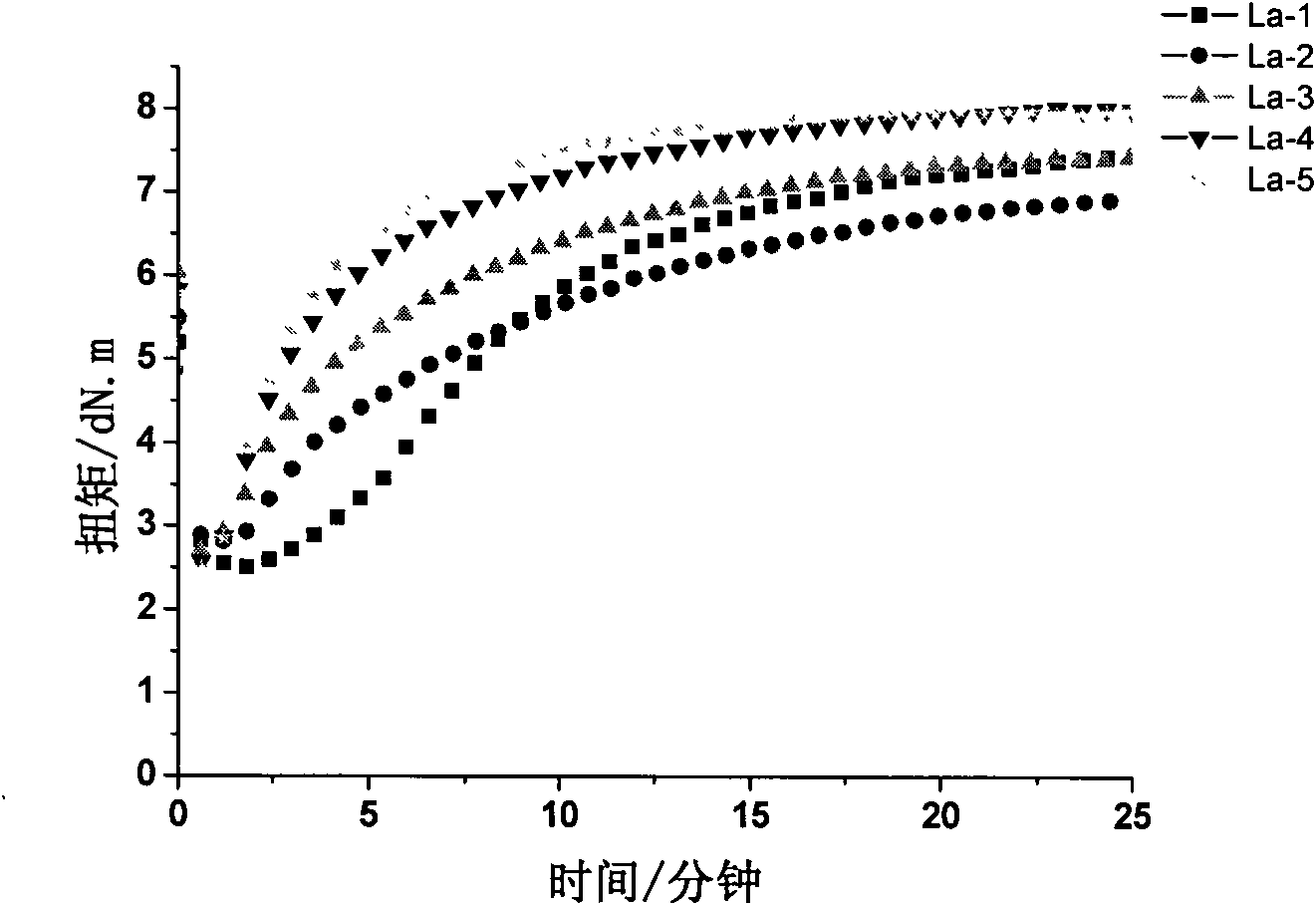
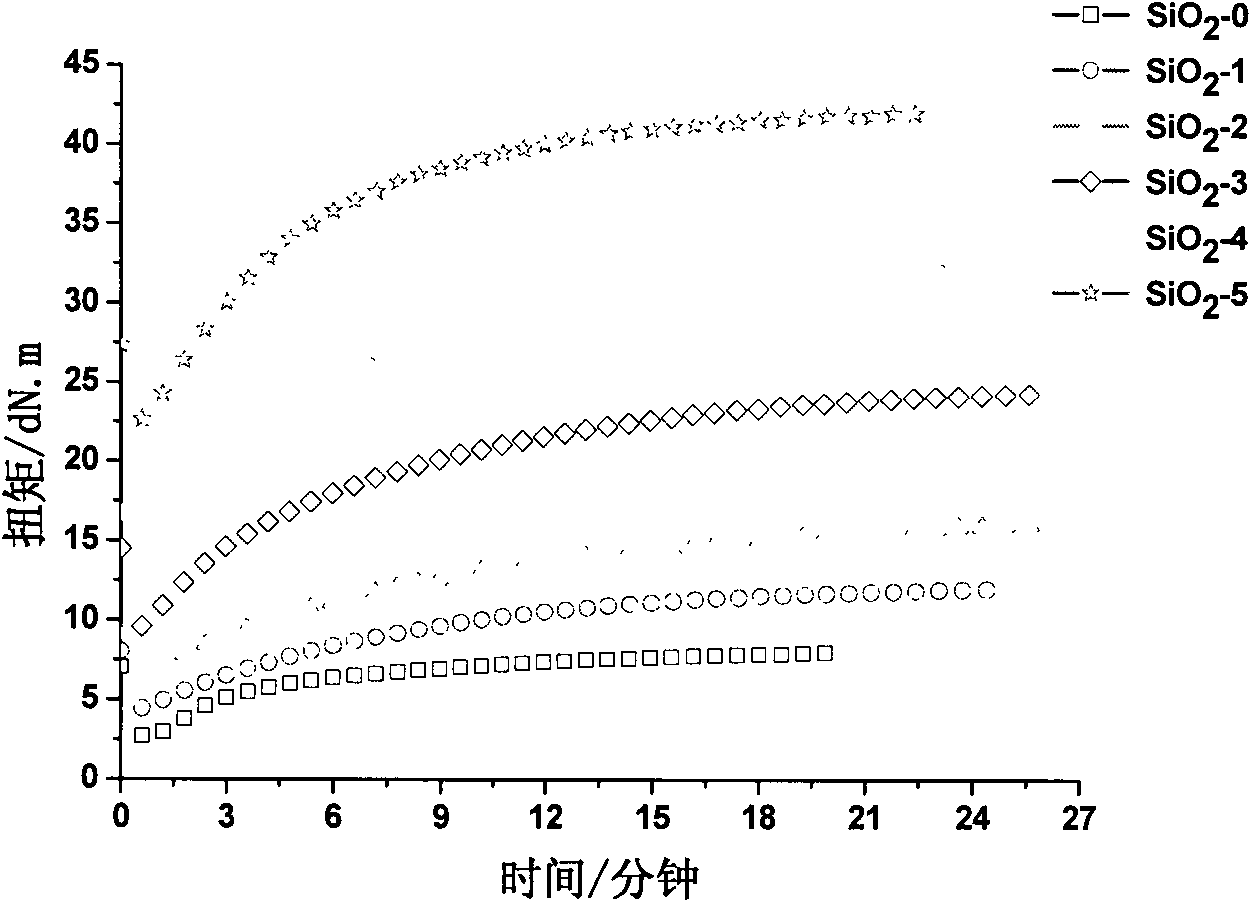
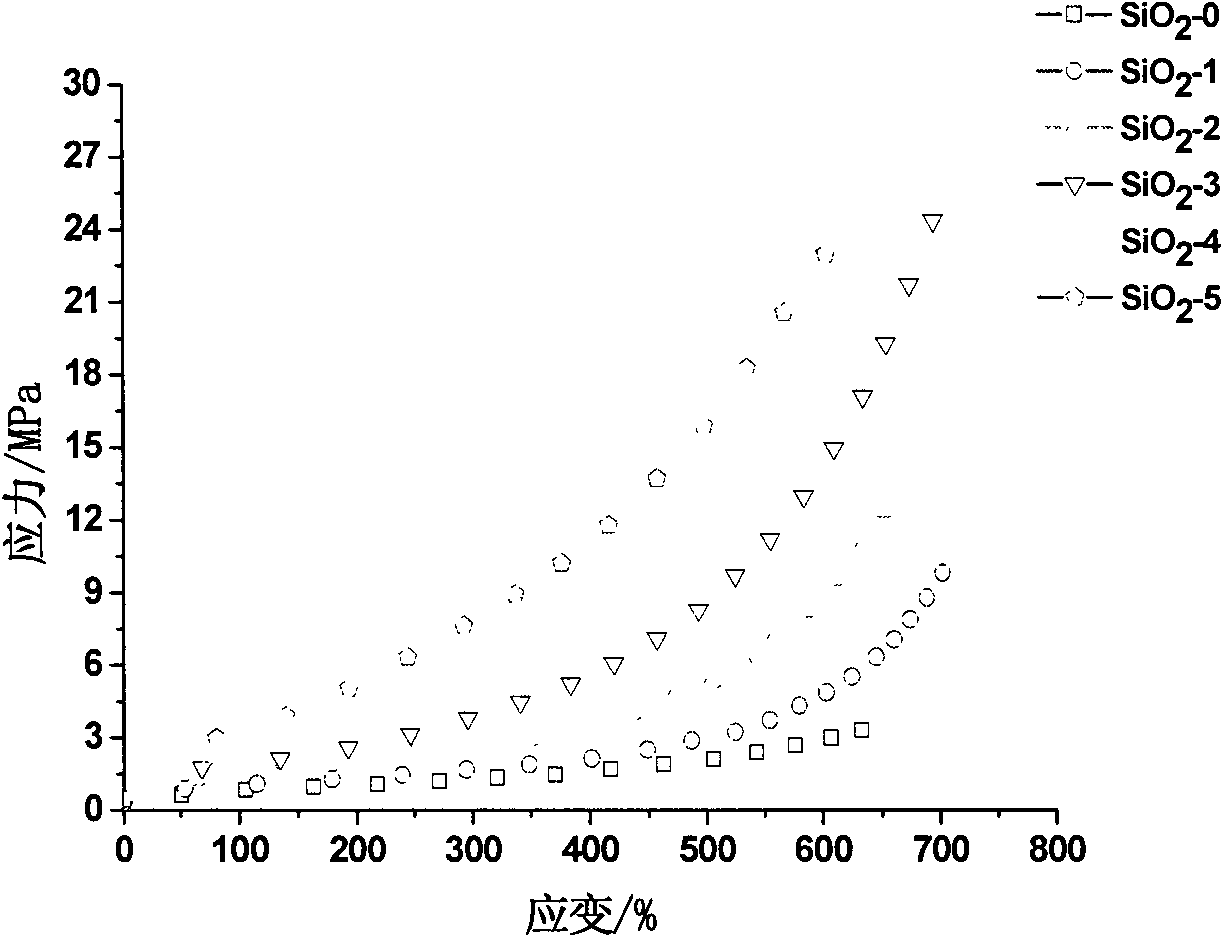
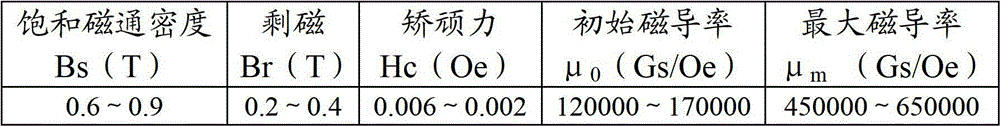
Patents
Literature
306 results about "Promethium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of only two radioactive elements that are followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3.
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
Layered thermal barrier coatings containing lanthanide series oxides for improved resistance to CMAS degradation
InactiveUS20070160859A1Avoid damageElimination of expensiveBlade accessoriesEfficient propulsion technologiesReaction layerCerium
A coating applied as a two layer system. The outer layer is an oxide of a group IV metal selected from the group consisting of zirconium oxide, hafnium oxide and combinations thereof, which are doped with an effective amount of a lanthanum series oxide. These metal oxides doped with a lanthanum series addition comprises a high weight percentage of the outer coating. As used herein, lanthanum series means an element selected from the group consisting of lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu) and combinations thereof, and lanthanum series oxides are oxides of these elements. When the zirconium oxide is doped with an effective amount of a lanthanum series oxide, a dense reaction layer is formed at the interface of the outer layer of TBC and the CMAS. This dense reaction layer prevents CMAS infiltration below it. The second layer, or inner layer underlying the outer layer, comprises a layer of partially stabilized zirconium oxide.
Owner:GENERAL ELECTRIC CO
High performance lithium ion battery anode material lithium manganate and preparation method thereof
The invention provides a high performance lithium ion battery anode material lithium manganate and a preparation method of the material. The lithium manganate is a doped lithium manganate LiMn2-yXy04 which is doped with one kind or a plurality of other metal elements X, wherein X element is at least one kind selected form the group of aluminium, lithium, fluorine, silver, copper, chromium, zinc, titanium, bismuth, germanium, gallium, zirconium, stannum, silicon, cobalt, nickel, vanadium, magnesium, calcium, strontium, barium and rare earth elements lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium, and y is larger than 0 but less than or equal to 0.11. The lithium ion battery anode material lithium manganate provided in the invention has extraordinary charge and discharge cycle performance both in the environments of normal temperature and high temperature. According to the invention, the preparation method of the material is a solid phase method, the operation is simple and controllable and the cost is low so that it is easy to realize large-scale productions.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Methods for making barrier coatings comprising taggants and components having the same
Methods for making barrier coatings including a taggant involving providing a barrier coating, and adding from about 0.01 mol % to about 30 mol % of a taggant to the barrier coating wherein the taggant comprises a rare earth element selected from lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, ytterbium, and lutetium, salts thereof, silicates thereof, oxides thereof, zirconates thereof, hafnates thereof, titanates thereof, tantalates thereof, cerates thereof, aluminates thereof, aluminosilicates thereof, phophates thereof, niobates thereof, borates thereof, and combinations thereof.
