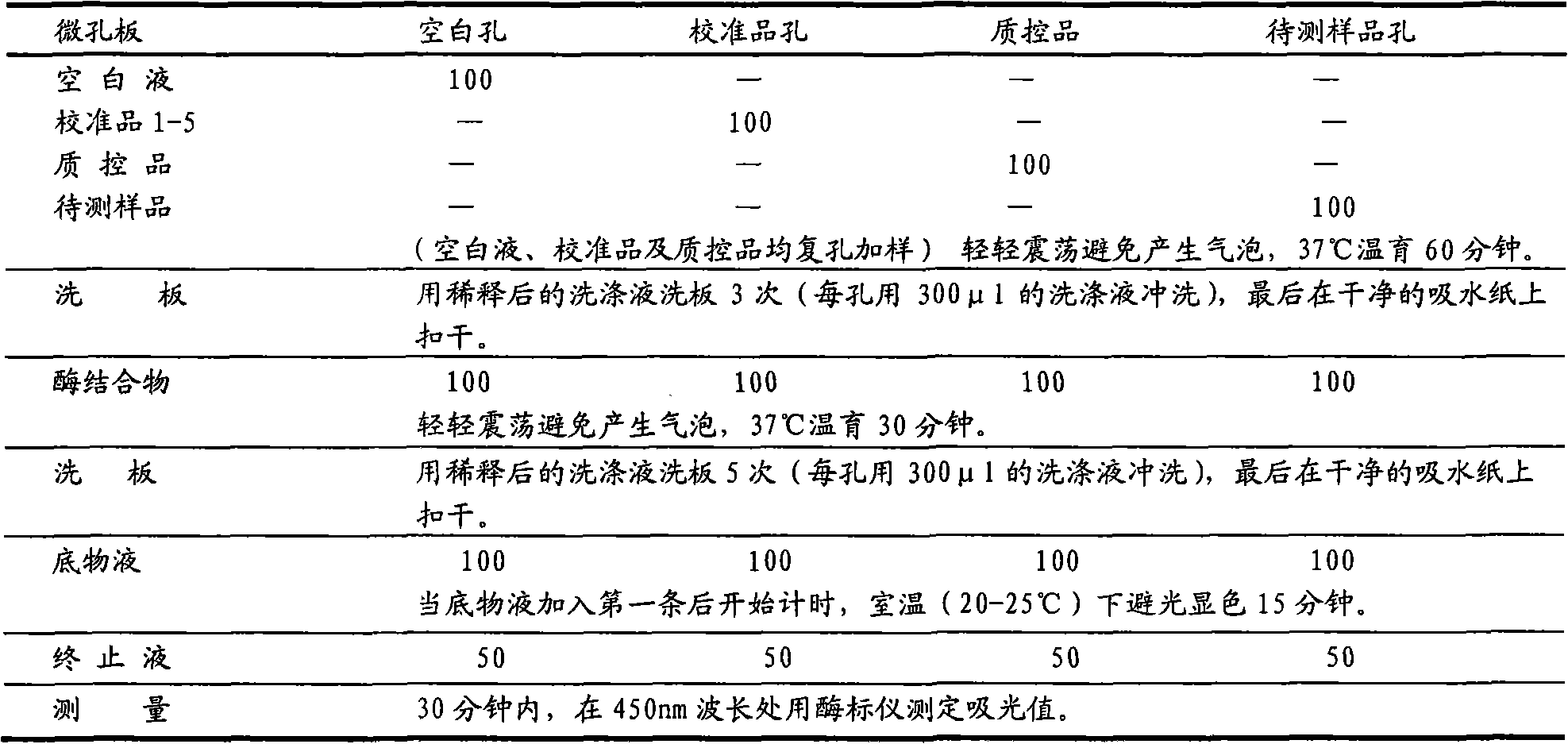
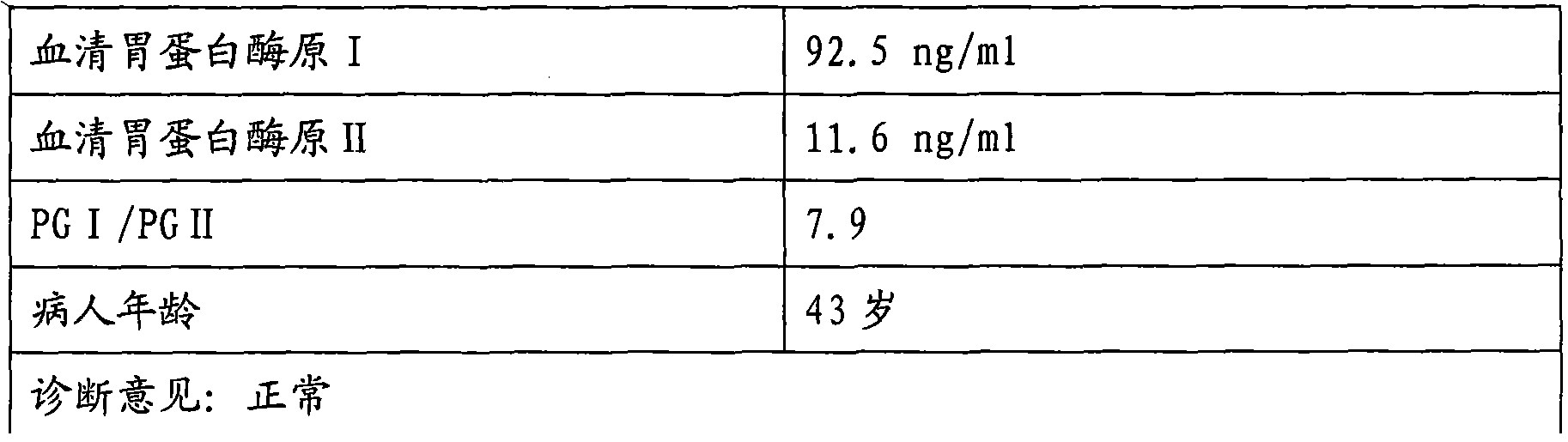
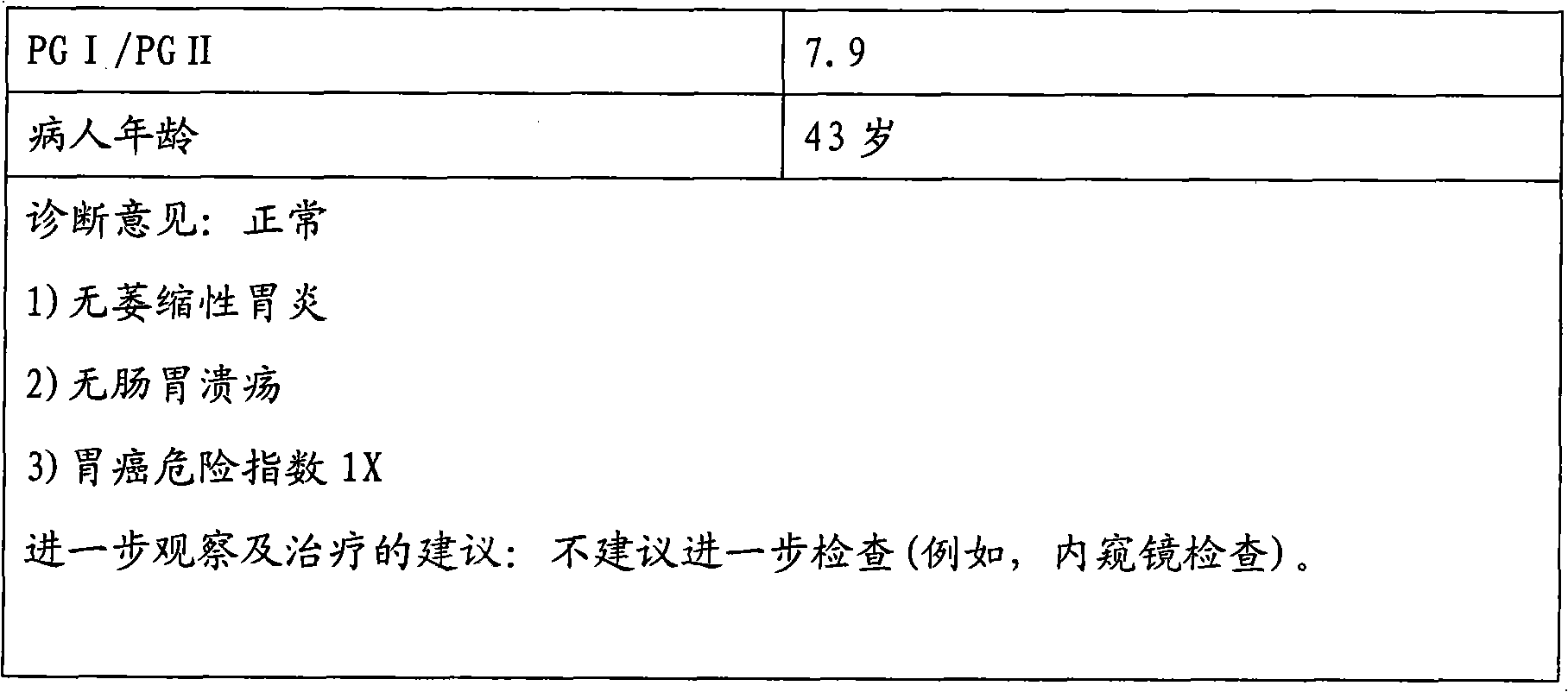
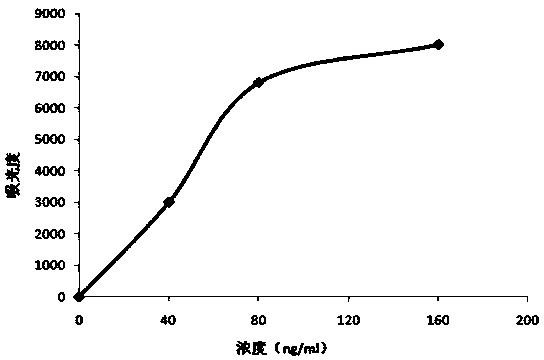
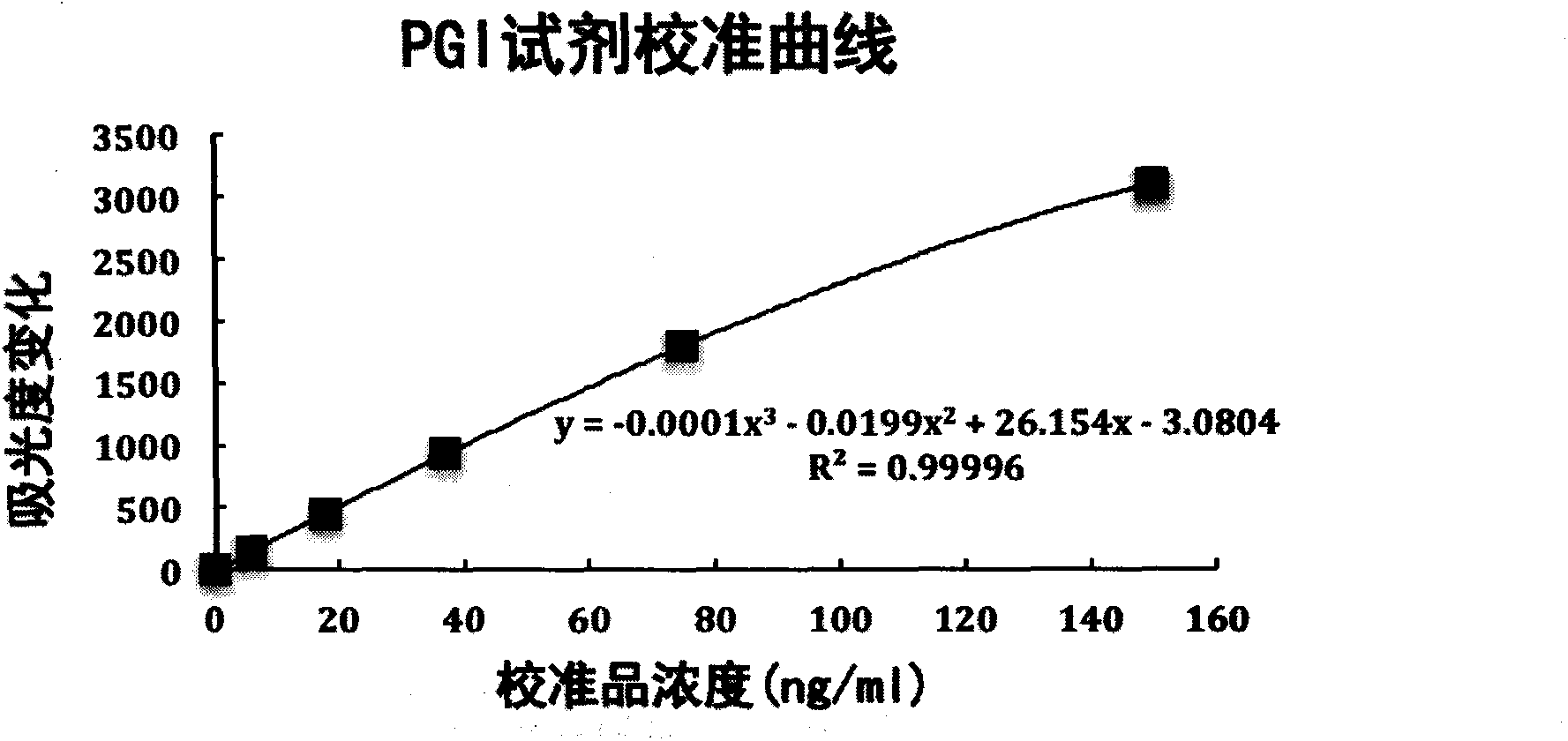
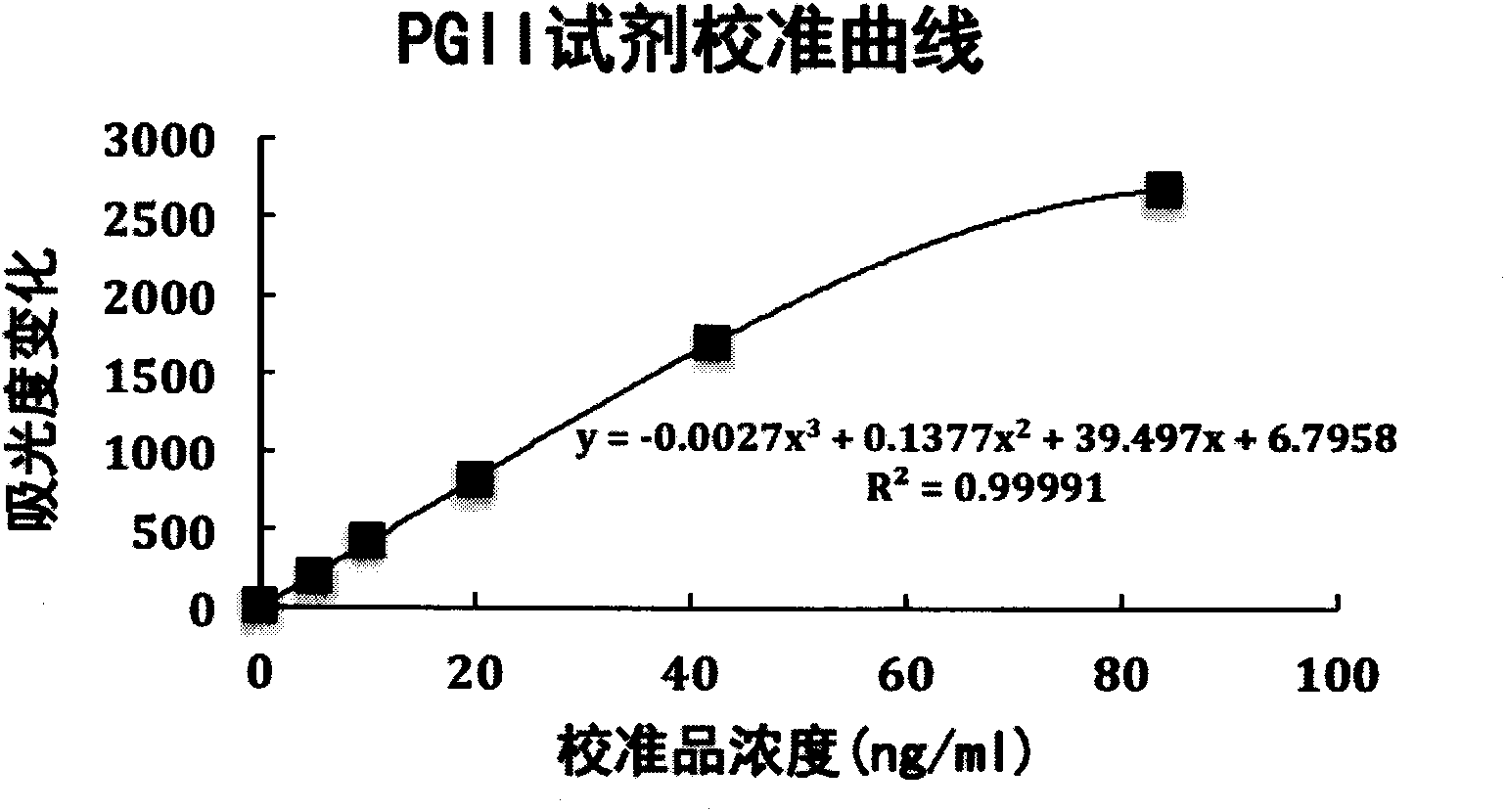
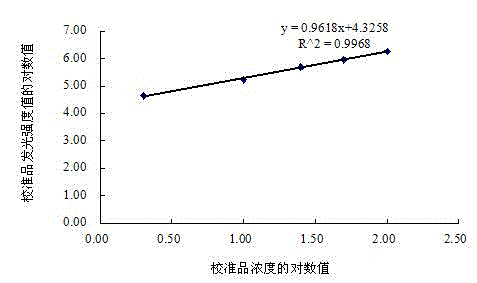
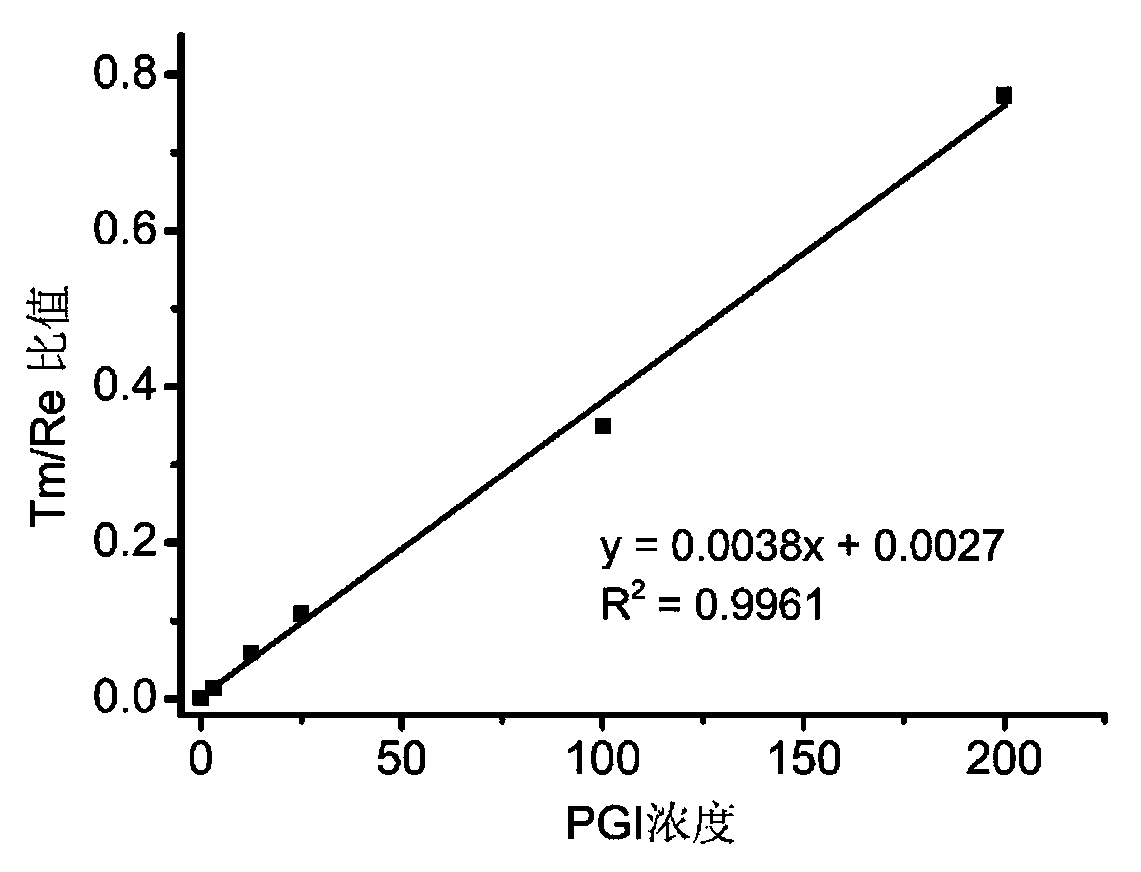
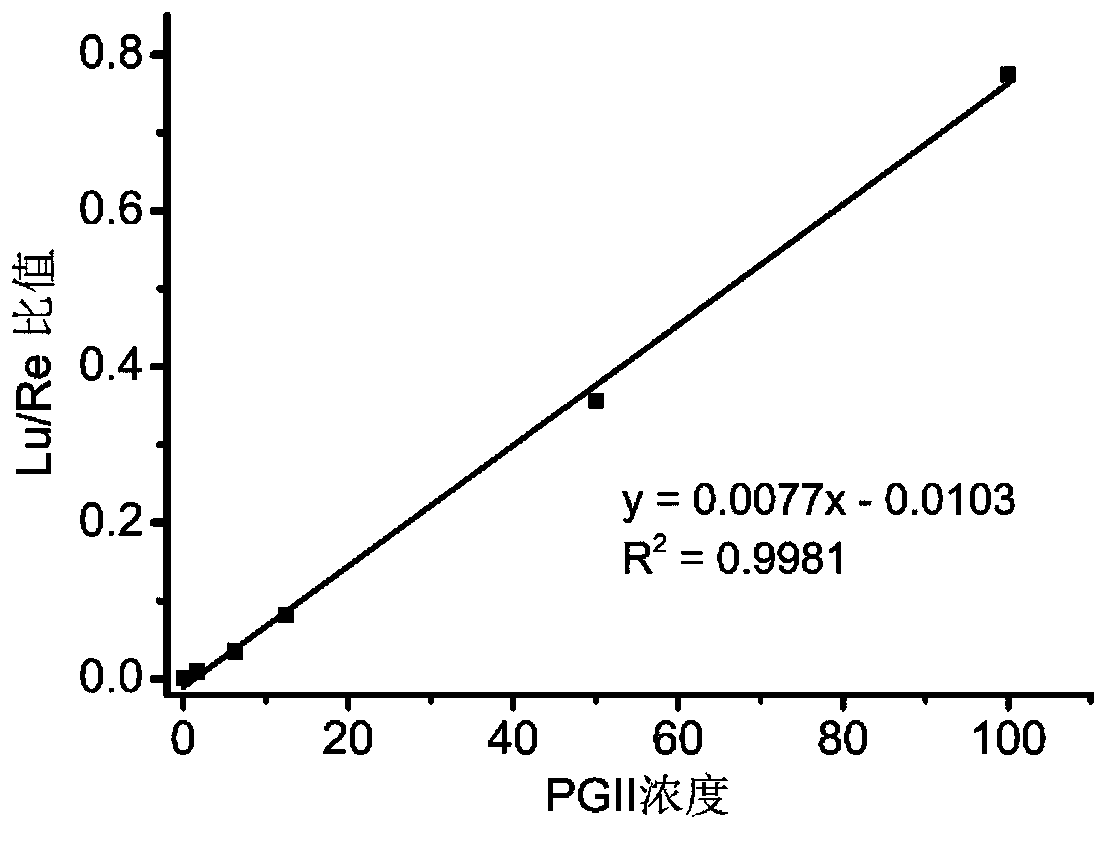
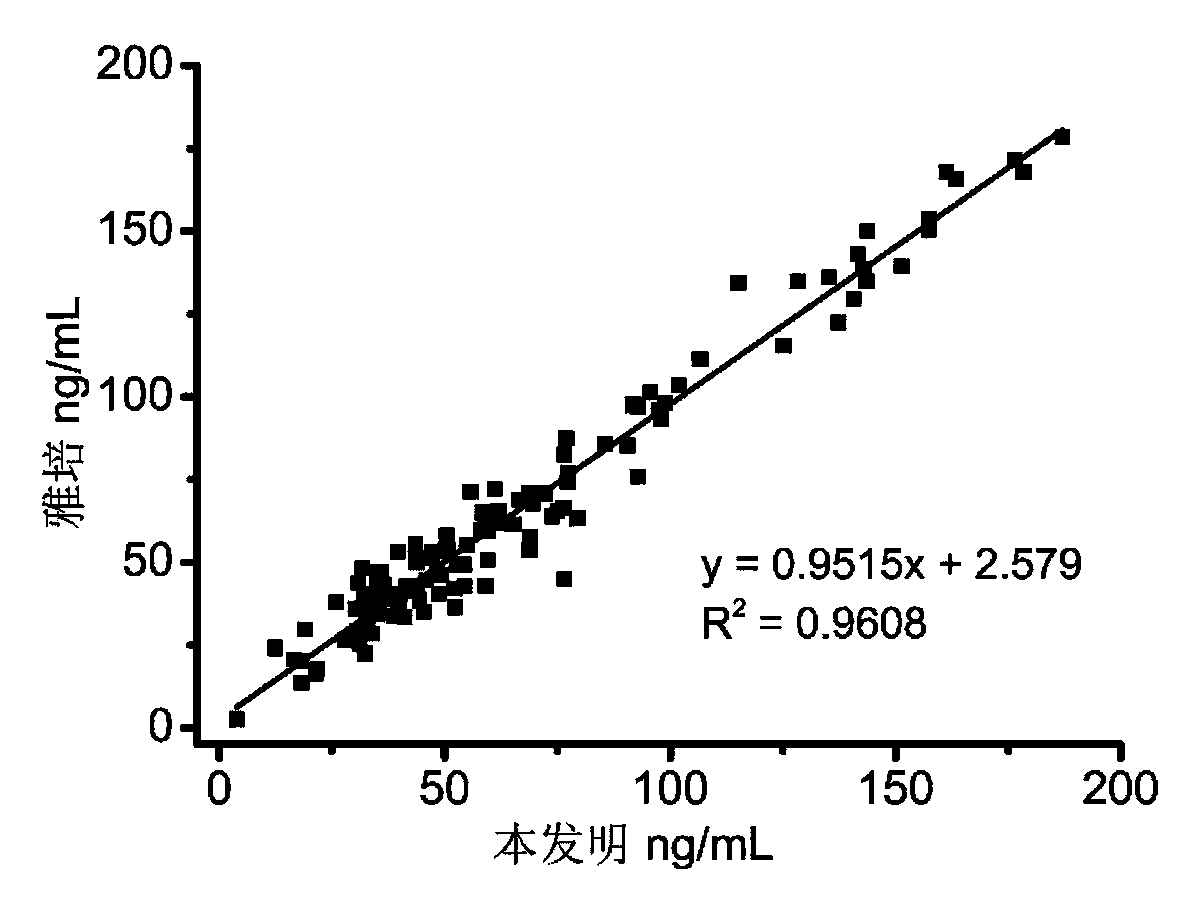
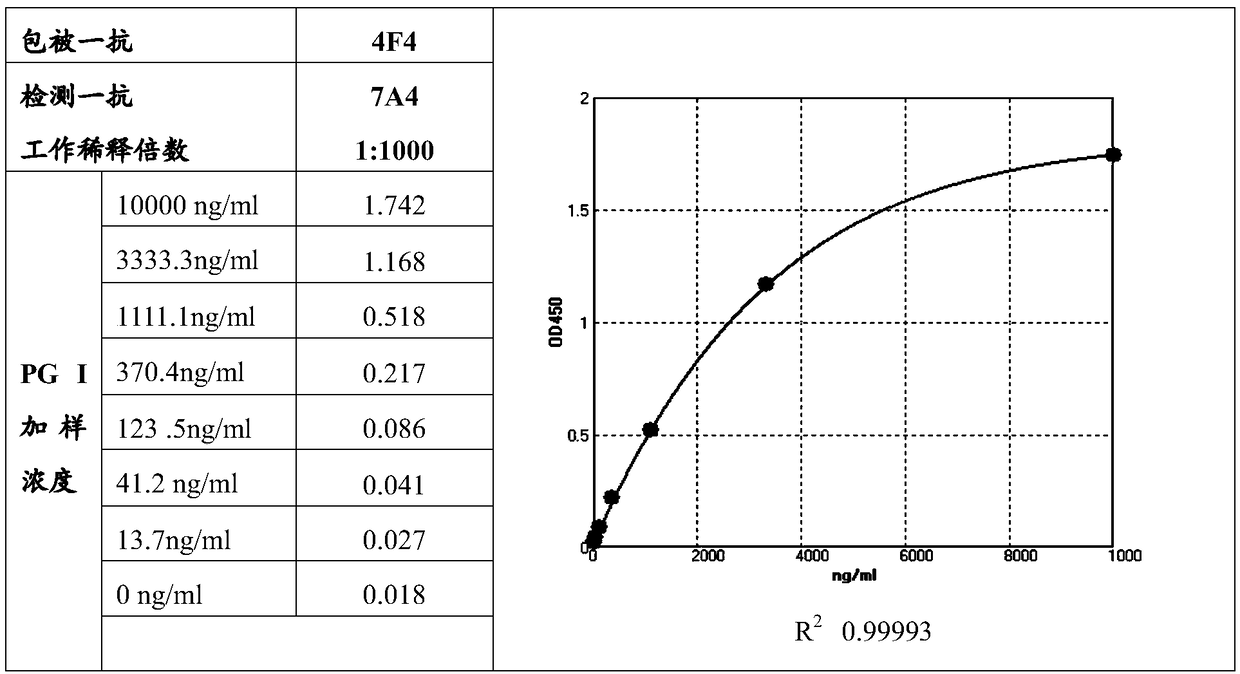
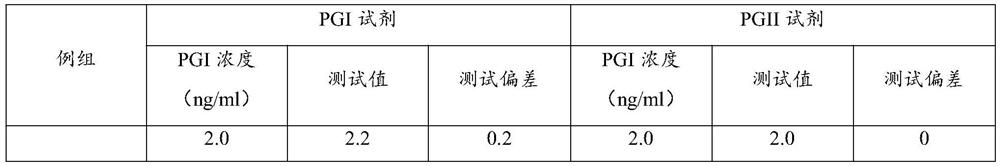
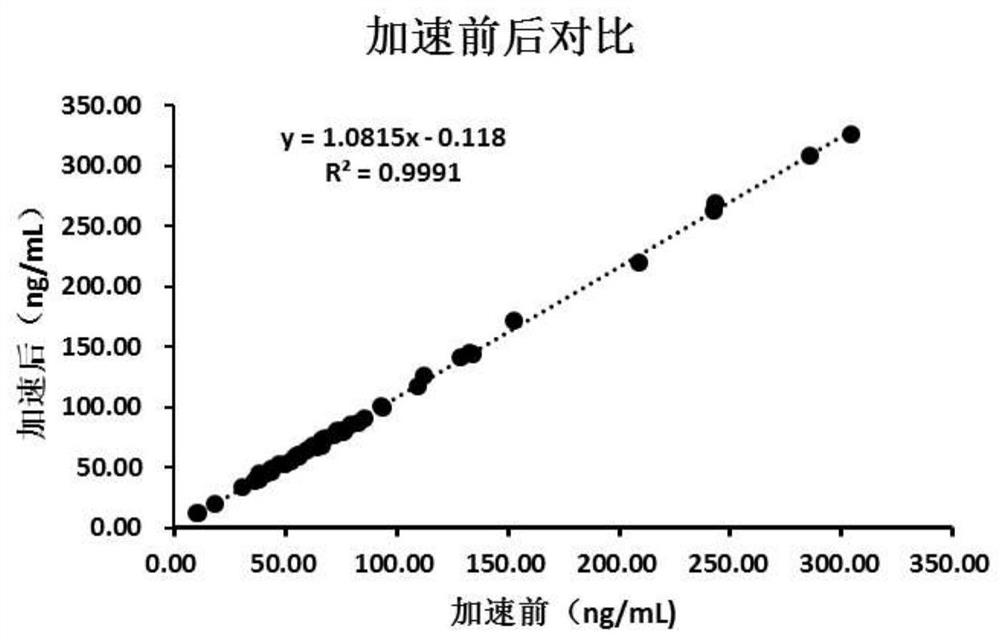
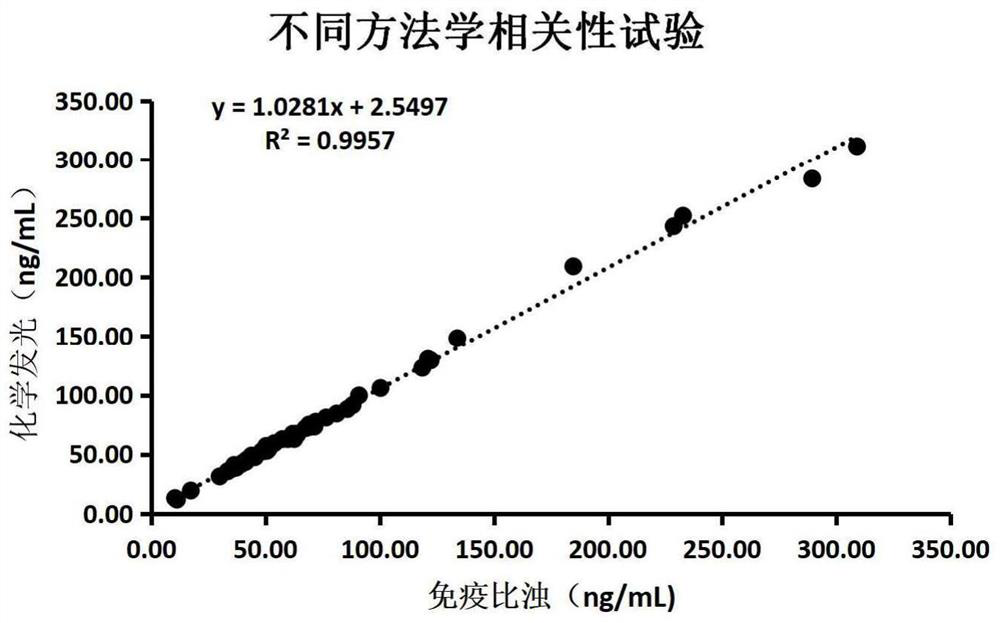
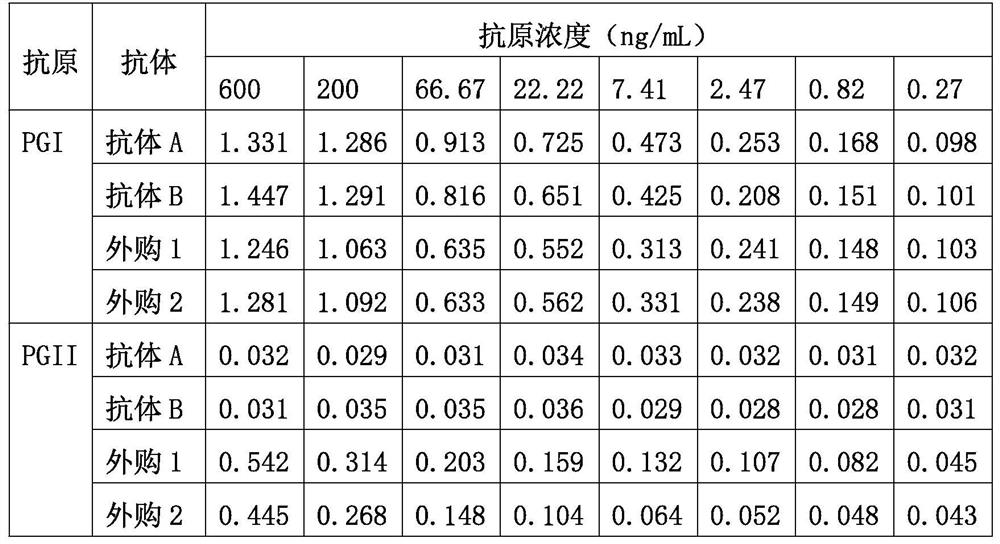
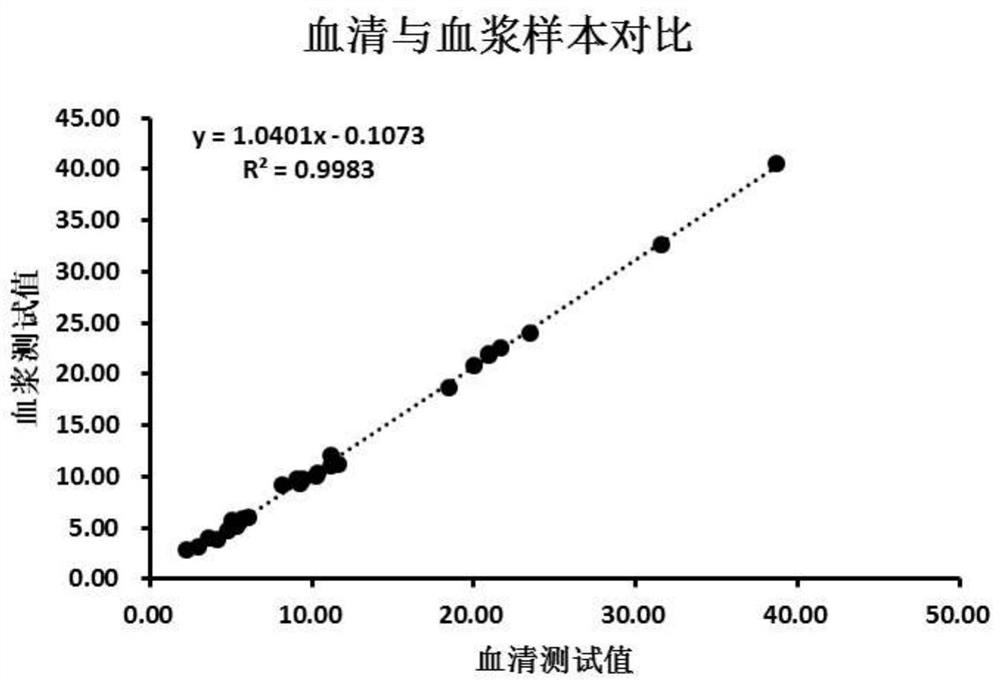
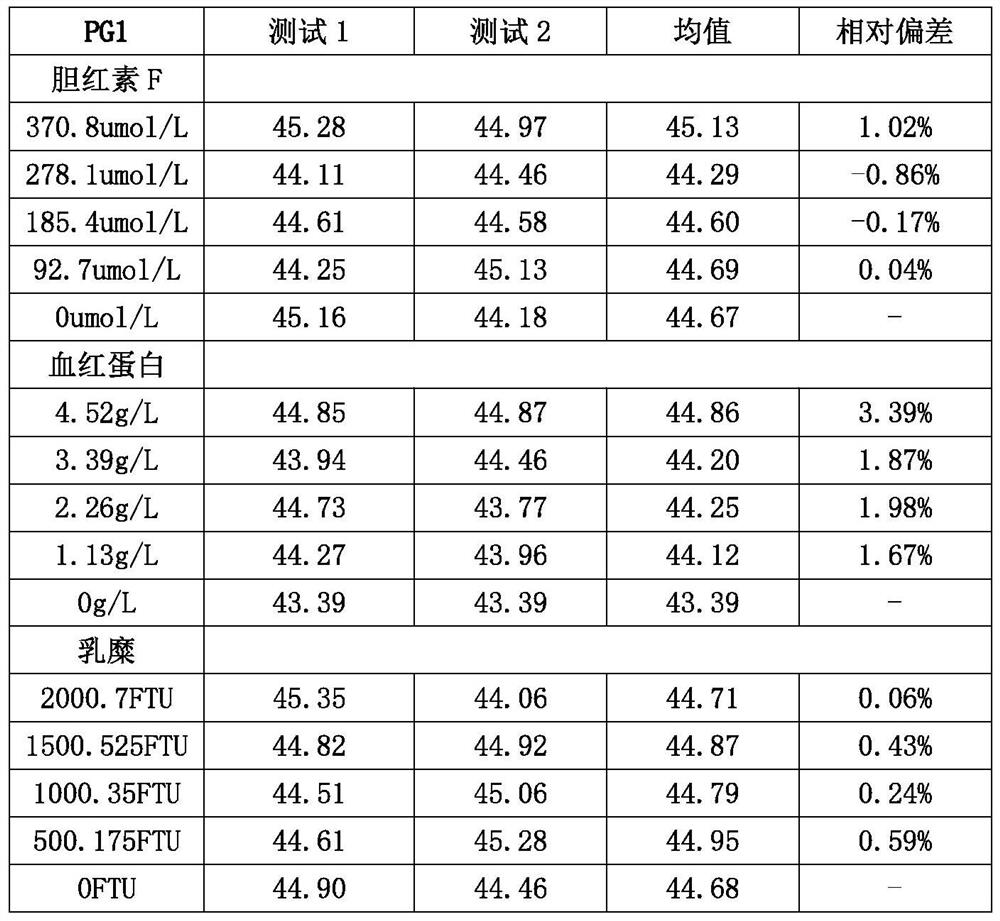
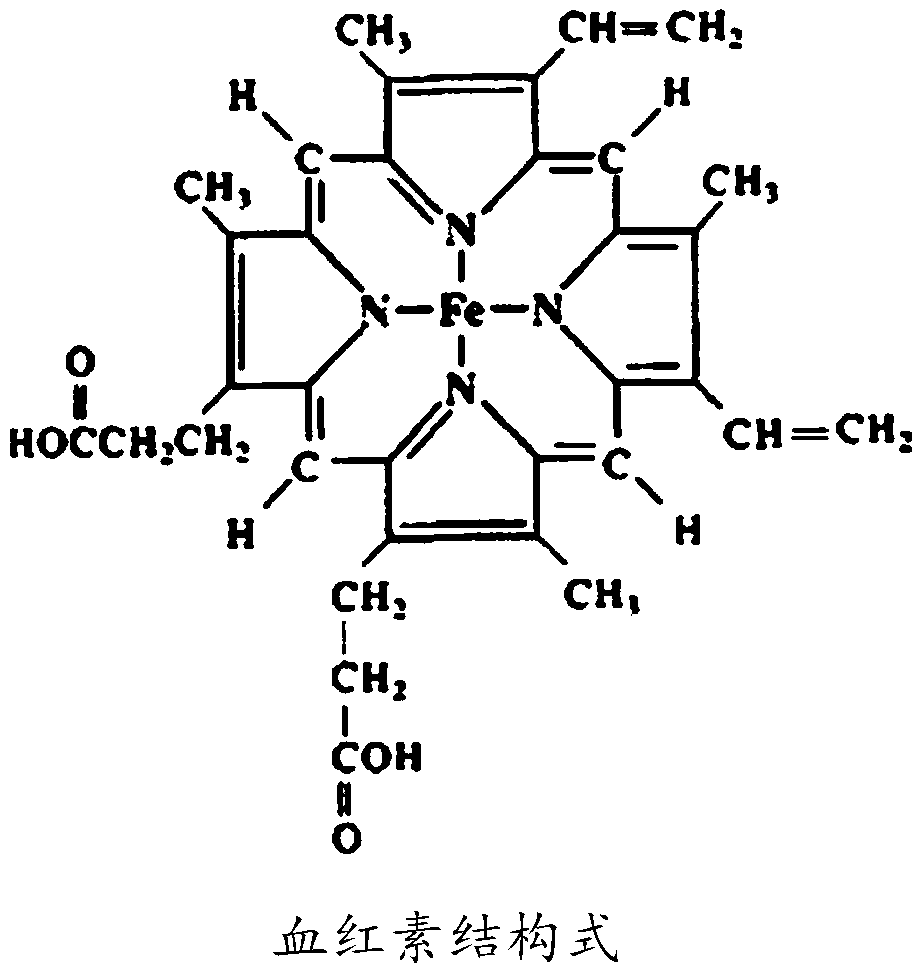
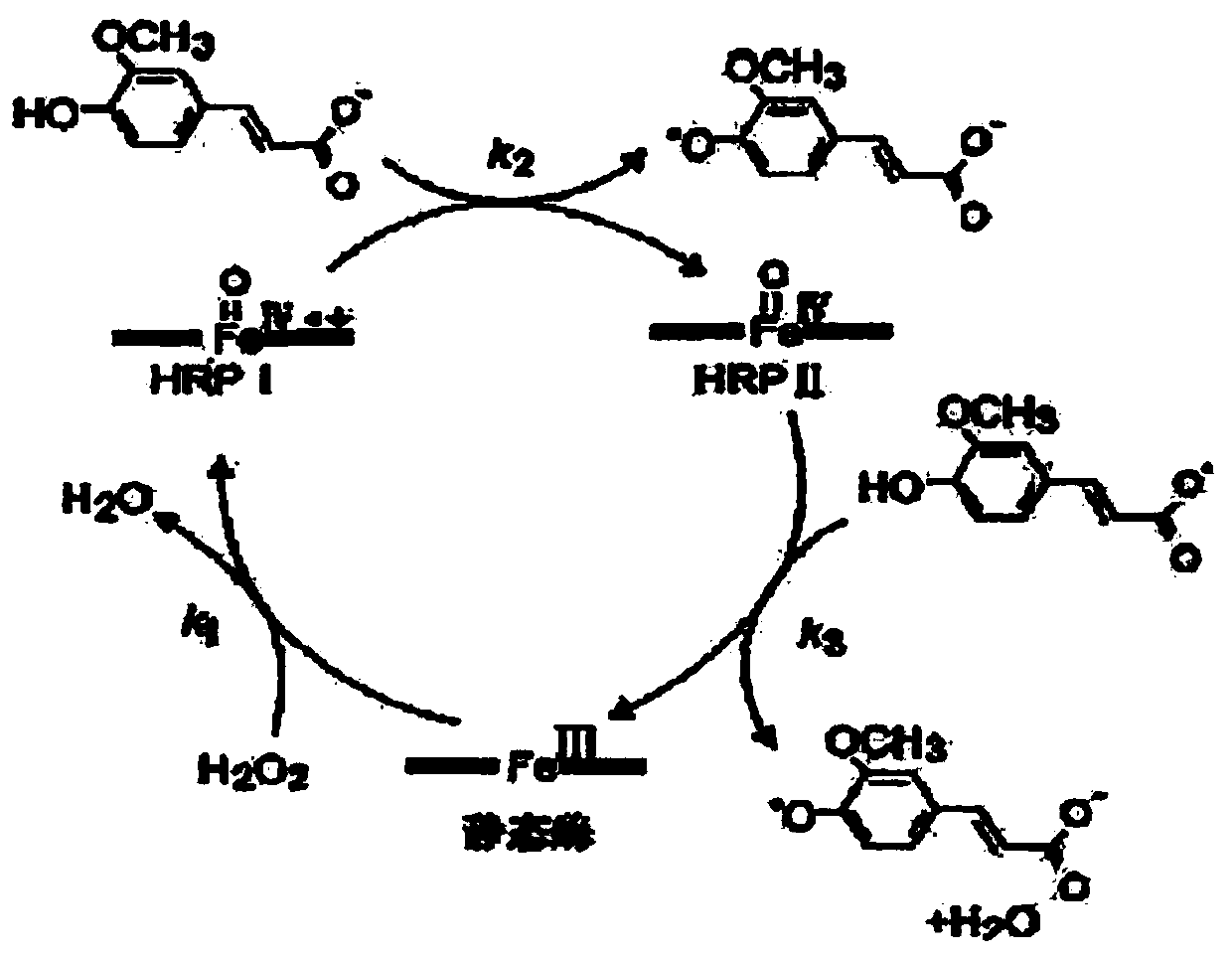
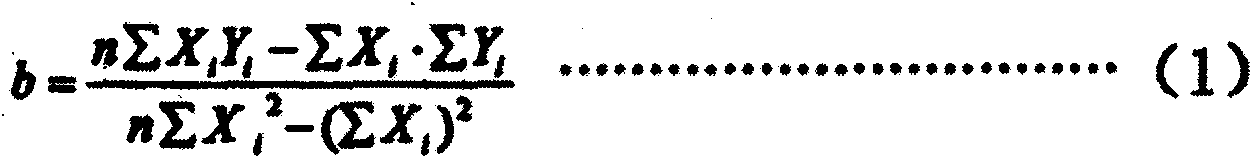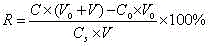Patents
Literature
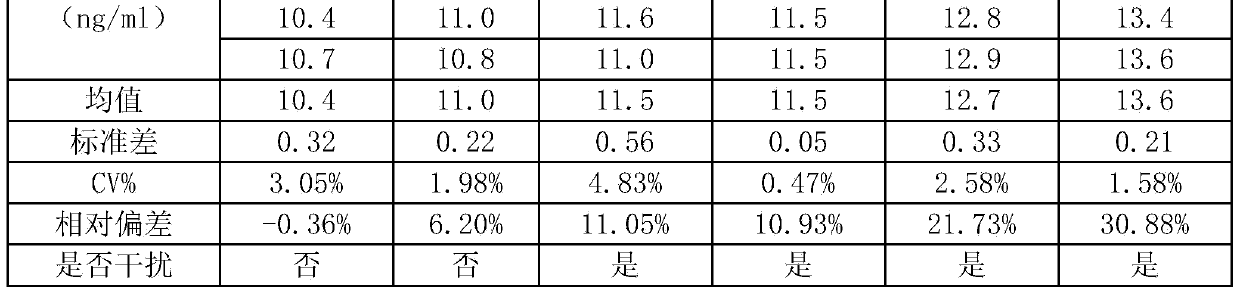
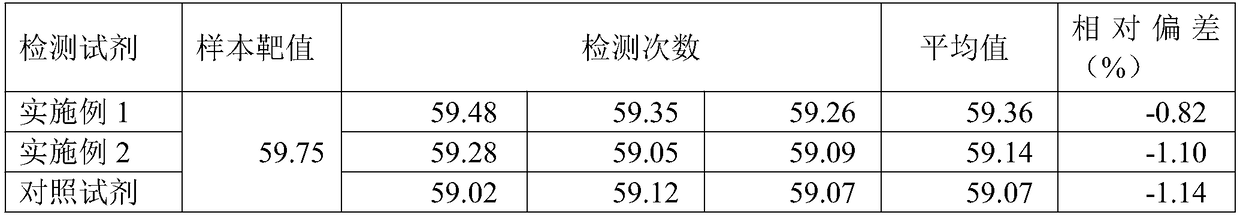
78 results about "Pepsinogen I" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enzyme linked immunosorbent assay kit for combined diagnosis of gastrosis or evaluation of gastric cancer risks
InactiveCN102087279AIncreased sensitivityImprove featuresComponent separationTissue cultureAntigenPepsinogen I
The invention discloses an enzyme linked immunosorbent assay kit for combined diagnosis of the gastrosis or evaluation of gastric cancer risks and a preparation method thereof. The kit comprises a micropore plate coated with an antibody against a pepsin antigen I or an antibody against a pepsin antigen II, an enzyme labeled antibody, a color-developing