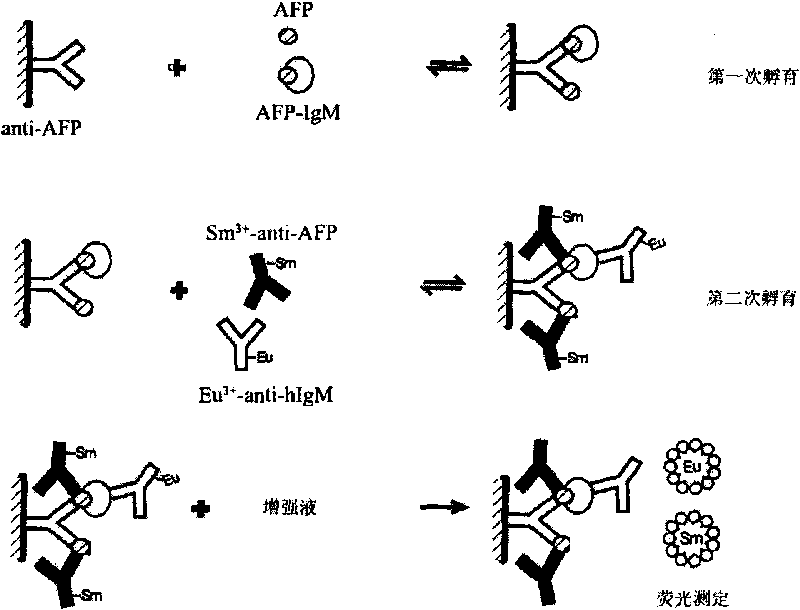Patents
Literature
144 results about "Time resolved fluorescence immunoassay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
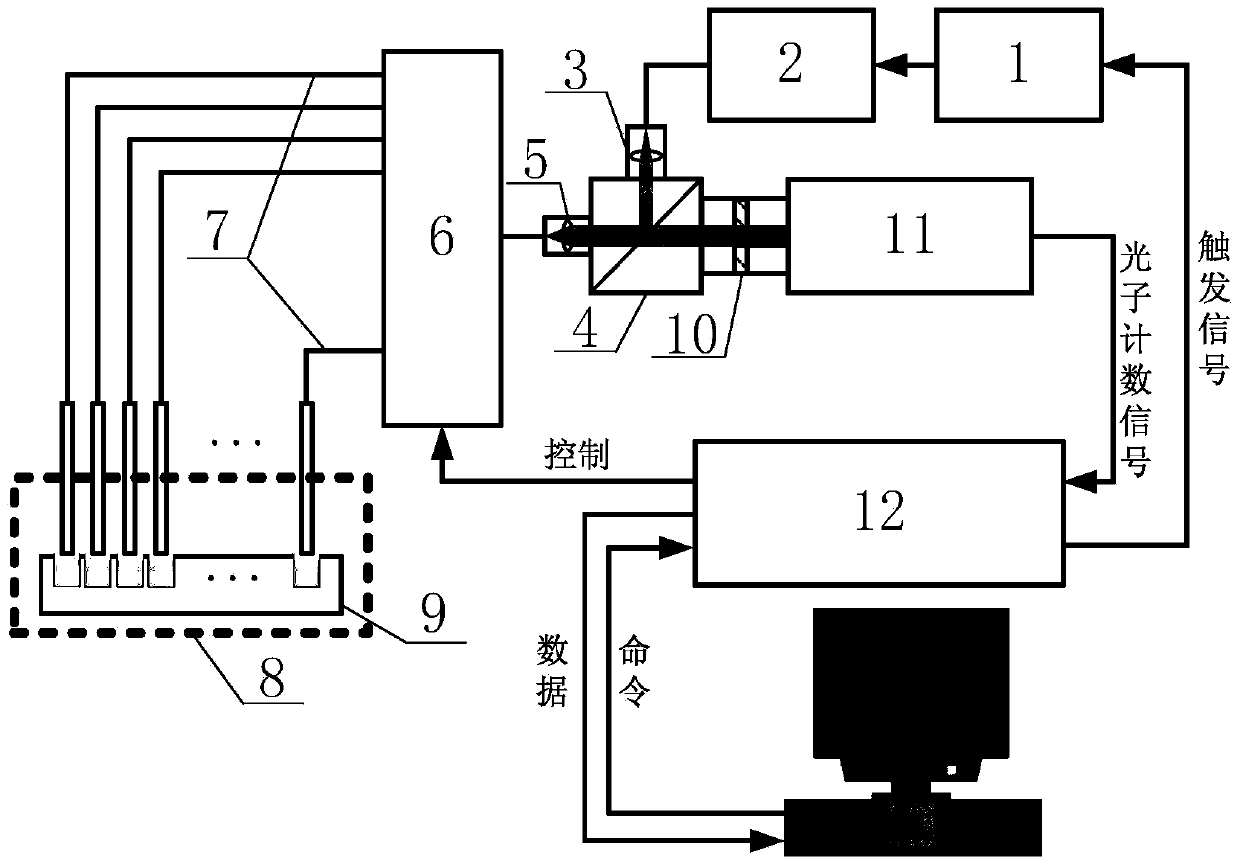
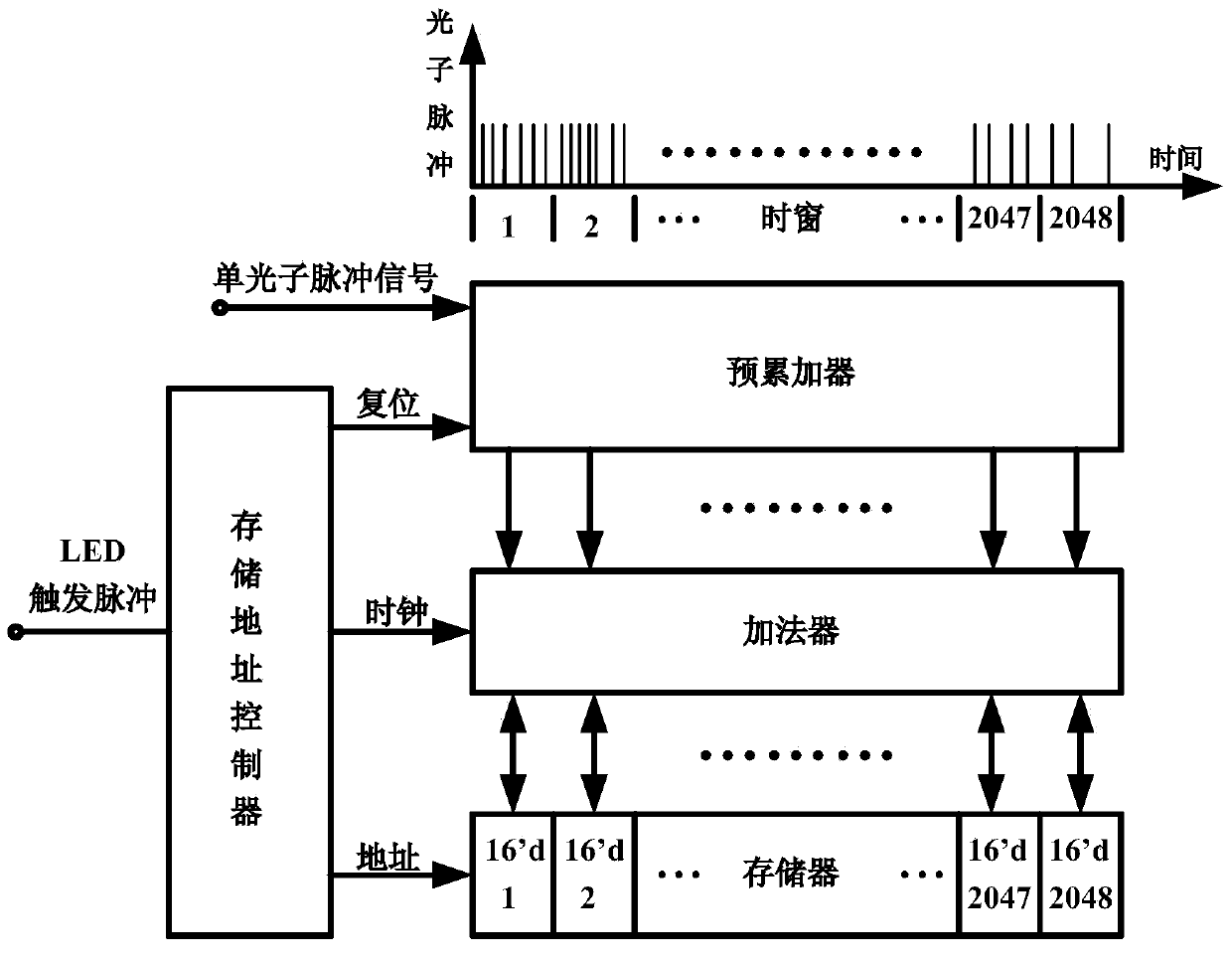
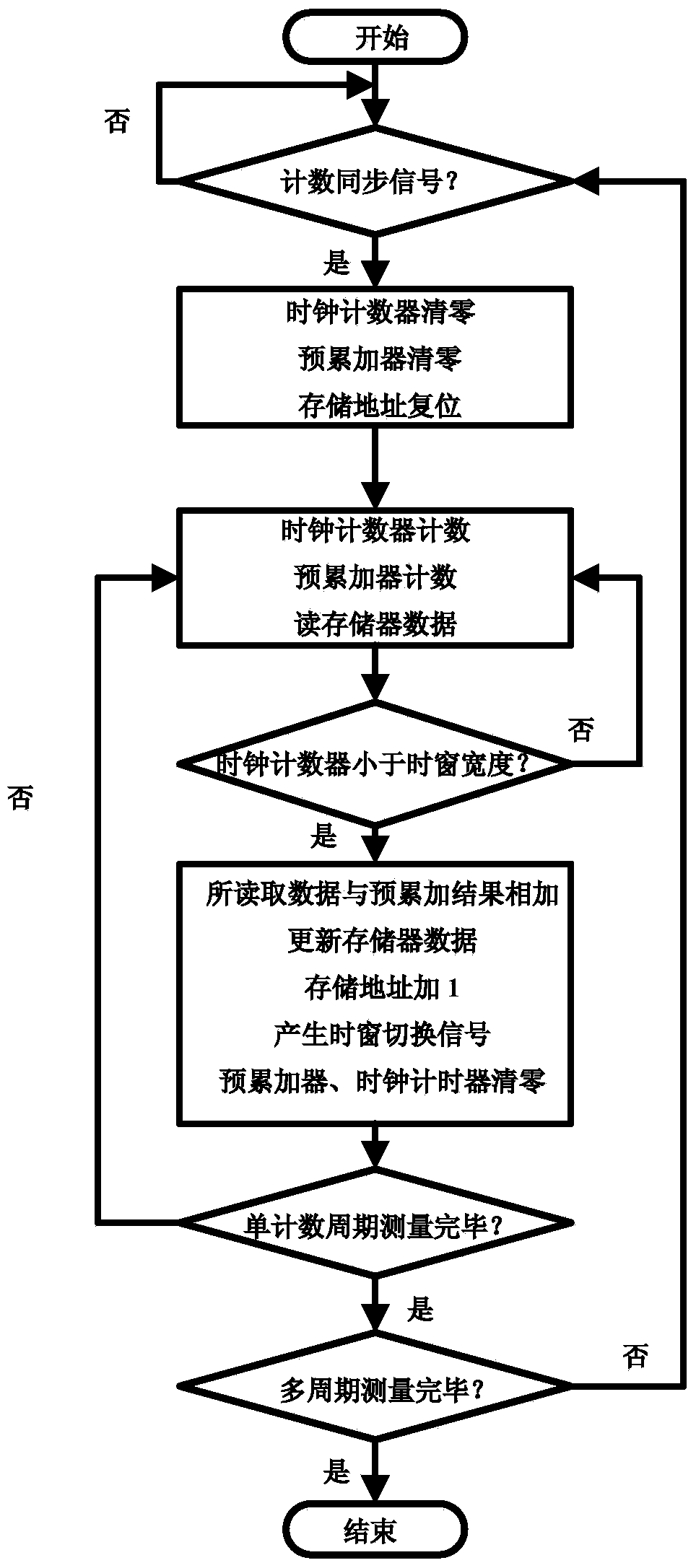
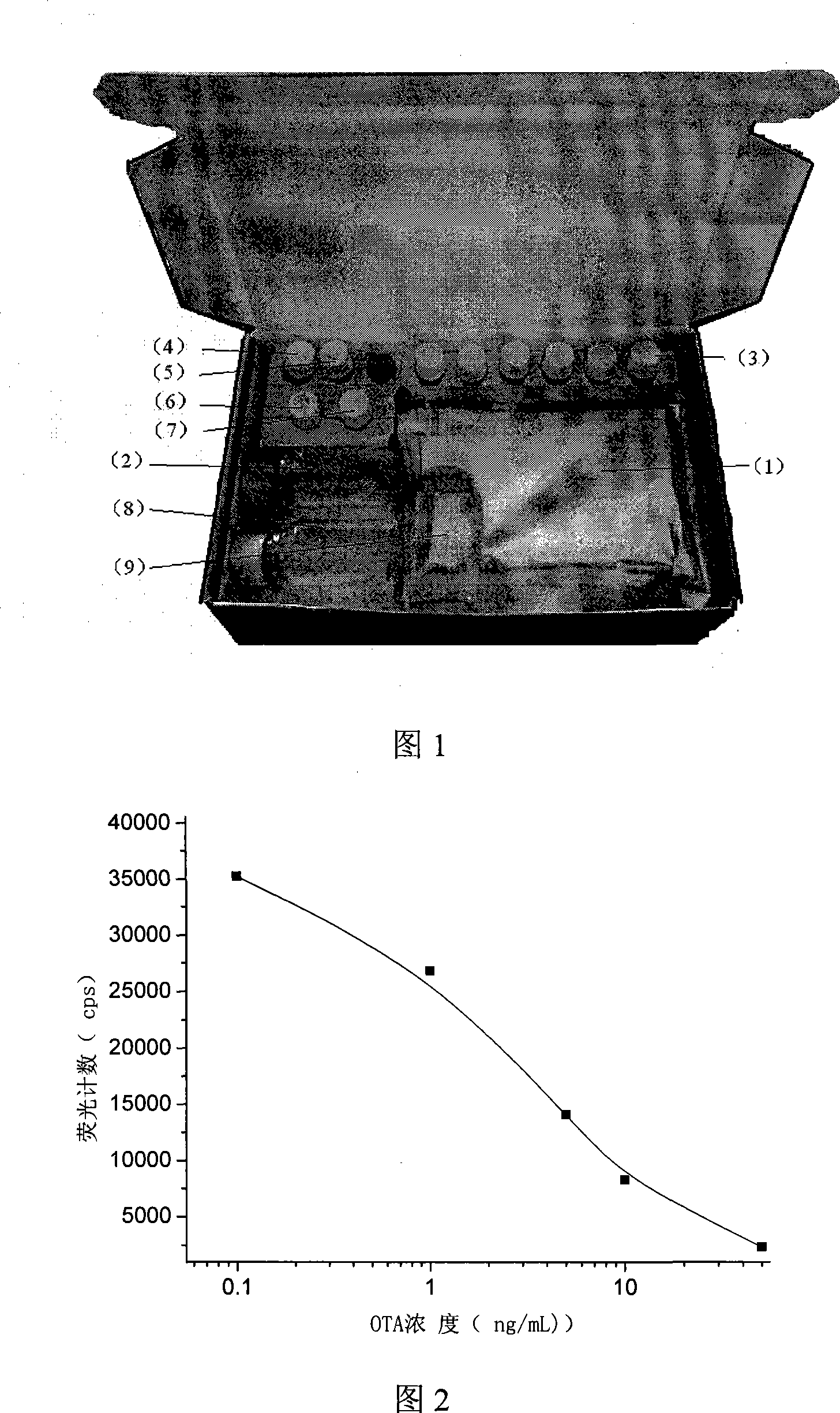
Photon counting multi-channel time-resolved fluorescence immunoassay system and counting method
InactiveCN103728446AAvoid interferenceEasy to assembleBiological testingFluorescence/phosphorescenceTime resolved fluorescence immunoassayTime mark
The invention belongs to the technical field of fluorescence immunoassay and relates to a photon counting multi-channel time-resolved fluorescence immunoassay system which comprises a light source module, a detection module and a time marked photon counting analysis / control module. At the same time, the invention relates to a counting method adopted by the system. The counting method of exciting a sample to be detected with pulse light, sending a fluorescence signal generated by the sample after excitation into a PMT (Photo Multiplier Tube) photon counting head and outputting a pulse signal comprises the following steps of taking cycles of the excitation pulse light as counting cycles, dividing each counting cycle into a plurality of time intervals with the same width, accumulating photons detected after sending excitation light pulses of each counting cycle into a memory corresponding to a corresponding time window, that is marking the positions of the photons in the pulse cycles with the intervals where the time windows are located, and uploading data in the memory into an upper computer after counting for the cycles. The multi-channel time-resolved fluorescence immunoassay system is low in cost and has very high sensitivity.
Owner:TIANJIN UNIV
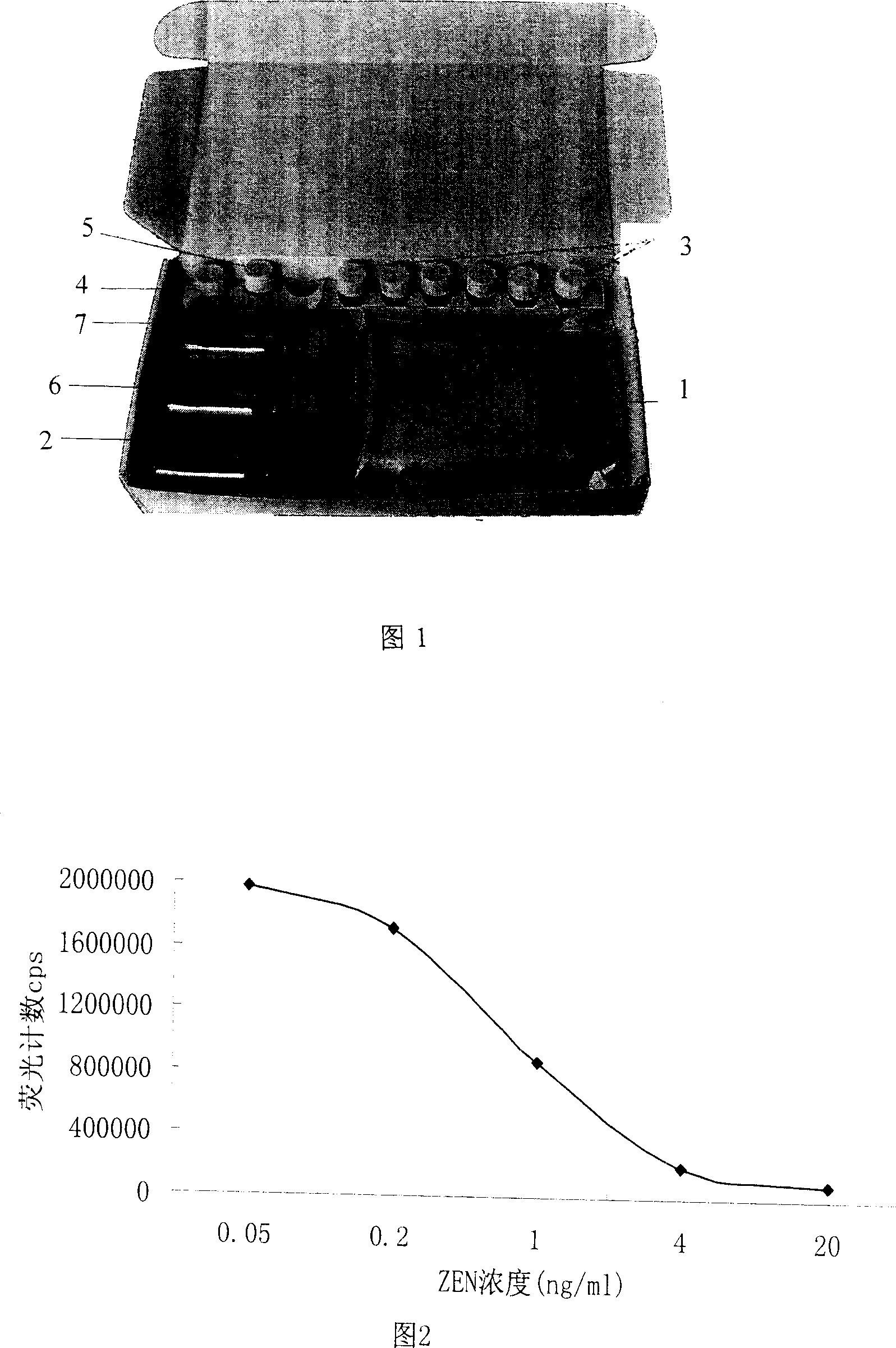
Reagent kit for testing corn zeranol and testing method thereof
ActiveCN1963506ASimple structureEasy to useColor/spectral properties measurementsTime resolved fluorescence immunoassayUltimate tensile strength
This invention relates to one agent case and its test method for corn zeranol, which is based on label immune reaction, wherein, the micro hole board comprises ZEN-BSA added with ZEN antibody; the free ZEN and micro board ZEN-BSA competes for ZEN antibody and the free ZEN antibody is removed with labeled sheep antipest antibody; the labeled immune reaction labeled antibody is removed; after adding strengthen liquid it uses time resolution fluorescence device to test the intensity cps with its concentration reverse to ZEN concentration to determine the ZEN content by comparing standard curve and sample.
