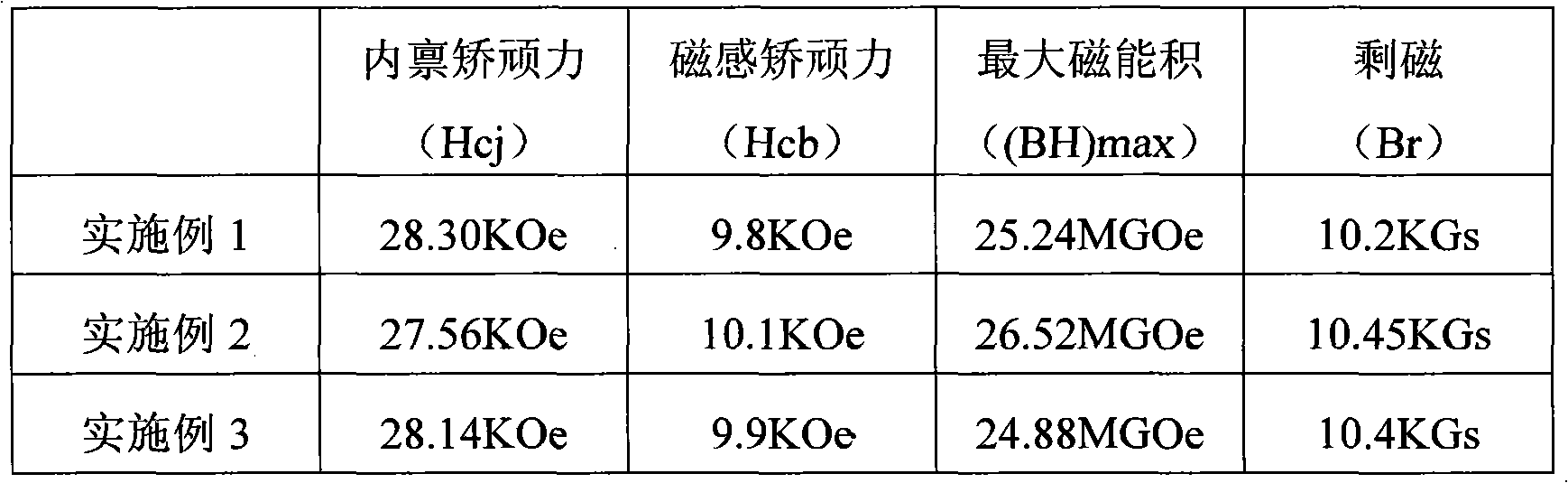
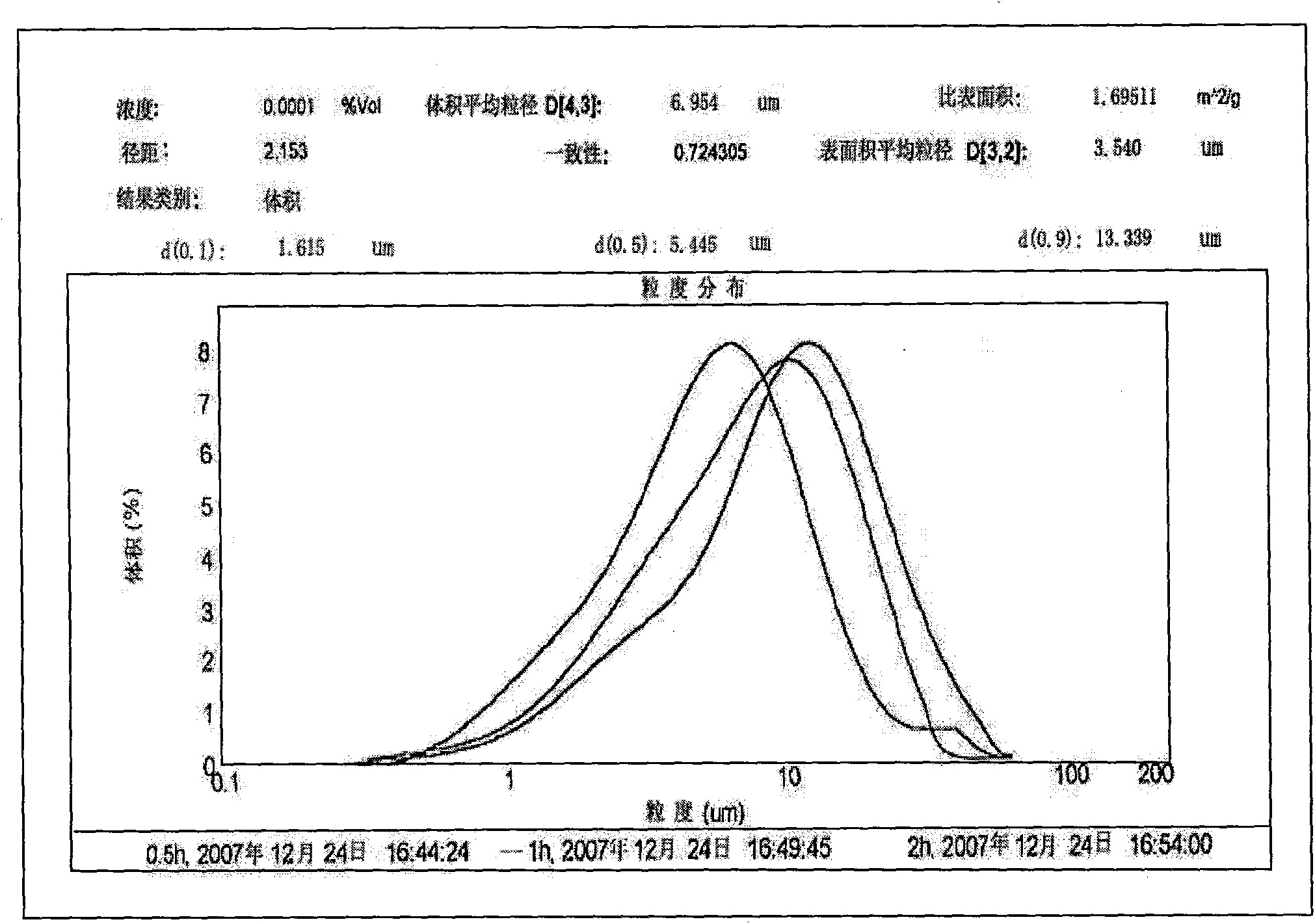
Patents
Literature
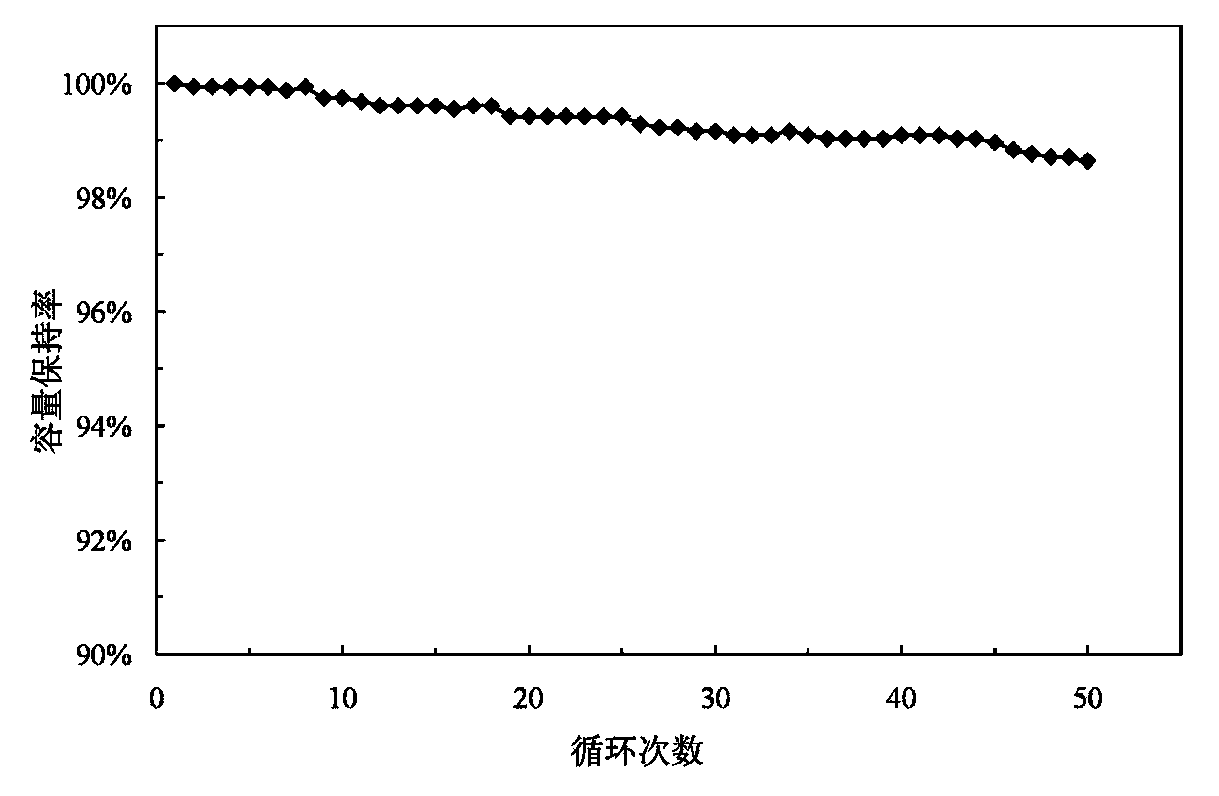
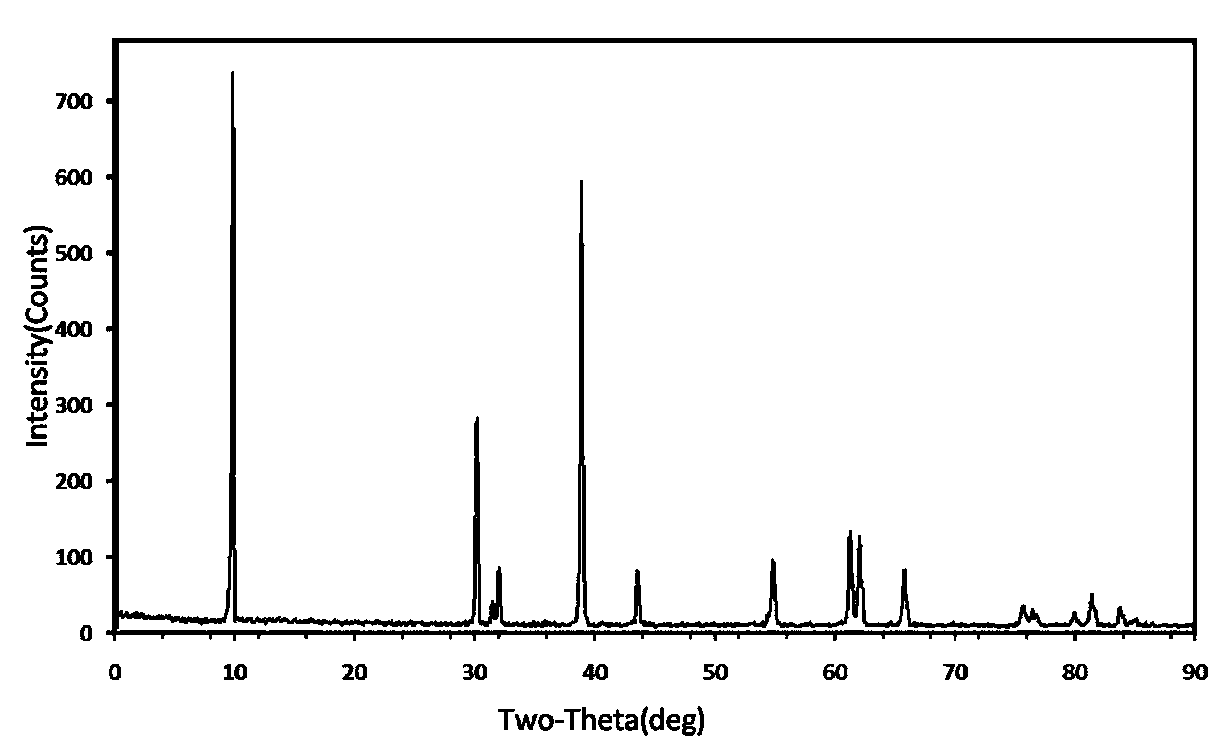
1739 results about "Samarium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Samarium is a chemical element with the symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3. Compounds of samarium(II) are also known, most notably the monoxide SmO, monochalcogenides SmS, SmSe and SmTe, as well as samarium(II) iodide. The last compound is a common reducing agent in chemical synthesis. Samarium has no significant biological role but is only slightly toxic.
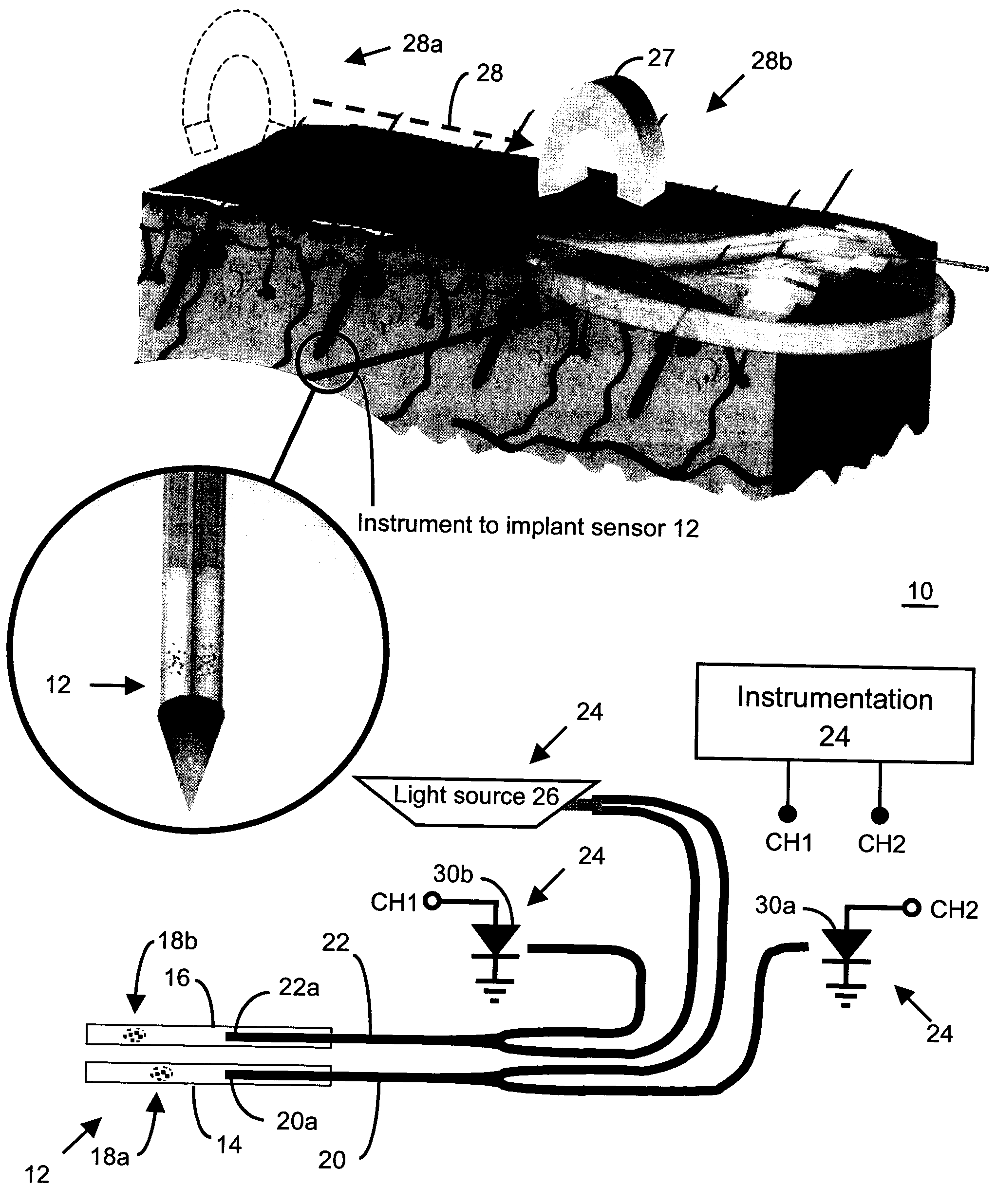
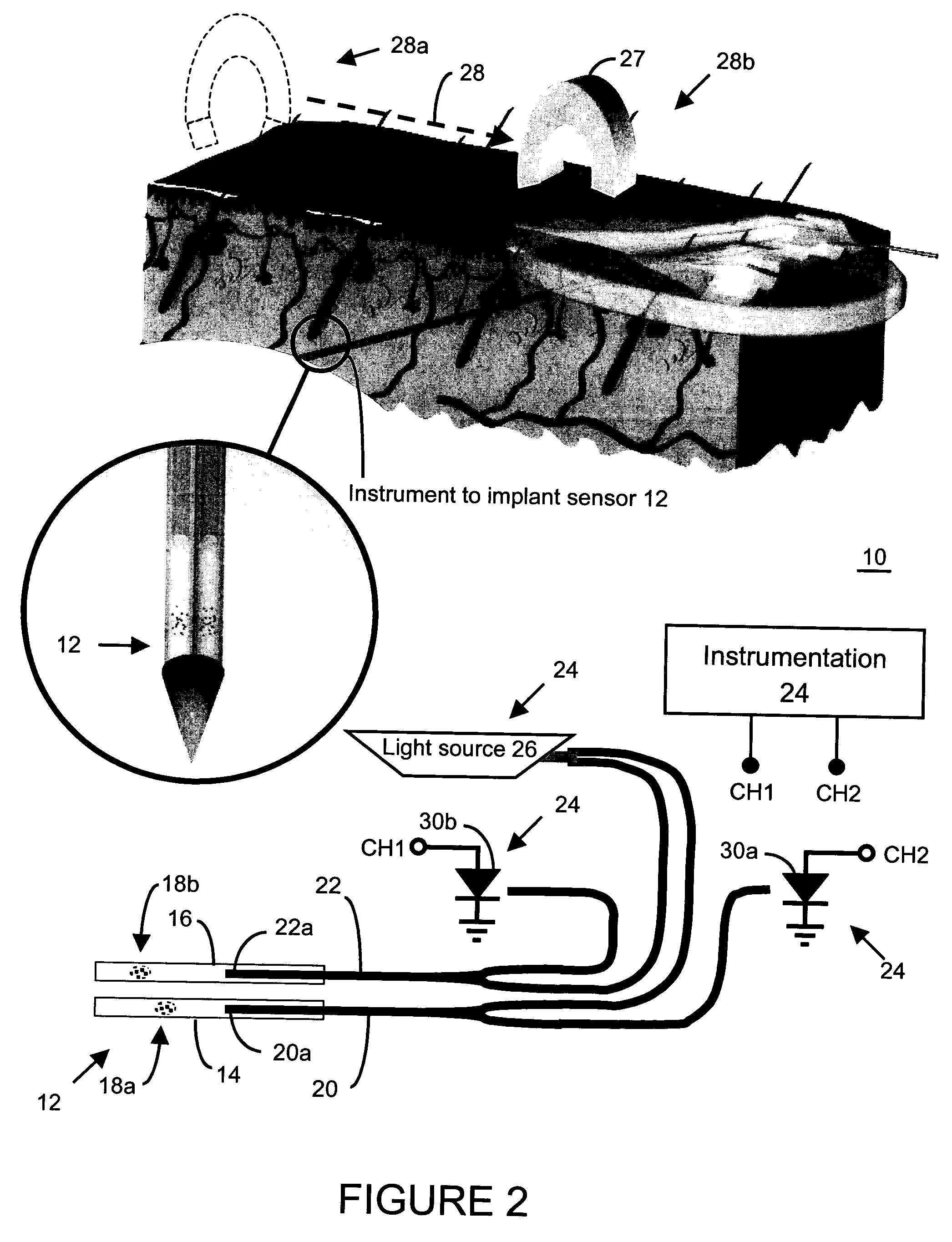
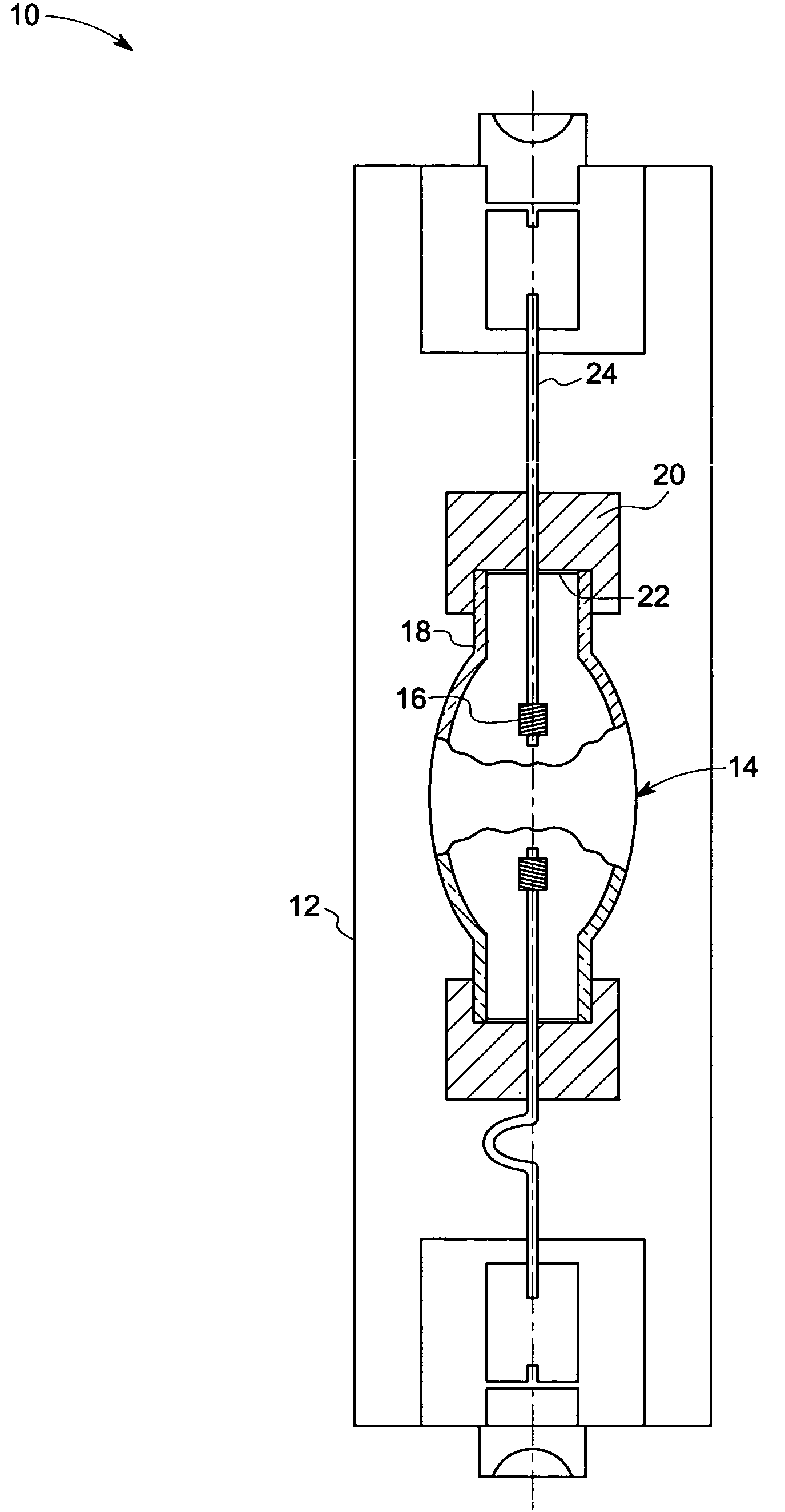
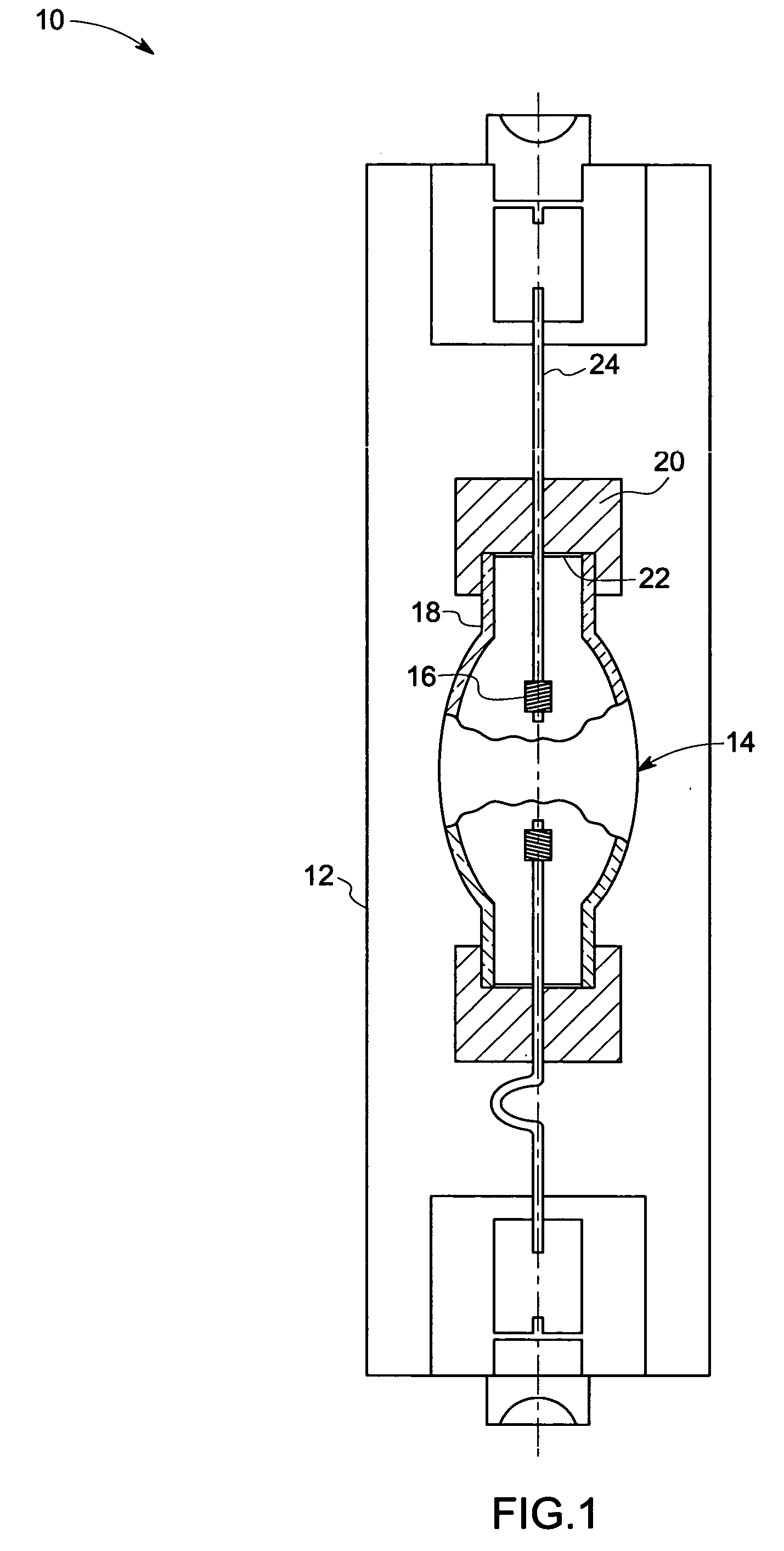
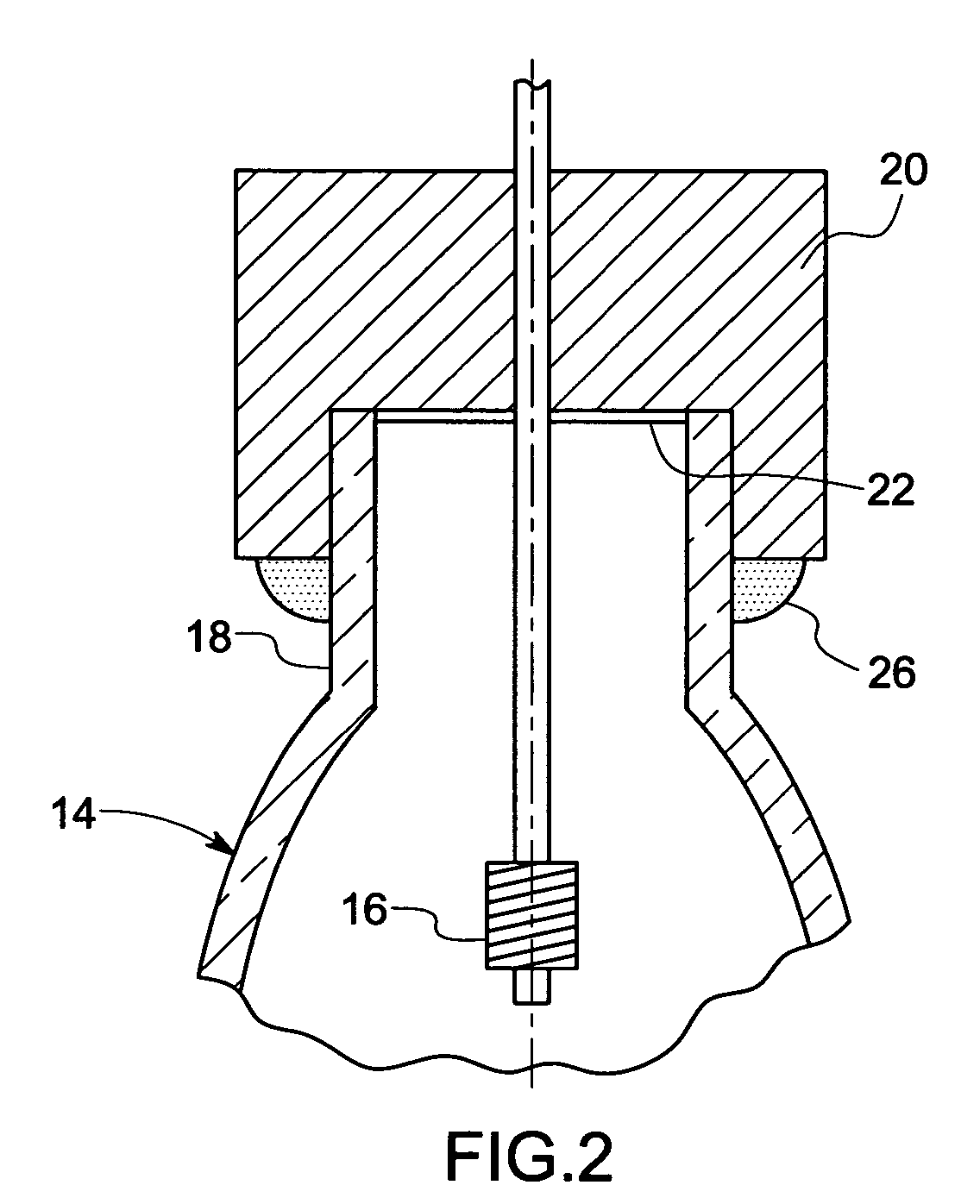
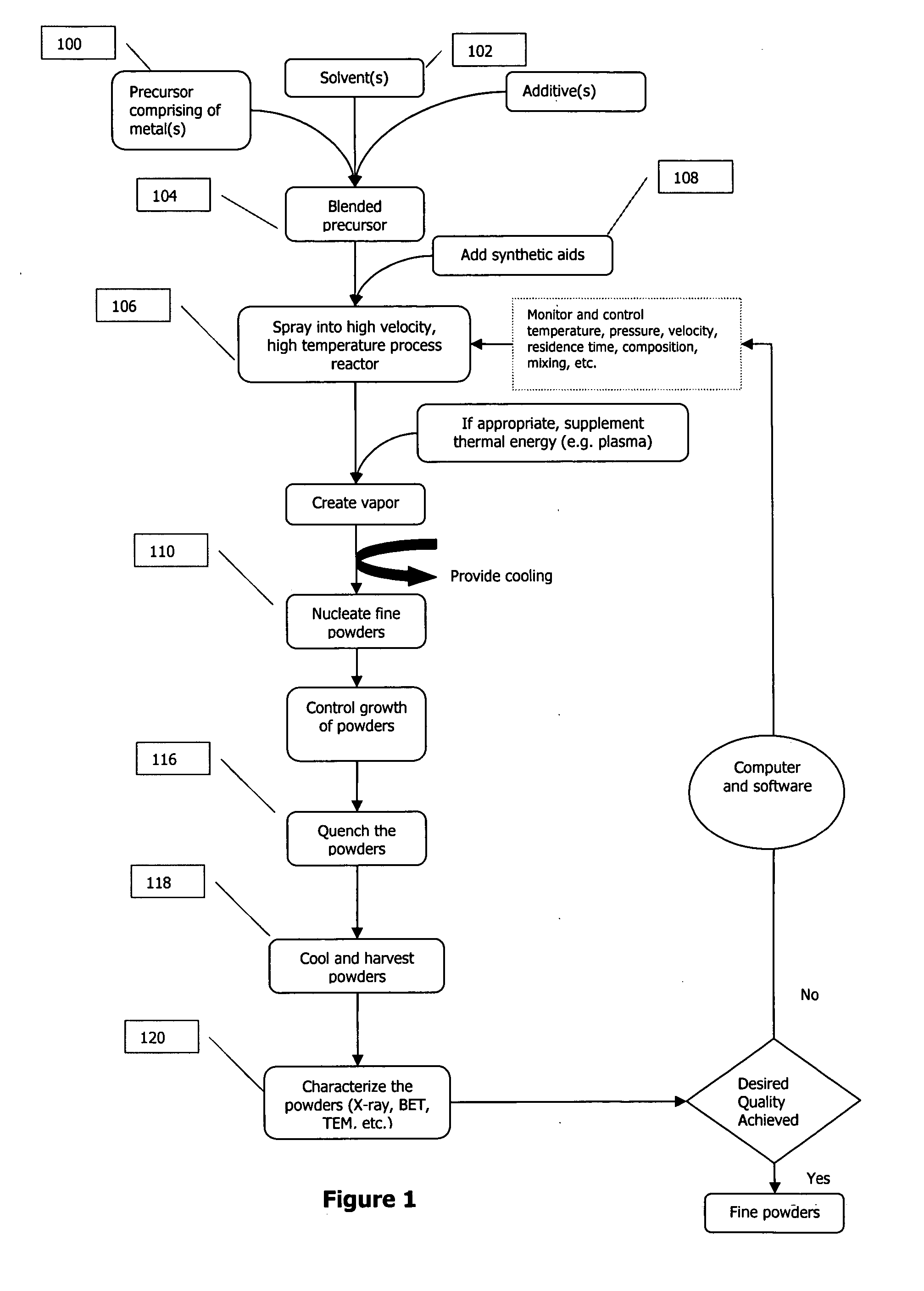
Method and apparatus for analyte sensing
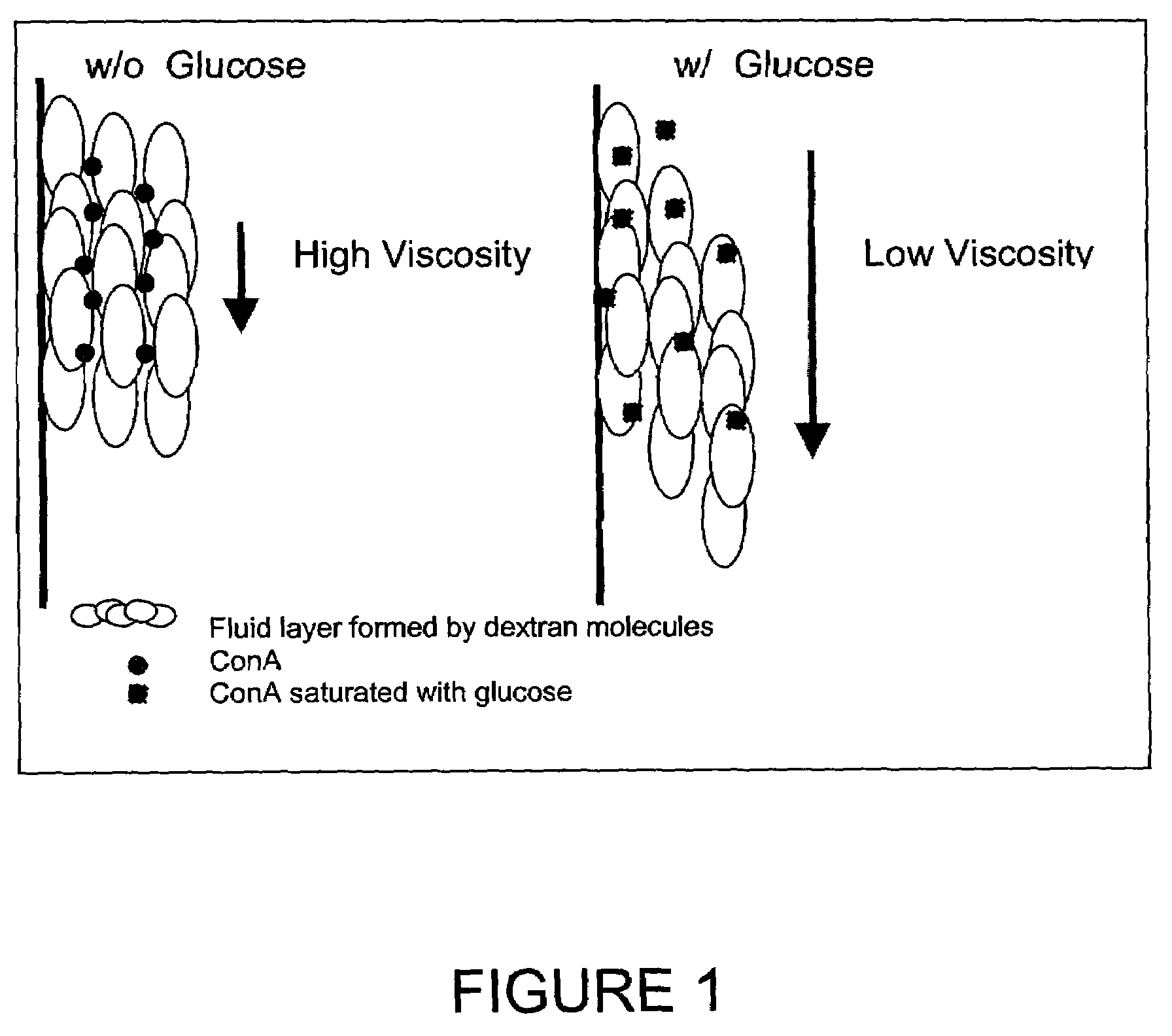
InactiveUS7226414B2Reducing dispersion viscosityLow viscosityCatheterDiagnostic recording/measuringRare-earth elementEngineering
In one aspect, the present invention is directed to a glucose sensing device for implantation within subcutaneous tissue of an animal body. In one embodiment, the glucose sensing device includes a first chamber containing first magnetic particles and a hydrocolloid solution (for example, ConA-dextran hydrocolloid) wherein the first magnetic particles are dispersed in the hydrocolloid solution. In operation, glucose within the animal may enter and exit the first chamber and the hydrocolloid solution changes in response to the presence or concentration of glucose within the first chamber. The sensing device also includes a reference chamber containing second magnetic particles and a reference solution wherein the second magnetic particles are dispersed in the reference solution. The reference solution (for example, oil or alcohol compounds) includes a known or fixed viscosity. The reference solution may also be a hydrocolloid solution (for example, ConA-dextran hydrocolloid). The first and / or second magnetic particles may include amine-terminated particles, at least one rare earth element (for example, neodymium or samarium), and / or a ferromagnetic material.