agent, a stop solution and a concentrated cleaning solution, wherein the pepsin antigen I or the pepsin antigen II is a natural protein obtained from extraction of human gastric mucosa tissue. The kit disclosed by the invention adopts a mouse immunized with pepsinogen I and pepsinogen II which are separated from human gastric mucosa to prepare immunogen of a monoclonal antibody, the used standard sample also adopts the pepsin antigen I or the pepsin antigen II separated from the human gastric mucosa, thereby the defects caused by adopting different structures of animal pepsinogen and human pepsinogen are filled. The kit can be used for accurately diagnosing the gastrosis or early gastric cancer and has the advantages of high sensitivity, strong specificity, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Latex immunoturbidimetry pepsinogen I detection kit for eliminating chyle interference
ActiveCN103698525AStrong interference abilityHigh sensitivityBiological material analysisBiological testingPepsinogen ITurbidity
The invention relates to the technical field of medical examination, and particularly relates to a latex immunoturbidimetry serum pepsinogen I detection kit for eliminating chyle interference. The kit provided by the invention comprises (1) a pepsinogen I calibrator; (2) a reagent 1 with a preset chyle remover; and (3) a reagent 2 containing the monoclonal antibody and polyclonal antibody coated latex particles against human pepsinogen I. According to the kit provided by the invention, the combination reaction between a substrate to be detected in a sample and a specific