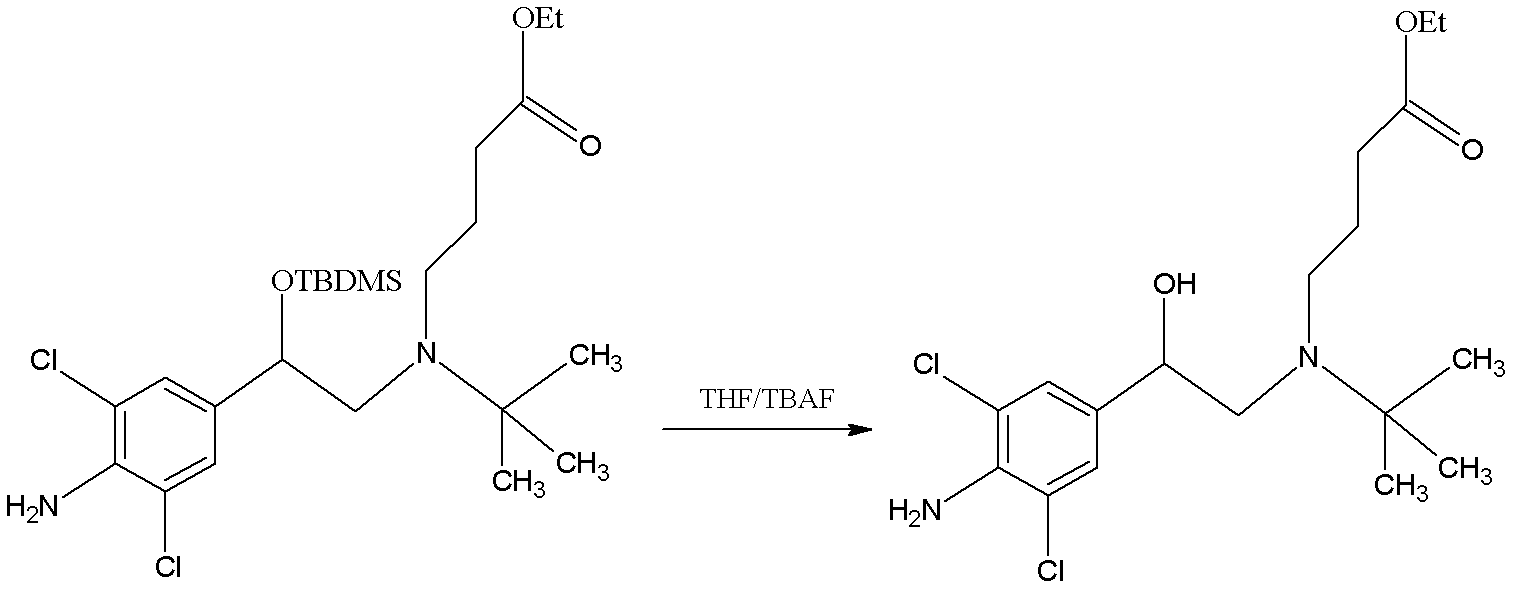
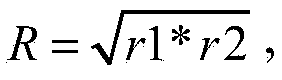Patents
Literature
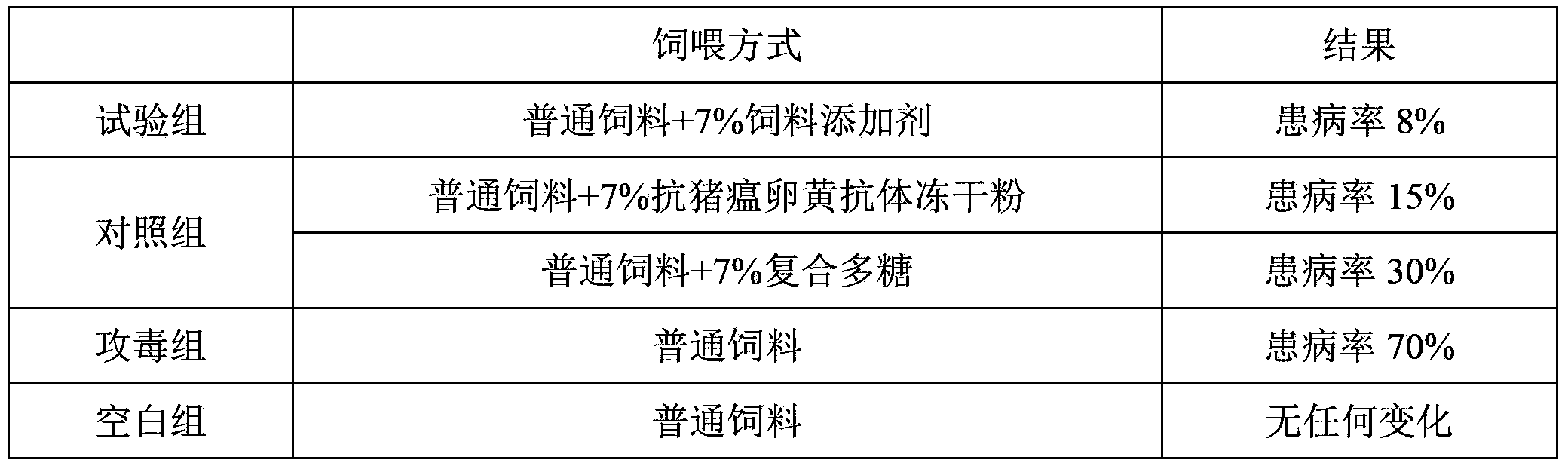
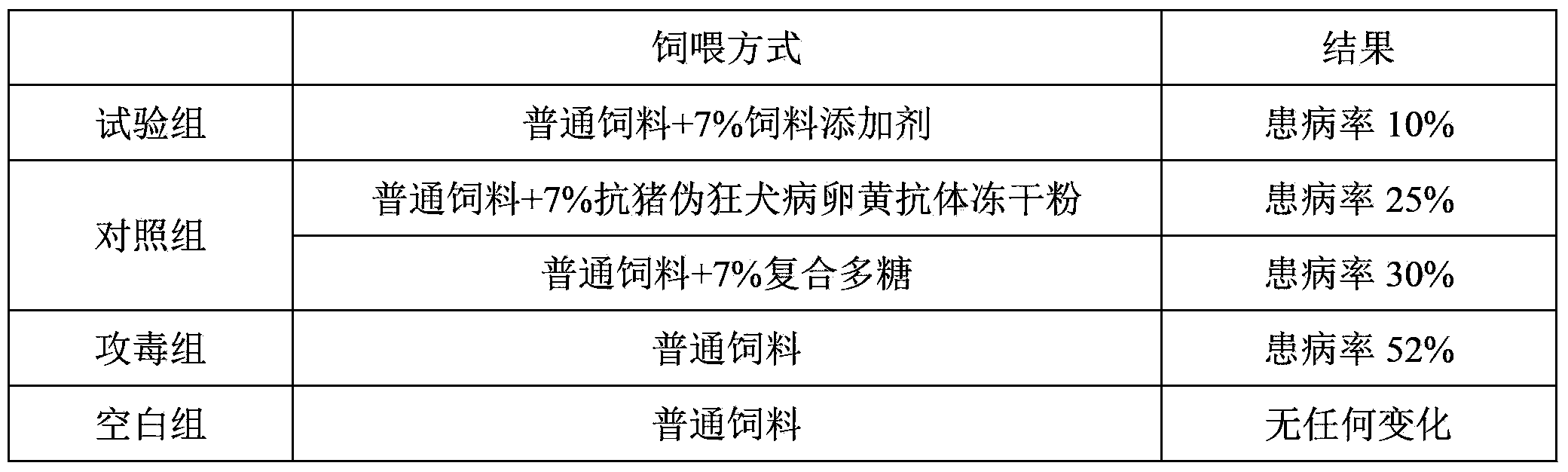
300results about How to "High antibody titer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
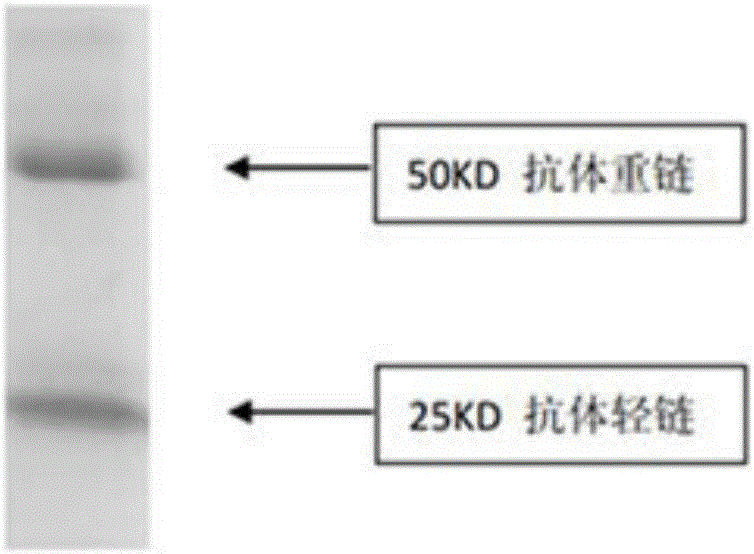
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
Tilapia streptococcus agalactiae IgM antibody capture ELISA detection kit
ActiveCN104198741AHigh potencyHigh detection sensitivityBiological testingPolyclonal antibodiesStreptococcus agalactiae
The invention discloses a tilapia streptococcus agalactiae IgM antibody capture ELISA detection kit. An anti-tilapia IgM specific monoclonal antibody is secreted from an anti-tilapia IgM hybridoma cell strain 2H5 / B5 assigned the accession number CCTCC No:C201277. The kit includes an ELISA plate which is coated by the monoclonal antibody, an antigen tilapia streptococcus agalactiae SIP fusion protein, an IgG-HRP enzyme marker of an SIP fusion protein rabbit polyclonal antibody, an antibody diluent, a washing solution, a coloring reagent, a stop solution, a positive control and a negative control. The anti-tilapia IgM specific monoclonal antibody is high in titer and specificity. The kit is simple in operation, is high in sensitivity, is strong in specificity, can be used in detection of a tilapia streptococcus agalactiae IgM antibody and has a quite high application value.
