Patents
Literature
593 results about "Agglutination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Agglutination is a linguistic process pertaining to derivational morphology in which complex words are formed by stringing together morphemes without changing them in spelling or phonetics. Languages that use agglutination widely are called agglutinative languages. An example of such a language is Turkish, where for example, the word evlerinizden, or "from your houses", consists of the morphemes ev-ler-iniz-den with the meanings house-plural-your-from.
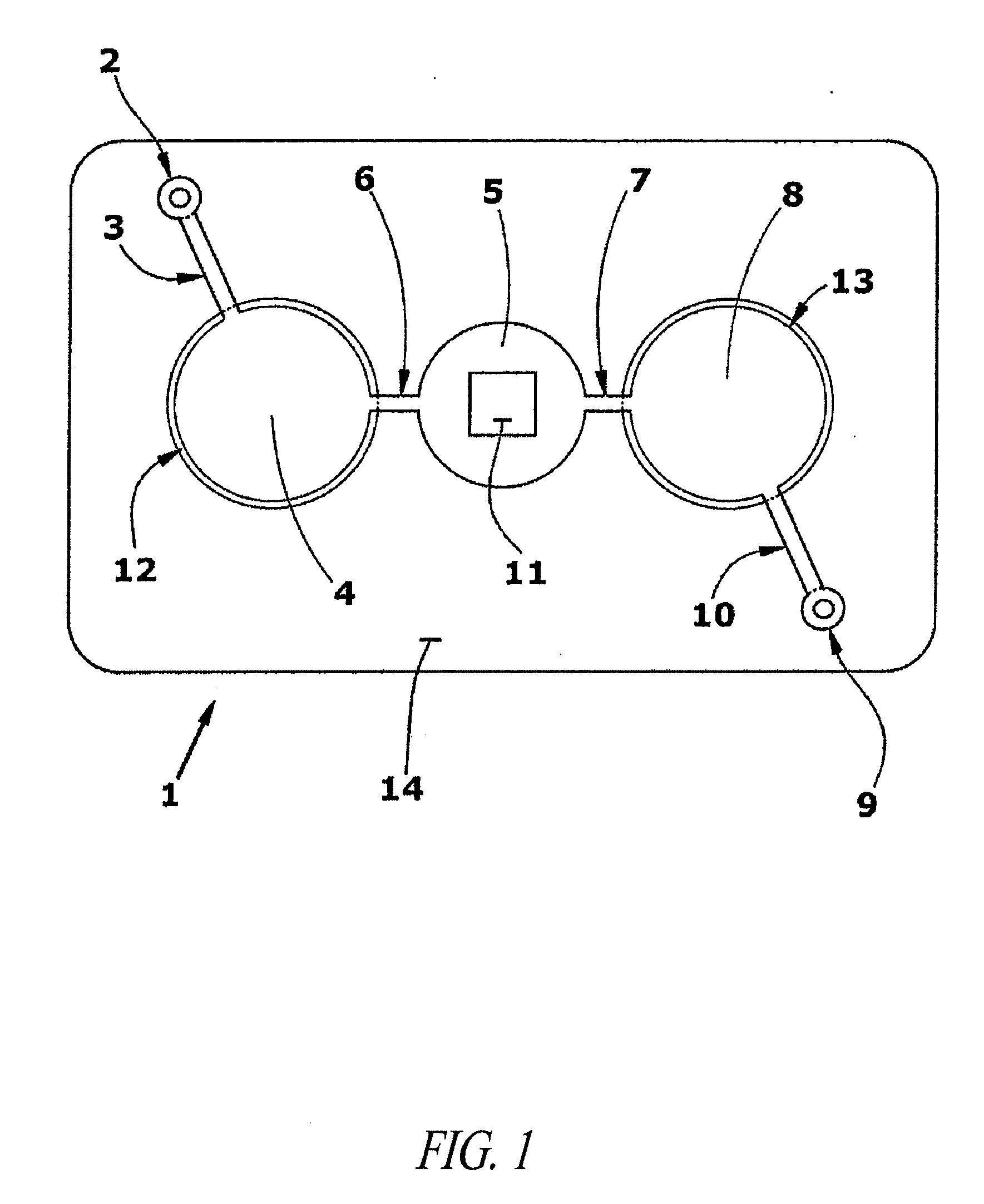
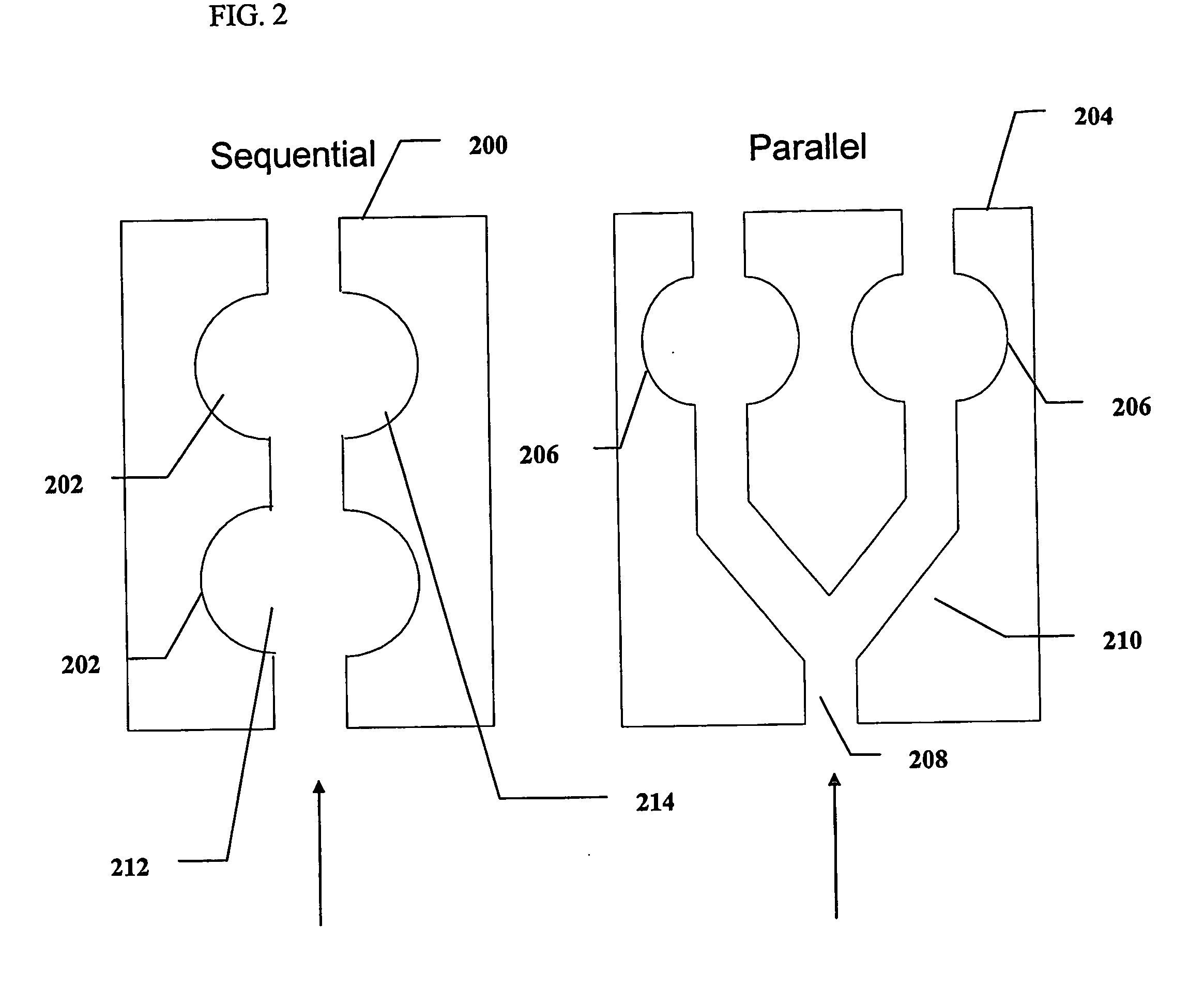
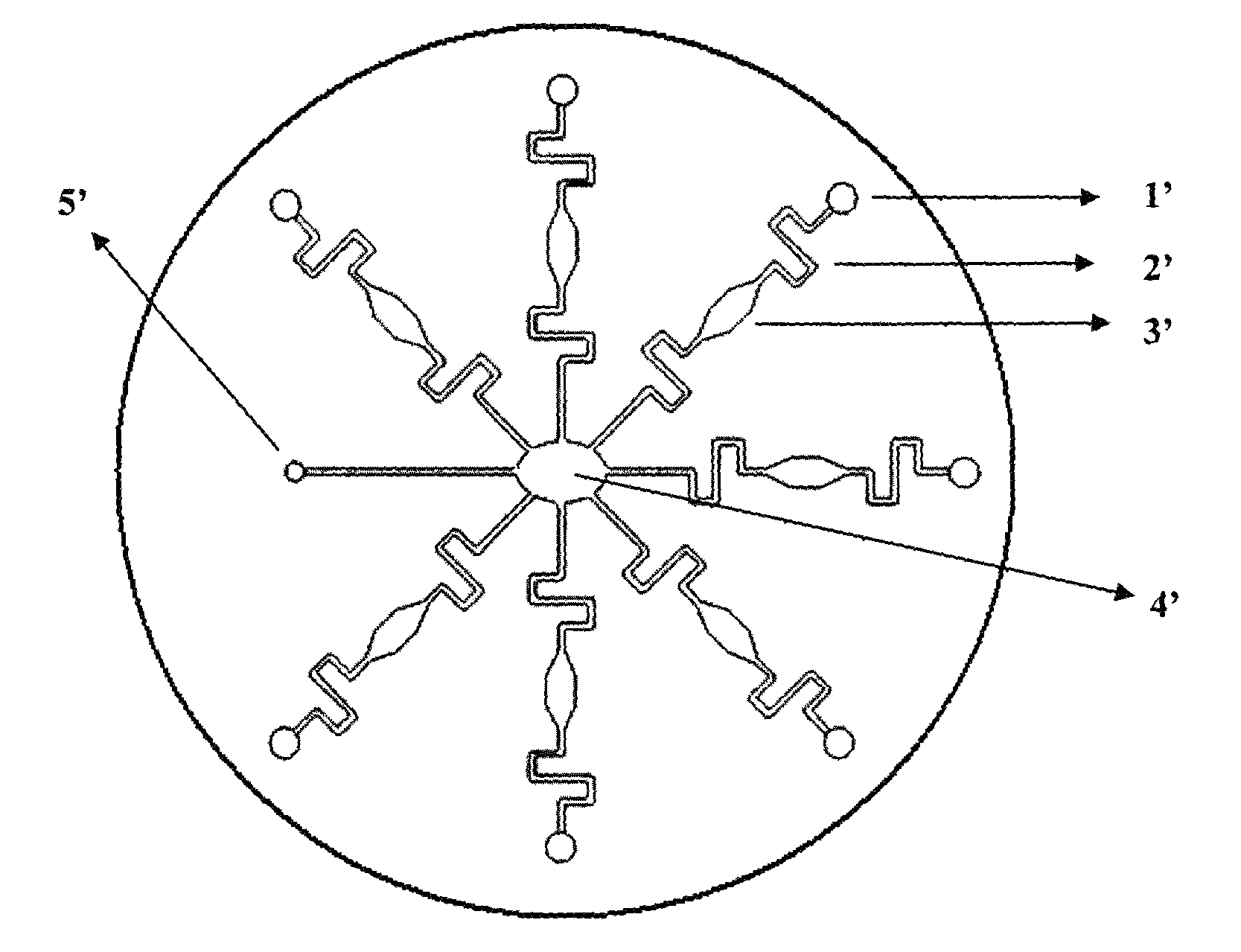
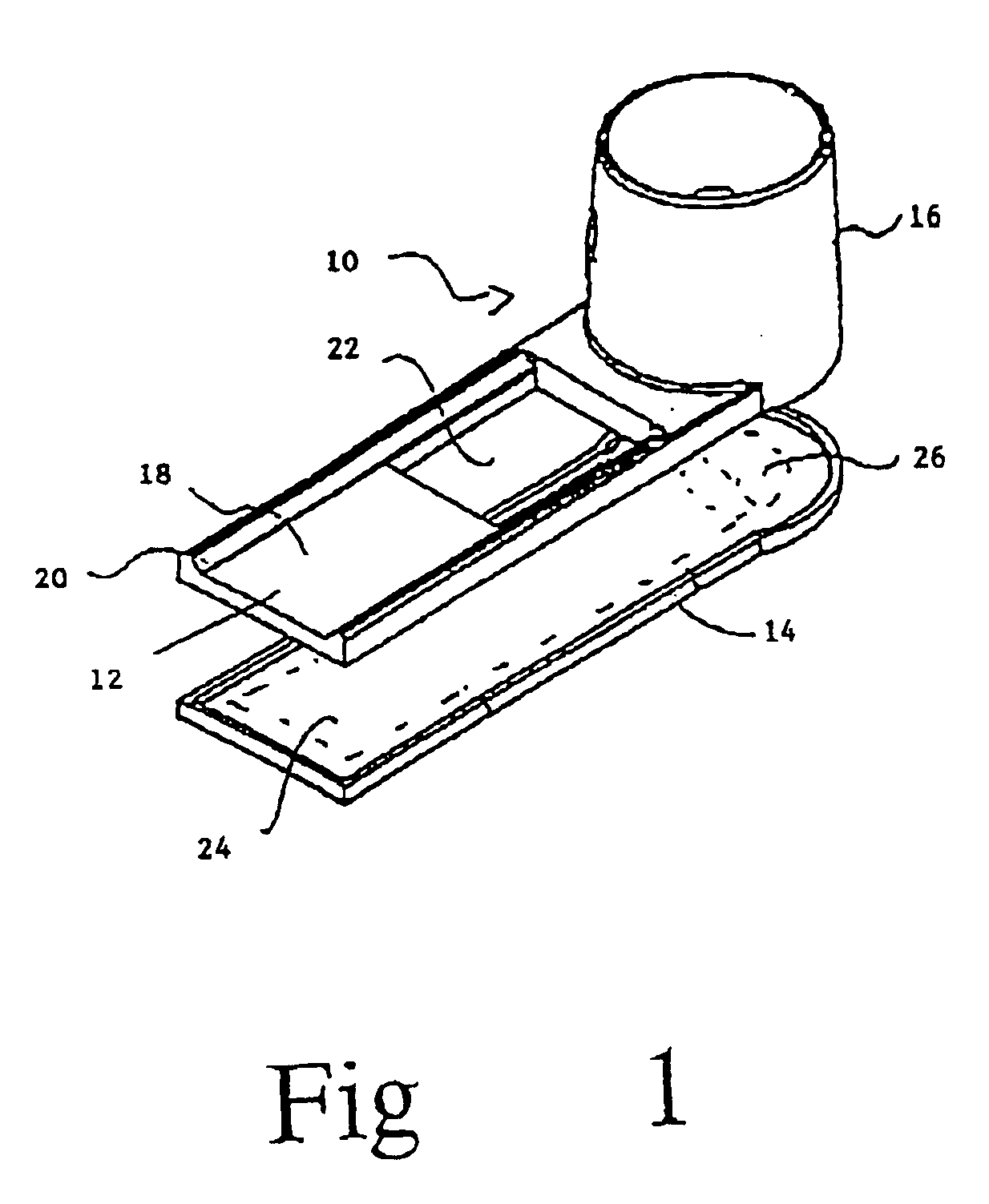
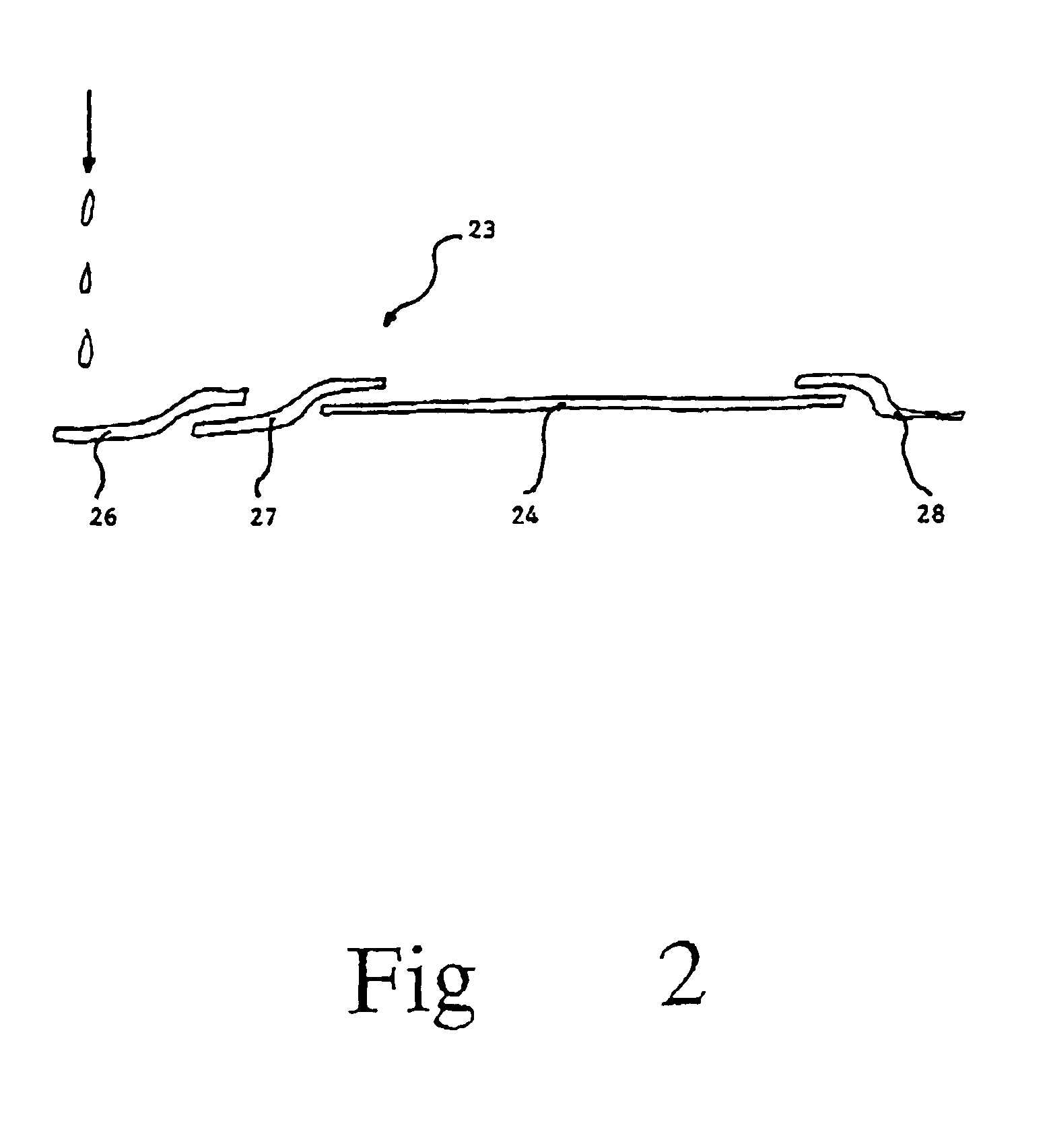