Owner:GENERAL ELECTRIC CO
CMAS resistant thermal barrier coating
ActiveUS20070172703A1Reduce componentsReduces sand related distressMolten spray coatingBlade accessoriesIndiumCerium
A turbine engine component is provided which has a substrate and a thermal barrier coating applied over the substrate. The thermal barrier coating comprises alternating layers of yttria-stabilized zirconia and a molten silicate resistant material. The molten silicate resistant outer layer may be formed from at least one oxide of a material selected from the group consisting of lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, indium, zirconium, hafnium, and titanium or may be formed from a gadolinia-stabilized zirconia. If desired, a metallic bond coat may be present between the substrate and the thermal barrier coating system. A method for forming the thermal barrier coating system of the present invention is described.
Owner:RTX CORP
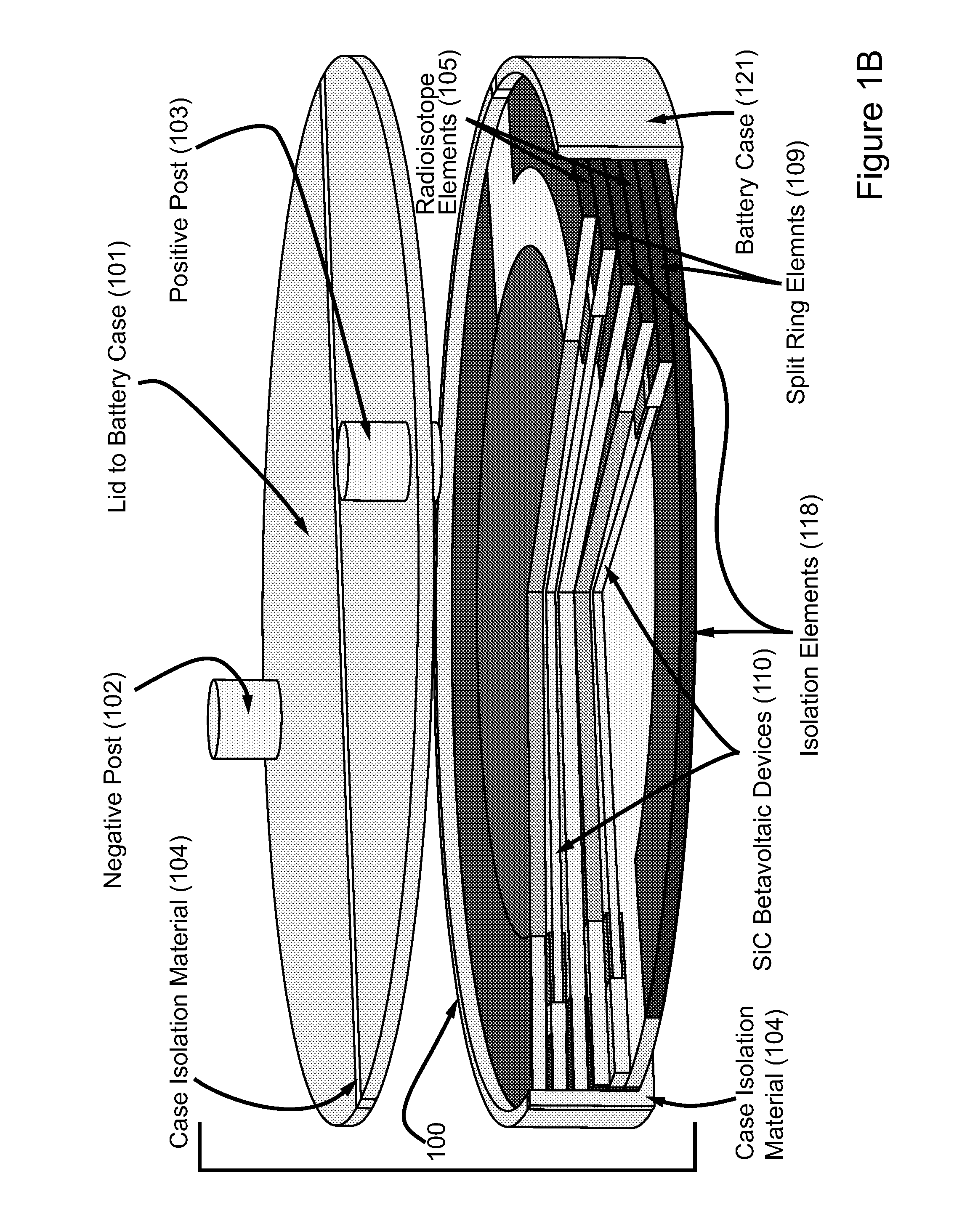
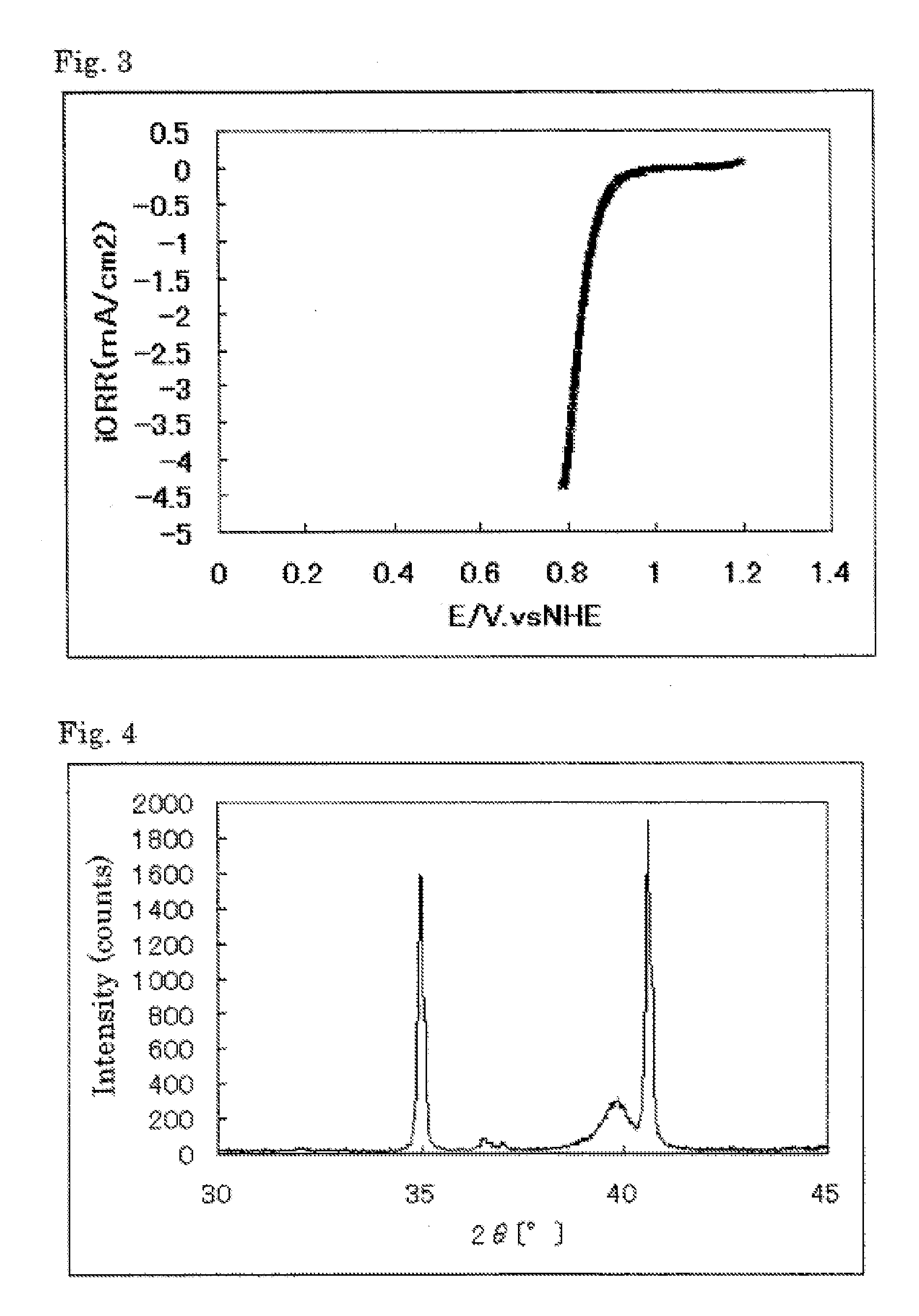
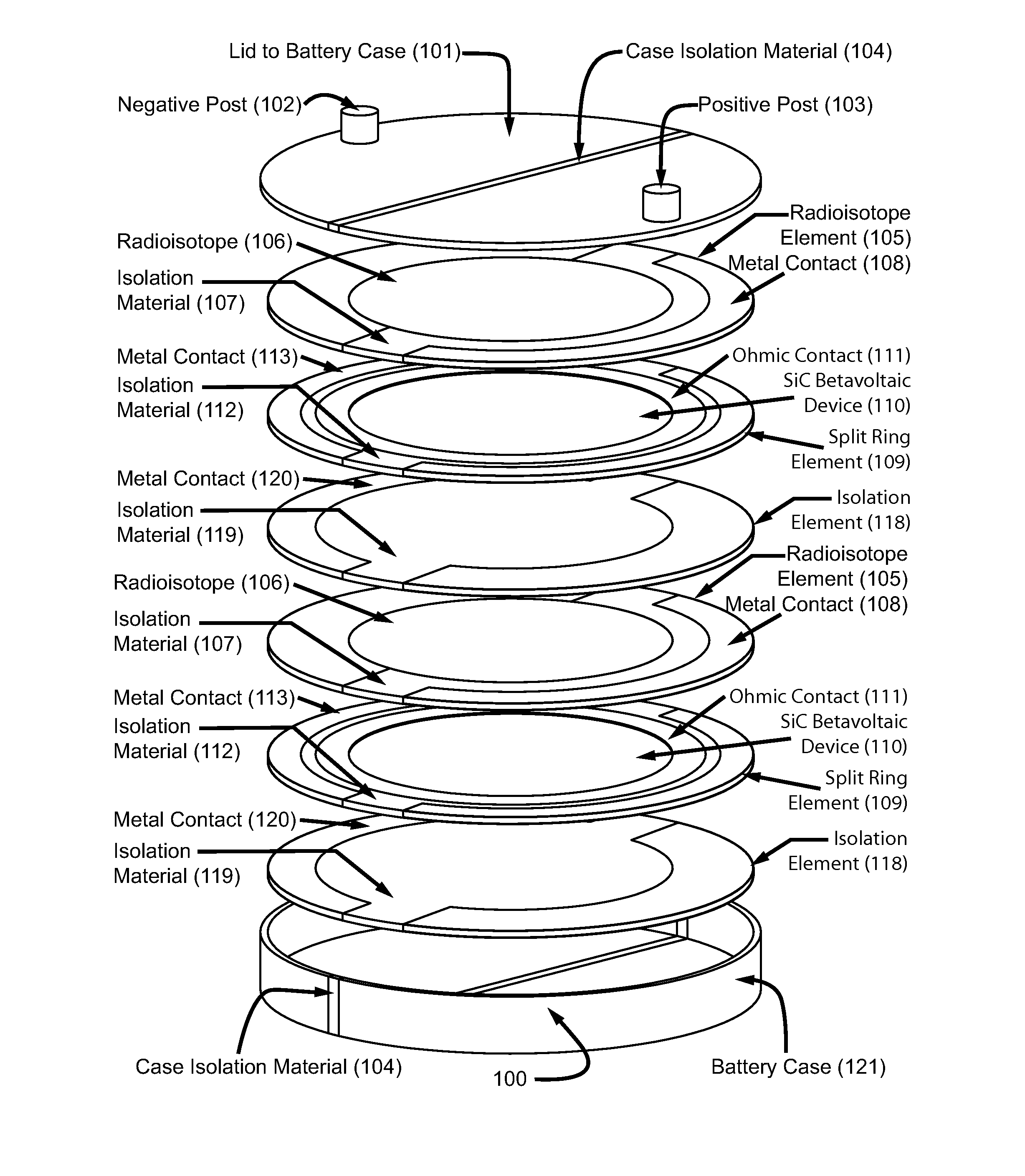
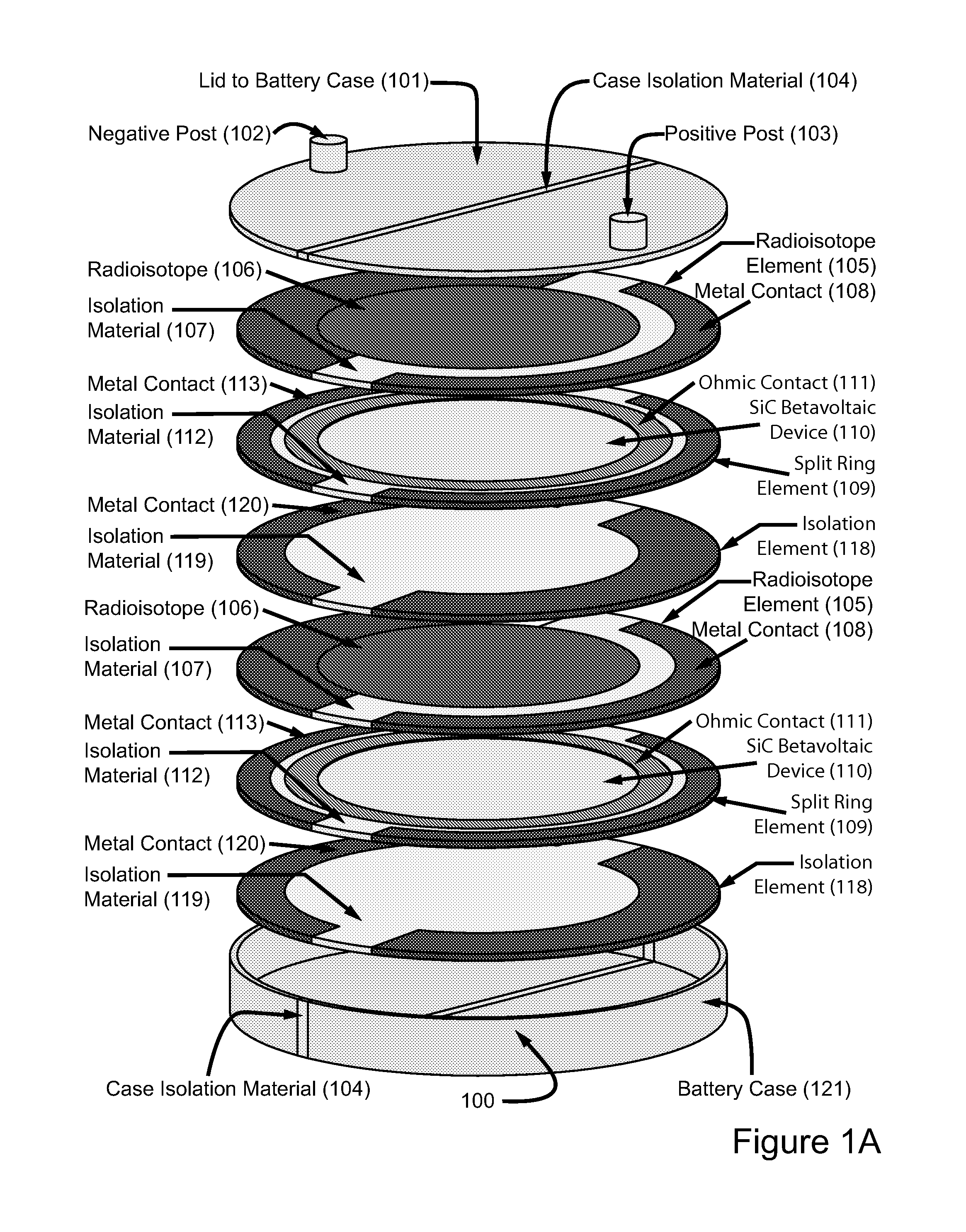
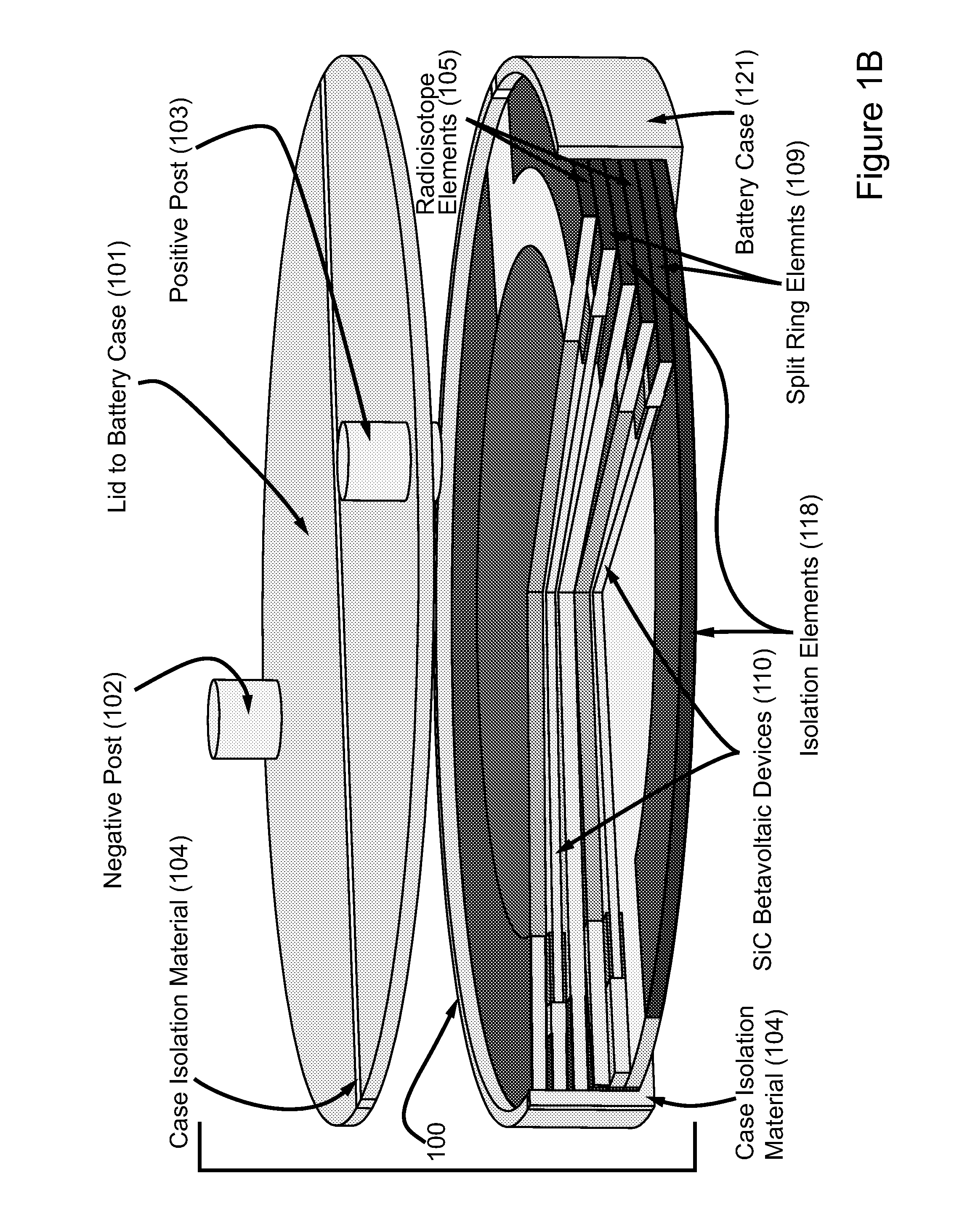
High power density betavoltaic battery
ActiveUS20110031572A1Improve power densitySolid-state devicesSemiconductor/solid-state device manufacturingBetavoltaicsSemiconductor materials
To increase total power in a betavoltaic device, it is desirable to have greater radioisotope material and / or semiconductor surface area, rather than greater radioisotope material volume. An example of this invention is a high power density betavoltaic battery. In one example of this invention, tritium is used as a fuel source. In other examples, radioisotopes, such as Nickel-63, Phosphorus-33 or promethium, may be used. The semiconductor used in this invention may include, but is not limited to, Si, GaAs, GaP, GaN, diamond, and SiC. For example (for purposes of illustration / example, only), tritium will be referenced as an exemplary fuel source, and SiC will be referenced as an exemplary semiconductor material. Other variations and examples are also discussed and given.
Owner:WIDETRONIX
Nanoparticles of rare earth oxides
ActiveUS20070104629A1Increase volumeLow cost productionMaterial nanotechnologyLanthanum oxide/hydroxidesCeriumScandium
Rare earth compositions comprising nanoparticles, methods of making nanoparticles, and methods of using nanoparticles are described. The compositions of the nanomaterials discussed may include scandium (Sc), yttrium (Y), lanthanum(La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium(Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). The nanoparticles can be used to make organometallics, nitrates, and hydroxides. The nanoparticles can be used in a variety of applications, such as pigments, catalysts, polishing agents, coatings, electroceramics, catalysts, optics, phosphors, and detectors.
Owner:PPG IND OHIO INC
Catalyst carrier, catalyst and process for producing the same
InactiveUS20100331172A1Improve heat resistanceHigh catalytic ability without increasing the specific surface areaCatalyst carriersCell electrodesIndiumCerium
The present invention provides a catalyst carrier having excellent durability and capable of attaining high catalytic ability without increasing the specific surface area thereof, and a catalyst obtainable by using the catalyst carrier. The catalyst carrier of the present invention comprises a metal oxycarbonitride, preferably the metal contained in the metal oxycarbonitride comprises at least one selected from the group consisting of niobium, tin, indium, platinum, tantalum, zirconium, copper, iron, tungsten, chromium, molybdenum, hafnium, titanium, vanadium, cobalt, manganese, cerium, mercury, plutonium, gold, silver, iridium, palladium, yttrium, ruthenium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, and nickel. Moreover, the catalyst of the present invention comprises the catalyst carrier and a catalyst metal supported on the catalyst carrier.