Owner:BIOTEX
Single phosphor for creating white light with high luminosity and high CRI in a UV LED device
InactiveUS6853131B2Avoids and reduces problemGas-filled discharge tubesDischarge tube luminescnet screensX-rayUltraviolet
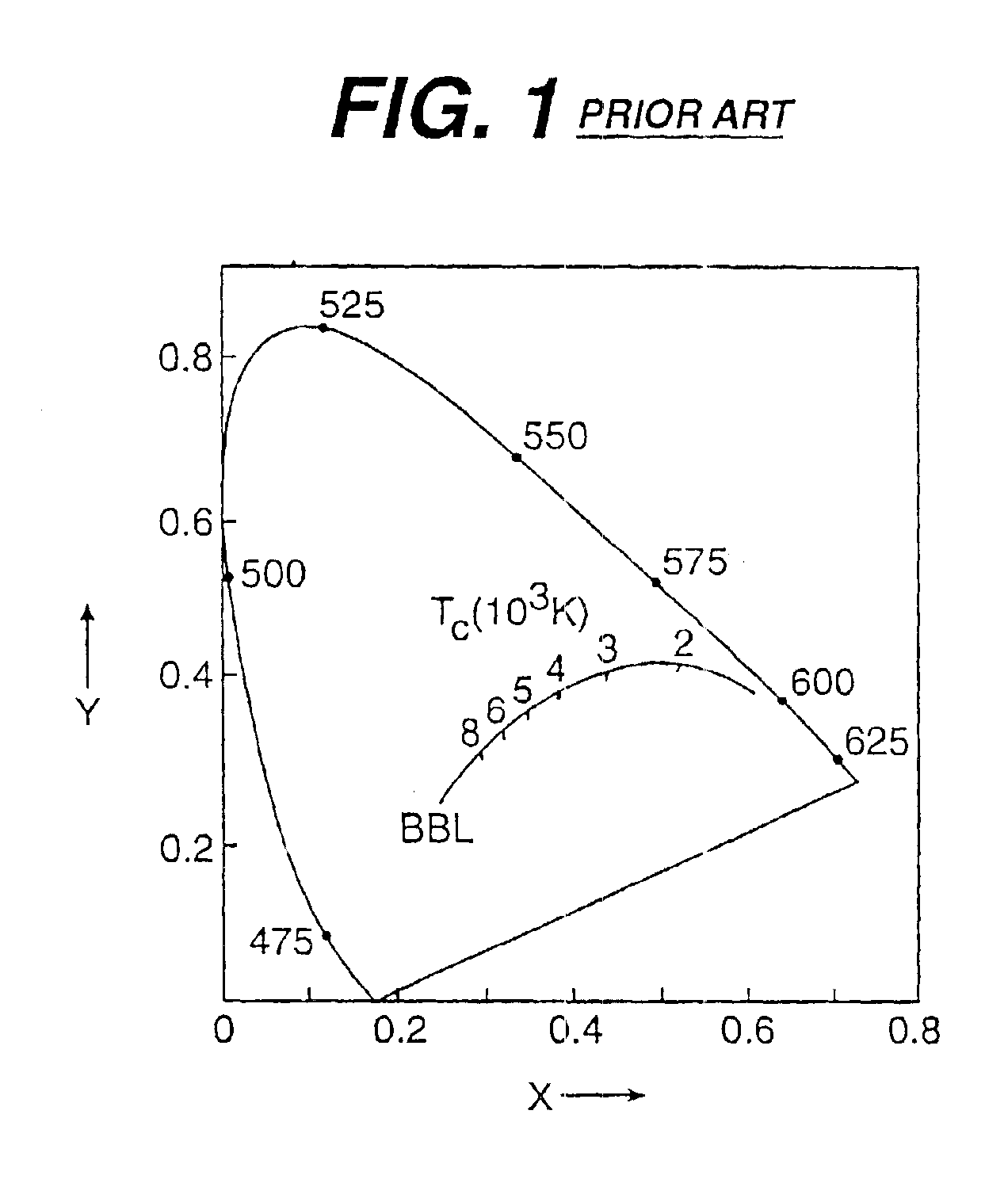
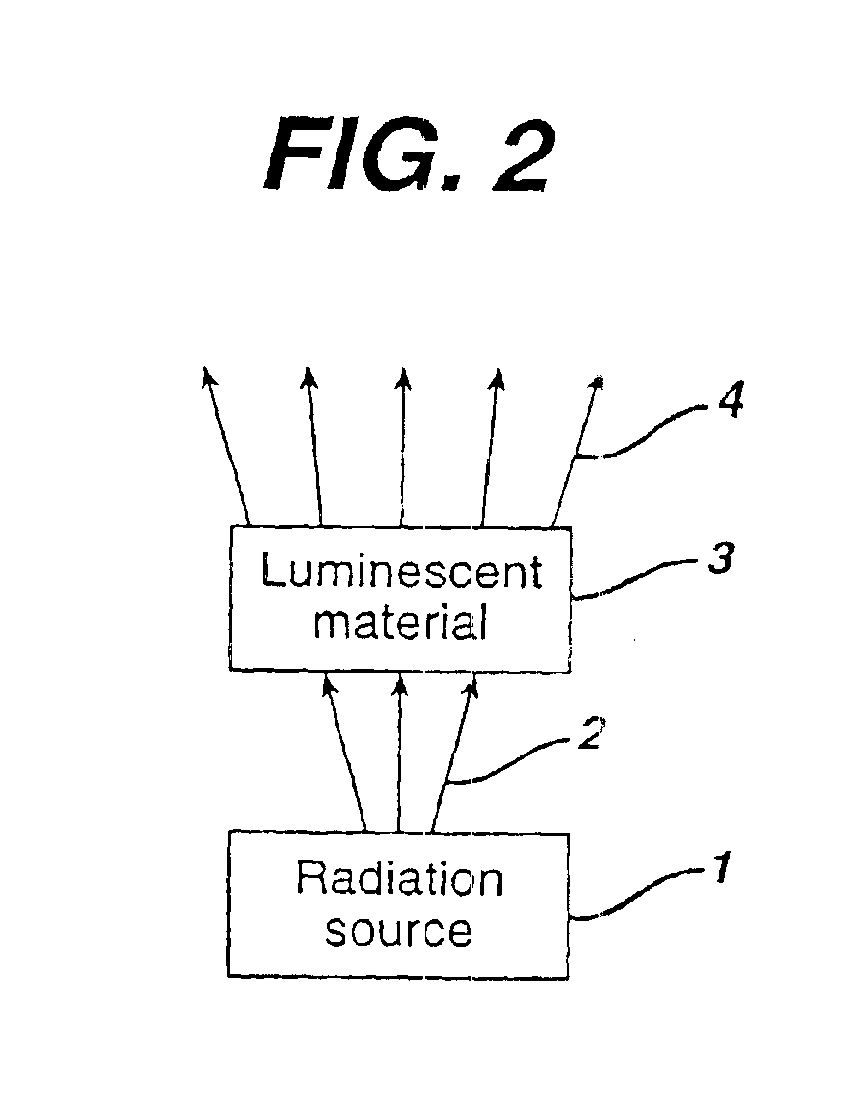
There is provided a white light illumination system. The illumination system includes a radiation source which emits either ultra-violet (UV) or x-ray radiation. The illumination system also includes a luminescent material which absorbs the UV or x-ray radiation and emits the white light. The luminescent material has composition A2−2xNa1+xExD2V3O12. A may be calcium, barium, strontium, or combinations of these three elements. E may be europium, dysprosium, samarium, thulium, or erbium, or combinations thereof. D may be magnesium or zinc, or combinations thereof. The value of x ranges from 0.01 to 0.3, inclusive.
Owner:GENERAL ELECTRIC CO
Layered thermal barrier coatings containing lanthanide series oxides for improved resistance to CMAS degradation
InactiveUS20070160859A1Avoid damageElimination of expensiveBlade accessoriesEfficient propulsion technologiesReaction layerCerium
A coating applied as a two layer system. The outer layer is an oxide of a group IV metal selected from the group consisting of zirconium oxide, hafnium oxide and combinations thereof, which are doped with an effective amount of a lanthanum series oxide. These metal oxides doped with a lanthanum series addition comprises a high weight percentage of the outer coating. As used herein, lanthanum series means an element selected from the group consisting of lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu) and combinations thereof, and lanthanum series oxides are oxides of these elements. When the zirconium oxide is doped with an effective amount of a lanthanum series oxide, a dense reaction layer is formed at the interface of the outer layer of TBC and the CMAS. This dense reaction layer prevents CMAS infiltration below it. The second layer, or inner layer underlying the outer layer, comprises a layer of partially stabilized zirconium oxide.
Owner:GENERAL ELECTRIC CO
Catalyst and method for converting low molecular weight paraffinic hydrocarbons into alkenes and organic compounds with carbon numbers of 2 or more
InactiveUS20070083073A1Reduce the amount requiredPromotes oxidative couplingHydrogenHeterogenous catalyst chemical elementsCarbon numberOxygen
A catalyst and process for formation of hydrocarbons having carbon numbers of two or greater, the result of both oxidative coupling of methane (“OCM”), and other reforming reactions of OCM end products. An OCM catalyst has a structure represented by formula ABTiO3, wherein A is samarium or tin, B is barium; the reforming catalysts a composition represented by formula XYZ, wherein X is a metal from Group IA, Group IIA or Group VIIIA, or not present, Y a metal from Group VA, Group VIA, Group VIIA or Group VIIIA, Z chosen from oxygen, silica, silicalite and alumina. The inventive catalyst comprises an OCM catalyst and a reforming catalyst blended together; when used in a reactor effects an increased yield of hydrocarbons having a carbon number greater than 2 (in excess of 27%-30%, first pass rate of methane conversion about 50%) than occurs under OCM conditions alone.
Owner:HRD CORP
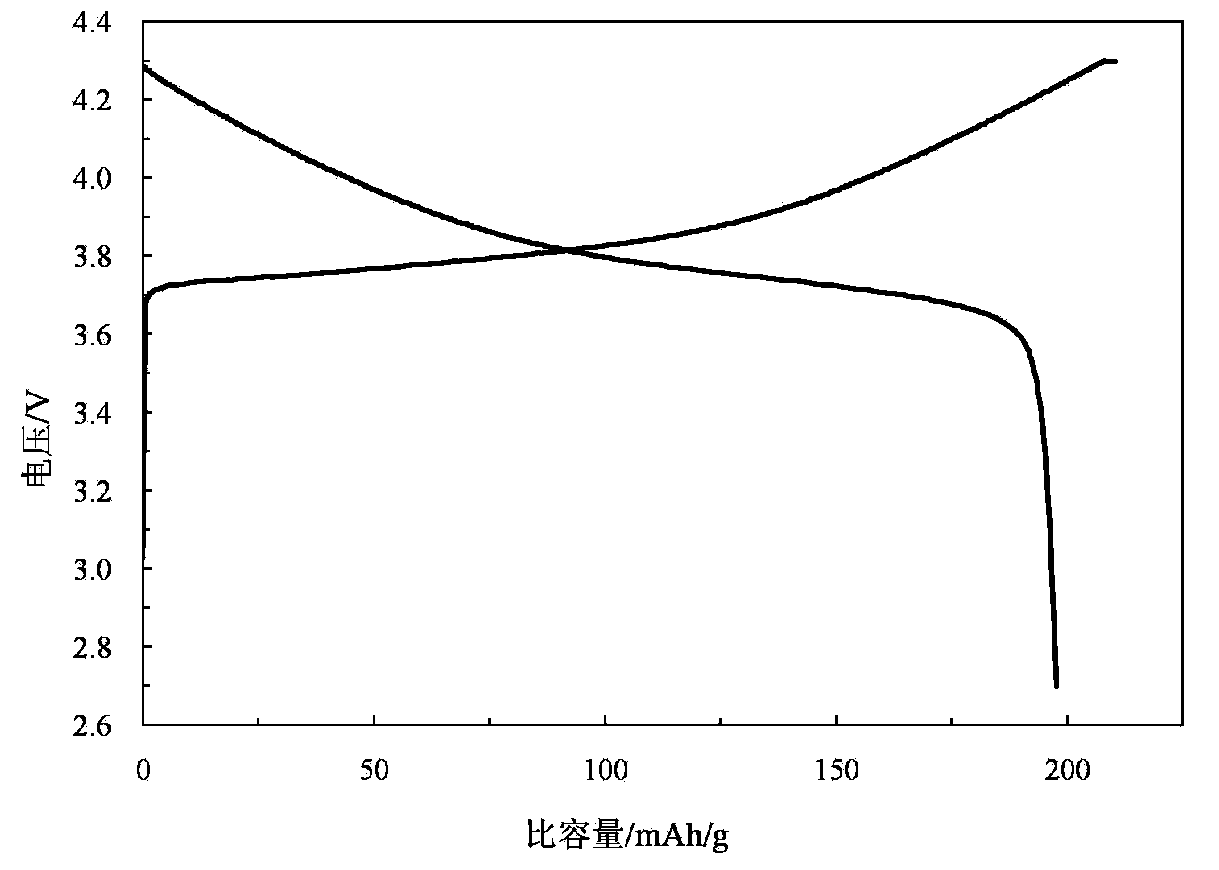
High performance lithium ion battery anode material lithium manganate and preparation method thereof
The invention provides a high performance lithium ion battery anode material lithium manganate and a preparation method of the material. The lithium manganate is a doped lithium manganate LiMn2-yXy04 which is doped with one kind or a plurality of other metal elements X, wherein X element is at least one kind selected form the group of aluminium, lithium, fluorine, silver, copper, chromium, zinc, titanium, bismuth, germanium, gallium, zirconium, stannum, silicon, cobalt, nickel, vanadium, magnesium, calcium, strontium, barium and rare earth elements lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium, and y is larger than 0 but less than or equal to 0.11. The lithium ion battery anode material lithium manganate provided in the invention has extraordinary charge and discharge cycle performance both in the environments of normal temperature and high temperature. According to the invention, the preparation method of the material is a solid phase method, the operation is simple and controllable and the cost is low so that it is easy to realize large-scale productions.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
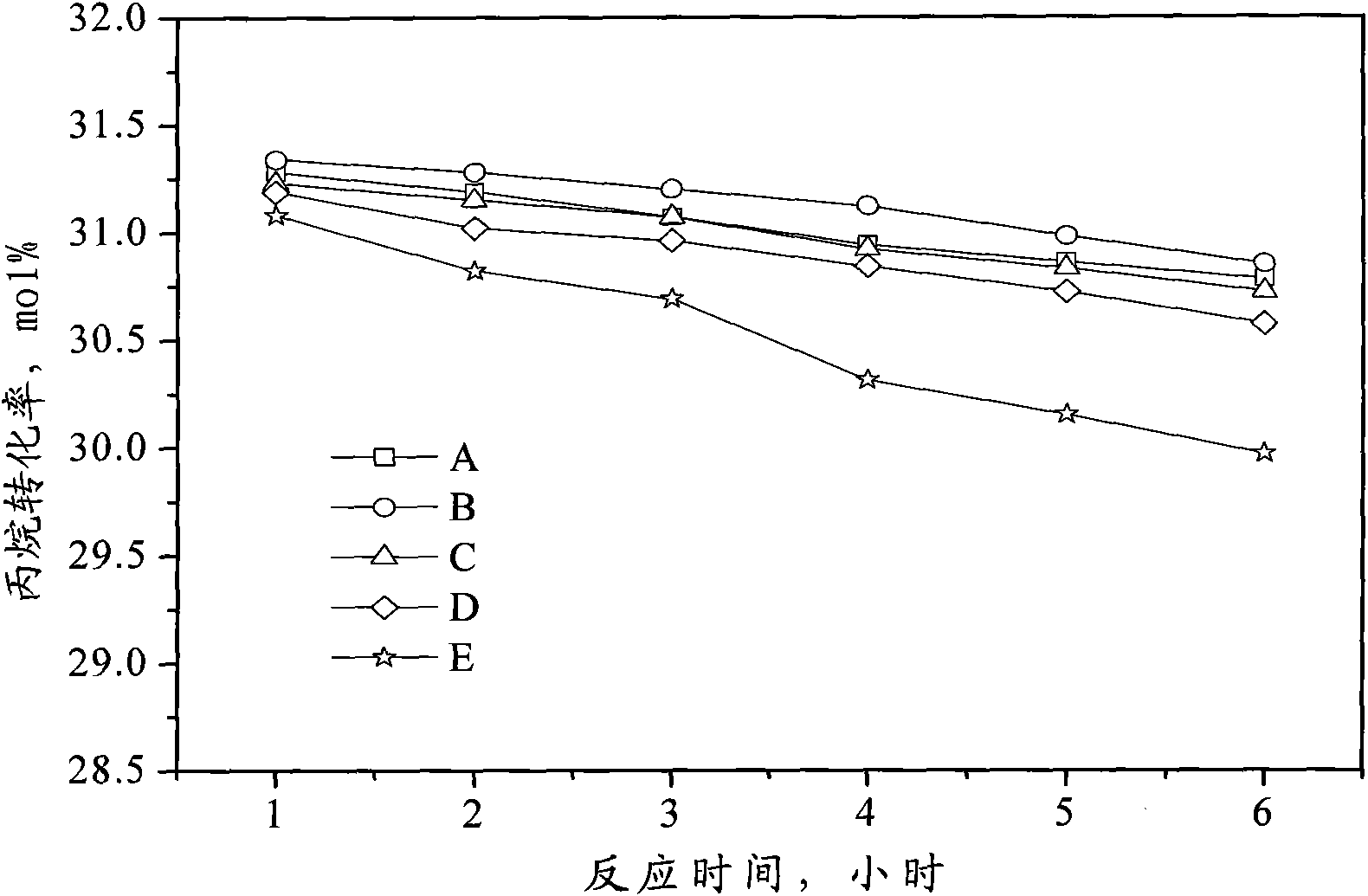
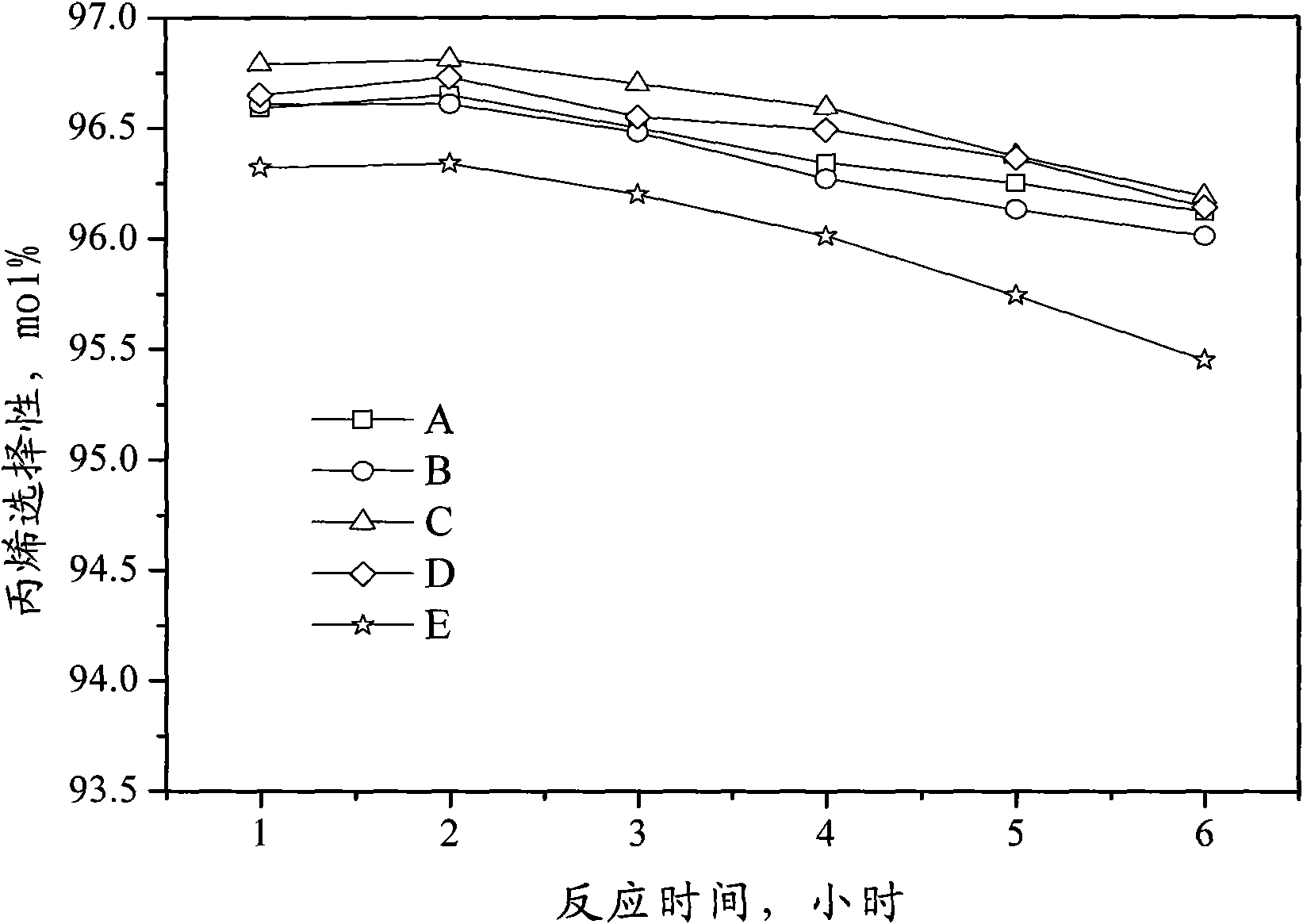
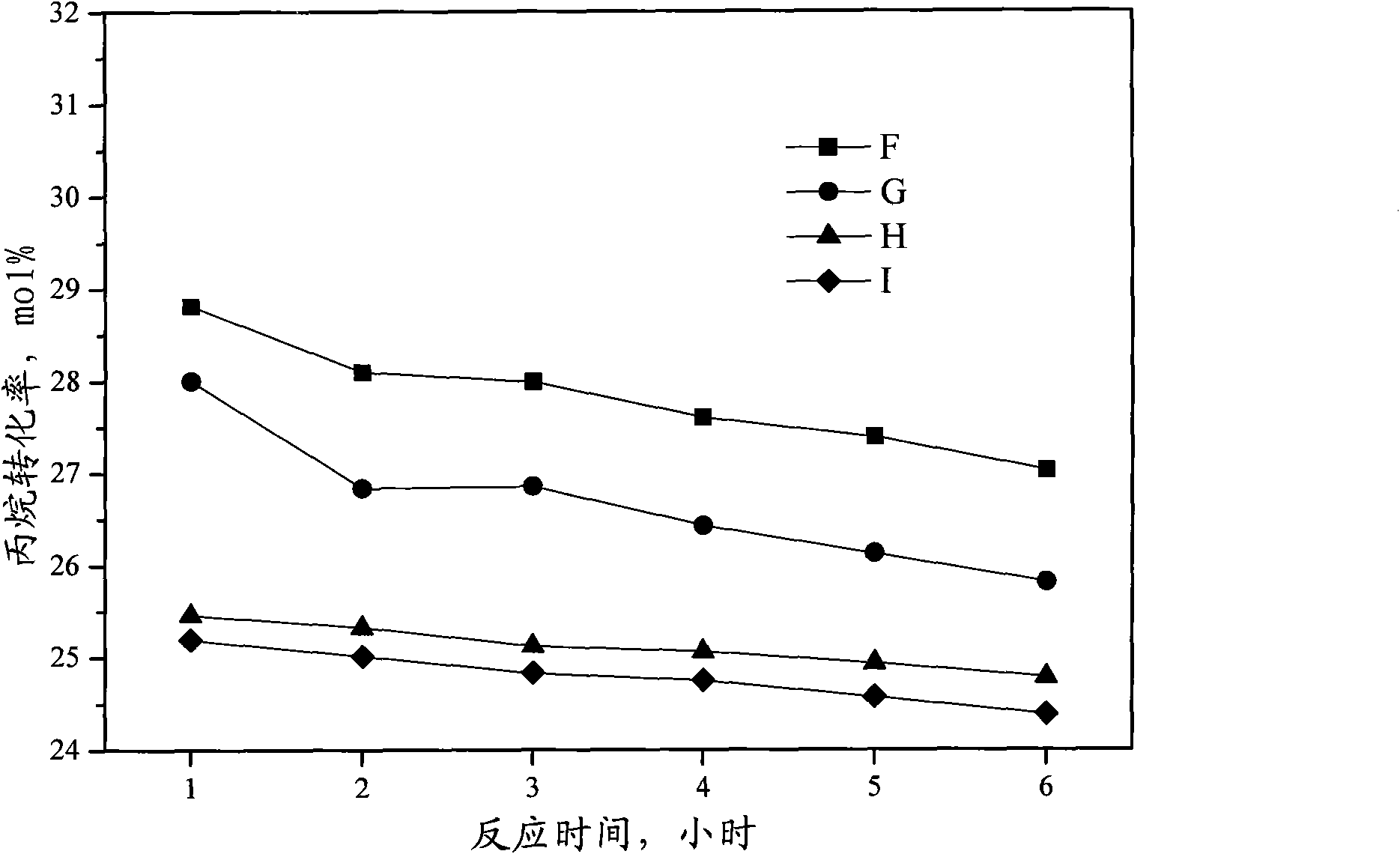
Catalyst for preparing propylene by dehydrogenating propane as well as preparation method and applications thereof
ActiveCN102049267AHigh activityReduce carbon depositionPhysical/chemical process catalystsHydrocarbonsHalogenActive component
The invention relates to a catalyst for preparing propylene by dehydrogenating propane, which comprises an alumina supporter and active components in the following contents by using the alumina supporter as a calculation basis: 0.1-2.0 percent by mass of platinum group metals, 0.1-2.0 percent by mass of IVA group metals, 0.5-5.0 percent by mass of potassium, 0.2-5.0 percent by mass of cerium or samarium, and 0.3-10 percent by mass of halogen. The catalyst is used in the reaction for preparing propylene by dehydrogenating propane. The catalyst has higher conversion rate of propane, higher selectivity of propylene and good regeneration performance.