Methods and devices for microfluidic point-of-care immunoassays
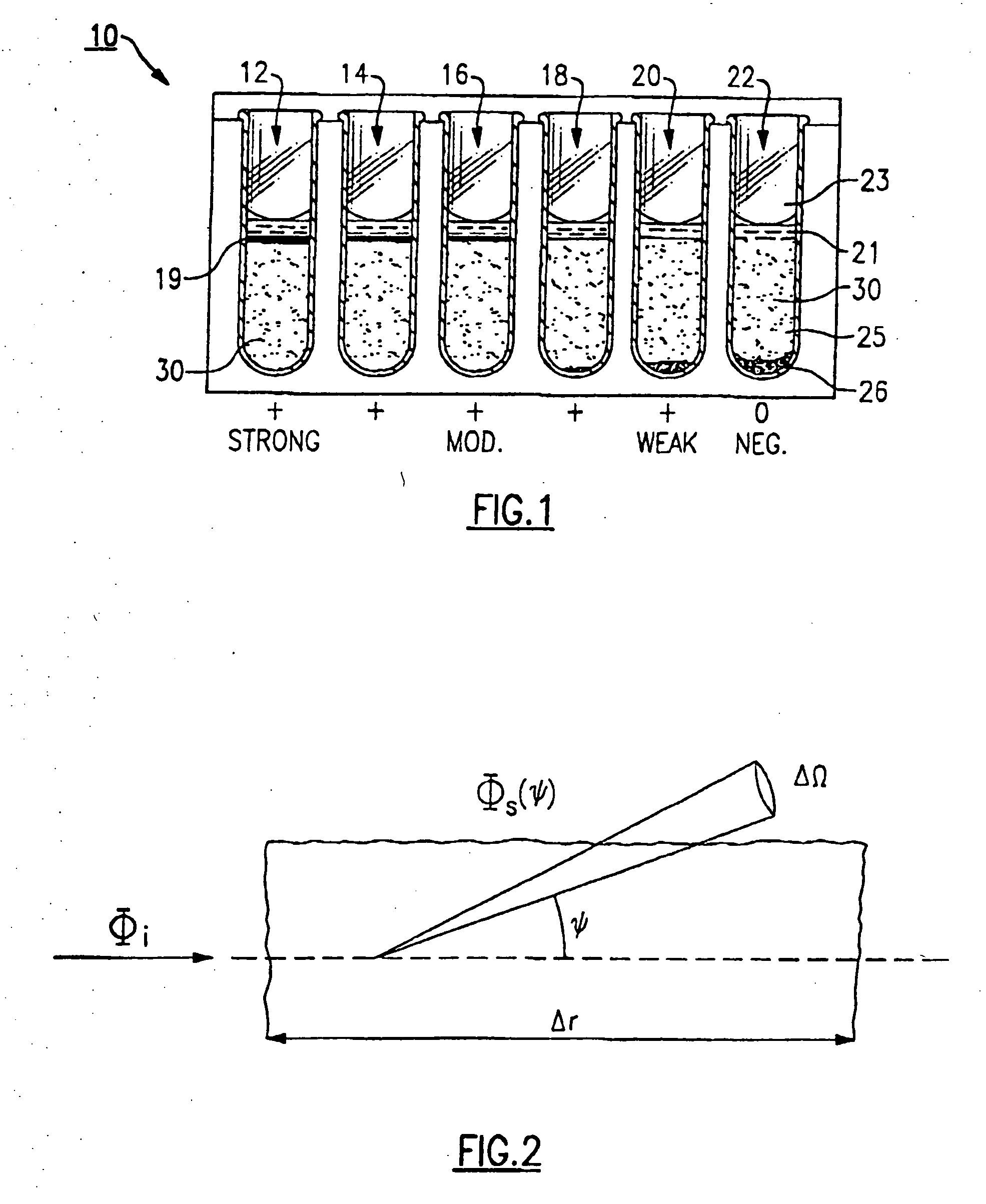
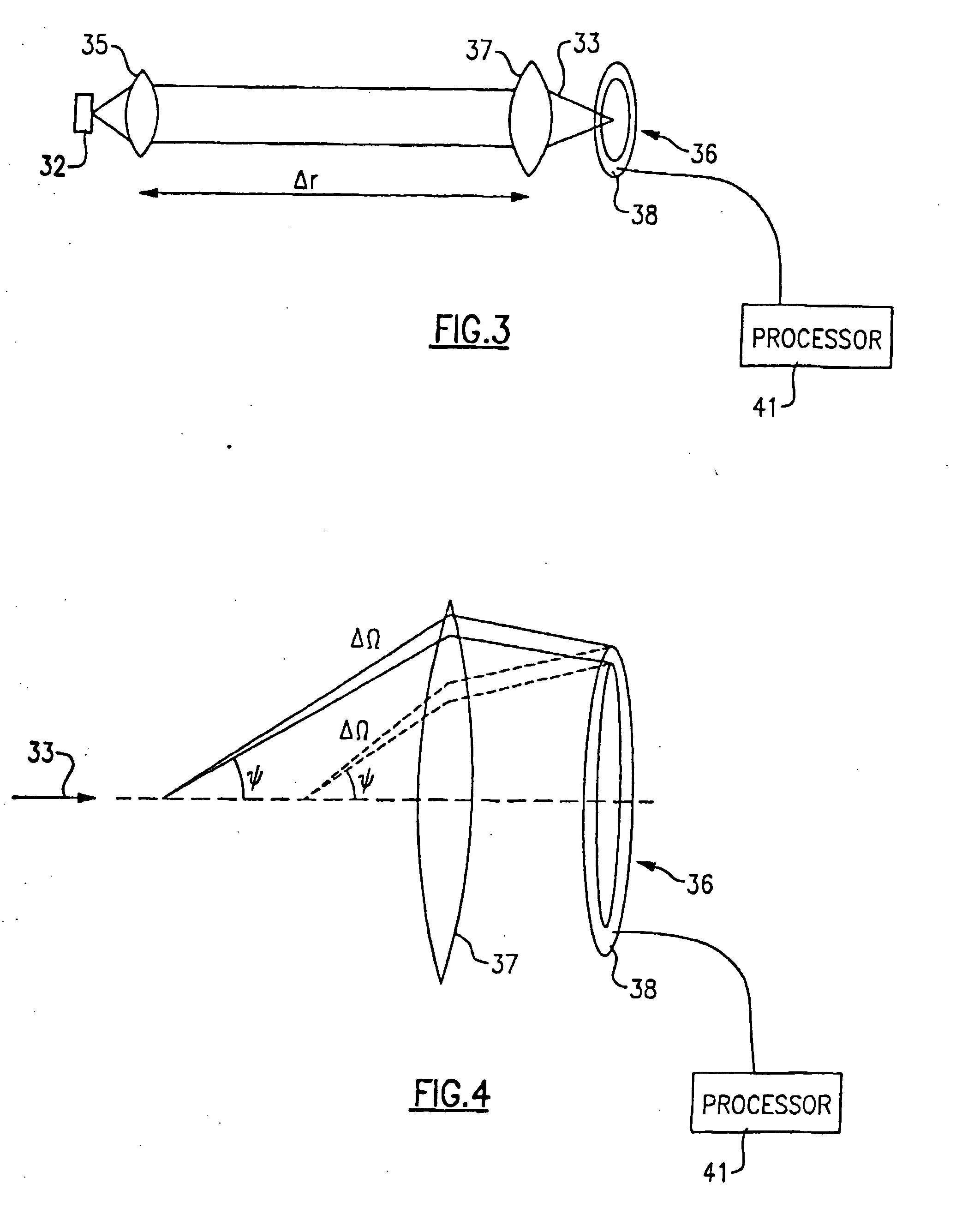
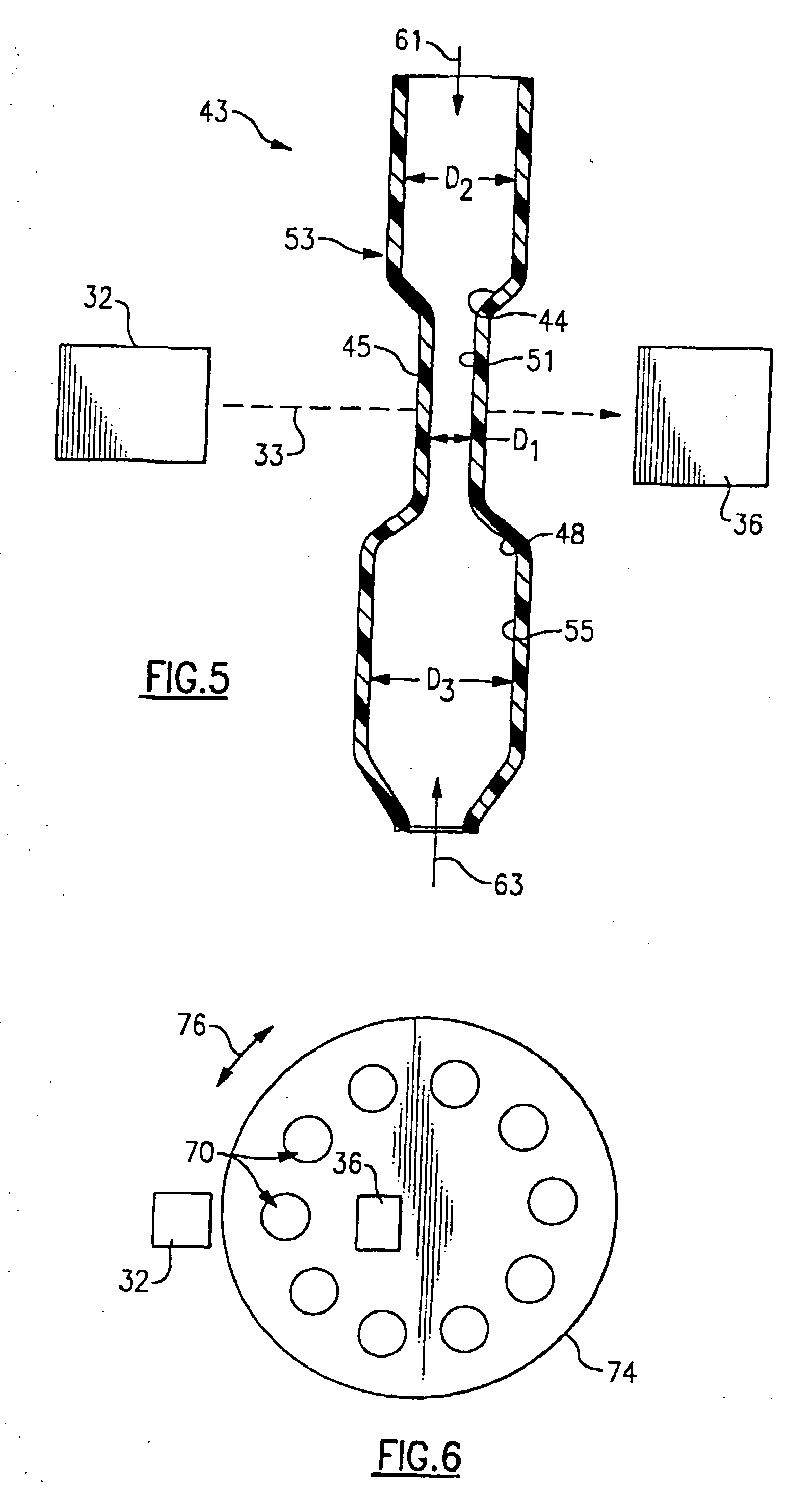
ActiveUS20090181411A1Endpoint detectionReduce incubation timeBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
Methods and devices for microfluidic point-of-care immunoassays
ActiveUS8110392B2Reduce incubation timeMean flow velocityBioreactor/fermenter combinationsShaking/oscillating/vibrating mixersPoint of careSystems design
Microfluidic methods and devices for heterogeneous binding and agglutination assays are disclosed, with improvements relating to mixing and to reagent and sample manipulation in systems designed for safe handling of clinical test samples.
