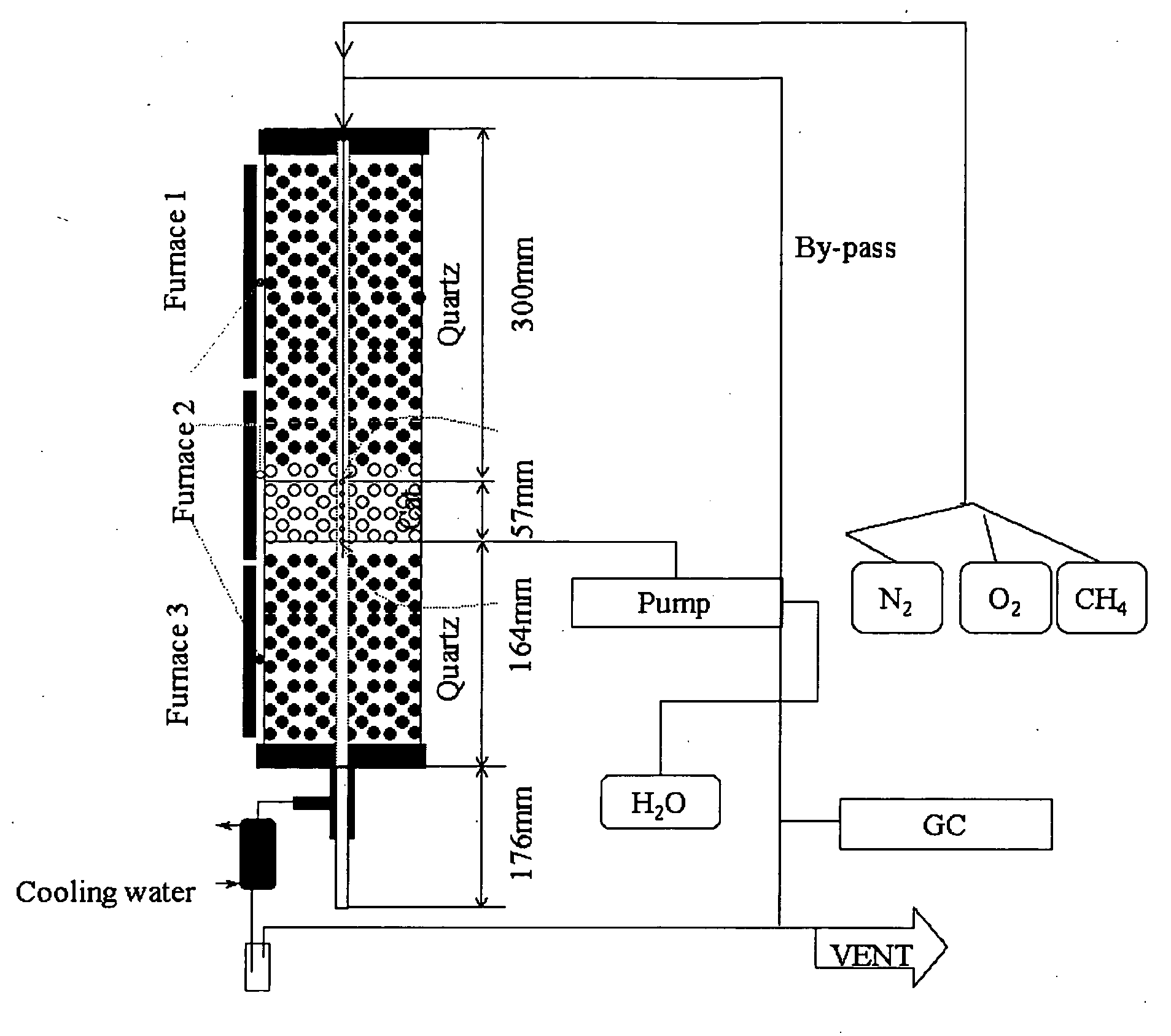
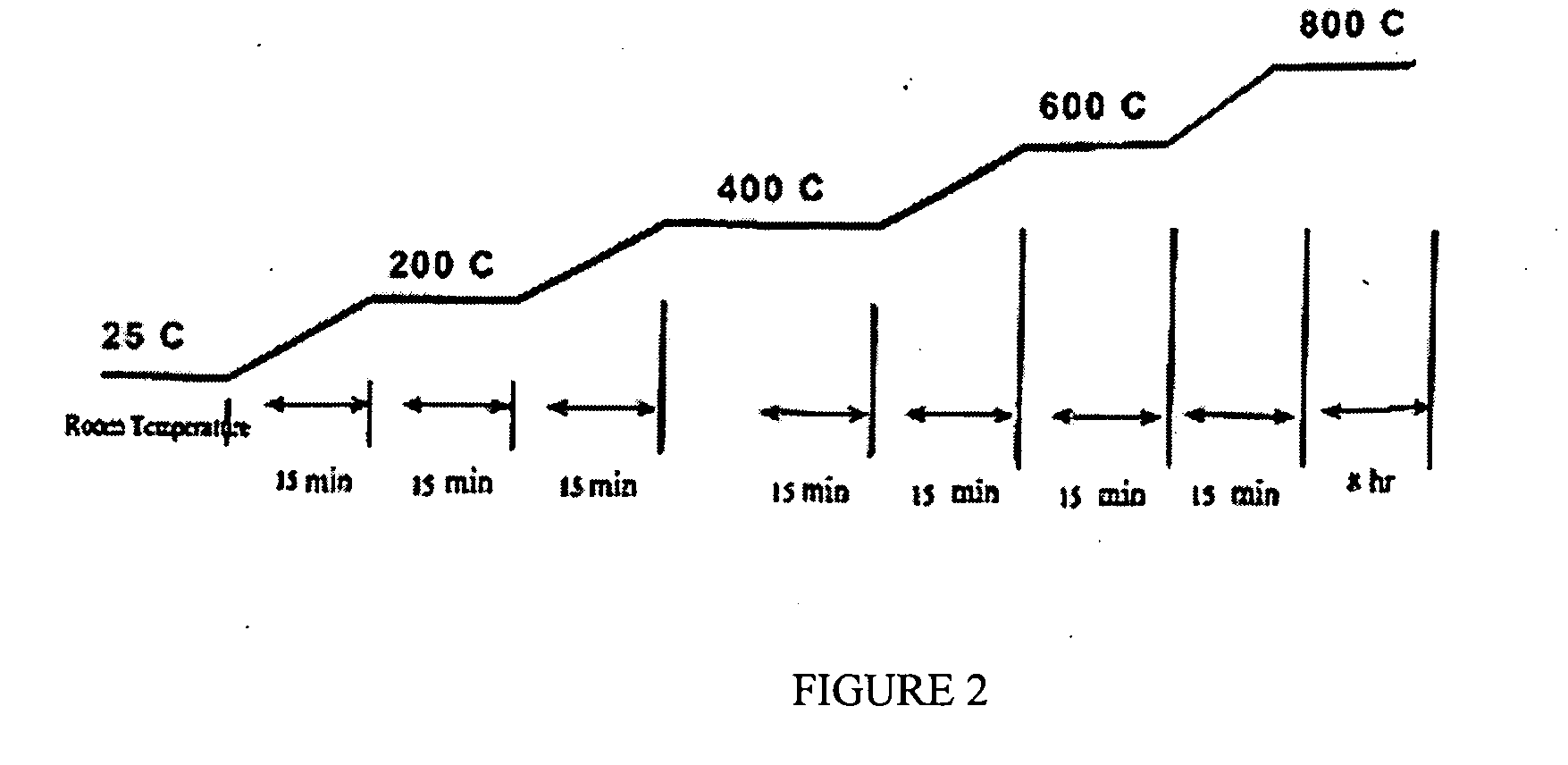
Catalyst and method for converting low molecular weight paraffinic hydrocarbons into alkenes and organic compounds with carbon numbers of 2 or more
a technology of low molecular weight paraffinic hydrocarbons and catalysts, which is applied in the direction of hydrocarbon oil treatment products, physical/chemical process catalysts, bulk chemical production, etc., can solve the problems of unattractive economic use, waste of resources, and difficulty in utilizing gas resources. , to achieve the effect of reducing the number of carbon dioxide emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effects of a Mixed Catalyst on Methane Conversion
[0116] This example used an OCM / EPC catalyst prepared by combining catalyst component # 14 (SnBaTiO3) from Table 1 with catalyst component # 15 (NaMo / SiO2) as shown in Table 2.
[0117] Catalyst component #14 was prepared using the sol-sol method described in Example 1, and paragraphs 25 through 38 of U.S. Patent App. Pub. No. 2004 / 0220053 A1 (Bagherzadeh et al, incorporated by reference herein in its entirety). Briefly, an aqueous slurry containing the metal salts is prepared, a polymeric binder added to the slurry to form a paste, which is then dried, crushed to a size appropriate for the reactor into which the catalyst will be used, and then calcined. After calcining, the calcined material was pressed, crushed and sieved to a sized appropriate for the reactor.
[0118] Catalyst component #15 (NaMo / SiO2) was prepared using the Sol-Sol method by mixing water and 1.36 gr methyl 2-hydroxy ethyl cellulose (Tylose from SE Tylose GmbH & Co. ...
example 2
Effects of an Alternate Mixed Catalyst on Methane Conversion
[0121] This example used an OCM / EPC catalyst prepared by combining catalyst component # 14 (SnBaTiO3) from Table 1 with catalyst component # 19 (KV on SiO2, 5% K, 10% V, 85% SiO2) as shown in Table 2.