Owner:SHOWA DENKO KK
High power density betavoltaic battery
ActiveUS20110298071A9Solid-state devicesSemiconductor/solid-state device manufacturingBetavoltaicsSemiconductor materials
To increase total power in a betavoltaic device, it is desirable to have greater radioisotope material and / or semiconductor surface area, rather than greater radioisotope material volume. An example of this invention is a high power density betavoltaic battery. In one example of this invention, tritium is used as a fuel source. In other examples, radioisotopes, such as Nickel-63, Phosphorus-33 or promethium, may be used. The semiconductor used in this invention may include, but is not limited to, Si, GaAs, GaP, GaN, diamond, and SiC. For example (for purposes of illustration / example, only), tritium will be referenced as an exemplary fuel source, and SiC will be referenced as an exemplary semiconductor material. Other variations and examples are also discussed and given.
Owner:WIDETRONIX
Nanoparticles of rare earth oxides
ActiveUS7229600B2Increase volumeLow cost productionMaterial nanotechnologyLanthanum oxide/hydroxidesCeriumScandium
Rare earth compositions comprising nanoparticles, methods of making nanoparticles, and methods of using nanoparticles are described. The compositions of the nanomaterials discussed may include scandium (Sc), yttrium (Y), lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu). The nanoparticles can be used to make organometallics, nitrates, and hydroxides. The nanoparticles can be used in a variety of applications, such as pigments, catalysts, polishing agents, coatings, electroceramics, catalysts, optics, phosphors, and detectors.
Owner:PPG IND OHIO INC
Abradable Compositions and Methods for CMC Shrouds
Coating systems on a surface of a CMC component, such as a CMC shroud, are provided. The coating system can include an environmental barrier coating on the surface of the CMC component and an abradable coating on the environmental barrier coating and defining an external surface opposite of the environmental barrier coating. The abradable coating includes a compound having the formula: Ln2ABO8, where Ln comprises scandium, yttrium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, or mixtures thereof; A comprises Si, Ti, Ge, or a combination thereof; and B comprises Mo, W, or a combination thereof. In one embodiment, the abradable coating has a first coefficient of thermal expansion at an interface with the environmental barrier coating that changes to a second coefficient of thermal expansion at its external surface. Methods are also provided for applying an abradable coating onto a CMC component.
Owner:GENERAL ELECTRIC CO
Method for preparing Ce-Zr based nano rare earth composite oxide
InactiveCN101058443ANo emissionsReduce energy consumptionCatalyst carriersRare earth metal compoundsRare-earth elementCerium
The invention discloses a making method of cerium-zirconium based nanometer composite oxide, which comprises the following steps: adopting low-chlorine zirconium carbonate and low-chlorine cerium carbonate as main raw material; adding rare earth element such as lanthanum, promethium, yttrium, neodymium and so on; proceeding solid-solid reaction to prepare; obtaining the solid solution with cubic crystal. The fresh specific area of cerium-zirconium based nanometer composite oxide is 100-150m2 / g with aged specific surface area at 45-60m2 / g after sintering 2h under 900 deg. c, which simplifies the technical course and equipment with low energy consumption.