Owner:福建省淡水水产研究所
Preparation and applications of clenbuterol monoclonal antibody
ActiveCN103012593AHigh recovery rateProcessing method saves timeOrganic compound preparationImmunoglobulinsAssayPharmaceutical drug
The present invention provides a clenbuterol monoclonal antibody and applications thereof. The present invention discloses a clenbuterol monoclonal antibody preparation method, wherein clenbuterol hapten is synthesized, the synthesized clenbuterol hapten and carrier protein are coupled to obtain clenbuterol antigen, and the clenbuterol antigen is adopted to immunize animals to obtain a high specificity monoclonal antibody. The present invention further provides a method for application of the clenbuterol monoclonal antibody in a clenbuterol enzyme-linked immunosorbent assay kit to detect clenbuterol. The present invention further provides a method for application of the clenbuterol monoclonal antibody in a clenbuterol colloidal gold test paper card to detect clenbuterol. The prepared clenbuterol monoclonal antibody has characteristics of high specificity and low cost, wherein the clenbuterol drug detection clenbuterol enzyme-linked immunosorbent assay kit prepared by using the clenbuterol monoclonal antibody and the clenbuterol drug detection clenbuterol colloidal gold test paper card prepared by using the clenbuterol monoclonal antibody have characteristics of convenient operation, high specificity, high sensitivity, high accuracy, high precision, fast detection and the like.
Owner:BEIJING KWINBON BIOTECH
Hybridoma cell strain 7G11 and antibody thereof
ActiveCN106701688AHigh antibody titerStrong specificityTissue cultureImmunoglobulins against fungi/algae/lichensAlternaria helianthiHybridoma cell
The invention relates to a hybridoma cell strain 7G11 and an antibody thereof. The hybridoma cell strain 7G11 is delivered to the China Center for Type Culture Collection for collection on December 27, 2016, with the collection number of CCTCC NO: C201703. The hybridoma cell strain 7G11 can generate a monoclonal antibody capable of resisting streptospora acid, can specifically detect the streptospora acid and has important significance for detection of the streptospora acid.
Owner:SOUTHWEST UNIVERSITY
Aluminium adjuvant as well as preparation method and application thereof
InactiveCN102583460ARapid immune responseHigh antibody titerMaterial nanotechnologyAntibody medical ingredientsAdjuvantPhosphate
The invention provides a preparation method of an aluminium adjuvant. The method comprises the step that water solution of aluminium salt and / or phosphate is contacted with water solution of sodium hydroxide and / or ammonia, and is characterized in that the pH value is constant during the whole process that the water solution of aluminium salt and / or phosphate is contacted with the water solution of sodium hydroxide and / or ammonia. The invention further provides an aluminium adjuvant obtained through the preparation method and the application of the aluminium adjuvant on vaccine. The preparation method of aluminium adjuvant has the advantage that the obtained product is uniform in structure, stable in property and reliable in quality; and the aluminium adjuvant prepared through the method can adsorb more vaccine.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Compound immunoenhancement agent, vaccine for birds and method for preparing compound immunoenhancement agent
ActiveCN102743750AShorten the immune window periodShort immune windowAntiviralsImmunological disordersBird fluDipeptide
The invention relates to a compound immunoenhancement agent and an application thereof on preparing a vaccine for birds. The compound immunoenhancement agent contains 5ng-10mg / mL of poly IC, 10ng-10mg / mL of muramyl dipeptide, 10ng-5mg / mL of levamisole, 10ng-5mg / mL of resiquimod and 10ng-5mg / mL of imiquimod. After the immunoenhancement agent and H5 hypotype bird flu inactivated vaccine are mixed together, antibody can be produced one week earlier, and the antibody titer is improved above 1.8log2. After chicken are immunized through H5 hypotype bird flu inactivated vaccine, H9 hypotype bird flu vaccine or infectious bronchitis vaccine which contains the compound vaccine immunoenhancement agent, the window phases of the antibody production are shortened, the antibody titer of the vaccine is improved, the immunization duration is prolonged, and the probability of infection disease is reduced.
Owner:南京国创生物技术研究院有限公司
Method for preparing infectious chicken Fabricius bursa refined yolk cryodesiccation antibody
InactiveCN101113176AEmergency prevention is goodGood treatment effectEgg immunoglobulinsAntiviralsAntigenYolk
A preparation method of a purified vitelline freezing antibody for chickens infectious bursal disease is characterized in that: high purity antigens are adopted to vaccinate IBDV B87 to SPF chickens, then velvet urine, idiosome, velvet urine membrence are acquired, grinded, filtered and inactivated to antigens, and the steps are: producing immune eggs, collecting high immune egg, separating vitelline, diluting with distilled water, inactivating, extraction, decentering, inactivating, freezing and checking; the eggs is radiated by Co60 to kill viruses and bacterium, and finally froze by novel immuno-enhancer - Astragali Polysaccharoses and preserved under a temperature of 2-8 DEG C. Composite drugs for prevention and therapy are prepared by adopting the vitelline antibody for chickens infectious bursal disease as major components and cooperating with a novel immuno-enhancer, to decrease the jeopardy of the chicken breeding industry, and improve the living level of people and benefit mankind.
Owner:PU LIKE BIO ENG
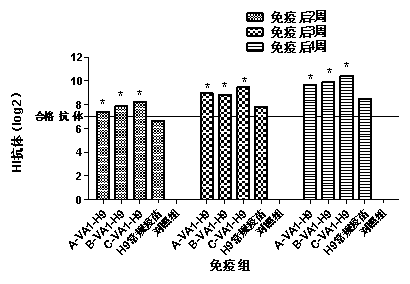
Fusion protein of SVV and FMDV, encoding gene of fusion protein, expression vector, cell line, engineering bacteria, vaccine and application
The invention relates to the technical field of biomedicine, and particularly provides a fusion protein of an SVV and an FMDV, an encoding gene of the fusion protein, an expression vector, a cell line, engineering bacteria, a vaccine and application. The fusion protein is obtained by replacing an epitope capable of inducing the body to produce a neutralizing antibody with a decoy epitope and comprises SVV VP1 protein fragments, FMDV VP1 protein fragments and complement C3d protein fragments. The fusion protein combines antigens for inducing the body to produce the neutralizing antibody, of twopathogens of SVV and FMDV, and the safety of the antigens is improved while the antigenic epitopes of the SVV and the FMDV are reserved. The complement C3d molecules can excite nonspecific body fluidand cellular immune reactions in the body, and play an important role in increasing the antibody titer of the vaccine, and activating the cellular immune response of the body. The vaccine prepared bymeans of the fusion protein is safe and effective, and hydroa and foot-and-mouth diseases can be effectively prevented.
Owner:天康生物制药有限公司
Conjugate of monophosphate A (MPLA) and carbohydrate antigen Globo H and preparation method and application thereof
InactiveCN109432415AReach killHigh antibody titerCancer antigen ingredientsCarrier-bound antigen/hapten ingredientsAntigenAdjuvant
The invention relates to a conjugate of monophosphate A (MPLA) and carbohydrate antigen Globo H and capable of serving as an antitumor vaccine and a preparation method and application thereof. The conjugate can be prepared through a chemical method and has the advantages of definite structure, clear composition and adjuvant needlessness. In addition, the invention further provides the applicationof the conjugate in preparing drug for treating or preventing cancer.
Owner:广州粤美医药科技有限公司
Preparation method for duck viral hepatitis refine yolk antibody
ActiveCN101607994ADoes not affect antibody titerNo chemical residueEgg immunoglobulinsImmunoglobulins against virusesYolkAntigen
The invention relates to a preparation method for a duck viral hepatitis refine yolk antibody, which comprises the following steps: (1) preparation of virus seed for production; (2) preparation of venom for preparing antigen; (3) preparation of oil-emulsion inactivated vaccine; (4) preparation of eggs from hype-immunized chickens; (5) separation of yolk; (6) inactivation I and extraction; (7) inactivation II, collation and deep filtration; (8) inactivation III and degerming filtration; and (9) mixing and package, wherein the collected sterile filtered liquid is subject to liver neutralizing antibody titer determination; the antibody is diluted until the neutralizing antibody titer of the antibody is 1:256-1:1,024 by using 0.015mol / L of sterilized PBS with the pH of 7.2; 0.02 volume percent of tween-80 as a stabilizer is added in the antibody, and the mixture is evenly mixed and sub-packaged; and the obtained product is sterile and quantitative sub-packaged. The product has the advantages of high purity, strong specificity, easy preservation, no residue and the like, and has extremely good effect of preventing and treating the duck viral hepatitis.
Owner:PU LIKE BIO ENG
Feed capable of improving oxidation resistance of hen
InactiveCN106333127AImprove antioxidant capacityImprove immunityFood processingAnimal feeding stuffCalcium bicarbonateVegetable oil
The invention discloses a feed capable of improving oxidation resistance of a hen. The feed comprises the following raw materials: maize meal, soya bean meal, citrus pulp, blood meal, fish meal, seaweed meal, earthworm powder, peanut meal, rice bran, bone meal, a rape seed cake, calcium hydrogen, corn gluten meal, calcium hydrophosphate, shell powder, vegetable oil, natural zeolite, calcium bicarbonate, traditional Chinese medicine extraction powder, beta-glucan, cellulase, a premix and an amino acid mixture. The feed capable of improving the oxidation resistance of the hen contains rich protein, starch, cellulose and microelements, and can promote growth of the hen and the digestive ability and oxidation resistance of the hen.