Owner:无锡市江原实业技贸有限公司
Reagent box for detecting clenbuterol hydrochloride and its detection method
InactiveCN1793927ASimple structureEasy to useChemiluminescene/bioluminescenceTime resolved fluorescence immunoassayBiology
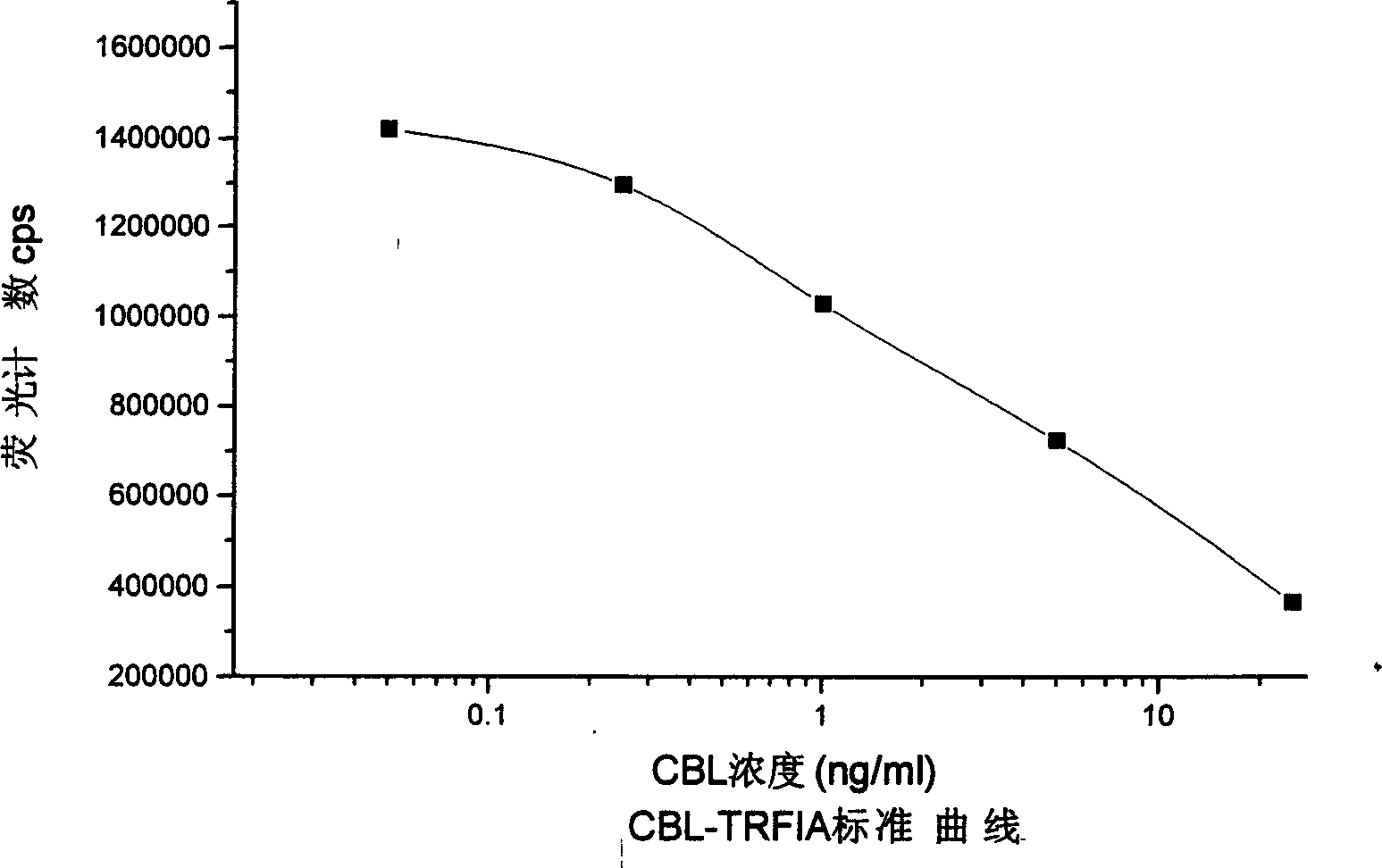
A method for detecting CBL includes competing CBL antibody by tree CBL with CBL ¿C OVA on micro hole board, washing off unconnected CBL antibody, adding EU3+ - sheep resisting rabbit antibody and washing off unconnected EU3+ - sheep resisting rabbit antibody, adding intensified liquid and using time ¿C identification luminoscope to determine its fluorescence intensity, presenting fluorescence intensity to CBL concentration in sample to be inverse ratio, comparing with standard curve to obtain CBL content in sample. The reagent kit for realizing said method is also disclosed.
Owner:JIANGNAN UNIV
Detection reagent kit and detection method for diethyl stilbestrol
InactiveCN101482562ASimple structureEasy to useMaterial analysisTime resolved fluorescence immunoassayCarrier protein
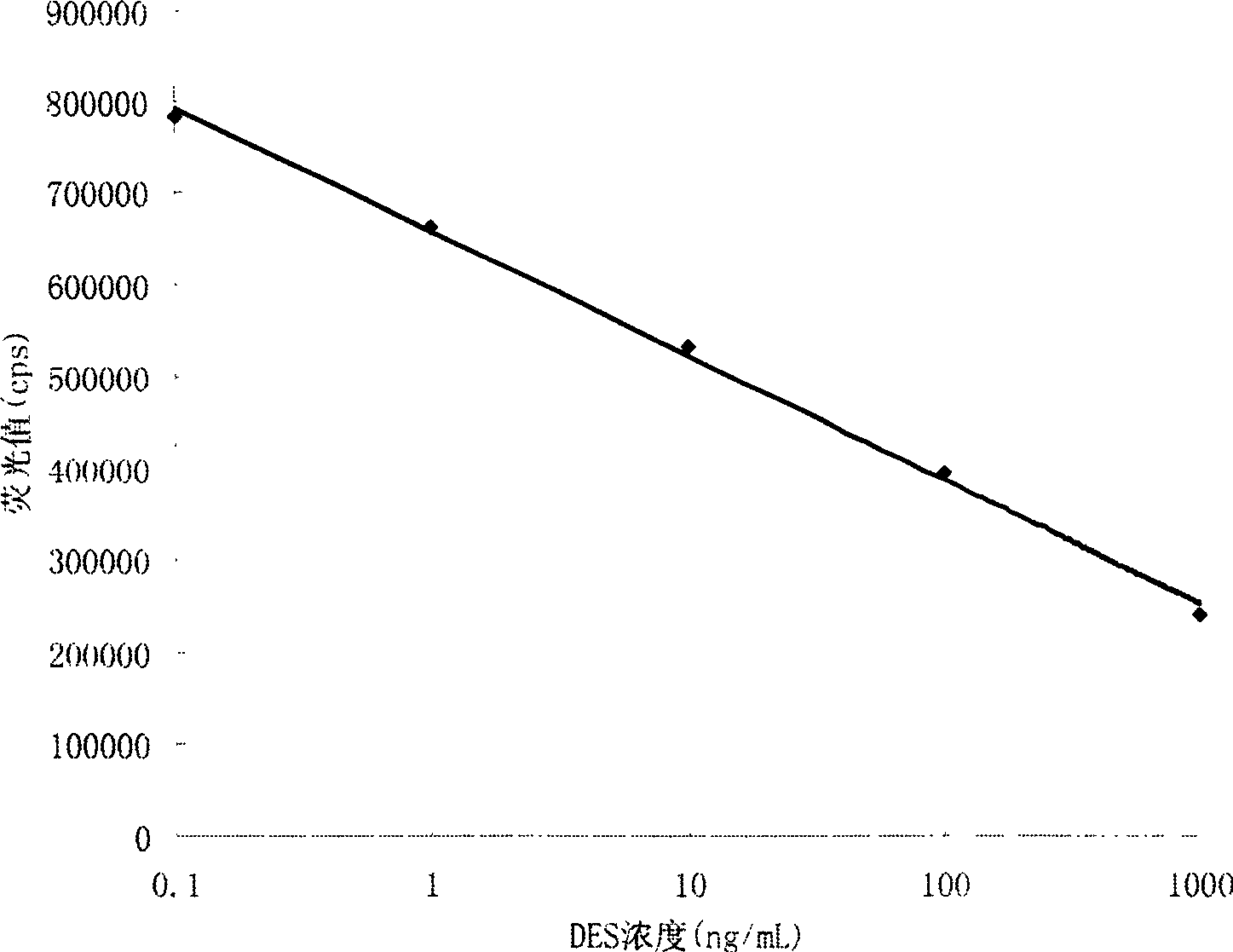
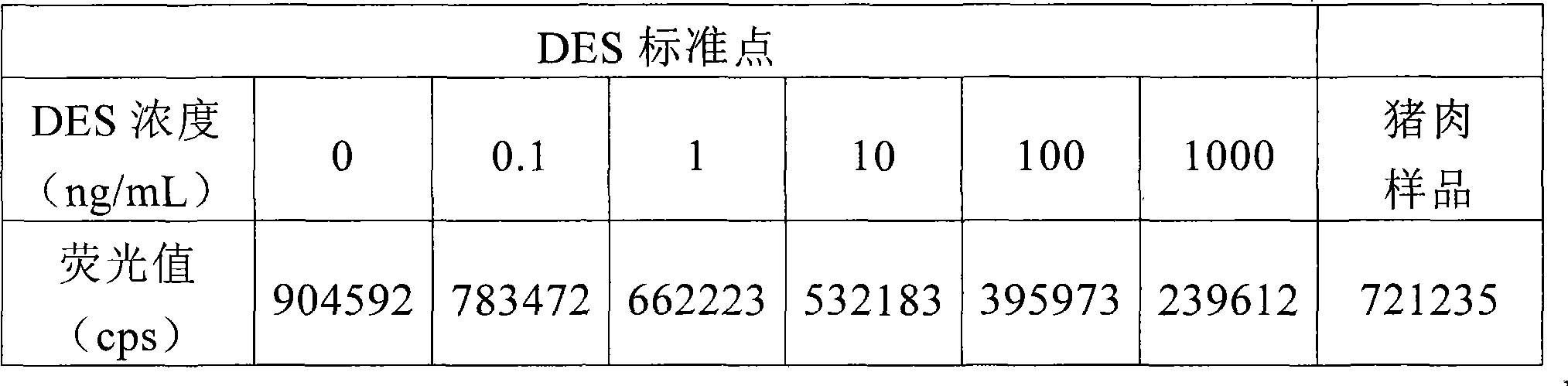
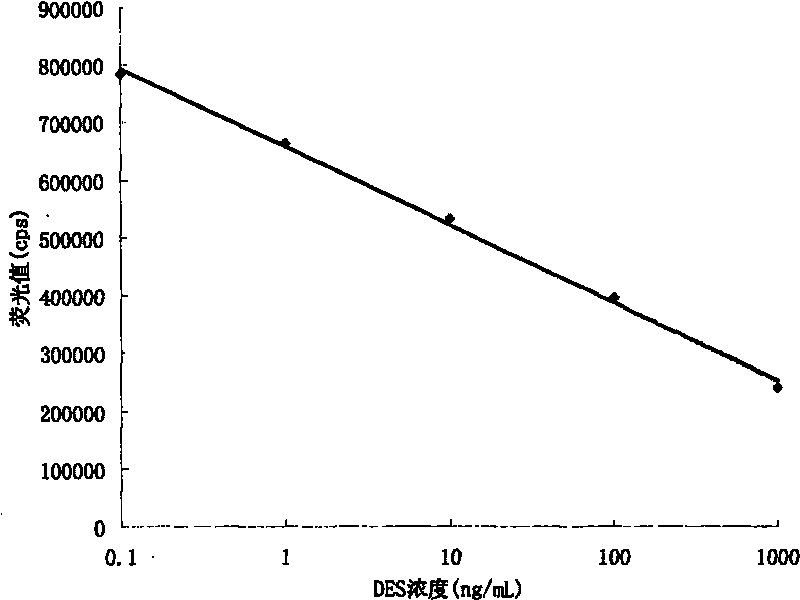
The invention provides a kit for detecting diethylstilbertrol and detection method thereof, belonging the the time resolution fluoroimmunoassay (TRFIA) technical field, capable of detecting the content of diethylstilbestrol (DES) in animal-source food, blood, urine and feedstuff. The kit uses the TRFIA to detect the DES and the mark immune response is the base of the detection. A micropore plate is coated by DES-carrier protein and added with DES standard or sample and then added with DES antibody. The free DES and the DES-carrier protein on the micropore plate complete the DES antibody and the DES antibody which is not bonded with the free DES or the DES-carrier protein is cleared away and added with the EU3+-goat anti-rabbit antibody, after labeling the immune reaction, the non-bonded EU3+-goat anti-rabbit antibody is cleared away. After adding the reinforcement liquid, the fluorescence intensity cps is detected using a time resolution fluorescence instrument and the fluorescence intensity is inversely proportional to the DES concentration in sample and the content of DES in sample can be determined according to the standard curve. The DES detection kit has features of simple structure, convenient use, cheapness, high sensitivity up to 1ng / mL.
Owner:JIANSGU INST OF MICROBIOLOGY
Protein chip reagent kit and method for comprehensively detecting lung cancer marker
InactiveCN103033619AReduce detection errorImprove accuracyFluorescence/phosphorescenceSquamous CarcinomasAntibody antigen reactions
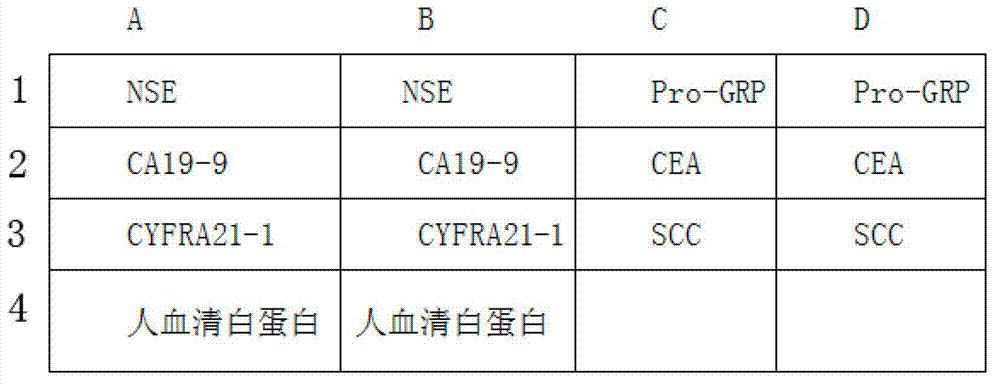
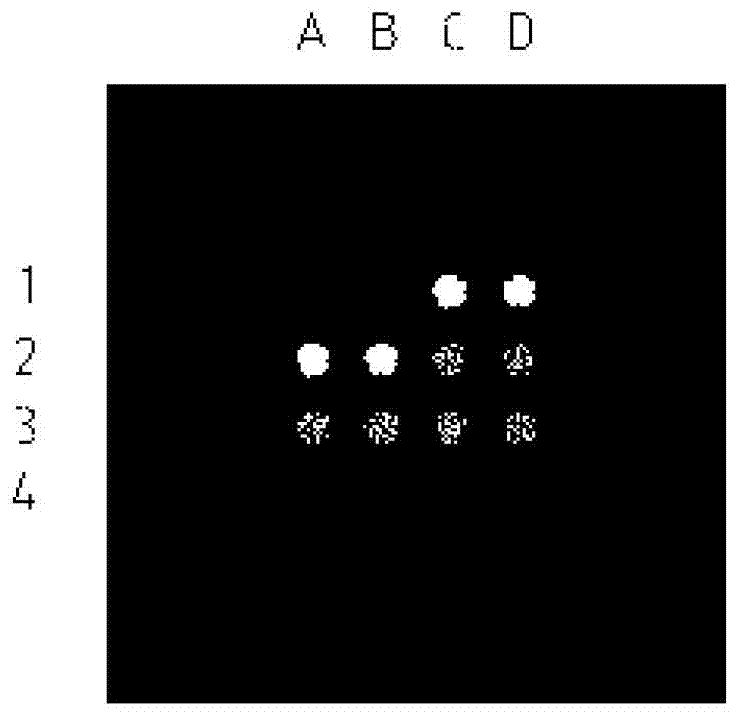
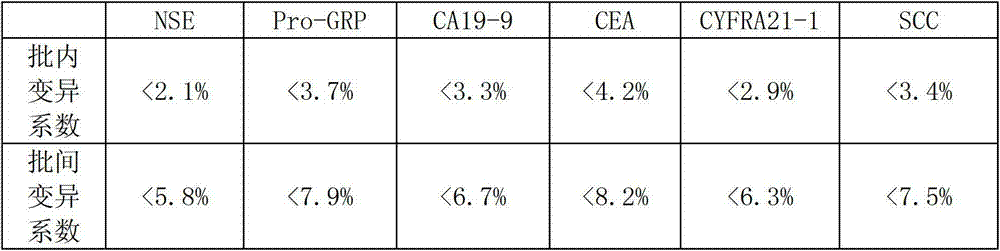
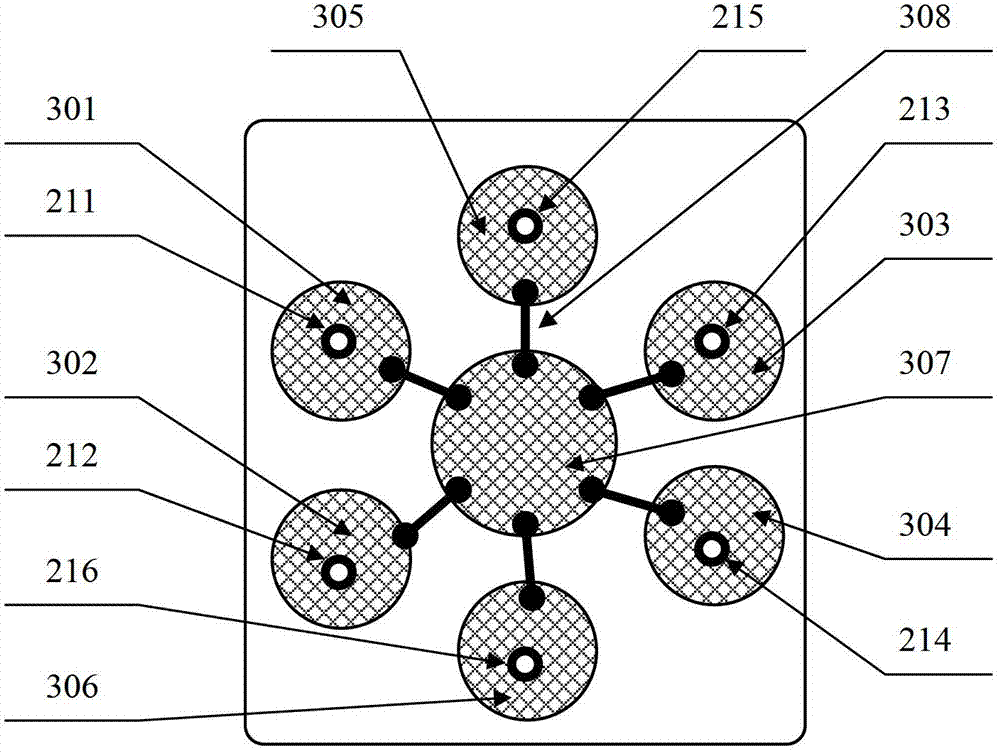
The invention belongs to the technical field of biology and relates to a protein chip reagent kit and a method for comprehensively detecting a lung cancer marker. The protein chip reagent kit for comprehensively detecting the lung cancer marker comprises: (1) a chip (1) and (2) a reaction agent and a detection agent, wherein a plurality of specific antibodies are simultaneously fixed on the chip, can generate antibody-antigen reaction with the lung cancer marker and are fixed on a bottom film of the chip to form a plurality of independent recognition sites; and the reaction agent and the detection agent are used for detecting whether a matter capable of generating antibody-antigen reaction with the specific antibodies exists in a sample to be detected or not through a TRFIA (Time Resolved Fluorescence Immunoassay) method. The reagent kit and the method have the beneficial effects that the six indexes of NSE (Neuron Specific Enolase), SCC (Squamous Cell Carcinoma), CEA (Carcino Embryonie Antigen), CA (Carbonic Anhydrase) 19-9, CYFR (Cytokeratin Fragment) A21-1 and pro-GRP (Glass Reinforced Plastic) can be simultaneously detected, the detection accuracy is improved, the repetitive experimental steps are reduced, and the time and the cost are saved.