Owner:EAST CHINA UNIV OF SCI & TECH
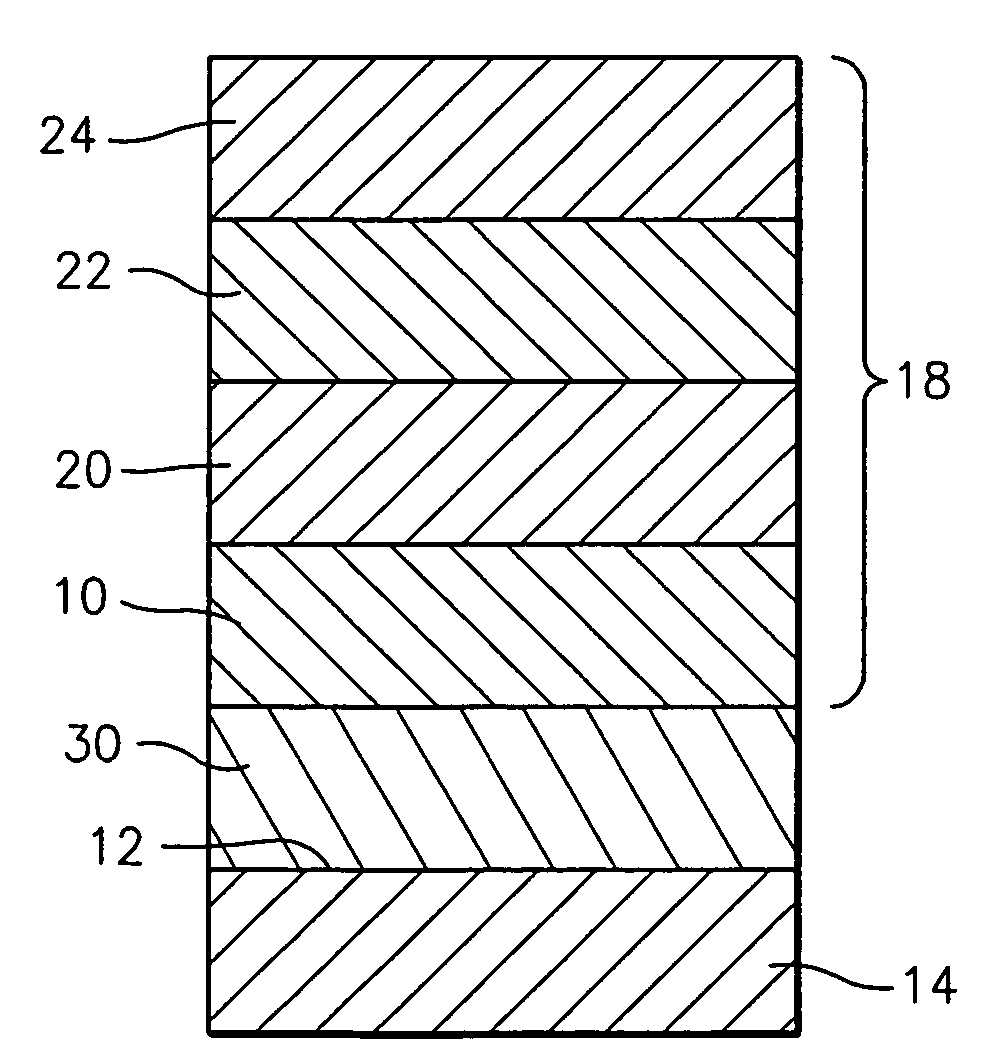
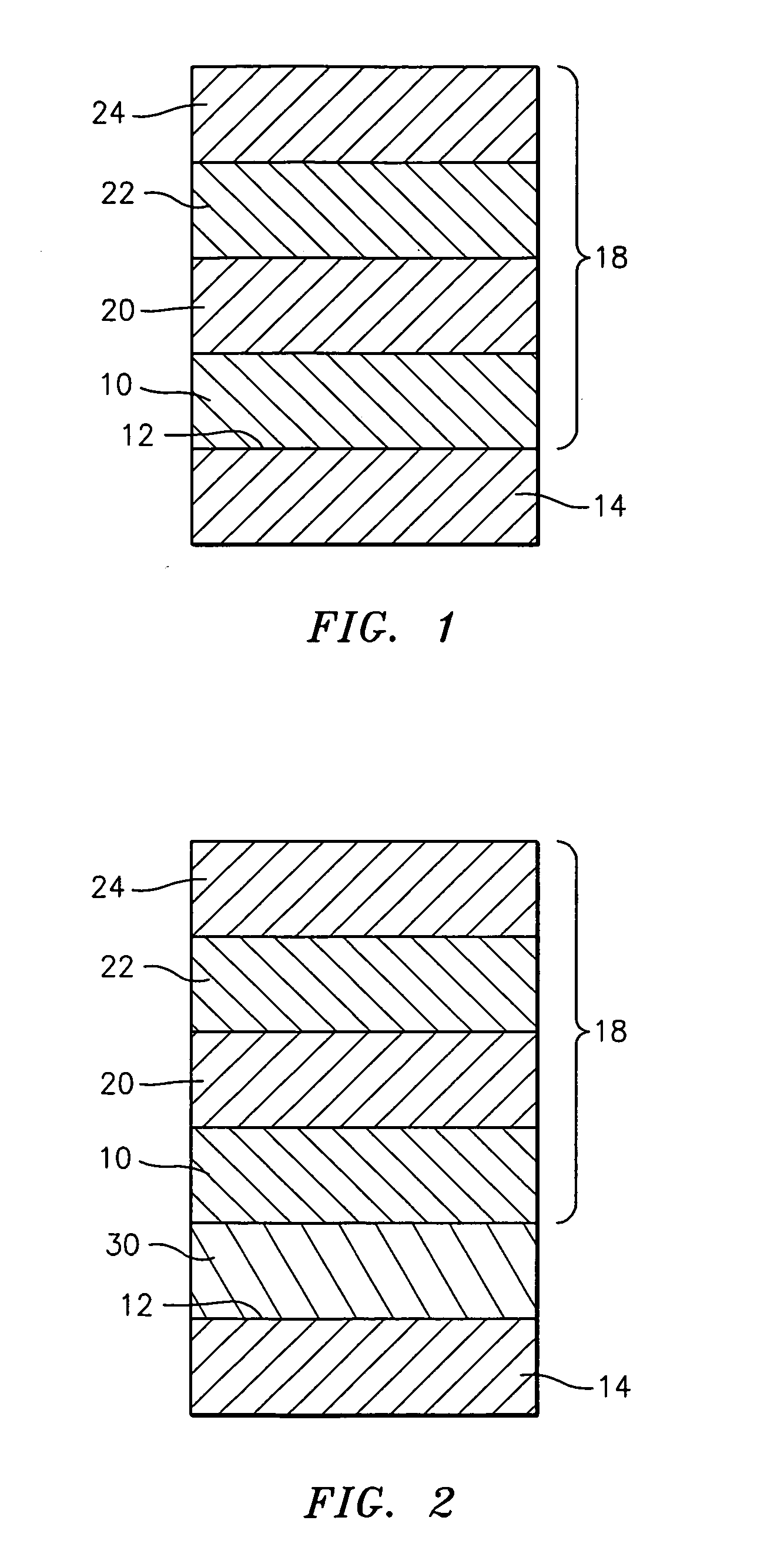
Cmas resistant thermal barrier coating
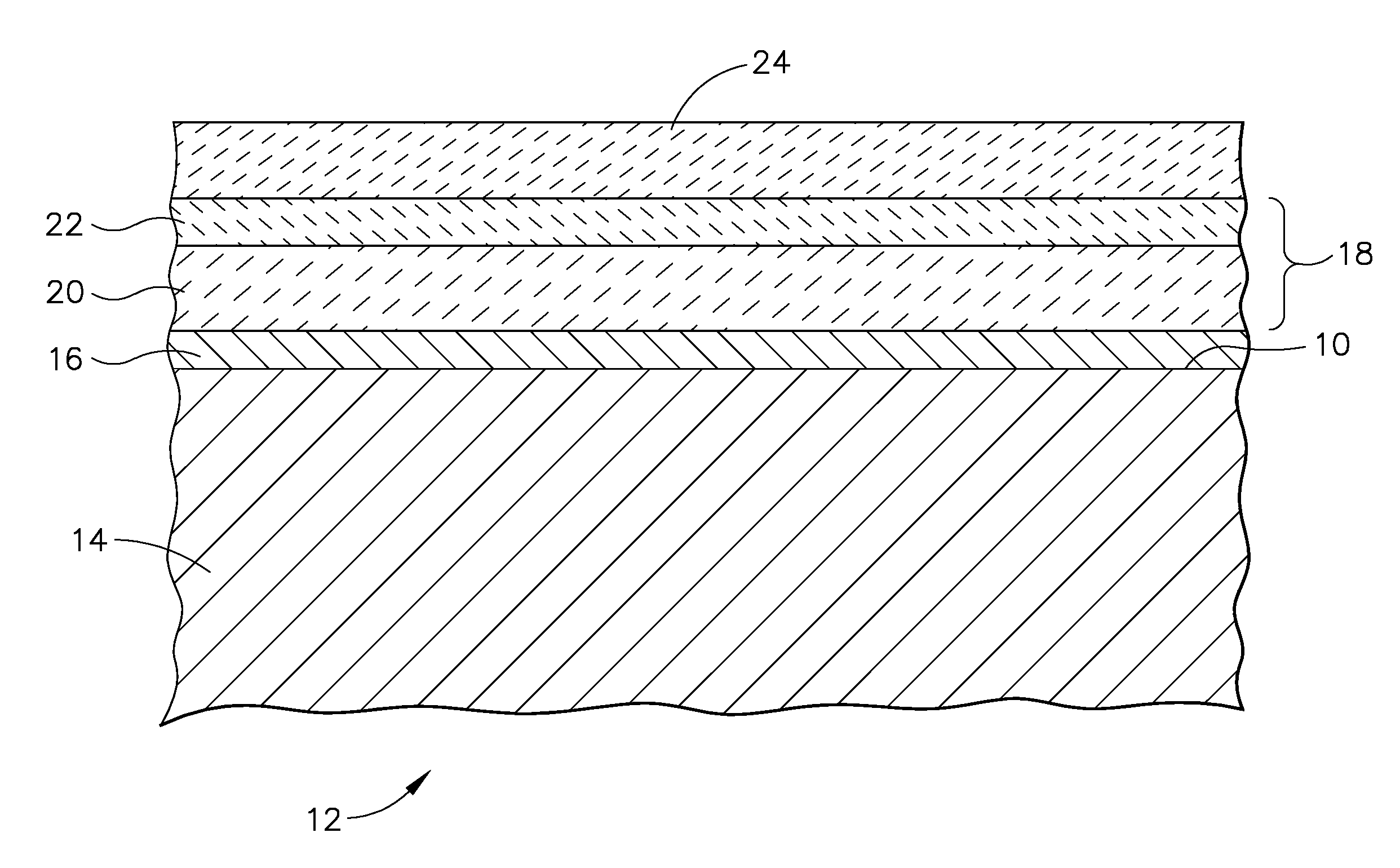
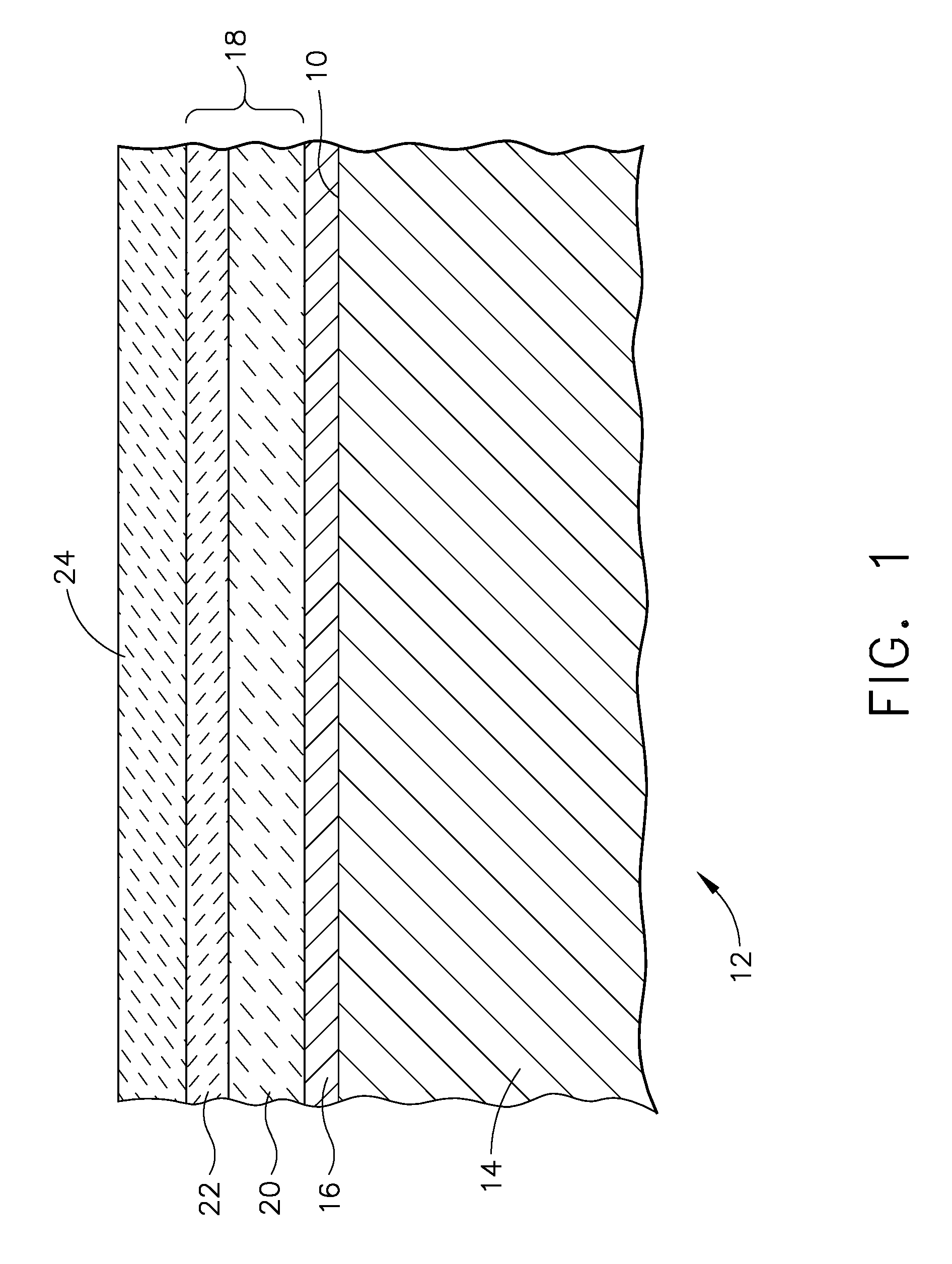
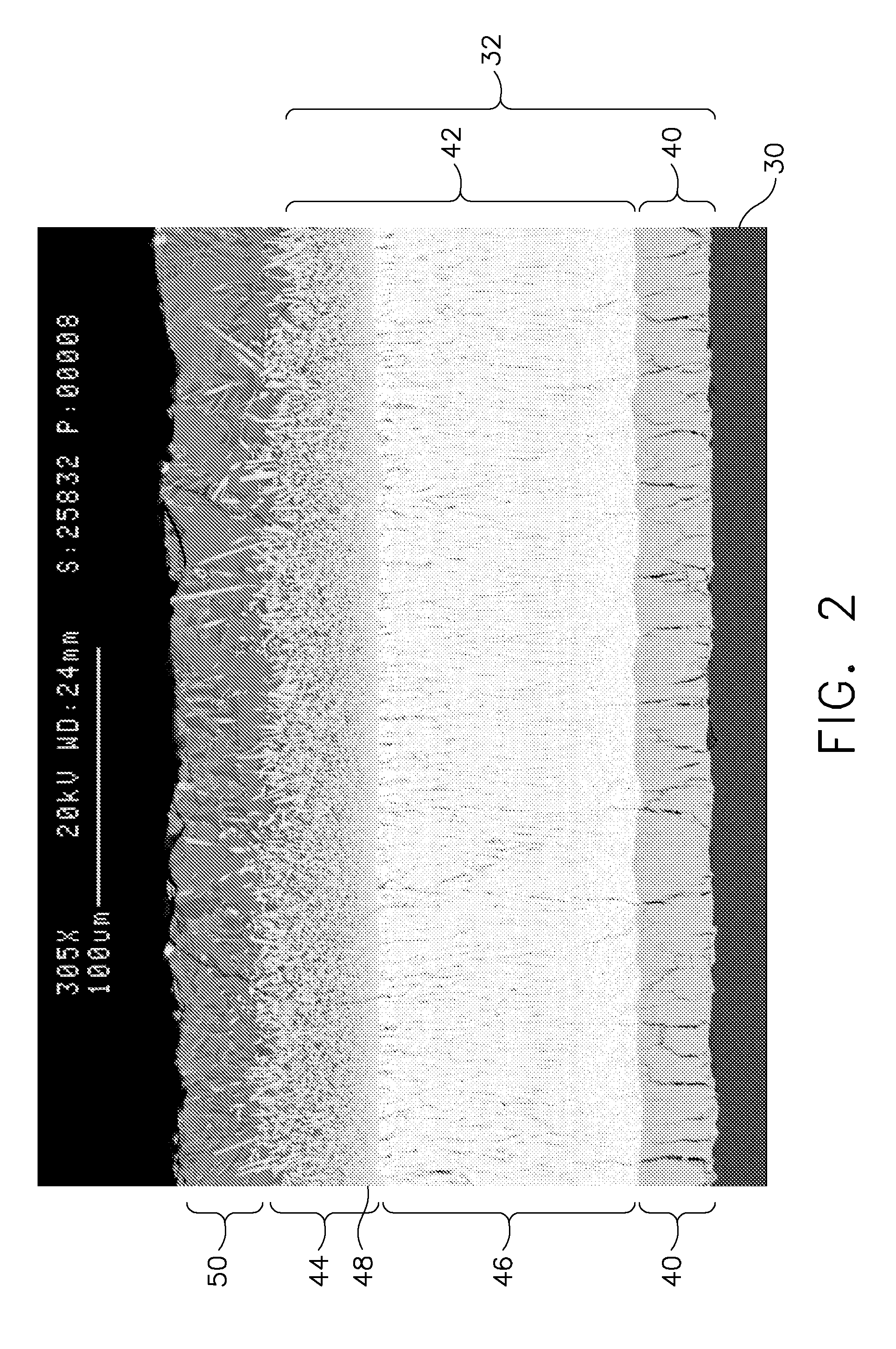
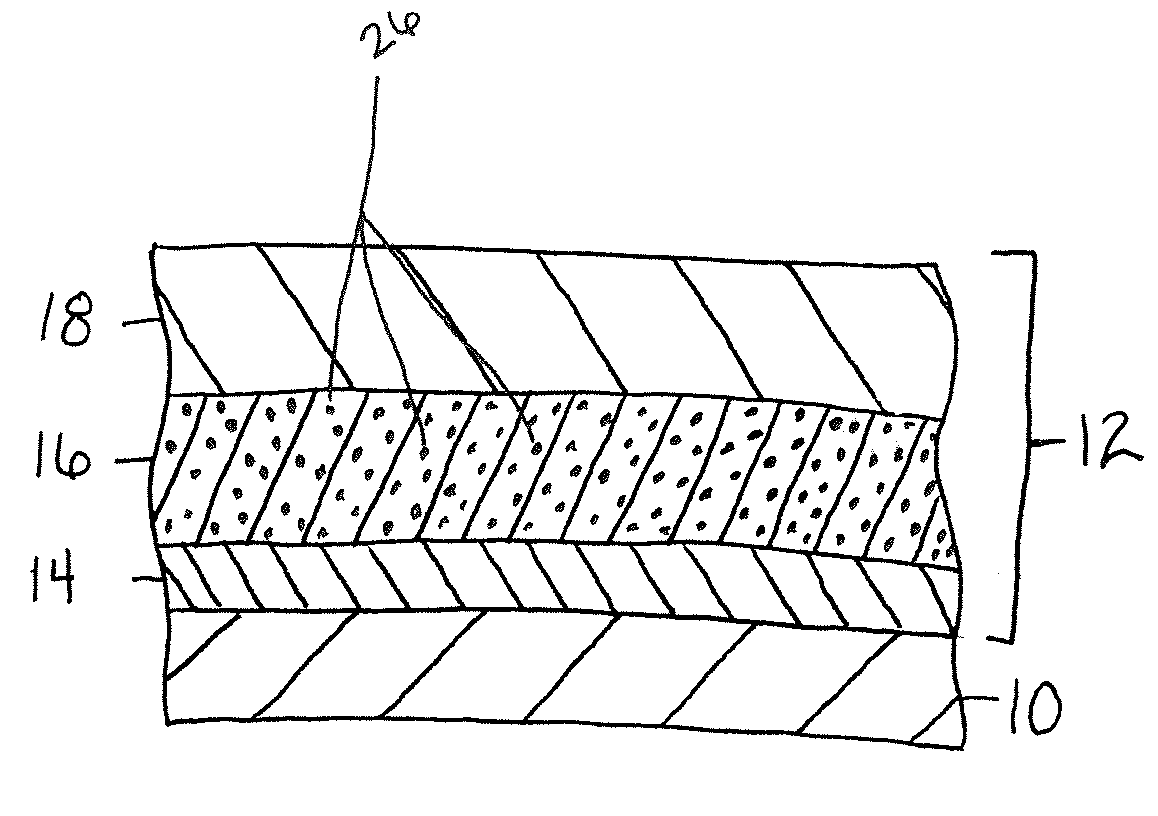
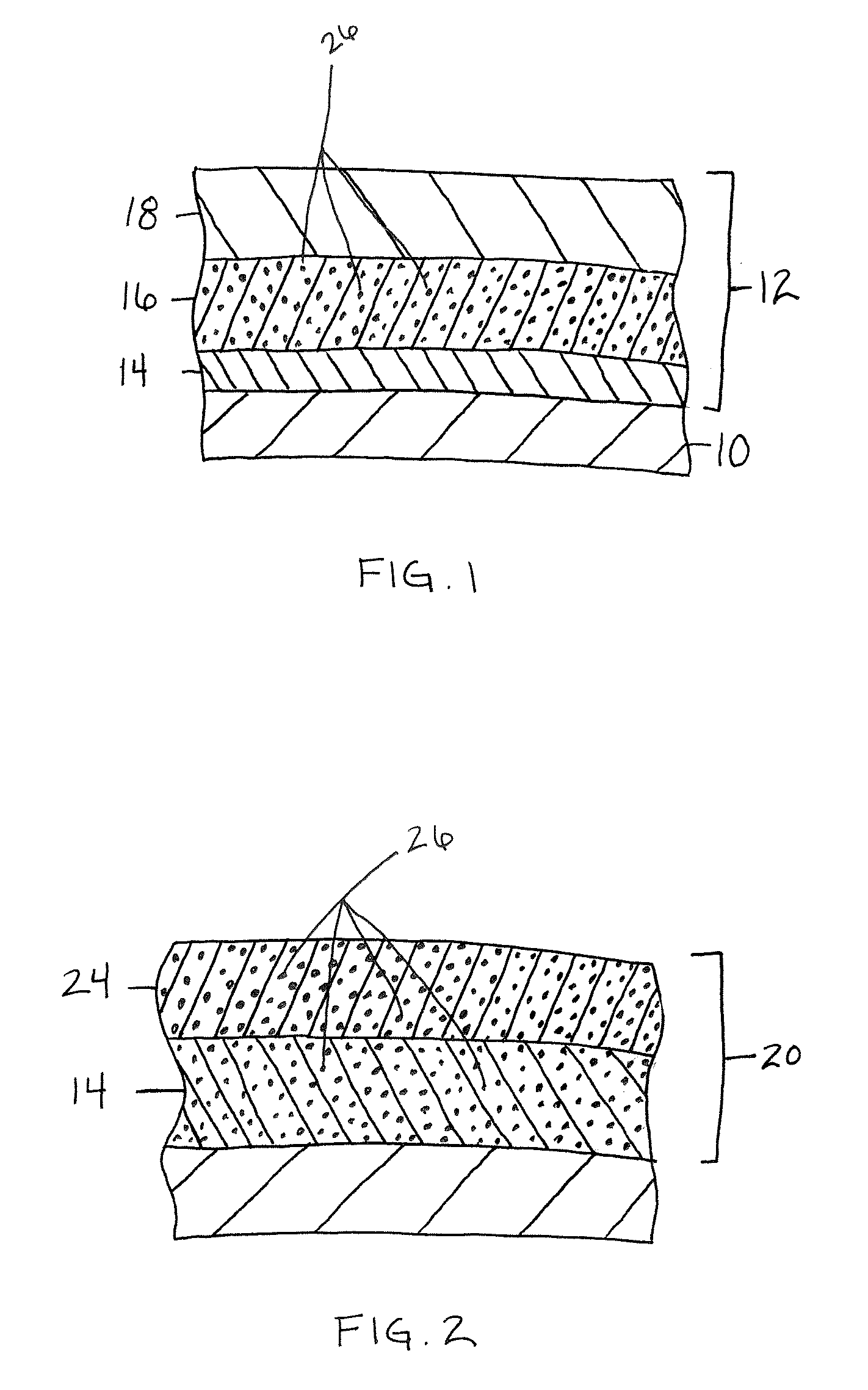
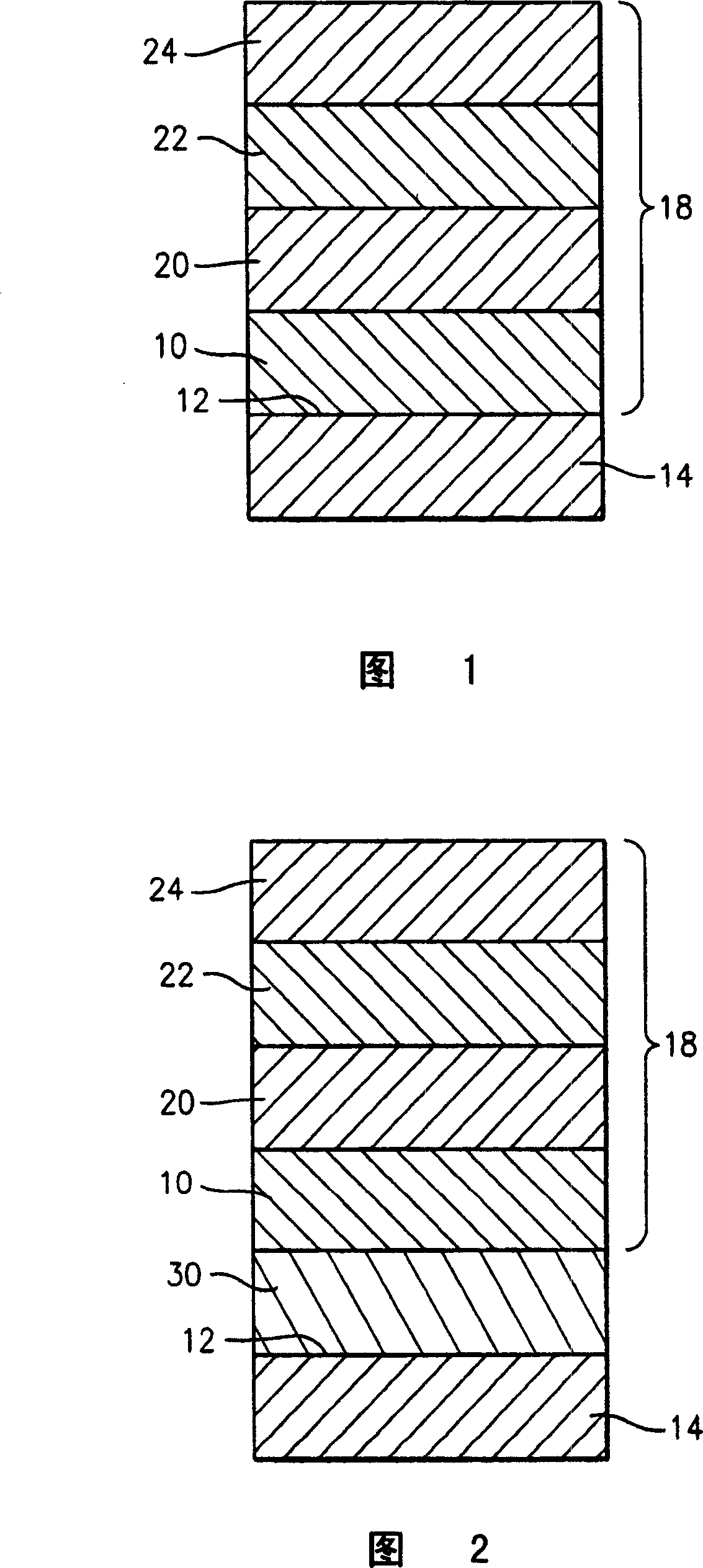
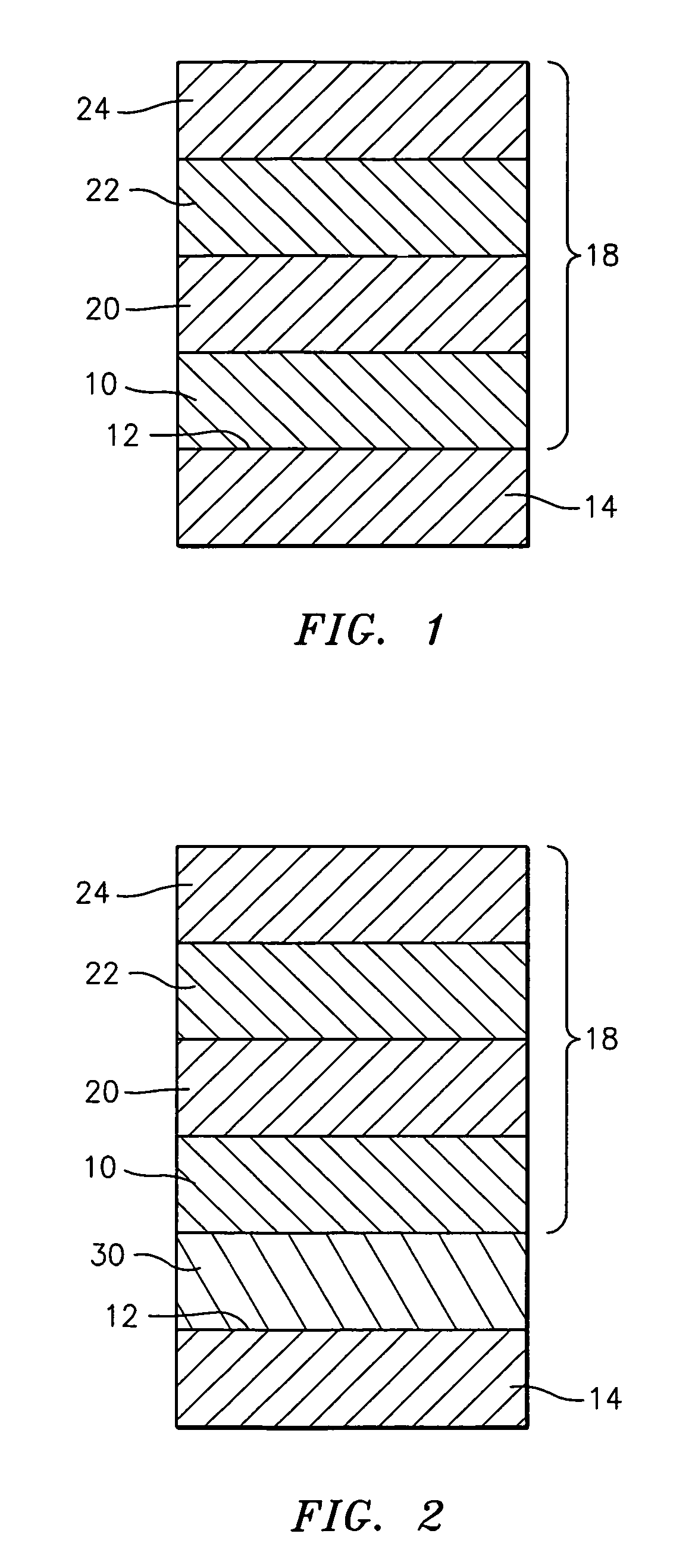
A turbine engine component is provided which has a substrate and a thermal barrier coating (18) applied over the substrate (14). The thermal barrier coating comprises alternating layers (10, 20, 22, 24) of yttria-stabilized zirconia and a molten silicate resistant material. The molten silicate resistant outer layer (24) may be formed from at least one oxide of a material selected from the group consisting of lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, indium, zirconium, hafnium, and titanium or may be formed from a gadolinia-stabilized zirconia. If desired, a metallic bond coat may be present between the substrate and the thermal barrier coating system. A method for forming the thermal barrier coating system of the present invention is described.
Owner:UNITED TECH CORP
Rare earth doped hydroxyfluorapatite monocrystal nanometer wire and preparation method thereof
InactiveCN102249205ALow costThe synthesis process is simpleMaterial nanotechnologyCoatingsNanowirePhosphate
The invention provides a rare earth doped hydroxyfluorapatite monocrystal nanometer wire and a preparation method thereof. The preparation method comprises the following steps: under an alkaline condition, enabling calcium nitrate, phosphate, sodium fluoride and rare earth nitrate to react with one another, thereby acquiring a hydroxyfluorapatite monocrystal nanometer wire, wherein the rare earthnitrate is at least one of lanthanum nitrate, cerous nitrate, praseodymium nitrate, neodymium nitrate, promethium nitrate, samarium nitrate, europium nitrate, gadolinium nitrate, terbium nitrate, dysprosium