Owner:全椒县金凤凰禽业养殖专业合作社
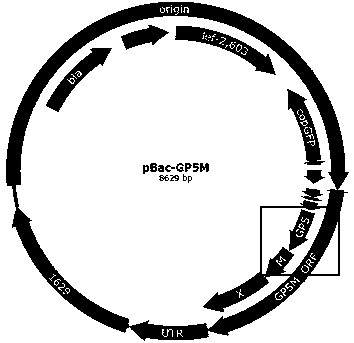
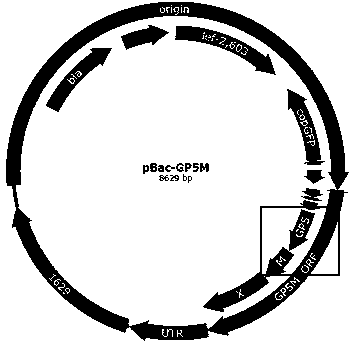
Recombinant PRRSV virus-like particles having immunogenicity and preparation thereof
ActiveCN109385435ABroad-spectrum cross-immunogenicityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsSpecific immunityTransfer vector
The invention discloses recombinant porcine reproductive and respiratory syndrome virus (PRRSV) virus-like particles (VLP) and a preparation method and an application thereof. Based on comparative analysis of GP5 of a PRRSV epidemic strain and an M gene sequence, a GP5 and M tandem sequence GP5M is synthesized artificially, the synthesized GP5M gene sequence is cloned into a vector with a pBAC5 plasmid as a skeleton, the baculovirus transfer vector pBAC-PRRSVGP5M is obtained, the recombinant bacmid rBacmid-GP5M is obtained, sf9 cells are transfected with the bacmid, and the recombinant baculovirus Ac-PRRSVGP5M is obtained. The PRRSV GP5 and M protein are expressed efficiently by the recombinant baculovirus, and the virus-like particles are formed. A subunit vaccine prepared by the proteinexpressed by the recombinant baculovirus can induce a body to produce a specific immune response after immunizing animals and can protect the pig body against the strong poison attacking of porcine reproductive and respiratory syndrome virus.
Owner:陕西诺威利华生物科技有限公司
Preparation method of diethylstilbestrol antibody
InactiveCN101914157AEasy to prepareLow costSerum immunoglobulinsImmunoglobulins against hormonesBovine serum albuminDiethylstilbestrol
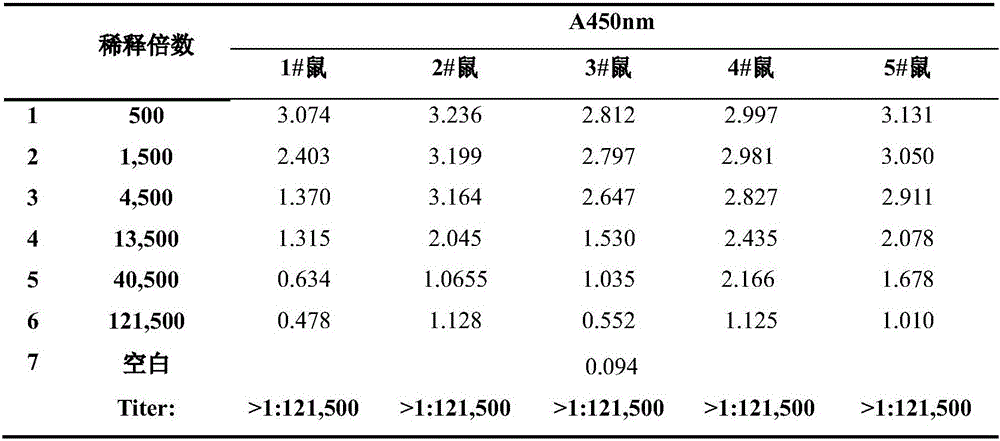
The invention discloses a preparation method of a diethylstilbestrol antibody. The preparation method of the diethylstilbestrol antibody comprises the following steps of: (1) performing a reaction of diethylstilbestrol and 4-bromine butyric acid hexylester, exposing a carboxyl group by saponifying and hydrolyzing, and synthesizing diethylstilbestrol artificial haptene; (2) performing a reaction of the diethylstilbestrol artificial haptene and bovine serum albumin by a mixed anhydride method to obtain diethylstilbestrol artificial immunogen; and (3) emulsifying the diethylstilbestrol artificial immunogen and Freund's complete adjuvant (initial immunization) or Freund's incomplete adjuvant, subcutaneously injecting a rabbit, and separating blood serum when the antibody titer is stable to obtain the diethylstilbestrol antibody. The preparation method of the diethylstilbestrol antibody has the advantages of simpleness, convenience and low cost; and the prepared diethylstilbestrol antibody has high specificity, high antibody titer and indirect ELISA experimental antibody titer of over 1 million, can reduce detection cost when used for immunologically detecting diethylstilbestrol, and is easy to promote and use.
Owner:SOUTH CHINA AGRI UNIV