Owner:PERKINELMER HEALTH SCIENCES INC
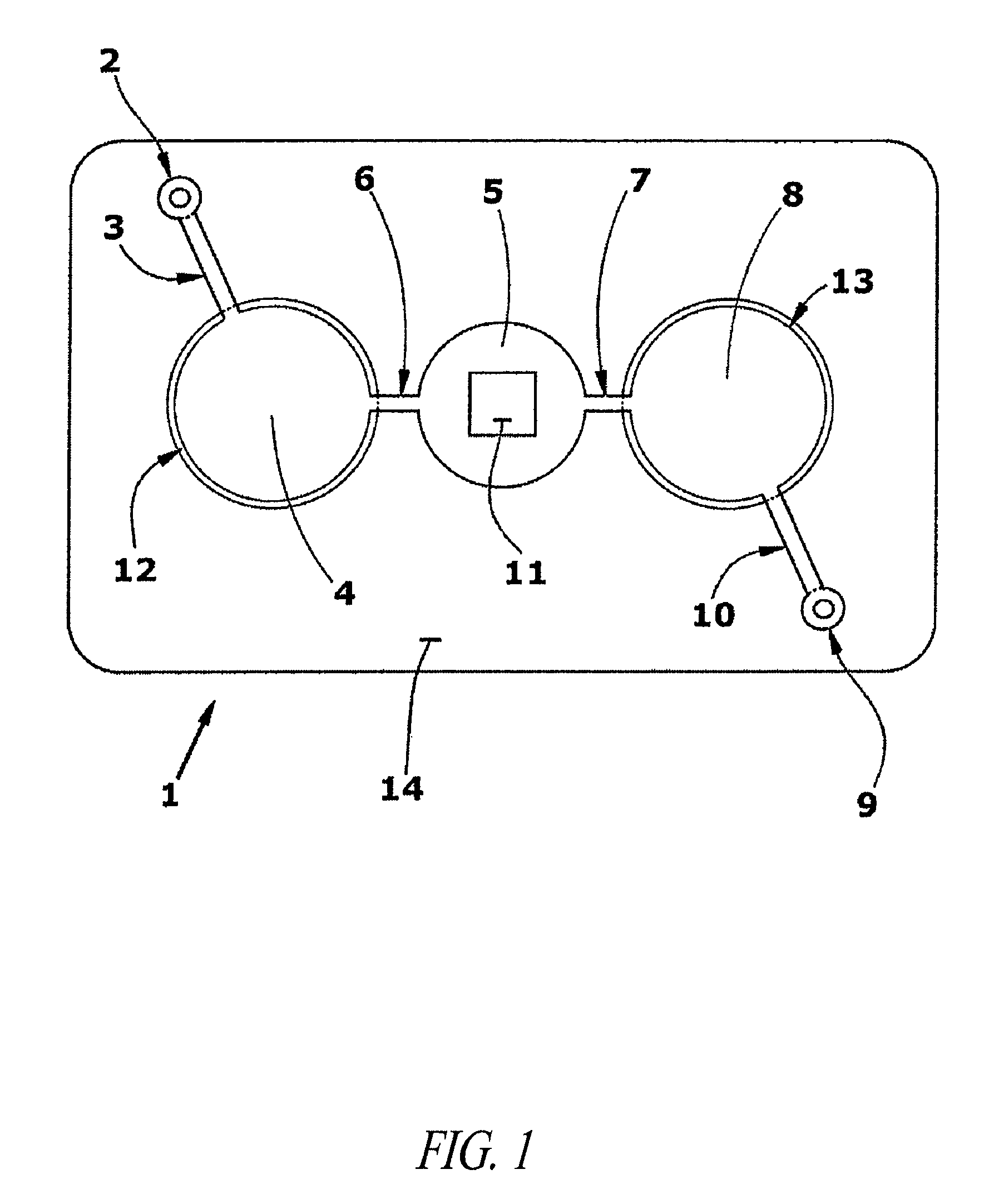
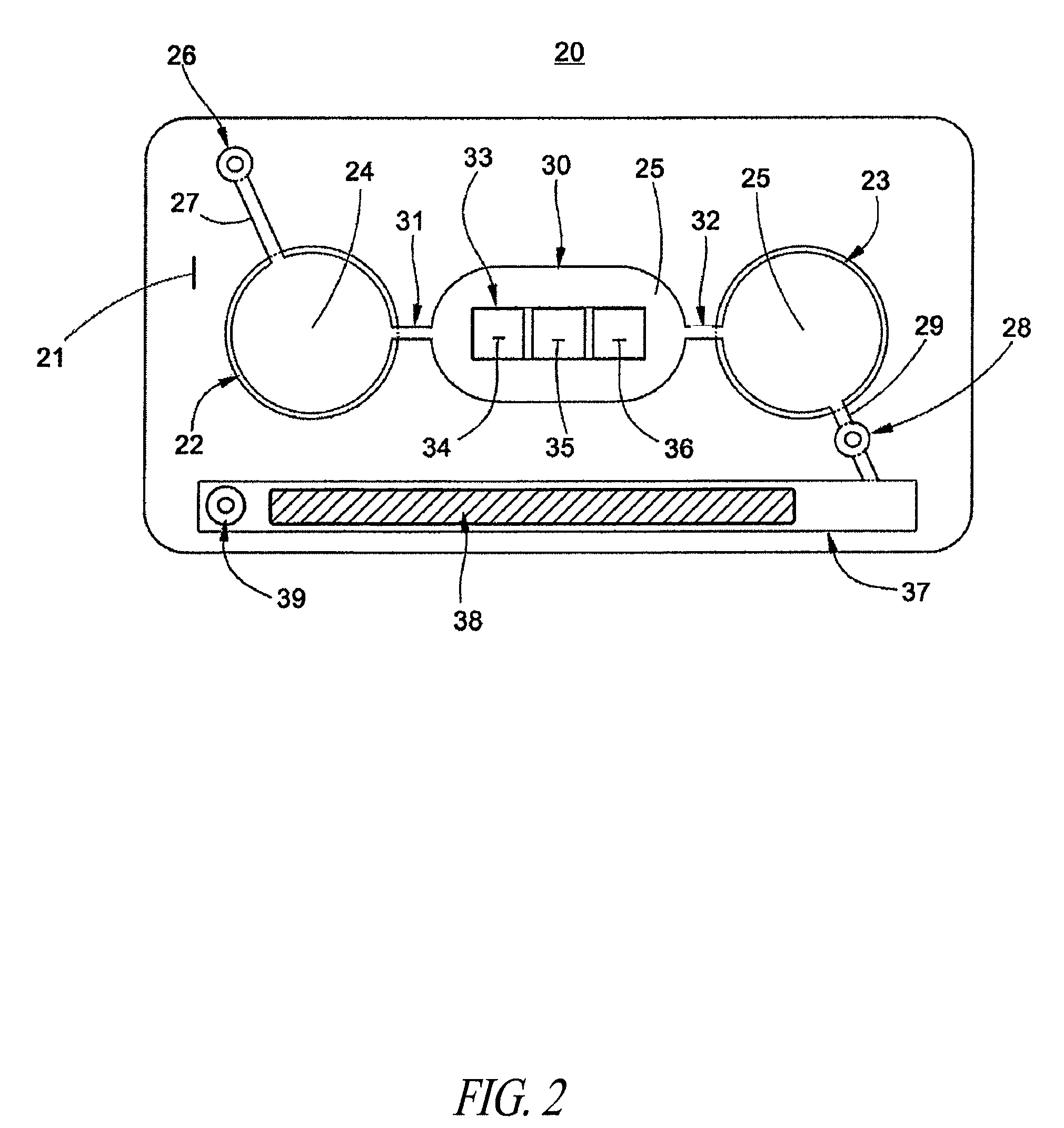
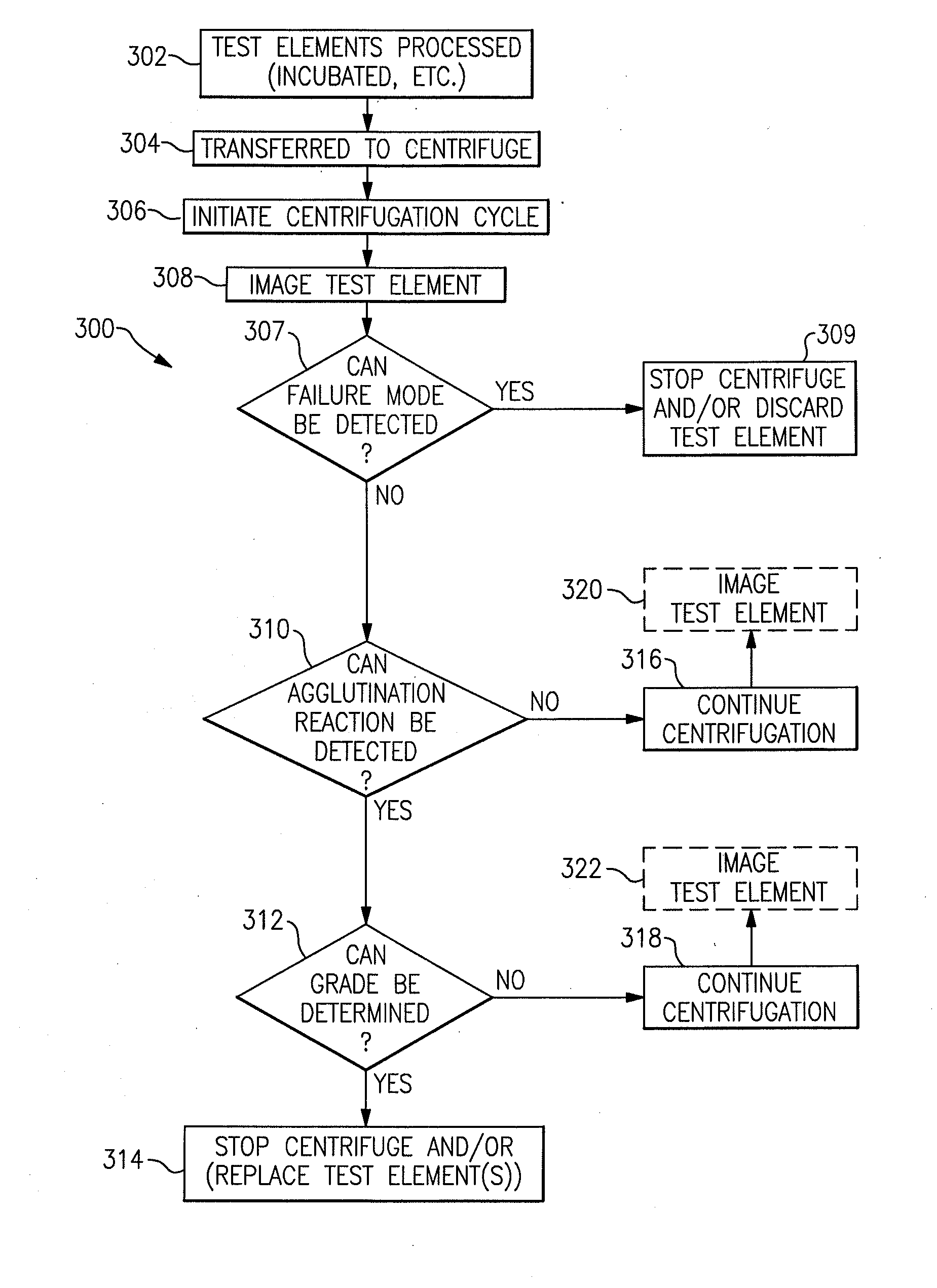
Immunodiagnostic test apparatus having at least one imager to provide agglutination evaluations during centrifugration cycle
InactiveUS20090274348A1Save a lot of timeSimple designCharacter and pattern recognitionMonitoring particle agglomerationCentrifugationEngineering
An immunodiagnostic testing apparatus includes a centrifuge and an imager disposed in relation to the centrifuge wherein at least one image is captured of at least one test element in advance of a complete centrifugation period in order to provide predictive data concerning the presence of an agglutination reaction or failure mode of the apparatus or test element.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Microfluidic apparatus and methods for performing blood typing and crossmatching
ActiveUS8318439B2Increase ratingsEasy to distinguishBioreactor/fermenter combinationsBiocideAntigenAntibody-mediated agglutination
Owner:PERKINELMER HEALTH SCIENCES INC
Multi-channel multi-target ultra-optical spectrum imaging method and system based on digital micro lens device
InactiveCN101303291AReduce dataAccurate identificationColor/spectral properties measurementsGratingData acquisition
The invention discloses a multichannel multiobjective hyper-spectral imaging method based on numerical microscopes, characterized in that, an object is imaged on a slit plane, an emergent light is collimate into a parallel light, split into ultraviolet light, infrared light and visible light, which are diffracted into the dispersion through the respective split grating diffraction, then is focused on the corresponding numerical microscope; the turning state of the numerical microscope is controlled by the computer, and the emergent light of the on-state is projected on the detector, used for image and post-treatment through the data acquisition treatment. The device is arranged with a multi-agglutination prism of the dichroism filter, and the dispersion of the ultraviolet light, the infrared light and the visible light is realized. The method realizes the image information acquisition of the triband of infrared light, visible light and ultraviolet light, solves the problems that the spectrum imaging data are too large without affecting the spectrographic detection quality of the target area, and is in favor of realizing the multiobjective identification and the real-time track.
Owner:SUZHOU UNIV
Immunoassay method for lysed whole blood
InactiveUS6030845AIntuitive effectEasy to separateMaterial analysis by optical meansBiological testingAntigenAgglutination
An immunoassay method in which blood can be measured even without pretreatment by a centrifuge etc. In the present invention, antibodies or antigens in a sample are subjected to agglutination reaction with insoluble carriers onto which antigens or antibodies specifically reacting with the antibodies or antigens in the sample have been imrobilized and the resulting agglutination mixture is determined for the change in its absorbance or in its scattered light by irradiation with light, wherein said sample is whole blood and the whole blood is forcibly lysed.
Owner:HORIBA LTD
Photometric determination of coagulation time in undiluted whole blood
InactiveUS20060110283A1Easy to implementEasy to operateMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceAgglutinationBlood coagulations
A device, system and method for photometric detection of coagulation in whole blood. The present invention is easy to implement and operate. Furthermore, the present invention has the advantage of being considered to fulfill the desired standard of using photometry for measuring blood coagulation. Also, a photometric coagulation test device for whole blood specimens according to the present invention provides medical accuracy to the home user and, at the same time, is simple to construct. The present invention is also useful for detecting and determining blood agglutination, for example as the results of a serological reaction with an antibody.
Owner:INVERNESS MEDICAL SWITZERLAND GMBH
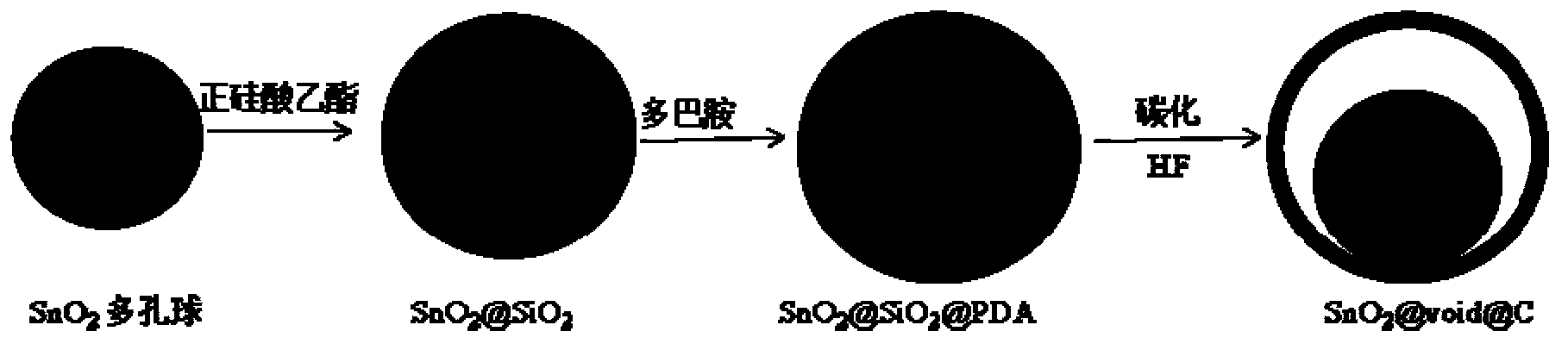
Yolk-shell structure tin dioxide-nitrogen-doped carbon material and preparation method thereof
ActiveCN103367719AImprove conductivityImprove electrochemical cycle stabilityMaterial nanotechnologyCell electrodesTin dioxideYolk
The invention relates to a yolk-shell structure tin dioxide-nitrogen-doped carbon material and a preparation method thereof, belonging to the technical field of lithium ion battery electrode material. The yolk-shell structure SnO2@void@N-C material takes porous submicron tin dioxide SnO2 as a core and has the diameter of 200-400 nanometers; nitrogen-doped carbon (N-C) is taken as a shell, and the thickness of the shell is 15-20 nanometers; a cavity has the inner diameter of 300-500 nanometers; in the N-C shell, the mass percent of N element is 8-12%. The porous SnO2 core shortens the lithium ion diffusion path; the volume change of SnO2 can be effectively buffered by a gap between the SnO2 core and a carbon layer in the charge-discharge process, and the N-C can effectively improve the electrical conductivity of the material, so that the yolk-shell structure tin dioxide-nitrogen-doped carbon material has excellent electrochemical cycle stability. The thickness of the carbon layer can be regulated and controlled by controlling the concentration of dopamine or the auto-agglutination time, and the size of the gap can be regulated and controlled by controlling the quantity of tetraethoxysilane; the preparation method can well control the structure of the material, and is simple in technology and convenient to operate.
Owner:BEIJING UNIV OF CHEM TECH
Lactobacillus plantarum and preparation method thereof for high-density culture and freeze-drying bacteria powder
InactiveCN102864096AStrong toleranceIncrease vitalityBacteriaMicroorganism based processesFreeze-dryingAgglutination
The invention discloses a lactobacillus plantarum and a preparation method thereof for high-density culture and freeze-drying bacteria powder, and has the technical scheme that the lactobacillus plantarum (Lactobacillus plantarum P8) is probiotics separated from the traditional fermentation milk product (yoghurt) of the Urat Middle Banner of Inner Mongolia Bayan Nur. The probiotics has the characteristics of artificial gastric juice and artificial digestive juice tolerance, cholate tolerance, intestinal agglutination action and common pathogenic entero bacteria inhibition. The bacterial strain of the bacteria is collected in the China general microbiological culture collection center with the collection number of CGMCC (China general microbiological culture collection center) No. 5468 on 18th, Nov., 2011.