nitrate, holmium nitrate, erbium nitrate, thulium nitrate, ytterbium nitrate and lutecium nitrate. A monocrystal nanometer wire prepared by using the method can be further processed into a repairing material for biologic sclerous tissues, such as artificial bone, bone cement, and the like, and can be used for the basic research and clinical practice in the fields related to biological materials, organizational engineering, and the like.
Owner:TSINGHUA UNIV
Method and reactor for cracking hydrocarbon
ActiveUS20110295051A1Reduces and eliminates build-upThermal non-catalytic crackingHydrocarbon oil cracking processIndiumCerium
A method for cracking hydrocarbon, comprises: providing steam and hydrocarbon; and feeding steam and hydrocarbon into a reactor accessible to hydrocarbon and comprising a perovskite material of formula AaBbCcDdO3-δ, wherein 0<a<1.2, 0≦b≦1.2, 0.9<a+b≦1.2, 0<c<1.2, 0≦d≦1.2, 0.9<c+d≦1.2, −0.5<δ<0.5; A is selected from calcium, strontium, barium, and any combination thereof; B is selected from lithium, sodium, potassium, rubidium and any combination thereof; C is selected from cerium, zirconium, antimony, praseodymium, titanium, chromium, manganese, ferrum, cobalt, nickel, gallium, tin, terbium and any combination thereof; and D is selected from lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, ebium, thulium, ytterbium, lutetium, scandium, titanium, vanadium, chromium, manganese, ferrum, cobalt, nickel, copper, zinc, yttrium, zirconium, niobium, molybdenum, technetium, ruthenium, rhodium, palladium, silver, cadmium, hafnium, tantalum, tungsten, rhenium, osmium, iridium, platinum, gold, gallium, indium, tin, antimony and any combination thereof.
Owner:BL TECH INC
High-strength steel plate for light goods shelves and forging process thereof