Owner:河南生生医疗器械有限公司
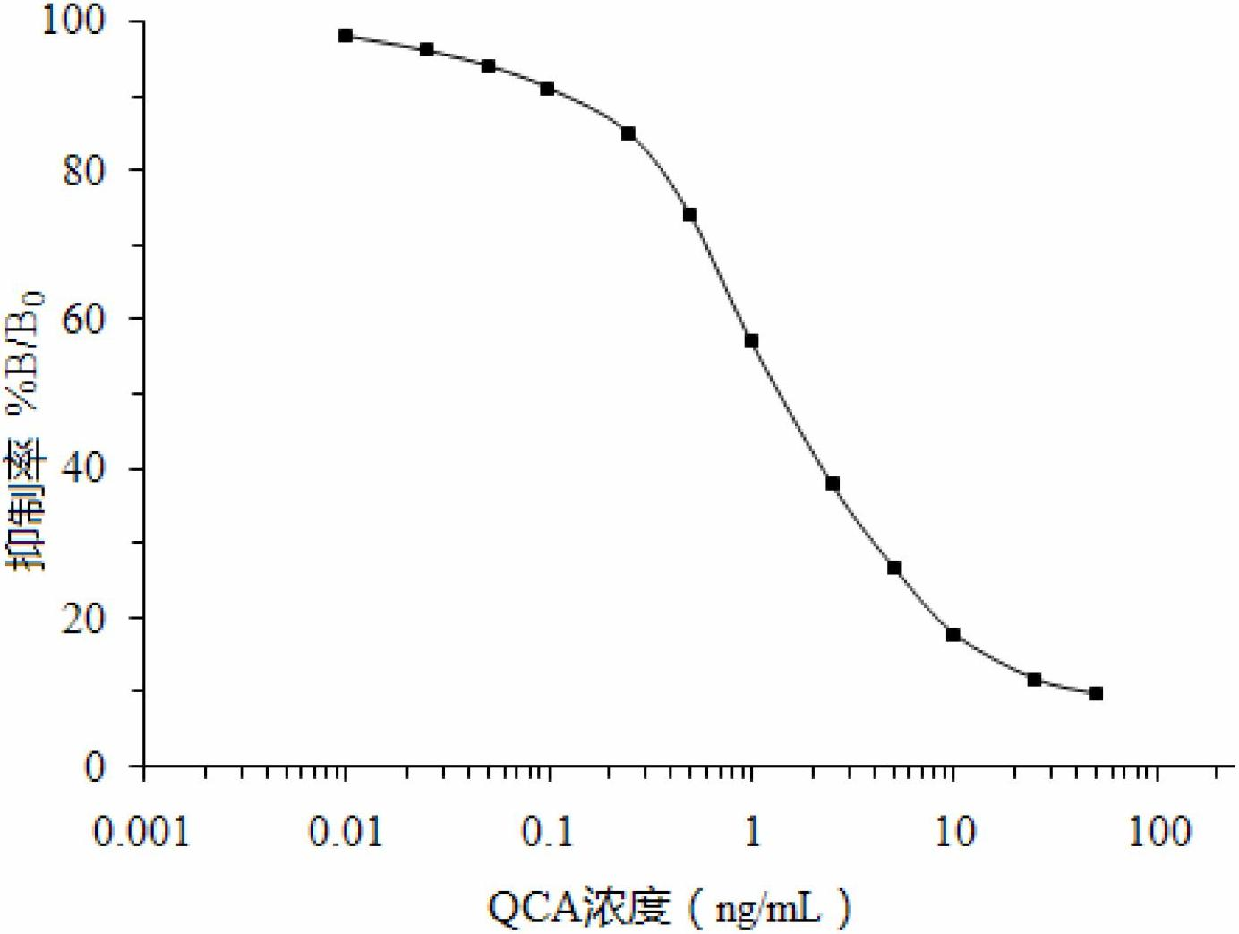
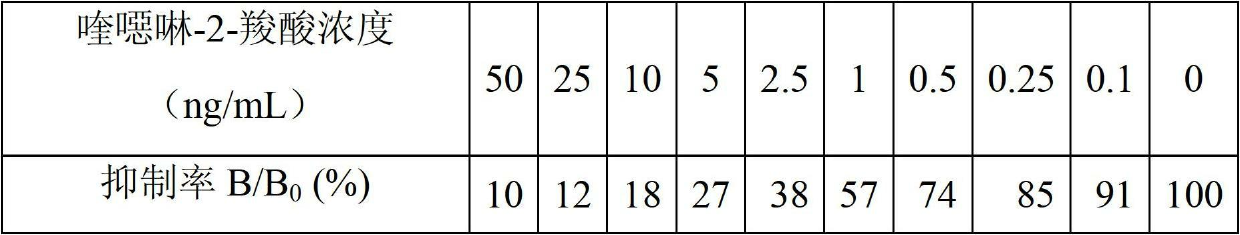
Detecting reagent kit used for detecting quinoxalinone-2-carboxylic acid and method
InactiveCN102654500AEasy to handleDetection suitable forMaterial analysisFreeze-dryingCarboxylic acid
The invention discloses a time-resolved fluorescence immunoassay reagent kit which is used for detecting quinoxalinone-2-carboxylic acid and a detection method of the time-resolved fluorescence immunoassay reagent kit. The reagent kit comprises a porous coated plate, a buffer solution, quinoxalinone-2-carboxylic acid standard, freeze-dried antibodies of quinoxalinone-2-carboxylic acid, europium-marked goat anti mouse antibodies, a cleaning solution and an enhancement solution. By measuring fluorescence intensity of counts per second (cps), the content of quinoxalinone-2-carboxylic acid in samples is calculated by a standard curve. The kit provided by the invention has the advantages of simple structure, easiness in assembly, corrosion resistance, light weight, low cost, wide application and the like. A valve core has good centering performance and can move flexibly and reliably and can play a good water-plugging role.
Owner:CHONGQING ACADEMY OF SCI & TECH
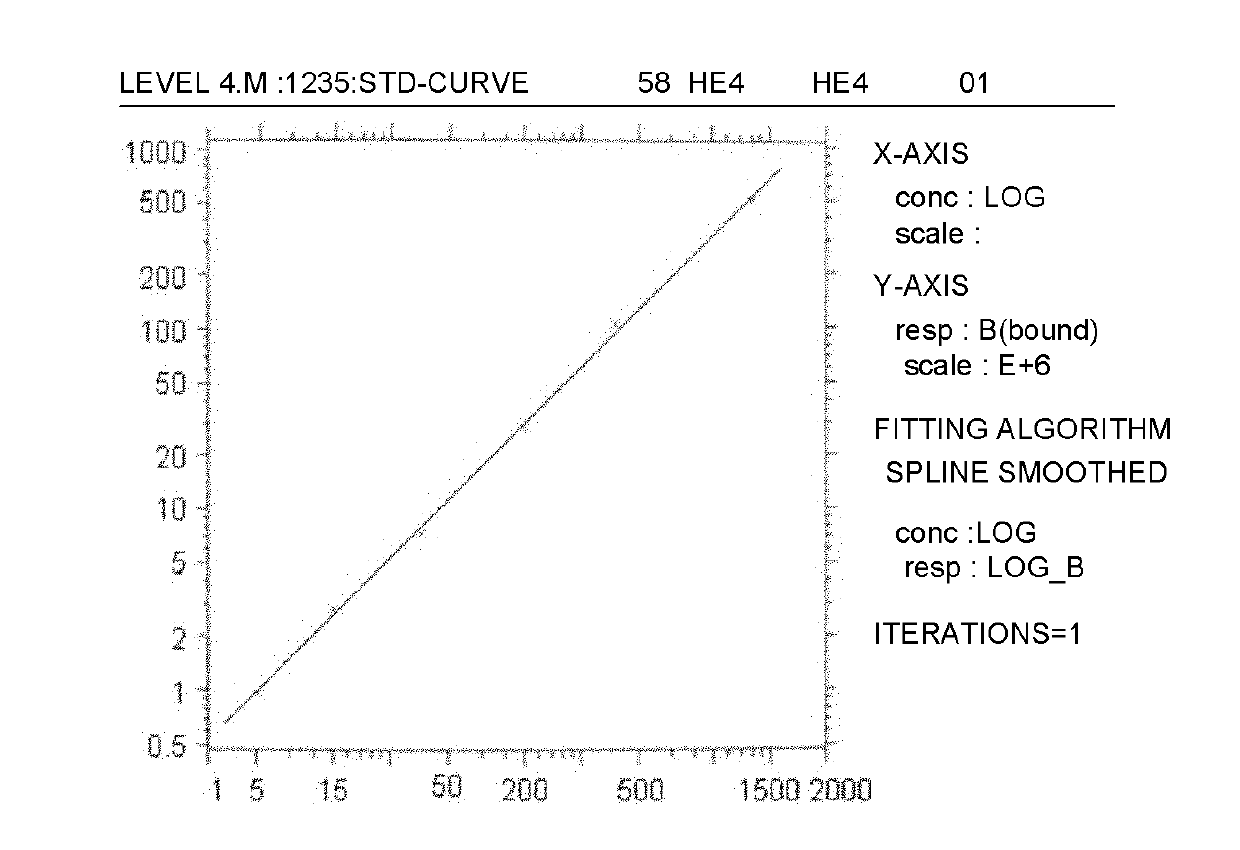
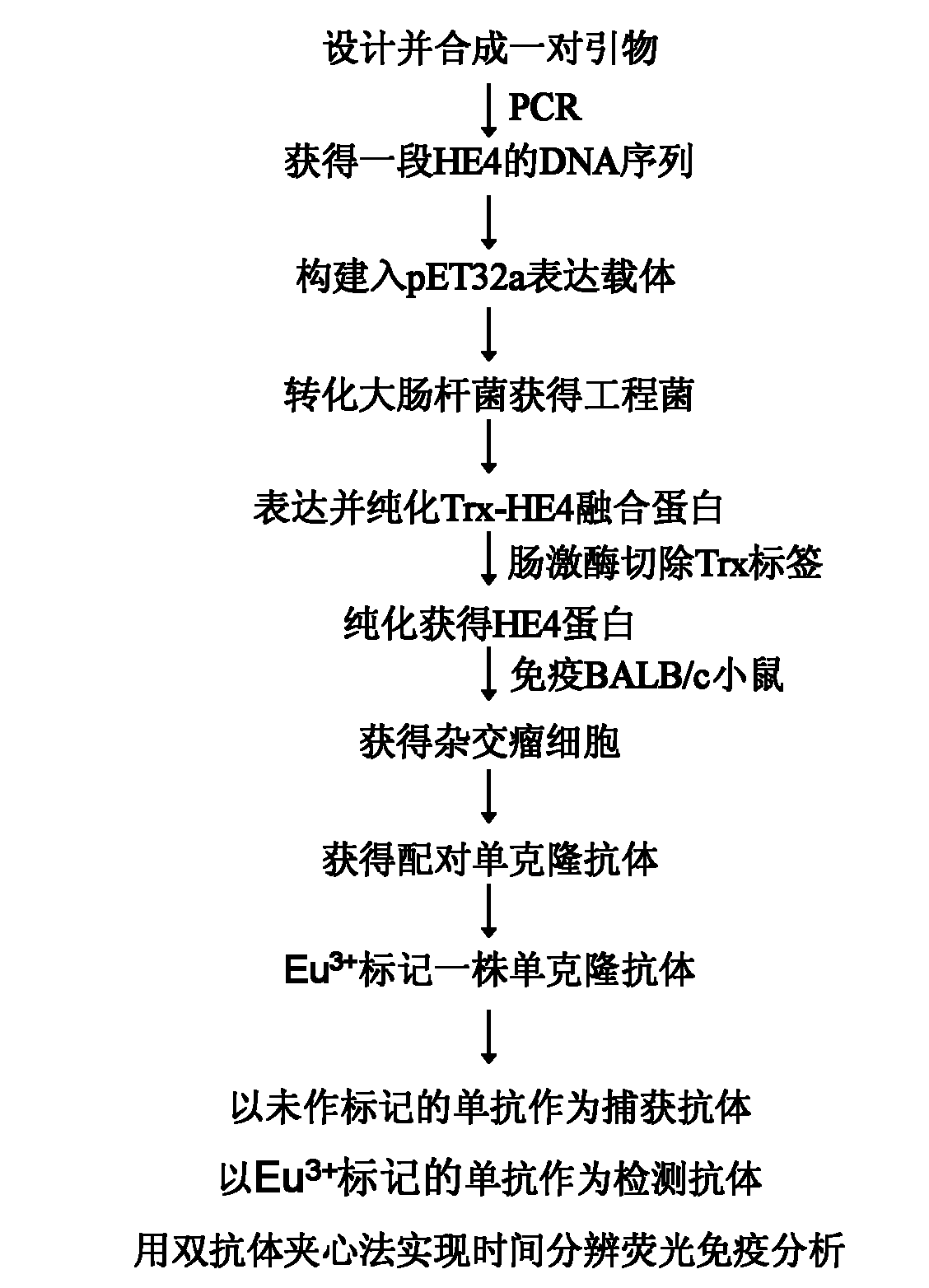
Time-resolved fluorescence (TRF) immunized detection kit of ovarian cancer tumor marker HE4
InactiveCN101949937AImmunoglobulins against animals/humansBiological testingRare-earth elementBALB/c
The invention relates to an analysis and detection technology of a human ovarian cancer tumor marker (human epididymis protein 4, HE4), in particular to a time-resolved fluorescence (TRF) immunized detection method and a kit of HE4, which is used for clinical auxiliary diagnosis, curative effect observation and prognosis judgment of the ovarian cancer. The invention comprises the following contents: constructing HE4 recombinant plasmids; expressing and purifying HE4 protein; immunizing BALB / c mice by the purified HE4 protein to prepare monoclonal antibodies; matching the obtained monoclonal antibodies to obtain two hybrid tumor cell strains (5A3 and 6C2) for secreting monoclonal antibodies of different epitopes of HE4 antigens, and marking the 5A3 monoclonal antibodies by the rare earth element Eu3+; and taking the unmarked 6C2 monoclonal antibodies as capture antibodies for coating a solid phase carrier, and taking the 5A3 monoclonal antibodies marked with the Eu3+ as detection antibodies to establish a double-antibody sandwich method for detecting HE4, thereby realizing the TRF immunized analysis of the invention.
Owner:大连美亿德生物科技有限公司
Anti-AKR1B10 protein monoclonal antibody and applications thereof
ActiveCN104650234AImmunoglobulins against enzymesMaterial analysisAntigenTime resolved fluorescence immunoassay
The invention relates to the field of medical biotechnology, and particularly relates to anti-human tumor specific antigen ketoreductase 1B10 (AKR1B10) protein monoclonal antibody, and a time resolved fluorescence immunoassay (TRFIA) kit used for screening, diagnosis, efficacy judgment, prognosis evaluation or recurrence monitoring of cancers.
Owner:湖南莱拓福生物科技有限公司
Double-labeling time-resolved fluorescence immunoassay method and kit for HIV (human immunodeficiency virus) antibody and HIV-1p24 antigen
InactiveCN103869073ARealize traceabilityEase of evaluationBiological material analysisBiotin-streptavidin complexTime resolved fluorescence immunoassay
The invention discloses a double-labeling time-resolved fluorescence immunoassay method and kit for HIV (human immunodeficiency virus) antibody-HIV-1p24 antigen. The analytical method mainly comprises the steps of preparing a solid phase carrier coated with an HIV recombinant antigen and an HIV-1p24 monoclonal antibody simultaneously; preparing biotin-labeled HIV-1p24 monoclonal antibody; preparing lanthanide 1-labeled HIV recombinant antigen; preparing lanthanide 2-labeled streptavidin; adding a calibrator containing HIV standard antibody and HIV-1p24 standard antigen or a sample to be tested into the solid phase carrier coated with the antigen and the antibody, adding the biotin-labeled HIV-1p24 antibody, incubating, washing, then adding the lanthanide 1-labeled HIV antigen and the lanthanide 2-labeled streptavidin, incubating again, washing, and adding enhancement solution for fluorescence detection. The analytical method overcomes the difficulty that the antigen and the antibody cannot be distinguished in the existing joint detection for HIV antigen and antibody, realizes simultaneous and quantitative detection of the HIV antibody and the HIV-1p24 antigen and therefore shortens the window phase of HIV detection.
Owner:GUANGZHOU FENGHUA BIOENG
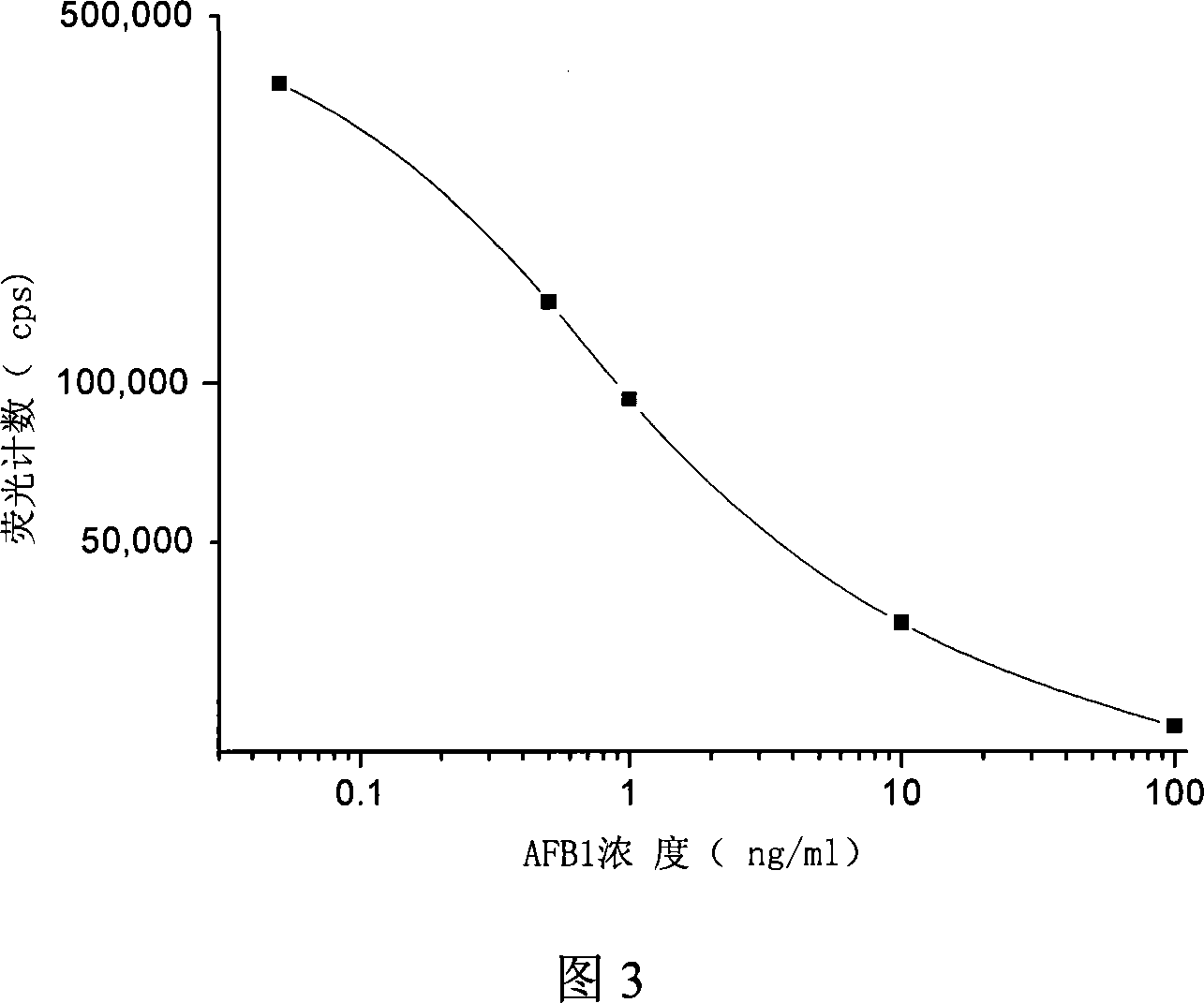
Reagent box and detection for ochracin A
InactiveCN1614422ASimple structureEasy to useFluorescence/phosphorescenceTime resolved fluorescence immunoassayAntibody
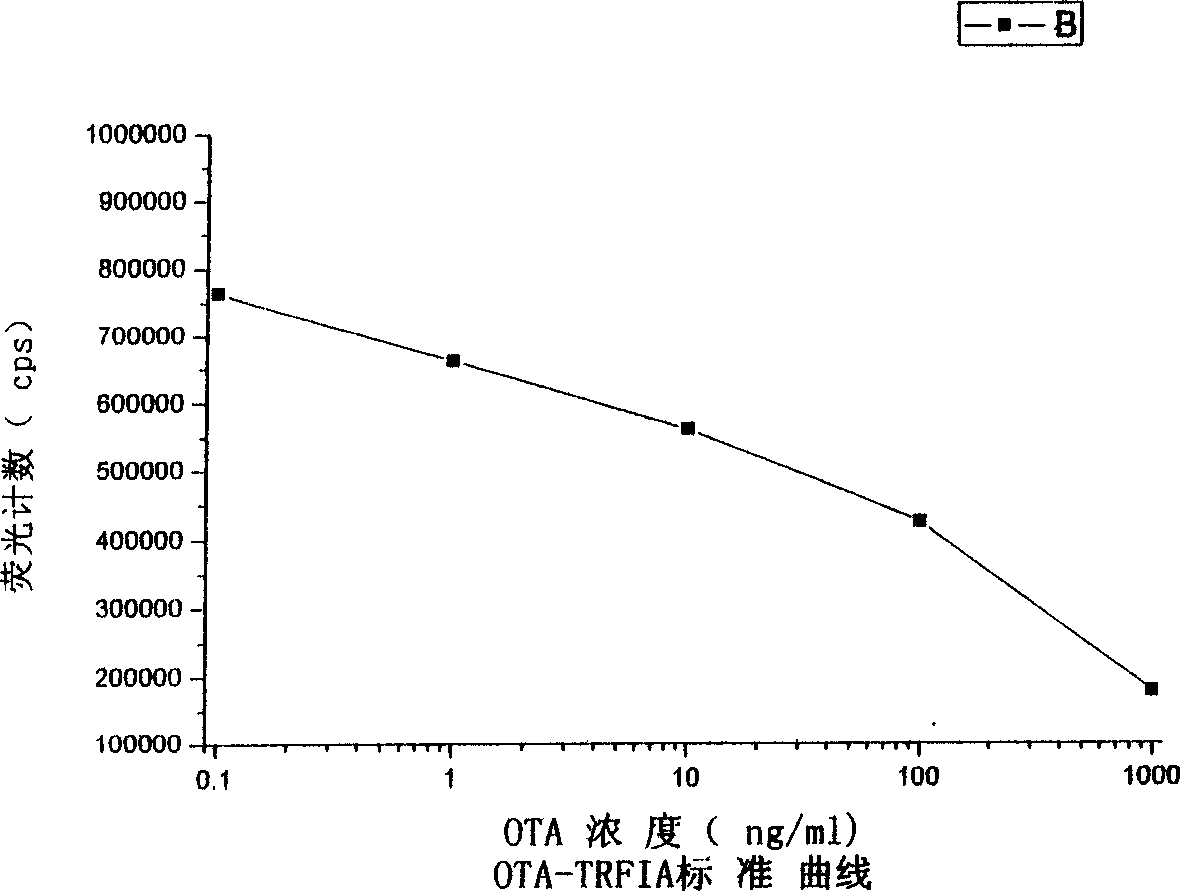
A detection method includes encapsulating microhole with OTA-BSA, adding OTA standard or sample and OTA antibody, washing out unconnected OTA antibody and EU3+ yangkangtu antiody, adding intensifier to determine fluorescent intensity CPS by time resolution fluorometer, presenting inverse ratio of fluorescent intensity to OTA concentration, comparing to standard curve for obtaining OTA concentration in sample. The kit applying TRFIA to detect OTA based on labelling immune reaction is also disclosed.