Owner:北京和美科盛生物技术有限公司
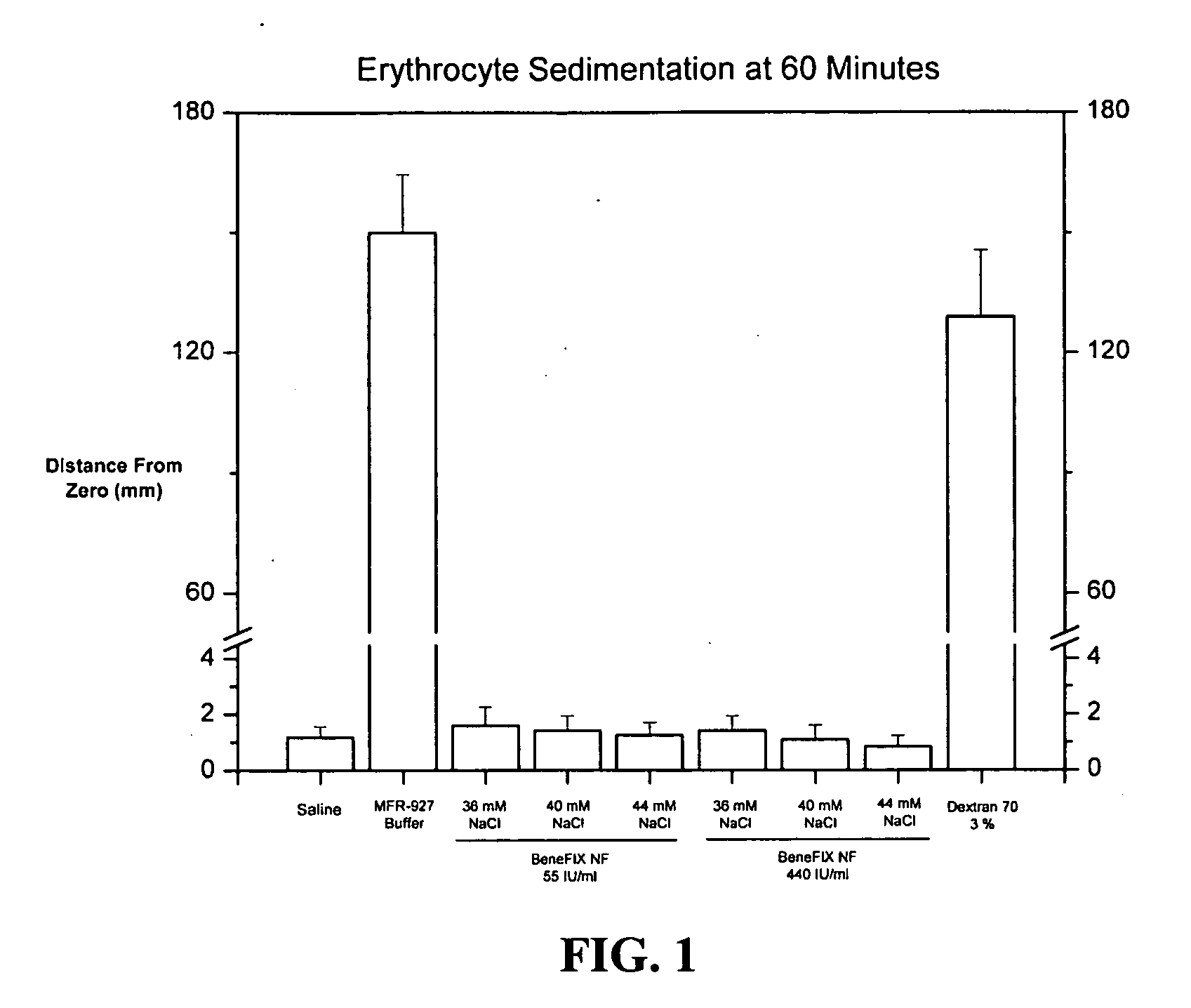
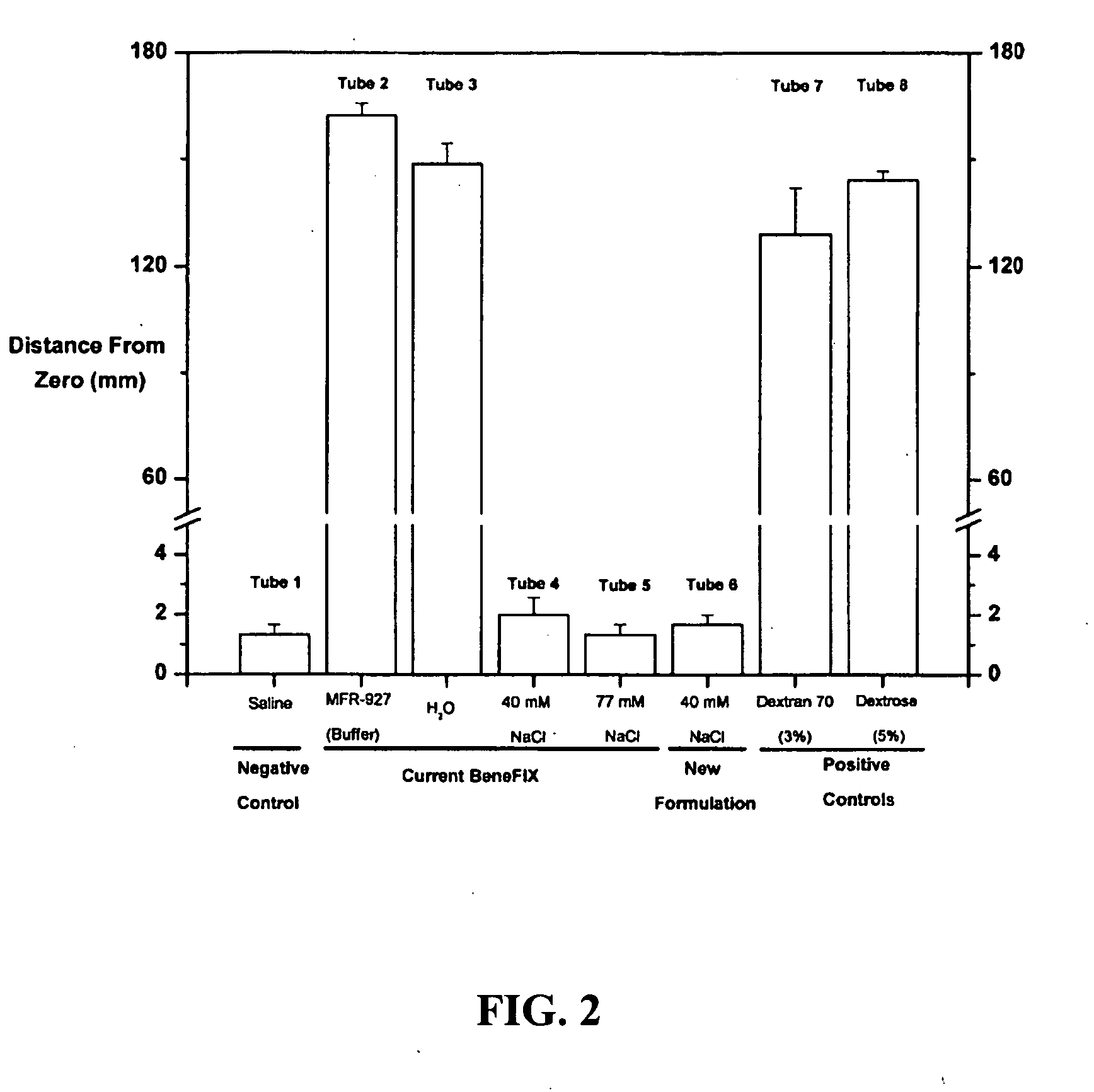
Sodium chloride solution for drug reconstitution or dilution
InactiveUS20070135343A1Prevent agglutinationIncrease ionic strengthBiocidePeptide/protein ingredientsHemolysisPresent method
The invention provides methods for preparing pharmaceutical formulations for injection such that upon injection the formulation does not cause erythrocyte agglutination, hemolysis, and / or cell shrinkage. To prevent agglutination, a pharmaceutical formulation ready for injection needs to have a sufficient ionic strength. To prevent hemolysis or cell shrinkage, a pharmaceutical formulation ready for injection needs to be about isotonic with respect to plasma. The invention provides methods that prepare pharmaceutical formulations for injection that have both the sufficient ionic strength to prevent agglutination and the requisite tonicity to prevent significant hemolysis or cell dehydration or shrinkage. The present methods involve the use of sodium chloride solutions that are about 25 mM to about 150 mM for reconstituting lyophilized cakes (or other non-liquid pharmaceutical formulations) into solution or for diluting pharmaceutical formulation solutions.
Owner:WYETH LLC
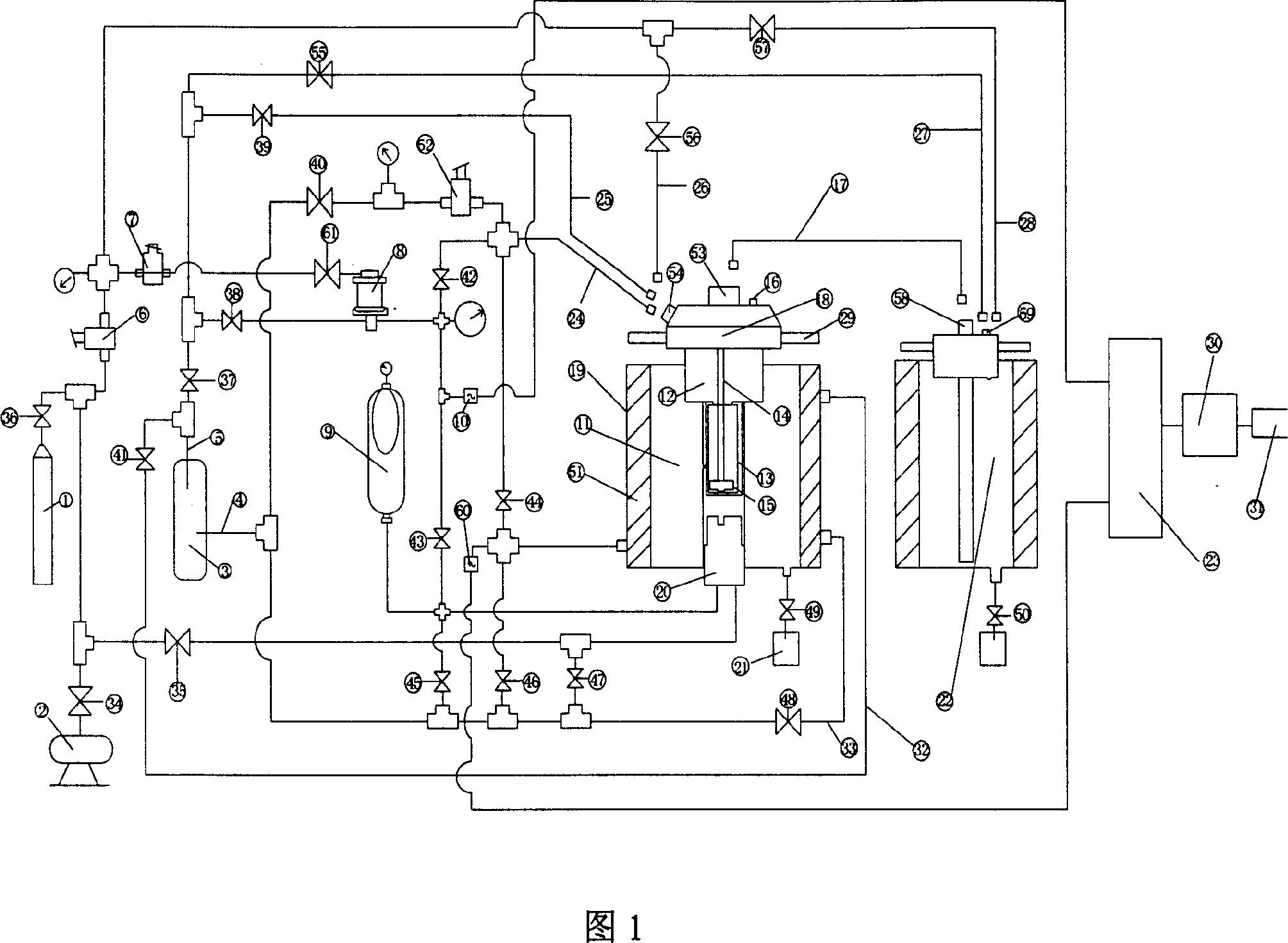
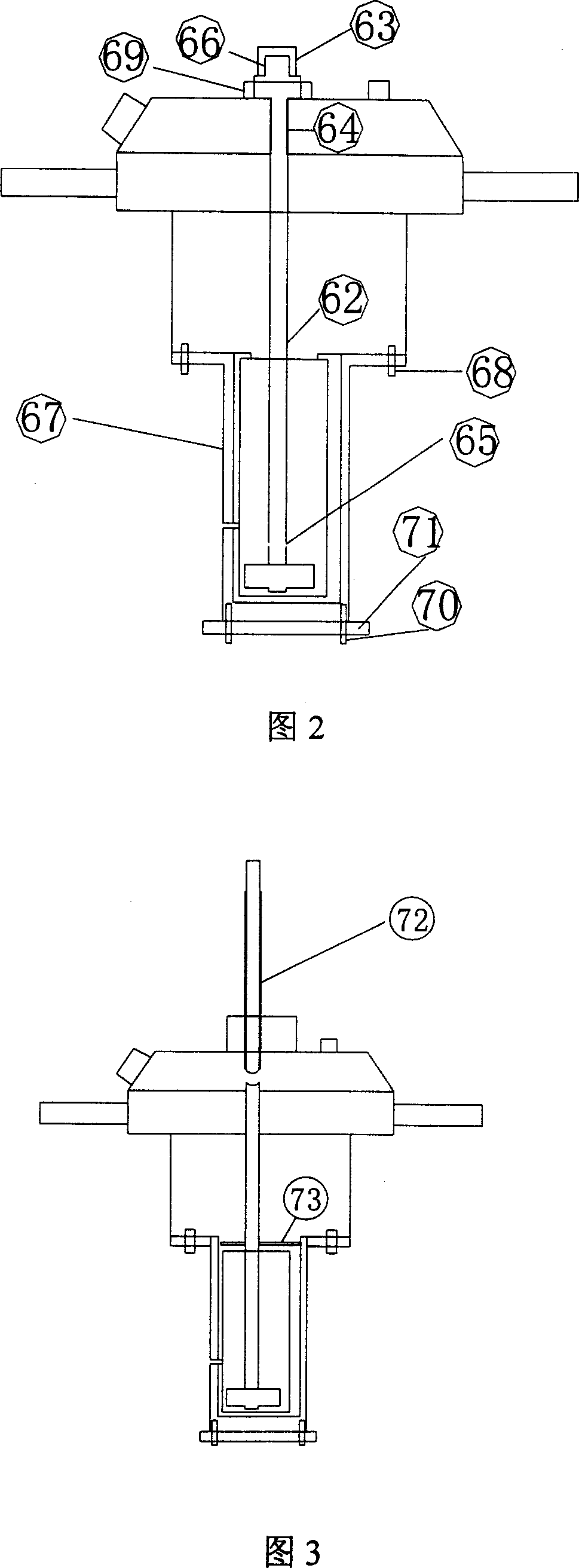
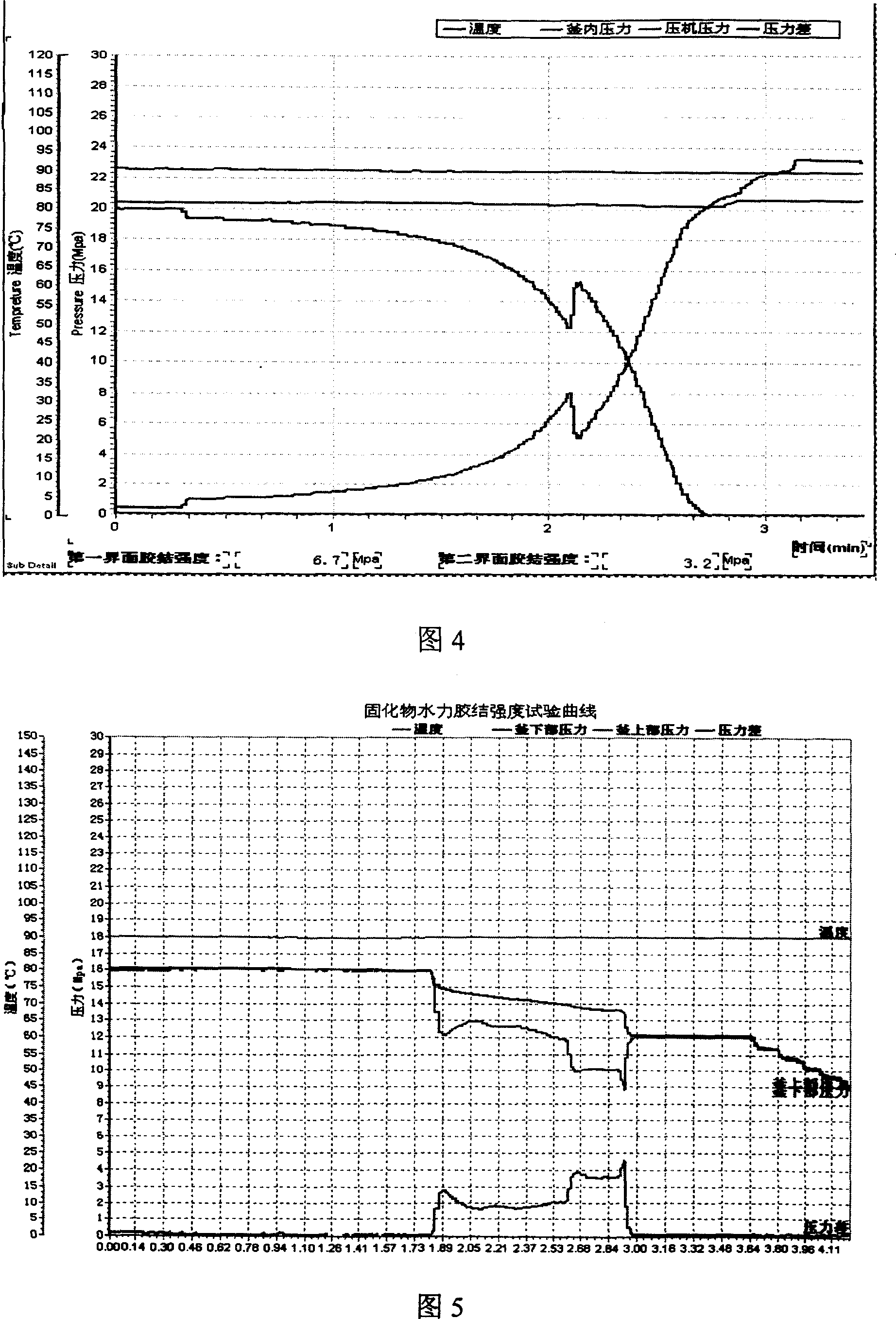
High-temperature high-pressure clay cake interface agglutination simulating-estimating device
ActiveCN101109281AOptimize formulation designImprove construction qualitySealing/packingPressure systemWell drilling
The invention provides a lab device for evaluating the hi-pressure and hi-temperature cementing quality of mud cakes, which essentially comprises a hi-temp and hi-pressure vessel 19, a simulated wellhole 13, an intermediate container 22, a heating and a cooling system, a pressure source and a pressure system, a data acquisition controller 23, a computer 30 and an output equipment 31. The function of the invention is to simulate the in-well pressure and temperature condition, realize such continuous experimental operations as well-hole type filtering of drilling fluid, rinsing and replacing of drilling fluid, injecting of well-solidifying fluid and curing of well-solidifying fluid, etc., simulate and evaluate the impact of in-well drilling fluid and the existing of mud cake on the cementing quality of the interface of the well-solidifying fluid, evaluate the flushing effects of different flushing fluids on circular space, measure the shearing and cementing strength of the interface between the flushing fluid and the circular space, measure the hydraulic cementing strength of the interface between the flushing fluid and the circular space, and provide experimental data for the optimal selection and engineering design for the drilling fluid and well-solidifying fluid in well drilling in oil field.
Owner:CHINA PETROLEUM & CHEM CORP +1
Patient sample classification based upon low angle light scattering
InactiveUS20070054405A1Effective qualitative determinationEfficient determinationParticle size analysisSedimentation analysisBlood typingAgglutination
An apparatus for classifying a liquid patient sample includes at least one sample container having a quantity of a sample that is aggressively acted upon so as to create a flow field. A measurement mechanism includes at least one low angle light emitter aligned with a measurement window of the at least one sample container and a detector oppositely disposed relative to the measurement window. Measurement of the scattered light detects particle characteristics of a moving flow field from the sample to determine, for example, the amount of agglutination of the sample so as to perform blood typing or other classifications without spatial separation.