The invention discloses a high-strength steel plate for light goods shelves and a forging process thereof. The high-strength steel plate for the light goods shelves is composed of, by weight percentage, 0.22-0.24% of carbon, 0.32-0.38% of boron, 0.52-0.54% of palladium, 0.02-0.04% of selenium, 0.02-0.04% of arsenic, 3.23-3.25% of rubidium, 0.45-0.48% of vanadium, 5.36-5.39% of titanium, 6.63-6.67% of copper, 4.57-4.59% of tin, 8.7-8.9% of chromium, 0.06-0.08% of molybdenum, 8.78-8.79% of nickel, 0.36-0.38% of barium, 3.42-3.44% of magnesium, 3.21-3.23% of neodymium, 0.25-0.35% of promethium, 0.45-0.48% of praseodymium, 1.2-1.5% of holmium, 0.04-0.06% of dysprosium, balancing Fe and inevitable impurities.
Owner:SUZHOU KESHENG STORAGE & LOGISTICS EQUIP
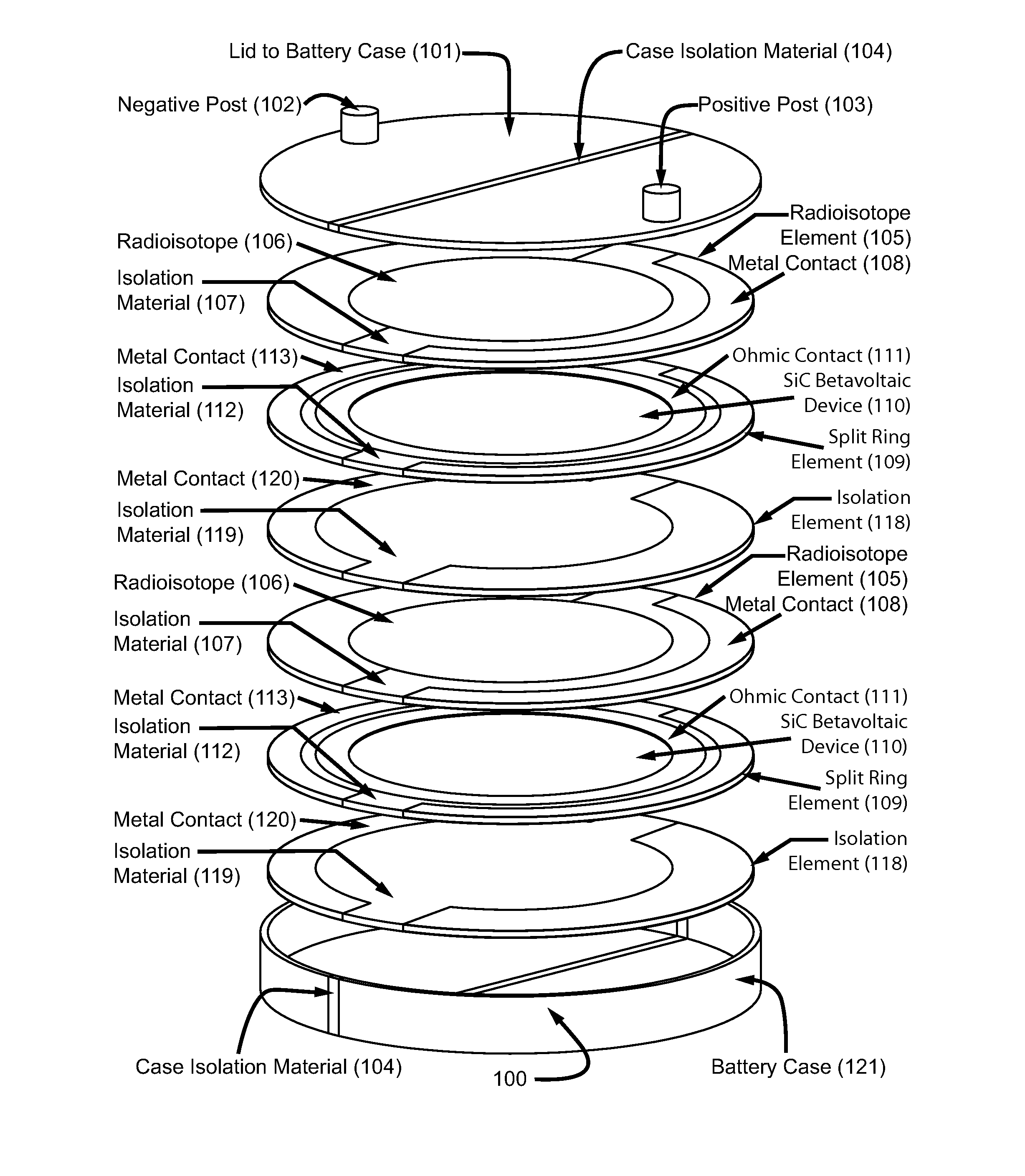
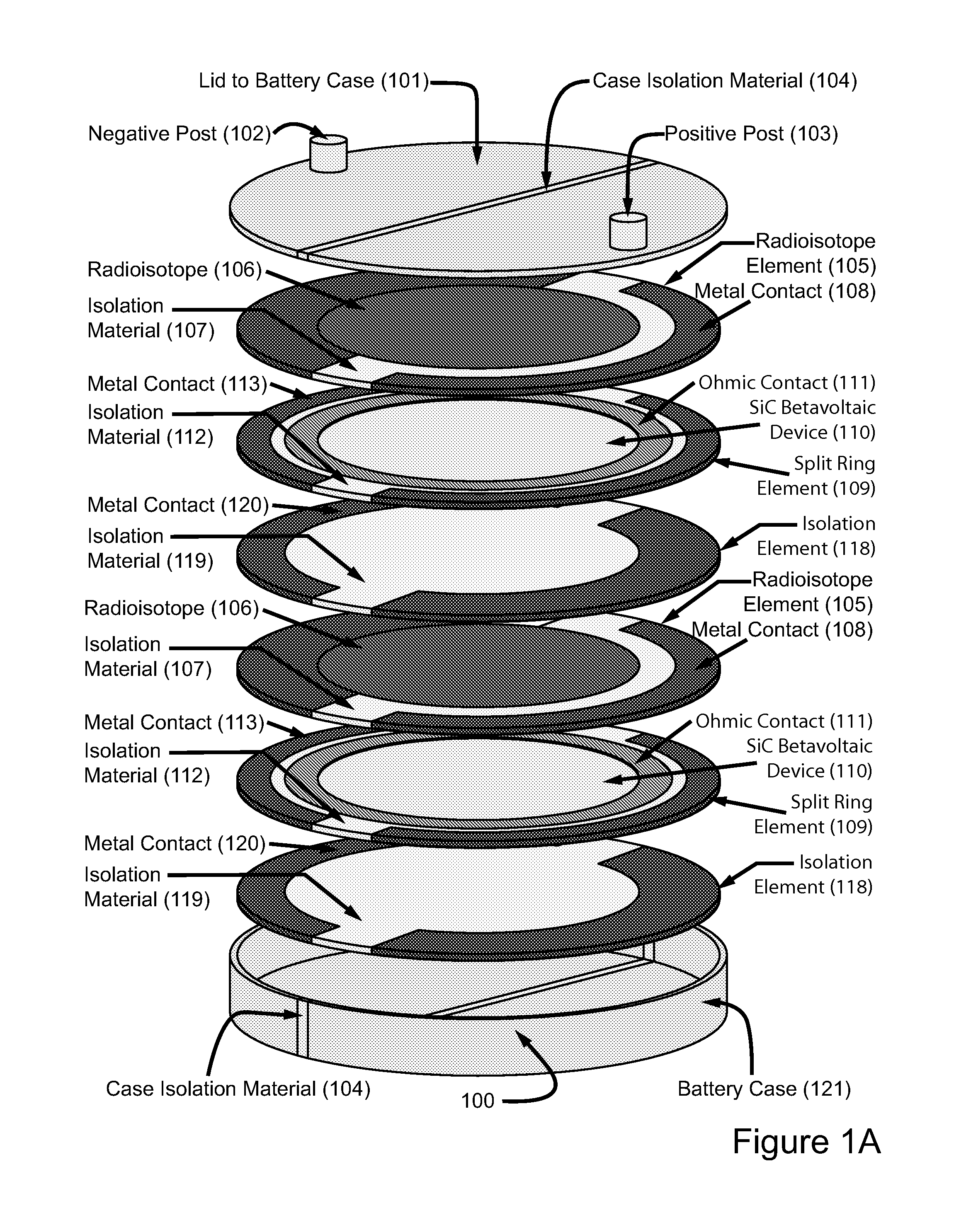
Betavoltaic battery with a shallow junction and a method for making same
ActiveUS8017412B2Reduce recombinationSemiconductor/solid-state device manufacturingRadiation electrical energyBetavoltaicsHigh energy
This is a novel SiC betavoltaic device (as an example) which comprises one or more “ultra shallow” P+N− SiC junctions and a pillared or planar device surface (as an example). Junctions are deemed “ultra shallow”, since the thin junction layer (which is proximal to the device's radioactive source) is only 300 nm to 5 nm thick (as an example). In one example, tritium is used as a fuel source. In other embodiments, radioisotopes (such as Nickel-63, promethium or phosphorus-33) may be used. Low energy beta sources, such as tritium, emit low energy beta-electrons that penetrate very shallow distances (as shallow as 5 nm) in semiconductors, including SiC, and can result in electron-hole pair creation near the surface of a semiconductor device rather than pair creation in a device's depletion region. By contrast, as a high energy electron penetrates a semiconductor device surface, such as a diode surface, it produces electron hole-pairs that can be collected at (by drift) and near (by diffusion) the depletion region of the device. This is a betavoltaic device, made of ultra-shallow junctions, which allows such penetration of emitted lower energy electrons, thus, reducing or eliminating losses through electron-hole pair recombination at the surface.