Owner:JIANGNAN UNIV
Time-resolved fluorescence immunoassay method fro detecting Dkk-1 and kit thereof
InactiveCN101398433AHigh speedImprove reliabilityBiological testingTime resolved fluorescence immunoassayFluorescence
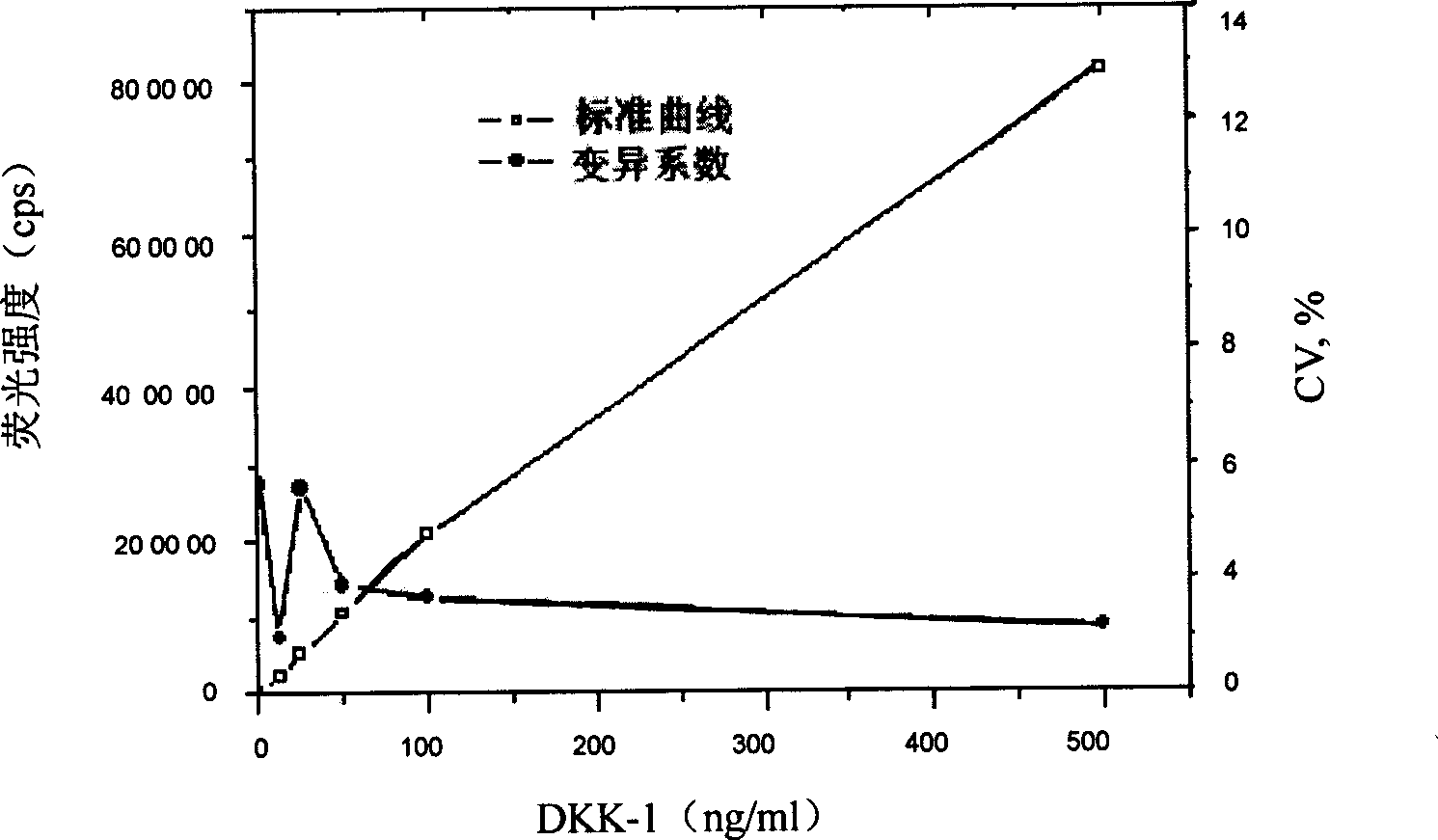
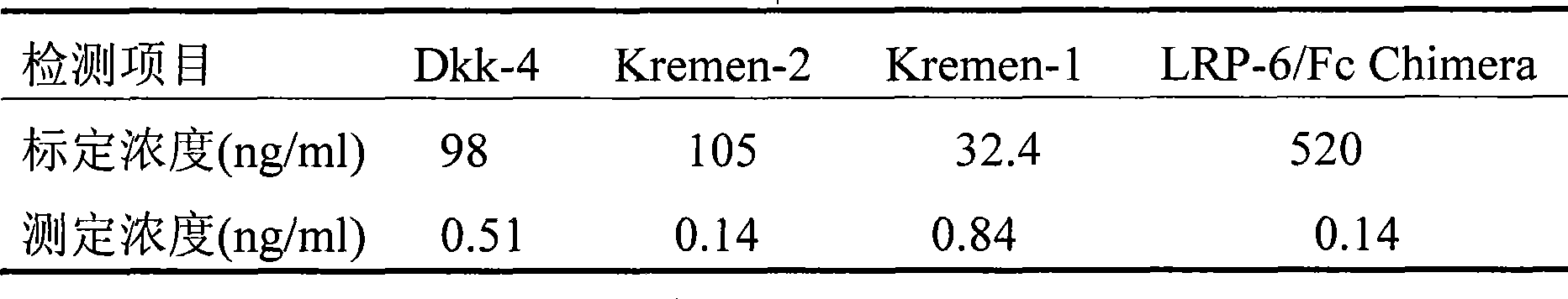
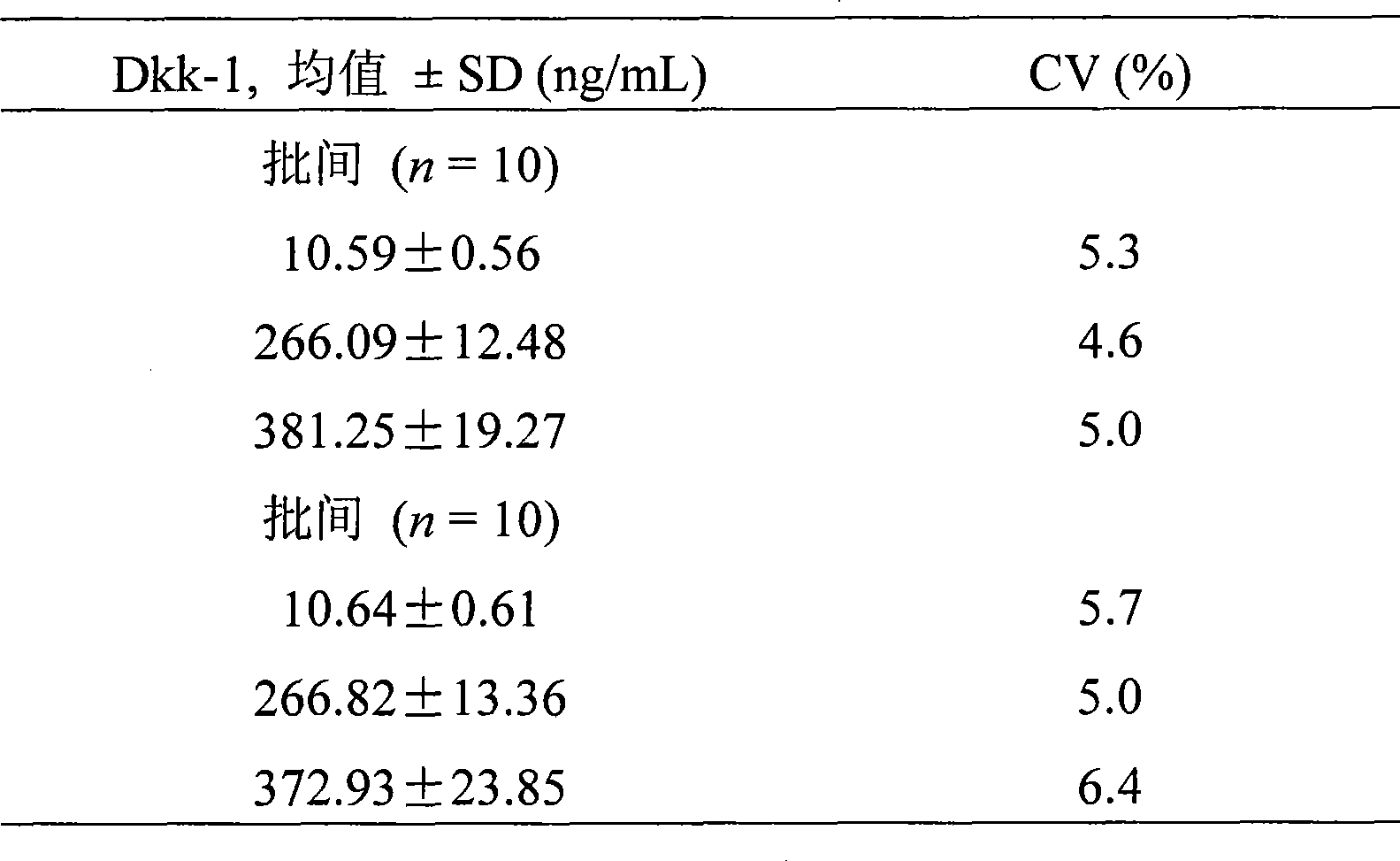
The invention discloses a time-resolved fluoroimmunoassay used for detecting Dkk1 and a kit thereof. The fluoroimmunoassay includes the following steps: 1. solid-phase anti-body preparing; 2) europium ion marker antibody preparing; and 3) the assay method: based on the double anti-body sandwiched immunoreaction. The invention has the advantages of relatively high sensitivity, specificity and stability on detection of Dkk1; the invention also has a supermatic analytical system which can not only enhance the speed of clinical test results, but also significantly reduce the personal errors and improve the reliability of the test results.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
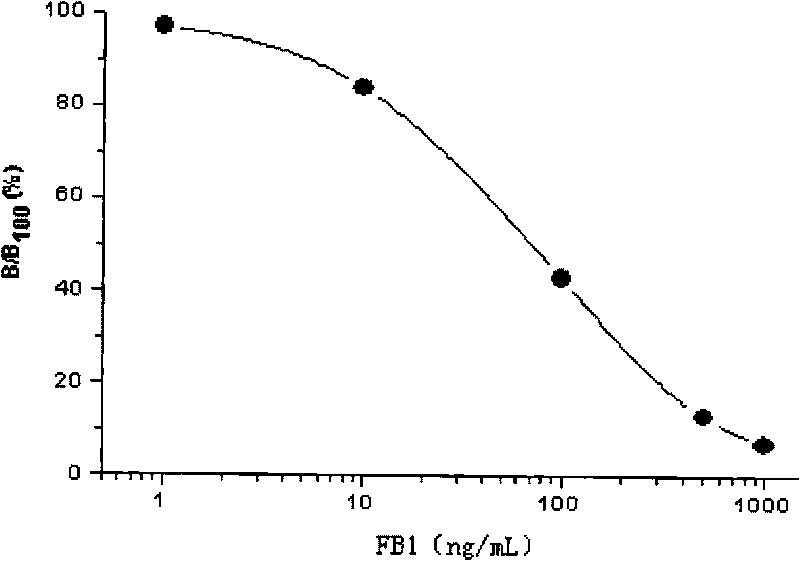
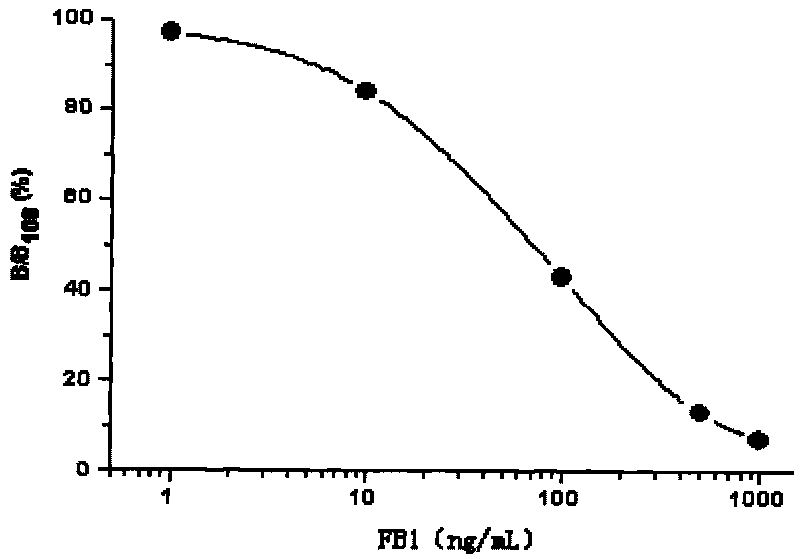
Time-resolved fluoroimmunoassay kit for detecting fumonisins B1 and detection method thereof
InactiveCN101699293ASimple structureEasy to useFluorescence/phosphorescenceTime resolved fluorescence immunoassayFumonisin B1
The invention discloses a time-resolved fluorescence immunoassay kit for detecting fumonisins B1 and a detection method thereof, belonging to the technical field of time-resolved fluoroimmunoassay (TRFIA) for detecting the content of FB1 in grain, fodder and food. The prepared kit adopts the TRFIA to detect the FB1. A micropore plate is coated with FB1-BSA, is added with FB1 standard or sample, and is added with FB1 monoclonal antibody. Dissociative FB1 and the FB1-BSA on the micropore plate compete for the FB1 monoclonal antibody, unconnected FB1 monoclonal antibody is removed by means of washing, Eu3+ -sheep anti-mouse antibody is added, and the unconnected Eu3+ -sheep anti-mouse antibody is removed by means of washing after labeled immune reaction. After adding strengthening liquid, fluorescence intensity (cps) is detected by a time-resolved fluoroimmunoassay apparatus, wherein the fluorescence intensity is in inverse proportion to the concentration of the FB1, and the content in the FB1 can be ensured by contrasting a standard curve. The kit has simple structure, convenient use, low price and high sensitivity more than 0.05ng / mL.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
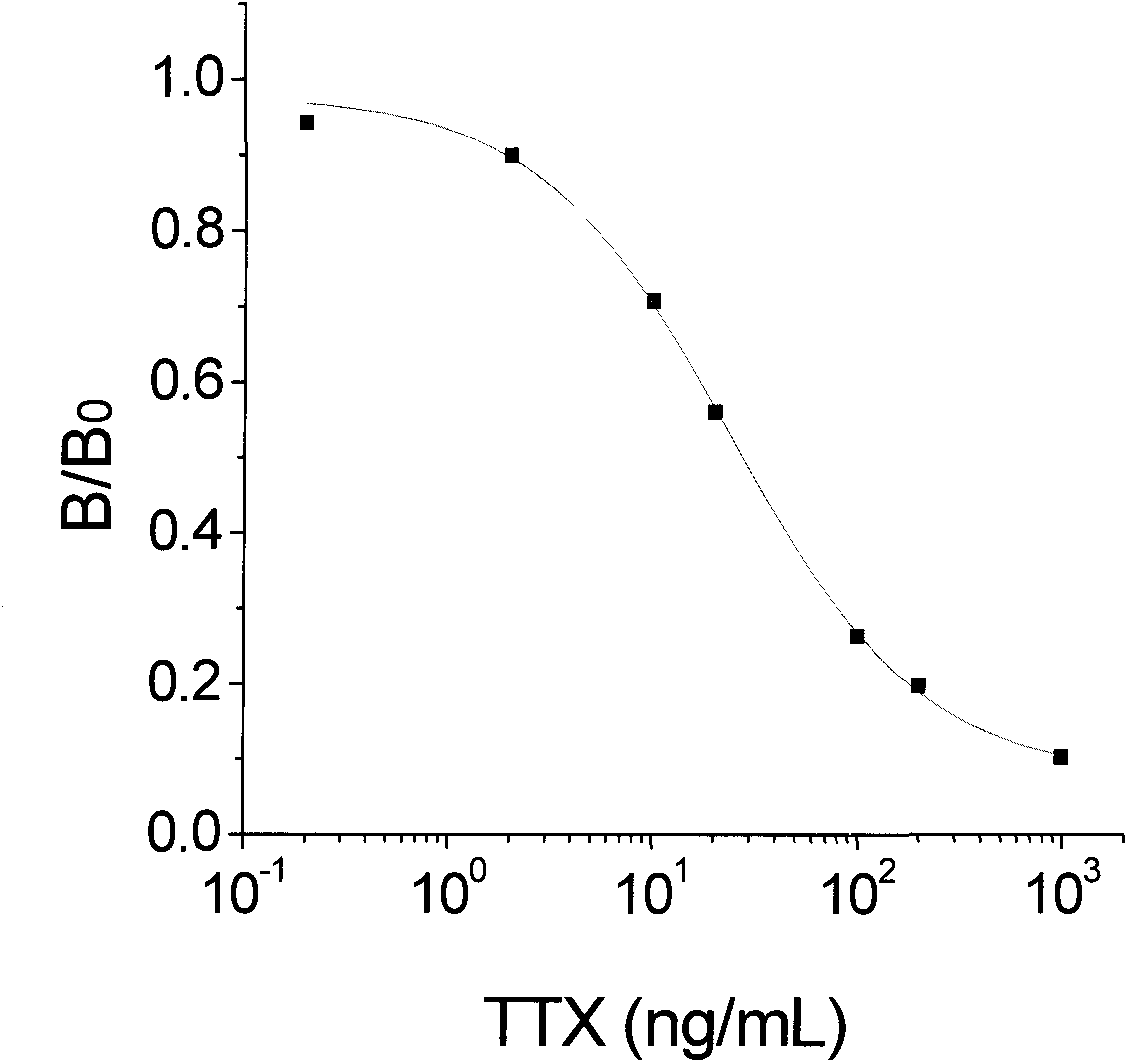
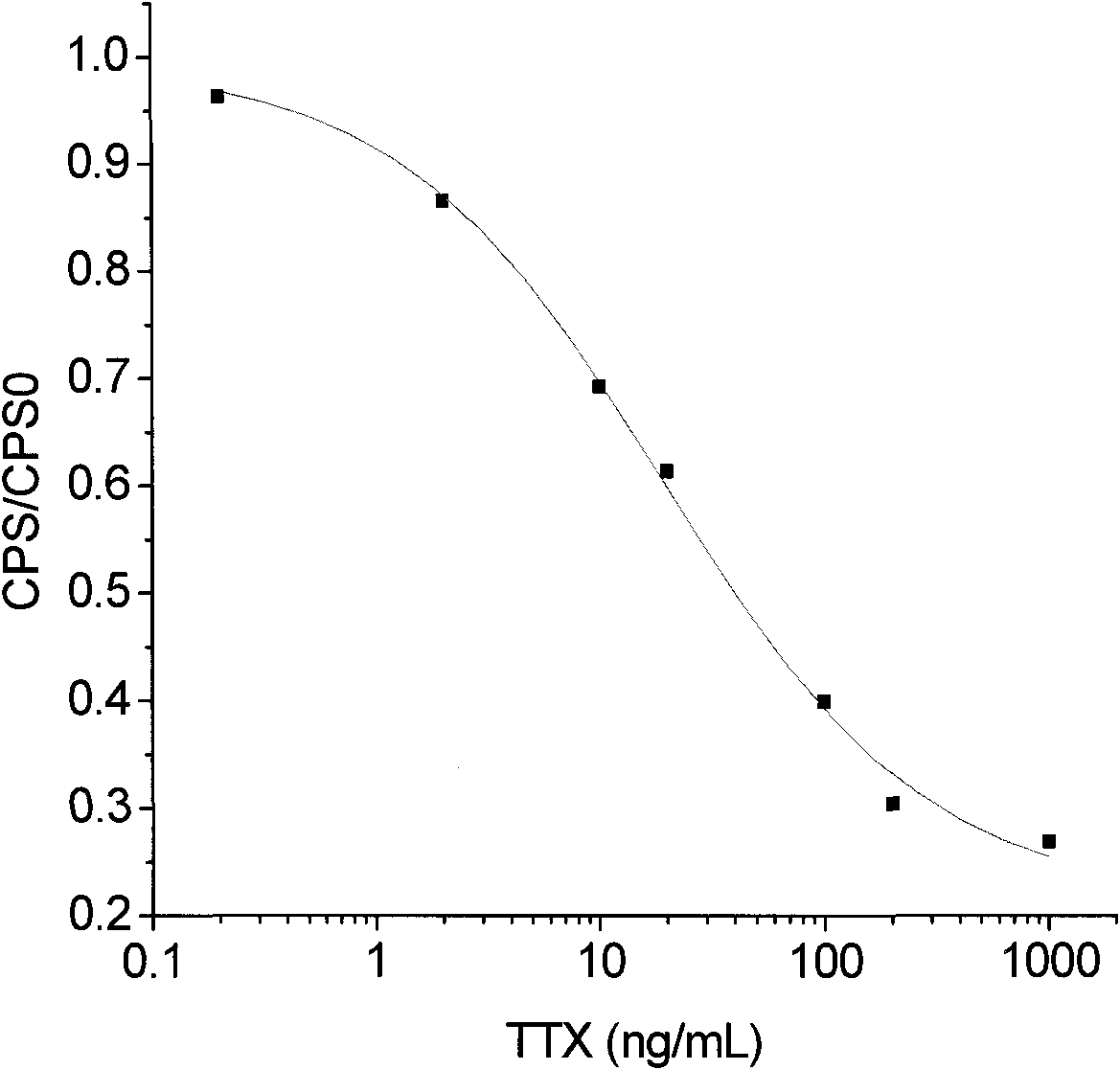
Artificial antigen of tetraodotoxin and corresponding specific antibody and preparation method and application thereof
InactiveCN101781365AQuick checkSensitive detectionImmunoglobulinsAnimals/human peptidesTime resolved fluorescence immunoassayCarrier protein
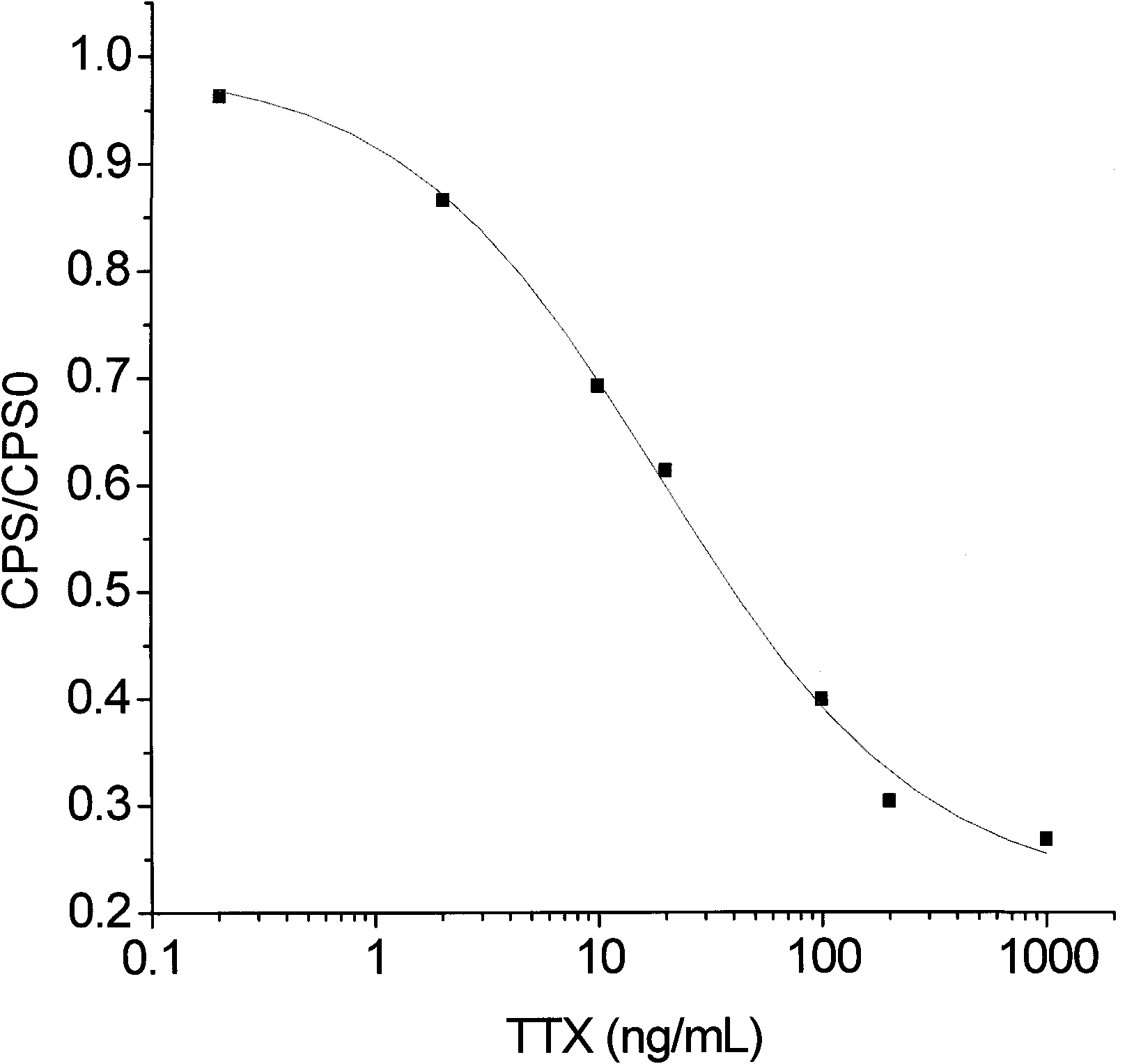
The invention discloses an artificial antigen of tetraodotoxin and a corresponding specific antibody and a preparation method and an application thereof. In the artificial antigen of the tetraodotoxin, the mass ratio of the tetraodotoxin to carrier protein is 1:10. In the invention, the artificial antigen is prepared from the tetraodotoxin which is coupled with carrier protein by a Mannich method, and the specific antibody is prepared from the artificial antigen immunization animals. The invention can be used for the extraction and purification of the artificial antigen aiming at the specific antibody of the artificial antigen of the tetraodotoxin, and can also be used for setting time-resolved immunofluorometric assay or an enzyme linked immunosorbent assay to detect the tetraodotoxin quickly and sensitively.
Owner:SOUTH CHINA AGRI UNIV
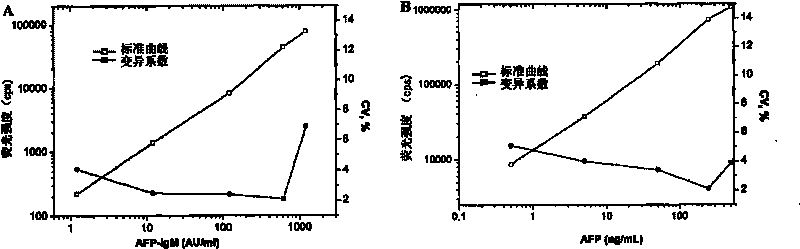
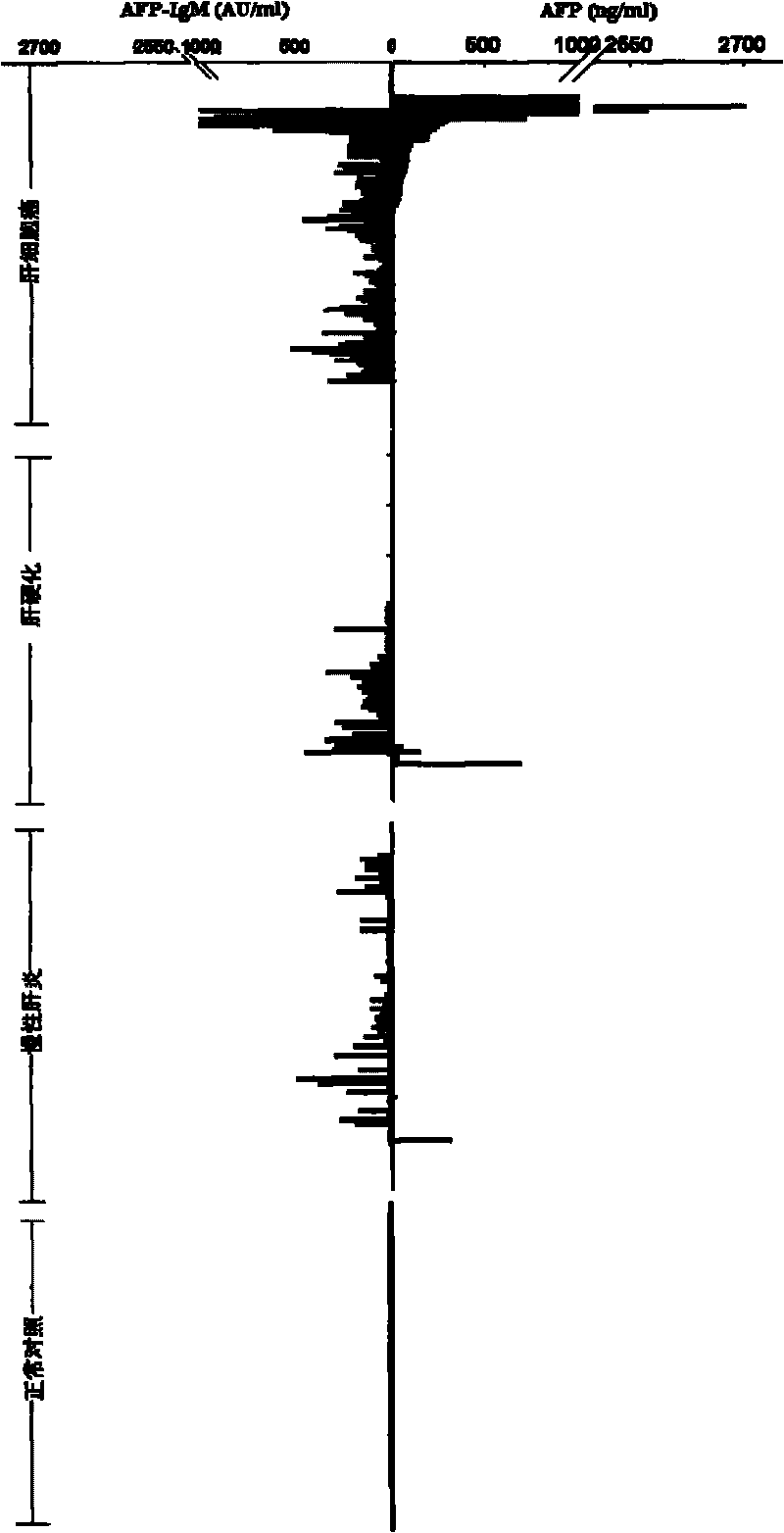
TRFIA for synchronously detecting AFP and AFP-IgM and reagent kit thereof
InactiveCN101750502AImprove diagnostic efficiencyLow costBiological testingEpitopeTime resolved fluorescence immunoassay