antibody in the reagent is amplified through the latex agglutination effect; with the given wavelength, the turbidity formed by the reaction is related to the content of the substrate to be detected, thus the content of the substrate to be detected is calculated. The kit provided by the invention is used for detecting the content of pepsinogen I in human serum, has high sensitivity and good specificity, and can eliminate chyle interference in the sample; moreover, the kit is simple and quick to operate and has high practicability and wide application range.
Owner:北京万泰德瑞诊断技术有限公司
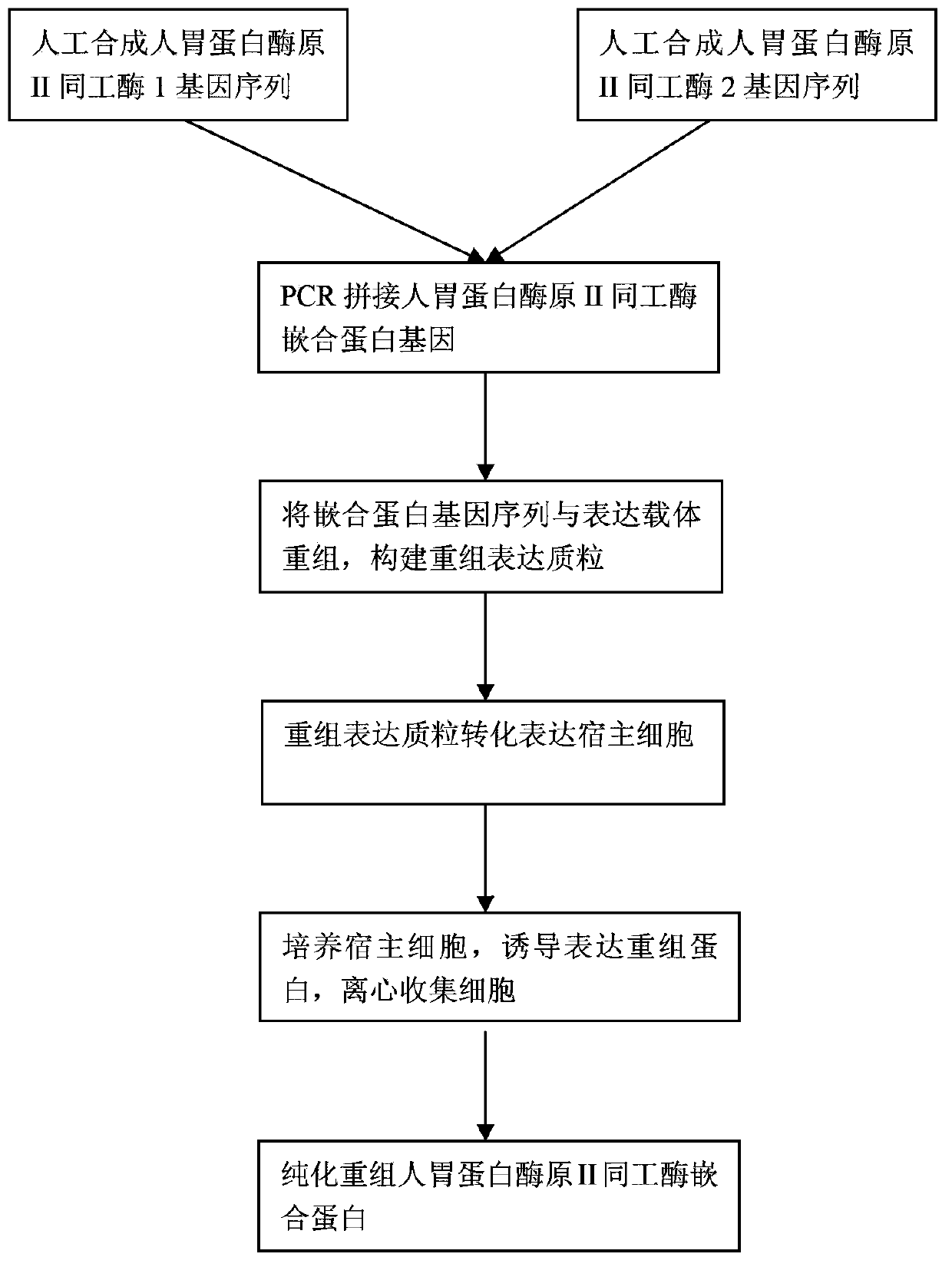
Recombinant human pepsinogen II isozyme chimeric protein, and preparation method and applications thereof
ActiveCN103387971AHigh sensitivityIncrease catch rateHydrolasesSerum immunoglobulinsPepsinogen IIIsozyme
The invention discloses a recombinant human pepsinogen II isozyme chimeric protein, and a preparation method and applications thereof. The preparation method comprises the steps as follows: by taking gene sequences of two isozymes of recombinant human pepsinogen II as a template, carrying out PCR (polymerase chain reaction) splicing to obtain a chimeric protein coding gene sequence, constructing recombinant expression plasmids, transforming the screened positive clone plasmids into expression host cells, screening an efficiently-expressed recombinant chimeric protein strain, carrying out enlargement culture on the cells and inducing expression chimeric protein, and purifying to obtain the recombinant human pepsinogen II isozyme chimeric protein. The invention further