Owner:CHINA PETROLEUM & CHEM CORP +1
Rare-earth doping modified lithium ion battery ternary positive electrode material and preparation method thereof
ActiveCN103855384AEase of industrial productionImprove charge and discharge efficiencyCell electrodesSecondary cellsCarbon compositesNickel salt
The invention relates to a rare-earth doping modified lithium ion battery ternary positive electrode material and a preparation method of the rare-earth doping modified lithium ion battery ternary positive electrode material. The chemical general formula of the material is as follows: LiNiaCo<1-a-b>MnbRxO2 / M, wherein a is more than 0 and less than 1, b is more than 0 and less than 1, (1-a-b) is more than 0 and less than 1, x is more than 0.005 and less than 0.1, R is one or more of rare-earth lanthanum, cerium, praseodymium and samarium, and M is a composite cladding layer of oxide of aluminum, titanium or magnesium and carbon. The soluble metal nickel salt, cobalt salt, manganese salt and rare-earth compound are mixed to prepare a mixed salt solution, the mixed salt solution is reacted with a mixed alkaline solution prepared by mixing NaOH and ammonium hydroxide, after the reaction solution is filtered, washed and dried, the obtained product is uniformly mixed with lithium salt powder to be ball milled, then the mixture is calcined at the high temperature and coated with the composite cladding layer of the aluminum, titanium or magnesium oxide and carbon, and finally the calcined mixture is calcined at a constant temperature to obtain the rare-earth doping modified lithium ion battery ternary positive electrode material. After doping the rare earth, the metal oxide and carbon composite cladding layer, which are cheap and easy to obtain, are adopted, so that the cycling performance and the rate performance can be improved, and the charging-discharging efficiency of the material also can be improved.
Owner:ZHEJIANG MEIDARUI NEW MATERIAL TECH CO LTD
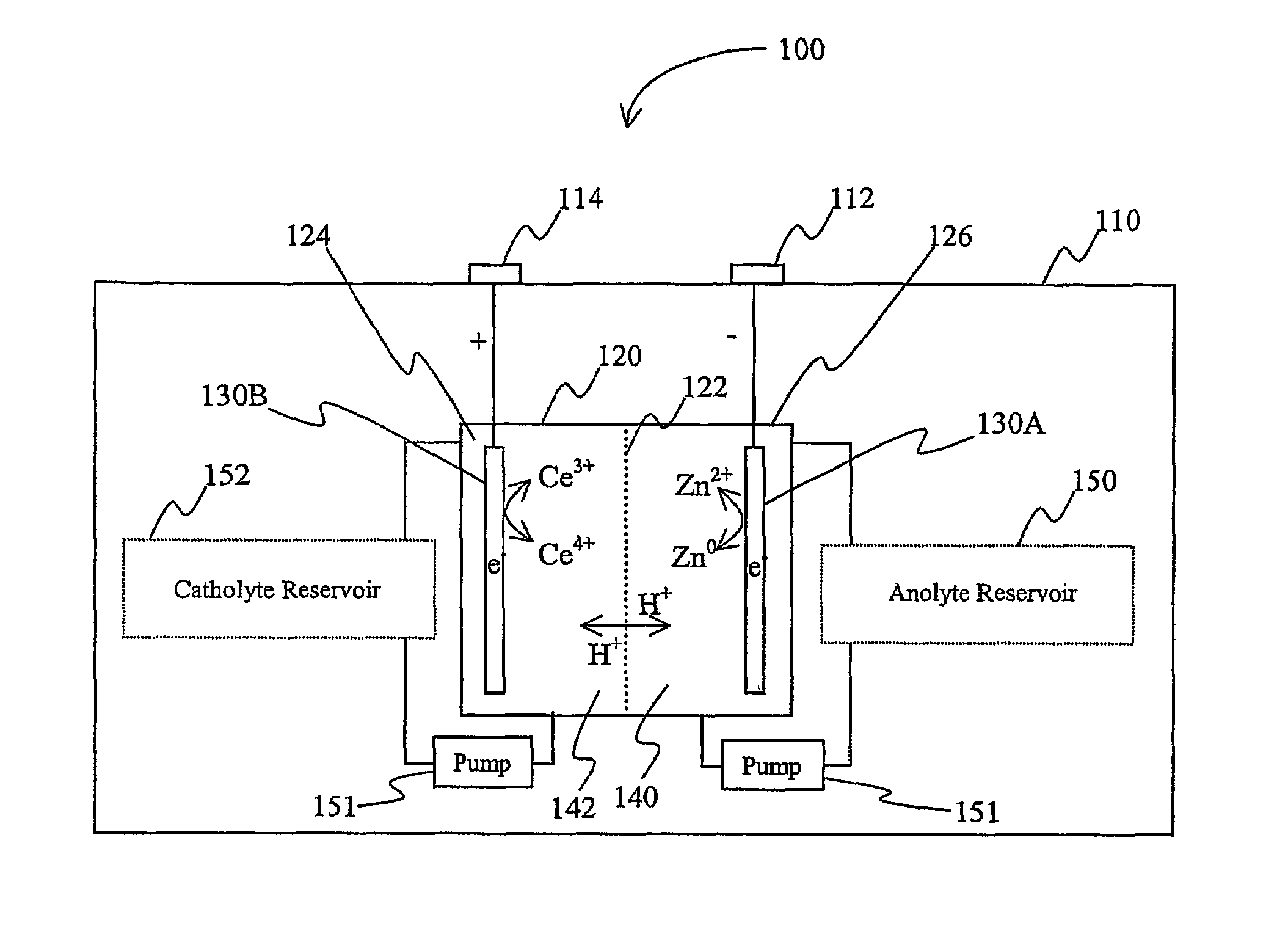
Lanthanide batteries
InactiveUS7252905B2Wide capacity rangeLarge capacityAlkaline accumulatorsSolid electrolyte cellsLanthanideCerium
A battery (100) comprises an electrolyte in which a lanthanide and zinc form a redox pair. Preferred electrolytes are acid electrolytes, and most preferably comprise methane sulfonic acid, and it is further contemplated that suitable electrolytes may include at least two lanthanides. Contemplated lanthanides include cerium, praseodymium, neodymium, terbium, and dysprosium, and further contemplate lanthanides are samarium, europium, thulium and ytterbium.
Owner:PLURION LTD
Ceramic bonding composition, method of making, and article of manufacture incorporating the same
A ceramic bonding composition comprises a first oxide and at least a second oxide having a formula of Me2O3; wherein the first oxide is selected from the group consisting of aluminum oxide, scandium oxide, and combinations thereof; Me is selected from the group consisting of yttrium, lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and combinations thereof. The ceramic bonding composition can further comprise silica. An article of manufacture comprising at least two members attached together with the ceramic bonding composition.
Owner:GENERAL ELECTRIC CO
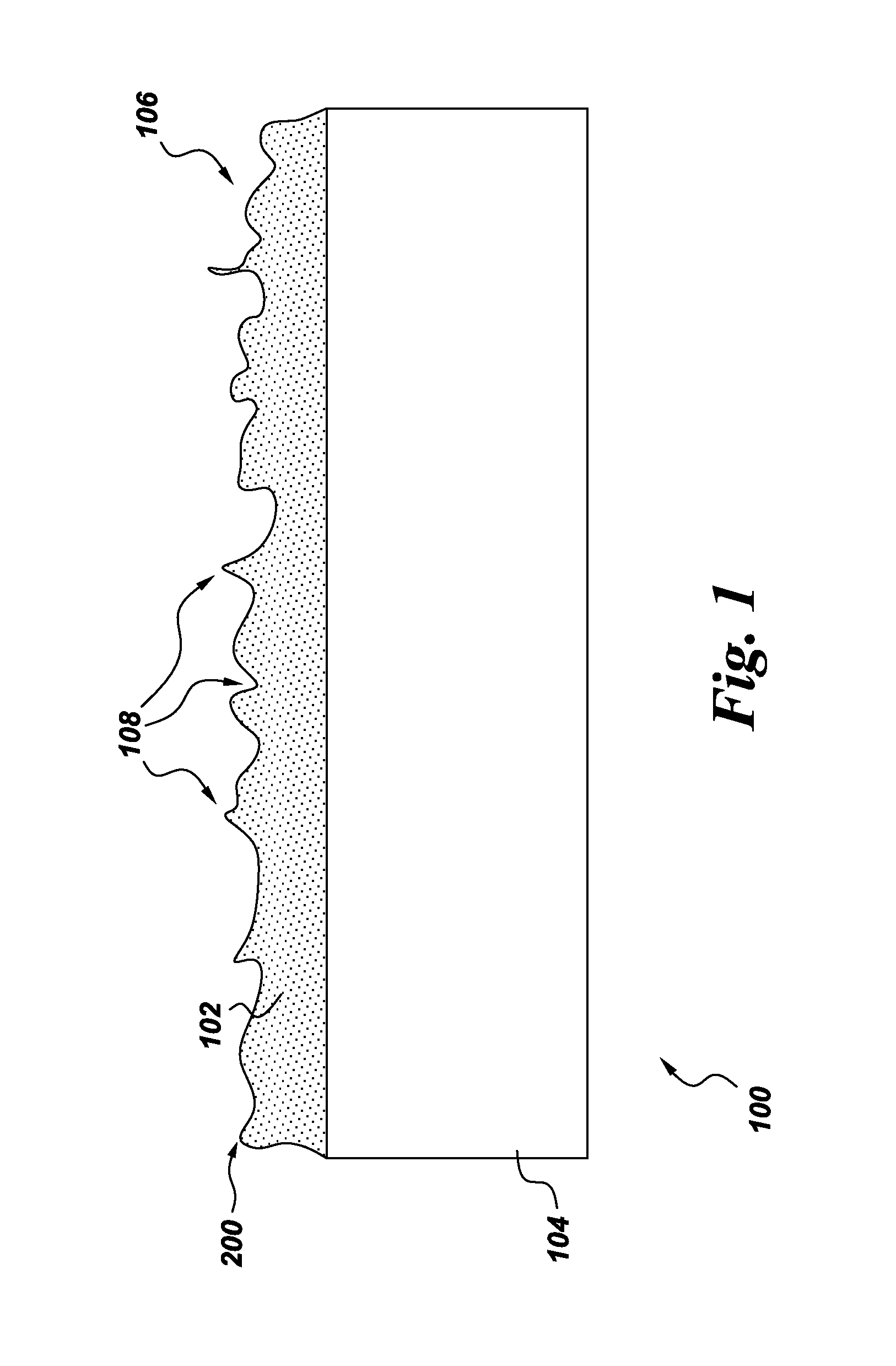
Methods for making barrier coatings comprising taggants and components having the same
Methods for making barrier coatings including a taggant involving providing a barrier coating, and adding from about 0.01 mol % to about 30 mol % of a taggant to the barrier coating wherein the taggant comprises a rare earth element selected from lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, ytterbium, and lutetium, salts thereof, silicates thereof, oxides thereof, zirconates thereof, hafnates thereof, titanates thereof, tantalates thereof, cerates thereof, aluminates thereof, aluminosilicates thereof, phophates thereof, niobates thereof, borates thereof, and combinations thereof.