The invention discloses a time resolution fluorescence immunity analysis (TRFIA) method for synchronously detecting AFP and AFP-IgM and a reagent kit thereof. The reagent kit comprises the following reagents: 1) an AFP monoclonal antibody of a first epitope; 2) an AFP monoclonal antibody of a second epitope marked by a lanthanide element; 3) an IgM monoclonal antibody marked by another lanthanide element; 4) AFP / AFP-IgM mixed standard products; 5) a buffer solution; 6) washing liquid; and 7) enhancing liquid, wherein the lanthanide elements are selected from europium and samarium, and the AFP monoclonal antibody of the second epitope and the AFP monoclonal antibody of the first epitope have different epitopes. The invention adopts the TRFIA with the high sensitivity, the method for synchronously detecting AFP and AFP-IgM is established, the and the invention has the advantages of high sensitivity, strong specificity, good stability and simple operation, can realize the high atomization, can improve the speed of the clinical examination, can greatly reduce the artificial error, can improve the reliability of the detected results, and greatly improves the diagnosis efficiency.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Method for screening single chain antibodies of Microcystin-LR and verification thereof
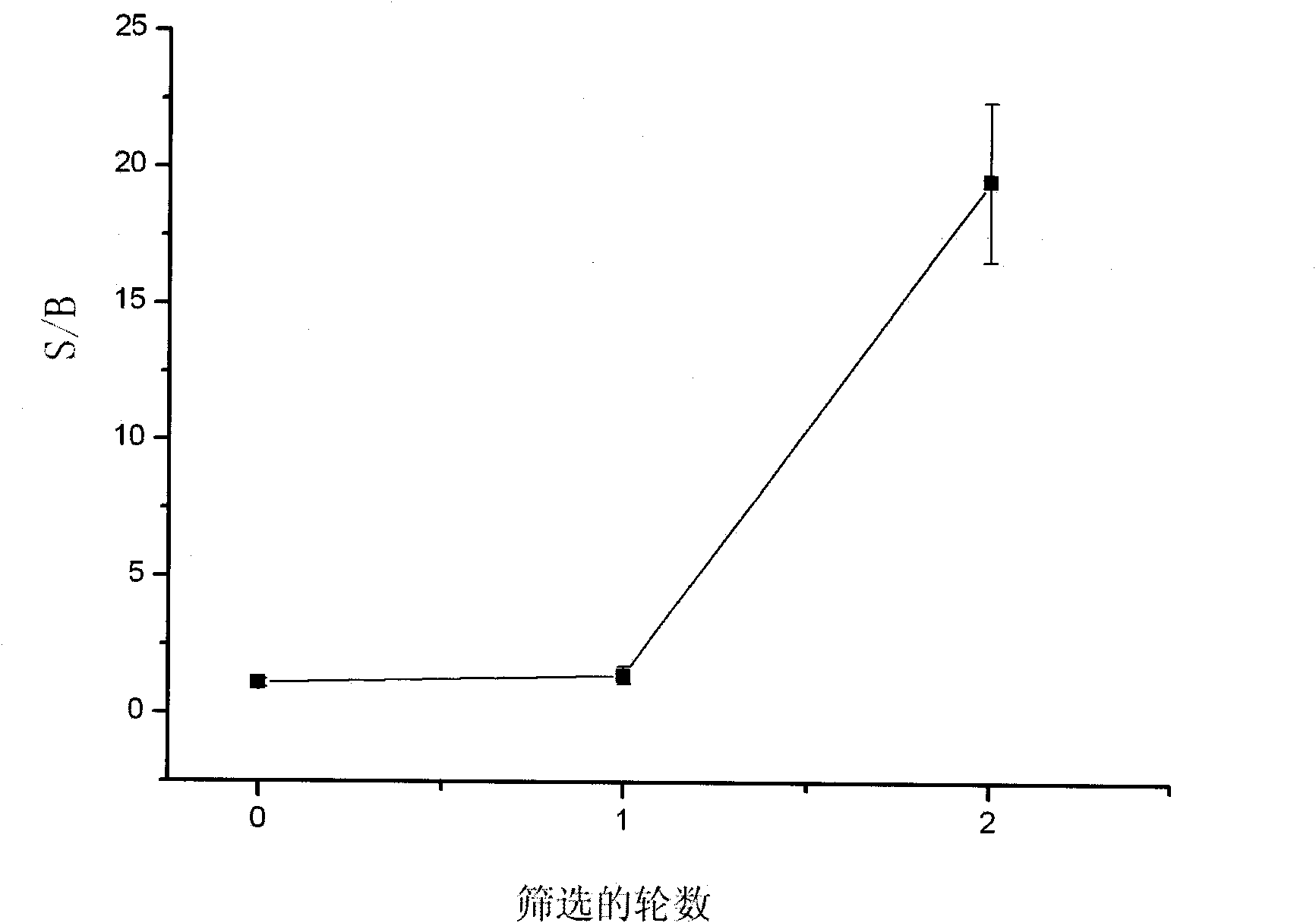
InactiveCN101857866AEasy to filterEasy to identifyImmunoglobulins against fungi/algae/lichensBiological testingEscherichia coliEnzyme digestion
The invention relates to a method for screening single chain antibodies of Microcystin-LR and verification thereof. The method comprises the following steps: carrying out two rounds of affinity screening on the biotinylated Microcystin-LR in a human source synthetic antibody library by using an avidin labeled magnetic bead and a negative screening method; extracting total plasmid DNA from phage colonies produced in the second round, carrying out enzyme digestion with enzyme Sfi I, recycling gel to obtain single chain antibody genes, connecting the single chain antibody genes with soluble expression carrier pAK100CL which is processed by enzyme digestion in the same way, and electrically transforming the connected carrier into colibacillus XL1-Blue to obtain soluble expression single chain antibodies; and verifying the soluble expression single chain antibodies by using a competitive time-resolved fluorescence immune analytical method. The invention has the advantage of quick, simple and convenient screening, and can well expose the Microcystin-LR three-dimensional structure into the incubation system. The verification on the screening result has the advantages of high detection signal and strong anti-interference capacity against stroma.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
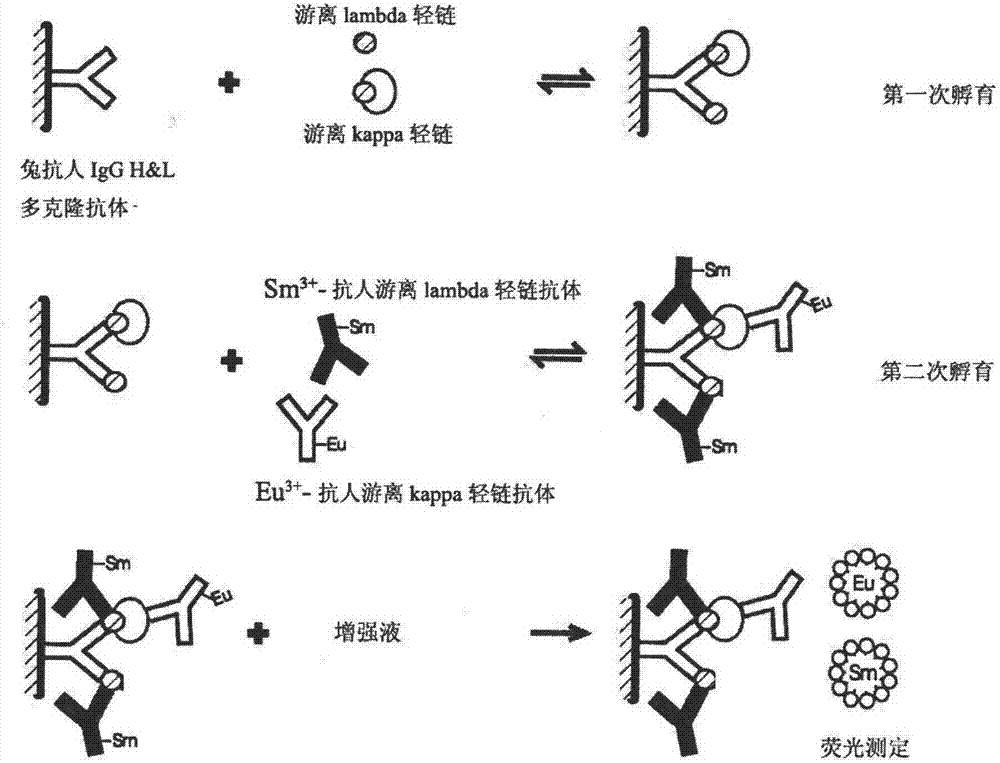
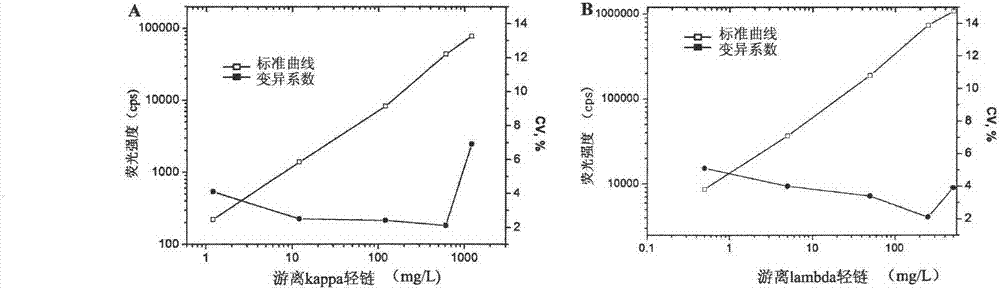
Novel detection method and kit for synchronously detecting concentration of free kappa light chain and free lambda light chain
InactiveCN102735845ALow costHigh speedBiological testingTime resolved fluorescence immunoassayHeavy chain
The invention discloses a time-resolved fluoroimmunoassay (TRFIA) kit and a detection method thereof for synchronously detecting the concentration of a free kappa light chain and a free lambda light chain. The kit comprises: 1) an antibody synchronously aiming at a heavy chain and a light chain of a human antibody, 2) an anti-free kappa light chain antibody labelled by lanthanide, 3) an anti-free lambda light chain antibody labelled by another lanthanide, 4) a standard substance of the free kappa light chain and a standard substance of the free lambda light chain, 5) a buffer, 6) a washing liquid, and 7) an enhancement solution. According to the invention, the antibody synchronously aiming at the heavy chain and the light chain of the human antibody is used as a capture antibody, other two antibodies respectively aiming at the free kappa light chain and the free lambda light chain are used as the labelled antibodies, double labeling TRFIA detection principles are used, thus the novel detection method for synchronously detecting the concentration of the free kappa light chain and the free lambda light chain is established. The method provided by the invention has the advantages of high sensitivity, strong specificity and good stability, and can realize high degrees of automation.