Owner:ORTHO-CLINICAL DIAGNOSTICS
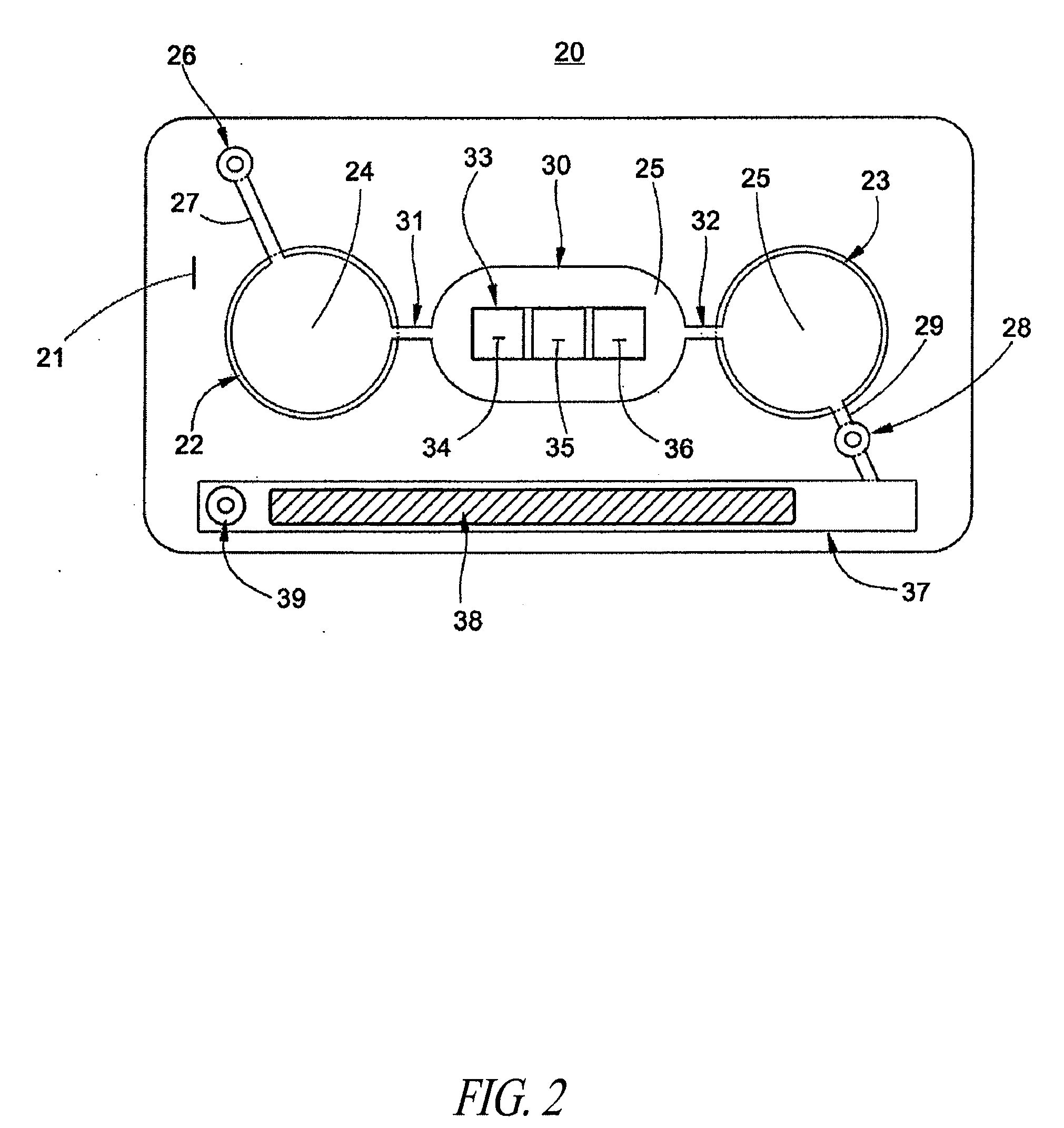
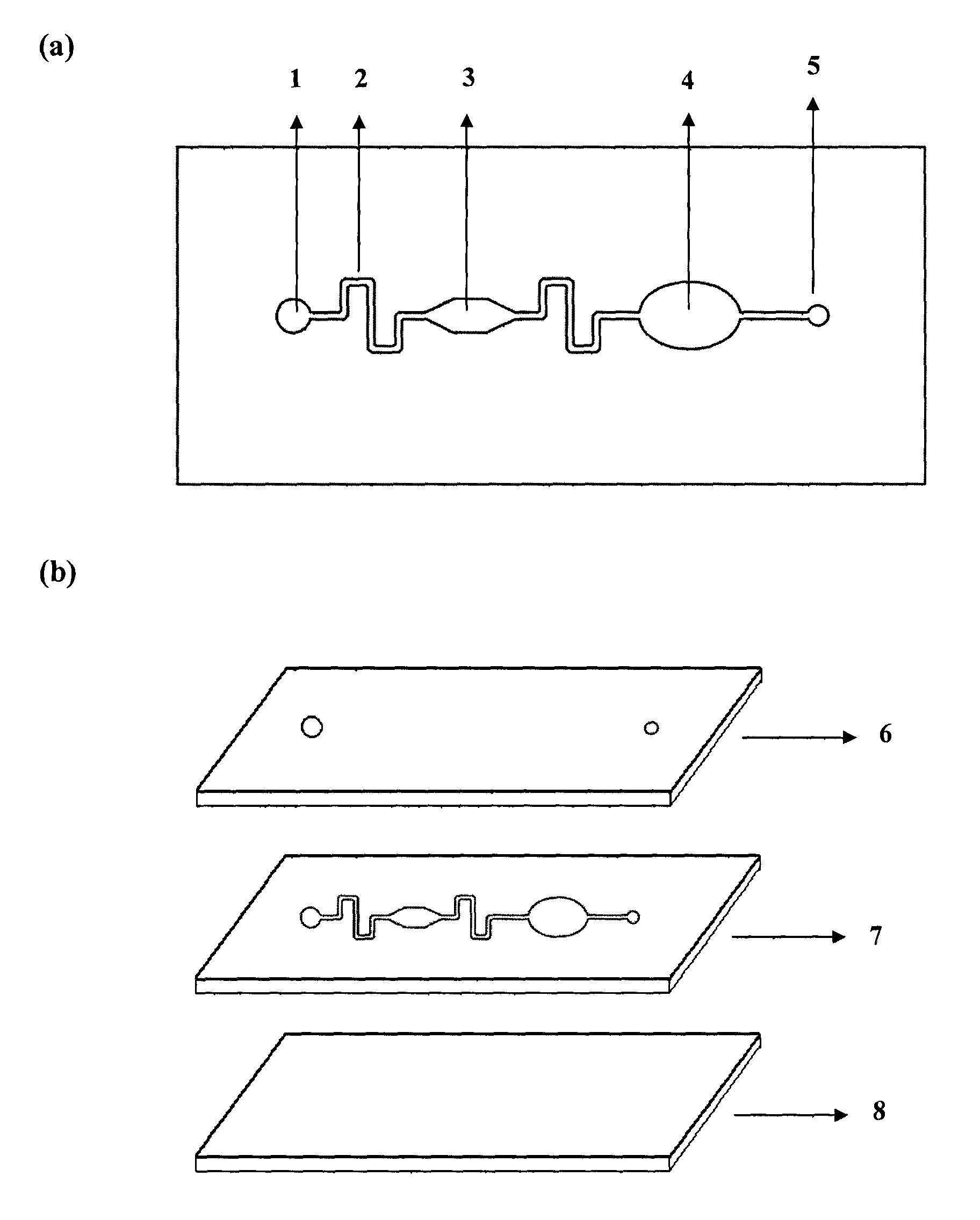
Micro-fluidic chip for glycosylated hemoglobin immunodetection
The invention provides a micro-fluidic chip for glycosylated hemoglobin immunodetection in serum, comprising a sample introduction pool, a micro-fluid channel, a reaction tank, a detection tank, a waste liquor tank and a pump valve interface, wherein the sample introduction pool, the micro-fluid channel, the water liquor tank and the pump valve interface are serially connected by the micro-liquid channel; antigens or antibodies necessary for protein detection are fixed in the reaction and detection tanks in advance, a serum sample solution to be detected successively flows in the reaction tank by virtue of the sample introduction pool and the micro-fluid channel by an externally connected pump valve system, completes antigen or antibody specific reaction in the reaction tank and agglomerates, and the reaction product is subjected to absorbancy analysis to obtain the immune agglutination reaction and detection of the sample to be detected. The chip is convenient for sample introduction, less in sample consumption, high in reaction efficiency and short in detection time.
Owner:ZHEJIANG PUSHKANG BIOTECHNOLOGY CO LTD
Reagent for an immunoassay
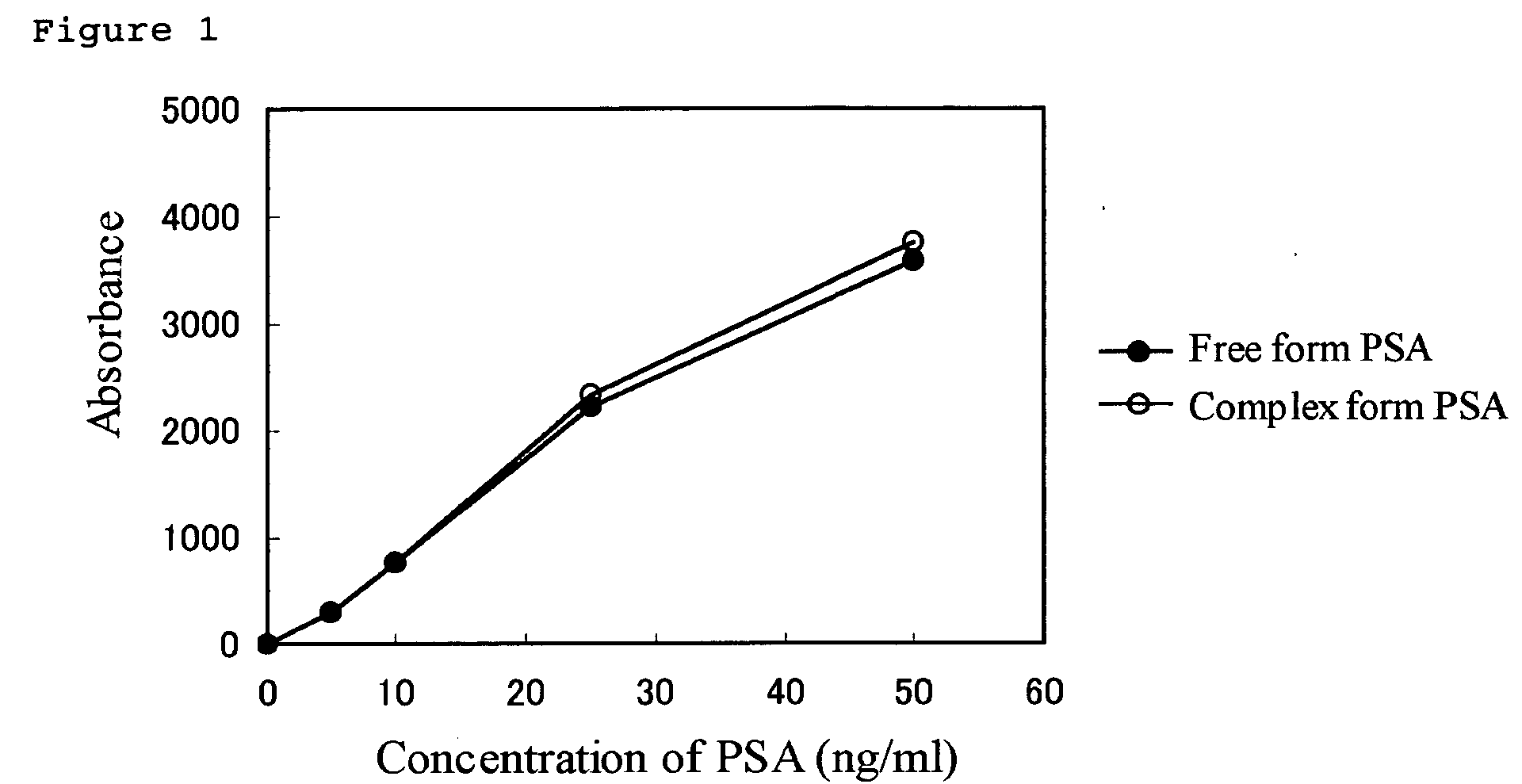
InactiveUS20050069967A1Highly sensitive and highly preciseAccurate measurementBiological testingParticle suspension analysisFree formMonoclonal antibody
The present invention relates to (1) A reagent for an immunoassay of a target substance existing in a free form and a bound form in a specimen, comprising a latex 1 which is immobilized with a monoclonal antibody 1 for the target substance, and a latex 2 which has a different mean particle size from the latex 1 and is immobilized with a monoclonal antibody 2 having a different recognition site for the target substance from the antibody 1, (2) An immunoassay method comprising reacting the target substance with the reagent of (1) and determining an amount of the substance based on the result of an agglutination reaction among the target substance, the latex1 and the latex2, and (3) A reagent kit comprising a reagent of (1) and a reagent containing an agglutination accelerator for an antigen-antibody reaction.
Owner:WAKO PURE CHEMICAL INDUSTRIES
Surface modification method for polymer separation membrane
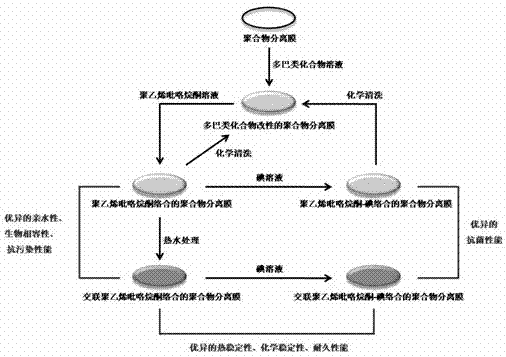
ActiveCN103041721AModification equipment is simpleShorten the production cycleSemi-permeable membranesPolymer scienceAgglutination
The invention discloses a surface modification method for a polymer separation membrane. Under the water solution condition, a dopa compound is easily oxidized by dissolved oxygen in water to generate auto-agglutination-composite reaction in order to generate a firmly attached dopa compound composite layer on the surface of the polymer separation membrane; the dopa compound composite layer contains rich catechol radicals which can form the multi-point hydrogen bonding effect with lactam radicals in polyvinylpyrrolidone, and the polyvinylpyrrolidone is firmly complexed on the surface of the separation membrane; and the polymer separation membrane immobilized with povidone-iodine on the surface can be prepared through the complexation between the polyvinylpyrrolidone and iodine. The method has simple technique, and is suitable for the polymer separation membranes with different materials and shapes; and the modified polymer separation membrane has excellent hydrophily, blood compatibility, contamination resistance and bacterial resistance, so that the important significance is given to improve the comprehensive performance of the polymer separation membrane.
Owner:ZHEJIANG UNIV
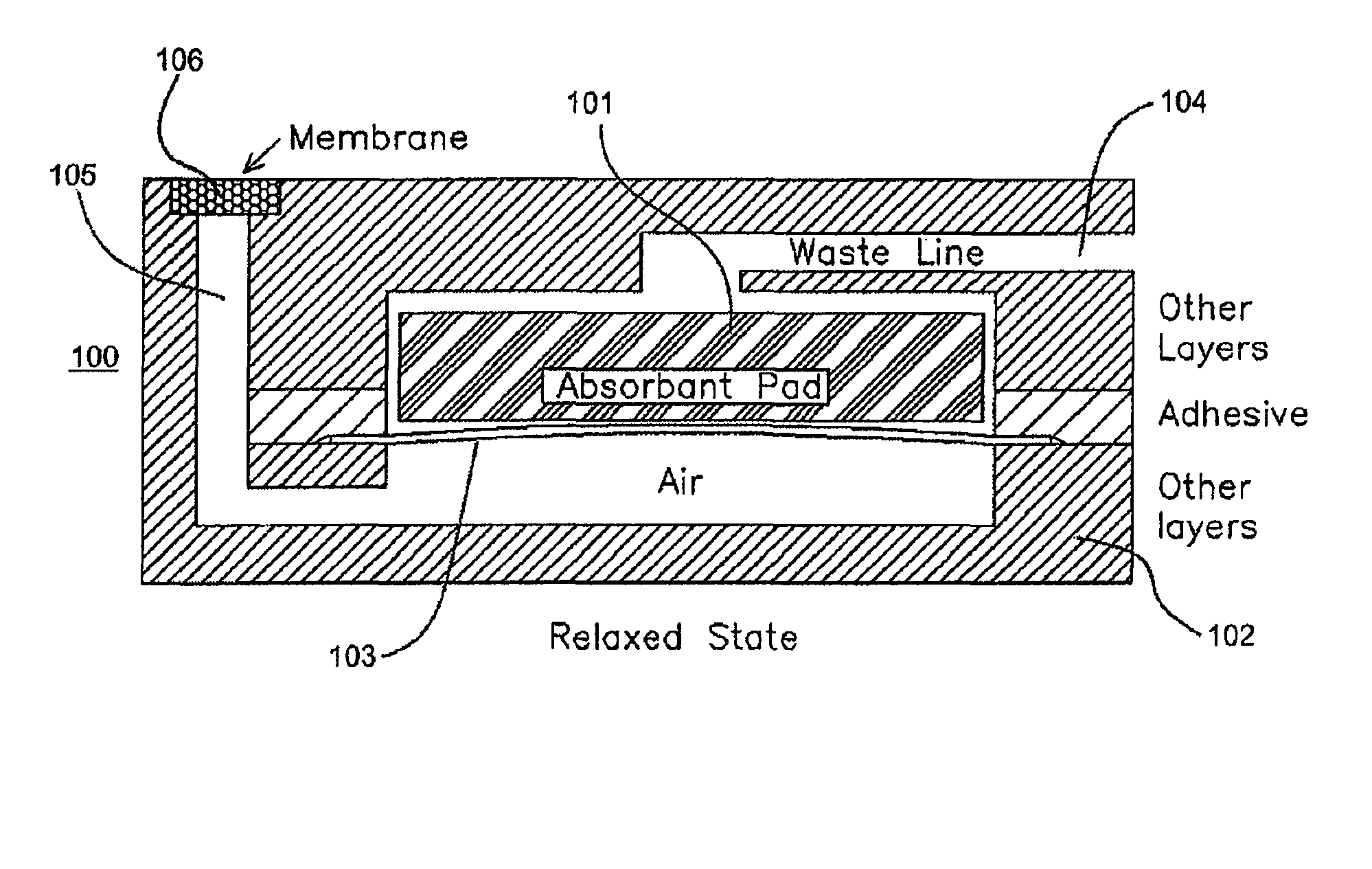
Screening arrangment for screening immunoassay tests and agglutination tests
InactiveUS7070920B2Bioreactor/fermenter combinationsBiological substance pretreatmentsPhotovoltaic detectorsPhotodetector
A screening device for performing an immunoassay test to detect the presence of a compound in a body fluid. The device includes a holder for removably receiving a membrane to which the fluid has been applied. A light is directed to the membrane. A photodetector measures the concentration of the light reflected back from the membrane. Specifically, the concentrations of reflected light from a control zone and a test zone are measured. Signals representative of the measured light concentrations are applied to a processor. If a specified concentration of predetermined light from a control zone on the membrane is detected, the processor considers the test to be successful. In the test is successful, the processor, based upon the measured concentration of reflected light from the test zone, generates data representative of the presence of the compound.