Owner:GENERAL ELECTRIC CO
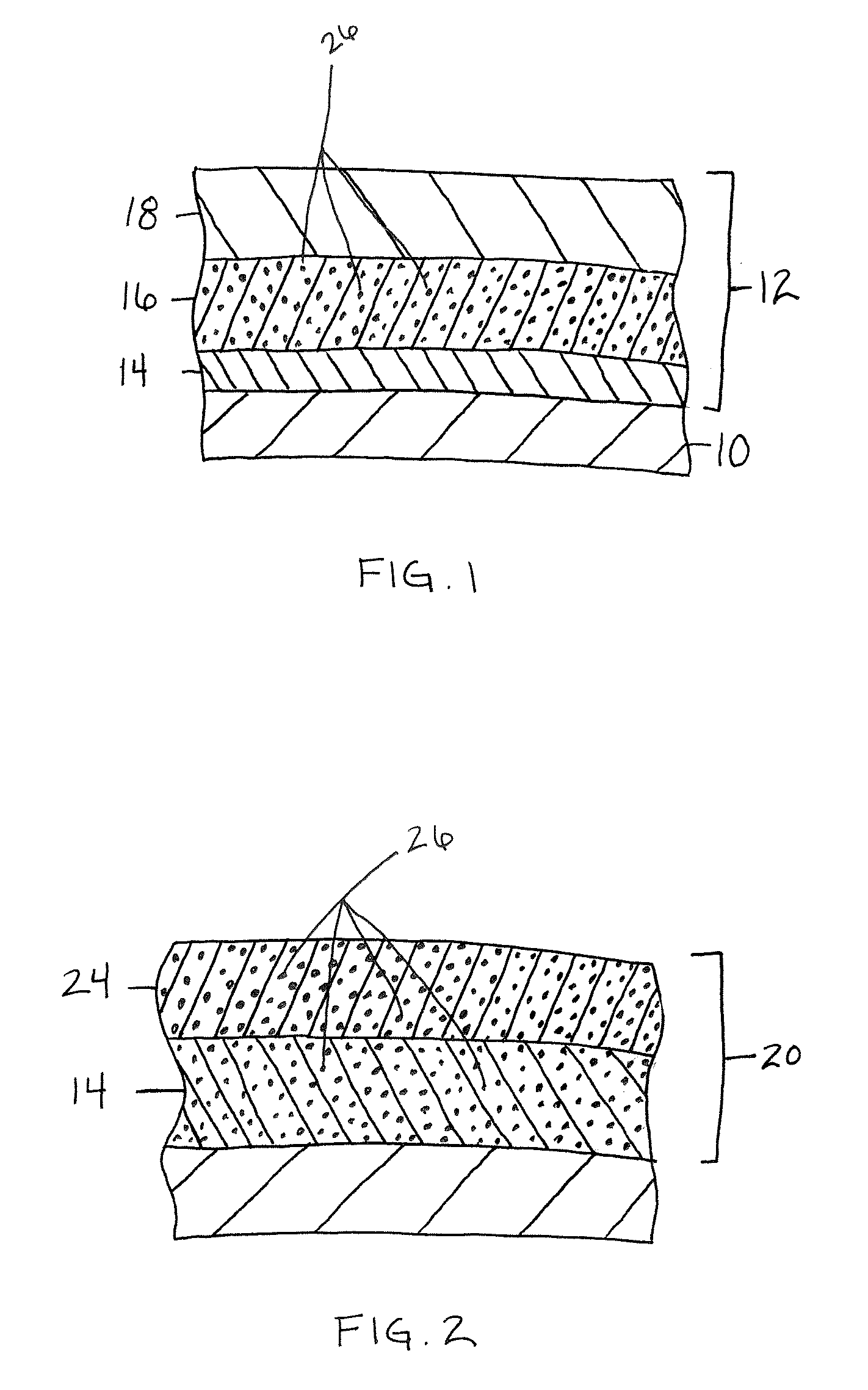
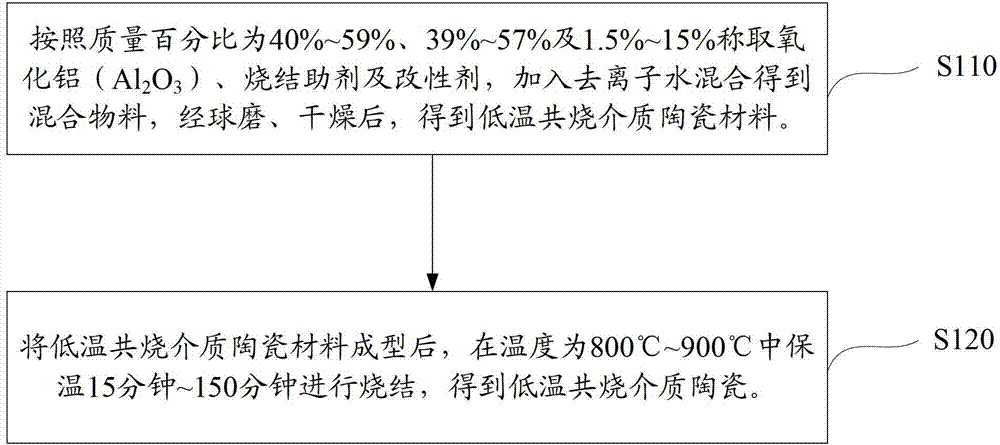
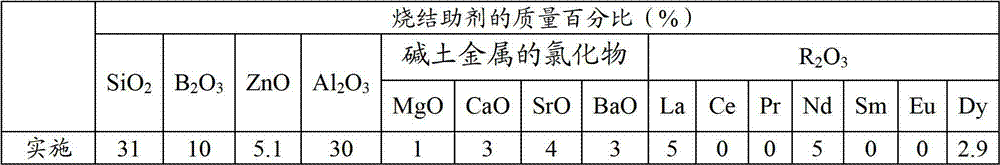
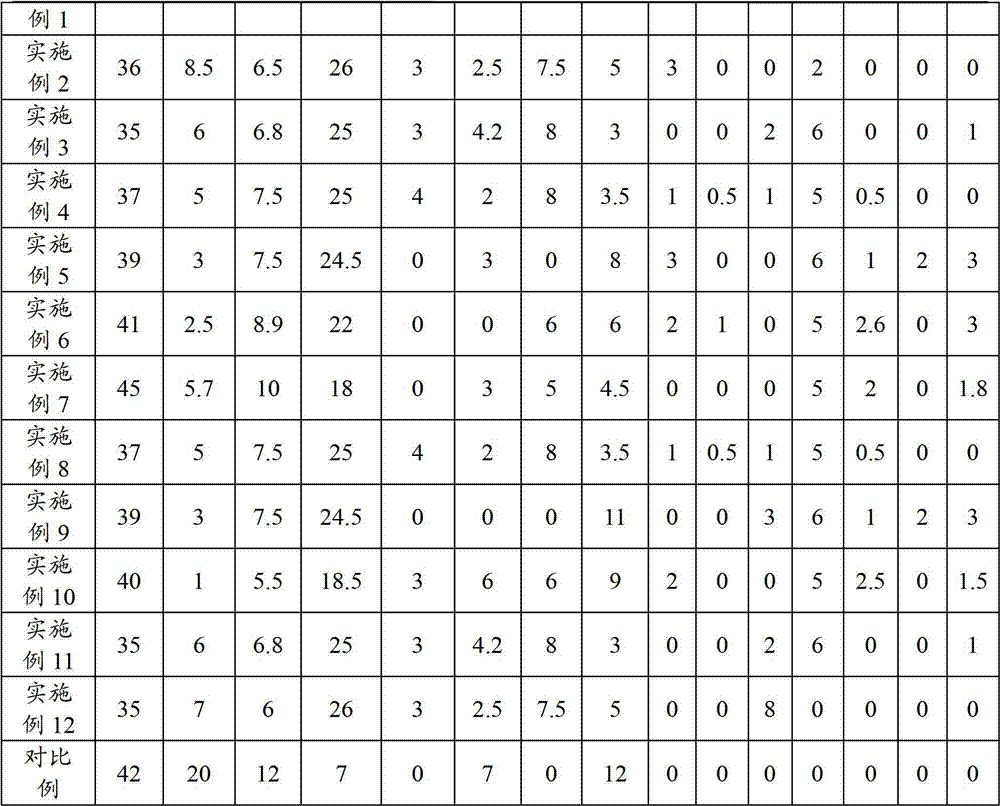
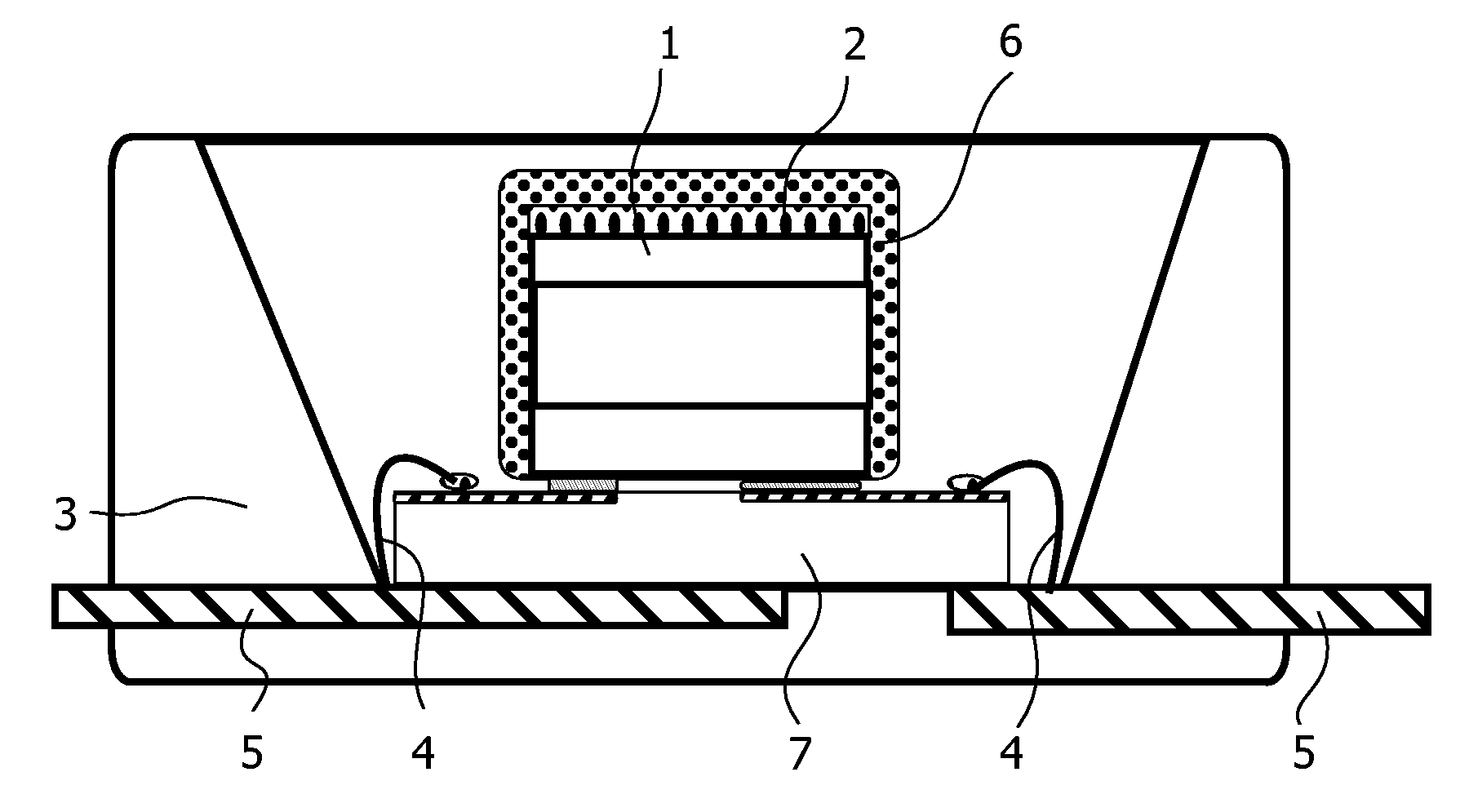
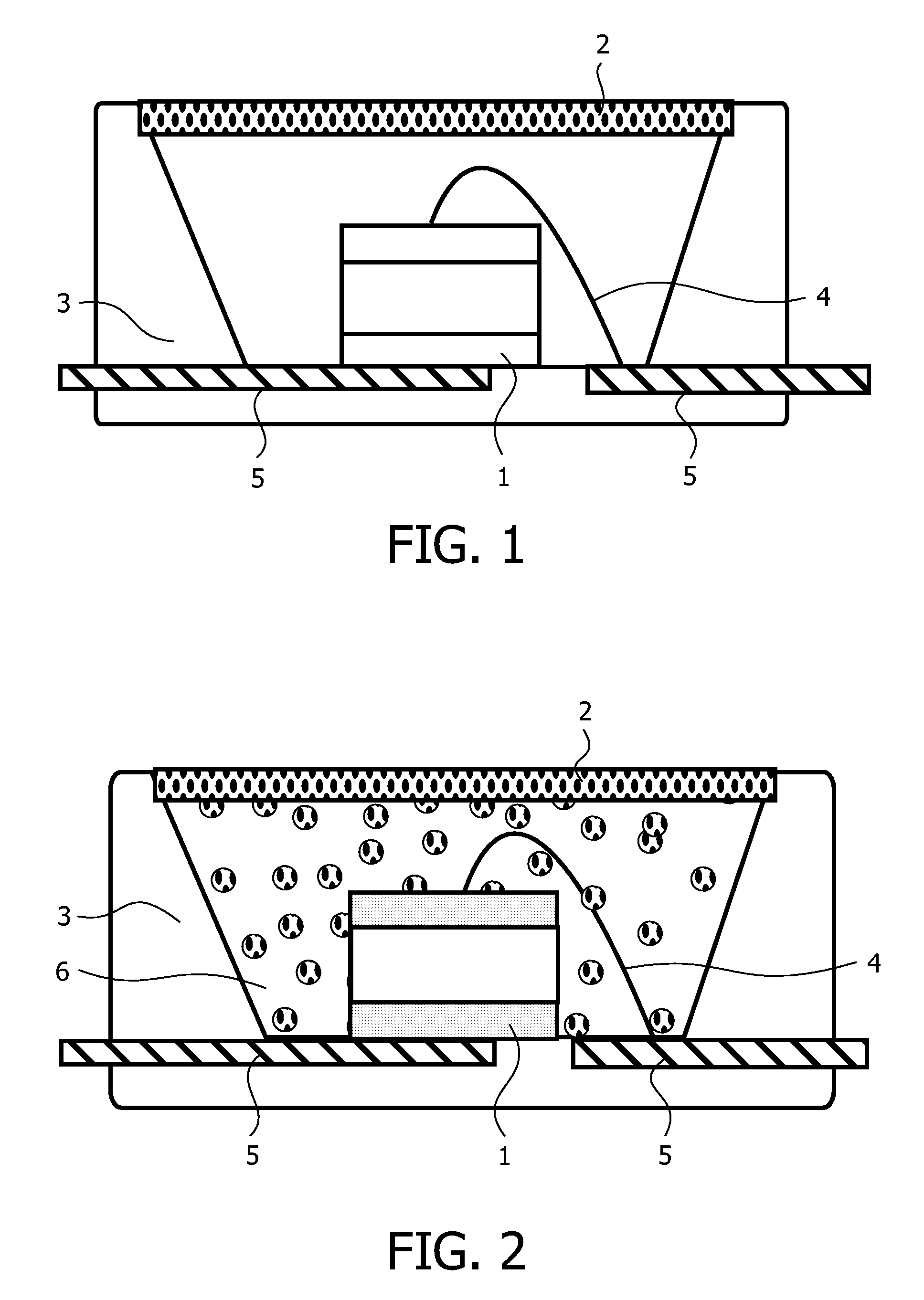
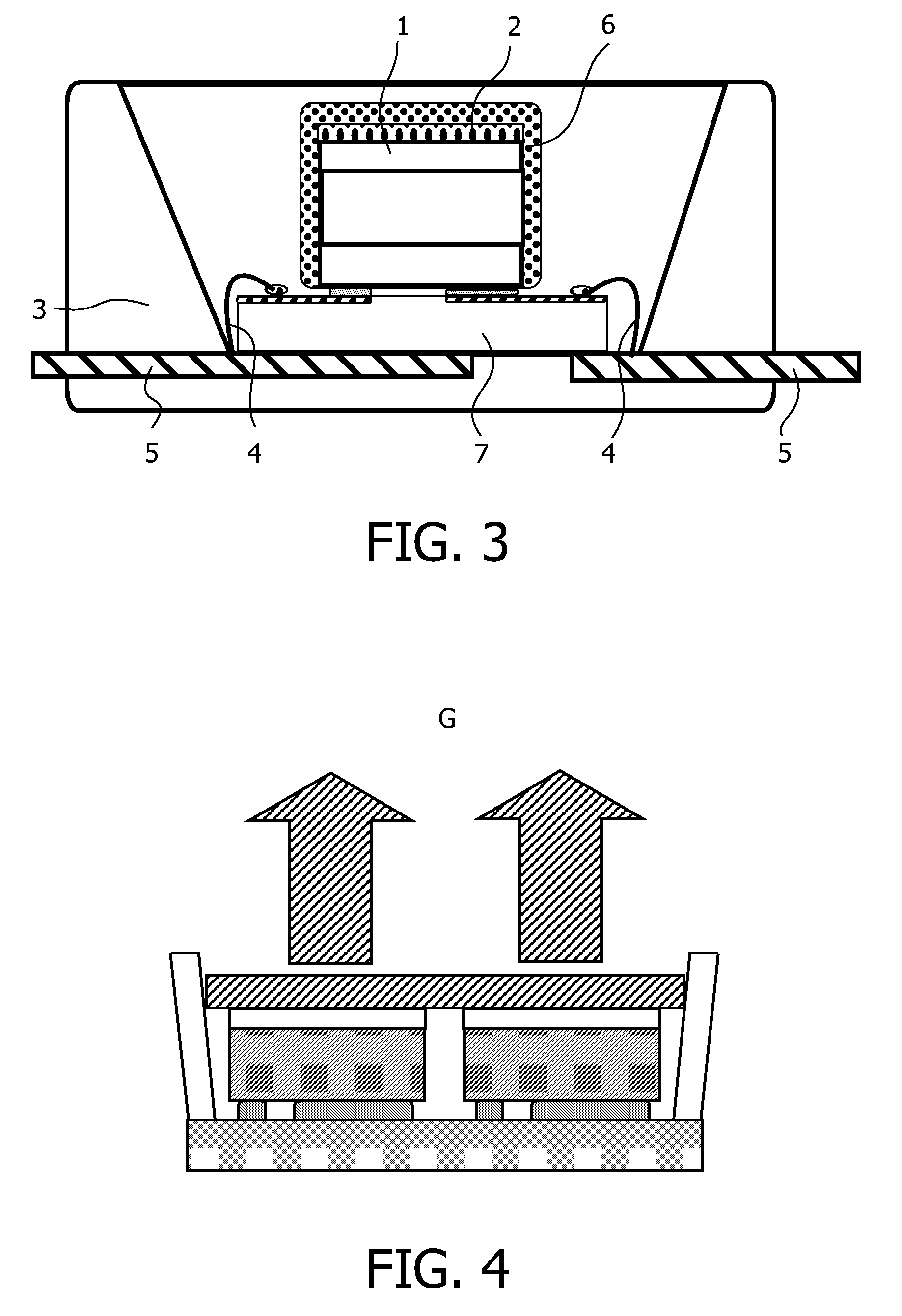
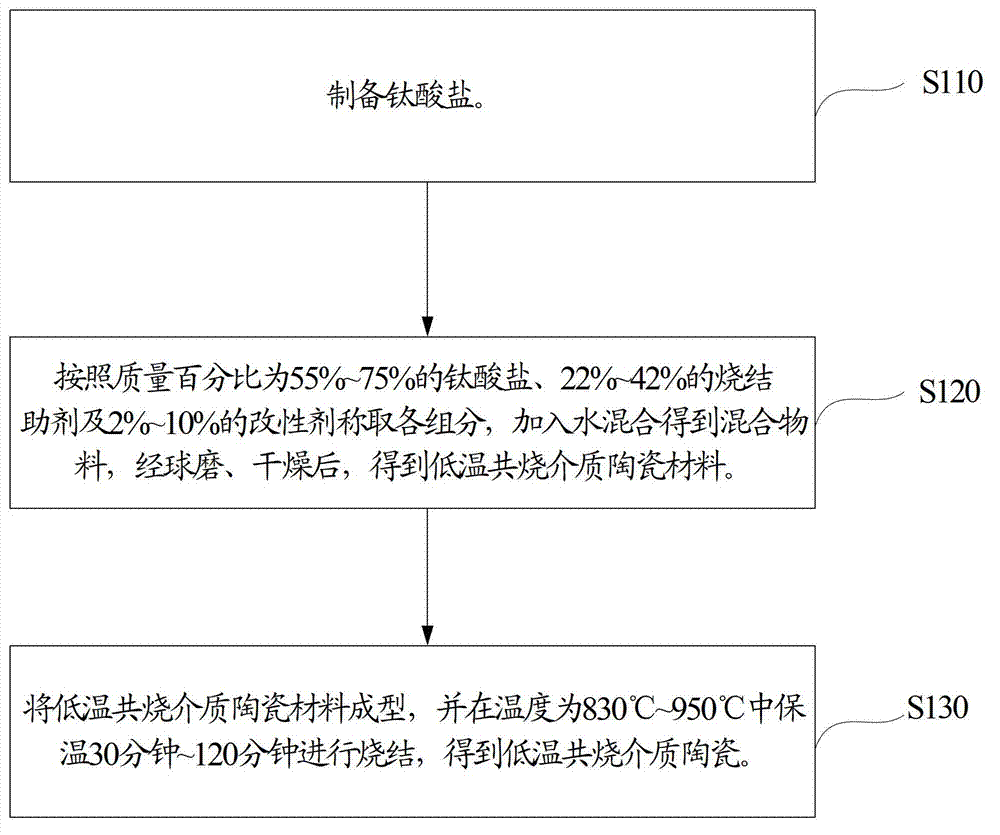
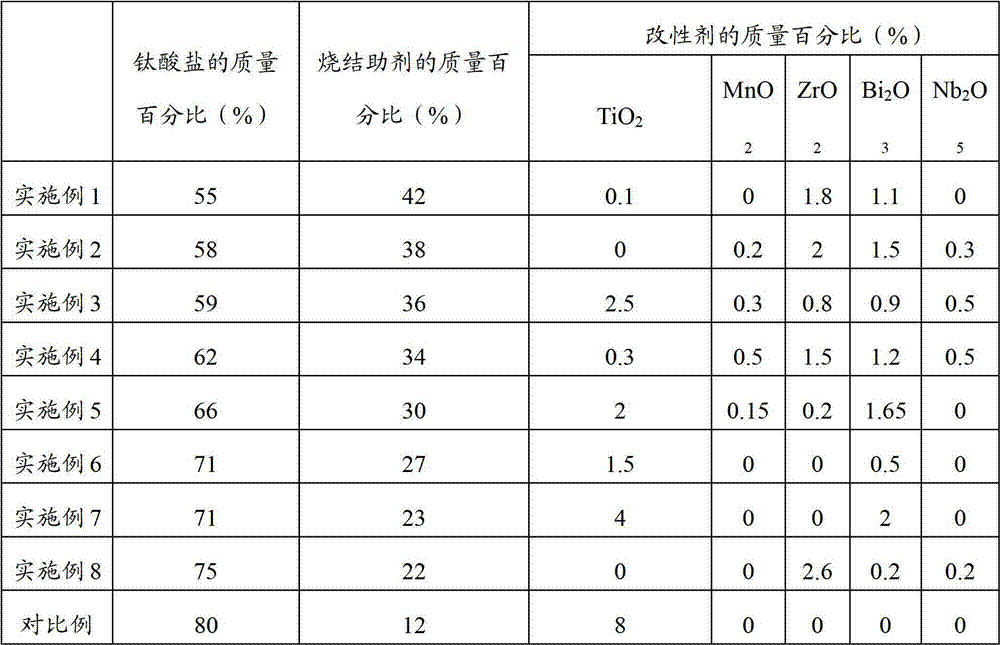
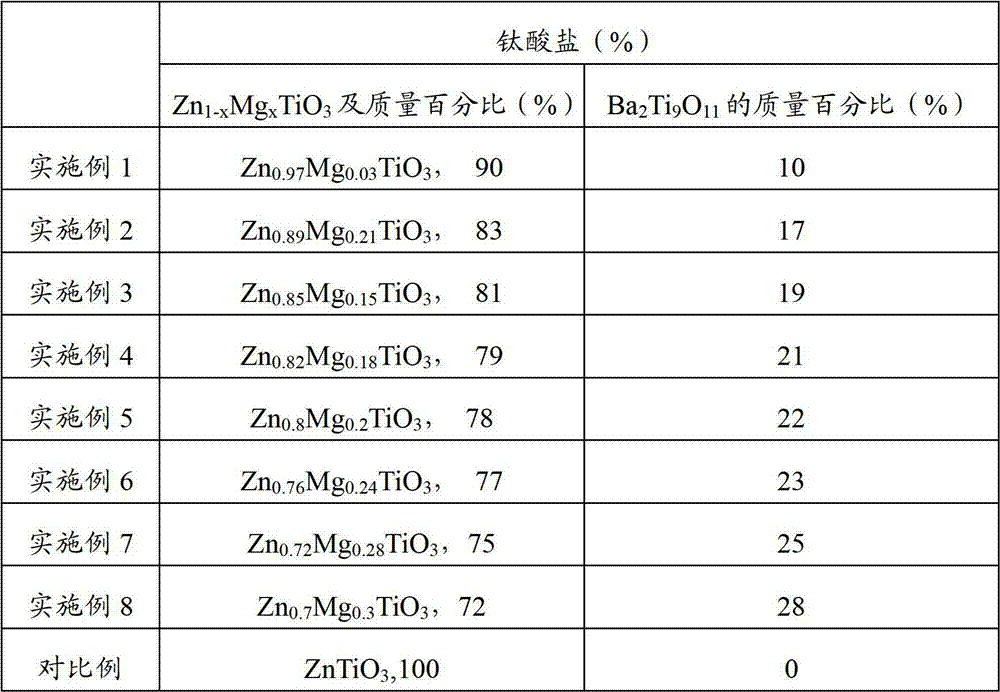
Method, sintering aid and materials for preparation of low-temperature cofired medium ceramic and application
A sintering aid for a low-temperature cofired medium ceramic material is composed of, by weight, 31%-45% of silicon dioxide, 1%-10% of boron oxide, 5.1%-10% of zinc oxide, 18%-30% of aluminum oxide, 11%-24% of alkaline earth metallic oxide and 5%-15% of oxide with the general formula of R2O3, wherein R refers to at least one of lanthanum, cerium, praseodymium, neodymium, samarium, europium and dysprosium, and the alkaline earth metallic oxide refers to one of magnesium oxide, calcium oxide, barium oxide and strontium oxide. Adding the sintering aid into the low-temperature cofired medium ceramic material enables the prepared low-temperature cofired medium ceramic to have excellent thermal mechanical performance and dielectric performance. In addition, the invention provides the low-temperature cofired medium ceramic material and application thereof and a method for preparing the low-temperature cofired medium ceramic.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG
CMAS resistant thermal barrier coating
ActiveUS20070172703A1Reduce componentsReduces sand related distressMolten spray coatingBlade accessoriesIndiumCerium
A turbine engine component is provided which has a substrate and a thermal barrier coating applied over the substrate. The thermal barrier coating comprises alternating layers of yttria-stabilized zirconia and a molten silicate resistant material. The molten silicate resistant outer layer may be formed from at least one oxide of a material selected from the group consisting of lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, indium, zirconium, hafnium, and titanium or may be formed from a gadolinia-stabilized zirconia. If desired, a metallic bond coat may be present between the substrate and the thermal barrier coating system. A method for forming the thermal barrier coating system of the present invention is described.