Owner:WIDETRONIX
CMAS resistant thermal barrier coating
A turbine engine component is provided which has a substrate and a thermal barrier coating applied over the substrate. The thermal barrier coating comprises alternating layers of yttria-stabilized zirconia and a molten silicate resistant material. The molten silicate resistant outer layer may be formed from at least one oxide of a material selected from the group consisting of lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, indium, zirconium, hafnium, and titanium or may be formed from a gadolinia-stabilized zirconia. If desired, a metallic bond coat may be present between the substrate and the thermal barrier coating system. A method for forming the thermal barrier coating system of the present invention is described.
Owner:RTX CORP
Method for producing a titanium-base alloy having an oxide dispersion therein
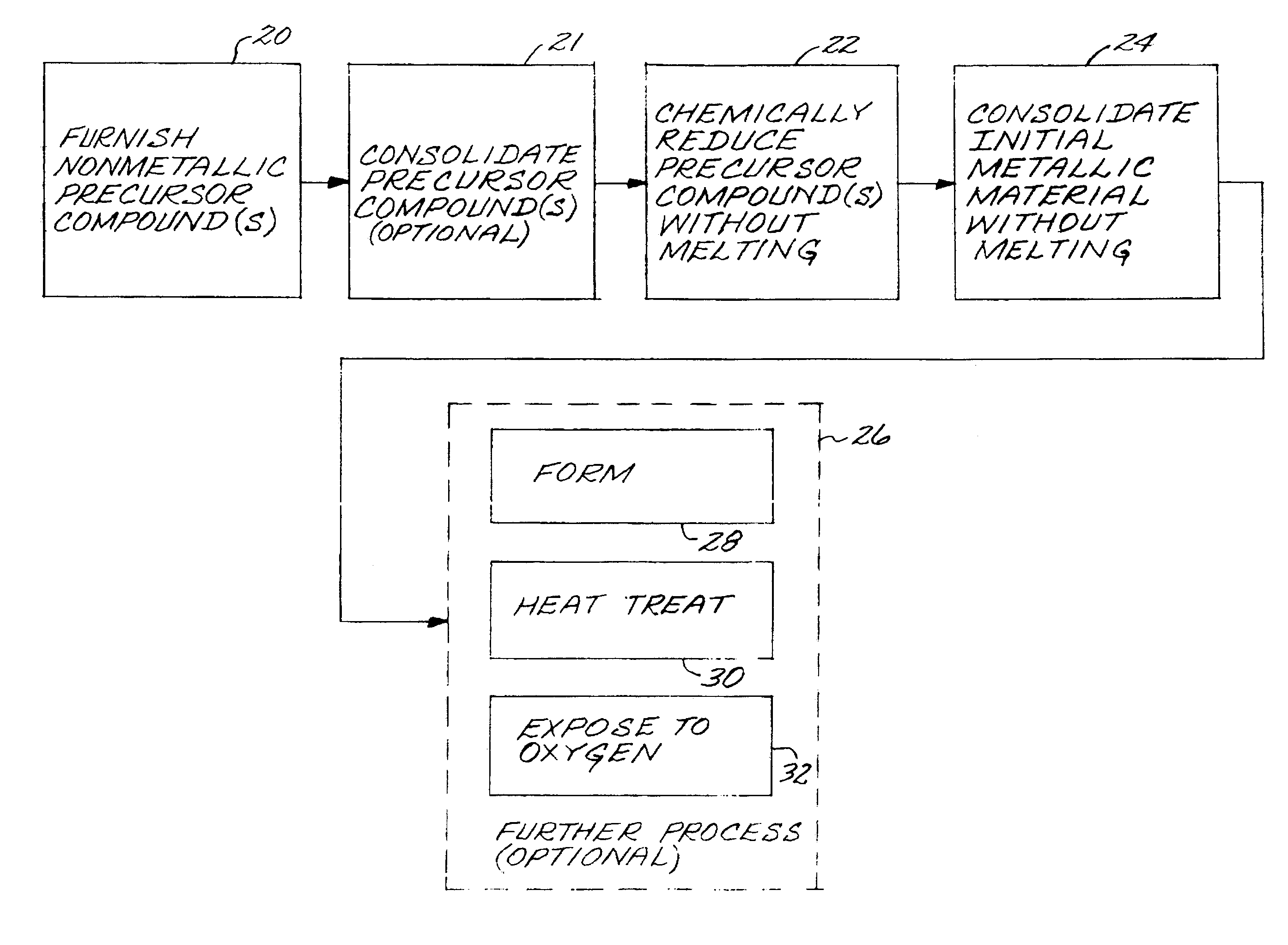
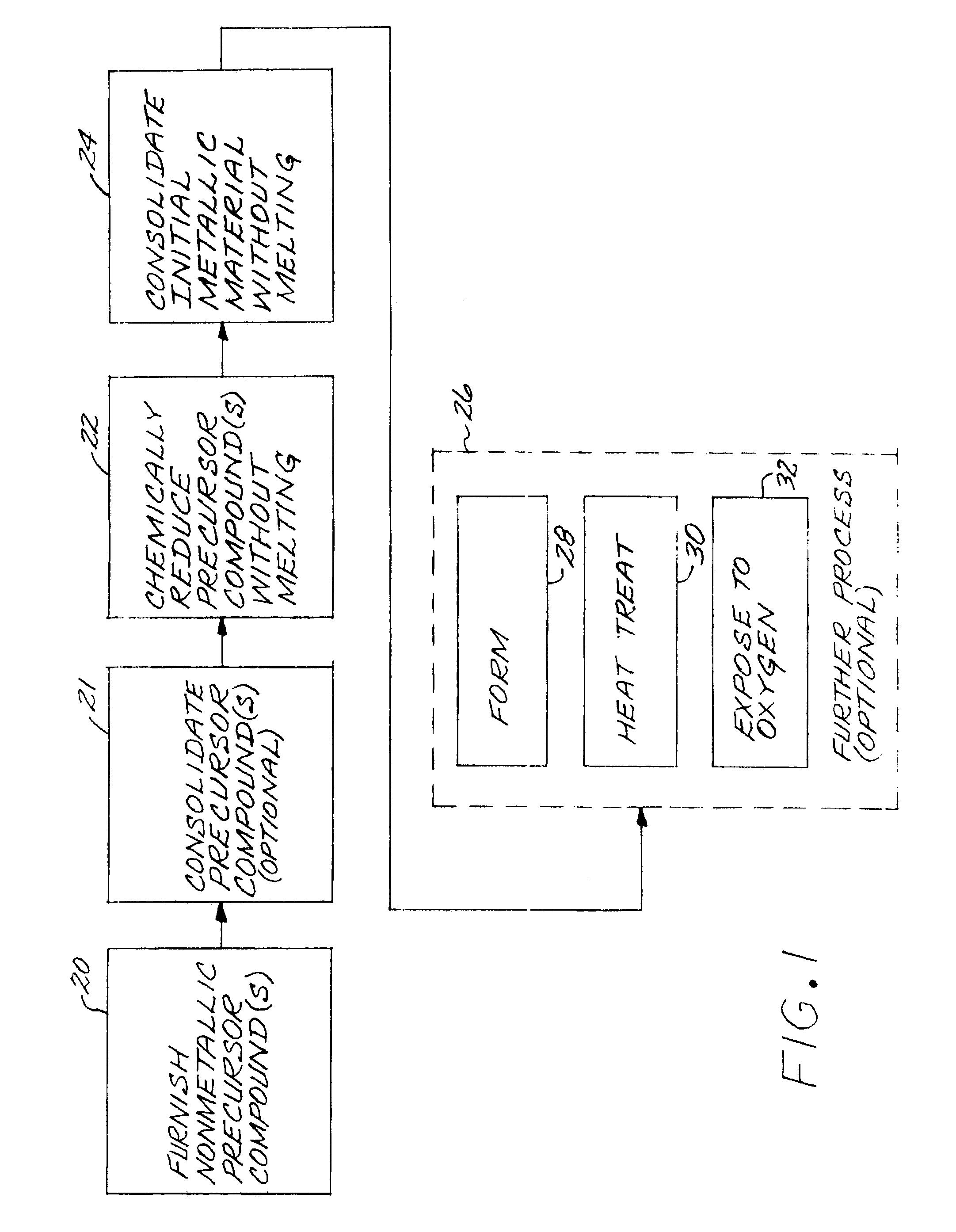
A metallic article is prepared by first furnishing at least one nonmetallic precursor compound, wherein all of the nonmetallic precursor compounds collectively containing the constituent elements of the metallic article in their respective constituent-element proportions. The constituent elements together form a titanium-base alloy having a stable-oxide-forming additive element therein, such as magnesium, calcium, scandium, yttrium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium, and mixtures thereof. The stable-oxide-forming additive element forms a stable oxide in a titanium-based alloy. At least one additive element is present at a level greater than its room-temperature solid solubility limit in the titanium-base alloy. The precursor compounds are chemically reduced to produce an alloy material, without melting the alloy material. The alloy material may be consolidated. The alloy material, or consolidated metallic article, is thereafter desirably exposed to an oxygen-containing environment at a temperature greater than room temperature.