discloses the applications of the recombinant human pepsinogen II isozyme chimeric protein in preparing monoclonal antibodies and multiresistant serums or pepsinogen II kit calibration products. A great amount of stably expressed recombinant human pepsinogen II isozyme chimeric protein can be produced by utilizing a genetic engineering technology, and one protein has two chimeric protein sequences of the human pepsinogen II.
Owner:常州爱复康生物科技有限公司
United quantitative detection test paper of gastric cancer risk markers and preparation method
The invention relates to united quantitative detection test paper of gastric cancer risk markers and a preparation method. The gastric cancer risk markers include pepsinogen I, pepsinogen II and gastrin 17. A test paper strip comprises a sample pad, a gastric cancer risk marker antibody magnetic rare earth fluorescent microsphere labelling pad, a coating membrane and a water absorption pad, wherein three gastric cancer risk marker quantitative detection lines and a quality control region C line are arranged on the coating membrane. After tested substances in samples are concentrated through amagnetic field, a low-background high-sensitivity fluorescence immunochromatographic method is utilized for detecting the tested substances, and the problem of low sensitivity of a POCT type product is solved. The problem that when blood samples are detected through an immunity lateral chromatography reagent, in order to reduce the interference of other substances, usually the samples are tested after being diluted, so that the sensitivity is slightly low can be solved.