Owner:RTX CORP
Samarium-cobalt permanent magnet material and preparation method thereof
The invention provides a samarium-cobalt permanent magnet material, which consists of the following components in percentage by mass: 23 to 25.5 percent of samarium, 44 to 50 percent of cobalt, 14 to 20 percent of iron, 3 to 8 percent of copper, 2 to 4 percent of zirconium and 0.5 to 2 percent of heavy rare earth element. The invention also provides a method for preparing the samarium-cobalt permanent magnet material, which comprises the following steps of: mixing; smelting alloys; preparing magnetic power; orienting and forming; sintering and performing solid solution; and ageing. The samarium-cobalt permanent magnet material has the characteristic of high coercive force and can effectively meet the requirement of the field of high and new technology on the high coercive force of a rare earth permanent magnet material.
Owner:NINGBO STAR MATERIALS HI TECH
Samarium-cobalt sintered magnet material and preparation method thereof
InactiveCN101882494ALower temperature coefficient of remanenceLower flux temperature coefficientMagnetic materialsRare earthCobalt
The invention discloses a 2:17 type samarium-cobalt sintered magnet material and a preparation method thereof. The 2:17 type samarium-cobalt sintered magnet material comprises the following raw materials in percentage by mass: 10 to 25 percent of samarium, 45 to 55 percent of cobalt, 10 to 20 percent of iron, 3 to 9 percent of copper, 1 to 3 percent of zirconium and 5 to 15 percent of at least one heavy rear earth element. Due to the selection and the proportion of the raw materials and the innovation of the sintering process, a microstructure of the magnet material is optimized, and the aims of reducing the temperature coefficient of a magnet and simultaneously maintaining higher magnetic energy product of the magnet are fulfilled, wherein the magnetic energy product of the prepared magnet is 14 to 25 MGsOe, the residual magnetism temperature coefficient is about -0.005 to -0.03 percent per DEG C, and a lower magnetic flux temperature coefficient is maintained in an environment at the temperature of between -35 and 300 DEG C.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
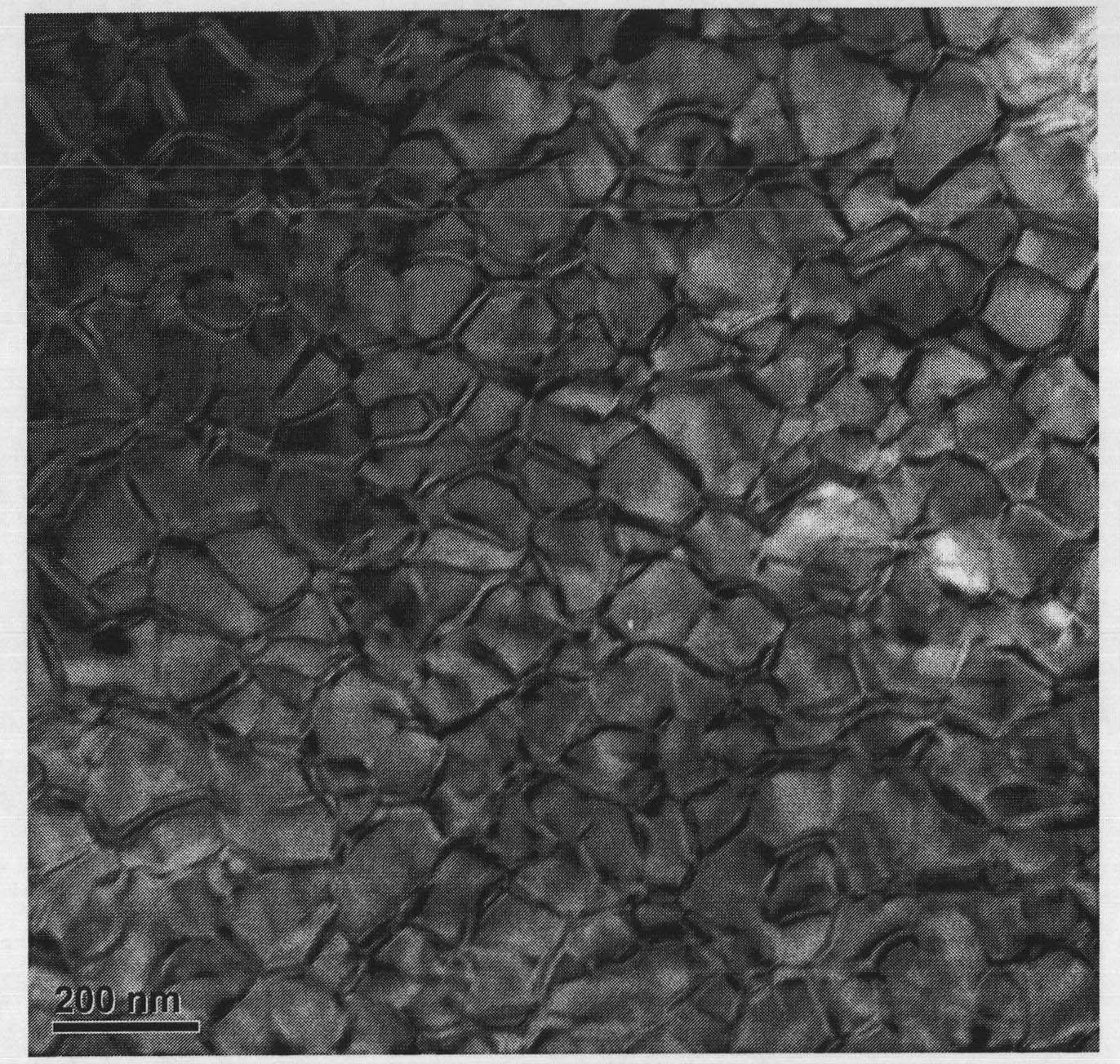
Nanoparticles of rare earth oxides
ActiveUS20070104629A1Increase volumeLow cost productionMaterial nanotechnologyLanthanum oxide/hydroxidesCeriumScandium
Rare earth compositions comprising nanoparticles, methods of making nanoparticles, and methods of using nanoparticles are described. The compositions of the nanomaterials discussed may include scandium (Sc), yttrium (Y), lanthanum(La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium(Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). The nanoparticles can be used to make organometallics, nitrates, and hydroxides. The nanoparticles can be used in a variety of applications, such as pigments, catalysts, polishing agents, coatings, electroceramics, catalysts, optics, phosphors, and detectors.
Owner:PPG IND OHIO INC
Coproduction of hydrofluoroolefins
InactiveUS7687670B2Preparation by hydrogen halide split-offHalogenated hydrocarbon separation/purificationCeriumCobalt
Disclosed is a process for the co-manufacture of the hydrofluoroolefins HFC-1225ye and HFC-1234yf. The process comprises contacting a blend of 1,1,1,2,3,3-hexafluoropropane and 1,1,1,2,3-pentafluoropropane at a temperature of from about 200° C. to about 500° C. with a catalyst, optionally in the presence of an inert gas. The catalyst includes, but is not limited to, aluminum fluoride; fluorided alumina; metals on aluminum fluoride; metals on fluorided alumina; oxides, fluorides, and oxyfluorides of magnesium, zinc and mixtures of magnesium and zinc and / or aluminum; lanthanum oxide and fluorided lanthanum oxide; chromium oxides, fluorided chromium oxides, and cubic chromium trifluoride; carbon, acid-washed carbon, activated carbon, three dimensional matrix carbonaceous materials; and metal compounds supported on carbon. The metal compounds are oxides, fluorides, and oxyfluorides of at least one metal selected from the group consisting of sodium, potassium, rubidium, cesium, yttrium, lanthanum, cerium, praseodymium, neodymium, samarium, chromium, iron, cobalt, rhodium, nickel, copper, zinc, and mixtures thereof. The product hydrofluoroolefins are separated from unreacted hydrofluorocarbons and hydrogen fluoride. In another embodiment, the unreacted hydrofluorocarbons optionally may be recirculated back through the process.
Owner:EI DU PONT DE NEMOURS & CO
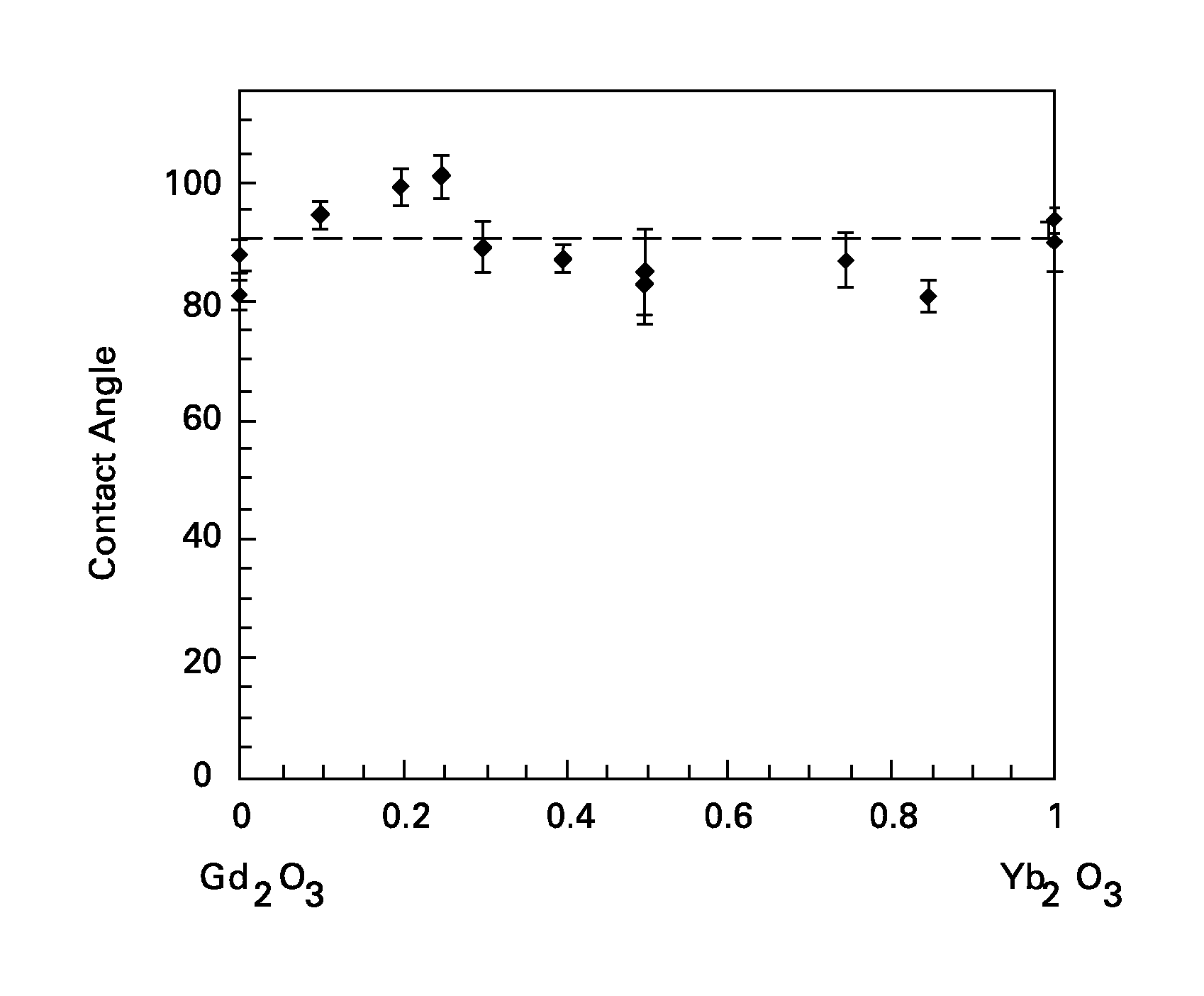
Wetting resistant materials and articles made therewith
Ceramic materials with relatively high resistance to wetting by various liquids, such as water, are presented, along with articles made with these materials. The oxide materials described herein as a class typically contain one or more of ytterbia (Yb2O3) and europia (Eu2O3). The oxides may further contain other additives, such as oxides of gadolinium (Gd), samarium (Sm), dysprosium (Dy), or terbium (Tb). In certain embodiments the oxide, in addition to the ytterbia and / or europia, further comprises lanthanum (La), praseodymium (Pr), or neodymium (Nd).
Owner:GENERAL ELECTRIC CO
Catalyst carrier, catalyst and process for producing the same
InactiveUS20100331172A1Improve heat resistanceHigh catalytic ability without increasing the specific surface areaCatalyst carriersCell electrodesIndiumCerium
The present invention provides a catalyst carrier having excellent durability and capable of attaining high catalytic ability without increasing the specific surface area thereof, and a catalyst obtainable by using the catalyst carrier. The catalyst carrier of the present invention comprises a metal oxycarbonitride, preferably the metal contained in the metal oxycarbonitride comprises at least one selected from the group consisting of niobium, tin, indium, platinum, tantalum, zirconium, copper, iron, tungsten, chromium, molybdenum, hafnium, titanium, vanadium, cobalt, manganese, cerium, mercury, plutonium, gold, silver, iridium, palladium, yttrium, ruthenium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and nickel. Moreover, the catalyst of the present invention comprises the catalyst carrier and a catalyst metal supported on the catalyst carrier.
Owner:SHOWA DENKO KK
Catalyst and Catalyst Structure for Reduction of Nitrogen Oxides, and Method for Catalytic Reduction of Nitrogen Oxides
InactiveUS20090084090A1Increased durabilityNo deteriorationGas treatmentMolecular sieve catalystsCobaltPt element
The invention provides a catalyst and a catalyst structure for catalytic reduction of nitrogen oxides contained in exhaust gas wherein fuel is supplied and subjected to combustion under periodic rich / lean conditions and the resulting exhaust gases are brought into contact therewith, which catalyst comprises:an outer catalyst layer comprising an outer catalyst component A which comprises at least one selected from a solid acid, and a solid acid supporting oxides and / or ions of at least one element selected from vanadium, tungsten, molybdenum, copper, iron, cobalt, nickel and manganese, andan inner catalyst layer comprising an inner catalyst component which comprises at least one noble metal catalyst component B selected from platinum, rhodium, palladium and an oxide thereof and a catalyst component C comprising ceria or praseodymium oxide or a mixture of oxides and / or a composite oxide of at least two elements selected from cerium, zirconium, praseodymium, neodymium, terbium, samarium, gadolinium and lanthanum. The invention further provides a method for catalytic reduction of nitrogen oxides by contacting the nitrogen oxides with the catalyst.
Owner:VALTION TEKNILLINEN TUTKIMUSKESKUS +1
Niobium powder, sintered body thereof, and capacitor using the same
InactiveUS20020064476A1Improve heat resistanceLarge capacitance per unit weightTransportation and packagingMetal-working apparatusIndiumCerium
A niobium powder comprising at least one element selected from the group consisting of chromium, molybdenum, tungsten, boron, aluminum, gallium, indium, thallium, cerium, neodymium, titanium, rhenium, ruthenium, rhodium, palladium, silver, zinc, silicon, germanium, tin, phosphorus, arsenic, bismuth, rubidium, cesium, magnesium, strontium, barium, scandium, yttrium, lanthanum, praseodymium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, hafnium, vanadium, osmium, iridium, platinum, gold, cadmium, mercury, lead, selenium and tellurium; a sintered body of the niobium powder; and a capacitor comprising a sintered body as one electrode, a dielectric material formed on the surface of the sintered body, and counter electrode provided on the dielectric material.
Owner:SHOWA DENKO KK
Anti-carbon-deposition Ni-based catalyst for hydrogen production by methane steam reforming and preparation method thereof
ActiveCN103752319AMeet activityMeet service life requirementsHydrogenMetal/metal-oxides/metal-hydroxide catalystsSteam reformingWater vapor
The invention relates to an anti-carbon-deposition Ni-based catalyst for hydrogen production by methane steam reforming and a preparation method thereof. By taking lanthanum nitrate, praseodymium nitrate, samarium nitrate, yttrium nitrate, zirconium nitrate, zirconium carbonate, zirconium oxychloride, and the like as precursors and taking ammonia as a precipitant, a pyrochlore composite oxide is prepared through using a coprecipitation method; and then the pyrochlore composite oxide is mixed with alumina by using a mechanical mixing method so as to obtain a pyrochlore alumina composite carrier. Nickel nitrate, nickel chloride, nickel sulfate, nickel oxalate and the like serving as nickel sources are loaded on the pyrochlore alumina composite carrier through direct immersion. The loading capacity of nickel in the catalyst accounts for 5-30% of the weight of the catalyst, the pyrochlore content of the catalyst is 5-50%, and the alumina content of the catalyst is 20-90%. By taking the pyrochlore alumina composite oxide as a carrier, the reaction activity and anti-carbon-deposition performance of the catalyst can be greatly increased; the preparation method of the catalyst is simple; and the catalyst has excellent catalytic activity and stability to methane steam reforming in a stationary bed.