Novel reagent and kit for renal injury monitoring
ActiveCN102775486AStrong specificityHigh antibody titerImmunoglobulins against animals/humansBiological testingPolyclonal antibodiesRenal injury
The invention relates to a novel reagent and a kit for renal injury monitoring. The inventor identifies critical antigenic determinants from full-length NGAL (neutrophil gelatinase-associated lipocalin). And a short peptide mixture containing the critical antigenic determinants is adopted as an immunogen to obtain a specific polyclonal antibody against NGAL. The antigen fragments and its polyclonal antibody provided in the invention have the advantages of simple preparation method, high titer, strong specificity, and high sensitivity.
Owner:SHANGHAI SUNFORY BIOPHARM INC
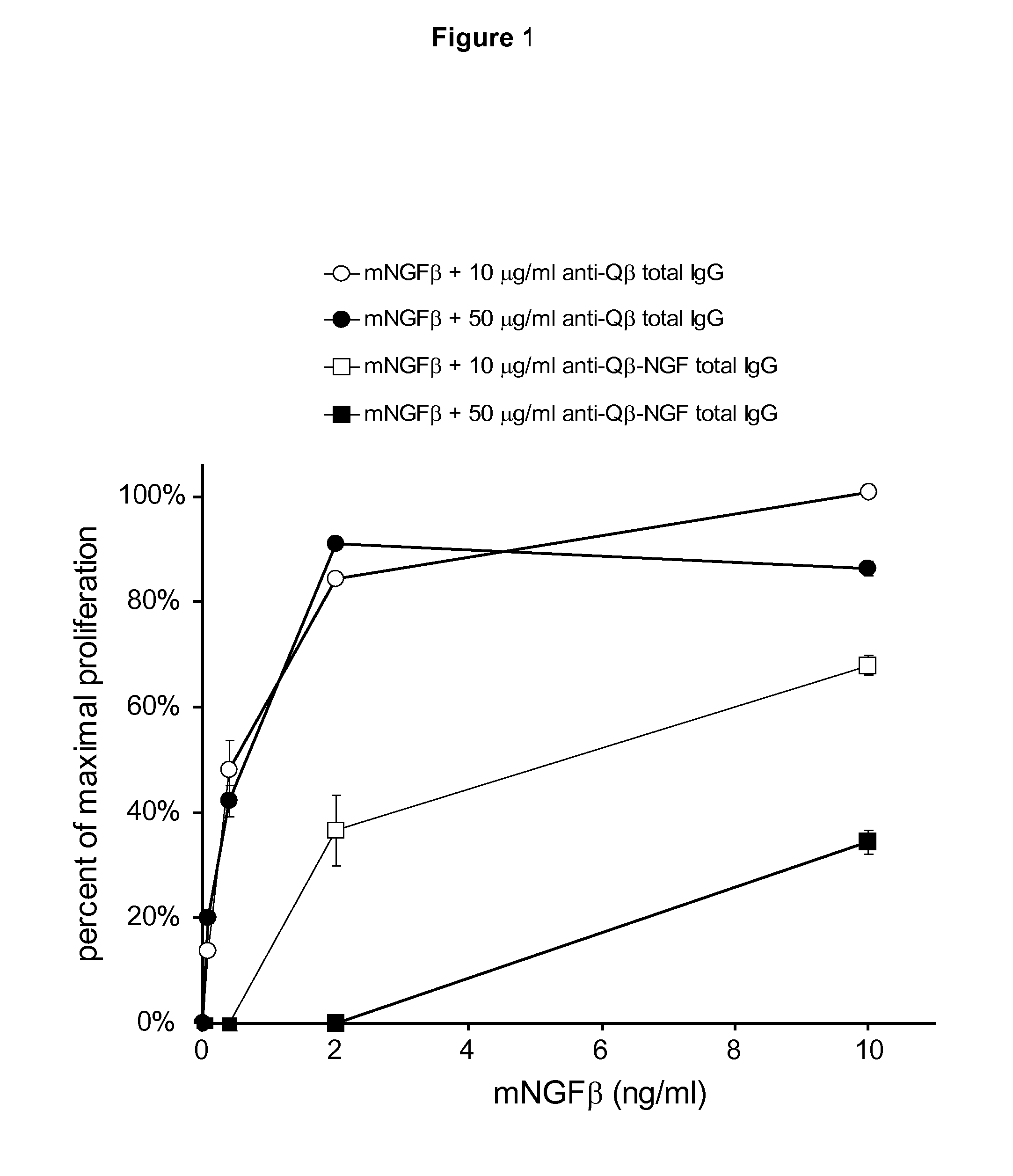
Nerve Growth Factor Conjugates and Uses Thereof
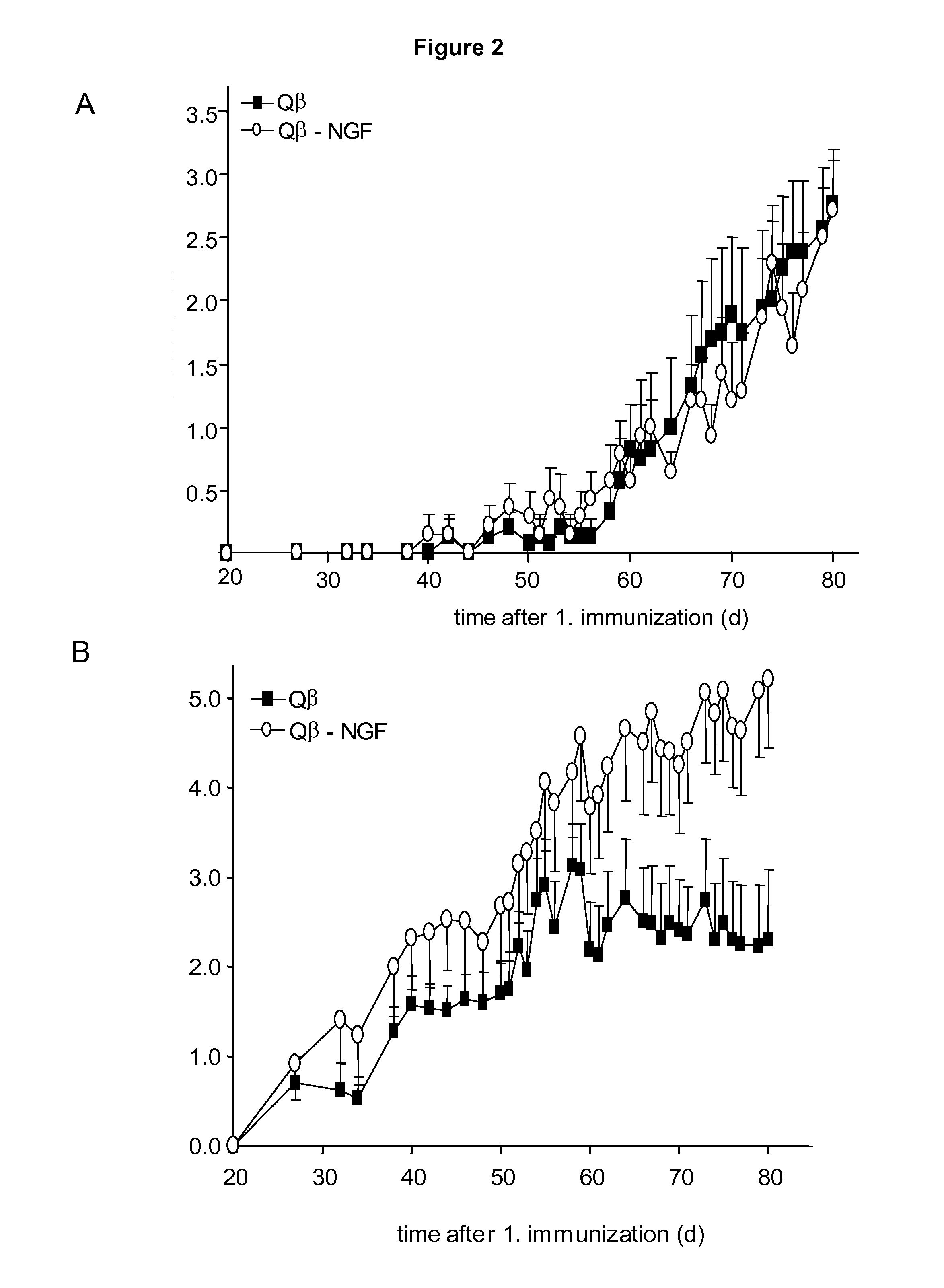
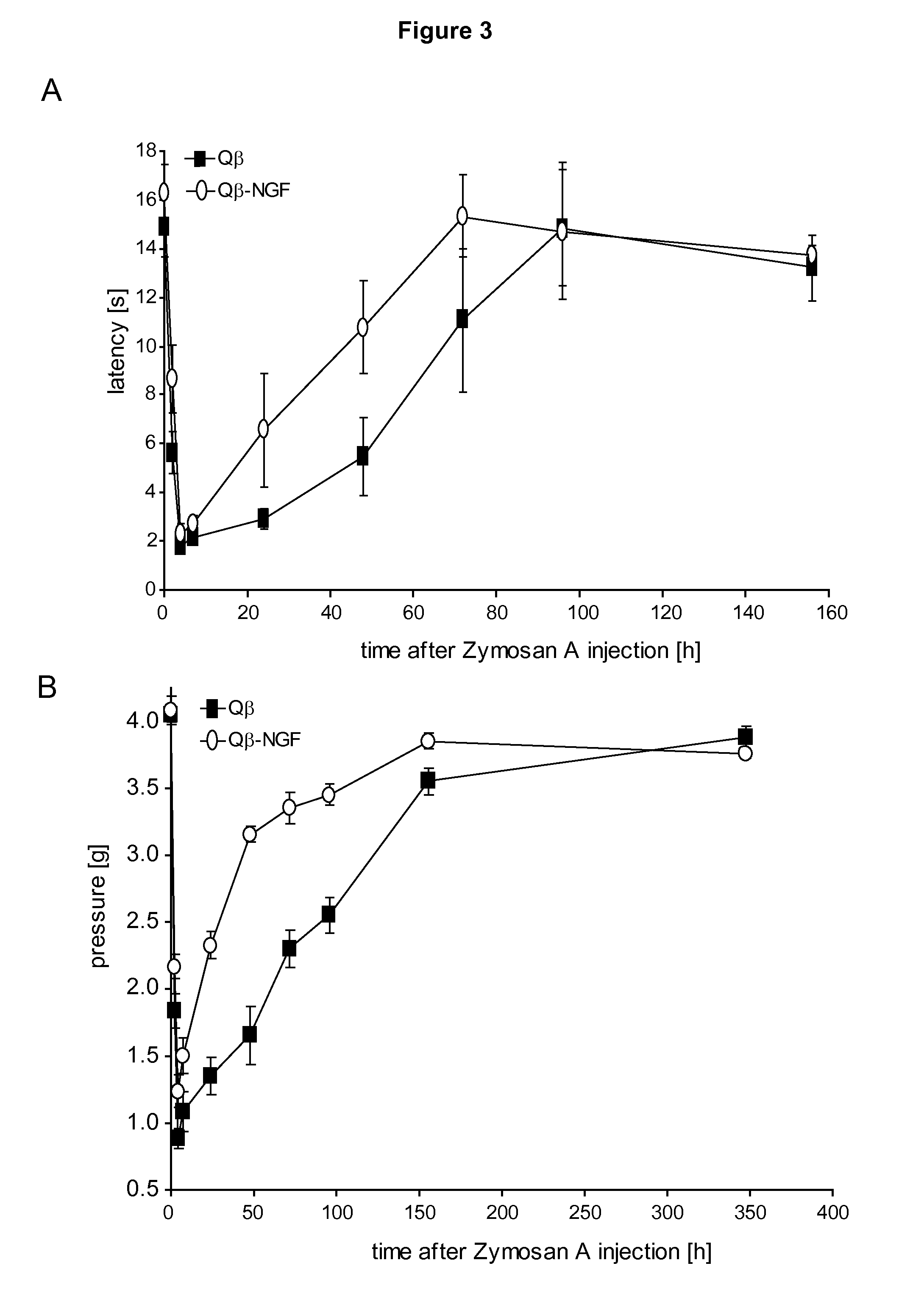
InactiveUS20110212122A1Reduce needReduce painNervous disorderPeptide/protein ingredientsImmunologic functionVirus-like particle
The present invention is in the fields of medicine, public health, immunology, molecular biology and virology. The invention provides composition comprising a virus-like particle (VLP) linked to at least one antigen, wherein said antigen is NGF antigen. The invention also provides a process for producing the composition. The compositions of this invention are useful in the production of vaccines, in particular, for the treatment of pain. Moreover, the compositions of the invention induce efficient immune responses, in particular antibody responses.
Owner:CYTOS BIOTECHNOLOGY AG
Glycyrrhetinic acid nano-liposome for improving immune function of livestock and poultry and its preparation method
InactiveCN103181895AGreen and safeReduced bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsWater bathsVeterinary Drugs
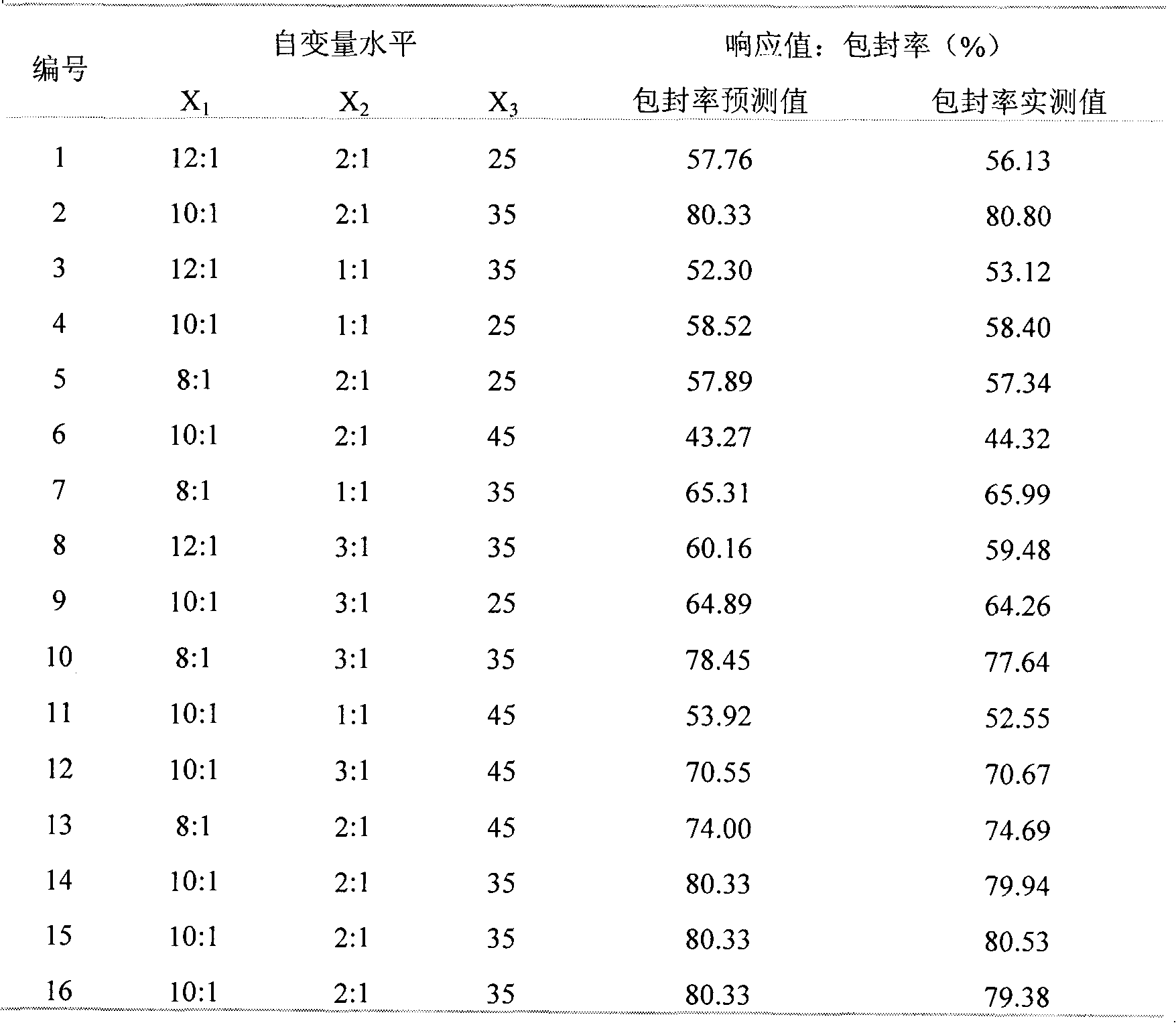
Belonging to the fields of preparation technologies and immune application of herbal veterinary drugs, the invention relates to a glycyrrhetinic acid nano-liposome for improving the immune function of livestock and poultry and its preparation method. A film dispersion technique is employed to prepare the glycyrrhetinic acid nano-liposome. With the encapsulation rate as an index, a response surface technique is adopted to study the following 3 factors: a lipid-drug ratio, a film material ratio and a water bath temperature, and preferable selection is conducted to determine the optimal preparation conditions as: a lipid-drug ratio of 9.0:1, a film material ratio of 2.5:1, and a water bath temperature of 31DEG C. The obtained glycyrrhetinic acid nano-liposome has a high encapsulation rate and strong immunoenhancement activity. The glycyrrhetinic acid nano-liposome provided in the invention significantly enhances the immunoenhancement activity of glycyrrhetinic acid.