Owner:广州华澳生物科技有限公司
Pepsinogen, helicobacter pylori antibody and gastrin 17 detection method and kit thereof
InactiveCN107271669AQualitative and quantitative detectionFast and highly sensitive assayDisease diagnosisBiological testingPepsinogen IHelicobacter pylori Antibody
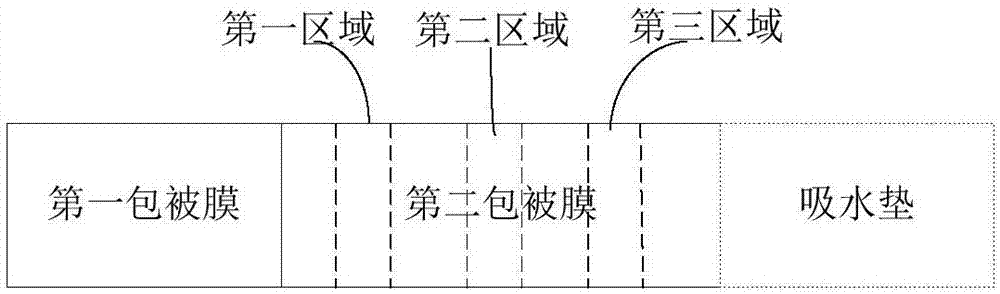
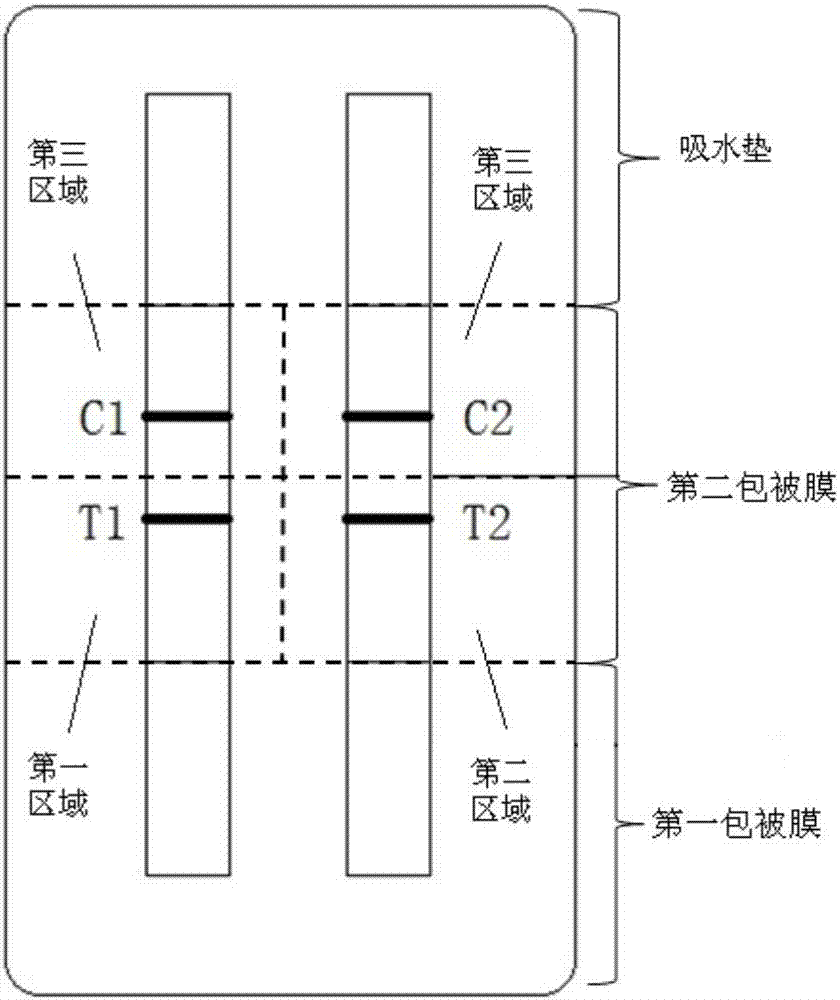
The invention provides a kit, application of the kit in detection of pepsinogen I, pepsinogen II, a helicobacter pylori antibody and gastrin 17 and a method for detecting the pepsinogen I, the pepsinogen II, the helicobacter pylori antibody and the gastrin 17 by using the kit. The kit comprises a first coating film and a second coating film, wherein at least one region in the first coating film is coated with a fluorescent microsphere-labeled pepsinogen I antibody, a fluorescent microsphere-labeled pepsinogen II antibody, a first fluorescent microsphere-labeled helicobacter pylori antigen and a fluorescent microsphere-labeled gastrin 17 antibody; the second coating film comprises a first region, a second region, a third region, a fourth region and a fifth region which are separated; a formed fluorescent microsphere material comprises a polystyrene-methyl methacrylate copolymer. The kit and the method which are provided by the invention have the advantages of high sensitivity, high specificity, quickness, simplicity, capability of realizing objective measurement and the like.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Pepsinogen I and II combined detection method and kit thereof
InactiveCN104502591ARealize joint quantitative detection in vitroAccurately determine the qualityBiological testingFluorescence/phosphorescencePepsinogen IFluorescence
The invention relates to a pepsinogen I and II combined detection method and a kit, and especially relates to a dual wavelength fluorescence immunity chromatography combined detection method of pepsinogen I and II and a detection kit thereof. The method comprises the following steps: 1)preparing an immunity chromatography test strip; 2)preparing a freeze-dried probe; 3)preparing a sample weak solution; and 4)examining the sample. The pepsinogen I and II combined detection method and the kit have the advantages of high sensitivity, high accuracy, simple operation and low cost.
Owner:江苏宏泰格尔生物医学工程有限公司
Latex enhanced immunoturbidimetry assay kit for pepsinogen I/II
InactiveCN104111335AHigh detection sensitivityEasy to operateDisease diagnosisBiological testingPepsinogen IPepsinogen II
The invention relates to a kit for measurement of the contents of pepsinogen I (PGI) and pepsinogen II (PGII) in serum. To overcome a technical problem, the invention provides the PGI and PGII content measurement kit applicable to a nephelometric instrument or fully automatic biochemical analyzer. The kit comprises the following components: a, R1, which is composed of a buffer solution, an accelerator and a surfactant, with the balance being purified water; b, R2-I, which is composed of a buffer solution and a latex microsphere binding with an antibody against human PGI; c, R2-II, which is composed of a buffer solution and a latex microsphere binding with an antibody against human PGII; d, a calibrator 1, which is composed of a buffer solution, a stabilizing agent, an antiseptic and a certain amount of pure recombinant PGI protein, with the balance being purified water; and e, a calibrator 2, which is composed of a buffer solution, a stabilizing agent, an antiseptic and a certain amount of pure recombinant PGII protein, with the balance being purified water Through combination of the above-mentioned reagents, the kit can rapidly measure the contents of PGI and PGII in serum.
Owner:唐勇
Chemiluminescent quantitative determination kit for pepsinogen II and preparation method of chemiluminescent quantitative determination kit
InactiveCN104569415AStrong specificityHigh sensitivityChemiluminescene/bioluminescencePepsinogen IPepsinogen II
The invention relates to the field of immunodetection, in particular to a chemiluminescent quantitative determination kit for pepsinogen II and a preparation method of the chemiluminescent quantitative determination kit. The chemiluminescent quantitative determination kit for the pepsinogen II comprises (1) a pepsinogen II antibody coated micropore plate, (2) an HRP-labeled pepsinogen II antibody, (3) series pepsinogen II calibration materials diluted by a calibration material diluent, (4) a luminescent substrate A, (5) a luminescent substrate B and (6) a solid washing liquid. The kit disclosed by the invention is capable of detecting the content of the pepsinogen II in serum; and the level of the pepsinogen II in the serum is in positively relevant to atrophic gastritis and peptic ulcer; in treatment of peptic ulcer, the treatment result can be judged by monitoring the change of the degermed pepsinogen; and the chemiluminescent quantitative determination kit is noninvasive, simple and fast in detection method, high in sensitivity, wide in detection range and the like.
Owner:HENAN MAINCARE BIOLOGICAL TECH
Screening method for gastritis
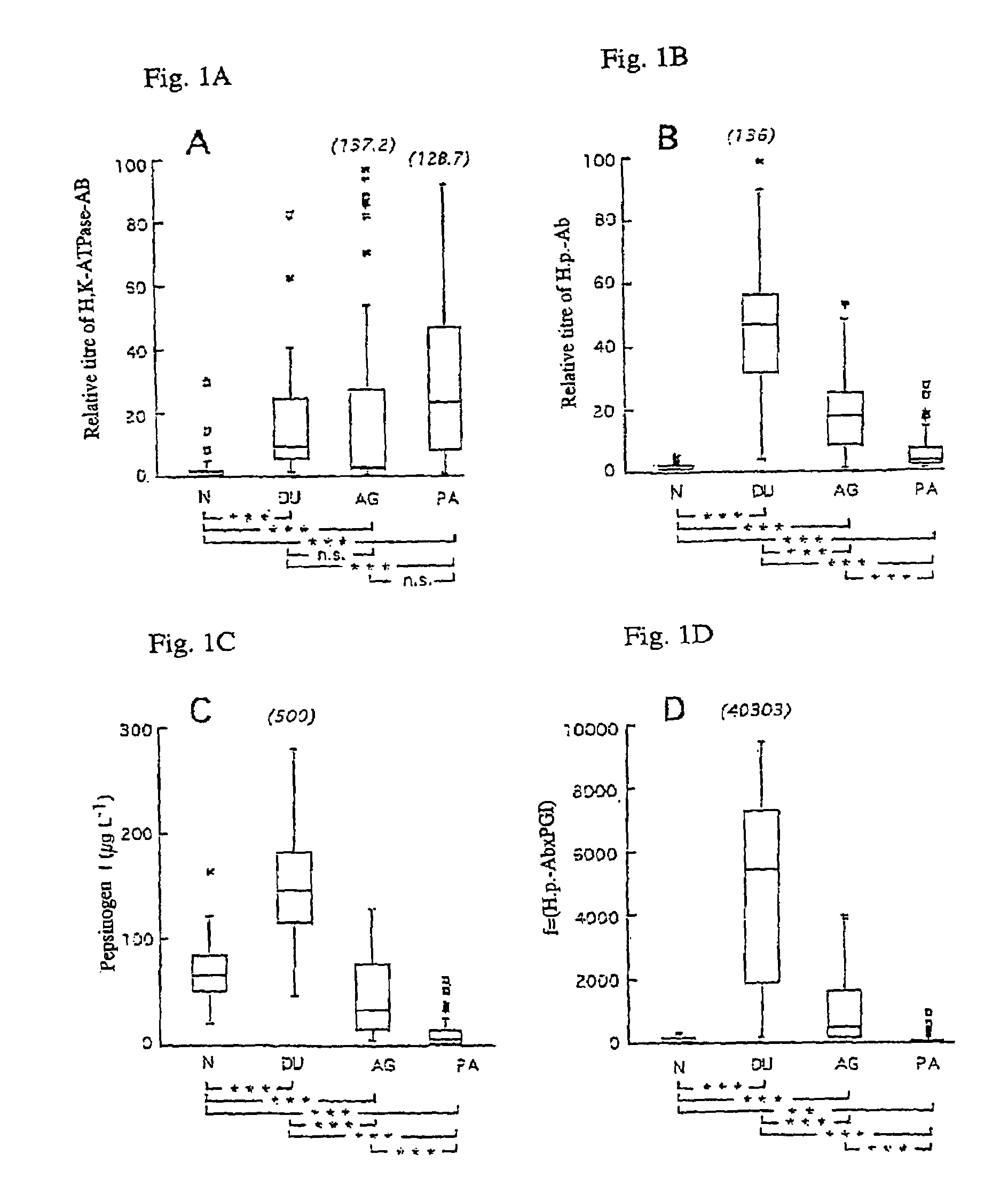
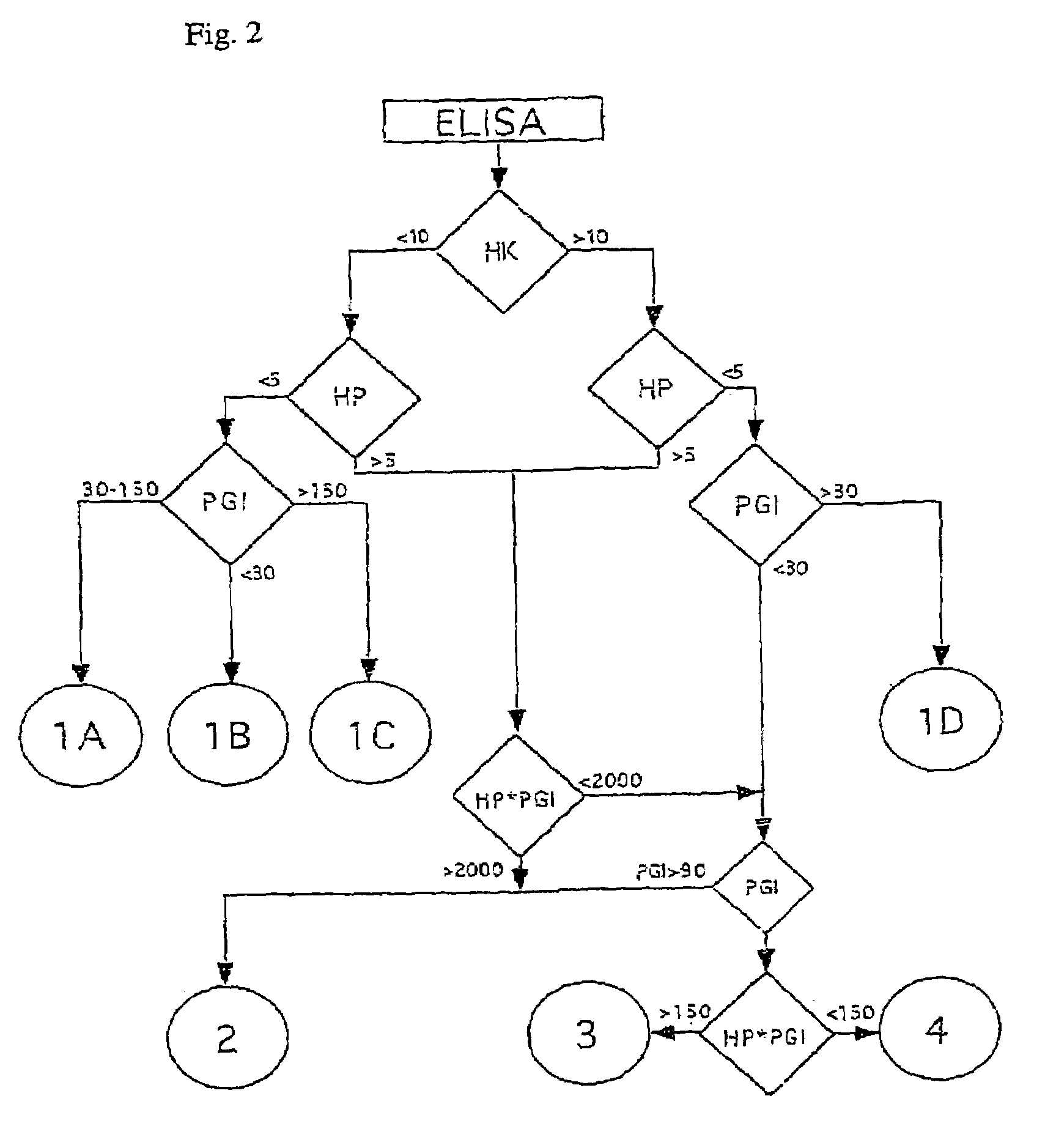
A screening method for gastritis is provided. The method involves the serological measurement of at least two of the following analytes: H,K-ATPase antibodies, Helicobacter pylori antibodies and the concentration of pepsinogen I; and the evaluation of the results in comparison with results from normal individuals. The evaluation scheme provides an initial, non-invasive identification of individuals with various forms of gastritis.