Owner:NANCHANG UNIV +1
Illumination System Comprising a Red-Emitting Ceramic Luminescence Converter
InactiveUS20080191609A1Suitable light extraction efficiencySuitable transparencyDischarge tube luminescnet screensLamp detailsScandiumAntimony
An illumination system, comprising a radiation source and a monolithic ceramic luminescence converter comprising at least one phosphor capable of absorbing a part of light emitted by the radiation source and emitting light of wavelength different from that of the absorbed light; wherein said at least one phosphor is an europium(III)-activated rare earth metal sesquioxide of general formula (YY-x-XEx)2-z(EU1-a-3Aa)z, wherein RE is selected from the group of gadolinium, scandium, and lutetium, A is selected from the group of bismuth, antimony, dysprosium, samarium, thulium, and erbium, 0≦x<1, 0.001≦z≦0.2; and 0≦a<1 can provide light sources having high luminosity and color-rendering index, especially in conjunction with a light emitting diode as a radiation source. The invention is also concerned with an amber to red-emitting a monolithic ceramic luminescence converter comprising an europium(III)-activated rare earth metal sesquioxide of general formula (Y1-x-REx)2-zO3:(Eu1-aAa)Z, wherein RE is selected from the group of gadolinium, scandium, and lutetium, A is selected from the group of dysprosium, samarium, thulium, and erbium, 0≦x<1, 0.001≦z≦; and 0≦a<1.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Method for preparing low-temperature cofired dielectric ceramic and material and sintering aid of low-temperature cofired dielectric ceramic
The invention relates to a sintering aid for a low-temperature cofired dielectric ceramic material. The sintering aid comprises the following components in percentage by mass: 40 to 55 percent of silicon dioxide, 5 to 16 percent of boron oxide, 12 to 17 percent of zinc oxide, 5 to 15 percent of aluminum oxide, 3 to 10 percent of lithium oxide, 0 to 5 percent of copper oxide, 0 to 5 percent of cobaltosic oxide and 3 to 8 percent of oxides of which the general formula is R2O3, wherein R may be at least one of lanthanum, neodymium, samarium and dysprosium. By the sintering aid for the low-temperature cofired dielectric ceramic material, the low-temperature cofired dielectric ceramic material can be sintered at temperature of between 830 and 950 DEG C, and the dielectric performance of the low-temperature cofired dielectric ceramic is improved effectively. In addition, the invention also provides the low-temperature cofired dielectric ceramic material and a method for preparing the low-temperature cofired dielectric ceramic.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG
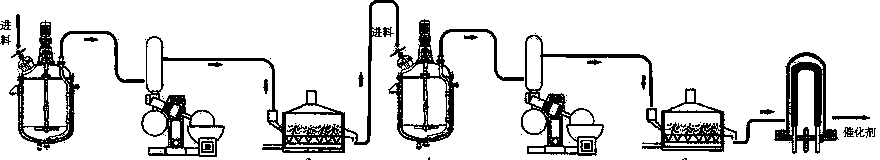
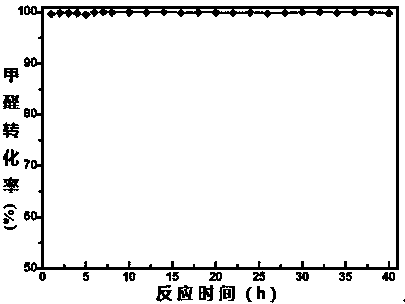
Catalyst for complete catalytic oxidation of indoor low concentration formaldehyde at room temperature
InactiveCN104162425AGood formaldehyde catalytic oxidation activityHigh activityDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsDispersityRare-earth element
The invention discloses a catalyst for complete catalytic oxidation of indoor low concentration formaldehyde at room temperature, the catalyst is characterized by comprising a nano metal oxide, a noble metal component and an assistant component, the nano metal oxide is used as a carrier, the noble metal component is at least one from platinum, palladium, ruthenium, rhodium, gold and silver, and the assistant is one or more than one substance from rare earth elements lanthanum, cerium, praseodymium, samarium, yttrium and neodymium, one or more than one kind of. The catalyst has the advantages that: the catalyst uses one or two rare earth modified nano inorganic oxide carrier, and is helpful to improve the dispersity and formaldehyde catalytic oxidation activity of metal nanoparticles. Therefore, under the circumstances of maintaining the same catalytic activity, the amount of noble metals can be reduced, and the cost of the catalyst can be reduced. The catalyst can completely oxidize formaldehyde at room temperature, does not need the help of optical, electric and other complex external auxiliary equipment. The catalyst can catalytically oxidize indoor low concentration harmful gas formaldehyde into non-toxic harmless carbon dioxide and water at room temperature.
Owner:BEGOOD TECH
Dehydrogenation catalyst applicable to raw gas rich in carbon monoxide, and preparation and application thereof
InactiveCN102974344AEasy to operateHigh activityCarbon monoxideMetal/metal-oxides/metal-hydroxide catalystsDehydrogenationCerium
The invention relates to gas purification techniques and specifically to a dehydrogenation catalyst applicable to raw gas rich in carbon monoxide and preparation and application thereof. Palladium is used as an active component of the catalyst, auxiliary agents for the catalyst are two to four selected from the group consisting of silver, zinc, lanthanum, cerium, samarium, praseodymium, iron, stannum, manganese, calcium, magnesium, tungsten and molybdenum, and a carrier of the catalyst is alumina. The catalyst realizes selective catalytic oxidation removal of hydrogen in the raw gas rich in carbon monoxide by directing using synergism of palladium and oxides of a plurality of the auxiliary agents. Components of the catalyst comprise, based on the weight of the carrier, 0.05 to 1% of the active component and 0.5 to 5% of the total auxiliary agents. According to the invention, the catalyst has high activity and stability even when the application amount of a precious metal is small; through usage of the catalyst in a gas source rich in carbon monoxide, outlet hydrogen content is less than 100 ppm, outlet oxygen content is less than 0.1%, and purifying indexes of hydrogen and oxygen in reaction tail gas still accord with requirements after continuous operation for 1000 hours.
Owner:DALIAN CATALYTIC ENG TECH
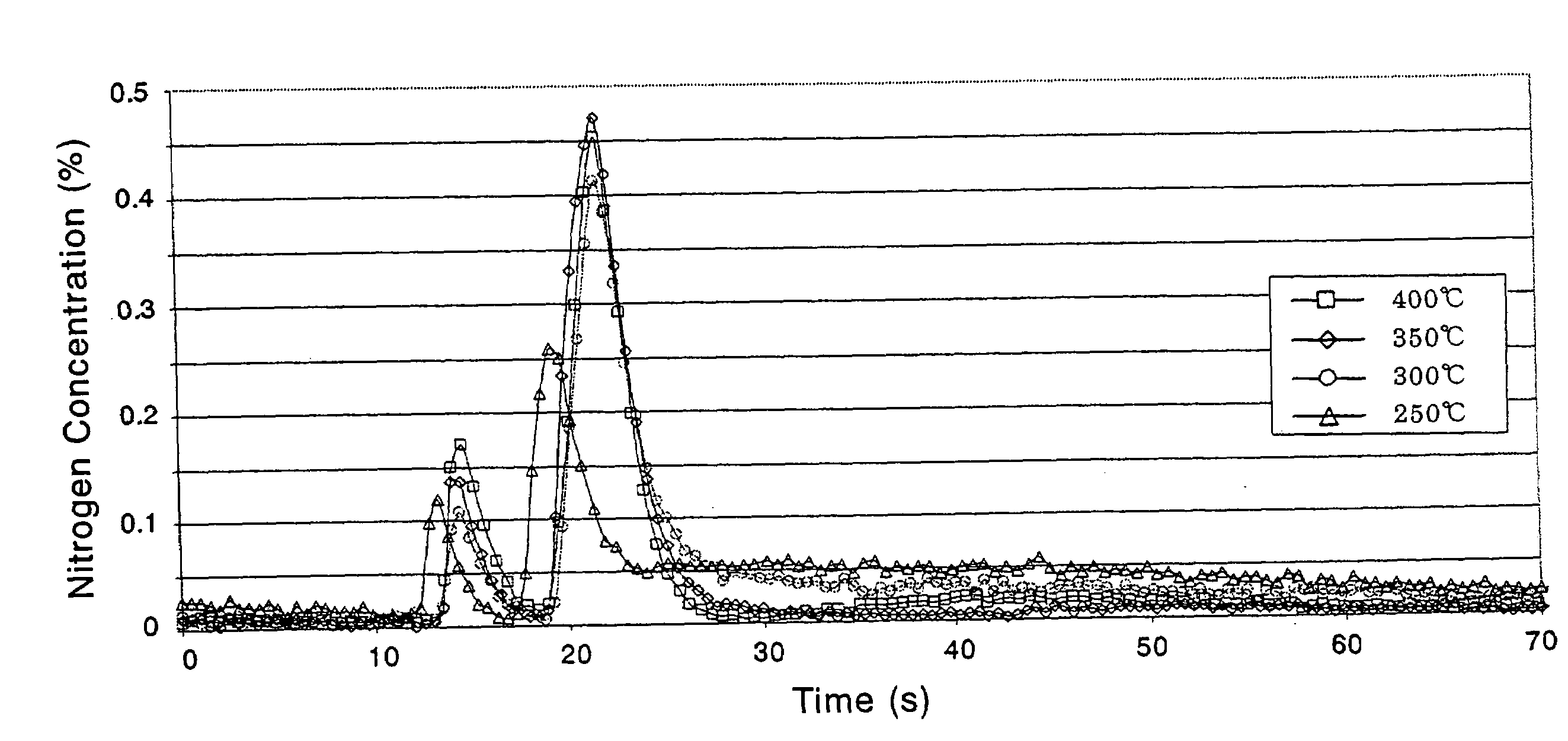
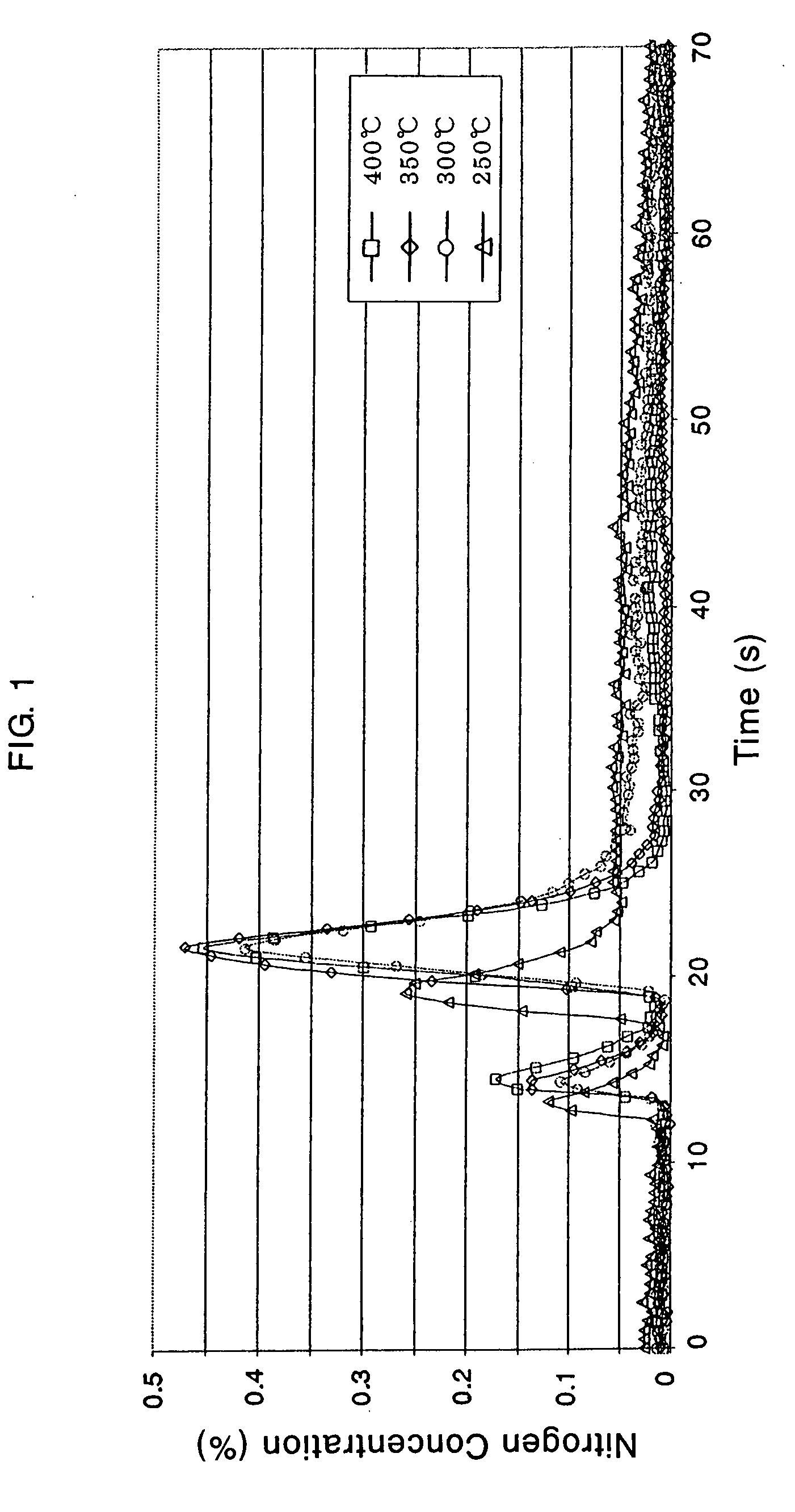
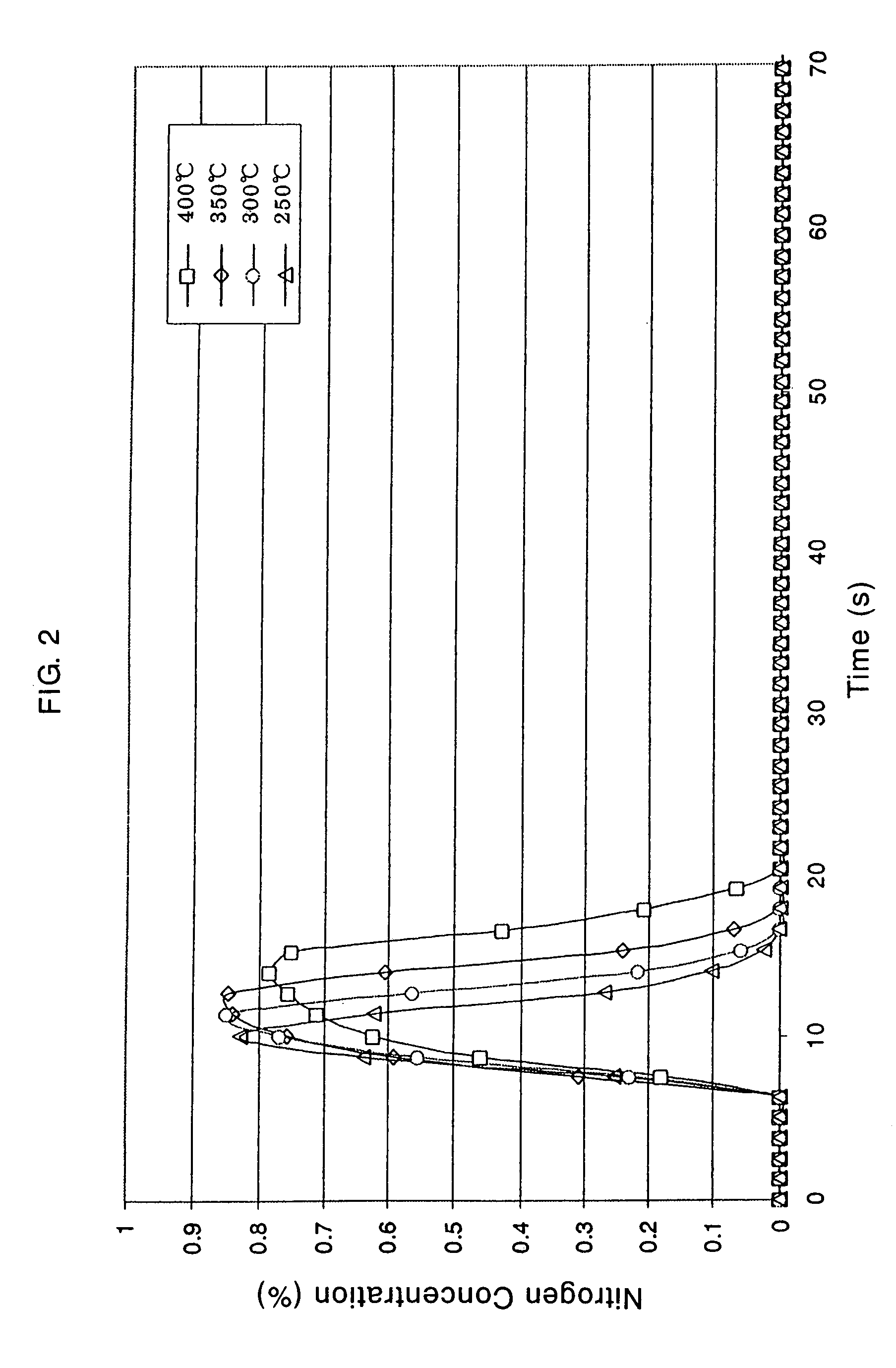
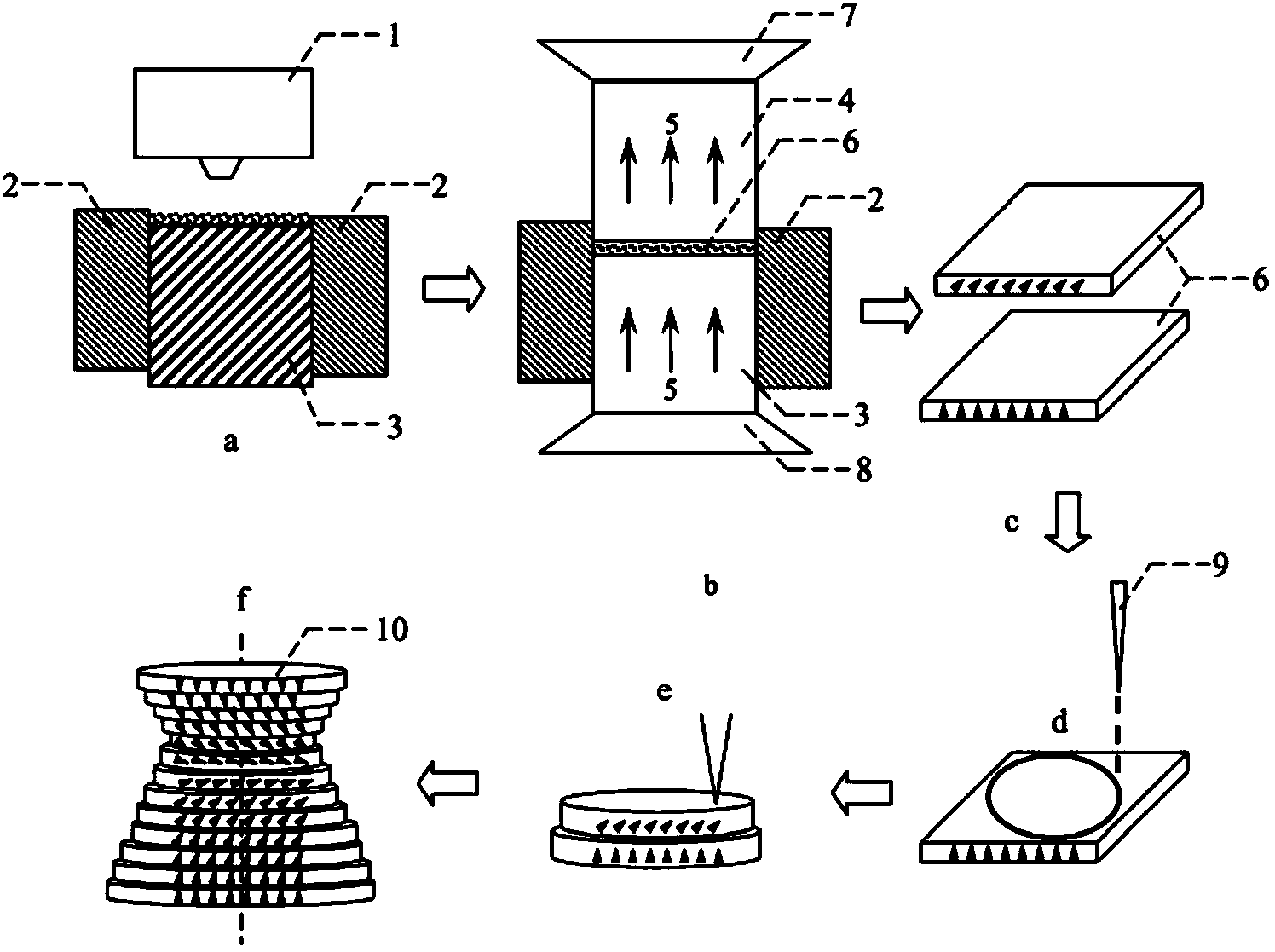
Method of preparing samarium iron nitrogen permanent amgnet material using mechanical alloying
Final mass percent of SmFeN permanent magnetic material is prepared according to 25-30%Sm and 70-75%Fe. In order to increase infiltration capacity of nitrogen, element such as Cr, Ni, Co etc. with mass percent 0.1-2% is added. The mixed material powder is ball milled for 1- 20 hrs. The ball mill machine stops for 5- 20 minutes in every 5 - 30 minutes in order to prevent high temperature in tank. Its advantages are high nitrogen content, compact structure, simple equipment, easy of operation, high efficiency and low cost.