Owner:SHANGHAI HUJING BIO TECH CO LTD +1
Double rare earth coordination compound, Ag at SiO2 fluorescent nano particle doped with the same and preparation method thereof
InactiveCN101864298AUniform sizeGood dispersionLuminescent compositionsTime resolved fluorescence immunoassayRare earth
The invention relates to a double rare earth coordination compound, an Ag at SiO2 fluorescent nano particle doped with the same and a preparation method thereof. The fluorescent nano particle takes Ag doped with the double rare earth coordination compound Eu3<+> / Tb3<+>-PABA-DTPA-APTMS as an inner core; silicon dioxide with a mesh structure is covered on the surface of the inner core; an active amino group is arranged on the surface of the silicon dioxide, wherein the ratio of the double rare earth coordination Eu3<+> / Tb3<+>-PABA-DTPA-APTMS to the Ag is 1:0.176-0.2; and the mass ratio of the inner core to the silicon dioxide is 1:5-12 and each milligram of nano particle contains 595-630nmol of amino groups. The fluorescence intensity of Eu3<+> and Tb3<+> in the nano particle at the maximum transmitting peak is improved by 3.0 and 3.4 times compared with the fluorescence intensity of a SiO2 fluorescent nano particle doped with the Eu3<+> / Tb3<+>-PABA-DTPA-APTMS without an Ag core; the prepared nano particle is regularly spherical, is uniform in size with the particle size of 120+ / -5nm and has favorable monodispersity and light stability; and an amino group is arranged on the surface of the nano particle and directly reacts with a biomolecule without surface modification. The nano particle is expected to be used as a novel rare earth fluorescent probe which is applied to the time distinguishing fluorescent immunoassay for high-sensitivity detection, a biosensor, a biological chip and the like.
Owner:SHANGHAI UNIV
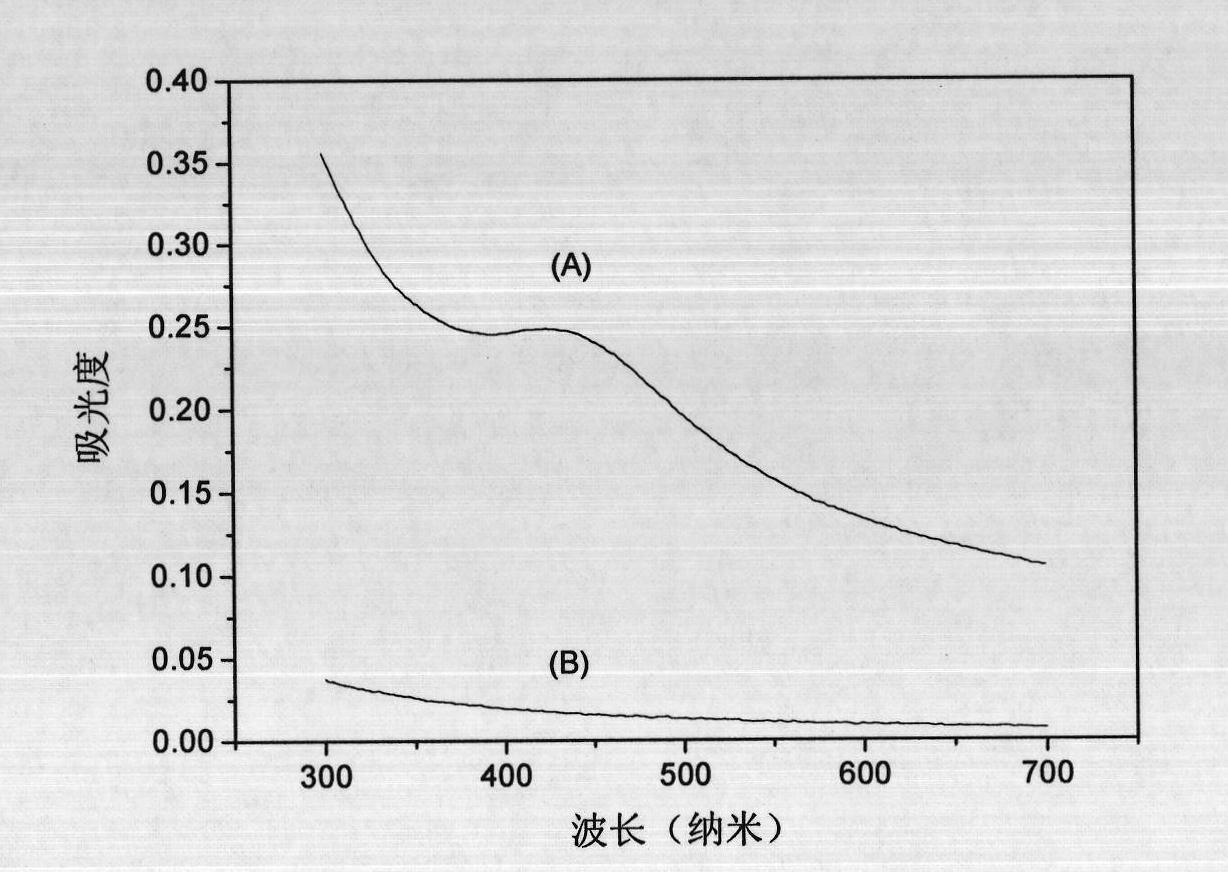
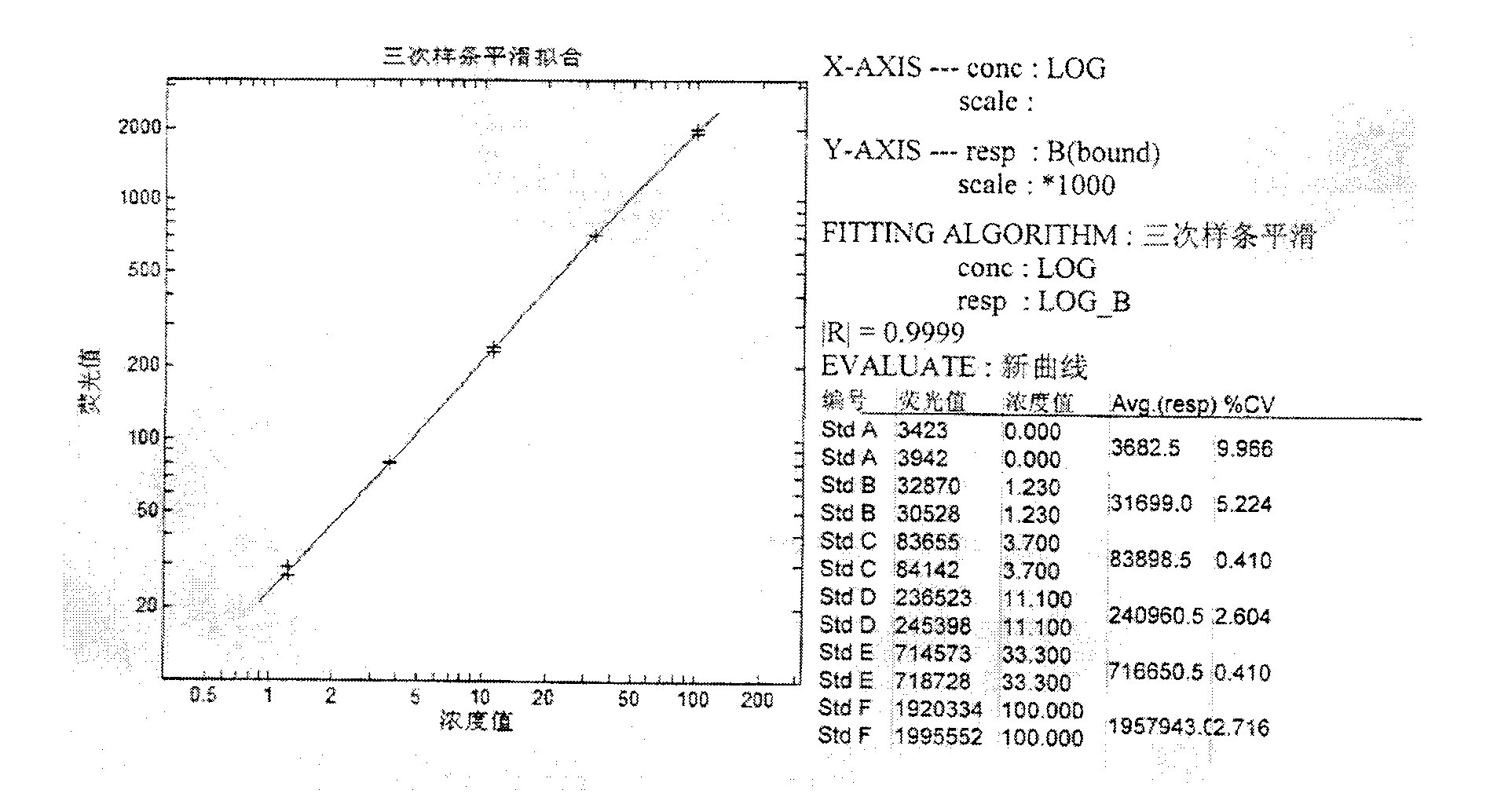
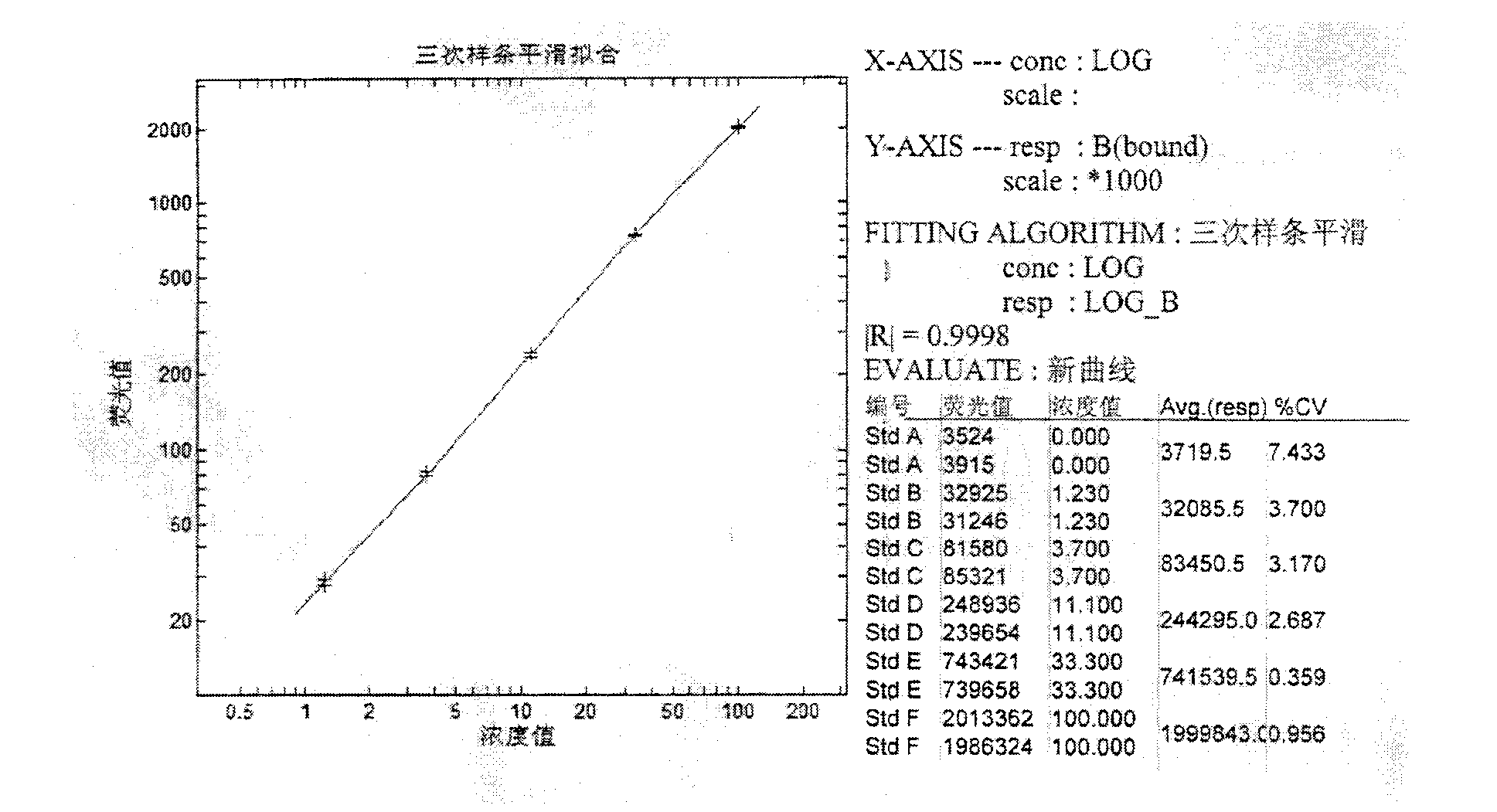
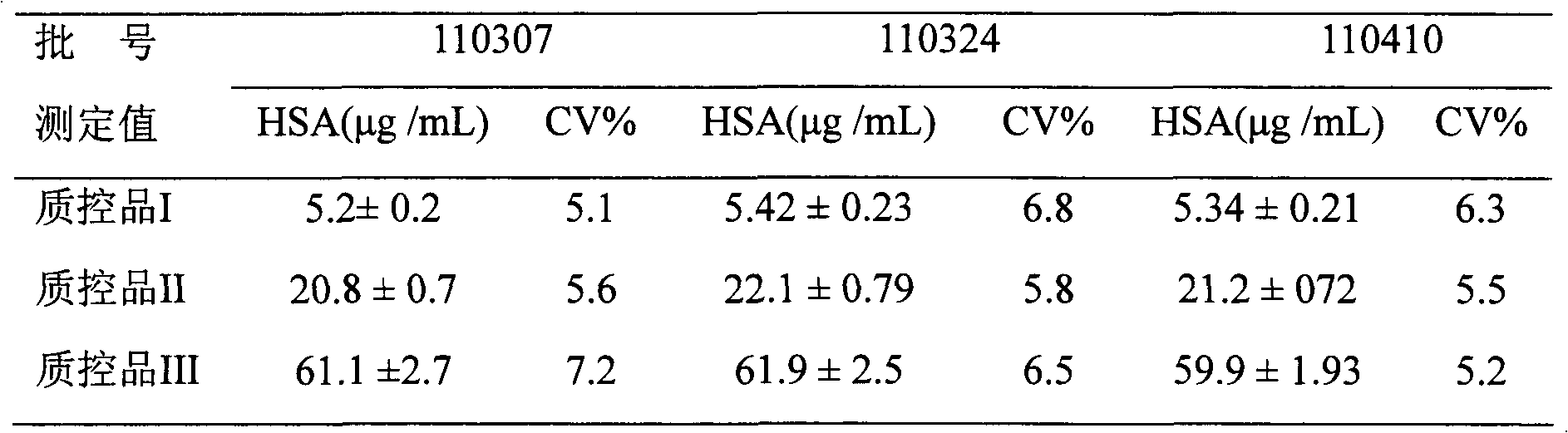
Human serum albumin (HSA) quantitative detection kit based on time-resolved fluorescence immunoassay technology
The invention discloses a human serum albumin (HSA) quantitative detection kit based on a time-resolved fluorescence immunoassay (TRFIA) technology. The kit comprises: a 96-pore microporous reaction plate coated with anti-HSA antibody, a calibrator, europium-labeled anti-HSA antibody, an assay buffer, a washing solution, and an enhancing solution. With a double-antibody sandwich immune reaction, a monoclonal antibody composition with coated anti-HSA antibody-HSA molecule-europium-labeled anti-HAS is formed; the enhancing solution is added, and value measuring is carried out by using a time resolution instrument. The method provided by the invention has the technical advantages of TRFIA, and can satisfy the requirement of quantitative detection of high-concentration (microgram-grade) HSA in biological product production. Therefore, the dilution of a product requiring detection during application is avoided, such that working time is greatly reduced, and working efficiency is greatly improved.
