Pepsinogen I and pepsinogen II combined detection kit and application thereof
A pepsinogen and combined detection technology, which is applied in the field of pepsinogen Ⅰ and pepsinogen Ⅱ combined detection kits, can solve the problems of time-consuming and laborious work, cumbersome work, and the inability to realize multi-index simultaneous detection, so as to shorten the time and reduce the The cost of testing and the effect of stable and reliable testing results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Pepsinogen I (PGI) and Pepsinogen II (PGII) Combined Detection Kit
[0051] 1. Pepsinogen Ⅰ (PG Ⅰ) and Pepsinogen Ⅱ (PG Ⅱ) combined detection kit consists of the following components:
[0052] Magnetic beads coated with anti-PGⅠ monoclonal antibody (PGⅠ8015 antibody) and magnetic beads coated with anti-PGⅡ monoclonal antibody (PGⅡ8101 antibody);
[0053] Rare earth element thulium (Tm) labeled anti-PGⅠ monoclonal antibody (PGⅠ8003 antibody) and rare earth element lutetium (Lu) labeled anti-PGⅡ monoclonal antibody (PGⅡ8103 antibody);
[0054] PG composite calibrator;
[0055] PG composite quality control;
[0056] Dissociation solution;
[0057] Immunowashing solution.
[0058] The preparation method of above-mentioned each component is as follows:
[0059] 1. The preparation method of magnetic beads coated with anti-PGⅠ monoclonal antibody (PGⅠ8015 antibody) and magnetic beads coated with anti-PGⅡ monoclonal antibody (PGⅡ8101 antibody), comprising the fol...
Embodiment 2
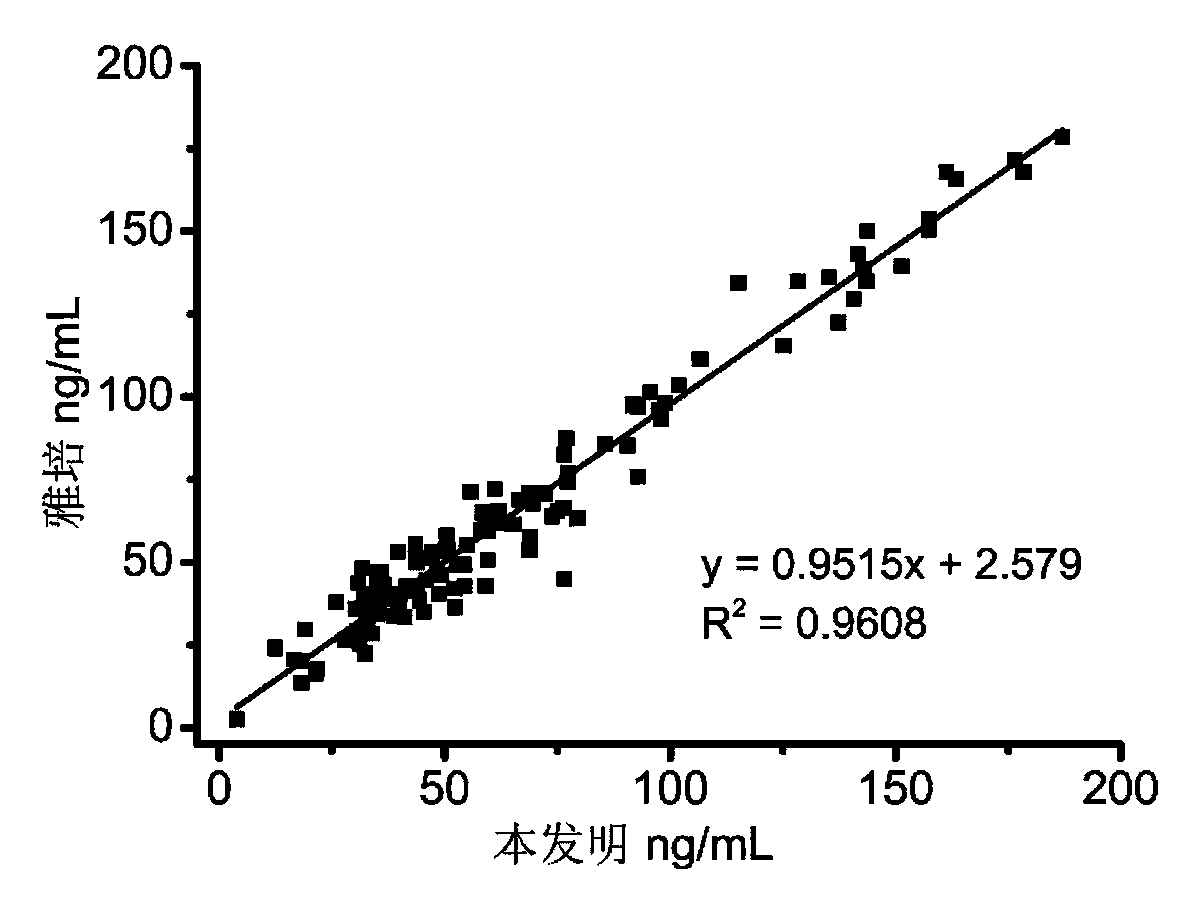
[0103] Example 2 Performance testing and application of pepsinogen Ⅰ (PGⅠ) and pepsinogen Ⅱ (PGⅡ) combined detection kit
[0104] 1. Performance indicators
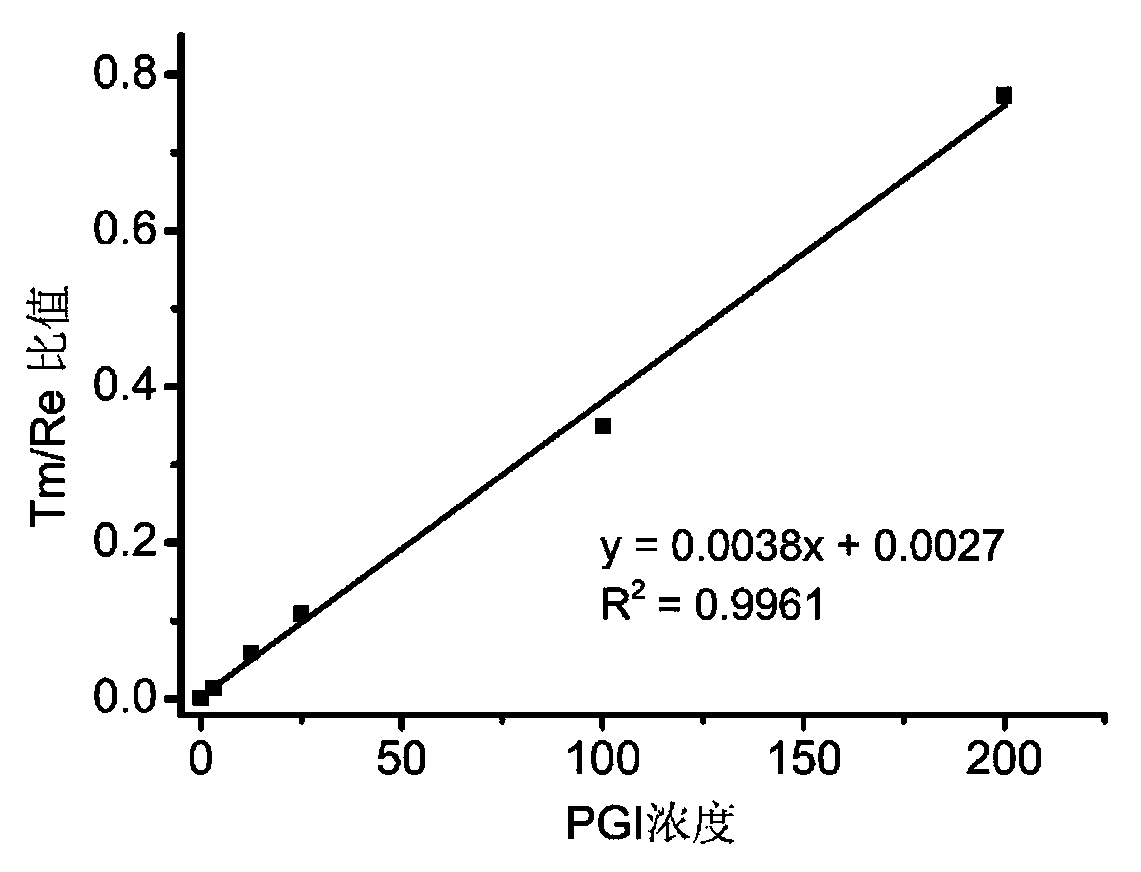
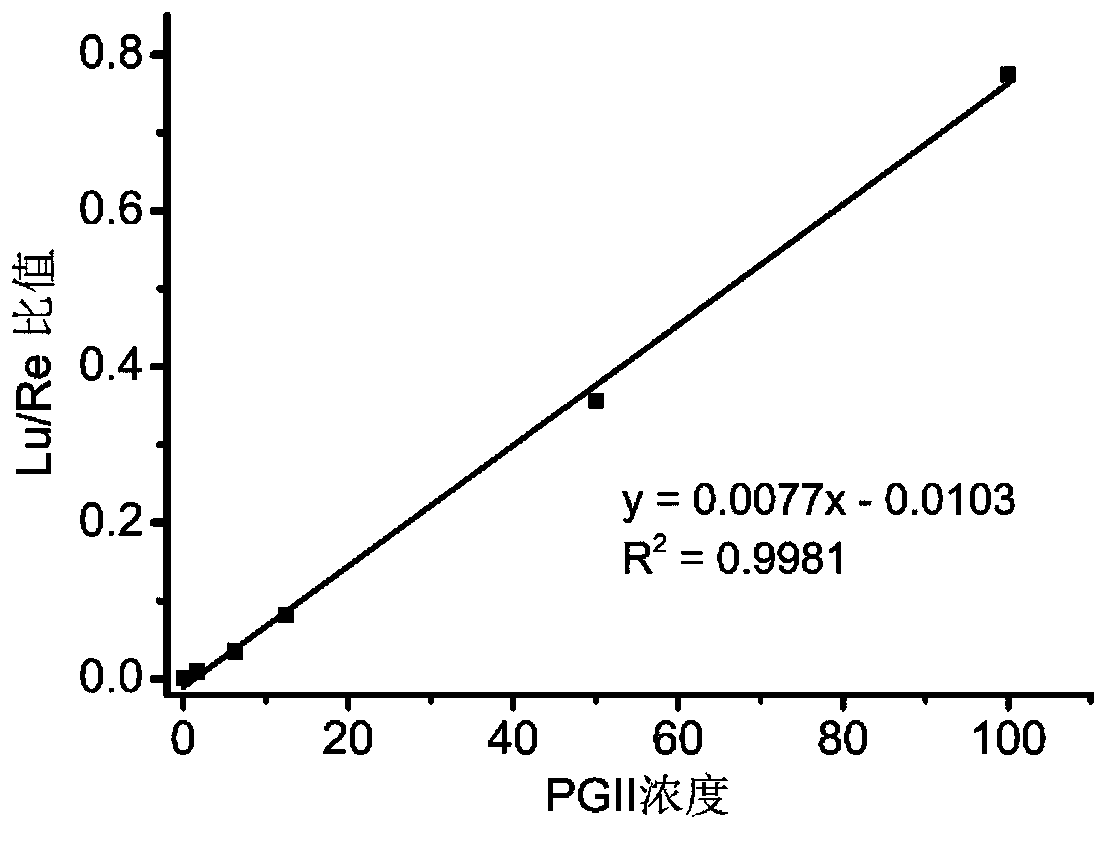
[0105] 1. Minimum detection limit
[0106] According to "step B in the step 2 in embodiment 1) detection test sample method", detect with zero concentration PG composite calibrator solution (i.e. PG composite calibrator solution A in the embodiment) as the sample to be tested, repeat detection 20 times, Obtain the response value of the 20 detection results-the corresponding ion count value per second (cps), calculate the mean (M) and standard deviation (SD), and obtain M+2SD. The adjacent concentration PG composite calibrator solution (i.e. PG composite calibrator solution B) is used as the sample to be tested for detection, the detection is repeated 3 times, and the average value is taken to obtain the response value-corresponding ion counts per second (cps).
[0107] According to the concentration-cps results between ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com