Owner:UNIV OF SCI & TECH BEIJING
Flexible rare-earth cementing magnet and method of manufacturing the same
InactiveCN101465188AImprove magnetic propertiesIncreased anisotropyInorganic material magnetismInductances/transformers/magnets manufactureRare earthNitrogen
The invention provides a flexible rare earth bonded magnet and a manufacturing method thereof. Based on weight percentage, the flexible rare earth bonded magnet comprises 85%-92% of compound permanent magnet powders, 6%-10% of binder and 2% -5% of processing aid; wherein, the compound permanent magnet powders comprise anisotropic samarium iron nitrogen permanent magnet powders, anisotropic Nd-Fe-N permanent magnet powders and anisotropic NdFeB powders. The flexible rare earth bonded magnet is obtained through extrusion molding and orientation field application and is provided with high magnetic properties and good flexibility, thereby broadening the application scope of flexible magnets and extending the magnetic property space of the flexible magnet system; therefore, the flexible rare earth bonded magnet can meet the requirements of high-tech products on the magnetic properties and flexibility of magnets.
Owner:BEIKUANG MAGNETS FUYANG CO LTD
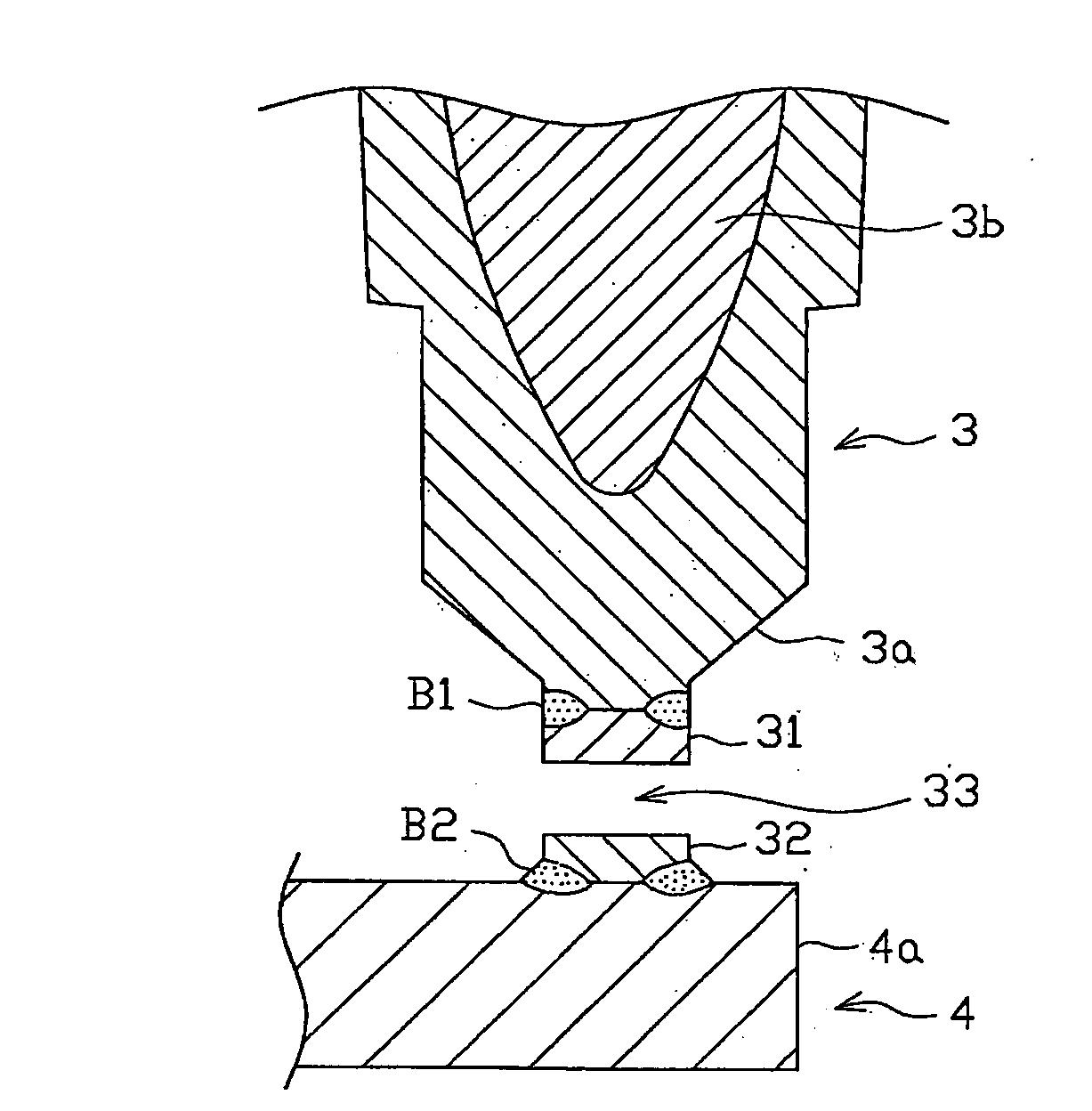
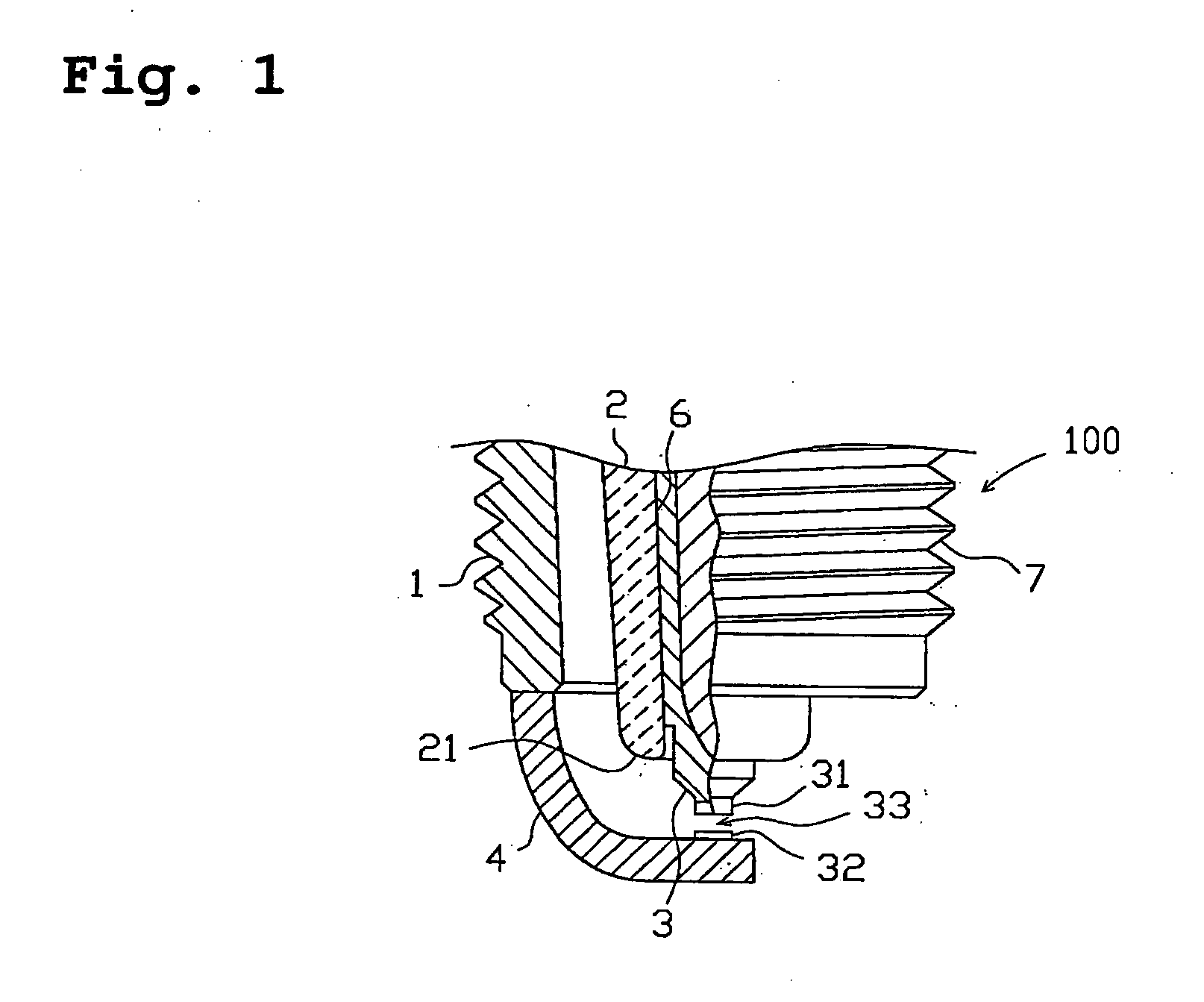
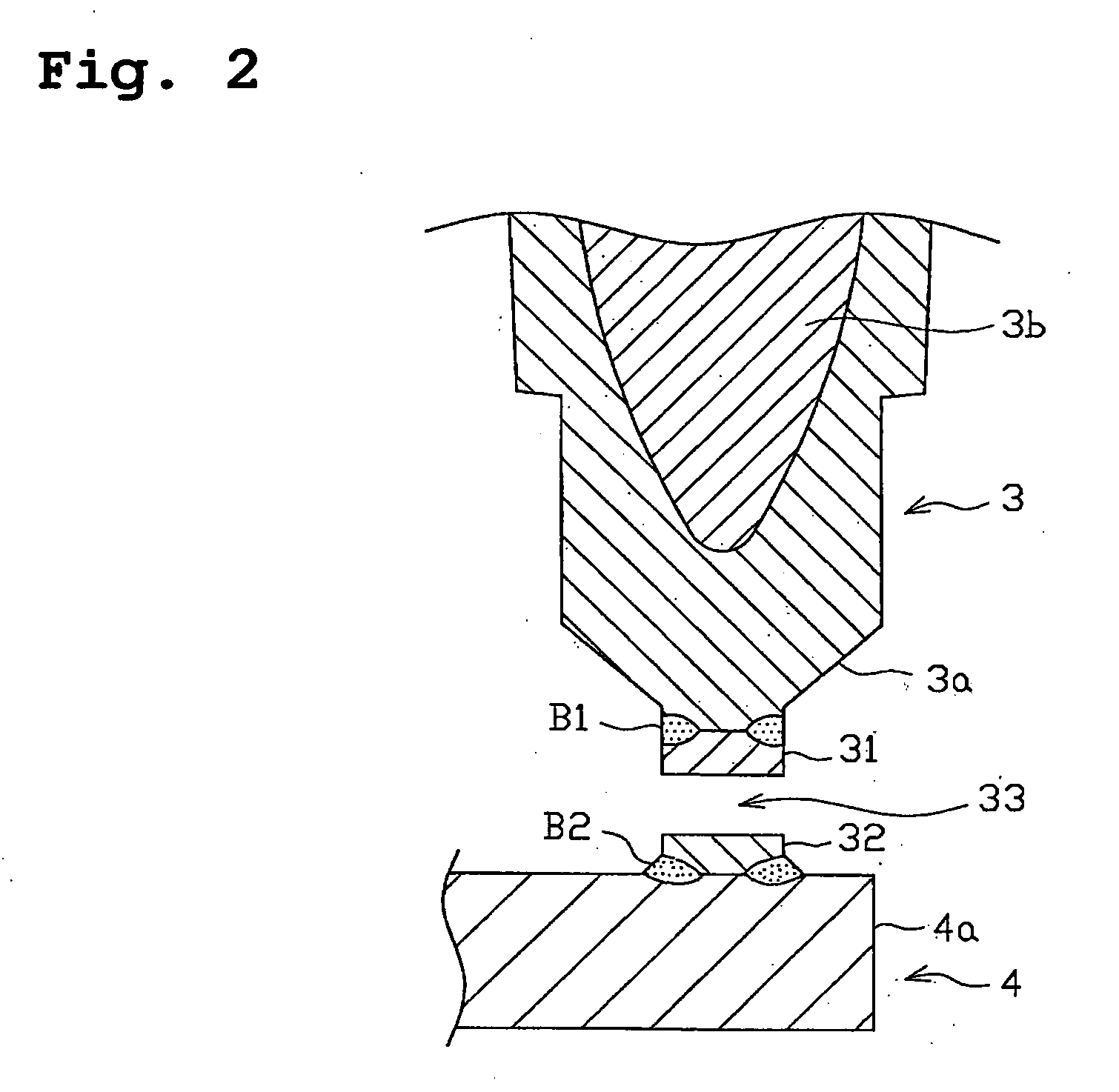
Spark plug for internal-combustion engines
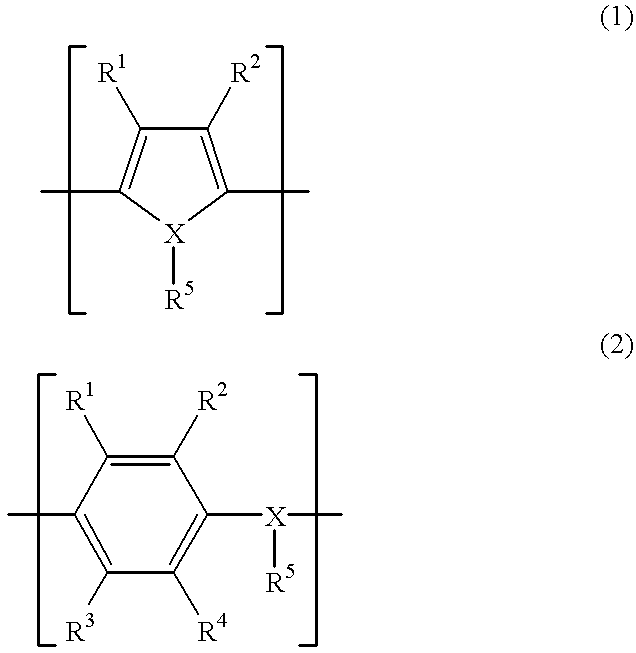
A spark plug for an internal-combustion engine is provided wherein the central and ground electrodes exhibit a long service life and wherein the fatigue strength at high temperatures is improved. The ground electrode is made from an alloy comprised of nickel (Ni) as a primary component, chromium: 20-30% by weight, iron: 7-20% by weight, aluminum: 1-3% by weight, titanium: 0.05-0.5% by weight, manganese: not higher than 0.1% by weight, silicon: not higher than 0.1% by weight, and carbon: not higher than 0.5% by weight. The alloy further includes at least one specific element selected from zirconium, yttrium, neodymium, cerium, lanthanum and samarium. Further, the total content of the specific element group is 5% or more of the aluminum content and is not higher than 1% by weight.
Owner:NGK SPARK PLUG CO LTD
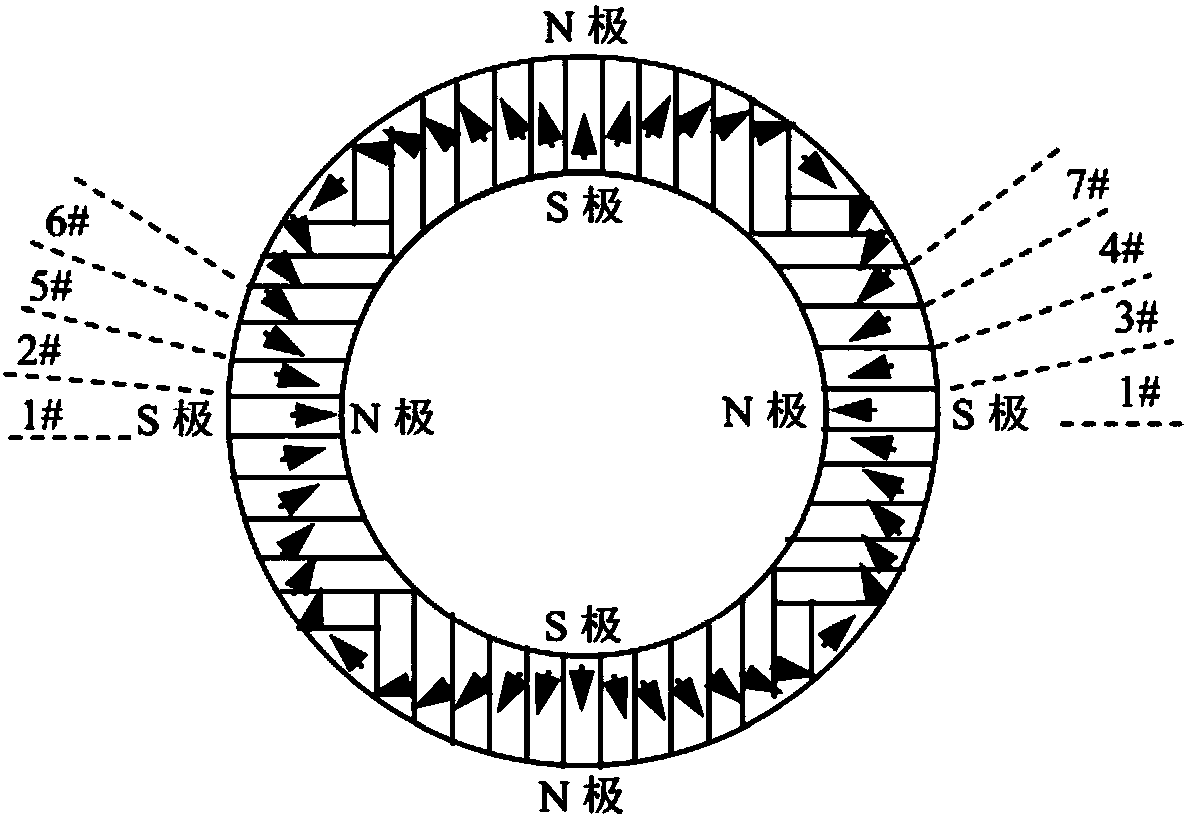
Magnetic field orientation three-dimensional printing anisotropic bonded permanent magnet and preparation method thereof
ActiveCN104269265AAchieve regulationElimination of magnetic interaction forcesInductances/transformers/magnets manufactureRare earthNitrogen
The invention relates to the technical field of rare earth permanent magnetic materials, in particular to a magnetic field orientation three-dimensional printing anisotropic bonded permanent magnet and a preparation method thereof. The magnetic field orientation three-dimensional printing anisotropic bonded permanent magnet, adopts magnet powder having magnetocrystalline anisotropy, comprises one or a plurality of anisotropic neodymium iron boron magnetic powder, samarium cobalt magnetic powder and samarium iron nitrogen powder and is a semi-continuous or continuous orientation changed anisotropic bonded permanent magnet. The magnetic field orientation three-dimensional printing anisotropic bonded permanent magnet is prepared by the following steps of powder filling, orienting and forming into sheet layers, thermal demagnetizing, cutting the sheet layers into required-shaped unit sheet layers, stacking and solidifying the unit sheet layers layer by layer and magnetizing. According to the magnetic field orientation three-dimensional printing anisotropic bonded permanent magnet and the preparation method thereof, preparation of the three-dimensional printing anisotropic bonded permanent magnet is achieved, adjustment of the orientation and the order degree of powder in the sheet layers are achieved by adjusting the magnetic field direction and or the magnetic field strength, the defect that orientation of the traditional bonded permanent magnet cannot be changed is overcame, and continuous or semi-continuous change of magnetic orientation in the same three-dimensional entity is achieved.
Owner:CENT IRON & STEEL RES INST
Nanoparticles of rare earth oxides
ActiveUS7229600B2Increase volumeLow cost productionMaterial nanotechnologyLanthanum oxide/hydroxidesCeriumScandium
Rare earth compositions comprising nanoparticles, methods of making nanoparticles, and methods of using nanoparticles are described. The compositions of the nanomaterials discussed may include scandium (Sc), yttrium (Y), lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). The nanoparticles can be used to make organometallics, nitrates, and hydroxides. The nanoparticles can be used in a variety of applications, such as pigments, catalysts, polishing agents, coatings, electroceramics, catalysts, optics, phosphors, and detectors.
Owner:PPG IND OHIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com