Owner:GENERAL ELECTRIC CO
Rare earth rubber vulcanization accelerator, and preparation method and application thereof
ActiveCN101962452ASignificant vulcanization acceleration effectAccelerated vulcanization acceleration effectOrganic chemistryRare-earth elementVulcanization
The invention discloses a rare earth rubber vulcanization accelerator, and a preparation method and application thereof. The accelerator is prepared by compounding rare earth ions and carboxylic acid substituted dithiocarbamate, wherein the rare earth ions may be one or the mixture of more than one of 14 rare earth elements, except promethium which is a radioactive element, with the atomic numbers of 57 to 71 in the periodic table of elements. The rare earth vulcanization accelerator of the invention is added into a rubber formula without adding the vulcanization active agents of stearic acid and zinc oxide to achieve good vulcanization effect and prepare a rubber composite material with high comprehensive mechanical properties which are remarkably superior to those of the accelerator zinc diethyl dithiocarbamate (ZDC) commonly used in industry at present in the same proportion, is a carbamate rare earth compound of primary amine, does not produce nitrite in a vulcanization process, and is environmentally-friendly.
Owner:SOUTH CHINA UNIV OF TECH
Rear earth-contained high-permeability Ni-Fe soft magnetic alloy, preparation method and purposes thereof
ActiveCN102723158AGood alloying effectImprove the original microstructureMagnetic materialsCeriumMechanical property
The invention belongs to the field of metallic functional materials, and particularly relates to a rear earth-contained high-permeability Ni-Fe soft magnetic alloy, a preparation method and purposes thereof. The alloy as weight percentage comprises the following components: 40-83 percent nickel (Ni), 0.38-0.56 percent manganese (Mn), 0.24-4.0 percent silicon (Si), 3.0-5.7 percent molybdenum (Mo), 2.0-8.2 percent copper (Cu), 0.02-15 percent rare earth element Re in lanthanide series, carbon less than 0.015 percent, phosphorus less than 0.015 percent and sulfur less than 0.015 percent, as well as iron and inevitable impurities, which occupy the less percentage, wherein the Re is at least one of the elements among lanthanum (La), cerium (Ce), seodymium (Pr), neodymium (Nd), promethium (Pm), Samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm),Ytterbium (Yb), lutetium (Lu), Scandium (Sc) and yttrium (Y) which are the rare earth elements in the lanthanide series. According to the invention, the suitable rare earth elements are added and the components of the alloy are reasonably adjusted, the Ni-Fe alloy has good alloying effect and improves the original microscopic structure, thereby having improved integrated magnetic property and mechanical property.
Owner:昀诺能源科技(江苏)有限公司
Wear-resistant metal coating for hydraulic cylinder and preparation method of wear-resistant metal coating
InactiveCN104789906AHigh strengthImprove toughnessHeat inorganic powder coatingTitanium zirconiumManganese
The invention discloses a wear-resistant metal coating for a hydraulic cylinder and a preparation method of the wear-resistant metal coating. The wear-resistant metal coating for the hydraulic cylinder comprises the following components in mass percent: 0.25-0.27% of carbon, 1.26-1.28% of magnesium, 2.25-2.28% of silicon, 1.23-1.25% of vanadium, 17.33-17.35% of nickel, 0.13-0.15% of manganese, 0.22-0.25% of scandium, 2.84-2.86% of copper, 2.56-2.58% of selenium, 0.72-0.74% of tungsten, 2.72-2.75% of titanium, 0.42-0.45% of zirconium, 2.45-2.48% of lanthanum, 3.54-3.56% of neodymium, 2.33-2.35% of promethium, 1.22-1.24% of europium, 4.42-4.45% of gadolinium, 3.21-3.24% of an auxiliary, and the balance of iron.
Owner:SUZHOU JIN YUAN OIL MACHINERY
Durable reactive thermal barrier coatings
ActiveUS7662489B2Reduces sand related distressBlade accessoriesVacuum evaporation coatingIndiumLutetium
A turbine engine component is provided which has a substrate and a thermal barrier coating applied over the substrate. The thermal barrier coating comprises at least one layer of a first material selected from the group consisting of a zirconate, a hafnate, a titanate, and mixtures thereof, which first material has been mixed with, and contains, from about 25 to 99 wt % of at least one oxide. The at least one oxide comprises at least one oxide of a material selected from the group consisting of lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, scandium, indium, and yttrium. If desired, a metallic bond coat may be present between the substrate and the thermal barrier coating system. A method for forming the thermal barrier coating system of the present invention is described.
Owner:RTX CORP
Method and reactor for cracking hydrocarbon
ActiveUS9499747B2Reduces and eliminates build-upThermal non-catalytic crackingHydrocarbon by dehydrogenationIndiumHafnium
A method for cracking hydrocarbon, comprises: providing steam and hydrocarbon; and feeding steam and hydrocarbon into a reactor accessible to hydrocarbon and comprising a perovskite material of formula AaBbCcDdO3-δ, wherein 0<a<1.2, 0≦b≦1.2, 0.9<a+b≦1.2, 0<c<1.2, 0≦d≦1.2, 0.9<c+d≦1.2, −0.5<δ<0.5; A is selected from calcium, strontium, barium, and any combination thereof; B is selected from lithium, sodium, potassium, rubidium and any combination thereof; C is selected from cerium, zirconium, antimony, praseodymium, titanium, chromium, manganese, ferrum, cobalt, nickel, gallium, tin, terbium and any combination thereof; and D is selected from lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, ebium, thulium, ytterbium, lutetium, scandium, titanium, vanadium, chromium, manganese, ferrum, cobalt, nickel, copper, zinc, yttrium, zirconium, niobium, molybdenum, technetium, ruthenium, rhodium, palladium, silver, cadmium, hafnium, tantalum, tungsten, rhenium, osmium, iridium, platinum, gold, gallium, indium, tin, antimony and any combination thereof.
Owner:BL TECH INC
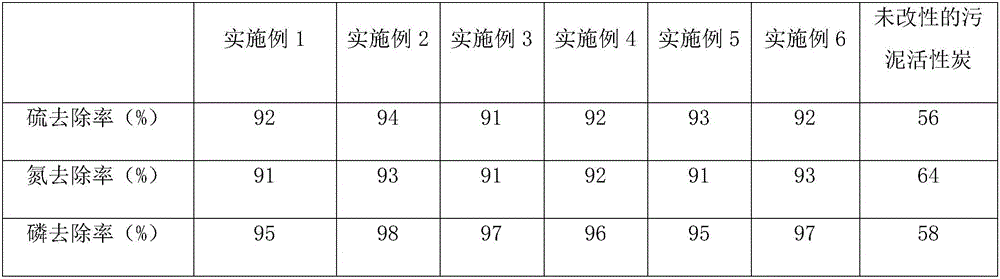
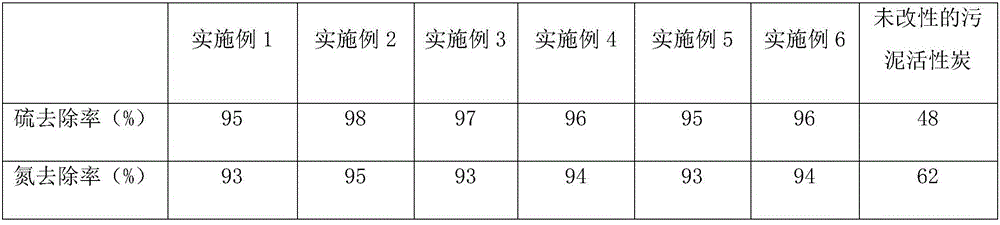
Rare metal-based sludge activated carbon and application thereof in removal of sulfur, ammonia and phosphorus
ActiveCN106345411AImprove adsorption capacityAchieve separationOther chemical processesWater contaminantsActivated sludgePorosity
The invention provides a rare metal-based sludge activated carbon and application thereof in the removal of sulfur, ammonia and phosphorus. The rare metal-based sludge activated carbon includes sludge activated carbon and metal elements such as manganese, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium and the like, and is specifically prepared by a method comprising the following steps: mixing pyrolusite powder with activated sludge, adding a zinc chloride compounded activating agent into the mixture, activating the mixture at a high temperature, carbonizing the mixture to obtain pyrolusite compounded activated sludge, mixing the activated sludge with rare earth oxide powder and a chelating agent after the activated sludge is modified by potassium hydroxide solution, heating the mixture in a microwave oven, carbonizing the mixture to obtain rare earth compounded activated sludge, and mixing the compounded activated sludge to obtain a product. The rare metal-based sludge activated carbon prepared by the method has the advantages of high activity, high porosity, uniform pore size distribution, high adsorption capacity, capability of chelating a plurality of metals and microorganisms, high capacity of adsorbing sulfur, ammonia and phosphorus and high rate of removing the sulfur, the ammonia and the phosphorus in sewage and waste.
Owner:徐海燕
Anticorrosion coating for metal surface and preparation method of anticorrosion coating
InactiveCN104789958AHigh strengthImprove corrosion resistanceHeat inorganic powder coatingNiobiumManganese
The invention discloses an anticorrosion coating for a metal surface and a preparation method of the anticorrosion coating. The anticorrosion coating comprises the following components in mass percent: 0.23-0.25% of carbon, 4.62-4.65% of manganese, 7.52-7.58% of chromium, 6.43-6.45% of vanadium, 8.52-8.55% of nickel, 0.51-0.55% of strontium, 1.24-1.27% of niobium, 1.42-1.45% of molybdenum, 2.22-2.24% of zirconium, 0.25-0.29% of tungsten, 4.32-4.35% of zinc, 3.45-3.48% of lanthanum, 12.27-12.29% of neodymium, 5.43-5.45% of promethium, 2.2-2.4% of gadolinium, 3.62-3.65% of terbium, 3.5-3.8% of an auxiliary, and the balance of iron.
Owner:SUZHOU TONGMING MACHINERY
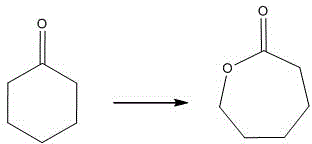
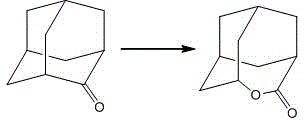
Solid acid catalyst as well as preparation method and application of solid acid catalyst
InactiveCN104588049AEasy to prepareSuitable for industrial mass productionPhysical/chemical process catalystsOrganic chemistryPtru catalystLutetium
The invention provides a solid acid catalyst as well as a preparation method and an application of the solid acid catalyst. The catalyst is applied to the Baeyer-Villiger oxidation reaction to catalyze the conversion of cyclic ketones into lactones; the catalyst has the advantages of high active site utilization rate, good selectivity, good stability, and easy recycling. The solid acid catalyst is composed of two parts, namely the active ingredient of the catalyst and a carrier, wherein the carrier is zirconium hydrogen phosphate nanosheet; the active ingredient is one or the combination of several of lanthanum, yttrium, aluminum, neodymium, cerium, chromium, zinc, indium, tin, scandium, praseodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium metal ions; the molar ratio of the metal component to the zirconium hydrogen phosphate in the solid acid catalyst is (1: 5) to (5: 1).
Owner:NANJING UNIV OF INFORMATION SCI & TECH
Process for the preparation of promoted calcium-aluminate supported catalysts
A promoted calcium-alumina supported reforming catalyst that is particularly useful for reforming reactions where low H2 / CO ratio synthesis gas, such as less than 2.3 is generated directly is disclosed. The catalyst comprises from about 25 wt % to about 98 wt % alumina, from about 0.5 wt % to about 35 wt % calcium oxide, from about 0.01 wt % to about 35 wt % of a promoter, and from about 0.05 wt % to about 30 wt % of an active metal. The promoter is selected from the group consisting of titanium, zirconium, yttrium, niobium, elements of the lanthanum-series, such as, without limitation, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, ytterbium, and combinations thereof. The active metal is selected from the group consisting of nickel, cobalt, rhodium, ruthenium, palladium, platinum, iridium and combinations thereof as active metal, wherein the calcium oxide is combined with the alumina to form aluminum-rich calcium aluminates.
Owner:CLARIANT INT LTD
Tin oxide exhaust catalyst supports and catalysts stable at high temperatures
InactiveUS7056856B2Low costImprove efficiencyInternal combustion piston enginesDispersed particle separationCeriumInternal combustion engine
A tin-oxide based three-way catalytic material employing precious metals that is stable at exhaust gas temperatures of internal combustion engines when the tin oxide lattice includes any of several rare earth oxide stabilizers in the lanthanide series. The rare-earth elements are the 15 lanthanide elements with atomic numbers 57 through 71 that are in Group IIIA of the Periodic Table: lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium. Such tin-oxide based catalytic materials may also beneficially include zirconium oxide and / or yttrium oxide. The invention is useful in providing three-way catalytic exhaust converters for internal combustion engines.
Owner:AIRFLOW CATALYST SYST
High-melt-strength polypropylene material, and preparation method and application thereof
The invention discloses a high-melt-strength polypropylene material, and a preparation method and application thereof. The polypropylene material is prepared from 65-99.79 parts of polypropylene, 0.01-10 parts of initiator, 0.1-10 parts of polyfunctional group monomer, 0.1-5 parts of free radical stabilizer and 0-10 parts of inert processing assistant, wherein the free radical stabilizer is a lanthanide rare-earth oxide, carboxylate of lanthanide rare-earth element, naphthenate complex of lanthanide rare-earth element or dithiocarbamic acid rare-earth compound. The lanthanide rare-earth element does not include promethium. The proportion of the polypropylene, initiator and polyfunctional group monomer is controlled, the grafting reaction time is prolonged, and the rare-earth substances are utilized to inhibit the degradation of the polypropylene and prolong the service life of the free radical, thereby obtaining long-branched-chain high-melt-strength polypropylene with stable performance and enhancing the service performance of the conventional polypropylene. The method disclosed by the invention is easy to operate and convenient to control, and can easily implement industrial production.
Owner:KINGFA SCI & TECH CO LTD +2
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com