Owner:COZART BIOSCIENCE LTD
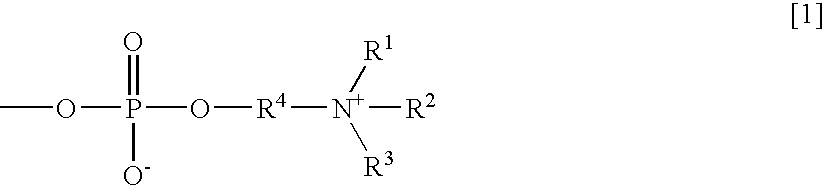
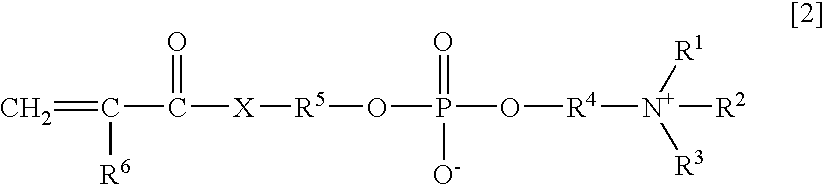
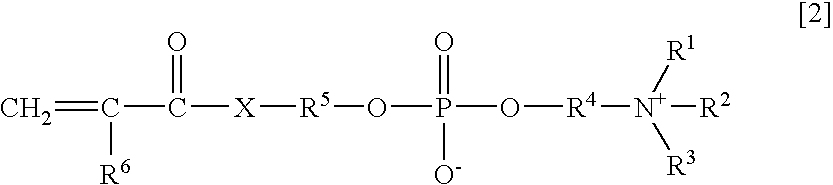
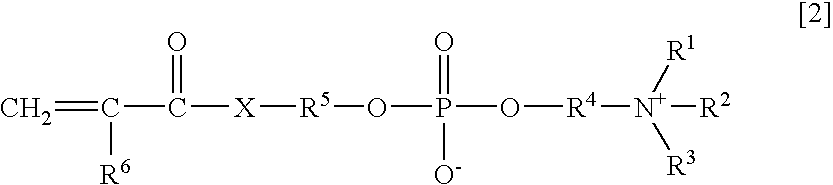
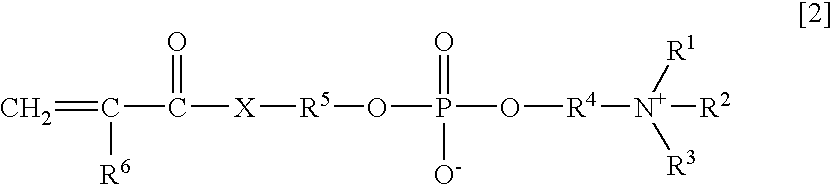
Agglutination accelerator for immunological measurement
An object of the present invention is to provide an immunoassay of PSA using an agglutination accelerator, which has an agglutination accelerating effect equal to or stronger than the known agglutination accelerator; hardly generates non-specific turbidity; and hardly generates salting out even in a solution with a high salt concentration. The present invention relates to an immunoassay of a prostate-specific antigen comprising performing an antigen-antibody reaction in the presence of a polymer having a monomer unit derived from a monomer represented by the following general formula [2]: (wherein R<1>-R<3 >are each independently a hydrogen atom or an alkyl group optionally having a hydroxyl group; R<4 >is an alkylene group; R<5 >is an alkylene group optionally having a substituent and optionally having an oxygen atom in a chain; R<6 >is a hydrogen atom or a methyl group, and X is an oxygen atom or a -NH- group), and a kit of reagent for an immunoassay comprising a reagent containing an agglutination accelerator for the immunoassay.
Owner:FUJIFILM WAKO PURE CHEM CORP
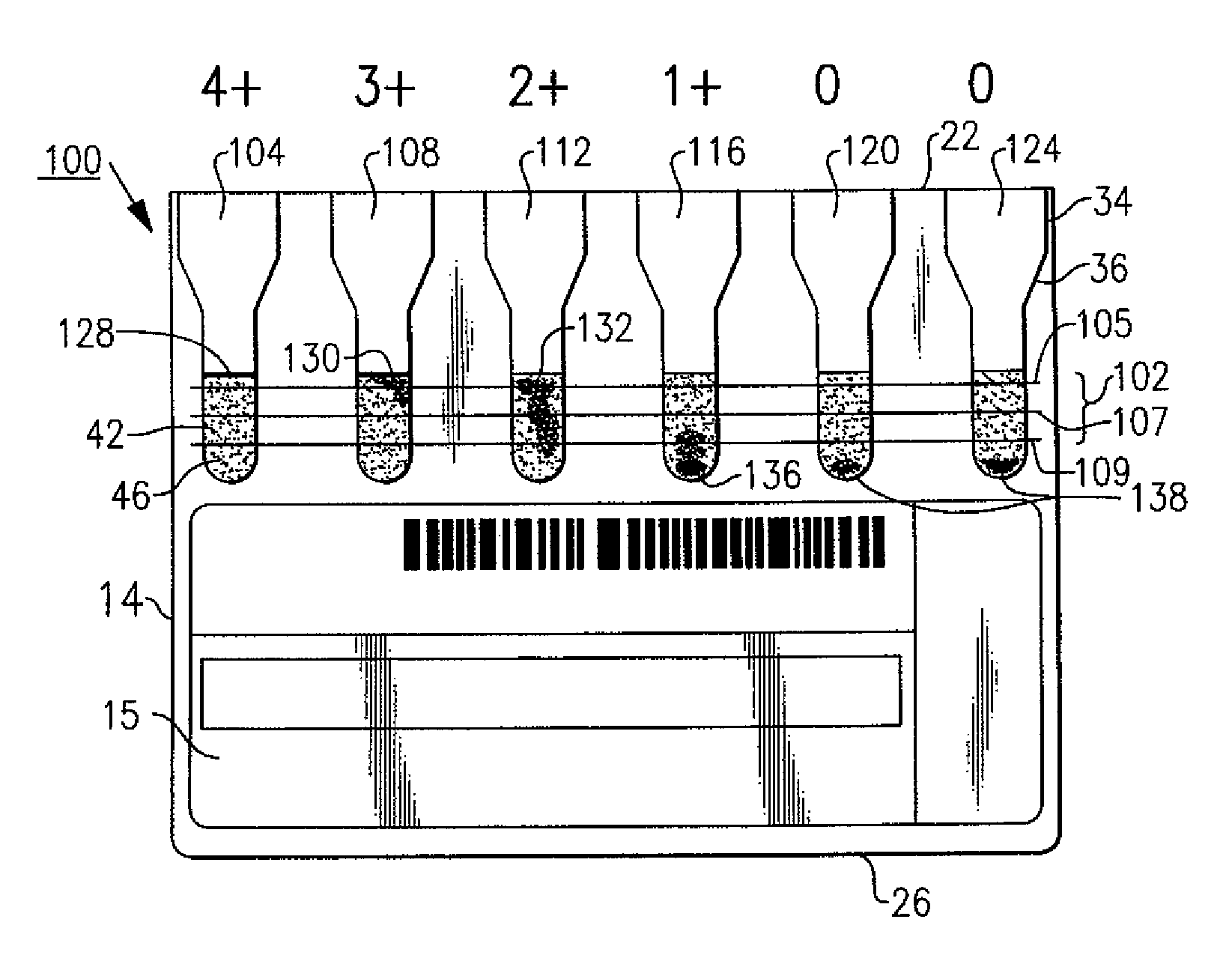
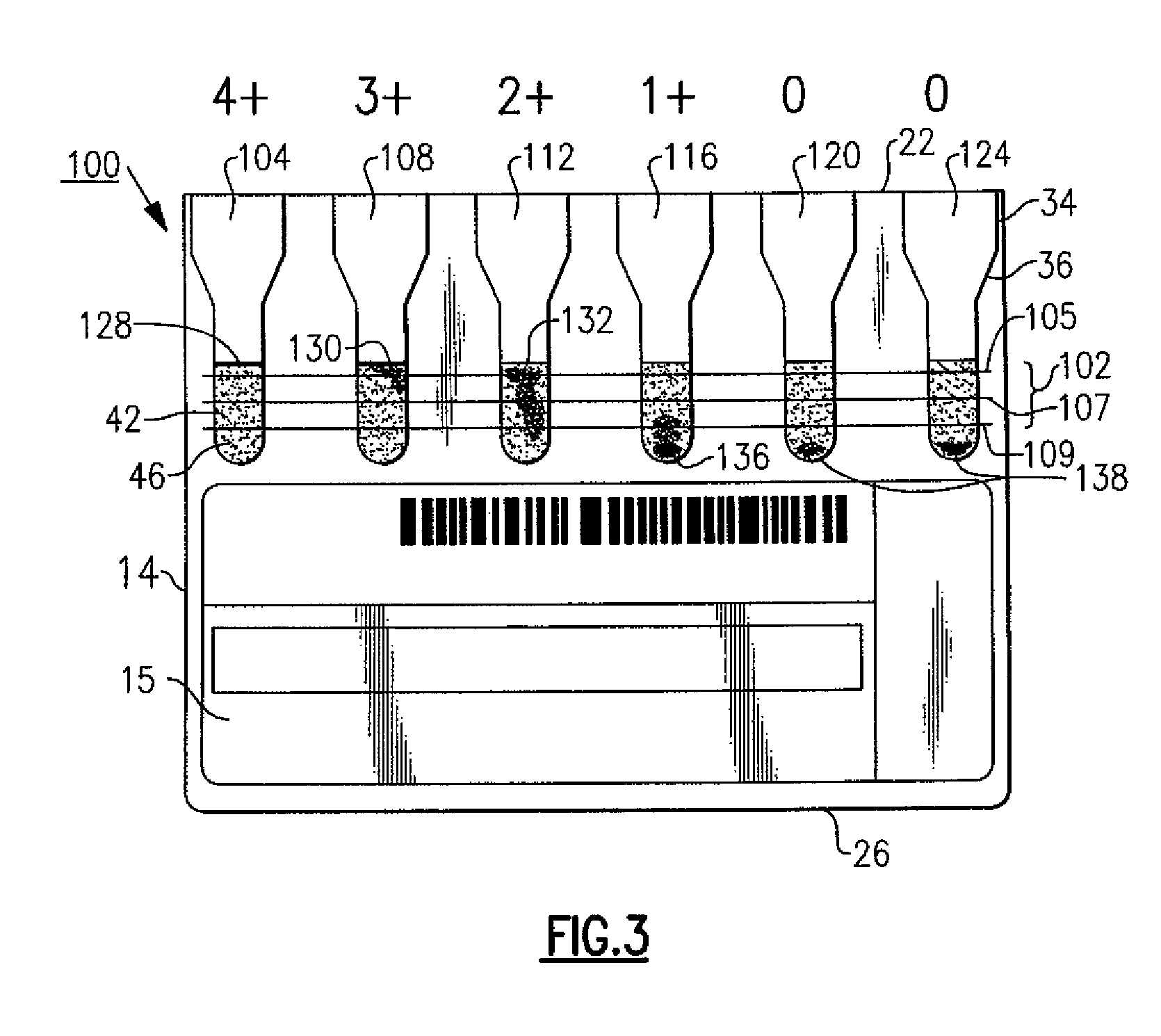
Immunodiagnostic test cards having indicating indicia
ActiveUS8058073B2Improve visualizationEasily identifiableBioreactor/fermenter combinationsBiological substance pretreatmentsTest cardEngineering
An immunodiagnostic test card includes a plurality of transparent chambers wherein each chamber includes a quantity of testing material that combines with a patient sample, when mixed, to produce an agglutination reaction. A plurality of indicia are disposed to aid in the manufacture and determining the usability of the cards prior to test and also in objectively grading the agglutination reactions that are formed or lack of agglutination.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Oil-soluble pesticide nano capsules and preparation method thereof
InactiveCN101461358AMild conditionsEase of industrial productionBiocideAnimal repellantsCypermethrinAbamectin
Disclosed is an oil soluble pesticide nm capsule and preparation method, having weight ration of: pesticide: 0.5-4%; high molecular compound: 8-25%; mixed-surfactant: 8-20%; cosurfactant 3-10%; crosslinking agent 0.5-1.0%; organic solvent 3-10%; water: added to 100%, wherein the high molecular compound is selected from sodium lignosulfonates, chitosan, gum arabic or glutin, one or two is / are selected for agglutination reaction, forming capsule skin of the nm capsule; the capsule core is pesticide bulk drug, selected from diflubenzuron, emamectin benzoate, abamectin, imidacloprid, ivermectin or highly active cypermethrin. The mixed-surfactant adopts nonionic surfactant and anionic surfactant for compound. The pesticide nm controlled release preparation prepared by the invention is easy in storage, high in stability, large in drug-loading rate and high in dispersion.
Owner:NANJING NORMAL UNIVERSITY
Method for fast testing human ABO/Rh/MN blood type and kit
ActiveCN101603967AQuick, easy and accurate identificationEasy to operateBiological testingRed blood cellGroup A - blood
The invention relates to a method for fast testing human ABO / Rh / MN blood type and a kit used in the method. The method and the testing kit follow the principle of immunochromatography and realize the blood type test by observing whether residual red substances resulted from the agglutination of erythrocytes exist or not after the antigen antibody reaction of blood type substances. The method has simple operation and can avoid various defects in present ordinary blood type testing methods. In the method, the loading amount of tested blood sample is no more than 10 microliter, and the whole test process lasts no more than 2 minutes.
Owner:INTEC PROD INC
Integrated tunable micro-antenna with small electrical dimensions and manufacturing method thereof
InactiveUS20080158069A1Size of antenna becomes largeReduce antenna sizeSimultaneous aerial operationsRadiating elements structural formsGround planeAgglutination
The present invention describes a tunable micro-antenna of reduced electrical dimensions. This antenna consists of the agglutination of several substrate layers (11), identical or distinct, with electrical, thermal and mechanical properties compatible with the manufacturing processes of integrated circuits. These layers are interleaved with metallic sheets (12) that are interconnected by metallized walls or vias, in such a way that they form a radiating structure (15) and a ground plane (14). The radiating structure includes slots in one or more levels, therefore allowing a greater reduction of the antennas' electrical length. These slots may include switches, used to render the antenna tunable. Since the entire manufacturing method is compatible with the wafer level packaging technology, the micro-antenna is easily integrable in microsystems requiring wireless communication
Owner:UNIVERSITY OF MINHO
Preparation method for SnO2/SnS2 heterostructure photocatalyst
InactiveCN102671676AComposite uniformContact stabilityPhysical/chemical process catalystsIon exchangeAgglutination
The invention discloses a preparation method for SnO2 / SnS2 heterostructure photocatalyst. The preparation method includes following steps: (1) preparing SnO2 nanometer particles by an alcohothermal method; and (2) and performing in-situ synthesis for SnO2 / SnS2 into heterostructure in an ion exchange method. The preparation method has the advantages that a preparation process is simple and convenient, controllability of reaction conditions is high, more importantly, SnO2 and SnS2 can be uniformly compounded, and accordingly more stable contact is provided to conduct charge and prevent auto-agglutination. When illuminated by visual light (>420nm), the SnO2 / SnS2 heterostructure photocatalyst has good photocatalysis activity, the photocatalysis activity is the best after the SnO2 / SnS2 heterostructure photocatalyst is heated by a kettle for 30 hours, the degradation degree of the SnO2 / SnS2 heterostructure photocatalyst can basically reach 100% after the SnO2 / SnS2 heterostructure photocatalyst is heated by the kettle for 4 hours, and the preparation method has a potential application value in the aspect of comprehensive ecological improvement, particularly dye wastewater pollution treatment.
Owner:SHANGHAI NORMAL UNIVERSITY
Latex immunoturbidimetry pepsinogen I detection kit for eliminating chyle interference
ActiveCN103698525AStrong interference abilityHigh sensitivityBiological material analysisBiological testingPepsinogen ITurbidity
The invention relates to the technical field of medical examination, and particularly relates to a latex immunoturbidimetry serum pepsinogen I detection kit for eliminating chyle interference. The kit provided by the invention comprises (1) a pepsinogen I calibrator; (2) a reagent 1 with a preset chyle remover; and (3) a reagent 2 containing the monoclonal antibody and polyclonal antibody coated latex particles against human pepsinogen I. According to the kit provided by the invention, the combination reaction between a substrate to be detected in a sample and a specific antibody in the reagent is amplified through the latex agglutination effect; with the given wavelength, the turbidity formed by the reaction is related to the content of the substrate to be detected, thus the content of the substrate to be detected is calculated. The kit provided by the invention is used for detecting the content of pepsinogen I in human serum, has high sensitivity and good specificity, and can eliminate chyle interference in the sample; moreover, the kit is simple and quick to operate and has high practicability and wide application range.
Owner:北京万泰德瑞诊断技术有限公司
Method and device for ultrasound assisted particle agglutination assay
InactiveUS20090053688A1Accurate assessmentSensitive detectionBioreactor/fermenter combinationsBiological substance pretreatmentsActive matterAgglutination assay
Ultrasound-assisted particle agglutination assay methods and apparatuses are described based on first providing a standing wave ultrasound field at a resonance frequency of a test liquid in a resonator cell containing microparticles covered with a binding agent with high affinity to an analyte sought to be detected by the assay test. Formation of the specifically-bound and nonspecifically-bound aggregates of these microparticles is then followed by effective stirring of the liquid with swept-frequency sonication causing disintegration of nonspecifically-bound aggregates and leaving specifically-bound aggregates in place for further detection and measurement. The methods and devices of the invention allow significant improvement in the sensitivity and specificity of agglutination tests and are advantageously applicable to detecting various proteins, DNA, RNA and other biologically active substances. Specific examples are provided.
