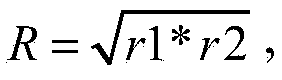Avian influenza H9 subtype inactivated vaccine, preparation method and application thereof
A technology of inactivated vaccines and avian influenza, applied in biochemical equipment and methods, methods based on microorganisms, pharmaceutical formulations, etc., can solve the problem that vaccines cannot be formally produced, sold, and widely promoted, and cannot guarantee safety and immune protection to achieve the effects of good protection against viruses, stable potency, and strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Research on the biological characteristics of H9 subtype avian influenza virus HZ strain and FJ strain
[0025] The H9 subtype avian influenza virus HZ strain and FJ strain described in the present invention were isolated from diseased materials collected from diseased chicken flocks in the Huzhou area of Zhejiang Province and Putian area of Fujian Province in 2010 respectively, and were identified by sequencing. H9 subtype of avian influenza virus. Its biological characteristics are studied as follows: 1) Virus seed passage: the primary seed virus (E1 generation) of HZ strain and FJ strain was diluted to 10 with sterilized normal saline. -4 , inoculate 9-day-old SPF chicken embryos into the allantoic cavity, 0.2 mL per embryo, continue to incubate at 37°C for 96 hours, harvest dead and surviving chicken embryos within 24 to 96 hours, harvest chicken embryo liquid one by one after freezing overnight Detect its HA titer, mix the chicken embryo fluid te...
Embodiment 2
[0053] Embodiment 2: Safety research of avian influenza H9 subtype inactivated vaccine
[0054] A kind of preparation method of avian influenza H9 subtype inactivated vaccine is characterized in that comprising the steps:
[0055] 1) Preparation of H9 subtype avian influenza virus HZ strain venom: make H9 subtype avian influenza virus HZ strain with normal saline for 10 -4 Dilute, inoculate 9-11 day-old SPF chicken embryos, aseptically harvest chicken embryo allantoic fluid with HA≥8log2 that died within 24-96 hours and survived after 96 hours as H9 subtype avian influenza virus HZ strain venom;
[0056] 2) Preparation of H9 subtype avian influenza virus FJ strain venom: make H9 subtype avian influenza virus FJ strain with normal saline for 10 -4 Dilute, inoculate 9-11 day-old SPF chicken embryos, aseptically harvest chicken embryo allantoic fluid with HA≥8log2 that died within 24-96 hours and survived after 96 hours as H9 subtype avian influenza virus FJ strain venom;
[00...
Embodiment 3
[0068] Embodiment 3: Avian influenza H9 subtype inactivated vaccine is to SPF chicken immune effect test
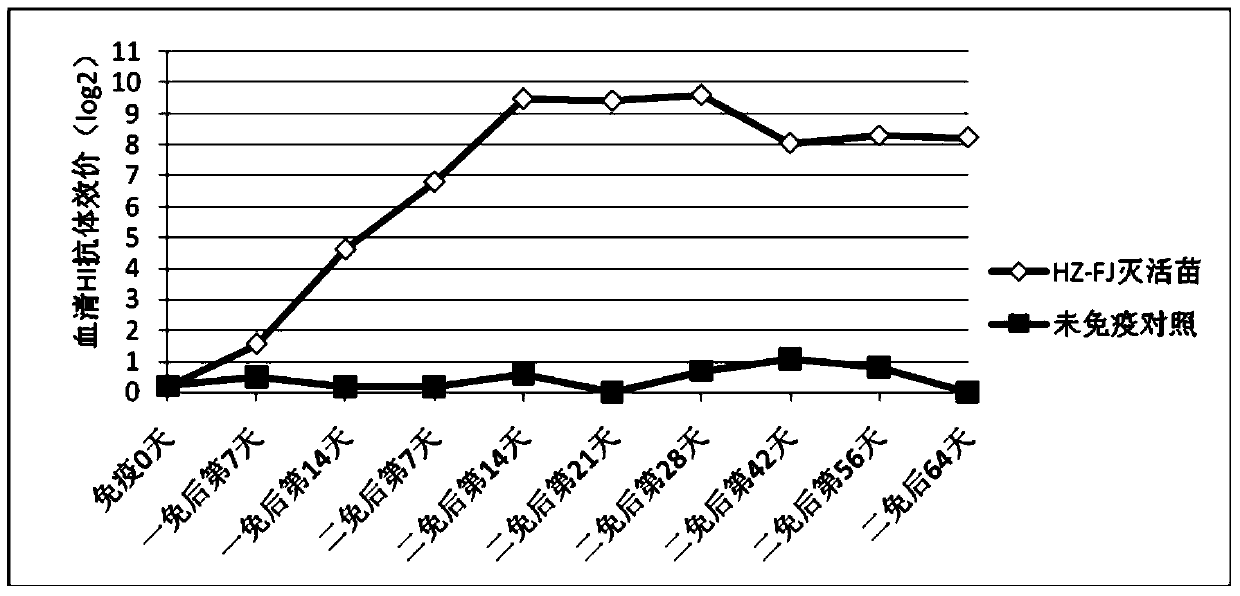
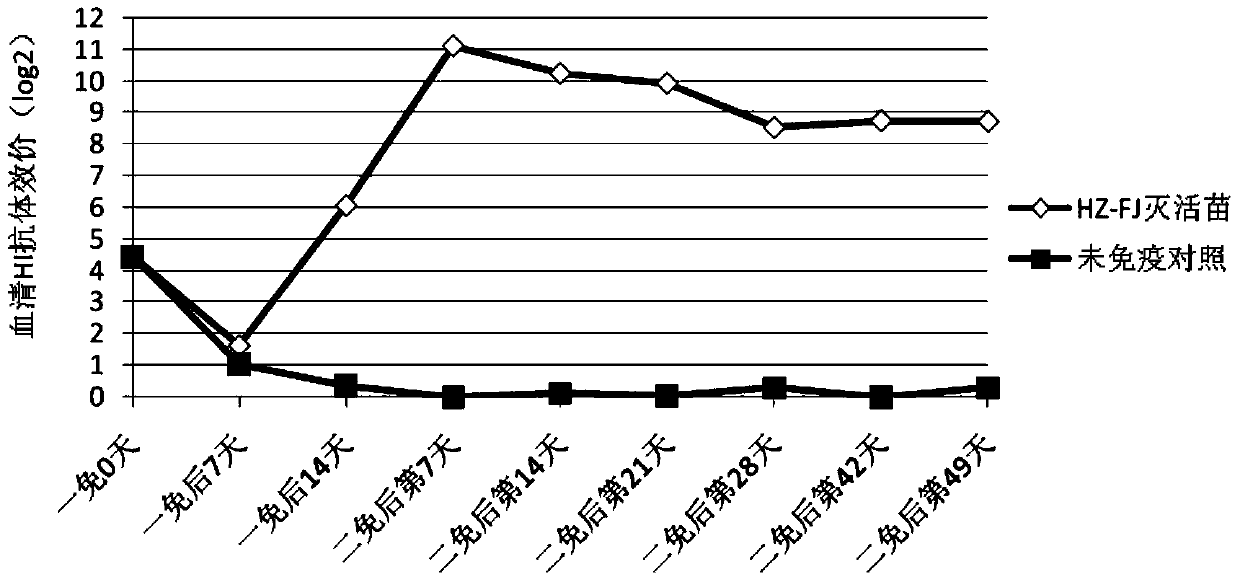
[0069] Prepare avian influenza H9 subtype inactivated vaccine according to the method of Example 2, immunize 21-day-old SPF chickens with the prepared avian influenza H9 subtype inactivated vaccine, 0.3mL / only, take blood 21 days after immunization, separate serum, measure HI Antibody titer, at the same time, the subwing veins were used to attack the isolated strains of AIV H9 subtypes in different places, and the challenge dose was 0.2mL / bird. On the 5th day after the challenge, the throat swabs of each chicken were collected and inoculated through the allantoic cavity Viruses were isolated from SPF chicken embryos aged 9-10 days. The throat swab sample of each chicken was inoculated into the allantoic cavity of 5 SPF chicken embryos aged 9-10 days, and incubated for 96 hours. Regardless of the dead embryos or live embryos, the erythrocyte agglutination value of chicken ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com