Owner:NANJING AGRICULTURAL UNIVERSITY
Preparation method and method of hog cholera-ring-mixed antigen, hog cholera-ring subunit vaccines and preparation method thereof
PendingCN109091669AHigh potencyLow immunogenicitySsRNA viruses positive-senseViral antigen ingredientsAntigenPorcine Circoviruses
The invention provides a preparation method and method of hog cholera-ring-mixed antigen, hog cholera-ring subunit vaccines and a preparation method thereof, and relates to the technical field of biological products. The method comprises the following step of inoculating virus mixing liquid of hog cholera E2 protein recombinant viruses and porcine circovirus II-type Cap protein recombinant virusesinto a cell culture for culturing to obtain the mixed antigen. The preparation method of the hog cholera-ring-mixed antigen, hog cholera-ring subunit vaccines relieves the complicated working procedures that in a conventional technology, two kinds of viruses are cultured in batches and compounded after being gained. The valence of antibody after the vaccines prepared from the mixed antigen are used for vaccine immunity is high.
Owner:TECON BIOLOGY CO LTD
Avian influenza H9 subtype inactivated vaccine, preparation method and application thereof
ActiveCN103736088AGood protection against poisonImprove securityMicroorganism based processesAntiviralsOil adjuvantAvian influenza virus
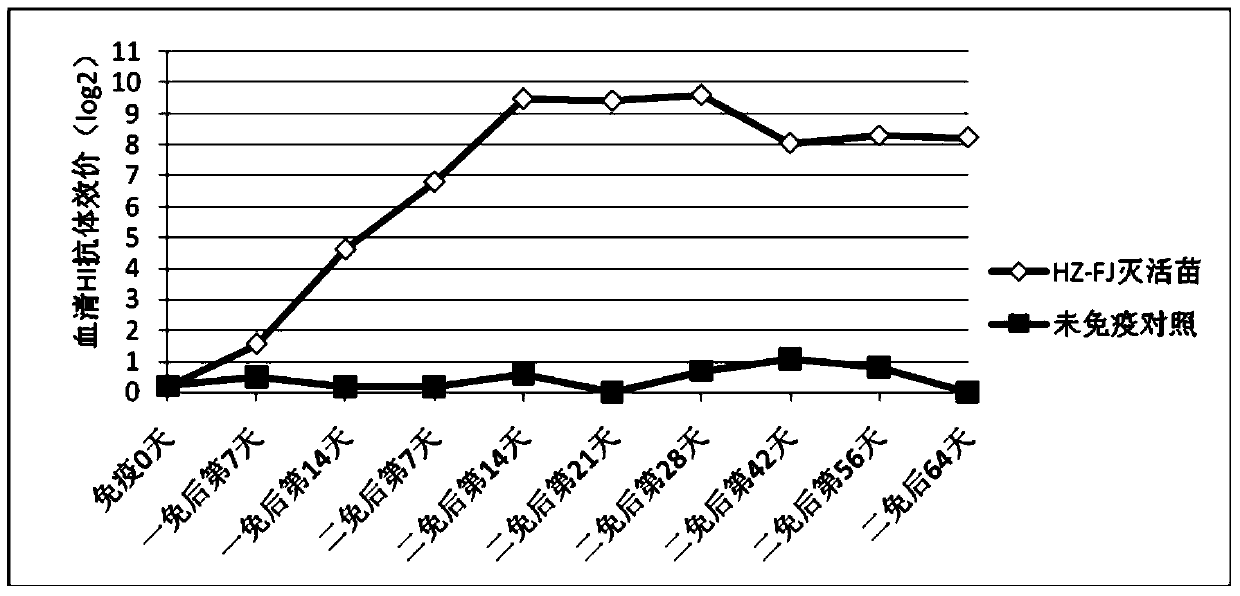
The present invention relates to an avian influenza H9 subtype inactivated vaccine, which is formed by mixing two strains of inactivated H9 subtype avian influenza viruses and an oil adjuvant, wherein the two strains of the inactivated H9 subtype avian influenza viruses are respectively the HZ strain and the FJ strain, the HA gene sequence of the HZ strain is represented by SEQ ID NO:1, and the HA gene sequence of the FJ strain is represented by SEQ ID NO:2. According to the preparation method, the virus solution of the HZ strain and the virus solution of the FJ strain of the H9 subtype avian influenza viruses are prepared and inactivated, the oil phase solution and the water phase solution are prepared, and emulsification is performed to obtain the finished product. According to the present invention, the two strains of the H9 subtype avian influenza viruses separated from different places are utilized to prepare the inactivated vaccine with characteristics of strong immunogenicity and good cross-protection property, wherein the inactivated vaccine can be used for prevention of chicken H9 subtype avian influenza diseases. In addition, after the inactivated vaccine is adopted to immunize chicken, the antibody titer is high so as to make the chicken have good virus challenge protection property on the H9 subtype strains epidemic in different places, the safety is high, and the efficacy is stable.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA
Alpha thymosin peptides as vaccine enhancers
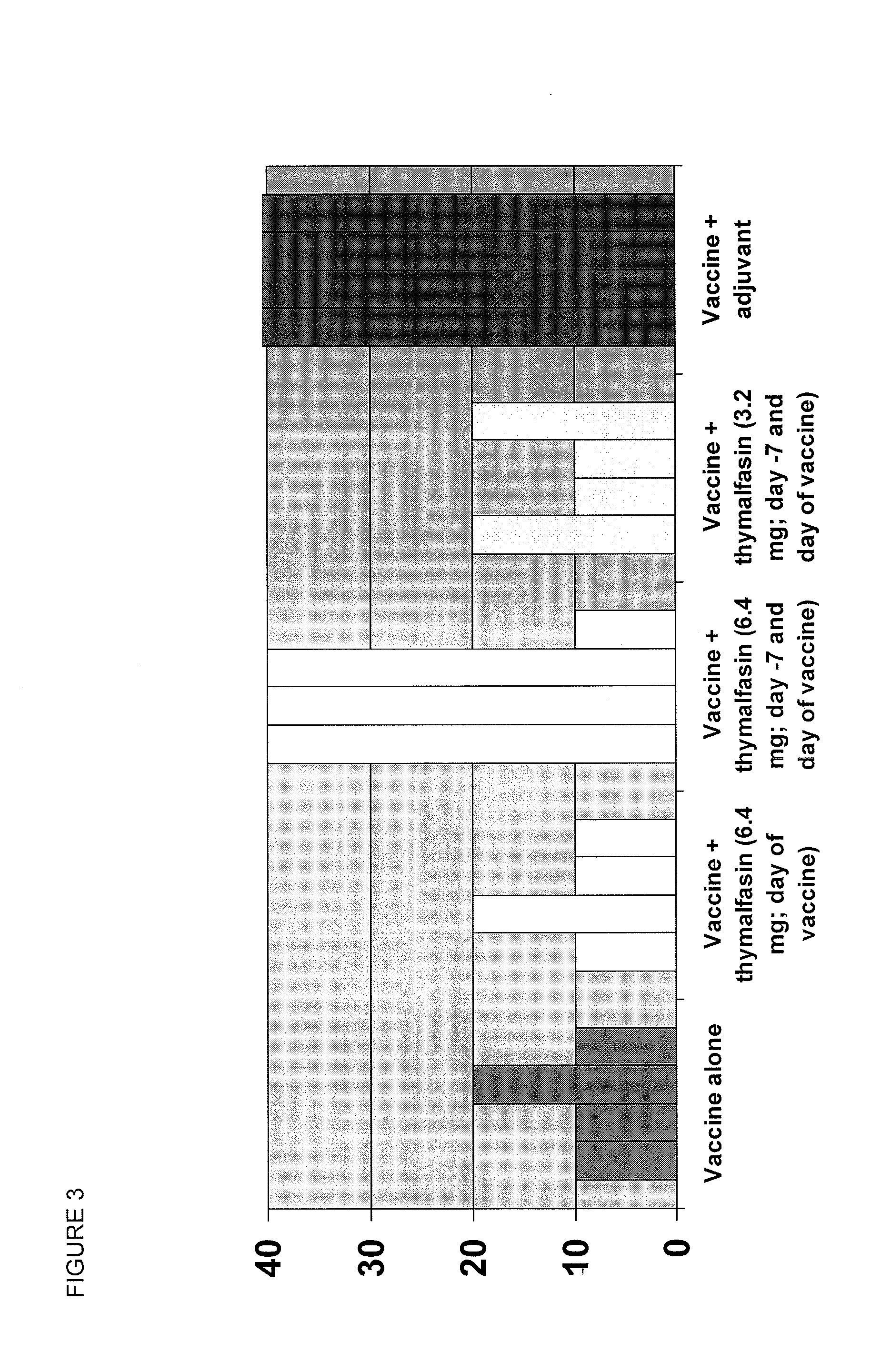
ActiveUS20100285060A1Enhancing vaccine effectivenessEnhance vaccine effectivenessSsRNA viruses negative-senseBacterial antigen ingredientsRegimenImmunodeficiency
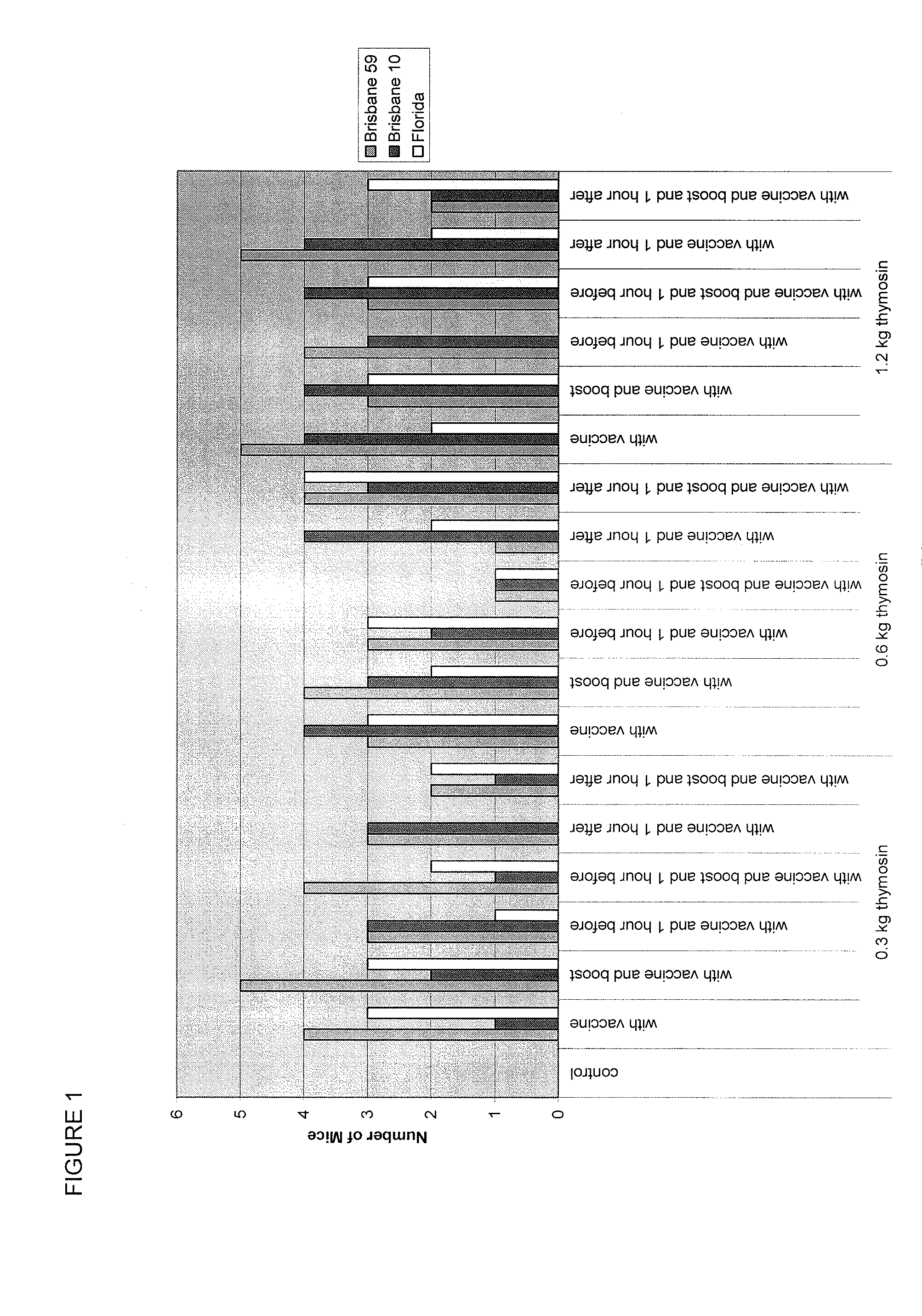