Owner:ATROPHUS
Pepsinogen I/pepsinogen II detection kit
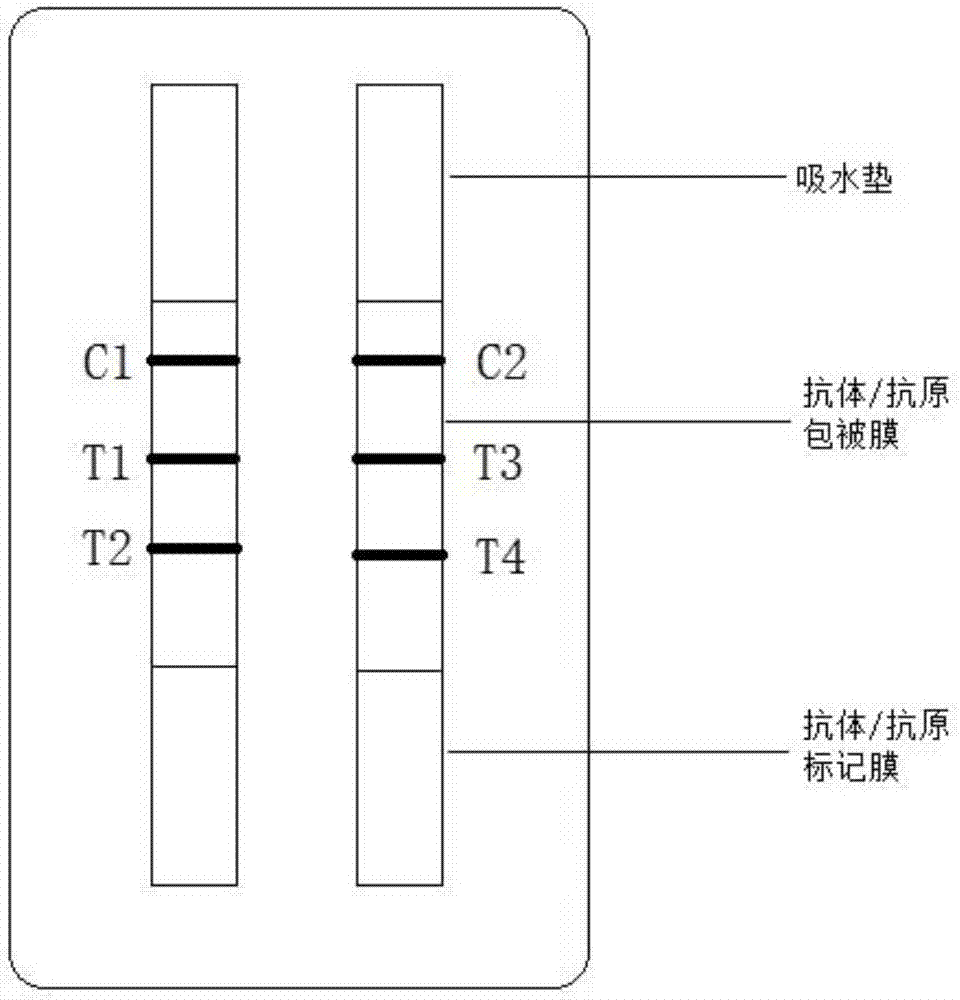
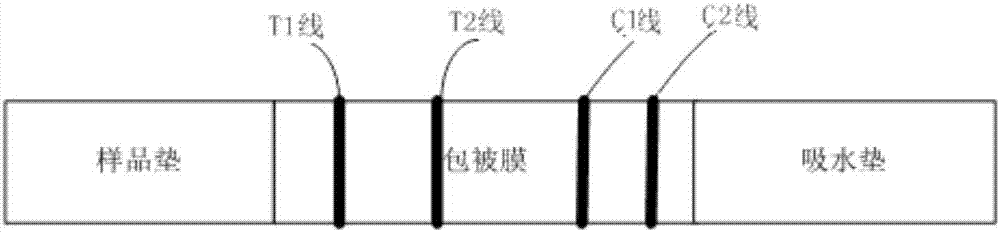
The invention provides a pepsinogen I / pepsinogen II detection kit, and belongs to the technical field of fluorescence immunochromatography in medical immunology. The pepsinogen I / pepsinogen II detection kitwhich is a detection kit of fluorescence quantitative immunochromatography, comprises a detection card, and is characterized in that the detection card is provided with a PVC board, a sample pad, a combination pad, a chromatography membrane and a water absorption paper in sequence; and the chromatographic membrane comprises a detection region and a quality control region, a detection regionof an anti-PG I monoclonal antibody and a PG II monoclonal antibody are coated with the detection region, and a sheep anti-mouse IgG antibody is coated with the quality control region. Test samples ofthe reagent kit are human serum, plasma or whole blood samples, and good detection specificity, higher sensitivity, simple and convenient operation and stable fluorescent markers are achieved to ensure the accuracy of the detection.
Owner:ZHEJIANG JUKANG BIOENG CO LTD
Kit for measuring pepsinogen I/II content of human serum
InactiveCN105067815AHigh detection sensitivityEasy to operateMaterial analysisPepsinogen IPolyethylene glycol
The invention relates to a kit for measuring the pepsinogen I / II content of human serum. The kit comprises reagents R1 and R2 and a calibration solution, wherein the reagent R1 is a buffer solution of which the PH value is 7.2 to 8.6, the reagent R2 is an anti-human PGI antibody latex reagent or anti-human PGII antibody latex reagent, and a standard substance is recombinant protein containing quantitative PGI or PGII or natural PGI or PGII extracted from the human serum. The kit is characterized by further comprising a reagent R3, wherein the reagent R3 is used for eliminating chyle and is composed of octyl methoxycinnamate, polyoxypropylene polyoxyethylene propylene glycol ether and polyethylene glycol-sulfate chitosan enzyme. The kit has the advantages of high detection sensitivity and strong anti-chyle interference capability and is suitable for detecting severe chyle.
Owner:ZHEJIANG KAICHENG BIOTECH
Pepsinogen I detection kit and preparation method thereof
The invention discloses a pepsinogen I detection kit and a preparation method thereof. The pepsinogen I detection kit is composed of the following components: PGI antibody-coating magnetic microspheres, a PGI calibrator, a PGI antibody marker bound to lanthanides, an analytical buffer, a cleaning solution, an enhancement solution and an RFID card. Based on combination of the magnetic microspheresand a time-resolved immunoassay method, the drawbacks of long physical adsorption reaction time and slow detection result of an enzyme label plate are overcome, and the reaction time is greatly shortened; besides, the advantages of high accuracy, high sensitivity, strong specificity, wide linear range, and stable and convenient detection of a time-resolved detection technology can also be realized. The magnetic microspheres are coated with corresponding antigens or antibodies, due to the stereoscopic characteristic of the magnetic microspheres, the contact surface area of an immune response isgreatly increased, the detection time is greatly shortened, and further optimization is made for the traditional two-step method detection, pepsinogen I detection only requires a one-step method, anda result can be obtained within 20 minutes.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Pepsinogen II detection kit and preparation method thereof
PendingCN111122866AElimination of chylolysisGuaranteed stabilityDisease diagnosisPepsinogen IPepsinogen II
The invention discloses a pepsinogen II detection kit, which comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from 50-150 mmol / L of sodium phosphate buffer solution, 2-3 g / Lof bovine serum albumin, methylisothiazolinone with the concentration of 2-3%, 1.0-5.0 g / L of fatty alcohol and ethylene oxide condensate, 3-5 mmol / L of magnesium chloride and 20-30 mmol / L of ammonium formate, and the balance of deionized water.; and the reagent R2 is prepared from 1.3-2 mg / ml of pepsinogen II antibody emulsion solution, 2-3 g / L of bovine serum albumin, methylisothiazolinone withthe concentration of 2-3%, and the balance of deionized water. The prepared pepsinogen II detection kit and the preparation method of the pepsinogen II detection kit have the advantages that the chyle problem can be effectively solved, the anti-interference capability is high, and meanwhile, the stability and the sensitivity are high.
Owner:浙江强盛生物科技有限公司
Pepsinogen I (PGI) detection kit and detection method thereof
InactiveCN108398555AEnhanced detection signalHigh detection sensitivityBiological material analysisColor/spectral properties measurementsPepsinogen IPhosphate
The invention discloses a pepsinogen I (PGI) detection kit and a detection method thereof. The detection kit is prepared from a reagent R1 and a reagent R2, wherein the reagent 1 is prepared from 100mmoL / L of phosphate buffer solution, 0.5g / L of Proclinc 300, 10g / L of polyethylene glycol 6000, 1g / L of triton 100 and 10g / L of TRIS; the reagent R2 is prepared from 100mmoL / L of phosphate buffer solution, 1.3mg / mL of latex particle anti-human PGI antibody fixation emulsion (anti-human pepsinogen I (mouse) monoclonal antibody sensitive emulsus solution, 0.5g / L of Proclinc 300, 10g / L of bovine serumalbumin (BSA) and 90g / L of glycerium. The detection method comprises the following steps: separating a serum sample, adding the reagent R1 into the serum sample, evenly mixing, incubating for 3 to 5min, adding the R2, incubating 30s, detecting an absorbance value A1 under 700nm wavelength, detecting an absorbance value A2 under the 700nm wavelength after 5min and calculating a content of the pepsinogen I according to serum standard substance data. The detection kit disclosed by the invention obviously improves flexibility and stability of testing reagents and has the characteristics of high flexibility, high accuracy, stable reagents and the like.
Owner:广州市伊川生物科技有限公司
Pepsinogen I and pepsinogen II detection method and kit thereof
ActiveCN107132359AStrong specificityHigh detection sensitivityFluorescence/phosphorescencePepsinogen IPolystyrene
The invention discloses a kit and a method for detecting pepsinogen I and pepsinogen II. The kit comprises a first coating film and a second coating film, wherein one end of the first coating film is connected with one end of the second coating film; at least one region in the first coating film is coated with a fluorescent microsphere labeled pepsinogen I antibody and a fluorescent microsphere labeled pepsinogen II antibody; the second coating film comprises a first region, a second region and a third region, which are separated from one another; a material for forming a fluorescent microsphere comprises a polystyrene-methyl methacrylate copolymer. The kit and the method, disclosed by the invention, have the advantages of high sensitivity, strong specificity, rapidness, simplicity and convenience, and can be used for realizing objectification determination and the like.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Pepsinogen I and pepsinogen II combined detection kit and application thereof
PendingCN111398591AShorten the timeReduce testing costsDisease diagnosisPepsinogen ITime resolved fluorescence immunoassay
The invention discloses a pepsinogen I and pepsinogen II combined detection kit and application thereof. The invention discloses a pepsinogen I and II combined detection kit for the first time. The pepsinogen I and II combined detection kit comprises a coating antibody and a labeled antibody, wherein the coating antibody is an anti-PGI monoclonal antibody named as a monoclonal antibody IA and an anti-PGII monoclonal antibody named as a monoclonal antibody IIA; wherein the labeled antibody is an anti-PG I monoclonal antibody named as a monoclonal antibody IB and an anti-PG II monoclonal antibody named as a monoclonal antibody IIB. The invention further discloses application of the kit. According to the invention, the pepsinogen I and II combined detection kit is combined with a high-sensitivity inductively coupled plasma mass spectrometry detection technology on the basis of time-resolved fluorescence immunoassay. Detection of two indexes of PGI and PGII can be completed at the same time in one cycle, the time for clinically obtaining a PGI value and a PGI / PGII ratio result is shortened, and the detection cost is greatly reduced.
Owner:北京清分稳同科技有限公司
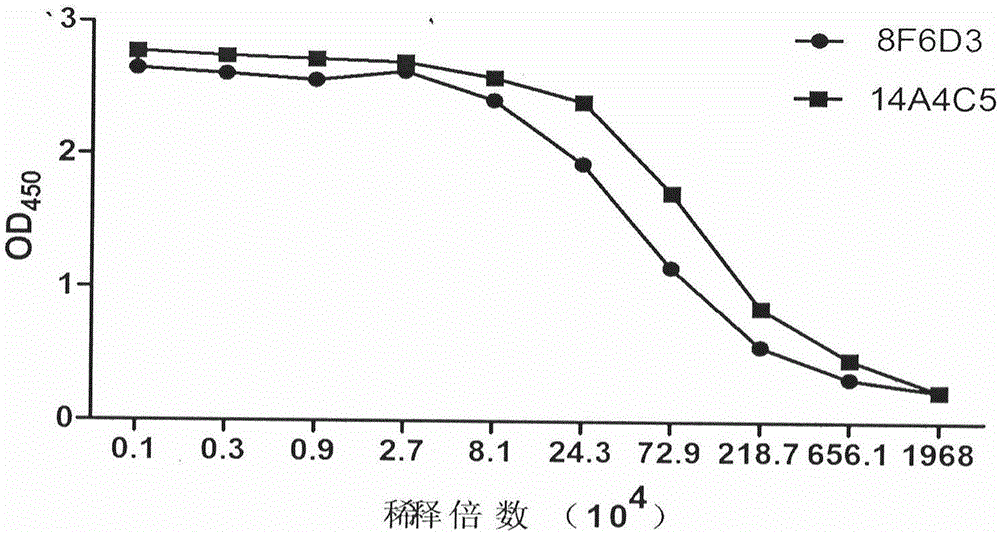
Preparation method of pepsinogen I paired monoclonal antibody
ActiveCN108395476AIncrease chanceImprove linearityTissue cultureImmunoglobulins against enzymesBALB/cPepsinogen I
The invention discloses a preparation method of a pepsinogen I paired monoclonal antibody. The preparation method of the pepsinogen I paired monoclonal antibody comprises the following steps: (1) immunizing BALB / c mice with pepsinogen I protein as an antigen, and selecting immunized mice with high serum antibody titer for cell fusion, screening a high-titer positive cell line, preparing ascites, and purifying to obtain a monoclonal antibody; and (2) mixing the pepsinogen I protein with the monoclonal antibody obtained in the step (1) to prepare antigen-monoclonal antibody immune complex immunized BALB / c mice, selecting mice with high serum antibody titer for cell fusion, screening a positive cell line having no competitive inhibition effect with the monoclonal antibody of the step (1), andpurifying the secreted monoclonal antibody to obtain a monoclonal antibody paired with the monoclonal antibody of the step (1). The method effectively improves the probability of obtaining the pairedmonoclonal antibody of the pepsinogen I antigen.