Owner:DAAN GENE CO LTD
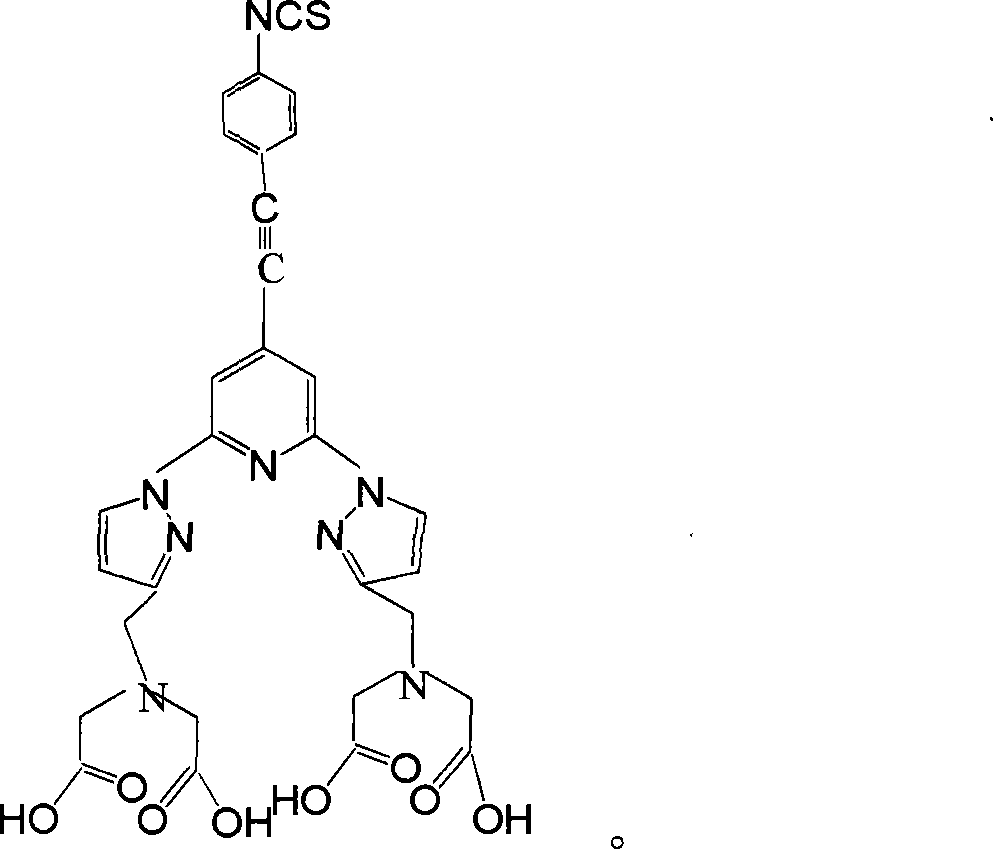
Reagent kit for simultaneously detecting ochratoxin A and aspergillus flavus toxin B1 and its detection method
InactiveCN101241131ASimple structureEasy to useMaterial analysisTime resolved fluorescence immunoassayMonoclonal antibody
A reagent kit for detecting ochratoxin A and aflatoxin B1 at the same time and method thereof are provided, which belongs to fields of time-resolved fluoroimmunoassay. Micro plate is coated with OTA-BSA, AFB1-BSA, adding OTA, AFB1 standard or sample, and then adding OTA monoclonal antibody, AFB1 polyclonal antibody. Dissociated OTA, AFB1 and OTA-BSA, AFB1-BSA on micro plates competing corresponding antibody, and antibody which haven't be connected is eliminated after washing, adding Sm3+-goat anti rat and Eu3+- goat anti rabbit, labeled antibody haven't be connected after immune reaction are eliminated after washing. After adding enhancement solution, detecting Eu and scythe fluorescence intensity cps respectively which are inversely proportional to intensity of OTA and AFB1 in sample respectively by time resolved luminoscope, comparing standard curve and the content of OAT and AFB1 in sample is determined. The reagent kits of present invention has merits of simple structure, convenient operation and low cost, two examining results of OAT and AFB1are obtained by one operation, the present invention is used for detecting of grain, feed and food.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
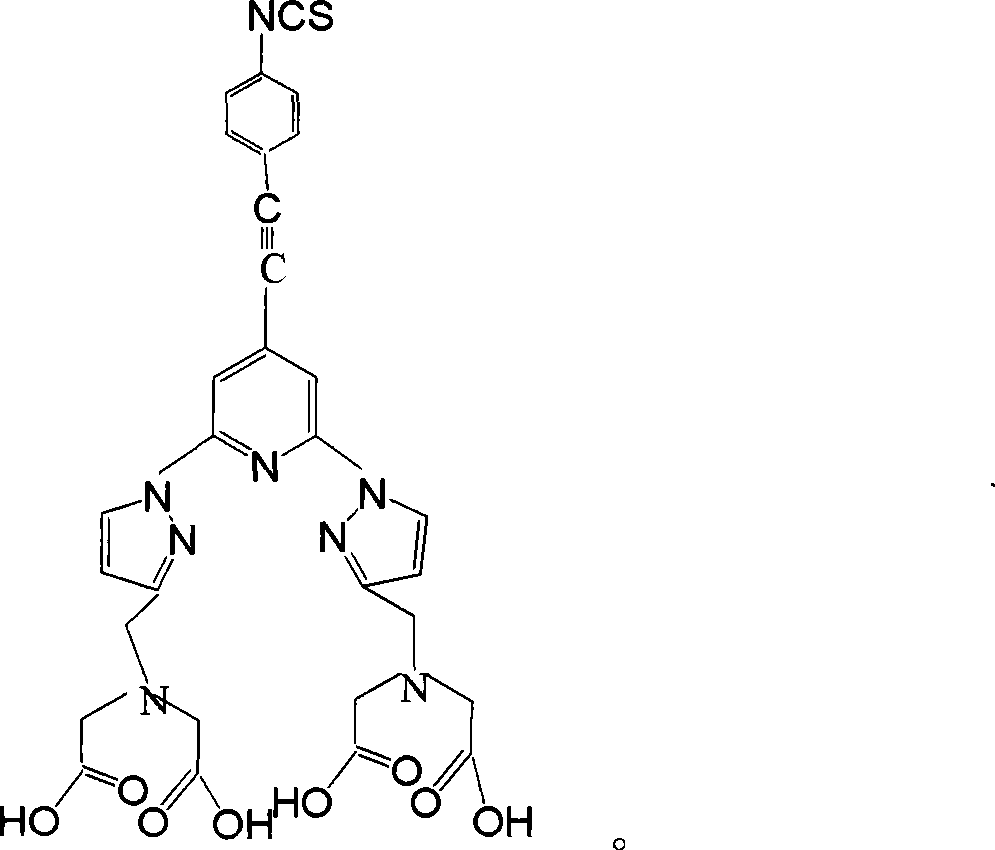
Homogeneous phase time discrimination fluorescence immunity analysis chelating agent and its preparing method
InactiveCN101221169AAdvantage designAdvantage structureBiological testingLuminescent compositionsSolubilityTriplet state
The invention relates to a homogeneous time resolved fluorescence immunoassay chelating agent and the preparation method thereof. The chelating agent is N, N, N', N'-((2, 6-di(3'-aminomethyl)-1-pyrazole)-4-(4-isothiocyanate(phenylacetylene)-pyridine))-tetraacetic acid. A luminous group is 2, 6-di(pyrazole) pyridine; the invention has high triplet-state energy level, the excitation wavelength after the combination with rare earth ions is 320nm, the emission wavelength is 520 to 620nm, the luminous service life is 2.99ms, the quantum yield is 0.58, the invention is in line with the requirements of homogeneous TRFIA to be combined with phenylacetylene at the pyridine 4-position, and the acetylene bond can block protein macromolecules from consuming luminous energy. The phenyl 4-linking isothiocyanate at the pyridine 4-position can be coupled with protein without injury, so as to be conductive to the high-efficient measurement of the luminescence of the chelating agent. The polyammonia polycarboxyl structure of 11 coordination site can exclude the quenching function of water molecules on rare earth luminescence and improve the solubility of the chelating agent in water and the dynamic stability of the chelating agent in the water r solution.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for detecting olaquindox by directly-competing TRFIA (Time Resolved Fluorescence Immunoassay)
InactiveCN104569404AShort detection timeHigh average recoveryMaterial analysis by optical meansBiological testingTime resolved fluorescence immunoassayAntigen
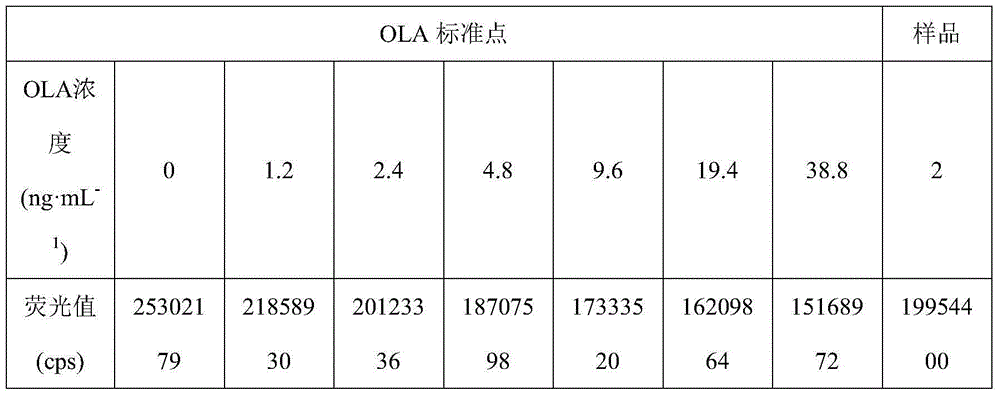
The invention discloses a method for detecting olaquindox by a directly-competing TRFIA (Time Resolved Fluorescence Immunoassay). The method comprises the following steps of: respectively adding standard olaquindox solution or treated sample solution and europium-labeled monoclonal antibodies into each micropore of an antigen precoating strip, mixing to be uniform, incubating for 1 hour at the temperature of 37 DEG C, and washing the micropores for 3-5 times by using washing liquid; adding 200muL of enhancement solution into each micropore, and carrying out light-proof oscillating incubation for 10 minutes at the temperature of 37 DEG C; determining the fluorescence intensity value cps by using a time resolution meter; drawing a standard curve; calculating out the corresponding concentration of the olaquindox from the standard curve according to the cpsx / cps0 value of each sample, multiplying by the corresponding dilution ratio and calculating out the actual concentration of the olaquindox in the sample. The method disclosed by the invention has the advantages that the operation is simple and convenient, the sensitivity is high, the stability is good and the lowest detection limit can reach 0.83ng.mL<-1>.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Microfluidic time-resolved fluorescence immunoassay device and application thereof
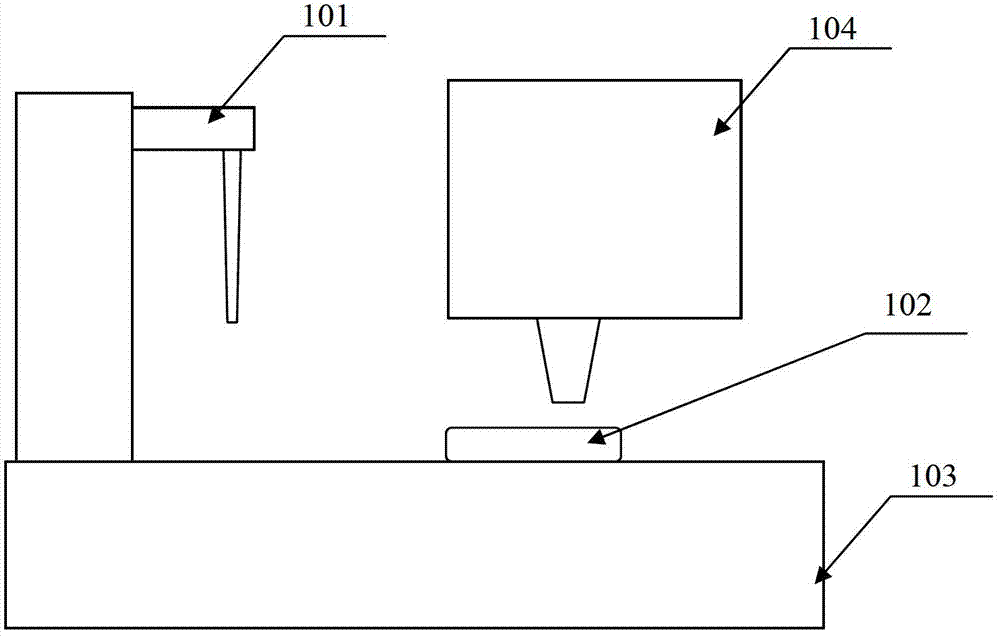
ActiveCN103207169ASimple structureSimple detection systemLaboratory glasswaresFluorescence/phosphorescenceTime resolved fluorescence immunoassayFluorescence
The invention provides a microfluidic time-resolved fluorescence immunoassay device. The device comprises a sample injection unit (101), a microfluidic control unit (103), a detection unit (104) and a microfluidic chip (102) adopting a membrane pump drive mode, and the microfluidic chip (102) is positioned in a microfluidic chip carrier on an analysis workbench of the microfluidic control unit (103). The microfluidic time-resolved fluorescence immunoassay device has the advantages that by adopting the microfluidic technology, a detection system is simplified; and a microplate oscillator and a microplate washer are omitted, a microfluidic control system is simple in structure, optimized, free of mechanical movements and structures of cleaning, vibration, transmission and the like, and automation is achieved conveniently.
Owner:BEIJING BOHUI INNOVATION TECH
Lanthanide-series compound and preparation method and application thereof
ActiveCN105218570AImprove stabilityIncrease brightnessGroup 3/13 element organic compoundsFluorescence/phosphorescenceTime resolved fluorescence immunoassayChemical structure
The invention provides a lanthanide-series compound and a preparation method and an application thereof; the lanthanide-series compound has the chemical structural formula described in the description. The lanthanide-series compound has no inversion center, is beneficial for the compound to generate higher-brightness fluorescence, and enables a strongest emission peak to be used in a fluorescence analysis peak of time-resolved fluorescence immunoassay; and at the same time, the compound has relatively strong stability, and still can be used for marking and testing after being placed for a certain time.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI +1
Full-automatic magnetic-bead time resolution fluorescence immunoassay analyzer
ActiveCN109142708AReduce dosageReduce waiting timeBiological testingTime resolved fluorescence immunoassayFluorescence immunoassay analyzer
The invention discloses a full-automatic magnetic-bead time resolution fluorescence immunoassay analyzer. The analyzer is divided into an inner chamber and an outer chamber by a rack; an electronic element box is fixed on the upper layer of the inner chamber, a dark chamber shell is positioned on the lower layer of the inner chamber, a dark chamber door device is arranged on the rack, and a dark chamber space of which one side is openable is formed by the dark chamber door device and the dark chamber shell; a tray module is positioned on the lower layer of the outer chamber, and a plurality ofreagent strips can be placed on the tray module, and are conveyed to the lower layer of the inner chamber or are withdrawn to the lower layer of the outer chamber; a puncturing liquid relief module is positioned on the upper layer of the outer chamber, can automatically pierce the film of the top surface of each reagent strip, and automatically suck or discharge liquid in the reagent; a scanningand value reading module is positioned on the lower layer of the inner chamber, is positioned in the dark chamber shell, and is used for triggering and reading fluorescence information of the reagentstrips after incubation; and by reasonable arrangement of the inner chamber and the outer chamber and in combination with compact and scientific design of the related functional module, the structureis simple and compact, the cost is low, furthermore, the degree of automation of the integral instrument is increased, and thus, the full-automatic magnetic-bead time resolution fluorescence immunoassay analyzer is quite suitable for being used and popularized in China medical units.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
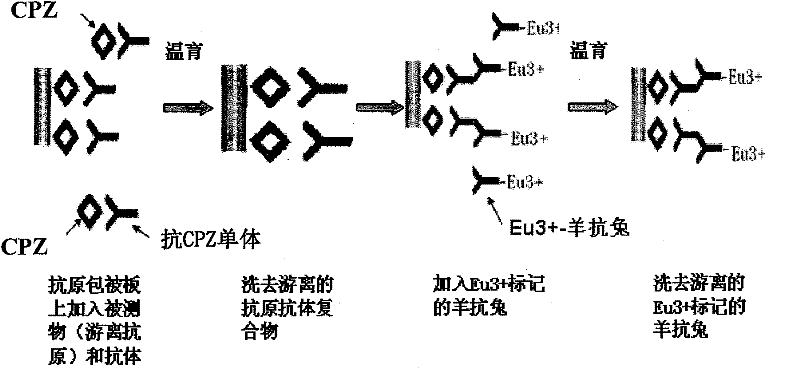
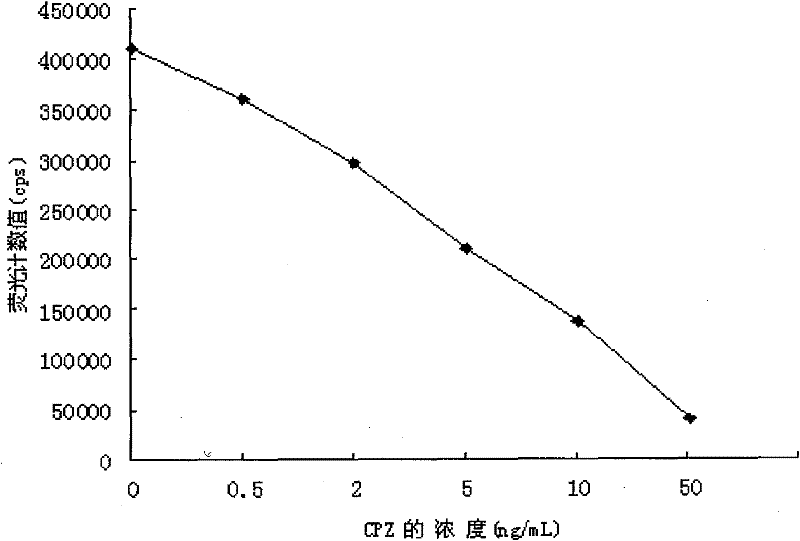
Time resolved fluoroimmunoassay kit for detecting chlorpromazine and detecting method thereof
InactiveCN102236012ABiological testingFluorescence/phosphorescenceTime resolved fluorescence immunoassayBiology
The invention provides a kit for detecting chlorpromazine (CPZ) and a detecting method thereof, belonging to the technical field of time resolved fluoroimmunoassay (TRFIA) and used for detecting the content of CPZ in meat, fish and other animal derived foods. The kit prepared by the method is used for detecting CPZ by using TRFIA, and the detection is based on a labeled immunoreaction. A microporous plate is coated with CPZ-OVA (ovalbumin), a CPZ standard or sample is added, and a CPZ antibody is added; free CPZ competes for the CPZ antibody with the CPZ-OVA on the microporous plate, the CPZ antibody which is not connected is washed off and removed, Eu3+- antiMIgG is added, and the Eu3+- antiMIgG which is not connected is washed off and removed after the labeled immunoreaction; after an enhancing solution is added, the fluorescence intensity cps of the enhancing solution is detected by using a time-resolved fluorescence spectrofluorometer, the fluorescence intensity is in inverse proportion to the concentration of CPZ in the sample; and the content of CPZ in the detected sample can be determined in comparison with a standard curve. The kit for detecting CPZ provided by the invention has the advantages of simple structure, convenience in use, low cost and high sensitivity.
Owner:FOOD INSPECTION CENT OF CIQ SHENZHEN
Time resolution fluorescence immunoassay method based on magnetic separation
InactiveCN109298177AHigh sensitivityExtended storage timeBiological testingFluorescence/phosphorescenceAntigenMagnetic bead
The invention provides a time resolution fluorescence immunoassay method based on magnetic separation; the method mainly comprises the following steps of firstly, coupling magnetic beads with an antibody to form immunomagnetic beads; meanwhile, enabling time resolution fluorescent microsphere to be coupled with antibody to form immunofluorescent microspheres; then enabling the immunomagnetic beadsand the immunofluorescent microspheres and the antigen in a sample to be oscillated and incubated in a reaction tube to form an immunomagnetic bead-antigen-immunofluorescent microsphere compound; andfinally, testing the fluorescence intensity, emitted by excitation of the compound at 360 nm excitation, by a time resolution instrument, wherein a standard curve is used as reference for determiningthe amount of the antigen in the sample. According to the analysis method, the reaction time is greatly shortened, and the detection efficiency and sensitivity are improved.