The present invention provides methods of vaccination as well as pharmaceutical combinations and kits for enhancing vaccine effectiveness, including for immunodeficient or immunecompromised patients, including non-responders and low-responders to vaccination. As disclosed herein, the invention relates to administering a vaccine and a regimen of thymosin alpha peptide so as to provide higher antibody titers, speed the development of such antibody titers, and / or to provide for a longer duration of such antibody titers, thereby providing a greater protective effect. In another aspect, the invention allows for reducing a vaccine dose, such as an influenza vaccine dose, by administration of a thymosin peptide regimen.
Owner:SCICLONE PHARM INT LTD
Preparation method of immune-enhanced recombinant PRRSV virus-like particle subunit vaccine
ActiveCN109402145ABroad-spectrum cross-immunogenicityImproving immunogenicityViral antigen ingredientsVirus peptidesBaculovirus expressionVirus-like particle
The invention discloses a preparation method of an immune-enhanced recombinant PRRSV virus-like particle subunit vaccine. The subunit vaccine is prepared from PRRSV virus-like particles and compound immunological adjuvants. By a genetic engineering means, a PRRSV GP5-M gene is modified, and PRRSV virus-like particles with high immunogenicity are prepared by constructing a rhabdovirus expression vector. In addition, by improving the immunological adjuvants, the immuno-enhanced recombinant PRRSV virus-like particle subunit vaccine is obtained, and the subunit vaccine has better immunization effects.
Owner:陕西诺威利华生物科技有限公司
HPV egg yolk antibody and application thereof in preparing drug for treating HPV infection
ActiveCN109666685AAchieve transmembrane transportSolving the problem that leaves cervical cancer with no effective solutionOrganic active ingredientsEgg immunoglobulinsPharmaceutical medicineAntibacterial activity
The invention belongs to the field of biomedicine, and particularly relates to an egg yolk antibody of HPV E6 / E7 recombined minicircle DNA and application of the antibody in preparing a drug for treating HPV infection. The egg yolk antibody is obtained by utilizing HPV E6 / E7 recombined minicircle DNA as a nuclear acid antigen for direct immunization of a laying hen. The egg yolk antibody can be used for preparing the drug for treating HPV infection. The egg yolk antibody is prepared into freeze-dried powder and subjected to superfine grinding, after lipidosome encapsulating, a nano-liposome encapsulating the egg yolk antibody is prepared, the antibody enters cells in a transmembrane mode, the high-activity anti-HPV antibody is input to a patient with positive HPV, and E6 / E7 protein in an HPV virus inside the cells is directly neutralized. The antibody is combined with antibacterial active ingredients (antibacterial compounds or gynecologic inflammation pathogen specific antibodies) andacceptable pharmaceutical carriers to be prepared into vagina spraying agents, vagina washing liquor, vagina gels, vagina soft and hard capsules, vagina suppositories, vagina membranes, vagina tablets, effervescent tablets, ointment, creams and the like, and can also be prepared into lozenges, chewable tablets, chewing gums and the like for both male and female.