Owner:BEIJING LEADMAN BIOCHEM
Pepsinogen I detection kit and preparation method thereof
PendingCN111122867AElimination of chylolysisGuaranteed stabilityDisease diagnosisPepsinogen IAntiendomysial antibodies
The invention discloses a pepsinogen I detection kit, which comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from 50-150 mmol / L of sodium phosphate buffer solution, 2-3 g / Lof bovine serum albumin, methylisothiazolinone with the concentration of 2-3%, 1.0-5.0 g / L of fatty alcohol and ethylene oxide condensate, 3-5 mmol / L of magnesium chloride and 20-30 mmol / L of ammoniumformate, and the balance of deionized water.; and the reagent R2 is prepared from 1.3-2 mg / ml of pepsinogen I antibody emulsion solution, 2-3 g / L of bovine serum albumin, methylisothiazolinone with the concentration of 2-3%, and the balance of deionized water. The prepared pepsinogen I detection kit and the preparation method of the pepsinogen I detection kit have the advantages that the chyle problem can be effectively solved, the anti-interference capability is high, and meanwhile, the stability and the sensitivity are high.
Owner:浙江强盛生物科技有限公司
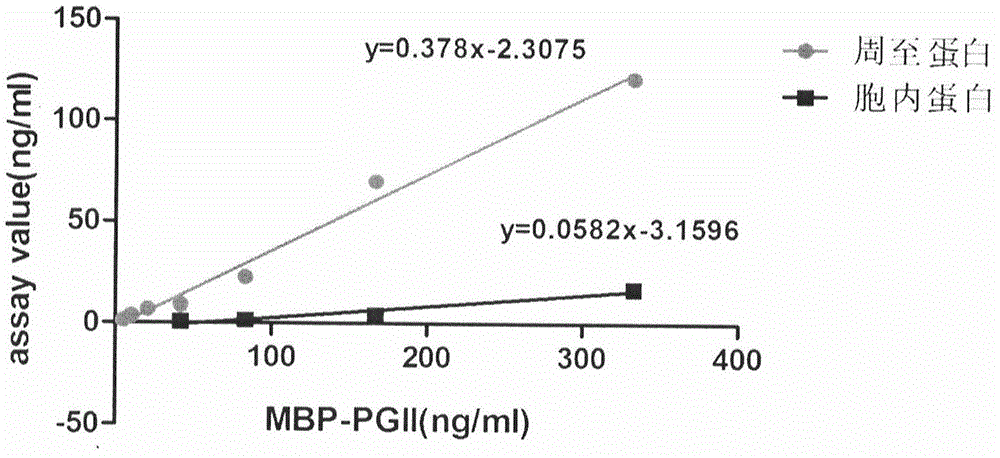
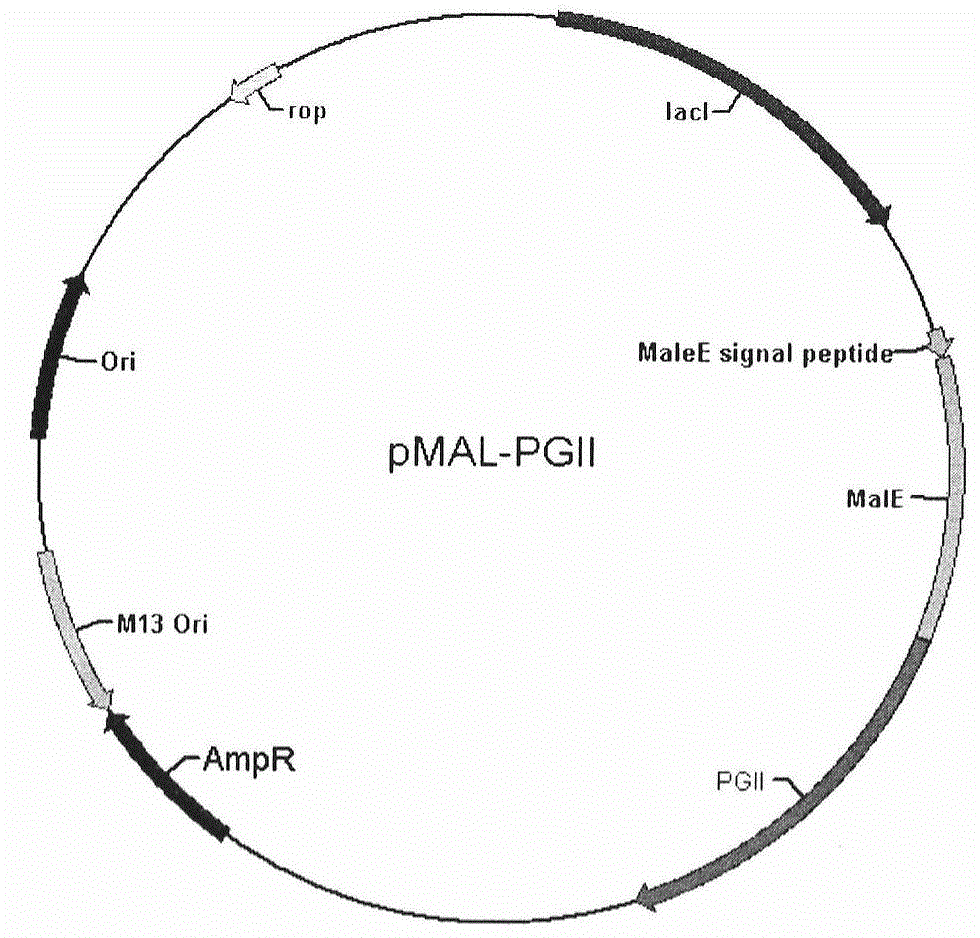
Soluble secretory expression of PG II-MBP fusion protein and application thereof
The invention belongs to the technical field of bioengineering and particularly relates to soluble secretory expression of a fusion protein of a human pepsinogen II (PG II) and a maltose binding protein (MBP) in escherichia coli, provides a preparation method of the fusion protein, and also discloses application of a recombinant BMP-PG II fusion protein to preparation of monoclonal antibodies and calibration materials of pepsinogen II kits. Through the adoption of elements such as a Ptac promoter, an malE signal peptide and the maltose-binding protein, the soluble secretory expression of PG II from periphery to inside of escherichia coli is implemented; the immunogen activity of the recombinant human PG II protein from a prokaryotic expression system is greatly improved; and the preparation cost of the recombinant PG II is effectively reduced.
Owner:BEIJING JIAWAN BIOTECH CO LTD
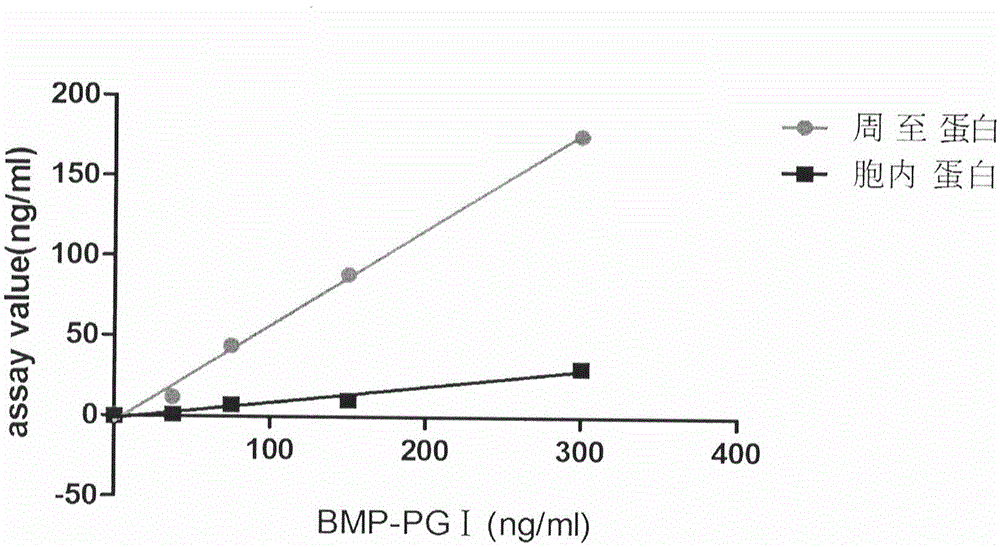
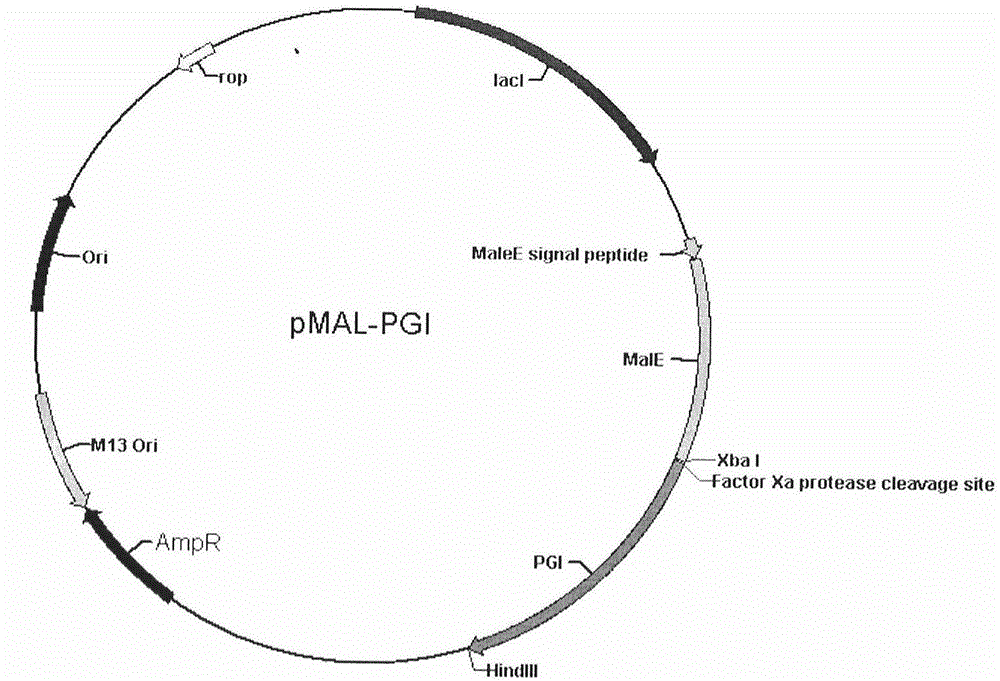
Human pepsinogen I (PGI)-maltose-binding protein (MBP) fusion protein soluble secretory expression and use
The invention belongs to the technical field of bioengineering, relates to soluble secretory expression of a human pepsinogen I (PGI)-maltose-binding protein (MBP) fusion protein in escherichia coli and provides a preparation method of the fusion protein. The invention also discloses a use of the recombinant BMP-PGI fusion protein in preparation of a monoclonal antibody and a pepsinogen I kit calibrator. Through use of a Ptas promoter, a malE signal peptide and a maltose binding protein, soluble secretory expression of PGI in escherichia coli is realized so that immunogen activity of the recombinant PGI protein of a prokaryotic expression system is greatly improved and a recombinant PGI preparation cost is effectively reduced.
Owner:BEIJING JIAWAN BIOTECH CO LTD
Fluorescent microsphere detection device for gastric function and gastric cancer risk and preparation method thereof
ActiveCN111638369ASimple structureNovel ideaDisease diagnosisBiological testingPepsinogen ICellulose
The invention relates to a fluorescent microsphere detection device for gastric function and gastric cancer risk and a preparation method thereof, and belongs to the field of medical detection equipment. The device is prepared by adhering a nitrocellulose membrane with solid phase containing high-specificity gastrin 17, pepsinogen I, pepsinogen II antibody, anti-human IgG antibody, anti-human IgMantibody and goat-anti-mouse IgG polyclonal antibody, glass fibers adsorbed with fluorescent microsphere labeled gastrin 17, pepsinogen I, pepsinogen II antibodies and helicobacter pylori urease antigens, a sample pad, absorbent paper and other auxiliary materials. The reaction sensitivity is effectively improved; under the same threshold value, the dosage of the immunofluorescence microspheres can be reduced, the cost is saved; five gastric cancer risk markers including gastrin 17, pepsinogen I, pepsinogen II, helicobacter pylori urease IgG antibody and helicobacter pylori urease IgM antibodyin a specimen can be detected at the same time, and the complexity of production operation is not increased. The detection test paper is high in sensitivity, strong in specificity and strong in practicability.
Owner:JILIN PROVINCE GERUISITE BIOTECHNOLOGY CO LTD
Composite quality control product for gastric function detection and detection kit
InactiveCN111024964AMeet stability requirementsShelf life stableDisease diagnosisBiological testingPepsinogen IPepsinogen II
The invention relates to a pepsinogen I, pepsinogen II and gastrin-17 composite quality control product. The composite quality control material comprises a protein preserving solution, and pepsinogenI, pepsinogen II and gastrin-17 which are dissolved in the protein preserving solution, wherein the total volume of the protein preserving solution is calculated; wherein the protein preserving solution contains 0.8 to 3.5 g / L of AEP-HBC, 0.002 to 0.006 g / L of fibrinogen, 0.05 to 0.15 g / L of gelatin, 2 to 8 g / L of trehalose, 0.05 to 0.25 g / L of tetrapolyphosphate, 5-25 mmol / L of 2-(N-morpholine) ethanesulfonic acid, 0.5-4 g / L of ethylenediaminetetraacetic acid or ethylenediaminetetraacetic acid salt, 1-20 mmol / L of cysteine, methionine or arginine and 0.05-0.10% of a preservative,. The pepsinogen I, pepsinogen II and gastrin-17 composite quality control product is initiated at home, and can be stably stored for 14 months at the temperature of 2-8 DEG C. The invention further discloses a preparation method of the pepsinogen I, pepsinogen II and gastrin-17 composite quality control product. The invention also provides a detection kit containing the composite quality control product, andthe detection kit can be used for clinical detection of gastric functions.