Owner:江苏美克医学技术有限公司
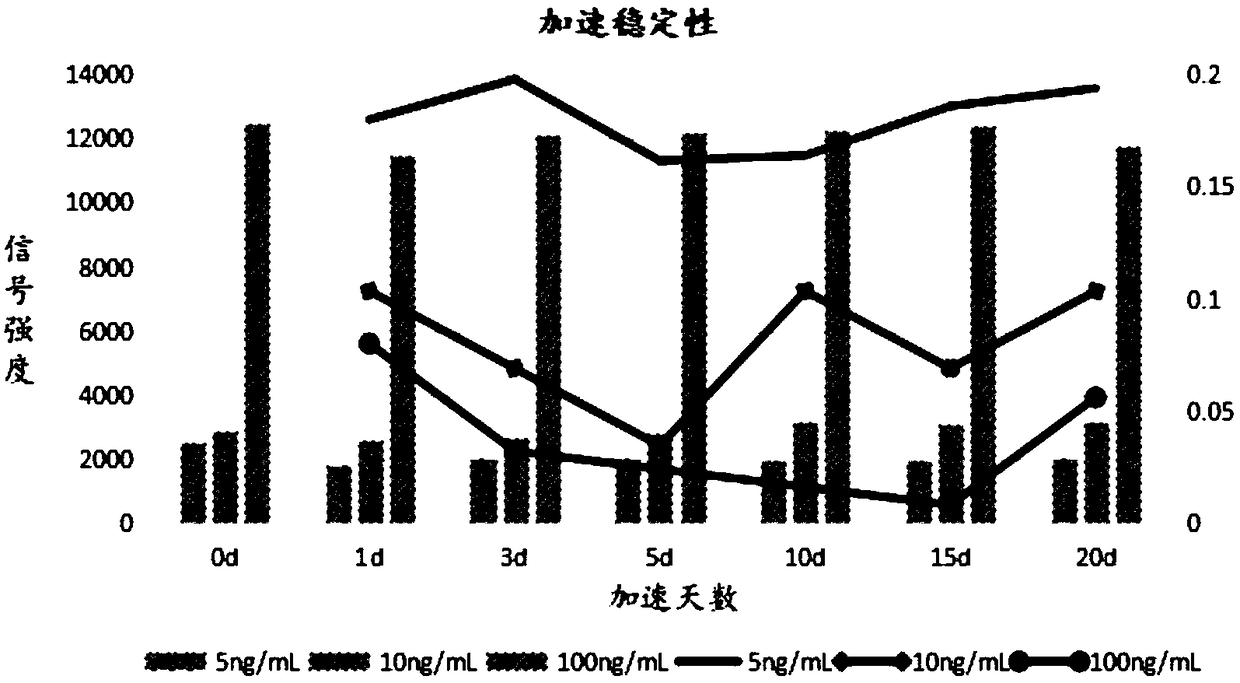
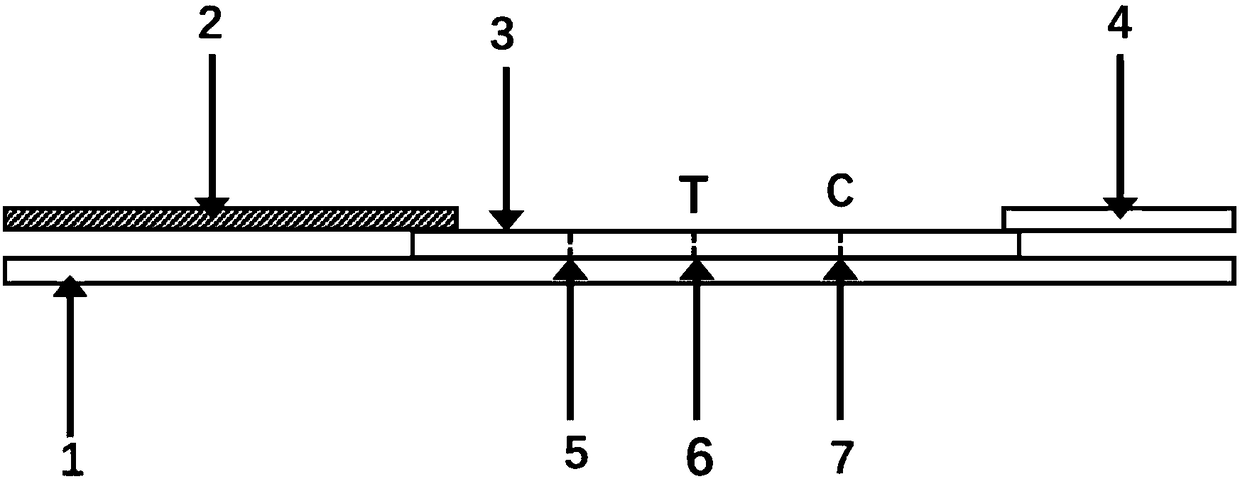
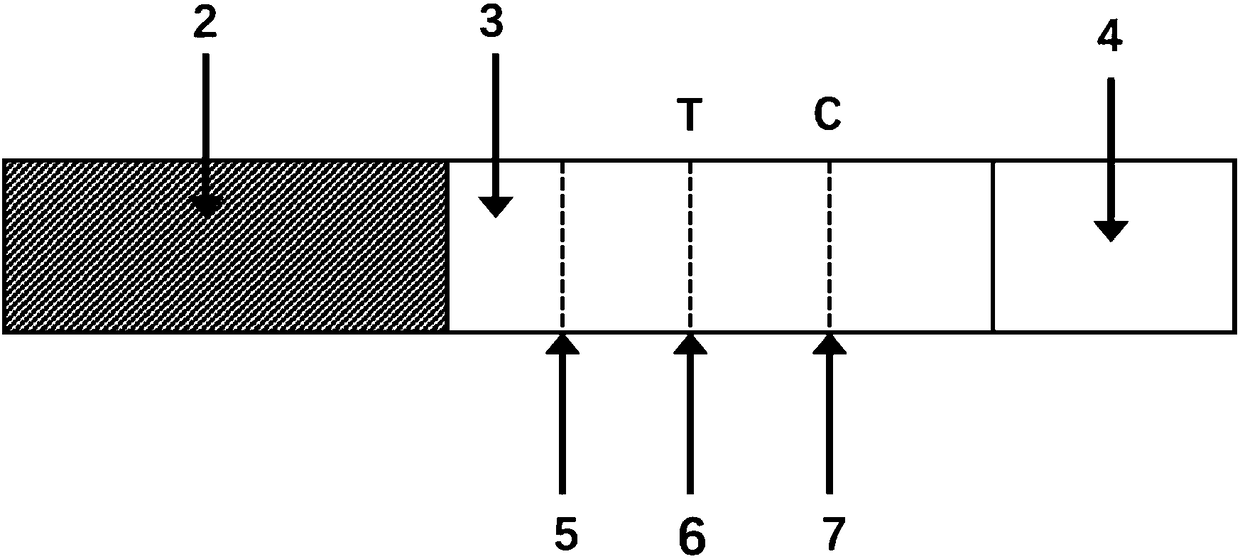
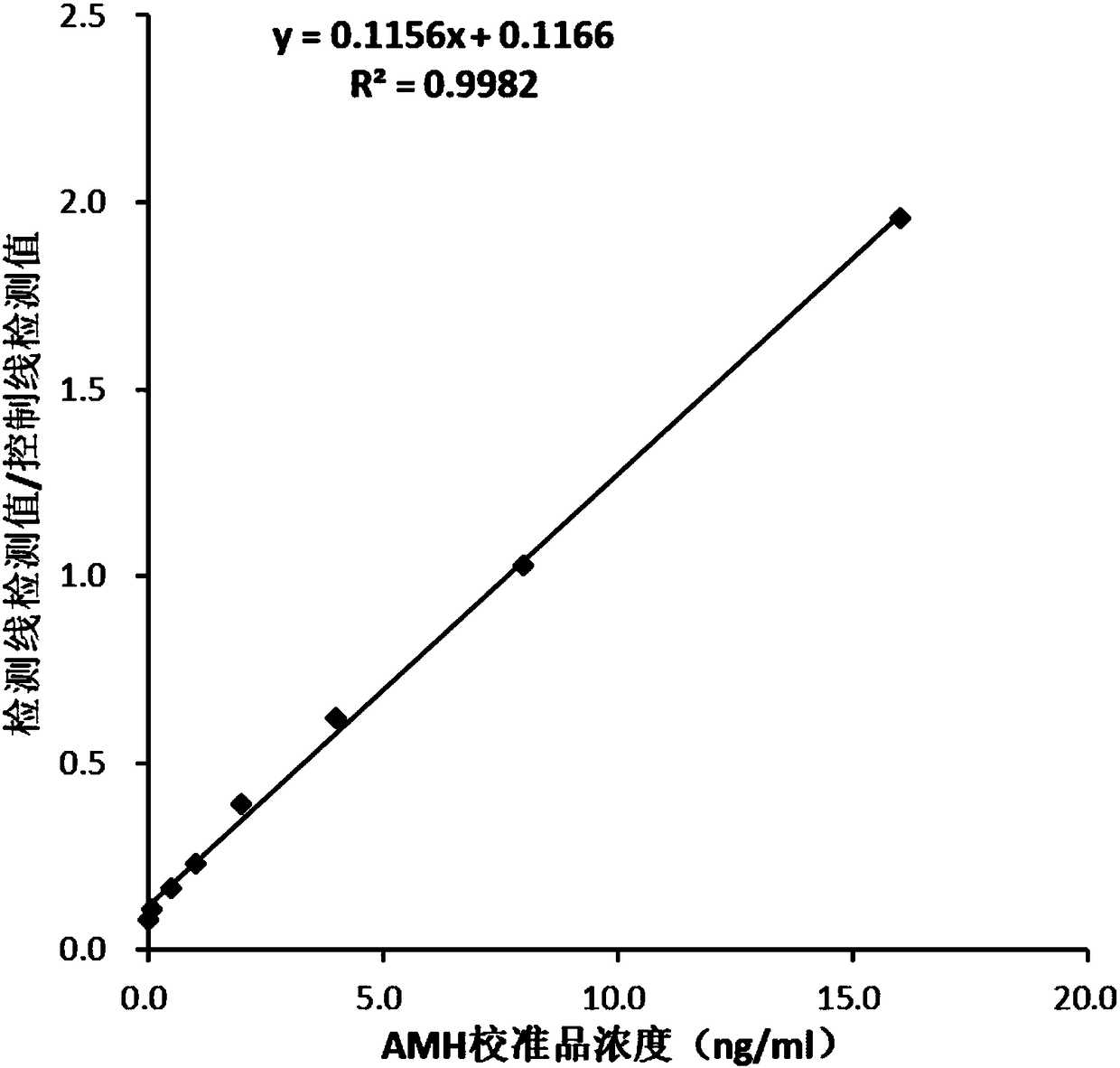
Test strip, kit and method for detecting AMH (anti-mullerian hormone) with TRFIA (time-resolved fluorescence immunoassay)
PendingCN108195815AAvoid fluorescence interferenceStrong specificityBiological material analysisBiological testingTime resolved fluorescence immunoassayBuffer solution
The invention relates to a test strip, a kit and a method for detecting AMH (anti-mullerian hormone) with TRFIA (time-resolved fluorescence immunoassay). The test strip comprises a PVC bottom plate, awhole blood filter pad, an antibody carrying membrane and absorbent paper. The kit comprises the test strip for detecting the AMH with the TRFIA, a sample buffer solution and an ID card containing anAMH standard curve. The kit can meet the requirement of single-person and small-batch detection of AMH and has the advantages of high sensitivity, high specificity, small blood amount, short detection time, operation convenience and detection result accuracy and reliability.
Owner:贵州立知健生物科技有限公司
Testing kit for enrofloxacin and testing method thereof
InactiveCN101710117ASimple structureEasy to useMaterial analysisTime resolved fluorescence immunoassayTest sample
A testing kit for enrofloxacin and a testing method thereof belong to the TRFIA field, used for testing the ENR content in animal-based food, blood, urine and forage. The kit prepared in the invention uses RRFIA to detect ENR, wherein the testing base is marking immunization response. A micro-hole plate is coated by ENR-carrier protein, is added with ENR standard or sample and ENR antibody. Dissociative ENR competes against ENR-carrier protein on the micro-hole plate for ENR antibody. The unconnected ENR antibody is washed off and then Eu3+- goat antibody to rabbit is added. Unconnected Eu3+- goat antibody to rabbit is washed off after marking immunization response. Fluorescence intensity cps is tested by using a time-resolved fluoroimmunoassay instrument after adding enhancing liquid. The fluorescence intensity has inverse ratio to the ENR concentration in sample. The ENR content in the tested sample can be immediately confirmed by comparing standard curve. The kit for testing ENR of the invention has simple structure, convenient use, low cost, and high sensitivity which can reach to 0.01ng / ml.
Owner:JIANSGU INST OF MICROBIOLOGY
Reagent box for detecting aftatoxin B and detecting method thereof
InactiveCN1673747ASimple structureEasy to useMaterial analysisTime resolved fluorescence immunoassayTest sample
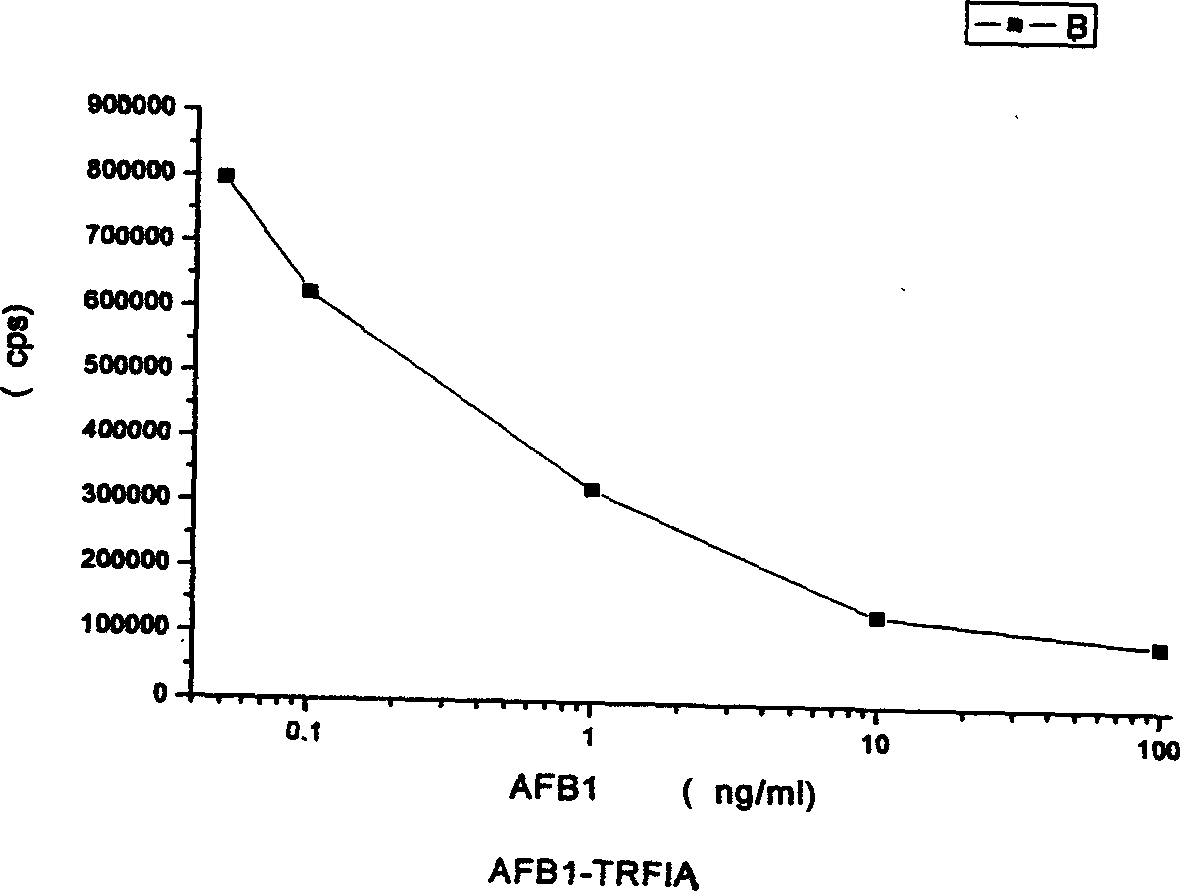
The aflatoxin B1 detecting kit and its detecting method belongs to the field of time-resolved fluorescent immunoassay (TRFIA) technology, and is used in the detection of aflatoxin B1 (AFB1) content in food and feed. The kit of the present invention detects AFB1 in TRFIA based on marker immune reaction. The microporous board has coated AFB1-HRP, added AFB1 standard or sample, and added AFB1 antibody. The free AFB1 and the AFB1-HRP in the microporous board compete AFB1 antibody, un-connected AFB1 antibody is washed off, EU3+ sheep anti-rabbit antibody is added and un-connected EU3+ sheep anti-rabbit antibody after marker immune reaction is washed off. After reinforcing solution is added, the fluorescence strength cps, which is proportional inversely to AFB1 concentration in the sample, is determined with time-resolved fluorescent instrument to determine the AFB1 content in the tested sample via comparison with standard curve.
Owner:JIANGNAN UNIV
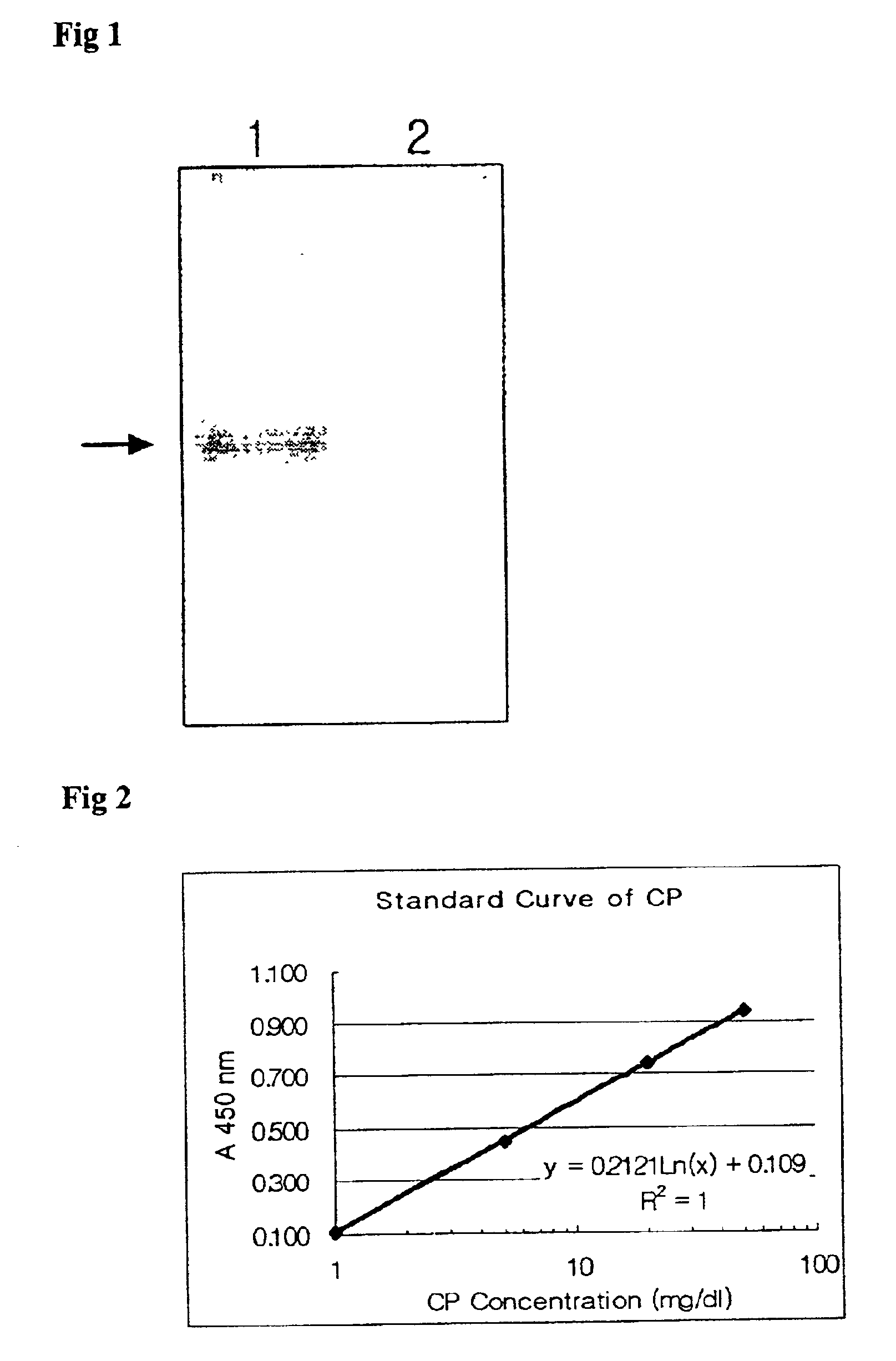
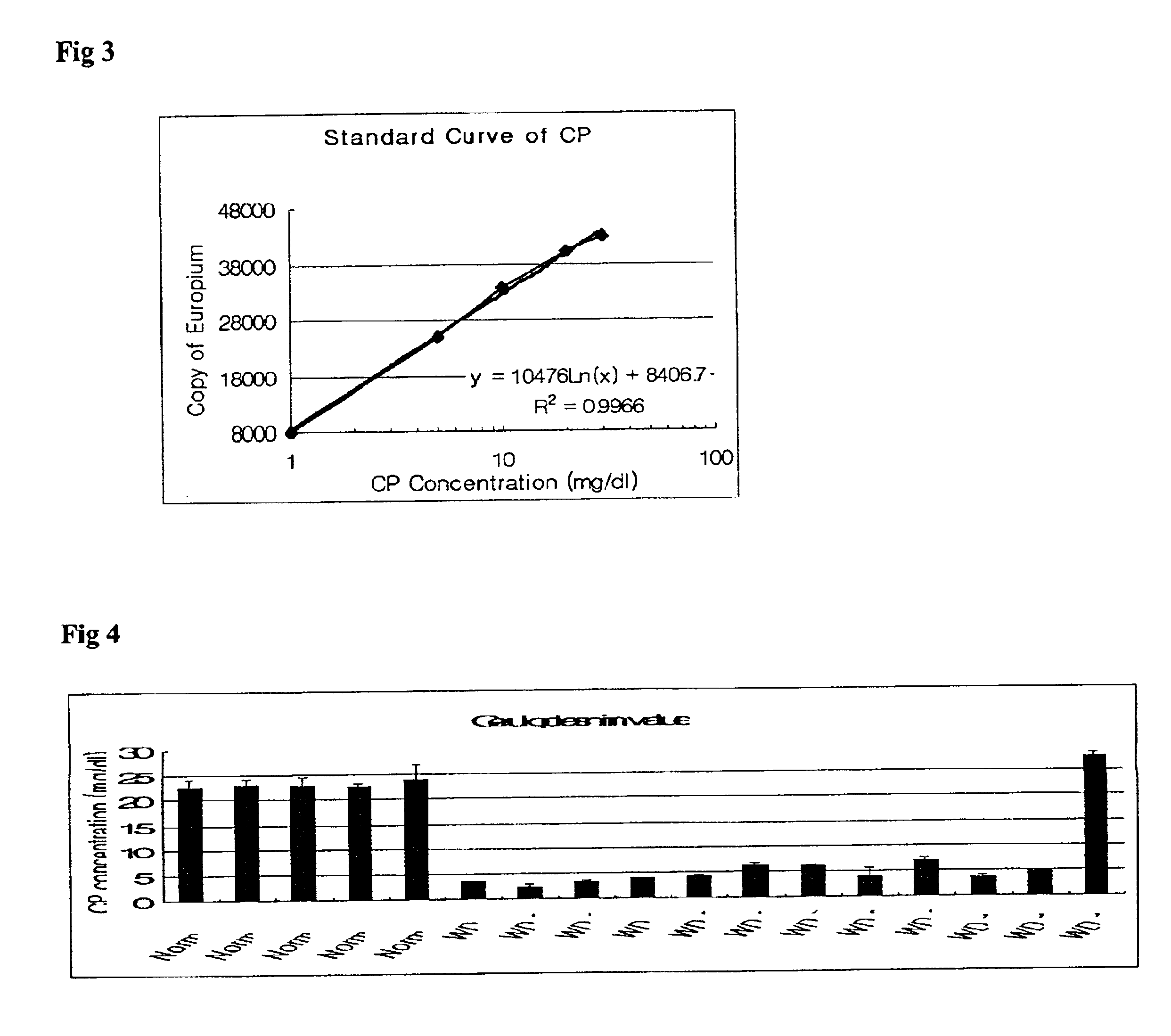
Method of measuring ceruloplasmin concentration in a blood spot, kit and method of diagnosing Wilson's disease
InactiveUS6806044B2Dissociation-enhanced time-resolved fluoroimmunoassayEarly detectionMicrobiological testing/measurementEnzymologyDiseaseTime resolved fluorescence immunoassay
The present invention relates to a method for measuring a holoceruloplasmin concentration, and more particularly, to a method for measuring a holoceruloplasmin in a blood spot based on a standard concentration curve obtained through an enzyme-linked immunosorbent assay (ELISA) or a dissociation-enhanced time-resolved fluoroimmunoassay using a holoceruloplasmin-specific polyclonal antibody and a holoceruloplasmin-specific monoclonal antibody.
Owner:ZENOVAC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com