Owner:江苏润洁生物科技有限公司
Preparation and freeze-dried storage method of pseudorabies standard positive serum
ActiveCN103864931AFully neutralizedLong storage timeSerum immunoglobulinsImmunoglobulins against animals/humansMonosodium glutamateFreeze-drying
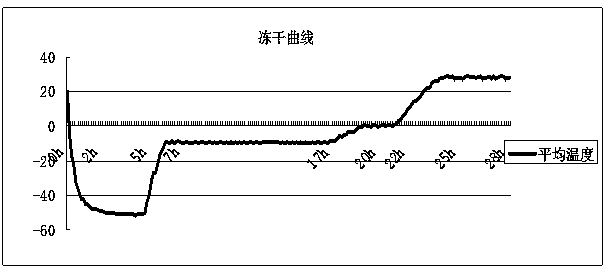
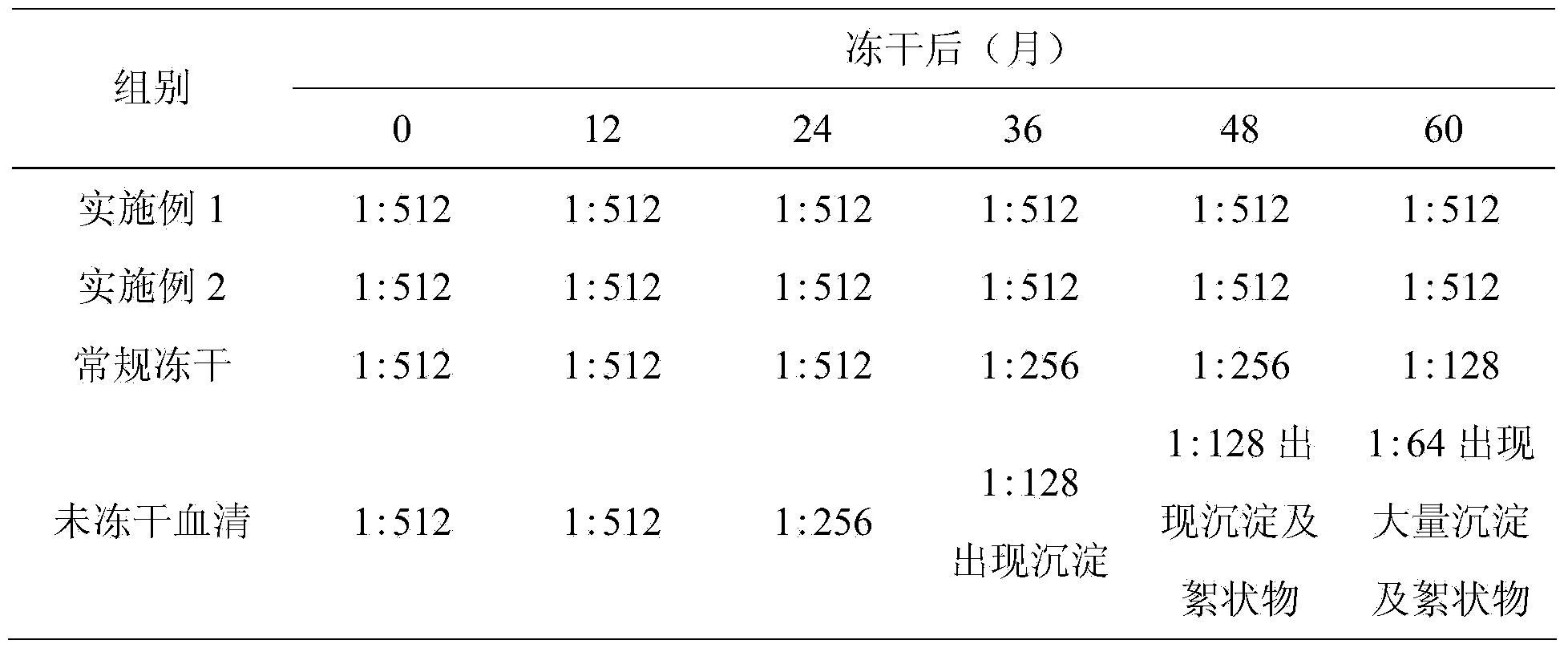
The invention discloses a preparation and freeze-dried storage method of pseudorabies standard positive serum. The preparation comprises the following steps: immunizing a rabbit twice with an inactivated PRV (pseudorabies virus) solution, then, immunizing the rabbit once with the inactivated PRV solution, and collecting the serum; adding a protective agent sodium chloride, trehalose, monosodium glutamate and L-arginine into the serum, fully dissolving, filtering to remove bacteria, and subpackaging; adding the subpackaged serum into a freeze dryer box, and performing freeze-drying according to a freeze-drying curve shown as fig.1 described in the specification to obtain the pseudorabies standard positive serum. For the pseudorabies standard positive serum obtained by the method disclosed by the invention, PRV neutralizing antibody titer is more than 1:512, the antibody titer remains unchanged after the pseudorabies standard positive serum is stored for 60 months at 2-8 DEG C, so that the pseudorabies standard positive serum has good stability, can fully neutralize pseudorabies virus in vaccine exogenous virus and specificity detection, and provides a good technical support for quality inspection of pseudorabies virus vaccines.
Owner:WUHAN CHOPPER BIOLOGY
Composition for resisting swine foot-and-mouth disease, freeze-dried powder, and preparation method and use of freeze-dried powder
InactiveCN104162162AImprove prevention and control functionImproving the Ability to Control Pig Foot-and-Mouth DiseasePowder deliveryPeptide/protein ingredientsYolkFreeze-drying
The invention discloses a composition for resisting swine foot-and-mouth disease, freeze-dried powder, and a preparation method and use of the freeze-dried powder, and belongs to the technical field of veterinary feed additives. The composition comprises, by weight, 95-100 parts of a swine foot-and-mouth disease-resistant yolk antibody and 1-5 parts of a swine foot-and-mouth disease-resistant transfer factor. The freeze-dried powder comprises, by weight, 95-97 parts of the swine foot-and-mouth disease-resistant yolk antibody, 1-2 parts of the swine foot-and-mouth disease-resistant transfer factor, 0.2-0.3 parts of an inactivator, 0.01-0.02 parts of an antiseptic and 2.05-3.6 parts of a heat-resistant freeze-dried protective agent, and has the advantages of low preservation conditions, long preservation time, high antibody titer and good biological activity. Through combined use of the swine foot-and-mouth disease-resistant yolk antibody and the swine foot-and-mouth disease-resistant transfer factor, the freeze-dried powder has effects of preventing and treating swine foot-and-mouth disease, improves swine immunity and survival rate, is convenient for use and has a low cost.
Owner:HENAN UNITED INVE FEEDSTUFF
PEDV (porcine epidemic diarrhea virus), inactivated vaccine and preparation method of inactivated vaccine
ActiveCN106591244AEffective protectionImproving immunogenicityAntiviralsViruses/bacteriophagesOil phaseVirus strain
The invention provides a PEDV (porcine epidemic diarrhea virus), an inactivated vaccine and a preparation method of the inactivated vaccine and belongs to the field of bioengineering. The collection registration number of a PEDV NJ strain is CGMCC NO.13283. The invention provides an application of the virus strain in preparation of a PED vaccine, a preparation method of a virus solution of the virus strain as well as the obtained virus solution and the inactivated vaccine. The preparation method of the inactivated vaccine comprises steps as follows: the inactivated virus solution and tween-80 in the volume ratio being 96:4 are mixed uniformly, and a water phase is obtained; white oil for injection and span-80 are mixed uniformly in the volume ratio being 96:4 and an oil phase is obtained; the water phase and the oil phase are mixed in the volume ratio being 1:(2-3) and then emulsified. The NJ strain is a recent prevalent virus, has good immunogenicity, adapts to cell culture and can be subjected to large-scale fermenting culture, and pregnant sows inoculated with the inactivated vaccine of the virus strain can effectively protect newly-born suckling pigs during toxicity attacking by the recent prevalent virus.
Owner:JIANGSU ACAD OF AGRI SCI
Infectious bursal disease virus Vero cell-adapted strain and application thereof
InactiveCN103923885AStable proliferationImprove securityViral antigen ingredientsMicroorganism based processesOil emulsionEmbryo
The invention provides an infectious bursal disease virus Vero cell-adapted strain and belongs to the field of bioengineering. The related infectious bursal disease virus Vero cell-adapted strain is named Ck / Jiangsu / NJ-23 / 2008 and has a collection No. of CGMCCNO.8852. The infectious bursal disease virus Vero cell-adapted strain which can efficiently multiply on serum-free cultured Vero cell is finally obtained through wild strain separation, chick embryo passage, Vero cell passage adaption; the infectious bursal disease virus Vero cell-adapted strain is subjected to continuous passage culture on the serum-free cultured Vero cell and TCID50 can be kept to be higher than 108.5 / mL. Virus culture solution is inactivated and prepared into oil emulsion; after the prepared oil emulsion is used to immunize chicken, detection proves that the prepared oil emulsion has good immunogenicity. The infectious bursal disease virus (IBDV) strain and the production process thereof are simple, safe and efficient and suitable for industrial culture.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Highly pathogenic H7N9 avian influenza virus, vaccine, detection reagent, and preparation methods of virus and vaccine
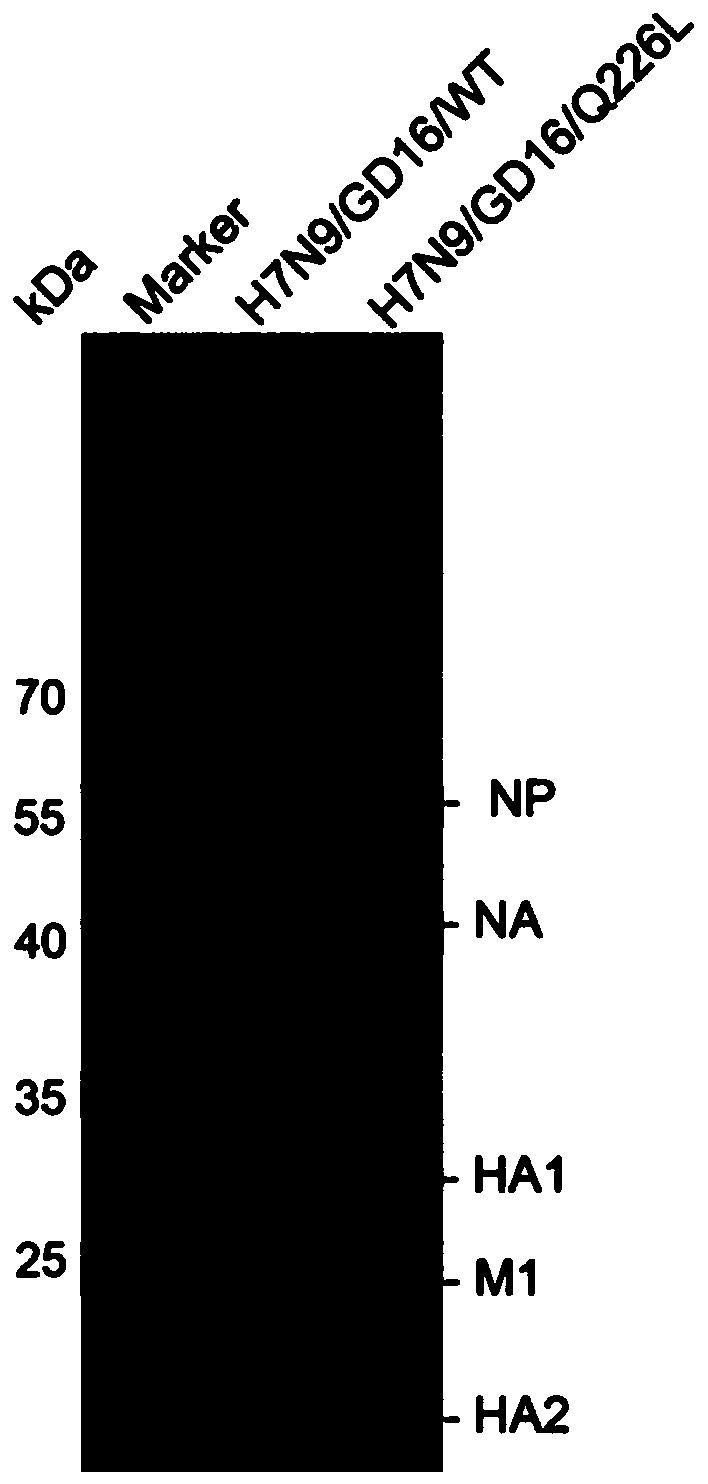
InactiveCN110172452AHigh biosecurityDoes not affect immunogenicitySsRNA viruses negative-senseViral antigen ingredientsHighly pathogenicAvian influenza virus
The invention discloses a preparation method of highly pathogenic H7N9 avian influenza virus, and the method can improve immunogenicity of virus. The preparation method comprises the following steps:preparing a Q226L mutated HA gene: preparing the gene sequence of full-length HA protein of Q226L-mutated highly pathogenic H7N9 avian influenza virus to obtain the Q226L mutated HA gene; rescuing thevirus: adopting the Q226L mutated HA gene to rescue the recombinant influenza virus. The invention further discloses the highly pathogenic H7N9 avian influenza virus prepared by the method, a vaccineprepared by using the highly pathogenic H7N9 avian influenza virus and a preparation method of the vaccine, and a detection reagent prepared by using the highly pathogenic H7N9 avian influenza virus.The preparation method of the highly pathogenic H7N9 avian influenza virus improves the biosecurity of the recombinant virus and immunogenicity.