Owner:深圳市蔚景生物科技有限公司
Pepsinogen I detection kit
InactiveCN109307766AEliminates heterophile antibodiesImprove anti-interference abilityChemiluminescene/bioluminescenceBiological testingPepsinogen IAntigen
The invention discloses a pepsinogen I detection kit, which comprises a magnetic particle coated with a pepsinogen I monoclonal antibody, enzyme diluent which contains HRP (Horse Radish Peroxidase) and labels the pepsinogen I monoclonal antibody, a calibration product which contains a pepsinogen I antigen, and sample diluent, wherein the sample diluent contains blocking agent ingredients, and theblocking agent is one or a plurality of human anti-mouse antibodies used for neutralizing HAMA (Human Anti-Mouse Antibody) interference. The pepsinogen I detection kit has the advantages that a high-sensitivity chemiluminiscence technology and a magnetic particle preparation technology are combined, and an AutoLumo full-automatic detection analyzer is assorted to realize detection full automation.Meanwhile, blocking agent ingredients are added into the sample diluent, so that heterophile antibodies in the sample can be eliminated, and the anti-interference ability of the kit is greatly improved.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Method for Predicting the State of the Gastric Mucosa
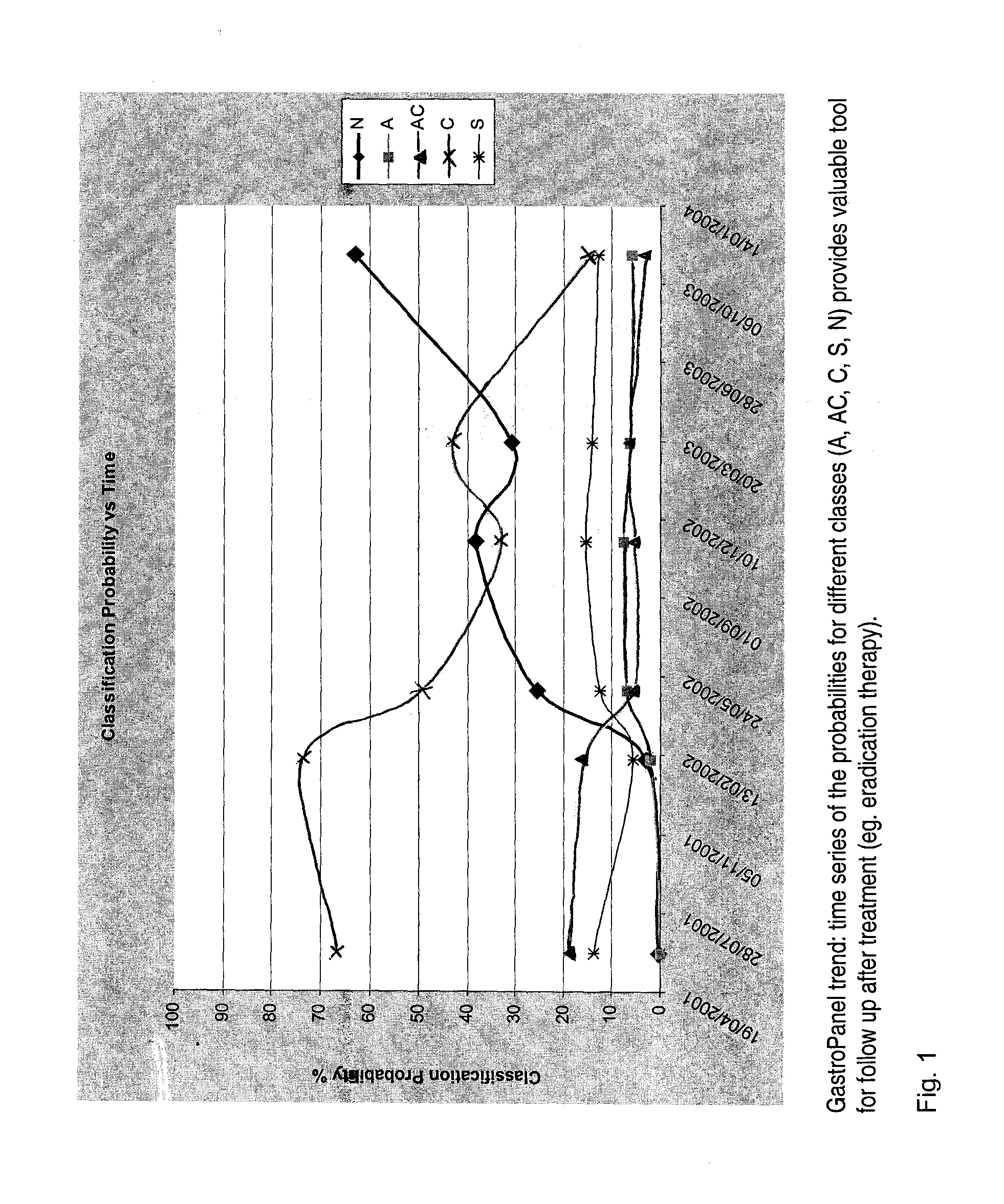
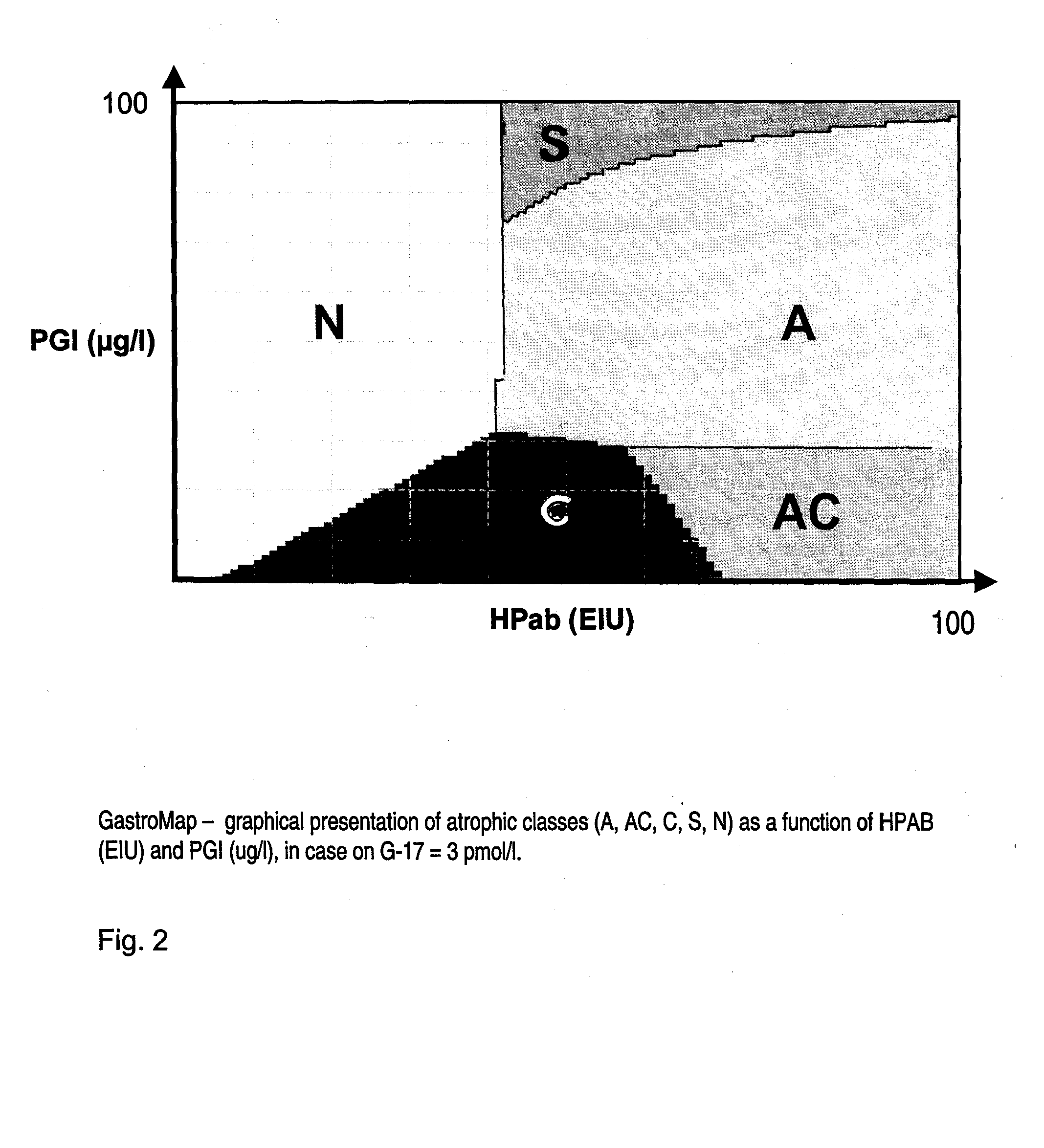
InactiveUS20080162101A1Analogue computers for chemical processesDisease diagnosisPepsinogen IData processing system
The present invention is directed to a method for assessing or predicting the state of the gastric mucosa in a subject by determining, in said subject, the probability for the gastric mucosa belonging to at least one gastric mucosa class, the method comprising: measuring, from a sample of said subject, the pepsinogen I (PGI) and gastrin-17 (G-17) analyte concentrations, as well as determining the presence or concentration of a marker for Helicobacter pylori; entering the data so obtained in a data processing system comprising an operating system, a database and means for transceiving and processing data, the said data processing system being adapted to determine the probability for the gastric mucosa belonging to the at least one gastric mucosa class, based on the data entered as well as on predefined clinical data in the database, the information so generated being indicative of the state of the gastric mucosa in said subject.
Owner:BIOHIT OY FI
Pepsinogen I/II determination kit and preparation method thereof
ActiveCN112098654AEasy to storeAvoid reunionBiological material analysisBiological testingPepsinogen ISucrose
The invention provides a pepsinogen I / II determination kit and a preparation method thereof. A magnetic bead diluent in the kit comprises 0.04-0.06 mol / L of HEPES, 9-11 g / L of protein, 1-4 g / L of a protective agent, 29-31 g / L of sucrose, 1.5-2.5 g / L of S17 and 0.5-1.5 g / L of a preservative, and the pH value is 7.07.5. The enzyme-labeled diluent comprises 0.04 to 0.06 mol / L of MES, 19 to 21 g / L ofcasein, 39 to 41 g / L of sucrose, 1 to 5 g / L of a dispersant, 0.05 to 0.15 g / L of a blocking agent, 0.13 to 0.14 g / L of ZnCl2, 1 to 1.5 g / L of MgCl2, 1.5 to 2.5 g / L of Tween 20, 0.5 to 1.5 g / L of a preservative and 8 to 9 g / L of NaCl, the PH value is 6.0-6.5; and the stability is high.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Pepsinogen I monoclonal antibody and application thereof
ActiveCN113004412AHigh sensitivityHigh precisionBiological material analysisImmunoglobulins against enzymesPepsinogen IPepsinogen II
The invention relates to the technical field of biology, in particular to a pepsinogen I monoclonal antibody and application thereof, and discloses a group of pepsinogen I monoclonal antibodies which can be used in a matched mode. The pepsinogen I monoclonal antibodies have high specificity and stability when being combined with PGI, cannot generate cross reaction with pepsinogen II, and are suitable for various methodologies, and high-sensitivity and high-precision detection of PGI is realized.
Owner:重庆艾生斯生物工程有限公司
Pepsinogen II monoclonal antibody and application thereof
ActiveCN113045666AWith anti-interference abilityAccurate measurementDisease diagnosisImmunoglobulins against enzymesPepsinogen IPepsinogen II
The invention relates to the technical field of biology, in particular relates to a pepsinogen II monoclonal antibody and application thereof, and discloses a group of pepsinogen II monoclonal antibodies which can be used in cooperation, which can be applied to different methodologies, accurate measurement of PGII is achieved, interference of other impurities can be avoided, and high-specificity binding is achieved. The monoclonal antibody provided by the invention can assist in research and development of medical diagnostic reagents, and has great significance.
Owner:重庆艾生斯生物工程有限公司
Stable protein solution, preparation method thereof and detection kit
InactiveCN111024959AImprove stabilityAvoid sex changeBiological material analysisBiological testingProtein solutionPepsinogen I
The invention provides a stable protein solution. The protein solution comprises a buffer solution and a protein containing a heme auxiliary group or a protein conjugate of the protein containing theheme auxiliary group and other proteins. Ferrous ions in the heme auxiliary group are combined with carbon monoxide, so that the protein or the protein conjugate can be prevented from being oxidized and denatured in the storage process, and the stability of the protein solution is improved. The protein solution may also include other stabilizers. The protein solution disclosed by the invention canbe stably stored for at least one year at 2-8 DEG C, has good stability, and can be used for preparing a chemiluminescence detection kit or an enzyme-linked immunosorbent assay kit, such as a detection kit for detecting gastrin 17, pepsinogen I and pepsinogen II.
Owner:深圳市蔚景生物科技有限公司
Chemiluminiscence detection kit for diagnosing gastropathy in combined manner and preparation and application method thereof
The invention discloses a chemiluminiscence detection kit for diagnosing gastropathy in a combined manner. The detection kit is mainly used for performing pepsinogen I detection and pepsinogen II detection. On the basis of the detection kit, the invention further provides a marker combined standard value, which is applicable to the detection kit and can provide reference for gastropathy detection, for detection. The detection kit has the advantages that the detection kit is wide in linearity range, high in specificity, convenient to operate, and the like in terms of gastropathy diagnosis and early gastric cancer screening; in addition, the detection kit is accurate and detailed in gastropathy diagnosis and high in market popularization value, and damage, caused by X-rays, on human bodies and the inconvenience of a gastroscope are avoided.
Owner:山东康华生物医疗科技股份有限公司
Nile tilapia cholecystokinin and coding nucleic acid thereof and application of functional octopeptide
InactiveCN102241761AReduce manufacturing costEfficient use ofAnimal feeding stuffAccessory food factorsAmylasePepsinogen I
The invention provides Nile tilapia cholecystokinin and a nucleic acid sequence, a cloning method and application thereof. In the invention, the amino acid sequence for coding the Nile tilapia cholecystokinin is shown as SEQ ID NO:1. The invention also provides application of a CCK(Cholecystokinin)-8 octopeptide sequence in digestion ingestion, and the fact that the CCK-8 polypeptide can promote pyloric caecum to secrete amylase, trypsin and pepsinogen and promote hypothalamus to secrete NPY (neuropeptide Y) and orexin is discovered after being abdominally injected into tilapia. The invention provides a theoretical basis for clarifying an effect of CCK in a fish digestion and ingestion process and provides some gene resources for developing novel fish feed additives in the future.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com