[0122] Catalyst component # 14 was prepared as described in Example 1.
[0123] Catalyst component #19 (KV on SiO2) was prepared using the Sol-Sol method by mixing water and 1.36 gr methyl 2-hydroxy ethyl cellulose (Tylose from SE Tylose GmbH & Co. KG in Wiesbaden, Germany) and the potassium and vanadium components. To this mix is added 13.9 gm of vinyl acetate-butyl acrylate copolymer as an organic binder. The weight % of the components was 5 wt % potassium, 10 wt % vanadium and 85 wt % SiO2. The mixture is dried and calcined at 800° C. for 8 hours.
[0124] Catalyst component # 14 was combined with catalyst component # 19 in a 90:10 wt % ratio. The combined catalyst was mechanically mixed to a uniform consistency and pressed to...
example 3
Effects of a Catalyst Prepared Only by the Sol-Gel Method on Methane Conversion
[0126] This example used an OCM catalyst which was only made by the sol-gel technique. The catalyst composition is that shown in Table 1 as #1 (SmBaTiO3). The catalyst was prepared using oxides of samarium (Sm) and barium (Ba) in the forms of SmO3 and BaO, respectively, and TiCl4 (all sourced from Sigma-Aldrich, St. Louis, Mo.), mixed in separate containers, each with 400 cc propionic acid and refluxed for 5 hours at 130 degrees C. The ratio of metal components was calculated based on the resulting perovskite crystal (SmBaTiO3) containing titanium in the form of an octahedron and having equal molar ratios as indicated in Table 1. The individual solubilized metal organic components were combined and then the solvent evaporated to form a gel. The gel was dried and calcined at 800 degrees C. for 8 hours.
[0127] Since this catalyst is an oxidation catalyst, there are endothermic reactions occurring when expo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com