Owner:GUANGZHOU MEDICAL UNIV
Use of immunological stimulant compound(ISCOMs)in fish immunity by oral administration and dipping bath method
InactiveCN1879880AImprove the ability of proliferation and transformationHigh antibody titerImmunological disordersAntibody medical ingredientsStimulantT lymphocyte
The invention relates to a bath or oral application of immune activate compound in the fish immunity, wherein the invention has the advantages that: said compound ISCOMs is used to bath immunity to improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection; when in the same dosage, the antibody level is higher than non-adjuvant group and the ISM1312 group; and the ISCOMs used in oral can improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI +3
Feed additive for resisting viral diseases of pigs, preparation method and application
InactiveCN104161180AStrong targetingImprove biological activityOrganic active ingredientsAnimal feeding stuffYolkDisease
The invention discloses a feed additive for resisting viral diseases of pigs, a preparation method and application. The feed additive comprises the following components in parts by weight: 2-5 parts of freeze-dried powder of yolk antibodies for resisting the viral diseases of pigs, 5-10 parts of astragalus membranaceus polysaccharides, 5-10 parts of Chinses angelica polysaccharides, 10-20 parts of Chinese wolfberry polysaccharides, and 9-31 parts of licorice root polysaccharides. The feed additive for resisting the viral diseases of the pigs, disclosed by the invention, is compounded by the freeze-dried powder of the yolk antibodies for resisting the viral diseases of the pigs and composite polysaccharides consisting of the astragalus membranaceus polysaccharides, the chinses angelica polysaccharides, the Chinese wolfberry polysaccharides and the licorice root polysaccharides, and the feed additive is added to daily feeds, so that the organism immunity and the disease resistance can be improved. Experimental results indicate that after the feed additive disclosed by the invention is added, the effect of preventing the viral diseases of the pigs is obviously improved, and is obviously better than the effect of individually adding the freeze-dried powder of the yolk antibodies or the composite polysaccharide.
Owner:HENAN UNITED INVE FEEDSTUFF
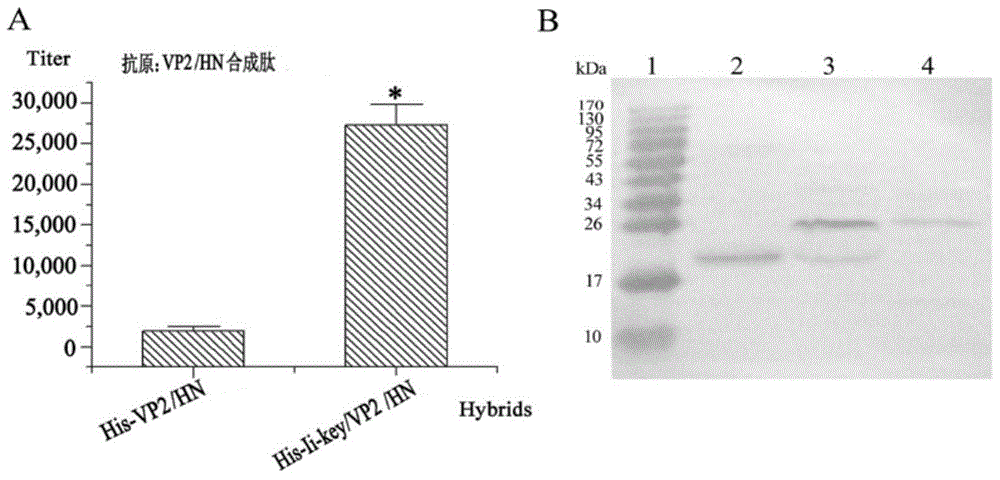
Preparation method of chimeric vaccine by using Ii-Key active tetrapeptide carrying Fabricius bursa VP2 and newcastle disease HN antigen peptide epitope
ActiveCN103933560AHigh antibody titerStrong specificityViral antigen ingredientsAntiviralsBALB/cEscherichia coli
The invention discloses a preparation method of chimeric vaccine by using Ii-Key active tetrapeptide carrying Fabricius bursa VP2 and newcastle disease HN antigen peptide epitope, which is characterized in that the chimera gene of Ii-Key and chicken infectious Fabricius bursa VP2 peptide epitope (197-209) and newcastle disease HN antigen peptide epitope (345-353) is artificially synthesized, a pET-32a prokaryotic expression vector is respectively inserted, then escherichia coli Rosetta is conversed, and chimera gene is purified through a nickel affinity column chromatography, an enzyme-linked immunosordent assay(ELISA) is employed for detecting antibody level in mice with recombinant protein immunization SPF grade BALb / c; and vaccine is compared with a simple VP2197-209 / HN345-353 series epitope control group. The antibody titer of the chimeric vaccine group based on active Ii-Key is increased by 13.72 times on average. The chimeric vaccine has the characteristics of strong specialty, easy preparation and good immunization effect.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Gene vaccine for preventing and treating tumors and preparation method and application of gene vaccine
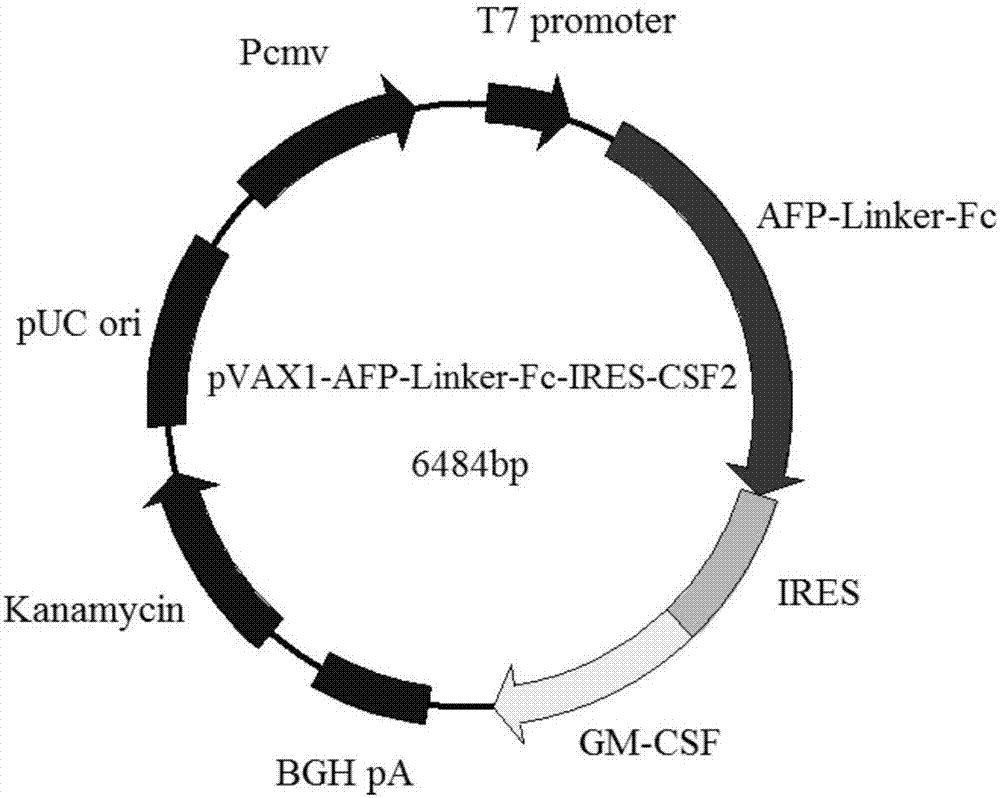
ActiveCN107281475AExtended half-lifeImprove stabilityNucleic acid vectorCancer antigen ingredientsSpecific immunityWilms' tumor
The invention discloses a gene vaccine for preventing and treating tumors. The gene vaccine comprises a recombinant gene eukaryotic expression vector carrying a human AFP gene, a human IgG1 Fc fragment gene and a human CSF2 gene at the same time. The gene vaccine can be applied to gene immunotherapy and prevention of diseases, such as a liver cancer, and is capable of developing the immunomodulatory effect of cytokines and generating a specific immune response through targeting to the diseases, such as the liver cancer; and the number of components of the vaccine is also reduced by adopting a dual-gene co-expression strategy. The test result shows that the vaccine is capable of normally expressing a target gene in an in-vitro human cell line and capable of significantly inhibiting division and proliferation of liver cancer cells under an in-vitro condition.
Owner:杭州贝罗康生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com