Owner:ALLIED INNOVATIVE SYST
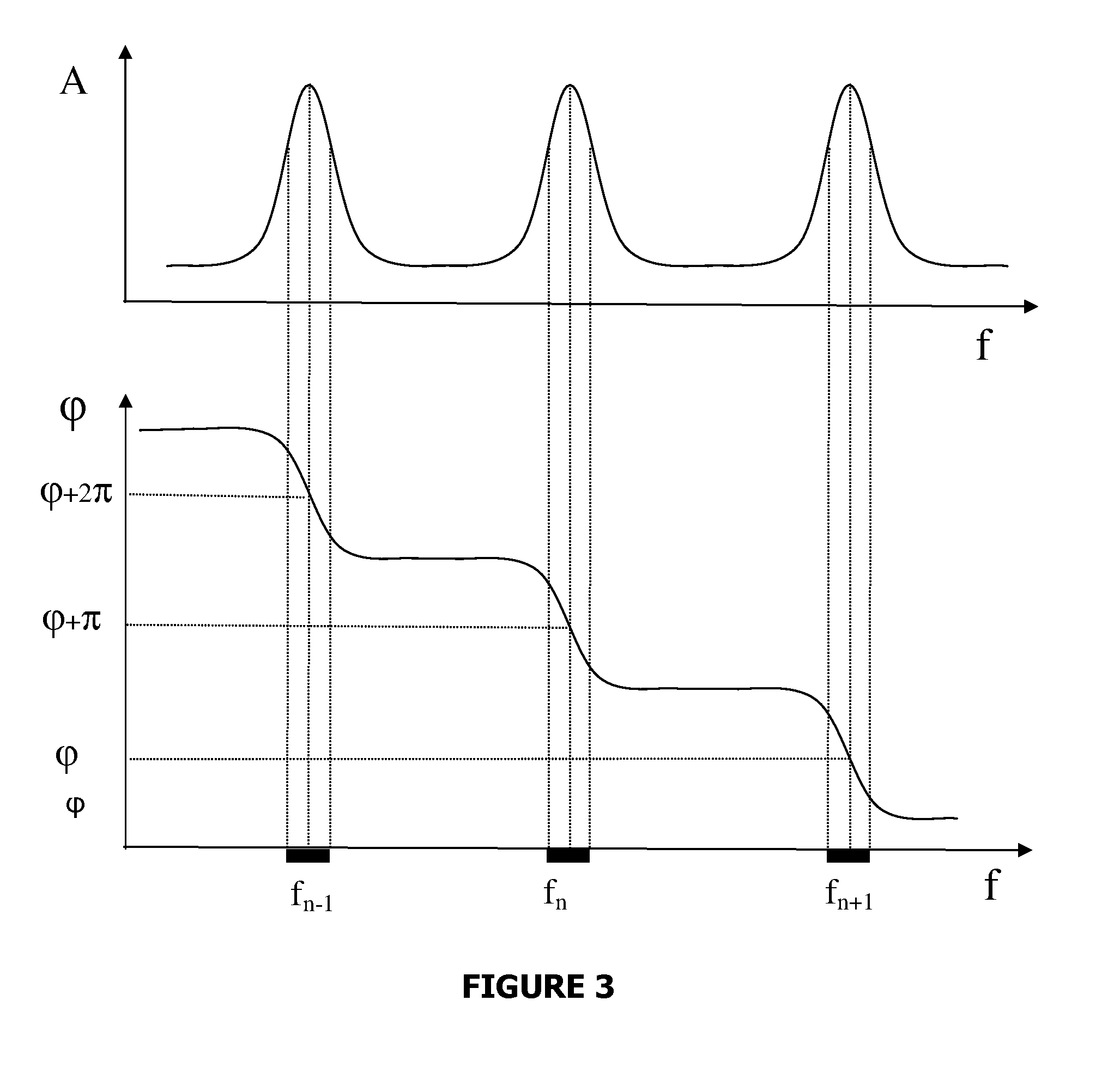
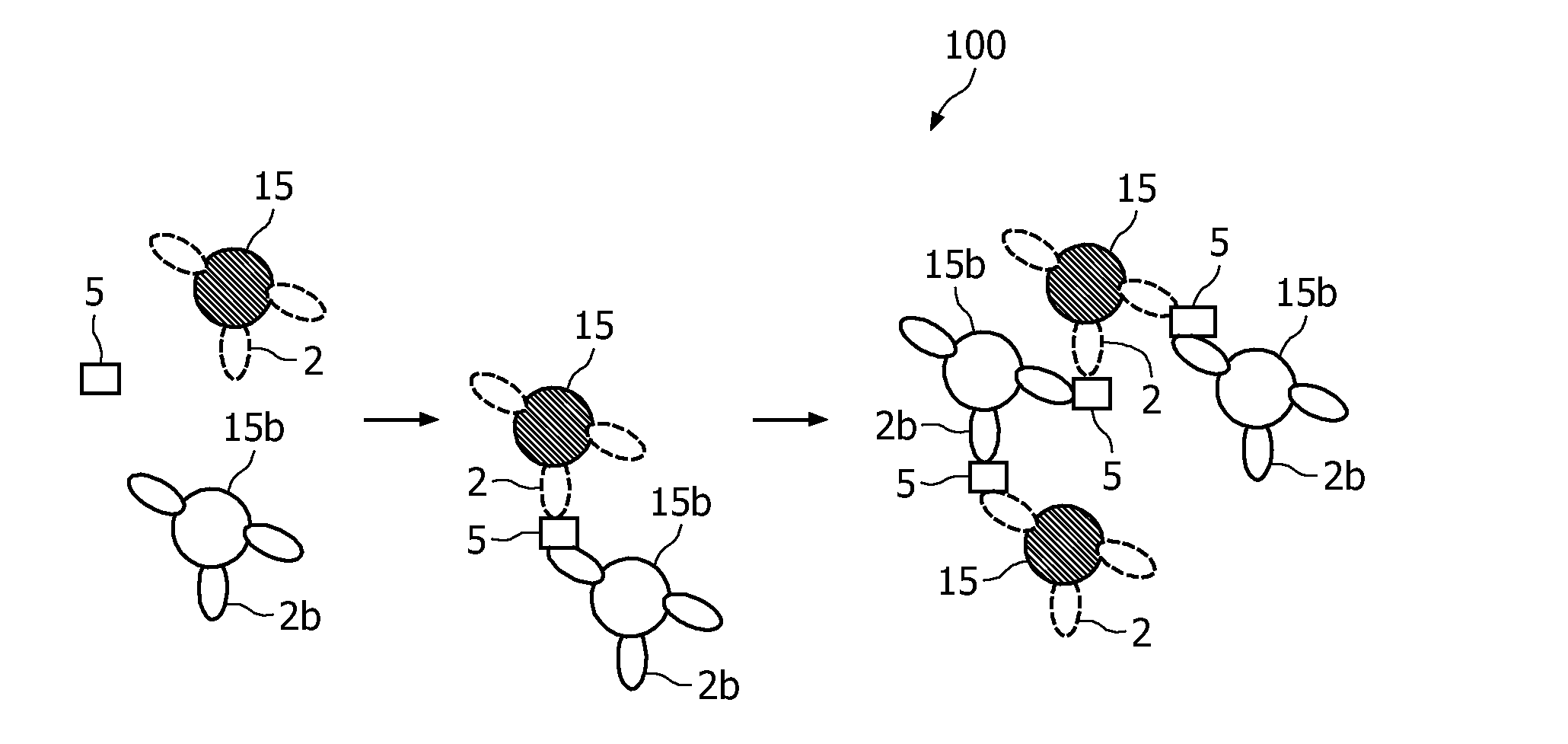
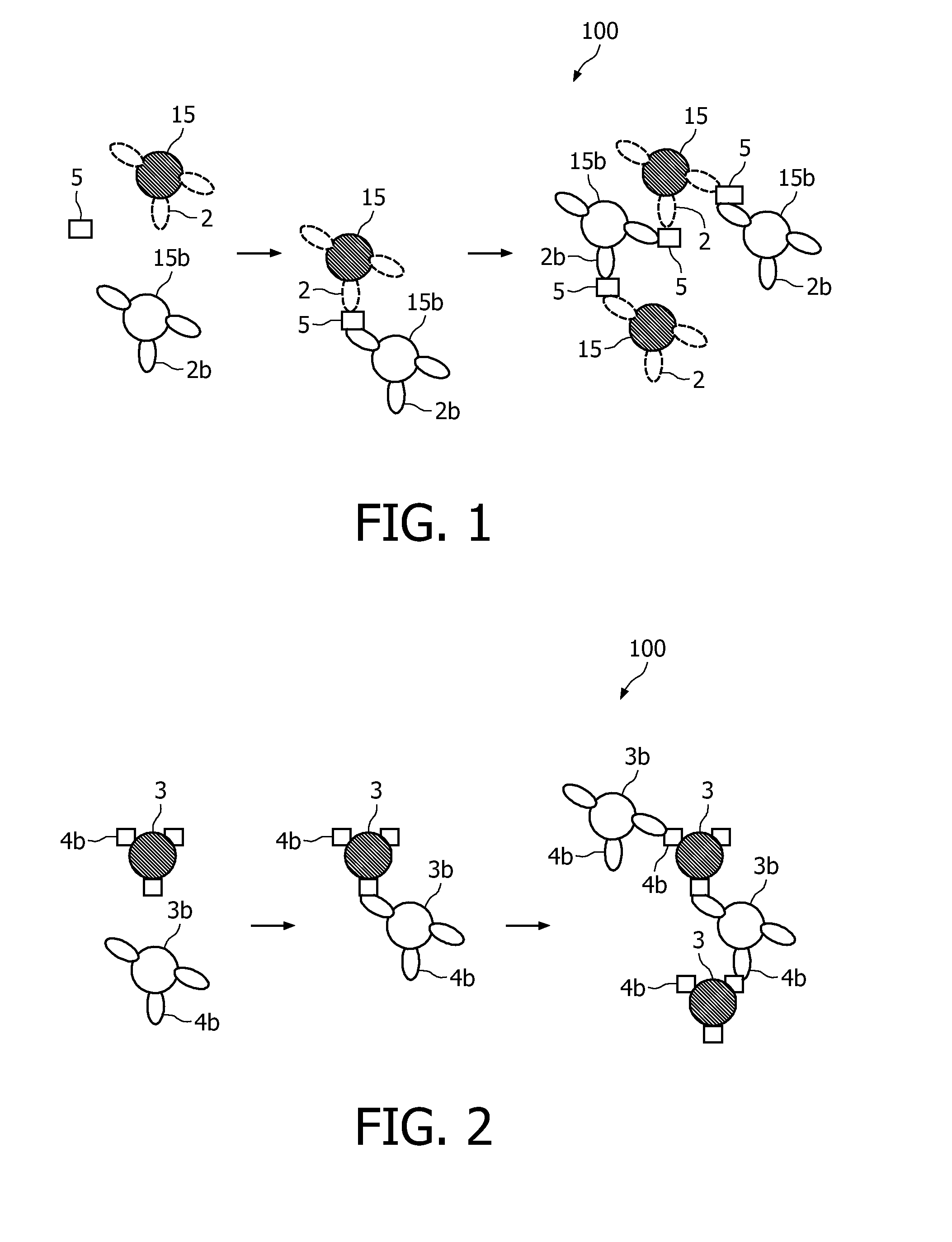
Measuring agglutination parameters
ActiveUS20100033158A1Mitigate, alleviate or eliminate one or moreImprove throughputBioreactor/fermenter combinationsNanomagnetismAgglutination assayAgglutination
A method and system are described for measuring agglutination in a target-induced agglutination assay with one or more magnetic particles performed in a reaction chamber. After the magnetic particles (3, 15), which are capable of binding to a target (5) are provided in the assay, an agglutination process resulting in agglutinated particles (100) comprising at least one magnetic particle is performed. The method then further comprises applying an alternating current magnetic field (HAC) to the assay and—measuring an effect of the HAC on the one or more magnetic particles (3,15) unattached to any surface. The measured effect is indicative of one or more agglutination parameters.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
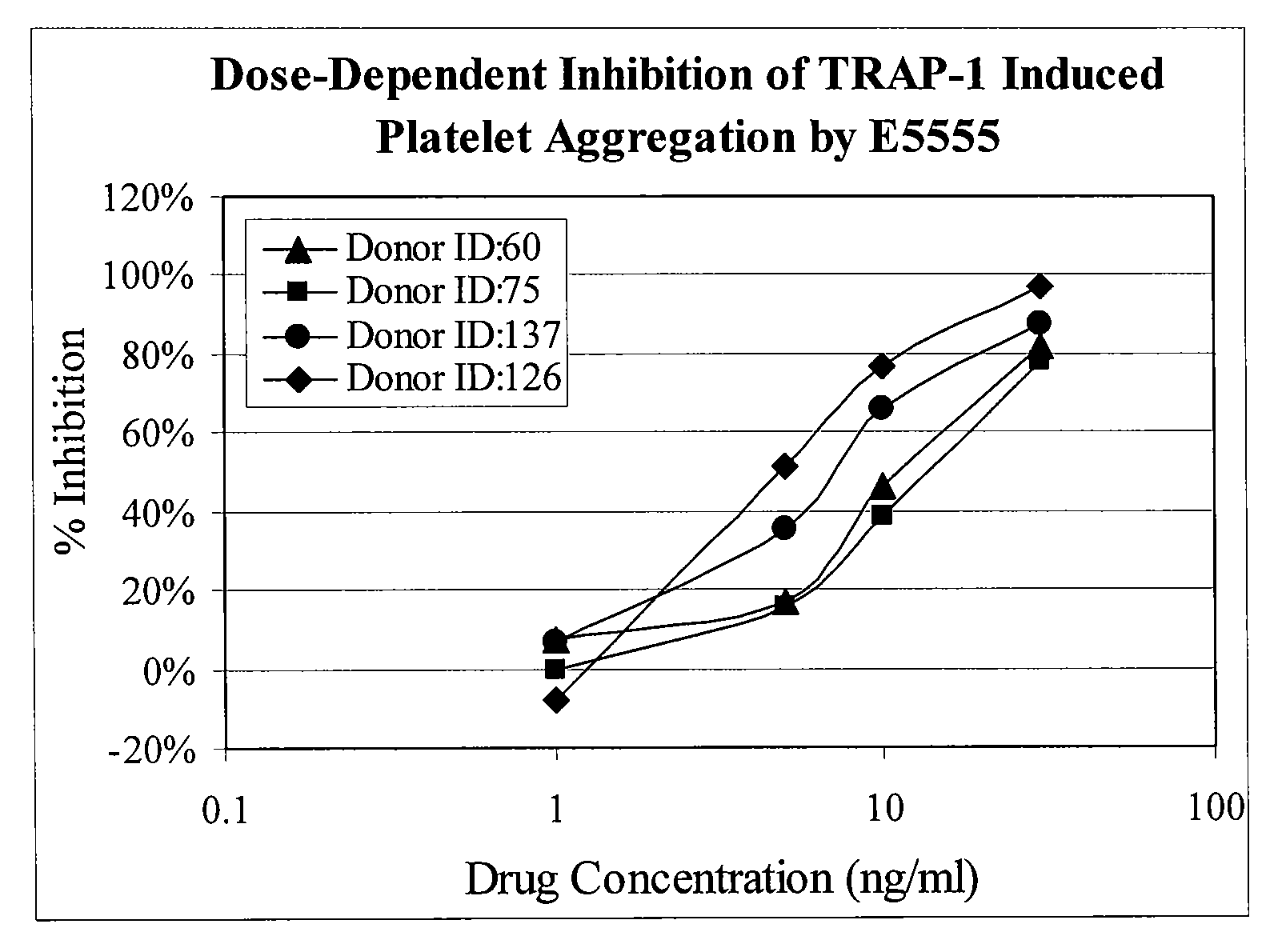
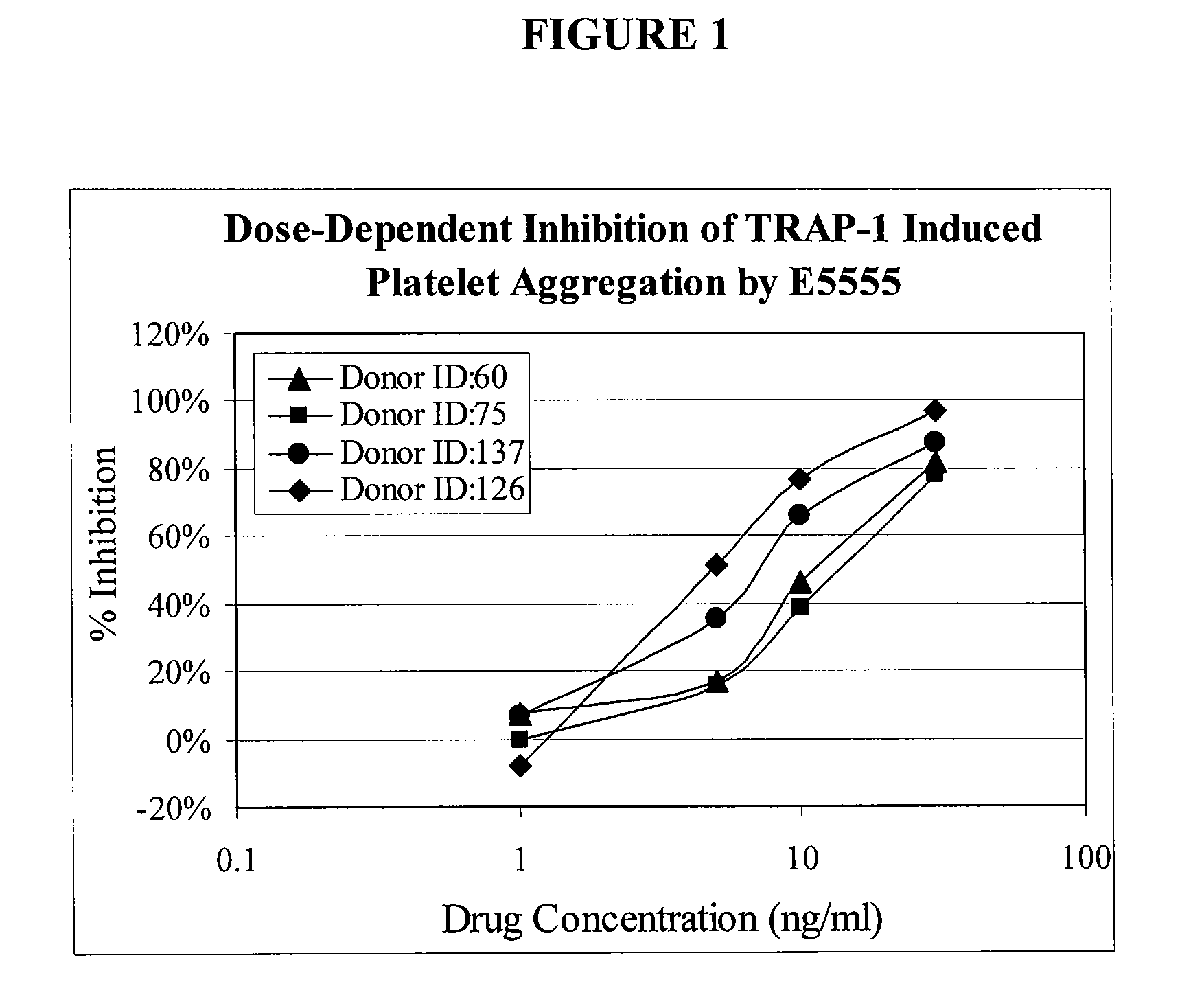
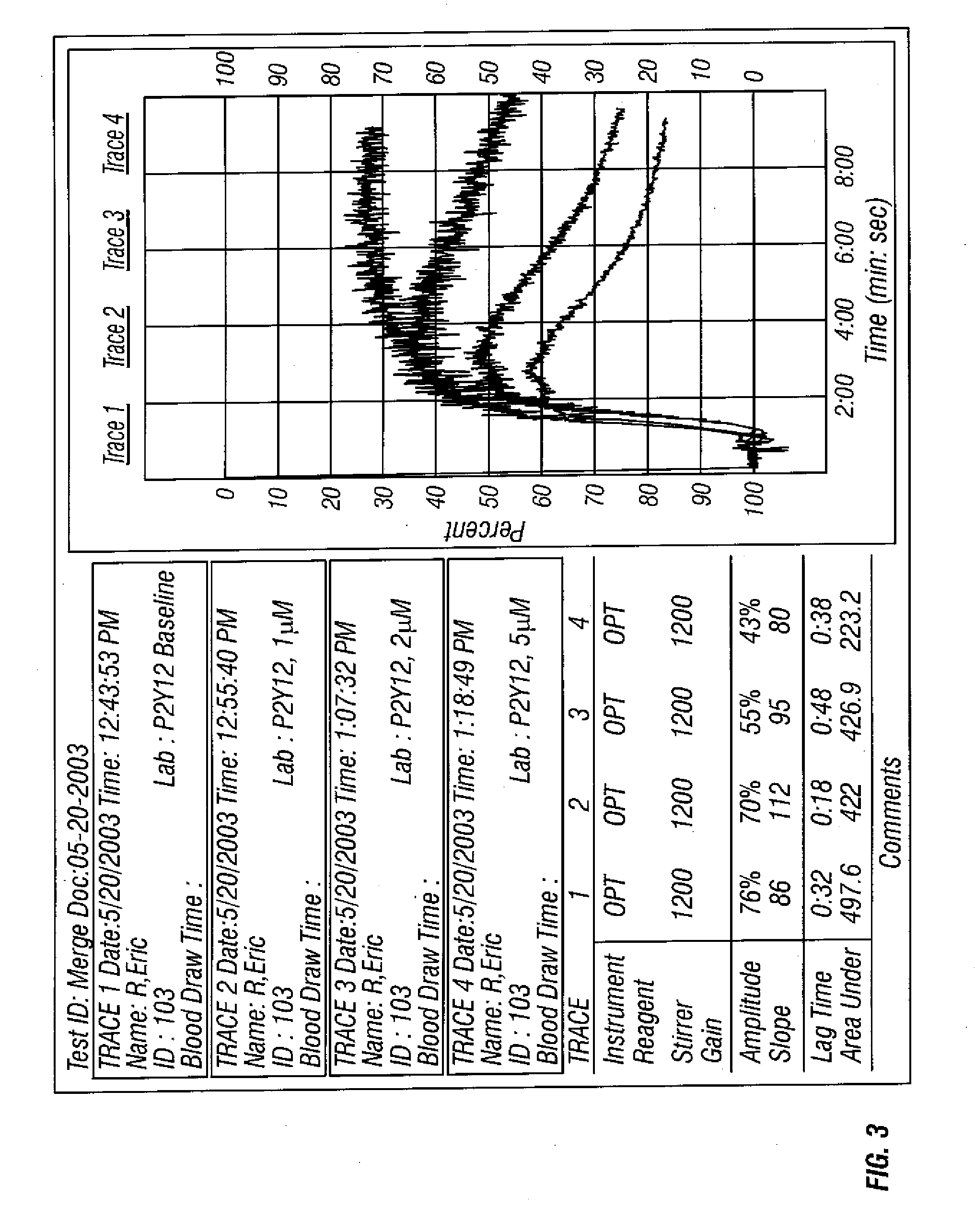
Methods of measuring inhibition of platelet aggregation by thrombin receptor antagonists
InactiveUS20080299587A1Microbiological testing/measurementBiological testingThrombin receptor antagonistNK1 receptor antagonist
A method is provided for measuring inhibition of platelet aggregation by a thrombin receptor antagonist. First, a blood sample is obtained from a patient treated with a thrombin receptor antagonist. The blood sample is mixed in combination with particles including an immobilized GPIIb / IIIa receptor ligand and a thrombin receptor activator. The combination is then incubated under conditions suitable for agglutinating the particles, and platelet-mediated agglutination is assessed in the mixture. The absence of agglutination indicates that the patient has reduced ability to form platelet thrombi in response to the thrombin receptor antagonist treatment. Also provided is a kit for measuring inhibition of platelet aggregation by a thrombin receptor antagonist that includes a GPIIb / IIIa receptor ligand immobilized on a particle, a thrombin receptor activator, an anticoagulant, and a buffer to maintain the anticoagulated blood in a condition suitable for platelet aggregation.
Owner:ACCUMETRICS INC
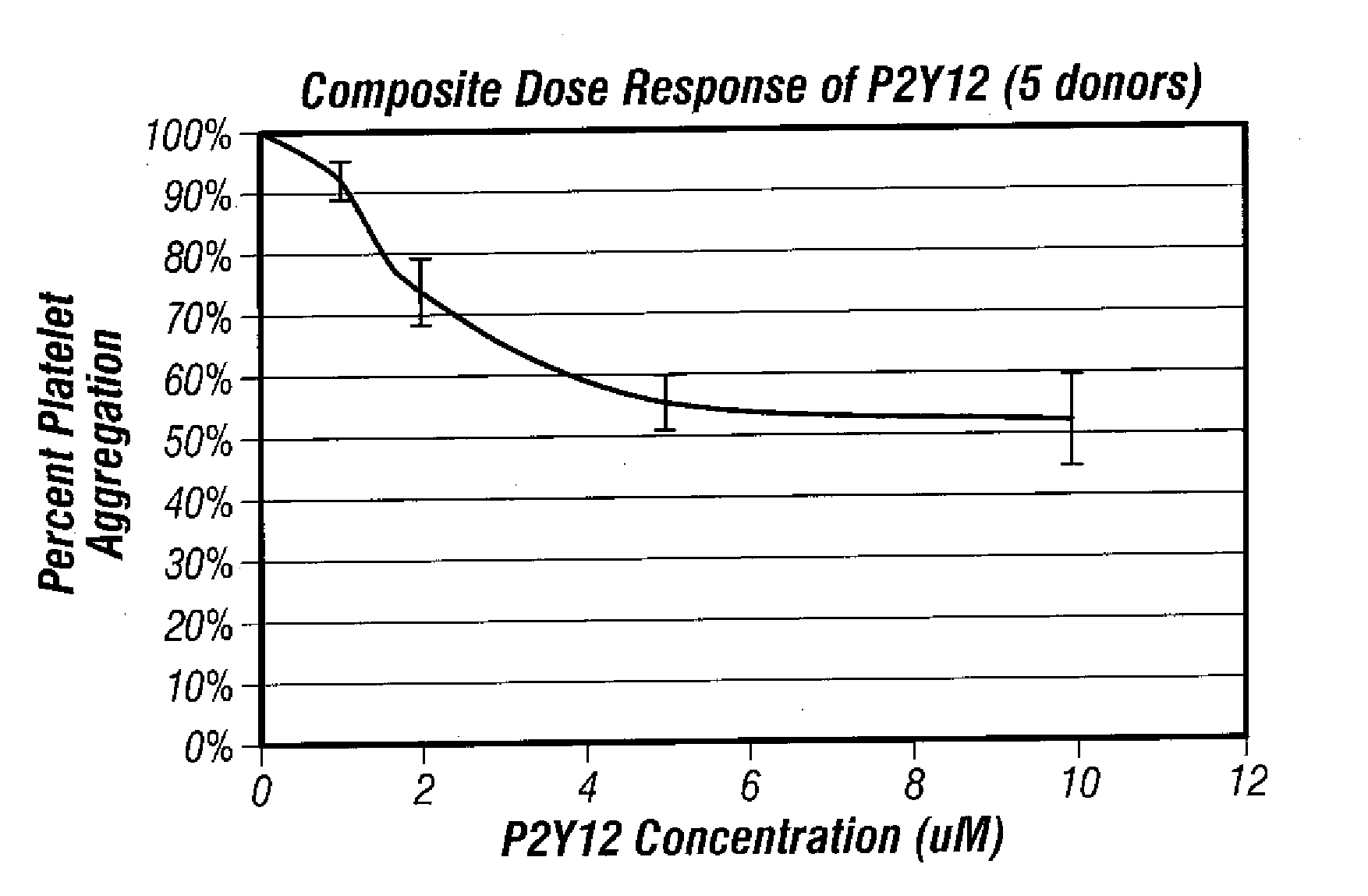
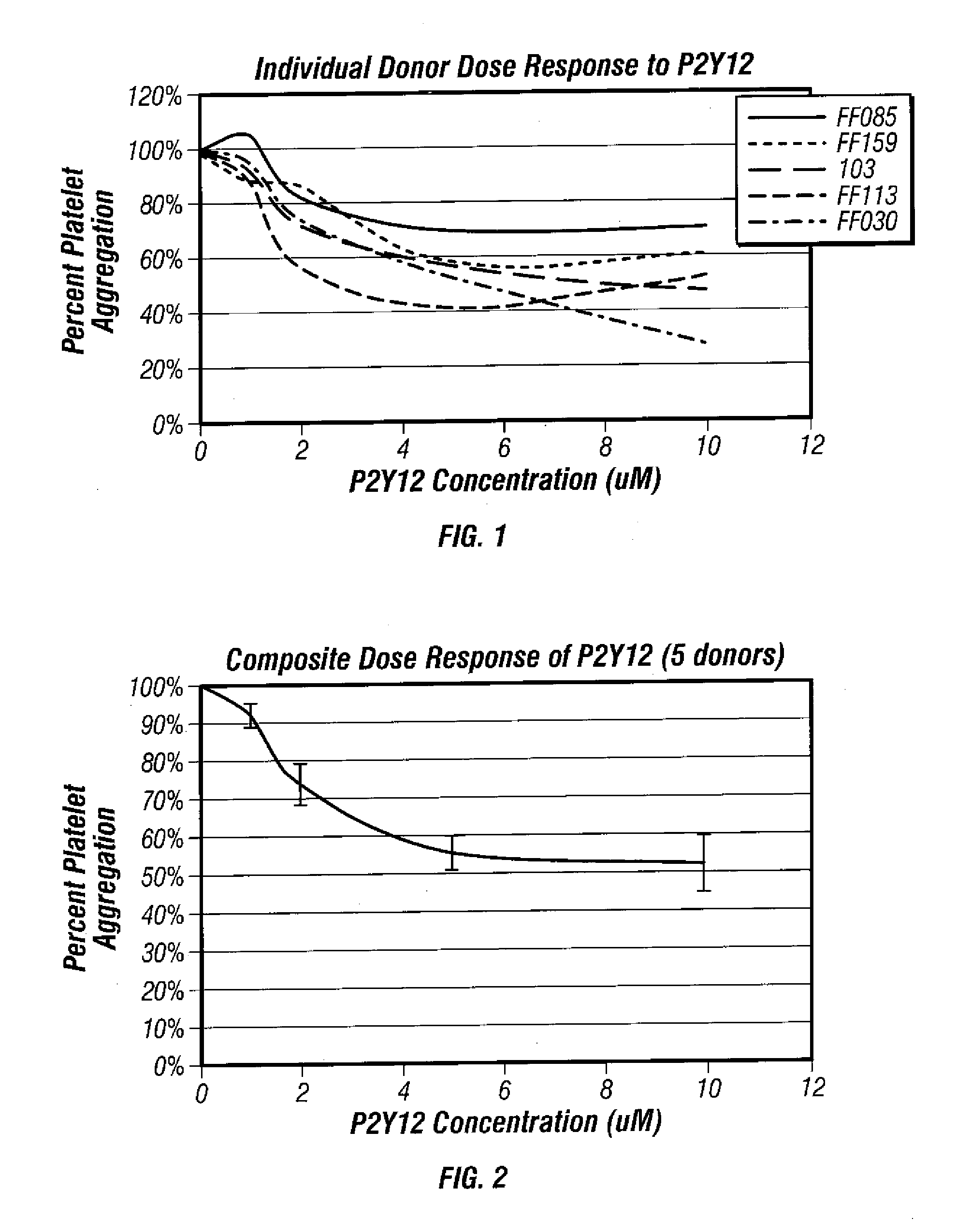
Methods for measuring platelet reactivity of patients that have received drug eluting stents
InactiveUS20070243632A1Reduce capacityEnhance signal transductionElcosanoid active ingredientsMicrobiological testing/measurementThrombusReceptor activation
A method is providing for measuring platelet reactivity of a PCI patient that has a (DES). A blood sample is obtained from the patient. The blood sample is mixed in combination with 1) an anticoagulant; 2) sufficient buffer to maintain the pH and salt concentration of the anticoagulated blood within a range suitable for platelet aggregation; 3) a platelet GPIIb / IIIa receptor ligand immobilized on a solid surface; 4) one or more agents to enhance a signal transduction pathway and 5) a receptor activator. The combination is incubated under conditions for agglutinating particles. Platelet-mediated agglutination is assessed in the mixture. The absence of agglutination indicates that the patient has reduced ability to form platelet thrombi.
Owner:ACCUMETRICS INC
CD47 nanobody and application thereof
ActiveCN110144009AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMedicineAgglutination
The invention relates to the field of biomedicine, discloses a CD47 nanobody and an application thereof, and particularly discloses a blocking type nanobody for integrin-associated protein (CD47) andderived protein thereof. Particularly, the invention discloses an integrin-associated protein (CD47) binding molecule and an application thereof, particularly in treatment and / or prevention or diagnosis of CD47 associated diseases such as tumor. The related CD47 nanobody can effectively block interaction between the CD47 and a ligand SIRPa thereof, and the antibody cannot cause human erythrocyte agglutination.
Owner:SHANGHAI NOVAMAB BIOPHARM CO LTD
SiO2-PS nuclear shell structure ceramic coating diaphragm and preparation method and application thereof
ActiveCN108735953AImprove bindingGood dispersionCell seperators/membranes/diaphragms/spacersSecondary cellsPolyolefinSilanes
The invention relates to the technical field of the lithium ion battery diaphragm production, and specifically relates to a SiO2-PS nuclear shell structure ceramic diaphragm coating. A preparation method of the SiO2-PS nuclear shell structure ceramic diaphragm coating comprises the following steps: after modifying the monodispersed nanometer SiO2-particle in-situ grafted gamma-methacryloyloxy trimethoxy silane (MPS) prepared through the St-ber method, coating the monodispersed nanometer modified SiO2 particles by adopting the polyvinyl pyrrolidone ethanol solution and styrene-azodiisobutyronitrile mixed solution, thereby forming the nuclear shell structure with stable combination effect, and preparing the SiO2-PS nuclear shell structure ceramic diaphragm coating slurry; the nuclear shell SiO2-PS composite particle with good dispersibility can be uniformly dispersed in the coating slurry system and cannot produce excessive agglutination, and can form the coating not hindering the polyolefin porous diaphragm gap after being coated on the polyolefin porous diaphragm, and has good combining effect with the polyolefin porous diaphragm, and the membrane has good mechanical performance onthe whole; the diaphragm has stable membrane form under high-temperature state, the SiO2 of the nuclear layer is free from ceramic powder falling and blocking diaphragm duct under the coating of theglass-state polymer when the heat shrinkage phenomenon of the diaphragm caused by the high temperature is effectively blocked, and the security of the lithium ion battery is improved.
Owner:安徽美芯新材料有限公司
Immunological analytical method and device for the determination of advanced glycosylation end products (AGEs)
The invention provides an immunological analytical method and devices for determination of the advanced glycosylation end products (AGEs), comprising a reagents for determining the AGEs, which comprises a displaying carrier suspension and the antigen or antibody immobilized on the surface of the said displaying carrier; and a test strip, comprising of: a base plate, and constitutive parts provided on the said base plate. After infusing the said reagent into the said test strip, the immunological reaction of the AGEs antigen or antibody can be determined based on the agglutination phenomenon or accompanied changes of absorbance or color and the presence or raised level of the AGEs in the diabetic patient can be known accordingly such that the practitioner can prevent the occurrence of the complications, or block further the progression of the complications at the earlier stage.
Owner:LIU YUNG HSIANG +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com